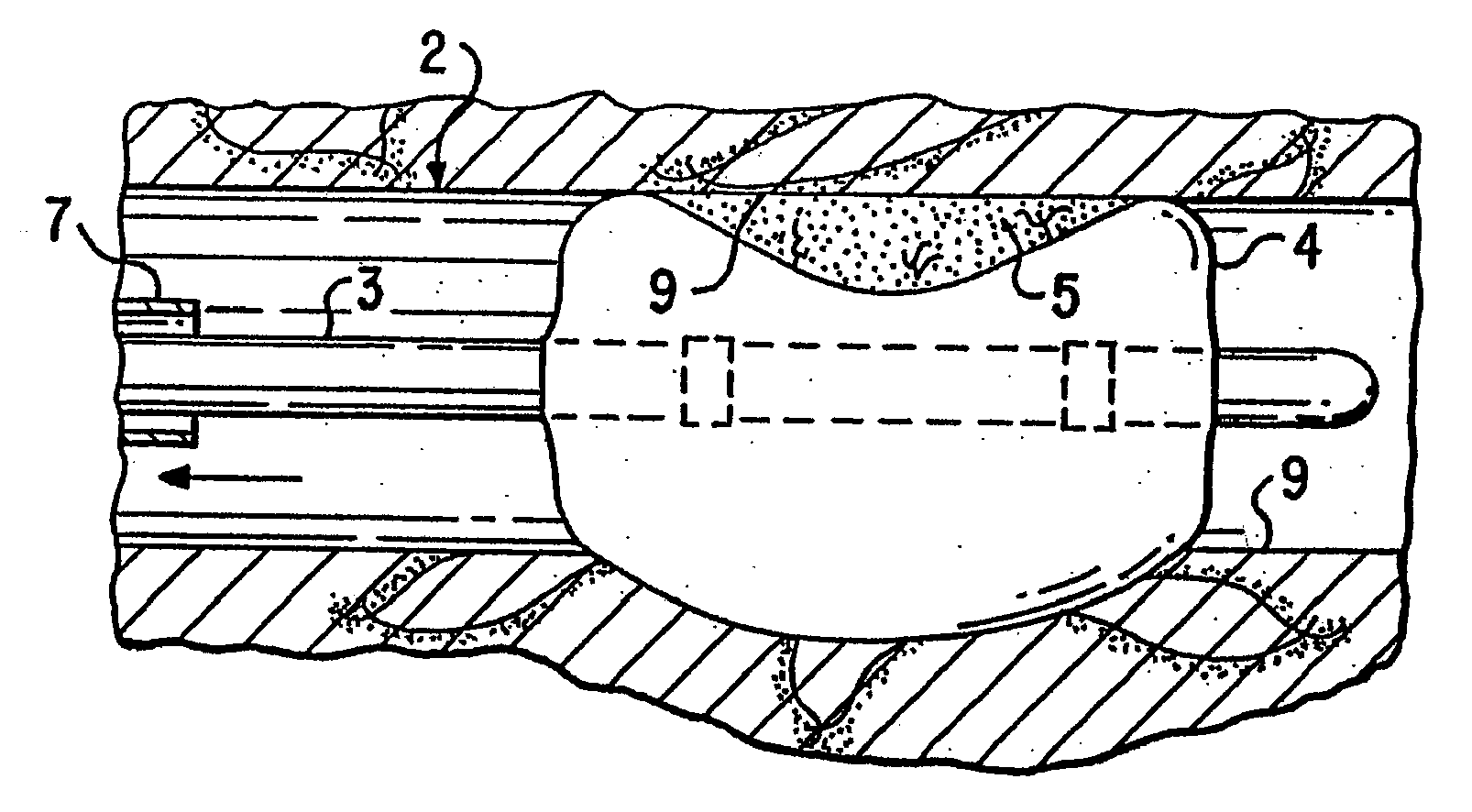
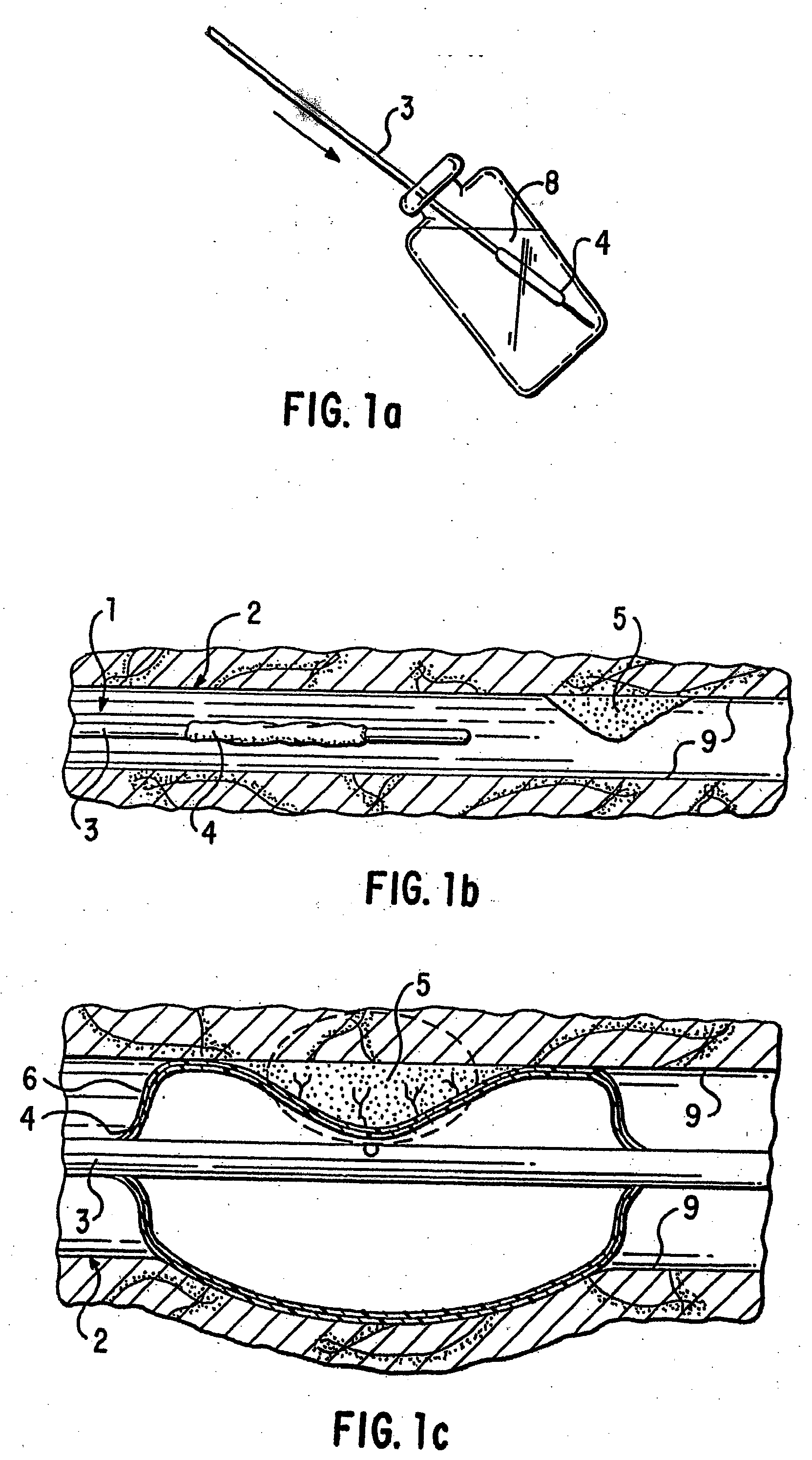
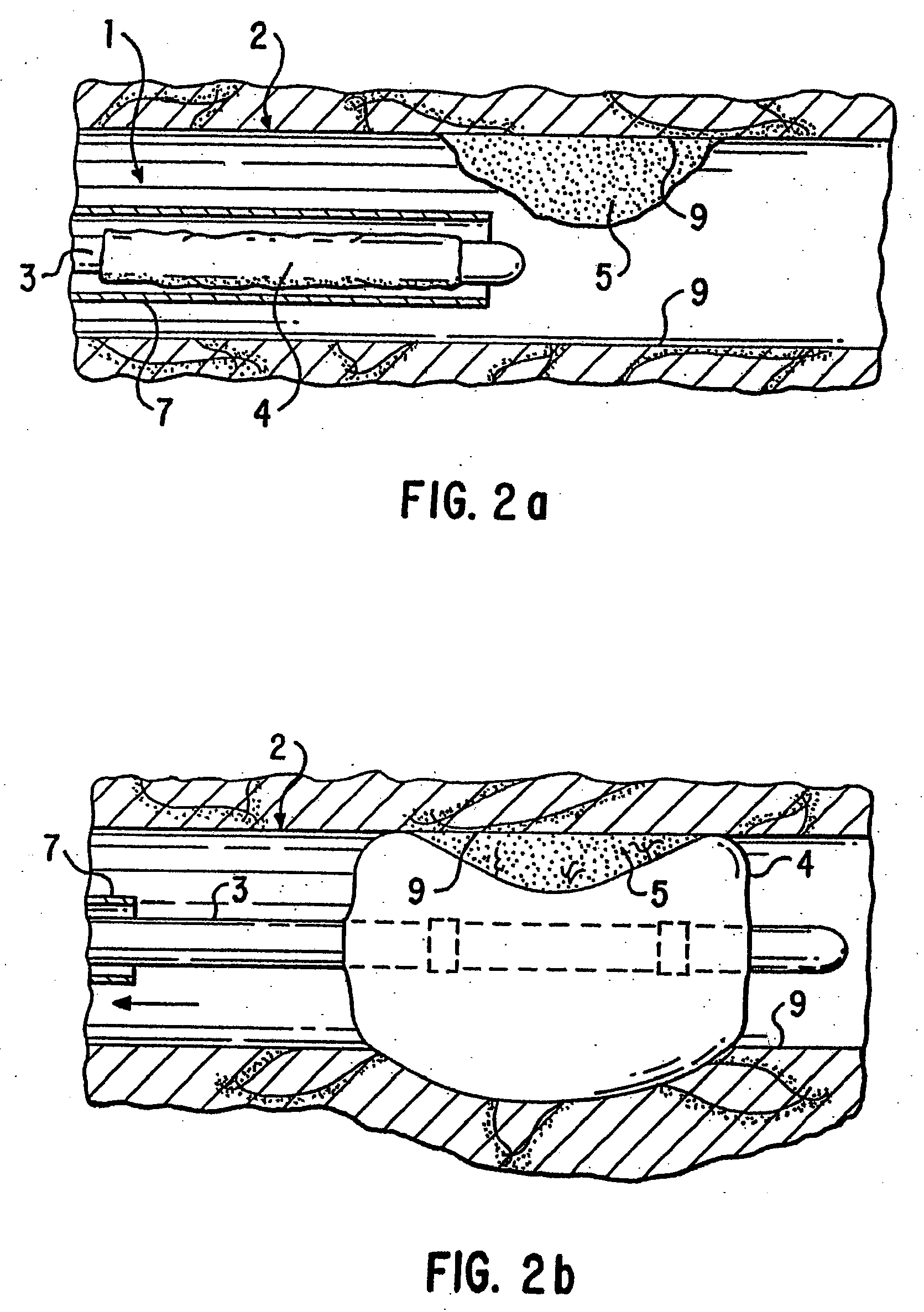
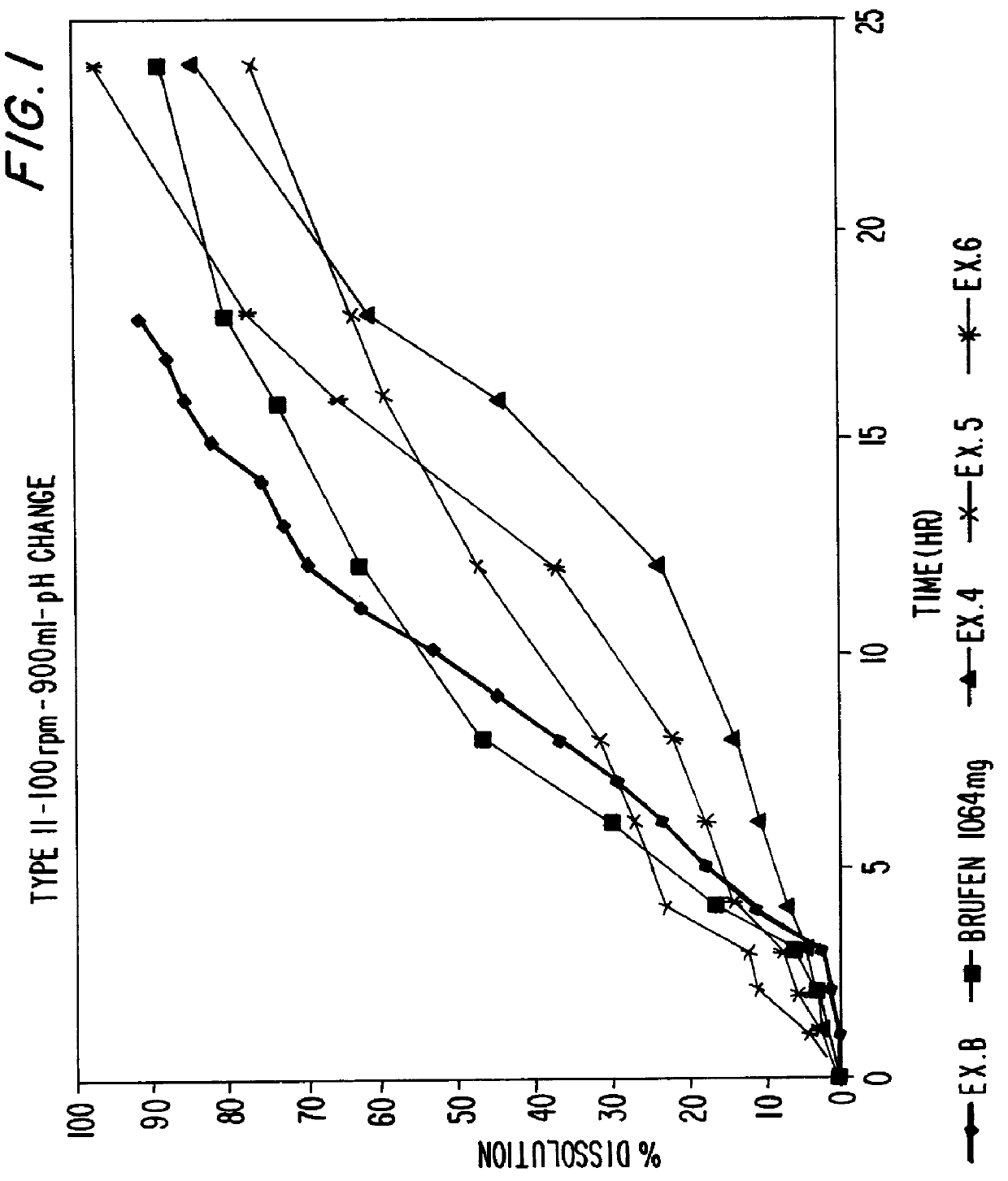
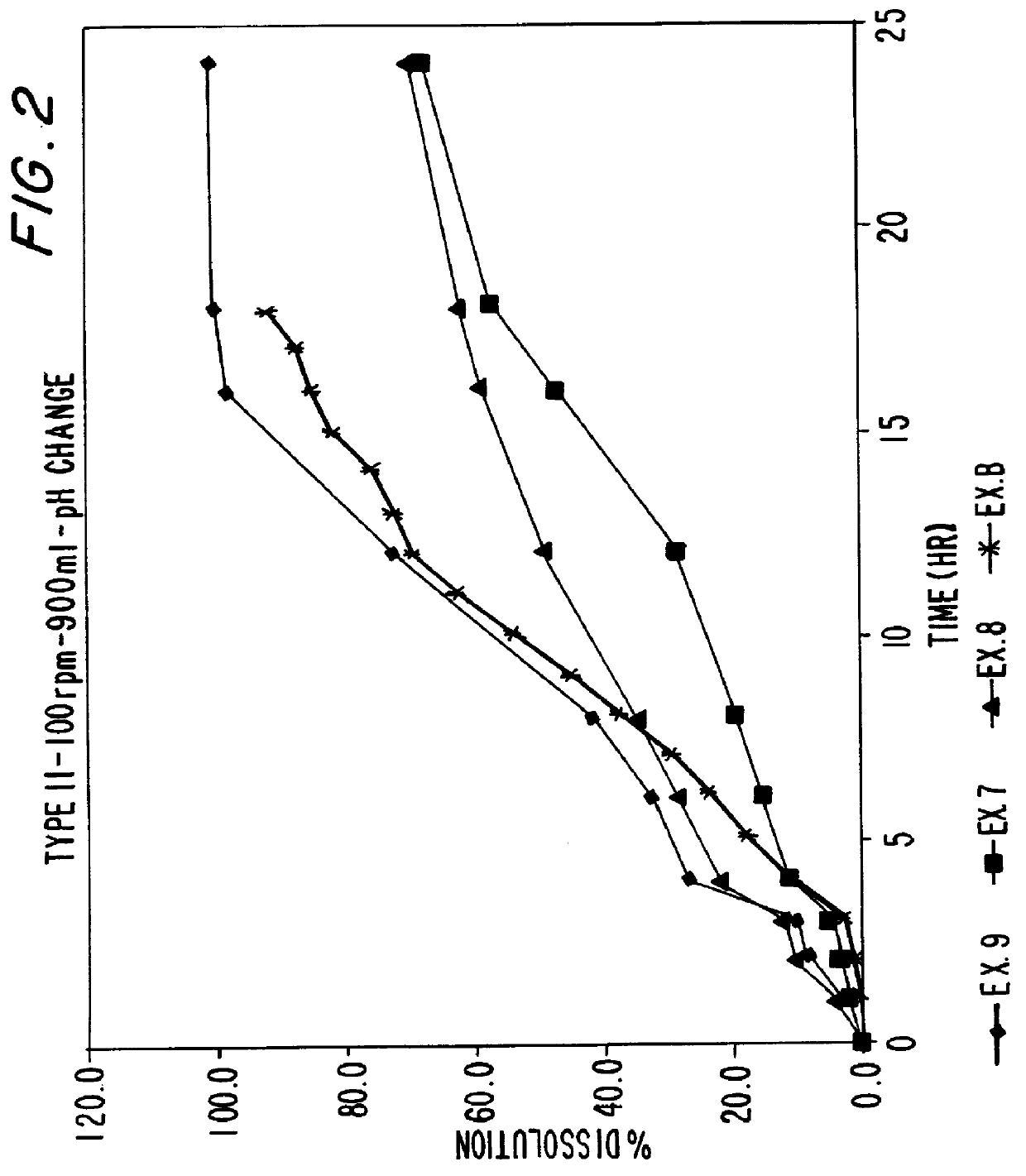
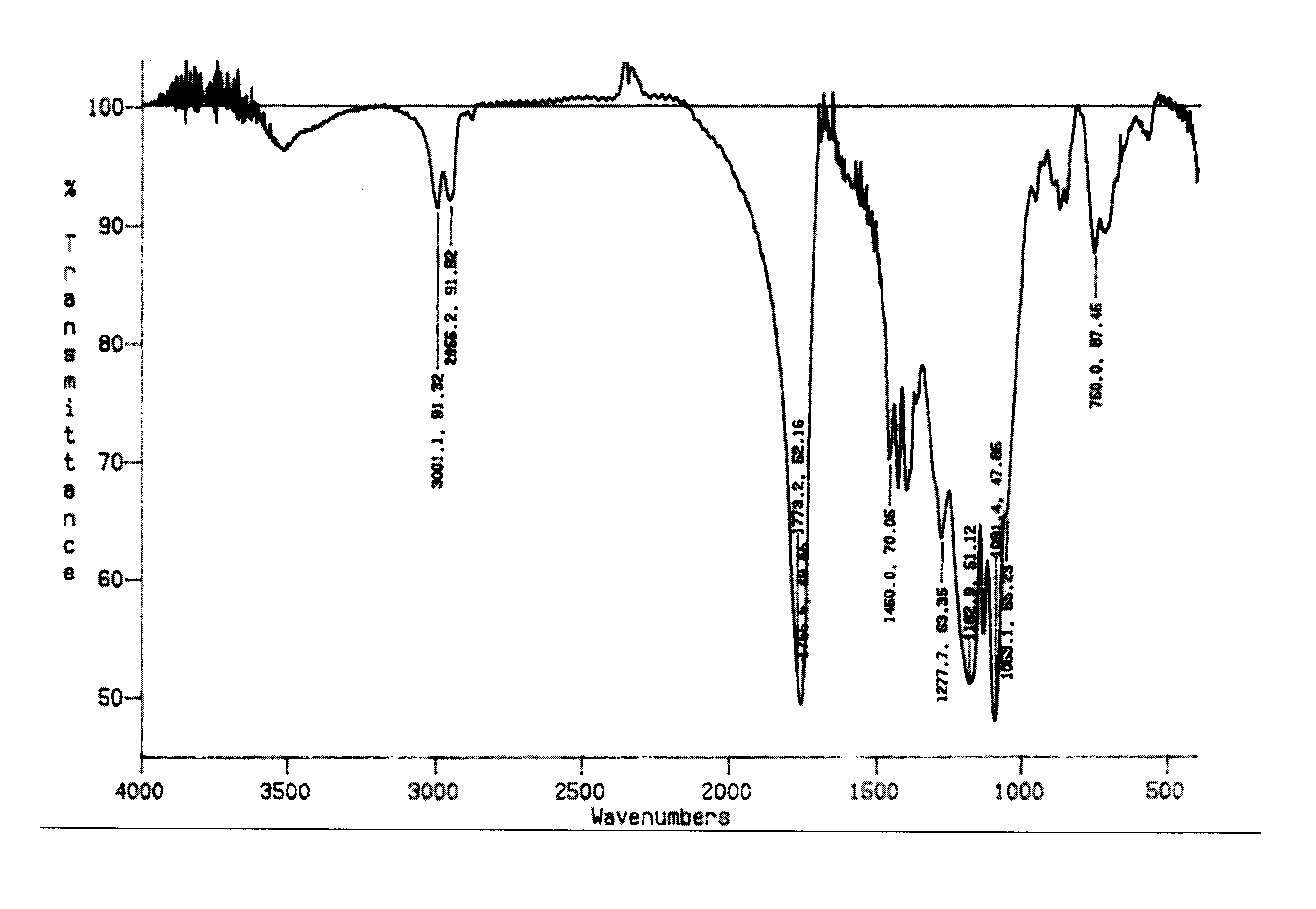
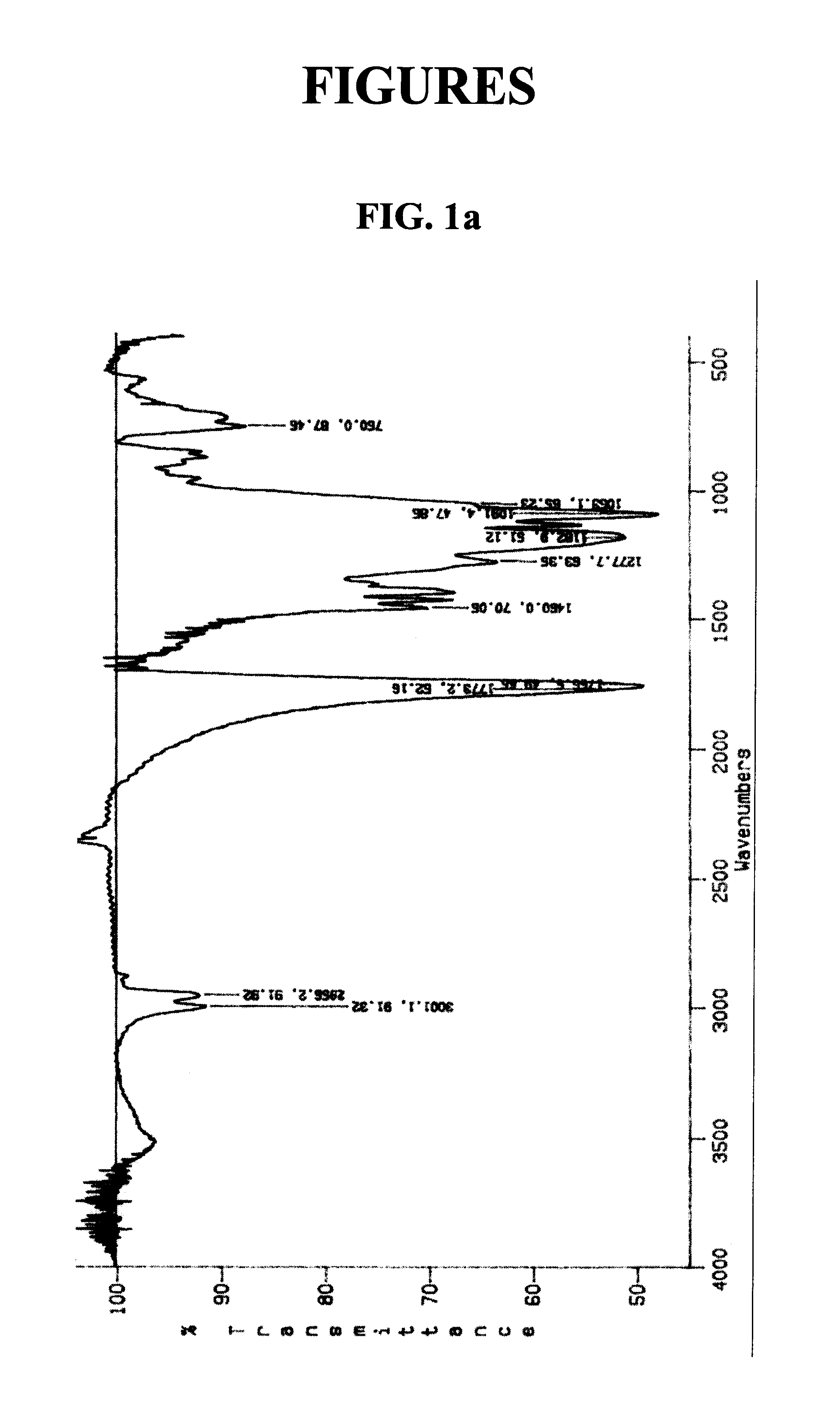
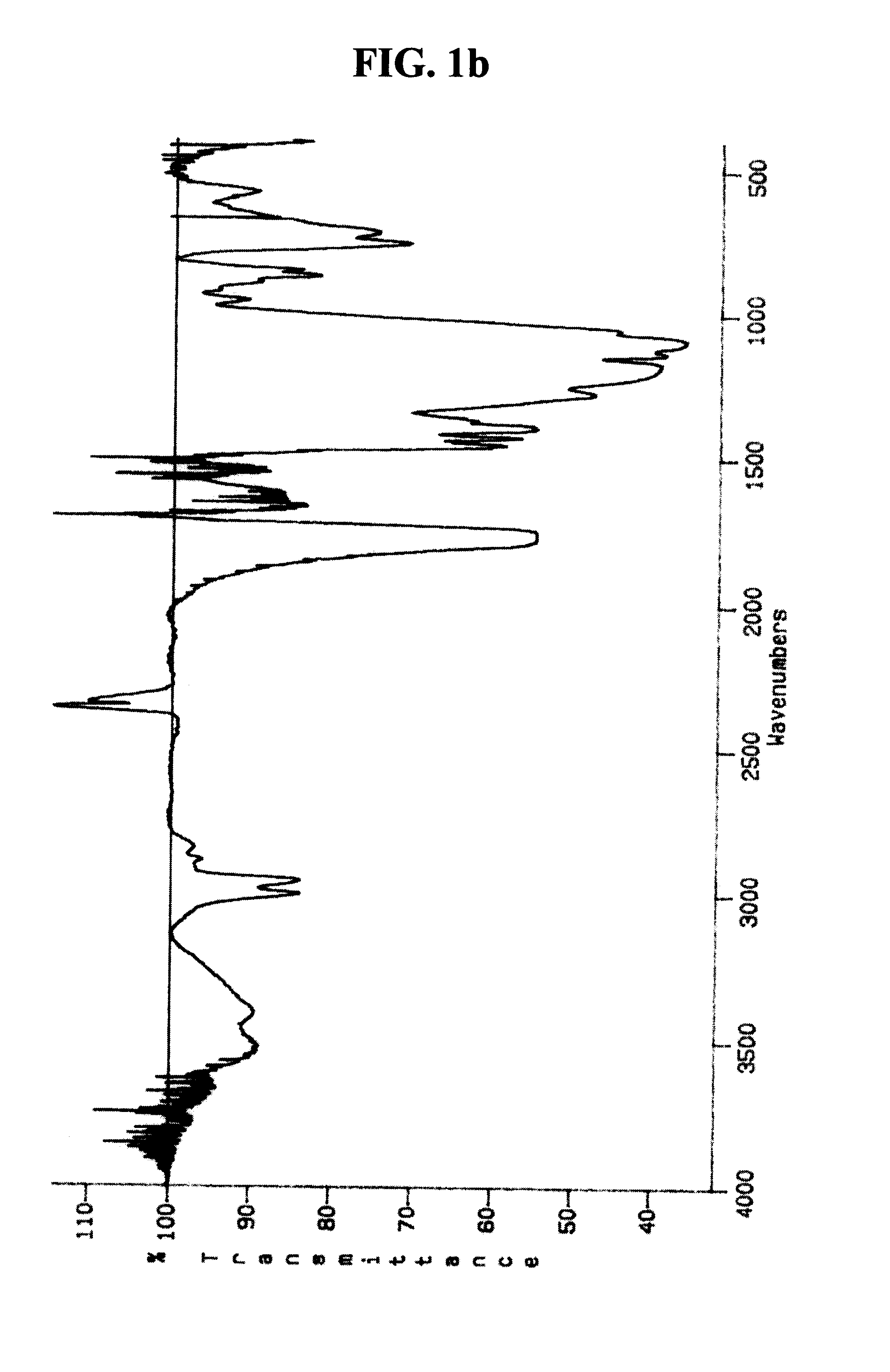
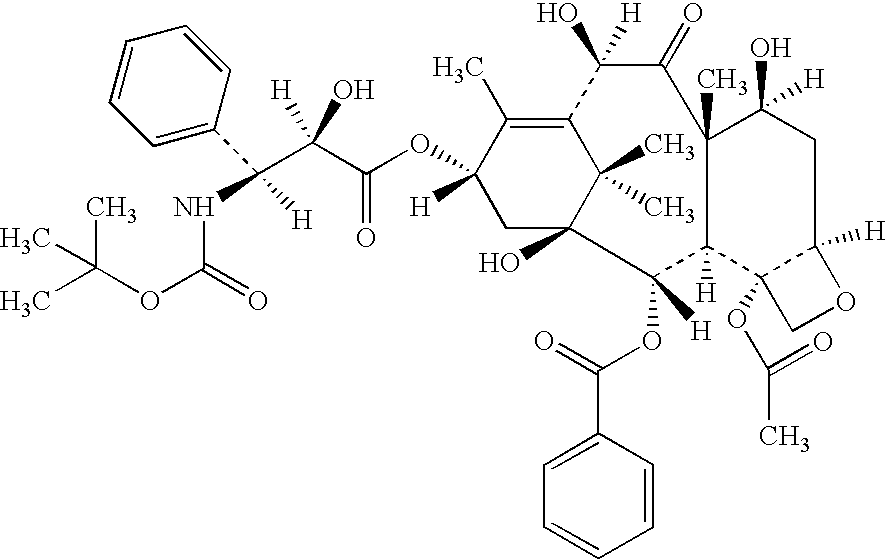
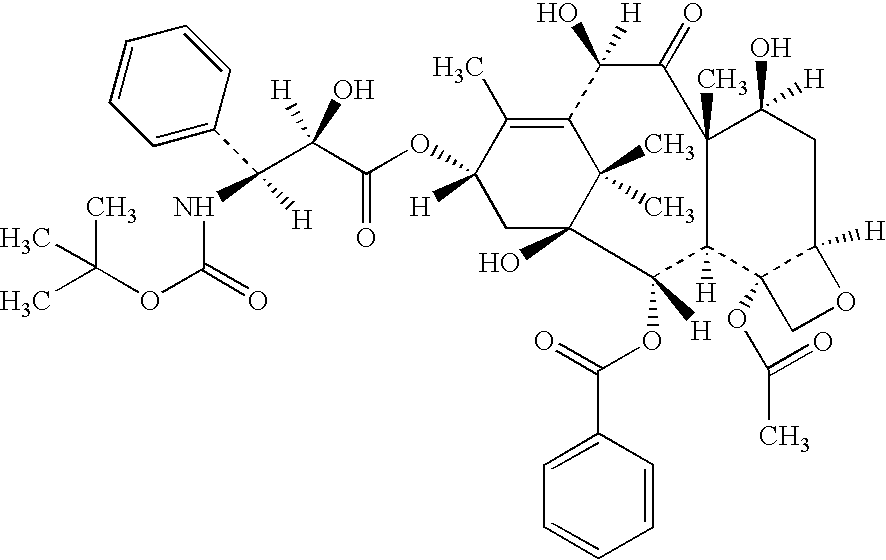
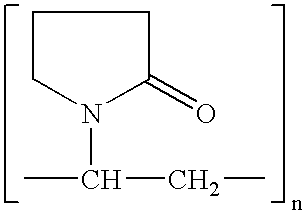
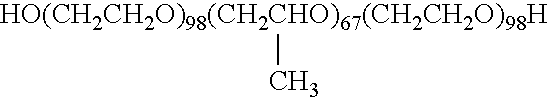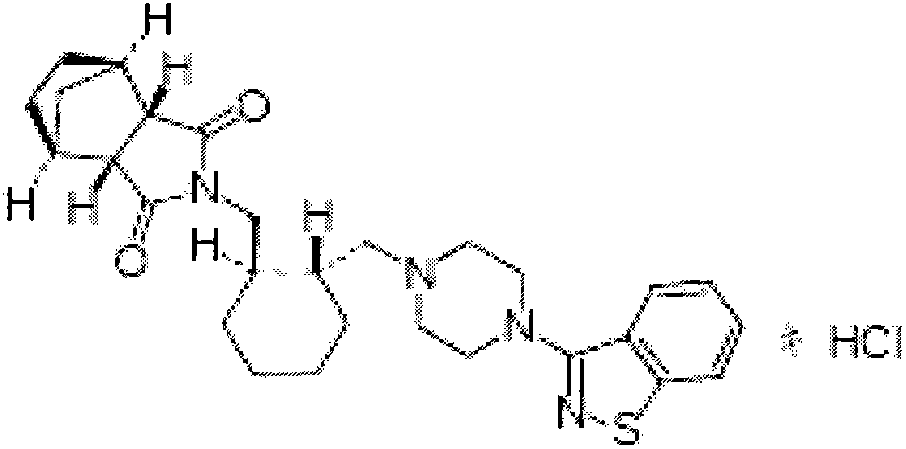Patents
Literature
279 results about "Insoluble drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Insoluble drugs make up an overwhelming 40-50% of existing drug molecules and 90% of newly identified molecules. This results in low bioavailability and difficult drug administration. Nano-sized drug particles have proven potential in a variety of applications.
Loading and release of water-insoluble drugs
Owner:BOSTON SCI SCIMED INC
Sustained release matrix for high-dose insoluble drugs
Sustained release dosage forms of high dose insoluble drugs such as ibuprofen and methods for their manufacture are disclosed.
Owner:PENWEST PHARMA CO
Amphiphilic biodegradable block copolymers and self-assembled polymer aggregates formed from the same in aqueous milieu
InactiveUS6569528B2Glass/slag layered productsWood layered productsPolyesterCritical micelle concentration
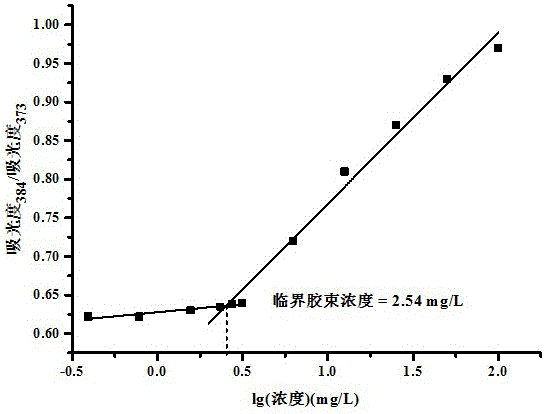
There are provided amphiphilic biodegradable block copolymers comprising polyethylenimine (PEI) as a hydrophilic block and aliphatic polyesters as a hydrophobic block, which can form various size of polymer aggregates and have very low critical micelle concentration, approximately 10-3 g / l in comparison with low-molecular-weight micelle, and self-assembled polymer aggregates formed from the block copolymers in aqueous milieu, which can be applied to solubilization of insoluble drug and a delivery system of proteins, genes or drugs.
Owner:AMOREPACIFIC CORP
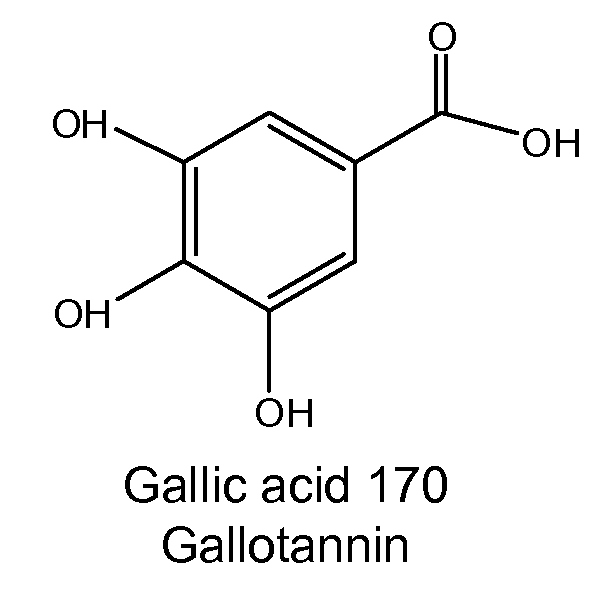
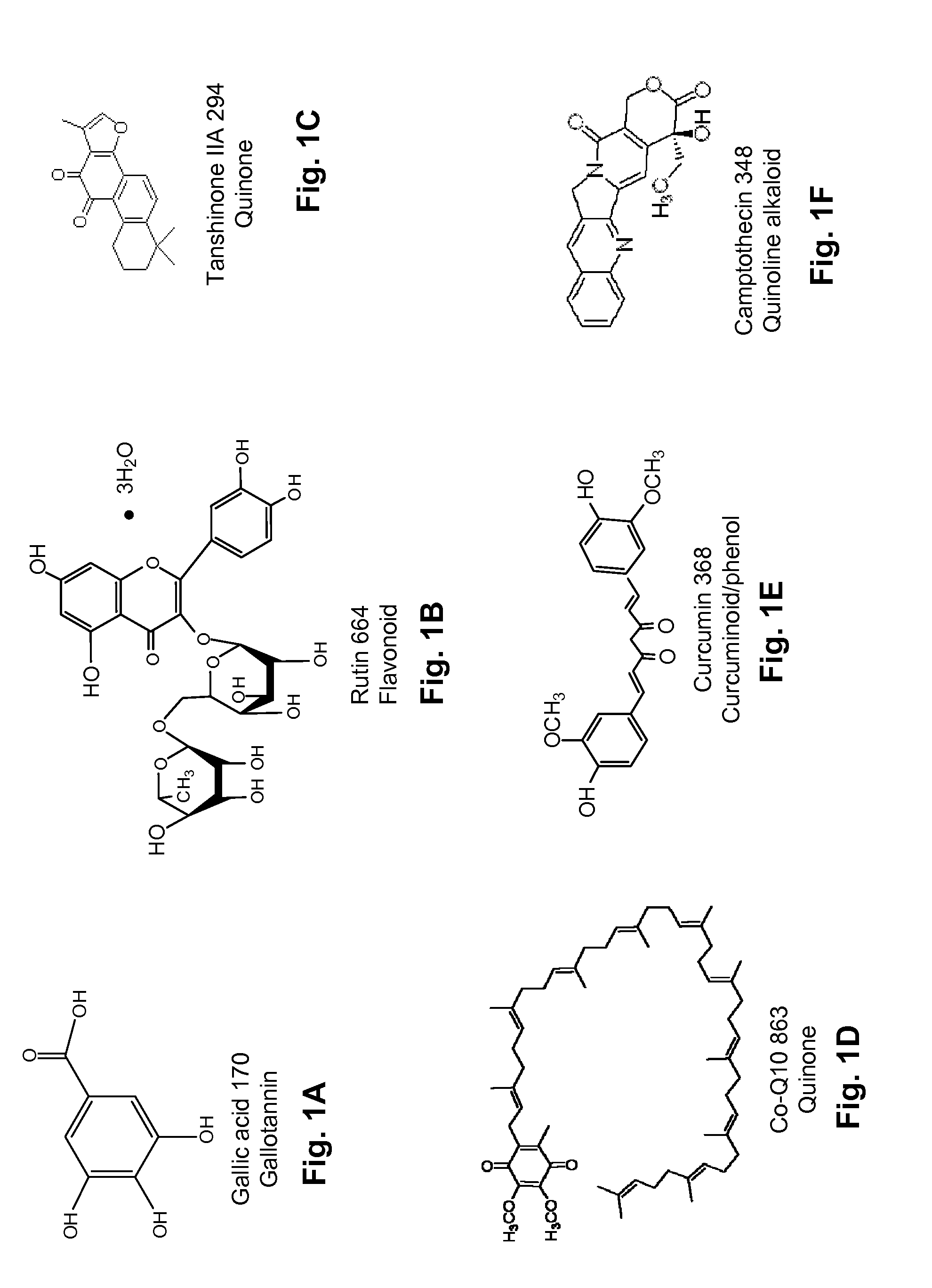
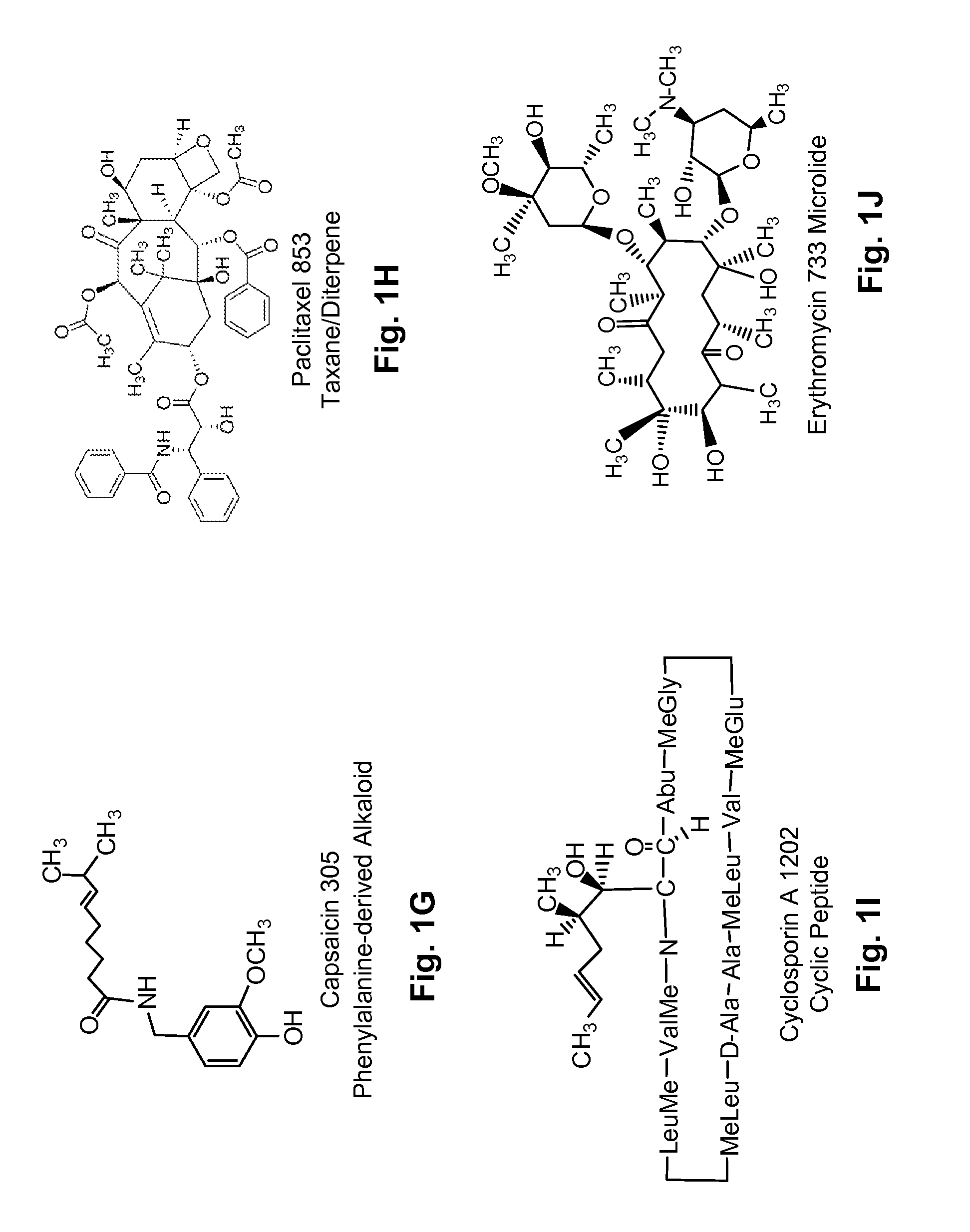
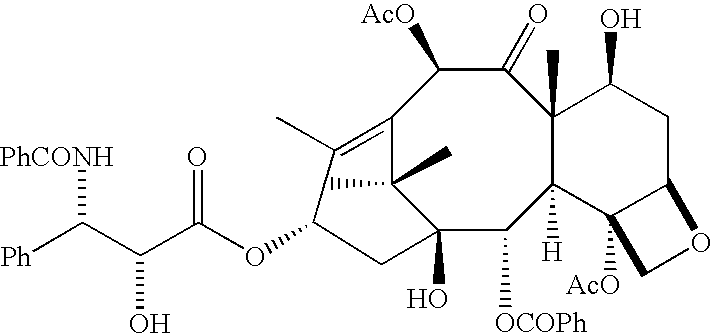
Diterpene Glycosides as Natural Solubilizers
InactiveUS20110033525A1Improve solubilityRetain activityBiocideHydroxy compound active ingredientsItraconazoleCapsaicin
Several diterpene glycosides (e.g., rubusoside, rebaudioside, steviol monoside and stevioside) were discovered to enhance the solubility of a number of pharmaceutically and medicinally important compounds, including but not limited to, paclitaxel, camptothecin, curcumin, tanshinone HA, capsaicin, cyclosporine, erythromycin, nystatin, itraconazole, and celecoxib. The use of the diterpene glycoside rubusoside increased solubility in all tested compounds. The diterpene glycosides are a naturally occurring class of water solubility-enhancing compounds that are non-toxic and that will be useful as new complexing agents or excipients in the pharmaceutical, agricultural (e.g., solubilizing pesticides), cosmetic and food industries. Aqueous solutions by using rubusoside to increase the solubility of otherwise insoluble drugs will have several new routes of administration. In addition, aqueous solutions of therapeutic compounds with rubusoside were shown to retain the known pharmacological activity of the compounds.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Low oil emulsion compositions for delivering taxoids and other insoluble drugs
ActiveUS20060067952A1Reduce oil contentNot hyperallergenicOrganic active ingredientsBiocideOil emulsionWater insoluble
The present invention provides injectable oil-in-water emulsions of taxoid drugs or other water insoluble drugs. The present invention also provides methods for preparing and using such oil-in-water emulsions.
Owner:CHEN ANDREW XIAN
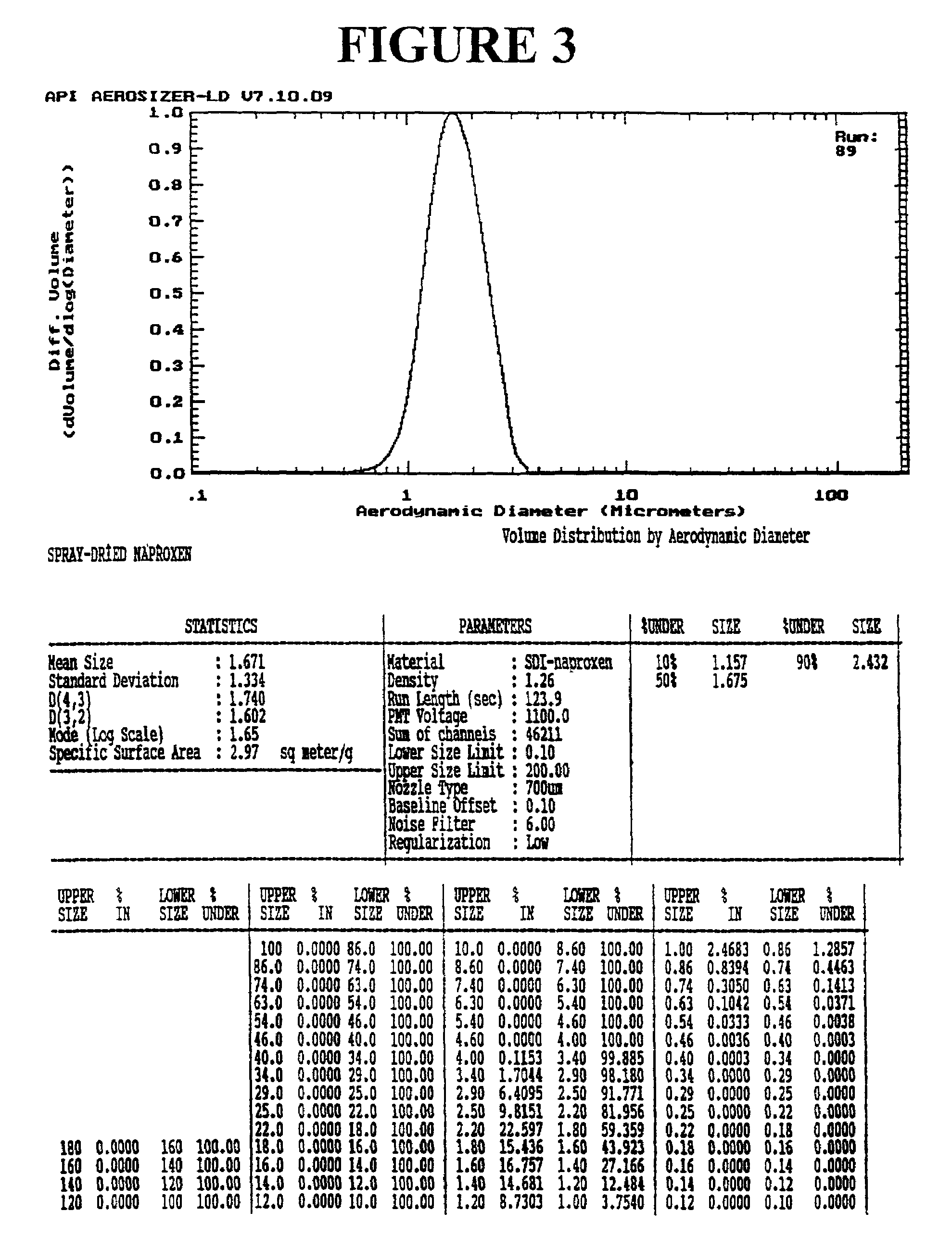
Preparation of drug particles using evaporation precipitation into aqueous solutions
A method for preparing poorly water soluble drug particles is disclosed. The method comprises dissolving a drug in at least one organic solvent to form a drug / organic mixture, spraying the drug / organic mixture into an aqueous solution and concurrently evaporating the organic solvent in the presence of the aqueous solution to form an aqueous dispersion of the drug particles. The resulting drug particles are in the nanometer to micrometer size range and show enhanced dissolution rates and reduced crystallinity when compared to the unprocessed drug. The present invention additionally contemplates products and processes for new drug formulations of insoluble drug particles having high dissolution rates and extremely high drug-to-excipient ratios.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances
ActiveUS20100008985A1Disperse fastEfficient packagingBiocidePowder deliveryWater insolubleProphylactic treatment
One aspect of the invention relates to a pharmaceutical dosage unit for sublingual, buccal, pulmonary or oral administration, said dosage unit having a weight of 20-500 mg and comprising 1-80 Wt. % of a microgranulate that is distributed throughout a solid hydrophilic matrix; said microgranulate being characterised in that it: has a volume weighted average diameter of 5-100 m; contains at least 0.01 wt. %, preferably at least 0.1 wt. % of one or more water-insoluble pharmaceutically active substances; contains at least 10 wt. %, preferably at least 20 wt. % of an emulsifier component; and is capable of forming a micro-emulsion upon contact with saliva or water. The dosage units of the present invention achieve the inherent benefits of oral delivery whilst at the same time realising a high transmucosal absorption rate of the cannabinoids contained therein. Other aspects of the present invention relate to the use of the aforementioned dosage units in the therapeutic or prophylactic treatment and to a process for the manufacture of said dosage units.
Owner:ECHO PHARM BV (NL)
Liquid Compositions of Insoluble Drugs and Preparation Methods Thereof
ActiveUS20130156853A1Good biocompatibilityEliminate hidden dangersBiocideCarbohydrate active ingredientsPhospholipidSolvent
A liquid composition of an insoluble medicament and a preparation method thereof are disclosed. The composition includes insoluble medicament, oil for injection, phospholipid, and solvent; the percentage by weight of each component is as follows: insoluble medicament 0.01-10%, oil for injection 0%-20%, phospholipid 10-80%, solvent 20-89%. The preparation method for the composition includes the following steps: dissolving an insoluble medicament into solvent or oil for injection or a mixture thereof firstly, and then adding other components, and mixing uniformly; or dissolving an insoluble medicament into a mixture of other components, and mixing uniformly; or dissolving an insoluble medicament into part of solvent firstly, and then adding into a mixed solvent of other components and the remaining solvent, and mixing uniformly.
Owner:PEKING UNIV
Drug coating providing high drug loading and methods for providing same
InactiveUS20050112195A1High viscosityEasy to produceBiocideNervous disorderDrug coatingInsoluble drug
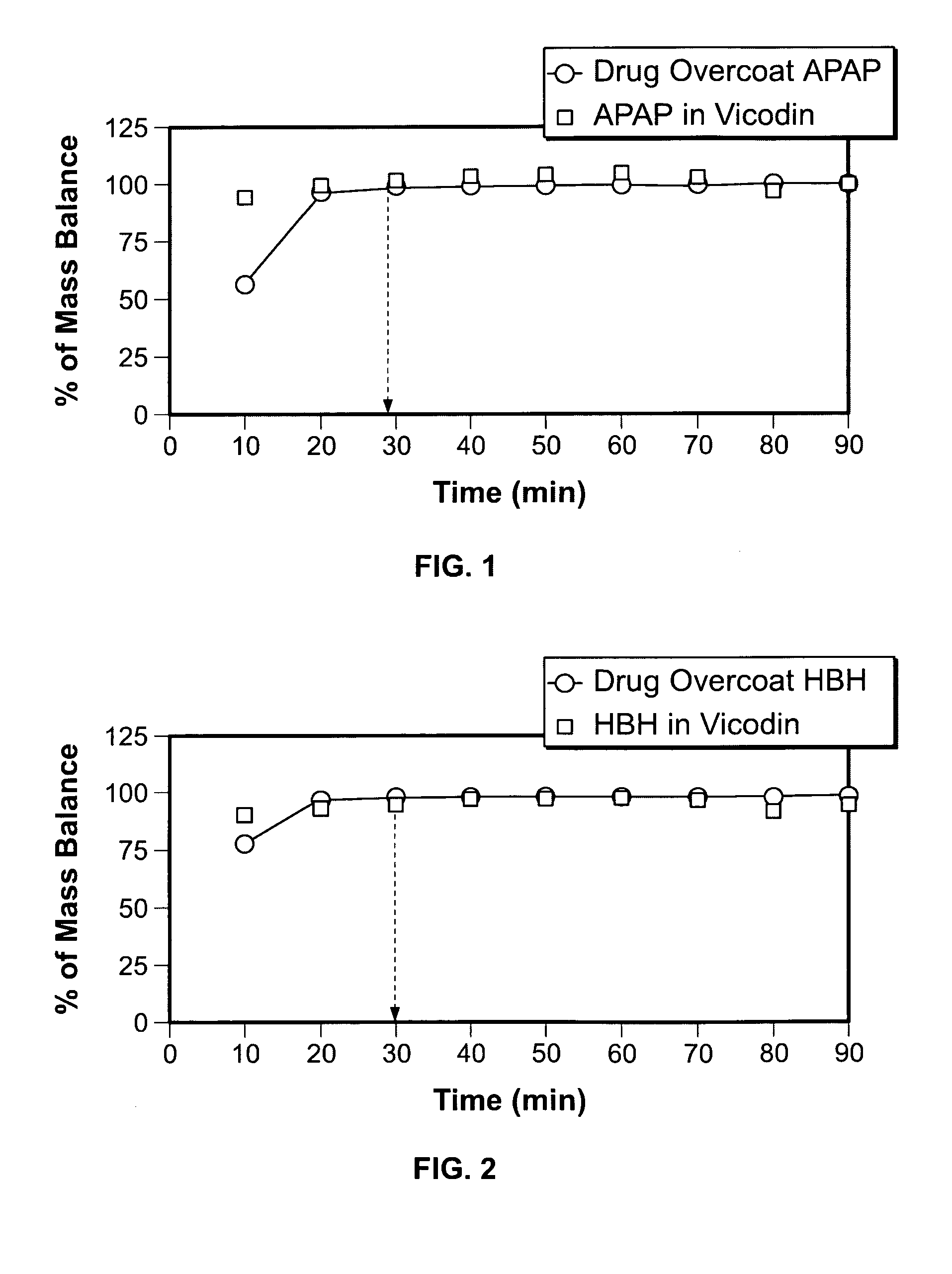
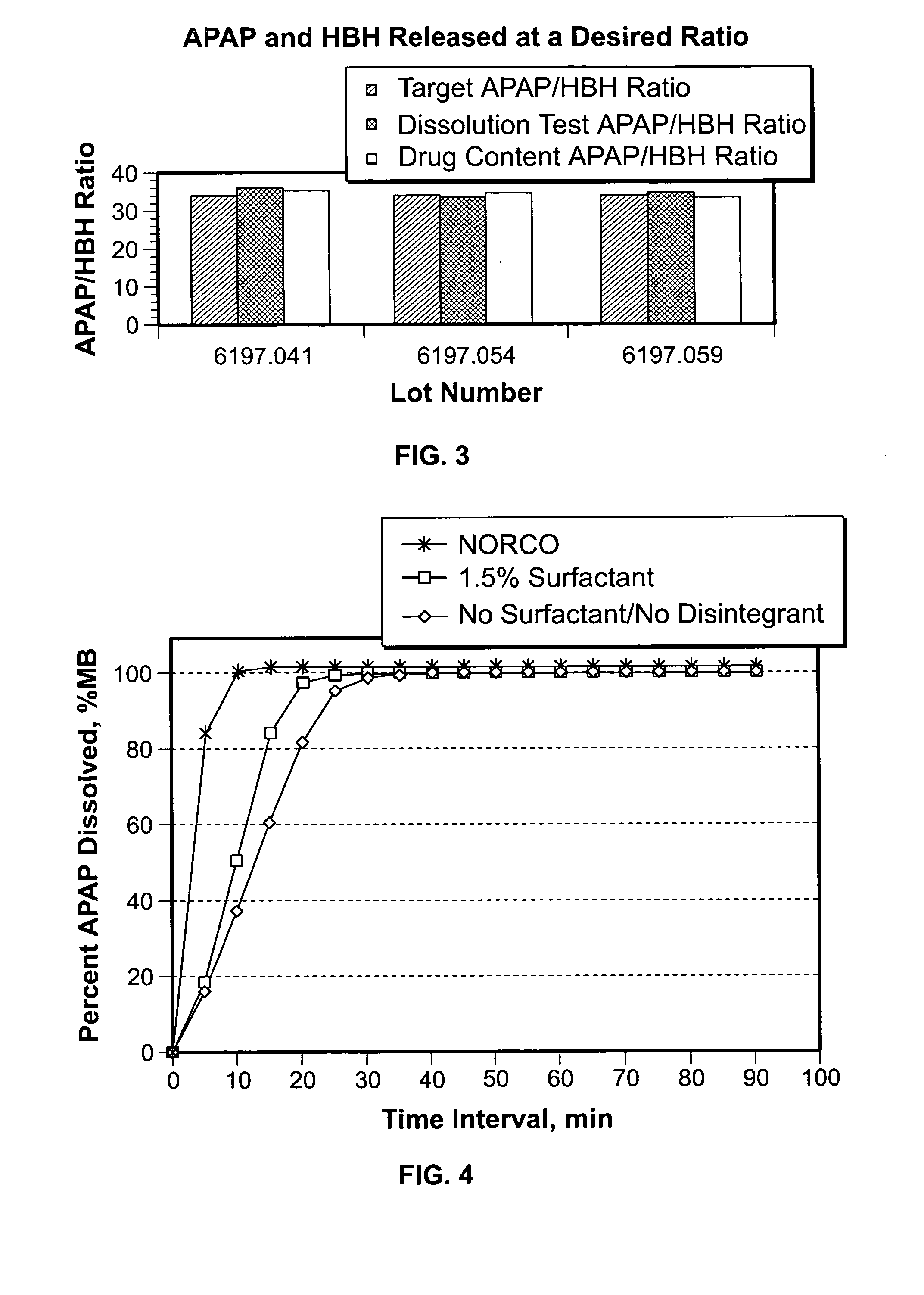
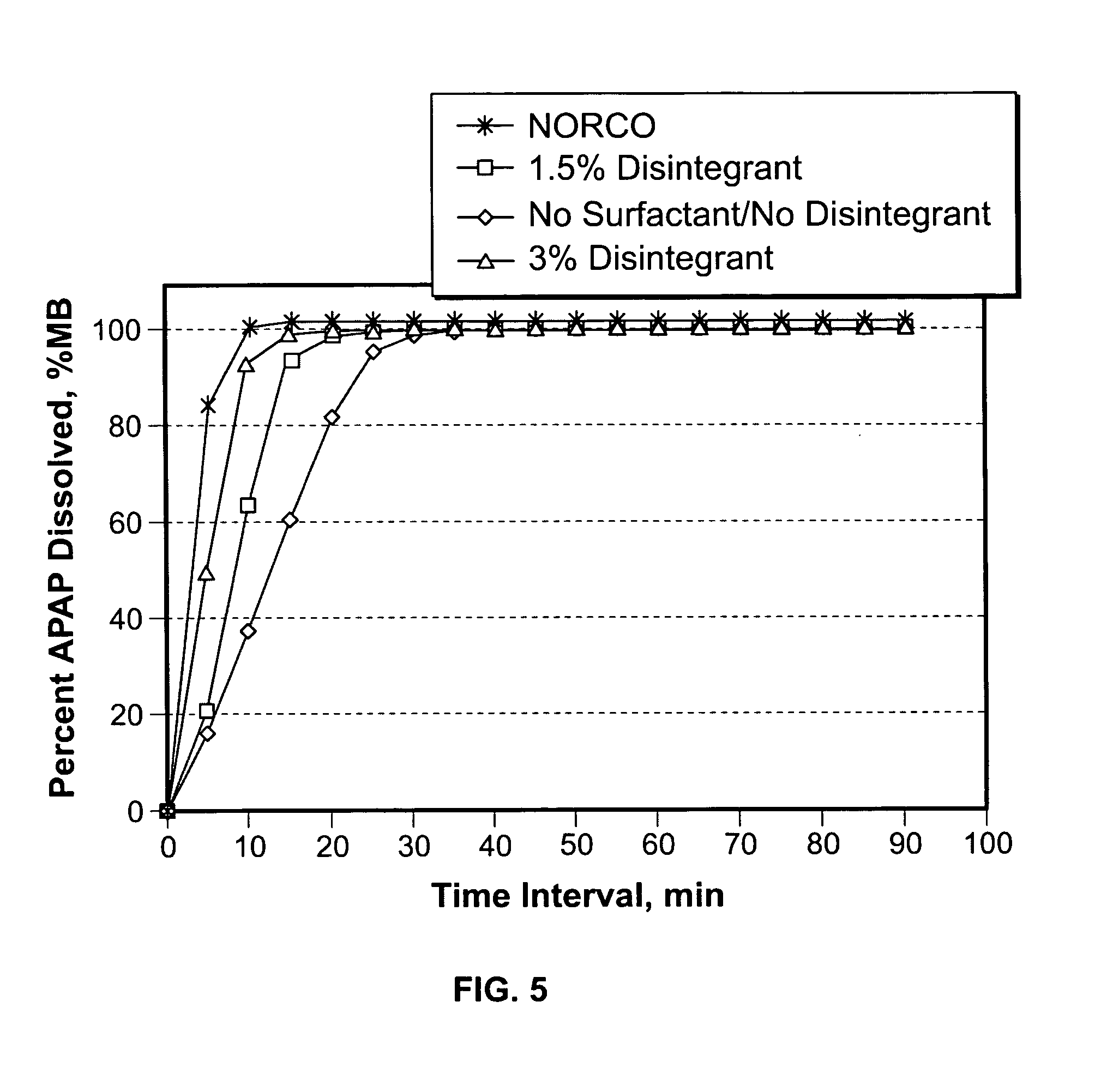
The present invention is directed to aqueous drug coatings that include at least one insoluble drug, wherein the drug accounts for about 85 wt % to about 97 wt % of the drug coatings. A drug coating according to the present invention may include only one insoluble drug, two or more insoluble drugs, or one or more insoluble drugs in combination with one or more soluble drugs. The present invention also includes drug coating formulations suitable for providing drug coatings according to the present invention and dosage forms that include a drug coating according to the present invention.
Owner:ALZA CORP
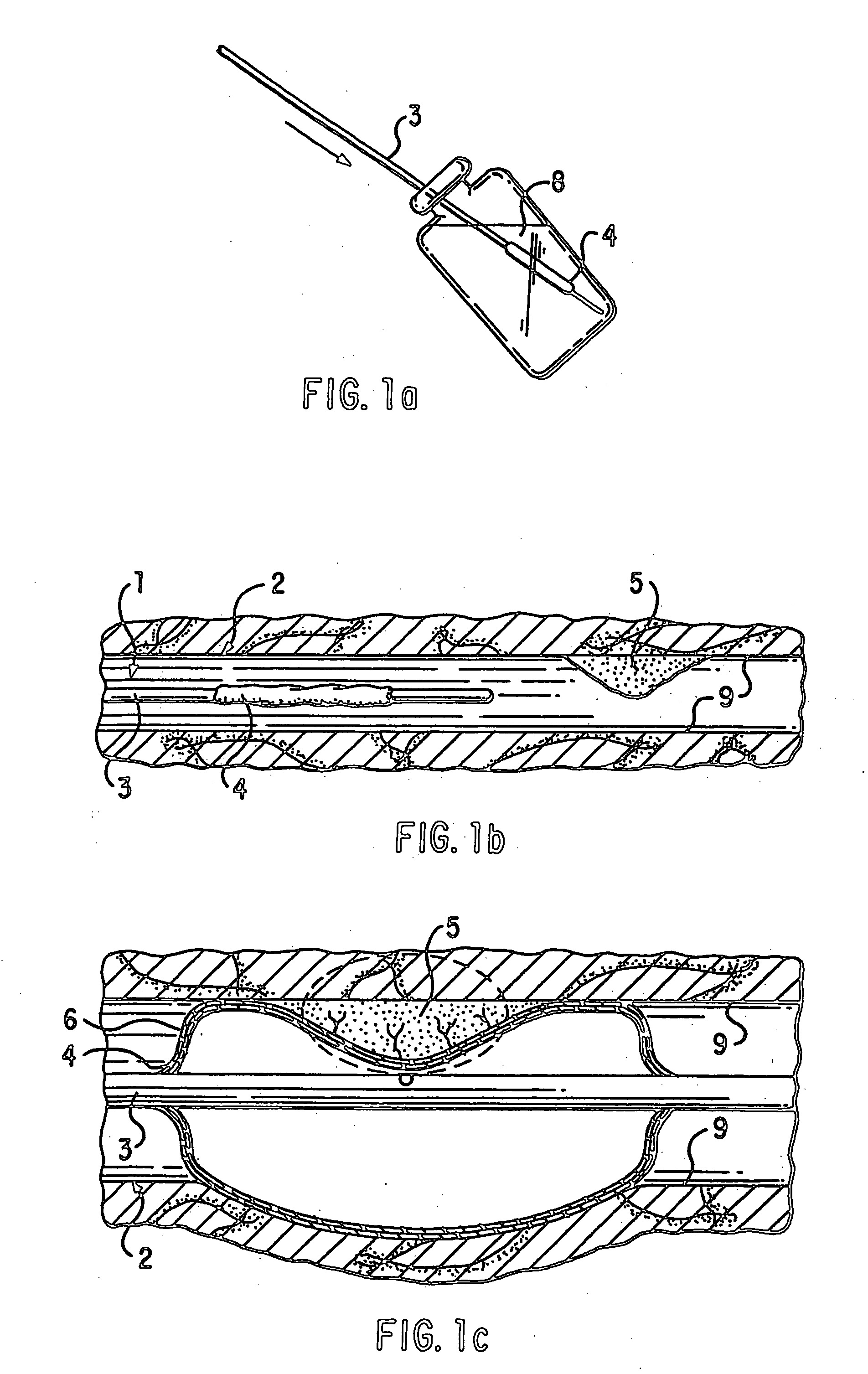
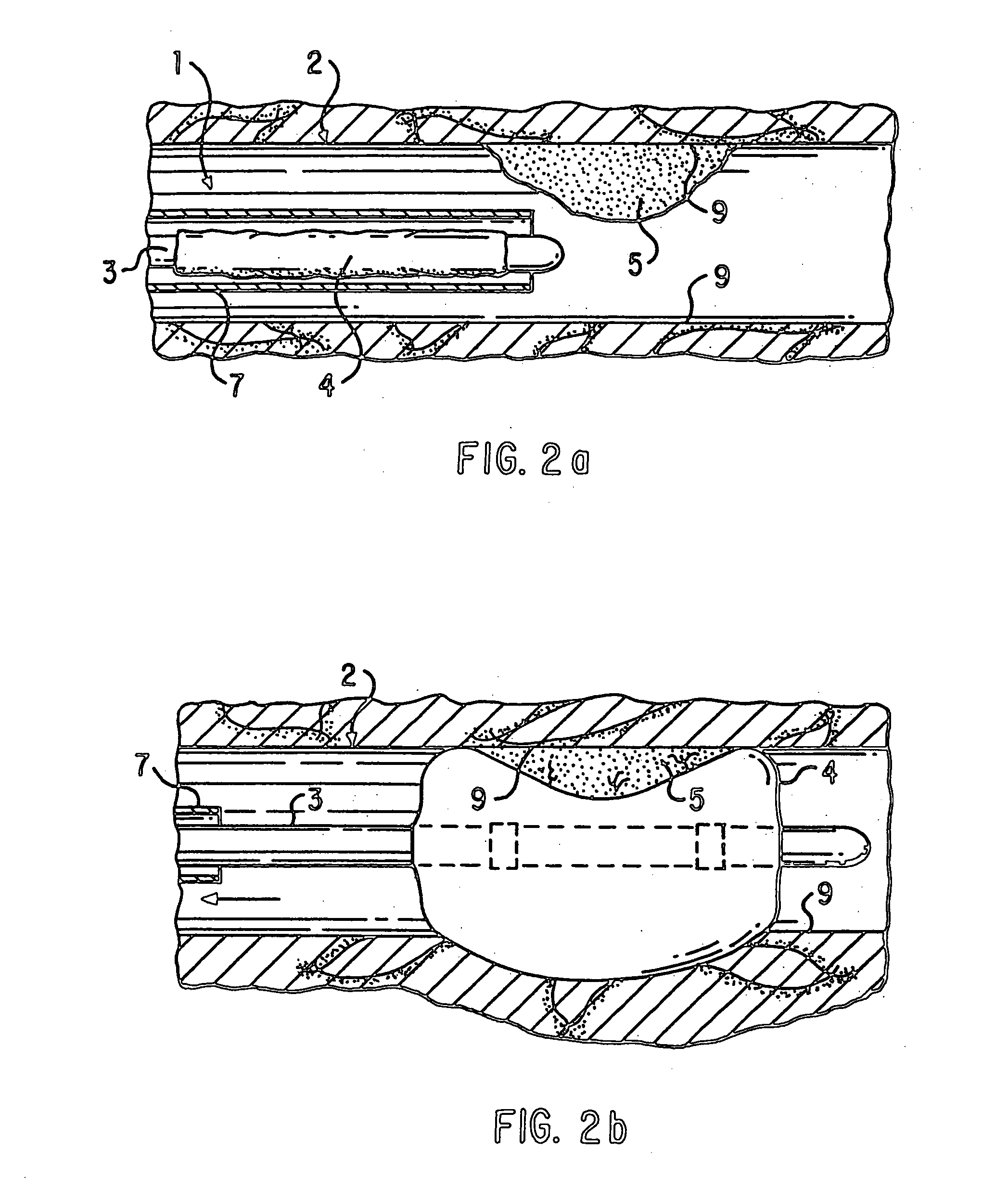
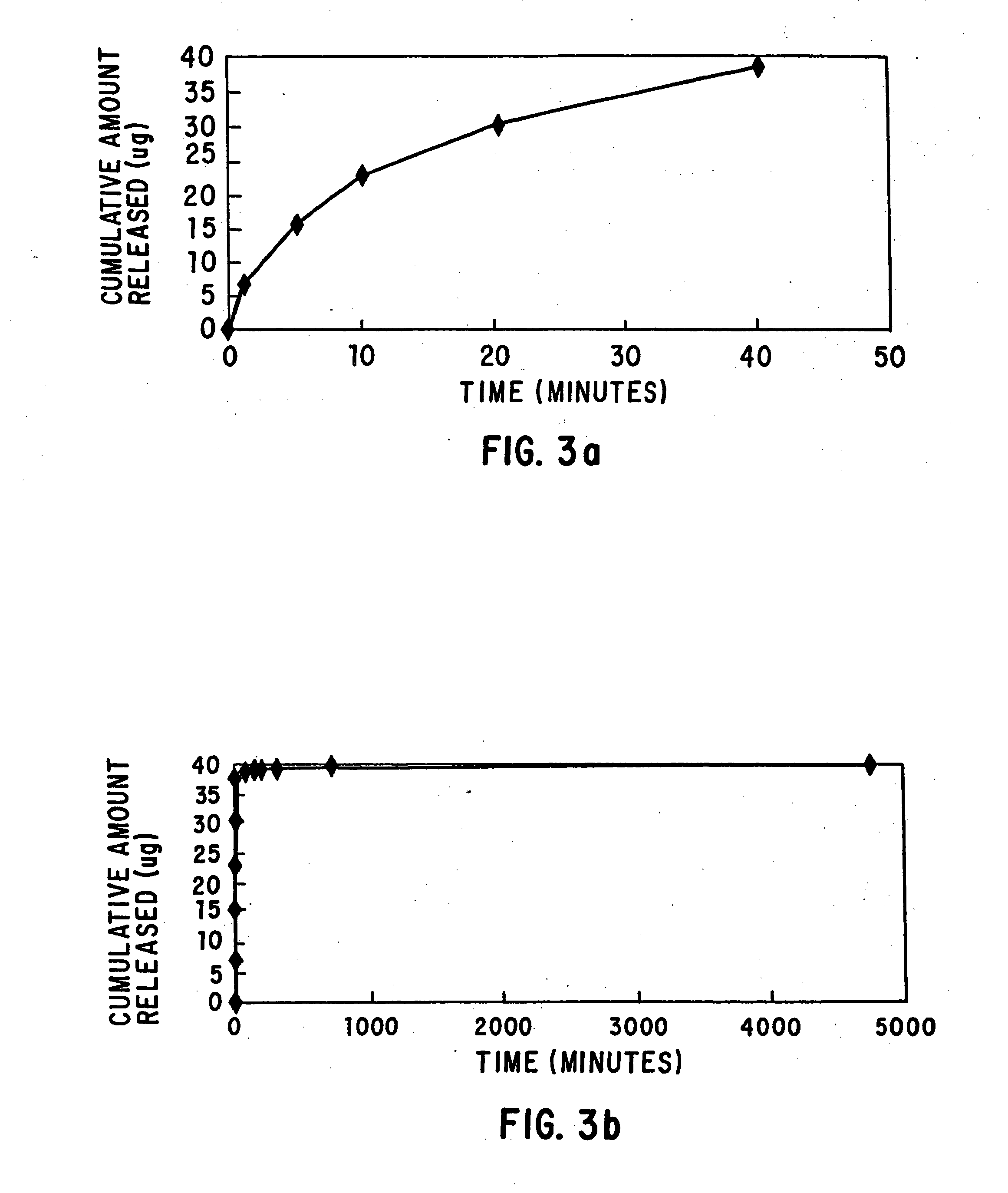
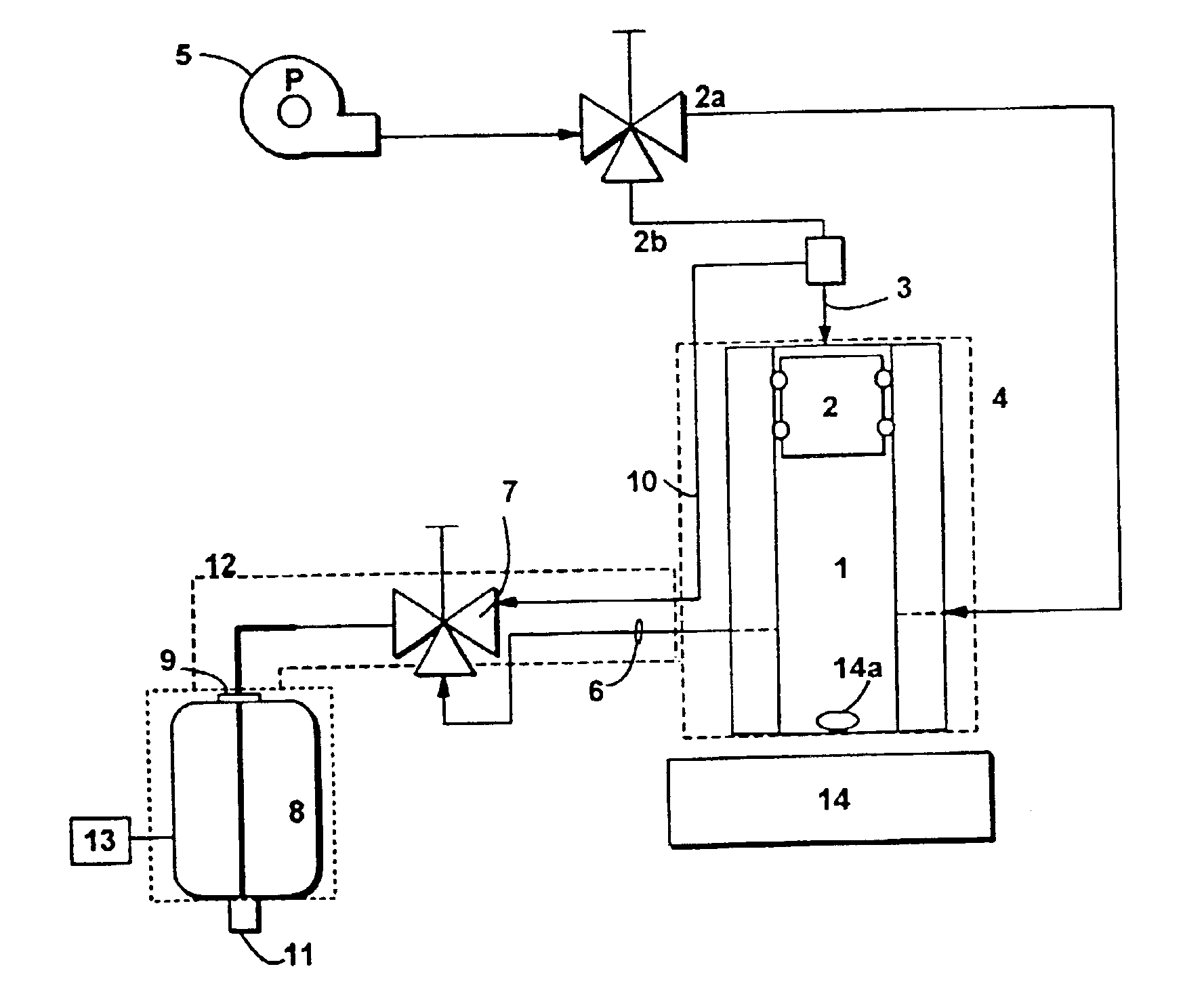
Loading and release of water-insoluble drugs
A medical device, polymer composition, and method for delivering substantially water-insoluble drugs to tissue at desired locations within the body. At least a portion of the exterior surface of the medical device is provided with a polymer coating. Incorporated in the polymer coating is a solution of at least one substantially water-insoluble drug in a volatile organic solvent. The medical device is positioned to a desired target location within the body, whereupon the drug diffuses out of the polymer coating.
Owner:SCI MED LIFE SYST
Polymer micelle lyophilized agent encapsulating insoluble antitumor drug
ActiveCN102218027ASmall toxicityGood biocompatibilityOrganic active ingredientsPharmaceutical delivery mechanismPolyesterSide effect
The invention belongs to the field of pharmaceutical agents, relates to a polymer micelle lyophilized agent encapsulating an insoluble antitumor drug as well as a preparation method and an application thereof. The polymer micelle lyophilized agent is prepared by carrying out molecular self-assembly on a methoxy poly(ethylene glycol) 2000-polyester block copolymer to form micelles, and then encapsulating the insoluble antitumor drug in a hydrophobic core formed by the polyester. The lyophilized agent has high encapsulation rate, high drug loading and small particle size, can significantly improve the water solubility of the insoluble drug and result in passive targeting of more antitumor drugs to concentrate in the tumor tissues, thus improving an anti-tumor treatment effect and reducing the toxic and side effects of drugs, and can be used to prepare the drugs used for the treatment of lung cancer, intestinal cancer, mammary cancer, ovarian cancer, etc. The lyophilized agent can also be quickly dissolved and dispersed to form a transparent micellar solution after water for injection, normal saline solution and the like are added, and is used for the preparation of the drugs for treating primary intestinal cell carcinoma.
Owner:上海谊众药业股份有限公司
Transdermal delivery system for water insoluble drugs
InactiveUS7395111B2Minimal irritationMinimal sensitizationOrganic active ingredientsBiocideWater insolubleWater insoluble drug
Owner:SYNERON MEDICAL LTD
Preparation of indissoluble medicament nano granule
InactiveCN101322682AIncrease Saturation SolubilityPassive targetingPowder deliveryPharmaceutical non-active ingredientsSide effectNanocrystal
The invention relates to the technical field of medicine, in particular to a preparation method of nano-particles of insoluble drugs. The method includes the following steps: (1) dissolving the drugs in a first solvent (good solvent) to form solution, (2) blending the solution with a second solvent (poor solvent) to form premixed suspension, (3) applying energy on the premixed suspension to form the nano-particles, the average effective grain diameter of which is less than 2Mum. By adopting the technology combining micro-deposition and homogenization, the invention suspends the drugs in poor solvent (usually water) in a form of pure nano-crystal, thus solving the problem that solution is difficult to be prepared since the drugs are difficult to be dissolved in water and oil; compared with the corresponding injection for intravenous infusion and the oral preparations such as tablets, capsules, and the like, the preparation method of the invention can lower adverse reaction, reduce toxic and side effect, improve bioavailability, has sustained-release effect and is convenient to be used by patients.
Owner:KANGYA OF NINGXIA PHARMA +1
Application of solid dispersion to preparation of veterinary drugs
ActiveCN101919804AImprove palatabilityFast initial dissolutionPowder deliveryPharmaceutical non-active ingredientsInsoluble drugVeterinary medicine
The invention discloses a solid dispersion of veterinary drugs, a preparation method thereof and application of the solid dispersion to the preparation of veterinary drugs. The solid dispersion can improve the dissolving speed and the solubility of insoluble drugs, enhances the absorption and the bioavailability of drugs and covers unpleasant odor and taste of drugs. Since the effective components of the solid dispersion and the drugs prepared by the invention are covered in carriers, the stability of the drugs, including the veterinary drugs, can be improved.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE +1
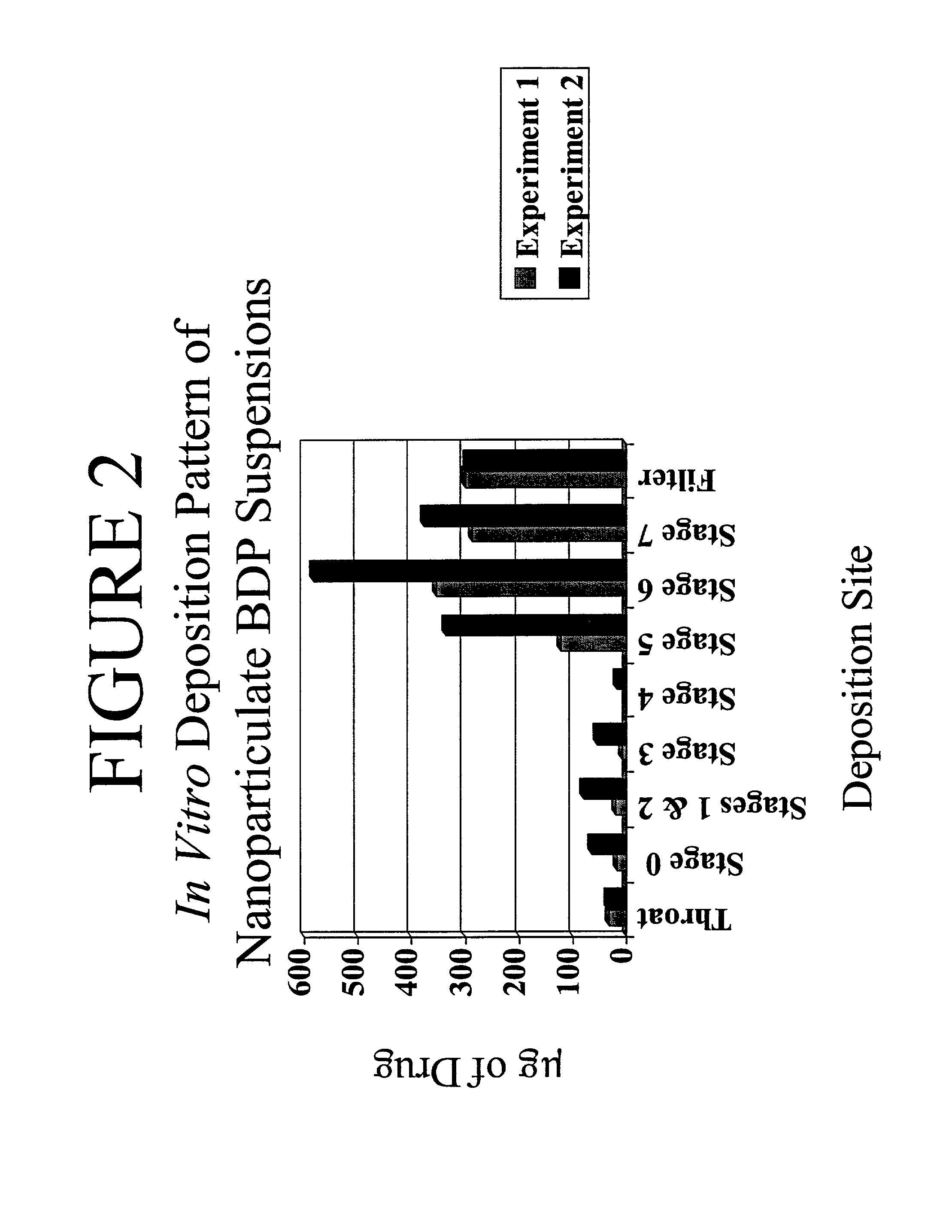
Dry powder aerosols of nanoparticulate drugs
InactiveUS7521068B2Permit deliveryEasy to atomizeAntibacterial agentsOrganic active ingredientsNanoparticleAerosol delivery
There invention discloses aqueous dispersions of nanoparticulate aerosol formulations, dry powder nanoparticulate aerosol formulation, propellant-based aerosol formulations, methods of using the formulations in aerosol delivery devices, and methods of making such formulations. The nanoparticles of the aqueous dispersions or dry powder formulations comprise insoluble drug particles having a surface modifier on the surface thereof.
Owner:ALKERMES PHARMA IRELAND LTD
Sustained release heterodisperse hydrogel systems for insoluble drugs
A sustained release pharmaceutical formulation includes a sustained release excipient including a gelling agent, an inert pharmaceutical diluent, an optional cationic cross-linking agent, and a medicament having moderate to poor solubility is disclosed. In certain embodiments, the sustained release excipient is granulated with a solution or suspension of a hydrophobic polymer in an amount effective to slow the hydration of the gelling agent when the formulation is exposed to an environmental fluid. In another embodiment, the tablet is coated with a hydrophobic polymer.
Owner:PENWEST PHARMA CO
Insoluble drug delivery
InactiveUS6974593B2Robust and scalableWide rangeOrganic active ingredientsPowder deliveryWater insolubleWater insoluble drug
Particles of water insoluble biologically active compounds, particularly water-insoluble drugs, with an average size of 100 nm to about 300 nm, are prepared by dissolving the compound in a solution then spraying the solution into compressed gaz, liquid or supercritical fluid in the presence of appropriate surface modifiers.
Owner:JAGOTEC AG
Amphiphilic biodegradable block copolymers and self-assembled polymer aggregates formed from the same in aqueous milieu
InactiveUS20030009004A1Glass/slag layered productsWood layered productsPolyesterCritical micelle concentration
There are provided amphiphilic biodegradable block copolymers comprising polyethylenimine (PEI) as a hydrophilic block and aliphatic polyesters as a hydrophobic block, which can form various size of polymer aggregates and have very low critical micelle concentration, approximately 10-3 g / l in comparison with low-molecular-weight micelle, and self-assembled polymer aggregates formed from the block copolymers in aqueous milieu, which can be applied to solubilization of insoluble drug and a delivery system of proteins, genes or drugs.
Owner:AMOREPACIFIC CORP
Preparation and application of insoluble drug-entrapped poloxamer/amphiphilic polysaccharide mixed micelle
InactiveCN102626518AOvercome the problems of high critical micelle concentration and low drug loadingImprove oral bioavailabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMixed micelleCytochrome p450 enzyme
The invention discloses preparation and application of an insoluble drug-entrapped poloxamer / amphiphilic polysaccharide mixed micelle. The insoluble drug-entrapped poloxamer / amphiphilic polysaccharide mixed micelle is prepared through a dialysis method or a solvent evaporation method. The mixed micelle is low in critical micelle concentration, is high in drug-loading rate, is capable of obviously prolonging the stabilization time and has the long-circulation function of a nanomicelle and has dual functions of restraining the metabolism of P-glycoprotein and cytochrome P450 enzyme and is capable of increasing the bioavailability of oral administration. The mixed micelle is simple in preparation method, is mature in process and is high in yield and can be prepared into preparations for the oral administration, such as tablets, capsules, pills and syrups.
Owner:CHINA PHARM UNIV
High-stability polyethylene glycol-polyester polymer and application thereof
ActiveCN103601878AImprove hydrophobicityHigh drug loadingPharmaceutical non-active ingredientsPolyesterPolymer science
The invention belongs to the field of medical technology, relates to a high-stability polyethylene glycol-polyester polymer and an application thereof, and particularly relates to an amphiphilic block copolymer having a hydrophilic block and a hydrophobic block with terminal hydroxyl, wherein the terminal hydroxyl of the hydrophobic block is replaced by a cholic acid group. The hydrophilic A block is any one of ethylene glycol monomethyl ether, polyethylene glycol, polyvinyl alcohol and polyvinylpyrrolidone; the hydrophobic B block is any one of polylactide, polylactide-co-glycolide, polyglycollide, polycaprolactone, polylactide-co-caprolactone, polyglycollide-co-caprolactone and polylactide-co-glycolide-cocaprolactone. The polymer provided by the invention can spontaneously form high-stability micelle in a waterborne medium, and can be used as a carrier of various water-insoluble drugs.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of novel hard-soluble medicine liposome
InactiveCN101385715AWell mixedSimple production processPharmaceutical non-active ingredientsLiposomal deliveryOrganic solventDiluent
The invention provides a preparation method of a novel insoluble drug liposome without the application of an organic solvent in the preparation process. The invention names the method as a water soluble surfactant dispersion method. The preparation method comprises the following steps: A. an insoluble drug is dissolved or suspended or mixed in a water soluble surfactant; B. lipid substances, a water soluble diluent and the water soluble surfactant which is dissolved or suspended or mixed with the insoluble drug for forming the liposome are evenly mixed; C. the appropriate amount of water or water solution is added in the mixture which is obtained in step B, the even mixing is carried out, and the high pressure homogenization or the high pressure homogenization is carried out, thereby obtaining liposome suspension; D. other liposome components can be added by adopting the appropriate method in any step of the steps of A, B and C according to the physical and the chemical properties, if the insoluble drug liposome does not contain other liposome components, the liposome components can not be added.
Owner:CHINA PHARM UNIV
Preparation method and applications of mesoporous carbon
InactiveCN103086346ALarge specific surface areaUniform particle sizePowder deliveryCarbon preparation/purificationSucroseSide effect
The invention belongs to the technical field of medicine, and relates to mesoporous carbon and a preparation process thereof, and an application of the mesoporous carbon in insoluble drug delivery systems. According to the process, a hard template method is adopted to prepare the mesoporous carbon, wherein mesoporous silica is adopted as a template, sucrose is adopted as a carbon source, and sulfuric acid is adopted as a catalyst to prepare the mesoporous carbon, wherein the prepared mesoporous carbon has characteristics of large specific surface area, stable property, no toxic-side effect and good biocompatibility, and can be adopted as a insoluble drug carrier. According to the present invention, a solvent method and a melting method are adopted to carry out drug embedding and adsorption so as to achieve uniform dispersion of the drug in the pores and on the surface of the carrier; with the drug delivery system, water solubility of insoluble drugs can be significantly enhanced, and in vitro dissolution rate and oral bioavailability can be improved.
Owner:SHENYANG PHARMA UNIVERSITY
Nano-structural lipid carrier pharmaceutical composition and preparation method thereof
InactiveCN105708799ANo harmGood physiological compatibilityPowder deliveryMetabolism disorderLipid formationLiquid state
The invention discloses a nano-structural lipid carrier pharmaceutical composition and a preparation method thereof. The pharmaceutical composition consists of the following components in percentage by weight: 0.02-1% of an insoluble drug, 0.3-15% of a solid-state lipid material, 0.5-20% of a liquid-state liquid material, 0.8-10% of a fat-soluble emulsifier, 1-15% of a water-soluble emulsifier and 39-97.38% of an aqueous solvent. The nano-structural lipid carrier pharmaceutical composition prepared by the invention can greatly overcome the shortcomings of the insoluble drug which is not easy to dissolve in water, low in oral bioavailability and the like. The preparation method is simple and controllable, good in repeatability, and an available drug delivery system is provided for the insoluble drug.
Owner:金银秀 +1
Preparation method for amphiphilic segmented copolymer with pH value and temperature sensitivities
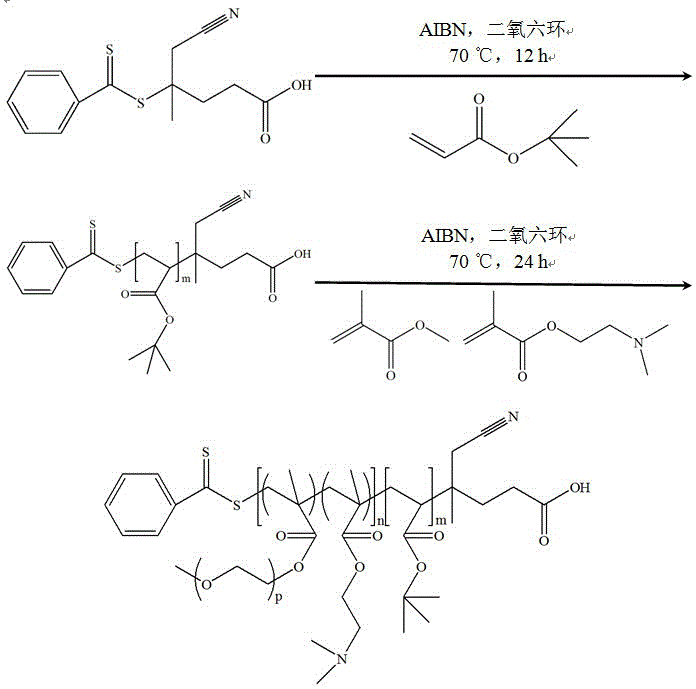
The invention relates to a preparation method for an amphiphilic segmented copolymer with pH value and temperature sensitivities. The preparation method comprises the following steps: by adopting a reversible addition-fragmentation chain transfer polymerization process, synthesizing a macromolecular chain transfer agent namely tert-butyl polyacrylate, and subjecting the tert-butyl polyacrylate with dimethylaminoethyl methacrylate and ethylene glycol monomethyl ether methacrylate to the reversible addition-fragmentation chain transfer (RAFT) polymerization process again so as to synthesize the amphiphilic segmented copolymer P(tBA)-b-P(DMAEMA-co-PEGMA. The segmented copolymer provided by the invention can self-assemble to form micelles in an aqueous solution, and has pH value and temperature sensitivities, wherein the micelles have a critical pH value of 7 and a critical temperature of 37.5 DEG C. The preparation method provided by the invention has the advantages of high yield, relatively wide applicable monomer range, low requirements on reaction conditions, simple and convenient operation, greenness and environmental friendliness; and the amphiphilic segmented copolymer prepared by using the method provided by the invention has double pH value and temperature sensitivities and broad application prospects, and can be applied to the chemical production fields like dye adsorption, the environment protection fields like heavy metal pollution treatment, and the biomedical fields like controllable release of insoluble drugs.
Owner:TONGJI UNIV
Novel nano preparation with stable protein and preparation method and use thereof
InactiveCN101385857AShort operating timeStable productionOrganic active ingredientsMacromolecular non-active ingredientsWater insolubleFree protein
The invention discloses a novel nano preparation with stable protein, as well as a preparation method and a purpose thereof. The invention is characterized in that: a protein coating is formed from albumin and other materials containing sulphydryl or disulfide bond through the cross linking of the disulfide bond; the protein coating contains free protein or protein derivatives which associate(s) with the protein coating, wherein, part of insoluble drugs are contained in the protein coating and part of such drugs are associated with the free protein or the protein derivatives, and the average diameter of protein coating particles is not more than 1 micron. The purpose of the preparation is to send water insoluble drugs into the bodies of living things.
Owner:CHINA PHARM UNIV
Preparation method of insoluble drug nanosuspension
ActiveCN107281100ASimple preparation processGood reproducibilitySolution deliveryPharmaceutical non-active ingredientsOrganic solventMass ratio
The invention discloses a preparation method of insoluble drug nanosuspension. The preparation method comprises the following steps: firstly, dispersing an insoluble drug into an aqueous solution containing a stabilizer to obtain an initial suspension, wherein the mass ratio of the insoluble drug to the stabilizer is 2.5-1 to 8-1; secondly, carrying out high-speed shearing on the initial suspension obtained in the first step to obtain coarse suspension; thirdly, carrying out micro-jet high-pressure homogenizing on the coarse suspension obtained in the second step to obtain the insoluble drug nanosuspension, wherein the drug concentration of the insoluble drug nanosuspension is 150 to 300mg / mL. The preparation method disclosed by the invention has the advantages of no use of an organic solvent, simple preparation technology, good repeatability and good safety; in addition, the drug concentration of the prepared insoluble drug nanosuspension can reach 150 to 300mg / mL, the particle size of the drug is small and the distribution of the drug is uniform.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Lurasidone hydrochloride orally-disintegrating tablet preparation and preparation method thereof
InactiveCN103054824AOrganic active ingredientsNervous disorderLurasidone HydrochlorideOrally disintegrating tablet
The invention belongs to the technical field of medicine, and relates to a lurasidone hydrochloride orally-disintegrating tablet preparation and a preparation method thereof. The orally-disintegrating tablet comprises the following components in mass percent: 5-60 percent of lurasidone hydrochloride, 25-80 percent of filling material, 5-20 percent of disintegrating agent, 0.02-0.15 percent of wetting agent, 0.2-5 percent of flavoring agent and 0.5-2 percent of lubricating agent. The invention aims to provide a lurasidone hydrochloride orally-disintegrating tablet with simple preparation process, low cost, convenience in use and quick action to the indications; and compared with the conventional preparation for oral use, the lurasidone hydrochloride orally-disintegrating tablet preparation can reduce the difficulty in swallowing, improve the compliance, is suitable for special populations such as the elderly, children, patients with difficulty in swallowing and mental patients, can be fully disintegrated within 60 s, can be disintegrated into extremely fine powder, is higher in drug dissolution rate and quick in absorption, and can improve the bioavailability of insoluble drugs, for example, lurasidone hydrochloride.
Owner:BEIJING VENTUREPHARM BIOTECH
Beta-cyclodextrin based pH responsive star polymer, micelle and composite material
ActiveCN105017445ARealize the combinationImprove treatment efficiencyOrganic active ingredientsX-ray constrast preparationsWater insolubleTumor chemotherapy
Belonging to the technical field of biomedical polymer materials, the invention discloses a beta-cyclodextrin based amphiphilic pH responsive star polymer and a preparation method thereof, a micelle system based thereon, a composite material and application thereof. The polymer has a structure shown as the formula (1) in the specification, wherein x=3-20, y=2-30, z=5-35, and n=2-10. The polymer can be reduced in situ to obtain nanogold with small particle size and good stability. The invention also provides a micelle system based on the polymer, the micelle system has strengthened entrapment ability to water-insoluble drugs, can efficiently load hydrophobic drugs, gold nanoparticles and other substances, realizes the combination of tumor imaging diagnosis and tumor chemotherapy, and improves the therapeutic efficiency of cancer. And the polymer is easy to control the proportion of each block, and can be applied to preparation of the water-insoluble drug loaded micelle system to satisfy the release requirements of different drugs.
Owner:SOUTH CHINA UNIV OF TECH
Transdermal delivery system for water insoluble drugs
InactiveUS20050287217A1Minimal irritationImprove drug solubilityBiocideOrganic active ingredientsPharmaceutical drugWater insoluble drug
The present invention provides a system for transdermal delivery of water insoluble drugs and methods using the same. The system includes a pharmaceutical composition of a water insoluble drug and a carrier molecule that enhances the solubility of the drug in aqueous solution, a medical patch containing the same and an apparatus that generates hydrophilic micro-channels in an area of skin of a subject using the composition or patch. The system preferably avoids the use of penetration enhancers and is particularly useful for transdermal delivery of steroids.
Owner:SYNERON MEDICAL LTD
Liquid compositions of insoluble drugs and preparation methods thereof
ActiveUS9339553B2Good biocompatibilityEliminate hidden dangersBiocideCarbohydrate active ingredientsPhospholipidSolvent
A liquid composition of an insoluble medicament and a preparation method thereof are disclosed. The composition includes insoluble medicament, oil for injection, phospholipid, and solvent; the percentage by weight of each component is as follows: insoluble medicament 0.01-10%, oil for injection 0%-20%, phospholipid 10-80%, solvent 20-89%. The preparation method for the composition includes the following steps: dissolving an insoluble medicament into solvent or oil for injection or a mixture thereof firstly, and then adding other components, and mixing uniformly; or dissolving an insoluble medicament into a mixture of other components, and mixing uniformly; or dissolving an insoluble medicament into part of solvent firstly, and then adding into a mixed solvent of other components and the remaining solvent, and mixing uniformly.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com