Preparation method of novel hard-soluble medicine liposome
A technology of insoluble drugs and liposomes, which is applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of no residue, high risk, environmental pollution, etc., and achieve particle control diameter size, stable product quality, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Prescription: Paclitaxel 1g
[0038] Soy Lecithin 30g
[0039] Tween 80 1g
[0040] Sodium deoxycholate 1g
[0041] Cholesterol 3g
[0042] Mannitol 60g
[0043] water 600mL
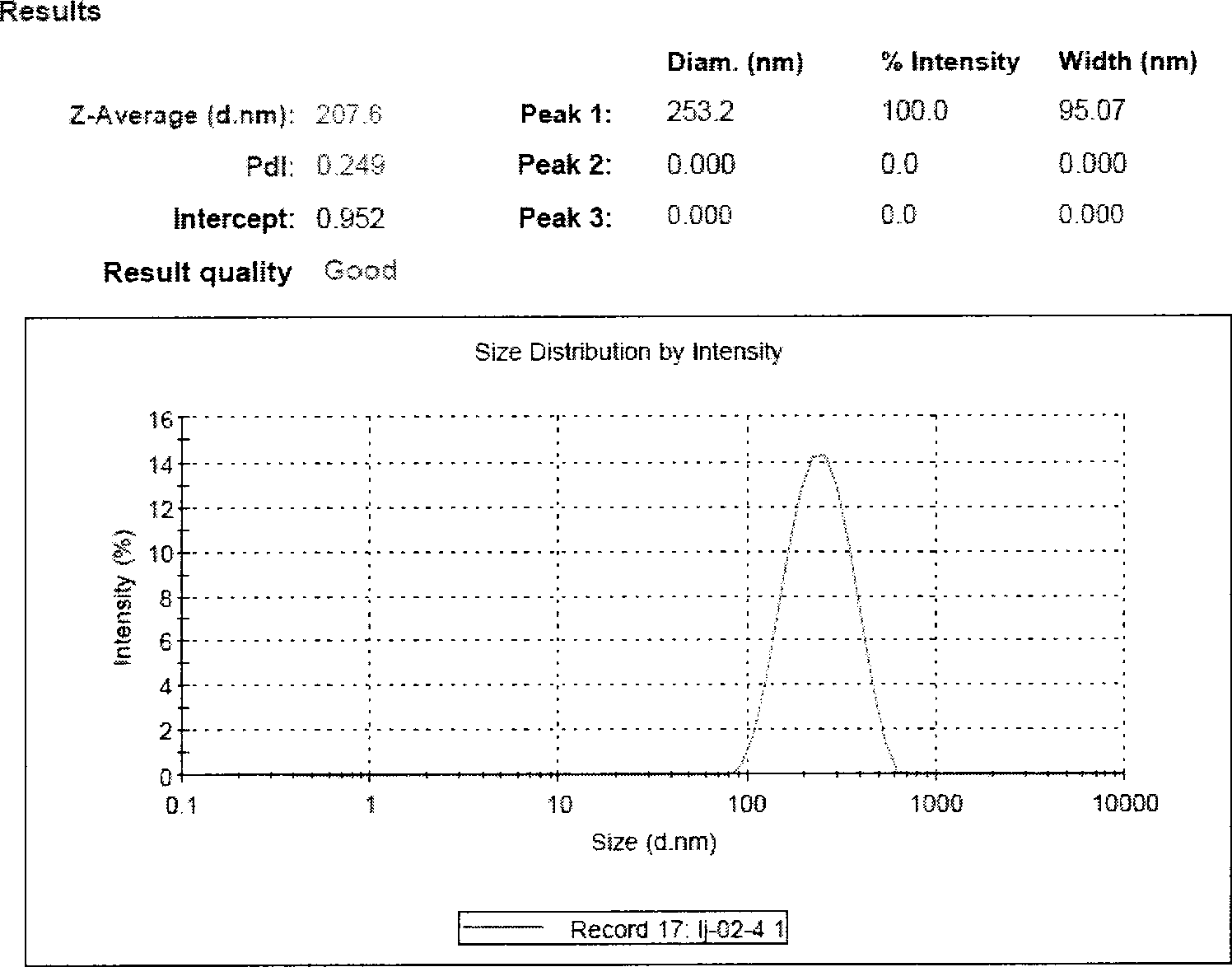
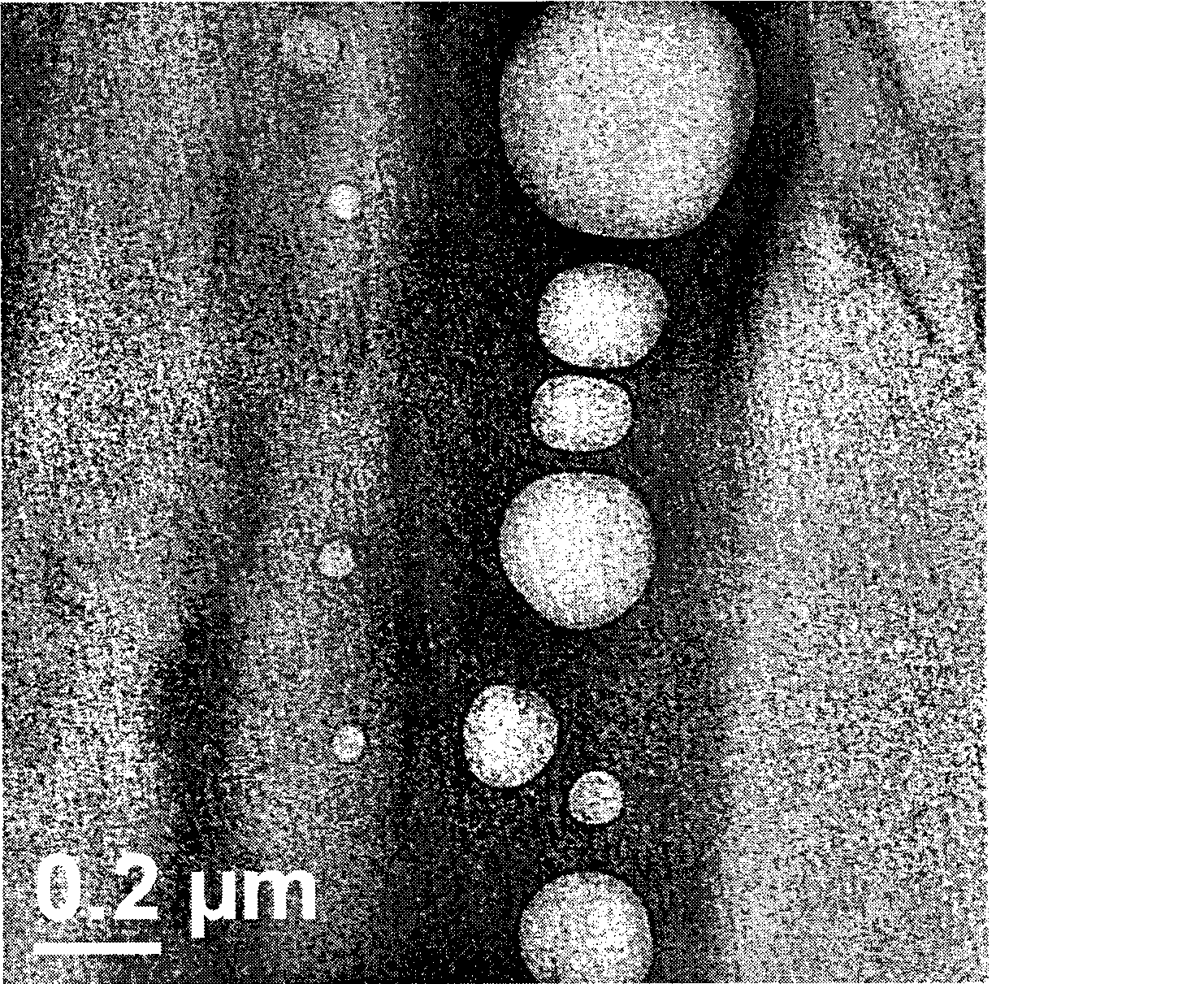
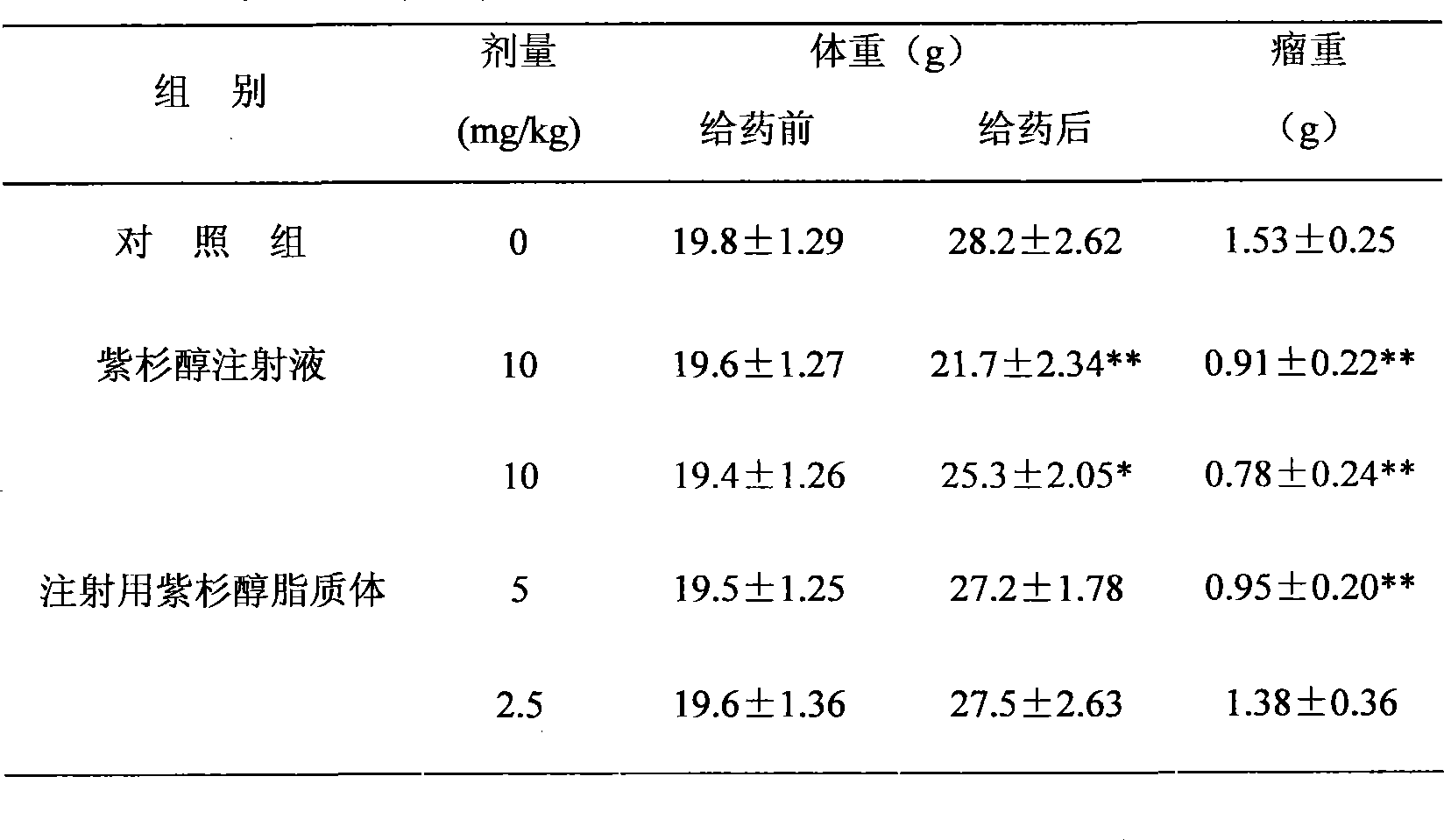
[0044] Preparation process: mix paclitaxel, Tween 80, and sodium deoxycholate uniformly to obtain mixture a; grind soybean lecithin, cholesterol and mannitol (water-soluble diluent) together until uniformly mixed to obtain mixture b; mix mixture a and b Grind until mixed uniformly to obtain mixture c, add 600mL of water for injection, and use a colloid mill for shearing and mixing; after mixing evenly, perform high-pressure homogeneous circulation to obtain a paclitaxel liposome suspension with an average particle size of 90%.
Embodiment 2
[0046] Prescription: Paclitaxel 1g
[0047] Soy Lecithin 30g
[0048] Tween 80 3g
[0049] Mannitol 60g
[0050] water 1000mL
[0051] Preparation process: Mix paclitaxel and Tween 80 evenly to obtain mixture a; grind soybean lecithin and mannitol (water-soluble diluent) together until mixed evenly, pass nitrogen protection during grinding, and keep the temperature of the material below 20°C through a condensing device , to obtain mixture b; grind mixture a and b until they are evenly mixed, pass nitrogen protection during the grinding, and keep the material temperature below 20°C through a condensing device to obtain mixture c, add 1000mL water for injection, and shear at high speed until uniformly mixed. During the cutting process, the temperature of the material was kept below 20°C by a condensing device, and nitrogen was protected; after mixing evenly, a high-pressure homogenization cycle was carried out, and then treated with a liposome extruder to obtain a paclitaxel ...
Embodiment 3
[0053] Prescription: Paclitaxel 1g
[0054] Soy Lecithin 30g
[0055] Tween 80 3g
[0056] Vitamin E 0.1g
[0057] Mannitol 60g
[0058] water 1000mL
[0059] Preparation process: Mix paclitaxel and Tween 80 evenly to obtain mixture a; grind soybean lecithin, vitamin E (antioxidant) and mannitol (water-soluble diluent) together until mixed evenly, pass nitrogen protection during the grinding, pass condensation The device keeps the temperature of the material below 20°C to obtain mixture b; grind the mixture a and b until they are evenly mixed, pass nitrogen protection during the grinding, and keep the temperature of the material below 20°C through the condensation device to obtain mixture c, add 1000mL of water for injection, high-speed Shear until the mixture is uniform. During the high-speed shearing process, the temperature of the material is kept below 20°C through the condensing device, and the nitrogen is protected; after the mixture is uniform, the high-pressure hom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com