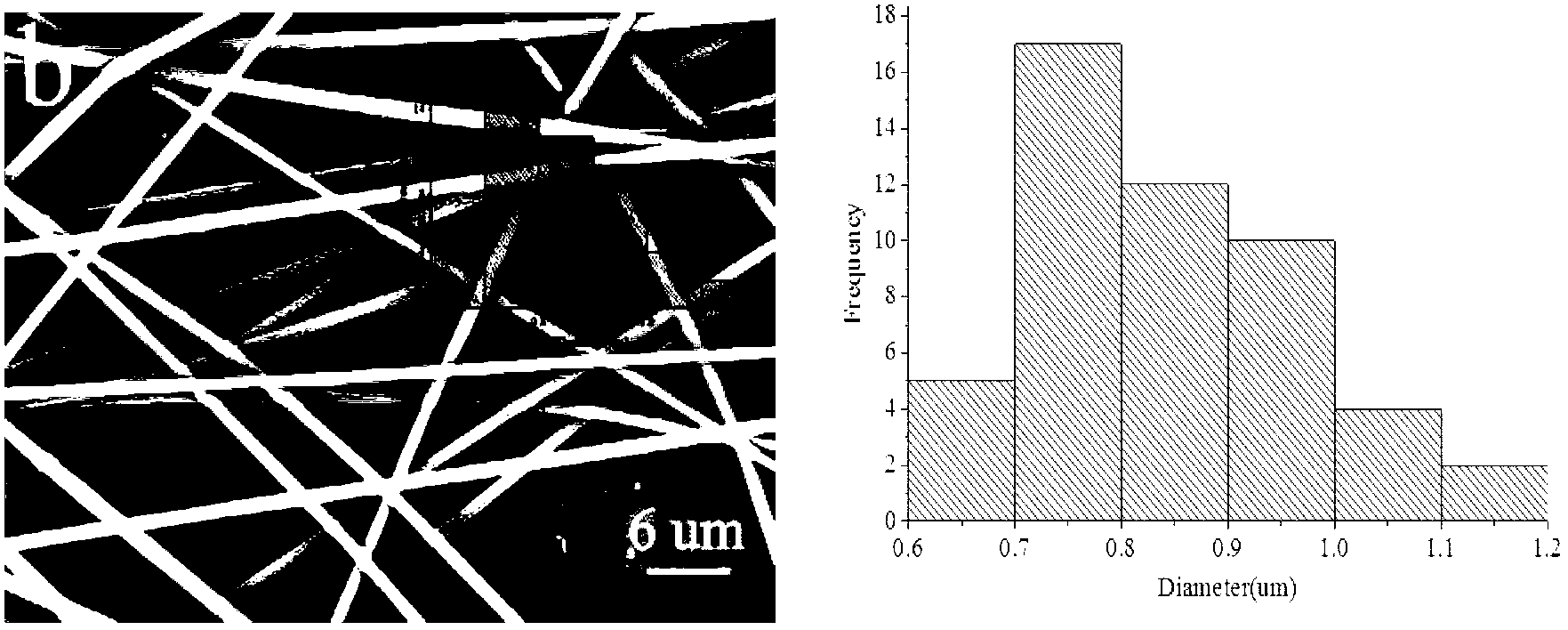
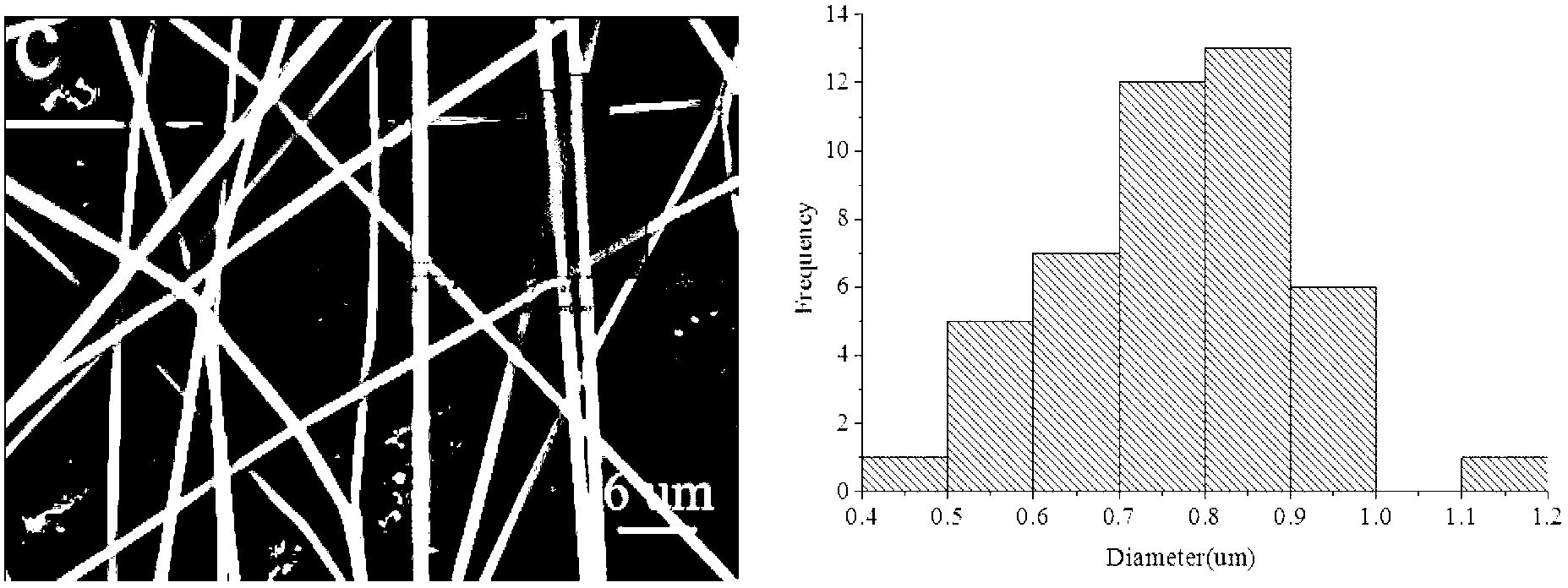
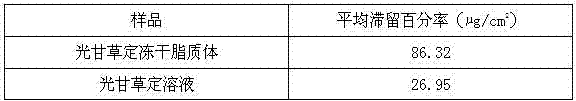
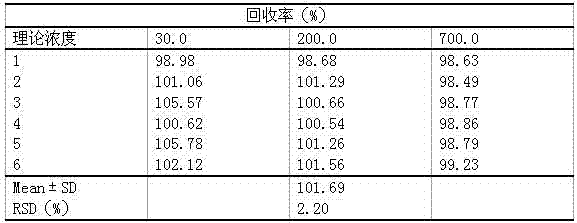
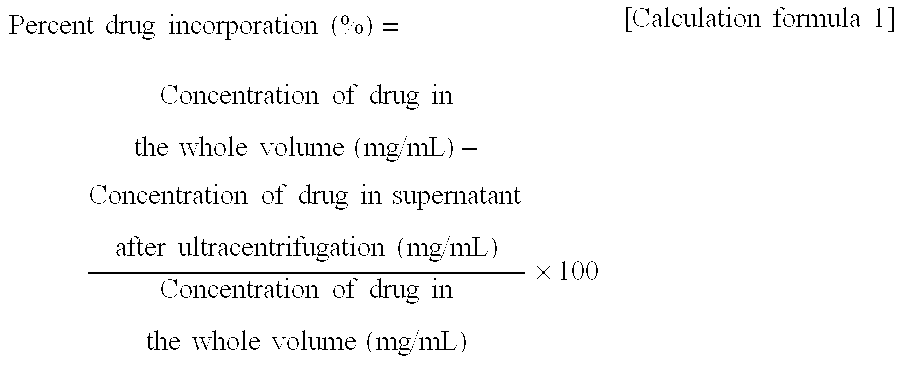Patents
Literature
164 results about "Liposome suspension" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Self forming, thermodynamically stable liposomes and their applications
InactiveUS6958160B1Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsSelf formingLipid composition
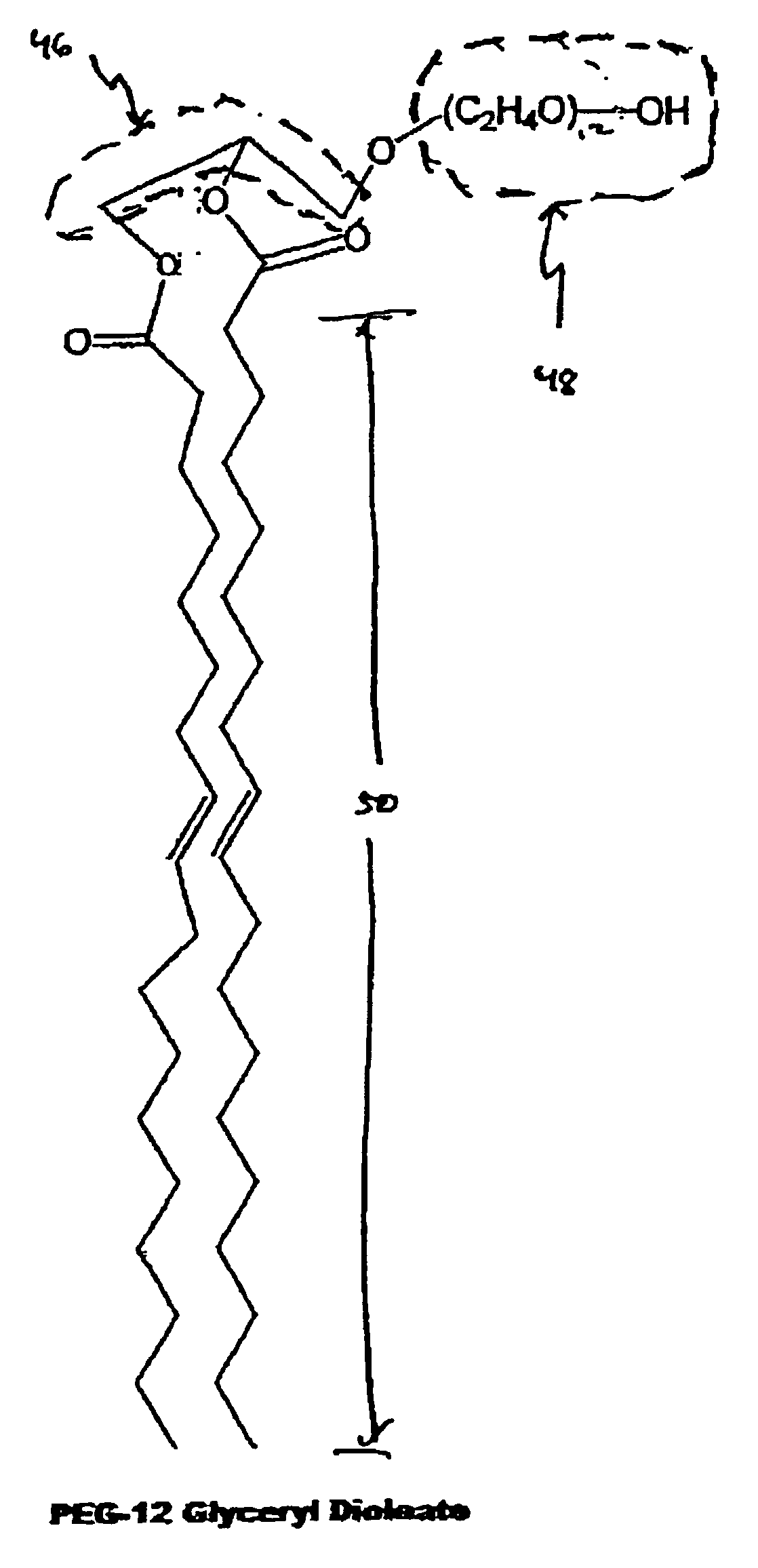
A liposome suspension forms spontaneously upon adding a lipid composition to an aqueous solution. The liposomes include diacylglycerol-PEG compounds. The melting point of the diacylglycerol-PEG is below about 40 degrees C., and the acyl chains of the diacylglycerol-PEG are greater than or equal to 14 carbons in length. Such liposome suspensions are useful for a variety of purposes, including the delivery of therapeutic agents.
Owner:BIOZONE LAB
Method of pulmonary administration of an agent
A method for administering a therapeutic or diagnostic agent to a subject is described. The method includes providing a suspension of liposomes comprised of one or more of vesicle-forming lipids selected from (i) a vesicle-forming lipid derivatized with a hydrophilic polymer and (ii) a neutral lipopolymer, said liposomes being associated with said therapeutic or diagnostic agent, forming an aerosol of said liposome suspension; and administering the aerosol to the subject by inhalation. The liposome formulation delivers intact liposomal particles to the respiratory tract of said subject to form a depot of therapeutic agent therein with no observable provocation of an immune response, as measured by neutrophil or macrophage cell count in the lung after administration.
Owner:ALZA CORP
Spray freeze dried liposomal ciprofloxacin powder aerosol drug delivery
A powder for inhalatory aerosol delivery, the powder having: spray freeze dried liposome particles with a biologically active agent, such as an antibiotic, encapsulated within a phospholipid, and a method of producing a powder for inhalatory aerosol delivery, the method including the steps of: mixing a biologically active agent with a phospholipid to form a liquid liposome suspension; and spray freeze drying the liposome suspension to form particles of powder.
Owner:WARREN H FINLAY +2
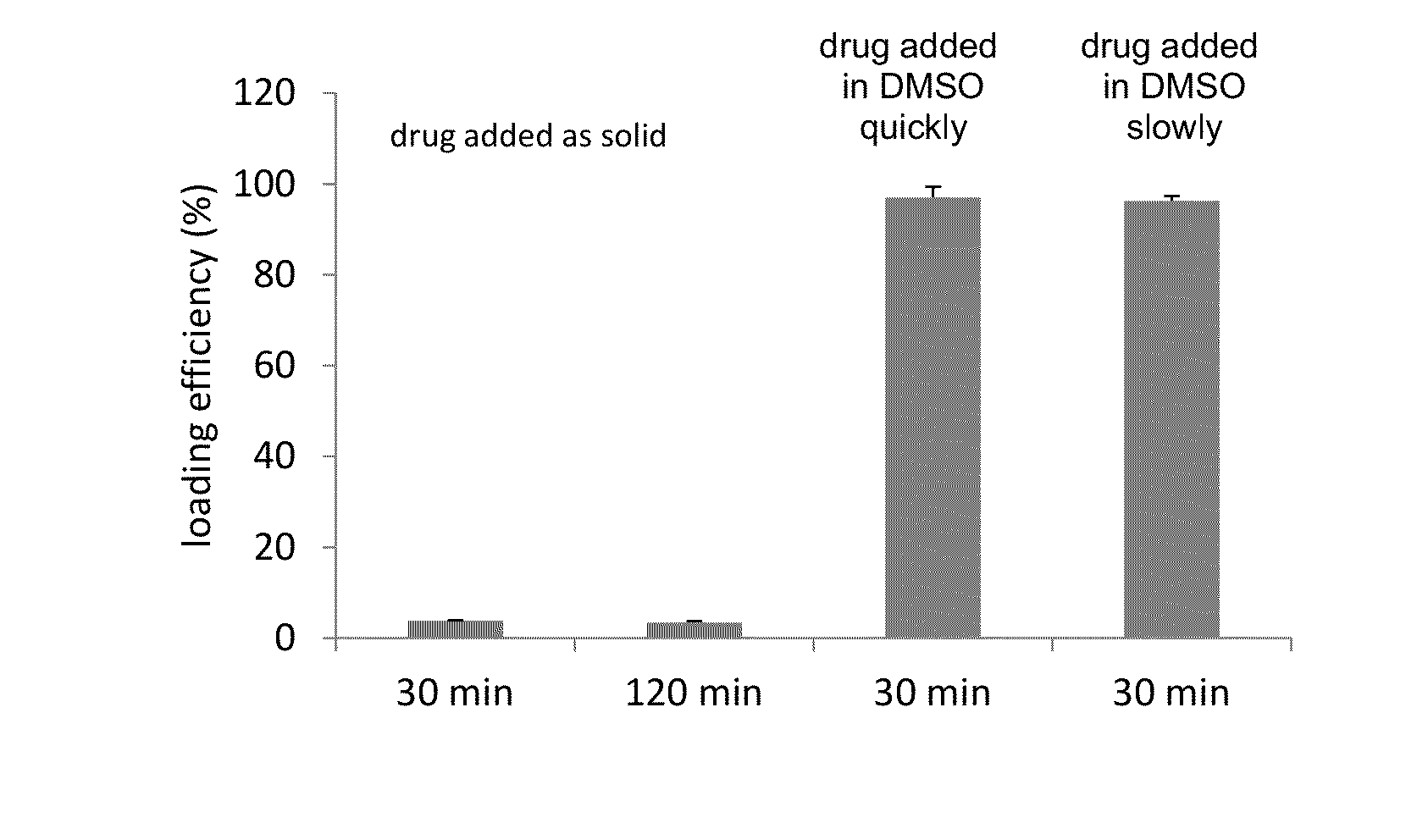
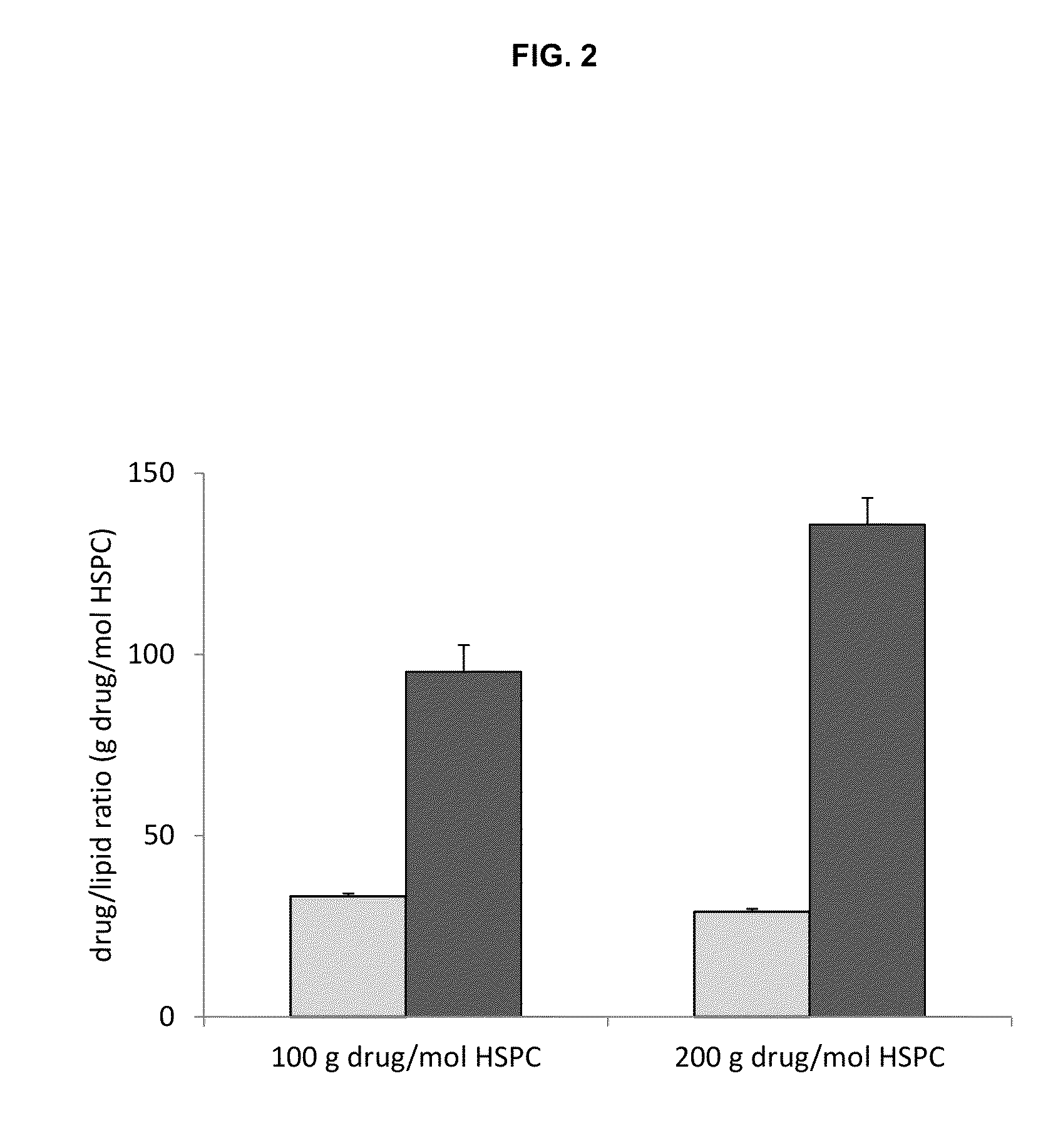
Remote loading of sparingly water-soluble drugs into liposomes
InactiveUS20140220111A1Raise the ratioCost efficiencyOrganic active ingredientsInorganic non-active ingredientsLipid formationDisease
The present invention provides liposome compositions containing sparingly soluble drugs that are used to treat life-threatening diseases. A preferred method of encapsulating a drug inside a liposome is by remote or active loading. Remote loading of a drug into liposomes containing a transmembrane electrochemical gradient is initiated by co-mixing a liposome suspension with a solution of drug, whereby the neutral form of the compound freely enters the liposome and becomes electrostatically charged thereby preventing the reverse transfer out of the liposome. There is a continuous build-up of compound within the liposome interior until the electrochemical gradient is dissipated or all the drug is encapsulated in the liposome. However, this process as described in the literature has been limited to drugs that are freely soluble in aqueous solution or solubilized as a water-soluble complex. This invention describes compositions and methods for remote loading drugs with low water solubility (<2 mg / mL). In the preferred embodiment the drug in the solubilizing agent is mixed with the liposomes in aqueous suspension so that the concentration of solubilizing agent is lowered to below its capacity to completely solubilize the drug. This results in the drug precipitating but remote loading capability is retained. The process is scalable and, in liposomes in which the lipid composition and remote loading agent are optimized, the resulting drug-loaded liposomes are characterized by a high drug-to-lipid ratios and prolonged drug retention when the liposome encapsulated drug is administered to a subject.
Owner:ZONEONE PHARMA
Polymer coated vitamin E liposome and preparation method thereof
InactiveCN101780041AReduce the degree of oxidationHigh biological potencyPowder deliveryOrganic active ingredientsPolymer scienceFreeze-drying
The invention relates to a polymer coated vitamin E liposome and a preparation method thereof. The preparation method of the invention comprises the following steps: preparing a vitamin E liposome suspension; adding a polymer into the suspension for coating to obtain a polymer coated vitamin E liposome suspension; obtaining a polymer coated vitamin E proliposome solid powder by spray drying or freeze drying; and before use, adding a proper amount of distilled water as required to obtain the polymer coated vitamin E liposome suspension with the entrapment rate of 82-94%. The polymer coated vitamin E liposome of the invention can obviously reduce the degree of oxidation of vitamin E, reduce the percolation rate, delay the release of the vitamin E in vivo, prolong the cycling time in vivo, and be prepared into the coated type proliposome solid powder which can be more conveniently packed, stored, transported and used.
Owner:NANCHANG UNIV
Method for preparing natural material-liposome composite nanofiber based on electrostatic spinning technology
InactiveCN102797074ALow priceEasy to operateMonocomponent protein artificial filamentFilament/thread formingFiberCross-link
The invention relates to a method for preparing a natural material-liposome composite nanofiber based on electrostatic spinning technology. The method comprises the following steps of: (1) adding lecithin, cholesterol and octadecylamine into a reaction vessel, then adding anhydrous alcohol, stirring for dissolving, and finally depressurizing to remove alcohol so as to obtain a liposome membrane; (2) adding deionized water into the reaction vessel containing the liposome membrane, stirring at room temperature, and then carrying out ultrasonic treatment so as to obtain a liposome suspension with uniform particle sizes; (3) preparing a spinning solution containing natural materials by taking the liposome suspension as a solvent, and then carrying out electrostatic spinning so as to obtain a nanofiber; and (4) fumigating and cross-linking the nanofiber in a genipin solution or alcohol steam. The method provided by the invention has the advantages of simplicity in operation, low cost of raw materials, mild reaction conditions and good biocompatibility; and a composite nanofiber bracket prepared by the method provided by the invention can control the release of genes, growth factors and various medicaments, has stable performance, is easy to preserve, and has wide application prospects.
Owner:DONGHUA UNIV
Method for preparing antioxidation gelatine membrane containing tea polyphenol nano lipidosome
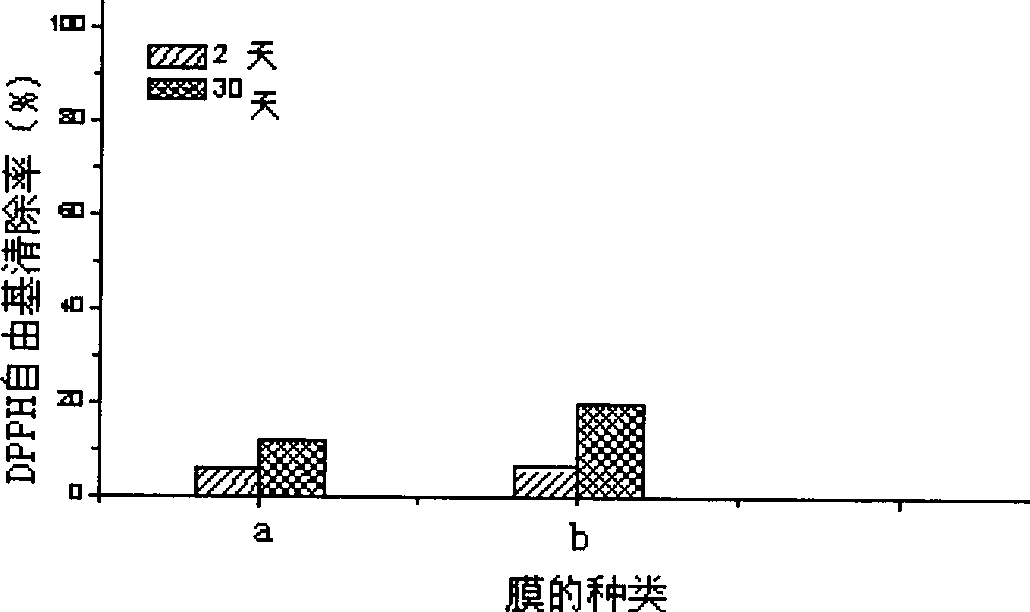
InactiveCN101461534AControl releaseImprove antioxidant capacityFood shapingFood preparationPhenolic content in teaGlycerol
The invention relates to preparation of an oxidation resistant gelatin film containing a tea polyphenol nano-liposome, pertains to the application technology field of nano-liposome controlled release and oxidation resistance. The oxidation resistant gelatin film component comprises tea polyphenol nano-liposome, gelatin and glycerin. The preparation includes dissolving gelatin and glycerin into deionized water in proportion to produce a gelatin solution, charging a predetermined amount of tea polyphenol nano-liposome suspension, mixing uniformly, hypersonic de-gassing, and removing dried film spreading on the organic glass board. The prepared oxidation resistant gelatin film has oxidation resistance greatly improved comparing with the simple gelatin film phase, and oxidation resistance of the gelatin film is capable of lasting for longer time due to protection function of the liposome to the tea polyphenol and slowly-releasing of the tea polyphenol. The gelatin film can be biologically degraded, without environment pollution during application process; has functions of preventing loss of flavor substance in the food, preventing food oxidation, so as to reach the effect of anti-staling and prolonging storage period.
Owner:JIANGNAN UNIV
Remote loading of sparingly water-soluble drugs into liposomes
InactiveUS20140220110A1Raise the ratioCost efficiencyBiocideInorganic non-active ingredientsDiseaseElectrochemical gradient
The present invention provides liposome compositions containing sparingly soluble drugs that are used to treat life-threatening diseases. A preferred method of encapsulating a drug inside a liposome is by remote or active loading. Remote loading of a drug into liposomes containing a transmembrane electrochemical gradient is initiated by co-mixing a liposome suspension with a solution of drug, whereby the neutral form of the compound freely enters the liposome and becomes electrostatically charged thereby preventing the reverse transfer out of the liposome. There is a continuous build-up of compound within the liposome interior until the electrochemical gradient is dissipated or all the drug is encapsulated in the liposome. However, this process as described in the literature has been limited to drugs that are freely soluble in aqueous solution or solubilized as a water-soluble complex. This invention describes compositions and methods for remote loading drugs with low water solubility (<2 mg / mL). In the preferred embodiment the drug in the solubilizing agent is mixed with the liposomes in aqueous suspension so that the concentration of solubilizing agent is lowered to below its capacity to completely solubilize the drug. This results in the drug precipitating but remote loading capability is retained. The process is scalable and, in liposomes in which the lipid composition and remote loading agent are optimized, the resulting drug-loaded liposomes are characterized by a high drug-to-lipid ratios and prolonged drug retention when the liposome encapsulated drug is administered to a subject.
Owner:ZONEONE PHARMA
Preparation method of novel hard-soluble medicine liposome
InactiveCN101385715AWell mixedSimple production processPharmaceutical non-active ingredientsLiposomal deliveryOrganic solventDiluent
The invention provides a preparation method of a novel insoluble drug liposome without the application of an organic solvent in the preparation process. The invention names the method as a water soluble surfactant dispersion method. The preparation method comprises the following steps: A. an insoluble drug is dissolved or suspended or mixed in a water soluble surfactant; B. lipid substances, a water soluble diluent and the water soluble surfactant which is dissolved or suspended or mixed with the insoluble drug for forming the liposome are evenly mixed; C. the appropriate amount of water or water solution is added in the mixture which is obtained in step B, the even mixing is carried out, and the high pressure homogenization or the high pressure homogenization is carried out, thereby obtaining liposome suspension; D. other liposome components can be added by adopting the appropriate method in any step of the steps of A, B and C according to the physical and the chemical properties, if the insoluble drug liposome does not contain other liposome components, the liposome components can not be added.
Owner:CHINA PHARM UNIV
Terpene carrier
InactiveUS20160309774A1Good carrierEnhance the beneficial effectTobacco treatmentHydroxy compound active ingredientsBULK ACTIVE INGREDIENTActive ingredient
Owner:WAND MICHAEL D
Process for producing liposome suspension and product containing liposome suspension produced thereby
A process for the large scale production of a liposome suspension, in which three selected lipid compounds in a predetermined ratio are dissolved in an alcohol solvent to form a mixture, which, in turn, is directly admixed with an aqueous ammonium sulfate solution in a predetermined ratio. The resultant mixture is subjected to a pore-extrusion treatment, followed by dialyzing the pore-extruded mixture with a 5% to 15% sucrose aqueous solution, such that a liposome suspension containing liposome particles suspended in the liposome suspension is obtained. The thus obtained liposome suspension can be used to encapsulate a selected drug, in particular doxorubicin.
Owner:TTY BIOPHARM
Method of treating insulin resistance, adult onset diabetes and metabolic syndrome x
InactiveUS20050287197A1Little riskReduce absorptionNervous disorderPeptide/protein ingredientsCreatine kinaseCreatine kinase.MB
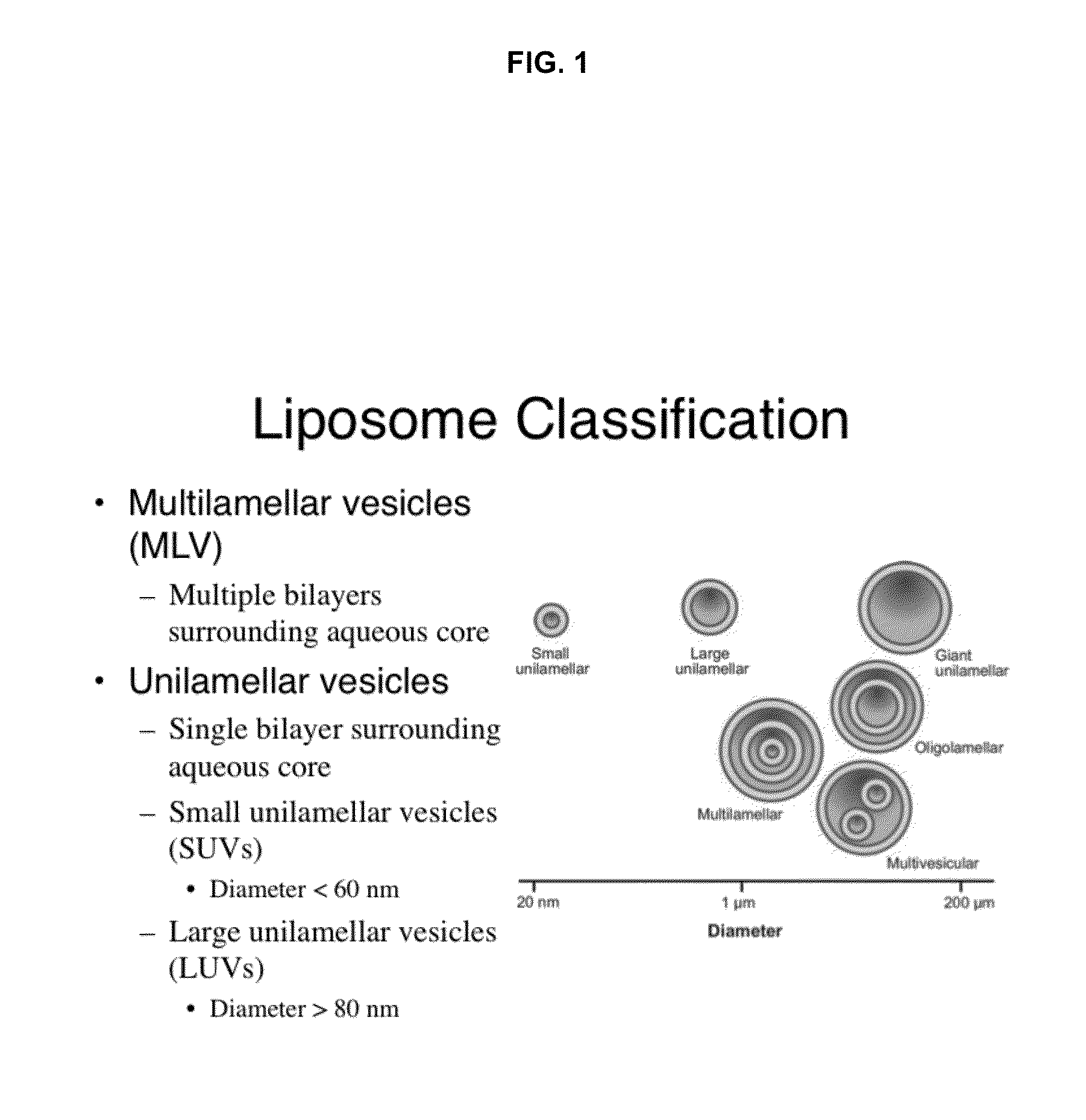
A method of treating insulin resistance, adult onset diabetes, and metabolic syndrome X and its related complications, in mammalian subject is accomplished by intravenously administering to a mammalian subject, a therapeutically effective amount of a liposomal suspension of lipoprotein small unilamellar vesicles (SUVs) comprising predominantly phospholipids. The liposomal suspension is administered over a period of time, whereby in the levels of some or all of blood glucose, insulin, total cholesterol, LDL cholesterol, triglyceride, creatine kinase (CK), creatine kinase-MB (CK-MB), Hb-A1c, lipoprotein (a), SGOT and SGPT fall back within the normal range or are significantly reduced.
Owner:KURTZ SEYMOUR J
Method for production of liposome preparation
InactiveUS20100021531A1Induces no irregularity in the amount of incorporated drugShorten production timeLiposomal deliveryLipid degradationMembrane configuration
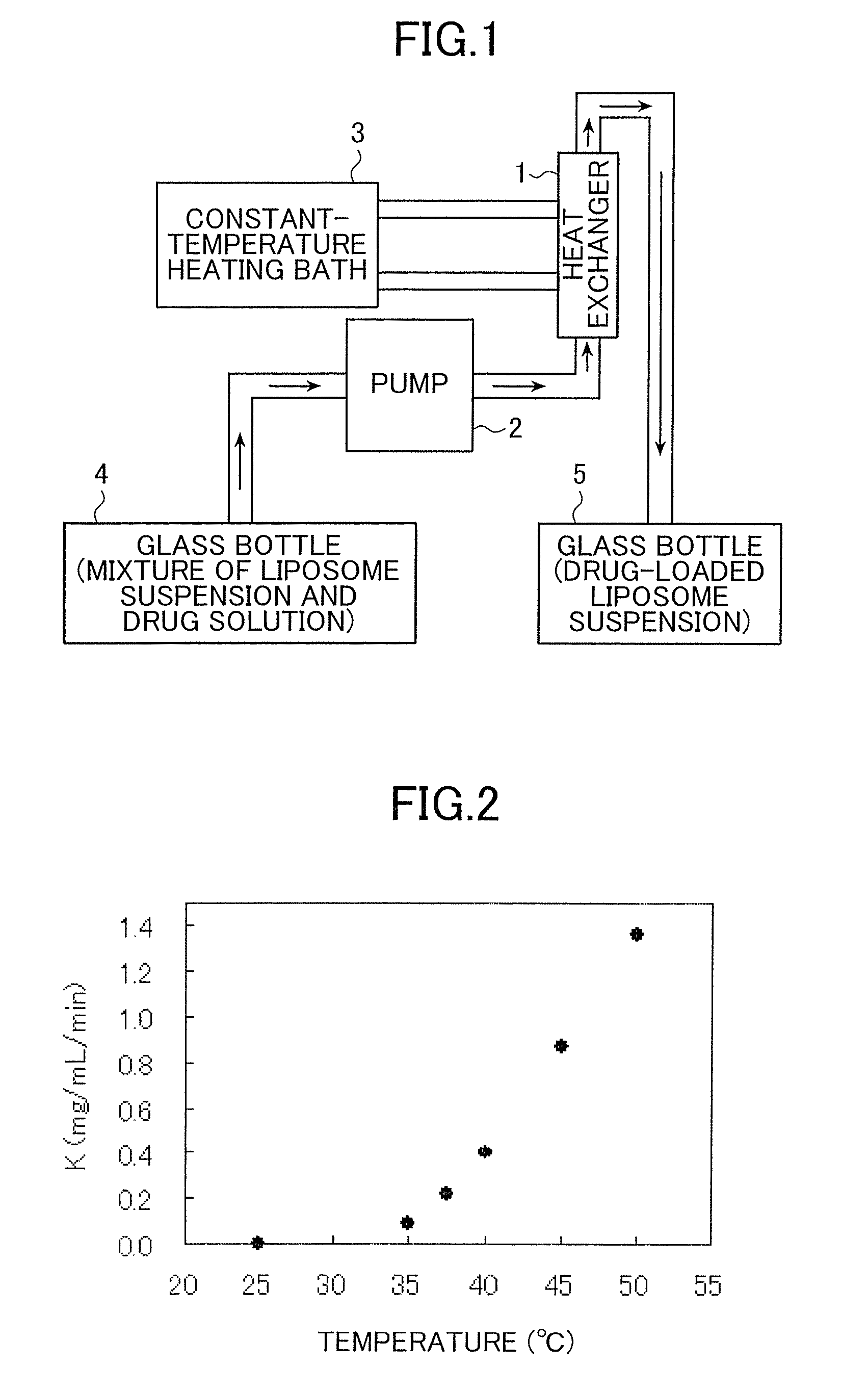
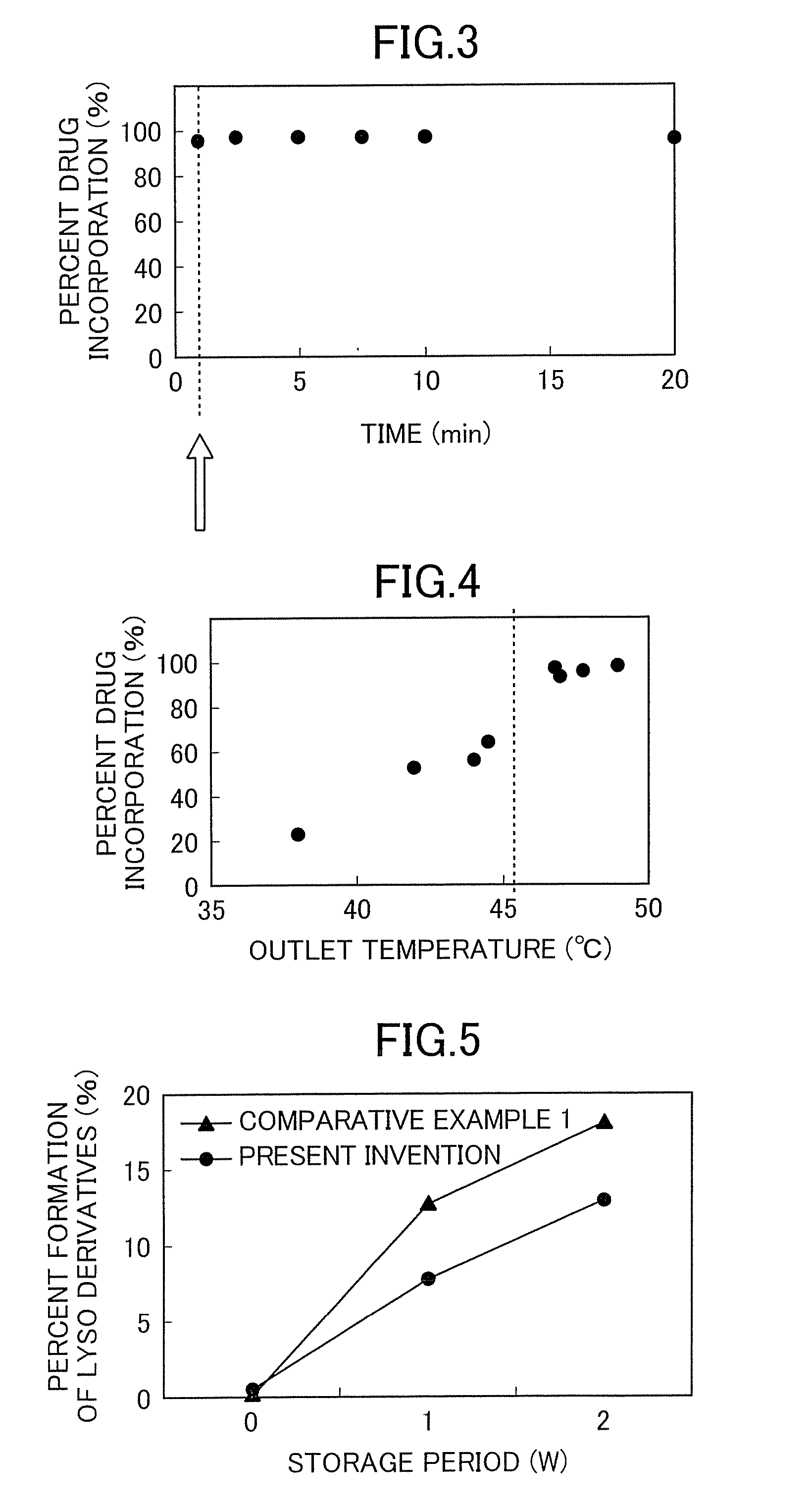
Disclosed is a method which permits simple and easy production of a stable, high-quality liposome preparation suppressed in lipid degradation. This method can significantly shorten production time and can achieve a substantial cost cut-down in medium- to large-scale production, and can also attain the incorporation of a drug in uniform amounts. Specifically disclosed is a method for producing a liposome preparation by using a remote loading method. This method includes a drug incorporation step that heats a mixture of a suspension of liposomes and a drug, the mixture having been prepared beforehand, by rapid heating means to a temperature from not lower than a phase transition point of membranes of the liposomes to not higher than 80° C. to incorporate the drug into the liposomes.
Owner:TERUMO KK
Human umbilical cord MSC (mesenchymal stem cell) serum-free medium liposome freeze-dried powder as well as preparation method and application thereof
ActiveCN106176563AReduce immune rejectionExtended shelf lifeCosmetic preparationsToilet preparationsFreeze thawingLipid formation
The invention discloses human umbilical cord MSC (mesenchymal stem cell) serum-free medium liposome freeze-dried powder, which consists of 1-3% of a human umbilical cord MSC serum-free medium active protein ingredient, 15-60% of phospholipid, 1-12% of cholesterol, 1-25% of Tween-80, 0.5-10% of a lipid-soluble antioxidant, 10-60% of a cryoprotectant and 1-5% of water. The invention also discloses a preparation method of the liposome freeze-dried powder, wherein the preparation method comprises the following steps: firstly, dissolving the phospholipid, the cholesterol, the Tween-80 and the lipid-soluble antioxidant in absolute ethyl alcohol, so that a raw material solution is obtained; then, adding the raw material solution to a buffer solution which contains the cryoprotectant and conducting hydrating; after conducting hydrating, removing all ethanol, and conducting ultrasonic granulation, so that a blank liposome suspension is obtained; and conducting an encapsulation reaction by adding a human umbilical cord MSC serum-free medium to the blank liposome suspension, conducting film filtration and granulation on a finished product, and sequentially implementing freeze-thawing and freeze-drying, so that the liposome freeze-dried powder is obtained. The liposome freeze-dried powder disclosed by the invention, which is directly added to cosmetics after being hydrated, has a function of delaying aging. The liposome freeze-dried powder disclosed by the invention is long in shelf life, good in cosmetic effect and free from immunological rejection.
Owner:西安艾尔菲生物科技有限公司
Submicron liposome suspensions obtained from preliposome lyophilizates
InactiveUS7238366B1Severe adverse effect on liposome integrityShorten hydration timePowder deliveryTetracycline active ingredientsLipid formationLiposome Vesicle
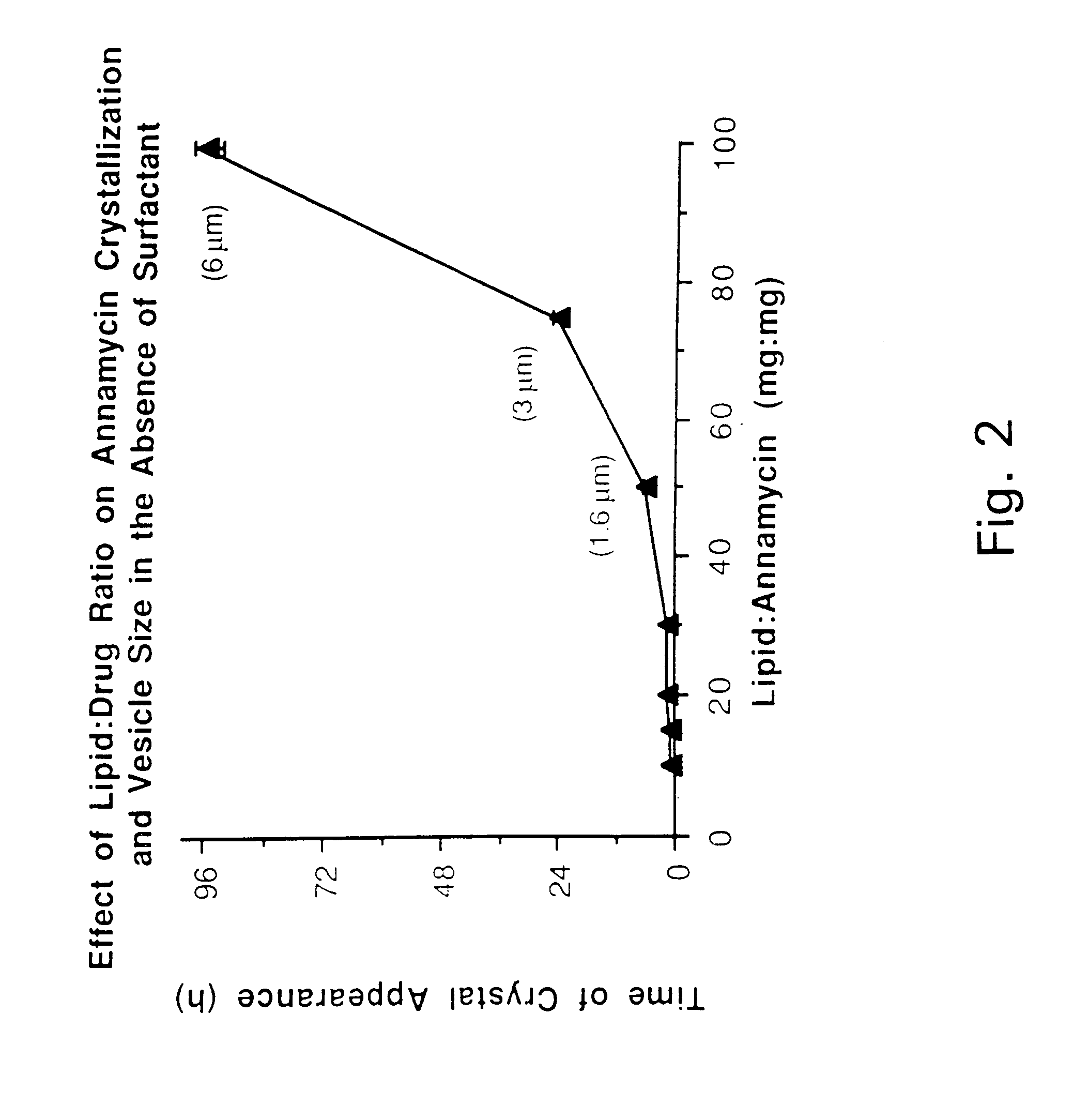
This invention provides an aqueous / t-butanol solvent-system, facile reconstitute, submicron-reconsitiute preliposome-lyophilaye and method of its preparation and use.In one embodiment this entails a modified method for the preparation of a submicron and stable liposome formulation of the non-cross-resistant anthracycline Annamycin is described. The optimal lipid composition was DMPC:DMPG at a 7:3 molar ratio and the optimal lipid:drug weight ratio 50:1. The selected formulation is a preliposome lyophilized powder that contains the phospholipids, Annamycin, and 1.7 mg Tween 20 per mg of Annamycin. The liposome suspension is obtained on the day of use by adding normal saline at 37° C. (1 ml per mg Annamycin) and hand-shaking for one minute. The presence of Tween 20 is essential in shortening the reconstitution step (from >2 hours to 1 minute), avoiding the early formation of free drug crystals, and reducing the median particle size (from 1.5 μm to 0.15-0.20 μm) without destruction of the liposome vesicles. The chemical stability of the preliposome powder at room temperature was >3 months and the chemical and physical stability of the liposome suspension at room temperature >24 hours. The in vitro cytotoxicity of the formulation was equivalent to that prepared by the standard evaporation method. The results of the study indicate that small amounts of surfactant may be used to enhance the reconstitution step and reduce the liposome size of lyophilized liposome formulations of lipophilic drugs.
Owner:BOARD OF REGENTS
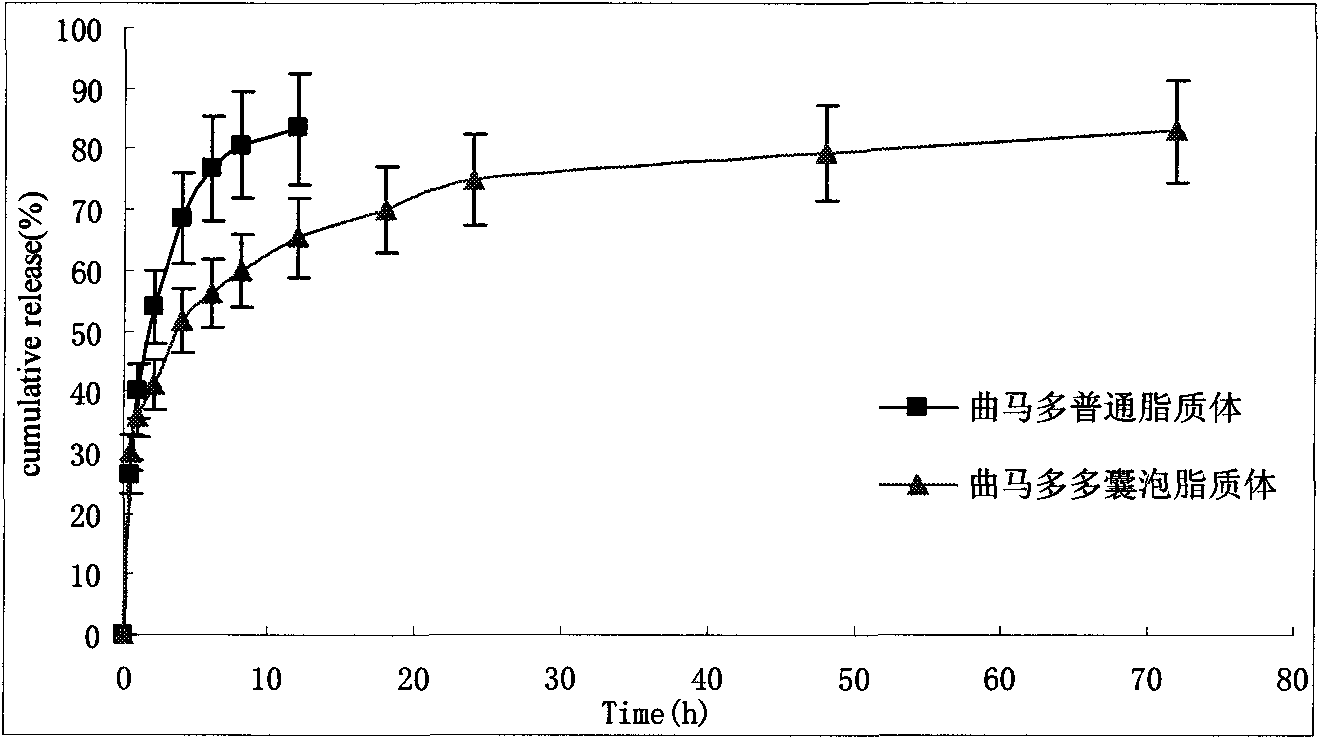
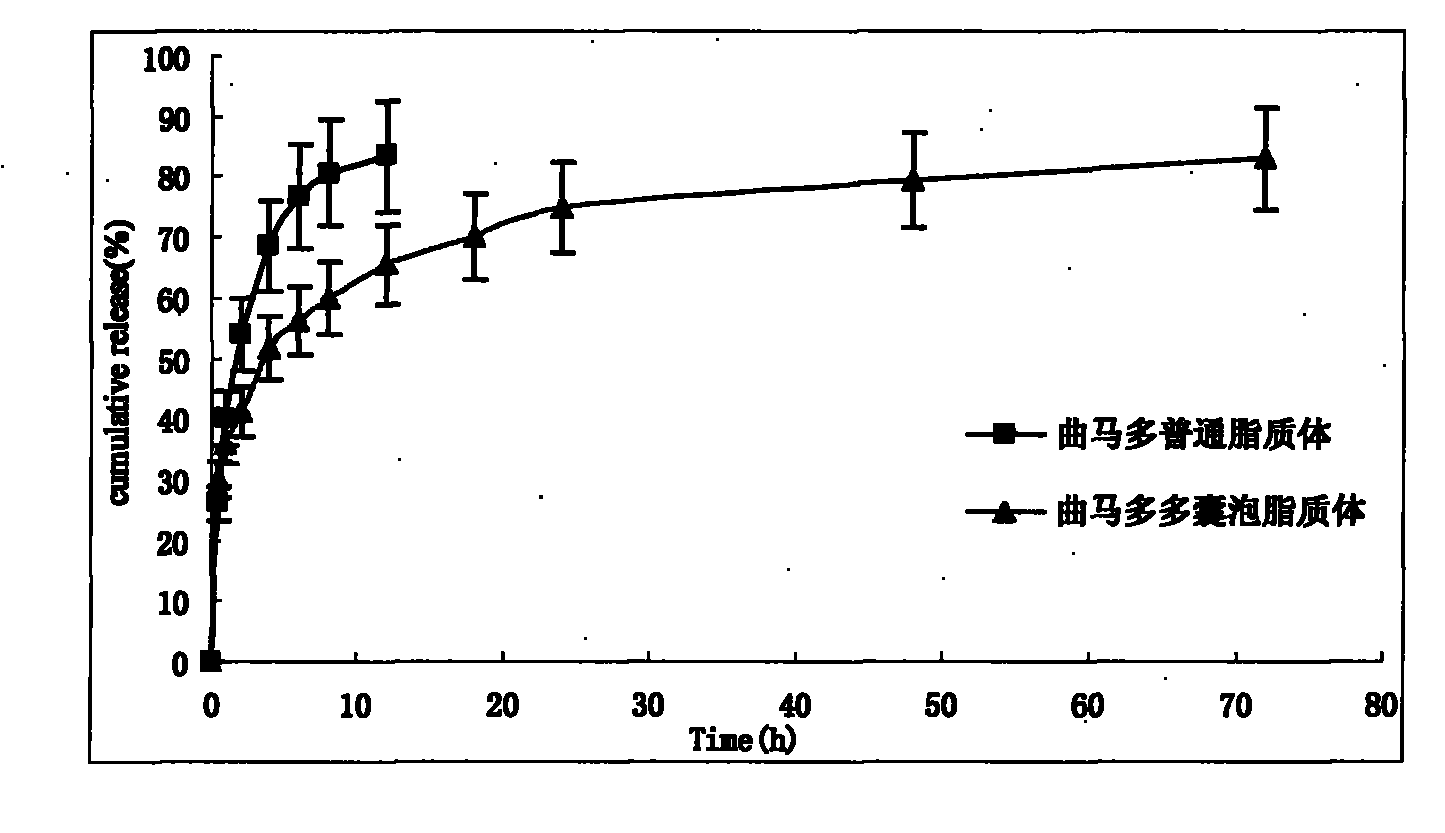
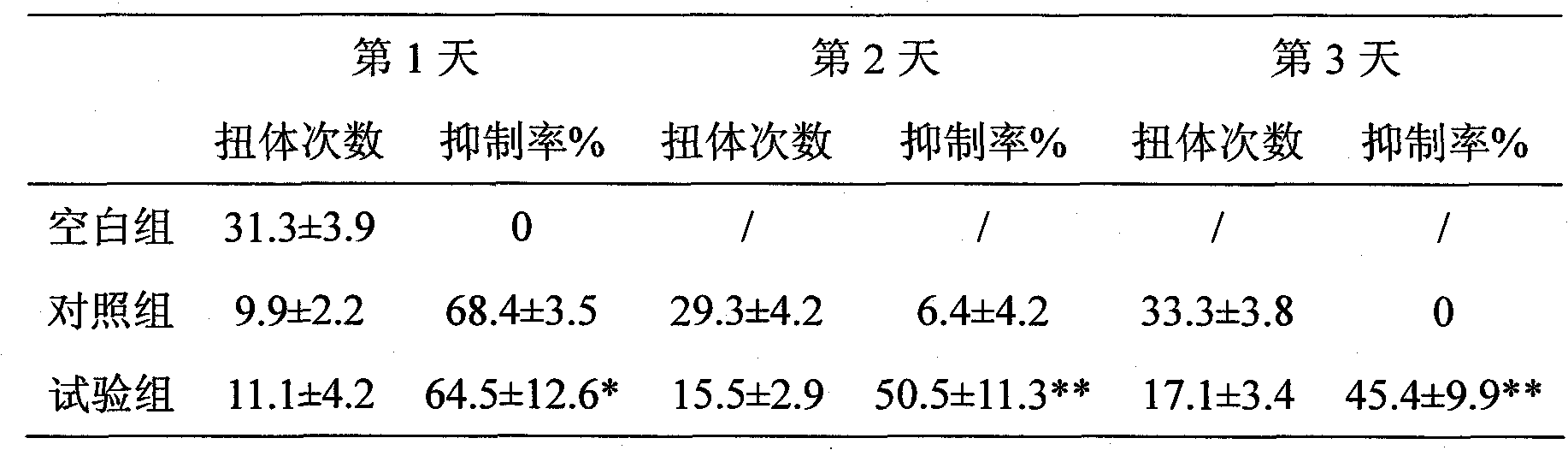
Tramadol multivesicular liposome and preparation method thereof
InactiveCN101780039AFirmly connectedHigh encapsulation efficiencyOrganic active ingredientsNervous disorderCholesterolNitrogen gas
The invention discloses a tramadol multivesicular liposome and a preparation method thereof. The preparation method comprises the following steps of: 1, dissolving phospholipids, cholesterol and neutral lipids into organic solvents to obtain a mixture which serves as an organic phase; 2, preparing 10 to 500mmol / L tramadol solution which serves as an internal water phase; 3, adding the internal water phase with the same volume as that of an organic phase into the organic phase, and mixing and emulsifying the mixture to obtain water-in-oil primary emulsion; 4, preparing an external water phase containing amino acid and osmotic modulators and / or surfactants, and adding the external water phase of which the volume is 2 to 10 times that of the water-in-oil primary emulsion into the water-in-oil primary emulsion, stirring the mixture to form oil-in-water type double emulsion; 5, adding the emulsion into the solution of the amino acid, introducing nitrogen or carbon dioxide into the mixed solution to remove the organic solvent from the emulsion to obtain suspension; 6, dissolving the suspension into the solution of amino acid, centrifuging and taking lower liposome suspension to obtain the tramadol multivesicular liposome. The prepared tramadol multivesicular liposome has the advantages of higher encapsulation efficiency, good slow release effect, and longer analgesic effect.
Owner:NANJING HAILING TRADITIONAL CHINESE MEDICINE RES CO LTD +2
Liposome suspensions, method for preparing the same, and application thereof
InactiveUS20150174070A1Simple preparation procedureSmall particle sizeBiocideCarbohydrate active ingredientsParticle-size distributionSmall particles
A method for preparing the liposome suspensions comprising liposomes with small particle size and uniform particle size distribution by performing an injection process in combination with a one-step extrusion, and further the liposome suspensions obtainable by this method as well as drug-encapsulating liposomes as well as a system for preparing the said liposome suspensions are disclosed.
Owner:PHARMOSA BIOPHARM INC
Method for preparing suspension of rhodiola root nanoliposome
InactiveCN1951391AImprove targetingAchieve sustained releaseOrganic active ingredientsAntinoxious agentsOrganic solventCholesterol
The invention relates to a method for preparing gadol glycosides nanometer liposome suspension. Wherein, said suspension comprises the extractive of gadol as gadol glycosides and the nanometer liposome packing the gadol glycosides. The inventive production comprises that: dissolving the lecithin and cholesterol into absolute ether, depressurizing and vaporizing the organic solvent; adding gadol glycosides phosphate buffer with some surface activator, expanding, and dispersing at ultrasonic wave, to obtain the final suspension. The average grainness of product is lower than 100nm; the package rate of gadol glycosides can reach 25-35%.
Owner:JIANGNAN UNIV
Method for the treatment and/or prevention of oral allergic symptions of the lips due to oral contact with a food allergen
InactiveUS20130030009A1Increase contact timePromote absorptionBiocideOrganic chemistryAllergic symptomsAllergic reaction
A method of applying a topical preparation of at least one of a mast cell stabilizer, an antihistamine, and a leukotriene inhibitor is disclosed for prevention and / or treatment of oral allergy syndrome of the lips, including lip itchiness and / or swelling. For example, topical application of Cromolyn Sodium to the lips can be used to prevent and / or treat allergic reaction to consumption or other contact with raw fruits and / or raw vegetables. The topical administration can be performed by using applicator devices that apply at least one of a mast cell stabilizer and an antihistamine in the form of a liquid, or a gel, or a butter, or a wax-like solid, or a liposome suspension. Applicator devices can include at least one of: a roller, a brush, a sponge, a swab, a tube, a lipstick. The taste of the at least one of a mast cell stabilizer and an antihistamine can be masked by flavors.
Owner:HARISH ZIV
Preparation method of radix rehmanniae polysaccharide liposome
ActiveCN103536534AEffectively maintain effective concentrationImprove bioavailabilityOrganic active ingredientsAntipyreticIce waterPhosphate
The invention discloses a preparation method of radix rehmanniae polysaccharide liposome, which comprises the following steps: taking soybean lecithin, cholesterol and Tween 80, dissolving in a solvent, and carrying out ultrasonic dissolution; carrying out vacuum evaporation at 30-70 DEG C to remove the organic solvent, and adding aether for dissolution; introducing a radix rehmanniae polysaccharide PBS (phosphate buffer solution) into a reaction vessel to form a two-phase system, and carrying out ultrasonic treatment in an ice water bath to form a stable W / O emulsion; carrying out vacuum evaporation at 30-70 DEG C to form a colloid, adding the PBS, and continuing rotary evaporation to remove the organic solvent; carrying out ultrasonic treatment in an ultrasonic cell disruptor until the radix rehmanniae polysaccharide is fused in liposome, thereby obtaining a radix rehmanniae polysaccharide liposome suspension; and extruding the suspension respectively through microporous filter membranes to obtain the radix rehmanniae polysaccharide liposome. The radix rehmanniae polysaccharide is prepared into the liposome for the first time, and the preparation method of the radix rehmanniae polysaccharide liposome is a reversed-phase evaporation process; by optimizing the response surface, the method can maximally save the raw material and obtain higher drug entrapment efficiency; and by combining the ultrasonic action, the prepared liposome has higher uniformity and higher stability.
Owner:NANJING AGRICULTURAL UNIVERSITY
Probiotics liposome and preparation method thereof
InactiveCN110122564AProtection from environmental influences such as stomach acidImprove stabilityMilk preparationAntipyreticLipid filmFiltration
The invention discloses a probiotics liposome and a preparation method thereof. The particle size of the probiotics liposome is (143.87+ / -10.58)-(161.53+ / -11.62)nm, and the encapsulation rate of probiotics in the probiotics liposome is 40-57.14%. The method includes steps: (1) preparing a lipid film; (2) preparing phosphate buffer solution; (3) dissolving probiotics into the phosphate buffer solution to form mixed solution 2, and preheating; (4) forming liposome suspension; (5) subjecting the liposome suspension to ultrasonic treatment, filtration and hydration to obtain the probiotics liposome. The probiotics liposome is prepared aiming at the problem of proneness to inactivation of probiotics in a digestion process of the human body, stability and activity of encapsulated probiotics in the human body are improved, the survival rate and setting quantity of probiotics in the intestinal tract are increased, and accordingly probiotic effects of probiotics can be truly achieved.
Owner:SOUTH CHINA UNIV OF TECH
Recombinant human epidermal growth factor cationic liposome and preparation method thereof
InactiveCN102949345AImprove tissue compatibilityIncrease cell affinitySenses disorderPeptide/protein ingredientsNiosomeCitrate buffer
The invention relates to a recombinant human epidermal growth factor cationic liposome and a preparation method thereof. The recombinant human epidermal growth factor cationic liposome comprises a recombinant human epidermal growth factor, a cationic liposome and a growth factor protective agent, wherein a part of the recombinant human epidermal growth factor is encapsulated by the cationic liposome. The preparation method comprises the following steps of: step 1. dissolving a panniculus adiposus material and a cationic additive by an absolute ethyl alcohol, carrying out rotary evaporation to get rid of the alcohol to form uniform panniculus adiposus, and adding a citrate buffer solution and hydrating to obtain a crude liposome suspension liquid; step 2. carrying out ultrasonic dispersion to process the crude liposome suspension liquid, and straightening particles by microfiltration membranes to obtain the blank liposome; and step 3. adding the blank liposome solution after mixing a recombinant human epidermal growth factor solution with a growth factor protective agent solution, adjusting pH to 6.0-8.0, and carrying out incubation processing to obtain the recombinant human epidermal growth factor cationic liposome. According to the preparation method, the human epidermal growth factor can be encapsulated by the cationic liposome so that the stability of rhEGF, the intermiscibility of tissues and the affinity of cells can be increased.
Owner:SHENZHEN POLYTECHNIC
Strychnine composite phospholipid liposome, and preparation method thereof and use in pharmacy
InactiveCN101406455AImprove stabilityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsUltrafiltrationCholesterol
The invention discloses a strychnine compound phospholipid liposome which is prepared by two types of phospholipids, strychnine and cholesterol in certain weight ratio. The preparation method for the strychnine compound phospholipid liposome comprises the following steps: (1) dissolving the first type phospholipid, the second class phospholipid and the cholesterol in absolute ethyl alcohol, and injecting the aqueous solution of ammonia sulfate into the mixture ; (2) removing the alcohol through heating or decompression method; (3) carrying out ultrasonic processing or high-pressure emulsion homogenization to reduce the grain size of the liposome; (4) removing the ammonia sulphate which is not encapsulated by the liposome through a dialysis method, an ultrafiltration method or a gel column chromatographic method so as to form blank liposome suspension; and (5) adding the strychnine into the suspension, and carrying out moderate incubation so as to enable the strychnine to be encapsulated by the liposome. The strychnine compound phospholipid liposome has significant characteristics of high encapsulating rate of the liposome for the strychnine, greatly improved stability, no easy leakage, and remarkably improved medicine carrying capacity. The results of tumor resisting experiments show that the cancer resisting effect of the strychnine compound phospholipid liposome is remarkably higher than that of a common single phospholipid liposome.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for preparing self-assembly ketoprofen liposome by electrostatic spinning technology
InactiveCN102697727AAchieve self-assemblyUniform and stable particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsBiocompatibility TestingSolvent
The invention relates to a method for preparing self-assembly ketoprofen liposome by an electrostatic spinning technology. The method comprises the following steps of: (1) adding polyvinylpyrrolidone, soybean lecithin and ketoprofen into a mixed solvent while stirring, continuously stirring for 1 to 2 hours until the mixed solvent is completely swelled, oscillating until the mixed solvent is completely dissolved, making solution transparent, and performing ultrasonic processing for degassing to obtain spinning solution; (2) performing electrostatic spinning by means of the spinning solution, and drying collected electrostatically-spun fibrous membranes in vacuum; and (3) dissolving the electrostatically-spun fibrous membranes in double distilled water to obtain liposome suspending liquid, performing ultrasonic processing, and thus obtaining the ketoprofen liposome. The method is easy to operate, has low time consumption and is suitable for large-scale production; the used raw materials are inexpensive and readily available; and the ketoprofen liposome has high degradability and biocompatibility and has the potential when applied to subsequent related experimental analysis.
Owner:DONGHUA UNIV
Glabridin freeze-dried liposome and preparation method thereof
InactiveCN104721138AImprove skin penetrationStrong in vitro transdermal absorption performanceOrganic active ingredientsMetabolism disorderPhosphateFreeze-drying
The invention relates to glabridin freeze-dried liposome and a preparation method thereof. The glabridin freeze-dried liposome comprises, by weight, 65-70 parts of soybean phospholipid, 30-35 parts of cholesterol, 0.5-3.0 parts of glabridin, 1000-1500 parts of anhydrous ethanol and 70-120 parts of a phosphate buffer. The preparation method comprises the following steps of 1, weighing soybean phospholipid and cholesterol, putting the soybean phospholipid and cholesterol in an eggplant-shaped bottle, and dissolving the soybean phospholipid and cholesterol in anhydrous ethanol, 2, carrying out decompression on the eggplant-shaped bottle to evaporate the solvent, 3, preparing a glabridin solution, 4, preparing a glabridin phosphate solution, 5, preparing a glabridin freeze-dried liposome suspension, 6, preparing glabridin freeze-dried liposome by a freeze-drying method, and 7, carrying out vacuum-pumping by a freeze-drier for 5-10min so that water is completely evaporated and the glabridin freeze-dried liposome is obtained. The glabridin freeze-dried liposome has a reasonable design, has very strong external transdermal absorption performances and provides experiment basis for a bio-pharmaceutical industry.
Owner:天津芸熙生物技术有限公司
Method for preparing nicotinamide-coated multivesicular liposomes
InactiveCN106619149AImprove stabilityLess irritatingCosmetic preparationsToilet preparationsOrganic solventEmulsion
The invention relates to a method for preparing nicotinamide-coated multivesicular liposomes. The nicotinamide-coated multivesicular liposomes are prepared from phospholipid, neutral lipid, cholesterol, an organic solvent, a hydrophilic emulsifier, nicotinamide, amino acid, an osmotic pressure regulator and deionized water. The method comprises the steps: dissolving the phospholipid, the cholesterol and the neutral lipid in the organic solvent, so as to obtain an organic phase; uniformly mixing the nicotinamide, the amino acid, the osmotic pressure regulator and the deionized water, so as to obtain an internal water phase; homogenizing the organic phase component, and adding the internal water phase component into the organic phase component, so as to prepare a W / O primary emulsion; mixing the hydrophilic emulsifier, the amino acid, the osmotic pressure regulator and the deionized water, and carrying out dispersing, so as to obtain an external water phase; homogenizing the external water phase component, adding the W / O primary emulsion component into the external water phase component, carrying out homogenizing, then, carrying out ultrasonic treatment, and then, cooling the mixture to room temperature with stirring, so as to form a W / O / W multiple emulsion; dispersing the multiple emulsion into an amino acid solution, introducing nitrogen gas into the solution to remove the organic solvent, thereby obtaining a nicotinamide-coated multivesicular liposome suspension. According to the method, the stability of the nicotinamide is improved.
Owner:SHANGHAI INST OF TECH
Preparation method of cyclodextrin inclusion compound liposome with whitening effect
ActiveCN104306269AIncrease brightnessOvercome technical defects of poor compatibilityCosmetic preparationsToilet preparationsLaminaria OchroleucaSucrose
The invention relates to a preparation method of a cyclodextrin inclusion compound liposome with a whitening effect. The method comprises the following steps: A, weighing extract of haematococcus pluvialis and extract of laminaria ochroleuca to prepare an ethanol solution; B, weighing hydroxypropyl-beta-cyclodextrin to prepare an aqueous solution of cyclodextrin; C, uniformly mixing the ethanol solution and the aqueous solution of cyclodextrin, centrifuging to filter out precipitates and carrying out liquid drying to obtain a cyclodextrin inclusion compound; D, weighing hydrogenated polydecene, hydrogenated lecithin and sucrose dilaurate, mixing and heating to obtain a liquid oil phase; E, weighing the cyclodextrin inclusion compound, palmaria palmata extract, pancratium zeylanicum extract, daisy extract and deionized water, mixing and heating to obtain a liquid water phase; F, adding the liquid oil phase into the liquid water phase to carry out homogenizing so as to obtain liposome suspension; G, freezing and drying to obtain the cyclodextrin inclusion compound liposome with the whitening effect. According to the preparation method, various active matters can be simultaneously loaded; the technical defect of poor compatibility between various active matters is overcome; percutaneous permeability and slow-release property of the active matters are improved.
Owner:PROYA COSMETICS
Remote loading of sparingly water-soluble drugs into liposomes
ActiveUS20160324780A1Raise the ratioCost efficiencyOrganic active ingredientsInorganic non-active ingredientsDiseaseElectrochemical gradient
The present invention provides liposome compositions containing sparingly soluble drugs that are used to treat life-threatening diseases. A preferred method of encapsulating a drug inside a liposome is by remote or active loading. Remote loading of a drug into liposomes containing a transmembrane electrochemical gradient is initiated by co-mixing a liposome suspension with a solution of drug, whereby the neutral form of the compound freely enters the liposome and becomes electrostatically charged thereby preventing the reverse transfer out of the liposome. There is a continuous build-up of compound within the liposome interior until the electrochemical gradient is dissipated or all the drug is encapsulated in the liposome. However, this process as described in the literature has been limited to drugs that are freely soluble in aqueous solution or solubilized as a water-soluble complex. This invention describes compositions and methods for remote loading drugs with low water solubility (<2 mg / mL). In the preferred embodiment the drug in the solubilizing agent is mixed with the liposomes in aqueous suspension so that the concentration of solubilizing agent is lowered to below its capacity to completely solubilize the drug. This results in the drug precipitating but remote loading capability is retained. The process is scalable and, in liposomes in which the lipid composition and remote loading agent are optimized, the resulting drug-loaded liposomes are characterized by a high drug-to-lipid ratios and prolonged drug retention when the liposome encapsulated drug is administered to a subject.
Owner:CELATOR PHARMA INC
Polymer-coated oleanolic acid liposome and preparation method thereof
InactiveCN103381143AImprove bioavailabilityImprove stabilityOrganic active ingredientsDigestive systemParticulatesFreeze-drying
The invention belongs to the technical field of medicine and relates to a polymer-coated oleanolic acid liposome and a preparation method thereof. The preparation method comprises the following steps of preparing an oleanolic acid liposome suspension, adding a polymer into the oleanolic acid liposome suspension, carrying out coating to obtain a polymer-coated oleanolic acid liposome suspension, and carrying out spray drying or freeze drying to obtain polymer-coated oleanolic acid liposome powder, wherein before use, hydration reconstruction is carried out according to requirements. The polymer-coated oleanolic acid liposome utilizes long-circulating characteristics, biological adhesion and penetration promotion of the coating material, improves drug encapsulation efficiency and liposome stability, prolongs in-vivo residence and cycle time of particulates, promotes intercellular transmembrane transport of the particulates, improves oral bioavailability of oleanolic acid, is processed into solid powder, and is convenient for package, storage, transport and use.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com