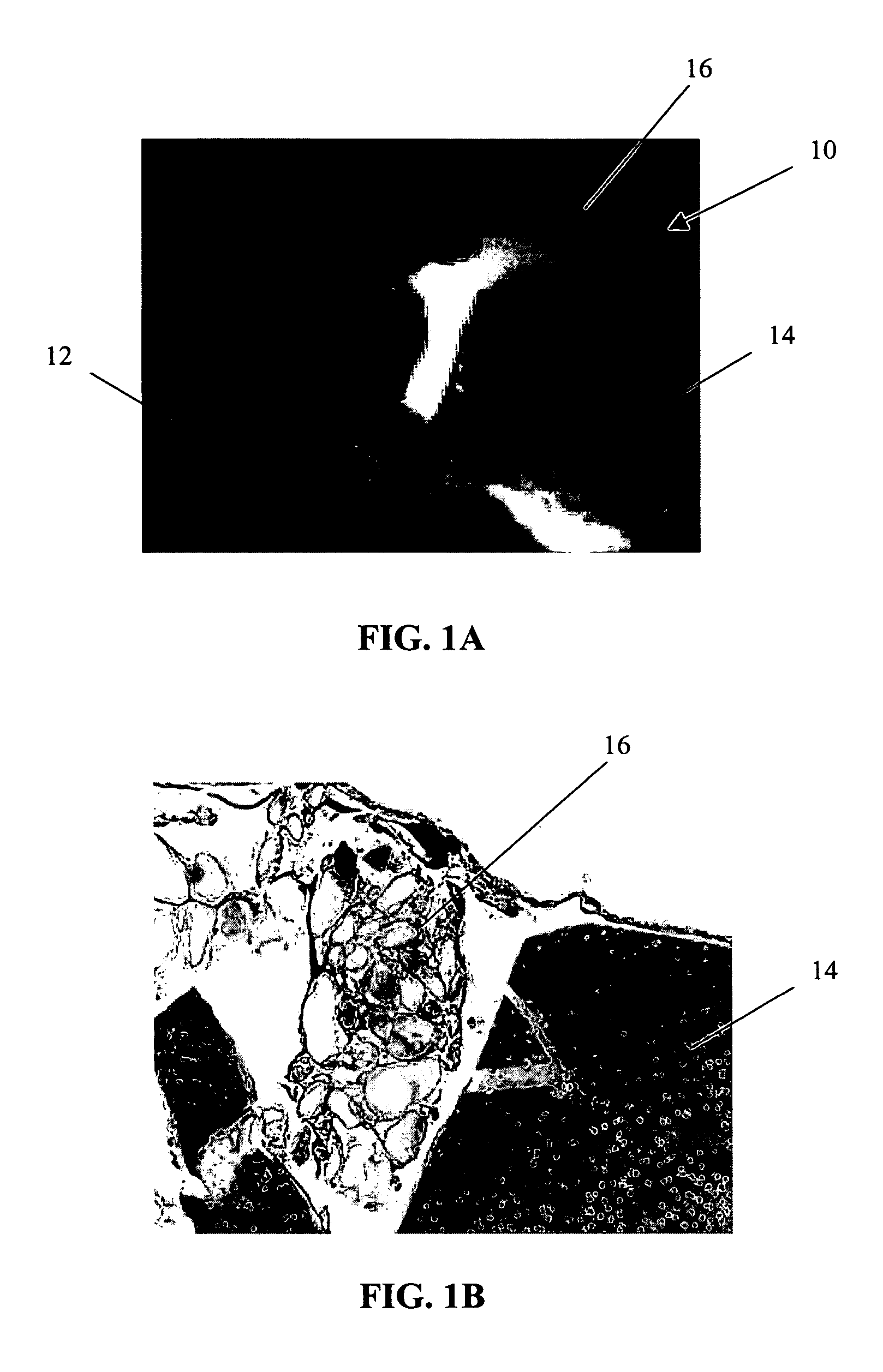
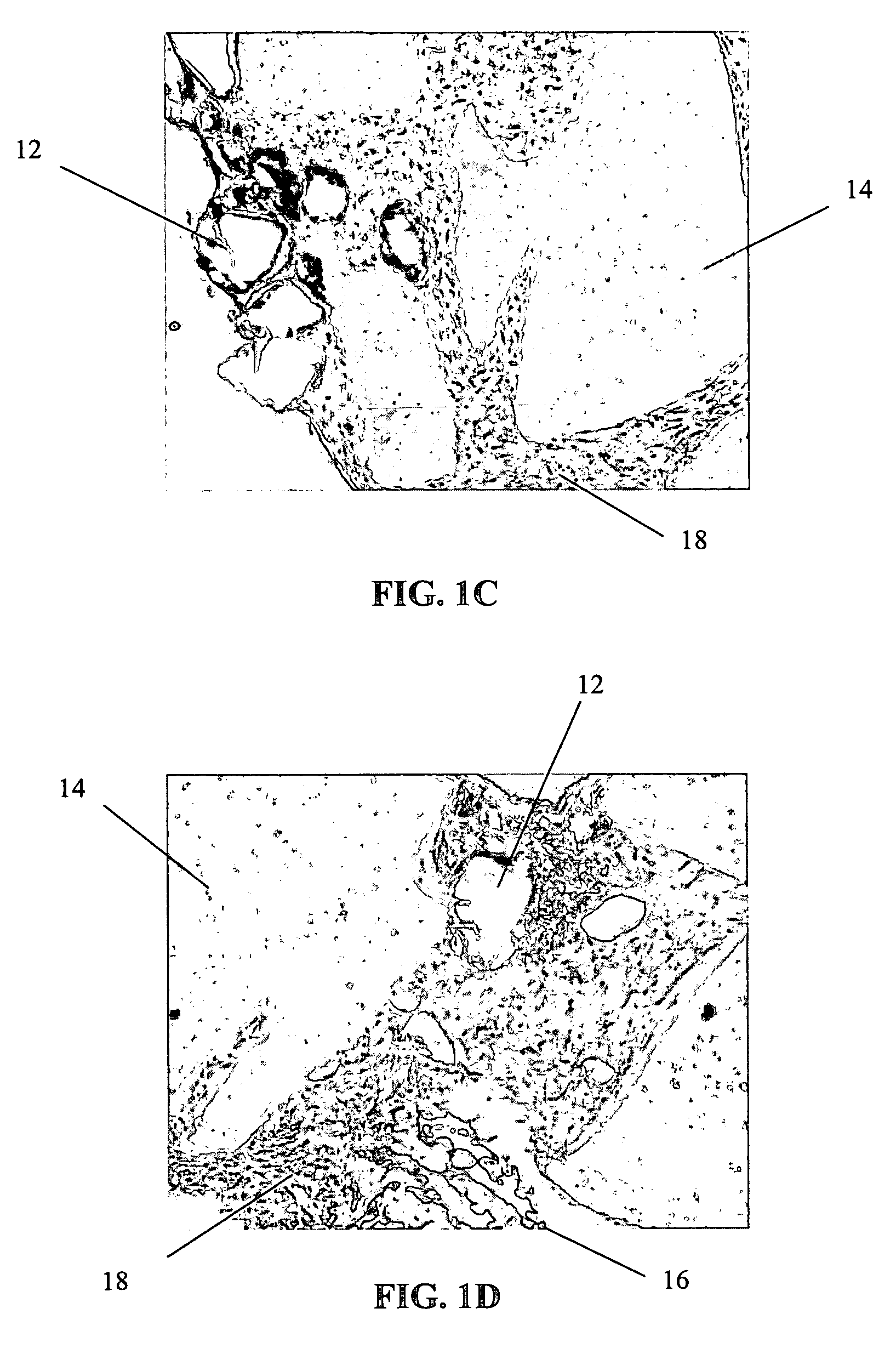
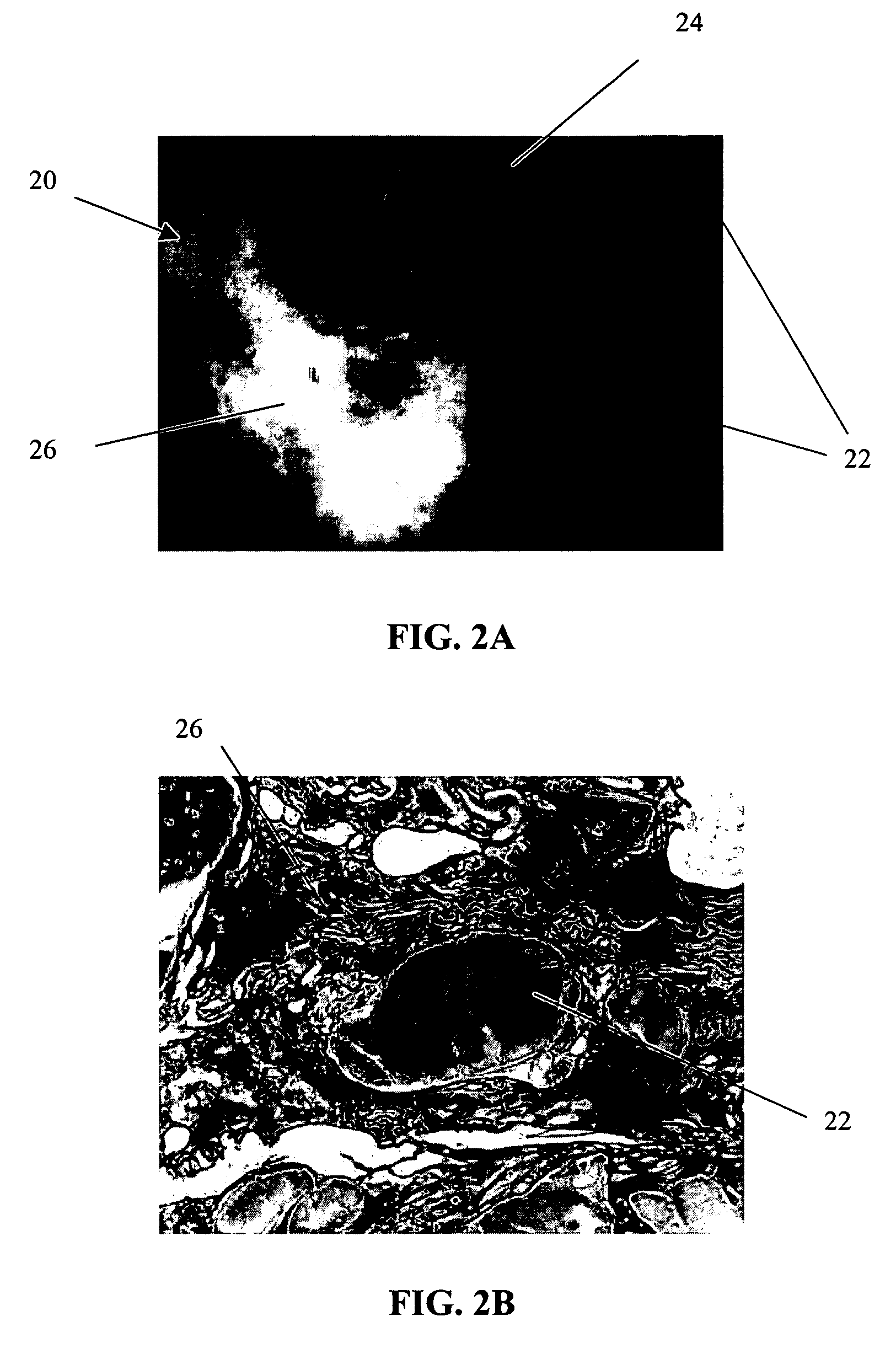
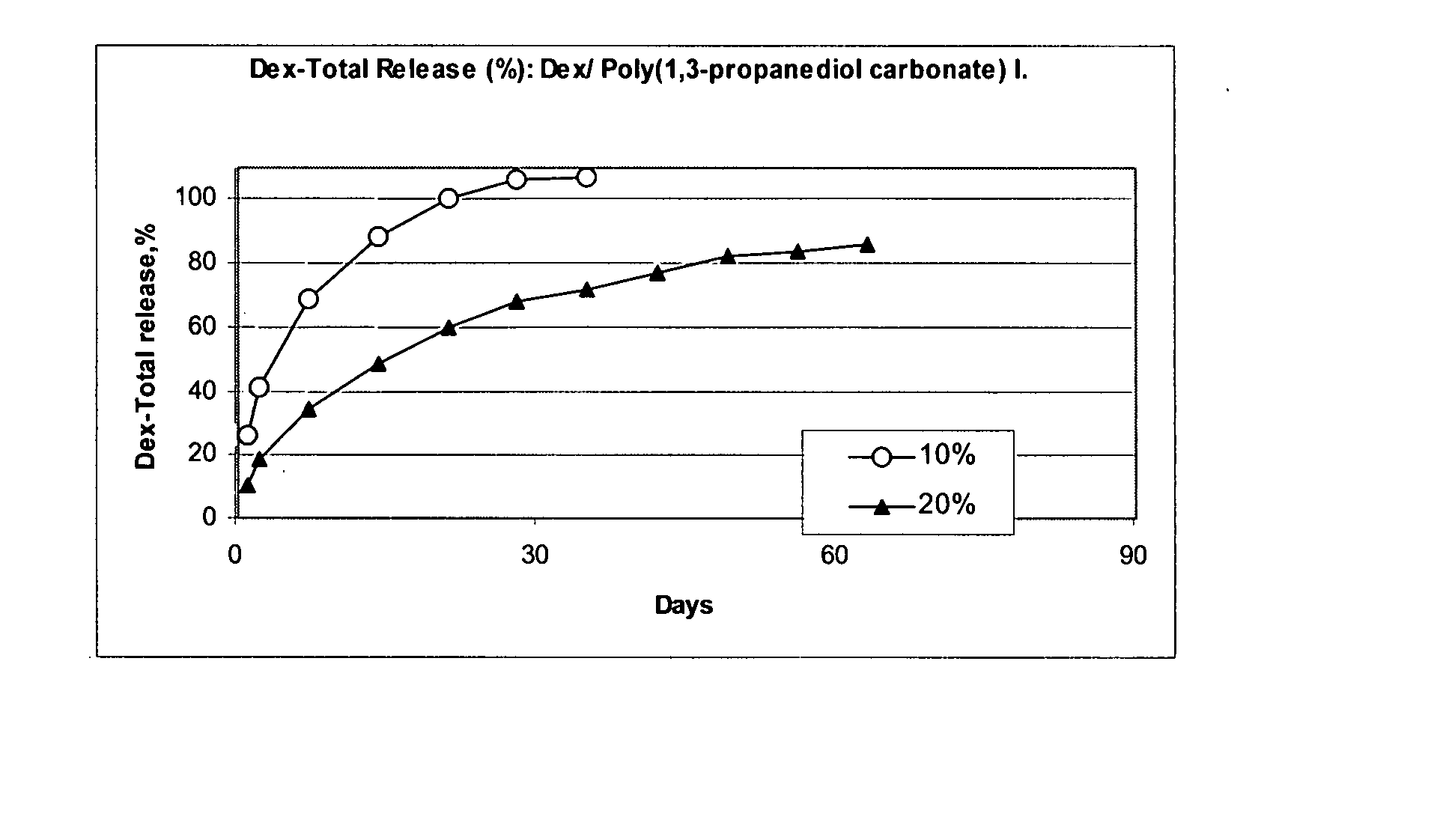
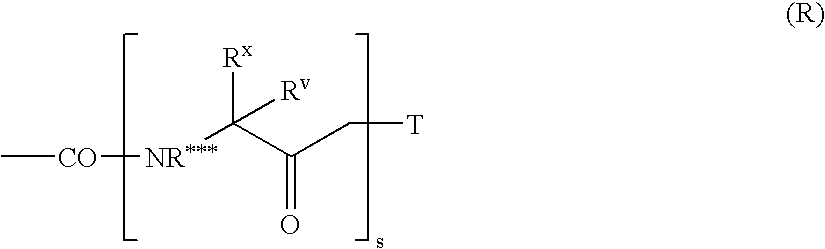
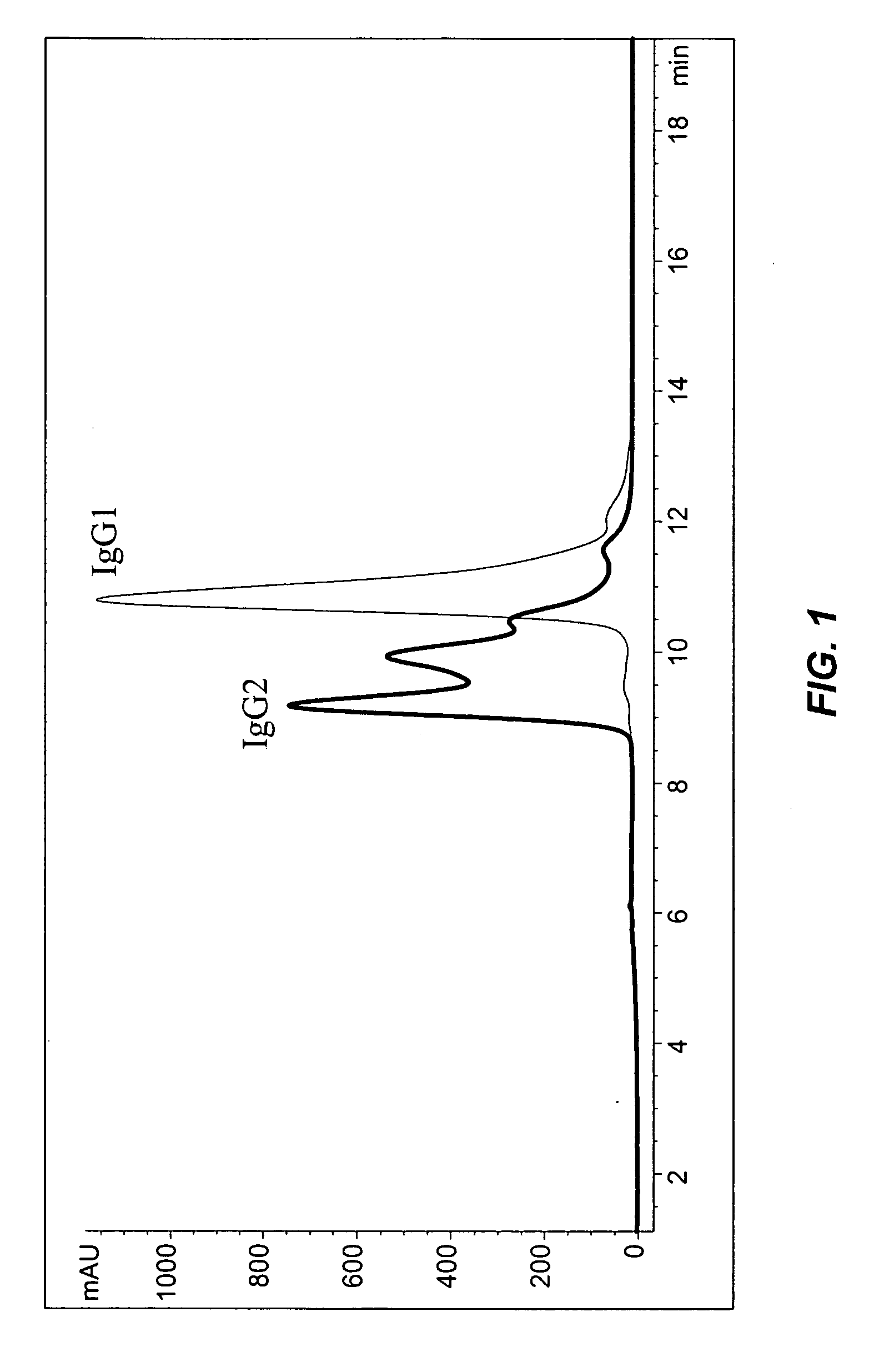
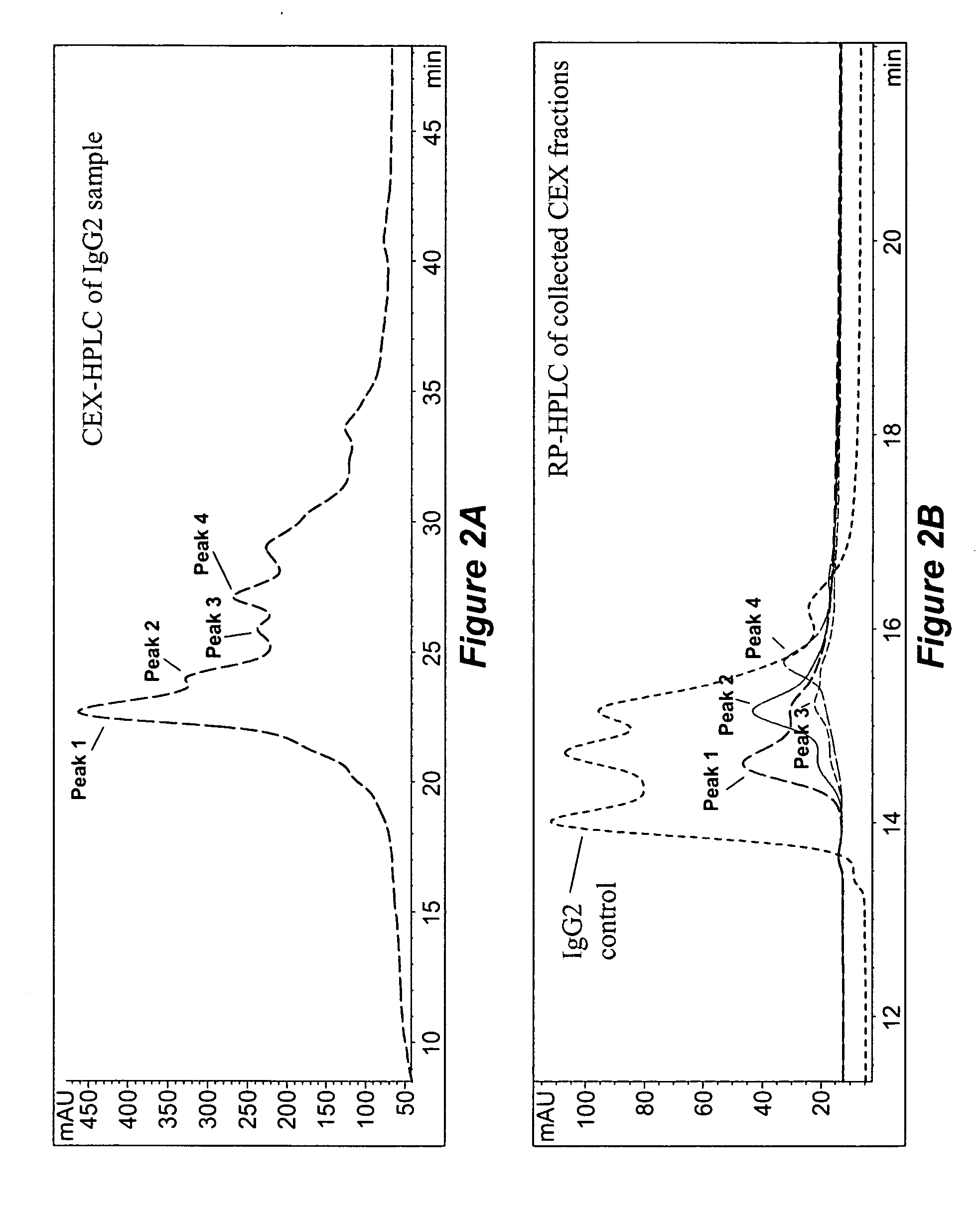
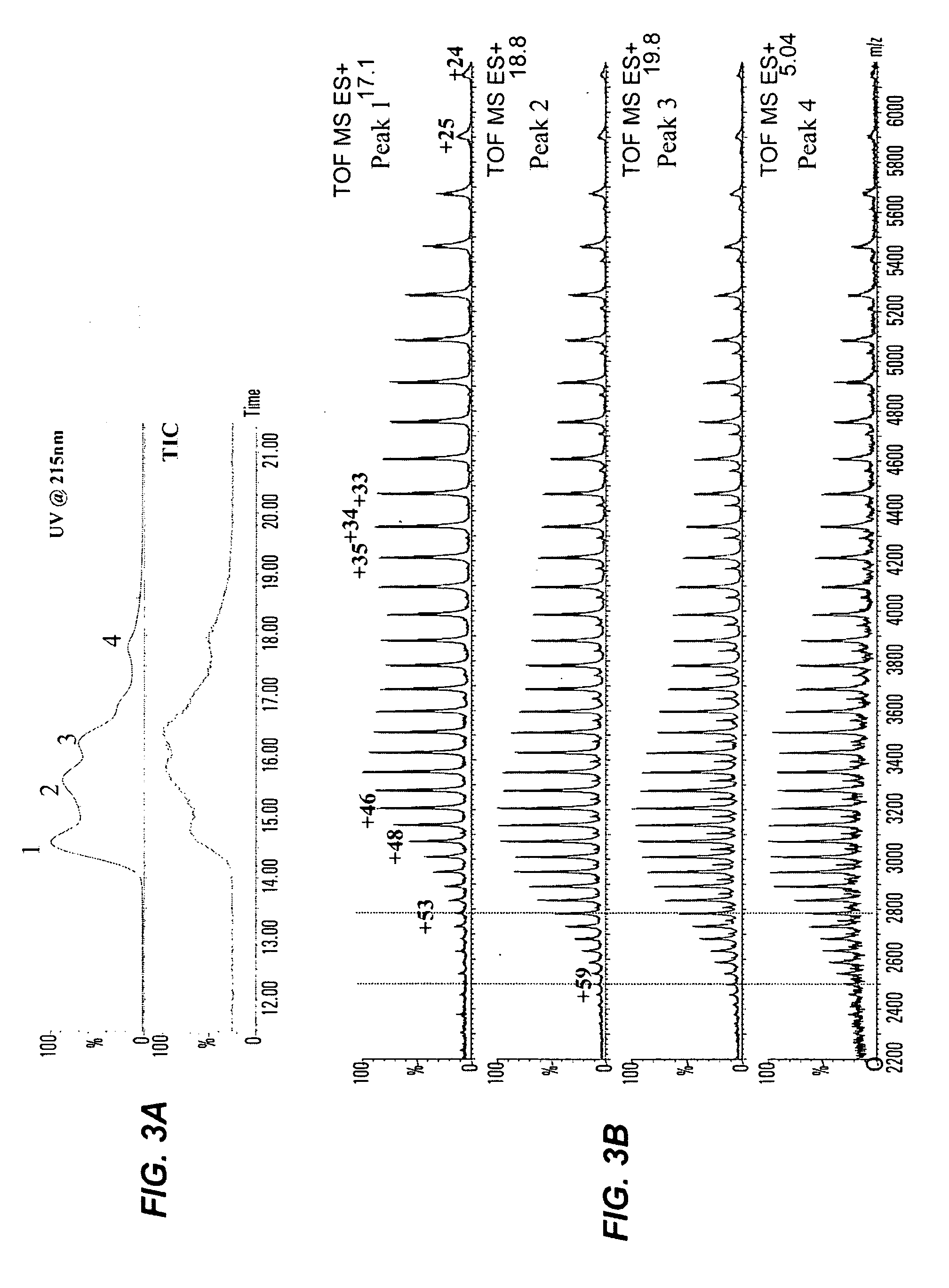
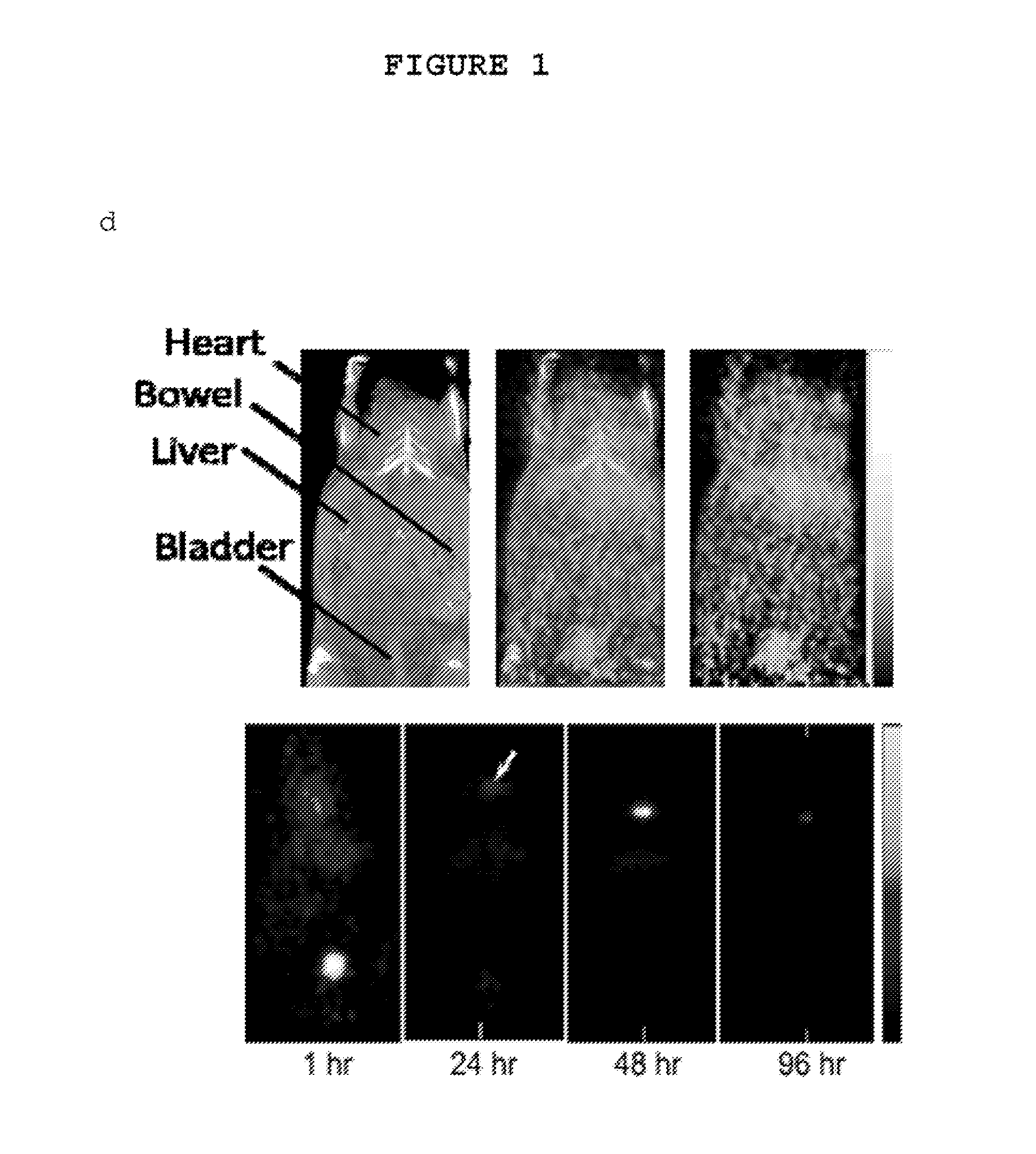
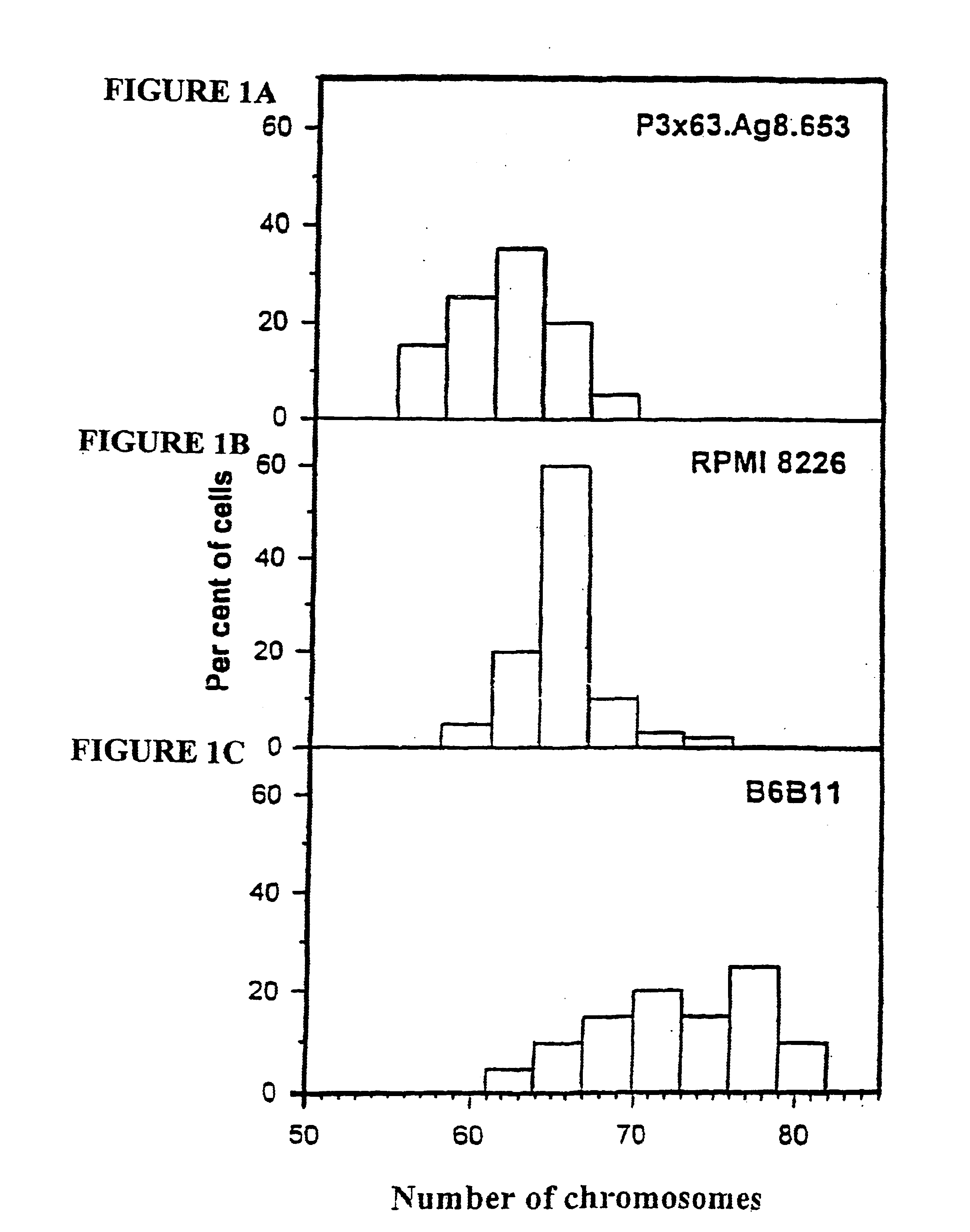
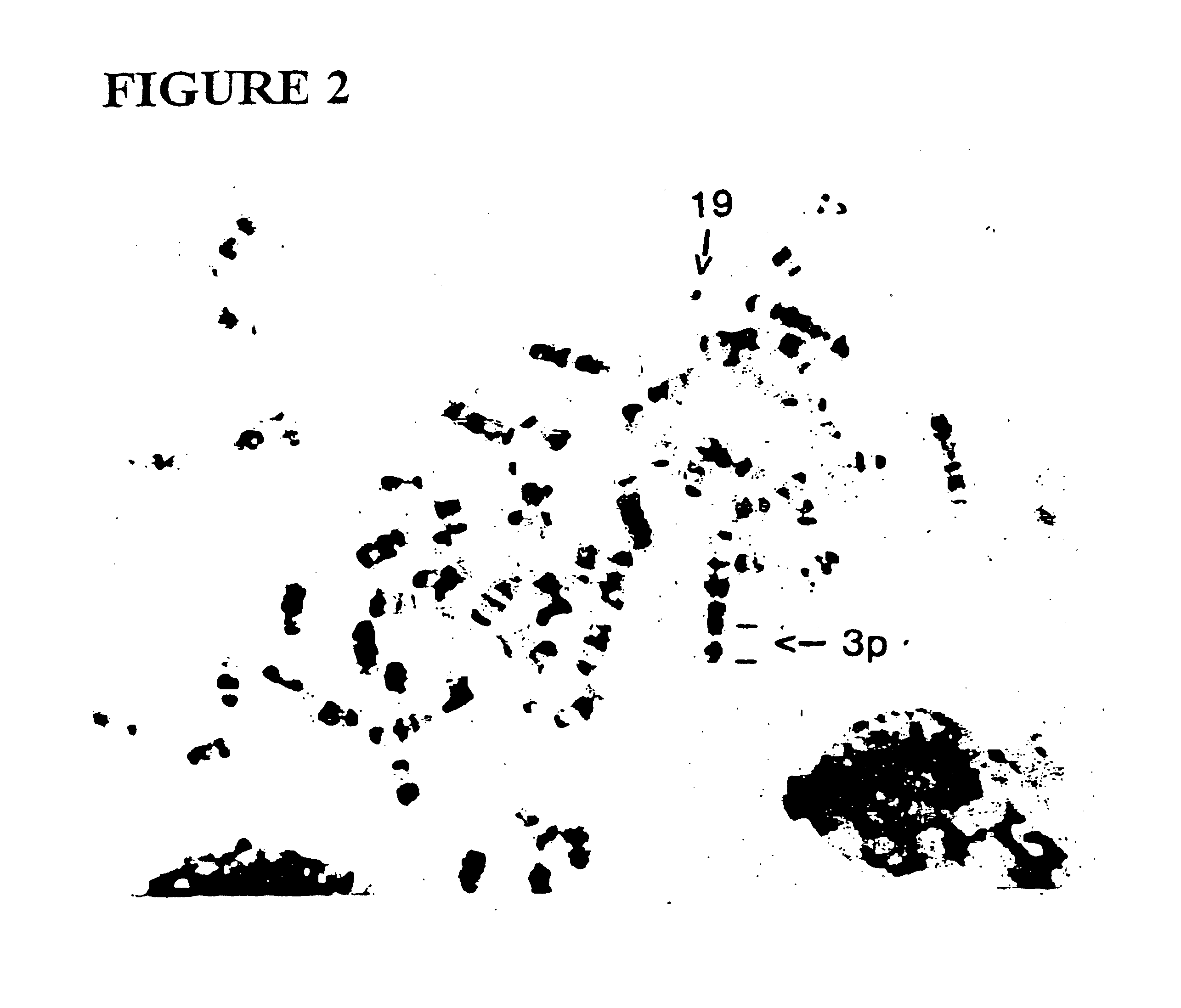
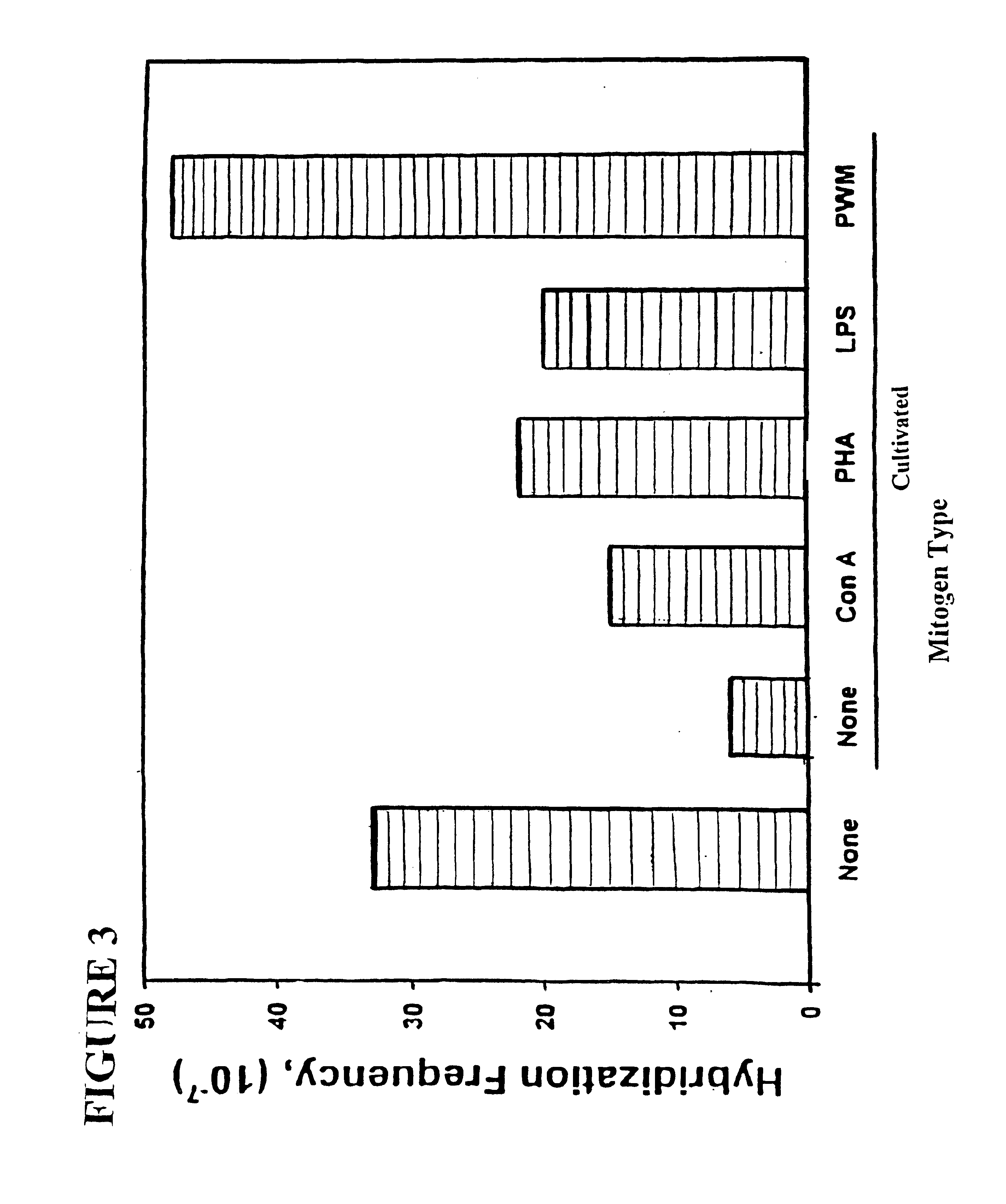
Patents
Literature
26698results about "Antinoxious agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
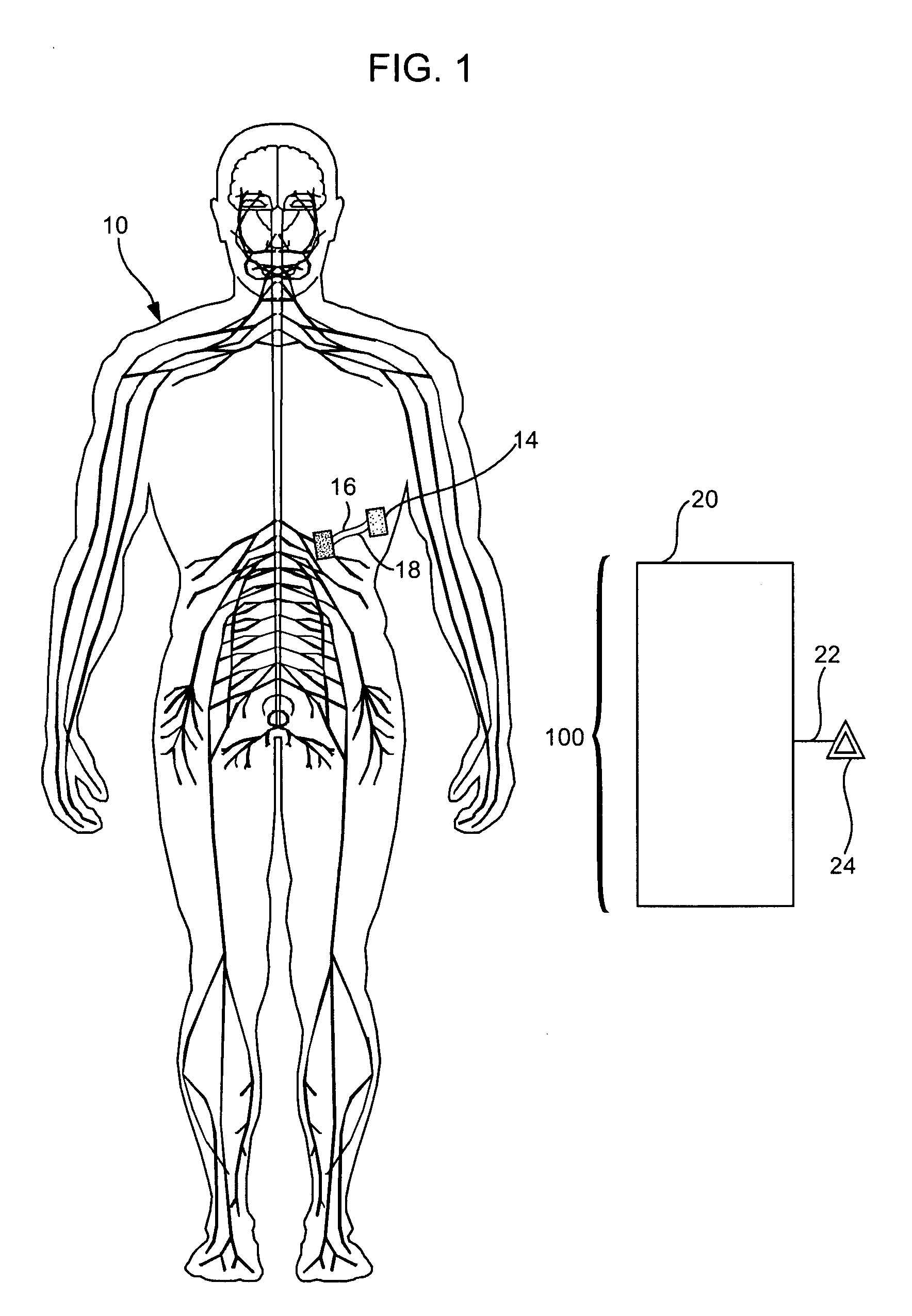
Treatment of conditions through modulation of the autonomic nervous system
InactiveUS20050153885A1Effective treatmentOrganic active ingredientsNervous disorderNervous systemMedicine
Methods are provided for treating a subject for a condition caused by an abnormality in the subject's autonomic nervous system. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is pharmacologically modulated with at least one aldosterone antagonist in a manner that is effective to treat the subject for the condition. Also provided are systems and kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
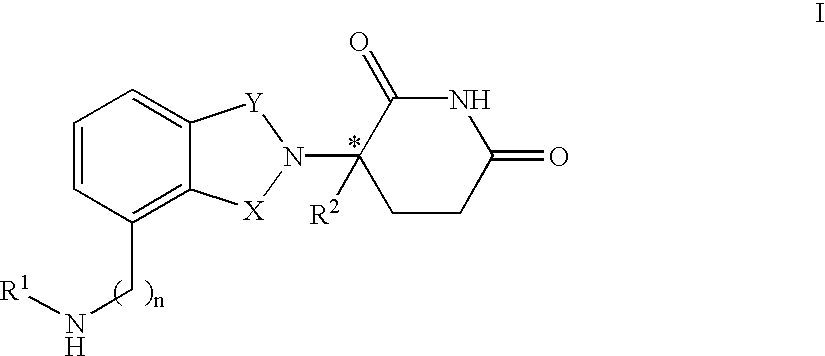
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
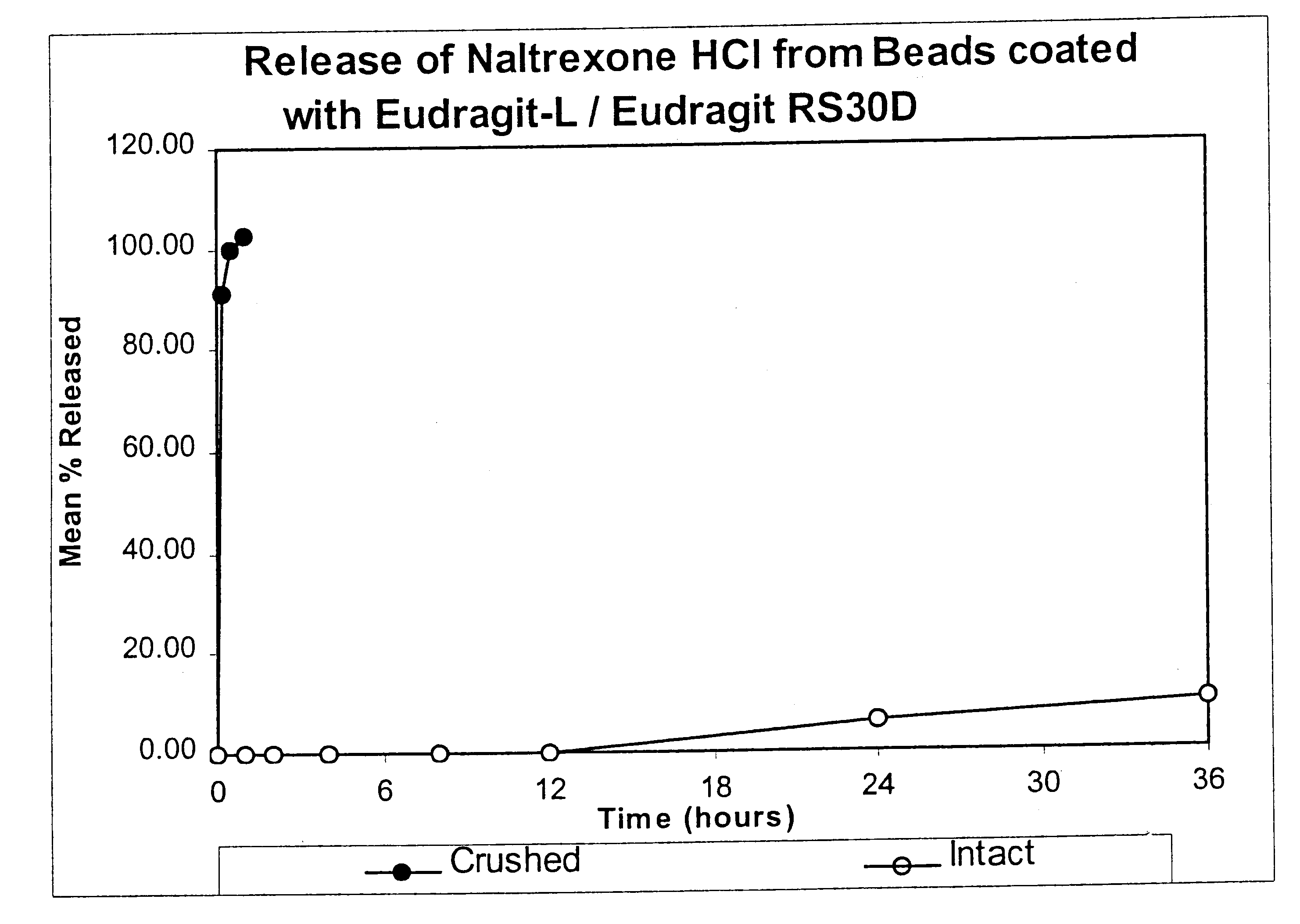
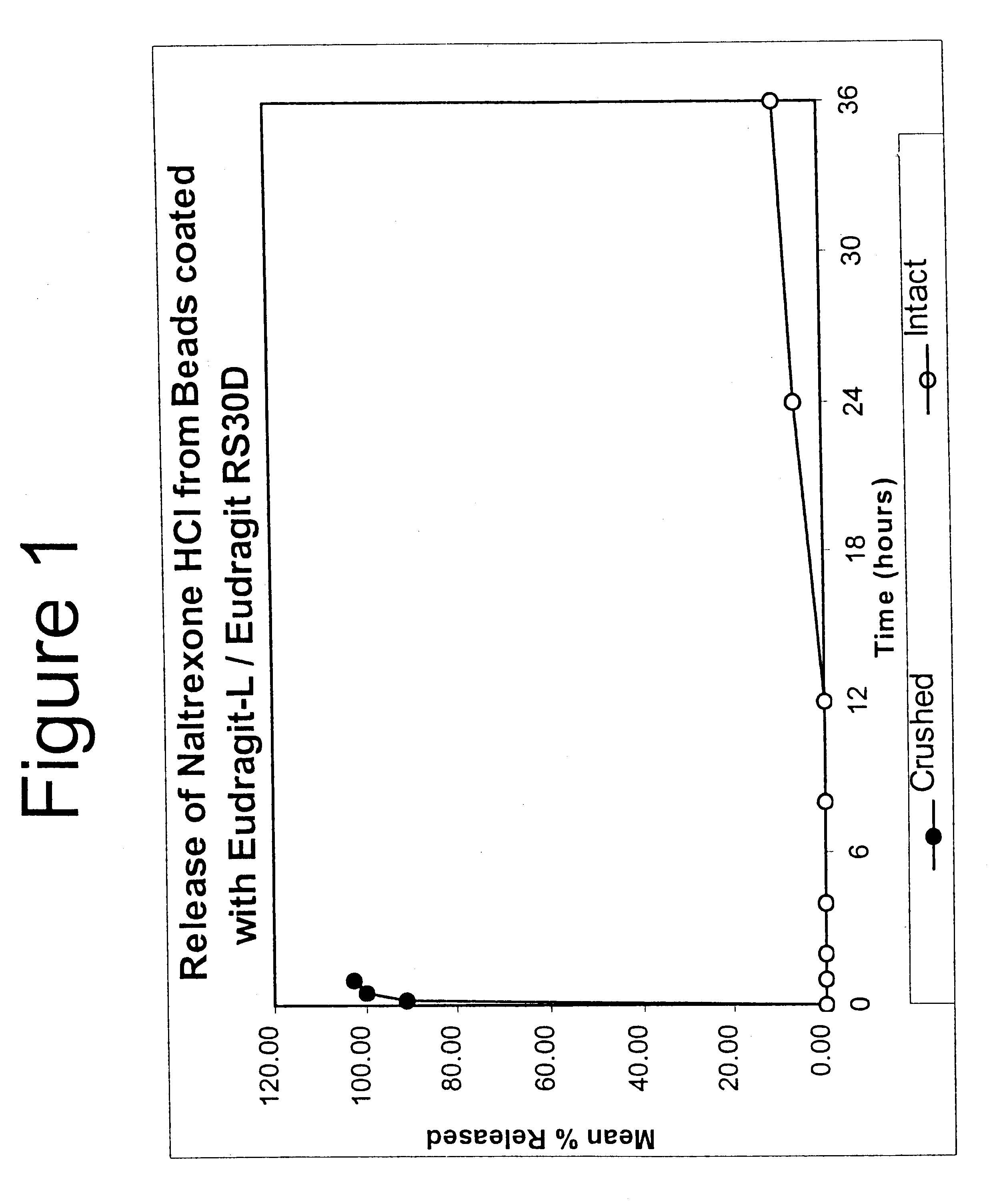
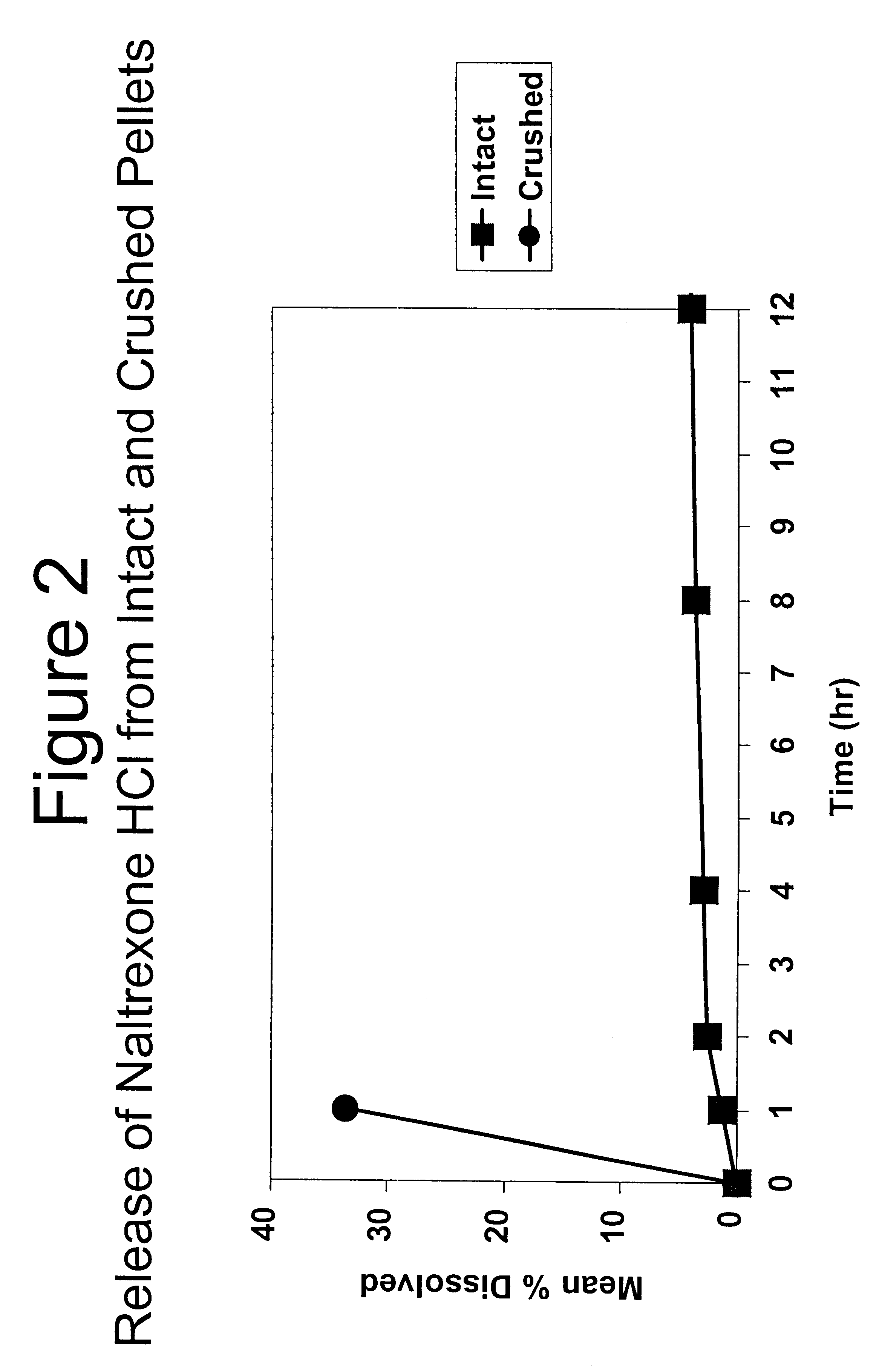
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Cartilage and bone repair and regeneration using postpartum-derived cells
Cells derived from postpartum tissue and methods for their isolation and induction to differentiate to cells of a chondrogenic or osteogenic phenotype are provided by the invention. The invention further provides cultures and compositions of the postpartum-derived cells and products related thereto. The postpartum-derived cells of the invention and products related thereto have a plethora of uses, including but not limited to research, diagnostic, and therapeutic applications, for example, in the treatment of bone and cartilage conditions.
Owner:DEPUY SYNTHES PROD INC
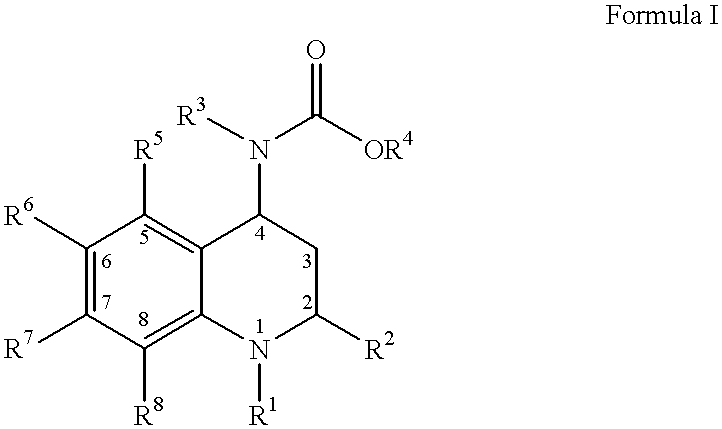
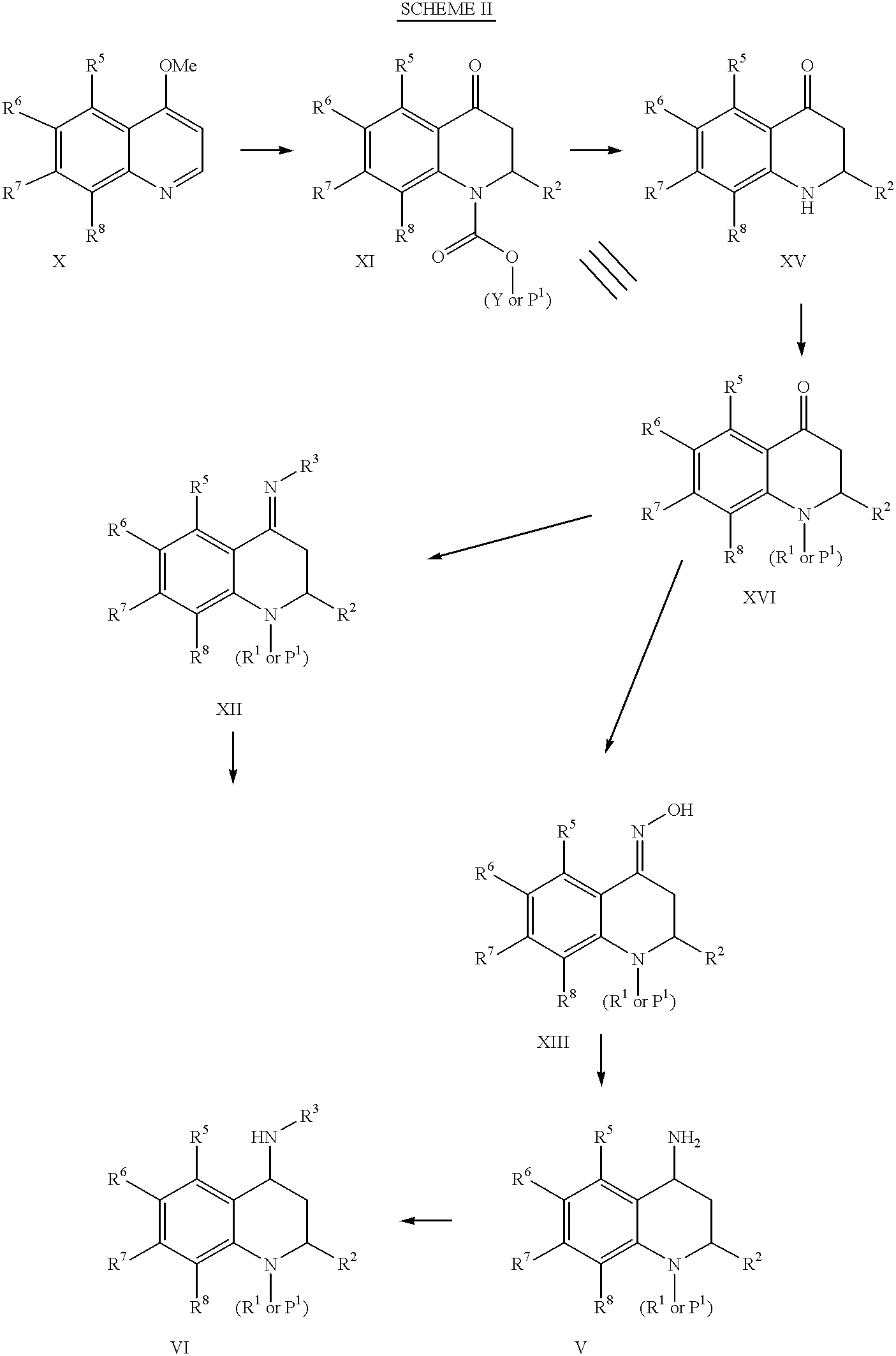
4-Carboxyamino-2-substituted-1,2,3,4-tetrahydroquinolines
Cholesteryl ester transfer protein inhibitors, pharmaceutical compositions containing such inhibitors and the use of such inhibitors to elevate certain plasma lipid levels, including high density lipoprotein-cholesterol and to lower certain other plasma lipid levels, such as LDL-cholesterol and triglycerides and accordingly to treat diseases which are exacerbated by low levels of HDL cholesterol and / or high levels of LDL-cholesterol and triglycerides, such as atherosclerosis and cardiovascular diseases in some mammals, including humans.
Owner:PFIZER INC
Combination Products
InactiveUS20080020018A1Sufficient reliefExtended stayAntibacterial agentsOrganic active ingredientsMethyl xanthineBULK ACTIVE INGREDIENT
A pharmaceutical formulation comprises a plurality of seamless minicapsules having a diameter of from 0.5 mm to 5 mm, at least some of the minicapsules containing a methyxanthine as one active ingredient, and at least some of the minicapsules containing a corticosteriod as another active ingredient.
Owner:SIGMOID PHARM LIMITED
Compositions and methods of delivery of pharmacological agents
InactiveUS20050004002A1Reducing one or more side effectsInhibiting oxidation in the pharmaceutical compositionAntibacterial agentsOrganic active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serum albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
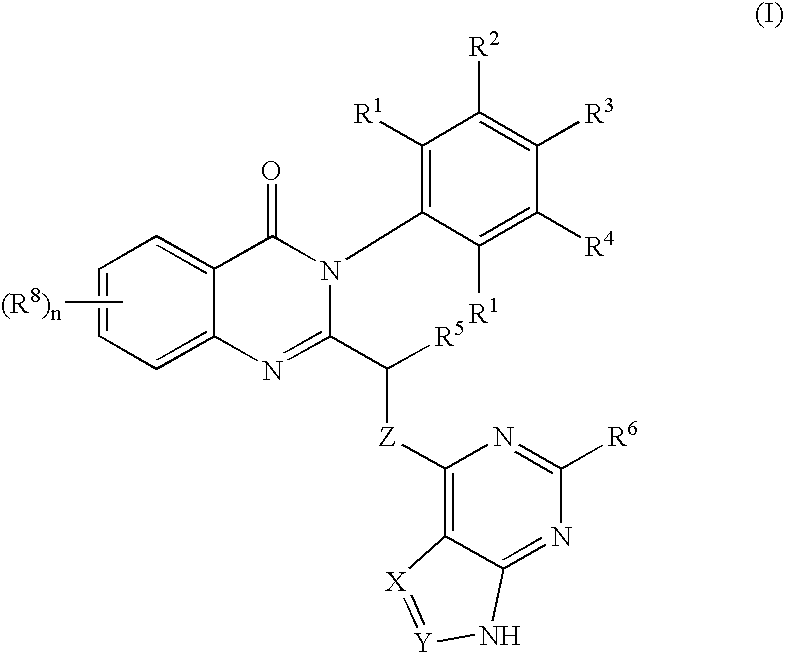
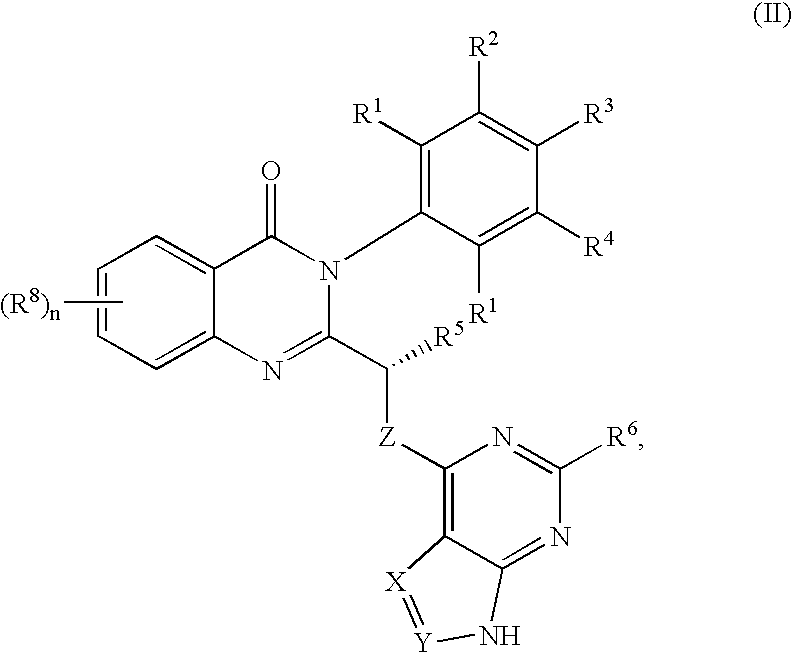
Quinazolinones as inhibitors of human phosphatidylinositol 3-kinase delta
ActiveUS7932260B2Inhibit growth and proliferationInhibit growthBiocideSenses disorderLeukocyte functionWhite blood cell
The invention provides a class of substituted quinazolinone compounds and methods of treating diseases mediated by PI3Kδ activity. The disclosed compounds are useful in treating diseases such as bone-resorption disorders; and cancer, especially hematopoietic cancers, lymphomas, multiple myelomas and leukemia. The compounds are also useful in disrupting or inhibiting cellular processes such as leukocyte function or accumulation, neutrophils function, lymphocyte proliferation, and endogenous immune responses.
Owner:ICOS CORP
Soft tissue repair and regeneration using postpartum-derived cells
ActiveUS20050058629A1Reduce productionReduce risk of rejectionSenses disorderPeptide/protein ingredientsSoft tissue repairSupporting cell
Cells derived from postpartum tissue having the potential to support cells of and / or differentiate to cells of a soft tissue lineage, and methods of preparation and use of those postpartum tissue-derived cells, are provided by the invention. The invention also provides methods for the use of such postpartum-derived cells and products related thereto in therapies for conditions of soft tissue.
Owner:DEPUY SYNTHES PROD INC
Crystalline anti-hTNFalpha antibodies
The present invention relates to a batch crystallization method for crystallizing an anti-hTNFalpha antibody which allows the production of said antibody on an industrial scale; antibody crystals as obtained according to said method; compositions containing said crystals as well as methods of use of said crystals and compositions.
Owner:ABBVIE BIOTECHNOLOGY LTD
Orally dissolving films
ActiveUS20060198873A1Improve complianceRapid and sustained and combination nicotine craving reliefBiocideNervous disorderActive agentPharmacology
Rapidly dissolving, oral film preparations for rapid release of an active agent in the oral cavity, in particular, rapidly dissolving oral films comprising a nicotine active which achieve good transbuccal absorption and provide nicotine craving relief to an individual are disclosed herein.
Owner:GLAXO SMITHKLINE LLC
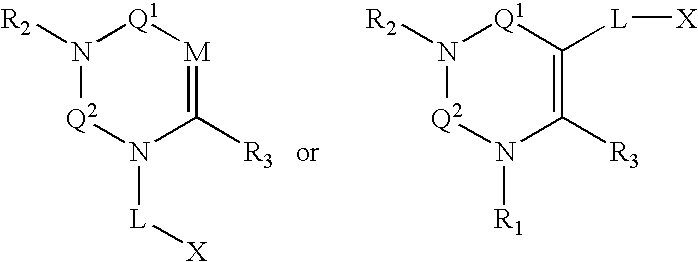
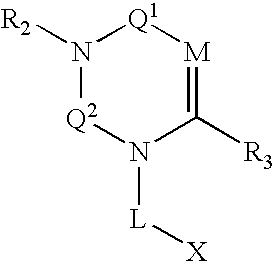
Dipeptidyl peptidase inhibitors
Compounds, pharmaceuticals, kits and methods are provided for use with DPP-IV and other S9 proteases that comprise a compound comprising: wherein M is N or CR4; Q1 and Q2 are each independently selected from the group consisting of CO, SO, SO2, and C═NR9; and each L, X, R1, R2, and R3 are as defined herein.
Owner:TAKEDA PHARMA CO LTD
Postpartum-derived cells for use in treatment of disease of the heart and circulatory system
Cells derived from postpartum tissue are disclosed along with methods for their therapeutic use in diseases of the heart or circulatory system are disclosed. Cells may be used therapeuticall in either differentiated or undifferentiated forms, in homogenous cultures, or as populations with other cells, and in conjunction with other bioactive factors.
Owner:DEPUY SYNTHES PROD INC
Conformable tissue repair implant capable of injection delivery
A conformable tissue implant is provided for use in repairing or augmenting a tissue defect or injury site. The tissue implant contains a tissue carrier matrix comprising a plurality of biocompatible, bioresorbable granules and at least one tissue fragment in association with the granules. The tissue fragment contains one or more viable cells that can migrate from the tissue and populate the tissue carrier matrix. Also provided is a method for injectably delivering the tissue implant.
Owner:DEPUY SYNTHES PROD INC
Conveniently implantable sustained release drug compositions
InactiveUS20080038316A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Cartilage and bone repair and regeneration using postpartum-derived cells
Cells derived from postpartum tissue and methods for their isolation and induction to differentiate to cells of a chondrogenic or osteogenic phenotype are provided by the invention. The invention further provides cultures and compositions of the postpartum-derived cells and products related thereto. The postpartum-derived cells of the invention and products related thereto have a plethora of uses, including but not limited to research, diagnostic, and therapeutic applications, for example, in the treatment of bone and cartilage conditions.
Owner:ETHICON INC
Topical Pharmaceutical Foam Composition
InactiveUS20070154402A1Reduced intensity of colorReduce odor intensityAntibacterial agentsBiocideAlcohol freeActive agent
A stable topical alcohol-free aerosol foam containing one or more keratolytic agents is provided. The foam-forming formulation is an emulsion which contains an HFA propellant and one or more keratolytic agents. The emulsion has an oil phase and an aqueous, i.e. water-containing, phase. The active agent(s) may be present in either phase of the emulsion or dispersed in the emulsion. The oil phase may consist at least in part of the HFA propellant. Either or both of the oil phase and the aqueous phase may contain one or more surfactants, emulsifiers, emulsion stabilizers, buffers, and / or other excipients. The foam is stable on the skin, for example, for at least 5 minutes at body temperature, preferably at least 20 minutes at body temperature, and disappears into the skin upon rubbing or after prolonged standing. In one embodiment, the formulation contains an HFA propellant which does not contain additional co-solvents or co-propellants. The formulations demonstrate reduced intensity of the odor and / or color associated with the keratolytic agent(s) as compared to conventional formulations containing keratolytic agents.
Owner:PRECISION DERMATOLOGY
Methods of increasing endogenous erythropoietin (EPO)
InactiveUS20030153503A1Increase endogenous EPOIncrease endogenous EPO levelOrganic active ingredientsPeptide/protein ingredientsIn vivoBiology
The present invention relates to methods for treating erythropoietin-associated conditions by increasing endogenous erythropoietin in vitro and in vivo. Methods for treating, pretreating or preconditioning, or preventing erythropoietin-associated conditions are also included. Compounds for use in these methods are provided, as are methods of identifying such compounds.
Owner:FIBROGEN INC
Methods for refolding of recombinant antibodies
ActiveUS20060194280A1Efficient and economic productionImproved pharmaceuticalImmunological disordersFermentationRecombinant antibodiesCoupling reagent
The present invention is generally directed to methods of producing an increase in the enrichment or recovery of preferred forms of IgG proteins. More particularly, the invention relates to subjecting preparations of such recombinant IgG proteins with a reduction / oxidation coupling reagent and optionally a chaotropic agent.
Owner:AMGEN INC
Methods and compositions for treatment of free radical injury
InactiveUS20060121016A1Reduction of tissue level oxidative damage toBiocideDipeptide ingredientsAntioxidantCell membrane
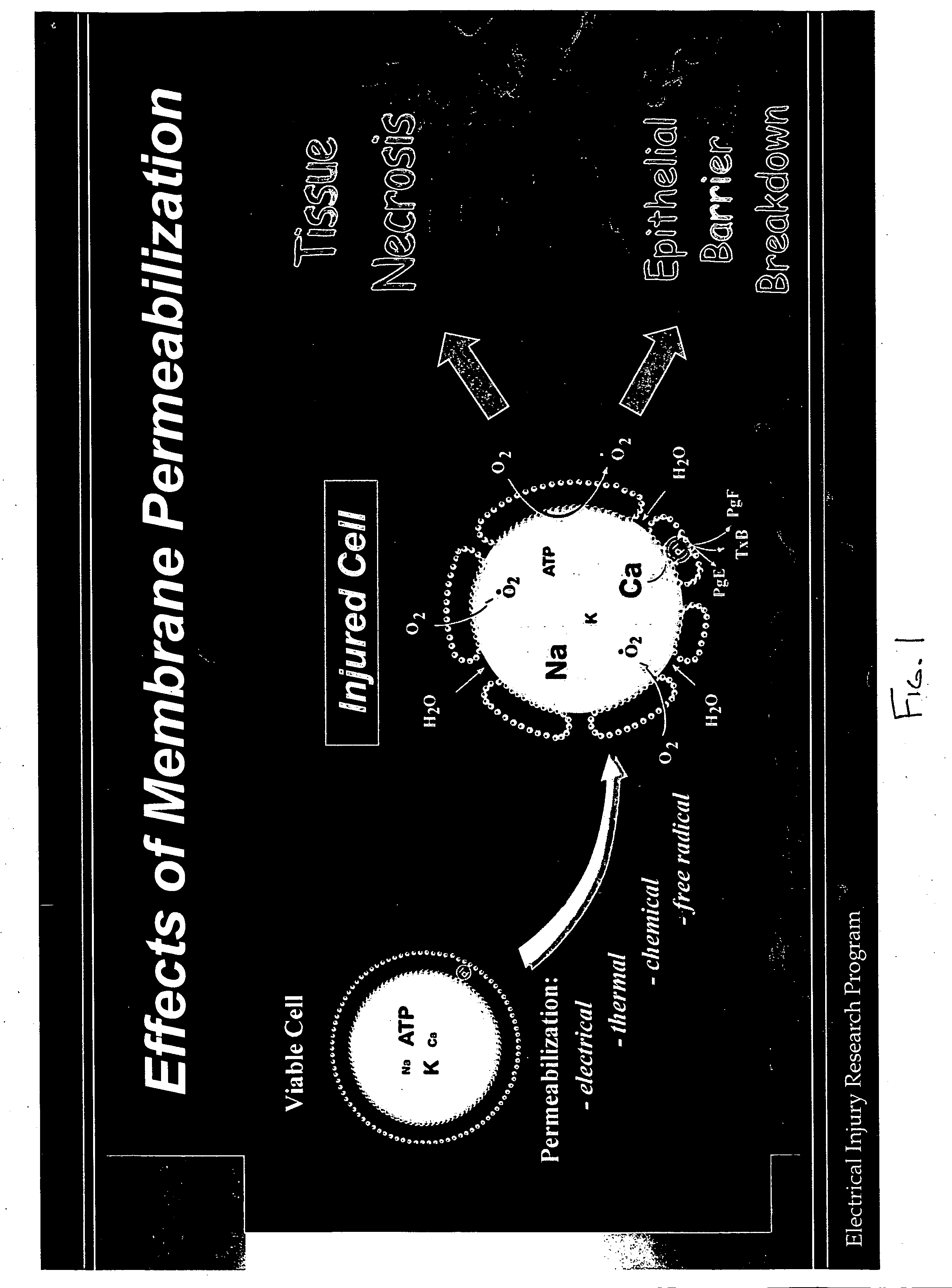
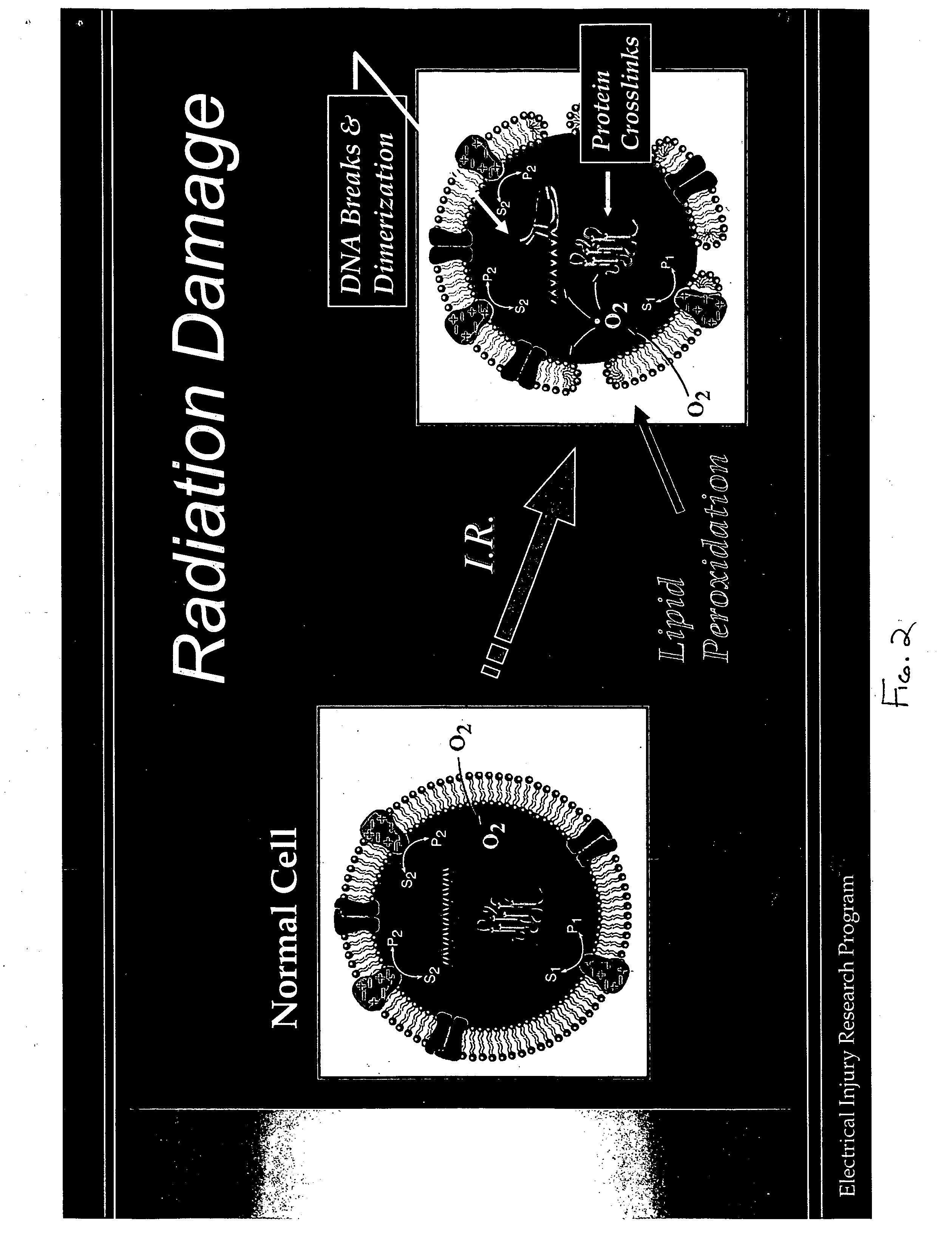
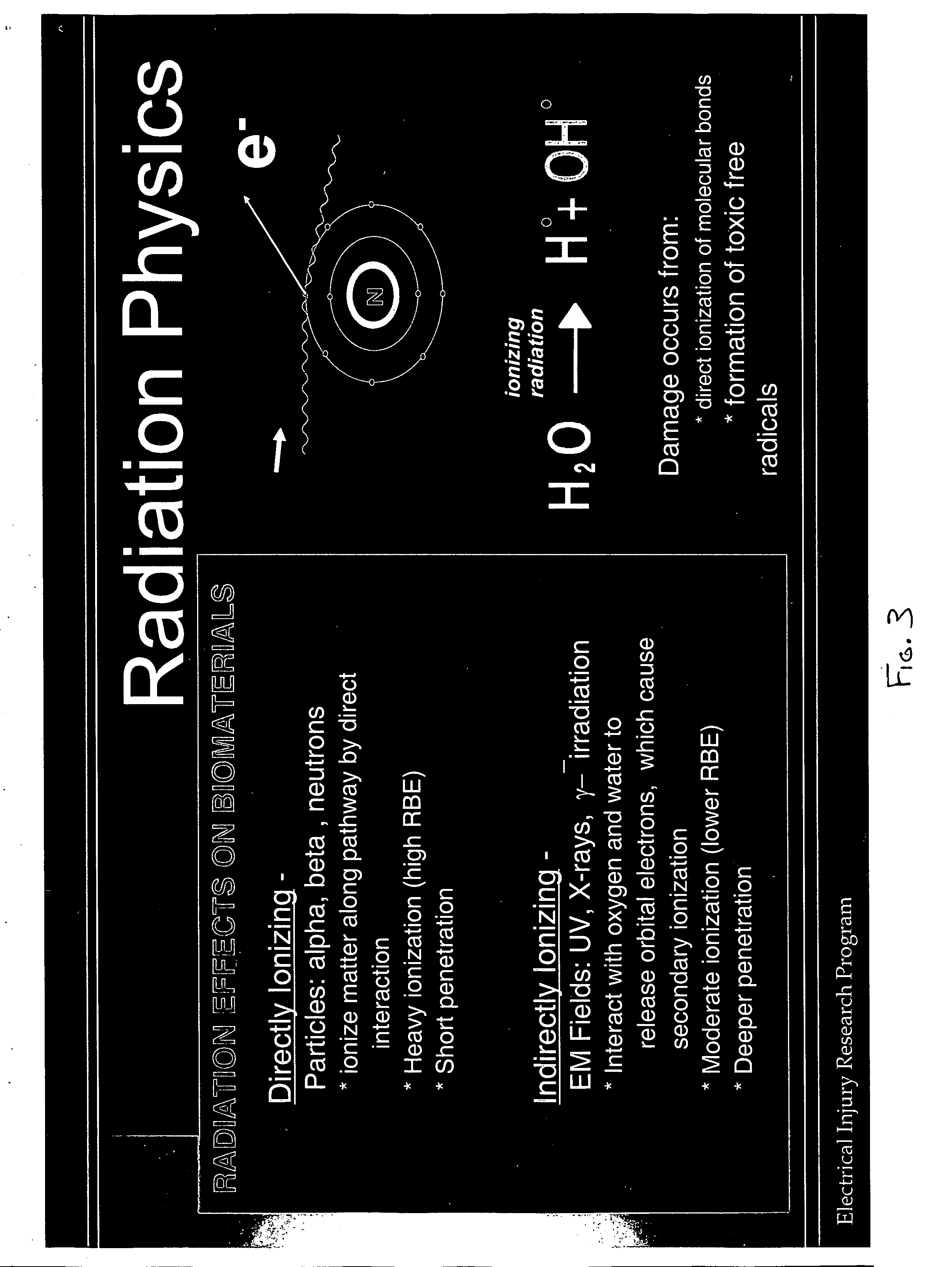
Therapeutic methods and compositions useful for the prevention and / or treatment of cellular membrane damage leading to or resulting from peroxidation of the cellular membrane and a breakdown of the barrier function of the cellular membrane. A therapeutic composition includes a combination of a membrane sealing sealing surfactant and a cofactor treatment consisting of an antioxidant and a cellular energy store. To affect this goal, the permeability of damaged cellular membranes is reestablished by the membrane sealing surfactant, effectively “sealing” the injured membranes. To facilitate rapid tissue recovery, cellular energy levels can be reestablished through addition of a cellular energy source such as, for example, MgCl2-ATP which, serves a further dual benefit of improving the cellular ion balance. Addition of an antioxidant eliminates the generation of Reactive Oxygen intermediates and enhances the metabolism of free radicals.
Owner:MAROON BIOTECH
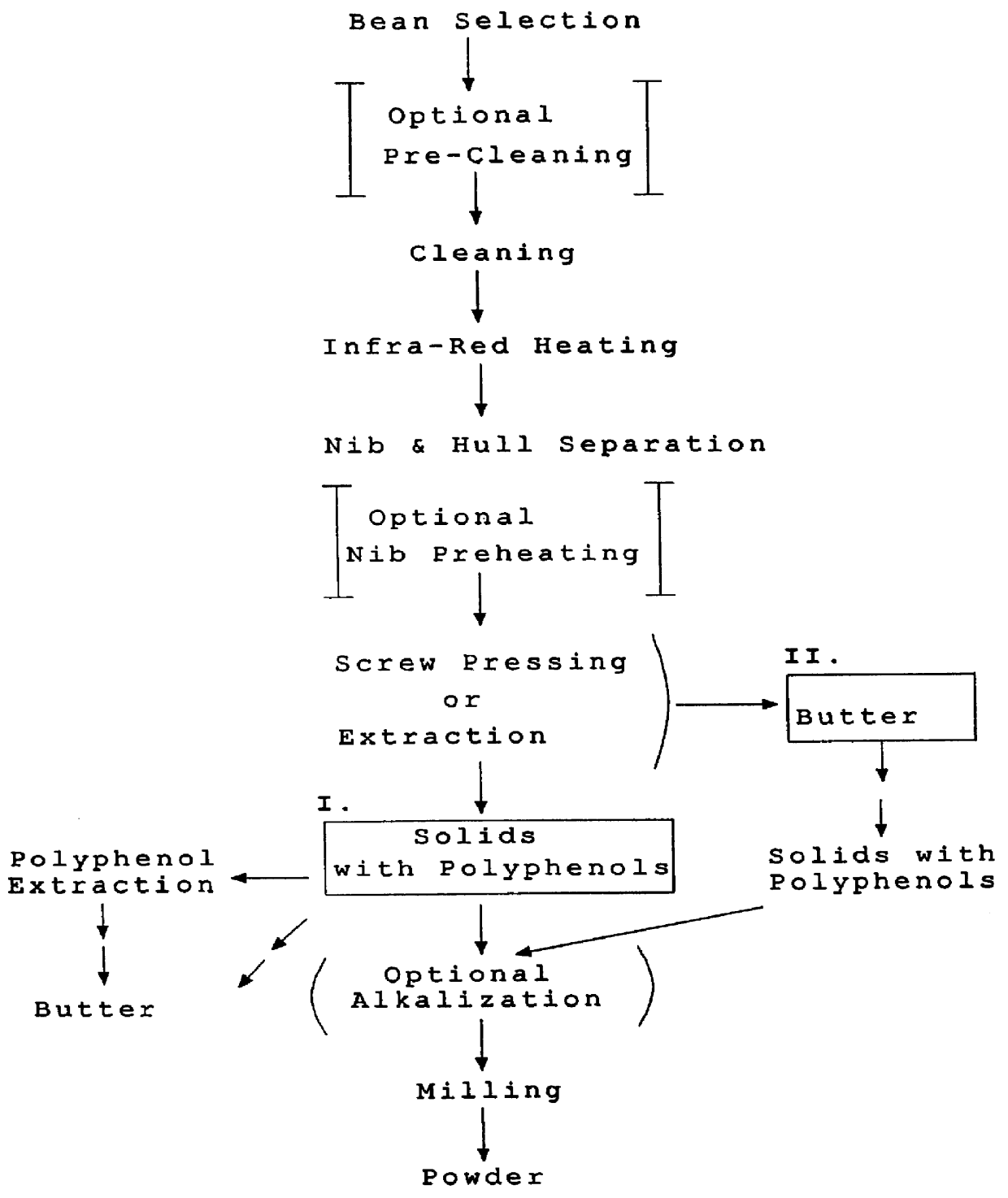
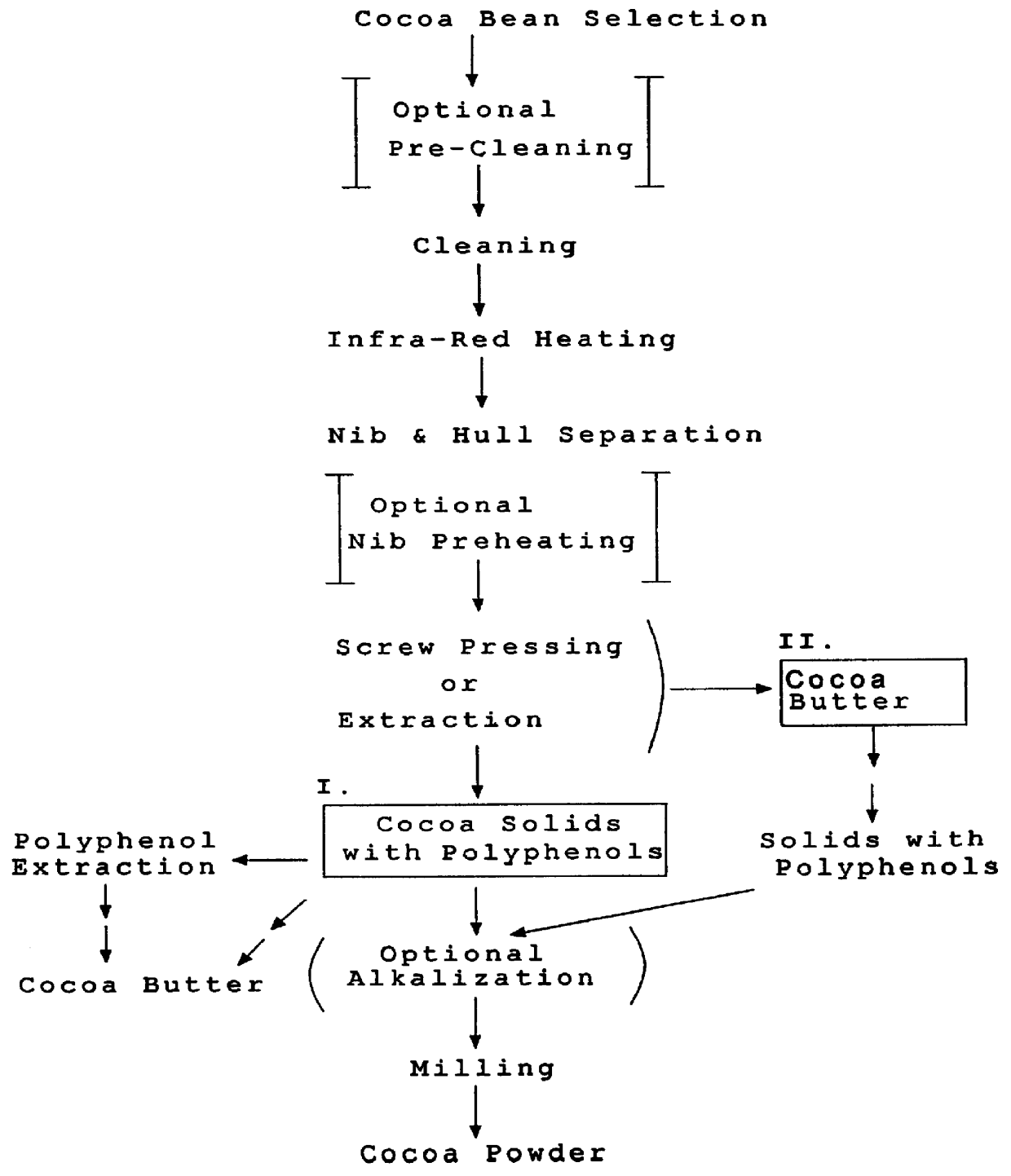
Method for producing fat and/or solids from cocoa beans
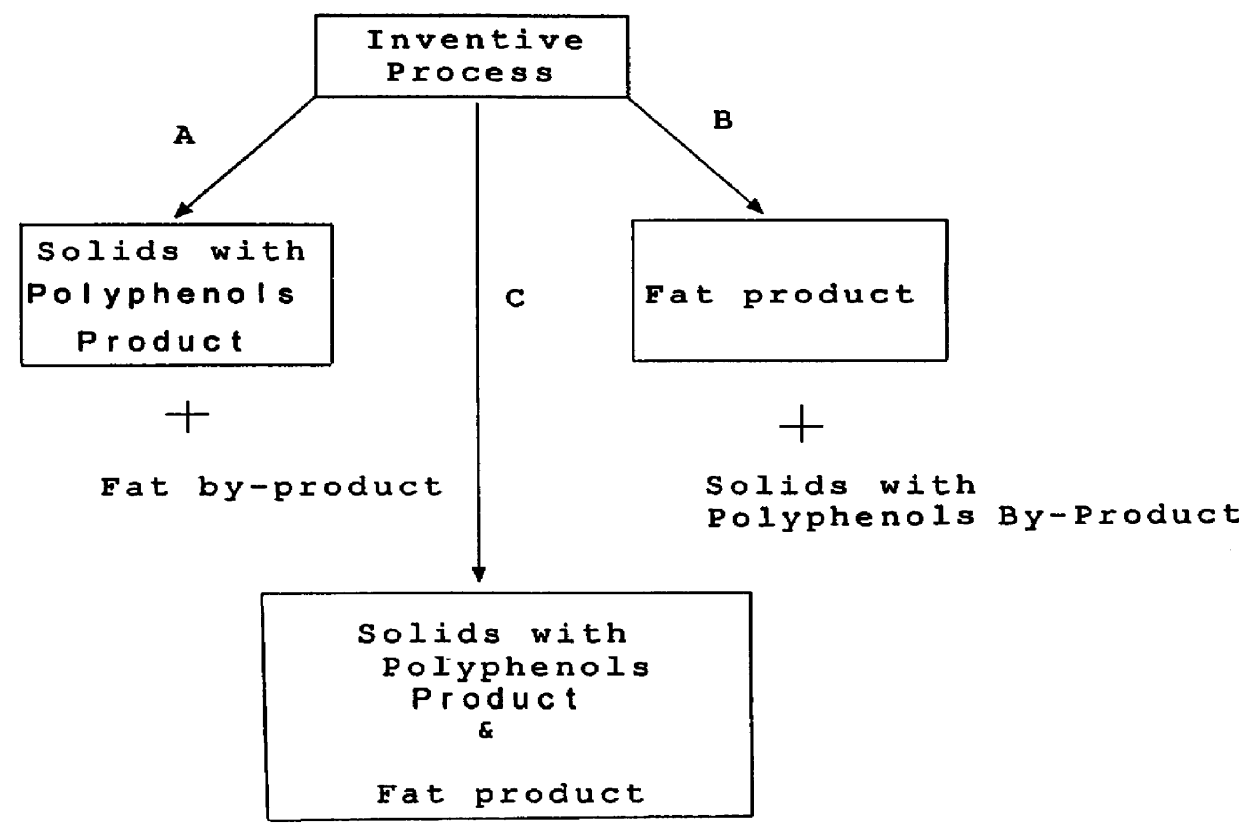
InactiveUS6015913AHighly conserved levelReduce moisture contentBiocideDough treatmentPolyphenolCOCOA BEAN
The present invention is directed to a method of processing a fat-containing bean, e.g., cocoa beans, for producing solids comprising active polyphenols and / or fat-containing products, comprising extracting the fat to produce solids and fat-containing products. Additionally, the inventive method also provides cocoa compositions comprising at least one active polyphenol, wherein the concentration of the polyphenol(s) with respect to the nonfat solids is conserved with respect to the concentration of the active polyphenol(s) in the bean from which the compositions are derived.
Owner:MARS INC +1
Powders for inhalation
InactiveUS6045828AControl cohesivenessGood estimateBiocideOrganic active ingredientsLipid formationInhalation
PCT No. PCT / SE95 / 01560 Sec. 371 Date Mar. 20, 1996 Sec. 102(e) Date Mar. 20, 1996 PCT Filed Dec. 20, 1995 PCT Pub. No. WO96 / 19199 PCT Pub. Date Jun. 27, 1996A proliposome powder, said powder comprising in a single phase discrete particles of a biologically active component together with a lipid or mixture of lipids having a phase transition temperature of below 37 DEG C. and a process for the manufacture of a proliposome powder for inhalation.
Owner:ASTRAZENECA AB
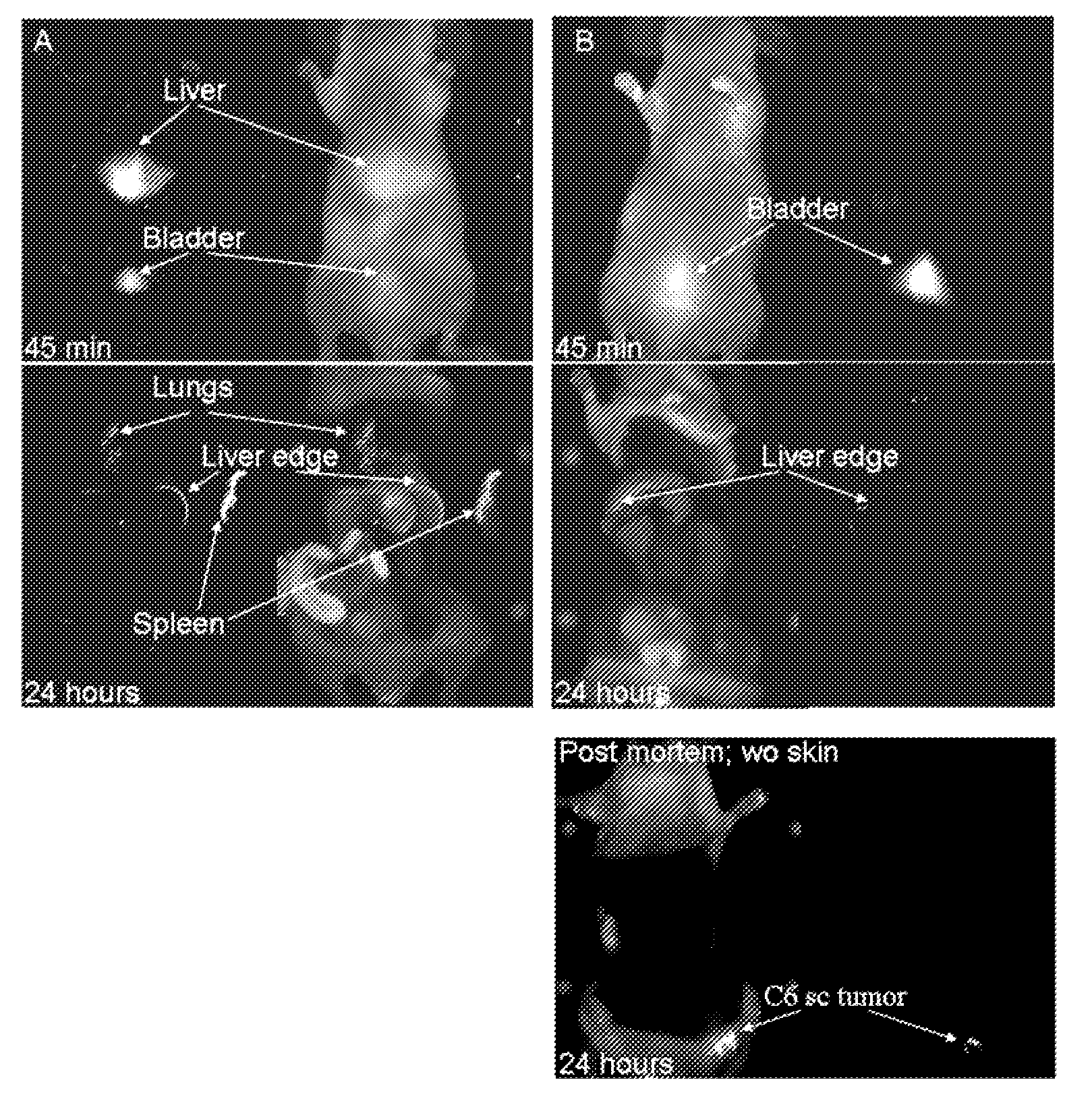
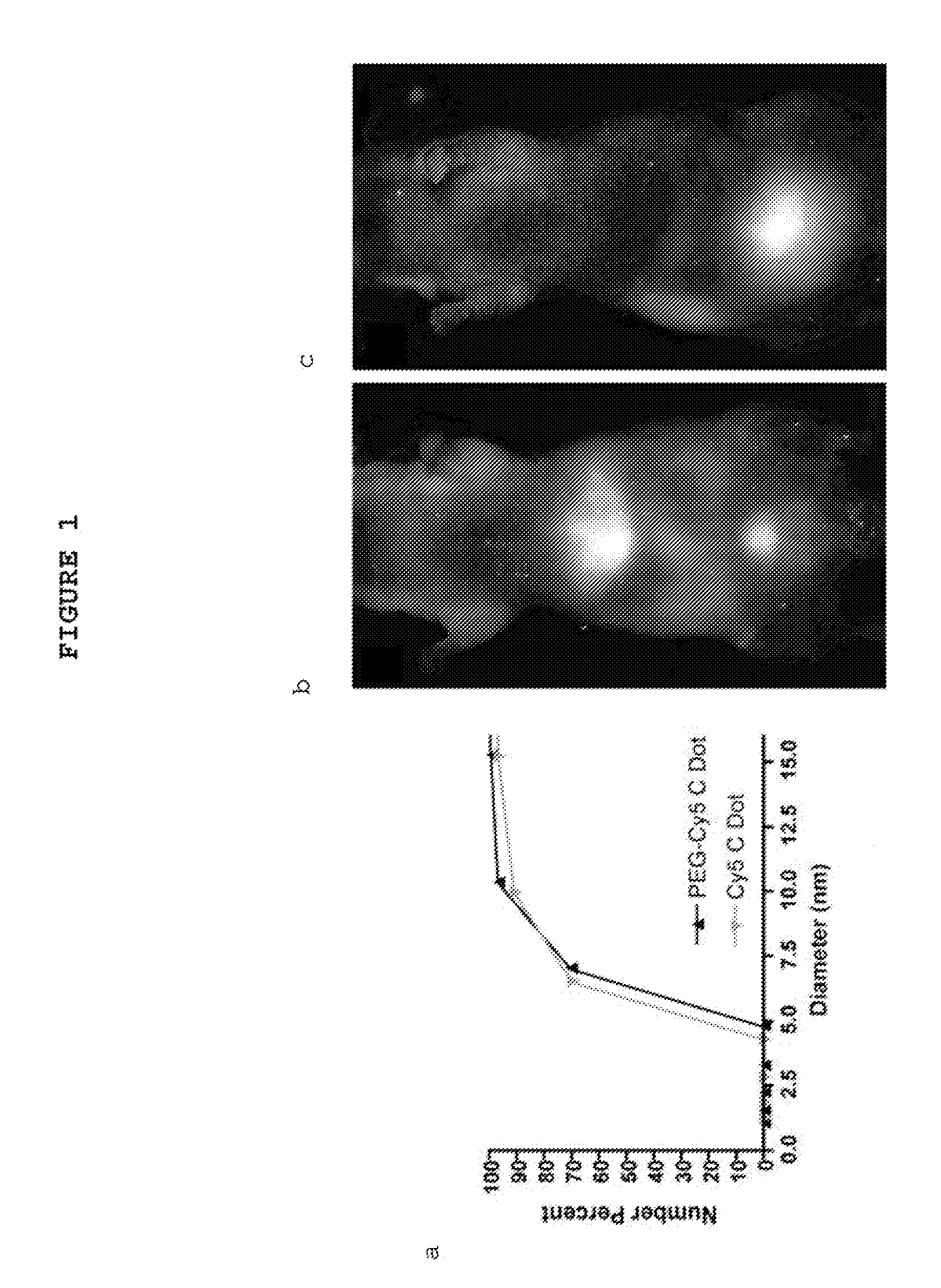
Fluorescent silica-based nanoparticles
ActiveUS20130039848A1Ultrasonic/sonic/infrasonic diagnosticsAntibacterial agentsDiseaseCellular component
The present invention provides a fluorescent silica-based nanoparticle that allows for precise detection, characterization, monitoring and treatment of a disease such as cancer The nanoparticle has a fluorescent compound positioned within the nanoparticle, and has greater brightness and fluorescent quantum yield than the free fluorescent compound To facilitate efficient urinary excretion of the nanoparticle, it may be coated with an organic polymer, such as polyethylene glycol) (PEG) The small size of the nanoparticle, the silica base and the organic polymer coating minimizes the toxicity of the nanoparticle when administered in vivo The nanoparticle may further be conjugated to a ligand capable of binding to a cellular component associated with the specific cell type, such as a tumor marker A therapeutic agent may be attached to the nanoparticle Radionuclides / radiometals or paramagnetic ions may be conjugated to the nanoparticle to permit the nanoparticle to be detectable by various imaging techniques.
Owner:CORNELL UNIVERSITY +1
Tumor-associated marker
This invention provides monoclonal antibody-producing hybridomas designated 27.F7 and 27.B1. The invention also provides methods for detecting TIP-2 antigen-bearing cancer cells in a sample, detecting the presence of TIP-2 antigen, optionally on the surface of cancer cells, immunohistochemical screening of a tissue section for the presence of TIP-2 antigen bearing cancer cells, diagnosing cancer in a subject, monitoring progression of cancer wherein the cancer cells are TIP-2 antigen-bearing cells, delivering exogenous material to TIP-2 antigen-bearing cancer cells of a human subject, and treating cancer in a human subject. This invention further provides a kit for detecting the presence of TIP-2 antigen-bearing cancer cells. This invention also provides isolated peptides having the amino acid sequences Lys Leu Leu Gly Gly Gln Ile Gly Leu (SEQ ID No:3) and Ser Leu Leu Gly Cys Arg His Tyr Glu Val (SEQ ID NO:4).
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
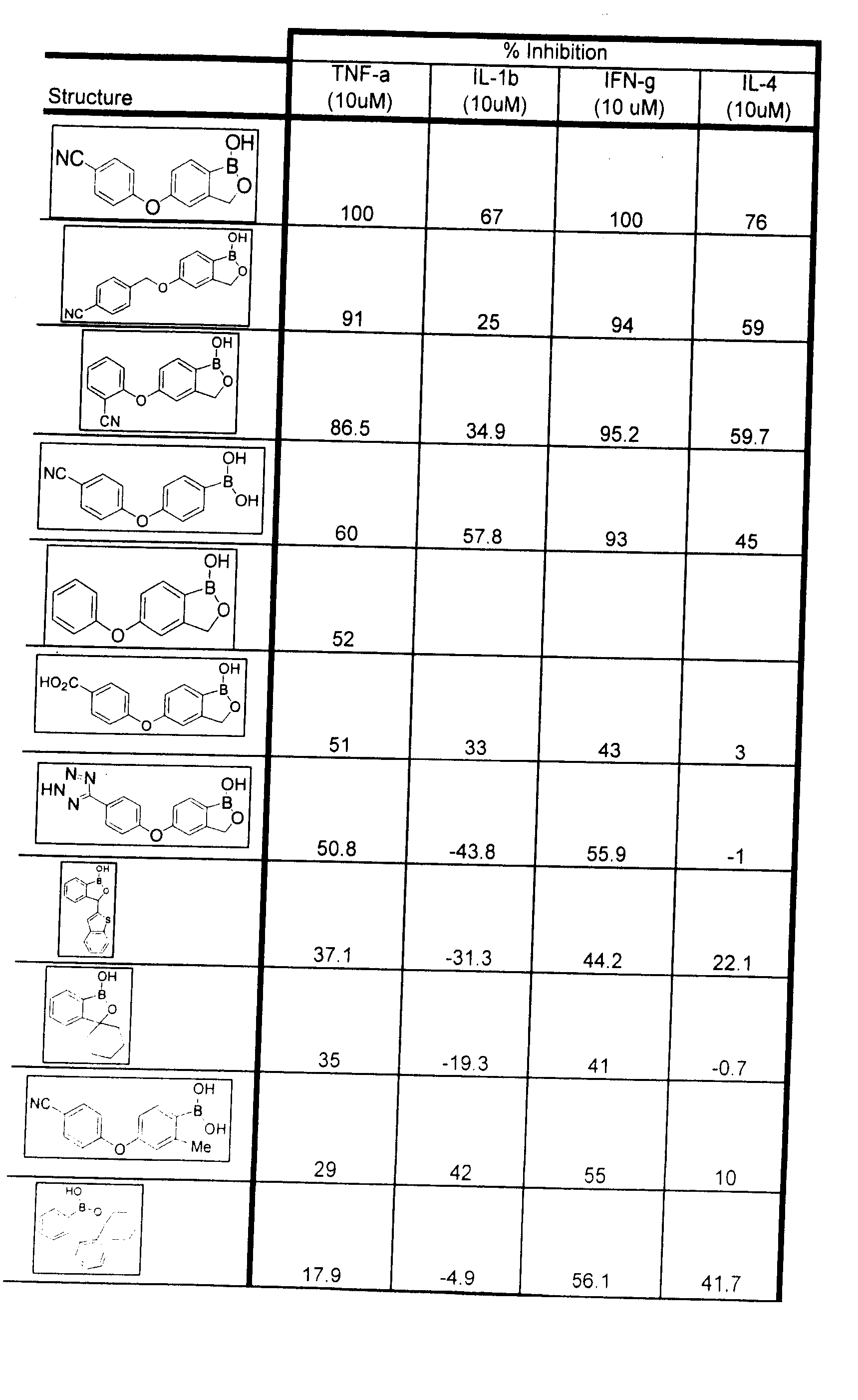
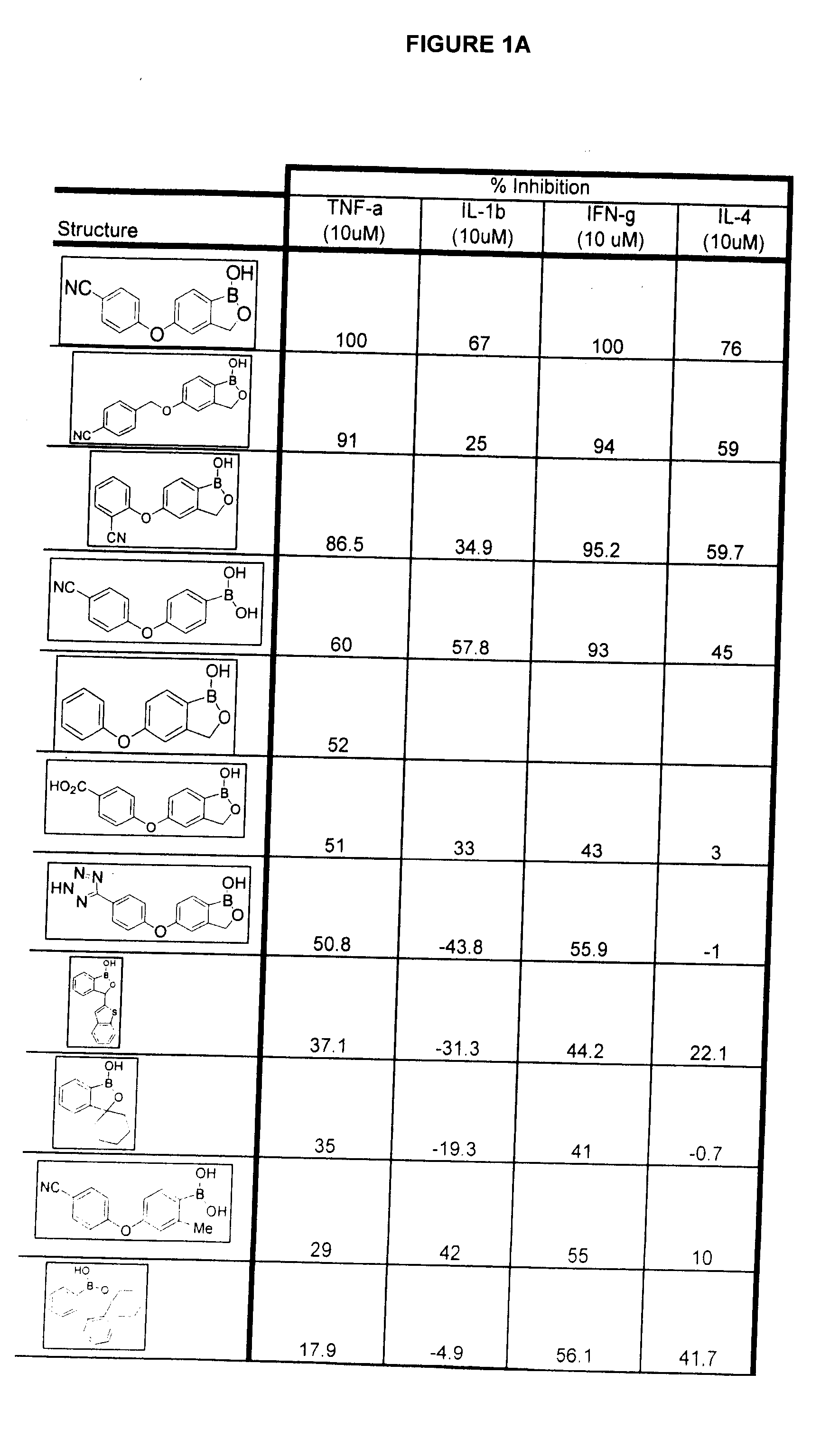
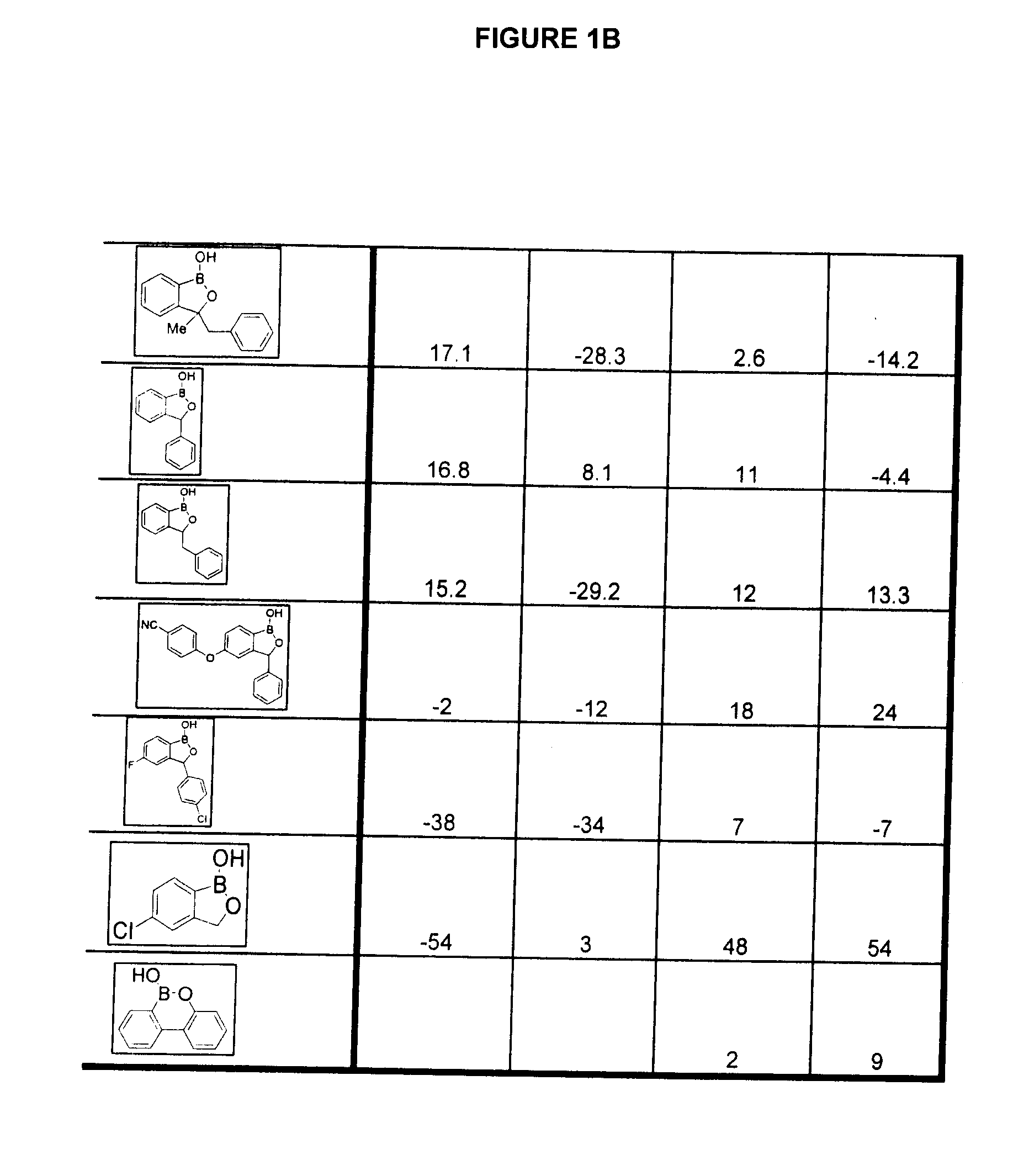
Boron-containing small molecules as Anti-inflammatory agents
Methods of treating anti-inflammatory conditions through the use of boron-containing small molecules are disclosed.
Owner:ANACOR PHARMA INC
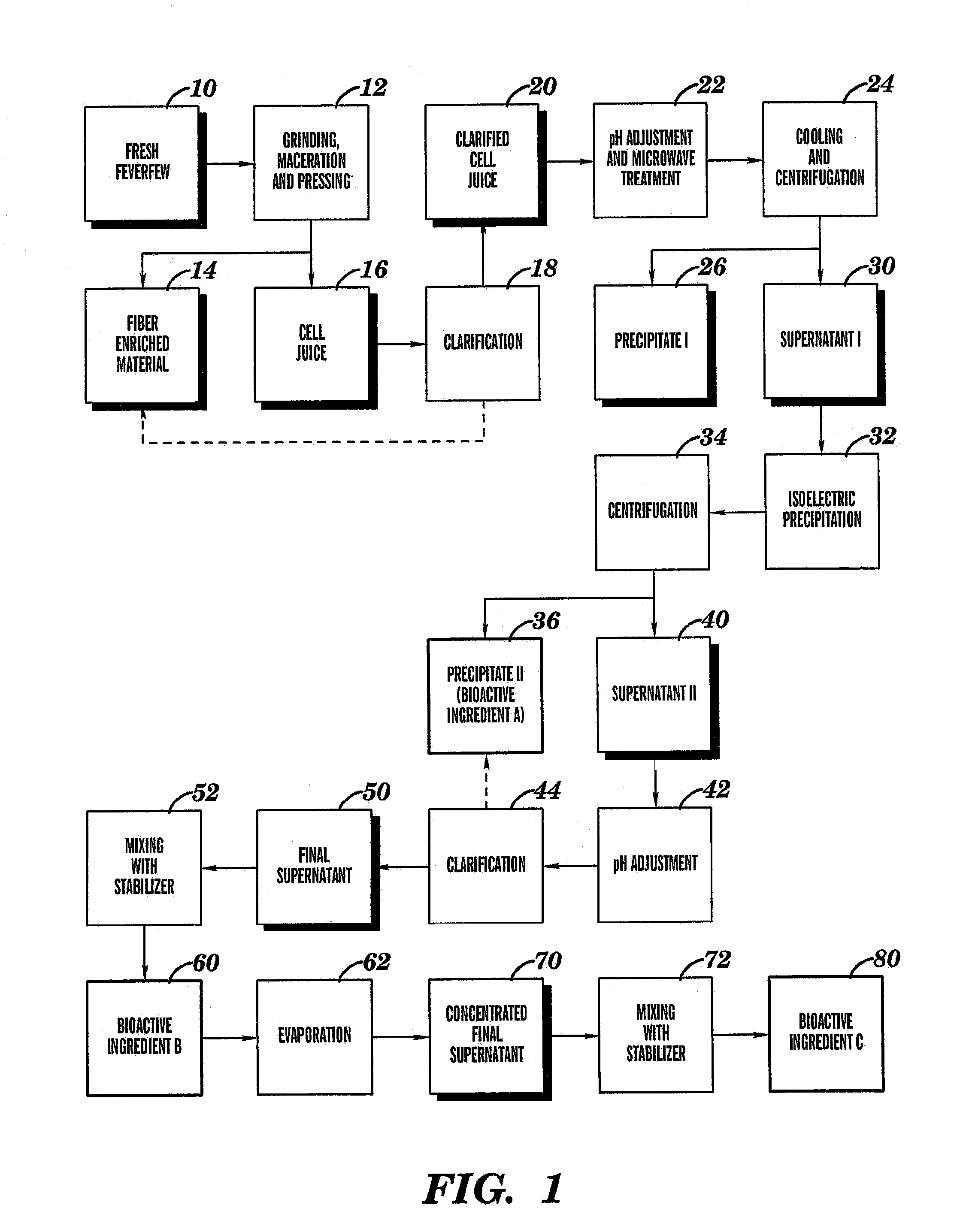
Parthenolide free bioactive ingredients from feverfew (Tanacetum parthenium) and processes for their production
ActiveUS7537791B2Inhibitory activityProtection from damageAntibacterial agentsBiocideTanacetum partheniumAdditive ingredient
The present invention relates to bioactive ingredients that include isolated bioactive fractions derived from cell juice of fresh biomass of a feverfew (Tanacetum parthenium) plant. The bioactive fractions are either free of or substantially free of α-unsaturated γ-lactones (e.g., parthenolide). Further, the bioactive fractions have anti-inflammatory and antioxidant activity. The present invention also relates to a method for isolating a bioactive fraction that is derived from cell juice of fresh biomass of a feverfew (Tanacetum parthenium) plant and that is at least substantially free of α-unsaturated γ-lactones (e.g., parthenolide). The present invention also relates to a method for preparing a stabilized cell juice serum fraction and a stabilized concentrated cell juice serum fraction that are free of α-unsaturated γ-lactones (e.g., parthenolide). The present invention also relates to a bioactive composition that includes a mixture of one or more of the isolated bioactive fractions of the present invention.
Owner:ISP INVESTMENTS LLC
Stabilization of hypoxia inducible factor (HIF) alpha
InactiveUS20030176317A1Improve heart functionPromote healingOrganic active ingredientsPeptide/protein ingredientsMedicineBiochemistry
The present invention relates to methods of stabilizing the alpha subunit of hypoxia inducible factor (HIF). The invention further relates to methods of preventing, pretreating, or treating conditions associated with HIF, including ischemic and hypoxic conditions. Compounds for use in these methods are also provided.
Owner:FIBROGEN INC
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20040102429A1Minimize and prevent irritationReduce transmissionAntibacterial agentsOrganic active ingredientsHigh concentrationFungicide
The addition of low concentrations of combinations of water-soluble organic salts of zinc to gels, creams, lotions or ointments can increase the ability of these products to reduce or prevent exogenous irritants from causing irritation of the underlying substrate. The addition of low concentrations of combinations of water-soluble organic zinc salts to these gels, creams, lotions or ointments also can reduce the irritation of skin or mucous membranes caused by the addition of potentially-irritating substances such as spermicides, microbicides, fungicides or other therapeutic agents to the gel, cream, lotion or ointment. The advantages of this anti-irritant approach over others, which generally employ high concentrations of single zinc salts, are the reduced potential for zinc toxicity, the reduced potential for toxicity related to zinc itself, and the preservation of the desirable biological properties of potentially-irritating therapeutic substances added to the gel, cream, lotion or ointment.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com