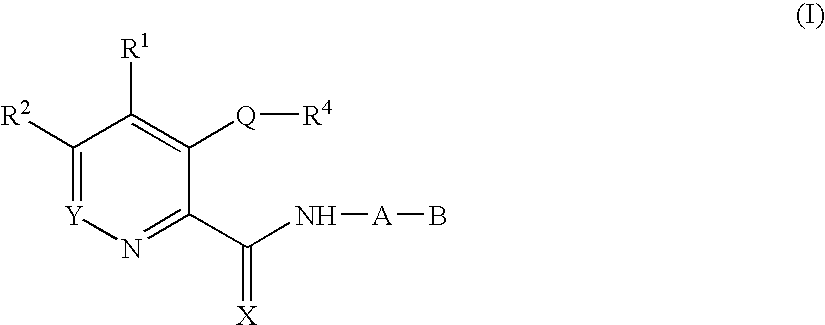
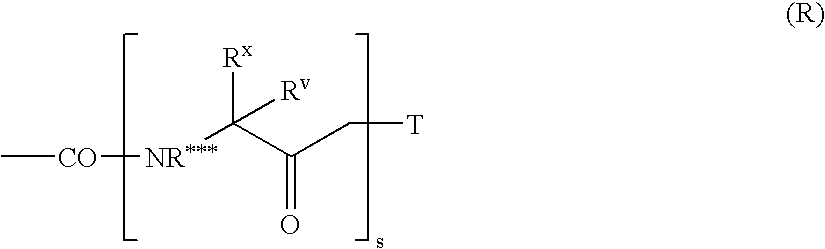
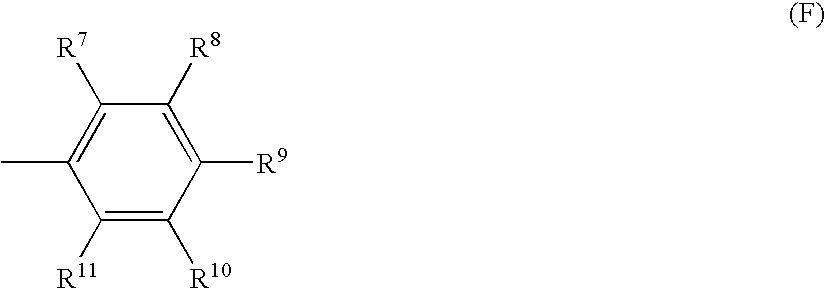Methods of increasing endogenous erythropoietin (EPO)
a technology of endogenous erythropoietin and erythropoietin, which is applied in the direction of depsipeptides, peptide/protein ingredients, chemical treatment enzyme inactivation, etc., can solve the problems of bone marrow destruction, difficulty in breathing and heart abnormalities, and decrease in quality of life, so as to increase endogenous epo, increase endogenous epo levels, and reduce the need for allogenogenous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Increase in Endogenous Erythropoietin Levels in Vitro
[0218] Human cells derived from hepatocarcinoma (Hep3B) tissue (see, e.g., American Type Culture Collection, Manassas Va.) were seeded into 35 mm culture dishes and grown at 37.degree. C., 20% 02, 5% CO.sub.2 in Minimal Essential Medium (MEM), Earle's balanced salt solution (Mediatech Inc., Herndon Va.), 2 mM L-glutamine, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, and 10% FBS. When cell layers reached confluence, the media was replaced with OPTI-MEM media (Invitrogen Life Technologies, Carlsbad Calif.) and cell layers were incubated for approximately 24 hours in 20% O.sub.2, 5% CO.sub.2 at 37.degree. C. A compound of the invention (one of compounds A to I) or 1% DMSO (negative control) was then added to existing media and incubation was continued overnight.
[0219] Following incubation, the conditioned media was collected from cell cultures and analyzed for erythropoietin expression using a QUANTIKINE immunoassay (R&D S...
example 2
Increase in Endogenous Erythropoietin Levels in Vivo
experiment i
[0220] Experiment I
[0221] Twelve Swiss Webster male mice (30-32 g) were obtained from Simonsen, Inc. (Gilroy Calif.), and treated by oral gavage two times per day for 2.5 days (5 doses) with a 4 ml / kg volume of either 0.5% carboxymethyl cellulose (CMC; Sigma-Aldrich, St. Louis Mo.) (0 mg / kg / day) or 2.5% Compound C (25 mg / ml in 0.5% CMC) (200 mg / kg / day). Four hours after the final dose, animals were anesthetized with isoflurane and two blood samples were collected from the abdominal vein. One blood sample was collected into a MICROTAINER serum separator tube (Becton-Dickinson, Franklin Lakes N.J.), and incubated at room temperature for 30 minutes, centrifuged at 8,000 rpm at 4.degree. C. for 10 minutes. The serum fraction was then processed and analyzed for erythropoietin (EPO) expression using a QUANTIKINE immunoassay (R&D Systems) according to the manufacturer's instructions. The second blood sample was collected into a MICROTAINER EDTA-2K tube (Becton-Dickinson) for hematocrit ana...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com