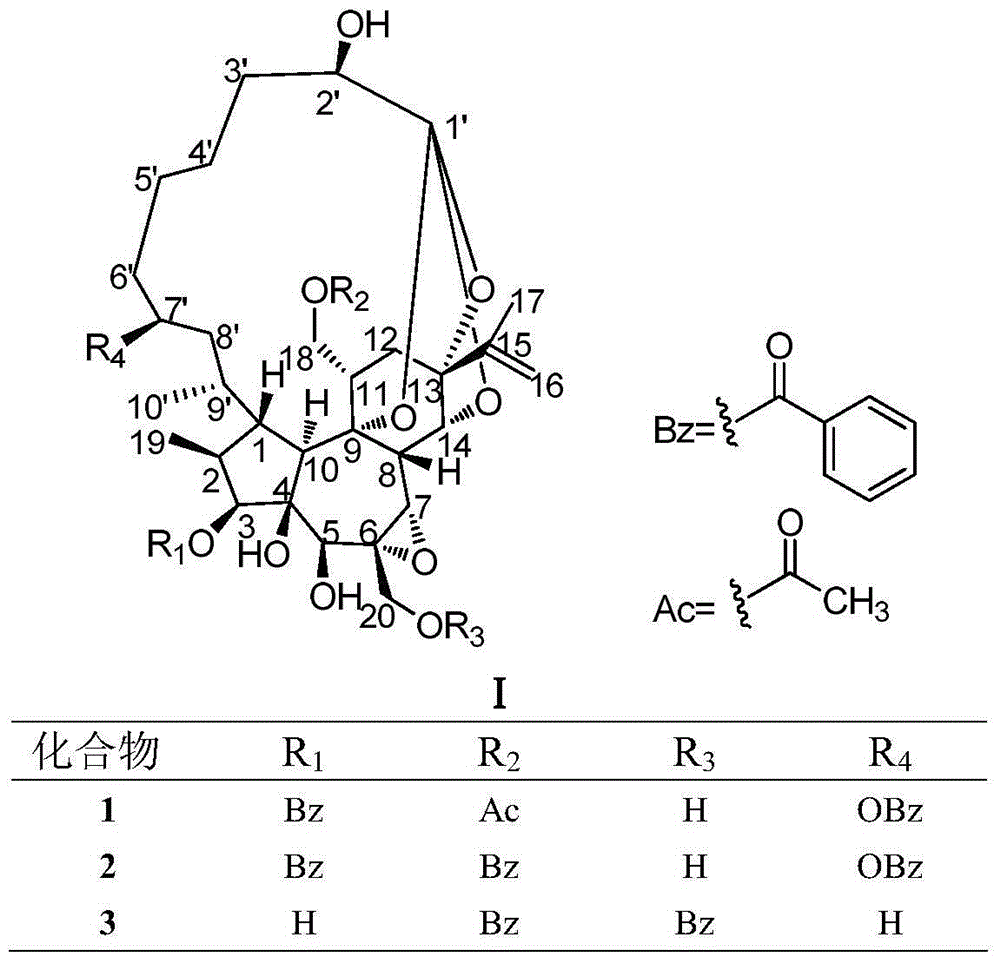
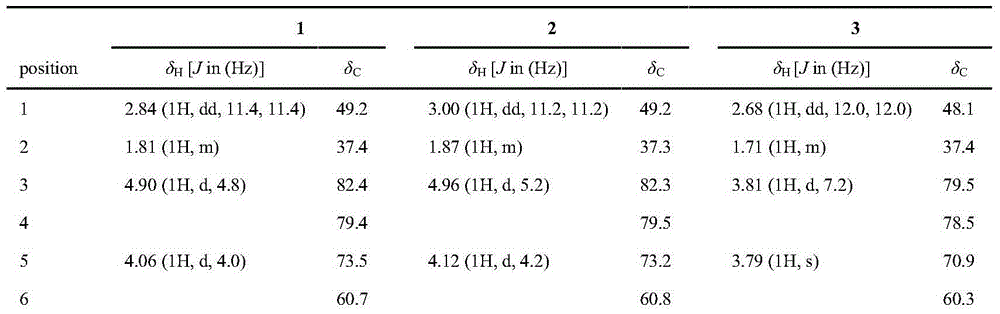
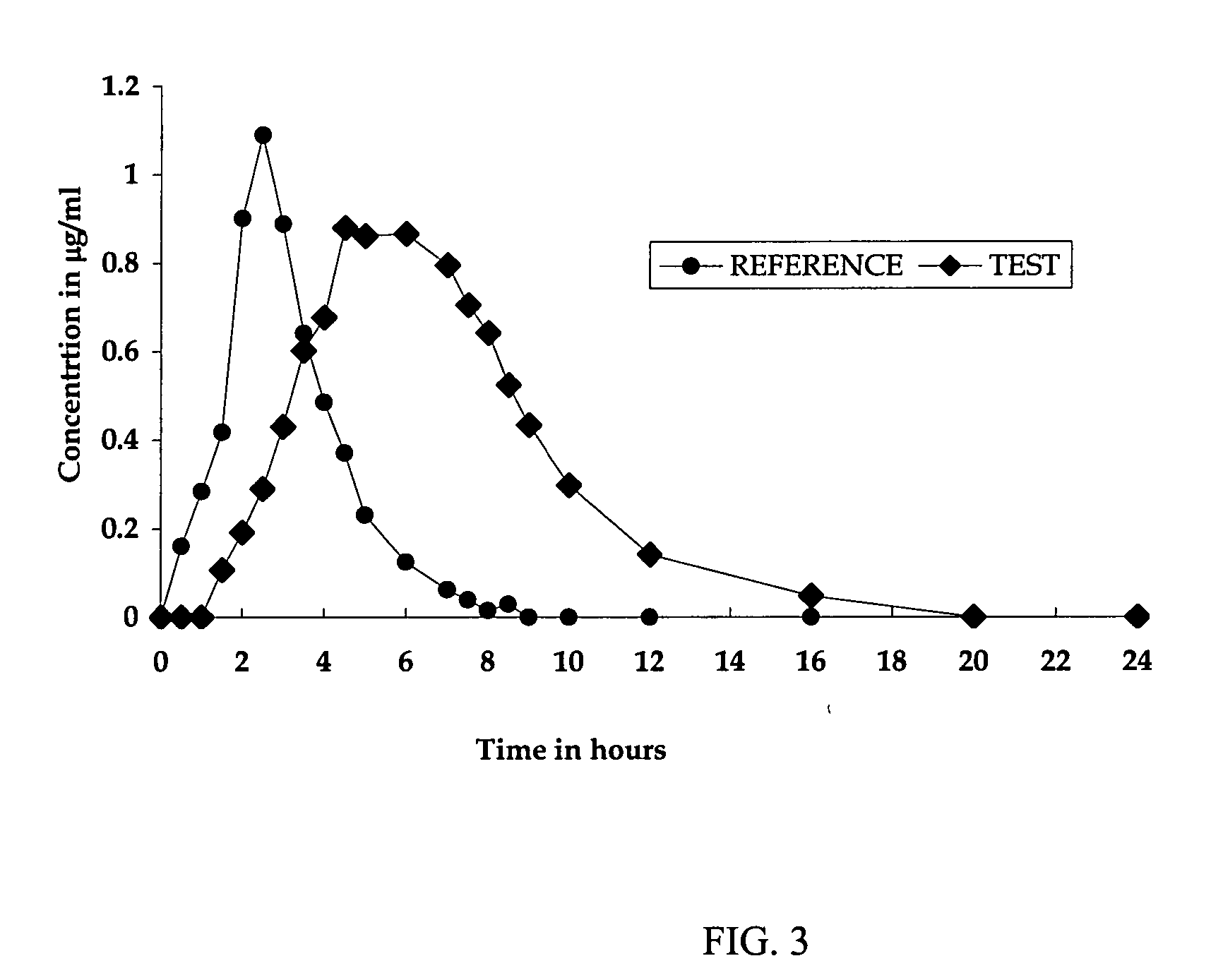
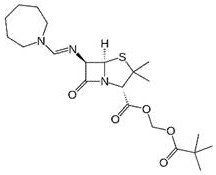
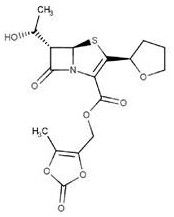
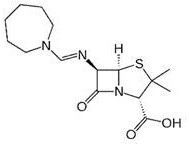
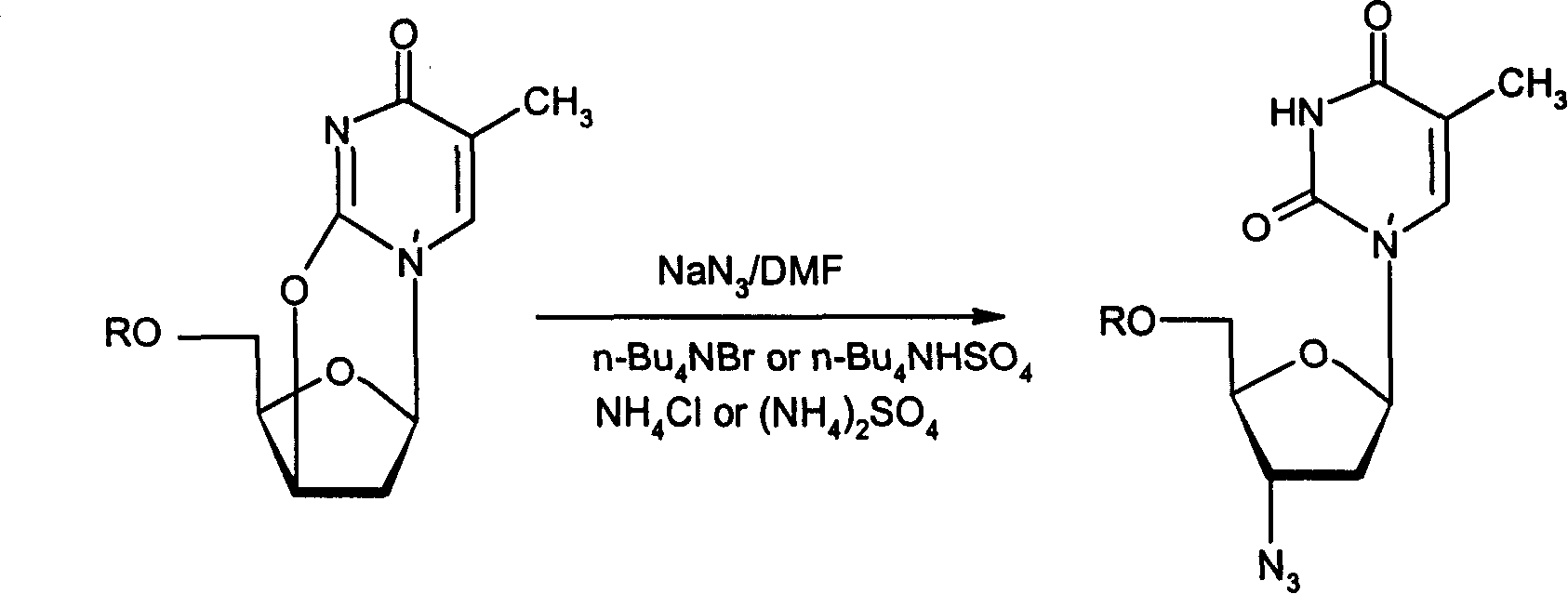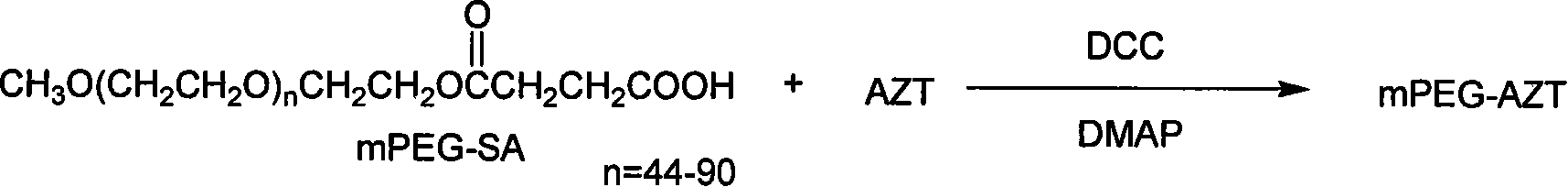Patents
Literature
92 results about "Zidovudine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This drug is used with other HIV medications to help control HIV infection.
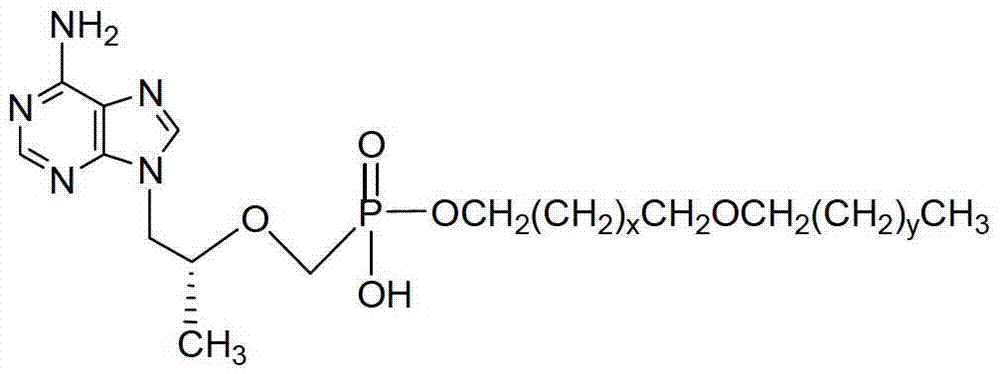
Tenofovir disoproxil compounds, and preparation method and application thereof in anti-virus aspects
ActiveCN103224530AInhibition of replication activityImprove attributesOrganic active ingredientsGroup 5/15 element organic compoundsSolubilityAnti virus
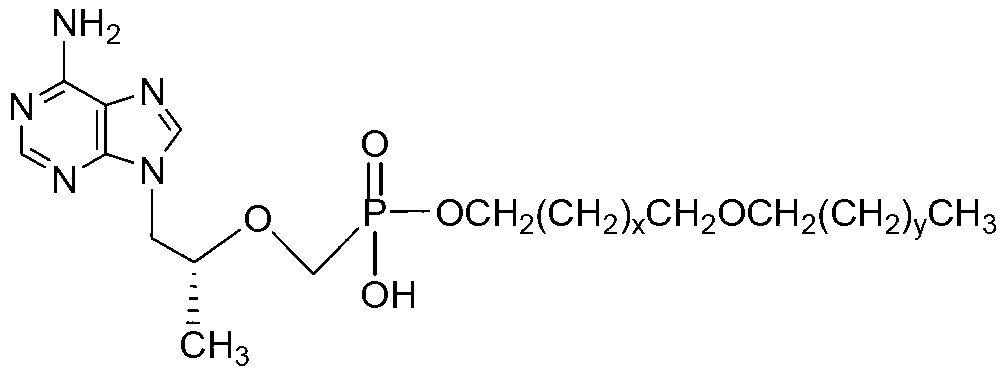
The invention discloses a group of tenofovir disoproxil compounds with activity for inhibiting HIV-1 / HBV virus replication and pharmaceutically acceptable salts thereof, and a preparation method and pharmaceutical applications thereof. The group of the compounds have a general formula I, wherein X=H, Y=H, R1=-CH2(CH2)mCH2O(CH2)nCH3, m=0-4, n=10-20, and R2, R3 and R4 are respectively described in the specification. The invention also discloses a pharmaceutical composition containing the group of the compounds. Experiments show that one of the compounds has the advantages that an activity for inhibiting HIV-1 virus replication is 20 times that of a positive control medicine zidovudine (AZT), 1,000 times that of TDF that is the best medicine for treating Aids and about 9 times that of CMX157 in a clinical stage, and lipid solubility is about 2 times that of CMX157. Experiments also show that the compounds provided by the invention have the activity for inhibiting HBV virus replication, and can be used for development of drugs for treating the Aids and hepatitis B.
Owner:洛阳聚慧新材料科技有限公司 +2
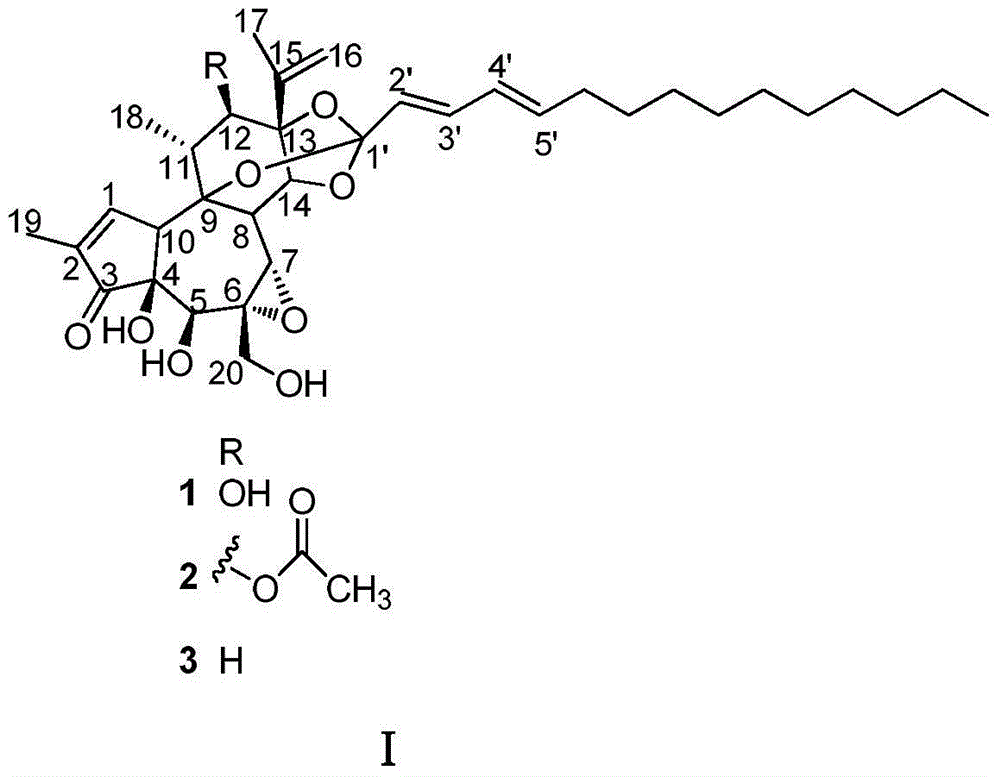
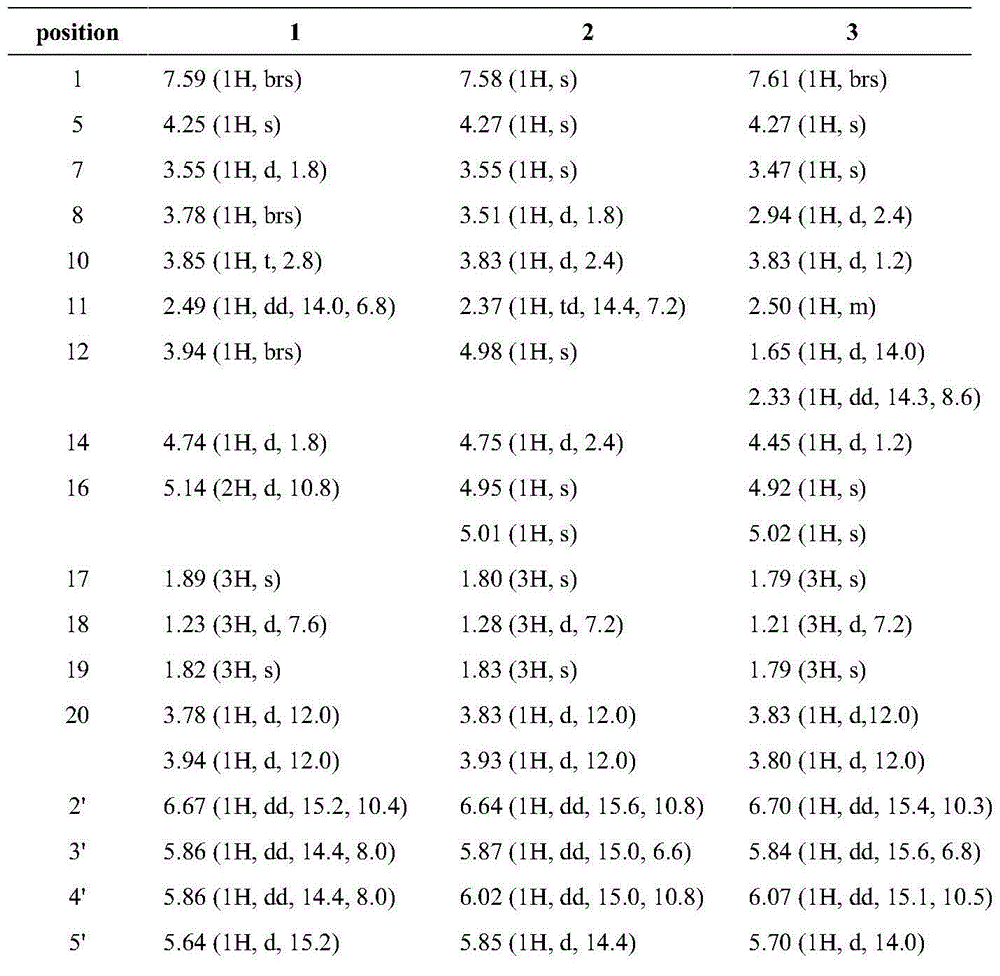
1-alkylated daphnane diterpene and application thereof to preparation of anti-HIV drugs
The invention belongs to the field of traditional Chinese medicine production, and relates to 1-alkylated daphnane diterpene and application thereof to preparation of anti-HIV drugs. 1-alkylated daphnane diterpene is a compound extracted from S. chamaejasme of the Stellera genus; tests prove that 1-alkylated daphnane diterpene is remarkable in anti-HIV activity, and the EC50 value is lower than 0.001 [mu]M; 1-alkylated daphnane diterpene is relatively low in cytotoxicity, and the CC50 value is higher than 11 [mu]M; compared with a positive control drug zidovudine, 1-alkylated daphnane diterpene has more obvious advantages; the 1-alkylated daphnane diterpene compound can serve as an active ingredient for preparation of the anti-HIV drugs.
Owner:FUDAN UNIV
Novel HIV integrase inhibitors and HIV therapy based on drug combinations including integrase inhibitors
InactiveUS20050049242A1Strong synergyBiocideAnimal repellantsCombination drug therapyResistant virus
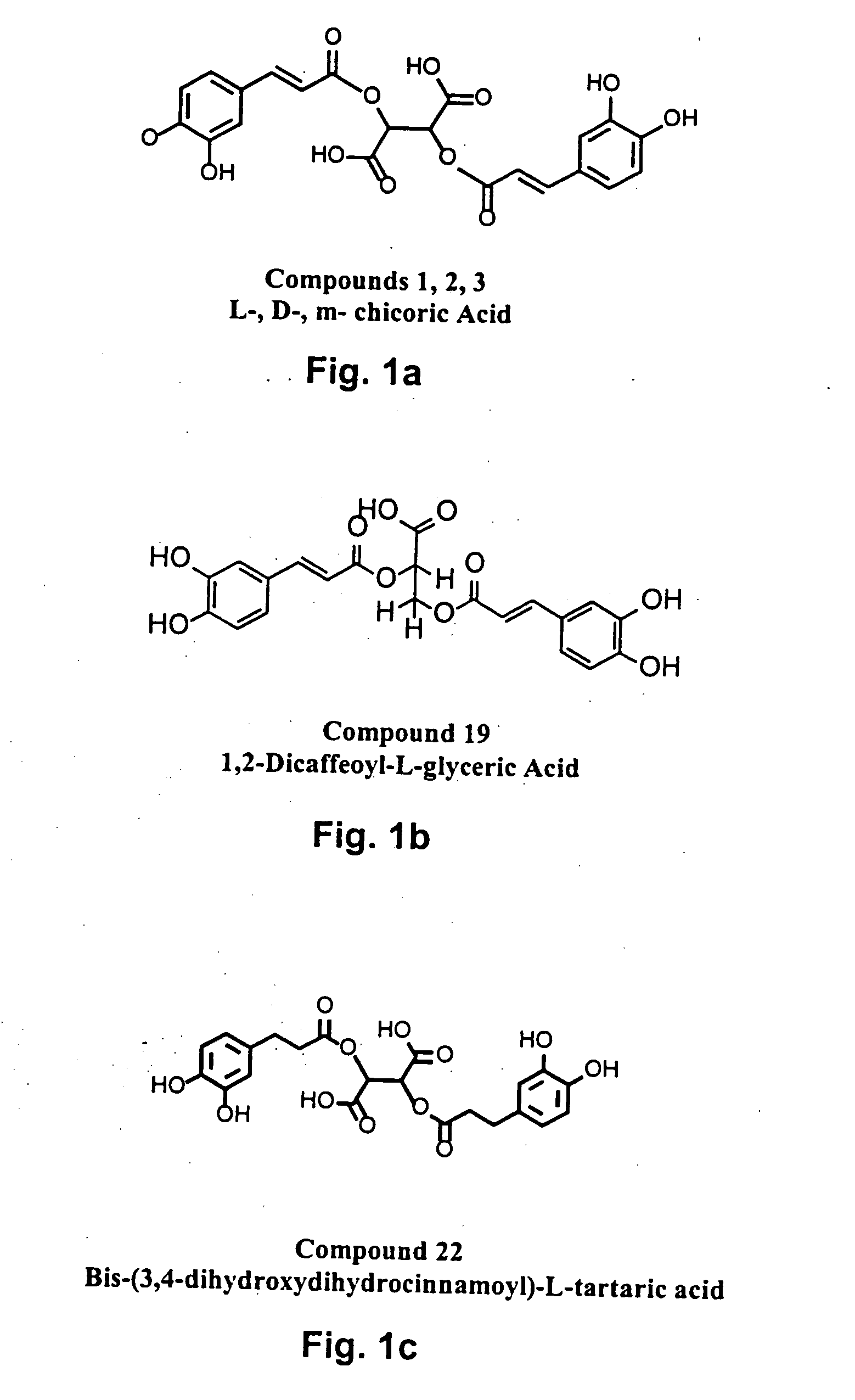
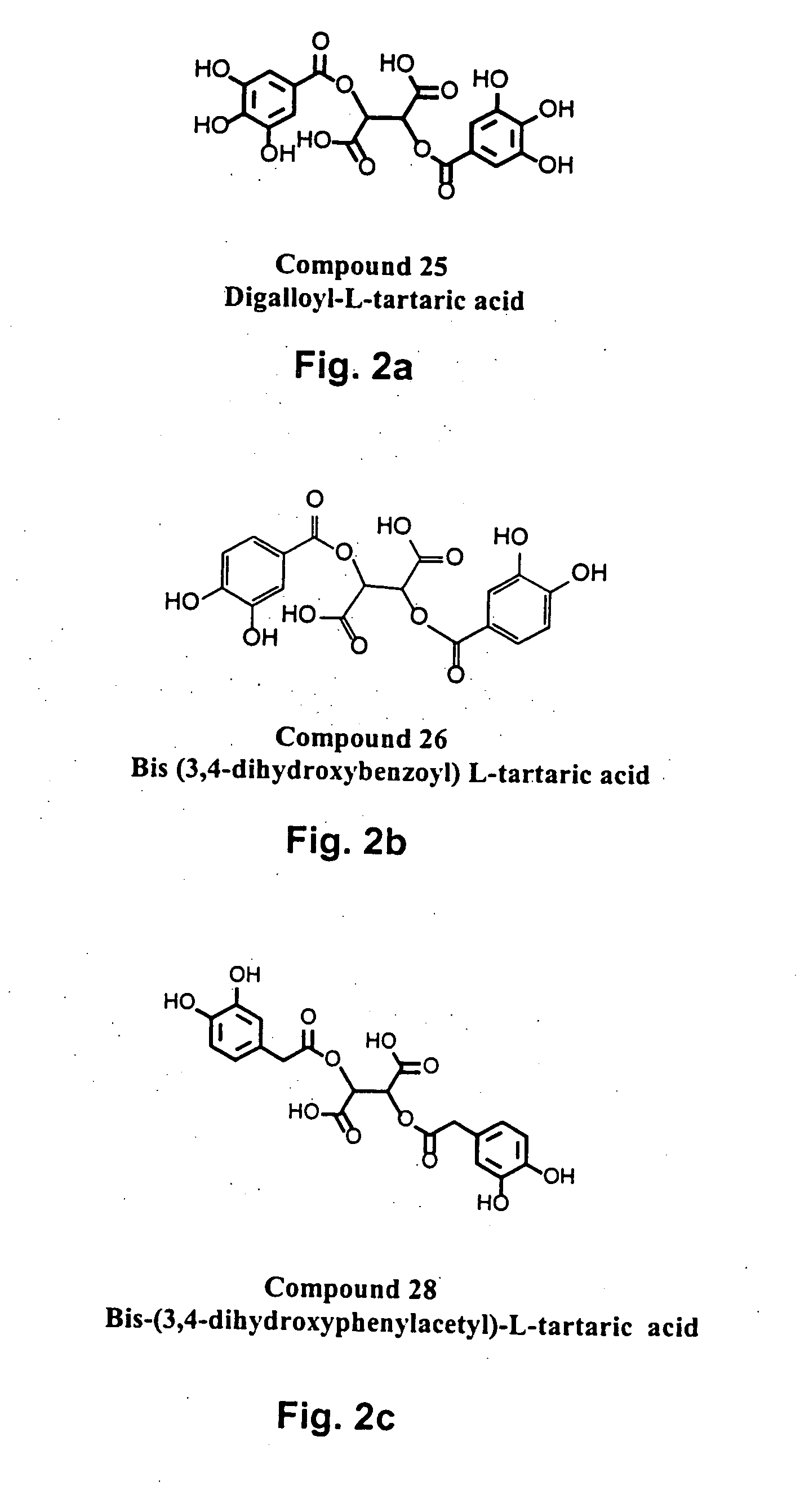
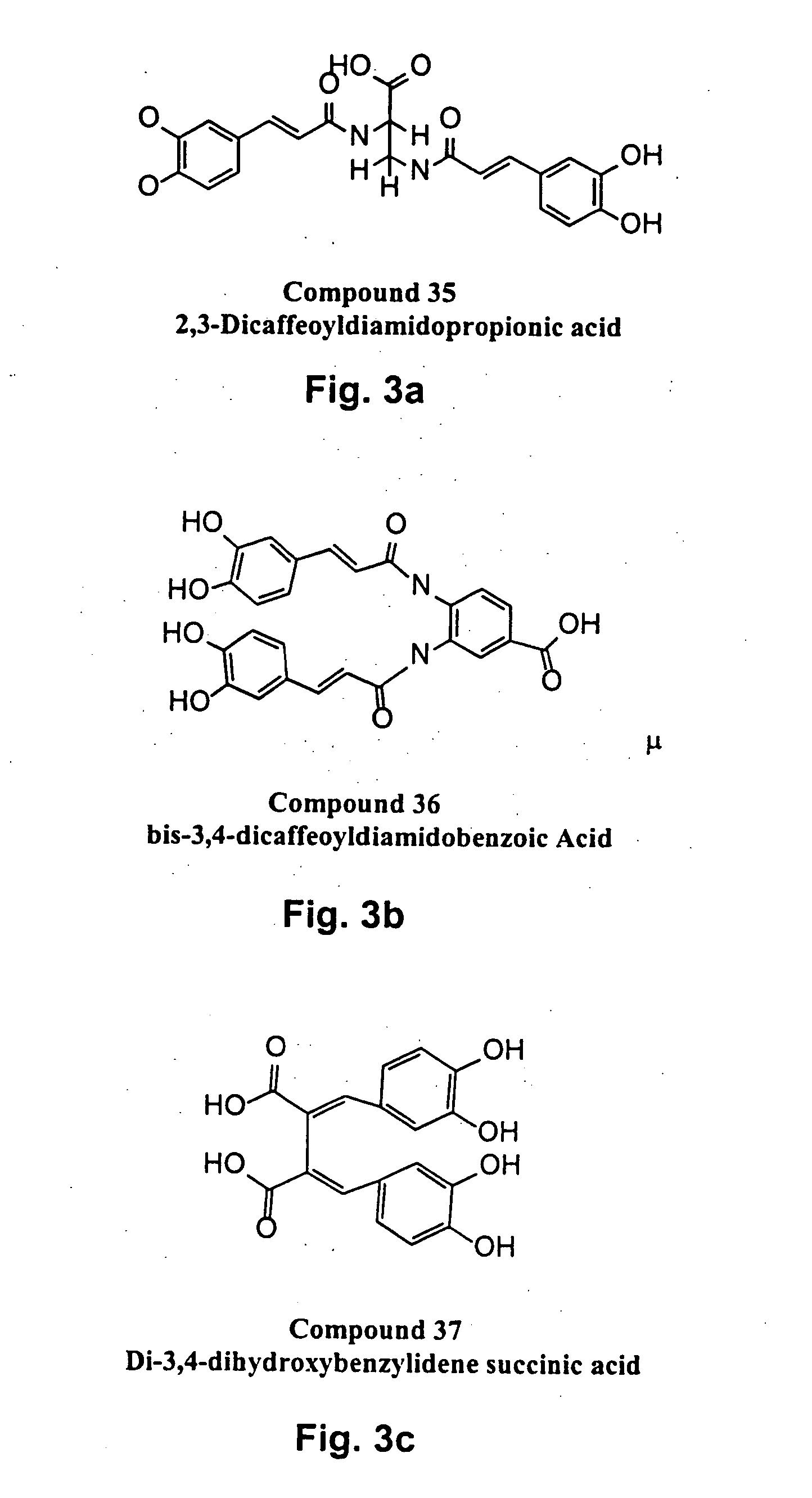
The present invention includes a group of novel compounds that are demonstrated to potently and selectively inhibit HIV integrase (IN) activity in vitro and to potently inhibit HIV replication in live, cultured cells at non-toxic concentrations. The novel compounds disclosed include 2,3-di(3,4-dihydroxy-dihydroxydihydrocinnamoyl)-L-tartaric acid, 2,3-di-(3,4-dihydroxybenzoyl)-L-tartaric acid, 2,3-di-(3,4-dihydroxyphenylacetyl)-L-tartaric acid, 2,3-di-(3,4,5-trihydroxybenzoyl-L-tartaric acid, 2,3-dicaffeoyldiamidopropionic acid, 1,2,-dicaffeoyl-L-glyceric acid, bis,-3,4-dicaffeoyldiamidobenzoic acid, di-3,4-dihydroxybenzylidene succinic acid, di-3,4-dihydrodihydroxybenzylidine succinic acid, 2,3-dicaffeoyl-L-serine, bis-dicaffeoyl-L-isoserine and 1,4-dicaffeoyl-L-lysine. Tests of integrase inhibitors with 2′,3′-dideoxycytidine, zidovudine and nelfinavir (protease inhibitor) indicated a potent synergy against reverse transcriptase inhibitor resistant virus. The potential benefit from the addition of integrase inhibitors to combination drug therapies is significant.
Owner:ROBINSON W EDWARD JR +2
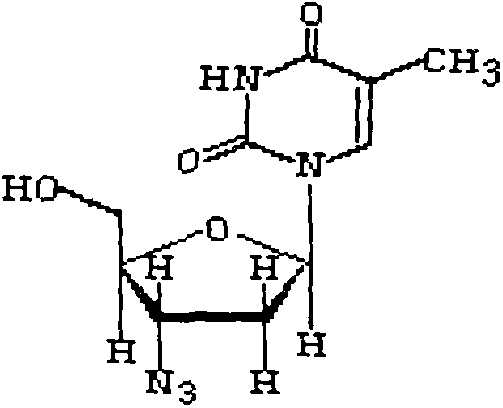
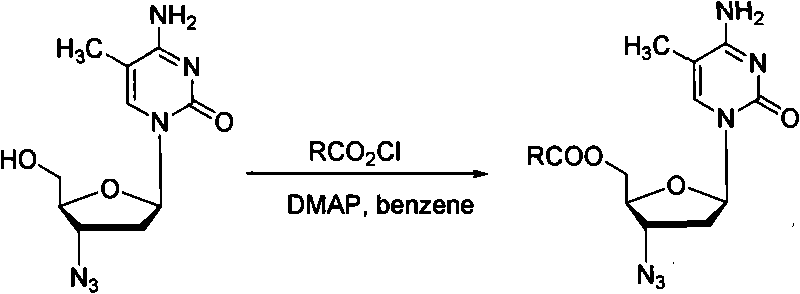
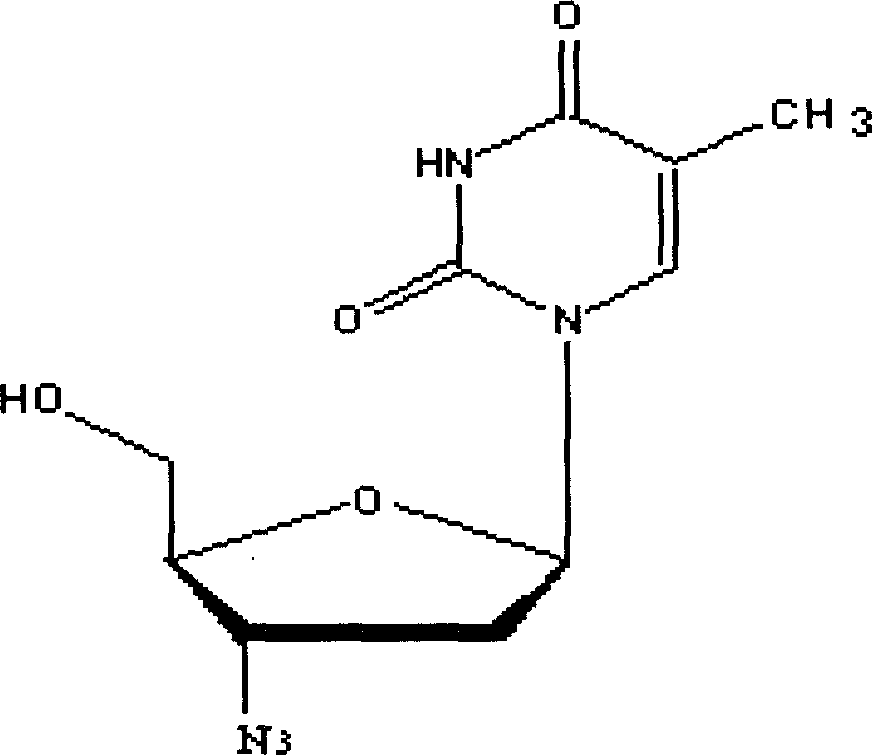
Intermediate for synthesizing azidothimidine, preparation thereof and use in azidothimidine synthesis
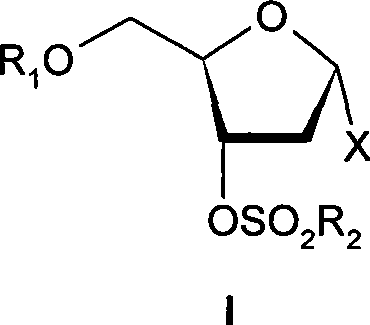
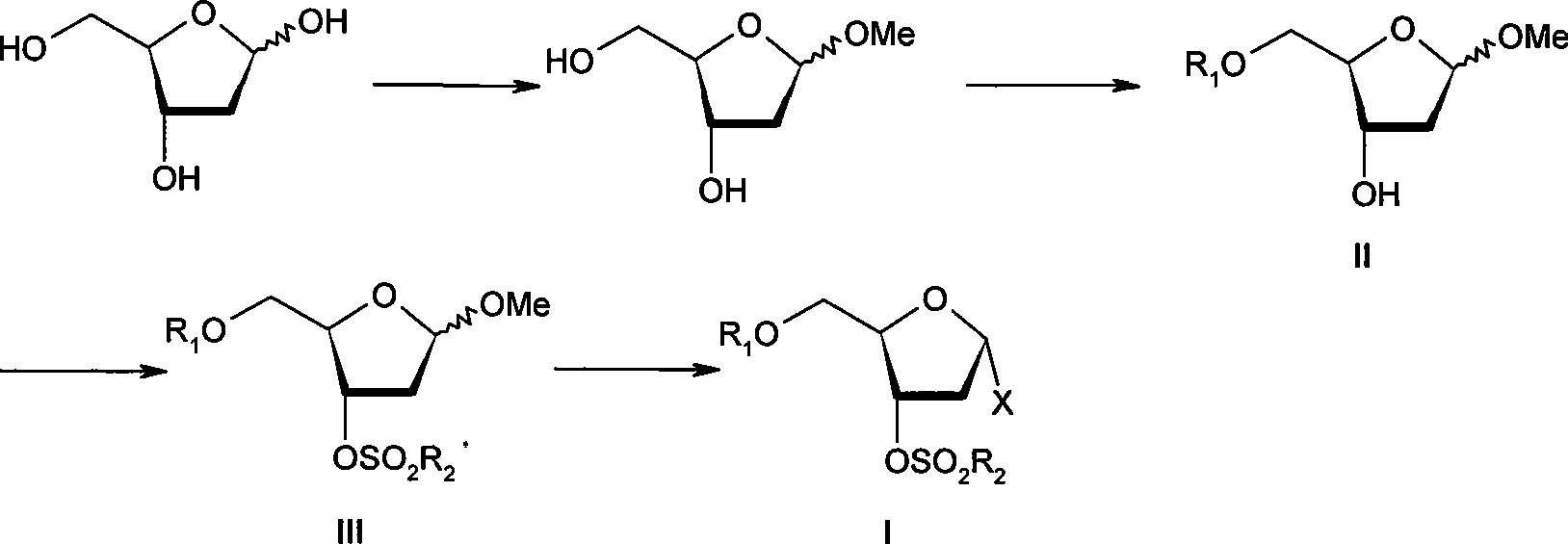
ActiveCN101376667AHigh stereoselectivityMild reaction conditionsEsterified saccharide compoundsSugar derivativesArylHalogen
The invention discloses an intermediate with a following structural formula (I) for synthesizing zidovudine, wherein R1 is a hydroxyl protective group, R2 is C1-C12 alkyl or C6-C30 aryl, and X is halogen. The invention also discloses a preparation method of the intermediate and application thereof on synthesizing zidovudine. The method for synthesizing zidovudine from the intermediate has the advantages of mild reaction conditions, short lines, high yield, good intermediate stereoselectivity, low cost, and no need of Beta-thymidine intermediate, and is suitable for the industrial production.
Owner:JIANGSU PUXIN PHARMA CO LTD
Potent combinations of zidovudine and drugs that select for the K65R mutation in the HIV polymerase
InactiveCN101878032ALow toxicityImprove absolute antiviral effectAntiviralsCarbohydrate active ingredientsNucleoside Reverse Transcriptase InhibitorRetroviral infection
Owner:EMORY UNIVERSITY
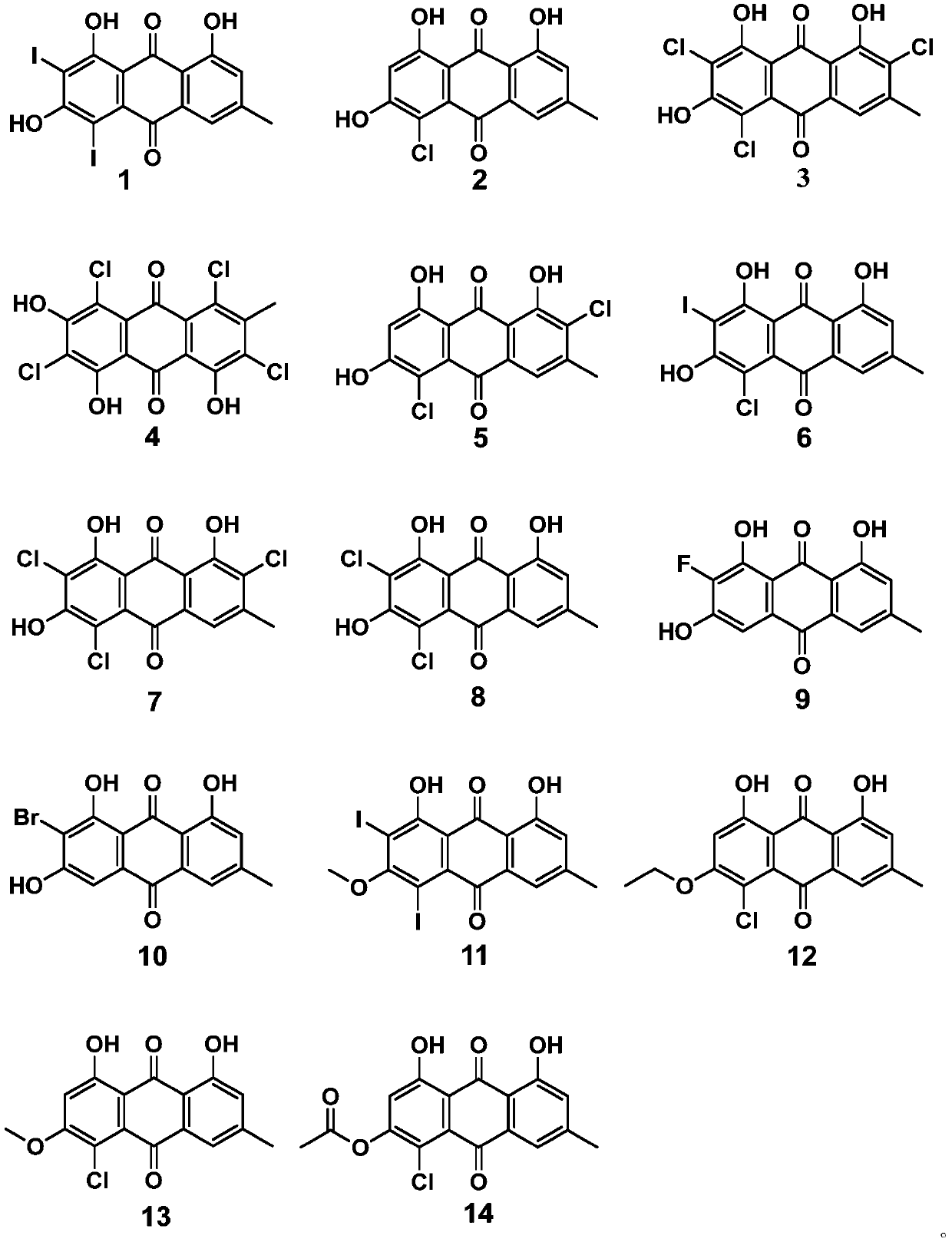
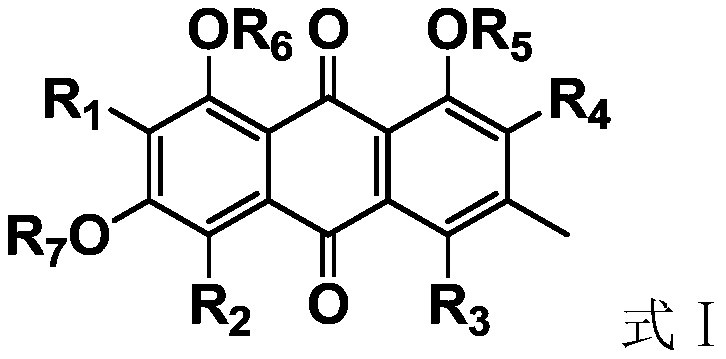
Antiviral anthraquinone derivative and application thereof
ActiveCN111233650AInhibitory activityHigh activityOrganic chemistryAntiviralsAnthraquinonesAntiviral drug
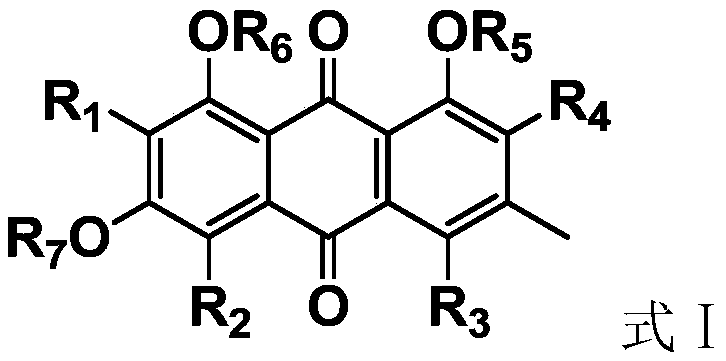
The invention discloses an antiviral anthraquinone derivative and application thereof, relates to the technical field of medicines, and solves the technical problem of lack of novel antiviral drugs inthe prior art. The antiviral anthraquinone derivative has a structure shown as a formula I, wherein R1, R2, R3 and R4 are -F, -Cl, -Br, -I or -NO2, R5, R6 and R7 are -H, -CH3, -CH2CH3 or -COCH3, andwhen R5, R6 and R7 are H, R1, R2, R3 and R4 are not H at the same time. Experiments prove that the antiviral anthraquinone derivative provided by the invention has universality of an antiviral effect,compared with clinical first-line drugs, an antiviral drug prepared from the antiviral anthraquinone derivative provided by the invention has the advantages that the activity of the antiviral anthraquinone derivative is more remarkable, and the effect of the antiviral drug is superior to those of ribavirin, zidovudine and acyclovir.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
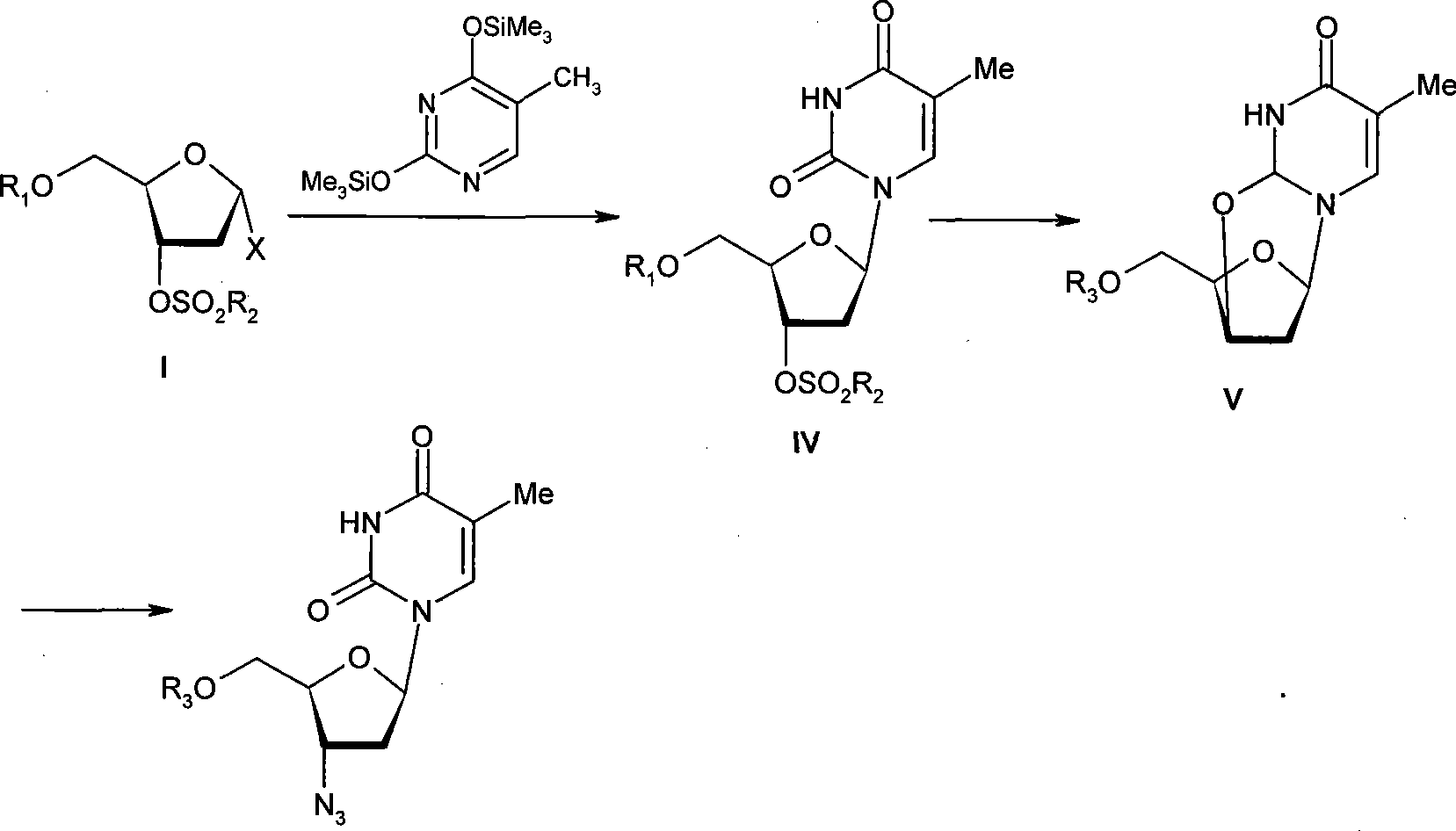
Method for preparing Zidovudine azide intermediate by phase transition method
ActiveCN101190934AMild operating conditionsShort reaction timeSugar derivativesCombinatorial chemistrySodium azide
The invention discloses a method which adopts a phase transfer method to prepare Zidovudine azide intermediate, comprising the following steps: Zidovudine oxy-bridged compound reacts with sodium azide in the solution, in which a phase transfer catalyst and a phase transfer ancillary reagent exist, and then the Zidovudine azide intermediate is collected from the reaction product. The method of the invention has moderate operational conditions, short reaction time and a reaction yield of 91 percent, thereby increasing production hidden dangers greatly.
Owner:SHANGHAI DESANO CHEM PHARMA +1
Tenofovir disoproxil fumarate compounds, preparation method and application to antiviral field
ActiveCN103242366AImprove attributesIncreased toxicityOrganic active ingredientsGroup 5/15 element organic compoundsSolubilityTherapy HIV
The invention discloses a group of tenofovir disoproxil fumarate compounds with activity for inhibiting HIV(human immunodeficiency virus) / HBV(Hepatitis B Virus) replication, a preparation method and pharmaceutical application of the group of tenofovir disoproxil fumarate compounds. The compounds have a formula I, wherein X=H, Y=H; R1=-CH2(CH2)mCH2OCH2(CH2)nCH3, wherein m ranges from 0 to 4, and n ranges from 10 to 20; and R2=-OCH2OC(O)OCH(CH3)2. The invention also discloses a pharmaceutical composition containing the compounds. Shown by the experiment, the activity of one of the compounds for inhibiting the replication of HIV-1 (Human Immunodeficiency Virus-1) is 4.5 times that of azidothymidine (AZT), about 250 times that of the tenofovir disoproxil fumarate (TDF) which is the best medicine for treating acquired immunodeficiency syndrome at present, and 1.37 times that of the medicine CMX157 (Cefmenoxime) which comes into the clinical stage, and the lipid solubility of the compound is about 2 times that of the CMX157; the compounds also have the activity for inhibiting the replication of HBV (Hepatitis B Virus); and the compounds can be applied to the development of medicines for treating the acquired immune deficiency syndrome / hepatitis B.
Owner:洛阳聚慧新材料科技有限公司 +2
Anti-HIV medicine zidovudine powder injection and its preparation method thereof
InactiveCN1404840AFew accessoriesSimple production processOrganic active ingredientsAntiviralsAqueous solutionZidovudine
The present invention discloses a medicine for resisting HIV-zidovudine powder injection and its preparation method. Said injection comprises zidovudine and sodium hydroxide according to their weight ratio of 3:1-10:1, and pH value of its aqueous solution is 9-12. Said invention not only possesses simple production process and convenient transportation, but also its stability is good, quality is high and storage time is long.
Owner:刘万忠
Application of fenugreek biflavone glycosides for preparing anti-virus or/and anti-tumor drugs
ActiveCN101982171ASignificant anti-influenza virus effectExcellent anti-influenza effectOrganic active ingredientsOrganic chemistryAnti virusIn vivo
The invention provides an anti-virus or / and anti-tumor drug and a preparation method thereof and an application of fenugreek biflavone glycosides for preparing anti-virus or / and anti-tumor drugs. The invention provides the application of the chemical component fenugreek biflavone glycosides in fenugreek seeds as drugs for the first time, and uses the fenugreek biflavone glycosides for preparing anti-virus drugs and anti-tumor drugs for the first time. In vivo and in vitro experiment research shows that fenugreek biflavone glycoside tablets or fenugreek biflavone glycoside capsules of the invention have obvious effect on resisting influenza viruses, and the anti-influenza drug effect of the fenugreek biflavone glycoside tablets or fenugreek biflavone glycoside capsules of the invention is superior to the anti-influenza drug effect of the existing Western medicine ribavirin tablets. The anti-HBV effect of the fenugreek biflavone glycoside tablets or fenugreek biflavone glycoside capsules is superior to the anti-HBV effect of kurarinone capsules, and the anti-HIV effect of the fenugreek biflavone glycoside tablets or fenugreek biflavone glycoside capsules is superior to the anti-HIV effect of zidovudine tablets. The fenugreek biflavone glycoside tablets or fenugreek biflavone glycoside capsules have obvious effect on resisting proliferation of liver cancer, gastric cancer, intestinal cancer, lung cancer and cervical cancer cells, and the anti-tumor effect of the fenugreek biflavone glycoside tablets or fenugreek biflavone glycoside capsules is superior to the anti-tumor effect of the existing chemical component preparation hydroxycamptothecine injection.
Owner:XINJIANG INST OF MATERIA MEDICA
Compound anti-AIDS pharmaceutic preparation
InactiveCN103417564APromote dissolutionQuick effectOrganic active ingredientsAntiviralsPolyethylene glycolDrug administration
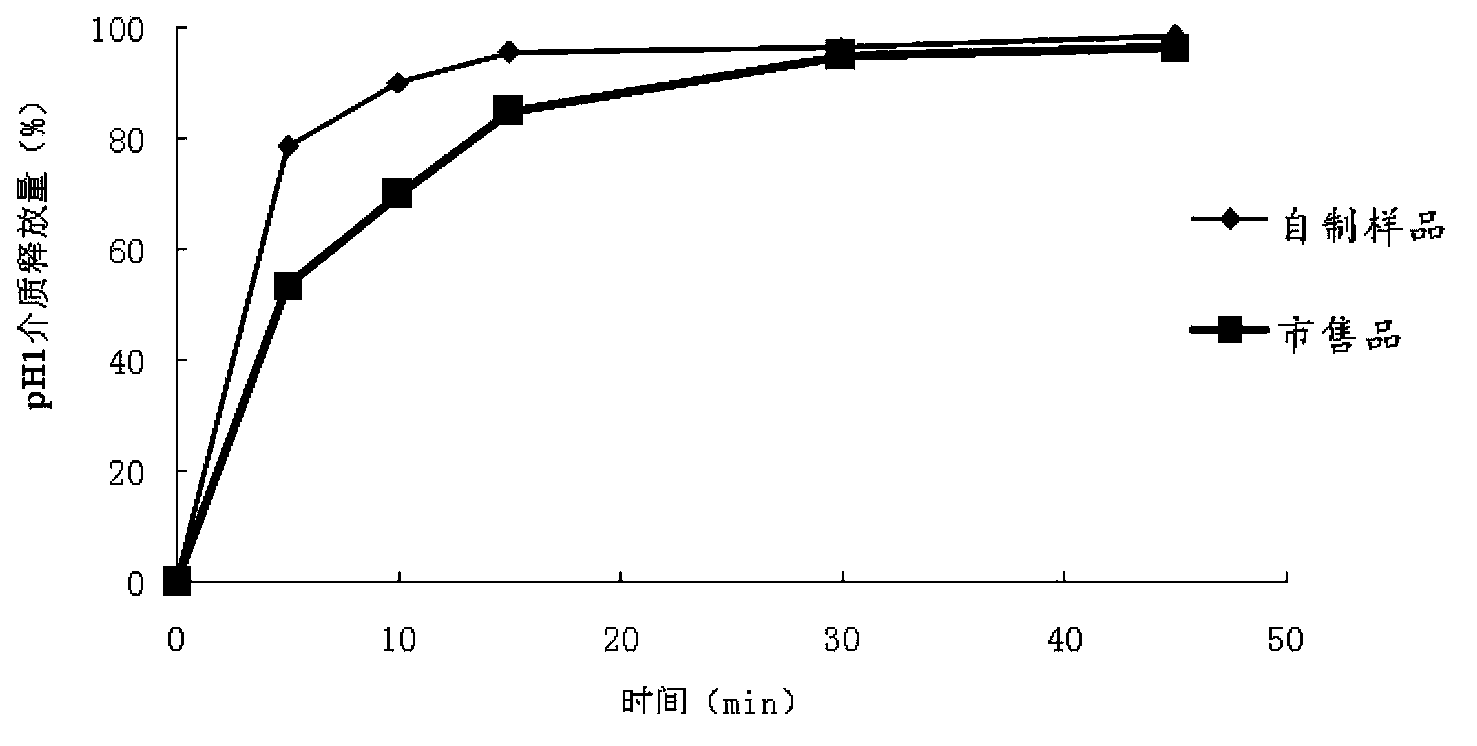
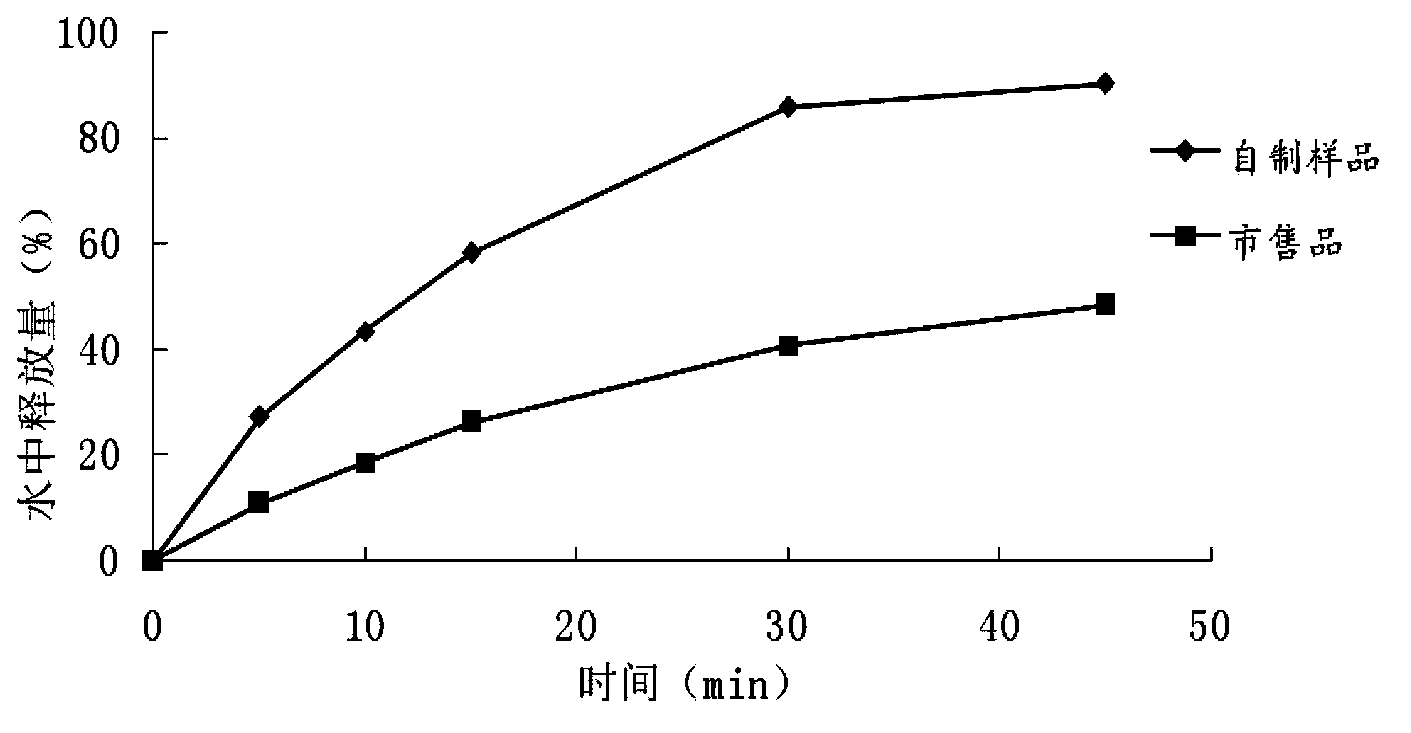
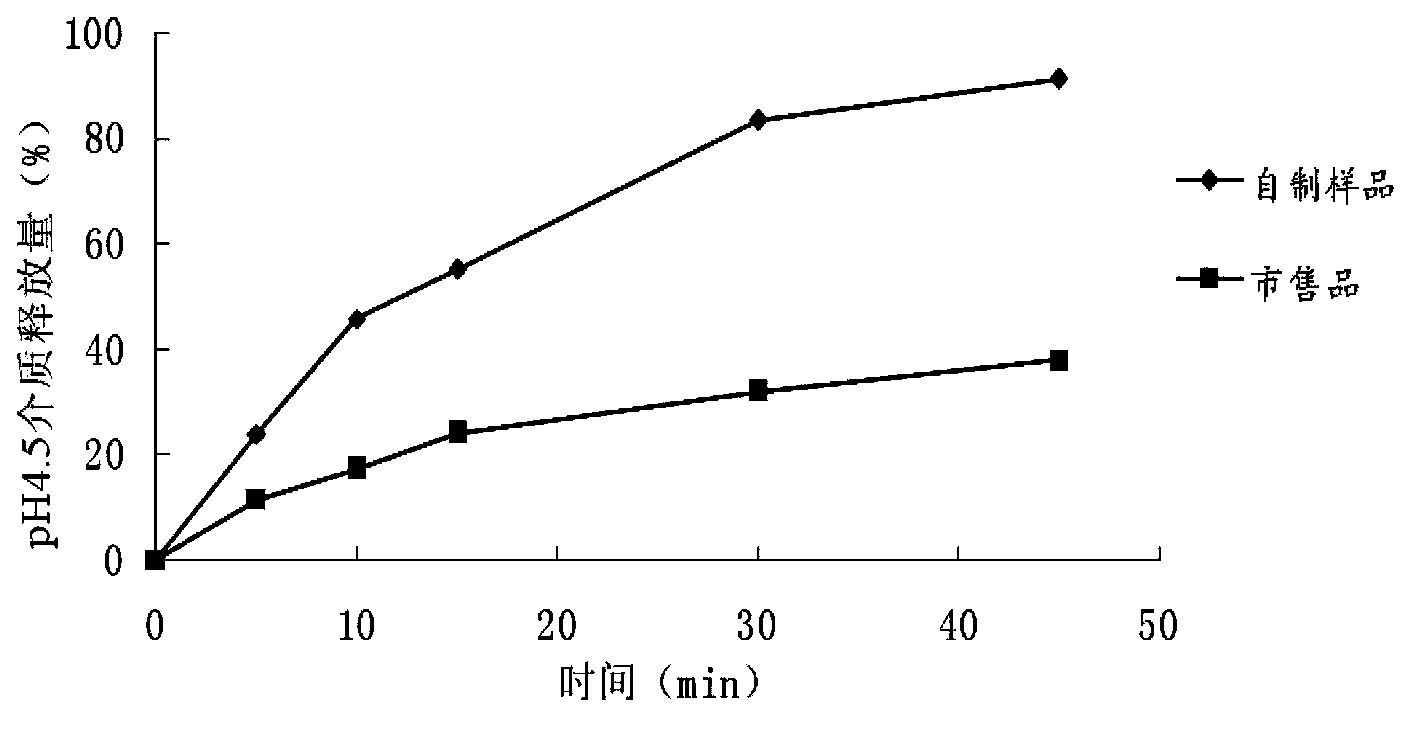
The invention belongs to the field of pharmaceutic preparations, and discloses a compound anti-AIDS pharmaceutic preparation with high drug stability and dissolvability. The compound anti-AIDS pharmaceutic preparation is prepared from the following ingredients: zidovudine, lamivudine, nevirapine, methylcellulose, polyethylene glycol 6000, proper disintegrating agents, lubricant, filler and the like. The compound anti-AIDS pharmaceutic preparation has the advantages that the drug stability and the dissolvability are high; the onset is rapid; the carrying and the drug administration are convenient.
Owner:UNIV OF SHANGHAI FOR SCI & TECH +1
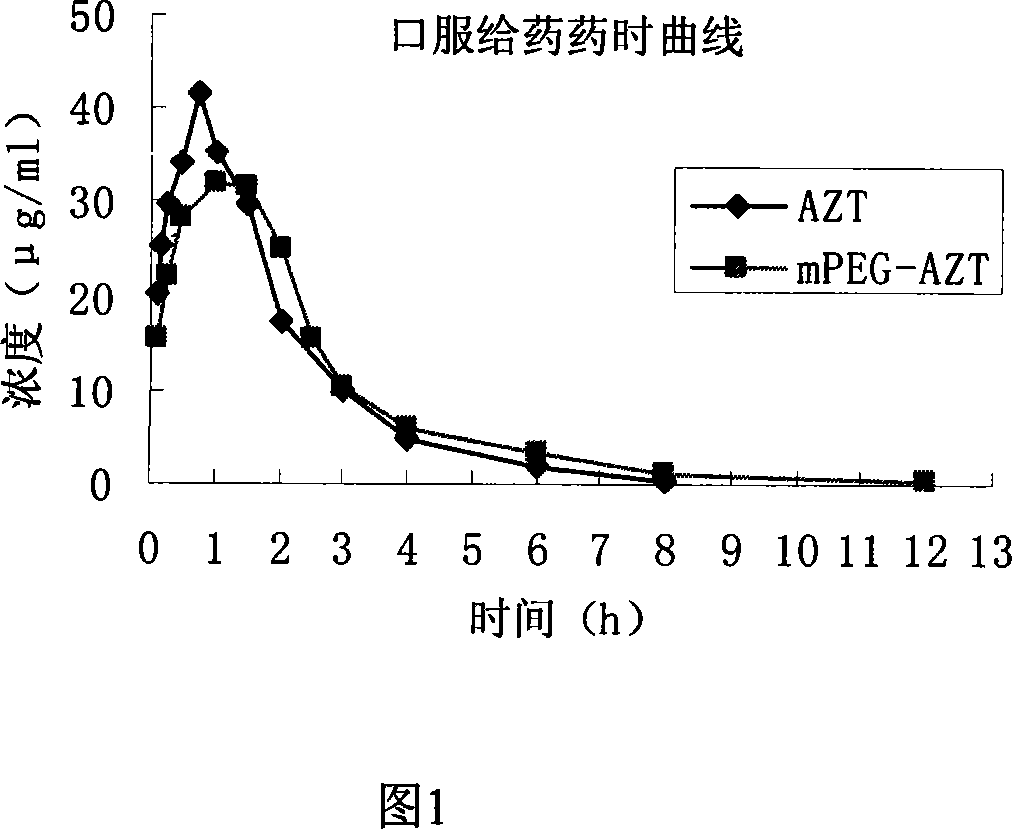
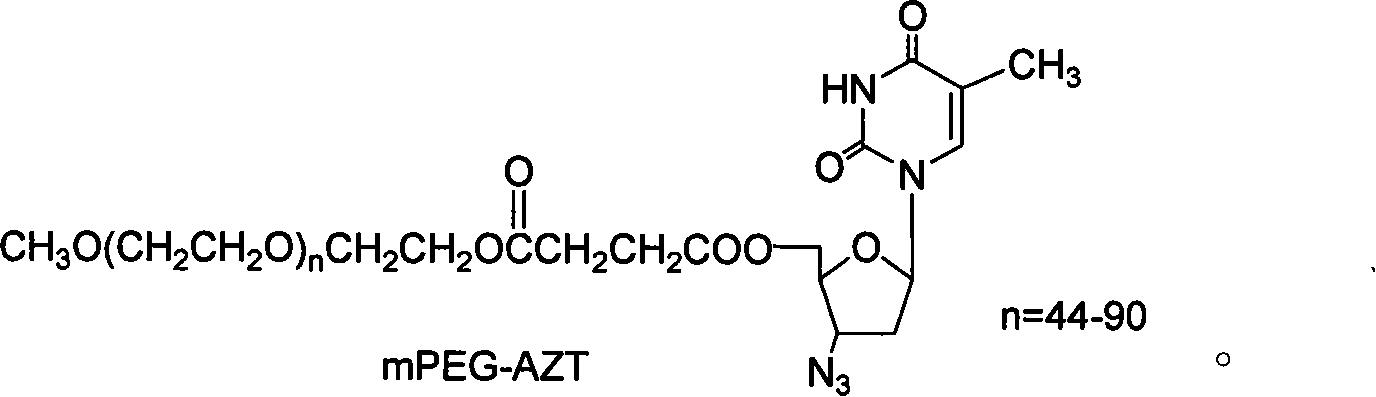
Polyesthylene glycol modified zidovudine conjugate and its prepn process and application
InactiveCN101066459AExtended half-lifeOrganic active ingredientsAntiviralsMonomethoxypolyethylene glycolPolymer science
The present invention relates to one kind of polyethylene glycol modified zidovudine conjugate and its preparation process and application. The polyethylene glycol modified zidovudine conjugate with the structure as shown is prepared through dissolving zidovudine, p-dimethyl aminopyridine and dicyclohexyl carbon diimide in proper amount of dichloromethane, dropping equi-molar ratio dichloromethane solution of mPEG-SA to react, filtering, adding cold ether in 45-55 times volume into the filtrate to obtain white precipitate, filtering to separate the precipitate, and drying to obtain precursor methoxy polyethylene glycol zidovudine conjugate for preparing anti-AIDS medicine.
Owner:江西润泽药业有限公司
Pharmaceutical compositions of antiretrovirals
The present invention relates to the stable pharmaceutical dosage forms of combination of antiretroviral agents. More particularly, the present invention relates to stable pharmaceutical dosage forms of Lamivudine, Zidovudine and Nevirapine, prepared by granulation technology.
Owner:AUROBINDO PHARMA LTD
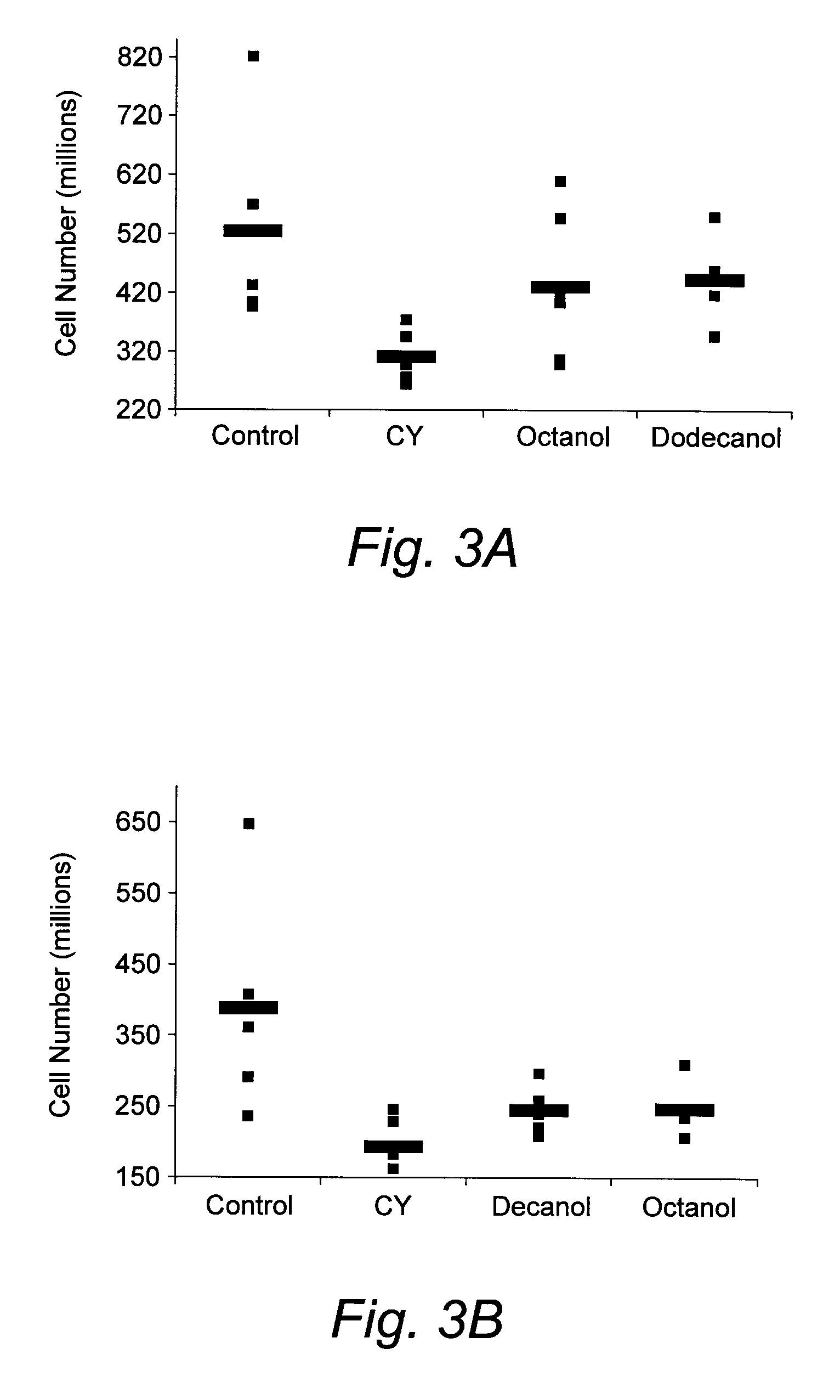
Medium-Chain Length Fatty Alcohols as Stimulators of Hematopoiesis
InactiveUS20080051324A1Good curative effectReducing and eliminating chemotherapyBiocideHydroxy compound active ingredientsProgenitorNutritional deficiency
Owner:PROMETIC PHARMA SMT LTD
Anti-AIDS (Acquired Immune Deficiency Syndrome) compound preparation and preparation method thereof
The invention discloses an anti-AIDS (Acquired Immune Deficiency Syndrome) compound preparation and a preparation method thereof. In particular, the compound preparation comprises 1) safe zidovudine in effective amount for treatment or pharmaceutically acceptable derivatives; 2) safe lamivudine in effective amount for treatment or pharmaceutically acceptable derivatives; 3) safe nevirapine in effective amount for treatment or pharmaceutically acceptable derivatives; 4) a disintegrating agent; and 5) pharmaceutically acceptable carriers or auxiliary materials beside the disintegrating agent, wherein the content of the disintegrating agent is 4-15wt% of total weight the compound preparation. The compound preparation is quick to disintegrate and adequate to dissolve. The invention further discloses a method for preparing the compound preparation.
Owner:SHANGHAI DESANO BIO PHARM CO LTD +1
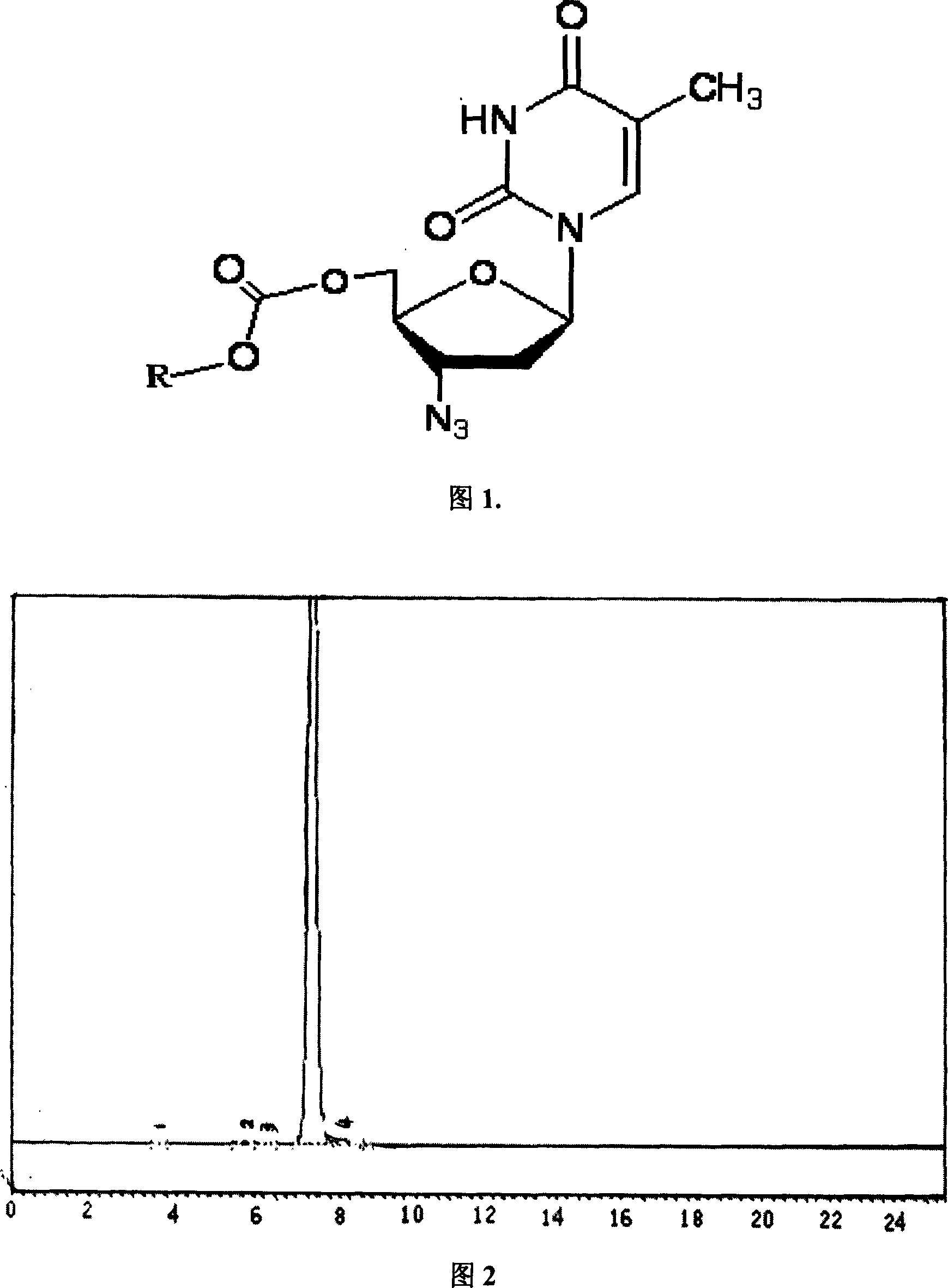
Preparing method of Azidothimidine cholesterol carbonate, preparation and analytical method thereof
InactiveCN101016322AImprove medication safetyGood treatment effectSugar derivativesSugar derivatives preparationSide effectTreatment effect
The invention discloses a new synthesizing method of Azidothimidine carbonic cholesterol ester (or other esters of sterol) as drug primer, which is characterized by the following: adopting Azidothimidine and cholesterol to form chloroformate cholesterol ester; fitting for multiple agents; establishing internal and external analytic method; detecting medical kinetics in the mouse; obtaining the product with half-decay time at 4.9h; lengthening AZT acting time; reducing side-effect; targeting AIDS enriched organs and tissues.
Owner:SHENYANG PHARMA UNIVERSITY
Suppository for controlling sexual spread of AIDS
InactiveCN1357331ASmall side effectsImprove bioavailabilityOrganic active ingredientsAntiviralsSide effectPolyethylene glycol
The suppository for controlling sexual spread of AIDS contains Zidovudine and substrate and each grain of the suppository may also contains Aciclovir 0-500 mg, Amphotercin 0-50 mg, Miconazole 0-300 mg, surfactant 0-100 mg and deodorant 0-100 mg. It is applied locally to urogenital system and this results in less toxic side effect and high biological utilization. Its Aciclovir is converted in vivo into triphophate compound to interfere the duplication of herpes simple xvirus DNA and to inhibit herpes virus, cytomegalo virus and EB virus; Amphotercin is used for treating vaginalmucous membrane infection; and Miconazole has broad-spectrum antifungal function.
Owner:WUHAN UNIV
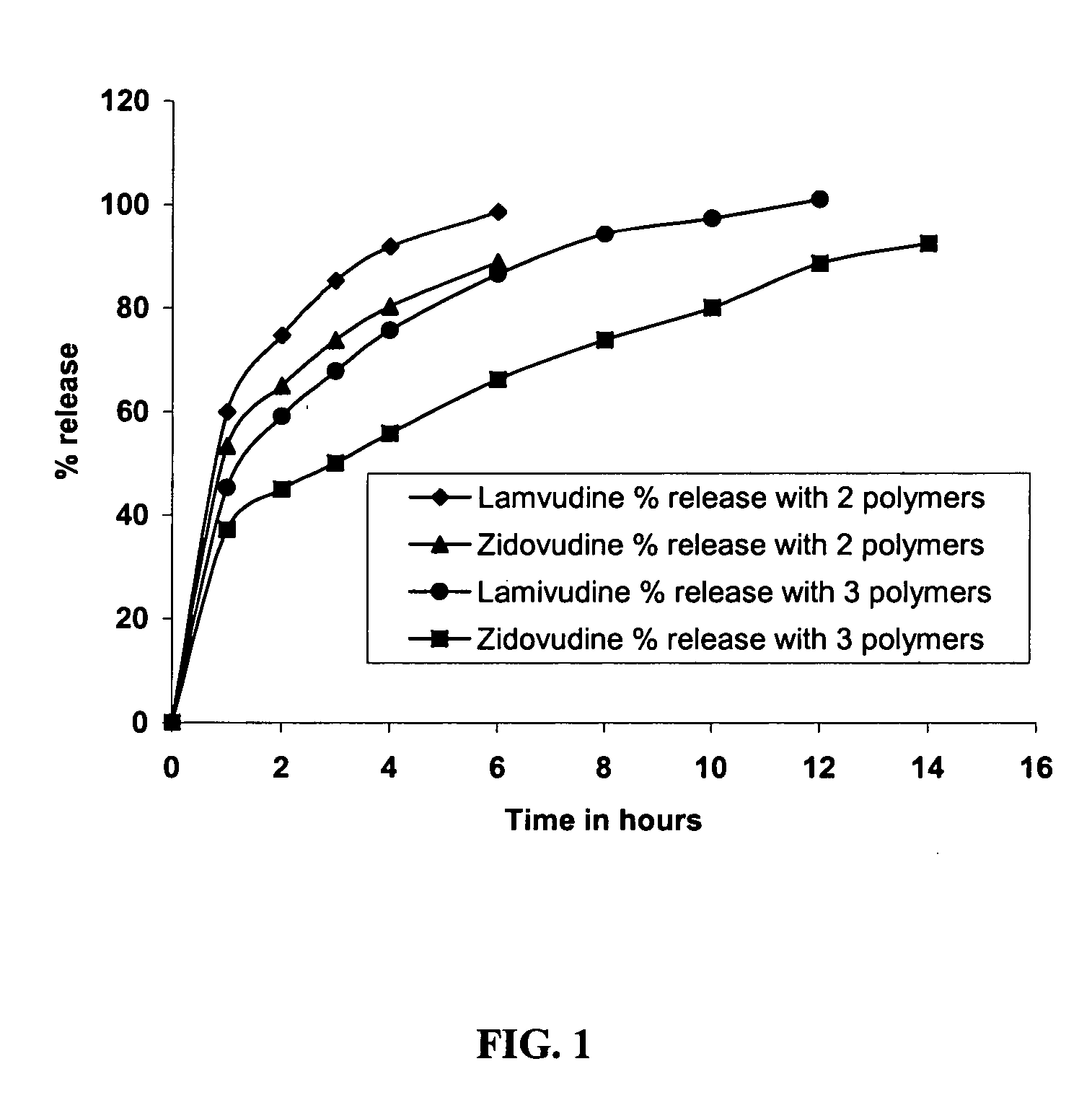
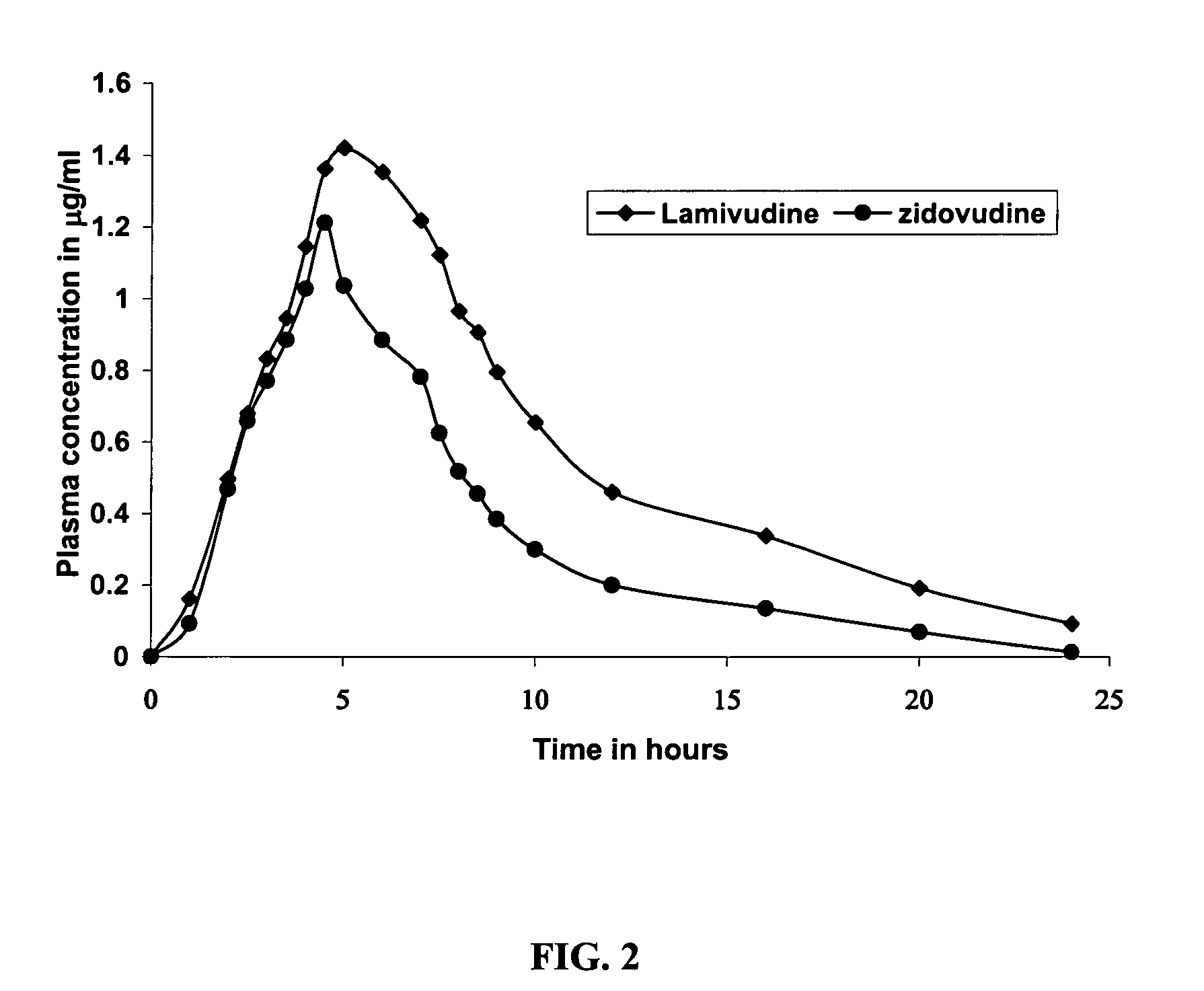
Controlled Release Pharmaceutical Composition and a Process for Preparing the Same
InactiveUS20070231385A1Reduce frequencyReduce pill loadBiocideAntiviralsNucleoside Reverse Transcriptase InhibitorControlled release
A three-drug antiretrovial pharmaceutical composition having a selective combination of a controlled release active formulation and an immediate release active formulation for once daily administration. The composition provides desired dosages of the actives lamivudine, zidovudine or pharmaceutically acceptable derivatives thereof, and the immediate release formulation including at least one selective Non-nucleoside Reverse Transcriptase Inhibitor (NNRTI), preferably nevirapine or a pharmaceutically acceptable derivative thereof along with pharmaceutically acceptable excipients. The once daily composition would favour patient compliance and effective treatment. A method of reducing the pill burden in a patient suffering from HIV infection and / or Acquired Immunodeffieciency Syndrome by administering a once daily dose of the three-drug antiretroviral pharmaceutical composition.
Owner:LUPIN LTD
Process for purifying zidovudine palmitate raw materials and preparations thereof
InactiveCN1827632AImprove medication safetyOrganic active ingredientsSugar derivativesOrganic solventEmulsion
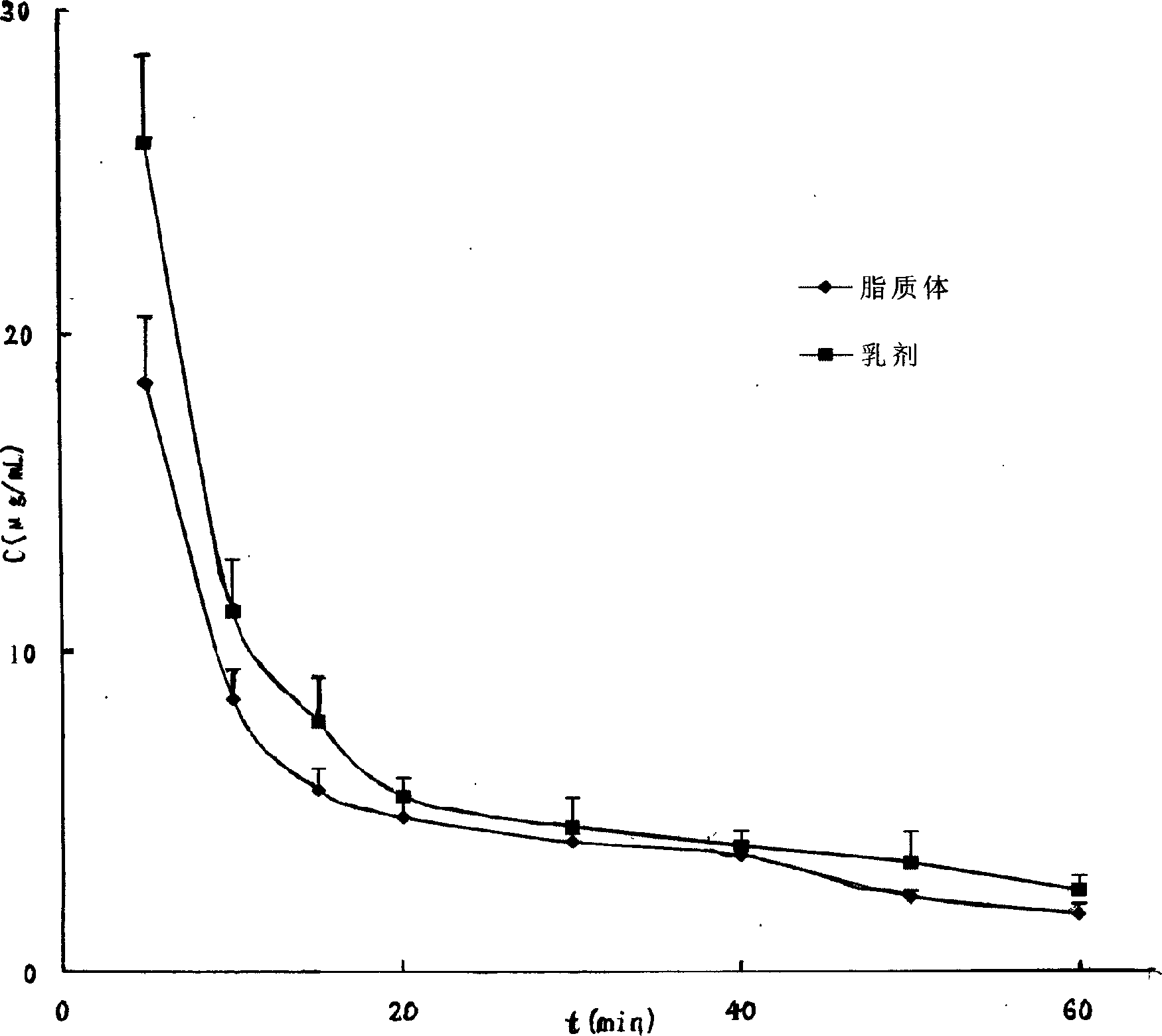
The invention relates to a refining method for Azidothimidine palmitate (or stearate) stock and its preparation, which sets up a decontamination method for Azidothimidine palmitate and improves the medicinal satefty. The main technical project consists of dissolving the stock of Azidothimidine palmitate or stearate with right amount of organic solvents, adding adsorbent or not, heat-preserving at the indoor temperature of 80 DEG C and stirring for 5-500 minutes, filtering to remove the adsorbent, adjusting a low-temperature to 5--50 DEG C and dispensing for 1-100 hours, collecting the precipitate, and drying to obtain the target product. The purified Azidothimidine palmitate stock is white or similar to white, and its purity quotient is more than 99 % checked by HPLC. The purified Azidothimidine palmitate or stearate stock can further prepare various preparations which include emulsion, liposome, hard or soft capsule, tablet, granula and turbid agent. The internal pharmacokinetics after being injected by Azidothimidine palmitate is studied by the said liposome and emulsion prepared by the high-mass Azidothimidine palmitate stock, which provides an authentic criteria for clinical application.
Owner:SHENYANG PHARMA UNIVERSITY
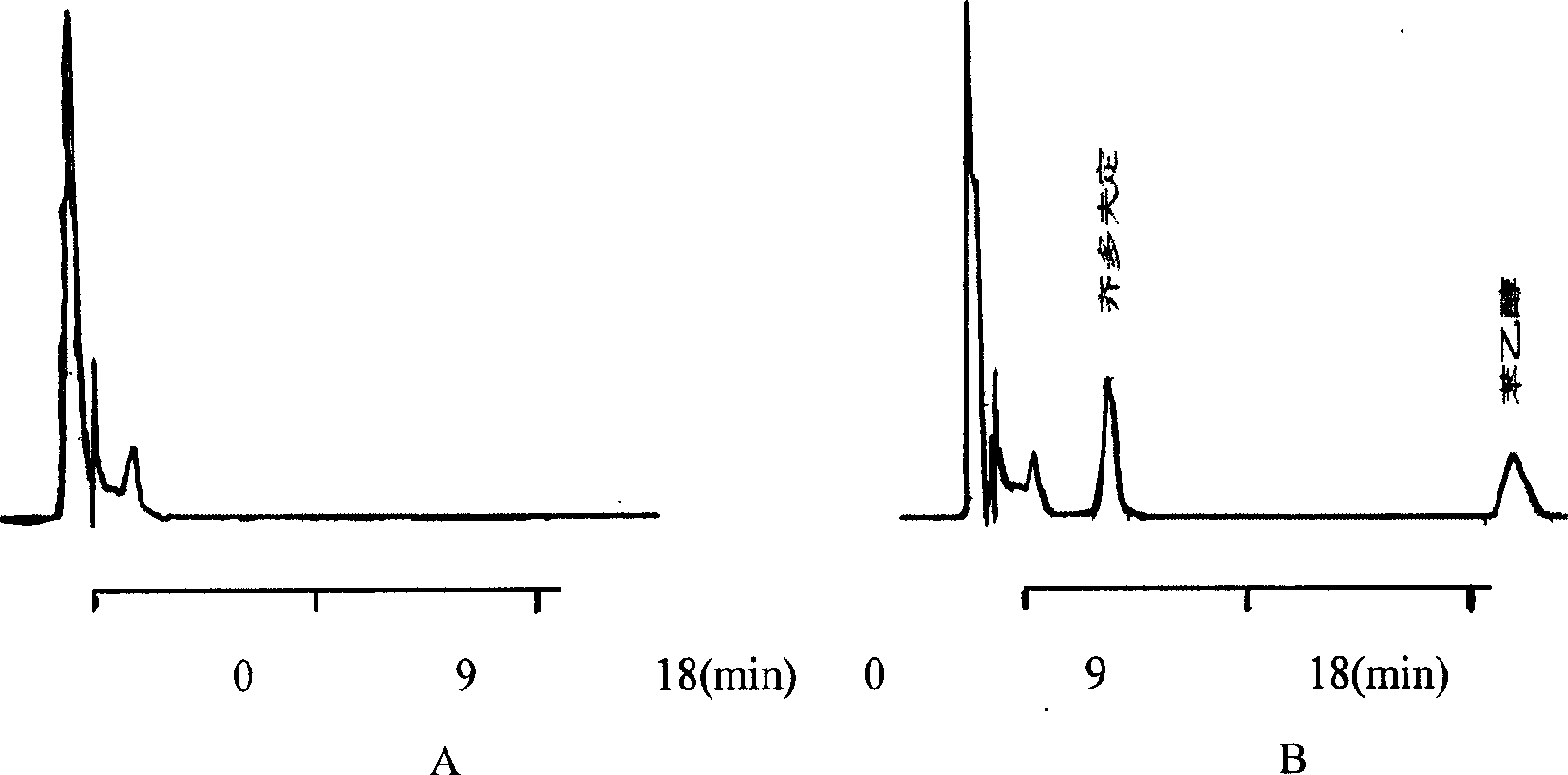
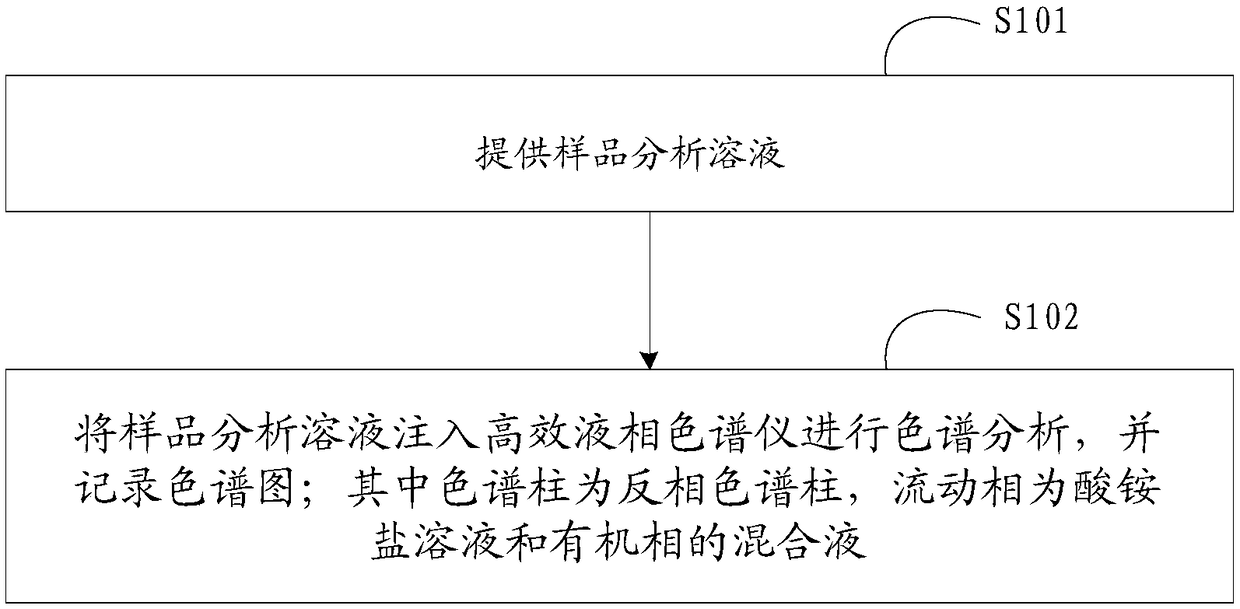
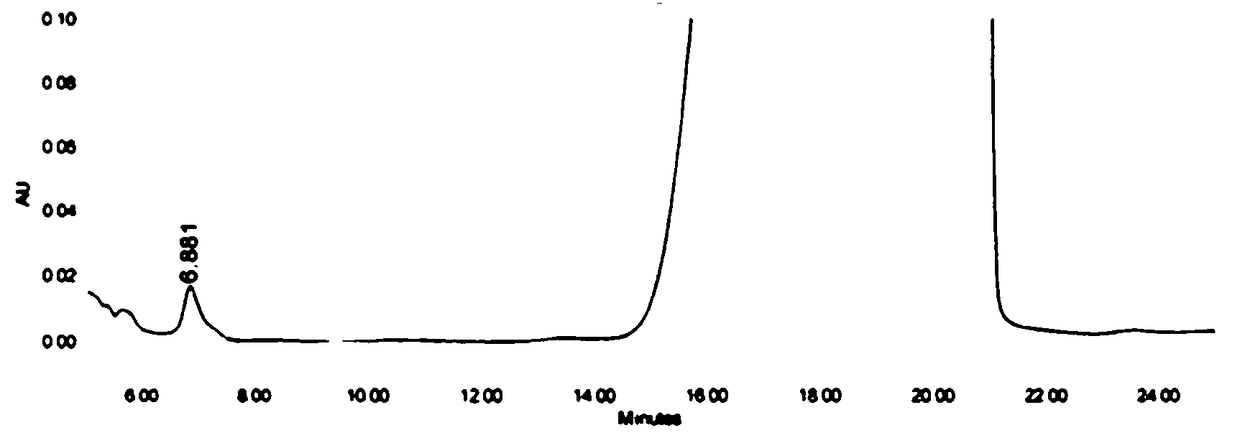
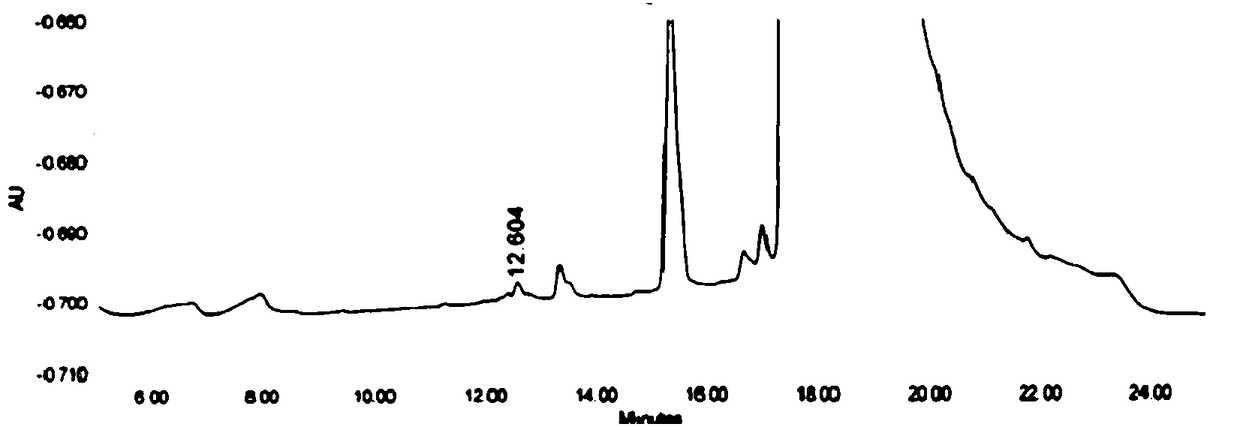
High-performance liquid detection method for genotoxic impurities of zidovudine
InactiveCN109212068AEasy to separateEasy to detectComponent separationGenotoxic impuritiesSalt solution
The present application discloses a high-performance liquid detection method for the genotoxic impurities of the zidovudine. The method comprises the following steps of: providing a sample analysis solution; injecting the sample analysis solution into a high performance liquid chromatograph for chromatographic analysis, and recording a chromatogram; a chromatography column is a reverse phase chromatography column, and a mobile phase is a mixture of an acid ammonium salt solution and the organic phase. According to the high-performance liquid detection method for the genotoxic impurities of thezidovudine, the detection separation and the detection limit of the genotoxic impurities can be improved by the means.
Owner:SHANGHAI SHYNDEC PHARMA HAIMEN CO LTD
Long acting compositions comprising zidovudine and lamivudine
A pharmaceutical composition in the form of a tablet for controlled release of active ingredient(s) comprises lamivudione, zidovudine or combination of lamivudine and zidovudine or their pharmaceutically acceptable derivatives, and a mixture of hydrophilic polymers selected from the group consisting of at least one hydroxypropyl methylcellulose, at least one sodium alginate and at least one guar gum as controlled release matrix and a pharmaceutically acceptable calcium salt as a matrix stabilizer. The composition may also contain one or more of a water soluble and / or water dispersible diluent, wherein the quantities of the hydrophilic polymers, the calcium salt and water soluble and / or water dispersible diluents are such that the therapeutically effective active ingredient(s) is released at a rate suitable for once daily administration of the pharmaceutical composition. The tablets may be coated with a water soluble polymeric film coat.
Owner:LUPIN LTD
Combination comprising zidovudine and an antimicrobial compound
PendingCN112218633AAntibacterial agentsHeterocyclic compound active ingredientsCefalexinPharmaceutical medicine
The invention provides a combination comprising zidovudine or a pharmaceutically acceptable derivative thereof and an antimicrobial compound selected from nitrofurantoin, mecillinam, fosfomycin, cephalexin and faropenem, or a pharmaceutically acceptable derivative or prodrug thereof. These combinations are particularly useful for the treatment of microbial infections.
Owner:HELPERBY THERAPEUTICS LTD
Zidovudine-lamivudine tablet easy to dissolve out and preparation method thereof
ActiveCN103908465APromote dissolutionAvoid hindranceOrganic active ingredientsAntiviralsLow-substituted hydroxypropylcelluloseZidovudine lamivudine
The invention relates to a zidovudine-lamivudine tablet easy to dissolve out and a preparation method thereof. The zidovudine-lamivudine tablet comprises the following raw materials in parts by weight: 300 parts of zidovudine, 150 parts of lamivudine, 40-60 parts of lactose, 40-60 parts of microcrystalline cellulose, 155-170 parts of a 3% low substituted hydroxypropyl cellulose aqueous solution, and 6-8 parts of magnesium stearate.
Owner:ANHUI BIOCHEM BIO PHARMA
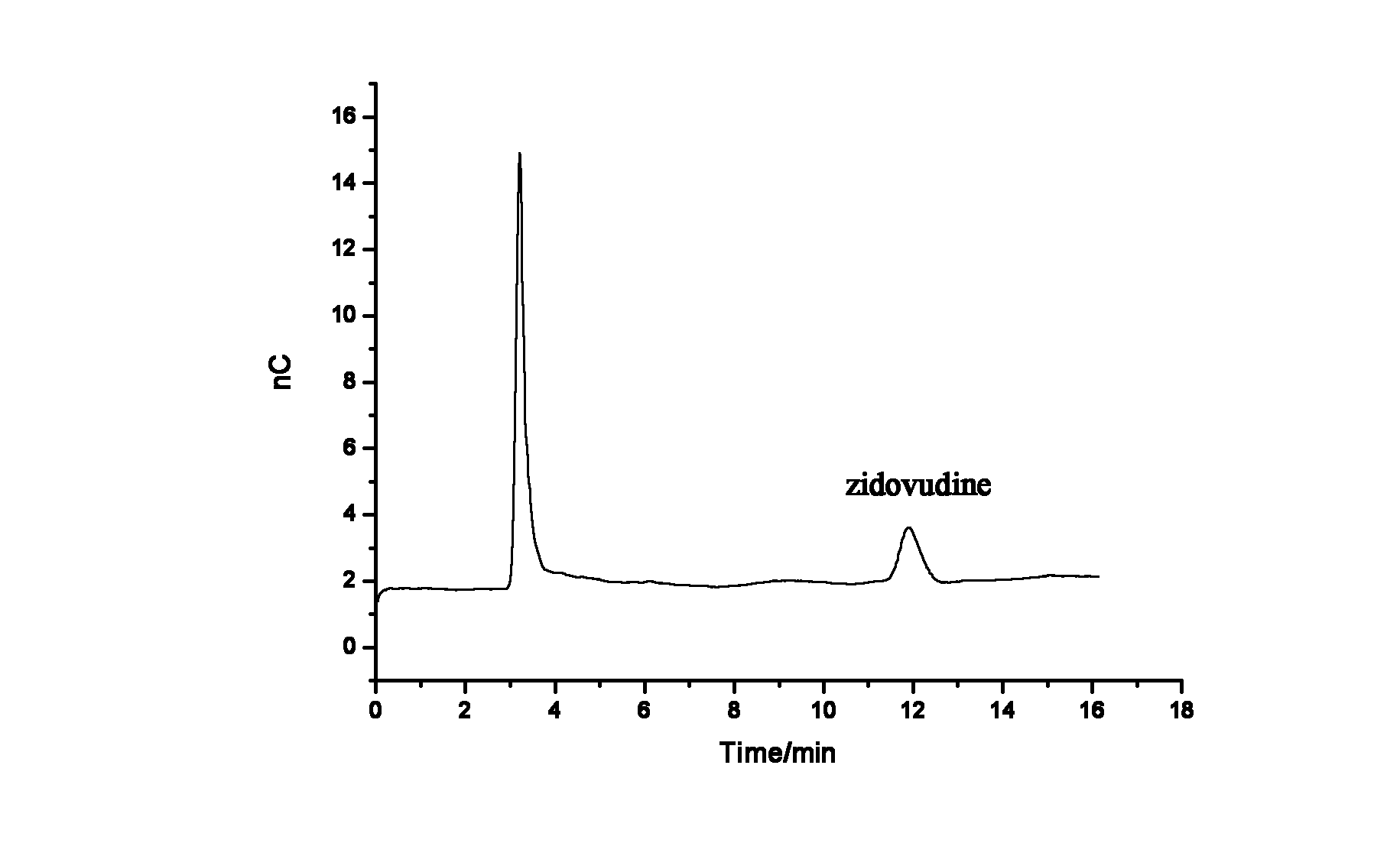
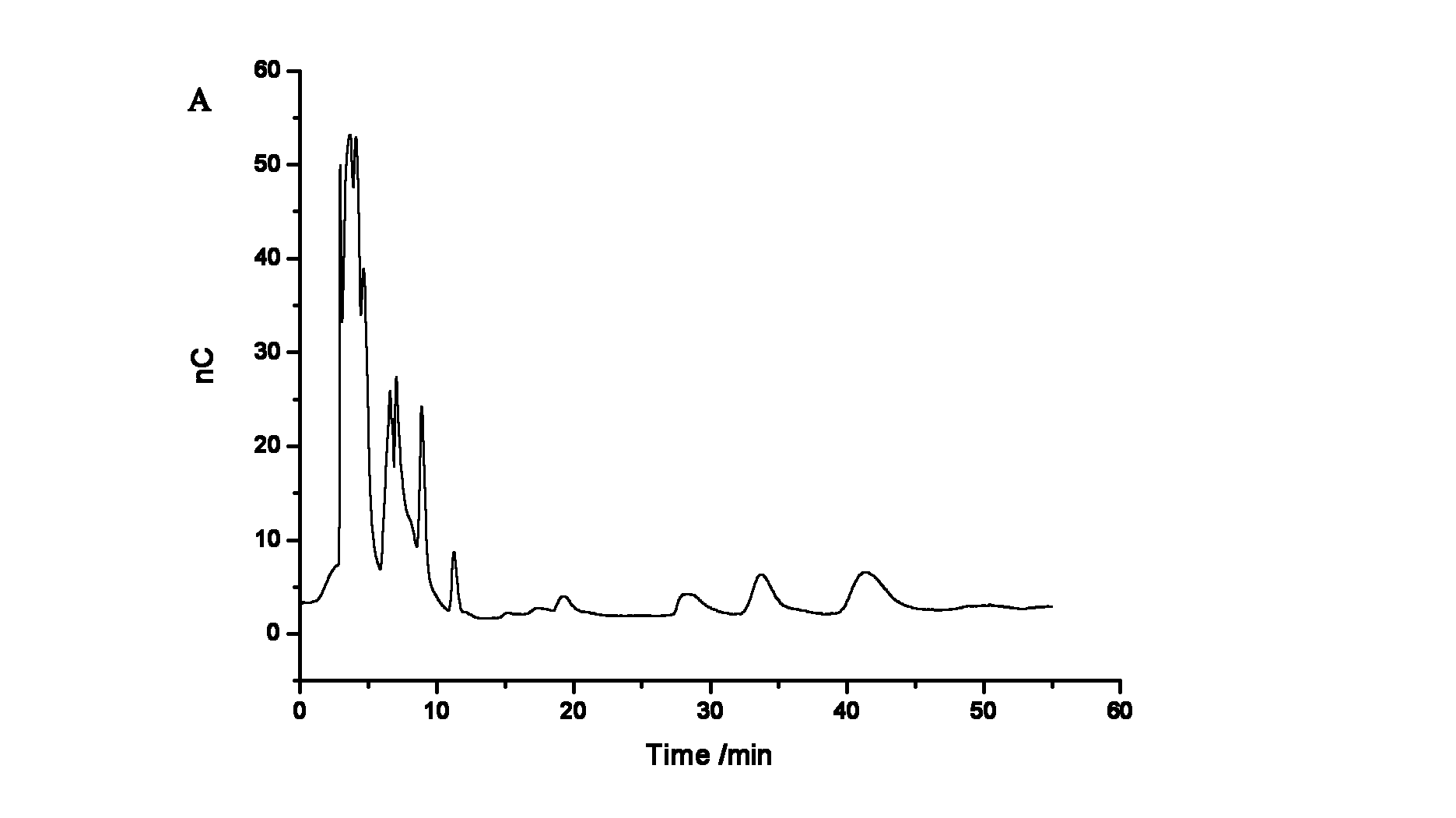
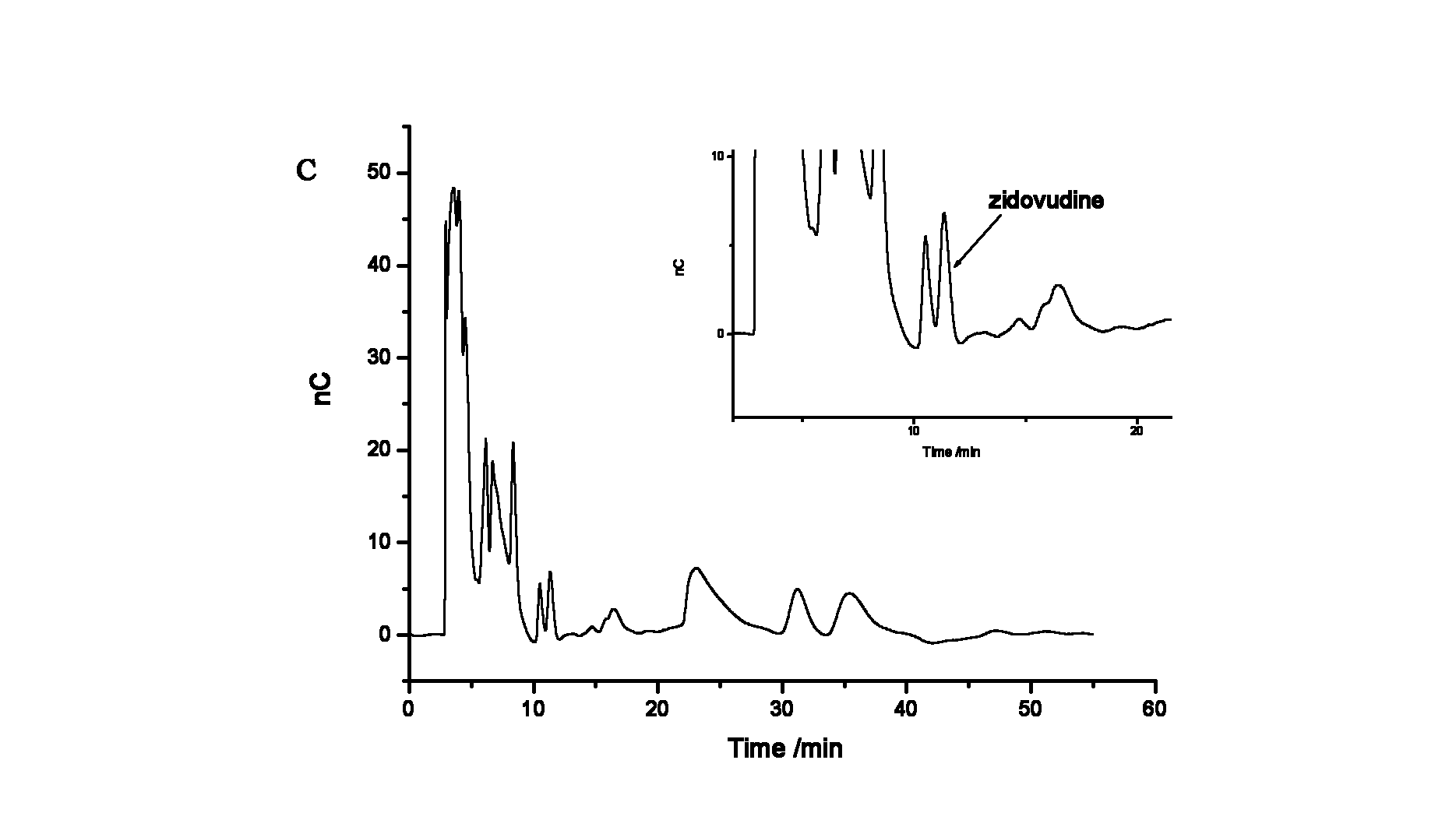
Method for ion chromatography separation integrated pulsed amperometric detection and analysis of antiviral drug zidovudine
InactiveCN102539571AGood linear relationshipGood separation analysisComponent separationElectrochemical detectorChromatographic separation
The invention relates to a method for ion chromatography separation and analysis of antiviral drug zidovudine, in particular to a detection method for isocratic separation integrated pulsed amperometric detection and analysis of antiviral drug zidovudine. The method comprises the following steps of: actual sample treatment, baseline surevying and mapping, sample introduction and ion exchange, elution and electrochemical detection and analysis. The method has the beneficial effects that the antiviral drug zidovudine can be subjected to good separation and analysis, and the relative standard deviation of retention time, peak height and peak area is less than 2.0 percent; a sample is directly injected into an ion chromatography separation column for separation, and a spectrogram with high sensitivity can be obtained from zidovudine solution outputted by the chromatography separation column by an electrochemical detection pool via an electrochemical detector, so that the technical process is greatly simplified; and the method can also be used for detecting the content of the antiviral drug zidovudine in a blood sample.
Owner:ZHEJIANG UNIV
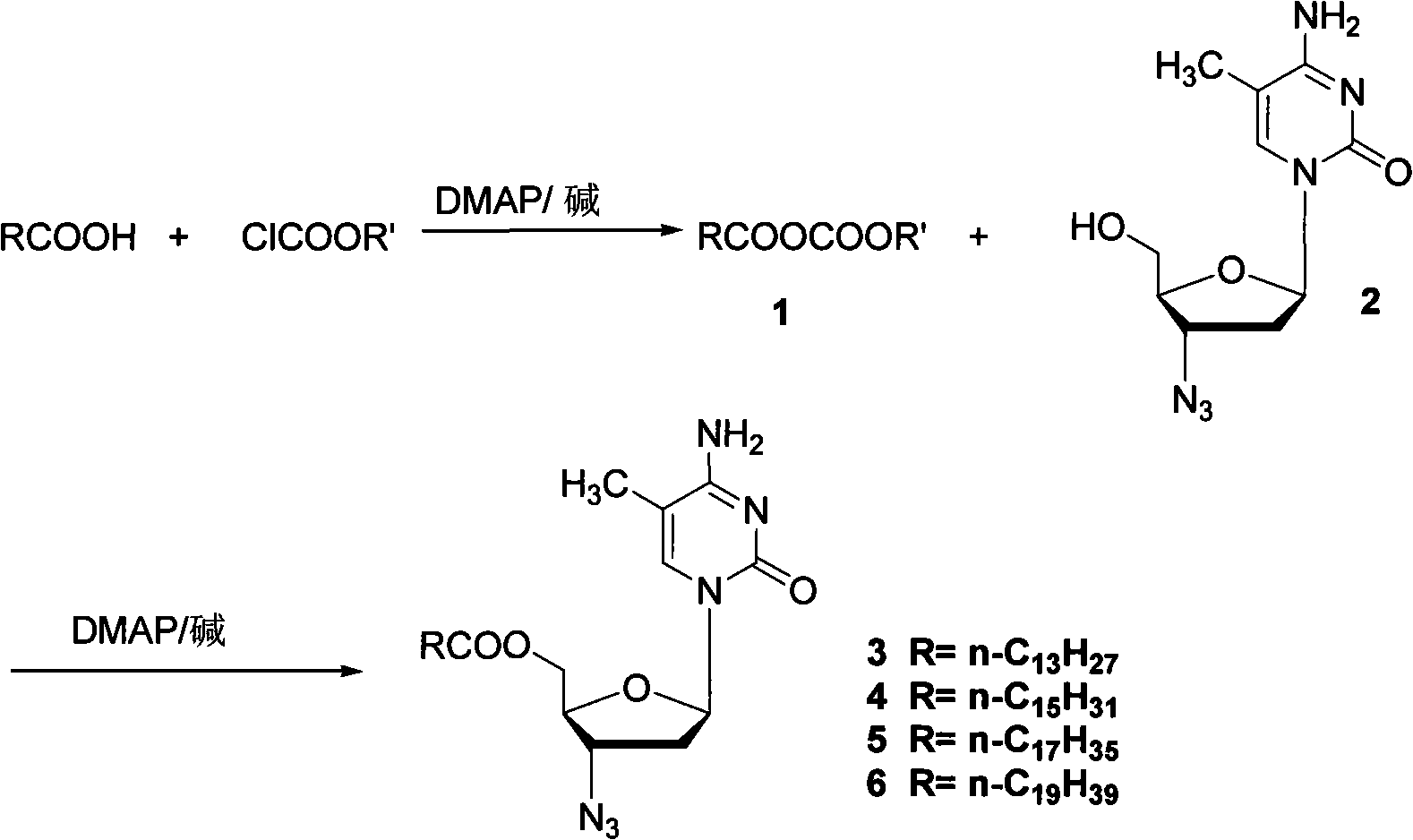
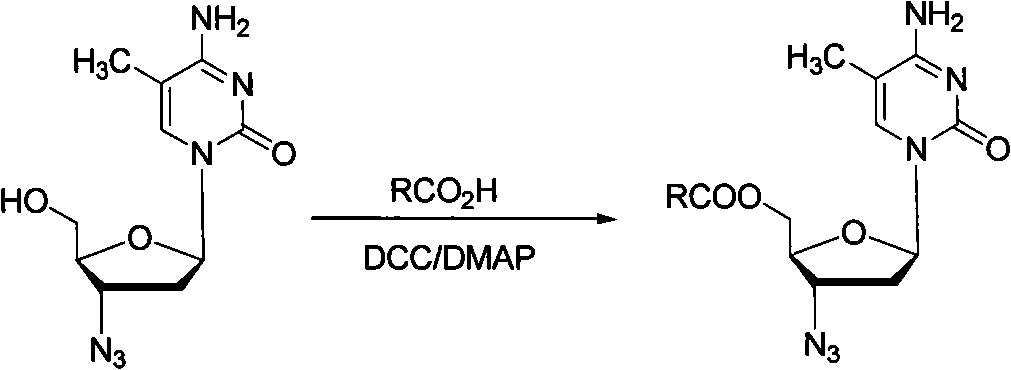
Method for synthesizing higher fatty acid zidovudine ester
InactiveCN101260135AReduce pollutionLow costSugar derivativesAntineoplastic agentsOrganic solventSynthesis methods
The invention provides a synthesis method for a higher fatty acid zidovudine ester, relating to a zidovudine ester, in particular to a synthesis method for a novel prodrug higher fatty acid zidovudine ester; the invention provides the synthesis method for the higher fatty acid zidovudine ester, which is high in yield and suitable for the industrialized production. The method is that higher fatty acid and N, N-dimethyl-4-aminopyridine are dissolved in an organic solvent, and the organic solvent is added with chloro formate and alkali for reaction, so that the mixed anhydride is obtained; zidovudine, the N, N-dimethyl-4-aminopyridine and the alkali are added to the solvent, and the mixed anhydride is added to the solvent for reaction, and the higher fatty acid zidovudine ester is obtained.
Owner:XIAMEN UNIV
Application of daphnetoxin diterpene in preparing anti-HIV drug
InactiveCN105287497ARich medicinal resourcesGood anti-HIV activityOrganic active ingredientsOrganic chemistryCytotoxicityBULK ACTIVE INGREDIENT
The invention belongs to the field of traditional Chinese medicine production and relates to application of daphnetoxin diterpene in preparing anti-HIV drug. A daphnetoxin diterpene compound is extracted from S.chamaejasme which is a Stellera plant; experiments verify that the compound has high anti-HIV activity, and results of pharmacological experiment comparison between the compound and zidovudine serving as positive contrast drug show that the compound has strong anti-HIV effect and is low in effective concentration and cytotoxicity. The daphnetoxin diterpene compound can serve as an active ingredient to be used for preparing the anti-HIV drug.
Owner:FUDAN UNIV
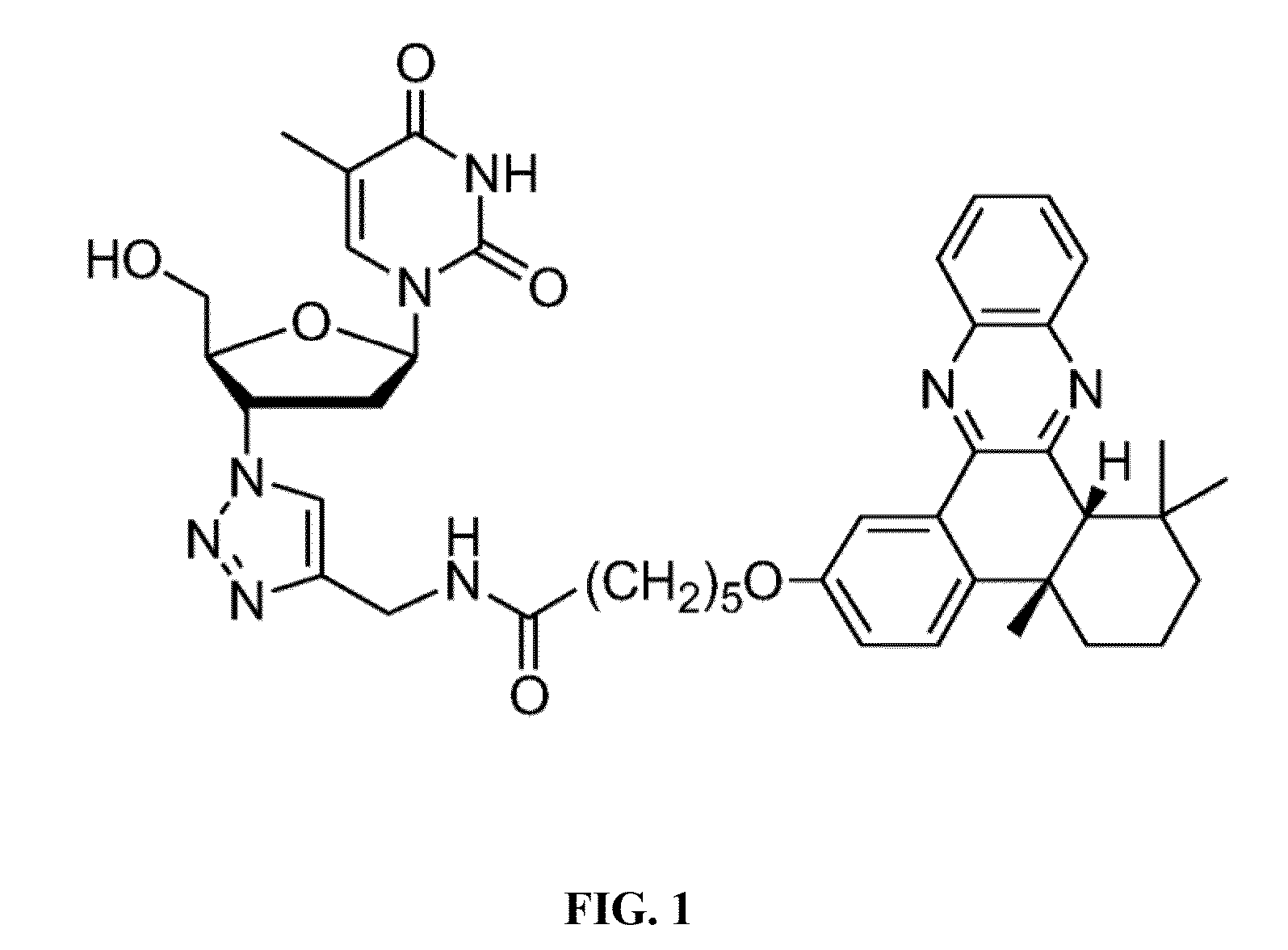
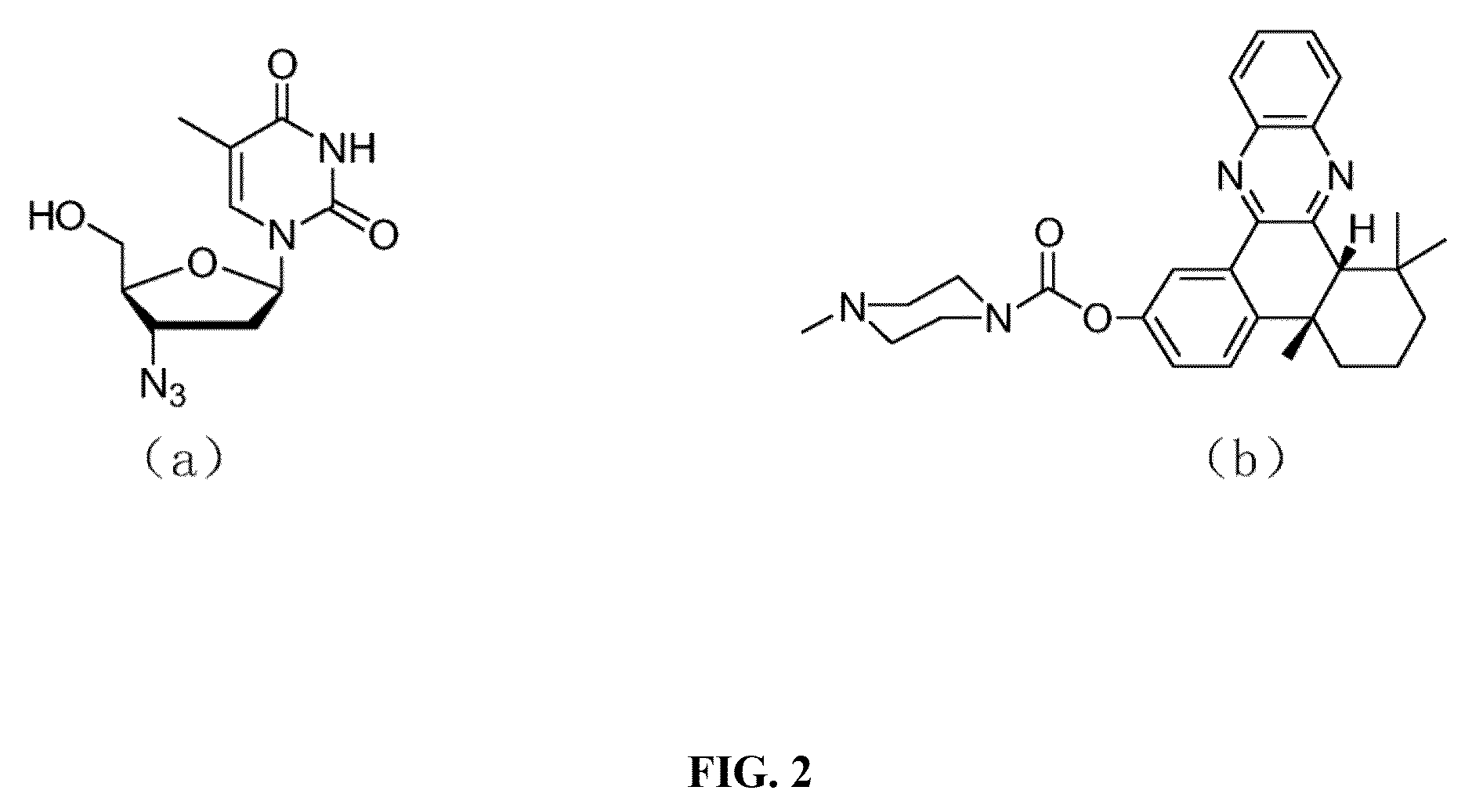
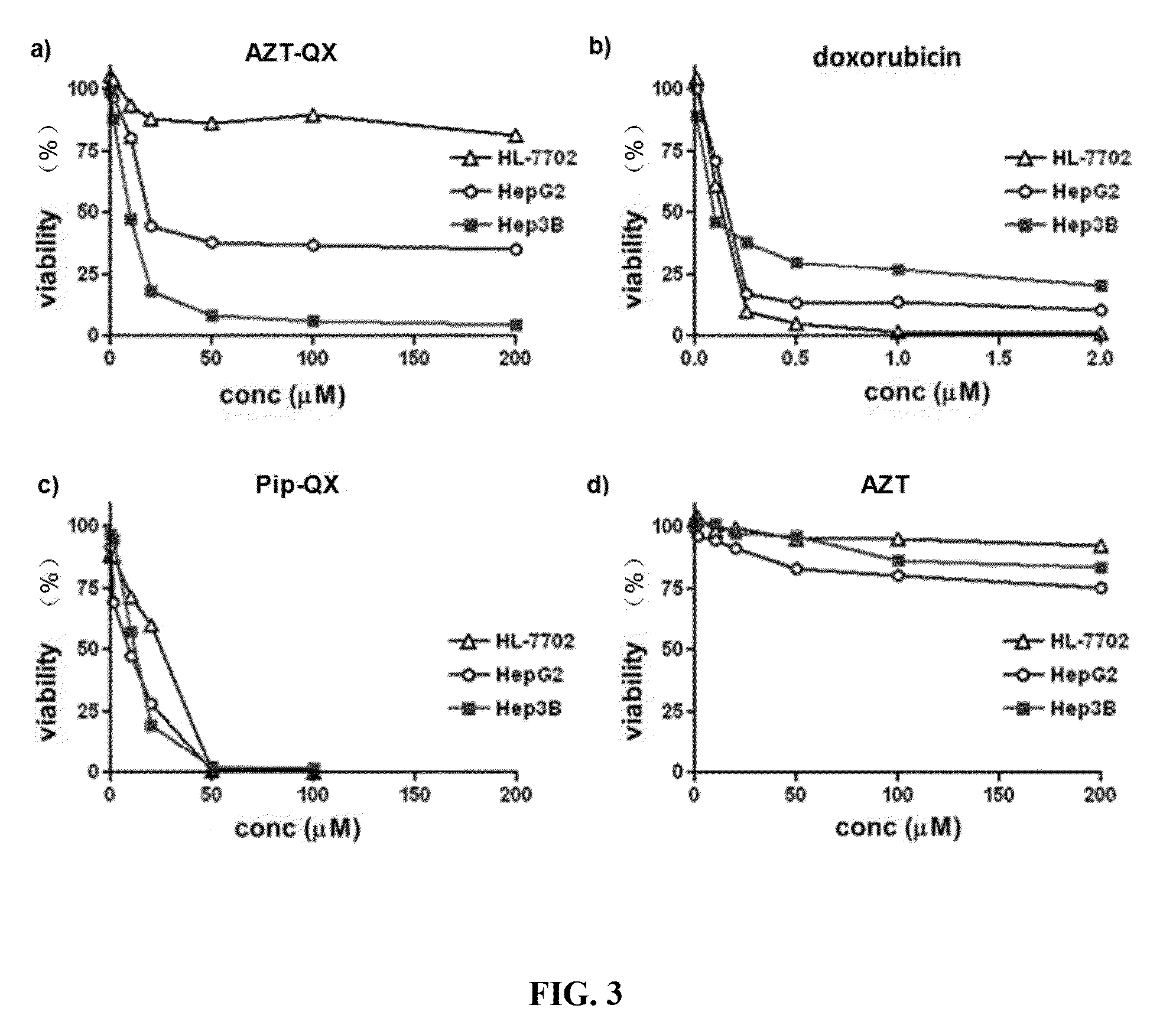
Azidothymidine quinoline conjugated compound, preparation method therefor and application thereof in anti-hepatoma therapy
The invention provides the Zidovudine-conjugated quinoline compound N-((1-(2-(hydroxy-methyl)-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-tetrahydrofuran-3-yl)-1H-1,2,3-triazol-4-yl)methyl)-6-(4b,8,8-trimethyl-4b,5,6,7,8,8a-hexahydrodibenzo[a,c]phenazin-2-yloxy)hexanamide. The compound can selectively kill hepatoma cells, especially hepatoma cells with hepatitis B, and inhibit the growth of subcutaneous tumors in mice, but has no significant toxicity to normal liver cells. Experiments confirm that the compound has an anti-hepatoma effect and can be used in preparation of anti-hepatoma drugs.
Owner:HUAZHONG UNIV OF SCI & TECH
Preparation method of ingenane-type diterpenoid and application of same in pharmacy
ActiveCN110540504AThe ingredients are clearIncrease contentAntiviralsCarboxylic acid esters separation/purificationPositive controlCytotoxicity
The invention relates to a preparation method of ingenane-type diterpenoid and application of the same in preparation of anti-HIV drugs, belonging to the field of Chinese pharmaceutical manufacturing.According to the method disclosed by the invention, ingenane-type diterpenoid components are enriched in the Euphorbia plant--Euphoria ebracteolata, the contents of main active ingredients are determined and anti-HIV activity tests are carried out. Results show that anti-HIV activity obviously increases with the increase of the content of the ingenane-type diterpenoid components; when the contentof the total ingenane-type diterpenoids in the enriched components is greater than 47%, extremely strong anti-HIV activity is obtained, the value of EC50 reaches 0.0063 [mu]g / mL, cytotoxicity is relatively small (CC50=14), and a selection index (SI) is greater than 2000; and the ingenane-type diterpenoid has obvious advantages compared with the positive control drug zidovudine. The invention establishes the method for rapidly enriching the total ingenane-type diterpenoids in Euphoria ebracteolata, and the enriched total ingenane-type diterpenoids can be used for preparing anti-HIV drugs.
Owner:FUDAN UNIV
Medicinal composition containing zidovudine, valproic acid or its salt
InactiveCN1762377AEvade attackReduce hiddenAntiviralsAnhydride/acid/halide active ingredientsImmunodeficiency virusZidovudine
The invention relates to a therapeutic combination containing Azidothimidine and dipropylacetic acid or its salts, wherein the formulation of the medicinal composition comprises (by weight percentage) Azidothimidine 0.1-95%, dipropylacetic acid or its salts 1.0-95%, and medicinal auxiliary material 4-98.9%. Each unit of the dosage preferably comprises Azidothimidine 10-600mg, dipropylacetic acid or its salts 50-500mg, and pharmaceutically acceptable auxiliary materials amounting to 15-40% of the total weight of the unit dosage. The combination can be used for the treatment of human immunodeficiency virus (HIV) infection.
Owner:北京明新高科技发展有限公司
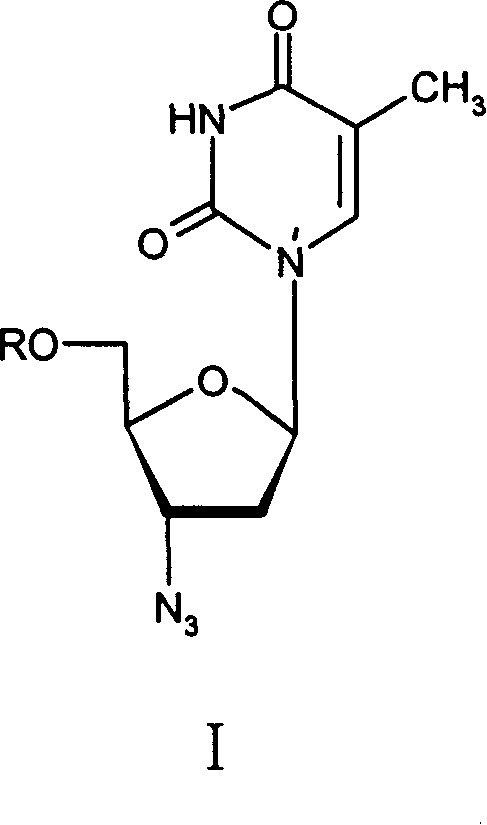
Macroheterocyclic nucleoside derivatives and their analogues, production and use thereof
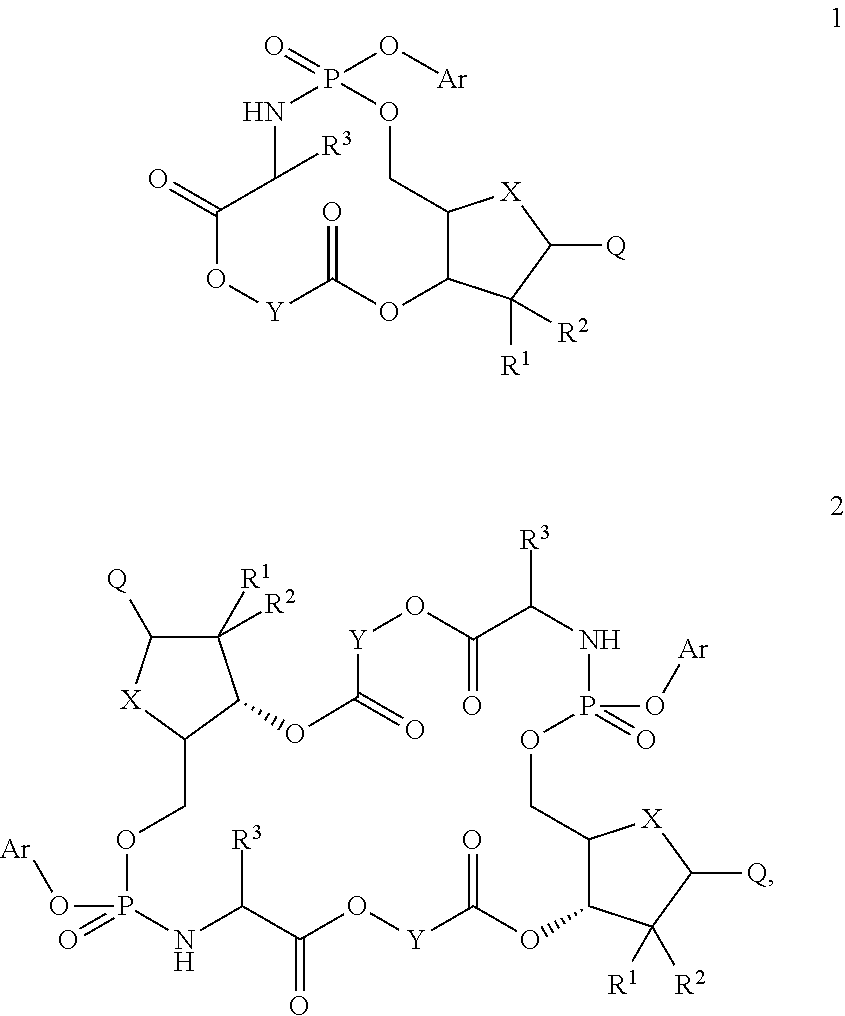
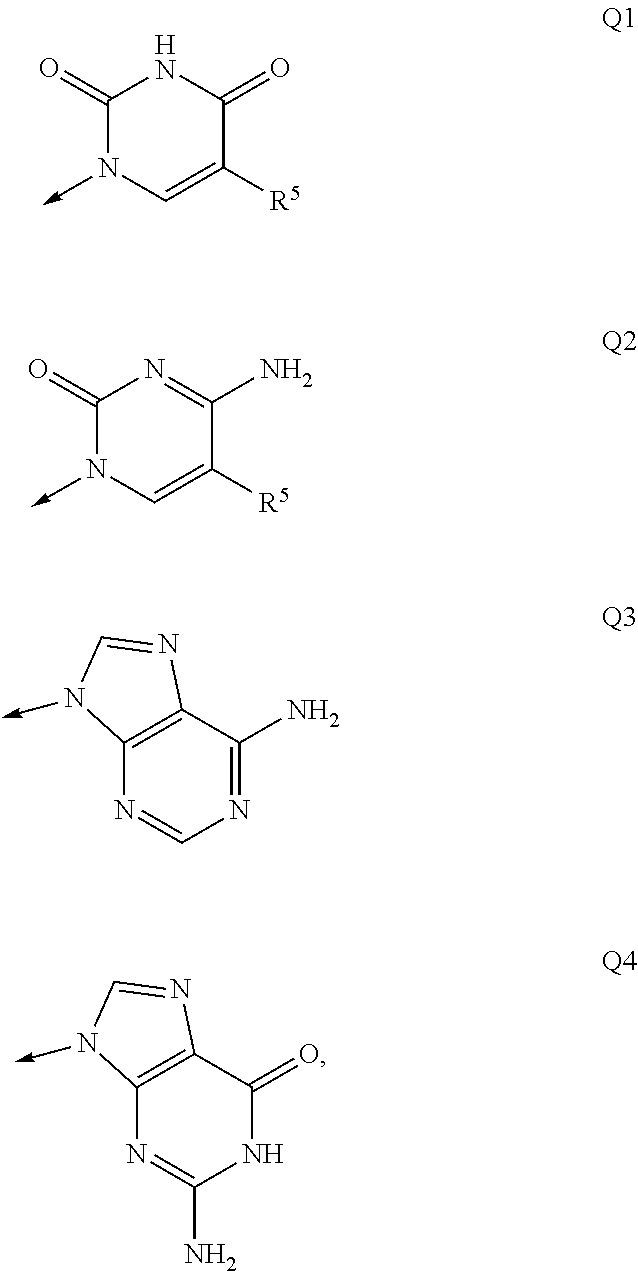
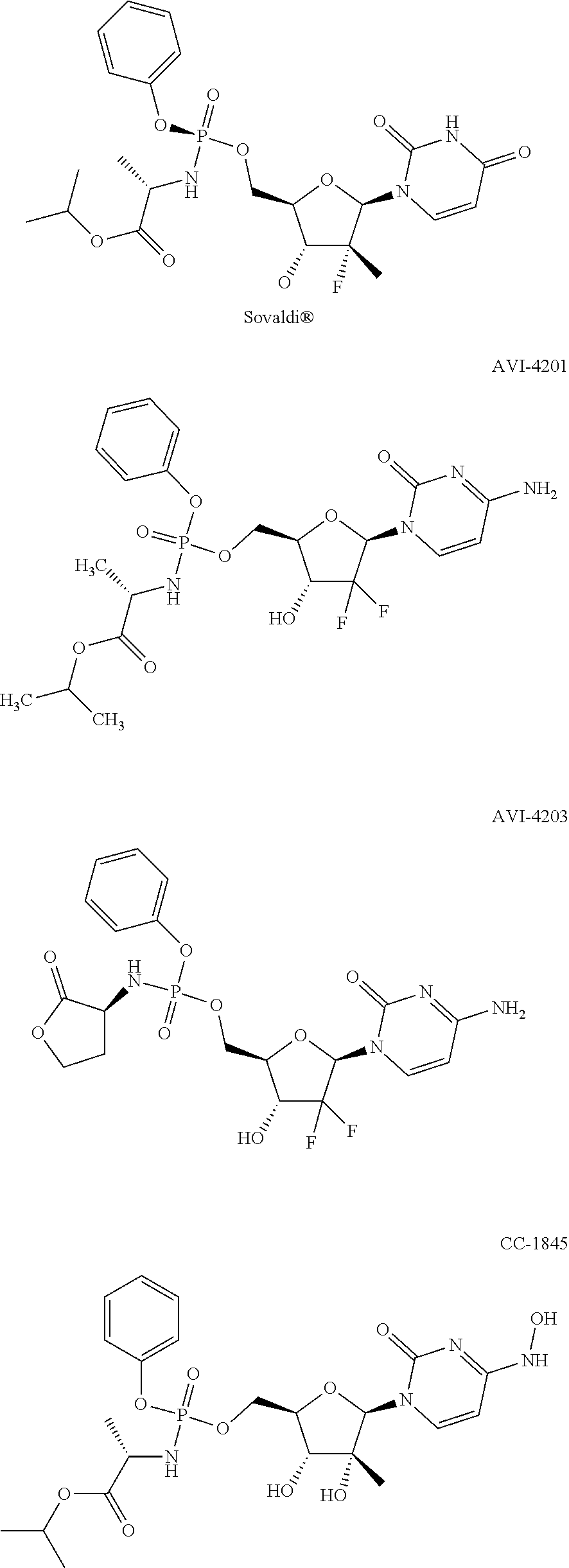
Nucleosides and nucleotides (nucleos(t)ides) have been in clinical use for almost 50 years and have become cornerstones of treatment for patients with viral infections or cancer. The approval of several additional drugs over the past decade demonstrates that this family still possesses strong potential. Therefore nucleos(t)ide are of great interest as promising chemotherapeutic agents, including: 2′-deoxy-L-uridine (CAS 31501-19-6), 2′-deoxy-D-uridine (CAS 951-78-0), telbivudine (CAS 3424-98-4), zidovudine (AZT, CAS 30516-87-1), trifluridine (CAS 70-00-8), clevudine (CAS 163252-36-6), PSI-6206 (CAS 863329-66-2), 2′-(S)-2′-chloro-2′-deoxy-2′-fluorouridine (CAS 1673560-41-2), ND06954 (CAS 114248-23-6), stavudine (CAS 3056-17-5), 5-ethynyltavudine (Festinavir, CAS 634907-30-5), torcitabine (CAS 40093-94-5), (−)-beta-D-(2R,4R)-dioxolane-thymine (DOT, 1-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-5-methyl-2,4(1H,3H)-pyrimidinedione, CAS No. 127658-07-5), 2-(6-amino-purin-9-yl)-ethanol (CAS 707-99-3), 2′-C-methylcytidine (CAS 20724-73-6), PSI-6130 (CAS 817204-33-4), gemcitabine (CAS 95058-81-4), 2′-chloro-2′-deoxy-2′-fluorocytidine (CAS 1786426-19-4), 2′,2′-dichloro-2′-deoxycytidine (CAS 1703785-65-2), 2′-C-methylcytidine (CAS 20724-73-6), PSI-6130 (CAS 817204-33-4), lamivudine (3TC, CAS 134678-17-4), emtricitabine (CAS 143491-57-0), 2′-deoxyadenosine (CAS 958-09-8), 2′-deoxy-β-L-adenosine (CAS 14365-45-8), 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine (CAS 865363-93-5), didanosine (CAS 69655-05-6), entecavir (CAS 209216-23-9), FMCA (CAS 1307273-70-6), dioxolane-G (DOG, CAS 145514-01-8), β-D-2′-deoxy-2′-(R)-fluoro-2′-β-C-methylguanosine (CAS No 817204-45-8), abacavir (ABC, CAS 136470-78-5), dioxolane-A (DOA, CAS #145514-02-9), [(2R,4R)-4-(6-cyclopropylamino-purin-9-yl)-[1,3]dioxolan-2-yl]-methanol (CAS 1446751-04-7), amdoxovir (AMDX, CAS 145514-04-1), (R)-1-(6-amino-purin-9-yl)-propan-2-ol (CAS 14047-28-0), and [(2S,5R)-5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yl]-methanol.Macroheterocyclic nucleoside derivative and its analog of the general formula 1 or general formula 2, a stereoisomer, isotope-enriched analog, pharmaceutically acceptable salt, hydrate, solvate, or crystalline or polymorphic form thereof,wherein:Ar is aryl or hetaryl;R1 and R2 are not necessarily the same substituents selected from H, F, Cl, CH3, OH;R3 is H or CH3;X is oxygen or ethanediyl-1,1 (C═CH2);Y is CH(R4)(CH2)k, CH(R4)(CH2)mC(O)O(CH2)n;R4 is H or CH3;k has a value from zero to six;m has a value from zero to two;n has a value of one to four;Q is a radical selected from Q1-Q4;wherein: R5 is the substituent selected from H, F, Cl, CH3, OH;the arrow (→) indicates the location, joined by Q1-Q4.
Owner:ALLA CHEM LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com