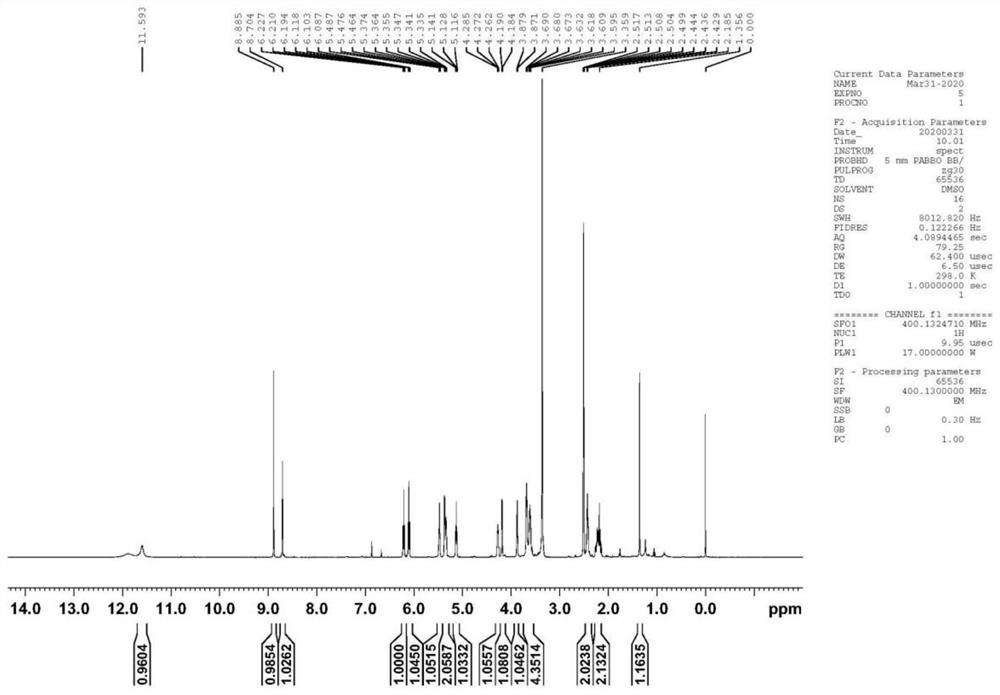
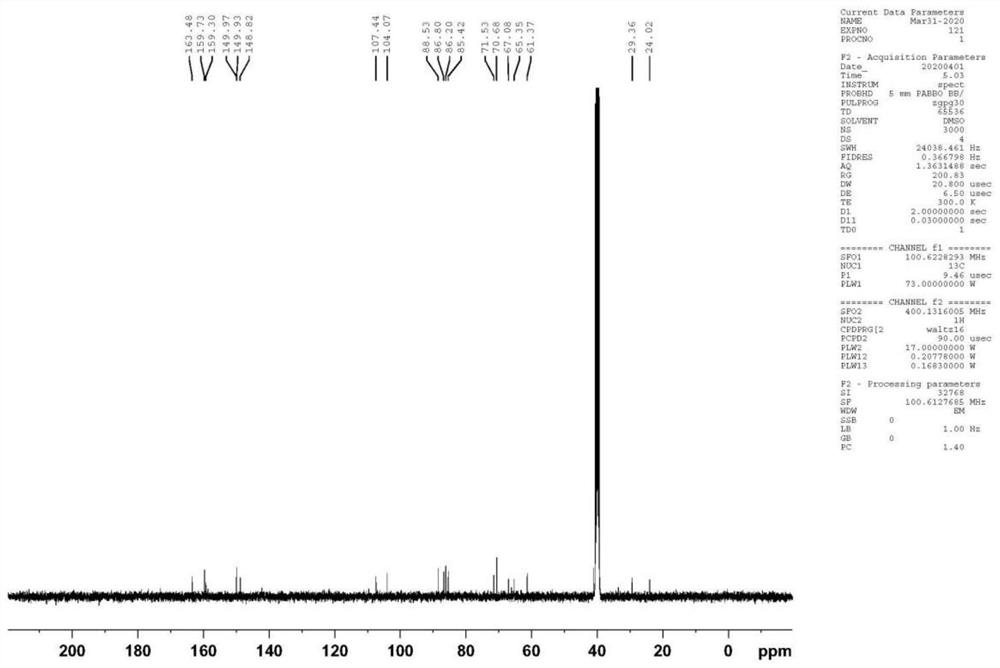
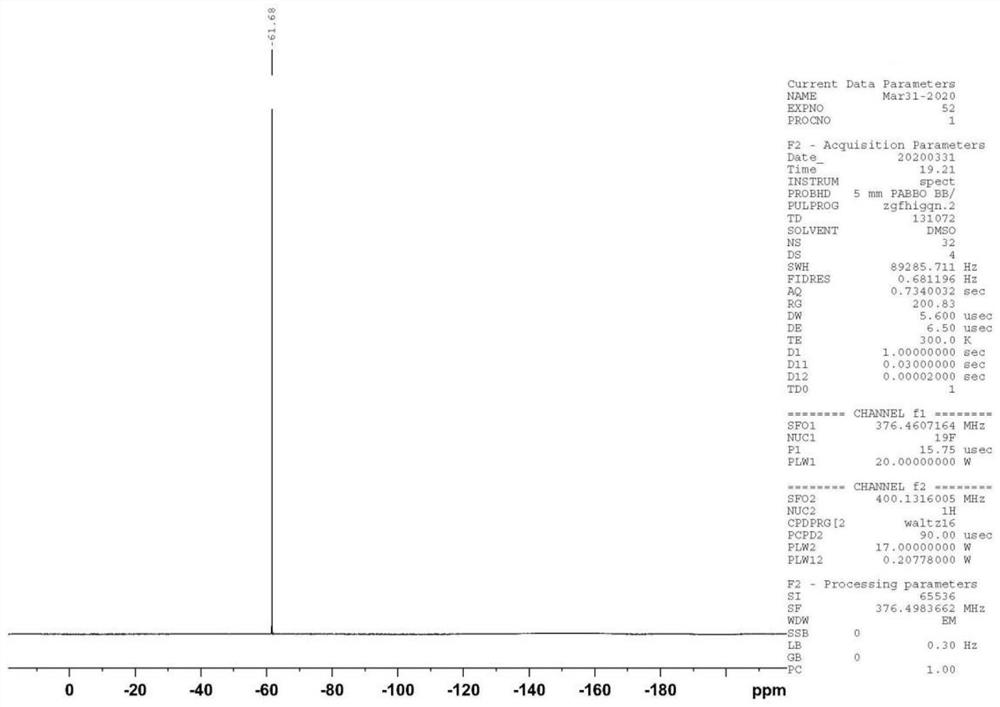
Patents
Literature
34 results about "Trifluridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat herpes infection of the eye.
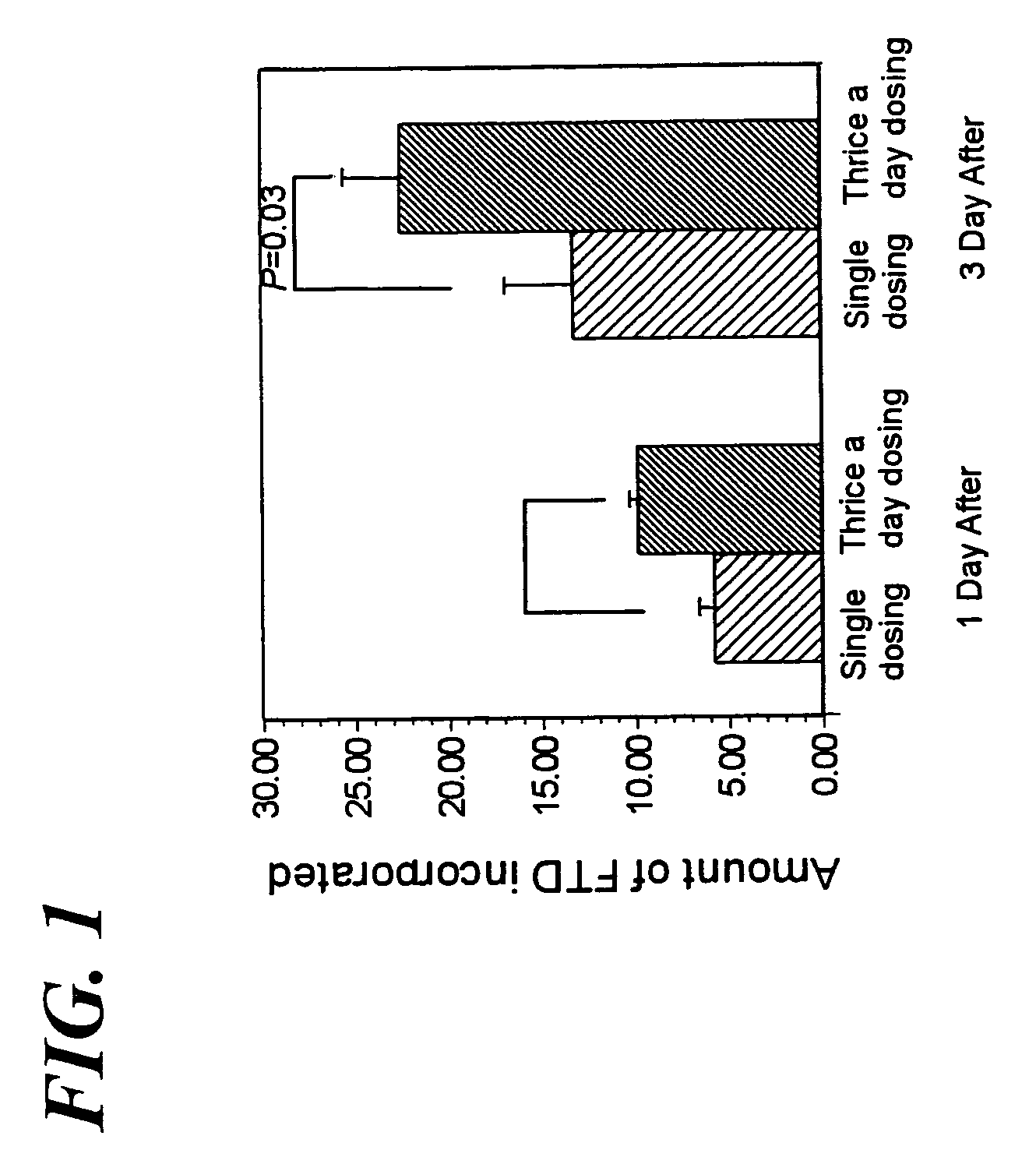
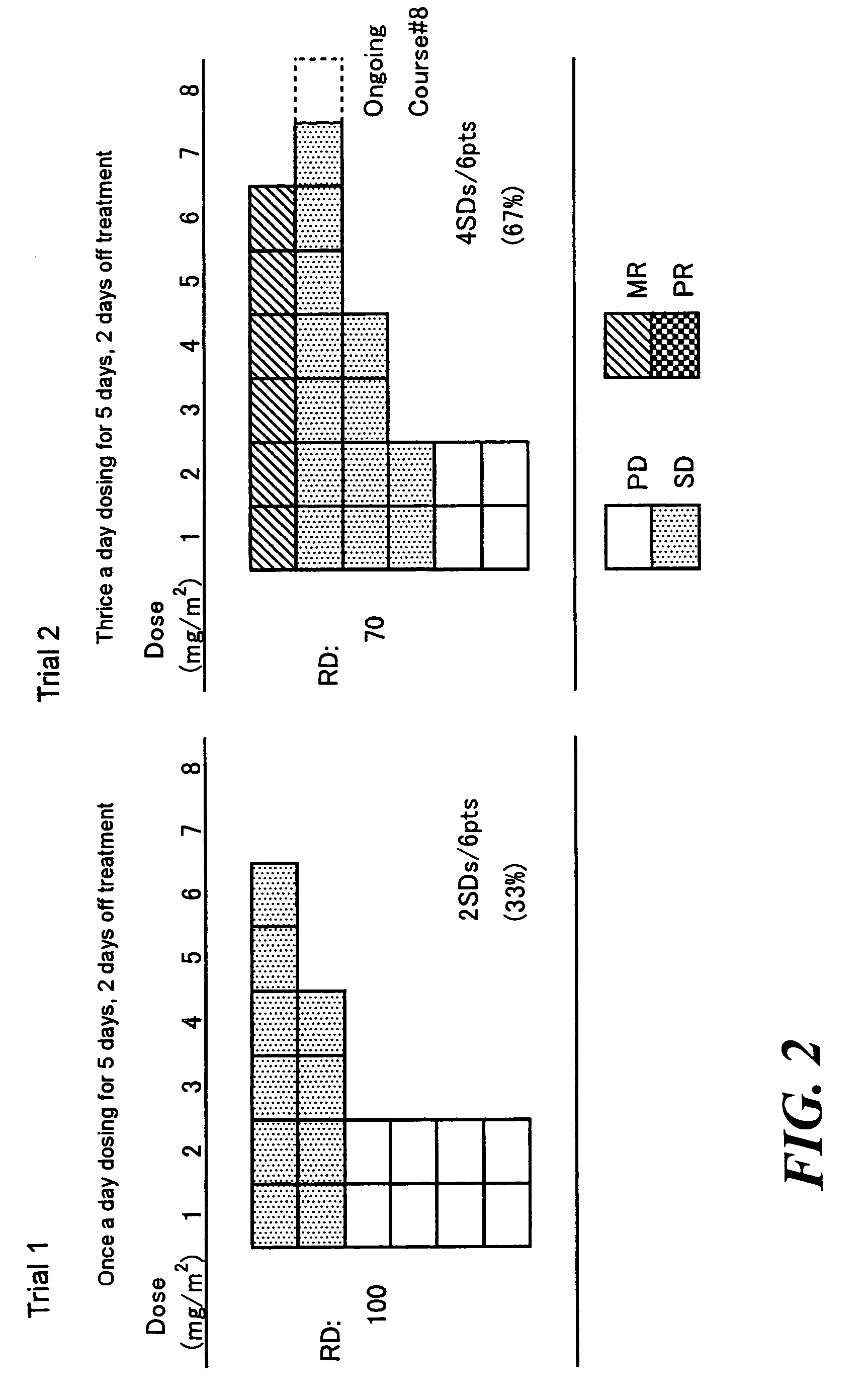
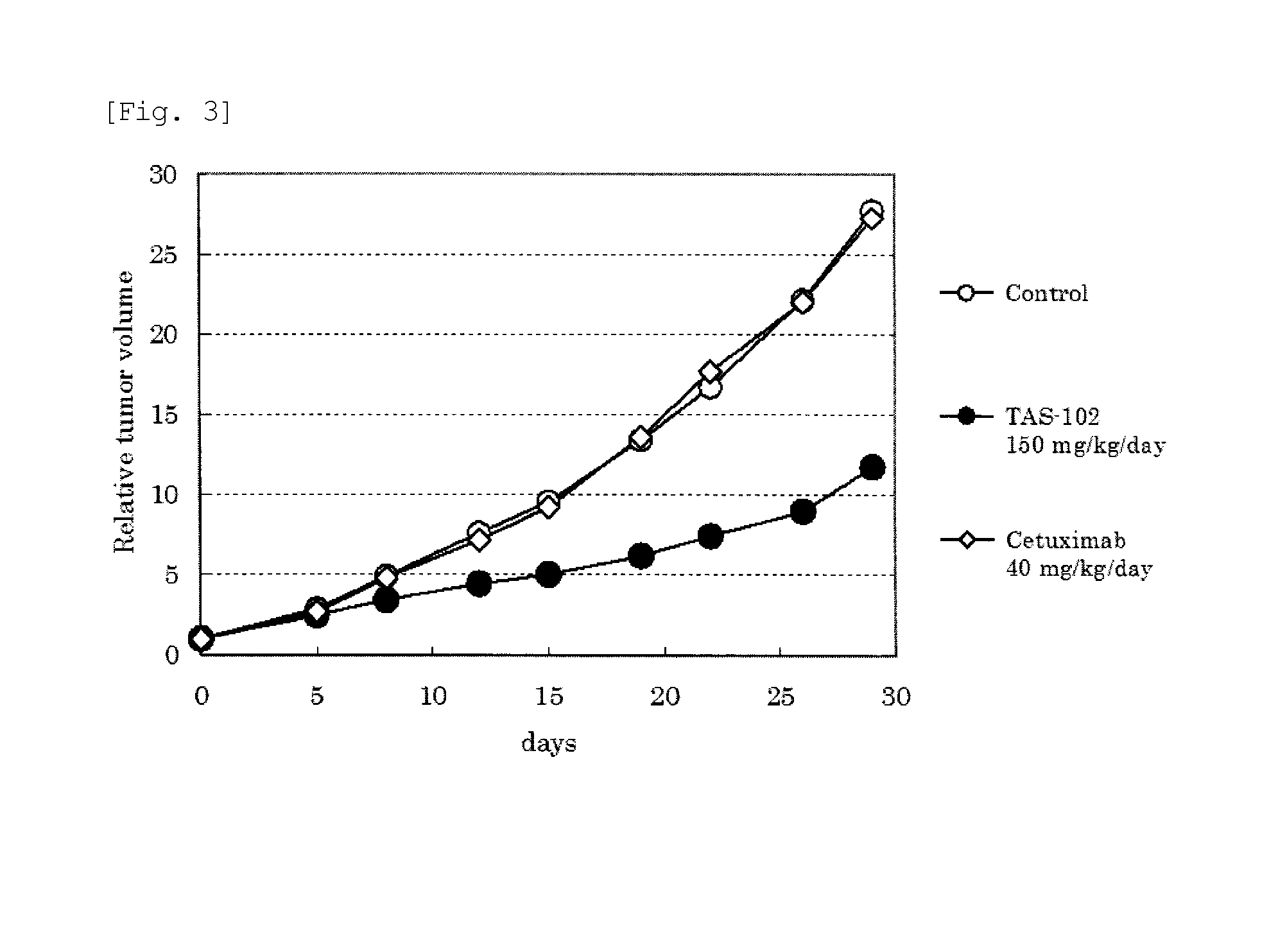
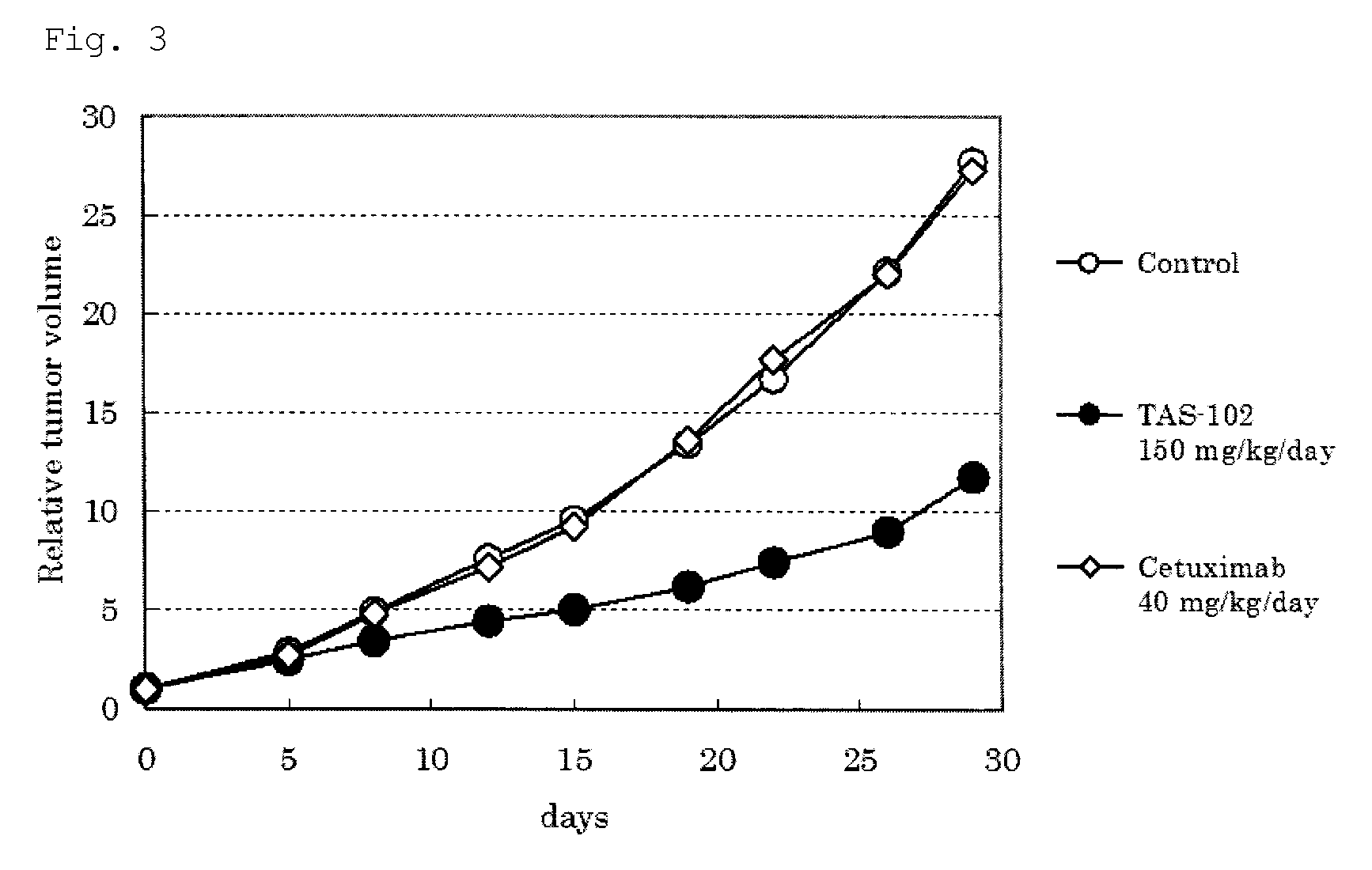
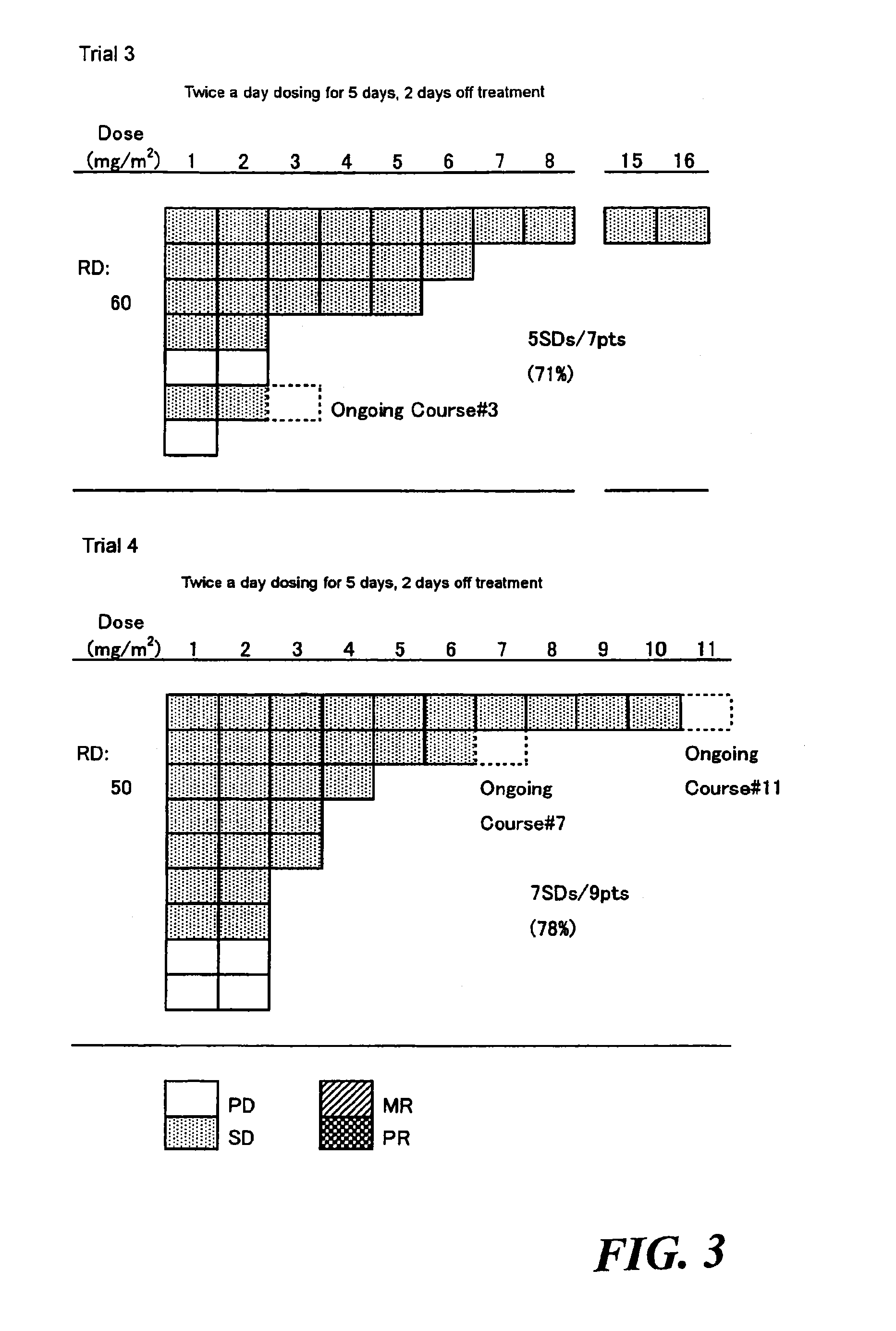
Method of administrating an anticancer drug containing alpha, alpha, alpha-trifluorothymidine and thymidine phosphorylase inhibitor
ActiveUS20060167031A1Good treatment effectImprove anti-tumor activityOrganic active ingredientsBiocideMedicineTrifluridine
The present invention relates to a method for treating a cancer comprising orally administering a composition containing α,α,α-trifluorothymidine (FTD) and 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl)uracil hydrochloride in a molar ratio of 1:0.5 at a dose of 20 to 80 mg / m2 / day in terms of FTD in 2 to 4 divided portions per to patients in need of the treatment.
Owner:TAIHO PHARMA CO LTD
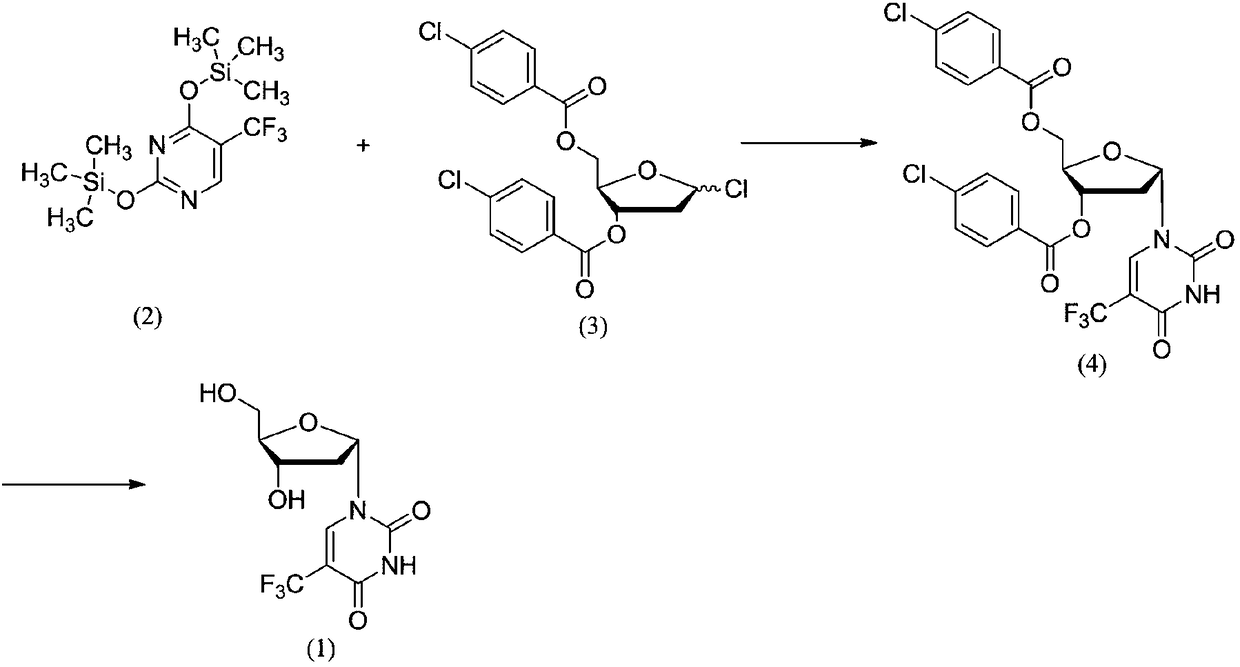
Trifluridine intermediate and preparation method of trifluridine
ActiveCN105461772ASolve pollutionSolve productivitySugar derivativesSugar derivatives preparationLewis acid catalysisHomogeneous catalysis
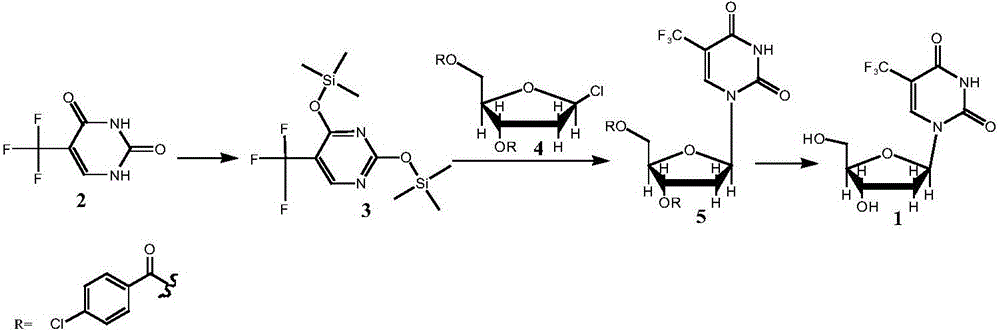
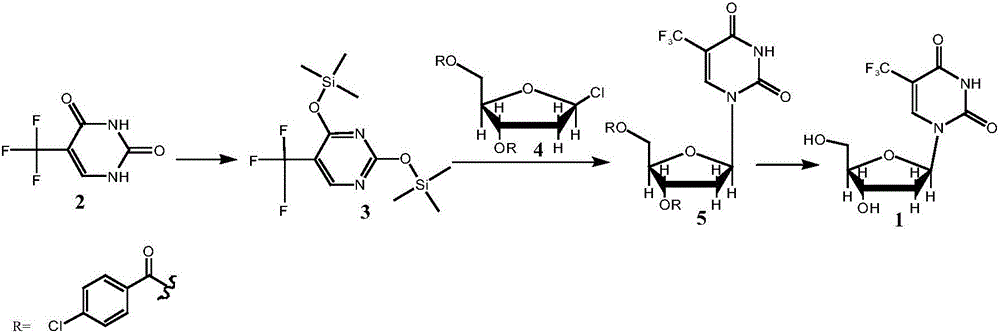
The invention provides a preparation method of a trifluridine intermediate. Under the action of an acidic resin catalyst, 1-chloro-2-deoxy-3,5-di-O-p-chlorobenzoyl-D-ribose and 5-trifluoromethyl-2,4-bis(trimethylsilaneoxy)pyrimidine undergo a condensation reaction so as to obtain the trifluridine intermediate. According to the invention, a heterogeneous catalysis technology is utilized, acidic resin is used as a catalyst, and a traditional Lewis acid catalyst is replaced. Under the precondition of guaranteeing that product quality is controllable, a production technology is greatly improved. Catalytic efficiency is high, and conditions are mild. Purity of the prepared 1-(2'-deoxy-3,5-di-O-p-chlorobenzoyl-beta-D-furanose)-5-trifluoromethyluracil is greatly raised, and the problem that the use of the Lewis acid catalyst leads to severe post-treatment emulsification and environmental pollution and is not beneficial to industrial production is also effectively solved.
Owner:SINOPHARM A THINK PHARMA
Method of administrating an anticancer drug containing α, α, α-trifluorothymidine and thymidine phosphorylase inhibitor
ActiveUS7799783B2Improve anti-tumor activityGood treatment effectOrganic active ingredientsBiocideTrifluridineAnticancer drug
The present invention relates to a method for treating a cancer comprising orally administering a composition containing α,α,α-trifluorothymidine (FTD) and 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl)uracil hydrochloride in a molar ratio of 1:0.5 at a dose of 20 to 80 mg / m2 / day in terms of FTD in 2 to 4 divided portions per to patients in need of the treatment.
Owner:TAIHO PHARMA CO LTD
Trifluridine compound and medicine composition thereof
ActiveCN105198947AImprove solubilityImprove stabilityOrganic active ingredientsSugar derivativesSolubilityChemical compound
The invention belongs to the technical field of pharmaceutical chemical engineering, and particularly discloses a novel crystal form Trifluridine compound, a preparation method and medicine composition thereof. The Trifluridine novel crystal form is good in stability and solubility; the preparation method is relatively good in reproducibility, the operation is simple and convenient, the period is short, the cost is low, damage to the environment and staff is little, and the preparation method is suitable for industrial large-scale production, and has quite important application value in medicine preparation.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation method of high purity trifluridine
InactiveCN106220699AReduce generationHigh puritySugar derivativesSugar derivatives preparationSodium methoxideTrimethylsilyl chloride
The invention provides a preparation method of high purity trifluridine. The method is as below: reacting a raw material of a compound 2 with HMDS under the action of trimethylchlorosilane to obtain a compound 3; subjecting the compound 3 and a compound 4 to condensation in the presence of a catalyst copper difluoride; conducting ethanol recrystallization to obtain a compound 5; and finally conducting deprotection on the compound 5 under the effect of sodium methylate; and recrystallizing by a mixed solvent of ethanol and acetone (1:1) to obtain the high purity object compound 1. The method has the advantages of high purity of the product, simpleness, easy purification and less industrial pollution.
Owner:HARVEST PHARMA HUNAN CO LTD
Flufenacet preparation method
InactiveCN107721948AAchieve recyclingReasonable process designOrganic chemistryReaction rateReaction temperature
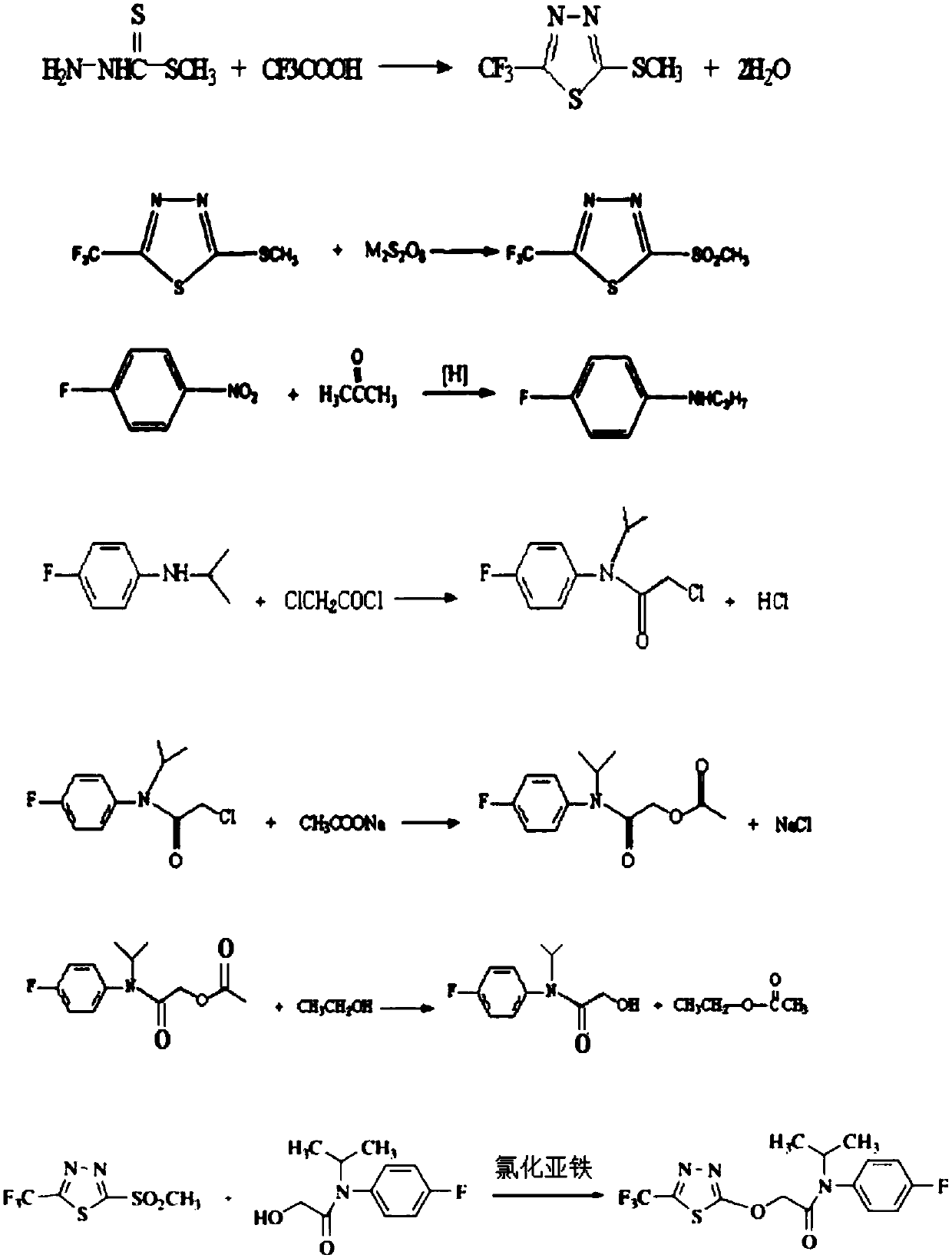
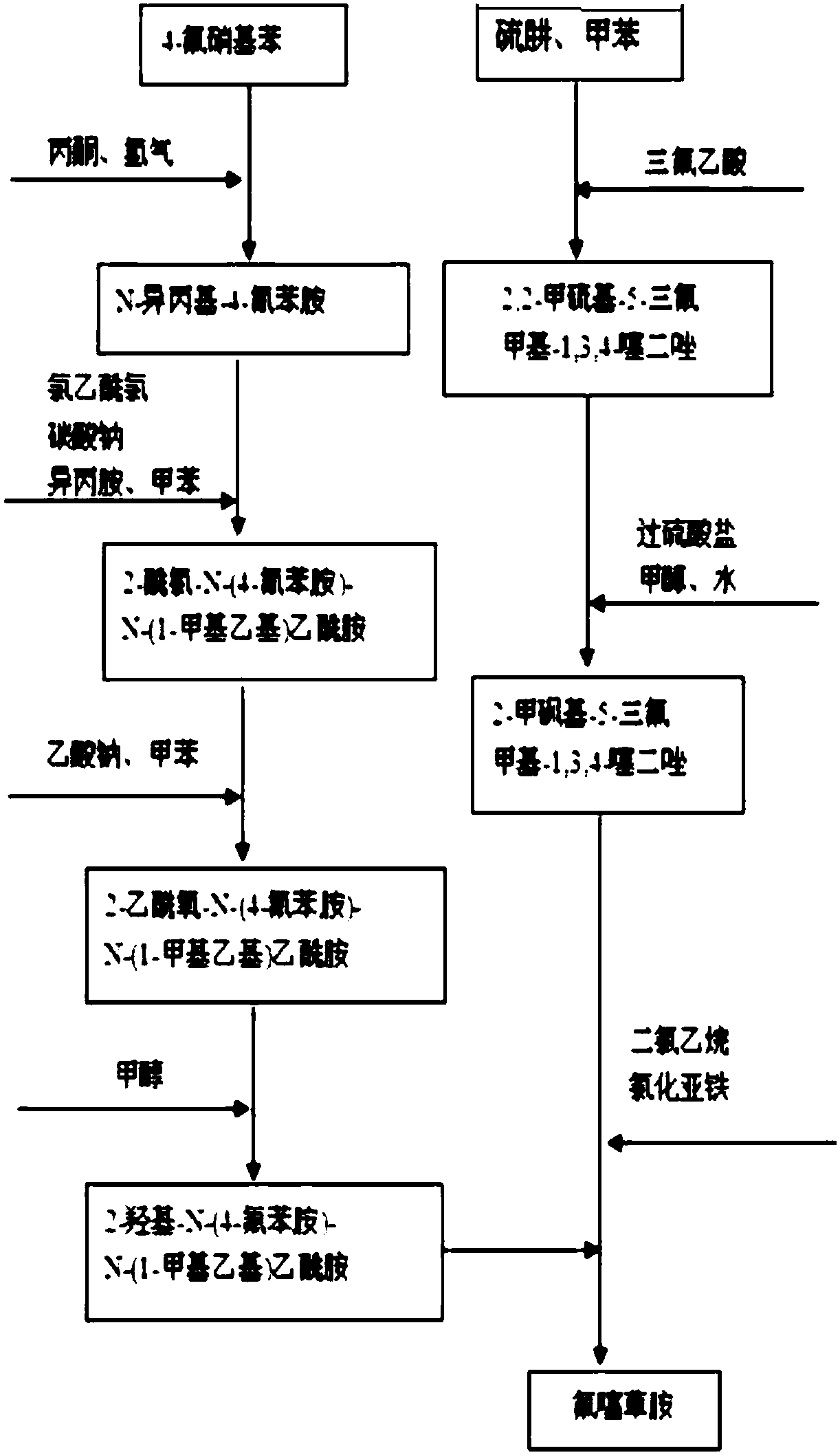
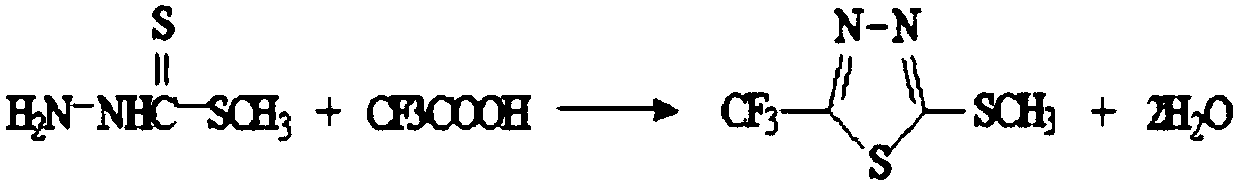
The invention discloses a flufenacet preparation method, which comprises synthesis of 2-methylsulfonyl-5-trifluoromethyl-1,3,4-thiadiazole, synthesis of 2-hydroxy-N-(4-fluoroaniline)-N-(1-methylethyl)acetamide and synthesis of 4'-fluoro-N-isopropyl-2-[5-(trifluridine)-1,3,4-thiadiazole-2-imide]acetamide. The optimal flufenacet preparation method is screened by a large number of experiments, the whole process is reasonable in design, particularly the steps of screening optimal reaction conditions and the optimal amount ratio, reaction temperature, reaction time and the like of reaction raw materials, the reaction yield (capable of reaching 90 percent or more) can be greatly increased, side reaction can be reduced, the reaction rate can be increased, the reaction raw materials can be recycled, the production cost is greatly reduced, and a broad application prospect is achieved.
Owner:江苏绿叶农化有限公司
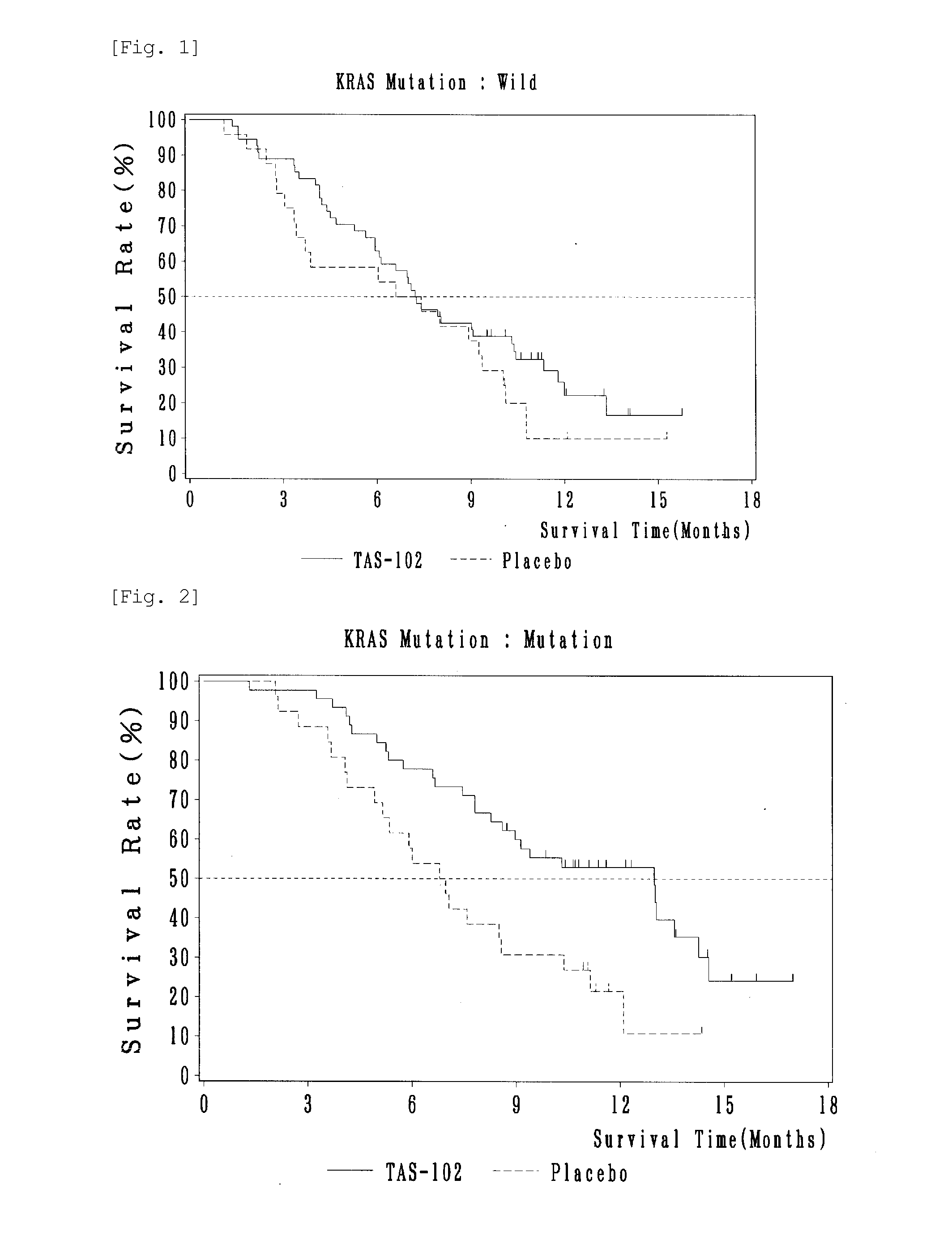
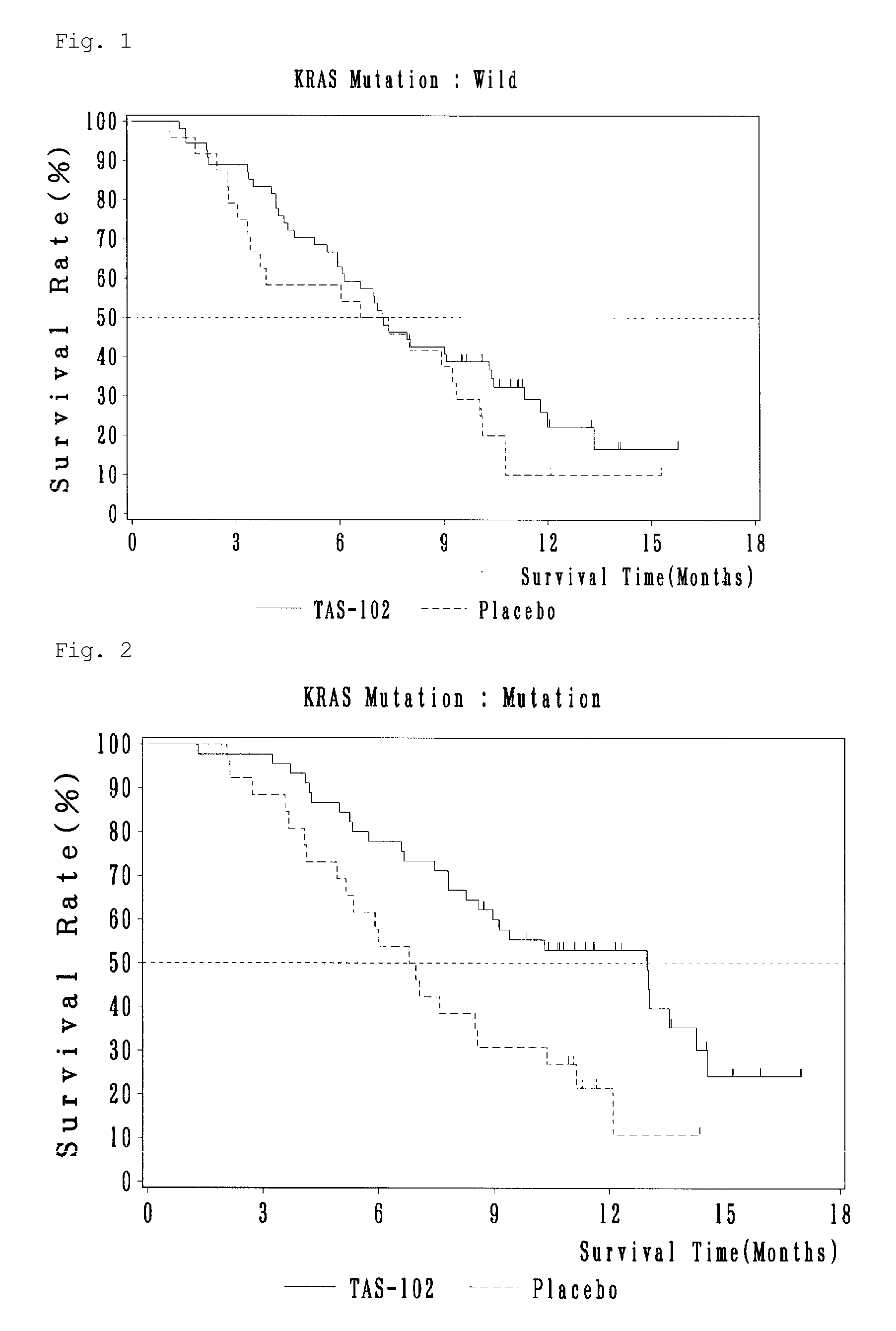
Antitumor agent and therapeutic effect prediction method for patients with kras-mutated colorectal cancer
ActiveUS20140213602A1Remarkable effectImprove survivalOrganic active ingredientsBiocideUracilCurative effect
This invention provides a method for predicting a therapeutic effect of chemotherapy that uses an antitumor agent comprising α,α,α-trifluorothymidine and 5-chloro-6-(1-(2-iminopyrrolidinyl) methyl)uracil hydrochloride at a molar ratio of 1:0.5 on a colorectal cancer patient,the method comprising:(1) detecting the presence or absence of KRAS gene mutation in a biological sample obtained from the patient; and(2) predicting that the patient is likely to sufficiently respond to the chemotherapy, when KRAS gene mutation is detected in Step (1).
Owner:TAIHO PHARMA CO LTD
Oral administrable pharmaceutical composition
InactiveUS20140356431A1Secured formulation stabilityStable storageBiocideCarbohydrate active ingredientsHigh humidityBULK ACTIVE INGREDIENT
The present invention provides an FTD and TPI-containing orally administrable pharmaceutical composition which can be orally administered and is stable even under high-humidity conditions.An orally administrable pharmaceutical composition which comprises α,α,α-trifluorothymidine and 5-chloro-6-(2-iminopyrrolidine-1-yl)methyl-2,4(1H,3H)-pyrimidine dione hydrochloride as active ingredients and additives having a critical relative humidity of 85% or more at 25° C. as an excipient.
Owner:TAIHO PHARMA CO LTD
Preparation method of trifluridine
ActiveCN104761602AReduce the amount of feedMild reaction conditionsSugar derivativesSugar derivatives preparationClinical efficacyDeoxyuridine
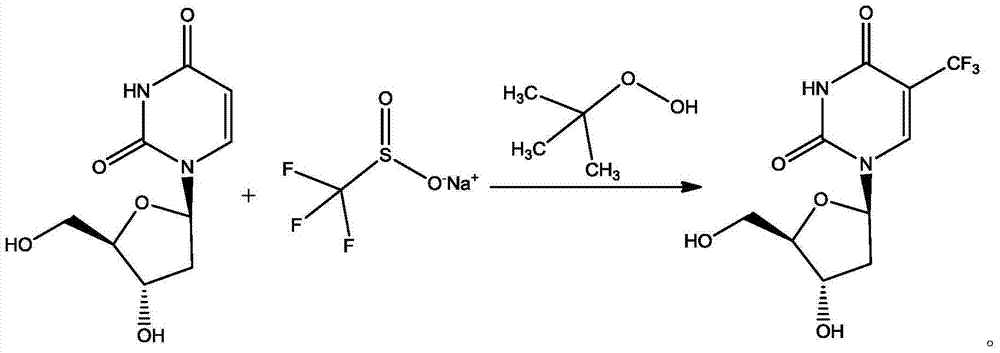
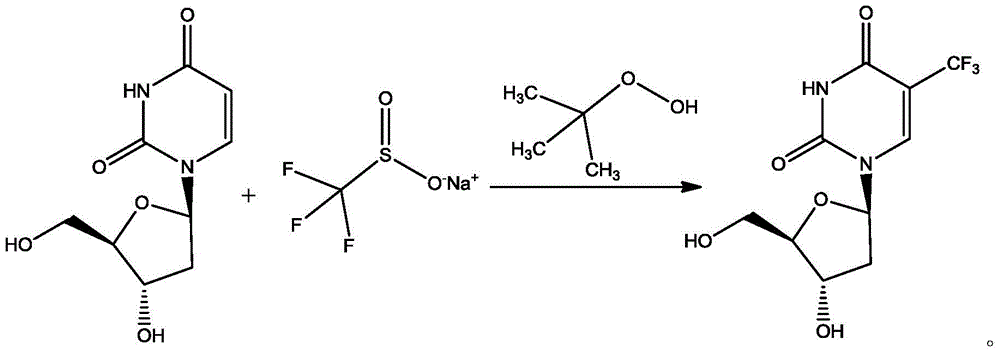
The invention discloses a preparation method of trifluridine. The method comprises the specific steps of: adding 2'-deoxyuridine and trifluoromethyl sulfinate sodium to a reaction solvent, stirring and cooling to - 5 to - 3 DEG C, introducing of nitrogen for protection, stirring to dissolve, dropwise adding tert-butyl hydroperoxide, controlling the temperature at less than 5 DEG C, heating to 60-65 DEG C for reaction, and conducting posttreatment after the reaction to obtain a finished product. The method provided by the invention has mild reaction conditions, simple operation, little side reaction, short reaction time, great reduction of feeding amount of tert-butyl hydroperoxide, great saving of the production cost, and high yield and high purity of the product, and is especially applicable to industrial production, and has significance to the quality control of medicine and clinical curative effect.
Owner:SHANDONG CHENGCHUANG BLUE OCEAN PHARM TECH CO LTD
Anti-HIV compositions
InactiveUS6596720B1Potent anti-HIV activityEliminate side effectsOrganic active ingredientsSugar derivativesAnti hiv activityMedicinal chemistry
The present invention provides:an anti-HIV composition comprising at least one member selected from the group consisting of trifluridine and derivatives thereof, and a pharmaceutically acceptable carrier;an anti-HIV composition comprising (a) at least one member selected from the group consisting of trifluridine and derivatives thereof, (b) a thymidine phosphorylase inhibitor, and a pharmaceutically acceptable carrier; anda composition for potentiating the anti-HIV activity of trifluridine and derivatives thereof, comprising a thymidine phosphorylase inhibitor and a pharmaceutically acceptable carrier.
Owner:TAIHO PHARMA CO LTD
Oral pharmaceutical composition
InactiveUS20140363512A1Quality improvementPowder deliveryBiocideBULK ACTIVE INGREDIENTActive ingredient
The present invention provides an FTD and TPI-containing oral pharmaceutical composition which can be orally administered and is stable even under high humidity conditions.An oral pharmaceutical composition comprising α,α,α-trifluorothymidine and 5-chloro-6-(2-iminopyrrolidine-1-yl)methyl-2,4(1H,3H)-pyrimidine dione hydrochloride as an active ingredient; and being substantially free of an additive comprising a metal salt.
Owner:TAIHO PHARMA CO LTD
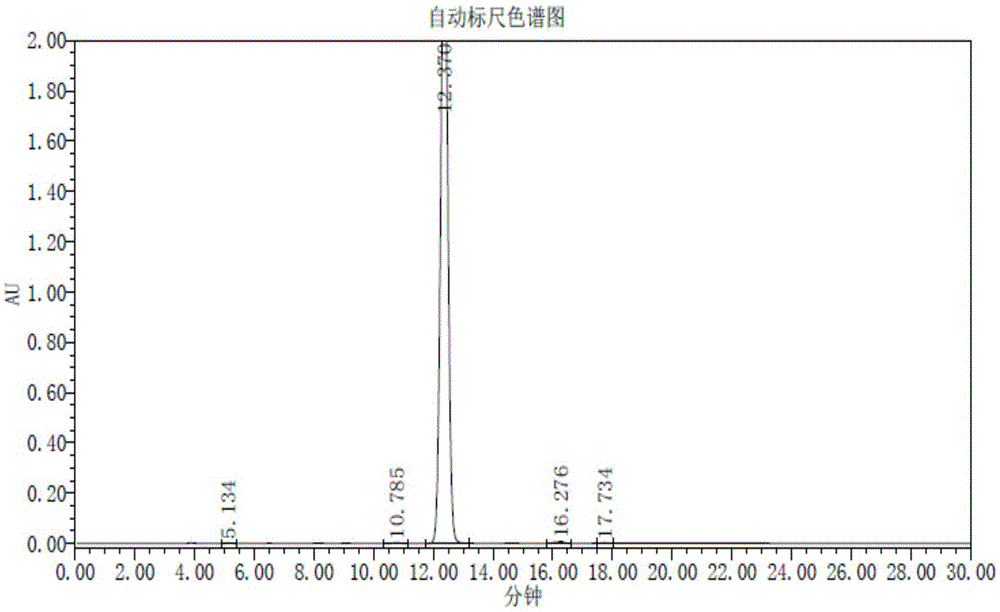
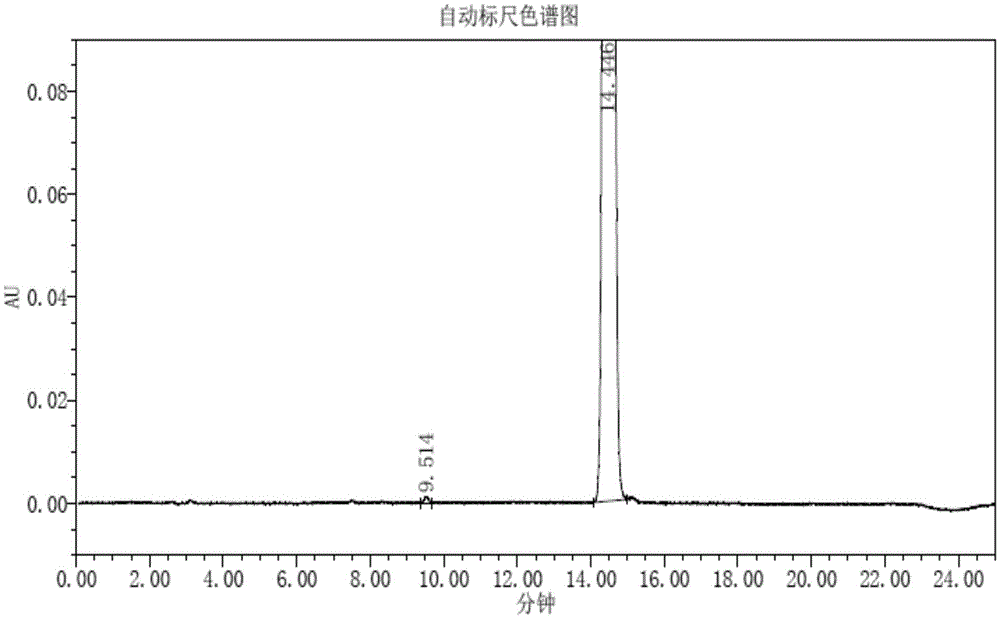
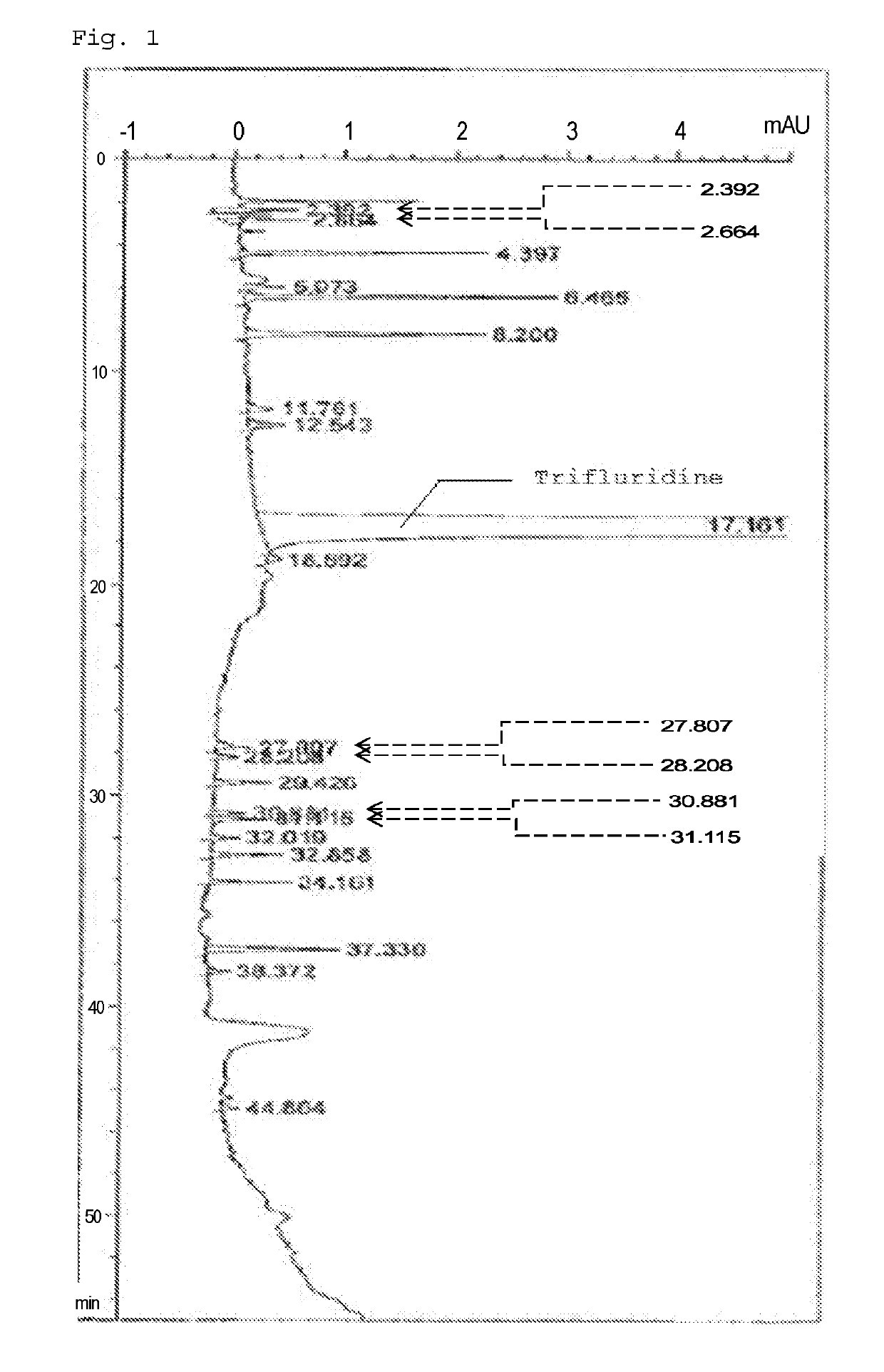
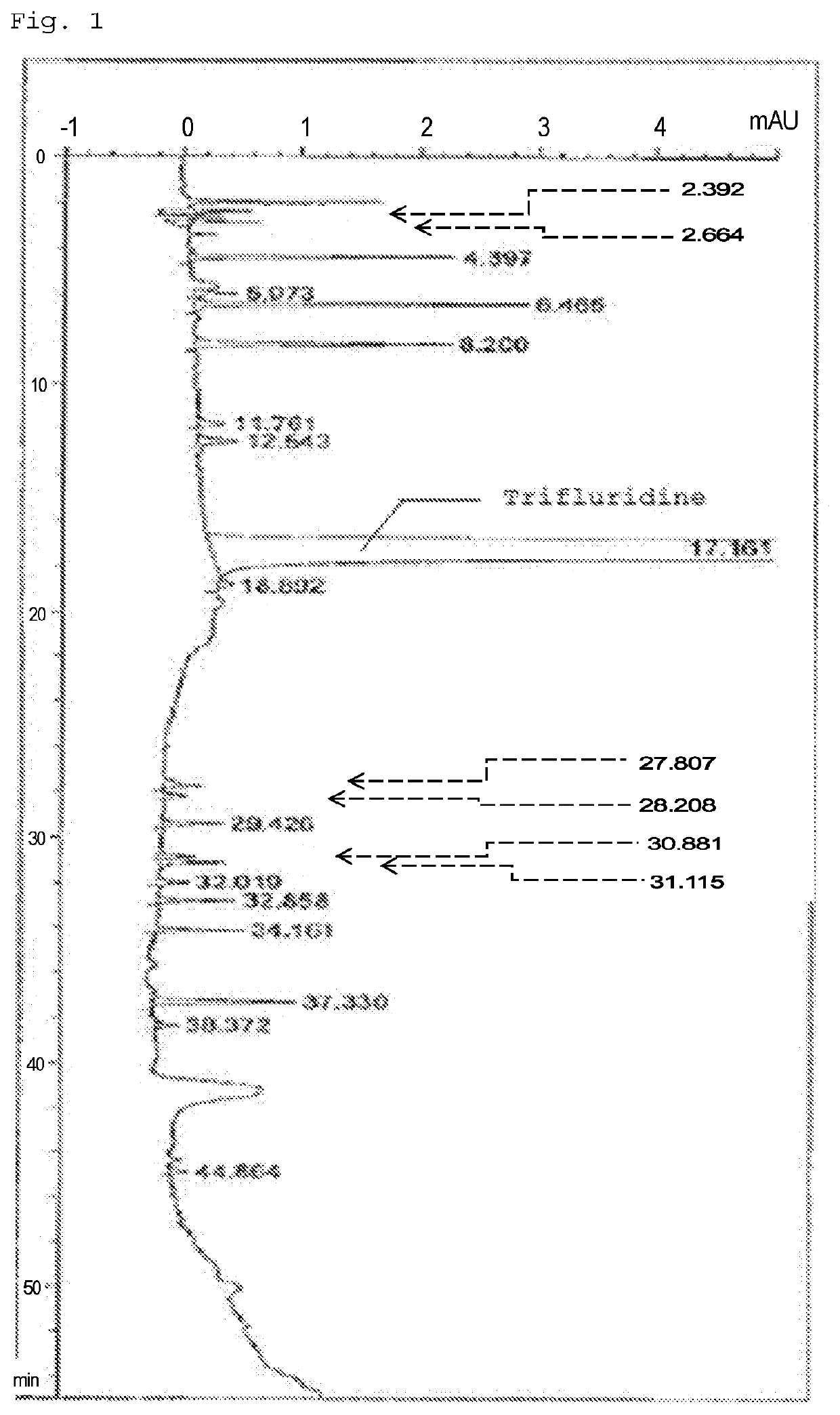
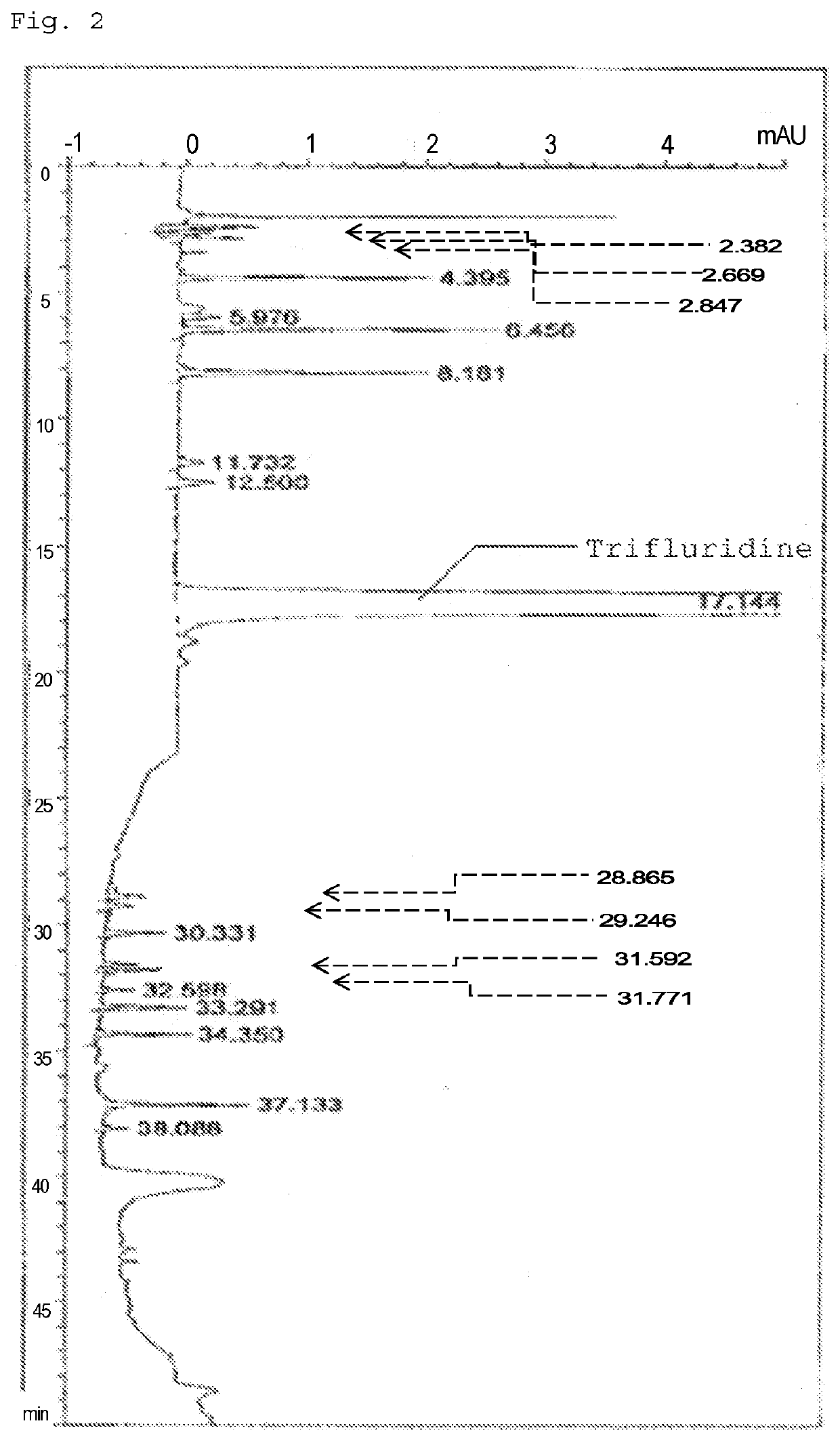
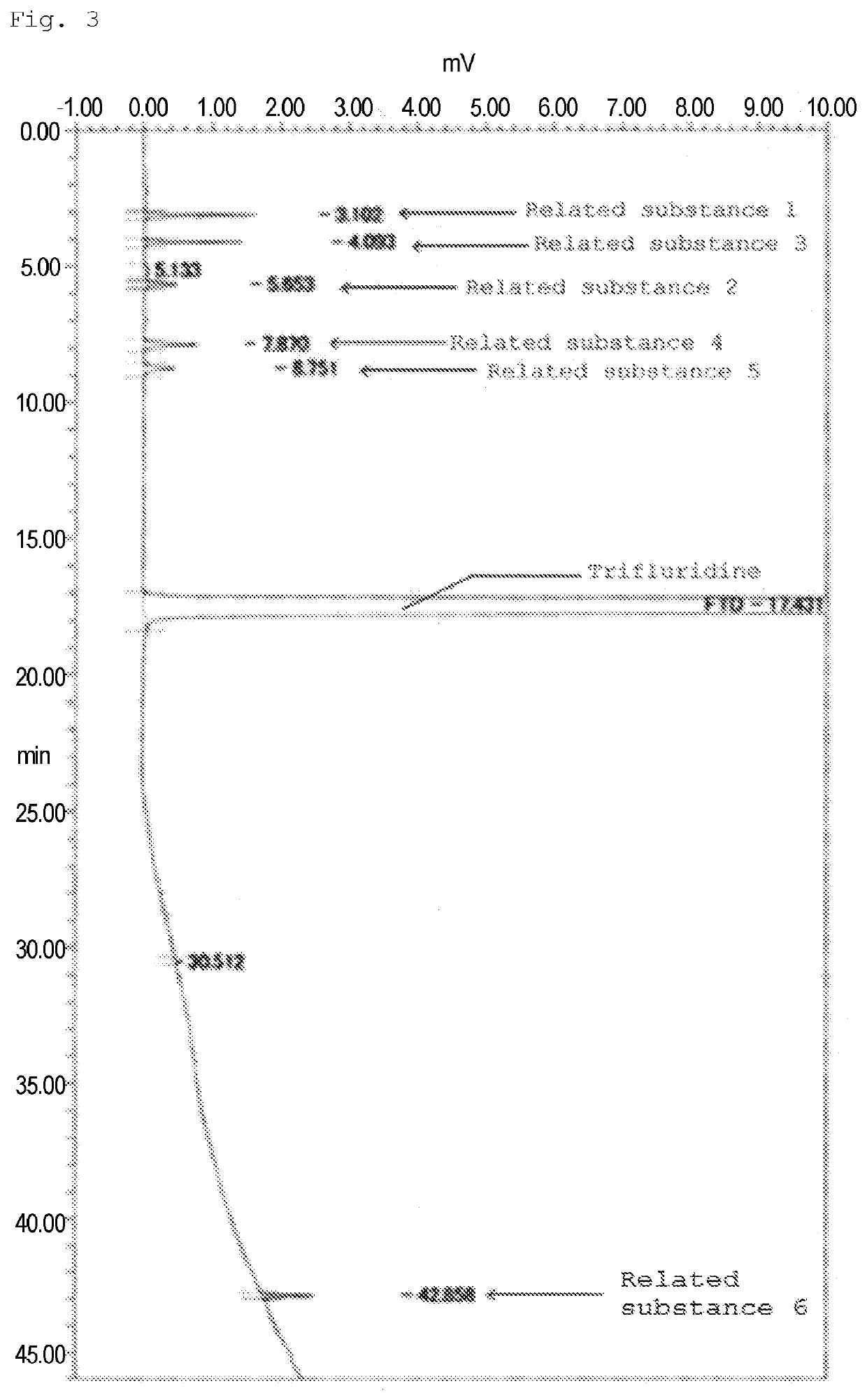
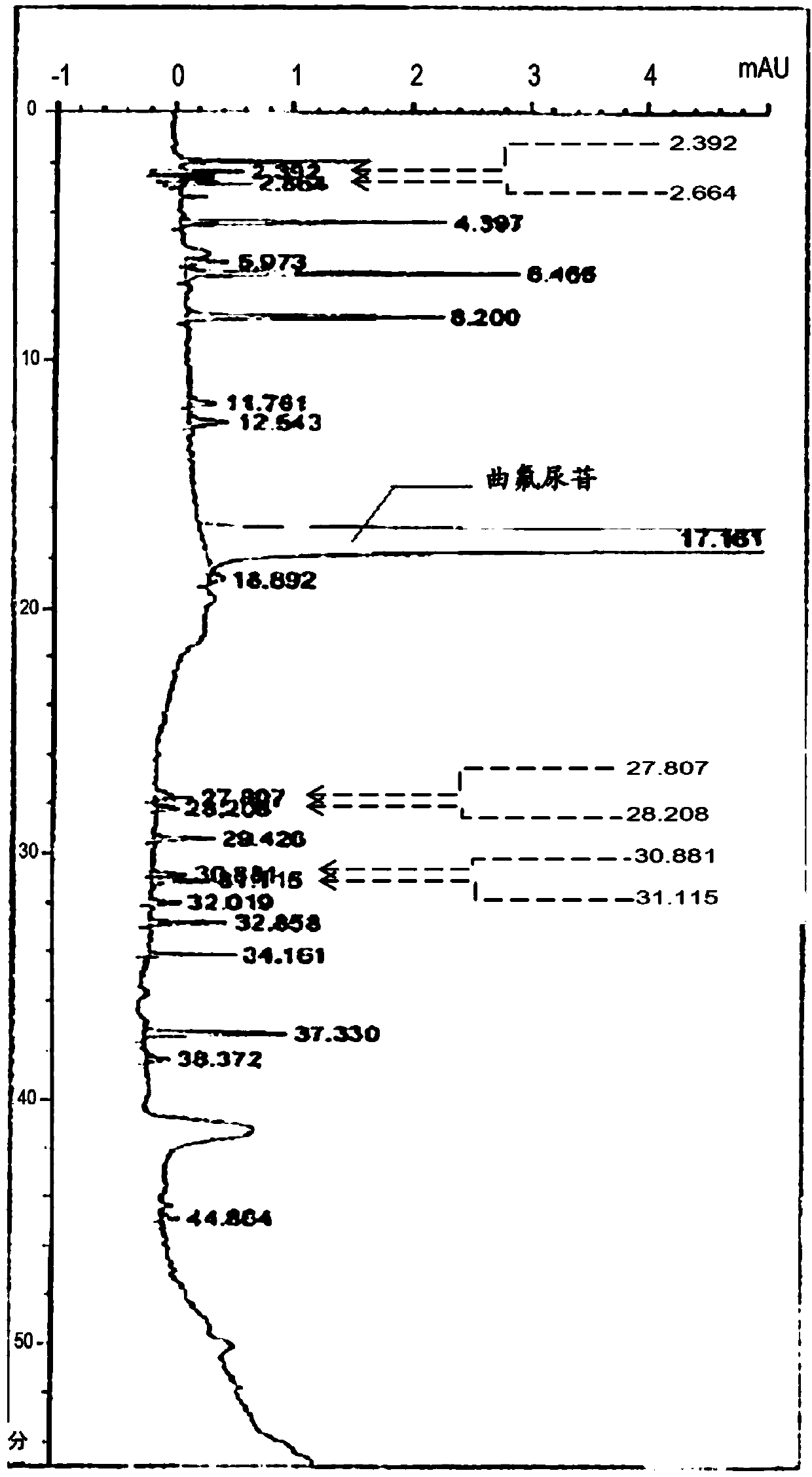
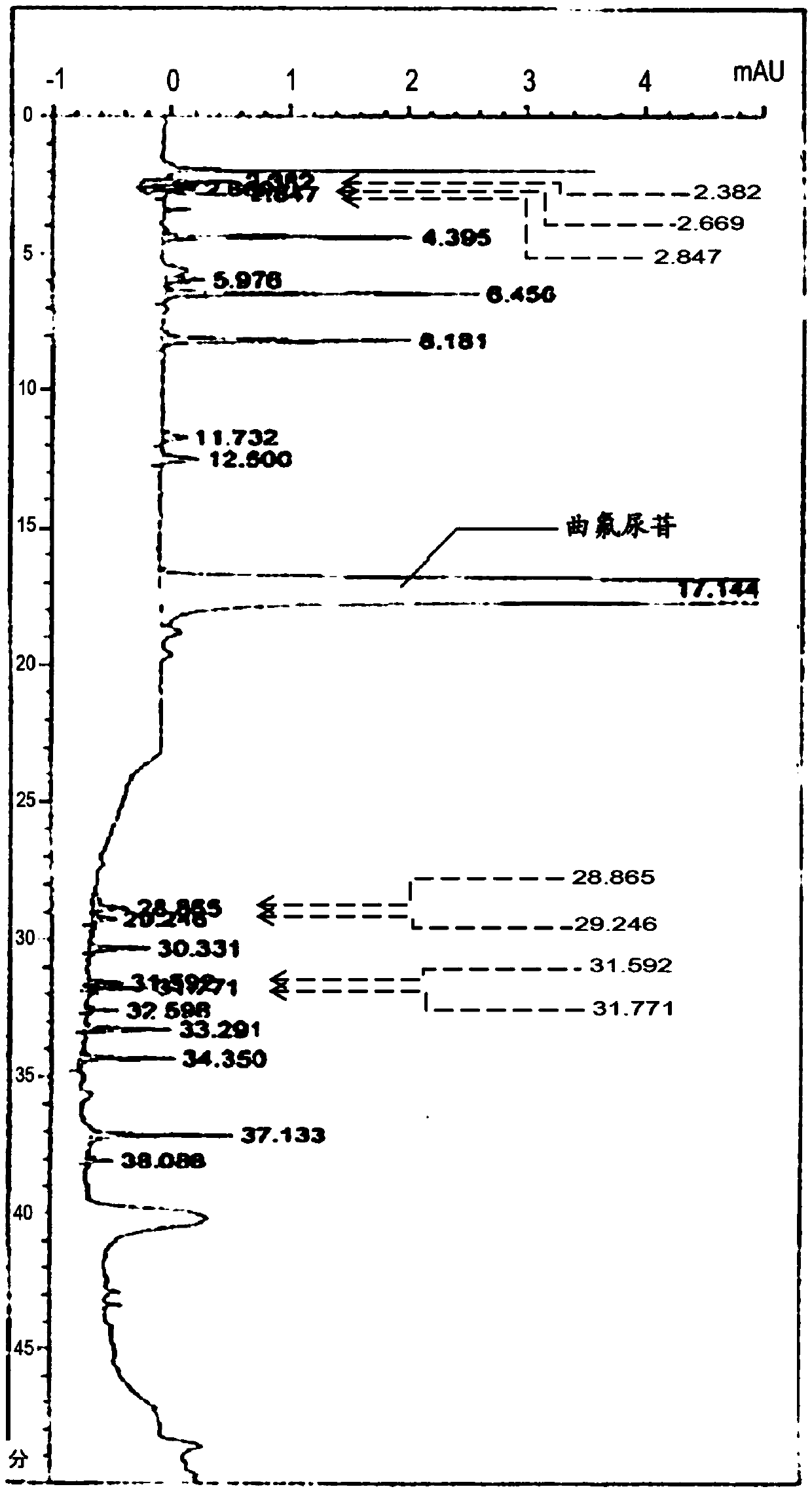
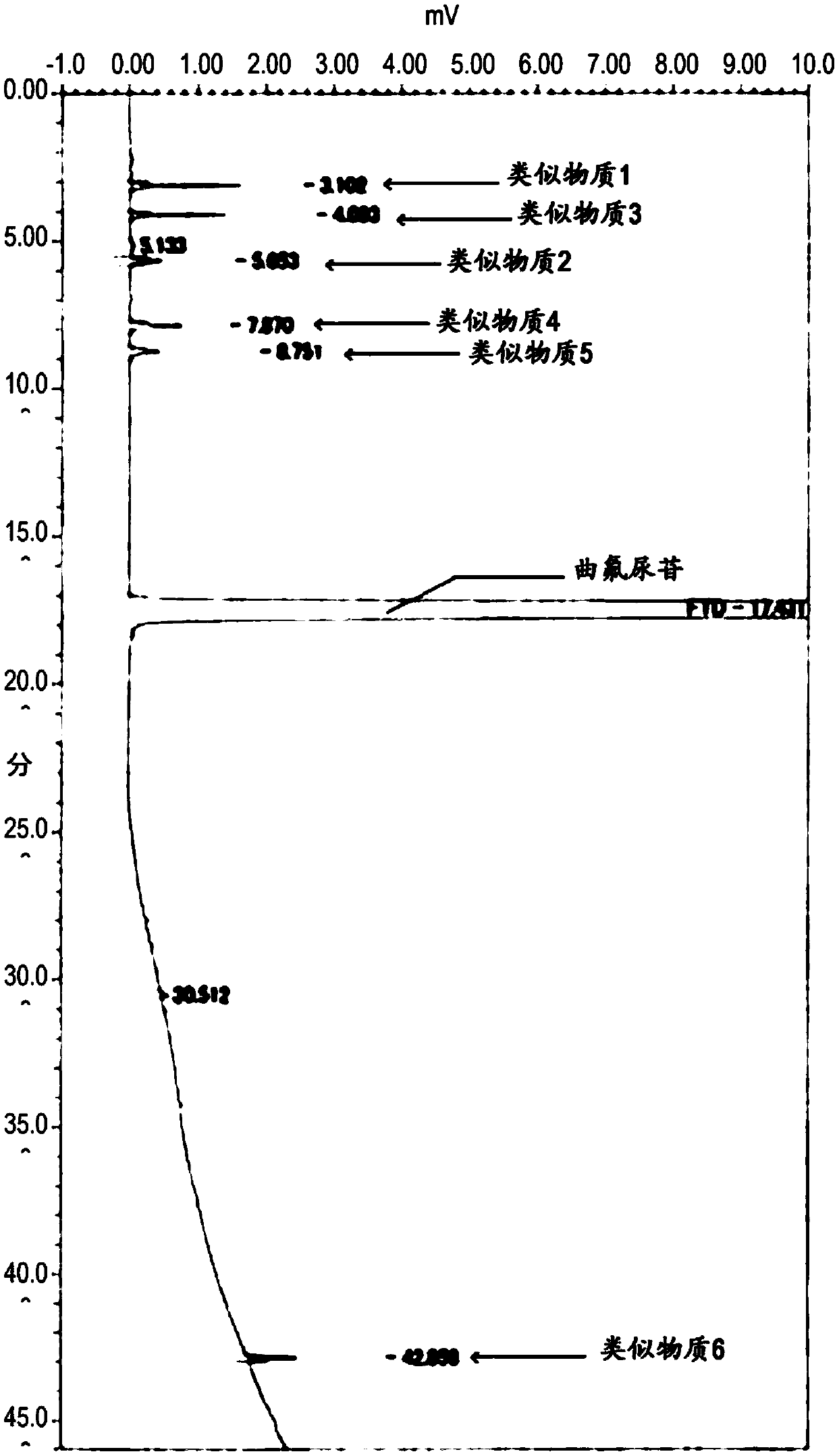
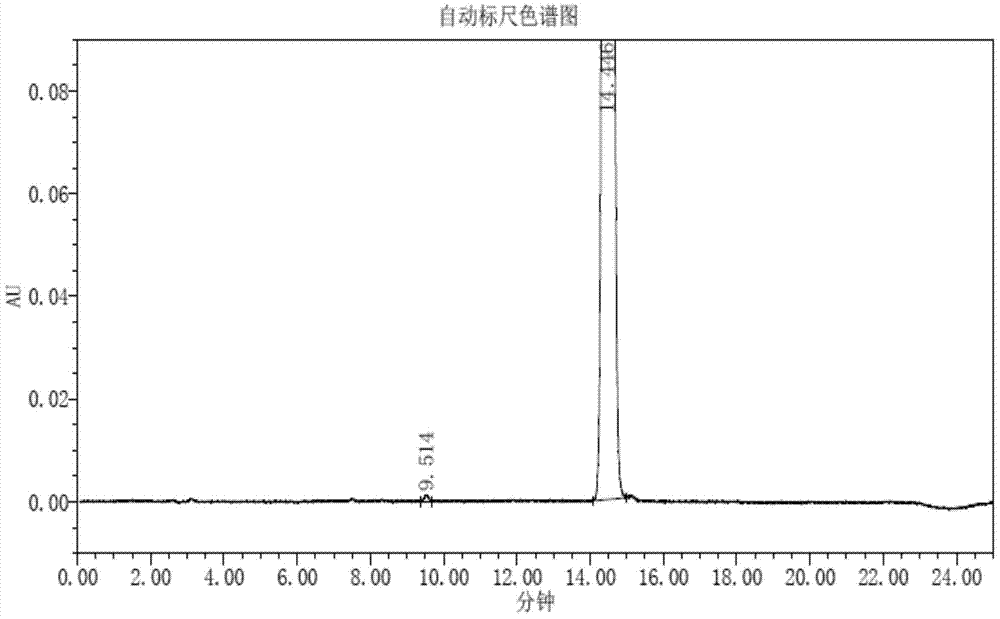
Method for detecting trifluridine-related substance by high-performance liquid chromatography
ActiveUS20190212309A1Efficient detectionQuality assuranceSugar derivativesComponent separationElutionTwo step
The present invention provides a novel method that is capable of detecting a trifluridine-related substance from a sample containing trifluridine or a salt thereof by high-performance liquid chromatography comprising two steps that are performed under gradient conditions. More specifically, the method is for detecting a trifluridine-related substance, the method comprising the step of subjecting a sample containing trifluridine or a salt thereof to high-performance liquid chromatography using a mobile phase composed of an organic phase and an aqueous phase, wherein the step of high-performance liquid chromatography comprises steps 1 and 2 that satisfy the following requirements: Step 1: the percentage of the organic phase in the entire mobile phase is 1 to 14% by volume; and Step 2: after step 1, elution is performed by applying a gradient of increasing the percentage of the organic phase in the entire mobile phase.
Owner:TAIHO PHARMA CO LTD
Antitumor agent and therapeutic effect prediction method for patients with KRAS-mutated colorectal cancer
ActiveUS9371380B2Remarkable effectImprove survivalOrganic active ingredientsMicrobiological testing/measurementUracilCurative effect
This invention provides a method for predicting a therapeutic effect of chemotherapy that uses an antitumor agent comprising α,α,α-trifluorothymidine and 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl)uracil hydrochloride at a molar ratio of 1:0.5 on a colorectal cancer patient,the method comprising:(1) detecting the presence or absence of KRAS gene mutation in a biological sample obtained from the patient; and(2) predicting that the patient is likely to sufficiently respond to the chemotherapy, when KRAS gene mutation is detected in Step (1).
Owner:TAIHO PHARMA CO LTD
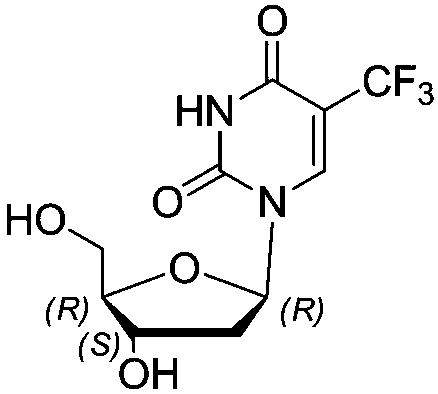
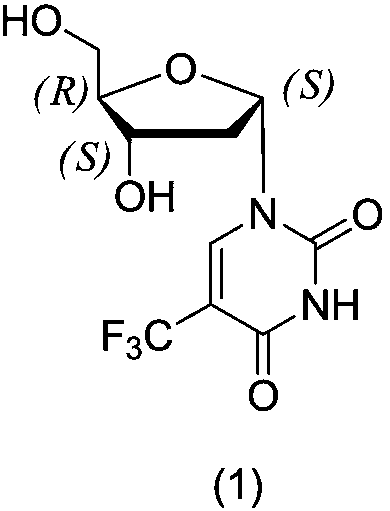
Method for synthesizing trifluridine process impurity
InactiveCN109021048AImprove finished product qualityPrecise positioningSugar derivativesOrganic chemistry methodsUracilRibose
The invention discloses a method for synthesizing a trifluridine impurity 1-((2S,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-(trifluoromethyl)pyrimidine-2,4(1H,3H)-dione, and belongs tothe technical field of chemical pharmacy. The method comprises the following steps: condensing starting raw materials 5-trifluoromethyl-2,4-bis(trimethylsilyloxy)uracil (2) and 1-chloro-2-deoxy-3,5-di-4-chlorobenzoyl-D-ribose (3) to prepare 3',5'-di(4-chlorobenzoyl)-2'-deoxy-alpha-D-ribose-5-trifluoromethyluracil (4); and hydrolyzing the compound (4) to generate 1-((2S,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-(trifluoromethyl)pyrimidine-2,4(1H,3H)-dione (1). The highly-pure trifluridine impurity synthesized in the invention can be used as an impurity standard product in the detection and analysis of finished trifluridine, so the accurate localization and qualitative diagnosis of the impurity in the detection and analysis of the finished trifluridine are improved, and theenhancement of the control of the impurity is benefited, thereby the quality of the finished trifluridine is improved. The method has the advantages of cheap and easily available raw materials, and simplicity in operation, and allows the yield of the obtained product to be (40 + / - 5)% and the HPLC purity of the product to be equal to or more than 98%.
Owner:江苏悦兴医药技术有限公司
Improved method of preparation of trifluridine
ActiveCN107652341ASugar derivativesSugar derivatives preparationTrifluoromethylationSecurity properties
The invention relates to a method for preparing trifluridine (Viroptic). The method comprises the following main steps: by using uridine (a compound shown in a formula II) as a starting raw material,sequentially carrying out the steps such as esterification and halogenating reaction of hydroxyl, reduction reaction, trifluoromethylation reaction and protective group removal reaction so as to obtain a target. The method provided by the invention has the advantages that the use of expensive raw materials and the use of reagents with high toxicity and capability of polluting an environment are avoided and reaction conditions required in all steps are mild; therefore, the invention provides a preparation method of trifluridine with environmental protection property, security property and a commercial preparation value.
Owner:SHANGHAI ZHAOWEI TECH DEV +1
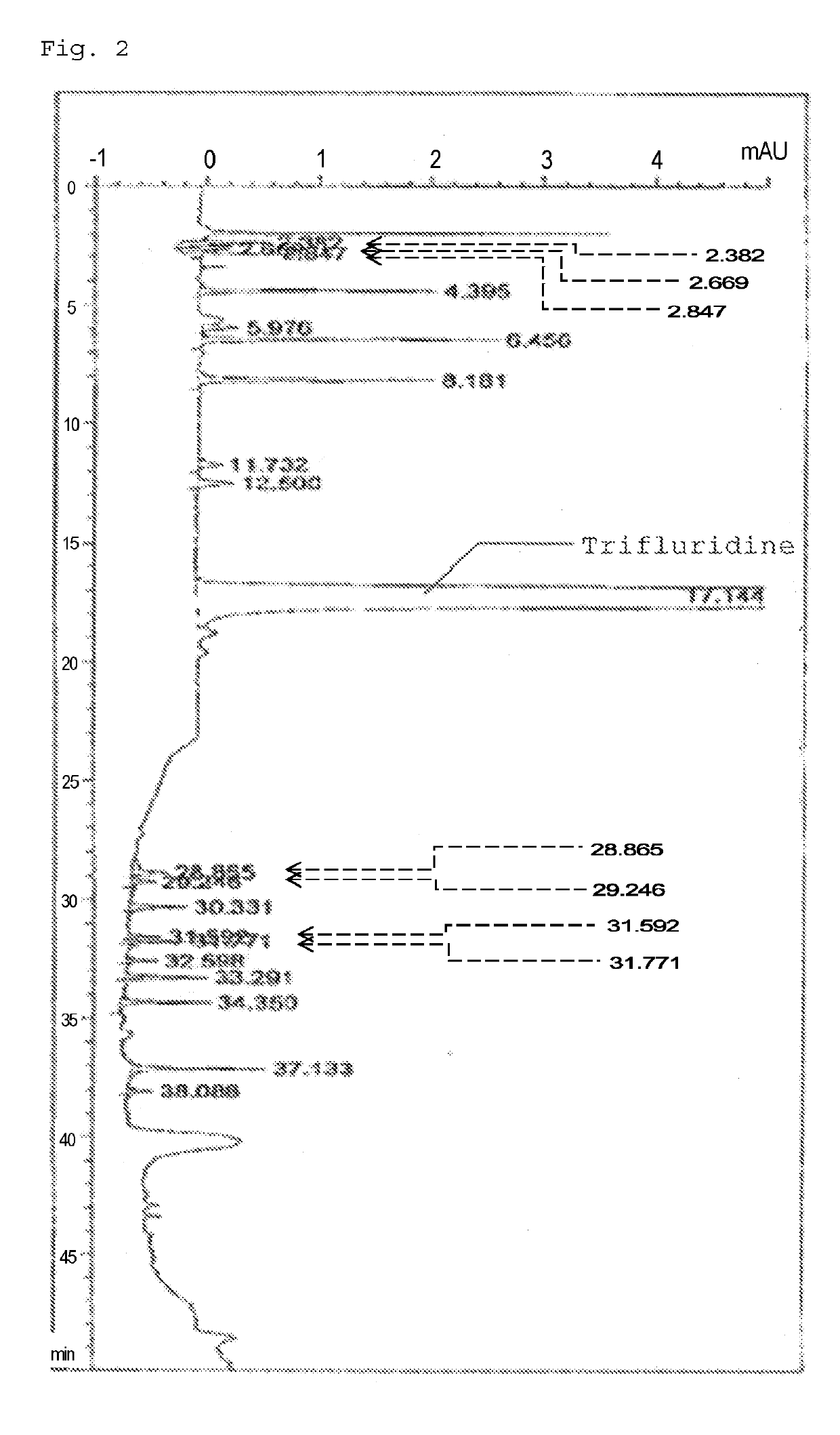
Method for detecting trifluridine-related substance by high-performance liquid chromatography
ActiveUS20190369063A1Efficient detectionQuality assuranceSugar derivativesComponent separationElutionTwo step
The present invention provides a novel method that is capable of detecting a trifluridine-related substance from a sample containing trifluridine or a salt thereof by high-performance liquid chromatography comprising two steps that are performed under gradient conditions. More specifically, the method is for detecting a trifluridine-related substance, the method comprising the step of subjecting a sample containing trifluridine or a salt thereof to high-performance liquid chromatography using a mobile phase composed of an organic phase and an aqueous phase, wherein the step of high-performance liquid chromatography comprises steps 1 and 2 that satisfy the following requirements: Step 1: the percentage of the organic phase in the entire mobile phase is 1 to 14% by volume; and Step 2: after step 1, elution is performed by applying a gradient of increasing the percentage of the organic phase in the entire mobile phase.
Owner:TAIHO PHARMA CO LTD
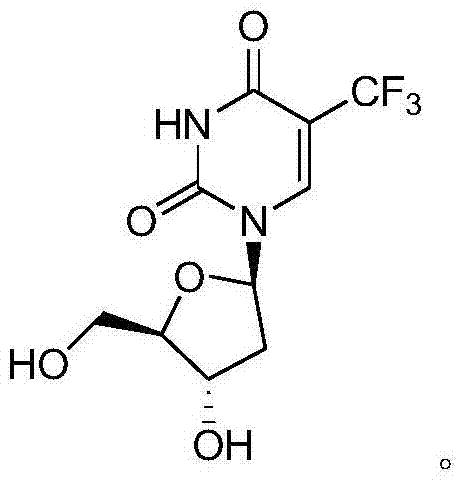
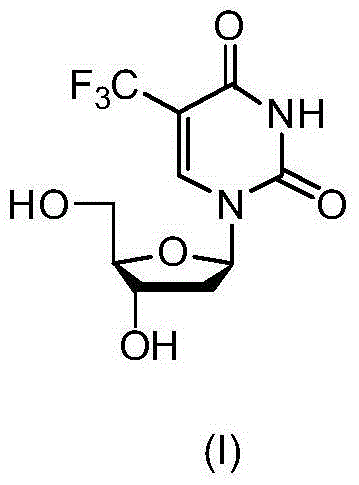
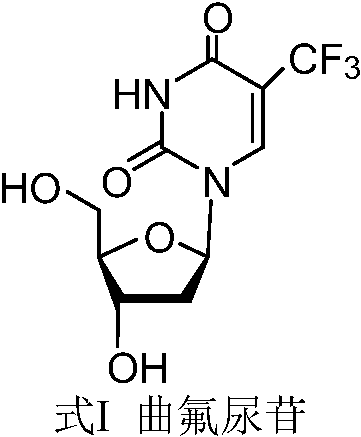
New crystal form of trifluridine, and preparation method thereof
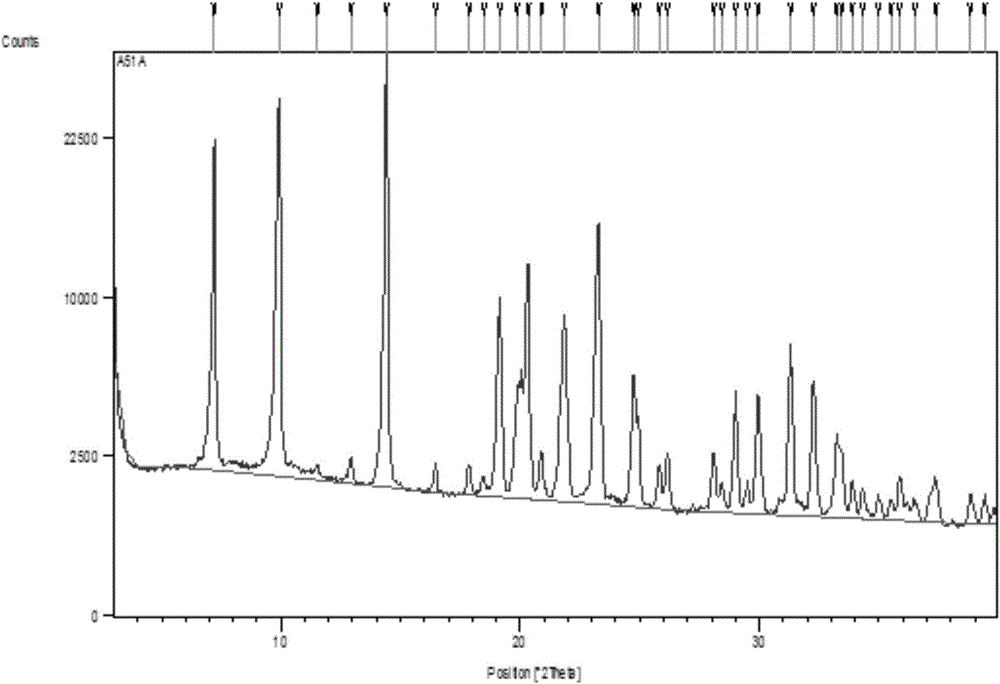
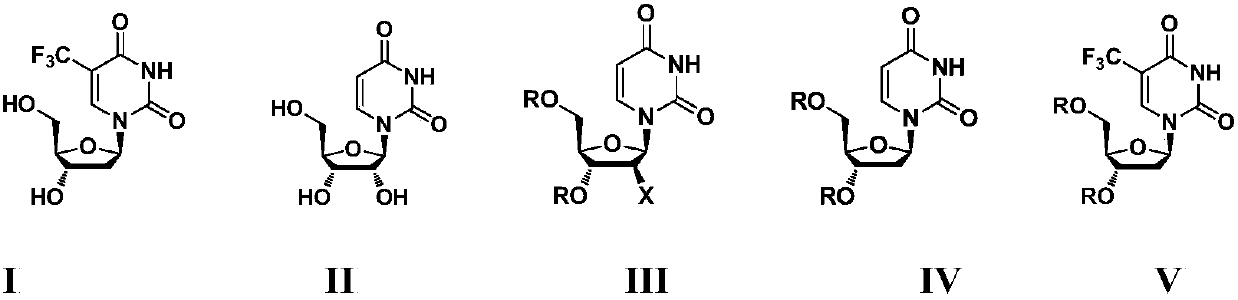
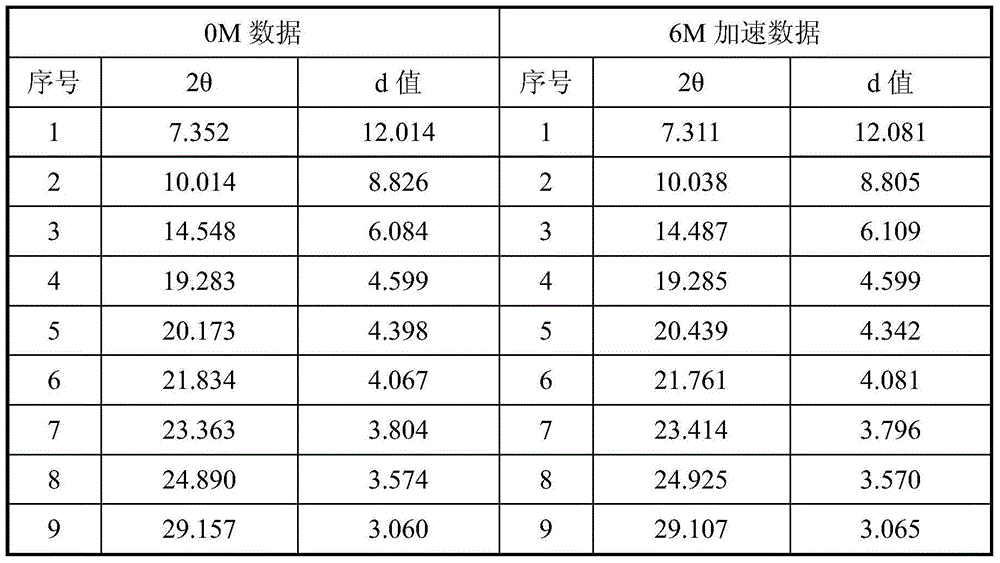
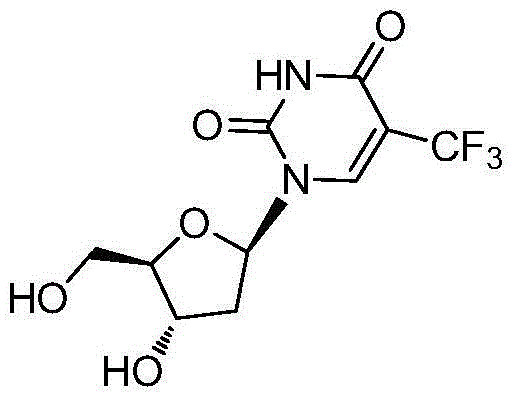
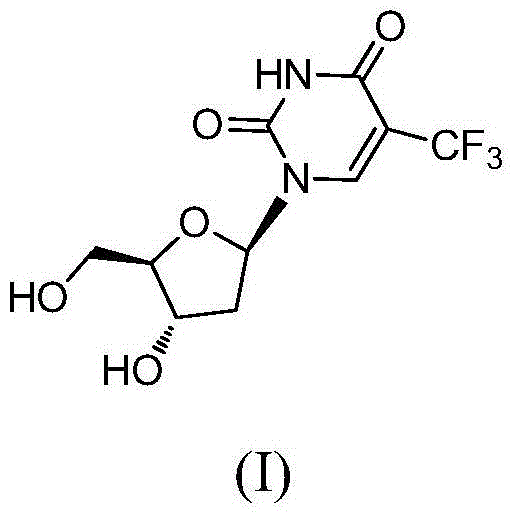
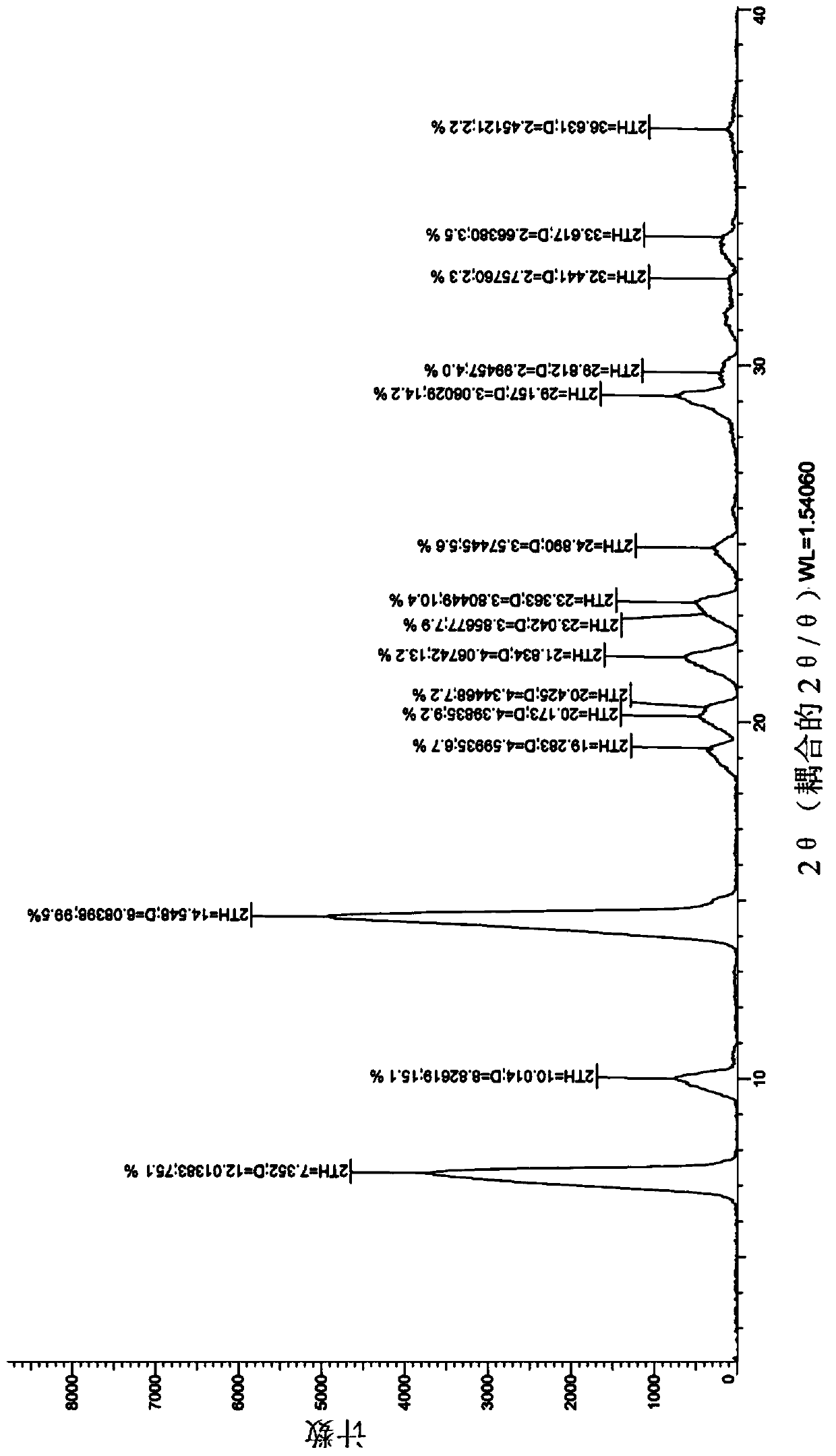
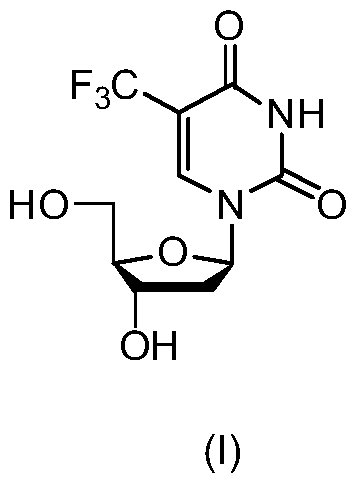
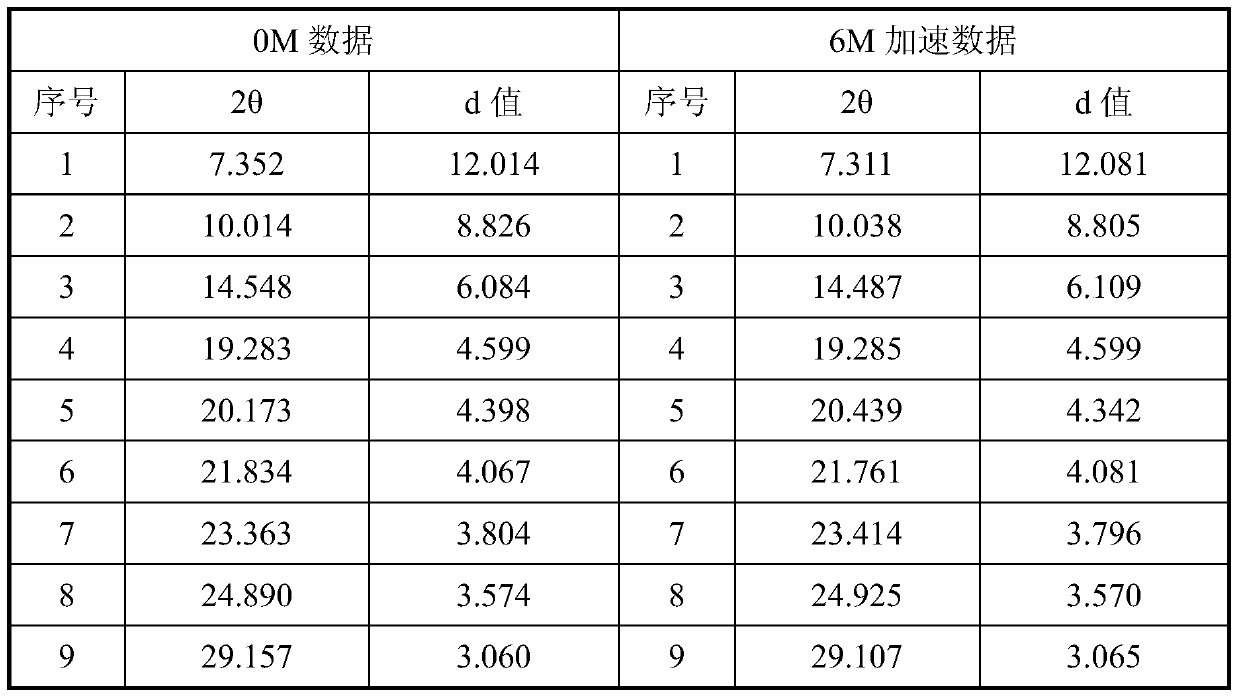
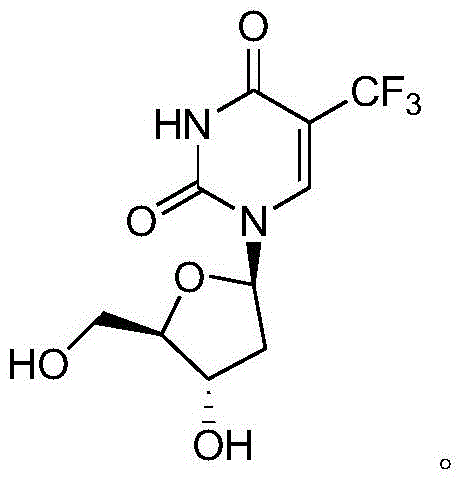
The present invention discloses a new crystal form of trifluridine, and a preparation method thereof, and particularly relates to a new 5-trifluoromethyl-2'-deoxyuridine crystal form represented by a formula (I), and a preparation method thereof, wherein the characteristic peaks of the crystal form in the X-ray powder diffraction spectrum are positioned at (a) 7.3 DEG, (b) 10.0 DEG, (c) 14.5 DEG and (d) 23.4 DEG C by adopting 2[theta] (2[theta]+ / -0.2 DEG) to represent. According to the present invention, the crystal form has characteristics of stable characteristics and good repeatability, and is suitable for drug development. The formula I is defined in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD
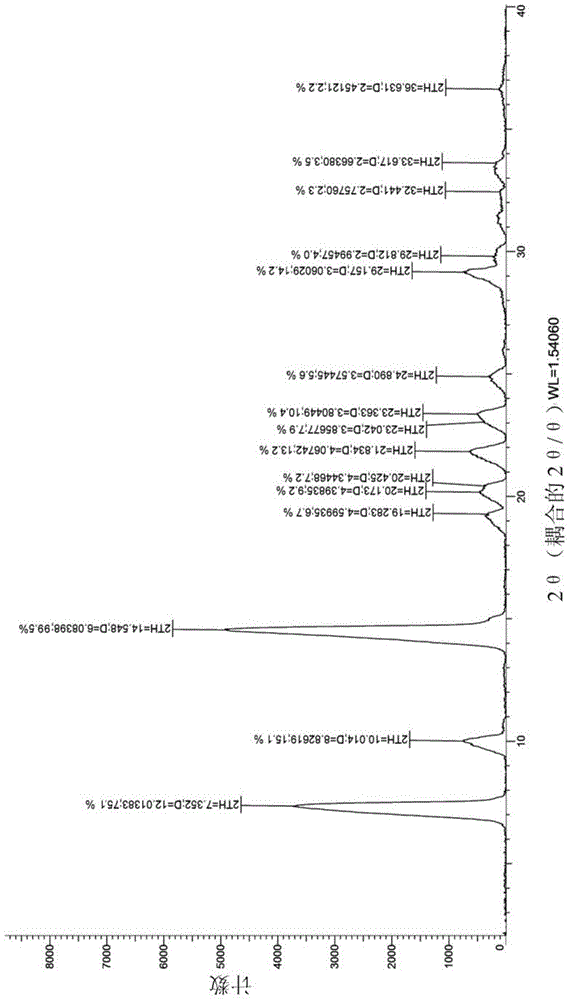
Novel trifluridine crystal form and preparation method thereof
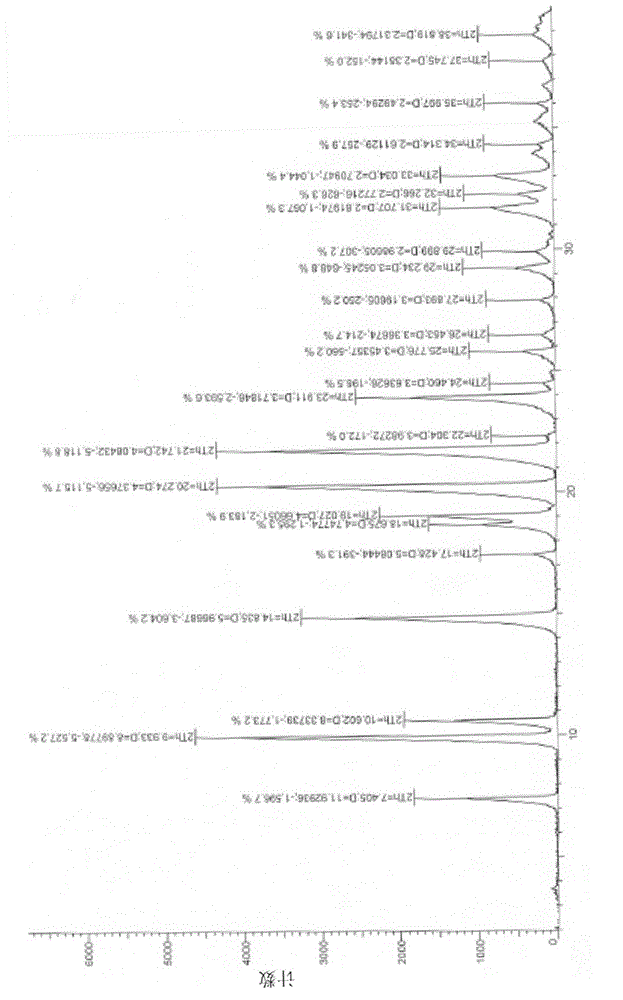
InactiveCN105198946AMeet application needsThe crystal form is stableSugar derivativesSugar derivatives preparationX-rayProtic solvent
The invention relates to a novel trifluridine crystal form and a preparation method thereof. The trifluridine crystal form II shown in the formula (I) is characterized in that a CuKa ray is used as the feature X-ray for powder determination, and characteristic peaks are shown when the 2Q diffraction angle is located at the following positions such as 7.405, 9.933, 10.602, 14.865, 17.428, 18.675, 19.027, 20.274 and 23.911. The preparation method of the trifluridine crystal form II shown in the formula (II) comprises the step that trifluridine is crystallized in a supersaturated solution with protic solvent or aprotic solvent at the temperature of 0-30 DEG C, so that the crystal form II is obtained. The property of the crystal form is stable, and the crystal form meets the development requirement for pharmaceutical preparations (please see the specification for the formula).
Owner:JIANGSU HANSOH PHARMA CO LTD
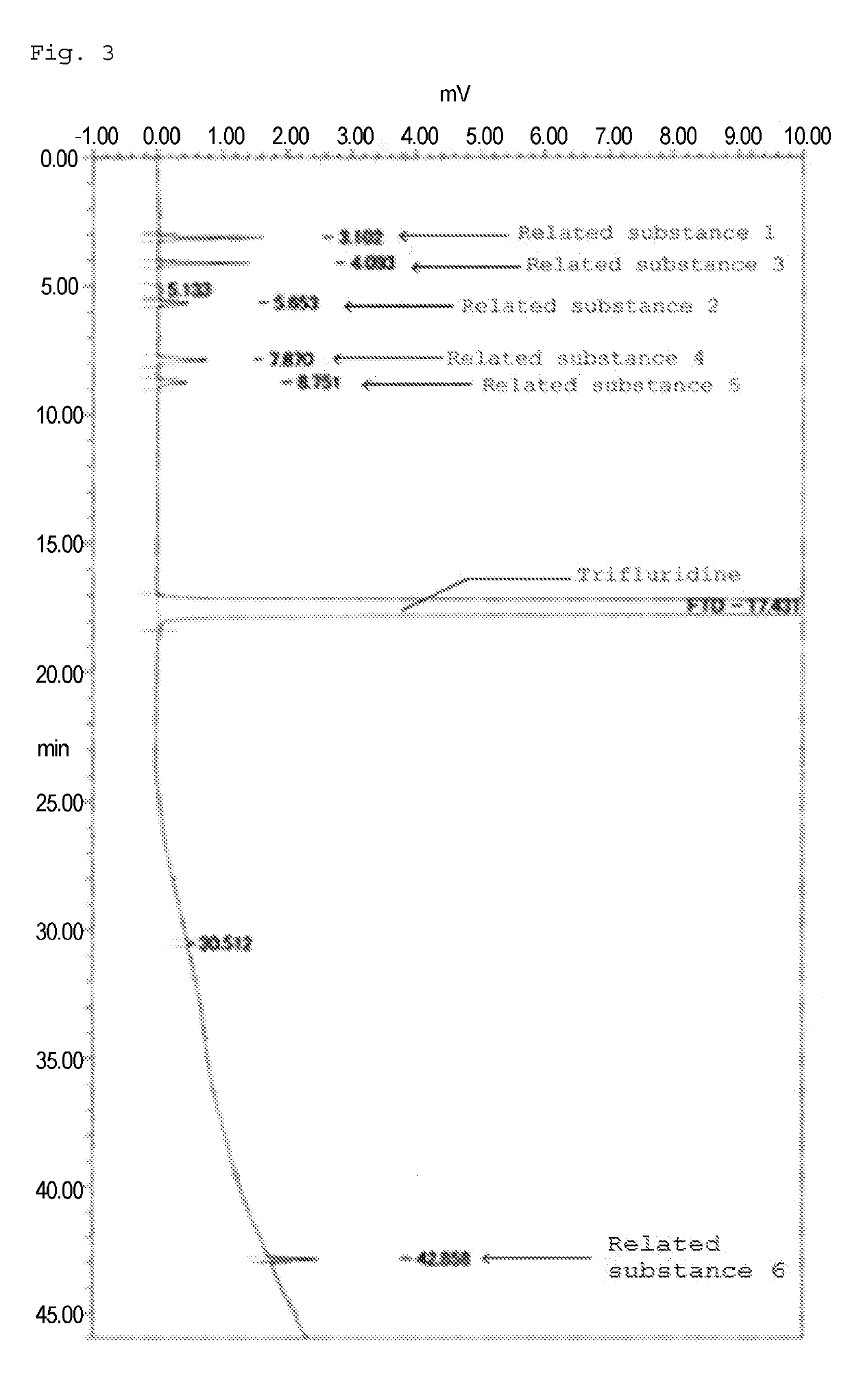
METHOD FOR DETECTING similar SUBSTANCE derived from TRIFLURIDINE
The present invention relates to a method for detecting a similar substance derived from trefluuridine. The present invention provides a novel method that is capable of detecting a trifluridine-related substance from a sample containing trifluridine or a salt thereof by high-performance liquid chromatography comprising two steps that are performed under gradient conditions. The solution of the invention is the method for detecting the similar substance derived from the trefluuridine, and the method comprises the step of subjecting a sample containing trifluridine or a salt thereof to high-performance liquid chromatography using a mobile phase composed of an organic phase and an aqueous phase, wherein the step of high-performance liquid chromatography comprises steps 1 and 2 that satisfy the following requirements: the step 1: the percentage of the organic phase in the entire mobile phase is 1 to 14% by volume; and the step 2: after step 1, a gradient is set to increase the percentage of the organic phase relative to the entire mobile phase.
Owner:TAIHO PHARMA CO LTD
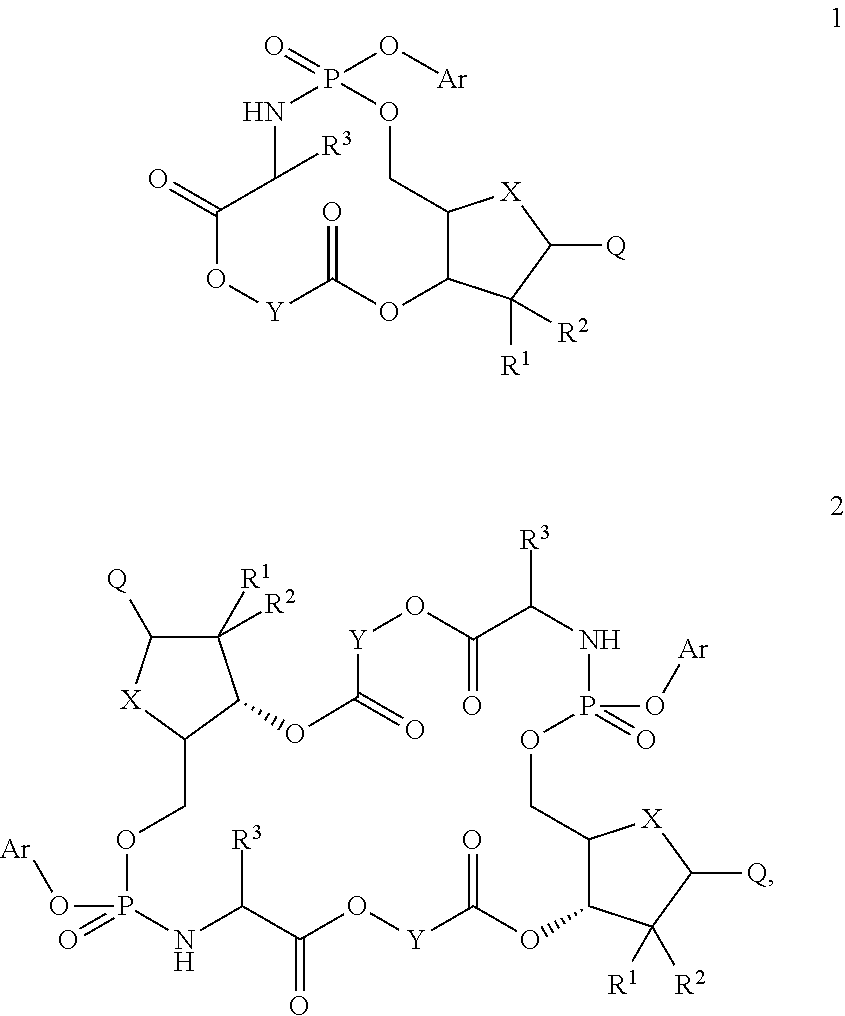
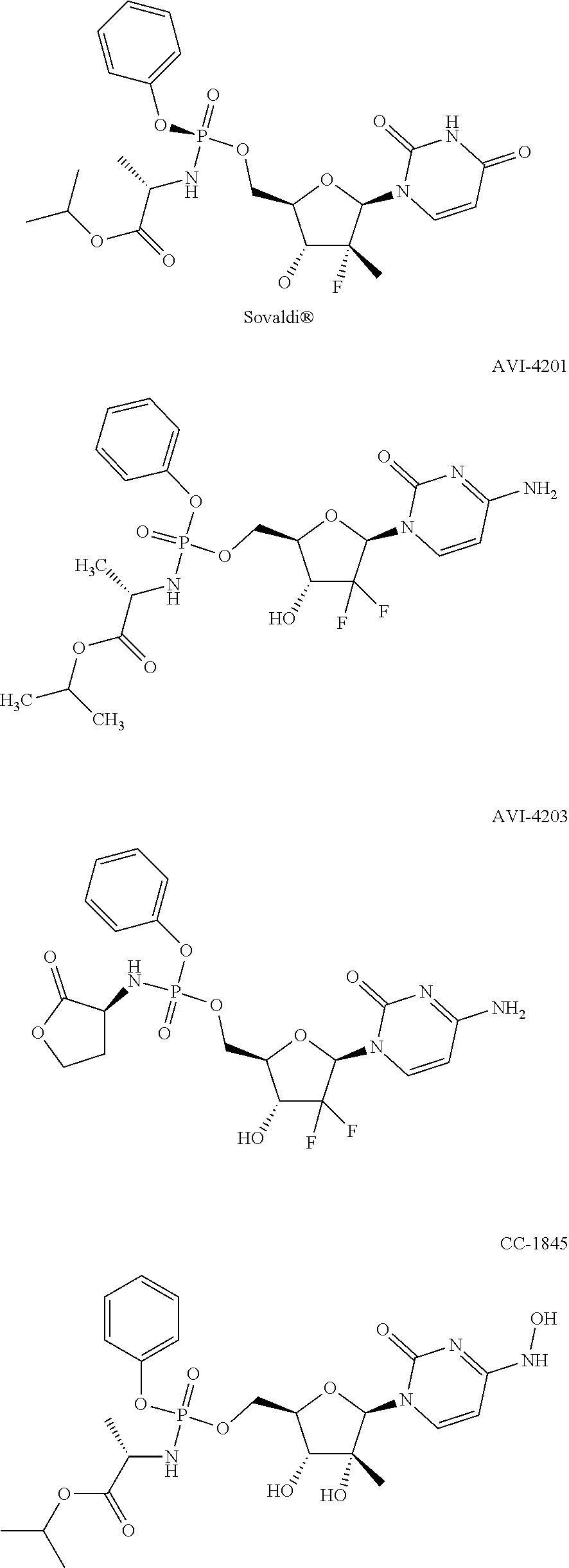
Macroheterocyclic nucleoside derivatives and their analogues, production and use thereof
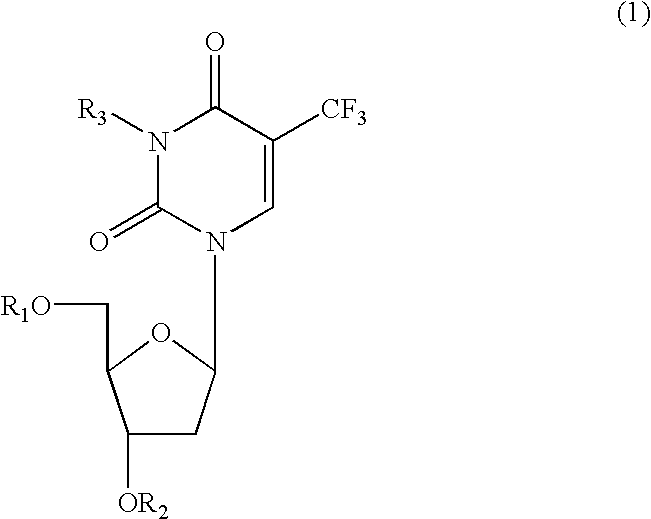
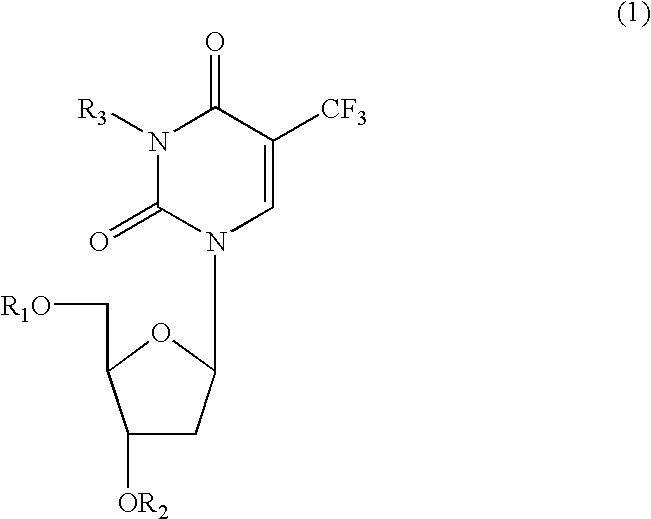
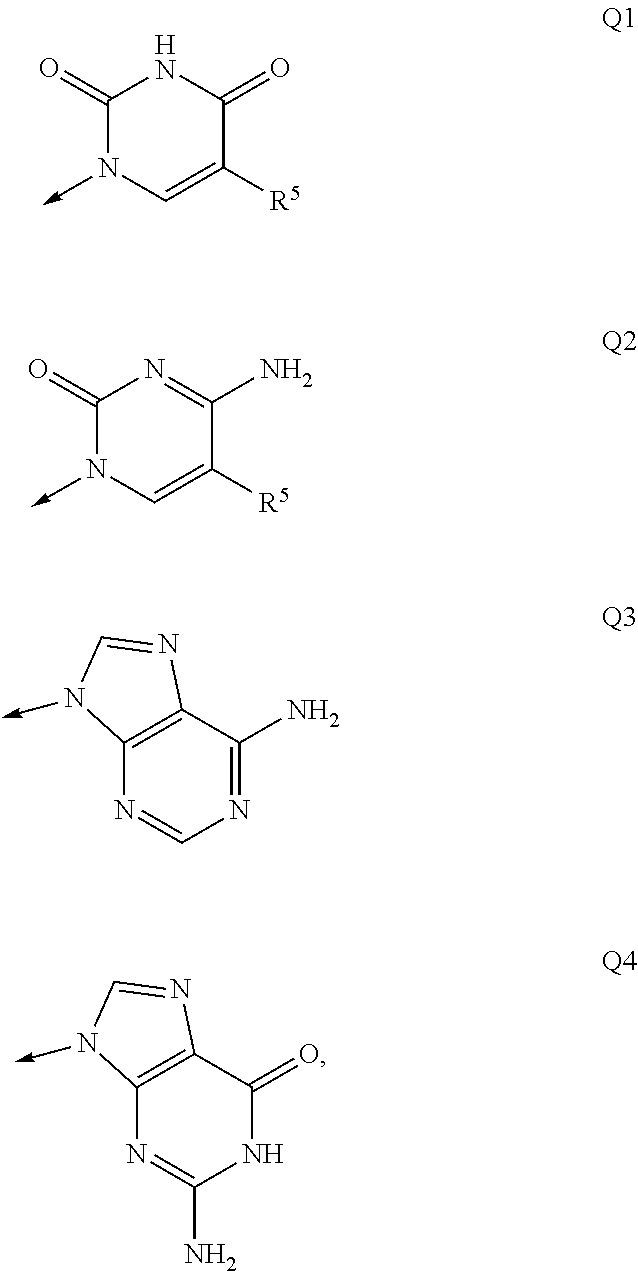
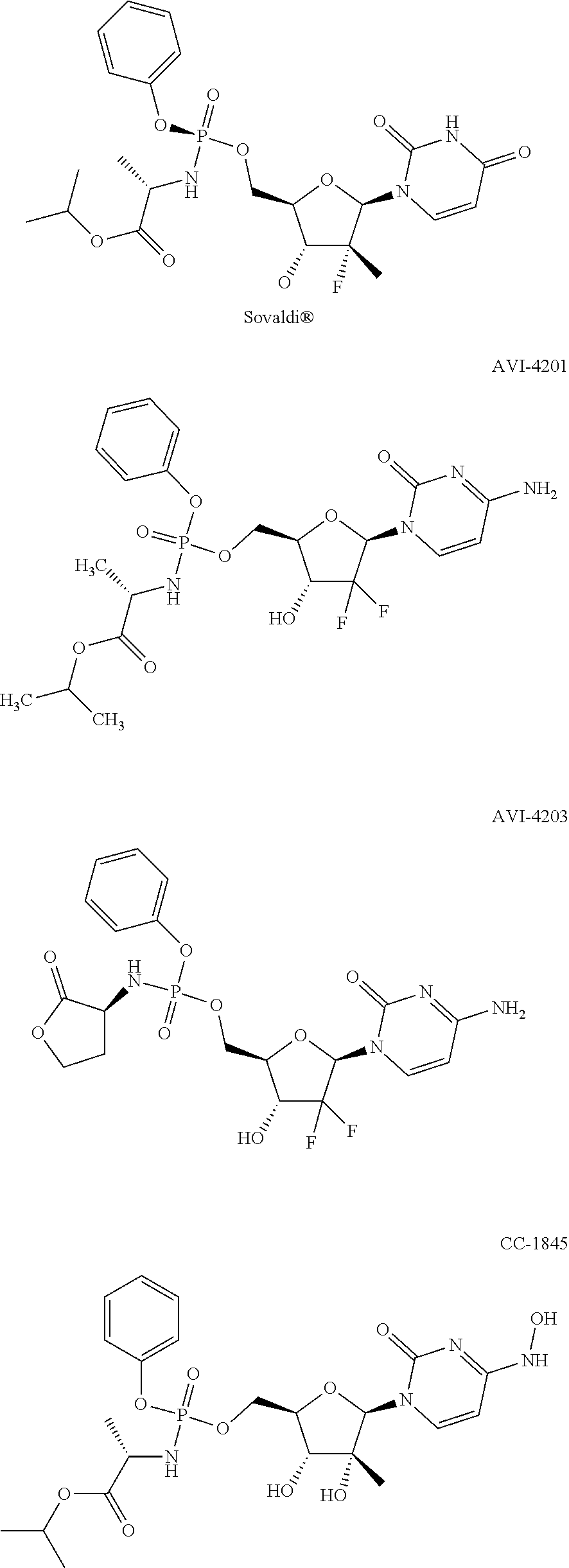
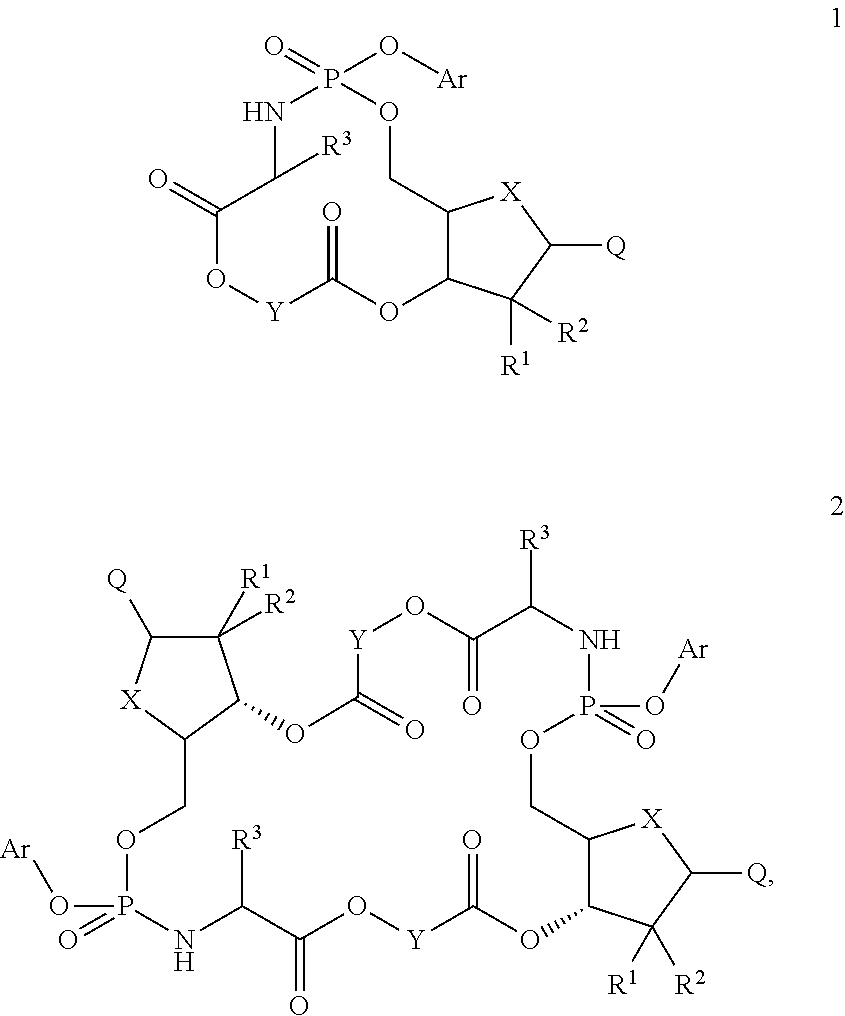
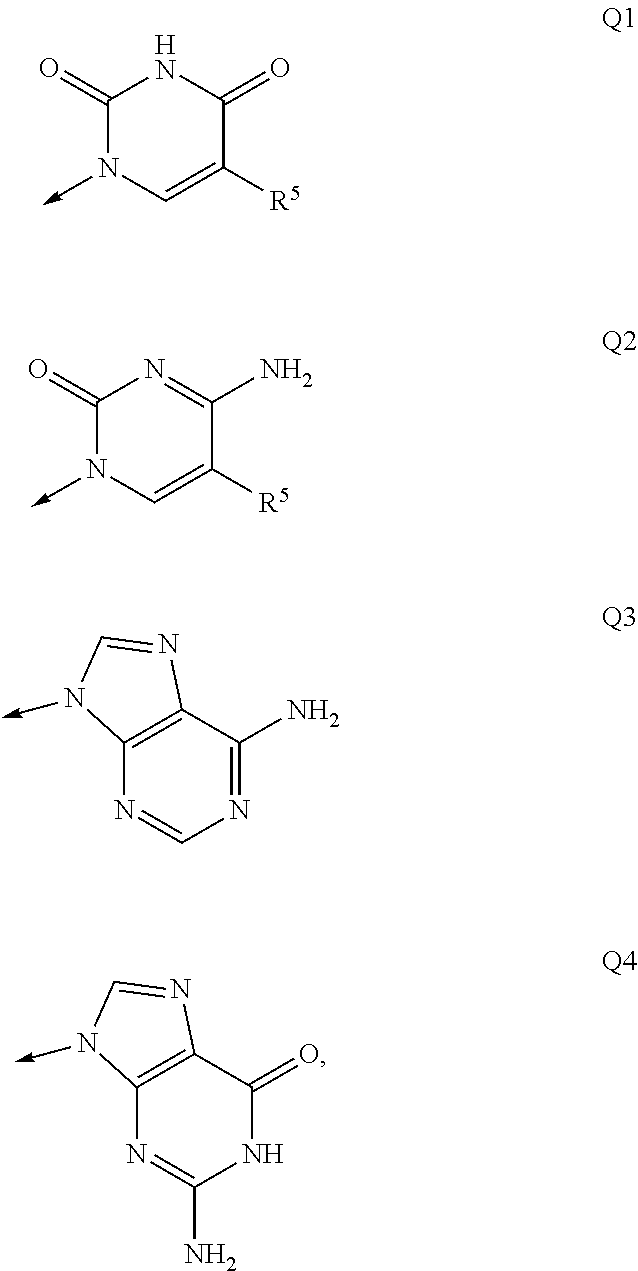
Nucleosides and nucleotides (nucleos(t)ides) have been in clinical use for almost 50 years and have become cornerstones of treatment for patients with viral infections or cancer. The approval of several additional drugs over the past decade demonstrates that this family still possesses strong potential. Therefore nucleos(t)ide are of great interest as promising chemotherapeutic agents, including: 2′-deoxy-L-uridine (CAS 31501-19-6), 2′-deoxy-D-uridine (CAS 951-78-0), telbivudine (CAS 3424-98-4), zidovudine (AZT, CAS 30516-87-1), trifluridine (CAS 70-00-8), clevudine (CAS 163252-36-6), PSI-6206 (CAS 863329-66-2), 2′-(S)-2′-chloro-2′-deoxy-2′-fluorouridine (CAS 1673560-41-2), ND06954 (CAS 114248-23-6), stavudine (CAS 3056-17-5), 5-ethynyltavudine (Festinavir, CAS 634907-30-5), torcitabine (CAS 40093-94-5), (−)-beta-D-(2R,4R)-dioxolane-thymine (DOT, 1-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-5-methyl-2,4(1H,3H)-pyrimidinedione, CAS No. 127658-07-5), 2-(6-amino-purin-9-yl)-ethanol (CAS 707-99-3), 2′-C-methylcytidine (CAS 20724-73-6), PSI-6130 (CAS 817204-33-4), gemcitabine (CAS 95058-81-4), 2′-chloro-2′-deoxy-2′-fluorocytidine (CAS 1786426-19-4), 2′,2′-dichloro-2′-deoxycytidine (CAS 1703785-65-2), 2′-C-methylcytidine (CAS 20724-73-6), PSI-6130 (CAS 817204-33-4), lamivudine (3TC, CAS 134678-17-4), emtricitabine (CAS 143491-57-0), 2′-deoxyadenosine (CAS 958-09-8), 2′-deoxy-β-L-adenosine (CAS 14365-45-8), 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine (CAS 865363-93-5), didanosine (CAS 69655-05-6), entecavir (CAS 209216-23-9), FMCA (CAS 1307273-70-6), dioxolane-G (DOG, CAS 145514-01-8), β-D-2′-deoxy-2′-(R)-fluoro-2′-β-C-methylguanosine (CAS No 817204-45-8), abacavir (ABC, CAS 136470-78-5), dioxolane-A (DOA, CAS #145514-02-9), [(2R,4R)-4-(6-cyclopropylamino-purin-9-yl)-[1,3]dioxolan-2-yl]-methanol (CAS 1446751-04-7), amdoxovir (AMDX, CAS 145514-04-1), (R)-1-(6-amino-purin-9-yl)-propan-2-ol (CAS 14047-28-0), and [(2S,5R)-5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yl]-methanol.Macroheterocyclic nucleoside derivative and its analog of the general formula 1 or general formula 2, a stereoisomer, isotope-enriched analog, pharmaceutically acceptable salt, hydrate, solvate, or crystalline or polymorphic form thereof,wherein:Ar is aryl or hetaryl;R1 and R2 are not necessarily the same substituents selected from H, F, Cl, CH3, OH;R3 is H or CH3;X is oxygen or ethanediyl-1,1 (C═CH2);Y is CH(R4)(CH2)k, CH(R4)(CH2)mC(O)O(CH2)n;R4 is H or CH3;k has a value from zero to six;m has a value from zero to two;n has a value of one to four;Q is a radical selected from Q1-Q4;wherein: R5 is the substituent selected from H, F, Cl, CH3, OH;the arrow (→) indicates the location, joined by Q1-Q4.
Owner:ALLA CHEM LLC
Macroheterocyclic nucleoside derivatives and their analogues, production and use thereof
InactiveUS20180099989A1Reduce absorptionOrganic active ingredientsSugar derivativesEmtricitabinePharmaceutical medicine
Nucleosides and nucleotides (nucleos(t)ides) have been in clinical use for almost 50 years and have become cornerstones of treatment for patients with viral infections or cancer. The approval of several additional drugs over the past decade demonstrates that this family still possesses strong potential. Therefore nucleos(t)ide are of great interest as promising chemotherapeutic agents, including: 2′-deoxy-L-uridine (CAS No 31501-19-6), 2′-deoxy-D-uridine (CAS No 951-78-0), telbivudine (CAS No 3424-98-4), zidovudine (AZT, CAS No 30516-87-1), trifluridine (CAS No 70-00-8), clevudine (CAS No 163252-36-6), PSI-6206 (CAS No 863329-66-2), 2′-(5)-2′-chloro-2′-deoxy-2′-fluorouridine (CAS No 1673560-41-2), ND06954 (CAS No 114248-23-6), stavudine (CAS No 3056-17-5), 5-ethynyltavudine (Festinavir, CAS No 634907-30-5), torcitabine (CAS No 40093-94-5), (−)-beta-D-(2R,4R)-dioxolane-thymine (DOT, 1-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-5-methyl-2,4 (1H,3H)-pyrimidinedione, CAS No. 127658-07-5), 2-(6-amino-purin-9-yl)-ethanol (CAS No 707-99-3), 2′-C-methylcytidine (CAS No 20724-73-6), PSI-6130 (CAS No 817204-33-4), gemcitabine (CAS No 95058-81-4), 2′-chloro-2′-deoxy-2′-fluorocytidine (CAS No 1786426-19-4), 2′,2′-dichloro-2′-deoxycytidine (CAS No 1703785-65-2), 2′-C-methylcytidine (CAS No 20724-73-6), PSI-6130 (CAS No 817204-33-4), lamivudine (3TC, CAS No 134678-17-4), emtricitabine (CAS No 143491-57-0), 2′-deoxyadenosine (CAS No 958-09-8), 2′-deoxy-β-L-adenosine (CAS No 14365-45-8), 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine (CAS No 865363-93-5), didanosine (CAS No 69655-05-6), entecavir (CAS No 209216-23-9), FMCA (CAS No 1307273-70-6), dioxolane-G (DOG, CAS No 145514-01-8), β-D-2′-deoxy-2′-(R)-fluoro-2′-β-C-methylguanosine (CAS No 817204-45-8), abacavir (ABC, CAS No 136470-78-5), dioxolane-A (DOA, CAS #145514-02-9), [(2R,4R)-4-(6-cyclopropylamino-purin-9-yl)-[1,3]dioxolan-2-yl]-methanol (CAS No 1446751-04-7), amdoxovir (AMDX, CAS No 145514-04-1), (R)-1-(6-amino-purin-9-yl)-propan-2-ol (CAS No 14047-28-0), and [(2S,5R)-5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yl]-methanol.Macroheterocyclic nucleoside derivative and its analogue of the general formula 1 or general formula 2, a stereoisomer, isotope-enriched analogue, pharmaceutically acceptable salt, hydrate, solvate, or crystalline or polymorphic form thereof,wherein:Ar is aryl or hetaryl;R1 and R2 are not necessarily the same substituents selected from H, F, Cl, CH3, OH;R3 is H or CH3;X is oxygen or ethanediyl-1,1 (C═CH2);Y is CH(R4)(CH2)k, CH(R4)(CH2)mC(O)O(CH2)n;R4 is H or CH3;k has a value from zero to six;m has a value from zero to two;n has a value of one to four;Q is a radical selected from Q1-Q4;wherein: R5 is the substituent selected from H, F, Cl, CH3, OH;the arrow (→) indicates the location, joined by Q1-Q4.
Owner:ALLA CHEM LLC
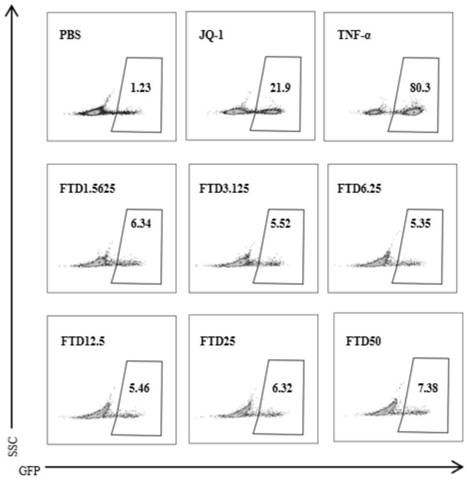
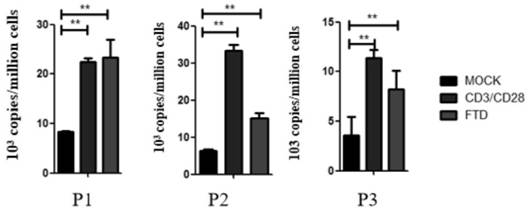
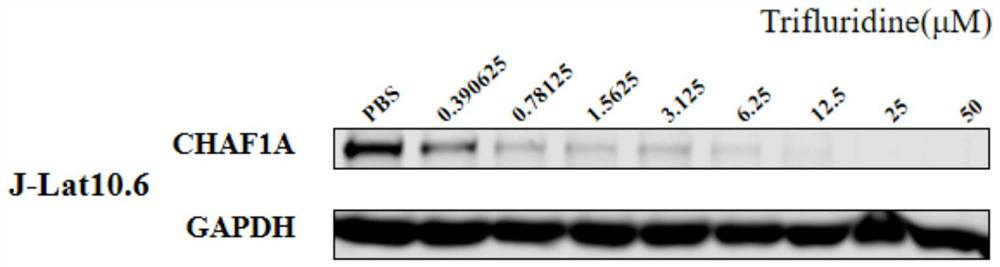
Application of trifluridine in preparation of HIV-1 drug
InactiveCN112569250ALow toxicityImprove securityOrganic active ingredientsAntiviralsPharmaceutical drugTrifluridine
The invention discloses an application of trifluridine in preparation of an HIV-1 drug, and provides a new application of trifluridine as an HIV-1 latent infection activator, and the trifluridine hasa very good activation effect on HIV-1 latent infection. As trifluridine is a clinically used drug, trifluridine is low in toxicity and good in safety, but the related application of trifluridine serving as an HIV-1 latent infection activator is not reported yet at present. Trifluridine has important research and development value and development significance in the aspect of activation of HIV-1 latent infection.
Owner:SUN YAT SEN UNIV
New crystal form of trifluridine and preparation method thereof
The present invention discloses a new crystal form of trifluridine, and a preparation method thereof, and particularly relates to a new 5-trifluoromethyl-2'-deoxyuridine crystal form represented by a formula (I), and a preparation method thereof, wherein the characteristic peaks of the crystal form in the X-ray powder diffraction spectrum are positioned at (a) 7.3 DEG, (b) 10.0 DEG, (c) 14.5 DEG and (d) 23.4 DEG C by adopting 2[theta] (2[theta]+ / -0.2 DEG) to represent. According to the present invention, the crystal form has characteristics of stable characteristics and good repeatability, and is suitable for drug development. The formula I is defined in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD
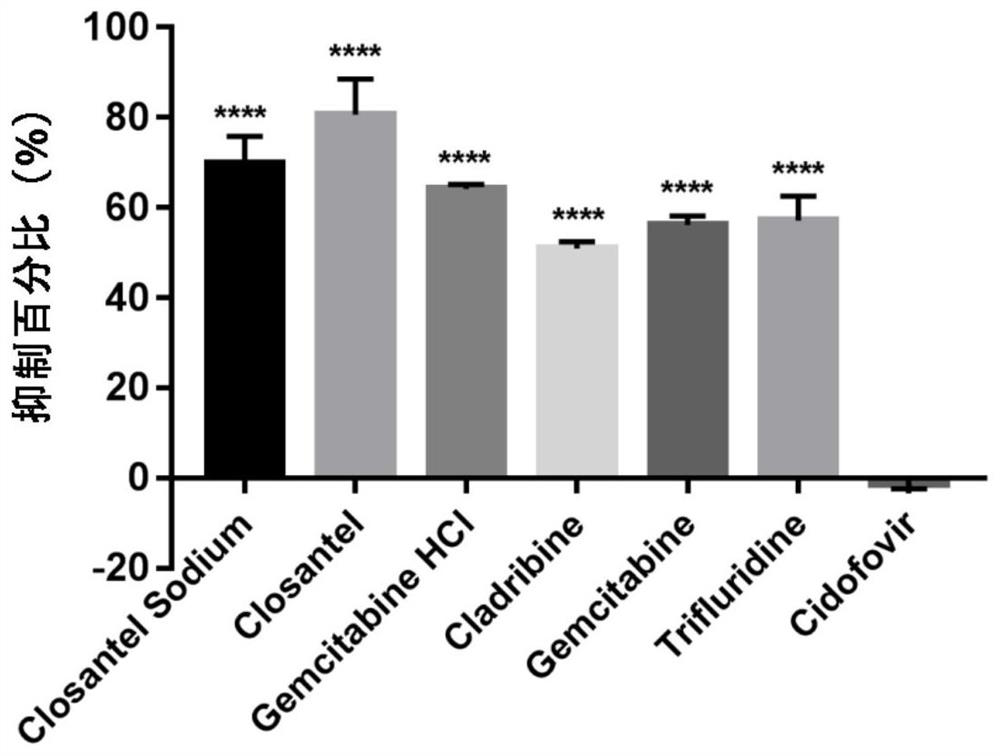
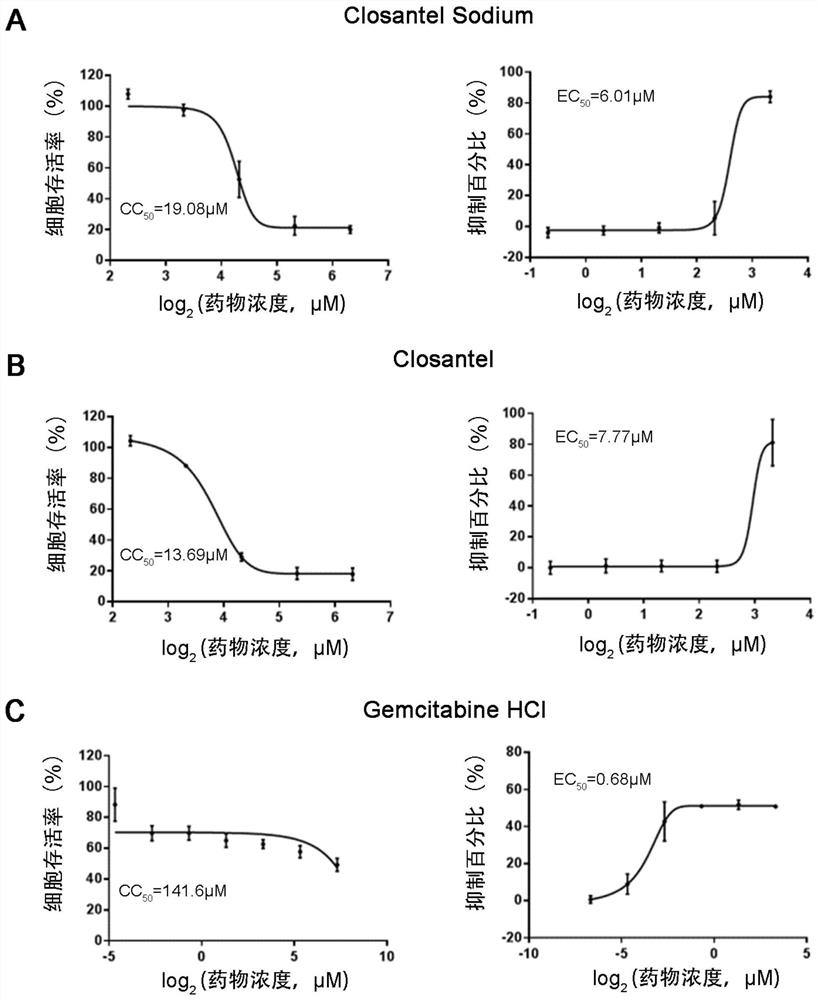
Application of 6 Small Molecule Drugs in Inhibiting Canine Parvovirus
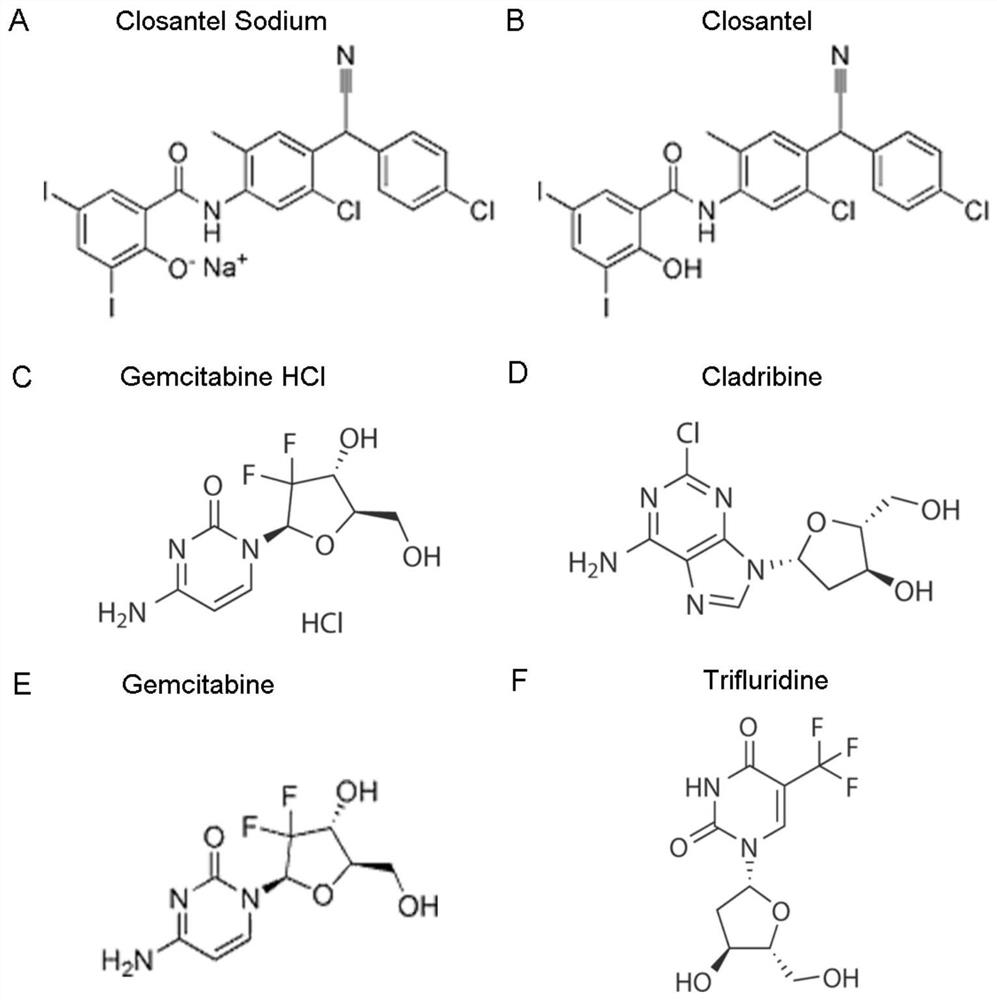
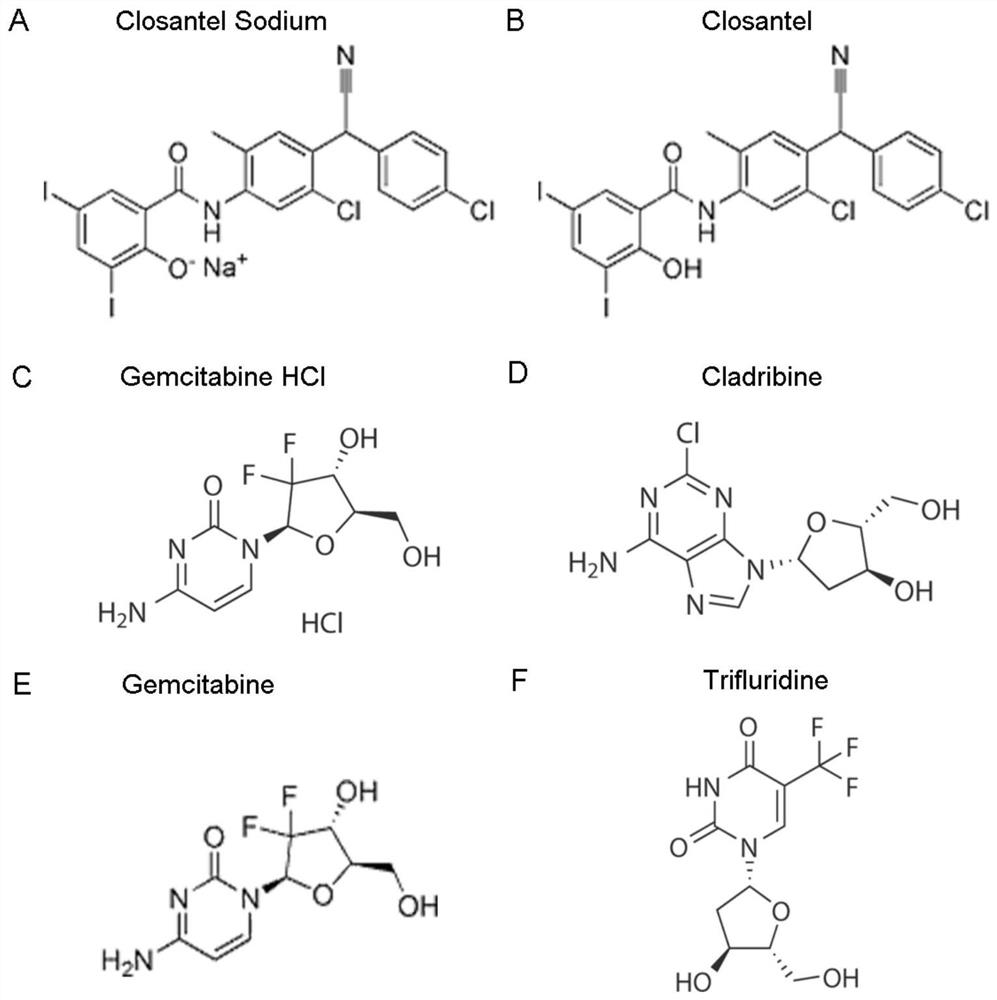
ActiveCN111840308BAntiviralsNitrile/isonitrile active ingredientsCLOSANTEL SODIUMPharmaceutical drug
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
A kind of preparation method of trifluridine intermediate and trifluridine
ActiveCN105461772BSolve pollutionProduct quality is easy to controlSugar derivativesSugar derivatives preparationLewis acid catalysisRibose
The invention provides a preparation method of a trifluridine intermediate. Under the action of an acidic resin catalyst, 1-chloro-2-deoxy-3,5-di-O-p-chlorobenzoyl-D-ribose and 5-trifluoromethyl-2,4-bis(trimethylsilaneoxy)pyrimidine undergo a condensation reaction so as to obtain the trifluridine intermediate. According to the invention, a heterogeneous catalysis technology is utilized, acidic resin is used as a catalyst, and a traditional Lewis acid catalyst is replaced. Under the precondition of guaranteeing that product quality is controllable, a production technology is greatly improved. Catalytic efficiency is high, and conditions are mild. Purity of the prepared 1-(2'-deoxy-3,5-di-O-p-chlorobenzoyl-beta-D-furanose)-5-trifluoromethyluracil is greatly raised, and the problem that the use of the Lewis acid catalyst leads to severe post-treatment emulsification and environmental pollution and is not beneficial to industrial production is also effectively solved.
Owner:SINOPHARM A THINK PHARMA
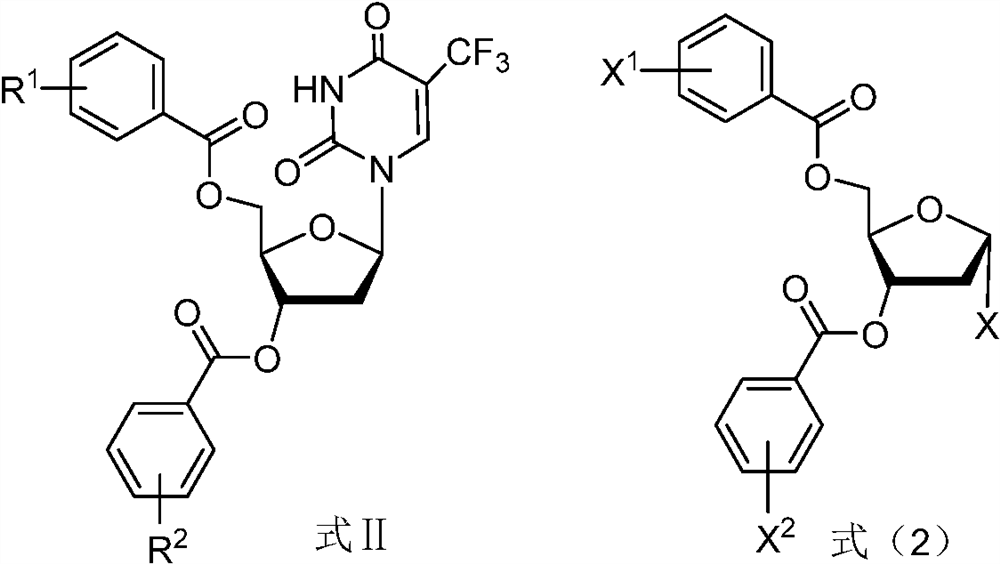
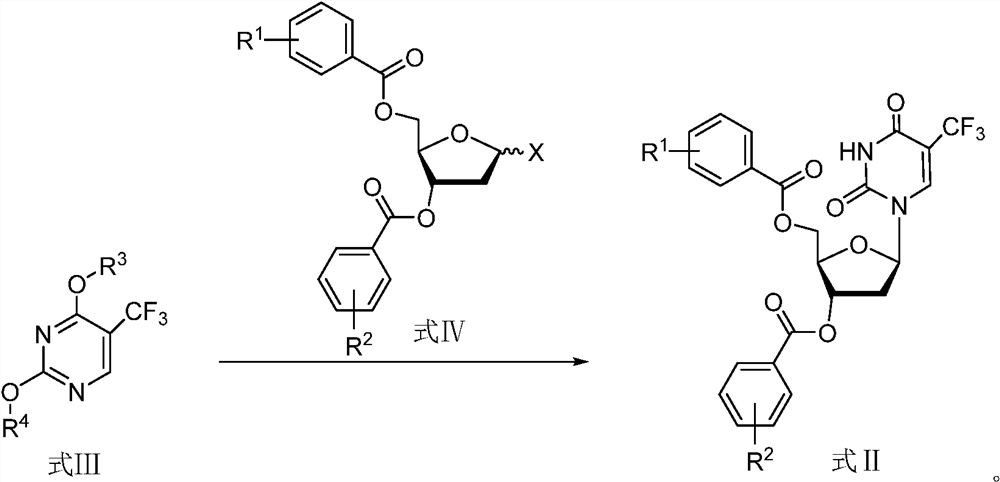
A kind of preparation method of trifluridine
ActiveCN112424211BSugar derivativesSugar derivatives preparationCombinatorial chemistryOrganic chemistry
The present application relates to a preparation method of trifluridine, comprising reacting a compound of formula III and a compound of formula IV in a first solvent in the presence of an acid to obtain a compound of formula II, and further reacting to obtain trifluridine.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
A kind of preparation method of trifluridine
ActiveCN104761602BReduce the amount of feedMild reaction conditionsSugar derivativesSugar derivatives preparationClinical efficacySolvent
The invention discloses a preparation method of trifluridine. The method comprises the specific steps of: adding 2'-deoxyuridine and trifluoromethyl sulfinate sodium to a reaction solvent, stirring and cooling to - 5 to - 3 DEG C, introducing of nitrogen for protection, stirring to dissolve, dropwise adding tert-butyl hydroperoxide, controlling the temperature at less than 5 DEG C, heating to 60-65 DEG C for reaction, and conducting posttreatment after the reaction to obtain a finished product. The method provided by the invention has mild reaction conditions, simple operation, little side reaction, short reaction time, great reduction of feeding amount of tert-butyl hydroperoxide, great saving of the production cost, and high yield and high purity of the product, and is especially applicable to industrial production, and has significance to the quality control of medicine and clinical curative effect.
Owner:SHANDONG CHENGCHUANG BLUE OCEAN PHARM TECH CO LTD
Method of administrating an anticancer drug containing α, α, α-trifluorothymidine and thymidine phosphorylase inhibitor
ActiveUSRE46284E1Improve anti-tumor activityGood treatment effectOrganic active ingredientsAntineoplastic agentsTrifluridineHydrochloride
The present invention relates to a method for treating a cancer comprising orally administering a composition containing α,α,α-trifluorothymidine (FTD) and 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl)uracil hydrochloride in a molar ratio of 1:0.5 at a dose of 20 to 80 mg / m2 / day in terms of FTD in 2 to 4 divided portions per to patients in need of the treatment.
Owner:TAIHO PHARMA CO LTD
Application of six small molecule drugs in inhibition of canine parvovirus
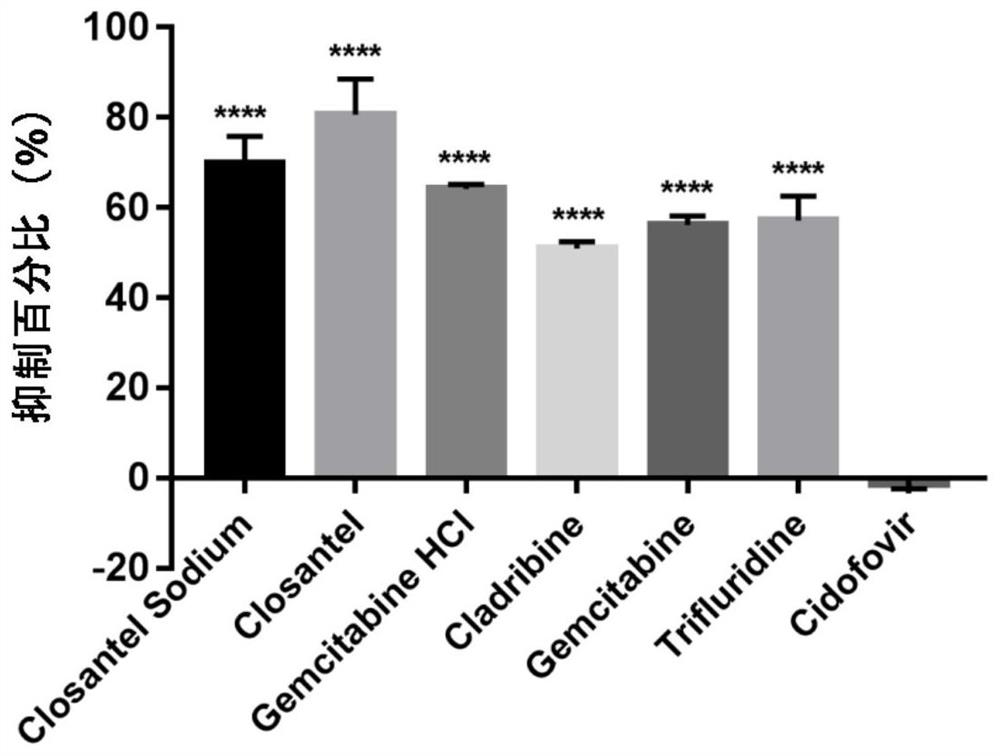
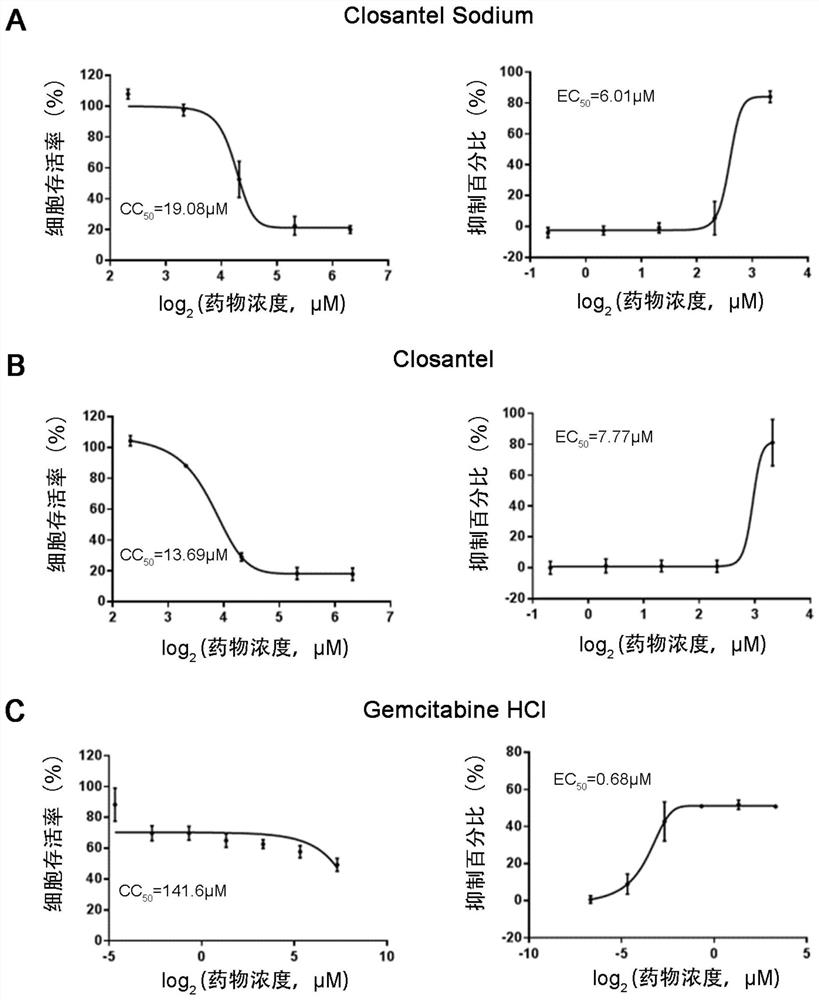
The invention relates to new application of known compounds, in particular to an application of six small molecule drugs in inhibition of canine parvovirus. The invention provides the application of closantel, Closantel Sodium, gemcitabine HCl, Trifluridine, Gemcitabine and Cladribine in preparation of drugs for inhibiting replication of canine parvovirus, t active components of the medicine comprise Closantel, Closantel Sodium, Gemcitabine HCl, Trifluridine, Gemcitabine and / or Cladribine, and the structural formula of the medicine is shown in the specification. The data prove that the six small molecule drugs have very excellent inhibition effects on CPV replication, and can inhibit expression of different genotypes of CPV virus VP2 proteins.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Trifluridine impurity compound and preparation method thereof
PendingCN114380876AHigh reaction yieldHigh puritySugar derivativesSugar derivatives preparationCarboxylic acidPharmaceutical Substances
The invention belongs to the technical field of medicine synthesis, and particularly relates to a trifluridine impurity compound and a preparation method thereof. The preparation method comprises the following steps: esterifying 1-((2S, 4S, 5R)-4-acetoxy-5-(acetoxymethyl) tetrahydrofuran-2-yl)-2, 4-dioxo-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylic acid serving as a raw material and hydroxyl at the 5-position of a glycosyl group of trifluridine under the action of Novozyme 435, and then removing a hydroxyl protecting group on the glycosyl group to prepare the trifluridine impurity compound. The synthesis method provided by the invention is simple, and the trifluridine impurity compound obtained by the method is high in purity and high in yield after recrystallization precipitation, and can be used as an impurity reference substance in a trifluridine finished product detection standard.
Owner:LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com