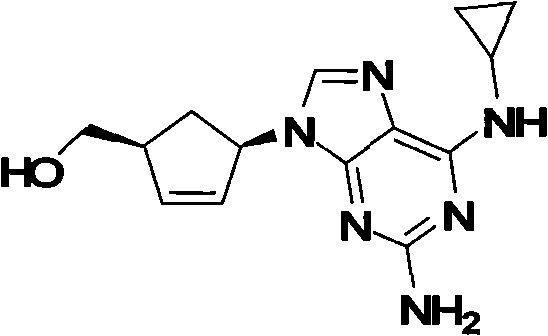
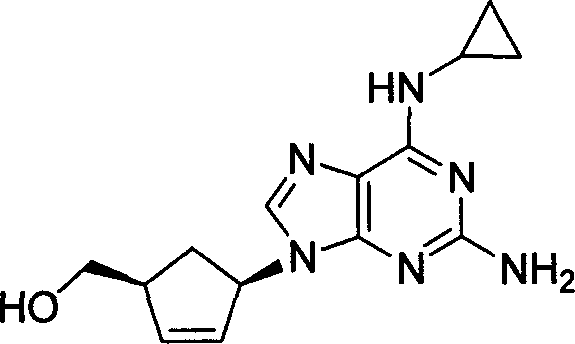
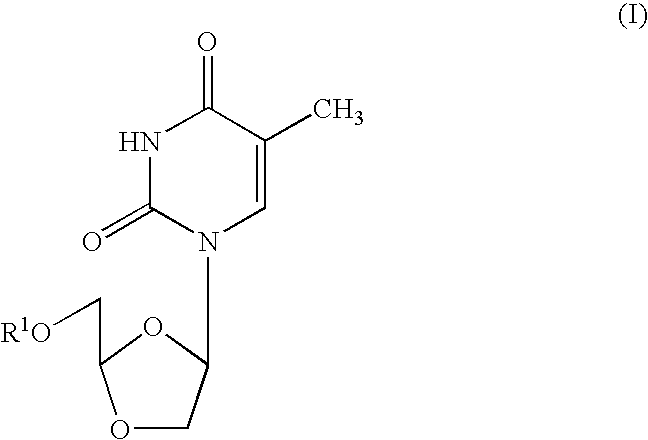
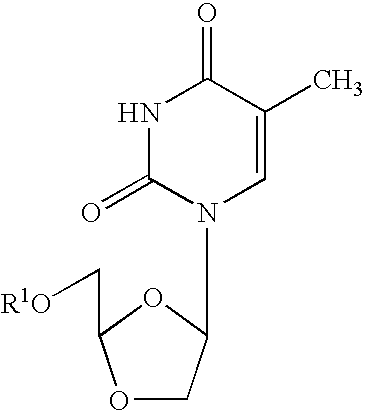
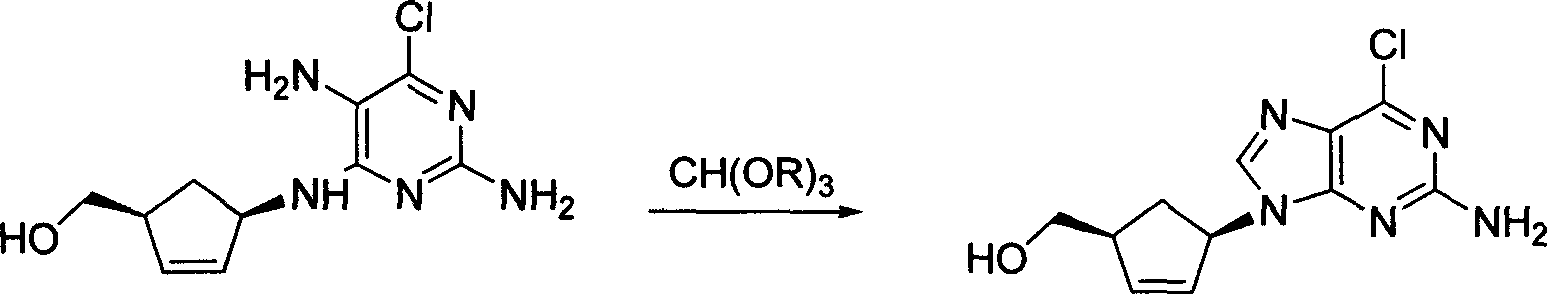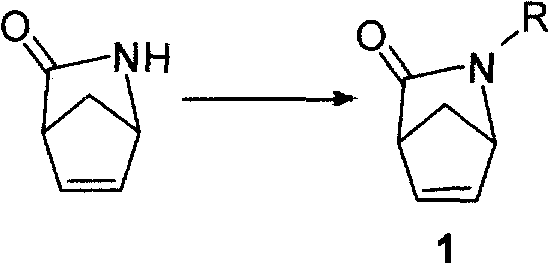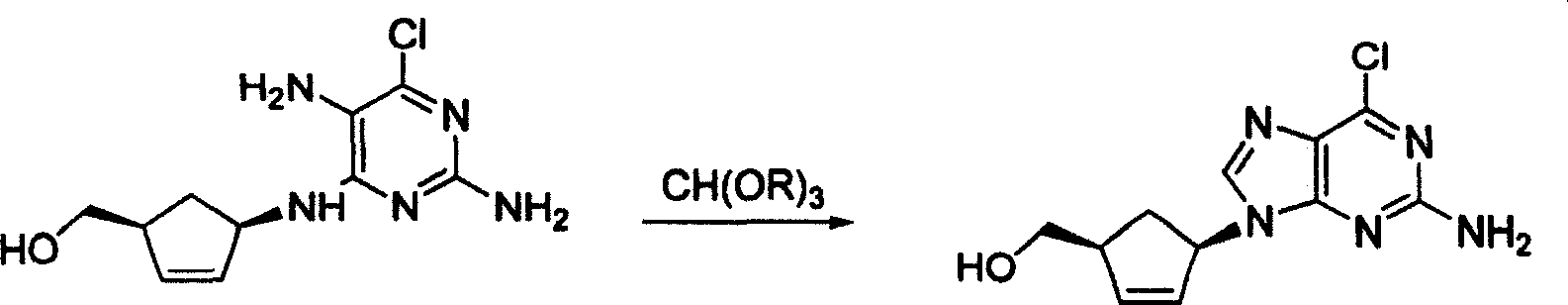Patents
Literature
40 results about "Abacavir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This drug is used with other HIV medications to help control HIV infection.
Pharmaceutical compositions comprising abacavir and lamivudine
InactiveUS20050171127A1High drug loadingMaintain good propertiesBiocideAntiviralsCyclopenteneImmunodeficiency virus
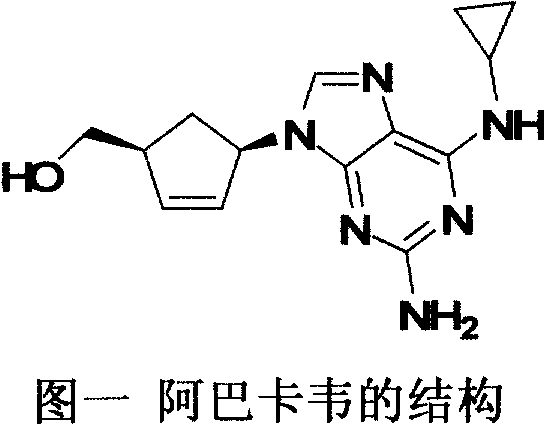
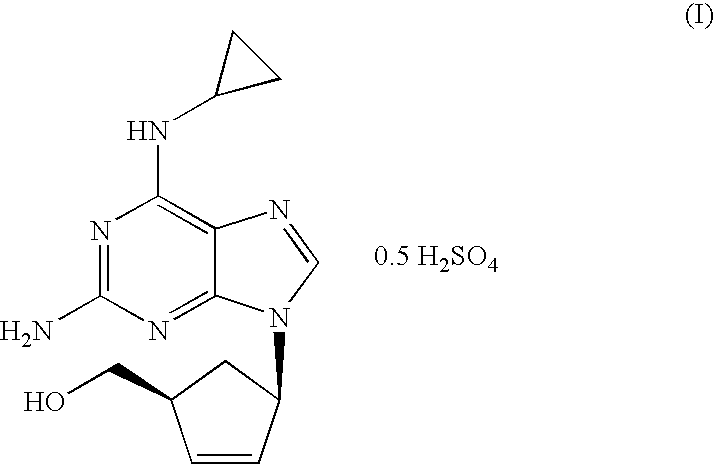
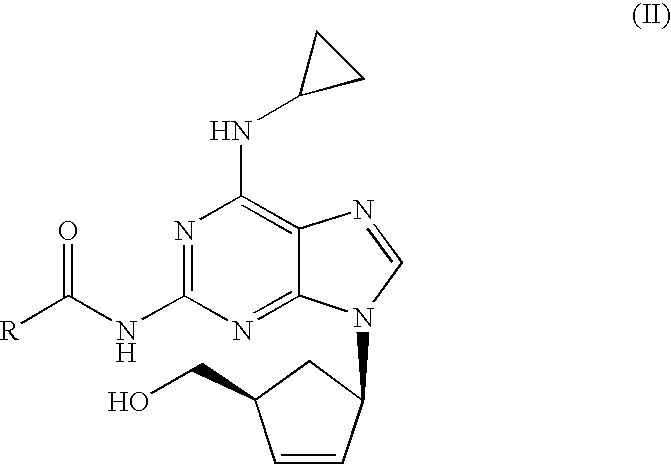
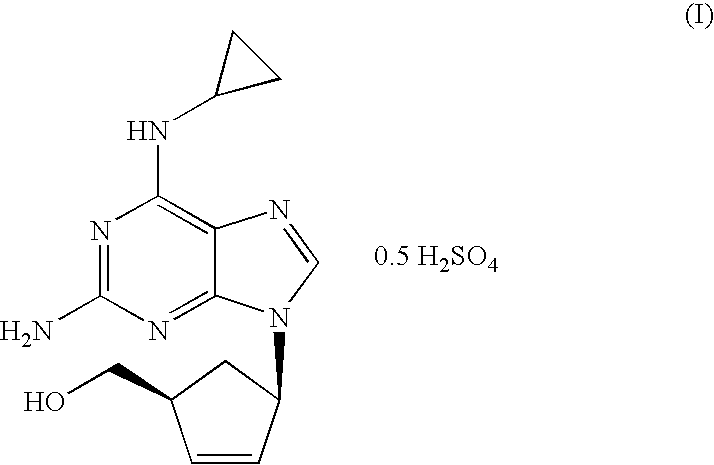
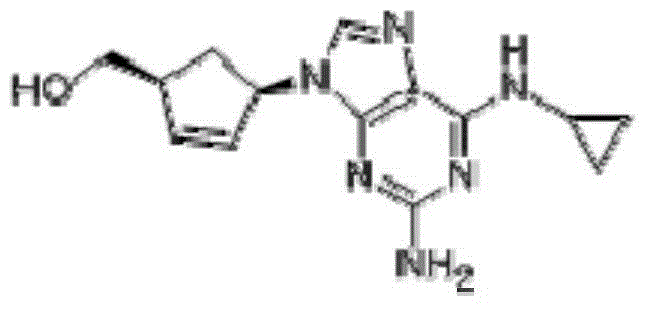
A pharmaceutical composition comprising (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol and (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one, in an amount which achieves antiviral efficacy, a process for the preparation of such a composition, and a method of inhibiting human immunodeficiency virus (HIV) which comprises administering such a composition to an HIV infected patient is disclosed.
Owner:SMITHKLINE BECKMAN CORP
Potent combinations of zidovudine and drugs that select for the K65R mutation in the HIV polymerase
InactiveCN101878032ALow toxicityImprove absolute antiviral effectAntiviralsCarbohydrate active ingredientsNucleoside Reverse Transcriptase InhibitorRetroviral infection
Owner:EMORY UNIVERSITY
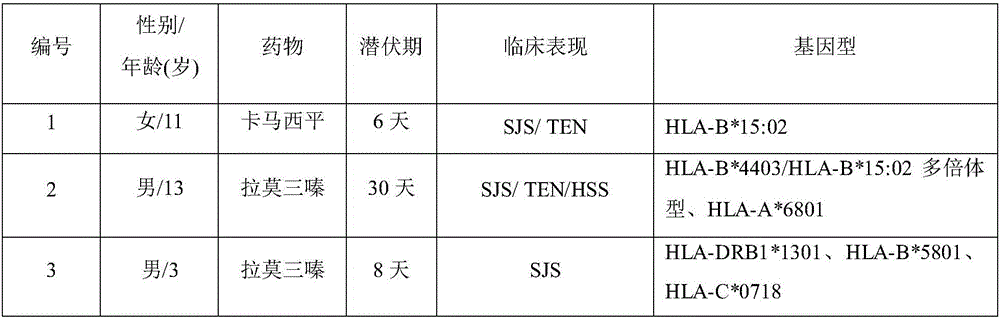
Biomarker for forecasting severe drug-induced cutaneous adverse reaction of child patient and application
The invention discloses a biomarker for forecasting severe drug-induced cutaneous adverse reaction of a child patient and an application. The biomarker is capable of forecasting the risk of severe cutaneous adverse reaction of the child patient using beta-lactam antibiotics such as penicillin, cephalosporin, carbamazepine, lamotrigine, oxcarbazepine, phenytoin, allopurinol, nevirapine, abacavir, methazolamide and dapsone.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
Synthetic method for chiral carbocyclic ring intermediate of abacavir
ActiveCN101284811AShort reaction timeHigh yieldOrganic chemistryChemical recyclingCross-linkOrganic solvent
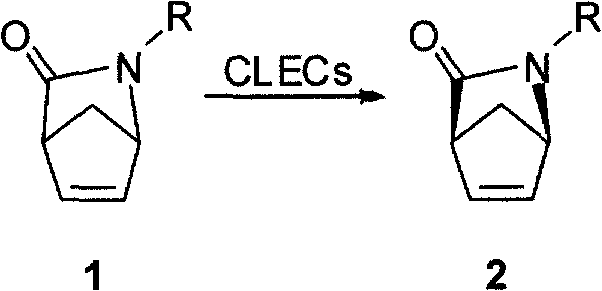
The invention discloses a method for synthesizing a chiral carbon ring intermediate of Abacavir. The steps are as follows: firstly, an intermediate 1 is prepared by 2-azabicyclo[2.2.1]hept-5- en-3-one, 1 mol of intermediate 1 is dissolved into 500 to 2000ml of organic solvent, the organic solvent is added into phosphate buffer solution of bacillus subtilis proteinase cross-linked enzyme crystals; the system is maintained between 15 and 35 DEG C and is mechanically stirred for 5 to 10 hours, the stirring speed is between 100 and 500r / m; the reaction is stopped, and the bacillus subtilis proteinase cross-linked enzyme crystals are reclaimed after the filtration; another 1000 to 2000ml of organic solvent is used to extract the filtrate for 3 to 6 times; organic phases are merged and dried by drying agent; and the target compound is obtained through reduced pressure distillation or column chromatography. The method has the advantages that: the method has short reaction time and high yield, the obtained product has high optical purity, and the catalyst can be reclaimed and reused.
Owner:苏州国镝医药科技有限公司
Process for preparing optics pure abacavir
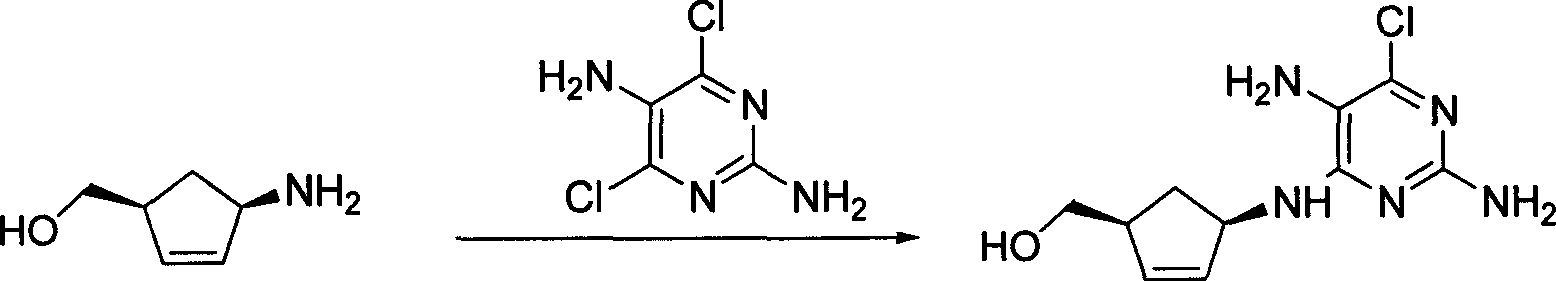
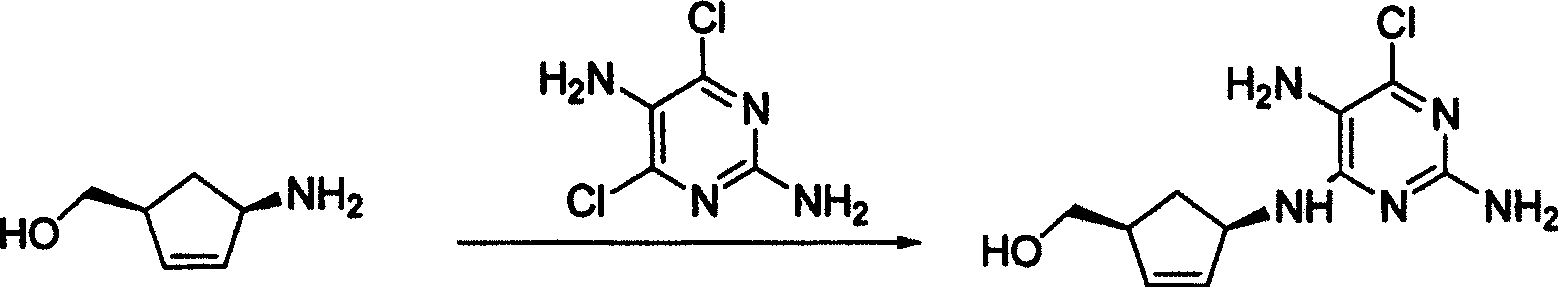
A process for preparing the optical-purity (99.9% ee) Abakawei features that its initial raw materials are (1S,4R)-4-amino-2-cyclopentyl-1-methanol and 2,5-diamino- 4,6-dichloropyrimidine.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Pharmaceutical composition for use in prevention or treatment of cancer
ActiveUS20160000796A1Raise the ratioEliminate side effectsBiocideAntiviralsBULK ACTIVE INGREDIENTT-cell leukemia
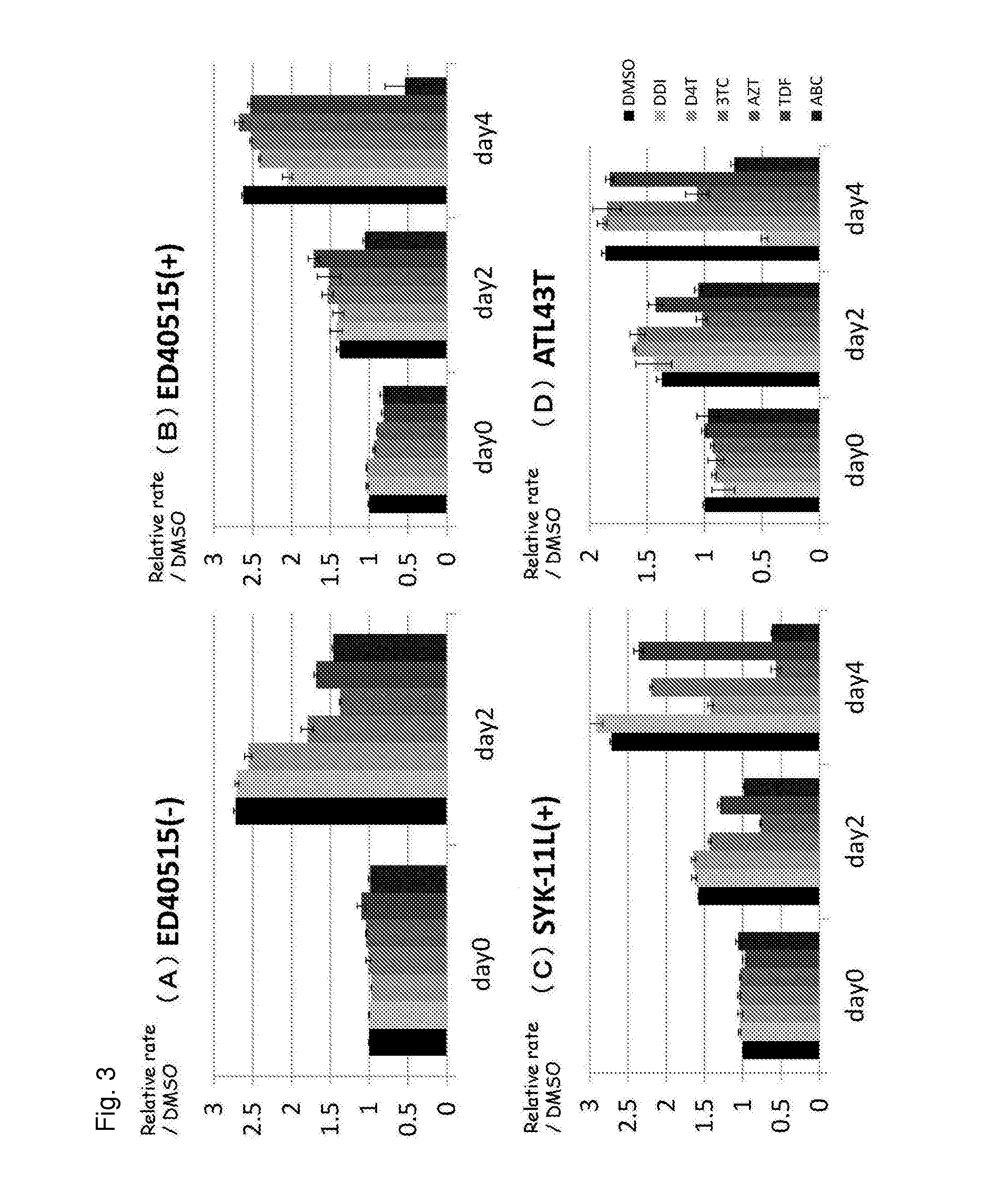
Abacavir, a nucleoside analogue reverse transcriptase inhibitor, has been found to exhibit an anti-cancer activity on ATL cells in vitro without inhibiting DNA replication of normal cells. Abacavir or a pharmaceutically acceptable derivative thereof is useful as an active ingredient of a pharmaceutical composition for use in the prevention or the treatment of cancer, in particular a cancer whose DNA repair system is impaired such as breast cancer or adult T-cell leukemia.
Owner:KYOTO UNIV
Method of screening for drug hypersensitivity reaction
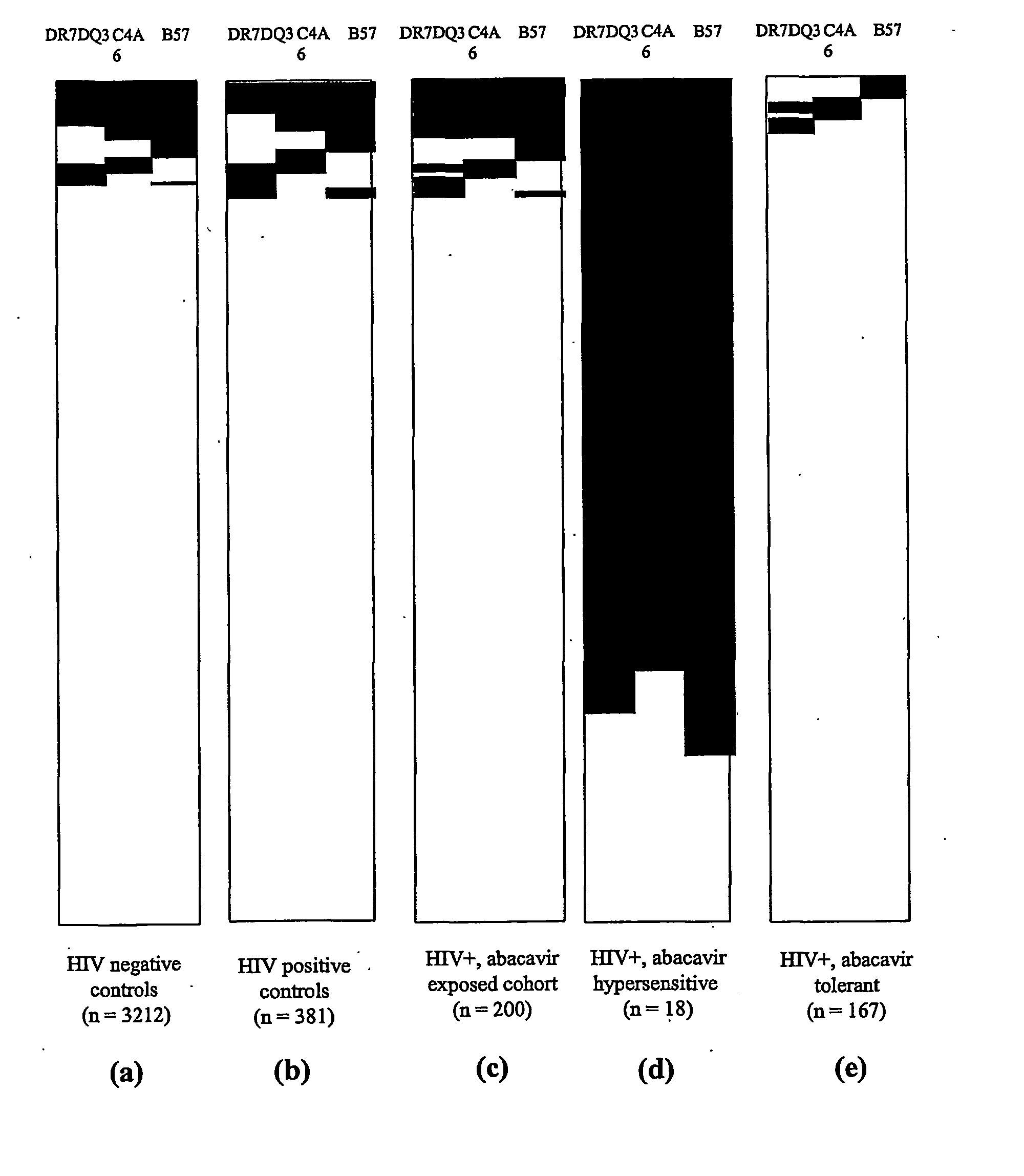
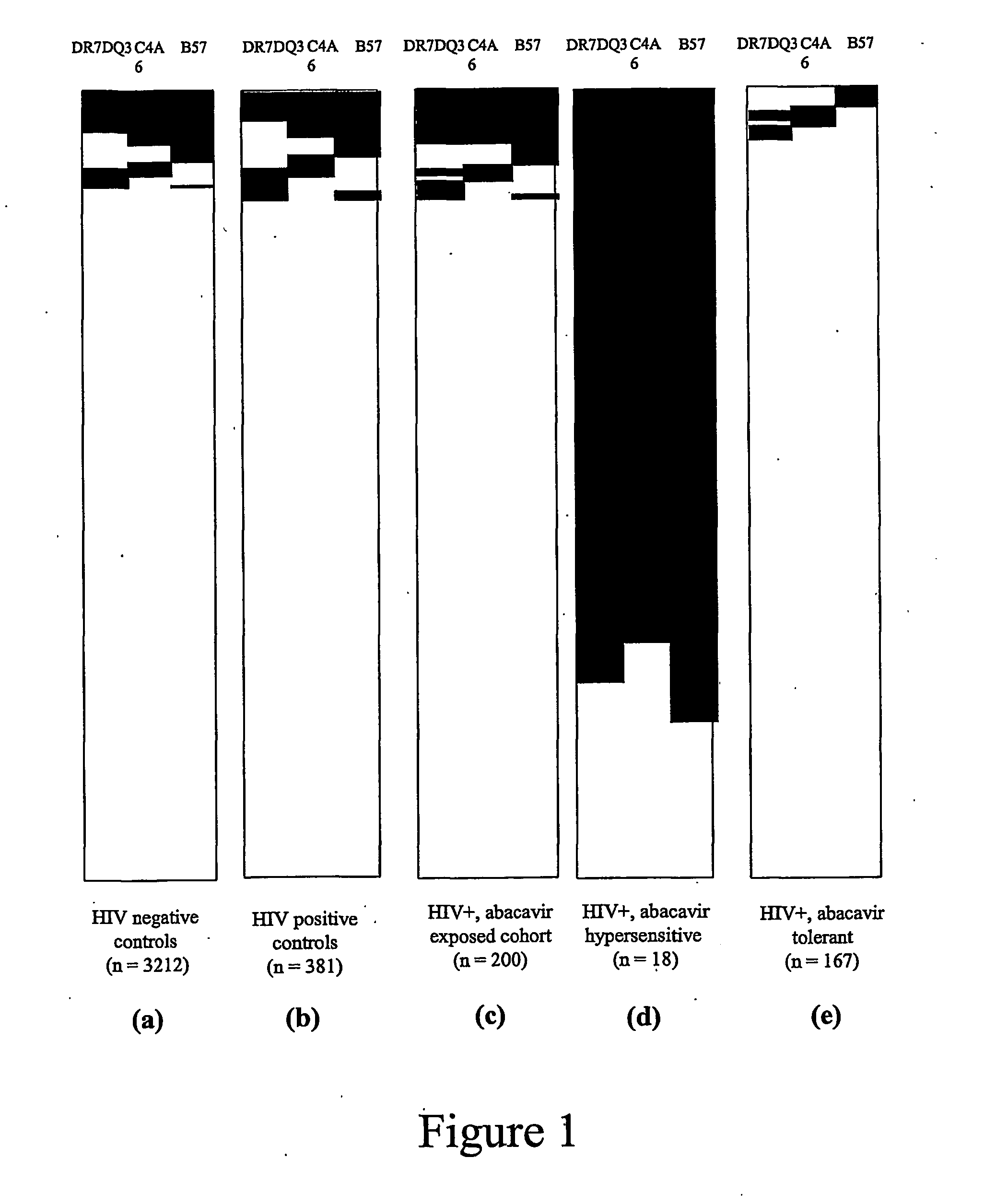
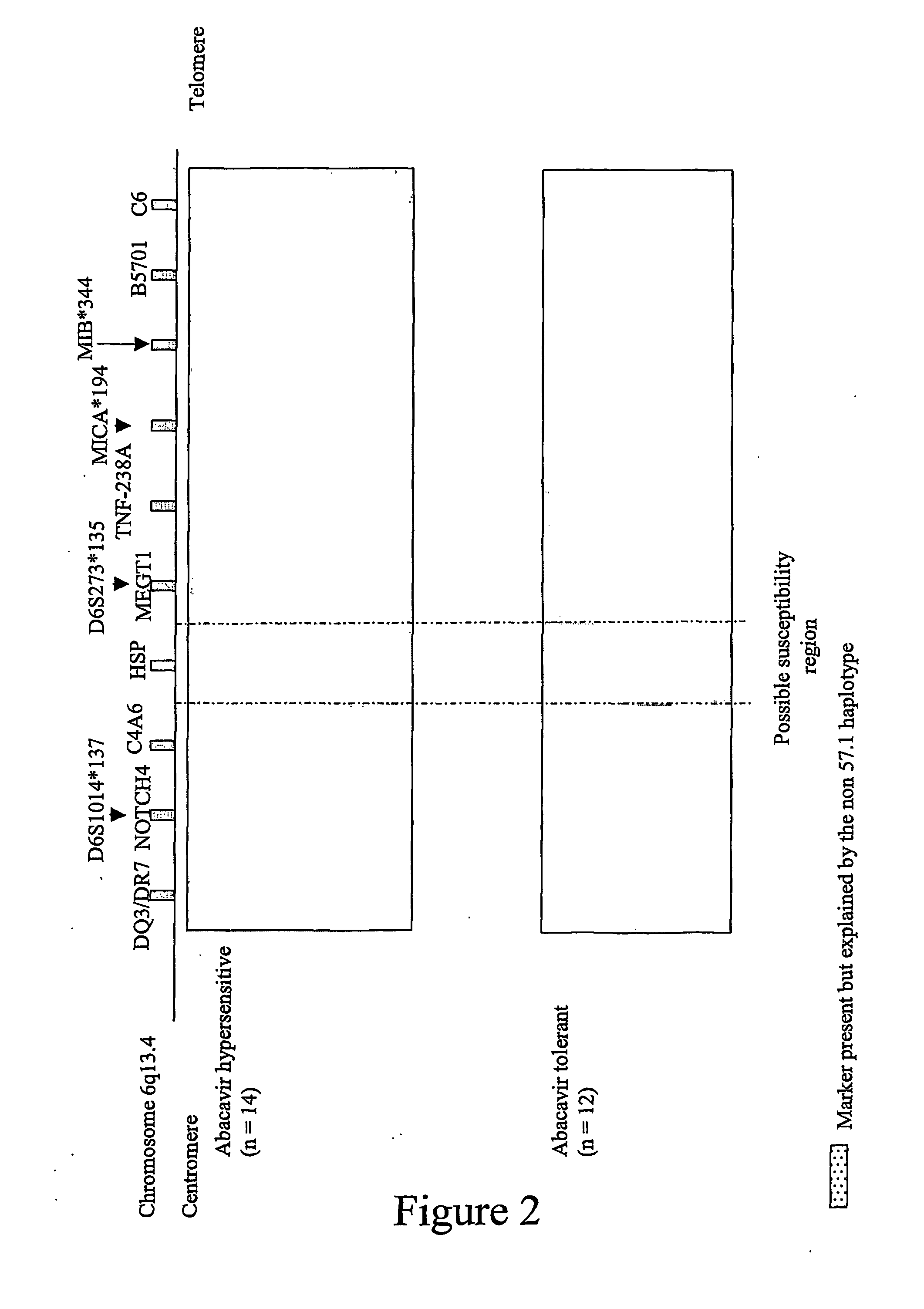
ActiveUS20070148641A9High incidenceIncreased riskSugar derivativesMicrobiological testing/measurementHypersensitive responseDrug allergy
Methods of assessing the risk of clinical signs of hypersensitivity reaction to nucleoside antiviral compounds, including abacavir, are described. The methods include genotyping subjects for polymorphisms in the TNFα gene, the class 1 HLA genes, or a combination of both the TNFα and HLA genes.
Owner:VIIV HEALTHCARE UK LTD
Method for identification and determination of hypersensitivity of a patient to abacavir
Owner:EPIPOP
Method for preparing abacavir chiral intermediate by biological split
InactiveCN101875959ANo protectionImprove economyMicroorganism based processesOn/in organic carrierWater bathsAbacavir
The invention discloses a method for preparing an abacavir chiral intermediate by biological split, and in particular relates to application of Pseudomonas cepacia DSM9959 immobilized cells in preparing the abacavir chiral intermediate by the biological split. 5 to 50g of immobilized cells are used for each 100mL of reaction system, and are converted for 24 to 48 hours under the conditions that the concentration of the racemic 2-azabicyclo-[2,21]-heptane-5-ene-3-one substrate is 10 to 500g / L, 50mM potassium phosphate buffer solution has the pH of 7.5 and a 25 DEG C water bath shake has the rotating speed of 200rpm; and the maximum conversion rate is 48.5 percent and the e.e value is more than 99.5 percent. The reaction liquid is filtered and the residual immobilized cells are obtained; the residual immobilized cells can be repeatedly used for more than 5 times, and the conversion rate is still more than 35 percent and the e.e value is more than 99.5 percent; and compared with free cells for conversion, the immobilized cells have the activity more than half of that of the free cells. The method has the advantages of simple preparation of catalyst, repeated use of the catalyst for more than 5 times, mild reaction conditions, no substrate protection, simplified reaction steps, and the enantiomeric excess of the product of more than 99.5 percent, so the method has good economy and technical feasibility.
Owner:JIANGXI CHIBANG PHARMA +1
Synthetic method for chiral carbocyclic ring intermediate of abacavir
ActiveCN101284811BShort reaction timeHigh yieldOrganic chemistryChemical recyclingSubtilisinPtru catalyst
The invention discloses a method for synthesizing a chiral carbon ring intermediate of Abacavir. The steps are as follows: firstly, an intermediate 1 is prepared by 2-azabicyclo[2.2.1]hept-5- en-3-one, 1 mol of intermediate 1 is dissolved into 500 to 2000ml of organic solvent, the organic solvent is added into phosphate buffer solution of bacillus subtilis proteinase cross-linked enzyme crystals;the system is maintained between 15 and 35 DEG C and is mechanically stirred for 5 to 10 hours, the stirring speed is between 100 and 500r / m; the reaction is stopped, and the bacillus subtilis proteinase cross-linked enzyme crystals are reclaimed after the filtration; another 1000 to 2000ml of organic solvent is used to extract the filtrate for 3 to 6 times; organic phases are merged and dried bydrying agent; and the target compound is obtained through reduced pressure distillation or column chromatography. The method has the advantages that: the method has short reaction time and high yield,the obtained product has high optical purity, and the catalyst can be reclaimed and reused.
Owner:苏州国镝医药科技有限公司
Quadruple therapy useful for treating persons afflicted with the human immunodeficiency virus (HIV)
InactiveUS20150305983A1Reduce in quantityMaintain curative effectComputer controlDrug and medicationsEmtricitabineNucleotide
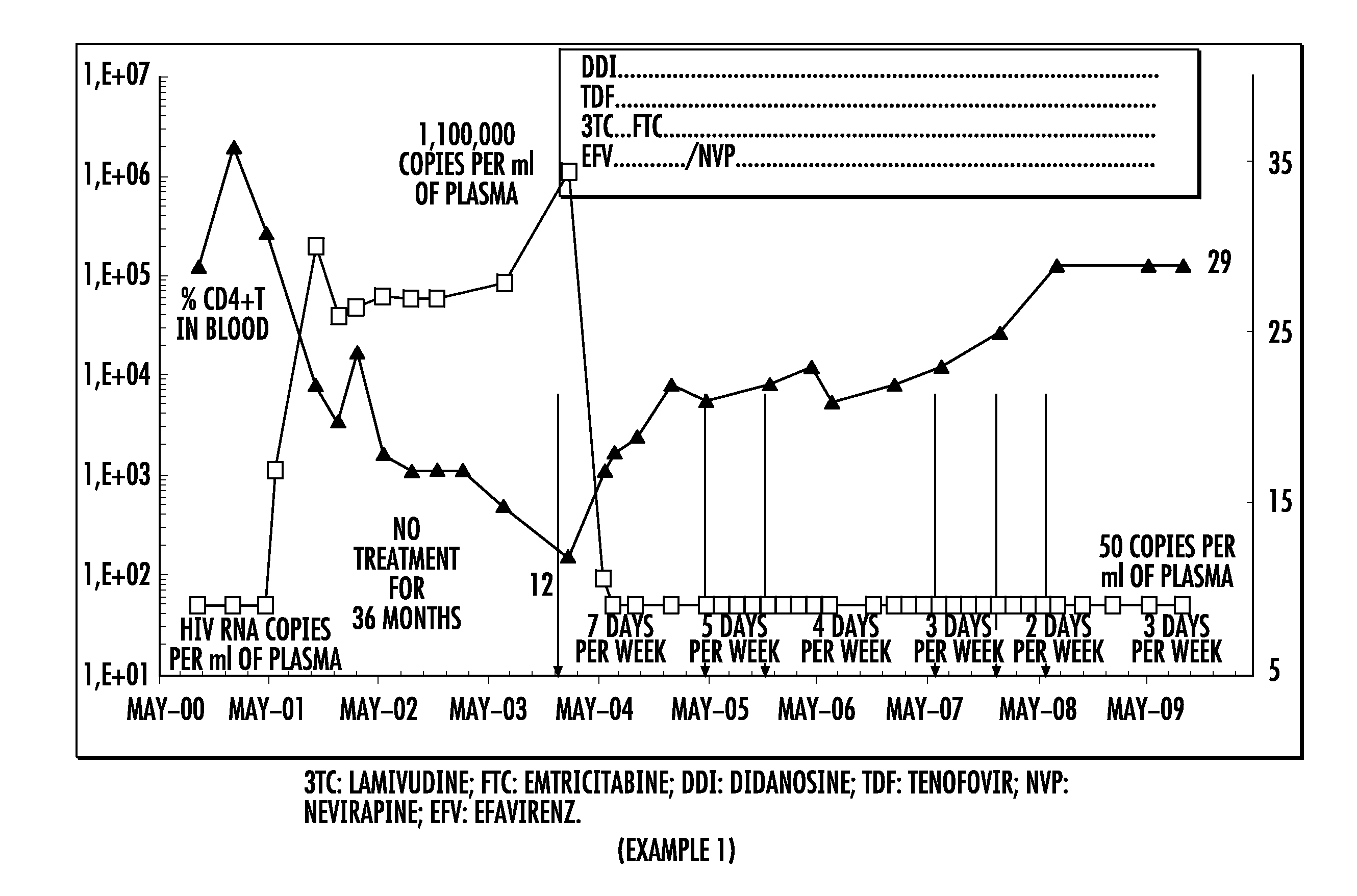
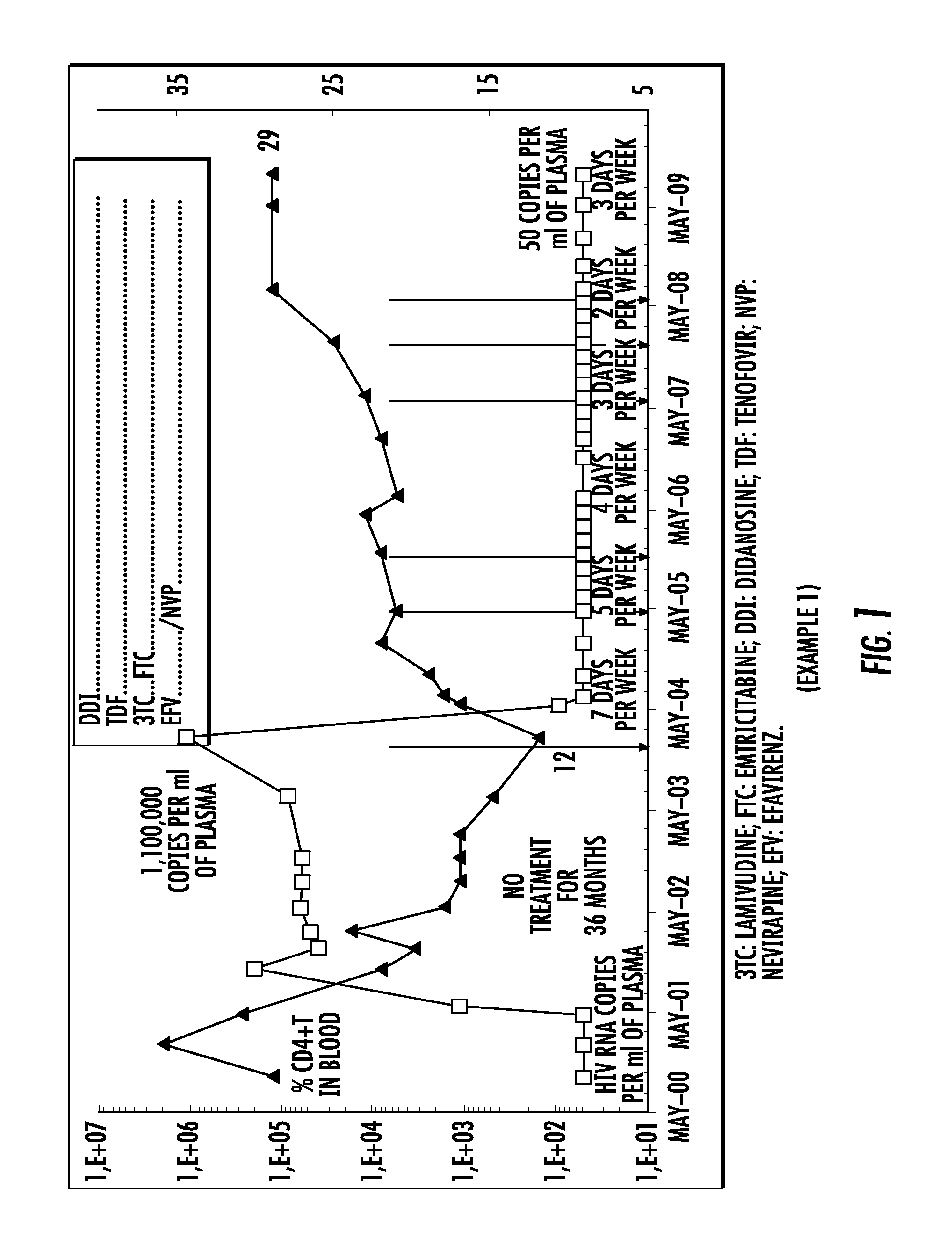
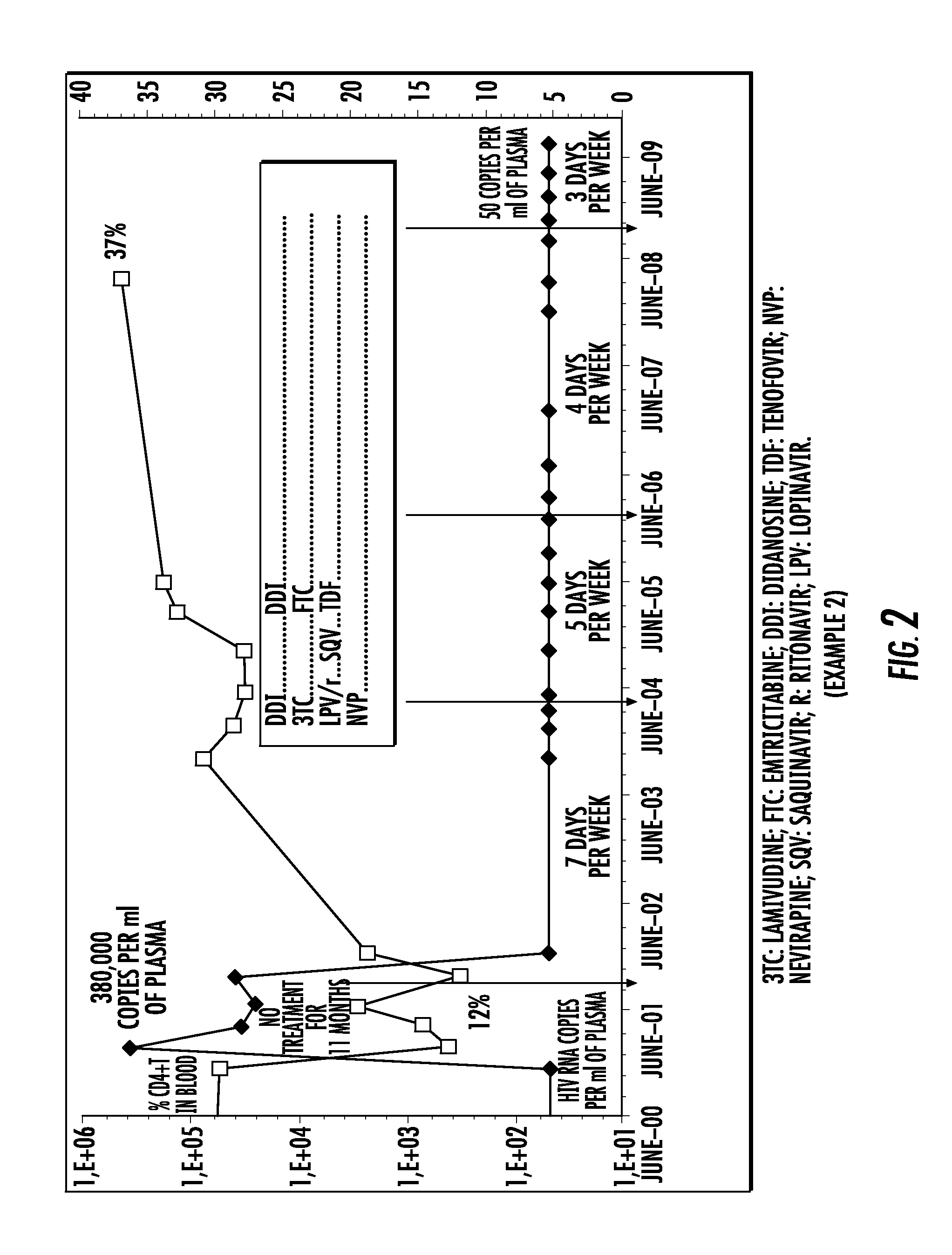
The present invention relates to a pharmaceutical composition for treating the human immunodeficiency virus (HIV) in a human being, comprising four active principles selected as being: one nucleoside inhibitor of reverse transcriptase (NRTI) selected from lamivudine and emtricitabine; two nucleoside or nucleotide inhibitor of reverse transcriptase (NRTI) selected from didanosine, abacavir and tenofovir; and the fourth active principle is selected from (i) a non-nucleoside inhibitor of reverse transcriptase (NNRTI) selected from nevirapine, efavirenz and etravirine; or (ii) a protease inhibitor selected from atazanavir, lopinavir, saquinavir, ritonavir, indinavir, amprenavir, nelfinavir, fosamprenavir, tipranavir and darunavir.The present invention also relates to an electronic portable pillbox comprising a multidrug therapy for treating the immunodeficiency virus (HIV) in human beings allowing improving the observance of medication intake.
Owner:UNIV VERSAILLES SAINT QUENTIN EN YVELINES
Primer pair and probe for detecting ABC (Abacavir) drug-resistant mutation sites of AIDS (Acquired immune deficiency syndrome) curing drugs and application of primer pair and probe
PendingCN110079635AStrong specificityEnrichmentMicrobiological testing/measurementDNA/RNA fragmentationPol genesSocial benefits
The invention discloses a primer pair and probe for detecting ABC (Abacavir) drug-resistant mutation sites of AIDS (Acquired immune deficiency syndrome) curing drugs. The primer pair and probe are characterized by comprising an ARMS (Amplification refractory mutation system) primer and a Taqman probe for detecting the mutation sites of K65R, K70E, L74V, L74I, Y115F and Q151M at the 65th, 70th, 74th, 115th and 151th sites of a pol gene of HIV-1 (Human immunodeficiency virus) viral RNA. The invention further provides application of the primer pair and probe in the detection of the ABC major drug-resistant mutation sites of K65R, K70E, L74V, L74I, Y115F and Q151M. According to the primer pair and probe disclosed by the invention, a kit is high in detection sensitivity, good in specificity andlow in detection cost; medication guidance is provided for the treatment of clinical AIDS patients; individualized treatment of the AIDS patients is realized; the effectiveness of the drugs can be improved; the survival time of the AIDS patients is prolonged; wide application prospect and social benefit are realized.
Owner:JIANGSU FAST BIOTECH
Process for the Preparation of Abacavir
InactiveUS20100004446A1Short reaction timeLow formation of impurityOrganic chemistryBulk chemical productionAlcoholAbacavir
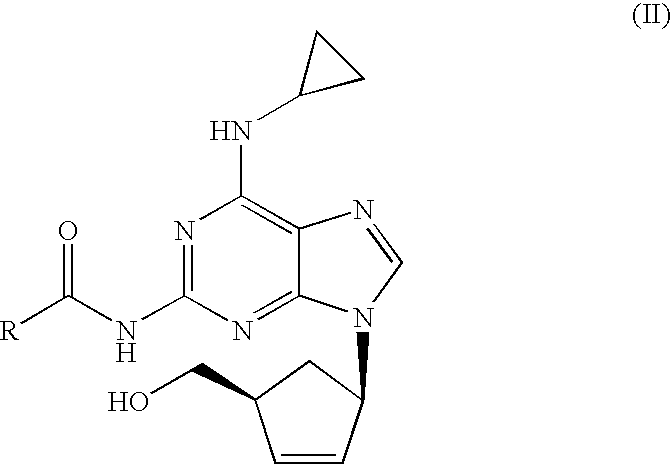
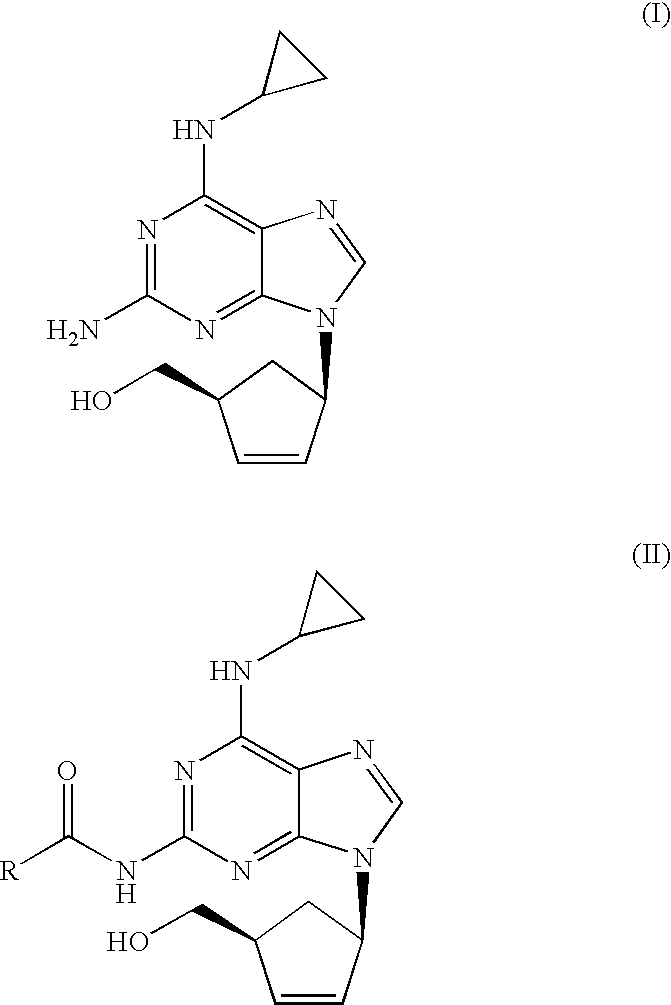
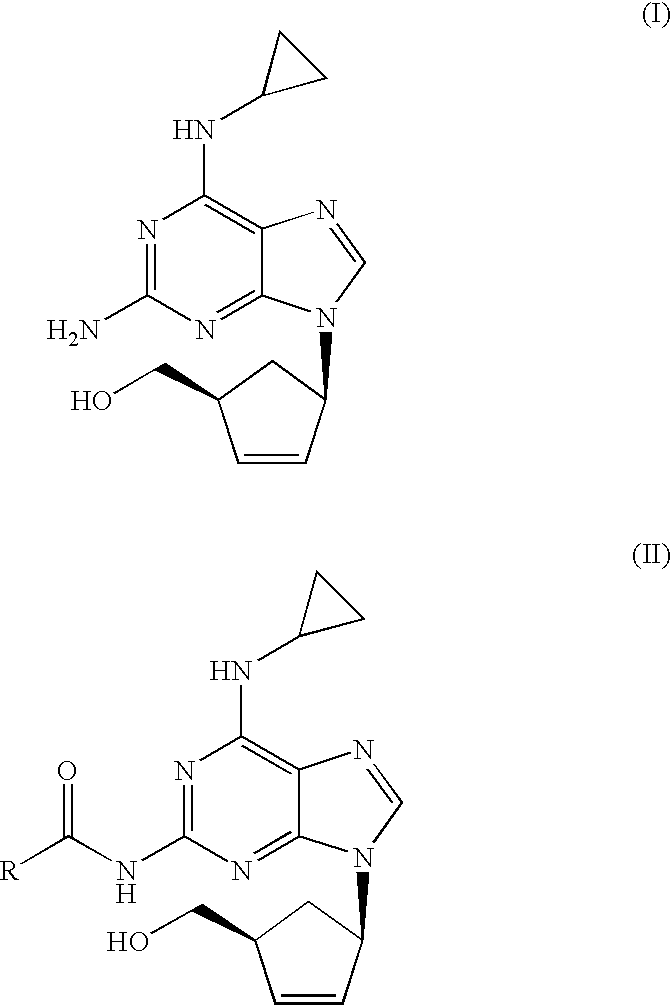
Process for removal of the amino protective group of a N-acylated [(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-cyclopent-2-enyl]methanol of formula (II) where R═H or a (C1-C4)-alkyl, using an inorganic base in a mixture of water and alcohol, to yield abacavir or its salts. The process proceeds very fast and the product can be obtained in high yield and purity.
Owner:ESTEVE QUIMICA
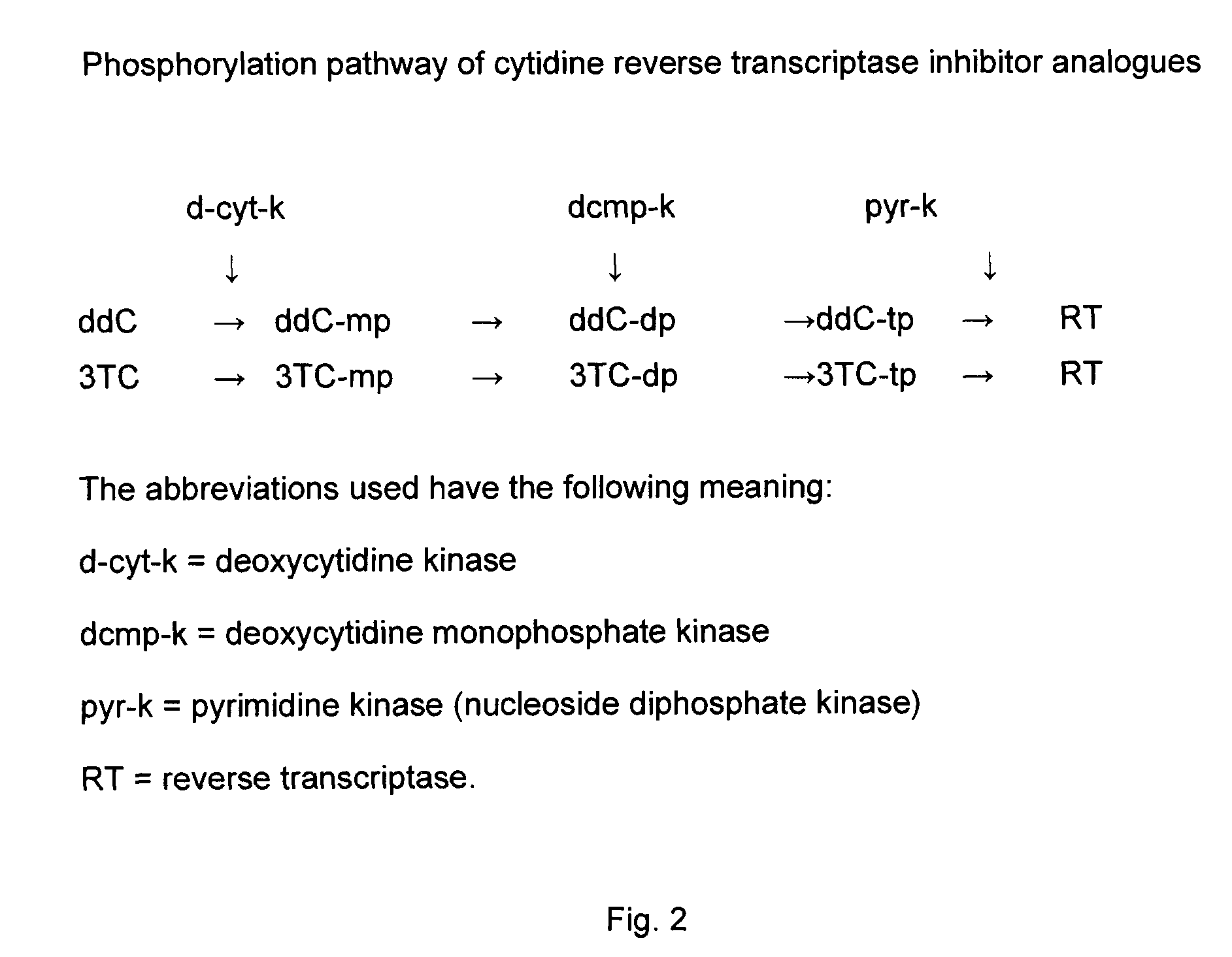
Zalcitabine (Ddc) Boosted Lamivudine (3Tc) Compositions for Antiretroviral Therapy
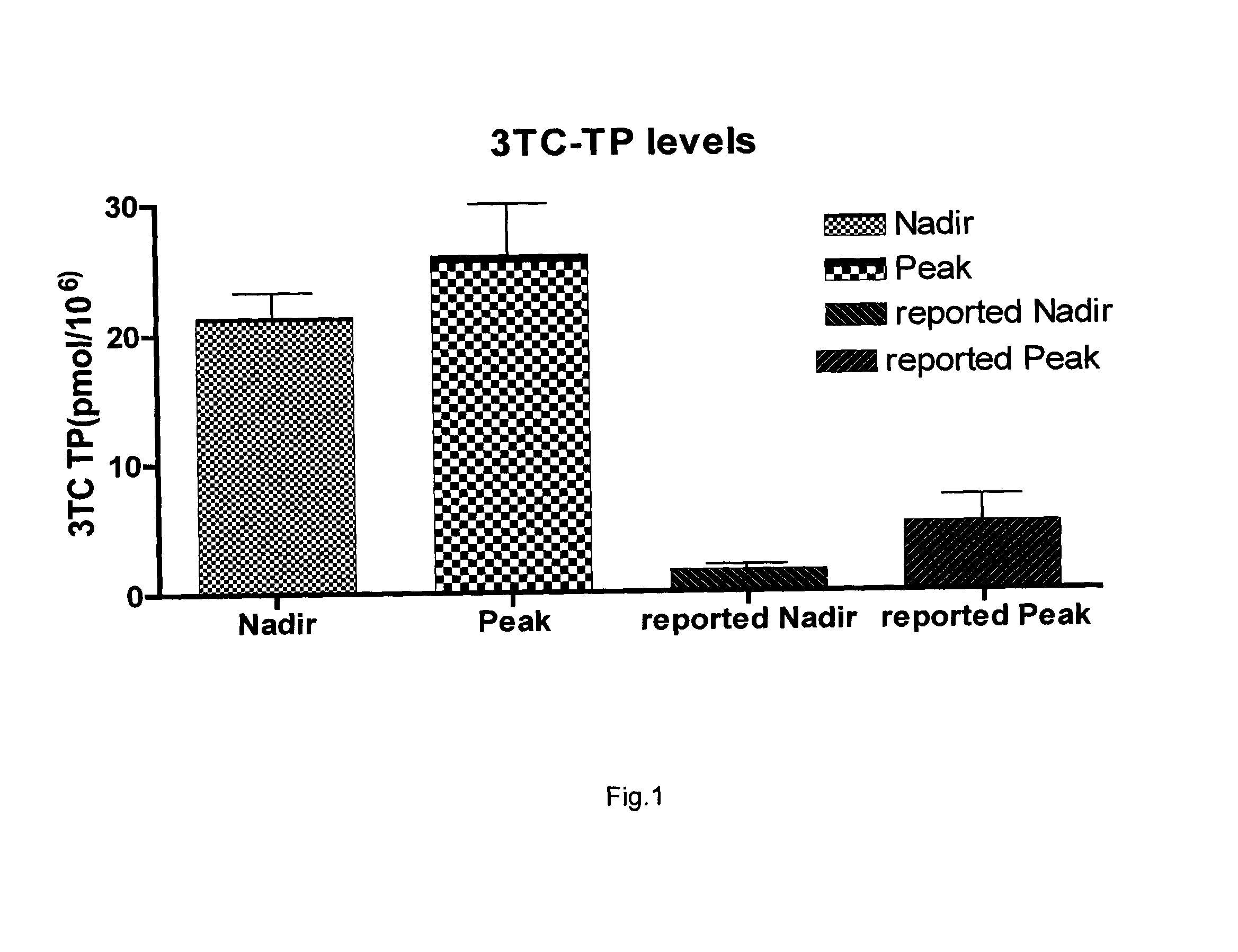
ActiveUS20080214590A1High activityImprovement of TC antiretroviral activityOrganic active ingredientsBiocideTolerabilitySide effect
Boosted cytidine analogue reverse transcriptase inhibitor antiretroviral compound is a new therapeutic anti HIV option, in combination with another drug such as a NRTI or a protease inhibitor. It's heightened and sustained antiretroviral potency is due to the increased intracellular level of 3TC triphosphate, the active form of 3TC. This effect is obtained by combining 3TC, in usual doses, with a reduced dose of ddC, in the same pharmaceutical formulation. The product could be administered twice or even once daily, which is convenient, and does not increase the pill burden for the patient. The reduced ddC dosage prevents the occurrence of ddC related side effects. Other cytidine derivatives (racemic or negative enantiomers) could have the same effects as ddC and could probably be combined with 3TC, and have the same effect. On the other hand, low dose ddC may also increase the intracellular levels of other cytidine derivatives as it does for 3TC. Boosted cytidine analogue reverse transcriptase inhibitor antiretroviral compound could also be formulated in combination with another drug such as another NRTI (e.g. abacavir) or any protease inhibitor in the same capsule or tablet. This approach offers a dual anti-HIV therapy that is as efficacious as the routine triple therapy. In this way the HIV treatment cost could be significantly reduced which is imperative for resource-poor settings. This new formulation is convenient and well tolerated with no additional toxicity than that of the combining drug (NRTI or protease inhibitor) and 3TC. Moreover, this will enable a larger number of patients to benefit from the already known 3TC effects. It will also increase the 3TC effects in those organs or HIV sanctuaries with usually reduced 3TC concentrations or activity. It could be indicated in both the initial as well as in salvage HIV therapy. It could also be used for therapy optimization or simplification. Moreover, in combination with another NRTI such as abacavir, or even alone, it could be beneficial for reducing the HIV harm in resource-poor settings.
Owner:TOMA EMIL
Process for the preparation of abacavir
Process for the preparation of abacavir, or its salts or its solvates comprising the step of reacting a compound (IV) where R1 is a (C1-C4)-alkyl radical with anhydrous hydrochloric acid / (C1-C6)-alcohol, and then with tri(C1-C4)-alkyl orthoformate, in the absence of water. The preparation process may include further steps of reacting the compound obtained with cyclopropylamine and subsequently hydrolysis to yield abacavir.
Owner:ESTEVE QUIMICA
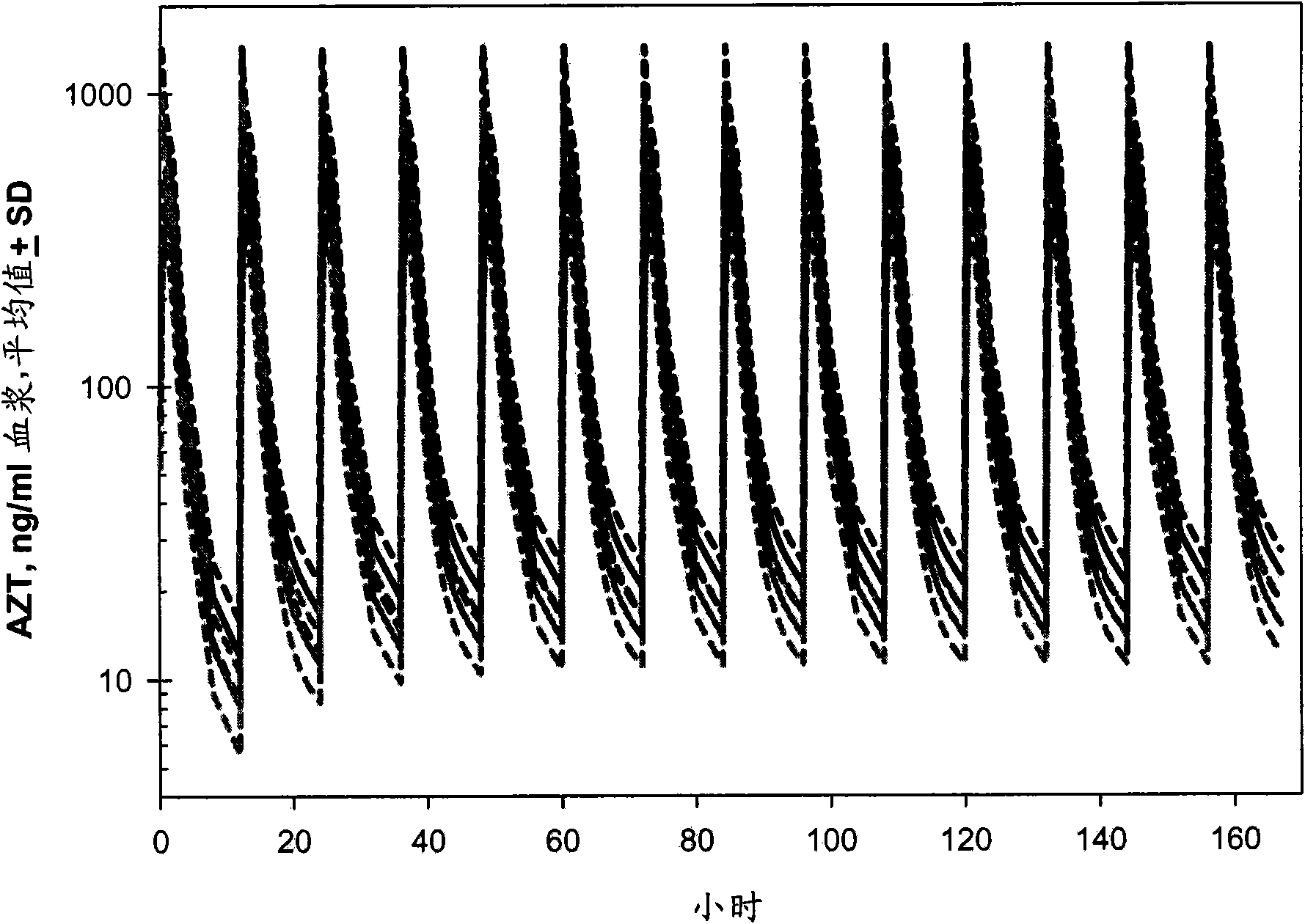
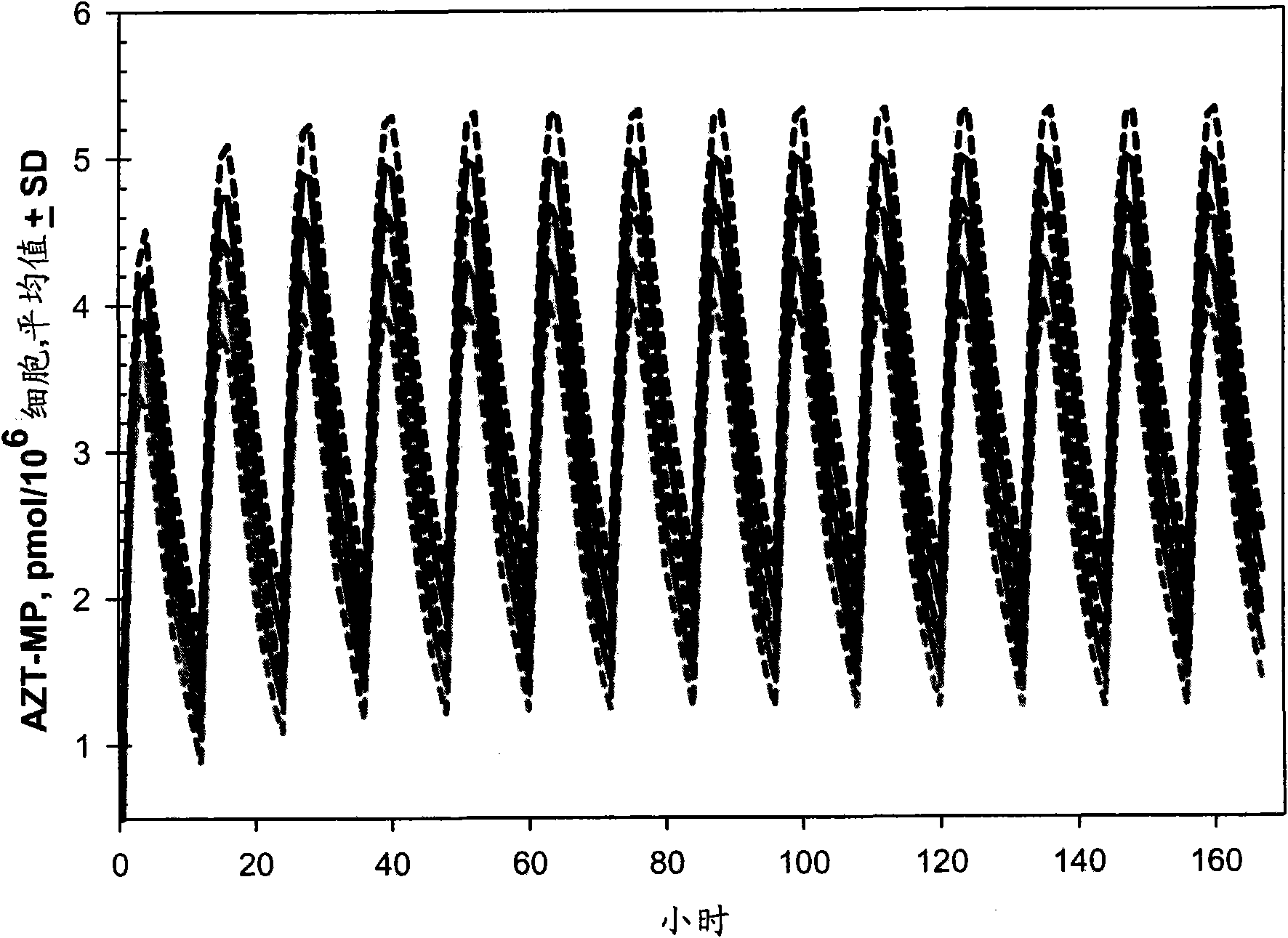
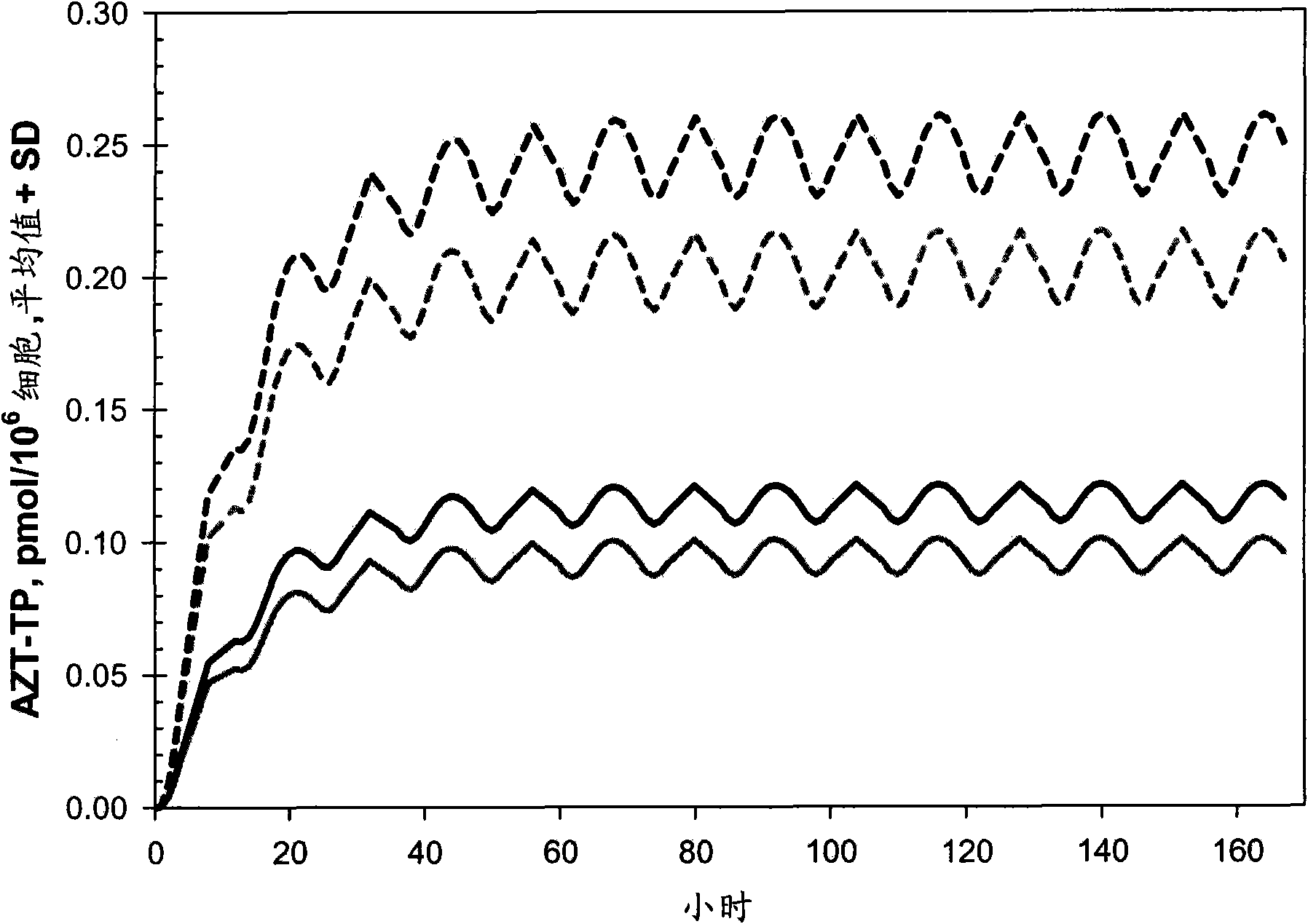
Macroheterocyclic nucleoside derivatives and their analogues, production and use thereof
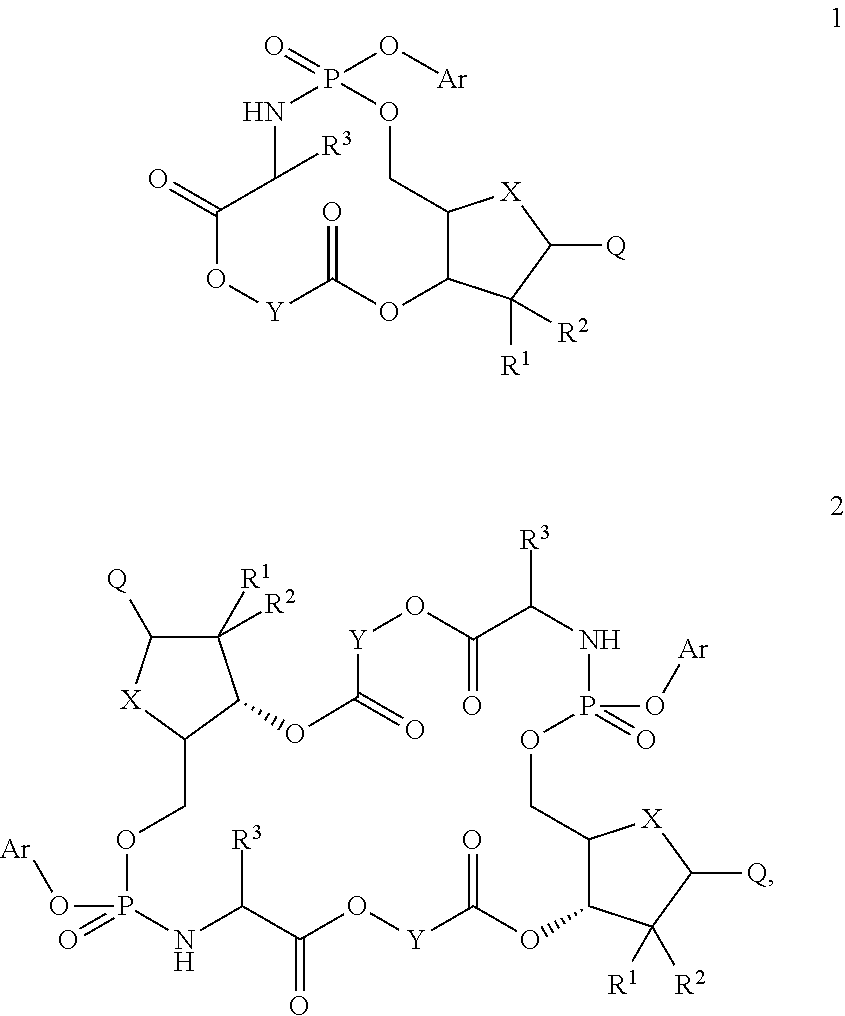
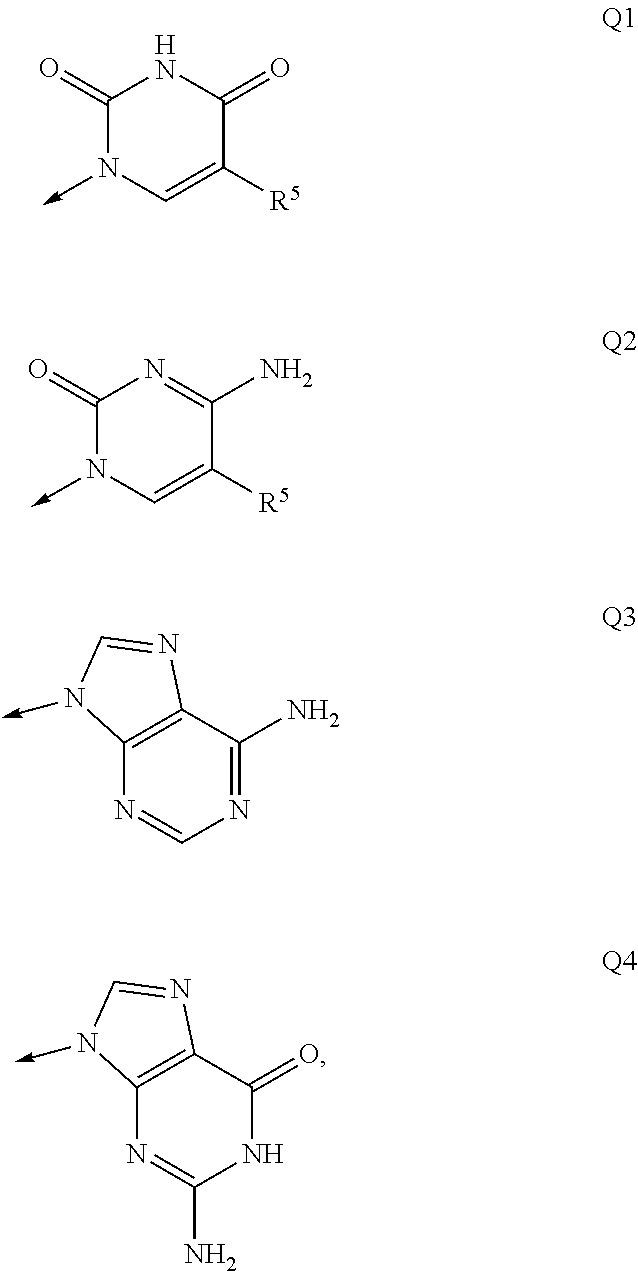
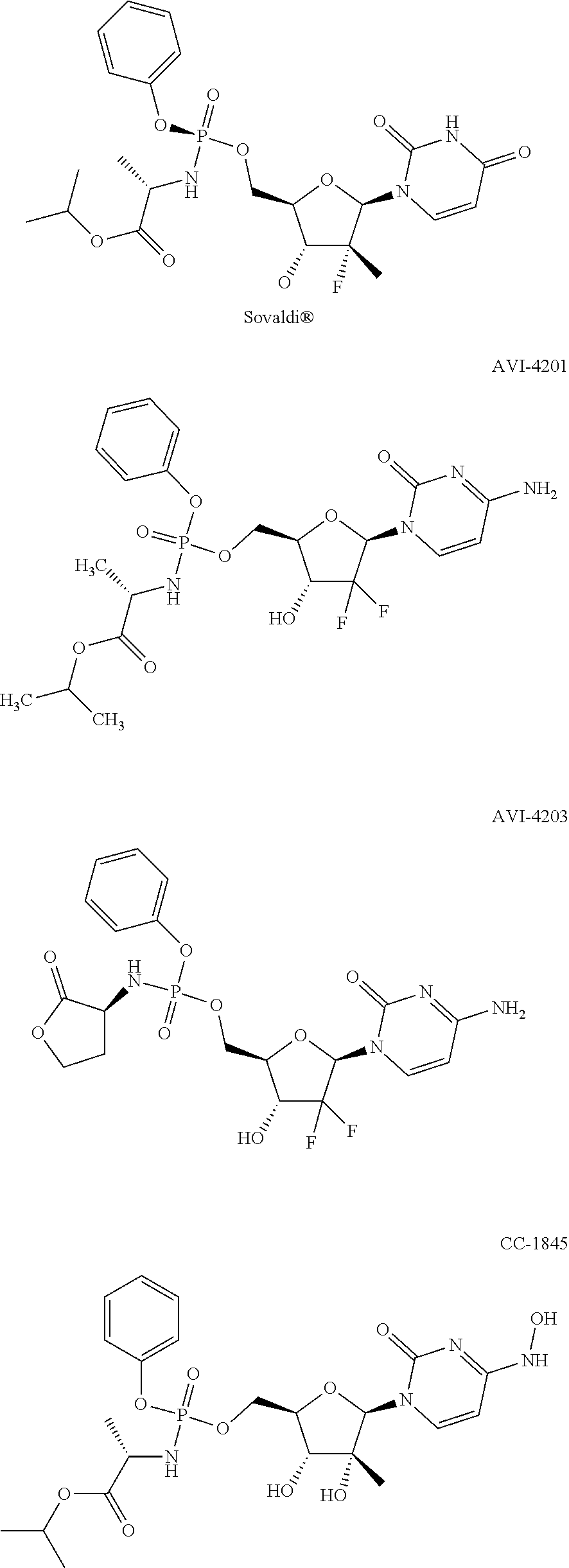
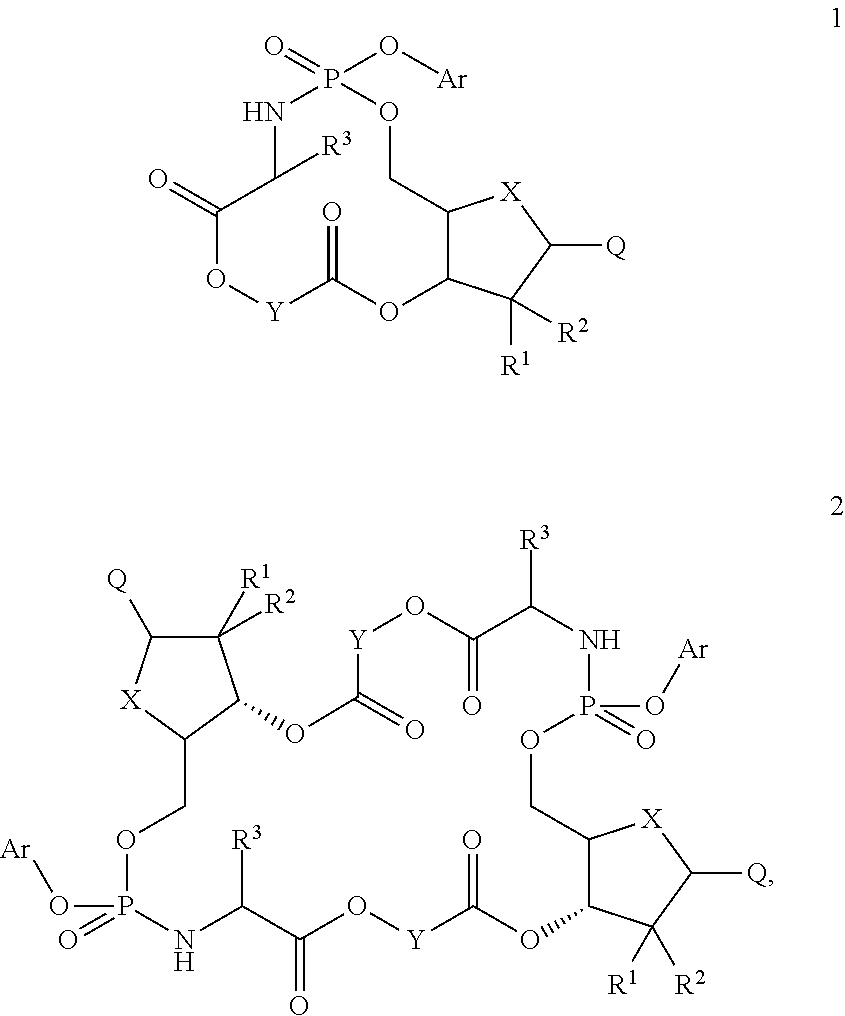
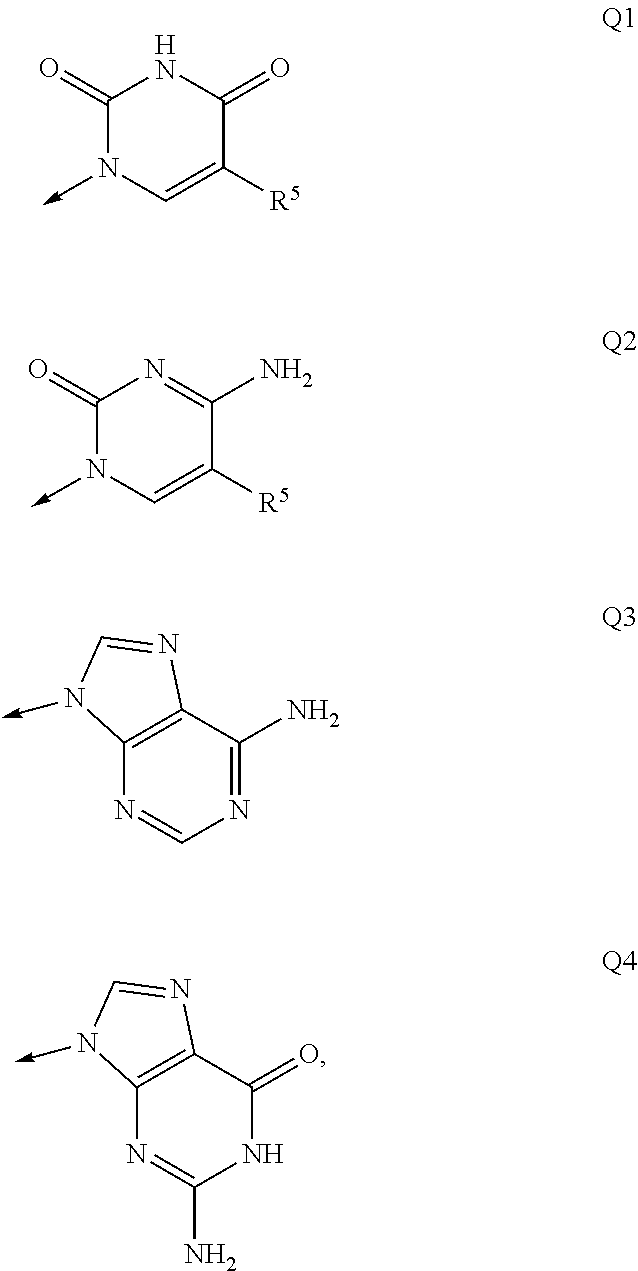
Nucleosides and nucleotides (nucleos(t)ides) have been in clinical use for almost 50 years and have become cornerstones of treatment for patients with viral infections or cancer. The approval of several additional drugs over the past decade demonstrates that this family still possesses strong potential. Therefore nucleos(t)ide are of great interest as promising chemotherapeutic agents, including: 2′-deoxy-L-uridine (CAS 31501-19-6), 2′-deoxy-D-uridine (CAS 951-78-0), telbivudine (CAS 3424-98-4), zidovudine (AZT, CAS 30516-87-1), trifluridine (CAS 70-00-8), clevudine (CAS 163252-36-6), PSI-6206 (CAS 863329-66-2), 2′-(S)-2′-chloro-2′-deoxy-2′-fluorouridine (CAS 1673560-41-2), ND06954 (CAS 114248-23-6), stavudine (CAS 3056-17-5), 5-ethynyltavudine (Festinavir, CAS 634907-30-5), torcitabine (CAS 40093-94-5), (−)-beta-D-(2R,4R)-dioxolane-thymine (DOT, 1-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-5-methyl-2,4(1H,3H)-pyrimidinedione, CAS No. 127658-07-5), 2-(6-amino-purin-9-yl)-ethanol (CAS 707-99-3), 2′-C-methylcytidine (CAS 20724-73-6), PSI-6130 (CAS 817204-33-4), gemcitabine (CAS 95058-81-4), 2′-chloro-2′-deoxy-2′-fluorocytidine (CAS 1786426-19-4), 2′,2′-dichloro-2′-deoxycytidine (CAS 1703785-65-2), 2′-C-methylcytidine (CAS 20724-73-6), PSI-6130 (CAS 817204-33-4), lamivudine (3TC, CAS 134678-17-4), emtricitabine (CAS 143491-57-0), 2′-deoxyadenosine (CAS 958-09-8), 2′-deoxy-β-L-adenosine (CAS 14365-45-8), 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine (CAS 865363-93-5), didanosine (CAS 69655-05-6), entecavir (CAS 209216-23-9), FMCA (CAS 1307273-70-6), dioxolane-G (DOG, CAS 145514-01-8), β-D-2′-deoxy-2′-(R)-fluoro-2′-β-C-methylguanosine (CAS No 817204-45-8), abacavir (ABC, CAS 136470-78-5), dioxolane-A (DOA, CAS #145514-02-9), [(2R,4R)-4-(6-cyclopropylamino-purin-9-yl)-[1,3]dioxolan-2-yl]-methanol (CAS 1446751-04-7), amdoxovir (AMDX, CAS 145514-04-1), (R)-1-(6-amino-purin-9-yl)-propan-2-ol (CAS 14047-28-0), and [(2S,5R)-5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yl]-methanol.Macroheterocyclic nucleoside derivative and its analog of the general formula 1 or general formula 2, a stereoisomer, isotope-enriched analog, pharmaceutically acceptable salt, hydrate, solvate, or crystalline or polymorphic form thereof,wherein:Ar is aryl or hetaryl;R1 and R2 are not necessarily the same substituents selected from H, F, Cl, CH3, OH;R3 is H or CH3;X is oxygen or ethanediyl-1,1 (C═CH2);Y is CH(R4)(CH2)k, CH(R4)(CH2)mC(O)O(CH2)n;R4 is H or CH3;k has a value from zero to six;m has a value from zero to two;n has a value of one to four;Q is a radical selected from Q1-Q4;wherein: R5 is the substituent selected from H, F, Cl, CH3, OH;the arrow (→) indicates the location, joined by Q1-Q4.
Owner:ALLA CHEM LLC
Method of screening for drug hypersensitivity reaction
ActiveUS7550261B2High incidenceIncreased riskSugar derivativesMicrobiological testing/measurementAbacavirDrug allergy
Methods of assessing the risk of clinical signs of hypersensitivity reaction to nucleoside antiviral compounds, including abacavir, are described. The methods include genotyping subjects for polymorphisms in the TNFα gene, the class 1 HLA genes, or a combination of both the TNFα and HLA genes.
Owner:VIIV HEALTHCARE UK LTD
Dioxolane thymine and combinations for use against 3tc/azt resistant strains of hiv
InactiveUS20050209196A1Inhibits growth and replication and elaborationBiocideSugar derivativesDelavirdinePhosphate
The present invention relates to the use of a dioxolane thymine compound according to the chemical structure of Formula (I): where R1 is H, an acyl group, a C1-C20 alkyl or ether group, a phosphate, diphosphate, triphosphate or phosphodiester group, for use in the treatment of HIV infections which exhibit resistance to 3TC and / or AZT. Preferably, compounds according to the present invention are combined with at least one anti-HIV agent which inhibits HIV by a mechanism other than through the inhibition of thymidine kinase (TK). These agents include those selected from among nucleoside reverse transcriptase inhibitors (NRTI), non-nucloeoside reverse transcriptase inhibitors, protease inhibitors, fusion inhibitors, among others. These agents are generally selected from the group consisting of 3TC (Lamivudine), AZT (Zidovudine), (−)-FTC, ddI (Didanosine), ddC (zalcitabine), abacavir (ABC), tenofovir (PMPA), D-D4FC (Reverset), D4T (Stavudine), Racivir, L-D4FC, NVP (Nevirapine), DLV (Delavirdine), EFV (Efavirenz), SQVM (Saquinavir mesylate), RTV (Ritonavir), IDV (Indinavir), SQV (Saquinavir), NFV (Nelfinavir), APV (Amprenavir), LPV (Lopinavir), fuseon and mixtures thereof. The TK dependent agents, such as AZT and D4T, may be used in combination with one of the dioloxane thymine compounds according to the present invention, but the use of such agents may be less preferred. In preferred compositions according to the present invention, R1 is preferably H or a C2-C18 acyl group or a monophosphate group. Pharmaceutical compositions and methods of reducing the likelihood that a patient at risk for contract an HIV infection will contract the infection are other aspects of the present invention.
Owner:UNIV OF GEORGIA RES FOUND INC +1
Process for the preparation of abacavir
InactiveUS8183370B2Low formation of impurityShort reaction timeOrganic chemistryBulk chemical productionAlcoholAbacavir
Owner:ESTEVE QUIMICA
Macroheterocyclic nucleoside derivatives and their analogues, production and use thereof
InactiveUS20180099989A1Reduce absorptionOrganic active ingredientsSugar derivativesEmtricitabinePharmaceutical medicine
Nucleosides and nucleotides (nucleos(t)ides) have been in clinical use for almost 50 years and have become cornerstones of treatment for patients with viral infections or cancer. The approval of several additional drugs over the past decade demonstrates that this family still possesses strong potential. Therefore nucleos(t)ide are of great interest as promising chemotherapeutic agents, including: 2′-deoxy-L-uridine (CAS No 31501-19-6), 2′-deoxy-D-uridine (CAS No 951-78-0), telbivudine (CAS No 3424-98-4), zidovudine (AZT, CAS No 30516-87-1), trifluridine (CAS No 70-00-8), clevudine (CAS No 163252-36-6), PSI-6206 (CAS No 863329-66-2), 2′-(5)-2′-chloro-2′-deoxy-2′-fluorouridine (CAS No 1673560-41-2), ND06954 (CAS No 114248-23-6), stavudine (CAS No 3056-17-5), 5-ethynyltavudine (Festinavir, CAS No 634907-30-5), torcitabine (CAS No 40093-94-5), (−)-beta-D-(2R,4R)-dioxolane-thymine (DOT, 1-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-5-methyl-2,4 (1H,3H)-pyrimidinedione, CAS No. 127658-07-5), 2-(6-amino-purin-9-yl)-ethanol (CAS No 707-99-3), 2′-C-methylcytidine (CAS No 20724-73-6), PSI-6130 (CAS No 817204-33-4), gemcitabine (CAS No 95058-81-4), 2′-chloro-2′-deoxy-2′-fluorocytidine (CAS No 1786426-19-4), 2′,2′-dichloro-2′-deoxycytidine (CAS No 1703785-65-2), 2′-C-methylcytidine (CAS No 20724-73-6), PSI-6130 (CAS No 817204-33-4), lamivudine (3TC, CAS No 134678-17-4), emtricitabine (CAS No 143491-57-0), 2′-deoxyadenosine (CAS No 958-09-8), 2′-deoxy-β-L-adenosine (CAS No 14365-45-8), 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine (CAS No 865363-93-5), didanosine (CAS No 69655-05-6), entecavir (CAS No 209216-23-9), FMCA (CAS No 1307273-70-6), dioxolane-G (DOG, CAS No 145514-01-8), β-D-2′-deoxy-2′-(R)-fluoro-2′-β-C-methylguanosine (CAS No 817204-45-8), abacavir (ABC, CAS No 136470-78-5), dioxolane-A (DOA, CAS #145514-02-9), [(2R,4R)-4-(6-cyclopropylamino-purin-9-yl)-[1,3]dioxolan-2-yl]-methanol (CAS No 1446751-04-7), amdoxovir (AMDX, CAS No 145514-04-1), (R)-1-(6-amino-purin-9-yl)-propan-2-ol (CAS No 14047-28-0), and [(2S,5R)-5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yl]-methanol.Macroheterocyclic nucleoside derivative and its analogue of the general formula 1 or general formula 2, a stereoisomer, isotope-enriched analogue, pharmaceutically acceptable salt, hydrate, solvate, or crystalline or polymorphic form thereof,wherein:Ar is aryl or hetaryl;R1 and R2 are not necessarily the same substituents selected from H, F, Cl, CH3, OH;R3 is H or CH3;X is oxygen or ethanediyl-1,1 (C═CH2);Y is CH(R4)(CH2)k, CH(R4)(CH2)mC(O)O(CH2)n;R4 is H or CH3;k has a value from zero to six;m has a value from zero to two;n has a value of one to four;Q is a radical selected from Q1-Q4;wherein: R5 is the substituent selected from H, F, Cl, CH3, OH;the arrow (→) indicates the location, joined by Q1-Q4.
Owner:ALLA CHEM LLC
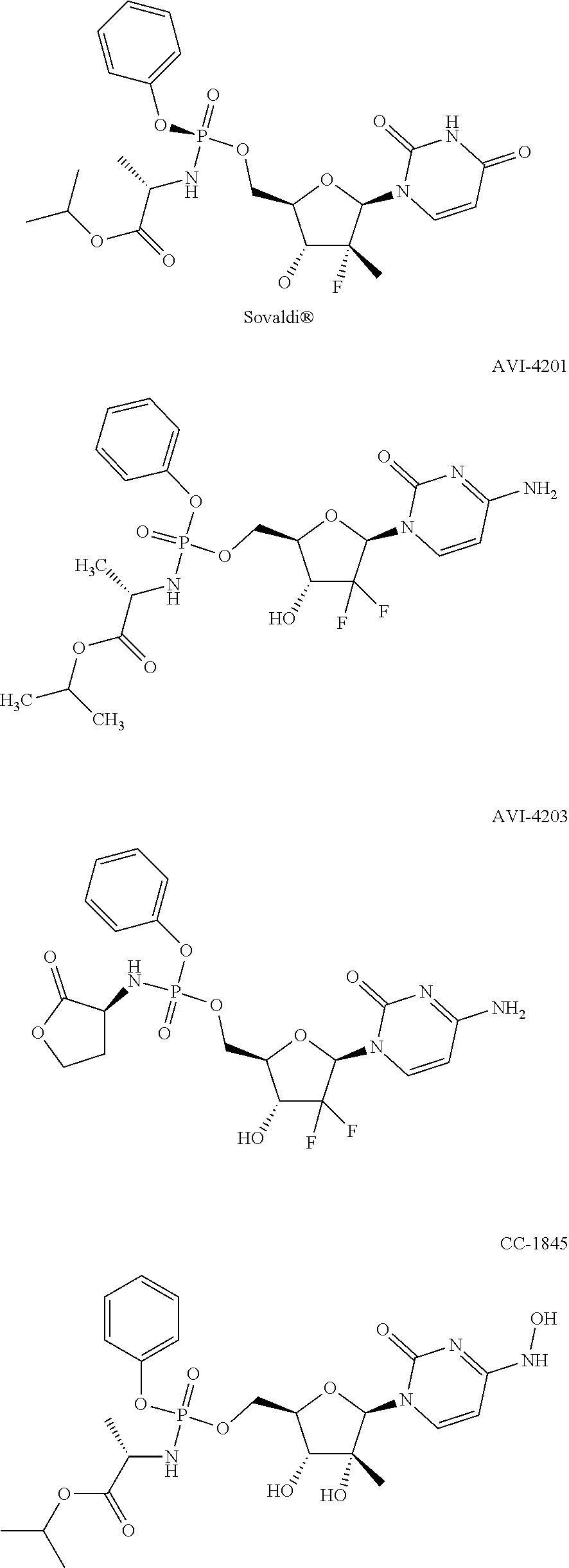
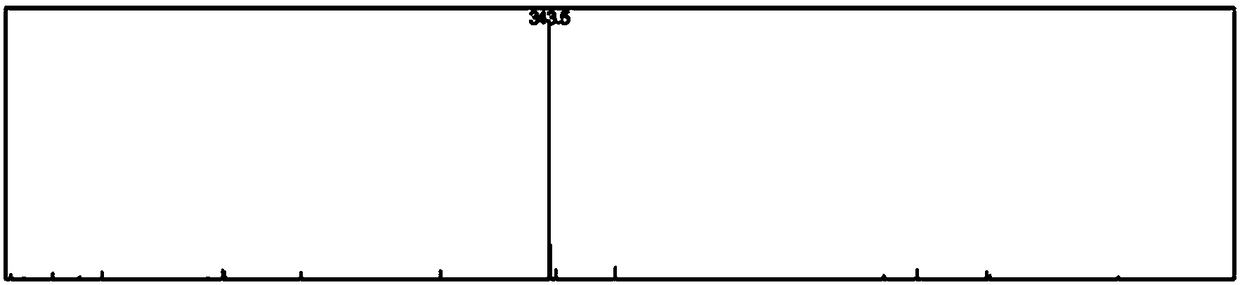
High-performance liquid detection method for impurity of abacavir
The present application discloses a high-performance liquid detection method for an impurity of abacavir. The structural formula of the impurity is formula, wherein R is an alkyl group, an alkenyl group, an alkynyl group, an aryl group, a hydroxyl group, a halogen group, a hydroxyalkyl group, a carboxyl group or an ester group. The method comprises the following steps: providing a sample analysissolution; injecting the sample analysis solution into a high-performance liquid chromatograph for chromatographic analysis and recording a chromatogram map, wherein the chromatographic column used isa reversed phase chromatographic column, and the mobile phase used is a mixed liquor of an acid solution and a solvent phase. In the above manner, the high-performance liquid detection method for an impurity of abacavir in the present application can improve the degree of separation and accuracy of detection.
Owner:SHANGHAI SHYNDEC PHARMA HAIMEN CO LTD
A kind of abacavir individualized drug detection kit and method thereof
ActiveCN105219857BAvoid cross contaminationStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationHLA-BLoop-mediated isothermal amplification
The invention provides an abacavir individualized medication detection kit and method and belongs to the technical field of abacavir medication detection. The Allele-Specific LAMP (loop-mediated isothermal amplification) technology is adopted, specific primers are designed aiming at a G allele of rs2395029, and whether a patient carries HLA (human leukocyte antigen)-B*5701 allele is judged according to the relevance of the G allele of rs2395029 and HLA-B*5701. After detection, whether a positive reaction is performed can be judged through observation of the state change, namely, whether precipitation or color change exists or not, of a reaction system, judgment can be performed with naked eyes, additional device equipment is not needed, and easiness and feasibility are realized.
Owner:BEIJING JINQI BIOLOGICAL TECH CO LTD
Detection method for the concentration of metacavir and its metabolites in biological samples
Owner:GUANGZHOU YIPINHONG PHARMA +4
A method for preparing optics pure abacavir
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
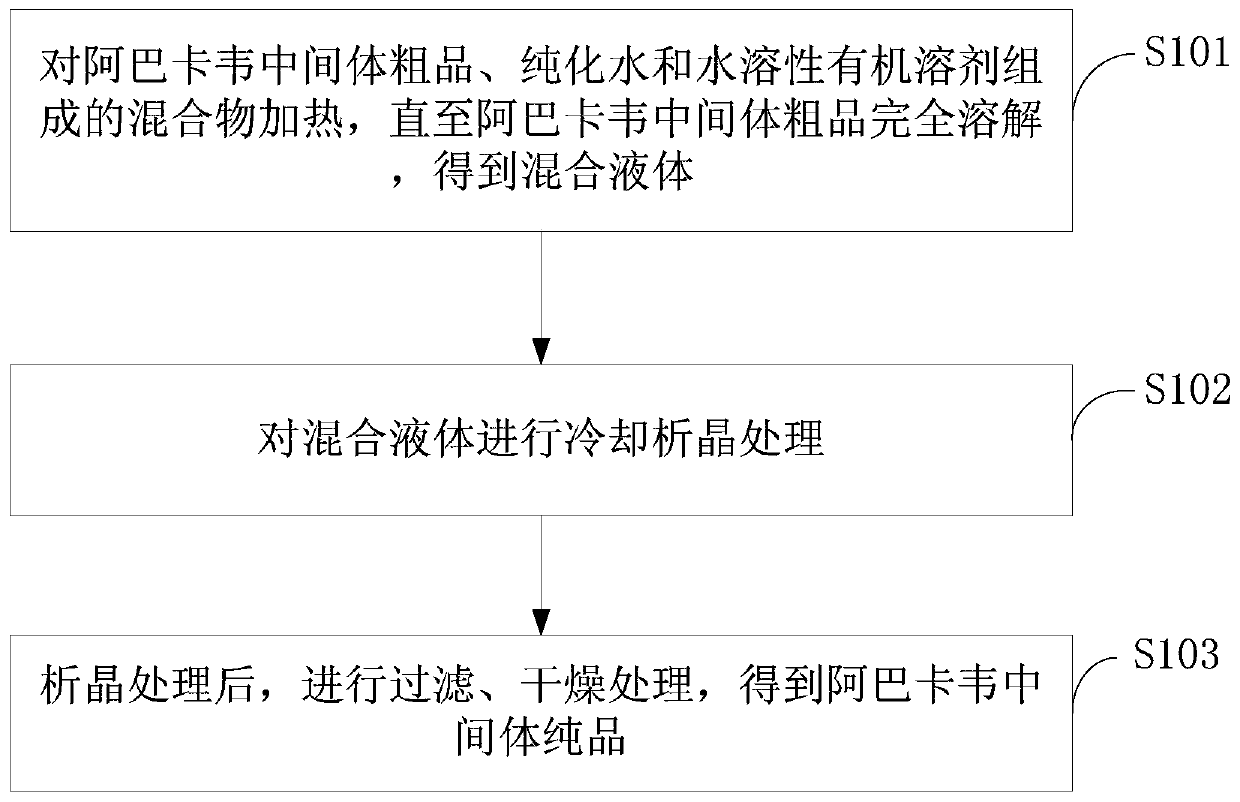
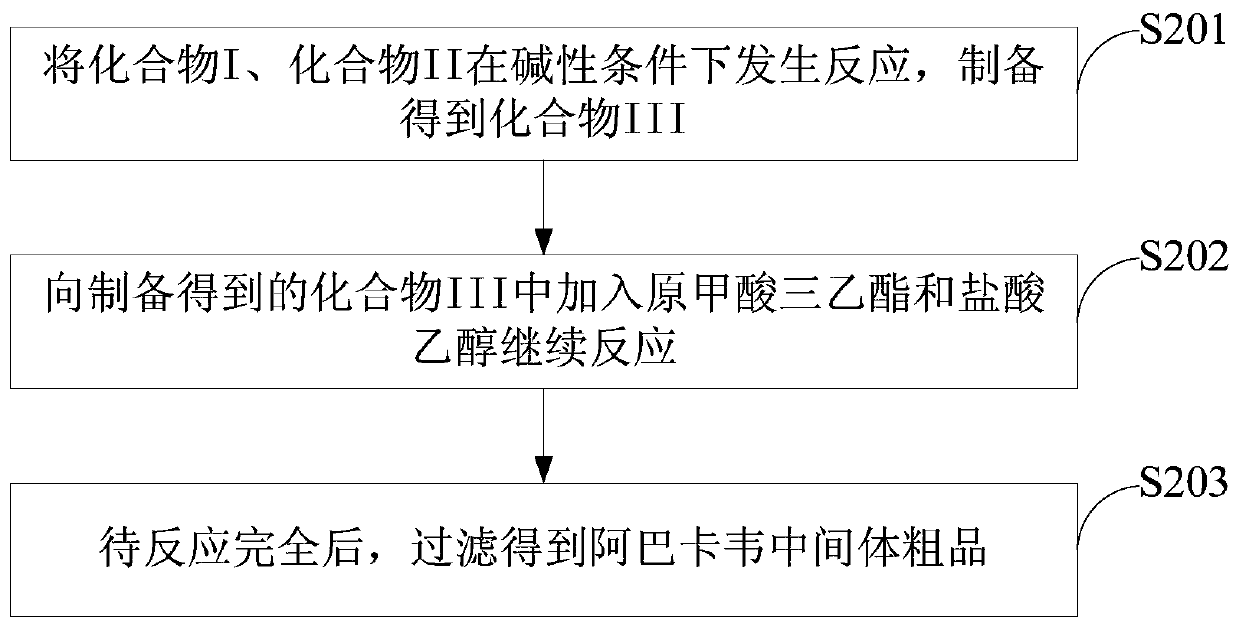
A kind of abacavir intermediate and purification method thereof
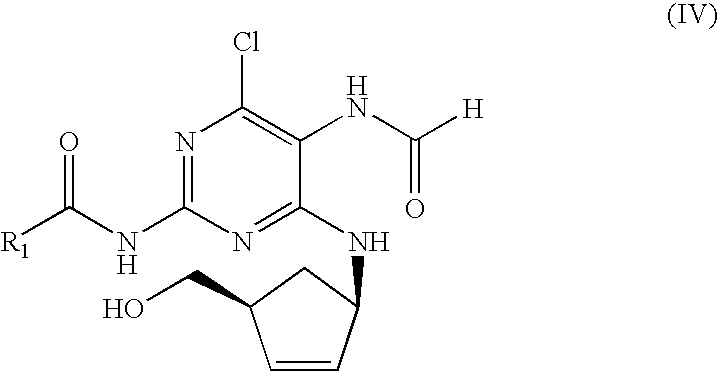
The invention discloses an abacavir intermediate and a method for purifying the same. The method includes heating mixtures with abacavir intermediate crude products, purified water and water-soluble organic solvents until the abacavir intermediate crude products are completely dissolved so as to obtain mixed liquid; carrying out cooling and crystallization treatment on the mixed liquid; carrying out filtering and drying treatment after crystallization treatment is carried out so as to obtain abacavir intermediate pure products. A structural formula of the abacavir intermediate is shown as a formula IV. The abacavir intermediate and the method have the advantage that the method is easy and convenient to implement and is suitable for industrial production.
Owner:SHANGHAI SHYNDEC PHARMA HAIMEN CO LTD +1
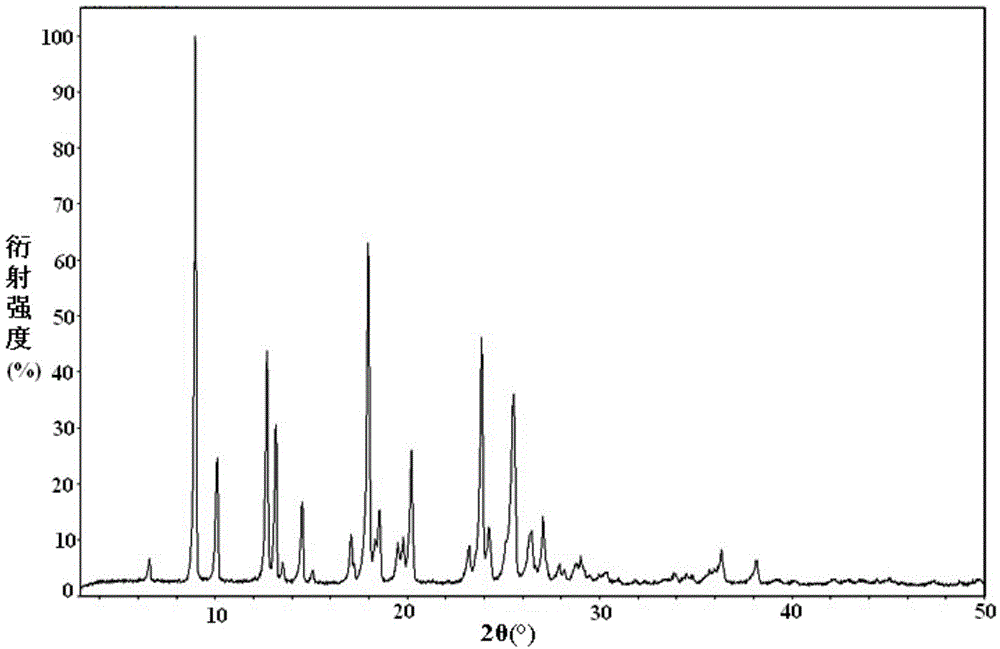
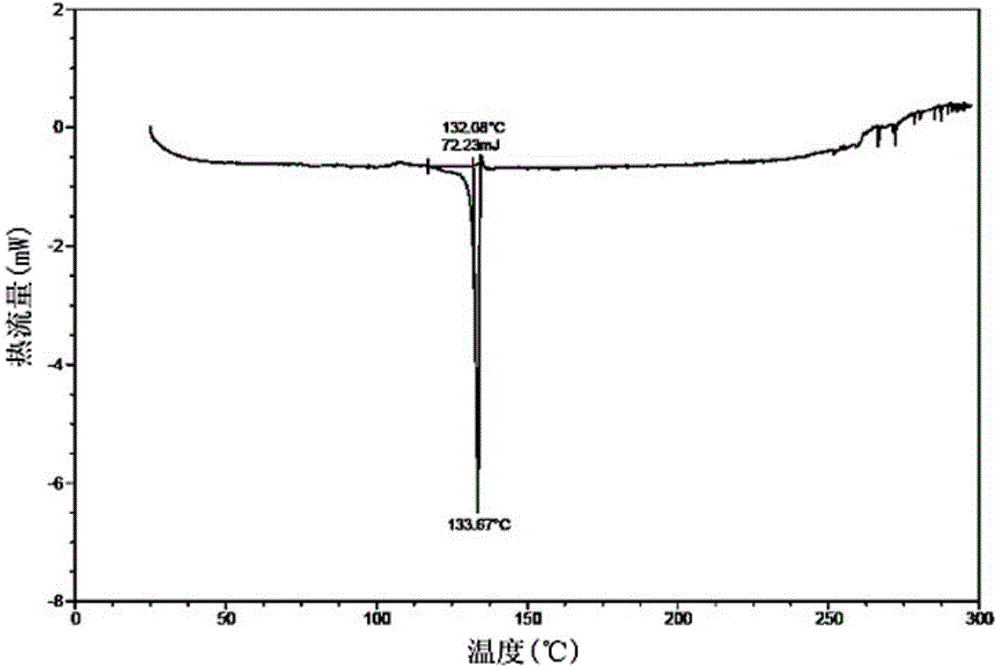
Abacavir crystal and preparation method thereof
The invention discloses an abacavir crystal and a preparation method thereof. Under powder X-ray diffraction, the crystal has main characteristic peaks at the diffraction angle 2[theta] of 9.0 degrees, 10.1 degrees, 12.7 degrees, 13.2 degrees, 17.8 degrees, 20.2 degrees, 23.8 degrees and 25.5 degrees, wherein test error is + / - 0.2 degrees. The preparation method comprises the following steps: adding an abacavir raw material to an organic solvent, increasing the temperature until the solution is clear, dropwisely adding an anti-solvent at 50-60 DEG C, and then performing temperature-maintained stirring for 1-2 h, finally reducing the temperature to room temperature, collecting the separated-out crystal and drying the crystal. The abacavir crystal has stable form, is easy to separate, is high in purity and is stable in quality. The preparation method is simple in process, is easy to operate and achieve in large scale, and is beneficial to development and large-scale production of a follow-up preparation.
Owner:SHANGHAI DESANO CHEM PHARMA +1
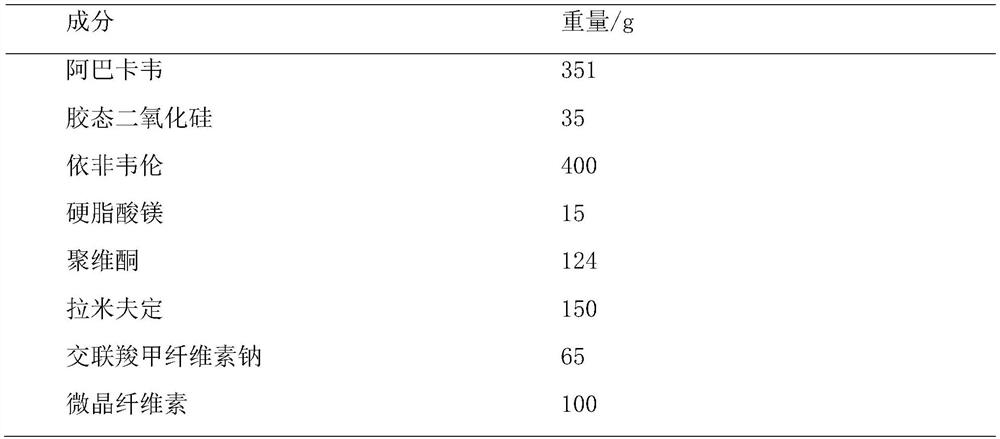
Abacavir, lamivudine and efavirenz compound tablet and preparation method thereof
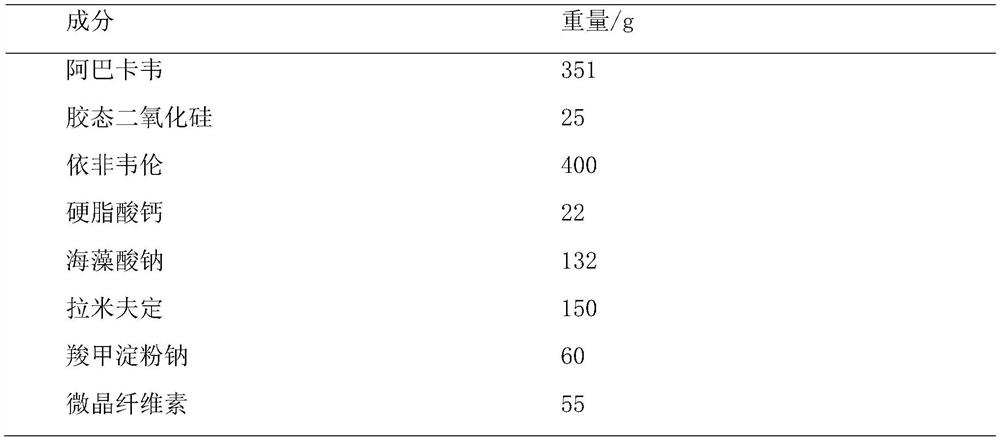
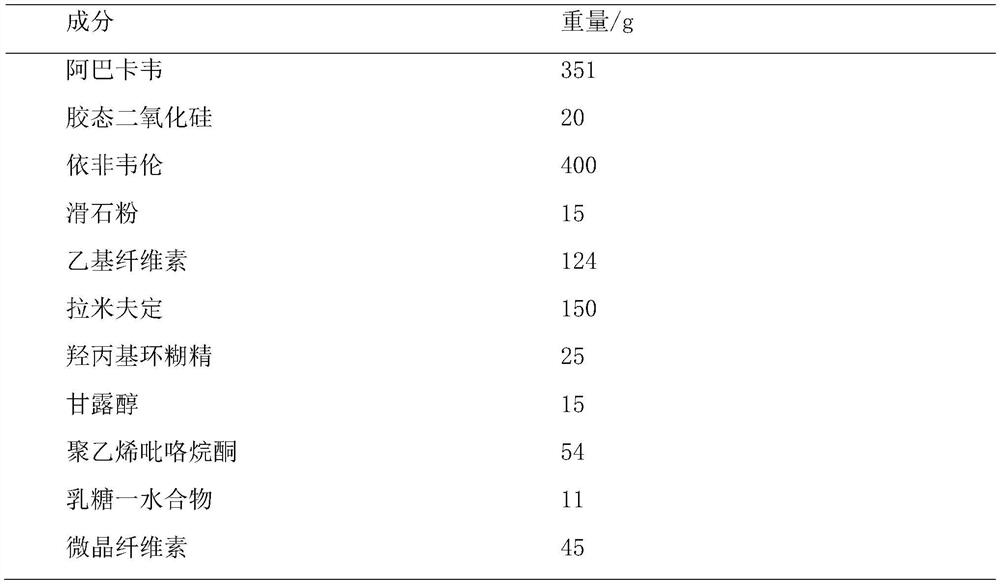
ActiveCN114404377APromote dissolutionDissolution inhibitionInorganic non-active ingredientsAntiviralsAbacavirPharmaceutical Aids
The invention provides abacavir, lamivudine and efavirenz compound tablets and a preparation method, the compound tablets are formed by mixing and tabletting 11-15 parts of first particles, 9-12 parts of second particles and 0.8-2 parts of a flow aid, and the weight ratio of the first particles to the second particles is (1-1.5): 1. According to the compound tablet, abacavir is coated with efavirenz to prepare first particles, efavirenz and lamivudine are mixed to prepare second particles, efavirenz is contained on the surfaces of the particles, only the flow aid is added for mixing, the dosage of auxiliary materials is small, and the uniformity of the tablet is good.
Owner:ANHUI BIOCHEM BIO PHARMA
A kind of high performance liquid phase detection method of the impurity of abacavir
The application discloses a high performance liquid phase detection method for impurities of abacavir. The structural formula of the impurities is wherein, R is an alkyl group, an alkenyl group, an alkynyl group, an aryl group, a hydroxyl group, a halogen, a hydroxyalkyl group, a carboxyl group or an ester The method includes: providing a sample analysis solution; injecting the sample analysis solution into a high-performance liquid chromatograph for chromatographic analysis, and recording the chromatogram; wherein the chromatographic column used is a reversed-phase chromatographic column, and the mobile phase used is acid solution and A mixture of solvent phases. Through the above method, the present application can improve the separation and accuracy of detection.
Owner:SHANGHAI SHYNDEC PHARMA HAIMEN CO LTD
Treatment method of by-product after enzymatic resolution of gamma-lactam
InactiveCN112341350AAvoid wastingImprove use valueOrganic compound preparationAmino-carboxyl compound preparationCyclopenteneMethyl carbamate
The invention provides a treatment method of a by-product after enzymatic resolution of gamma-lactam. The treatment method comprises the following steps of carrying out racemization catalytic reactionon the by-product (+)1-amino-4-formate-2-cyclopentene, then adding methanol, tartaric acid, thionyl chloride and methanol, carrying out a reaction at 65 DEG C, then carrying out vacuum concentrationuntil no fraction exists, adding dichloromethane and water, carrying out stirring for dissolving, then carrying out standing for layering, and washing the separated dichloromethane layer with drinkingwater so as to finally prepare the (-)1-amino-4-methyl formate-2-cyclopentene dichloromethane solution. The method has the beneficial effects that the (-)1-amino-4-formate-2-cyclopentene dichloromethane solution prepared by the method is esterified to obtain the abacavir key intermediate (-)1-amino-4-methyl formate-2-cyclopentene, so that the use value is greatly improved.
Owner:SHANGHAI SHYNDEC PHARMA HAIMEN CO LTD
Crystalline form of abacavir that is essentially free of solvent
InactiveUS9056864B2Improve featuresGood processing characteristicsBiocideOrganic chemistryCrystallographyChemical compound
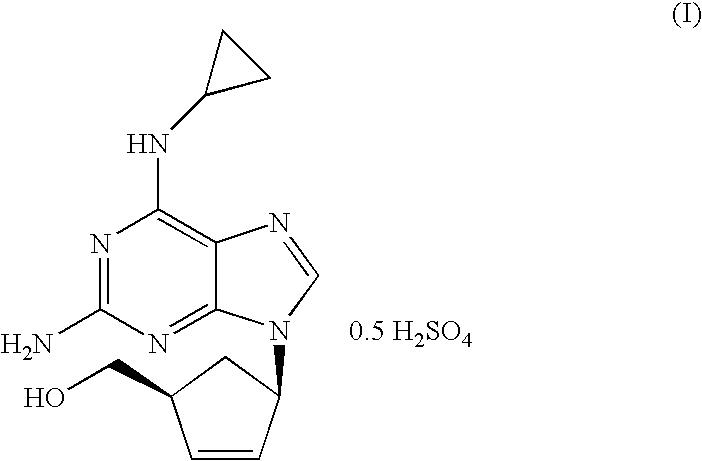
Crystalline form of abacavir that is essentially free of solvent of formula (I), in particular crystalline Form I, and its preparation process which comprises the following steps: a) crystallizing abacavir from a solution of said compound in a (C1-C4)-alcohol, dichloromethane, acetonitrile / water, or mixtures thereof; b) isolating the crystalline form of abacavir that appears in the prior step; and c) removing the solvent from the crystalline form of abacavir thus obtained. Crystalline Form I can also be obtained by dispersion of abacavir in acetonitrile. The crystalline form of abacavir that is essentially free of solvent is useful for the preparation of pharmaceutical compositions for use in the treatment and / or prophylaxis of HIV infections.
Owner:ESTEVE QUIMICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com