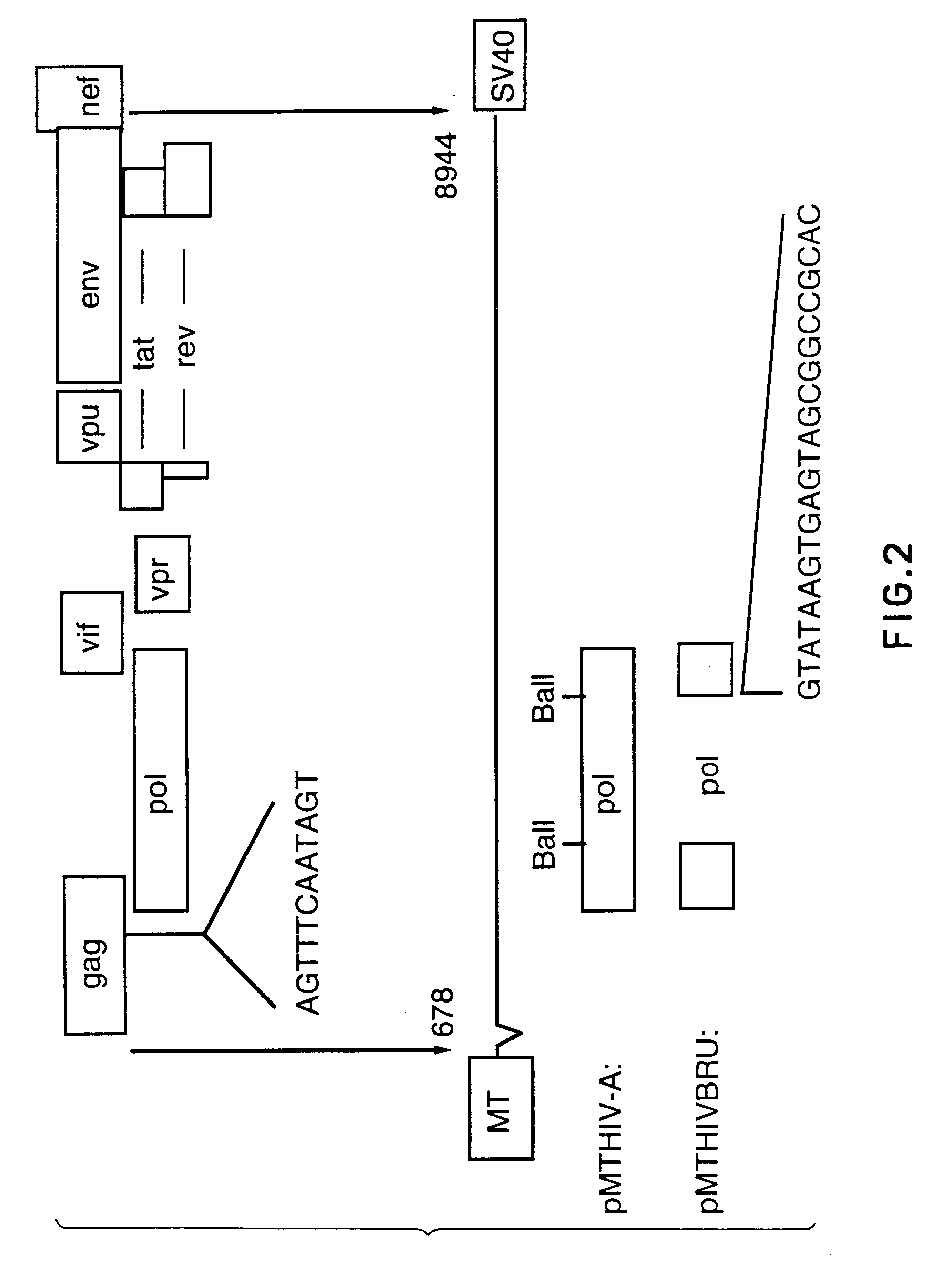
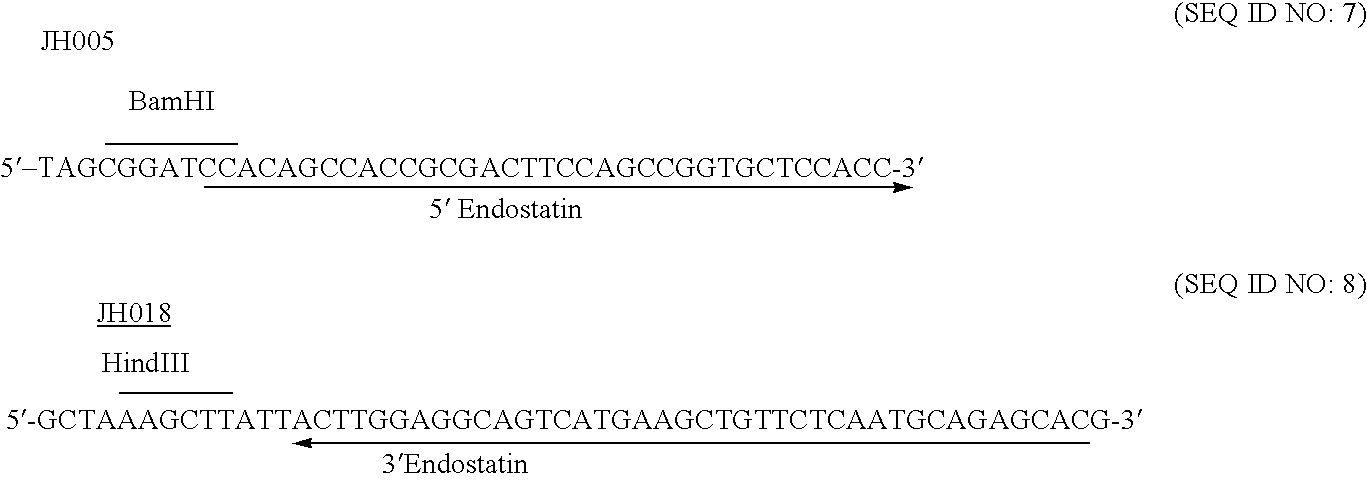Patents
Literature
169 results about "Anti retro viral" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of antiretroviral. : acting, used, or effective against retroviruses. All four drugs, which inhibit HIV protease and thus interfere with viral maturation and replication, are the most potent antiretroviral agents available to treat patients with HIV disease.
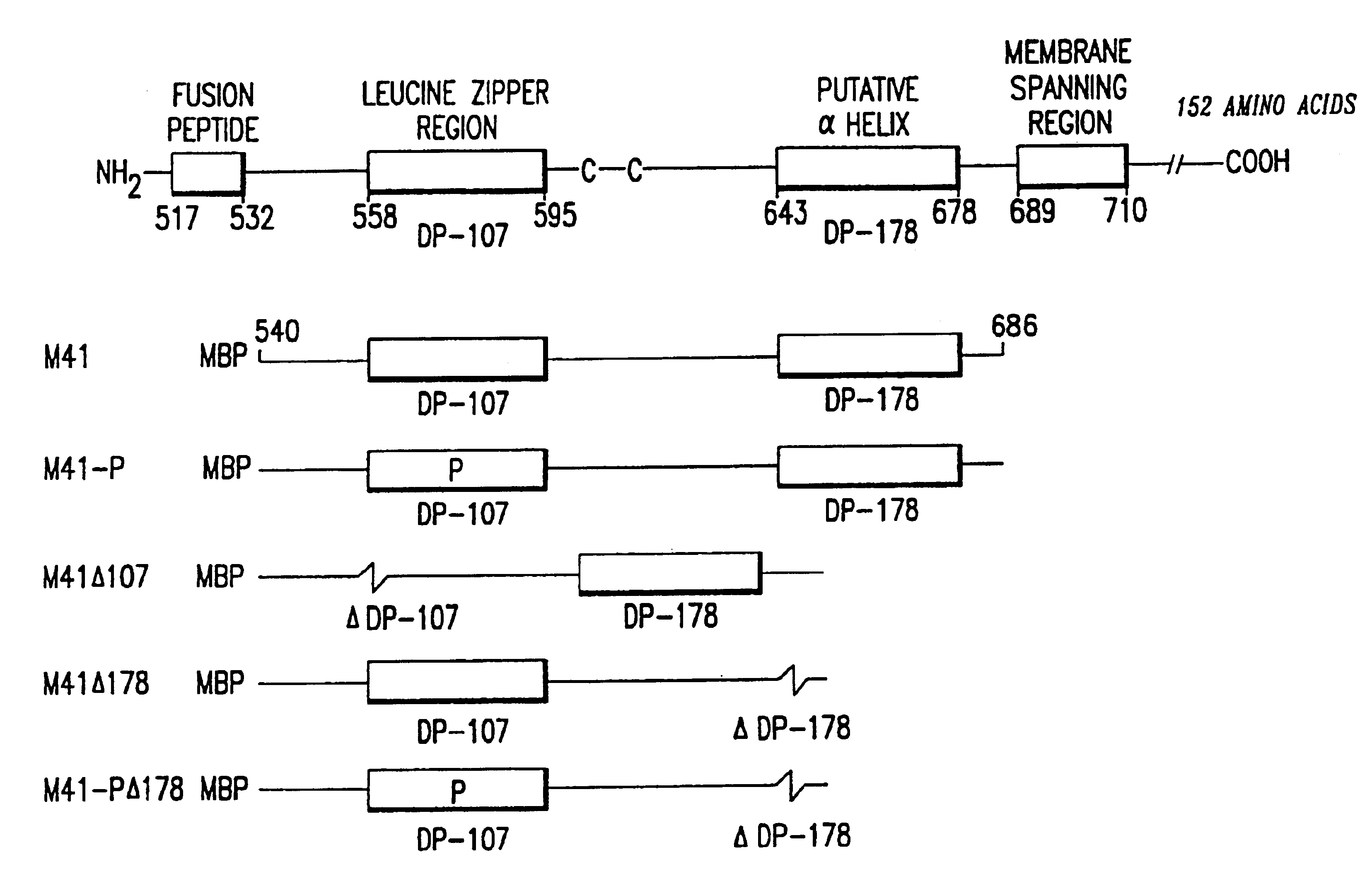
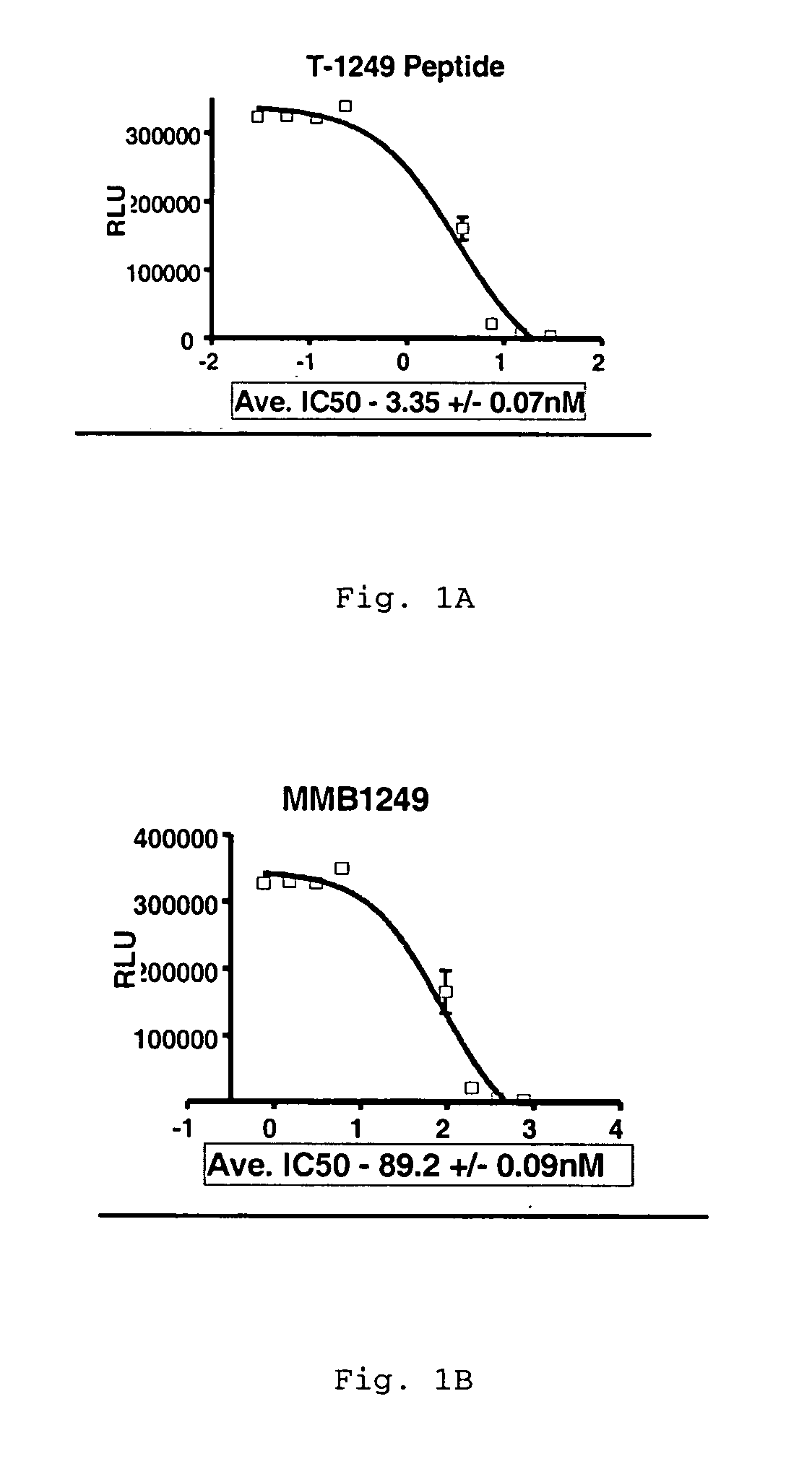
Measles virus peptides with antifusogenic and antiviral activities
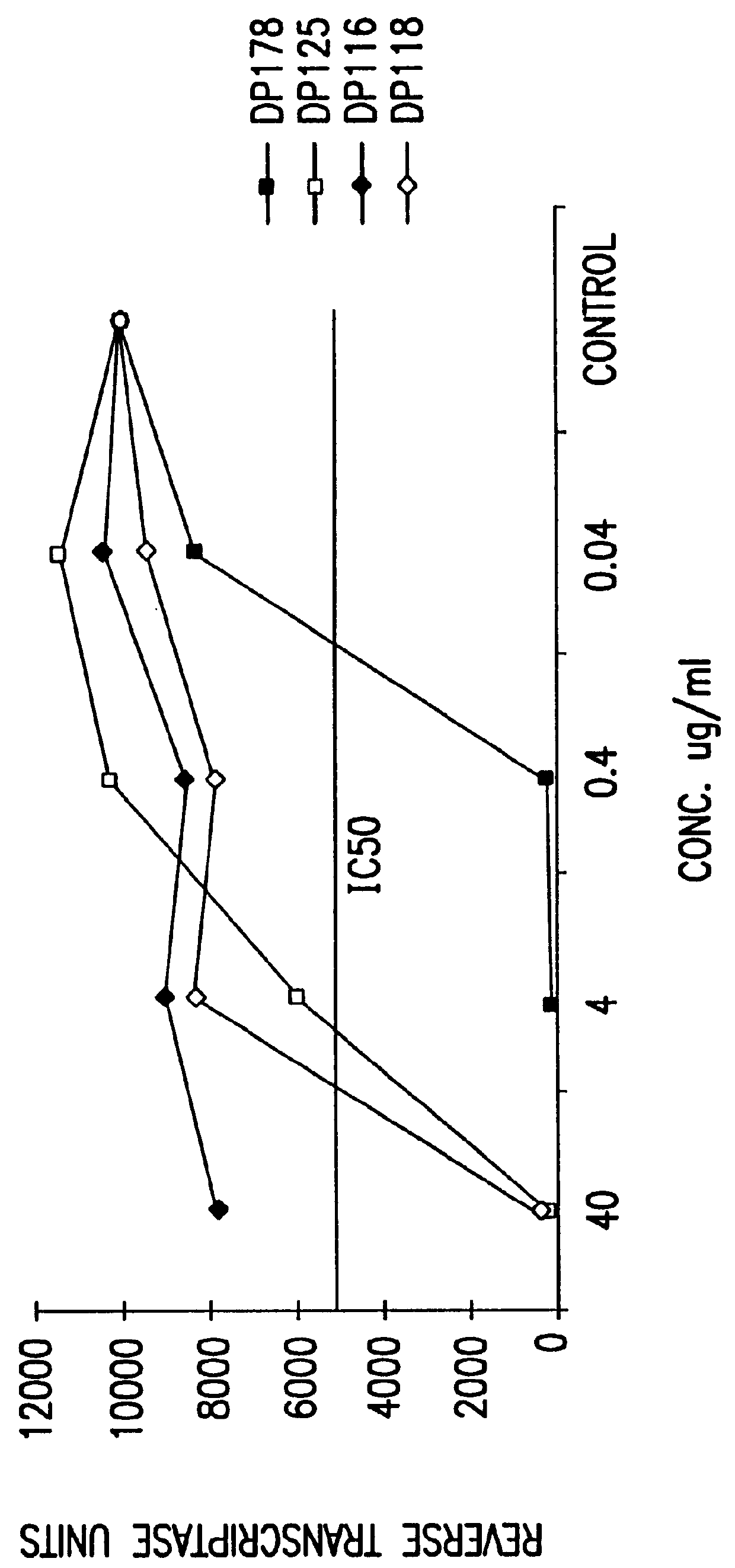
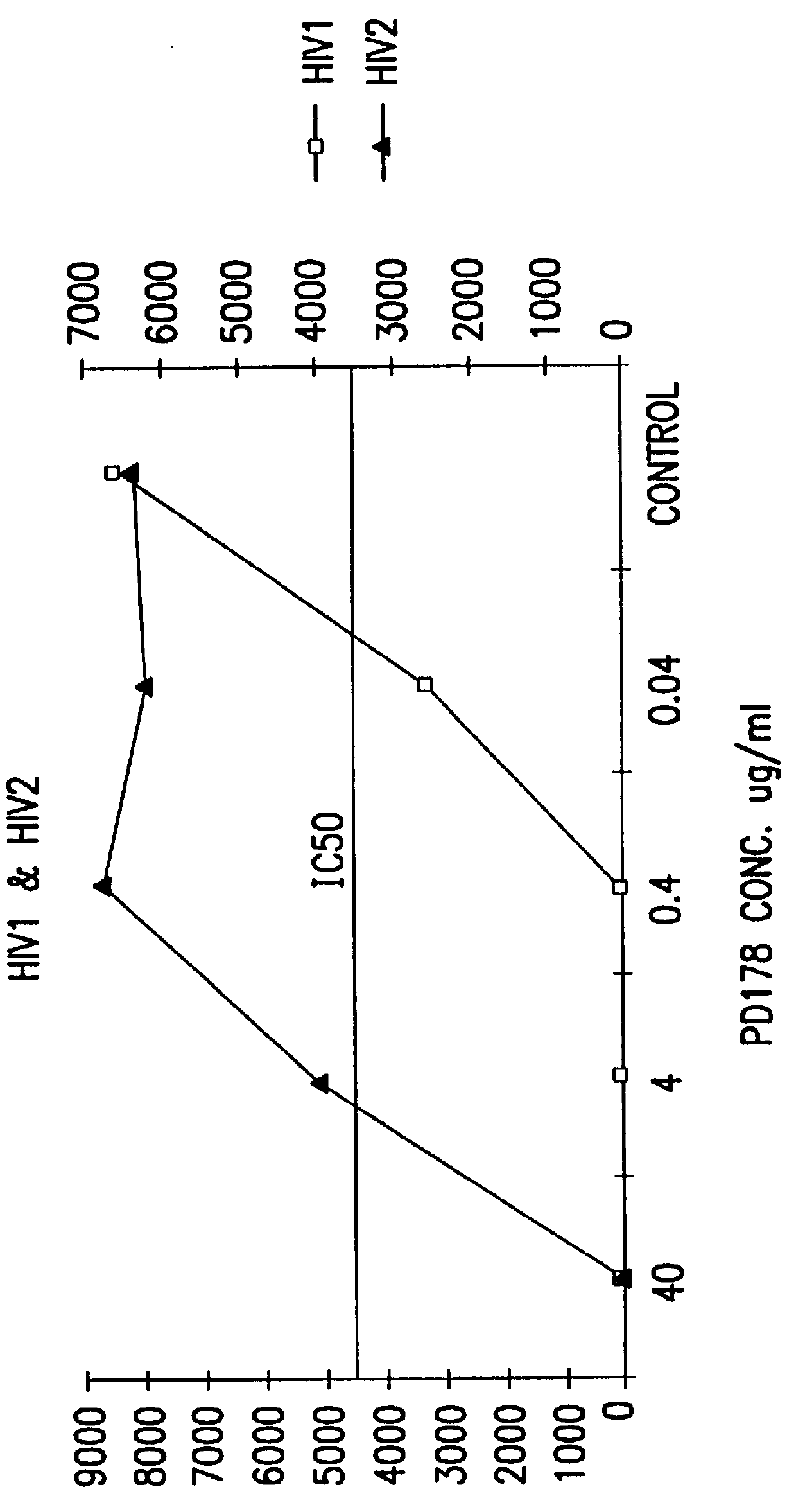
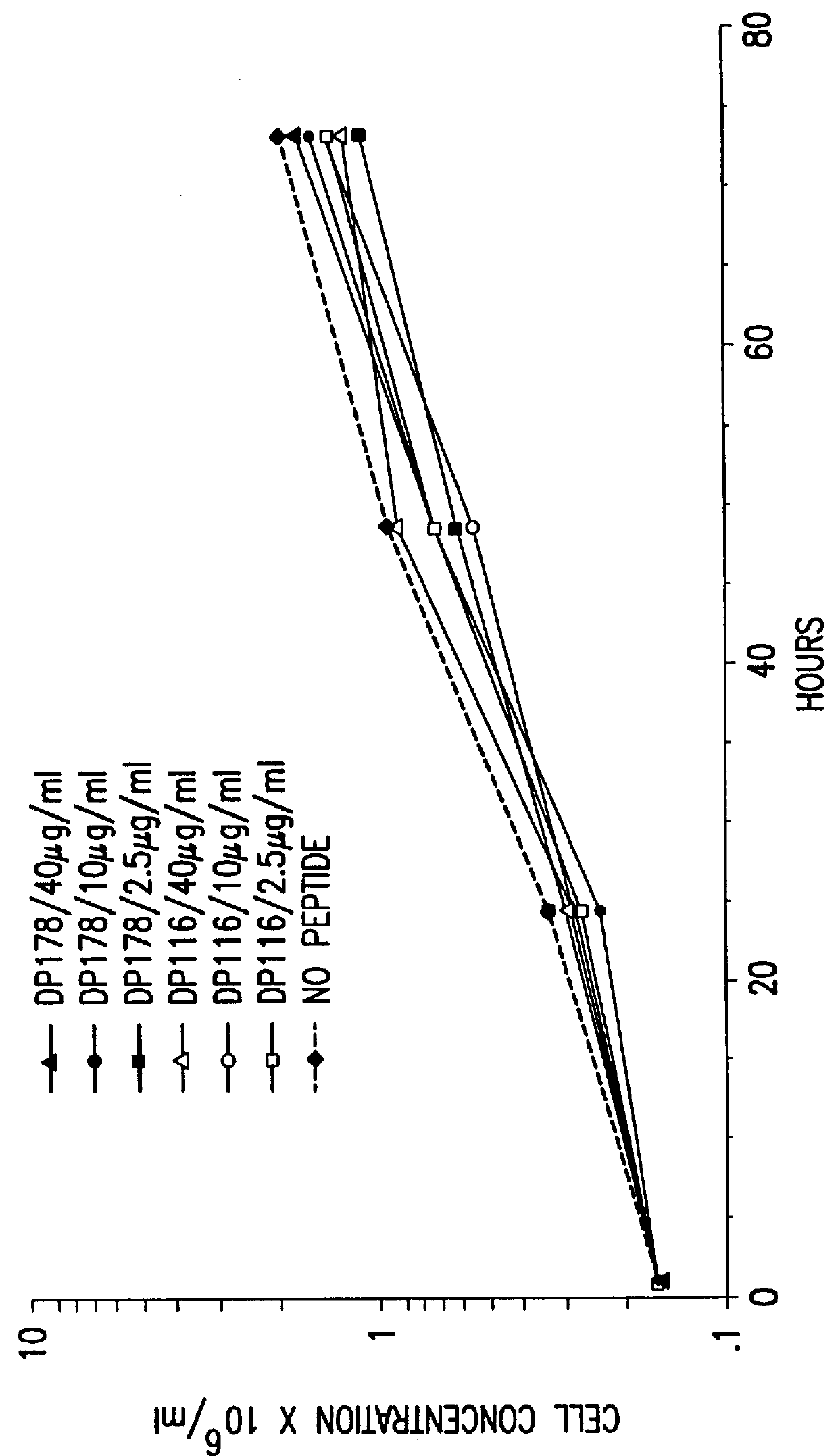
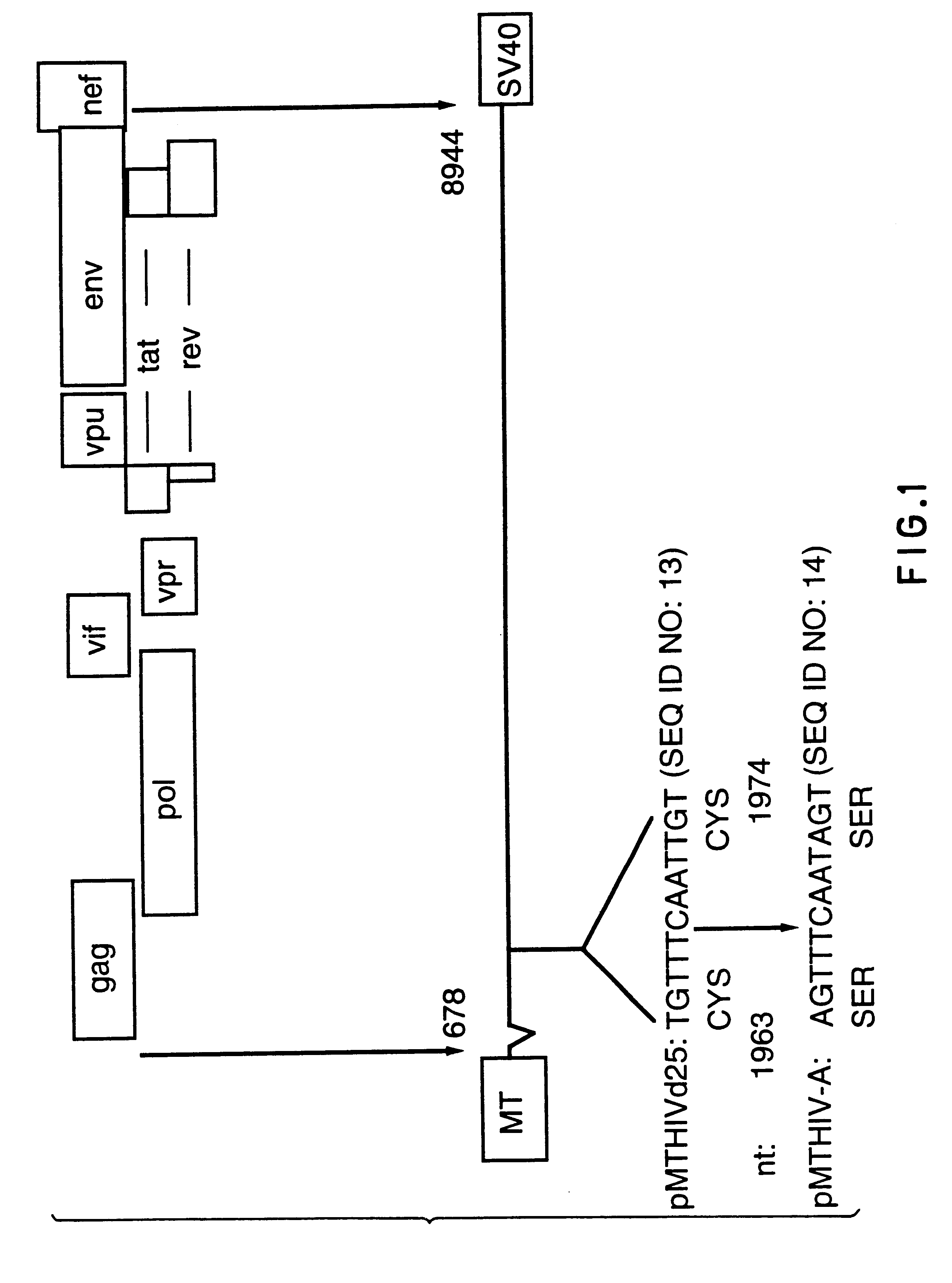
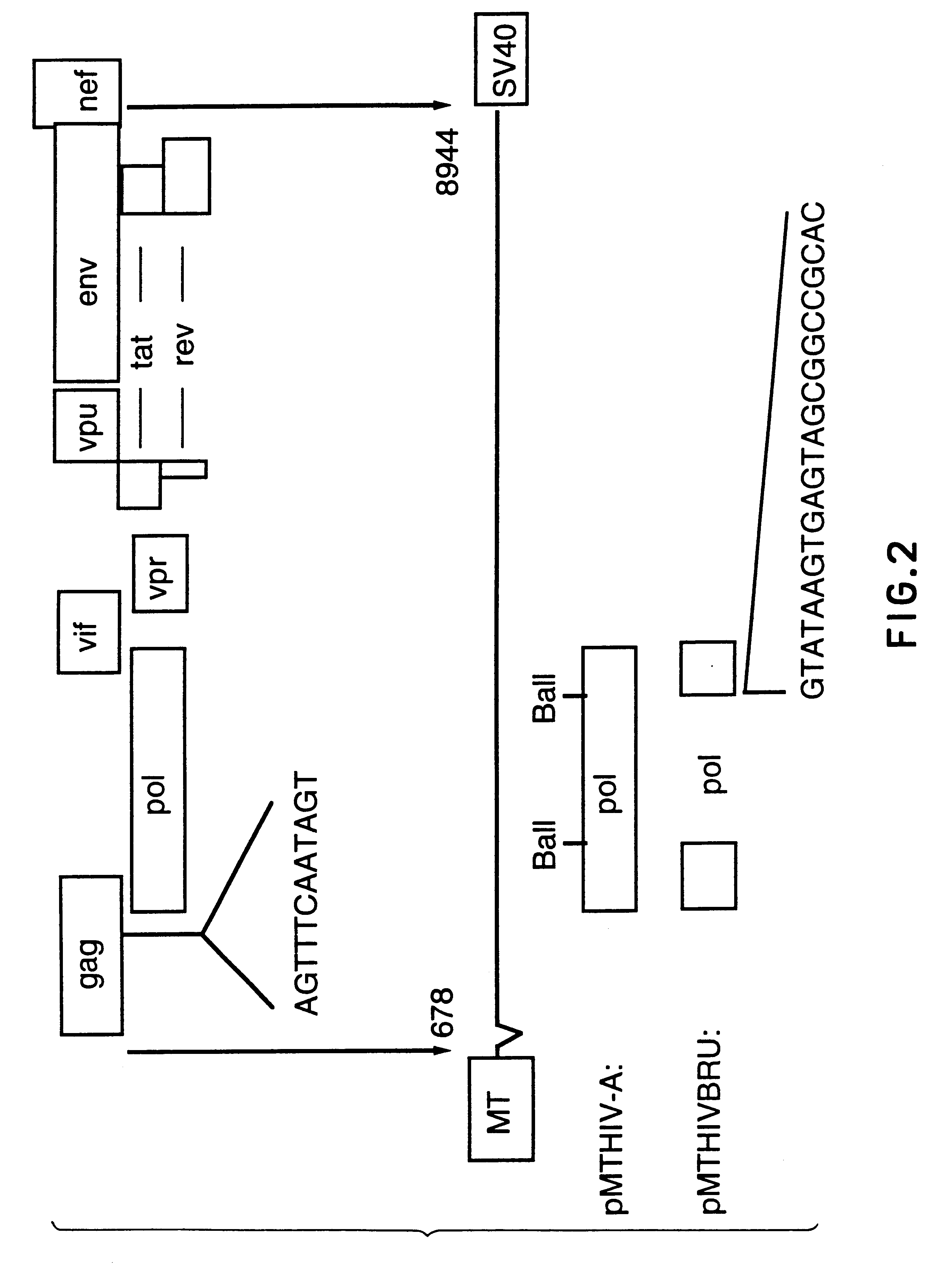
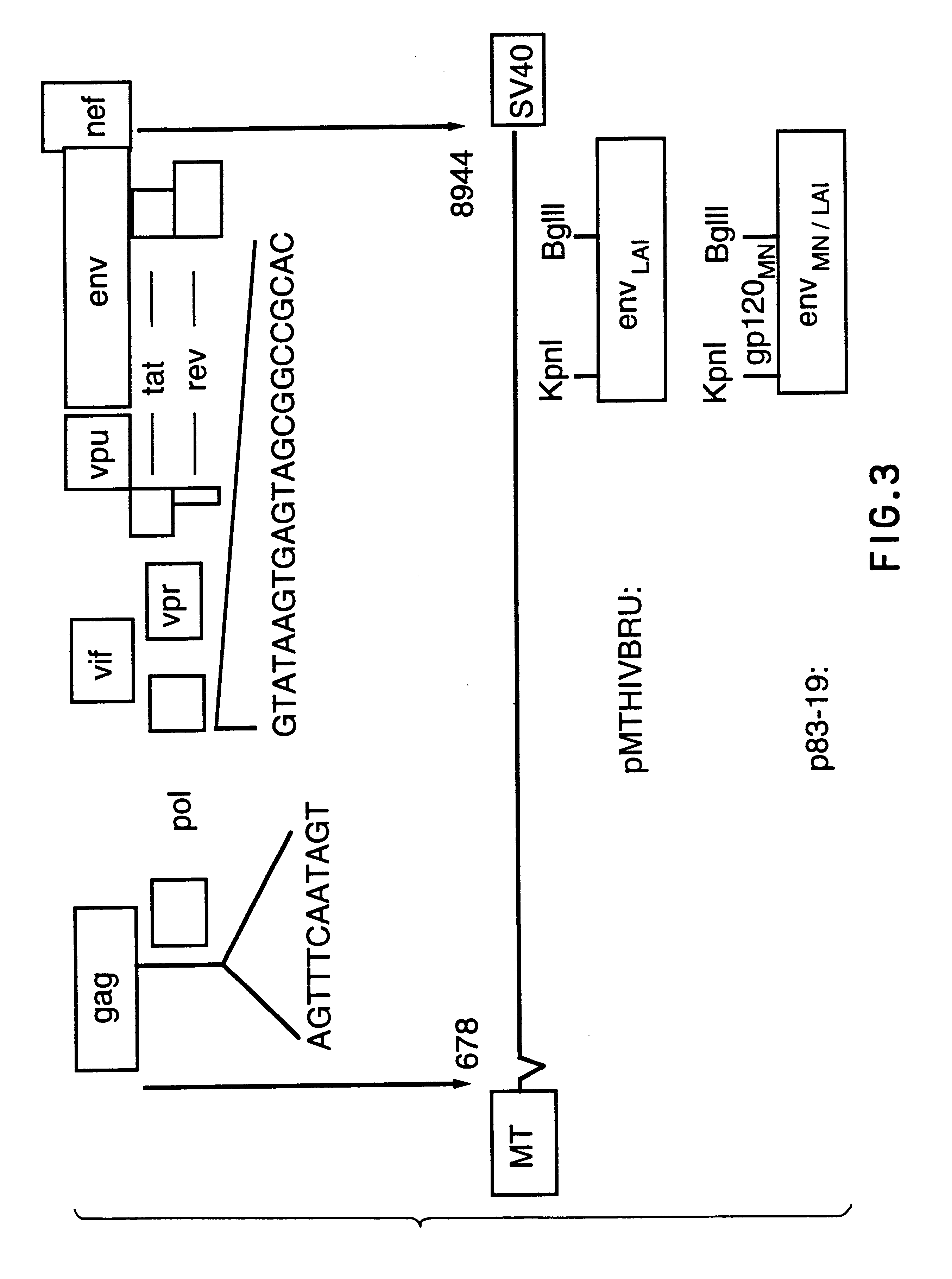
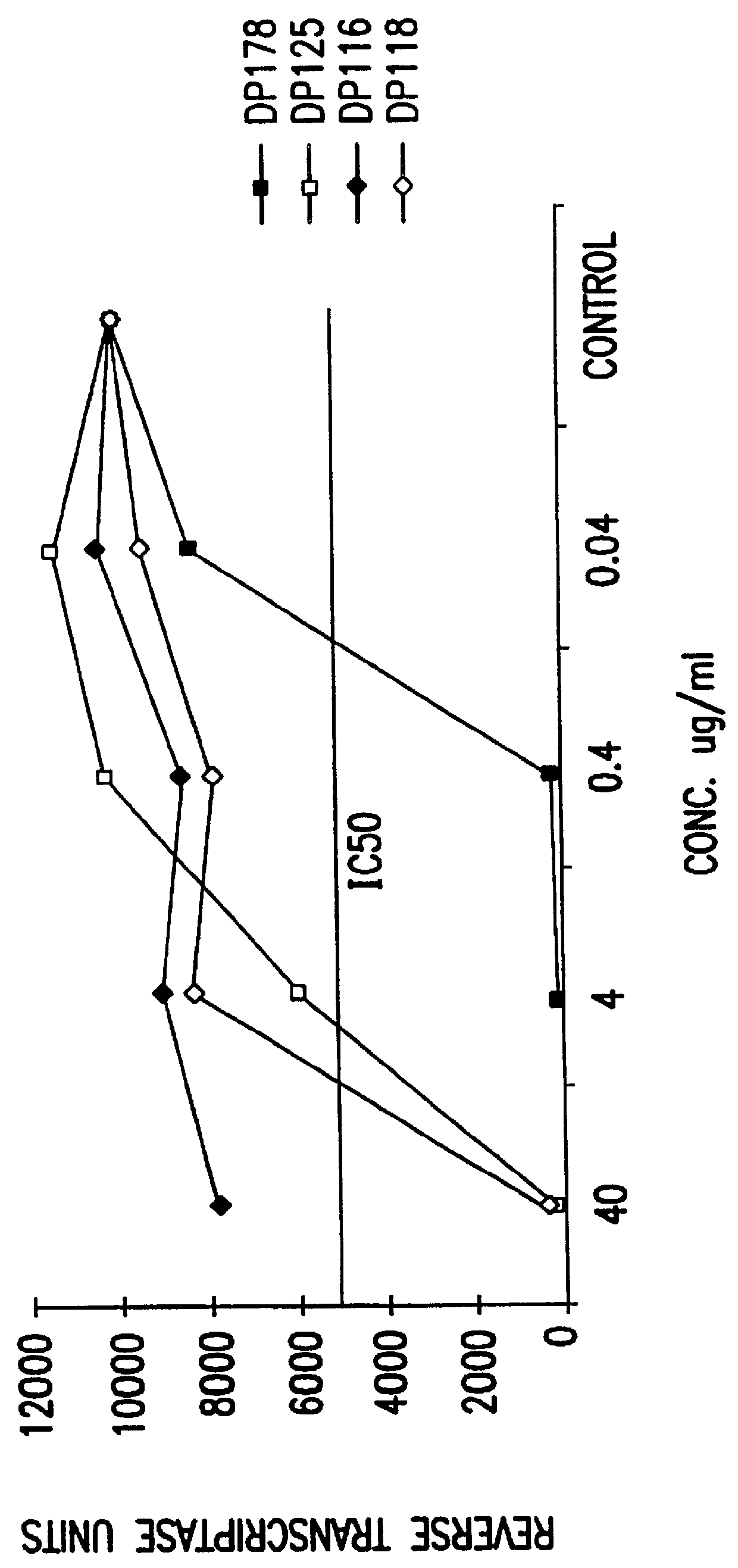
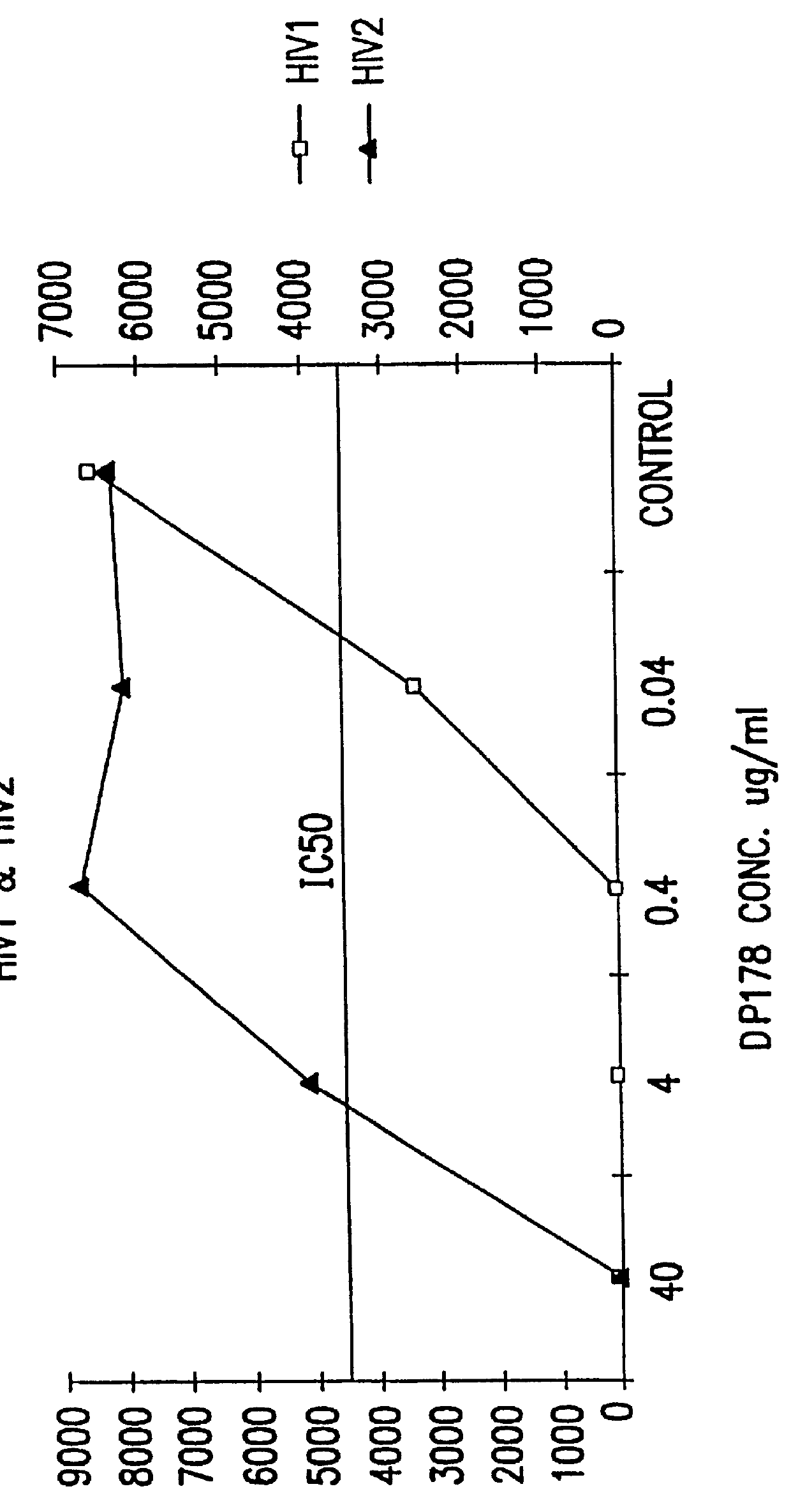
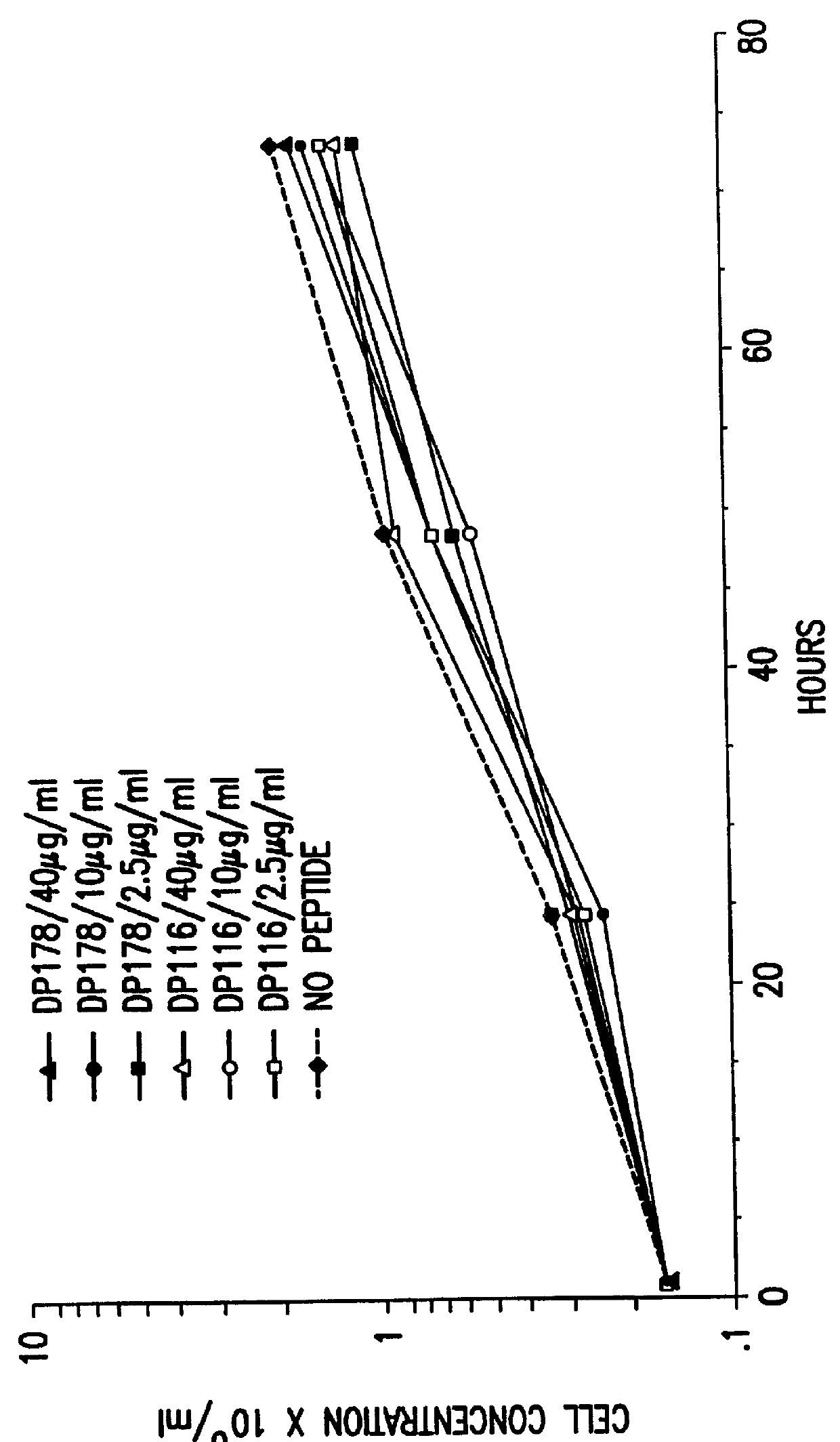
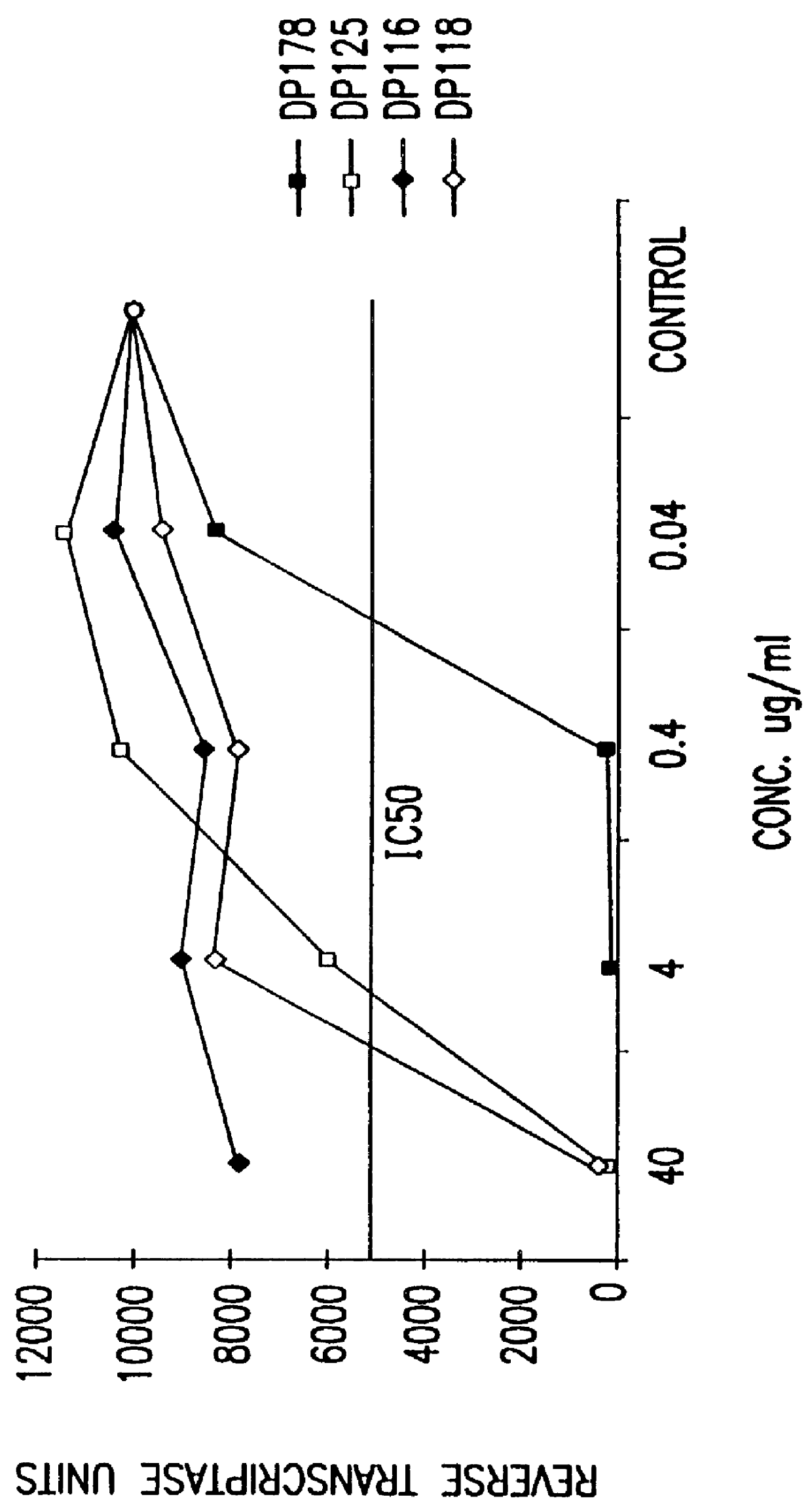
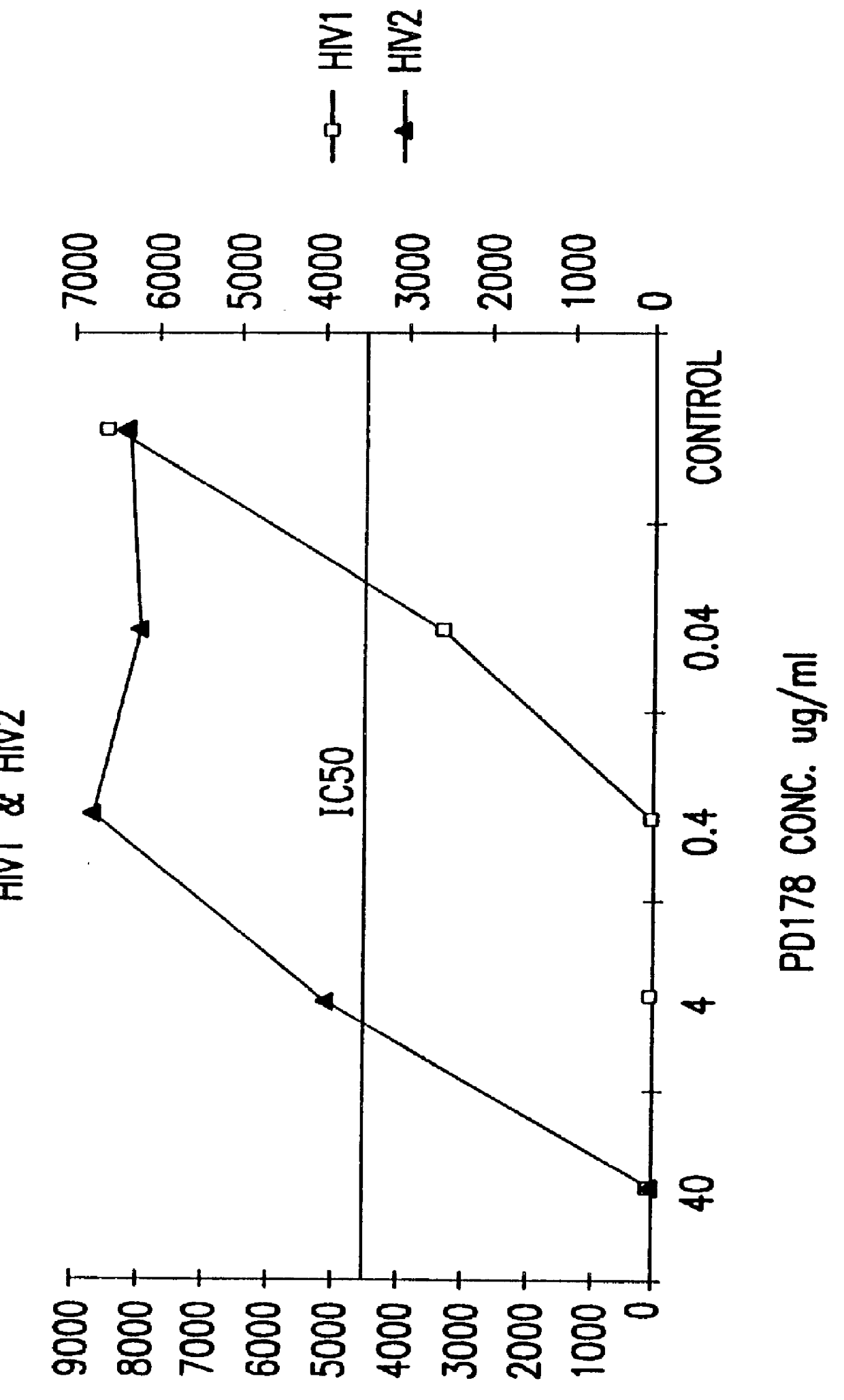
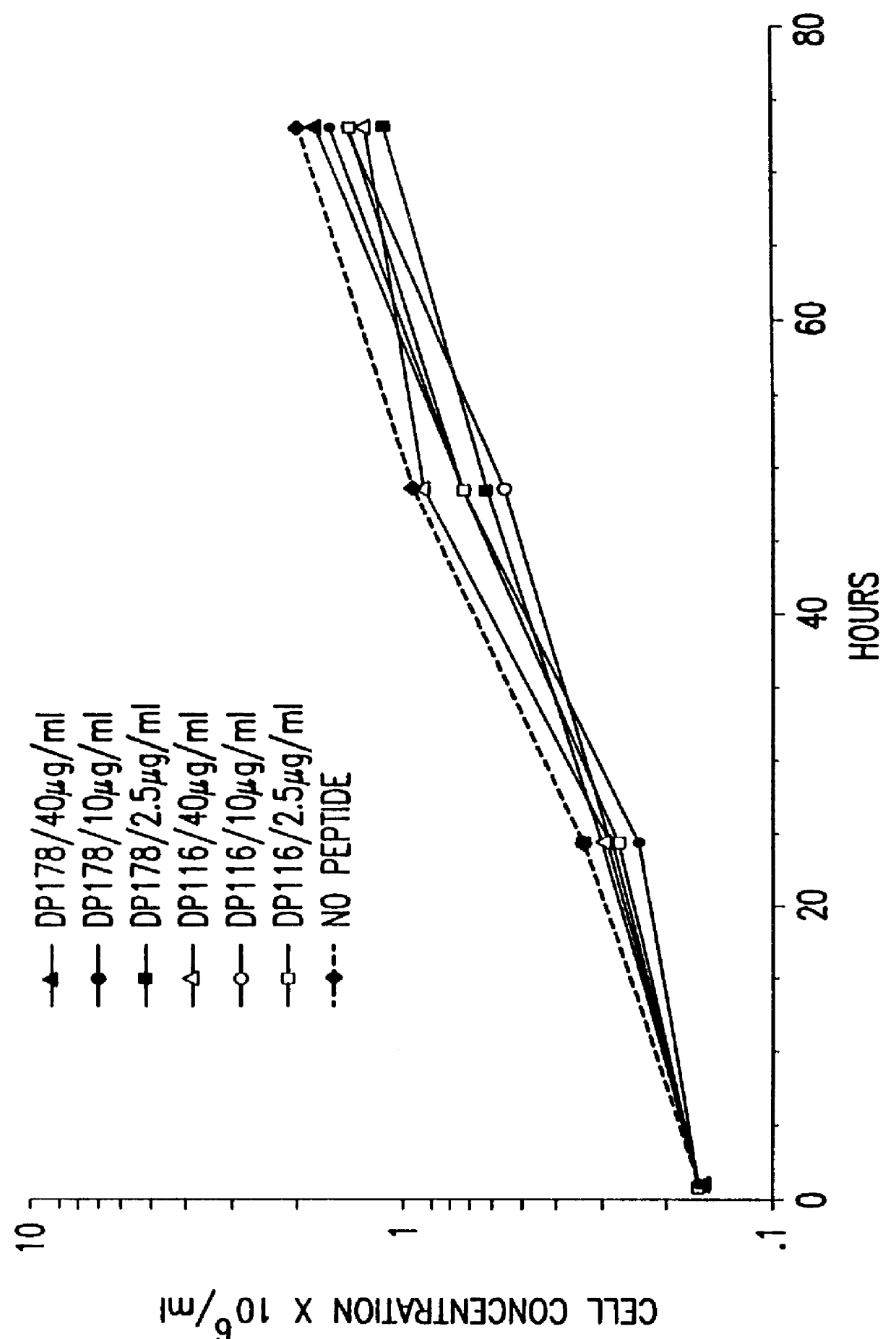
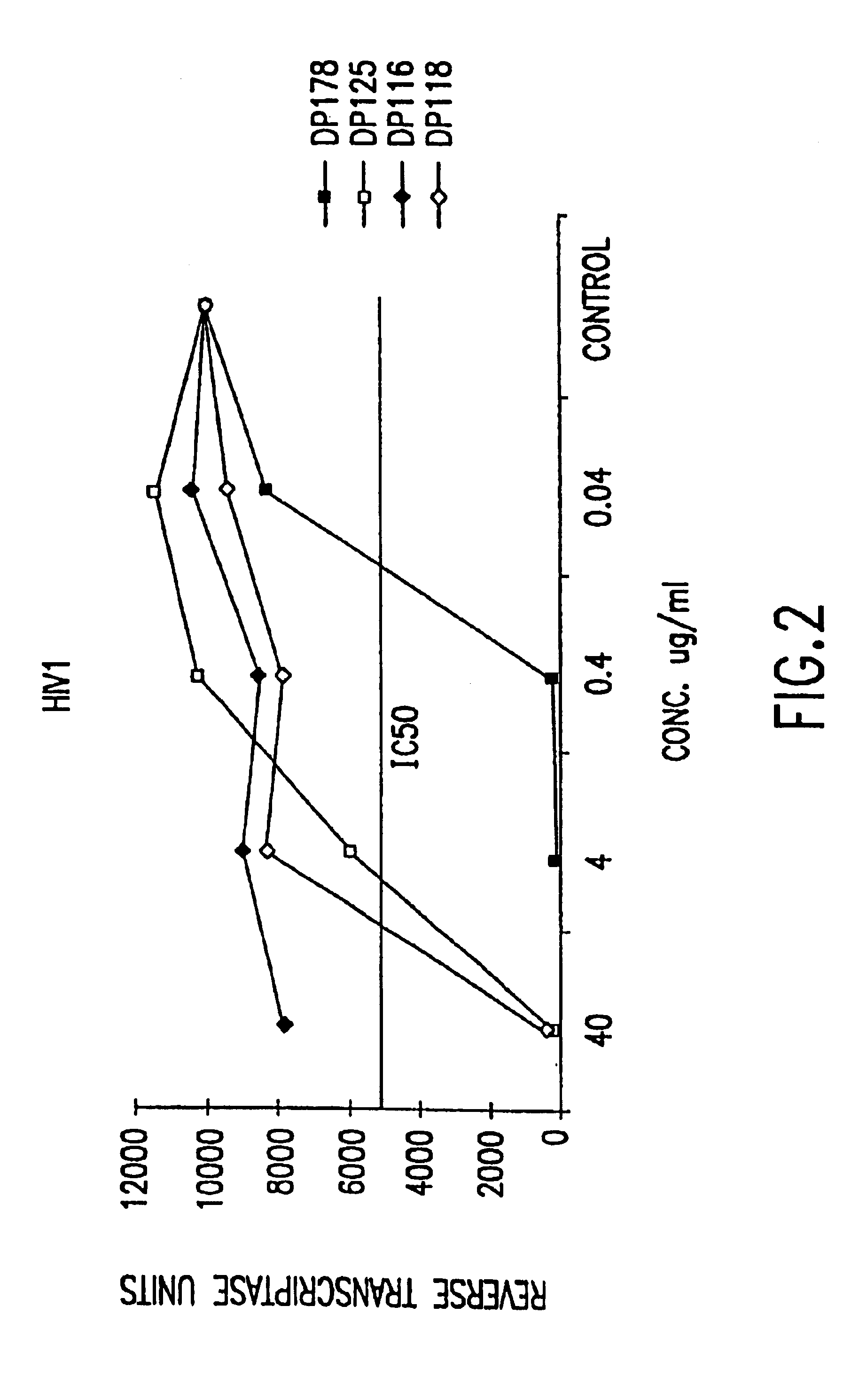
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:TRIMERIS
Antigenically-marked non-infectious retrovirus-like particles
InactiveUS6291157B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:CONNAUGHT LAB
Synthetic peptide inhibitors of HIV transmission
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP-178 (SEQ ID:1) ptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP-178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:DUKE UNIV
Screening assays for compounds that inhibit membrane fusion-associated events
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:TRIMERIS
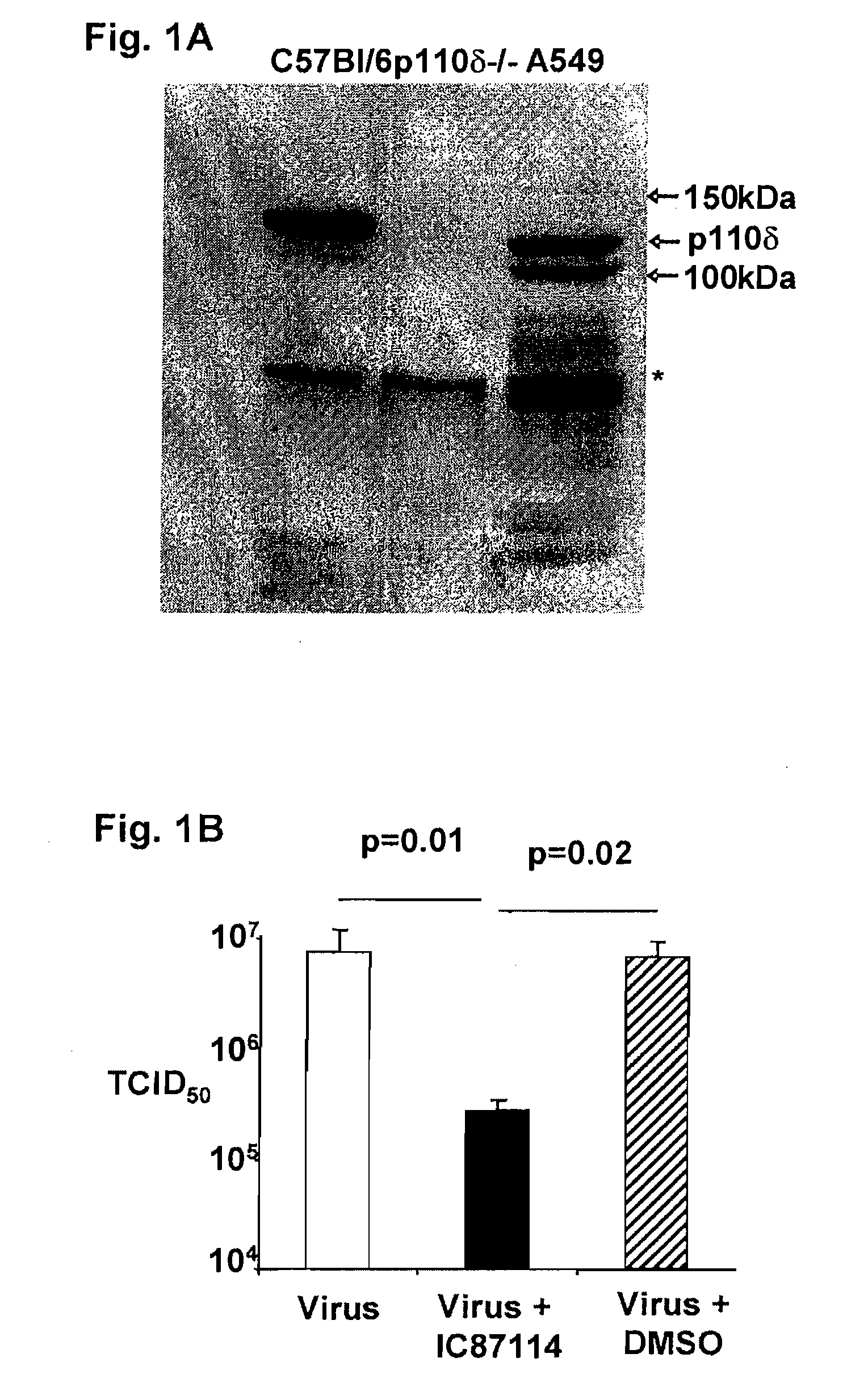
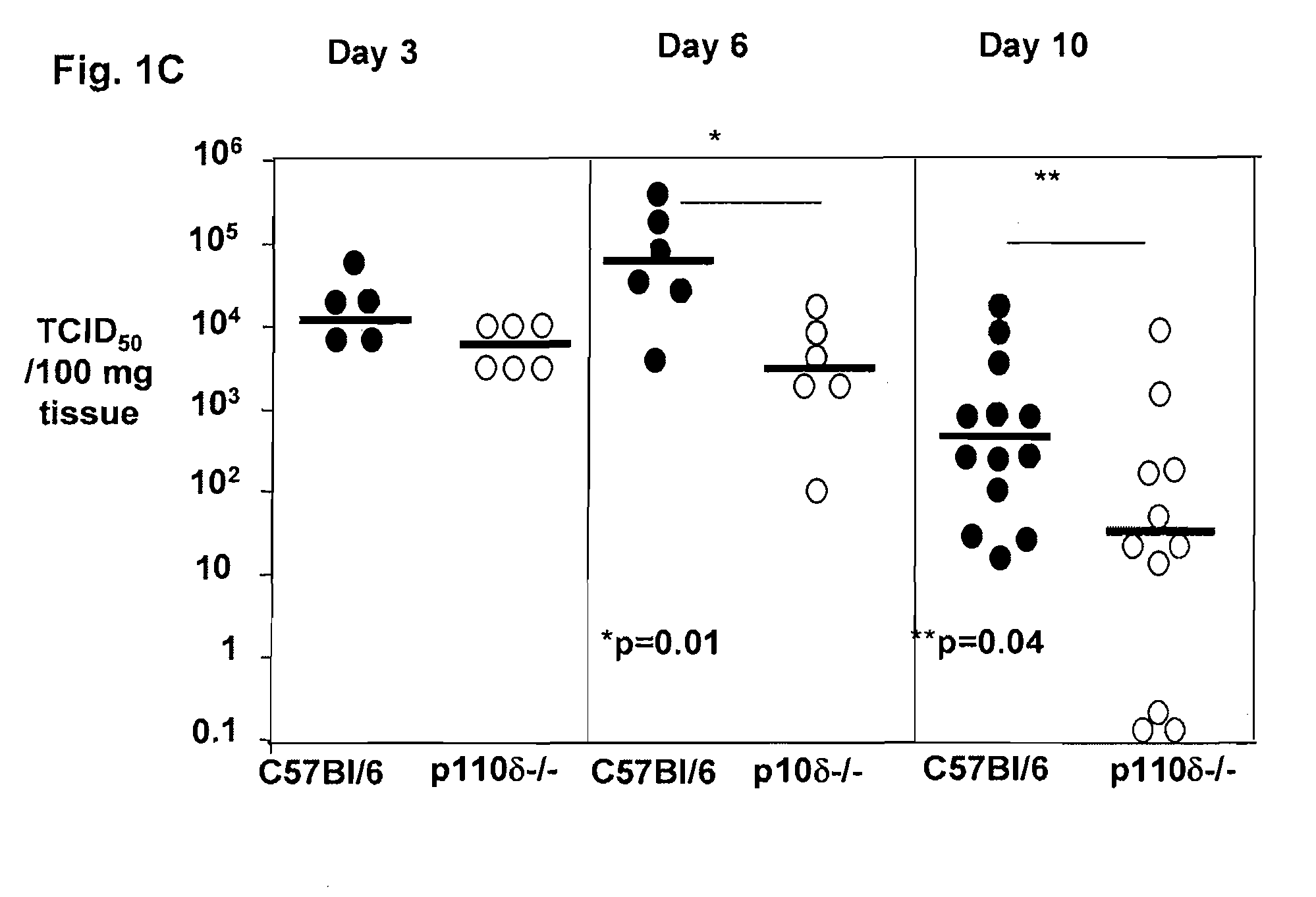
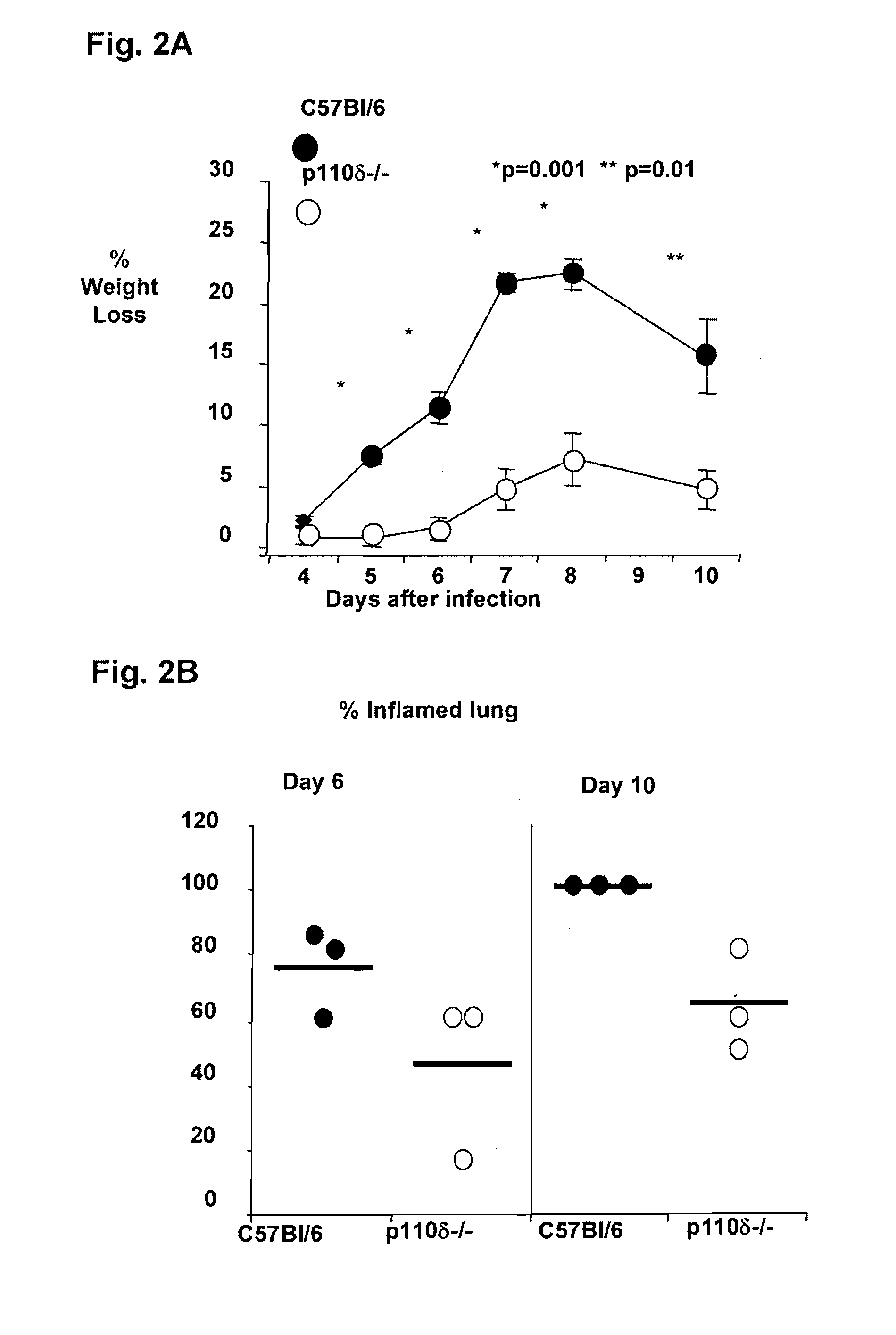
Role of PI3K p110 delta Signaling in Retroviral Infection and Replication
InactiveUS20110135655A1Avoid infectionSsRNA viruses negative-senseBiocideVirus-RetrovirusRetroviral infection
The invention includes compositions and methods for regulating PI3K p110 delta as an anti-retroviral therapy. The invention includes inhibiting p110 delta, a component of PI3K p110 delta signaling pathway, or any combination thereof in a cell as an anti-retroviral therapeutic approach for treating a retroviral infection, for example HIV. The invention includes a method of modulating PI3K p110 delta in a cell infected with a retrovirus by contacting the cell with an effective amount of a composition comprising an inhibitor of PI3K p110 delta.
Owner:DREXEL UNIV +1
Combination therapy comprising the use of protein kinase C modulators and Histone Deacetylase inhibitors for treating HIV-1 latency
InactiveUS20100166806A1Adverse propertyPrevent HIV-1-induced cytotoxicityBiocideOrganic chemistryReverse transcriptaseHydroxamic acid
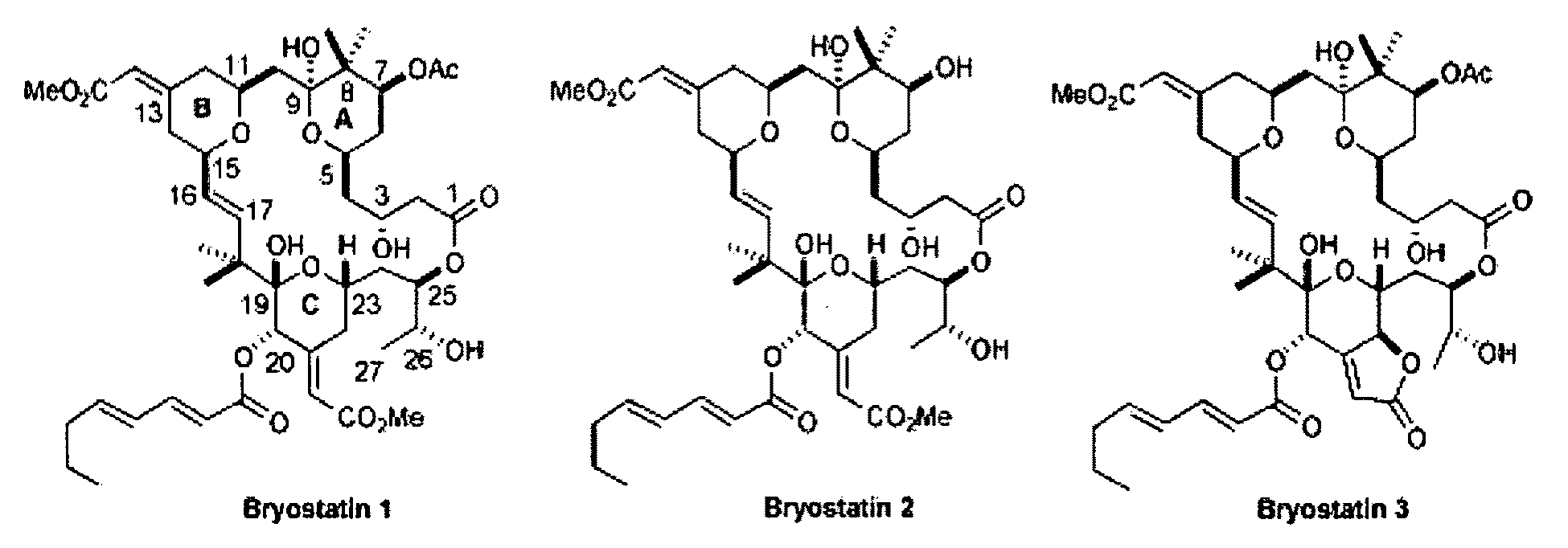
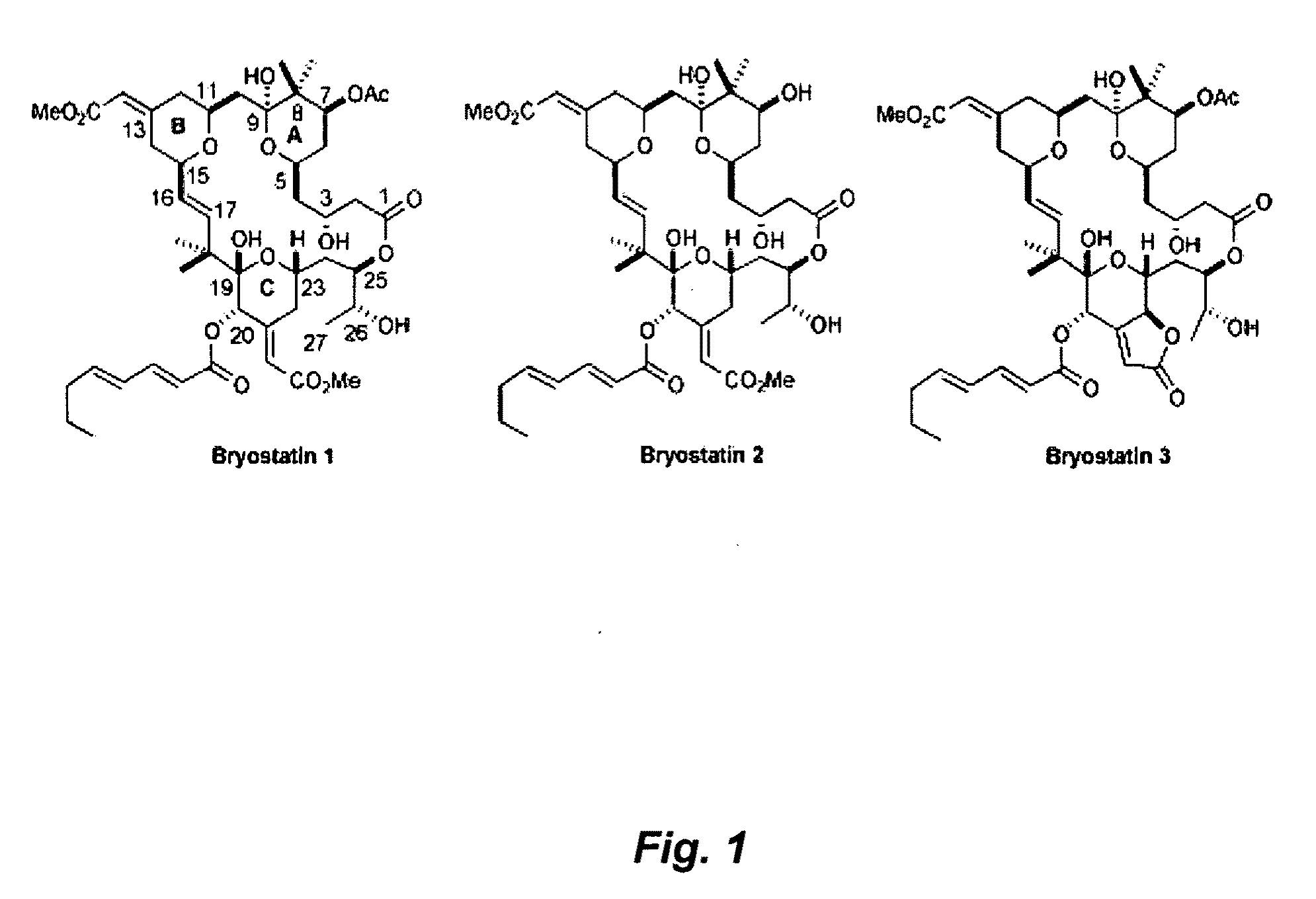
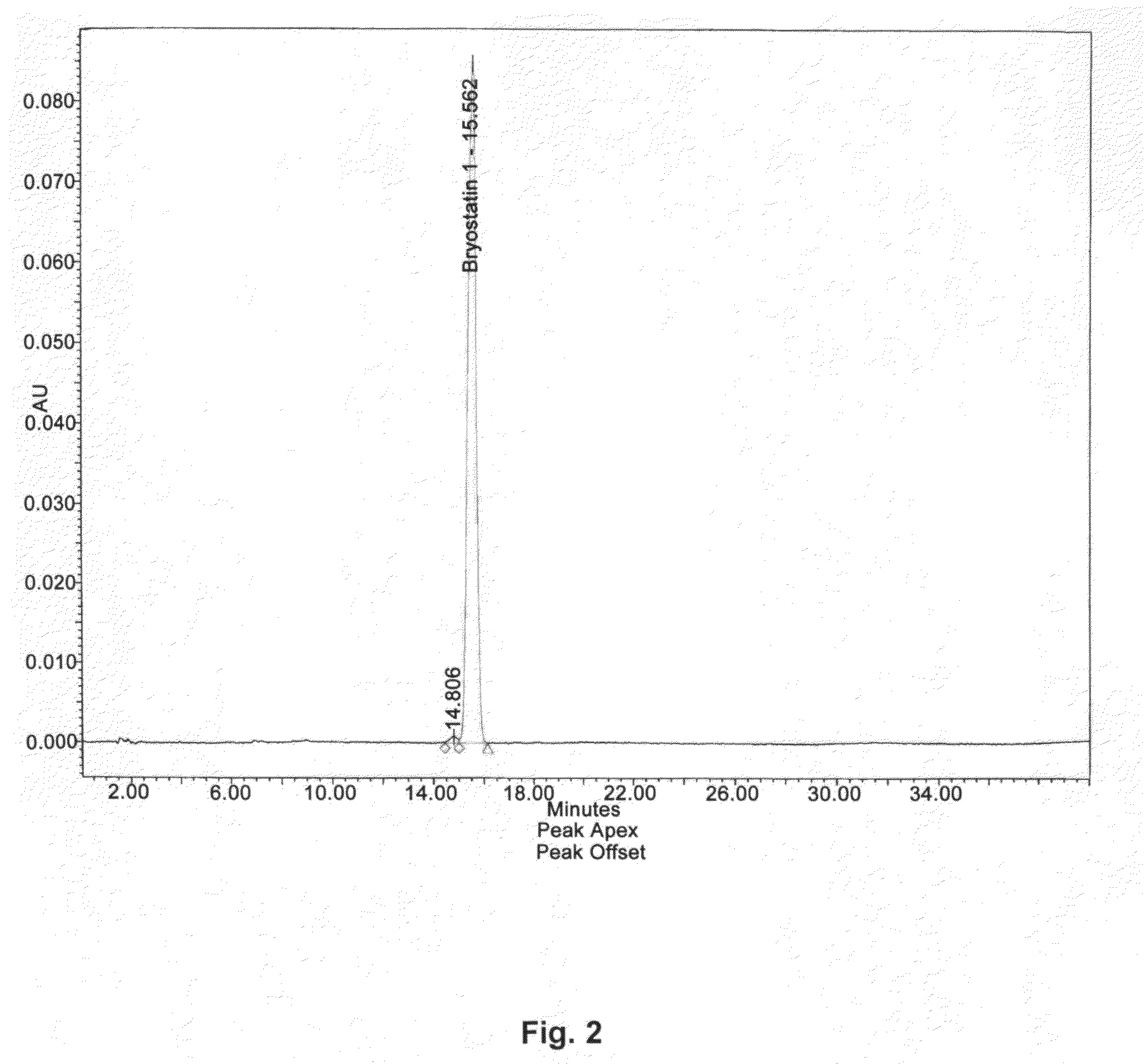
The invention relates to a combination of treatments, more particularly a combination treatment for HIV-1 infection. The present invention is directed to the use of bryostatin-1 and their natural and synthetic derivatives for AIDS therapy, in particular to the use of bryostatins in combination with other active drugs such as Histone Deacetylases (HDACs) inhibitors and anti-retrovirals, for the treatment of HIV-1 latency. According to the present invention, we provide a combination therapy for the treatment of HIV-1 latency which employs bryostatin-1 (and analogues) and one of the following HDAC inhibitors; valproic acid, butyrate derivatives, hydroxamic acids and benzamides. While HDACi can be used in continuous dosing protocol, bryostatins can be used following a cyclical dosing protocol. Bryostatins can be formulated in pharmaceutical acceptable carriers including nanoparticles, phospholipids nanosomes and / or biodegradable polymer nanospheres. This combination therapy needs to be used in patients treated with antiretroviral therapy (HIV-1 protease inhibitors, HIV-1 reverse transcriptase inhibitors, HIV-1 integrase inhibitors, CCR5 co-receptor inhibitors and fusion inhibitors).
Owner:APHIOS
Diagnostic kits comprising genetically engineered human immunodeficiency virus-like particles containing heterologous antigenic markers
InactiveUS6342228B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesHeterologous
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:AVENTIS PASTEUR LTD
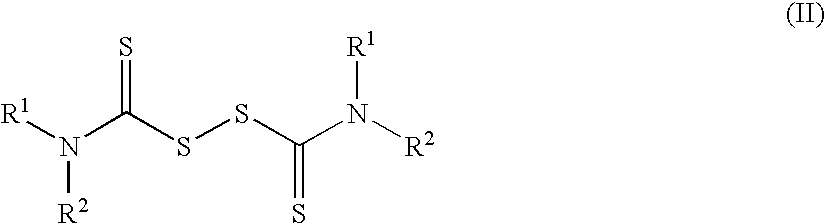
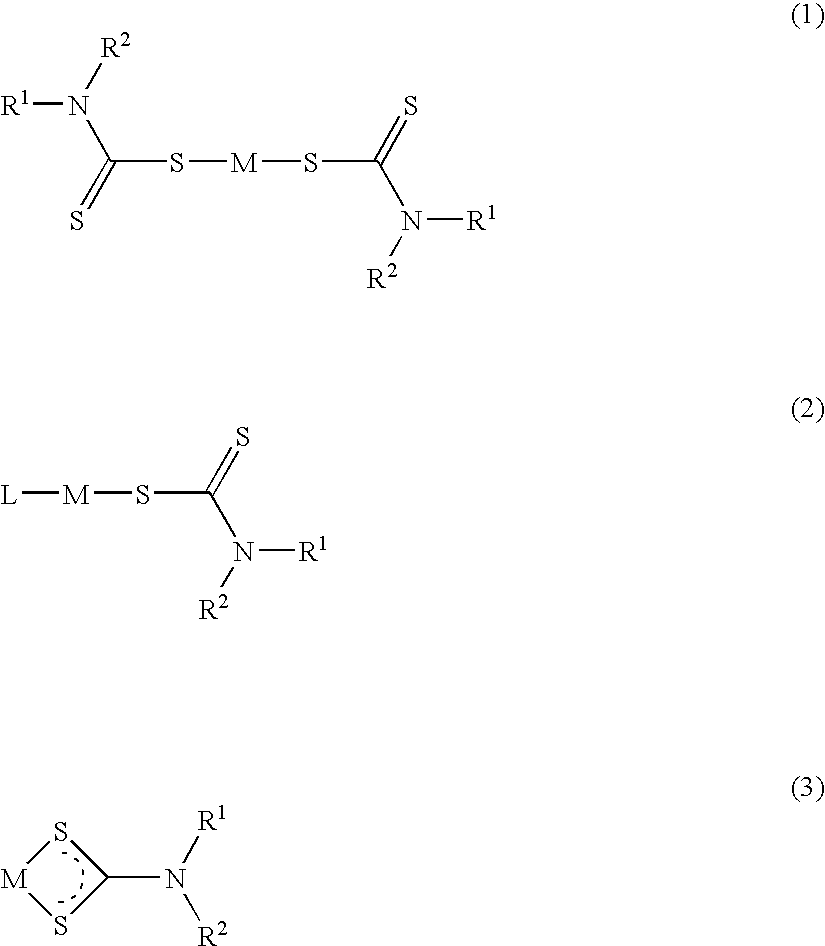
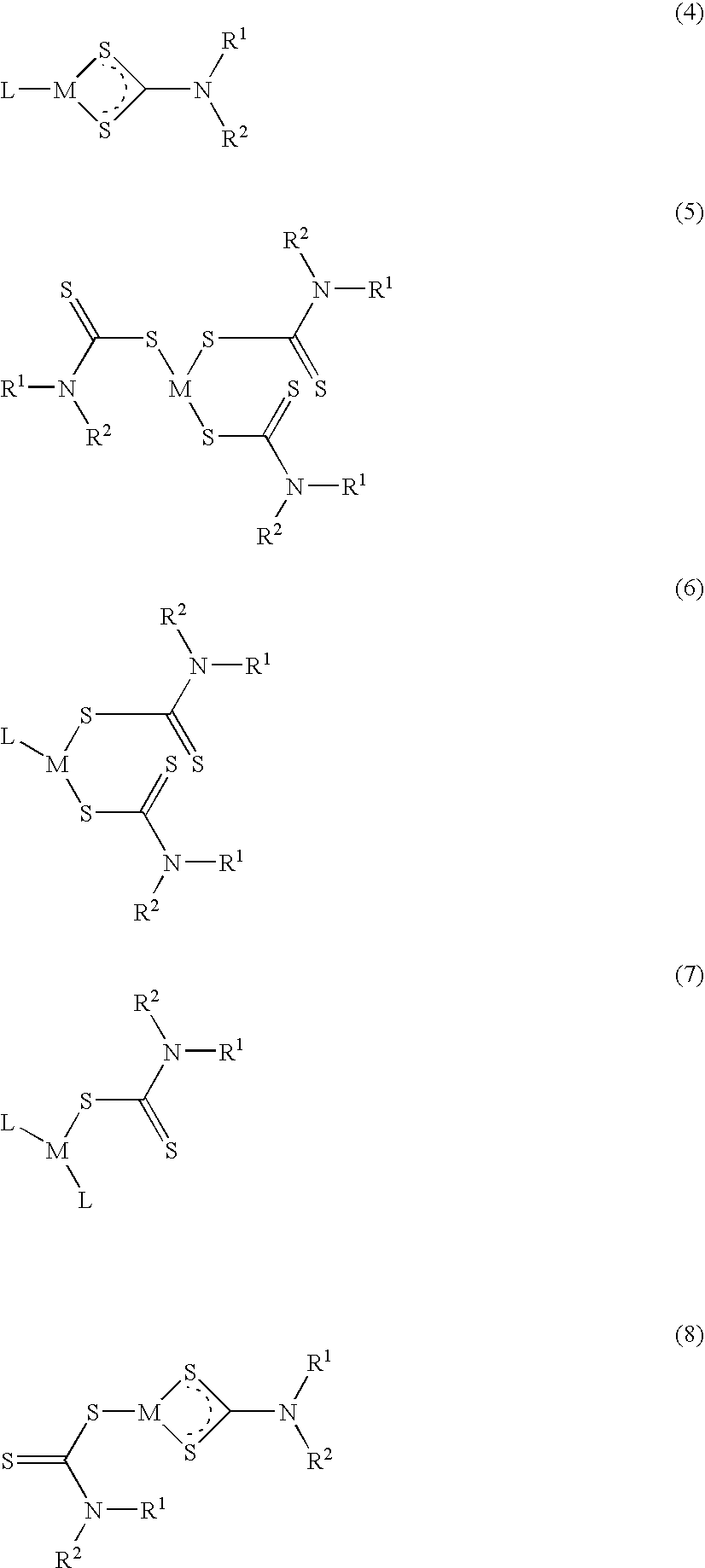
Method of treating cancer using dithiocarbamate derivatives
InactiveUS20050096304A1Readily available easily used treatmentInhibitionHeavy metal active ingredientsBiocideAdjuvantAnticarcinogen
The invention encompasses neutral dithiocarbamate metal compounds and methods of treating cancer using such compounds, along with methods for sensitizing AIDS / HIV patients to anti-retroviral therapy by blocking the P-glycoprotein membrane toxin extrusion pump using such compounds. Compounds inhibit the growth of cancer cells of a variety of cell types. A method is presented for using the neutral compounds disclosed herein, amongst other uses disclosed herein, to reduce tumor growth, and to potentiate the effect of other anticancer agents. The invention also encompasses pharmaceutical compositions comprising the neutral compounds and a pharmaceutically acceptable excipient, diluent, solubilizer, solvent, adjuvant or carrier, or a mixture thereof.
Owner:AAIPHARMA SERVCIES CORP
Combined therapy for treatment of HIV infection
InactiveUS7094413B2Good curative effectReduce resistanceBiocideSugar derivativesImmunodeficiency virusGastrointestinal complications
The present invention relates to pharmaceutical preparations and methods for treating individuals infected with the human immunodeficiency virus (HIV). The pharmaceutical preparations comprise an immunomodulating agent and a anti-retroviral compound. The pharmaceutical preparations are used to treat HIV infected patients, particularly for gastrointestinal complications arising from viral infection. In addition, the pharmaceutical preparations of the present invention have the effect of raising the levels of CD4+ single positive and CD4+ and CD8+ double positive T cells, thus promoting restoration and normalization of the immune system following HIV infection.
Owner:SANGSTAT MEDICAL +1
Antigentically-marked non-infectious retrovirus-like particles
InactiveUS6518030B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:AVENTIS PASTEUR LTD
Methods for inducing an immune response against human immunodeficiency virus infection in subjects undergoing antiretroviral treatment
ActiveUS20180064803A1Measurable immune responseMaintain viremic controlViral antigen ingredientsAntiviralsImmunodeficiency virusVaccinia
Methods for inducing an immune response against Human Immunodeficiency Virus (HIV) in HIV-infected subjects undergoing antiretroviral therapy (ART) are described. The methods include administering an adenovirus vector primer vaccine and a modified vaccinia virus (MVA) vector booster vaccine encoding mosaic HIV antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +3
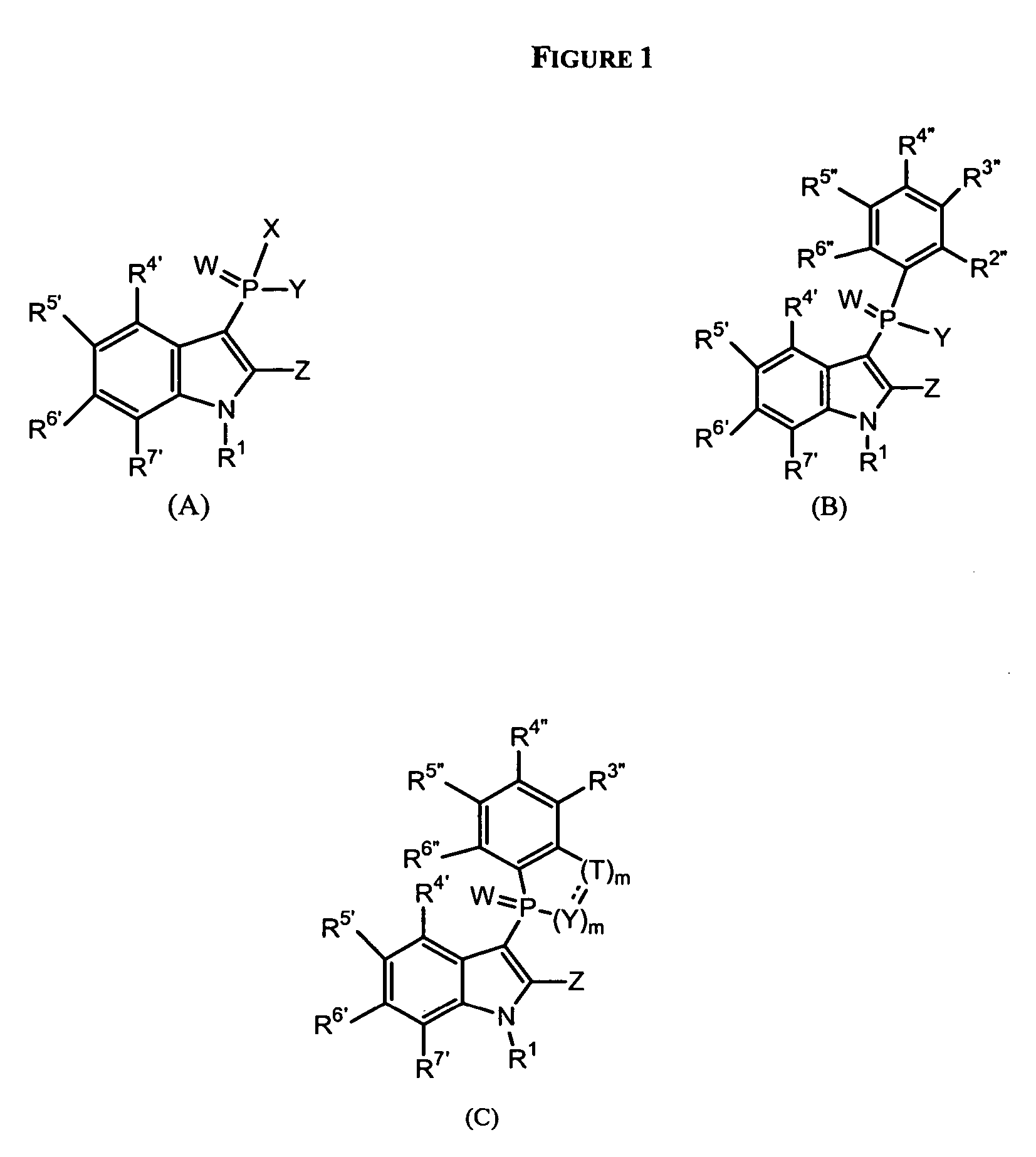
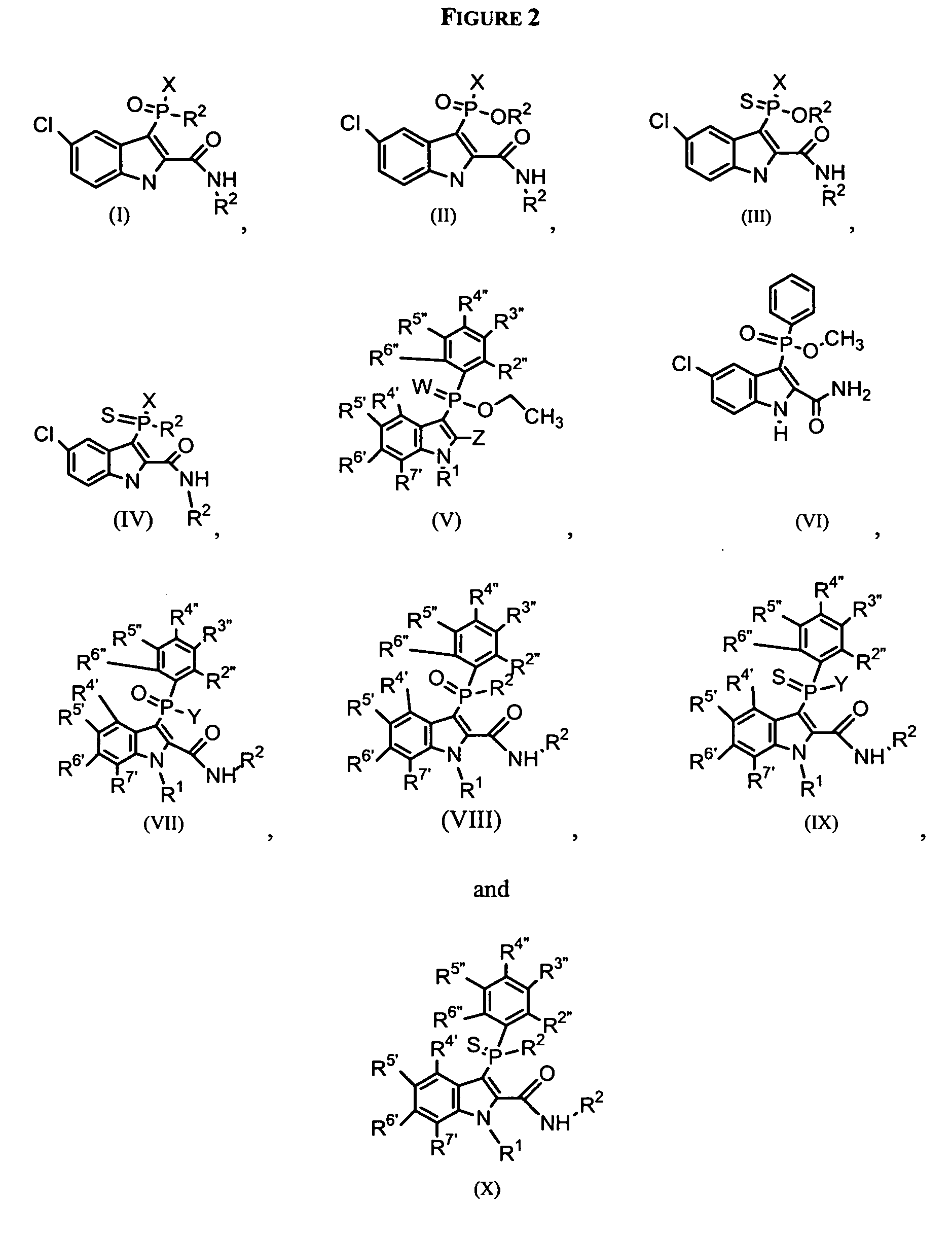
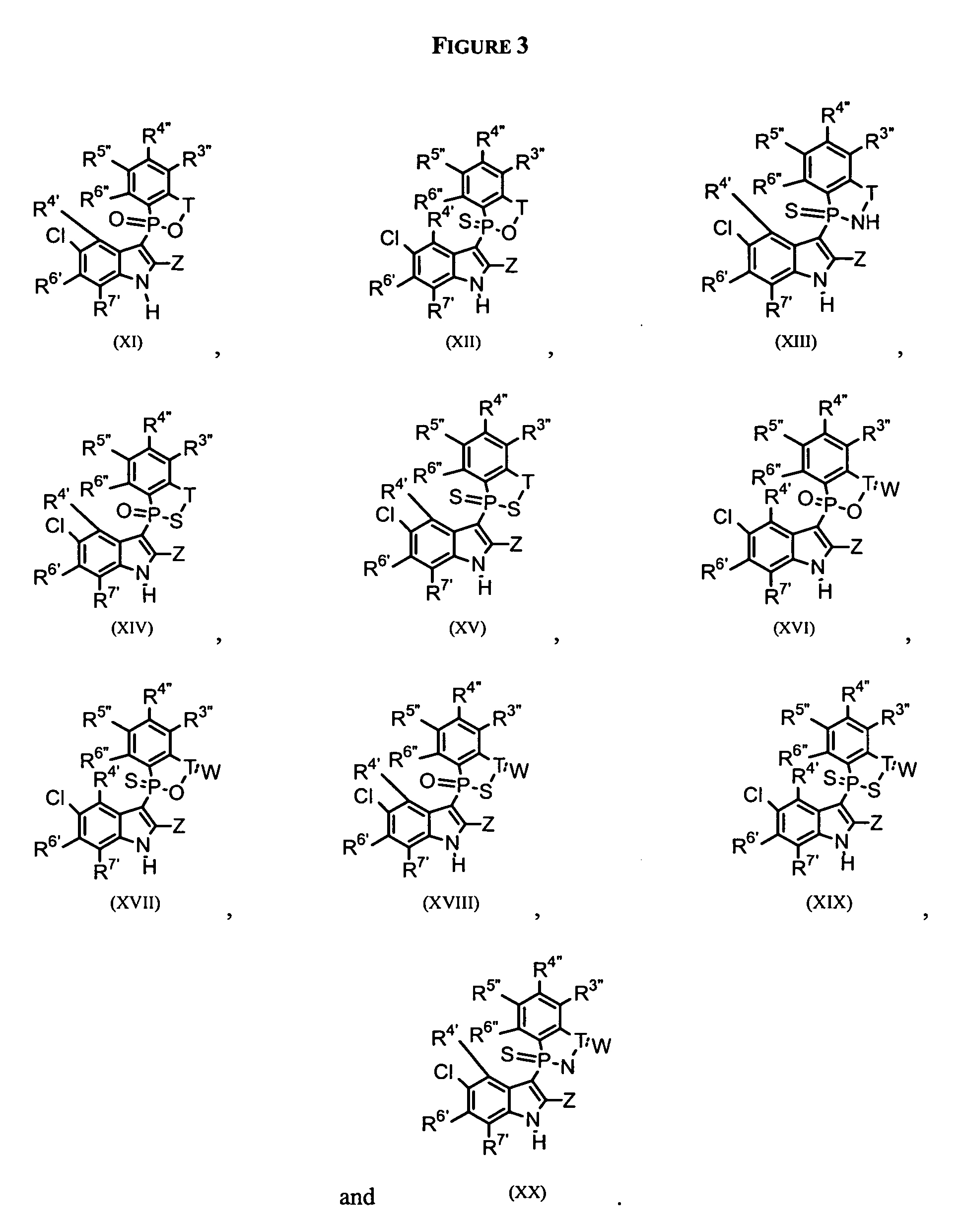
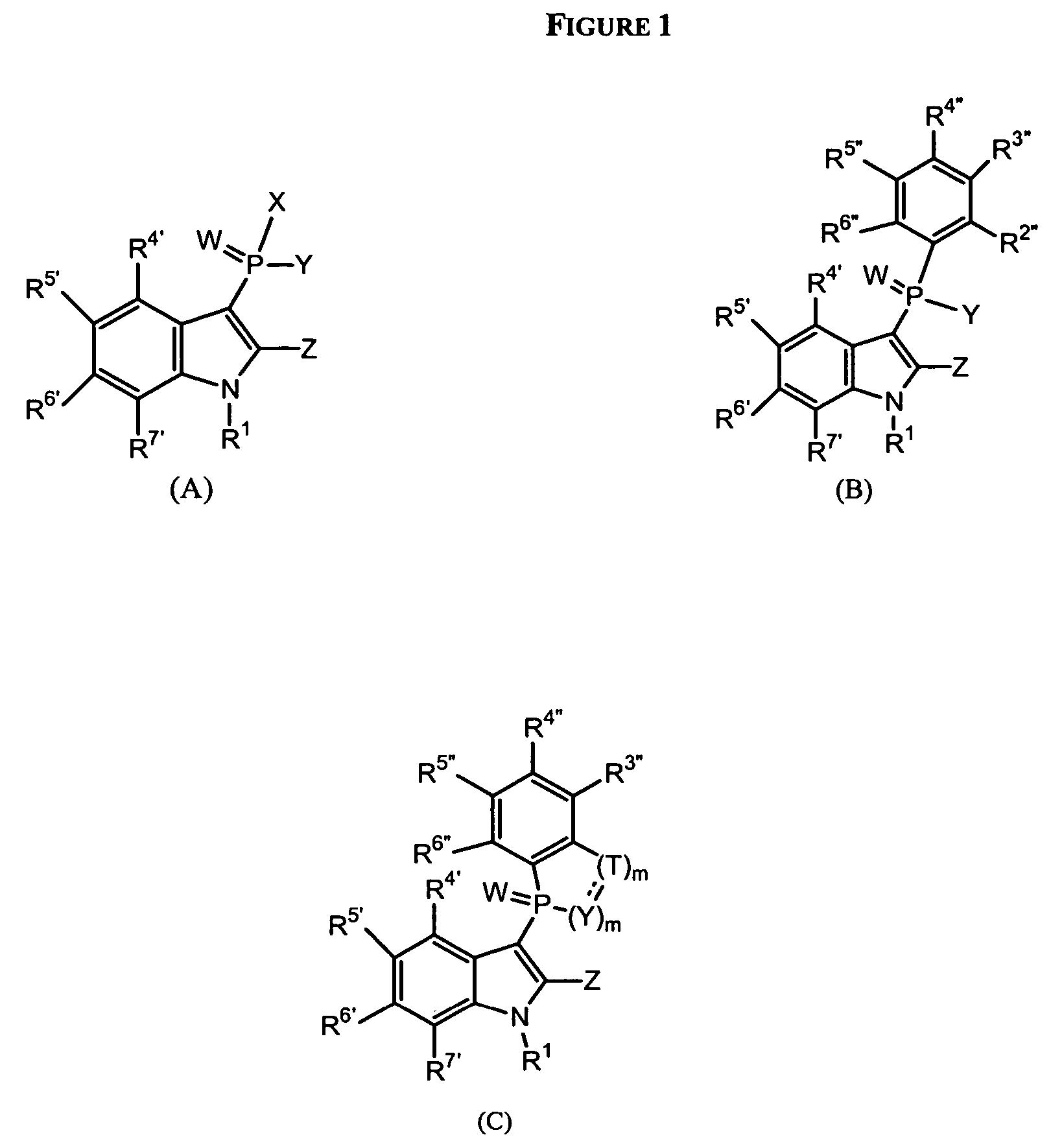
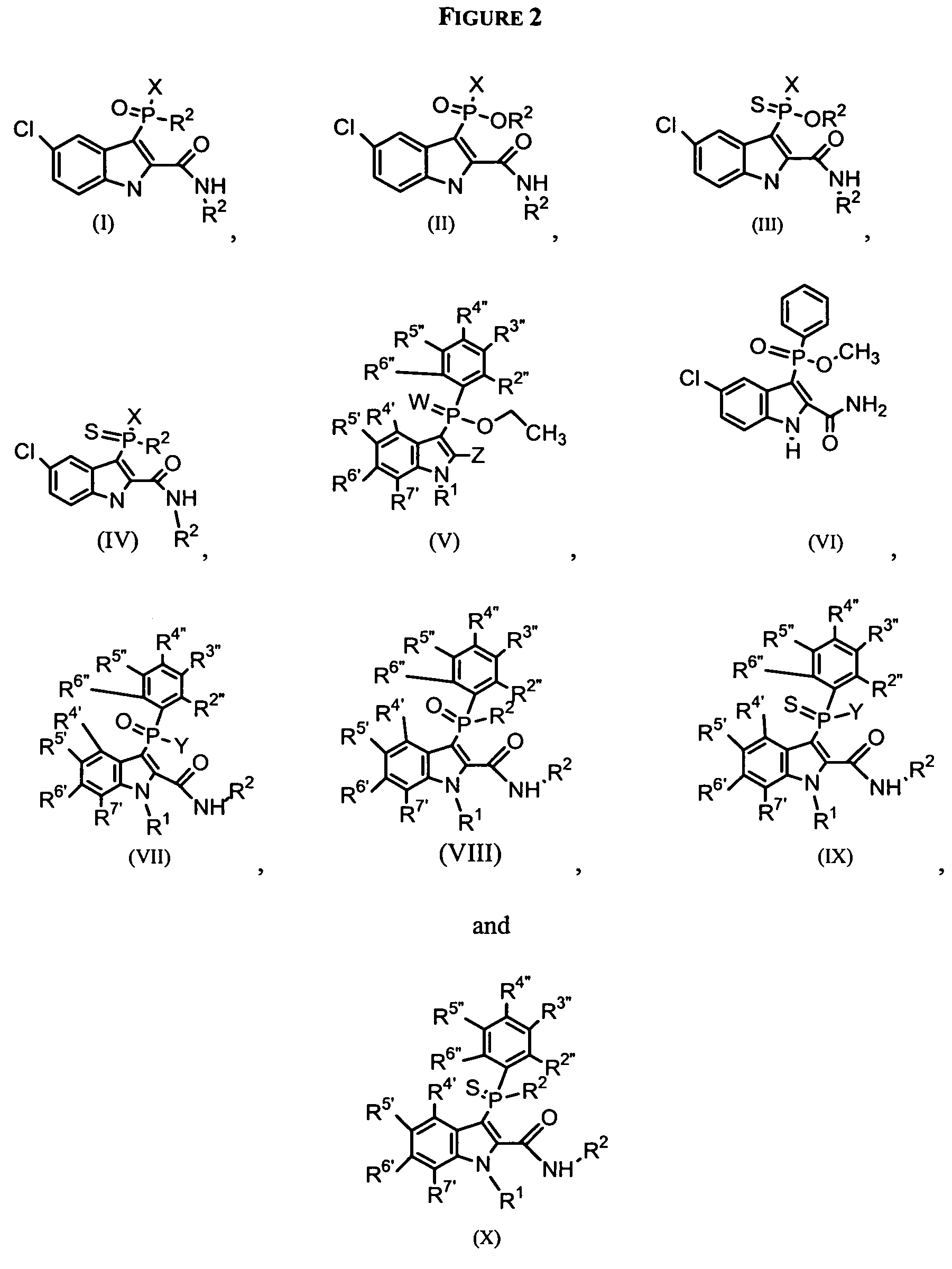
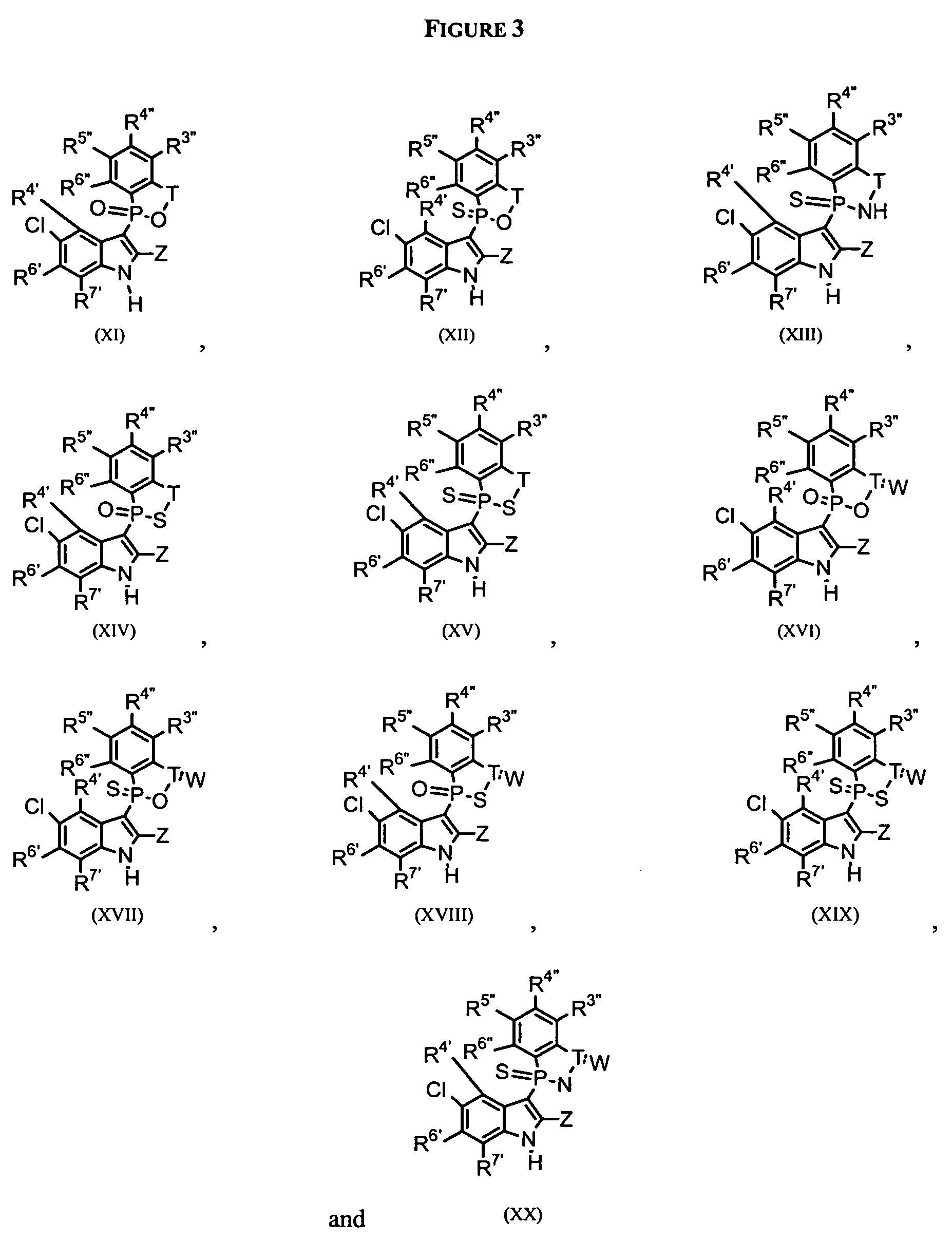
Phospho-indoles as HIV inhibitors
Owner:INDENIX PHARM LLC
Phospho-indoles as HIV inhibitors
Owner:INDENIX PHARM LLC
Polyoxometalate compounds as antiviral agents
Polyoxometalate compounds which exhibit anti-retroviral activity are disclosed. Compounds with anti-retroviral activity include those having the following general molecular formulas:M7PW11O39 M8SiW11O39 M9HSiW9O34 M8HPW9O34 M10(TM)4(PW9O34)2 M16(TM)4(P2W15O56)2 M14[NaP5W30O110]M12(TM)3(PW9O34)2 M6P2W18O62 [0001]wherein M is an alkali metal, NH4+ or other common monocation or soluble dication, or any combination of the above provided adequate water solubility is exhibited, or histidinium ion, argininium ion, or lysinium ion or any dication of a dipeptide or oligopeptide with 2 protonated basic amino acid residues, or any combination of these monocations or dications with each other or with any common inorganic cation, and[0002]TM is a divalent transition metal ion, such as Mn, Fe, Co, Ni, Cu and Zn.
Owner:SCHINAZI RAYMOND F +1
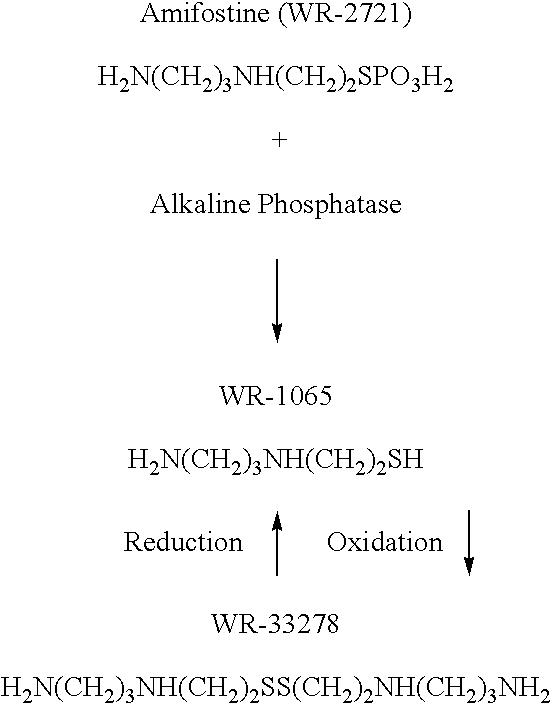
Organic thiophosphate antiretroviral agents
InactiveUS20090239817A1Reducing and preventing effectPromote repairBiocideSulfur/selenium/tellurium active ingredientsImmunodeficiency virusAmifostine
A method for the prevention or treatment of human immunodeficiency virus infection by administering an effective amount of amifostine, phosphonol, or similar compound to an individual in need is provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
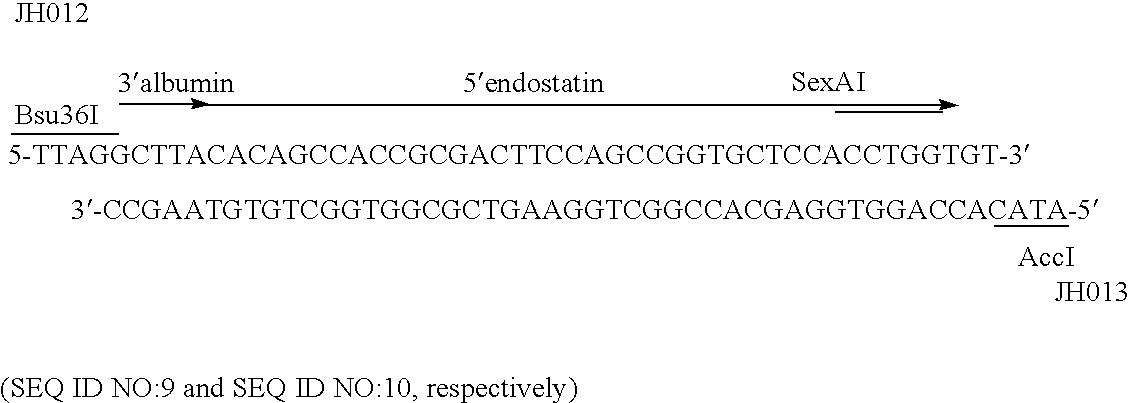
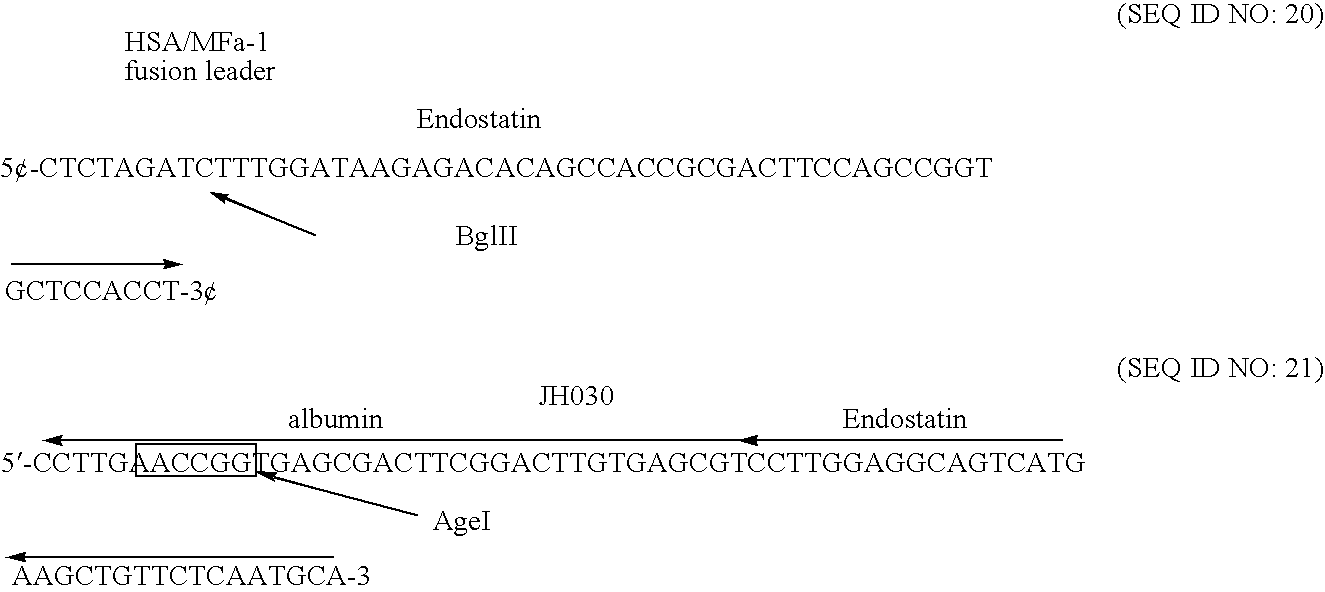
Albumin-fused anti-angiogenesis peptides
InactiveUS20060122374A1High activityExtended half-lifeOrganic active ingredientsFungiAbnormal tissue growthLymphatic Spread
The invention relates to proteins comprising angiogenesis inhibiting peptides, such as endostatin peptides (including, but not limited to, fragments and variants thereof), which exhibit anti-retroviral activity, fused or conjugated to albumin (including, but not limited to fragments or variants of albumin). These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins exhibit extended shelf-life and / or extended or therapeutic activity in solution. The invention encompasses therapeutic albumin fusion proteins, compositions, pharmaceutical compositions, formulations and kits. The invention also encompasses nucleic acid molecules encoding the albumin fusion proteins of the invention, as well as vectors containing these nucleic acuds, host cells transformed with these nucleic acids and vectors, and methods of making the albumin fusion proteins of the invention using these nucleic acids, vectors, and / or host cells. The invention also relates to compositions and methods for inhibiting proliferation of vascular endothelial cells and tumor aniogenesis induced cell fusion. The invention further relates to compositions and methods preventing growth of, or promoting regression of, primary tumors and metastases; and for treating cancer, diabetic retinophathy, progressive macular degeneration or rheumatoid arthritis.
Owner:NOVOZYMES BIOPHARMA DK AS
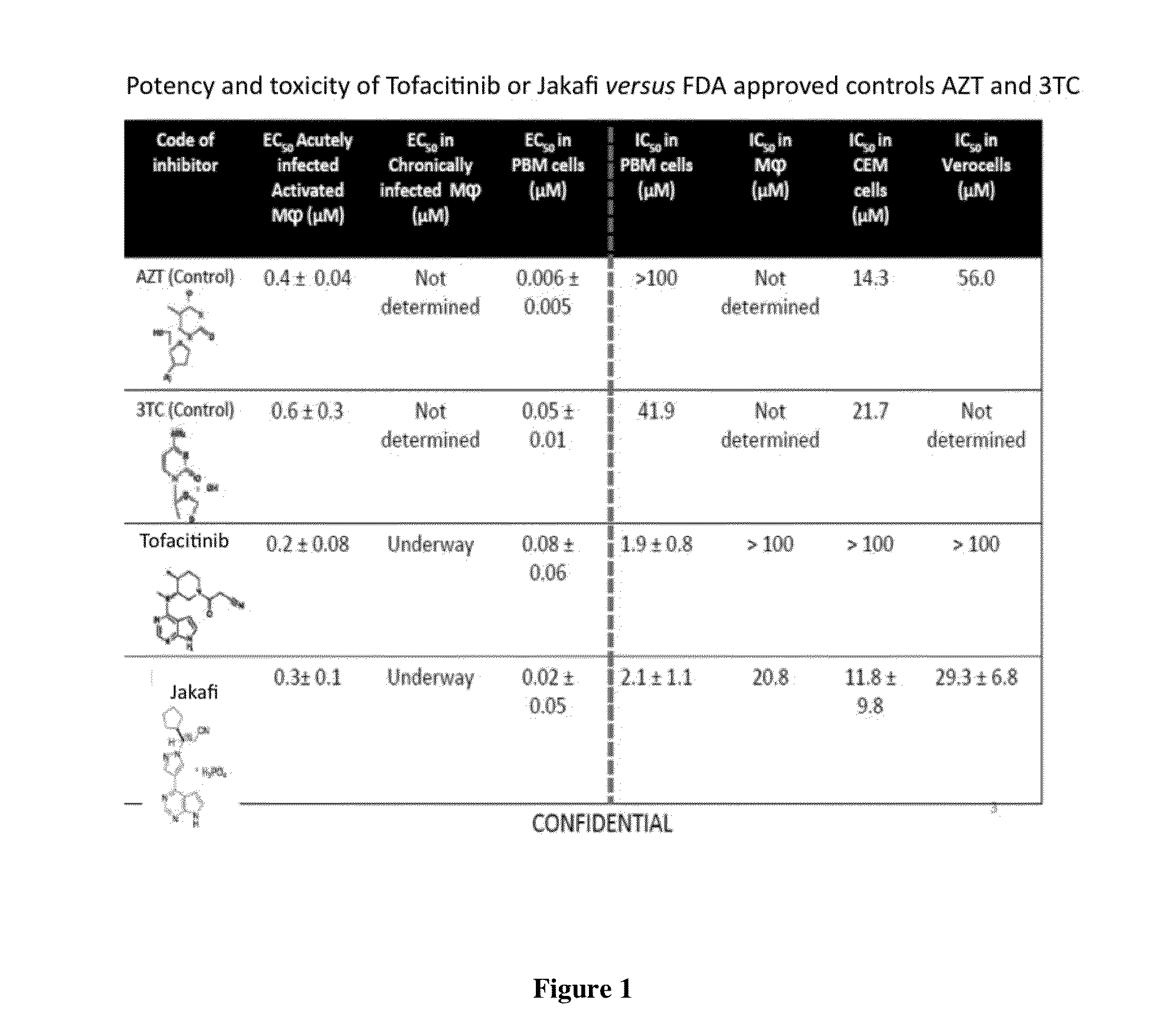
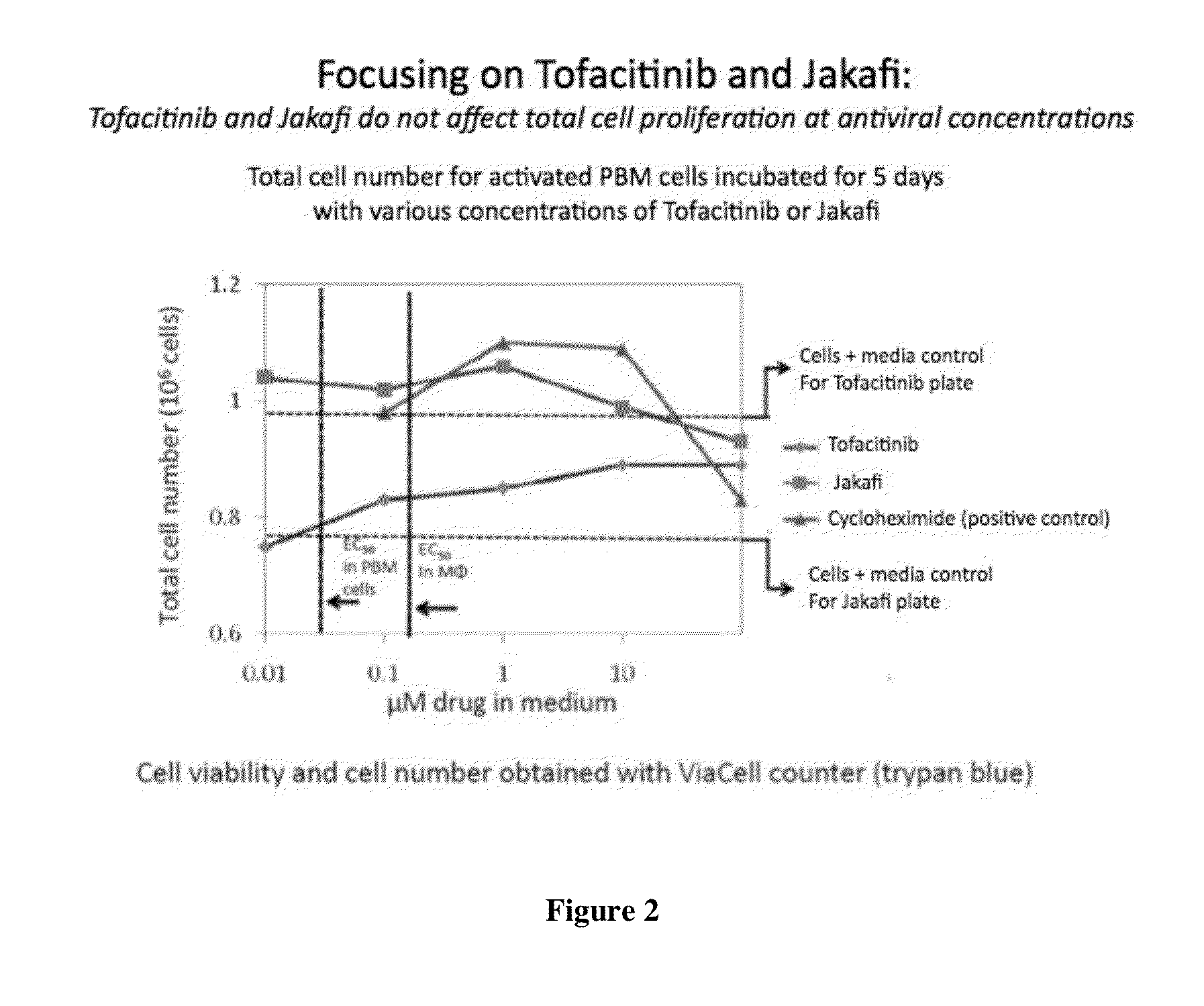
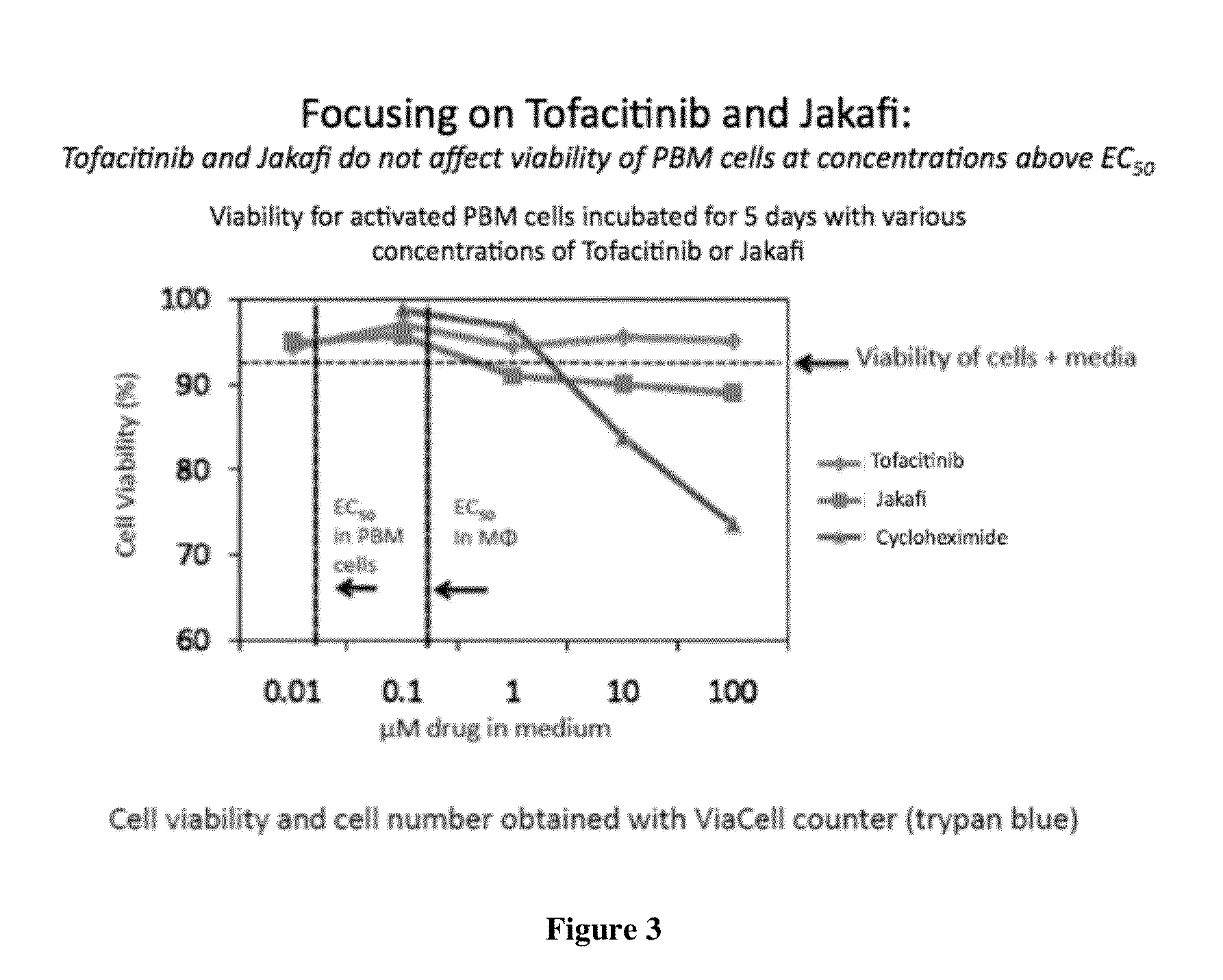
Antiviral jak inhibitors useful in treating or preventing retroviral and other viral infections
ActiveUS20140328793A1Improve their absolute antiviral effectLow toxicityBiocidePeptide/protein ingredientsProteinase inhibitorThymidine
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
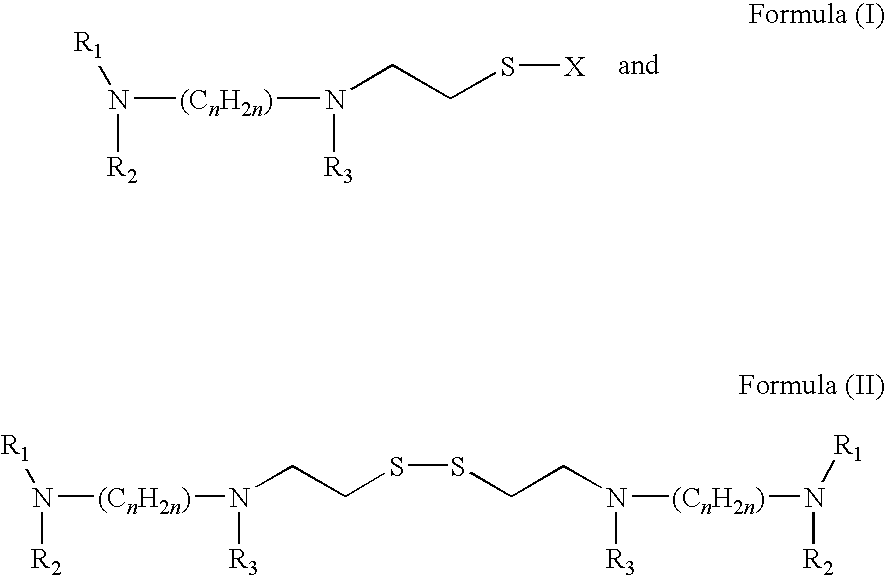
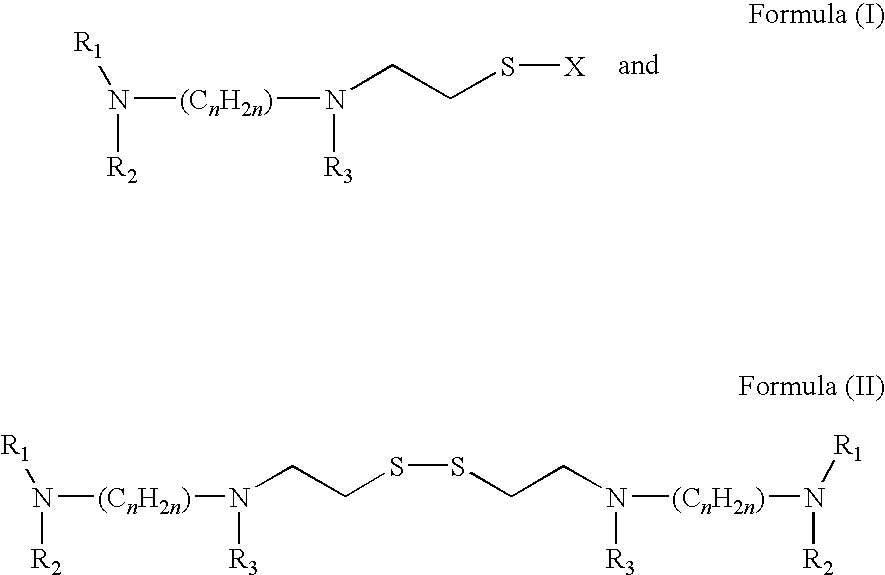
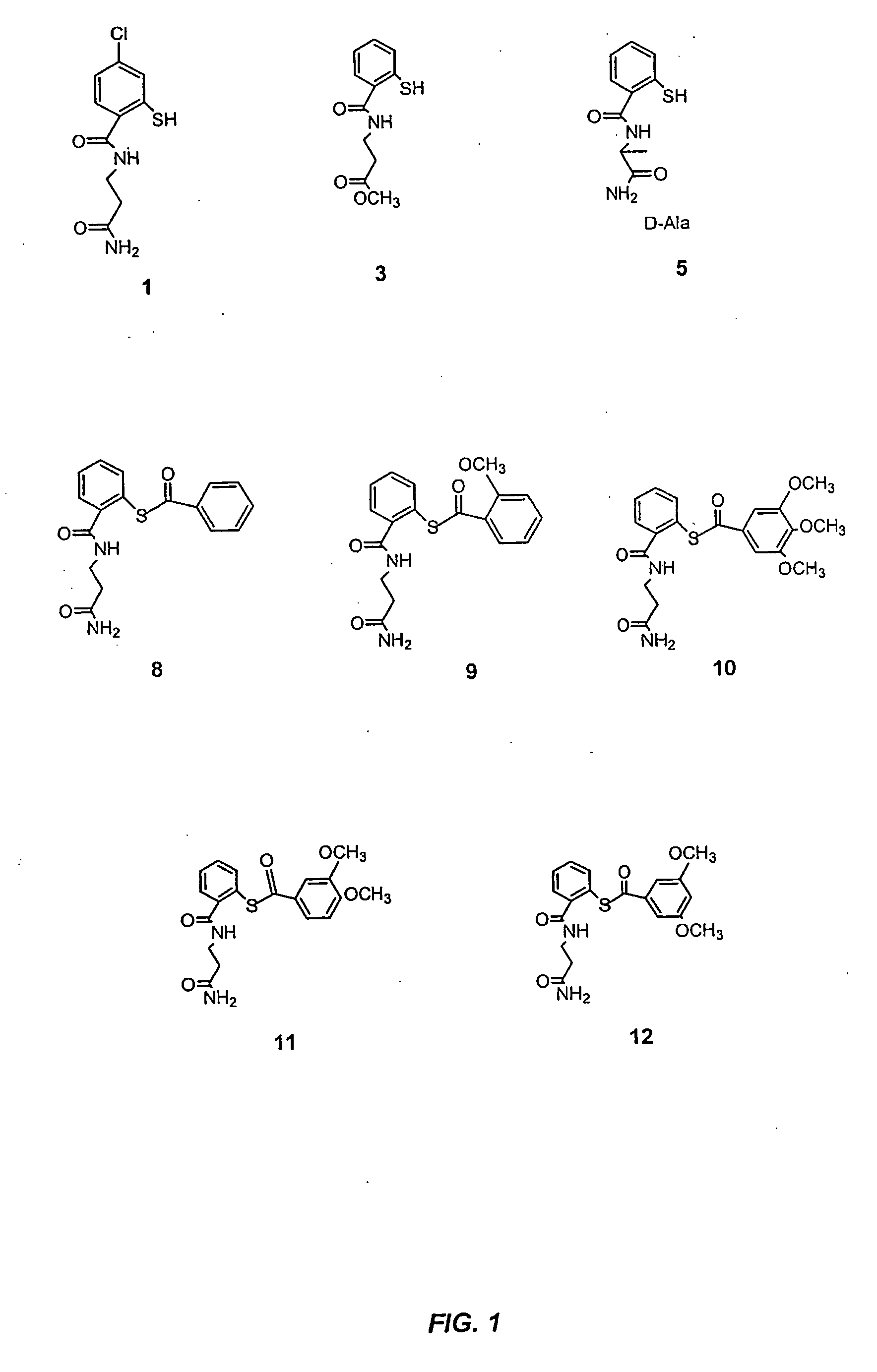
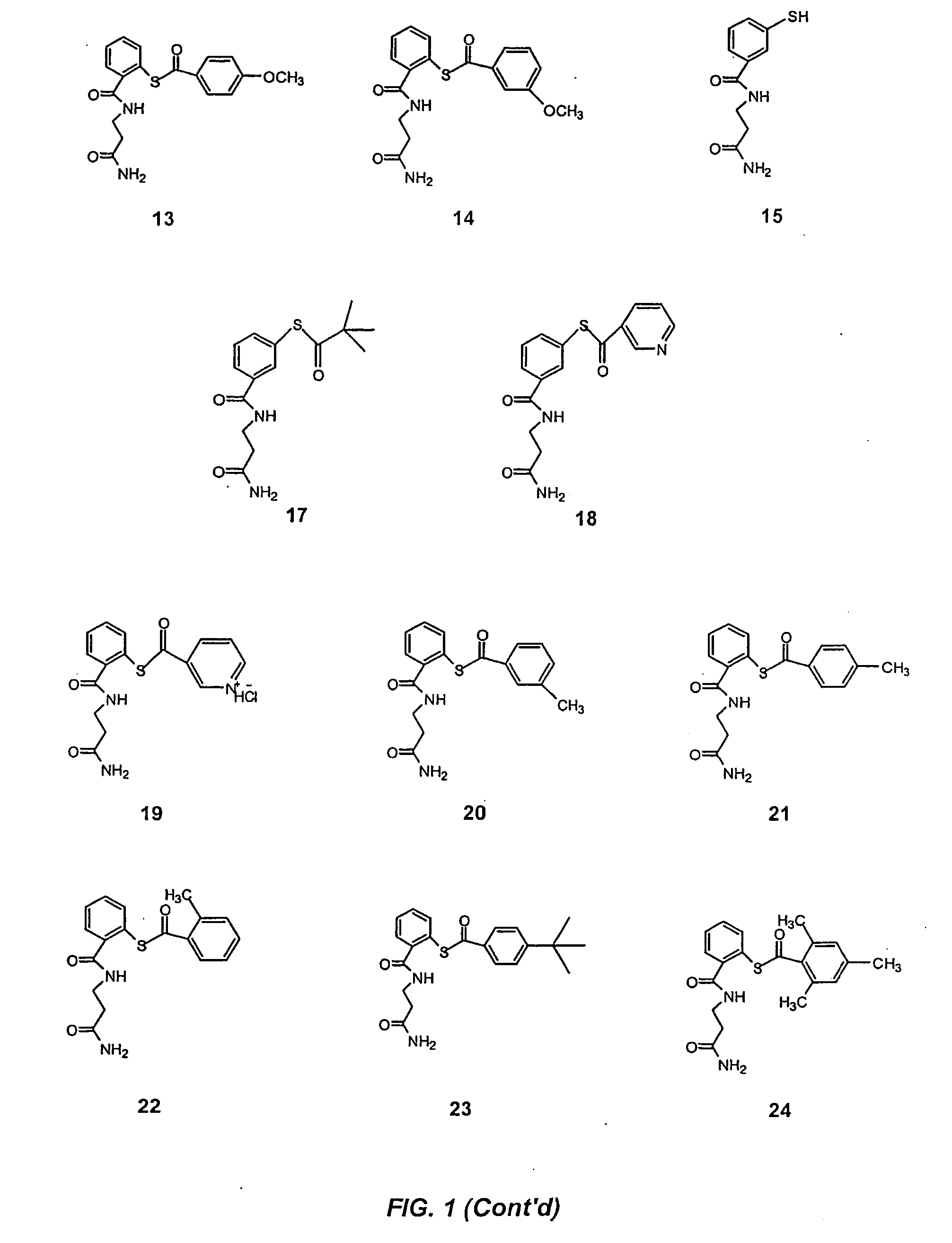
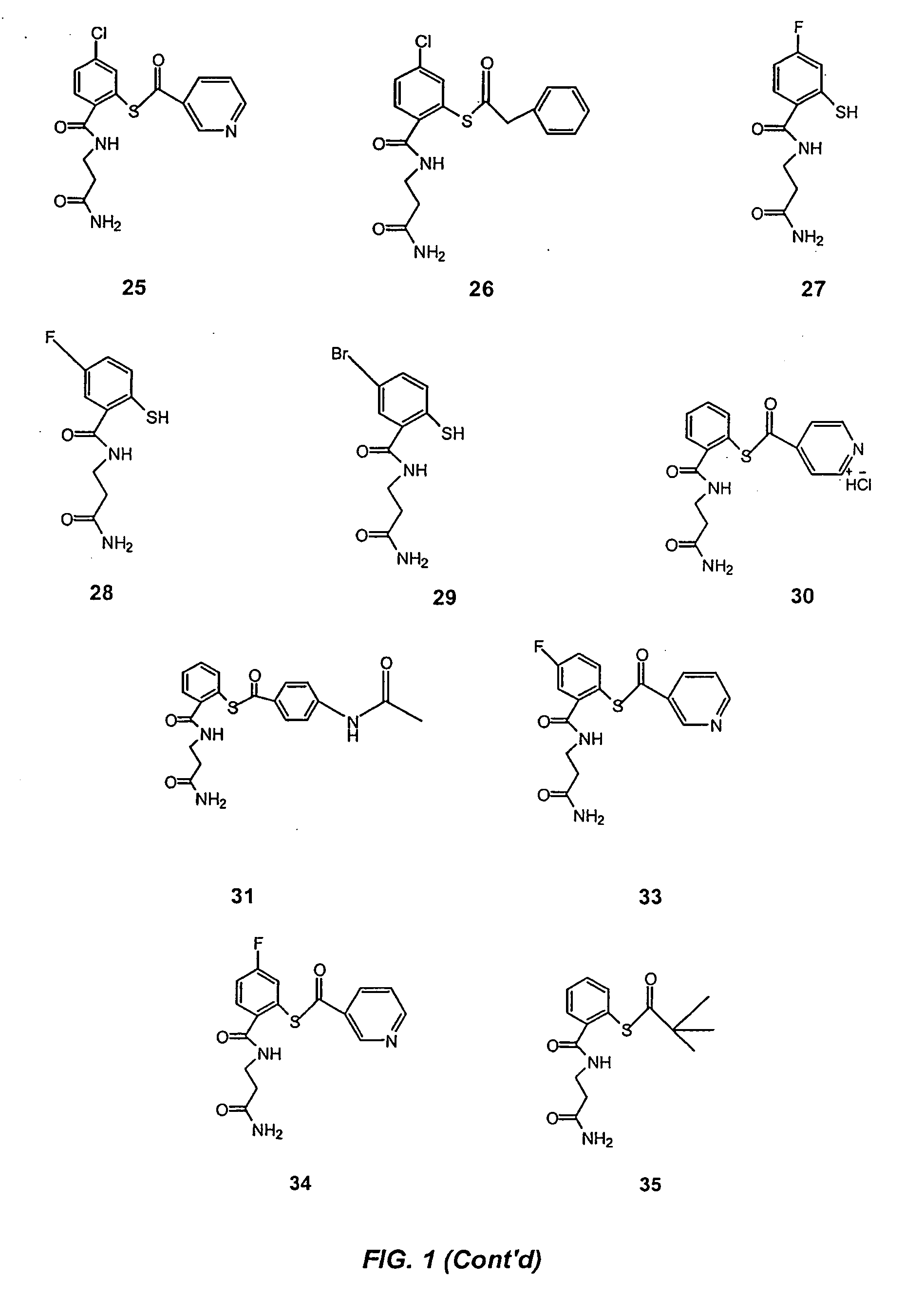
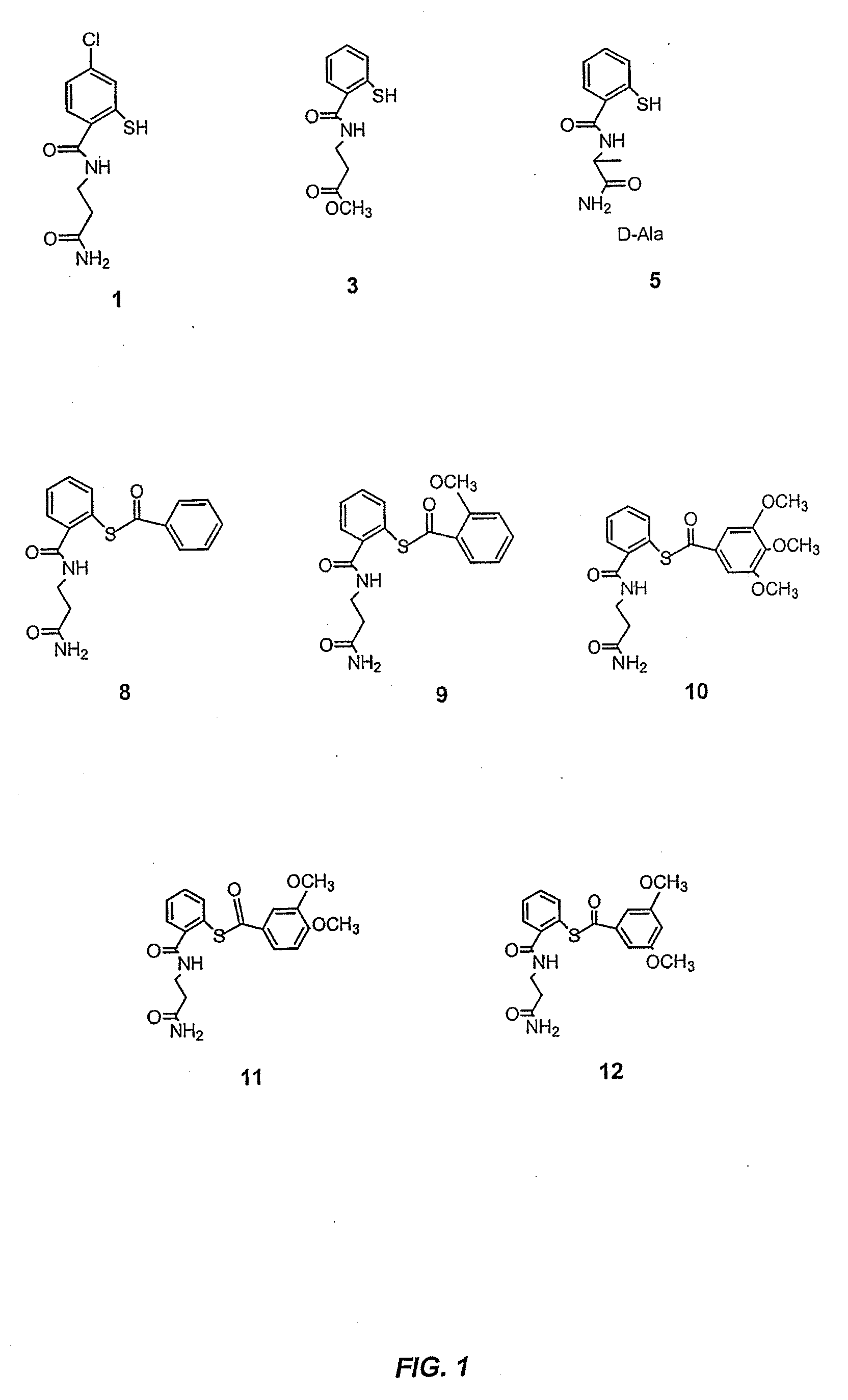
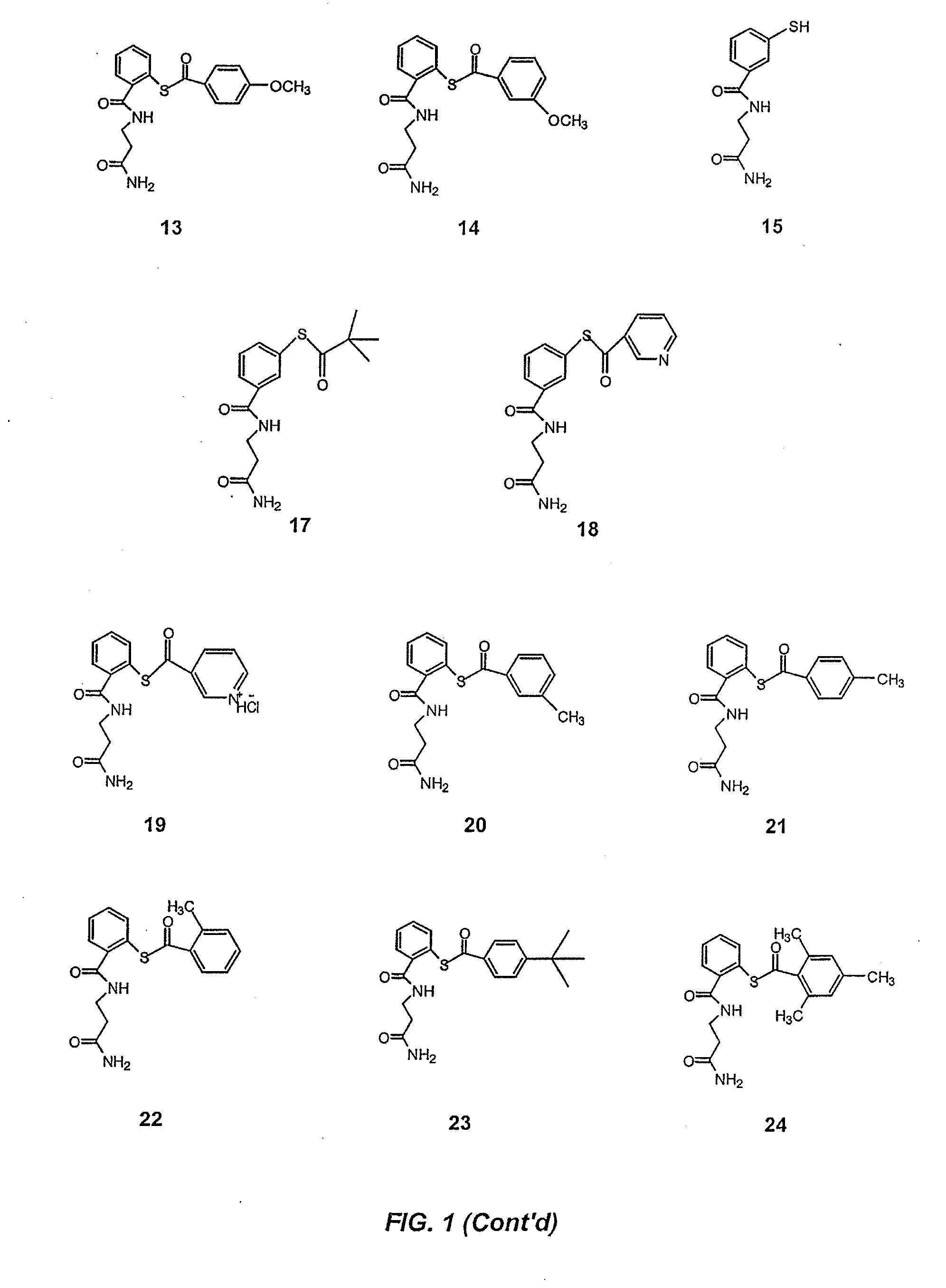
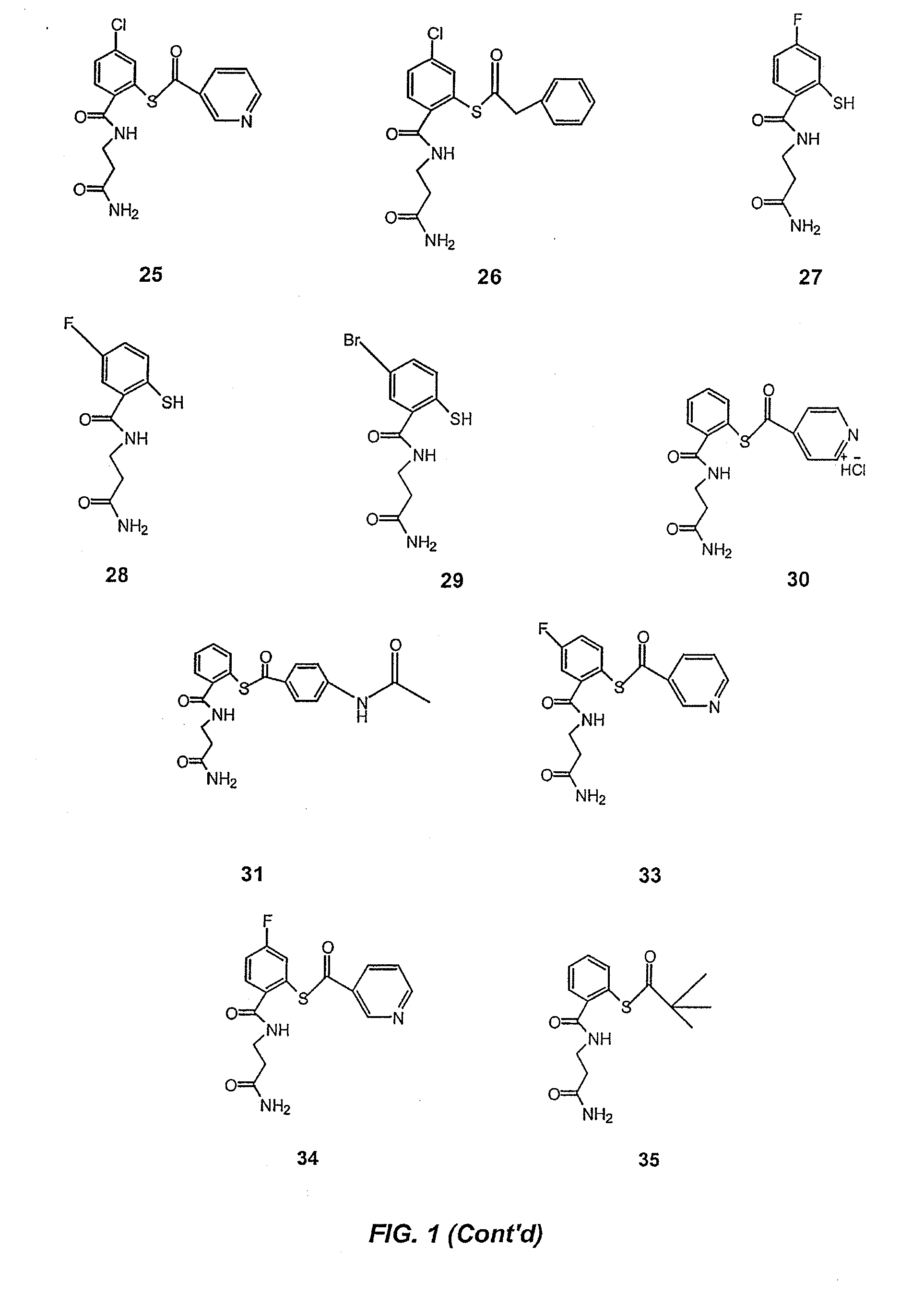
Acylthiols and component thiol compositions as anti-hiv and anti-retroviral agents
Certain thiol and acylthiol compounds inhibit retrovirus growth by attacking the highly conserved zinc finger regions of essential viral proteins. These compounds, compositions containing them, and methods of using them to treat retroviral infections such as HIV are described. These compounds are also useful for preparation of vaccines comprised of inactivated retroviruses such as HIV, prevention of the transmission of such retroviruses, and detection of retroviral proteins.
Owner:UNITED STATES OF AMERICA
Methods and compositions for inhibition of membrane fusion-associated events, including HIV transmission
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:TRIMERIS
Pharmaceutical formulation for use in hiv therapy
InactiveUS20090281132A1Reduce chance of degradationReduce chanceOrganic active ingredientsBiocideOrganic solventPharmaceutical formulation
The invention discloses a formulation prepared by granulating at least one anti-retro viral drug and at least one pharmaceutically acceptable additive, using an organic solvent; milling the product; finally processing the milled product to form tablets or capsules.
Owner:MATRIX LABORATORIES LTD
Enhanced method and composition for the treatment of hiv+ tuberculosis patients with Anti-retroviral drugs and liposomal encapsulation for delivery of reduced glutathione
InactiveUS20120244212A1Increase intracellular and extra cellular antioxidantsReduced glutathioneAntibacterial agentsBiocideNucleoside Reverse Transcriptase InhibitorDisease
The invention is the use of a therapeutically effective amount of glutathione (reduced) in a liposome encapsulation for oral administration to improve symptoms of illnesses that are related to tuberculosis and HIV and more generally viruses and for the treatment and prevention of virus, particularly HHV-6 and EBV, which liposomal encapsulation of glutathione (reduced) is referred to as liposomal glutathione. The application references specifically reduced glutathione and its importance, and how to stabilize it effectively so it can be taken orally, and need not be refrigerated. New uses for tuberculosis are discussed. The combination is proposed of reduced glutathione and Highly Active Anti-Retroviral Therapy having at least one pharmaceutical composition selected from the group of Nucleoside / tide Reverse Transcriptase Inhibitors (NRTIs), Protease Inhibitors (PIs), and Non-nucleoside Reverse Transcriptase Inhibitors (NnRTIs), and further anti-tuberculosis drugs.
Owner:GUILFORD FREDERICK TIMOTHY
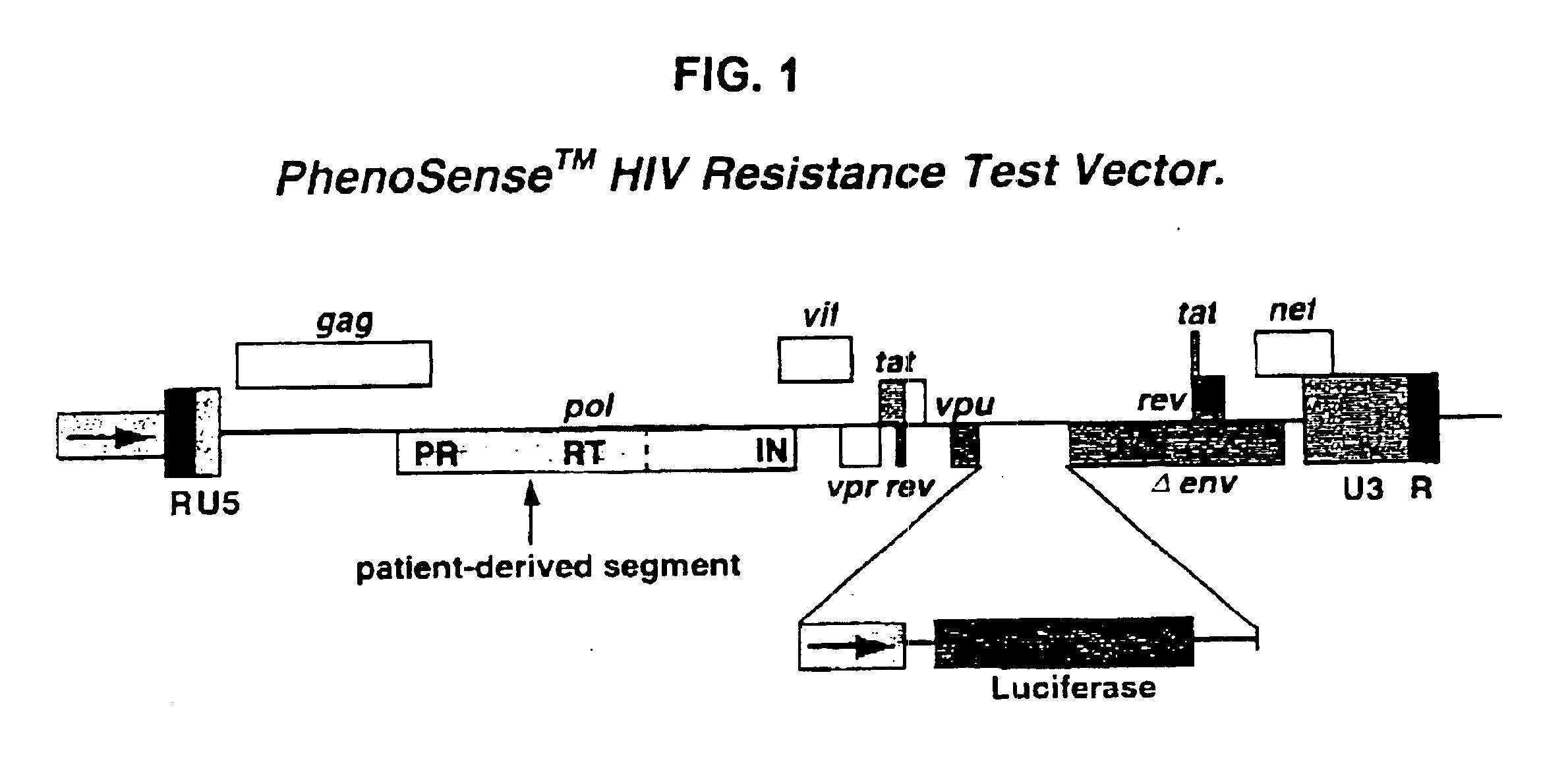
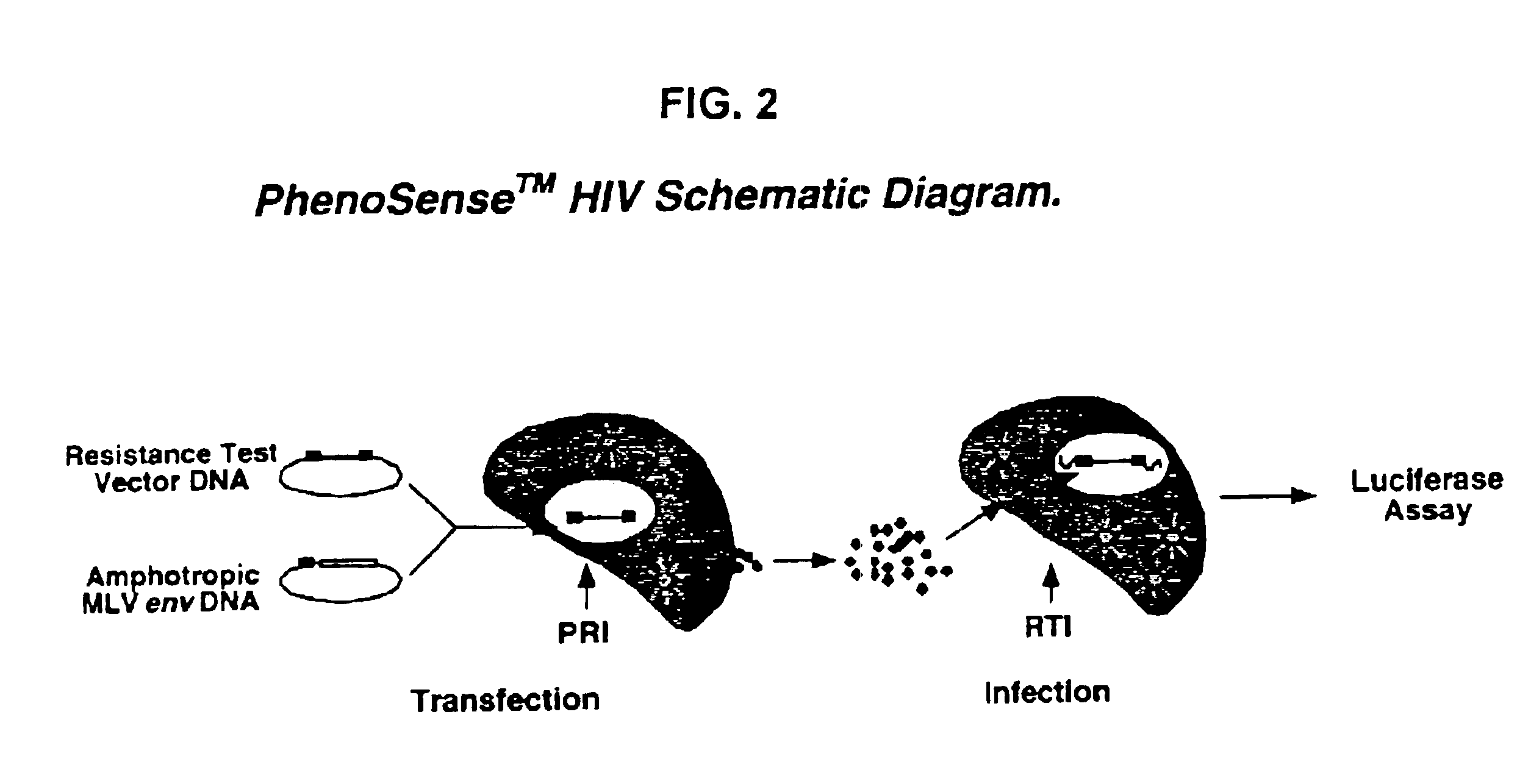
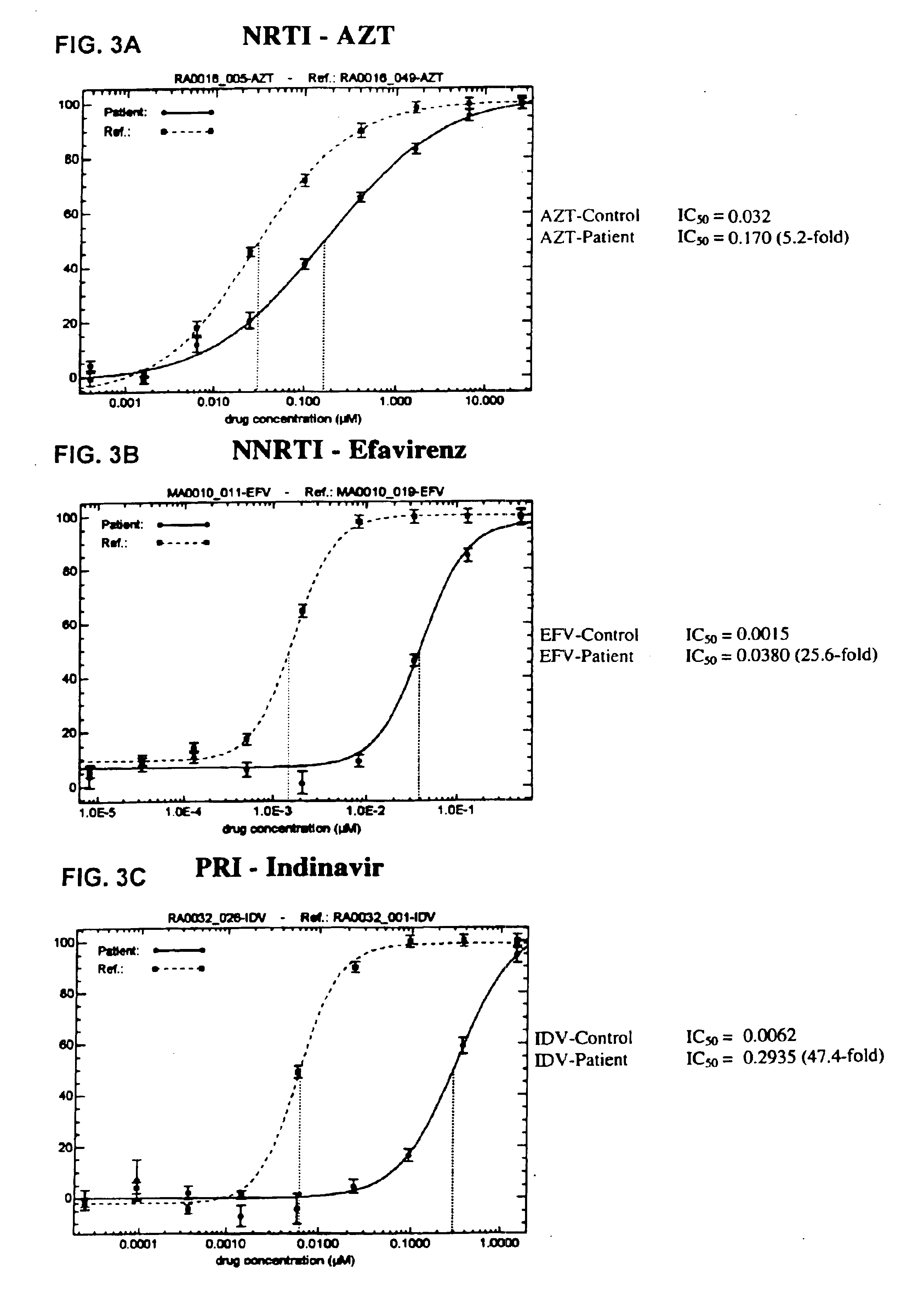
Means and methods for monitoring protease inhibitor antiretroviral therapy and guiding therapeutic decisions in the treatment of HIV/AIDS
InactiveUS6869759B1Identification and of fitnessSugar derivativesMicrobiological testing/measurementAcquired immunodeficiencyImmunodeficiency virus
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), particularly treatment regimens including a protease inhibitor. The invention further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy using phenotypic or genotypic susceptibility assays.
Owner:MONOGRAM BIOSCIENCES
Treatment of AIDS
InactiveUS20060194212A1Peptide/protein ingredientsMicrobiological testing/measurementTherapy HIVAntiretroviral therapy
The invention includes methods of treating HIV infection in a patient where the method includes administration of an antibody to TNF-alpha and an antibody to interferon-gamma to the patient and administering antiretroviral therapy to a patient. The invention further includes methods of treating HIV infection in a patient where the method comprises administration of an antibody to TNF-alpha and an antibody to alpha interferon to the patient and administering antiretroviral therapy to a patient. The invention further includes a method of treating HIV infection in a patient where the method includes administering an antibody to alpha interferon and antiretroviral therapy to a patient. The invention further includes a method of treating an HIV infection in a patient where the method comprises administering a chimeric TNF-alpha receptor and anti-retroviral therapy to a patient.
Owner:ADVANCED BIOTHERAPY
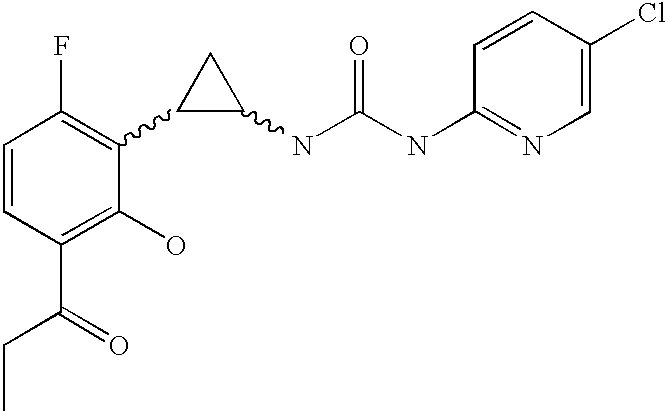
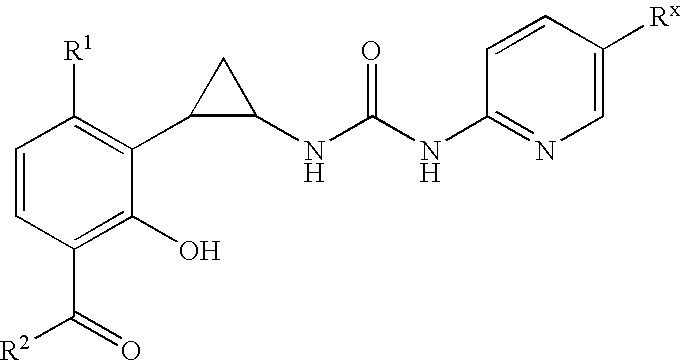
Antivirals
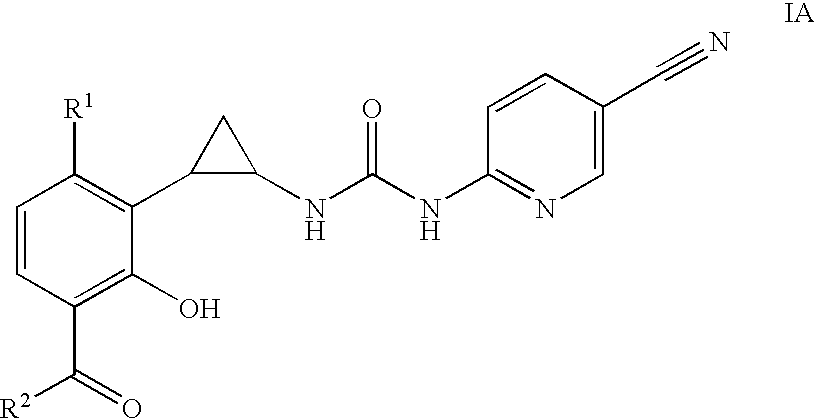
Compounds of formula (I): wherein Rx is cyano or bromo; R1 is halo; R2 is C1-C3 alkyl, and pharmaceutically acceptable salts and prodrugs thereof have activity as antiretrovirals.
Owner:MEDIVIR AB
Anti-retroviral agents, compositions, methods and uses
InactiveUS20050136061A1Preventing HIV infectionRelieve symptomsSenses disorderVirusesNucleotideCell membrane
HIV gp4l mimetibody polypeptides and encoding polynucleotides are disclosed. Methods of utilizing the polypeptides to reduce or inhibit HIV fusion with a cell membrane and entry into target cells are also disclosed.
Owner:CENTOCOR ORTHO BIOTECH
Anti-retroviral analysis by mass spectrometry
InactiveUS20050032042A1Fast and simple and accurateFast and simple and accurate analysisMicrobiological testing/measurementIsotope separationAnalyteMedicine
Methods for the simultaneous or sequential analysis and quantification of a plurality of antiretroviral analytes in a complex biological matrix by mass spectrometry are disclosed. The methods require minimal sample size, minimal preparation time and allow for rapid through-put. The system is particularly useful in therapeutic drug monitoring.
Owner:CHILDRENS NAT MEDICAL CENT
Acythiols and component thiol compositions as Anti-hiv and Anti-retroviral agents
Certain thiol and acylthiol compounds inhibit retrovirus growth by attacking the highly conserved zinc finger regions of essential viral proteins. These compounds, compositions containing them, and methods of using them to treat retroviral infections such as HIV are described. These compounds are also useful for preparation of vaccines comprised of inactivated retroviruses such as HIV, prevention of the transmission of such retroviruses, and detection of retroviral proteins.
Owner:UNITED STATES OF AMERICA
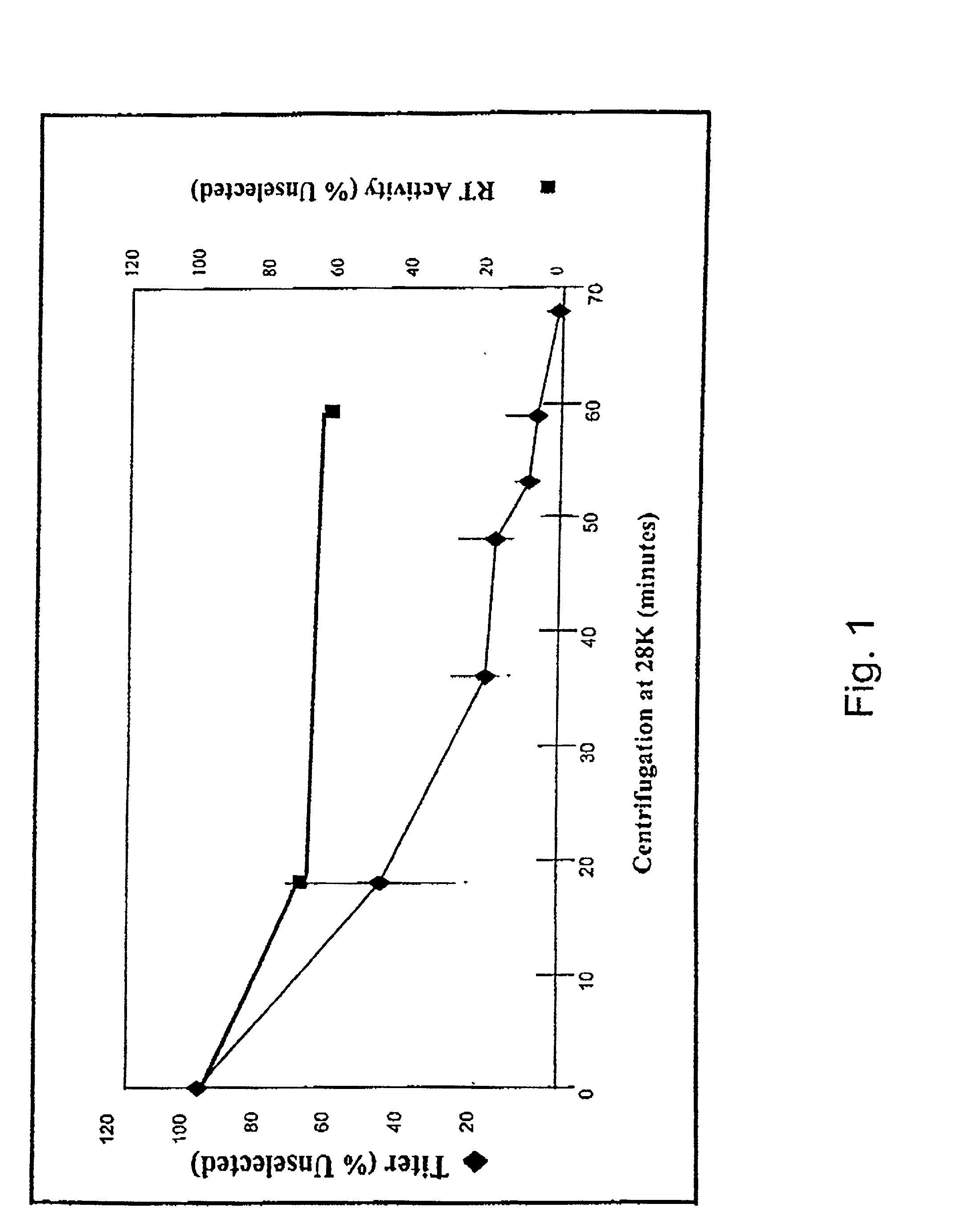
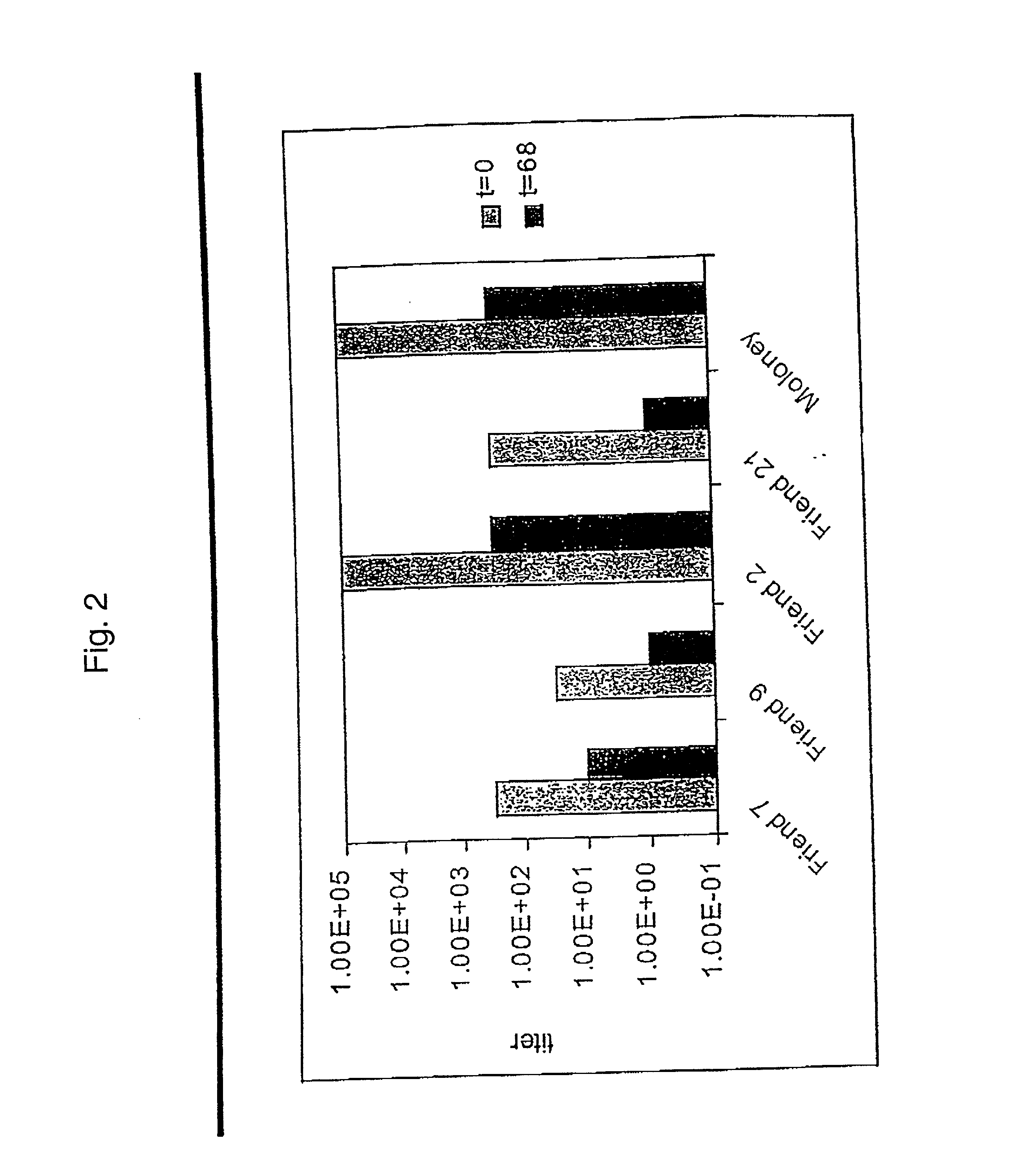
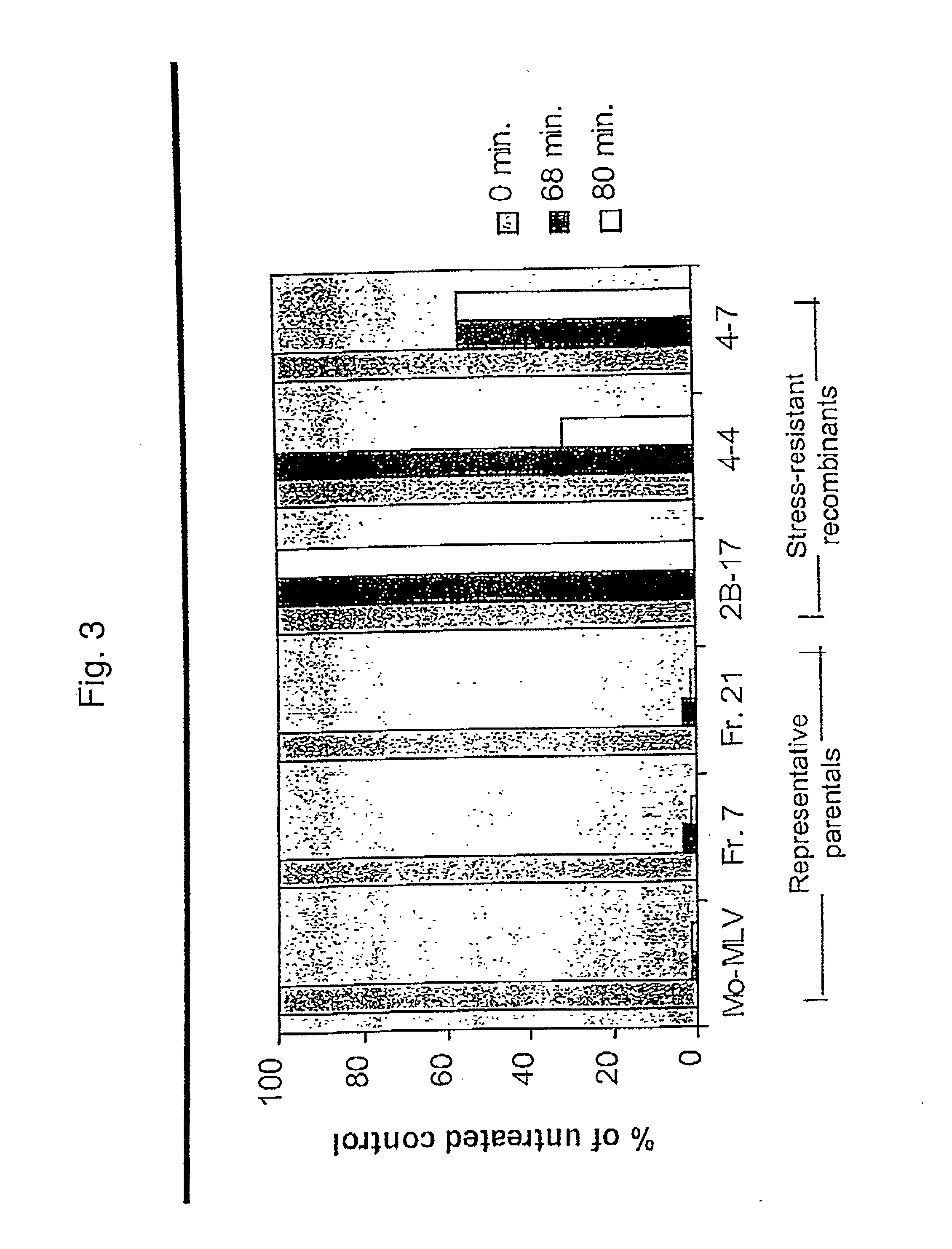
Stress resistant retroviruses
InactiveUS20020137889A1Peptide/protein ingredientsGenetic material ingredientsRetrovirusNucleic acid
Stress and / or shear resistant retrovirus envelope protein polypeptides and nucleic acids encoding such polypeptides, as well as fragments of such nucleic acids and polypeptides and compositions thereof, are provided. Retroviruses incorporating such polypeptides and methods of using stress resistant retrovirus envelope protein polypeptides and corresponding nucleic acids are also described.
Owner:MAXYGEN +1
Use of dsrnas in strategic therapeutic intervention of highly active antiretroviral therapy
In the treatment of HIV administration of dsRNA at an appropriate stage in highly active antiretroviral (HAART) therapy of HIV allows for the discontinuation of HAART by increasing the time to HIV rebound after stopping HAART.
Owner:HEMISPHERX BIOPHARMA
Modified venom and venom components as anti-retroviral agents
InactiveUS20060088858A1Avoid infectionInhibition of replicationPeptide/protein ingredientsMicrobiological testing/measurementFeline immunodeficiency virusAcquired immunodeficiency
The present invention relates to a class of proteins, and a method for treatment of neurological and viral diseases in humans and animals. More specifically it applies to the treatment of heretofore intractable diseases such as retro-viral infections including human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), feline immunodeficiency virus (FIV), bovine immunodeficiency virus (BIV) and equine acquired immunodeficiency virus (EAIV). The method of treatment comprises administering to the subject a disease mitigating amount of a detoxified modified venom composition.
Owner:RECEPTOPHARM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com