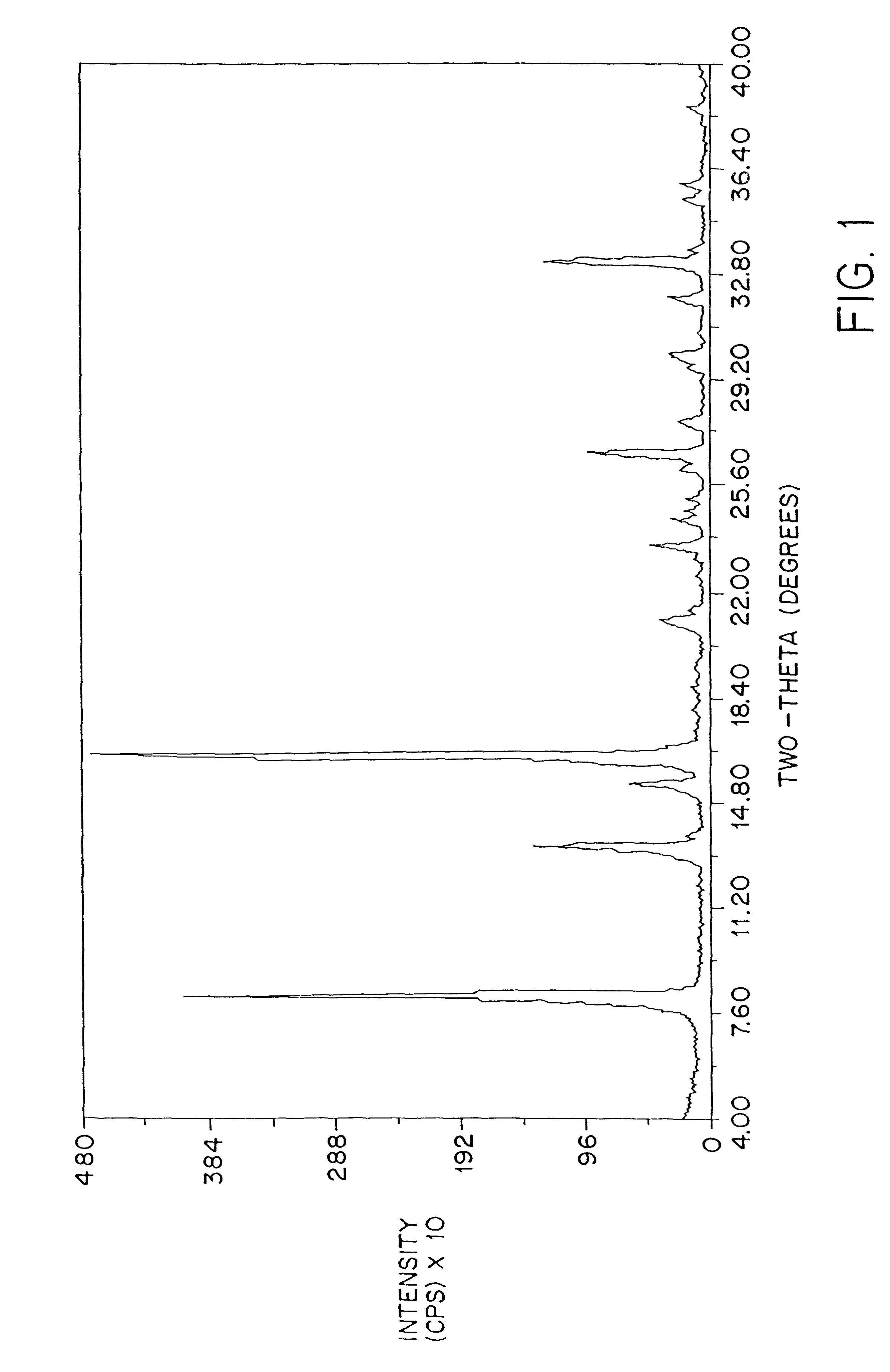
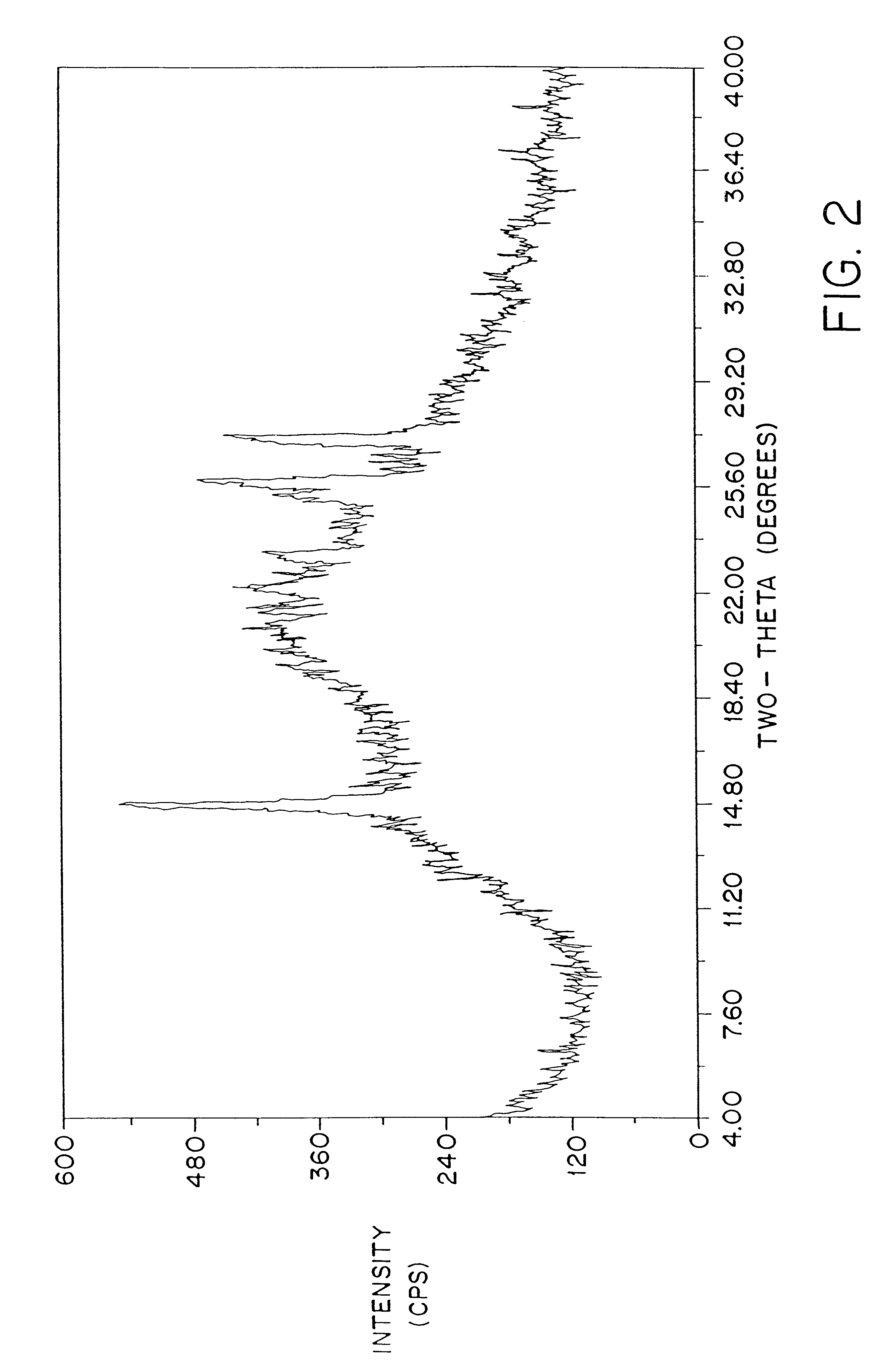
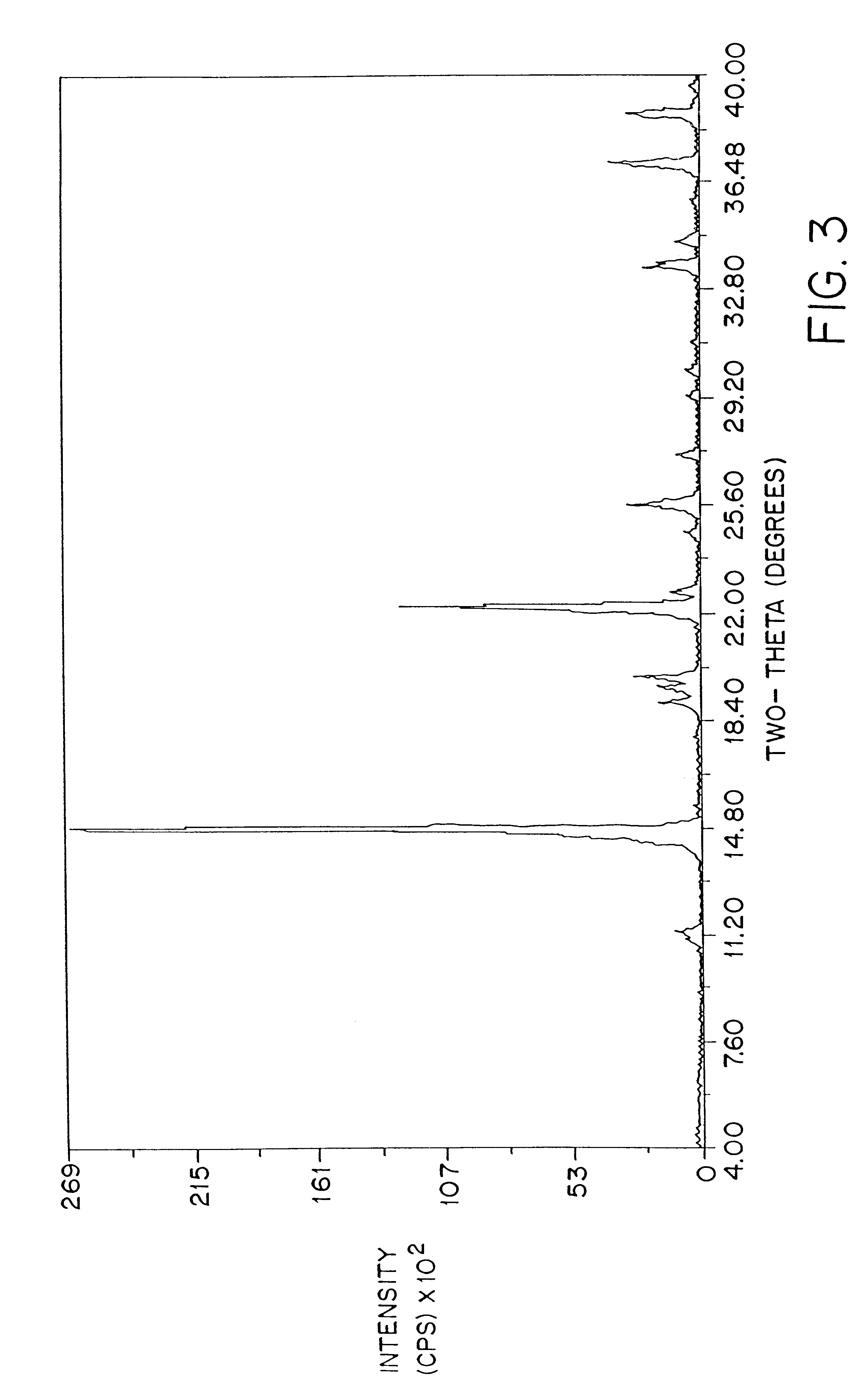
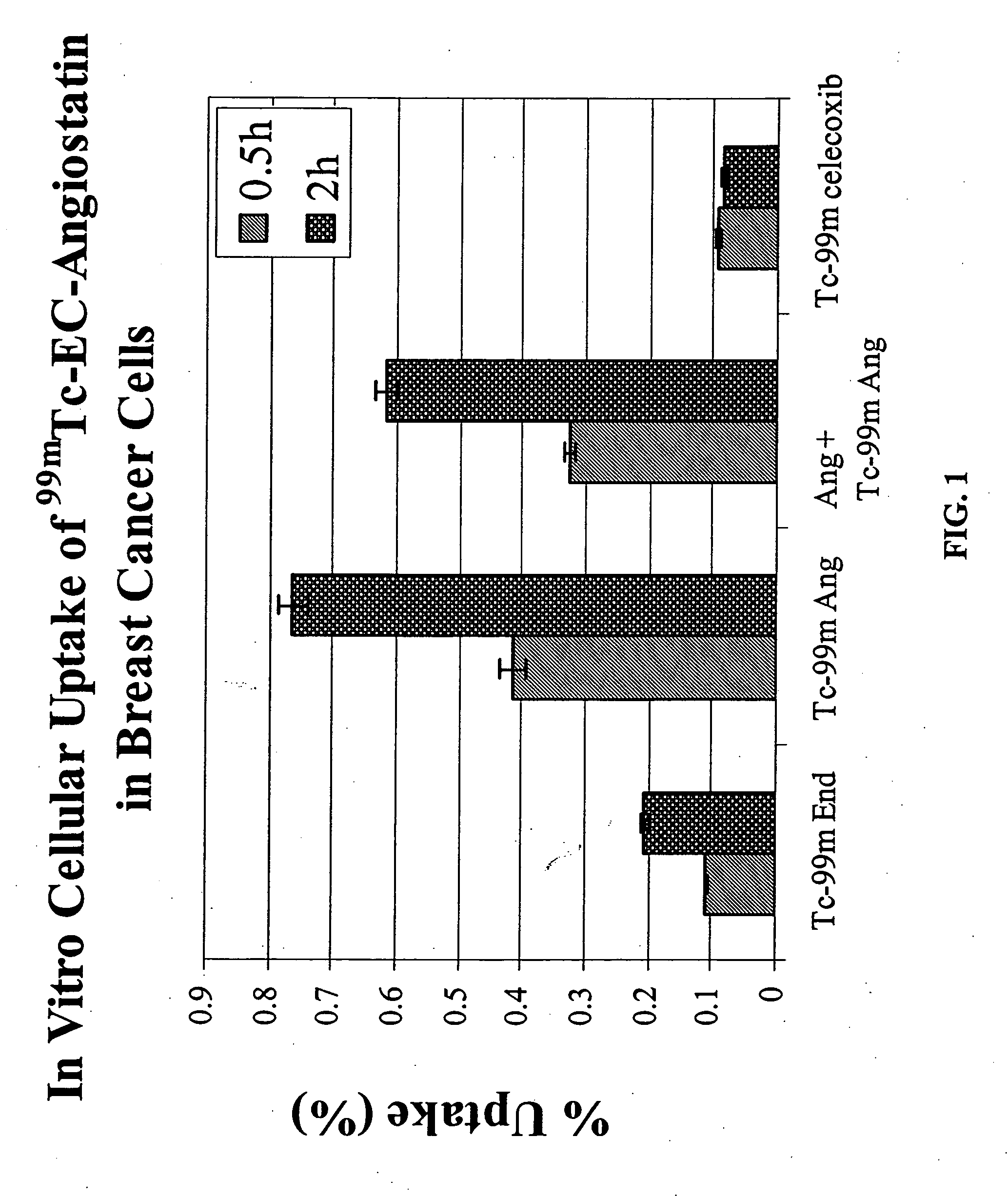
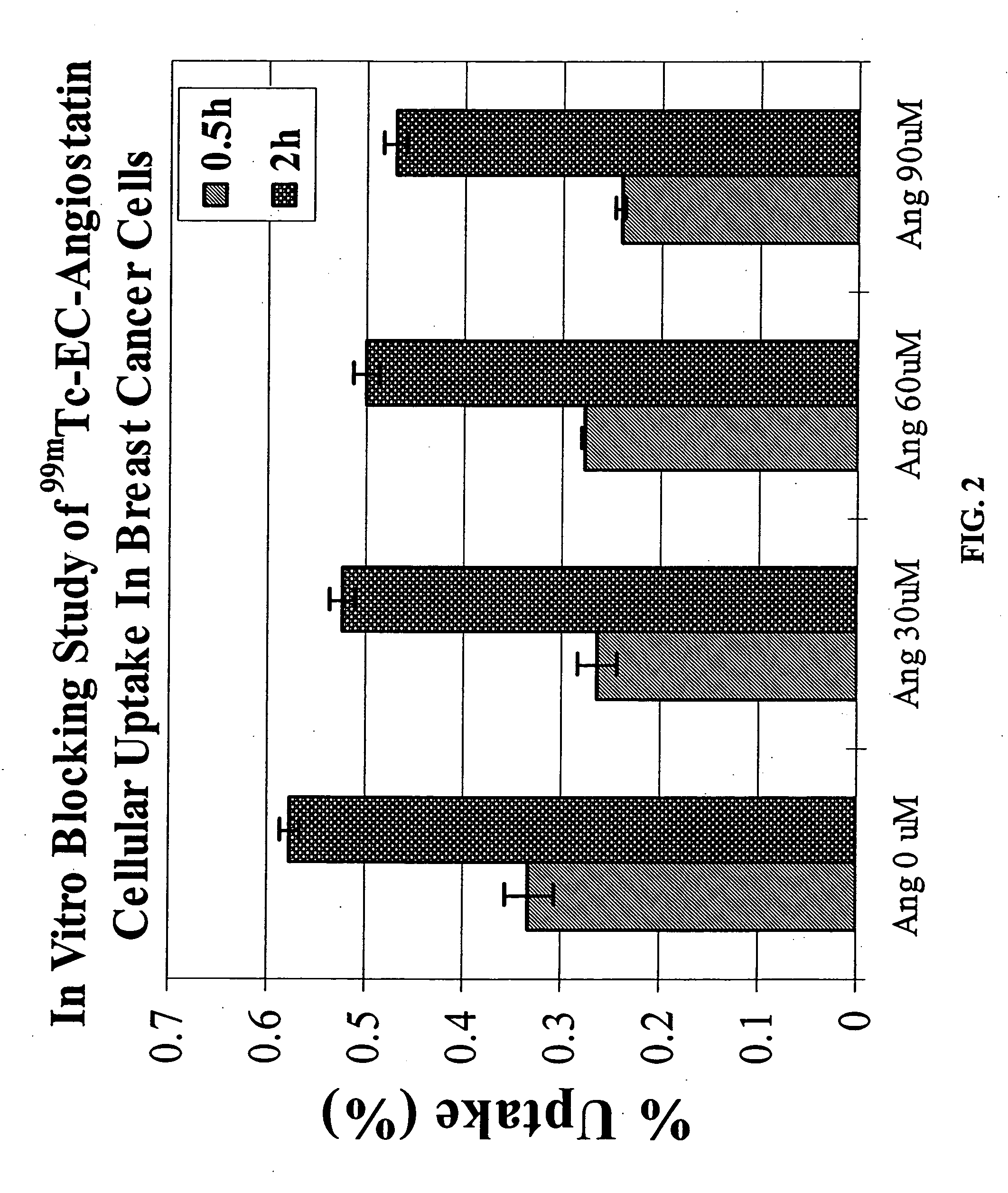
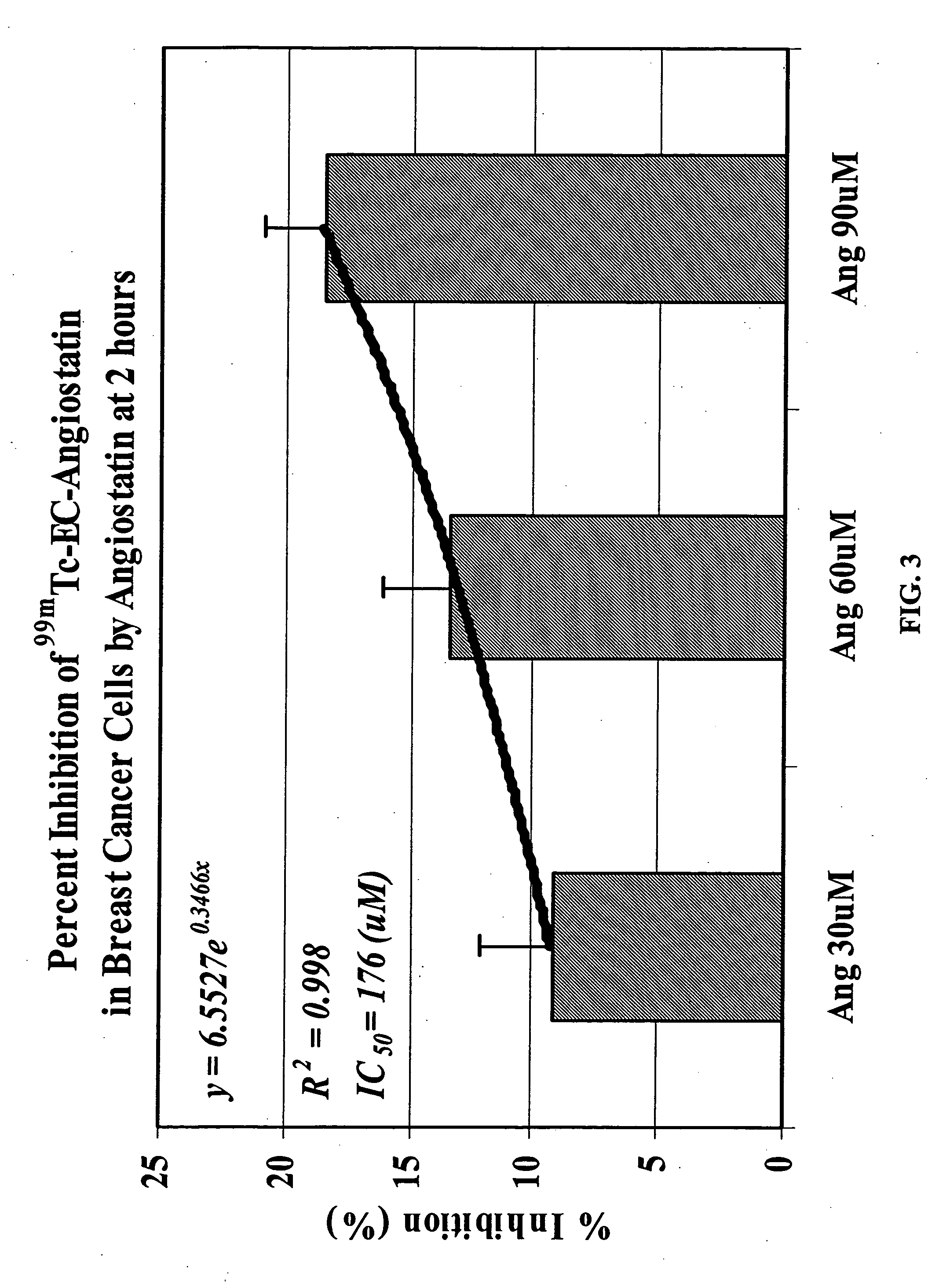
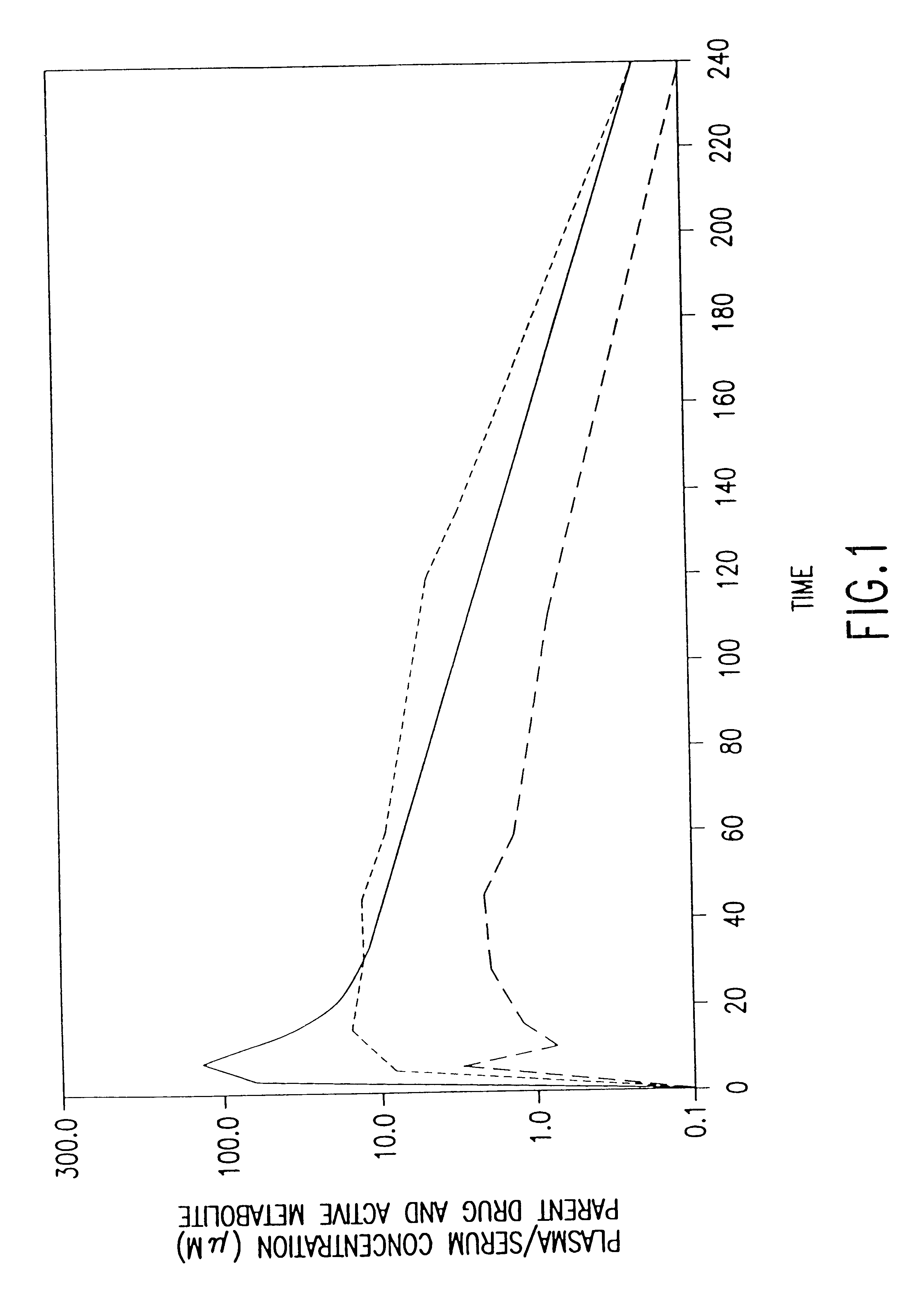
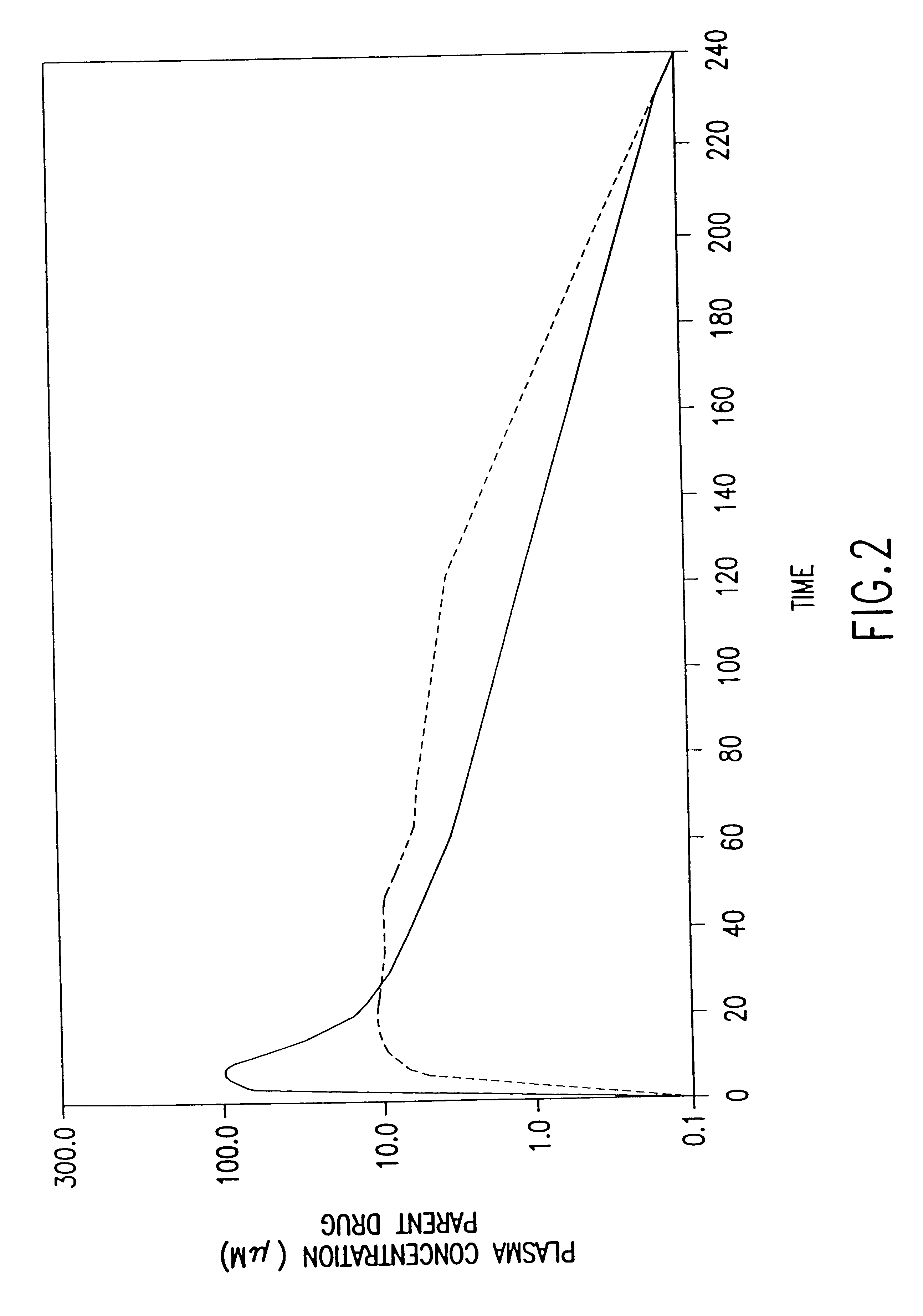
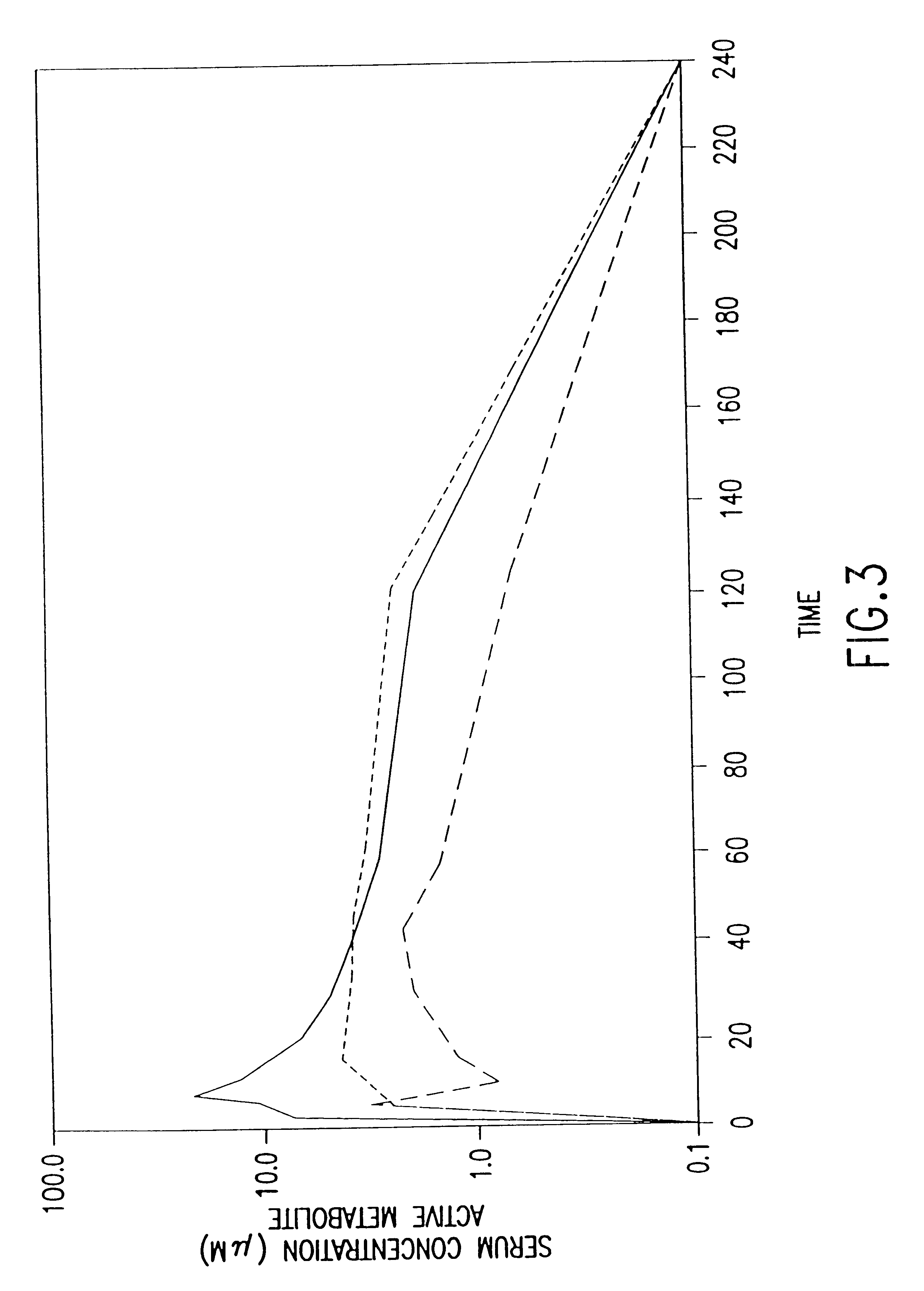
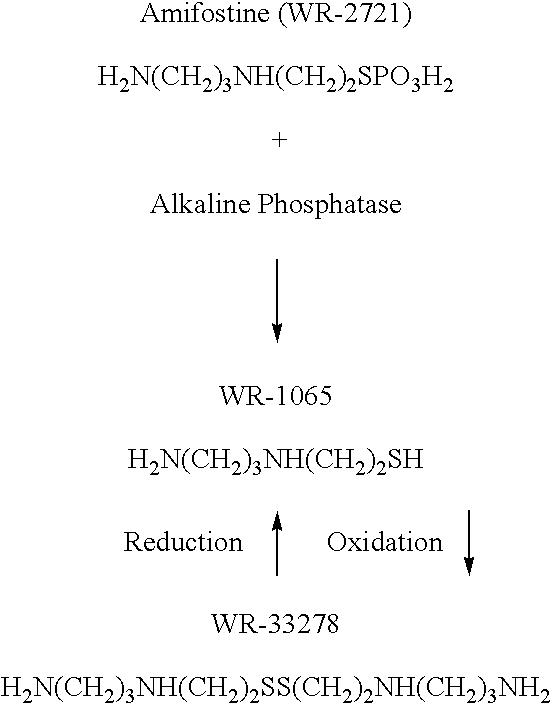
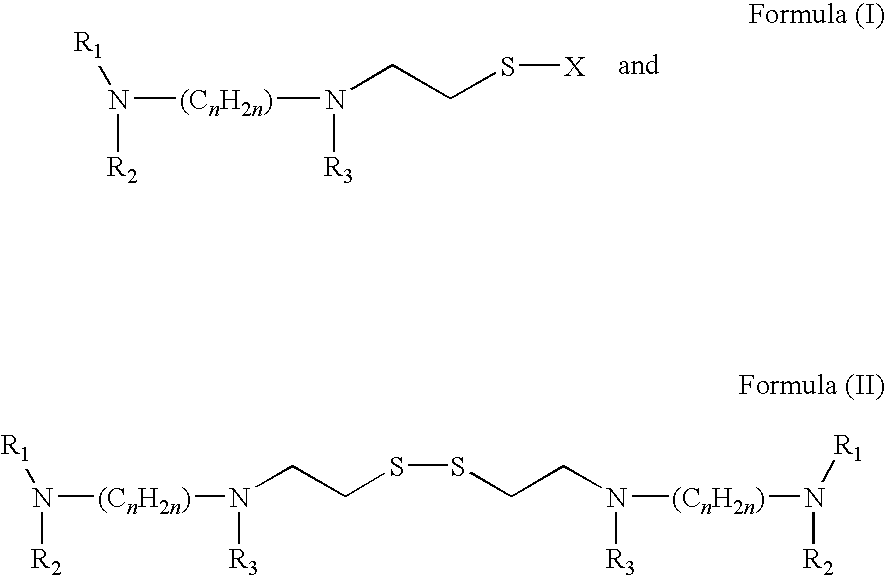
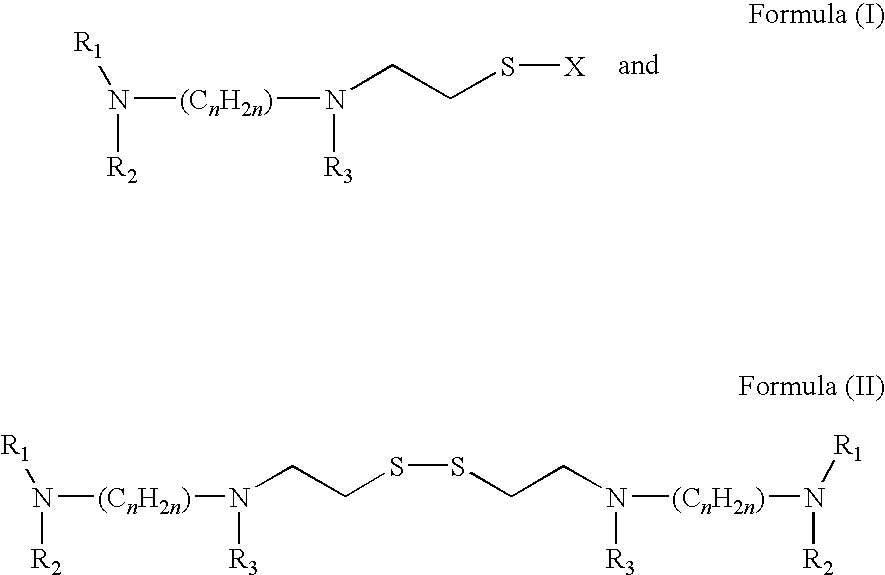
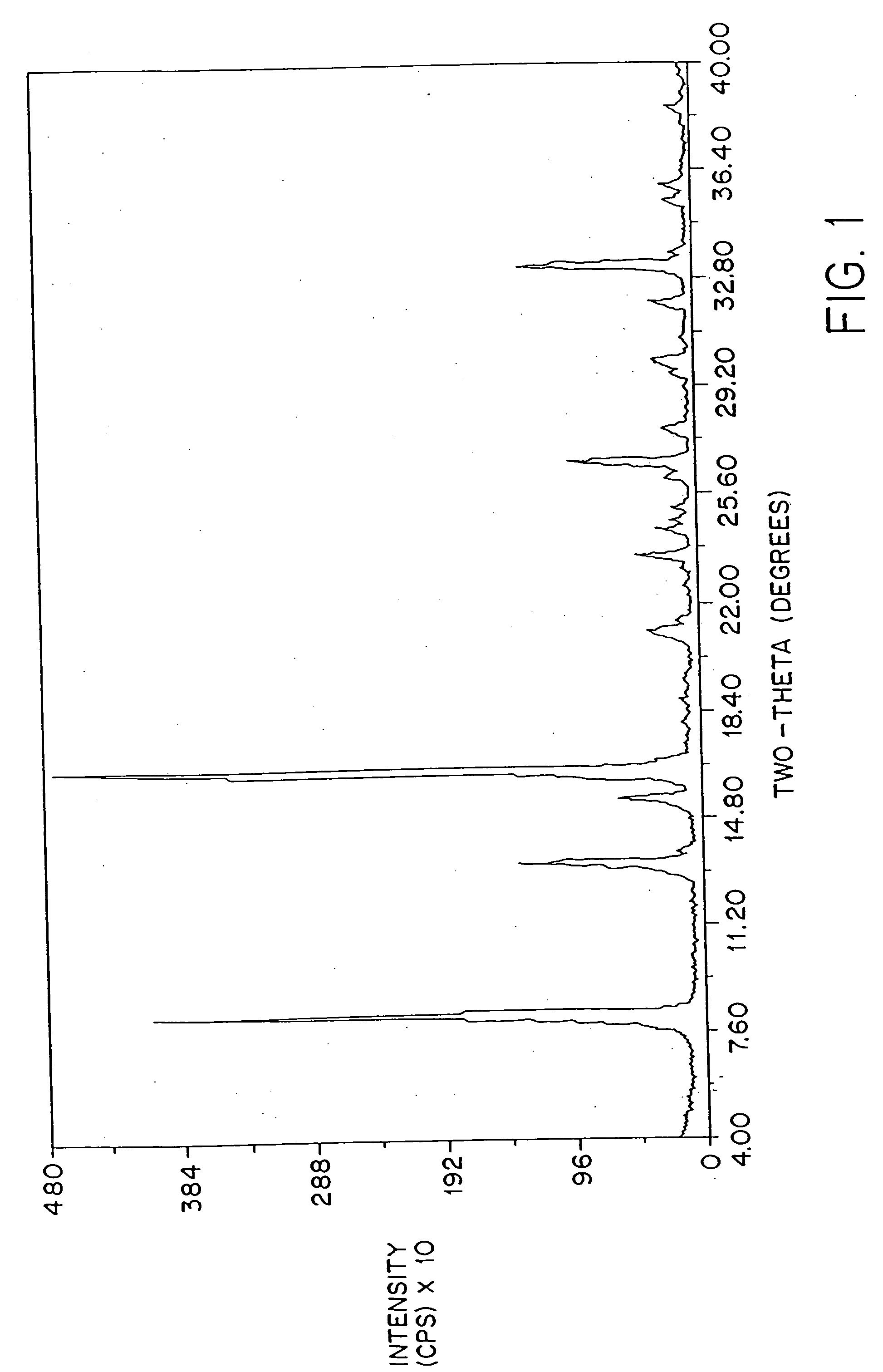
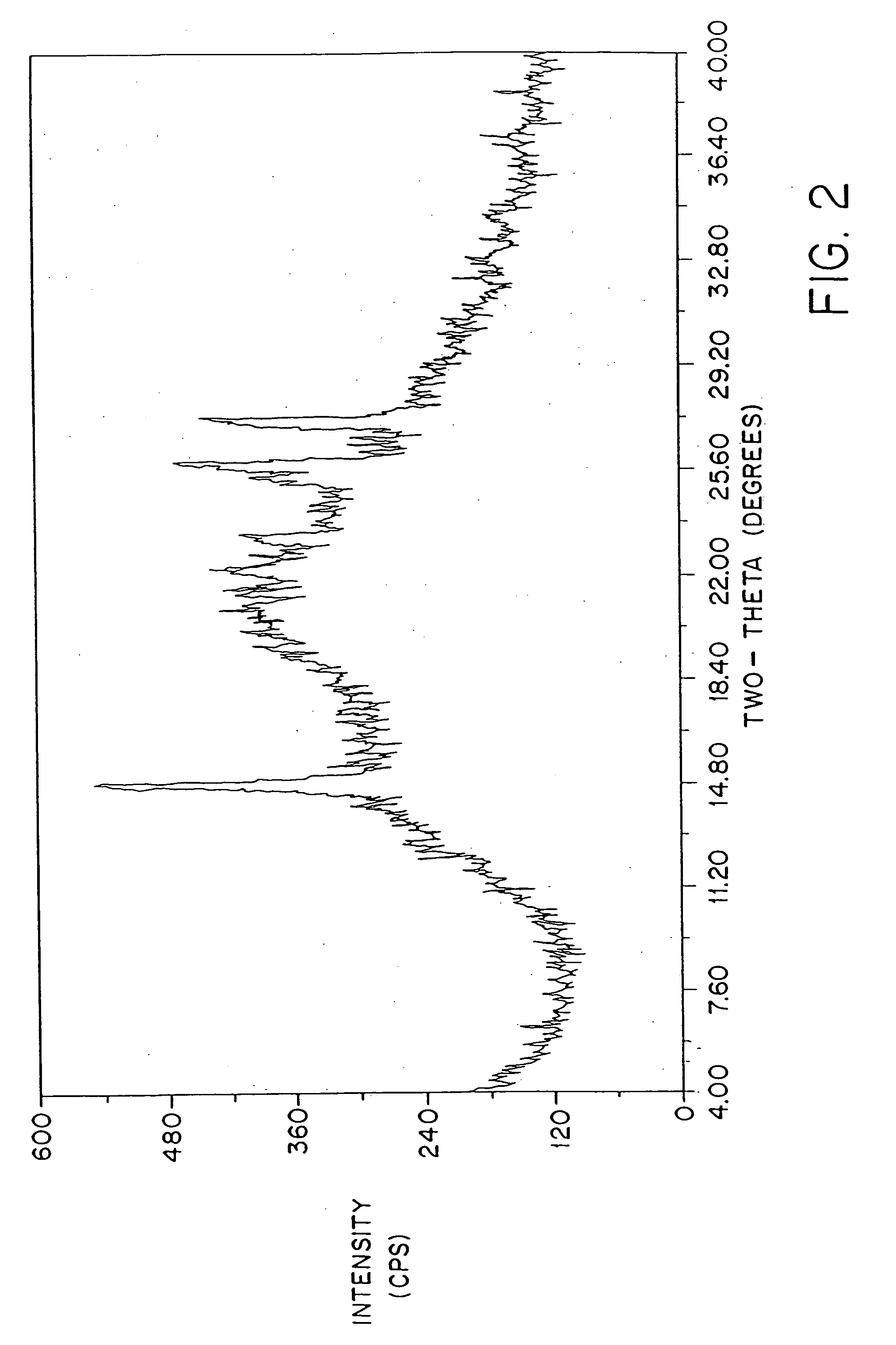
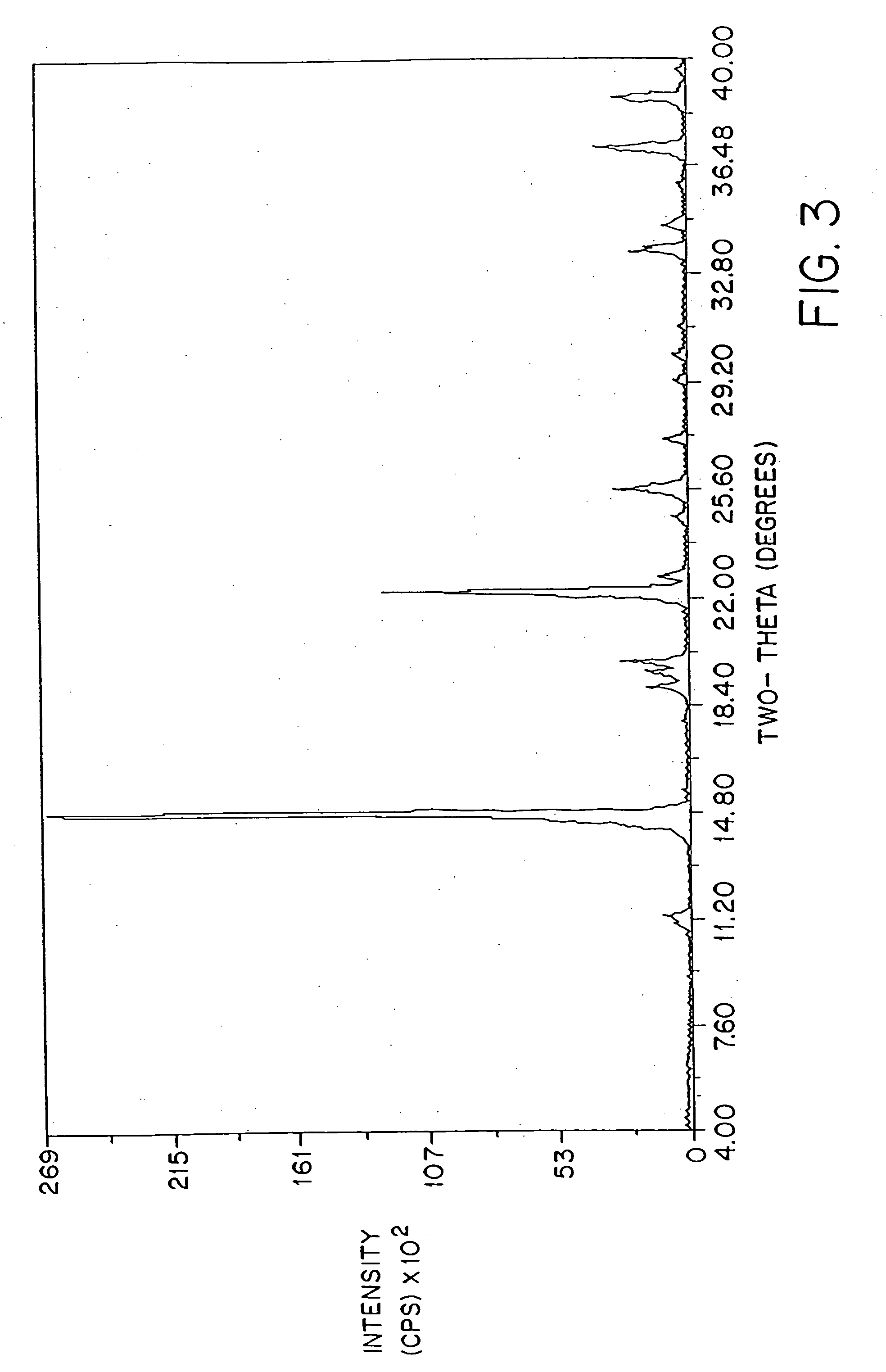
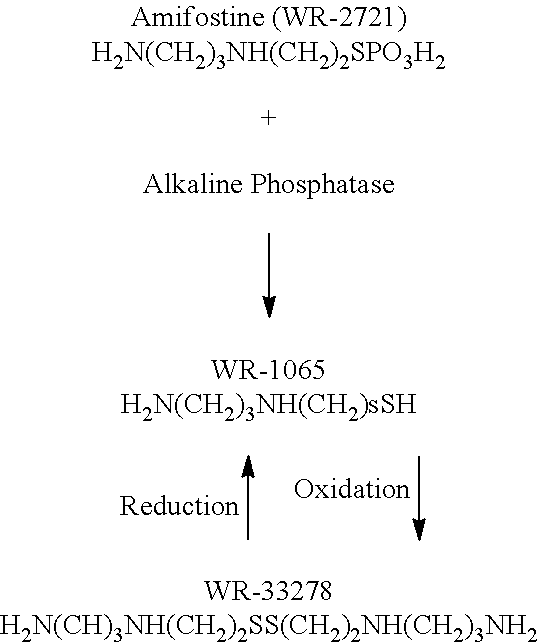
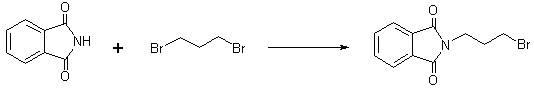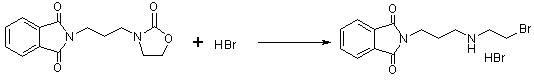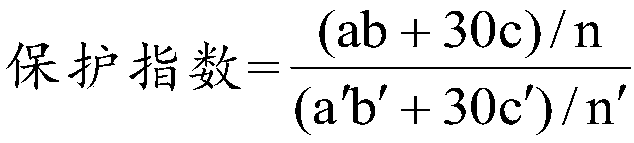Patents
Literature
52 results about "Amifostine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Amifostine is used to decrease the risk of kidney problems caused by treatment with a certain anti-cancer drug (cisplatin). It is also used to help prevent a certain side effect (dry mouth) caused by radiation treatment for head and neck cancer.
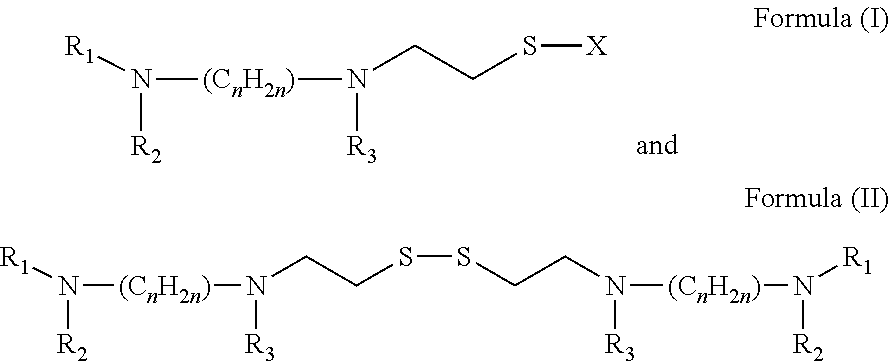
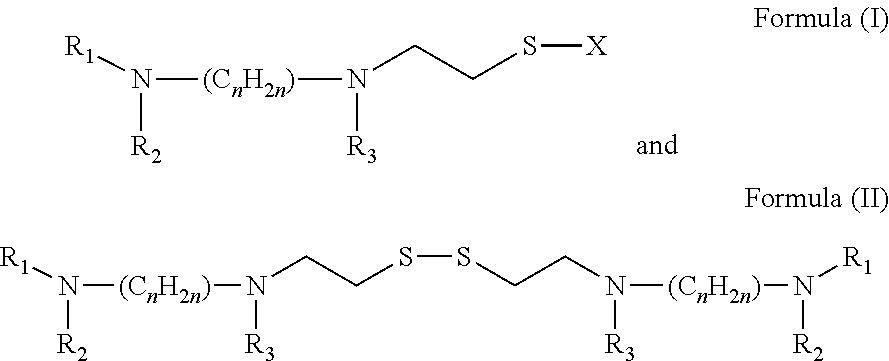
Topical administration of amifostine and related compounds
InactiveUS6239119B1Undesirable side-effectPrevent adverse side effectsBiocideNitro compound active ingredientsSide effectAmifostine
The present invention is directed to methods of treating or protecting mucosal tissue from damage associated with radiation and / or chemotherapeutic treatment of cancers, by the topical application of amifostine and related compounds. These methods avoid the side effects of systemically applied radio / chemo protectants. The invention is also directed to treatment and prevention of infections associated with mucositis by topical application of amifostine and related compounds.
Owner:CLINIGEN GRP PLC
Stable amorphous amifostine compositions and dosage form
The present invention relates to a sterile, stable dosage forms suitable for reconstitution and parenteral administration to a patient, said dosage form comprising an amorphous aminoalkyl dihydrogen phosphorothioate, and of amifostine in particular. The invention further relates to a method of preparing such a dosage form, which typically exhibits enhanced thermal stability as compared to existing vacuum dried amorphous amifostine.
Owner:CLINIGEN GRP PLC
N2S2 chelate-targeting ligand conjugates
ActiveUS20050129619A1Sufficient amountHybrid immunoglobulinsRadioactive preparation carriersAngiostatinAbnormal tissue growth
The invention provides, in a general sense, a new labeling strategy employing compounds that are are N2S2 chelates conjugated to a targeting ligand, wherein the targeting ligand is a disease cell cycle targeting compound, a tumor angiogenesis targeting ligand, a tumor apoptosis targeting ligand, a disease receptor targeting ligand, amifostine, angiostatin, monoclonal antibody C225, monoclonal antibody CD31, monoclonal antibody CD40, capecitabine, a COX-2 inhibitor, deoxycytidine, fullerene, herceptin, human serum albumin, lactose, leuteinizing hormone, pyridoxal, quinazoline, thalidomide, transferrin, or trimethyl lysine. The present invention also pertains to kits employing the compounds of interest, and methods of assessing the pharmacology of an agent of interest using the present compounds.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods for the administration of amifostine and related compounds
InactiveUS6573253B2Decrease and reduces undesirable side effect of compoundReduces or decreases the adverse or undesirable side effects sufferedBiocideEnergy modified materialsThiolSide effect
The present invention provides methods of administering aminoalkyl phosphorothioate and / or aminoalkyl thiol compounds to patients receiving radiation therapy in a manner that significantly reduces or decreases the adverse or undesirable side-effects of the compounds as compared with conventional intravenous administration.
Owner:CLINIGEN GRP PLC
Organic thiophosphate antiretroviral agents
InactiveUS20090239817A1Reducing and preventing effectPromote repairBiocideSulfur/selenium/tellurium active ingredientsImmunodeficiency virusAmifostine
A method for the prevention or treatment of human immunodeficiency virus infection by administering an effective amount of amifostine, phosphonol, or similar compound to an individual in need is provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Method for protection against tumor metastasis formation
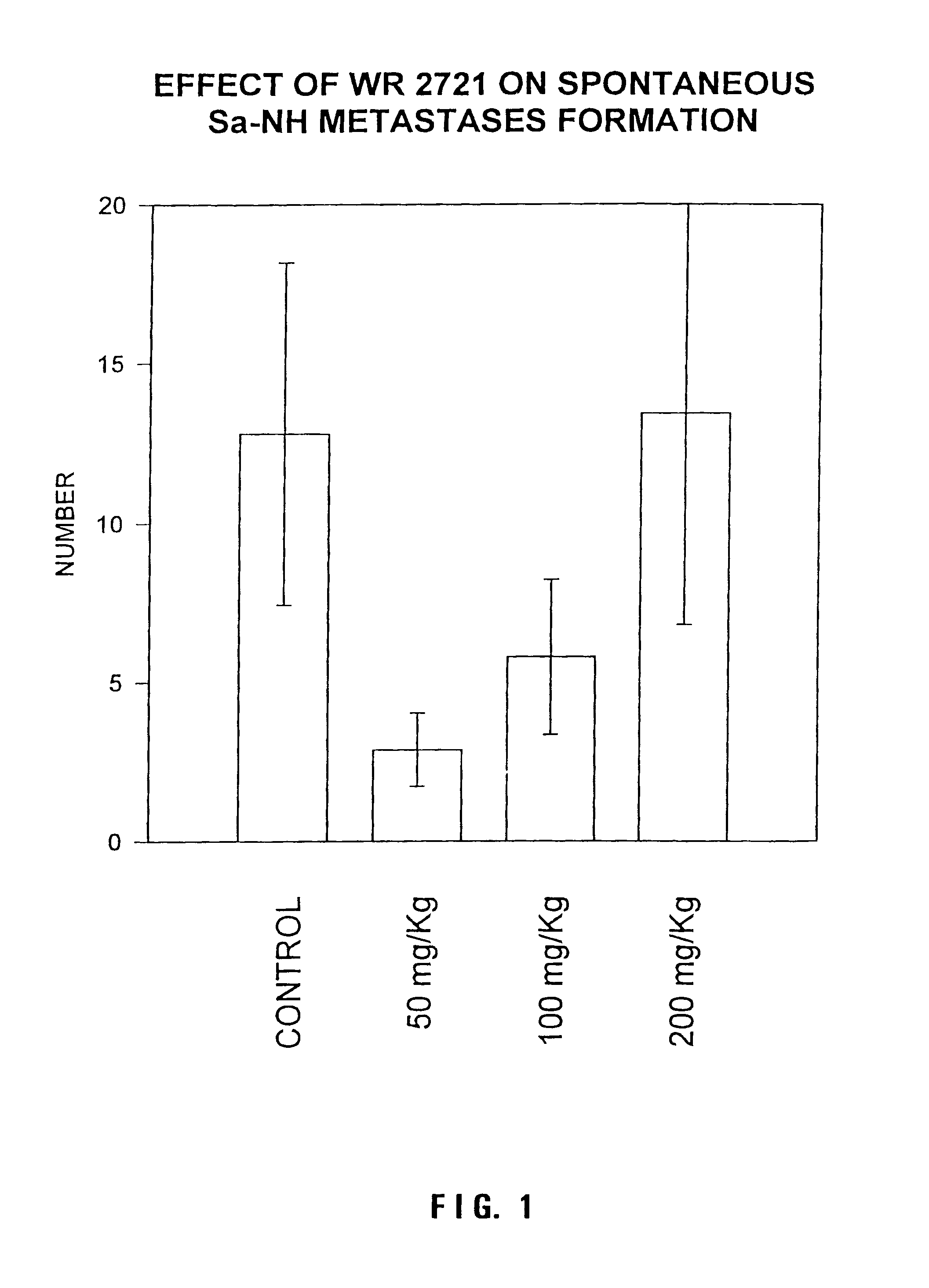
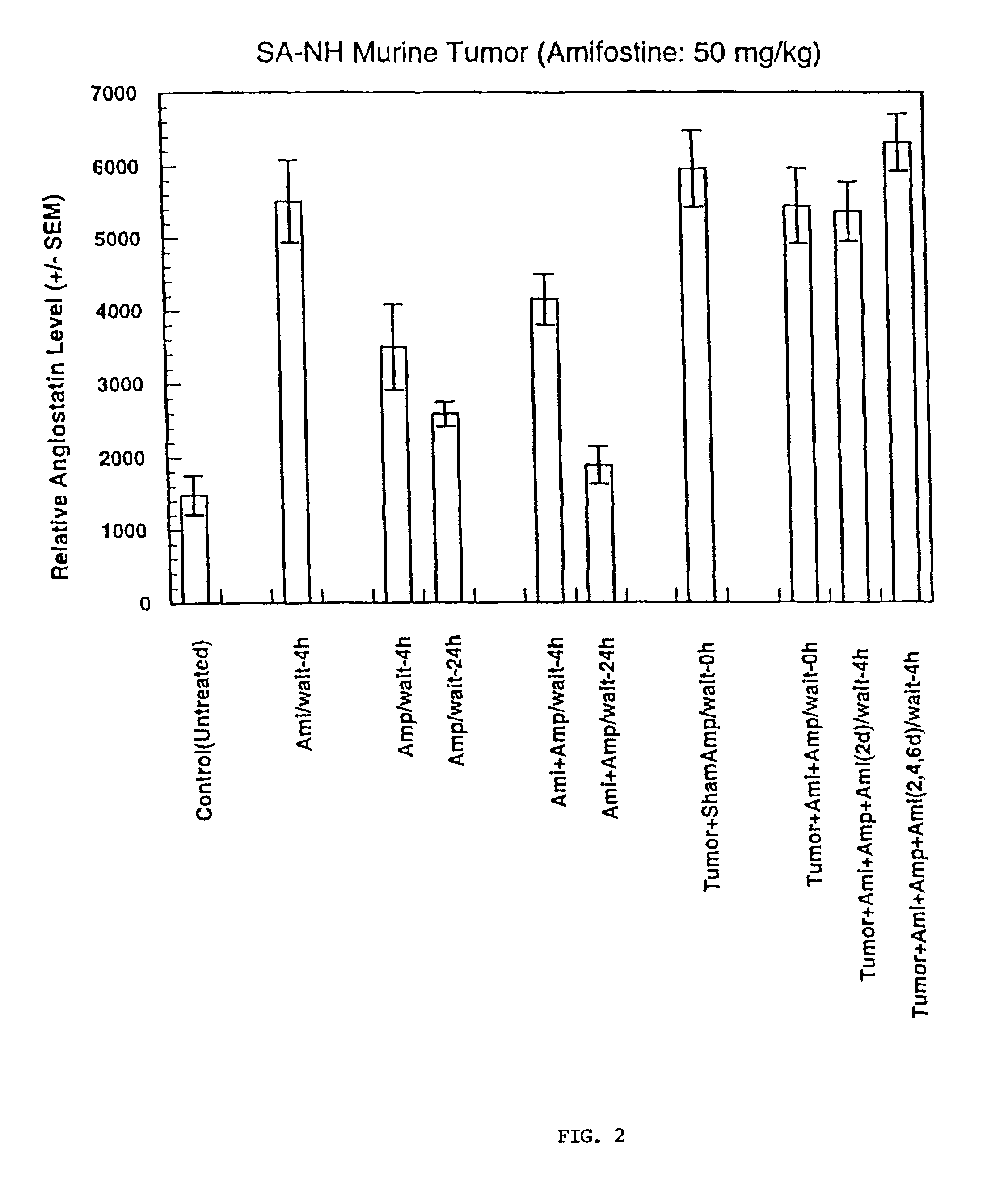
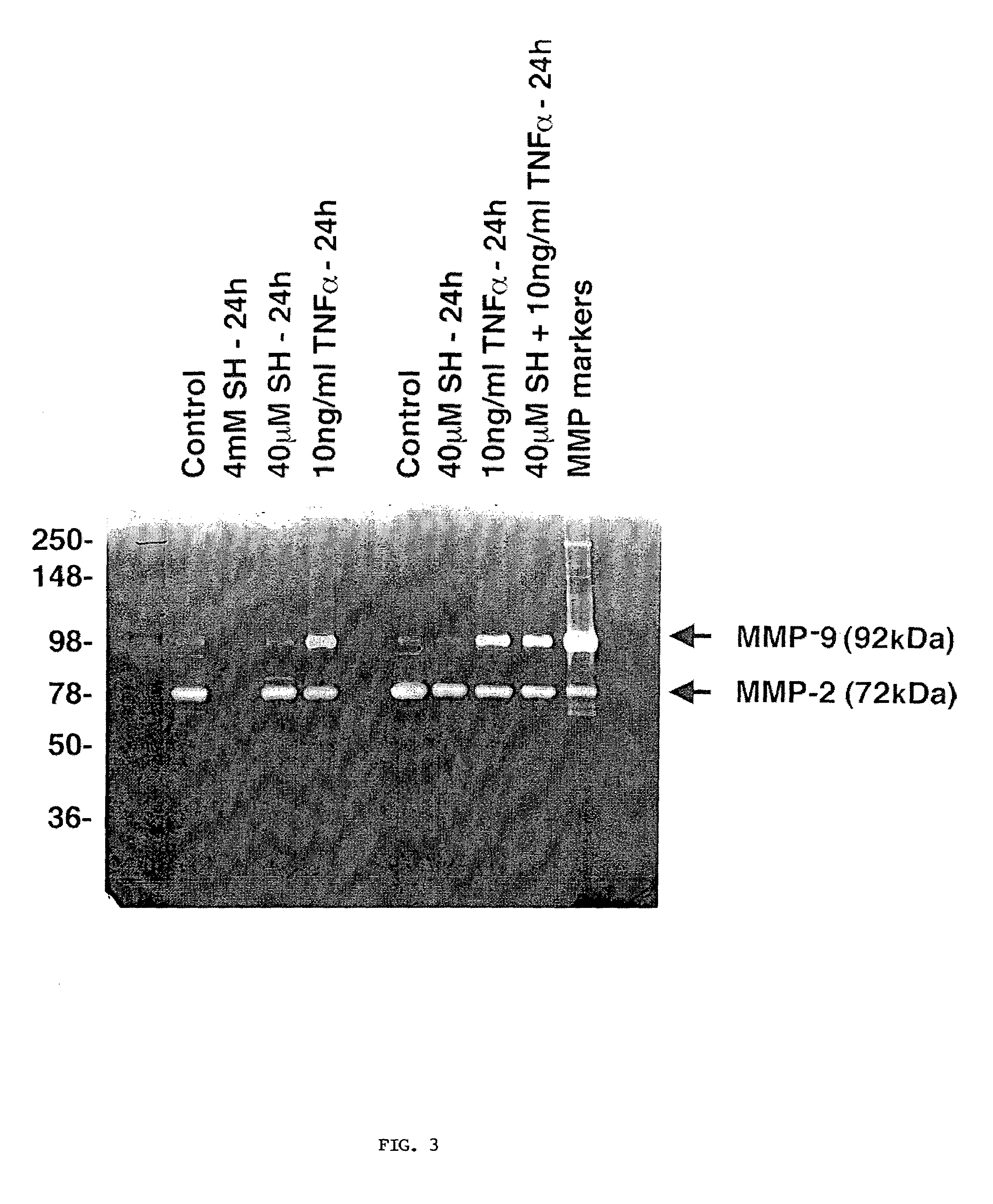
Methods and pharmaceuticals for inhibiting or preventing metastasis formation in animals, including humans, having primary tumors, through the administration of phosphorothioates including their thiol and disulfide metabolites are disclosed. These compounds stimulate angiostatin levels, inhibit matrix metalloproteinases (MMPs), and stimulate manganese superoxidase dismutase (MnSOD). Phosphorothioates, of which amifostine is an example, can be administered as a combination therapy with traditional cancer therapies, including chemotherapy, radiotherapy, surgery, immunotherapy, hormone therapy and gene-therapy. Inhibition or prevention of metastasis by phosphorothioates is independent of tumor type, including adenocarcinomas and sarcomas.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Method for the administration of amifostine and related compounds
InactiveUS6127351ADecrease and reduces undesirable side effect of compoundReduces or decreases the adverse or undesirable side effects sufferedBiocideAntinoxious agentsThiolSide effect
The present invention provides methods of administering aminoalkyl phosphorothioate and / or aminoalkyl thiol compounds to patients in a manner that significantly reduces or decreases the adverse or undesirable side-effects of the compounds as compared with conventional intravenous administration.
Owner:CLINIGEN GRP PLC
Topical administration of amifostine and related compounds
InactiveUS20030022867A1Undesirable side-effectSignificant antibacterial propertyBiocideNitro compound active ingredientsSide effectAmifostine
The present invention is directed to methods of treating or protecting mucosal tissue from damage associated with radiation and / or chemotherapeutic treatment of cancers, by the topical application of amifostine and related compounds. These methods avoid the side effects of systemically applied radio / chemo protectants. The invention is also directed to treatment and prevention of infections associated with mucositis by topical application of amifostine and related compounds.
Owner:CLINIGEN GRP PLC
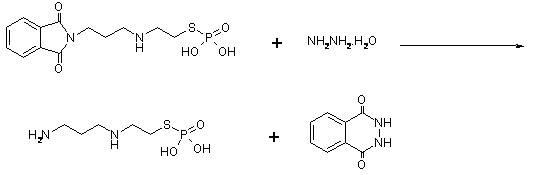
Methods for the treatment of nephro-disorders using aminothiol compounds
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +1
Methods for treatment of neuro- and nephro- disorders and therapeutic toxicities using aminothiol compounds
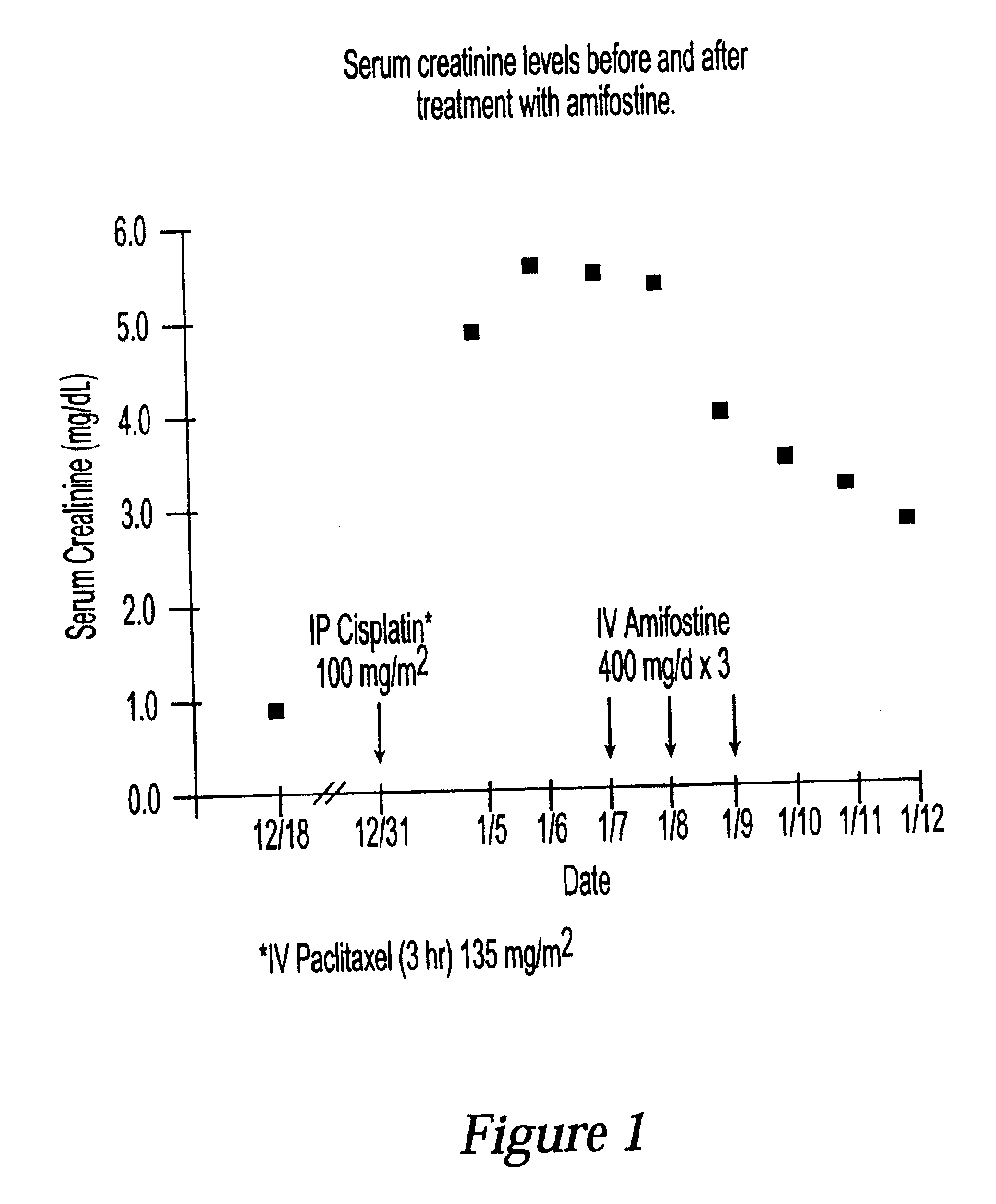
The present invention relates to new uses of S-2-(3-aminopropylamino)ethyl dihydrogen phosphorothioate, (amifostine) and other aminothiol compounds to treat and reverse toxicities caused by therapeutic agents, radiation treatment or diabetes. In particular, the invention provides a method for treating neurotoxicity and nephrotoxicity associated with the administration of chemotherapeutic agents.
Owner:CLINIGEN GRP PLC
Process for the preparation of (omega-aminoalkylamino)alkyl halides and conversion to amifostine
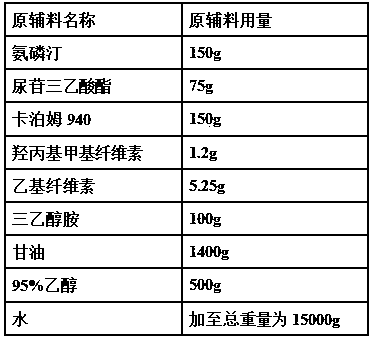
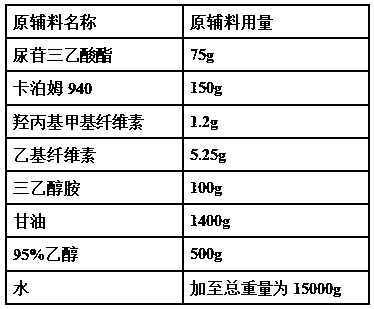
InactiveCN101321724AOrganic compound preparationGroup 5/15 element organic compoundsAlcoholAmifostine
The present invention relates to processes for the preparation of (Omega-aminoalkylainino)alkyl halides, their conversion to S-Omega-(Omega-aminoalkylamino)alkyl phosphothioates, and purification of the crystalline products of the reaction. The preparation process for the (Omega-aminoalkylamino)alkyl halides comprises contacting an appropriate alcohol with a brominating agent in the presence of a sulfone solvent under temperature and pressure conditions suitable to effect salt formation without subsequent premature precipitation. The process is especially useful for converting (Omega-aminoalkylamino) ethyl alcohol to amifostine.
Owner:ALBEMARLE CORP
Stable amifostine liquid concentrate
InactiveUS20080139515A1Reduce nephrotoxicityReducing xerostomiaBiocidePhosphorous compound active ingredientsAmifostineAqueous solution
A storage stable aqueous solution of amifostine at a pH of at least 10.0, in an amifostine concentration of about 50 to about 250 mg / l, which formulation is storage stable under refrigerated conditions.
Owner:SCIDOSE
Stable amorphous amifostine compositions and methods for the preparation and use of same
The present invention relates to sterile, stable dosage forms suitable for reconstitution and parenteral administration to a patient, said dosage form comprising an amorphous aminoalkyl dihydrogen phosphorothioate, and amifostine in particular. The invention further relates to a method of preparing such a dosage form, which typically exhibits enhanced thermal stability as compared to existing vacuum dried amorphous amifostine.
Owner:MEDIMMUNE ONCOLOGY
Treatment medicine capable of degrading high-polymer tumor and sustained-release medicine for normal cell protective agent
InactiveCN101444625AAvoid damageImprove protectionOrganic active ingredientsPharmaceutical delivery mechanismTumor targetTumor targeting
The invention discloses a treatment medicine capable of degrading high-polymer tumor and a sustained-release medicine for normal cell protective agent. The sustained-release medicine comprises one or more of the following sustained-release systems: (1) a tumor tissue and peripheral implanted medicine sustained-release system; (2) an absorbable nanometer medicine sustained-release system containing a lymphaden tracking medicine in lymph; (3) a nanometer medicine sustained-release system in blood or body fluid; (4) a nanometer medicine sustained-release system in a specific tissue, wherein, the specific tissue can be a liver, a spleen, a lung or marrow. The treatment medicine for tumor comprises one or more of chemotherapeutic medicine, chemotherapeutic sensitizer and tumor targeting medicine, and uses amifostine as the normal cell protective agent. The range of tumor treatment is applicable to any tumor through changing different treatment medicines. The invention is a novel medicine application platform, can be used by any new medicine besides the existing medicines, and plays a role in conquering cancer finally.
Owner:盛小禹
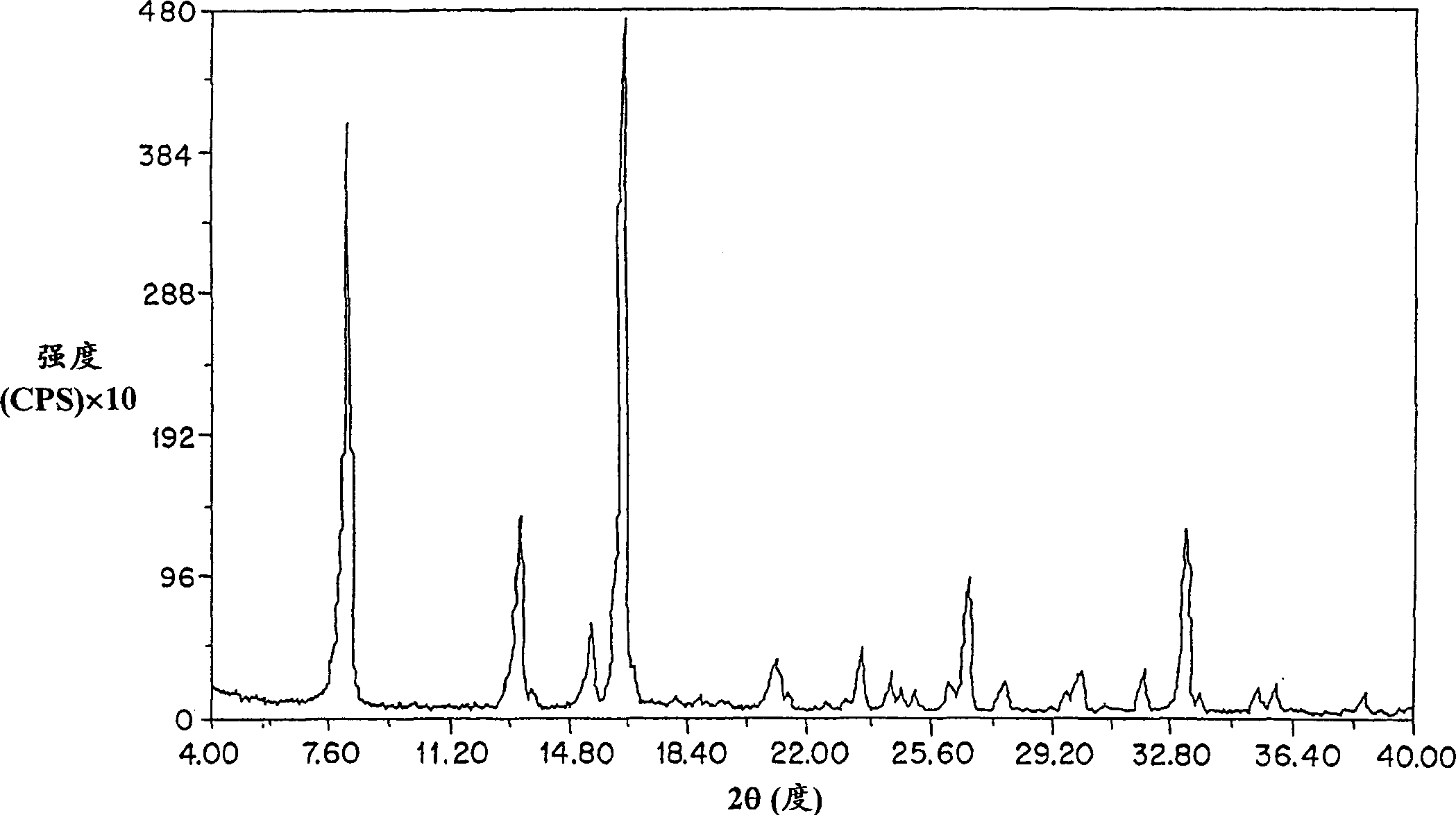
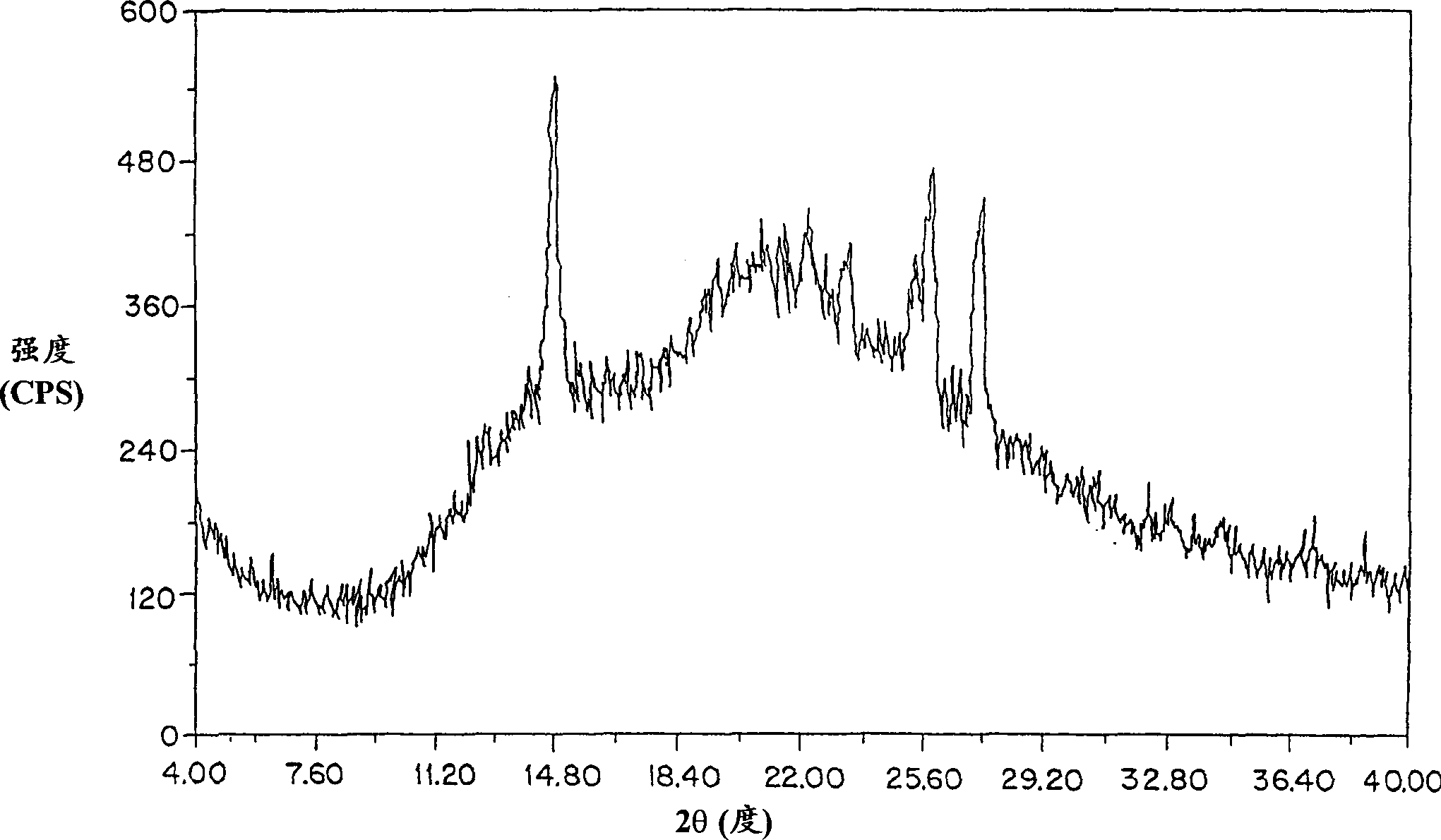
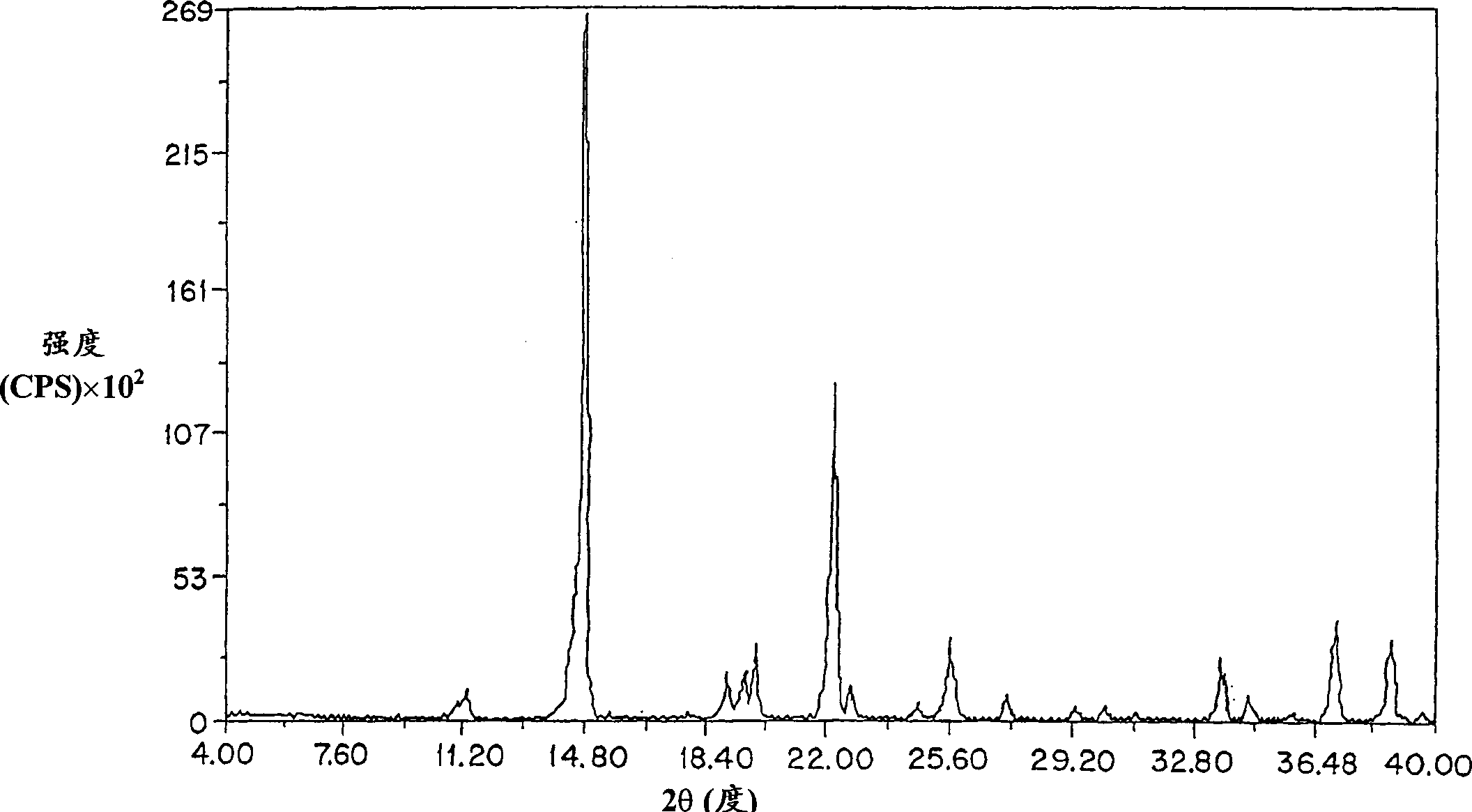
Method for preparing amifostine
ActiveCN102659836AEasy to produceReduce manufacturing costGroup 5/15 element organic compoundsOxazolidoneDibromopropane
The invention relates to a method for preparing amifostine, comprising the following steps: a) reacting phthalimide with 1,3-dibromopropane in dipolar aprotic solvents to genenrate N-(3-bromopropyl) phthalimide; and b)reacting N-(3-bromopropyl) phthalimide with 2-oxazolidinone in dipolar aprotic solvents to generate 2,2-[3-(2-carbonyl-3-oxazole)propyl]-1H-isoindole-1,3(2H)-diketone; and using the one-pot process to prepare the amifostine directly from the intermediate 2,2-[3-(2-carbonyl-3-oxazole)propyl]-1H-isoindole-1,3(2H)-diketone, thus the production process can be simplified, and the production cost can be saved. In addition, primary amine is obtained by hydrazinolysis, thus the reaction conditions are mild, the side effect is low, and the post-treatment is simple. The method has the characteristics of easy obtainment of raw materials, low price, safe and convenient usage, little pollution, easy transportation, and the like.
Owner:NANJING CHENGONG PHARM CO LTD
Freeze drying preparation of amifostine, and preparation method
ActiveCN1695626AImprove performanceEasy to usePowder deliveryOrganic active ingredientsFreeze-dryingCombinatorial chemistry
A freeze-dried amifostine is prepared from amifostine, acetone and the solution of pharmacologically acceptable excipient through prefreezing, sublimated drying, and drying again.
Owner:深圳市资福药业有限公司
Methods for the administration of amifostine and related compounds
InactiveUS7053072B2Affects efficacyReduce or decrease adverse or undesirable side effects sufferedBiocideEnergy modified materialsSide effectRadical radiotherapy
The present invention provides methods of administering amifostine, WR-1065, or a combination thereof, to patients receiving radiation therapy in a manner that significantly reduces or decreases the adverse or undesirable side-effects of the compounds as compared with conventional intravenous administration.
Owner:CLINIGEN GRP PLC
Amifostine slow-release microspheres for subcutaneous injection and preparation method thereof
ActiveCN105997889AAvoid disadvantagesUniform in vitro releaseOrganic active ingredientsAntinoxious agentsHypodermoclysisEmulsion
The invention discloses amifostine slow-release microspheres for subcutaneous injection and a preparation method thereof. A unique two-way emulsion-solution evaporation technology is adopted, the prepared amifostine slow-release microspheres for subcutaneous injection can reach relative constant and long-acting drug release in a subcutaneous injection scene, in-vitro release is even, release time can be as long as 300 hours or above, the protection function is stable, adverse factors brought by the fact that existing amifostine can only adopt an intravenous injection drug application mode are avoided, and the problem that drug release is not uniform and lasting through subcutaneous injection is also solved.
Owner:SHANGHAI PULMONARY HOSPITAL
Stable amorphous amifostine compositions and methods for the preparation and use of same
InactiveUS20050209200A1Increase processing costDifficult to useBiocideAntinoxious agentsThermal stabilityAmifostine
The present invention relates to a sterile, stable dosage forms suitable for reconstitution and parenteral administration to a patient, said dosage form comprising an amorphous aminoalkyl dihydrogen phosphorothioate, and of amifostine in particular. The invention further relates to a method of preparing such a dosage form, which typically exhibits enhanced thermal stability as compared to existing vacuum dried amorphous. amifostine.
Owner:CLINIGEN GRP PLC
Organic thiophosphate antiretroviral agents
InactiveUS20160374967A1Promote repairReduced mutagenesisBiocideSulfur/selenium/tellurium active ingredientsImmunodeficiency virusAmifostine
A method for the prevention or treatment of human immunodeficiency virus infection by administering an effective amount of amifostine, phosphonol, or similar compound to an individual in need is provided.
Owner:UNITED STATES OF AMERICA +1
Method for quickly and efficiently separating cartilage cells
ActiveCN106244529AHigh activityImprove protectionSkeletal/connective tissue cellsProteinase activityDecomposition
The invention relates to the technical field of in-vitro tissue cell separation, and particularly relates to a method for quickly and efficiently separating cartilage cells. A human serum albumin is added in the early digestion stage to buffer the decomposition effect of other extrinsic protease on type II collagenase, thus ensuring that the type II collagenase is not damaged and has high activity; and an amifostine solution is further added in the late digestion stage to favorably protect cartilage cells and effectively prevent the cartilage cells from being damaged in the digestion process, thereby being beneficial to obtaining more primary cartilage cells with higher activity.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
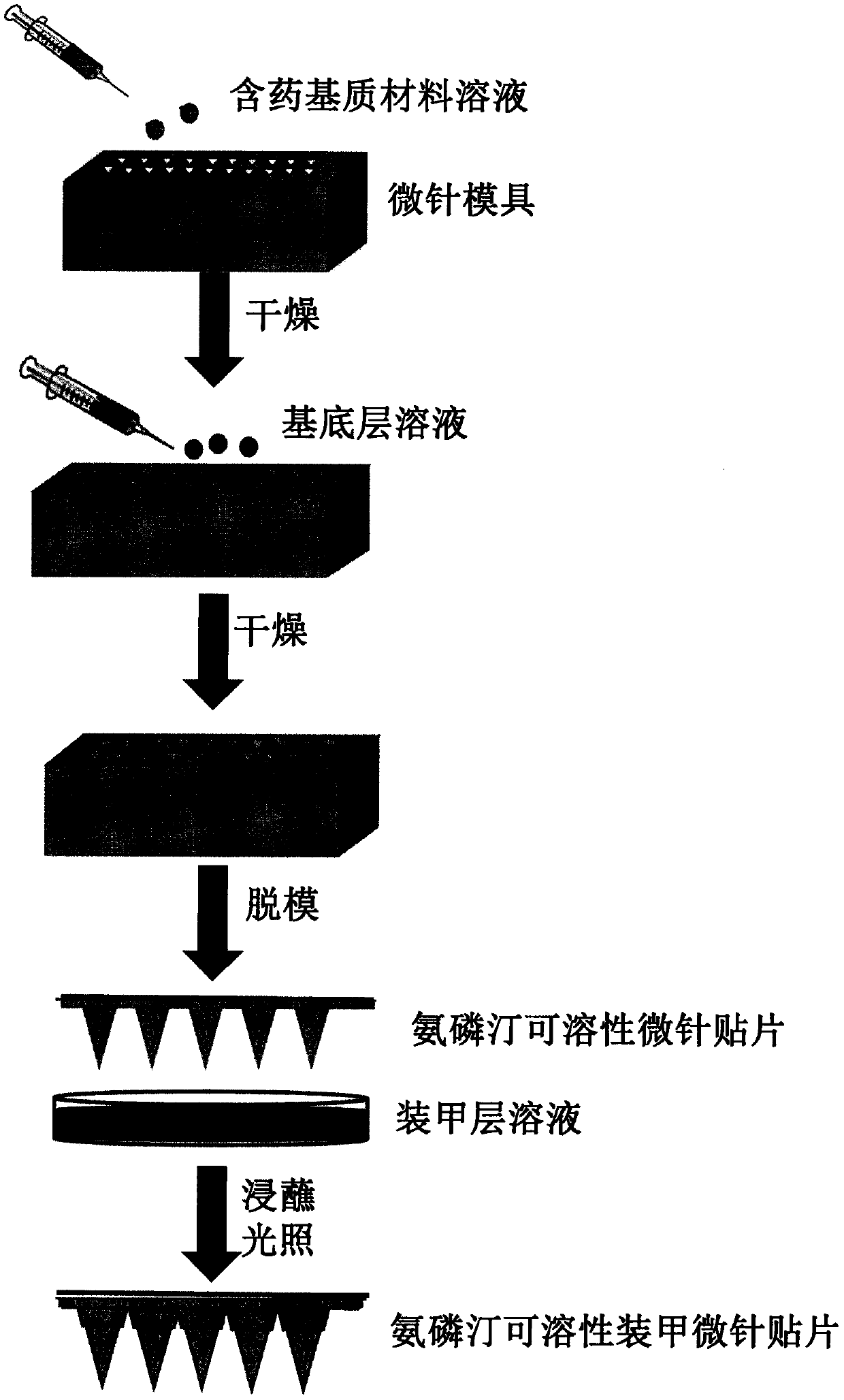
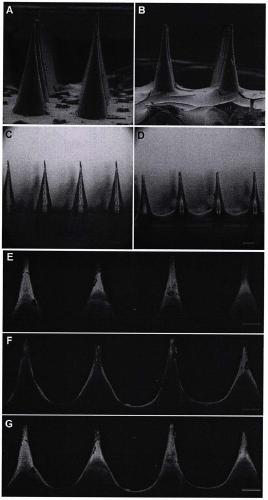
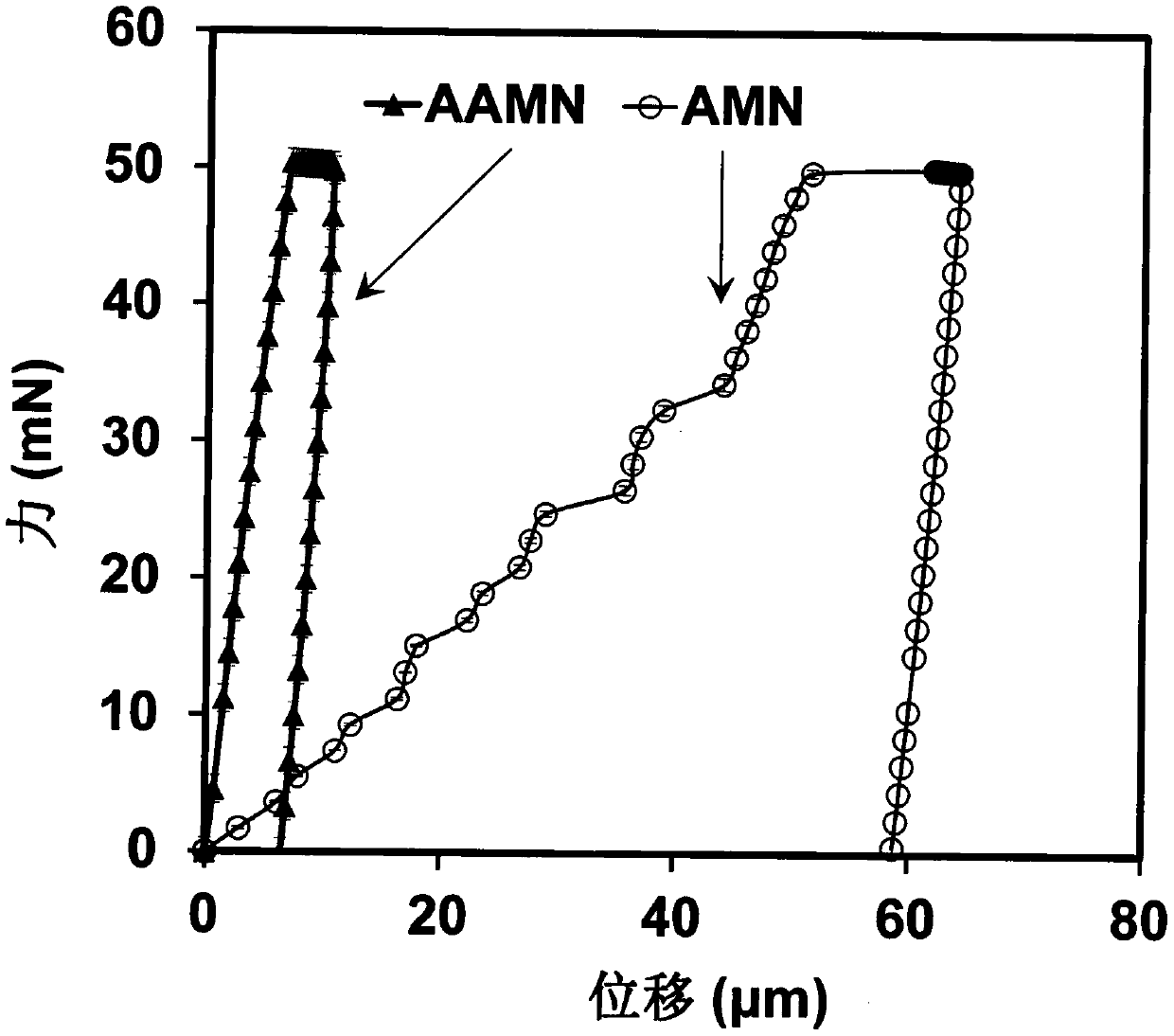
Amifostine soluble armored microneedle patch
InactiveCN111467300AAvoid breakingDifficult to penetrate the stratum corneumOrganic active ingredientsMicroneedlesPharmaceutical drugAmifostine
The invention discloses an amifostine soluble armored microneedle patch. The microneedle patch is composed of an amifostine soluble armored microneedle array and a substrate layer. The amifostine soluble armor microneedle structurally comprises a needle and an armor layer. The armored microneedle can greatly improve the drug dosage, does not influence the mechanical strength, can be smoothly inserted into the skin to implement transdermal drug delivery, achieves a long-time and high-efficiency cell protection effect, and can be used for radiation protection and chemotherapy protection.
Owner:ACADEMY OF MILITARY MEDICAL SCI
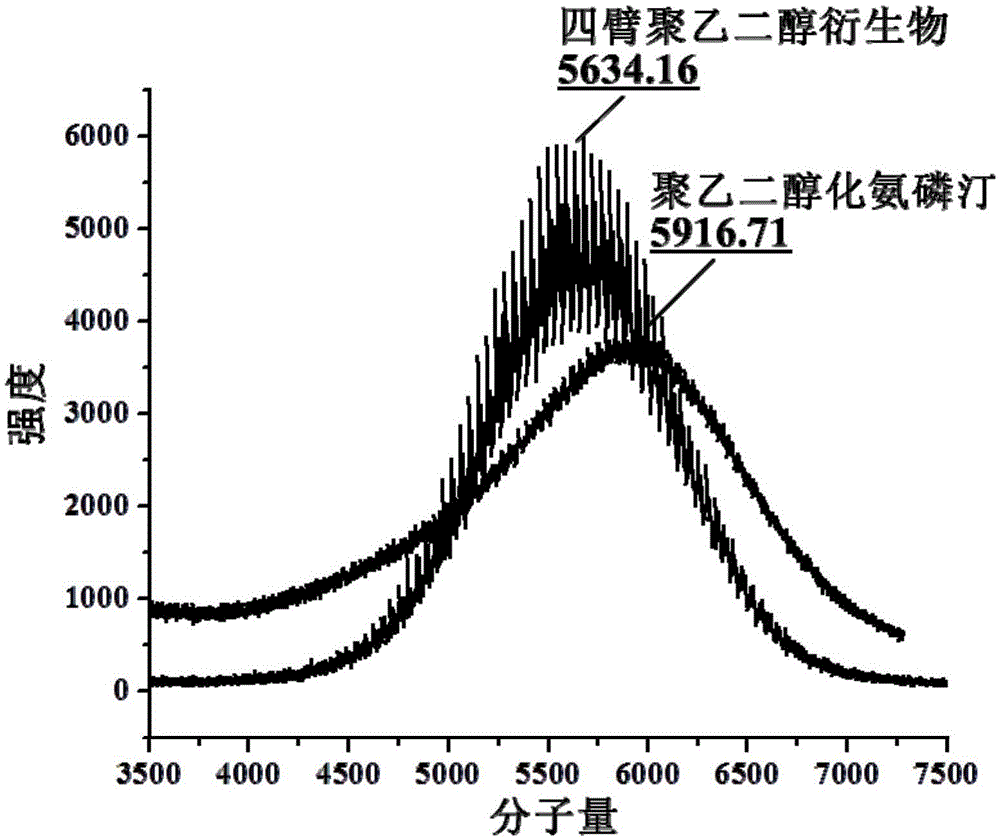
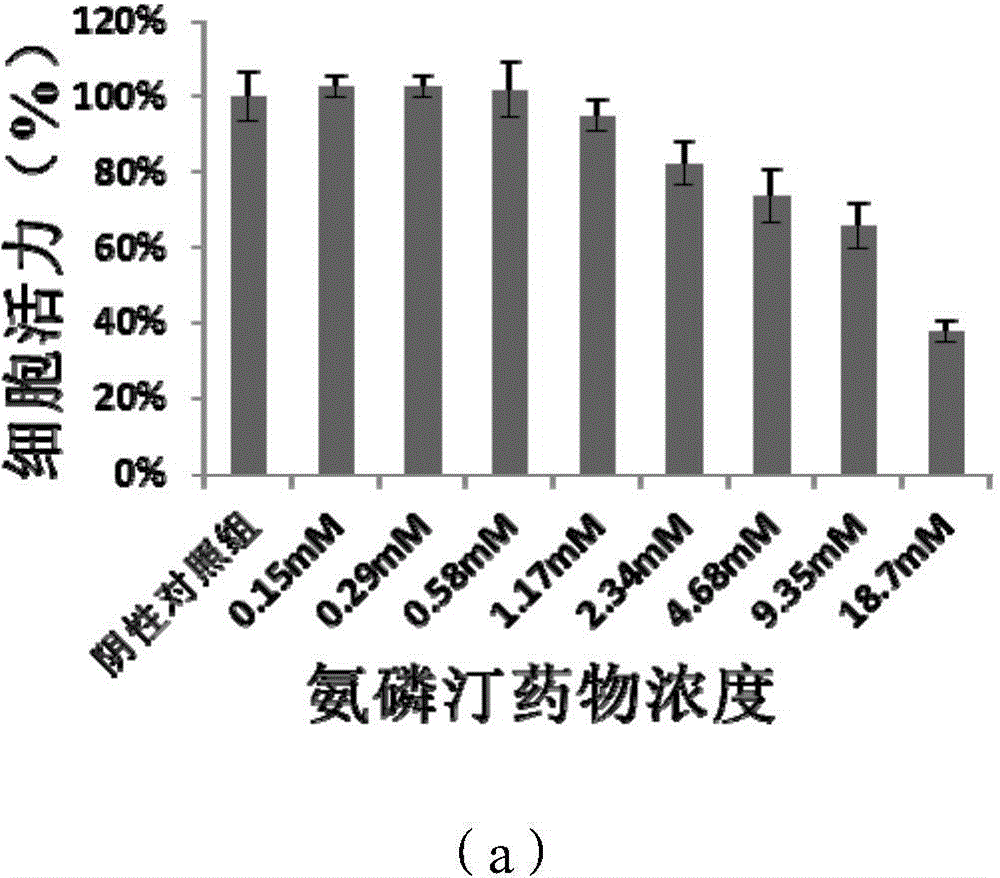
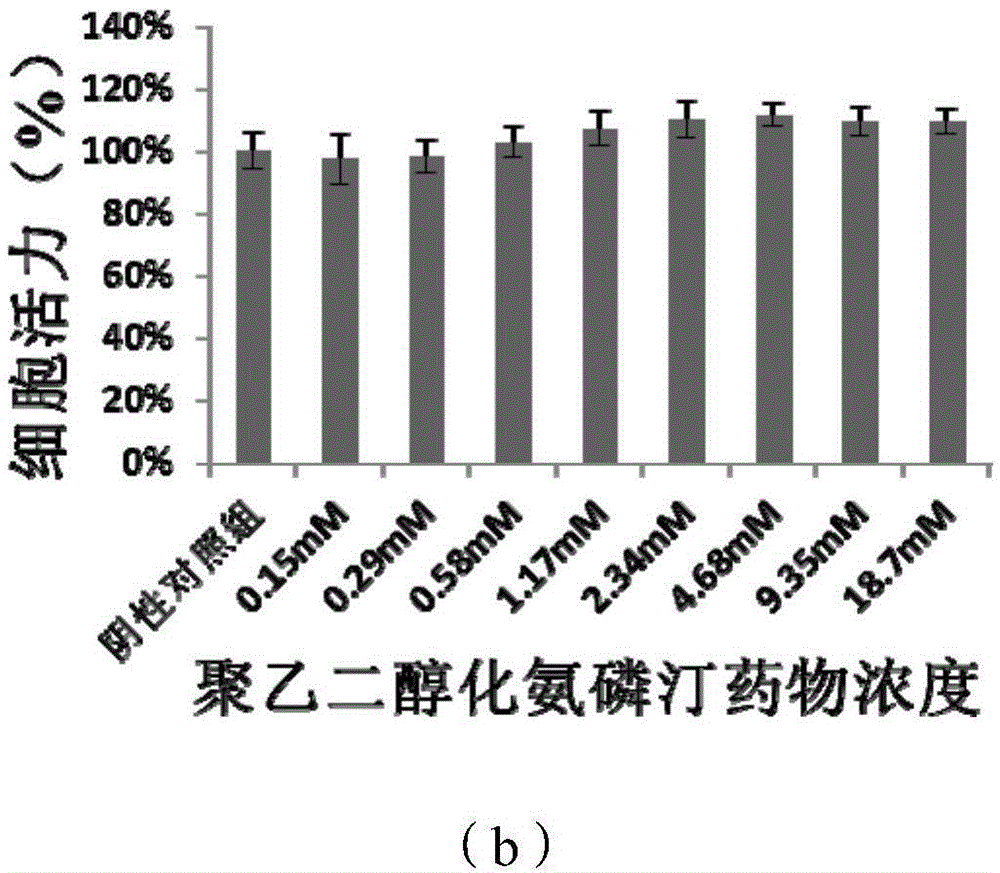
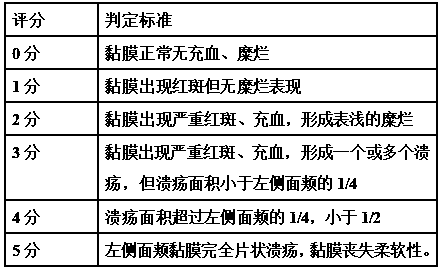
PEGylation modified amifostine as well as preparation method and use of PEGylation modified amifostine
ActiveCN104693433AExtended half-lifeImprove stabilityOrganic active ingredientsAntinoxious agentsAmifostineCellular level
The invention provides a PEGylation modified amifostine as well as a preparation method and use of the PEGylation modified amifostine. The polymer is of a structure formed by connecting the micromolecular medicine amifostine with polyethylene glycol (PEG) by use of a covalent bond, wherein the amino on the amifostine is in covalent linkage with the long chain of a PEG derivative by use of an amido bond. The invention also provides a preparation method of the PEGylation modified amifostine. The PEGylation modified amifostine also has an enhanced oxidation effect at the cellular level.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Transdermal preparation for treating oral mucositis caused by radiotherapy of tumor
ActiveCN107753490AGood treatment effectReduce adverse effectsOrganic active ingredientsAerosol deliveryUridine TriacetateLarge dose
The invention belongs to the field of medicine and particularly relates to a transdermal preparation for treating oral mucositis caused by radiotherapy of tumor. The transdermal preparation comprisesactive ingredients of 109-237 parts by weight of amifostine and 42-85 parts by weight of uridine triacetate. The transdermal preparation is administered externally at a daily dose level of milligrams,has a markedly improved effect on the oral mucositis caused by the radiotherapy, and can avoid the drop of blood pressure caused by large doses of amifostine hydrochloride to affect the health of patients with normal blood pressure or hypotension.
Owner:宋在明
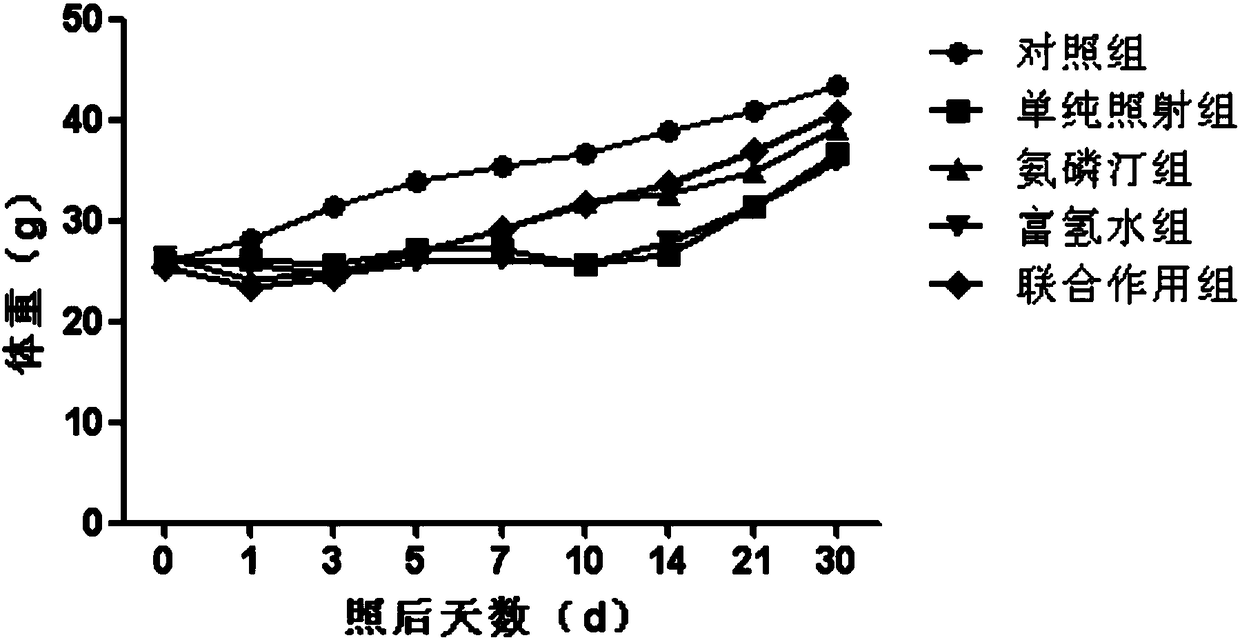
Application of combination of hydrogen-rich aqueous solution and amifostine to resistance of radiation damage
PendingCN109381481AGood resistance to radiation damageGood anti-radiation damage effectOrganic active ingredientsInorganic active ingredientsHydrogenAmifostine
Owner:CHINA INST FOR RADIATION PROTECTION
A fast and efficient method for isolating chondrocytes
ActiveCN106244529BHigh activityImprove protectionCell dissociation methodsSkeletal/connective tissue cellsDecompositionAmifostine
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Stable amorphous amifostine compositions and methods for the preparation and use of same
The present invention relates to sterile, stable dosage forms suitable for reconstitution and parenteral administration to a patient, said dosage form comprising an amorphous aminoalkyl dihydrogen phosphorothioate, and amifostine in particular. The invention further relates to a method of preparing such a dosage form, which typically exhibits enhanced thermal stability as compared to existing vacuum dried amorphous amifostine.
Owner:MEDIMMUNE ONCOLOGY
Application of AMF (amifostine) in tumors with high Cyclin D1 expression
ActiveCN104800231ASuppression detectionPrevent proliferationOrganic active ingredientsAntineoplastic agentsMegakaryoblastic leukemiaCyclin D1
The invention discloses an application of AMF (amifostine) in treatment of tumors with high Cyclin D1 expression. According to research, AMF inhibits tumor cell growth by inhibiting cancer gene CyclinD1 expression in a targeted manner and resists the tumors by inhibiting the CyclinD1 expression in a targeted manner, and the functions are successfully verified through megakaryocyte leukemia cell lines Dami; and AMF successfully cures mantle cell lymphoma with high Cyclin D1 expression and high leukocyte expression as characteristics in clinical practice.
Owner:潍坊峡山精准数基生物科技有限公司
Freeze drying preparation of amifostine, and preparation method
ActiveCN1305478CImprove performanceEasy to usePowder deliveryOrganic active ingredientsFreeze-dryingCombinatorial chemistry
A freeze-dried amifostine is prepared from amifostine, acetone and the solution of pharmacologically acceptable excipient through prefreezing, sublimated drying, and drying again.
Owner:深圳市资福药业有限公司
The application of amifostine in tumors with high expression of cyclin d1
ActiveCN104800231BPrevent proliferationOrganic active ingredientsAntineoplastic agentsCyclin D1Cancer gene
The invention discloses an application of AMF (amifostine) in treatment of tumors with high Cyclin D1 expression. According to research, AMF inhibits tumor cell growth by inhibiting cancer gene CyclinD1 expression in a targeted manner and resists the tumors by inhibiting the CyclinD1 expression in a targeted manner, and the functions are successfully verified through megakaryocyte leukemia cell lines Dami; and AMF successfully cures mantle cell lymphoma with high Cyclin D1 expression and high leukocyte expression as characteristics in clinical practice.
Owner:潍坊峡山精准数基生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com