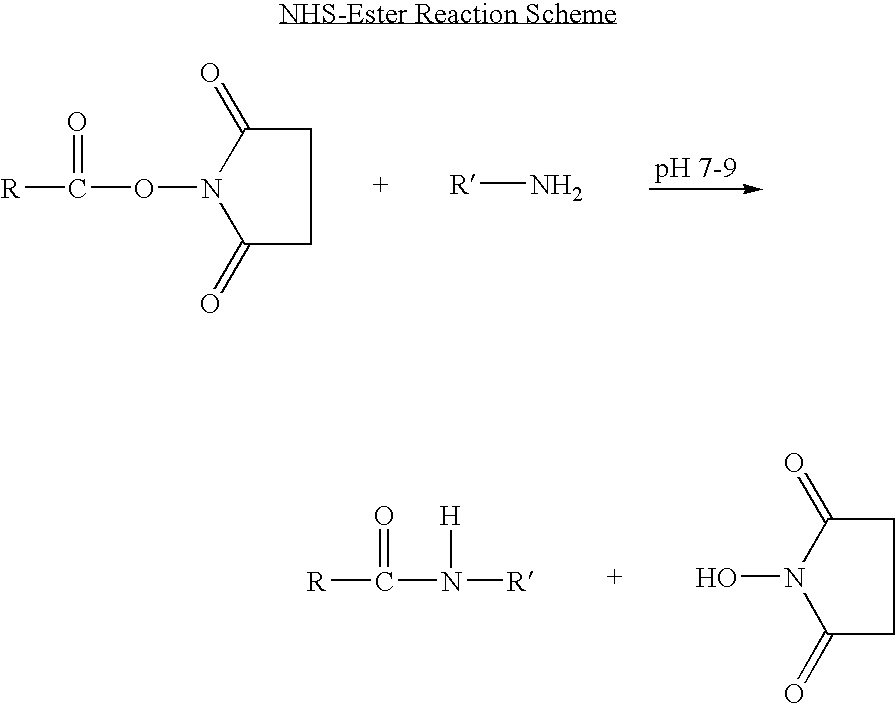
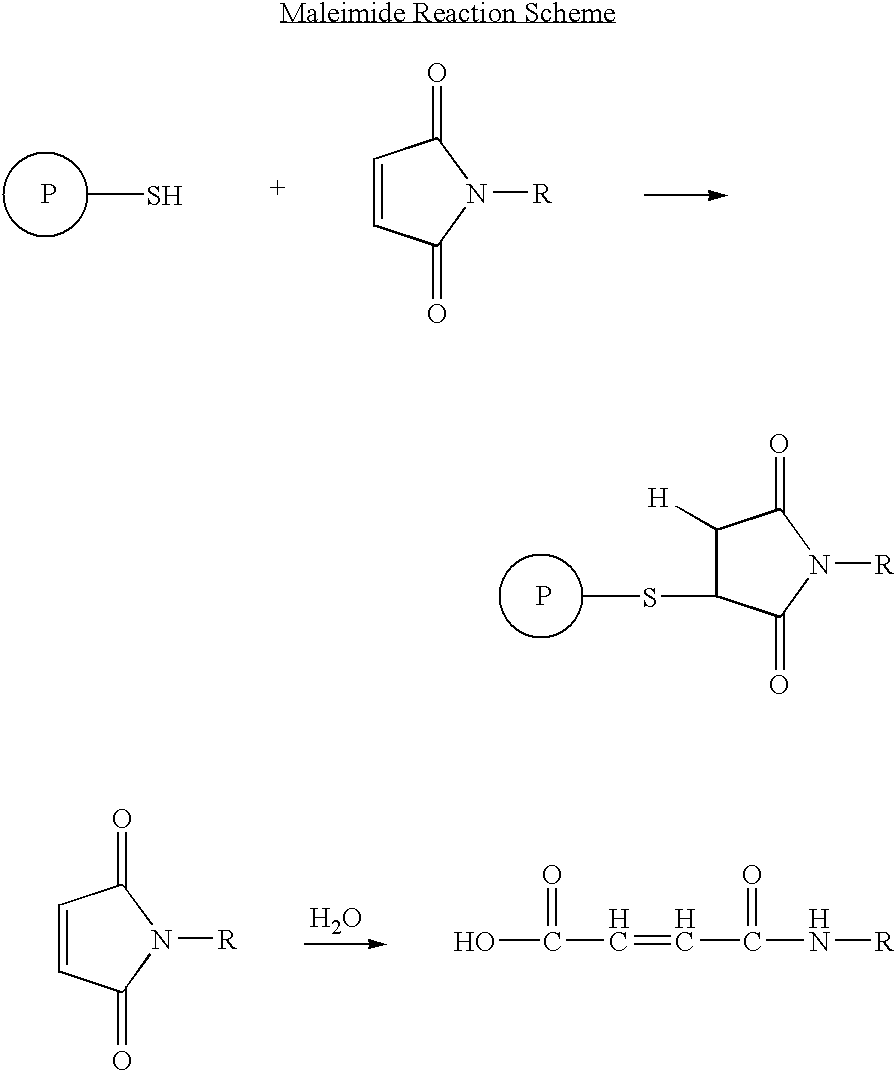
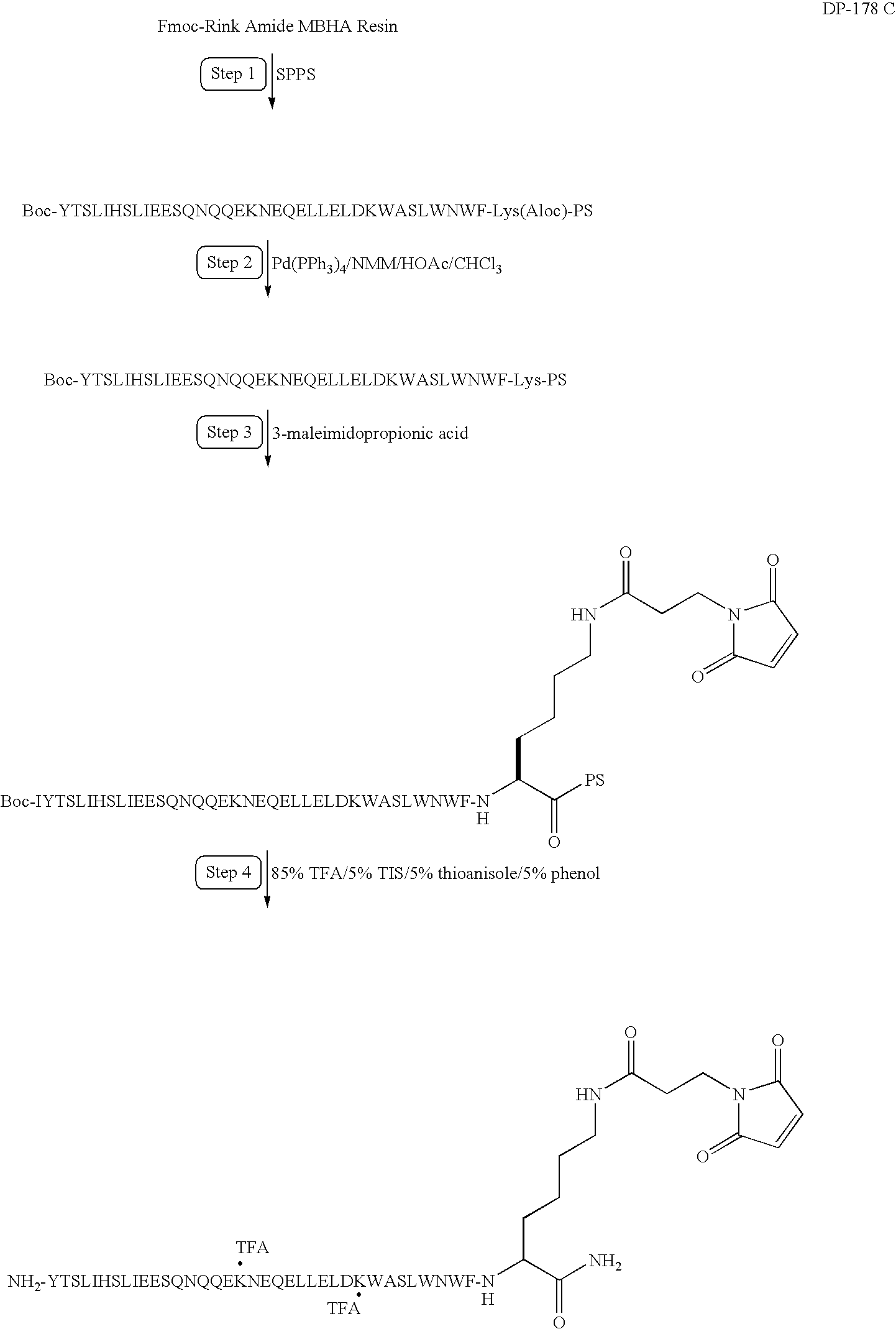
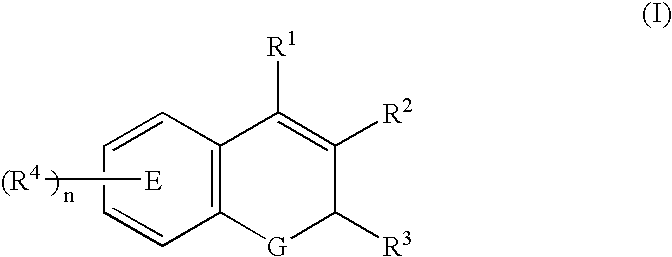
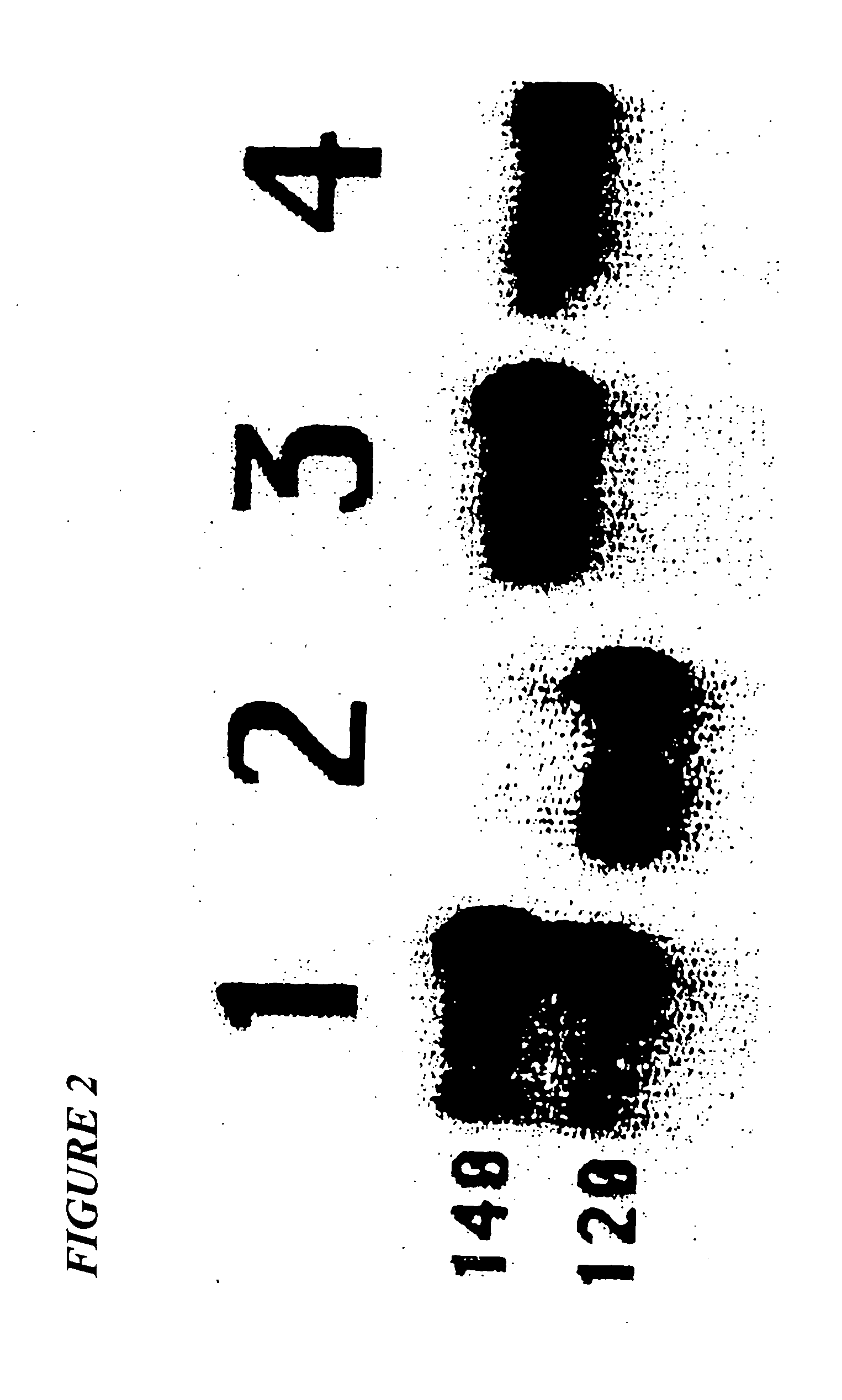
Patents
Literature
995 results about "Immunodeficiency virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The human immunodeficiency viruses (HIV) are two species of Lentivirus (a subgroup of retrovirus) that causes HIV infection and over time acquired immunodeficiency syndrome (AIDS). AIDS is a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive.
Method of eliminating inhibitory/instability regions from mRNA
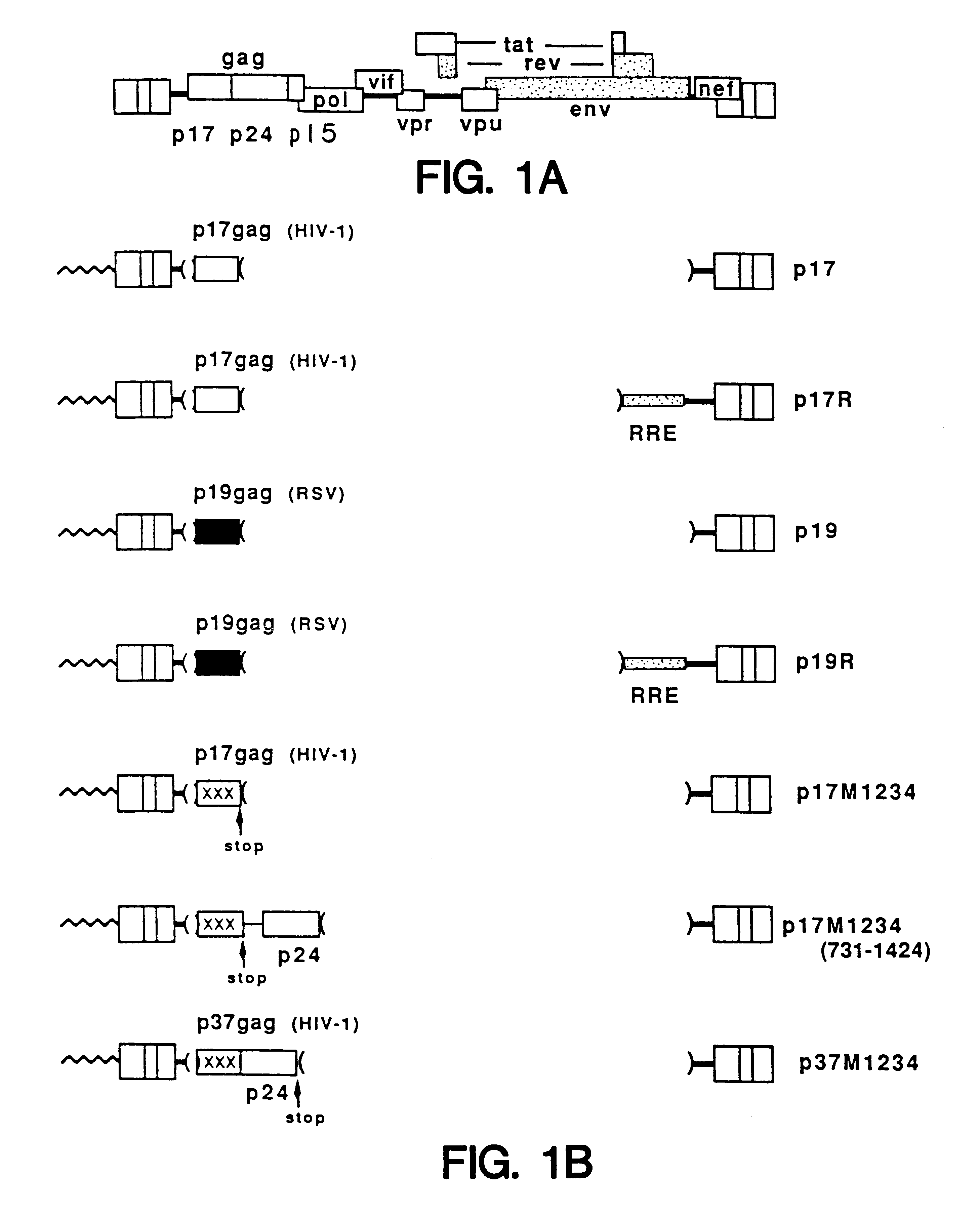
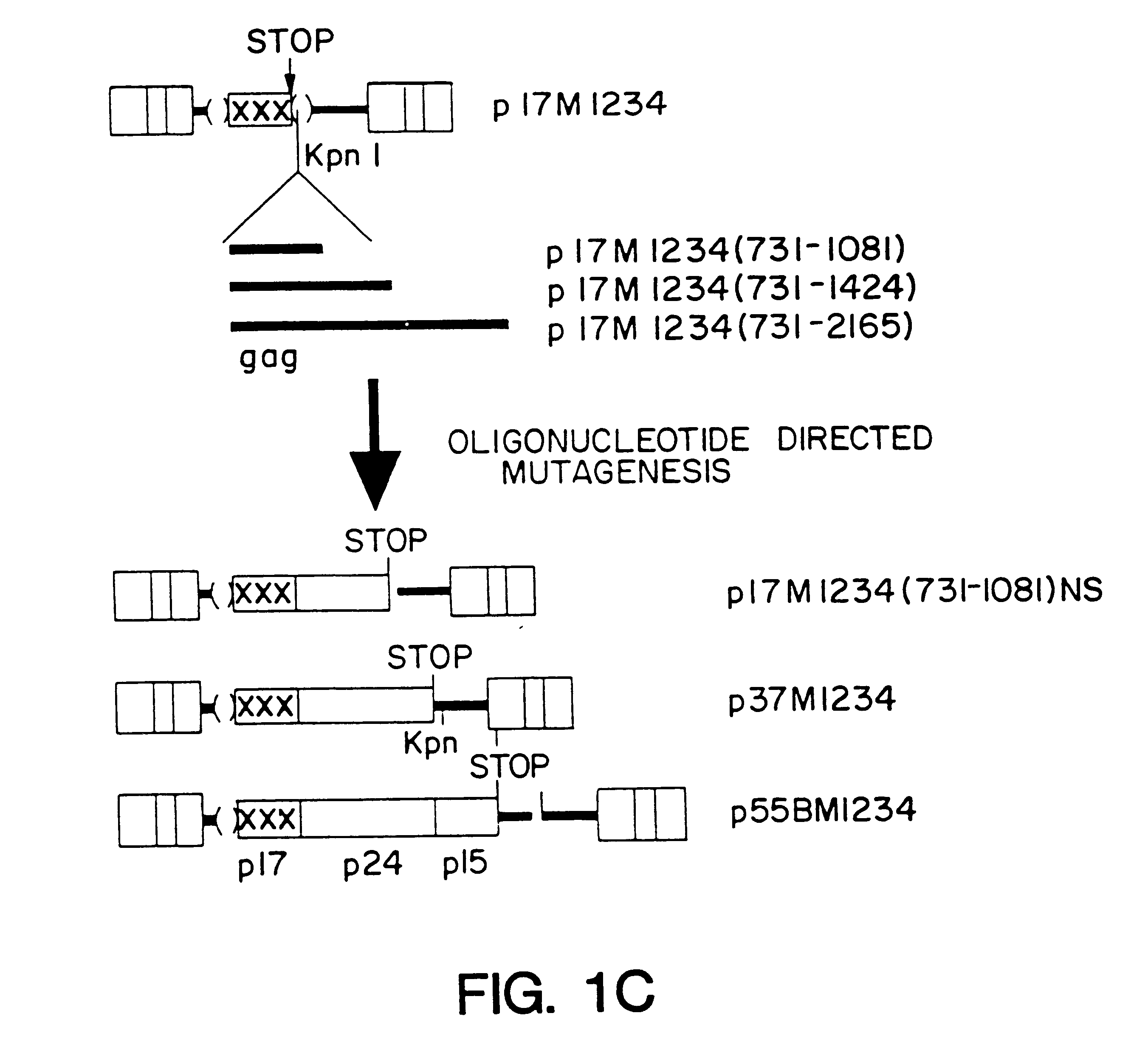
InactiveUS6174666B1Microbiological testing/measurementVirus peptidesImmunodeficiency virusInstability
A method of locating an inhibitory / instability sequence or sequences within the coding region of an mRNA and modifying the gene encoding that mRNA to remove these inhibitory / instability sequences by making clustered nucleotide substitutions without altering the coding capacity of the gene is disclosed. Constructs containing these mutated genes and host cells containing these constructs are also disclosed. The method and constructs are exemplified by the mutation of a Human Immunodeficiency Virus-1 Rev-dependent gag gene to a Rev-independent gag gene. Constructs useful in locating inhibitory / instability sequences within either the coding region or the 3' untranslated region of an mRNA are also disclosed.
Owner:UNITED STATES OF AMERICA
Polycyclic-carbamoylpyridone compounds and their pharmaceutical use
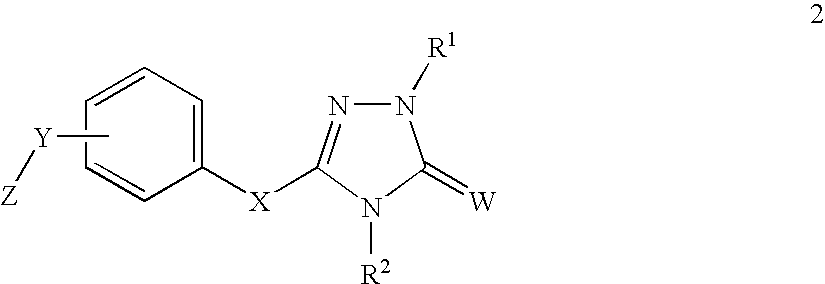
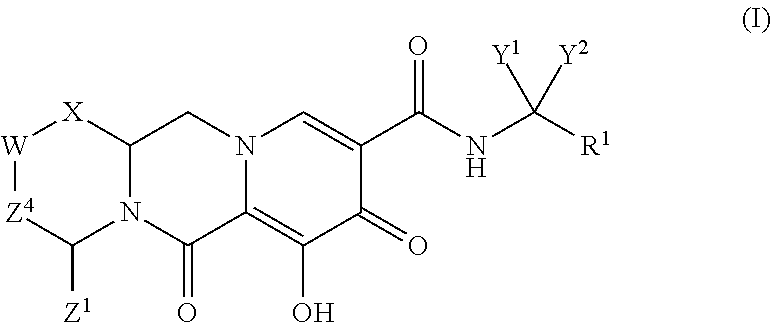
ActiveUS20140221356A1Inhibitory activityReduce HIV replicationBiocideOrganic chemistryImmunodeficiency virusAcyl group
Compounds for use in the treatment of human immunodeficiency virus (HIV) infection are disclosed. The compounds have the following Formula (I):including stereoisomers and pharmaceutically acceptable salts thereof, wherein R1, X, W, Y1, Y2, Z1, and Z4 are as defined herein. Methods associated with preparation and use of such compounds, as well as pharmaceutical compositions comprising such compounds, are also disclosed.
Owner:GILEAD SCI INC
Heterocyclic aspartyl protease inhibitors
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein W is a bond, —C(═S)—, —S(O)—, —S(O)2—, —C(═O)—, —O—, —C(R6)(R7)—, —N(R5)— or —C(═N(R5))—; X is —O—, —N(R5)— or —C(R6)(R7)—; provided that when X is —O—, U is not —O—, —S(O)—, —S(O)2—, —C(═O)— or —C(═NR5)—; U is a bond, —S(O)—, —S(O)2—, —C(O)—, —O—, —P(O)(OR15)—, —C(═NR5)—, —(C(R6)(R7))b— or —N(R5)—; wherein b is 1 or 2; provided that when W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, U is not —S(O)—, —S(O)2—, —O—, or —N(R5)—; provided that when X is —N(R5)— and W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, then U is not a bond; and R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:PHARMACOPEIA DRUG DISCOVERY +1
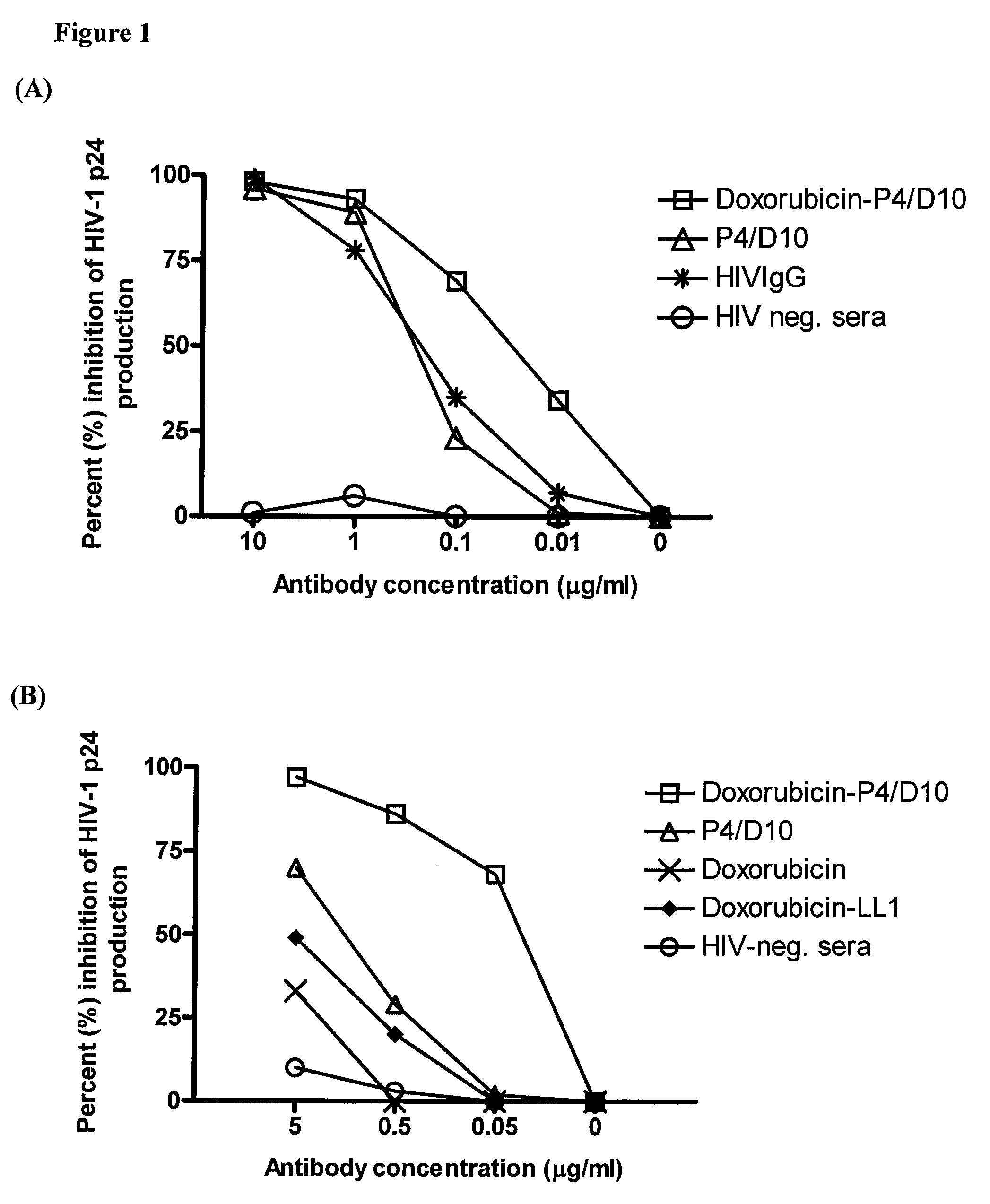
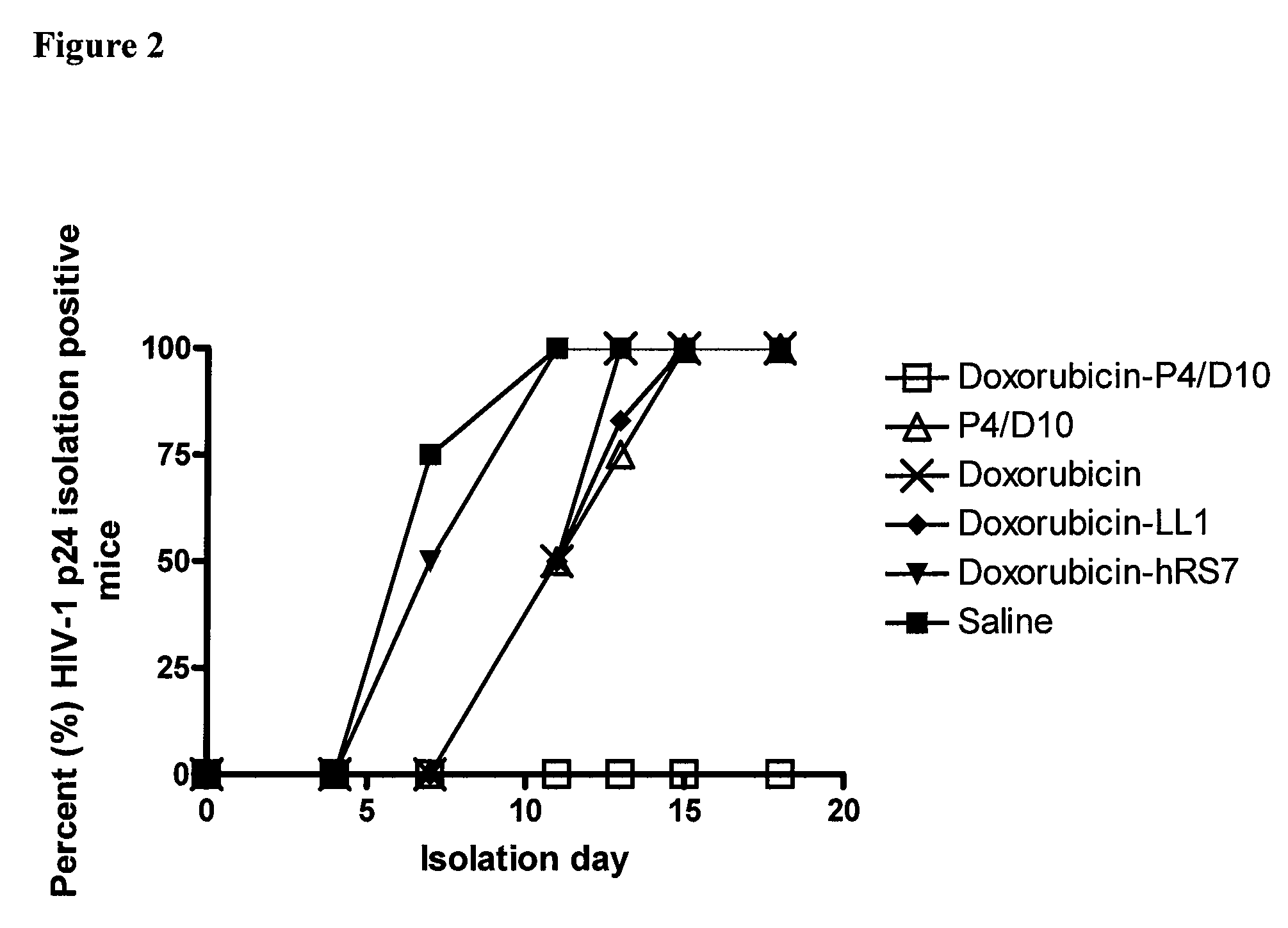
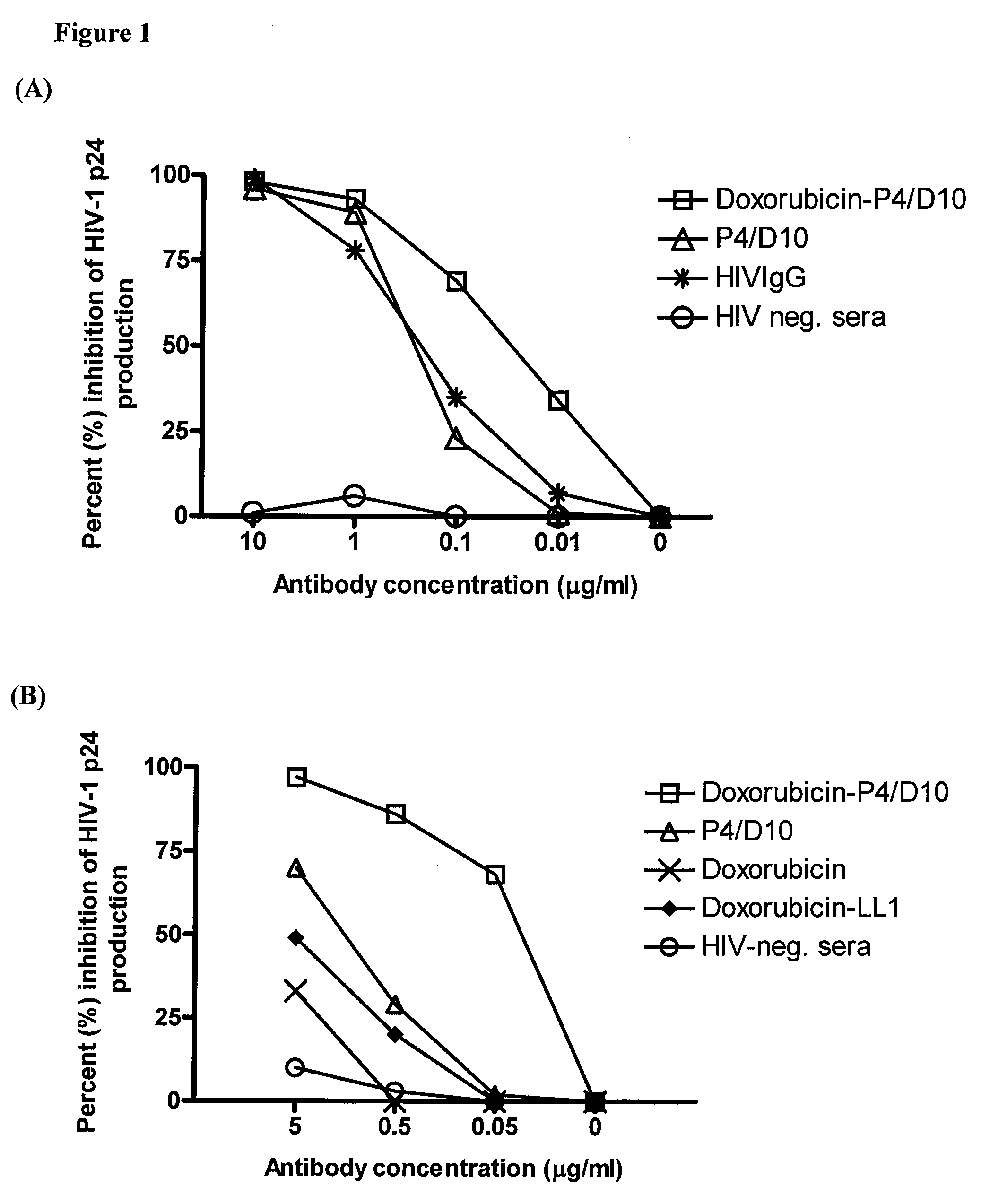
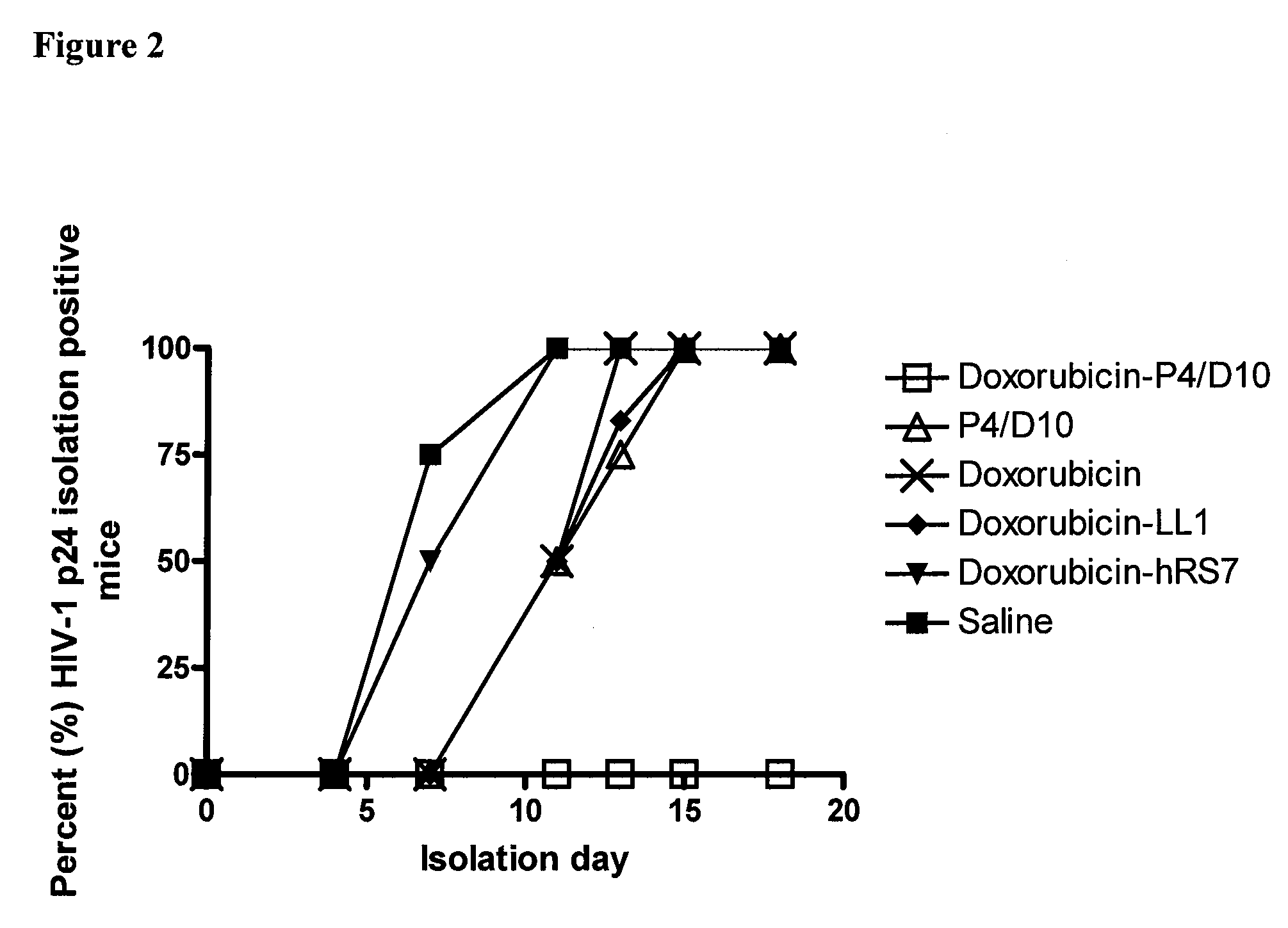
Methods and compositions for treatment of human immunodeficiency virus infection with conjugated antibodies or antibody fragments
ActiveUS20070264265A1Avoid infectionReduce eliminateOrganic active ingredientsAntiviralsDiagnostic agentBinding site
The present invention concerns methods and compositions for treatment of HIV infection in a subject. The compositions may comprise a targeting molecule against an HIV antigen, such as an anti-HIV antibody or antibody fragment. The anti-HIV antibody or fragment may be conjugated to a variety of cytotoxic agents, such as doxorubicin. In a preferred embodiment, the antibody or fragment is P4 / D10. Other embodiments may concern methods of imaging, detection or diagnosis of HIV infection in a subject using an anti-HIV antibody or fragment conjugated to a diagnostic agent. In alternative embodiments, a bispecific antibody with at least one binding site for an HIV antigen and at least one binding site for a carrier molecule may be administered, optionally followed by a clearing agent, followed by administration of a carrier molecule conjugated to a therapeutic agent.
Owner:IMMUNOMEDICS INC
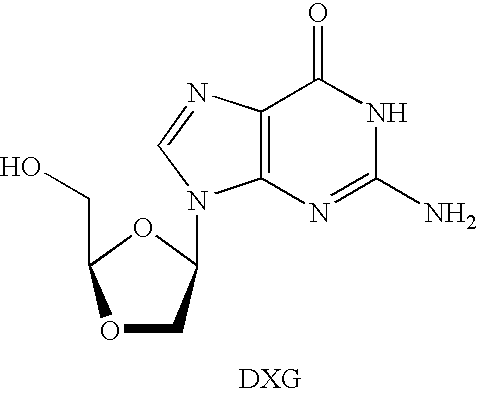
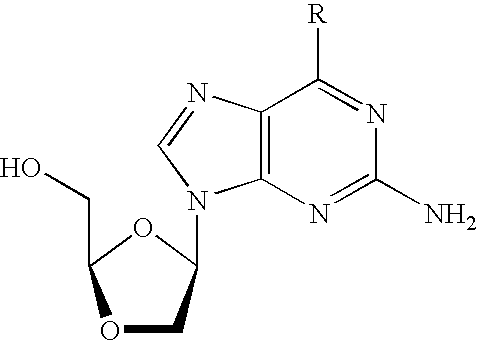
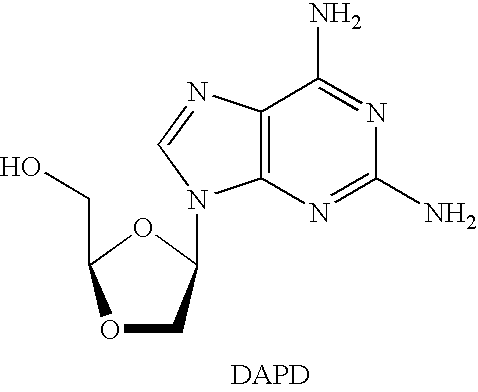
N4-acylcytosine-1,3-dioxolane nucleosides for treatment of viral infections
InactiveUS6908924B2Strong inhibitory activityBiocideOrganic active ingredientsImmunodeficiency virusHuman patient
The present invention is directed to a compound, method and composition of treating or preventing viral infections, in particular, human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infections, in human patients or other animal hosts, comprising the administration of N4-acylcytosine-1,3-dioxolane and pharmaceutically acceptable salts, prodrugs, and other derivatives thereof.
Owner:GILEAD PHARMASSET LLC
Nitrogenated heterocyclic derivative , and pharmaceutical agent comprising the derivative as active ingredient
InactiveUS20090131403A1Prevention and/or treatmentEasy to useBiocideSenses disorderAcquired immunodeficiencyAutoimmune condition
The compound represented by formula (I), a salt thereof, an N-oxide thereof, a solvate thereof, or a prodrug thereof specifically binds CCR5, so it is useful for preventing and / or treating CCR5-related diseases, for example, various inflammatory diseases (asthma, nephritis, nephropathy, hepatitis, arthritis, rheumatoid arthritis, rhinitis, conjunctivitis, ulcerative colitis, etc.), immunological diseases (autoimmune diseases, rejection in organ transplantation, immunosuppression, psoriasis, multiple sclerosis, etc.), infectious diseases (infection with human immunodeficiency virus, acquired immunodeficiency syndrome, etc.), allergic diseases (atopic dermatitis, urticaria, allergic bronchopulmonary aspergillosis, allergic eosinophilic gastroenteritis, etc.), ischemic reperfusion injury, acute respiratory distress syndrome, shock accompanying bacterial infection diabetes cancer metastasis and so on.Wherein all symbols in formula are as defined in the specification
Owner:ONO PHARMA CO LTD
Aspartyl protease inhibitors
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein W, R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:SCHERING CORP
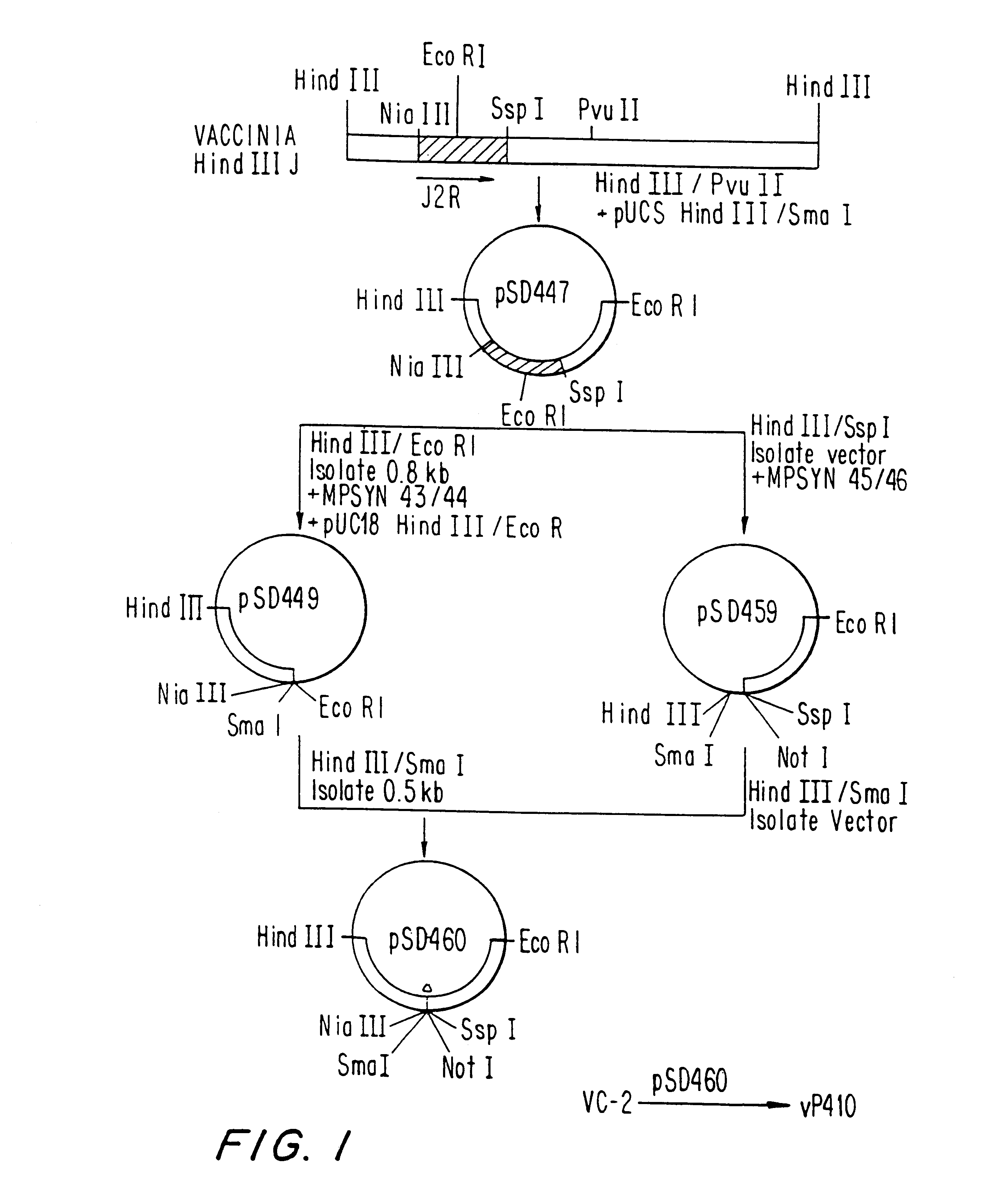
Immunodeficiency recombinant poxvirus
InactiveUS6596279B1SsRNA viruses negative-senseSsRNA viruses positive-senseFeline immunodeficiency virusCtl epitope
Attenuated recombinant viruses containing DNA encoding an immunodeficiency virus and / or CTL antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: HIV1gag(+pro)(IIIB), gp120(MN)(+transmembrane), nef(BRU)CTL, pol(IIIB)CTL, ELDKWA or LDKW epitopes, preferably HIV1gag(+pro)(IIIB), gp120(MN) (+transmembrane), two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL etpitopes; or two ELDKWA in gp120 V3 or another region or in gp160. The two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL epitopes are preferably CTL1, CTL2, pol1, pol2 and pol3. The recombinant viruses and gene products therefrom and antibodies generated by the viruses and gene products have several preventive, therapeutic and diagnostic uses. DNA from the recombinant viruses are useful as probes or, for generating PCR primers or for immunization. Also disclosed and claimed are HIV immunogens and modified gp160 and gp120.
Owner:VIROGENETICS
Non-lethal conditioning methods for the treatment of acquired immunodeficiency syndrome
InactiveUS6039684AGood conditionPromote resultsPeptide/protein ingredientsEnergy modified materialsAcquired immunodeficiencyImmunodeficiency virus
The present invention relates to the use of a non-lethal preparative regimen for the treatment of a patient with human immunodeficiency virus (HIV) disease. In a clinical trial, an HIV-positive patient was conditioned by non-lethal doses of irradiation and a chemotherapeutic drug prior to receiving donor bone marrow cells from a baboon. While long-term engraftment of donor cells was not observed, the non-lethal preparative conditioning regimen was able to reduce the viral burden and improved the clinical outcome. Such method is useful for treatment of patients with advanced acquired immunodeficiency syndrome (AIDS).
Owner:ALLEGHENY UNIV OF THE HEALTH SCI +1
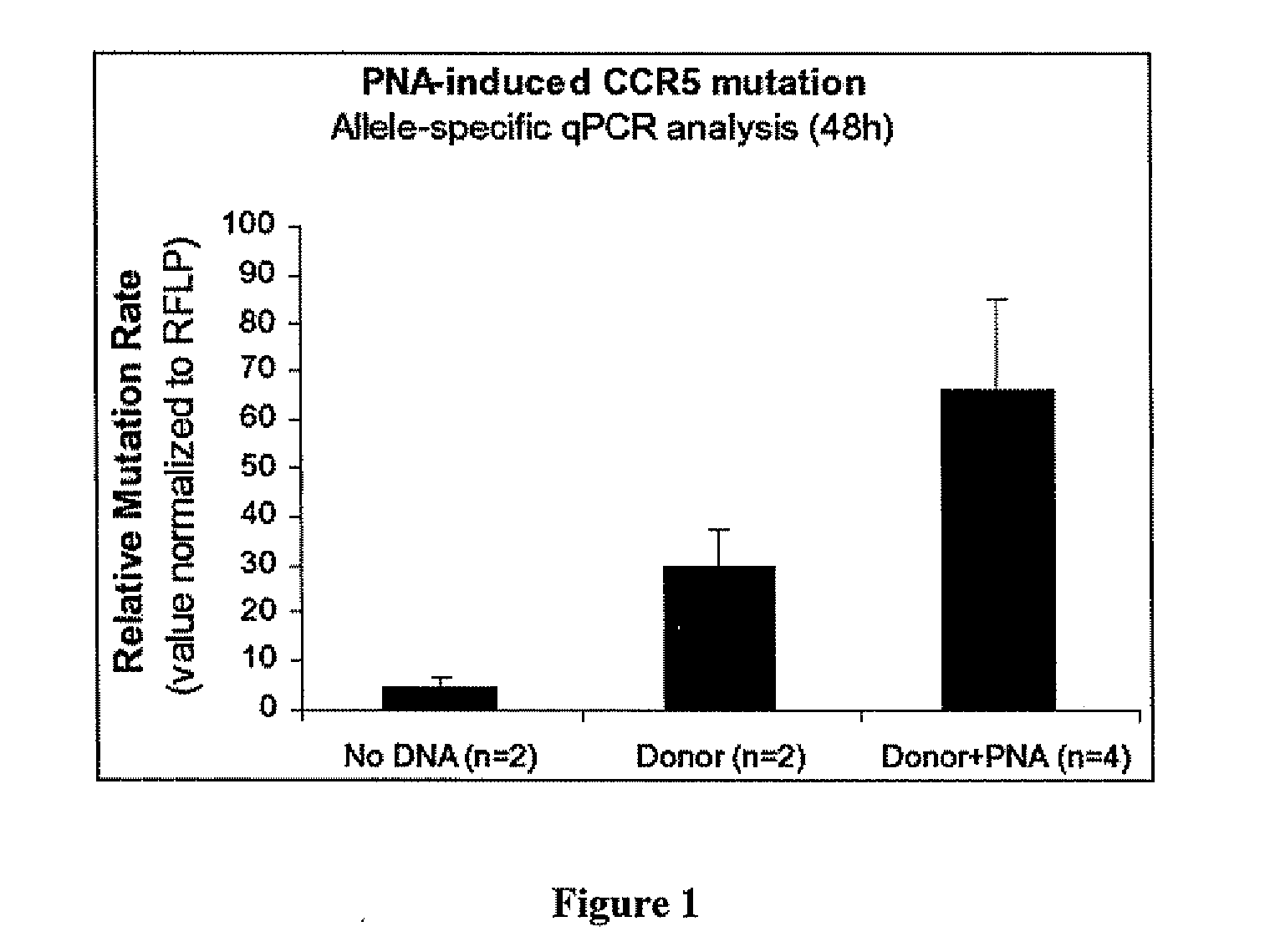
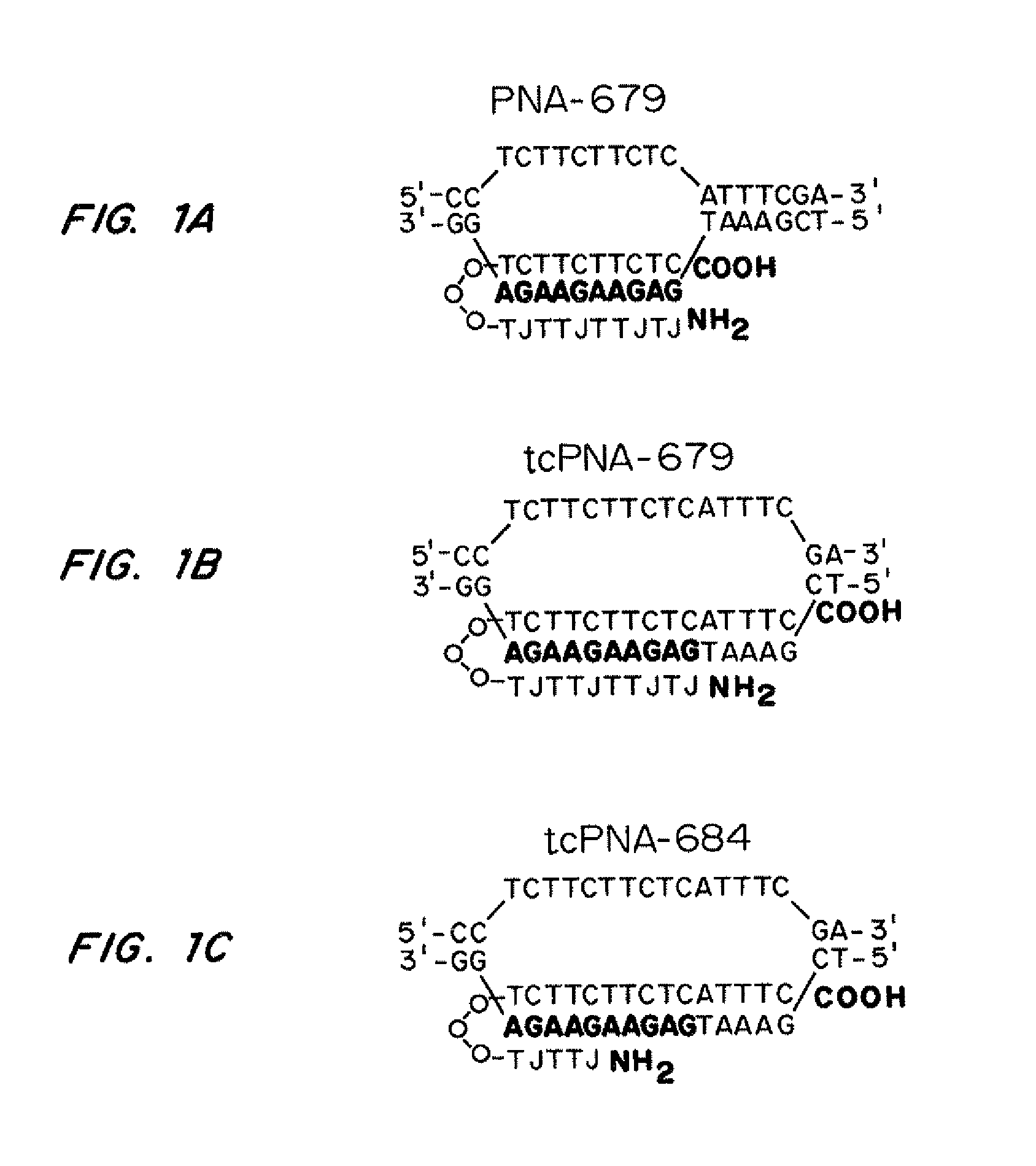
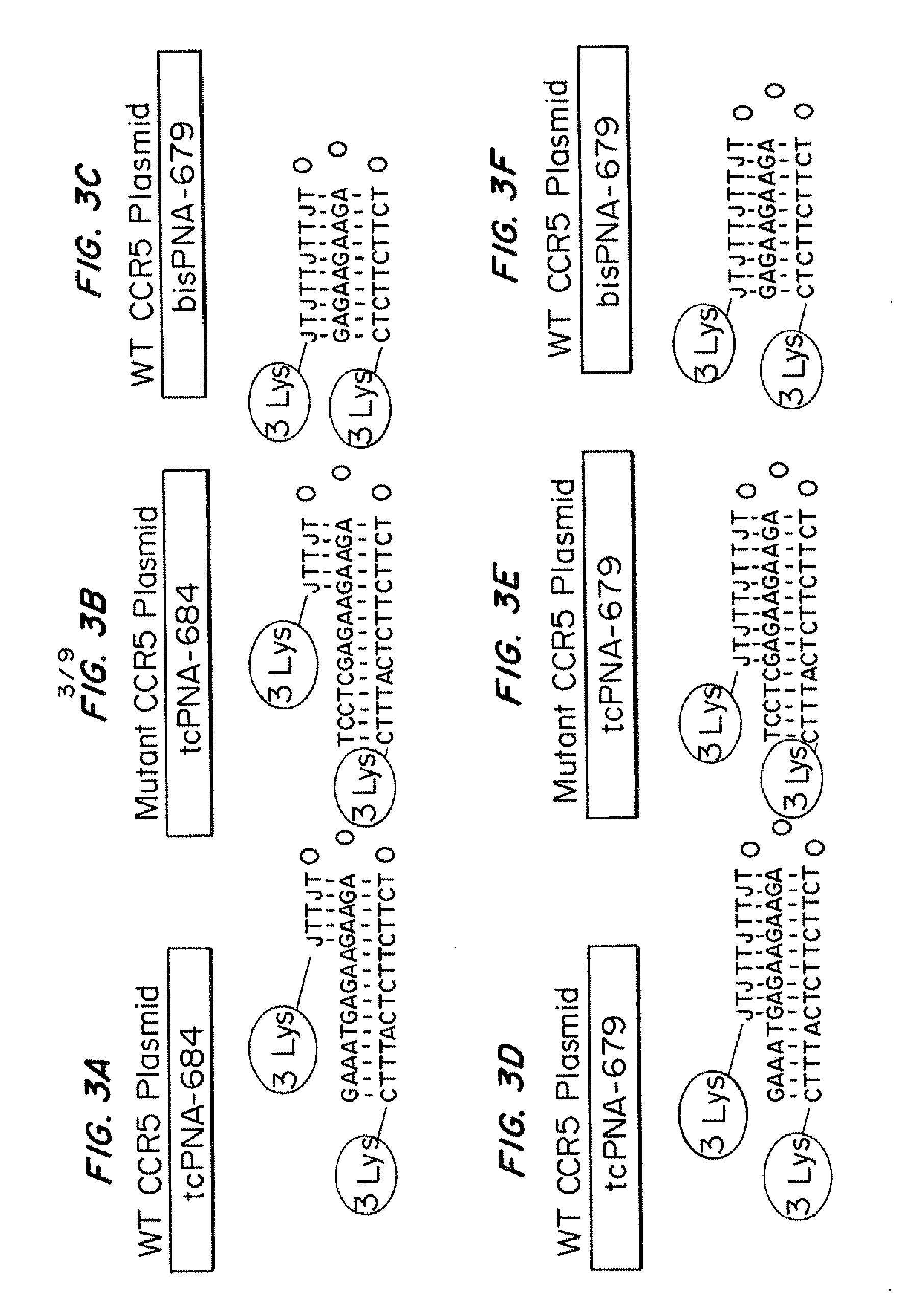
Compositions and methods for targeted inactivation of HIV cell surface receptors
InactiveUS20110262406A1Avoid infectionReduce viral loadBiocideOrganic active ingredientsImmunodeficiency virusTail
Compositions for targeted mutagenesis of cell surface receptors for HIV and methods of their use are provided herein. The compositions include triplex-forming molecules that displace the polypyrimidine strand of target duplex and form a triple-stranded structure and hybrid duplex in a sequence specific manner with the polypurine strand of the target duplex. The triplex-forming molecules include a mixed-sequence “tail” which increases the stringency of binding to the target duplex, improves the frequency of modification at the target site, and reduces the requirement for a polypurine:polypyrimidine stretch. Methods for using the triplex-forming molecules in combination with one or more donor oligonucleotides for targeted modification of sites within or adjacent to genes that encodes cell surface receptors for human immunodeficiency virus (HIV) are also disclosed. Methods for ex vivo and in vivo prophylaxis and therapy of HIV infection using the disclosed compositions are also provided.
Owner:YALE UNIV
Virus coat protein/receptor chimeras and methods of use
InactiveUS7311920B1Sufficient amountMethod can be usedCompound screeningApoptosis detectionCXCR4Immunodeficiency virus
The invention relates to chimeric molecules comprising a virus coat sequence and a receptor sequence that can interact with each other to form a complex that is capable of binding a co-receptor. Such chimeric molecules therefore exhibit functional properties characteristic of a receptor-coat protein complex and are useful as agents that inhibit virus infection of cells due to occupancy of co-receptor present on the cell, for example. In particular aspects, the chimeric polypeptide includes an immunodeficiency virus envelope polypeptide, such as that of HIV, SIV, FIV, FeLV, FPV and herpes virus. Receptor sequences suitable for use in a chimeric polypeptide include, for example, CCR5 and CXCR4 sequences.
Owner:MARYLAND BIOTECH INST UNIV OF
Long Acting Biologically Active Conjugates
InactiveUS20070207952A1High level of drugPoor adhesionBiocidePeptide/protein ingredientsImmunodeficiency virusIn vivo
The invention provides biologically active compounds that may be reacted with macromolecules, such as albumin, to form covalent linked complexes wherein the resulting complexes exhibit a desired biological activity in vivo. More specifically, the complexes are isolated complexes comprising a biologically active moiety covalently bound to a linking group and a protein. The complexes are prepared by conjugating a biologically active moiety, for example, a renin inhibitor or a viral fusion inhibitor peptide, with purified and isolated protein. The complexes have extended lifetimes in the bloodstream as compared to the unconjugated molecule, and exhibit biological activity for extended periods of time as compared to the unconjugated molecule. The invention also provides anti-viral compounds that are inhibitors of viral infection and / or exhibit anti-fusiogenic properties. In particular, this invention provides compounds having inhibiting activity against viruses such as human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) and that have extended duration of action for the treatment of viral infections.
Owner:SEQUOIA PHARMACEUTICALS INC
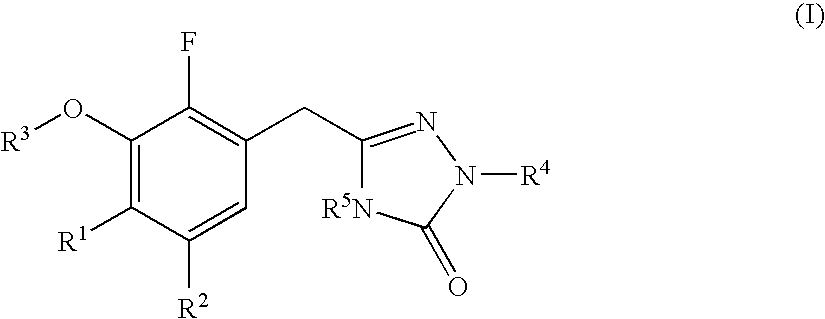
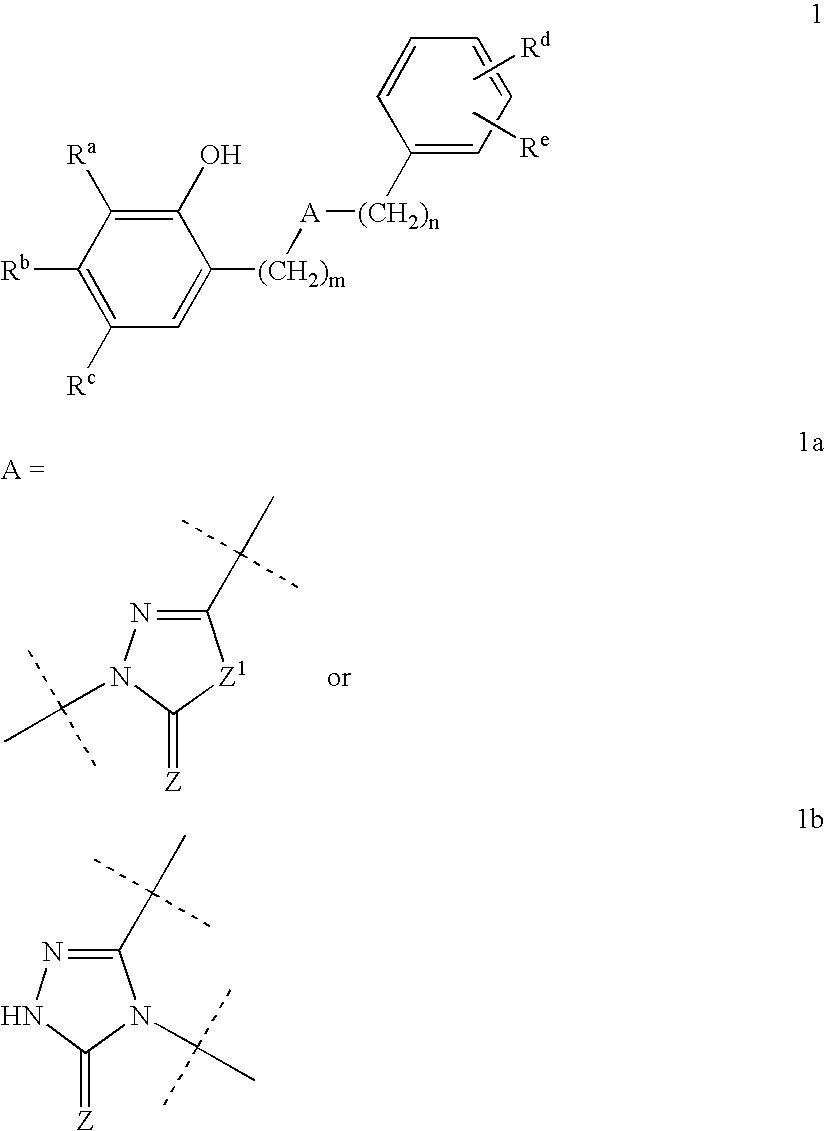
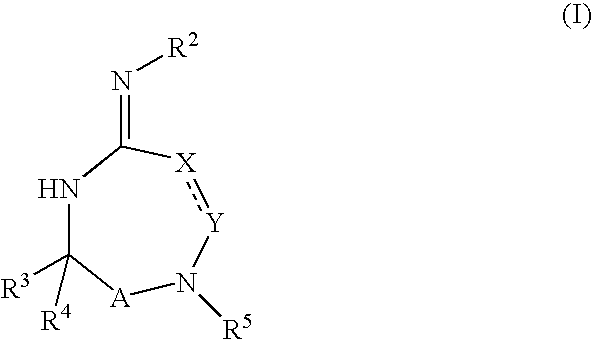
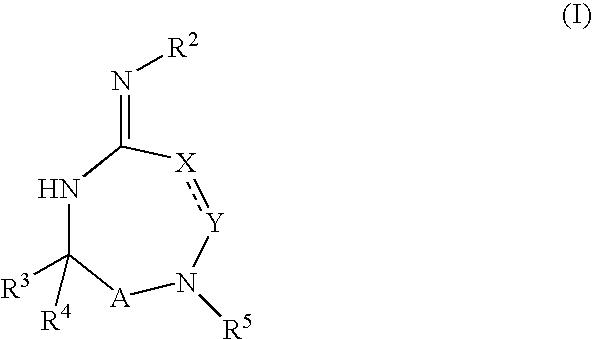
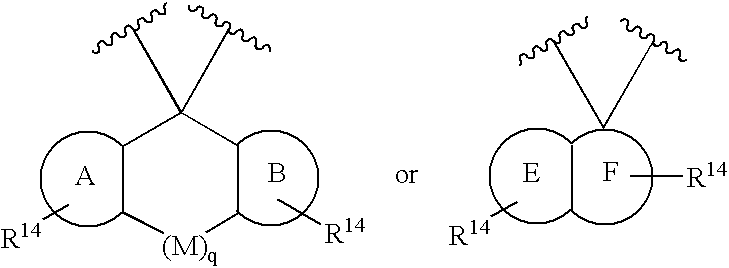
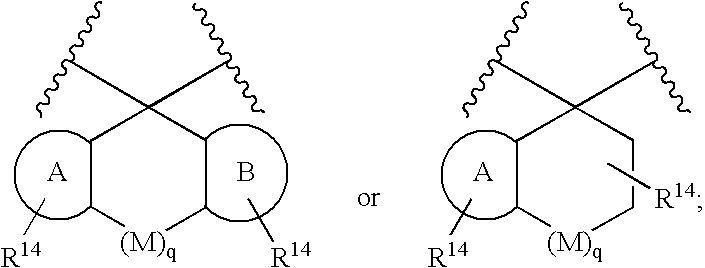
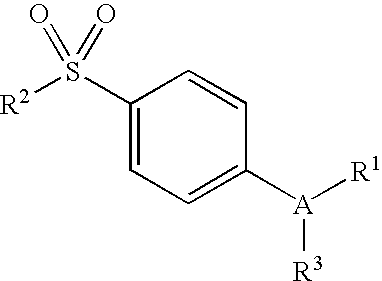
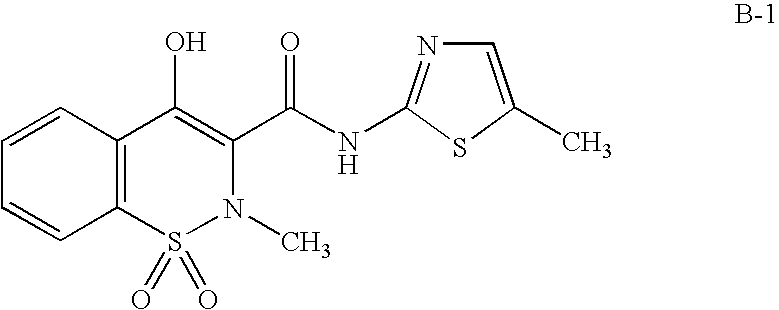
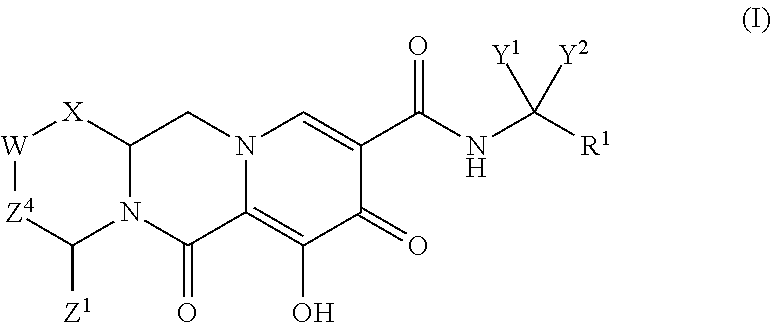
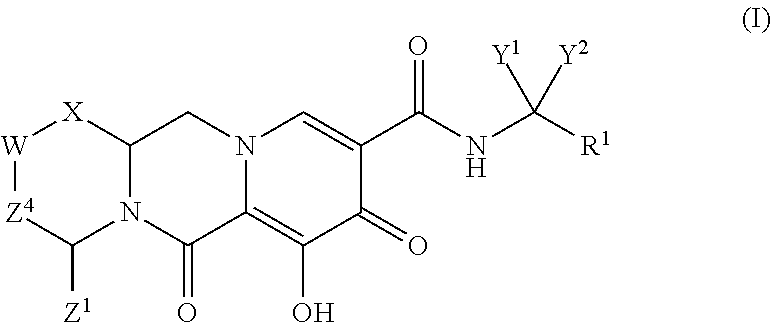
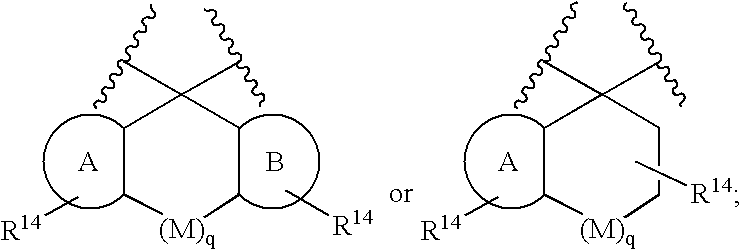
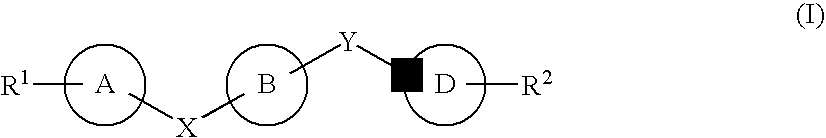
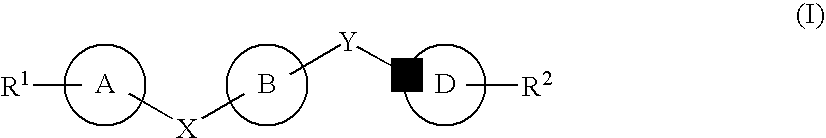
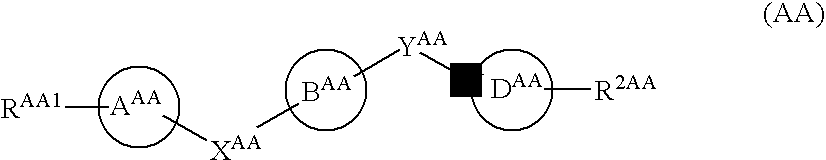
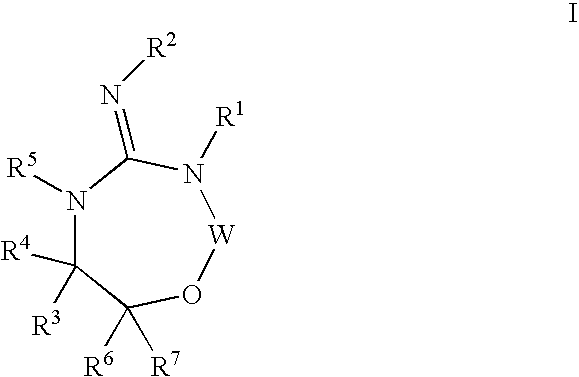
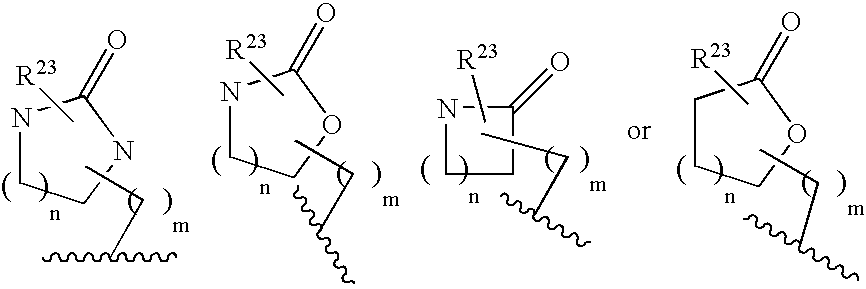
Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections
Owner:GILEAD SCI INC
ALVAC/FIV constructs
InactiveUS7255862B1Improve security levelViral antigen ingredientsGenetic material ingredientsFeline immunodeficiency virusHeterologous
Recombinants containing and expressing lentivirus, retrovirus or immunodeficiency virus DNA and methods for making and using the same are disclosed and claimed. In an exemplified embodiment, attenuated recombinant viruses containing DNA encoding a feline immunodeficiency virus epitope such as an antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinants can be NYVAC or ALVAC recombinants. The DNA can encode at least one of: Env, Gag, Pol, or combinations thereof such as Gag and Pol or protease or Env, Gag and Pol or protease. The recombinants and gene products therefrom and antibodies generated by them have several preventive, therapeutic and diagnostic uses. DNA from the recombinants are useful as probes or, for generating PCR primers or for immunization. The immunogenicity and protective efficacy of immunization protocols involving ALVAC-FIV and priming with a recombinant canarypox virus ALVAC-FIV vaccine followed by a booster immunization with inactivated FIV-infected celled vaccine (ICV) was evaluated against FIV challenge in cats and the protocol was shown to effectively induce FIV-specific protective immune responses. Further, it was found that immunized cats were fully protected from an initial challenge with a slightly heterologous FIV strain (50CID50) and were partially protected from a second challenge with a distinctly heterologous FIV strain (75CID50) given eight months after the initial challenge without any intervening booster.
Owner:VIROGENETICS
Methods and compositions for treatment of human immunodeficiency virus infection with conjugated antibodies or antibody fragments
ActiveUS8333971B2Avoid infectionReduce eliminateOrganic active ingredientsAntiviralsDiagnostic agentBinding site
The present invention concerns methods and compositions for treatment of HIV infection in a subject. The compositions may comprise a targeting molecule against an HIV antigen, such as an anti-HIV antibody or antibody fragment. The anti-HIV antibody or fragment may be conjugated to a variety of cytotoxic agents, such as doxorubicin. In a preferred embodiment, the antibody or fragment is P4 / D10. Other embodiments may concern methods of imaging, detection or diagnosis of HIV infection in a subject using an anti-HIV antibody or fragment conjugated to a diagnostic agent. In alternative embodiments, a bispecific antibody with at least one binding site for an HIV antigen and at least one binding site for a carrier molecule may be administered, optionally followed by a clearing agent, followed by administration of a carrier molecule conjugated to a therapeutic agent.
Owner:IMMUNOMEDICS INC
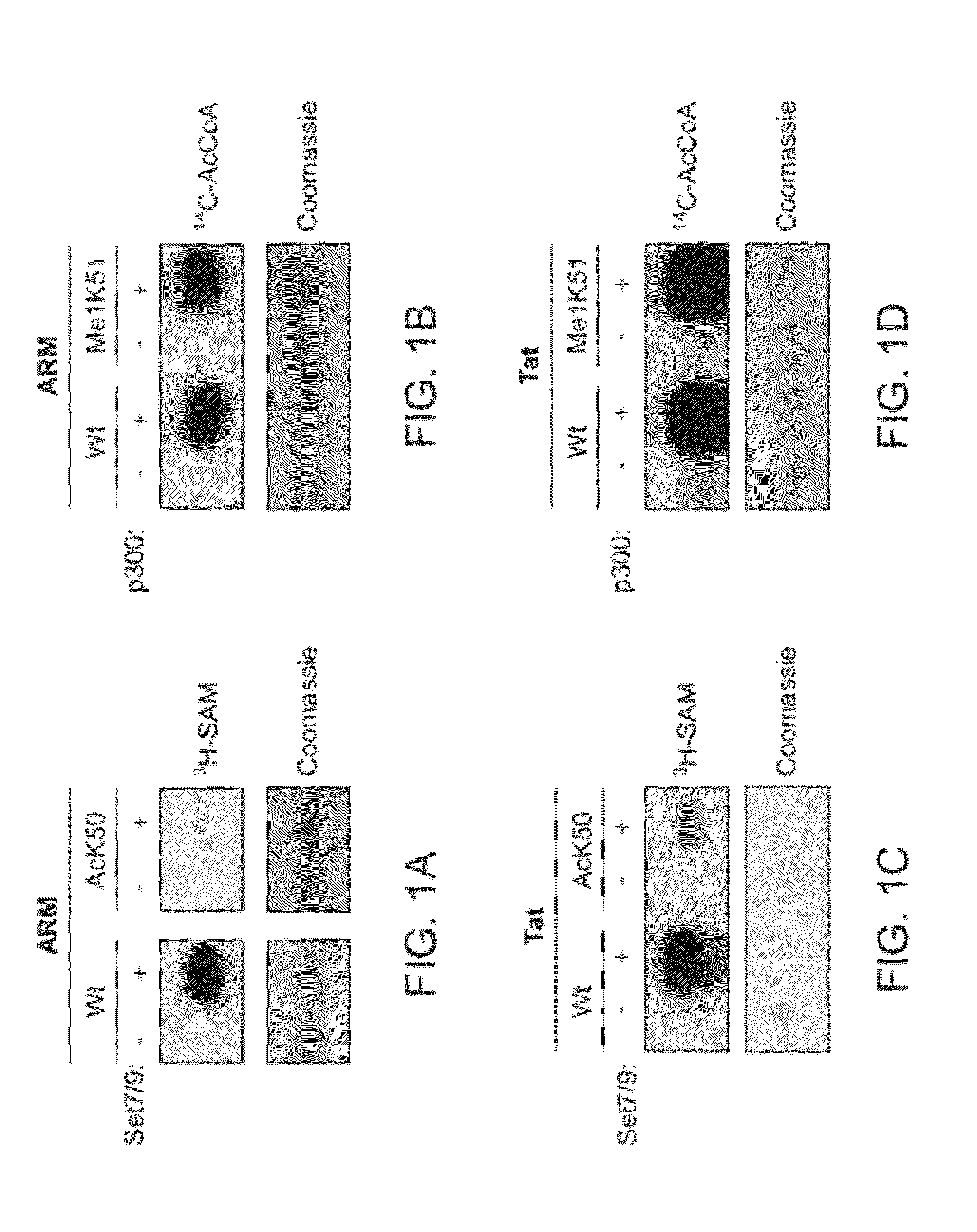
Compositions and Methods for Modulating Immunodeficiency Virus Transcription
The present disclosure provides methods of modulating immunodeficiency virus transcription, involving modulating enzymatic activity and / or levels of a lysine-specific demethylase-1 (LSD1) polypeptide and / or LSD1-mediated demethylation of methylated Tat. The present disclosure also provides method of identifying agents that modulate LSD1-mediated demethylation of a human immunodeficiency virus (HIV) Tat polypeptide.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
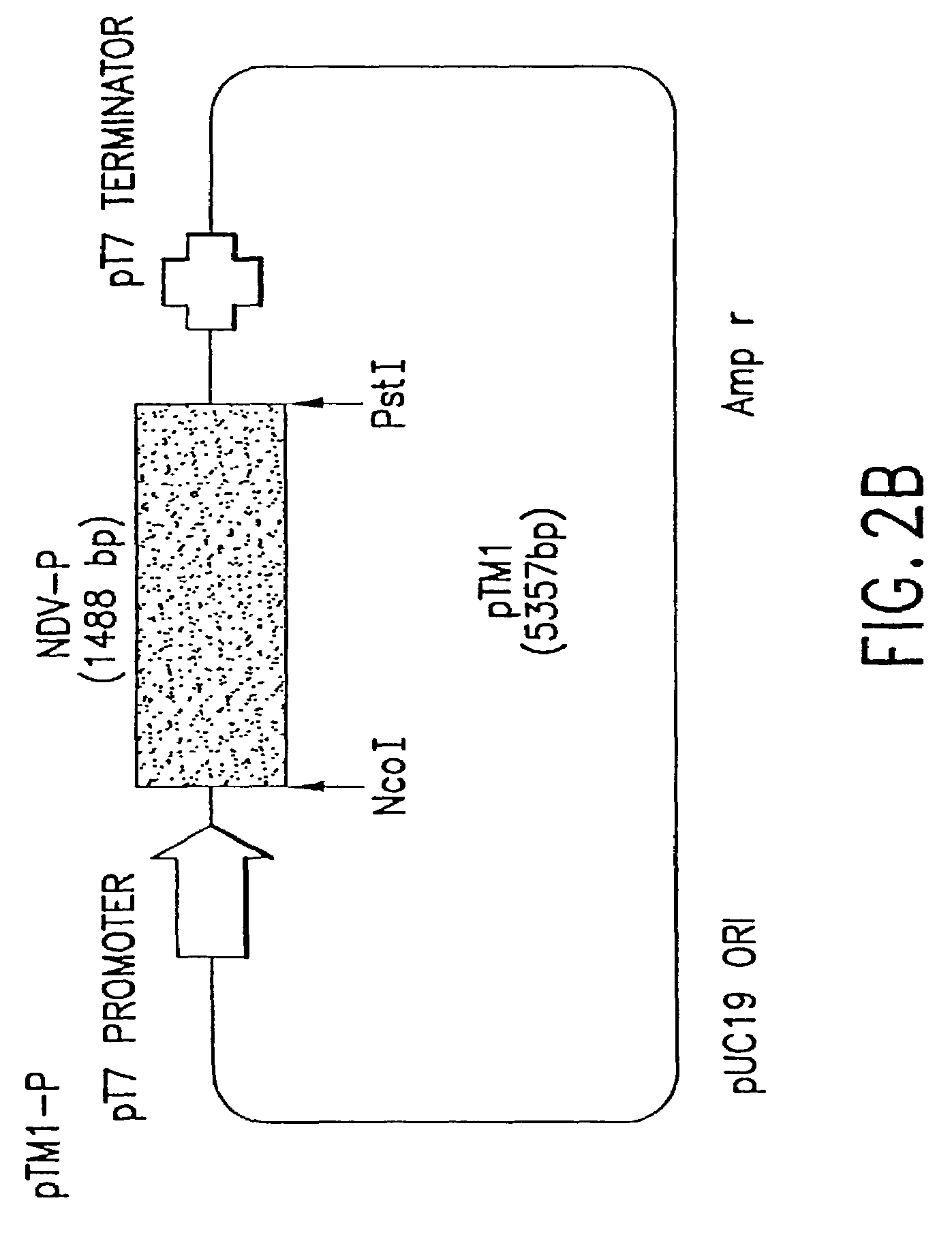
Recombinant Newcastle disease virus RNA expression systems and vaccines
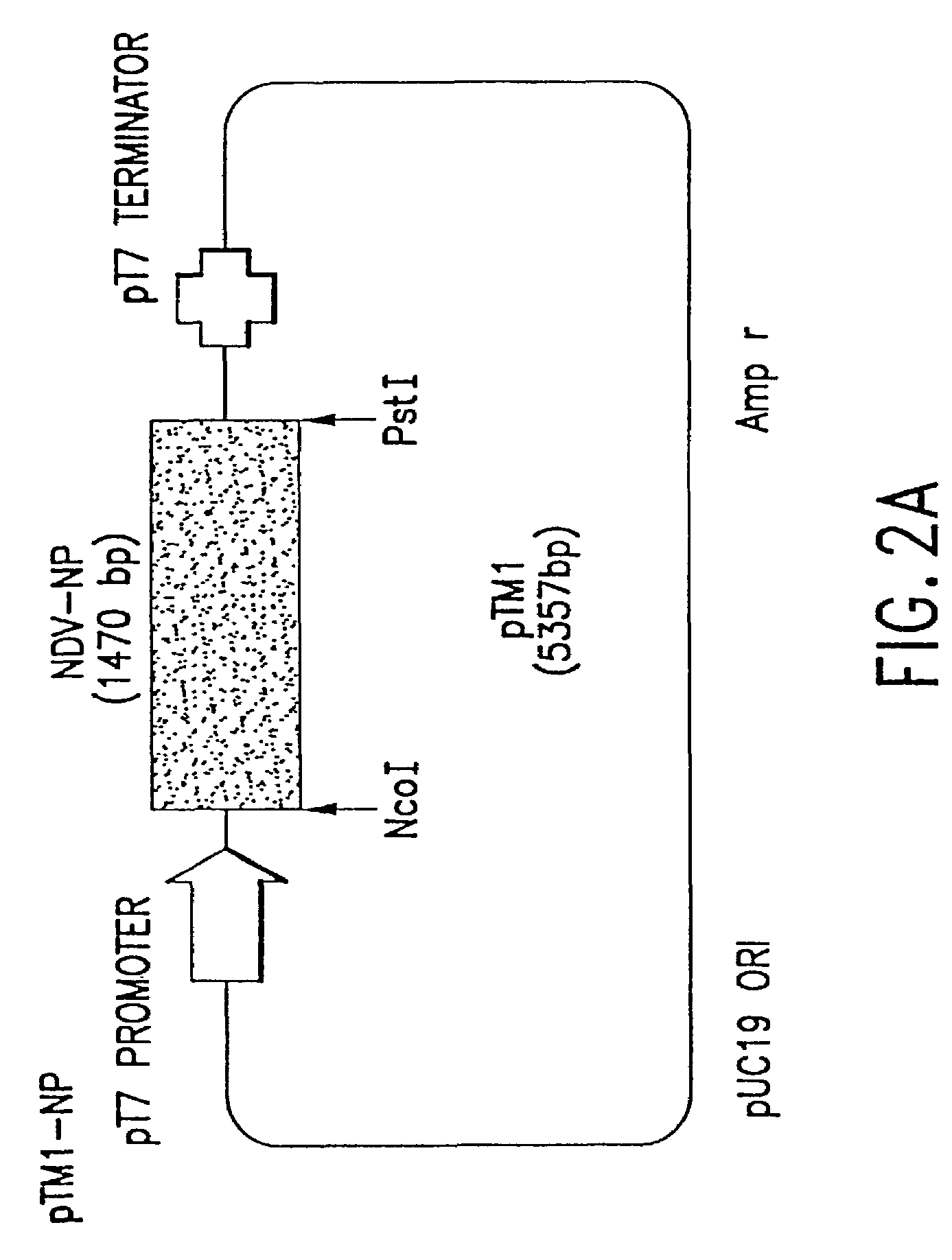
This invention relates to genetically engineered Newcastle disease viruses and viral vectors which express heterologous genes or mutated Newcastle disease viral genes or a combination of viral genes derived from different strains of Newcastle disease virus. The invention relates to the construction and use of recombinant negative strand NDV viral RNA templates which may be used with viral RNA-directed RNA polymerase to express heterologous gene products in appropriate host cells and / or to rescue the heterologous gene in virus particles. In a specific embodiment of the invention, the heterologous gene product is a peptide or protein derived from the genome of a human immunodeficiency virus. The RNA templates of the present invention may be prepared by transcription of appropriate DNA sequences using any DNA-directed RNA polymerase such as bacteriophage T7, T3, SP6 polymerase, or eukaryotic polymerase I.
Owner:MT SINAI SCHOOL OF MEDICINE
Compositions and methods for targeted inactivation of HIV cell surface receptors
InactiveUS20100172882A1Avoid infectionReduce viral loadAntibacterial agentsBiocideImmunodeficiency virusReceptor degradation
Compositions for targeted mutagenesis of cell surface receptors for HIV and methods of their use are provided herein. The compositions include triplex-forming molecules that bind to duplex DNA in a sequence specific manner at target sites to form triple-stranded structures. The triplex-forming molecules can be triplex-forming oligonucleotides (TFOs) or peptide nucleic acids (PNAs). The triplex-forming molecules are useful to induce site-specific homologous recombination in mammalian cells when used in combination with donor oligonucleotides. The triplex-forming molecules target sites within or adjacent to genes that encodes cell surface receptors for human immunodeficiency virus (HIV). This binding stimulates homologous recombination of a donor oligonucleotide to cause mutations in HIV cell surface receptor genes that result in one or more deficiencies in the ability of the encoded receptor to bind to HIV and allow its transport into the cell. Methods for ex vivo and in vivo prophylaxis and therapy of HIV infection using the disclosed compositions are also provided.
Owner:YALE UNIV
Heterocyclic antiviral compounds
The present invention relates to a compounds according to formula I, methods for treating diseases mediated by human immunodeficieny virus by administration of a compound according to formula I and pharmaceutical compositions for treating diseases mediated by human immunodeficieny virus containing a compound according to formula I where R1, R2, R3, R4, R5, are as defined herein.
Owner:ROCHE PALO ALTO LLC
Dual vector for inhibition of human immunodeficiency virus
The present invention provides an expression vector for preventing or inhibiting HIV entry, fusion or replication in mammalian cells. In particular, the invention provides a recombinant retroviral vector that encodes an inhibitor of a HIV co-receptor, such as CCR5 or CXCR4, and a protein that inhibits HIV fusion to target cells and / or HIV replication. Pharmaceutical compositions comprising such constructs and methods of use thereof to prevent or treat HIV infection in a patient are also disclosed.
Owner:RGT UNIV OF CALIFORNIA +1
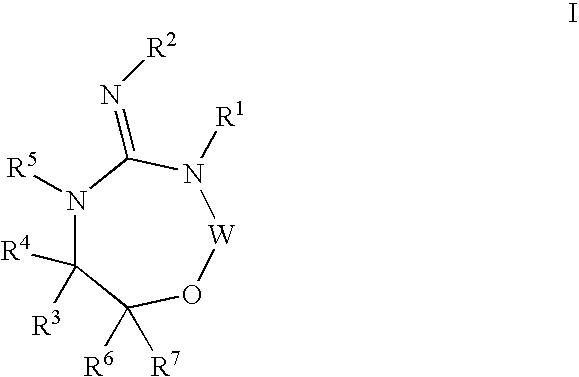
Preparation and use of compounds as aspartyl protease inhibitors
ActiveUS20070010667A1Nervous disorderMetabolism disorderImmunodeficiency virusNeuro-degenerative disease
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein A is a bond, —C(O)—, or —C(R3′)(R4′)—; X is —N(R1)— or —C(R6)(R7)—; Y is —S(O)2—, —C(═O)—, —PO(OR9) or —C(R6′R7′)—; is a single or double bond and R, R1, R2, R3, R4, R3′, R4′, R5, R6, R6′, R7 and R7′ are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:MERCK SHARP & DOHME LLC
Heterocyclic aspartyl protease inhibitors
Disclosed are compounds of the formula Ior a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, whereinW is a bond, —C(═S)—, —S(O)—, —S(O)2—, —C(═O)—, —O—, —C(R6)(R7)—, —N(R5)— or —C(═N(R5))—;X is —O—, —N(R5)— or —C(R6)(R7)—; provided that when X is —O—, U is not —O—, —S(O)—, —S(O)2—, —C(═O)— or —C(═NR5)—;U is a bond, —S(O)—, —S(O)2—, —C(O)—, —O—, —P(O)(OR15)—, —C(═NR5)—, —(C(R6)(R7))b— or —N(R5)—; wherein b is 1 or 2; provided that when W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, U is not —S(O)—, —S(O)2—, —O—, or —N(R5)—; provided that when X is —N(R5)— and W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, then U is not a bond;and R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I.Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes.Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:PHARMACOPEIA INC +1
Long-lasting antiviral fusion inhibitor peptide conjugates comprising albumin and human immunodeficiency virus (HIV) peptides
InactiveUS7582301B1Reduce sensitivityImprove in vivo stabilityPeptide/protein ingredientsVirus peptidesHalf-lifeImmunodeficiency virus
Peptides exhibiting anti-viral and anti-fusogenic activity are modified to provide greater stability and improved half-life in vivo. The selected peptides include fusion inhibitors DP178 and DP107 and related peptides and analogs thereof. The modified peptides are capable of forming covalent bonds with one or more blood components, preferably a mobile blood component.
Owner:CONJUCHEM
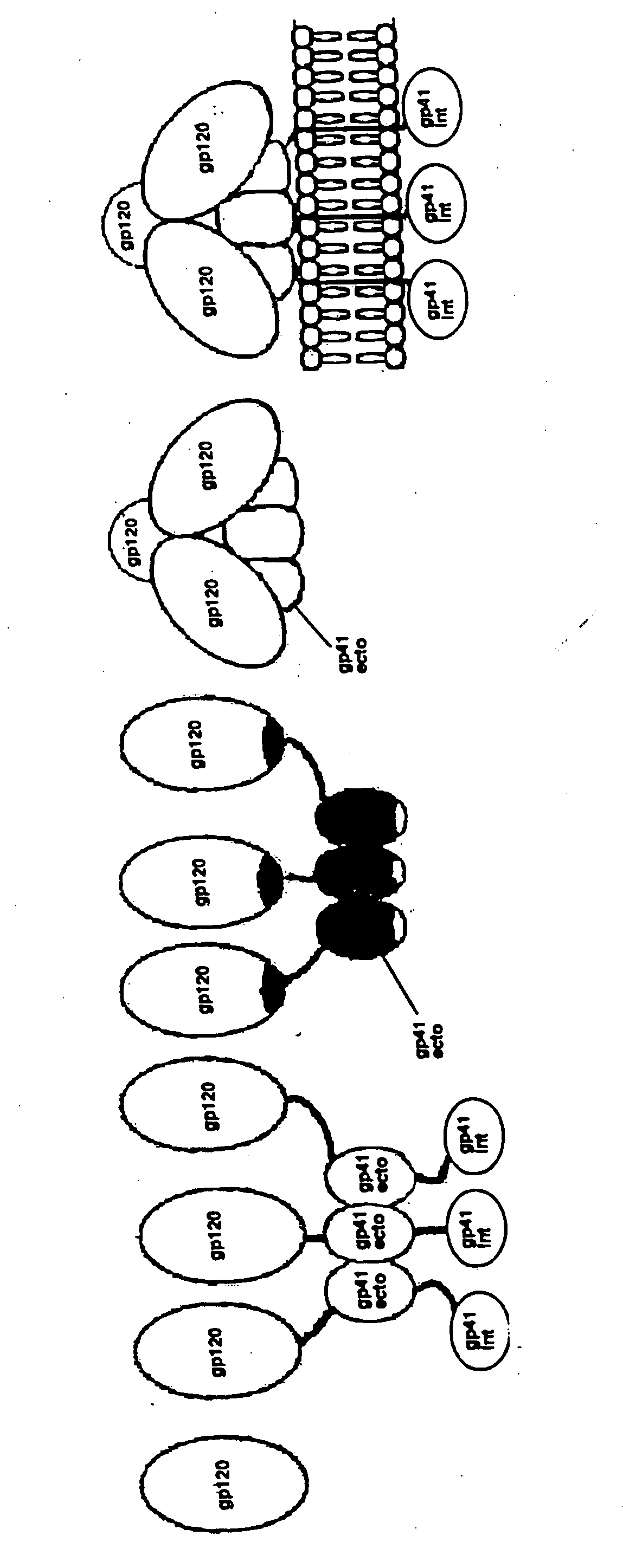
Stabilized human immunodeficiency virus (HIV) envelope (ENV) trimer vaccines and methods of using same
ActiveUS20140302080A1Reduce dissociationImprove stabilitySugar derivativesViral antigen ingredientsImmunodeficiency virusHuman immunodeficiency
The invention features stabilized human immunodeficiency virus (HIV) envelope (Env) trimers. The invention also features vaccines, nucleic acids, and vectors to deliver and / or facilitate production of the stabilized HIV Env trimers. In addition, the invention features methods of making and using the stabilized HIV Env trimers of the invention.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Methods and compositions for the treatment or prevention of human immunodeficiency virus and related conditions using cyclooxygenase-2 selective inhibitors and antiviral agents
The present invention provides compositions and methods for the treatment of human immunodeficiency virus (HIV) infection as well as HIV associated diseases and related disorders. More particularly, the invention provides a combination therapy for the treatment of HIV infection as well as HIV associated diseases and related disorders comprising the administration to a subject of an anti-human immunodeficiency virus agent in combination with a cyclooxygenase-2 selective inhibitor or an isomer or a pharmaceutically acceptable salt, ester, or prodrug thereof.
Owner:PHARMACIA CORP
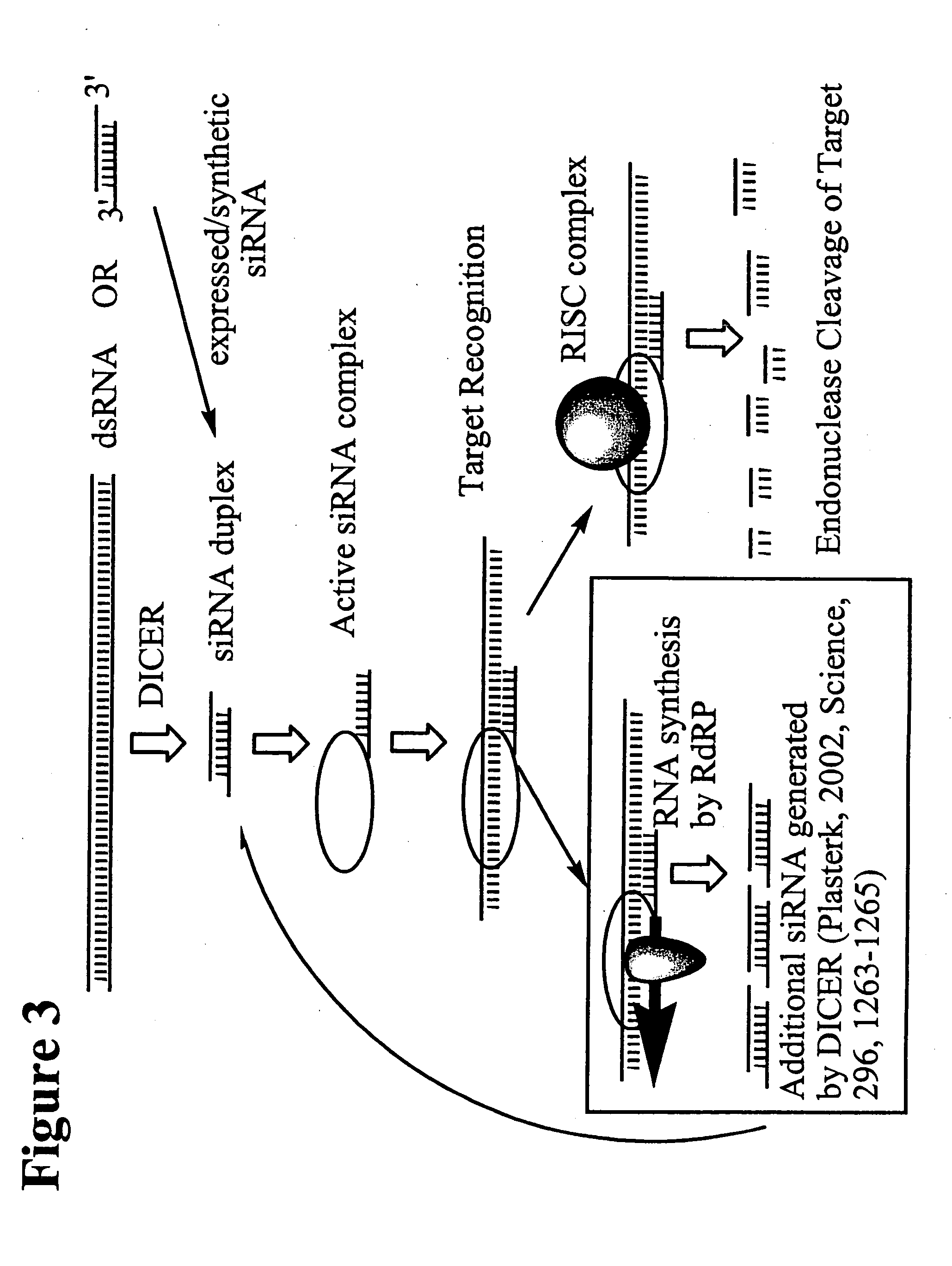
RNA interference mediated inhibition of human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050191618A1Improves various propertyImprove the immunitySugar derivativesMicrobiological testing/measurementDiseaseBiological body
This invention relates to compounds, compositions, and methods useful for modulating human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of human immunodeficiency virus (HIV) gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of HIV genes. The small nucleic acid molecules are useful in the treatment of HIV infection, AIDS, and / or diseaes and conditions related to HIV infection and / or AIDS in a subject or organism.
Owner:SIRNA THERAPEUTICS INC
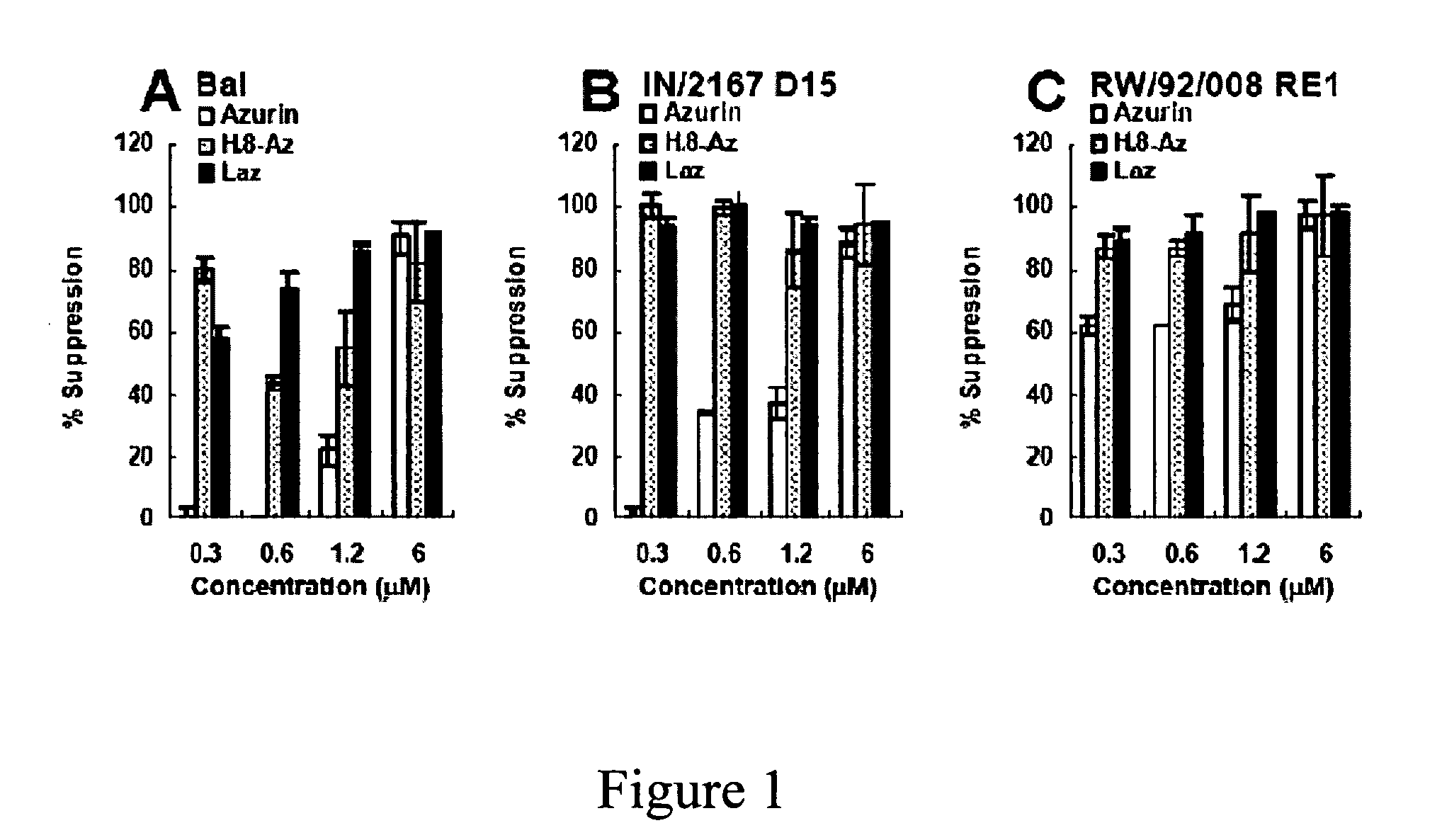
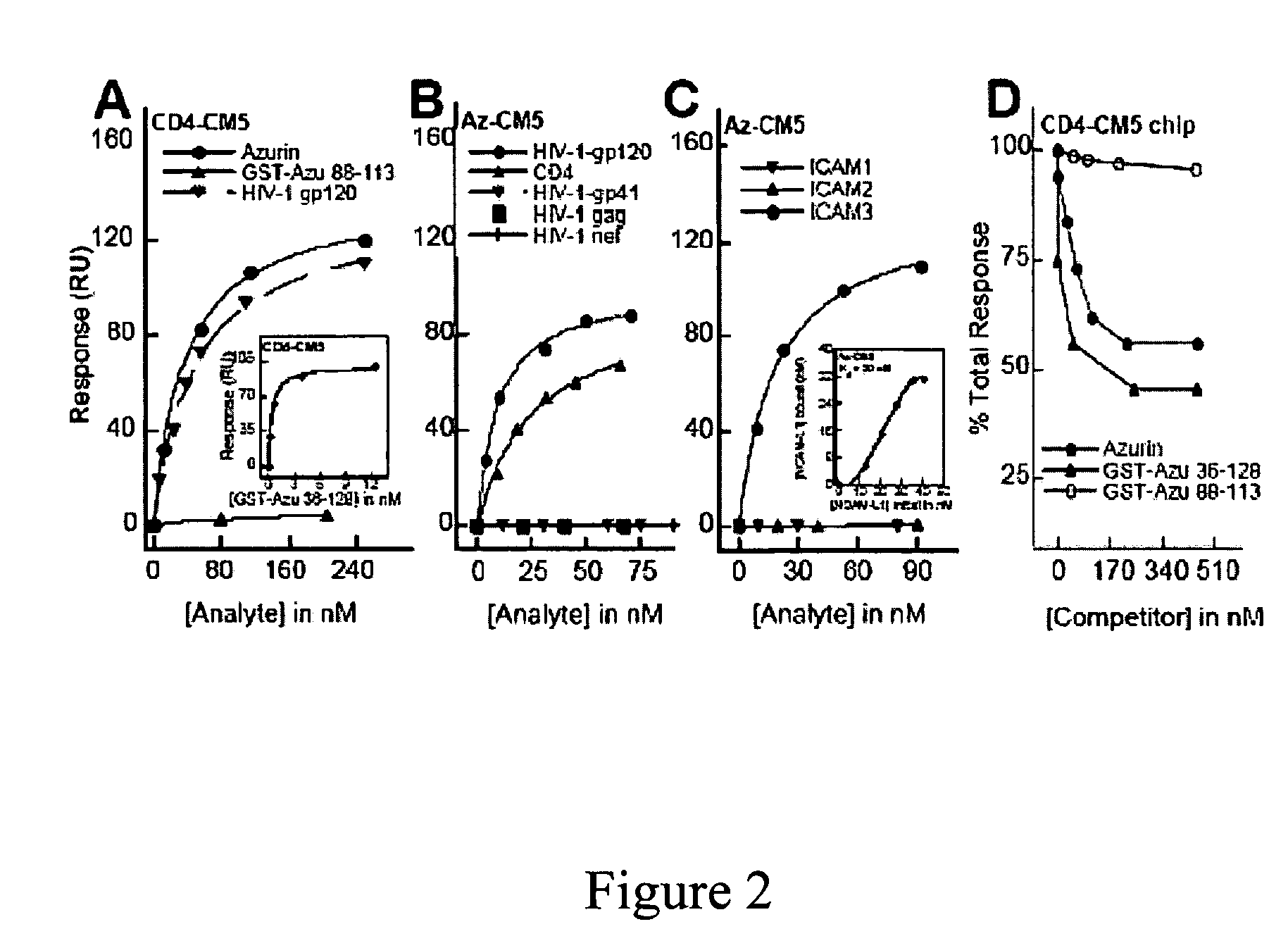
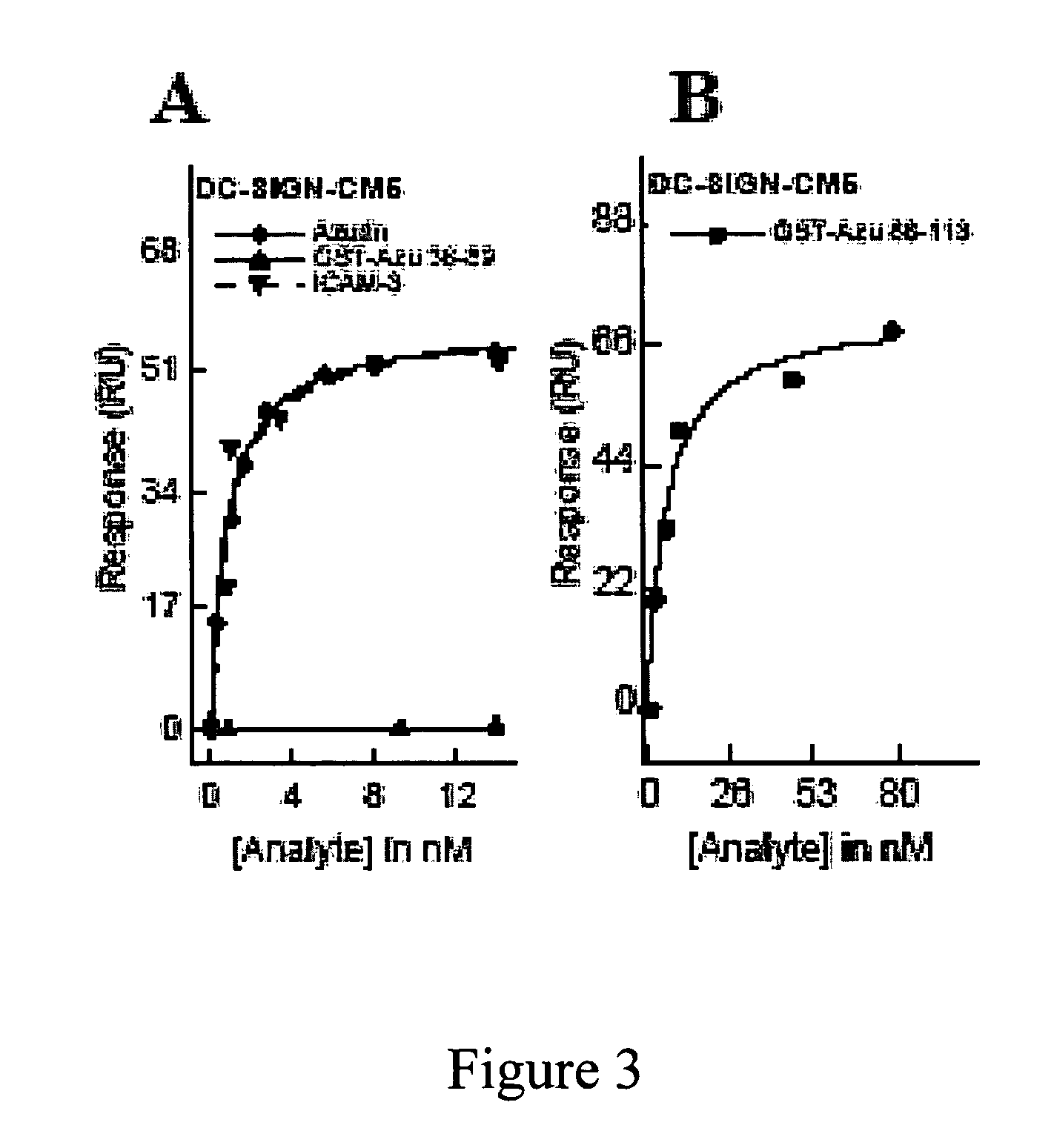
Compositions and methods for treating HIV infection with cupredoxin and cytochrome c
The present invention relates to cupredoxin, specifically Pseudomonas aeruginosa azurin, and / or Pseudomonas aeruginosa cytochrome C551 and their use in inhibiting of viral infection, and in particular infection of mammalian cells by the Human Immunodeficiency Virus (HIV). The invention also relates to variants and derivatives of cupredoxin and cytochrome c that retain the ability to inhibit viral infection, and in particular infection by the Human Immunodeficiency Virus (HIV). The invention also relates to research methods for studying viral and bacterial infection in mammalian cells.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
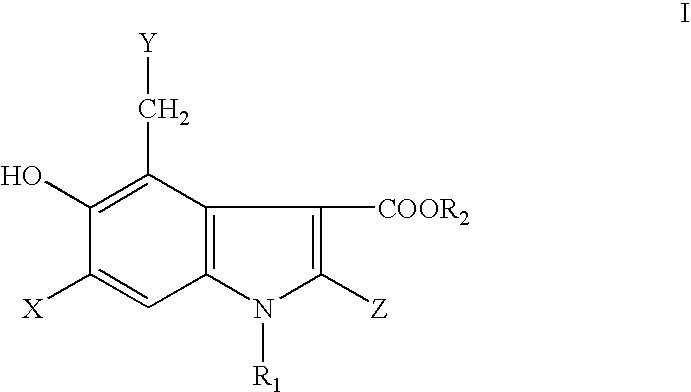
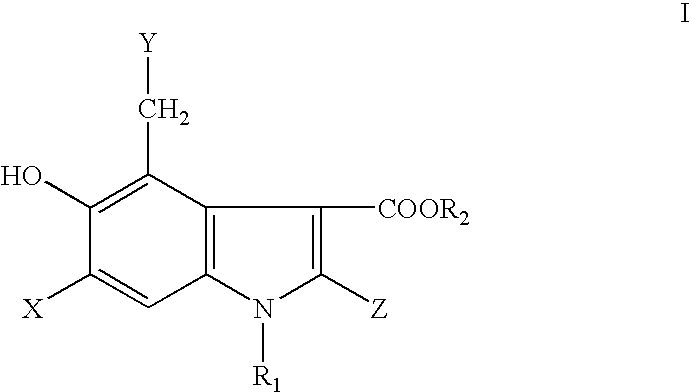
5-Hydroxyindole-3-Carboxylate Derivatives and Uses Thereof
InactiveUS20080249155A1Treatment and/or prophylaxisBiocideOrganic chemistry methodsImmunodeficiency virusMedicine
The present invention relates to 5-hydroxy-indole-3-carboxylate derivatives of formula I, or racemic mixture or optical isomers or pharmaceutically acceptable salts and / or hydrates thereof,wherein: substituents R1, R2, Z, X and Y are as defined in the description. The compounds of formula I can be useful for preparation of medicament for treatment and / or prophylaxis of virus infections, especially for preparation of medicament for anti-HBV (Hepatitis B virus) and anti-HIV (Human immunodeficiency virus).
Owner:SHENYANG PHARMA UNIVERSITY
Human immunodeficiency virus envelope clycoprotein mutants and uses thereof
This invention provides stable HIV-1 pre-fusion envelope glycoprotein trimeric complexes. This invention also provides related polypeptides and compositions comprising pharmaceutically acceptable particles and the trimeric complexes operably affixed thereto. This invention further provides related nucleic acids, vectors, host cells, compositions, production methods, and prophylactic and therapeutic methods.
Owner:CORNELL RES FOUNDATION INC +1
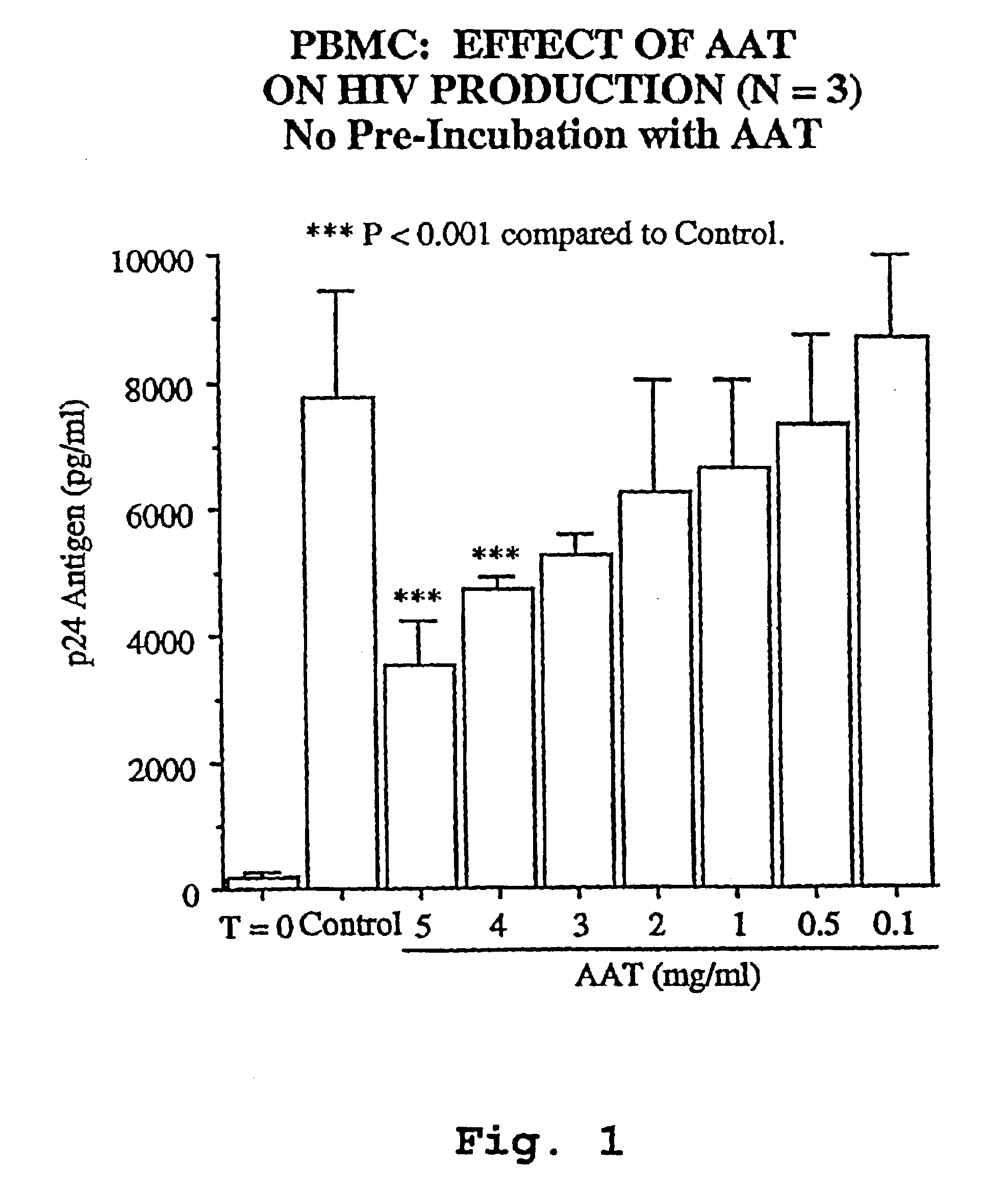
Inhibitors of serine protease activity, methods and compositions for treatment of viral infections
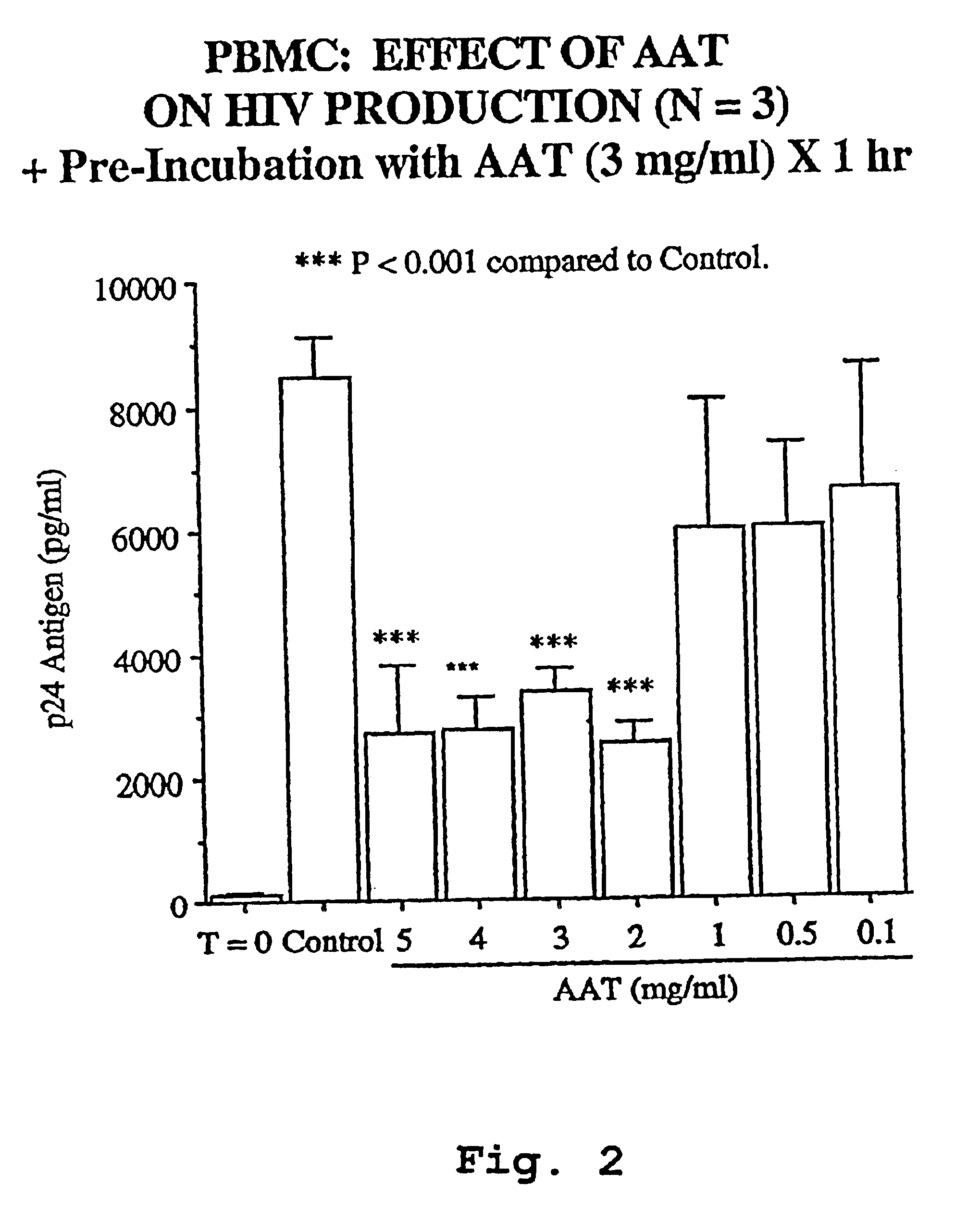
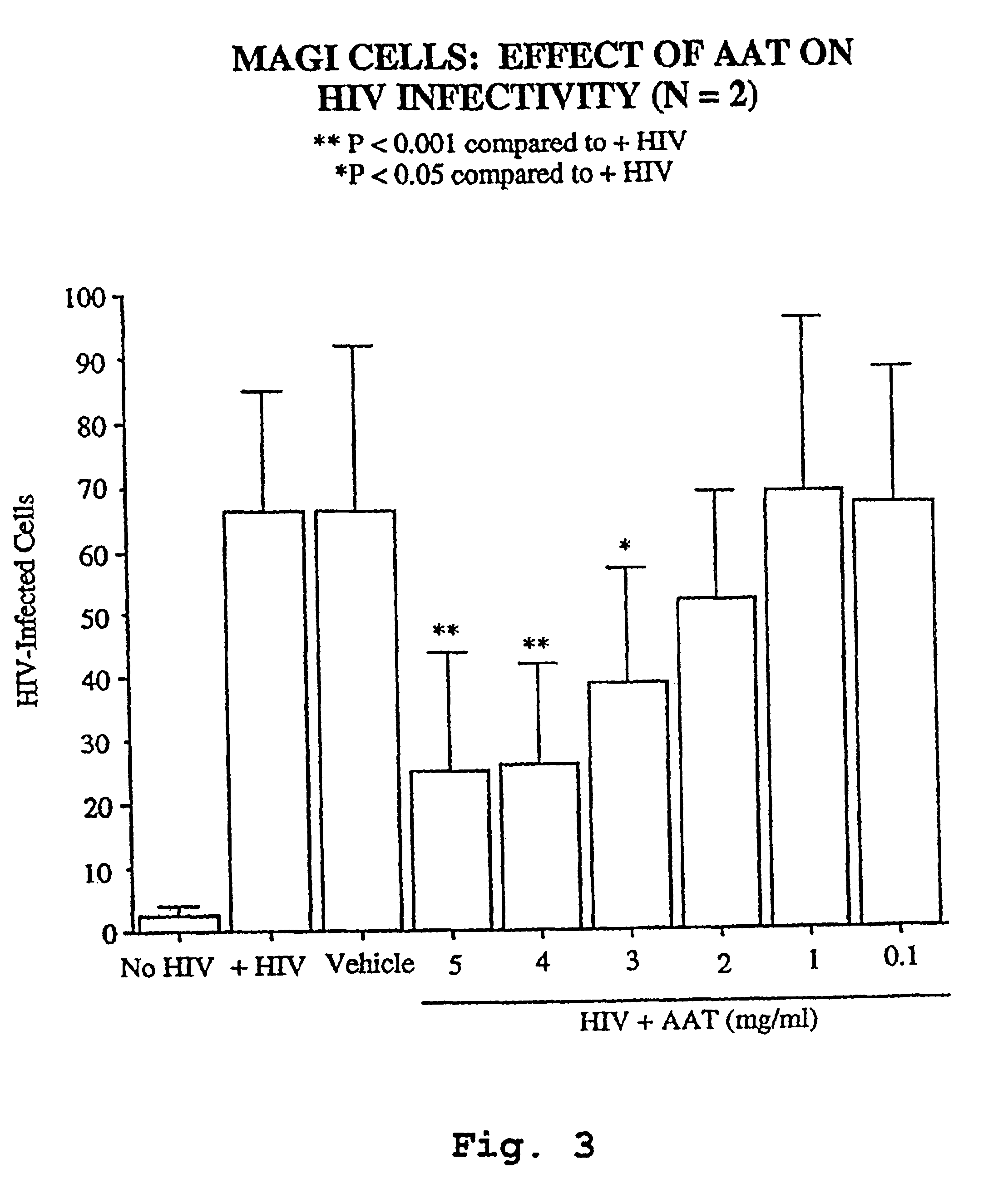
InactiveUS6849605B1Inhibit productionIncrease in p2 antigen productionBiocideDipeptide ingredientsImmunodeficiency virusRetroviral infection
A novel method of treating and preventing viral infection is provided. In particular a method of blocking viral infection facilitated by a serine proteolytic (SP) activity is disclosed, which consists of administering to a subject suffering or about to suffer from viral infection a therapeutically effective amount of a compound having a serine protease inhibitory or serpin activity. Among compounds are α1-antitrypsin (AAT), peptide derivatives from the carboxyterminal end of AAT, and man-made, synthetic compounds mimicking the action of such compounds. The preferred viral infections include retroviral infection such as human immunodeficiency virus (HIV) infection.
Owner:UNIV TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections](https://images-eureka.patsnap.com/patent_img/6c3f089e-e4cf-4756-aced-265f1c834861/US09216996-20151222-C00001.PNG)
![Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections](https://images-eureka.patsnap.com/patent_img/6c3f089e-e4cf-4756-aced-265f1c834861/US09216996-20151222-C00002.PNG)
![Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections Substituted 2,3,4,5,7,9,13,13a-octahydropyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepines and methods for treating viral infections](https://images-eureka.patsnap.com/patent_img/6c3f089e-e4cf-4756-aced-265f1c834861/US09216996-20151222-C00003.PNG)