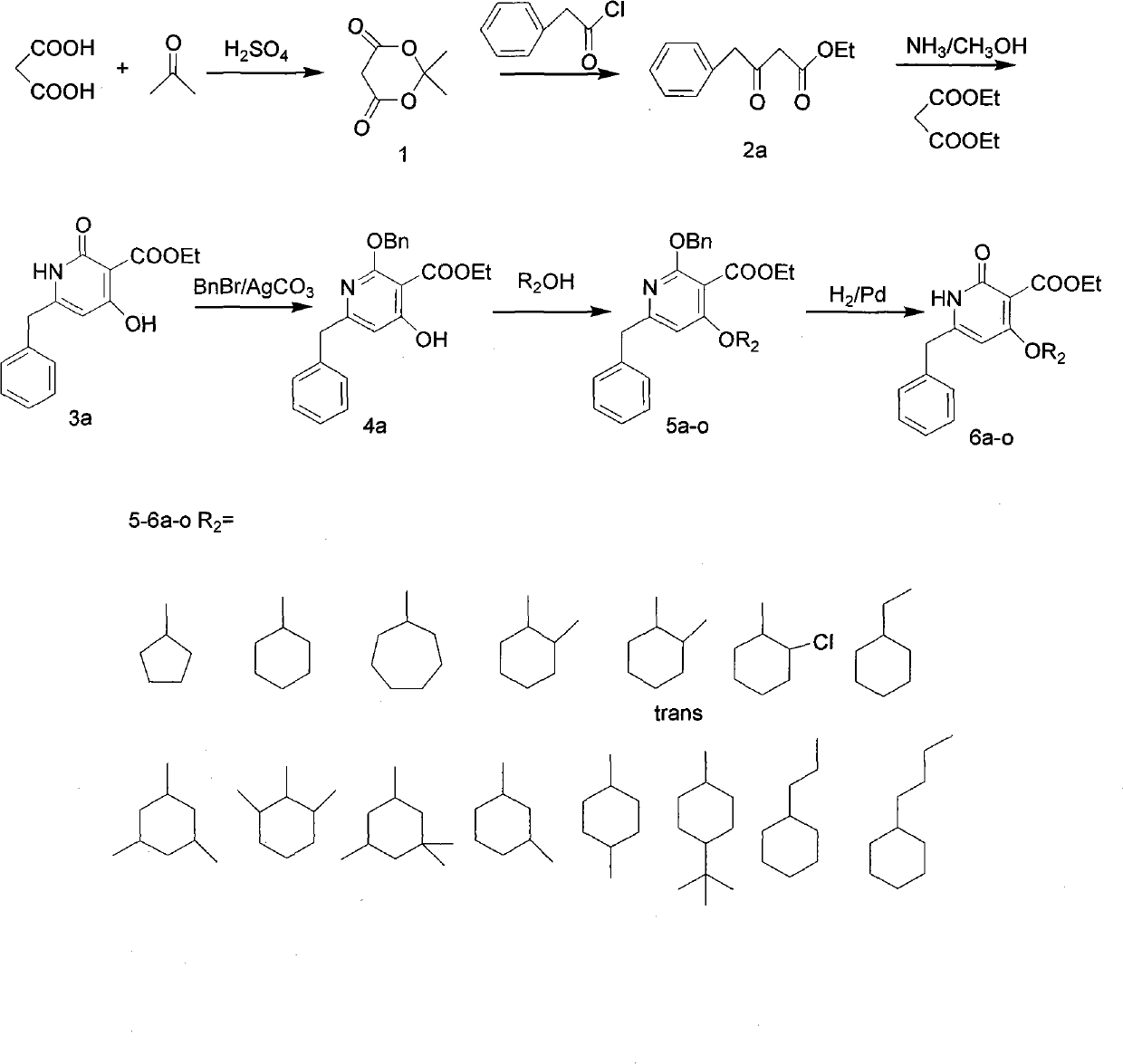
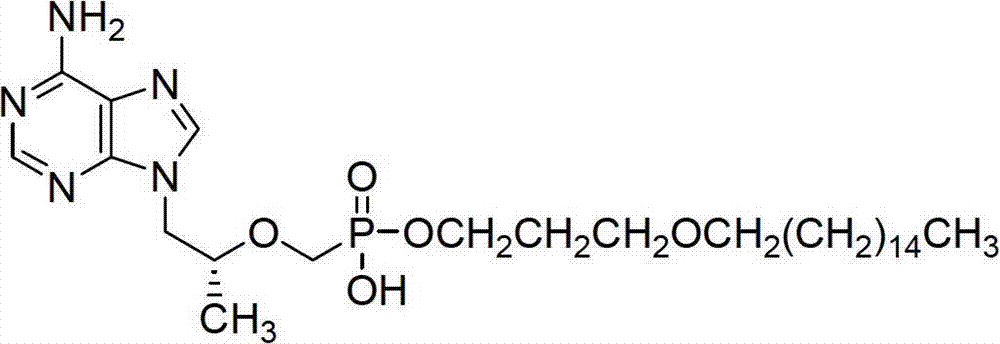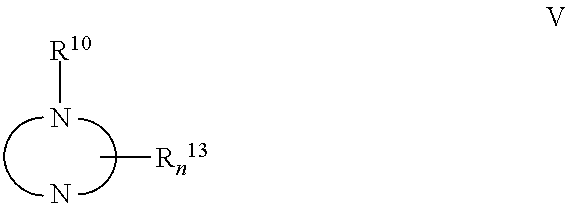Patents
Literature
100 results about "Human immunodeficiency virus 1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
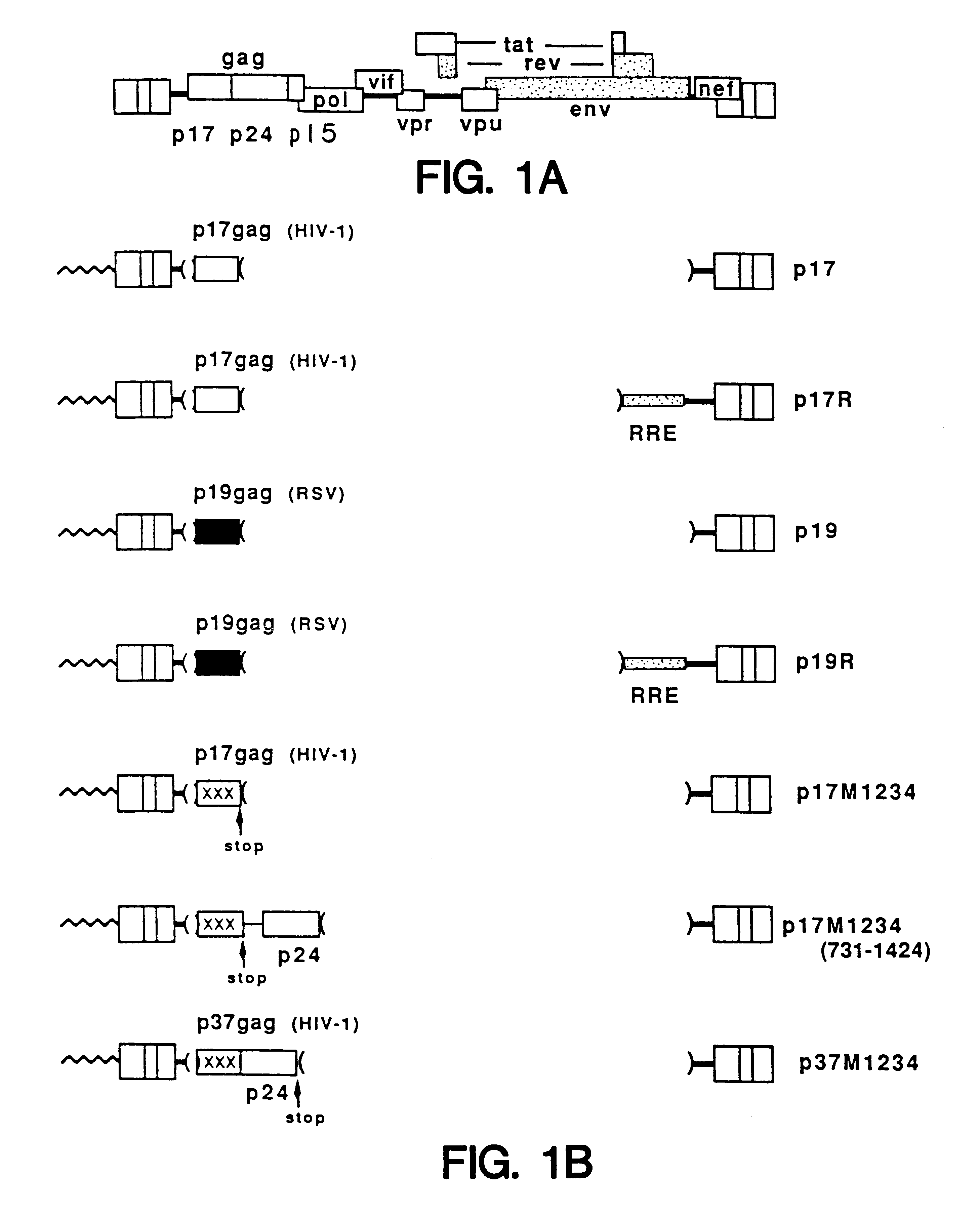
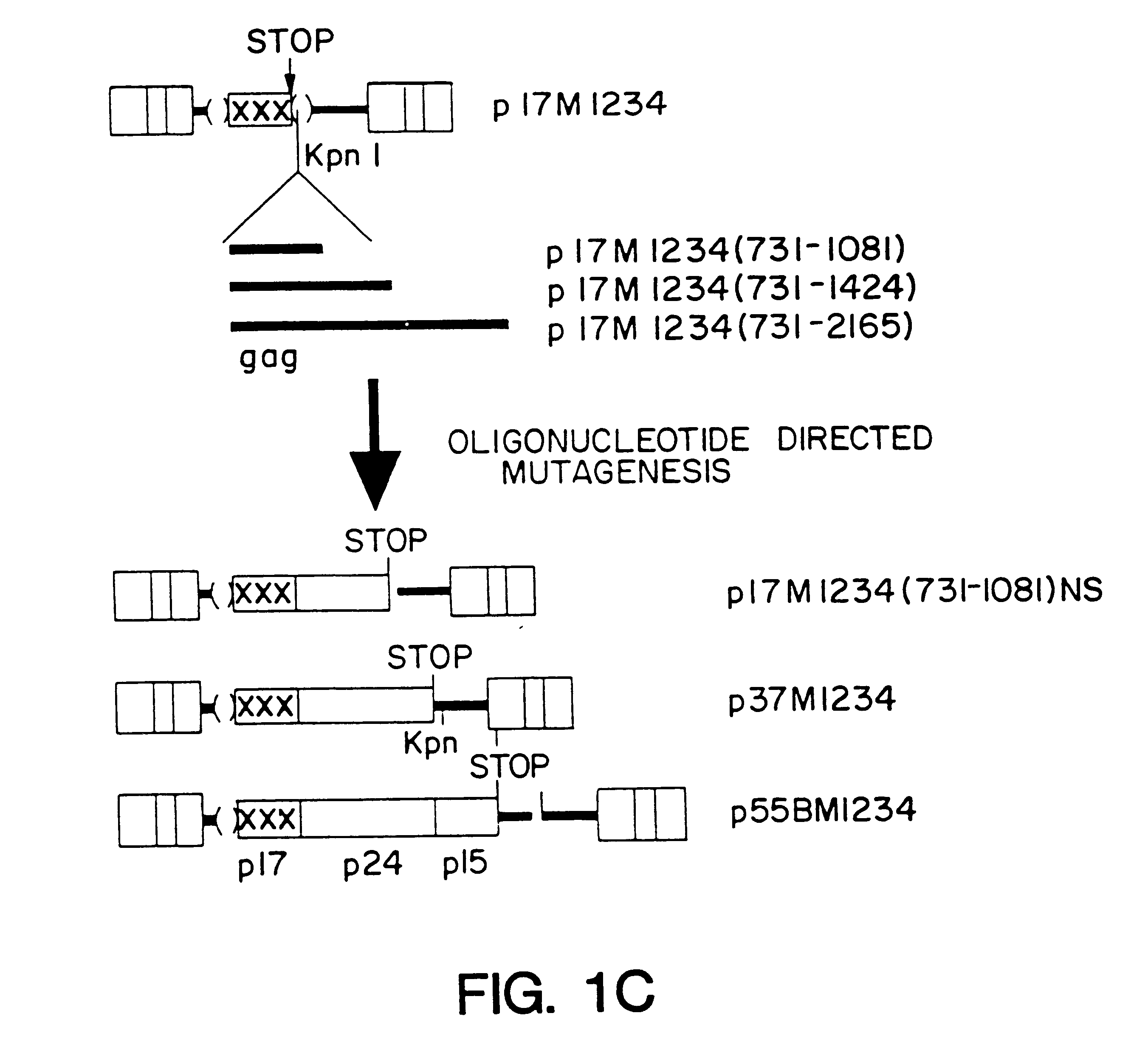
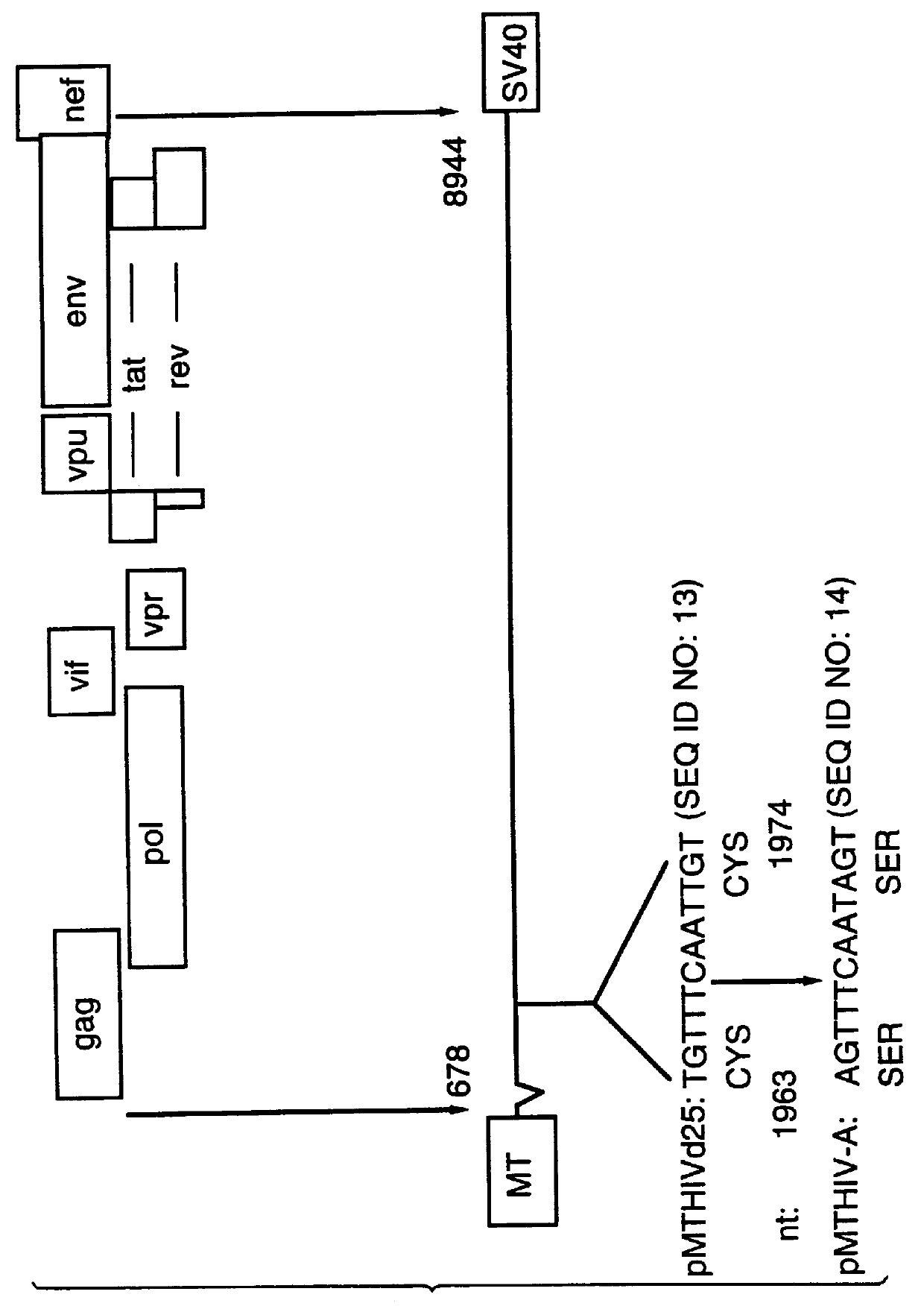
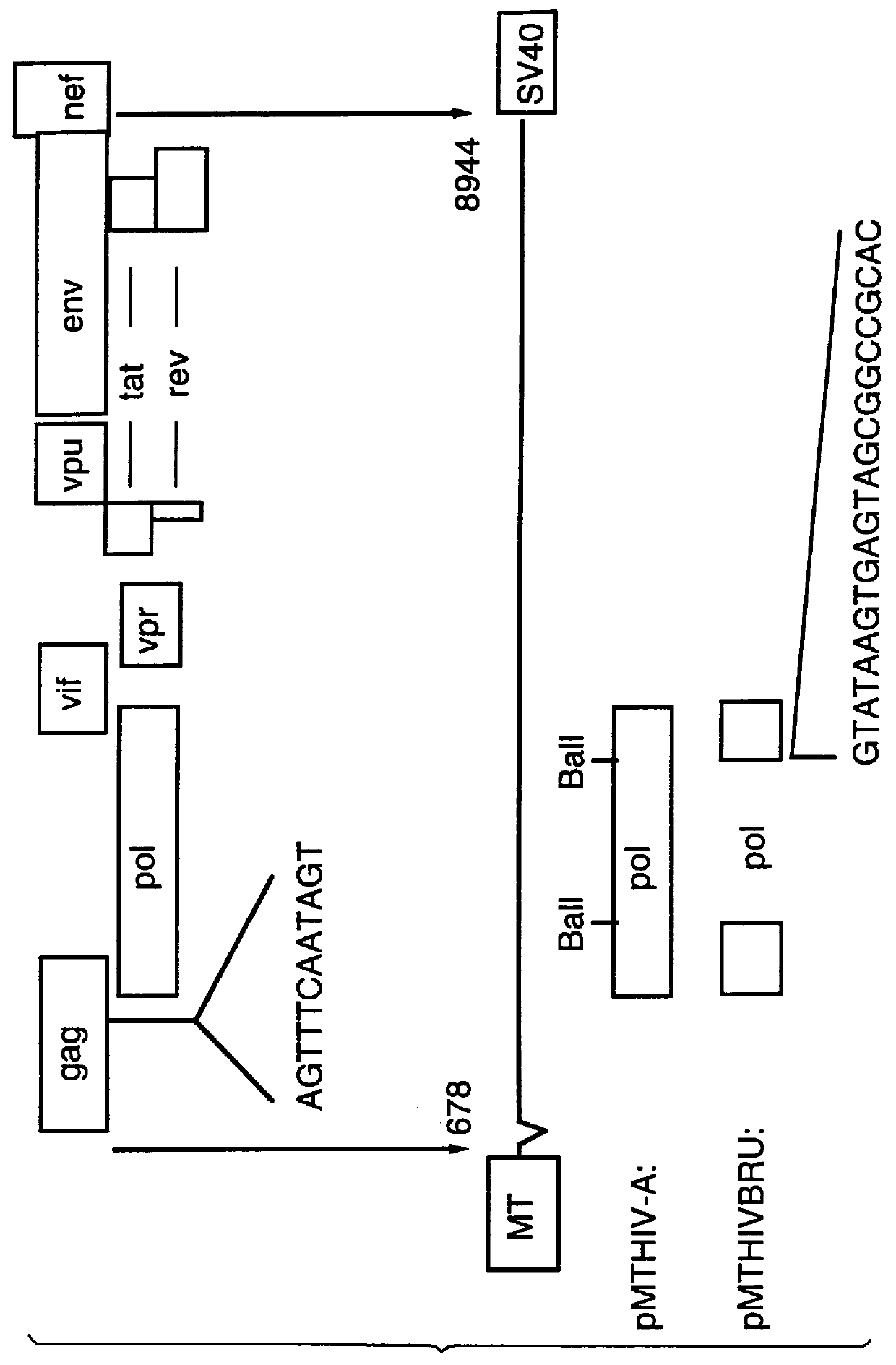
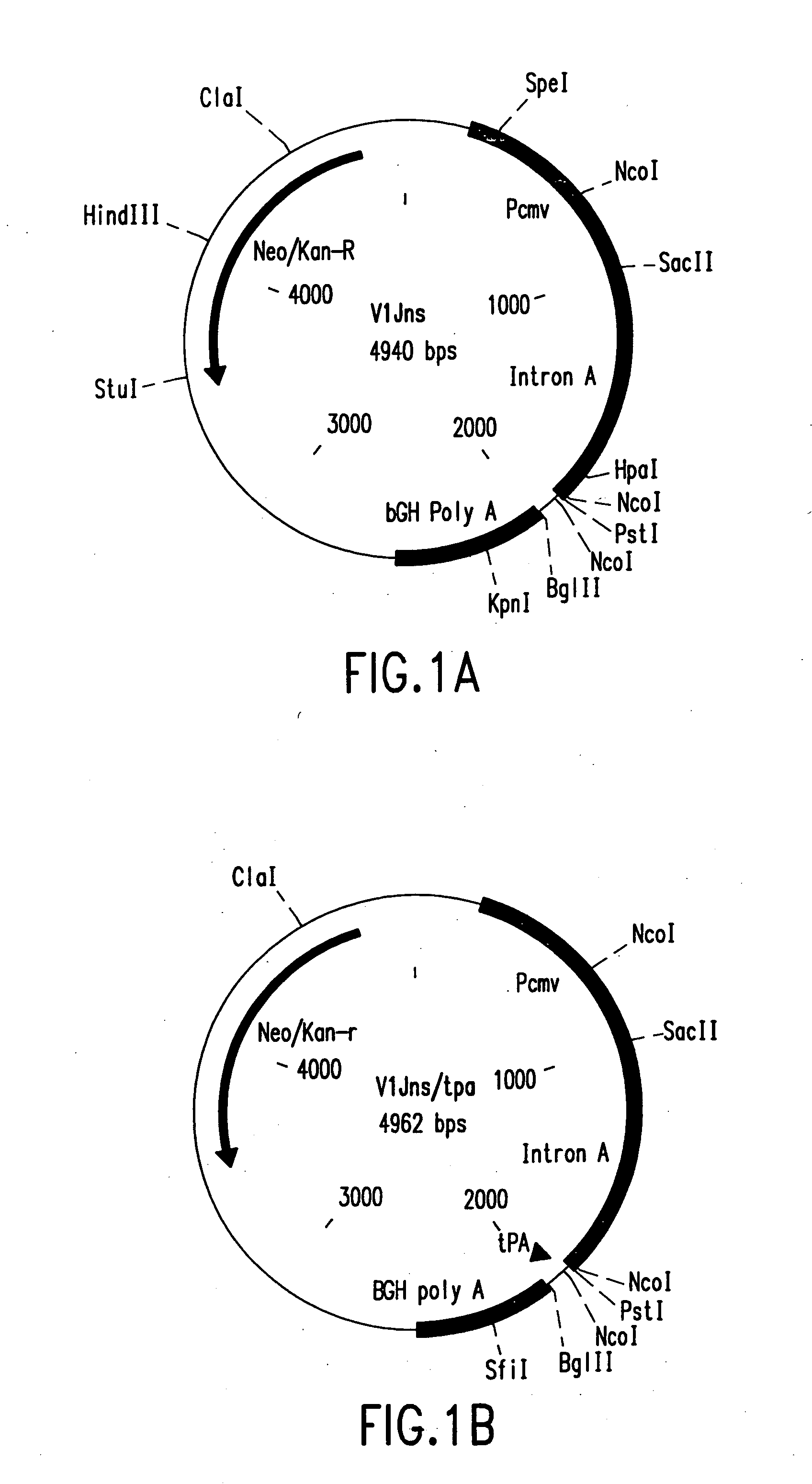
Method of eliminating inhibitory/instability regions from mRNA
InactiveUS6174666B1Microbiological testing/measurementVirus peptidesImmunodeficiency virusInstability
A method of locating an inhibitory / instability sequence or sequences within the coding region of an mRNA and modifying the gene encoding that mRNA to remove these inhibitory / instability sequences by making clustered nucleotide substitutions without altering the coding capacity of the gene is disclosed. Constructs containing these mutated genes and host cells containing these constructs are also disclosed. The method and constructs are exemplified by the mutation of a Human Immunodeficiency Virus-1 Rev-dependent gag gene to a Rev-independent gag gene. Constructs useful in locating inhibitory / instability sequences within either the coding region or the 3' untranslated region of an mRNA are also disclosed.
Owner:UNITED STATES OF AMERICA
Human immunodeficiency virus type 1 nucleic acids devoid of long terminal repeats capable of encoding for non-infectious, immunogenic, retrovirus-like particles
InactiveUS6080408AImprove efficiencyLow backgroundSugar derivativesViral antigen ingredientsPol genesReverse transcriptase activity
Non-infectious, retrovirus-like particles contain mutations to reduce gag-dependent RNA-packaging of the gag gene product, eliminate reverse transcriptase activity of the pol gene product, eliminate integrase activity of the pol gene product and eliminate RNase H activity of the pol gene product through genetic manipulation of the gag and pol genes. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in diagnosis.
Owner:CONNAUGHT LAB
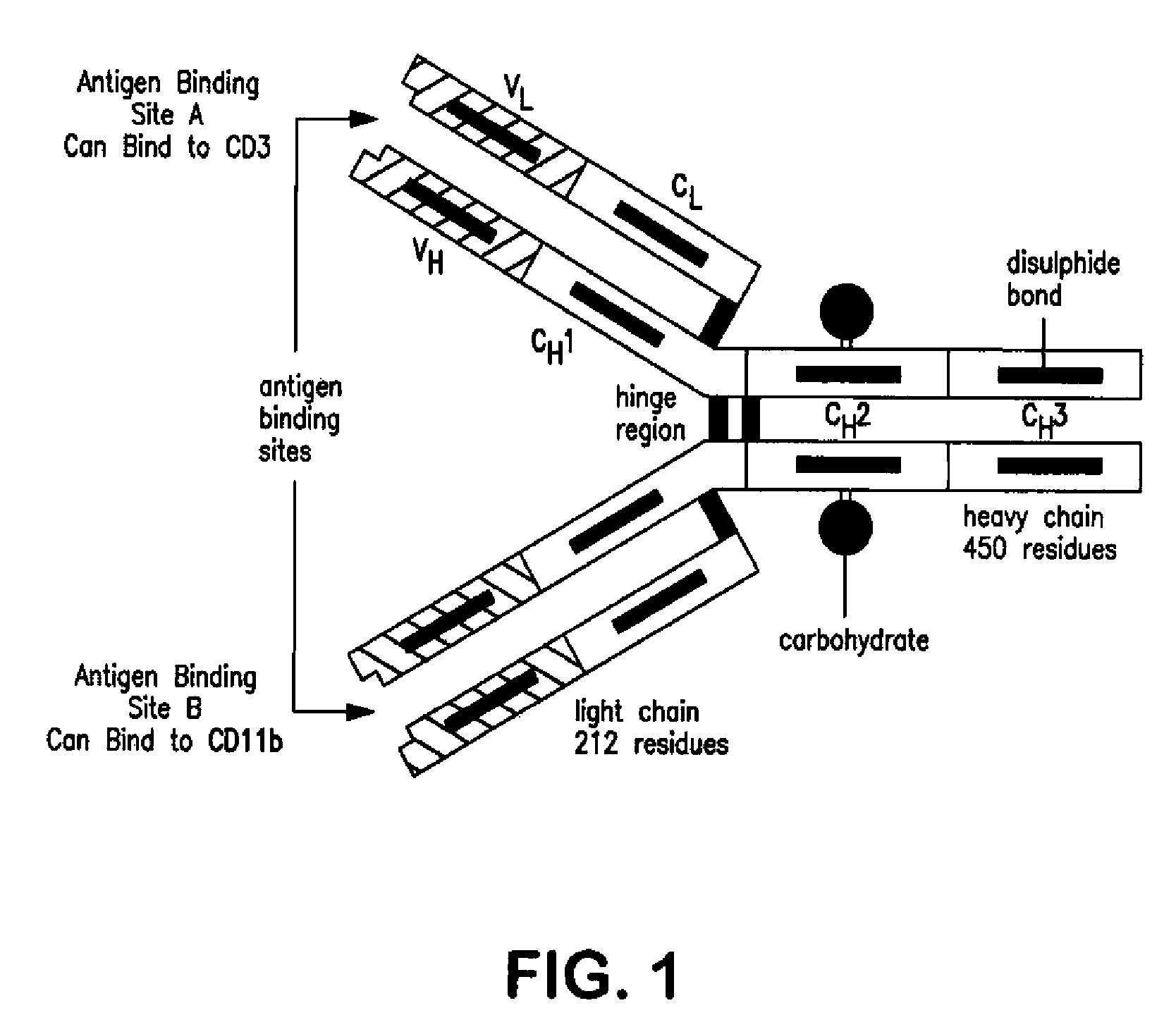
Bi-specific monoclonal antibody (specific for both CD3 and CD11b) therapeutic drug
ActiveUS7862813B2Deleterious effectPeptide/protein ingredientsDisease diagnosisHuman cancerBispecific monoclonal antibody
The present invention relates to the treatment of immune system abnormalities that can be found in lethal human cancers and also in the progressive Human Immunodeficiency Virus Type 1 (HIV-1) infections and provides medicaments to correct abnormalities in a subject with cancer or HIV-1-infected subjects, in order to allow the immune system to fight the cancer or HIV-1 infections. The present invention also discloses multivalent polypeptides which specifically bind to and enable destruction and / or inactivation of immune cells that have CD11b and CD3 on their surface, therefore dissipating the deleterious effects of the CD11b+ T cells.
Owner:BJORK JR ROBERT LAMAR
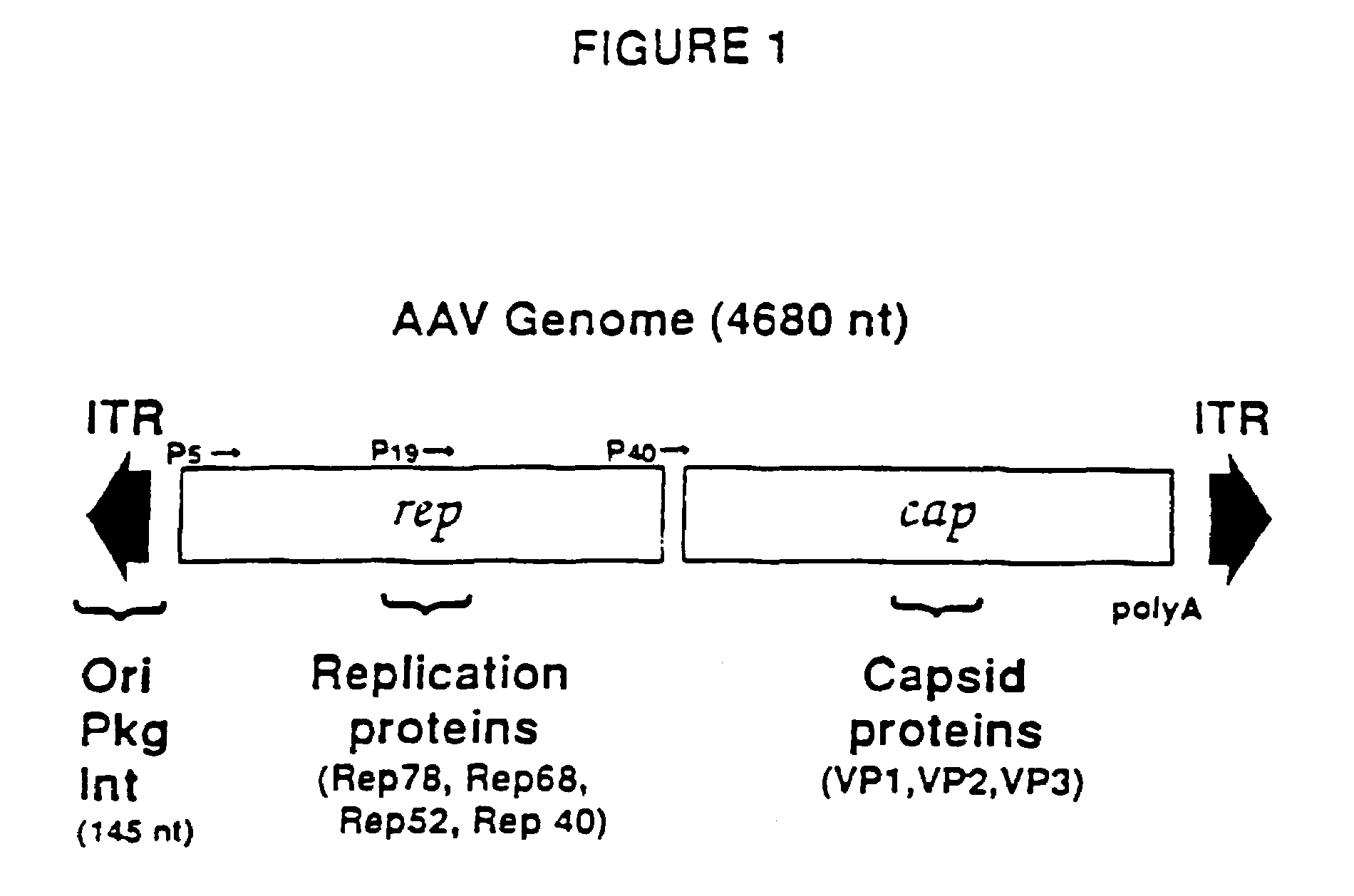
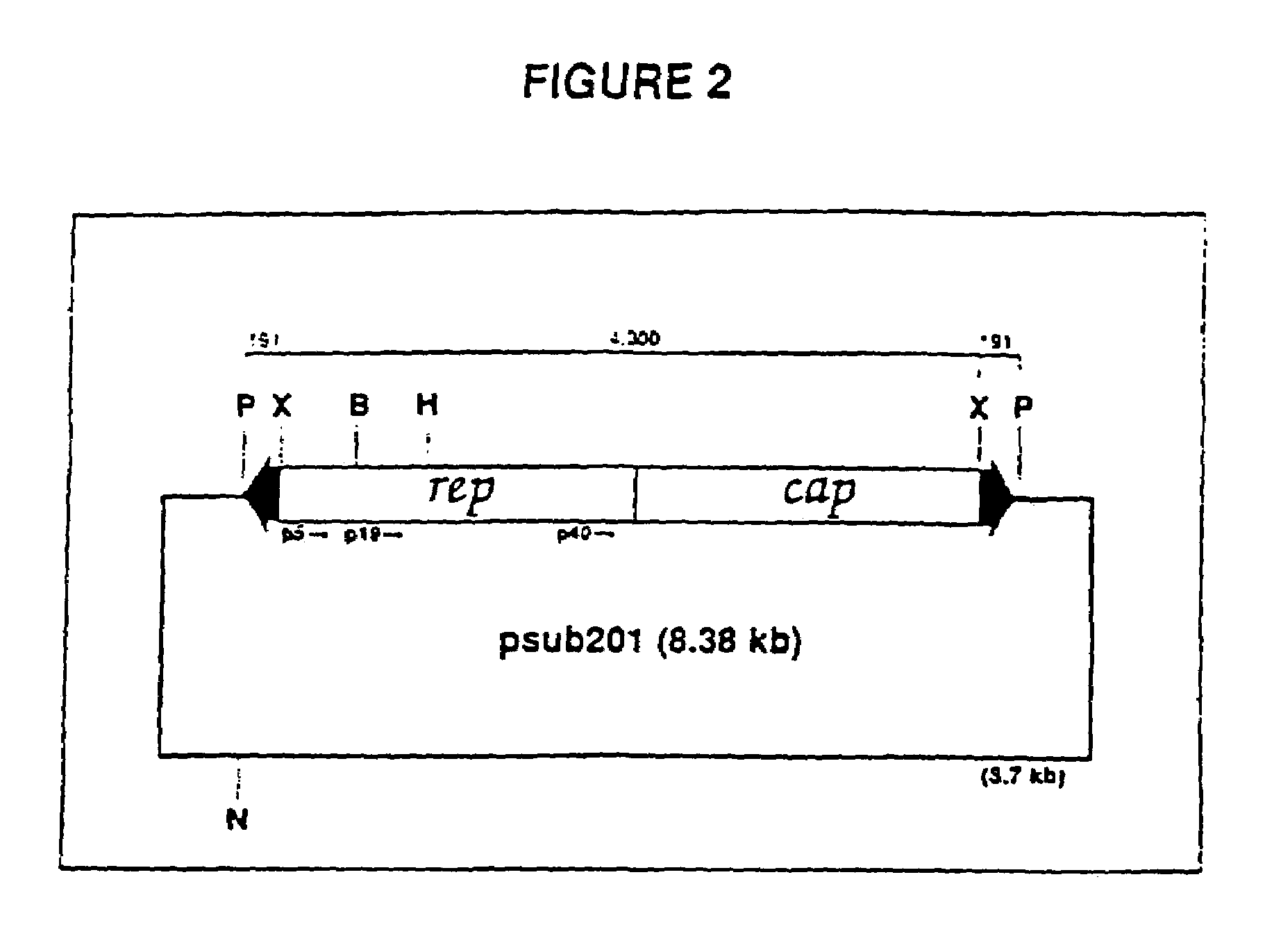
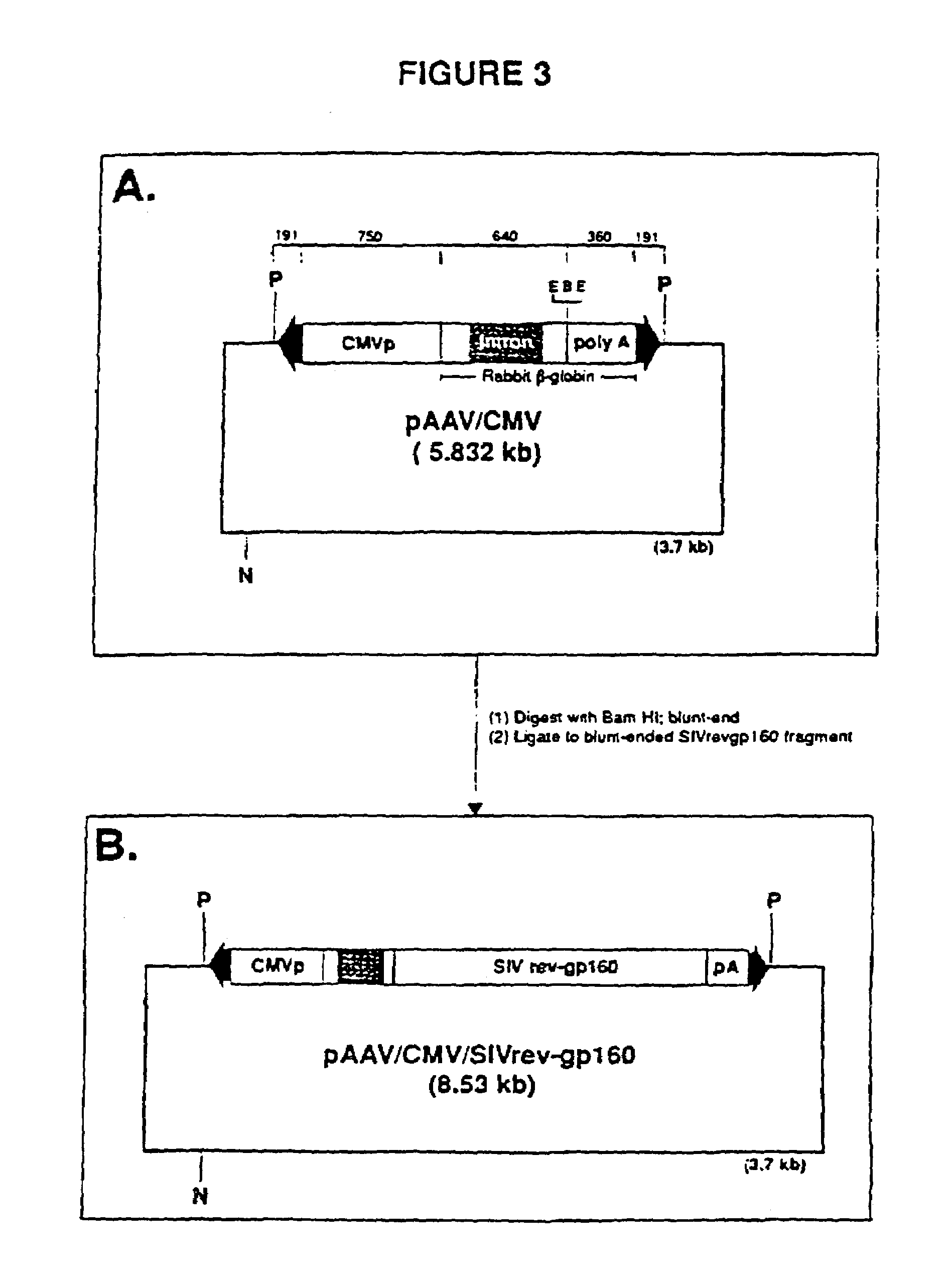
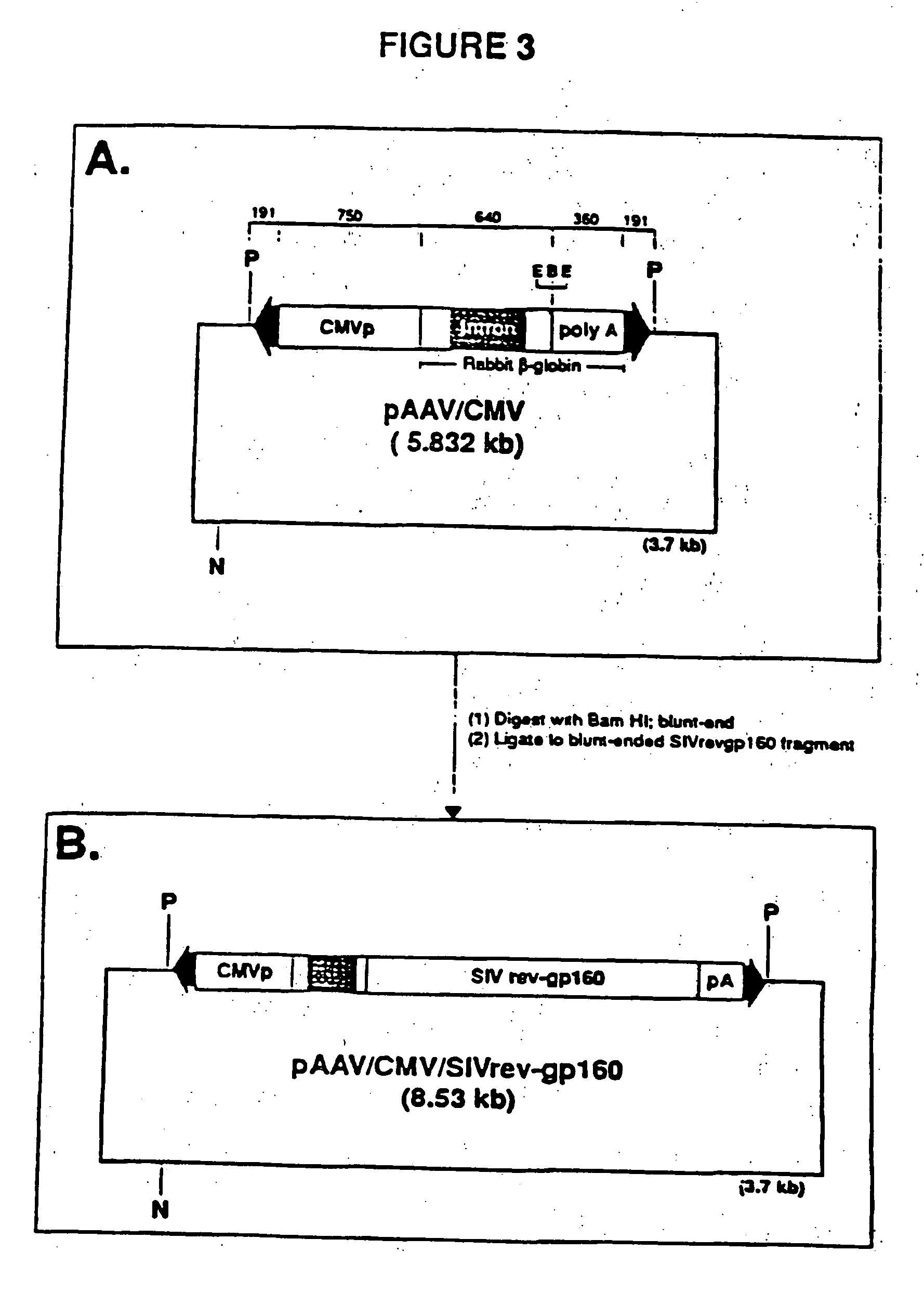
Adeno-associated virus materials and methods
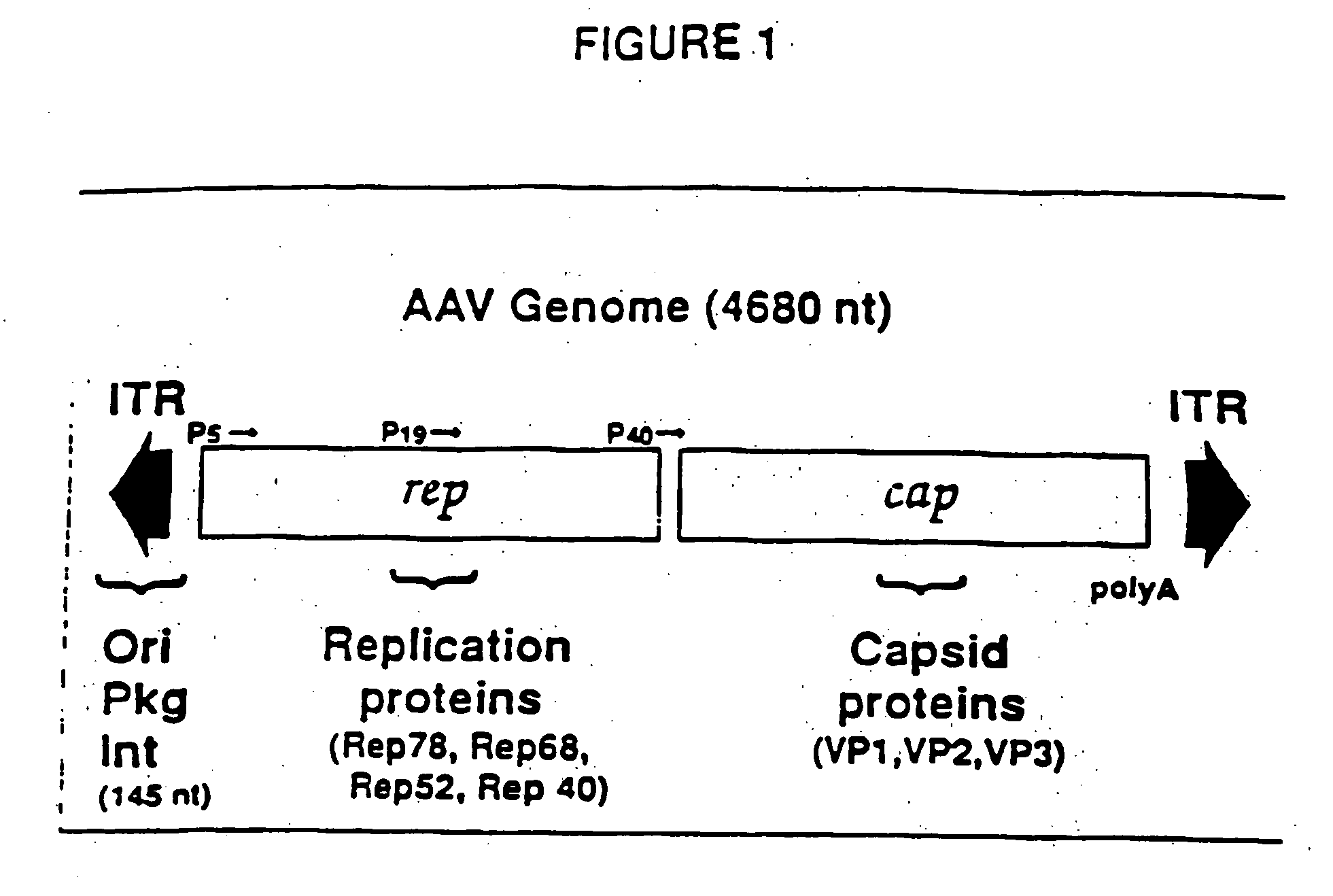
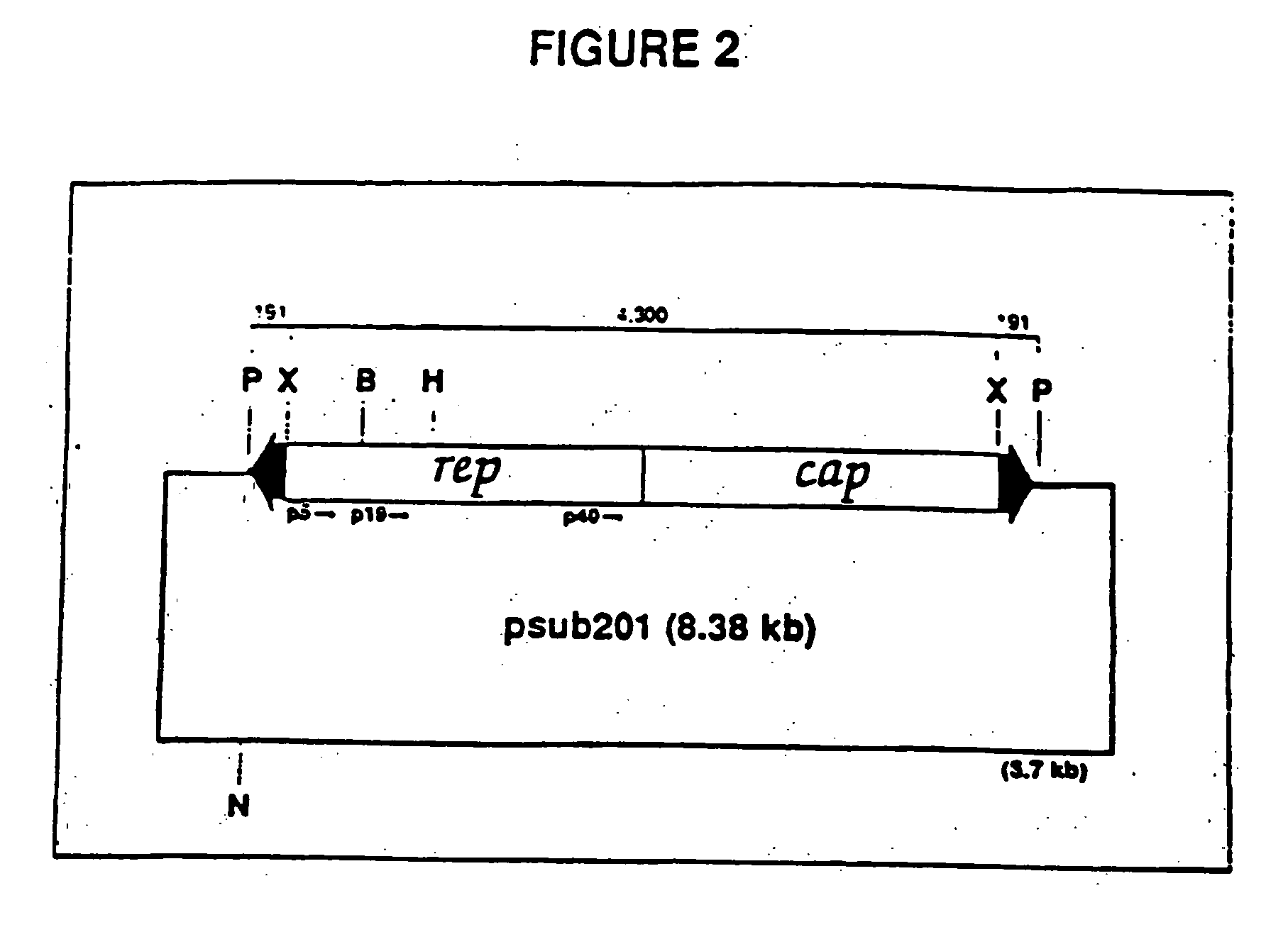
The present invention provides adeno-associated virus (AAV) materials and methods which are useful for DNA delivery to cells. More particularly, the invention provides recombinant AAV (rAAV) genomes, methods for packaging rAAV genomes, stable host cell lines producing rAAV and methods for delivering genes of interest to cells utilizing the rAAV. Particularly disclosed are rAAV useful in generating immunity to human immunodeficiency virus-1 and in therapeutic gene delivery for treatment of neurological disorders.
Owner:CHILDRENS HOSPITAL
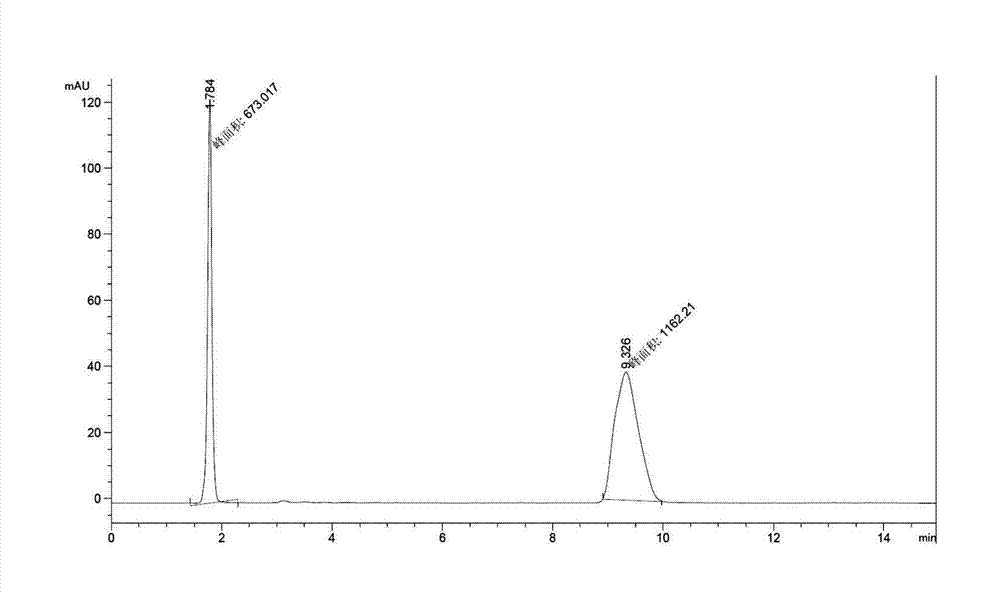
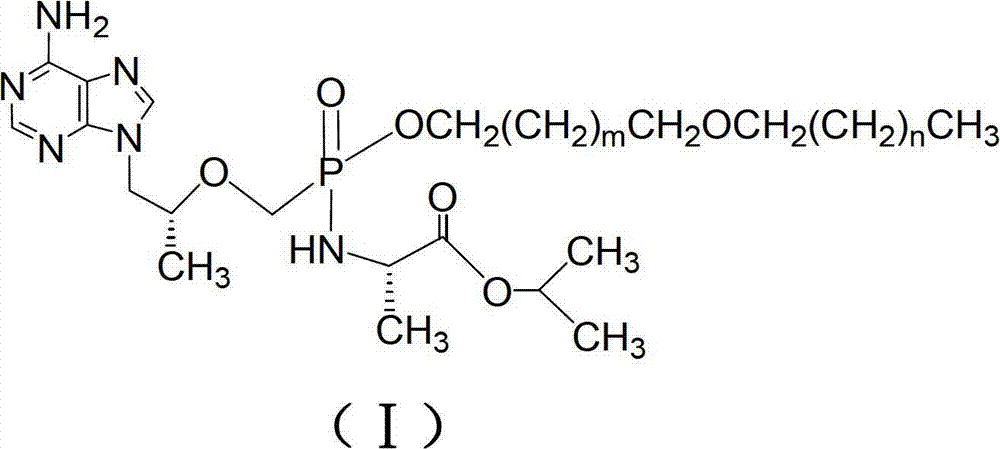
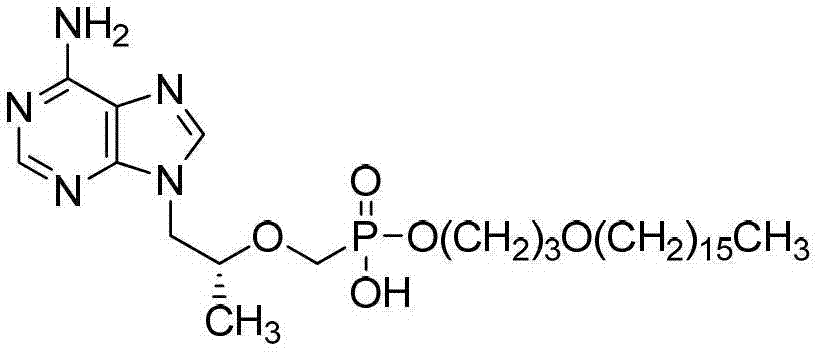
Tenofovir diester compounds with activity of inhibiting HIV-1 (human immunodeficiency virus-1) virus replication and preparation method and pharmaceutical use thereof
ActiveCN102786549AHigh activityLow cytotoxicityOrganic active ingredientsGroup 5/15 element organic compoundsTherapy HIVHuman immunodeficiency virus 1
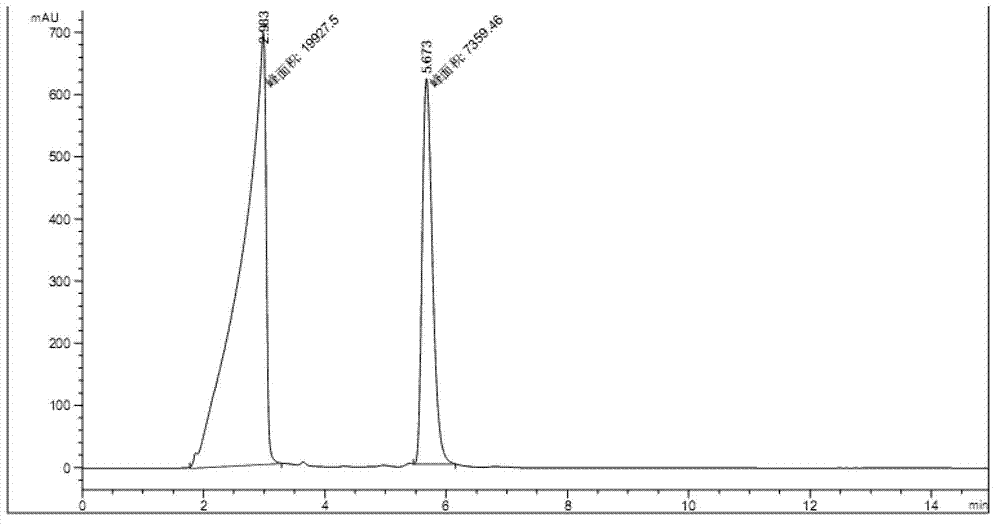
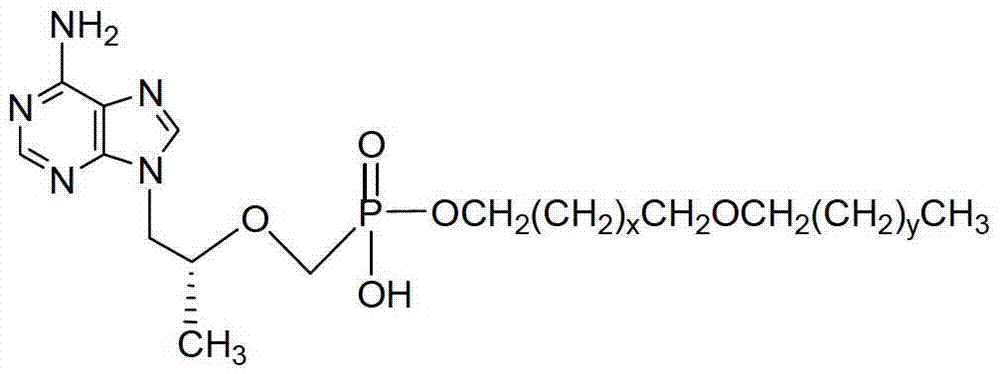
The invention discloses tenofovir diester compounds with activity of inhibiting HIV-1 (human immunodeficiency virus-1) virus replication and a preparation method and pharmaceutical use thereof. The structure of the compounds is shown in Formula (I), wherein m is 0-4, n is 12-16, and the structural formula is fixed when m is 1 and n is 14. The invention also discloses a preparation method of the compounds shown in the structural formula (I) and a pharmaceutical composition with the compounds. Tests show that the compounds have the activity of inhibiting HIV-1 replication and also much higher lipophilicity than the current HIV treatment drug tenofovir fumarate, and can be applied in development of drugs for treatment of HIV infection.
Owner:洛阳聚慧新材料科技有限公司 +2
Modulators of viral transcription, and methods and compositions therewith
The present invention is directed to a process for inhibiting the replication of human immunodeficiency virus-1 (HIV-1), by contacting a cell with at least one compound according to Formula I.The substituent groups R1, R2, R3, X, Y, Z, A and B are as defined above. Also contemplated is a method for treating or preventing a HIV-1 infection in a subject, by administering a therapeutically effective amount of at least one compound according to Formula I, as well as a method for modulating the activity of a cyclin dependent kinase (cdk) in a cell infected with HIV-1 using a Formula I compound.
Owner:GEORGE MASON UNIVERSITY
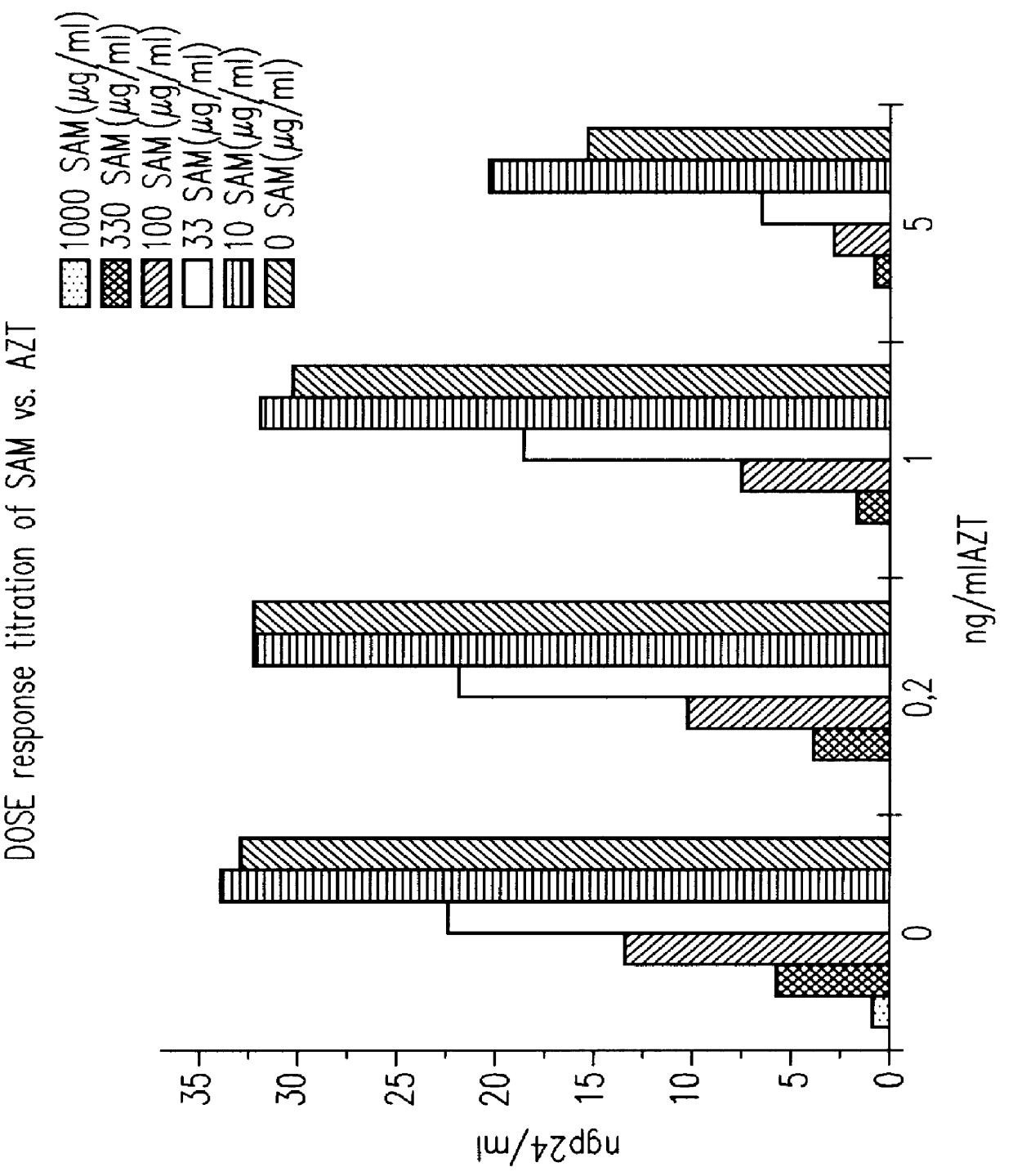
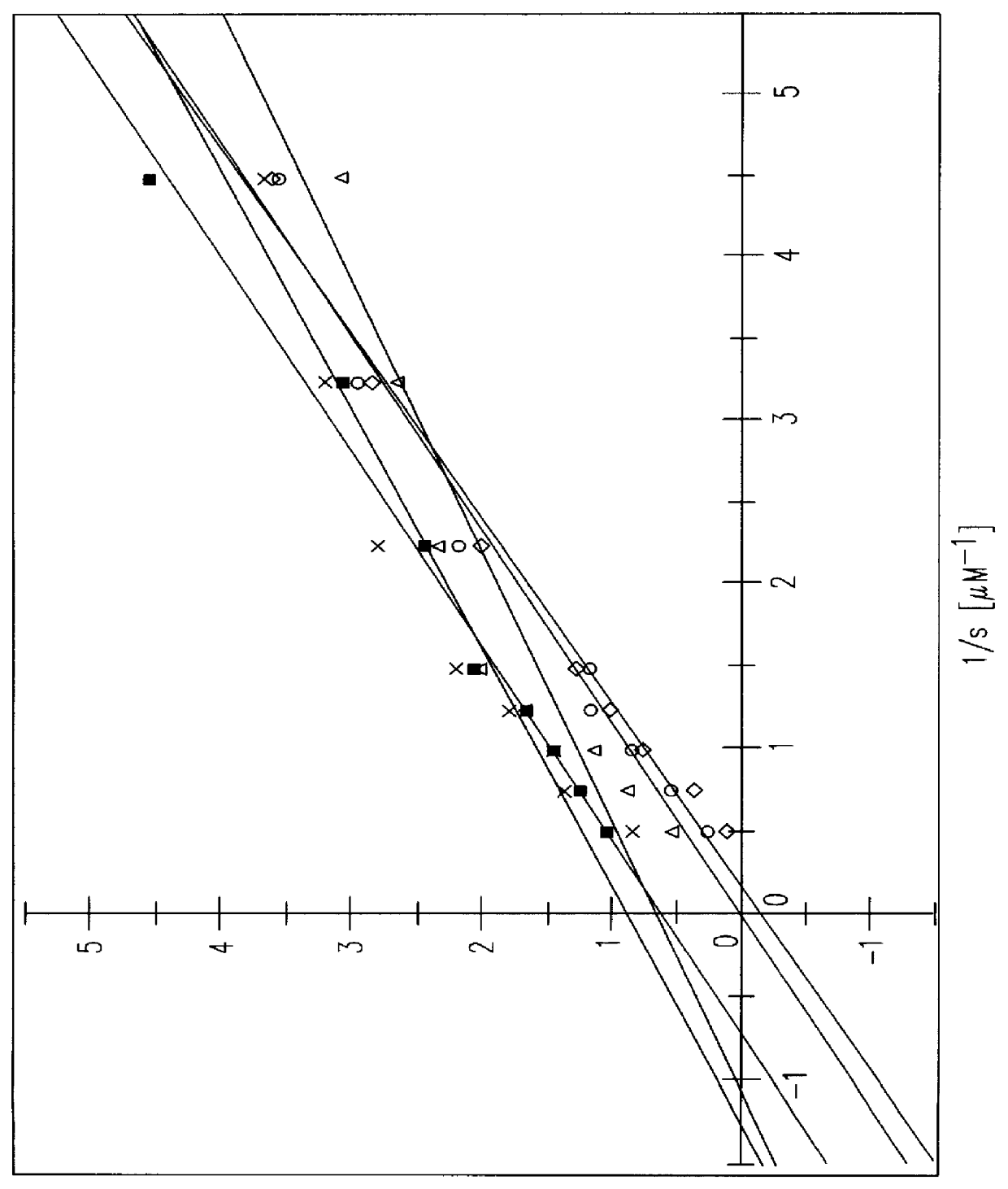
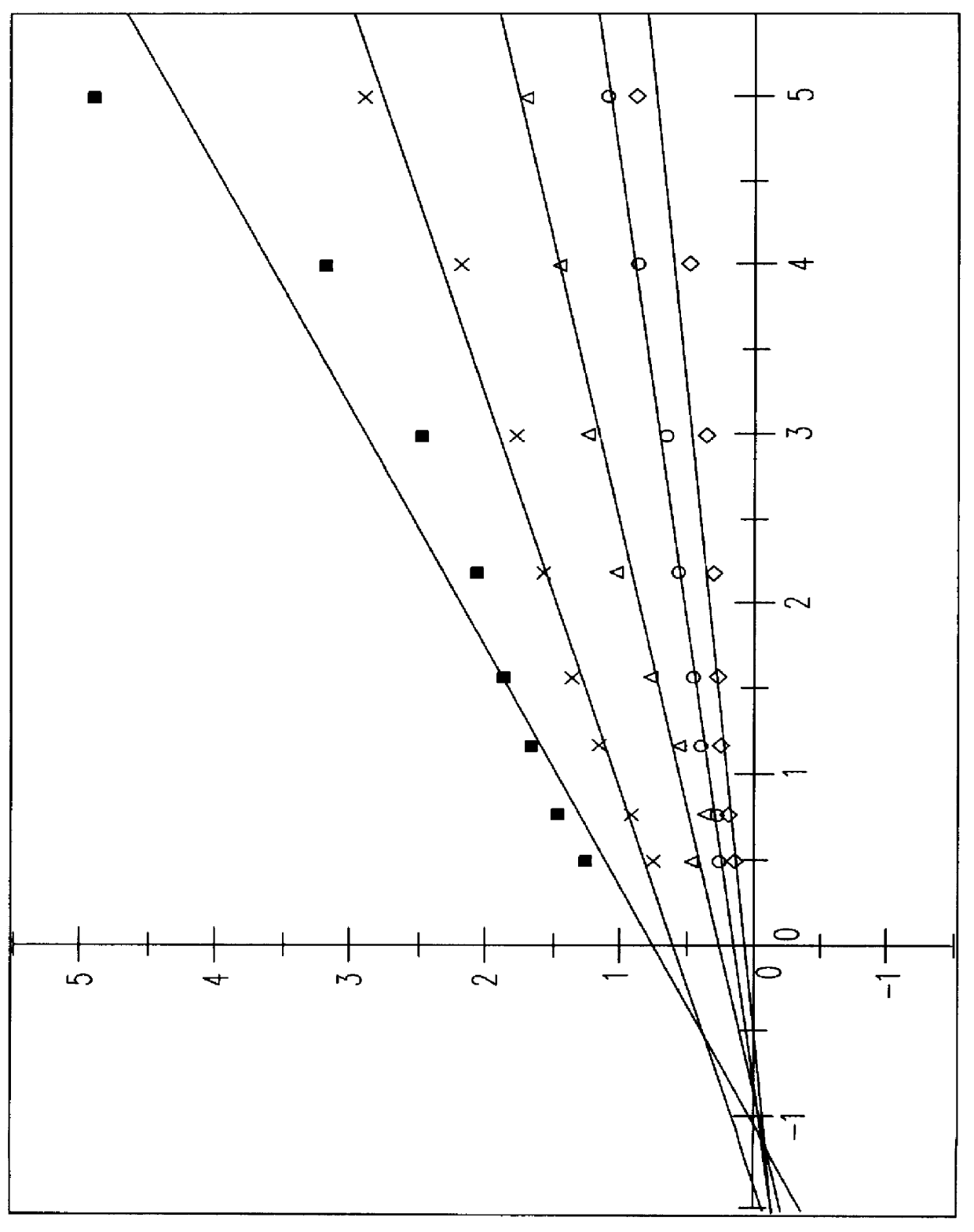
S-(+)-adenosylmethionine and 3'-azido-2', 3'-dideoxy-nucleoside complexes as potent inhibitors of HIV-replication
Molecular Complexes, comprising of S-(+)-adenosylmethionine and 3'-azido-2',3'-dideoxy nucleosides are prepared, and shown to have synergistic inhibitory effects on the replication of human-immunodeficiency virus 1 & 2 in vitro and in vivo, particularly on the reverse transcriptase, and having a high therapeutic index.
Owner:SYMBIO HERBORN GRP GMBH & CO
Methods to prevent vertical transmission of infectious diseases
InactiveUS20080269205A1Prevent vertical transmissionPrevent the spread of diseaseAntiviralsRadiation therapyEscherichia coliMonilinia laxa
The present invention presents a method of preventing vertical transmission of an infectious disease comprising: (a) applying a photosensitizing composition to host tissues of birth canal of a mother during the intrapartum period; and (b) applying light to the host tissues after the step (a) at a wavelength absorbed by the photosensitizing composition so as to inhibit or eliminate infectious disease microorganisms that come into contact with the host tissues. The infectious disease may be caused by human immunodeficiency virus type 1, hepatitis B virus, hepatitis C virus, Group B Streptococcus, cytomegalovirus, Listeria monocytogenes, Chiamydia trachomatis, Escherichia coli, herpes simplex virus, Epstein-Barr virus, Toxoplasma gondii, human papilloma virus, and Candida.
Owner:ONDINE INT
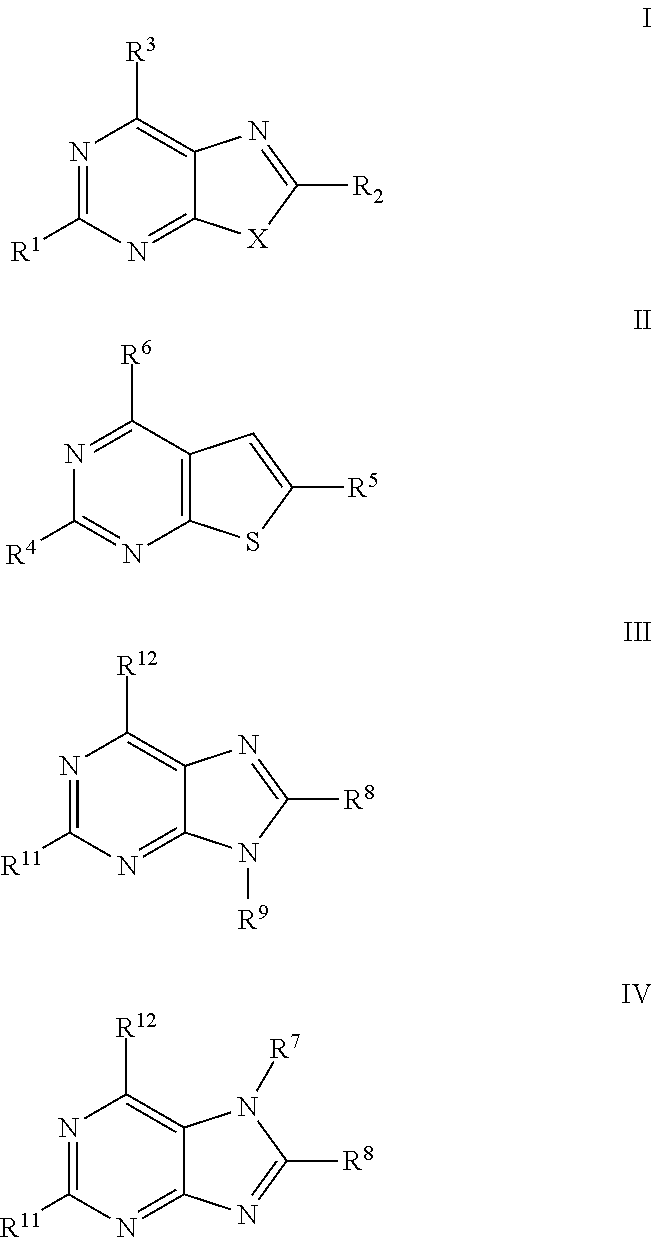
Antiviral Activity of Novel Bicyclic Heterocycles
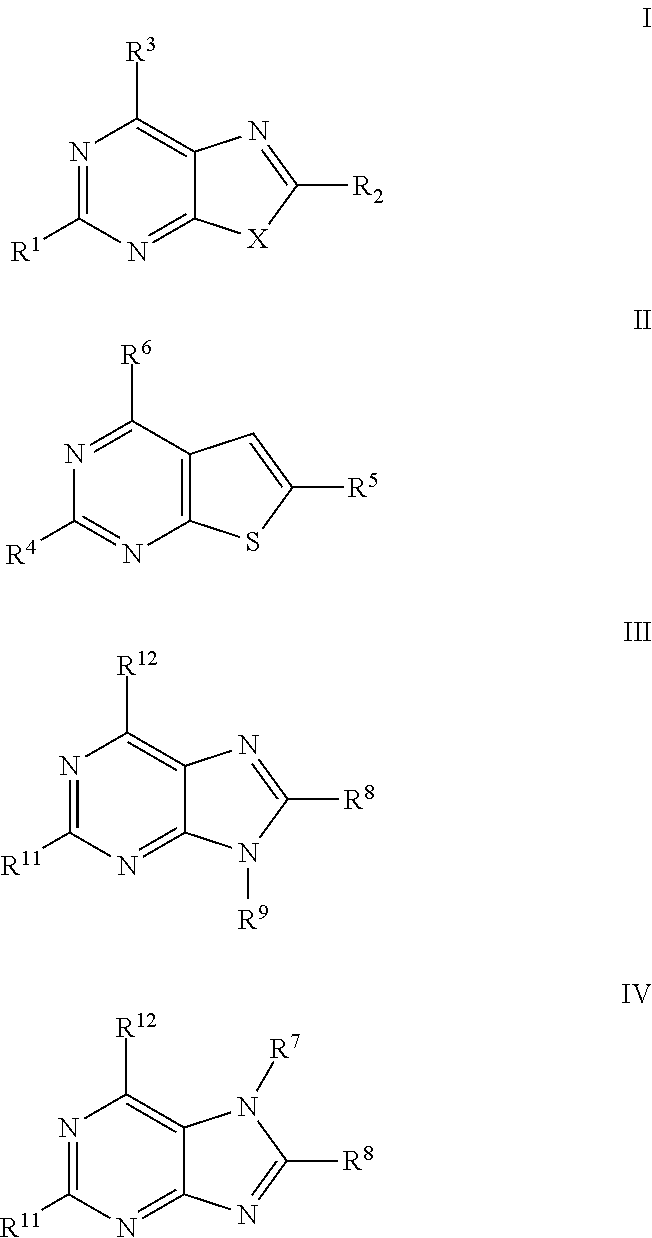
The present invention relates to compound of Formula I, II, III, or IV, and / or a pharmaceutical acceptable addition salt thereof and / or a stereoisomer thereof and / or a solvate thereof, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R11, and R12 are as defined in the claim 1 or as described in detail in the description of the invention, and to the use of said compounds to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections, particularly infections with RNA-viruses belonging to the family of the Retroviridae, the family of the Flaviviridae and the family of the Picornaviridae and more preferably infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus. The present invention also relates to pharmaceutical compositions of said compounds and the use of said pharmaceutical compositions to treat or prevent viral infections. The present invention further relates to the use of said compounds as biologically active ingredients, more specifically as medicaments for the treatment of viral disorders and pathologic conditions such as, but not limited to, viral infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus.
Owner:KATHOLIEKE UNIV LEUVEN
Borneol-curcumin liposome and preparation method and application thereof
ActiveCN102895188AImprove central drug effectImprove bioavailabilityNervous disorderKetone active ingredientsHydration reactionRotary evaporator
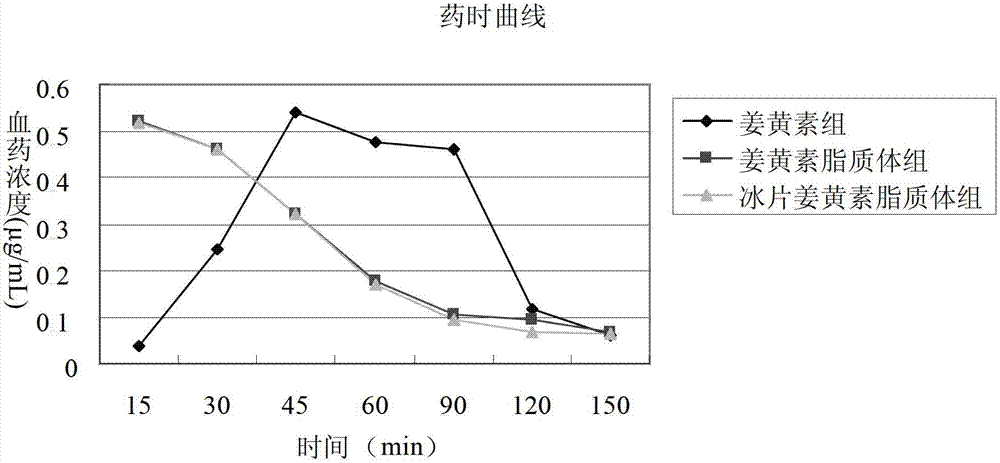
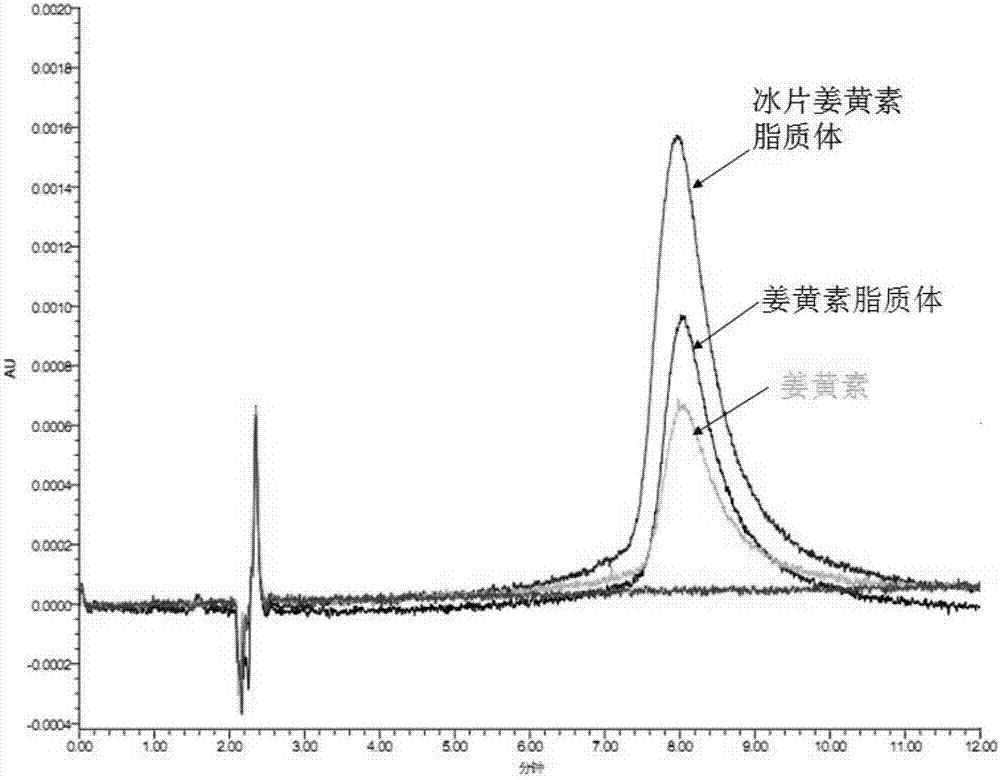
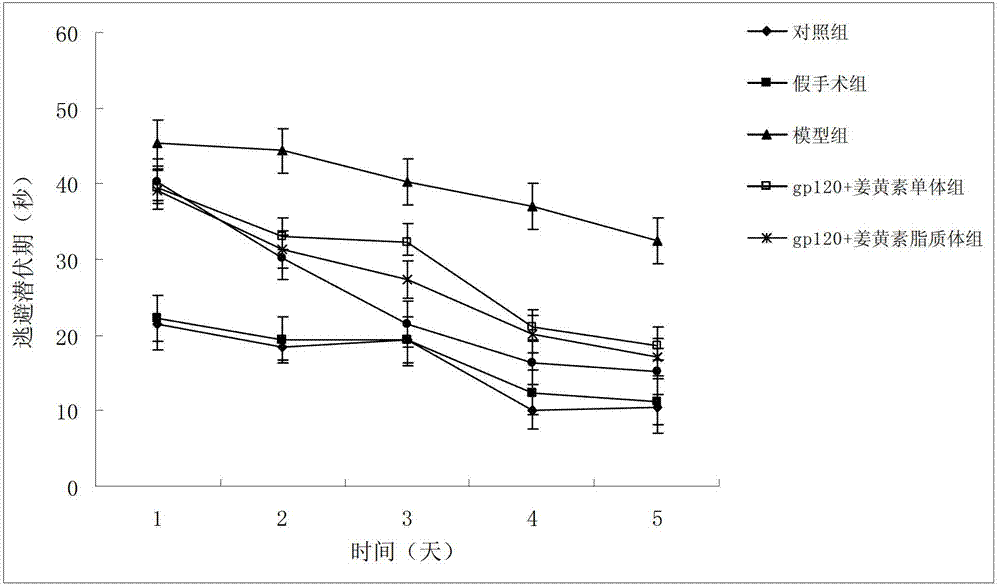
The invention belongs to the medicine field and provides a borneol-curcumin liposome, a preparation method of the borneol-curcumin liposome and an application of the borneol-curcumin liposome in preparing medicines for preventing human immunodeficiency virus-1(HIV-1) associated neurocognitive disorder. The borneol-curcumin liposome is prepared by, by mass, 270-330 parts of lecithin, 90-110 parts of cholesterol, 0.9-1.1 parts of curcumin and 0.09-0.11 part of borneol. The preparation method includes that the components and 0-11 parts by mass of oxidation protection agents are dissolved by absolute ethyl alcohol, a mixture is subjected to rotary evaporating on a rotary evaporator until ethanol is volatilized to form uniform films, a defined amount of phosphate buffer solution is added, the incubation is performed for three hours, a hydration reaction is undertaken to form an emulsion, then the ultrasound is performed for 10 minutes, and the emulsion passes through a 0.45 micrometer millipore filter to obtain uniform particles so that the borneol-curcumin liposome is obtained. The borneol-curcumin liposome can be prepared to various dosage forms of HIV-associated neurocognitive disorder (HAND) resistant medicines which are peroral, and the pertinence of the borneol-curcumin liposome for treating HAND is high, and the effect is good.
Owner:JINAN UNIVERSITY
Human immunodeficiency virus-1NASBA-ELISA (enzyme-linked immunosorbent assay) detection kit
InactiveCN103540684AGood repeatabilityShort detection time periodMicrobiological testing/measurementDNA/RNA fragmentationImmunodeficiency virusLong terminal repeat
The invention relates to a gene detection kit against human immunodeficiency virus-1 (HIV-1). The gene detection kit comprises a pair of amplification primers, a capture probe, a detection probe, an NASBA (National Association of State Boards of Accountancy) amplification reagent and an NASBA product detection reagent. The gene detection kit provided by the invention designs the primers against the long terminal repeat sequence of the HIV-1, establishes an NASBA fast detection method and is suitable for early diagnosis of HIV-1 infection.
Owner:薛健 +1
Jurkat-KI-R5 cell line and construction method and application thereof
ActiveCN106995821AEfficient knockoutSusceptible to infectionGenetically modified cellsPeptidesAIDS StudyHuman immunodeficiency virus 1
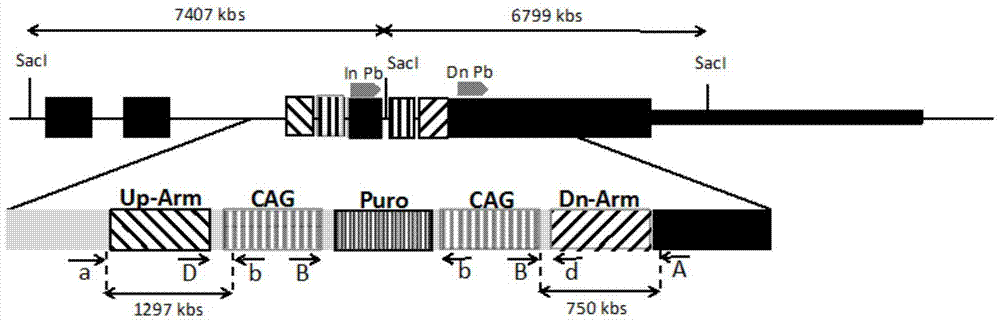
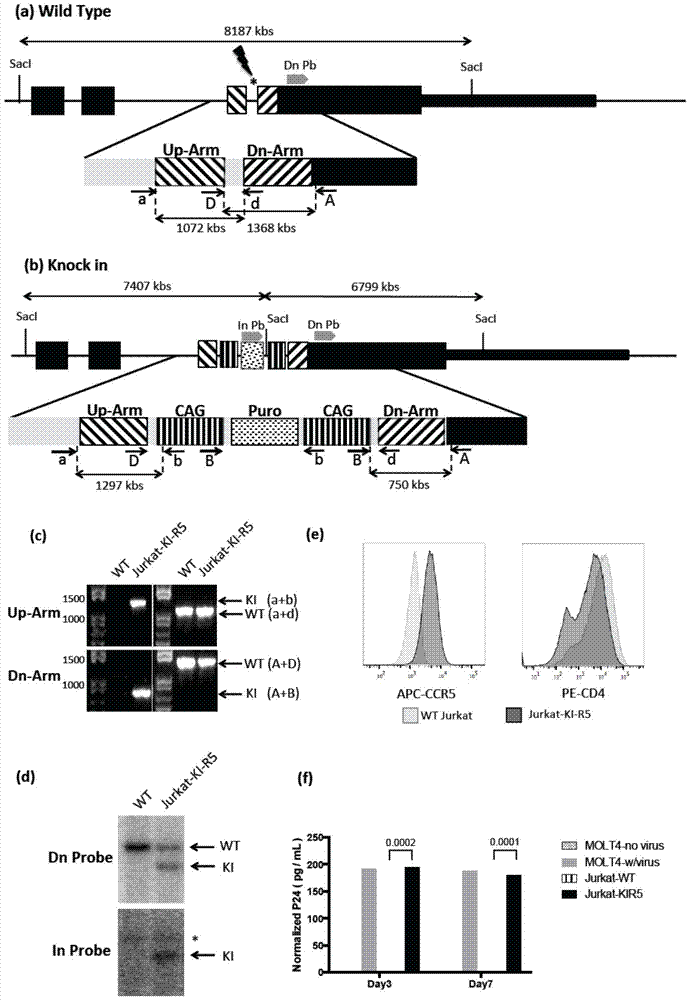
The invention relates to Jurkat-KI-R5 and a construction method and application thereof. A powerful enhancer / starter combination (CAG) is knocked into a starter area of a CCR5 gene of a CD4+Jurkat T cell by a CRISPR / Cas9 gene editing technique in a homologous recombination way; the name of the gene-modified cell system is Jurkat-KI-R5 cell. The Jurkat-KI-R5 cell has the advantages that a CAG starter of the Jurkat-KI-R5 is precisely recombined into the specified location of a CCR5 starter, and the high expression of the CCT5 is stably caused; oppisite to a maternal line Jurkat cell, the Jurkat-KI-R5 cell is easily infected by HIV-1 (human immunodeficiency virus-1); a cell platform urgently demanded by the HIV disease study is provided by the Jurkat-KI-R5 cell.
Owner:SHENZHEN CHILDRENS HOSPITAL
Nonnucleoside inhibitors of reverse transcriptase, composite binding pocket and methods for use thereof
InactiveUS20050153995A1Inhibition of RT activityPotent anti-HIV activityBiocideOrganic chemistryImmunodeficiency virusNon nucleoside inhibitor
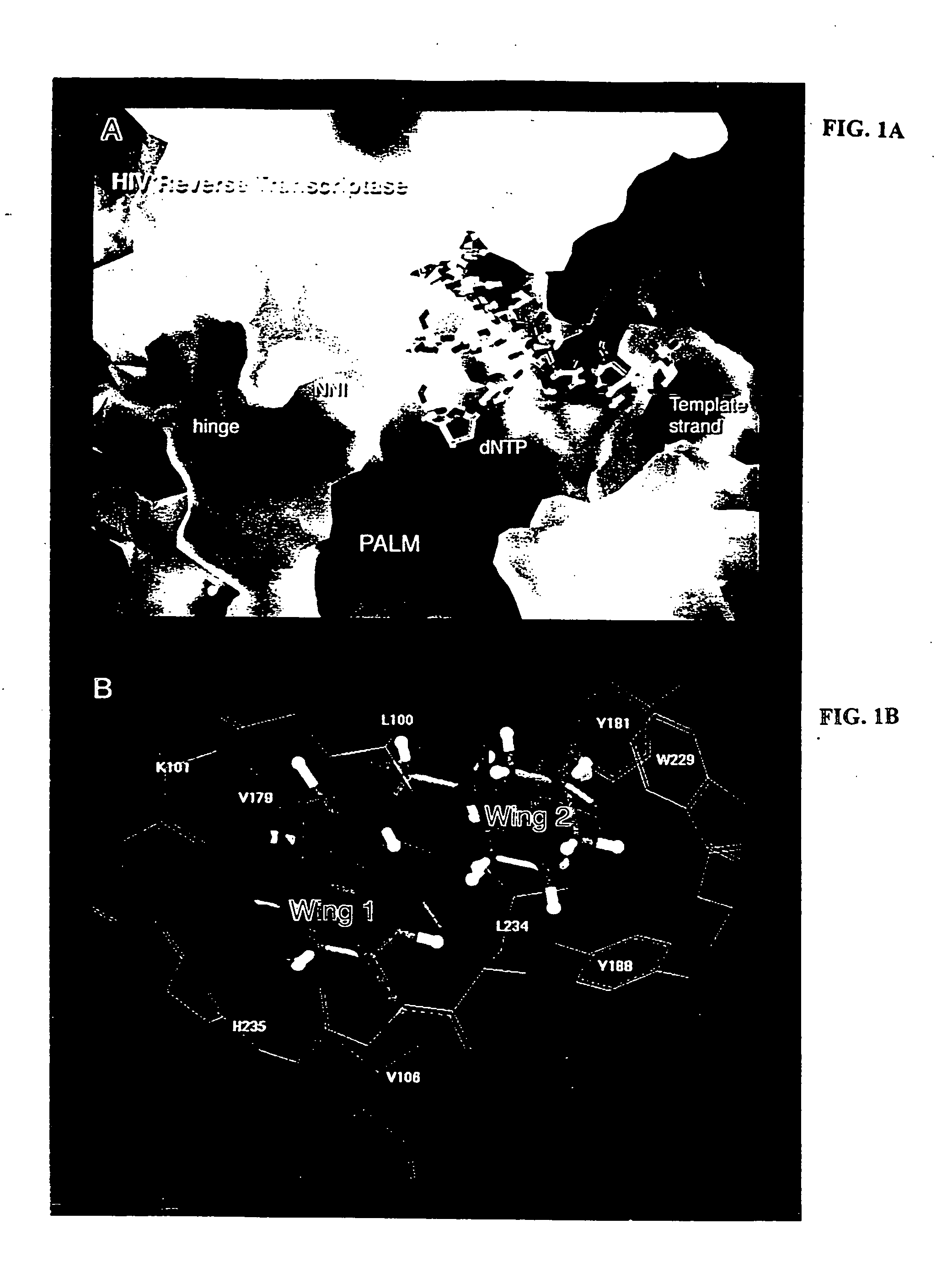
Novel compounds that are potent inhibitors of HIV reverse transcriptase (RT) are described in the invention. Thes novel compounds also inhibit replication of a retrovirus, such as human immunodeficiency virus-1 (HIV-1). The novel compounds of the invention include analogs and derivatives of phenethylthiazolylthiourea (PETT), of dihydroalkoxybenzyloxopyrimidine (DABO), and of 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine (HEPT). The invention additionally provides a composite HIV reverse-transcriptase (RT) nonnucleoside inhibitor (NNI) binding pocket constructed from a composite of multiple NNI-RT complexes The composite RT-NNI binding pocket provides a unique and useful tool for designing and identifying novel, potent inhibitors of reverse transcriptase.
Owner:PARKER HUGHES INST
Tenofovir disoproxil fumarate compounds, preparation method and application to antiviral field
ActiveCN103242366AImprove attributesIncreased toxicityOrganic active ingredientsGroup 5/15 element organic compoundsSolubilityTherapy HIV
The invention discloses a group of tenofovir disoproxil fumarate compounds with activity for inhibiting HIV(human immunodeficiency virus) / HBV(Hepatitis B Virus) replication, a preparation method and pharmaceutical application of the group of tenofovir disoproxil fumarate compounds. The compounds have a formula I, wherein X=H, Y=H; R1=-CH2(CH2)mCH2OCH2(CH2)nCH3, wherein m ranges from 0 to 4, and n ranges from 10 to 20; and R2=-OCH2OC(O)OCH(CH3)2. The invention also discloses a pharmaceutical composition containing the compounds. Shown by the experiment, the activity of one of the compounds for inhibiting the replication of HIV-1 (Human Immunodeficiency Virus-1) is 4.5 times that of azidothymidine (AZT), about 250 times that of the tenofovir disoproxil fumarate (TDF) which is the best medicine for treating acquired immunodeficiency syndrome at present, and 1.37 times that of the medicine CMX157 (Cefmenoxime) which comes into the clinical stage, and the lipid solubility of the compound is about 2 times that of the CMX157; the compounds also have the activity for inhibiting the replication of HBV (Hepatitis B Virus); and the compounds can be applied to the development of medicines for treating the acquired immune deficiency syndrome / hepatitis B.
Owner:洛阳聚慧新材料科技有限公司 +2
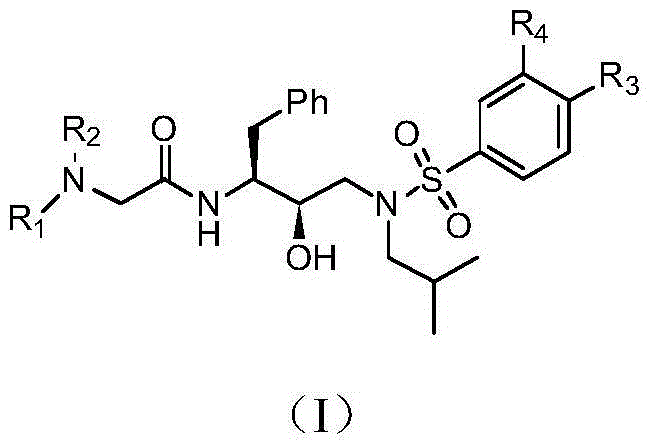
Tertiary amine analogical peptide derivative and application of tertiary amine analogical peptide derivative in inhibiting HIV-1 protease
The invention belongs to the technical field of medicine and relates to a tertiary amine derivative as shown as the general formula I, and pharmaceutically acceptable salt or a prodrug of the tertiary amine derivative. Results of experimental studies show that the tertiary amine derivative has higher capacity of inhibiting the activity of HIV-1 (human immunodeficiency virus-1) protease and is expected to be developed into an effective drug for resisting Aids.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
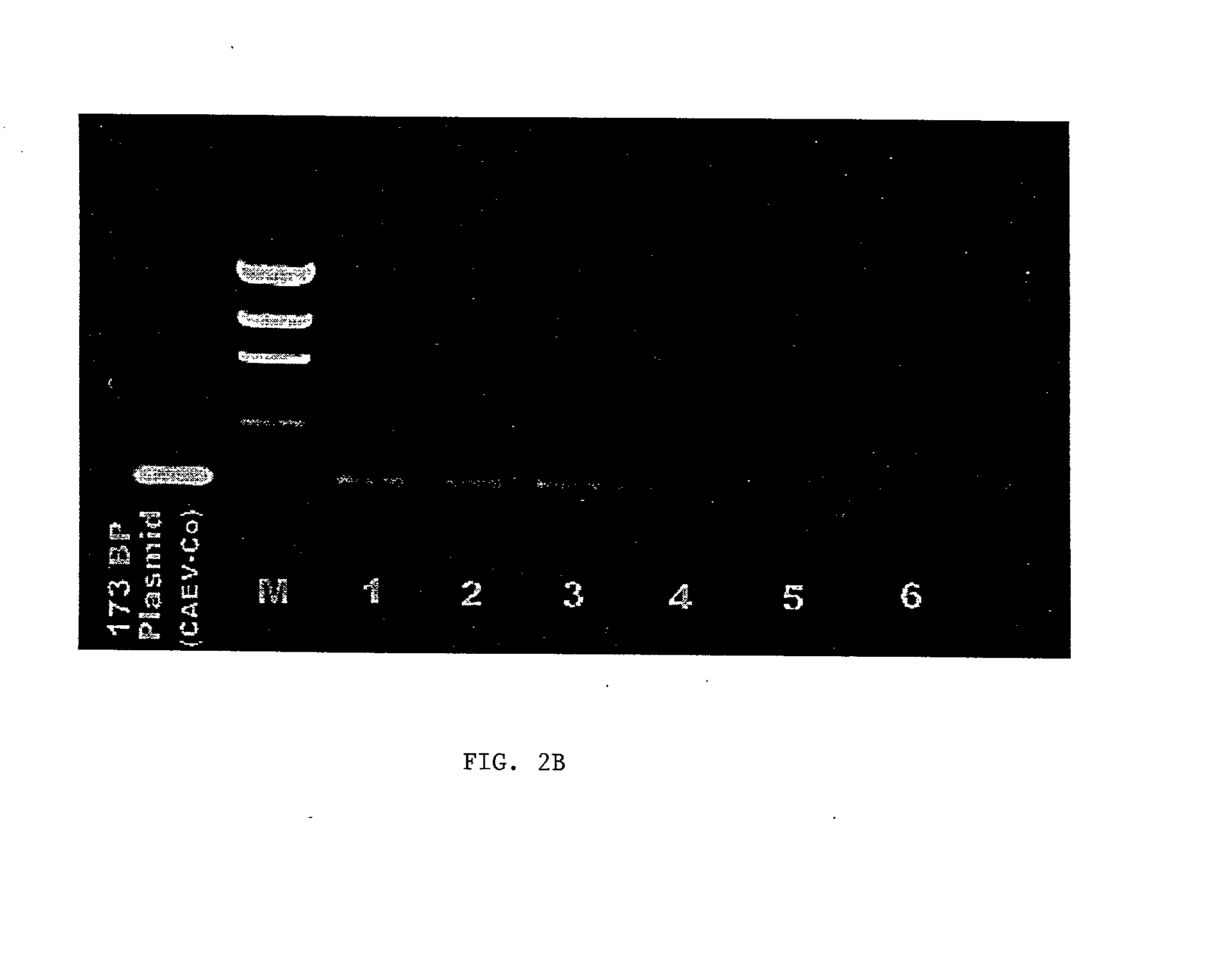
Viral chimeras comprised of caev and hiv-1 genetic elements
InactiveUS20010039669A1Virus peptidesImmunoglobulins against virusesVirus-RetrovirusImmunodeficiency virus
The present invention provides a polynucleotide comprising portions of the genomes of caprine arthritis-encephalitis virus and HIV-1, resulting in a chimeric retrovirus referred to as a "CHIV." The invention also provides a vaccine comprising a CHIV immunogen and a pharmaceutically acceptable carrier. A method of stimulating an immune response in an individual against human immunodeficiency virus-1 infection by administering a therapeutically effective amount of a CHIV immunogen is also provided. The invention further provides a method of stimulating an immune response in vitro by contacting a lymphocyte with a therapeutically effective amount of a CHIV immunogen.
Owner:UNIV OF SOUTHERN CALIFORNIA
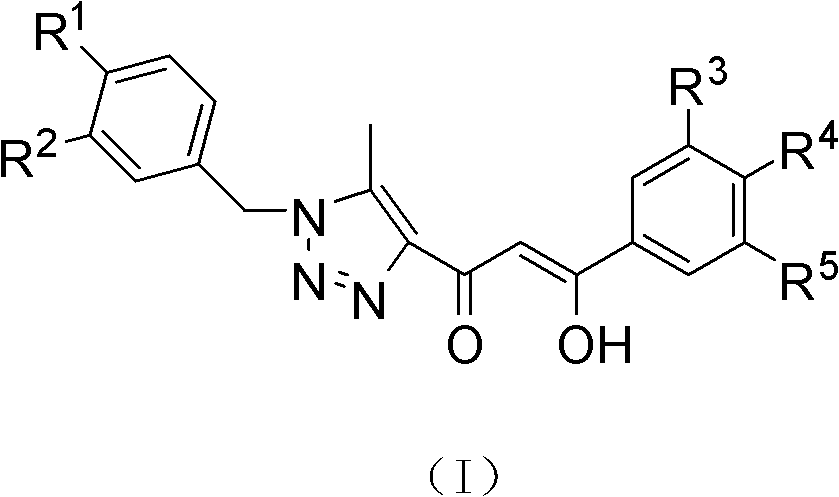
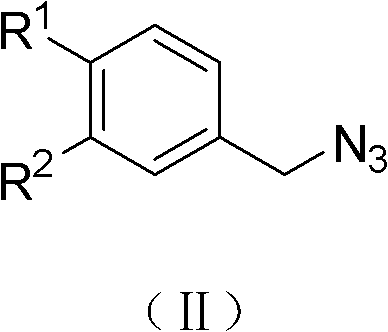
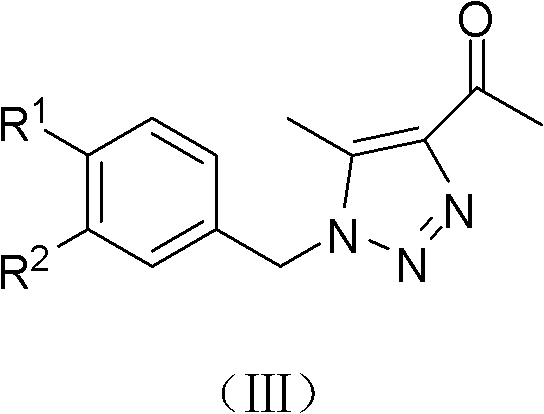
(Z)-1-(1-substituted benzyl-5-methyl-1H-1,2,3-triazole-4-yl)-3-substituted benzyl-3-hydroxy-2-propylene-1-ketone compound and preparation method and application thereof
InactiveCN102503900AMild reaction conditionsOrganic active ingredientsOrganic chemistryBenzoyl bromideCarboxylic acid
Owner:BEIJING UNIV OF TECH
Method for detection of HIV-1 nucleoside inhibitor drug resistance mutation and kit
InactiveCN103215378AHigh degree of integrationHigh sensitivityMicrobiological testing/measurementMicroorganism based processesNucleoside Reverse Transcriptase InhibitorImmunodeficiency virus
The invention relates to a method for detection of HIV-1 (human immunodeficiency virus-1) nucleoside inhibitor drug resistance mutation and a kit thereof. Specifically, the invention discloses a specific probe for detection of human immunodeficiency virus nucleoside reverse transcriptase inhibitor drug resistance mutation, and the probe includes at least one of: a probe for detection of reverse transcriptase gene M41L mutation, a probe for detection of reverse transcriptase gene A62V mutation, a probe for detection of reverse transcriptase gene K65R mutation, a probe for detection of reverse transcriptase gene D67N mutation, a probe for detection of reverse transcriptase gene 69INS insertion mutation, a probe for detection of reverse transcriptase gene K70R mutation, a probe for detection of reverse transcriptase gene L74V mutation, a probe for detection of reverse transcriptase gene V75I mutation, a probe for detection of reverse transcriptase gene F77L mutation, a probe for detection of reverse transcriptase gene Y115F mutation, a probe for detection of reverse transcriptase gene F116Y mutation, and the like.
Owner:ZHEJIANG UNIV
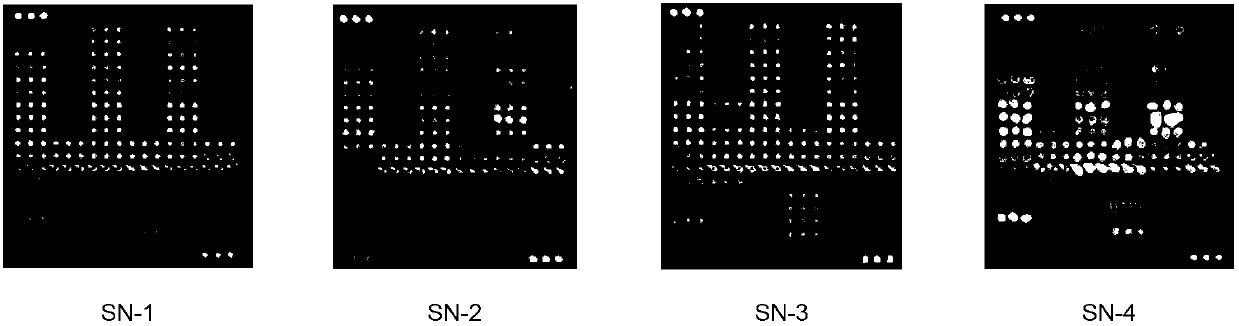
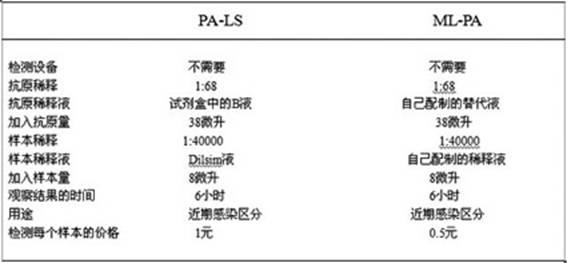
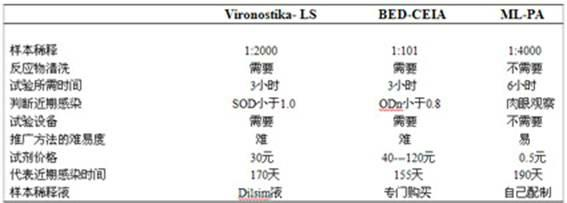
Blood serum or blood plasma diluting solution applied to detection method of trace gelatin particle agglutinated HIV-1 (Human Immunodeficiency Virus-1) recent infection
InactiveCN102608312ARealize independent productionRespond effectivelyMaterial analysisMedicineBovine serum albumin
The invention provides a blood serum or blood plasma sample diluting solution applied to a method for detecting trace gelatin particle agglutinated HIV-1 (Human Immunodeficiency Virus-1) recent infection, belonging to the field of a biological technology. The invention particularly provides a reagent applied to detecting the HIV-1 recent infection. The diluting solution is prepared from a buffering solution, TritonX100 with the concentration of 30%, NaN3 and bovine serum albumin by mixing according to a ratio. The diluting solution disclosed by the invention can completely replace a DilsimTM11 diluting solution and can be used for a detection method of the trace gelatin particle agglutinated HIV-1 recent infection.
Owner:李洪
A kind of camel velvet transfer protein and its preparation method and application
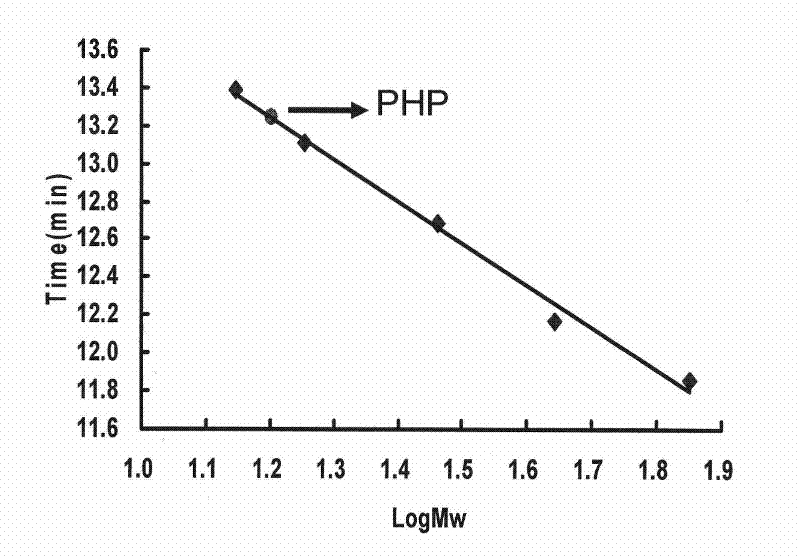
InactiveCN102286085AGood antifungal activityEnhanced inhibitory effectBiocidePeptide/protein ingredientsLipid formationAntifungal
The invention discloses a peganum harmala lipid transfer protein (PHP) and relates to the preparation and use of new protein with antifungal, anticancer and antivirus activities. The method uses seeds of a peganum harmala plant as raw materials and comprises the following steps: preparing a coarse product, performing cationic exchange chromatographic separation and purifying by using a molecular sieve; and gradually separating and collecting a purified component having antibacterial activity, dialyzing, desalting, freeze-drying and thus, obtaining purified PHP. In the invention, a filter paper disc agar diffusion method is adopted, and researches prove the protein has growth inhibiting effect on five plant pathogenic fungi, namely alternaria alternate, penicillium degitatum, rhizopus stuolonifer, magnaporthe grisea and penicillium italicum. An 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method proves that the protein has inhibiting effect on proliferation of human esophageal carcinoma cell Eca109, human cervical cancer cells HeLa, human stomach cancer cells MGC-7, mouse melanoma cells B16 and the like. The protein can inhibit the activity of human immunodeficiency virus 1 (HIV-1) reverse transcriptase. The protein can be used for developing medicines for resisting agricultural pathogenic fungi, tumors and viruses.
Owner:XINJIANG UNIVERSITY
Adeno-associated virus materials and methods
The present invention provides adeno-associated virus (AAV) materials and methods which are useful for DNA delivery to cells. More particularly, the invention provides recombinant AAV (rAAV) genomes, methods for packaging rAAV genomes, stable host cell lines producing rAAV and methods for delivering genes of interest to cells utilizing the rAAV. Particularly disclosed are rAAV useful in generating immunity to human immunodeficiency virus-1 and in therapeutic gene delivery for treatment of neurological disorders.
Owner:NATIONWIDE CHILDRENS HOSPITAL
Mesenchymal stem cell as well as establishing method and application of mesenchymal stem cell
ActiveCN104212766AEasy to getWeak implant responseGenetic engineeringFermentationHuman immunodeficiency virus 1Gp41
The invention relates to a mesenchymal stem cell. The cell carries an HIV-1 (Human Immunodeficiency Virus-1) receptor and an HIV-1 coreceptor. When BMSCs (Bone Marrow Mesenchymal Stems) are infected by HIV-1, an envelope protein gp120 on HIV-1 is combined with the HIV-1 receptor to ensure that gp41 on HIV-1 is exposed, the exposed gp41 is combined with the HIV-1 coreceptor to mediate HIV-1 to enter the BMSCs, and the reverse transcription and replication conditions of HIV-1 in the BMSCs are researched, so that the pathogenesis and pathological characteristics of HIV-1 / AIDS (Acquired Immune Deficiency Syndrome) and HIV-1-resistant drugs are researched.
Owner:GUILIN MEDICAL UNIVERSITY
Application of cortex magnolia officinal extract in preparing medicine for healing and preventing acquired immune deficiency syndrome
InactiveCN103127040AInhibitory activityHigh inhibition rateHydroxy compound active ingredientsAntiviralsHonokiolChemical compound
The invention provides application of lignin-class chemical compounds in preparing medicine for healing and preventing acquired immune deficiency syndrome, wherein the lignin-class chemical compounds are 6'-O-methyl honokiol, Randaiol, Magnolignan C and Strebluslignanol. The Strebluslignanol has good human immunodeficiency virus-1 (HIV-1) protease inhibitory activity, and the half maximal inhibitory concentration (IC50) of the Strebluslignanol reaches 35 %mM. The lignin-class chemical compounds are made from cortex magnolia officinal extract.
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
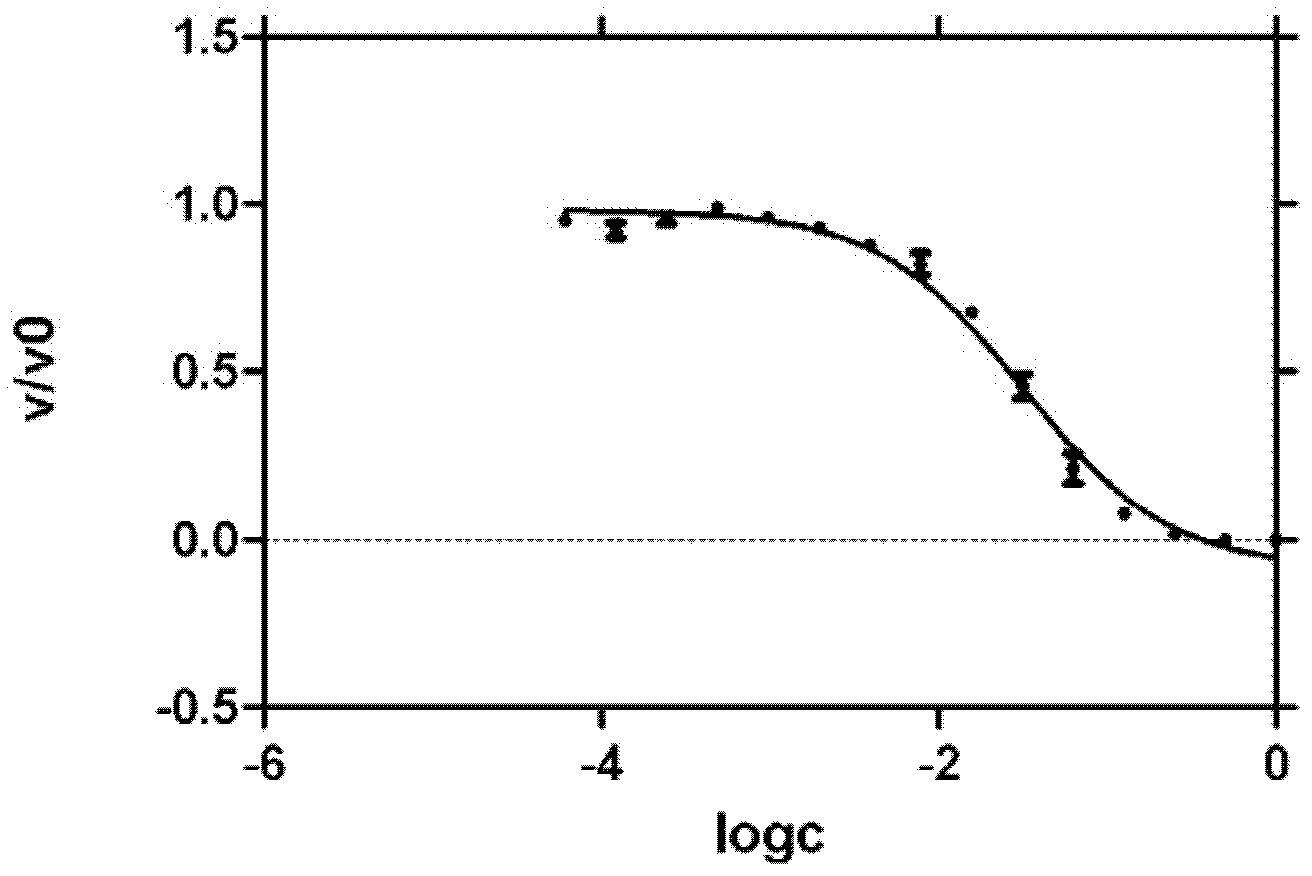
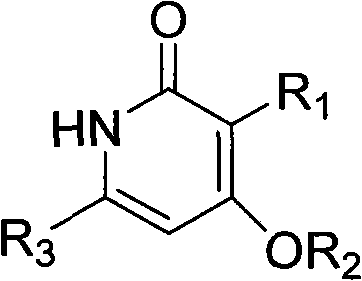
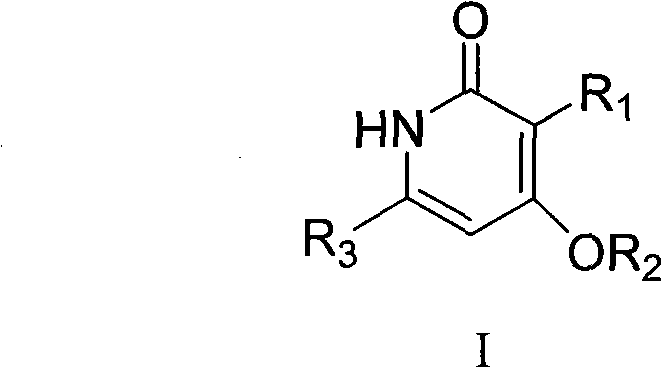
Preparation and application of novel pyridone human immunodeficiency virus-1 (HIV-1) reverse transcriptase inhibitor
InactiveCN102001994AOrganic active ingredientsOrganic chemistryNucleoside Reverse Transcriptase InhibitorHydrogen
The invention relates to a novel molecule with a relative structure, which is designed according to the bioisosteric principle and the theories of hydrogen bonding interaction and the like by using a non nucleoside human immunodeficiency virus-1 (HIV-1) reverse transcriptase inhibitor 4-(cyclohexyl methoxyl)-6-phenethyl-2(1H)-pyridone-3-carboxylic acid ethyl ester as a primer. In a general formula I, the definitions of groups are described in claims. Meanwhile, the invention relates to reverse transcriptase activity evaluation of a compound and application of the compound as the HIV-1 reverse transcriptase inhibitor. The conformation of the synthesized novel compound is more easily combined with HIV-1 reverse transcriptase, so that the novel compound is more favorable for inhibiting the activity of the reverse transcriptase and becomes the novel high-activity low-toxicity HIV-1 reverse transcriptase inhibitor.
Owner:PEKING UNIV +1
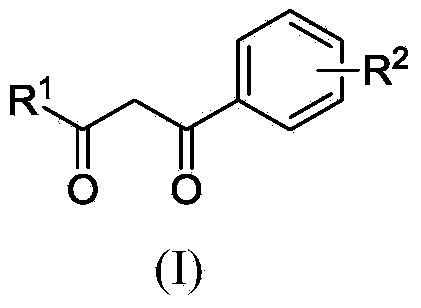
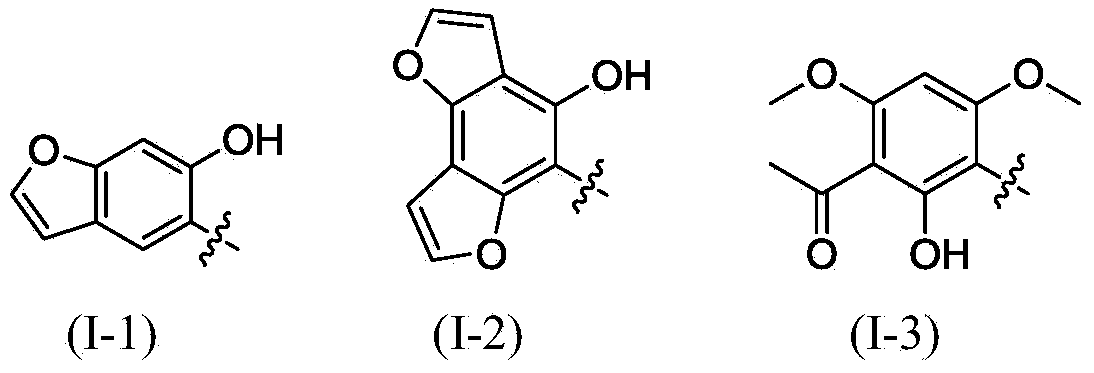
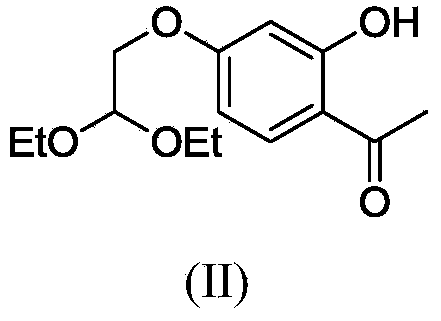
Polysubstituted aromatic diketone compounds as well as preparation method and application thereof
InactiveCN103804390AMild reaction conditionsSimple stepsOrganic compound preparationAntiviralsAcetyl chlorideHuman immunodeficiency virus 1
The invention relates to polysubstituted aromatic diketone compounds as shown in the formula (I) as well as a preparation method and application thereof. R1 in the formula (I) is a substituent group expressed by the formula (I-1), (I-2) or (I-3), and R2 is -H, -F, -Cl, -Br, -CH3 or -OCH3. When R1 is the formula (I-1), the preparation method comprises the following steps: with 2,4-dihydroxyacetophenone as the raw material, enabling the 2,4-dihydroxyacetophenone to be reacted with bromoacetaldehyde ethylene acetal to obtain 1-(4-(2,2-methoxyethoxy)-2-hydroxyphenyl) ethyl ketone; then, cyclizing and enabling the 1-(4-(2,2-methoxyethoxy)-2-hydroxyphenyl) ethyl ketone to be reacted with aryl chloride; finally, rearranging under an alkaline condition to obtain the polysubstituted aromatic diketone compounds. When R1 is the formula (I-2), the method disclosed by the invention is basically same as the method adopted when R1 is the formula (I-1). When R1 is the formula (I-3), the method comprises the following steps: with phloroglucinol as the raw material, methylating to obtain 1,3,5-trimethoxybenzene; then, enabling the 1,3,5-trimethoxybenzene to be reacted with acetyl chloride to obtain 1,1'-(2-hydroxy-4,6-dimethoxy-1,3-phenylene) diethyl ketone; next, enabling the 1,1'-(2-hydroxy-4,6-dimethoxy-1,3-phenylene) diethyl ketone to be reacted with aryl chloride; finally, rearranging under an alkaline condition to obtain the polysubstituted aromatic diketone compounds. The polysubstituted aromatic diketone compounds have an inhibiting effect for HIV-1 (Human Immunodeficiency Virus-1) integrase. The formula (I) is shown in the specification.
Owner:BEIJING UNIV OF TECH
Polynucleotide vaccines expressing codon optimized HIV-1 Pol and modified HIV-1 Pol
InactiveUS20060148750A1Low transmission rateLower Level RequirementsSugar derivativesGenetic material ingredientsTranscriptase activityOpen reading frame
Pharmaceutical compositions which comprise HIV Pol DNA vaccines are disclosed, along with the production and use of these DNA vaccines. The pol-based DNA vaccines of the invention are administered directly introduced into living vertebrate tissue, preferably humans, and preferably express inactivated versions of the HIV Pol protein devoid of protease, reverse transcriptase activity, RNase H activity and integrase activity, inducing a cellular immune response which specifically recognizes human immunodeficiency virus-1 (HIV-1). The DNA molecules which comprise the open reading frame of these DNA vaccines are synthetic DNA molecules encoding codon optimized HIV-1 Pol and codon optimized inactive derivatives of optimized HIV-1 Pol, including DNA molecules which encode inactive Pol proteins which comprise an amino terminal leader peptide.
Owner:MERCK & CO INC
Application of recombinant lentivirus to HIV (human immunodeficiency virus) phenotypic drug resistance detection
ActiveCN105648037ASmall side effectsLow costMicrobiological testing/measurementFermentationSide effectHuman immunodeficiency virus 1
The invention discloses application of recombinant lentivirus to HIV (human immunodeficiency virus) phenotypic drug resistance detection. The recombinant lentivirus is a package body obtained through cotransfection on a 293FT cell or 293T cell by a packaging plasmid, an envelope plasmid and a transferred plasmid, wherein the envelope plasmid is a psPAX2m-pol plasmid; the transferred plasmid is a pHAGE-CMV-Luc-IRES-ZsGreen plasmid. The recombinant lentivirus is safe and efficient; the toxic or side effect is small; the cost is low; when the recombinant lentivirus is applied to HIV phenotypic drug resistance detection, important reference basis can be provided for reasonably and effectively selecting anti-HIV medicine in clinics. The reporter genes Luciferase and ZsGreen of the transferred plasmid are positioned on pHAGE-CMV-Luc-IRES-ZsGreen; the expression of the reporter genes Luciferase and ZsGreen is controlled by a CMV (cytomegalovirus) promoter, so that the antiviral effect of an HIV-1 (human immunodeficiency virus-1) inhibitor can be simply obtained through the observation under an inverted fluorescence microscope or the determination of luciferase activity; after a pol region is imported, various cells can realize drug resistance detection by the recombinant lentivirus with the transferred plasmid; the universality is high.
Owner:广州佰芮慷生物科技有限公司
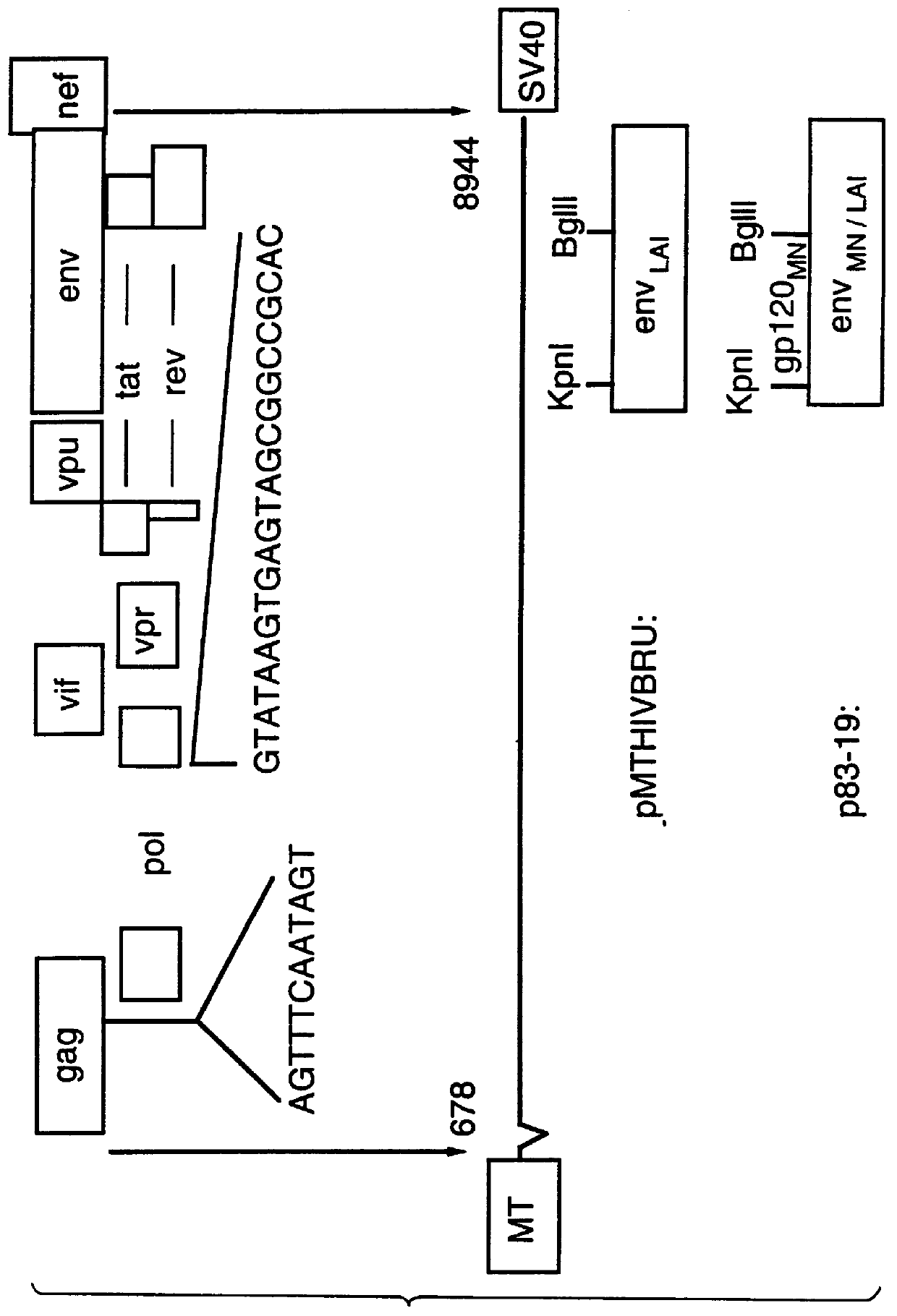
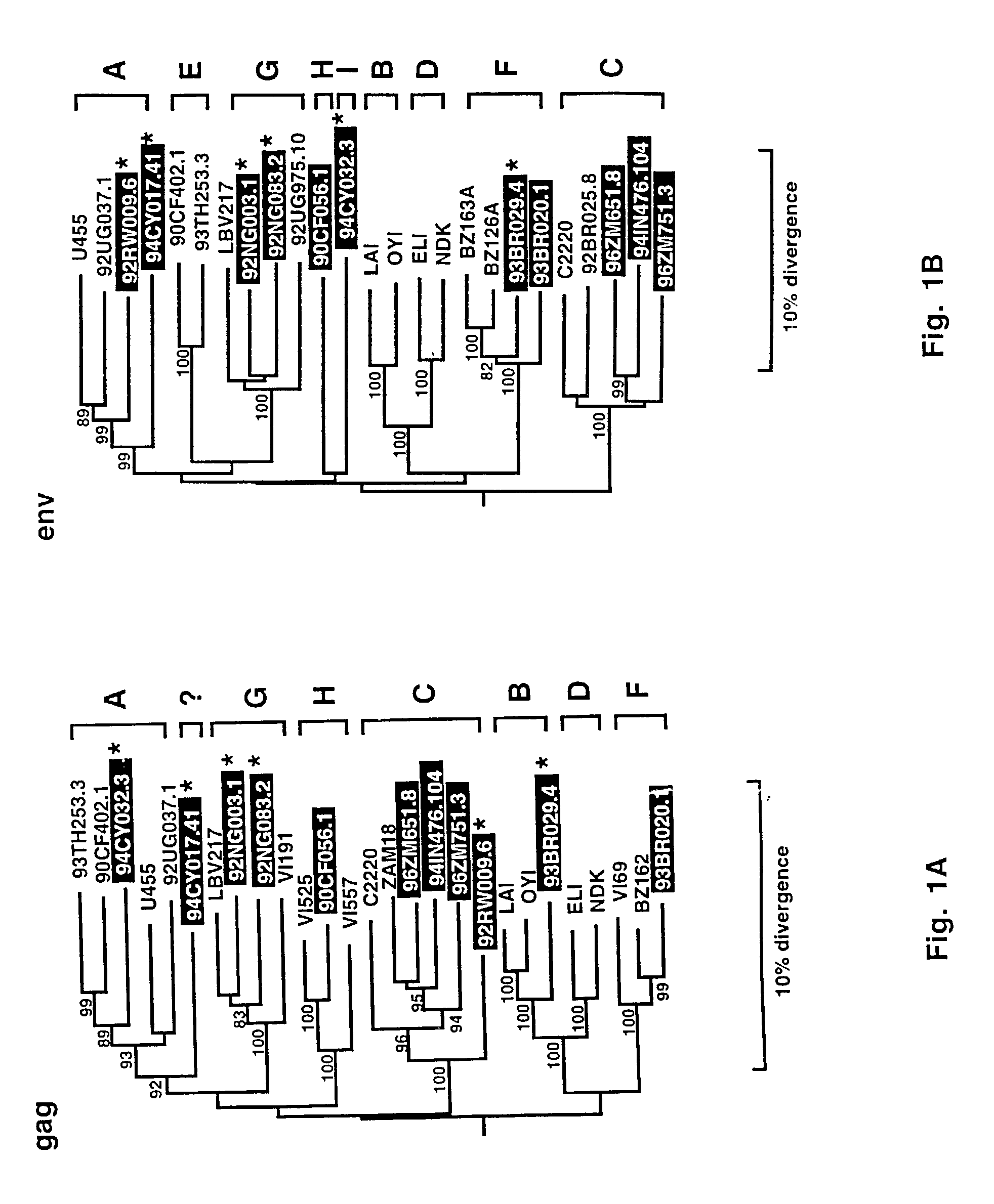
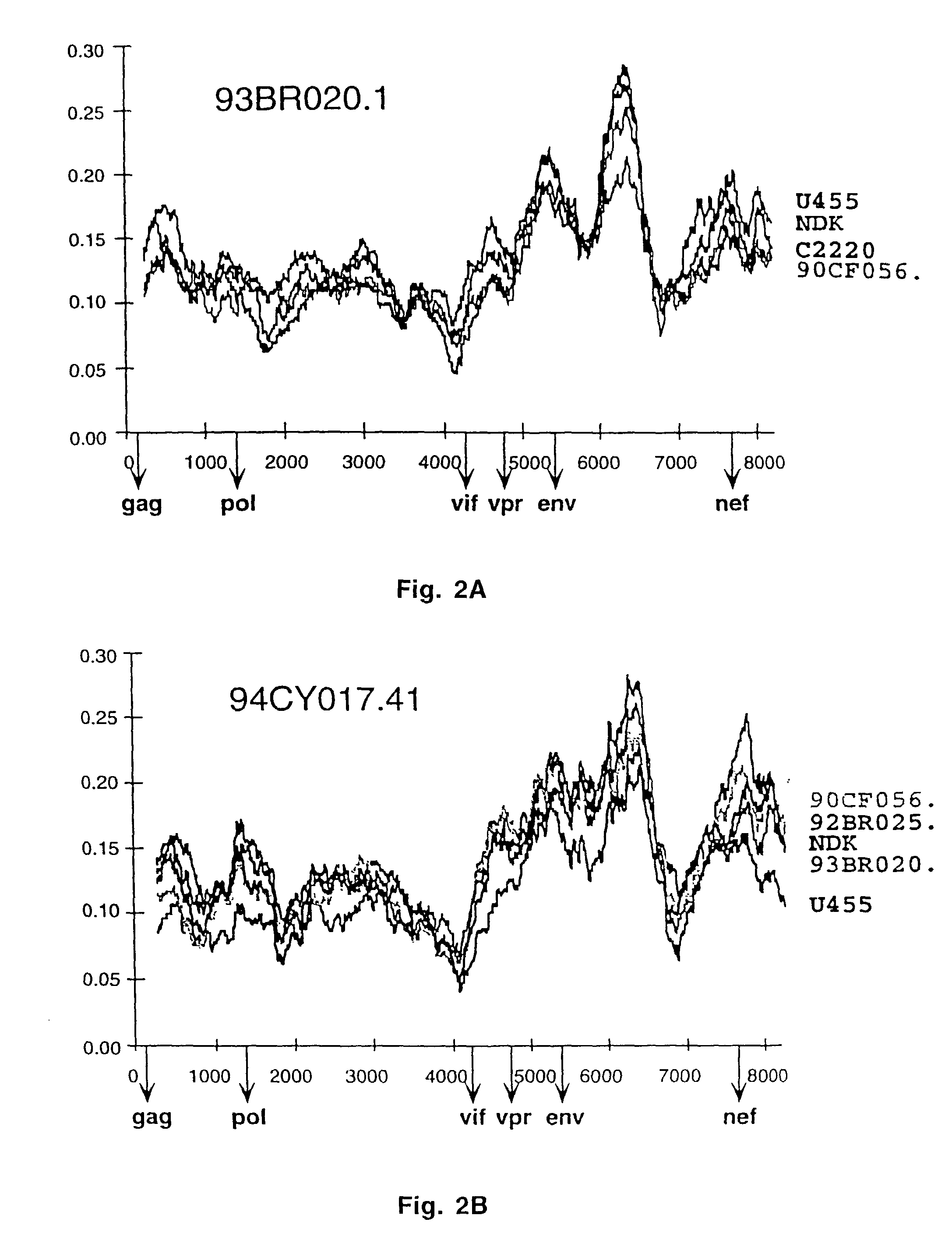
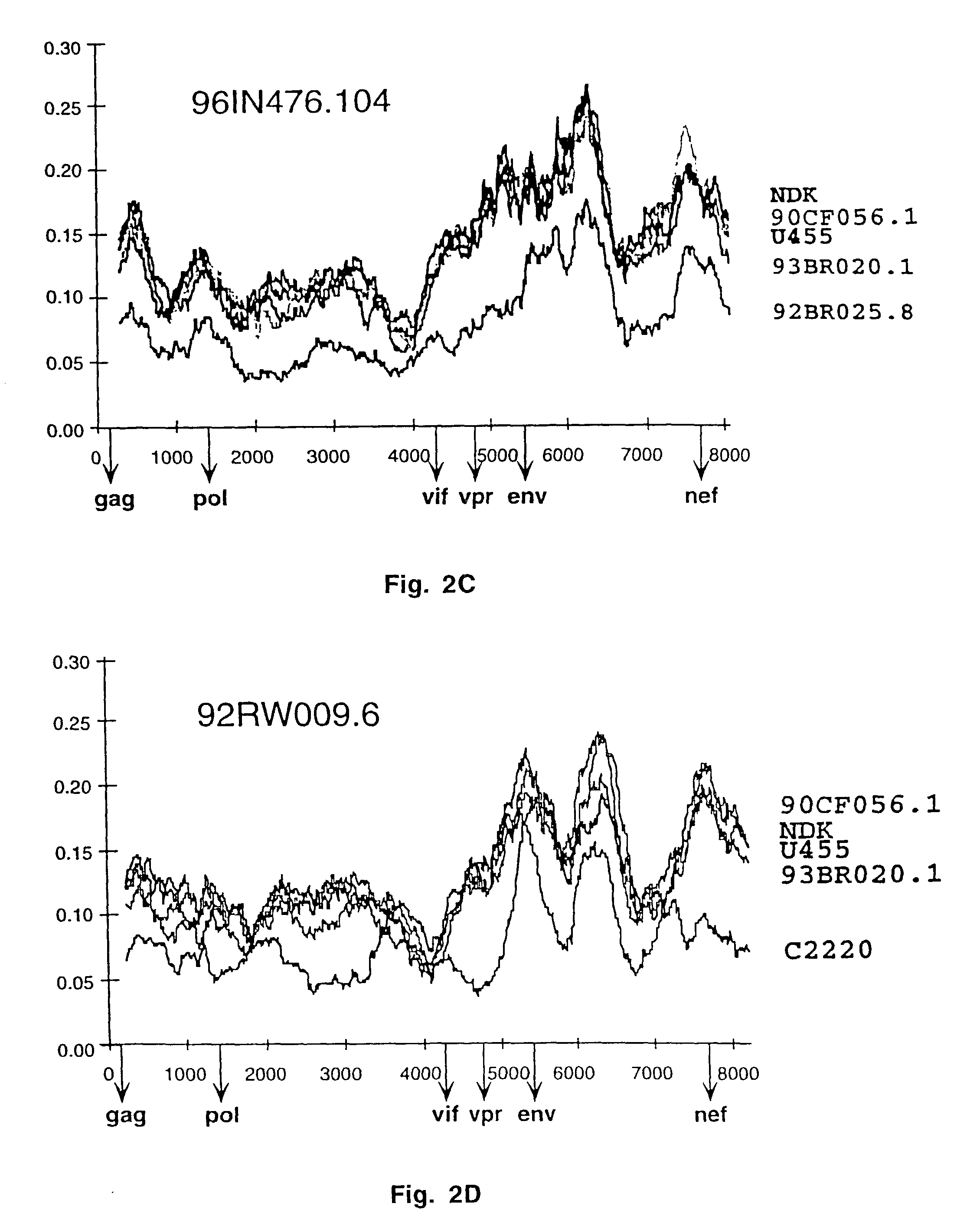
Reference clones and sequences for non-subtype B isolates of human immunodeficiency virus type 1
InactiveUS20030148266A1Readily availableEasy to useCosmetic preparationsVirusesHuman immunodeficiency virus 1Nucleotide sequencing
The nucleotide sequences of the genomes of eleven molecular clones for non-subtype B isolates of human immunodeficiency virus type 1 are disclosed. The invention relates to the nucleic acids and peptides encoded by and / or derived from these sequences and their use in diagnostic methods and as immunogens.
Owner:UAB RES FOUND
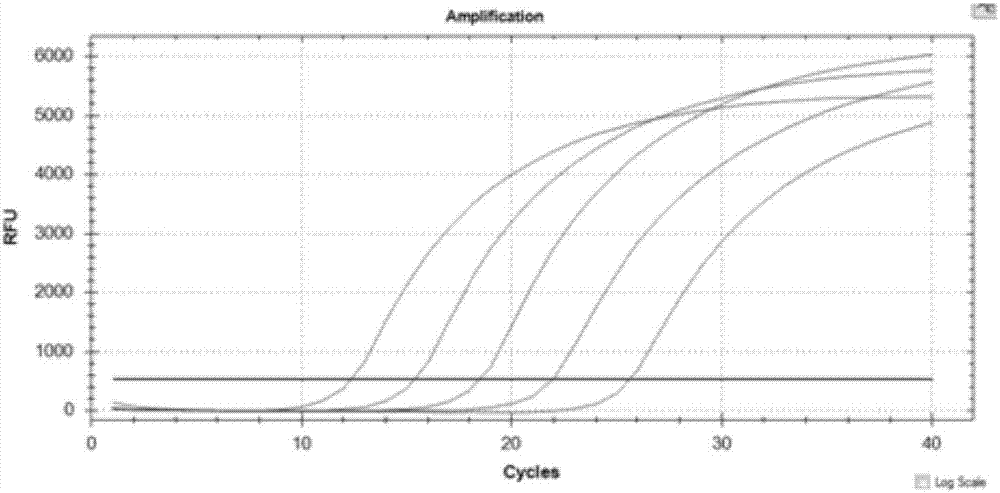
PCR primer group, probe and kit and detection method for detecting HIV (human immunodeficiency virus)-1 2-LTR DNA
ActiveCN107119148ASolve technical problems that cannot achieve quantitative detectionEasy to checkMicrobiological testing/measurementMicroorganism based processesNucleotideFluorescence
The invention belongs to the field of biotechnology, particularly relates to the field of virus detection, and more particularly relates to a PCR primer group, probe and kit and detection method for detecting human immunodeficiency virus-1 2-LTR DNA. The detection primer group provided by the invention comprises nucleotide sequences shown as SEQ ID NO: 1-6; and the detection probe comprises nucleotide sequences shown as SEQ ID NO: 7-8. The detection primer group provided by the invention is different from the conventional real-time fluorescence quantitative PCR; through introduction of 3 pairs of real-time fluorescent quantitative PCR primers, that is, 6 primers, adoption of multiple cycles, and increase of the primer annealing temperature, the PCR detection method for detecting the HIV-1 2-LTR DNA, provided by the invention, achieves extremely high sensitivity and specificity, and the minimum detection limit can reach 2 copies per million cells in each PCR; and moreover, when a quantitative cell reaction system is further added in the PCR reaction, the HIV-1 2-LTR DNA and the number of cells can be quantified at the same time in the same PCR reaction (in the conventional method, the cell count is additionally required).
Owner:GUANGZHOU SUPBIO BIO TECH & SCI
Human immunodeficiency virus type 1 (HIV-1) nucleic acid quantitative detection kit
ActiveCN109576397AHigh sensitivityEasy to useMicrobiological testing/measurementMicroorganism based processesHiv 1 rnaFluorescence
The invention provides a human immunodeficiency virus type 1 (HIV-1) nucleic acid quantitative detection kit. Specifically, according to the detection kit provided by the invention, HIV-1 RNA nucleicacid is identified and quantified through a real-time fluorescent polymerase chain reaction technology. The detection kit can be widely applied in multiple fields of HIV-induced AIDS window detection,clinical diagnosis, scientific research, efficacy tracking, inspection and quarantine, plague prevention and control and the like.
Owner:DAAN GENE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com