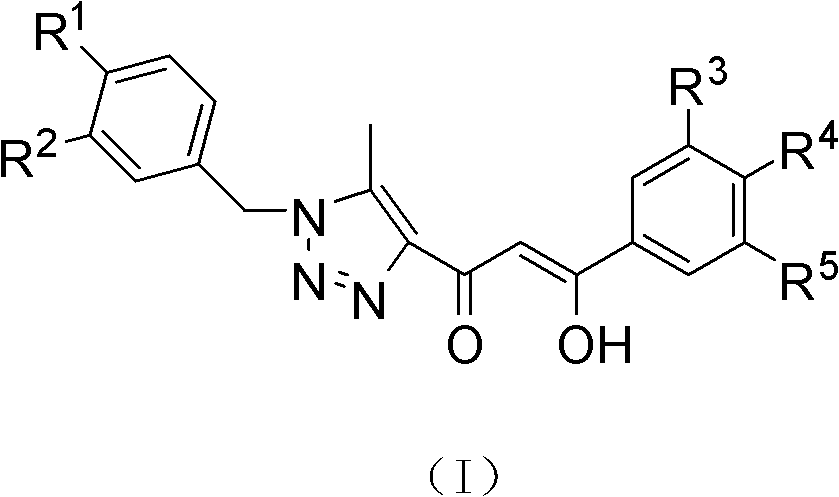
(Z)-1-(1-substituted benzyl-5-methyl-1H-1,2,3-triazole-4-yl)-3-substituted benzyl-3-hydroxy-2-propylene-1-ketone compound and preparation method and application thereof
A compound, benzyl technology, applied in the field of HIV integrase inhibitors, can solve the problem of easy reduction of enol, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
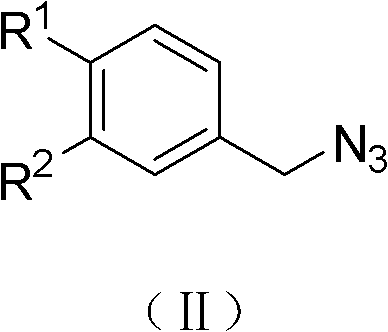
[0035](a) Synthesis of p-fluorobenzyl azide (1a)
[0036]
[0037] Add (1mmol, 0.189g) 4-fluorobenzyl bromide to a 50mL round bottom flask filled with 30mL acetone, stir at room temperature, then slowly add 10mL aqueous solution containing 2mmol sodium azide dropwise into the reaction system, and continue stirring at room temperature About 1 hour, TLC showed disappearance of starting material. The reaction solvent was removed under reduced pressure, dichloromethane extracted, anhydrous MgSO 4 Drying, desolvation.
[0038] The pure product is light yellow oily liquid, yield: 86%;
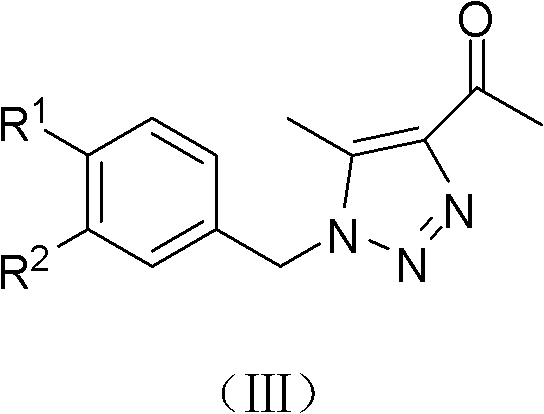
[0039] (b) Synthesis of 1-(1-(4-fluorobenzyl)-4-acetyl-5-methyl)-1H-1,2,3-triazole (2a)
[0040]
[0041] Place (10mmol, 1.51g) p-fluorobenzyl azide (1a), (10mmol, 1.0g) acetylacetone, (20mmol, 2.76g) anhydrous potassium carbonate in 15mL absolute ethanol and 15mL acetonitrile solution, stir and heat To 60°C, the reaction time was 2.5 hours, TLC showed that the starting material disappeared...
Embodiment 2
[0052] (a) Synthesis of p-fluorobenzyl azide (1a)
[0053] With embodiment 1.
[0054] (b) Synthesis of 1-(1-(4-fluorobenzyl)-4-acetyl-5-methyl)-1H-1,2,3-triazole (2a)
[0055] With embodiment 1.
[0056] (c) (Z)-1-(1-(4-fluorobenzyl)-5-methyl-1H-1,2,3-triazol-4-yl)-3-(4-methoxy Synthesis of substituted phenyl)-3-hydroxy-2-propen-1-one (3b)
[0057]
[0058] Under ice-bath conditions, (20mmol, 0.48g) sodium hydride was dissolved in 30mL dry tetrahydrofuran solution, and (10mmol, 2.33g) 10mL dry tetrahydrofuran solution of 1,2,3-triazole compound (2a) was added dropwise. After completion, (15mmol, 2.49g) 10mL dry tetrahydrofuran solution of methyl 4-methoxybenzoate was added dropwise, stirred and heated to 55°C, the reaction time was 3 hours, TLC showed that the raw material disappeared. After cooling, the reaction mixture was poured into ice water containing 5 mL of concentrated hydrochloric acid, extracted with 10 mL × 3 ethyl acetate, washed with brine, dried over anh...
Embodiment 3
[0069] (a) Synthesis of p-fluorobenzyl azide (1a)
[0070] With embodiment 1.
[0071] (b) Synthesis of 1-(1-(4-fluorobenzyl)-4-acetyl-5-methyl)-1H-1,2,3-triazole (2a)
[0072] With embodiment 1.
[0073] (c) (Z)-1-(1-(4-fluorobenzyl)-5-methyl-1H-1,2,3-triazol-4-yl)-3-(3,4-di Synthesis of methoxy-substituted phenyl)-3-hydroxy-2-propen-1-one (3c)
[0074]
[0075] Under ice-bath conditions, dissolve (20mmol, 0.48g) sodium hydride in 30mL dry tetrahydrofuran solution, add dropwise (10mmol, 2.33g) 10mL dry tetrahydrofuran solution of 1,2,3-triazole compound (2a), dropwise , followed by dropwise addition of (15mmol, 2.94g) 10mL dry tetrahydrofuran solution of methyl 3,4-dimethoxybenzoate, stirred and heated to 65°C, the reaction time was 2 hours, TLC showed that the raw material disappeared. After cooling, the reaction mixture was poured into ice water containing 5 mL of concentrated hydrochloric acid, extracted with 10 mL × 3 ethyl acetate, washed with brine, dried over an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com