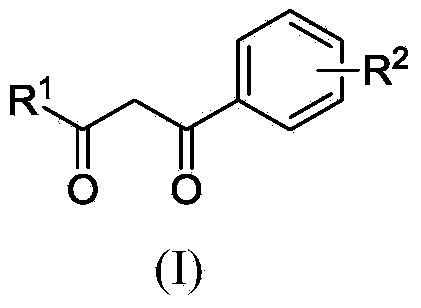
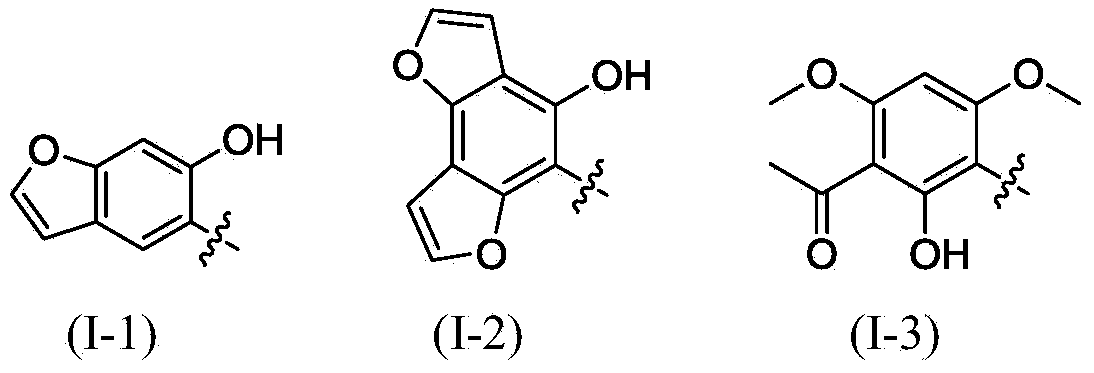
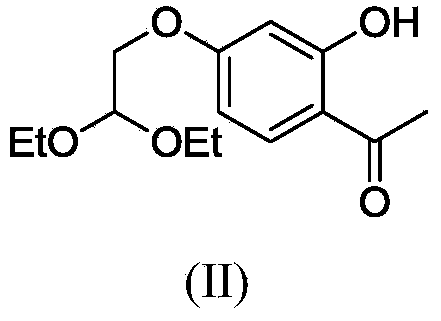
Polysubstituted aromatic diketone compounds as well as preparation method and application thereof
A technology for aromatic diketones and compounds, which is applied in the fields of polysubstituted aromatic diketones and their preparation and application, can solve problems such as limiting the application of HAART, toxic and side effects, and achieve the effects of mild reaction conditions and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Preparation of 1-(4-hydroxybenzo[1,2-b:3,4-b']difuran-5-yl)propyl-1,3-dione (1a)
[0054] (1) Preparation of 1-(2,4-(2,2-diethoxyethoxy)-6-hydroxyphenyl)ethanone
[0055]
[0056] Add 2,4,6-trihydroxyacetophenone (3g, 17.9mmol) into a 50mL two-necked flask, add 20mL of dry DMF and stir to dissolve, add K 2 CO 3 (9.84g, 71.3mmol), bromoacetaldehyde ethylene acetal (3mL, 18.8mmol), after stirring uniformly at room temperature, the reaction mixture was heated to reflux for 8h, poured into ice, stirred and cooled, extracted with ethyl acetate, and the organic phase was separated And with anhydrous MgSO 4 dry. The organic solvent was evaporated to dryness to obtain a dark brown oily liquid, which was separated and purified by column chromatography using petroleum ether: ethyl acetate = 8:1 as the eluent.
[0057] The pure product is a white solid, melting point: 60-62°C, yield: 60%.
[0058] 1 H NMR (400MHz, CDCl 3 ,δppm):1.19(t,12H,J=7.2Hz),2.58(s,1H),3...
Embodiment 2
[0079] Example 2: 1-(4-chlorophenyl)-3-(4-hydroxybenzo[1,2-b:3,4-b']difuran-5-yl)propyl-1,3- Preparation of diketone (2a)
[0080] (1), (2), (3) steps are with embodiment 1.
[0081] (4) 1-(4-chlorophenyl)-3-(4-hydroxybenzo[1,2-b:3,4-b']difuran-5-yl)propyl-1,3-di Preparation of ketone (2a)
[0082]
[0083] Add 2-acetylbenzodifuran-4-chlorobenzoate (0.846g, 2.39mmol) into a 50mL round bottom flask, add 15mL of acetone and stir to dissolve, add KOH (0.335g, 5.98mmol) and stir evenly, then Heated to reflux for 7 h under the protection of argon, cooled to room temperature, adjusted to pH=6 with 1M HCl, extracted with EtOAc, separated the organic phase and washed with anhydrous MgSO 4 dry. The organic solvent was evaporated to dryness to obtain a yellow solid, which was recrystallized from ethanol to obtain a pure product.
[0084] Melting point: 220-221°C, yield: 40%.
[0085] 1 H NMR (400MHz, CDCl 3 ,δppm):7.03(d,1H,J=2.0Hz),7.07(d,1H,J=2.0Hz),7.48(d,2H,J=8.8Hz),7.60(...
Embodiment 3
[0088]Example 3: Preparation of 1-(3-acetyl-2-hydroxyl-4,6-dimethoxyphenyl)-3-phenylpropane-1,3-dione (3a)
[0089] (1) Preparation of 1,3,5-trimethoxybenzene
[0090]
[0091] Phloroglucinol (0.162g, 1mmol) was dissolved in 20mL of acetone, adding K 2 CO 3 (0.55g, 4mmol), stirred for a while, then added dropwise dimethyl sulfate (0.2mL, 2mmol), stirred at room temperature for 6h, the raw material disappeared, the reaction solution was poured into water, extracted with ethyl acetate, the organic phase was separated and washed with anhydrous MgSO 4 dry. The solvent was spin-dried to obtain pure product;
[0092] The pure product is a white solid, melting point: 50-52°C, yield: 80%.
[0093] 1 H NMR (CDCl 3 ,400MHz,δppm):6.08(s,3H),3.77(s,9H).
[0094] (2) Preparation of 1,1'-(2-hydroxy-4,6-dimethoxy-1,3-phenylene) diethyl ketone
[0095]
[0096] 1,3,5-Trimethoxybenzene (2.02g, 12mmol) was dissolved in 150mL of dichloromethane, anhydrous AlCl was added 3 (5g, 37m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com