Patents
Literature
56 results about "Virus-Retrovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A retrovirus is a single-stranded positive-sense RNA virus with a DNA intermediate and, as an obligate parasite, targets a host cell. Once inside the host cell cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards).
Retrovirus and viral vectors
InactiveUS6635472B1Prevent and attenuate diseaseEnhance immune responseGenetic material ingredientsVirus peptidesVirus-RetrovirusDna viral
This invention relates to the fields of genetic engineering, virus replication and gene transfer. More specifically, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors, wherein an ori derived from a DNA virus capable of replicating in vertebrate cells is inserted into the retrovirus, allowing the retrovirus following the reverse transcription to efficiently replicate as extrachromosomal or episomal DNA without the necessity of integration into the host cell chromosome. Additionally, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors replicating episomally without aid of an ori and related elements. Also, this invention encompasses preventive, therapeutic, and diagnostic applications employing said constructs, viruses and vectors.
Owner:RUBICON LABS
Cocal vesiculovirus envelope pseudotyped retroviral vectors
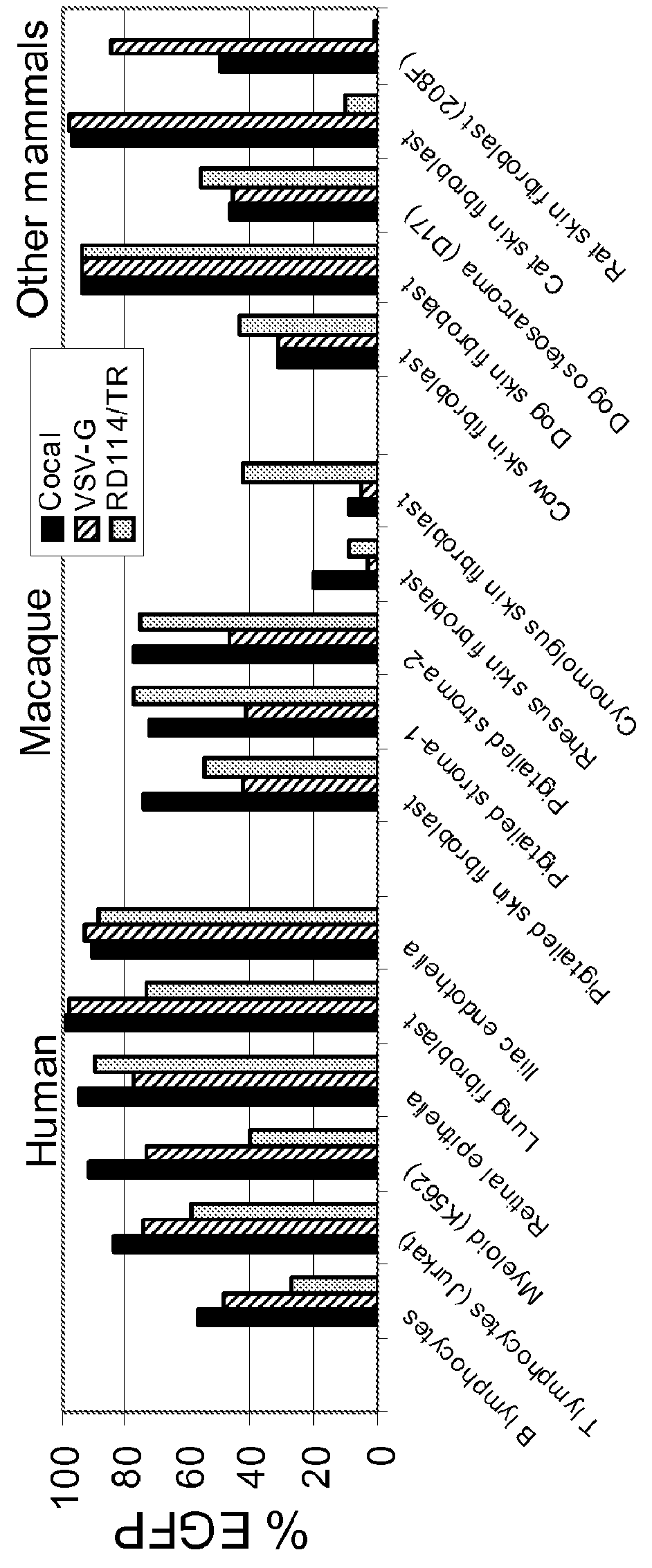
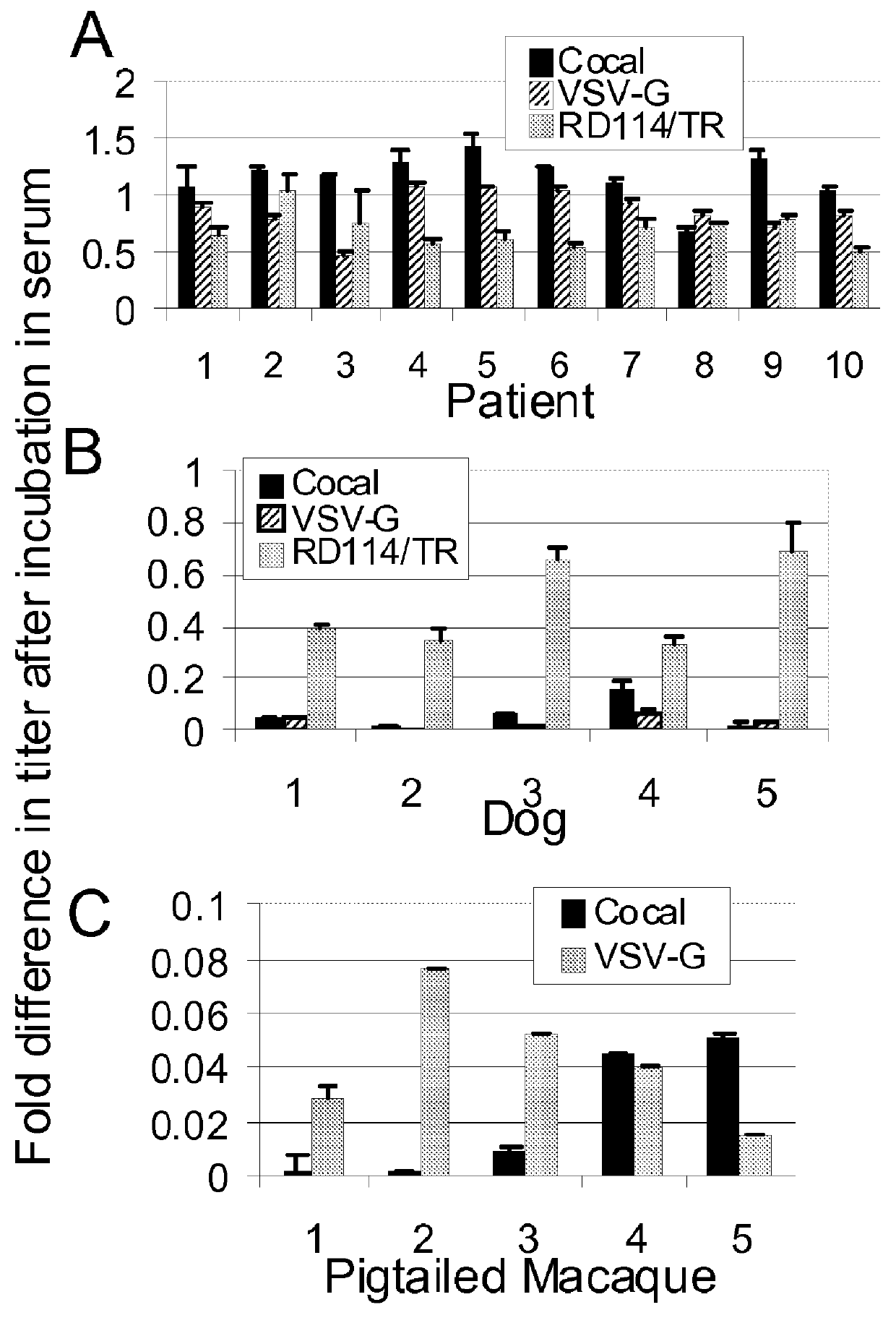
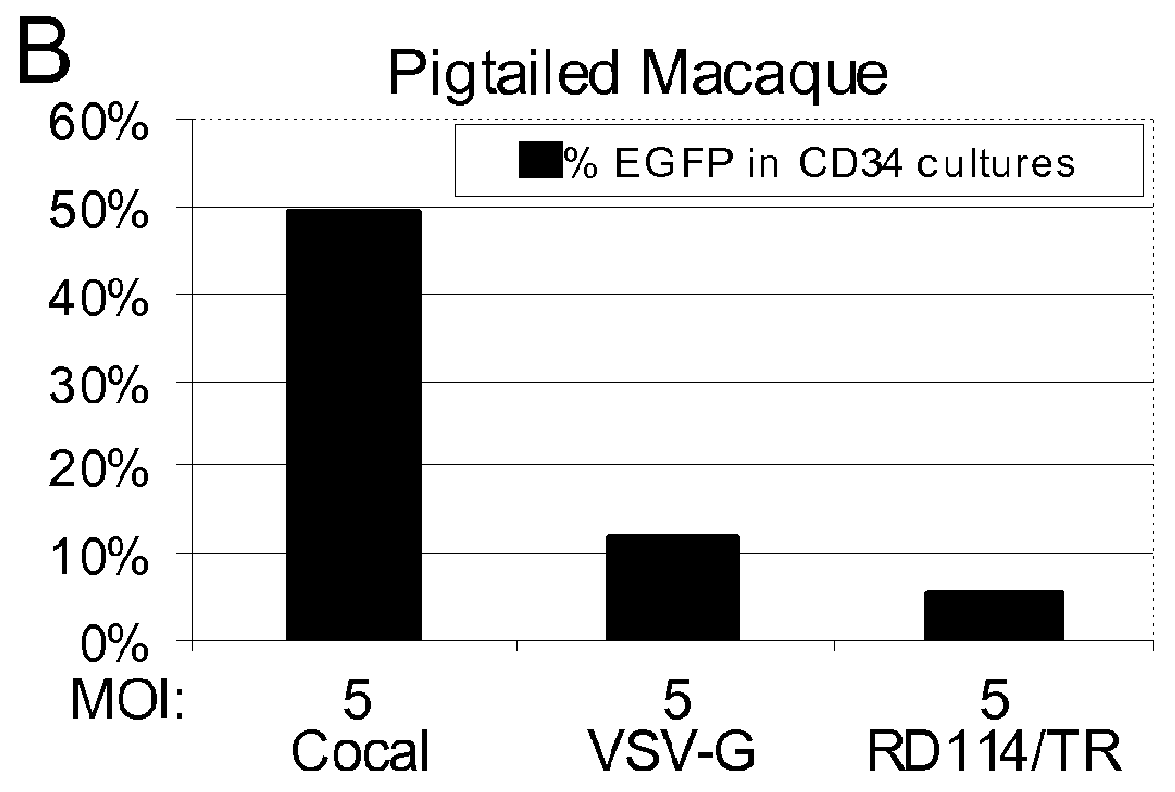
InactiveUS20120164118A1Increase serum stabilitySsRNA viruses negative-senseBiocideCocal vesiculovirusVirus-Retrovirus
Provided herein are Cocal vesiculovirus envelope pseudotyped retroviral vectors that exhibit high titers, broad species and cell-type tropism, and improved serum stability. Disclosed Cocal vesiculovirus envelope pseudotyped retroviral vectors may be suitably employed for gene therapy applications and, in particular, for the ex vivo and in vivo delivery of a gene of interest to a wide variety of target cells.
Owner:FRED HUTCHINSON CANCER RES CENT
Gene transfer method with the use of serum-free medium
InactiveUS6287864B1Effective maintenanceImprove efficiencyBiocideMicroorganismsVirus-RetrovirusGene transfer
A method for transferring a gene into target cells by a retrovirus with the use of serum-free medium. This method comprises infecting target cells with a retrovirus in serum-free medium optionally containing low-density lipoprotein and / or cytokines in the presence of a functional substance such as fibronectin in an amount effective in elevating the gene transfer efficiency of the retrovirus into the target cells by co-localizing the retrovirus and the target cells.
Owner:TAKARA HOLDINGS
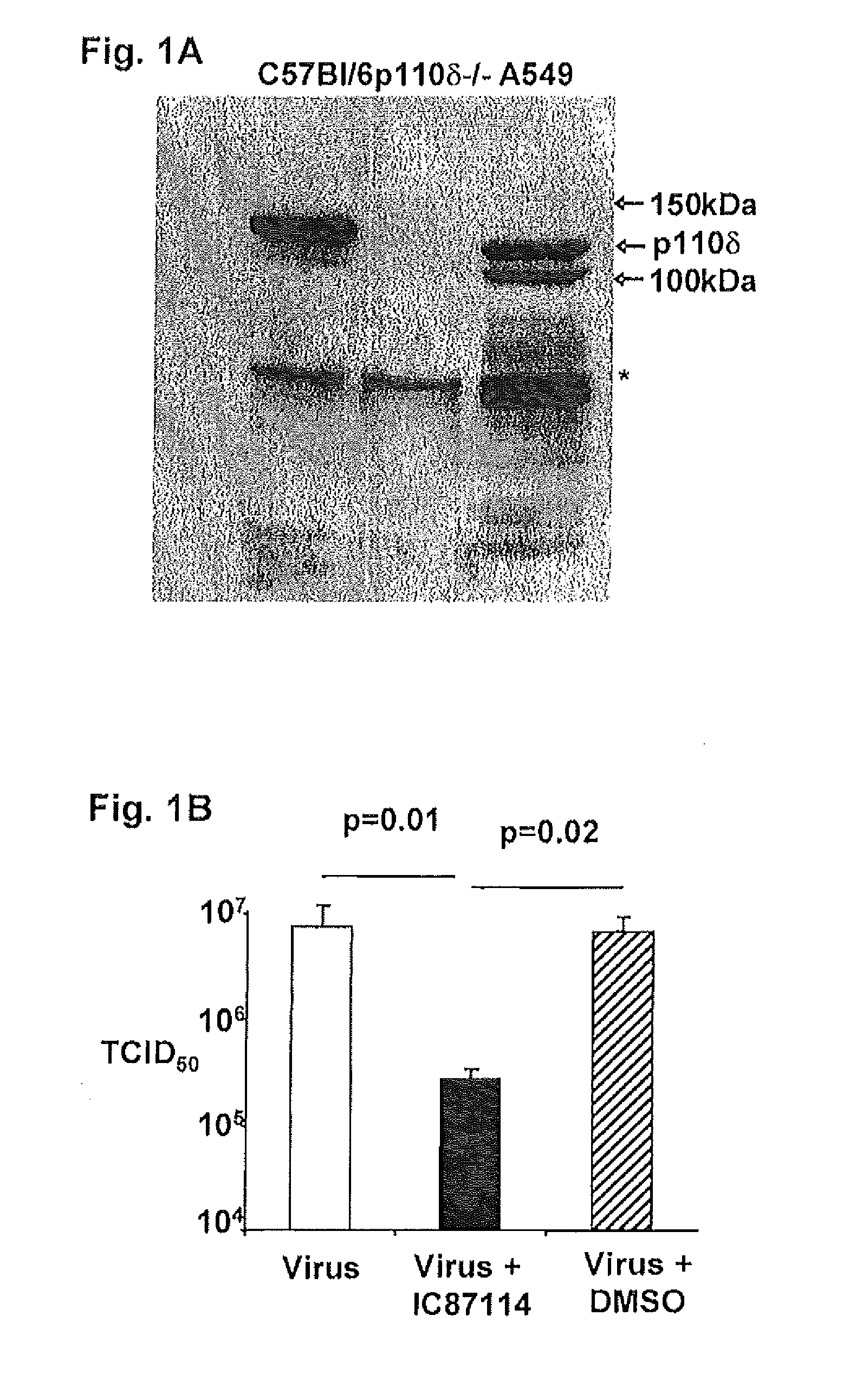
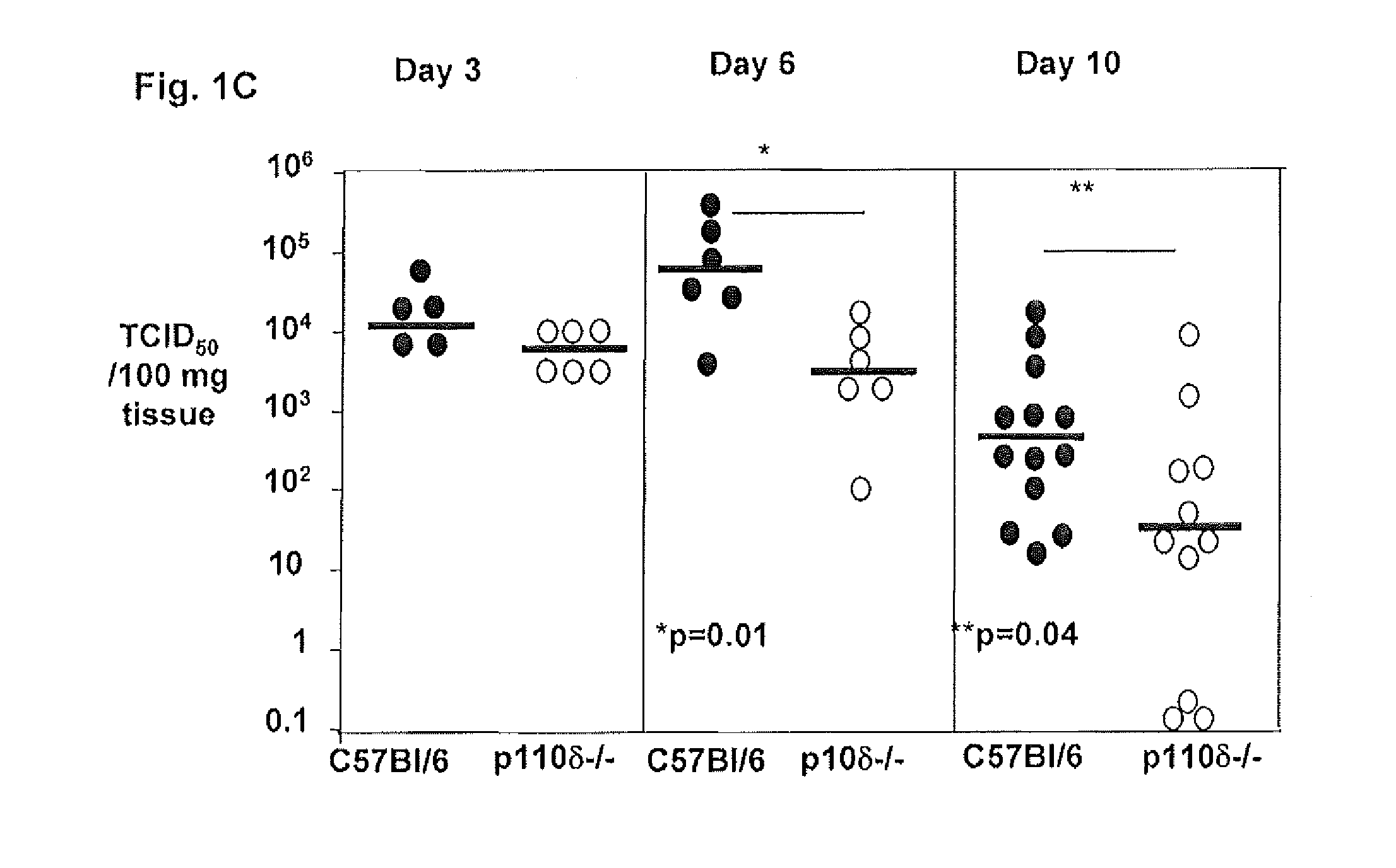
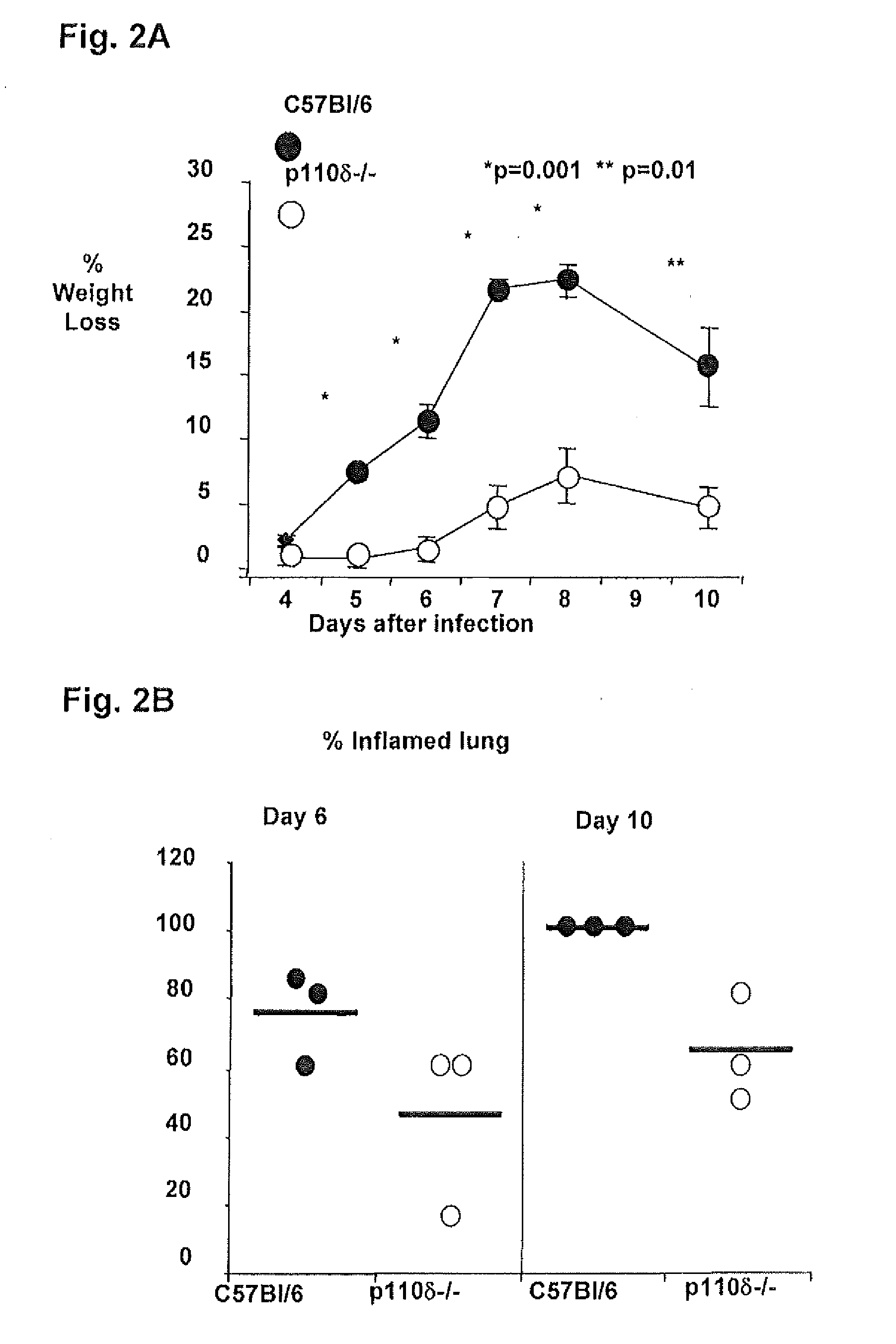
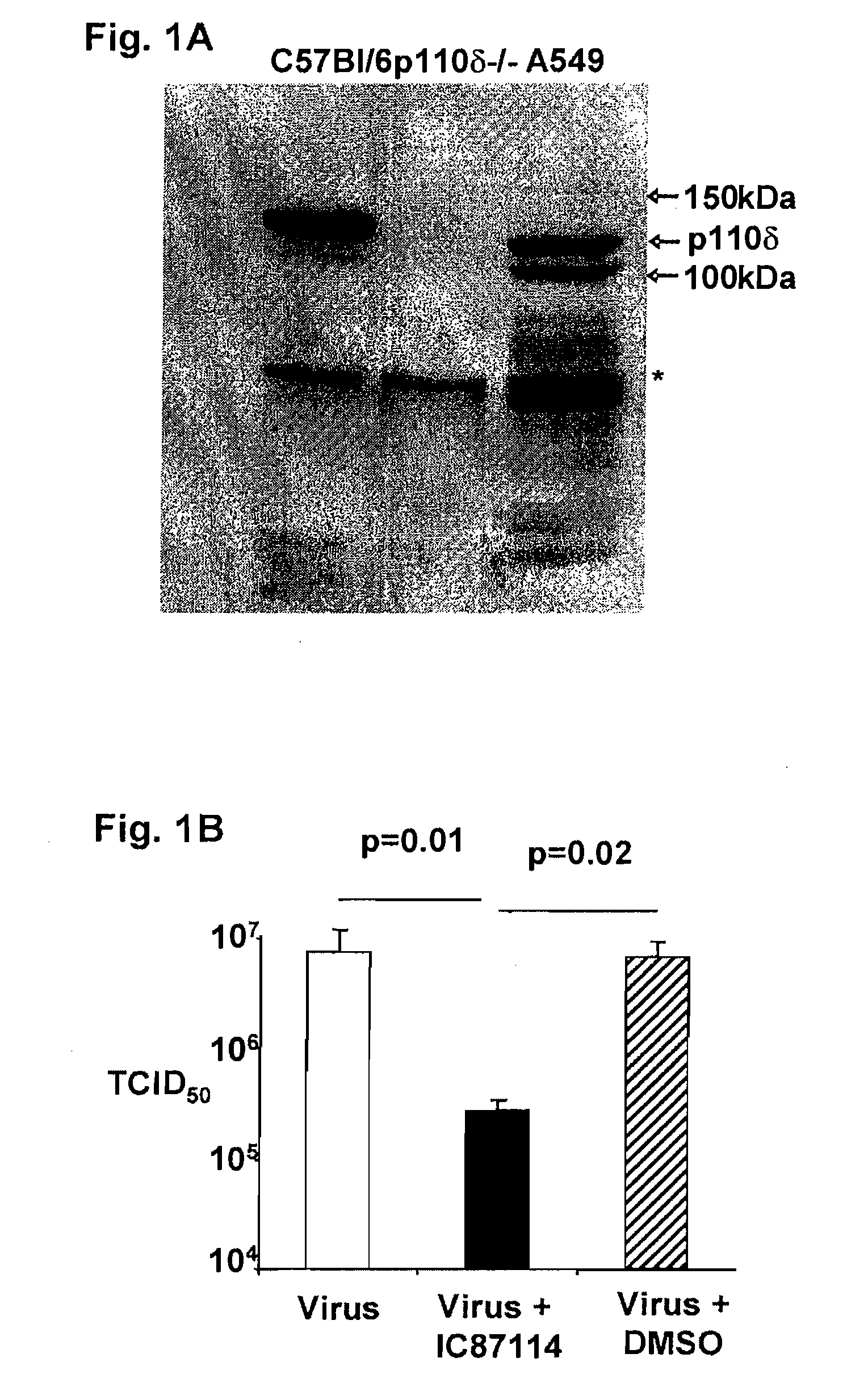
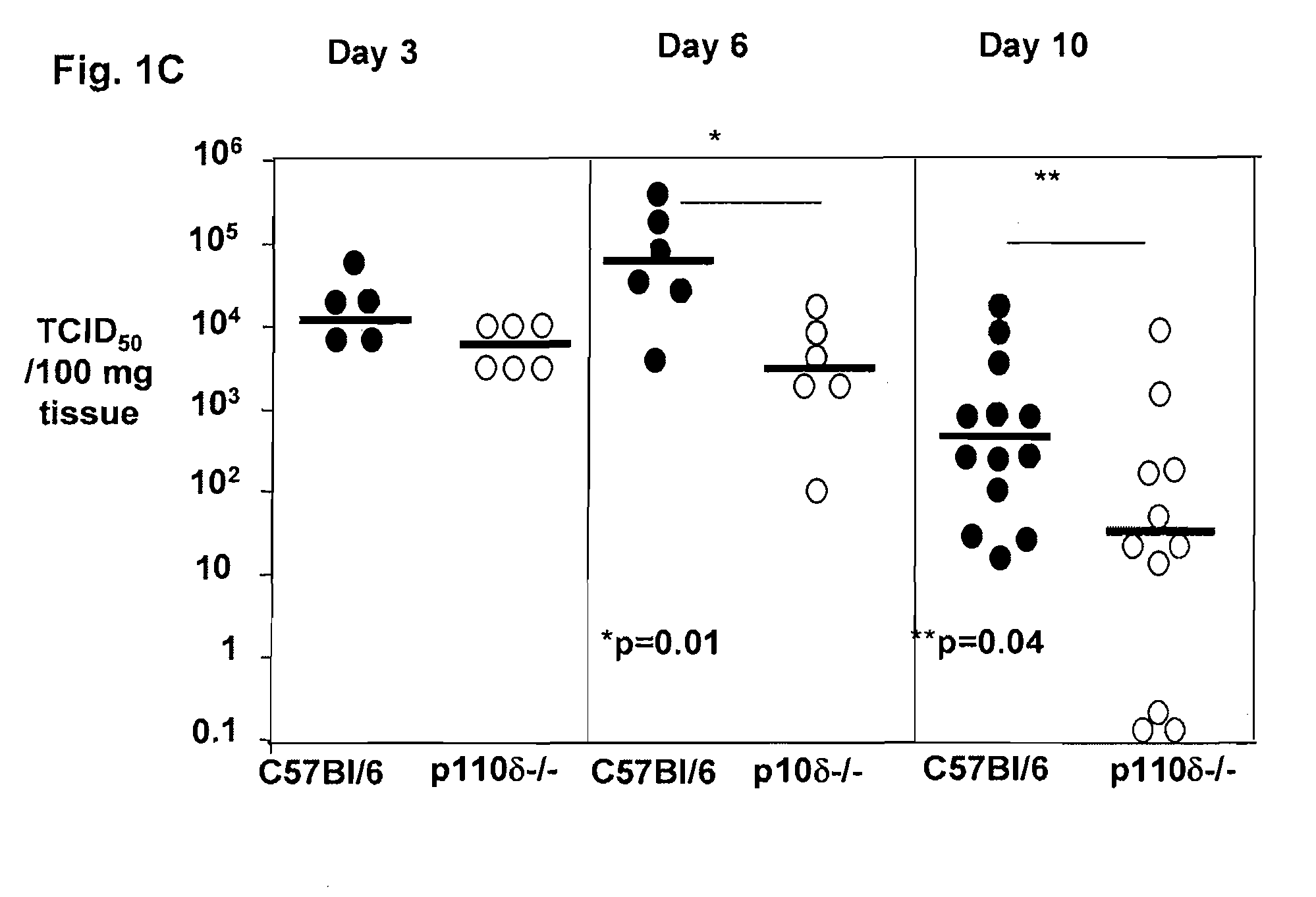
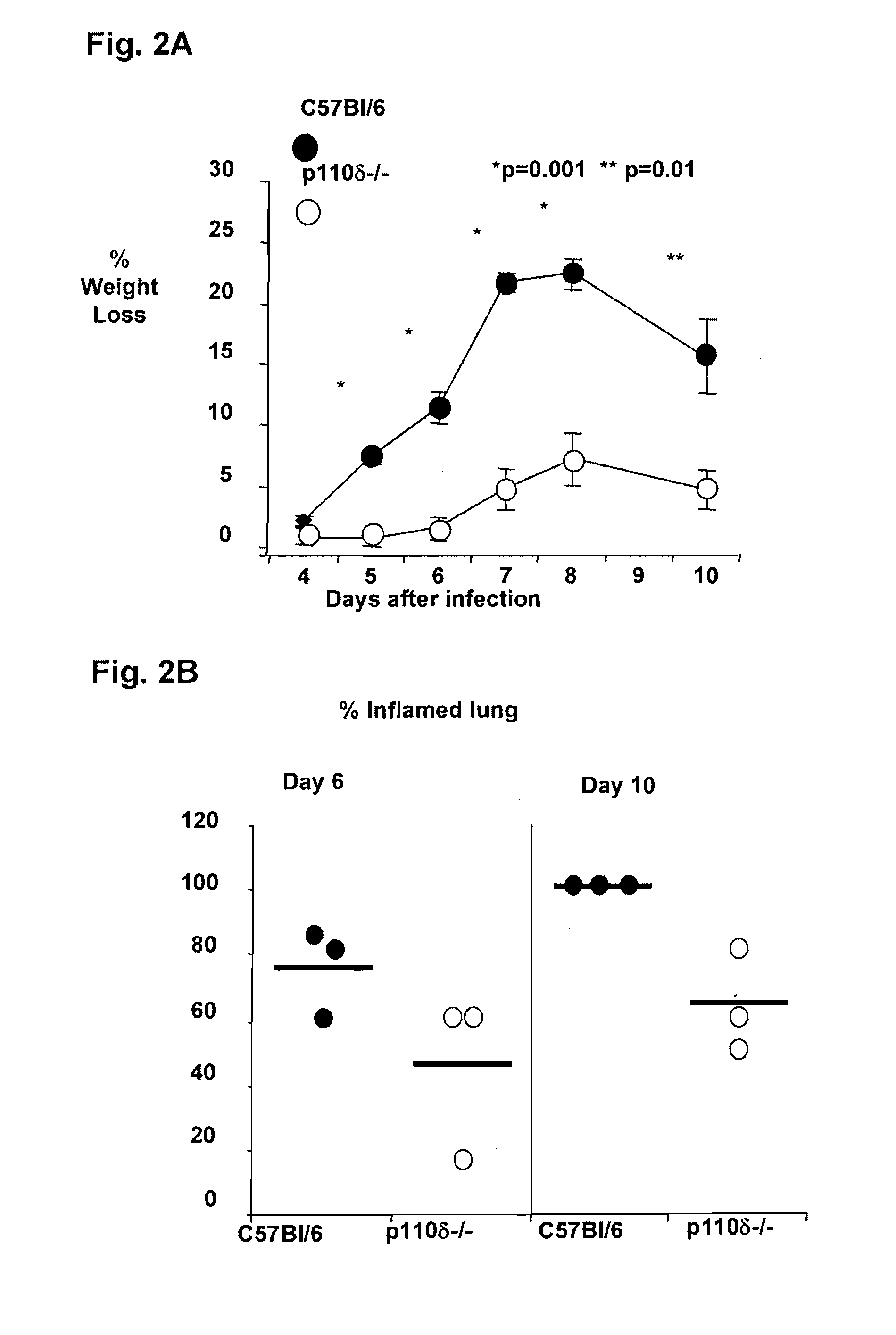
Role of PI3K p110 delta Signaling in Retroviral Infection and Replication
InactiveUS20110135655A1Avoid infectionSsRNA viruses negative-senseBiocideVirus-RetrovirusRetroviral infection
The invention includes compositions and methods for regulating PI3K p110 delta as an anti-retroviral therapy. The invention includes inhibiting p110 delta, a component of PI3K p110 delta signaling pathway, or any combination thereof in a cell as an anti-retroviral therapeutic approach for treating a retroviral infection, for example HIV. The invention includes a method of modulating PI3K p110 delta in a cell infected with a retrovirus by contacting the cell with an effective amount of a composition comprising an inhibitor of PI3K p110 delta.
Owner:DREXEL UNIV +1
Establishing method of immortal AM cell line
InactiveCN101798581AGuaranteed stable expressionConsistent with epithelial featuresFermentationGenetic engineeringTelomeraseCell activation
The invention provides an establishing method of an immortal AM cell line, which establishes the immortal AM cell line by constructing a retrovirus carrier to infect cell activation telomerase. The establishing method comprises the following processes: constructing the pLXSNneo-hTERT retrovirus carrier, and carrying out AM cell primary culture and AM cell immune cell chemical identification to establish a stable and reliable immortal AM cell line. The cell line meets the features of epithelial cells, can ensure the stable expression of a target gene and provides a valuable in-vitro model for researching AM development and invasion features.
Owner:陶谦
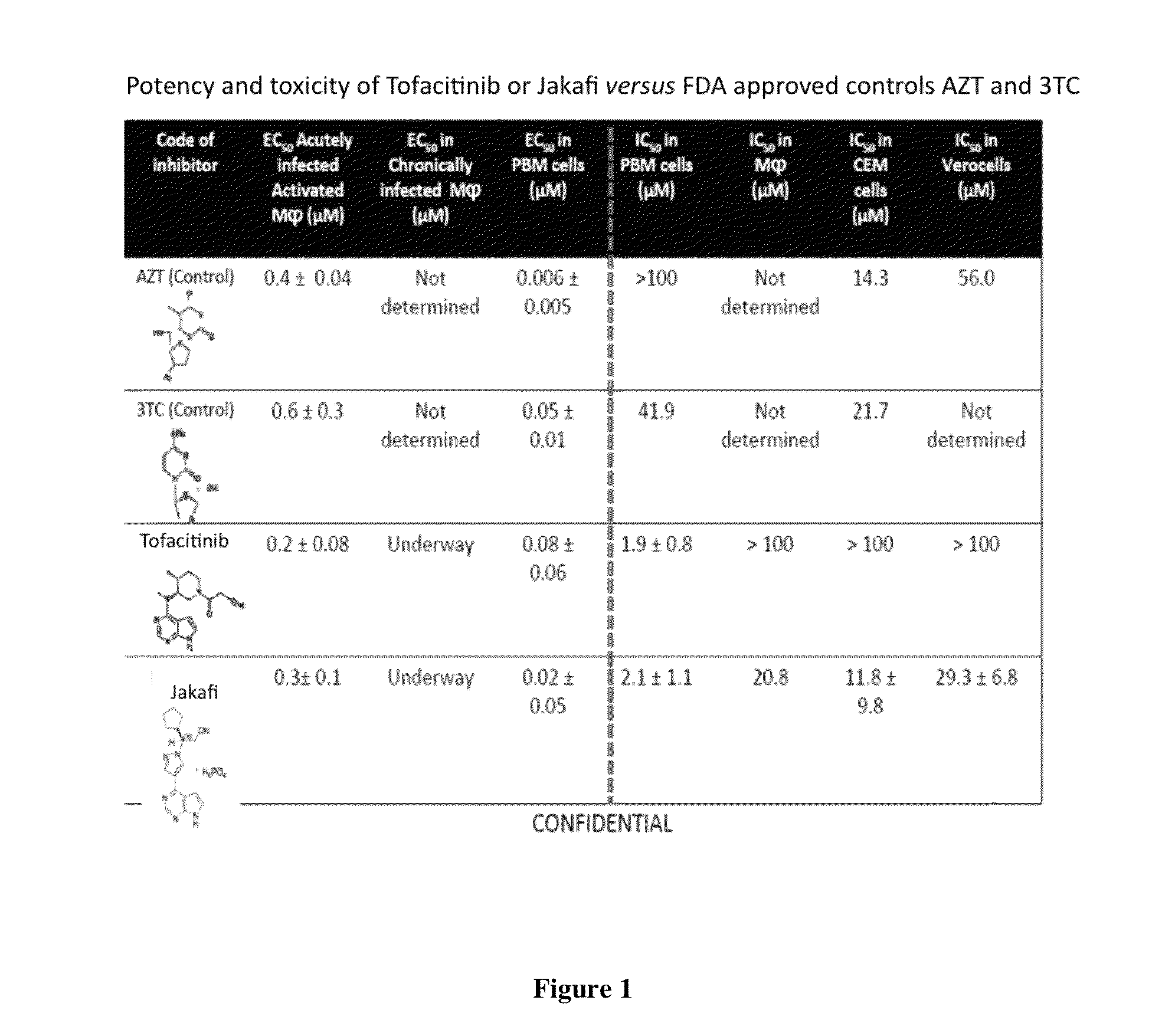
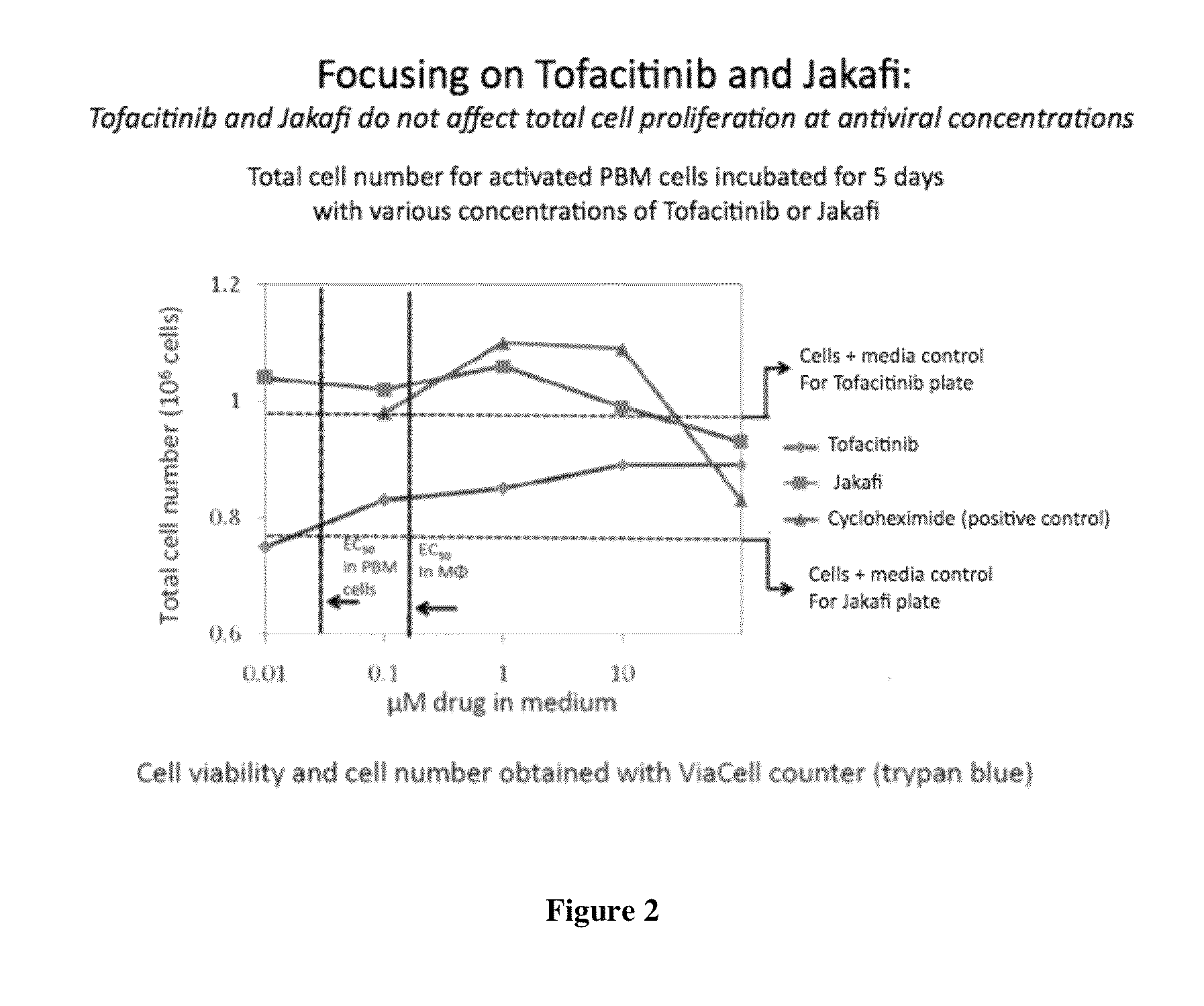
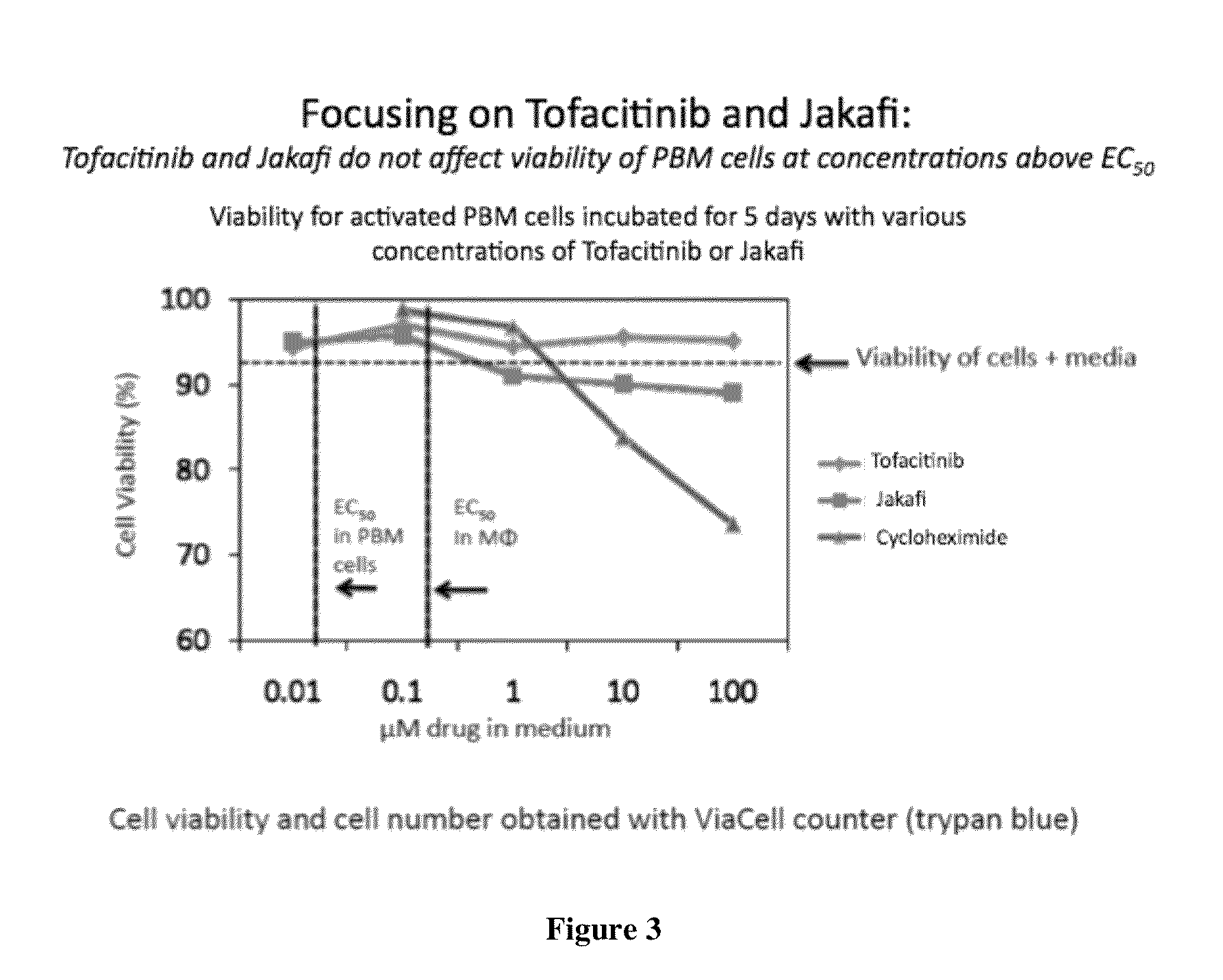
Antiviral jak inhibitors useful in treating or preventing retroviral and other viral infections
ActiveUS20140328793A1Improve their absolute antiviral effectLow toxicityBiocidePeptide/protein ingredientsProteinase inhibitorThymidine
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
New medicinal application of iridoid glycoside
ActiveCN104510747AReduce loadLower lung indexAntibacterial agentsOrganic active ingredientsViral MyocarditisInflammation
The invention belongs to the field of medicines and particularly relates to a new application of an iridoid glycoside compound, genipin-1-[beta]-D-gentiobioside, and a composition composed by compounding the genipin-1-[beta]-Dgentiobioside with other components as effective components for preparing antiviral drugs, antibacterial drugs, fever alleviating drugs, inflammation resistant drugs and anti-oxidizing drugs. The iridoid glycoside compound and the composition can be used for treating acute respiratory infection, flu, pneumonia, viral hepatitis type B, herpes zoster, herpes viral keratitis, viral myocarditis, Epstein-Barr virus infection, retrovirus infectious diseases and bacterial infectious diseases.
Owner:樊向德 +1
Host cells containing multiple integrating vectors
The present invention relates to the production of proteins in host cells, and more particularly to host cells containing multiple integrated copies of an integrating vector. Suitable integrating vectors for use in the present invention include retrovirus vectors, lentivirus vectors, transposon vectors, and adeno-associated virus vectors. Methods are provided in which the host cells are prepared by using the integrating vectors at a high multiplicity of infection. The host cells are useful for producing pharmaceutical proteins, variants of proteins for use in screening assays, and for direct use in high throughput screening.
Owner:CATALENT USA WOODSTOCK INC +3
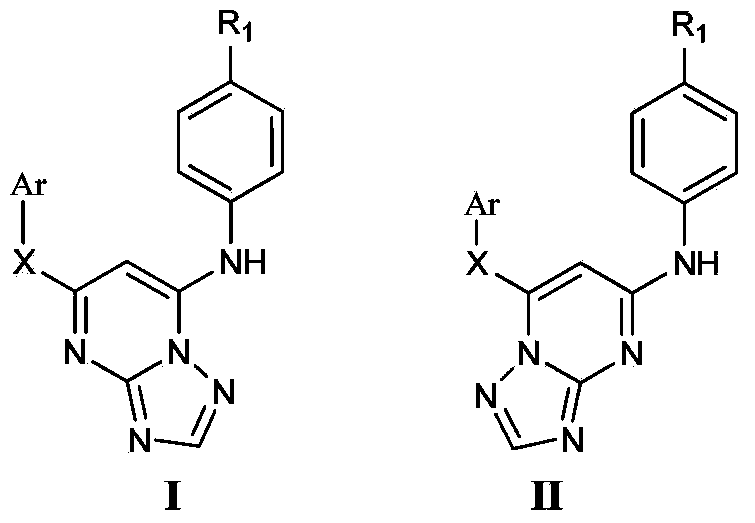
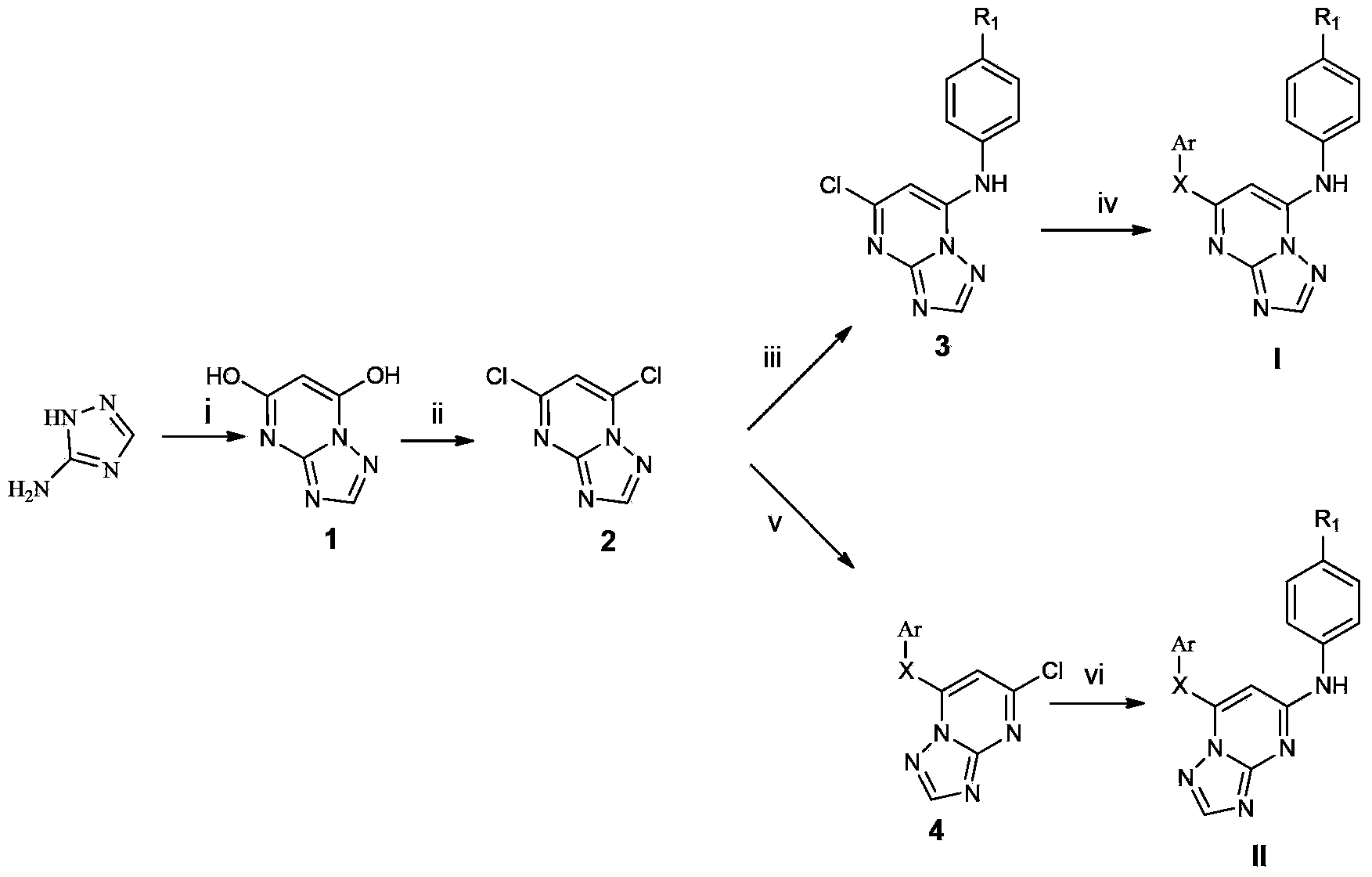
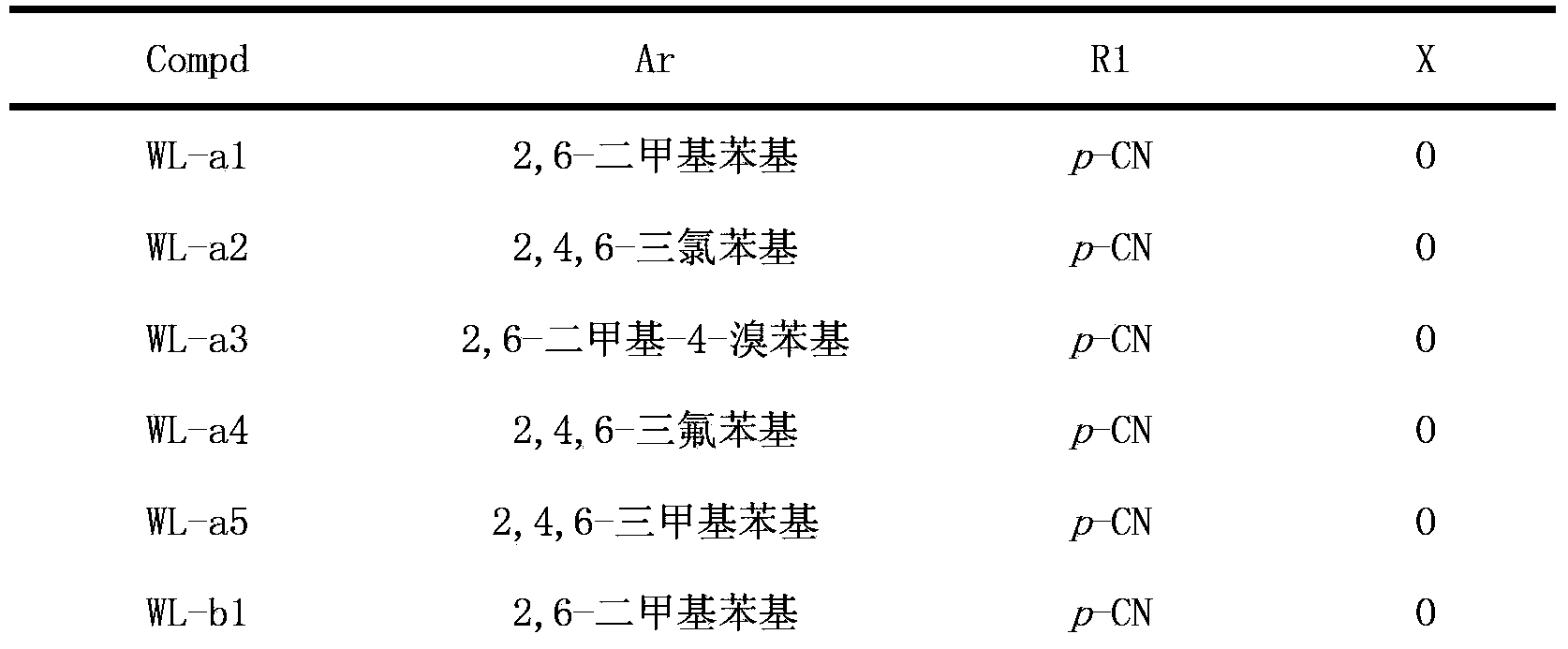
Triazolopyrimidine HIV-1 retrovirus inhibitor and its preparation method and application thereof
The invention relates to a triazolopyrimidine HIV-1 retrovirus inhibitor and its pharmaceutically acceptable salt, ester or prodrug, its preparation method and an application of one compound or a composition of more compounds in the preparation of anti-AIDS drugs.
Owner:SHANDONG UNIV
Kit with RT-LAMP nucleic acid test strips for detecting porcine epidemic diarrhea virus and applications of kit
InactiveCN103276103ALow costReduce use costMicrobiological testing/measurementMicroorganism based processesBetaineNucleic acid test
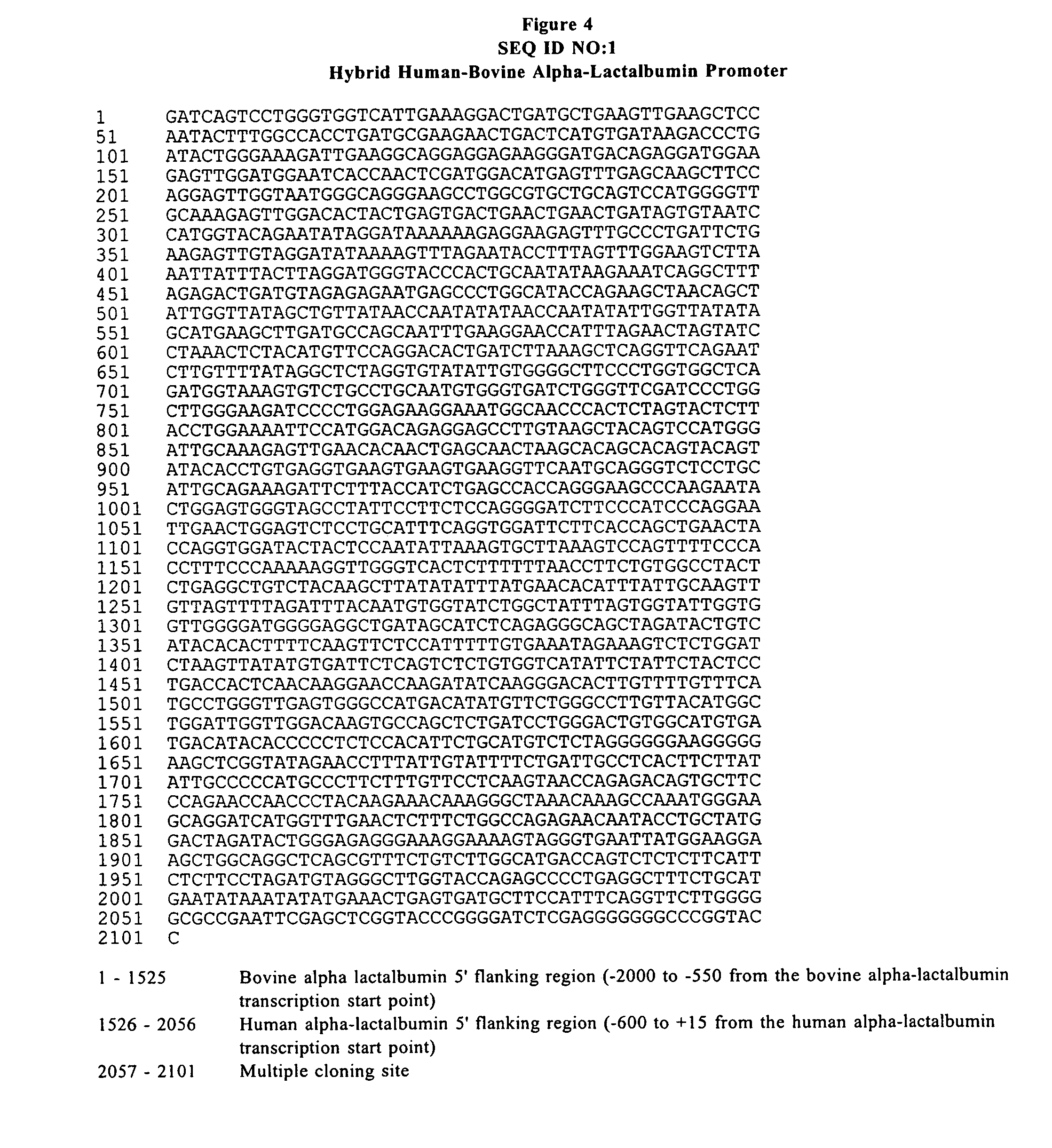
The invention discloses a kit with RT-LAMP nucleic acid test strips for detecting a porcine epidemic diarrhea virus and applications of the kit. The kit comprises a primer group of a nucleic acid represented by SEQ ID No. 1-6 and test strips for detection of nucleic acid. The usage of the kit is as follows: first preparing a RT-LAMP reaction system comprising an AMV retrovirus, a 1* reaction buffer, a strand displacement DNA polymerase, a dNTP mixture, betaine, MgSO4, a FIP primer, a BIP primer, a LoopF primer, a LoopB primer, a F3 primer, a B3 primer and RNA of a sample to be measured; carrying out a reaction at a constant temperature, after testing the obtained products by using the nucleic-acid-detecting test strip, judging and reading directly: the positive result is that two red strips appear, and one strip is in the detection zone while the other strip is in the control zone. The kit has advantages of simple operation, low cost, easy observation of the reaction result, good specificity, easy popularization and application in large scope and being extremely suitable for export quarantine, food hygiene and on-site detection in animal husbandry.
Owner:SOUTH CHINA AGRI UNIV
Recoverable immortalized hepatic cell line carrying double suicide genes and establishing method of recoverable immortalized hepatic cell line
ActiveCN104450620AAchieve knockoutRealize regulationGenetic engineeringFermentationHuman bodyProgenitor cell
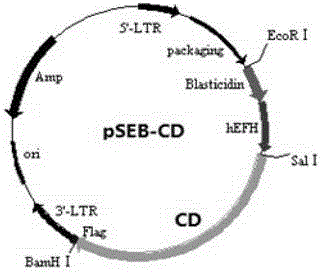
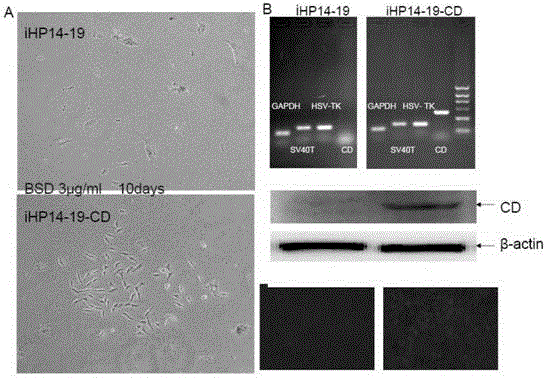
The invention provides an establishing method of a recoverable immortalized hepatic cell line. The establishing method comprises the following steps: separating a hepatic progenitor cell from liver in a mouse which is 12.5-14.5 days old in an embryonic period; guiding a retrovirus containing genes SV40T and HSV-TK into the hepatic progenitor cell; screening a monoclonal cell strain having a hepatic progenitor cell marker and having a function of differing to a mature hepatic cell; then guiding a retrovirus containing CD gene into the cell strain so as to obtain recoverable immortalized hepatic cell carrying double suicide genes. The cell strain can multiply in vitro to obtain the phenotype and functions of a normal hepatic cell, the safety of the immortalized cell becomes adjustable through controllable modes such as locus recombination and drug screening; when the cell is used in a human body, the biological safety of the immortalized cell is ensured to the greatest extent and the dangerousness of the immortalized cell is reduced; therefore, a reliable, safe and ideal hepatic cell material is provided for bioartificial liver technology.
Owner:重庆多沃生物科技有限公司
Method for retrovirus removal
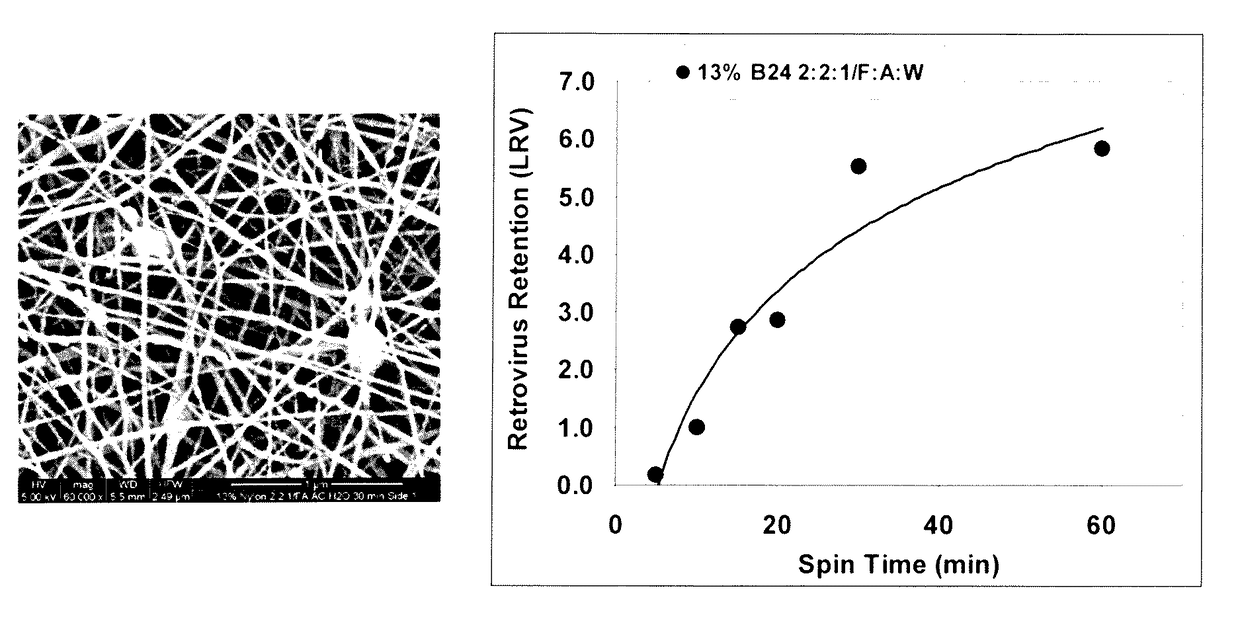
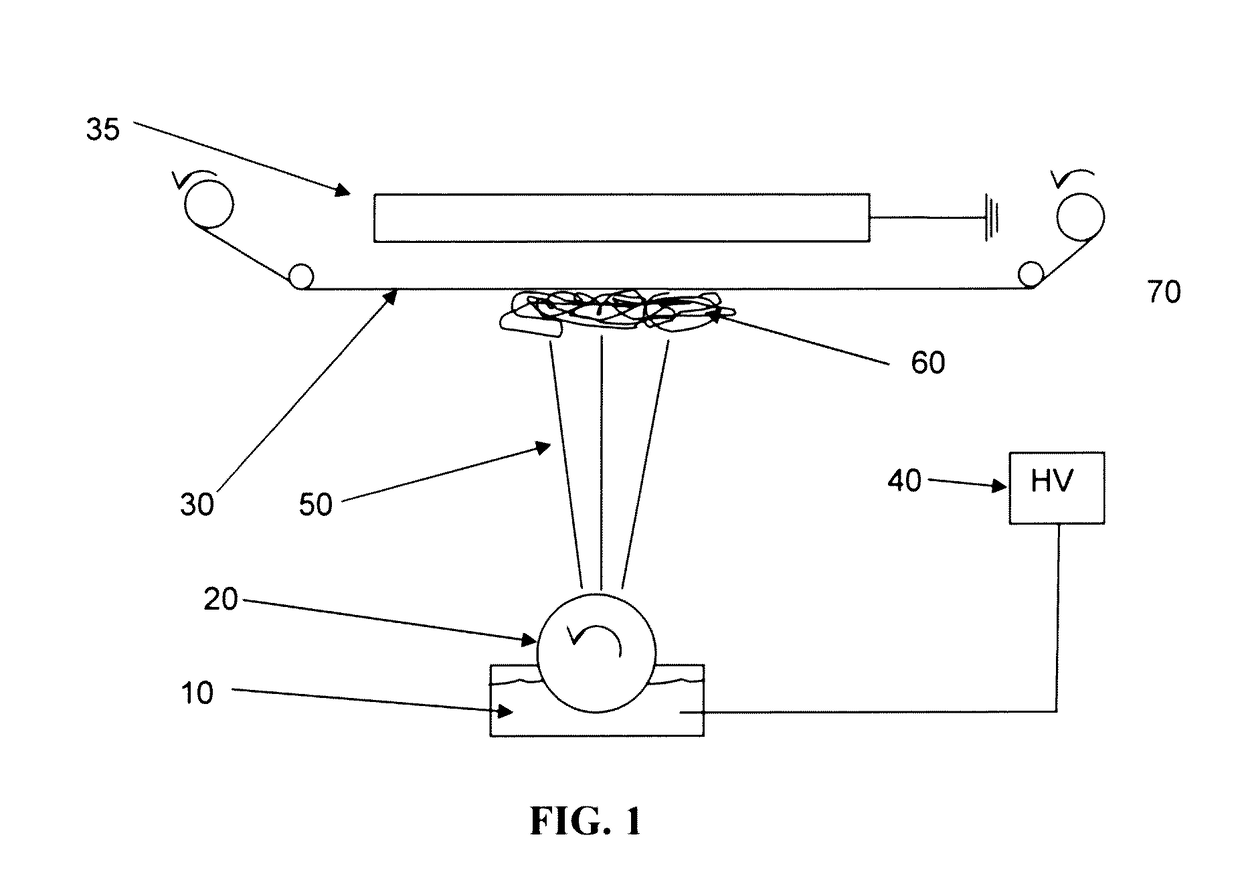
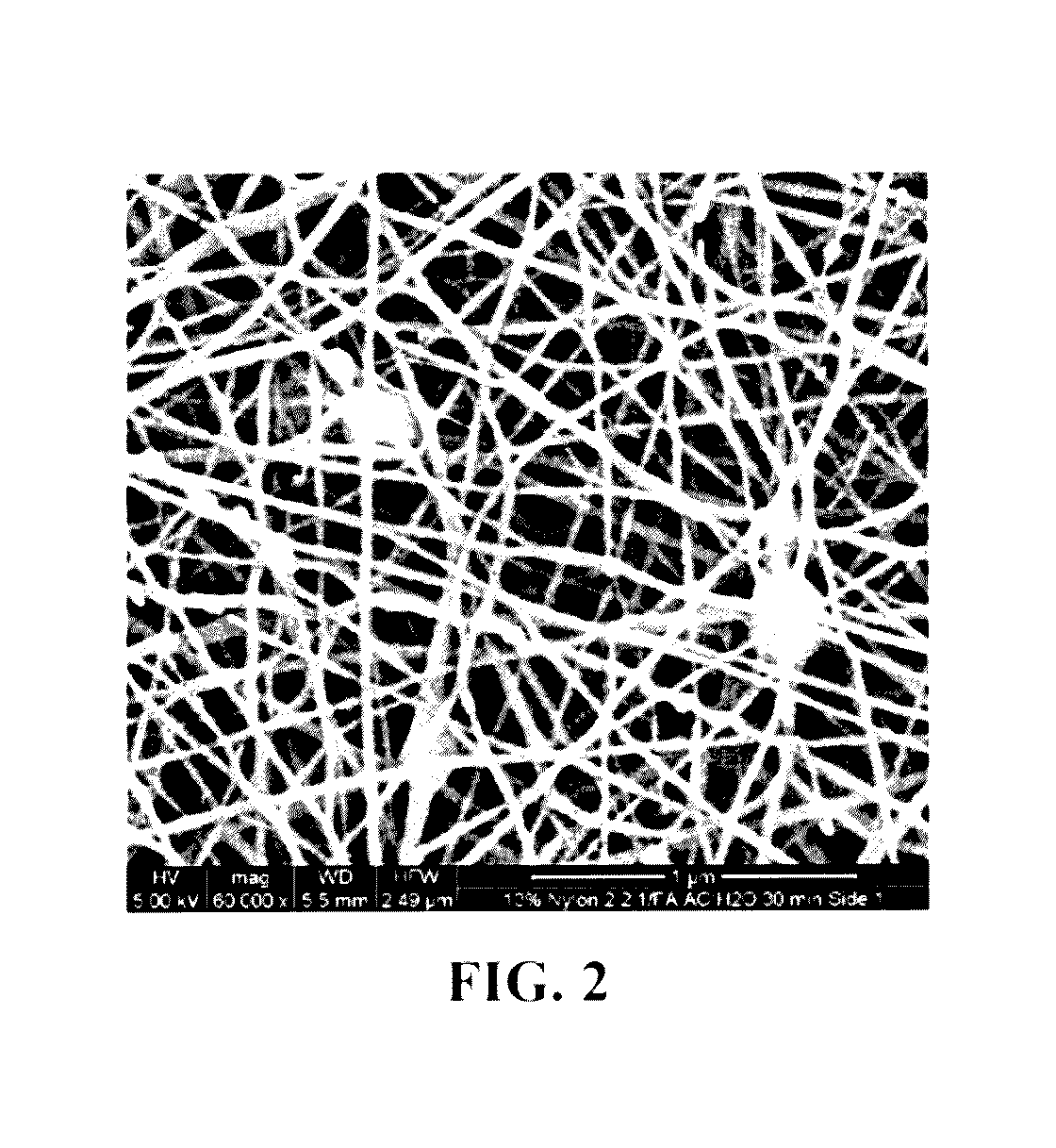
A method for removing retroviruses from liquid samples and a nanofiber containing liquid filtration medium that simultaneously exhibits high liquid permeability and high microorganism retention is disclosed. Retroviruses are removed from a liquid by passing the liquid through a porous nanofiber containing filtration medium having a retrovirus LRV greater than about 6, and the nanofiber(s) has a diameter from about 10 nm to about 100 nm. The filtration medium can be in the form of a fibrous electrospun polymeric nanofiber liquid filtration medium mat.
Owner:MILLIPORE CORP
Duplex-specific chimeric antigen receptor and application thereof
ActiveCN109748975AMultiple cytokinesAchieving specific killing effectMammal material medical ingredientsFermentationSingle-Chain AntibodiesAntigen receptors
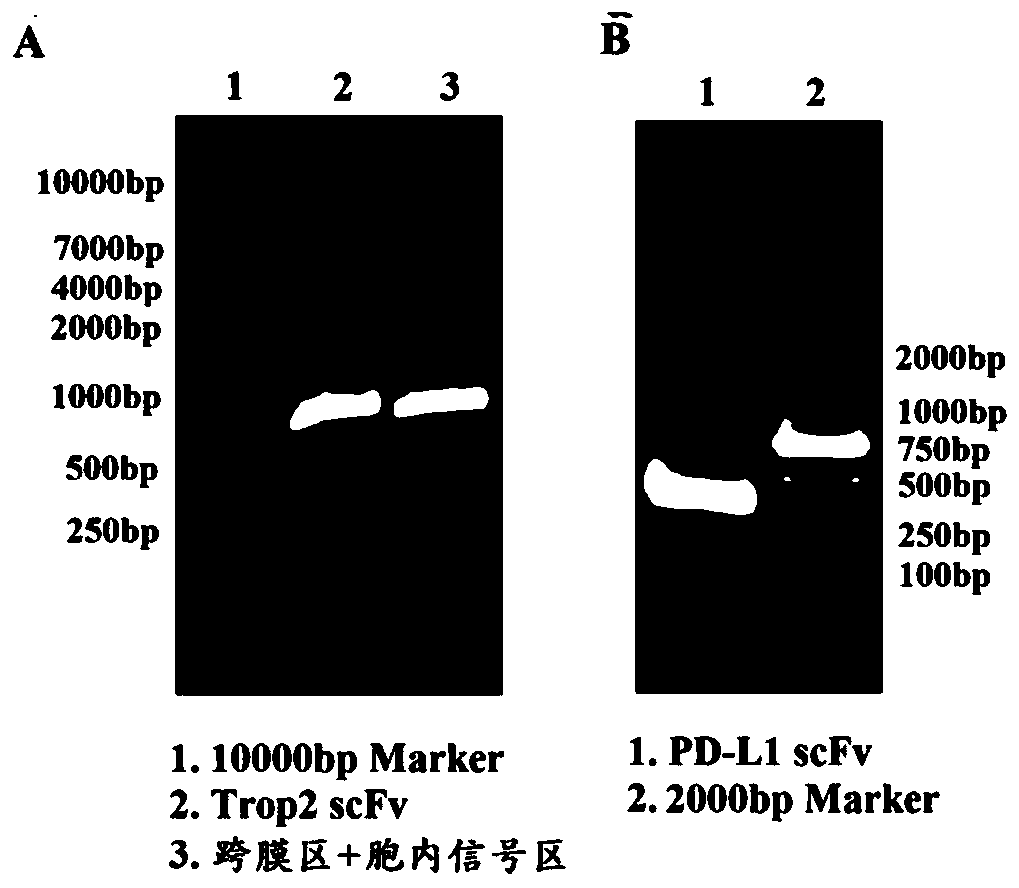
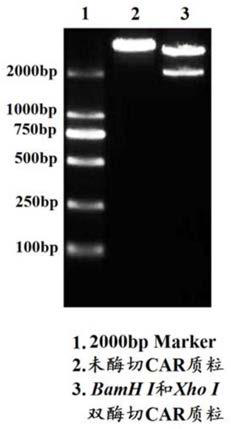
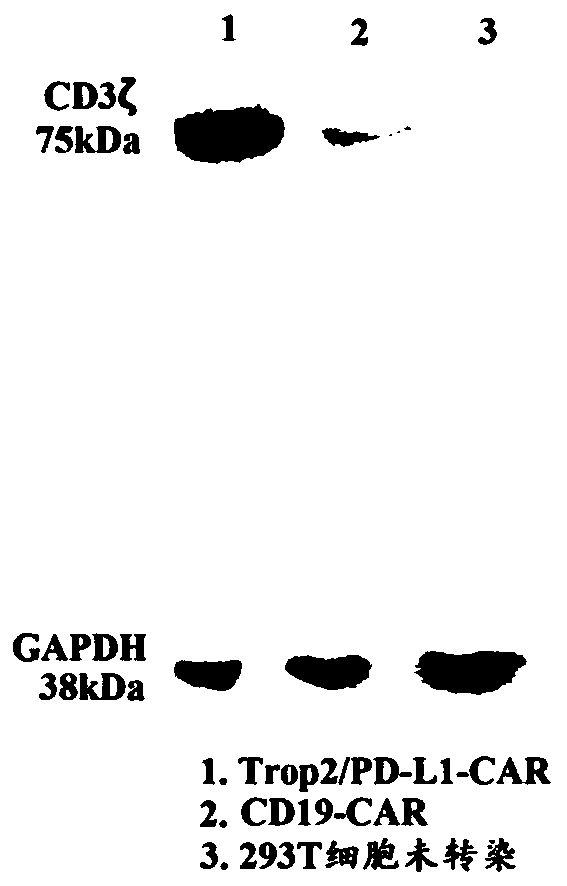
The invention relates to a duplex-specific chimeric antigen receptor and application thereof. The duplex-specific chimeric antigen receptor is composed of CD8alpha, a humanization anti-Trop2 and anti-PD-L1 single-chain antibody, a transmembrane region Cd8TM, a notch intracellular domain CD28, a notch intracellular domain CD137 and a notch intracellular domain CD3zeta in serial connection. According to the chimeric antigen receptor CD8alpha-Trop2 / PD-L1 scFv-CD8TM-CD28-CD137-CD3zeta, by adopting a retrovirus technology, T lymphocyte of the human can be infected in vitro, obtained CAR-T cells cansimultaneously express tumor cells of Trop 2 and PD-L1 antigens through partial specific identification of the single-chain antibody of CAR, the CAR-T cells are activated and release multiple kinds of cell factors, and the specific killing effect on the tumor cells is achieved.
Owner:NANJING FIRST HOSPITAL
Antiviral JAK inhibitors useful in treating or preventing retroviral and other viral infections
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
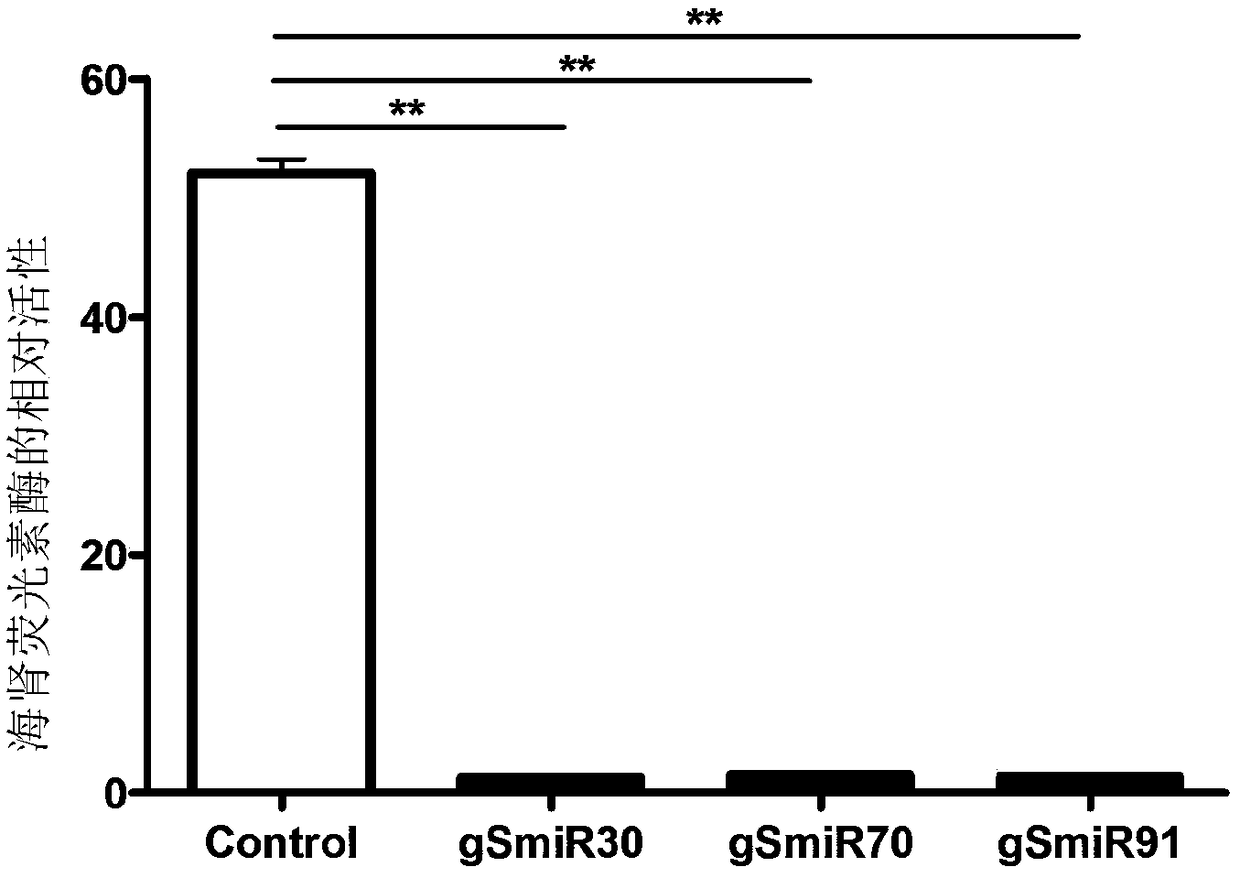
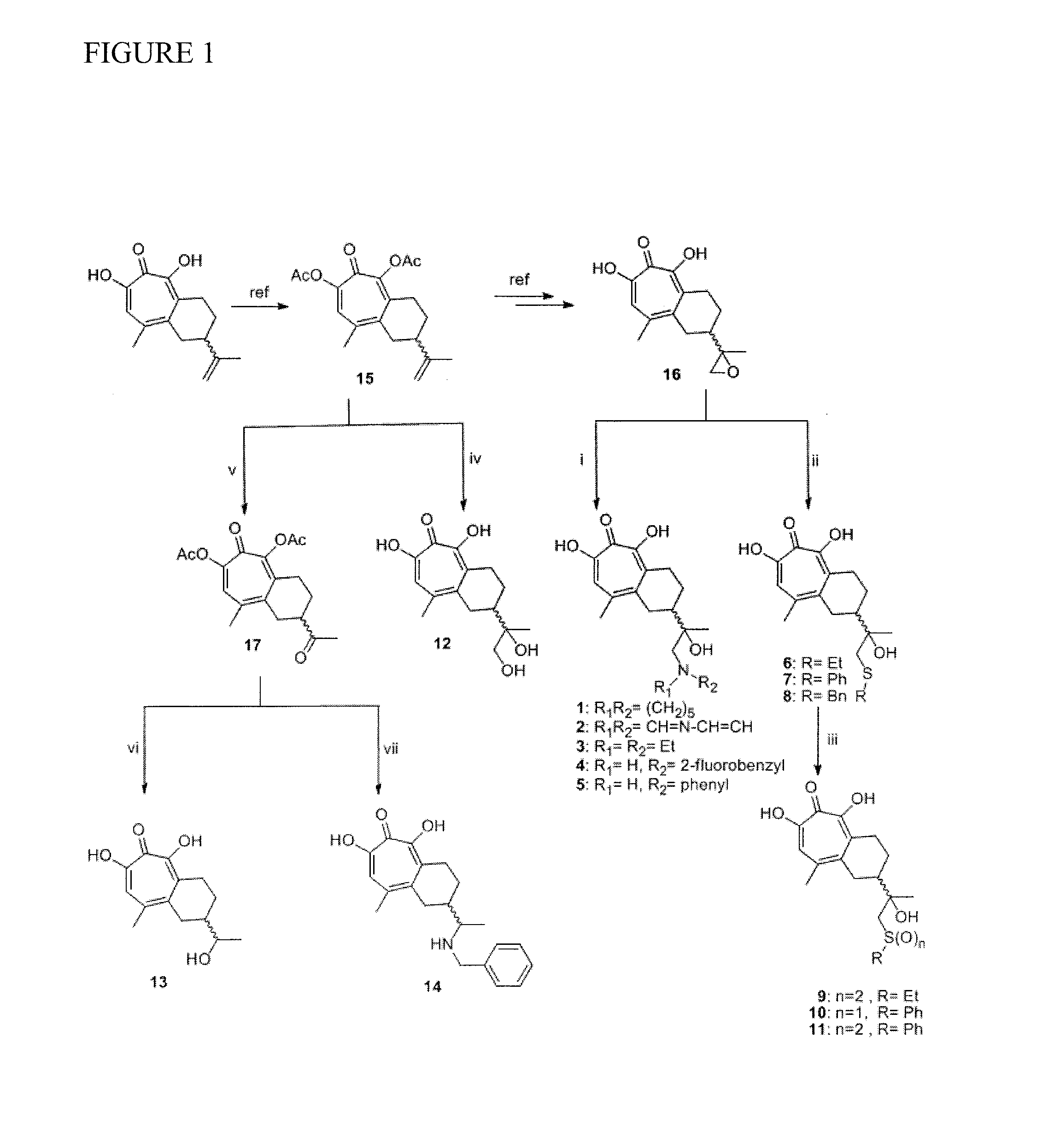
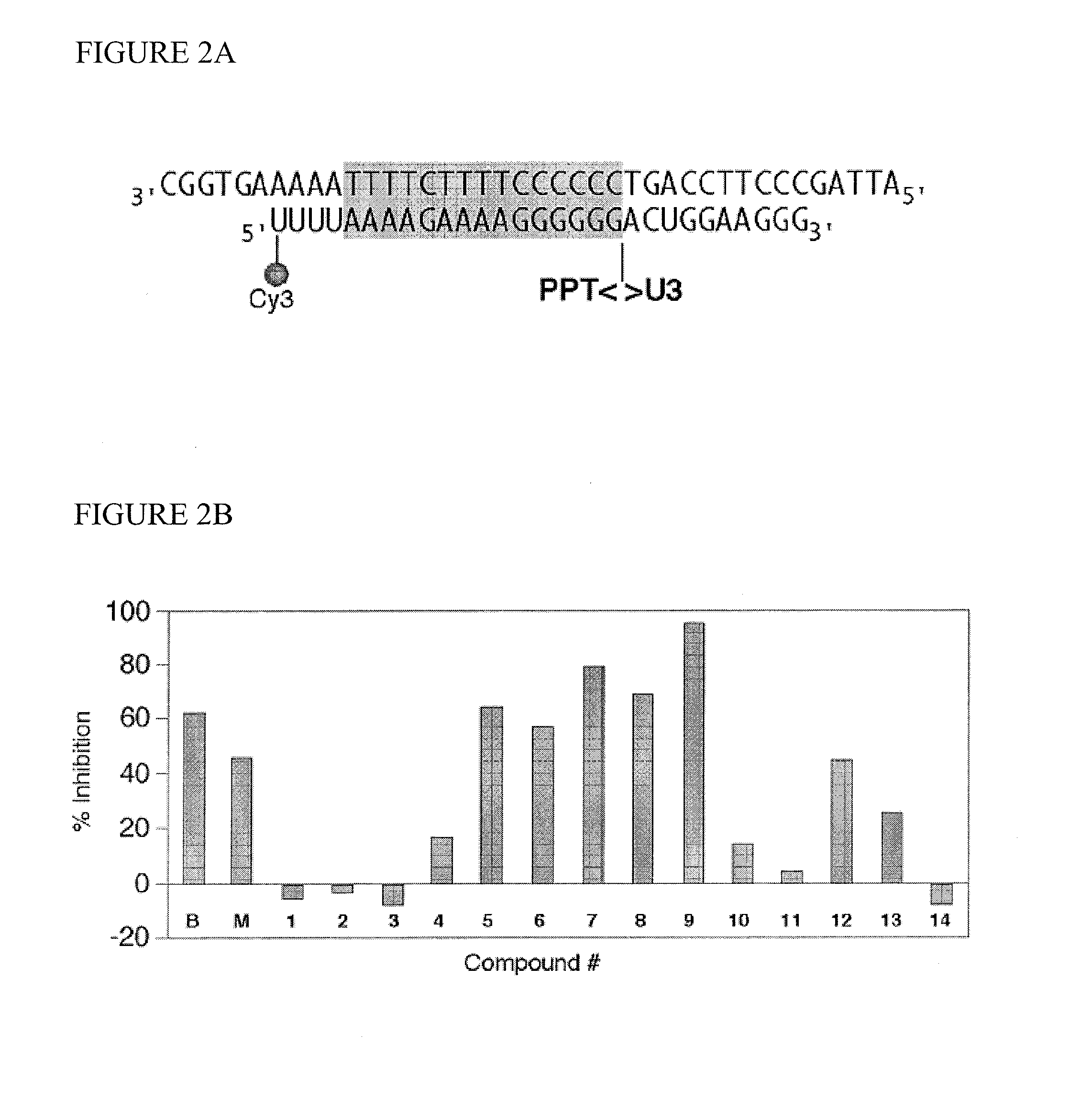
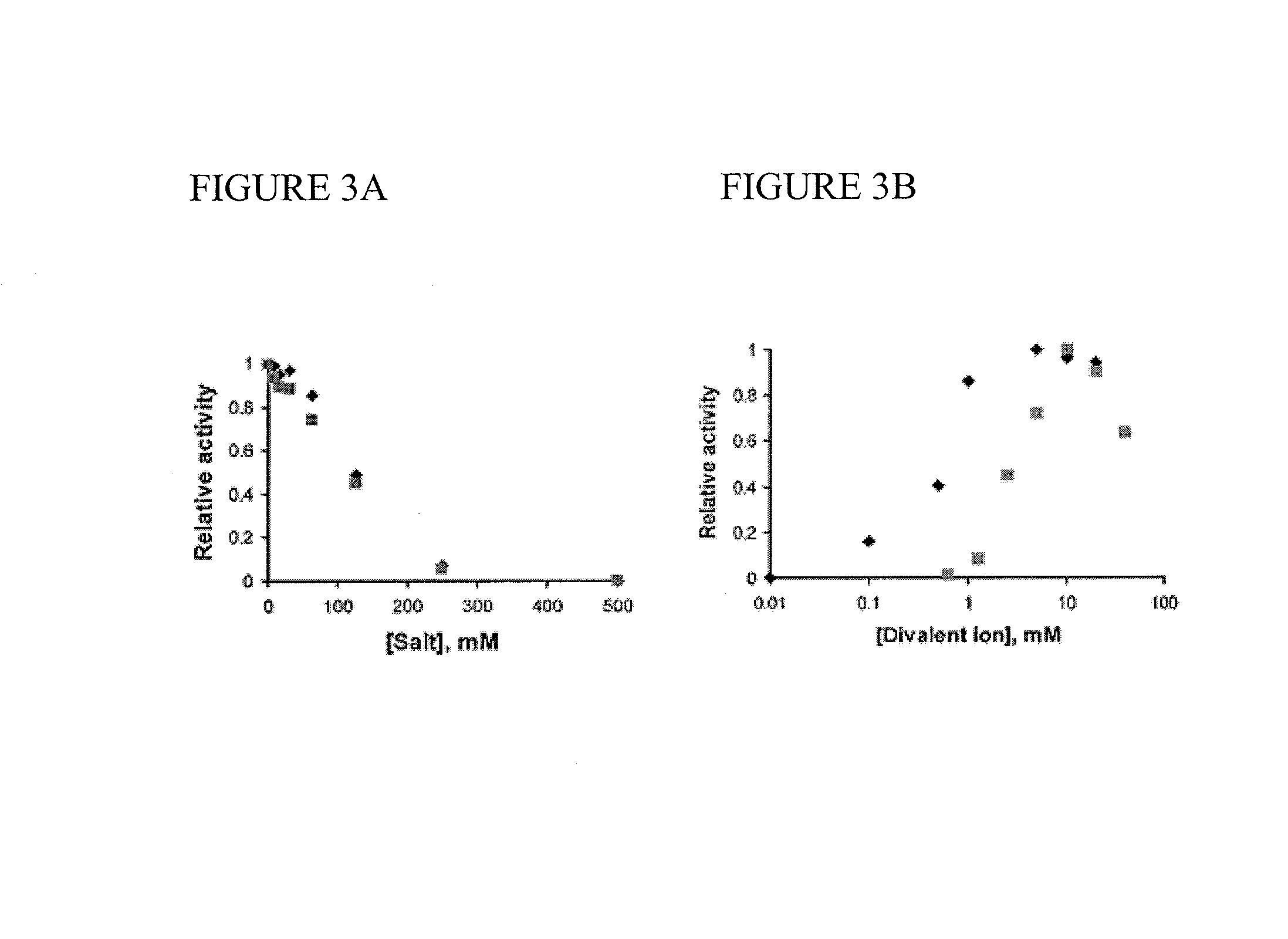
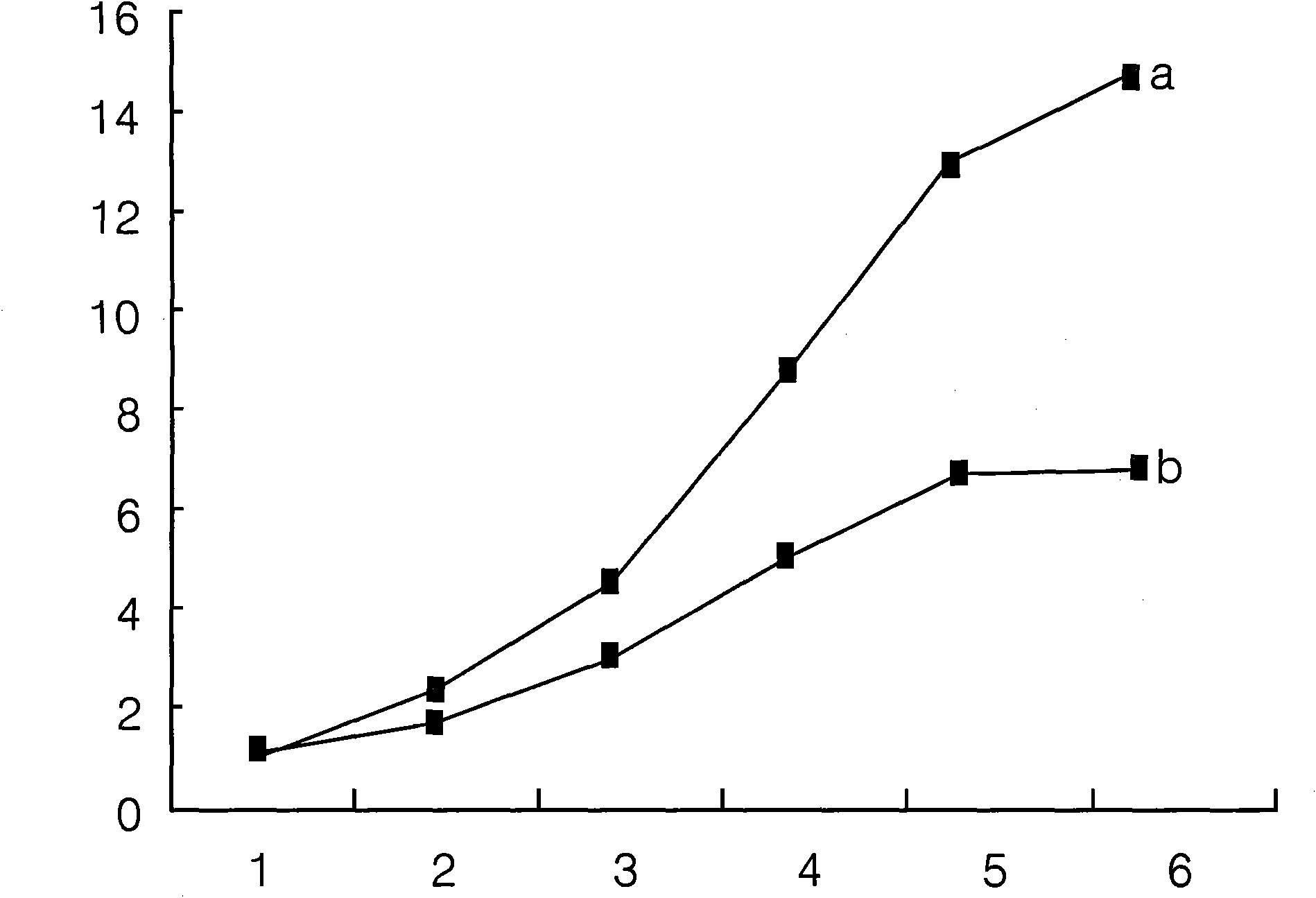
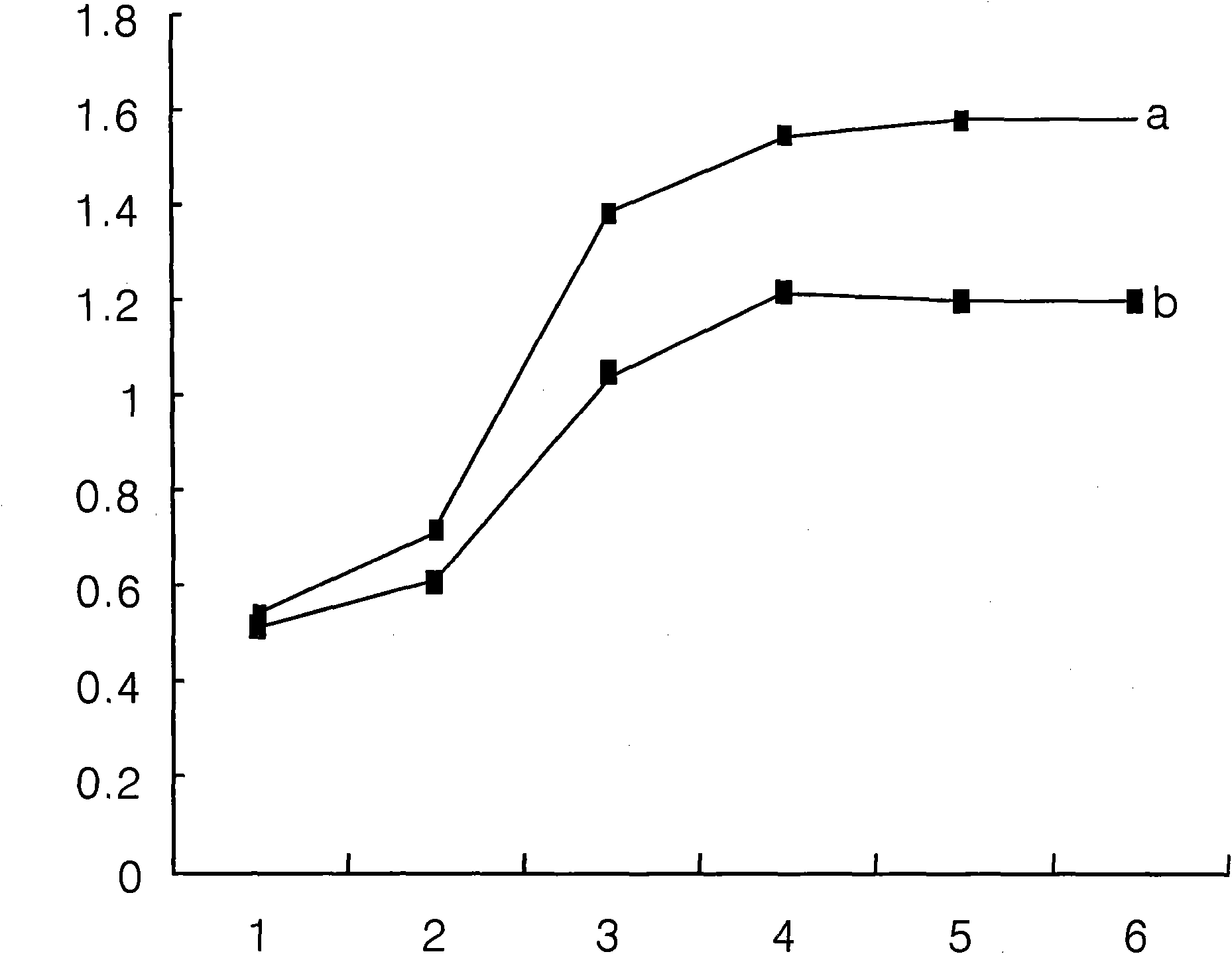
Use of 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid or salt thereof for treating retrovirus infection
The present invention provides a use of a therapeutically effective amount of 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (herein referred to as Compound I) or a pharmaceutically acceptable salt thereof, for the production of an agent for the treatment in a patient. The invention further provides a use of Compound I or a salt thereof for an agent for inhibition of integrase activity. Compound I or a salt thereof is also effective in inhibiting the replication of a retrovirus resistant to at least one anti-retroviral drug. In the use of the invention, Compound I or a salt thereof may be administered alone or in combination with at least one anti-retroviral drug other than Compound I or a salt thereof. The present invention also provides kits comprising Compound I or a salt thereof.
Owner:JAPAN TOBACCO INC
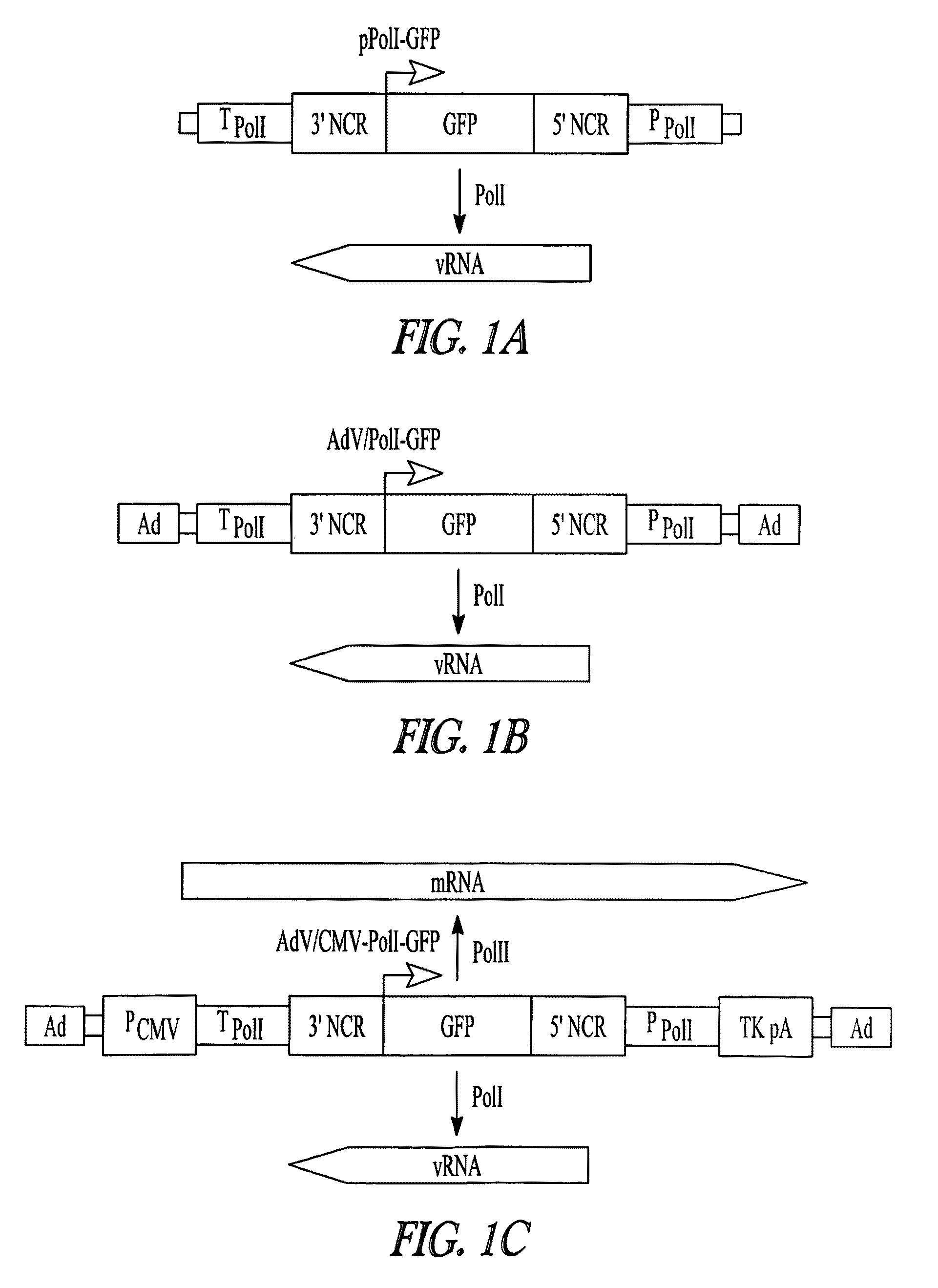
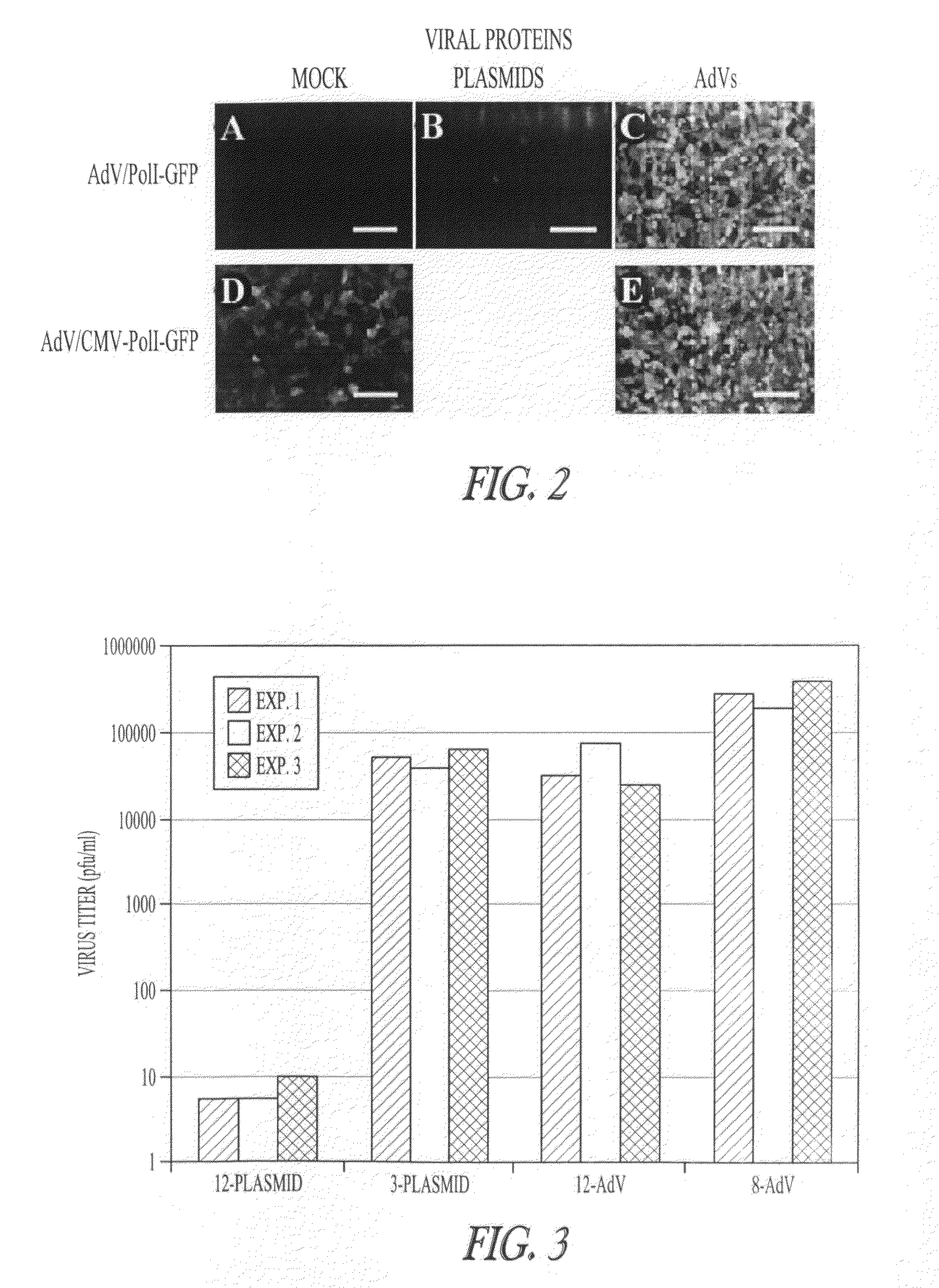
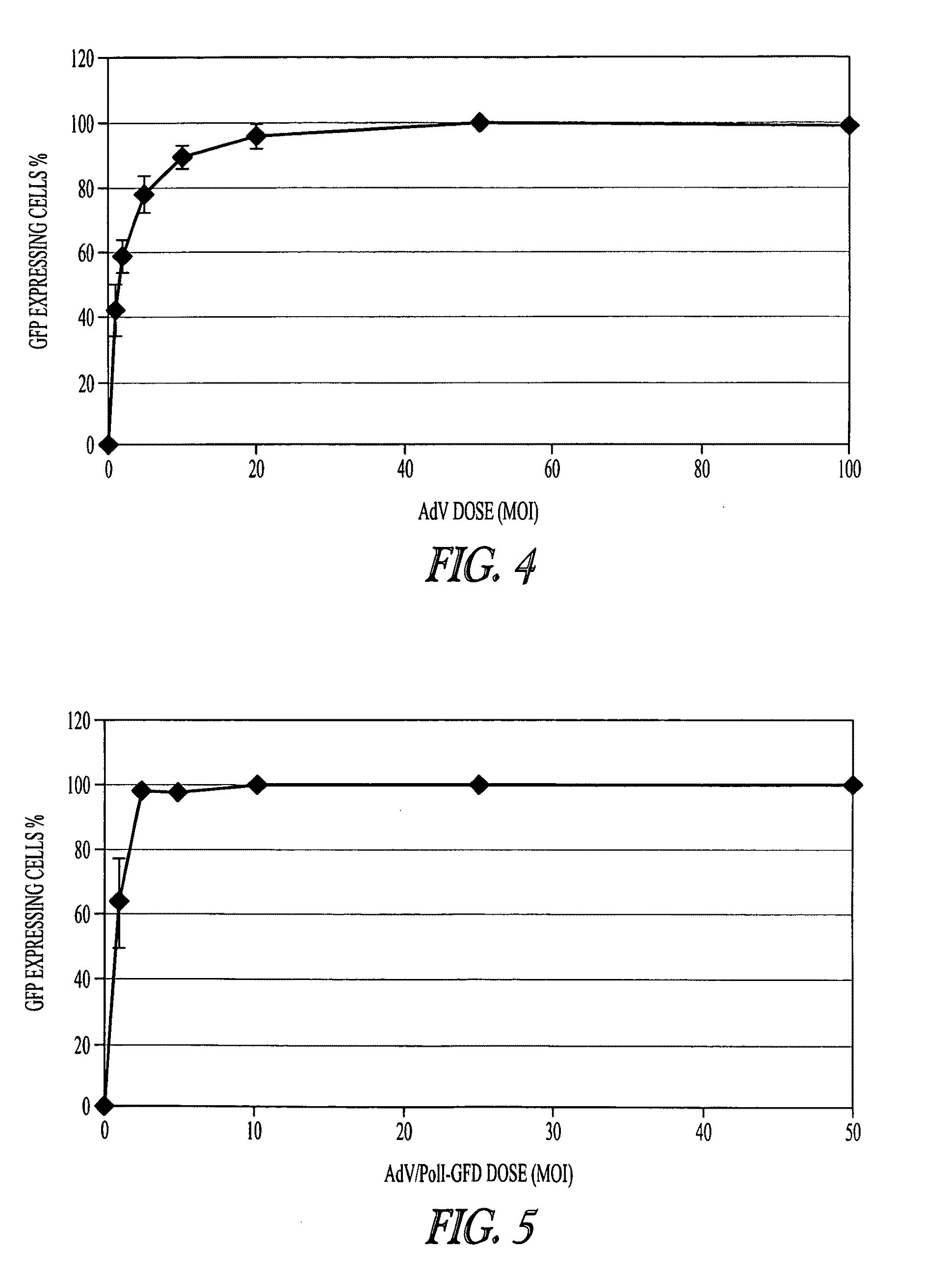
Adenoviral vectors for influenza virus production
ActiveUS8043856B2High expressionIncrease productionSsRNA viruses negative-senseAnimal cellsVirus-RetrovirusD'Aguilar virus
The invention provides adenovirus and retrovirus vectors useful to prepare influenza virus. Also provided is a canine RNA polymerase I promoter and vectors having that promoter.
Owner:WISCONSIN ALUMNI RES FOUND
RT-LAMP nucleic acid test strip kit for detection of porcine reproductive and respiratory syndrome virus, and applications
InactiveCN103276104ALow costReduce use costMicrobiological testing/measurementBetaineNucleic acid test
The invention discloses a RT-LAMP nucleic acid test strip kit for detection of a porcine reproductive and respiratory syndrome virus, and applications The kit comprises a primer combination with a nucleotide sequence represented by SEQ ID NO. 1-6 and nucleic acid detection test strips. The usage of the kit is as follows: first preparing RT-LAMP reaction system comprising an AMV retrovirus, a 1* reaction buffer, a Bst DNA polymerase, a dNTP mixture, betaine, MgSO4, a FIP primer, a BIP primer, a LoopF primer, a LoopB primer, a F3 primer, a B3 primer, and RNA of a sample to be measured; carrying out a reaction at a constant temperature, after testing the obtained products by using the nucleic-acid-detecting test strip, judging and reading directly: the positive result is that two red strips appear, and one strip is in the detection zone while the other strip is in the control zone. The kit has the advantages of simple operation, low cost, easy observation of the reaction result, good specificity, easy popularization and application in large scope and being extremely suitable for export quarantine, food hygiene and on-site detection in animal husbandry.
Owner:SOUTH CHINA AGRI UNIV
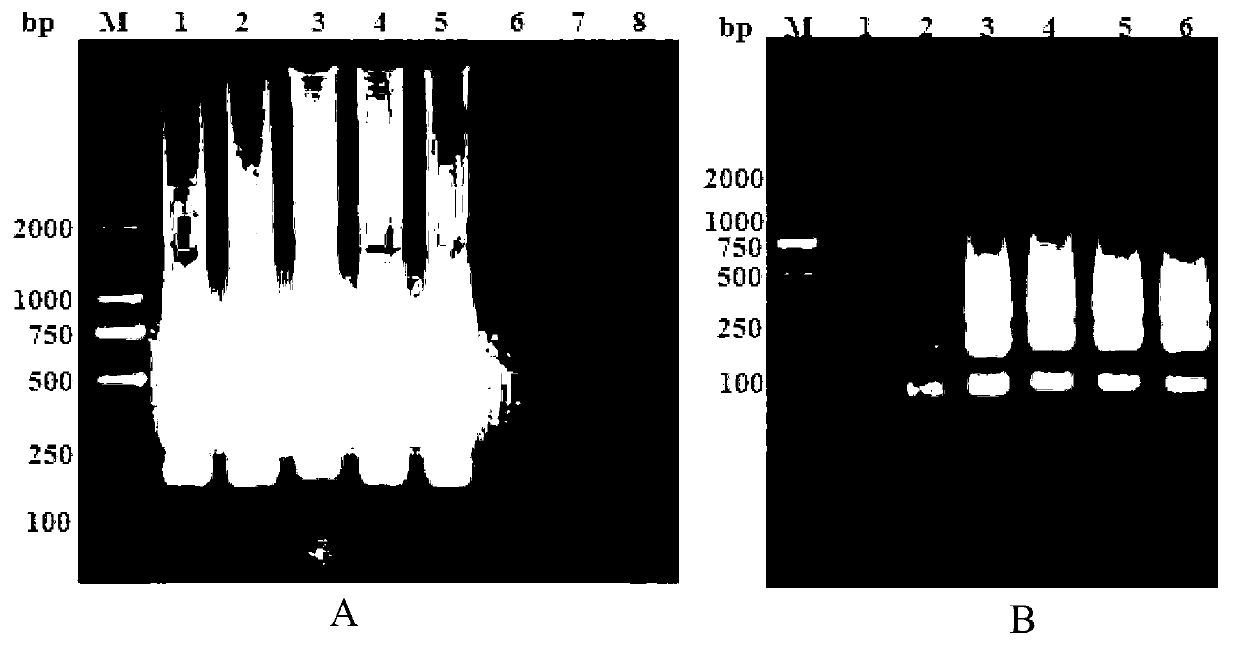
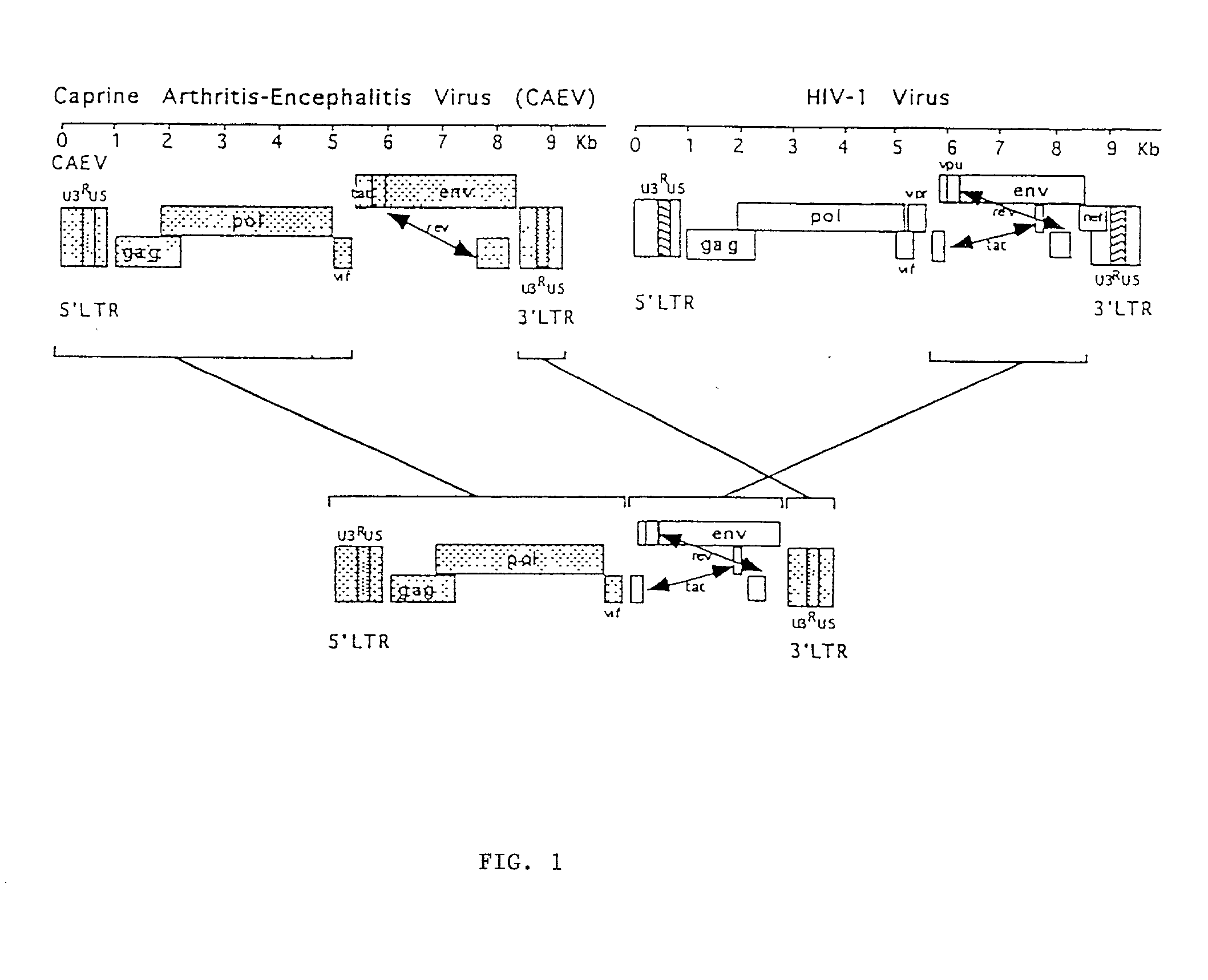
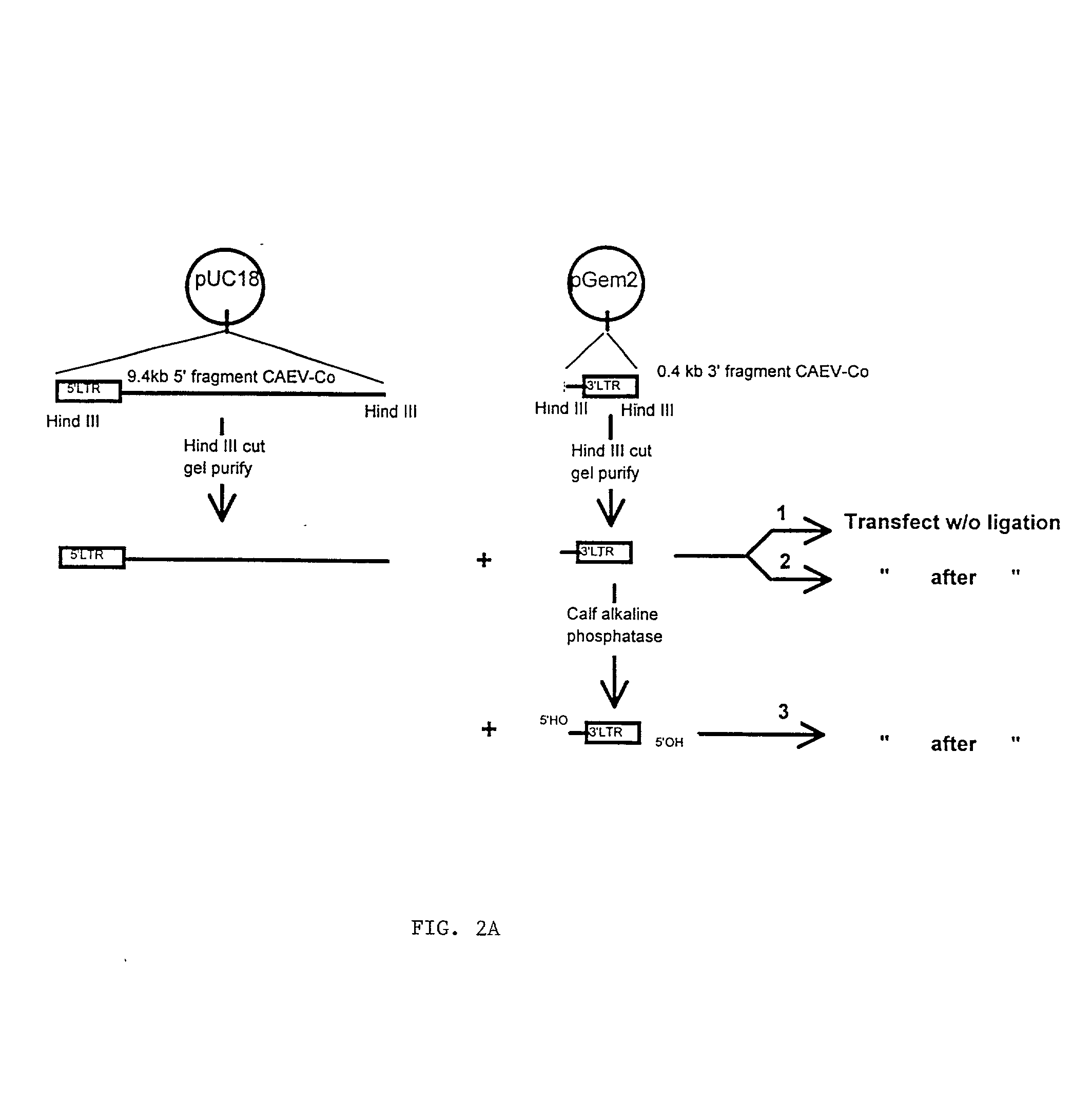
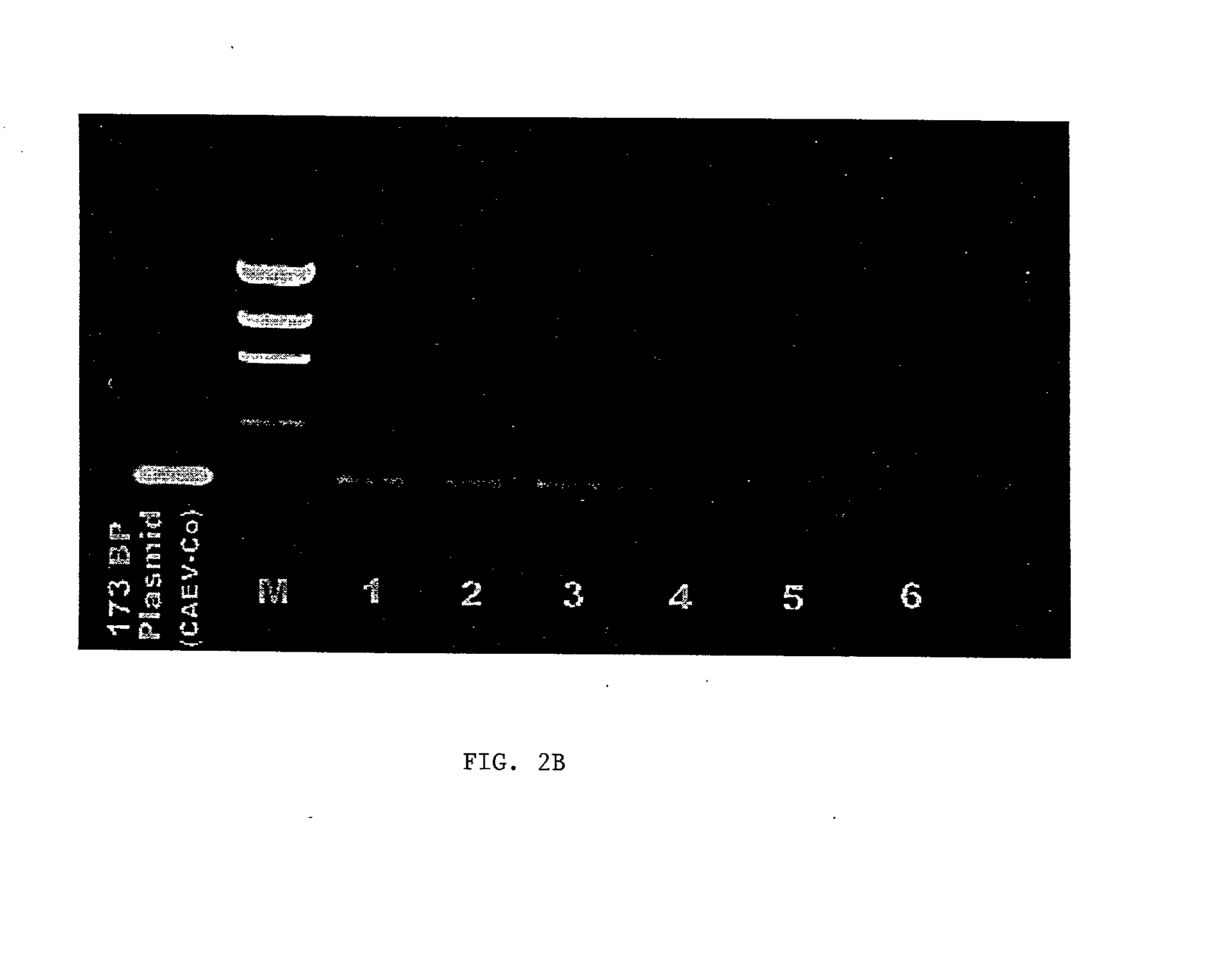
Viral chimeras comprised of caev and hiv-1 genetic elements
InactiveUS20010039669A1Virus peptidesImmunoglobulins against virusesVirus-RetrovirusImmunodeficiency virus
The present invention provides a polynucleotide comprising portions of the genomes of caprine arthritis-encephalitis virus and HIV-1, resulting in a chimeric retrovirus referred to as a "CHIV." The invention also provides a vaccine comprising a CHIV immunogen and a pharmaceutically acceptable carrier. A method of stimulating an immune response in an individual against human immunodeficiency virus-1 infection by administering a therapeutically effective amount of a CHIV immunogen is also provided. The invention further provides a method of stimulating an immune response in vitro by contacting a lymphocyte with a therapeutically effective amount of a CHIV immunogen.
Owner:UNIV OF SOUTHERN CALIFORNIA
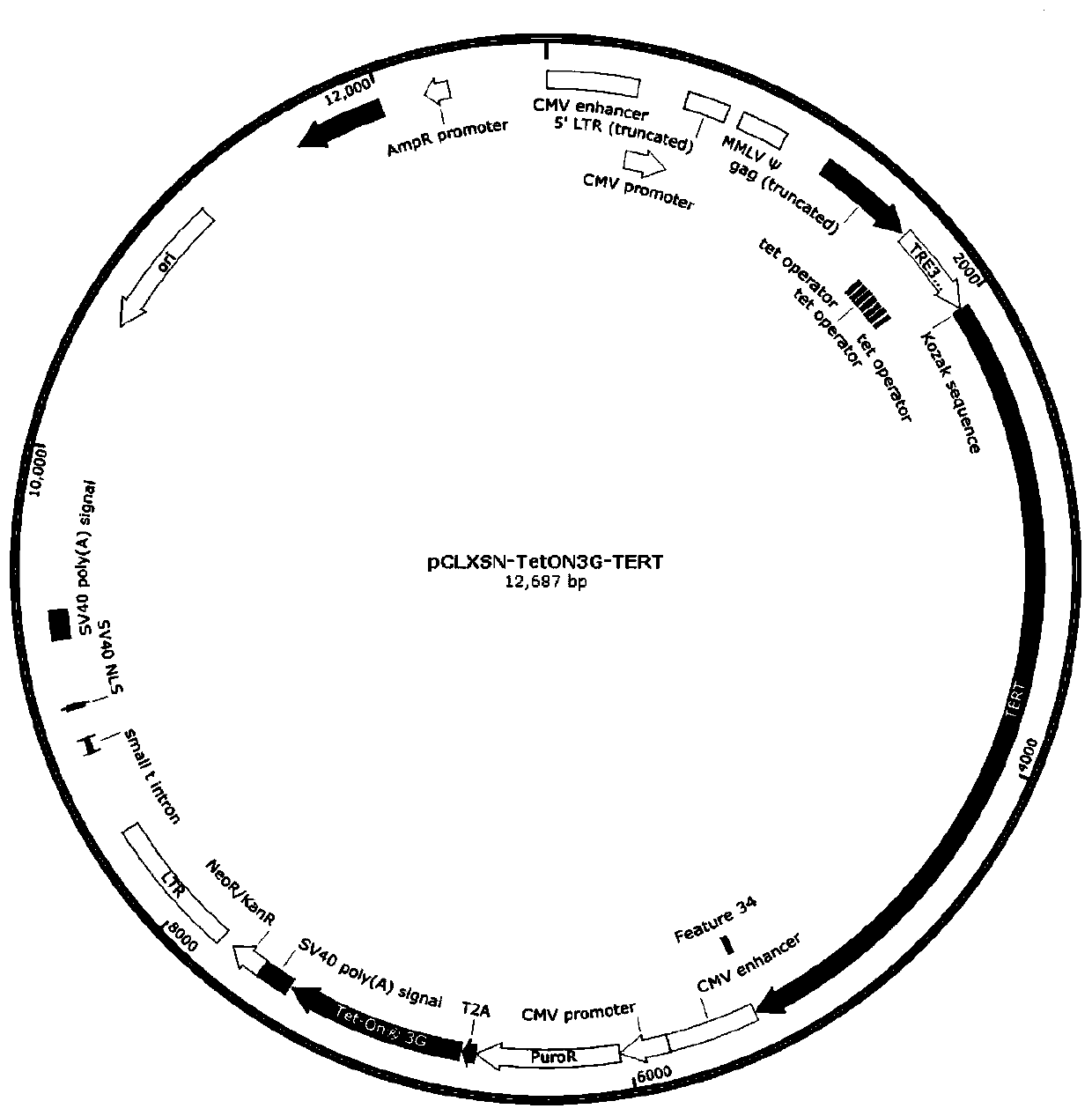
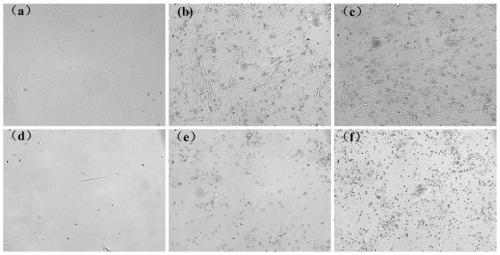
Controllable immortalized retrovirus vector and human umbilical cord mesenchymal stem cell and construction method thereof
PendingCN110106201AControllable expressionStable expressionFermentationVector-based foreign material introductionDiseaseVirus-Retrovirus
The invention belongs to the technical field of biology, and particularly relates to a controllable immortalized retrovirus vector and human umbilical cord mesenchymal stem cell and a construction method thereof. The construction method comprises the steps that a Tet-on 3G system is used for constructing a controllable immortalized retrovirus vector containing immortalized factors, the retrovirusis packaged for infecting the umbilical cord mesenchymal stem cell, expression of the immortalized factor gene is induced by adding an inductor, and the gene is converted into the immortalized cell with the high multiplication capacity; when the cell needs to be used for normal physiologic or disease treatment, the inductor is removed, the immortalized factor gene is in the transcription closed state, the cell recovers the characteristics of a primary umbilical cord mesenchymal stem cell, and the cell has the great important significance in gene function research or disease treatment.
Owner:广州辉园苑医药科技有限公司
Pharmaceutical Antiretroviral Combinations Comprising Lamivudine, Festinavir and Nevirapine
InactiveUS20150104511A1Easy to manufactureBiocidePowder deliveryAcquired immunodeficiencyVirus-Retrovirus
The present invention relates to a pharmaceutical antiretroviral composition comprising lamivudine, festinavir and nevirapine, to a process for preparing such a composition and to the use of such a composition for the treatment and / or prophylaxis of diseases caused by retroviruses, especially acquired immune deficiency syndrome or an HIV infection.
Owner:CIPLA LTD
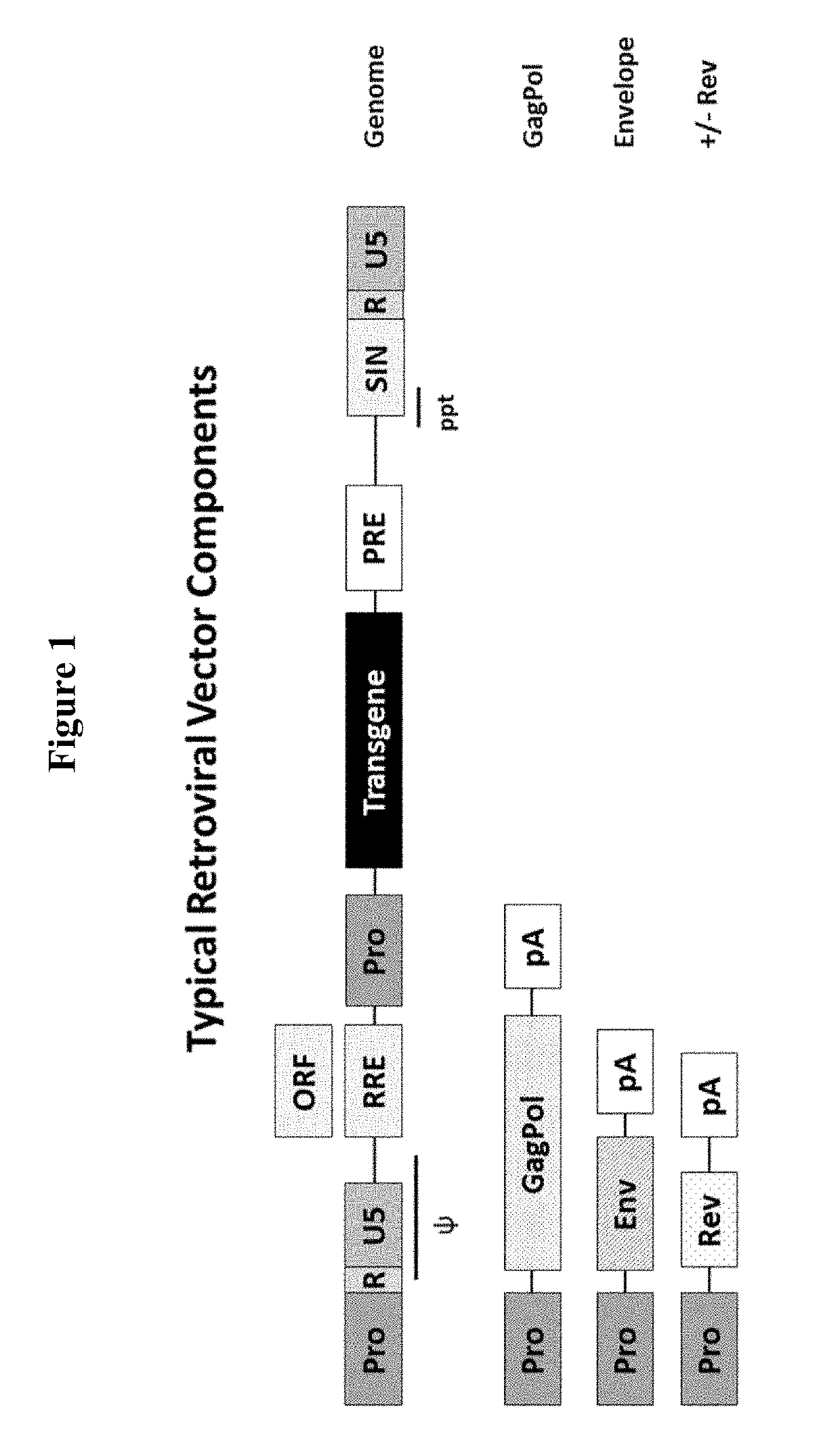
Nucleic acid constructs for producing retroviral vectors
InactiveUS20190055568A1Vector-based foreign material introductionDNA preparationVirus-RetrovirusCell Surface Proteins
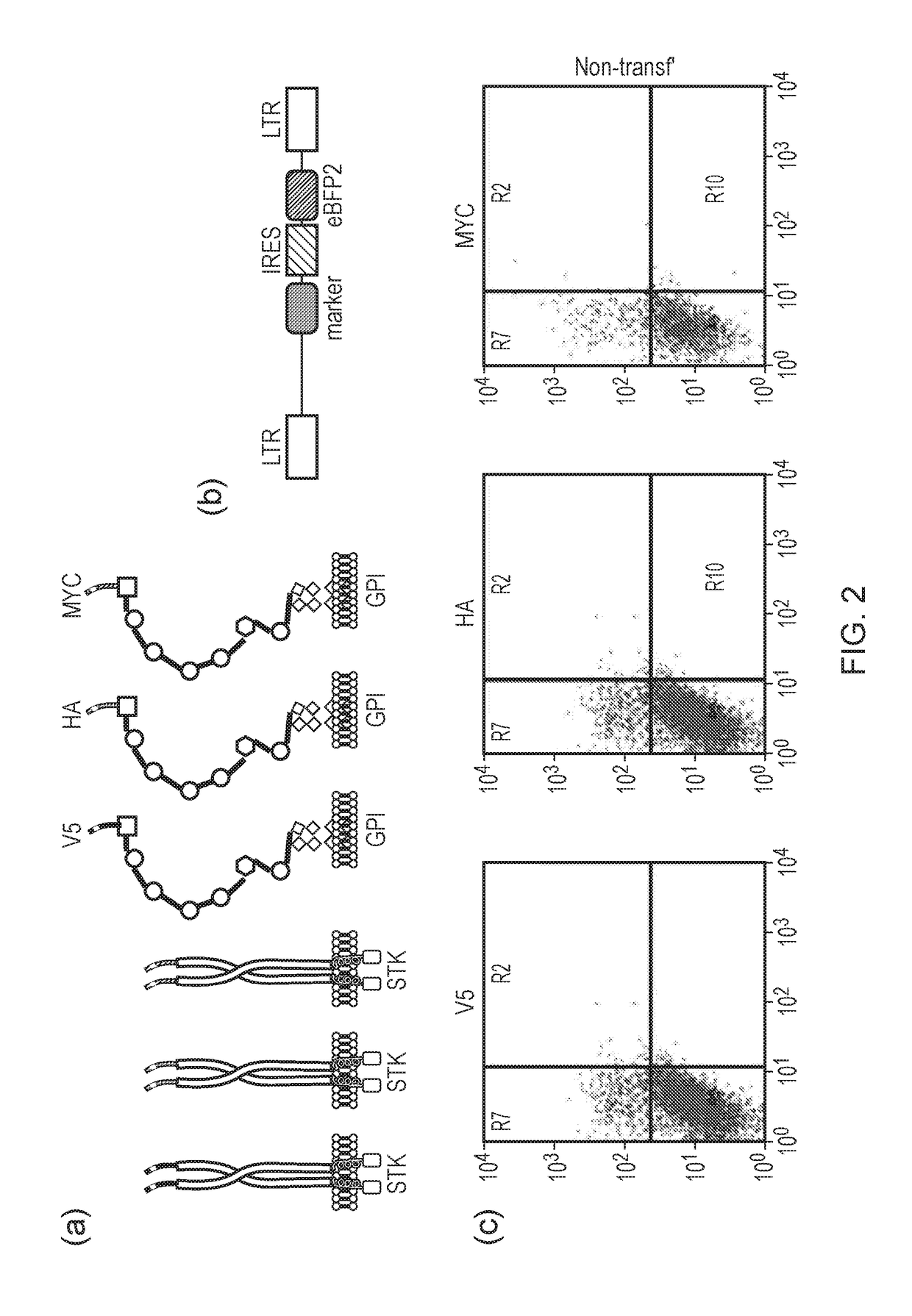
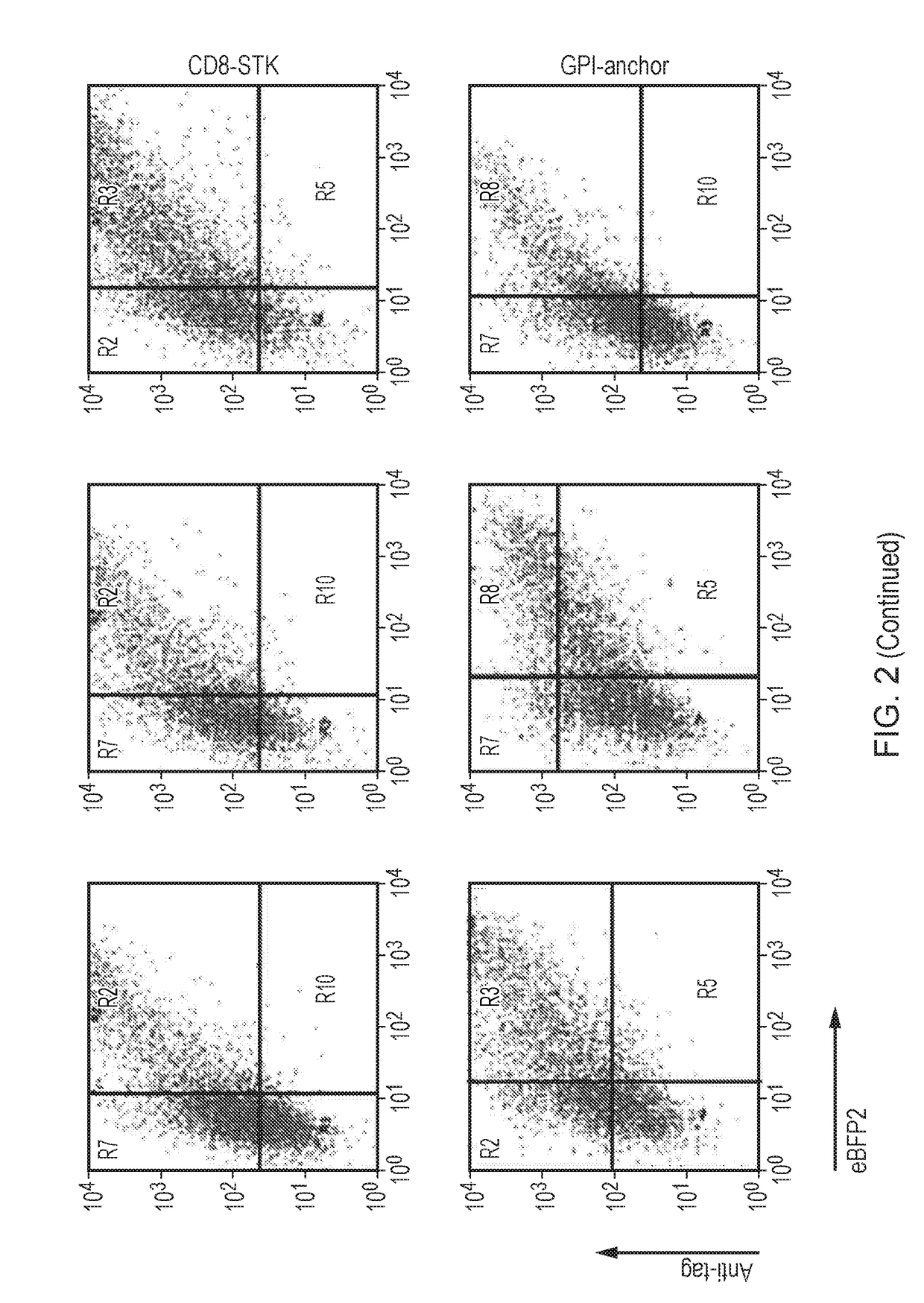
The present invention relates to a nucleic acid construct comprising: (i) a first nucleic acid sequence which either comprises a retroviral transfer vector or which encodes a retroviral protein; and (ii) a second nucleic acid sequence which encodes a detectable marker which is a cell surface protein comprising an extracellular domain and a membrane targeting domain.
Owner:UCL BUSINESS PLC
Retroviral Vector
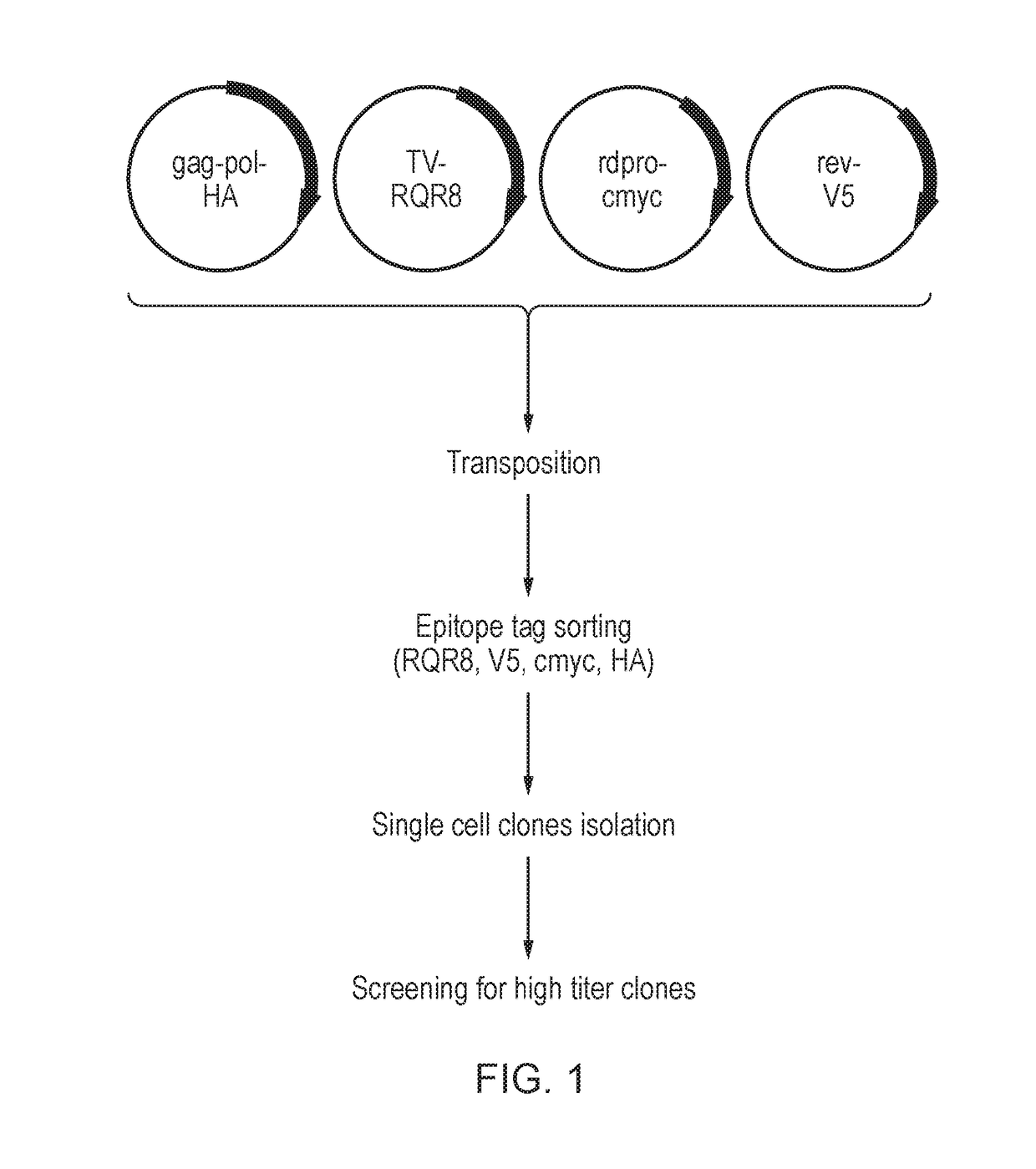
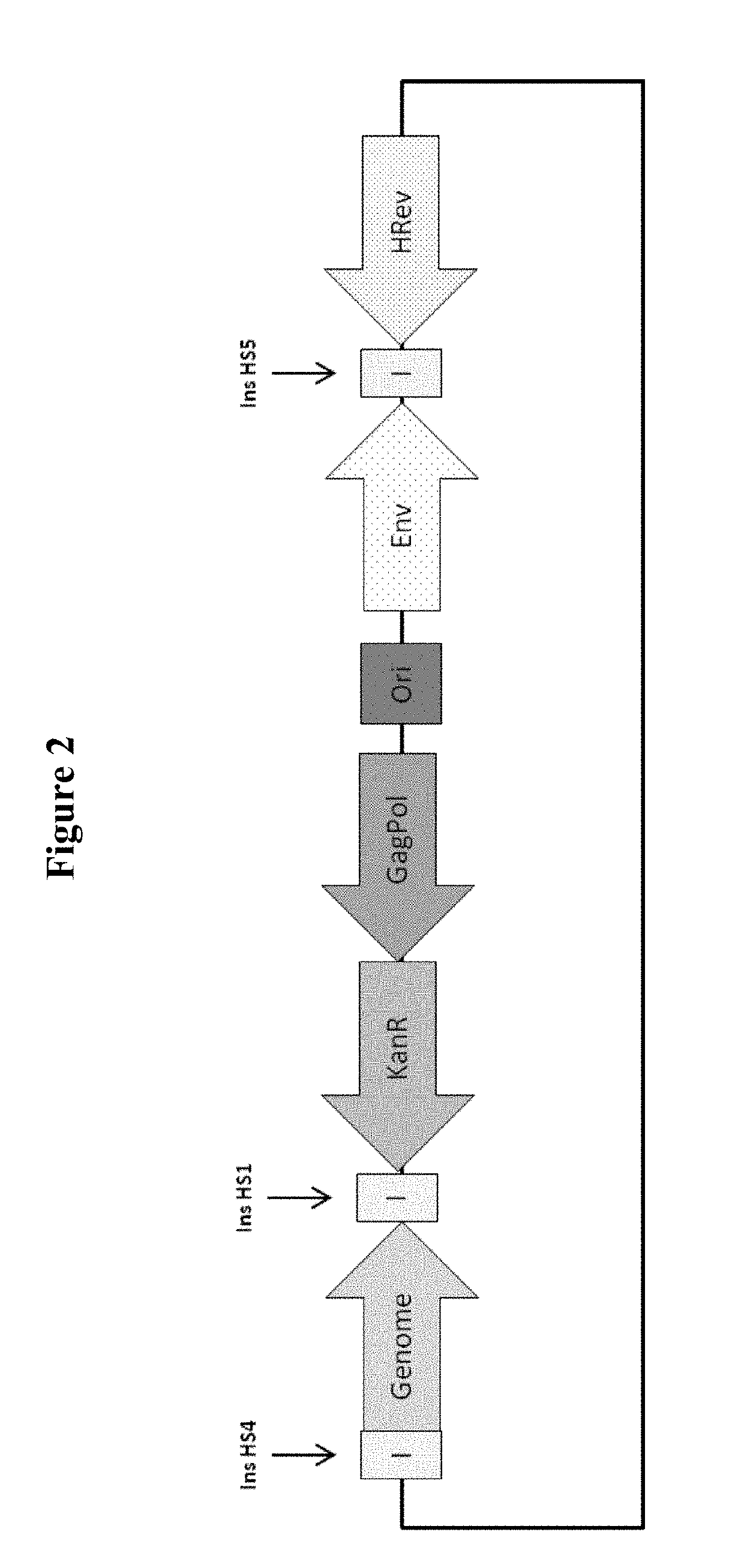
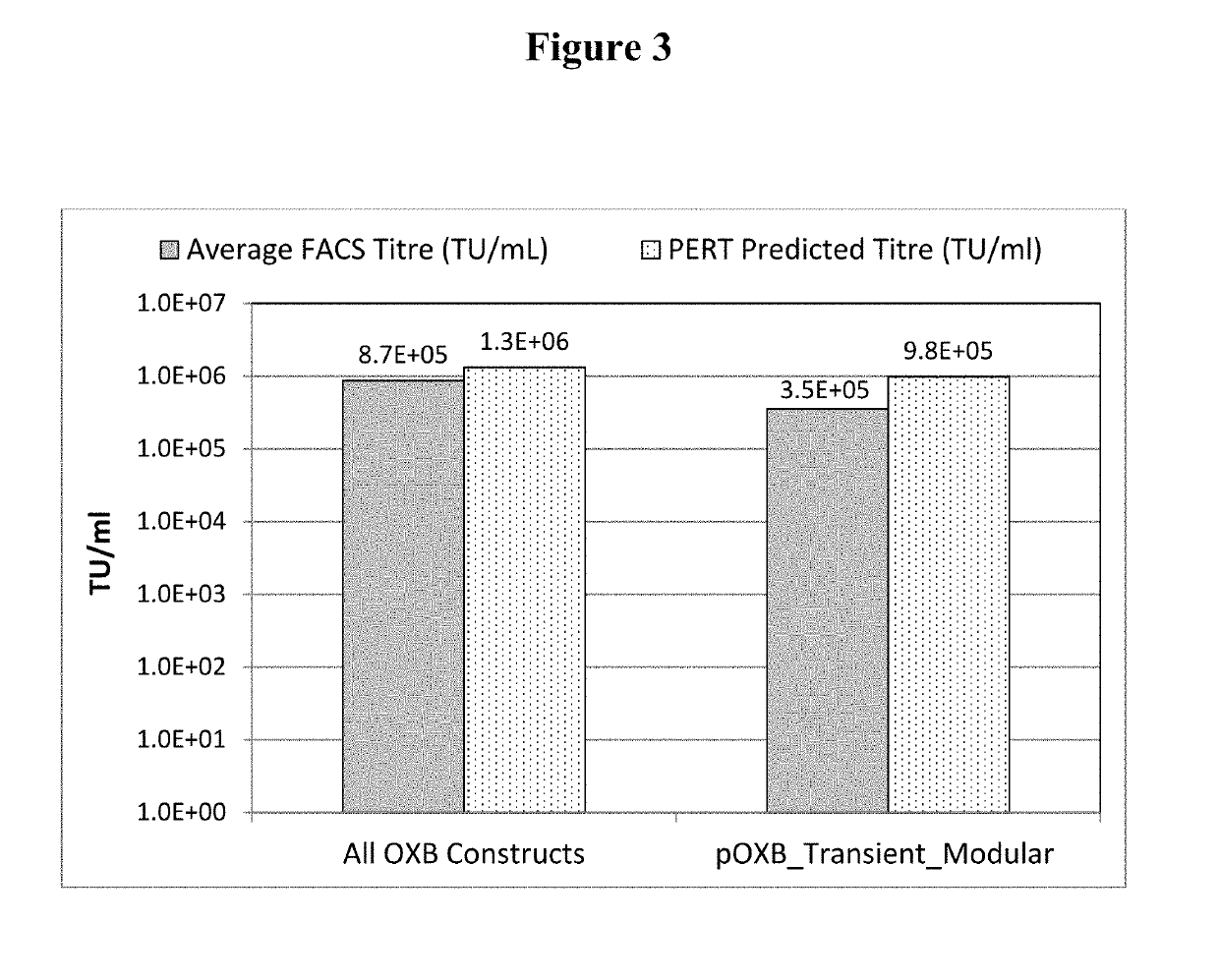
InactiveUS20190211358A1Generate efficientlyReduce usageVector-based foreign material introductionReverse transcribing RNA virusesVirus-RetrovirusNucleic acid sequencing
A cell for producing retroviral vectors comprising nucleic acid sequences encoding: i) gag-pol; ii) env; iii) the RNA genome of the retroviral vector; and iv) optionally rev, or a functional substitute thereof, wherein at least two nucleic acid sequences are located at the same genetic locus; and wherein the at least two nucleic acid sequences are in reverse and / or alternating orientations.
Owner:OXFORD BIOMEDICA (UK) LTD
MicroRNA for inhibiting expression of Sirt1 of chicken as well as recombinant over-expression plasmid and LMH cell line of microRNA
The invention discloses a microRNA for inhiting expression of Sirt1 of chicken, and also discloses a recombinant over-expression plasmid and specific application of the microRNA, and an LMH cell linefor constructing stable low-expression Sirt1 by utilizing over-expression miRNAs. Compared with the prior art, a target gene is interfered by artificially designed miRNA, and an over-expression vectorof the miRNA is directly and quickly constructed according to a simple PCR technology; pLNCX-pmirG provided by the invention is a very good vector for performing over-expression on the miRNA, retroviruses can be packaged in sequence, the cell line of stable over-expression miRNA can be quickly obtained by combining G418 pressurized screening and green fluorescent protein detecting methods; a reply mechanism of the Sirt1 that affecting the liver to a nutrition state can be disclosed by the research, and a unique hepatocyte subcloning system model is also provided for researching the functionsof human and animal livers in energy metabolism.
Owner:CHANGSHU INSTITUTE OF TECHNOLOGY
Tropolone compounds for treating or preventing retroviral infection
Disclosed are compounds that inhibit RNase H activity of retroviruses, for example, a compound of formula (I) wherein R1, R2, and R3 are as described herein, as well as pharmaceutically acceptable salts, solvates, stereoisomers, and prodrugs thereof. Pharmaceutical compositions comprising such compounds, as well as methods of use, and treatment or prevention of infection by human immunodeficiency virus (HIV).
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Method for directly determining non-separated small nucleic acid in biological sample, and detection kit thereof
ActiveCN104109708AImprove consistencyHigh sensitivityMicrobiological testing/measurementVirus-RetrovirusAntisense nucleic acid
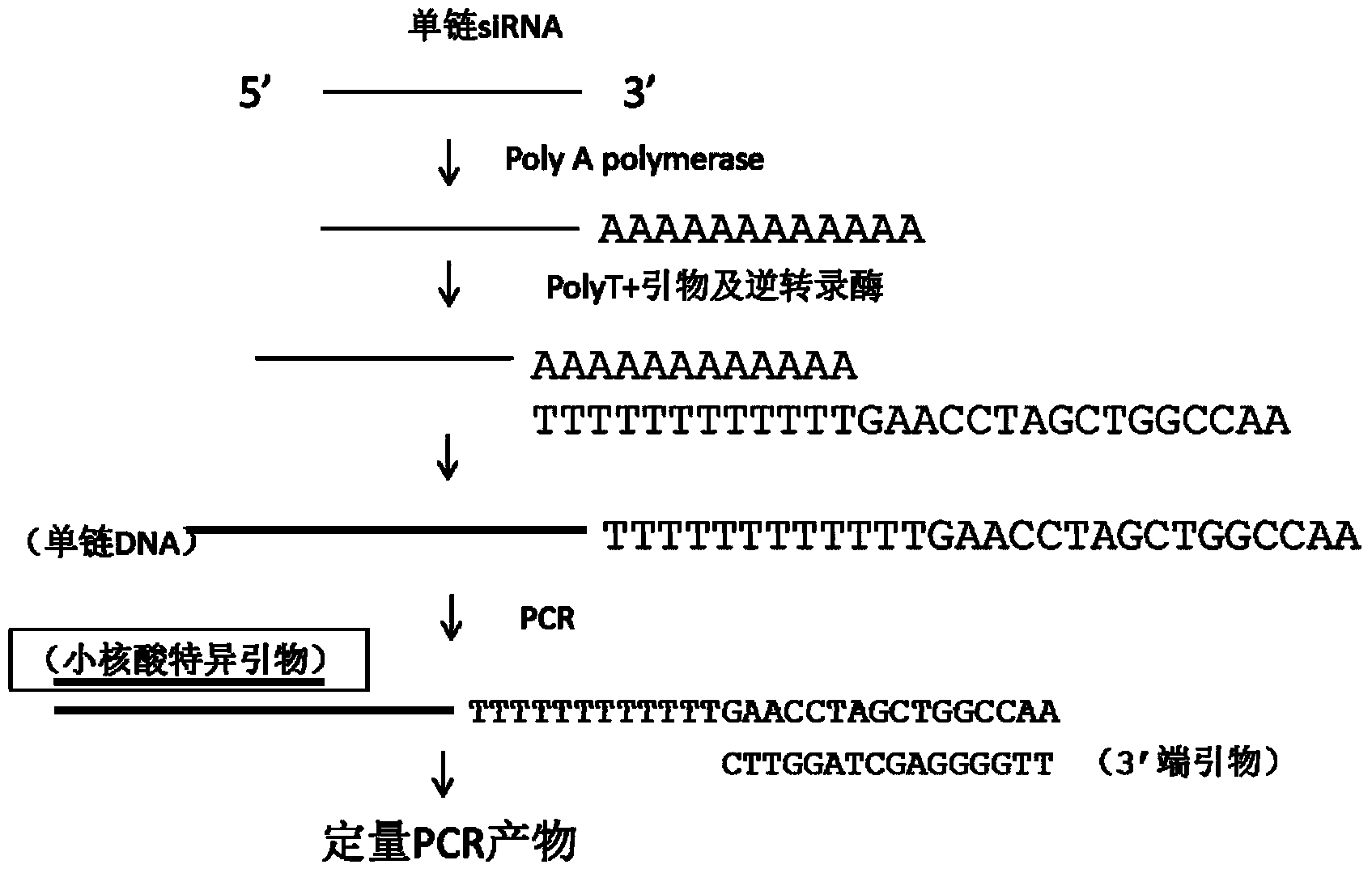
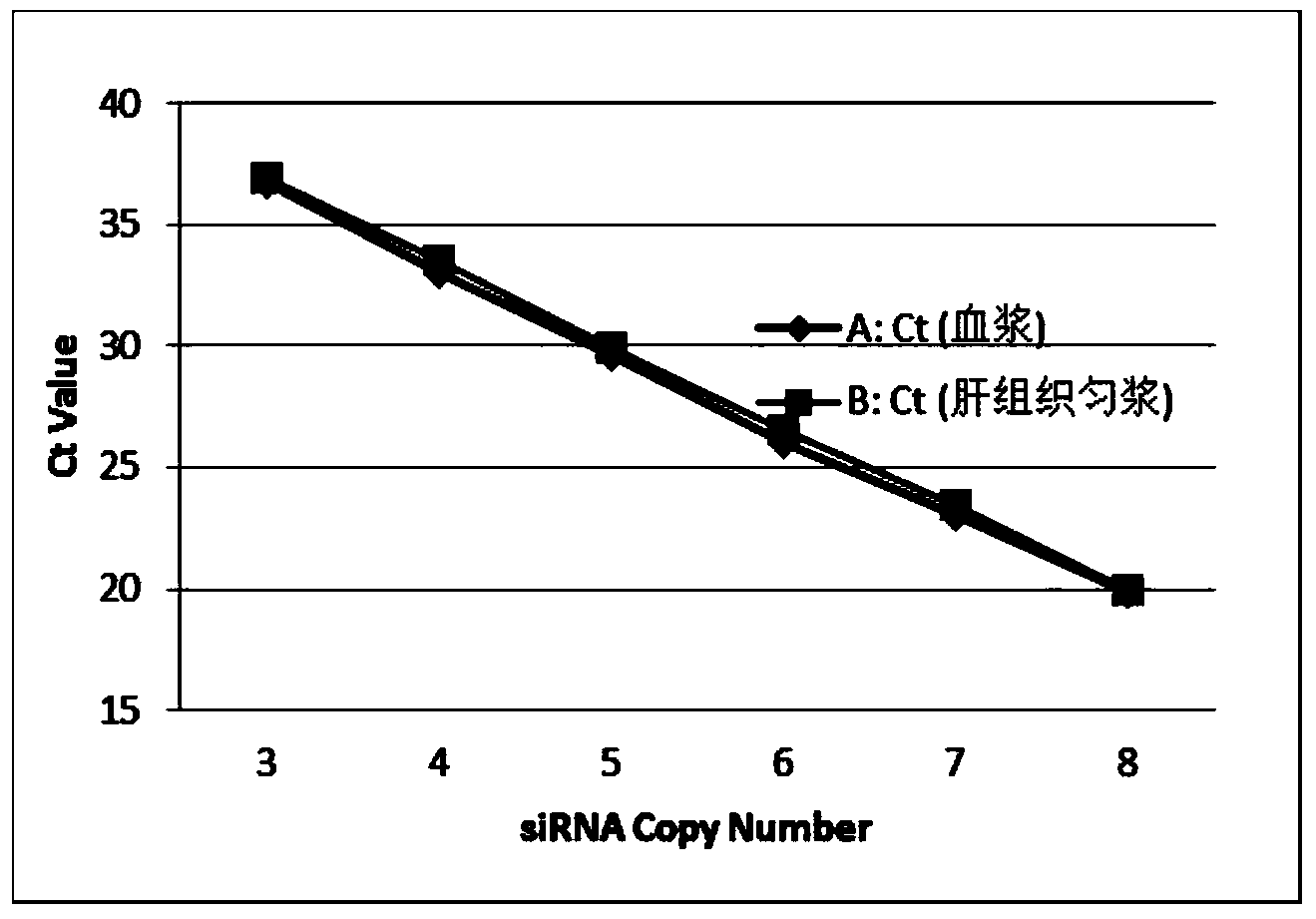
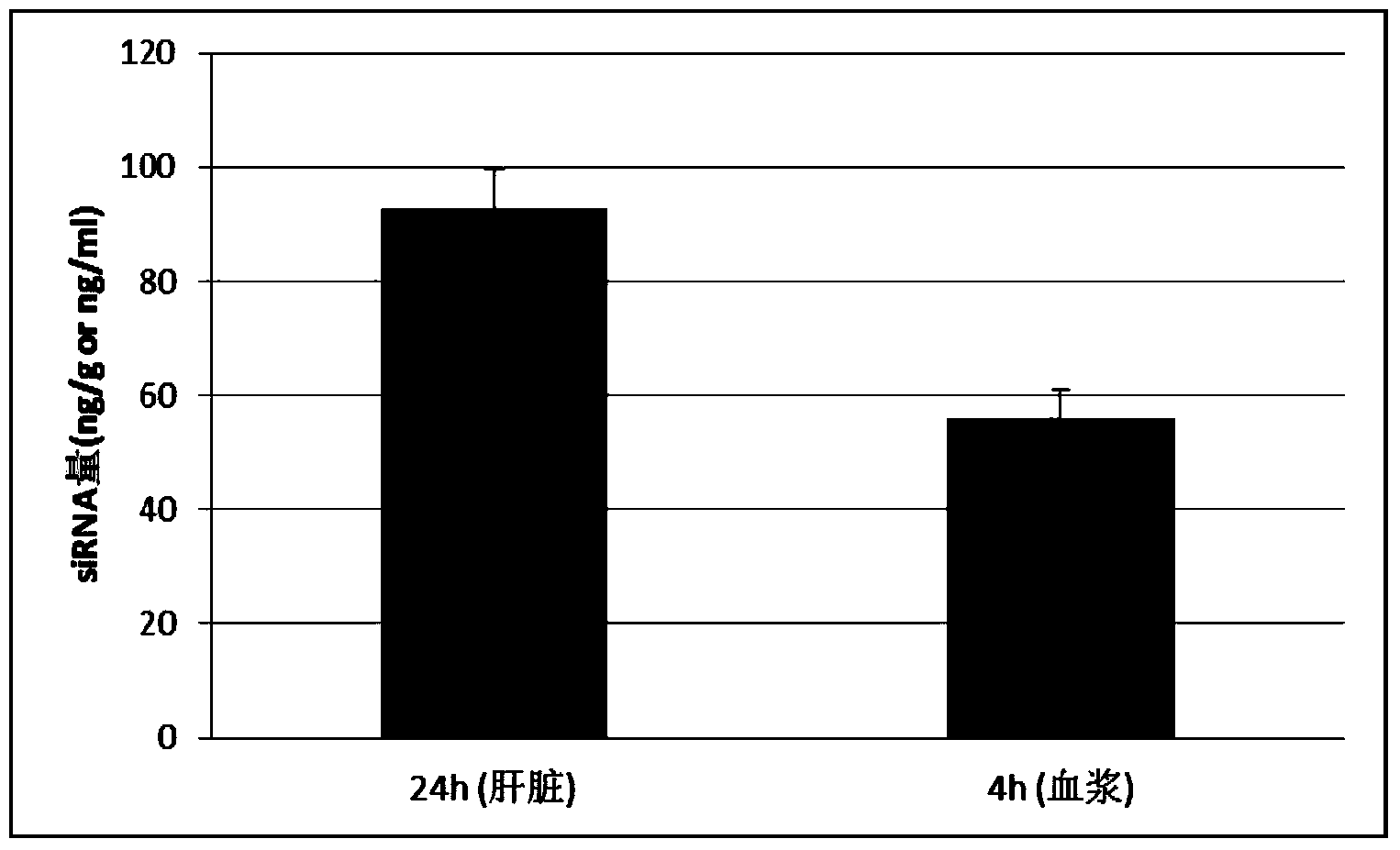
The invention discloses a method for directly determining a non-separated small nucleic acid in a biological sample. The method comprises the following steps: 1, taking the biological sample; 2, adding Ploy A to the 3'-terminal of a small nucleic acid; 2, reverse transcription reaction: adding oligomerized dT with a first primer and retrovirus to generate single strand cDNA with oligomerized T+ and a designed first primer; and 4, PCR amplification of quantitative small nucleic acid: adding a second primer completely or partially complementary to the sequence of the small nucleic acid, and a third primer completely or partially complementary to the first primer, and carrying out PCR to detect the amount of the quantitative small nucleic acid. The content of the small nucleic acid (like siRNA, miRNA, antisense nucleic acid and aptamer) in the biological sample is determined by directly using the biological sample without separating the small nucleic acid in the biological sample.
Owner:厦门成朴希晟股权投资合伙企业(有限合伙)
Pseudovirion particle and preparation method and application thereof
The invention discloses a pseudovirion particle and a preparation method and application thereof. The pseudovirion particle comprises RNA fragments of a monkey type D retrovirus encapsulated by MS2 phage capsid protein. The preparation method of the pseudovirion particle is simple, operation is easy, fineness of the obtained pseudovirion particle is high, and the pseudovirion particlecan be used as an external standard quality control product of a nuclein detecting method of monkey type D retrovirus detection. The method has the characteristics of high stability and nuclease resistance, a target template sequence exists only in the form of RNA, infectivity is avoided, and security is high.
Owner:海南出入境检验检疫局检验检疫技术中心
Compositions and Methods for Preventing or Treating Influenza Virus Infection
The invention includes compositions and methods for regulating PI3K p110 delta as an anti-retroviral therapy. The invention includes inhibiting p110 delta, a component of PI3K p110 delta signaling pathway, or any combination thereof in a cell as an anti-retroviral therapeutic approach for treating a retroviral infection, for example HIV. The invention includes a method of modulating PI3K p110 delta in a cell infected with a retrovirus by contacting the cell with an effective amount of a composition comprising an inhibitor of PI3K p110 delta.
Owner:DREXEL UNIV
Retrovirus detection method
InactiveCN105420413AHigh sensitivityImprove featuresMicrobiological testing/measurementMicroorganism based processesVirus-RetrovirusMonoclonal antibody
The invention discloses a retrovirus detection method. The method comprises the following steps: (1) selecting host cells that support the replication of the retrovirus; (2) culturing a sample to be tested with the host cells together; (3) collecting supernate, inoculating an indicating cell line, and detecting whether the supernate is polluted by retrovirus or not. According to the provided method, a co-culture method is used to detect the retrovirus, the sensitivity and specificity of retrovirus detection are greatly improved, compared with the conventional retrovirus detection method, the method can be widely used to detect biological products such as monoclonal antibody, in-vitro diagnosis products, and the like, and the method can also be used to detect the biological safety of the endogenous pollution elimination / inactivation step during the biological product production process.
Owner:SUZHOU YAOMING KANGDE INSPECTION TESTING +1
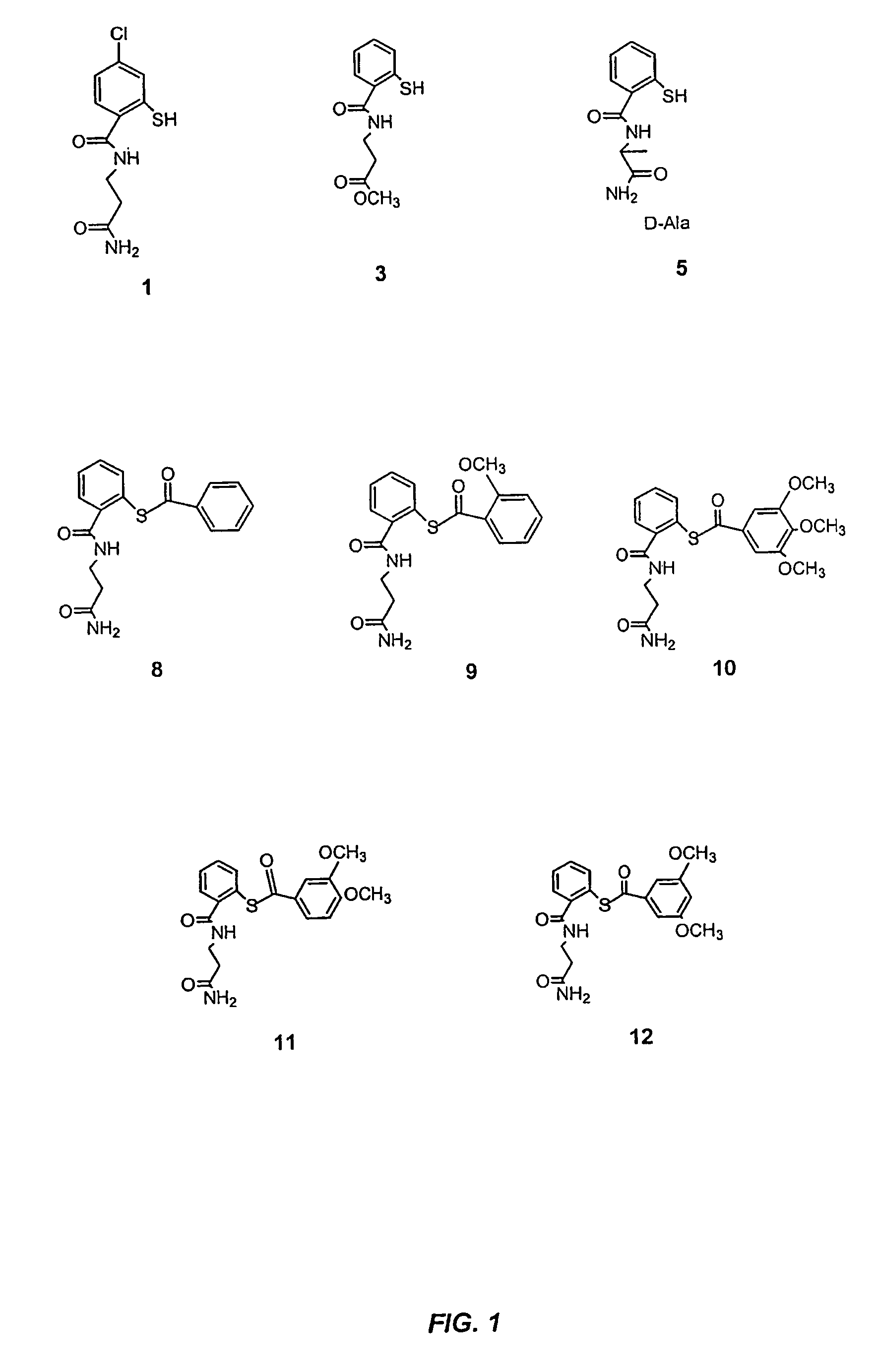
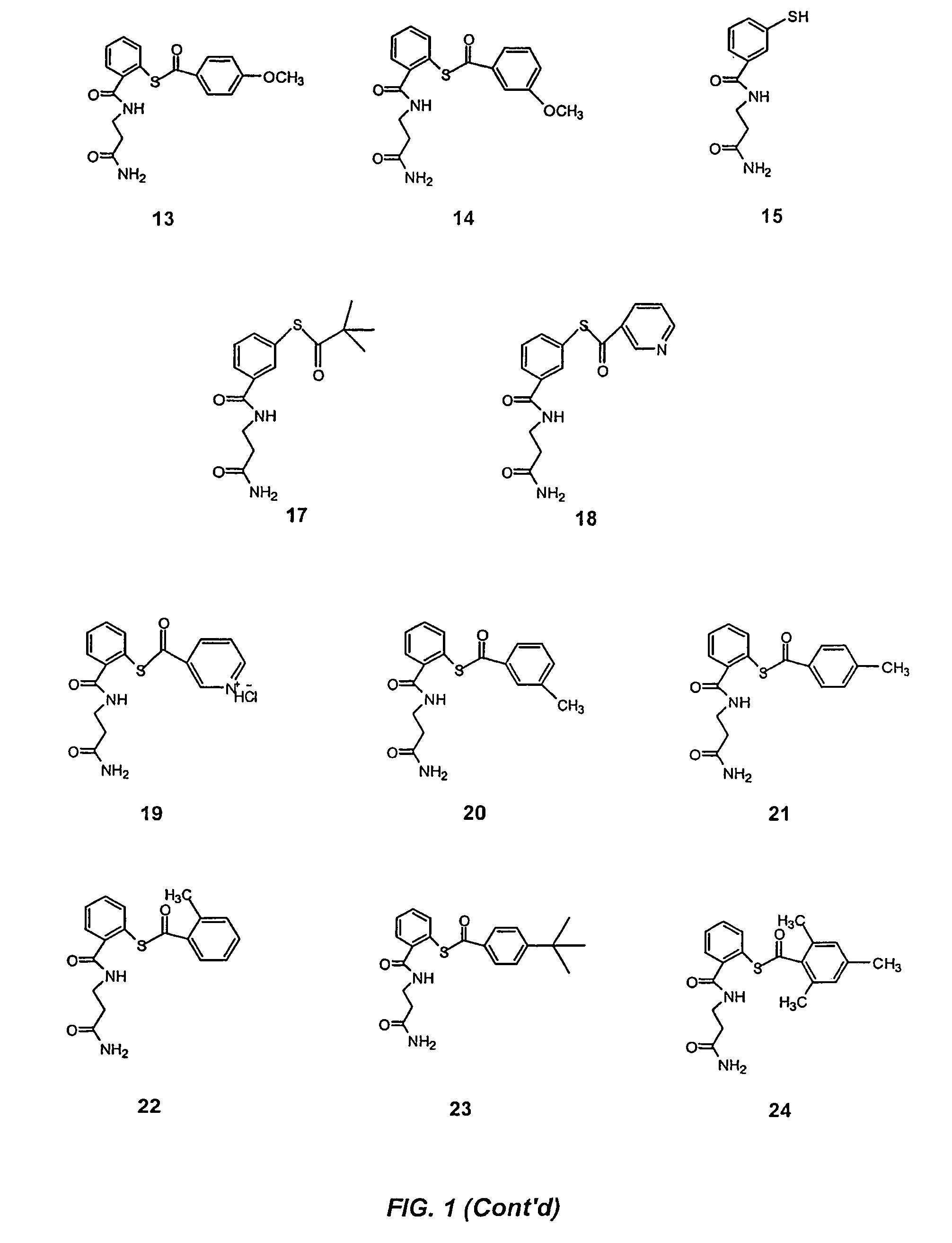
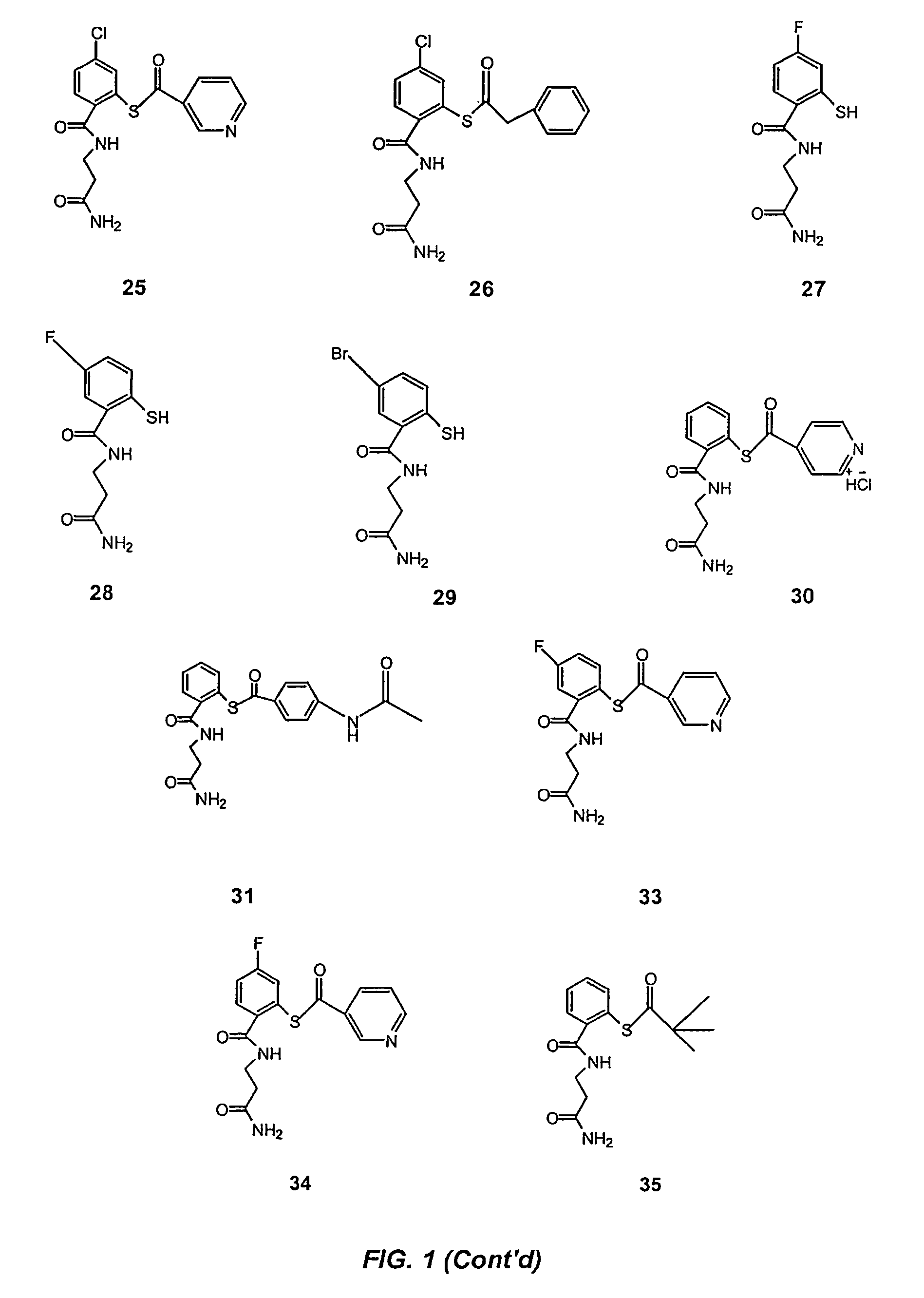
Acylthiols and component thiol compositions as anti-HIV and anti-retroviral agents
Certain thiol and acylthiol compounds inhibit retrovirus growth by attacking the highly conserved zinc finger regions of essential viral proteins. These compounds, compositions containing them, and methods of using them to treat retroviral infections such as HIV are described. These compounds are also useful for preparation of vaccines comprised of inactivated retroviruses such as HIV, prevention of the transmission of such retroviruses, and detection of retroviral proteins.
Owner:UNITED STATES OF AMERICA
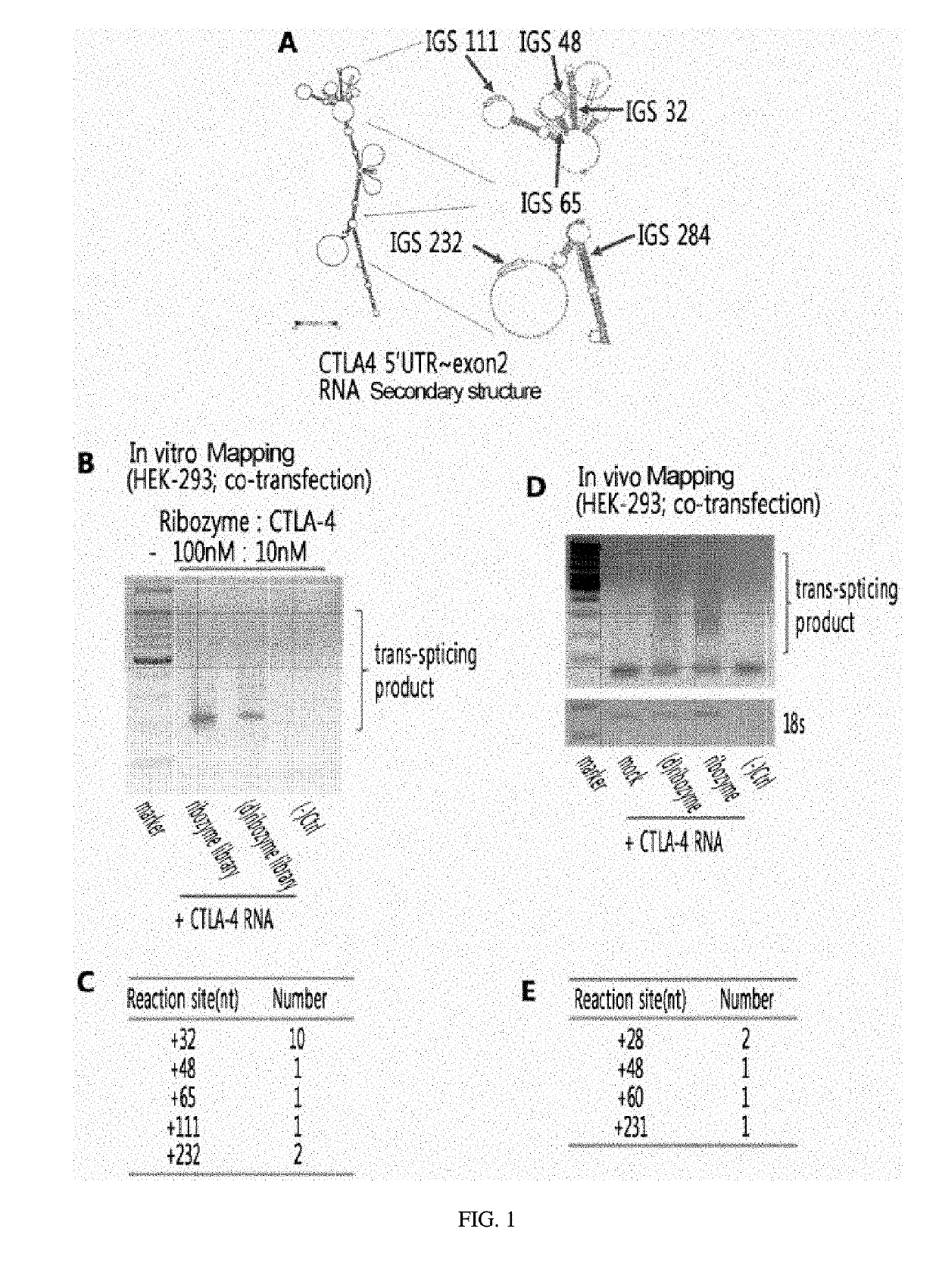
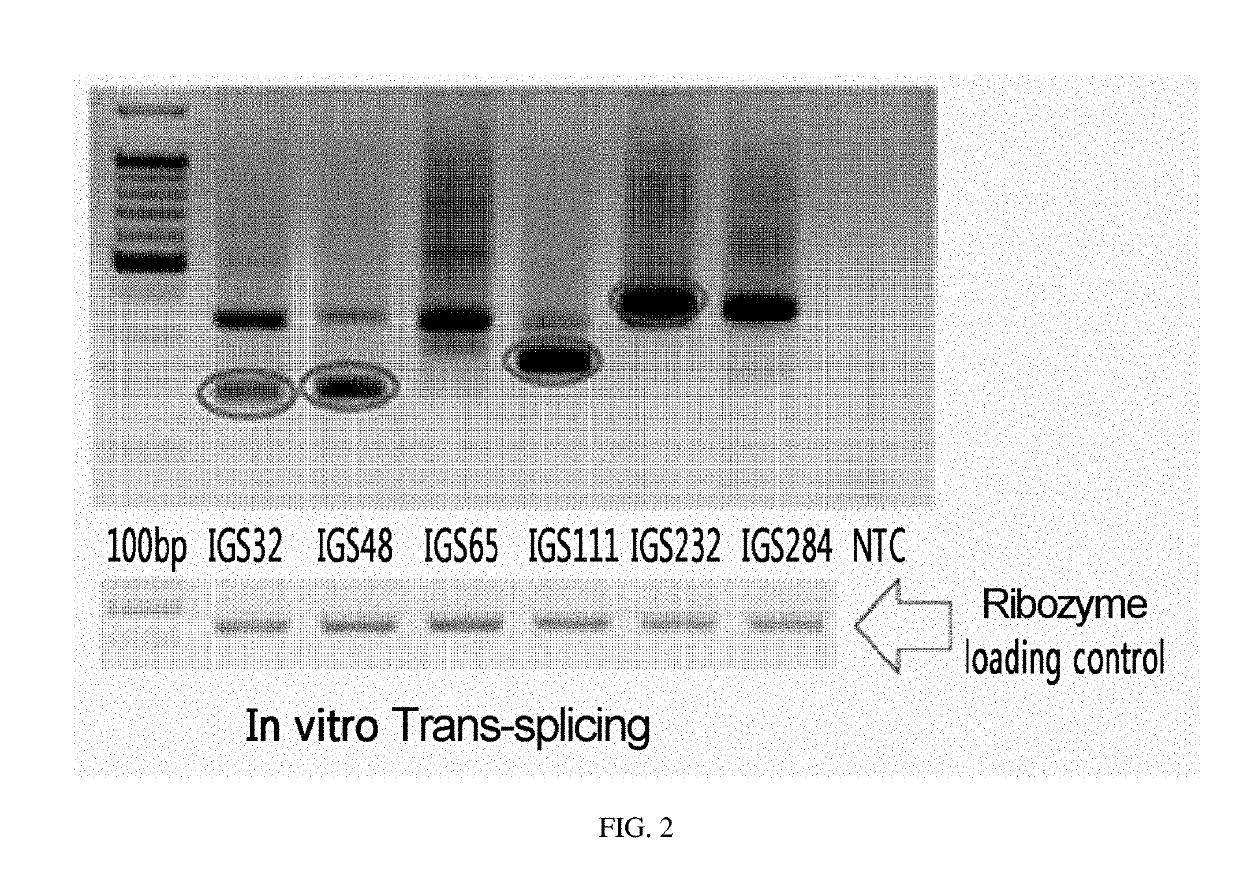
Ctla-4-targeting trans-splicing ribozyme for delivery of chimeric antigen receptor, and use thereof
ActiveUS20190225969A1Effective anti-cancer effectLow toxicityCell receptors/surface-antigens/surface-determinantsHydrolasesNucleotideAntigen receptors
The present invention relates to a recombinant vector, characterized by including a cytotoxic T-lymphocyte-associated protein-4 (CTLA-4)-targeting trans-splicing ribozyme expression cassette for delivery of chimeric antigen receptor, wherein the expression cassette includes: (i) a CTLA-4-targeting trans-splicing ribozyme; and (ii) a polynucleotide encoding a chimeric antigen receptor ligated to the 3′ exon of the ribozyme. The present invention also relates to a transformed cell into which the recombinant vector is introduced, a ribozyme expressed from the recombinant vector, a retrovirus expressing the ribozyme, and a T cell treated with the retrovirus. Furthermore, the present invention relates to a pharmaceutical composition for preventing or treating cancers, in which the pharmaceutical composition includes the recombinant vector, the transformed cell, the ribozyme, the retrovirus, the T cell, or a combination thereof; and a method for treating cancers, in which the method includes administering, to an individual in need thereof, the recombinant vector, the transformed cell, the ribozyme, the retrovirus, the T cell, or a combination thereof. The recombinant vector of the present invention and the ribozyme expressed therefrom become a gene-cell therapy which inhibits CTLA-4 on T cells which has been an obstacle in conventional anti-cancer therapies and, at the same time, enables anti-cancer treatment, thereby allowing more effective anti-cancer effects to be anticipated. Such a gene-cell therapy results in decreased toxicity in normal tissues and thus exhibits increased effects in both therapeutic efficacy and safety, which enables it to be widely utilized in the field of gene therapy in the future.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

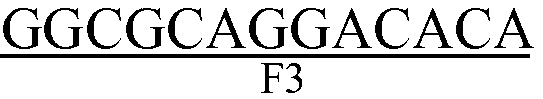
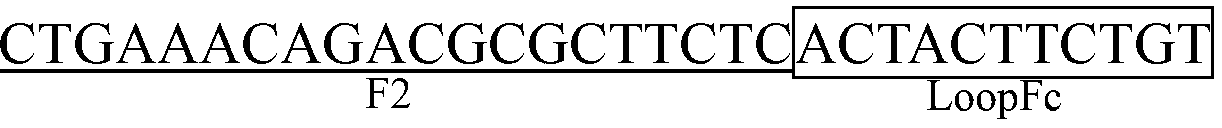

![Use of 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid or salt thereof for treating retrovirus infection Use of 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid or salt thereof for treating retrovirus infection](https://images-eureka.patsnap.com/patent_img/611600dc-1211-4e60-800c-801fe638b18a/US20090018162A1-20090115-C00001.png)
![Use of 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid or salt thereof for treating retrovirus infection Use of 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid or salt thereof for treating retrovirus infection](https://images-eureka.patsnap.com/patent_img/611600dc-1211-4e60-800c-801fe638b18a/US20090018162A1-20090115-C00002.png)