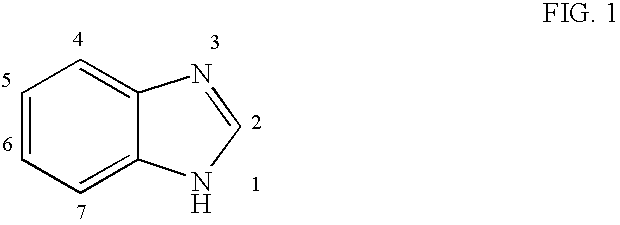Patents
Literature
193 results about "Respiratory infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pulmonary delivery of aminoglycosides
InactiveUS7368102B2Eliminate side effectsReduce in quantityBiocidePowder deliveryRespiratory infectionTopical treatment
Owner:BGP PROD OPERATIONS GMBH +1
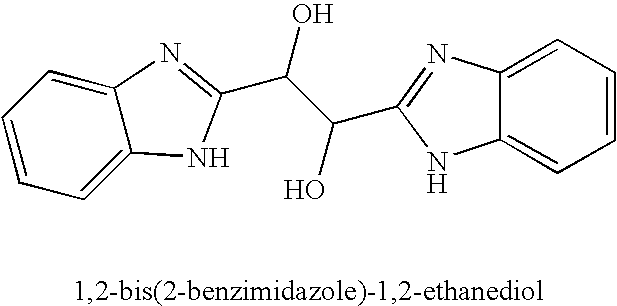
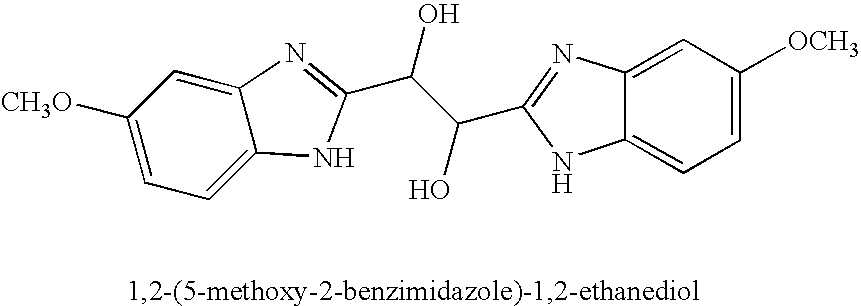
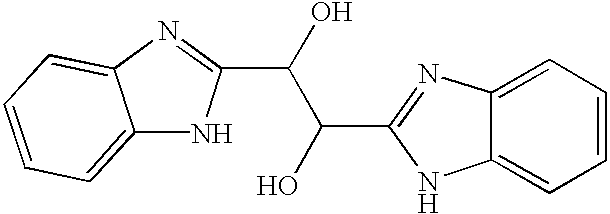
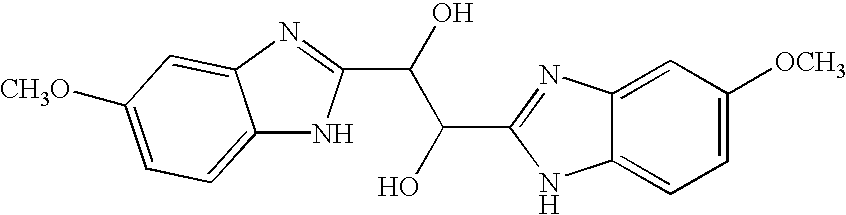
Hetero-substituted benzimidazole compounds and antiviral uses thereof
InactiveUS20040166137A1Many symptomReduce loadBiocideOrganic active ingredientsRespiratory infectionViral infection
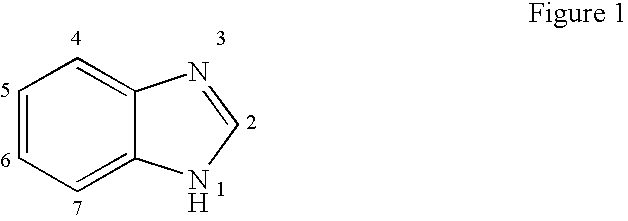
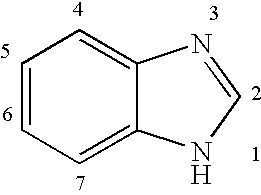
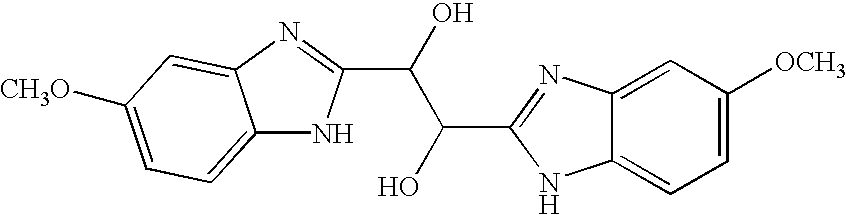
The present invention relates to novel hetero-substituted benzimidazole compounds that have useful antiviral activity. More specifically, the invention encompasses hetero-substituted benzimidazole compounds that inhibit membrane fusion associated events such as viral transmission, reduce viral load or otherwise treat viral infections. The invention also encompasses the use of hetero-substituted benzimidazole compounds as inhibitors of membrane fusion associated events, such as viral transmission. In another embodiment, the invention encompasses processes for making hetero-substituted benzimidazole compounds, methods of using the hetero-substituted benzimidazole compounds and compositions comprising the hetero-substituted benzimidazole compounds. Finally, the invention provides methods for treating, preventing or ameliorating symptoms associated with respiratory infection, particularly that caused by Respiratory Syncytial Virus utilizing the novel benzimidazole compounds of the invention.
Owner:TRIMERIS
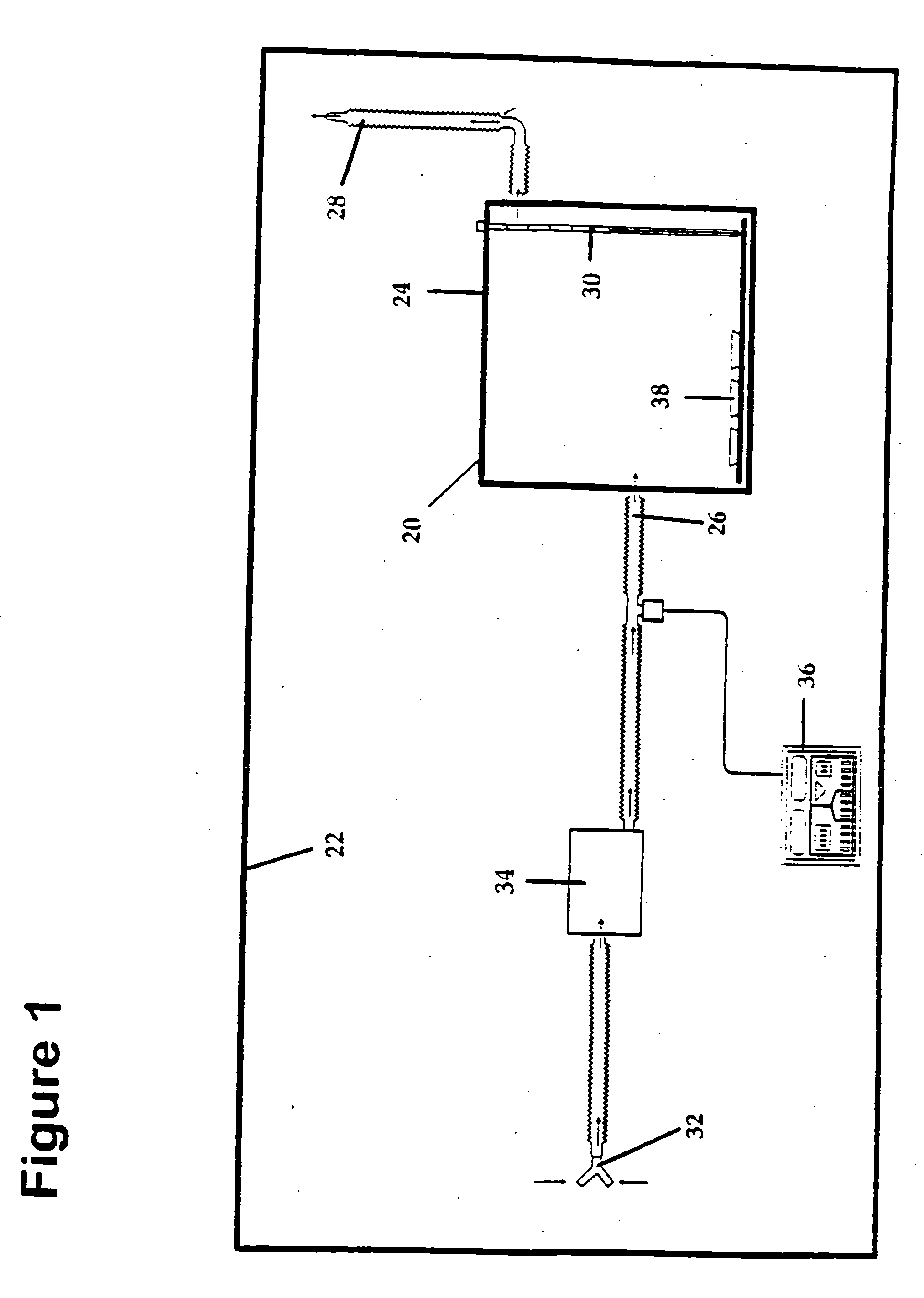
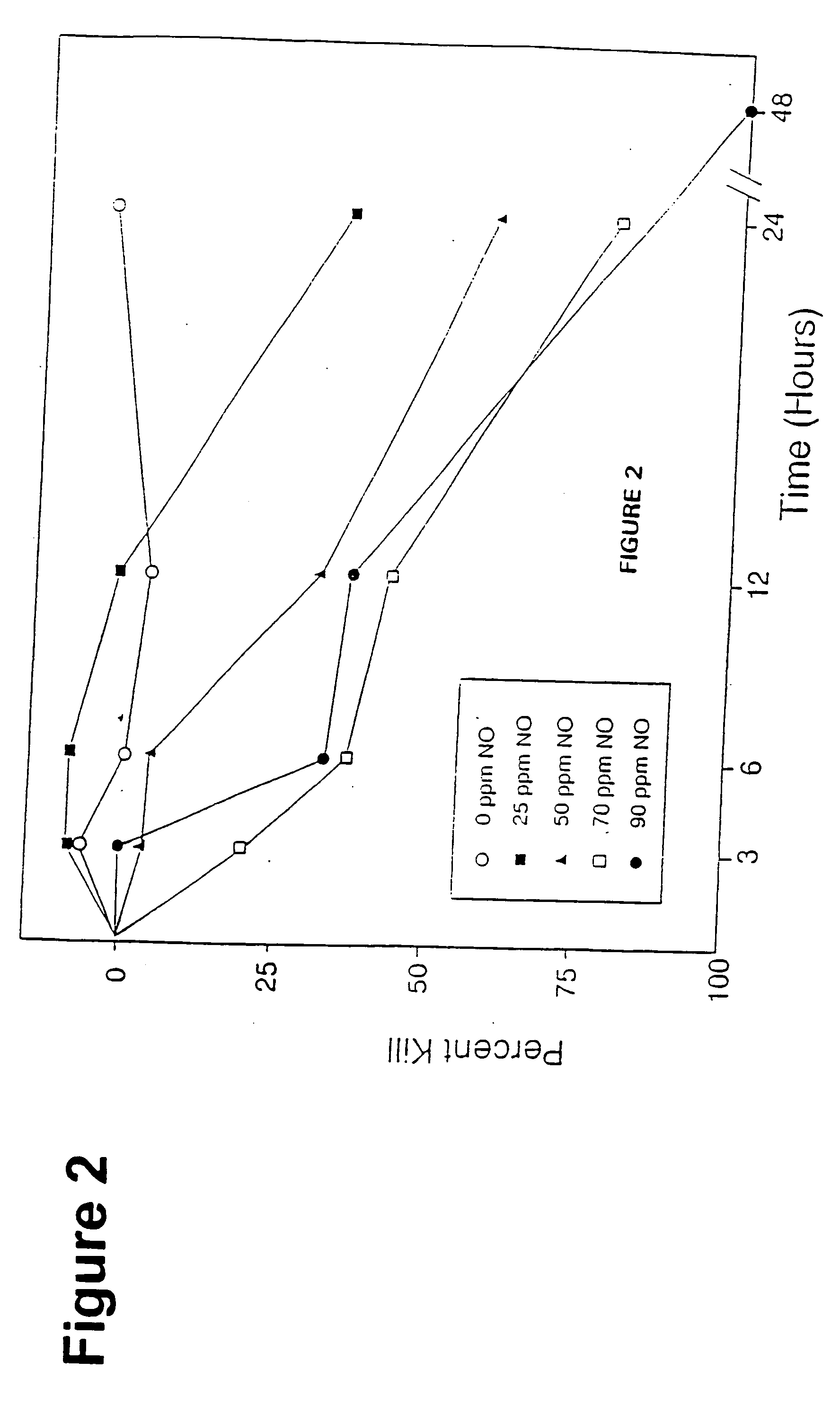
Method and apparatus for treatment of respiratory infections by nitric oxide inhalation
Methods for suppressing, killing, and inhibiting pathogenic cells, such as microorganisms associated with a respiratory infection within the respiratory tract of an animal are described. Methods include the step of exposing the pathogenic cells to an effective amount of nitric oxide, such as through inhalation of nitric oxide gas, in combination with traditional respiratory infection agents, such as antibiotics.
Owner:PULMONOX TECH
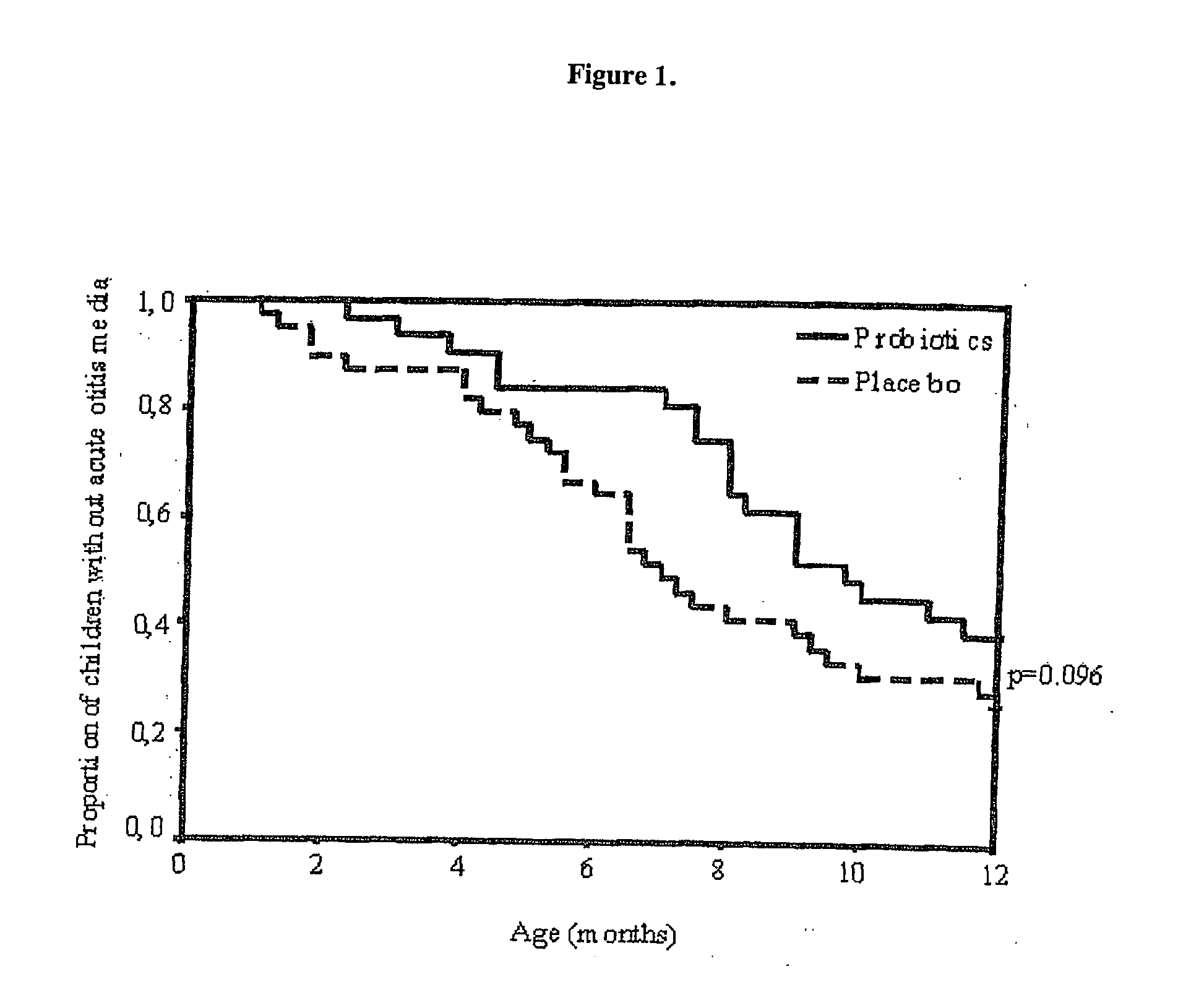
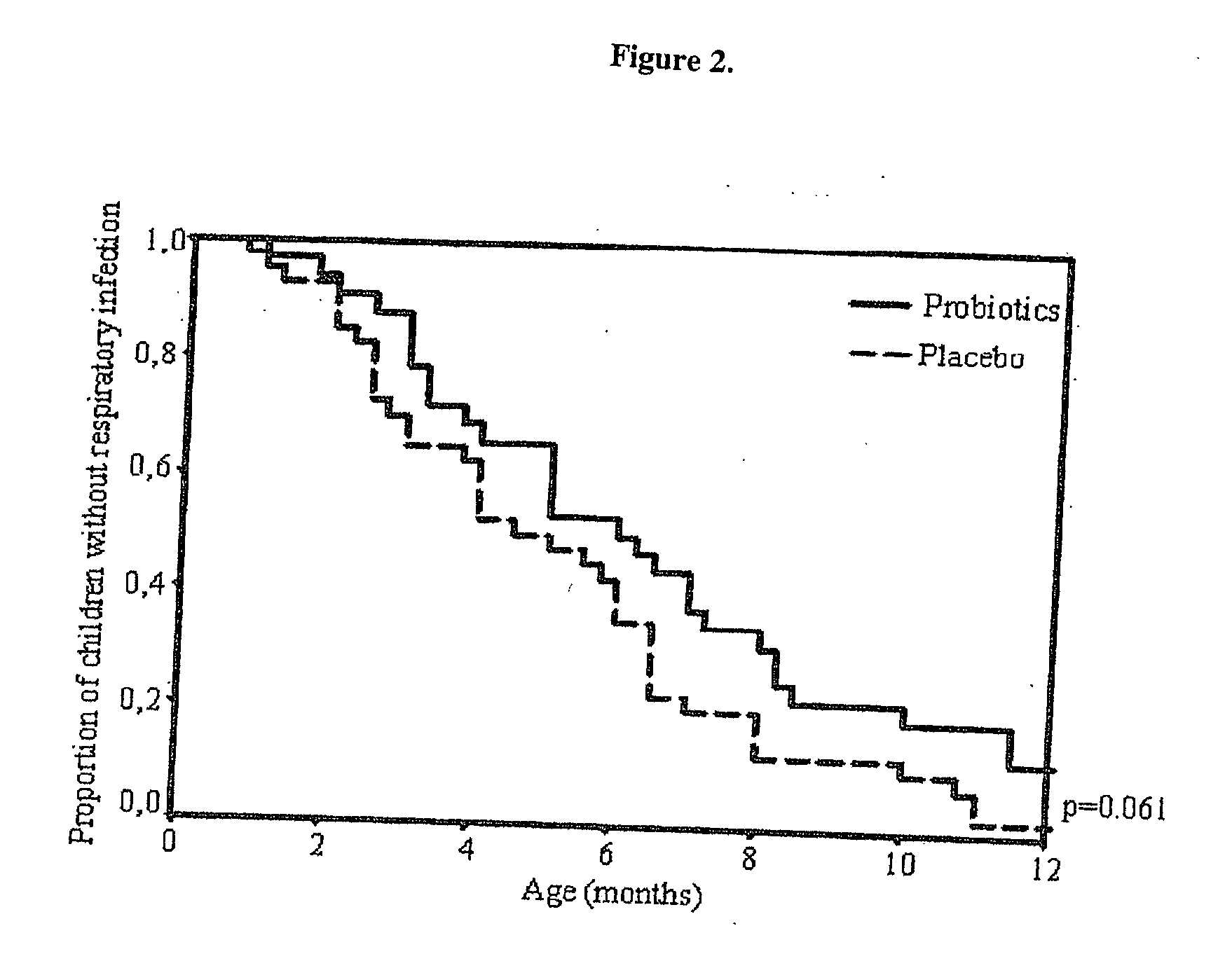
Method for preventing or treating respiratory infections and acute otitis media in infants
ActiveUS20060018890A1Easy adhesionPromote growthBiocideSenses disorderBifidobacteriumRespiratory infection
The present invention is directed to a novel method for preventing or treating respiratory infections and acute otitis media in infants. The method comprises the administration of a therapeutically effective amount of a Bifidobacteria strain and an adherence-promoting probiotic, such as LGG, to the infant.
Owner:MEAD JOHNSON NUTRITION
Respiratory infection treatment device
A device for treating an illness or infection in the respiratory tract of a body is provided. The device administers an antimicrobial mist, which coats the tissues in the respiratory tract where the infection is colonizing.
Owner:POPLAR +1
Anti-IL-9 antibody formulations and uses thereof
InactiveUS20050260204A1Low to undetectable levelEasy to manageAntibacterial agentsAntimycoticsDiseaseAntibody fragments
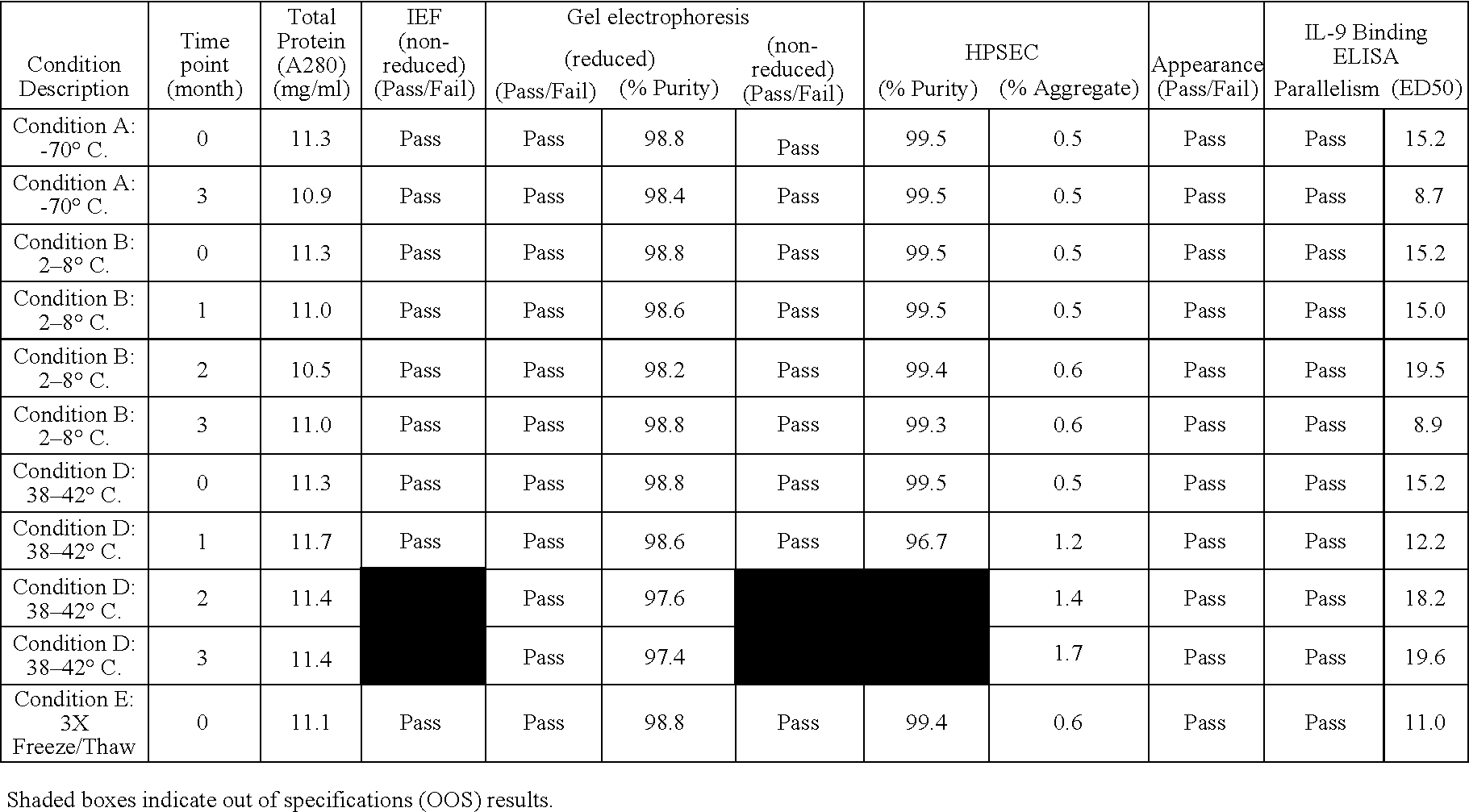
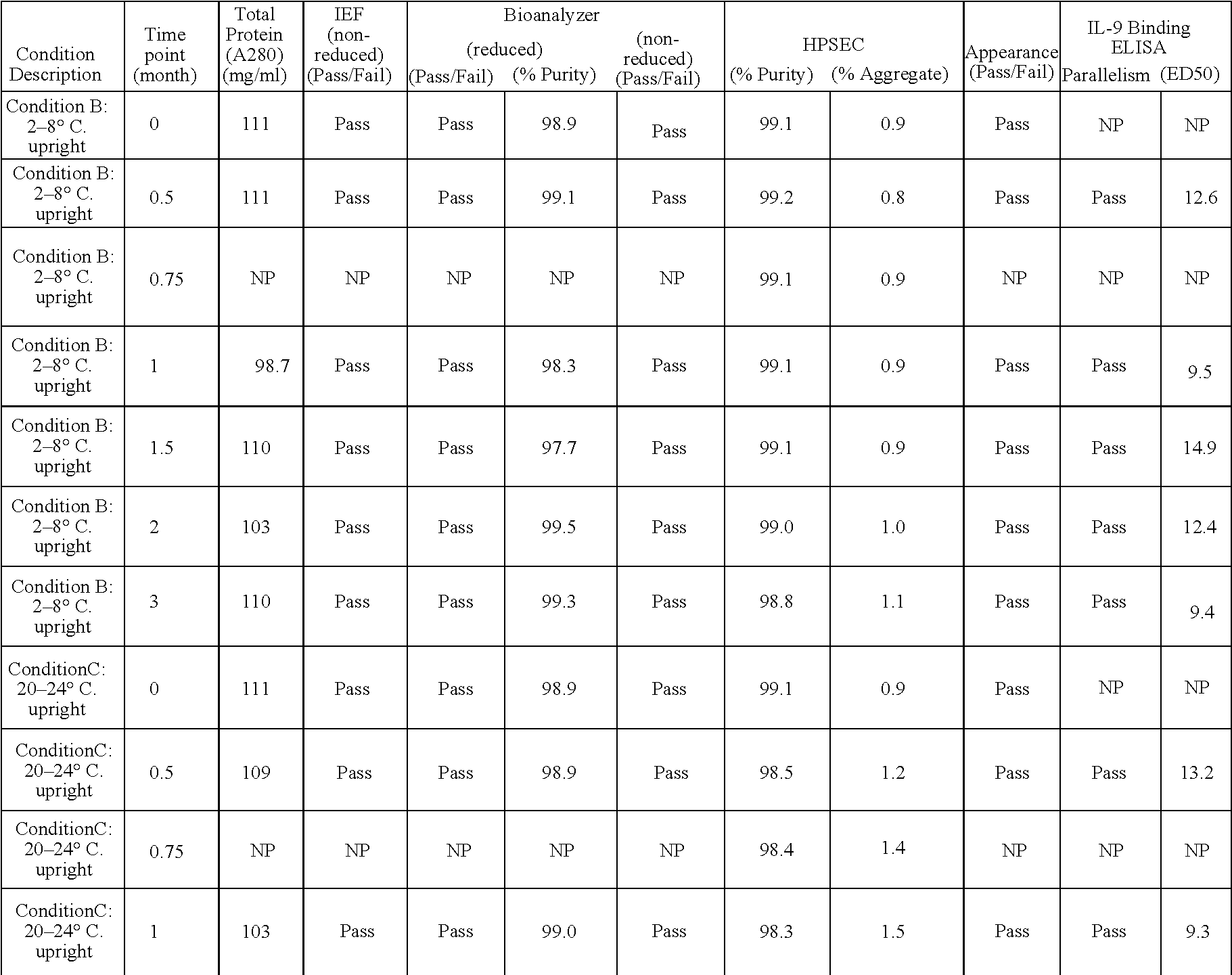
The present invention provides liquid formulations of antibodies or antibody fragments that immunospecifically bind to an IL-9 polypeptide, which formulations exhibit stability, low to undetectable levels of aggregation, and very little to no loss of the biological activities of the antibodies or antibody fragments, even during long periods of storage. In particular, the present invention provides liquid formulations of antibodies or fragments thereof that immunospecifically bind to an IL-9 polypeptide, which formulations are substantially free of surfactants, sugars, sugar alcohols, amino acids other than histidine (preferably with pKa values of less than 5 and above 7), and / or other common excipients. Furthermore, the invention provides methods of preventing, treating or ameliorating a disease or disorder associated with or characterized by aberrant expression and / or activity of an IL-9 polypeptide, a disease or disorder associated with or characterized by aberrant expression and / or activity of the IL-9R or one or more subunits thereof, an autoimmune disease, an inflammatory disease, a proliferative disease, or an infection (preferably, a respiratory infection), or one or more symptoms thereof, utilizing the liquid formulations of the present invention.
Owner:MEDIMMUNE LLC
Benzimidazole compounds and antiviral uses thereof
InactiveUS20030119754A1Reducing viral titer viralReduce viral loadBiocideSugar derivativesRespiratory infectionViral infection
Owner:TRIMERIS
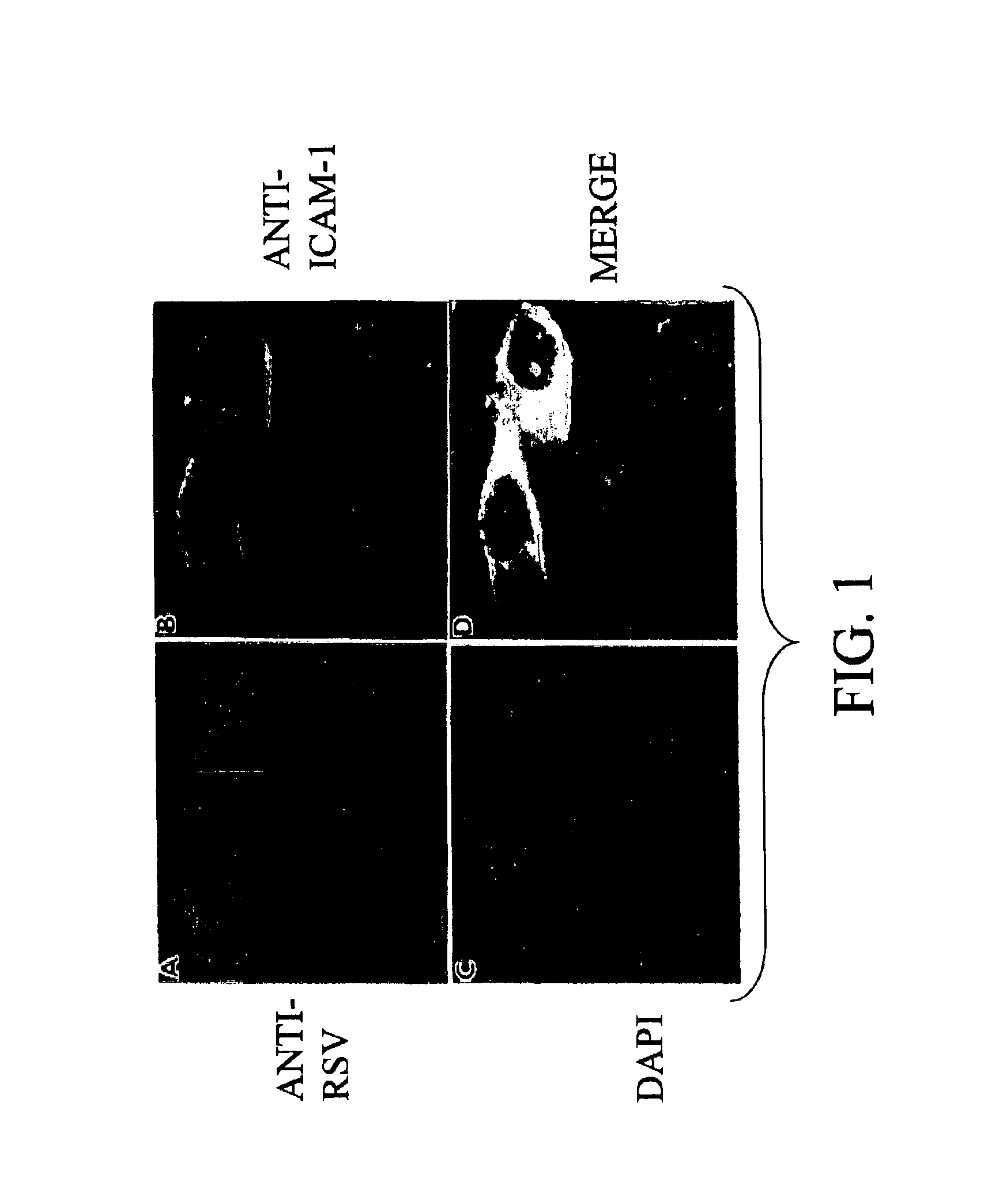
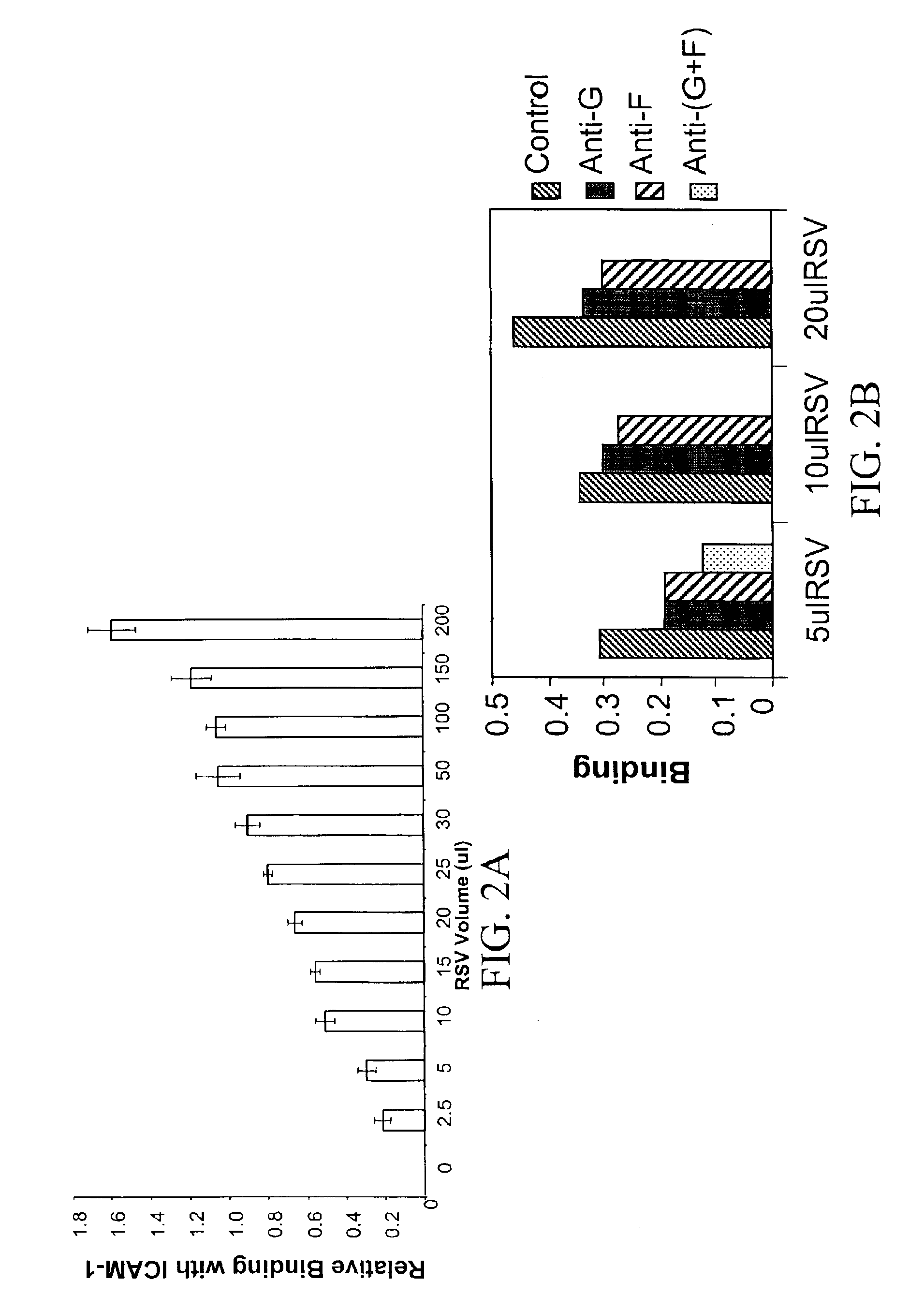
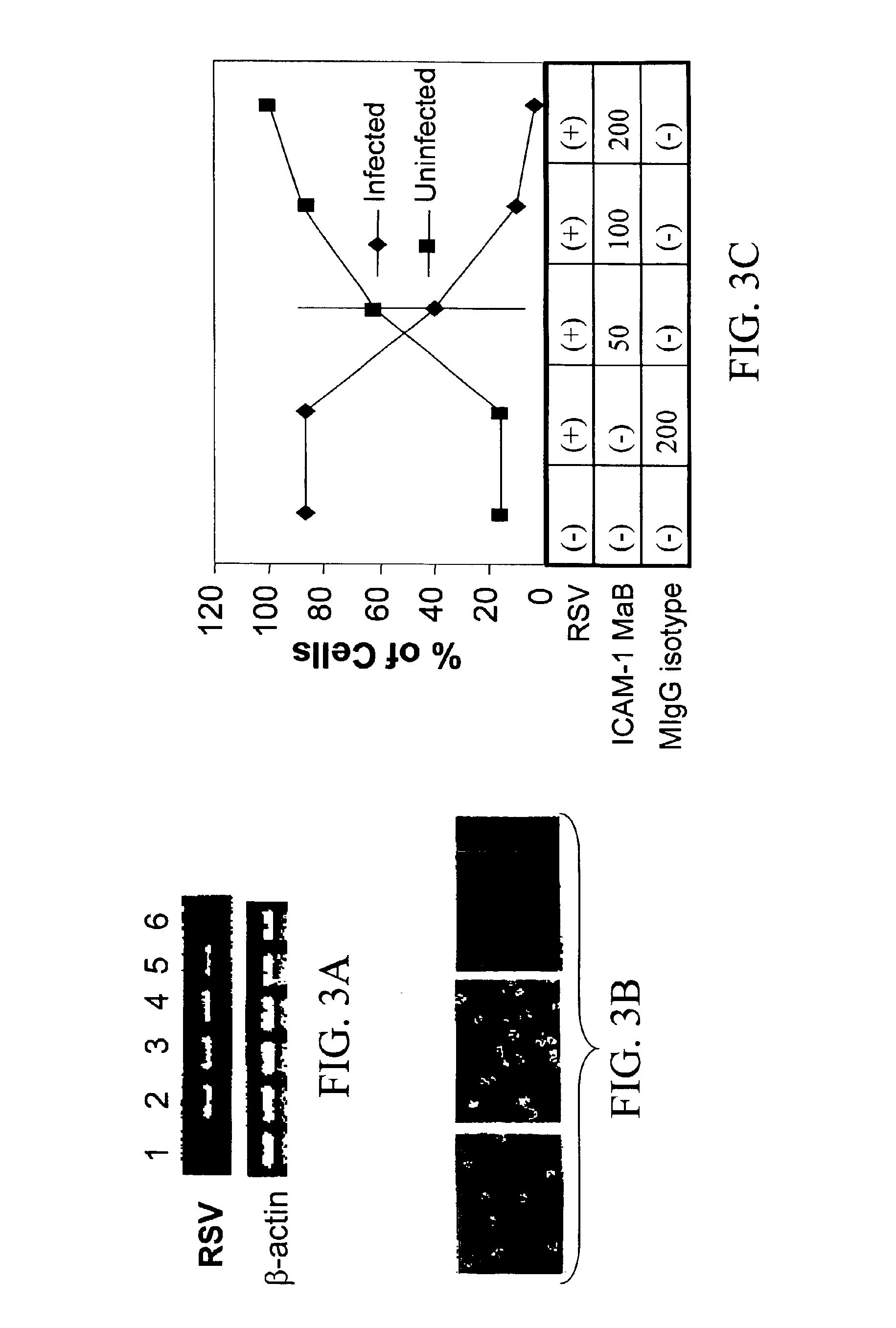
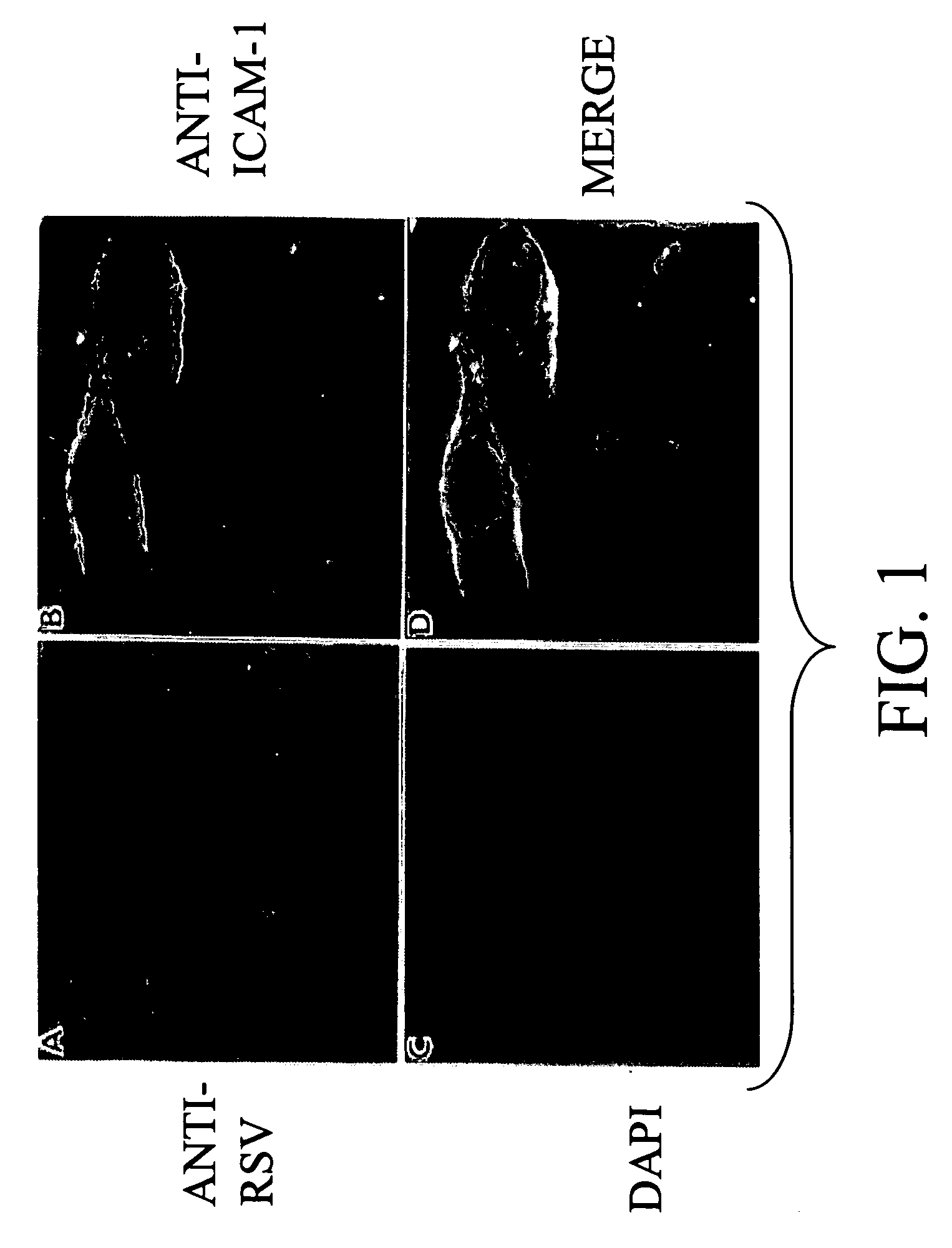
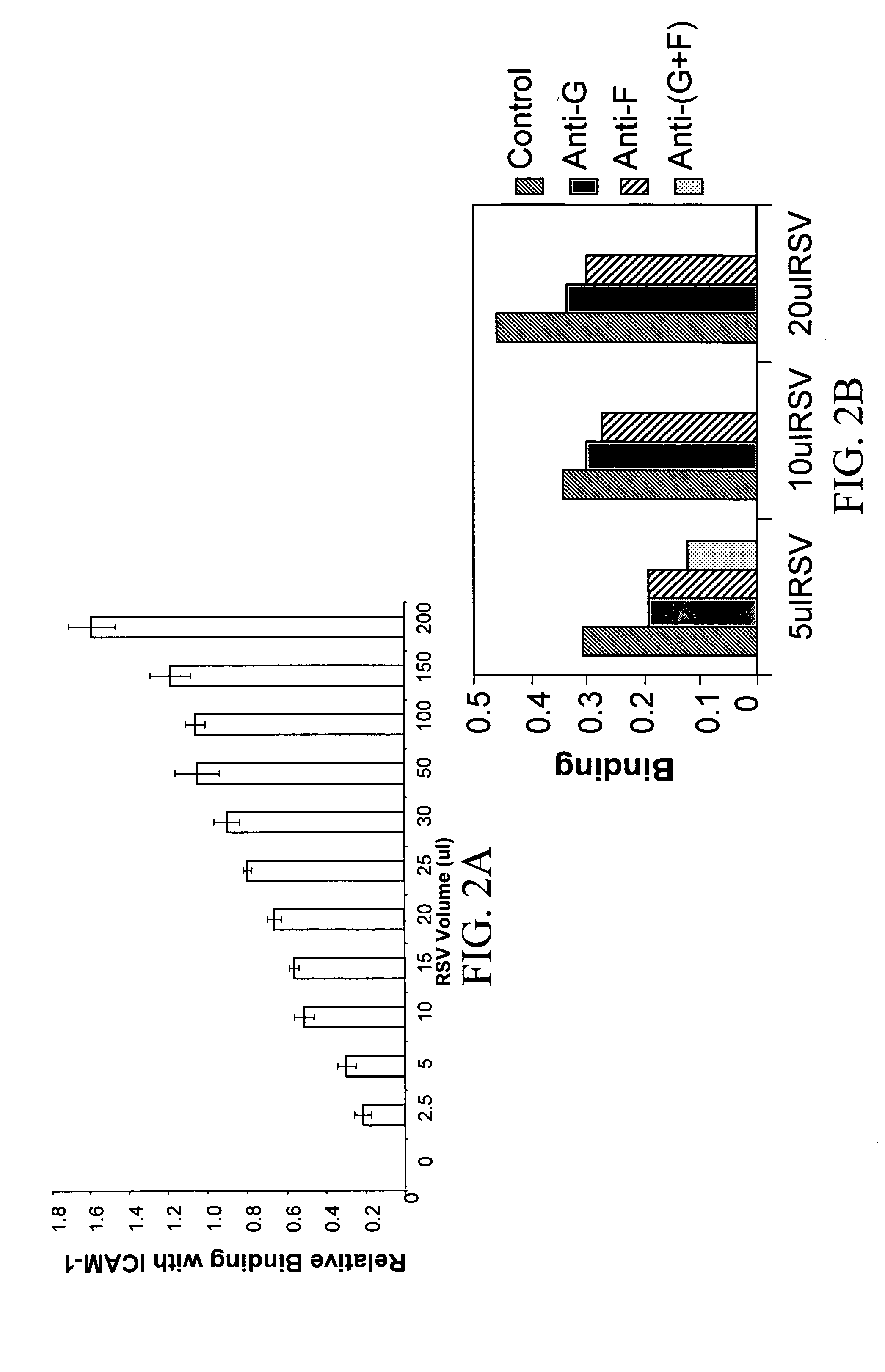
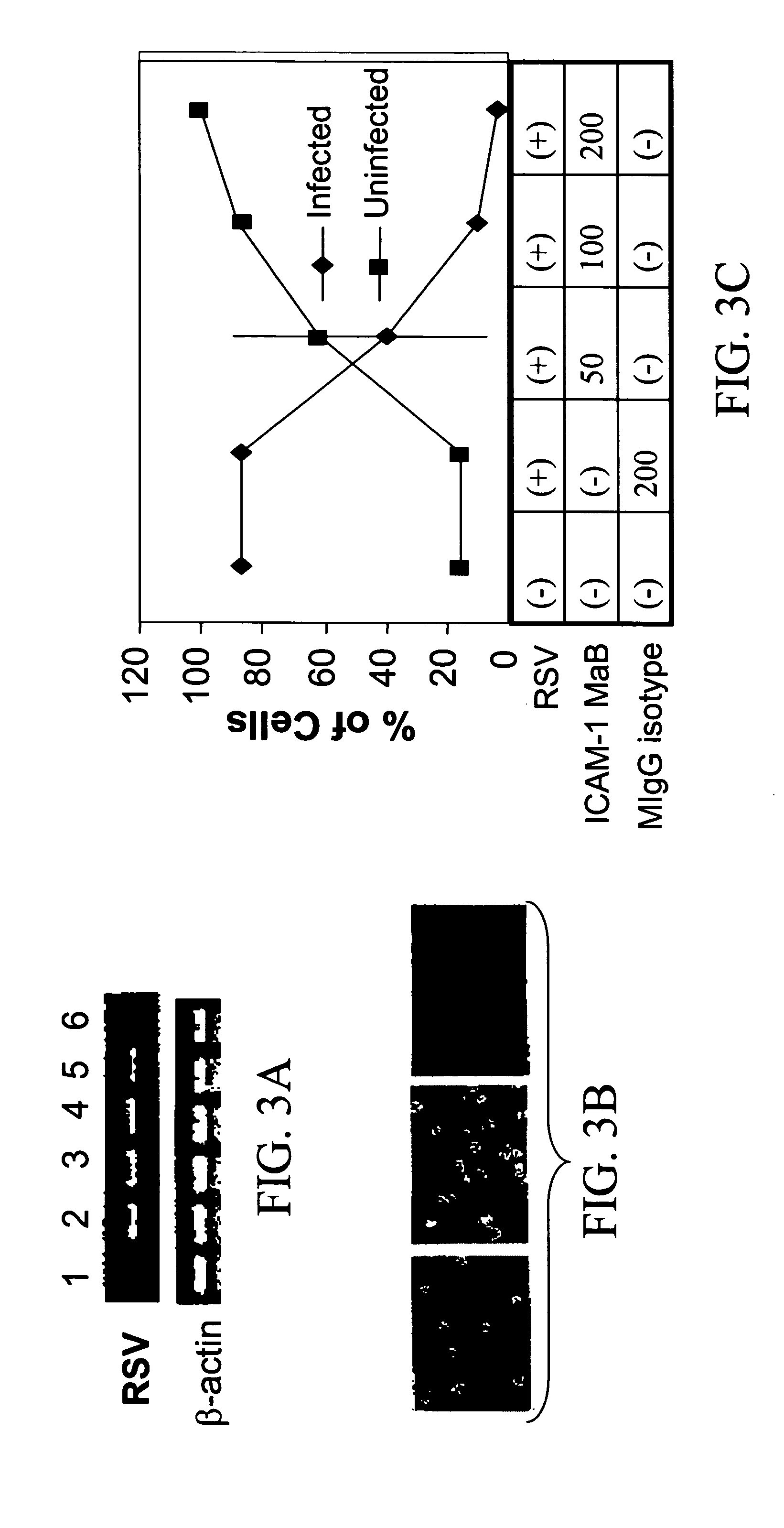
Interrupting the interaction of intercellular adhesion molecule-1 and respiratory syncytial virus for prevention and treatment of infection
InactiveUS6900299B1Avoid infectionDecreasing RSV bindingSpecial deliverySugar derivativesRespiratory infectionRSV Infections
There is provided a method of preventing a respiratory infection by administering an effective amount of an agent for regulating ICAM-1 expression. Also provided is a composition for the prevention of respiratory infection including an agent which regulates ICAM expression. method of preventing RSV infection by administering an effective amount of an agent that interferes with the binding of RSV to ICAM-1. A method of preventing RSV infection by administering an effective amount of an agent that down regulates the expression of ICAM-1, thereby decreasing RSV binding to ICAM-1 is also provided. There is provided a method of treating RSV infection by administering an effective amount of an agent for down regulating ICAM-1 expression. A method of blocking RSV-ICAM-1 interaction by administering an effective amount of agents for blocking ICAM sites of binding is provided. Also provided is a compound for blocking RSV-ICAM-1 interaction including an agent for blocking ICAM sites of binding.
Owner:UNIV OF SOUTH FLORIDA
Nanoemulsion therapeutic compositions and methods of using the same
ActiveUS20100203139A1Reduces and attenuates and prevents bacterial growthReduce generationAntibacterial agentsPowder deliveryRespiratory infectionPreventative Medicine
The present invention relates to therapeutic nanoemulsion compositions and to methods of utilizing the same. In particular, nanoemulsion compositions are described herein that find use in the treatment and / or prevention of infection (e.g., respiratory infection (e.g., associated with cystic fibrosis)), in burn wound management, and in immunogenic compositions (e.g., comprising a Burkholderia antigen) that generate an effective immune response (e.g., against a bacterial species of the genus Burkholderia) in a subject administered the immunogenic composition. Compositions and methods of the present invention find use in, among other things, clinical (e.g. therapeutic and preventative medicine), industrial, and research applications.
Owner:NANOBIO CORP +1
Methods of Preventing or Treating Respiratory Conditions
InactiveUS20090186038A1Reduce developmentAvoid and reduce side effectAntibacterial agentsAntimycoticsProtocol designRespiratory infection
Owner:MEDIMMUNE LLC
Anti-IL-9 antibody formulations
InactiveUS7691379B2Low to undetectable levelEasy to manageAntibacterial agentsAntimycoticsAntibody fragmentsAutoimmune disease
Owner:MEDIMMUNE LLC
Benzimidazole compounds and antiviral uses thereof
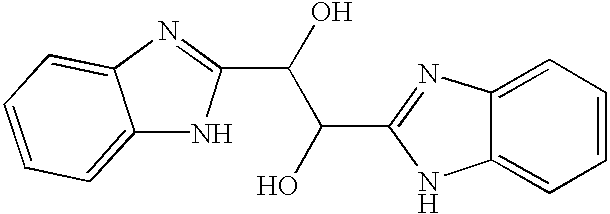
The present invention relates to novel benzimidazole compounds that have useful antiviral activity. More specifically, the invention encompasses benzimidazole compounds that inhibit membrane fusion associated events such as viral transmission, reduce viral load or otherwise treat viral infections. The invention also encompasses the use of benzimidazole compounds as inhibitors of membrane fusion associated events, such as viral transmission. In another embodiment, the invention encompasses processes for making benzimidazole compounds, methods of using the benzimidazole compounds and compositions comprising the benzimidazole compounds. Finally, the invention provides methods for treating, preventing or ameliorating symptoms associated with respiratory infection, particularly that caused by Respiratory Syncytial Virus utilizing the novel benzimidazole compounds of the invention.
Owner:TRIMERIS
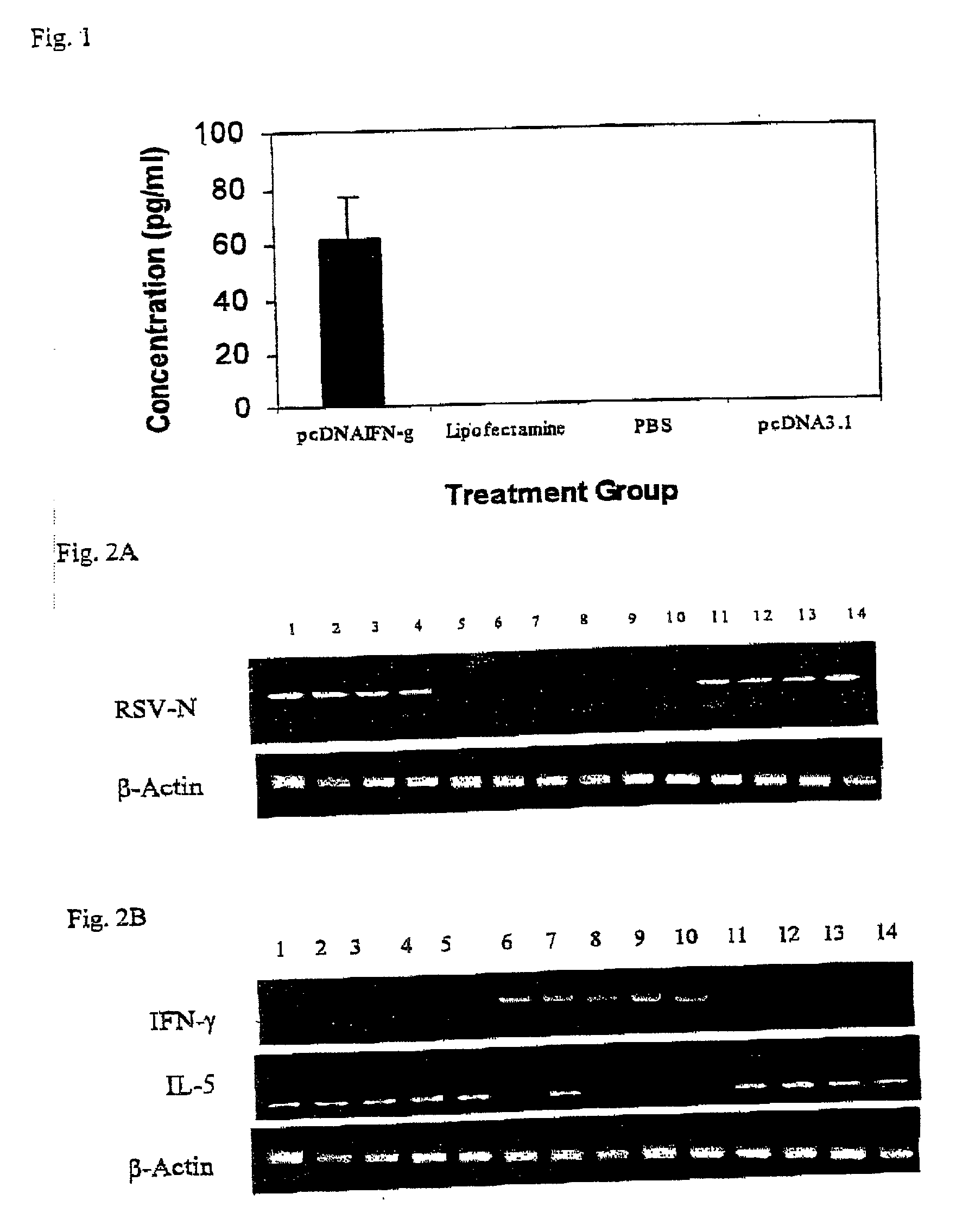
Method of intranasal gene transfer for protection against respiratory infection
A method of preventing a respiratory infection by administering DNA which encodes IFN is provided. Also provided is a therapy for the prevention of a respiratory infection containing DNA which encodes IFN.
Owner:SOUTH FLORIDA UNIVESITY OF
Multiple-channel test device, method for producing the same and use thereof
InactiveUS20070042444A1Improve storabilityLaboratory glasswaresMaterial analysisIgm antibodyCardiac infarction
The object of the invention is a multiple-channel test devise based on immunodiffusion and immunochromatography, which enables the simultaneous or parallel determination of several analytes. In the test devise, it is possible to group together different combinations of markers recognizing allergens, myocardial infarction markers, venereal disease analytes, blood screening analytes, respiratory infection producing agents, IgG, IgA and IgM antibodies, other infectious disease producing agents as well as various cancer markers. The multiple-channel test devise comprises a porous carrier material on which a channel network has been formed by etching the carrier material by laser to form a shaped figure that contains several channels. In the channels, various specific binding reagents have been immobilized, which enable the diagnoses of a target illness and / or syndrome. The sample application point is optionally provided with a filter and optionally contains a label mobilizable by the analyzable sample and a specific binding reagent. Also the method for the production of the test device and its use are disclosed in the invention.
Owner:ANI BIOTECH
Oligosaccharide composition for treating acute respiratory tract infections
ActiveUS20130236424A1Avoid symptomsReduce usageAntibacterial agentsOrganic active ingredientsFucosylationN-Acetyllactosamine
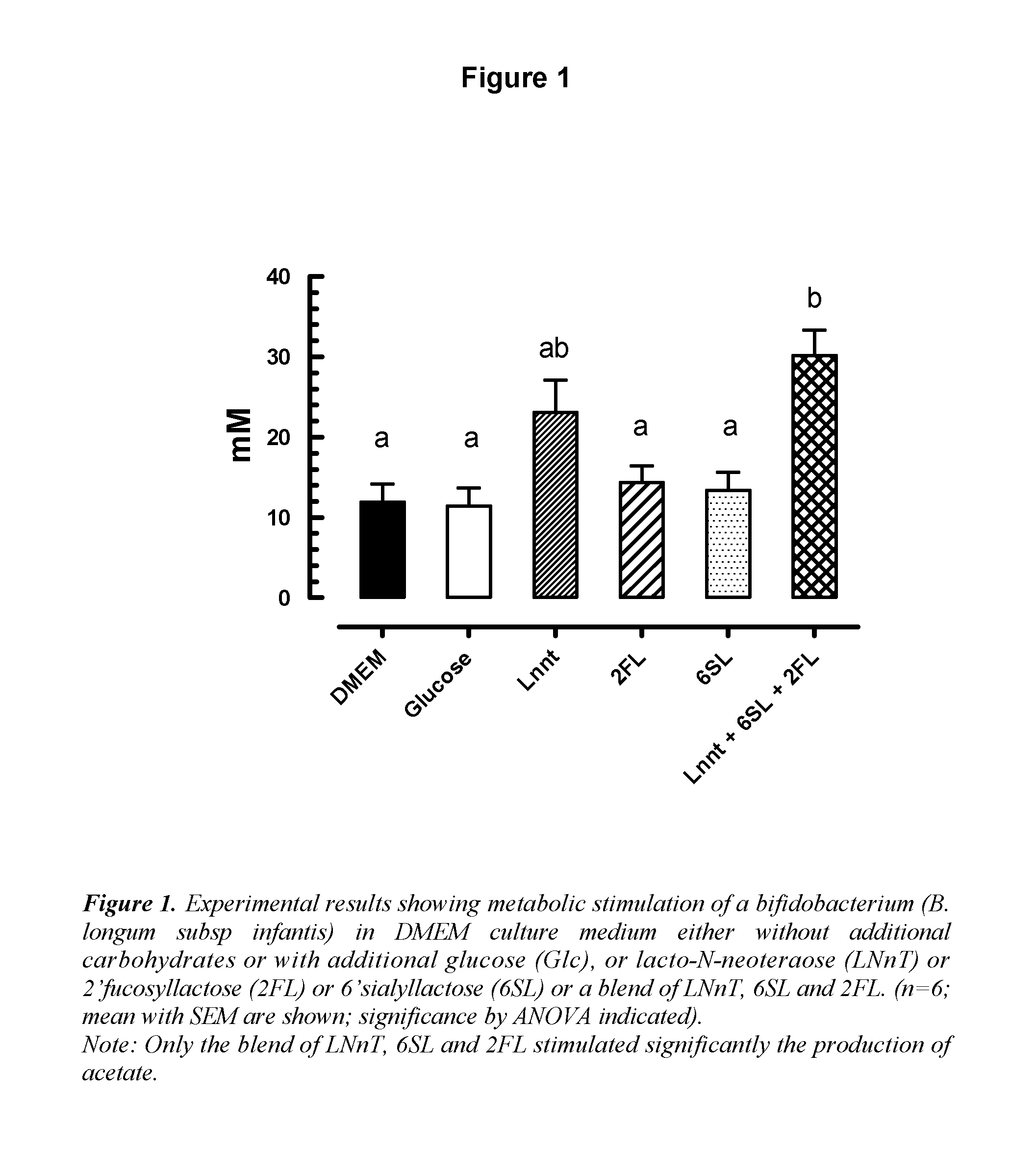
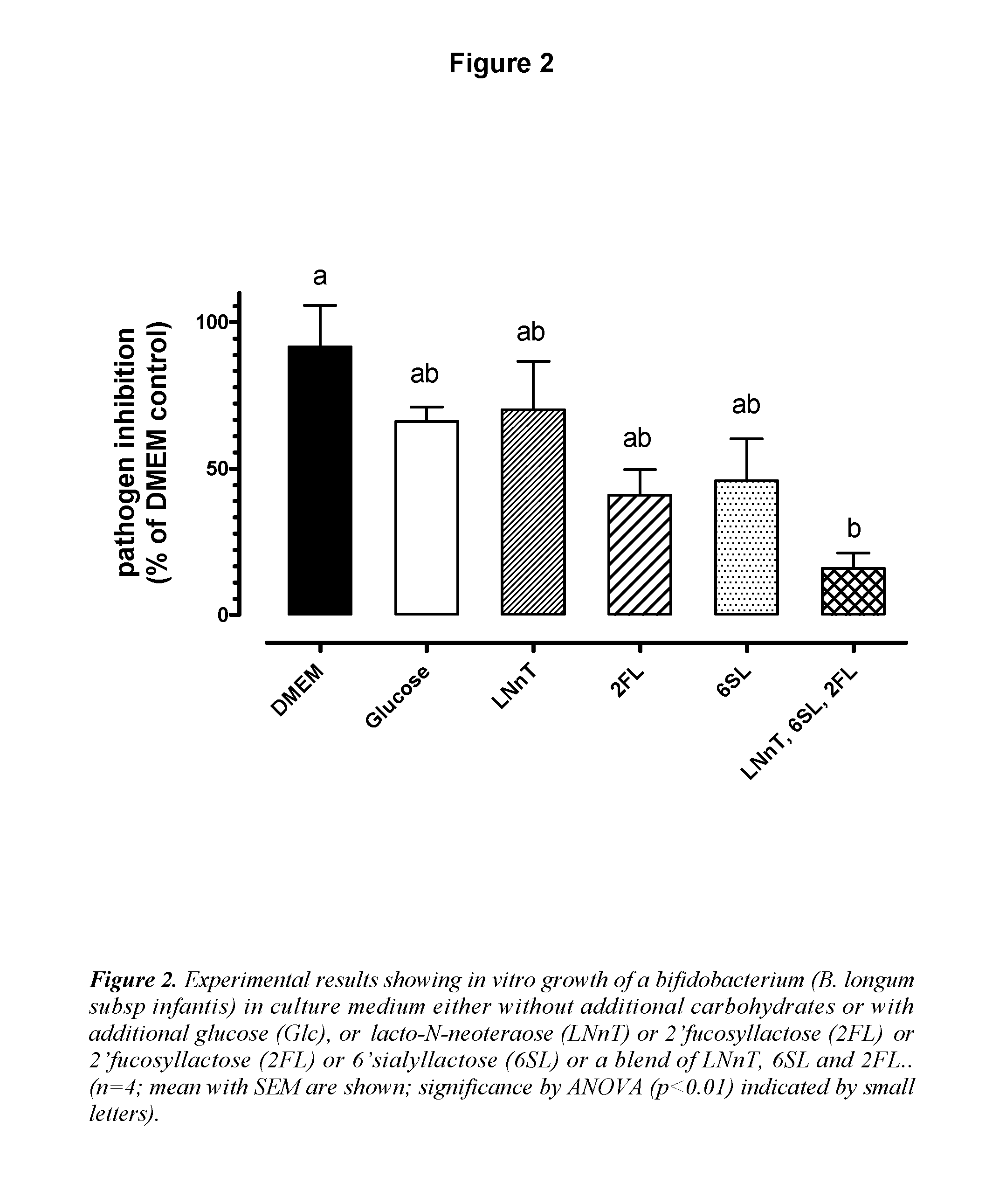
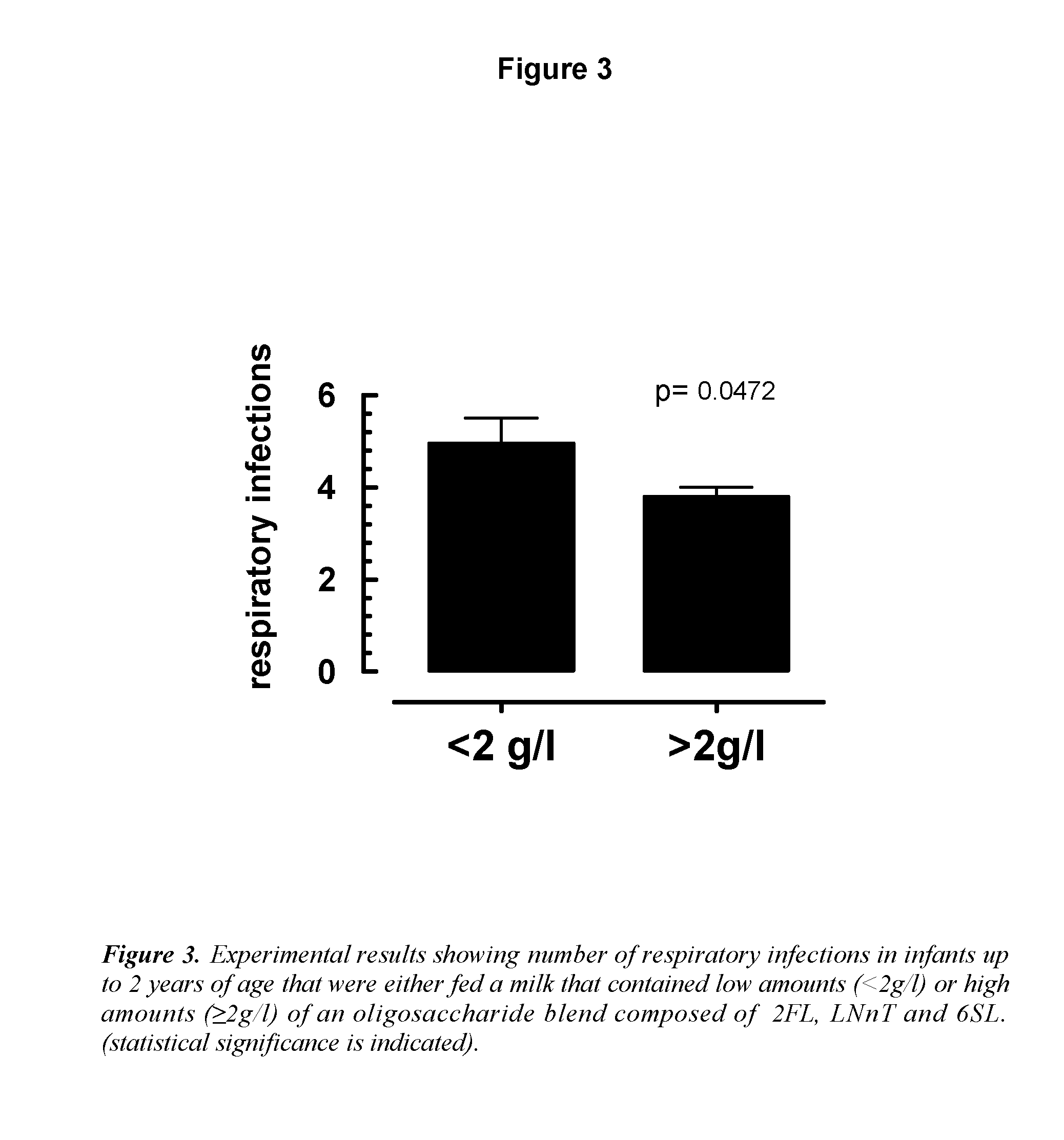
The inventions discloses a composition comprising at least one N-acetyl lactosamine, at least one sialylated oligosaccharide and at least one fucosylated oligosaccharide, for use in preventing acute respiratory infections (ARI) and / or relieving symptoms of said ARI infections. Preferably said composition is a starter infant formula. Said acute respiratory infection is in particular bronchiolitis or otitis.
Owner:SOC DES PROD NESTLE SA
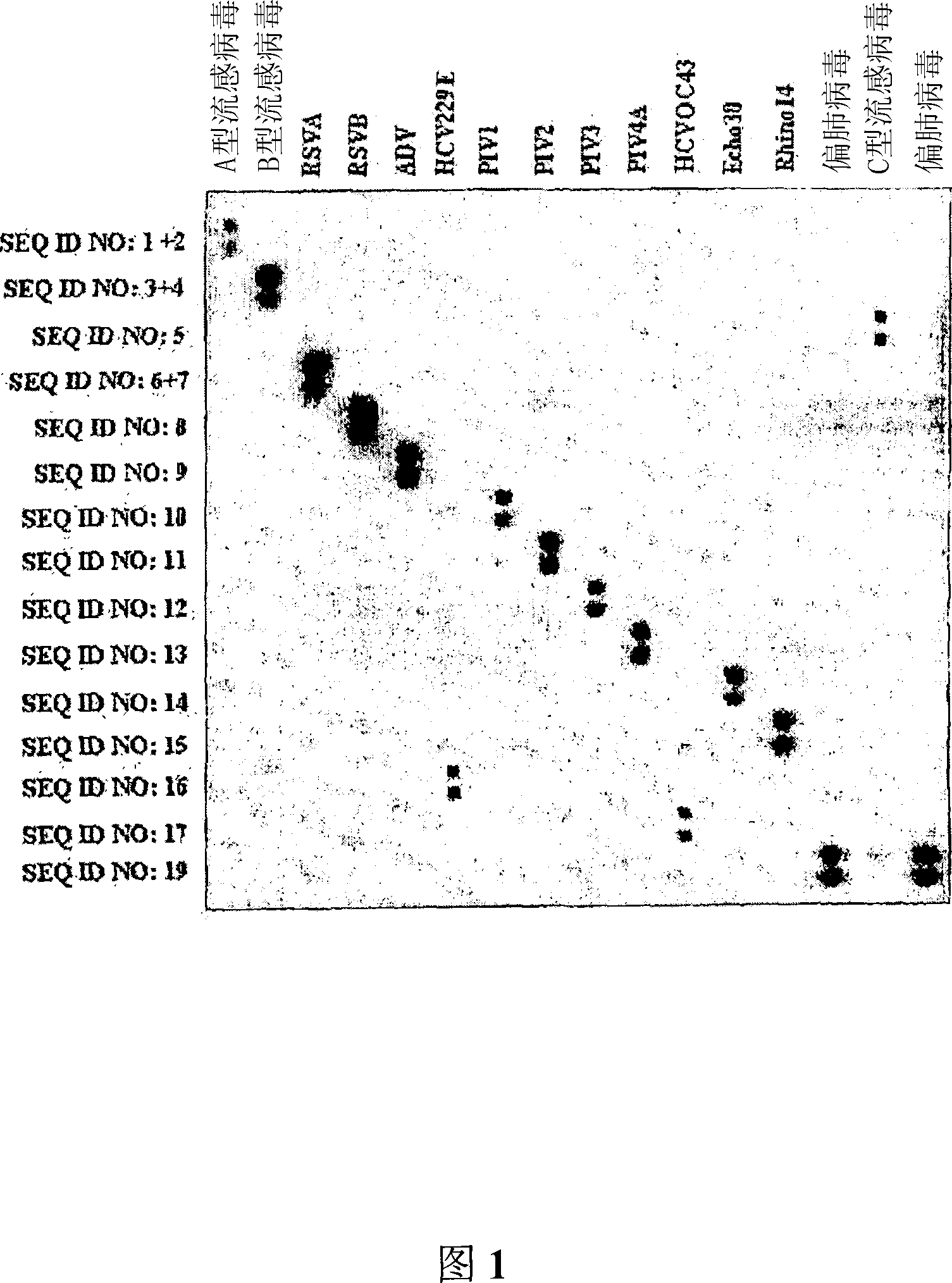
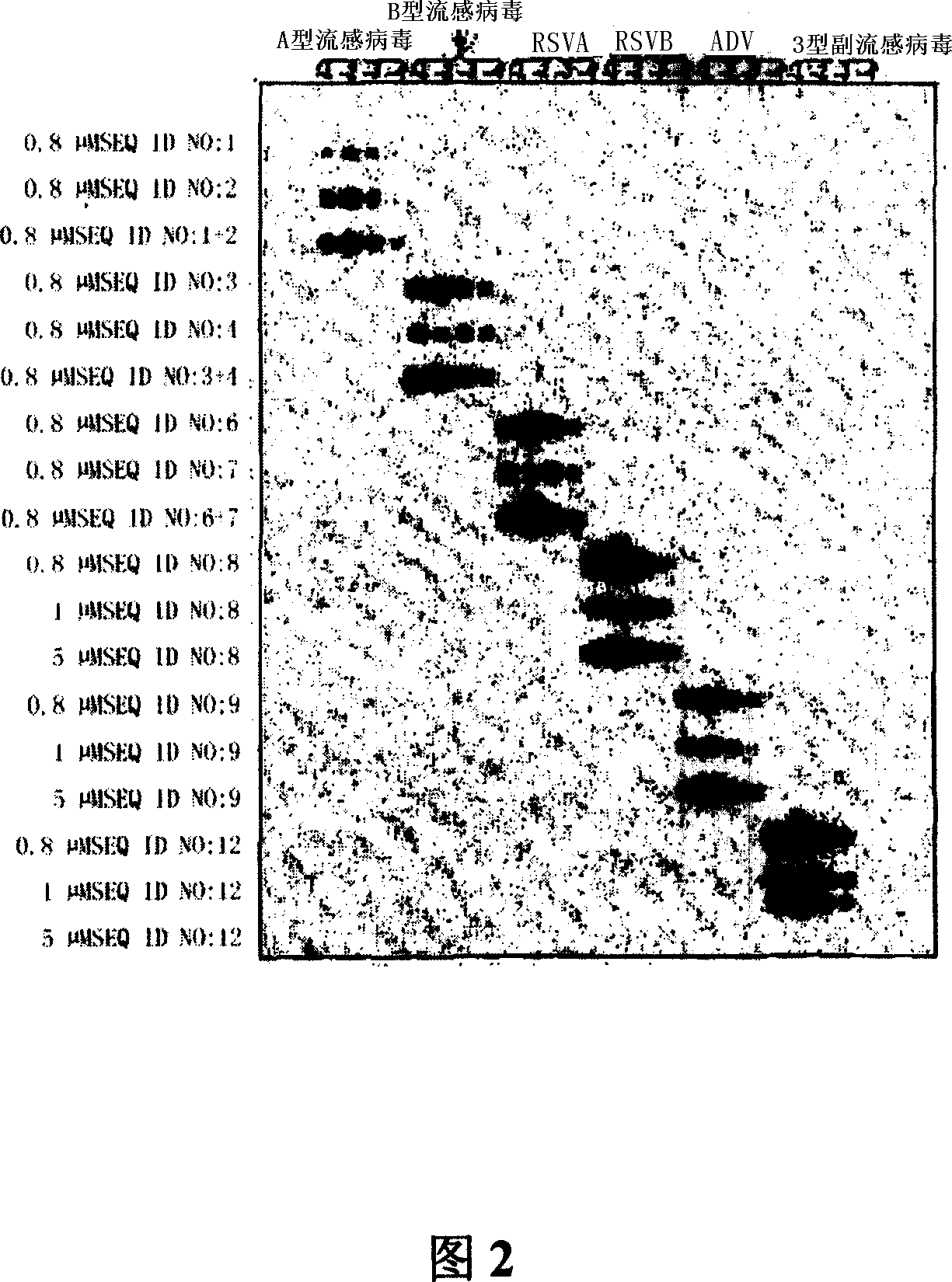
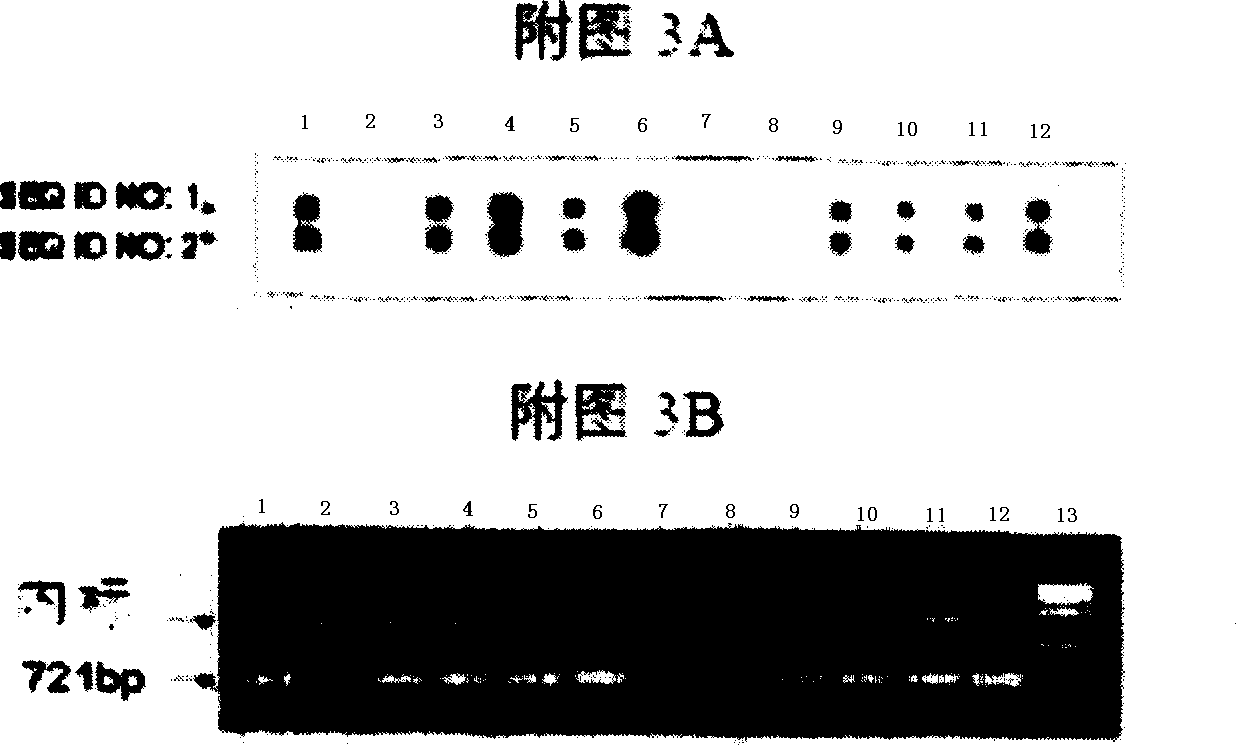
Probes and methods for the simultaneous detection and identification of multiple viruses that cause respiratory infections in humans
InactiveCN101107366AShorten the lengthSynthetic economyMicrobiological testing/measurementAgainst vector-borne diseasesEnterovirusSevere acute respiratory syndrome
The invention relates to probes and assays which are used for the simultaneous detection, in a single assay sample, of a plurality of nucleic acid sequences of viruses that cause respiratory infections in humans, selected from among influenza virus type A, influenza virus type B, influenza virus type C, human respiratory syncytial virus type A, human respiratory syncytial virus type B, human adenovirus, human parainfluenza virus type 1, human parainfluenza virus type 2, human parainfluenza virus type 3, human parainfluenza virus types 4A and 4B, enterovirus, rhinovirus, human coronavirus type 229E, human coronavirus type OC43, coronavirus that causes severe acute respiratory syndrome (SARS), human metapneumovirus and combinations thereof.
Owner:INST DE SALUD CARLOS III
New medicinal application of iridoid glycoside
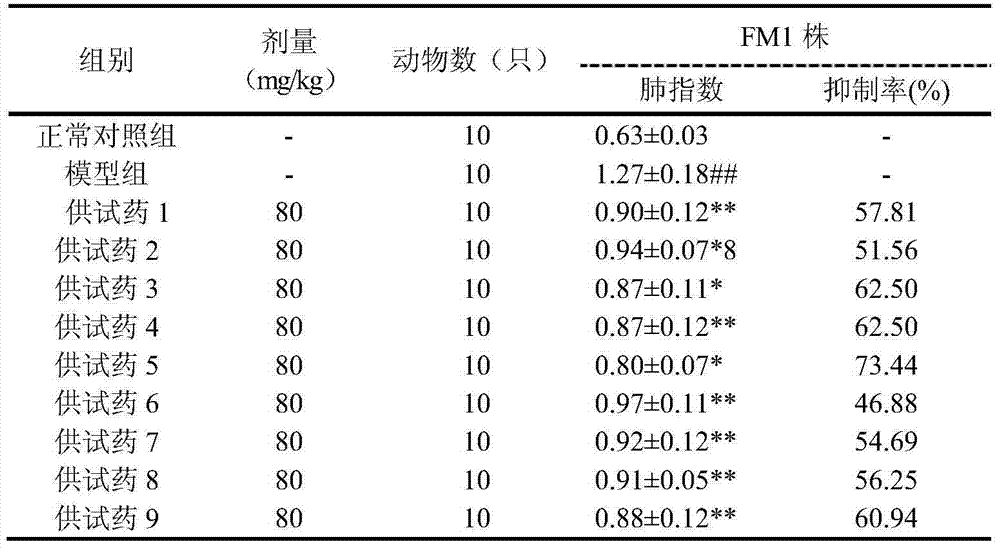
ActiveCN104510747AReduce loadLower lung indexAntibacterial agentsOrganic active ingredientsViral MyocarditisInflammation
The invention belongs to the field of medicines and particularly relates to a new application of an iridoid glycoside compound, genipin-1-[beta]-D-gentiobioside, and a composition composed by compounding the genipin-1-[beta]-Dgentiobioside with other components as effective components for preparing antiviral drugs, antibacterial drugs, fever alleviating drugs, inflammation resistant drugs and anti-oxidizing drugs. The iridoid glycoside compound and the composition can be used for treating acute respiratory infection, flu, pneumonia, viral hepatitis type B, herpes zoster, herpes viral keratitis, viral myocarditis, Epstein-Barr virus infection, retrovirus infectious diseases and bacterial infectious diseases.
Owner:樊向德 +1
Immunogenic Bordetella Bronchiseptica Compositions
PendingUS20130302369A1Avoid infectionAntibacterial agentsSsRNA viruses negative-senseRespiratory infectionBordetella
Provided herein are compositions, combinations, and methods comprising Bordetella bronchiseptica and isolated pertactin, which are effective in treating or preventing respiratory infections, such as kennel cough, in animals.
Owner:ZOETIS SERVICE LLC
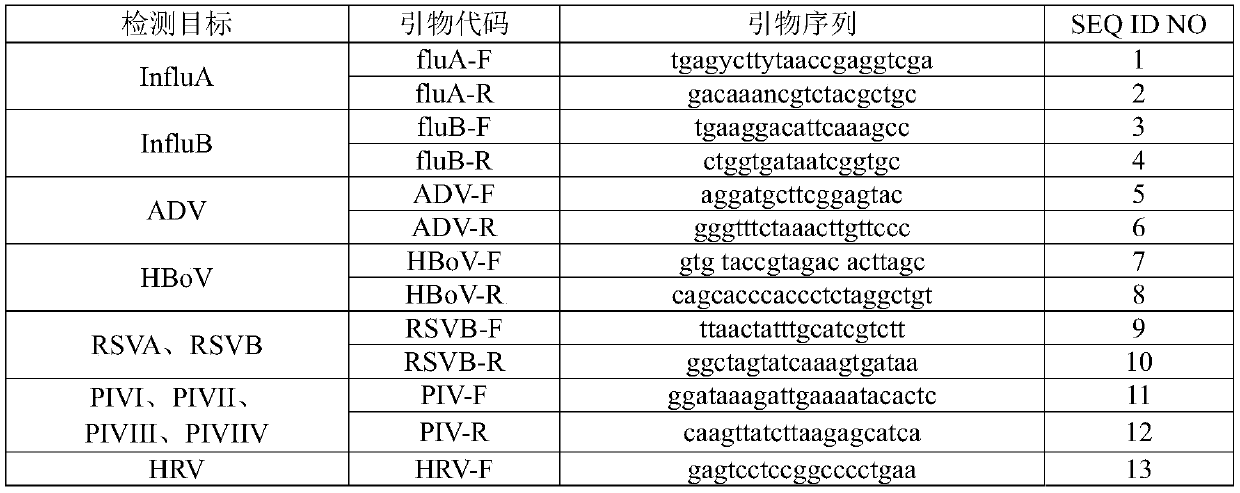
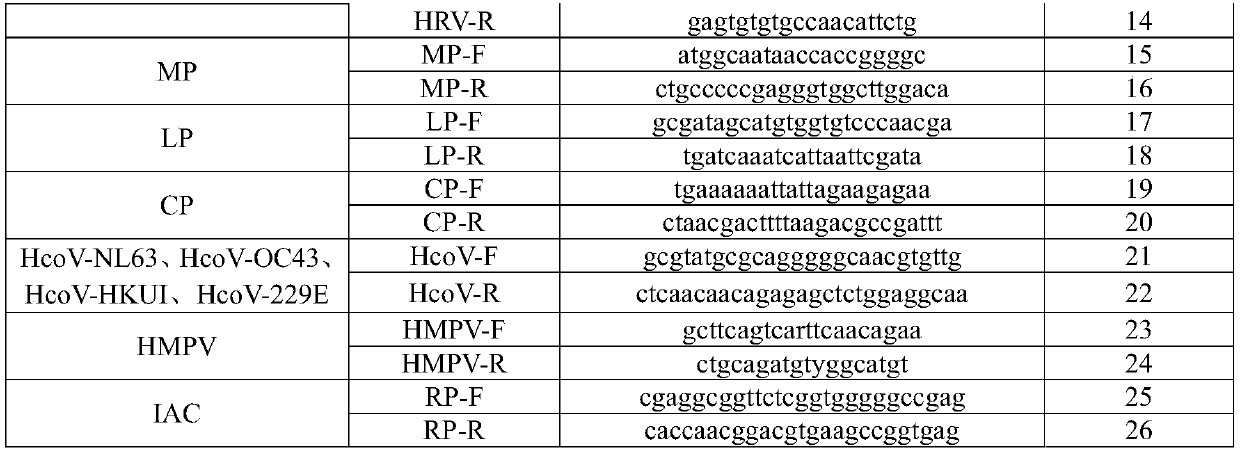
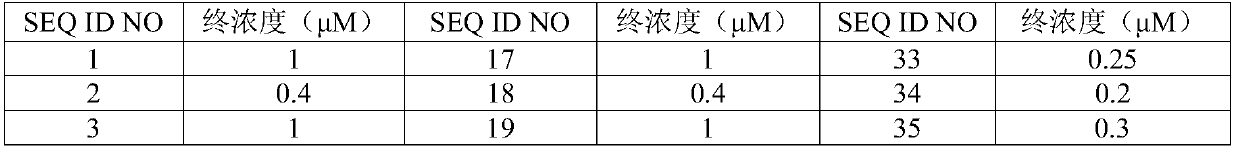
Nucleic acid reagent, kit, system and method for detecting respiratory infection pathogen
ActiveCN109609692AQuick result determinationComprehensive result determinationBioreactor/fermenter combinationsBiological substance pretreatmentsRespiratory infectionBiology
The present disclosure relates to a nucleic acid reagent, kit, system and method for detecting a respiratory infection pathogen. The nucleic acid reagent comprises primers shown as SEQ ID NO.1-24 andprobes shown as SEQ ID NO.27-45, wherein the primers and probes are stored separately from each other or arbitrarily mixed with each other. The present establishes the nucleic acid reagent, kits, system and method for detecting the respiratory infection pathogen by the primers and probes described above, and can realize rapid, comprehensive, sensitive, specific and automatic detection result determination.
Owner:北京卓诚惠生生物科技股份有限公司
Composite of traditional Chinese traditional medicine for clearing away heat and toxic, antiinflammatory, detumescence and preparation method thereof
ActiveCN101204424AHas swelling and dispelling stagnationSignificant effectAntiviralsGranular deliveryDigestive canalUpper urinary tract infection
The invention belongs to the field of traditional Chinese medicine technology, in particular to a traditional Chinese medicine combination of clearing away heat and toxic material, anti-inflammation and detumescence and a preparation method thereof. The components and the parts by weight of the traditional Chinese medicine combination are as follows: 2-5 shares of baikal skullcap root, 6-12 shares of dandelion herb, 1-4 shares of corydalis, 2-5 shares of dyers woad root. The invention can cure the respiratory infection, the infection of digestive canal, surgical infection and the urinary tract infection; and the invention can be made into pelletized granule type. As the correctives is added into the traditional Chinese medicine combination, the problem that the taste is affected by the corydalis is solved, and patients are easy to accept the medicine combination owing to the small dosage, the better absorption. The invention is a traditional Chinese medicine combination of clearing away heat and toxic material, anti-inflammation and detumescence which has the advantages that the quality can be controlled, the healing effect can be exerted fully, the dose is reasonable, the medicine is portable and is convenient for carrying, the invention has remarkable economic and social benefits and is suitable for industrial production.
Owner:TIANJIN ZHONGXIN PHARMA GRP CO LTD
Nutritional preparation comprising ribose and folic acid and medical use thereof
Trauma, surgery, inflammation, subfertility, lactation problems, gut disorders, infant nutrition, cancer, arthritis and other joint problems, vascular problems and cardio-or cerebro vascular problems, ischaemia, aging, impaired immune function, burns, sepsis, malnutrition, problems with liver or kidneys, malaria, cystic fibrosis, migraine, neurological problems, respiratory infections, improvement of sports results, muscle soreness, drug intoxication and pain can be treated with a nutritional composition containing effective amounts of ribose and folic acid, optionally combined with other components such as niacin, histidine, glutamine, orotate, vitamin B6 and other components.
Owner:NV NUTRICIA
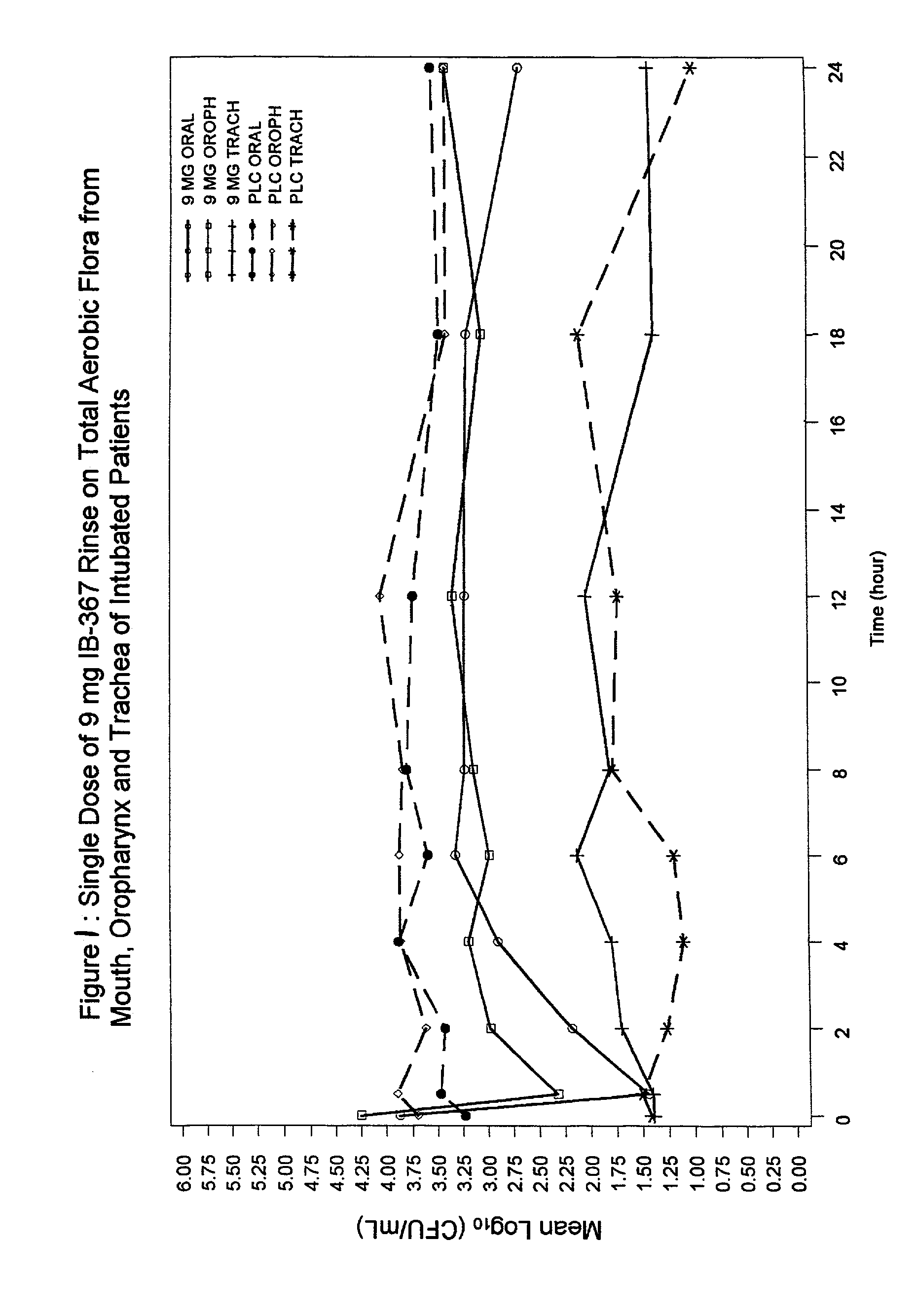
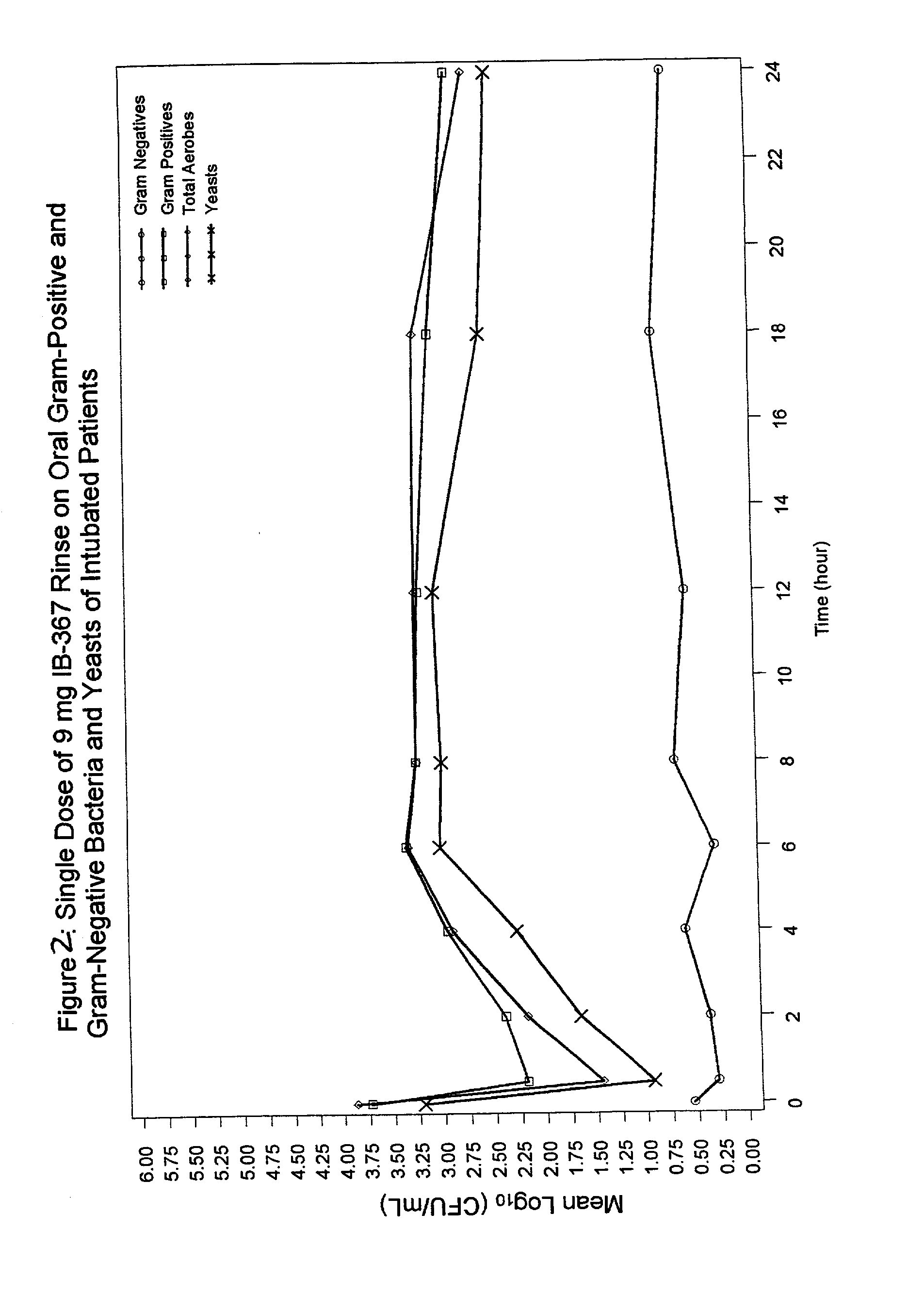
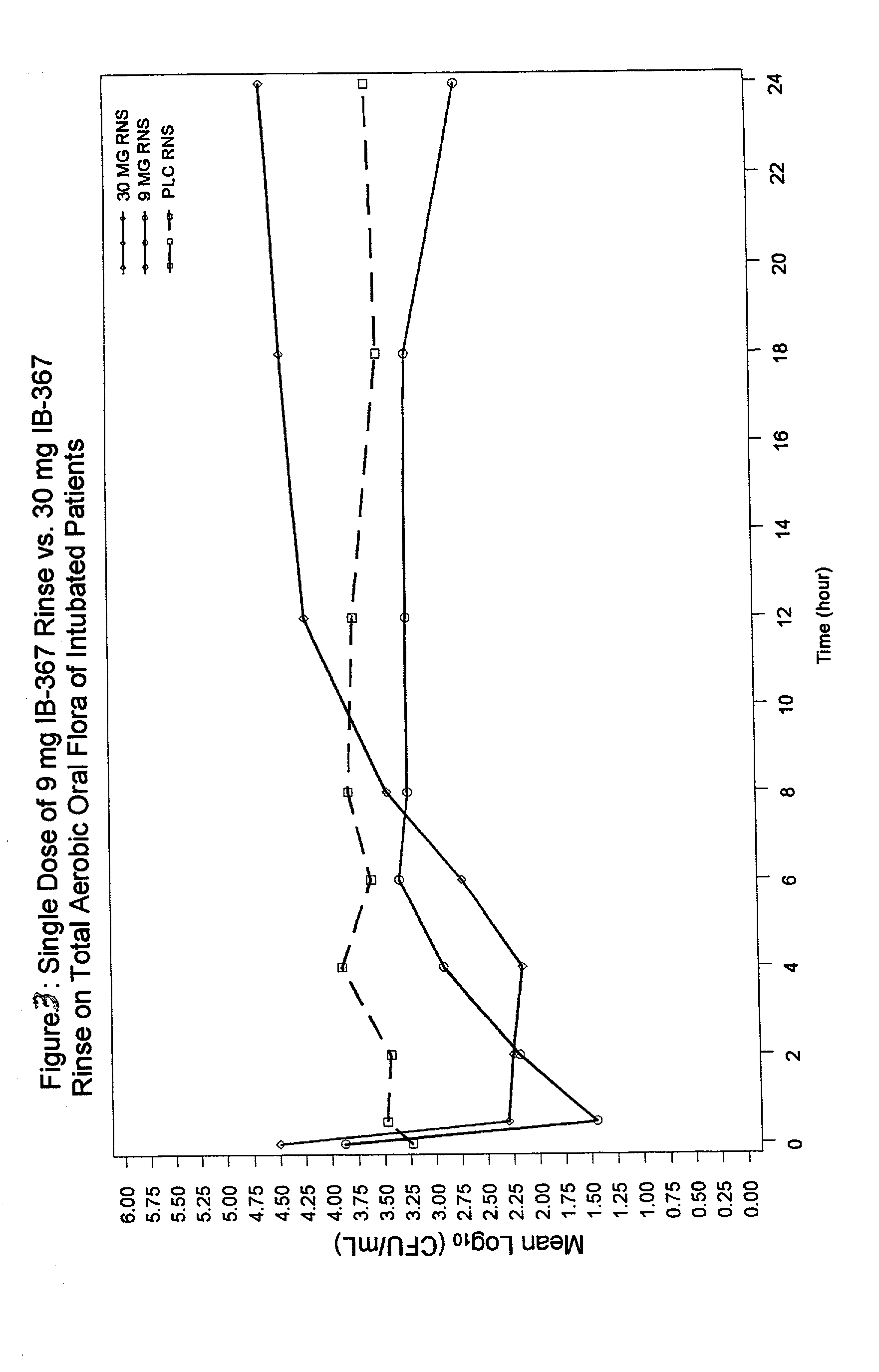
Methods of preventing ventilator associated pneumonia by oral administration of antimicrobial IB-367 peptides
InactiveUS20030073625A1Low costImprove the quality of lifeAntibacterial agentsBiocideProphylactic treatmentResistant strain
The present invention provides methods of preventing respiratory infections associated with intubation and / or mechanical ventilation, such as ventilator-associated pneumonia, in intubated patients. The method generally involves topical administration of a composition comprising an IB-367 peptide to the oral cavity of an intubated patient. As IB-367 peptides engender very little resistance, a significant advantage of the invention is that the prophylactic therapy may be applied without having to worry about creating resistant strains of pathogens.
Owner:RGT UNIV OF CALIFORNIA
Interrupting the interaction of intercellular adhesion molecule-1 and respiratory syncytial virus for prevention and treatment of infection
There is provided a method of preventing a respiratory infection by administering an effective amount of an agent for regulating ICAM-1 expression. Also provided is a composition for the prevention of respiratory infection including an agent which regulates ICAM expression. Also provided is a method of preventing RSV infection by administering an effective amount of an agent that interferes with the binding of RSV to ICAM-1. A method of preventing RSV infection by administering an effective amount of an agent that down regulates the expression of ICAM-1, thereby decreasing RSV binding to ICAM-1 is also provided. There is provided a method of treating RSV infection by administering an effective amount of an agent for down regulating ICAM-1 expression. A method of blocking RSV-ICAM-1 interaction by administering an effective amount of agents for blocking ICAM sites of binding is provided. Also provided is a compound for blocking RSV-ICAM-1 interaction including an agent for blocking ICAM sites of binding.
Owner:SOUTH FLORIDA UNIVESITY OF
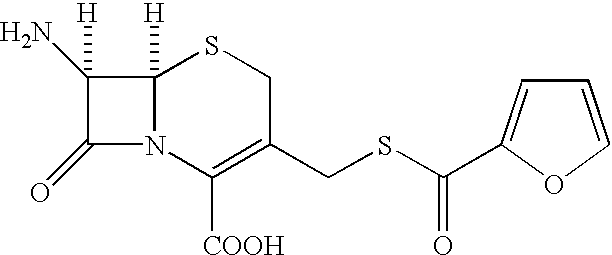
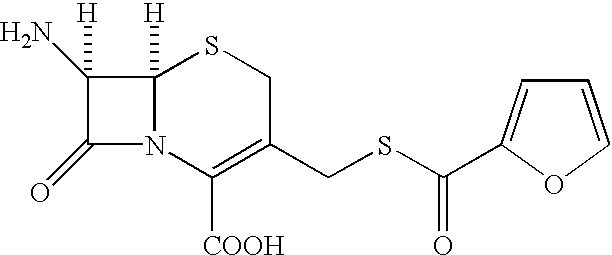
Cephalosporin compound and a process for its preparation
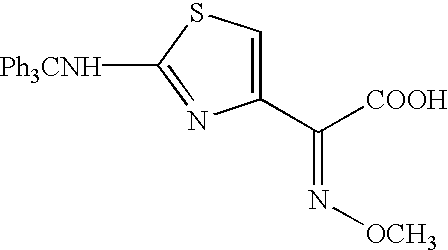
A process is disclosed for the preparation of 7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid of the formula This compound is useful as an intermediate for the preparation of ceftiofur cephalosporin antibiotic used for bovine respiratory infections.
Owner:AUROBINDO PHARMA LTD
Pathogenic bacteria inhalation toxicology experimental equipment for respiratory system
InactiveCN102670328AGuarantee the safety of lifePrevent and Eliminate Safety HazardsVeterinary instrumentsRespiratory infectionPathogenic bacteria
The invention provides pathogenic bacteria inhalation toxicology experimental equipment for a respiratory system. A closed cabin used for accommodating experimental animals is connected with a bacteria liquid aerosol atomization device capable of precisely controlling the bacteria carrying amount; the atomized aerosol is sent into the closed cabin to form a simulation environment with pathogenic bacteria; a transparent sealed door can be conveniently opened and closed to observe inside animals; the inside of the closed cabin can be sterilized and disinfected by an ultraviolet sterilizing lamp and an ozone sterilizer; after toxic exhaust gas is controlled by a solenoid-controlled valve and is disinfected by a high-temperature gas burning pipe, the toxic exhaust gas is pumped out via a negative-pressure pump; a gas environment monitoring and controlling device is arranged in the closed cabin; the closed cabin is connected with the negative-pressure pump, a compressed air source and the solenoid-controlled valve; a computer and a printer can observe and control according to a programmed program to finish an infection simulation experiment of the inhalation pathogenic bacteria; and an experiment process parameter is stored or printed. The pathogenic bacteria inhalation toxicology experimental equipment for the respiratory system has the effect that the safe and convenient simulation experiment equipment capable of precisely and quantitatively controlling the environment with the pathogenic bacteria is provided for the medical respiratory infection research.
Owner:TIANJIN HOPE IND & TRADE +1
Method for detecting lung infection pathogenic bacteria by adopting visual gene chip
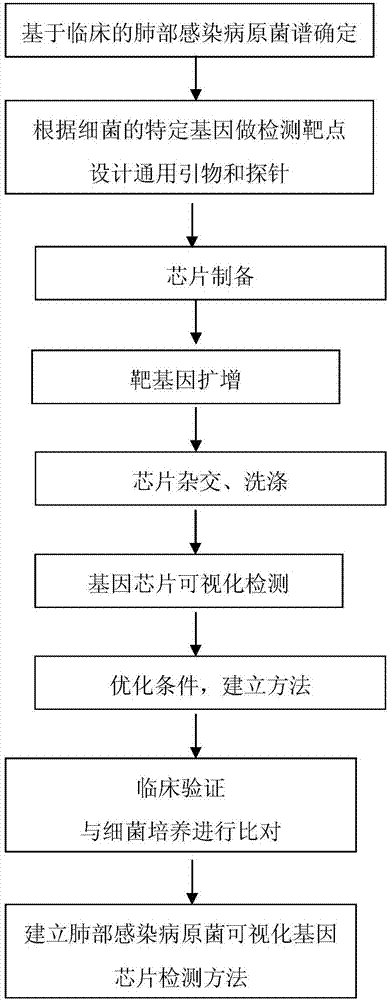
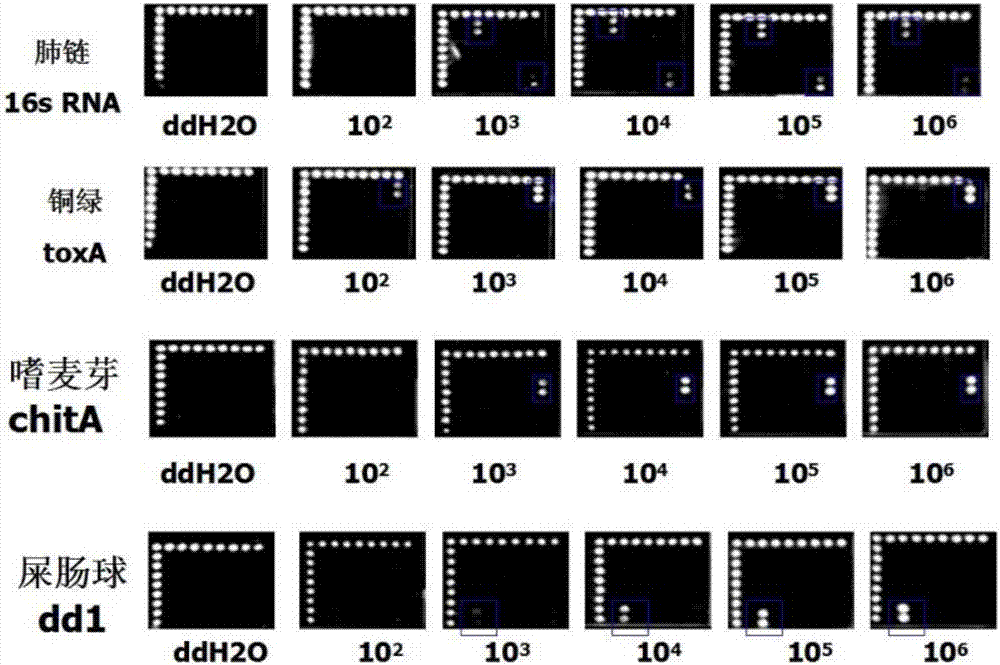
InactiveCN107164526AEfficient screening methodsMicrobiological testing/measurementAnti-infective therapyBiology
The invention discloses a method for detecting pathogenic bacteria of pulmonary infection by a visualized gene chip, comprising the following steps: S1 determining the spectrum of pathogenic bacteria of pulmonary infection based on clinical practice; S2 making the detection target according to the specific gene of the bacteria; S3 designing general primers and probes S4 chip preparation; S5 target gene amplification; S6 chip hybridization and washing; S7 gene chip visual detection; S8 optimization conditions, establishment of methods; S9 clinical verification; S10 comparison with bacterial culture; Chip detection. Beneficial effects of the present invention: realize rapid, sensitive, and high-throughput detection of important pathogenic microorganisms of common clinical respiratory infection flora, provide evidence for clinical disease diagnosis and anti-infection treatment of acute and severe respiratory infectious diseases; provide emergency, prevention and control Provide effective and efficient screening means and methods.
Owner:GENERAL HOSPITAL OF CHINESE PEOPLES ARMED POLICEFORCES
Modification of bioassays for detection of antigens characteristic of bacteria that are causative of ear and respiratory infections to eliminate false positive results caused by nasopharyngeal colonization of children
The present invention relates to modifying rapid immunochromatographic (“ICT”) tests for the detection of characteristic carbohydrate antigens of bacteria that are known to be causative of otitis media and respiratory diseases in children under the age of approximately 12 years. The test modifications involve either (1) reducing the total amount of antibodies to the carbohydrate antigen employed in each test, (2) adding at least one fixed “scrub” line located just prior to the capture line in the sample flow path to the prepared ICT test strip to “scrub” out an identical amount of target antigen from all bodily fluid test samples obtained from both colonized but otherwise healthy children and diseased children, or (3) combinations of (1) and (2).
Owner:ALERE SCARBOROUGH
Rescue negative pressure respiratory disinfection machine
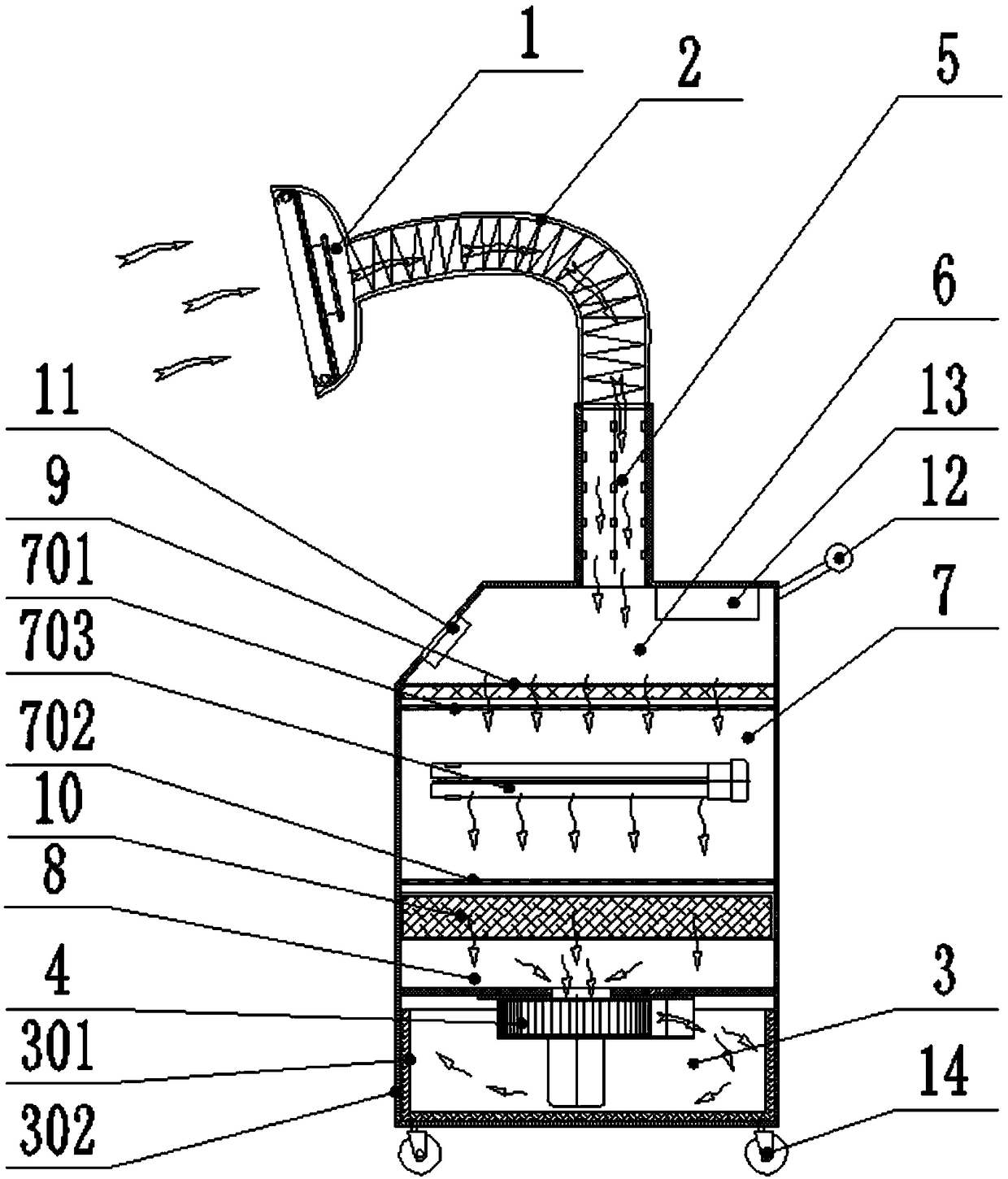
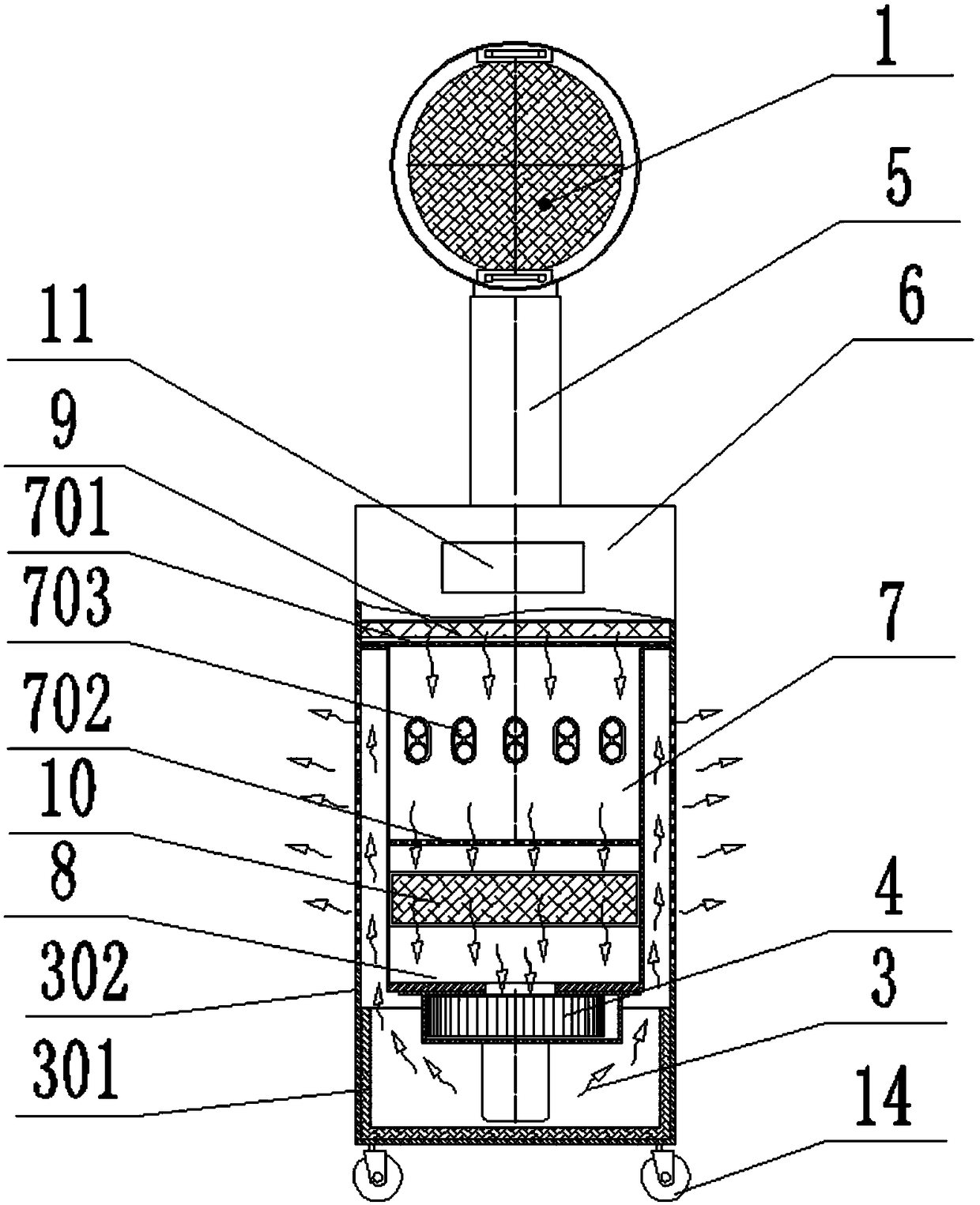
PendingCN109331212AReduce the chance of cross infectionGood disinfection and purification effectRadiationRespiratory infectionDrainage tubes
The invention relates to a rescue negative pressure respiratory disinfection machine which is characterized by comprising a respiratory drainage cover, a drainage tube, a multi-pass sterilization chamber, an air outlet chamber and a fan, wherein the respiratory drainage cover is used for collecting and draining the pathogenic gas exhaled by a patient, the drainage tube is used for introducing thegas drained by the respiratory drainage cover into the multi-pass sterilization chamber, the multi-pass sterilization chamber is used for multi-pass sterilization of the gas inside, the air outlet chamber is used for discharging the sterilized gas, the fan is used for forming negative pressure in the respiratory drainage cover, the drainage tube and the multi-pass sterilization chamber to drain the gas, and the sterilized gas is discharged from the air outlet chamber. The rescue negative pressure respiratory disinfection machine can quickly isolate and dynamically, safely and thoroughly sterilize and purify the gas exhaled from an infectious disease source during rescue, thereby realizing timely, convenient, safe, efficient and full-featured isolation and sterilization of the gas exhaled by respiratory infection patients.
Owner:JIANGSU JUGUANG PHOTOELECTRIC TECH
Modification of bioassays for detection of antigens characteristic of bacteria that are causative of ear and respiratory infections to eliminate false positive results caused by nasopharyngeal colonization of children
InactiveUS20050260694A1Reduce eliminateReduce morbidityBiological testingCarbohydrate antigenRespiratory disease
The present invention relates to modifying rapid immunochromatographic (“ICT”) tests for the detection of characteristic carbohydrate antigens of bacteria that are known to be causative of otitis media and respiratory diseases in children under the age of approximately 12 years. Children of this age group are also prone to nasopharyngeal colonization with the same bacteria, and urine samples taken from colonized, but otherwise healthy, children were shown to exhibit an unduly high incidence of test results that were false positive for the presence of disease. The test modifications, which maintain the test sensitivity unchanged and the test specificity at a value above 90% were developed to insure that healthy, albeit colonized, children were not medicated for disease the bacteria are known to cause. The modifications involve either (1) reducing the total amount of antibodies to the carbohydrate antigen employed in each test, (2) adding at least one fixed “scrub” line located just prior to the capture line in the sample flow path to the preprepared ICT test strip to “scrub” out an identical amount of target antigen from all bodily fluid test samples obtained from both colonized but otherwise healthy children and diseased children, or (3) combinations of (1) and (2).
Owner:ALERE SCARBOROUGH
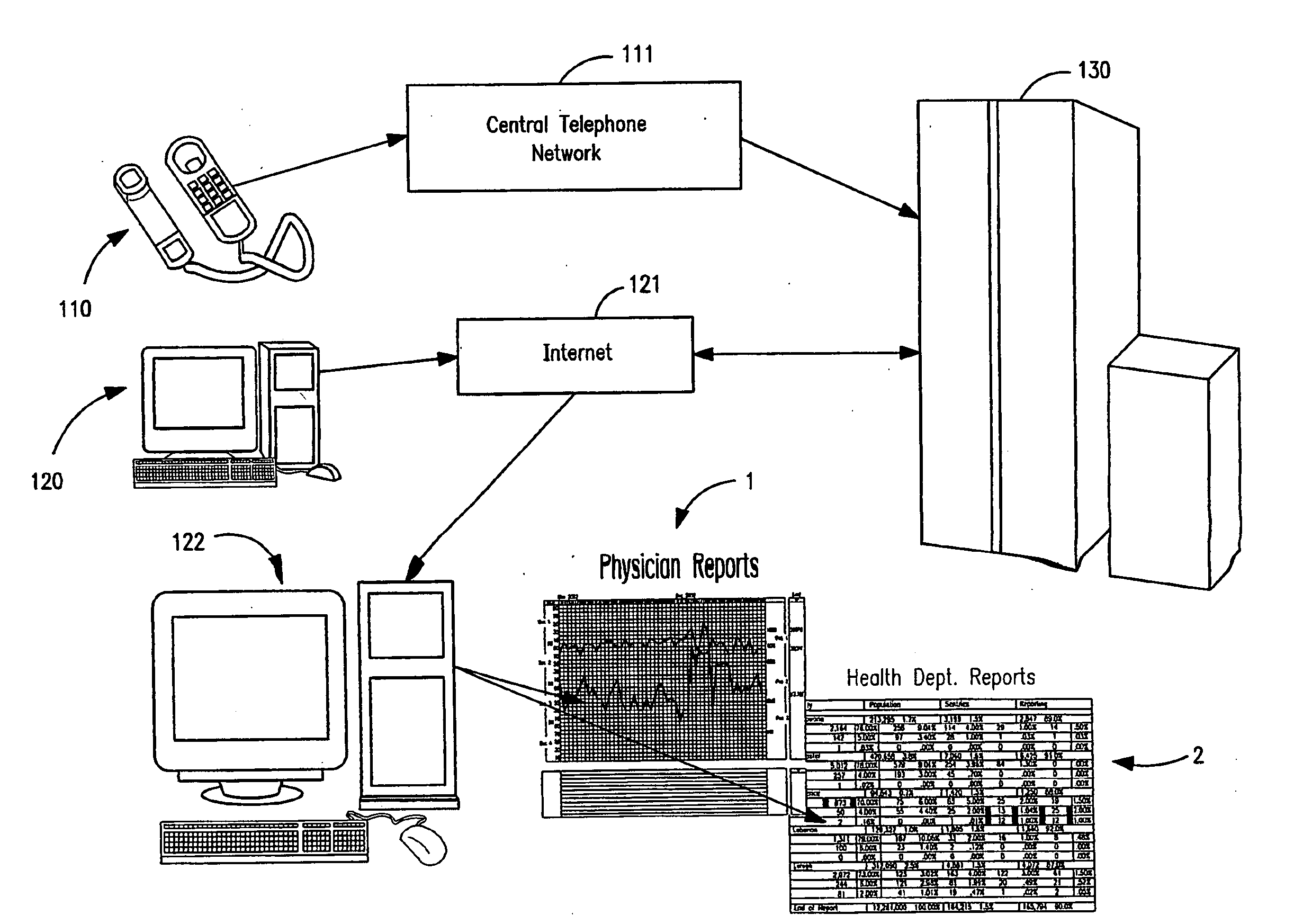
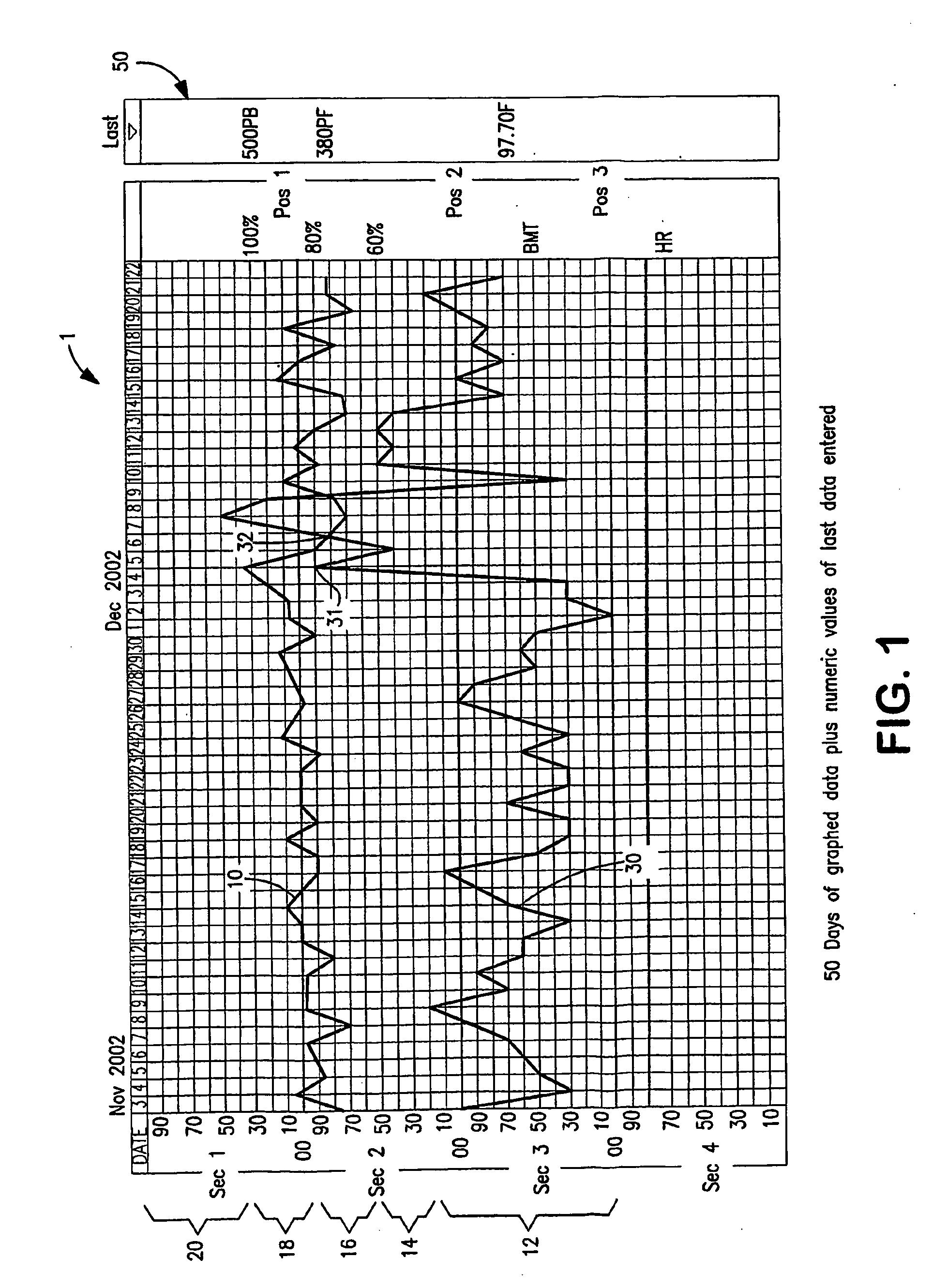
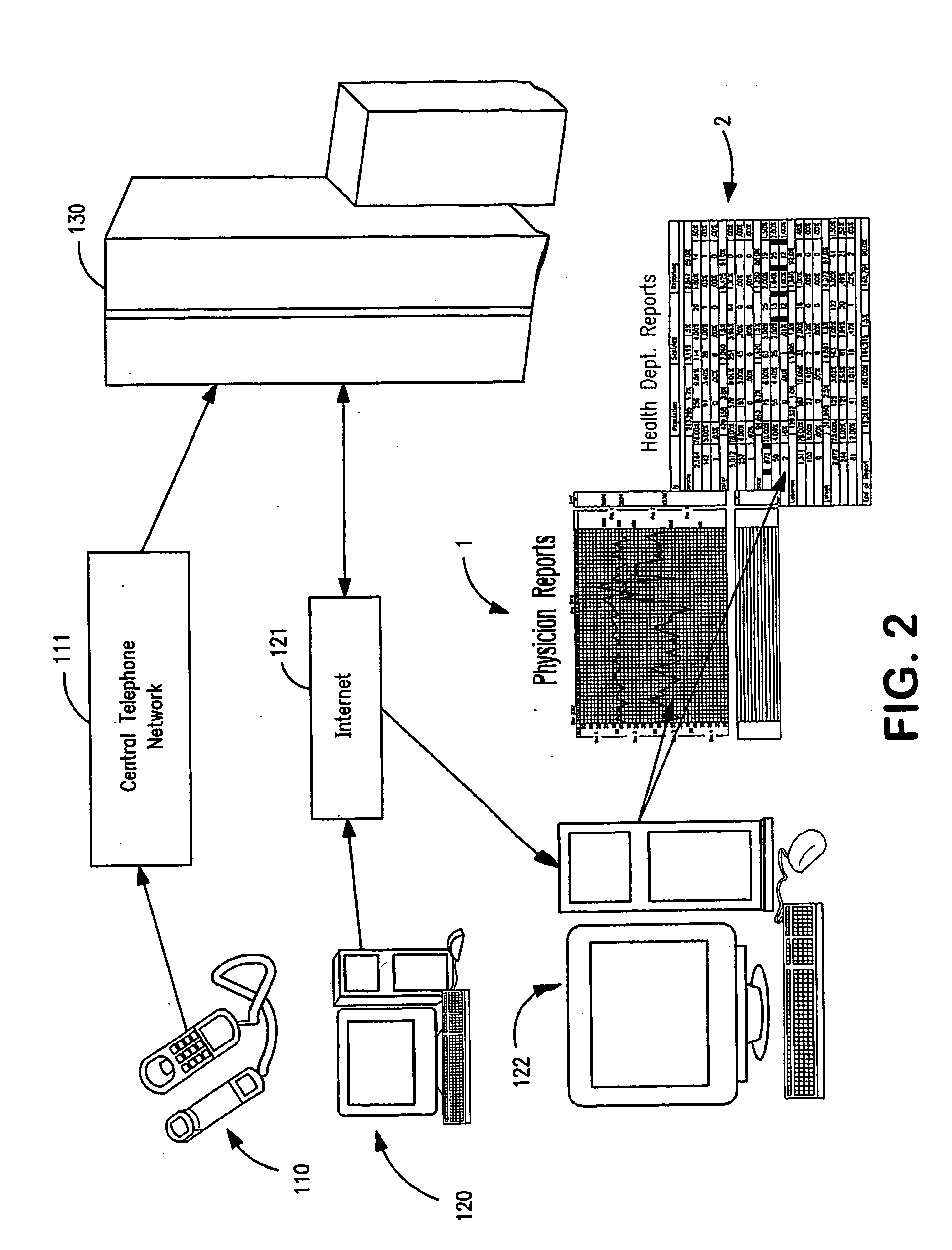
Apparatus and System for Predictive Health Monitoring
InactiveUS20080306352A1Other printing matterEpidemiological alert systemsRespiratory infectionPeak inspiratory flow
An apparatus and system are provided for monitoring an individual, a group or a community of individuals for respiratory infections. Predictive health data, such as waking peak expiratory flow rate (“WPF”) and basal metabolic temperature (“BMT”) are measured and charted to provide an indication of early stage respiratory infection. Data may be collected for target populations and reported to health agencies for use in combating the spread of infection. An apparatus is provided which can simultaneously measure WPF and BMT and other health-condition-related values and automatically transmit readings to a data collection and analysis program on a remote computer.
Owner:PREDICTIVE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com