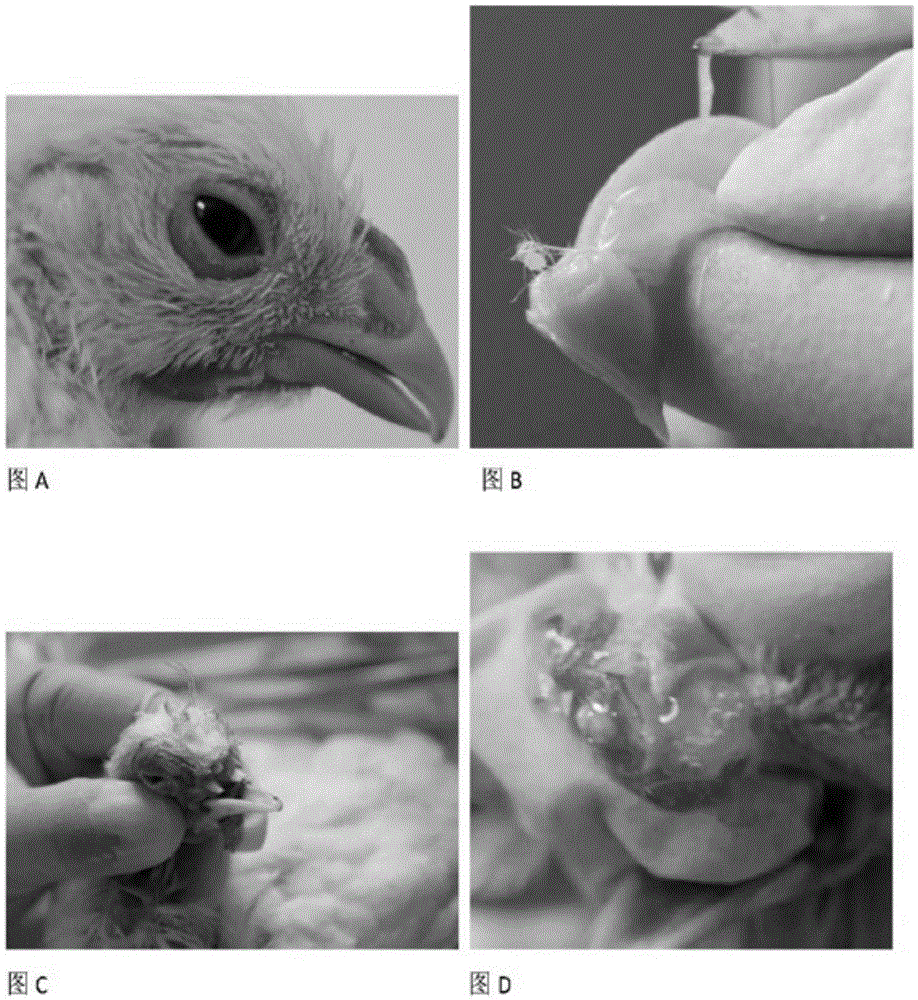
Patents
Literature
57 results about "Metapneumovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A genus of the subfamily PNEUMOVIRINAE, containing two members: Turkey rhinotracheitis virus and a human Metapneumovirus. Virions lack HEMAGGLUTININ and NEURAMINIDASE.
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences and methods for propagating virus
ActiveUS20050019891A1Narrow downSymptoms improvedSsRNA viruses negative-senseVirus peptidesNegative strandHeterologous
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. The invention also provide methods for propagating virus.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
InactiveUS20030232061A1Optimization orderImprove scalabilitySsRNA viruses negative-senseVectorsNegative strandAntigen
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
ActiveUS20040005544A1Narrow downSymptoms improvedOrganic active ingredientsFungiHeterologousNegative strand
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Virus causing respiratory tract illness in susceptible mammals
The invention relates to the field of virology. The invention provides an isolated essentially mammalian negative-sense single stranded RNA virus (MPV) within the sub-family Pneumovirinae of the family Paramyxoviridae and identifiable as phylogenetically corresponding to the genus Metapneumovirus and components thereof.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae , of the family Paramyxoviridae . The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Method for simultaneously detecting twelve kinds of common respiratory viruses
InactiveCN104342503ASave production costSave testing costMicrobiological testing/measurementMicroorganism based processesMultiplexInfluenza virus C
The invention discloses a method for simultaneously detecting twelve kinds of common respiratory viruses. According to the method, primers and probes are designed according to gene conservative areas of the twelve kinds of common respiratory viruses, namely influenza A virus, influenza B virus, influenza C virus, parainfluenza virus type 1, parainfluenza virus type 2, parainfluenza virus type 3, rhinovirus, Bocavirus, adenovirus, coronavirus, metapneumovirus and respiratory syncytial virus, nucleic acid fragments of samples to be measured are extracted for amplifying, and finally, the samples are separated by using a capillary electrophoresis method. The method disclosed by the invention has the advantages of low required sample size, high sensitivity and accuracy, good specificity and low cost; the defects that the conventional single tube multiplex fluorescence PCR (Polymerase Chain Reaction) detection primers are difficult to design, and multicolor fluorescence mutually intervenes and is not easy to part are overcome, the defects that a chip detection method is tedious in operation, high in detection cost and the like are also overcome, and a new method is provided for screening the respiratory viruses.
Owner:FUJIAN INT TRAVEL HEALTH CARE CENT +1
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences and methods for propagating virus
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. The invention also provide methods for propagating virus.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Acian Metapneumovirus antibody ELISA detection kit
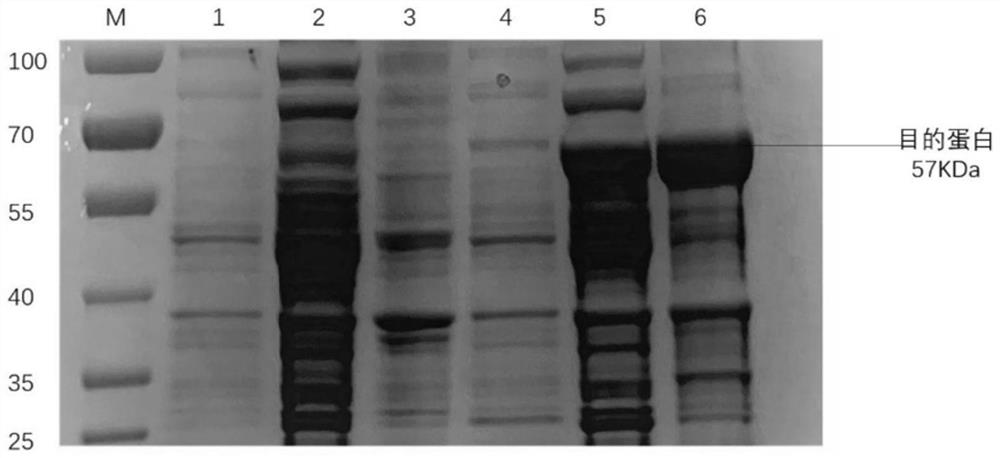
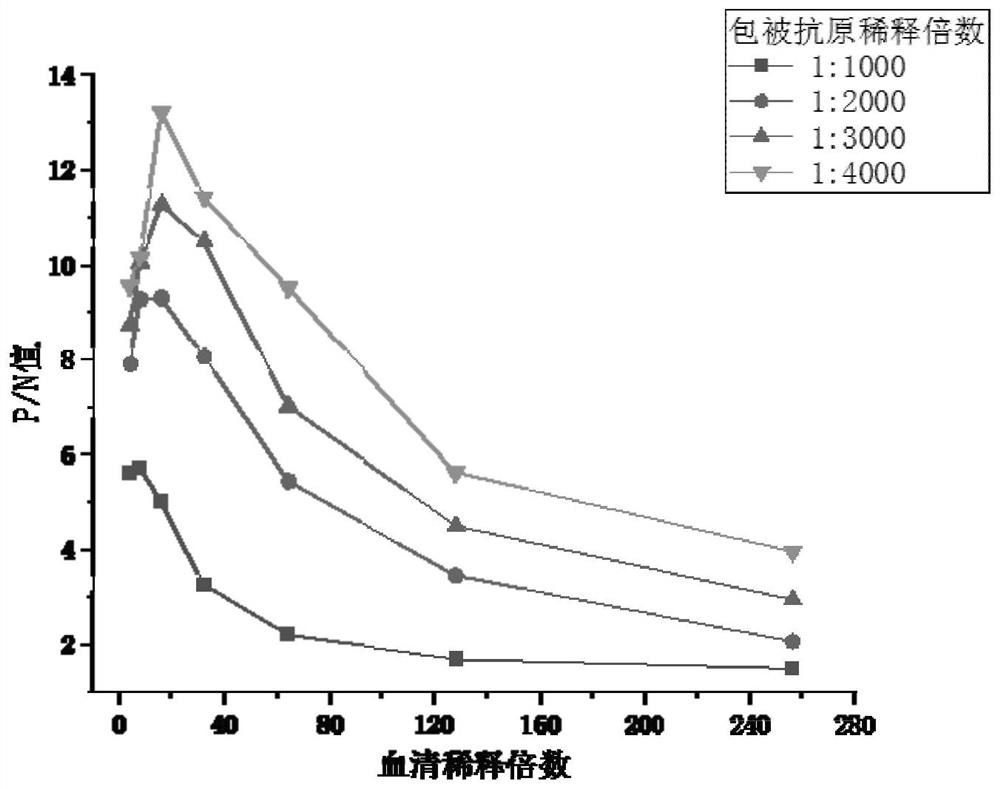
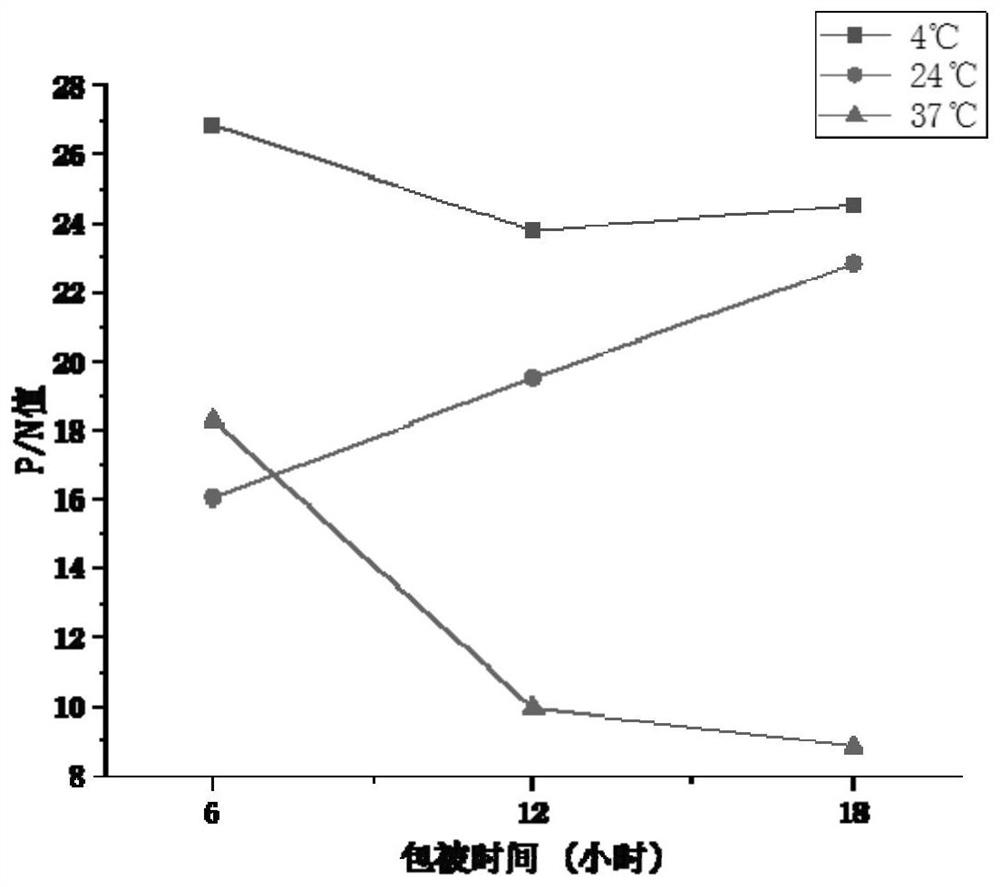
ActiveCN103360472ASolve the difficulty of purificationReactogenicDepsipeptidesBiological testingAntigenHuman metapneumovirus
The invention provides an Acian Metapneumovirus antibody ELISA detection kit. The kit is the ELISA kit established for detecting the Acian Metapneumovirus antibody on the basis of treating the monomer protein of the A subgroup Acian Metapneumovirus F protein as a coating antigen. The invention also provides a preparation method of the monomer protein of the coating antigen F protein for making the kit. The F protein prepared through the method has a good reactionogenicity, and the established ELISA detection kit provides an effective method for the early-stage diagnosis and detection of the Acian Metapneumovirus infection, and plays a substantial promotion effect in the prevention researches of the Acian Metapneumovirus infected diseases.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Live attenuated metapneumovirus strains and their use in vaccine formulations and chimeric metapneumovirus strains
The invention relates to an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae with one or more genetic modifications. The present invention also relates to the mutant components, i.e., nucleic acids and proteins, of these mutant mammalian MPVs. These mutant mMPV can be attenuated. These mutant mMPVs can encode non-native sequences. The invention further relates to vaccine formulations comprising the mMPV, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. In addition, the invention relates to chimeric viral RNA polymerase complex and assays using these chimeric RNA polymerase complexes. The chimeric RNA polymerase complexes of the invention are composed of different RNA polymerase components from different viruses of the family of paramyxoviridae.
Owner:VIRONOVATIVE
Avian metapneumovirus (aMPV) F protein polypeptide and application thereof
ActiveCN103739679AStrong positive signalHigh sensitivityImmunoglobulins against virusesHybrid peptidesF proteinMetapneumovirus
The invention provides an F protein polypeptide and an application thereof. The antigen polypeptide is obtained through bioinformatics analysis and comparison according to F protein gene sequences. The amino acid sequence of the antigen polypeptide is CTNAGSTAYYPNKDD. The antigen polypeptide has high conservative property and strong antigenicity, and monoclonal antibodies aiming at the Avian metapneumovirus (aMPV) F proteins can be generated by utilizing the polypeptide.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Metapneumovirus Immunogens and Related Materials and Methods
ActiveUS20160039884A1DetoxificationSsRNA viruses negative-senseSugar derivativesMetapneumovirusHuman metapneumovirus RNA
The present invention provides compositions useful to induce immune response to human metapneumovirus. Also provided are related materials and methods, including methods to induce an immune response in a mammal, comprising administering the compositions herein.
Owner:RES INST AT NATIONWIDE CHILDRENS HOSPITAL +1
Chicken C-type acian metapneumovirus strain(aMPV-JCX) and application thereof
The invention provides a chicken C-type acian metapneumovirus strain(aMPV-JCX) and application thereof. The preservation number of the aMPV / C-JCX strain is CGMCC No.10900. The virus is a cell culture adaptive strain, a preparation method of the cell culture adaptive strain comprises the steps that tissue grinding fluid of a sick chicken infected by aMPV / C-JCX which is firstly obtained through separation domestically is collected to be inoculated into single-layer Vero cells which grow being adherent to the wall, a cell culture serves as an inoculum for the next round of passage, sequentially continuous passage is conducted, and the cell culture adaptive strain is obtained. In the adaptive process, the early-generation culture cycle is 7-9 days, the culture cycle is shortened to 3-5 days when the 60th generation is cultured, and an obvious cytopathic effect can be observed. It is verified through a transmission electron microscope, indirect immunofluorescence and a western-blotting method that the aMPV / C-JCX can be effectively cultured. The titer 1 g TCID50 of the virus is equal to 10<-4.2> / 0.1 ml. It is verified through SPF chickens that the virus virulence is weak, good immunogenicity and protectiveness are achieved, and the cell-adapted virus is an ideal candidate strain for researching and producing viral vaccine.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Composition for detecting and typing respiratory tract related viruses, kit, use and method
InactiveCN111321253ALow costImprove throughputMicrobiological testing/measurementAgainst vector-borne diseasesMetapneumovirusRespiratory syncytial virus (RSV)
The invention relates to the field of detection of molecular biology and more specifically relates to detection on novel coronavirus 2019-nCoV, influenza A virus, influenza B virus, parainfluenza virus, respiratory adenovirus, respiratory syncytial virus and metapneumovirus. Meanwhile, the invention further provides a kit containing the composition, use of the composition and a method for detecting and typing respiratory tract related viruses. According to the composition provided by the invention, in combination with a fluorescent probe method and a melting curve method, the respiratory tractrelated viruses can be simultaneously detected and typed in one tube, the cost is low, the flux is high, the consumed time is short, and the efficiency of detection is greatly increased; and the operation is simple and convenient, and false positive and environmental pollution caused by crossing among samples is avoided due to single-tube operation.
Owner:SANSURE BIOTECH INC
Method for separating acian metapneumovirus
ActiveCN104911153AImprove in vitro culture conditionsImprove separation rateMicroorganism based processesViruses/bacteriophagesMixed cellHuman metapneumovirus
The invention provides a method for separating acian metapneumovirus, which adopts tracheas and lung tissues mixed cells of specific pathogen free (SPF) chick embryos which are incubated for 17-19 ages in days to cultivate the acian metapneumovirus. The method for separating the acian metapneumovirus successfully improves acian metapneumovirus (AMPV) vitro culture conditions through a separation and reproduction method of a same culture system and two kinds of mixed cells based on a traditional method that a clinical development sample is separated and cultured into a kind of sensitive cell at one time, obviously improves separation rate of AMPV, and reduces reproduction times.
Owner:YEBIO BIOENG OF QINGDAO
LAMP (Loop-medicated isothermal amplification) detection kit for metapneumovirus
ActiveCN102943130AQuick checkLow costMicrobiological testing/measurementMicroorganism based processesMetapneumovirusReverse transcription polymerase chain reaction
The invention discloses an LAMP (loop-medicated isothermal amplification) detection kit for metapneumovirus. The kit internally comprises a primer set formed by six primers designed aiming at eight regions of a metapneumovirus F gene conserved region, wherein the nucleotide sequence of the primer set is as shown in a sequence table from sequence 1 to sequence 6. The detection kit disclosed by the invention can be used for specifically detecting the metapneumovirus, wherein the minimum detection limit of the detection kit is 100fg / muL, and the sensitivity of the detection kit is 100 times as high as that of the normal RT-PCR (reverse transcription-polymerase chain reaction). The LAMP detection kit is quick to detect, low in cost, free from expensive apparatuses, simpler in operation method, and suitable for the quick detection in the clinic.
Owner:GUANGXI VETERINARY RES INST
Polypeptides and their use in treating metapneumovirus (MPV) infection
Polypeptides and compositions thereof are provided for treating or limiting metapneumovirus (MPV) infection, as well as methods for designing such polypeptides. In further aspects, methods of using said isolated polypeptides, VLPs or pharmaceutical compositions are provided, which include methods for treating a metapneumovirus (MPV) infection, methods for limiting development of an MPV infection, methods for generating an immune response in a subject, methods for monitoring an MPV-induced disease in a subject and / or monitoring response of the subject to immunization by an MPV vaccine, methods for detecting MPV binding antibodies, methods for producing MPV antibodies, and methods of preventing an MPV infection.
Owner:THE SCRIPPS RES INST
Nucleic acid for rapidly performing typing detection on avian metapneumovirus subgroup with RT-PCR and application of nucleic acid
ActiveCN107904326AThe test result is accurateReliable test resultsMicrobiological testing/measurementDNA/RNA fragmentationMetapneumovirusSubgroup C
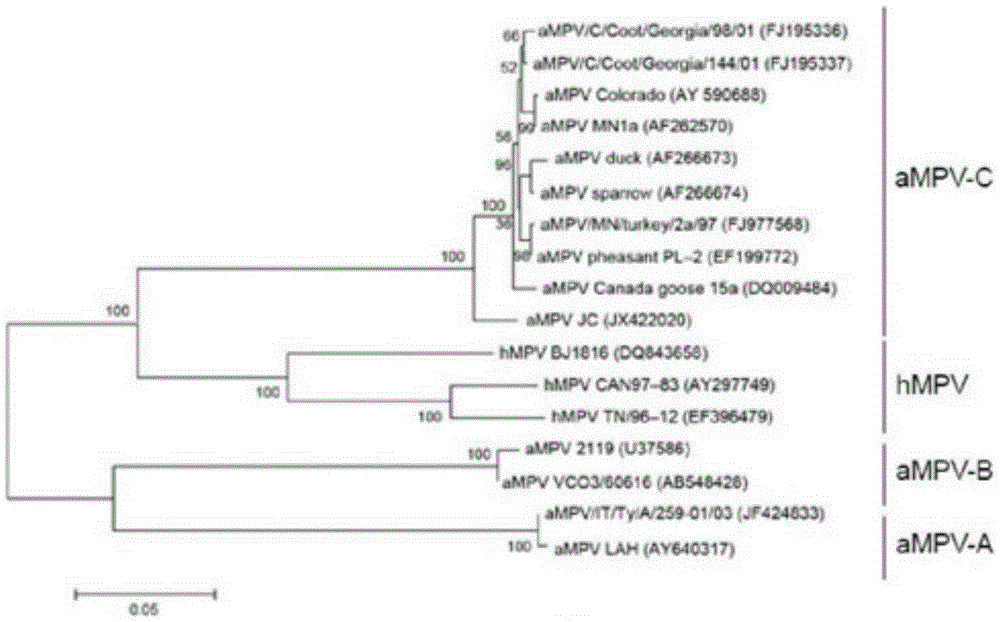
The invention belongs to the technical field of biology and particularly discloses a nucleic acid for rapidly performing typing detection on avian metapneumovirus subgroup with RT-PCR. The nucleic acid is characterized by comprising a primer pair 1 used for detecting avian metapneumovirus A, a primer pair 2 used for detecting avian metapneumovirus B, a primer pair 3 used for detecting avian metapneumovirus C and a primer pair 4 used for detecting avian metapneumovirus D, wherein sequences of the primer pair 1 are shown as SEQ ID No. 1 and SEQ ID No. 2, sequences of the primer pair 2 are shownas SEQ ID No. 3 and SEQ ID No. 4, sequences of the primer pair 3 are shown as SEQ ID No. 5 and SEQ ID No. 6, and sequences of the primer pair 4 are shown as SEQ ID No. 7 and SEQ ID No. 8. The nucleicacid can be used for respectively detecting an avian metapneumovirus subgroup A, an avian metapneumovirus subgroup B, an avian metapneumovirus subgroup C and an avian metapneumovirus subgroup D, the used primers are strong in specificity, no cross reaction exists among the primer pairs, and the primer pairs does not have cross reaction with other viruses.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Chicken C type avian metapneumovirus strain aMPV/C-JCZ and application thereof
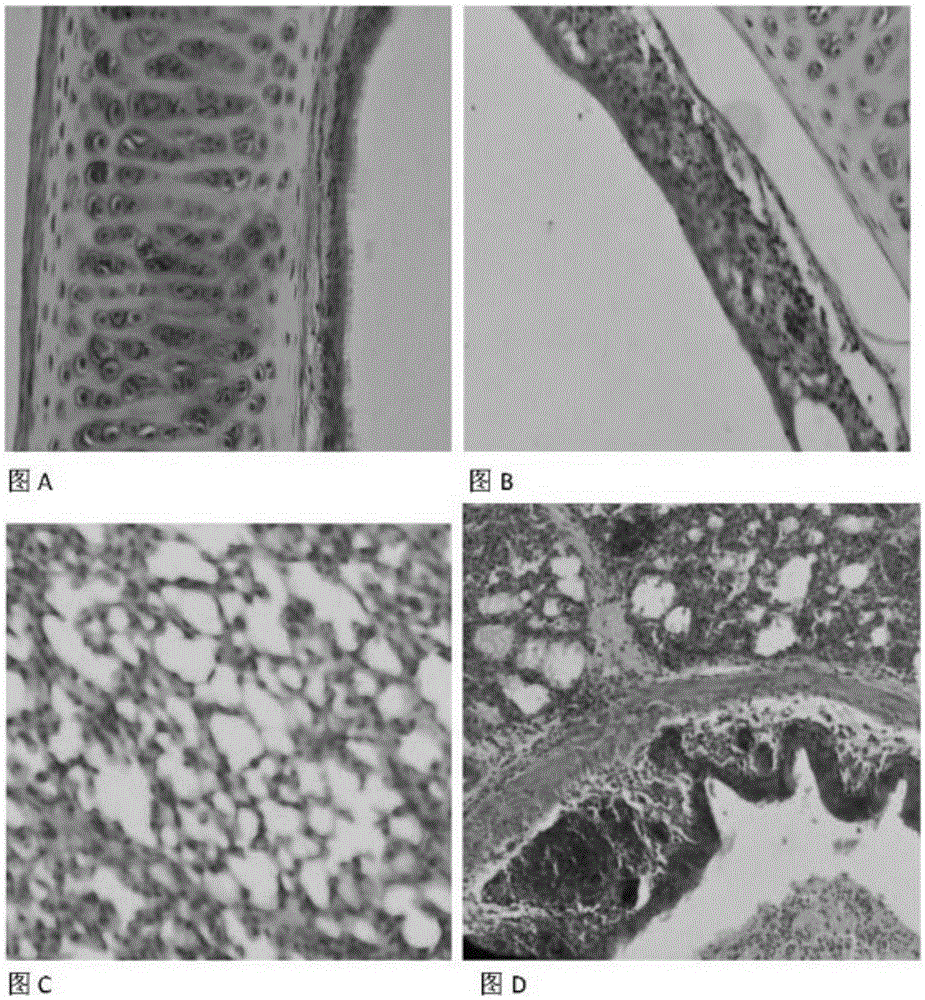
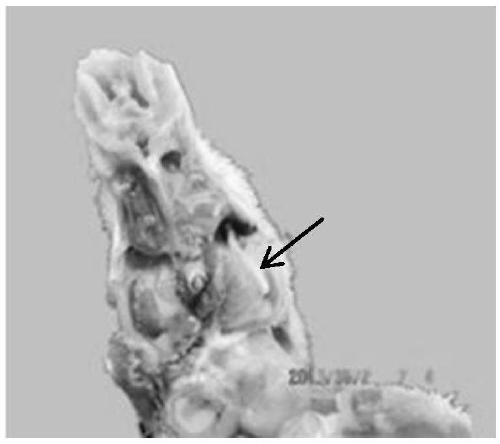
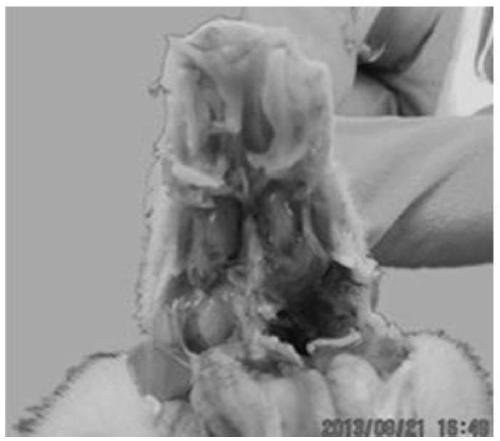
The invention provides a chicken C type avian metapneumovirus strain aMPV / C-JCZ and application thereof. The preservation number of the virus strain is CGMCC No.10889. The virus is a chicken-source avian metapneumovirus C subtype wild virus strain. When used for conducting chicken regression experiments on SPF chickens, the wild-isolated virus causes serious respiratory tract infection clinical symptoms and leads to trachea and lung pathological injuries, and M gene sequencing verifies that the virus is C subtype avian metapneumovirus. The titer of the virus is determined on SPF chicken embryos after the virus is diluted, and the experiment result shows that EID 50 is 10<-4.6> / 0.2 ml. It is the first time to isolate the chicken C type avian metapneumovirus at home, and the chicken C type avian metapneumovirus has large difference from A subtype or B subtype avian metapneumovirus in genotype and antigen. Direct supports are provided for further studying the pathogenic mechanism of aMPV and developing study on vaccine for effectively preventing and controlling the virus.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Attenuated strain for Muscovy duck aMPV (avian metapneumovirus)-C as well as preparation and application of attenuated strain
ActiveCN109402065AStable strainNo instabilitySsRNA viruses negative-senseViral antigen ingredientsAnimal virusMetapneumovirus
The invention belongs to the field of microbial animal viruses, and particularly relates to an attenuated strain for Muscovy duck aMPV (avian metapneumovirus)-C as well as preparation and applicationof the attenuated strain. The preservation number of the attenuated strain for Muscovy duck aMPV-C is CCTCC NO:V201823, the nucleotide sequence is shown as SEQ IDNO:1, and the amino acid sequences areshown as SEQ ID NO:2-SEQ ID NO:9. The attenuated strain for Muscovy duck aMPV-C is prepared from an aMPV-C S-01 strain by subculturing to the 50th generation. The attenuated strain for the Muscovy duck aMPV-C S-01 strain is obtained for the first time, and the foundation is laid for development of attenuated vaccines.
Owner:WENS FOOD GRP CO LTD
Fluorescent quantitative detection kit for typing identification of avian metapneumovirus
InactiveCN108130383ARealize same-plate PCR amplificationMicrobiological testing/measurementDNA/RNA fragmentationSpecific testFluorescence
The invention provides a fluorescent quantitative detection kit for typing identification of avian metapneumovirus. Firstly, through design and screening of specific fluorescent primers, optimizationof an FQ-PCR reaction system and conditions, and verification of specific tests, sensitivity tests and sensibility tests, a rapid, sensitive and specific identification detection method is establishedand is applied to clinical detection. Then, an ACTB FQ-PCR reaction system is established with a chicken ACTB gene as an internal reference; with compatible optimization with aMPV TaqMan-MGB PCR conditions, a relative quantitative FQ-PCR method of aMPV nucleic acid is established. Compared with detection methods of avian metapneumovirus in the prior art, the detection kit has higher degree of precision, can realize typing identification of different serotypes at the same time, and has a good application prospect.
Owner:TIANJIN RINGPU BIO TECH
Primer for detecting metapneumovirus, metapneumovirus kit and preparation method thereof
InactiveCN112725529AThe pre-processing process is simpleShort timeMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionMetapneumovirus
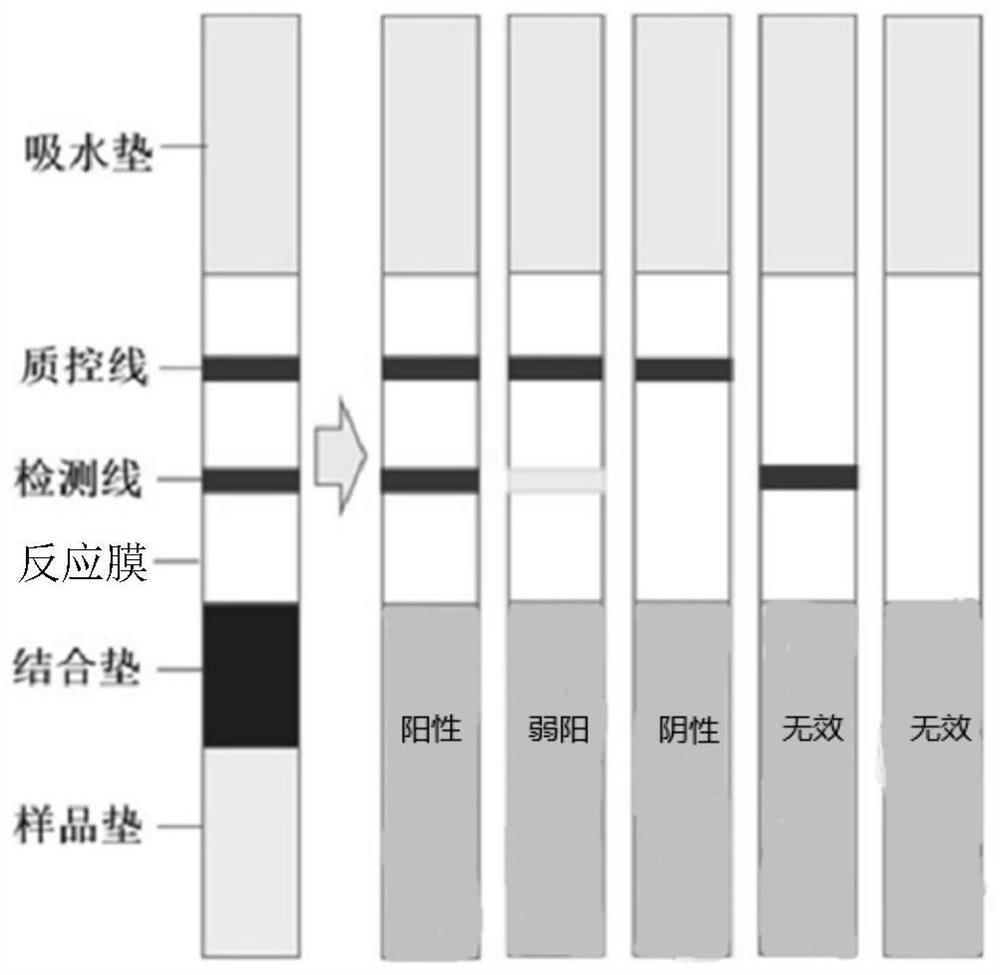
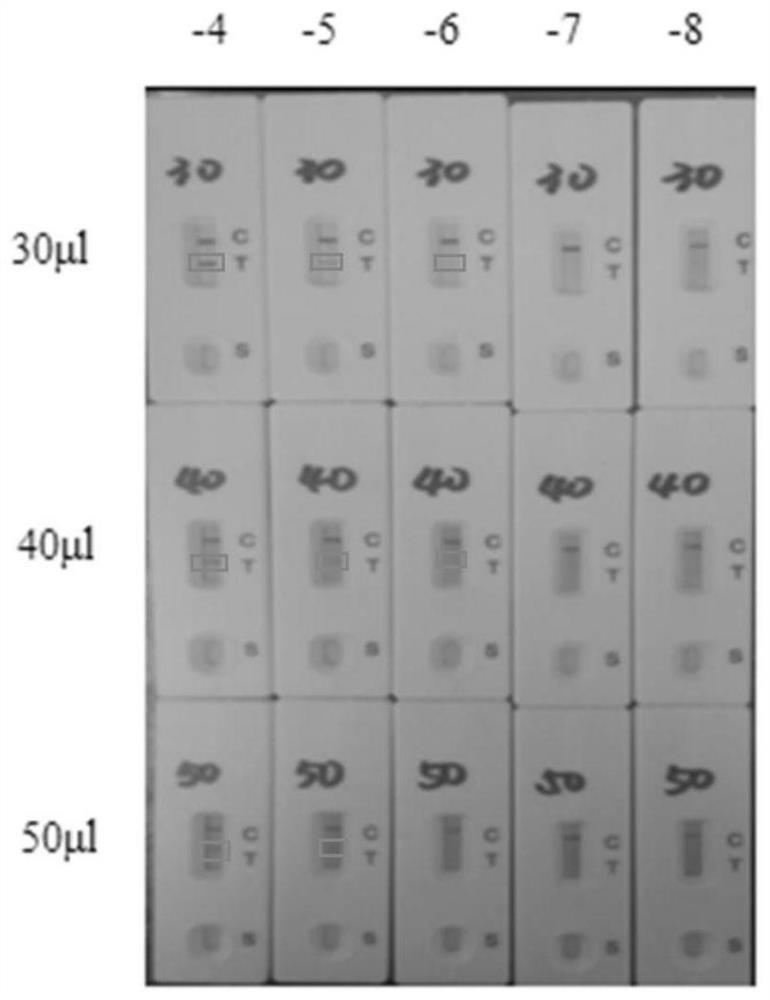
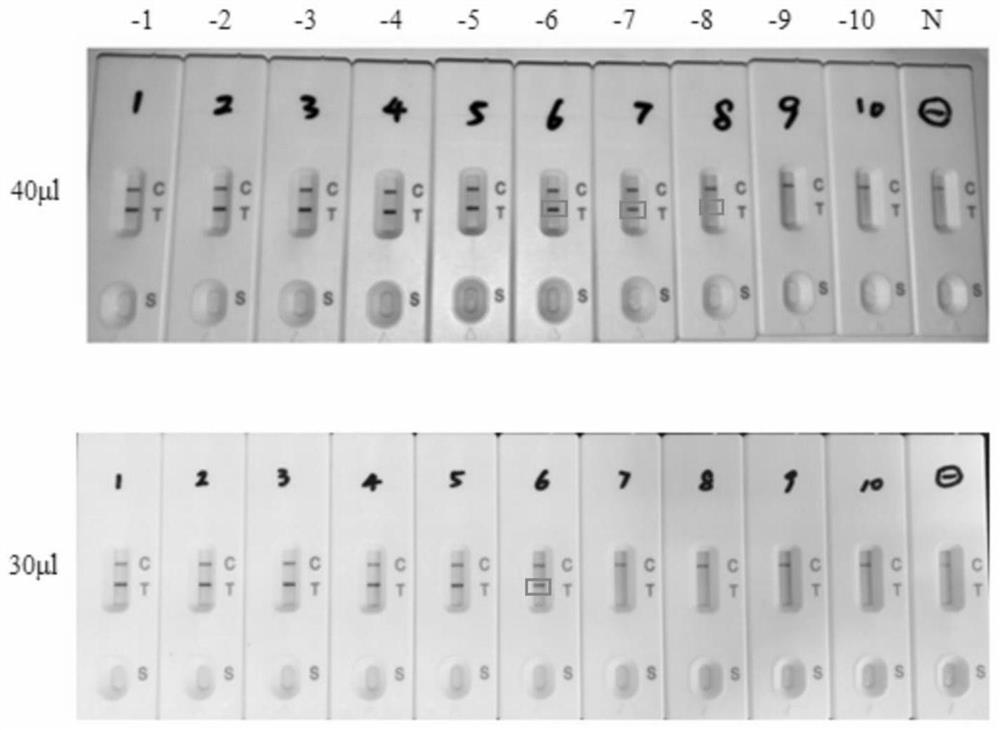
The invention provides a primer for detecting metapneumovirus. Nucleic acid detection of the metapneumovirus is carried out by specifically amplifying an N gene of C-type metapneumovirus. The invention further provides an metapneumovirus detection kit, visualization of a detection result is achieved through RTPCR amplification, nanogold tracing and double signal amplification, the kit is used for metapneumovirus detection, the detection lower limit can be greatly reduced, and meanwhile the specificity, sensitivity, accuracy and repeatability of virus detection are effectively improved. When the kit is used for detecting metapneumovirus, the pretreatment process of a sample is simple, consumed time is short, and a large number of samples can be detected simultaneously.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Antibodies Against Mammalian Metapneumovirus
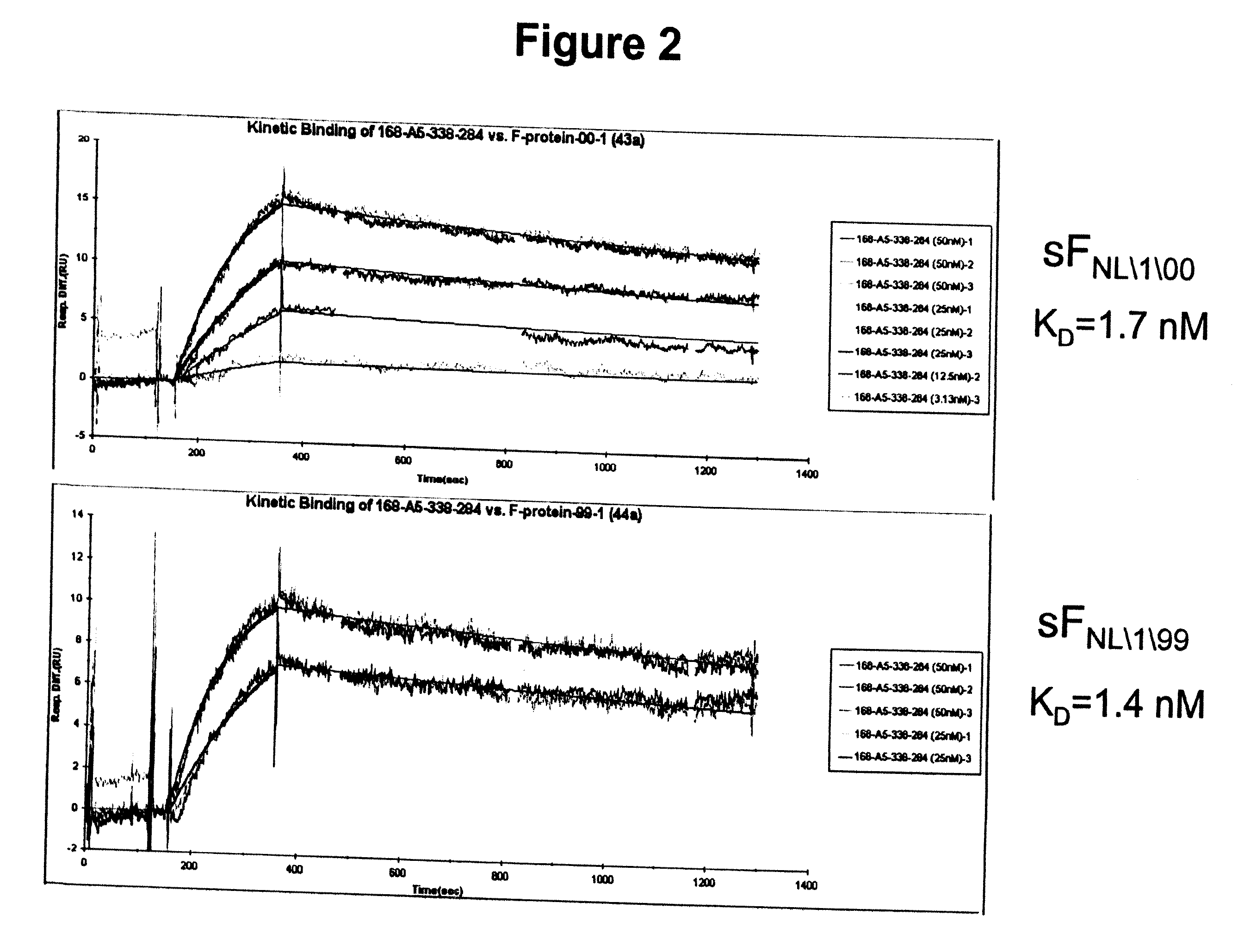
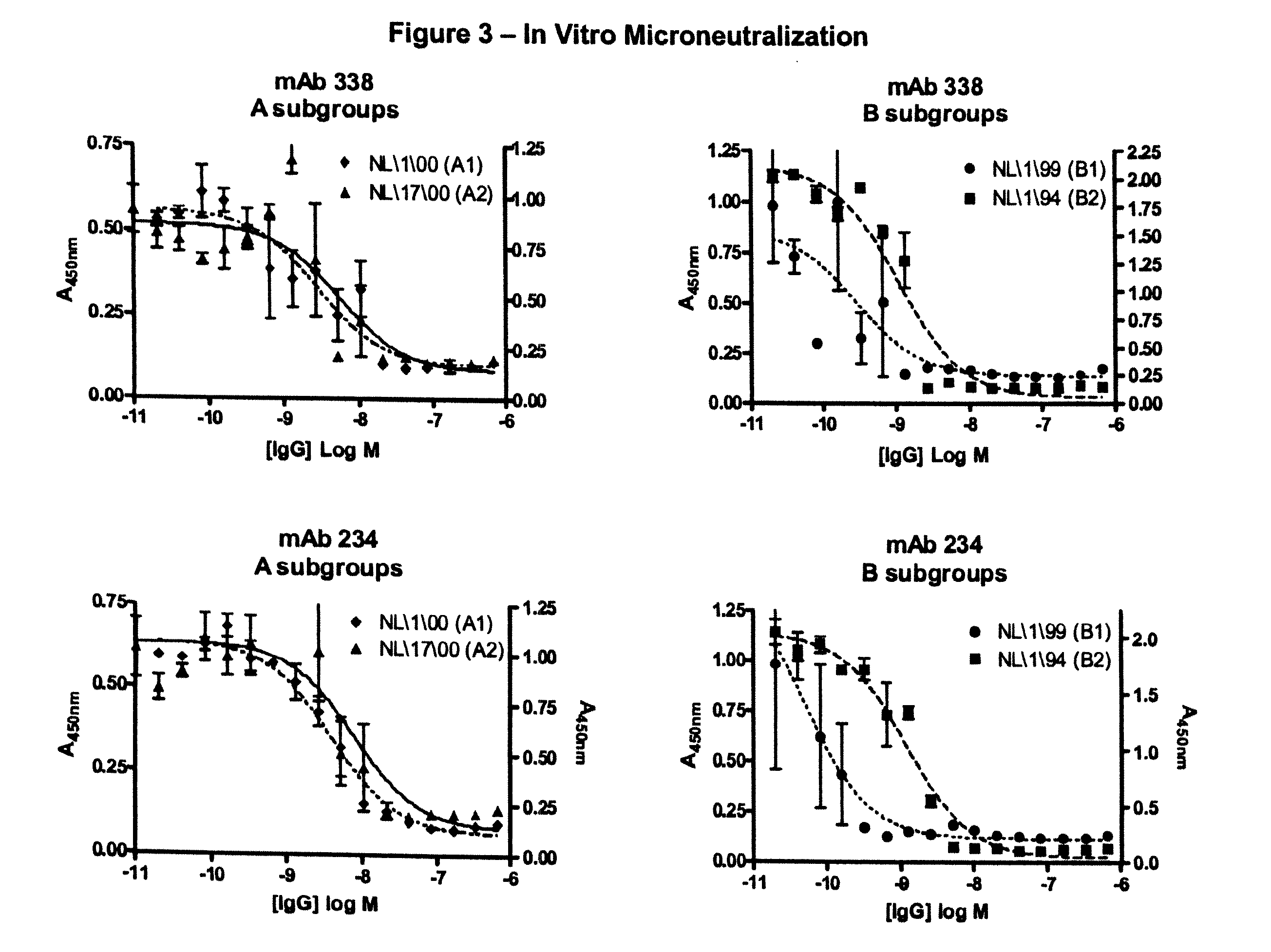
InactiveUS20100239585A1Reduce severityMany symptomImmunoglobulins against virusesAntiviralsF proteinMammal
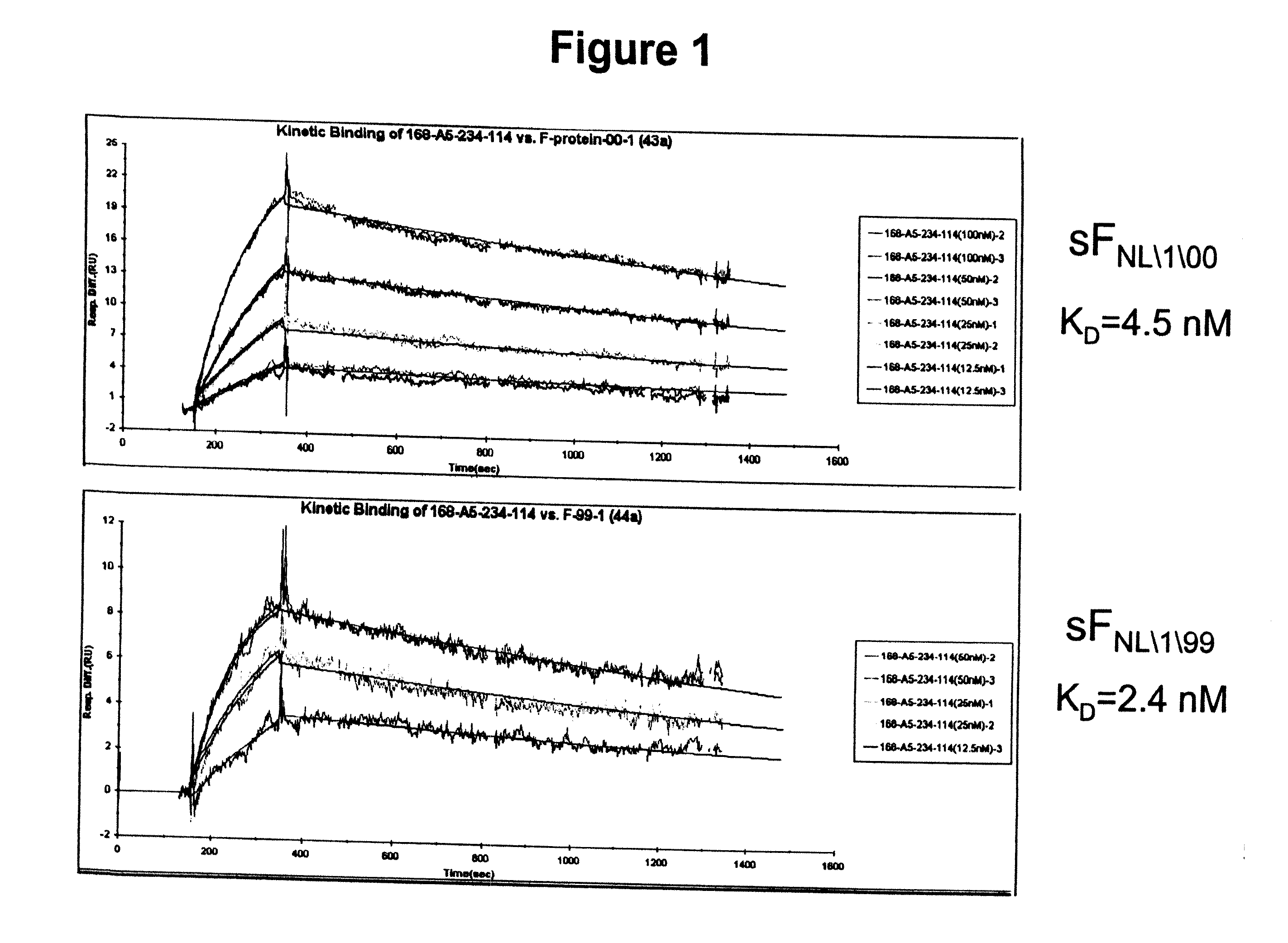
The present invention provides antibodies that immunospecifically bind to a polypeptide of a mammalian metapneumovirus, compositions comprising said antibodies, and methods for producing such antibodies. In particular, the invention provides monoclonal antibodies that immunospecifically bind to the F protein of human metapneumovirus and that neutralize human metapneumovirus. The invention also provides antibodies that cross-react with both the F protein of a mammalian metapneumovirus and the F protein of a mammalian respiratory syncytial virus and that neutralize both viruses. Further, the invention provides recombinant antibodies, such as humanized antibodies, against mammalian metapneumovirus, and methods for producing such recombinant antibodies. The invention further provides methods for treating, managing, ameliorating symptoms of and / or preventing infections with mammalian metapneumovirus, such as human metapneumovirus. The invention also provides antibodies that immunospecifically bind the F protein of avian pneumovirus. Antibodies that immunospecifically bind the F protein of avian pneumovirus are useful in the diagnosis and treatment of infections with avian pneumovirus.
Owner:MEDIMMUNE LLC
Serum-free full-suspension culture method of avian metapneumovirus and application of serum-free full-suspension culture method in vaccines
InactiveCN111961652AImprove adaptabilityGood viral contentSsRNA viruses negative-senseViral antigen ingredientsVirus CultivationNutrition
The invention relates to a serum-free full-suspension culture method of avian metapneumovirus and an application of the serum-free full-suspension culture method in vaccines. The method comprises thefollowing steps of performing continuous passage of an avian metapneumovirus strain in a suspension BHK-21 cell line to obtain virus adaptive cells, and taking an adaptive strain with the highest virus content as a basic strain; resuscitating suspension BHK-21 cells, and culturing the resuscitated suspension BHK-21 cells in a triangular shake flask by using a serum-free culture solution; adding the serum-free culture solution into a bioreactor, and inoculating seed cells into the bioreactor; continuously culturing the basic strain of the suspension BHK-21 cells; performing glucose monitoring every day after virus inoculation, and when the content of glucose in the culture solution is 1-5 mmol / L, supplementing a nutrition bag; in 72 hours after virus inoculation, performing sampling and calculating the cell viability, and harvesting a virus culture solution when the cell viability is lower than 60%; and repeatedly freezing and thawing the virus solution, performing centrifugation and performing storage. The suspension BHK-21 cells are adopted for culture, the virus adaptability is good, and the virus content is high; the operation cost is low and the product quality is stable; and the method is easy to operate and has industrial large-scale production feasibility.
Owner:WENS FOOD GRP CO LTD
Avian metapneumovirus antibody double-antigen sandwich method ELISA detection kit and application thereof
PendingCN114778810AHigh sensitivityIncreased sensitivityColor/spectral properties measurementsBiological testingAntigenAssay
The invention belongs to the technical field of avian metapneumovirus detection, and particularly relates to an avian metapneumovirus antibody double-antigen sandwich method ELISA (enzyme-linked immuno sorbent assay) detection kit and application thereof. The kit provided by the invention can accurately and sensitively detect the avian metapneumovirus neutralizing antibody in poultry whole blood or serum so as to qualitatively judge a sample. The kit has the advantages of being high in sensitivity, good in sensitivity and capable of detecting various poultry and subtypes at the same time, sample treatment is simple and rapid, time consumption is short, and cost is low. The invention provides a good solution for on-site detection of large-batch avian metapneumovirus samples, and provides an important means for prevention and control of avian metapneumovirus.
Owner:SOUTH CHINA AGRI UNIV
A kind of attenuated strain of muscovy duck C subtype avian metapneumovirus and its preparation and application
ActiveCN109402065BStable strainNo instabilitySsRNA viruses negative-senseViral antigen ingredientsAnimal virusNucleotide
The invention belongs to the field of microbial animal viruses, and in particular relates to an attenuated strain of muscovy duck subtype C avian metapneumovirus and its preparation and application. The attenuated Muscovy duck subtype C avian metapneumovirus strain of the present invention has a preservation number of CCTCC NO: V201823, a nucleotide sequence of SEQ ID NO: 1, and an amino acid sequence of SEQ ID NO: 2 to SEQ ID NO: 9. The attenuated muscovy duck subtype C avian metapneumovirus strain of the present invention is prepared by subculturing the C subtype avian metapneumovirus S-01 strain to 50 generations. The invention obtains the attenuated culture strain of Muscovy duck subtype C avian metapneumovirus S-01 strain for the first time, laying a foundation for the development of the attenuated vaccine.
Owner:WENS FOODSTUFF GRP CO LTD
Type B acian metapneumovirus subunit vaccine
ActiveCN105770884AGuaranteed immune effectImprove securitySsRNA viruses negative-senseViral antigen ingredientsPichia pastorisOil adjuvant
The invention provides a type B acian metapneumovirus subunit vaccine.The type B acian metapneumovirus subunit vaccine is prepared from an antigen and a vaccine adjuvant, wherein inactivated type B acian metapneumovirus F protein serves as the antigen, and the amino acid sequence of the type B acian metapneumovirus F protein is SEQ ID NO:1.According to the type B acian metapneumovirus subunit vaccine, genetic engineering pichia pastoris X33-F for expressing the type B acian metapneumovirus F protein is inoculated into a culture medium, the type B acian metapneumovirus F protein is efficiently expressed under the action of methyl alcohol, the type B acian metapneumovirus F protein is extracted and purified and then added into a formaldehyde solution to be inactivated, then an oil adjuvant is added for mixing emulsification, and the vaccine is prepared.The prepared vaccine can increase the level of an immunized antibody and improve the uniformity of the immunized antibody, and the immune effect of the vaccine can be ensured; the vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com