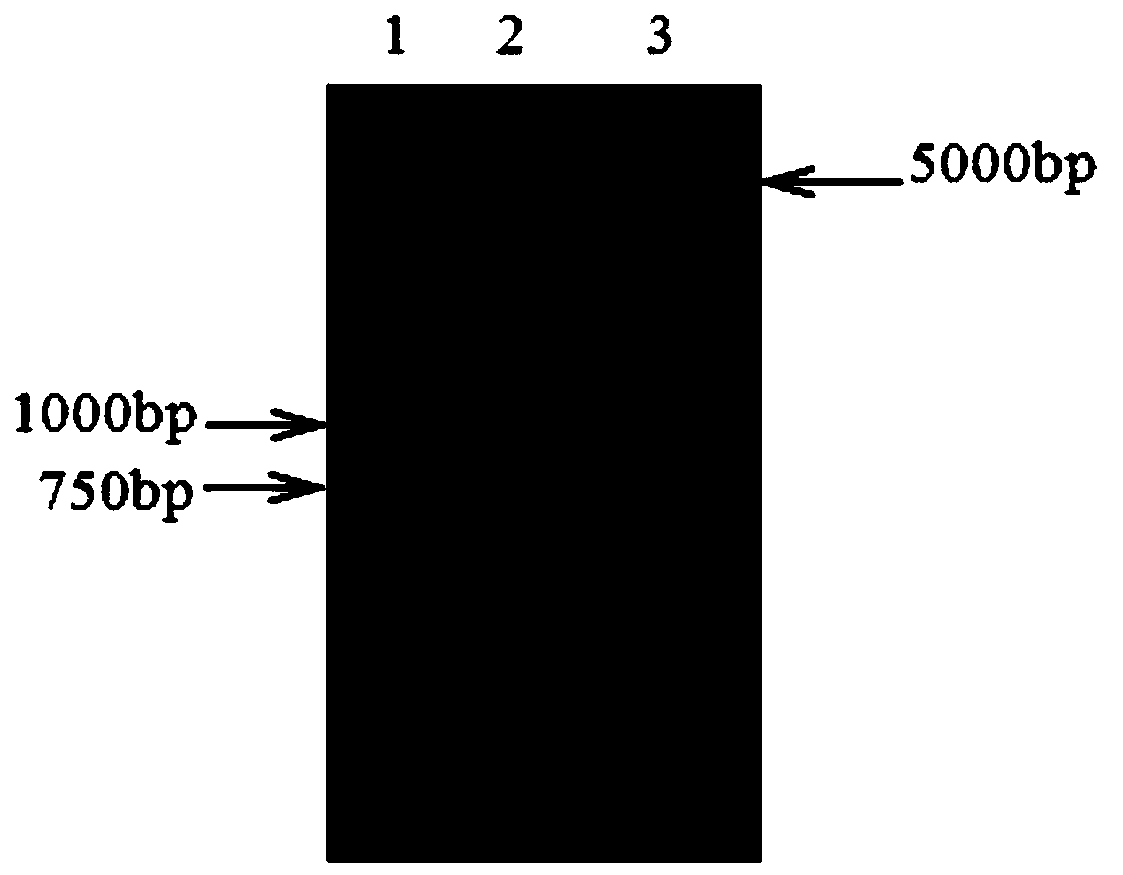
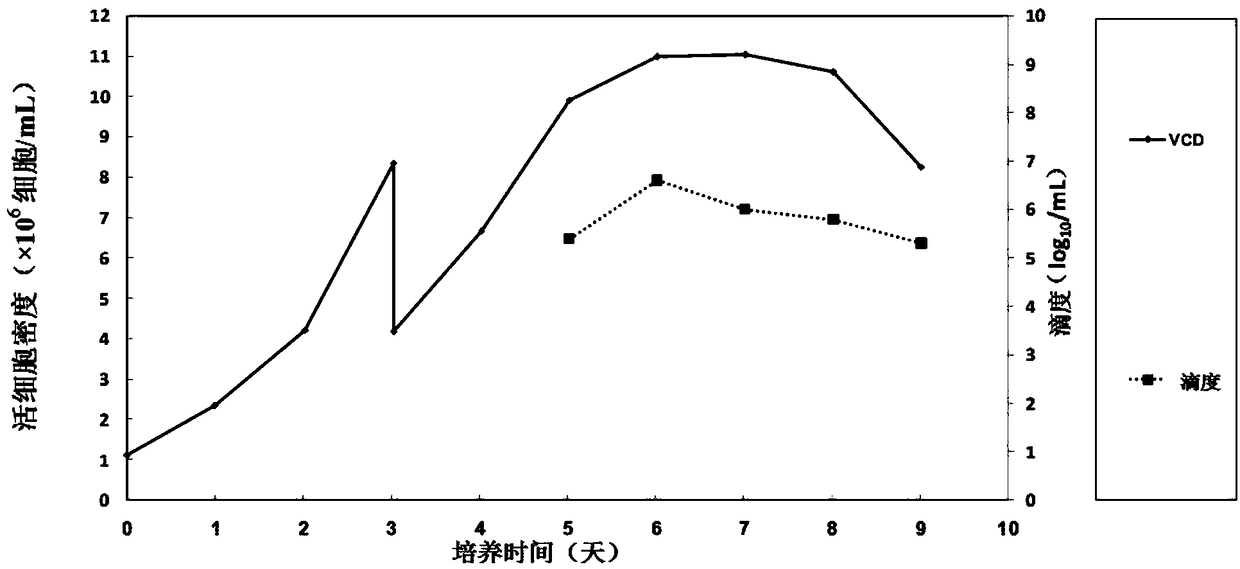
Patents
Literature
181 results about "Virus inoculation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inoculation is mainly done for production of vaccine of influenza virus, yellow fever, rabies. Most of avian viruses can be isolated using this method. Amniotic sac: Inoculation is mainly done for primary isolation of influenza virus and the mumps virus.
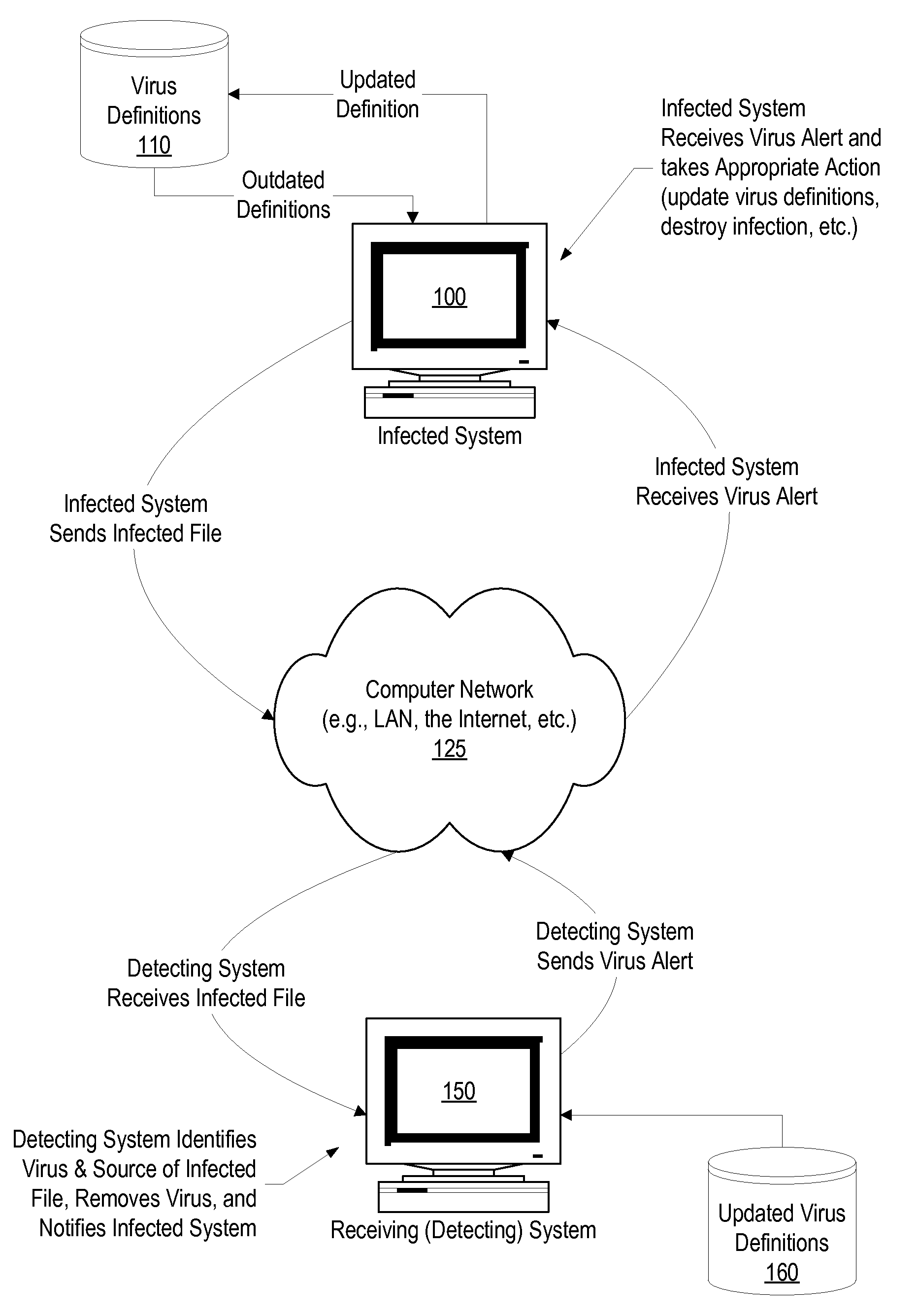
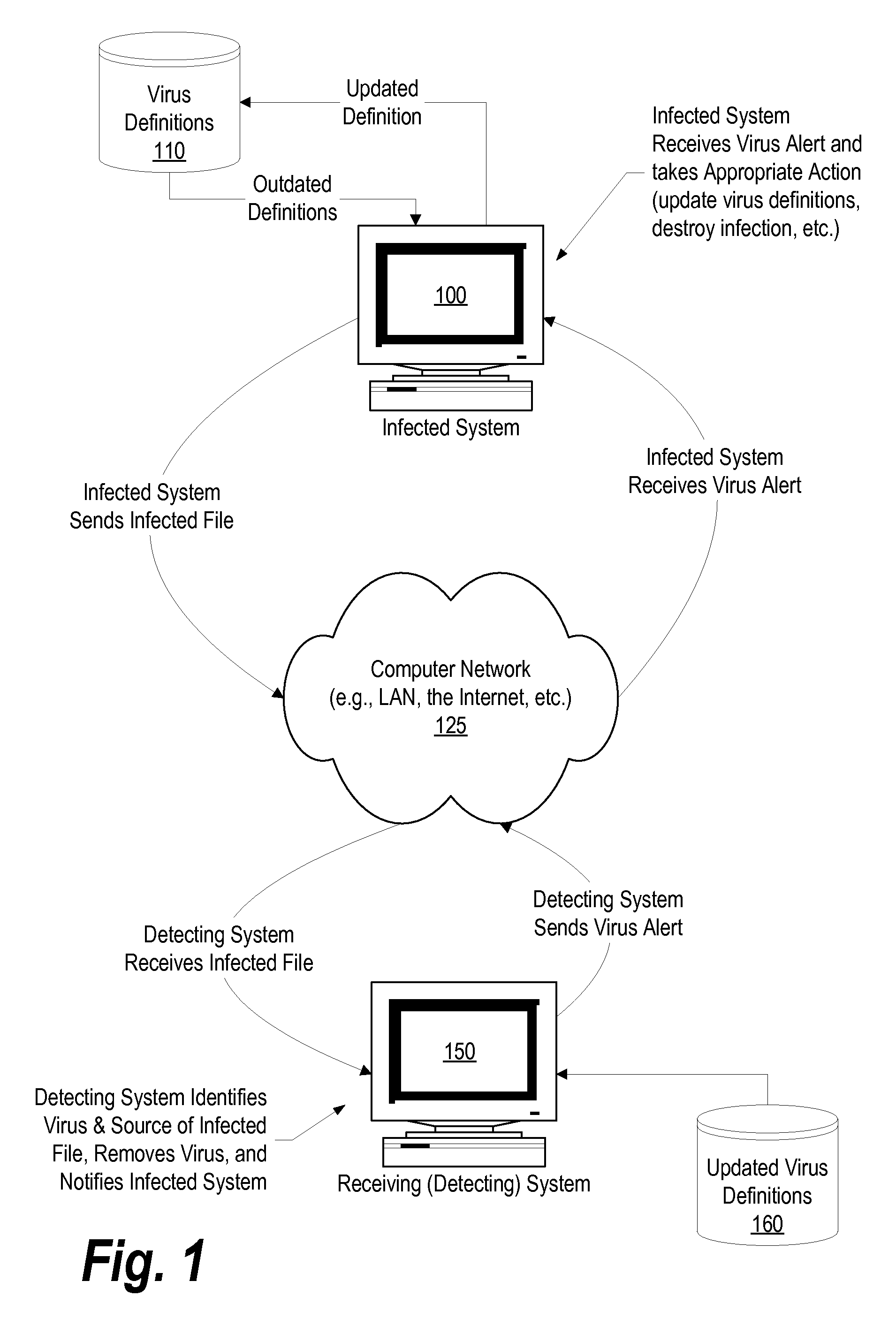
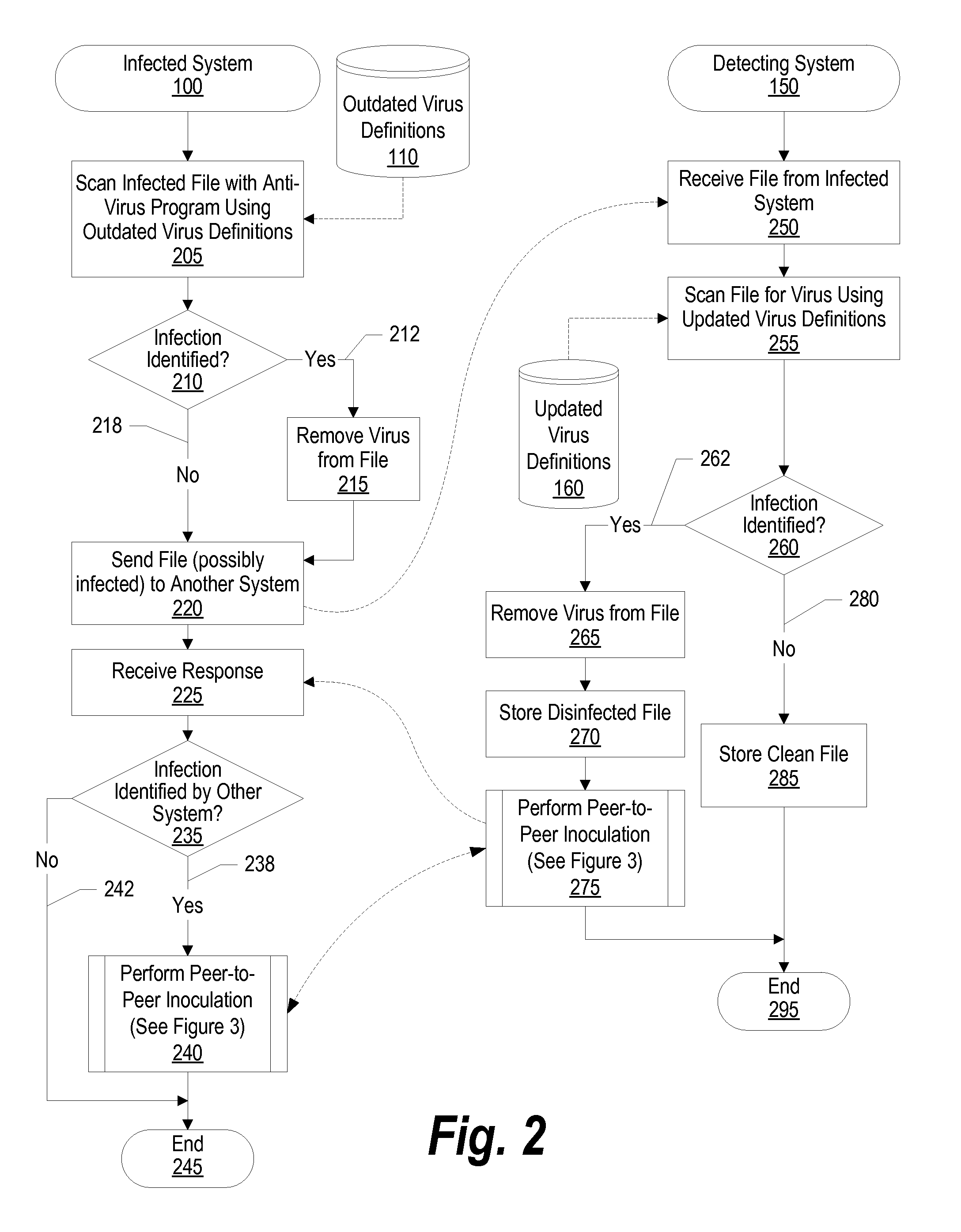
System and Method for Autonomic Peer-to-Peer Virus Inoculation
ActiveUS20080127347A1Memory loss protectionDigital data processing detailsComputerized systemComputer science
A system, method, and program product is provided that communicates virus information between a computer that detects a virus in a file (the detecting computer system) and the computer that sent the infected file (the infected computer system). When the infected computer system sends an infected file to the detecting computer system the detecting computer system detects the virus in the infected file, retrieves virus information corresponding to the virus (such as the name of the infected file, the identifier, or name, of the virus, the virus definitions used to identify the virus, and any instructions needed to eradicate the virus), and automatically sends the virus information back to the infected computer system over the network.
Owner:LENOVO PC INT
Method for producing pseudorabies attenuated vaccine by using bioreactor and pseudorabies attenuated vaccine product
ActiveCN101695572AImprove immune efficiencyIncrease growth densityMicroorganism based processesAntiviralsVaccine ProductionAntibiotic Y
The invention provides a method for producing a pseudorabies attenuated vaccine by using a bioreactor and a pseudorabies attenuated vaccine product. After being sterilized, the bioreactor and a micro carrier are inoculated with cells for producing the vaccine, and a cell growth medium is added for culture. A maintenance medium containing attenuated strains of pseudorabies viruses are inoculated into the bioreactor to continue culturing the cells. 2 to 3 days after virus inoculation, cell culture virus liquid is obtained and added with a stabilizer and antibiotics, and the cell culture virus liquid is refrigerated and dried under vacuum to obtain the pseudorabies attenuated vaccine. In the method, the cell density and virus concentration are improved greatly, the titer of the vaccine is improved, the side reactions, labor intensity and product cost are reduced, the monitoring performance of vaccine production is improved and uniform and stable product quality is guaranteed. The pseudorabies attenuated vaccine produced by the method has high safety, immune efficacy and good immune and protective effect against the attack by the virulent pseudorabies viruses.
Owner:广东永顺生物制药股份有限公司
Optimum condition for proliferating Guangxi epidemically representative strains of infectious bursal disease virus (IBDV) in chicken embryo
InactiveCN101792739AOptimizationMicroorganism based processesViruses/bacteriophagesEmbryoInfectious bursitis
The invention discloses an optimum condition for proliferating Guangxi epidemically representative strains of infectious bursal disease virus (IBDV) in chicken embryos. The optimum condition is an orthogonal design combination of seed virus inoculation route, inoculated embryo day age, inoculation density, embryo gathering time and gathering part when the virus candidate strain i.e. Guangxi epidemically representative strains 040124, BH11 and TSC-2(9), which are designed by biostatistics software, are proliferated in chicken embryos, and an optimum production parameter is explored to supply useful data to the commercial production of vaccines. The invention has the advantages of exploring the optimum condition and supplying useful data for the commercial production of the vaccines.
Owner:GUANGXI UNIV
Porcine reproductive and respiratory syndrome virus diluent and preparation method thereof
InactiveCN105505887APromote growthGuaranteed normal growthSsRNA viruses positive-senseMicroorganism based processesCymbopogon distansPhosphate
The invention provides a porcine and respiratory syndrome virus diluent and a preparation method thereof and belongs to the technical field of veterinary biological products. Every 1000 ml phosphate buffer of the diluent is prepared from, by weight, 10-30 g of amino acid combination, 10-30 g of compound traditional Chinese medicine polysaccharides and 10-30 g of liposome, wherein the amino acid combination comprises folic acid, riboflavin sodium phosphate, dexpanthenol and ascorbic acid, and the compound traditional Chinese medicine polysaccharides are extracted from pubescent angelica roots, Cymbopogon distans (Nees)A. Camus, chastetree fruit, butterflybush flowers, radix scrophulariae and red peony roots. The diluent has the advantages that after virus inoculation, cell growth can be promoted, and normal growth of cells can be maintained; viruses can infect the cells more easily after processing, and the probability that the viruses infect the cells is greatly increased; virus titer can be greatly increased in the same culture time, and virus content is increased by 10-100 times.
Owner:浙江美保龙生物技术有限公司
Cell culture method for duck flavivirus
InactiveCN102220290AStable proliferationObvious cytopathicViruses/bacteriophagesDiseaseCytopathic effect
The invention provides a cell culture method for duck flavivirus. The method comprises the following steps: inoculating to attach an anchorage-dependent kidney cell BHK21 of a baby hamster to the surface of a cell culture container to form a monolayer; inoculating the monolayer with virus; culturing the monolayer inoculated with the virus in an incubator with 5% CO2 at 37 DEG C; and continuously passing by taking a cell culture product as an inoculum for the next passage. According to the cell culture method provided by the invention, after the virus is continuously passed for 5-7 generations on the monolayer, stable multiplication can be obtained; and after the cell is inoculated for 3-4 days, an obvious cytopathic effect can be formed. The method provides a convenient and visual virus operating platform for studying the biological characteristics of the virus and the molecular basis of virus pathopoiesia and for the vaccine research developed for effectively preventing and controlling the disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Influenza virus subunit vaccine and preparation method thereof
InactiveCN104888212AHigh purityQuality improvementAntiviralsAntibody medical ingredientsHemagglutininAdjuvant
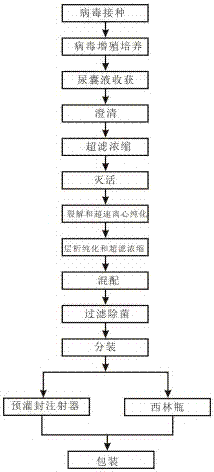
The invention discloses an influenza virus subunit vaccine and a preparation method thereof. The influenza virus subunit vaccine is formed by further purifying cracked viral proteins through a cracking agent and a new purification method; each agent of subunit vaccine comprises more than 80% of H1N1, H3N2 and B type influenza hemagglutinin, and does not comprise adjuvant, thiomersal or other corrosion removers. The invention further provides the preparation method of the influenza virus subunit vaccine. The preparation method of the influenza virus subunit vaccine comprises the following steps of virus inoculation, virus multiplication culture, allantonic fluid harvesting, clarification, inactivation, ultrafiltration and concentration, cracking and overspeed centrifugal purification, gel filtration chromatography purification, mixture, filtration sterilization, subpackage, packaging and the like. The influenza virus subunit vaccine can improve safety, eliminate untoward effects caused by adjuvant and eliminate the toxic and side effects caused by thiomersalate.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Applications of BHK-21 cell full-suspension culture technology in production of newcastle disease vaccine
The invention relates to applications of a BHK-21 cell full-suspension culture technology in production of a newcastle disease vaccine. A process of producing the newcastle disease vaccine by BHK-21 cell full-suspension culturing includes steps as follows: (1) a step of viral strain seed selection, namely a step of inoculating a monolayer BHK-21 cell with a newcastle disease vaccine virus seed cultured by a chick embryo, adding a virus maintenance medium, culturing to obtain a newcastle disease vaccine adapted to the BHK-21 cell, and performing system identification; (2) a step of domestication and seed selection of a suspension cell strain, namely a step of domesticating a full-suspension BHK21 cell used for culturing of the newcastle disease vaccine virus and establishing a basic seed; (3) a step of subjecting the suspension cell to enlarged cultivation; (4) a step of virus inoculation and harvest, namely a step of inoculating the newcastle disease vaccine virus adapted to the BHK-21 cell and harvesting a virus solution; and (5) a step of measuring the viral titer of the multiplicated newcastle disease vaccine virus and preparing the vaccine. According to the applications, culturing and production with chick embryos of the newcastle disease vaccine are changed into to large-scale culture and production with mammalian cells of the newcastle disease vaccine, the process of producing the newcastle disease vaccine is simplified, the production cost is reduced, and the yield and quality of the vaccine are largely improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Preparation method of inactivated vaccine for iridovirus of grouper
ActiveCN102178945AImproving immunogenicityHigh activityViral antigen ingredientsAntiviralsFreeze and thawEmbryo
The invention relates to a preparation method of an inactivated vaccine for the iridovirus of a grouper, comprising the following steps of: inoculating the iridovirus to embryonic cells of the grouper in a logarithmic phase by taking an embryonic fine system of the grouper as an amplification system of the iridovirus; repeatedly freezing and thawing and centrifugalizing after complete lesion, and inactivating an obtained iridovirus solution at 4 DEG C for 16 hours by using beta-propiolactone with the final concentration of 1:500 so as to obtain the inactivated iridovirus vaccine. The inactivated iridovirus vaccine is applied to a juvenile immunized Malabar grouper, and an iridovirus counteracting result shows that relative protection ratio is more than 90 percent after 15 days. The preparation method has the advantages of easiness and convenience for operation, simple equipment requirement and good repeatability and keeps good immunogenicity of the iridovirus under the precondition of efficiently inactivating the iridovirus, thereby having good immune protection effect; in addition, the invention can be used for the preventive immune of the grouper, thereby enhancing the survival rate and the culturing efficiency of cultured groupers.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method for producing concentrated inactivate vaccine for newcastle disease
ActiveCN101474401AGood concentration and stabilityHigh potencyViral antigen ingredientsAntiviralsAnti virusAntigen
The invention provides a method for producing improved concentrated inactivated vaccine against Newcastle disease. The method comprises the steps of virus inoculation passage, ultrafiltration and concentration, mixing water phase and oil phase and the like, wherein, a 30KD membrane is used for ultra-filtrating and concentrating, and the concentrated water phase and oil phase are mixed and emulsified in the proportion of 1 to 1 in a high pressure emulsification pump. The invention uses ultrafilter membrane concentration and antigen ingredient instead of the content of live virus, so that the chicken can produce strong immunity after being immunized by the vaccine for 10 to 14 days; the immunity period is 10 months; the anti-virus protective rate is 100 percent within the immunity period; and the invention has wide application prospect.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
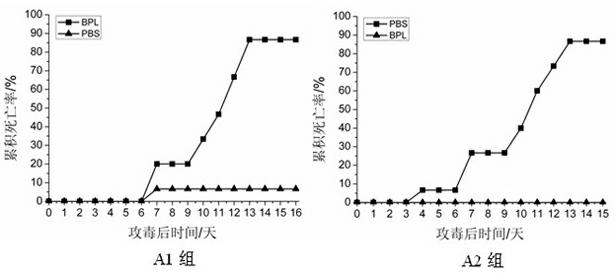
Rabbit hemorrhagic disease virus baculovirus vector and pasteurella multocida bivalent inactivated vaccine and preparation method thereof
ActiveCN108904796AFermentation culture process is matureImprove securityAntibacterial agentsSsRNA viruses positive-senseAdjuvantP. multocida
The invention relates to a rabbit hemorrhagic disease virus baculovirus vector and pasteurella multocida bivalent inactivated vaccine and a preparation method thereof, and belongs to the field of immune technology. Recombinant rabbit hemorrhagic disease virus VP60 baculovirus is inoculated into Sf9 insect cells and cultured at 27-28 DEG C. When cell lesion reaches 85% or more, a cell culture is harvested and inactivated, and the inactivated cell culture is used as a rabbit hemorrhagic disease virus antigen. Rabbit Pasteurella multocida capsular serotype A C51-17 strain is amplified and cultured, a bacterial solution is inactivated, and the inactivated bacterial solution is used as a Pasteurella multocida antigen. The rabbit hemorrhagic disease virus baculovirus vector and pasteurella multocida bivalent inactivated vaccine can be prepared by mixing the rabbit hemorrhagic disease virus antigen and the Pasteurella multocida antigen with adjuvants in proportion. The rabbit hemorrhagic disease virus baculovirus vector and pasteurella multocida bivalent inactivated vaccine has high safety, good immune effect and simple process, and can be used for preventing and controlling Rabbit Hemorrhagic Disease (Rabbit Plague) and Rabbit Pasteurella multocida.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Single clone antibody aiming at H3N2 dog flu virus HA2 protein
ActiveCN104293739AJudgment of toxicityStrong specificityImmunoglobulins against virusesAntiviralsAntigenNeutralizing antibody
The invention provides a single clone antibody aiming at H3N2 dog flu virus HA2 protein, belonging to the technical field of biologics. The single clone antibody is prepared by inoculating an H3N2 dog flu virus Jiangsu separated strain JS / 10 separated in 2010 with SPF chicken embryos of 10 days old, collecting allantoic fluid of which the blood clotting titer is greater than or equal to 6, purifying virus inoculation MDCK cells, performing intracutaneous multipoint injection to inoculate mice, after cell fusion, performing three times of ELISA to detect cell supernate that cell colonies are generated, selecting an anti-H3 type hybridization tumor cell strain mAbD7, and recognizing and identifying the antigen so as to verify that the antibody aims at conservative H2A protein, wherein the neutralizing antibody titer is up to 1:12800, and the antibody subclass is IgG2b. The single clone antibody aiming at H3N2 dog flu virus and HA2 protein, which is provided by the invention, provides an important immunological preparation for treating H3N2 dog flu virus infection and has a potential application prospect.
Owner:NANJING AGRICULTURAL UNIVERSITY
Tetravalent influenza virus subunit vaccine and preparation method thereof
ActiveCN111420044AReduce contentExtended shelf lifeSsRNA viruses negative-senseViral antigen ingredientsVirus multiplicationUltrafiltration
The invention belongs to the technical field of biology, and particularly relates to a tetravalent influenza virus subunit vaccine and a preparation method thereof. Each dose of the tetravalent influenza virus subunit vaccine contains H1N1, H3N2 and two types of B; and the tetravalent influenza virus subunit vaccine is prepared by virus inoculation, virus multiplication culture, allantoic fluid harvesting, clarification, inactivation, ultrafiltration concentration, cracking and ultracentrifugal purification, mixing, filtration sterilization, split charging and packaging, wherein the inactivation process comprises the following steps: firstly, adding a carboxymethyl glucan solution into monovalent virus harvesting liquid, and then adding formaldehyde for inactivation. According to the method disclosed by the invention, the content of free formaldehyde in the prepared tetravalent influenza virus subunit vaccine is reduced, the storage life of the vaccine is prolonged, and the antibody level after vaccine immunization is increased.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Porcine reproductive and respiratory syndrome virus Nsp2 protein nano-antibody and application thereof
ActiveCN111057145AImprove bindingSmall molecular weightImmunoglobulins against virusesAntiviralsCamelus bactrianusPig reproduction
The invention discloses a porcine reproductive and respiratory syndrome virus Nsp2 protein nano-antibody and an application thereof. The nano-antibody is named Nb12, and the amino acid sequence of thenano-antibody is represented by SEQ ID NO.3. A camel is immunized with a truncated expression Nsp2 recombinant protein, camel peripheral blood lymphocytes are separated, and an immunized bactrian camel VHH gene is amplified by using RT-PCR, the amplified gene is connected into a pCANTAB 5E phage vector to construct a bactrian camel heavy chain antibody variable region library, 44 Nsp2 specific nano-antibodies are screened out through three rounds of panning by utilizing a phage display technology, tests prove that the nano-antibodies can specifically react with a Nsp2 protein, and the Nb12 has strong binding force. Antiviral test results show that when the virus inoculation amount is 0.01 MOI, the nano-antibody Nb12 shows a virus replication inhibition effect on both PAM cells and Marc-145 cells. Therefore, a new technical means is provided for subsequent research of the PRRSV Nsp2 and prevention, control and diagnosis of the PRRSV.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
MDBK acclimation suspension method and two-stage virus production process
PendingCN108570454AHigh densityTo achieve growthSsRNA viruses positive-senseViral antigen ingredientsCytopathic effectSerum free
The invention relates to an MDBK cell acclimation suspension method and a two-stage virus production process. According to the MDBK cell acclimation suspension method, adherent MDBK cells are acclimated to adapt to serum-free suspension culture and used as host cells for producing viruses which can have cytopathic effect or sensitivity on suspension MDBK cells by two-stage culture virus inoculation; when the cells grow to 2.0-20.0*10<6> cells / mL, diluting with a production culture medium by 1-5 times is performed to reach a density range of 1.0-10.0*10<6> cells / mL, and then virus inoculation is carried out or virus inoculation is performed before dilution. The suspension MDBK cells are adaptive to suspension growth in the serum-free chemical-defined culture medium, and the two-stage virusproduction process is simple and easy in amplification; liquid changing is avoided, and high culture medium utilization efficiency is achieved; production and growth culture media can be identical ornot and can be mixed proportionally to realize cell secondary growth and viral expression promotion.
Owner:上海健士拜生物科技有限公司 +2
Method for preparing duck hemorrhagic ovaritis inactivated vaccines
ActiveCN103157102AHigh densityIncrease productionViral antigen ingredientsMicroorganism based processesTiterOrganism
The invention relates to a method for preparing duck hemorrhagic ovaritis inactivated vaccines, and belongs to the field of biotechnology. The method for preparing the duck hemorrhagic ovaritis inactivated vaccines includes the following steps: (1) culturing of cells used for vaccine preparation, (2) virus inoculation and culture, (3) virus liquid harvest, (4) virus liquid inactivation, and (5) vaccine preparation. Cells used for vaccine preparation are screened, matching degree between the cells and the virus is strengthened, a riptide perfusion type bioreactor culture system is used for improving multiplication titer and harvest yield of the virus, and a whole production process does not involve other biosafety and public health problems and is suitable for large scale production.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Full-suspension culture method for avian influenza virus
ActiveCN107904215ANon-tumorigenicEasy to useSsRNA viruses negative-senseRecovery/purificationVirus multiplicationAvian influenza virus
The invention discloses a full-suspension culture method for avian influenza virus. The full-suspension culture method comprises the following steps: step 1, taking EB66 cells for growth culture; step2, when the density of the EB66 cells grows to be suitable for virus inoculation, utilizing a fed-batch fluid-replacement virus inoculation technology to inoculate avian influenza virus into the EB66cells and performing virus multiplication culture; step 3, 24h after virus inoculation, sampling every 12h to determine HV titer of the virus and obtaining and storing the virus when the virus HA titer reaches to the maximum to obtain cultured avian influenza virus. According to the full-suspension culture method disclosed by the invention, the EB66 cells are utilized to perform avian influenza virus culture, so that the defect that a lot of miscellaneous protein is prone to being introduced into when a traditional chick embryo culture technology is utilized for production is overcome, and occurrence of chicken immunity side reaction is effectively reduced; meanwhile, titer and purity of the cultured avian influenza virus are improved; furthermore, production quality of the avian influenza vaccine is improved.
Owner:ZHAOQING INST OF BIOTECHNOLOGY CO LTD +2
Novel process for preparing influenza virus split vaccine
ActiveCN102133399AHigh yieldIncrease the effective antigen contentAntiviralsAntibody medical ingredientsHemagglutininPurification methods
The invention relates to a novel process for preparing influenza virus split vaccine, comprising the following steps of: (1) inoculating and culturing viruses: inoculating working seed lot viruses diluted to virus amount of 2.0-0.6LgEID50 / ml in chick embryo allantois, and culturing at 33-35 DEG C for 48-72h; (2), harvesting and inactivating viruses and concentrating a virus harvest liquid; (3) purifying and splitting viruses: adding TritonX-100 with volume ratio of final concentration of 0.3-0.5% and deoxysodium cholate in a purified virus liquid, mixing evenly and placing at 20-30 DEG C for 90-120min; (4) adding a PB split agent; and (5) sterilizing, filtering and then preparing a semi-finished product: diluting each stock solution to hemagglutinin content of 30micrograms / strain / ml by 0.01mol / L of PBS (Phosphate Buffered Saline) with a pH value of 7.2, and filtering by a filter membrane with aperture of 0.22micron to obtain a semi-finished product. The novel process for preparing influenza virus split vaccine has the beneficial effects that: due to the increase of a novel purification method, high yield can be achieved, effective antigen content can be improved and the contents of residual ovalbumin and split agent causing inoculation reaction can be greatly reduced.
Owner:ZHEJIANG TIANYUAN BIO PHARM CO LTD
Optimized technological method for amplifying recombinant adenovirus by using bioreactor
The invention relates to the biotechnology field and in particular relates to a comprehensively optimized technological method for amplifying recombinant adenovirus. The method is used for amplifying human embryonic kidney cells (HEK293) by using poly-fiber paper carriers through a bioreactor; in this way, the whole set of process flow of adenovirus replication and amplification is established. The optimized technological method provided by the invention comprises the following steps of: under the condition of comprehensively adapting to type 5 adenovirus replication and amplification system, utilizing a DMEM culture medium containing 10% of blood serum at the cell culture stage, utilizing a DMEM culture medium containing 20% of blood serum in 20 hours after virus inoculation absorption; employing a blood serum-free culture medium added with 0.25% of lactoalbumin hydrolysate at the virus collecting stage, and simultaneously, supplementing 2g / L glucose every 20 hours. The way of batch collection and batch liquid exchange is adopted; and the method enables the optimized process to achieve high virus titer on the basis of reducing the later-stage purification difficulty and meeting the requirements of biological products. Therefore, the optimized process established by the invention an efficient recombinant adenovirus amplification process having excellent repeatability, and is suitable for any bioreactor with the poly-fiber paper as the carriers to amplify the recombinant adenovirus.
Owner:ZHEJIANG PUKANG BIOTECH
Extractive and preparation containing same
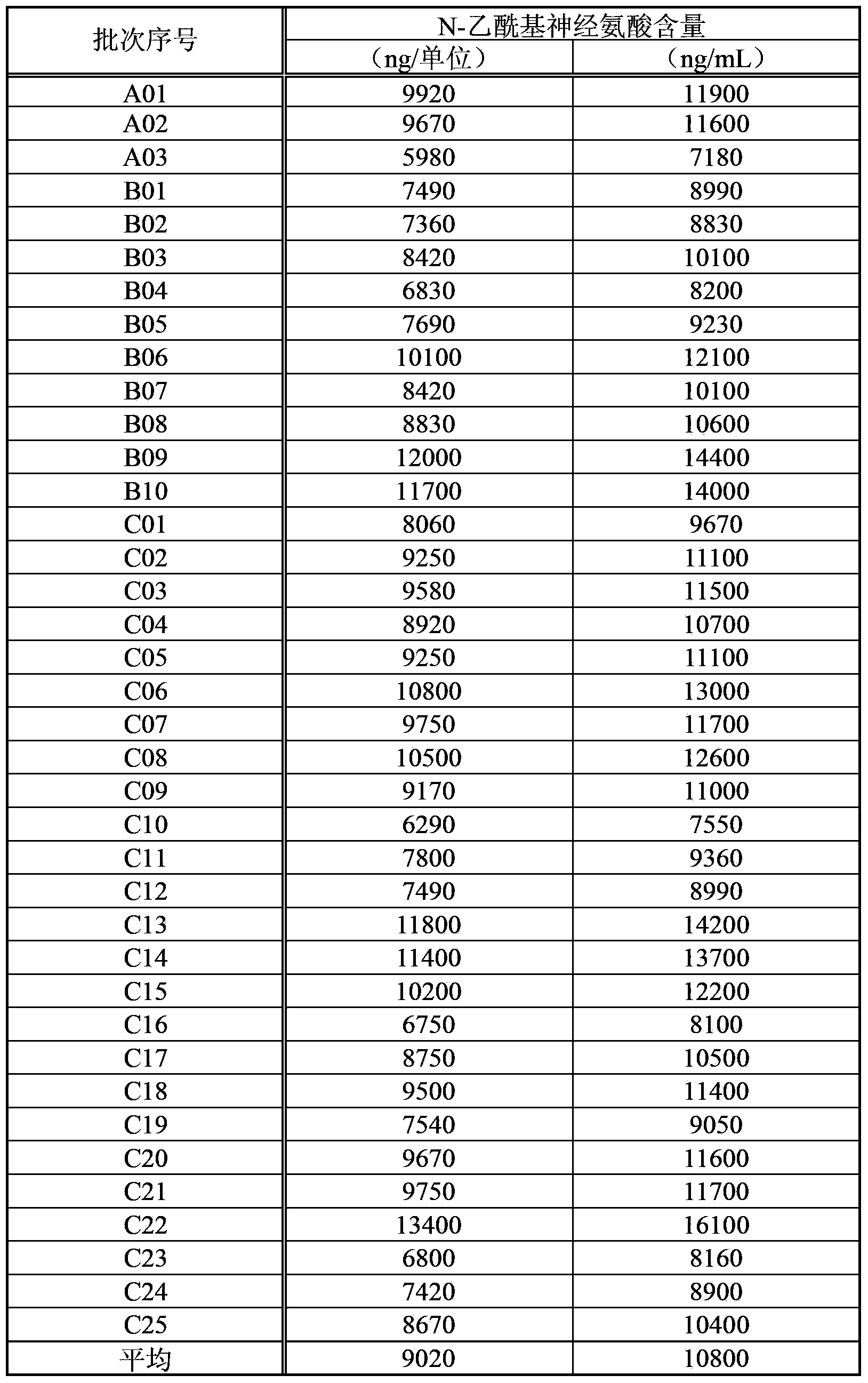
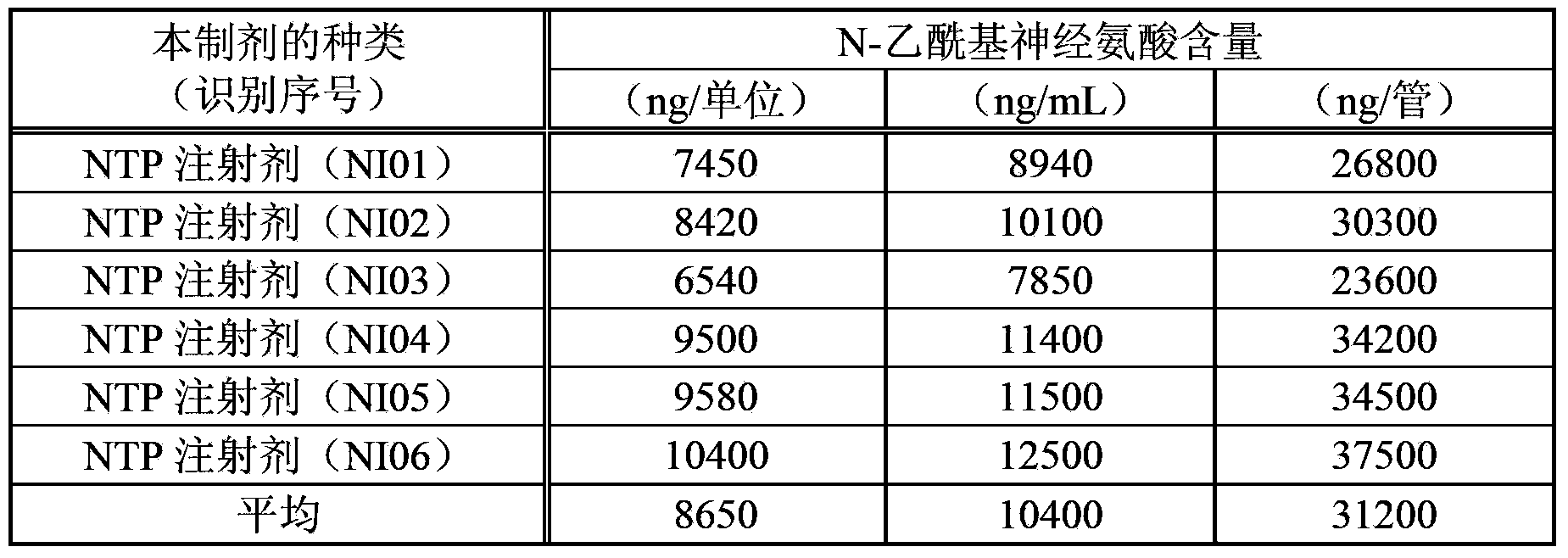
ActiveCN103357006AGuaranteed qualityOrganic active ingredientsSenses disorderMedicineNeuraminic acid
An objective of the invention is to provide a vaccinia virus inoculation rabbit inflammation skin extractive which is more stable in quality, and a preparation which contains the extractive as an effective constituent, etc. Amount of N-acetyl neuraminic acid which is contained in the vaccinia virus inoculation rabbit inflammation skin extractive and the preparation containing the extractive is used as an index, so that quality of each preparing batch of the extractive and the preparation is more stable. Effectiveness and security of the vaccinia virus inoculation rabbit inflammation skin extractive and the preparation containing the extractive which are made in the method and have more stable quality are ensured strictly, and the extractive and the preparation have very good usefulness.
Owner:NIPPON ZOKI PHARM CO LTD
PK15 cell acclimation suspension method and two-stage virus production process
PendingCN108570445ATo achieve growthTo achieve the role of replication and proliferationArtificial cell constructsViruses/bacteriophagesCytopathic effectChemical composition
The invention relates to a PK15 cell acclimation suspension method and a two-stage virus production process. According to the PK15 cell acclimation suspension method, PK15 cells are acclimated to adapt to serum-free suspension culture and used as host cells for producing viruses which can have cytopathic effect or sensitivity on suspension PK15 cells by two-stage culture virus inoculation; when the cells grow to 2.0-20.0*10<6> cells / mL, diluting with a production culture medium by 1-5 times is performed to reach a density range of 1.0-10.0*10<6> cells / mL, and then virus inoculation is carriedout or virus inoculation is performed before dilution. The suspension PK15 cells are adaptive to suspension growth in the serum-free chemical-defined culture medium, and the two-stage virus productionprocess is simple and easy in amplification; liquid changing is avoided, and high culture medium utilization efficiency is achieved; production and growth culture media can be identical or not and can be mixed proportionally to realize cell secondary growth and viral expression promotion.
Owner:上海健士拜生物科技有限公司 +2
Gene silencing method Si-VIGS (Seed imbibition-virus-induced gene silencing) in early stage of cotton
ActiveCN110172473AEasy to operateLong silencePlant peptidesVector-based foreign material introductionNicotiana tabacumSilencing gene
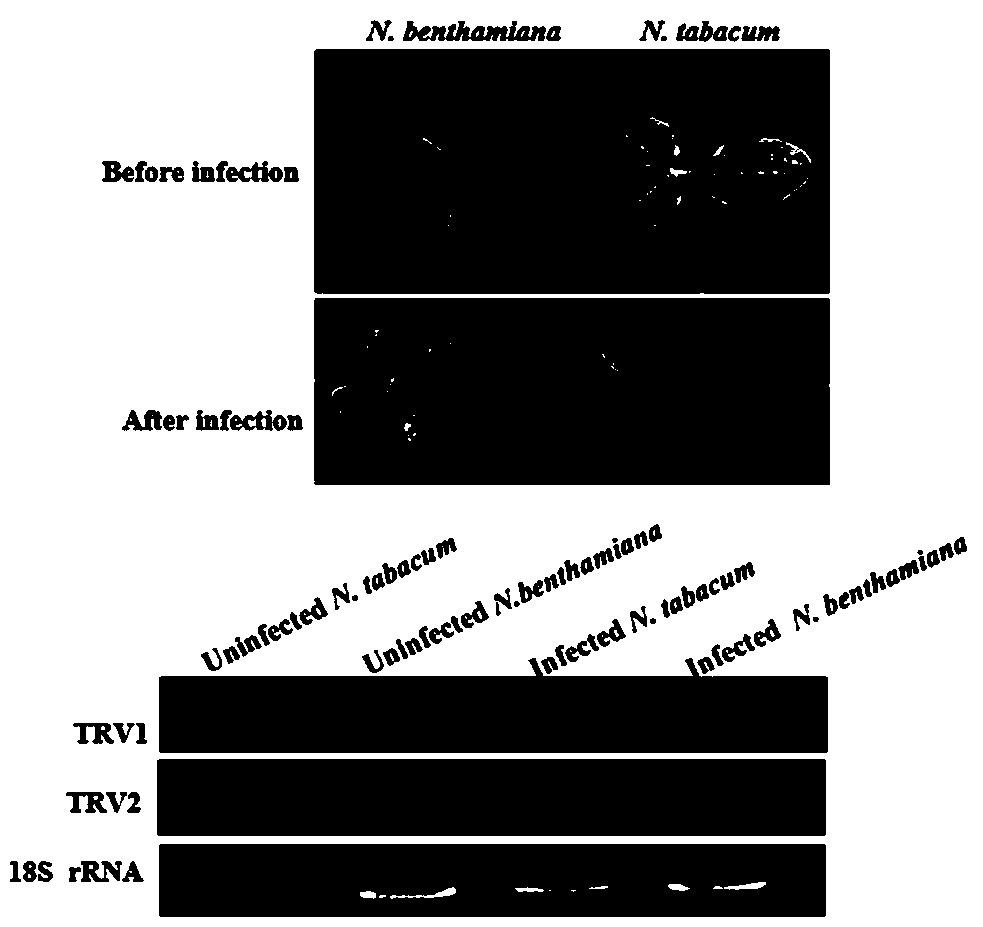
The invention belongs to the technical field of cotton breeding, and specifically relates to a gene silencing method in early germination and seedling stage of cotton. The method comprises the steps of preparing agrobacterium liquid for transfection, performing virus amplification by utilizing tobacco, infecting cotton seeds and the like. According to the invention, a cotton gene silencing technology is optimized and improved by taking a tobacco rattle virus (TRV) as a virus vector. It is proved by a result that a gene silencing phenotype can be obtained through inoculating cotton cotyledon orseeds with TRV homogenate or agrobacteria. Through comparing the similarities and differences of TRV homogenate and agrobacteria inoculation, it is discovered that through applying a seed imbibition(Si) method to inoculation of any infection liquid, the virus inoculation time can all be advanced to the inflorescence primordial formation stage of the seeds; functional genes during germination ofthe seeds can be efficiently silenced within 3 days; virus homogenate-mediated gene silencing can last till the seedling stage of the cotton. Si-VIGS (Seed imbibition-virus-induced gene silencing) involved in the invention can efficiently silence the genes from the early germination stage of the cotton; the operation is simple; the phenotypes are unified; a relatively good application prospect isshown.
Owner:ZHENGZHOU UNIV
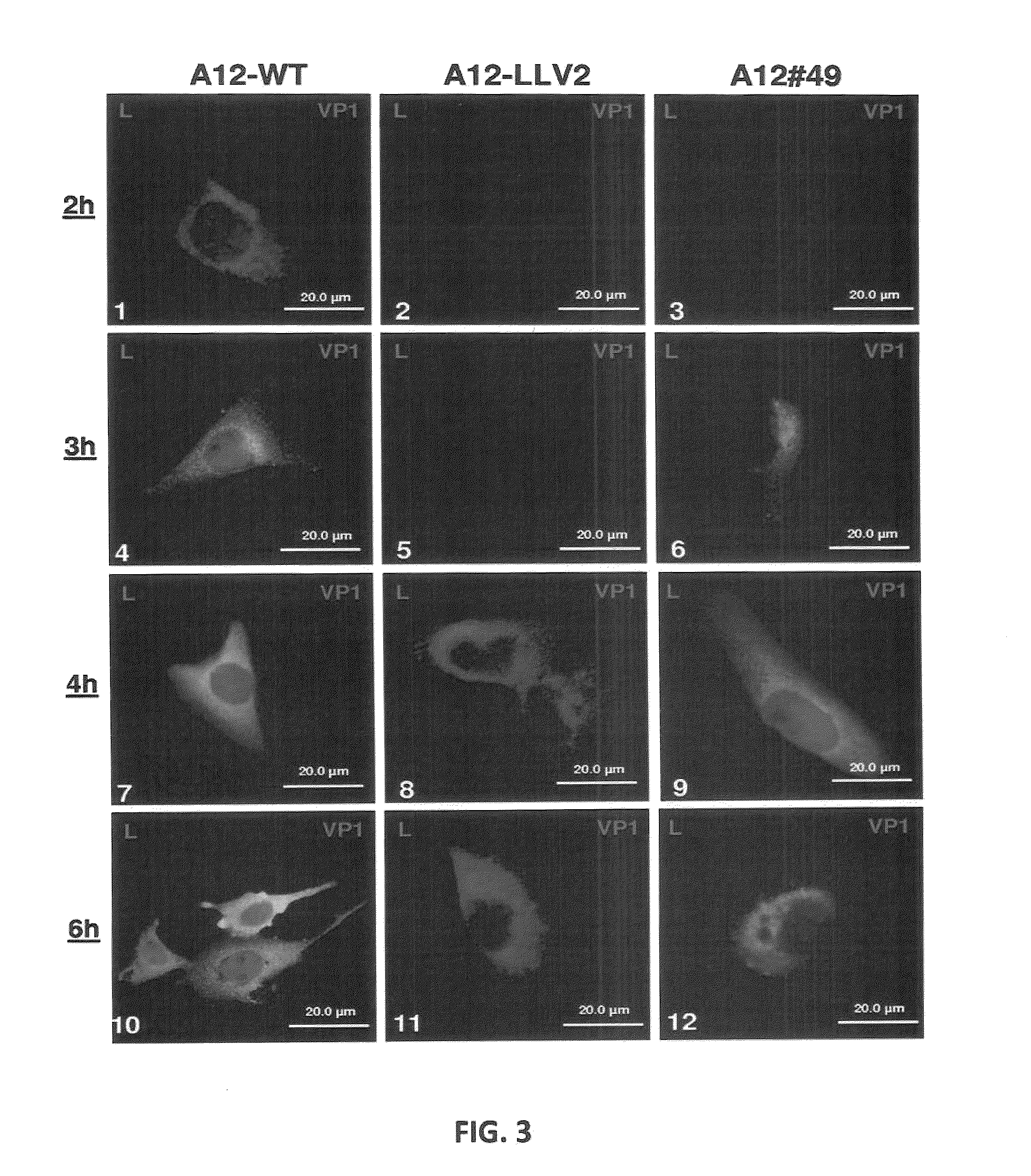
Recombinant live attenuated foot-and-mouth disease (FMD) vaccine containing mutations in the L protein coding region
ActiveUS8846057B2Reduce severityReduce probabilitySsRNA viruses positive-senseViral antigen ingredientsNeutralizing antibodyAttenuated Live Vaccine
Previously we have identified a conserved domain (SAP, for SAF-A / B, Acinus, and PIAS) in the foot-and-mouth disease virus (FMDV) leader (L) protein coding region that is required for proper sub-cellular localization and function. Mutation of isoleucine 55 and leucine 58 to alanine (I55A, L58A) within the SAP domain resulted in a viable virus that displayed a mild attenuated phenotype in cell culture, along with altered sub-cellular distribution of L and failure to induce degradation of the transcription factor nuclear factor kappa-B. Here we report that inoculation of swine and cattle with this mutant virus results in the absence of clinical disease, the induction of a significant FMDV-specific neutralizing antibody response, and protection against subsequent homologous virus challenge. Remarkably, swine vaccinated with SAP mutant virus are protected against wild type virus challenge as early as two days post-vaccination suggesting that a strong innate as well as adaptive immunity is elicited. This variant could serve as the basis for construction of a live-attenuated FMD vaccine candidate.
Owner:UNITED STATES OF AMERICA
Method for proliferating avian influenza virus on MDCK full suspension cells and application thereof
ActiveCN108753737AMutation Risk ReductionReduce accessSsRNA viruses negative-senseCulture processAvian influenza virusEmbryo
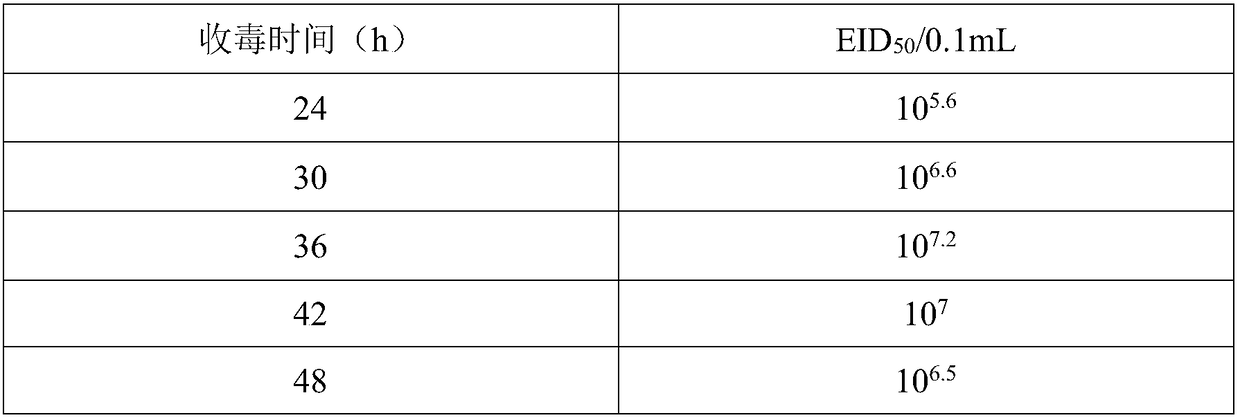
The invention relates to a method for proliferating avian influenza virus on MDCK full suspension cells and application thereof. The method comprises the steps of: inoculating, chicken embryo avian influenza virus according to MOI equal to 0.001 to 0.0001 when MDCK cells are cultured to 7.0*10<6> to 1.0*10<7> cells / mL in a full suspension manner; cultivating at pH value of 6.9 to 7.3, dissolved oxygen of 30 to 60%, and temperature of 32 to 37 DEG C after virus inoculation, and separating and purifying the virions, when the virus reaches a sufficiently high titer. The method, due to that fact that the seed virus does not need circulation, shortens the production cycle and greatly reduces the risk of virus variation. The HA of recombinant avian influenza virus cultivated by the method can beup to 1 to 1024, the virus content per 1 ml is more than or equal to 10<8.37>TCID<50>,the virus content per 0.1 ml is more than or equal to 10<8.17>EID<50>,, and the inoculation amount of the seed virus is reduced by 10 times or more.
Owner:吉林冠界生物技术有限公司
Method for preparing swine fever live vaccines
InactiveCN103157105AIncreased sensitivityUniform stateAntiviralsRecovery/purificationPublic healthTreatment fever
The invention relates to a method for preparing swine fever live vaccines, and belongs to the field of biotechnology. The method for preparing the swine fever live vaccines includes the following steps: (1) cell inoculation, (2) culturing of cells used for vaccine preparation, (3) virus inoculation, (4) virus culture and harvest, and (5) vaccine preparation. Cells used for vaccine preparation are screened, adaptability between the cells and virus is strengthened, a riptide perfusion type bioreactor culture system is used for improving multiplication titer and harvest yield of the virus, and a whole production process does not involve other biosafety and public health problems and is suitable for large scale production.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Method for preparing duck tembusu virus inactivated vaccine and duck tembusu virus inactivated vaccine
ActiveCN108721615AImprove adaptabilityHigh virus contentSsRNA viruses positive-senseViral antigen ingredientsDiseaseSide reaction
The invention discloses a method for preparing a duck tembusu virus inactivated vaccine and the duck tembusu virus inactivated vaccine. A cell line used for virus inoculation is EB66 cell line (a duckembryonic stem cell-derived cell strain), and a bioreactor is adopted for virus culture in a serum-free full suspension manner; and the method comprises the following steps: 1) breeding of virus species; 2) establishment of virus seed batches; 3) preparation of cell venom; 4) inactivation of viruses; and 5) emulsification and other steps to complete the preparation of the vaccine. The method provided by the invention has the characteristics that the prepared cell venom is high in virus content, the production process is stable, intelligent control is achieved, large-scale serum-free suspension culture is realized, the operation is easy, and the cost is low; and the prepared duck tembusu virus inactivated vaccine has the advantages of safety, low side reactions, high immune efficacy, smallbatch-to-batch difference, small number of tests, low cost and the like, and is an ideal vaccine for preventing the occurrence and the prevalence of a duck tembusu virus disease in the waterfowl industry.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Method for suspension culture of infectious bronchitis virus by continuous cell line
ActiveCN108300704AHigh titerImproving immunogenicitySsRNA viruses positive-senseRecovery/purificationInfectious laryngotracheitis virusInfectious bronchitis virus
The invention provides a method for suspension culture of an infectious bronchitis virus by a continuous cell line. The method comprises the following steps that 1, EB66 cells are taken for recovery and secondary culture; 2, the EB66 cells obtained in the step 1 are subjected to infectious bronchitis virus inoculation culture; 3, the EB66 cells after the virus inoculation are sampled every 6 to 12h; virus EID50 is determined; when the virus EID50 reaches the highest value, the virus is harvested and stored; the cultured infectious bronchitis virus is obtained. The EB66 cells are used as a culture medium of the infectious bronchitis virus; the EB66 cells providing a full suspension continuous cell line is used for performing efficient virus production; the culture titer of the infectious bronchitis virus is effectively improved, so that the large-scale culture of the infectious bronchitis virus vaccine is realized; the virus culture cost is reduced.
Owner:ZHAOQING INST OF BIOTECHNOLOGY CO LTD +3
Swine testicular clone cell line and production method of classical swine fever live vaccine
ActiveCN102965332AMicroorganism based processesAntiviralsBovine Viral Diarrhea VirusesInfection rate
The invention provides a highly classical swine fever virus infective swine testicular clone cell strain ST-B2, which has a preservation number of CCTCC NO.C2011101, and a preparation method of the swine testicular clone cell. The method comprises the steps of: 1) subjecting a swine testicular cell to limited dilution, conducting cell cloning and subcell cloning, thus obtaining a subcellular clone strain; and 2) selecting a subclone strain of the swine testicular cell with a highest classical swine fever virus infection rate, i.e. the highly infective swine testicular clone cell of the classical swine fever virus. The invention also provides a method for preparation of a high titer classical swine fever virus solution and a classical swine fever live vaccine from the swine testicular clone cell. The swine testicular clone cell provided in the invention has a high classical swine fever virus infection rate, and the classical swine fever vaccine produced from the swine testicular cell line ST clone cell strain ST-B2 by a virus-carrying and virus transmission technique has high virus titer, hard exposure to BVDV (bovine viral diarrhea virus) pollution and good pureness. By the virus-carrying and virus transmission technique, a resurgent cell can undergo continuous passage to at least 15 generations. The cell resurrection and virus inoculation times can be reduced, the production process is simplified, and the production efficiency is improved.
Owner:PU LIKE BIO ENG
Trivalent influenza virus subunit vaccine and preparation method thereof
InactiveCN107537030AHigh purityQuality improvementAntiviralsAntibody medical ingredientsHemagglutininSide effect
The invention discloses a trivalent influenza virus subunit vaccine and a preparation method thereof, wherein virus protein after lysis is further purified by using a lysis agent and a new purification method to prepare a tetravalent influenza virus subunit vaccine, the content of three influenza hemagglutinins such as influenza A virus H1N1, influenza A virus H3N2 and influenza B virus in each dose of the vaccine is more than 80%, and the trivalent influenza virus subunit vaccine does not contain adjuvant and does not contain thimerosal and other preservatives. The invention further providesa preparation method of the trivalent influenza virus subunit vaccine, wherein the preparation method comprises: virus inoculation, virus proliferation culture, allantoic fluid harvesting, clarification, ultra-filtration concentration, inactivation, lysis and ultracentrifugation purification, gel filtration chromatography purification (ultra-filtration), blending, filtration sterilization, sub-packaging, packaging and other steps. According to the present invention, the trivalent influenza virus subunit vaccine can improve the safety of the influenza vaccine, can eliminate the adverse reactioncaused by the adjuvant, and can eliminate the toxic-side effects caused by thimerosal.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Optimized process method for amplifying enterovirus type 71 by use of bioreactor
ActiveCN103215233AOptimize culture conditionsOptimization parametersMicroorganism based processesViruses/bacteriophagesEnterovirusHydrolysate
The invention relates to the field of biotechnology, and in particular relates to an optimized process method for amplifying the enterovirus type 71. The method adopts a poly-fiber paper scrap as a carrier and amplifies an African green monkey kidney cell by use of a bioreactor so as to establish a whole process flow for copying and amplifying the enterovirus type 71. Under a condition of completely adapting to an enterovirus type 71 copying and amplifying system, the method adopts a DMEM (dulbecco's minimum essential medium) containing 10% of serum in a cell culture stage; after virus inoculation and adsorption for 24 hours, the method adopts a DMEM containing 3-5% of serum; 0.5% of lactoalbumin hydrolysate is added by use of a serum-free medium in a virus harvesting stage, and 2g / L of glucose is supplemented every 24 hours; and on the basis of reducing the later-period purifying difficulty and adapting to the requirements for a biological product, the optimized process realizes a higher virus titer. The method provided by the invention has good repeatability and efficient enterovirus type 71 amplifying process, and can be used for amplifying the enterovirus type 71 by use of a bioreactor by taking a poly-fiber paper scrap as a carrier.
Owner:ZHEJIANG PUKANG BIOTECH
Quadrivalent influenza virus subunit vaccine and preparation method thereof
InactiveCN107537032AHigh purityQuality improvementAntiviralsViruses/bacteriophagesHemagglutininAdjuvant
The invention discloses a quadrivalent influenza virus subunit vaccine and a preparation method thereof, wherein virus protein after lysis is further purified by using a lysis agent and a new purification method to prepare the tetravalent influenza virus subunit vaccine, the content of four influenza hemagglutinins such as influenza A virus H1N1, influenza A virus H3N2 and two kinds of influenza Bviruses in each dose of the vaccine is more than 80%, and the quadrivalent influenza virus subunit vaccine does not contain adjuvant and does not contain thimerosal and other preservatives. The invention further provides a preparation method of the quadrivalent influenza virus subunit vaccine, wherein the preparation method comprises: virus inoculation, virus proliferation culture, allantoic fluid harvesting, clarification, ultra-filtration concentration, inactivation, lysis and ultracentrifugation purification, gel filtration chromatography purification (ultra-filtration), blending, filtration sterilization, sub-packaging, packaging and other steps. According to the present invention, the quadrivalent influenza virus subunit vaccine can improve the safety of the influenza vaccine, can eliminate the adverse reaction caused by the adjuvant, and can eliminate the toxic-side effects caused by thimerosal.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com