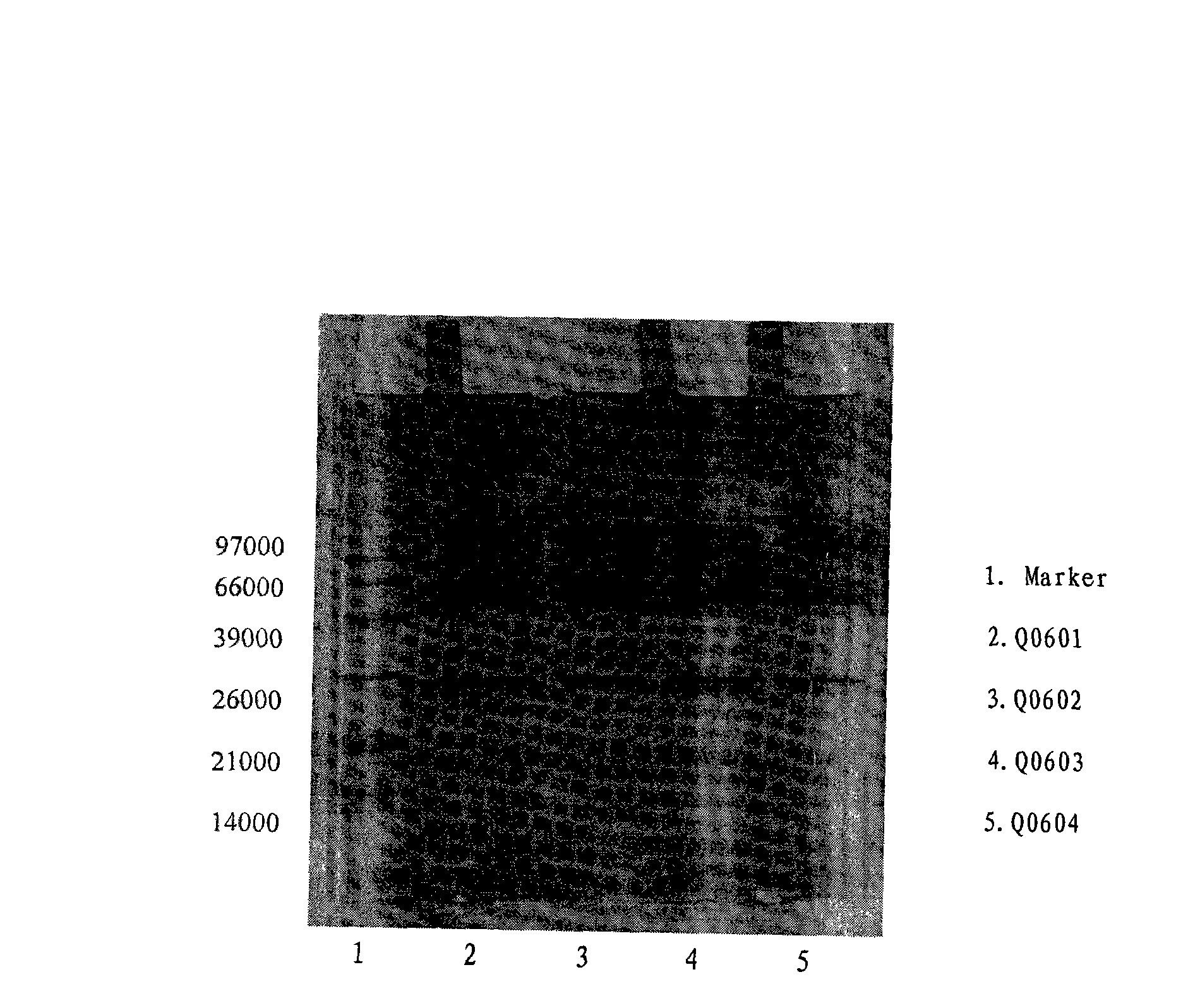
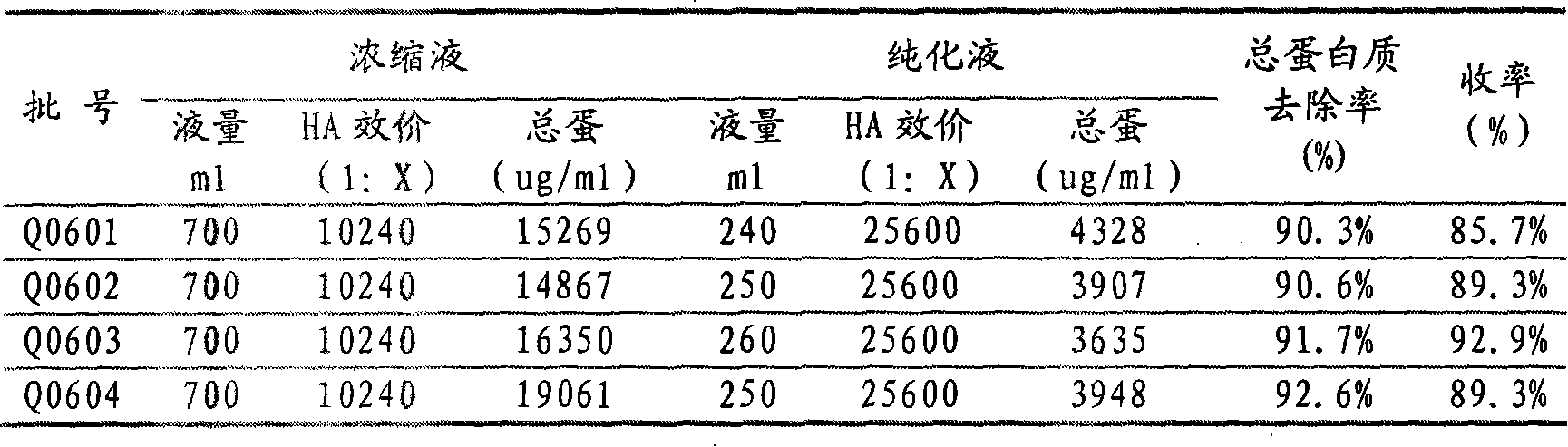
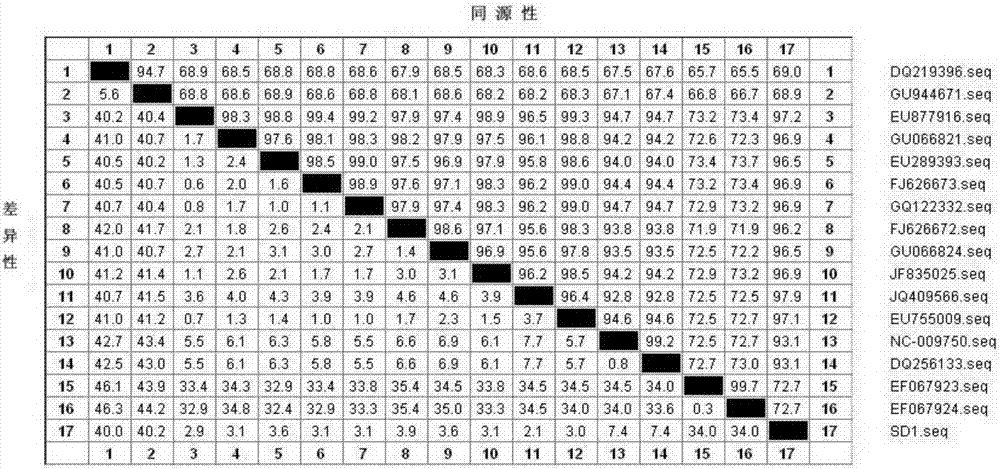
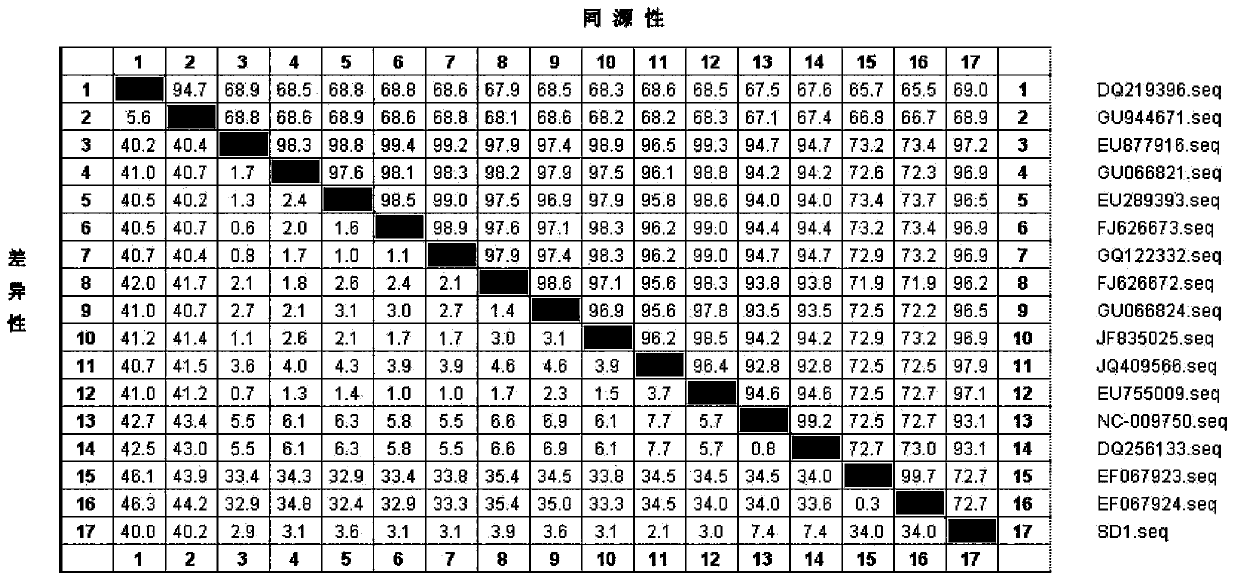
Patents
Literature
123 results about "Allantoic fluid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
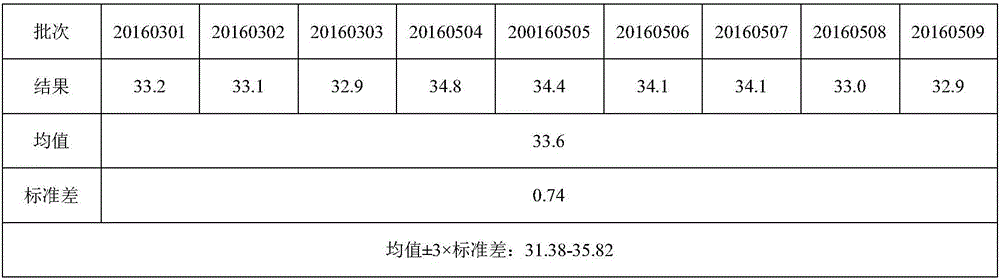
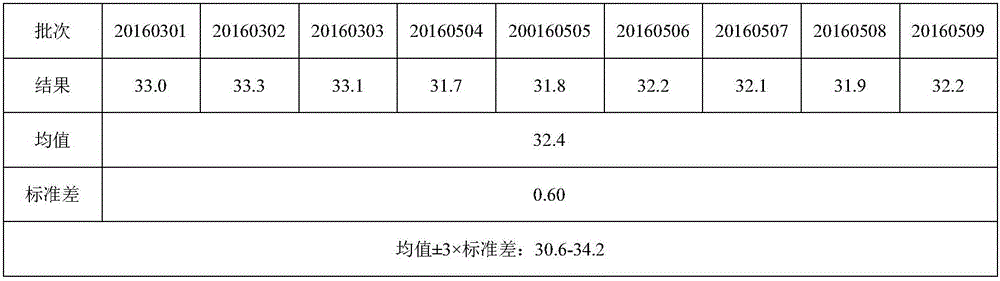
1. a liquid or gas; any liquid of the body. 2. composed of molecules which freely change their relative positions without separation of the mass. allantoic fluid. the fluid contained within the allantois.
Method for Producing the Flu Virus
The invention relates to a method for producing flu virus according to which:a) immunizing a hen by administering a flu vaccine to the hen,b) triggering embryogenesis in one or more eggs of the immunized hen,c) infecting the one or more embryonated eggs by inoculating a flu virus into the allantoic cavity of the eggs,d) incubating the one or more infected embryonated eggs under temperature and humidity conditions that allow replication of the virus, ande) harvesting the allantoic fluid of the one or more incubated eggs containing the virus.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC +1
Method for preparing anti-duck plague transfer factor
InactiveCN101293915AConvenient sourceNo pollution in the processAntiviralsPeptide preparation methodsAntigenOrganism
The invention discloses a preparation method of a natural high-effective biological active substance anti-duck plague virus transfer factor. The preparation method comprises the following steps of: inoculating duck plague virus into an allantoic fluid obtained from a 10-day-aged chick embryo, and preparing duck plague virus antigen; extracting the duck plague virus antigen; immunizing a pig with the extracted duck plague virus antigen; and extracting the anti-duck plague virus transfer factor from the immunized pig. The inventive anti-duck plague virus transfer factor can prevent duck plague and protect normal body cells from being infected by the duck plague virus, so as to reduce incidence rate.
Owner:TIANJIN SHENGJI GRP CO LTD
Novel muscovy duck reovirus disease vaccine and application thereof
InactiveCN102631674AImprove the level ofFight infectionViral antigen ingredientsAntiviralsDiseaseAdjuvant
The invention discloses a novel muscovy duck reovirus disease vaccine and an application of the novel muscovy duck reovirus disease vaccine. Firstly, a novel muscovy duck reovirus disease (NDRV)-JM85 plant is inoculated with a 11-day muscovy duck embryo proliferation virus by an allantoic cavity to obtain poison-containing allantoic fluid, or the NDRV-JM85 plant is inoculated with a muscovy duck embryo fibroblast cell monolayer; when cytopathy is above 75%, the cellular poison is harvested; after the virus is properly condensed and is inactivated by formaldehyde, the virus and white oil adjuvant are evenly mixed at a proper ratio to obtain the novel muscovy duck reovirus disease vaccine; and then the novel muscovy duck reovirus disease vaccine is used for preventing the novel muscovy duck reovirus disease. The inactivated vaccine is used for immunizing the breeding duck once, and each feather is applied with 1.0ml, immunization is boosted once in four weeks, and each feather is applied with 1.0ml.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Quadrivalent subunit influenza vaccine and preparation method thereof
InactiveCN106668854AReduce adverse reactionsImprove securitySsRNA viruses negative-senseViral antigen ingredientsPharmacySerotype
The invention relates to the field of biological pharmacy, and particularly discloses a quadrivalent subunit influenza vaccine and a preparation method thereof. Used virus seeds are respectively of an H1N1 type (NYMC X-179A), an H3N2 type (NIB-88), a BY type (B / Phuket), and a BV type (NYMC BX-35). The preparation method comprises the following steps: performing chick embryo cultivation on the virus seeds; preparing a mono-valent virus stock solution by the steps of allantoic fluid collection, clarification, concentration, purification and the like; and proportioning four mono-valent virus stock solutions with different serotypes according to certain dilution points to prepare a quadrivalent subunit influenza vaccine sample. A relatively low content of ovalbumin and relatively low residual quantities of formaldehyde and a cracking agent in the quadrivalent subunit influenza vaccine sample ensure that adverse reactions of a quadrivalent subunit vaccine are lower, and the safety of the vaccine is improved. Meanwhile, compared with an existing trivalent vaccine, the quadrivalent subunit vaccine additionally has a BV serotype, thereby having a broader protection scope for popularity of influenzas with different serotypes.
Owner:AB&B BIO TECH CO LTD JS
Virus production
InactiveUS20050186223A1Increase productionPromote localizationSsRNA viruses negative-senseAnimal cellsChick embryosAllantoic fluid
An improved process for recovery of virus from allantoic fluid of virus-infected chick embryos. Virus associated with granular and fibrous debris in the allantoic fluid can be disassociated from the debris and recovered, thereby increasing viral yield. Dissociation can be achieved by subjecting the virus-debris complex to conditions of increased salt concentrations, e.g., 0.5 M or greater.
Owner:MICROBIX BIOSYSTEMS INC
Virus production
InactiveUS7270990B2Increase productionSpeed up the processSsRNA viruses negative-senseAnimal cellsChick embryosViral infection
An improved process for recovery of virus from allantoic fluid of virus-infected chick embryos. Virus associated with granular and fibrous debris in the allantoic fluid can be disassociated from the debris and recovered, thereby increasing viral yield. Dissociation can be achieved by subjecting the virus-debris complex to conditions of increased salt concentrations, e.g., 0.5 M or greater.
Owner:MICROBIX BIOSYSTEMS INC
Preparation method and application of immunization preparation for controlling novel duck reovirus
InactiveCN102492661APrevent Hemorrhagic Necrotizing HepatitisEasy to manufactureViral antigen ingredientsMicroorganism based processesMaternal antibodyYolk
The invention discloses a novel duck reovirus strain NDRV-JM85 with a preservation number of CCTCC NO: V201127. The novel duck reovirus strain NDRV-JM85 is used as a virus seed to inoculate an allantoic cavity of a muscovy duck embryo and obtain a virus-containing allantoic fluid, or inoculate an MDEF cell to obtain cytotoxin when a CPE reaches 75%. The virus liquid is inactivated by formaldehyde and added with a white oil adjuvant, and the mixture is mixed thoroughly to from a safe and effective oil emulsion inactivated vaccine for novel duck reovirus. The vaccine is used to immunize a laying hen three times, with an immunization interval of 21 days and 1.0 ml for each hen at each time. Eggs laid 21-63 days after the three immunizations are gained, and extracted yolk and a proper amount of disinfected normal saline is prepared into a hyperimmune antibody preparation for the novel duck reovirus. A duckling without an NDRV maternal antibody is injected with the antibody preparation at 1-2 days old for 0.5ml / duckling, or a duckling with a maternal antibody is injected with the antibody preparation at 10 days old for 1.0 ml / duckling, so as to prevent and control the novel duck reovirus. The invention applies to large-scale production under a GMP condition.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +1
Purification technology applicable to mass production of human-used avian influenza vaccine
The invention belongs to the field of biotechnology and relates to the purification technology for avian influenza viruses, which is applicable to the mass production of human-used avian influenza vaccines. The purification technology is characterized in that: the avian influenza viruses are inoculated on a 9 to 11 days chicken embryo; the influenza viruses are cultured for 68 to 72 hours; and the allantoic fluid of the chicken embryo is sequentially collected, coarsely filtered, centrifugated with a continuous flow, ultrafiltered, condensed, centrifugated in a sucrose density gradient to be purified. The technology has the advantages of simple operation, small difference between batches, stable quality, high yield, low ovalbumin content and the like.
Owner:DALIAN ALEPH BIOMEDICAL
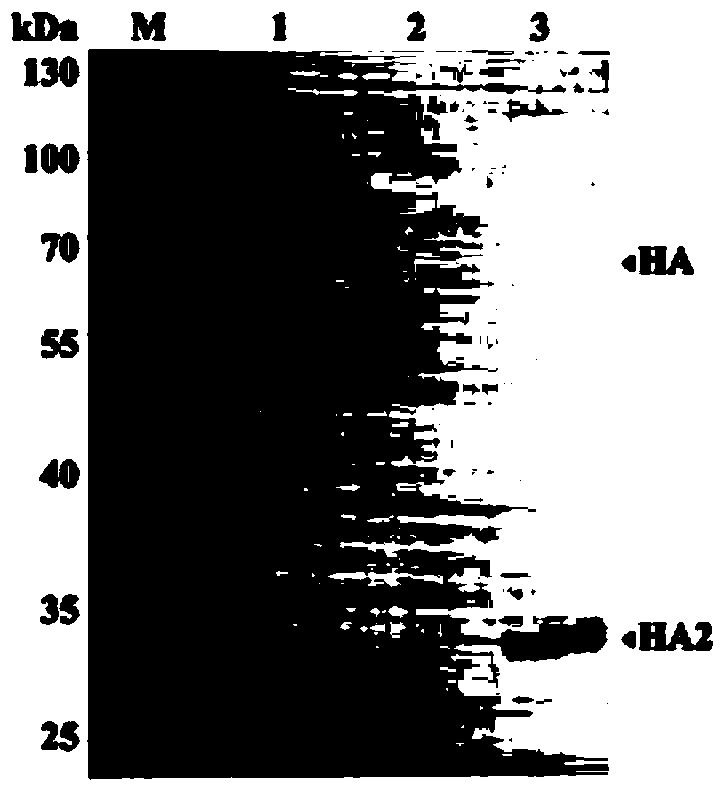
Single clone antibody aiming at H3N2 dog flu virus HA2 protein
ActiveCN104293739AJudgment of toxicityStrong specificityImmunoglobulins against virusesAntiviralsAntigenNeutralizing antibody
The invention provides a single clone antibody aiming at H3N2 dog flu virus HA2 protein, belonging to the technical field of biologics. The single clone antibody is prepared by inoculating an H3N2 dog flu virus Jiangsu separated strain JS / 10 separated in 2010 with SPF chicken embryos of 10 days old, collecting allantoic fluid of which the blood clotting titer is greater than or equal to 6, purifying virus inoculation MDCK cells, performing intracutaneous multipoint injection to inoculate mice, after cell fusion, performing three times of ELISA to detect cell supernate that cell colonies are generated, selecting an anti-H3 type hybridization tumor cell strain mAbD7, and recognizing and identifying the antigen so as to verify that the antibody aims at conservative H2A protein, wherein the neutralizing antibody titer is up to 1:12800, and the antibody subclass is IgG2b. The single clone antibody aiming at H3N2 dog flu virus and HA2 protein, which is provided by the invention, provides an important immunological preparation for treating H3N2 dog flu virus infection and has a potential application prospect.
Owner:NANJING AGRICULTURAL UNIVERSITY
Application of H3N2 canine influenza virus CGD1
ActiveCN103223162AReduce severityShorten the detox periodAntiviralsAntibody medical ingredientsTGE VACCINEMicrobiological culture
The invention belongs to the technical field of preparation of virus vaccine, and concretely discloses an application of an H3N2 canine influenza virus CGD1. According to the invention, three self-isolated, identified and preserved strains of H3N2 canine influenza virus CGD1, CGD2 and CGD3 are used to inoculate chick embryo for subcultring, and the CGD1 is found to be good in genetic stability. Therefore, the CGD1 is selected for plaque purification to breed an H3N2 canine influenza virus vaccine strain CIVGDYM1, and the vaccine strain has been preserved in China general microbiological culture collection center, with an accession number being CGMCCNO: 7218. By using the H3N2 canine influenza virus vaccine strain CIVGDYM1 to inoculate the chick embryo for virus breeding, and through gaining allantoic fluid of the chick embryo, inactivating, preparing vaccine and other steps, the safe and effective H3N2 canine influenza virus inactivated vaccine can be prepared.
Owner:SOUTH CHINA AGRI UNIV
Tetravalent influenza virus subunit vaccine and preparation method thereof
ActiveCN111420044AReduce contentExtended shelf lifeSsRNA viruses negative-senseViral antigen ingredientsVirus multiplicationUltrafiltration
The invention belongs to the technical field of biology, and particularly relates to a tetravalent influenza virus subunit vaccine and a preparation method thereof. Each dose of the tetravalent influenza virus subunit vaccine contains H1N1, H3N2 and two types of B; and the tetravalent influenza virus subunit vaccine is prepared by virus inoculation, virus multiplication culture, allantoic fluid harvesting, clarification, inactivation, ultrafiltration concentration, cracking and ultracentrifugal purification, mixing, filtration sterilization, split charging and packaging, wherein the inactivation process comprises the following steps: firstly, adding a carboxymethyl glucan solution into monovalent virus harvesting liquid, and then adding formaldehyde for inactivation. According to the method disclosed by the invention, the content of free formaldehyde in the prepared tetravalent influenza virus subunit vaccine is reduced, the storage life of the vaccine is prolonged, and the antibody level after vaccine immunization is increased.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Anti-tumor biological polysaccharide
The invention discloses an anti-tumor biological polysaccharide; the polysaccharide is obtained from chick embryo allantoic fluid and chick embryo through separating and purifying; the invention further discloses a specific purification technology of the polysaccharide, wherein the technology is to extract the biological polysaccharide by a method combined with salting-out and membrane technology. By adopting the technology disclosed by the invention, the purifying purity and the recovering rate of chick embryo allantoic fluid and chick embryo are greatly enhanced; through preclinical pharmacodynamic experiments, the biological polysaccharide has an exact killing ability to transplant human body tumors such as human primary liver cancer and ovarian cancer in nude mice, and can completely remove the transplant human body tumor cells from the nude mice after being fed with enough dosage and enough time. The polysaccharide disclosed by the invention is a biological active material with no toxicity and low side effect and is very safe; in addition, the polysaccharide is wide in drug administration route, simple in production process and high in recovering rate, and thus being a novel anti-tumor drug capable of providing great social benefit and economic benefit.
Owner:乔民 +1
Dual reverse transcription-polymerase chain reaction (RT-PCR) detection method for identifying H9N2 subtype avian influenza virus
InactiveCN103540680AStrong specificityRapid diagnosisMicrobiological testing/measurementMicroorganism based processesEpidemiologic surveyCoincidence
The invention discloses a dual reverse transcription-polymerase chain reaction (RT-PCR) detection method for identifying an H9N2 subtype avian influenza virus. A pair of primers is designed respectively according to HA genes and NA genes of the H9N2 subtype of avian influenza virus (AIV), the HA genes and NA genes of the H9N2 subtype AIV in a sample can be subjected to specific amplification, and lengths of target fragments are respectively 700bp (HA) and 423bp (NA). According to the method, cross reaction is avoided in subtype AIV such as H3N8, H4N6 and H5N8, Newcastle disease virus and infectious bronchitis virus of chicken; the lowest detection amount of allantoic fluid of the virus is 1*103.25EID50 / 100uL; compared with conventional methods such as hemagglutination inhibition of virus and a neuraminidase inhibition test, the method has the advantage that the coincidence rate of the identification result is 100 percent. A rapid, specific and sensitive detection means is provided for identifying the H9N2 subtype AIV. The detection method can be used for rapidly diagnosing diseases caused by the H9N2 subtype AIV and has good application prospects in aspects of clinical diagnosis and epidemiological investigation.
Owner:LIAOCHENG UNIV
Preparation method for goose-origin reovirus inactivated vaccine
InactiveCN106540250AInfection Prevention and ControlViral antigen ingredientsAntiviralsReovirus RNAOil phase
The invention provides a preparation method for a goose-origin reovirus inactivated vaccine. The preparation method comprises the following steps that a goose embryo is inoculated against reoviruses through an allantoic cavity, dead embryo allantoic fluids or live embryo allantoic fluids are collected within 24-144 h after incubation, and reovirus fluids are obtained; the reovirus fluids are inactivated through formaldehyde, Tween-80 is added to be mixed to serve as a water phase, and white oil, Span-80 and aluminium stearate are mixed to serve as an oil phase; and the water phase is added into the oil phase to be mixed uniformly, and the inactivated vaccine is obtained. According to the preparation method, the prepared goose-origin reovirus inactivated vaccine has high protective effects on a gosling, and is suitable for virus immunity of the gosling.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Inactivated vaccine of cow chlamydia, its preparation and inspection method
ActiveCN1698892AFight infectionInfection fromChlamydiaceae ingredientsAntiinfectivesMicrocosmic saltOil adjuvant
The invention relates to an inactivated vaccine of cow chlamydia, its preparation and the related inspection method during the vaccine preparation. The preparing process comprises diluting the Chlamydia psittaci SX 5 or NX with microcosmic salt buffering liquid or physiological saline, vaccinating to healthy chick embryo hatched at 37 deg. C for 6-7 days, harvesting vitelline membrane and allantois liquid of dead chick embryo after 72 hours as antigens, triturating the antigens, diluting and charging formaldehyde for deactivation, mixing the deactivated antigens with oil adjuvant by the proportion of 1:1, stirring homogeneously, carrying out homogeneous emulsion to obtain the vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Real-time fluorescence RT-PCR detection kit for H1N1 type A swine influenza virus and application of detection kit
InactiveCN104450956AStrong specificityEasy to operateMicrobiological testing/measurementMicroorganism based processesFluorescenceTissue sample
The invention discloses a fluorescent quantitative RT-PCR detection kit for an H1N1 type A swine influenza virus, and an application of the detection kit. Through multiple sequence alignment, a primer and a probe with high specificity for detecting the H1N1 type A swine influenza virus is designed aiming at conservative gene segments of the H1N1 (2009) type A swine influenza virus, a Eurasian avian-like H1N1 swine influenza virus, a classical type H1N1 swine influenza virus and a human-derived H1N1 swine influenza virus, and is applied to real-time fluorescence RT-PCR detection. An experiment result proves that the specific PCR primer and TaqMan fluorescence probe disclosed by the invention are high in specificity when being used for detecting the H1N1 type A swine influenza virus; the sensitivity can reach 2.6*10<-5>ng; tissue samples such as nasal swabs, lungs and tracheas of to-be-detected swinery can be detected; the chick embryo allantoic fluid can also be detected; the detection kit is simple to operate and easy to popularize; basic operation and application are facilitated; and the detection kit can become a useful detection tool for diagnosis of H1N1 type A swine influenza virus diseases and epidemiological survey.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Anti-H7N9 subtype avian influenza virus monoclonal antibody epitope as well as screening method and application thereof
The invention provides an anti-H7N9 subtype avian influenza virus monoclonal antibody epitope as well as a screening method and application thereof, belonging to the technical field of immunodetection. The screening method comprises the following steps: mixing wild type H7N9 subtype avian influenza virus liquid with a corresponding monoclonal antibody with a neutralizing property for incubation, and inoculating the mixture to an SPF chick embryo, so as to obtain allantoic fluid with a positive hemagglutination titer; and carrying out gradient dilution on the positive allantoic fluid, mixing the positive allantoic fluid with the monoclonal antibody for incubation, inoculating the mixture to the SPF chick embryo, determining a hemagglutination inhibition titer of the monoclonal antibody by selecting the allantoic fluid with the positive hemagglutination titer as an antigen, when the determined hemagglutination inhibition titer is lower than the hemagglutination inhibition titer of a wild type virus by 8log2, determining the positive allantoic fluid as an escape mutant of the wild type H7N9 subtype avian influenza virus, measuring an HA gene sequence of the positive allantoic fluid, and determining the epitope recognized by the monoclonal antibody. By virtue of the method, the specific epitope can be clearly screened; the method is simple, accurate and short in screening period.
Owner:YANGZHOU UNIV
Proliferation of H5 subtype avian influenza virus
PendingCN106754750AReduce manufacturing costIncrease productivityAntiviralsViruses/bacteriophagesAvian influenza virusMultivalent Vaccine
The invention provides a method for proliferation of an H5 subtype avian influenza virus. The H5 subtype avian influenza virus is diluted by a virus diluent and is inoculated into 10-day-old SPF chicken embryo and then the chicken embryo is cultured at 36 DEG C. The allantoic fluid of the chicken embryo not dead within 72 hours is collected and stored at 40 DEG C; the collected allantoic fluid is then diluted with the virus diluent in a ratio of 10 times. The virus liquid with a dilution degree of 10<6> -10<9> is selected to inoculate into a 10-day-old SPF chicken embryo and then the chicken embryo is cultured at 35.5 DEG C. The allantoic fluid of the chicken embryos not dead within 72 hours is collected and then mixed with the allantoic fluid of a high dilution degree and highest HA titer to obtain a seed liquid. The collected seed liquid is Continuously passed down and then is mixed with the allantoic fluid with a high dilution degree and the HA titer of 10log2, 9log2, and 10log2 to obtain the virus solution for the preparation of vaccine. The method of the invention can save the cost of preparing the vaccine and improve the production efficiency, so as to overcome the technical defects of low virus content in the existing strains for preparing vaccine and that the univalent vaccine can only be achieved by the concentration of the multivalent vaccine.
Owner:YEBIO BIOENG OF QINGDAO
Aptamer realizing specific binding with newcastle disease virus as well as screening method and applications of aptamer
ActiveCN107058329AImprove featuresHigh affinityDNA preparationMaterial analysisHN ProteinScreening method
The invention discloses an aptamer realizing specific binding with the newcastle disease virus as well as a screening method and applications of the aptamer. The aptamer is specific aptamer B53 obtained through screening by adopting the SELEX technology. Specifically, the random single stranded DNA library and primers are constructed and synthesized in vitro, the newcastle disease HN protein and the newcastle disease virus GM strain realizing prokaryotic expression are adopted as target molecules, after screening, PCR amplification, positive screening and inverse screening, the screened single stranded DNA library realizing specific binding with the newcastle disease virus GM strain is subjected to clone sequencing, and thus the specific aptamer B53 is obtained. The aptamer B53 has the capacity of inhibiting virus replication, virus blood clotting activity and plaque formation, can specifically recognize the newcastle disease virus HN protein and the newcastle disease virus GM strain, has no reaction to avian influenza virus, infectious bursal disease virus and SPF chick embryo allantoic fluid, and can be used for detecting the newcastle disease virus.
Owner:SOUTH CHINA AGRI UNIV
Avian influenza H9N2 subtype virus strain and application thereof
ActiveCN103789273AStrong specificityImprove featuresSerum immunoglobulinsMicroorganism based processesSerum igeAvian influenza virus
The invention provides an avian influenza H9N2 subtype virus strain with the preservation number of CGMCC No.6757. The avian influenza H9N2 subtype virus strain provided by the invention has favorable specificity and immunogenicity, the erythrocyte agglutination effect of allantoic fluid can not be inhibited by anti-NDV (Newcastle Disease Virus) positive serum, anti-EDS76 positive serum, anti-M41 positive serum, anti-H5 subtype avian influenza virus positive serum and anti-H7 subtype avian influenza virus positive serum, but can be inhibited by H9 subtype avian influenza virus positive serum, and immunized chicken can generate an HI (Hemagglutination Inbition) antibody which is specific for H9. The avian influenza H9N2 subtype virus strain provided by the invention can be used as a vaccine strain for preventing H9 subtype avian influenza of birds, and can be used for authenticating avian influenza viruses and researching epidemiology so as to have favorable market application prospects.
Owner:北京华都诗华生物制品有限公司
Method for preparing avian infectious brunchitis virus transfer resistant factor
InactiveCN101293913AConvenient sourceNo pollution in the processAntiviralsPeptide preparation methodsAntigenOrganism
The invention discloses a preparation method of a natural high-effective biological active substance anti-avian infectious bronchitis virus (IBV) transfer factor. The preparation method comprises the following steps of: inoculating IBV into an allantoic fluid obtained from a 10-day-aged chick embryo, and preparing IBV antigen; extracting the IBV antigen; immunizing a pig with the extracted IBV antigen; and extracting the anti-IBV transfer factor from the immunized pig. The inventive anti-IBV transfer factor can prevent IBV infection and protect normal body cells from being infected by the IBV, so as to reduce incidence rate.
Owner:TIANJIN SHENGJI GRP CO LTD
Trivalent influenza virus subunit vaccine and preparation method thereof
InactiveCN107537030AHigh purityQuality improvementAntiviralsAntibody medical ingredientsHemagglutininSide effect
The invention discloses a trivalent influenza virus subunit vaccine and a preparation method thereof, wherein virus protein after lysis is further purified by using a lysis agent and a new purification method to prepare a tetravalent influenza virus subunit vaccine, the content of three influenza hemagglutinins such as influenza A virus H1N1, influenza A virus H3N2 and influenza B virus in each dose of the vaccine is more than 80%, and the trivalent influenza virus subunit vaccine does not contain adjuvant and does not contain thimerosal and other preservatives. The invention further providesa preparation method of the trivalent influenza virus subunit vaccine, wherein the preparation method comprises: virus inoculation, virus proliferation culture, allantoic fluid harvesting, clarification, ultra-filtration concentration, inactivation, lysis and ultracentrifugation purification, gel filtration chromatography purification (ultra-filtration), blending, filtration sterilization, sub-packaging, packaging and other steps. According to the present invention, the trivalent influenza virus subunit vaccine can improve the safety of the influenza vaccine, can eliminate the adverse reactioncaused by the adjuvant, and can eliminate the toxic-side effects caused by thimerosal.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Quadrivalent influenza virus subunit vaccine and preparation method thereof
InactiveCN107537032AHigh purityQuality improvementAntiviralsViruses/bacteriophagesHemagglutininAdjuvant
The invention discloses a quadrivalent influenza virus subunit vaccine and a preparation method thereof, wherein virus protein after lysis is further purified by using a lysis agent and a new purification method to prepare the tetravalent influenza virus subunit vaccine, the content of four influenza hemagglutinins such as influenza A virus H1N1, influenza A virus H3N2 and two kinds of influenza Bviruses in each dose of the vaccine is more than 80%, and the quadrivalent influenza virus subunit vaccine does not contain adjuvant and does not contain thimerosal and other preservatives. The invention further provides a preparation method of the quadrivalent influenza virus subunit vaccine, wherein the preparation method comprises: virus inoculation, virus proliferation culture, allantoic fluid harvesting, clarification, ultra-filtration concentration, inactivation, lysis and ultracentrifugation purification, gel filtration chromatography purification (ultra-filtration), blending, filtration sterilization, sub-packaging, packaging and other steps. According to the present invention, the quadrivalent influenza virus subunit vaccine can improve the safety of the influenza vaccine, can eliminate the adverse reaction caused by the adjuvant, and can eliminate the toxic-side effects caused by thimerosal.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Infectious bronchitis virus QX type strain identifying and detecting kit
ActiveCN108950068ATo achieve the purpose of typing detectionEfficient detectionMicrobiological testing/measurementMicroorganism based processesGene typeGenotype
The invention relates to the technical field of bio-detection and aims to provide an infectious bronchitis virus QX type strain identifying and detecting kit. The kit comprises two pairs of primers which are separately universal primers for amplifying the infectious bronchitis virus and primers for specifically amplifying the QX gene type strain, wherein the sequences of the primers for specifically amplifying the QX gene type strain are as shown in SEQ ID NO: 5 and SEQ ID NO: 6. An effective RT-PCR detection method is established by means of the specific primers of QX genotype and a purpose of parting detection of the QX genotype can be achieved by the primary RT-PCR reaction is achieved. According to the kit, the two pairs of primers are added into a same PCR system in a same PCR reaction condition, so that IBV in a clinical sample or a chick embryo allantoic fluid can be detected effectively, and IBV of the QX genotype can be identified specifically. The detection method is quick, efficient and accurate, and has very good meaning in guiding selection of vaccines and quick control of IB.
Owner:ZHEJIANG UNIV
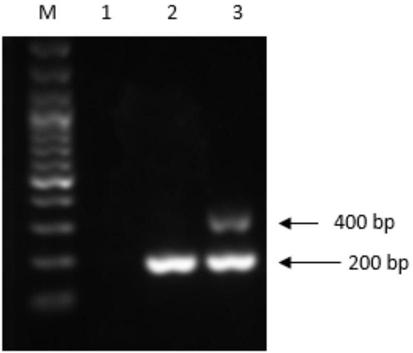
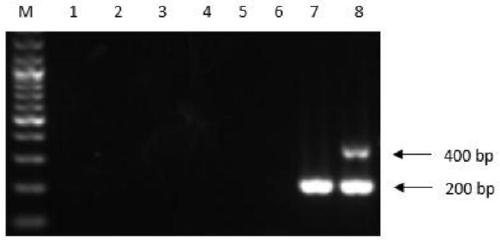
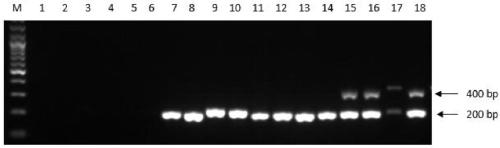
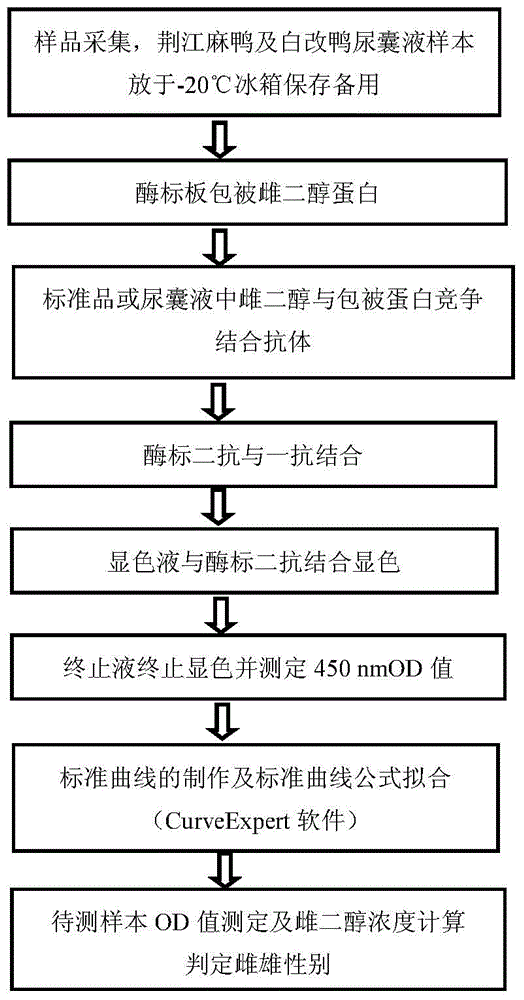
Duck embryo early sex identification method and special kit
ActiveCN104698173ADoes not affect normal developmentImprove welfareMaterial analysisDiseaseAnimal science
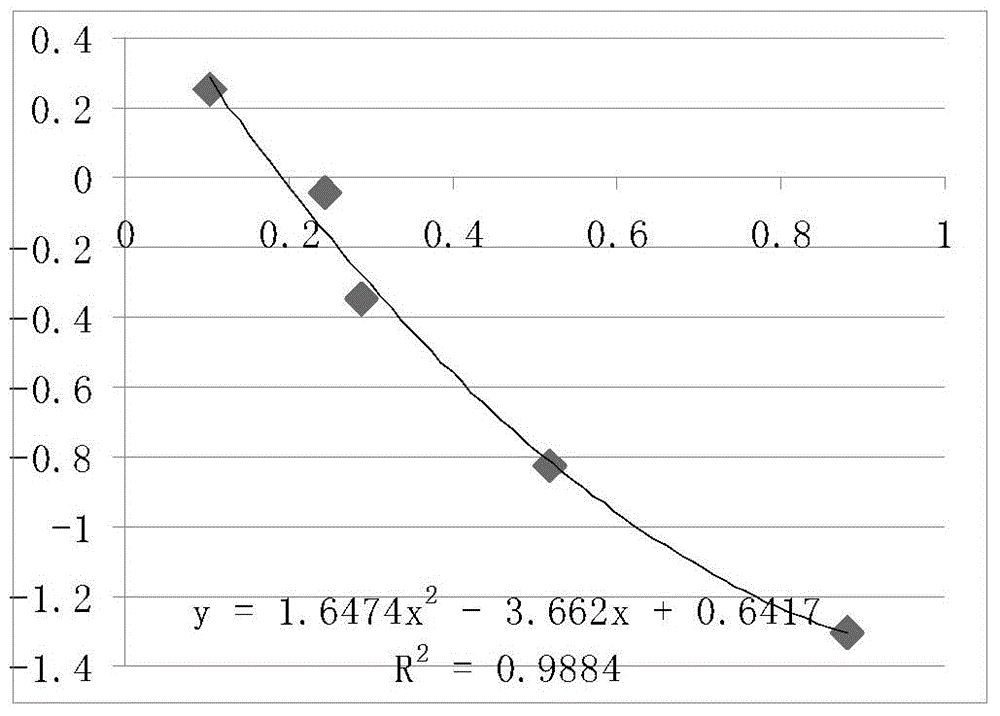
The invention belongs to the technical field of poultry sex detection, and in particular relates to a duck embryo early sex identification method and a special kit. The duck embryo early sex identification method is established by collecting a hatched early duck embryo allantoic fluid, making use of a monoclonal antibody for estradiol detection and carrying out antigen-antibody reaction. Male and female sexes are judged according to a characteristic that the content of estradiol in a female embryo is higher than 1ng / ml and the content of the estradiol in a male embryo is lower than 0.6ng / ml. The identification method disclosed by the invention can identify the female and male sexes in an early hatching period without an influence on the further development of the embryo; and the identification method is conducive to animal welfare and relieving the transmission of vertical diseases.
Owner:武汉赛维尔生物科技有限公司
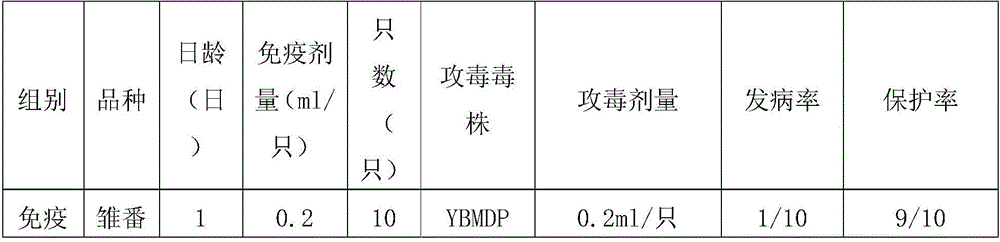
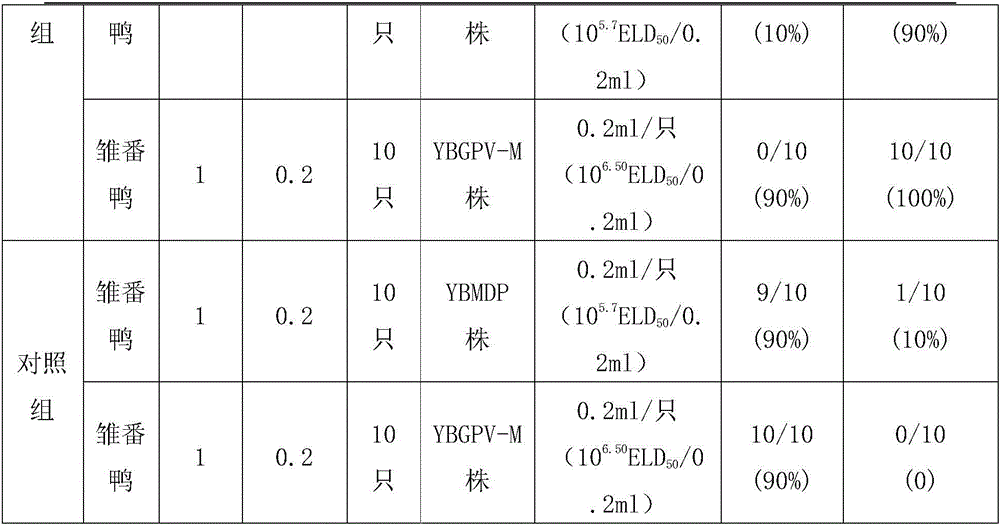
Muscovy duck parvovirus and gosling plague bivalent vaccine
ActiveCN105920596AImproving immunogenicityImprove securityViral antigen ingredientsAntiviralsMaternal antibodyImmunogenicity
The invention provides a Muscovy duck parvovirus and gosling plague bivalent vaccine. The antigens used by the vaccine is inactivated Muscovy duck parvoviruses and Muscovy duck-source gosling plague viruses, the preservation number of the Muscovy duck parvoviruses is CGMCC No. 8504, and the preservation number of the Muscovy duck-source gosling plague viruses is CCTCC No. V201620. A preparation method of the Muscovy duck parvovirus and gosling plague bivalent vaccine includes: the Muscovy duck parvovirus YBMDP strains and Muscovy duck-source gosling plague virus YBGPV-M strains which are high in virus content and good in immunogenicity are screened, infected embryos and allantoic fluid are collected after duck embryo inoculation, and oil emulsion adjuvant is added for emulsification and mixing to obtain the vaccine after homogenization, ultrafiltration and concentration, and formaldehyde solution inactivation. The prepared vaccine can immunize breeding Muscovy ducks and increase the level of two types of antibodies of the breeding Muscovy ducks at the same time, guarantee the offspring maternal antibody level of the breeding Muscovy ducks, and prevent the young Muscovy duck parvovirus diseases caused by the Muscovy duck parvoviruses and gosling plague virus infection caused by the Muscovy duck-source gosling plague viruses.
Owner:YEBIO BIOENG OF QINGDAO
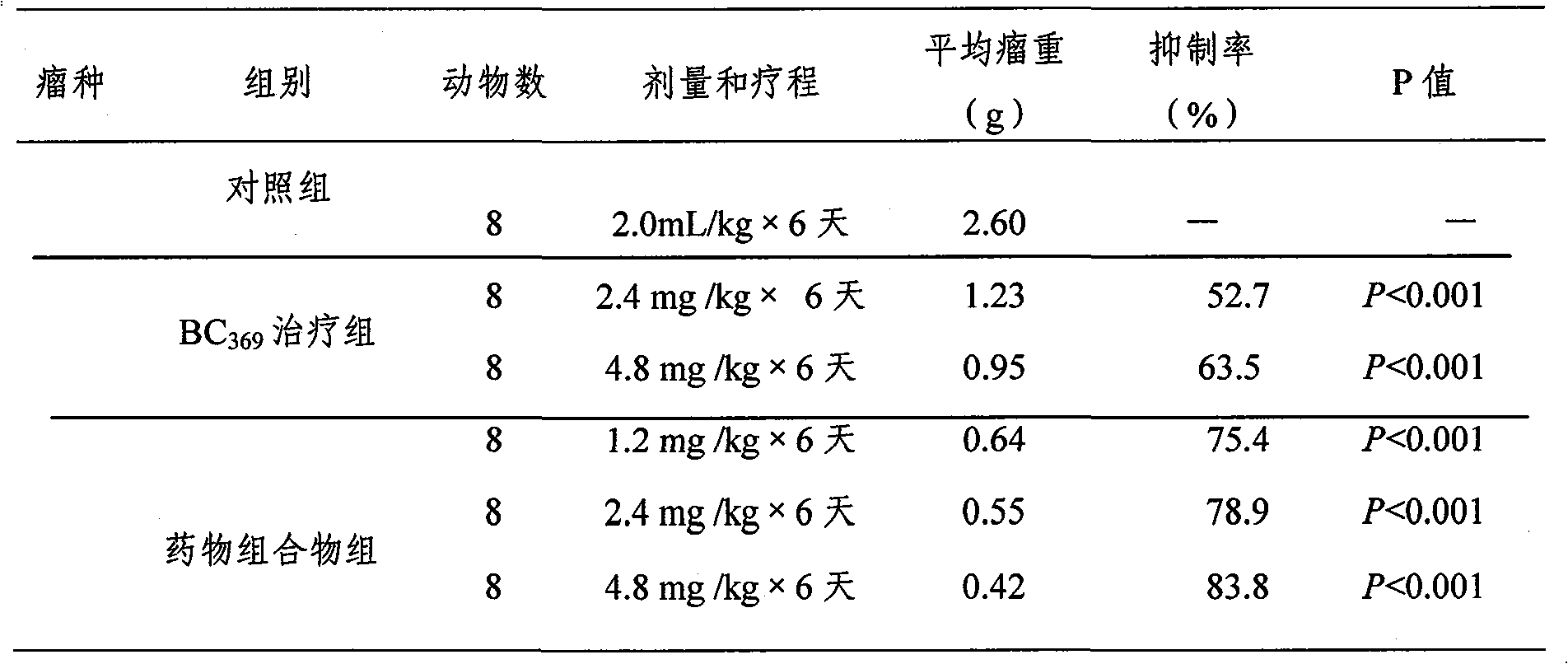
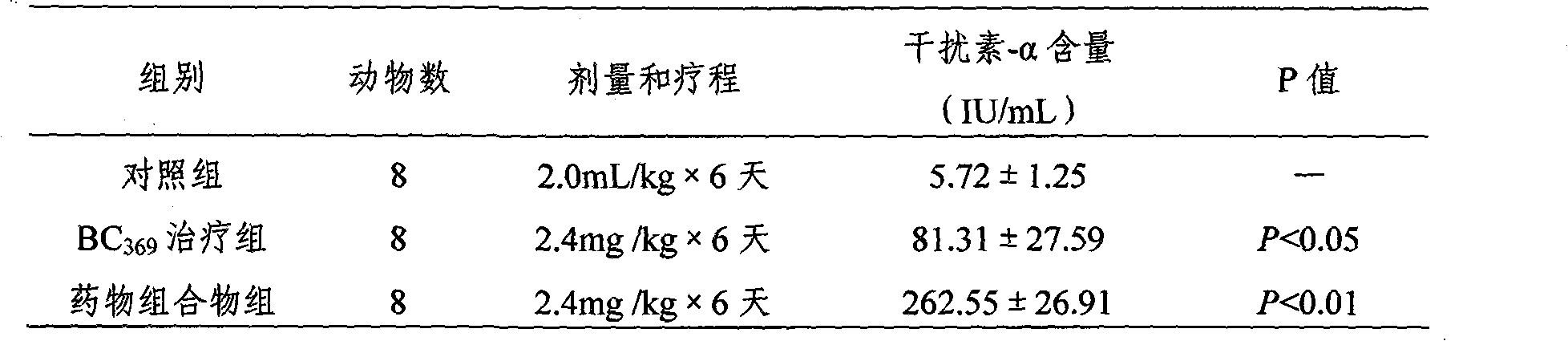
Novel medicinal composition and preparation method
InactiveCN101850110AIncreased anti-lung cancer effectSmall side effectsPeptide/protein ingredientsViral/bacteriophage medical ingredientsCholesterolGlycoprotein G
The invention relates to a novel medicinal composition for improving organism immune function and resisting tumor to address both the symptoms and root cause, and a preparation method. The medicinal composition comprises the following components: 1,500 milliliters of Newcastle disease virus allantoic fluid with a titer of 1:640, 2 milliliters of neuraminidase, 2 milliliters of nuclease, 1.08 grams of glycoprotein G, 10 grams of galactose, 1.0 milligrams of biotin, 5 grams of fucose, 15 grams of astragalus polysaccharide, 2.0 milligrams of sialidase, 2.0 milligrams of phospholipid, 10 milligrams of uracil, 5 milligrams of Vit C, 4.0 milligrams of cholesterol, 200 milligrams of ATP, 22 milliliters of 10 percent KCl, 3.6 grams of MgSO4.7H2O, and 10 milligrams of prothrombin. The experiments for pharmacodynamics, pharmacology and the like, show that the medicinal composition has good inhibiting and killing effects on mouse transplantable tumor such as Lewis lung carcinoma. Compared with the medicament of the conventional invention, the medicinal composition has more remarkable diffidence in the growth inhibiting effect on the mouse Lewis lung carcinoma. The medicinal composition has obvious effect, and is a novel biological anticancer preparation with good social and economic benefits.
Owner:天津泽世德生物医药有限公司
RT-PCR kit for detection of poultry source pedigree H3N2 subtype canine influenza virus and application thereof
ActiveCN103409559AFast identification testIncrease coverageMicrobiological testing/measurementMicroorganism based processesNucleotideNucleotide sequencing
The invention discloses an RT-PCR kit for detection of poultry source pedigree H3N2 subtype canine influenza virus and application thereof. The invention provides a primer pair for assisting detection of poultry source pedigree H3N2 subtype canine influenza virus. The nucleotide sequence of one primer is shown as a SEQ ID No.1, and the nucleotide sequence of the other primer is shown as a SEQ ID No. 2. Detection of poultry source pedigree H3N2 subtype canine influenza virus by the kit provided by the present invention is faster, and has higher singularity, sensibility and sensitivity than a traditional method. The kit can detect virus allantoic fluid as well as clinically acquired samples, and is easy for popularization as a useful tool for diagnosis and epidemiological investigation of poultry source pedigree H3N2 subtype canine influenza virus.
Owner:CHINA AGRI UNIV
Bivalent egg yolk antibody against DVH (duck virus hepatitis) as well as preparation method and application of bivalent egg yolk antibody
ActiveCN106854647AImprove securityEffective separation and purificationEgg immunoglobulinsSsRNA viruses positive-senseDuck hepatitis A virusYolk
The invention provides a bivalent egg yolk antibody against DVH (duck virus hepatitis) as well as a preparation method and an application of bivalent egg yolk antibody. The bivalent egg yolk antibody contains a DHAV (duck hepatitis A virus)-1 type egg yolk antibody against DVH and a DHAV-3 type egg yolk antibody against DVH. The preparation method comprises steps as follows: (1) a DHAV-1 type strain against DVH and a DHAV-3 type strain against DVH are inoculated with an SPF chick embryo and a susceptible duck embryo respectively, an allantoic fluid is obtained, obtained virus fluids are mixed in proportion and inactivated with formalin, and a vaccine is prepared; (2) laying hens are immunized with the vaccine, sampling is performed after immunization for measuring whether the neutralizing titer of DHAV-1 type and DHAV-3 type antigens and antibodies in hyperimmune egg yolk of chickens is larger than or equal to 1:8192, and later, hyperimmune eggs of the chickens are collected; (3) eggshells of the hyperimmune eggs are disinfected, isovolumetric distilled water is added after the egg yolk is collected, and the mixture is stirred and mixed uniformly and then is subjected to pasteurization at the low temperature; purification with an acidified distilled water method and purification with a caprylic acid method are performed; microfiltration and ultrafiltration are performed. The provided bivalent egg yolk antibody is low in cost and high in titer, DVH caused by DHAV-1 and DHAV-3 can be effectively controlled, and remarkable social benefits can be obtained.
Owner:PU LIKE BIO ENG
Duck viral hepatitis bivalent yolk antibody, preparation method and application thereof
InactiveCN103865884AImprove securityEffective separation and purificationEgg immunoglobulinsSsRNA viruses positive-senseYolkOctanoic Acids
The present invention provides a duck viral hepatitis bivalent yolk antibody and a preparation method thereof, wherein the bivalent yolk antibody comprises a duck viral hepatitis DHAV-1 type antibody and a duck viral hepatitis DHAV-3 type antibody. The preparation method comprises: (1) adopting a duck viral hepatitis DHAV-1 type strain and a duck viral hepatitis DHAV-3 type strain to respectively vaccinate SPF chicken embryo and susceptible duck embryo, harvesting allantoic fluid, mixing the harvested virus liquids according to a certain ratio, carrying out formaldehyde inactivation, and preparing a vaccine; (2) adopting the vaccine to immunize laying hens, sampling after immunization to determine whether the neutralizing titer of the anti-DHAV-1 type antigen antibody and the anti-DHAV-3 type antigen antibody in the chicken hyperimmune egg yolk is more than or equal to 1:8192, and collecting the hyperimmune egg of the chicken; and (3) disinfecting the eggshell of the hyperimmune egg, collecting the egg yolk, adding the equal volume of distilled water, uniformly stirring and mixing, carrying out low temperature pasteurization inactivation, adopting an acidification distilled water method to purify, adopting an octanoic acid method to purify, and carrying out micro-filtration and ultra-filtration. The duck viral hepatitis bivalent yolk antibody has characteristics of low cost and high titer, and can be provided for effectively controlling duck viral hepatitis caused by DHAV-1 and DHAV-3 so as to obtain significant social benefits.
Owner:PU LIKE BIO ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com