Anti-H7N9 subtype avian influenza virus monoclonal antibody epitope as well as screening method and application thereof
A monoclonal antibody, avian influenza virus technology, applied in the field of immune detection, can solve the problems of affecting binding, hindering the binding of the latter antibody to the antigen, unable to locate the antigen surface, etc., achieving the effect of simple method, rapid identification, and short screening period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] 1 method
[0076] 1. Preparation of 1H7N9 Subtype Avian Influenza Monoclonal Antibody
[0077] 1.1.1 Antigen preparation
[0078] 1.1.1.1 Virus amplification and inactivation
[0079] A / CHICKEN / JIANGSU / W1-8 / 2015 (H7N9 subtype avian influenza virus, isolated from cotton swab samples of chickens in the live poultry trading market by the Key Open Laboratory of Livestock and Poultry Infectious Diseases, Ministry of Agriculture, Yangzhou University) was sterile PBS conducts 10 -4 Dilute and inoculate 10-day-old SPF chicken embryos, inoculate chicken embryos with 0.2 mL of the diluted virus solution through the allantoic cavity, incubate at 35°C, discard dead chicken embryos within 24 hours, and place dead chicken embryos at 4°C overnight after 24 hours of inoculation. The allantoic fluid was collected, formalin was slowly added dropwise to a final concentration of 0.1%, placed at 4°C, 90 rpm, and shaken for 24 hours to inactivate, and finally the inactivated virus fluid was...
Embodiment 2
[0102] (1) Dilute the wild-type H7N9 subtype avian influenza virus (A / CHICKEN / JIANGSU / W1-8 / 2015) to 10 with PBS with a concentration of 0.01M and pH=7.4 6 EID 50 / 0.5ml, then take 0.5ml of monoclonal antibody ascites and the diluted viral allantoic fluid and mix them with room temperature for 1 hour.
[0103] (2) Inoculate four 10-day-old SPF chicken embryos with the above-mentioned virus and antibody mixture, inoculate 0.1ml of each chicken embryo, and culture at 35°C for 72h.
[0104] (3) Collect the allantoic fluid after 72 hours and measure its HA titer. The specific method is as follows:
[0105] Ⅰ. Add 25 μl of PBS to each well of a 96-well hemagglutination plate.
[0106] Ⅱ. Add 25 μl of virus to the first row of wells of the 96-well hemagglutination plate, dilute to the 11th well from left to right, and discard 25 μl. Well 12 was a negative control.
[0107] Ⅲ. Add 25μl PBS to each well.
[0108] Ⅳ. Add 25 μl of 1% red blood cells to each well, and the final volum...
Embodiment 4
[0130] Preparation of colloidal gold test strips
[0131] 1. Antibody purification effect identification
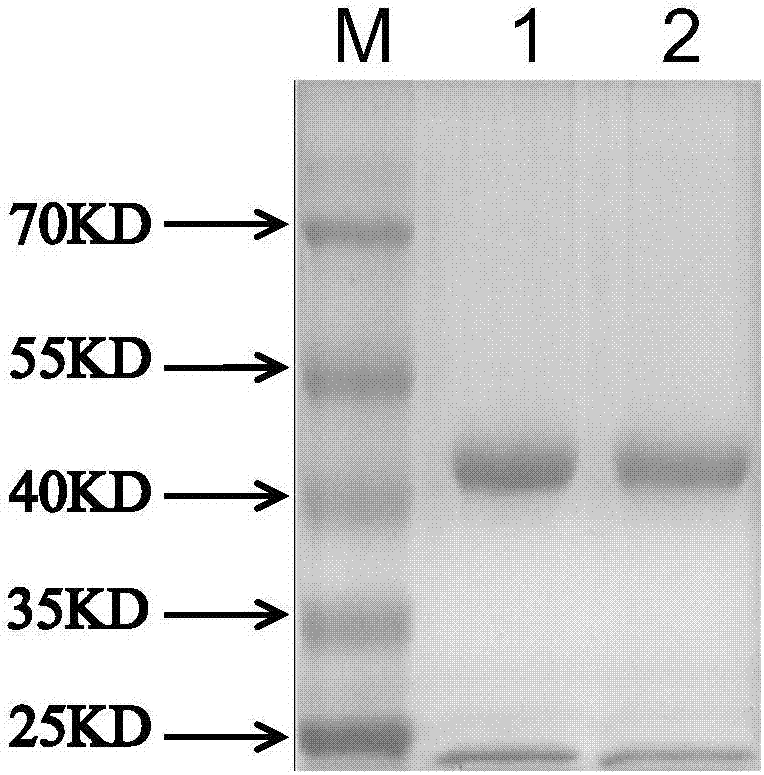
[0132] The 1B6 and 1A2 monoclonal antibodies were purified by Protein G affinity chromatography, and the concentrations were 1.231mg / mL and 2.663mg / mL, respectively. The processed samples were subjected to SDS-PAGE at a concentration of 0.5 mg / mL to observe the results. Such as figure 1 There are mainly two bands of heavy chain and light chain of immunoglobulin, among which M: protein molecular marker; 1 represents 1B6; 2 represents 1A2, and the purification effect is better.
[0133] 2. Optimal amount of protein labeled with colloidal gold
[0134] According to the color of the finger tube, it was found that the concentration started to be stable at 40 μl / ml, so the optimal amount of labeled monoclonal antibody was determined to be 40 μl / ml.
[0135] 3. Determination of the dilution factor of the coating antibody
[0136] Dilute the coated monoclonal antibody to 0.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com