Patents
Literature
809 results about "Avian influenza virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
H5 subtype avian flu virus hemagglutinin protein monoclonal antibody, and its preparing method and use
This invention relates to a monoclonal antibody capable of combining with H5 subtype avian influenza virus HA protein specifically, the hybridoma cell line secreting said antibody and a preparing method. The invention also relates to a serial test kit for testing H5 subtype avian influenza virus by the antibody and a bit of said antibody in the test sample of the virus and its usage in treatment.
Owner:XIAMEN UNIV
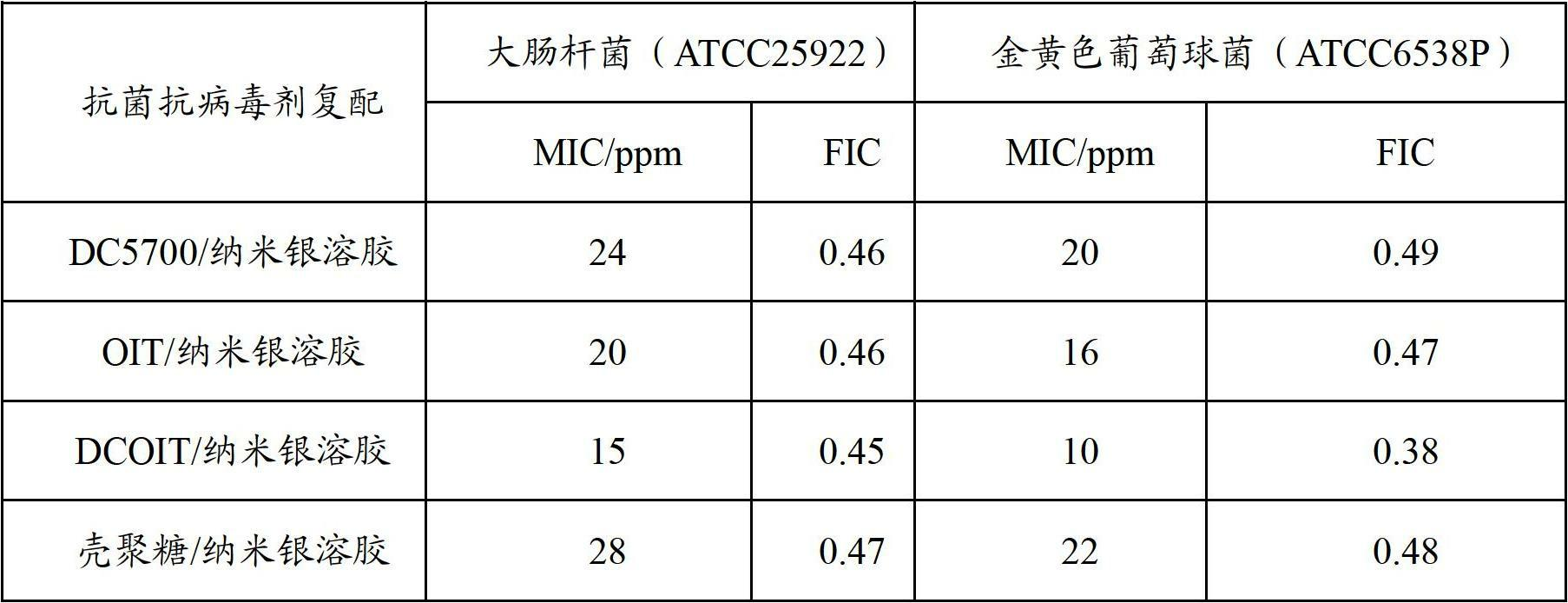
Antibacterial antiviral treating agent, preparation method and application thereof
ActiveCN102669179AImprove the safety of useSimple production processBiocideDisinfectantsAvian influenza virusSilver colloid
The invention relates to an efficient antibacterial antiviral treating agent, a preparation method and an application thereof. The antibacterial antiviral treating agent comprises a nanometer silver colloid, an organic anti-bacterial agent, a cosolvent, a polymer binder and water. According to the antibacterial antiviral treating agent, the use security is high, the quick-acting antibacterial rate of processed medical masks can reach to above 99%, the quick-acting killing effect on H1N1 influenza a virus and H5N1 avian influenza virus is excellent, and the antibacterial antiviral treating agent is non-toxic and harmless to skin and has an excellent long lasting effect.
Owner:BEIJING CHAMGO NANO TECH
Functional influenza virus like particles (VLPs)
ActiveUS8080255B2SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
The present invention discloses and claims virus like particles (VLPs) that express and / or contains seasonal influenza virus proteins, avian influenza virus proteins and / or influenza virus proteins from viruses with pandemic potential. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing substantial immunity to either seasonal and avian influenza, or at least one symptom thereof.
Owner:NOVAVAX
Functional influenza virus like particles (VLPs)
ActiveUS8506967B2SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
Owner:NOVAVAX
Monoclonal antibodies binding to avian influenza virus subtype h5 haemagglutinin and use thereof
InactiveUS20090068637A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHemagglutininAvian influenza virus
The present application provides monoclonal antibodies that specifically bind to the hemagglutinin of avian influenza virus subtype H5, as well as monoclonal antibodies capable of blocking at least 50% of the hemagglutinin binding activity of these monoclonal antibodies. Such antibodies are useful, for example, in the detection, diagnosis, prevention, and treatment of avian influenza virus. Also provided herein are hybridoma cell lines, isolated nucleic acid molecules, and short peptides related to the monoclonal antibodies provided herein, and pharmaceutical compositions and kits containing the monoclonal antibodies provided herein.
Owner:HX DIAGNOSTICS INC
Avian influenza viruses, vaccines, compositions, formulations, and methods
ActiveUS20080118531A1Reduces and eliminates disease transmissionInhibit growthCompounds screening/testingSsRNA viruses negative-senseHemagglutininAvian influenza virus
A vaccine composition and method which is effective in preventing or ameliorating Avian Influenza Virus infection is set forth herein. The vaccine contains at least two inactivated strains of avian influenza virus, wherein the combined hemagglutinin (HA) total is at least about 200 HA / dose of the vaccine composition, and wherein each of the strains presents at least about 128 HA / dose, and further wherein one of the strains has the same HA subtype as that of a challenge virus, and wherein at least one of the strains has a different NA subtype than the challenge virus.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +1
H9 subtype avian influenza virus isolate and vaccine composition prepared thereby
ActiveCN103789272AGood effectImprove immune efficiencyMicroorganism based processesAntiviralsHemagglutininAvian influenza virus
The invention discloses an avian influenza virus isolate belonging to an H9 subtype. An amino acid sequence of an HA1 structural domain of hemagglutinin has the following characteristic sites: 69-bit P, 180-bit A, 221-bit N and 236-bit R; the vaccine composition prepared by the H9 subtype avian influenza virus isolate with the following characteristic sites has good immune efficiency, and is superior to the vaccine prepared by the strain in the prior art in effect, cross protection can be provided for a popular wild strain, significant cross immunogen features are displayed, and the avian influenza virus isolate has a good application prospect in the aspect of preventing and treating poultry cross immune protection.
Owner:PU LIKE BIO ENG +1
Anti-fowl-plague Chinese medicine and preparing method
The present invention provides a Chinese medicine for resisting avian influenza virus and its preparation method. Said Chinese medicine is made up by using 9 Chinese medicinal materials of aspidium, isatis root, astragalus root, bupleurum root, forsythia fruit and others through a certain preparation process. Said invention also provides the concrete steps of its preparation process.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Functional influenza virus-like particles (VLPS)
InactiveUS20120207786A1SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
The present invention discloses and claims virus like particles (VLPs) that express and / or contains seasonal influenza virus proteins, avian influenza virus proteins and / or influenza virus proteins from viruses with pandemic potential. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing substantial immunity to either seasonal and avian influenza, or at least one symptom thereof.
Owner:NOVAVAX
GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases
ActiveCN102899424AStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationInfectious laryngotracheitisRespiratory tract disease
The invention discloses a GeXP rapid detection kit capable of simultaneously identifying nine pathogens of chicken respiratory tract diseases. The kit is used based on a GeXP system and comprises ten polymerase chain reaction (PCR) primer pairs; the kit is used for identifying and detecting avian influenza virus, H5, H7 and H9 subtype avian influenza virus, newcastle disease virus, infectious bronchitis, infectious laryngotracheitis, mycoplasma gallisepticum, bursa synovialis mycoplasma and haemophilus paragallinarum; and the kit is good in specificity, high in sensitivity and can detect 100 copy / mu l. Compared with an identifying result of the conventional experiment method of a pathogen separation and hemagglutination inhibition experiment or a serology experiment and the like, the GeXP rapid detection kit has the advantage that the coincidence rate reaches 100 percent. The kit is generally used for detecting the main chicken respiratory tract diseases and the pathogens thereof, so that a simple and high-flux detection kit and a detection system are provided, an actual requirement is met, and the application prospect is wide.
Owner:GUANGXI VETERINARY RES INST
Materials and methods for treating viral infections
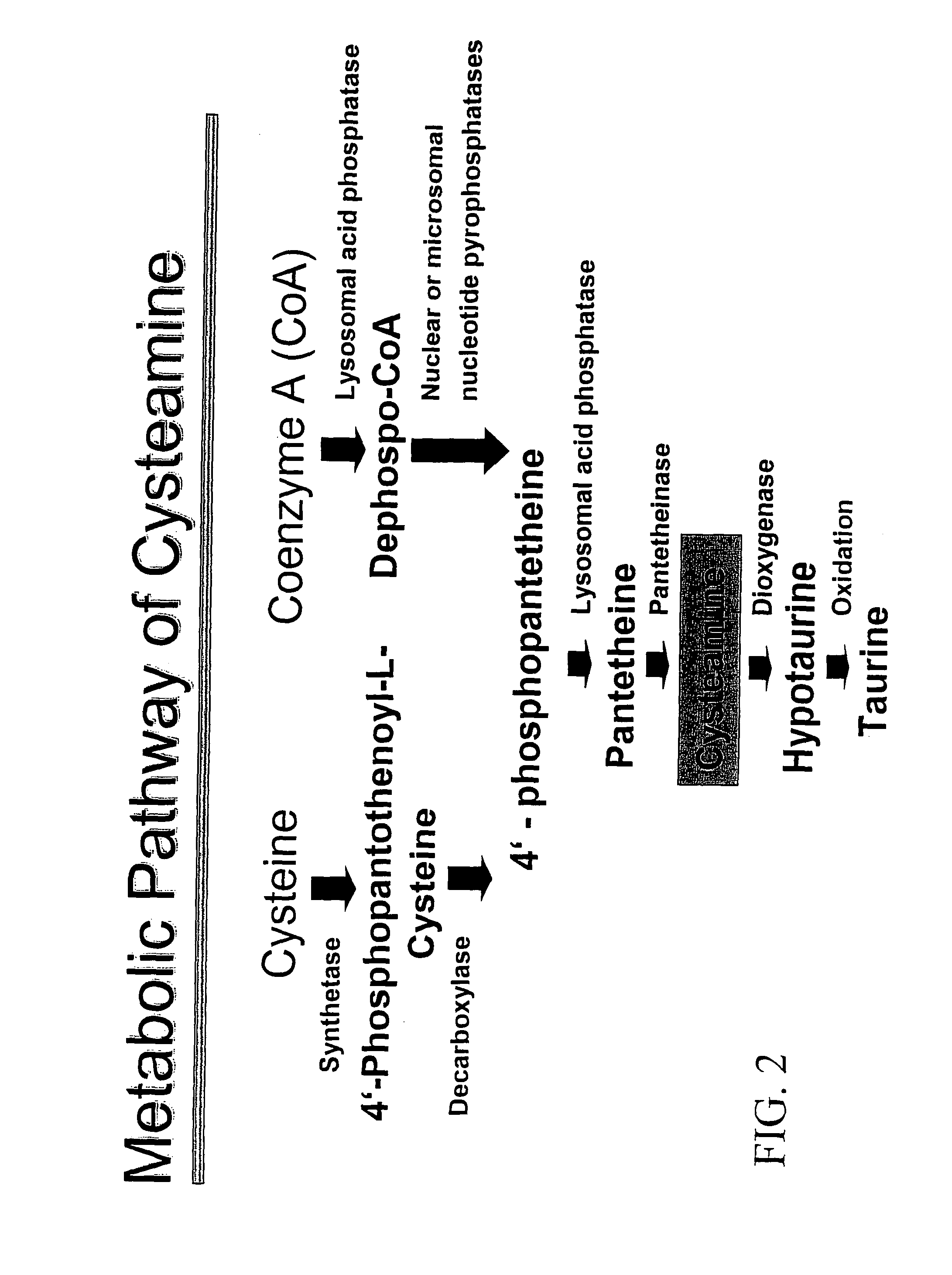
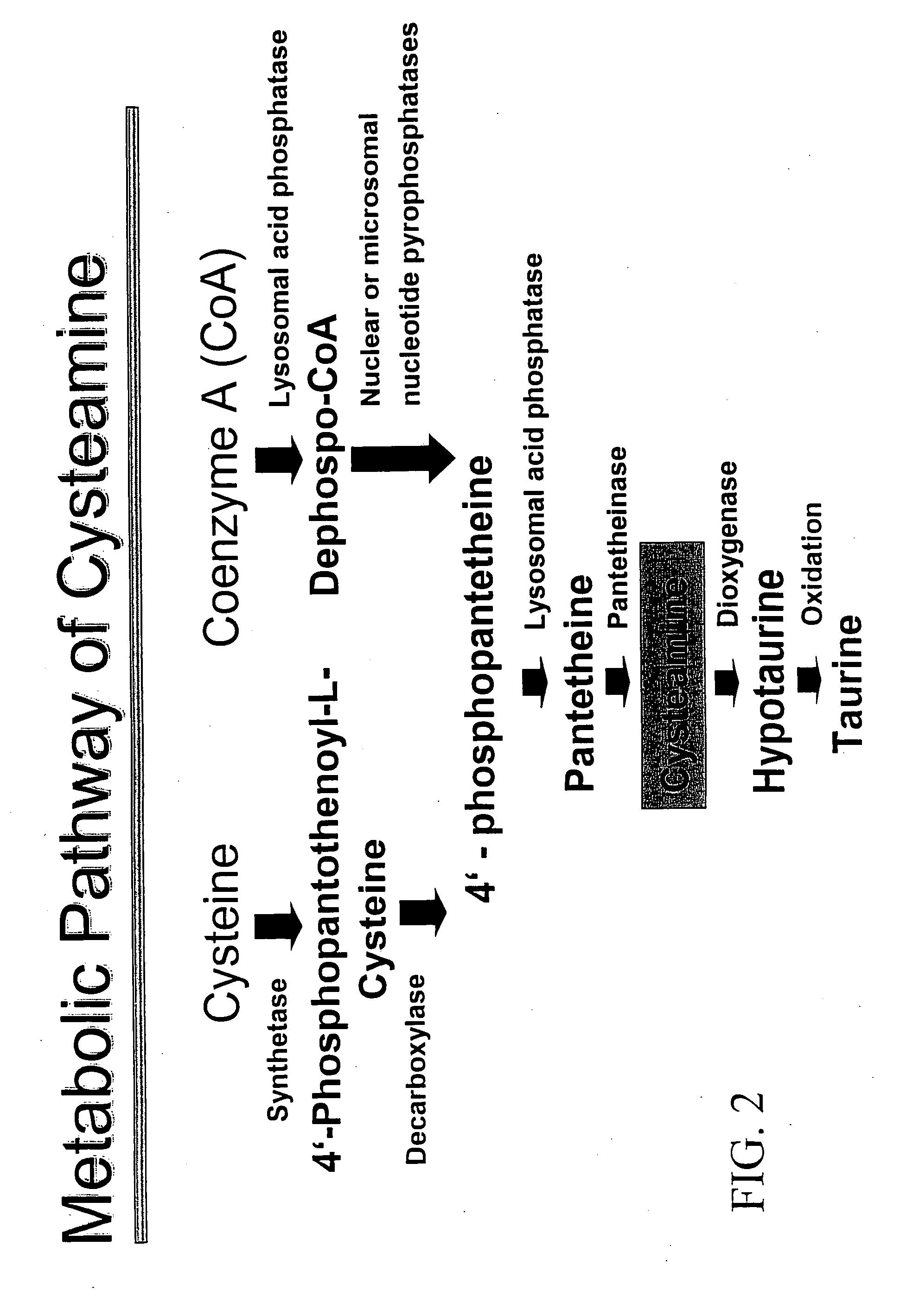
InactiveUS8415398B2Avoid infectionPrevent and delay developmentBiocideAntiviralsCysteamineAvian influenza virus
The subject invention provides materials and methods for treating various health conditions, including the prevention and / or treatment of a viral infection. In a preferred embodiment, a cysteamine compound is administered to a subject to treat an influenza virus infection. More preferably, a cysteamine compound is administered to a subject to treat influenza A, influenza B, influenza C virus infections, including avian influenza virus subtypes (such as H5N1 avian influenza virus).
Owner:OMEGA BIOPHARMA H K
Monoclonal antibodies binding to avian influenza virus subtype H5 haemagglutinin and uses thereof
Owner:XIAMEN UNIV +1
Gene encoding hemagglutinin protein of H5 avian influenza virus and its application
ActiveCN1632124AHigh level of immune responseImproving immunogenicityViral antigen ingredientsAntibody ingredientsHemagglutininHighly pathogenic
The present invention relates to an artificially synthesized gene optiHA containing codons for chicken partial tropism. Its reading frame contains 1707 bp nucleotides and encodes a total of 568 amino acids. The gene is compatible with H5 subtype highly pathogenic avian influenza virus A / Goose / GuangDong / 1 / 96(H5N1)[GD / 1 / 96(H5N1)]hemagglutinin (HA) gene has a nucleotide homology rate of 70%, an amino acid homology rate of 100%, and encodes the H5 subtype Hemagglutinin (HA) protein of avian influenza virus GD / 1 / 96 (H5N1). The invention also relates to the application of the gene as an immunogenic gene of H5 subtype influenza DNA vaccine and other genetic engineering vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
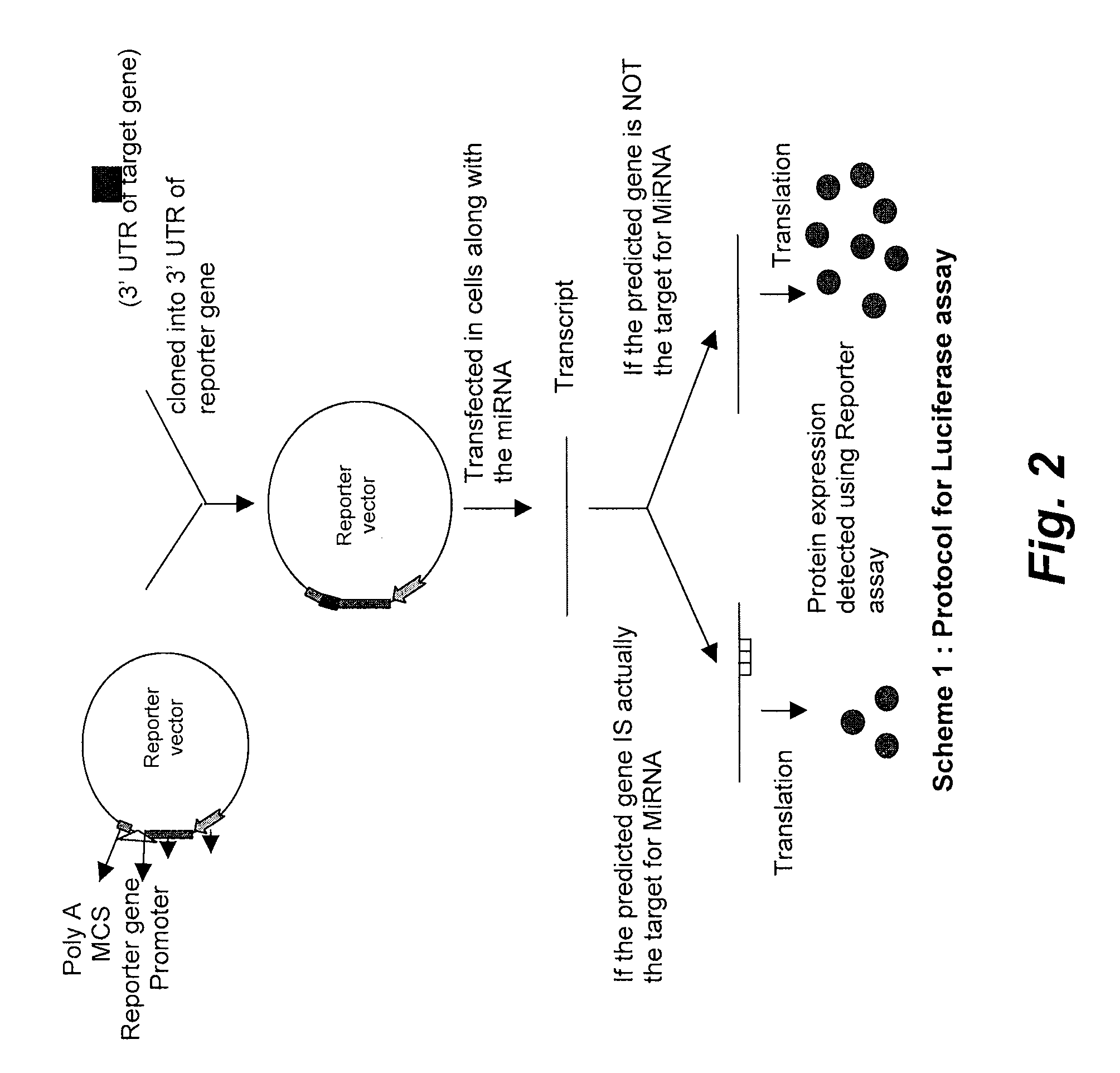
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
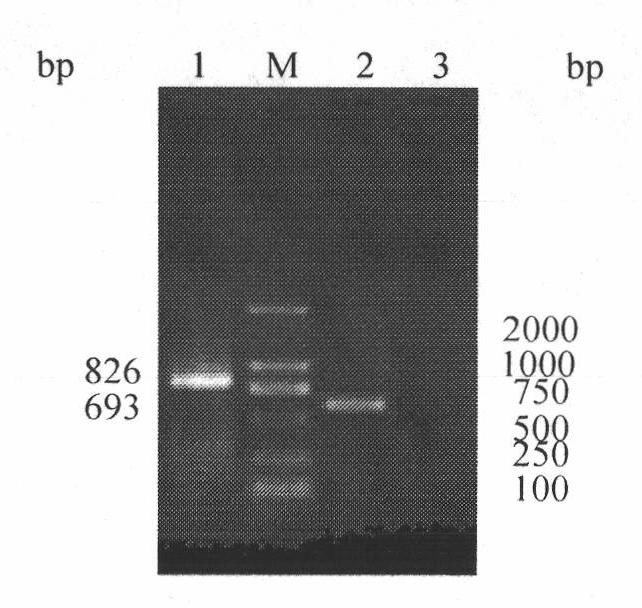
h5, h7, h9 subtype avian influenza virus detection kit
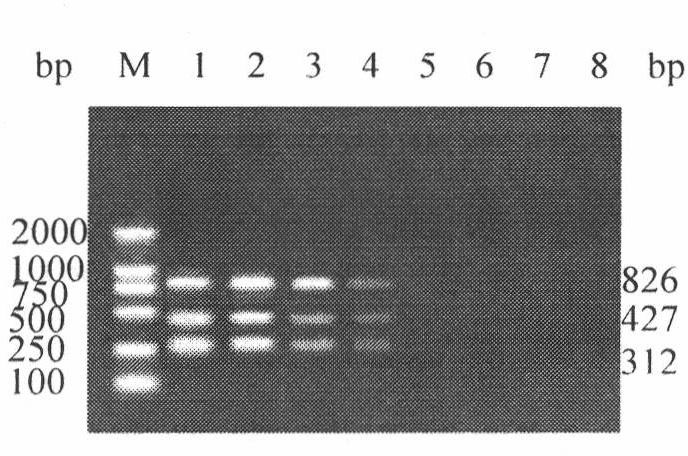
InactiveCN102260749AMicrobiological testing/measurementAvian influenza virusComplementary deoxyribonucleic acid
The invention discloses an H5, H7 and H9 subtype avian influenza virus detection kit. In the GenBank, three kinds of subtype HA gene sequences are searched, and specific primers are designed. By many tests and actual detections, three pairs of primers and reaction systems thereof are screened out and optimized, and the sizes of the amplified cDNA (complementary deoxyribonucleic acid) fragments are respectively 427bp, 312bp and 826bp. The result shows that by optimizing multiple RT-PCR (reverse transcription-polymerase chain reaction) amplification conditions, a multiple RT-PCR detection kit capable of detecting three kinds of subtype avian influenza viruses at the same time is prepared, and the minimum detectable quantity is 10pg. The detection kit does not have cross reaction to various kinds of chicken infectious diseases and normal structures, has strong specificity, and has consistent sensibility to three kinds of subtype detections. The avian influenza can be diagnosed in a shorttime in a primary laboratory and can be positioned in a subtype so as to gain the time for preventing and controlling the avian influenza, thus the spread of the avian influenza can be controlled in a small range as much as possible by adopting measures in time.
Owner:JILIN AGRICULTURAL UNIV
Use of a live attenuated Mycoplasma gallisepticum strain as a vaccine and vector for the protection of chickens and turkeys from respiratory disease
The present invention relates to a live cytadherence-deficient M. gallisepticum strain that does not express at least two of three proteins, the Gap-A molecule, crnA protein, and the 45 kDa protein, expressed by wildtype M. gallisepticum Strain R and its use as a vaccine for preventing and protecting birds, especially chickens and turkeys against the respiratory diseases attendant with wildtype Mycoplasma gallispeticum infection. The invention also relates to the use of the vaccine as a vector for the delivery of genes encoding protective antigens from other bacterial and viral avian pathogens, such as avian influenza virus. There is also disclosed a method for identifying the attenuated cytadherence-deficient M. gallisepticum Rhigh or a strain thereof.
Owner:UNIV OF CONNECTICUT
Vaccines for the rapid response to pandemic avian influenza
InactiveUS20070003576A1Reduce riskImprove the level ofSsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus influenza
The present invention relates to adenovirus-based vaccines against avian influenza viruses with pandemic potential. The present invention provides replication-defective adenoviral vectors, each having a nucleic acid encoding an influenza A polypeptide. When introduced into a subject, the expressed influenza A polypeptide induces the production of antibodies that bind to influenza. The present invention also provides methods for inducing an immune response in a subject. Subjects are administered a replication-defective adenoviral vector, wherein the vector has a nucleic acid encoding an influenza A polypeptide. When the vector is expressed in the subject, the influenza A polypeptide induces the subject to produce antibodies to influenza.
Owner:UNIVERSITY OF PITTSBURGH
Disinfectant with low corrosiveness
InactiveCN104621104AShort sterilization timeNo damageBiocideFungicidesBiotechnologyMedical equipment
The invention provides a disinfectant with low corrosiveness. The disinfectant comprises the following components by weight percent: 0.2-2.0% of ortho-phthalaldehyde, 1-10% of a synergist, 10-50% of a cosolvent, 0.5-5% of a buffering agent, 1-10% of a penetrant, 0.2-2% of a corrosion inhibitor, 0.1-5% of a chelating agent, 0.1-3% of a stabilizing agent and 45-80% of purified water. After the disinfectant is used, intestinal tract pathogenic bacteria, pyogenic coccus, hepatitis virus, H7N9 avian influenza virus, black variant spores of bacillus subtilis and other pathogenic microorganisms can be killed within a short time. Furthermore, the disinfectant adopts a slow release technique, thus having extremely low corrosiveness on metal and being very suitable for disinfecting medical equipment.
Owner:SHANDONG WEIGAO PHARM CO LTD
Monoclonal antibodies specific to the fusion peptide from hemagglutinin from influenza A viruses and uses thereof
ActiveUS8540995B2Effective protectionMicrobiological testing/measurementBiological material analysisHemagglutininHighly pathogenic
This invention relates to methods and products for the diagnosis, surveillance, prevention, and treatment of influenza A virus infections in animals and humans. More particularly, the invention relates to antibodies and related binding proteins for the detection, prevention and treatment of influenza A viruses. The monoclonal antibodies and related binding proteins of the invention are useful for the treatment of the highly pathogenic H5 subtypes of avian influenza virus (AIV).
Owner:TEMASEK LIFE SCIENCES LABORATORY
Preparation method of avian influenza virus growing in serum-free full-suspended cultured MDCK cells and obtained avian influenza virus
ActiveCN106011083AStable characteristicsGet rid of dependenceVertebrate cellsArtificial cell constructsSerum freeF1 generation
The invention discloses a preparation method of an avian influenza virus suitable for growing in a serum-free full-suspended cultured MDCK cell line and the avian influenza virus obtained through the method. The preparation method comprises the following steps of 1 preparation of the MDCK cells to be inoculated; 2 virus seed preparation, wherein a chick embryo source avian influenza virus is prepared; 3 F1 generation virus domestication; 4 F2 generation virus domestication, wherein a supernatant sample retained at the time point when the blood clotting titer of the avian influenza virus obtained in the F1 generation is highest is taken, and the step 3 is repeated; 5 F3 generation virus domestication, wherein the F3 generation virus culturing temperature is 35 DEG C; 6 F4 generation-F10 generation virus domestication, wherein the step 5 is repeated, and the domesticated avian influenza virus is obtained. According to the preparation method, through a domestication method, the avian influenza virus is directly domesticated to completely adapt to be efficiently reproduced on the serum-free full-suspended cultured MDCK cells from the mode of being cultured by a chick embryo, the domestication efficiency is high, the avian influenza virus can be efficiently infected and copied in the MDCK cells, and the virus characteristic is stable.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Sample pad processing liquid, H7 subtype avian influenza virus colloidal gold test strip and preparation method thereof
The invention discloses a sample pad processing liquid, a H7 subtype avian influenza virus colloidal gold test strip and a preparation method thereof, the processing liquid is prepared as follows: taking 8-20g of PEG(polyethylene glycol)-200, 11-25g of PVP (polyvinyl pyrrolidone), 10-45g of BSA (bovine serum albumin), 0.5-30ml of Triton X - 100, and 0.8-15ml of Twain-20, then using 5mM-10mM phosphate buffer solution to fix the volume to 1L, and adjusting the pH value to 9.5; and the colloidal gold test strip is formed by overlapping and pasting in turn a sample pad, a glass fiber membrane, a cellulose nitrate membrane and absorbent paper on a bottom plate, and the sample pad is processed by the processing liquid. The processing liquid can increase the sample pad hydrophilicity, contributes to the sample pad fast wetting, and promotes the chromatographic effect, aqueous solution formed by dissolving large molecules in water has suspension and dispersion effect, and can protect virus antigen in a sample. The test strip has the advantages of safe and simple operation, strong specificity, high sensitivity, and low rate of cross infection.
Owner:GUANGZHOU WONDFO BIOTECH
MAb-based Dot-ELISA method and assay kit for the detection of viruses
A monoclonal antibody-based Dot-ELISA assay for the rapid detection of animal viruses such as avian influenza virus. The assay includes applying a specimen suspected of containing an animal virus on a porous membrane and treating the specimen with a solution of citric acid or lactic acid and a solution containing a mucolytic agent and a detergent. The treated specimen is then contacted with a primary monoclonal antibody for detecting the virus. If present, the primary moncolonal antibody bind with an antigen of the animal virus specimen. The specimen is contacted with an anti-monoclonal antibody conjugate (secondary antibody) and incubated to facilitate binding of the antigen-bound monoclonal antibody to the conjugate. The bound conjugate and antigen-bound monoclonal antibody is contacted with a coloring reagent to allow visual detection of the presence of the animal virus in the specimen.
Owner:PENN STATE RES FOUND
Kit box used for detecting influenza virus in sample and detection method and application thereof
ActiveCN104062430ARealize the immune cascade effectSimple resultBiological material analysisImmunoglobulinsAvian influenza virusMonoclonal antibody
The invention provides a kit box used for detecting an influenza virus in a sample. The kit box comprises a buffer solution supply unit and a detection test strip. The detection test strip sequentially comprises a substrate supply area, a sample supply area and a detection area in the longitudinal direction. The substrate supply area comprises a substrate pad (3), a dry enzyme substrate is adsorbed to the substrate pad, and the substrate pad (3) makes contact with a nitrocellulose membrane (1). The sample supply area comprises an enzyme mark pad (2), a rat resistance avian influenza virus nucleoprotein antibody marking monoclonal antibody 1 is adsorbed to the enzyme mark pad (2), and the enzyme substrate can product a chromogenic reaction with enzymes marked on the marking monoclonal antibody 1. The detection area is provided with a rat resistance avian influenza virus nucleoprotein antibody fixed monoclonal antibody 2 in an immobilization mode. The invention further provides a method for detecting the influenza virus in the sample through the kit box. The kit box and the detection method of the kit box have the advantages of being rapid and accurate in detection, high in specificity, sensitive and good in stability.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Pure traditional Chinese medicine composition for treating avian influenza, preparing method and application thereof
InactiveCN101584806AGood inhibitory effectGood effectAntiviralsAluminium/calcium/magnesium active ingredientsSide effectAvian influenza virus
The present invention relates to a pure traditional Chinese medicine composition for treating avian influenza, which can be used for preventing or treating the avian influenza of chicken and duck. The pure traditional Chinese medicine composition is prepared by mixing a pure traditional Chinese medicine compound A, a pure traditional Chinese medicine compound B and a pure traditional Chinese medicine compound C, wherein the proportioning ranges are respectively as follows: 180g-420g, 80g-100g and 260-510g. The method for preparing the pure traditional Chinese medicine mainly comprises four steps of mixing, grinding, stirring and placing in an oxygen-deficient environment. In the specific application process, the proportion of the three pure traditional Chinese medicines by weight is 4: 1: 3. The medicine of the invention which is prepared by natural plant has the advantages of no side effect, ideal function, strong function, excellent safety, ideal effectiveness, convenient using and no effect for the egg laying rate of domestic birds of chicken, duct, etc. Furthermore the color of the egg is red and bright and the egg shell is smooth. The immunity of the domestic bird can be increased when the traditional Chinese medicine is used periodically. The traditional Chinese medicine of the invention develops a novel path for preventing the avian influenza virus.
Owner:韩四缺
Materials and methods for treating viral infections
InactiveUS20070135525A1Avoid infectionPrevent and delay developmentBiocideAntiviralsCysteamineAvian influenza virus
The subject invention provides materials and methods for treating various health conditions, including the prevention and / or treatment of a viral infection. In a preferred embodiment, a cysteamine compound is administered to a subject to treat an influenza virus infection. More preferably, a cysteamine compound is administered to a subject to treat influenza A, influenza B, influenza C virus infections, including avian influenza virus subtypes (such as H5N1 avian influenza virus).
Owner:OMEGA BIOPHARMA H K
Monoclone antibody of H5 subtype avian influenza virus haemagglutinin protein, or its combination liveness fragment and uses thereof
The invention provides a McAb that can be specifically binded with the HA protein of H5N1 avain influenza virus and a McAb that can prevent from at least 50 percent of McAb activity combining with the HA protein of H5N1 avain influenza virus, which can be used for detecting, diagnosing, preventing and treating the avain influenza virus, in particular to the H5N1 avain influenza virus. The invention also provides the relating hybridoma cell lines, the separated nucleic acid molecule and short peptide, as well as the drug combination, the medicine diagnosis equipment and the kit with the McAb included.
Owner:XIAMEN UNIV +1
Flu/human avian influenza virus detection gene chip and production method and use
InactiveCN101392302AShort detection timeImprove detection accuracyMicrobiological testing/measurementHighly pathogenicTyping
The invention discloses an influenza / human and avian influenza virus detection gene chip and a preparation method and an application thereof, 102 probes used for detecting A type, B type, H1N1 hipotype, H3N2 hipotype, H5N1 hipotype and H9N2 hipotype influenza viruses and 4 highly pathogenic differential diagnosis probes, as well as a process of sample treatment, hybridization, elution and chip identification which can carry out detection, typing or identification over the 6 types of viruses simultaneously and can identify the high pathogenicity of viruses. The invention not only greatly shortens the detection time of influenza viruses, but also improves the detection accuracy and provides a feasible technique support for the early warning mechanism of influenza epidemic situation in fields of clinical diagnosis, inspection quarantine, and the like.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
Monoclonal antibodies specific to hemagglutinin from influenza virus H5-subtype and uses thereof
ActiveUS8444986B2SsRNA viruses negative-senseMicrobiological testing/measurementHemagglutininAvian influenza virus
Monoclonal antibodies and related binding proteins that are specific for conformational epitopes of avian influenza virus K5 subtype hemagglutinin glycoprotein are provided. The antibodies can be used for the detection and treatment of H5 subtype AIV in specimens.
Owner:TEMASEK LIFE SCIENCES LABORATORY +1
Separation identification and purification process for chicken source H9N2 avian influenza virus strain and uses thereof
The invention relates to the field of animal virology, and provides separation identification and purification methods, biological characteristics and applications in biology of a recombinant fowl H9N2 avian influenza virus strain with preservation number being CCTCC NO:V200811; the differences between the virus and other separation strains are explained in term of molecular level; and the virus strain is proved to be capable of being used as candidate strain of avian influenza vaccines and as antigen of H9 subtype AIV hemoagglutination and hemoagglutination inhibition test (HA-HI). The strain has significance in knowing the molecular evolution and antigenic variation of fowl H9N2 avian influenza virus in China.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com