Patents
Literature
156 results about "Virus types" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Three types of viruses cause influenza, more commonly known as the flu. Influenza virus types A and B cause seasonal flu infections, which typically occur from late fall through early spring. Influenza type C infections occur far less frequently and typically cause a mild form of the illness.
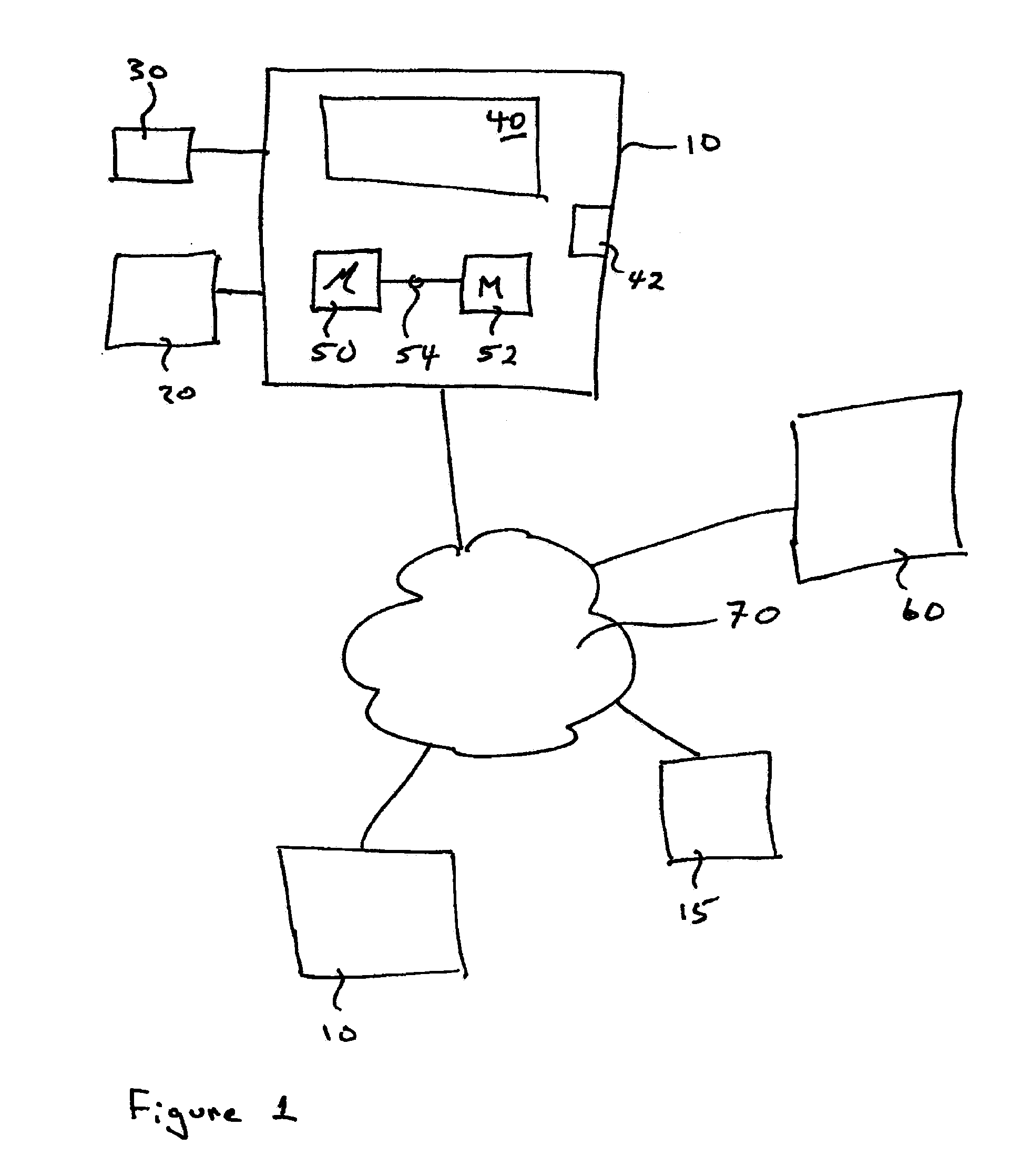
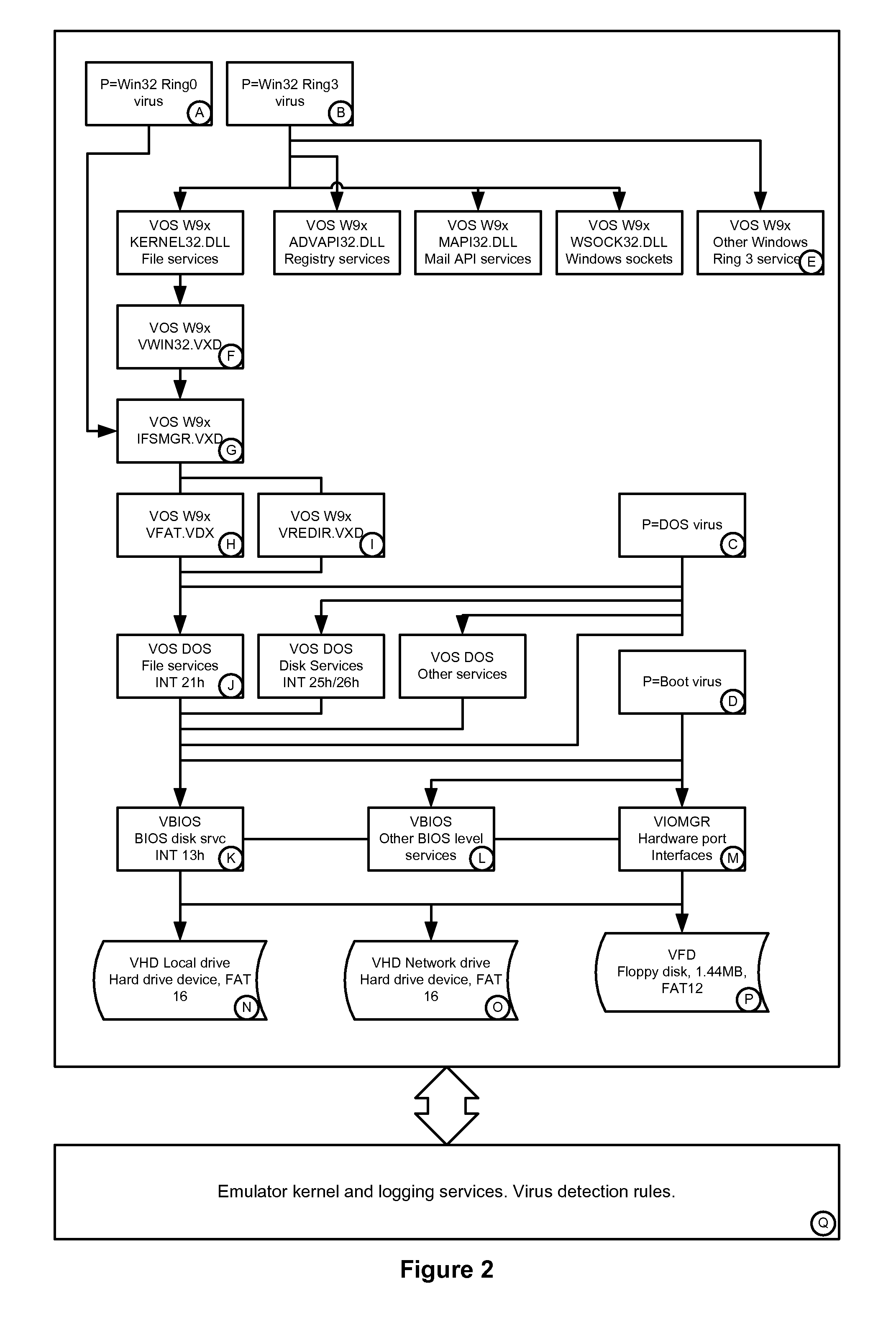
Simulated computer system for monitoring of software performance
InactiveUS7356736B2Reduce riskMemory loss protectionError detection/correctionVery low riskOperational system
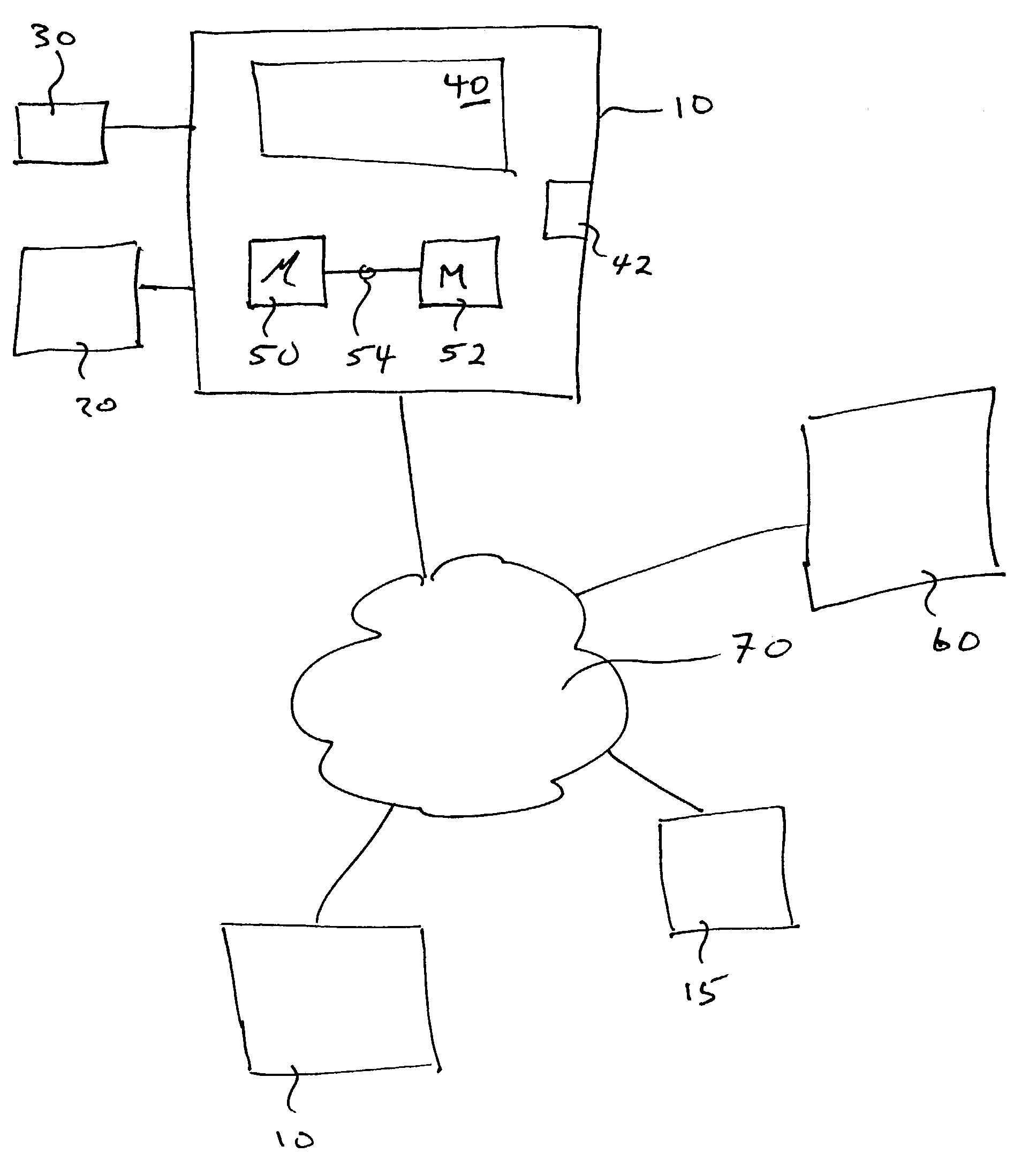
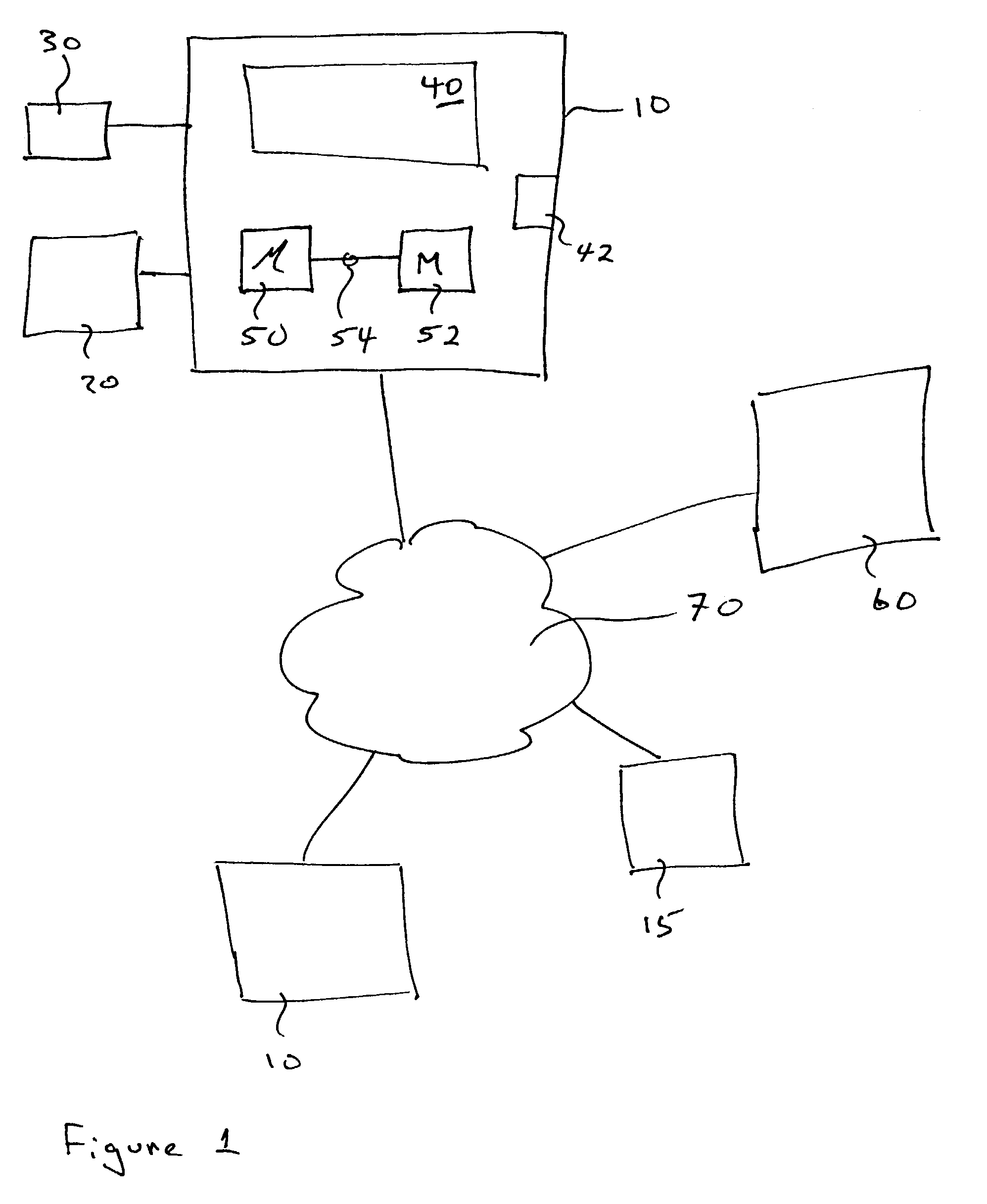
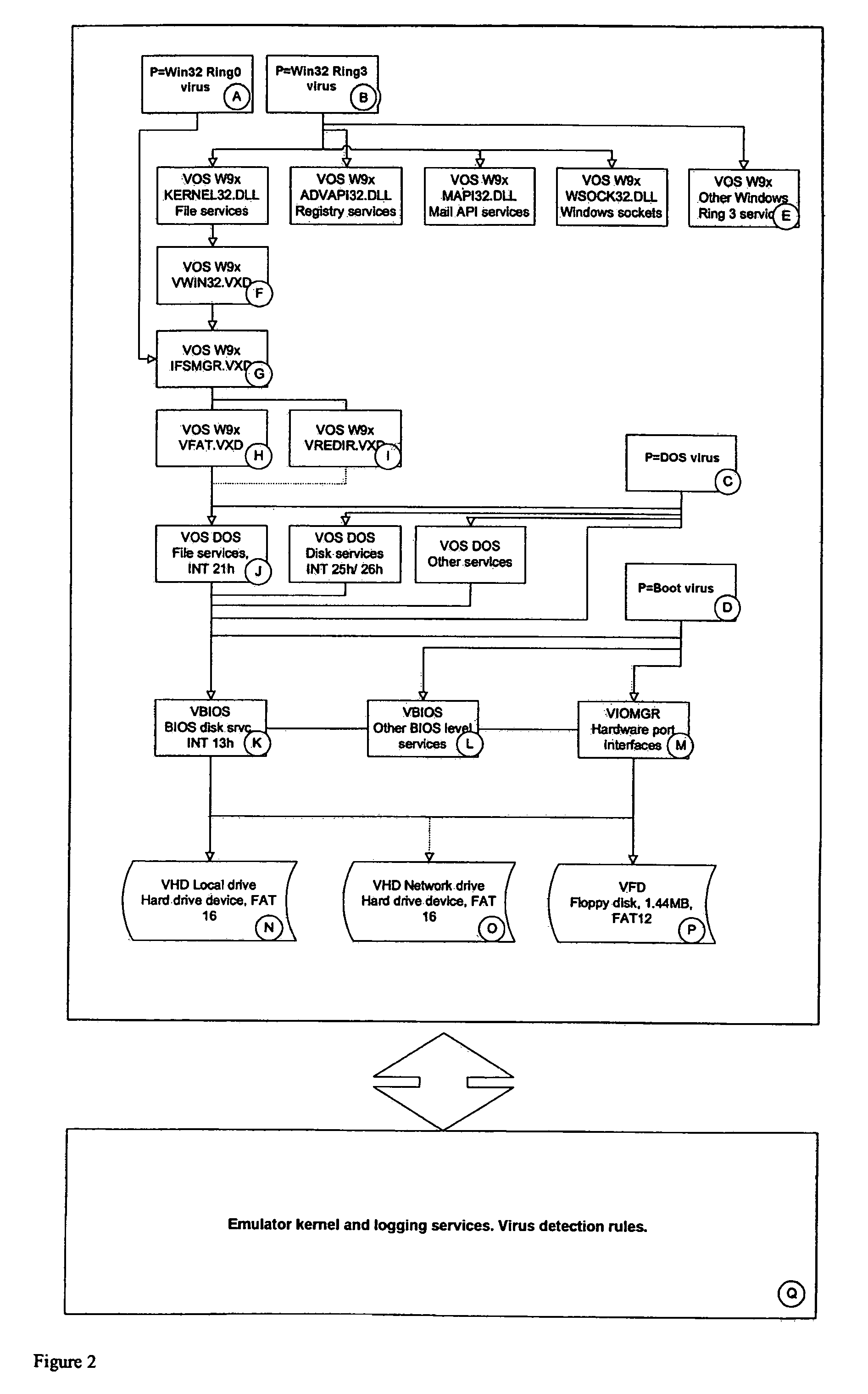
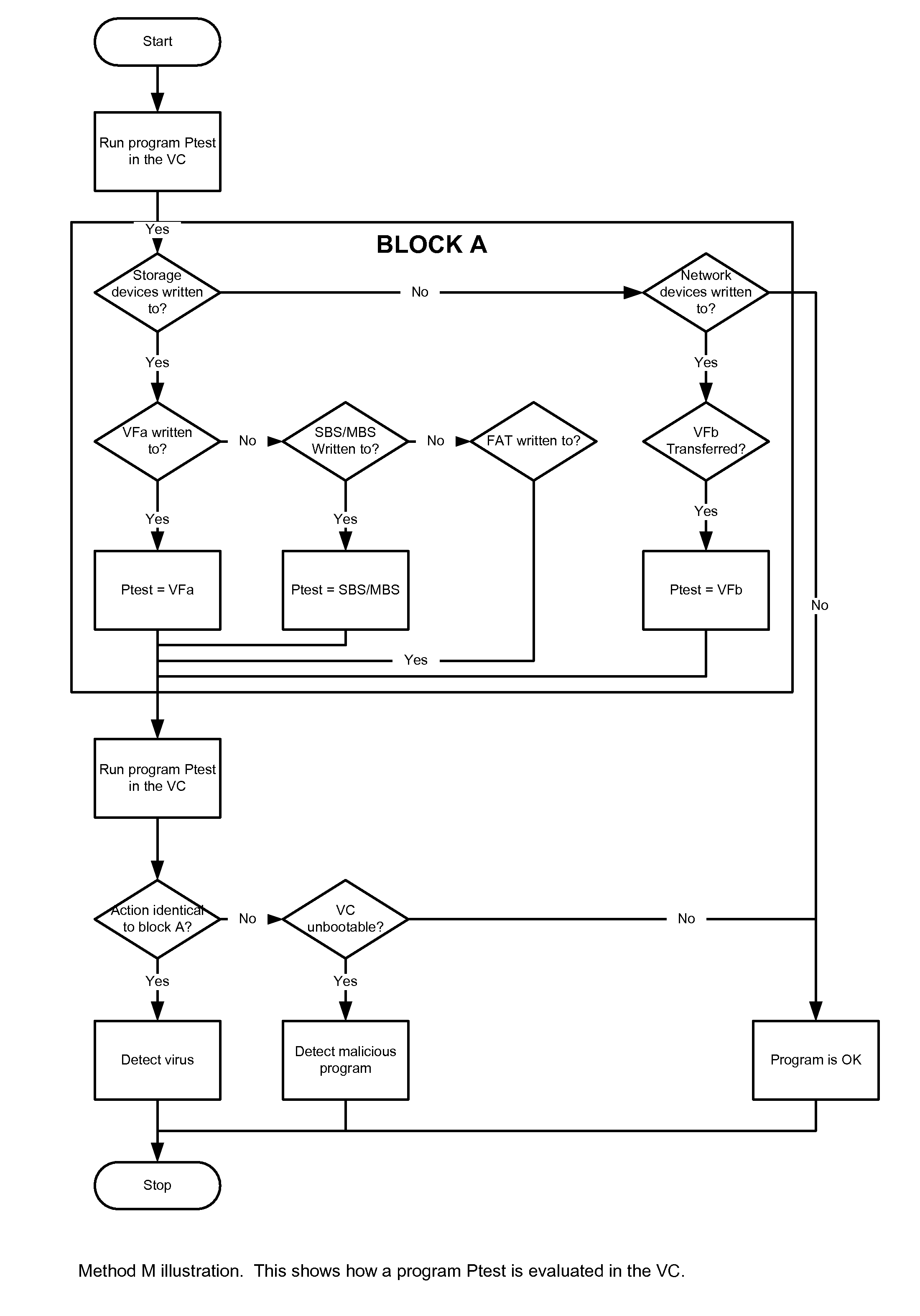
A system S is defined which is capable of simulating a computer (virtual computer, VC) for the purpose of software performance monitoring. The system is implemented as a set of software modules (SM) that can be exchanged to change the behavior of the VC. The VC is driven by a CPU emulator, and can run any operating system (virtual operating system, VOS) that is supported by the available SM's. The system is designed to log accesses to system resources and the nature of these accesses. The system is particularly useful for determining whether an executable or file contains an unknown virus, with a very low risk of false positives. Detected viruses include encrypted, polymorphic, metamorphic and other virus types.
Owner:CA TECH INC
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Simulated computer system for monitoring of software performance
InactiveUS20080201129A1Reduce riskMemory loss protectionError detection/correctionVery low riskOperational system
A system S is defined which is capable of simulating a computer (virtual computer, VC) for the purpose of software performance monitoring. The system is implemented as a set of software modules (SM) that can be exchanged to change the behavior of the VC. The VC is driven by a CPU emulator, and can run any operating system (virtual operating system, VOS) that is supported by the available SM's. The system is designed to log accesses to system resources and the nature of these accesses. The system is particularly useful for determining whether an executable or file contains an unknown virus, with a very low risk of false positives. Detected viruses include encrypted, polymorphic, metamorphic and other virus types.
Owner:CA TECH INC
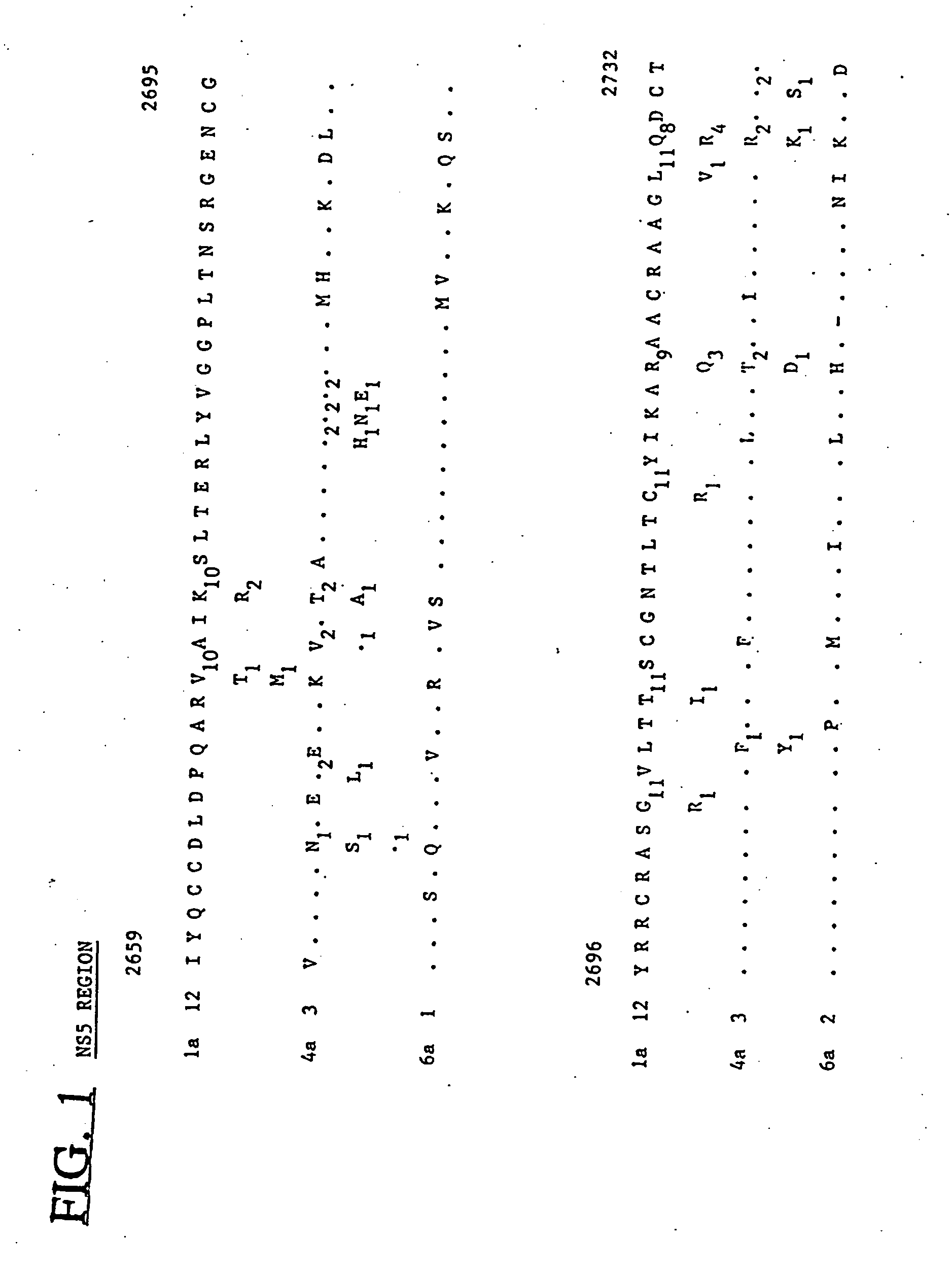
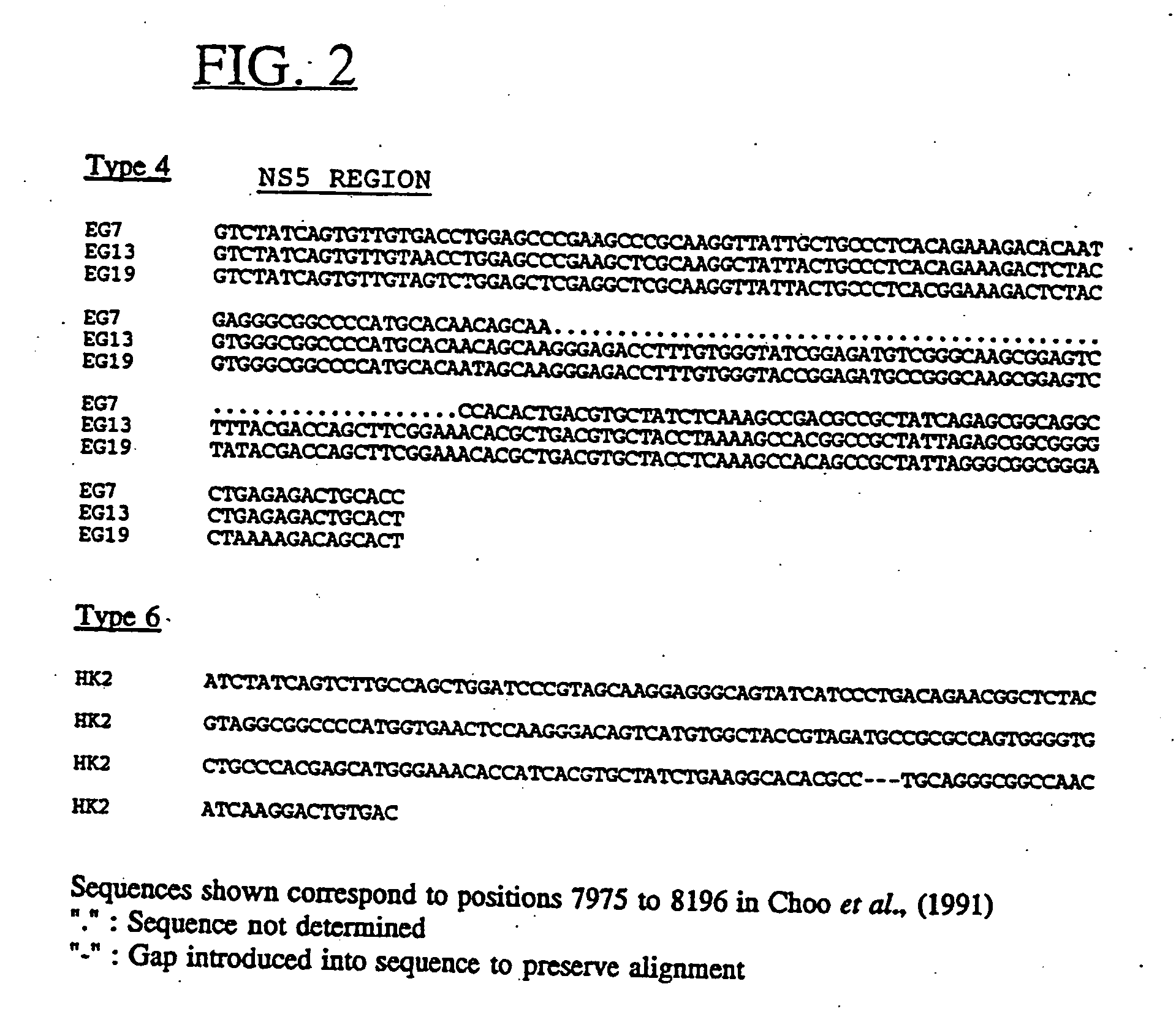
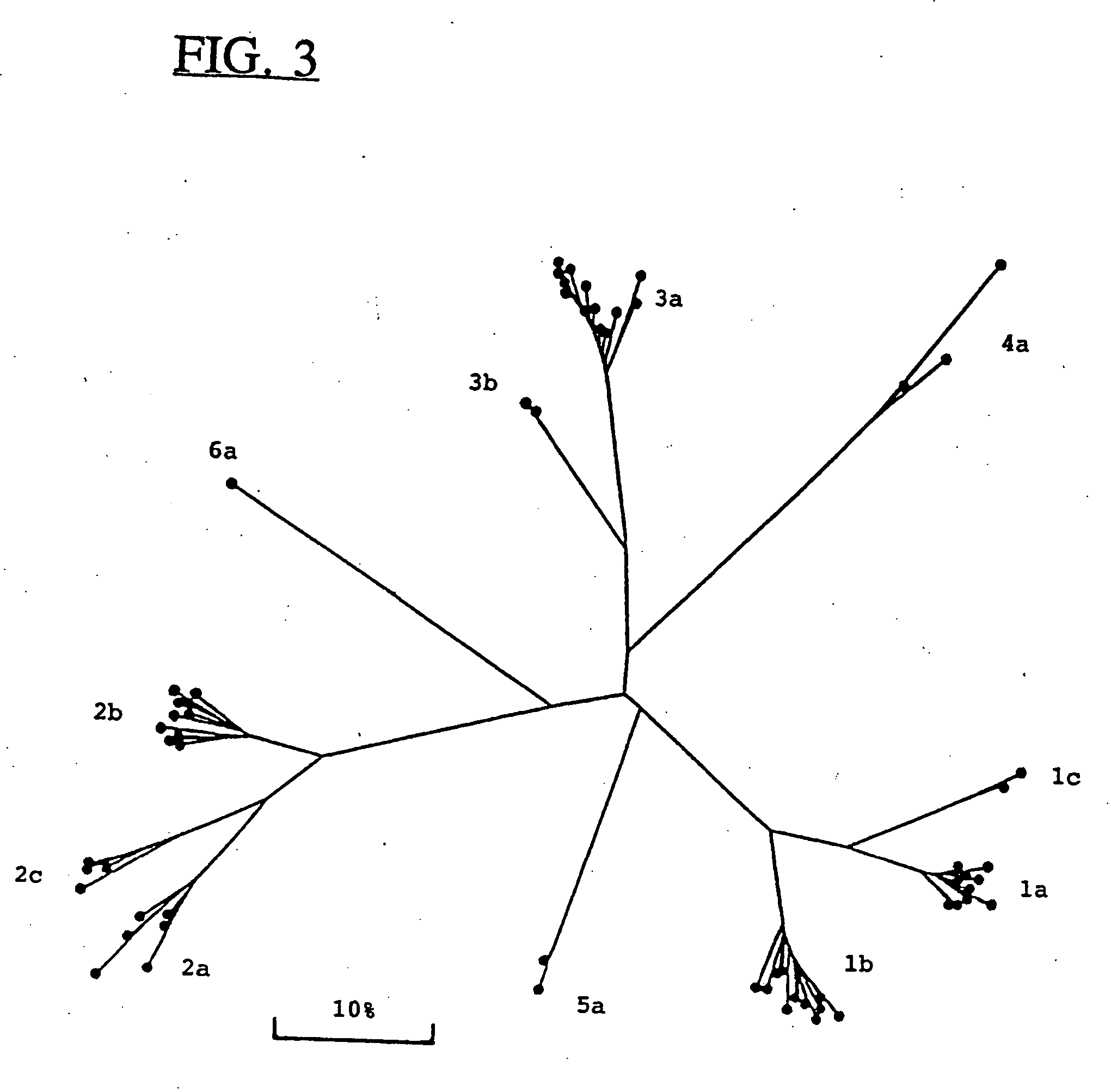
Hepatitis-C virus type 4, 5, and 6
InactiveUS6881821B2Good effectStimulate immune responseVirusesPeptide/protein ingredientsType specificGene
Newly elucidated sequences of hepatitis C virus type 4 and type 5 are described, together with those of a newly discovered type 6. Unique type-specific sequences in the NS4, NS5 and core regions enable HCV detection and genotyping into types 1 to 6. Antigenic peptides and immunoassays are described.
Owner:COMMON SERVICES AGENCY +1
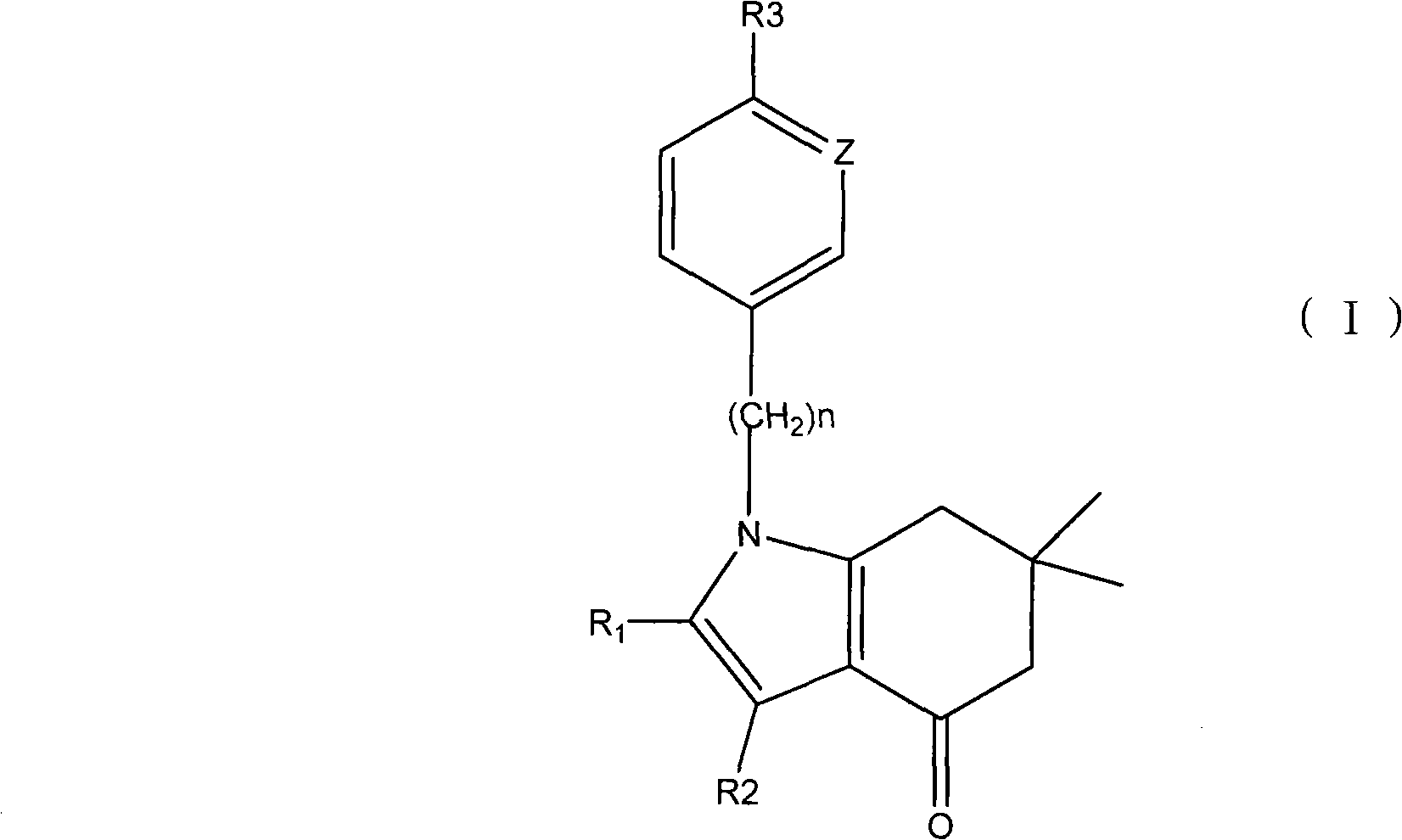
Applications of tetrahydroindolone/tetrahydroindazolone/tetrahydrocarbazole derivatives and salts thereof in preparation of antiviral medicine
The invention relates to an application of tetrahydroindolone / tetrahydroindazolone / tetrahydrocarbazole derivatives and salts thereof in the preparation of antiviral drugs. The tetrahydroindolone derivatives, the tetrahydroindazolone derivatives or the tetrahydrocarbazole derivatives, as well as the salts thereof of the invention have anti-viral functions, and the virus comprises I-typed and II-typed herpes virus, coxsackie virus type 3 (CVB3) and hepatitis B virus.
Owner:广州少伯控股集团有限公司 +1
Reagents and kits for detection of influenza virus and the like
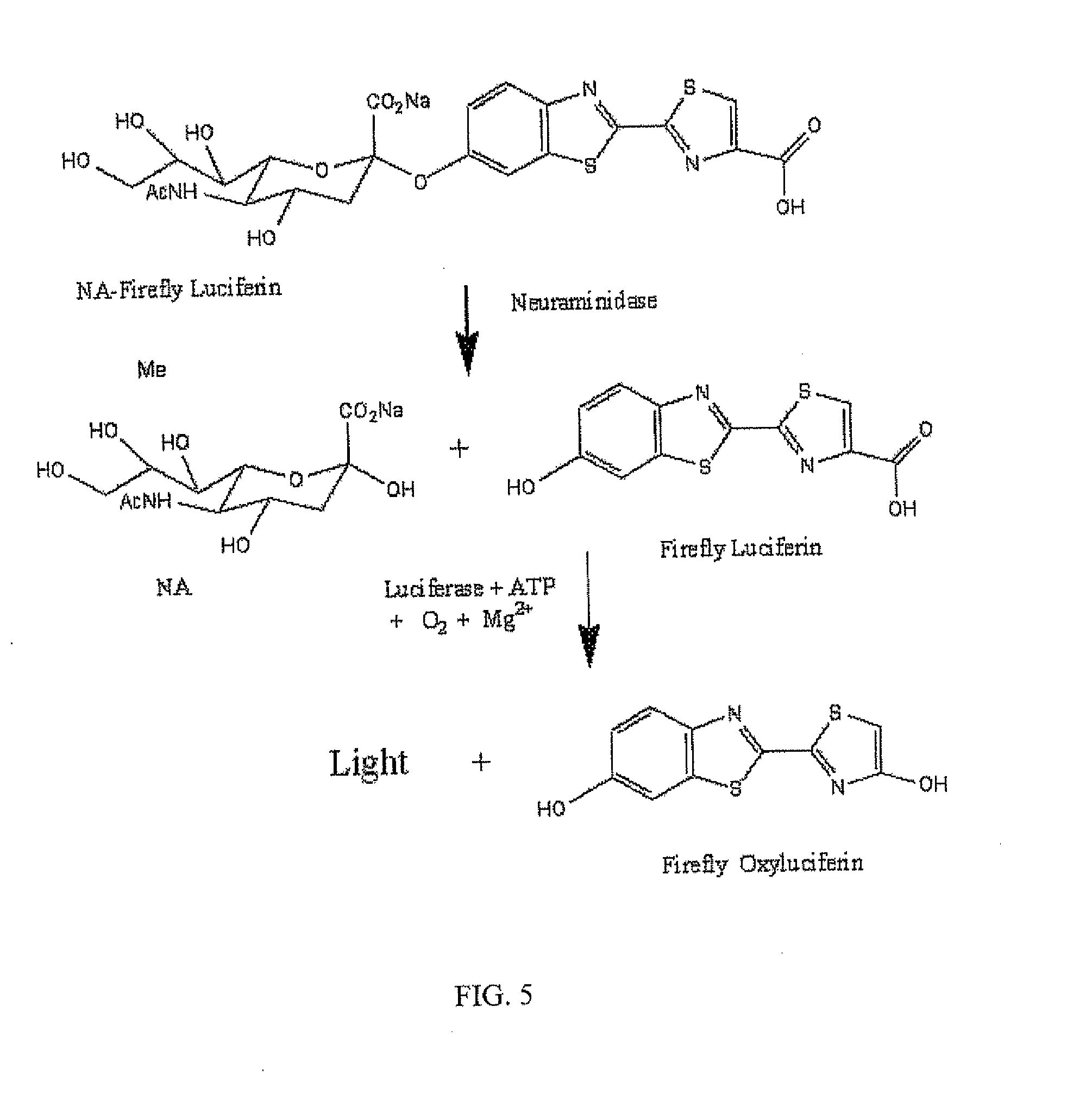
ActiveUS20110189655A1Simple and rapid and specific and sensitive detectionHigh detection sensitivitySugar derivativesMicrobiological testing/measurementNeuraminidaseFirefly luciferin
The present invention relates to reagents and methods for influenza virus detection. These reagents and methods disclosed in the present invention enable simple, rapid, specific and sensitive detection of influenza virus types A and B. These reagents are N-acetylneuraminic acid-firefly luciferin conjugates which can be cleaved by influenza virus neuraminidase.
Owner:CELLEX INC
Aav vector and assay for Anti-aav (adeno-associated virus) neutralizing antibodies
ActiveUS20160123990A1Microbiological testing/measurementGenetic material ingredientsAnti virusViral antibody
Virus vectors, virus particles, and methods and uses of screening for, detecting, analyzing and determining amounts of virus antibody, or neutralizing antibody activity of samples are provided. Such virus vectors, virus particles, and methods and uses are applicable to a broad range of virus types, such as lentiviruses, adenovirus, and adeno-associated virus (AAV) serotypes. Methods and uses include virus antibody screening, such as anti-virus immunoglobulins screened for, detected, analyzed and amounts determined
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Network information security protection system based on Internet
InactiveCN112187792AImprove securityReduce the risk of lossPlatform integrity maintainanceTransmissionThe InternetInformation security
The invention discloses a network information security protection system based on the Internet. The network information security protection system comprises a cloud platform, a registration and loginunit, a database, an information auditing unit, an intrusion detection module, a user management module, an alarm unit and a protection unit. The method comprises the following steps: detecting systemdata through an intrusion detection module, acquiring illegal access times, virus type quantity and vulnerability quantity of a system, acquiring a system intrusion coefficient Y through a formula, and comparing the system intrusion coefficient Y with an intrusion coefficient threshold value: if the system intrusion coefficient Y is less than or equal to the intrusion coefficient threshold value,judging that intrusion does not exist in the system; generating a system security signal and sending the system security signal to the cloud platform; if the system intrusion coefficient Y is greaterthan the intrusion coefficient threshold, judging that intrusion exists in the system, generating a system danger signal and sending the system danger signal to an alarm unit. After the system is detected, viruses are eliminated before data loss of the system, so that the data security is improved, and the risk of data loss is reduced.
Owner:安徽思珀特信息科技有限公司
Hepatitis-C virus type 4, 5 and 6
The present invention relates to a polynucleic acid composition comprising or consisting of at least one polynucleic acid containing 8 or more contiguous nucleotides corresponding to a nucleotide sequence from the region spanning positions 417 to 957 of the Core / E1 region of HCV type 3; and / or the region spanning positions 4664 to 4730 of the NS3 region of HCV type 3; and / or the region spanning positions 4892 to 5292 of the NS3 / 4 region of HCV type 3; and / or the region spanning positions 8023 to 8235 of the NS5 region of the BR36 subgroup of HCV type 3a; and / or the coding region of HCV type 4a starting at nucleotide 379 in the core region; and / or the coding region of HCV type 4; and / or the coding region of HCV type 5, with said nucleotide numbering being with respect to the numbering of HCV nucleic acids as shown in Table 1, and with said polynucleic acids containing at least one nucleotide difference with known HCV type 1, and / or HCV type 2 genomes in the above-indicated regions, or the complement thereof.
Owner:COMMON SERVICES AGENCY
Gene chip and method for detecting classical swine fever virus (CSFV), porcine circovirus virus type 2 (PCV-2) and porcine reproductive and respiratory syndrome virus (PRRSV)
InactiveCN102234693AStrong specificityEnsure consistencyMicrobiological testing/measurementFluorescence/phosphorescenceClassical swine fever virus CSFVDisease
The invention provides a gene chip detection method for a classical swine fever virus (CSFV), a porcine circovirus virus type 2 (PCV-2), a porcine reproductive and respiratory syndrome virus Europe type (PRRSVE) and a porcine reproductive and respiratory syndrome virus America type (PRRSVA). A gene chip is shown as a probe sequence in the table of the specification. The invention discloses a method for simultaneously detecting three diseases, namely classical swine fever (CSF), a porcine circovirus type 2 (PCV-2) and a porcine reproductive and respiratory syndrome (PRRS) by using the gene chip. By the method, the problems that the conventional detection technology is time-consuming and labor-consuming and has low specificity, or only can be used for detecting a single disease are solved. All probes provided by the invention are synthesized into a connecting amino group at a 5' end, a poly T connecting arm with the length of 15 bp is provided, and a result shows that fixing efficiency is relatively high. By a polymerase chain reaction (PCR) amplification technology with mark primers, various kinds of fluorescent mark deoxyribonucleic acid (DNA) complementary to corresponding probescan be amplified at a time, and the high specificity and sensitivity of nucleic acid hybridization in a solid-liquid phase can be ensured. In addition, by a mark method, detection time is greatly shortened, the cost of chip detection is lowered, and the detection method is more suitable to be popularized in clinical application.
Owner:CHONGQING UNIV OF TECH
Analytical method of components of fatty acid contained in listeria cells
InactiveCN101936960ABreaking the limits of taxonomyBreak the limitsComponent separationCytochemistryFatty acid
The invention relates to an analytical method of components of fatty acids contained in listeria cells, in particular to a method for carrying out bacteria classification by adopting a cell chemical analysis method, belonging to the technical field of biological engineering. The method mainly comprises the following steps of: rejuvenating listeria; respectively separating and purifying the listeria by using an OXA agar plate and a trypticase soy yeast extract agar plate; culturing the listeria of a single typical colony, and preparing into a bacterial suspension; carrying out inactivation processing by using formaldehyde; averagely filling the bacterial suspension processed by the formaldehyde into centrifuge tubes for washing; carrying out methyl esterification by using a mixed solution of hydrochloric acid and the formaldehyde; and carrying out GC-MS (Gas Chromatograph-Mass Spectrometer) analysis on the prepared fatty acids. The method can not only break through the limit of the traditional bacterial taxonomy and reduce the errors brought about by anthropic factors on the traditional morphological taxonomy, but also provide a strong and powerful tool for the classification and the identification of new strains and virus types; and in addition, the method can be used for identifying the listeria from other bacteria and foods.
Owner:HUBEI INSPECTION & QUARANTINE TECH CENT
A kind of o/asia type I foot-and-mouth disease virus bivalent genetic engineering polypeptide vaccine and its preparation method and application
InactiveCN102274496AEffective controlNo pollution in the processBacteriaMicroorganism based processesInclusion bodiesAdjuvant
The invention relates to an O / Asia I type foot and mouth disease virus bivalent genetic engineering polypeptide vaccine, its preparation method and its purpose. The method comprises the following steps: selecting two serotypes of O type and Asia I type, taking B cell determinant 15 amino acid fragments of VP1 and T-cell helper of VP4, performing a series connection, cloning without containing carrier protein, constructing O / Asia I gene engineering bacteria. An antigen protein product can be obtained after passing through the processes of high density fermenting, cell disrupting, inclusion body renaturating, fusion protein separating, and is homogenized with an adjuvant to form the O / Asia I type foot and mouth disease virus bivalent genetic engineering polypeptide vaccine. The vaccine of the present invention contains 2<n-1> polypeptide connected in series which is coded by a nucleic acid sequence shown in SEQ ID, wherein, n is an integer of 1-5. The invention has the advantages of good security and high immune efficacy, and can be used once in half year for immunization; and is suitable for large scale production and convenient preservation and transportation; and is capable of effectively preventing and controlling two serotypes foot and mouth disease of O type and Asia I type which is useful in our country; foot and mouth disease virus non-structural protein 3A.B. will not generate, so that the infective animals can be differentiated easily.
Owner:吴晓琰 +2
Oligonucleotide therapies for modulating the effects of herpesviruses
InactiveUS6310044B1Conveniently and desirably presentedFaster replicationPeptide/protein ingredientsGenetic material ingredientsOpen reading frameHerpesvirus infection
Compositions and methods are provided for the treatment and diagnosis of herpesvirus infections. In accordance with preferred embodiments, oligonucleotides are provided which are specifically hybridizable with RNA or DNA deriving from a gene corresponding to one of the open reading frames UL5, UL8, UL9, UL13, UL29, UL30, UL39, UL40, UL42 AND UL52 of herpes simplex virus type 1. The oligonucleotide comprises nucleotide units sufficient in identity and number to effect said specific hybridization. In other preferred embodiments, the oligonucleotides are specifically hybridizable with a translation initiation site; it is also preferred that they comprise the sequence CAT. Methods of treating animals suspected of being infected with herpesvirus comprising contacting the animal with an oligonucleotide specifically hybridizable with RNA or DNA deriving from one of the foregoing genes of the herpesvirus are disclosed. Methods for treatment of infections caused by herpes simplex virus type 1, herpes simplex virus type 2, cytomegalovirus, human herpes virus 6, Epstein Barr virus or varicella zoster virus are disclosed.
Owner:IONIS PHARMA INC
Human T-cell lymphotropic virus type II envelope protein and human monoclonal antibodies specific therefor
InactiveUS6270959B1Fast dynamicsLess-extensive cytopathic destructionMicrobiological testing/measurementVirus peptidesPolymerase LLymphocyte
Isolated and purified envelope protein of HTLV-I is provided devoid of non-envelope protein of HTLV-I and having substantially the same conformation as the envelope protein in native HTLV-I. The protein is produced recombinantly using a dual vaccinia / T7 polymerase system. Non-glycosylated and glycosylated forms of the protein are produced. Glycosylated forms are recognized by antibodies specific for the envelope protein of HTLV-I. Monoclonal antibodies are provided which are specific for the HTLV-I envelope protein and non-binding to HTLV-I envelope protein in denatured form. The HTLV-I envelope protein is cross-reactive with antibodies of HTLV-II and STLV. The envelope protein is useful in diagnosis of infection by HTLV-I and HTLV-II.
Owner:DEKABAN GREGORY A +2
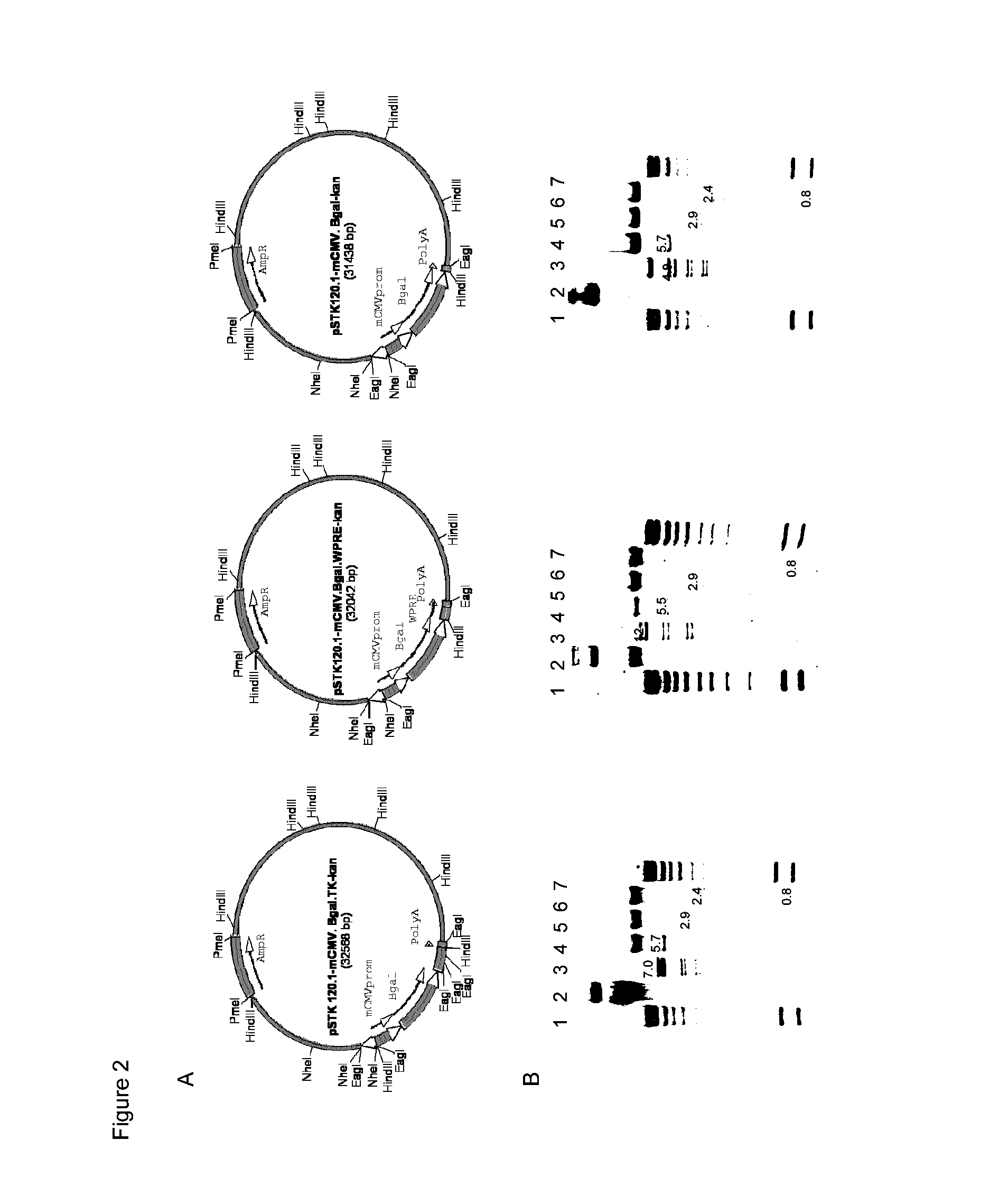
Adenoviral vector comprising herpes simplex virus type 1 thymidine kinase and a transgene for increasing the expression of the transgene
Compositions and methods useful in transgene expression are provided. Herpes simplex virus type 1 thymidine kinase sequences (“TK sequences”) are used to enhance transgene expression in first generation and high capacity adenoviral vectors. An mCMV promoter-driven β-galactosidase-expressing cassette is combined with TK sequences through direct fusion of the cDNA's. β-galactosidase (transgene) expression is enhanced independent of adenoviral vector selection. Methods of enhancing transgene expression employing the inventive adenoviral vectors are provided, along with pharmaceutical preparations comprising the inventive vectors and kits for enhanced transgene expression.
Owner:CEDARS SINAI MEDICAL CENT
CTL (Cytotoxic T Lymphocyte) epitope peptide of foot-and-mouth disease virus type O and screening method of CTL epitope peptide
ActiveCN103864905AImprove bindingConvenient researchSsRNA viruses positive-senseVirus peptidesCtl epitopeDisease
The invention discloses a CTL (Cytotoxic T Lymphocyte) epitope peptide of a foot-and-mouth disease virus type O as well as a screening method and application of the CTL epitope peptide. The CTL epitope peptide is composed of nine amino acid residues, and the amino acid sequence of the CTL epitope peptide is as follows: Ala-Thr-Arg-Val-Thr-Glu-Leu-Leu-Tyr. The epitope peptide has relatively strong combining capacity with SLA (Swineleukocyteantigen)-I proteins from various strains of swine and can induce cytotoxic immune response so as to be suitable for preparing vaccines for preventing and controlling foot-and-mouth disease viruses of various strains of swine and wide in application range. According to the invention, a CTL simulated epitope peptide of a foot-and-mouth disease virus is combined with a single-chain molecule of SLA-I of six strains of constructed swine in vitro, thus a polypeptide which can be combined with a complex can be screened through mass spectrum measurement; in addition, a simulated epitope peptide which can be induced to generate the immune response capacity of T cells is determined through ELISPOT (Enzyme-Linked Immunospot Assay) detection. The invention provides a method for screening and authenticating the CTL epitope of the foot-and-mouth disease virus in a large scale, and lays the foundation for researching and preparing a multi-epitope vaccine of a foot-and-mouth disease.
Owner:DALIAN UNIV
Cyprinid herpes virus type 2 CPA detection primer and application
ActiveCN106148332AEfficient detectionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationWater bathsNucleic acid detection
The invention discloses a cyprinid herpes virus type 2 CPA detection primer and application. A cyprinid herpes virus type 2 CPA detection kit comprises 10*ThermoPol Reaction Buffer, Bst DNA polymerase, dNTPs, a cross primer 1S, probe primers 2A and 3A, stripping primers 4S and 5A, MgSO4, Betaine and nucleic acid test strips. The primer has the advantages that of being easy and convenient to use, rapid, high in specificity and sensitivity, objective and visual in result judgment, low in cost, convenient to use and quite safe to people and environment. The cyprinid herpes virus type 2 CPA detection primer not only can be used in a special laboratory, but also can be applied in wild on-site rapid detection, and the cyprinid herpes virus type 2 in a sample can be accurately detected within 1.5 h through only one metal bath or water bath pot.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
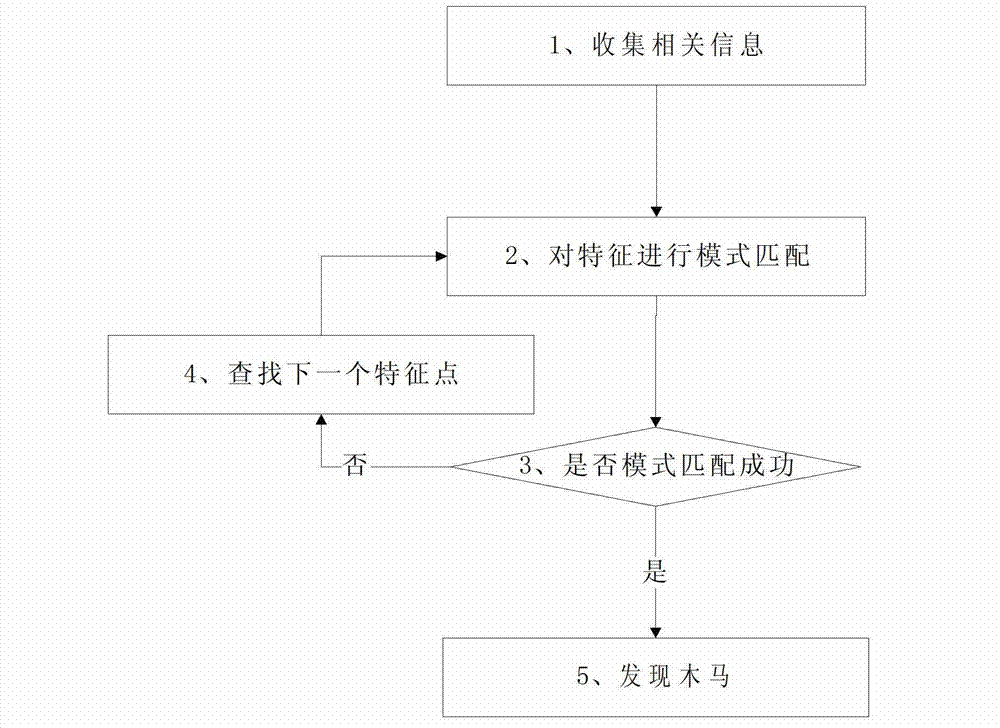
Trojan horse detection method based on Trojan horse virus type classification modeling
The invention discloses a Trojan horse detection method based on Trojan horse virus type classification modeling. The method comprises following steps of (1) classifying found Trojan horse according to characteristics; (2) forming a Trojan horse identification class library; (3) collecting characteristics of an operation system, identifying the Trojan horse through the Trojan horse identification class library in the step (2), and positioning in the belonging categories and characteristics of Trojan horse in; (4) positioning suspicious items; (5) according to the collected characteristics of the operation system in the step (3), conducting pattern matching in the class library through an algorithm to identify the Trojan horse in the system; (6) finding the same Trojan horse with pattern matching in the class library, and judging the Trojan horse to be detected. The method can conduct rapidly identification and analysis for existing Trojan horse in the system and particularly for the unknown novel Trojan horse. Compared with a traditional detection manner, detection capability for the Trojan horse, particularly for recognition and detection capability for the unknown novel Trojan horse has great improvement.
Owner:SICHUAN CINGHOO TECH
Genetic markers for discrimination and detection of viruses causing infectious aquatic organism diseases, and method of discriminating and detecting the viruses using the same
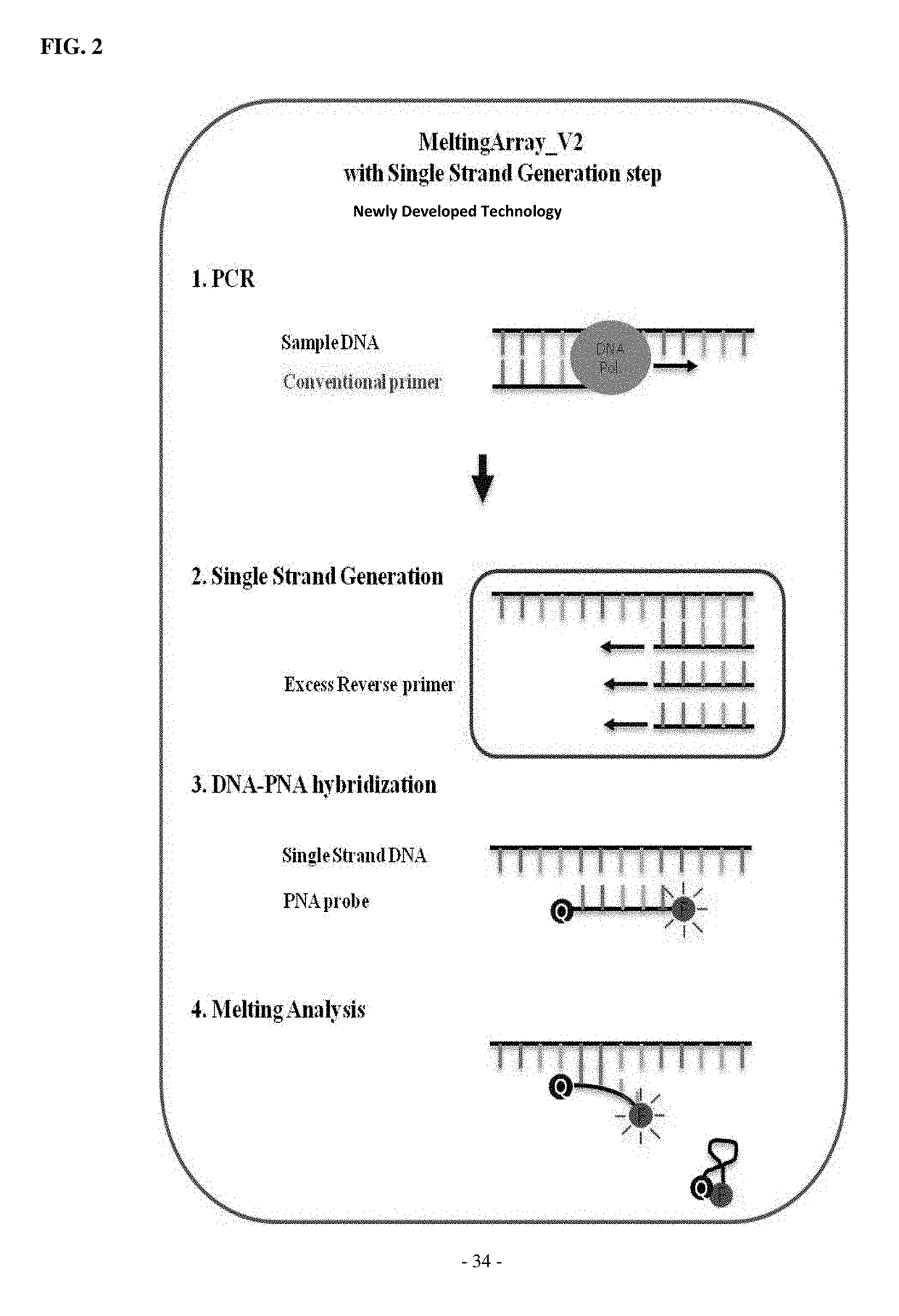
The present invention relates to genetic markers for discrimination and detection of viruses causing infectious aquatic organism diseases, and a method of discriminating and detecting the viruses using the same, and more particularly to a method for discriminating or detecting viruses causing infectious aquatic organism diseases, the method comprising: selecting and amplifying a DNA nucleotide sequence encoding a gene specific for viral hemorrhagic septicemia virus (VHSV), red sea bream iridovirus (RSIV) or infectious spleen and kidney necrosis virus (ISKNV), which is a virus causing red sea bream iridovirus disease, or Koi herpesvirus (KHV); hybridizing a peptide nucleic acid (PNA) that specifically recognizes the amplification product; controlling the temperature of the hybridization product to obtain a temperature-dependent melting curve; and discriminating the viral type or detecting whether or not fish would be infected with the viral type by analyzing the obtained melting curve to determine a melting temperature.
Owner:REPUBLIC OF KOREA (NAT FISHERIES RES & DEV INST)
Network protection method and device
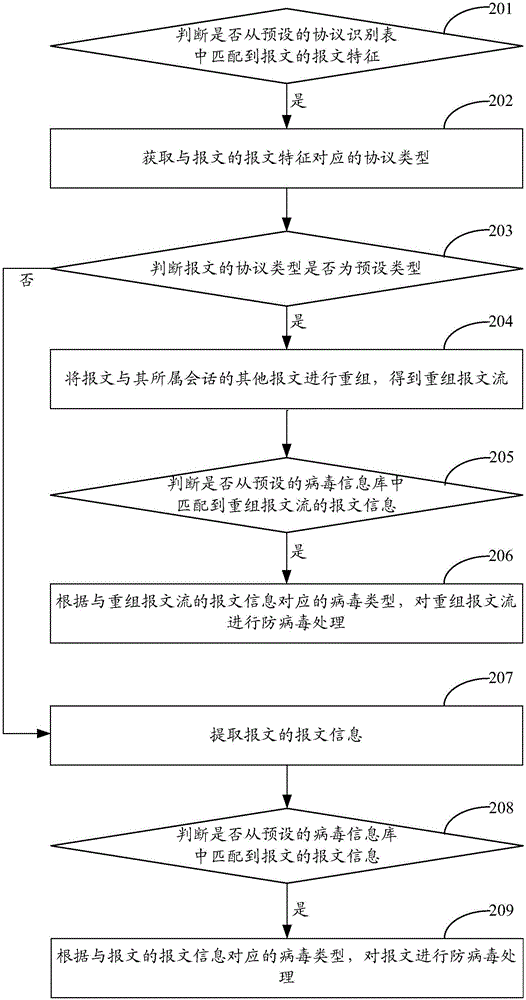
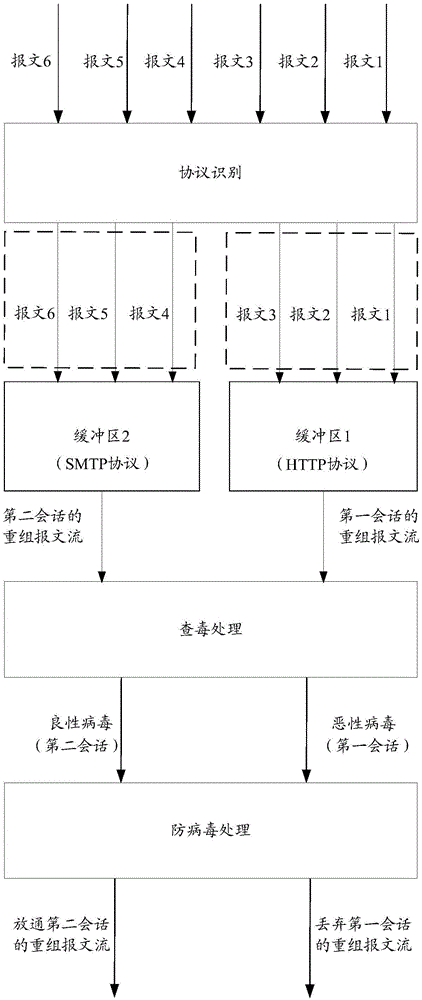
InactiveCN105939314AImprove accuracyReduce false alarm rateTransmissionInformation repositoryMessage flow
The invention provides a network protection method and a network protection device. The method comprises the steps of when receiving a message, judging that whether the protocol type of a message is a preset type; if yes, recombining the message with other messages of a session to which the message belongs, thus obtaining a recombined message flow; judging that whether message information matched with the recombined message flow exists in a preset virus information library which comprises correspondence between different message information and virus types; if yes, according to the virus type corresponding to the message information of the recombined message flow, implementing antivirus treatment on the recombined message flow. With the application of the method and device provided by the embodiment of the invention, the messages in the same session are recombined to obtain a recombined message flow, the recombined message flow is subjected to virus checking as a whole, thus being capable of avoiding that a virus cannot be accurately detected as the virus exists in the messages in a cross-packet, disordered or sliced manner, and thereby the accuracy of virus detection is improved, the false report rate or missing report rate is reduced, and guarantees are provided for network security.
Owner:HANGZHOU DPTECH TECH
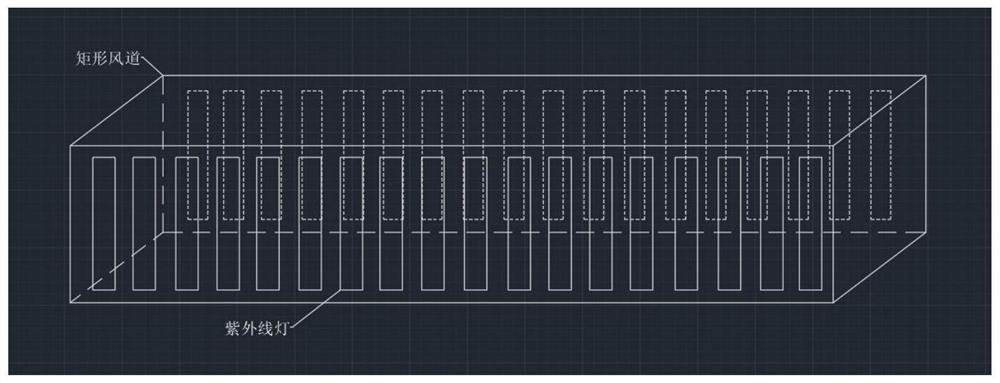
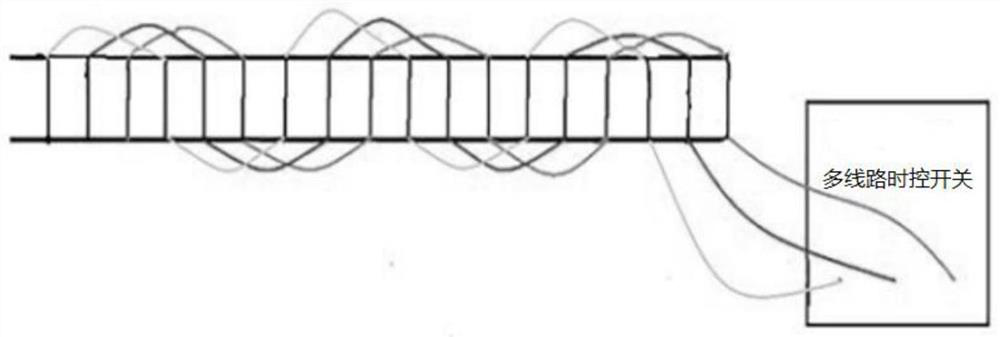
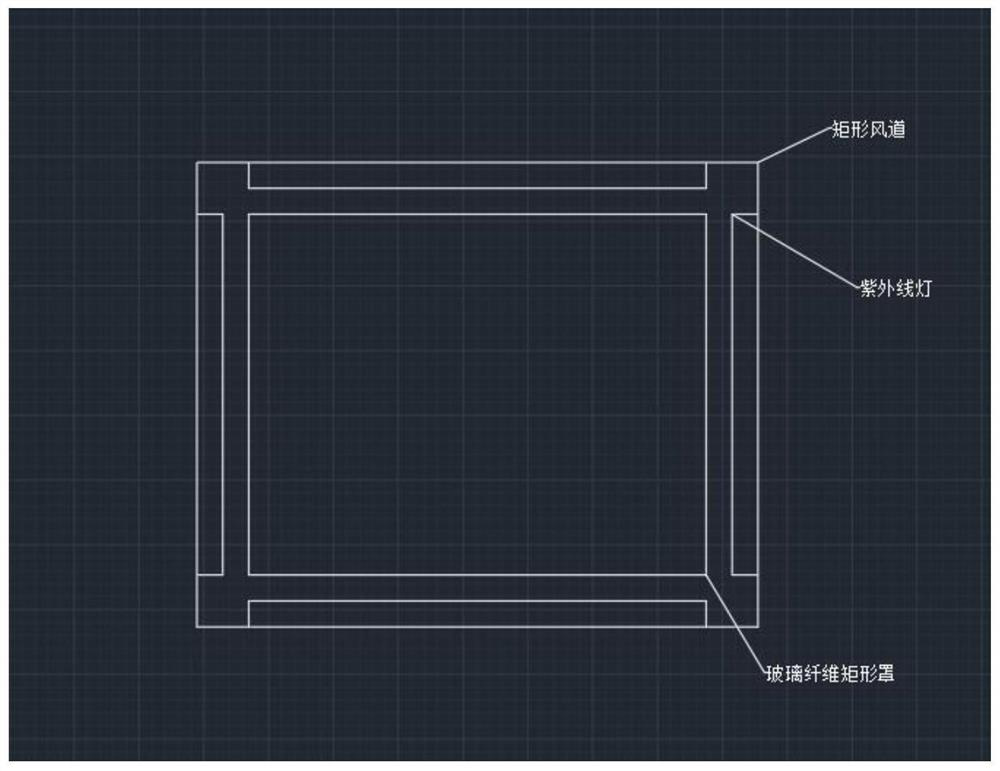
Automatically-controlled air conditioner pipeline ultraviolet disinfection and sterilization method and system
InactiveCN112524714AReduce cooling effectLow flow resistanceMechanical apparatusLighting and heating apparatusUltraviolet lightsSterilizing Units
The invention discloses an automatically-controlled air conditioner pipeline ultraviolet disinfection and sterilization method and system, and belongs to the technical field of central air conditionerpipeline ultraviolet disinfection and sterilization. Aiming at the problems of the non-ideal disinfection effect and the low disinfection speed of an existing disinfection device, the method comprises the steps that indoor viruses are collected on line, the types and concentrations of the viruses are judged, places where pollutants are not easy to discharge are judged through air distribution, and aerosol sampling points are arranged at the places; time and attenuation constants are introduced into a formula according to the virus types through a program, and irradiation intensity is calculated; the irradiation intensity of each point of a pipeline is calculated by using a computer through the formula, comparing is conducted to obtain a minimum value, the minimum value is compared with the obtained irradiation intensity, and the on-off state of an ultraviolet lamp is adjusted so as to kill the viruses. Computer program control is adopted, the intensity of the ultraviolet lamp meetingthe maximum bacterium killing concentration is worked out, automatic recognition and conversion are achieved through a multi-line time control switch, the sterilization effect is guaranteed, and meanwhile the energy can be saved to the maximum extent.
Owner:NORTHEAST FORESTRY UNIVERSITY
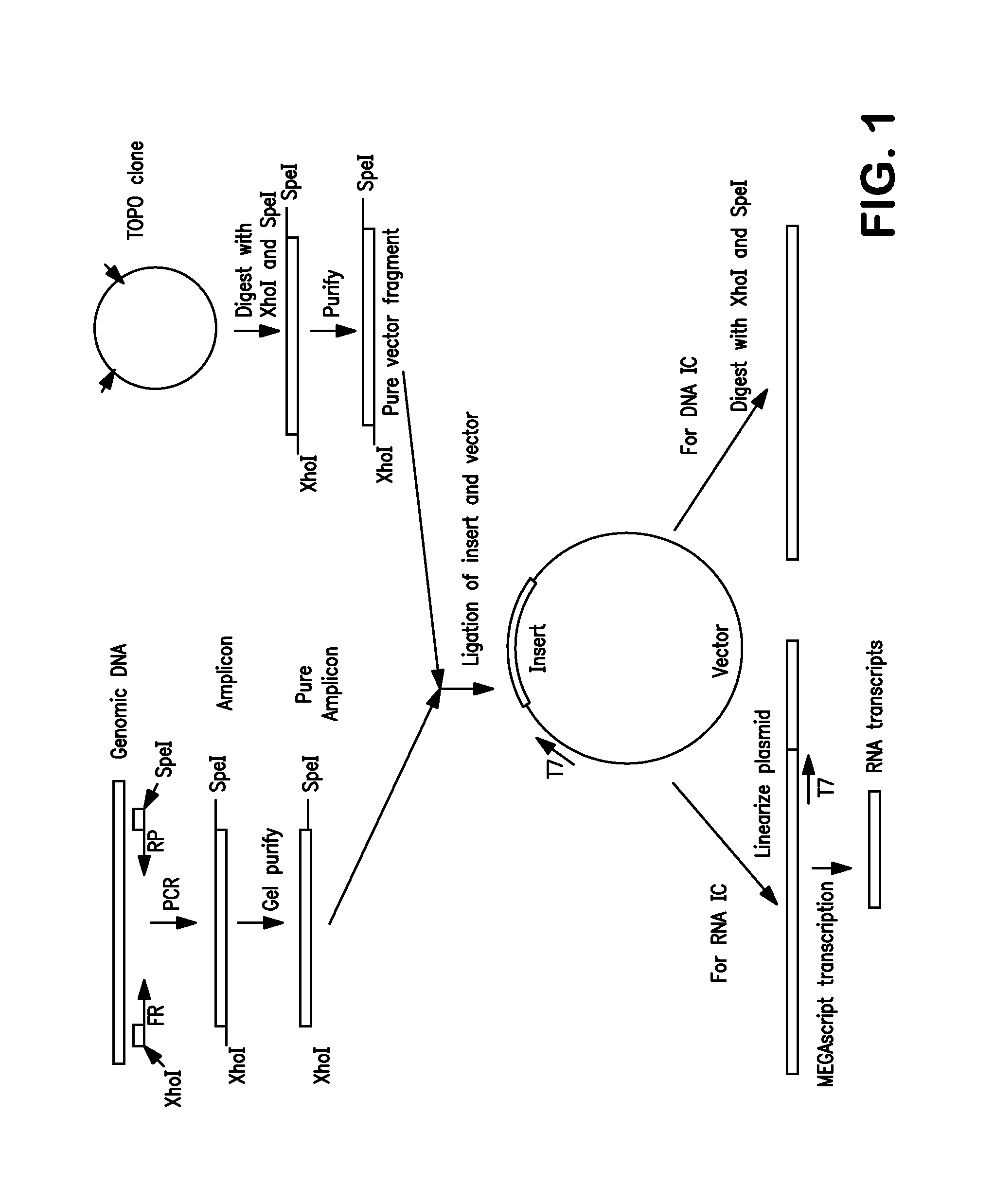
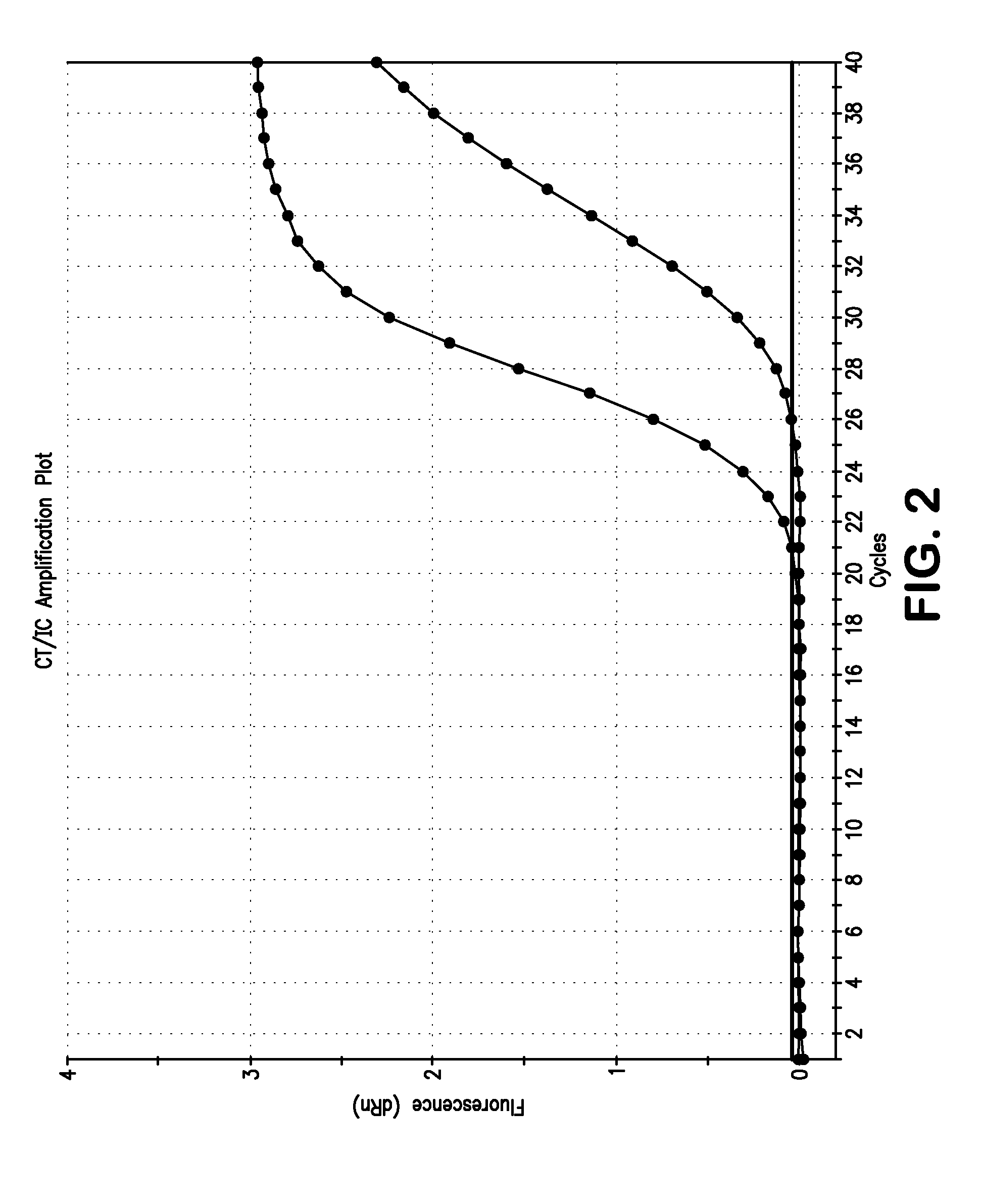
Non-Competitive Internal Controls for Use in Nucleic Acid Tests
InactiveUS20110003309A1Sugar derivativesMicrobiological testing/measurementNucleic acid testHuman immunodeficiency
Provided are non-competitive internal controls for use in nucleic acid tests (NATs), which are obtained from the organisms Methanobacterium thermoautrophicum (MET) and Zea mays (Corn). The non-competitive internal controls have utility in DNA and RNA NATs selected from Influenza A, Influenza B, parainfluenza viruses 1 to 4 (PIV-1 to PIV-4), respiratory syncytial virus type A (RSV A), RSV B, human metapneumovirus (hMPV), Chlamydia trachomatis (CT), and Neisseria gonorrhea (GC), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human Immunodeficiency Virus I (HIV-1), and Severe Acute Respiratory Syndrome (SARS).
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Coxsackie virus type A16 (CA16) real-time fluorescent nucleic acid isothermal amplification detection kit
ActiveCN103388032AReduce pollutionEfficient captureMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Throat swab
The invention discloses a Coxsackie virus type A16 (CA16) real-time fluorescent nucleic acid isothermal amplification detection kit comprising reagents such as a capturing probe, CA16 amplification primers T7 primer and nT7 primer, a CA16 detection probe, M-MLV reverse transcriptase, T7 RNA polymerase, and the like. The kit can be used for detecting CA16 RNA in throat swab or stool, and has the characteristics of high specificity, high sensitivity (reaching 10copies / reaction), low pollution (amplification product RNA can be easily degraded under natural environment), and fast detection (conventionally detection can be finished within 60min). The kit can perform important effect in clinical diagnosis of CA16 early-stage infection, and can be widely applied.
Owner:SHANGHAI RENDU BIOTECH
Chemical luminescence reagent kit for detecting herpes simplex virus type II IgM antibody
InactiveCN101368963AGuaranteed stabilityEasy to produceChemiluminescene/bioluminescenceImmune complex depositionIgm antibody
The invention provides a chemiluminescence immunoassay test kit and a preparing method thereof for detecting herpes simplex virus type II IgG antibody. The technical principle that the test kit adopts the capture method to detect herpes simplex virus type II IgG antibody is that: antihuman u link monoclonal antibody is absorbed into solid-phase carrier, and sample to be tested is added in, and then specific antigen and enzyme labeled antibody are added in after washing so as to form immune complex, finally chemiluminescence zymolyte liquid is added in, and the content of the IgM antibody can be obtained through measuring the chemiluminescence value. The enzyme is alkaline phosphatase, and the chemiluminescence zymolyte liquid contains 1,2-butyl dioxide derivatives. The test kit has the advantages that the operation is simple, the precision is better, the sample detecting result conforms the detecting result by the enzyme linked immunity analysis method.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
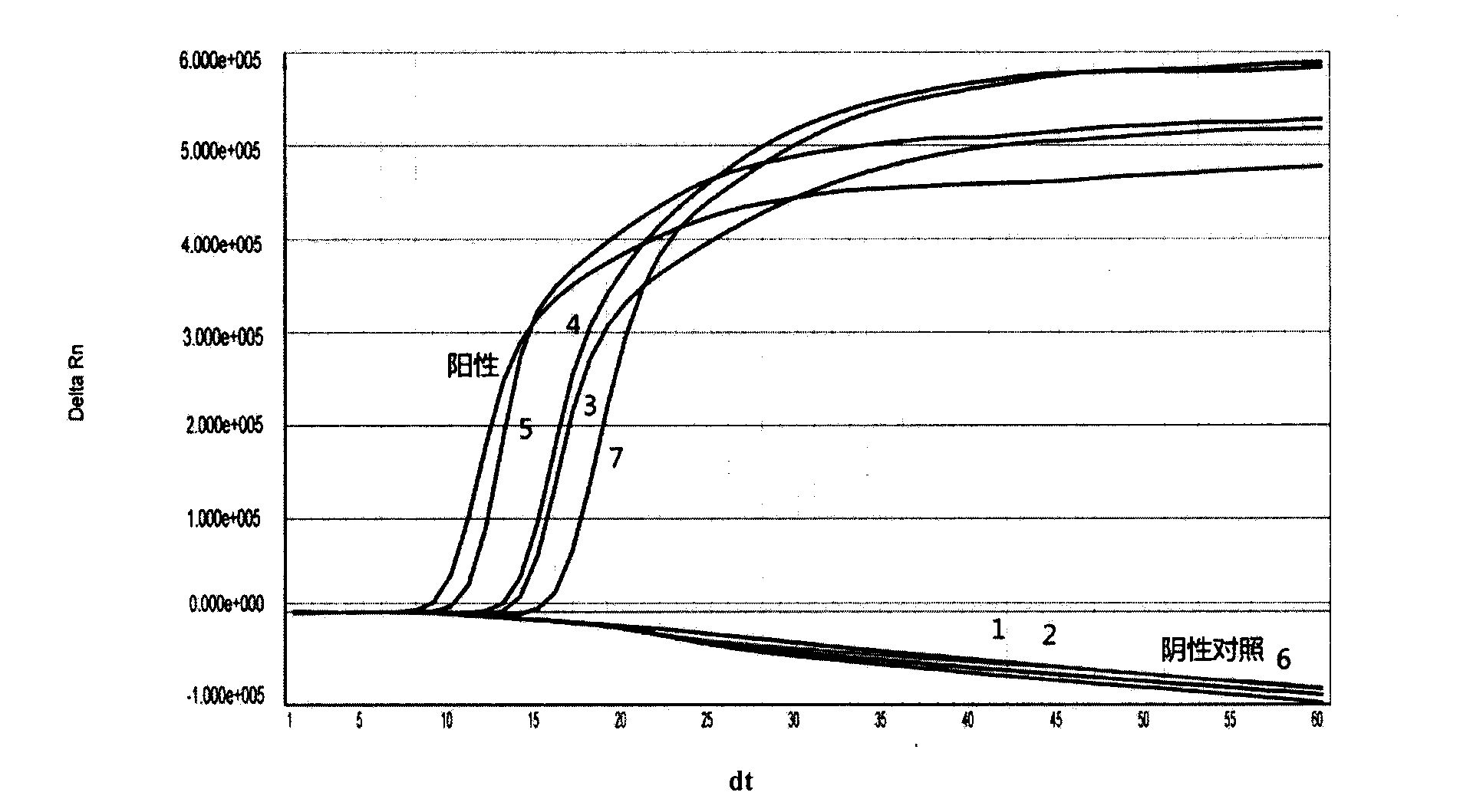
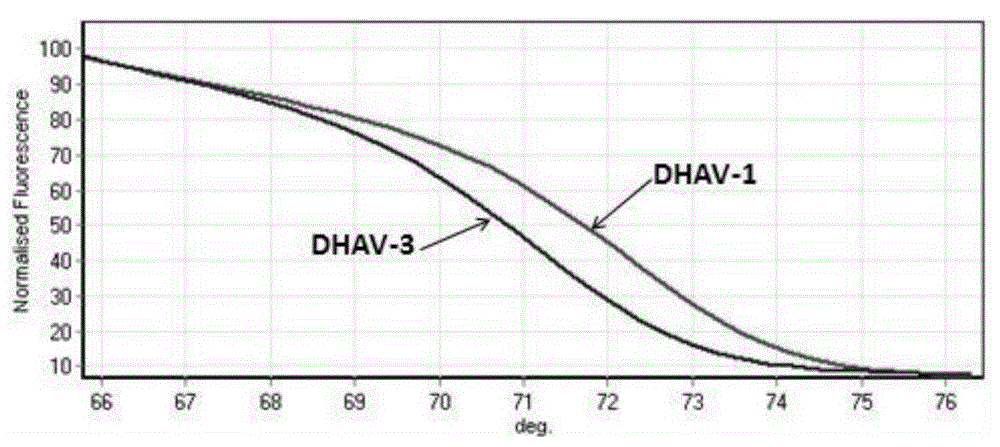
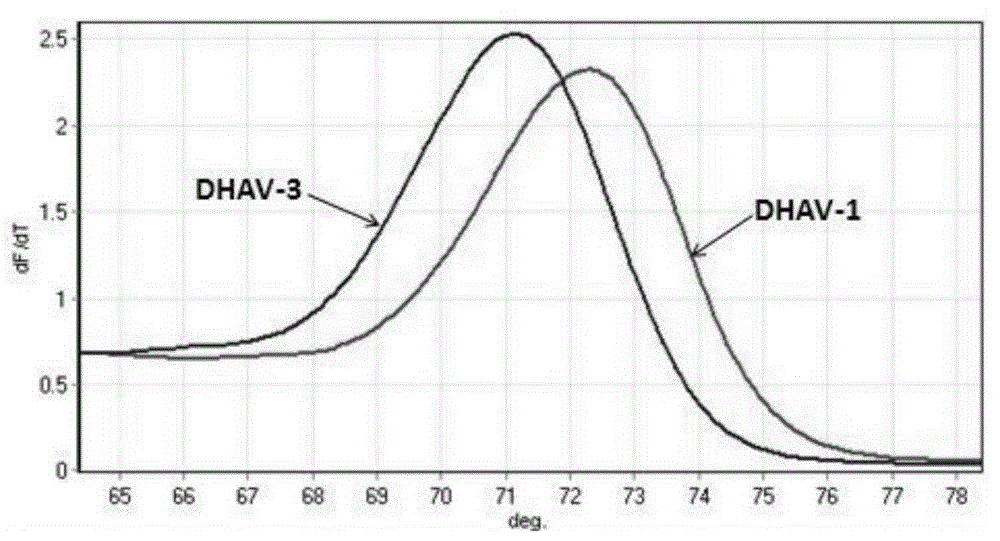
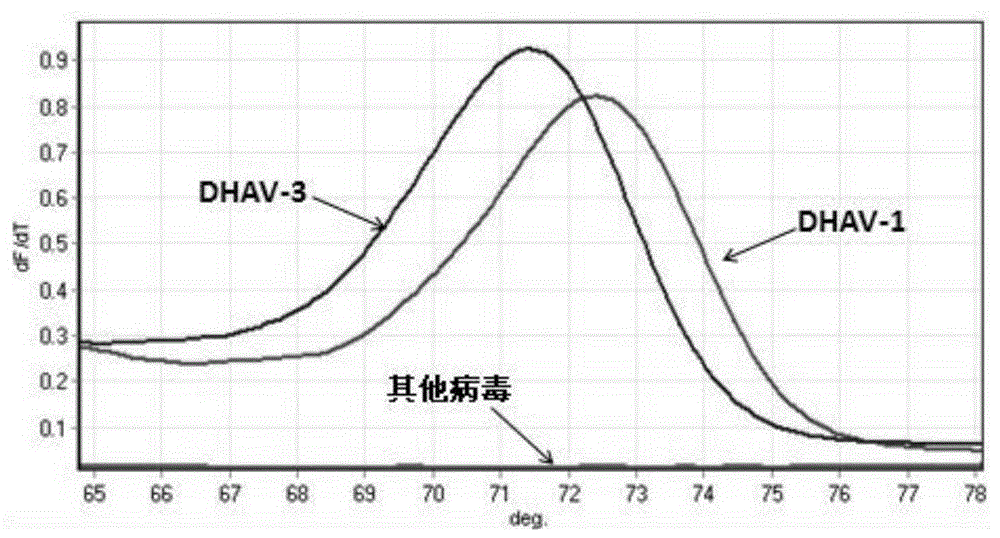
PCR-HRM primer and method for quickly distinguishing DHAV-1 from DHAV-3
ActiveCN105331740AEasy to operateIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesHepatovirusBiochemistry
The invention discloses a PCR-HRM primer and method for quickly distinguishing DHAV-1 from DHAV-3. According to the method, plasmid samples of corresponding target fragments of DHAV-1 and DHAV-3 are established firstly to serve as positive plasmids, then virus RNA is extracted from the samples and converted into cDNA through inverse transcription, and then cDNA is taken as a template and subjected to PCR-HRM amplification by means of the designed specific primer; HRM analysis is conducted on the amplification product, and virus type is determined. According to the method, operation is easy, and fluorescence saturable dye only needs to be added before ordinary PCR reaction; detection is quick, flux is high, the whole operation process lasts for about 3.5 h for PCR product detection of a 96 / 384 pore plate, and detection time is shortened greatly; cost is low, and it does not require multiple primers and specific probes; sensitivity is high, specificity and repeatability are high, and the PCR-HRM primer and method are suitable for being applied and popularized clinically.
Owner:梅州市桃花缘文化实业发展有限公司
Feline herpes virus type I gB-gD recombinant protein and preparation method and application thereof
PendingCN112457414AHigh sensitivityLow cross-reactivityBacteriaAntibody mimetics/scaffoldsAnimal virusI antibody
The invention discloses a feline herpes virus type I gB-gD recombinant protein, and belongs to the field of animal virus antibody detection. The recombinant protein comprises an amino acid sequence asshown in SEQ ID NO.1 or consists of an amino acid sequence as shown in SEQ ID NO.1. The invention further discloses a gene of the recombinant protein. The gene comprises a vector containing the gene,a host cell. The invention also discloses a preparation method of the recombinant protein and application of the recombinant protein in detection of feline herpes virus type I antibody. The recombinant protein is used for detecting the feline herpes virus type I antibody, is convenient and rapid, has high sensitivity, does not have cross reaction with other pathogens, has high specificity, and has huge clinical significance and wide application prospect.
Owner:杭州爱谨生物科技有限公司
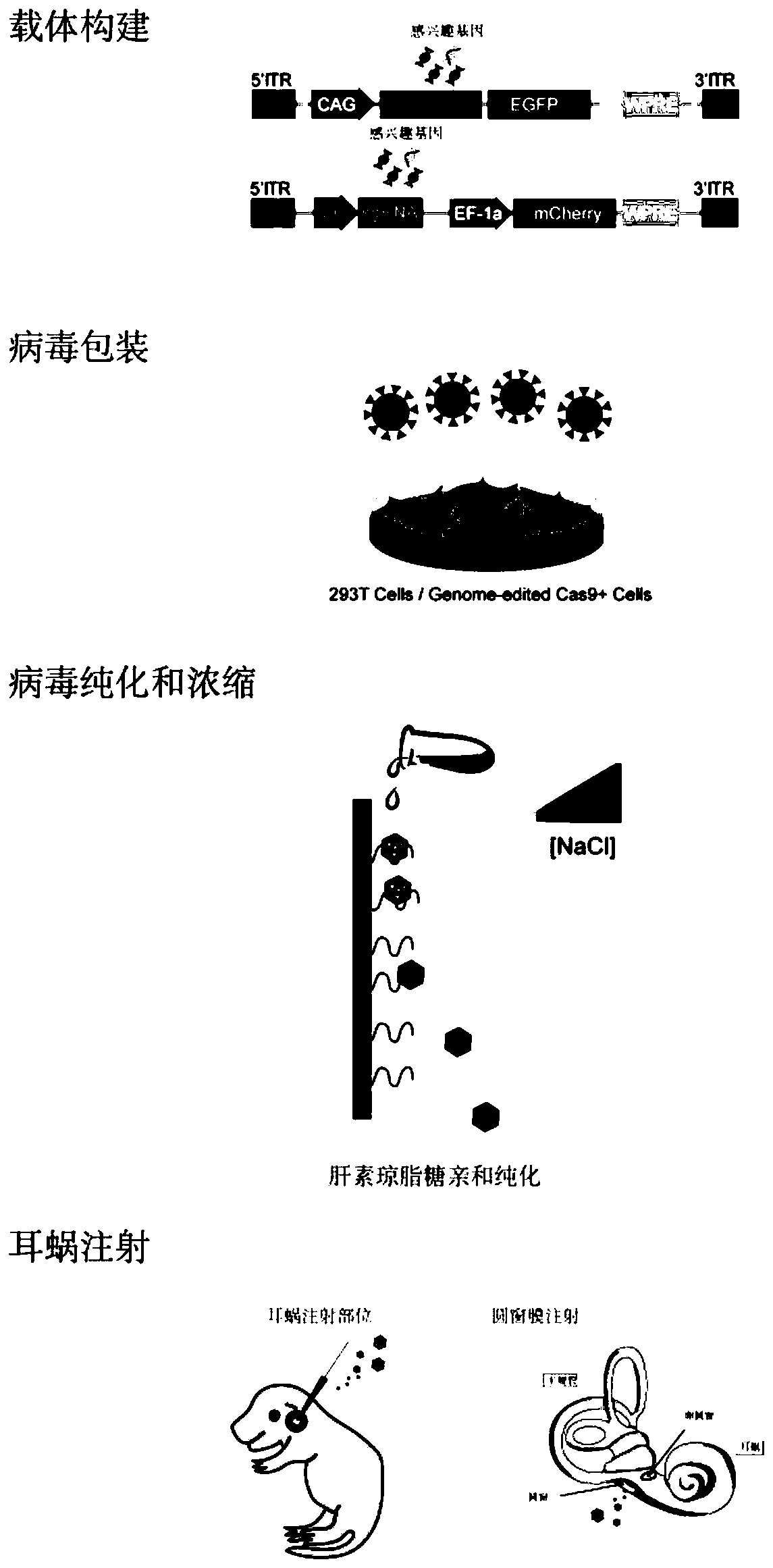
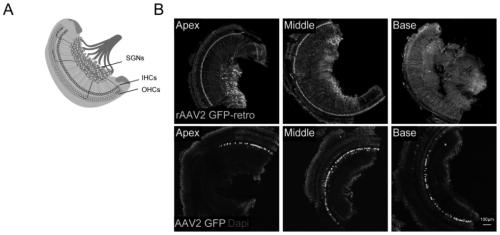
Application of retrograde recombinant adeno-associated virus type 2 in infected spiral neurons
ActiveCN110423778AEfficient infectionInfection specificitySenses disorderGenetically modified cellsDiseaseSensory neuron
The invention provides application of a retrograde recombinant adeno-associated virus type 2 in infected spiral neurons. The application is characterized in that the retrograde recombinant adeno-associated virus type 2 is injected into the cochlea by cochlear round window injection, and the retrograde recombinant adeno-associated virus type 2 is efficiently and specifically infects the spiral neurons. The invention provides a new usage of the recombinant adeno-associated virus type 2 in the infection of the cochlear spiral neurons, which has not been reported before, and the retrograde recombinant adeno-associated virus type 2 is capable of efficiently and specifically infecting the cochlear spiral neurons, and has good application prospects in the preparation of drugs for treating hereditary deafness and deafness related diseases, especially the drugs for auditory sensory neuron-spiral neuron related diseases.
Owner:SHANGHAI TECH UNIV
Non-competitive internal controls for use in nucleic acid tests
Provided are non-competitive internal controls for use in nucleic acid tests (NATs), which are obtained from the organisms Methanobacterium thermoautrophicum (MET) and Zea mays (Corn). The non-competitive internal controls have utility in DNA and RNA NATs selected from Influenza A, Influenza B, parainfluenza viruses 1 to 4 (PIV-1 to PIV-4), respiratory syncytial virus type A (RSV A), RSV B, human metapneumovirus (hMPV), Chlamydia trachomatis (CT), and Neisseria gonorrhea (GC), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human Immunodeficiency Virus I (HIV-1), and Severe Acute Respiratory Syndrome (SARS).
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Methods for preventing cattle reproductive diseases
InactiveUS20070154943A1Avoid infectionAntibacterial agentsSsRNA viruses negative-senseBovine Viral Diarrhea VirusesHeifer calf
The present invention relates to methods for treating or preventing diseases or disorders in a pregnant cow and calf nursing a pregnant cow caused by infection by Bovine Viral Diarrhea Virus (BVDV) Types 1 and 2, Bovine Herpes Virus Type-1 (BHV-1), Bovine Respiratory Syncytial Virus (BRSV), Parainfluenza Virus (PIV3), Campylobacter fetus, Leptospira canicola, Leptospira grippotyphosa, Leptospira hardj-prajitno, Leptospira icterohaemmorrhagiae, Leptospira hardjo-bovis and Leptospira pomona by administering to the animal an effective amount of a safe modified live viral combination vaccine further combined with a multivalent bacterin vaccine.
Owner:ZOETIS SERVICE LLC
In-vehicle air purification and humidity regulation system
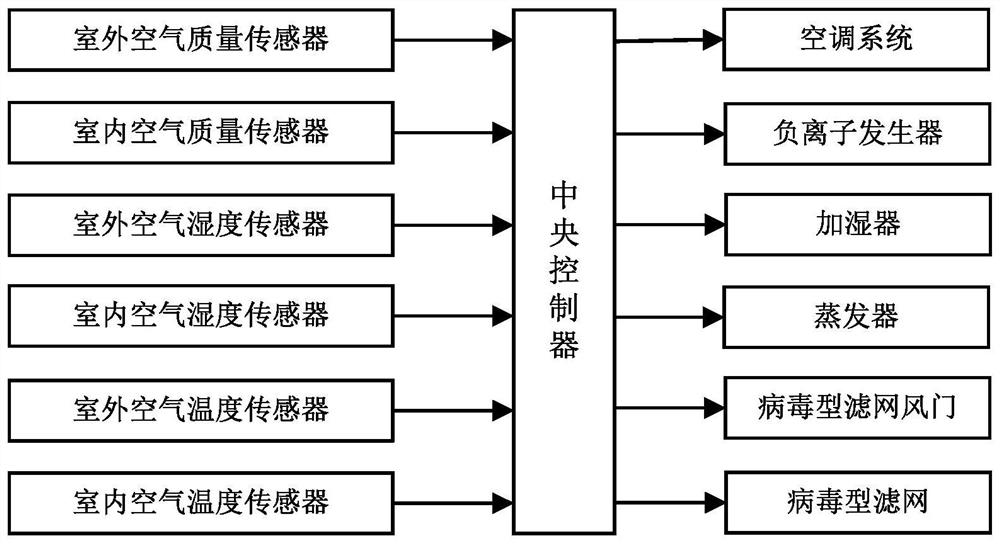
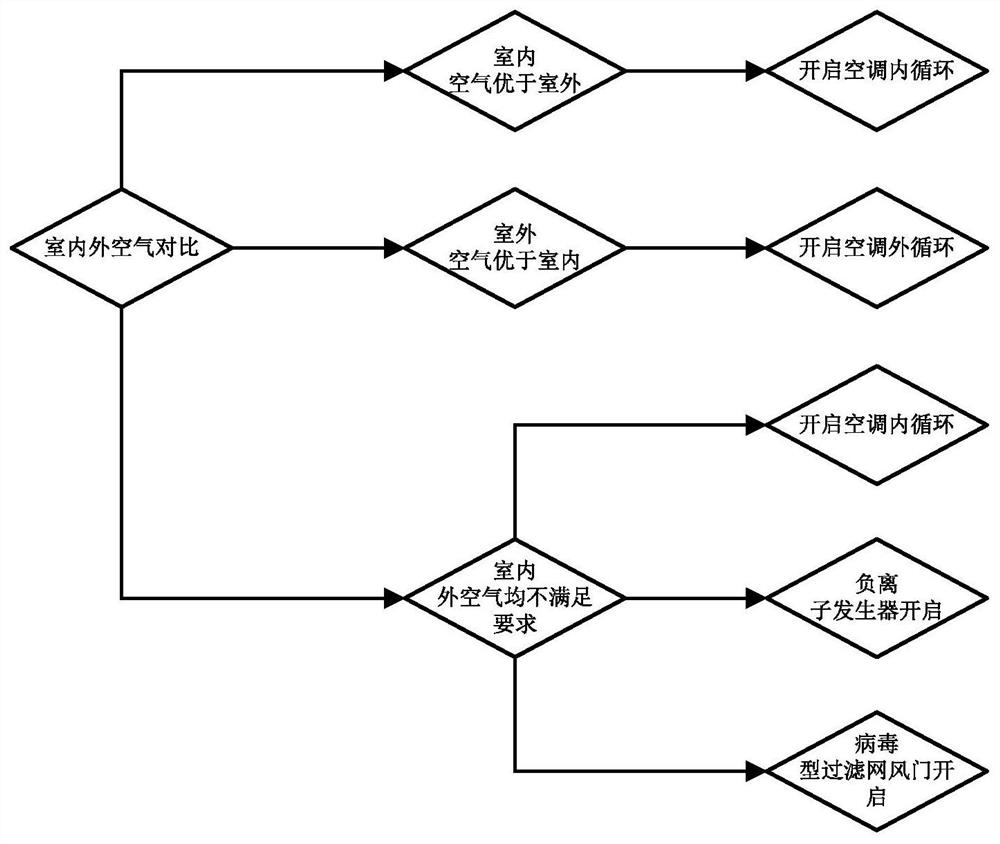
InactiveCN111959228AAdd air purification functionSimple structureAir-treating devicesVehicle heating/cooling devicesViral typeProcess engineering
The invention discloses an in-vehicle air purification and humidity adjustment system, and relates to the technical field of air quality adjustment. The in-vehicle air purification and humidity adjustment system comprises a sensor assembly, an adjustment assembly and a central controller, and the sensor assembly is used for collecting indoor and outdoor air quality, an air temperature and air humidity and sending the air quality, the air temperature and the air humidity to the central controller; the adjusting assembly comprises an air conditioning system, a negative-ion generator, a humidifier, an evaporator, a virus type filter screen and a virus type filter screen air door, wherein the negative-ion generator, the humidifier, the evaporator, the virus type filter screen and the virus type filter screen air door are located in the air conditioning system; the virus type filter screen air door is used for making air conditioner outlet air pass or bypass the virus type filter screen; and the central controller is used for driving one or more devices in the adjusting assembly to work based on the data sent by the sensor assembly so as to carry out air purification and humidity adjustment in a cab. Air purification and humidity adjustment in the cab can be effectively performed, and an overall structure and a control mode are simple.
Owner:DONGFENG COMML VEHICLE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com