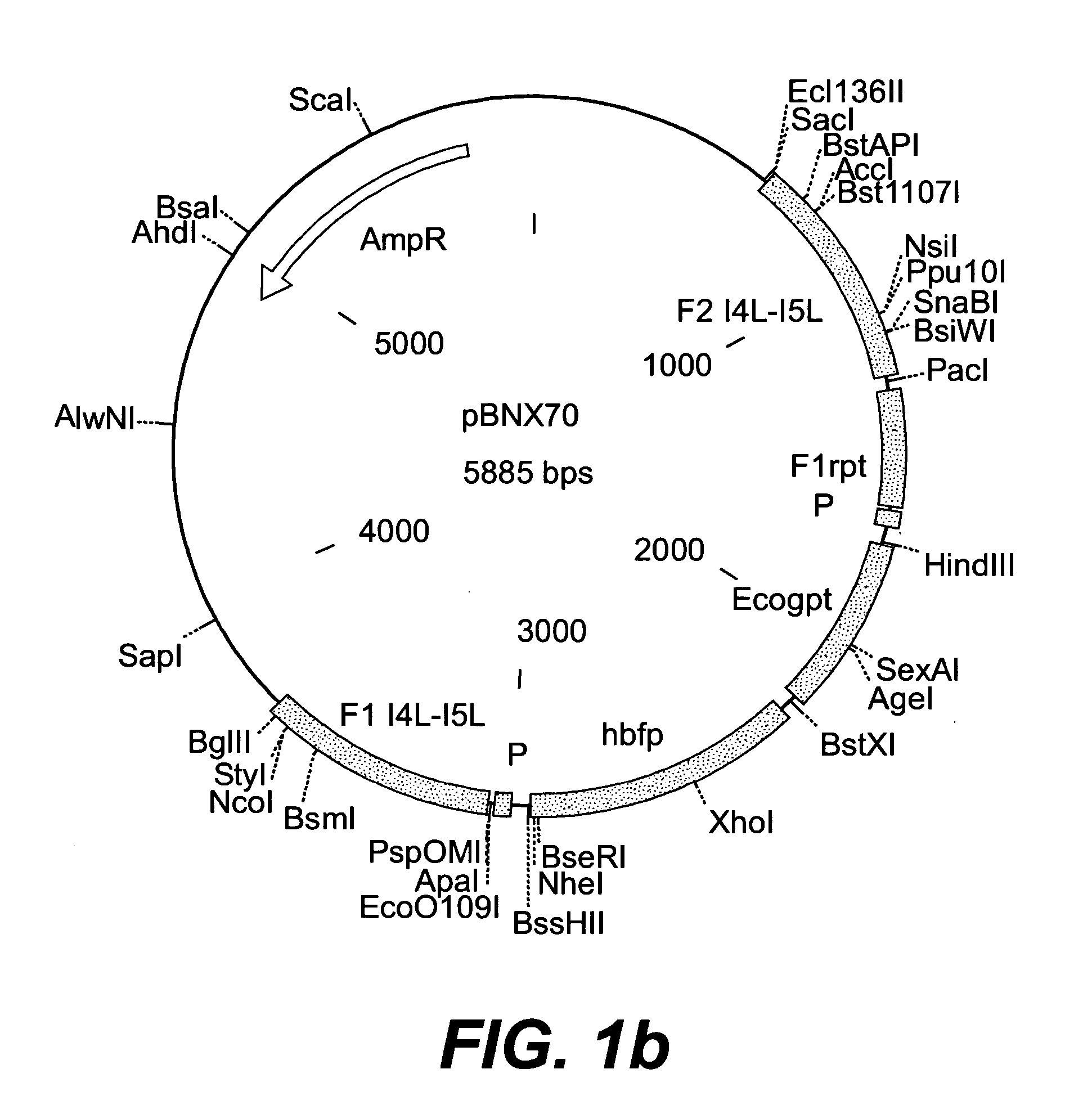
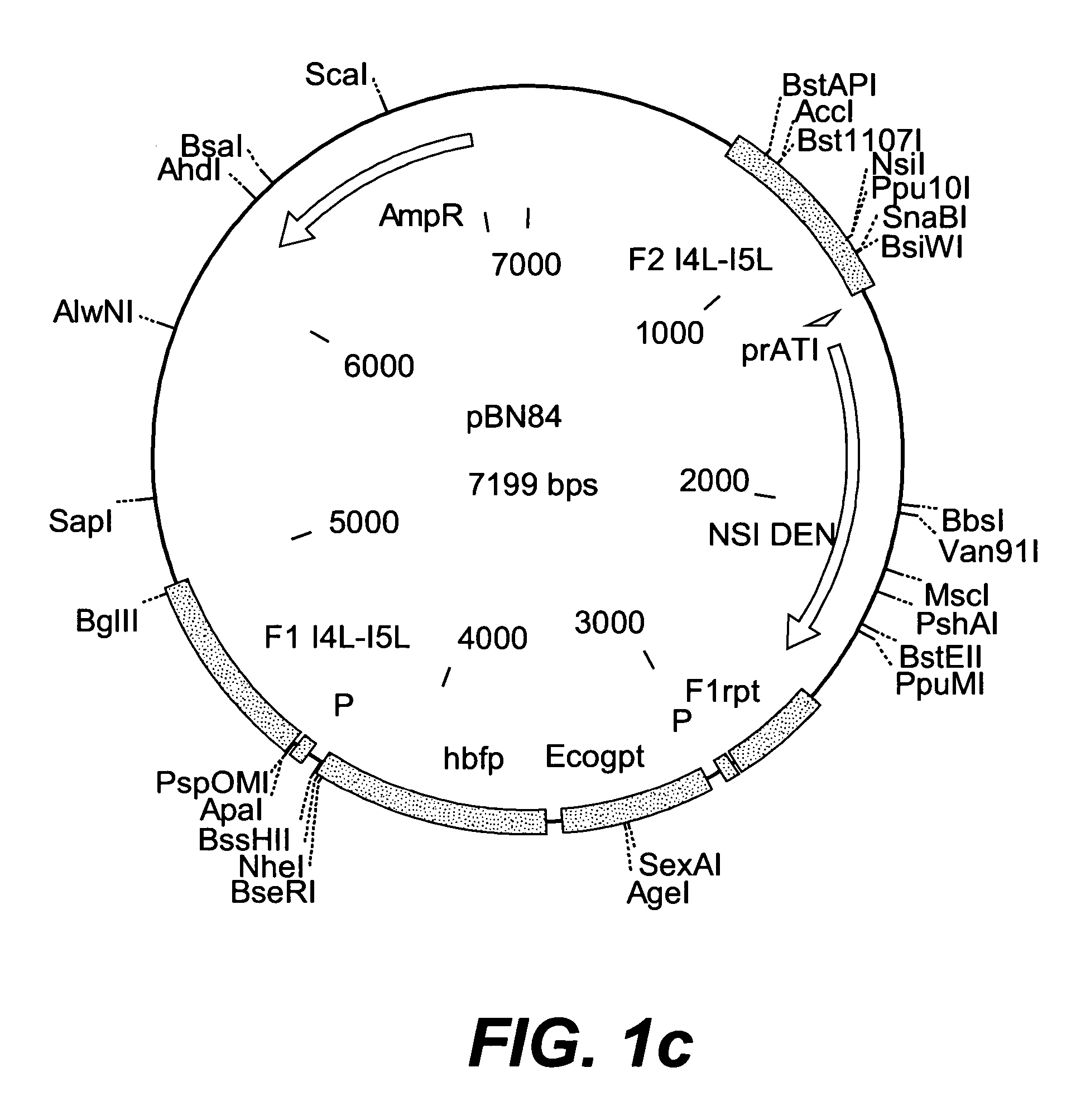
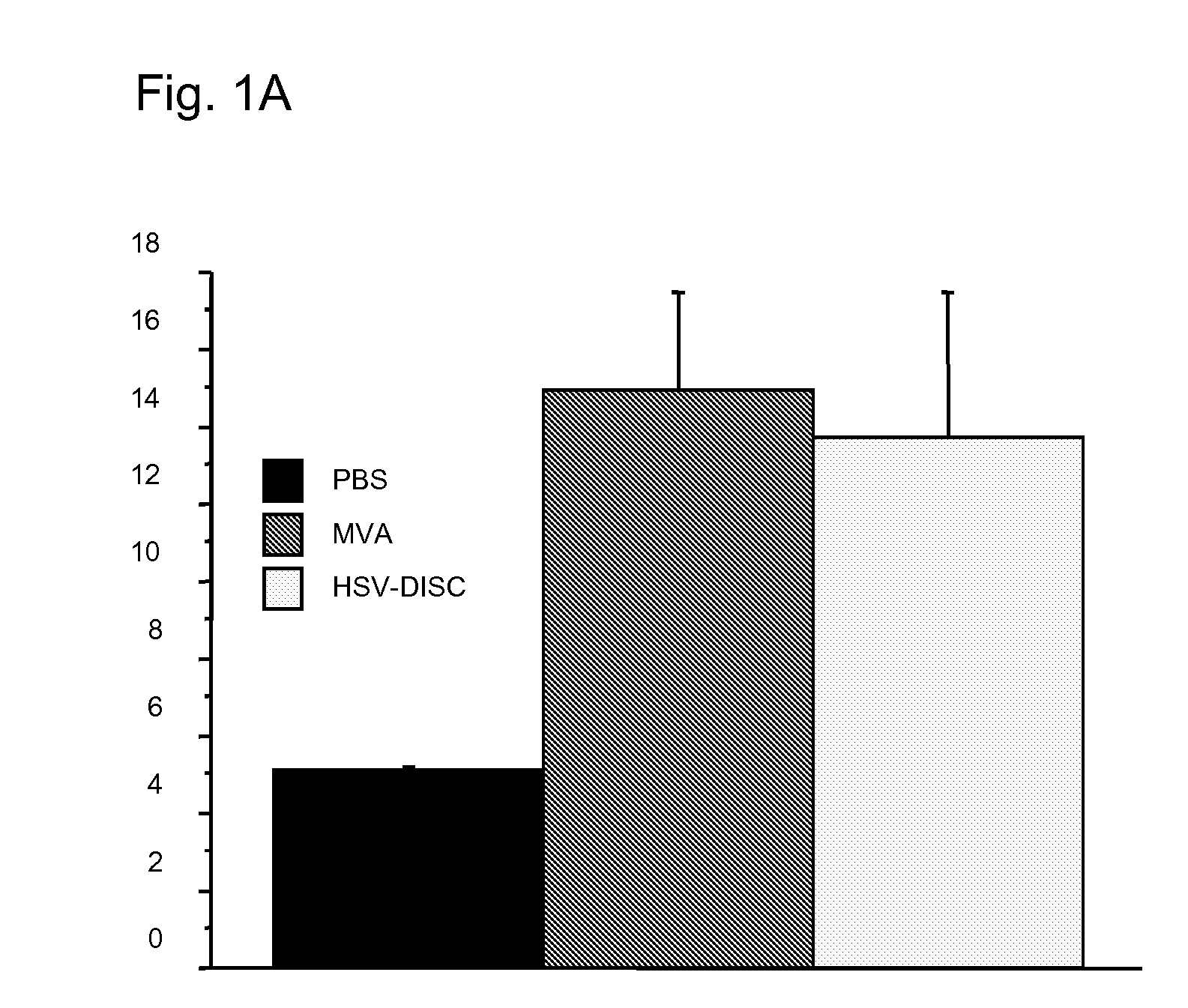
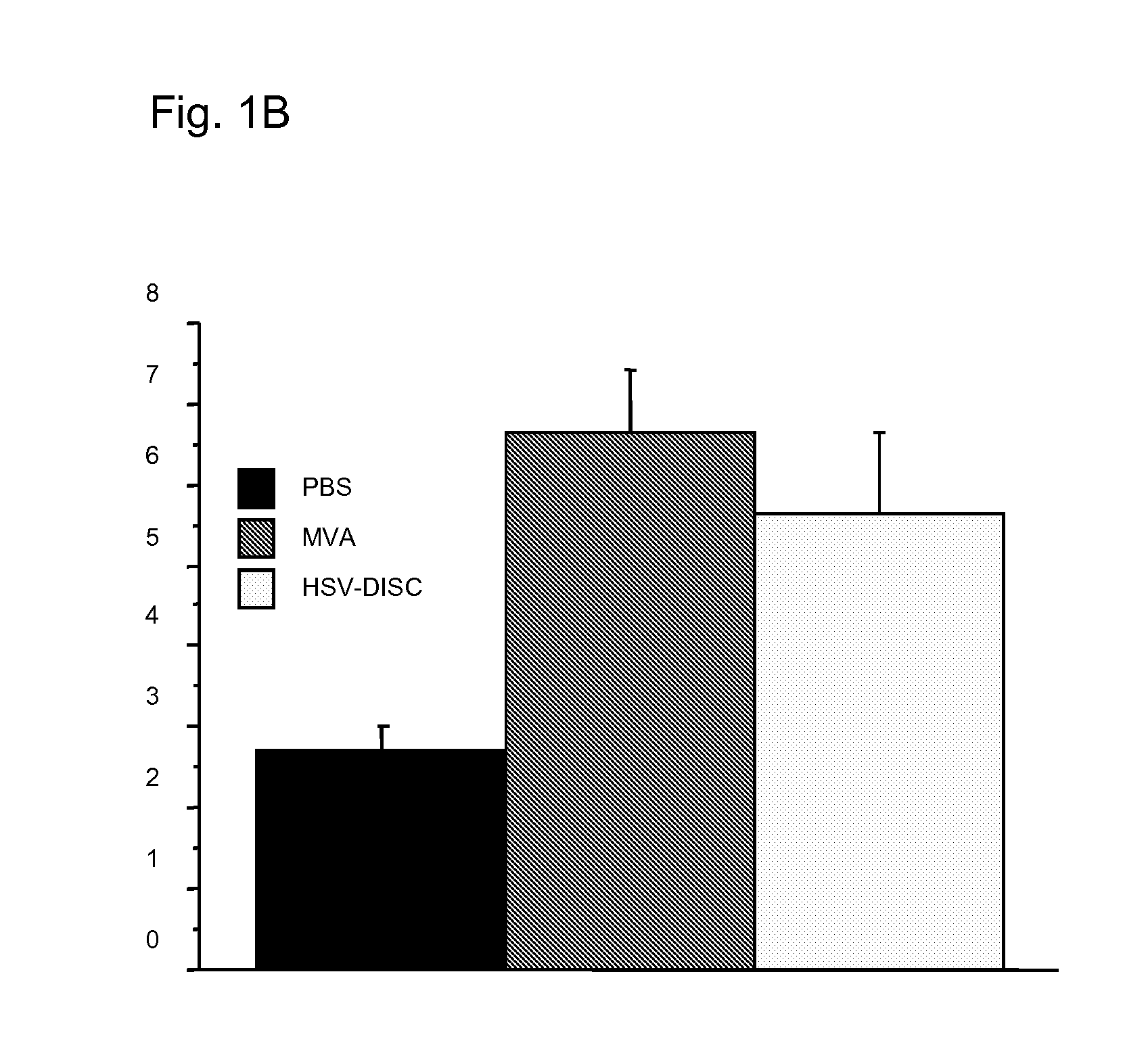
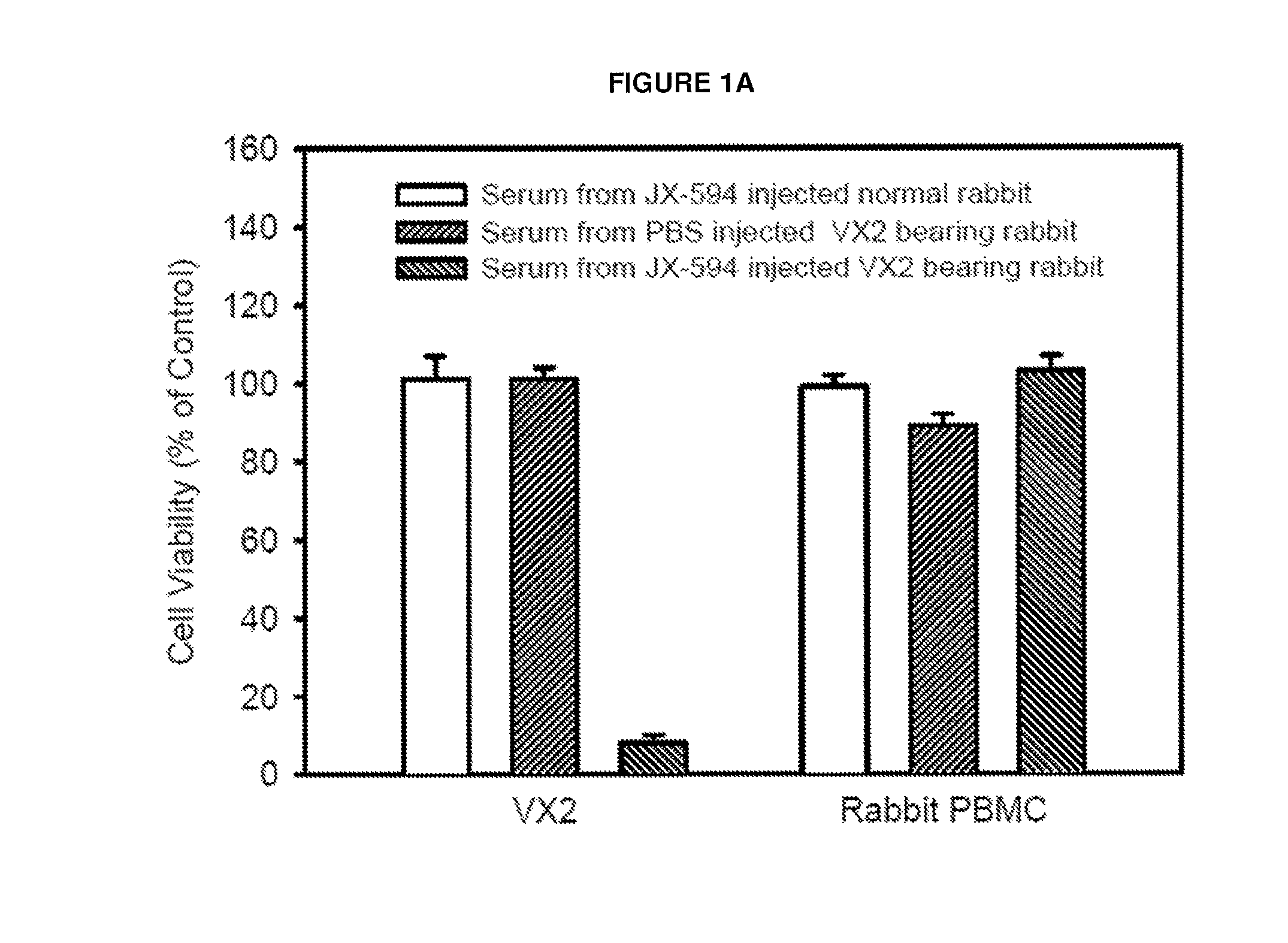
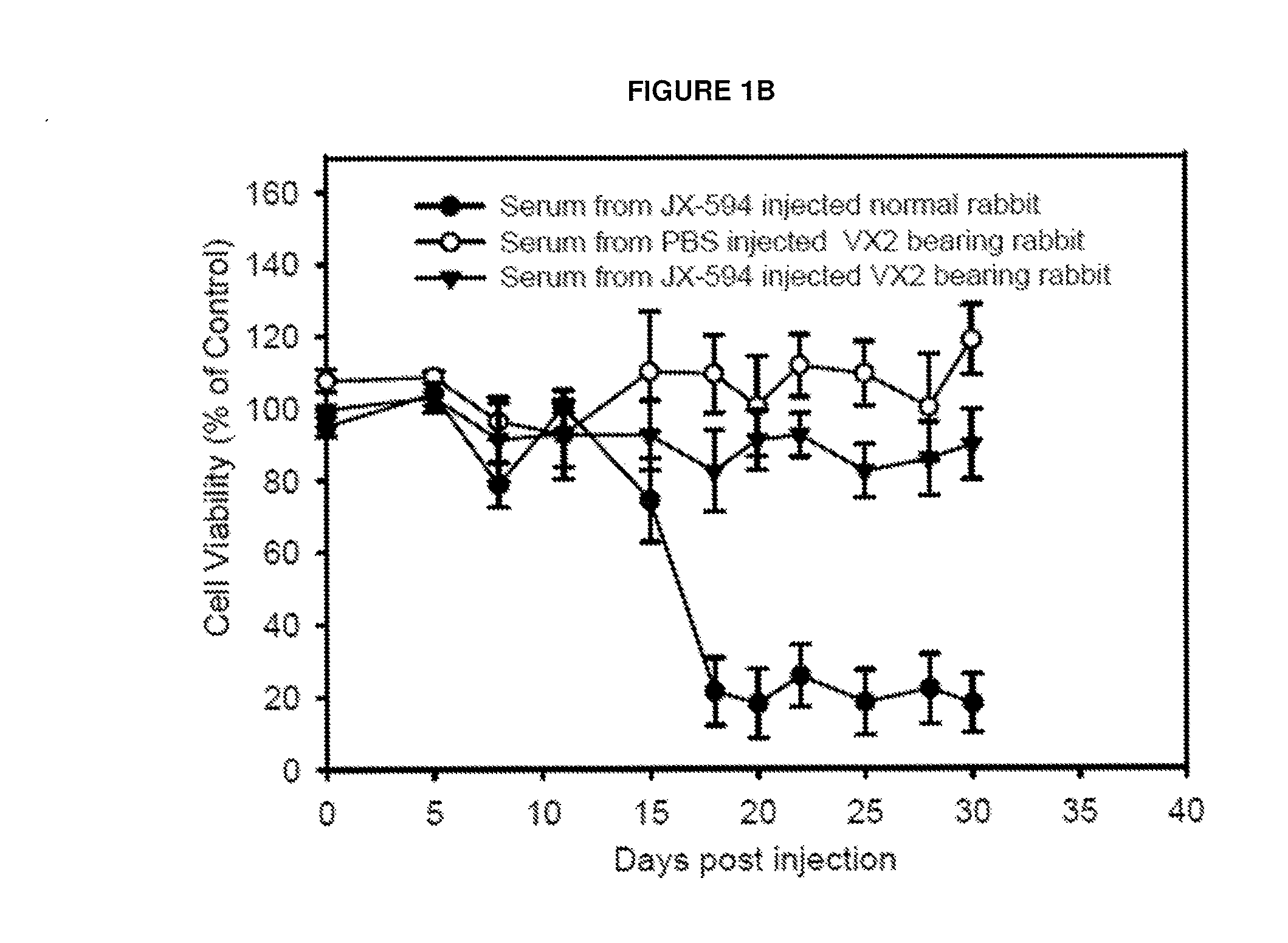
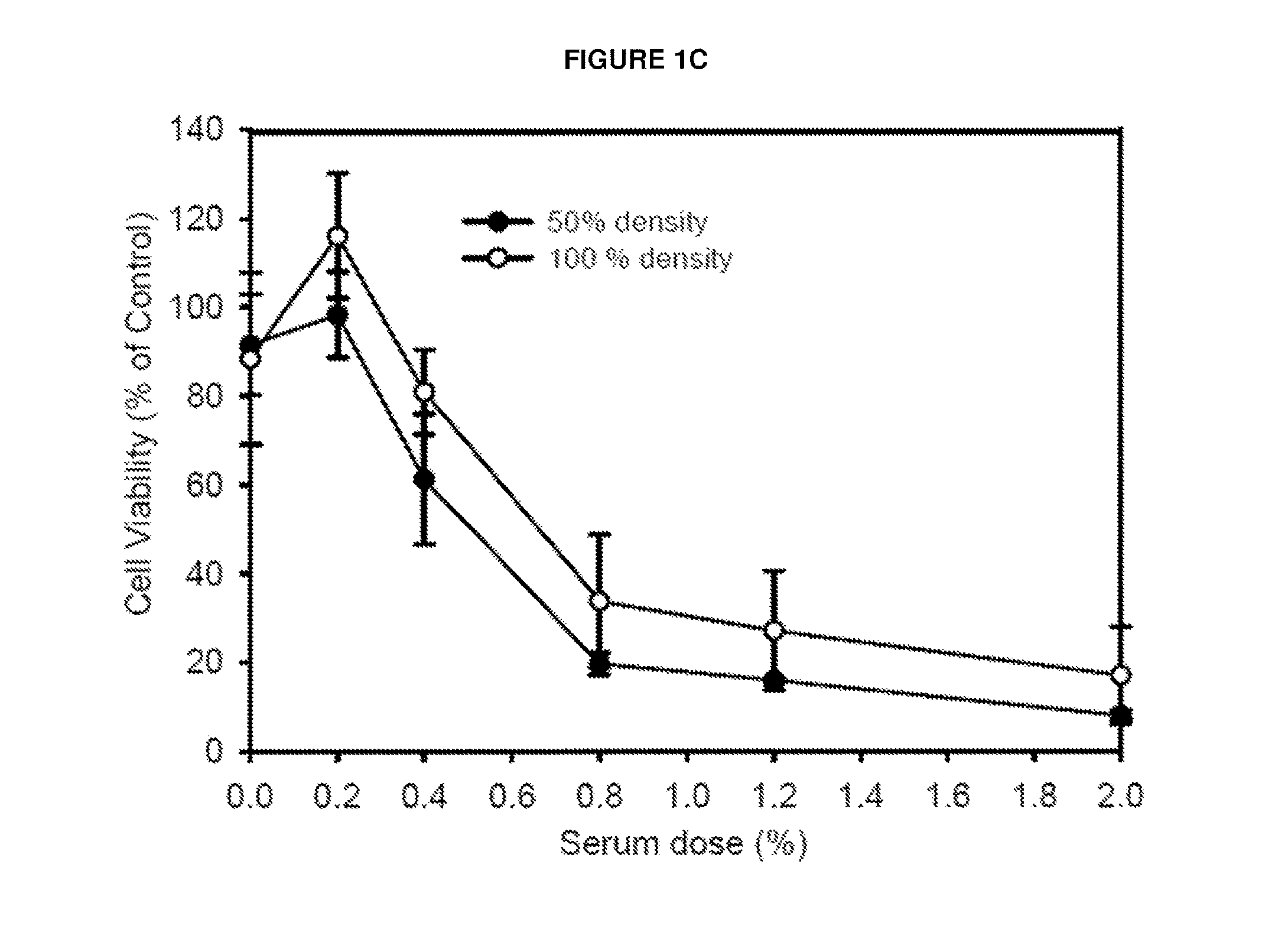
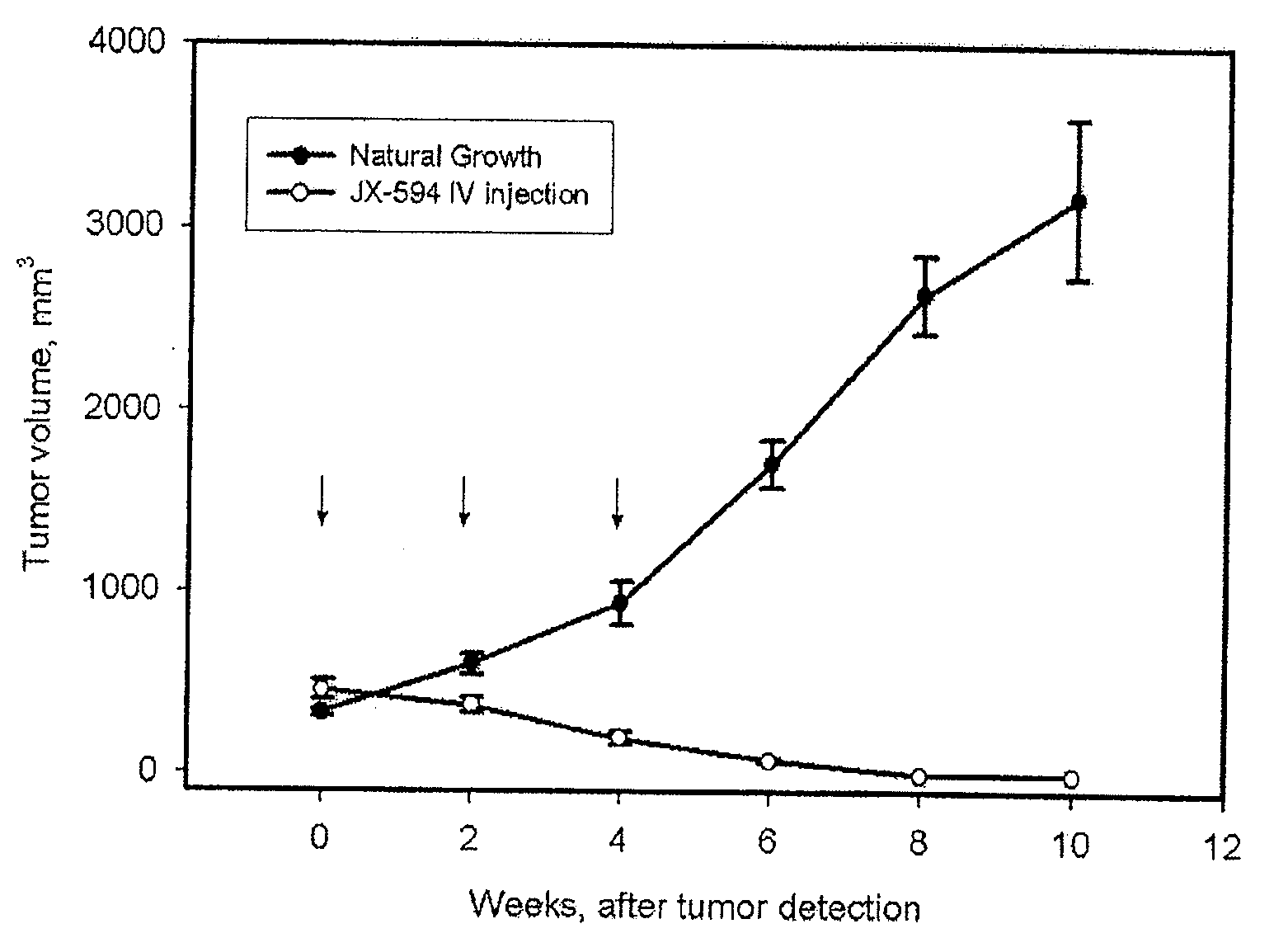
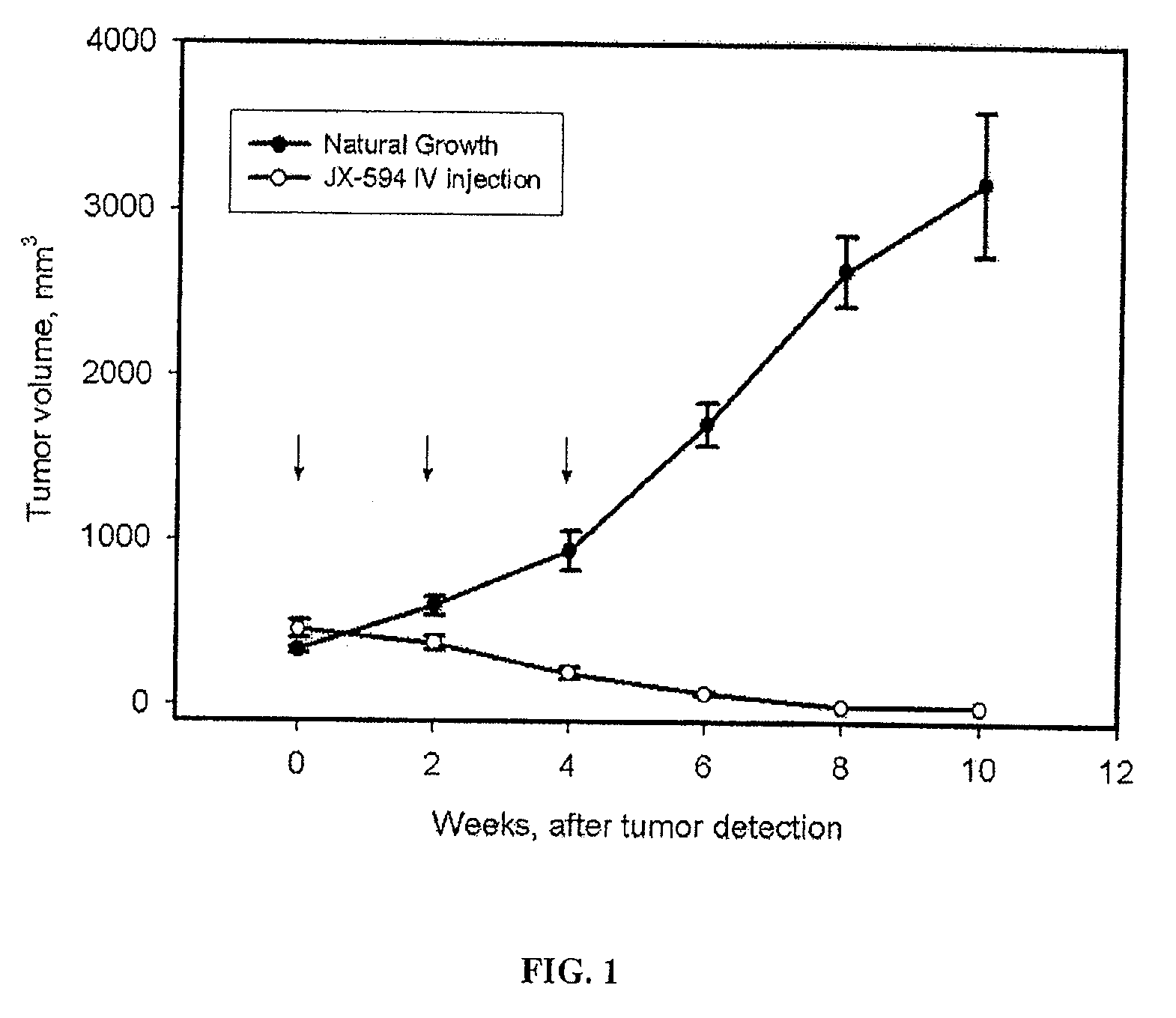
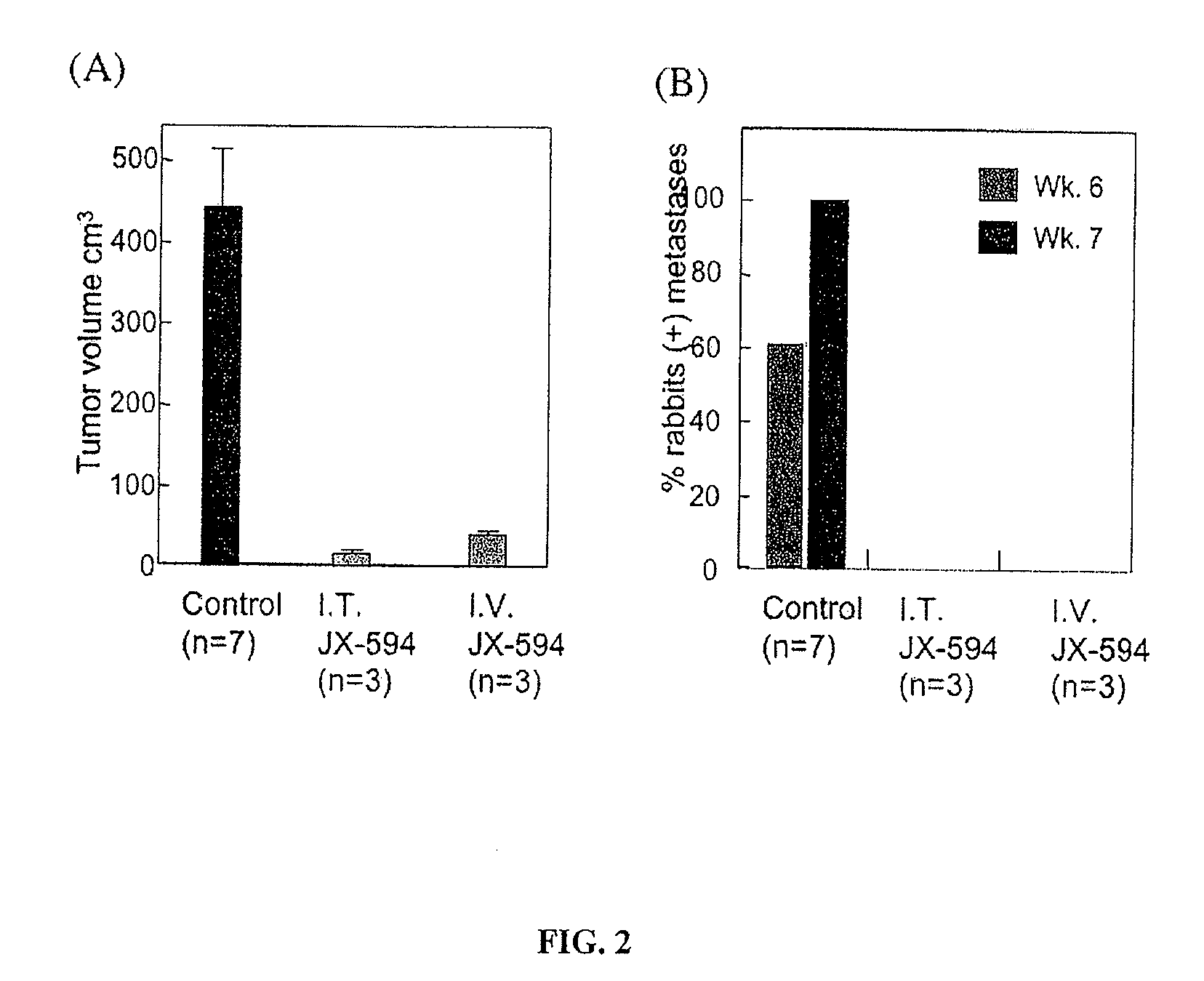
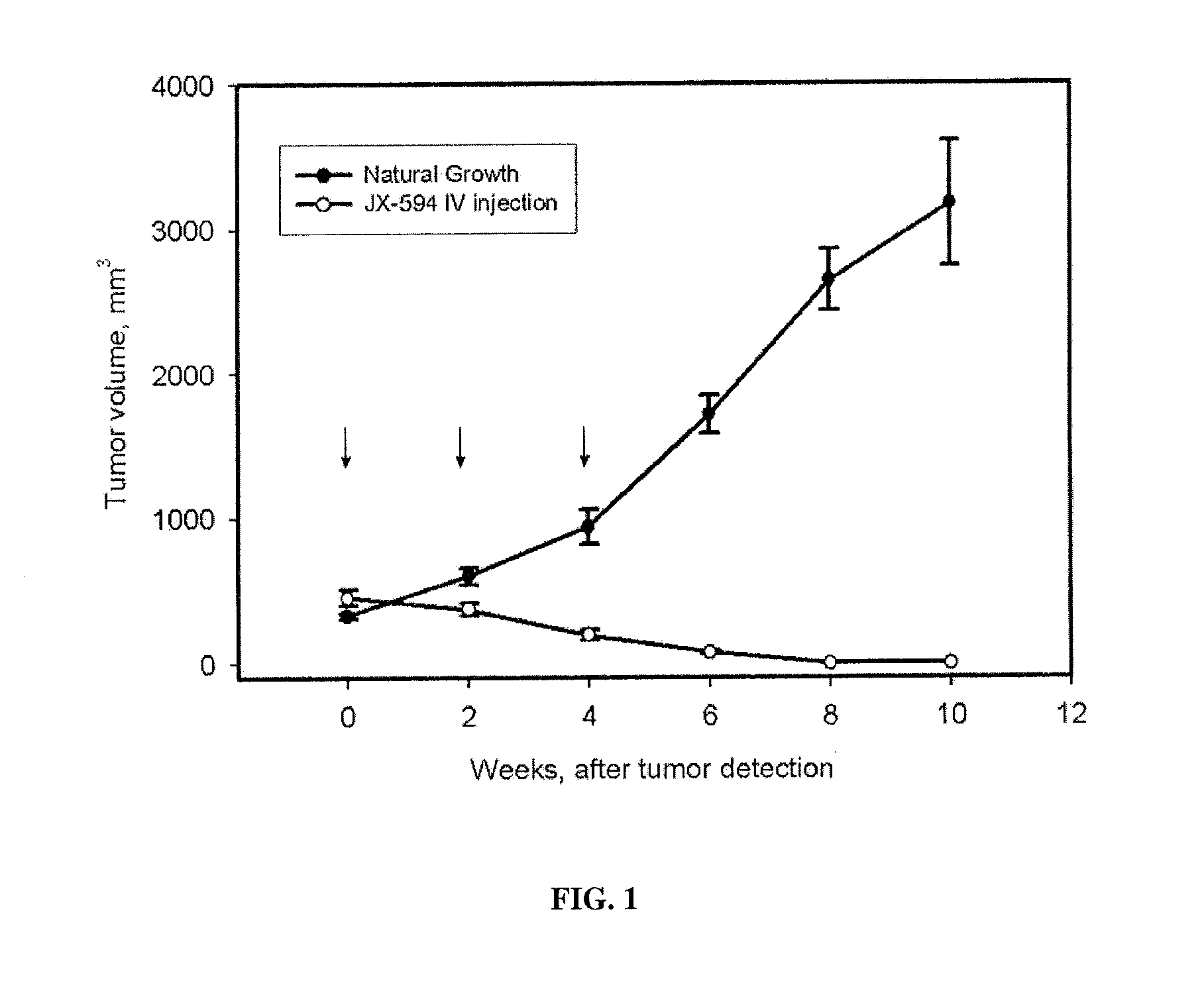
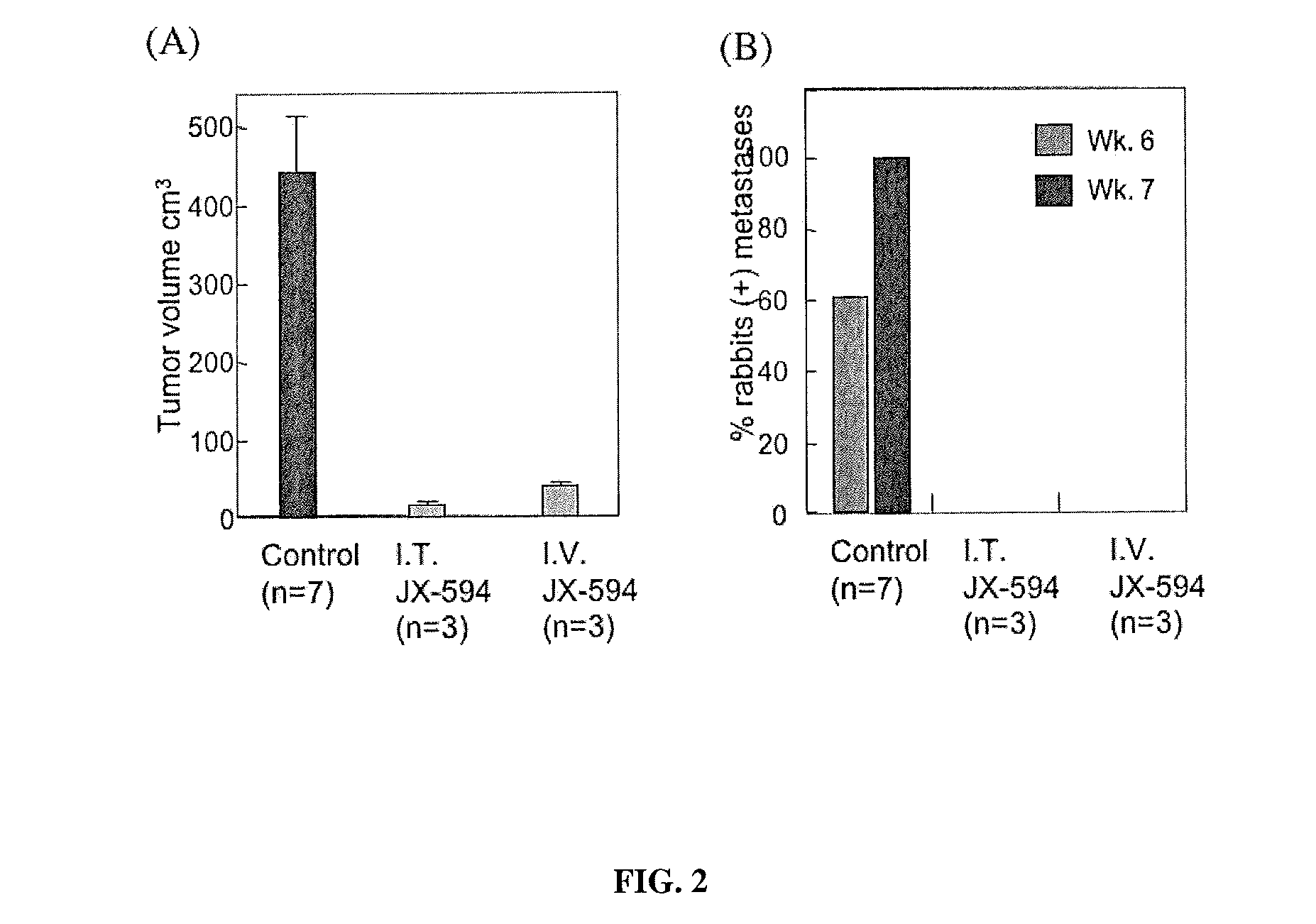
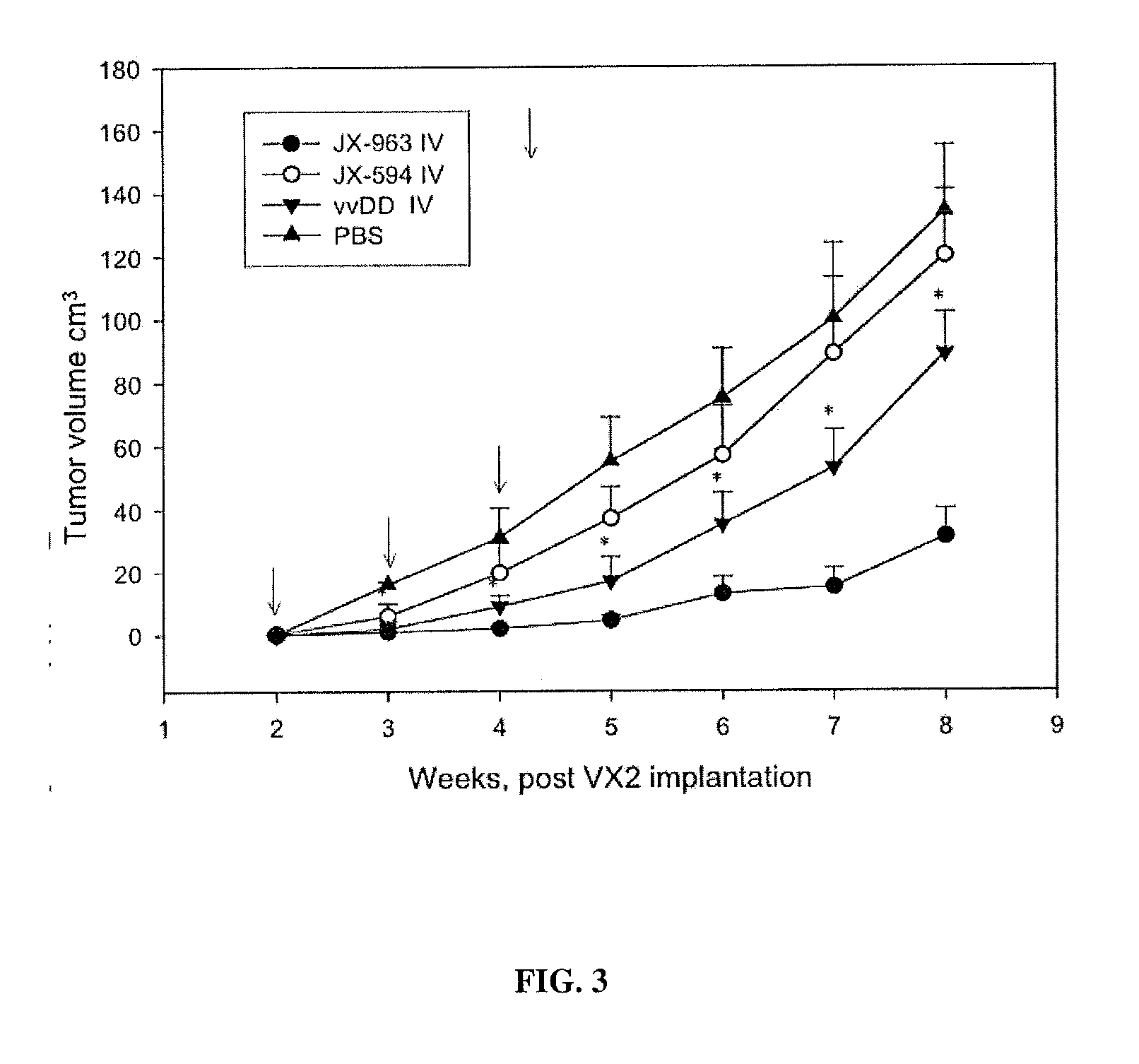
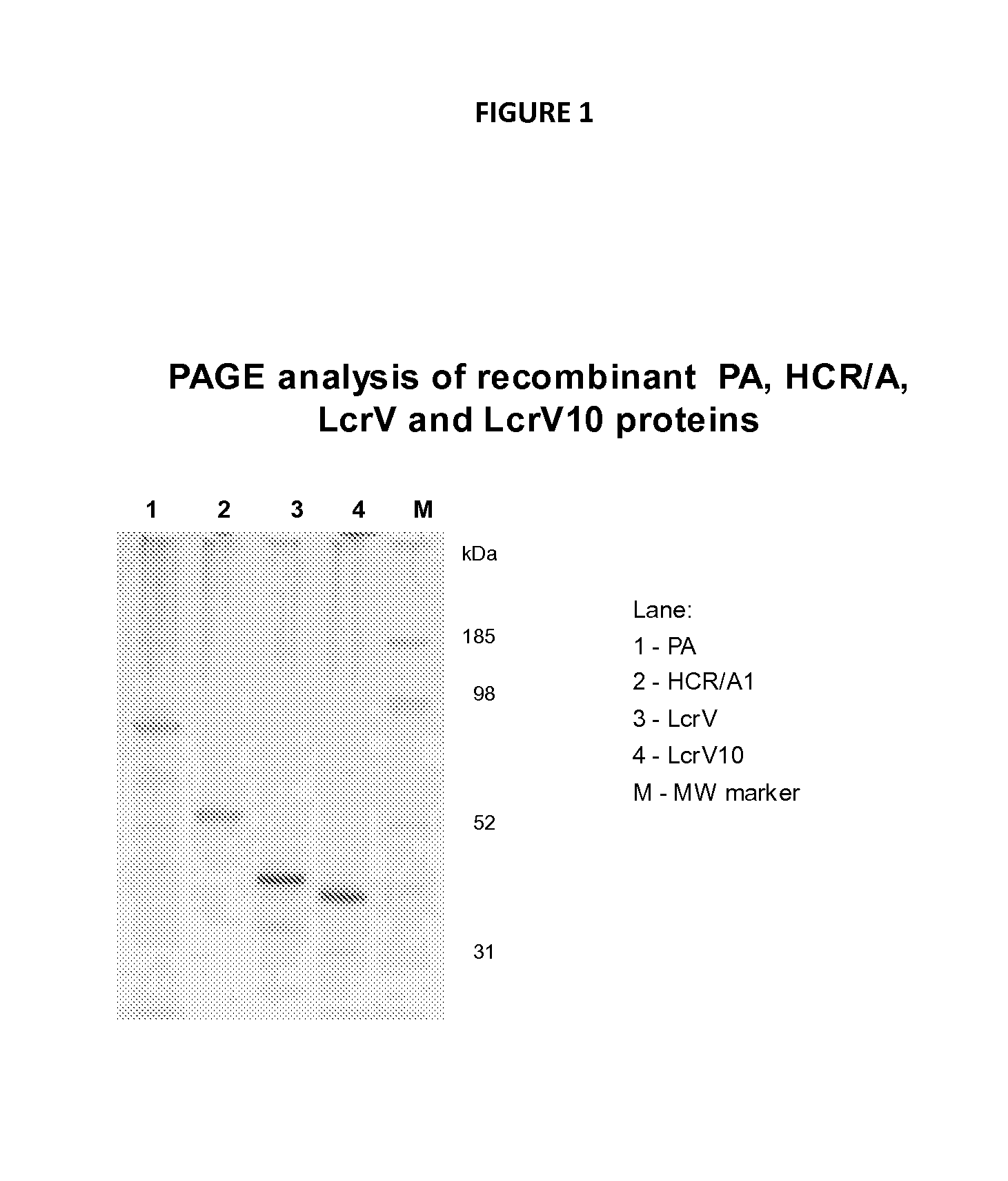
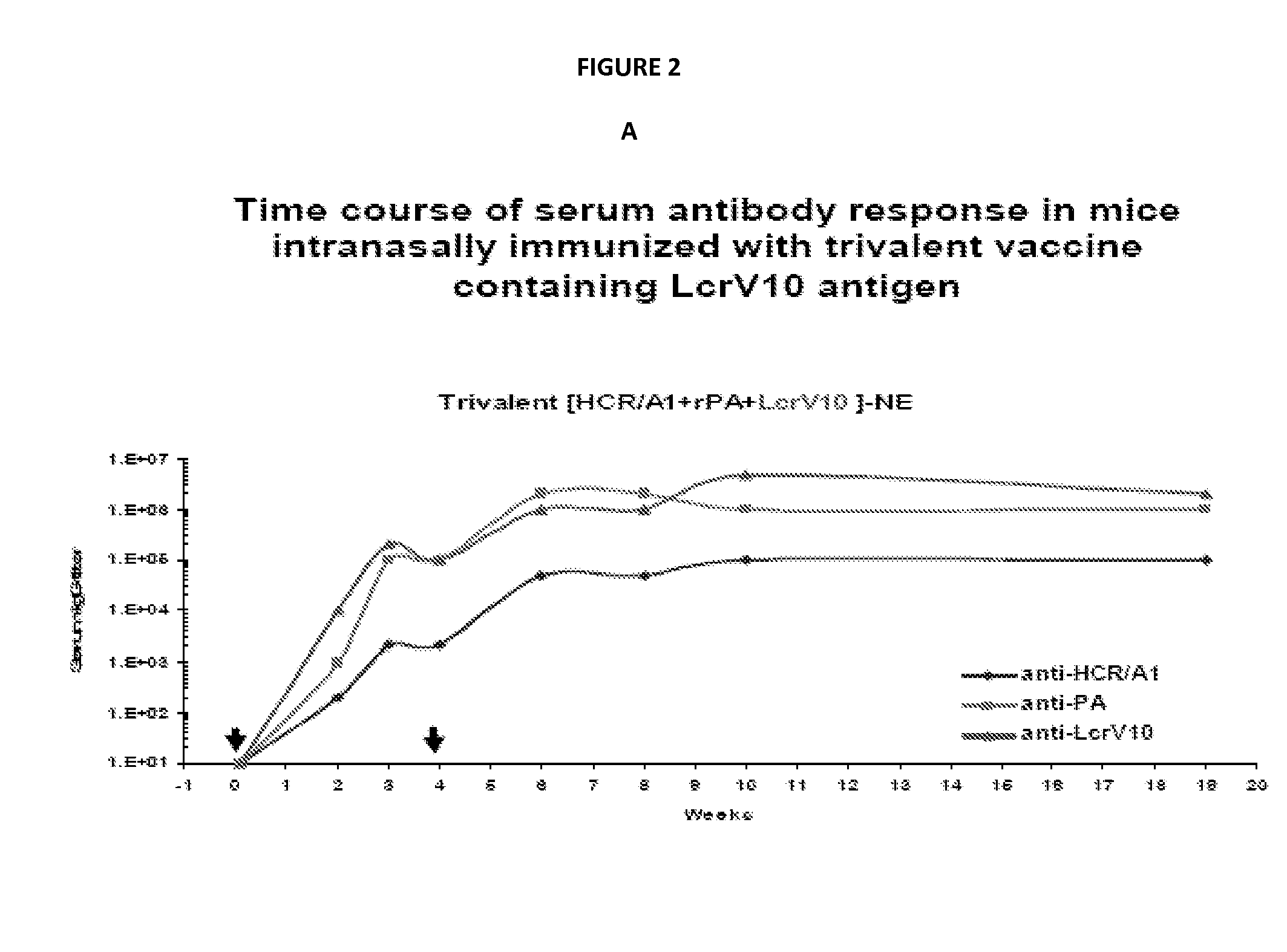
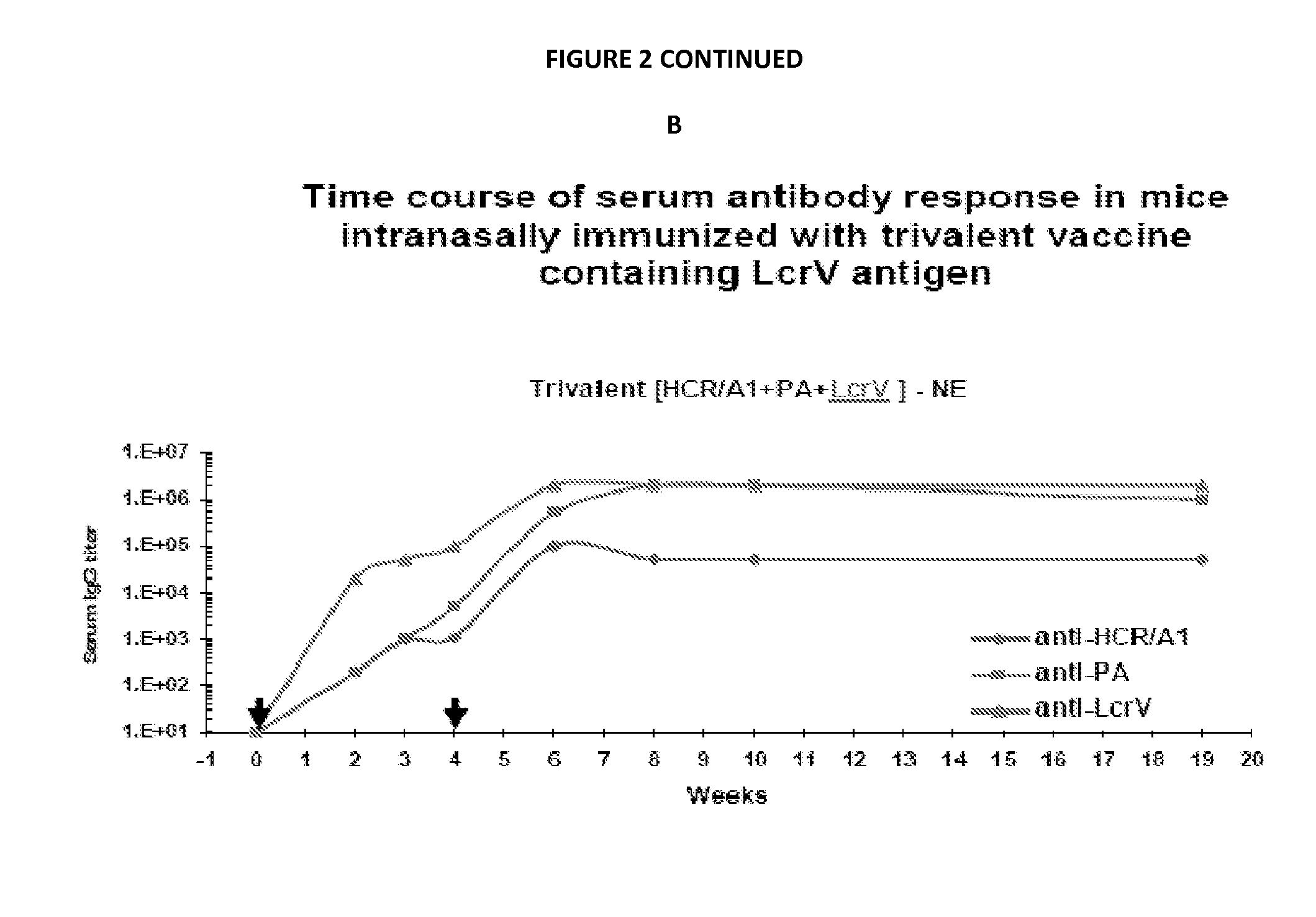
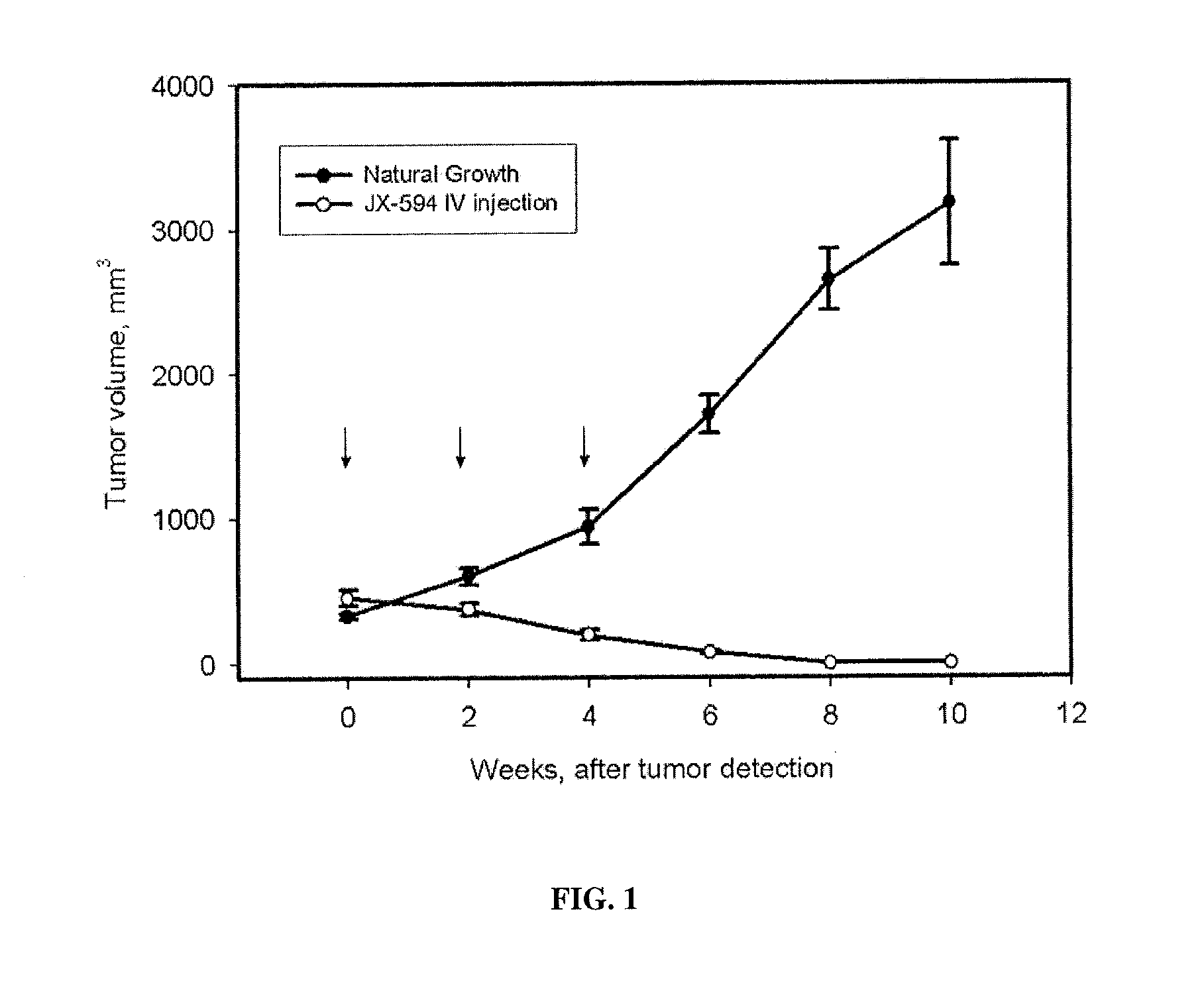
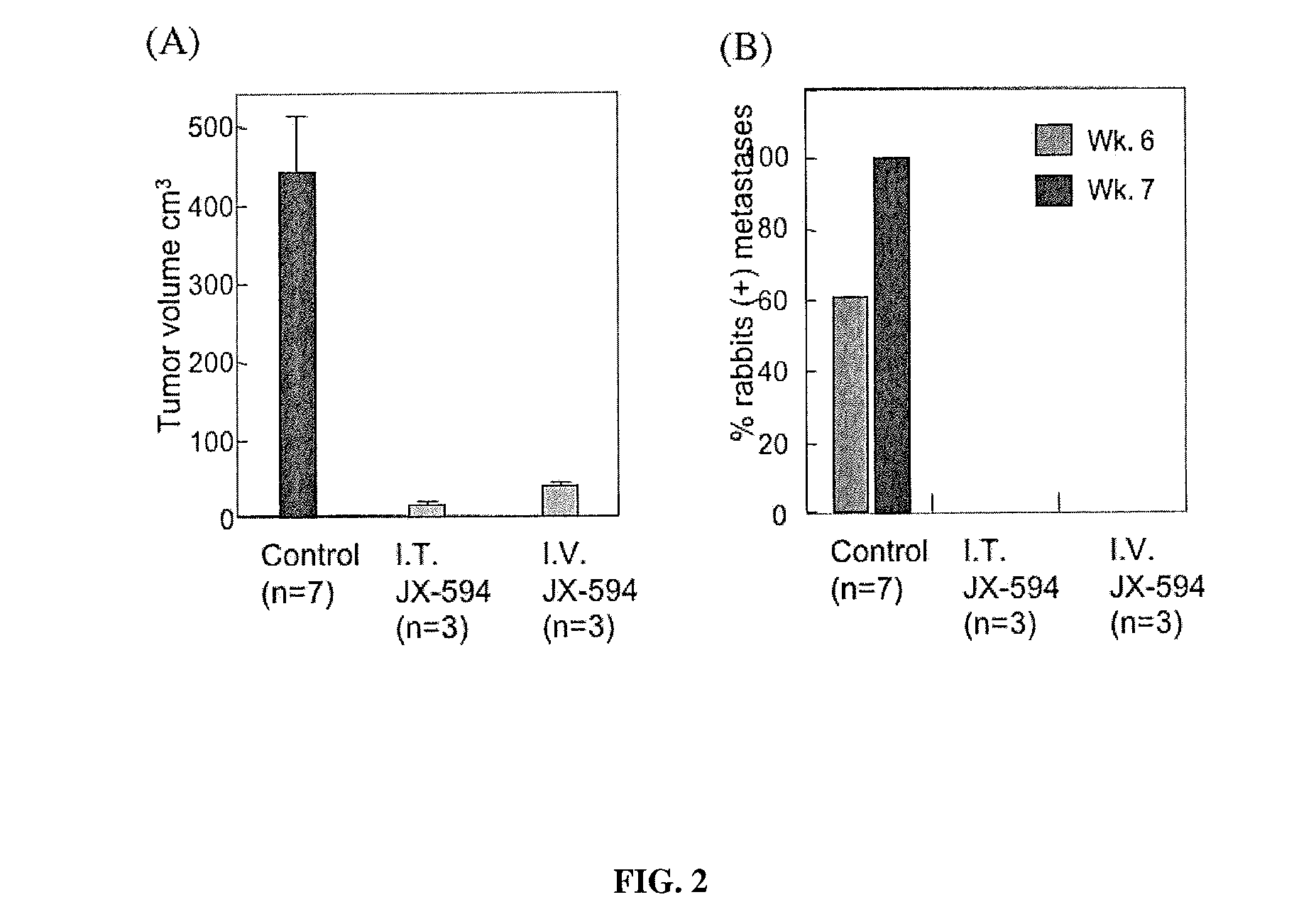
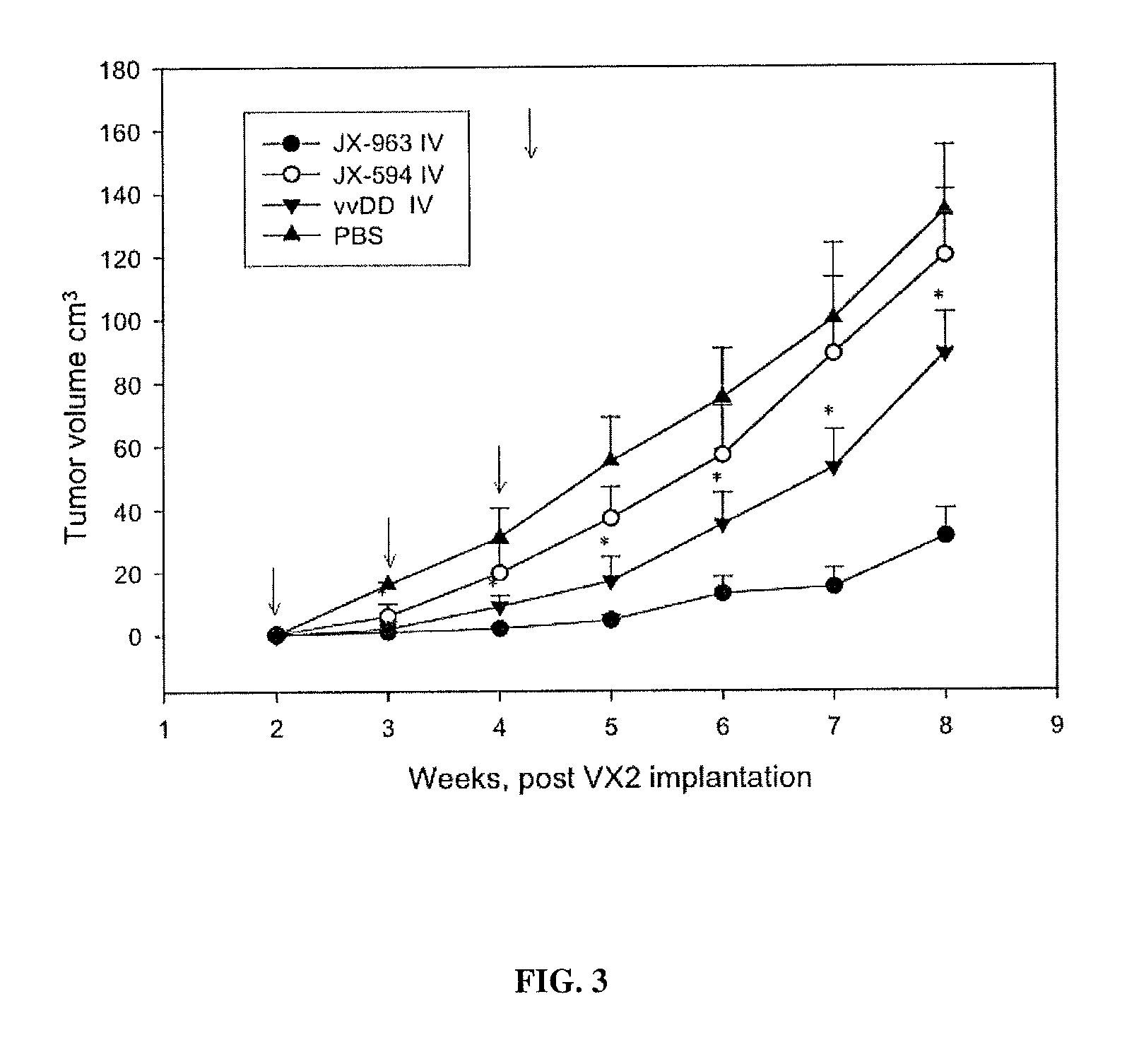
Patents
Literature
267 results about "Vaccinia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccinia virus (VACV or VV) is a large, complex, enveloped virus belonging to the poxvirus family. It has a linear, double-stranded DNA genome approximately 190 kbp in length, which encodes approximately 250 genes. The dimensions of the virion are roughly 360 × 270 × 250 nm, with a mass of approximately 5–10 fg.
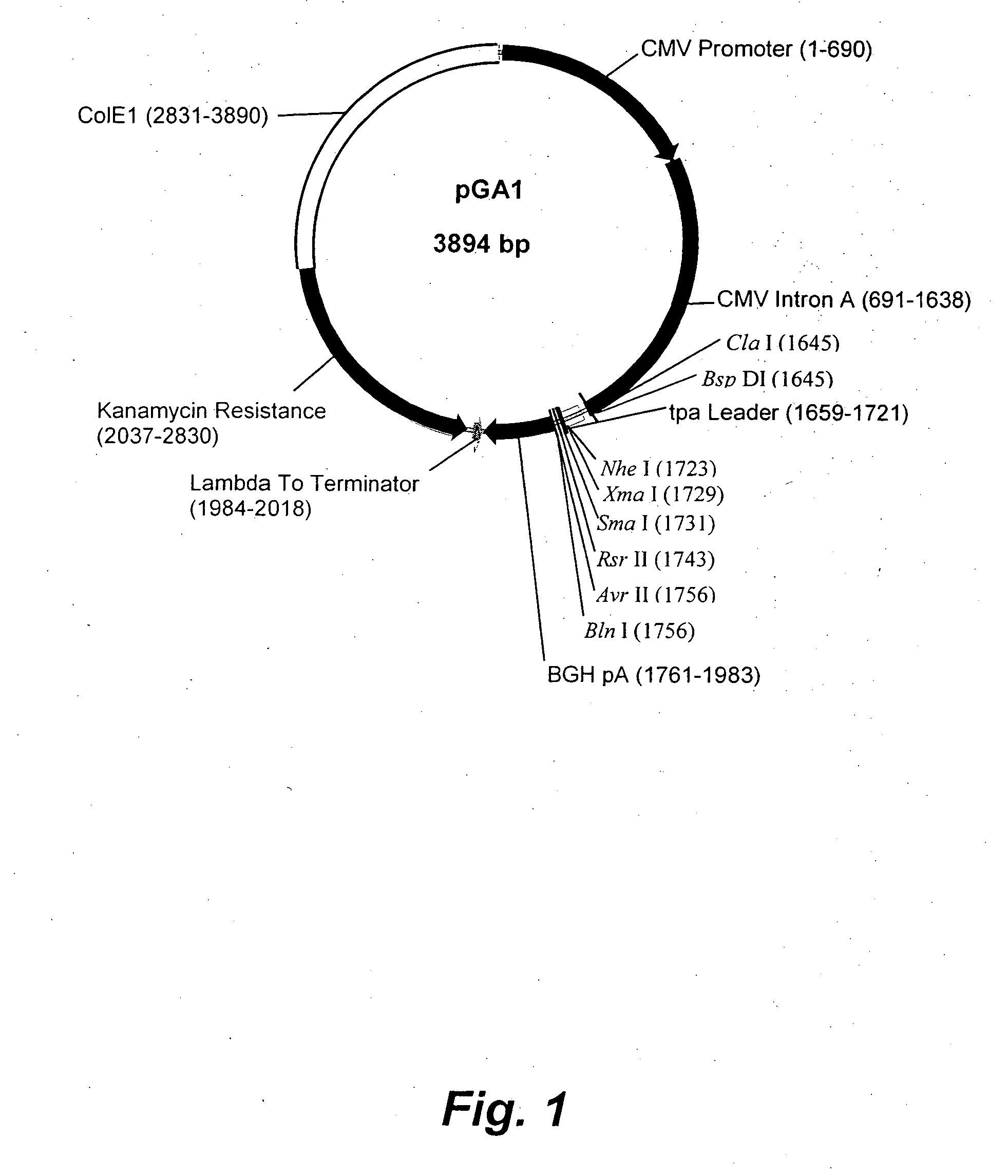
Vectors having enhanced expression and methods of making and uses thereof
InactiveUS6130066AHigh expressionIncrease transcriptionVectorsSugar derivativesOpen reading frameVaccinia
Disclosed and claimed are vectors having enhanced expression and methods for making and using them. Enhancement of expression is from substantially co-temporal expression of at least one first nucleic acid molecule and at least one second nucleic acid molecule. The second nucleic acid molecule encodes a transcription factor or a translation factor or a transcription factor and a translation factor. The contemporaneous expression can be from operably linking the first and second nucleic molecules to a single promoter, or from operably linking the first nucleic acid molecule to a first promoter and the second nucleic molecule to a second promoter wherein the first and second promoters function substantially contemporaneously. Thus, the first and second nucleic acid molecules can be at the same locus in the vector, or at different loci. The second nucleic acid molecule can encode: one transcription factor or more than one transcription factor; or one translation factor or more than one translation factor; or at least one transcription factor and at least one translation factor. The transcription factor can be from vaccinia H4L, D6, A7, G8R, A1L, A2L, H5R, or combinations thereof. The translation factor can be from a K3L open reading frame, an E3L open reading frame, a VAI RNA, an EBER RNA, a sigma 3 open reading frame, a TRBP open reading frame, or combinations thereof. The vector can be a poxvirus such as an attenuated poxvirus, e.g., NYVAC, or ALVAC.
Owner:VIROGENETICS
Recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS6998252B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses also are provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
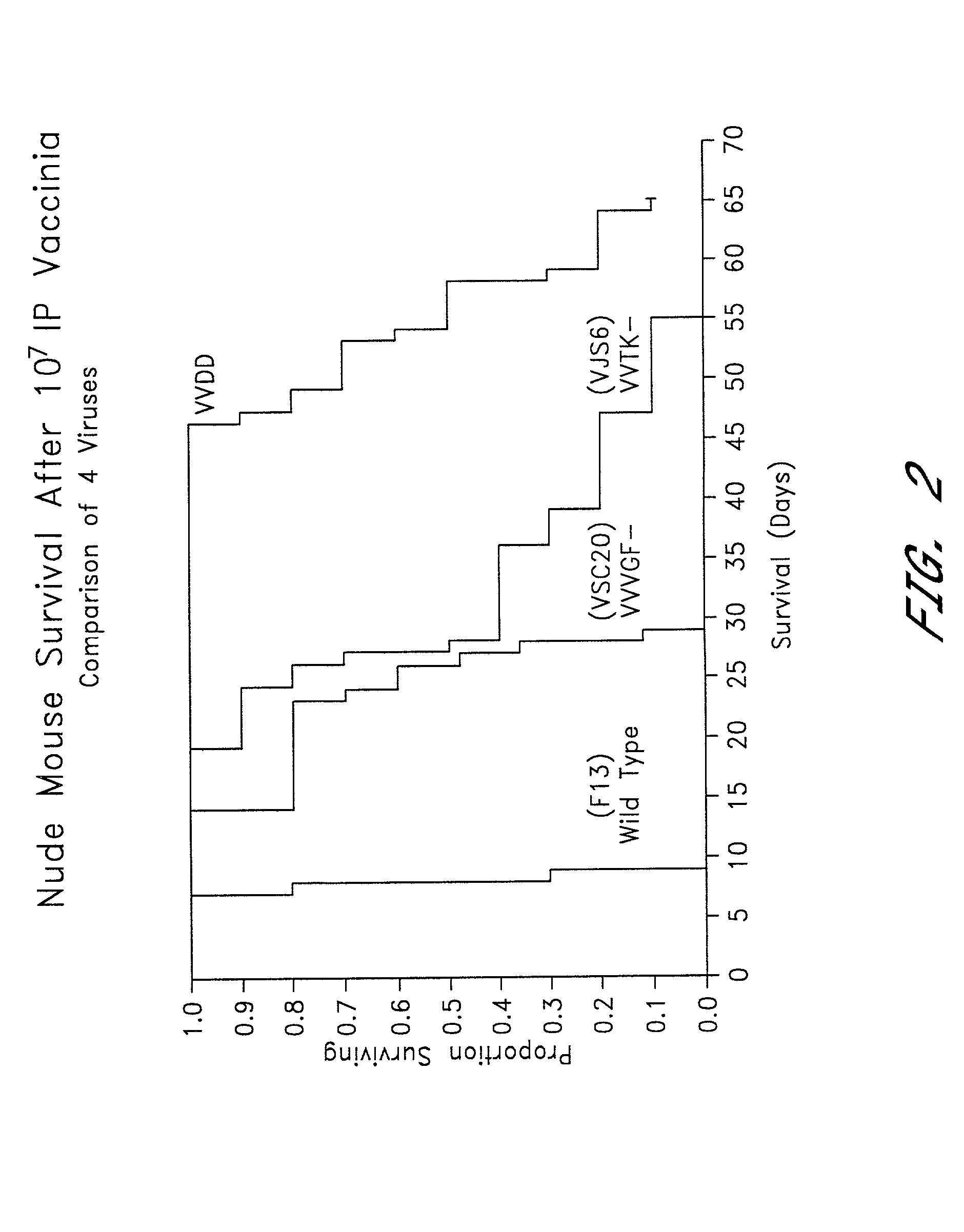
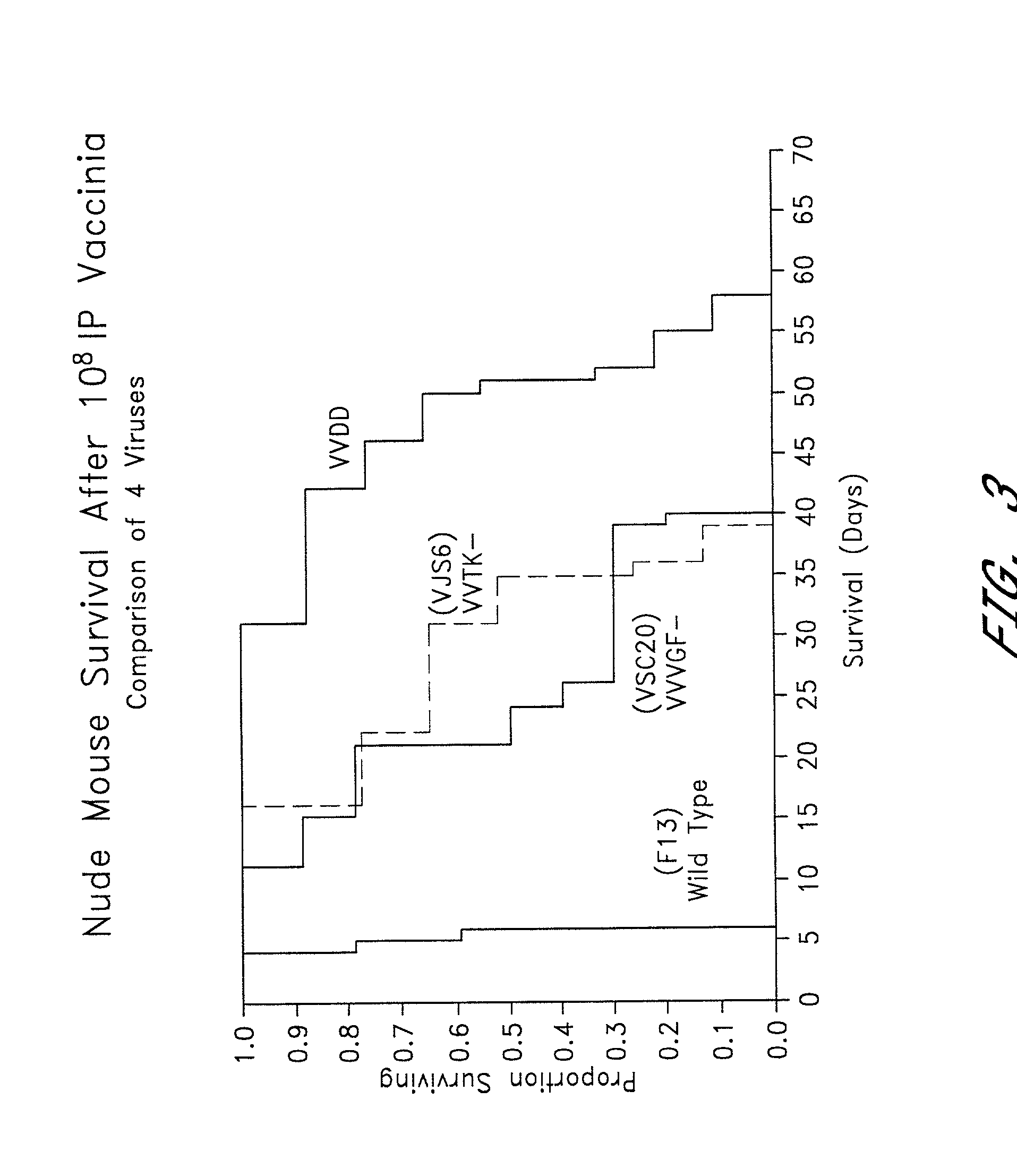
Combined growth factor-deleted and thymidine kinase-deleted vaccinia virus vector
A composition of matter comprising a vaccinia virus expression vector with a negative thymidine kinase phenotype and a negative vaccinia virus growth factor phenotype.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT HEALTH & HUMAN SERVICES THE
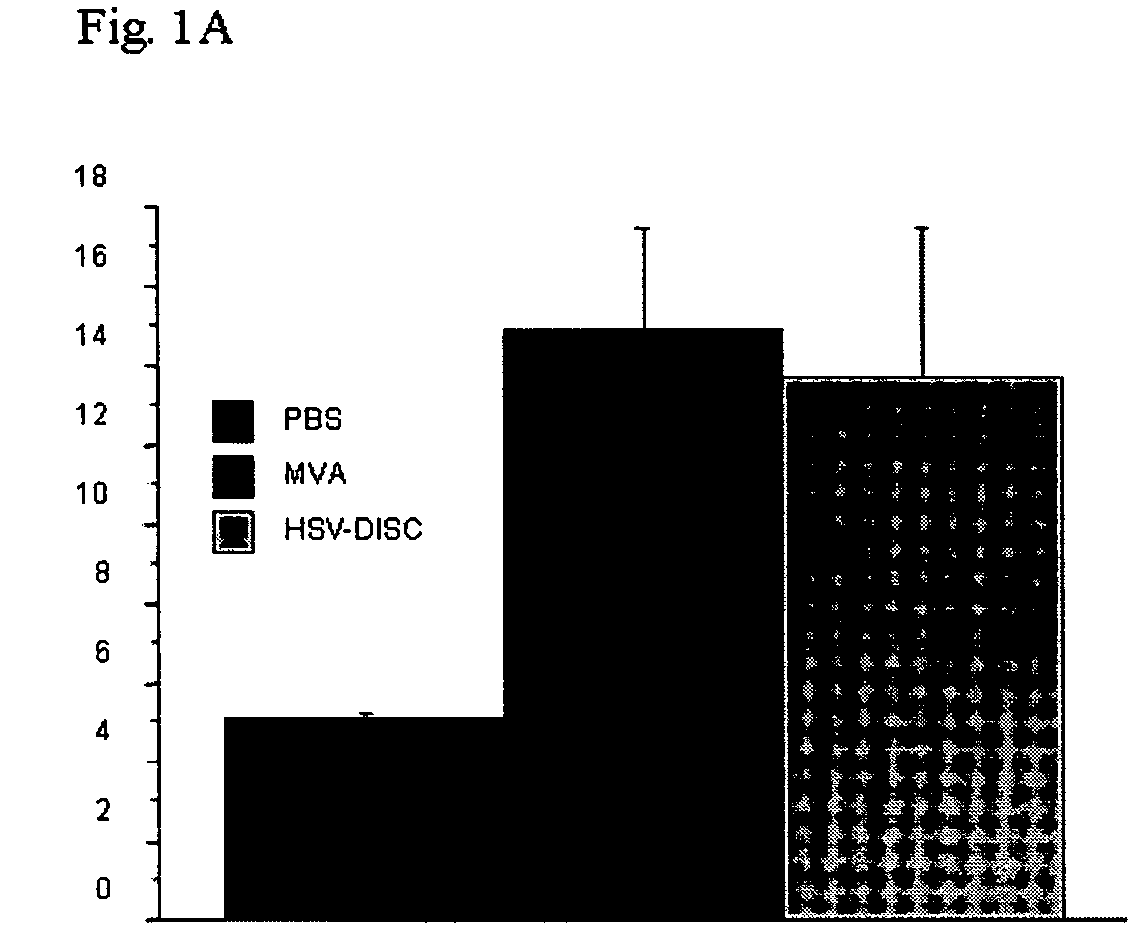
Modified vaccinia virus ankara for the vaccination of neonates
Owner:BAVARIAN NORDIC AS
Combined growth factor-deleted and thymidine kinase-deleted vaccinia virus vector
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT HEALTH & HUMAN SERVICES THE
Immunogenic compositions derived from poxviruses and methods of using same
Immunogenic compostions composed of poxvirus immunogens and related methods are disclosed. Specifically, immunogenic compostions useful in eliciting immune responses in animals are disclosed. In one embodiment the immunogenic compostions include viral antigens derived from vaccinia and / or variola that elicit cross-reactive immune responses. The immunogens can be made synthetically, by using recombinant DNA technology or derived from purified virus. Moreover, methods of using the immunogenic compostions are also disclosed.
Owner:MANNKIND CORP
Altered strain of the modified vaccinia virus ankara (mva)
InactiveUS20030013190A1Ease of mass productionEfficient and large-scale productionBiocideGenetic material ingredientsAdjuvantVirulent characteristics
The invention provides new strains of the Modified Vaccinia Virus Ankara (MVA) that have a strongly reduced virulence for most mammals, especially humans, but nevertheless grows in cells of a continuous cell line approved for the production of a therapeutic agent such as a vaccine. The invention also provides a method for producing said adapted MVA strains. The adapted MVA can be used e.g. for parenteral immunization, as a vector system, or in the active or inactivated from as an adjuvant or as a regulator of the unspecific components of the immune system.
Owner:BAVARIAN NORDIC AS
Modified Vaccinia virus Ankara for the vaccination of neonates
InactiveUS20060127984A1Promote maturitySafely and efficiently vaccinateViral antigen ingredientsMicrobiological testing/measurementAntigenEpidermal Dendritic Cells
The invention concern the use of a virus for the preparation of a medicament for the vaccination or treatment of a neonatal or prenatal animal, including a human, wherein the virus is capable of infecting the cells of the neonatal or prenatal animal, including a human, but not capable of being replicated to infectious progeny virus in the neonatal or prenatal animal, including a human. The virus is preferably a Modified Vaccinia Virus Ankara. In particular, the invention concerns the vaccination of neonates against infections with viruses belonging to the same virus group than the virus used for vaccination. Moreover, the invention concerns the vaccination of neonates against antigens selected from foreign antigens and tumour antigens, wherein the tumour antigen and / or the foreign antigen are different from the antigens associated with the virus. The invention further concerns the use of viruses as defined above to increase the level of factors which activate dendritic cells or their precursor cells and / or to increase the number of dendritic cells or their precursor cells and / or to increase the production and / or cellular content of an interferon (IFN) or IL-12.
Owner:BAVARIAN NORDIC AS
Method for Detection of Cancer Cells Using Virus
InactiveUS20090311664A1Microbiological testing/measurementMammal material medical ingredientsNon cancerCancer cell
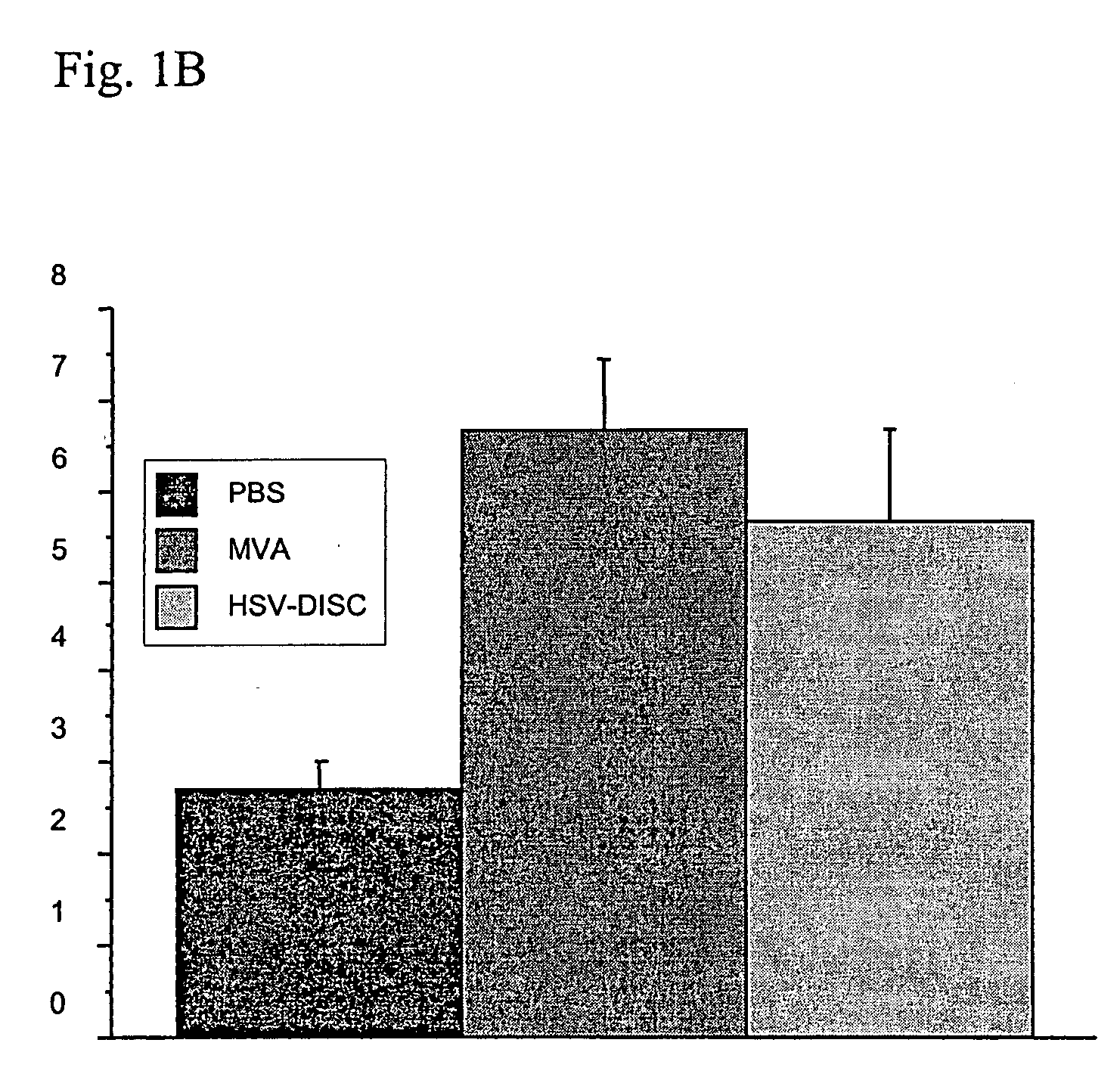
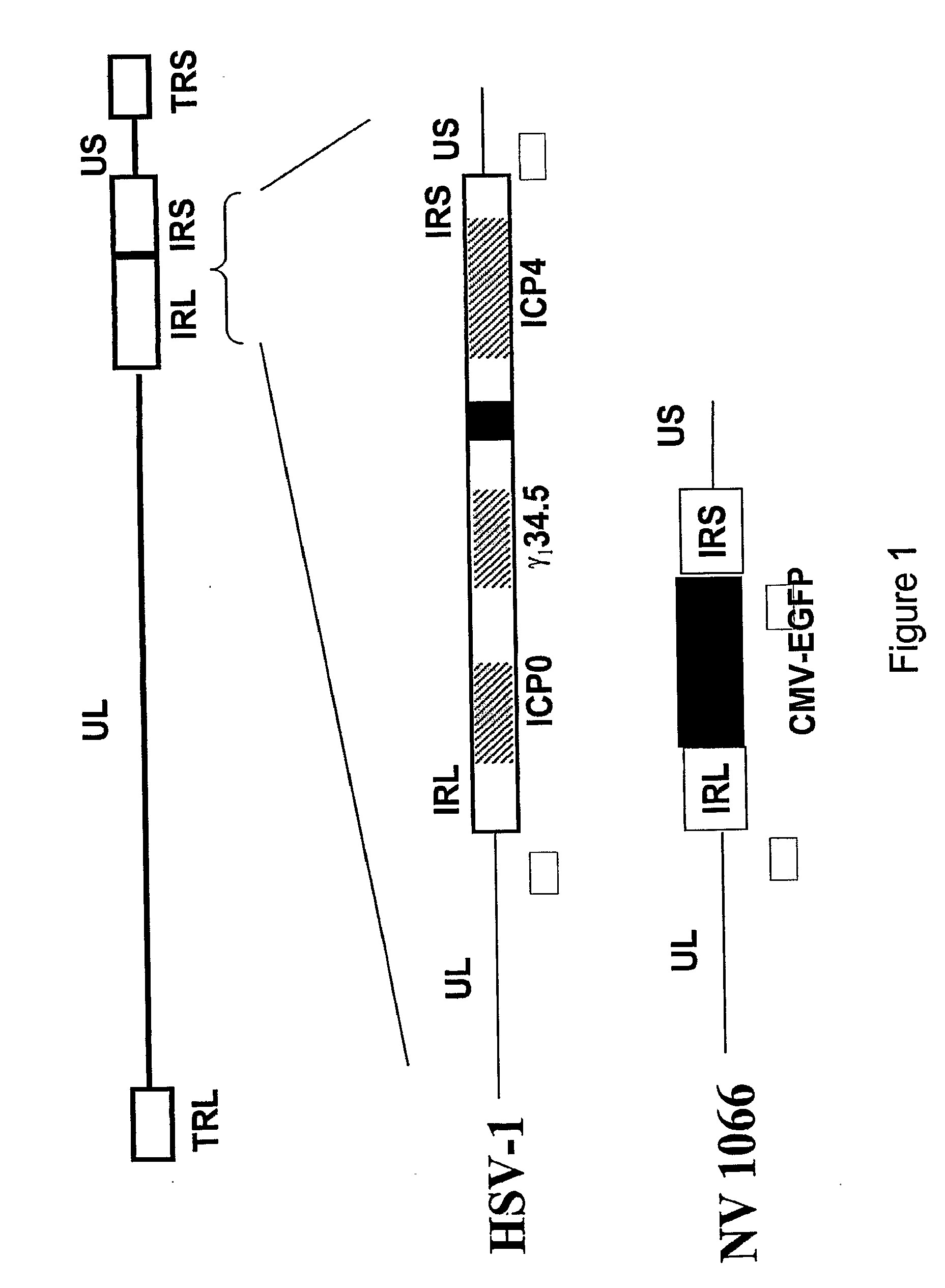
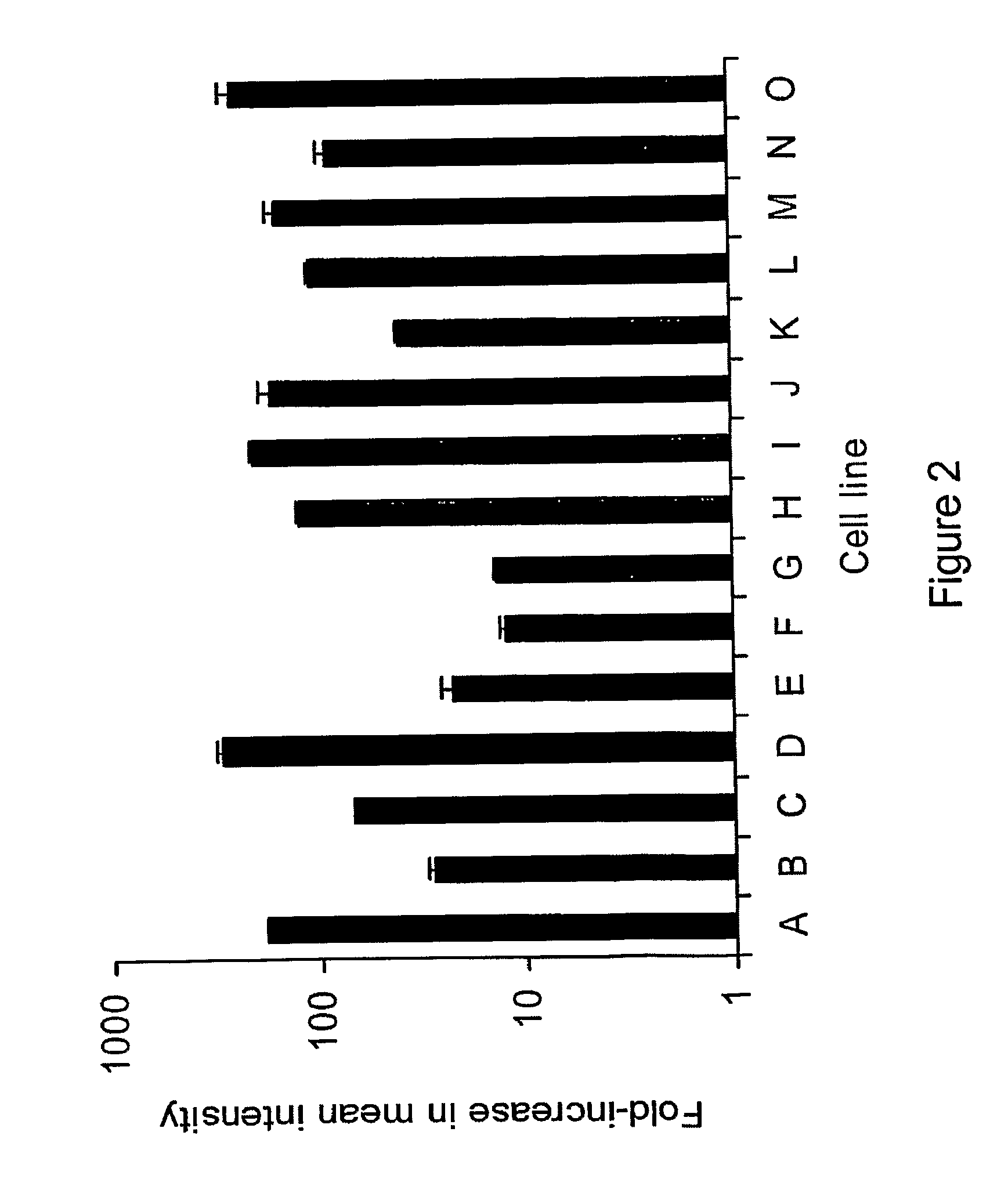
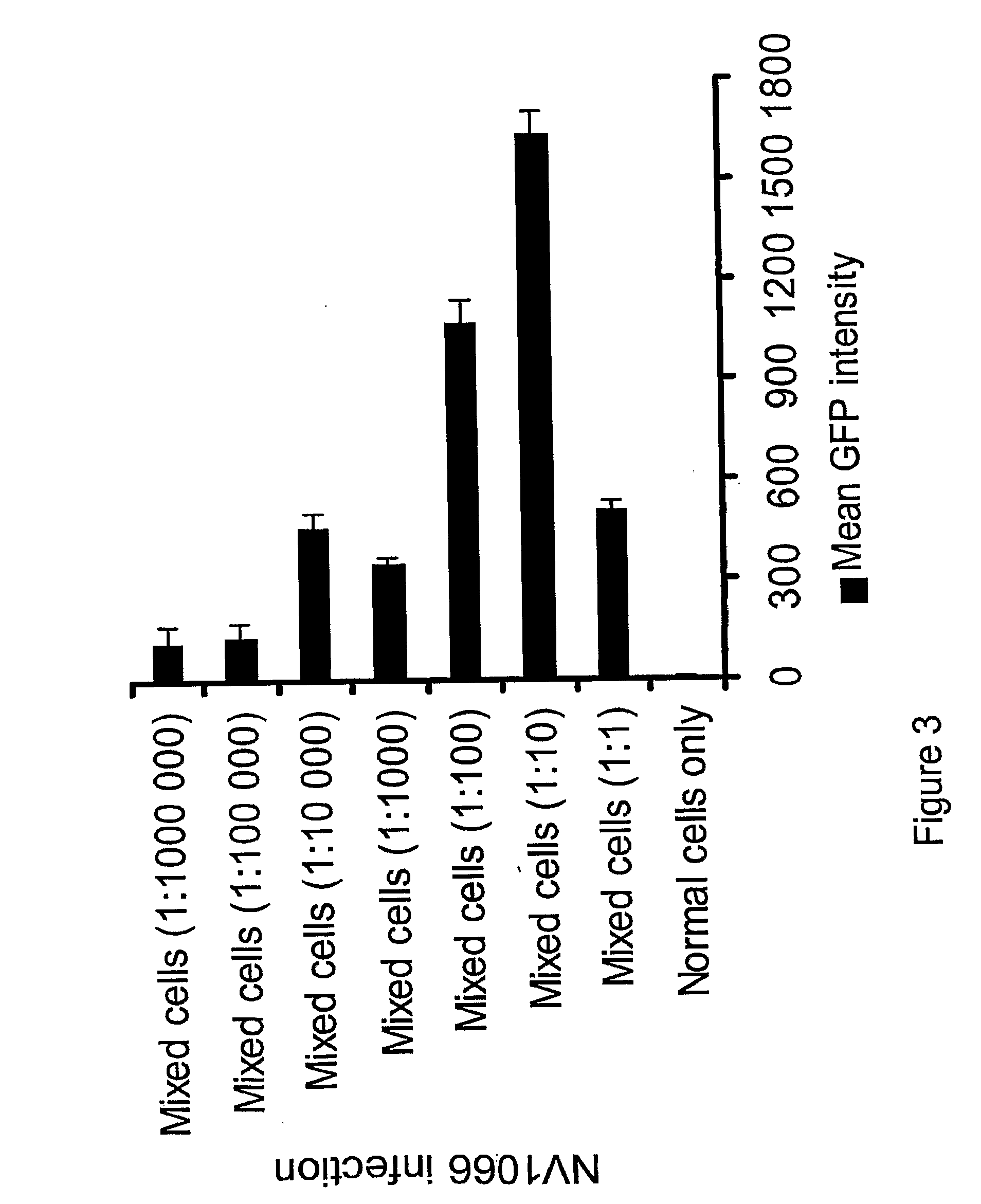
The invention relates to compositions and methods for cancer cell detection in bodily samples wherein a cancer cell can be detected within a mixed population of cancer cells and non-cancer cells. The invention also relates to compositions and methods that may be used in cancer cell detection, specifically viruses that are replication-competent conditional to a cancer cell, in particular an oncolytic herpes virus, such as NV 1066 and a vaccinia virus, such as GLV-1h68. Provided are methods and kits for using these viruses that preferentially replicate in cancer cells and may also preferentially infect cancer cells for specific identification of such cancer cells, even when a cancer cell is present, for example, at a ratio of one infected cancer cell in a background often thousand non-cancer cells, thus further providing a reproducible and sensitive screening method for cancer detection, monitoring and prognosis.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Rapid vaccinia antibody detection device, method and test kit
InactiveUS20040002063A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSerum igeTest sample
The invention relates to a rapid vaccinia antibody detection device, method and test kit for the detection of vaccinia antibody in a serum, plasma or whole blood test sample utilizing a purified vaccinia cell lysate as the capture antigen. The detection device operates on the basis of a 2-step flow-through format in use with a push buffer.
Owner:MEDMIRA
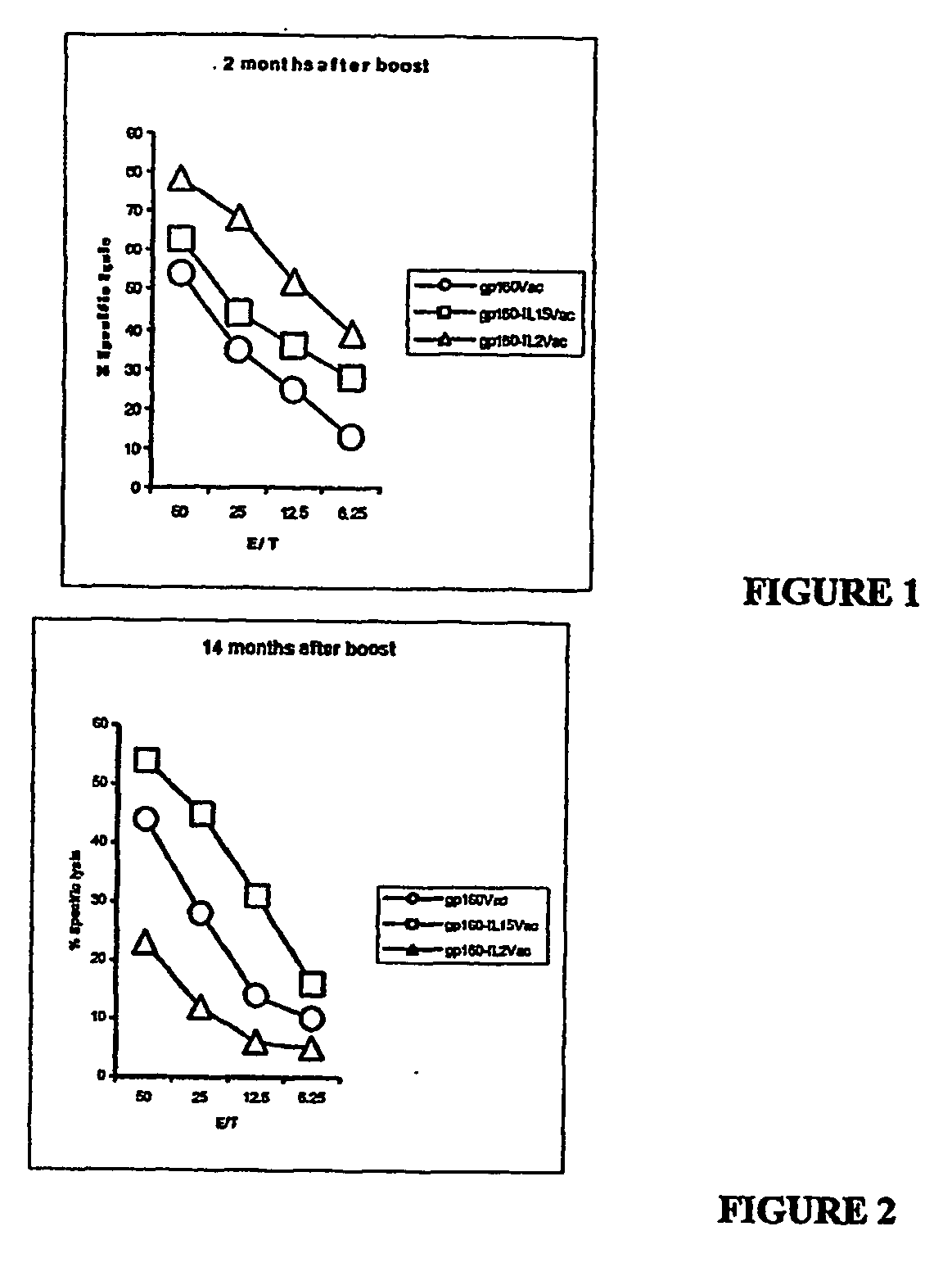
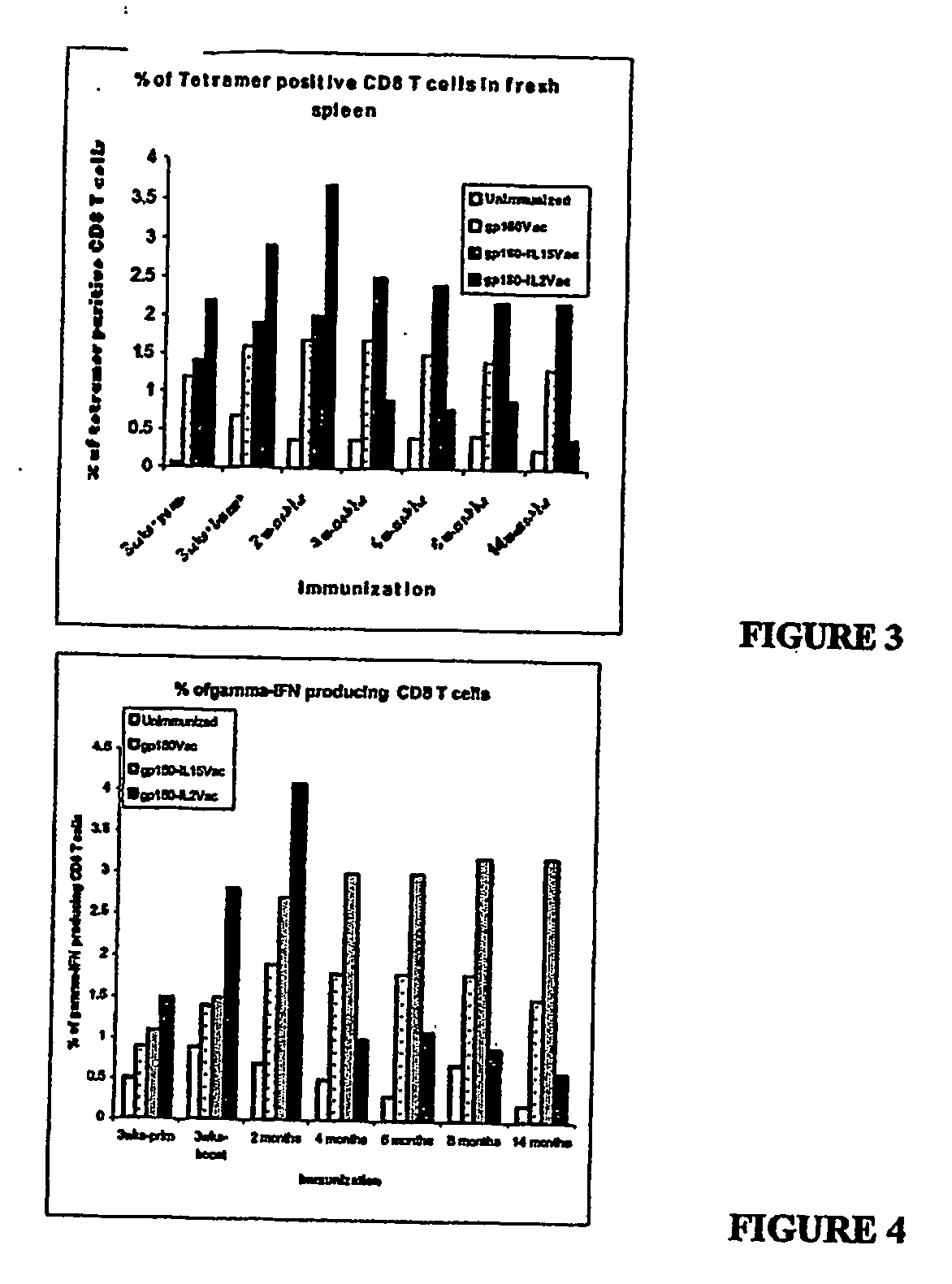
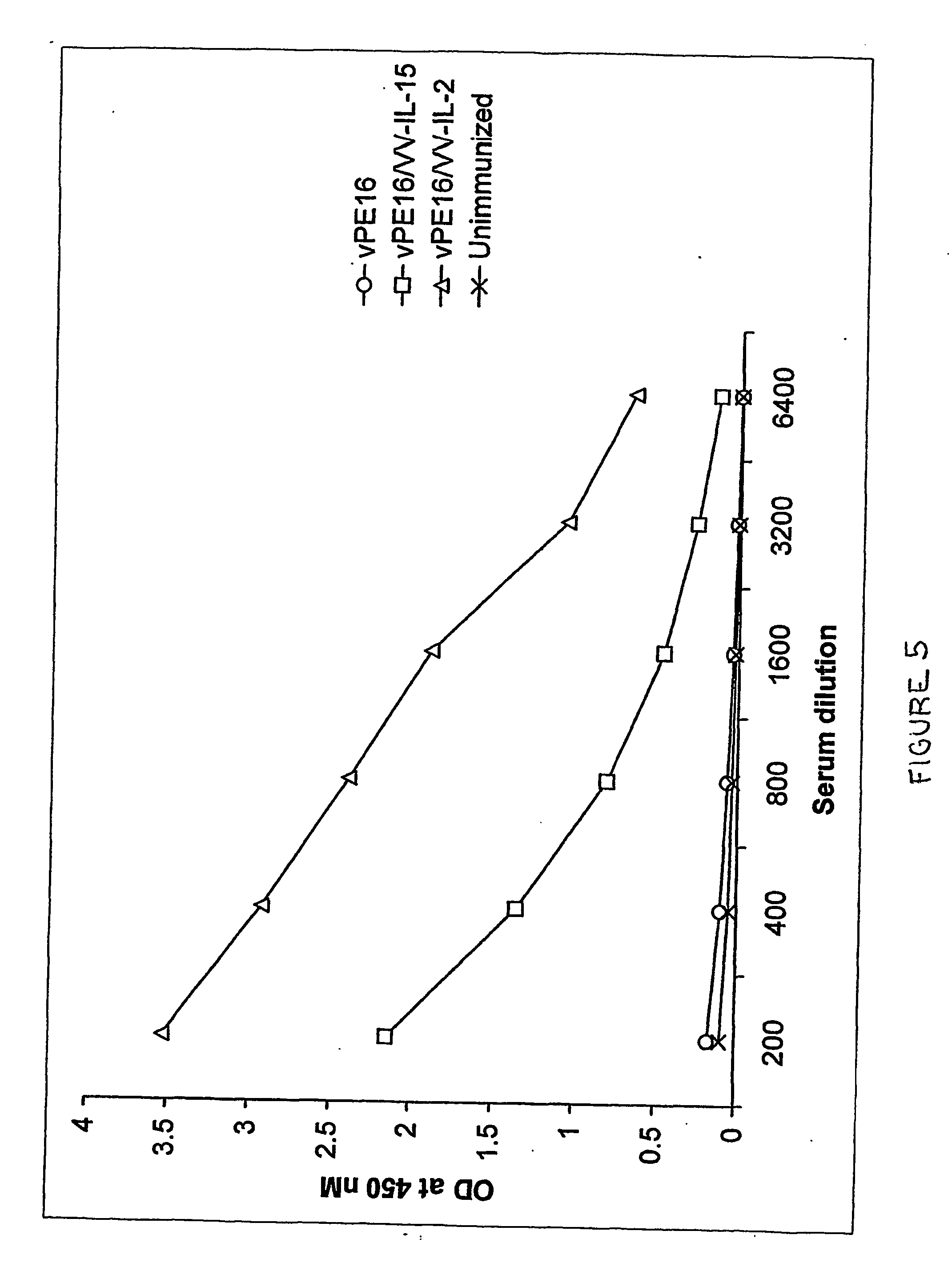
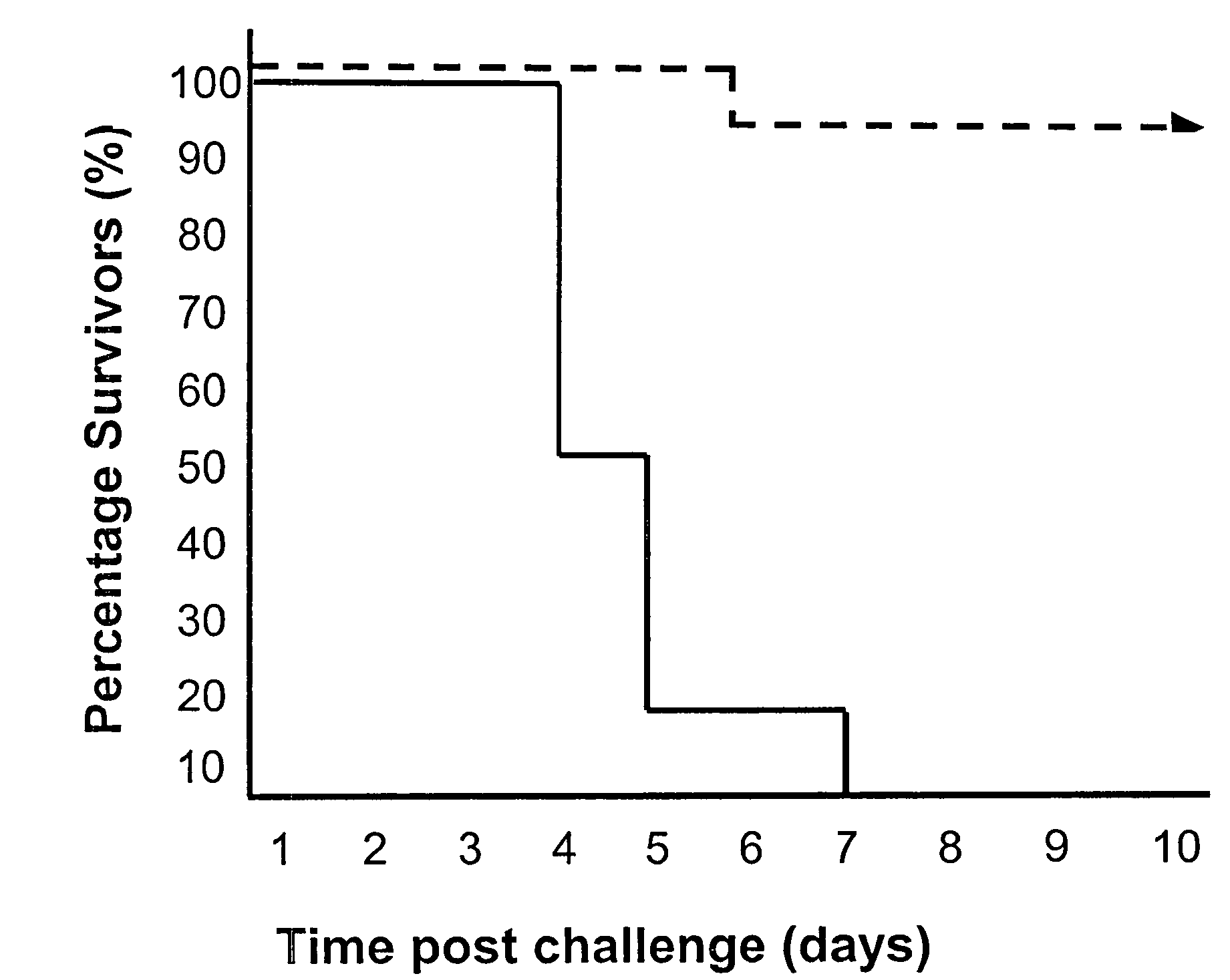
Recombinant vaccine viruses expressing il-15 and methods of using the same
InactiveUS20060147419A1Stimulates proliferationStimulates differentiationBiocideGenetic material ingredientsAdjuvantMammal
The invention is directed to compositions capable of augmenting the immunogenicity of a vaccine. The composition, or adjuvant, is administered to a mammal in need thereof in sequential or concurrent combination with a vaccine antigen. In one preferred aspect, the adjuvant is provided in the form of a recombinant poxvirus vector, such as a vaccinia virus vector, which comprises a nucleic acid sequence encoding IL-15.
Owner:UNITED STATES OF AMERICA
Modified vaccinia virus ankara for the vaccination of neonates
InactiveUS7628980B2Promote maturityBiocidePeptide/protein ingredientsAbnormal tissue growthDendritic cell
The invention relates inter alia to a method for inducing a long-term protection in an animal against foreign antigens and tumor antigens comprising the step of administering to the animal at least one factor selected from type I interferons and Flt-3, and to a method for inducing a long-term increase of the number of dendritic cells in an animal comprising the step of administering to the animal a factor selected from type I interferon and Flt-3 and to a method of inducing or enhancing the maturation and / or for the activation of the immune system of an animal comprising the step of administering to the animal a factor selected from type I interferon and Flt-3.
Owner:BAVARIAN NORDIC AS
Altered strain of the modified vaccinia virus ankara (MVA)
InactiveUS6682743B2Ease of mass productionEfficient and large-scale productionBiocideGenetic material ingredientsVector systemVirulent characteristics
The invention provides new strains of the Modified Vaccinia Virus Ankara (MVA) that have a strongly reduced virulence for most mammals, especially humans, but nevertheless grows in cells of a continuous cell line approved for the production of a therapeutic agent such as a vaccine. The invention also provides a method for producing said adapted MVA strains. The adapted MVA can be used e.g. for parenteral immunization, as a vector system, or in the active or inactivated from as an adjuvant or as a regulator of the unspecific components of the immune system.
Owner:BAVARIAN NORDIC AS
Compositions containing recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS7015024B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses, also are provided.
Owner:HEALTH & HUMAN SERVICES DEPT OF REPRESENTED BY THE SEC OF UNITED STATES OF AMERICA THE
Vaccinia virus for diagnosis and therapy of tumors
InactiveUS8642257B2Easy to identifyImprove securityUltrasonic/sonic/infrasonic diagnosticsVirusesAbnormal tissue growthCytotoxicity
Described are diagnostic and pharmaceutical compositions comprising a microorganism or cell containing a DNA sequence encoding a detectable protein or a protein capable of inducing a detectable signal, e.g. a luminescent or fluorescent protein, and, in a particular embodiment, furthermore (a) DNA sequence(s) encoding (a) protein(s) suitable for tumor therapy and / or elimination of metastatic tumors, e.g. a cytotoxic or cytostatic protein.
Owner:GENELUX
Method for the recovery and purification of poxviruses from infected cells
InactiveUS7056723B2Easy to controlImprove scalabilityViral antigen ingredientsAntiviralsInfected cellVaccinia
Owner:BAVARIAN NORDIC AS
Intergenic regions as novel sites for insertion of HIV DNA sequences in the genome of Modified Vaccinia virus Ankara
ActiveUS7501127B2Stably express multiple HIV proteinsRaise the possibilitySsRNA viruses positive-senseVirus peptidesVacciniaInsertion site
Owner:BAVARIAN NORDIC AS
Modified vaccinia virus ankara for the vaccination of neonates
InactiveUS7897156B2Improve the level ofIncrease the number ofAntibacterial agentsOrganic active ingredientsDendritic cellVaccination
Owner:BAVARIAN NORDIC AS
Methods for inducing an immune response against human immunodeficiency virus infection in subjects undergoing antiretroviral treatment
ActiveUS20180064803A1Measurable immune responseMaintain viremic controlViral antigen ingredientsAntiviralsImmunodeficiency virusVaccinia
Methods for inducing an immune response against Human Immunodeficiency Virus (HIV) in HIV-infected subjects undergoing antiretroviral therapy (ART) are described. The methods include administering an adenovirus vector primer vaccine and a modified vaccinia virus (MVA) vector booster vaccine encoding mosaic HIV antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +3
Methods and Compositions Concerning Poxviruses and Cancer
InactiveUS20090004723A1Less effectImprove clearanceBiocidePeptide/protein ingredientsCancer cellAnti viral response
The present invention concerns methods and compositions for the treatment of cancer and cancer cells using altered poxviruses, including a vaccinia virus that has been altered to generate a more effective therapeutic agent. Such poxviruses are engineered to be attenuated or weakened in their ability to affect normal cells. In some embodiments, methods and compositions involve poxviruses that possess mutations that result in poxviruses with diminished or eliminated capability to implement an antiviral response in a host. Poxviruses with these mutations in combination with other mutations can be employed for more effective treatment of cancer.
Owner:SILLAJEN BIOTHERAPEUTICS
Systemic treatment of metastatic and/or systemically-disseminated cancers using GM-CSF-expressing poxviruses
Owner:SILLAJEN BIOTHERAPEUTICS
Compositions and methods for generating an immune response
InactiveUS20070048861A1Increase typeSsRNA viruses negative-senseAntibody mimetics/scaffoldsImmunodeficiency virusEukaryotic plasmids
The present invention relates to novel plasmid constructs useful for the delivery of DNA vaccines. The present invention provides novel plasmids having a transcription cassette capable of directing the expression of a vaccine nucleic acid insert encoding immunogens derived from any pathogen, including fungi, bacteria and viruses. The present invention, however, is particularly useful for inducing in a patient an immune response against pathogenic viruses such as HIV, measles or influenza. Immunodeficiency virus vaccine inserts of the present invention express non-infectious HIV virus-like particles (VLP) bearing multiple viral epitopes. VLPs allow presentation of the epitopes to multiple histocompatability types, thereby reducing the possibility of the targeted virus escaping the immune response. Also described are methods for immunizing a patient by delivery of a novel plasmid of the present invention to the patient for expression of the vaccine insert therein. Optionally, the immunization protocol may include a booster vaccination that may be a live vector vaccine such as a recombinant pox virus or modified vaccinia Arbora vector. The booster live vaccine vector includes a transcription cassette expressing the same vaccine insert as the primary immunizing vector.
Owner:ROBINSON HARRIET L +11
Modified vaccinia virus ankara for the vaccination of neonates
InactiveUS20050260156A1Promote maturityBiocidePeptide/protein ingredientsDendritic cellNeonatal vaccination
The invention relates inter alia to a method for inducing a long-term protection in an animal against foreign antigens and tumor antigens comprising the step of administering to the animal at least one factor selected from type I interferons and Flt-3, and to a method for inducing a long-term increase of the number of dendritic cells in an animal comprising the step of administering to the animal a factor selected from type I interferon and Flt-3 and to a method of inducing or enhancing the maturation and / or for the activation of the immune system of an animal comprising the step of administering to the animal a factor selected from type I interferon and Flt-3.
Owner:BAVARIAN NORDIC AS
Generation of antibodies to tumor antigens and generation of tumor specific complement dependent cytotoxicity by administration of oncolytic vaccinia virus
The present invention relates to methods and compositions for use in inducing tumor-specific antibody mediated complement-dependent cytotoxic response in an animal having a tumor comprising administering to said animal a composition comprising a replication competent oncolytic virus wherein administration of the composition induces in the animal production of antibodies that mediate a CDC response specific to said tumor.
Owner:SILLAJEN +1
Systemic Treatment of Metastatic and/or Systemically-Disseminated Cancers using GM-CSF-Expressing Poxviruses
Owner:KIRN DAVID
Oncolytic vaccinia virus cancer therapy
ActiveUS20120276053A1Reduce cell viabilityBiocidePeptide/protein ingredientsVacciniaTumor vasculature
Embodiments of the invention are directed methods that include a thymidine kinase deficient vaccinia virus. The methods include administering the vaccinia virus at increased viral concentrations. Further aspects of the invention include methods for inducing oncolysis or collapse of tumor vasculature in a subject having a tumor comprising administering to a subject at least 1×108 infectious viral particles of a TK-deficient, GM-CSF-expressing, replication-competent vaccinia virus vector sufficient to induce oncolysis of cells in the tumor.
Owner:SILLAJEN BIOTHERAPEUTICS
Multivalent nanoemulsion vaccines
ActiveUS20120107349A1Antibacterial agentsBacterial antigen ingredientsVaccinationPaenibacillus lactis
The present invention provides methods and compositions for the stimulation of immune responses. Specifically, the present invention provides methods of inducing an immune response against one or a plurality of pathogens (e.g., vaccinia virus, H5N1 influenza virus, Bacillus anthracis, C. botulinum, Y. pestis, Hepatits B, and / or HIV, etc.) in a subject (e.g., a human subject) and compositions useful in such methods (e.g., immunogenic composition comprising nanoemulsion and one or a plurality of pathogens (e.g., inactivated by the nanoemulsion) and / or pathogen products and / or pathogen components). Compositions and methods of the present invention find use in, among other things, clinical (e.g. therapeutic and preventative medicine (e.g., vaccination)) and research applications
Owner:RGT UNIV OF MICHIGAN
Oncolytic vaccinia virus cancer therapy
Owner:SILLAJEN BIOTHERAPEUTICS
Remedy for fibromyalgia
ActiveUS20060051376A1Nervous disorderMicrobiological testing/measurementChronic Widespread PainSide effect
A therapeutic agent for fibromyalgia containing an extract from inflamed tissue inoculated with vaccinia virus as an active ingredient, use of the extract from inflamed tissue inoculated with vaccinia virus as the active ingredient for producing a medicinal composition for treating fibromyalgia, and a method of treating fibromyalgia which comprises administering a medicinal composition containing the extract from inflamed tissue inoculated with vaccinia virus as the active ingredient to a patient. The therapeutic agent containing the extract from inflamed tissue inoculated with vaccinia virus as the active ingredient is a novel therapeutic agent for fibromyalgia, no efficacious therapeutic agent for which has been known heretofore. Moreover, it is very useful as an effective drug having high safety with scarcely any side effects.
Owner:NIPPON ZOKI PHARM CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com