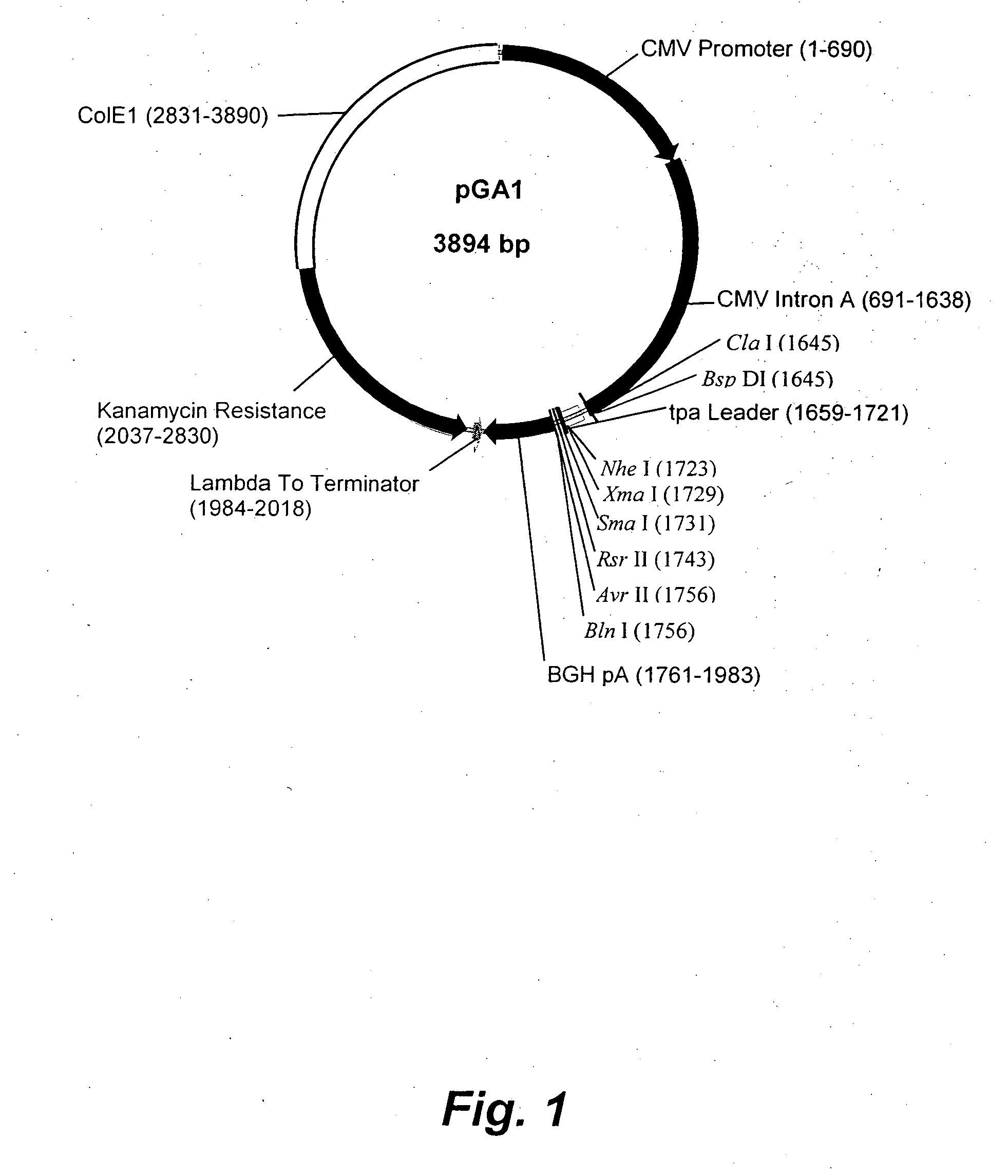
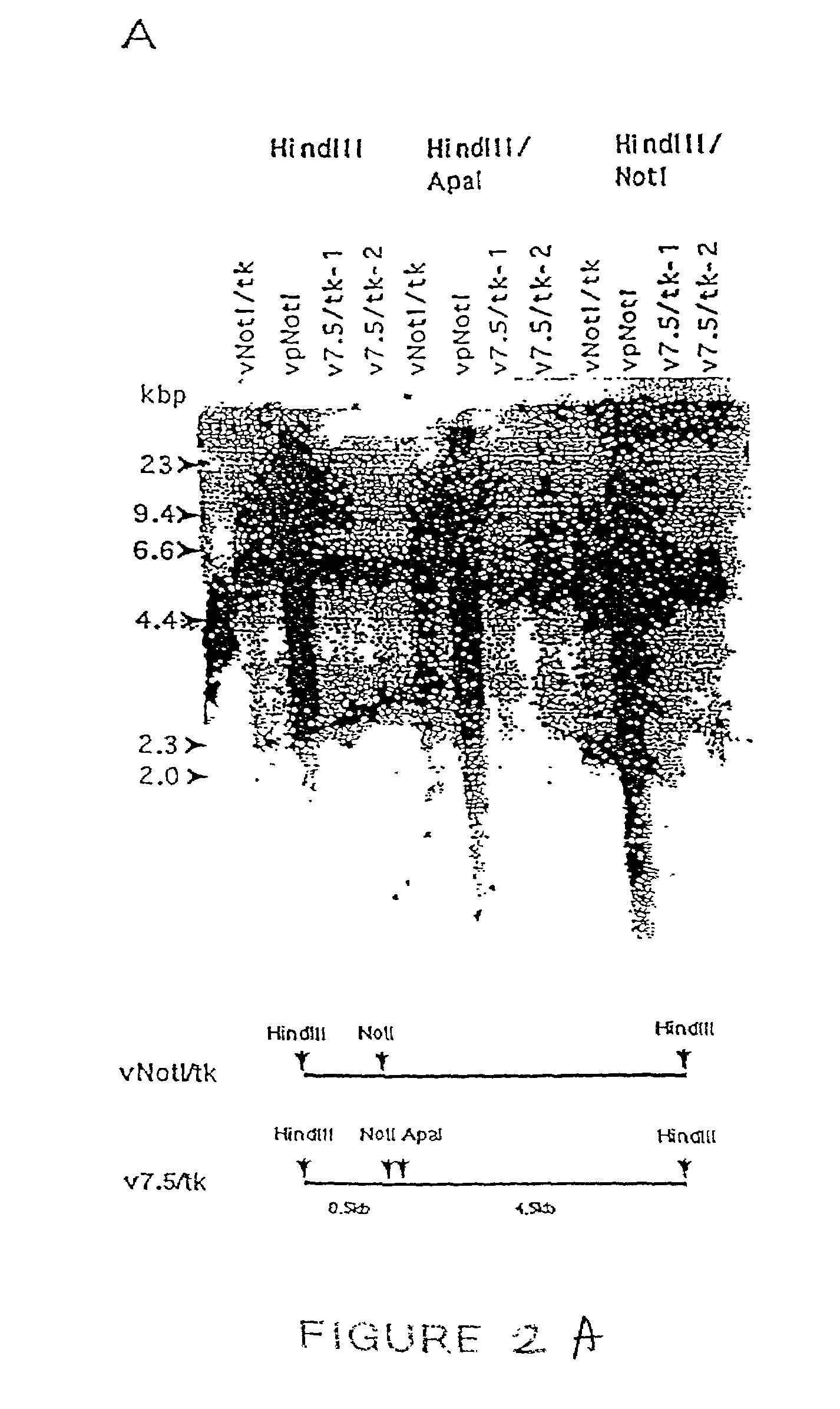
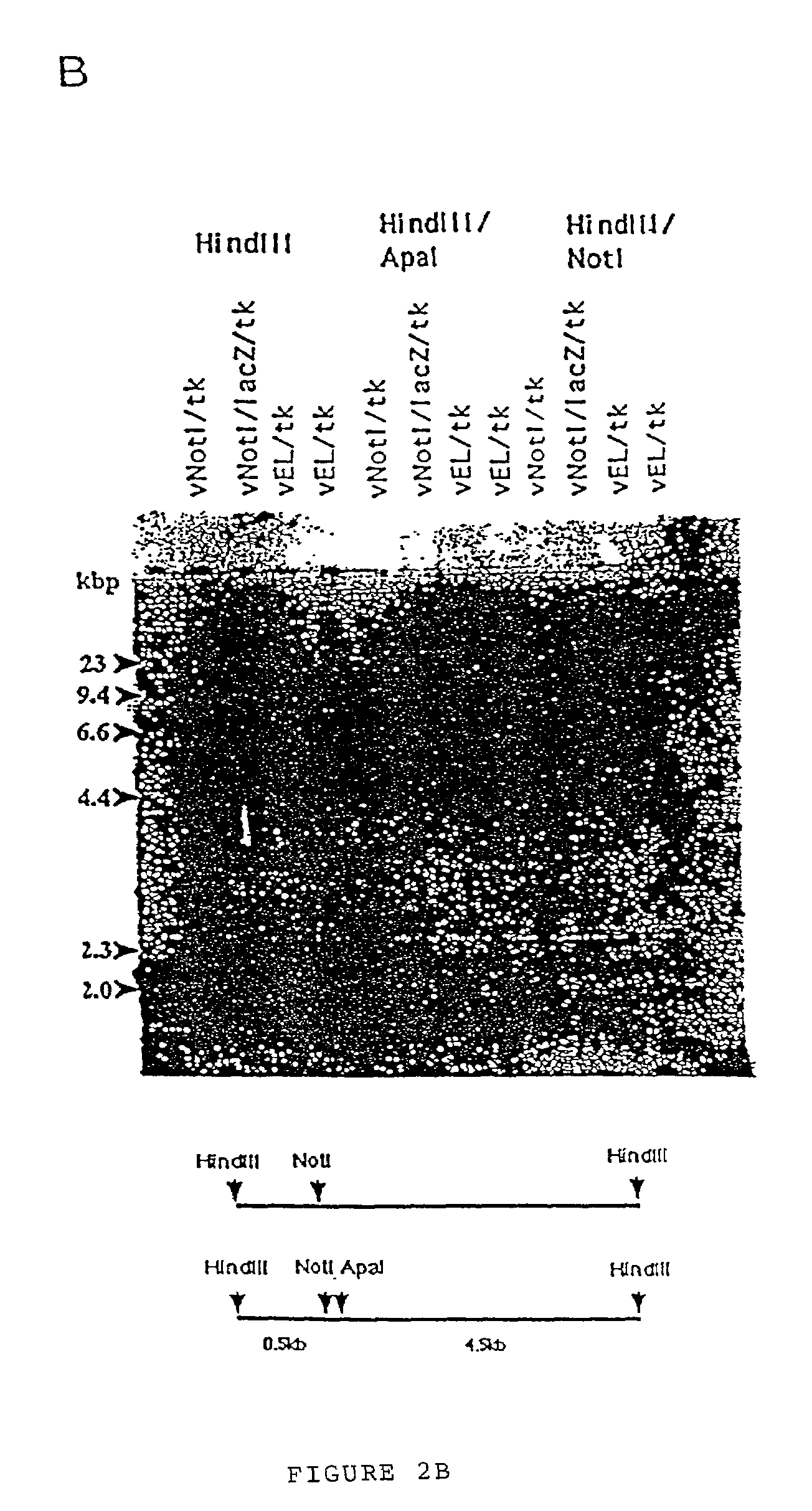
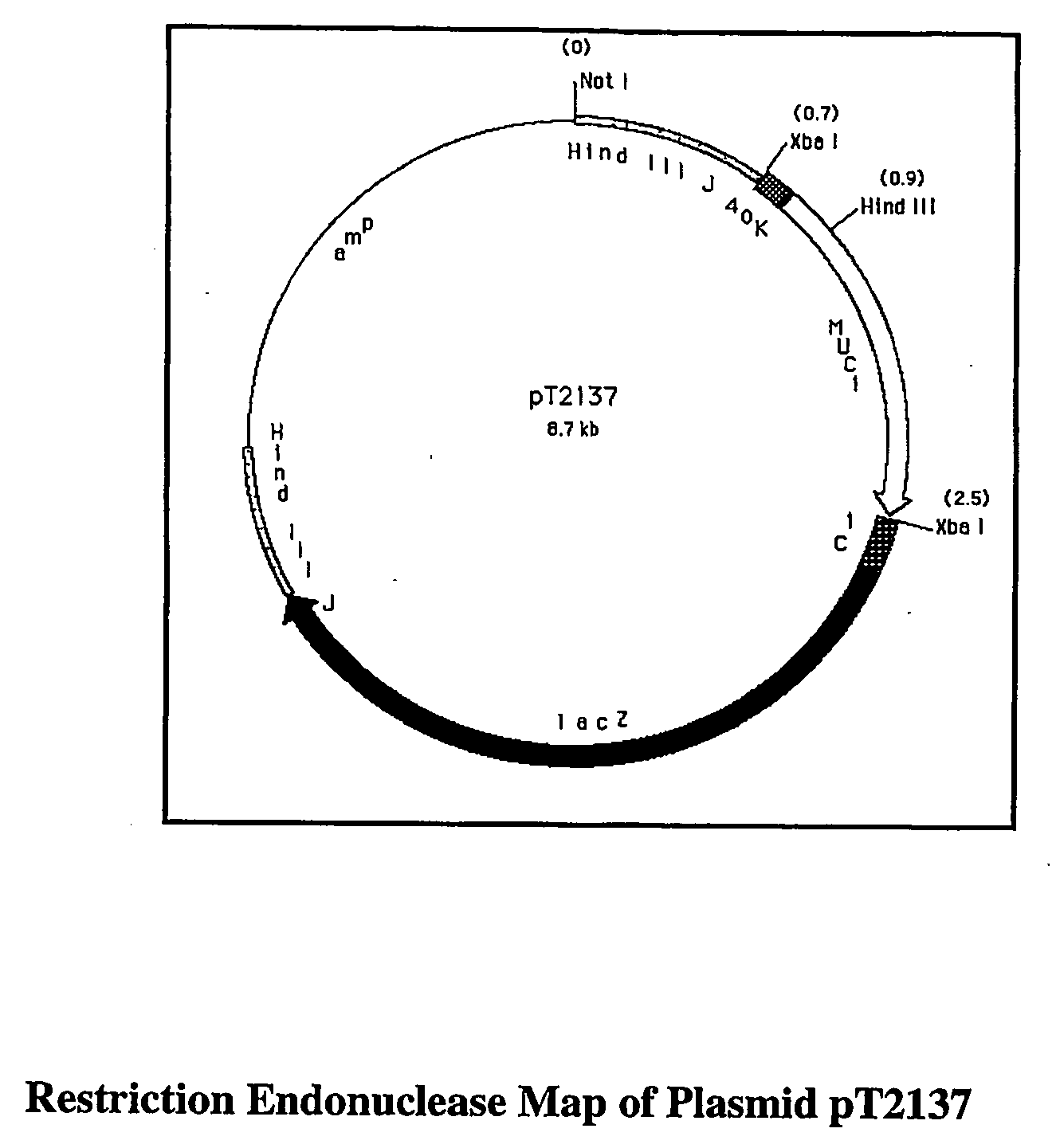
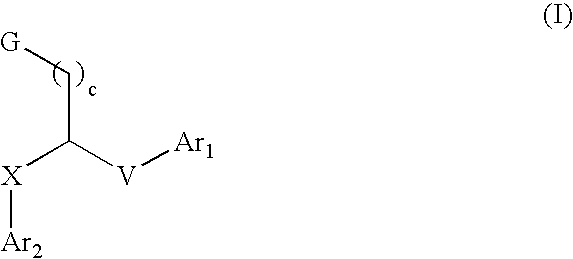Patents
Literature
284 results about "Pox virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alternative Title: Poxviridae. Poxvirus, (family Poxviridae), any of a group of viruses constituting the family Poxviridae, responsible for a wide range of pox diseases in humans and other animals.
Pox virus containing DNA encoding a cytokine and/or a tumor associated antigen
InactiveUS6265189B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Attenuated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least on of: human tumor necrosis factor; nuclear phosphoprotein p53, wildtype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GNCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:VIROGENETICS
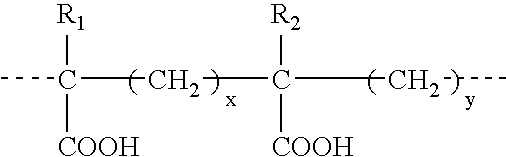
Aryl and heteroaryl compounds, compositions, and methods of use
This invention provides aryl and heteroaryl compounds of Formula (I) as described herein, and methods of their preparation. Also provided are pharmaceutical compositions made with the compounds of Formula (I) and methods for making such compositions. Compounds of Formula (I) may be useful for treating viral infections including orthopox viruses, either alone or in combination with other therapeutic agents.
Owner:VTV THERAPEUTICS LLC
Kits and methods for generating 5' capped RNA
InactiveUS20070281336A1Increase synthesisImprove efficiencySugar derivativesActivity regulationType specificPhosphoric acid
The present invention relates to kits and methods for efficiently generating 5′ capped RNA having a modified cap nucleotide and for use of such modified-nucleotide-capped RNA molecules. The invention is used to obtain novel compositions of such modified-nucleotide-capped RNA molecules. In particular, the present invention provides kits and methods for capping RNA using a modified cap nucleotide and a capping enzyme system, such as poxvirus capping enzyme. The present invention finds use for in vitro production of 5′-capped RNA having a modified cap nucleotide and for in vitro or in vivo production of polypeptides by in vitro or in vivo translation of such modified-nucleotide-capped RNA for a variety of research, therapeutic, and commercial applications. The invention also provides methods and kits for capturing or isolating uncapped RNA comprising primary RNA transcripts or RNA having a 5′-diphosphate, such as RNA synthesized in vitro or obtained from a biological source, including prokaryotic mRNA that is in a mixture with other prokaryotic and / or eukaryotic nucleic acids. The method for capturing modified-nucleotide-capped RNA also provides methods and kits for obtaining only type-specific or condition-specific modified-nucleotide-capped RNA by cap-dependent subtraction of that portion of the captured modified-nucleotide-capped RNA in cells of one type or condition that is the same as RNA in cells of another type or condition. The invention further provides methods and kits for using a capping enzyme system and modified cap nucleotides for labeling uncapped RNA comprising primary RNA transcripts or RNA having a 5′-diphosphate with detectable dye or enzyme moieties.
Owner:CELLSCRIPT
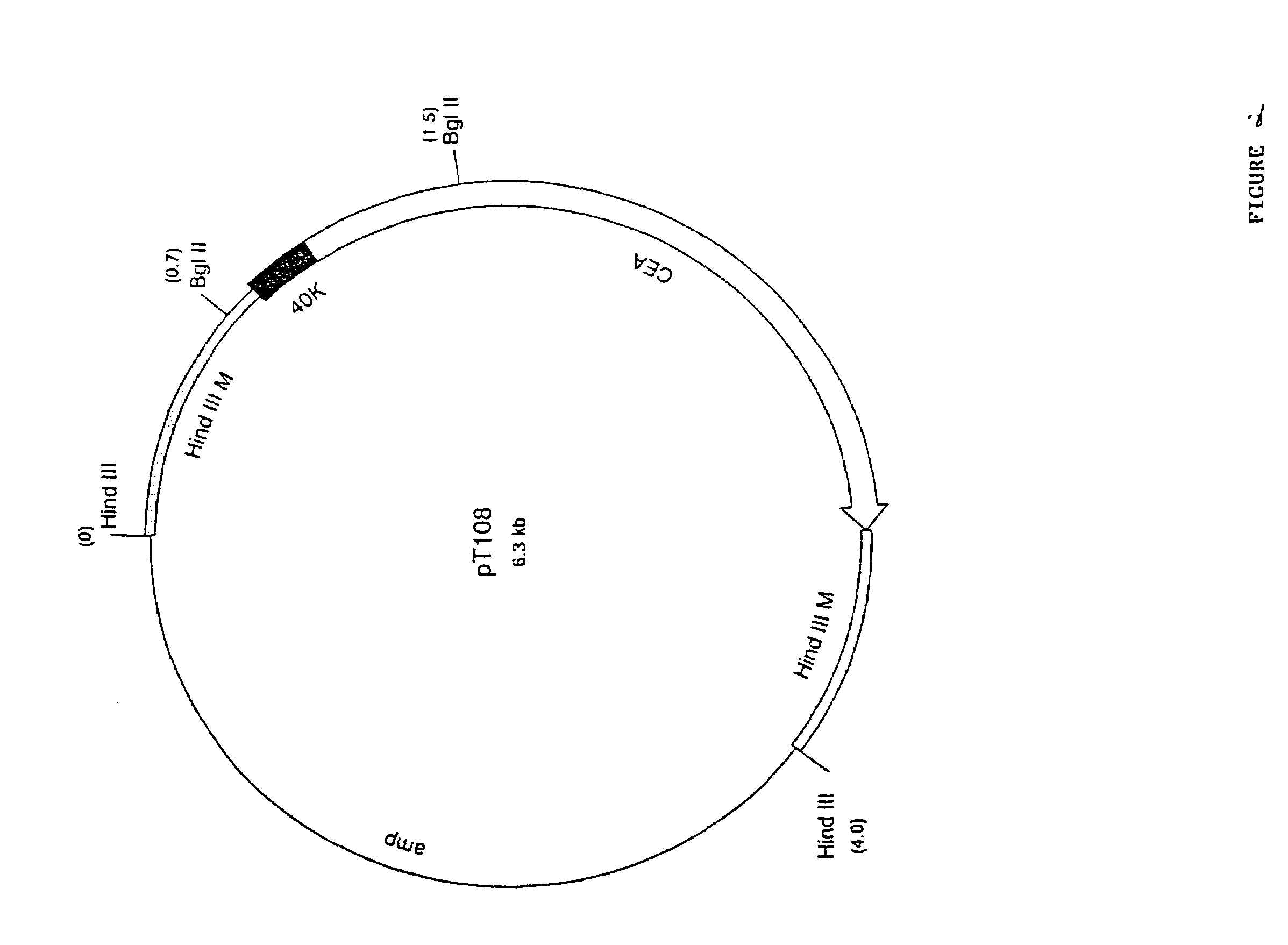
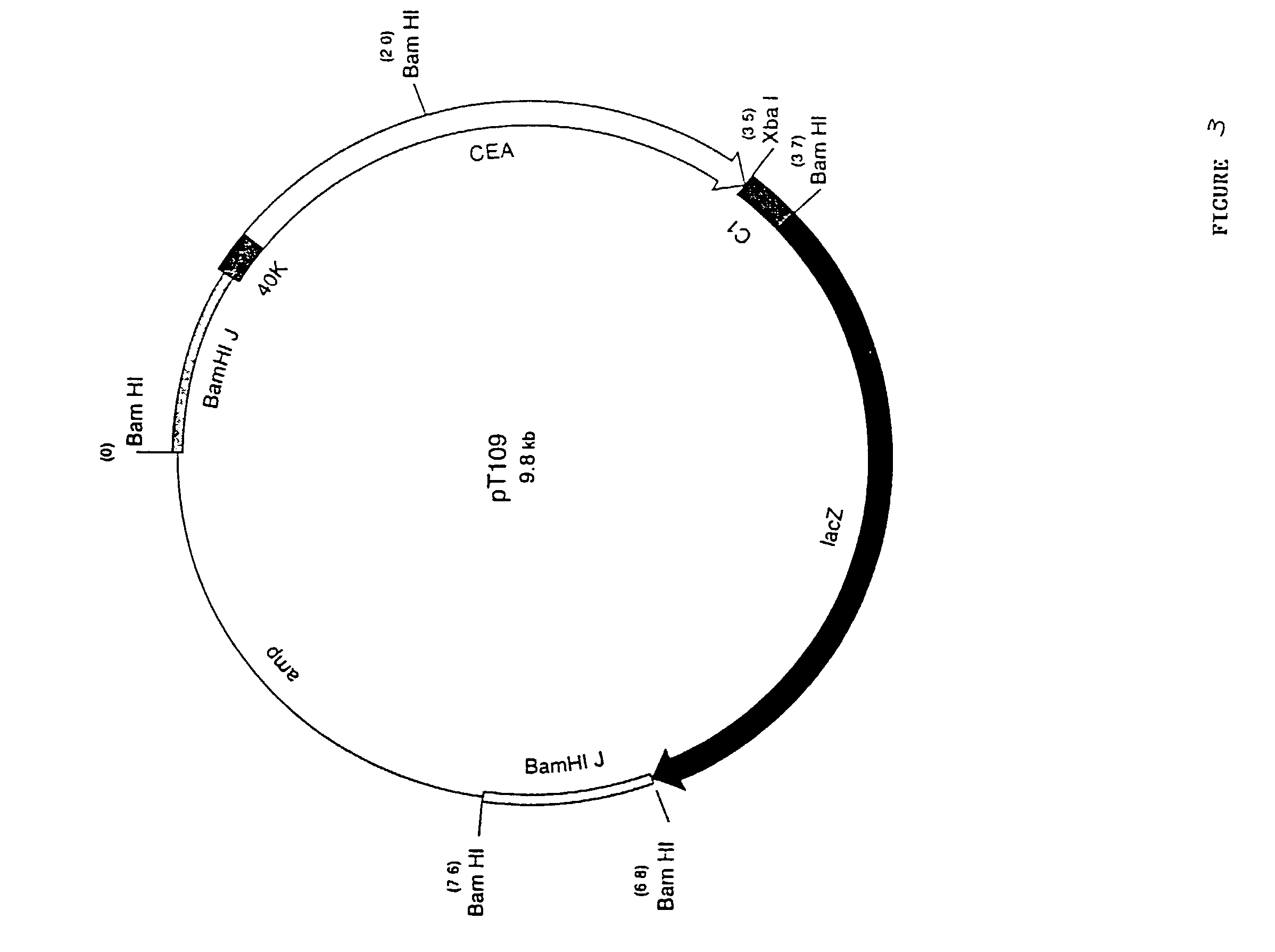
Pox virus comprising DNA sequences encoding CEA and B7 antigen
Attenutated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: human tumor necrosis factor; nuclear phosphoprotein p53, wiltype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GMCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:AVENTIS PASTEUR LTD
Recombinant vaccine against west nile virus
InactiveUS20030104008A1Inhibition effectEasy to storeSsRNA viruses positive-senseViral antigen ingredientsImmunogenicityNucleotide
Disclosed and claimed are immunogenic compositions to induce an immune response against West Nile (WN) virus, recombinants, for instance recombinant avipox viruses containing and expressing exogenous polynucleotide(s) from WN virus, and methods for making and using the same.
Owner:MERIAL SAS
Methods and reagents for vaccination which generate a CD8 T cell immune response
InactiveUS6663871B1Increase boost effectGood effectVirusesPeptide/protein ingredientsAntigenVaccination
New methods and reagents for vaccination are described which generate a CD8 T cell immune response against malarial and other antigens such as viral and tumour antigens. Novel vaccination regimes are described which employ a priming composition and a boosting composition, the boosting composition comprising a non-replicating or replication-impaired pox virus vector carrying at least one CD8 T cell epitope which is also present in the priming composition.
Owner:OXXON THERAPEUTICS LTD
Immunogenic compositions derived from poxviruses and methods of using same
Immunogenic compostions composed of poxvirus immunogens and related methods are disclosed. Specifically, immunogenic compostions useful in eliciting immune responses in animals are disclosed. In one embodiment the immunogenic compostions include viral antigens derived from vaccinia and / or variola that elicit cross-reactive immune responses. The immunogens can be made synthetically, by using recombinant DNA technology or derived from purified virus. Moreover, methods of using the immunogenic compostions are also disclosed.
Owner:MANNKIND CORP
Immunomodulatory compositions and uses therefor
The poxvirus proteins designated A41L and 130L bind to three receptor-like protein tyrosine phosphatases (RPTP), leukocyte common antigen related protein (LAR), RPTP-δ, and RPTP-σ, that are present on the cell surface of immune cells. When a host is infected with the poxvirus, binding of A41L to cell surface proteins on the host cells results in suppression of the immune response. The present invention provides agents such as antibodies, and antigen-binding fragments thereof, small molecules, aptamers, small interfering RNAs, and peptide-IgFc fusion polypeptides that interact with one or more of LAR, RPTP-δ, and RPTP-σ expressed by immune cells or interact with a polynucleotide encoding the RPTP. Also provided are RPTP Ig domain oligomers and Fc fusion polypeptides. Such agents are useful for treating an immunological disorder in a subject according to the methods described herein.
Owner:VIRAL LOGIC SYST TECH CORP
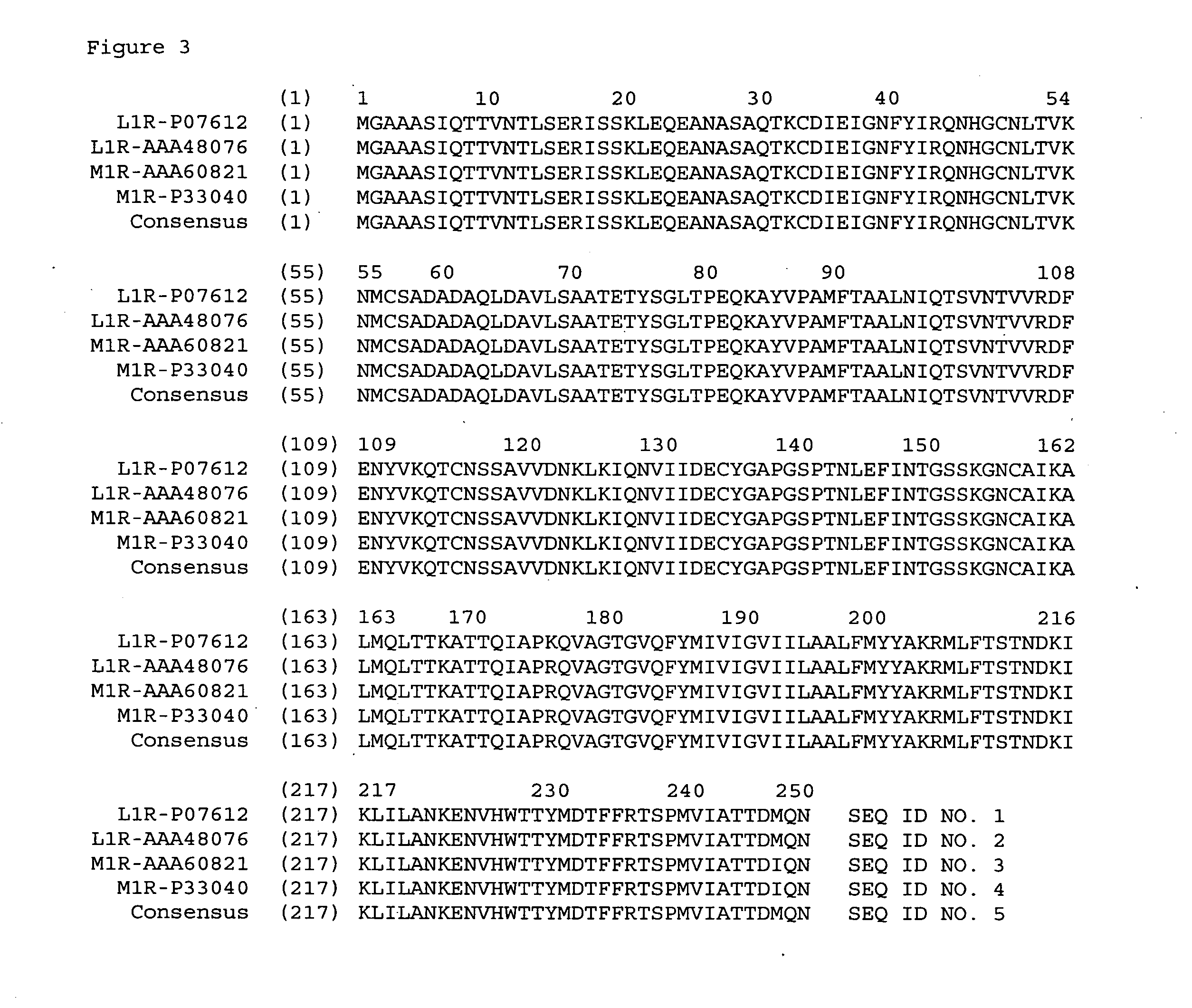
Avipox recombinants expressing foot and mouth disease virus genes
ActiveUS20050287672A1SsRNA viruses positive-senseViral antigen ingredientsGene productProtective immunity
The present invention relates to modified poxviral vectors and to methods of making and using the same. In particular, the invention relates to recombinant avipox that expresses gene products of foot and mouth disease virus (FMDV), and to compositions or vaccines that elicit immune responses directed to FMDV gene products and which can confer protective immunity against infection by FMDV.
Owner:MERIAL INC
Feline vaccines against avian influenza
InactiveUS20080107687A1Elicit immune responseSsRNA viruses negative-senseViral antigen ingredientsEpitopeViral Vaccine
The present invention encompasses influenza vaccines, in particular avian influenza vaccines. The vaccine may be a recombinant poxvirus vaccine or an inactivated vaccine. The invention also encompasses recombinant poxvirus vectors encoding and expressing avian influenza antigens, epitopes or immunogens which can be used to protect animals, in particular felids, against avian influenza.
Owner:MERIAL LTD
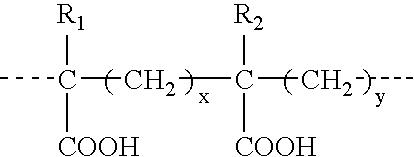
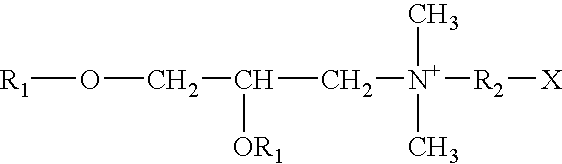
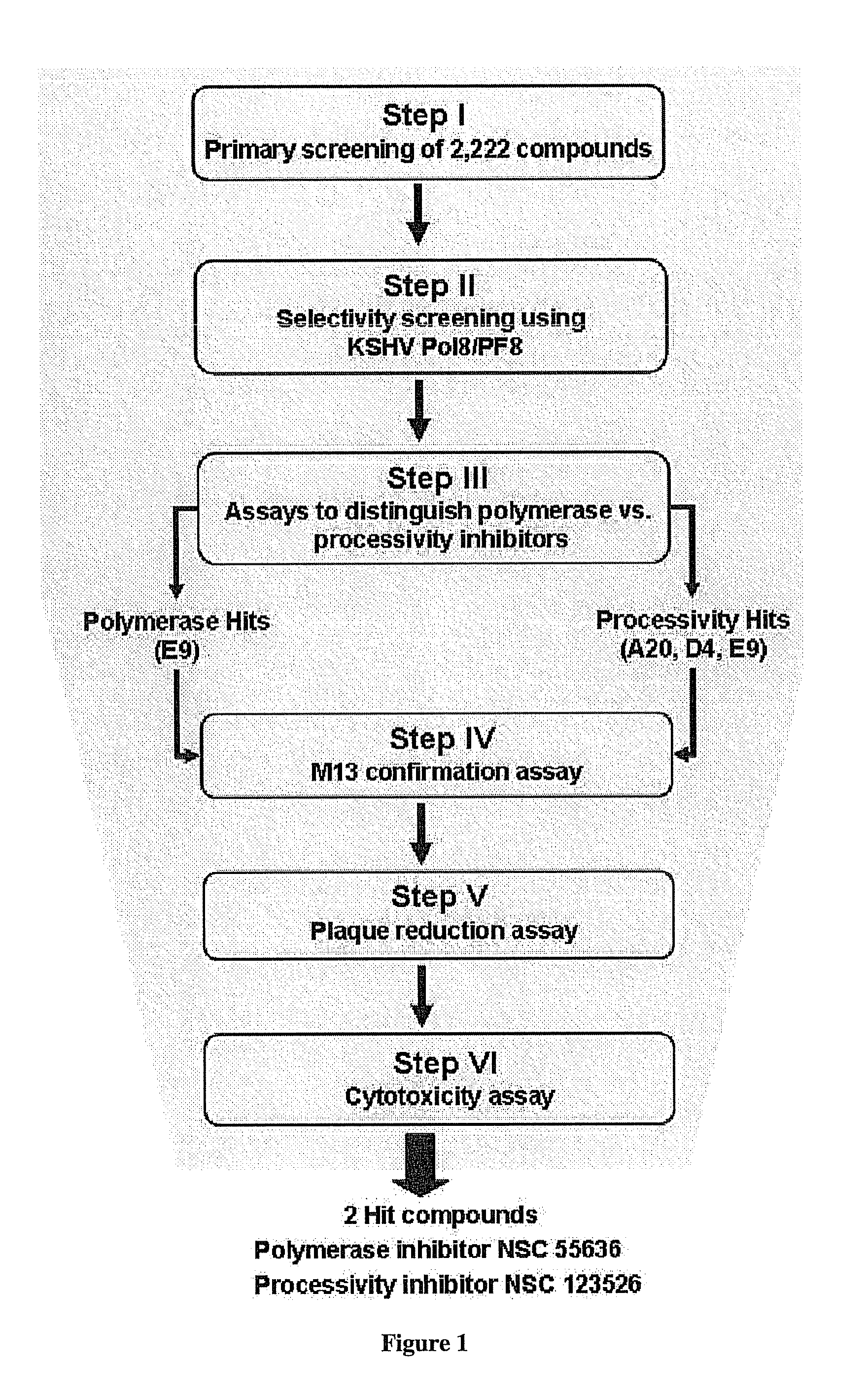
Therapeutic compounds for blocking DNA synthesis of pox viruses
ActiveUS20100035887A1Inhibition of replicationInhibit and reduce activityBiocideTetracycline active ingredientsMedicinePoxvirus Infections
This invention provides methods of inhibiting replication of a poxvirus by contacting a poxvirus with a compound having formula I, formula XXI, formula XXXII, or formula XLI which in turn reduce, inhibit, or abrogate poxvirus DNA polymerase activity and / or its interaction with its processivity factor. Formula I, formula XXI, formula XXXII, or formula XLI can be utilized to treat humans and animals suffering from a poxvirus infection. Pharmaceutical compositions for treating poxvirus infected subjects are also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
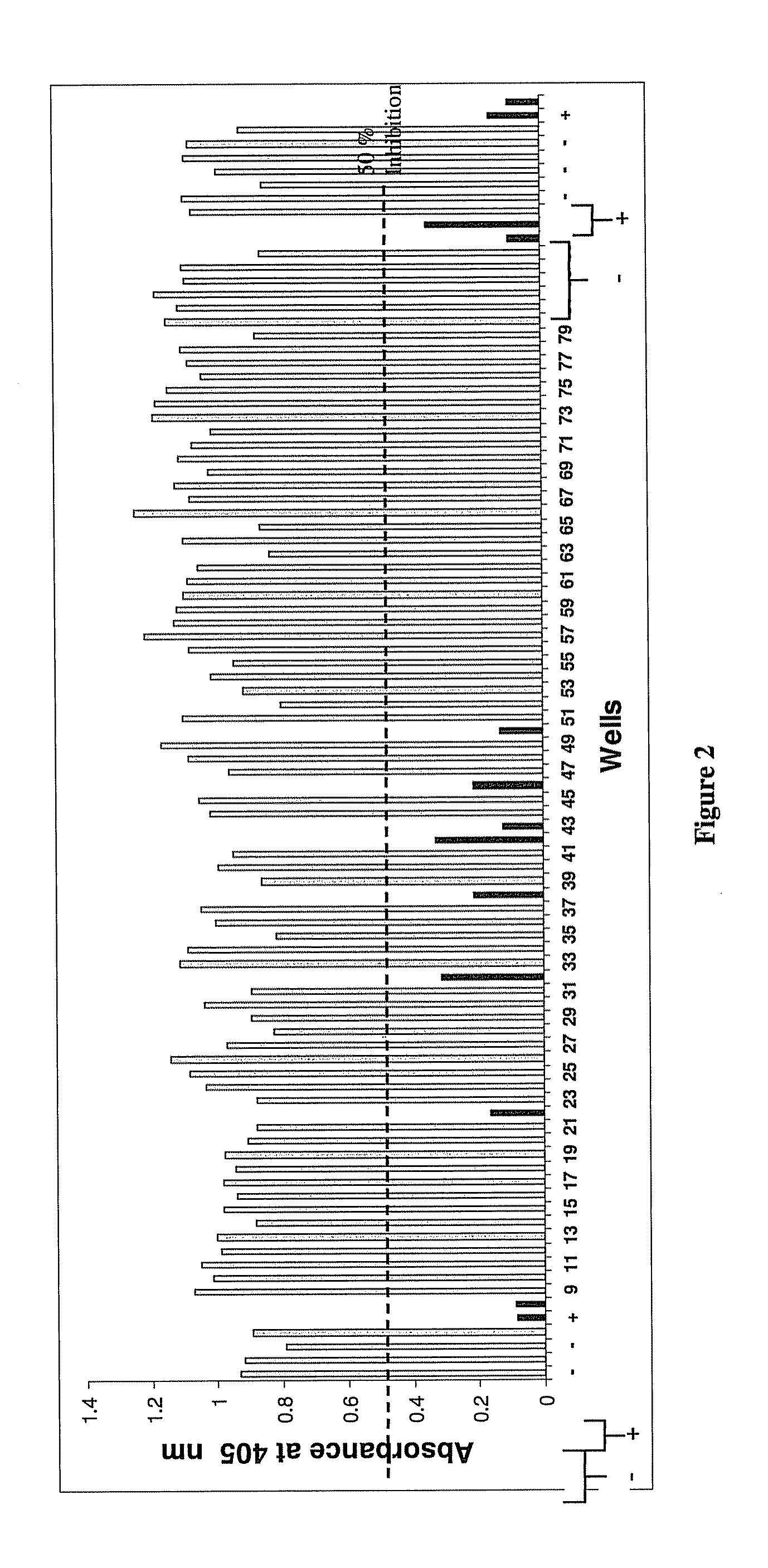
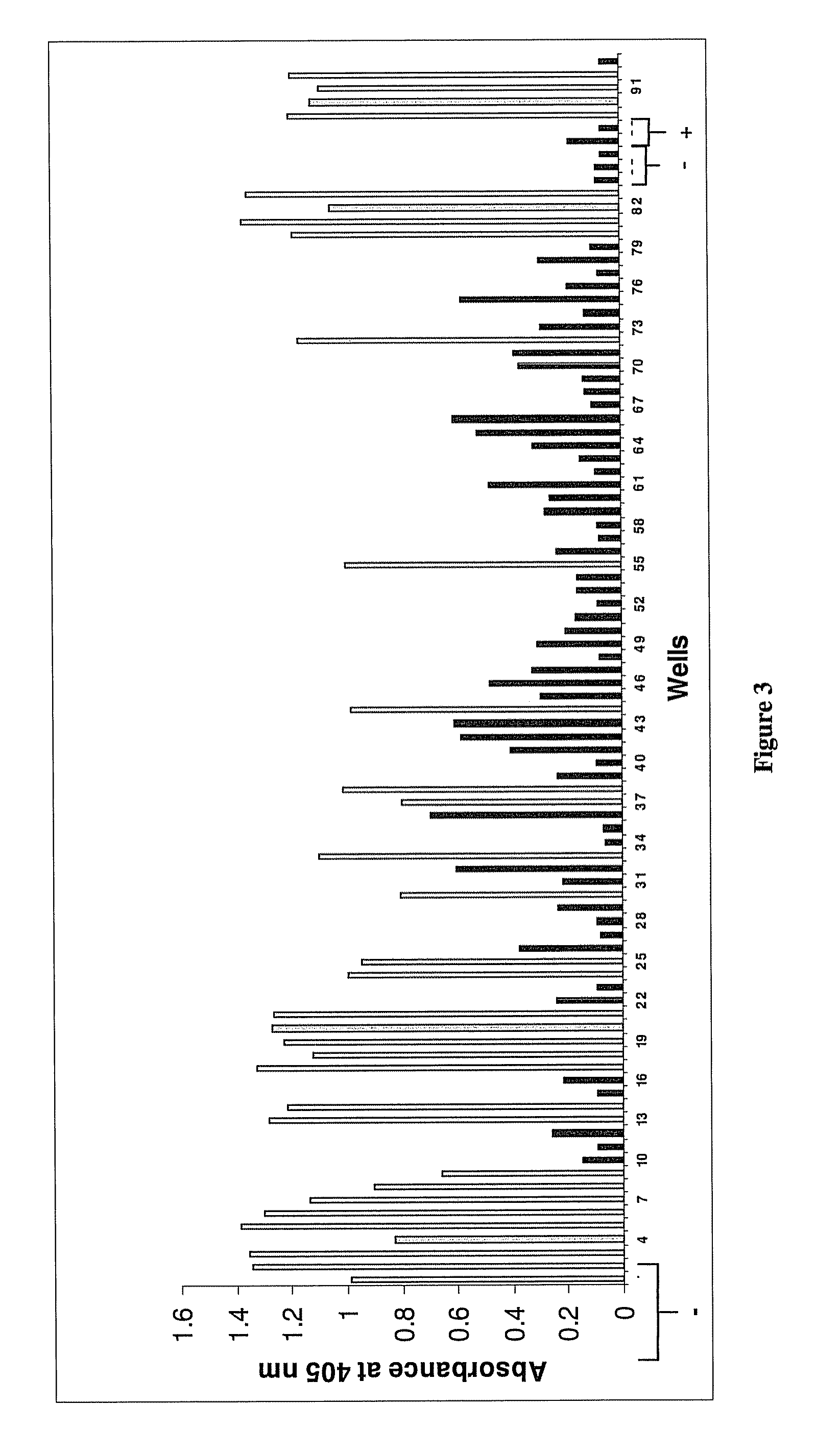
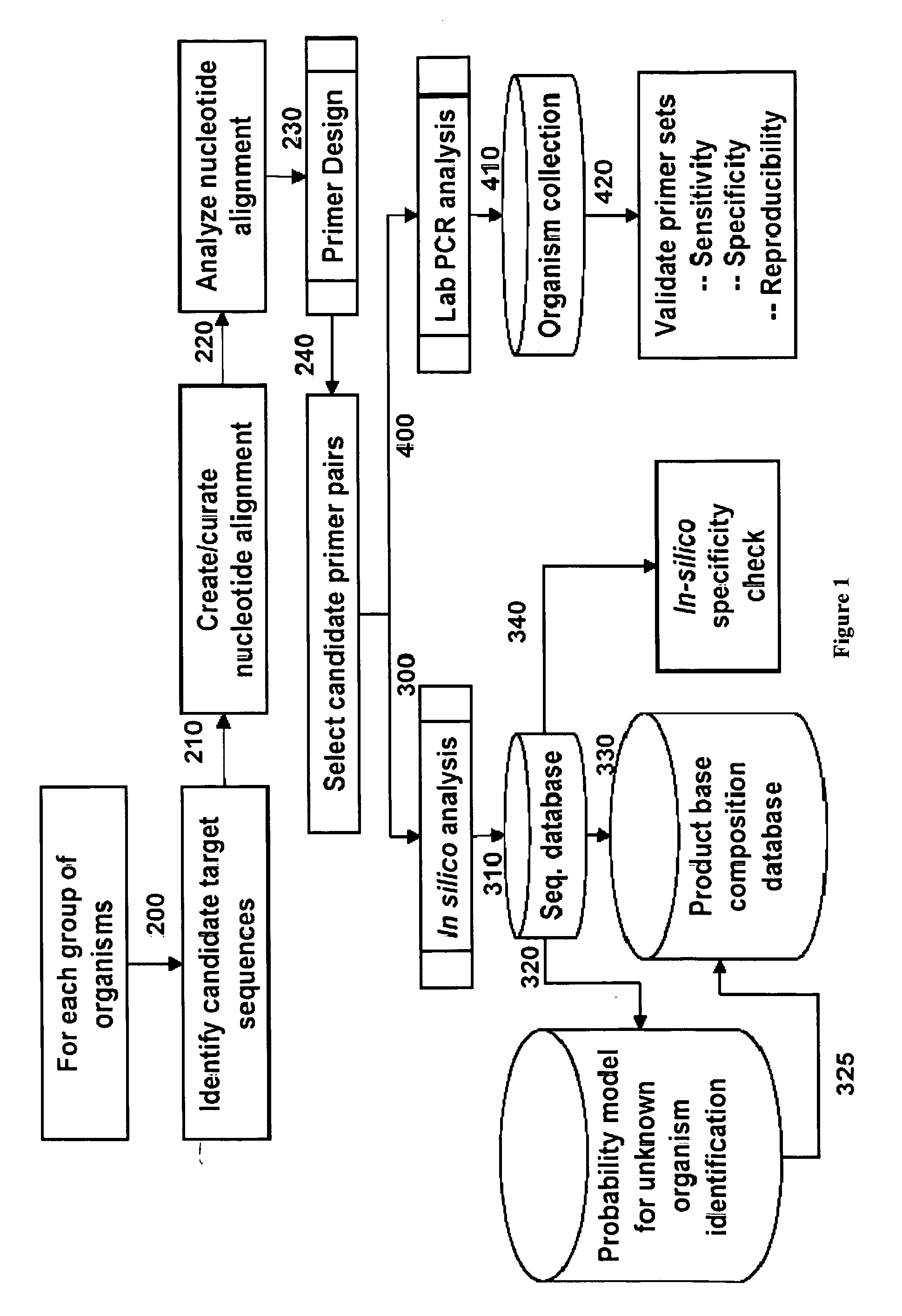
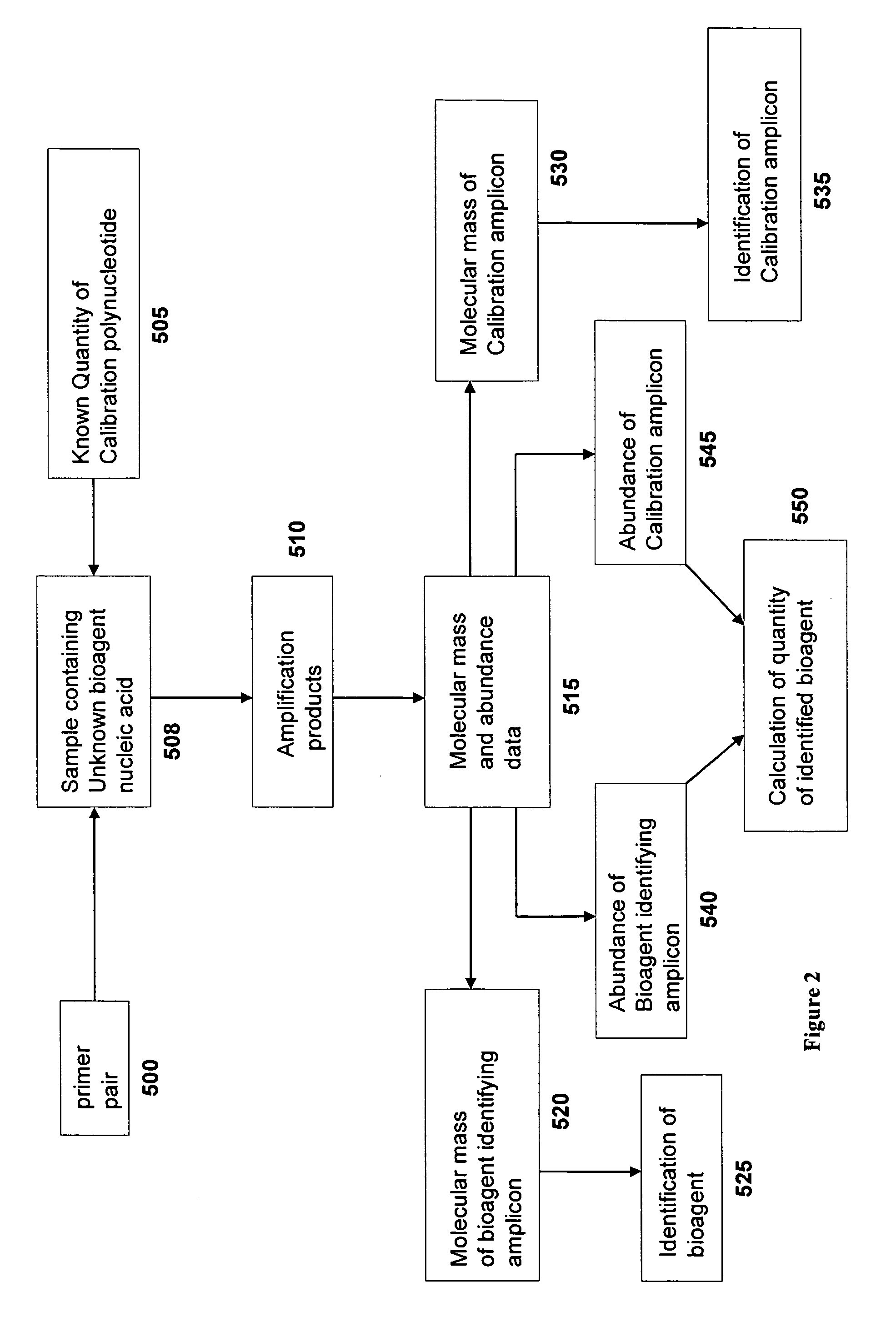
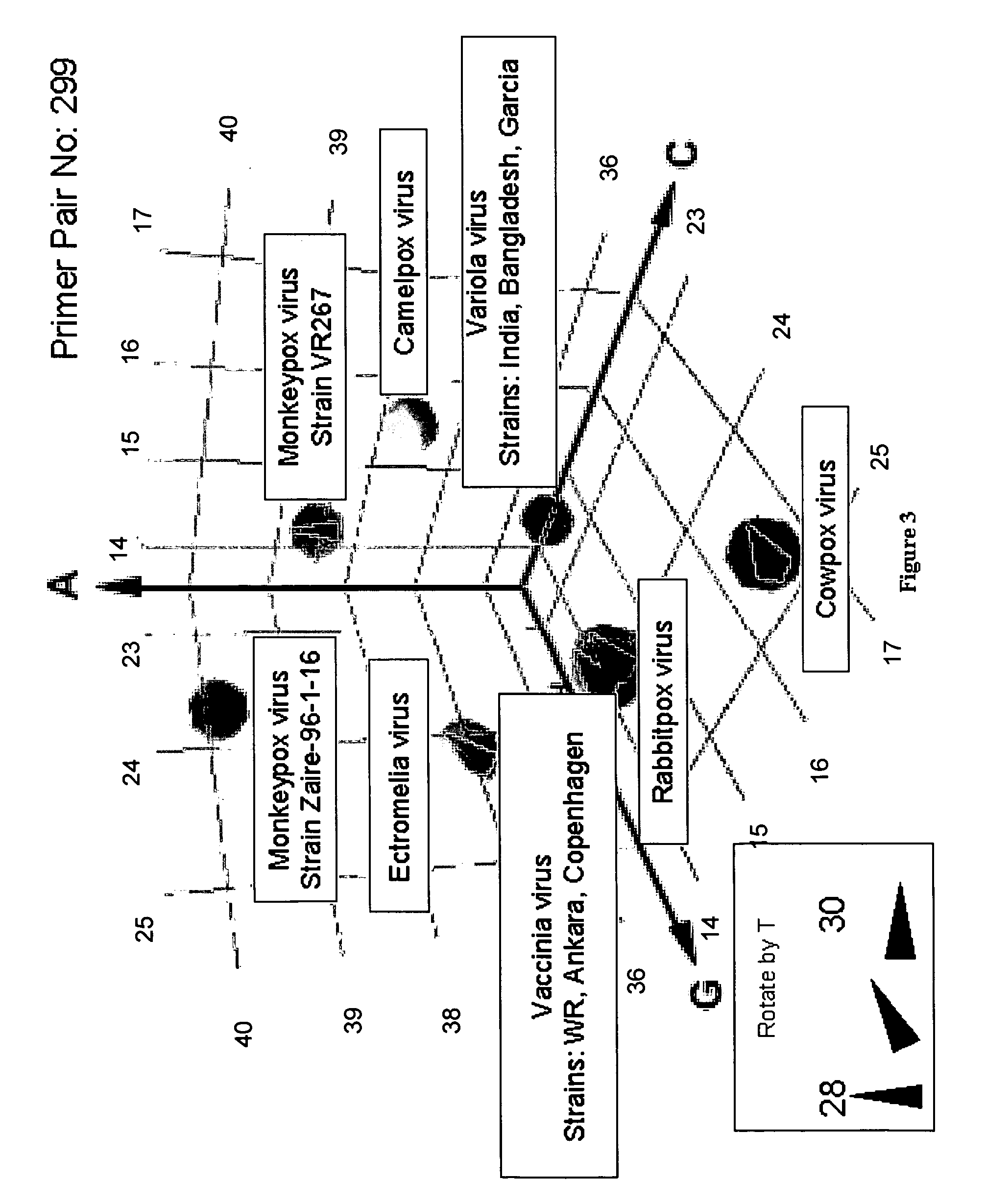
Compositions for use in identification of orthopoxviruses
InactiveUS20060275749A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
Oligonucleotide primers and compositions and kits containing the same for rapid identification of orthopoxviruses by amplification of a segment of viral nucleic acid followed by molecular mass analysis are provided.
Owner:IBIS BIOSCI
Kits and methods for generating 5' capped RNA
ActiveUS20140221248A1Easy to understandActivity regulationMicrobiological testing/measurementNucleotideEnzyme system
Owner:CELLSCRIPT
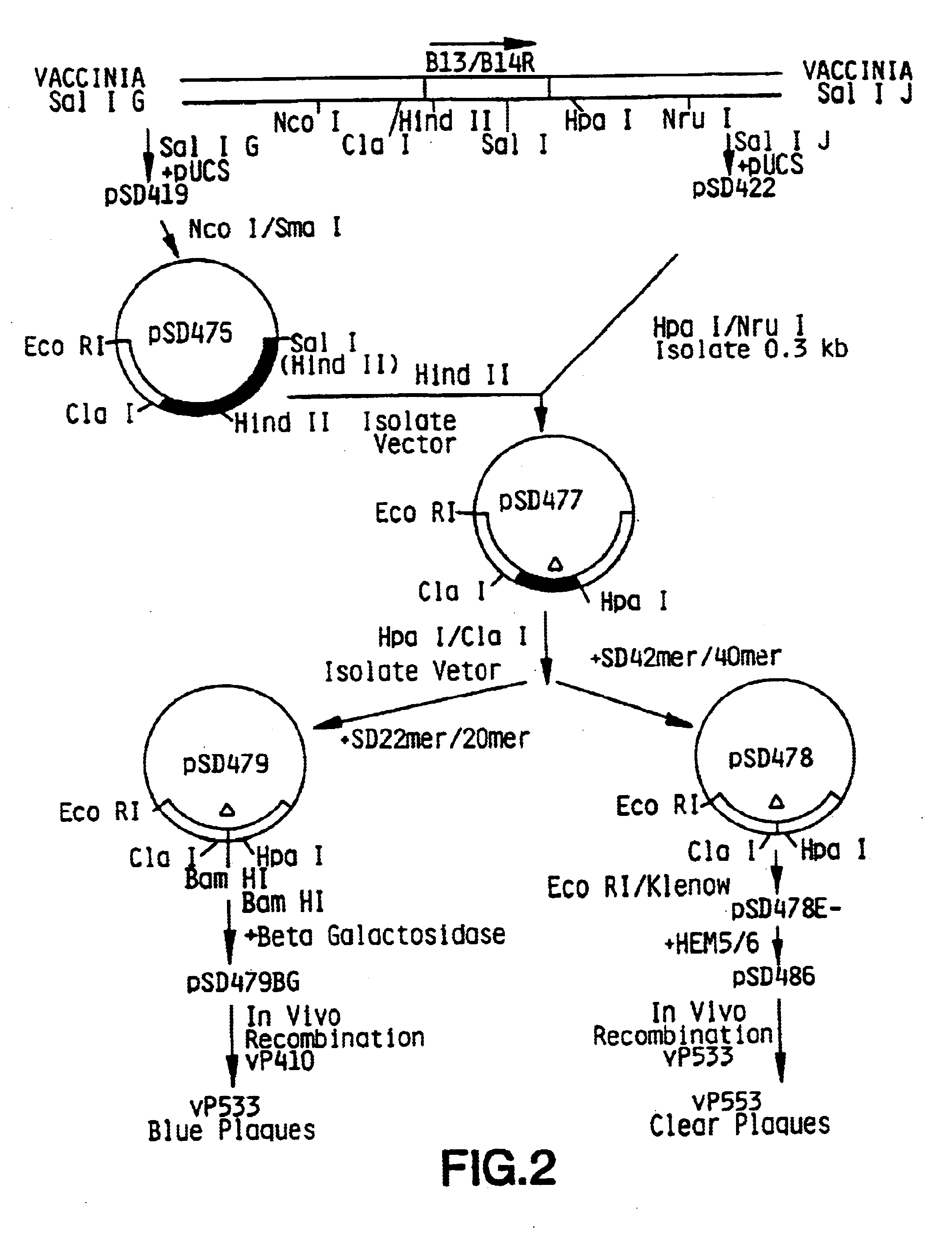
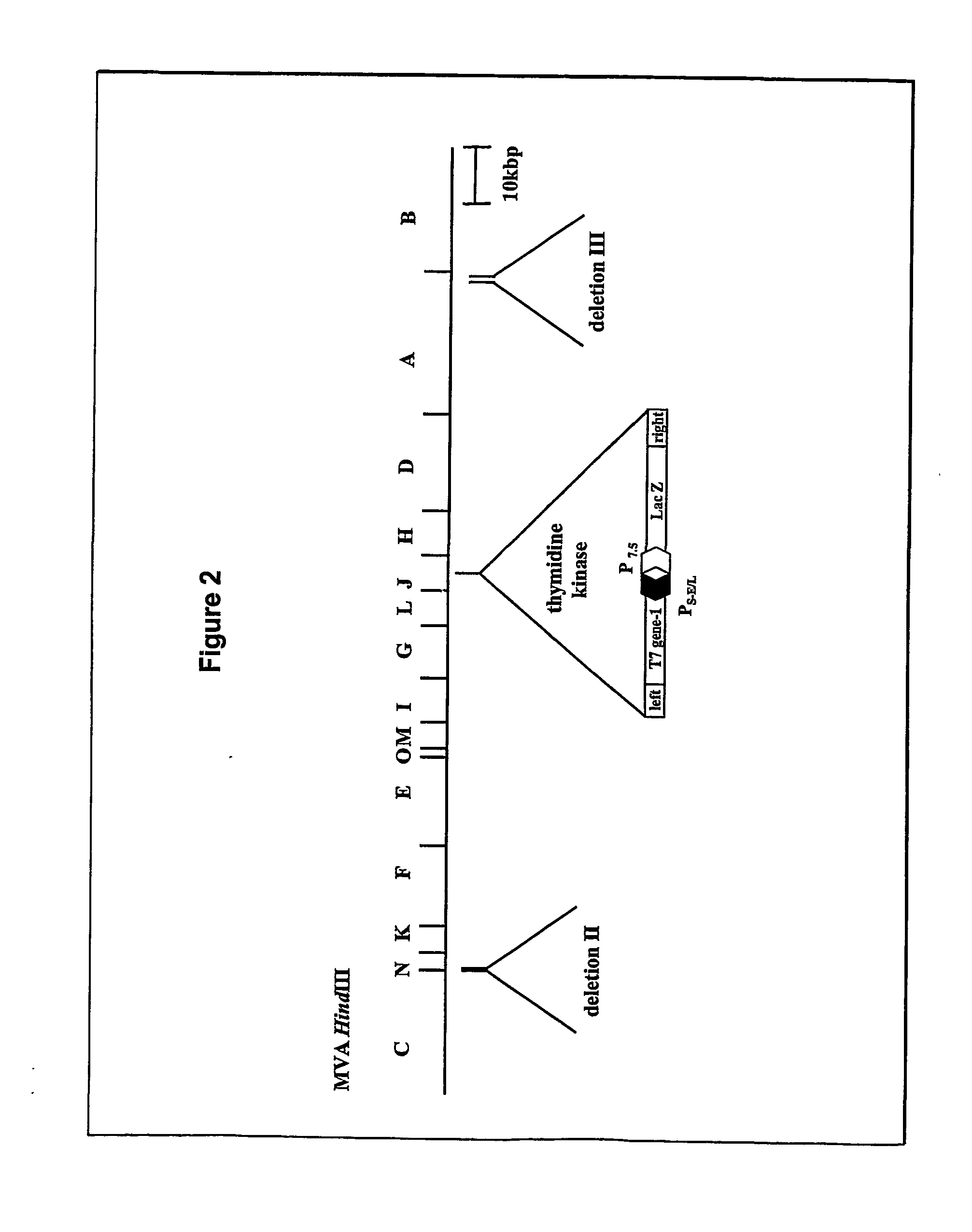
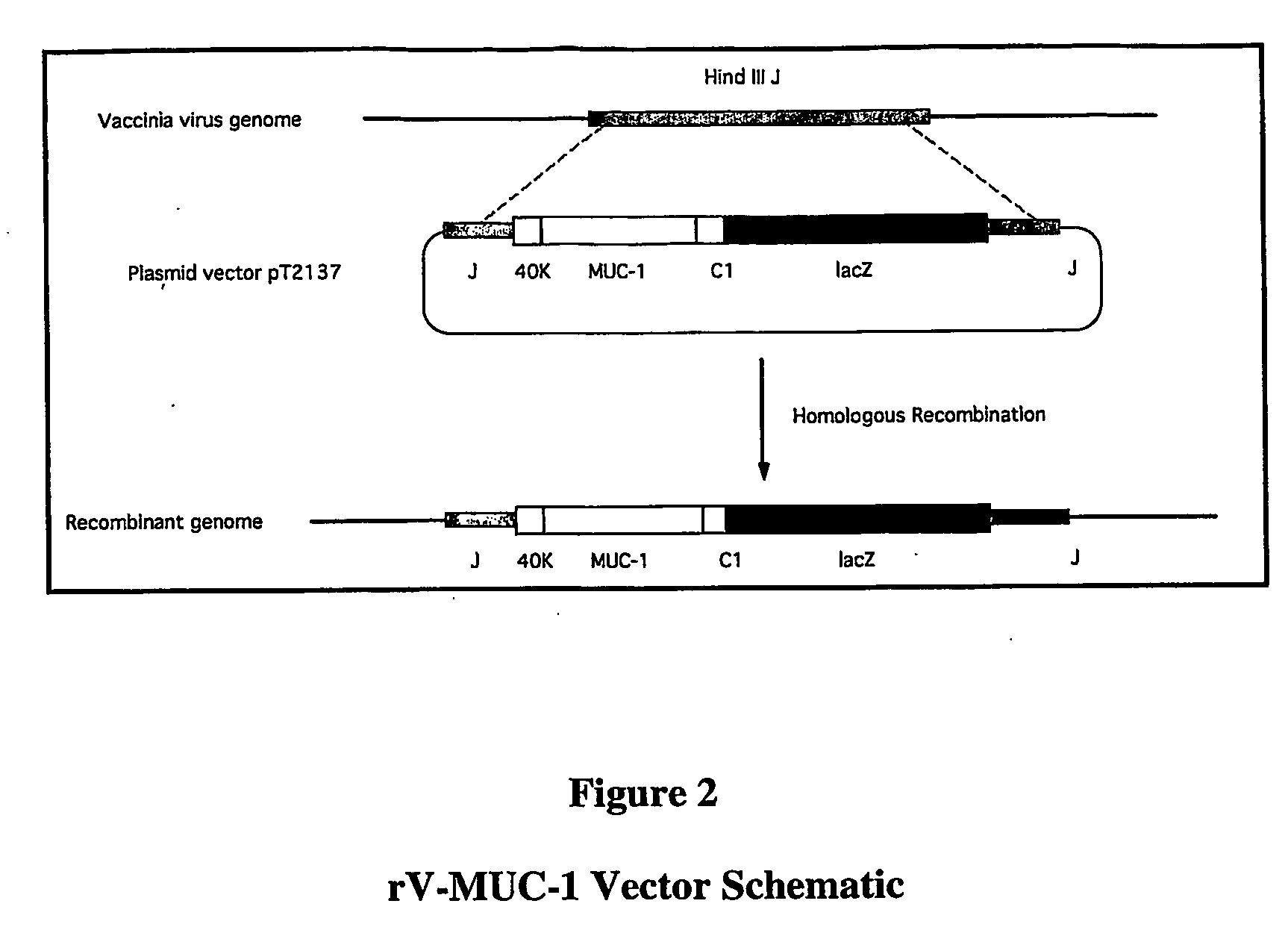
Vector for integration of heterologous sequences into poxviral genomes
The present invention provides a DNA vector comprising a nucleic acid sequence useful for inserting heterologous sequences into the genome of poxviruses by homologous recombination. The present invention relates also, inter alia, to recombinant poxvirses carrying heterologous coding sequences transferred by the vector according to the present invention.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
Recombinant pox virus for immunization against tumor-associated antigens
Recombinant pox viruses capable of expressing cell-encoded, tumor-associated antigens are disclosed. The recombinant viruses are useful for evoking an immune response against the antigen.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
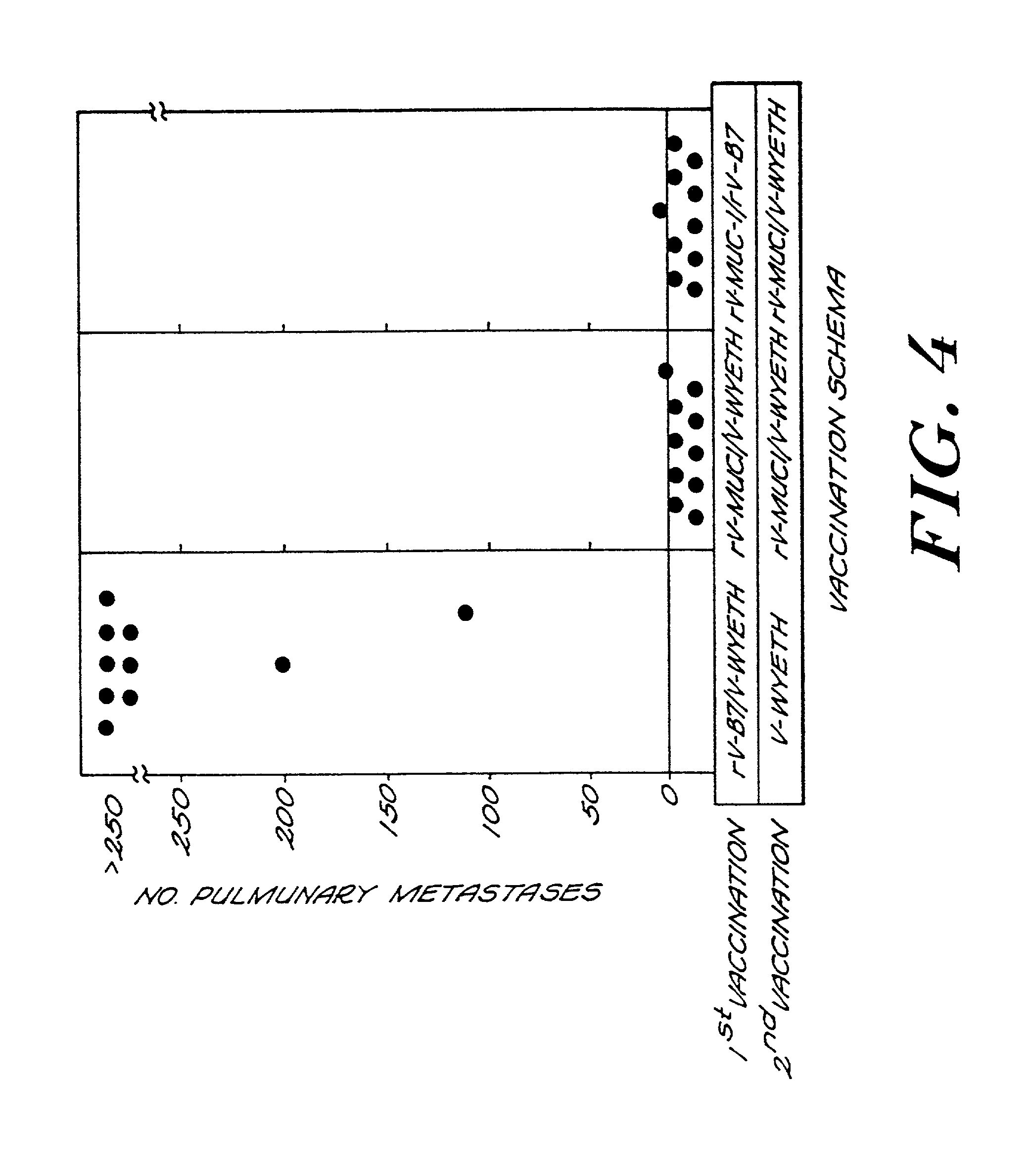
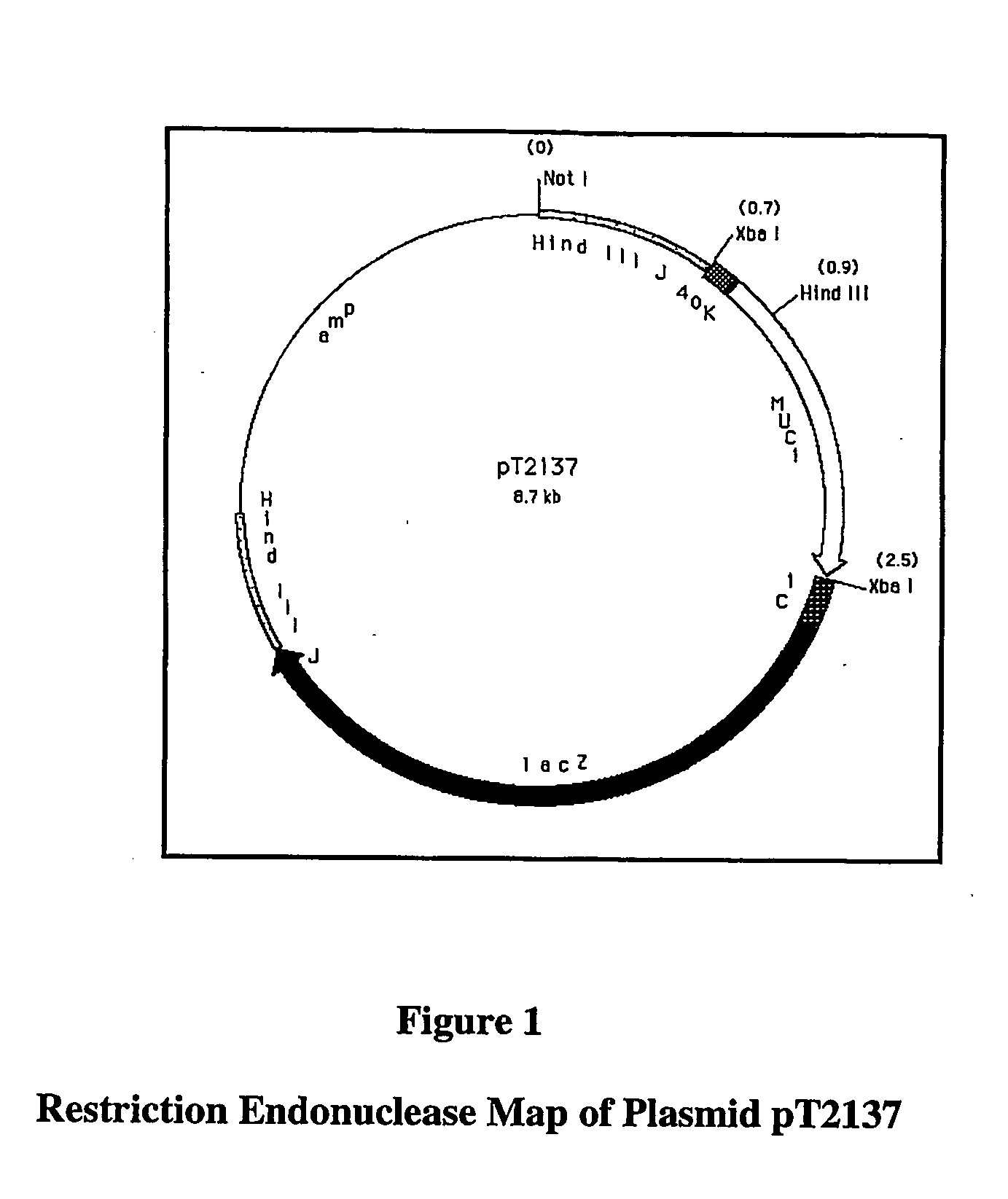
Recombinant pox virus for immunization against MUC1 tumor-associated antigen
Recombinant pox viruses capable of expressing an immunogenic fragment of the MUC1 tumor-associated antigen are disclosed. The recombinant viruses can be used as vaccines to prevent the establishment of or treat tumors or pre-tumorous cells expressing the MUC1 tumor-associated antigen. The vaccines can be provided as an admixture comprising: (1) a recombinant pox virus encoding the immunogenic fragment of the MUC1 tumor-associated antigen, and (2) a recombinant pox virus encoding a T-cell co-stimulatory factor. The vaccine admixture can be used, e.g., to prevent establishment of tumors or pre-tumorous cells expressing the MUC1 tumor-associated antigen. The MUC1 specific cytotoxic T-cells can be isolated and expanded and used in a method for treating a host having a tumor expressing MCU1 positive tumor cells.
Owner:UNITED STATES OF AMERICA
Method for the recovery and purification of poxviruses from infected cells
InactiveUS7056723B2Easy to controlImprove scalabilityViral antigen ingredientsAntiviralsInfected cellVaccinia
Owner:BAVARIAN NORDIC AS
Recombinant multivalent viral vaccine
InactiveUS7087234B1Elicit immune responseReadily apparentSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininViral Vaccine
The present invention relates to multivalent recombinant raccoon poxviruses, containing more than one exogenous gene inserted into either the thymidine kinase gene, the hemagglutinin gene, or a combination thereof. Disclosed is the use of the multivalent recombinant raccoon poxviruses as vaccines to immunize felines against subsequent challenge by feline pathogens. Also disclosed is a method of making a multivalent recombinant raccoon poxvirus by a recombination process involving the construction of an insertion vector into which the exogenous genes are inserted, and flanking the inserted genes are sequences which can recombine into the raccoon poxvirus thymidine kinase gene, or the hemagglutinin gene, or a combination thereof; introducing both the insertion vector containing the exogenous genes, and raccoon poxvirus into susceptible host cells; and selecting the recombinant raccoon poxvirus from the resultant plaques.
Owner:CORNELL RES FOUNDATION INC +1
Avipox recombinants expressing foot and mouth disease virus genes
ActiveUS7527960B2SsRNA viruses positive-senseViral antigen ingredientsGene productProtective immunity
The present invention relates to modified poxviral vectors and to methods of making and using the same. In particular, the invention relates to recombinant avipox that expresses gene products of foot and mouth disease virus (FMDV), and to compositions or vaccines that elicit immune responses directed to FMDV gene products and which can confer protective immunity against infection by FMDV.
Owner:MERIAL INC
Packaging of positive-strand rna virus replicon particles
InactiveUS20040029279A1Great titerProbability of generatingFungiSsRNA viruses positive-sensePharmaceutical formulationViral vector
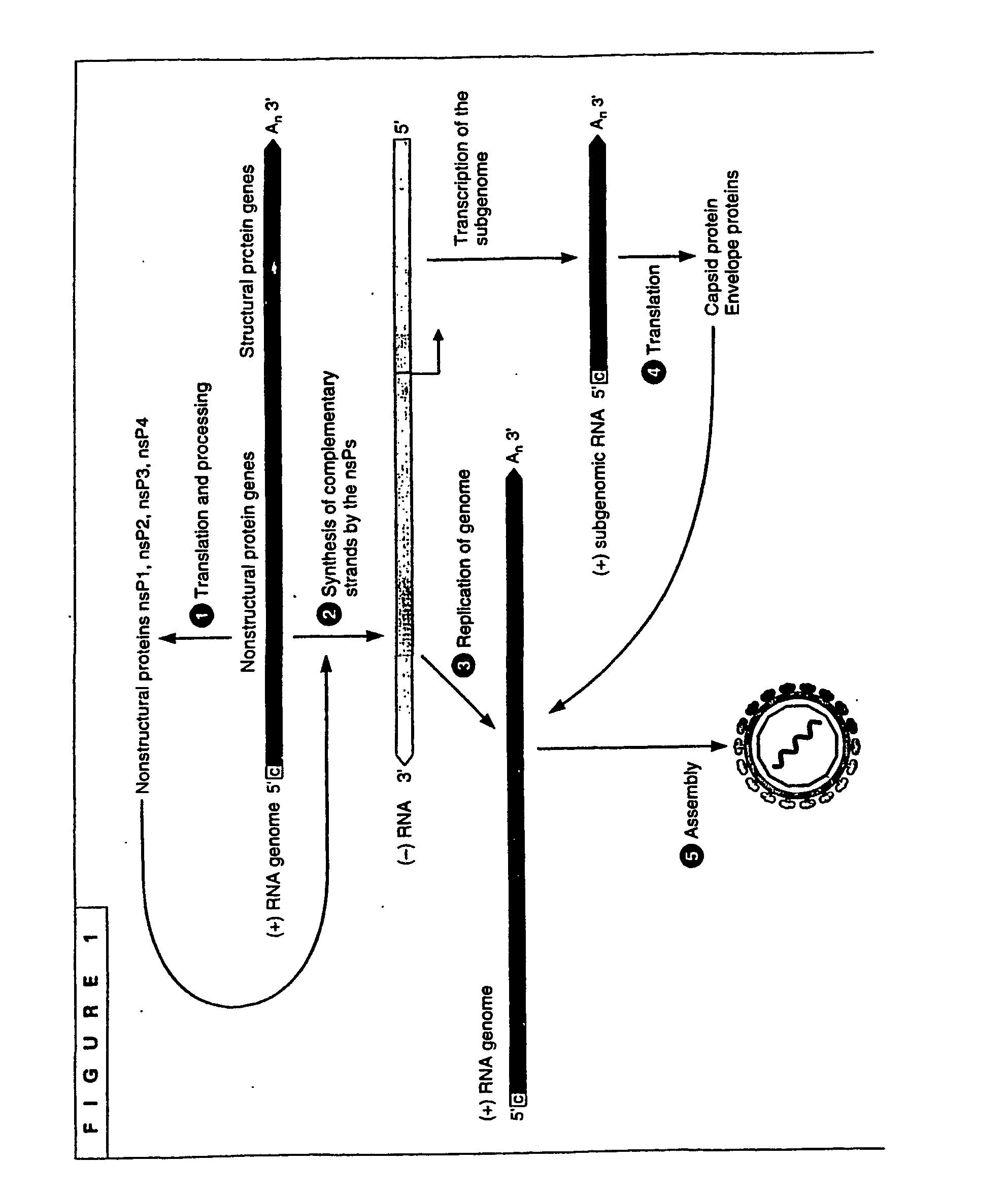
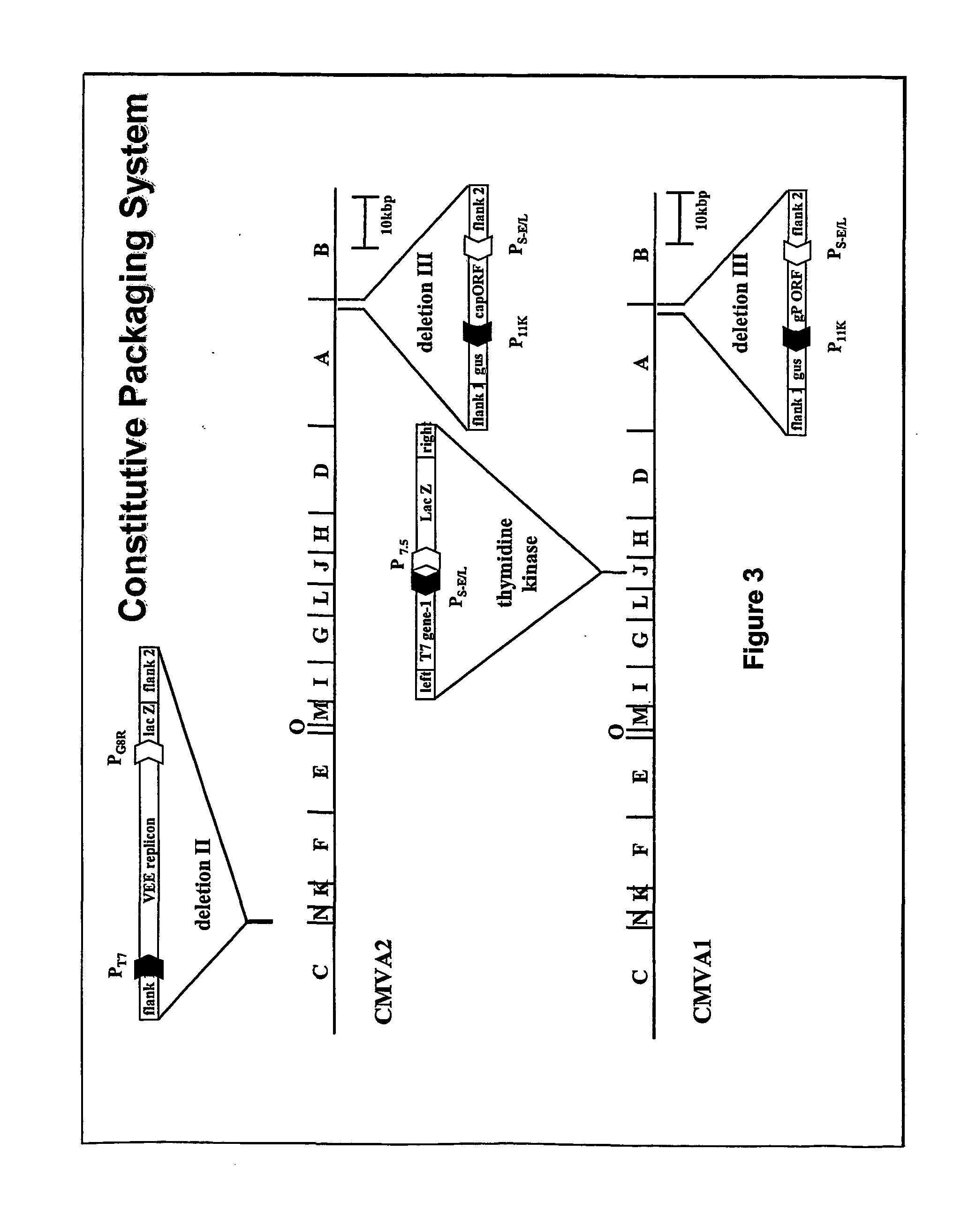
The invention generally relates to recombinant polynucleotides, positive-strand RNA virus (psRNAV) recombinant expression vectors, and packaging systems. The packaging systems are based on the expression of helper functions by coinfecting re-combinant poxvirus vectors comprising recombinant polynucleotides. Methods for obtaining psRNAV replicon particles using these packaging systems are disclosed. Immunogenic compositions and pharmaceutical formulations are provided that comprise replicon particles of the invention. Methods for generating an immune response or producing a pharmaceutical effect are also provided.
Owner:WYETH HOLDINGS CORP
Methods and Compositions Concerning Poxviruses and Cancer
InactiveUS20090004723A1Less effectImprove clearanceBiocidePeptide/protein ingredientsCancer cellAnti viral response
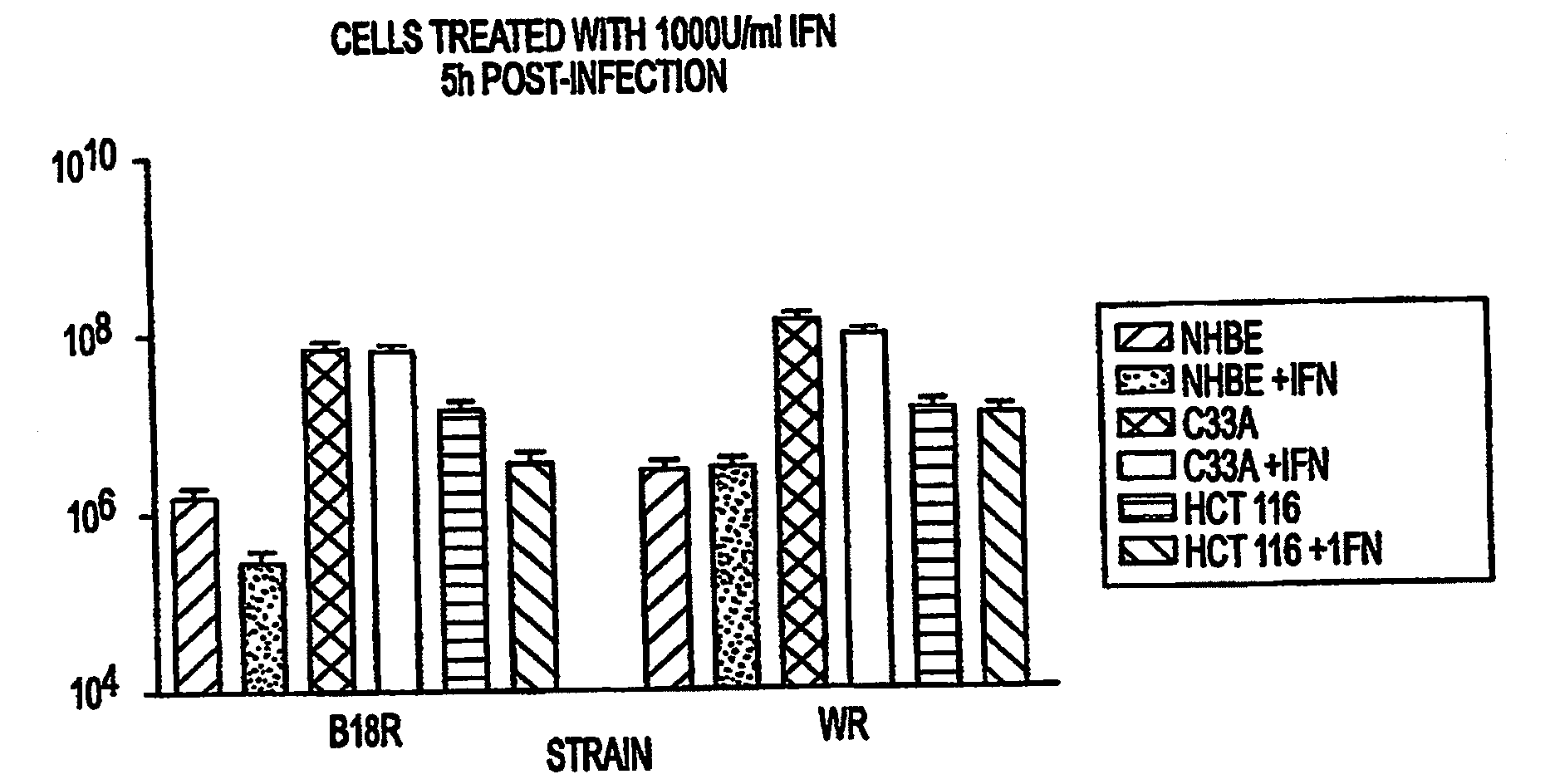
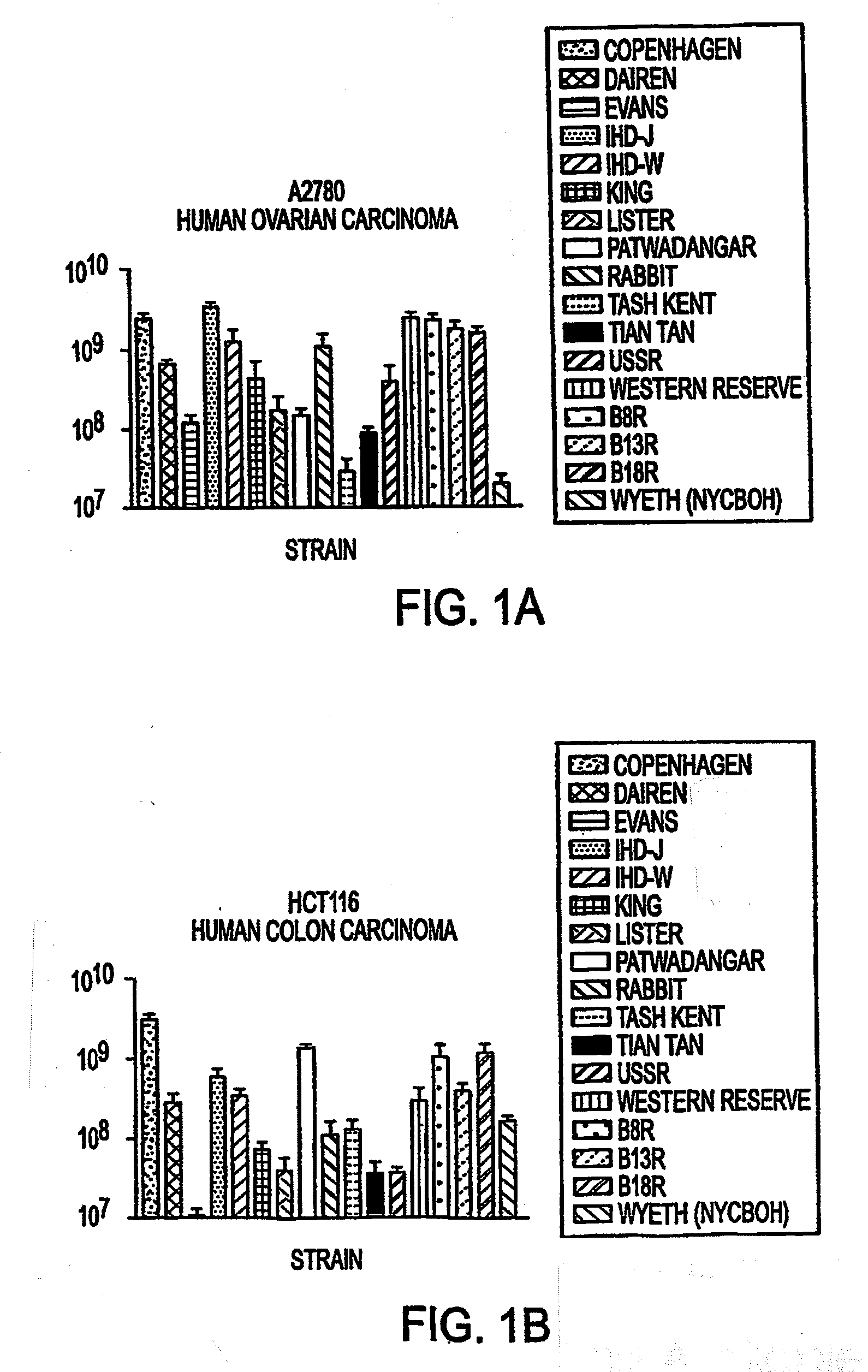
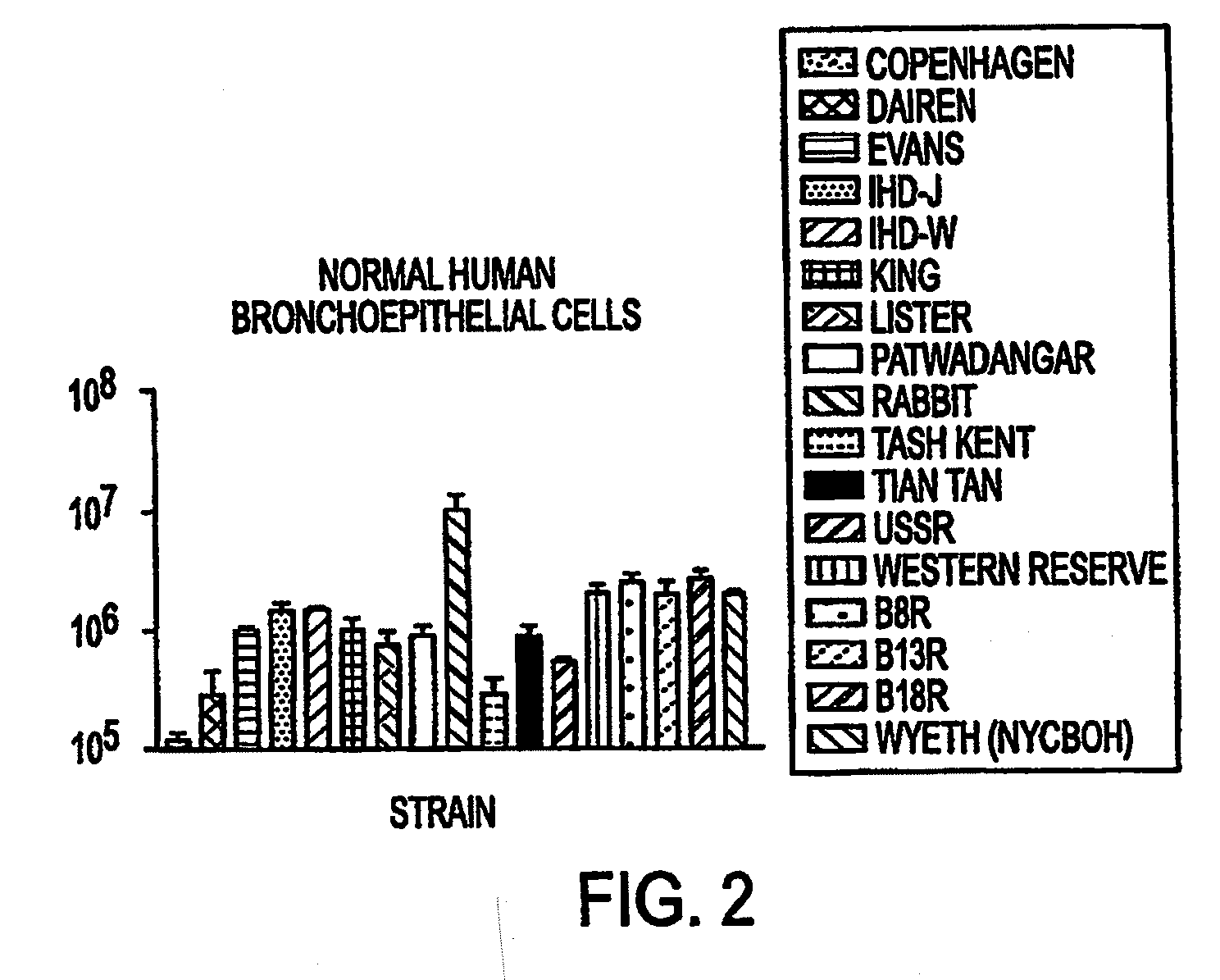
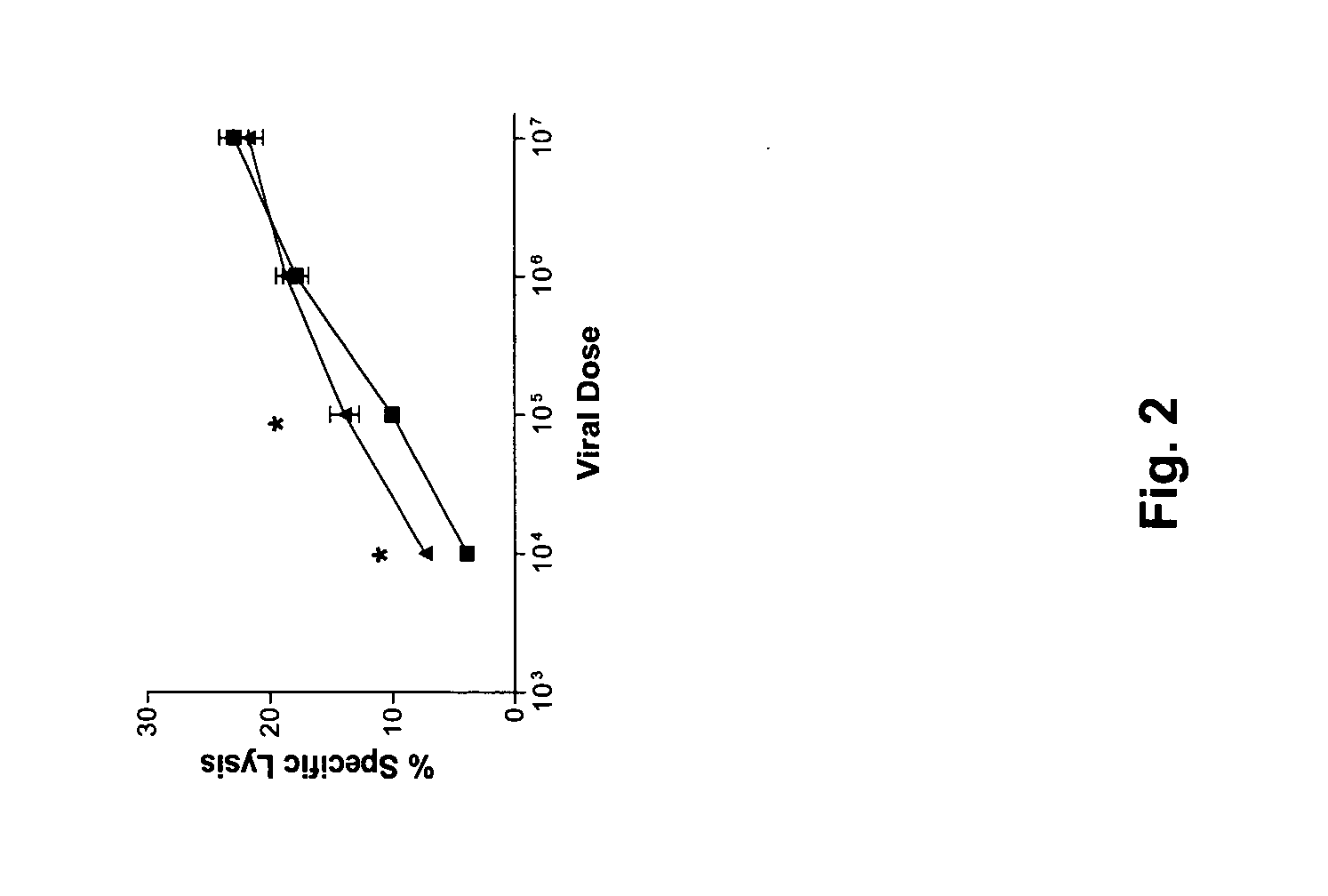
The present invention concerns methods and compositions for the treatment of cancer and cancer cells using altered poxviruses, including a vaccinia virus that has been altered to generate a more effective therapeutic agent. Such poxviruses are engineered to be attenuated or weakened in their ability to affect normal cells. In some embodiments, methods and compositions involve poxviruses that possess mutations that result in poxviruses with diminished or eliminated capability to implement an antiviral response in a host. Poxviruses with these mutations in combination with other mutations can be employed for more effective treatment of cancer.
Owner:SILLAJEN BIOTHERAPEUTICS
Novel recombinant poxvirus composition and uses thereof
InactiveUS20050281782A1Facilitated DiffusionPromote cell proliferationBiocideVirusesNucleic acid sequencingCD4 antigen
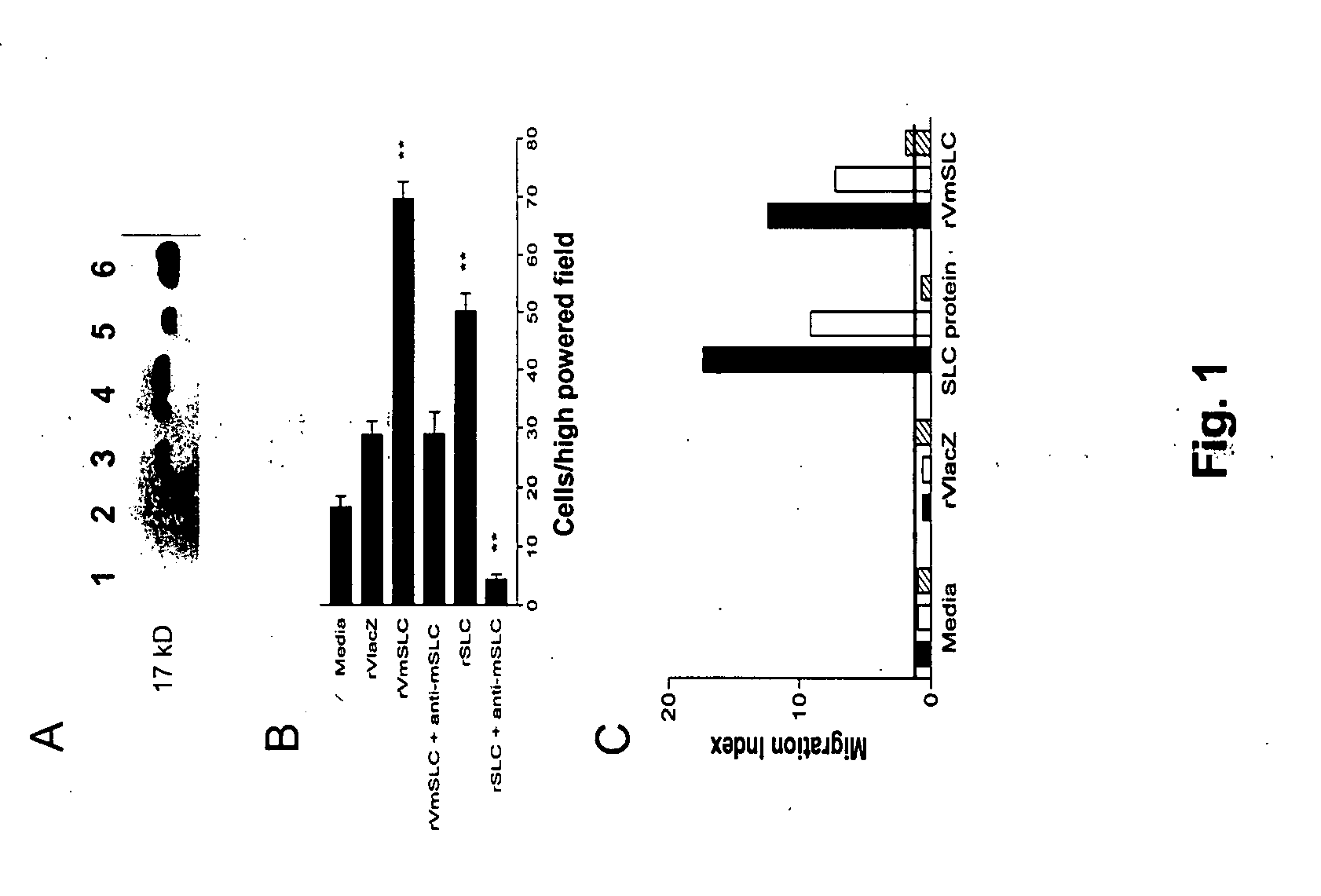
The present invention provides a recombinant pox virus composition comprising a nucleic acid sequence encoding chemokines as costimulatory molecules. The present invention further provides a host cell, a host animal, and a pharmaceutical composition comprising the recombinant pox virus composition. Also provided is a method for treating or preventing a neoplasm or infectious disease in a subject, using the pox virus composition and / or an SLC agent. Additionally, the present invention provides a method for promoting the proliferation of a CD4 T cell, comprising administering to the cell an SLC agent in an amount effective to directly promote the proliferation of the cell. Finally, the present invention provides a method for treating or preventing a neoplasm or infectious disease in a subject using cultured CD4 T cells.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Method for virus propagation
The present invention relates to a process for producing poxvirus, in particular Chordopoxvirus, wherein the poxvirus is cultivated at a temperature below 37° C. The process leads to increased virus propagation at the decreased temperature.
Owner:BAVARIAN NORDIC AS
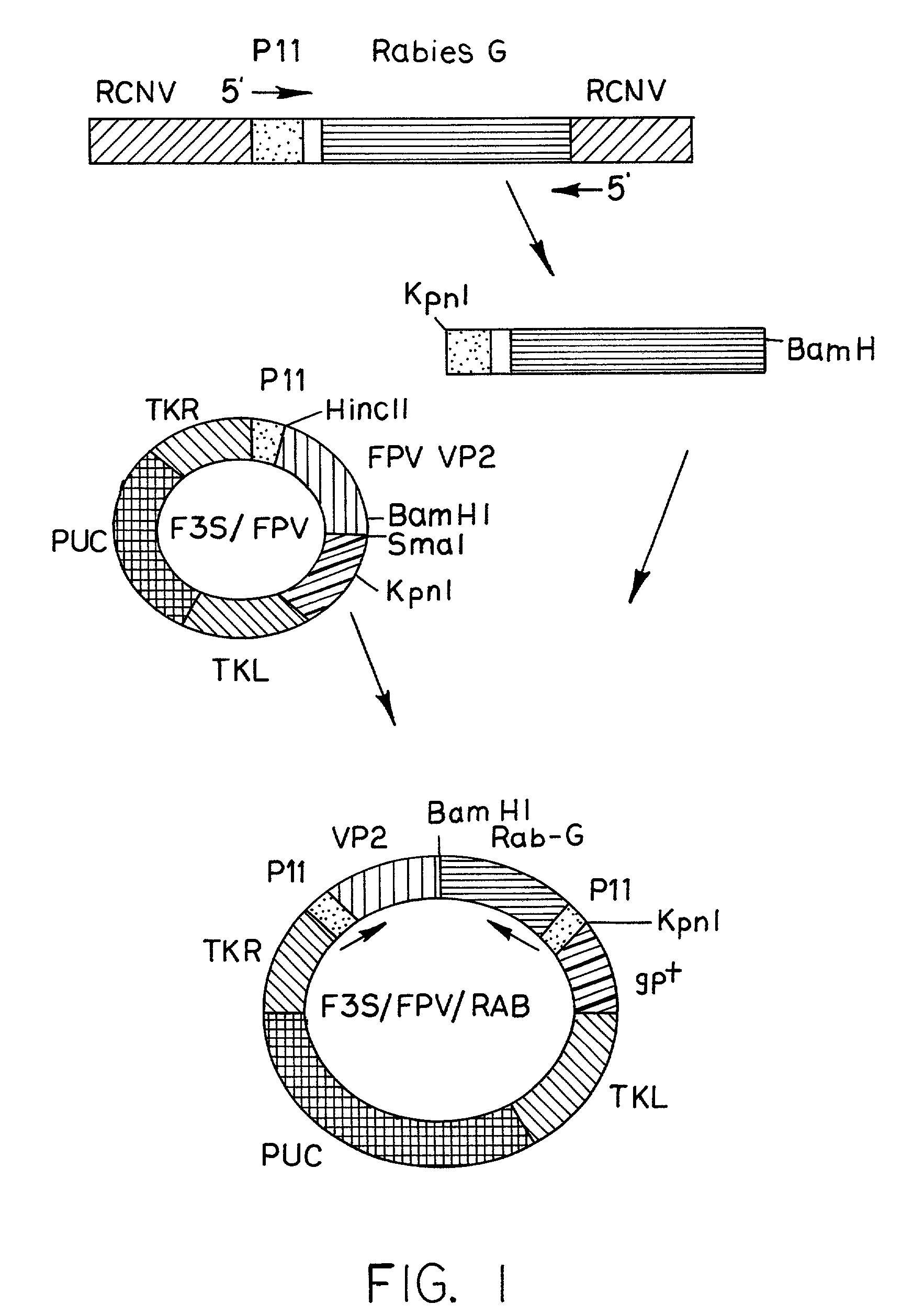
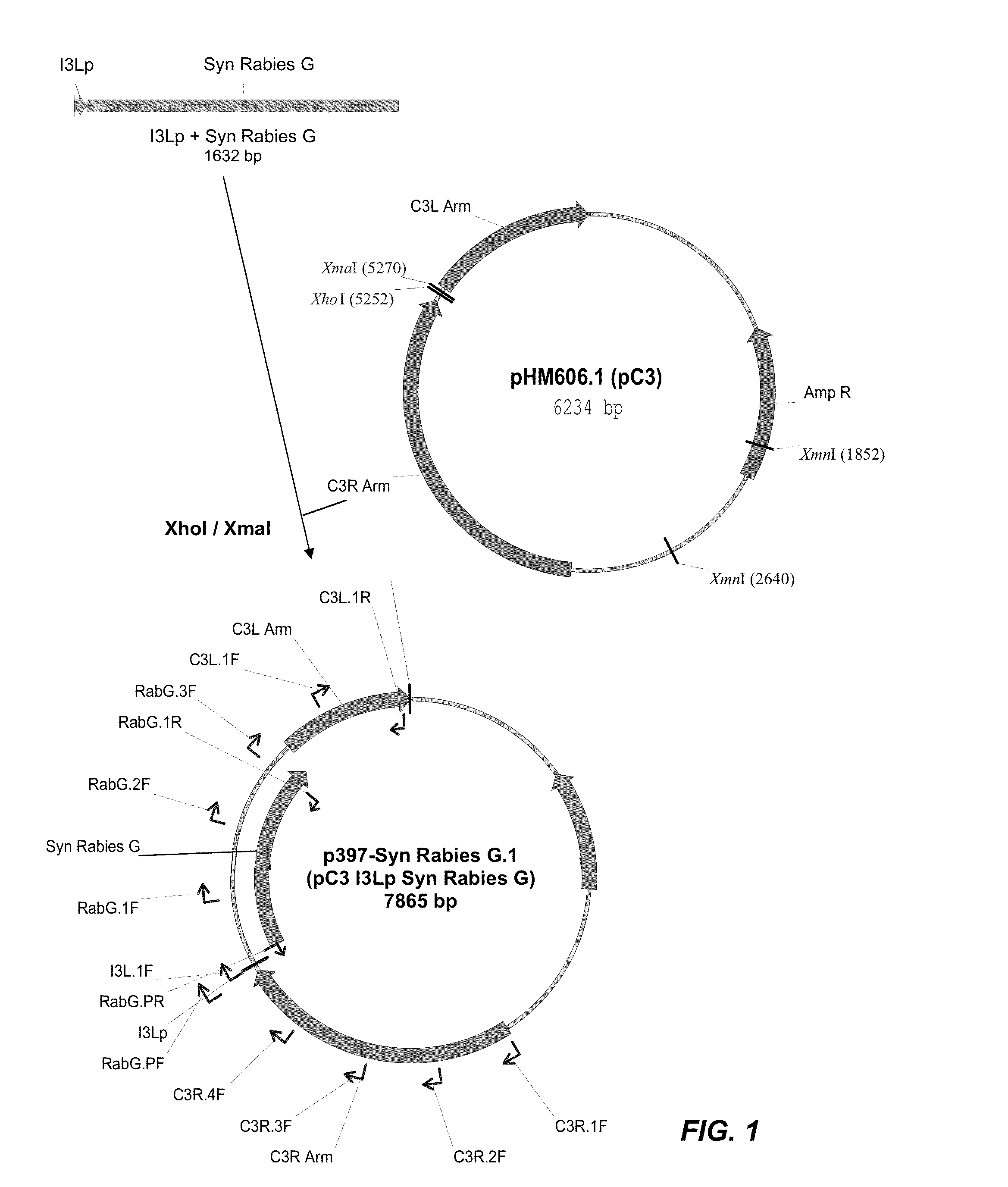
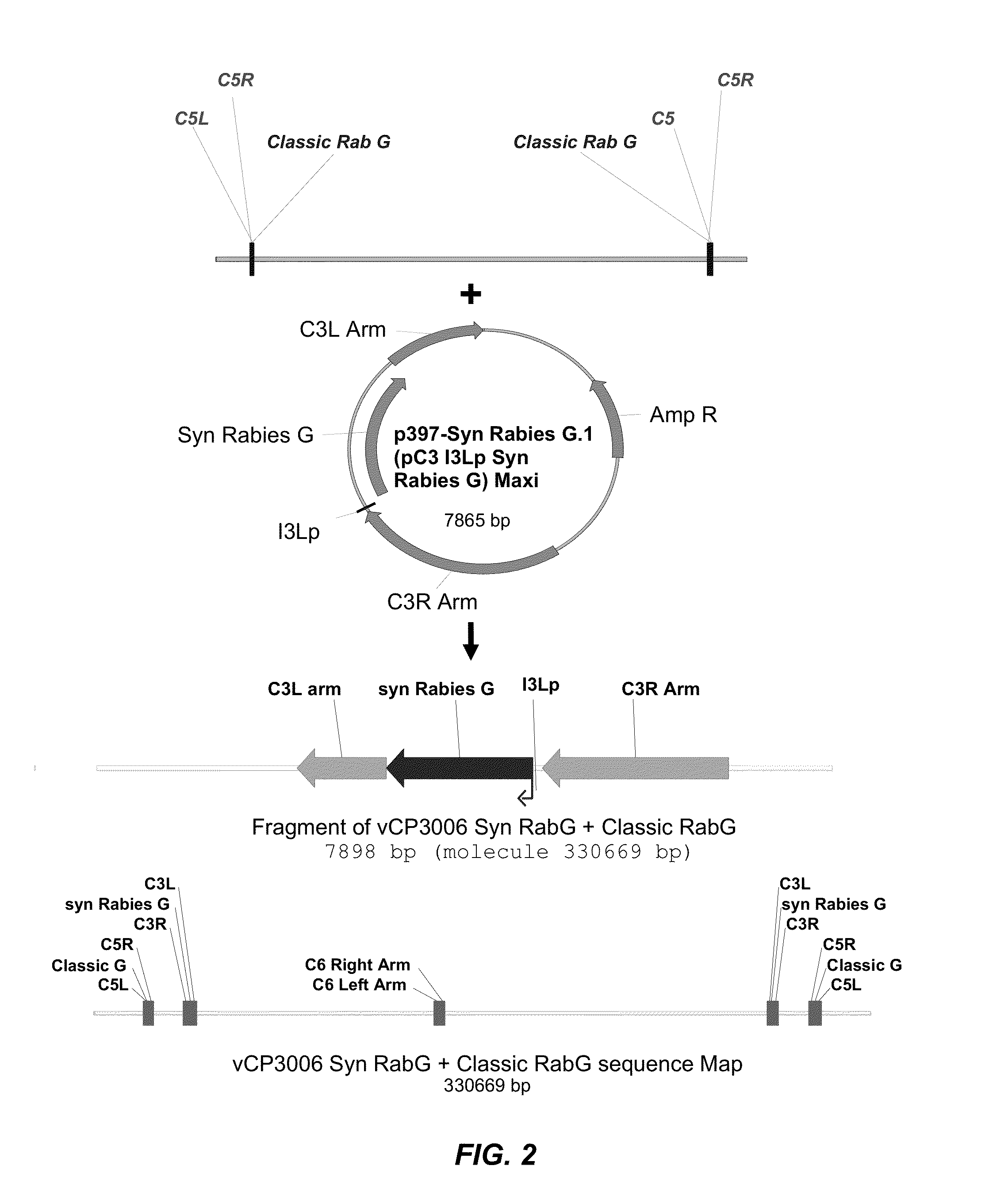
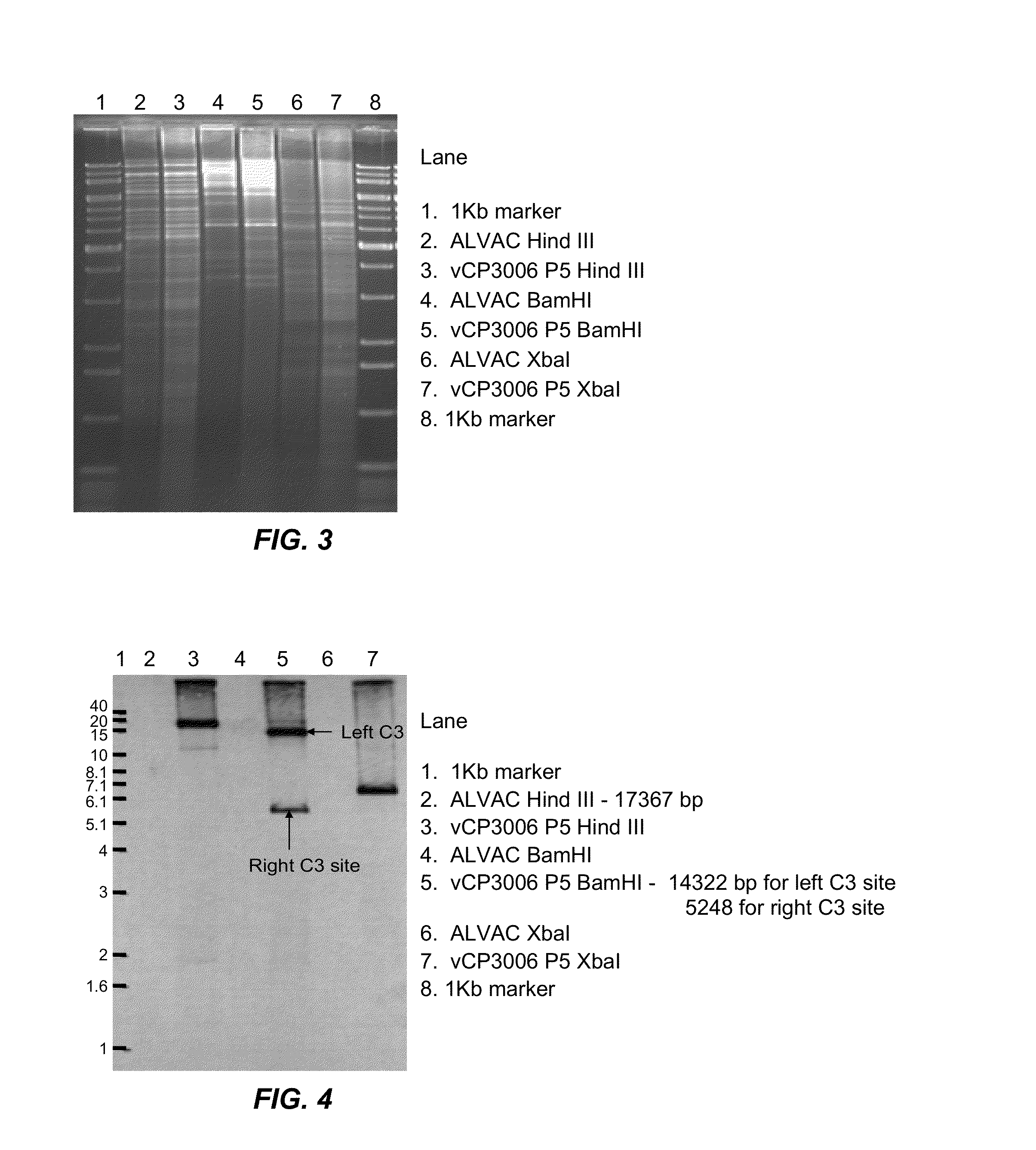
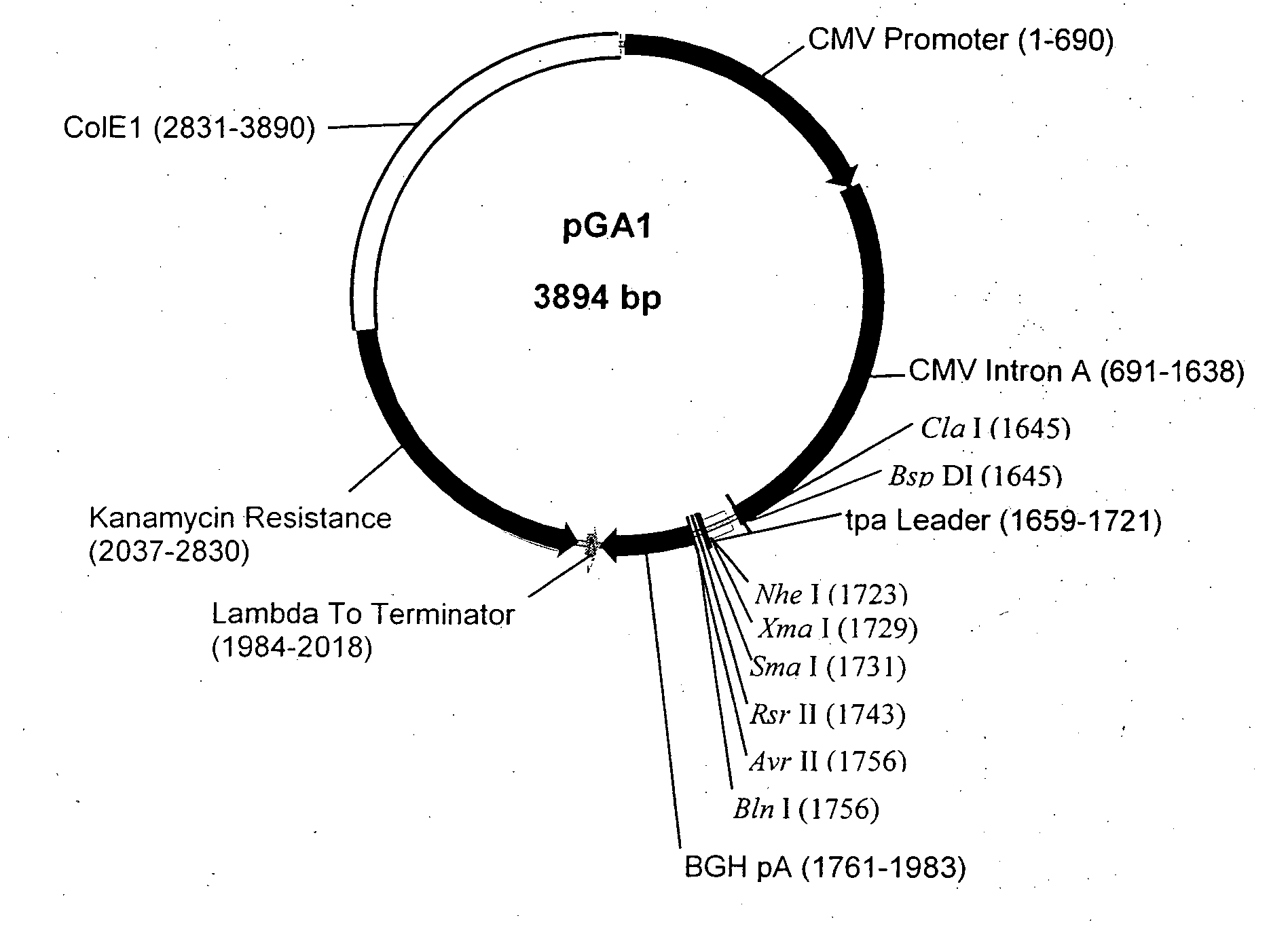
Recombinant Poxviral Vectors Expressing both Rabies and OX40 Proteins, and Vaccines Made Therefrom
The present invention provides vectors that contain and co-express in vivo or in vitro immunogenic polypeptides or antigens together with an OX40L polypeptide, which functions as a genetic adjuvant. Together, the immunogenic polypeptide and the OX40L polypeptide elicit an immune response in animal or human, which is greater than the immune response elicited by the immunogenic polypeptide alone. In a particular example, the invention provides vectors encoding a Rabies G immunogenic polypeptide and a canine OX40L genetic adjuvant, which vectors elicit strong immune responses in canine against rabies virus
Owner:MERIAL INC
Compositions and methods for generating an immune response
InactiveUS20070048861A1Increase typeSsRNA viruses negative-senseAntibody mimetics/scaffoldsImmunodeficiency virusEukaryotic plasmids
The present invention relates to novel plasmid constructs useful for the delivery of DNA vaccines. The present invention provides novel plasmids having a transcription cassette capable of directing the expression of a vaccine nucleic acid insert encoding immunogens derived from any pathogen, including fungi, bacteria and viruses. The present invention, however, is particularly useful for inducing in a patient an immune response against pathogenic viruses such as HIV, measles or influenza. Immunodeficiency virus vaccine inserts of the present invention express non-infectious HIV virus-like particles (VLP) bearing multiple viral epitopes. VLPs allow presentation of the epitopes to multiple histocompatability types, thereby reducing the possibility of the targeted virus escaping the immune response. Also described are methods for immunizing a patient by delivery of a novel plasmid of the present invention to the patient for expression of the vaccine insert therein. Optionally, the immunization protocol may include a booster vaccination that may be a live vector vaccine such as a recombinant pox virus or modified vaccinia Arbora vector. The booster live vaccine vector includes a transcription cassette expressing the same vaccine insert as the primary immunizing vector.
Owner:ROBINSON HARRIET L +11
Custom Vectors for Treating and Preventing Pancreatic Cancer
ActiveUS20080166367A1Improve abilitiesPreventing and delaying onsetSugar derivativesGenetic material ingredientsAntigenOncology
The present invention is directed to a system for treating individuals at risk of developing or suffering from pancreatic cancer. The system comprises administering to the individual a recombinant poxvirus, where the poxvirus contains a foreign nucleic acid encoding at least one pancreatic tumor associated antigen (PTAA).
Owner:REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES +1
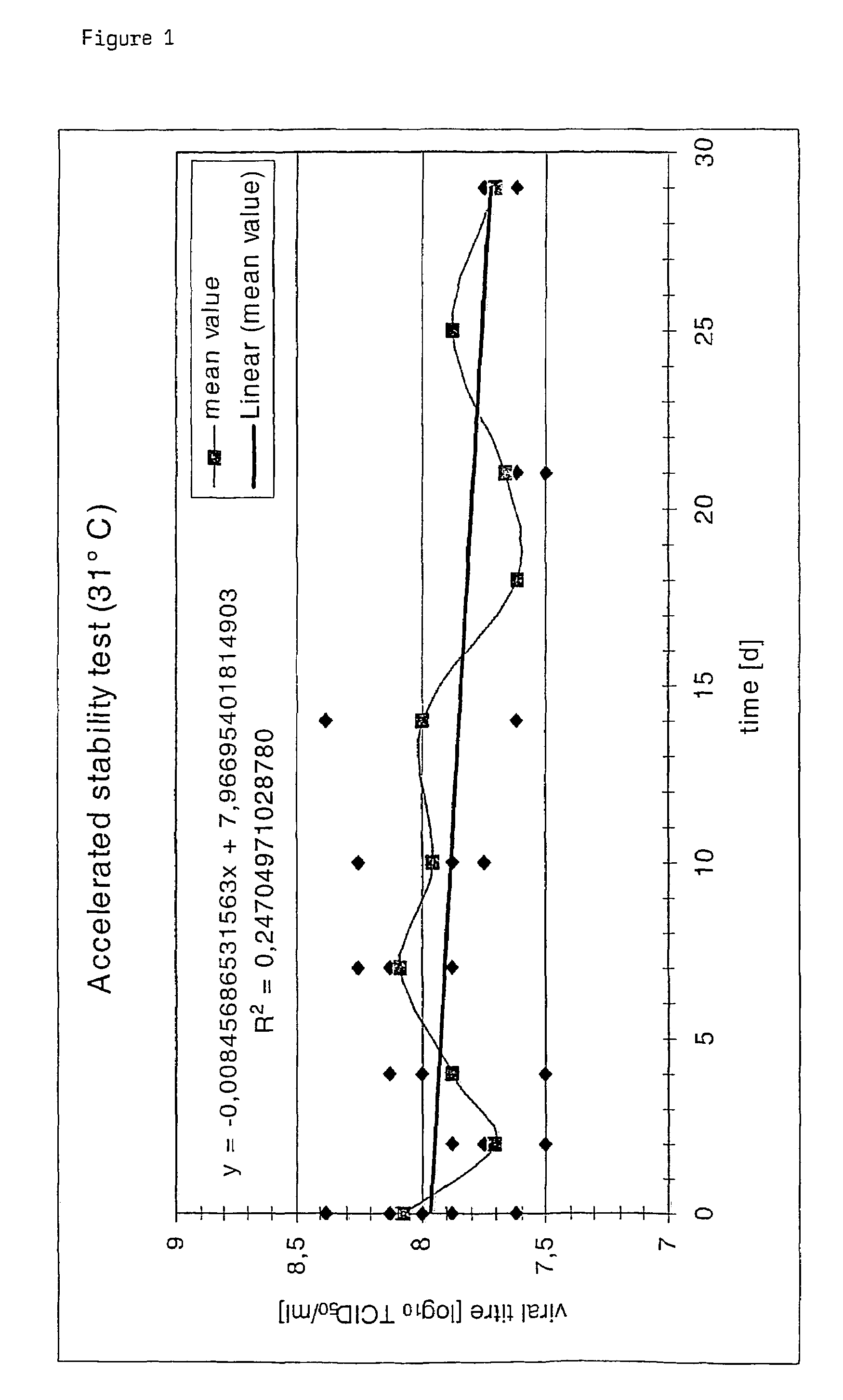
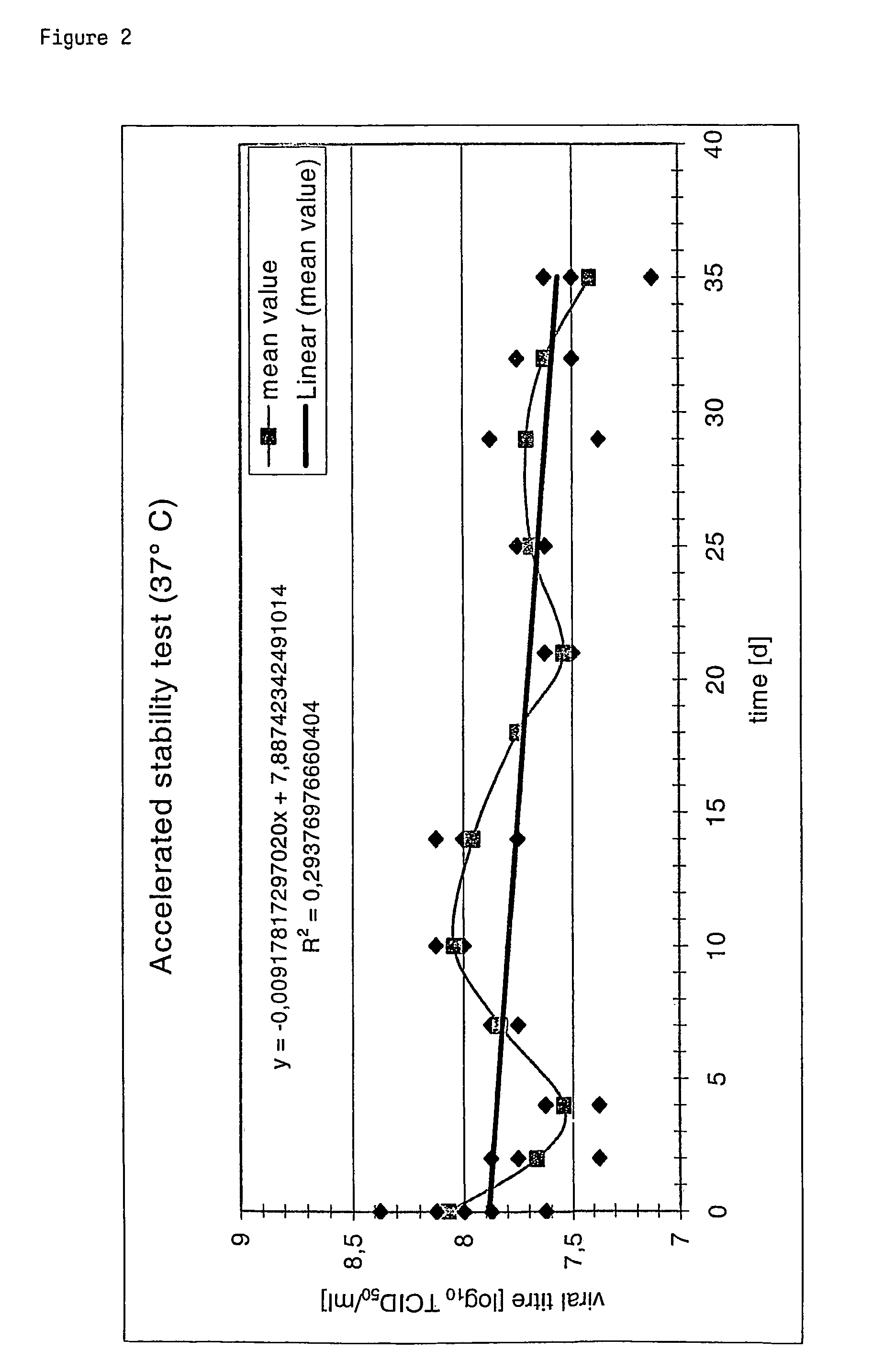
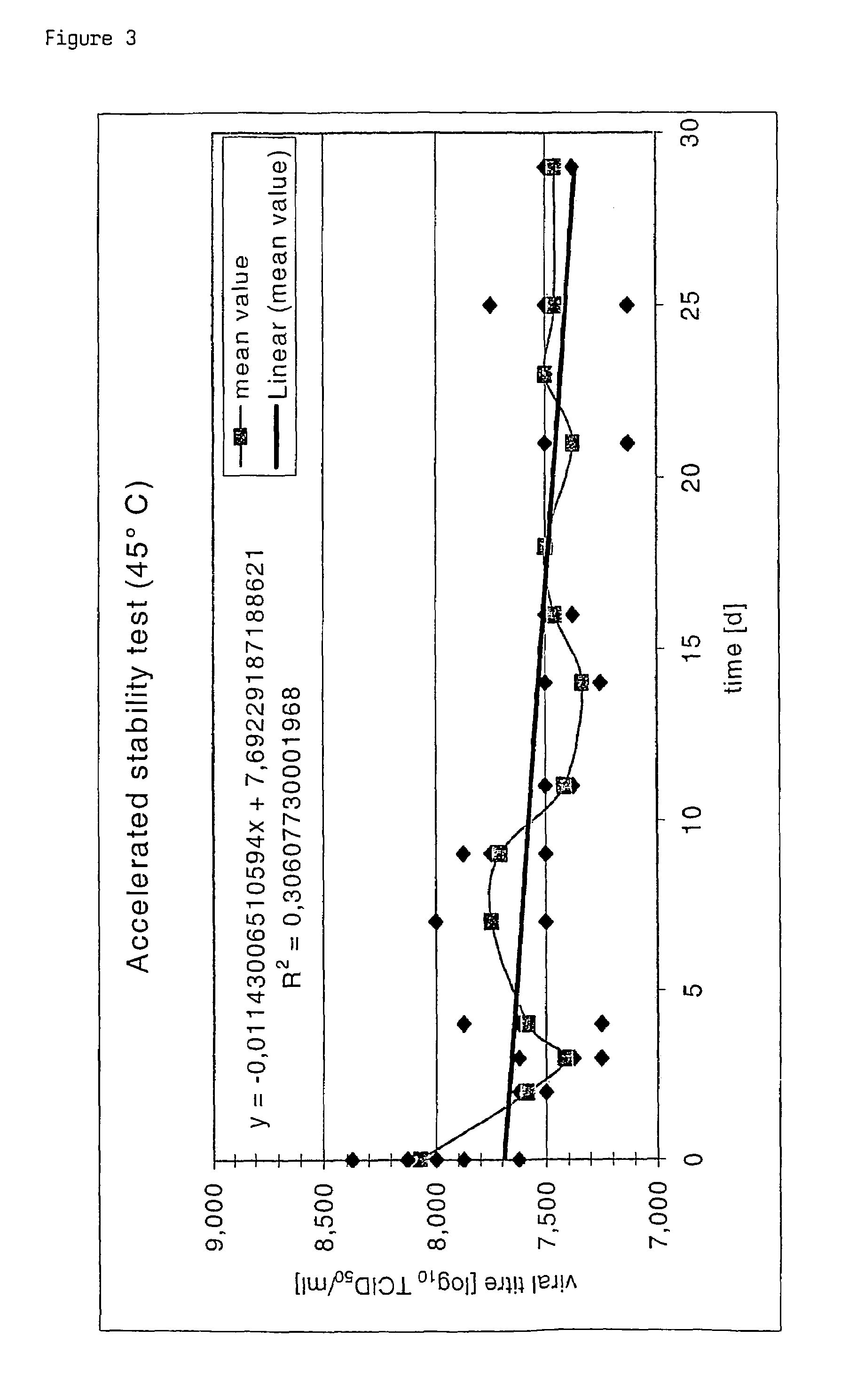
Poxvirus containing formulations and process for preparing stable poxvirus containing compositions
InactiveUS7094412B2Reduce the amount requiredPowder deliveryViral antigen ingredientsCapripoxvirusFreeze-drying
The present invention relates to a formulation, in particular an aqueous formulation comprising (i) a poxvirus of one of the genera orthopoxvirus, avipoxvirus, parapoxvirus, capripoxvirus and suipoxvirus, (ii) a disaccharide, (iii) a pharmaceutically acceptable polymer and optionally (iv) a buffer. The aqueous formulation is particularly suitable for freeze drying processes resulting in a stable, freeze-dried, poxvirus containing composition. The invention further concerns a method for preparing a freeze-dried, poxvirus containing composition and the thus obtained product.
Owner:BAVARIAN NORDIC AS
Chimeric vectors
ActiveUS20060073594A1Less cytopathicSsRNA viruses positive-senseNucleic acid vectorHeterologousViral vector
The present invention relates to chimeric vectors. More specifically, the invention relates to recombinant poxvirus vectors and viruses that are capable of expressing an alphaviral RNA replicon expressing a heterologous sequence of interest.
Owner:MERIAL INC
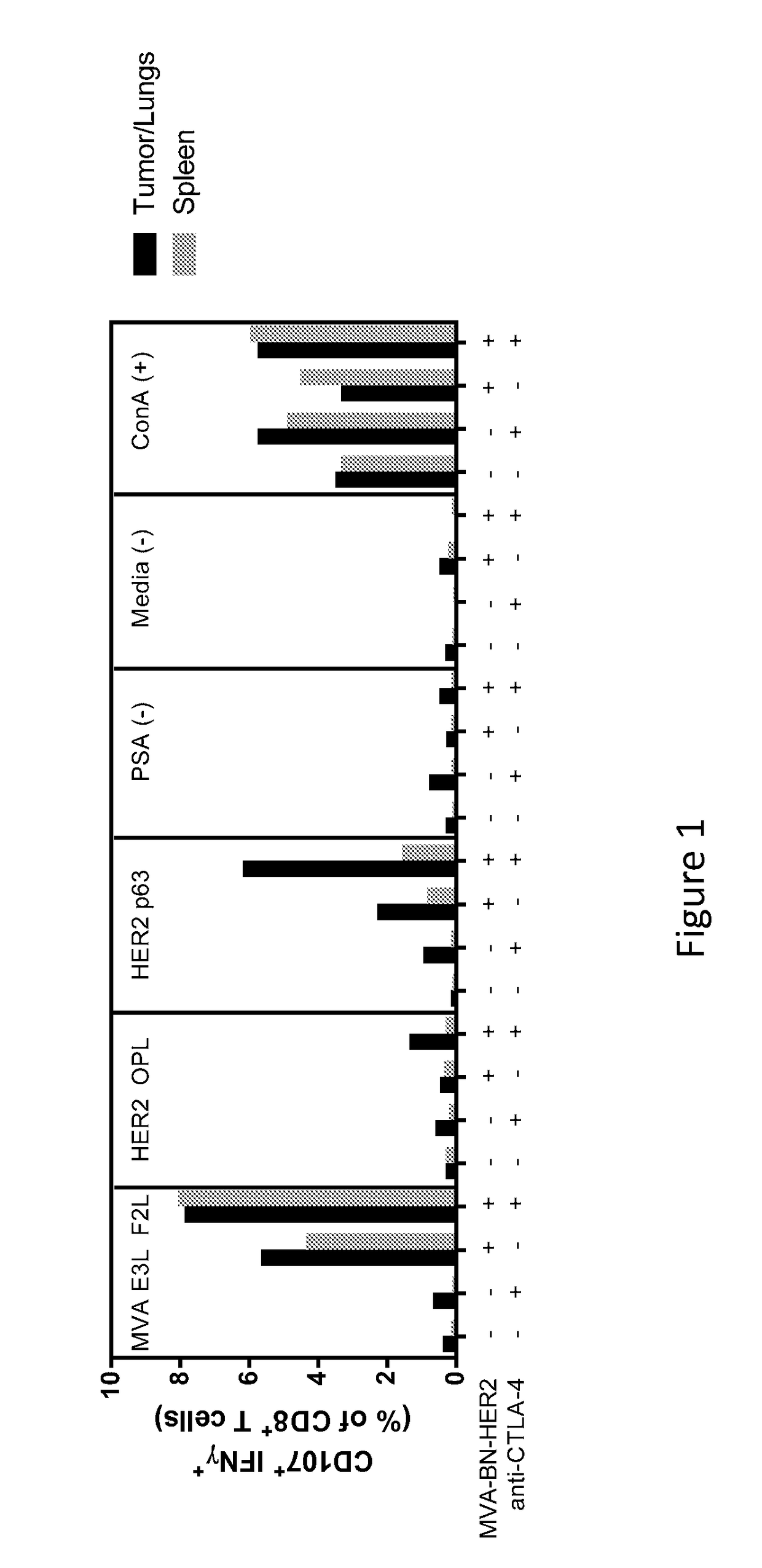
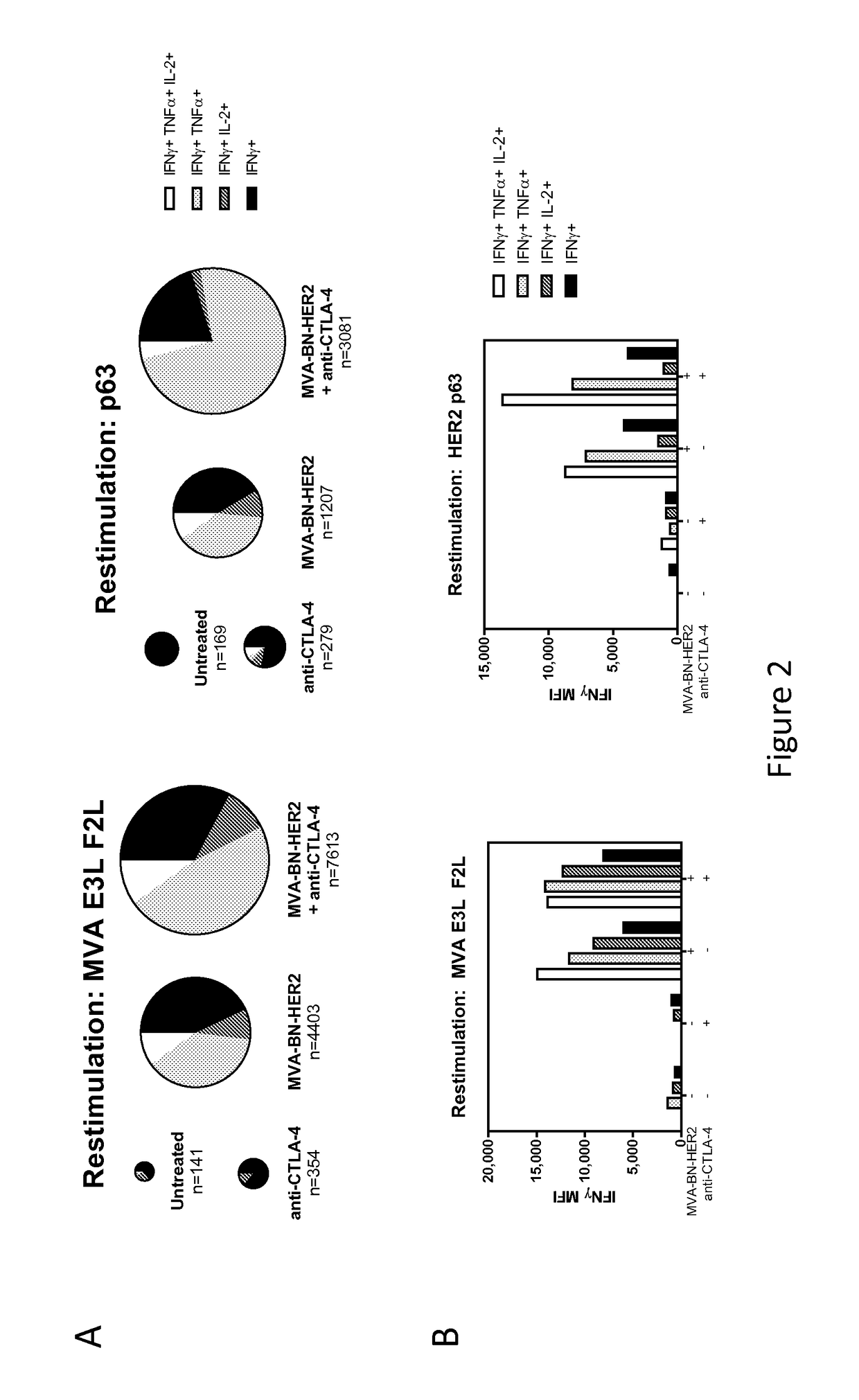
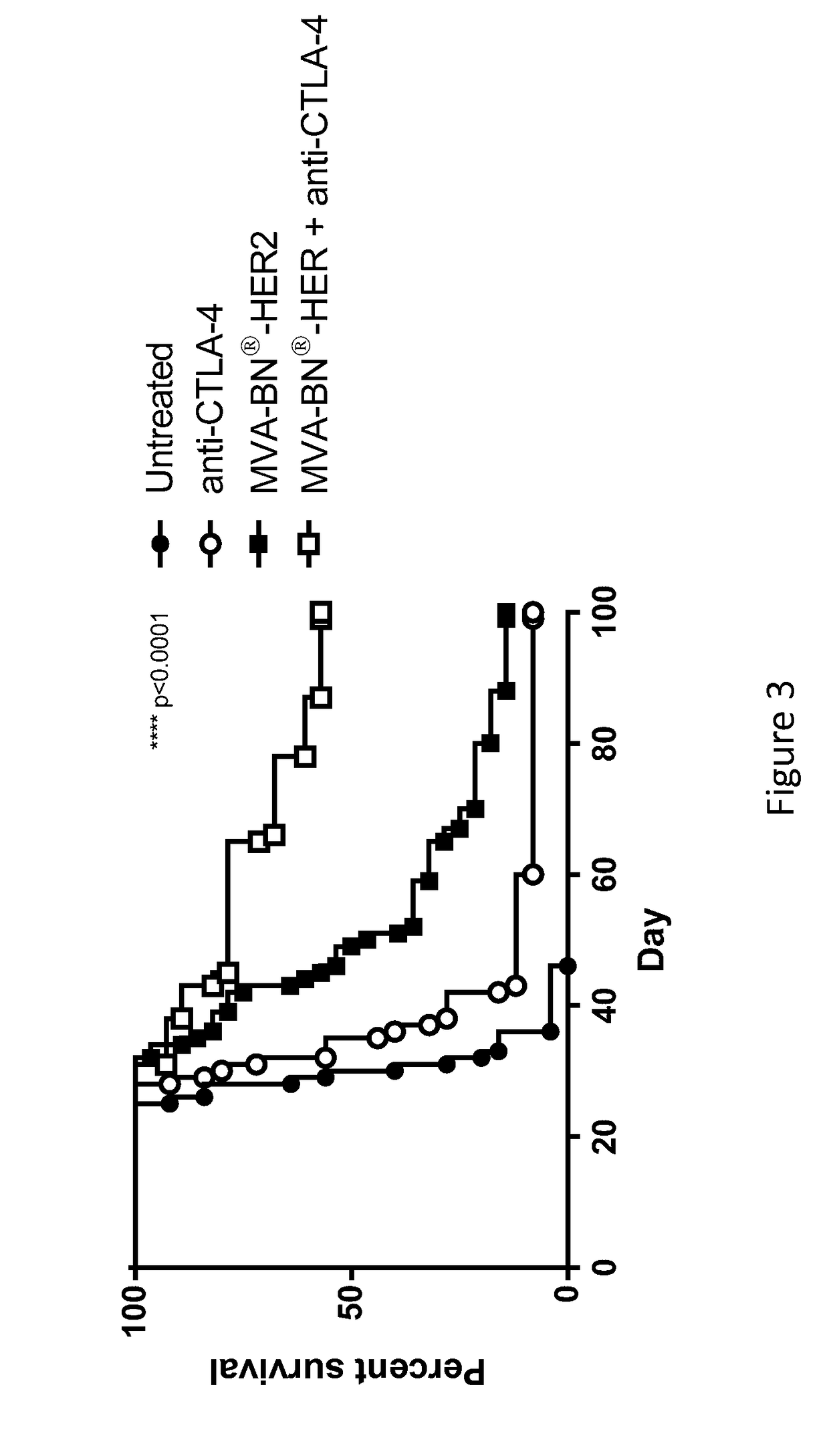
Combination Therapy for Treating Cancer with a Poxvirus Expressing a Tumor Antigen and an Antagonist and/or Agonist of an Immune Checkpoint Inhibitor
InactiveUS20170266270A1Good treatment effectImprove combination effectViral/bacteriophage medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsOncologyTumor antigen
The invention relates to compositions, kits, and methods for cancer therapy using recombinant poxviruses encoding a tumor-associated antigen in combination with antagonists or agonists of immune checkpoint inhibitors.
Owner:BAVARIAN NORDIC AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com