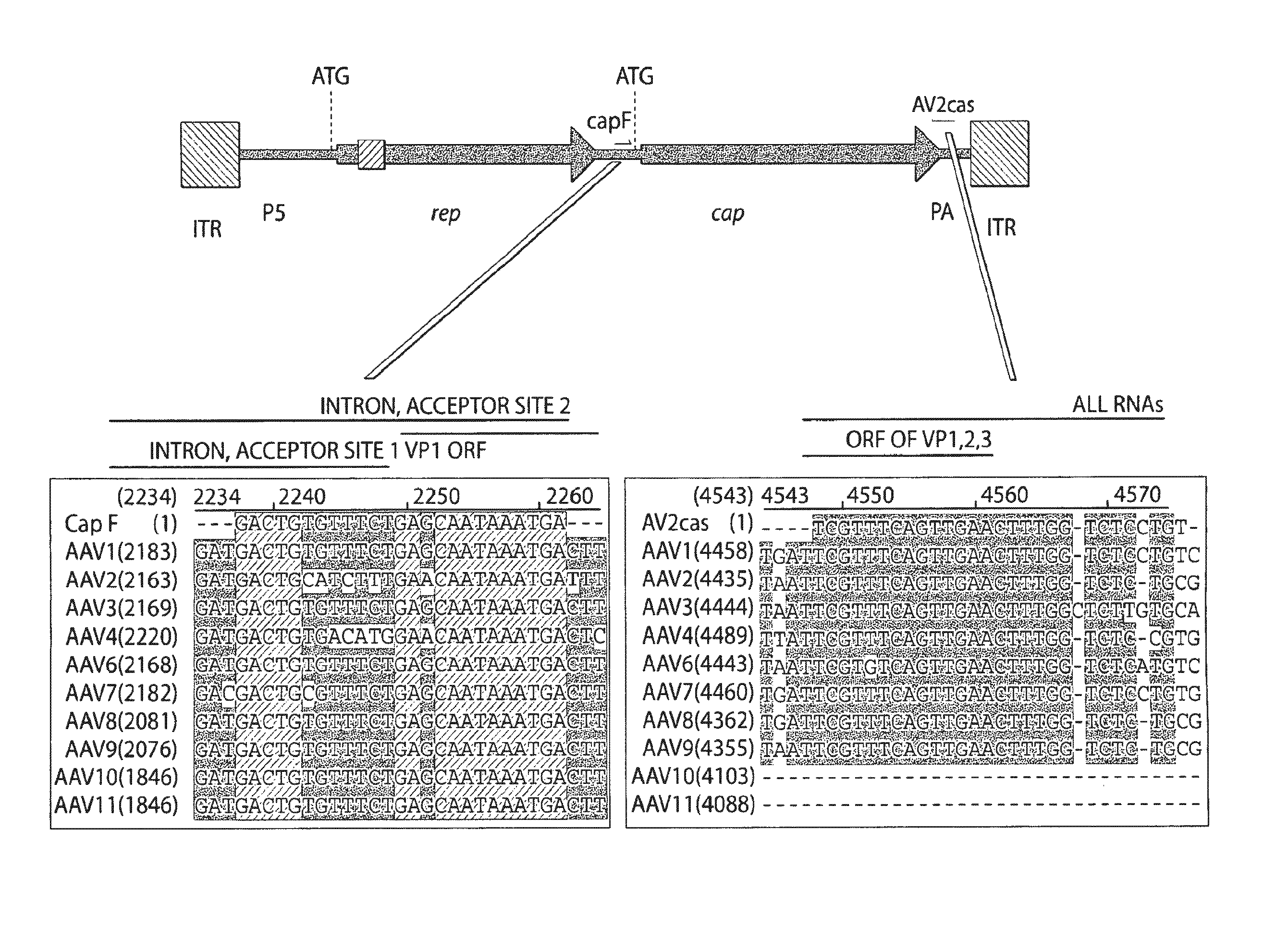
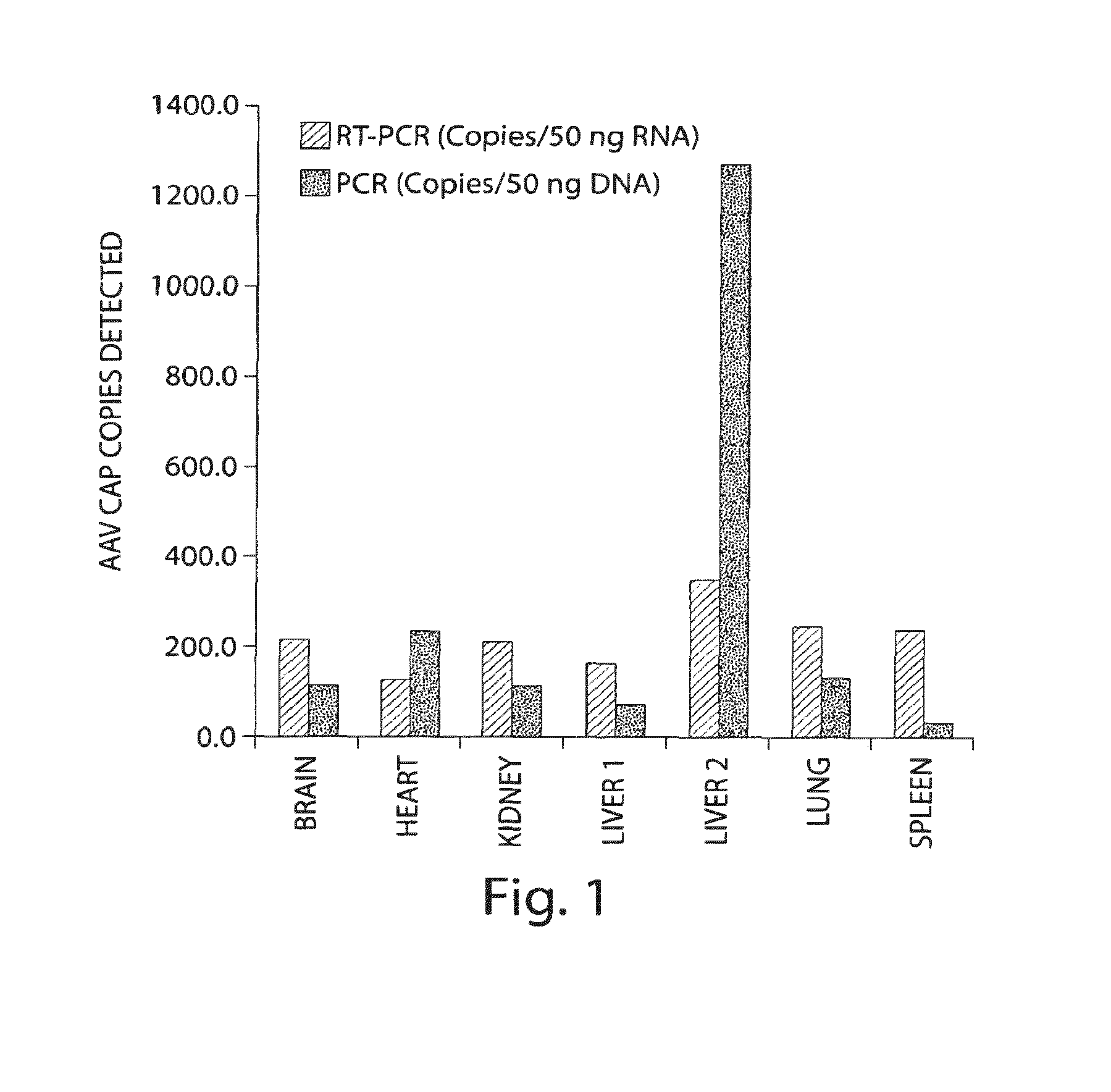
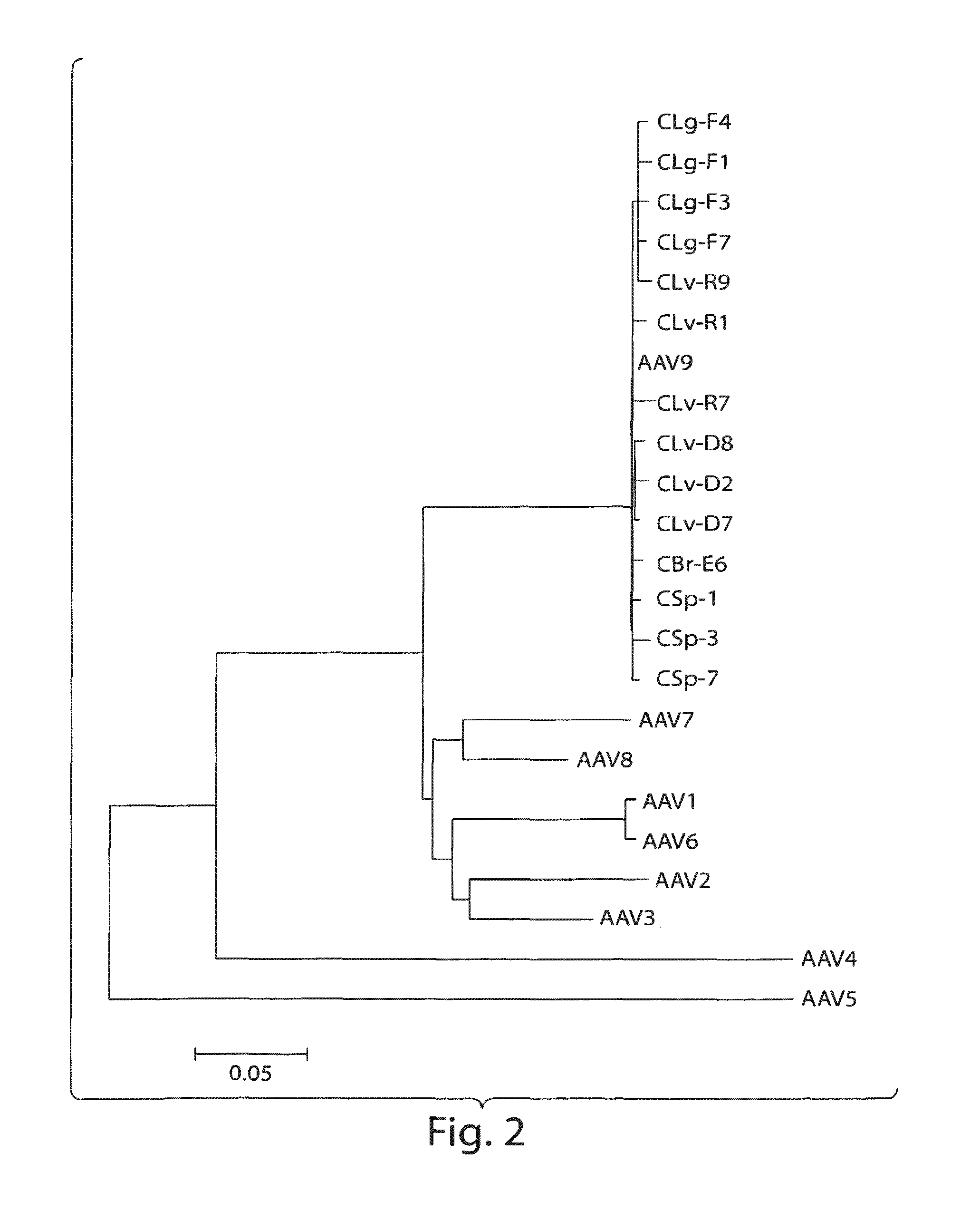
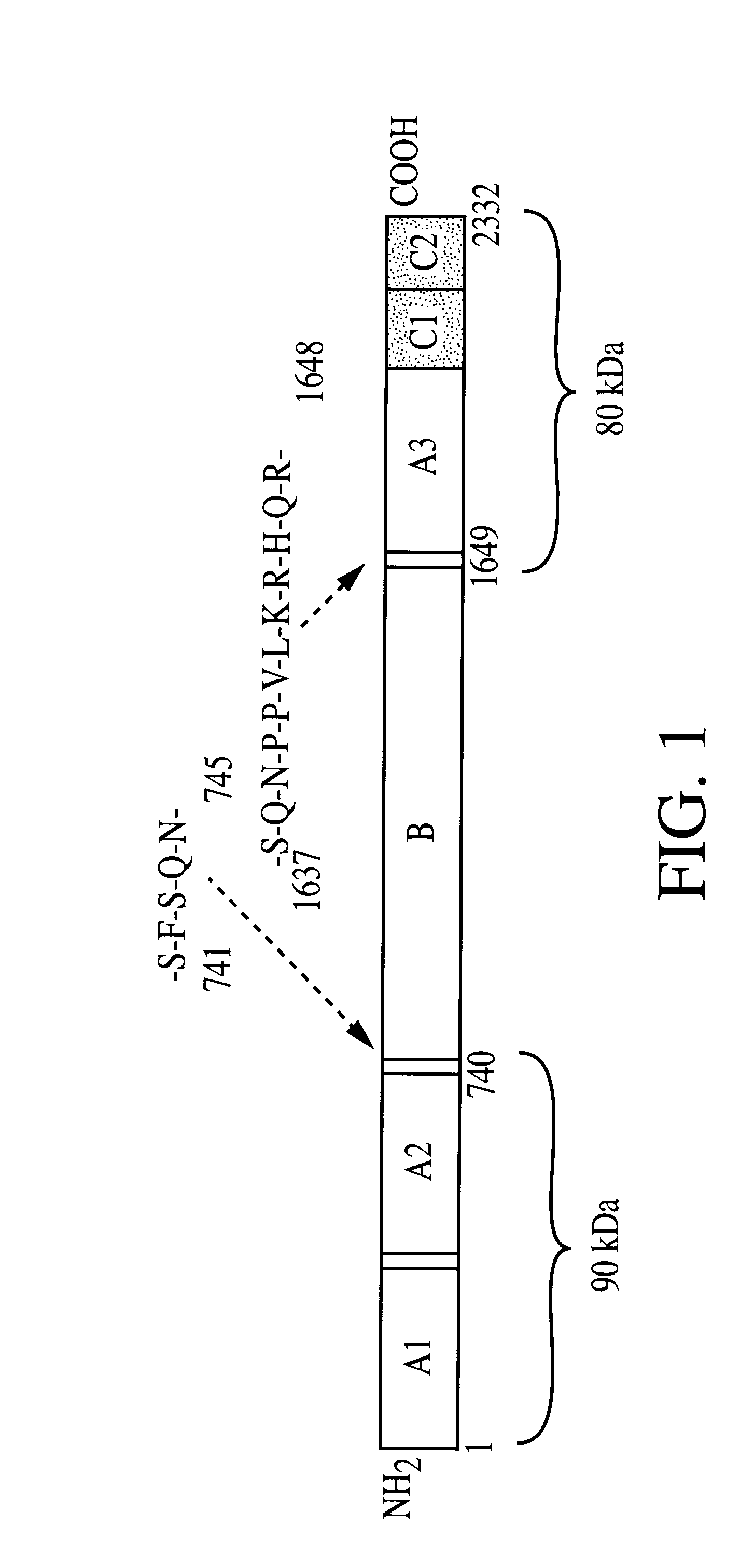
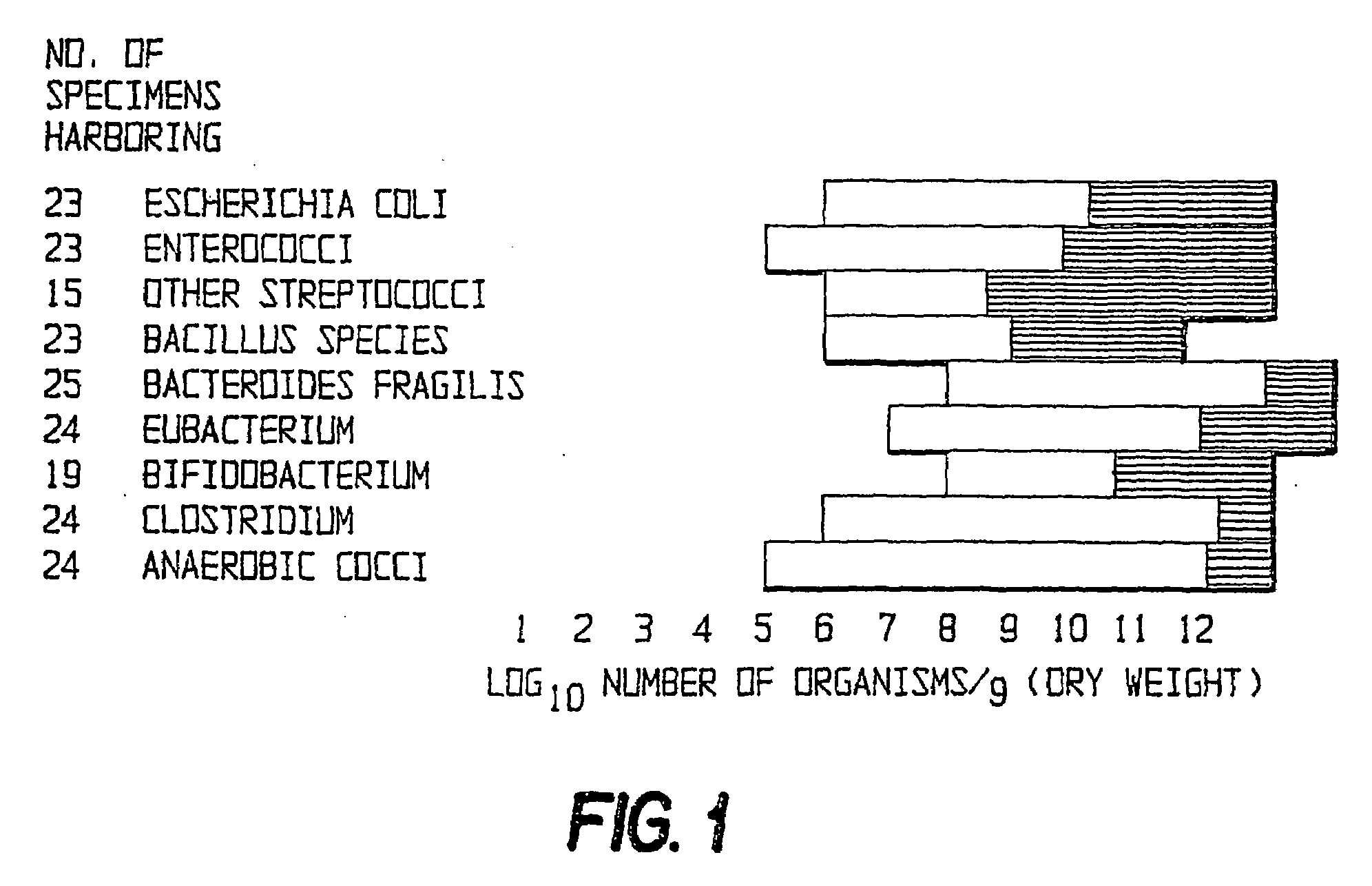
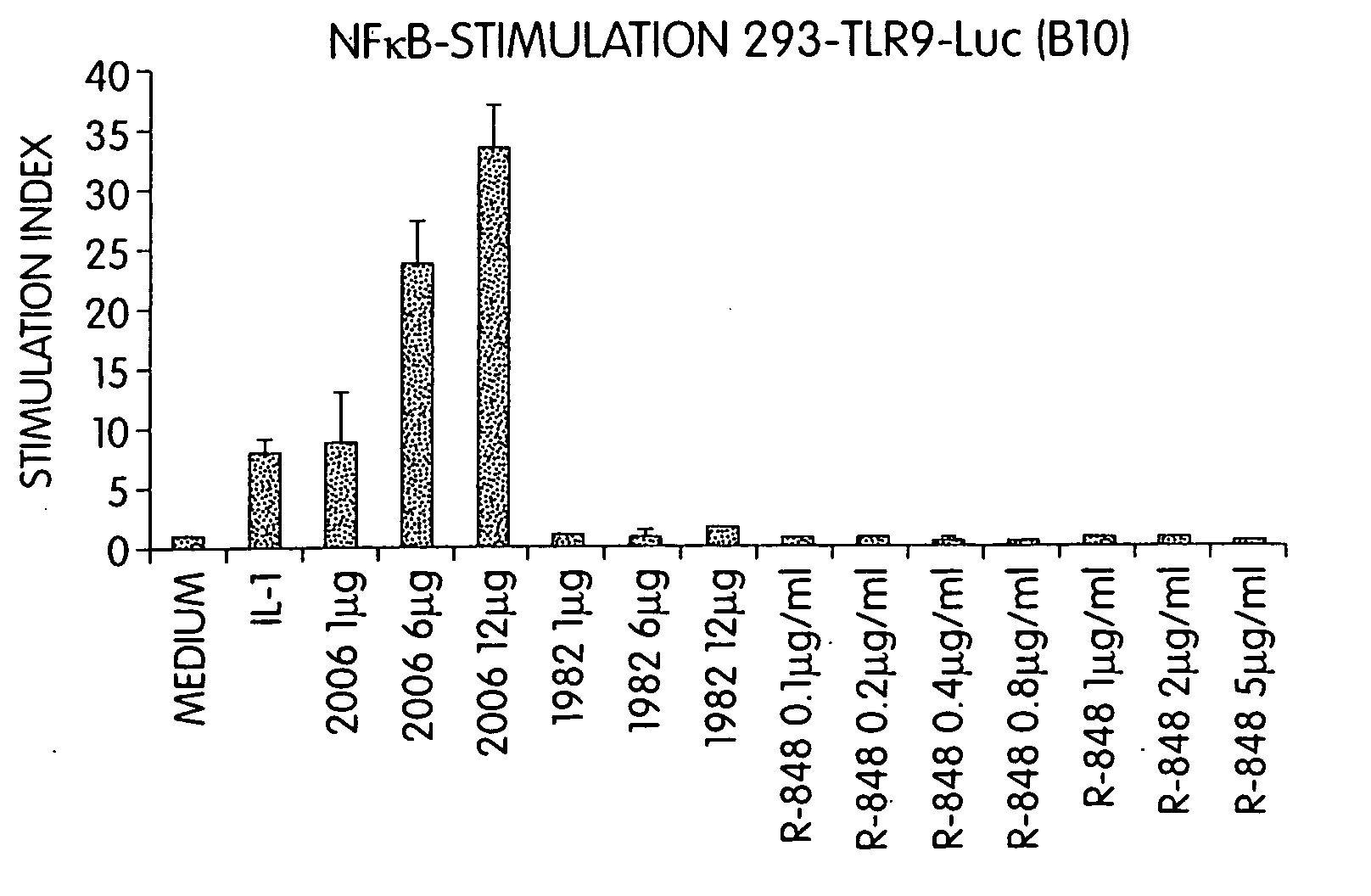
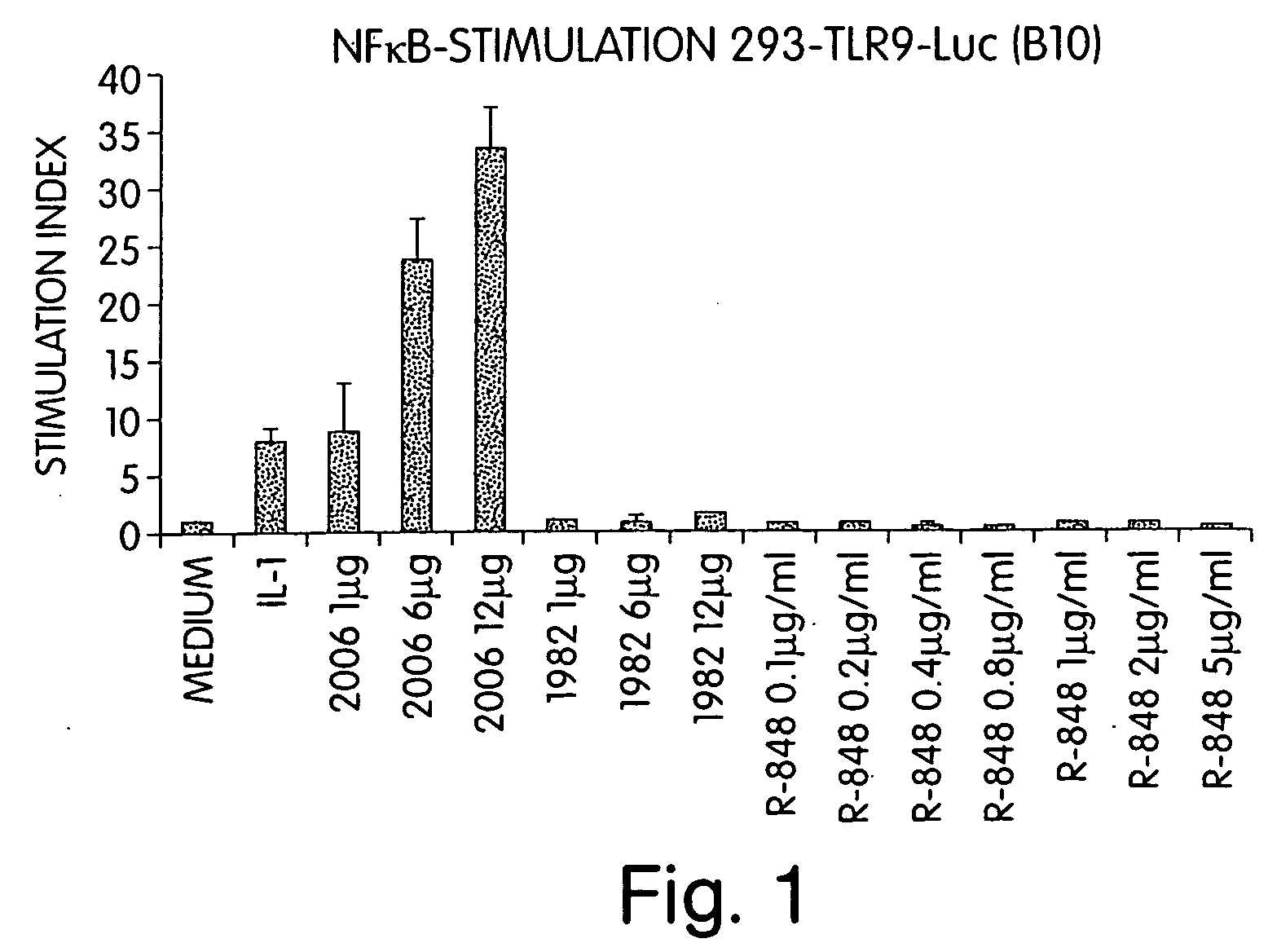
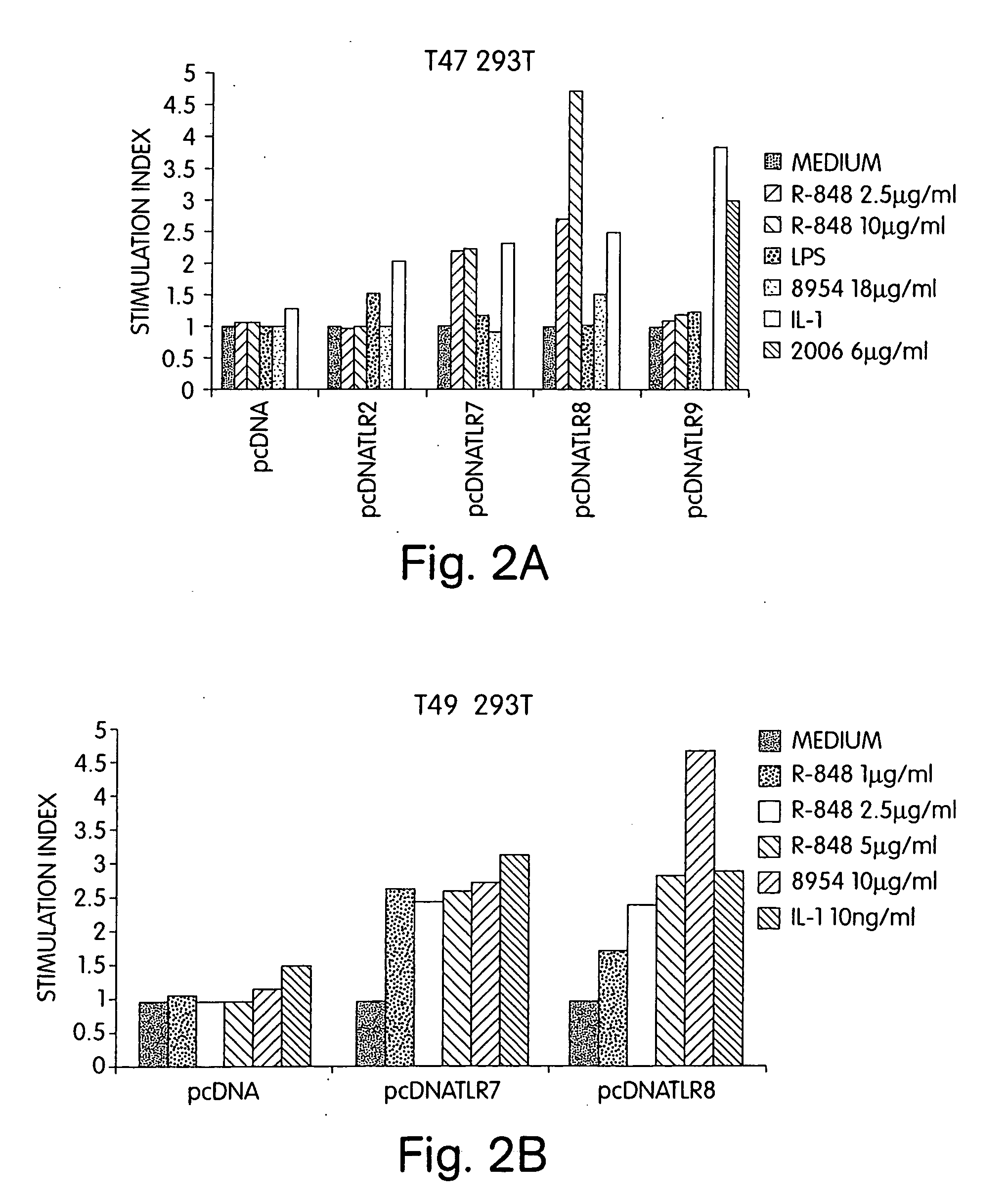
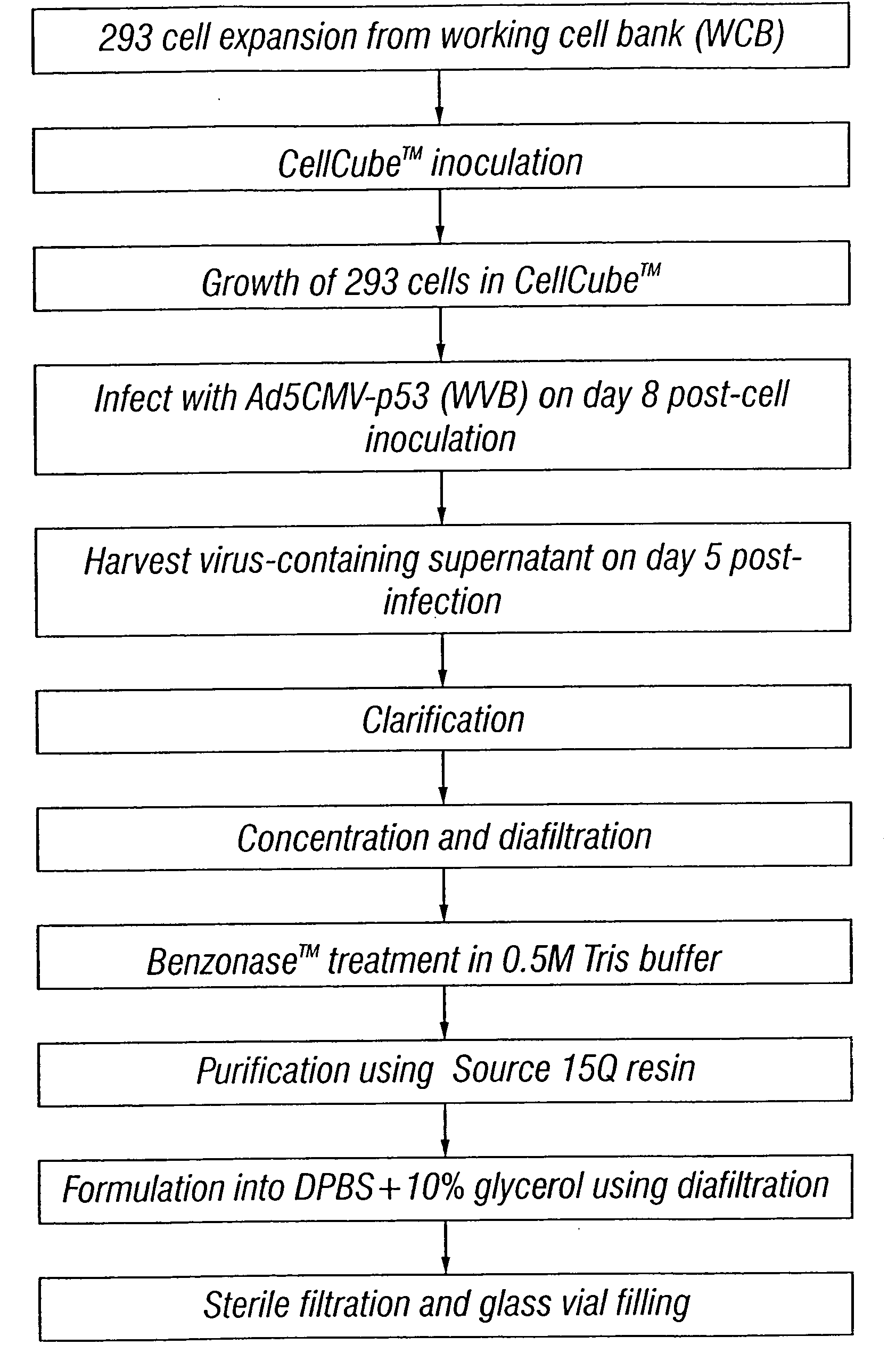
Patents
Literature
2575results about "Viral/bacteriophage medical ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
AAV's and uses thereof
The invention in some aspects relates to recombinant adeno-associated viruses having distinct tissue targeting capabilities. In some aspects, the invention relates to gene transfer methods using the recombinant adeno-associate viruses. In some aspects, the invention relates to isolated AAV capsid proteins and isolated nucleic acids encoding the same.
Owner:UNIV OF MASSACHUSETTS
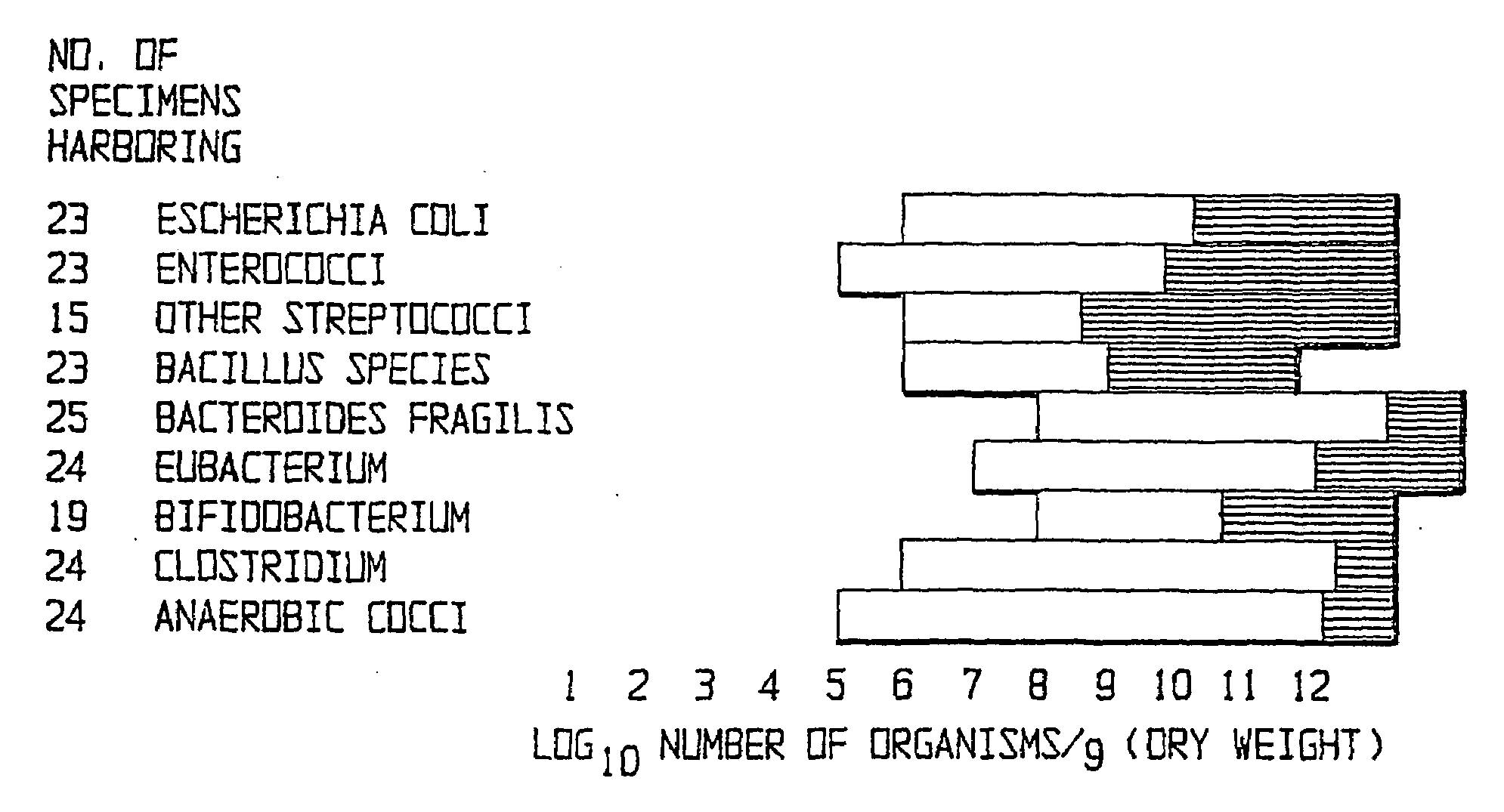
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Adeno-associated virus vectors for expression of factor VIII by target cells
InactiveUS6200560B1Easily transfectedConvenient platformBiocideFactor VIIHigh level expressionHuman cell
The present invention provides improved viral vectors useful for the expression of genes at high levels in human cells. In particular, the present invention provides recombinant adeno-associated vectors (AAV) suitable for gene therapy. These vectors are capable of delivering nucleic acid containing constructs which result in the production of full-length therapeutic levels of biologically active Factor VIII in the recipient individual in vivo. The present invention also provides pharmaceutical compositions comprising such AAV vectors, as well as methods for making and using these constructs.
Owner:GENZYME CORP
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Therapy of cancer by insect cells containing recombinant baculovirus encoding genes
Provided are compositions and methods of use for insect cells comprising baculovirus encoding non-surface expressed proteins and peptides. The claimed invention particularly relates to compositions comprising insect cells containing baculovirus that express cytokines. Such compositions may be administered by, for example, direct intratumoral injection into tumors in mammals, resulting in tumor reduction or recission. Another aspect of the claimed invention concerns methods of promoting resistance to the reoccurence of tumors in mammals who have undergone such tumor recission. In a specific aspect of the claimed invention, the mammals are human subjects presenting with various forms of cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Vector system
InactiveUS7259015B2Eliminate the effects ofInhibition of activationFungiNervous disorderVector systemTyrosine
Provided are retroviral vector genomes and vector systems comprising the genomes. In particular, a retroviral vector genome comprising two or more NOIs, operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and, optionally, Aromatic Amino Acid Dopa Decarboxylase are provided.
Owner:OXFORD BIOMEDICA (UK) LTD
Adeno-associated vectors for expression of factor VIII by target cells
InactiveUS6221349B1Easily transfectedConvenient platformBiocidePeptide/protein ingredientsHigh level expressionHuman cell
The present invention provides improved viral vectors useful for the expression of genes at high levels in human cells. In particular, the present invention provides adeno-associated vectors (AAV) suitable for gene therapy. These vectors are capable of delivering nucleic acid containing constructs which result in the production of full-length therapeutic levels of biologically active Factor VIII in the recipient individual in vivo. The present invention also provides pharmaceutical compositions comprising such AAV vectors, as well as methods for making and using these constructs.
Owner:GENZYME CORP
Vectors having enhanced expression and methods of making and uses thereof
InactiveUS6130066AHigh expressionIncrease transcriptionVectorsSugar derivativesOpen reading frameVaccinia
Disclosed and claimed are vectors having enhanced expression and methods for making and using them. Enhancement of expression is from substantially co-temporal expression of at least one first nucleic acid molecule and at least one second nucleic acid molecule. The second nucleic acid molecule encodes a transcription factor or a translation factor or a transcription factor and a translation factor. The contemporaneous expression can be from operably linking the first and second nucleic molecules to a single promoter, or from operably linking the first nucleic acid molecule to a first promoter and the second nucleic molecule to a second promoter wherein the first and second promoters function substantially contemporaneously. Thus, the first and second nucleic acid molecules can be at the same locus in the vector, or at different loci. The second nucleic acid molecule can encode: one transcription factor or more than one transcription factor; or one translation factor or more than one translation factor; or at least one transcription factor and at least one translation factor. The transcription factor can be from vaccinia H4L, D6, A7, G8R, A1L, A2L, H5R, or combinations thereof. The translation factor can be from a K3L open reading frame, an E3L open reading frame, a VAI RNA, an EBER RNA, a sigma 3 open reading frame, a TRBP open reading frame, or combinations thereof. The vector can be a poxvirus such as an attenuated poxvirus, e.g., NYVAC, or ALVAC.
Owner:VIROGENETICS
Vector system
InactiveUS20070025970A1Eliminate the effects ofInhibition of activationBiocideFungiVector systemTyrosine hydroxylase
The present invention relates to retroviral vector genomes and to vector systems comprising such genomes. In particular the present invention relates to a retroviral vector genome comprising two or more NOIs operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and optionally Aromatic Amino Acid Dopa Decarboxylase.
Owner:OXFORD BIOMEDICA (UK) LTD
Micrornas differentially expressed in pancreatic diseases and uses thereof
InactiveUS20090131348A1Reduce cell proliferationIncrease and decreasing cell proliferationOrganic active ingredientsAntipyreticDiseasePancreatic disease
The present invention concerns methods and compositions for identifying a miRNA profile for a particular condition, such as pancreatic disease, and using the profile in assessing the condition of a patient.
Owner:INTERPACE DIAGNOSTICS LLC
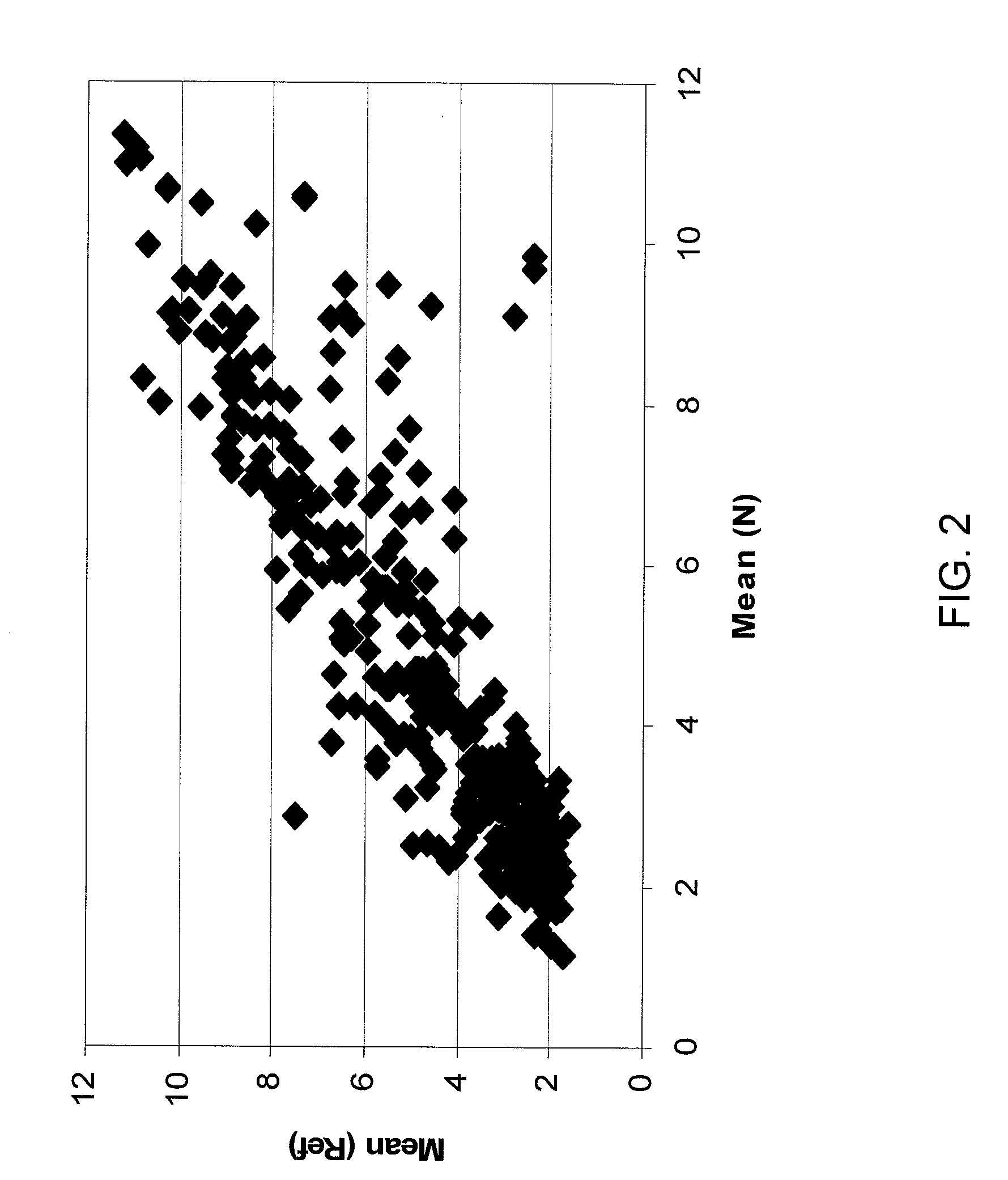
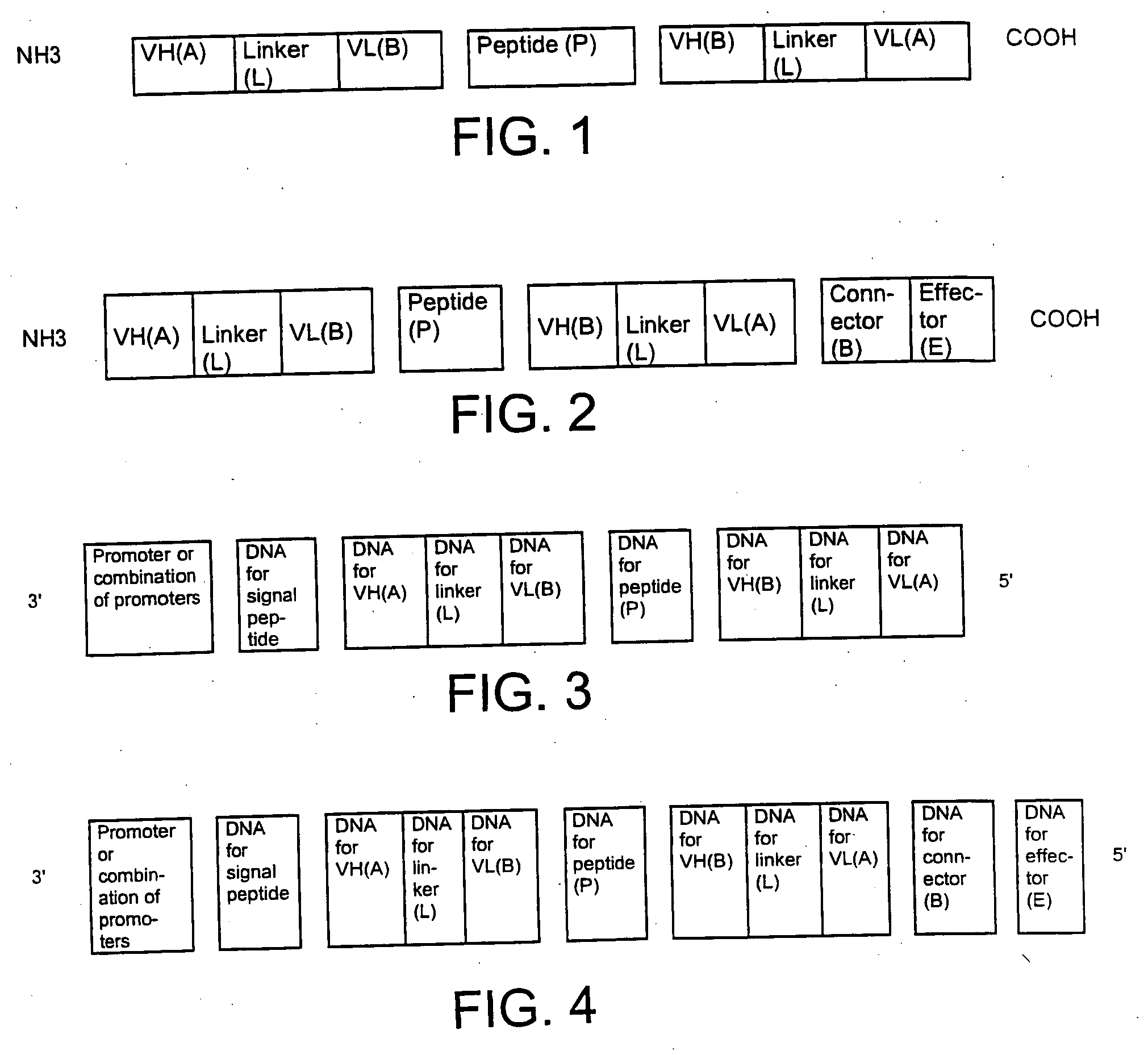
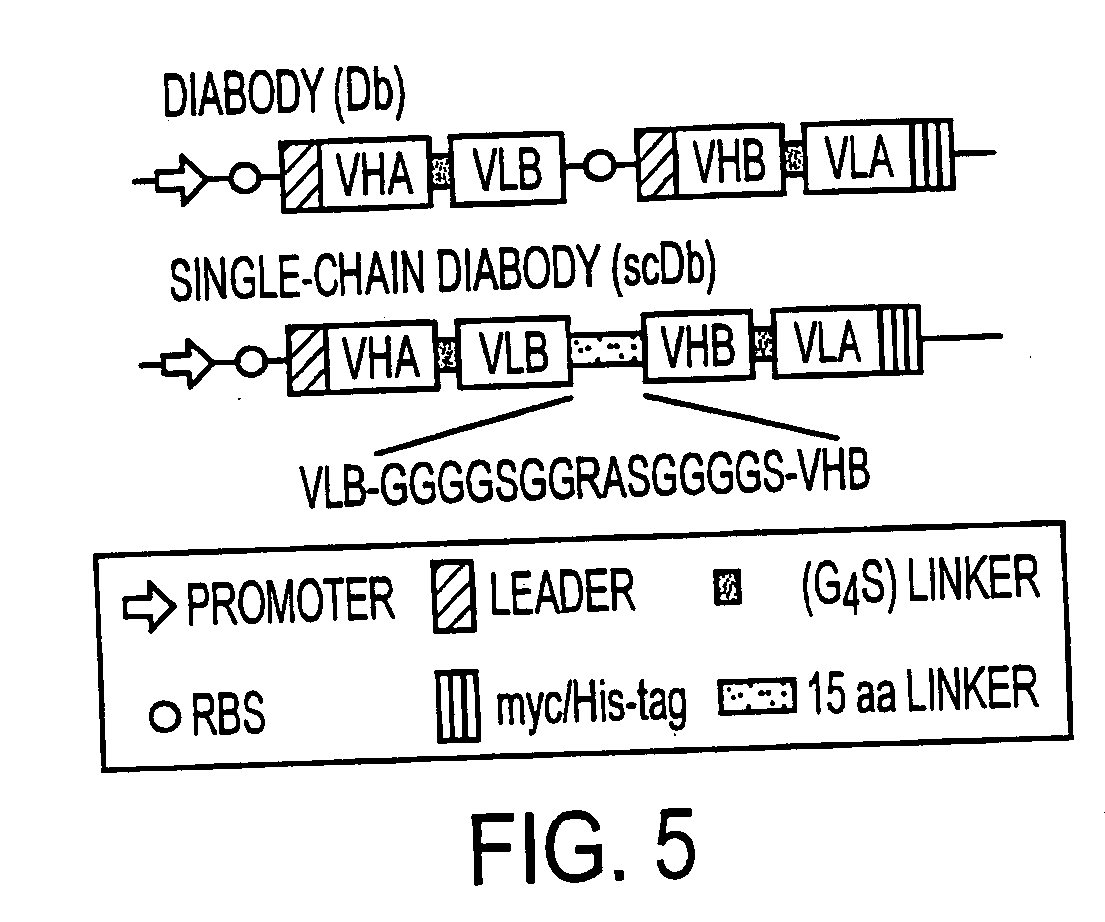
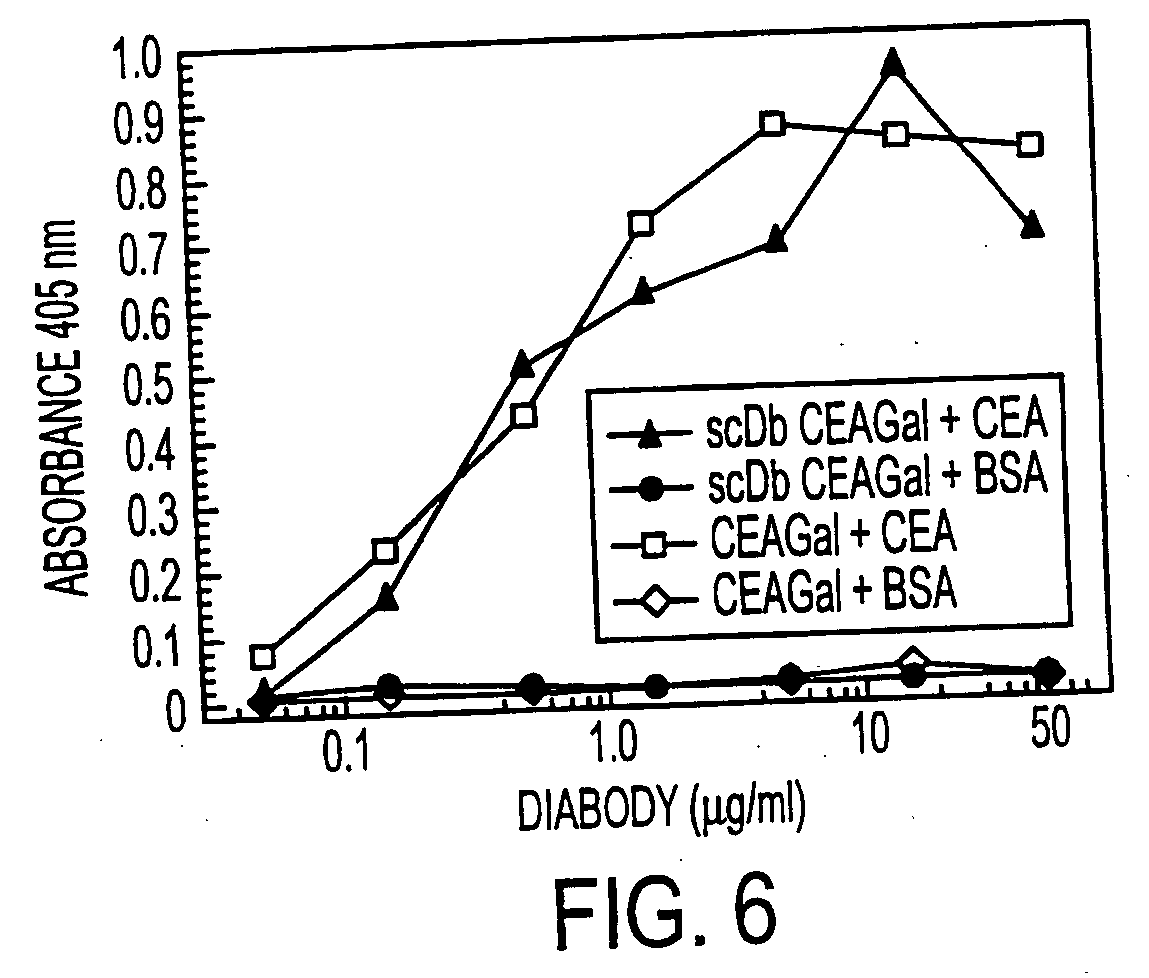
Single-chain multiple antigen-binding molecule, its preparation and use
InactiveUS20050004352A1Reduce dissociationLess complexOrganic active ingredientsFungiAntigen bindingVariable domain
The present invention relates to a single-chain, multiple antigen-binding molecule with diverse variable domains of a heavy and of a light chain of an immunoglobulin, which are connected in the form of a VH-VL construct, which are in turn connected together via a peptide, and to the preparation and use thereof as pharmaceutical or diagnostic aid.
Owner:AFFITECH RESEARCH AS
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20090202490A1Reduce the binding forceAltered infectivityOrganic active ingredientsBiocideCell type specificNeutralizing antibody
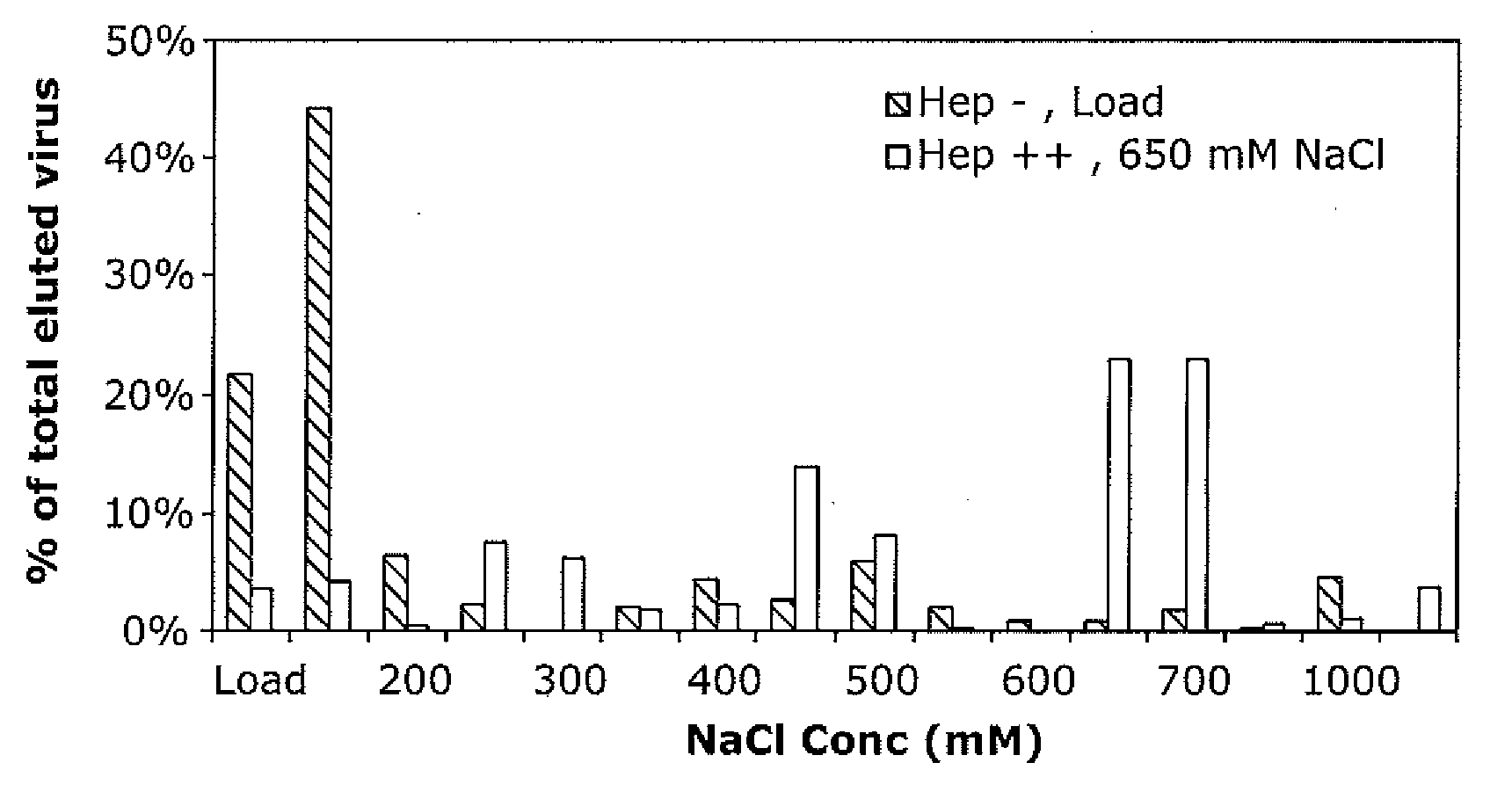
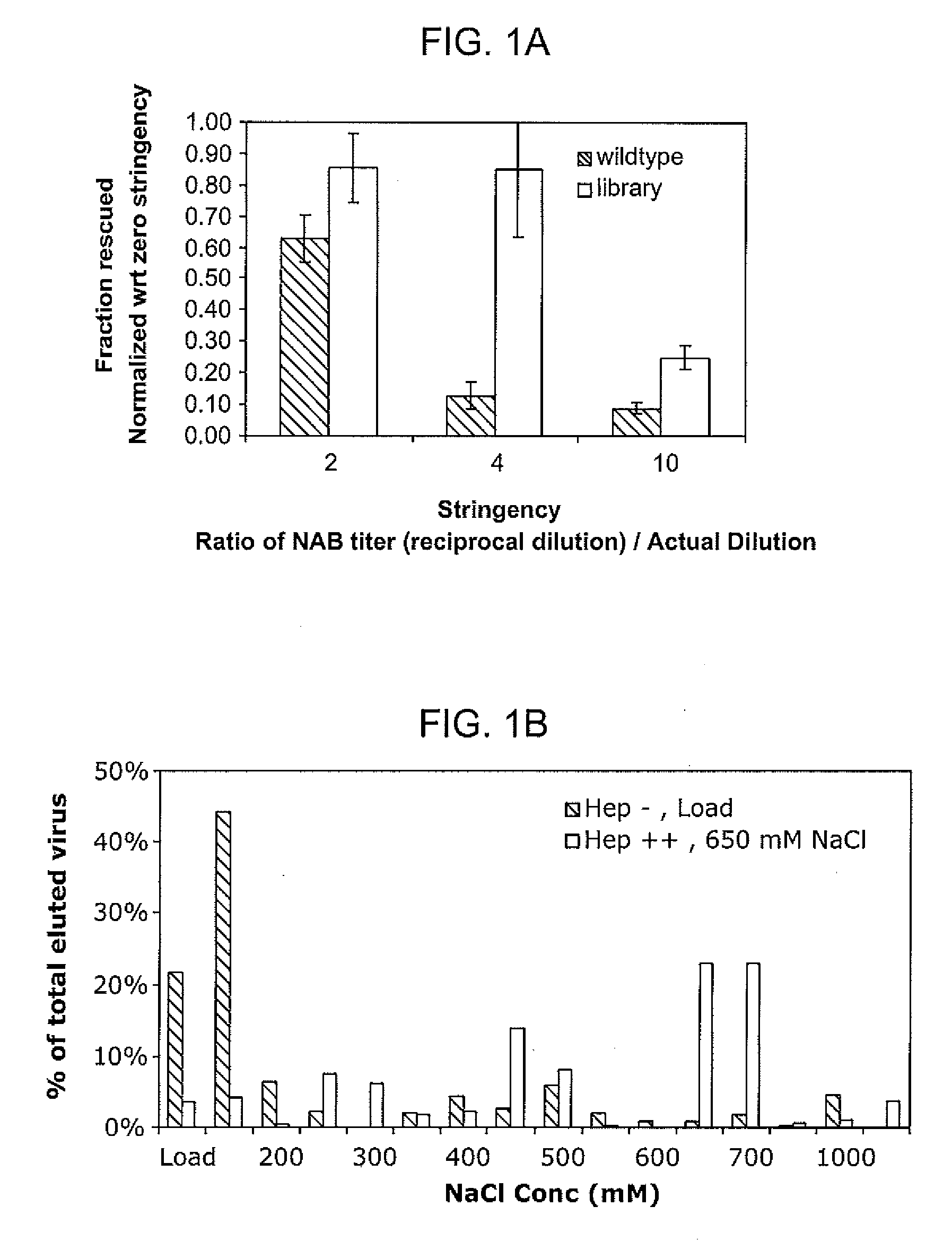
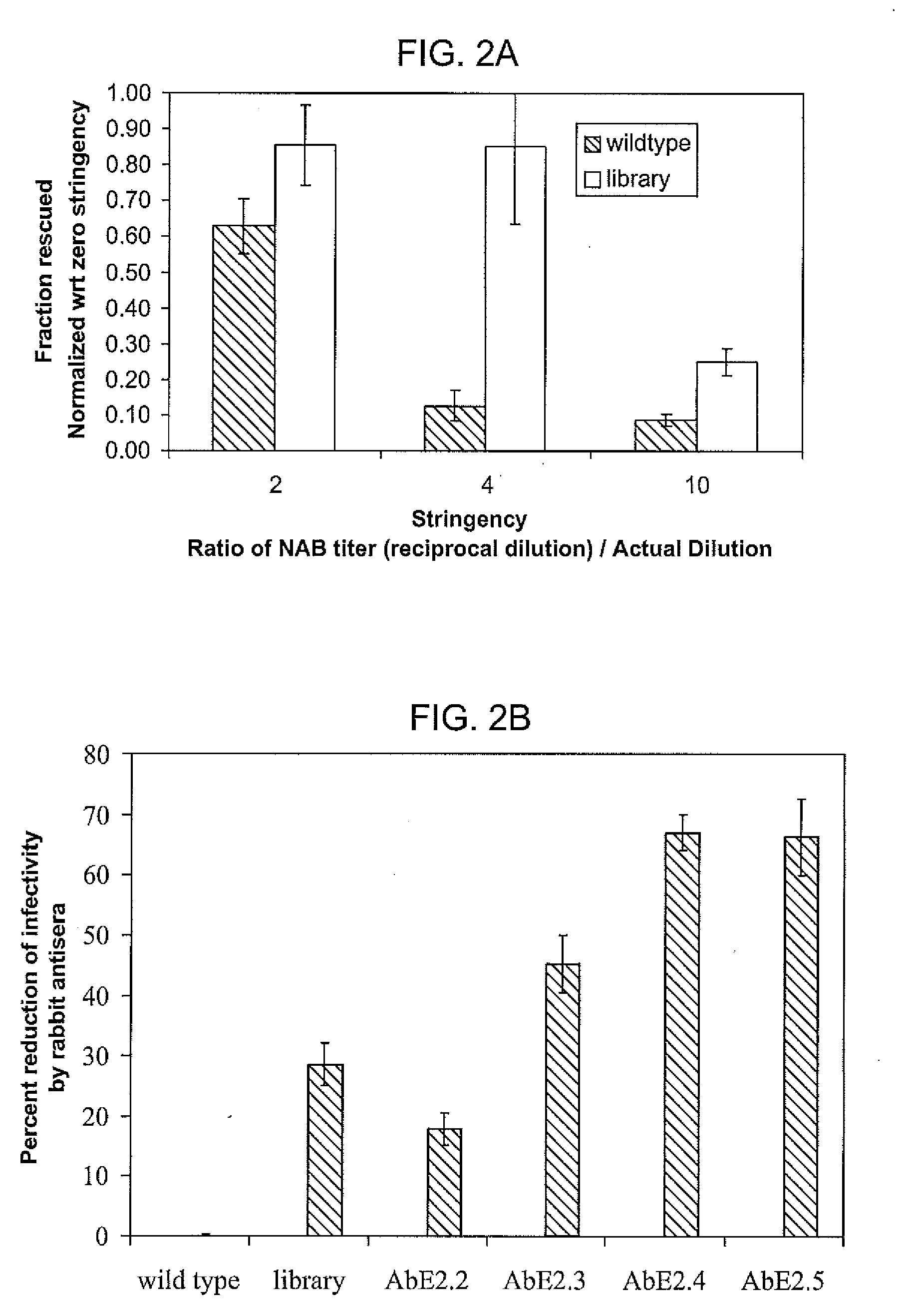
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:RGT UNIV OF CALIFORNIA +1
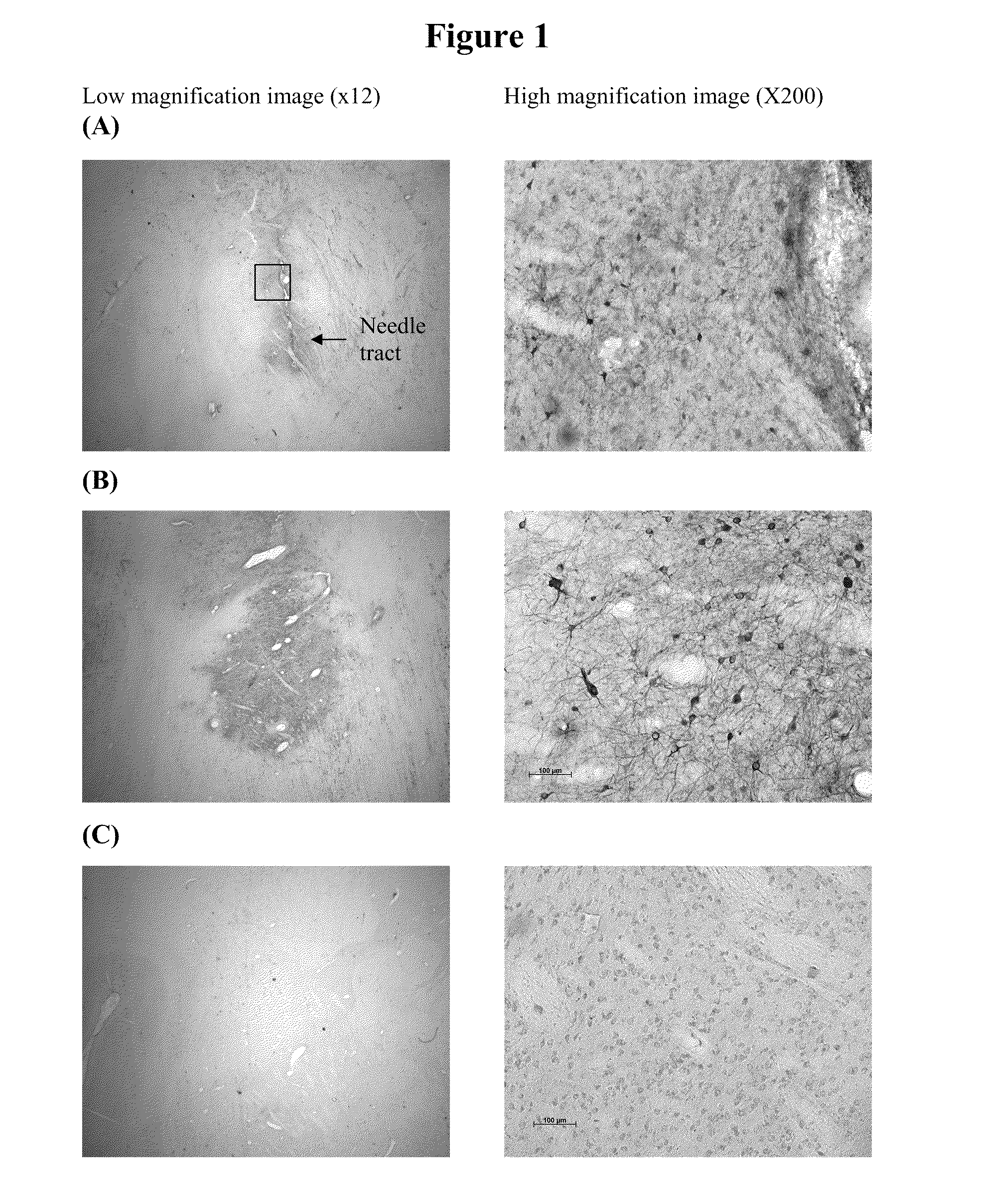
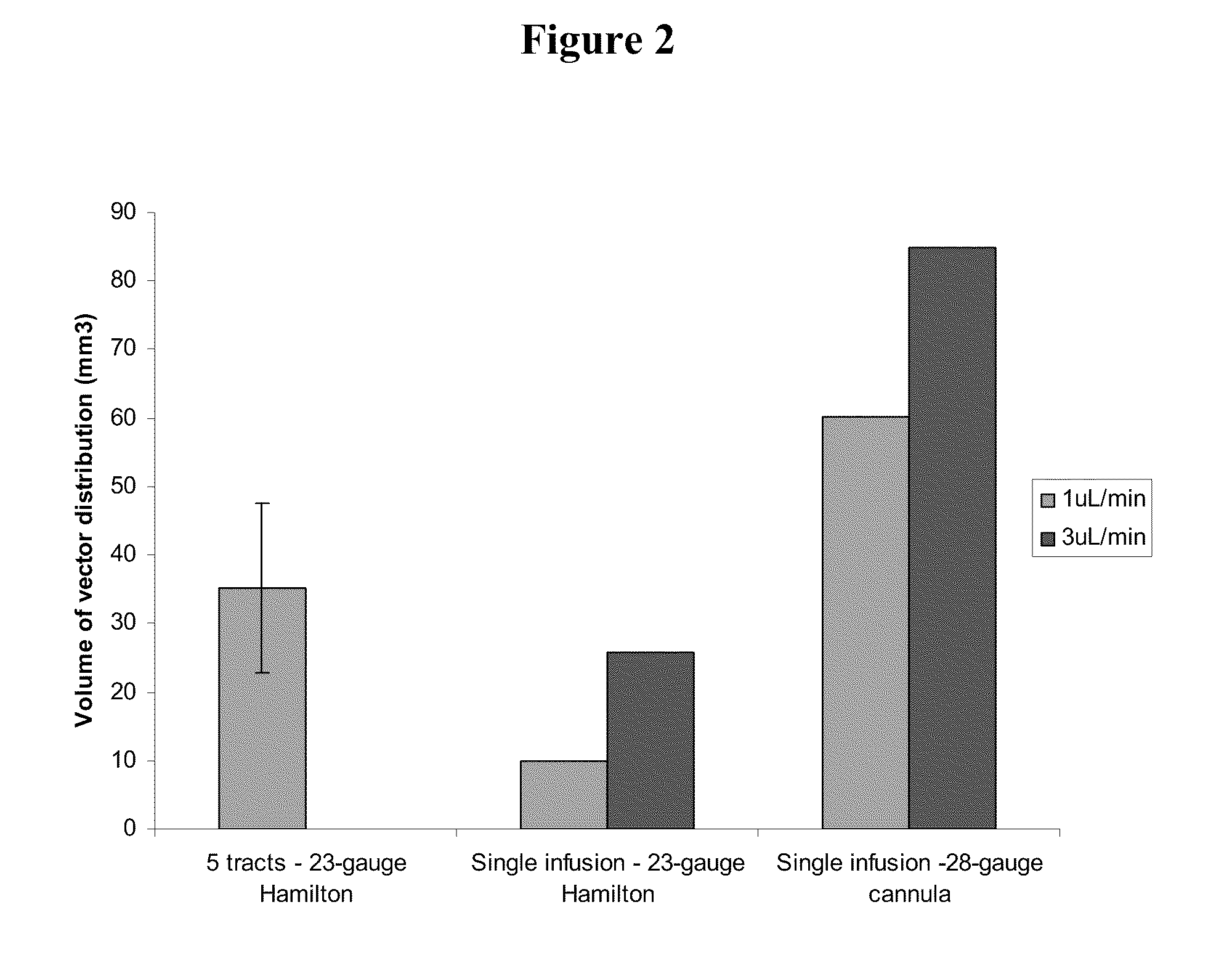
Method for vector delivery
InactiveUS20110293571A1Optimize volumeTotal volume of vector distribution in the brain wasBiocideOrganic active ingredientsNeuroscience
Owner:OXFORD BIOMEDICA (UK) LTD
Reduction of porcine circovirus-2 viral load with inactivated PCV-2
InactiveUS6517843B1Improving immunogenicityImprove performanceBiocideGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6th and 8th and / or the 10th and 13th weeks of gestation, can prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:QUEENS UNIV OF BELFAST +4
Method of treating gastrointestinal diseases associated with species of genus clostridium
The invention includes a method of treating gastrointestinal diseases associated with species of genus Clostridium such as clostridium deficit in human patients with gastrointestinal disorders having an etiological component such as a microbial agent producing a toxin where treated with an antimicrobial composition an amount effective to inhibit or eliminate the microbial agent. The antimicrobial composition in a form of probiotic mixture can be administrated alone or in combination with an antimicrobial agent, such as a bacteriophage which is specific for a bacterium producing toxin or antibiotics which are then used to eliminate or inhibit the clostridial species overgrown in a patient's gastrointestinal tract. Disorders that can be treated by the method of the invention include diarrhea or inflammatory bowel diseases such as colitis or Crohn's disease.
Owner:UNITED STATES OF AMERICA
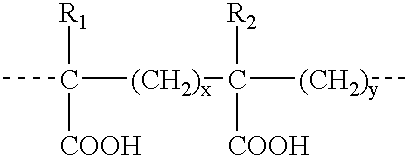
Microparticles for Oral Delivery
The invention provides microbeads containing oil-associated biologically active compounds and methods for their manufacture and use. The microbeads consist of a soluble complex of non-digestible polymer and emulsifier with oil-associated biologically active compounds embedded in a matrix of digestible polymer. The disclosed microbead complex protects the biologically active compounds, such as vitamins, fish oil and carotenoids, from oxidation, taste and odor degradation. The disclosed microbeads also provide protection from the stomach digestive distraction, and allows for the delivery of the biologically active compounds in the intestine.
Owner:INTERVET INC
Methods and products for enhancing immune responses using imidazoquinoline compounds
InactiveUS20060188913A1Good curative effectReduce doseCompound screeningApoptosis detectionTime scheduleDisease
The invention involves administration of an imidazoquinoline agent in combination with another therapeutic agent. The combination of drugs may be administered in synergistic amounts or in various dosages or at various time schedules. The invention also relates to kits and compositions concerning the combination of drugs. The combinations can be used to enhance ADCC, stimulate immune responses and / or patient and treat certain disorders.
Owner:COLEY PHARM GRP INC +2
Method for the production and purification of adenoviral vectors
InactiveUS20080050770A1Peptide/protein ingredientsGenetic material ingredientsSerum igeUltracentrifuge
Owner:JANSSEN VACCINES & PREVENTION BV
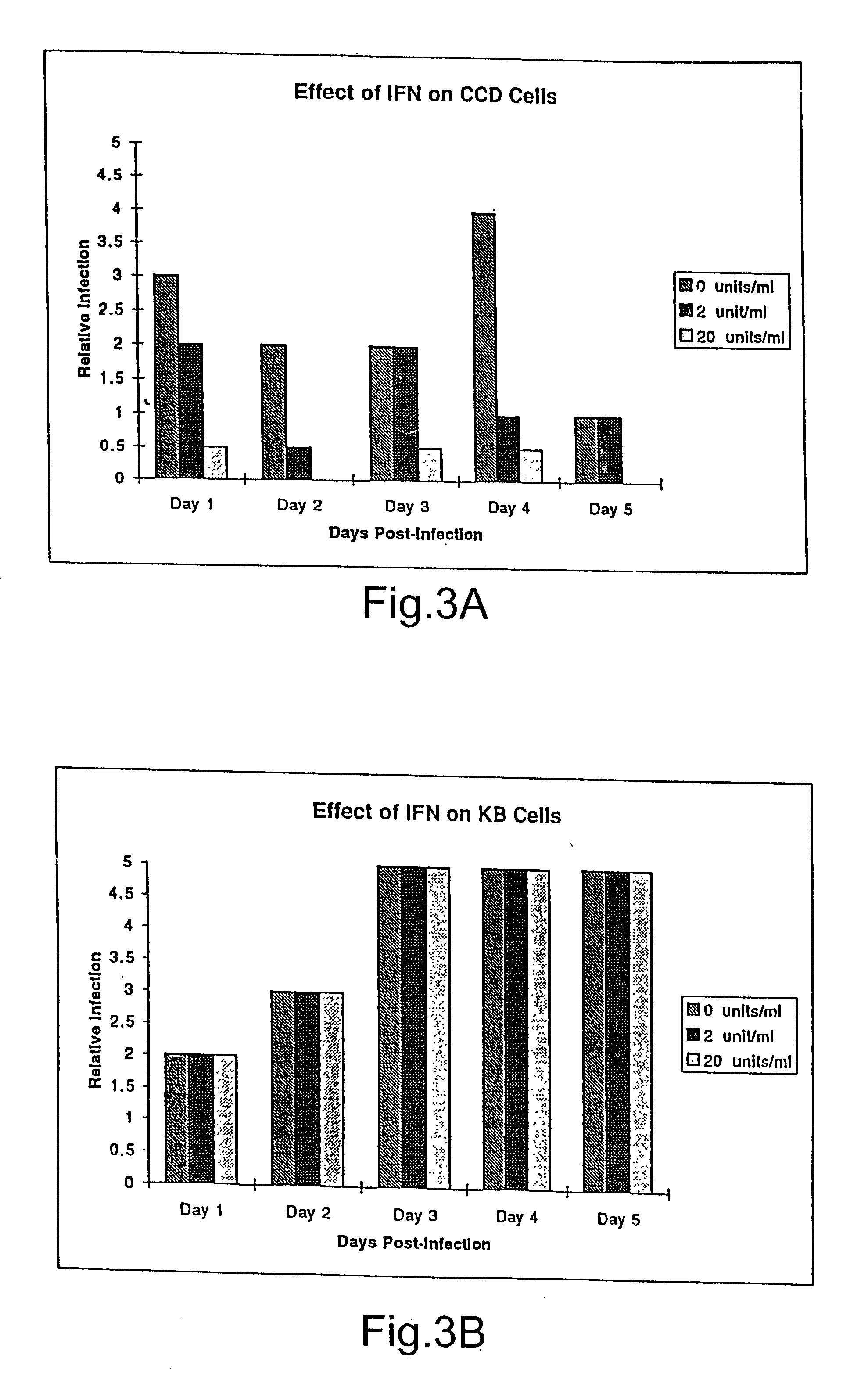
Treatment of neoplasms with viruses
InactiveUS20030044384A1Convenient treatmentReduce productionSsRNA viruses negative-senseBiocideDiseaseAnti viral response
The subject invention relates to viruses that are able to replicate and thereby kill neoplastic cells with a deficiency in the IFN-mediated antiviral response, and their use in treating neoplastic disease including cancer and large tumors. RNA and DNA viruses are useful in this regard. The invention also relates to methods for the selection, design, purification and use of such viruses for cancer therapy.
Owner:PRO VIRUS
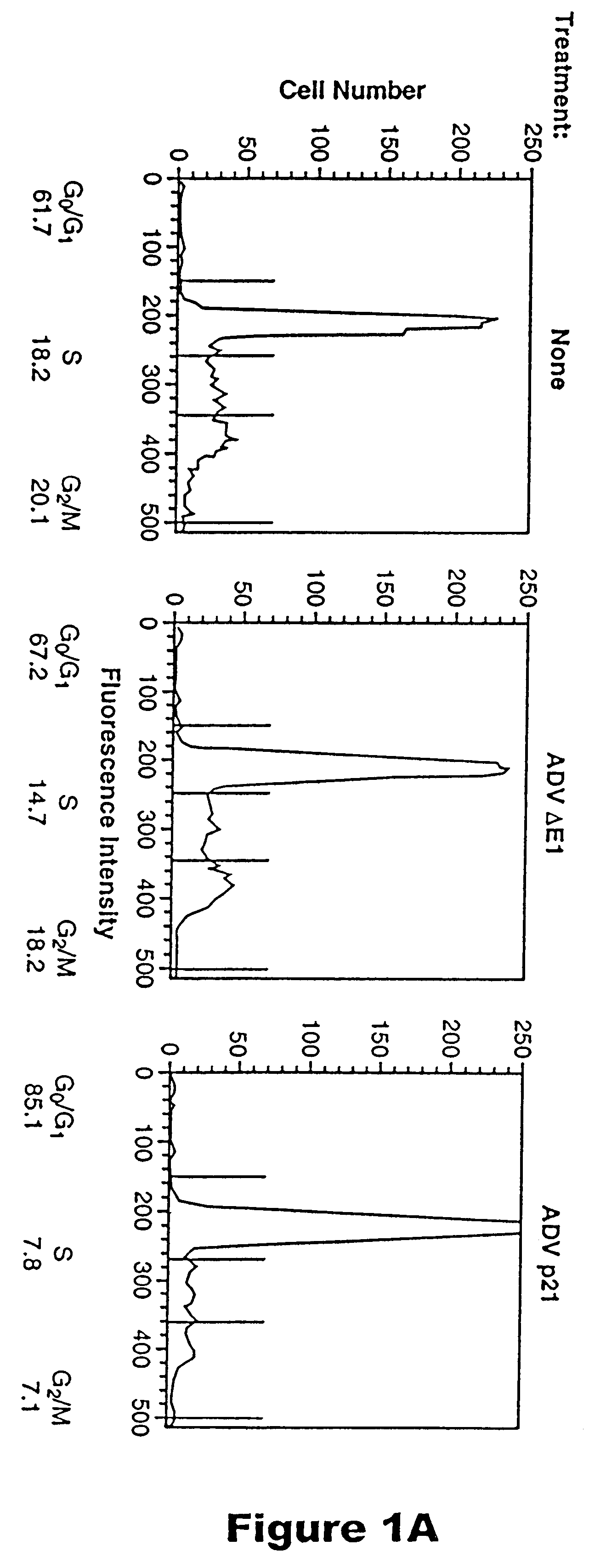
Methods for treating restenosis with p21
InactiveUS6218372B1Treating and preventing restenosis in vivoFacilitate immune recognitionBiocidePeptide/protein ingredientsPercent Diameter StenosisGene product
The p21 gene encodes a cyclin dependent kinase inhibitor which affects cell cycle progression, but the role of this gene product in altering tumor growth has not been established. The present inventors have now discovered that the growth of malignant cells in vivo is inhibited by expression of p21. Expression of p21 resulted in an accumulation of cells in G0 / G1, alteration in morphology, and cell differentiation.
Owner:RGT UNIV OF MICHIGAN
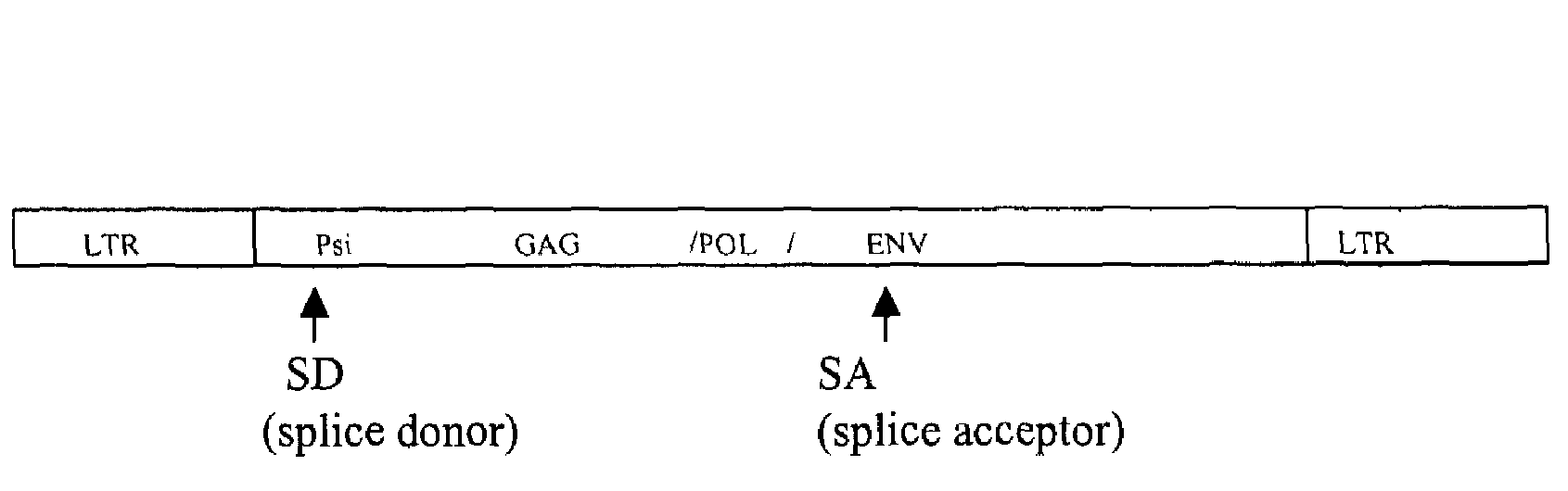
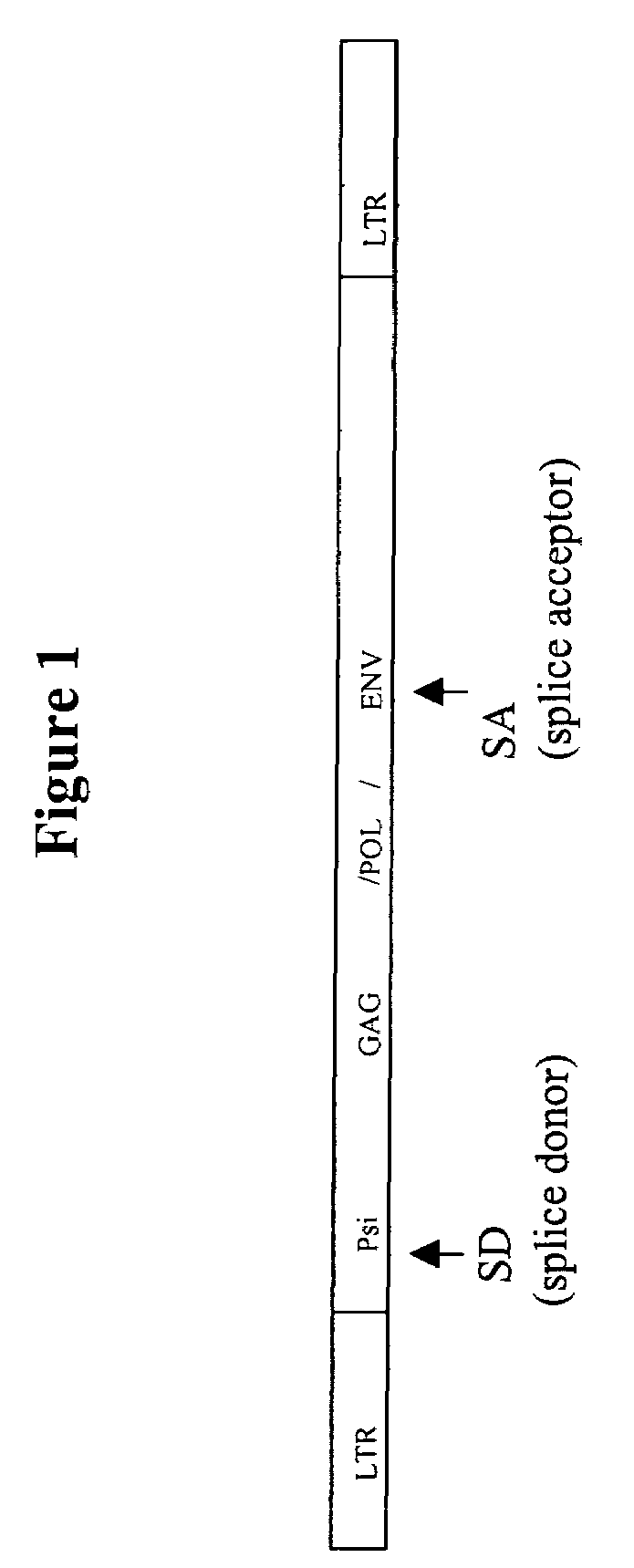
Retroviral vectors comprising a functional splice donor site and a functional splice acceptor site
InactiveUS7303910B2Efficient expressionIncrease rangeSsRNA viruses negative-senseFungiNucleotideNucleotide sequencing
A retroviral vector is described. The retroviral vector comprises a functional splice donor site and a functional splice acceptor site; wherein the functional splice donor site and the functional splice acceptor site flank a first nucleotide sequence of interest (“NOI”); wherein the functional splice donor site is upstream of the functional splice acceptor site; wherein the retroviral vector is derived from a retroviral pro-vector; wherein the retroviral pro-vector comprises a first nucleotide sequence (“NS”) capable of yielding the functional splice donor site and a second NS capable of yielding the functional splice acceptor site; wherein the first NS is downstream of the second NS; such that the retroviral vector is formed as a result of reverse transcription of the retroviral pro-vector.
Owner:OXFORD BIOMEDICA (UK) LTD
Method of reducing ecologically adverse changes of the gastro intestinal microbial flora in patients under treatment with medicaments
InactiveUS20020022019A1Reduce generationBiocideOrganic active ingredientsMicroorganismPresent method
A method for reducing ecologically adverse changes of the gastrointestinal micro-flora in patients under treatment with medicaments (which may also be referred to herein as the therapeutic compounds or medications) such as gastric acid reducing medicaments or antibiotics. A pharmaceutical product useful in the present method comprising a medicament and a probiotically active organism as a combined preparation presented in a commercial package unit.
Owner:CHR HANSEN AS
Light-Sensitive Ion-Passing Molecules
ActiveUS20130019325A1Dissuade depolarizationIncrease probabilityNervous disorderPeptide/protein ingredientsOptical stimulationLight activated
Disclosed are polynucleotides and methods for expressing light activated proteins in animal cells and altering an action potential of the cells by optical stimulation. The disclosure also provides animal cells and non-human animals comprising cells expressing the light-activated proteins.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
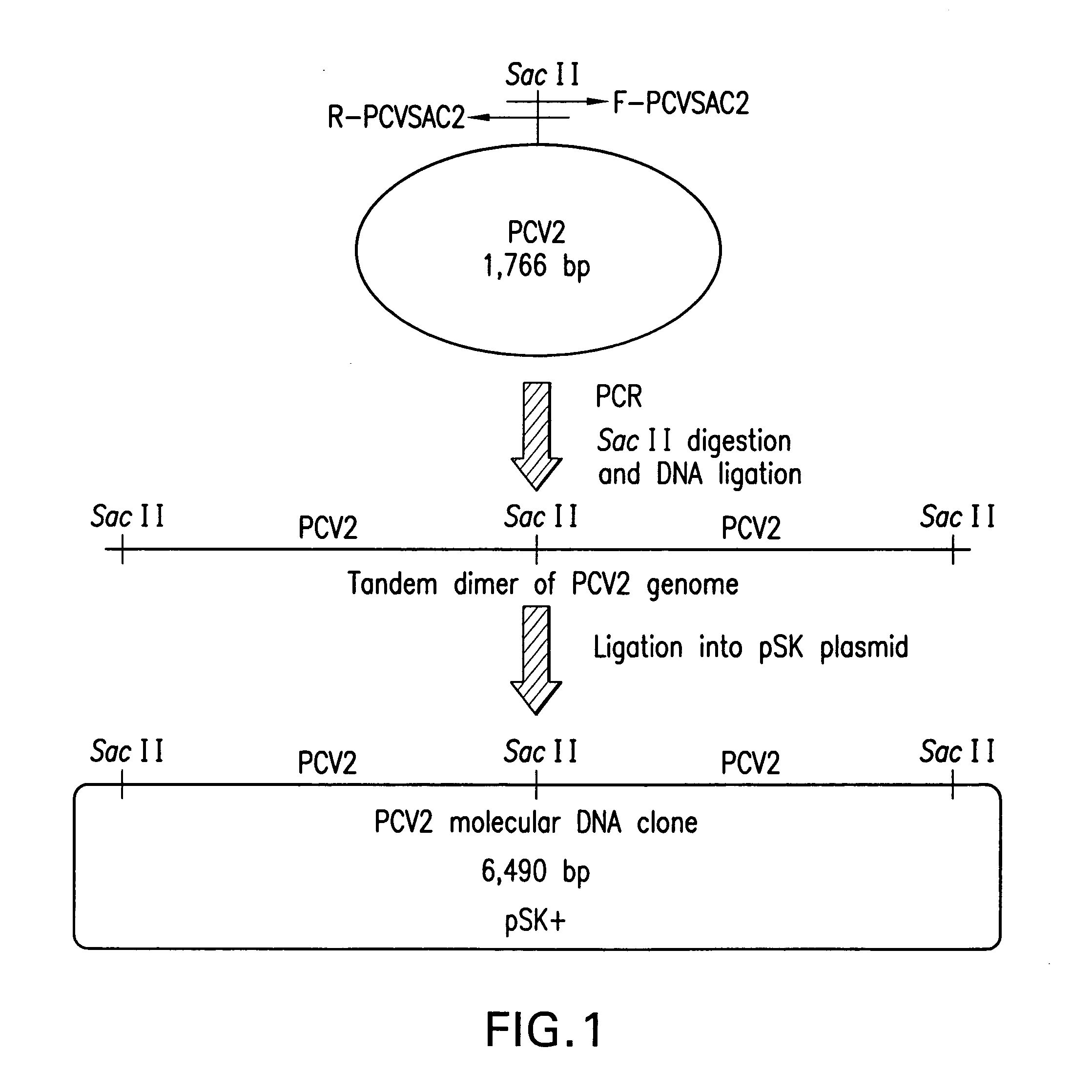
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
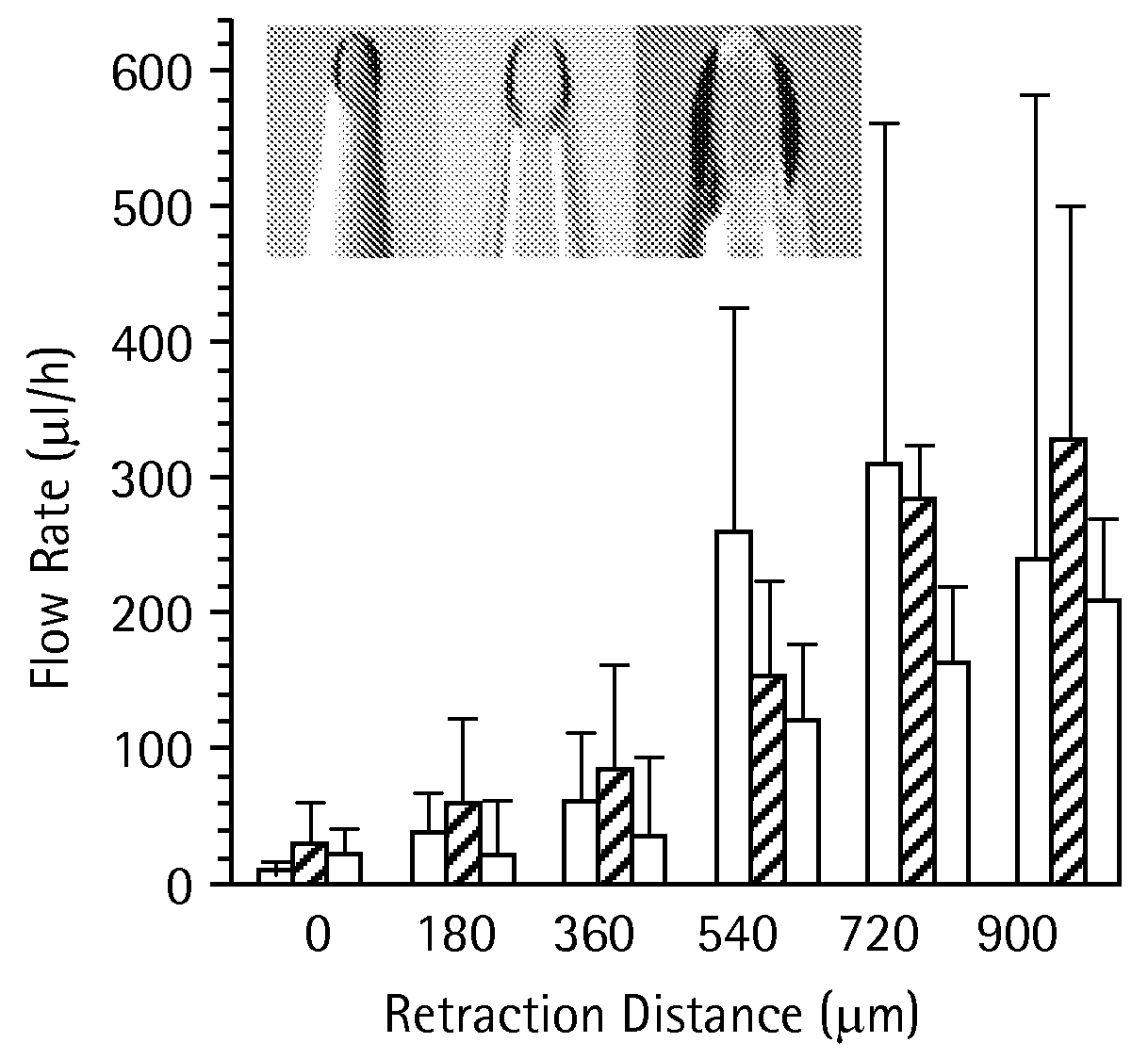
Microneedles and Methods for Microinfusion
Methods and devices are provided for delivering a drug to or withdrawing a fluid from a biological tissue, such the skin, sclera, cornea, and conjunctiva. One method includes the steps of inserting at least one microneedle into the biological tissue; partially retracting the at least one microneedle from the tissue; and then delivering at least one drug formulation into the biological tissue via the partially retracted at least one microneedle. The microneedle deforms and penetrates the biological tissue during the insertion step, and the retraction step at least partially relaxes the tissue deformation while maintaining at least part of the tissue penetration, facilitating drug delivery or fluid withdrawal.
Owner:GEORGIA TECH RES CORP
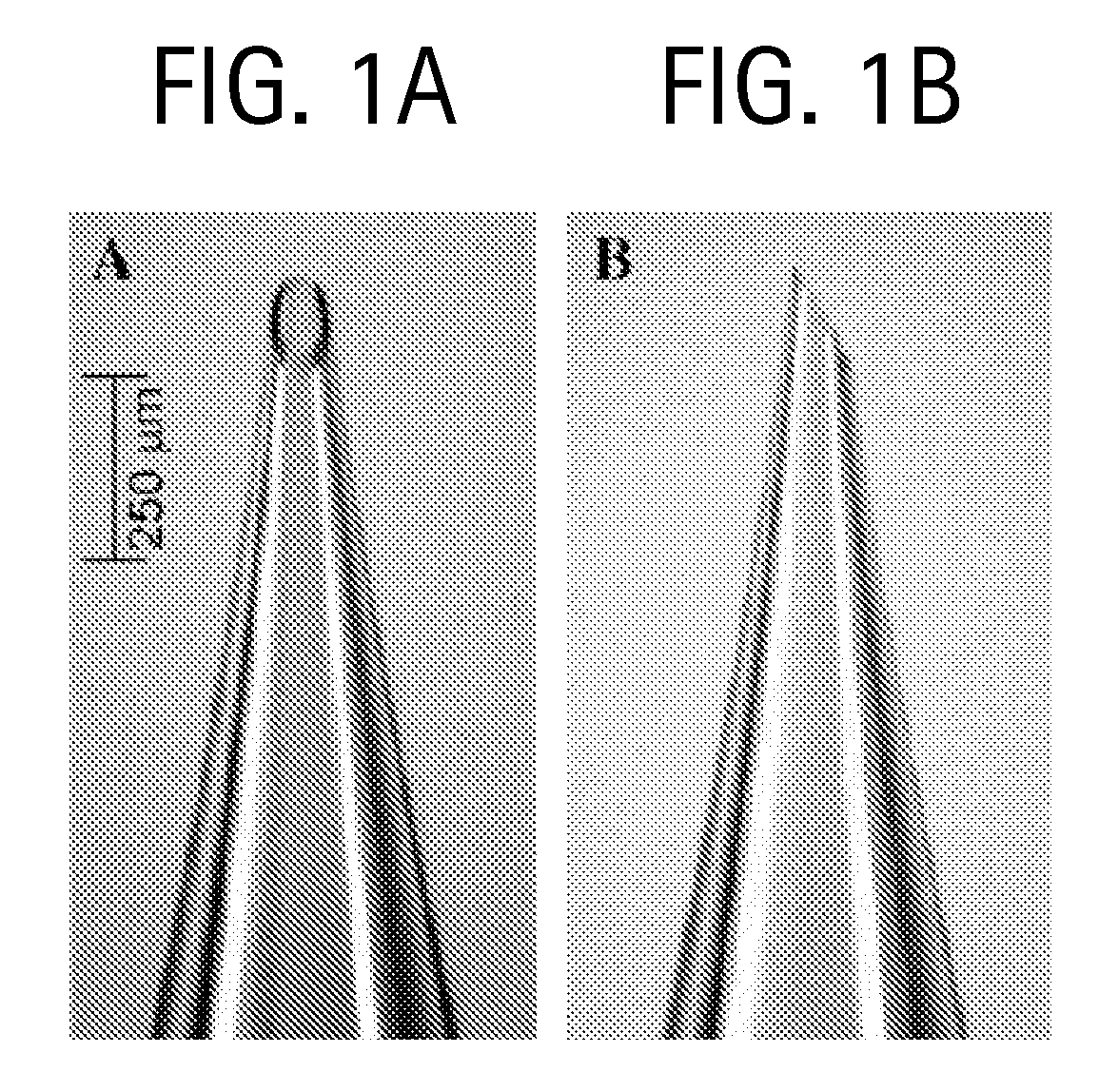
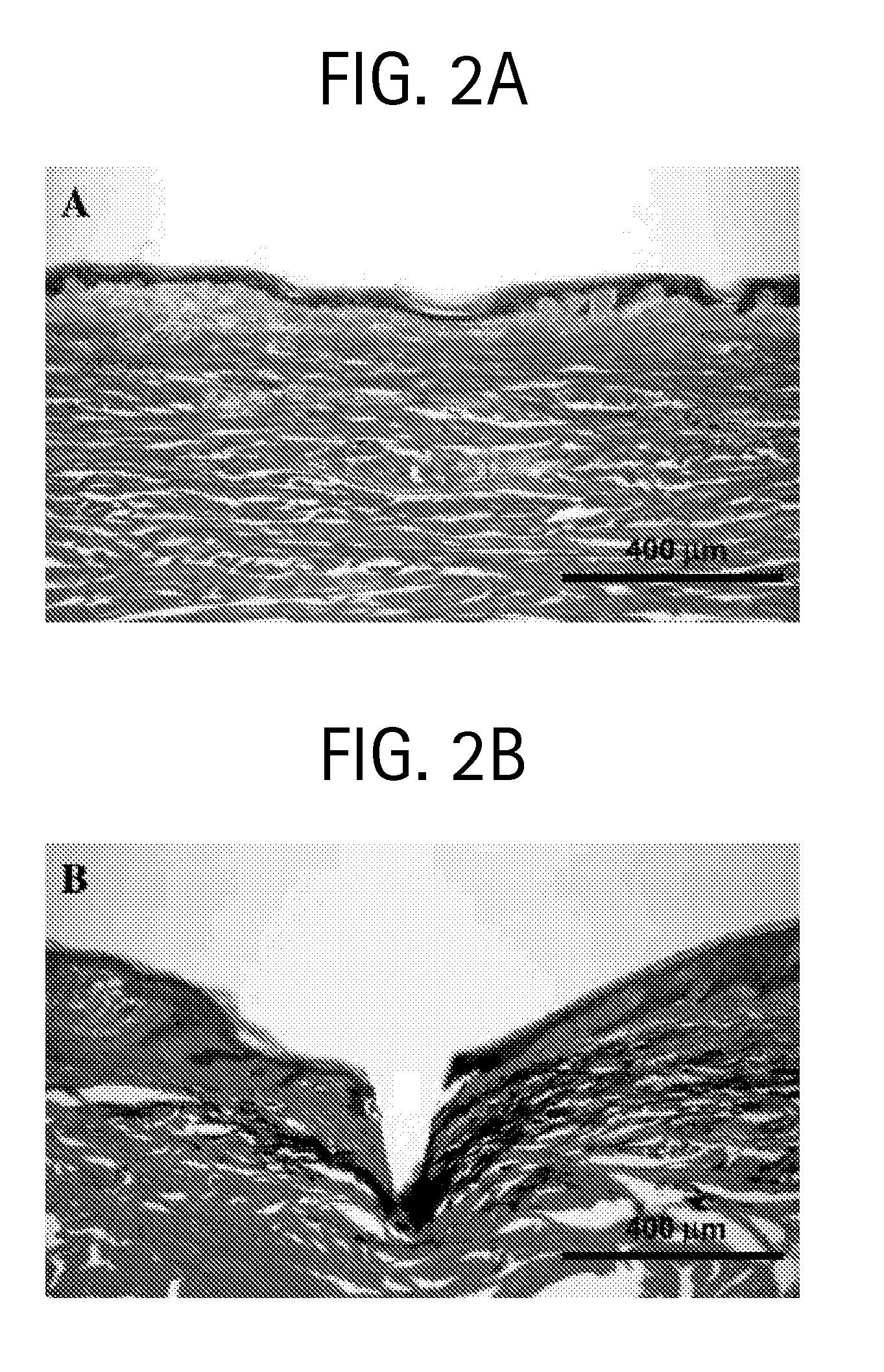
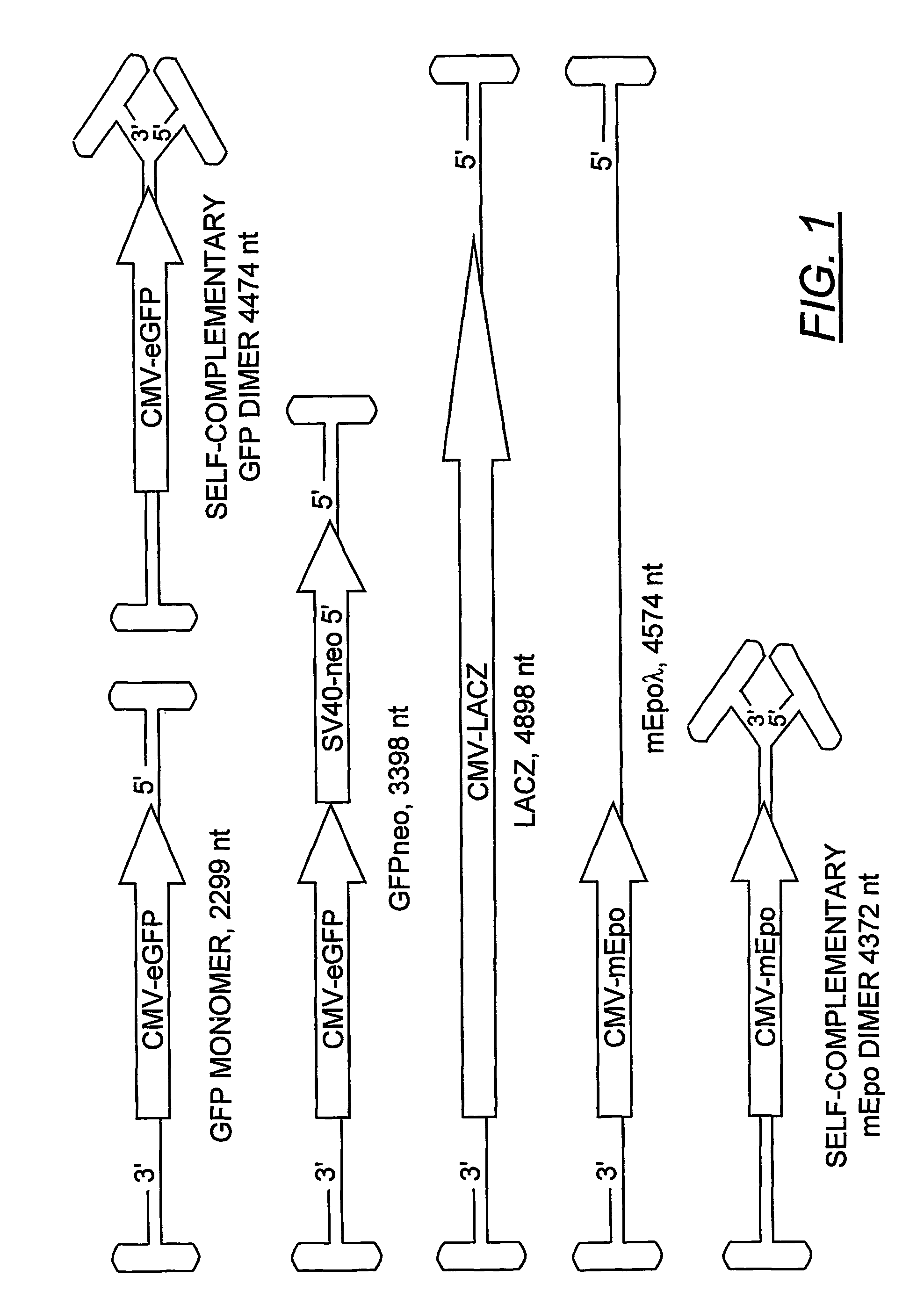
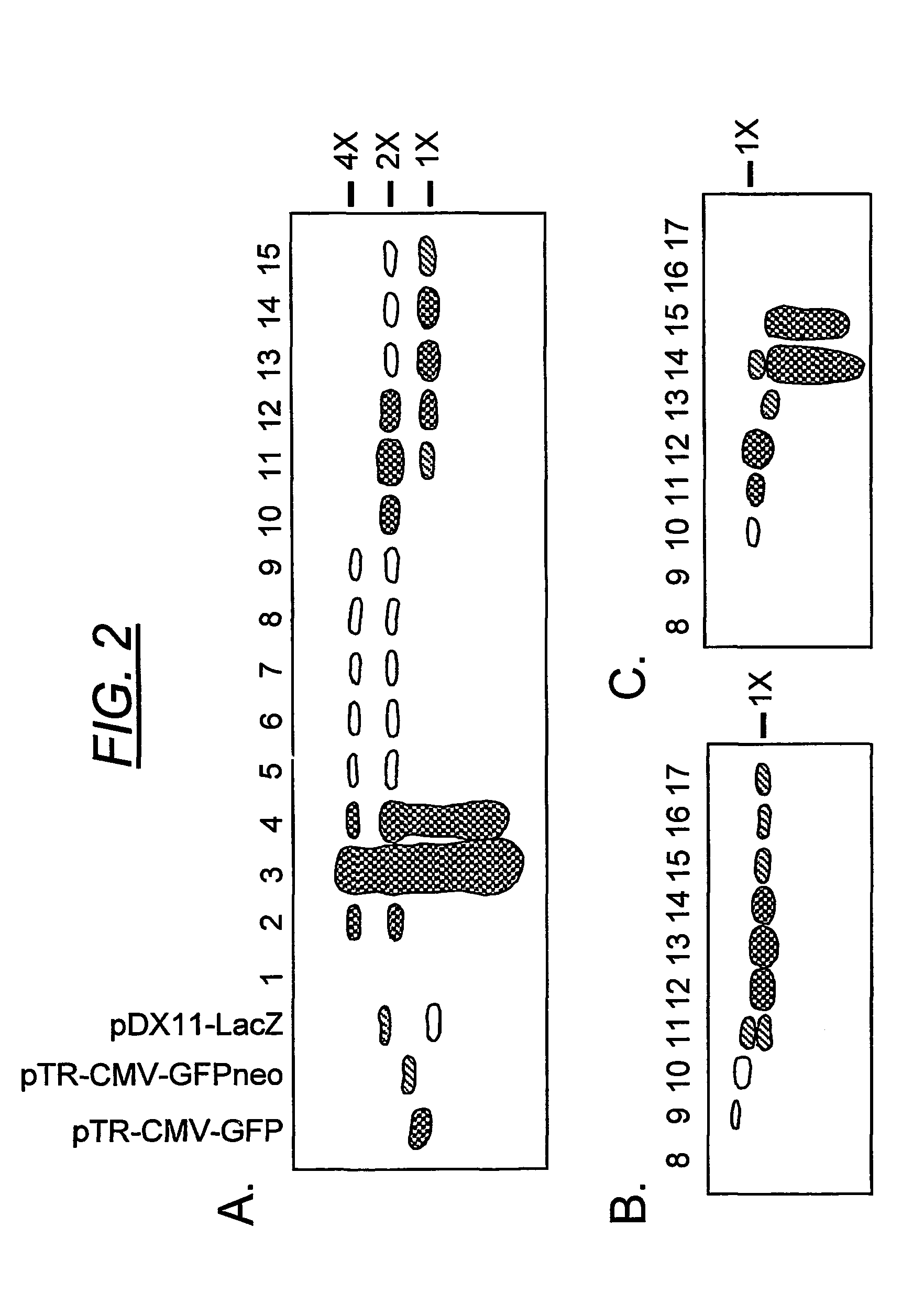
Duplexed parvovirus vectors
InactiveUS7465583B2High transduction efficiencyRapid onsetBiocidePeptide/protein ingredientsGeneticsViral vector
The present invention provides duplexed parvovirus vector genomes that are capable under appropriate conditions of forming a double-stranded molecule by intrastrand base-pairing. Also provided are duplexed parvovirus particles comprising the vector genome. Further disclosed are templates and methods for producing the duplexed vector genomes and duplexed parvovirus particles of the invention. Methods of administering these reagents to a cell or subject are also described. Preferably, the parvovirus capsid is an AAV capsid. It is further preferred that the vector genome comprises AAV terminal repeat sequences.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
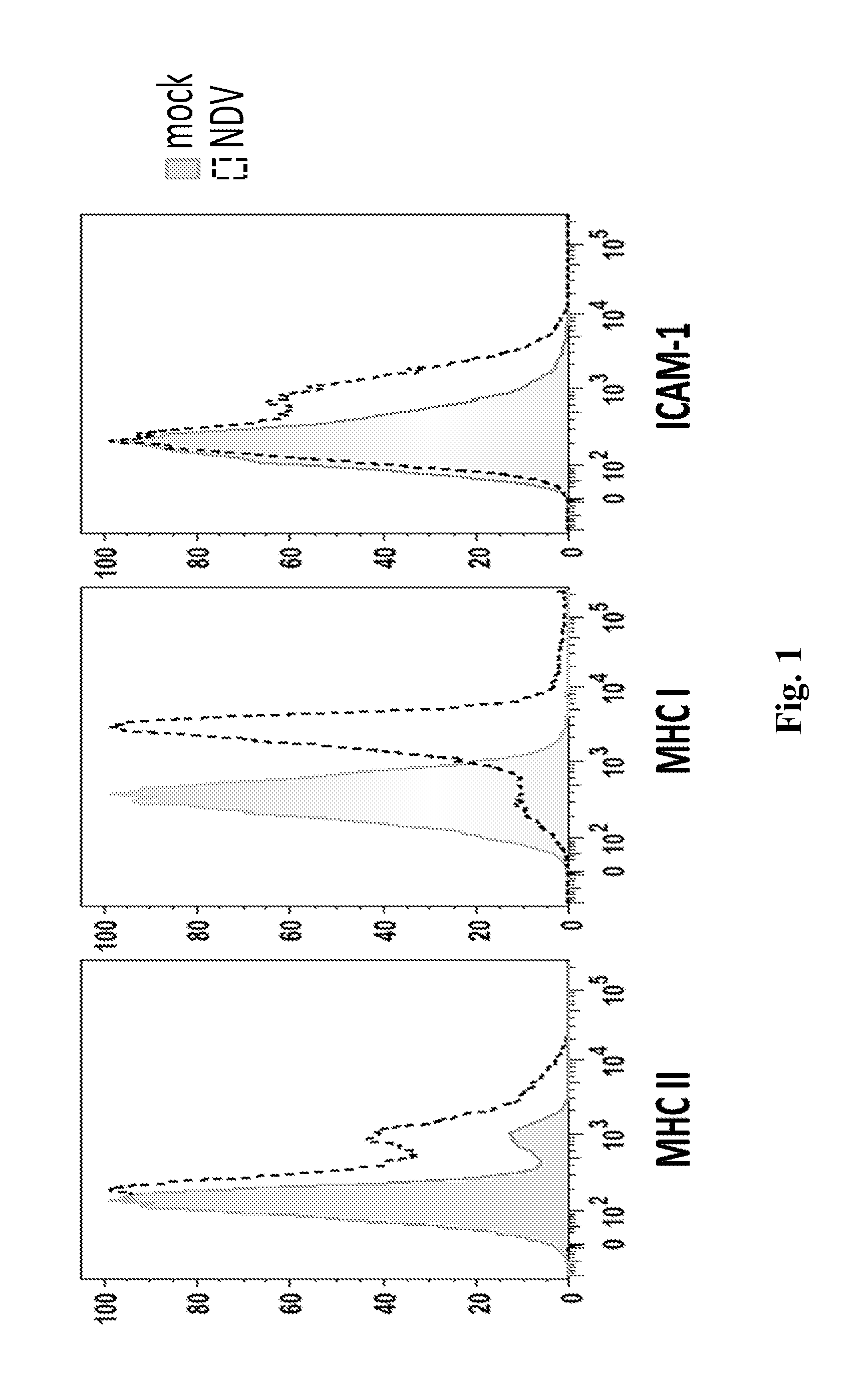
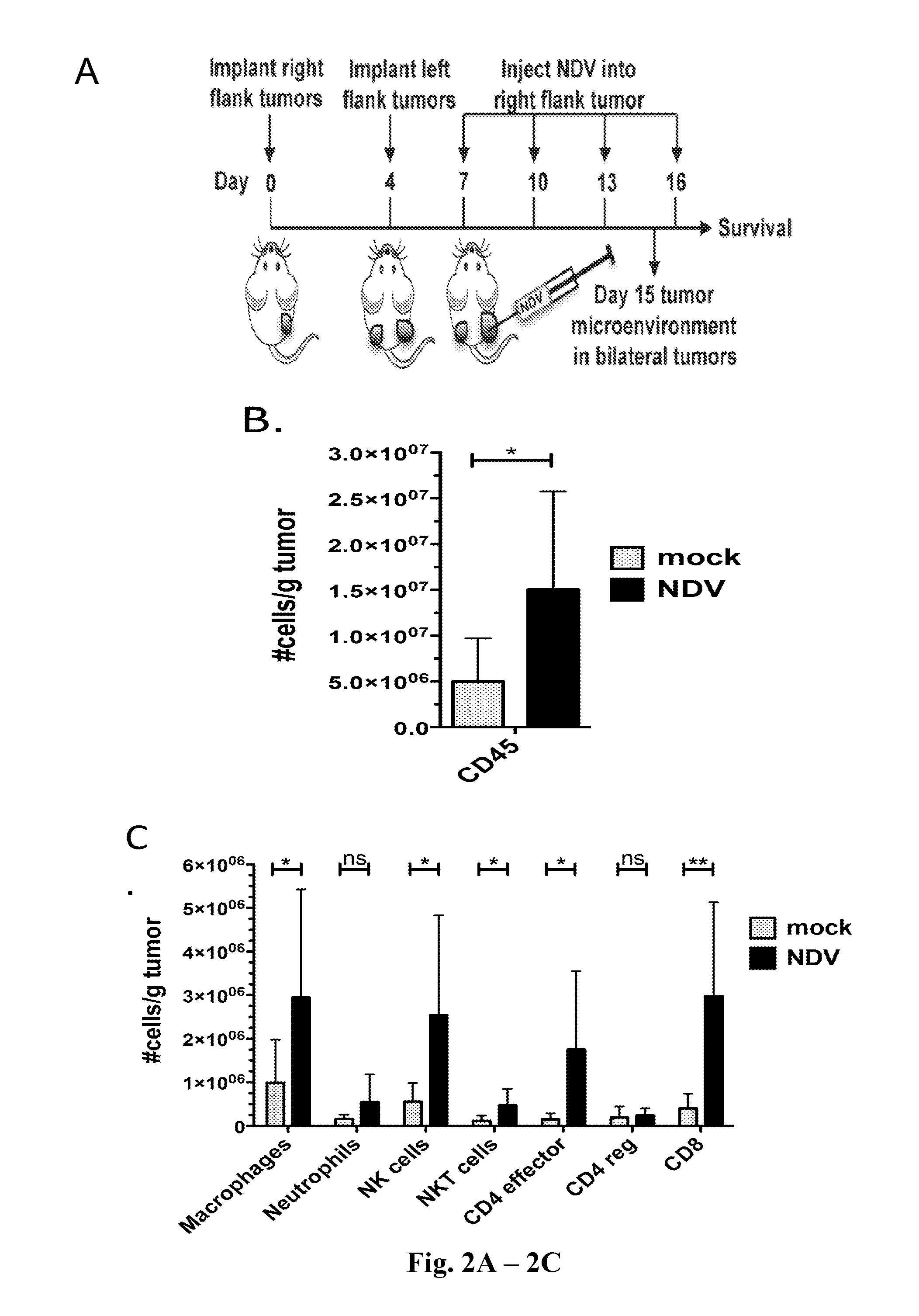
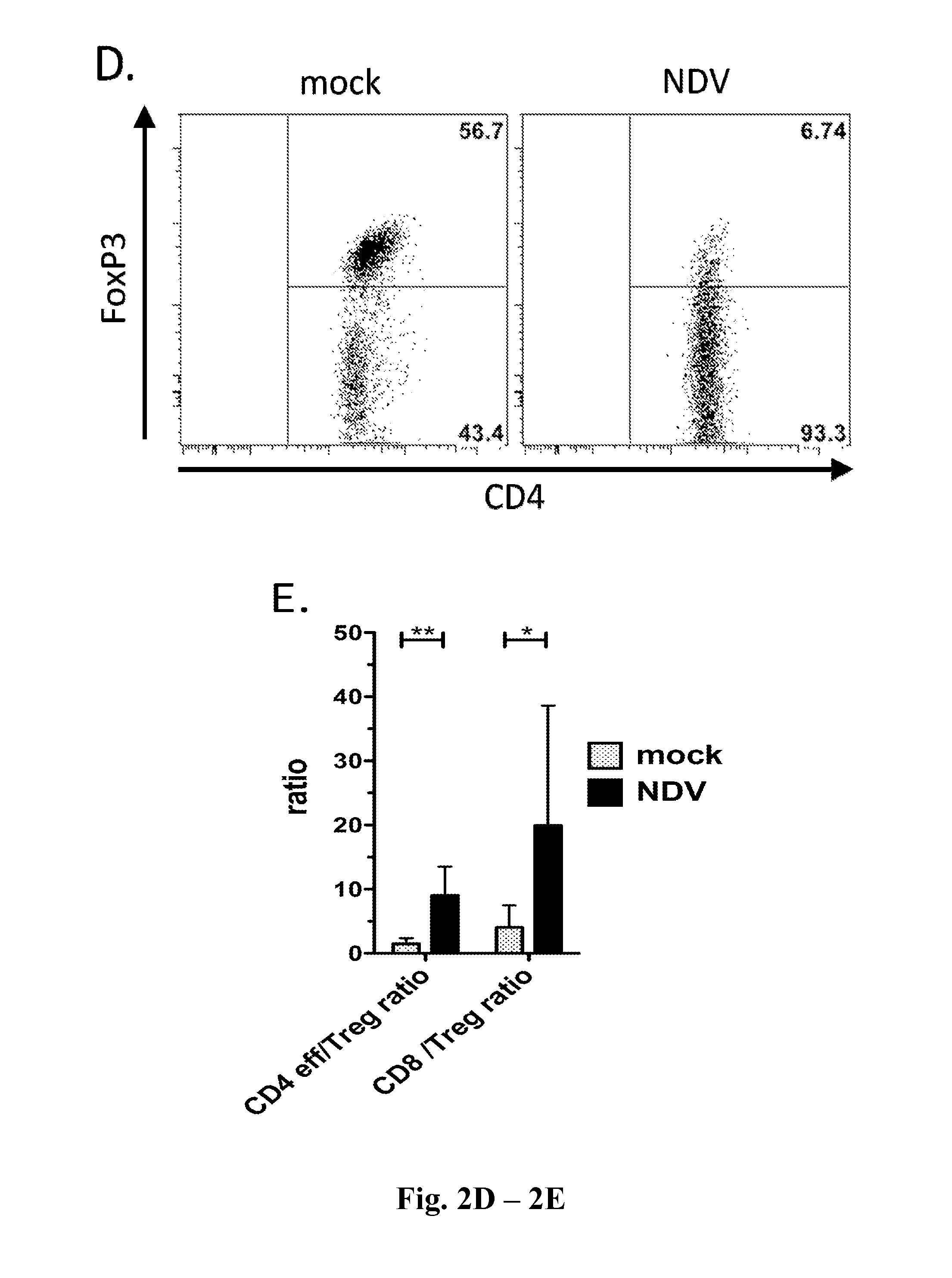
Newcastle Disease Viruses and Uses Thereof
InactiveUS20140271677A1Reduce severityPrevent relapseSsRNA viruses negative-senseViral antigen ingredientsNewcastle disease virus NDVAgonist
Described herein are chimeric Newcastle disease viruses engineered to express an agonist of a co-stimulatory signal of an immune cell and compositions comprising such viruses. Also described herein are chimeric Newcastle disease viruses engineered to express an antagonist of an inhibitory signal of an immune cell and compositions comprising such viruses. The chimeric Newcastle disease viruses and compositions are useful in the treatment of cancer. In addition, described herein are methods for treating cancer comprising administering Newcastle disease viruses in combination with an agonist of a co-stimulatory signal of an immune and / or an antagonist of an inhibitory signal of an immune cell.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Freeze dried fecal microbiota for use in fecal microbial transplantation
The present invention provides freeze-dried compositions that include an extract of human feces and a cryoprotectant, and methods for making and using such compositions, including methods for replacing or supplementing or modifying a subject's colon microbiota, and methods for treating a disease, pathological condition, and / or iatrogenic condition of the colon.
Owner:RGT UNIV OF MINNESOTA
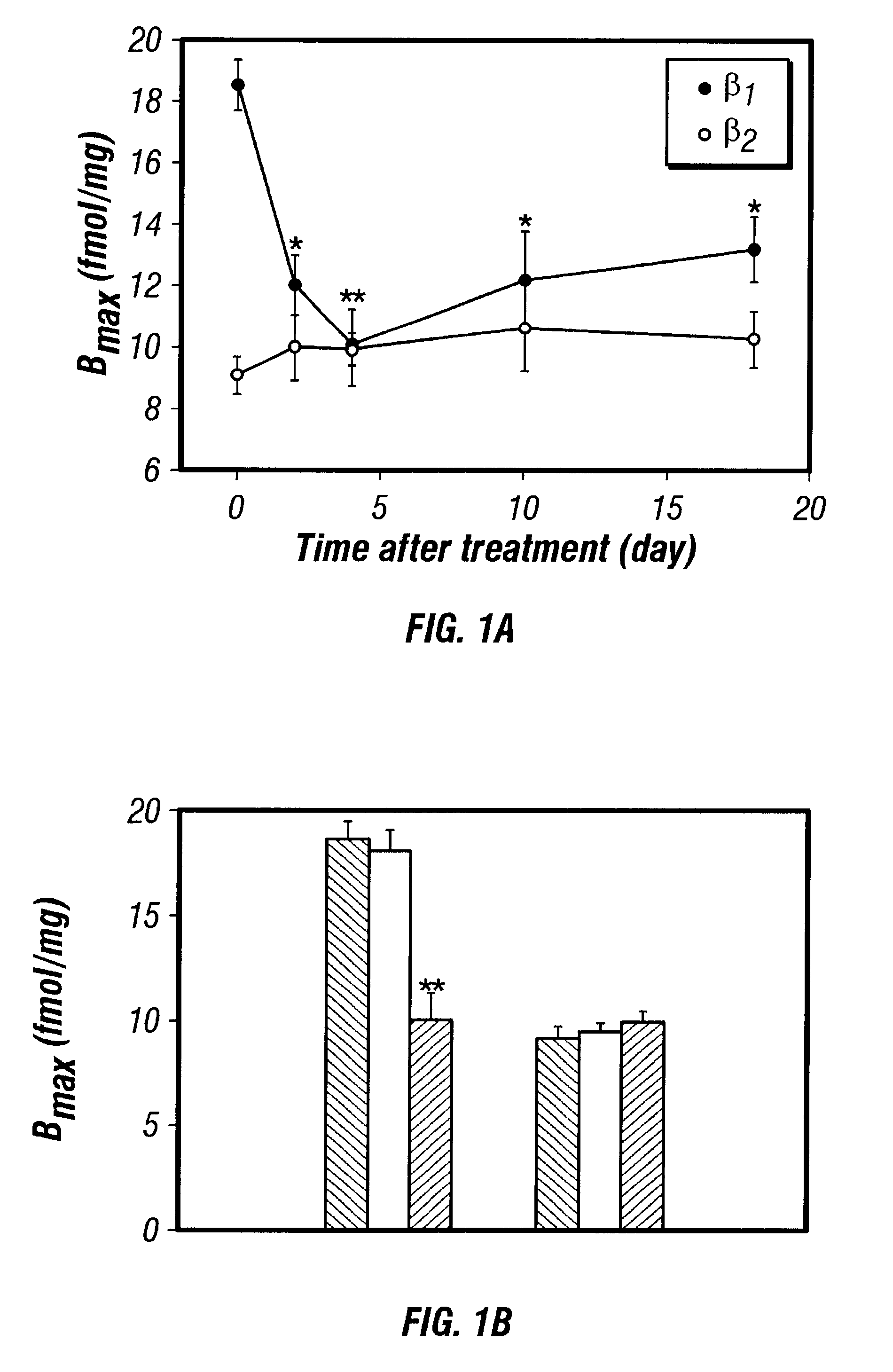
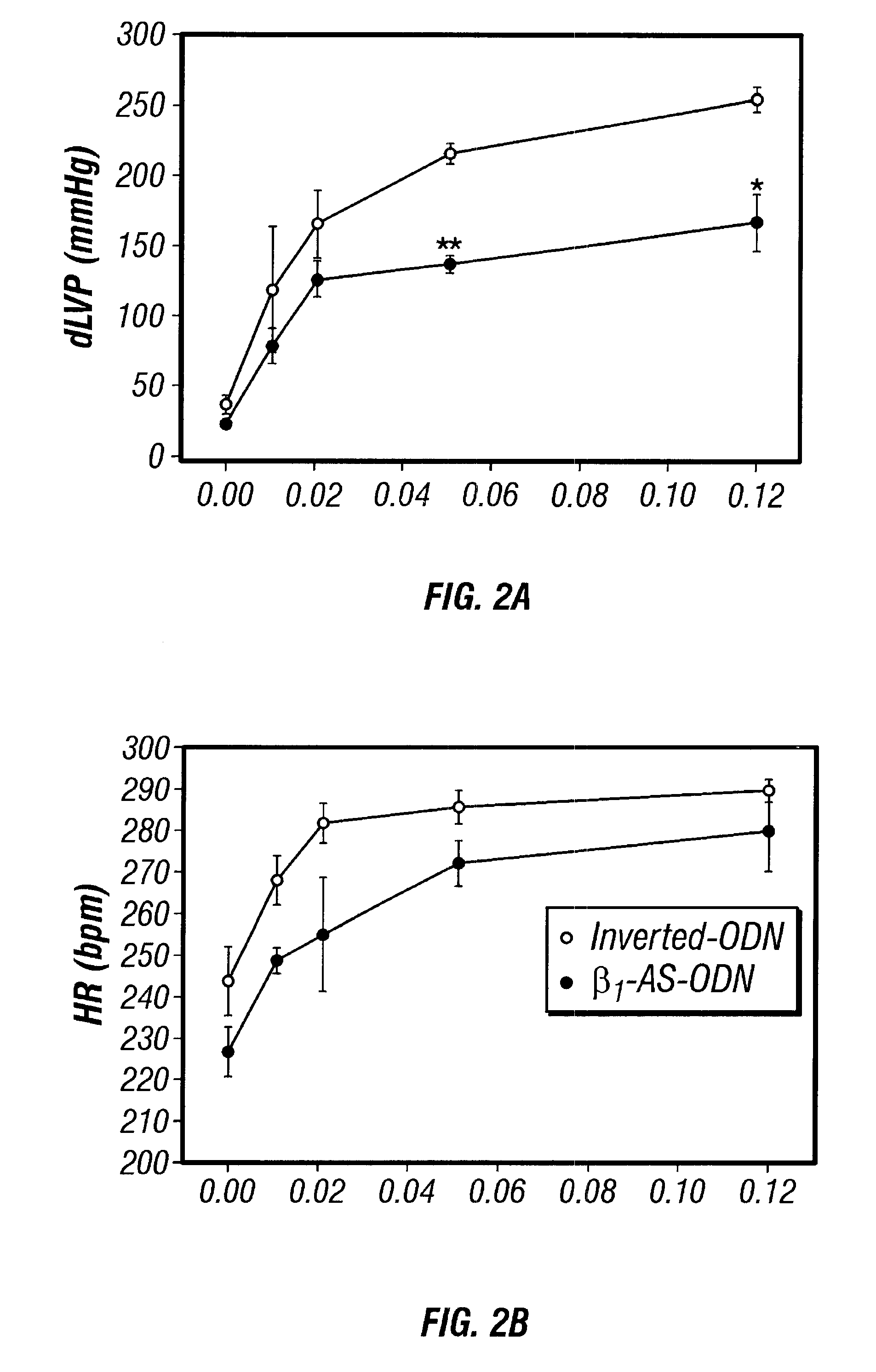
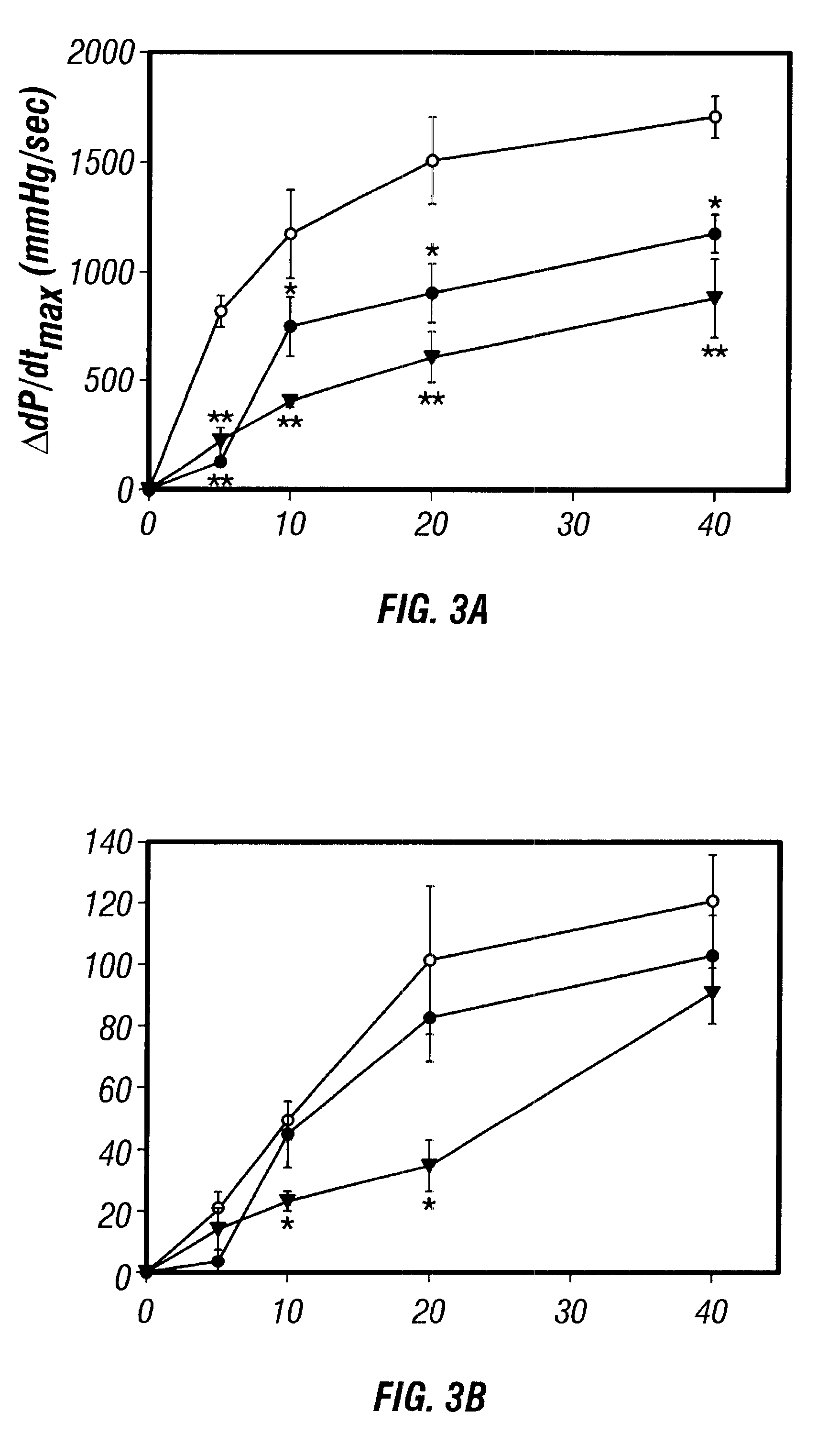
Antisense compositions targeted to beta1-adrenoceptor-specific mRNA and methods of use
InactiveUS6489307B1Reduce inhibitionInhibit and reduce expressionBiocideOrganic active ingredientsEccentric hypertrophyMammal
Disclosed are antisense oligonucleotide, polynucleotide, and peptide nucleic acid compounds that specifically bind to mammalian mRNA encoding a beta1-adrenoceptor polypeptide and that are useful in the control and / or treatment of cardiac dysfunction, hypertension, hypertrophy, myocardial ischemia, and other cardiovascular diseases in an affected mammal, and preferably, in a human subject. The antisense compounds disclosed herein, and pharmaceutical formulations thereof, provide sustained control of beta1-adrenoceptor expression over prolonged periods, and achieve therapeutic effects from as little as a single dose. Administration of these antisense compositions to approved animal models resulted in a decrease in blood pressure, but no significant change in heart rate. Use of such antisense compositions in the reduction of beta1-adrenoceptor polypeptides in a host cell expressing beta1-adrenoceptor-specific mRNA, and in the preparation of medicaments for treating human and animal diseases, and in particular, hypertension and other cardiac dysfunction is also disclosed.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Compositions and methods for the treatment of disease
InactiveUS20040209805A1Efficient transferAvoid cross contaminationOrganic active ingredientsPeptide/protein ingredientsAdjuvantMedicine
The present invention relates to pharmaceutical compositions for the treatment and / or prophylaxis of disease associated with fibrosis in a vertebrate, said composition comprising at least one activin antagonist, and optionally a pharmaceutically acceptable carrier, adjuvant and / or diluent. The invention also relates to methods of treatment of disease associated with fibrosis in a vertebrate, as well as methods for diagnosing such conditions, and kits therefor.
Owner:MONASH UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com