Method for vector delivery
a vector and brain technology, applied in the direction of viruses/bacteriophages, biocide, drug compositions, etc., can solve the problems of increased bleeding risk and other surgical complications, and achieve the effect of a larger volume of vector spread within the brain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparing Distribution of a Lentiviral Vector in the Putamen Following a) Conventional Delivery and b) Delivery Using a Single Site and a High Flow-Rate
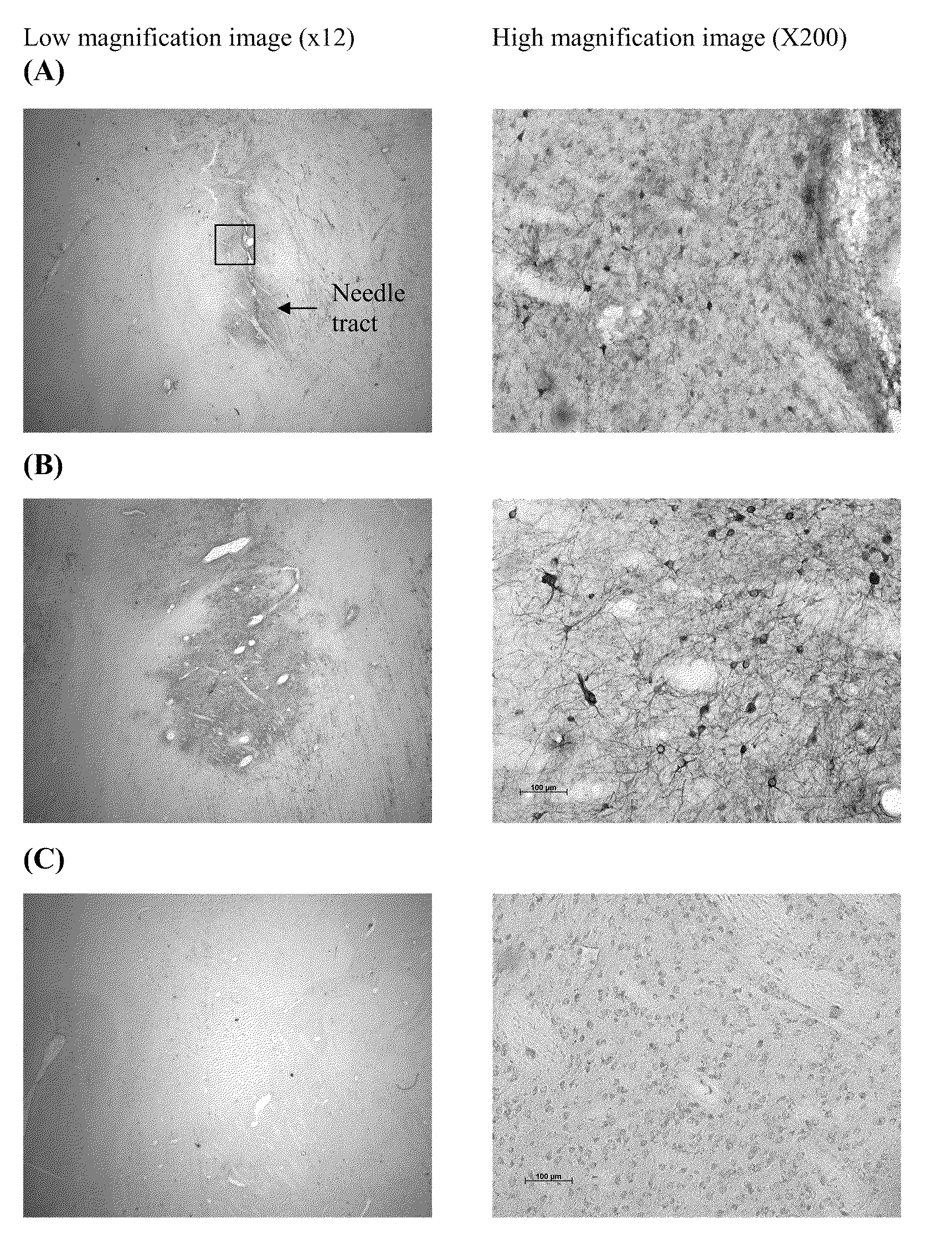
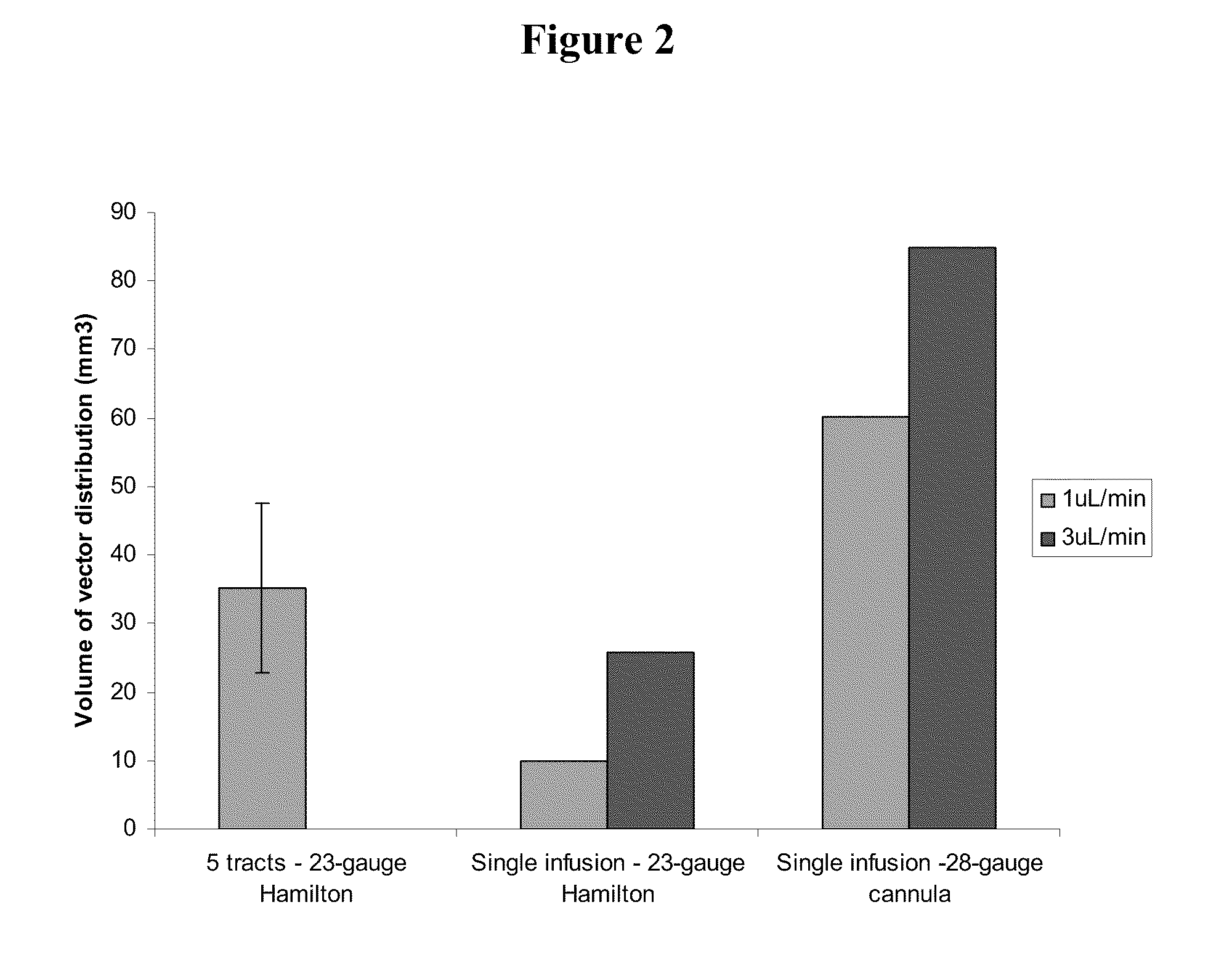
[0138]Administration of EIAV-LacZ vector suspension using the previously described technique (50 ∞L total volume vector administered to the putamen through 5 needle tracts using a Hamilton syringe and 23-gauge needle, each delivering 10 μL per tract (via three deposits) provided a moderate spread of vector in the dorso-ventral axis of the putamen that reached between 40 and 50% the depth of this brain structure (FIG. 1).
[0139]Spread of vector in the medio-lateral axis was very much less than with the dorso-ventral axis, with the vector only spreading around 1-2 mm at the greatest point. However, due to the positioning of the five needle tracts along the axis of the putamen, spread of vector along the rostro-caudal axis was around 7.8 mm in both of the two hemispheres that were administered vector by this paradigm.
[0140]In contrast, u...
example 2
Comparing Efficacy of a Lentiviral Vector for Parkinson's Disease Following Administration to the Motor Putamen of Parkinson's Disease Patients Using a) Conventional Delivery and b) Delivery Using a Reduced Number of Injection Sites with a Narrower Gauge Device and a High Flow-Rate
[0156]A phase I / II clinical trial is ongoing to evaluate the safety and efficacy of a lentiviral vector based treatment for Parkinson's disease, called ProSavin®. ProSavin® is an EIAV lentiviral vector that contains three genes which encode for enzymes in the dopamine biosynthesis pathway. As part of the trial the therapeutic potential of ProSavin® to correct symptoms of Parkinson's disease was evaluated using 1) the conventional method of administration and 2) a continuous method of infusion. The conventional method involved injection of a total volume of 125 μL of vector administered to each putamen through 5 needle tracts using a Hamilton syringe and 23-gauge needle and an administration rate of 1 μL / mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Flow rate | aaaaa | aaaaa |
| Flow rate | aaaaa | aaaaa |
| Flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com