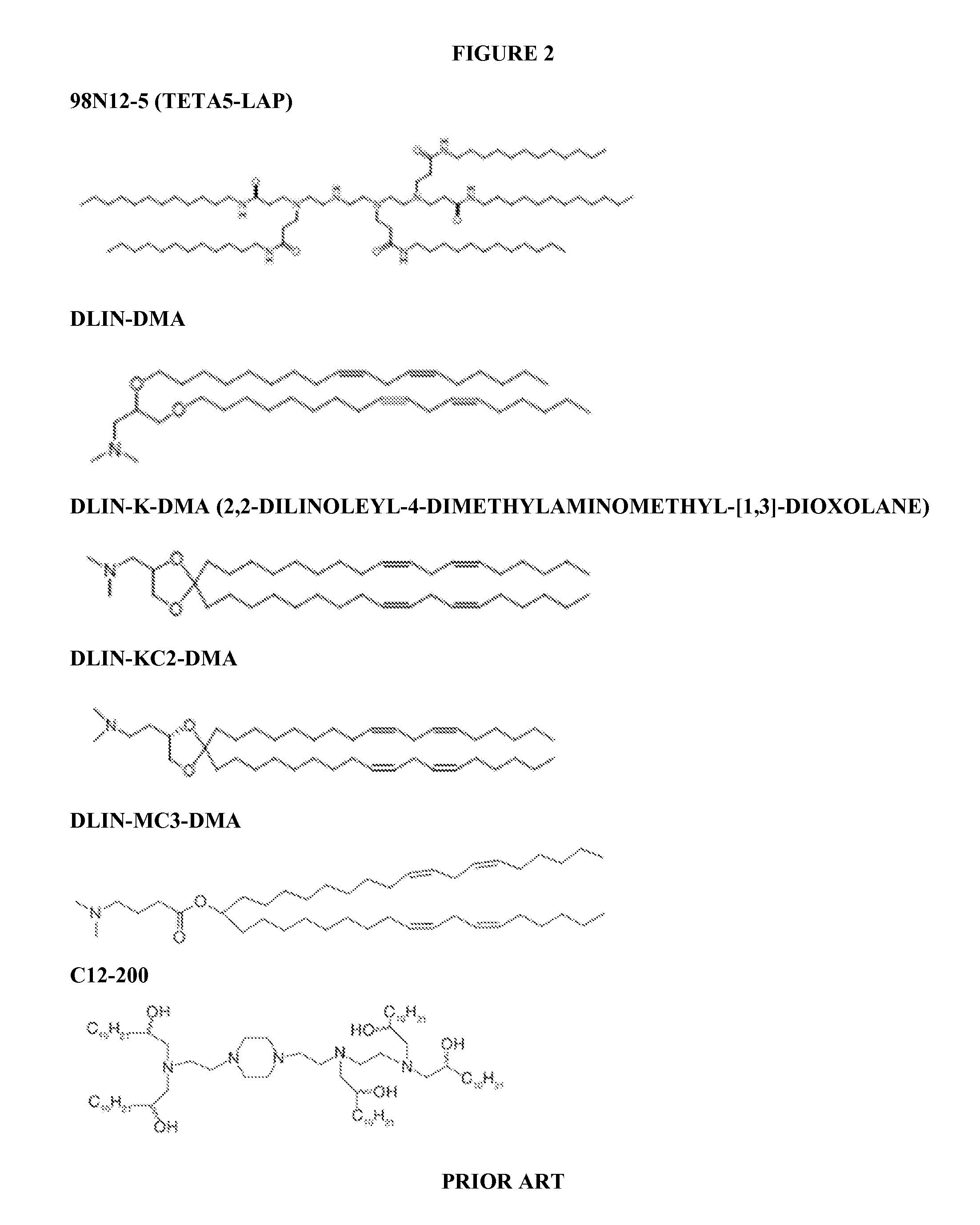
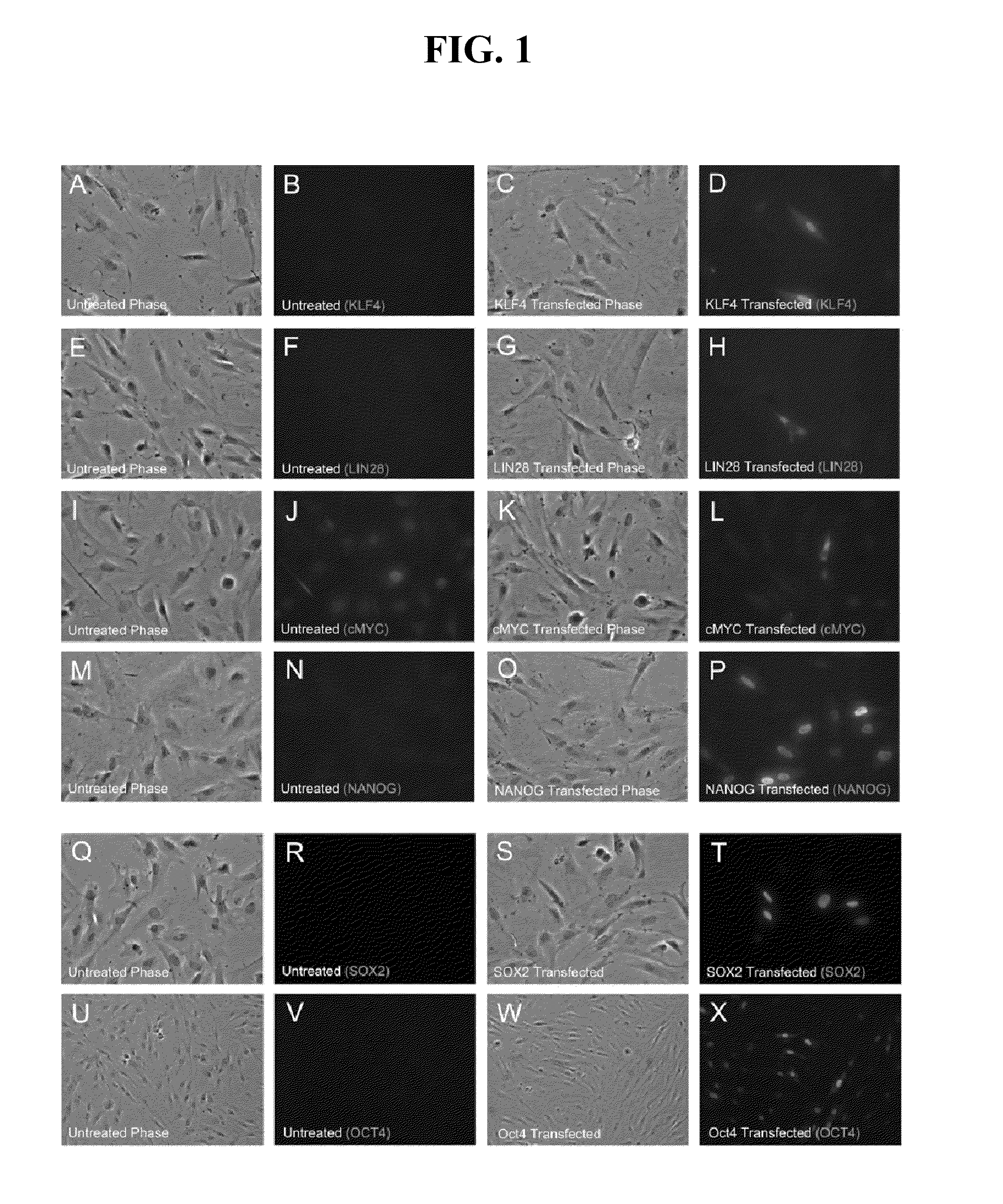
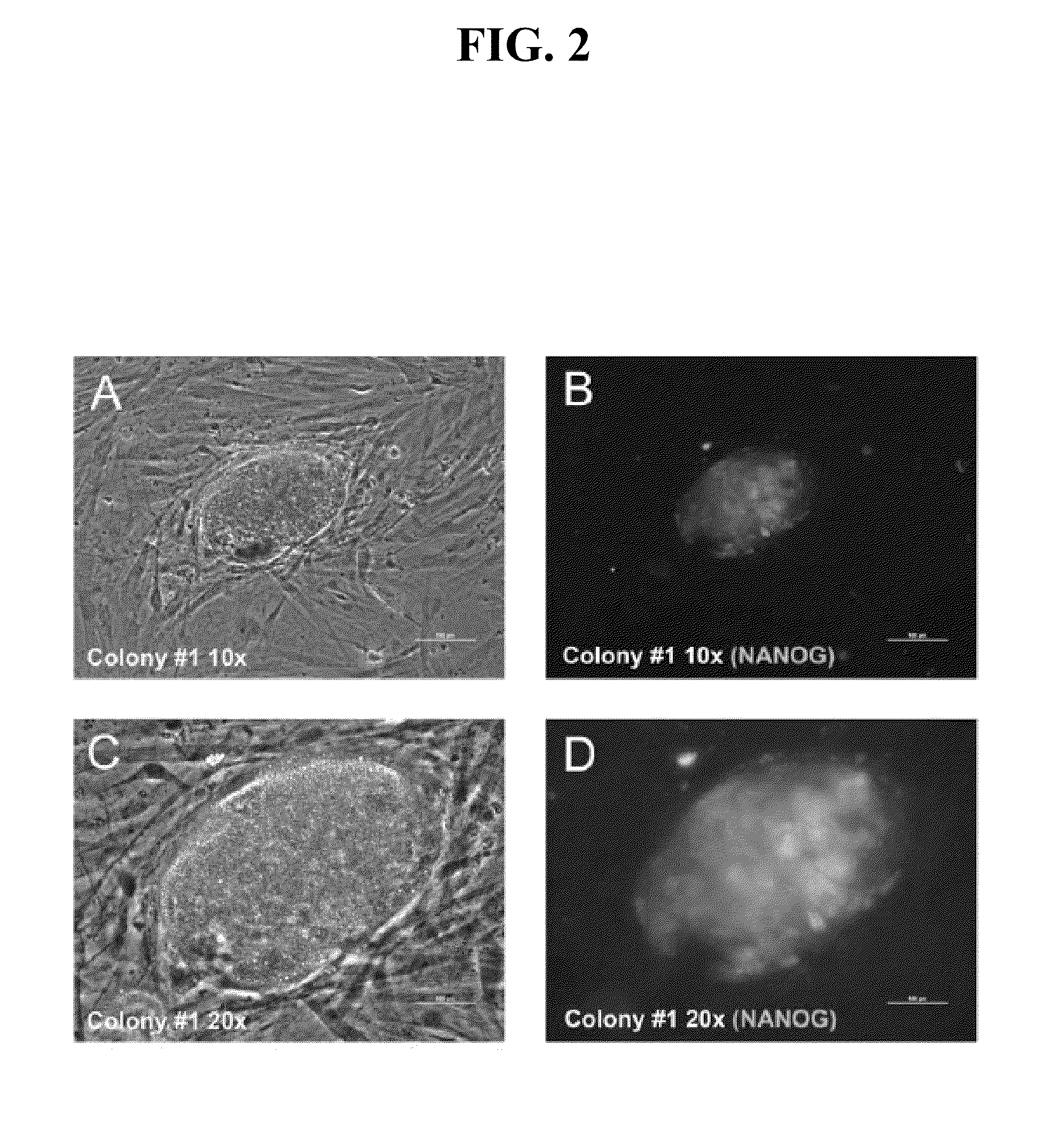
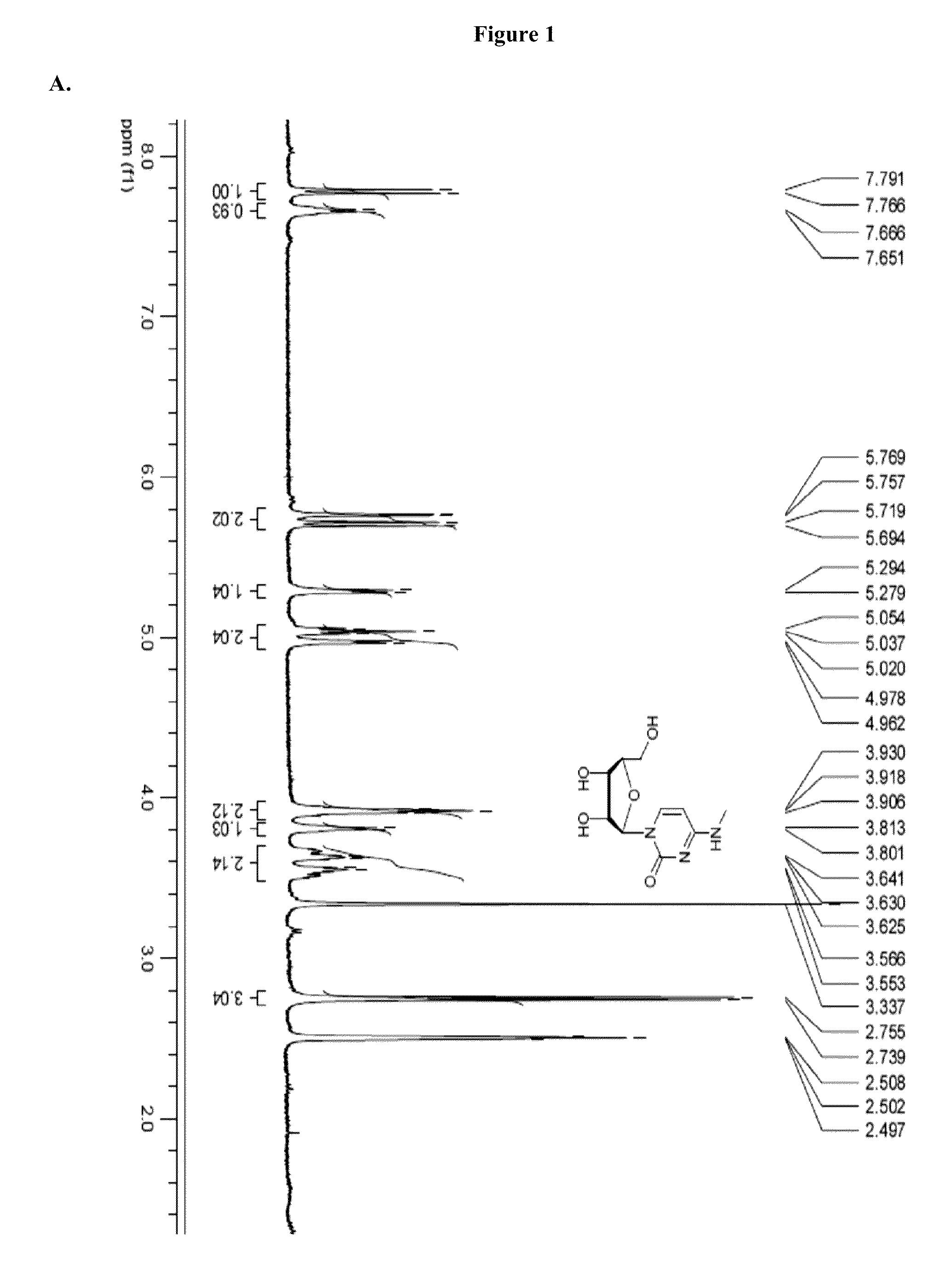
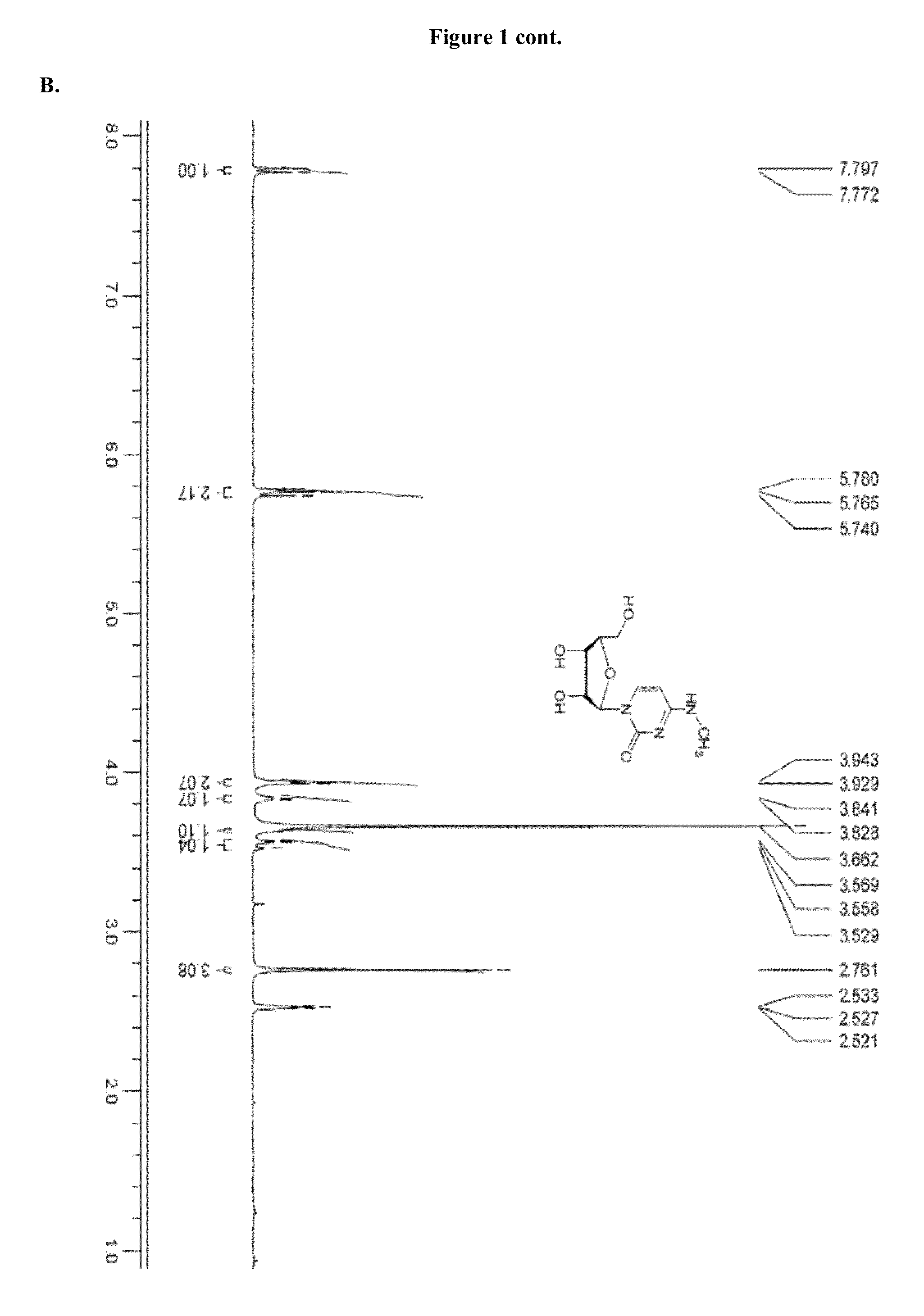
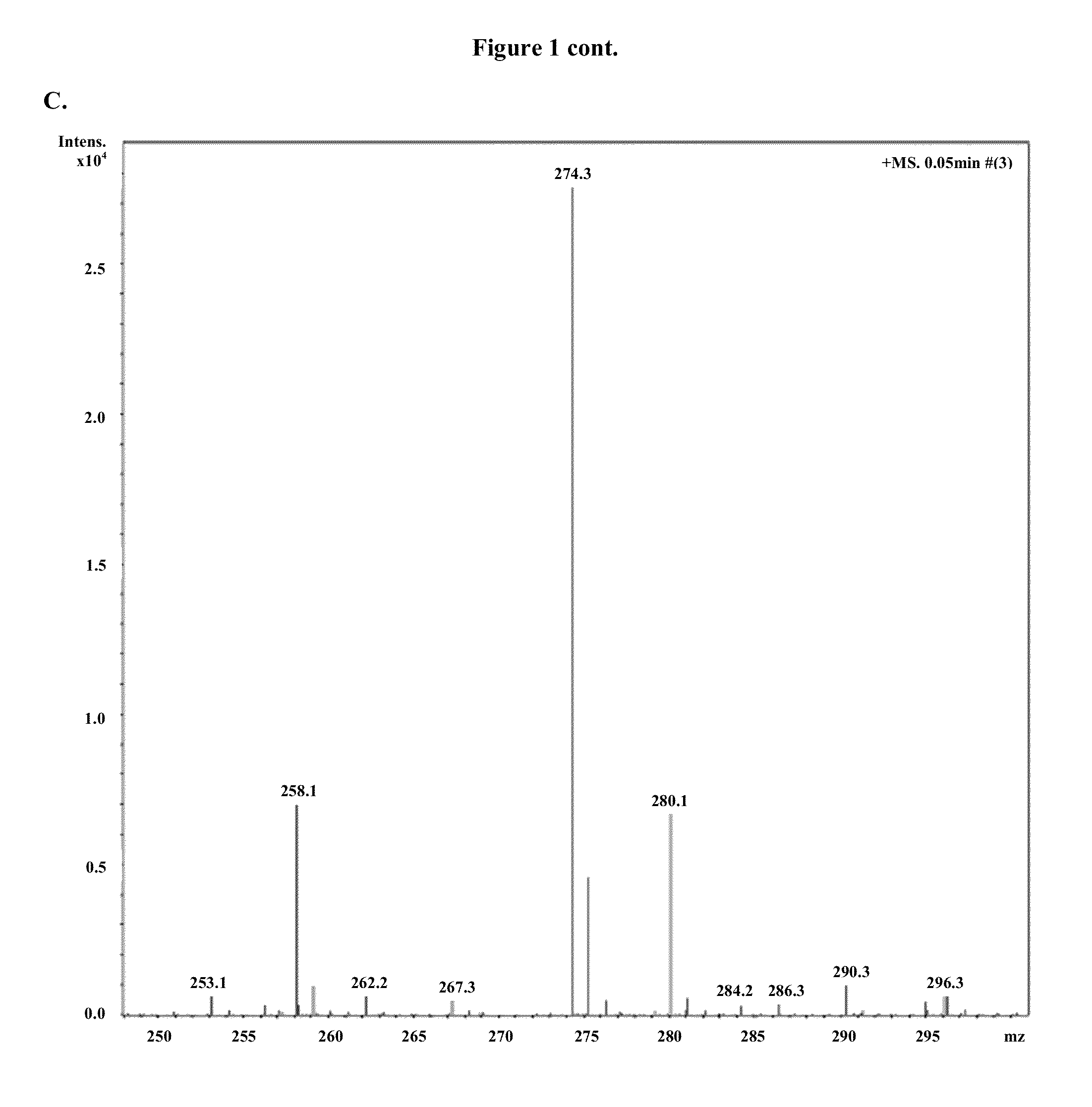
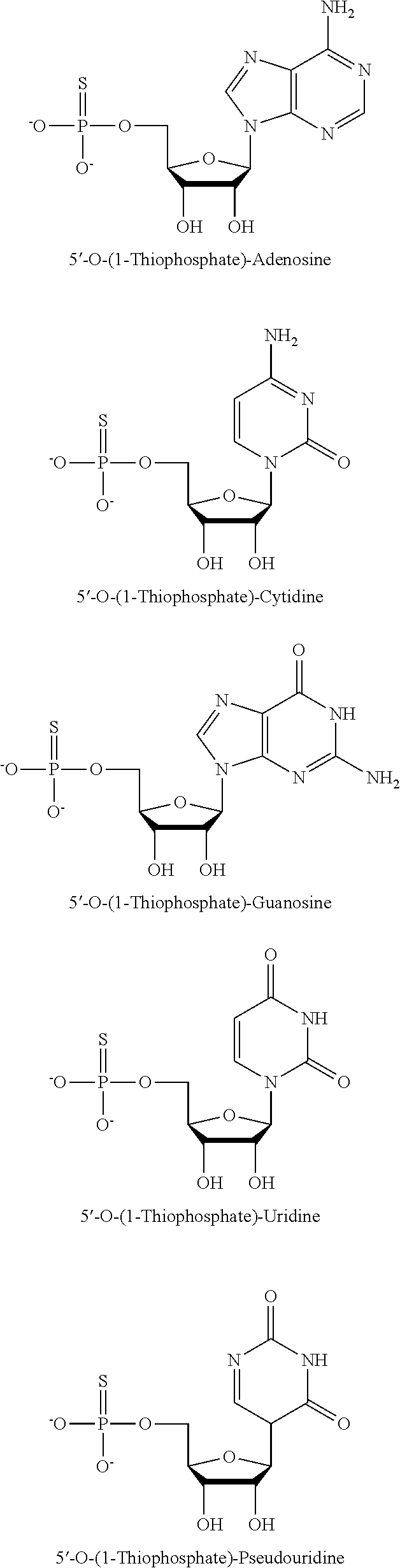
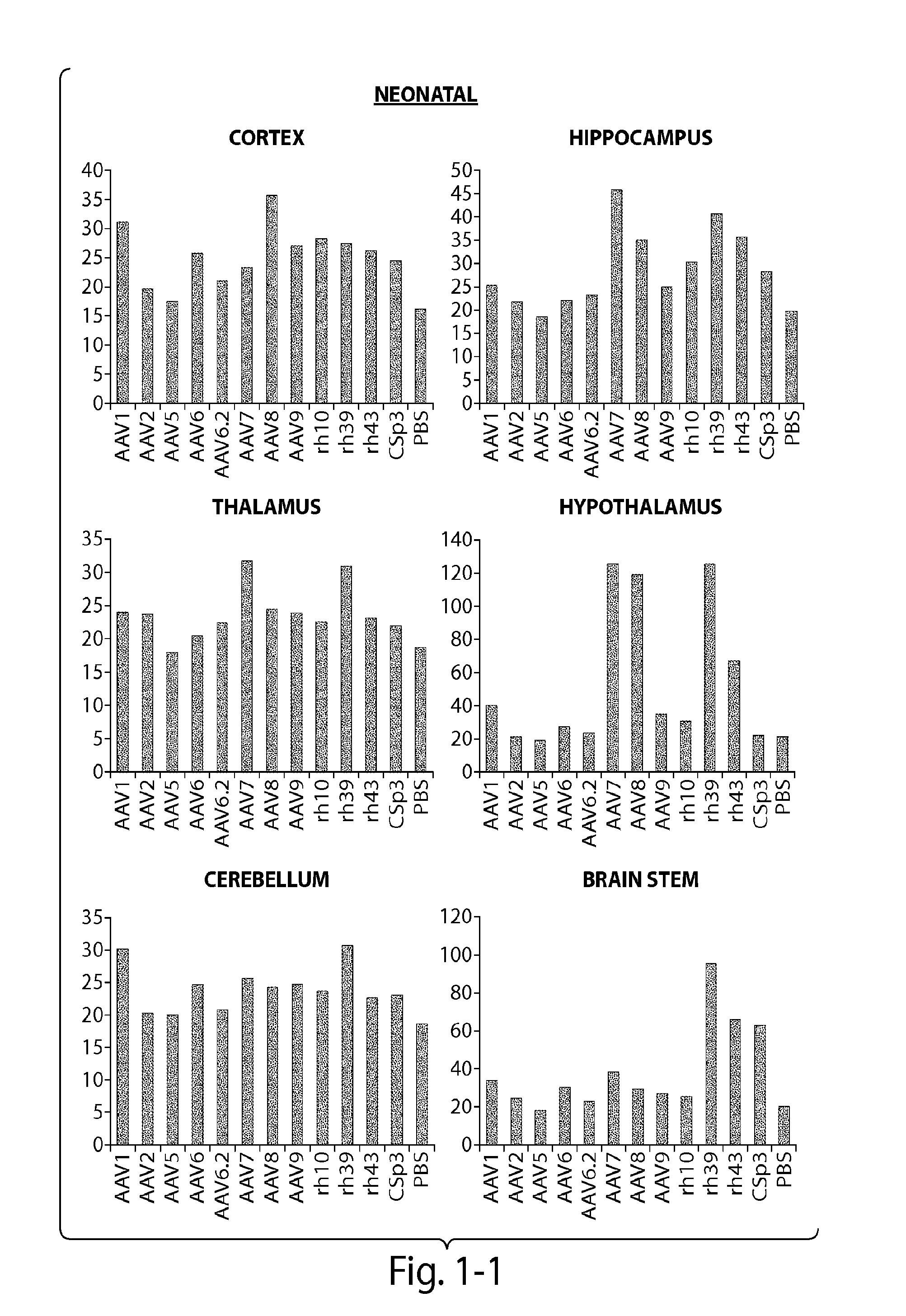
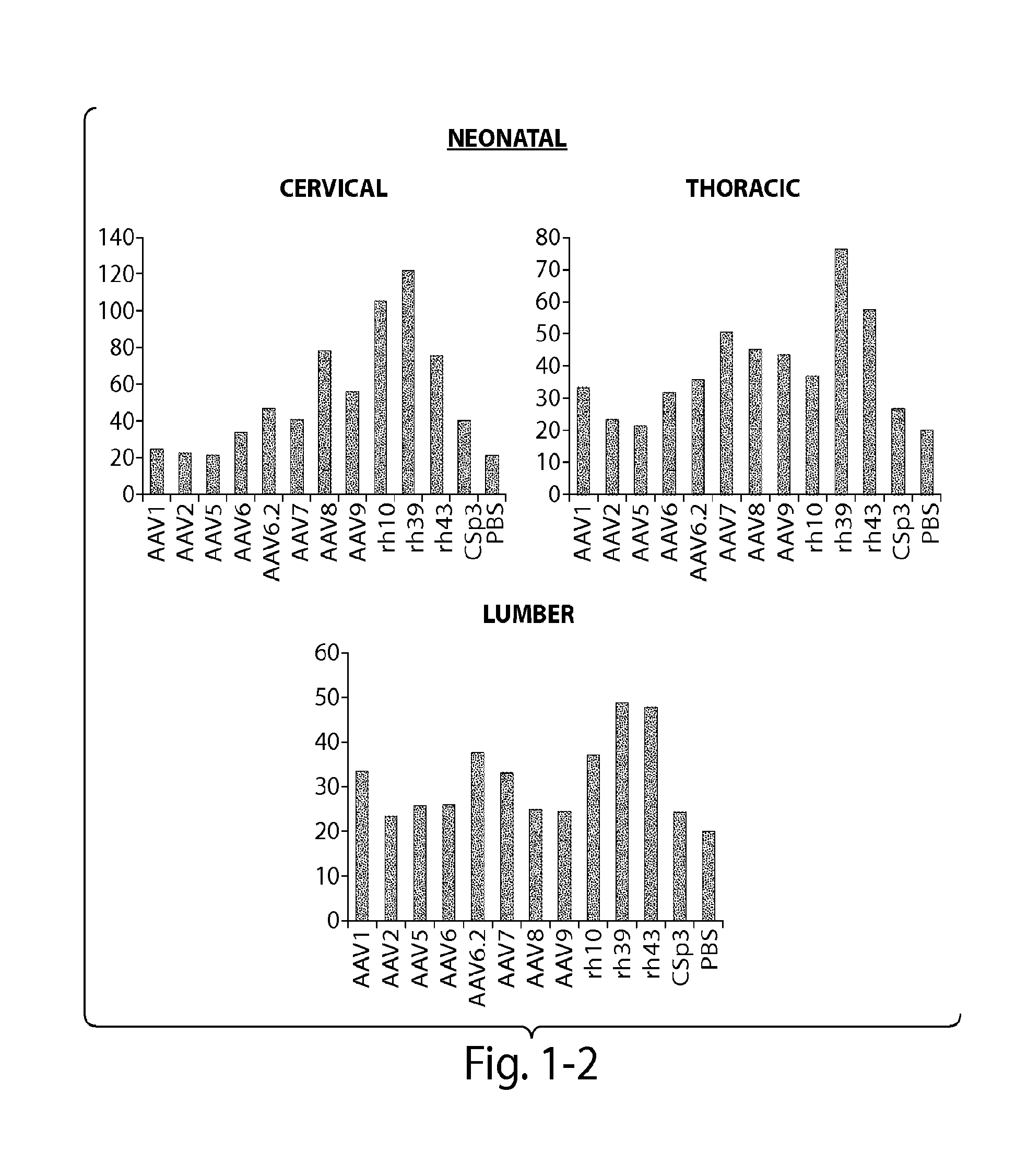
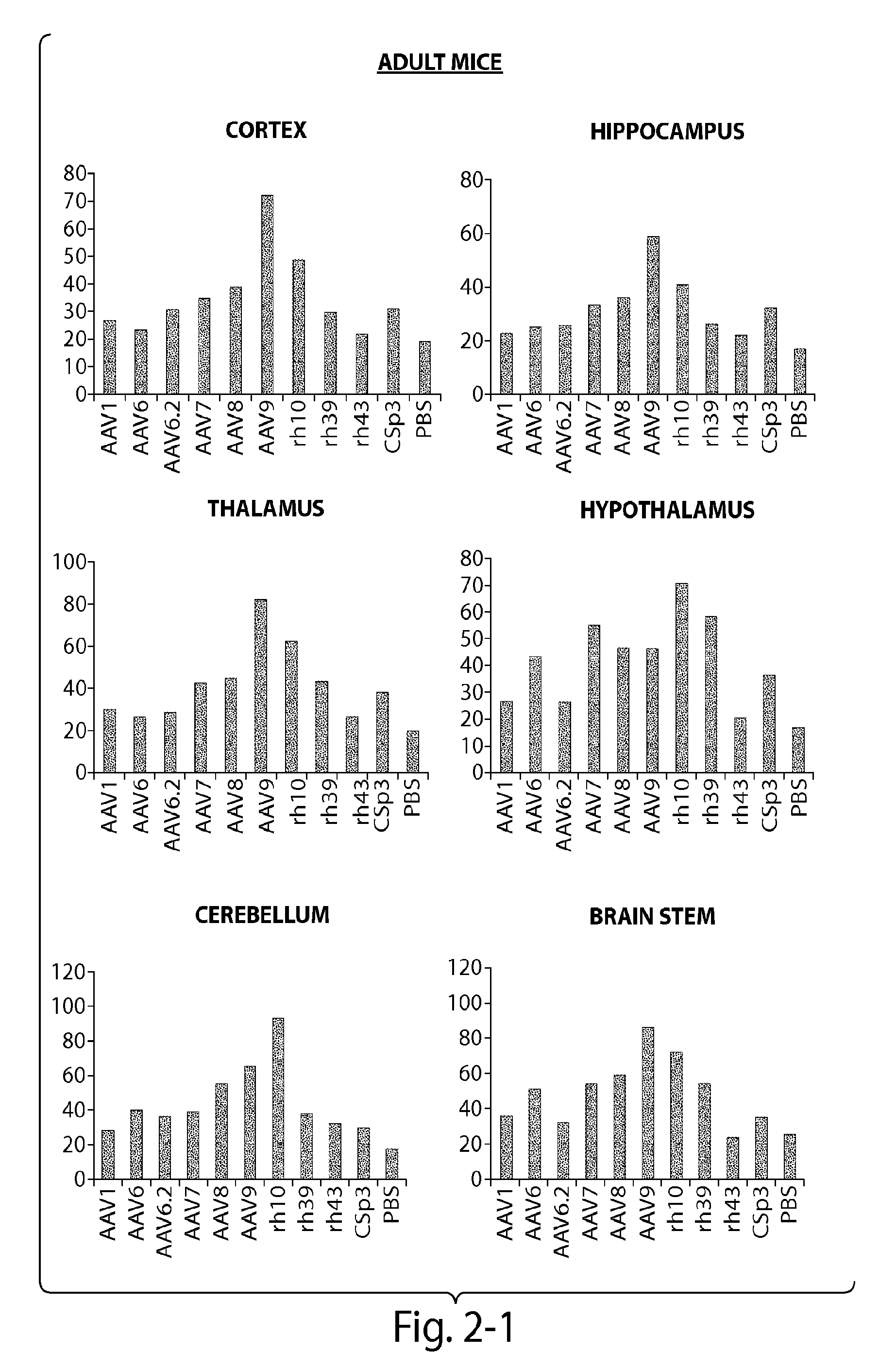
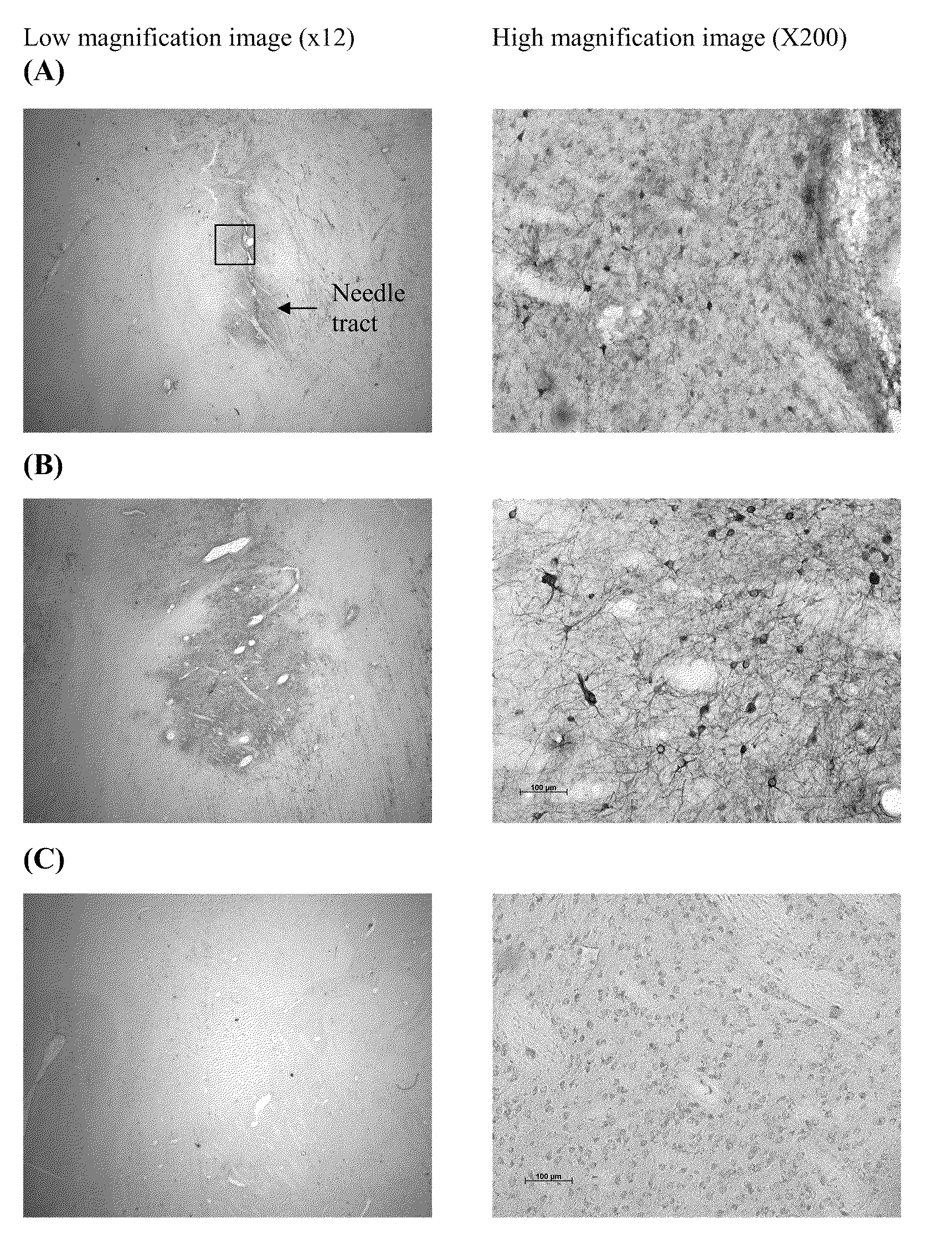
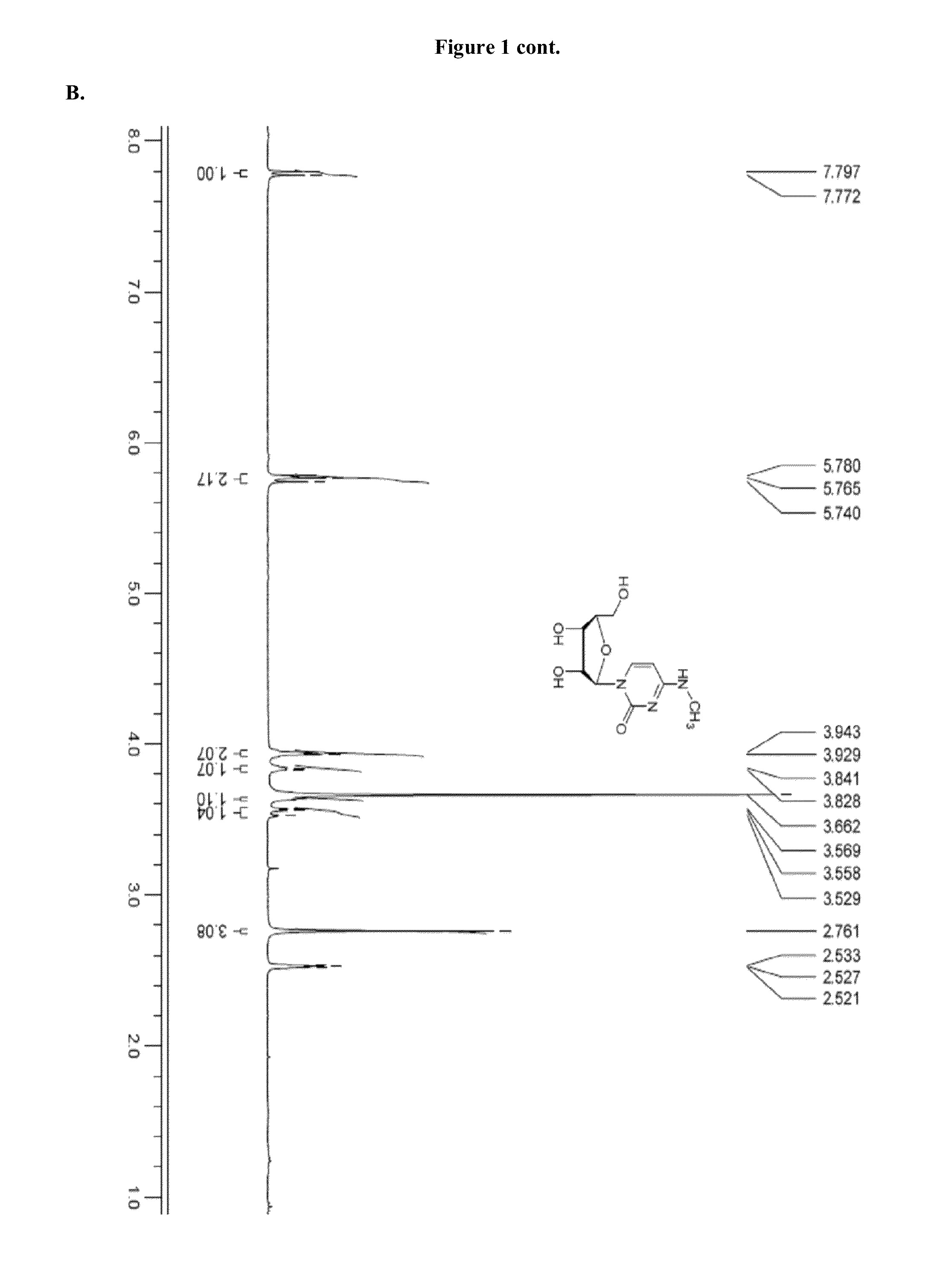
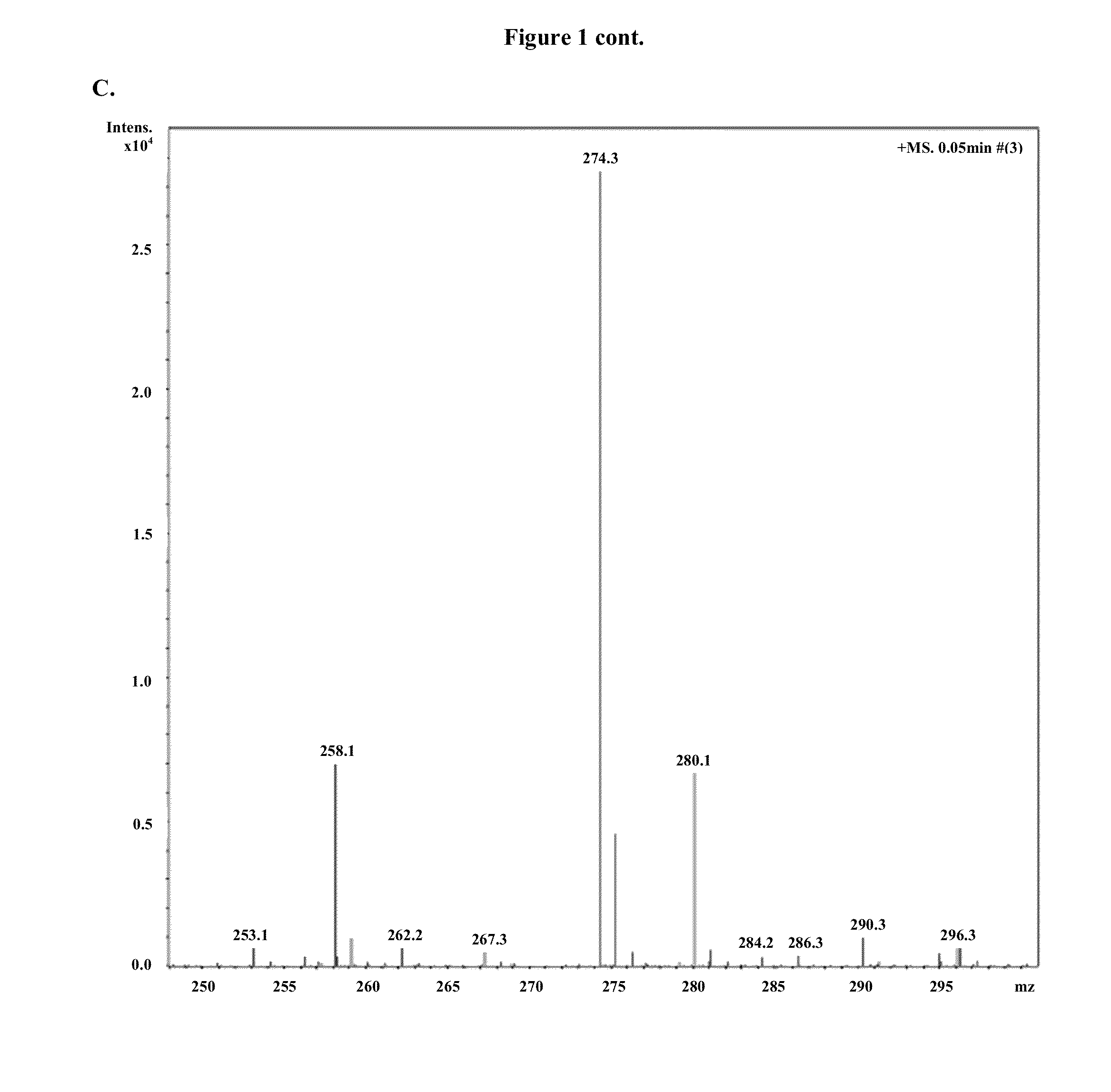
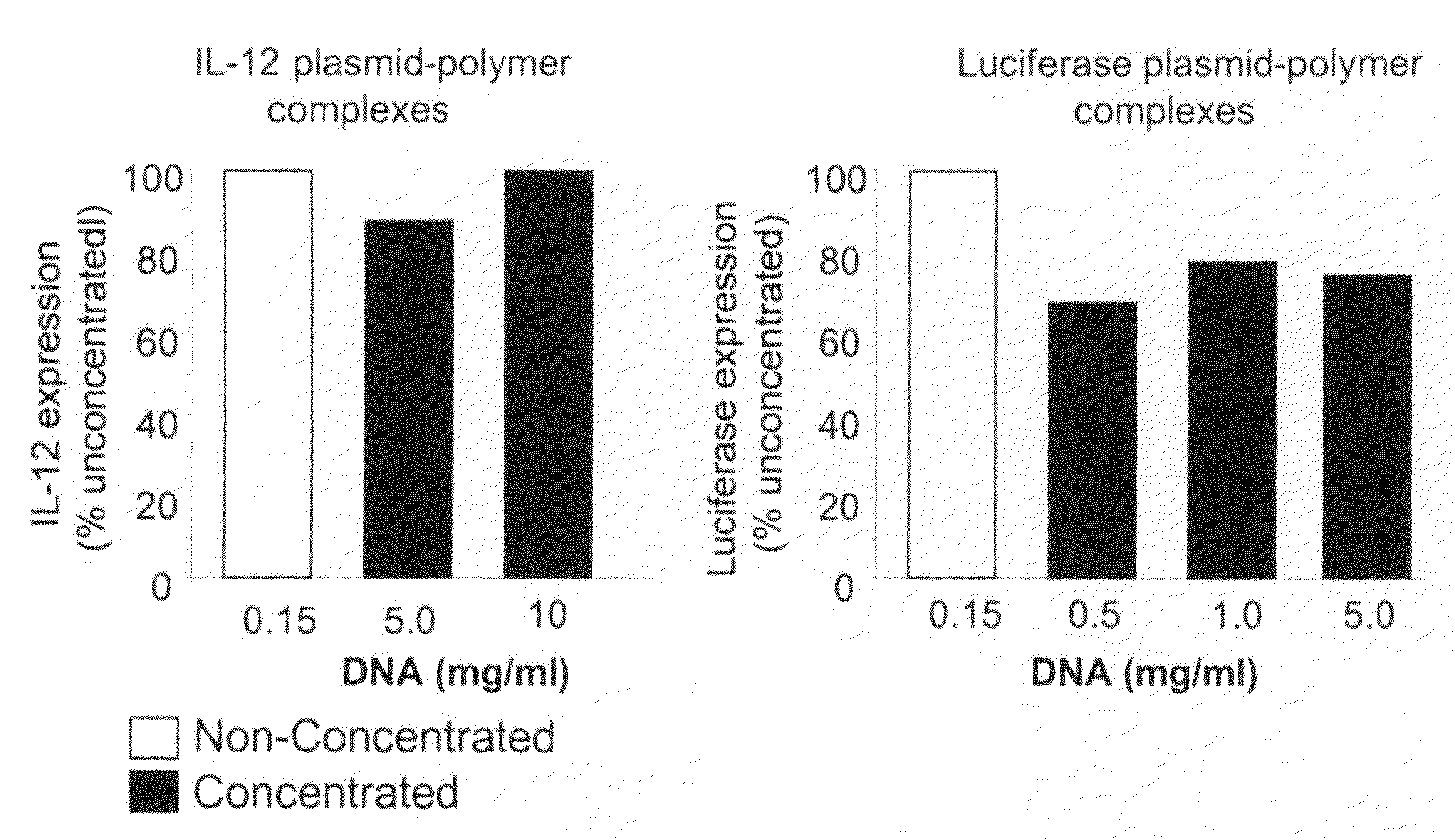
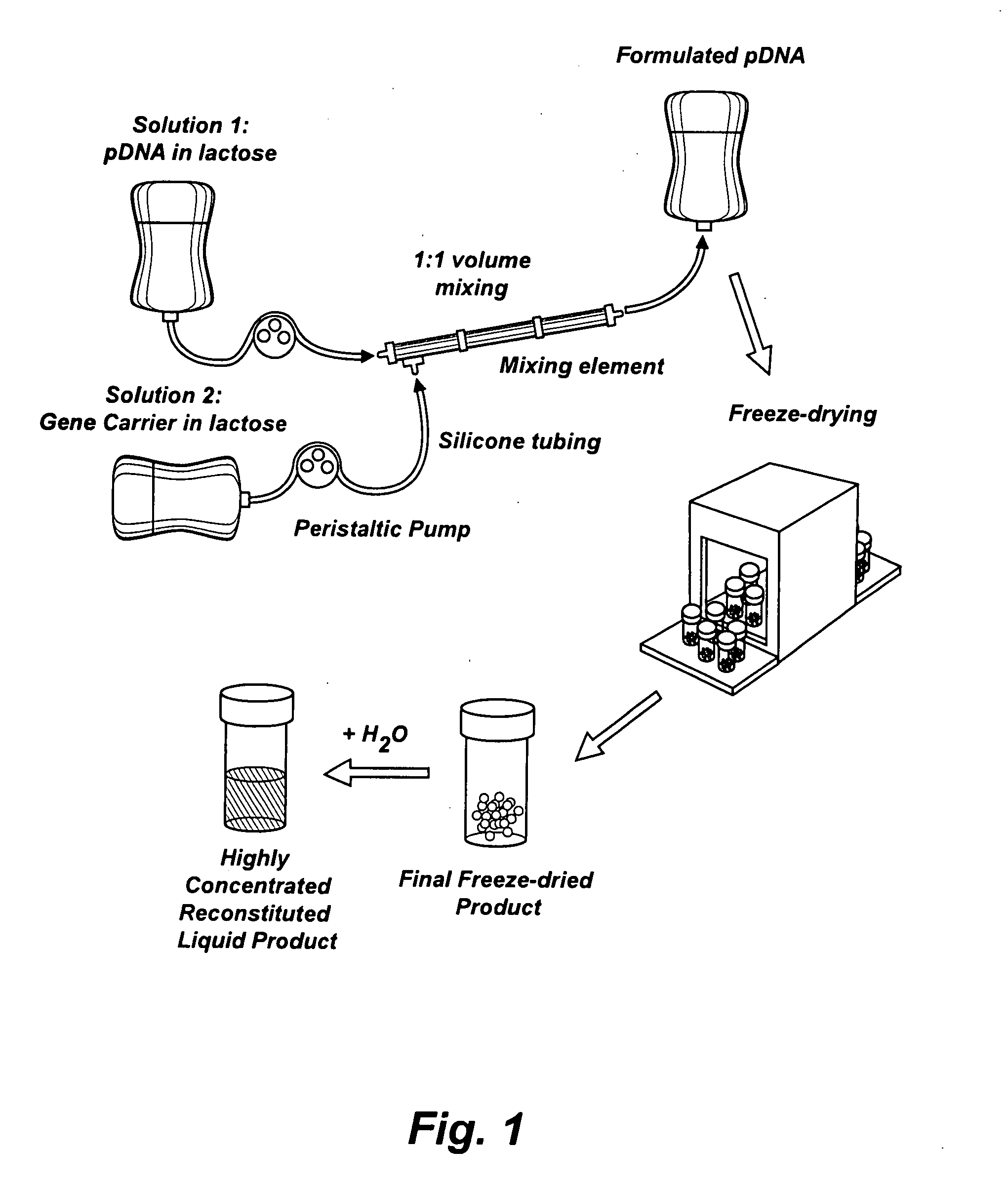
Patents
Literature
1019results about "Genetic material delivery route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
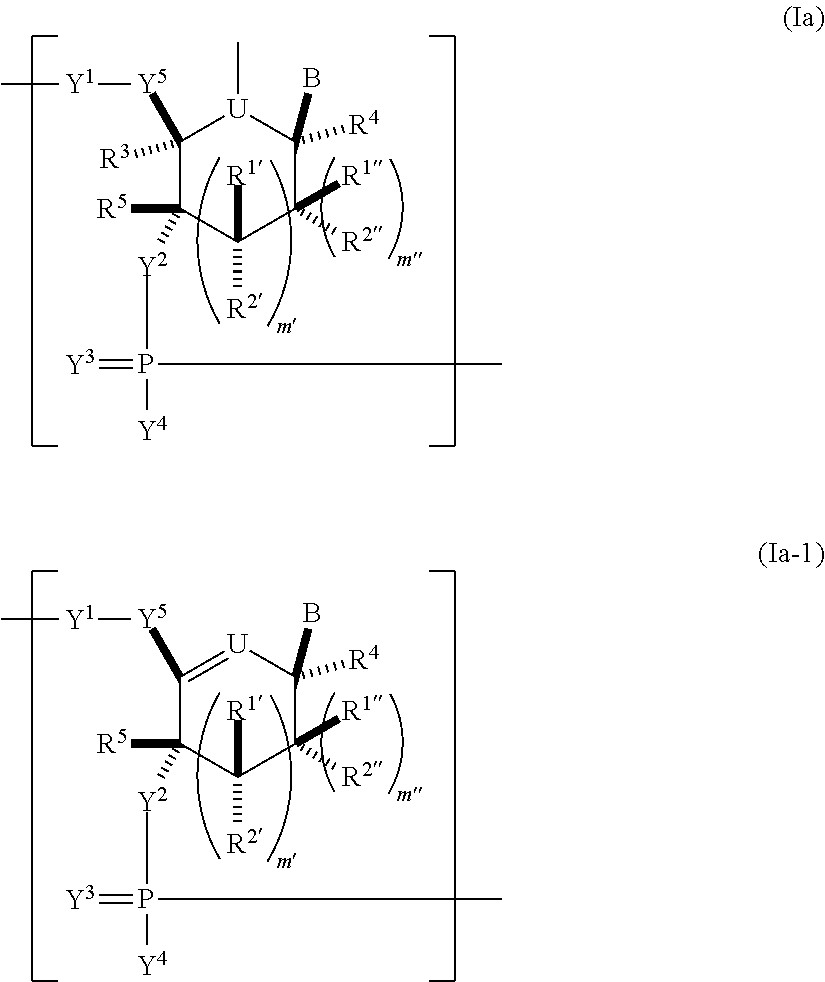
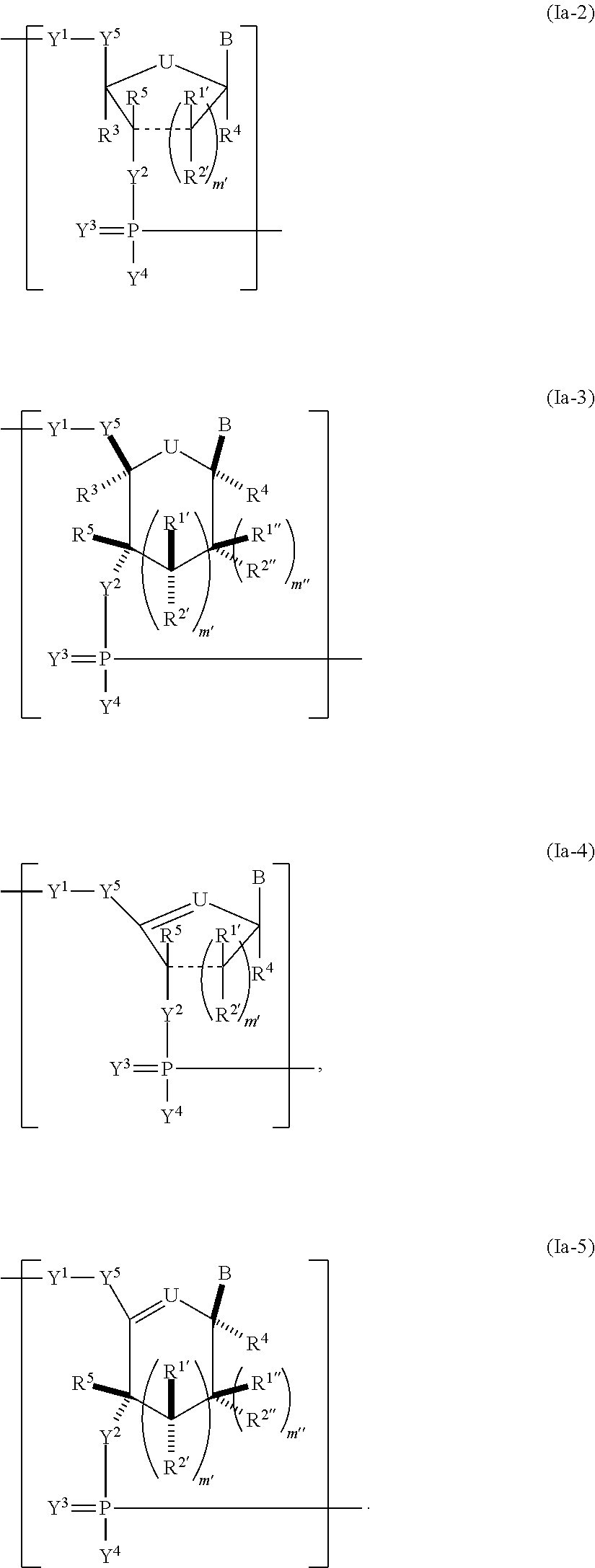
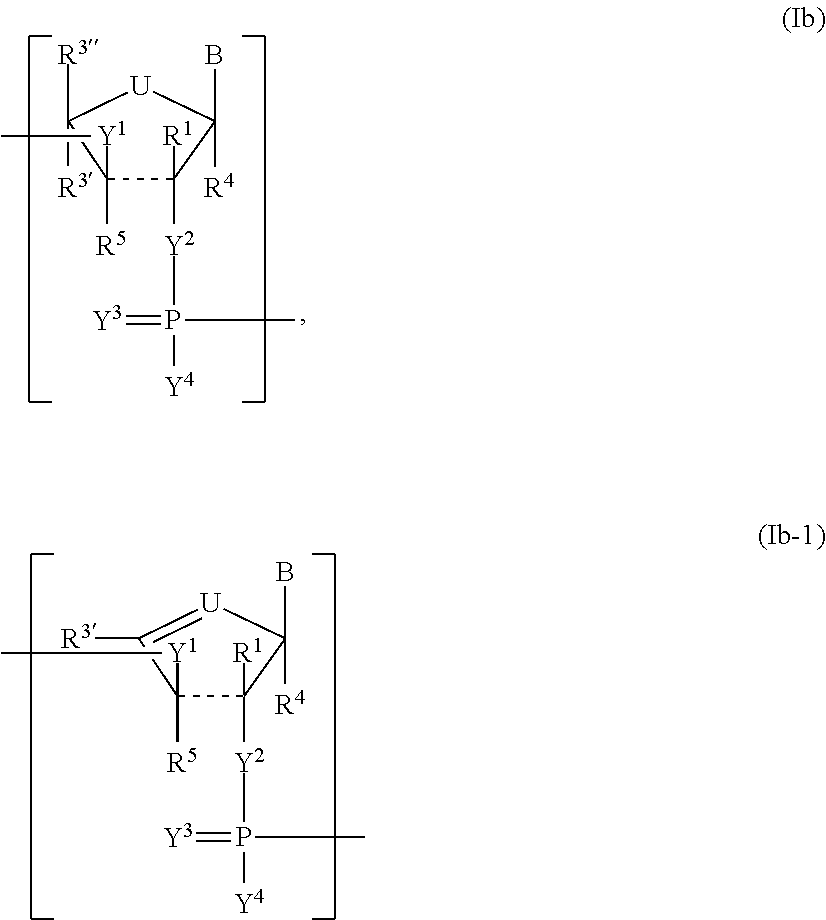
Pharmaceutical composition containing a stabilised mRNA optimised for translation in its coding regions
ActiveUS20050032730A1Overcome disadvantagesImprove efficiencyAntibacterial agentsVirusesTranslational efficiencyCoding region
The present invention relates to a pharmaceutical composition comprising a modified mRNA that is stabilised by sequence modifications and optimised for translation. The pharmaceutical composition according to the invention is particularly well suited for use as an inoculating agent, as well as a therapeutic agent for tissue regeneration. In addition, a process is described for determining sequence modifications that promote stabilisation and translational efficiency of modified mRNA of the invention.
Owner:CUREVAC SE
Nucleic Acid-Lipopolymer Compositions
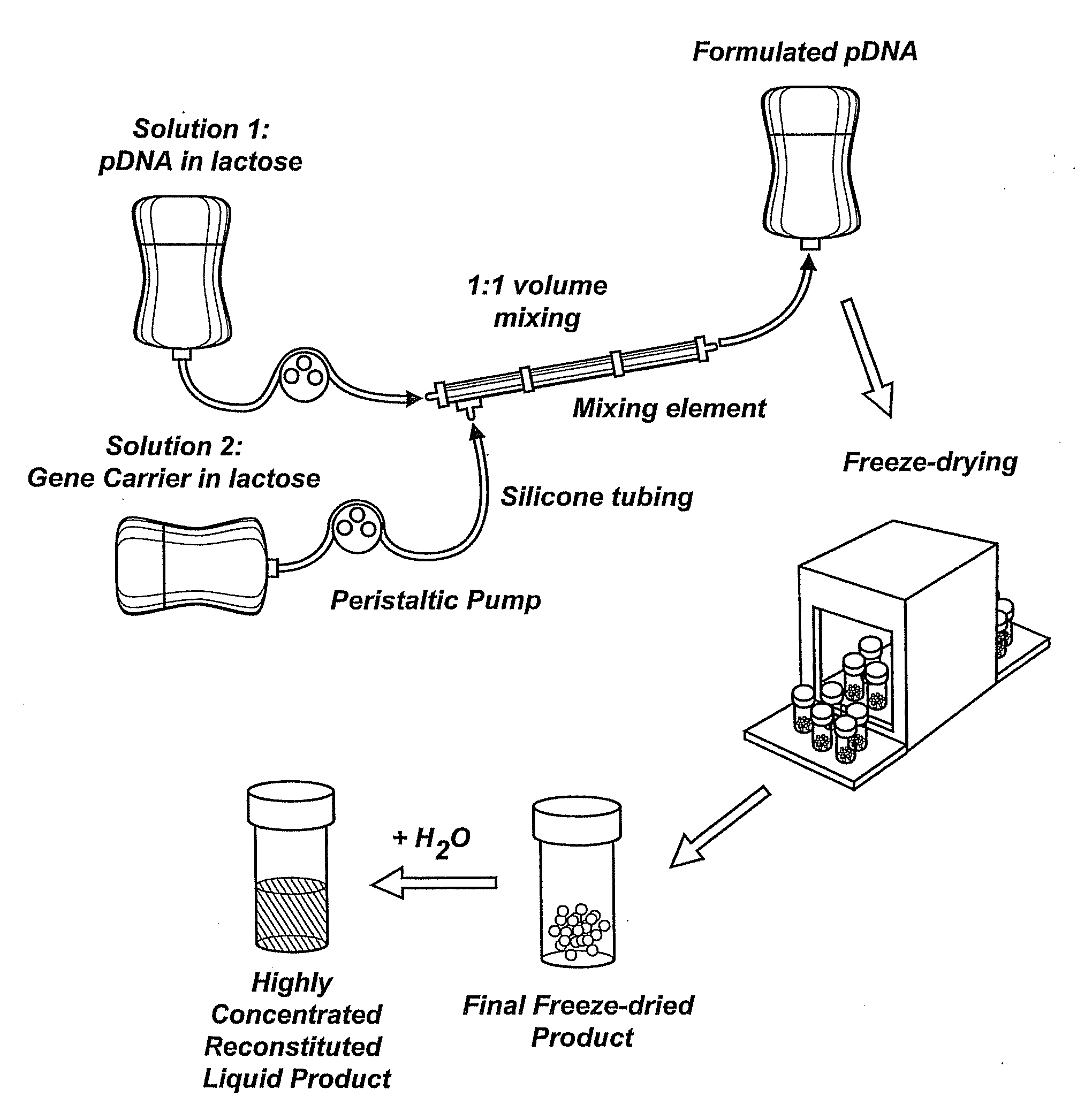
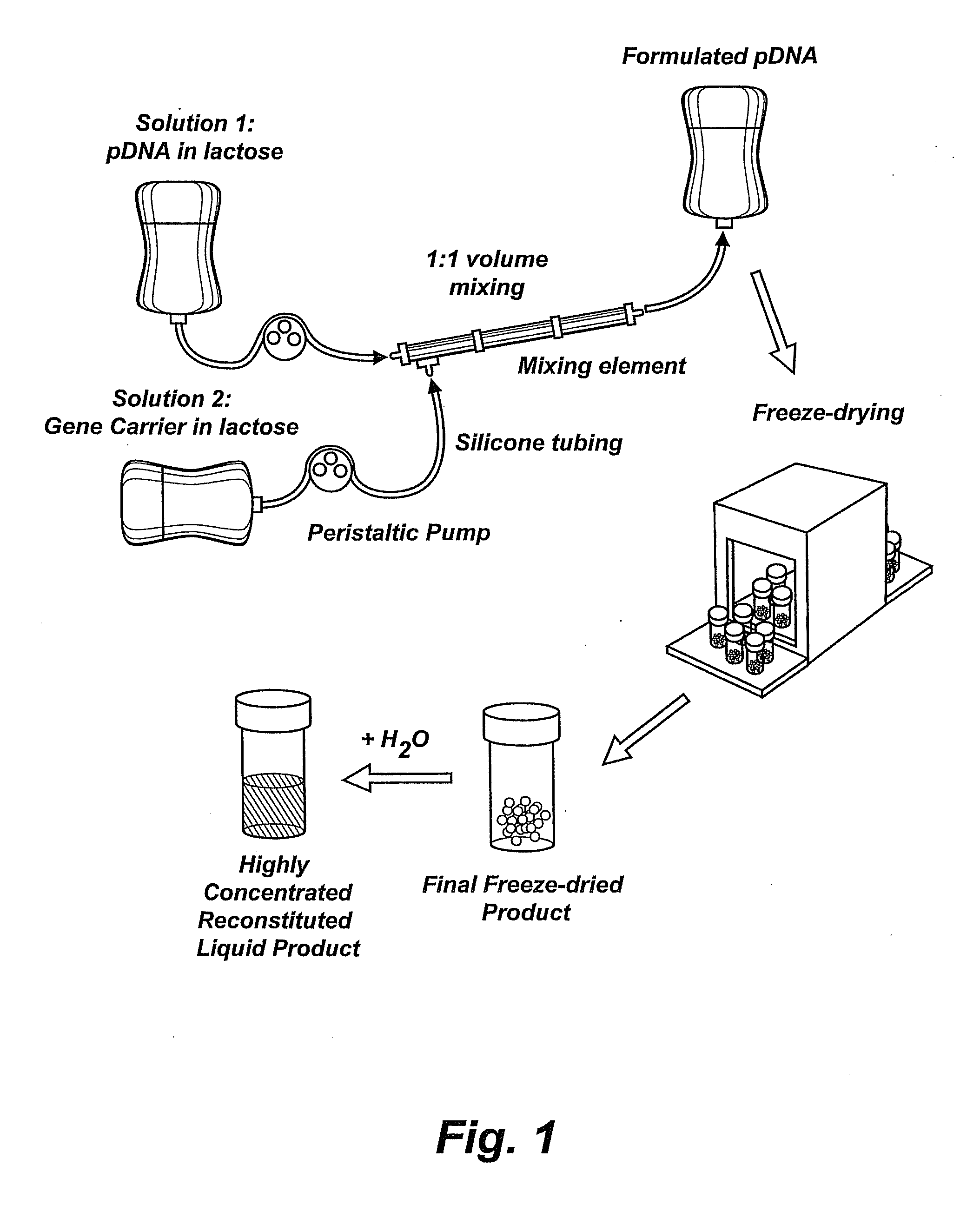
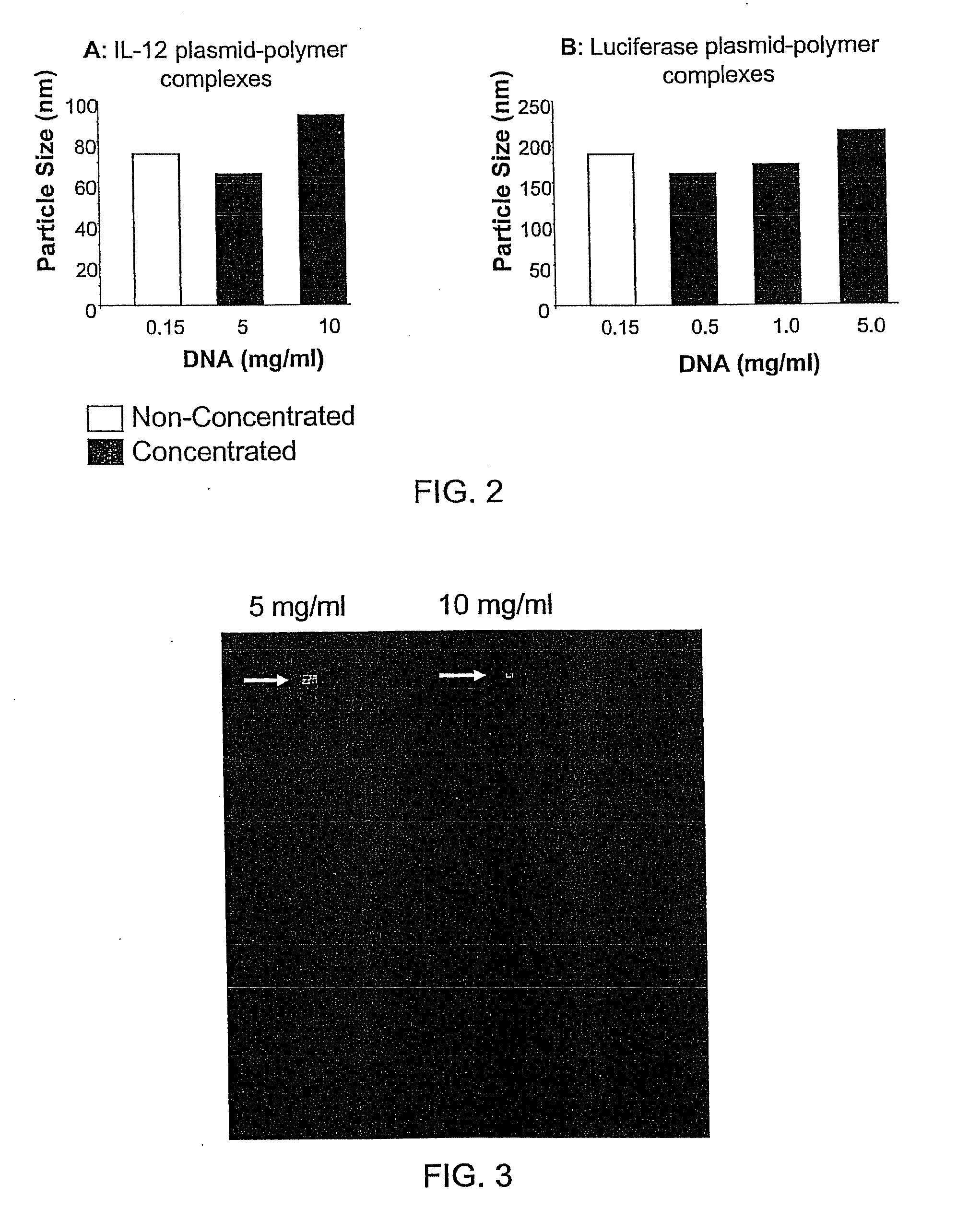
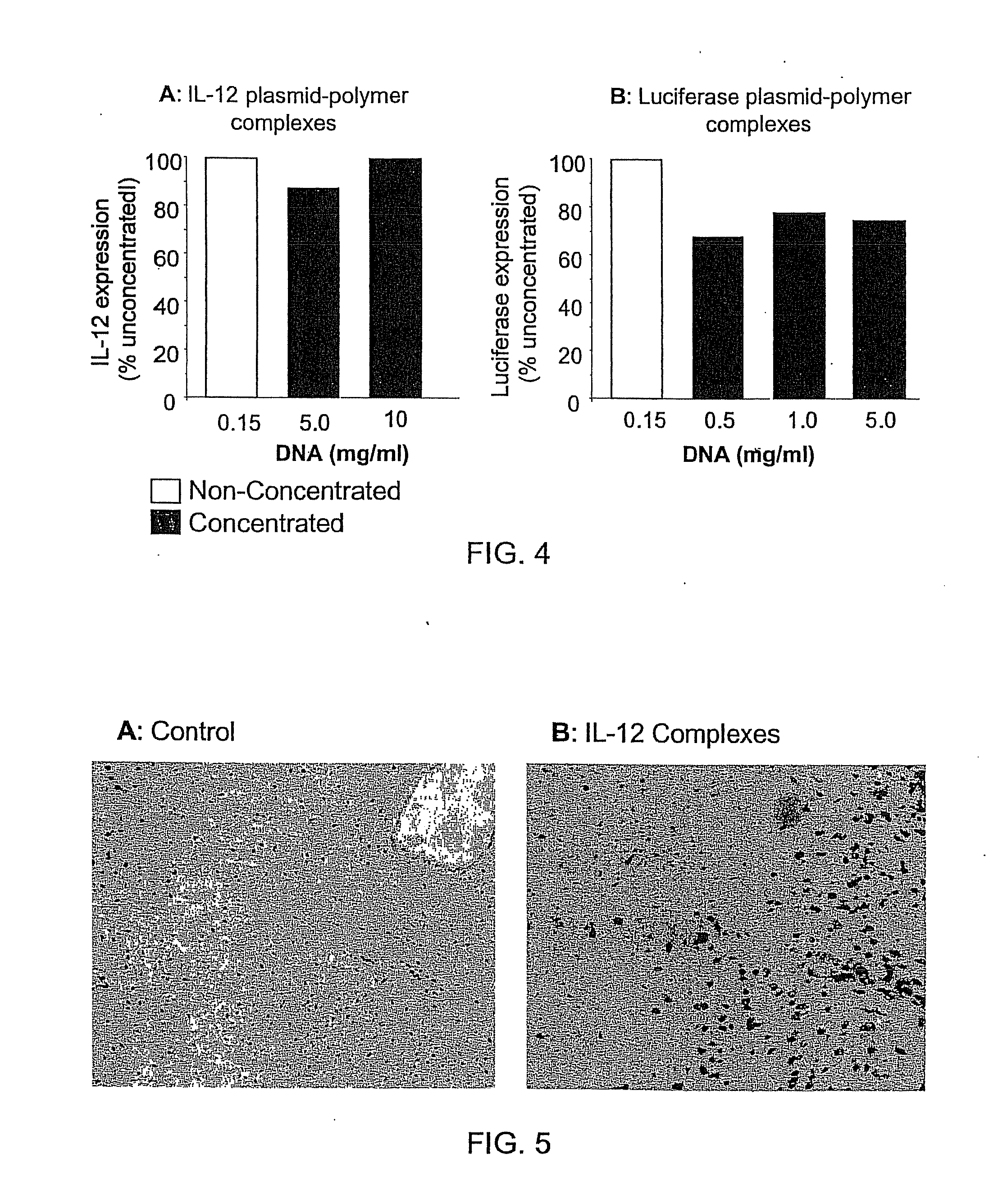
InactiveUS20090042829A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsCholesterolFiller Excipient
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
Modified polynucleotides for the production of secreted proteins
Owner:MODERNATX INC
RNA preparations comprising purified modified RNA for reprogramming cells
ActiveUS20110143397A1Promote growthArtificial cell constructsCell culture active agentsSingle strandSomatic cell
The present invention provides compositions and methods for reprogramming somatic cells using purified RNA preparations comprising single-strand mRNA encoding an iPS cell induction factor. The purified RNA preparations are preferably substantially free of RNA contaminant molecules that: i) would activate an immune response in the somatic cells, ii) would decrease expression of the single-stranded mRNA in the somatic cells, and / or iii) active RNA sensors in the somatic cells. In certain embodiments, the purified RNA preparations are substantially free of partial mRNAs, double-stranded RNAs, un-capped RNA molecules, and / or single-stranded run-on mRNAs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Modified nucleosides, nucleotides, and nucleic acids, and uses thereof
ActiveUS20130115272A1Reduce innate immune responseBiocidePeptide/protein ingredientsNucleotideModified nucleosides
The present disclosure provides modified nucleosides, nucleotides, and nucleic acids, and methods of using them.
Owner:MODERNATX INC
Engineered nucleic acids and methods of use thereof for non-human vertebrates
InactiveUS20140206752A1Sugar derivativesVector-based foreign material introductionCell biologyVertebrate
Provided are formulations, compositions, kits and methods for delivering biological moieties such as modified nucleic acids into cells to induce, reduce or modulate protein expression in non-human vertebrates.
Owner:MODERNA THERAPEUTICS INC
CNS targeting aav vectors and methods of use thereof
ActiveUS20130195801A1Widely distributedStable and nontoxic gene transferOrganic active ingredientsBiocideAdeno associate virusTransgene
Owner:UNIV OF MASSACHUSETTS
Method for vector delivery
InactiveUS20110293571A1Optimize volumeTotal volume of vector distribution in the brain wasBiocideOrganic active ingredientsNeuroscience
Owner:OXFORD BIOMEDICA (UK) LTD
PHARMACEUTICAL COMPOSITION CONTAINING A STABILISED mRNA OPTIMISED FOR TRANSLATION IN ITS CODING REGIONS
InactiveUS20100239608A1Improve efficiencyOvercome disadvantagesAntibacterial agentsOrganic active ingredientsCoding regionBiology
Owner:CUREVAC AG
Modified nucleosides, nucleotides, and nucleic acids, and uses thereof
The present disclosure provides modified nucleosides, nucleotides, and nucleic acids, and methods of using them.
Owner:MODERNA THERAPEUTICS INC
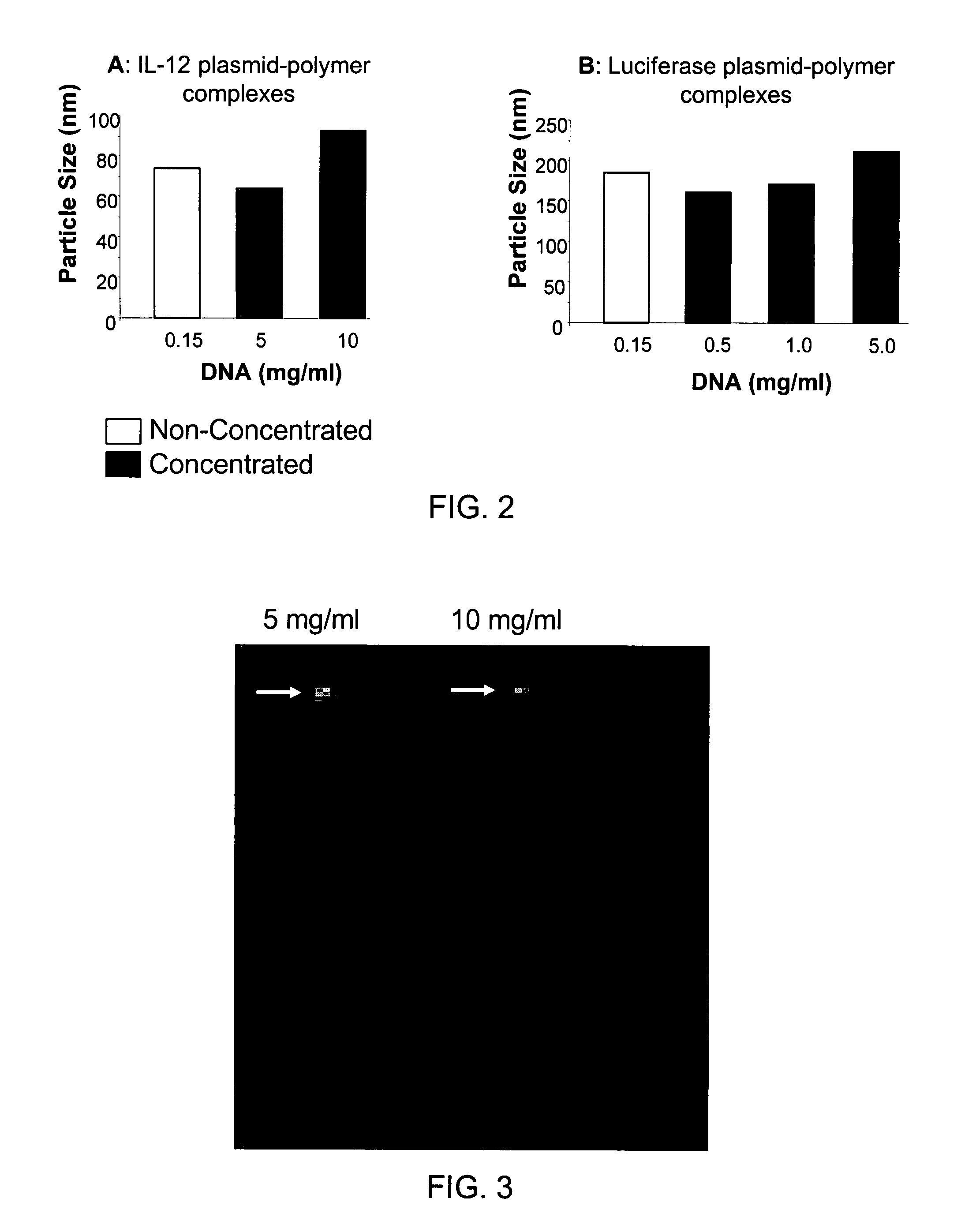
Composition, method of preparation & application of concentrated formulations of condensed nucleic acids with a cationic lipopolymer
UndeterminedUS20090042825A1Increase efficiency and dosing flexibilitySpecial deliveryPeptide/protein ingredientsFiller ExcipientCholesterol
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:EXPRESSION GENETICS INC
AAV vectors for gene delivery to the lung
ActiveUS7427396B2Efficient transductionDecreased immunoreactivityPowder deliveryBiocideGene deliveryVirosome
Owner:AVIGEN
Modified polynucleotides encoding cd28 molecule
Owner:MODERNA THERAPEUTICS INC
CNS targeting aav vectors and methods of use thereof
Owner:UNIV OF MASSACHUSETTS
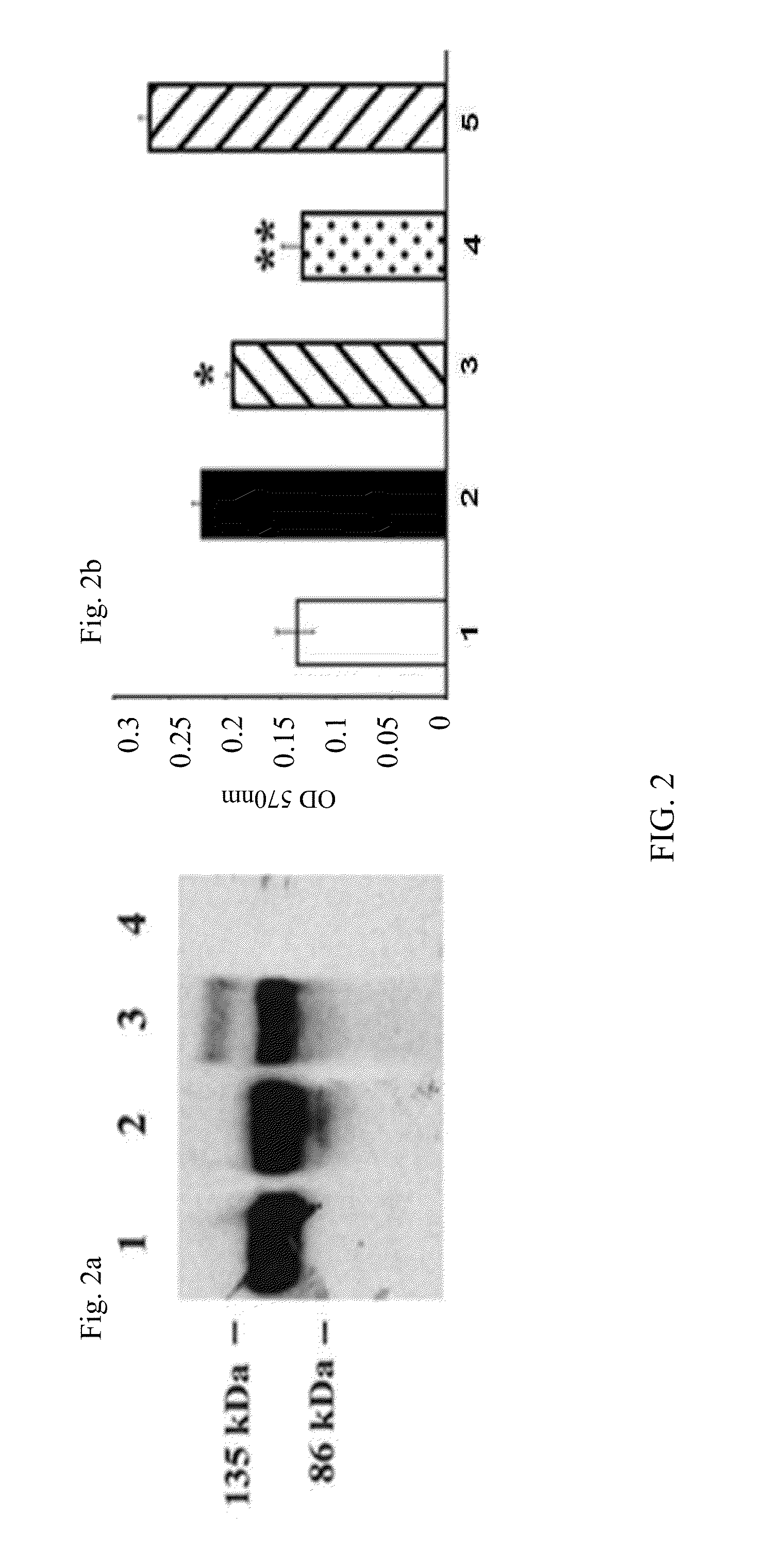
Methods for treating cancer using cytokine-expressing polynucleotides
InactiveUS7268120B1Improved in vivo polypeptide expressionMinimizing adverse side effectBiocideOrganic active ingredientsMammalSodium phosphates
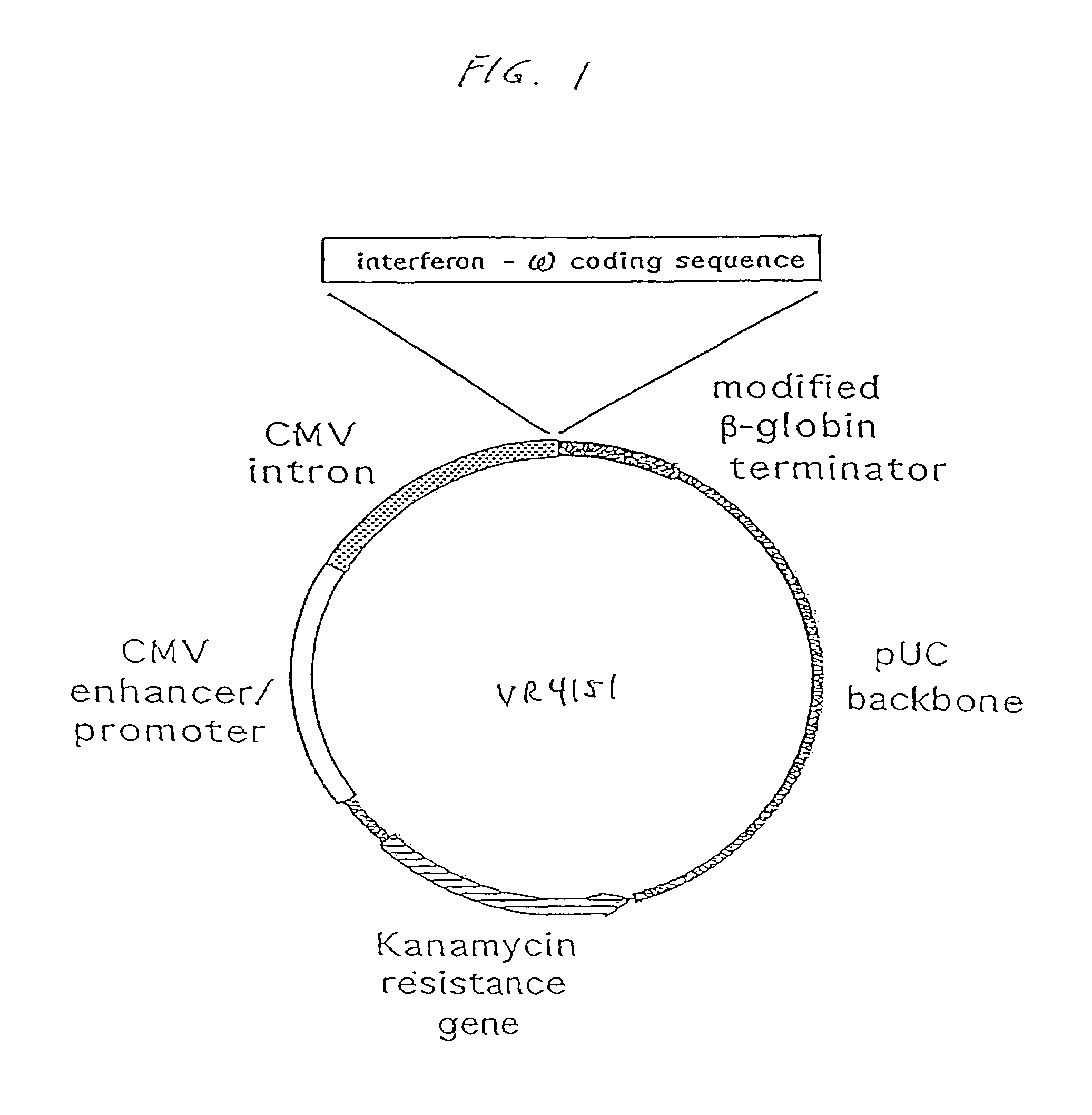
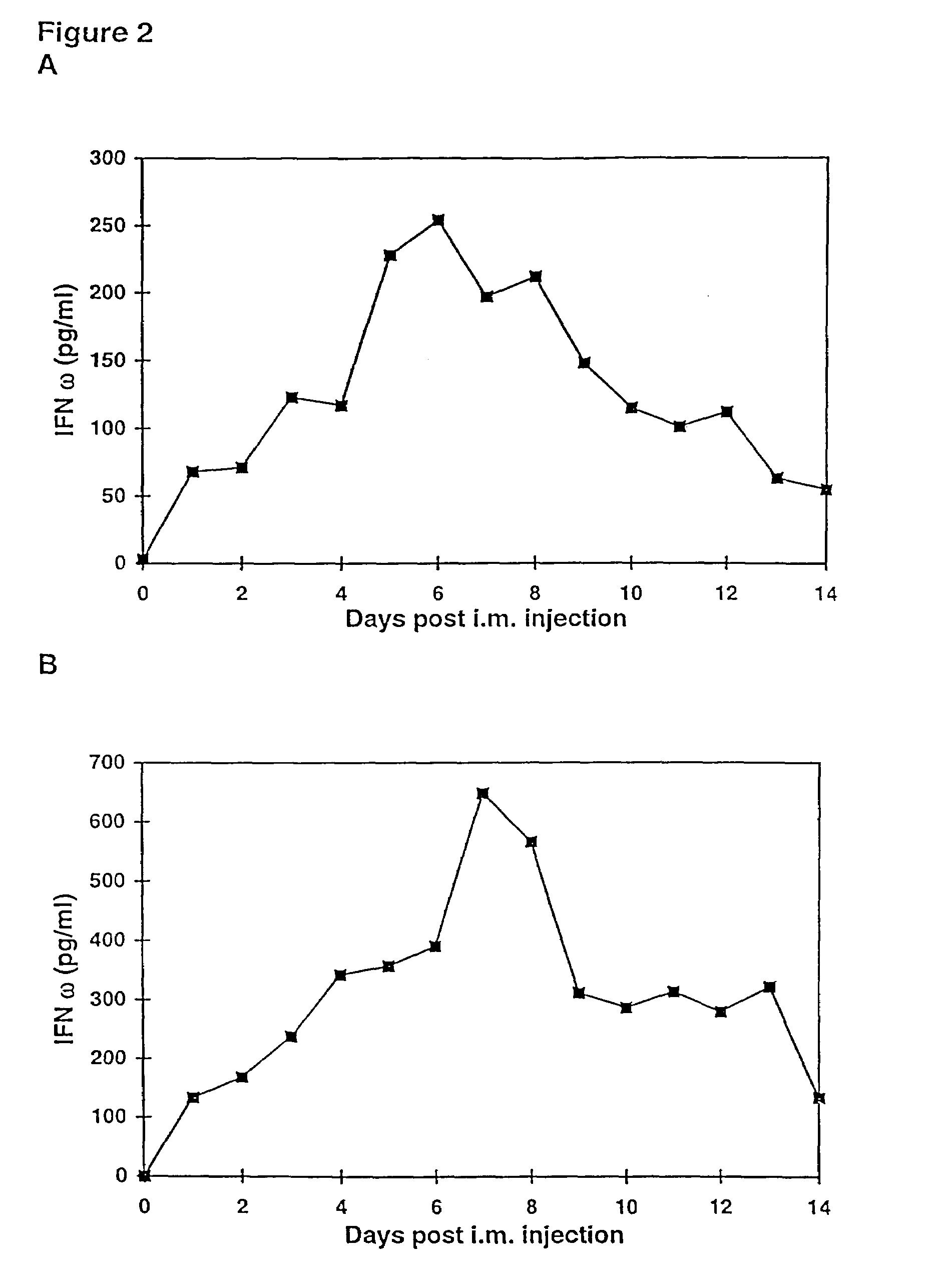
The present invention provides a pharmaceutical composition, comprising a non-infectious, non-integrating polynucleotide construct comprising a polynucleotide encoding an interferon ω and one or more cationic compounds. The present invention also provides methods of treating cancer in a mammal, comprising administering into a muscle of the mammal a non-infectious, non-integrating DNA polynucleotide construct comprising a polynucleotide encoding a cytokine. In addition, the present invention also relates to the methodology for selective transfection of malignant cells with polynucleotides expressing therapeutic or prophylactic molecules in intra-cavity tumor bearing mammals. More specifically, the present invention provides a methodology for the suppression of an intra-cavity dissemination of malignant cells, such as intraperitoneal dissemination. Furthermore, the invention relates to compositions and methods to deliver polynucleotides encoding polypeptides to vertebrate cells in vivo, where the composition comprises an aqueous solution of sodium phosphate.
Owner:VICAL INC
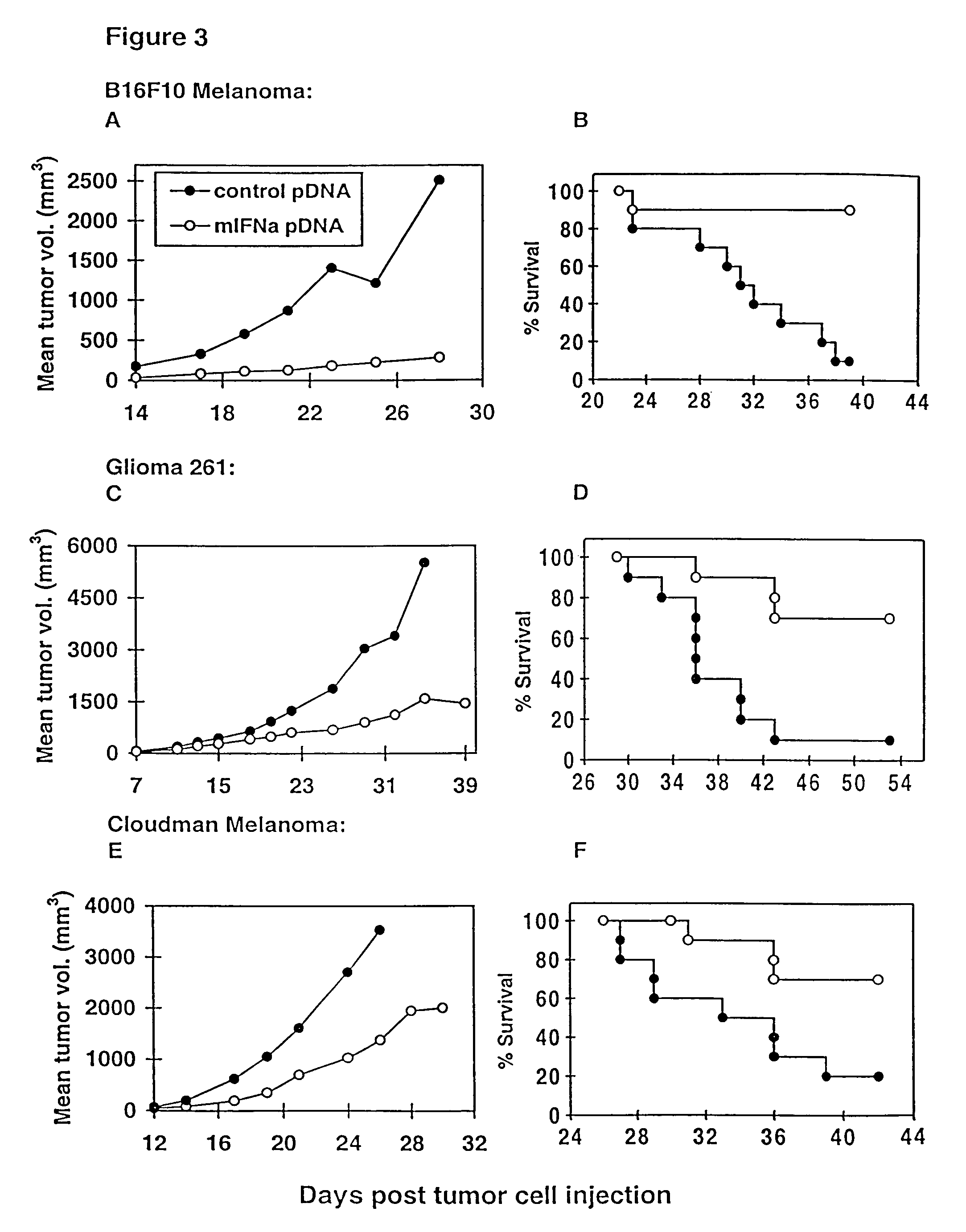
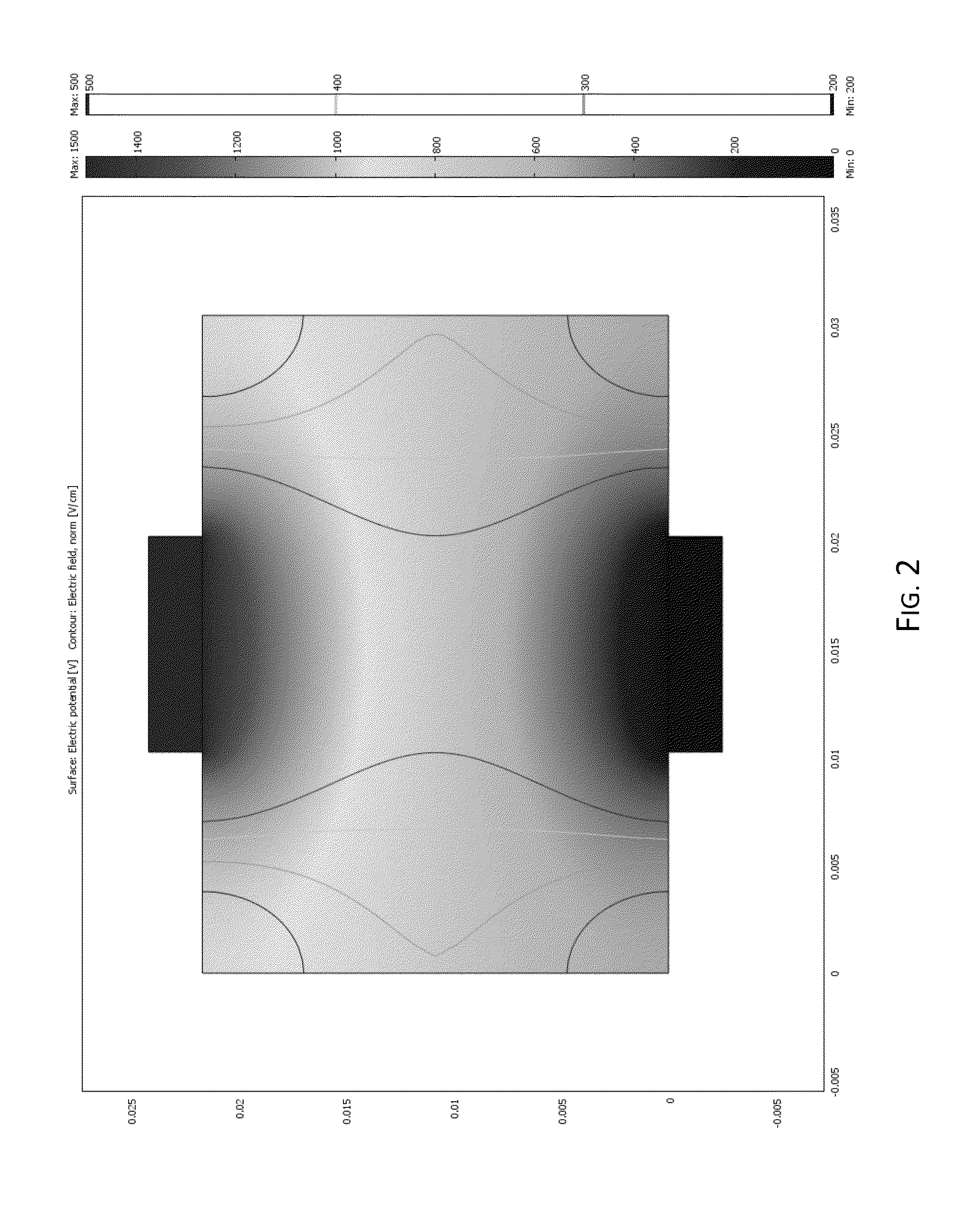
Irreversible electroporation using tissue vasculature to treat aberrant cell masses or create tissue scaffolds
ActiveUS20130253415A1Easy to storeImprove breathabilityElectrotherapyIntravenous devicesDiseaseNatural source
The present invention relates to the field of medical treatment of diseases and disorders, as well as the field of biomedical engineering. Embodiments of the invention relate to the delivery of Irreversible Electroporation (IRE) through the vasculature of organs to treat tumors embedded deep within the tissue or organ, or to decellularize organs to produce a scaffold from existing animal tissue with the existing vasculature intact. In particular, methods of administering non-thermal irreversible electroporation (IRE) in vivo are provided for the treatment of tumors located in vascularized tissues and organs. Embodiments of the invention further provide scaffolds and tissues from natural sources created using IRE ex vivo to remove cellular debris, maximize recellularization potential, and minimize foreign body immune response. The engineered tissues can be used in methods of treating subjects, such as those in need of tissue replacement or augmentation.
Owner:VIRGINIA TECH INTPROP INC
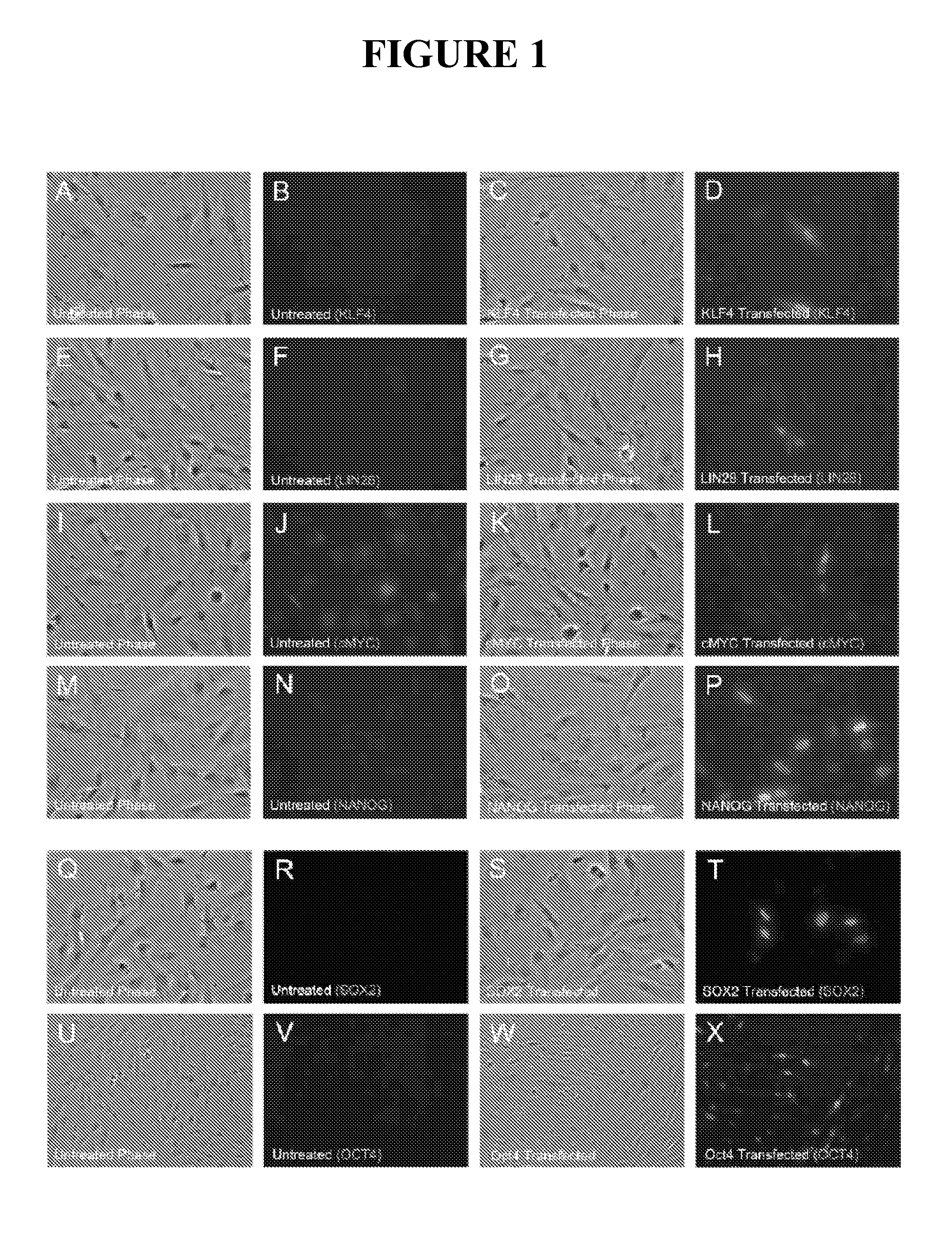
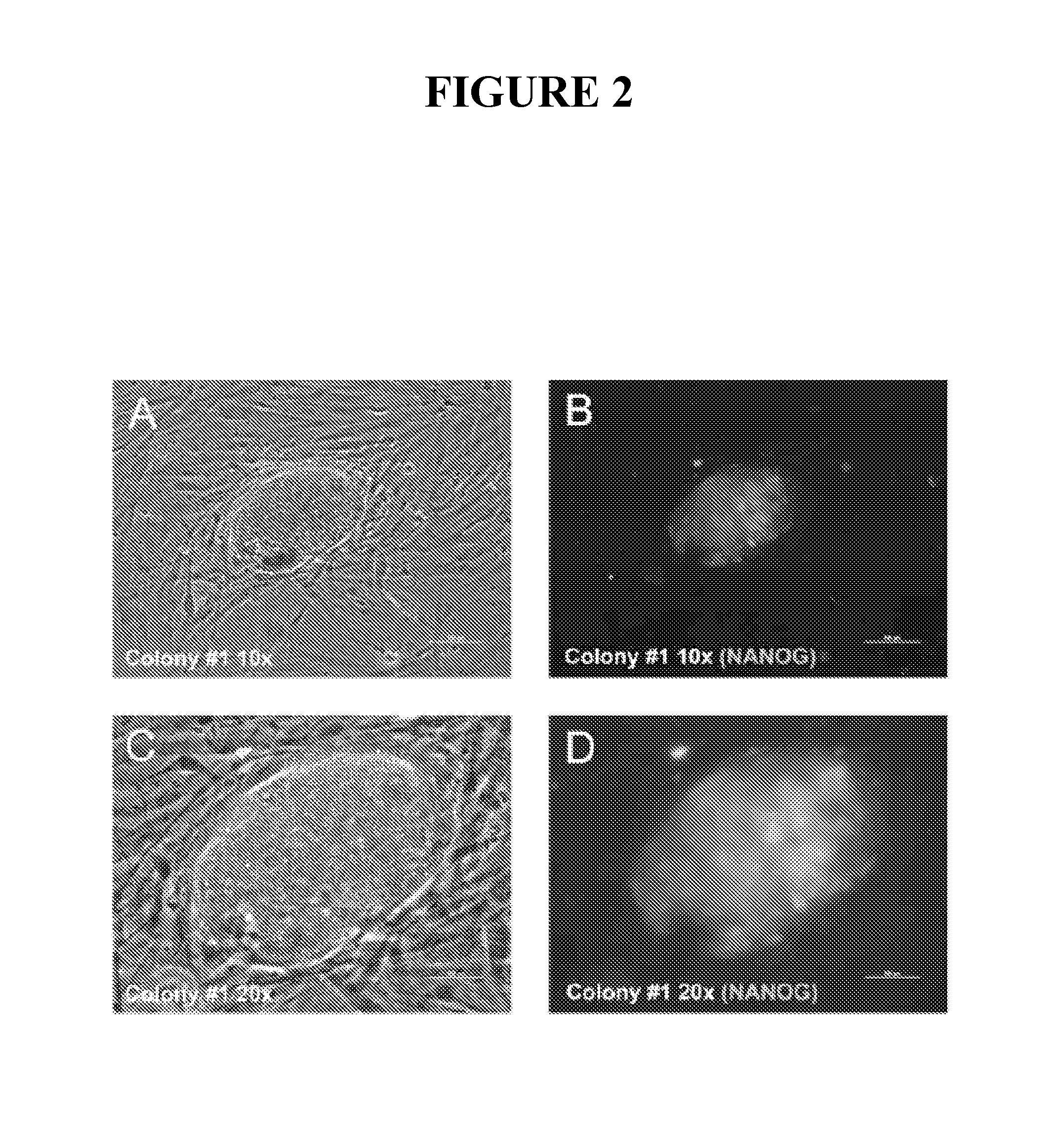
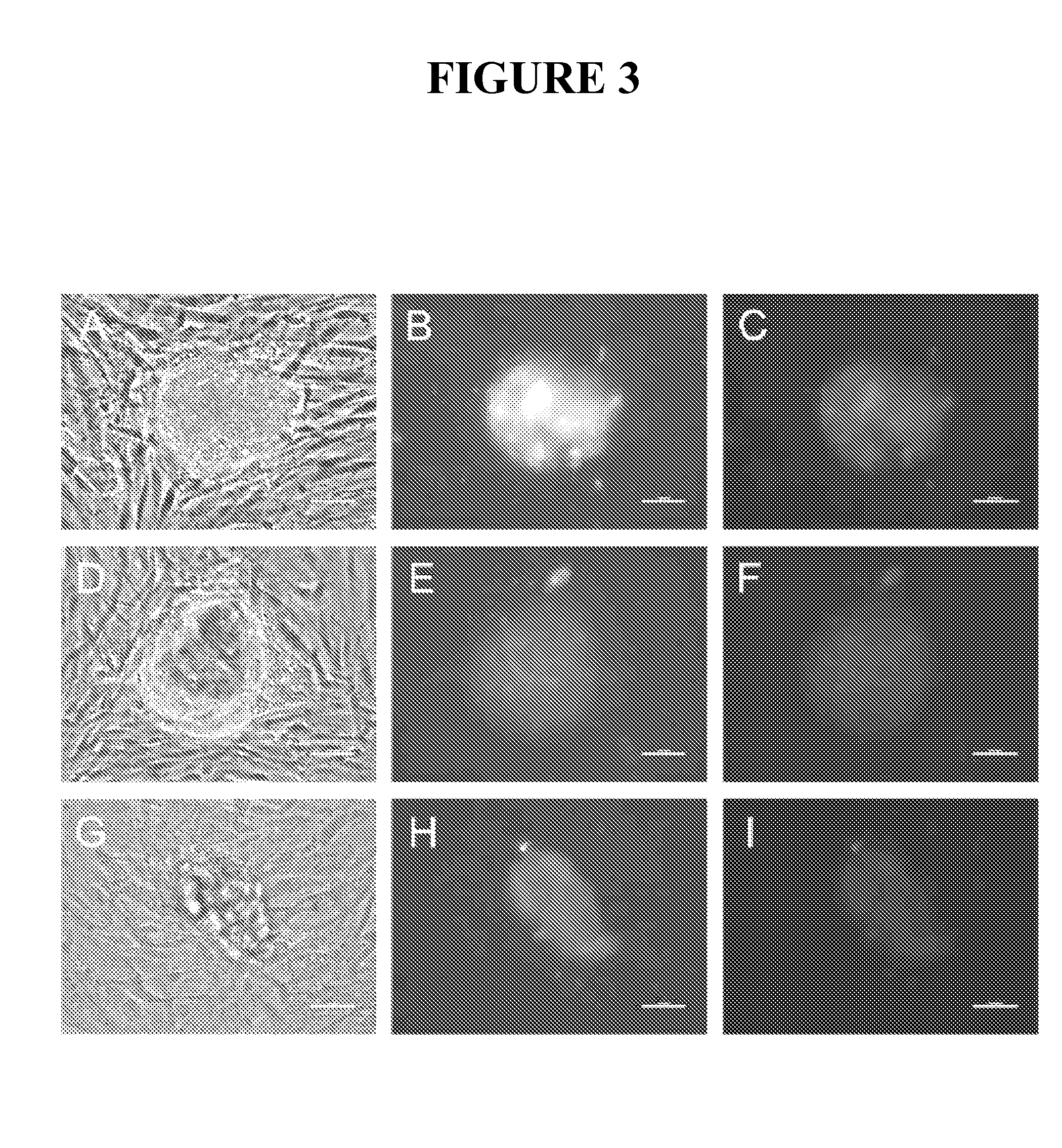
Compositions and methods for reprogramming eukaryotic cells
The present invention relates to methods for changing the state of differentiation of a eukaryotic cell, the methods comprising introducing mRNA encoding one or more reprogramming factors into a cell and maintaining the cell under conditions wherein the cell is viable and the mRNA that is introduced into the cell is expressed in sufficient amount and for sufficient time to generate a cell that exhibits a changed state of differentiation compared to the cell into which the mRNA was introduced, and compositions therefor. For example, the present invention provides mRNA molecules and methods for their use to reprogram human somatic cells into pluripotent stem cells.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Convection-enhanced delivery of AAV vectors
InactiveUS20020141980A1Efficient deliveryEfficient expressionBiocideOrganic active ingredientsDiseaseConvection-Enhanced Delivery
Methods of delivering viral vectors, particularly recombinant AAV virions, to the CNS are provided. Also provided are methods of treating Parkinson's Disease.
Owner:GENZYME CORP
Nucleic Acid-Lipopolymer Compositions
ActiveUS20130065942A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsFiller ExcipientCholesterol
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
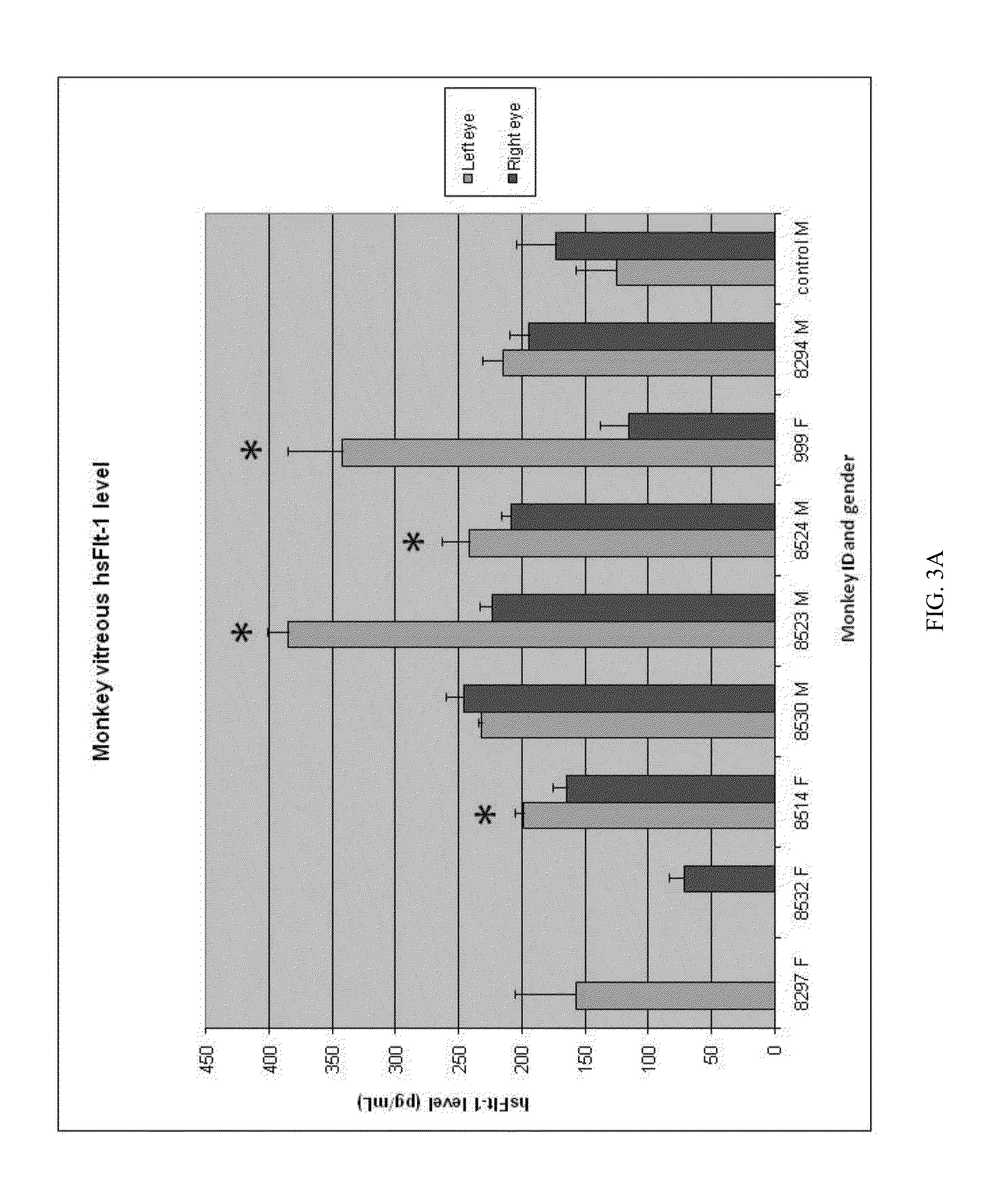
Treatment of AMD using aav sflt-1
InactiveUS20130323302A1Compounds screening/testingOrganic active ingredientsOcular neovascularizationMedicine
The present disclosure provides compositions and methods for the prevention or treatment of ocular neovascularization, such as AMD, in a human subject, by administering subretinally a pharmaceutical composition comprising a pharmaceutically effective amount of a vector comprising a nucleic acid encoding soluble Fms-related tyrosine kinase-1 (sFlt-1) protein to the human subject.
Owner:AVALANCHE AUSTRALIA
Compositions and methods for modulation of smn2 splicing in a subject
ActiveUS20120190728A1Increase inclusivenessOrganic active ingredientsSplicing alterationDiseasePharmaceutical drug
Disclosed herein are compounds, compositions and methods for modulating splicing of SMN2 mRNA in a subject. Also provided are uses of disclosed compounds and compositions in the manufacture of a medicament for treatment of diseases and disorders, including spinal muscular atrophy.
Owner:BIOGEN MA INC +1
Compositions and methods for reprogramming eukaryotic cells
The present invention relates to methods for changing the state of differentiation of a eukaryotic cell, the methods comprising introducing mRNA encoding one or more reprogramming factors into a cell and maintaining the cell under conditions wherein the cell is viable and the mRNA that is introduced into the cell is expressed in sufficient amount and for sufficient time to generate a cell that exhibits a changed state of differentiation compared to the cell into which the mRNA was introduced, and compositions therefor. For example, the present invention provides mRNA molecules and methods for their use to reprogram human somatic cells into pluripotent stem cells.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
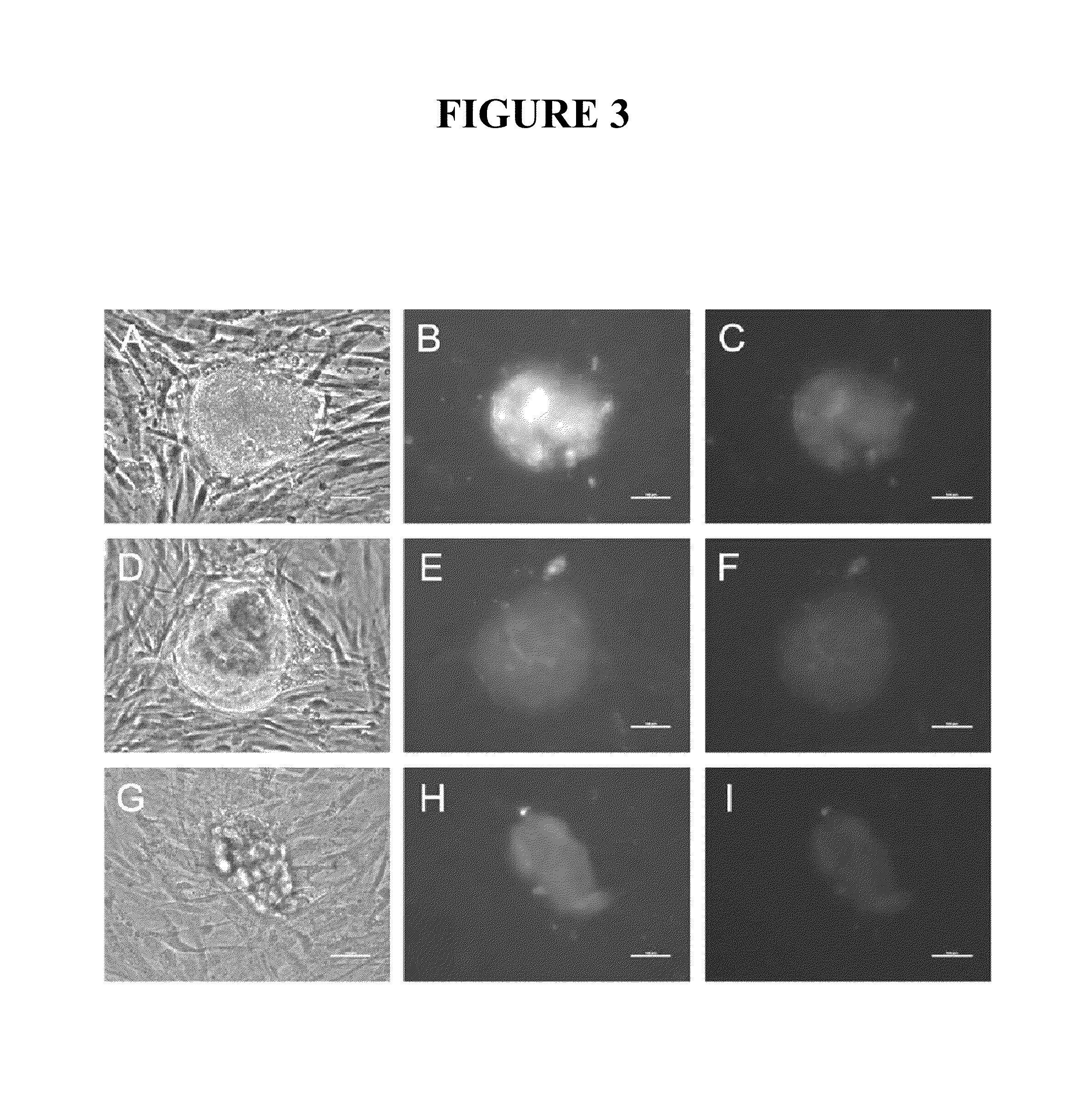
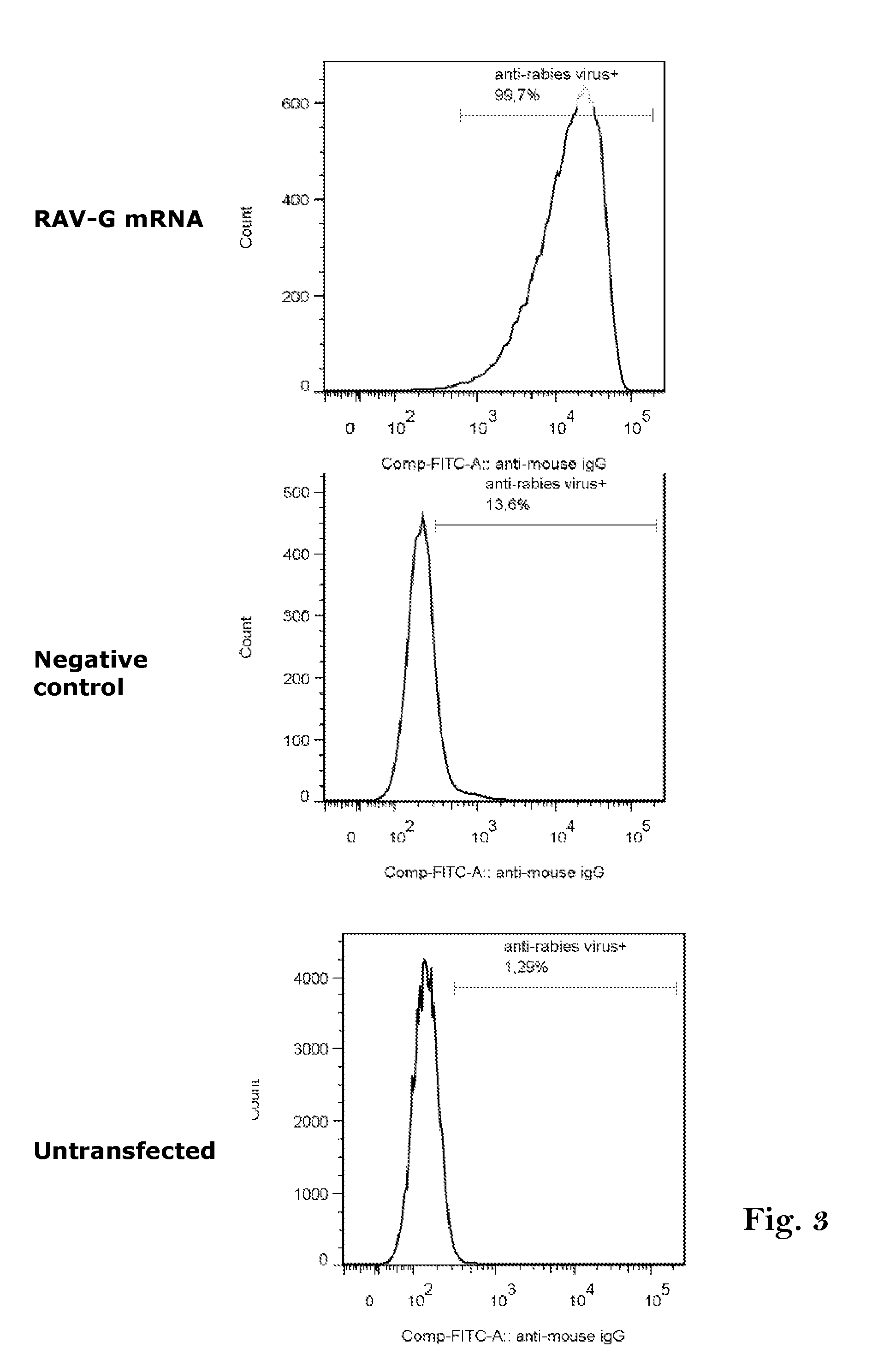
Rabies vaccine
ActiveUS20160166711A1Easy to identifyFaster and strong attackSsRNA viruses negative-senseVirus peptidesCoding regionRabies vaccine
The present invention relates to an mRNA sequence, comprising a coding region, encoding at least one antigenic peptide or protein of Rabies virus or a fragment, variant or derivative thereof. Additionally the present invention relates to a composition comprising a plurality of mRNA sequences comprising a coding region, encoding at least one antigenic peptide or protein of Rabies virus or a fragment, variant or derivative thereof.Furthermore it also discloses the use of the mRNA sequence or the composition comprising a plurality of mRNA sequences for the preparation of a pharmaceutical composition, especially a vaccine, e.g. for use in the prophylaxis or treatment of Rabies virus infections. The present invention further describes a method of treatment or prophylaxis of rabies using the mRNA sequence.
Owner:CUREVAC AG
Recombinant adeno-associated virus delivery of alpha-sarcoglycan polynucleotides
ActiveUS9434928B2High transduction efficiencyIncrease volumePeptide/protein ingredientsOther blood circulation devicesFhit genePolynucleotide
Owner:NATIONWIDE CHILDRENS HOSPITAL
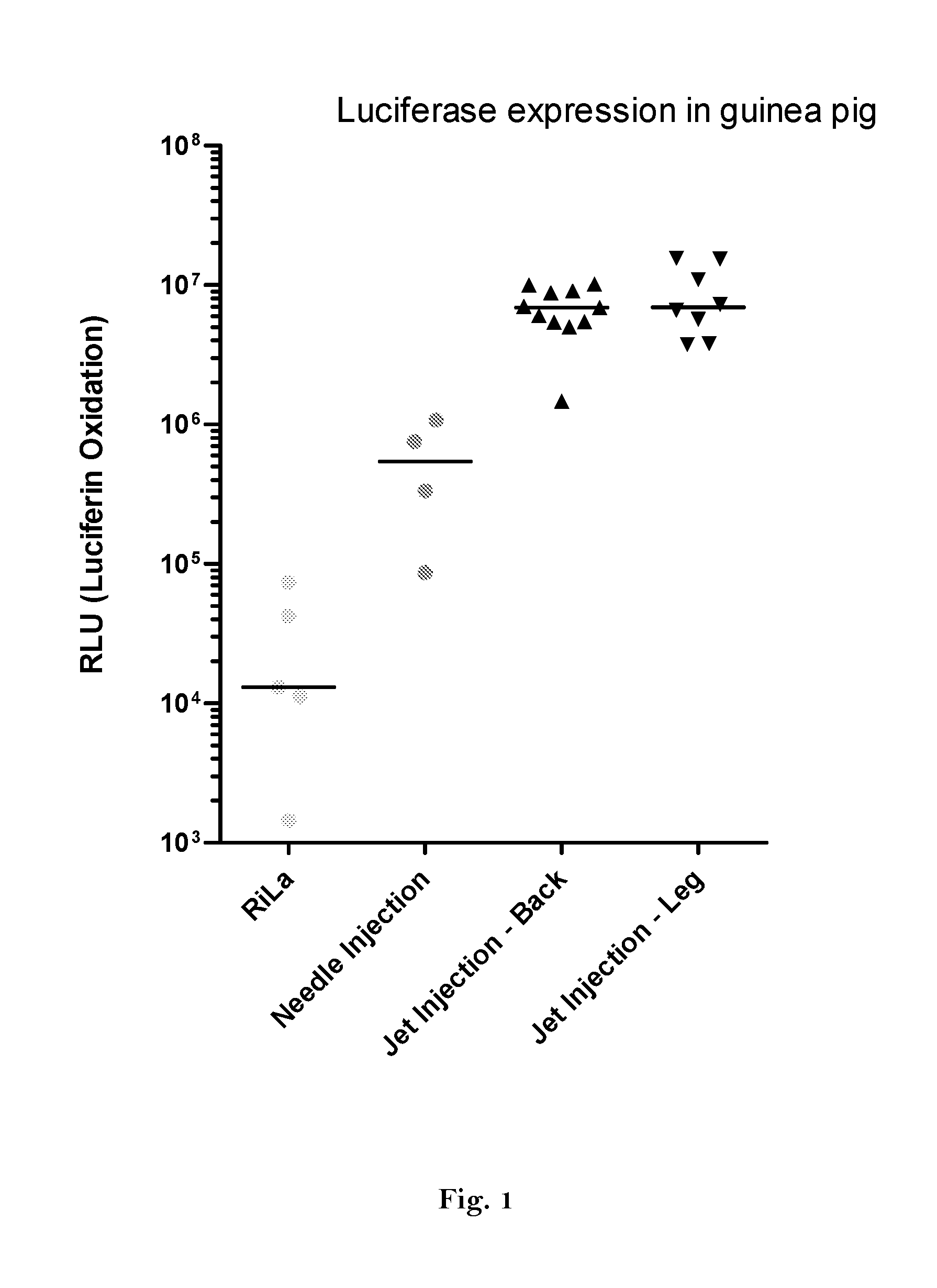
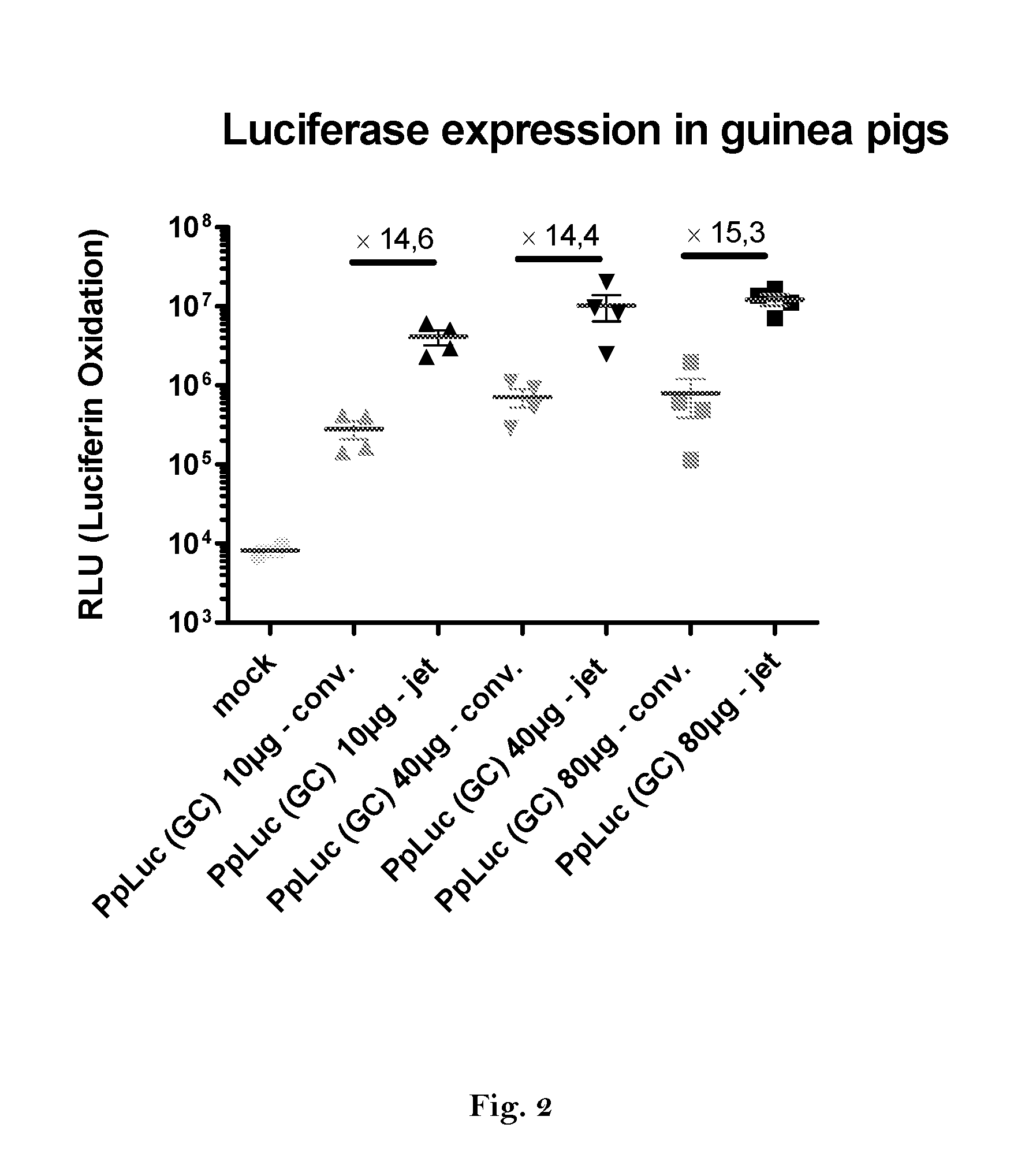
Method for increasing expression of rna-encoded proteins
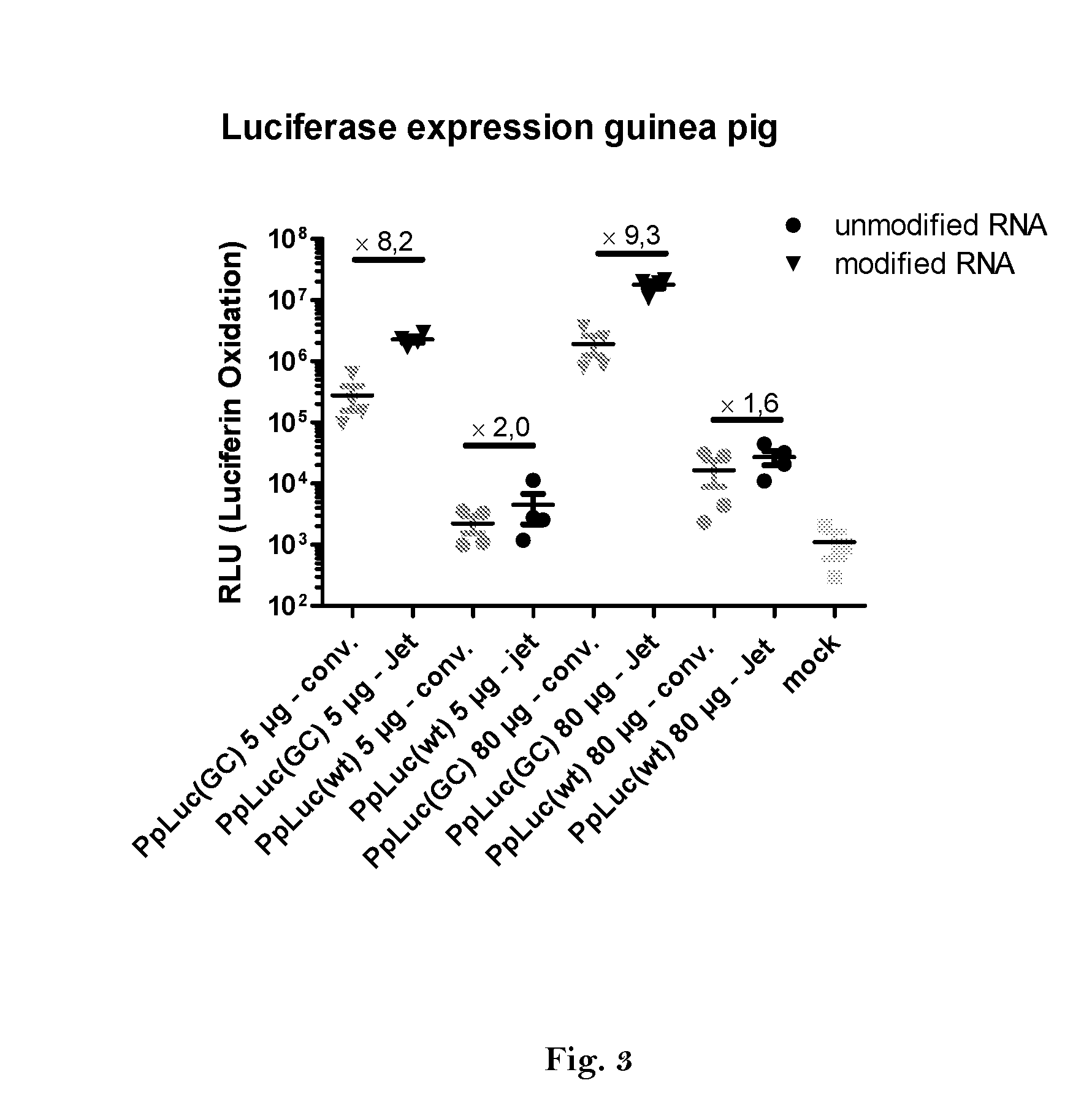
ActiveUS20160166710A1High expressionImprove securitySsRNA viruses negative-sensePeptide/protein ingredientsOpen reading frameJet injection
The invention relates to an RNA comprising at least one open reading frame (ORF) and comprising at least one modification, which increases the expression of the encoded peptide or protein. Furthermore, the invention relates to the medical use of such a modified RNA administered to a subject by jet injection. The invention relates further to a pharmaceutical composition and to a kit of parts comprising said modified RNA for administration by jet injection, preferably for use in the field of gene therapy and / or genetic vaccination. Additionally, the invention relates to a method for enhancing the (localized) expression of RNA-encoded peptides or proteins in the dermis or muscle (of a mammal) comprising administering the modified RNA by jet injection. And finally, the invention relates to a method of treatment comprising administering the modified RNA by jet injection to a subject in need thereof.
Owner:CUREVAC SE
Methods of administering vectors to synaptically connected neurons
InactiveUS20050032219A1Specific deliveryExcessive deliveryBiocidePeptide/protein ingredientsTreatment effectPrimary motor neuron
The present invention relates generally to efficient delivery of viral vectors to cells of the CNS, particularly useful in the treatment of neurodegenerative disorders and motor neuron diseases. The invention involves selecting a first population and a second population of synaptically connected neurons, wherein a therapeutic polypeptide is to be expressed in said second population of neurons; and administering rAAV virions comprising a therapeutic gene to said first subpopulation of neurons of said subject such that the rAAV virions are transported across a synapse between synaptically connected neurons. In another aspect the present invention also comprises the use of rAAV virions carrying a transgene in the preparation of a medicament for the treatment of a disease in a subject, wherein a first population and a second population of synaptically connected neurons are selected and a therapeutic polypeptide is to be expressed in said second population of neurons; and a medicament comprising recombinant adeno-associated virus (rAAV) virions is delivered to said first population of neurons of the subject, wherein said virions comprise a nucleic acid sequence that is expressible in transduced cells to provide a therapeutic effect in the subject, and wherein said rAAV virions are capable of transducing a synaptically connected neurons.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
RNA preparations comprising purified modified RNA for reprogramming cells
ActiveUS9012219B2Promote growthMicrobiological testing/measurementArtificial cell constructsGeneticsSingle strand
The present invention provides compositions and methods for reprogramming somatic cells using purified RNA preparations comprising single-strand mRNA encoding an iPS cell induction factor. The purified RNA preparations are preferably substantially free of RNA contaminant molecules that: i) would activate an immune response in the somatic cells, ii) would decrease expression of the single-stranded mRNA in the somatic cells, and / or iii) active RNA sensors in the somatic cells. In certain embodiments, the purified RNA preparations are substantially free of partial mRNAs, double-stranded RNAs, un-capped RNA molecules, and / or single-stranded run-on mRNAs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Magnetic pole matrices useful for tissue engineering and treatment of disease
A magnetic pole matrix chip facilitating the grinding of magnetic particles carrying matter effective for treating a disease or promoting tissue engineering to a disease site or a tissue engineering site, respectively
Owner:STEINBEIS TRANSFERZENT FUR HERZ KREISLAUFFORSCHUNG
Methods and compositions for targeting agents into and across the blood-brain barrier
This invention relates to modified nucleic acid compositions encoding therapeutic polypeptides and methods of producing the therapeutic polypeptides in cells.
Owner:MODERNATX INC
Gene or drug delivery system
The present invention includes compositions and methods for delivering one or more active agents in vivo by contacting a target organ or tissue with a microbubble encapsulated active agent comprising a neutrally charged lipid microbubble loaded with cationic liposomes comprising one or more active agents and selectively releasing the active agents at the target by exposing the microbubble at the target with ultrasound, wherein the active agents remain protected in the microbubble until selectively release at the target.
Owner:BAYLOR RES INST +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com