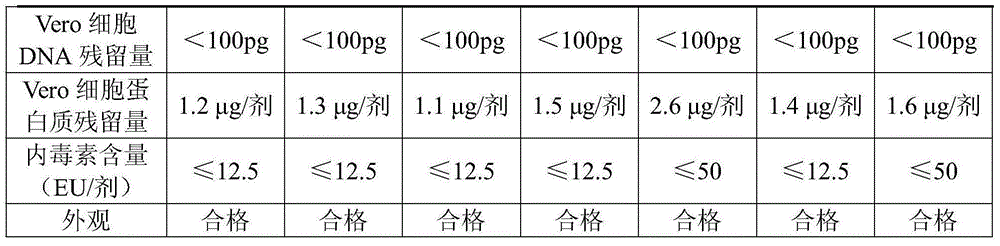
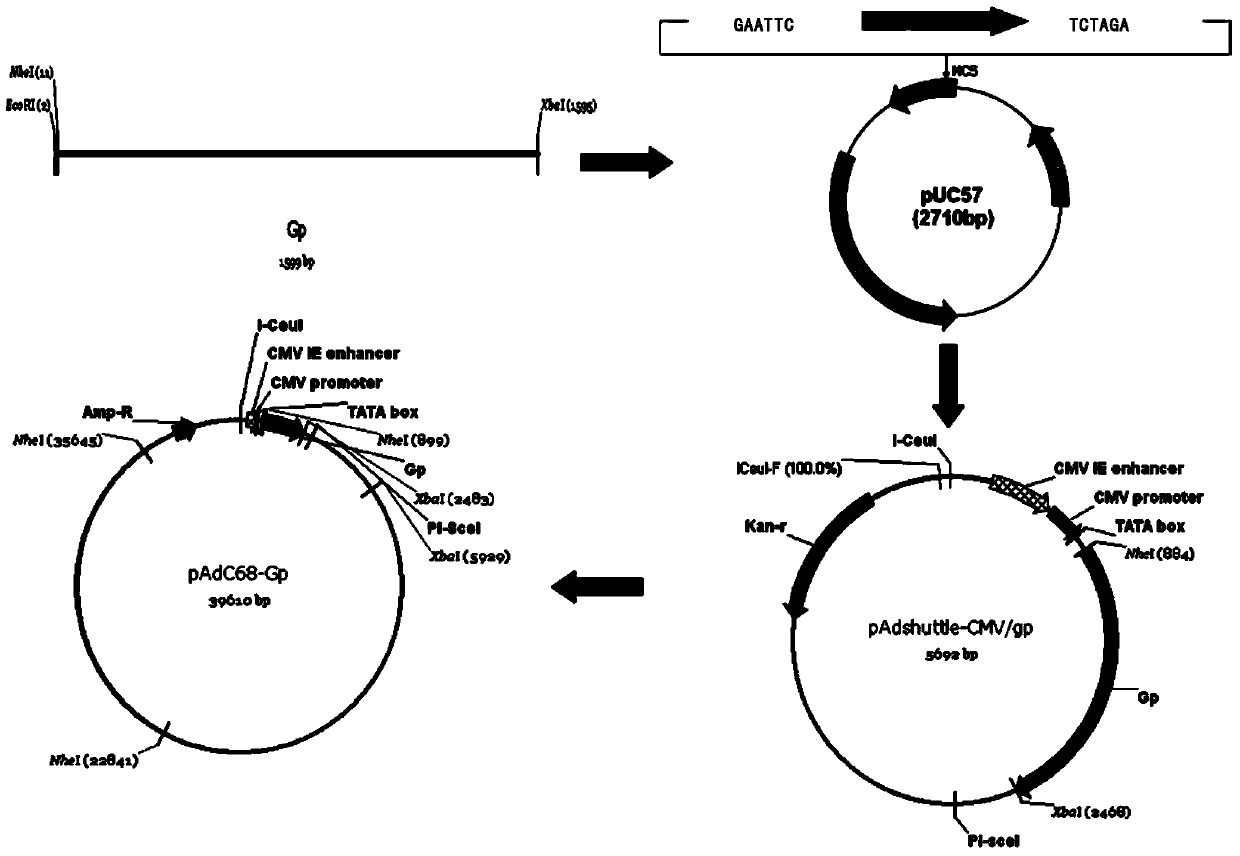
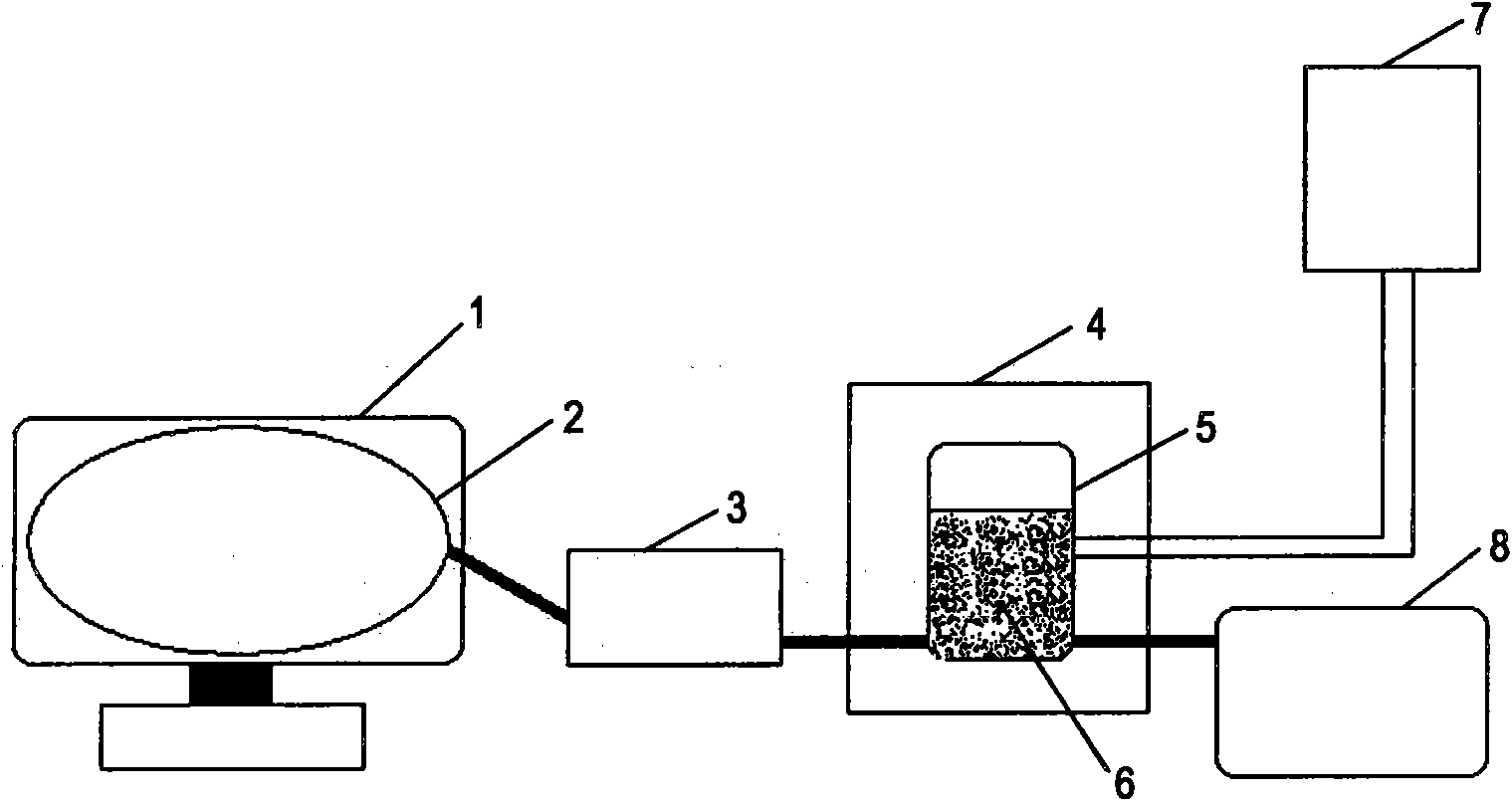
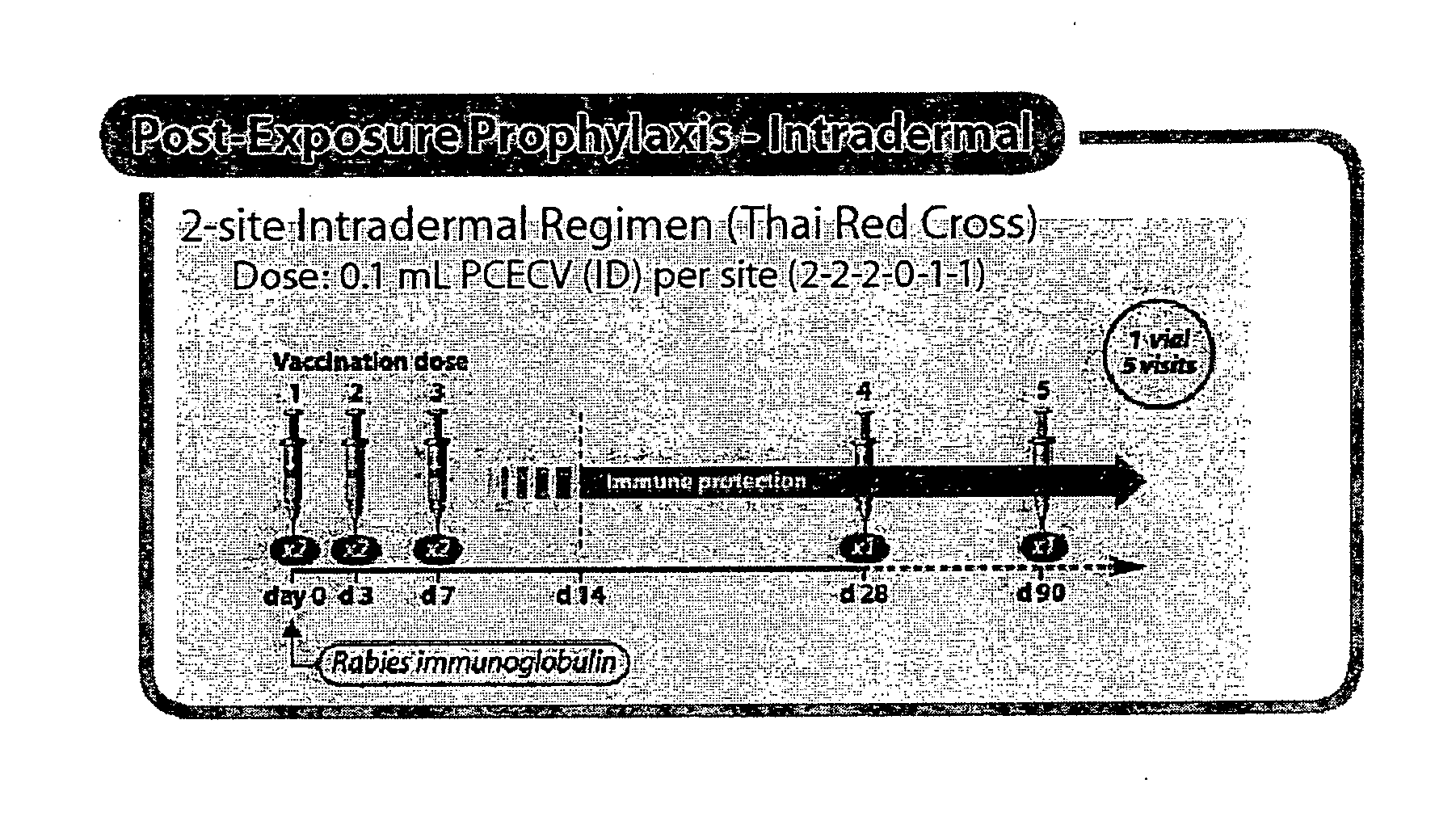
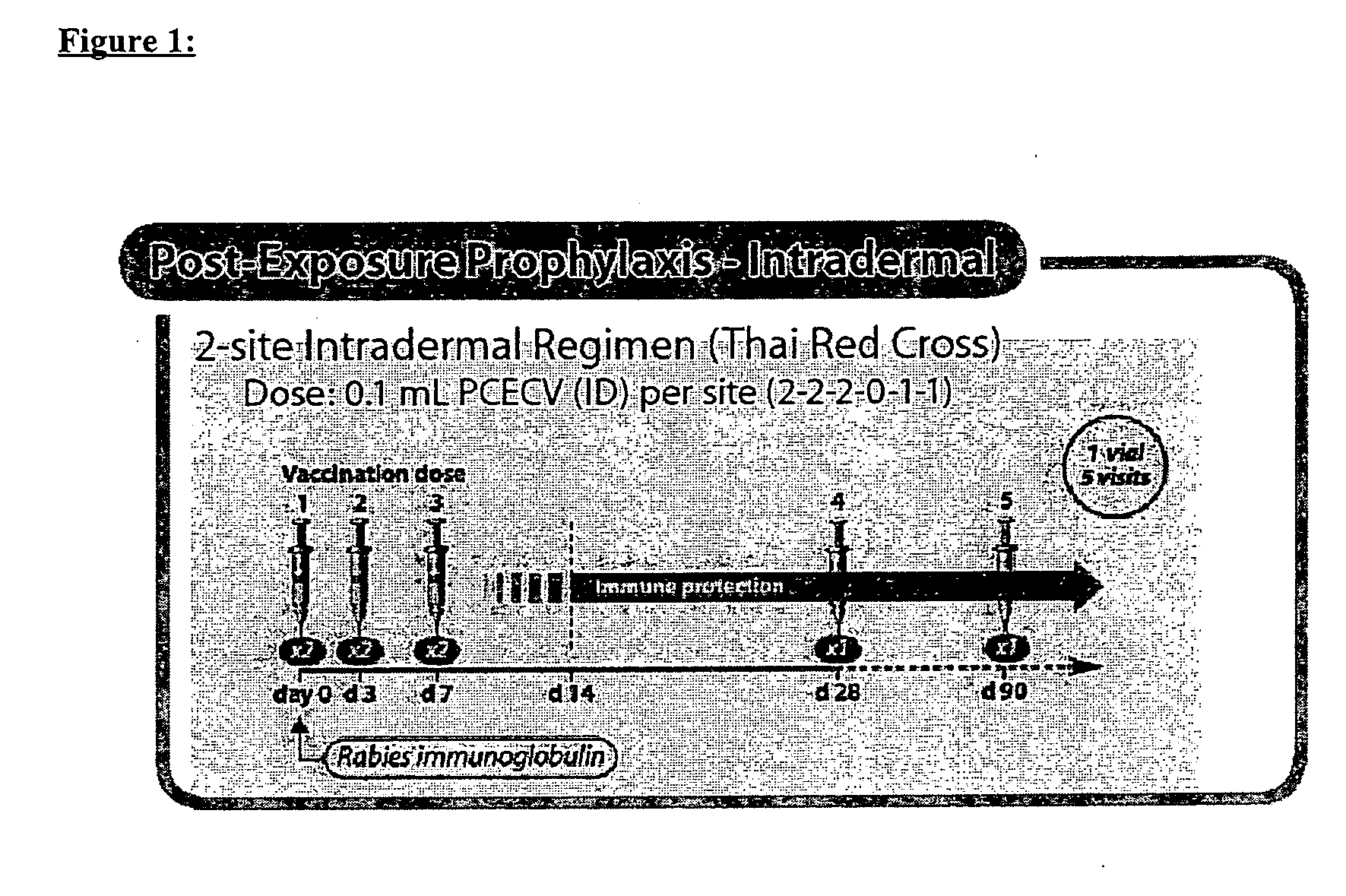
Patents
Literature
188 results about "Rabies vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rabies vaccine is a vaccine used to prevent rabies. There are a number of vaccines available that are both safe and effective. They can be used to prevent rabies before and for a period of time after exposure to the virus such as by a dog or bat bite. The immunity that develops is long lasting after a full course. Doses are usually given by injection into the skin or muscle. After exposure vaccination is typically used along with rabies immunoglobulin. It is recommended that those who are at high risk of exposure be vaccinated before potential exposure. Vaccines are effective in humans and other animals. Vaccinating dogs is very effective in preventing the spread of rabies to humans.
Rabies vaccine
ActiveUS20160166711A1Easy to identifyFaster and strong attackSsRNA viruses negative-senseVirus peptidesCoding regionRabies vaccine
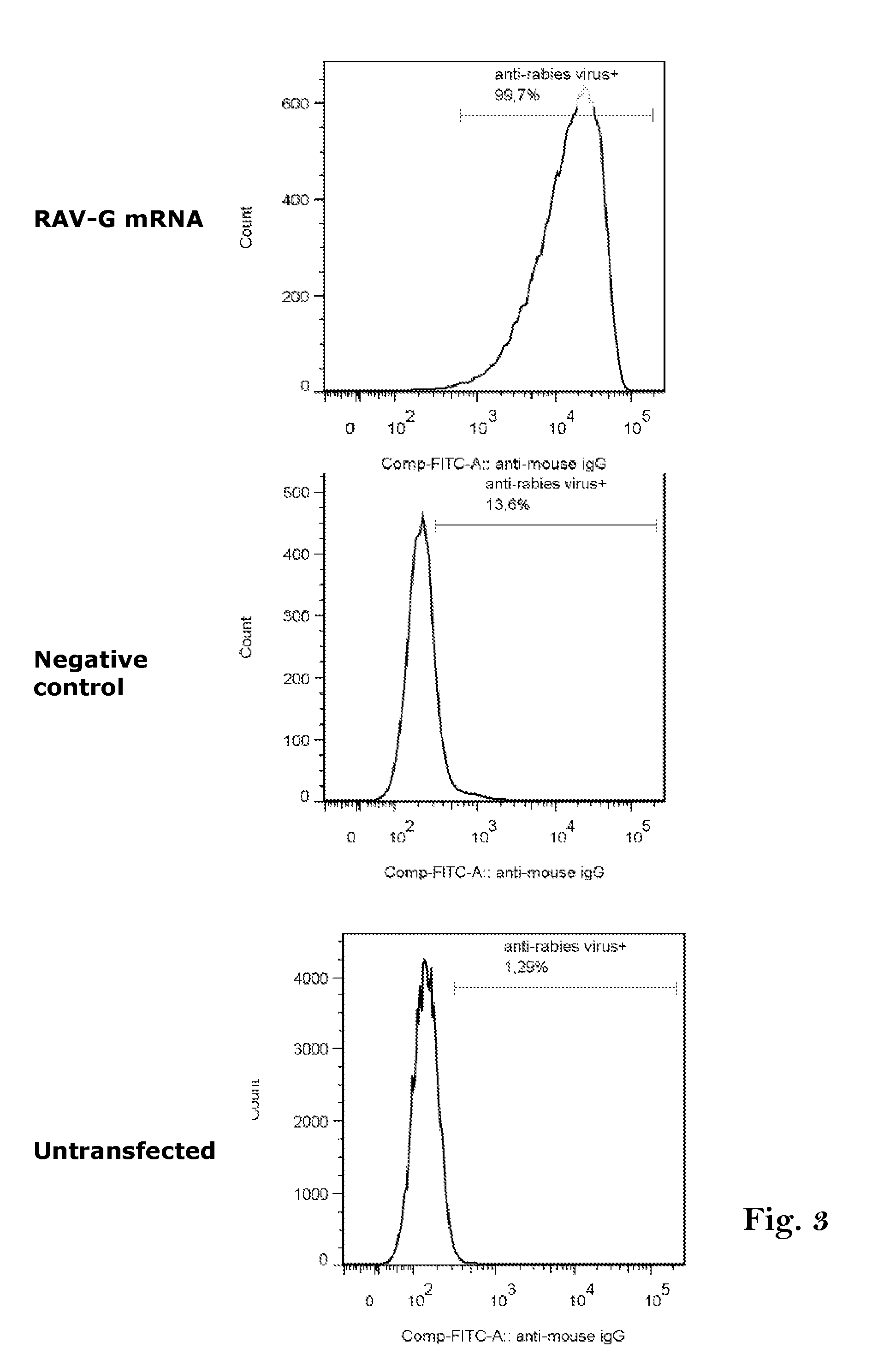
The present invention relates to an mRNA sequence, comprising a coding region, encoding at least one antigenic peptide or protein of Rabies virus or a fragment, variant or derivative thereof. Additionally the present invention relates to a composition comprising a plurality of mRNA sequences comprising a coding region, encoding at least one antigenic peptide or protein of Rabies virus or a fragment, variant or derivative thereof.Furthermore it also discloses the use of the mRNA sequence or the composition comprising a plurality of mRNA sequences for the preparation of a pharmaceutical composition, especially a vaccine, e.g. for use in the prophylaxis or treatment of Rabies virus infections. The present invention further describes a method of treatment or prophylaxis of rabies using the mRNA sequence.
Owner:CUREVAC AG
Method for purifying recombinant human serum albumin protein and application thereof
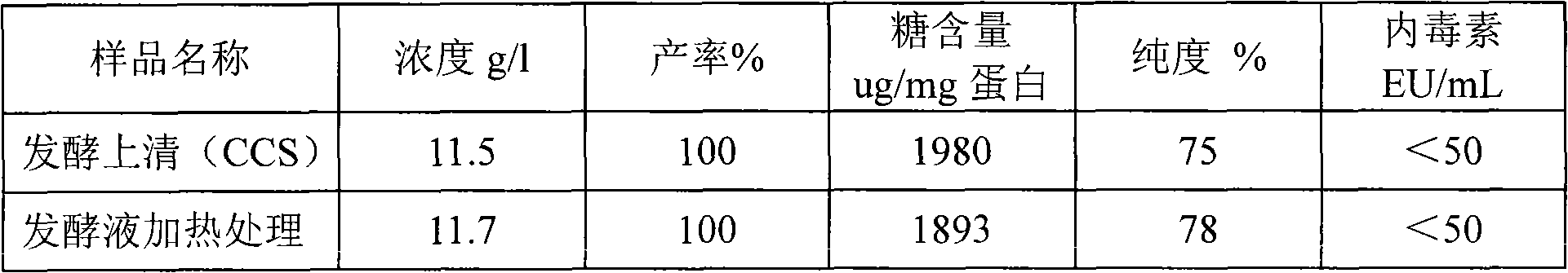
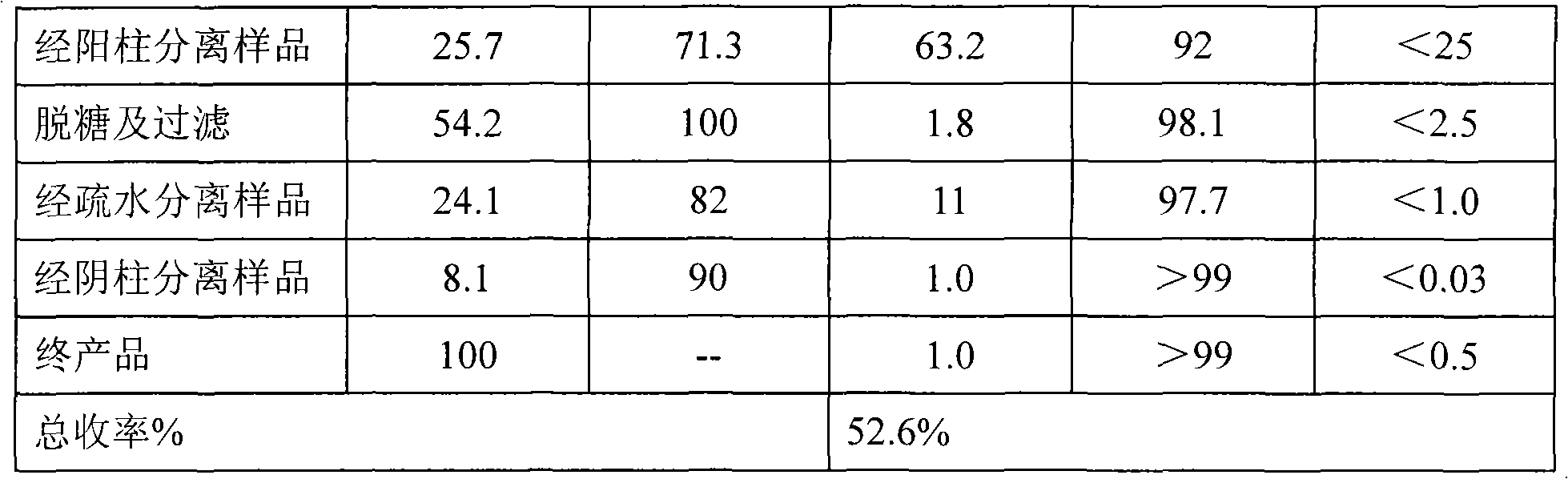
The present invention relates to a method for purifying recombinant human serum albumin (rHSA) protein. The method comprises the following steps: fermented liquid containing rHSA is processed by a ceramic membrane, supernatant liquid is orderly purified by high salt cation exchange chromatography, hydrophobic layer exchange chromatography and weak anion exchange chromatography, and purified rHSA is obtained. The present invention is characterized in that solution processed by high salt cation exchange chromatography is processed by borate and then filtered by hollow fibers. The rHSA obtained can be used for producing vaccines for humans against viruses with a cell culture method, particularly rabies vaccines.
Owner:NCPC NEW DRUG RES & DEV
Method for producing rabies viruses by suspension culture of BHK21 cells
InactiveCN101851608AHigh titerQuality improvementMicroorganism based processesViruses/bacteriophagesAutomatic controlCell culture media
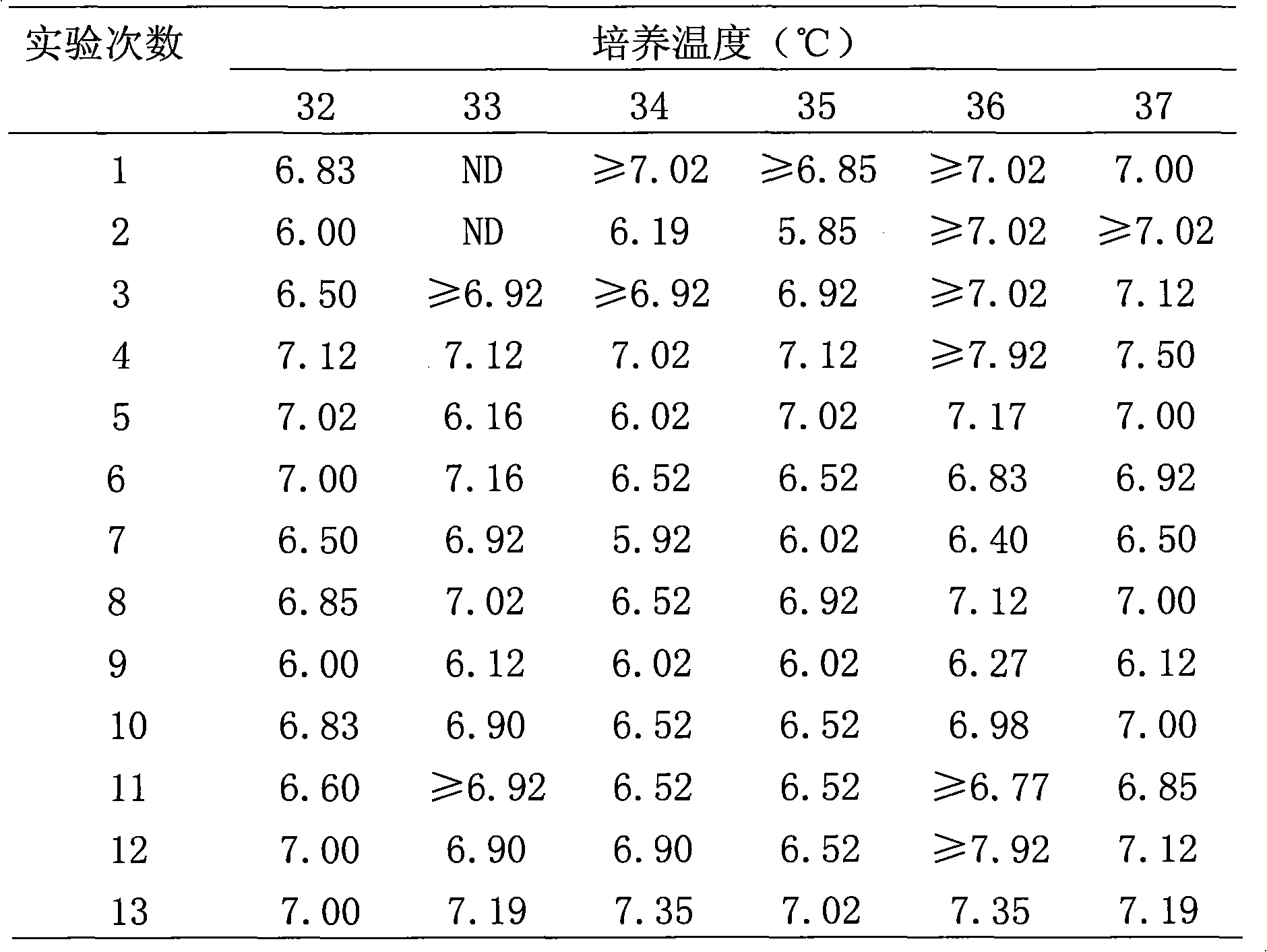
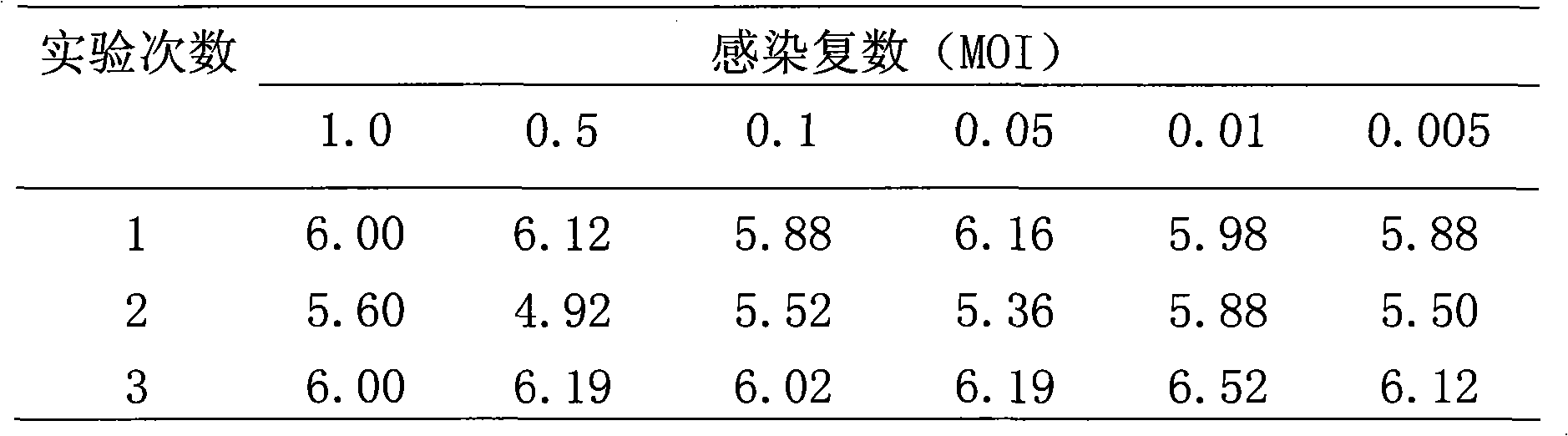
The invention provides a method for producing rabies viruses by the suspension culture of BHK21 cells, which comprises a step of performing suspension culture of BHK21 cells in bioreactor containing a BHK21 cell culture medium, wherein the conditions for the suspension culture in the step include a temperature of 32 to 36 DEG C, a pH value of 7.0 to 8.0 and a dissolved oxygen concentration of 30 to 50 percent; and the BHK21 cell culture medium comprises the components shown in a table 1. The method overcomes the biases of the prior art and realizes the production of the rabies viruses by the suspension culture of BHK21 cells in the bioreactor. The obtained rabies viruses can be used for producing rabies vaccine. Due to the automatic culture environment parameter control of the bioreactor, the cells grow and the viruses propagate in more favorable environments, the virus titer is improved and the large-scale automatic continuous production can be realized.
Owner:BEIJING SKYWING TECH CO LTD
Method for culturing baby hamster kidney (BHK) 21 cell in serum-free way, and vaccine preparation method
InactiveCN102115729AIncrease culture densityIncrease productivityMicroorganism based processesAntiviralsBiotechnologyHamster
The invention provides a method for culturing baby hamster kidney (BHK) 21 cell in a serum-free way, which leads the BHK 21 cell to be inoculated into a cell culture medium for culturing, wherein the cell culture medium comprises a basic culture medium and further comprises 100-200g / 100L of soy protein, 100-200g / 100L of pea protein, 100-200g / 100L of broad bean protein, 0-100g / 100L of potato protein, 0-200g / 100L of wheat gluten protein and 50-100g / 100L of rice protein by taking the volume of solvent of the culture medium as reference. In addition, the invention also provides a vaccine (such asrabies vaccine and foot-and-mouth disease vaccine) preparation method comprising the method for culturing the BHK 21 cell. The BHK 21 cell cultured by the culture medium containing the vegetable protein is high in culture density, beneficial to separating down-stream products of the cells, low in cost, small in batch difference, good in safety and suitable for producing virus host, expression vector and the like of biological products such as vaccine and the like.
Owner:BEIJING SKYWING TECH CO LTD
Vaccine protectant, hydrophobia vaccine and preparation method thereof
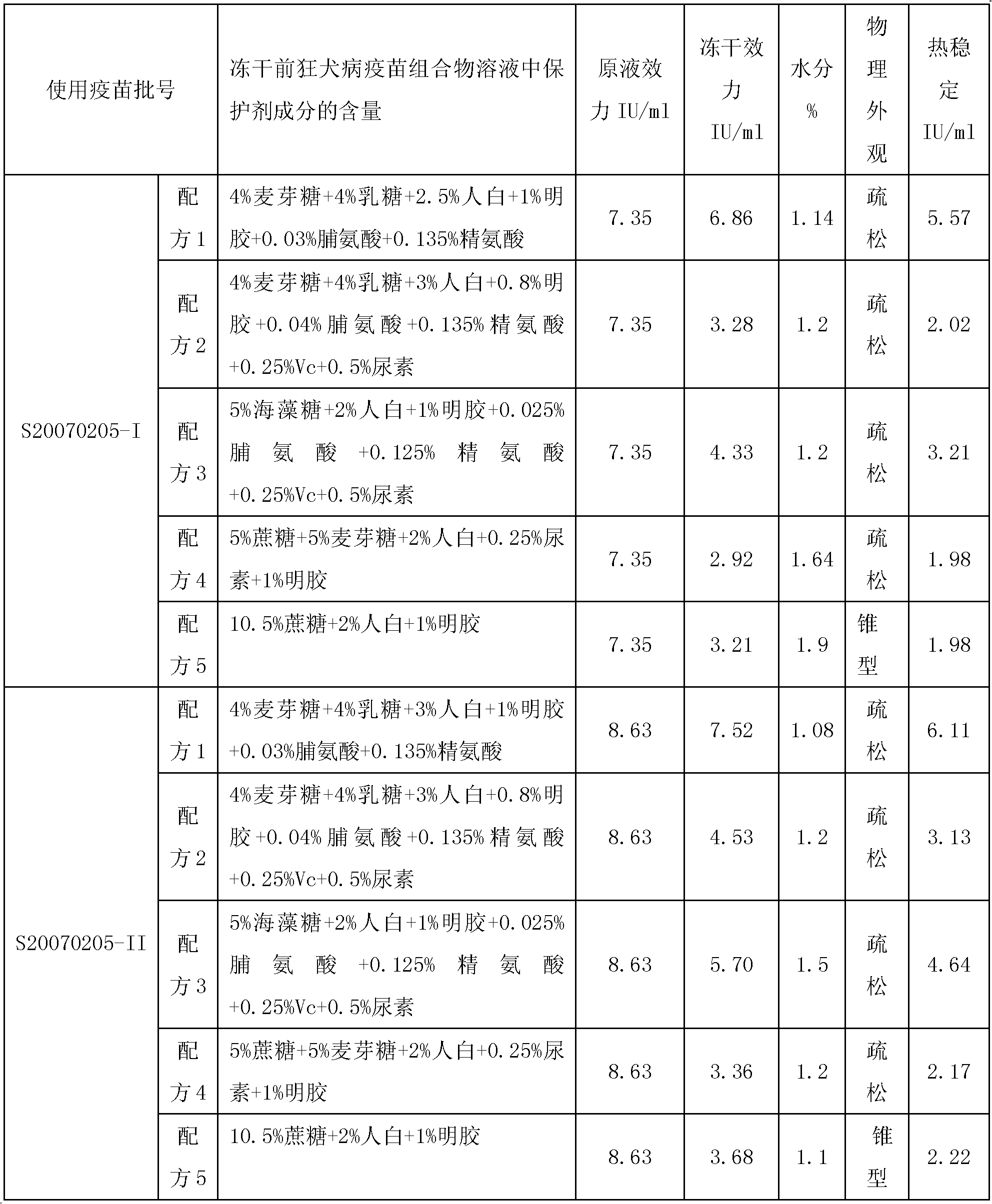
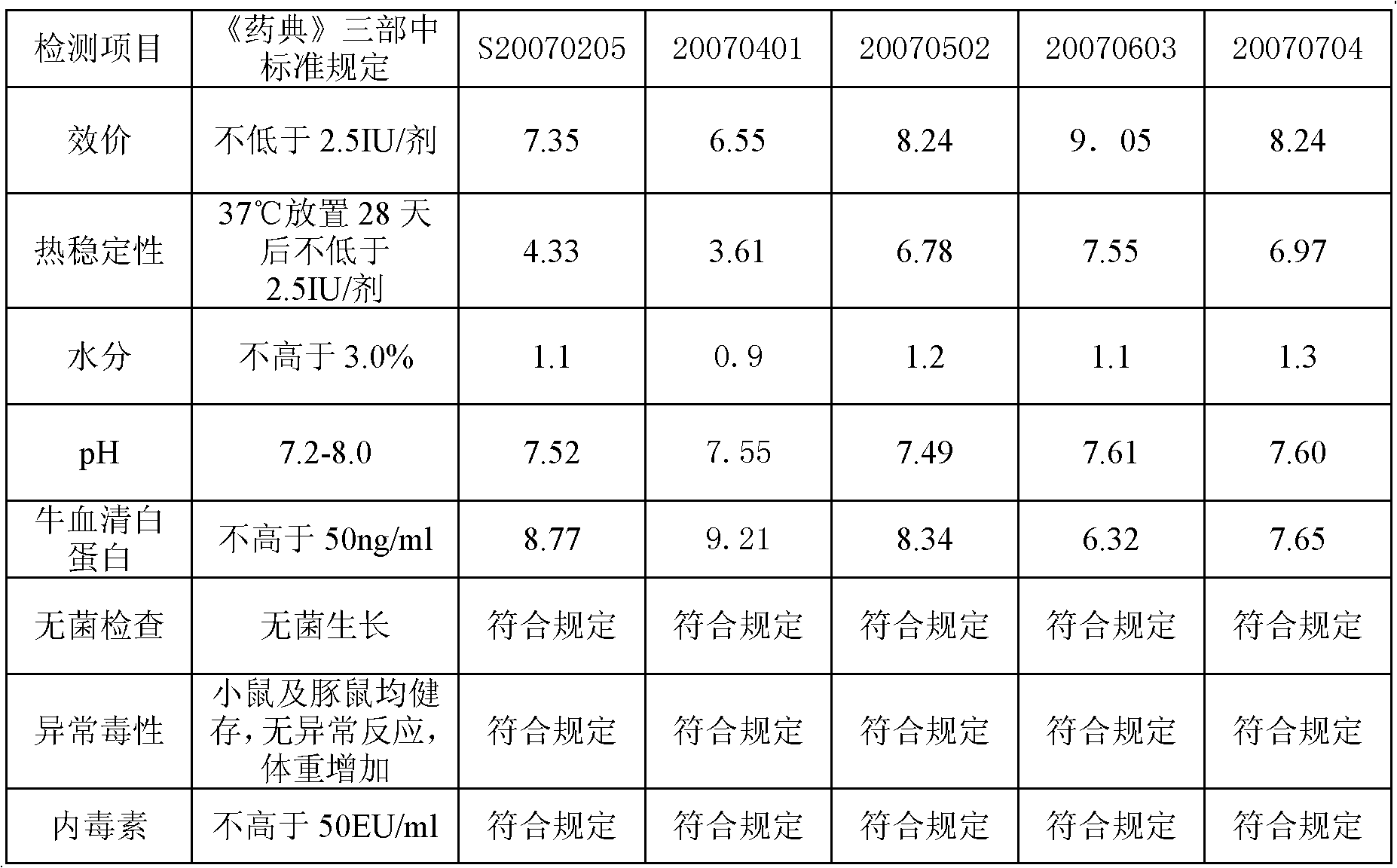
The invention provides a vaccine protectant, comprising the following components, by percentage composition: 50-80 parts of disaccharide, 20-30 parts of human albumin, 10 parts of gelatin and 1.5-1.65 parts of amino acid. The invention also provides a hydrophobia vaccine and a preparation method thereof. The vaccine protectant of the invention can effectively enhance thermal stability of a hydrophobia vaccine lyophilized preparation and has huge application value.
Owner:CHENGDU INST OF BIOLOGICAL PROD
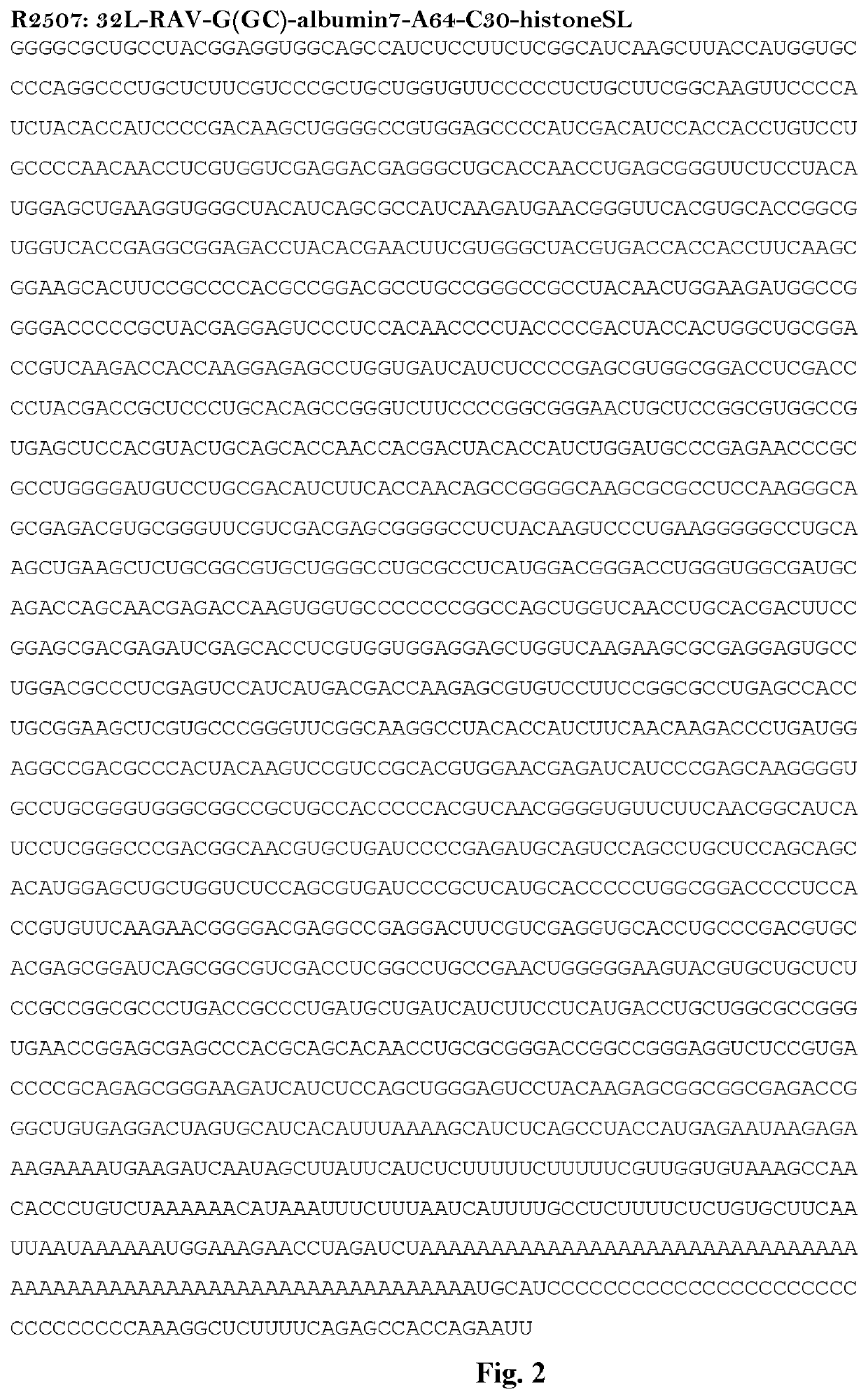
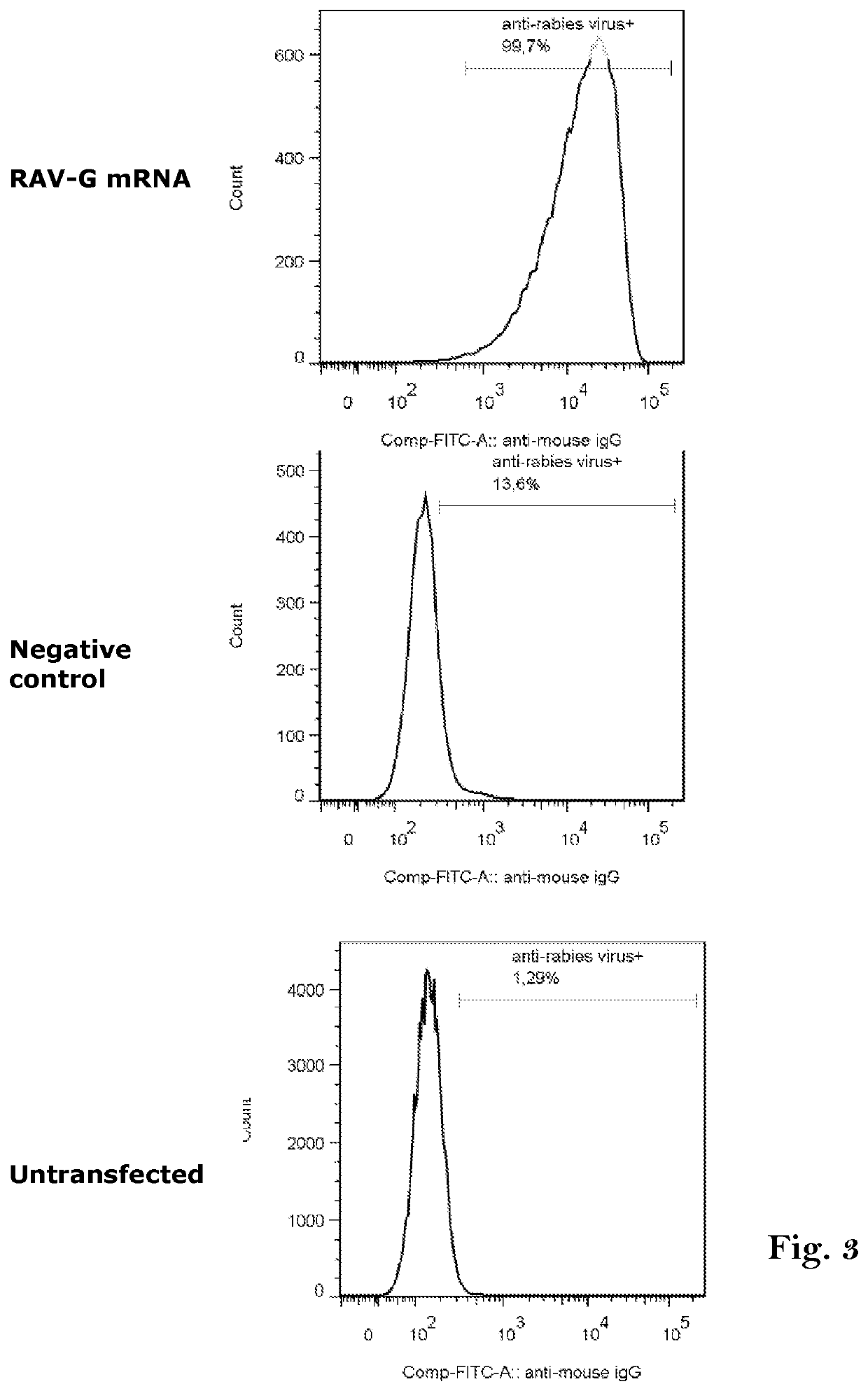
mRNA rabies vaccine
ActiveCN110714015AStable structureIncrease GC contentSsRNA viruses negative-sensePowder deliveryNucleotideNucleotide sequencing
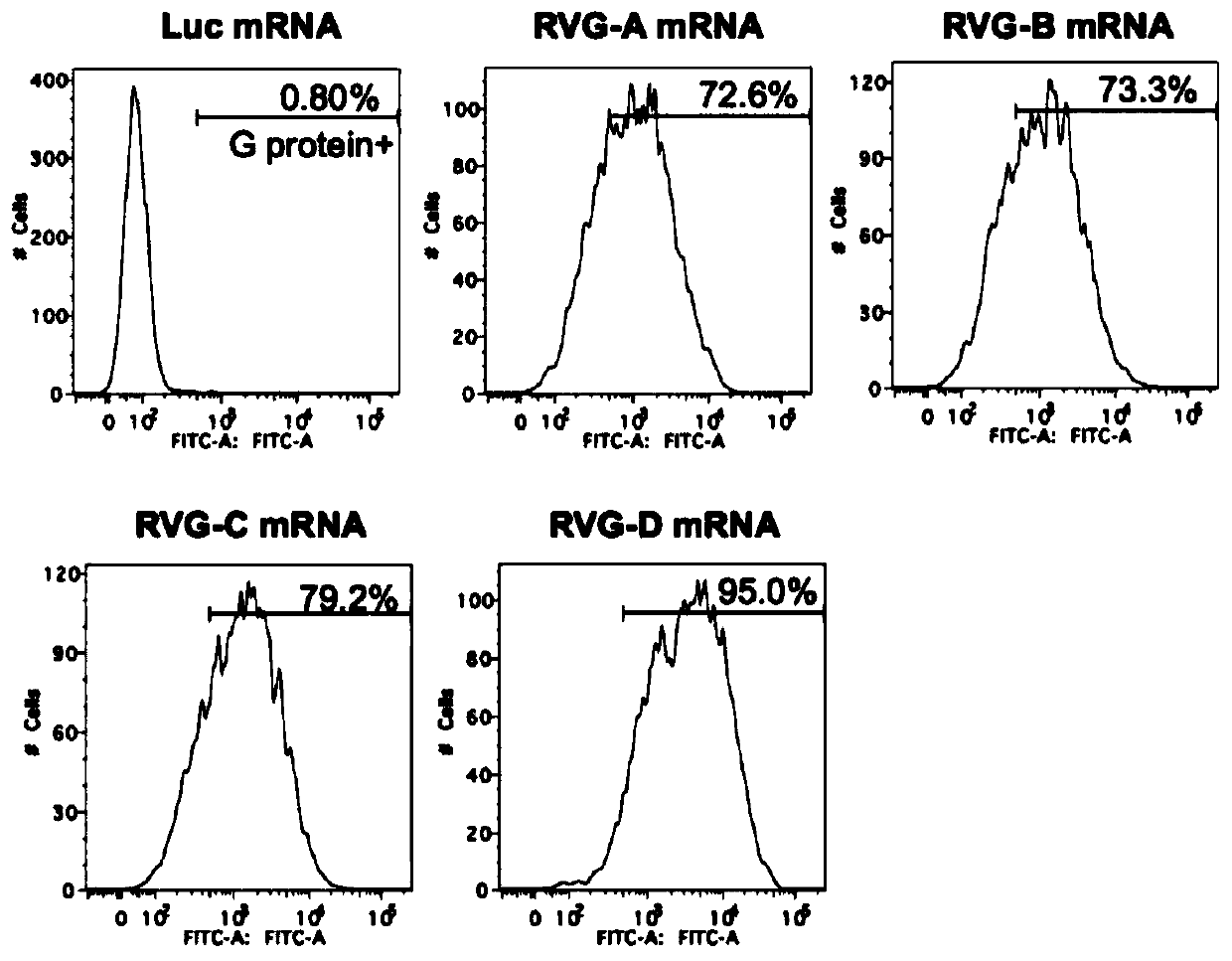
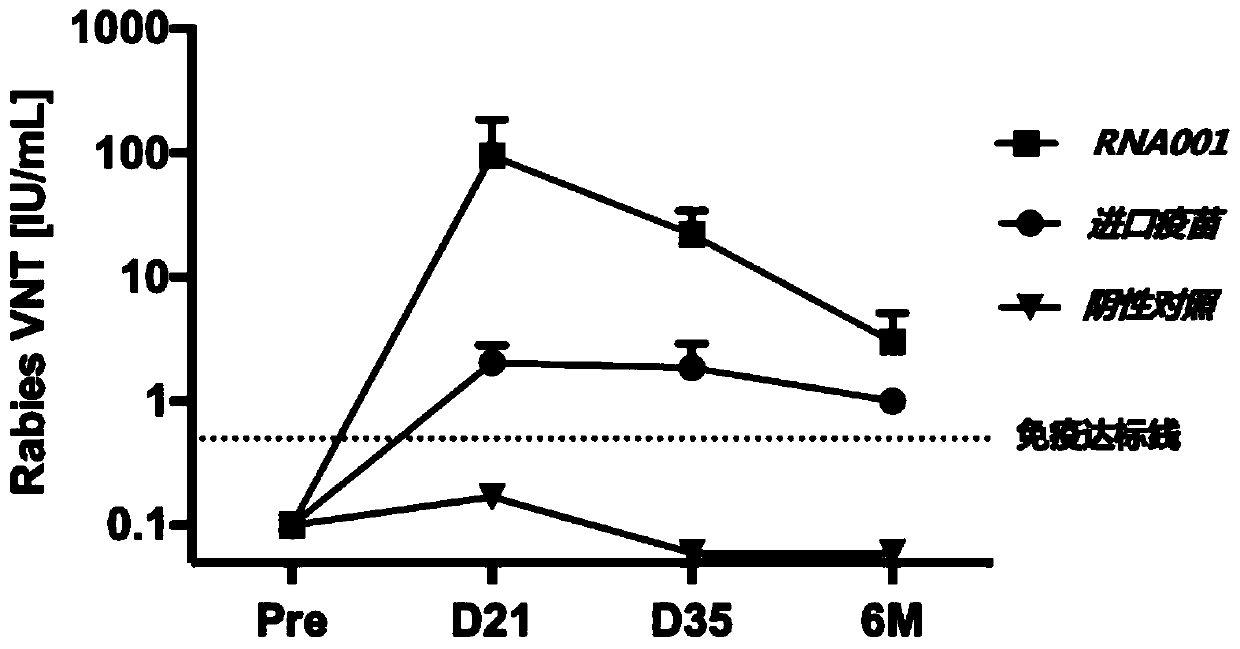
The present invention relates to the field of nucleic acid vaccines and particularly provides an mRNA rabies vaccine. A nucleotide sequence for transcribing the provided optimized RVG mRNA is shown asSEQ ID NO.1. A coding region of a rabies virus CTN-1 strain G protein (RVG) is optimized and an obtained nucleotide sequence is the optimized RVG mRNA sequence shown in the SEQ ID NO.1. The nucleotide sequence enables structure of the transcribed mRNA to be more stable and the target protein is more efficiently translated in mammals and humans. The provided rabies virus nucleic acid vaccine comprises a vaccine vector and the optimized RVG mRNA, achieves sufficient protection effects by using an extreme small dose, and is superior to existing rabies vaccine technologies in terms of safety andeffectiveness.
Owner:珠海丽凡达生物技术有限公司
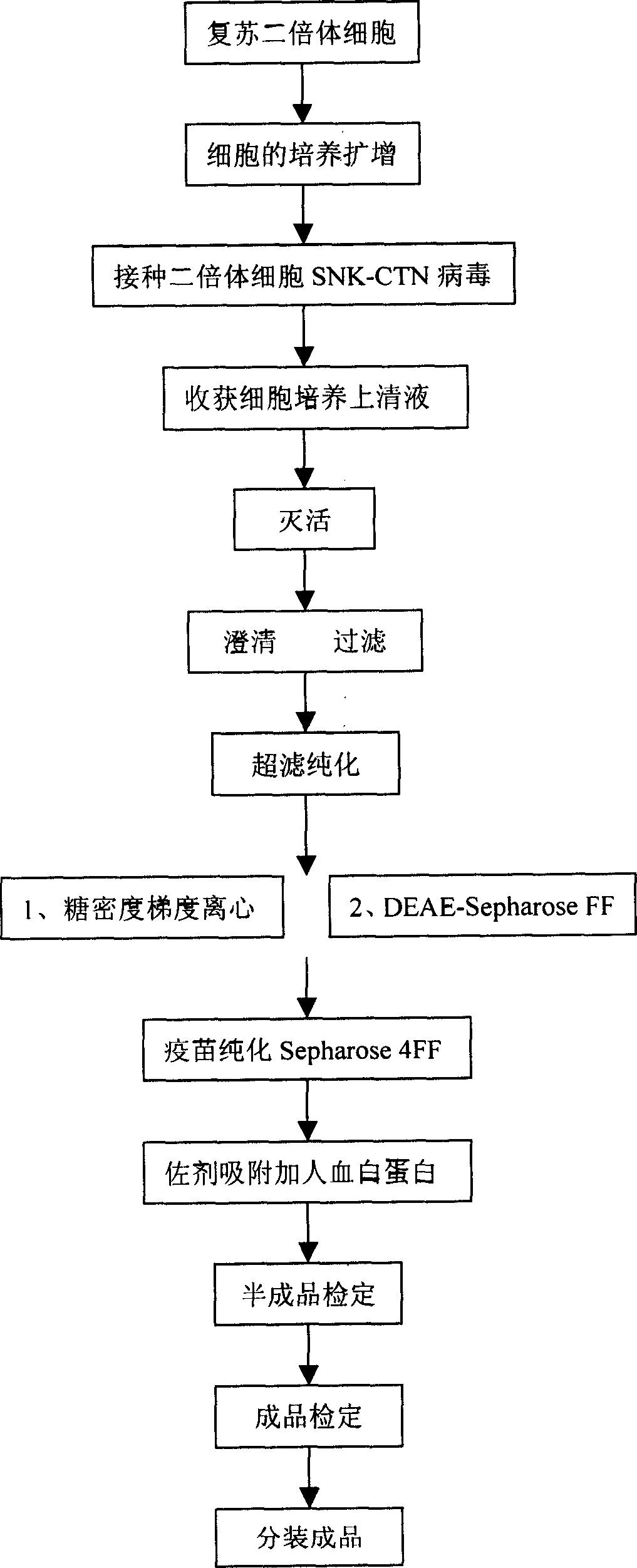
Human diploid cell rabies vaccine virus seed and preparation method thereof
ActiveCN102093983ASafeEffectiveMicroorganism based processesViruses/bacteriophagesBiotechnologyRabies
The invention relates to the field of biotechnology, in particular to a virus seed for producing vaccines for preventing human rabies by utilizing human diploid cells (KMB17) and a preparation method thereof. In the invention, a rabies fixed virus CTN-1V5 strain is continuously subcultured in the human diploid cells (KMB17), and a terminal dilution method is used for screening viruses with highertiter, thereby obtaining a rabies virus strain which is suitable for the human diploid cells (KMB17) and has good immunogenicity and heredity stability, and culturing a rabies vaccine virus seed (CTN-DK strain) capable of efficiently reproducing in the human diploid cells (KMB17). By using the virus seed for producing a human diploid cell (KMB17) rabies vaccine, the risk caused by residual heterogonous DNA (deoxyribonucleic acid) in the vaccine which is currently used at home can be effectively avoided, and the safety and practicability of the rabies vaccine in China are further improved, thus the invention has great social and economic benefits.
Owner:ZHEJIANG PUKANG BIOTECH
Method for producing rabies vaccine for human
The invention relates to a method for producing a rabies vaccine for human, comprising the following steps of: culturing human diploid cell lines by adopting a linear amplification technique of three or more levels of bioreactors; after the human diploid cell line in each level of bioreactor reaches 106 / ml, carrying out the vaccination on the next level of bioreactor; after the human diploid cell line in the last level of bioreactor grows on a microcarrier until the density reaches 106 / ml, vaccinating rabies virus strains; propagating viruses on cells by vaccinating the rabies virus strains,harvesting virus stock solutions, and inactivating, concentrating and purifying the harvested virus stock solutions to obtain the rabies vaccine for human. By using the linear amplification techniqueof the bioreactor to culture the human diploid cell lines, the cells do not contain exogenous pollution factors and tumorigenicity, and the residual DNA of the cells has no danger, and the rabies vaccine for human has the advantages of good immunizing effect and high safety and meets the requirement of large-scale industrial production of the rabies vaccine.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD +1
Diploid cell rabies vaccine and method for preparing purified rabies vaccine
InactiveCN101352570AIncreased antigenic activityHigh purityAntiviralsTissue cultureSerum free mediaSerum ige
The invention relates to a preparation method for a diploid cell rabies vaccine and a purified rabies vaccine, and the method uses a serum-free medium to culture human diploid cell rabies purified vaccine, thus greatly reducing anaphylactic reaction and being beneficial to purification.
Owner:崔栋
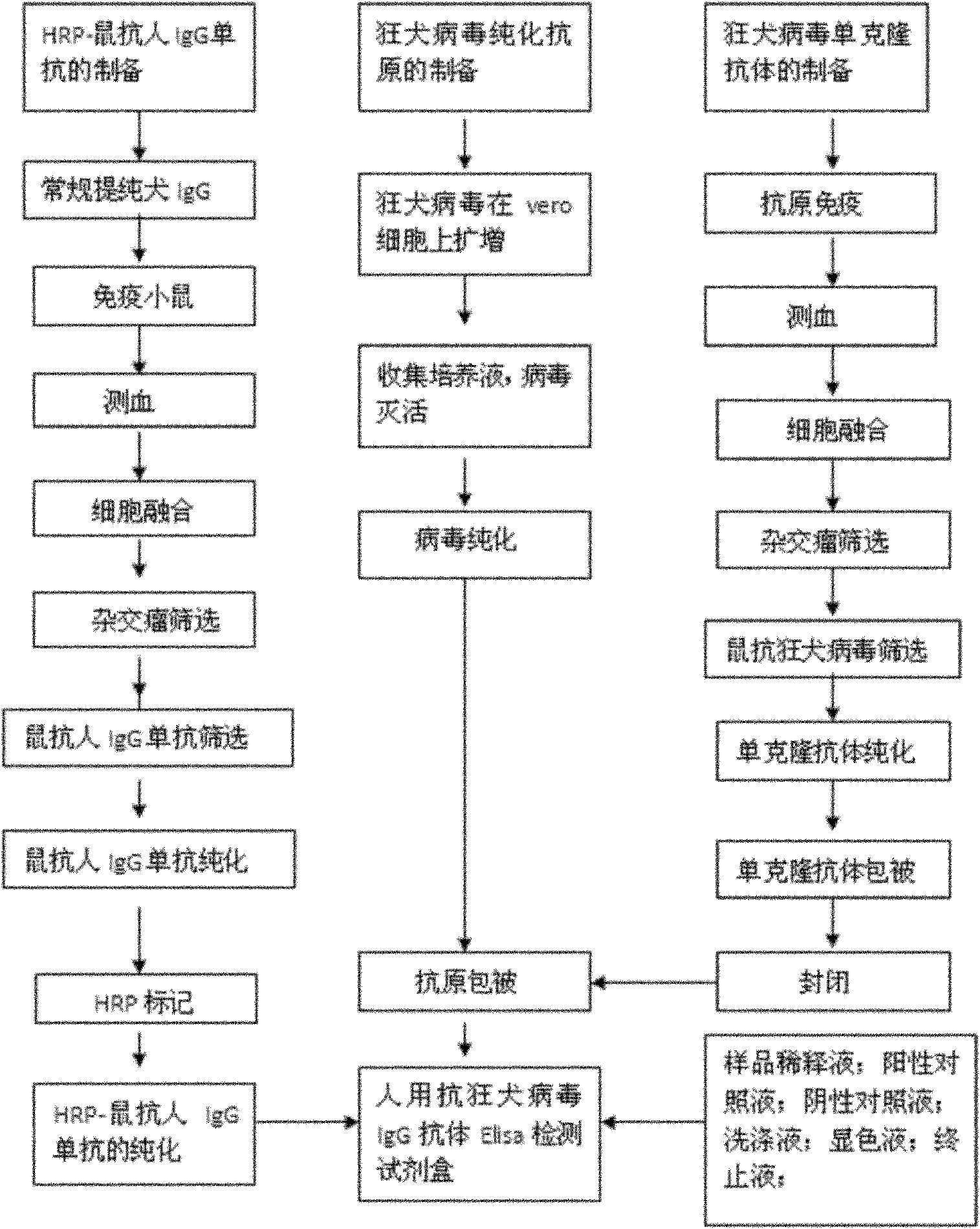
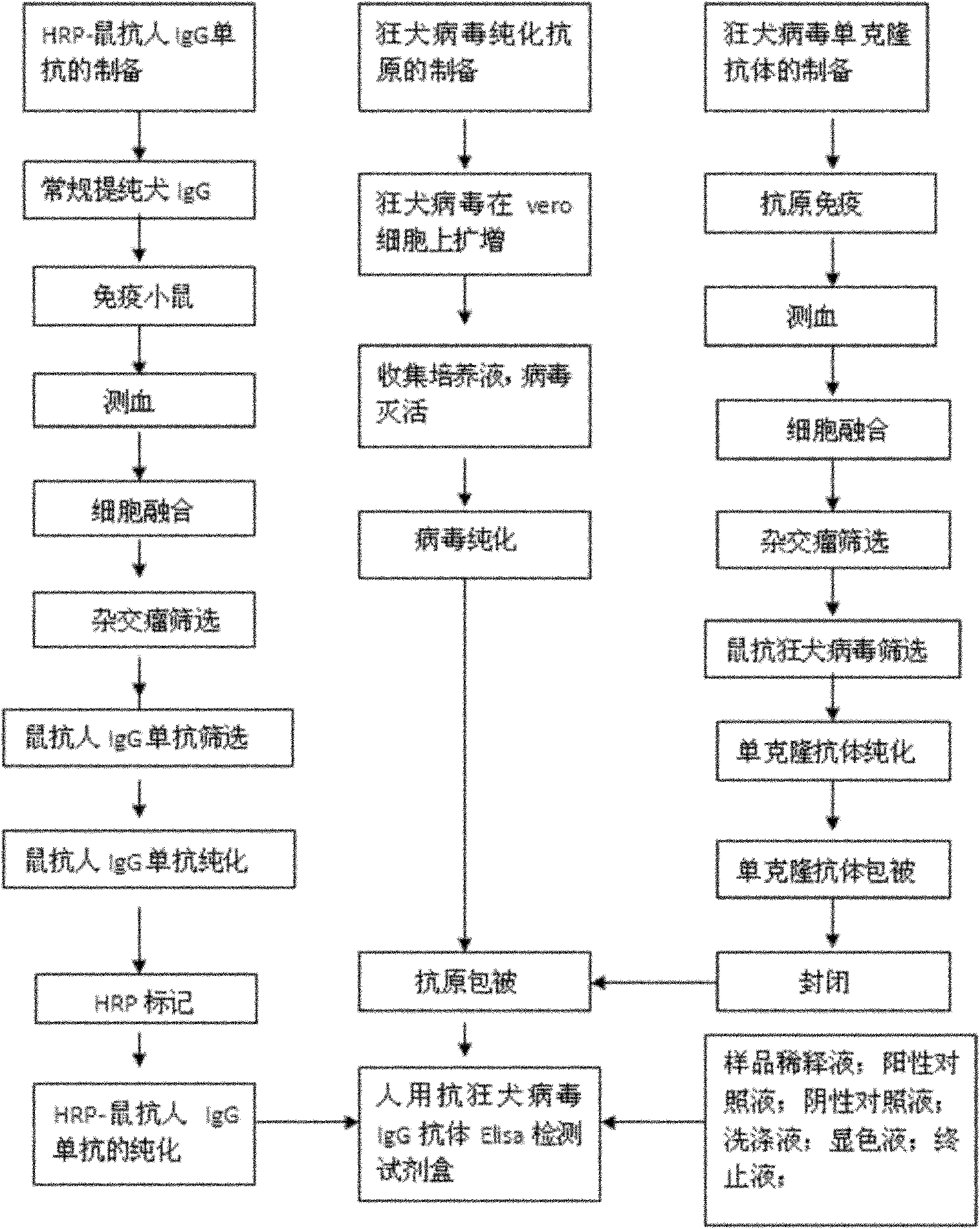
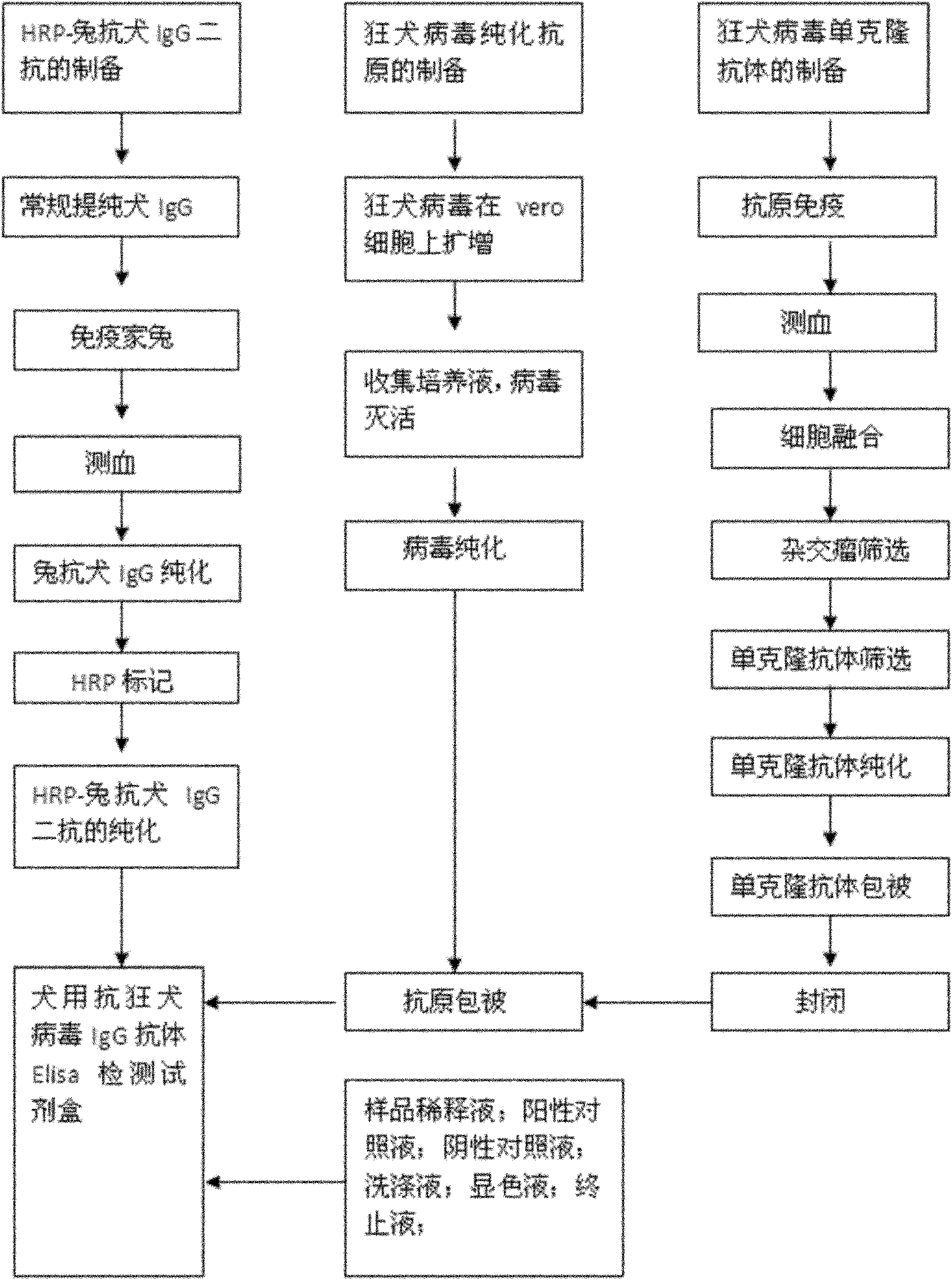
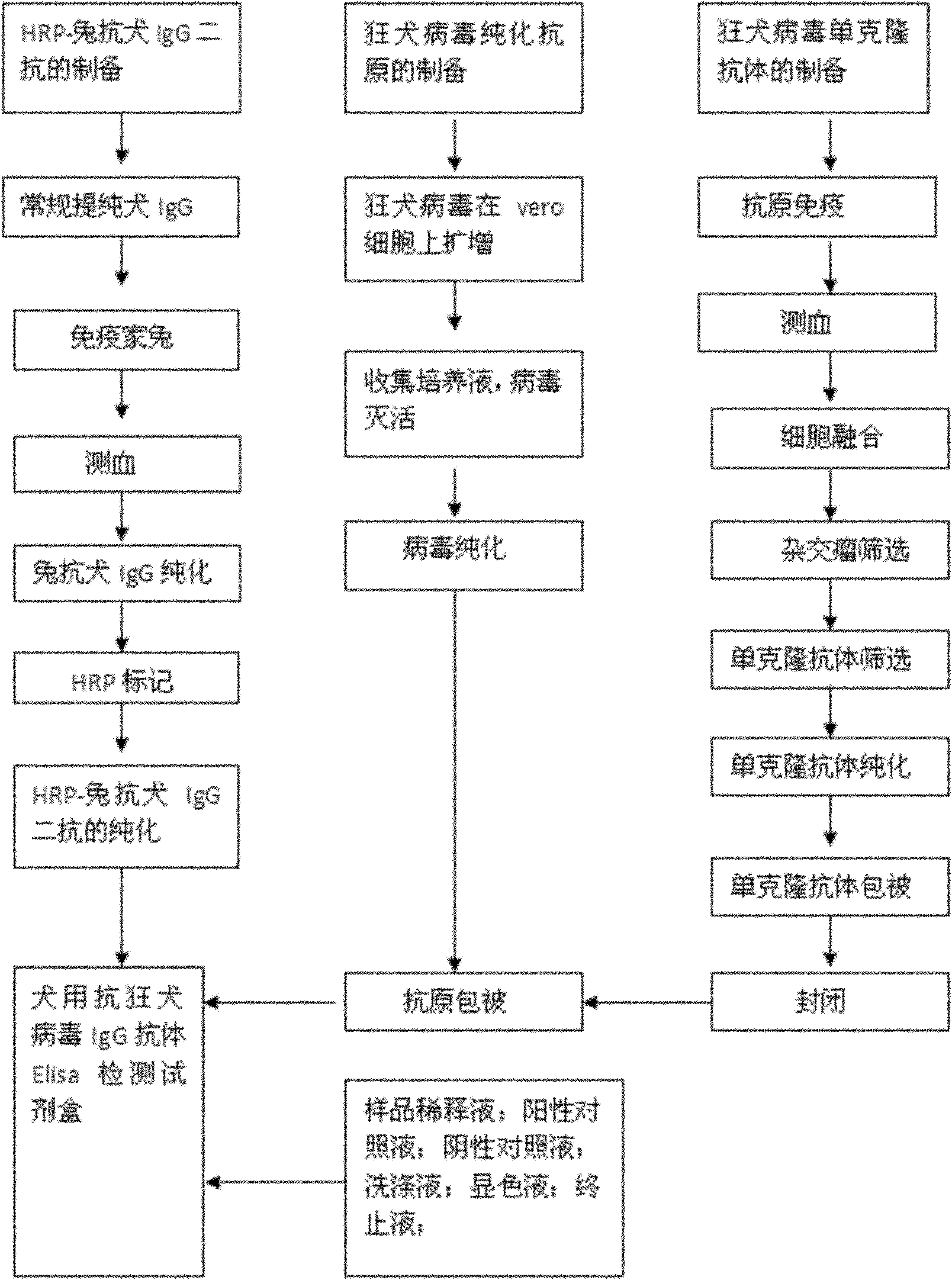
Human anti-rabies virus IgG antibody ELISA test kit
ActiveCN101936997AMake up for the shortcomings of low sensitivityHigh sensitivityDepsipeptidesMaterial analysisAntigenPositive control
The invention relates to a human anti-rabies virus IgG antibody ELISA test kit. An ELISA plate is firstly coated with an anti-rabies virus monoclonal antibody, wherein the coating buffer solution is a 0.05M carbonate buffer solution of which the pH value is 9.6, and the coating amount is 0.1-1ug per hole; a blocking solution is a BSA or skimmed milk of which the mass concentration is 1-10%; the ELISA plate is coated with a rabies virus purified antigen after being blocked, wherein the coating amount is 0.1-1ug per hole; a sample diluent is a 0.01mol / L phosphate buffer solution (PBS) which contains bovine serum albumin (BSA) with a mass concentration of 0.1-10% and NaN3 with a mass concentration of 0.01-0.05 and has a pH value of 7.2-7.4; an enzyme conjugate is a horse radish peroxidase-mouse anti-human IgG enzyme conjugate; a concentrated cleaning solution is a 0.01mol / L PBS which contains tween-20 with a volume concentration of 0.05% and has a pH value of 7.2-7.4; a zymolyte A solution is a 3,3'-5,5'-tetramethyl benzidine solution, and a zymolyte B solution is an oxydol solution; and a stop solution is a 1mol / L H2SO4 solution, and a positive control and a negative control are arranged in the kit. The specificity of the kit is up to 100%, and the sensitivity is 1:640. The kit is used for evaluating the immunity effect of humans inoculated with rabies vaccines.
Owner:WUHAN CHOPPER BIOLOGY
Freeze-dried rabies vaccine for humans and preparation method of vaccine
ActiveCN104826101AThe process steps are simpleEasy to operateInactivation/attenuationAntiviralsHuman useSide effect
The invention relates to a freeze-dried rabies vaccine for humans and a preparation method of the vaccine, relates to the field of vaccine production preparation technologies and aims at solving the problems that effective virus antigen expression content is low, the side effect rate of a vaccinator is high and vaccine yield and quality can not meet standard requirements as only a biological reactor is adopted for producing a rabies vaccine. The freeze-dried rabies vaccine for humans is obtained by inoculating aG strain rabies virus on Vero cells and sequentially carrying out ultrafiltration and concentration, separation and purification as well as freeze drying, wherein the packing volume of the freezed-dried rabies vaccine for human use is 0.5ml / dose, and during freeze drying, the adopted vaccine freeze-drying protecting agent comprises the following ingredients: 60-90g / l of trehalos, 6-14g / l of sodium glutamate, 3-6g / l of urea, 2-3g / l of L-arginine and 10g / l of 199 culture medium, and the vaccine freeze-drying protecting agent does not contain gelatin, human serum albumin or dextran. The freeze-dried rabies vaccine for humans has the advantages that cost is low, operation is easy, pollution is hardly produced, vaccine quality and yield are greatly improved, the content of impurities in a vaccine is reduced, allergy reactions are hardly caused, and vaccine safety is greatly improved.
Owner:江生(深圳)生物技术研发中心有限公司
Novel rabies vaccine and preparation method thereof
ActiveCN103468743ASsRNA viruses negative-senseViral antigen ingredientsRabies vaccineVaccine Immunogenicity
The invention relates to a novel rabies vaccine and a preparation method thereof. Based on a vaccine carrier for chimpanzee type adenovirus AdC68 genome, an inventor constructs a novel rabies vaccine carrier. The invention further provides a virus vaccine prepared from the vaccine carrier, which can efficiently express and has excellent immunogenicity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI +1
Rabies vaccine for human beings
InactiveCN102526722AGood immune effectImprove securityAntiviralsDepsipeptidesVirus ProteinTetanus toxoids
The invention provides a rabies vaccine for human beings, which contains an outer membrane fragment of a split rabies virus particle and tetanus toxoid; the outer membrane fragment comprises furcella and matrix protein, wherein each dosage of rabies vaccine for human beings contains 10-150 micrograms of virus protein; and the dosage of the tetanus toxoid is 3-10 Lf / dosage. Compared with the existing virus purification stock solution, the rabies vaccine for human beings contains less other proteins, has higher immunogenicity and effect, and is more secure for use.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Human diploid cell rabies vaccine and preparation method thereof
ActiveCN102671192AContains less impuritiesPerfect purification processMicroorganism based processesAntiviralsRabiesDiploid cells
The invention discloses a human diploid cell rabies vaccine and a preparation method thereof, and belongs to the field of vaccines. A CDKHBP-1 strain is inoculated into a human diploid cell WI-38, and the rabies vaccine is obtained through separation and purification. The human diploid cell does not contain any contaminants or oncogenicity, and is obvious superior to an animal cell serving as a medium for producing the vaccine; and the method is suitable for large-scale industrial production, a purification process is perfect, and the vaccine contains a few impurities and has high purity and immunogenicity.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Vaccination of skunks and/or mongooses against rabies
The present invention relates to recombinant anti-rabies vaccines and the oral administration of such vaccines to skunks and / or mongooses. Advantageously, the anti-rabies vaccine may comprise a recombinant vaccinia virus containing a rabies glycoprotein gene. The invention encompasses methods of vaccinating skunks and / or mongooses by administration of an anti-rabies vaccines which may comprise a recombinant vaccinia virus containing a rabies glycoprotein gene.
Owner:MERIAL LTD
Diploid-cell rabies vaccine and purified rabies vaccine, freeze-drying preparation and water injection thereof
InactiveCN1712068ATotal protein content decreasedHigh potencyAntibody medical ingredientsFreeze-dryingDiploid cells
Owner:崔栋
Cpg Single Strand Deoxynucleotides for Use as Adjuvant
InactiveUS20080003232A1Enhance immune responseReduce needOrganic active ingredientsSugar derivativesImmune effectsAdjuvant
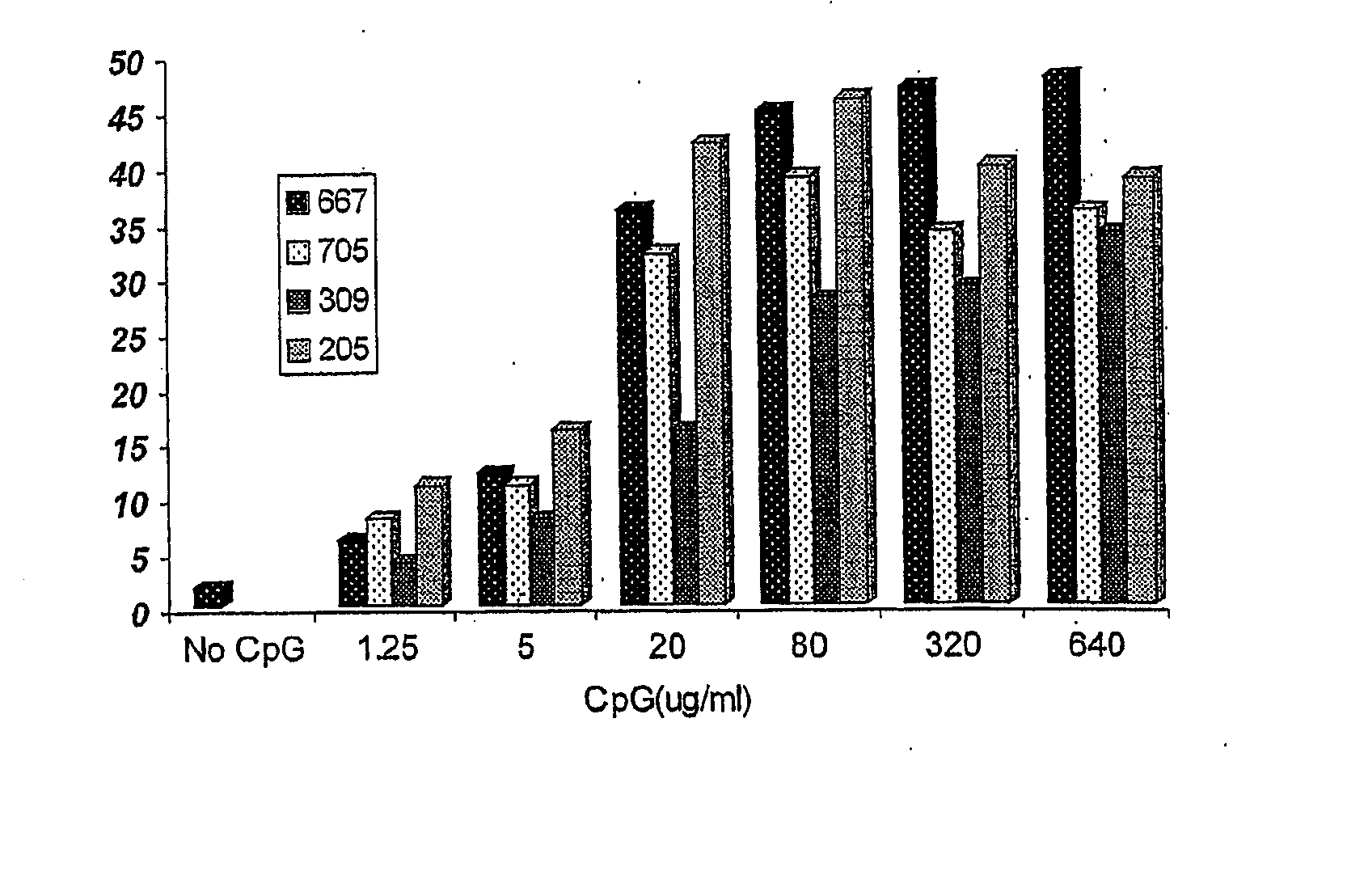
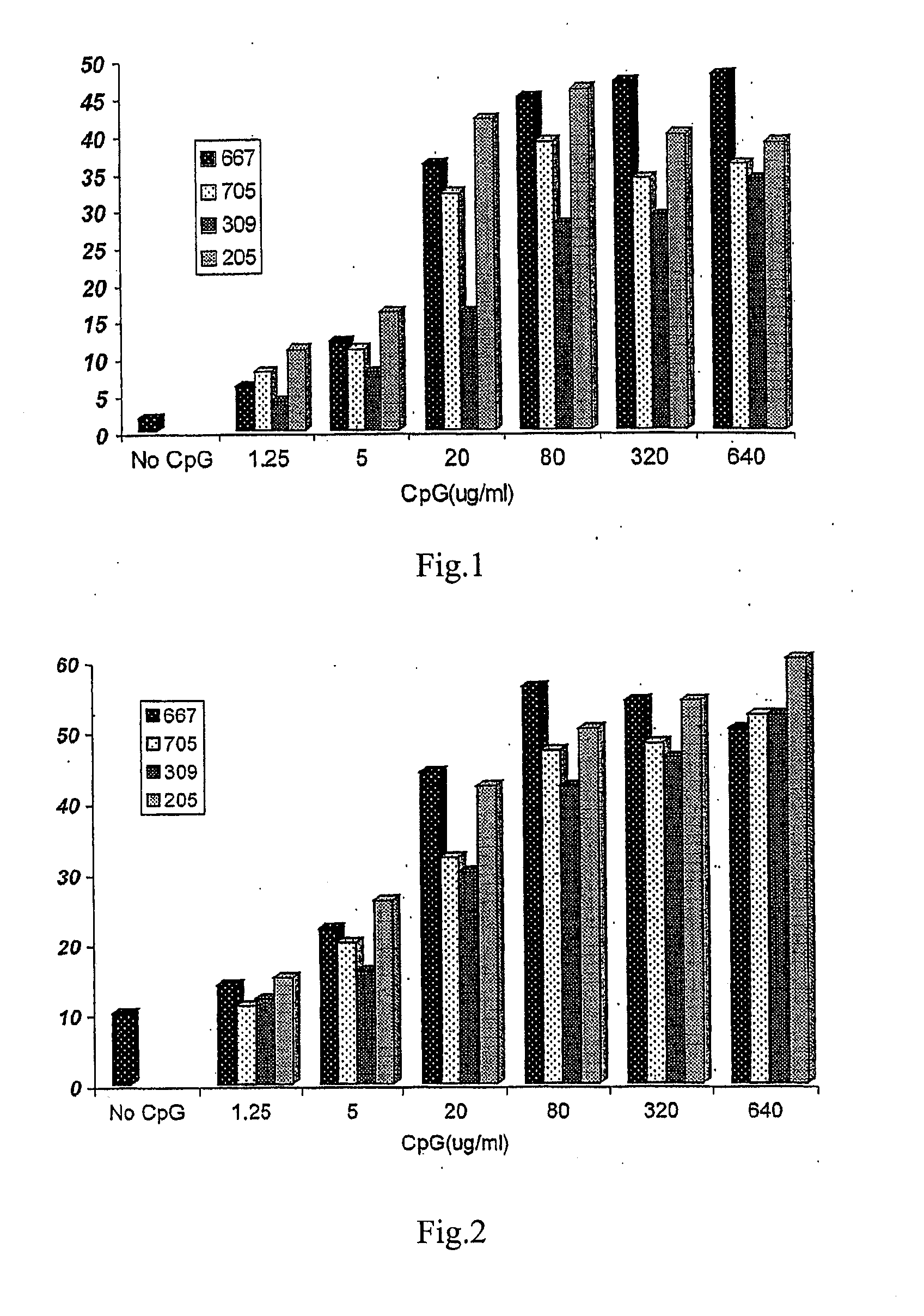
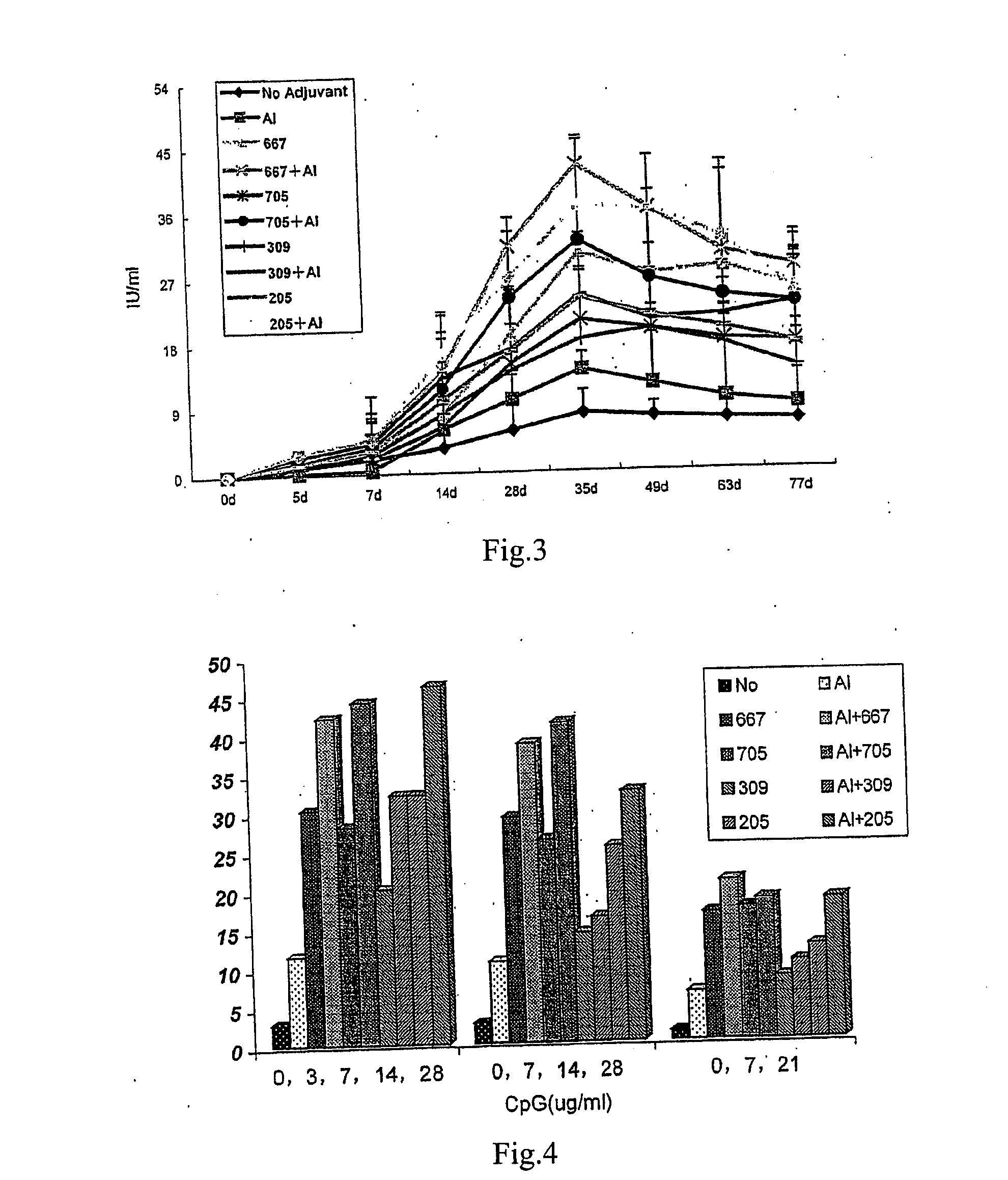
The present invention provides an adjuvant, which includes at least one single strand deoxynucleotide containing a CpG dinucleotide. The single strand deoxynucleotide comprises one or more CpG dinucleotides. When used in combination with rabies vaccine, HBV vaccine or other vaccines, the adjuvant can significantly improve the immune effect of the vaccine.
Owner:CHANGCHUN HUAPU BIOTECHNOLOGY CO LTD
Human vaccine for preventing hydrophobia and tetanus
ActiveCN102671194AIncreased potencyImprove immunityAntibacterial agentsBacterial antigen ingredientsRabiesTetanus toxoids
The invention discloses a human vaccine for preventing hydrophobia and tetanus, which belongs to the field of vaccines. The vaccine consists of a hydrophobia vaccine and a tetanus toxin, wherein the hydrophobia vaccine is obtained by inoculating a CDKHBP-1 strain onto a human diploid cell WI-38 and purifying. By using the hydrophobia vaccine and the tetanus toxin prepared with the preparation method disclosed by the invention together, immunity to hydrophobia and tetanus can be realized; and moreover, the tetanus toxin can be used for remarkably enhancing valence effect of the hydrophobia vaccine, plays a role in enhancing immunity, and is convenient for administration.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Preparation of rabies vaccine for human being
InactiveCN103182081AGood immune effectImprove securityBacterial antigen ingredientsAntiviralsRabiesAdjuvant
The invention provides rabies vaccine for human being. The vaccine comprises an outer membrane segment of cracked rabies complete viral particles and tetanus toxoid, and the outer membrane segment comprises spike and matrix protein. Each dose of the rabies vaccine comprises 10 to 100 micrograms of viral protein; the dosage of the tetanus toxoid is 2 Lf / dose to 10 Lf / dose; and the dosage of aluminum phosphate adjuvant is 0.5mg / dose. Compared with the existing virus purified stock solution, the rabies vaccine for human being has the advantages that the content of impurity protein is greatly reduced, the immunogenicity and the potency are high, and the rabies vaccine is safer to use.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Method for removing residual DNA from hydrophobia vaccine
The invention belongs to the technical field of biological engineering, in particular to a purification method during vaccine production, solving the problems that DNA is difficult to be removed and protein is easy to be polymerized and precipitated because column chromatography method is singly adopted during the previous production process of the hydrophobia vaccine. The method comprises the following steps: adopting a 750KD hollow fibre ultrafiltration column or a 300KD ultrafiltration membrane for concentrating virus harvested liquid or removing part of impurities, adopting non-restriction endonuclease for degrading DNA, and then removing nuclease by the method of ultrafiltration or column chromatography. The method is characterized by easy magnification, high product purity and obvious DNA removing effect, and greatly improves the safety of the vaccine.
Owner:CHANGCHUN ZHUOYI BIOLOGICAL CO LTD
Rabies virus resistant specific humanized antibody and application thereof
ActiveCN104193823AStrong neutralizing activityImmunoglobulins against virusesAntiviralsPhage antibodiesBacteriophage
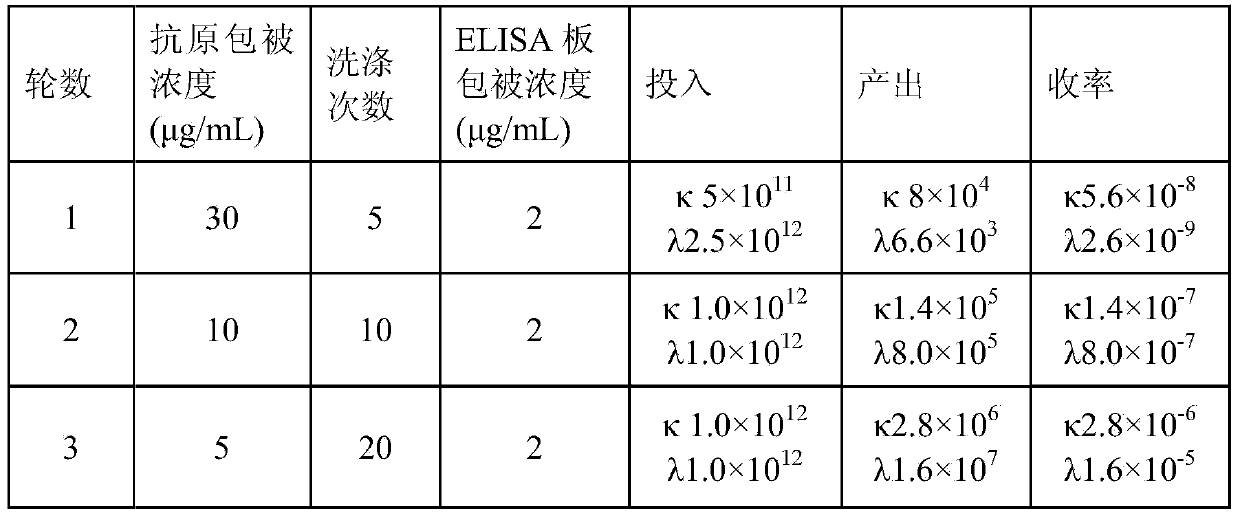
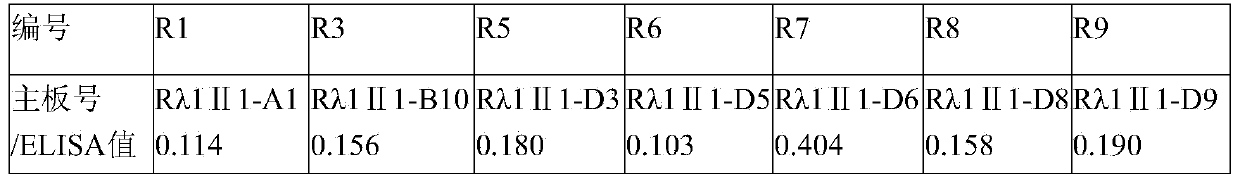
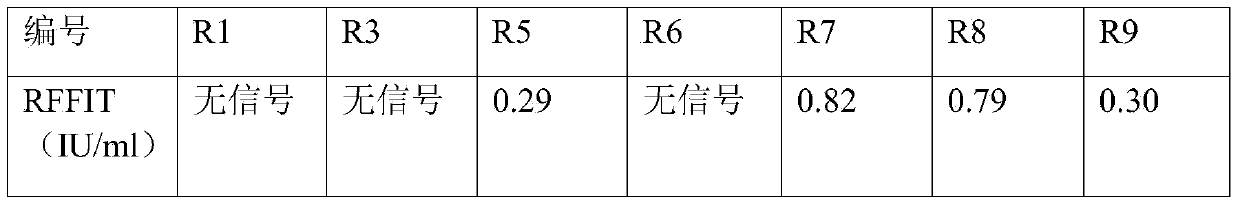
The invention aims at providing a rabies virus resistant neutralizing antibody, and particularly provides a humanized or completely humanized monoclonal antibody to meet the requirement for clinically diagnosing and / or treating rabies. A phage antibody library is prepared by adopting a phage antibody library technology and taking 32 parts of high-potency healthy human peripheral blood inoculated with rabies vaccines as a raw material; 7 ELISA positive antibodies are obtained through three rounds of screening from the phage antibody library; and furthermore, the neutralizing activity of the 7 ELISA positive antibodies is measured through an RFFIT method, wherein four ELISA positive antibodies, namely R5, R7, R8 and R9, have higher neutralizing activity in all. The rabies virus resistant neutralizing antibody with high affinity, which is provided by the invention, can be used for substituting ERIG and HRIG to carry out active and / or passive immune therapy on rabies virus seriously-exposed persons.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Animal rabies virus and vaccine and production method thereof
ActiveCN101979515AHigh infection efficiencyHigh titerInactivation/attenuationAntiviralsFreeze thawingAdjuvant
Owner:PULIKE BIOLOGICAL ENG INC
Freeze-dried rabies vaccine for human use and preparation method thereof
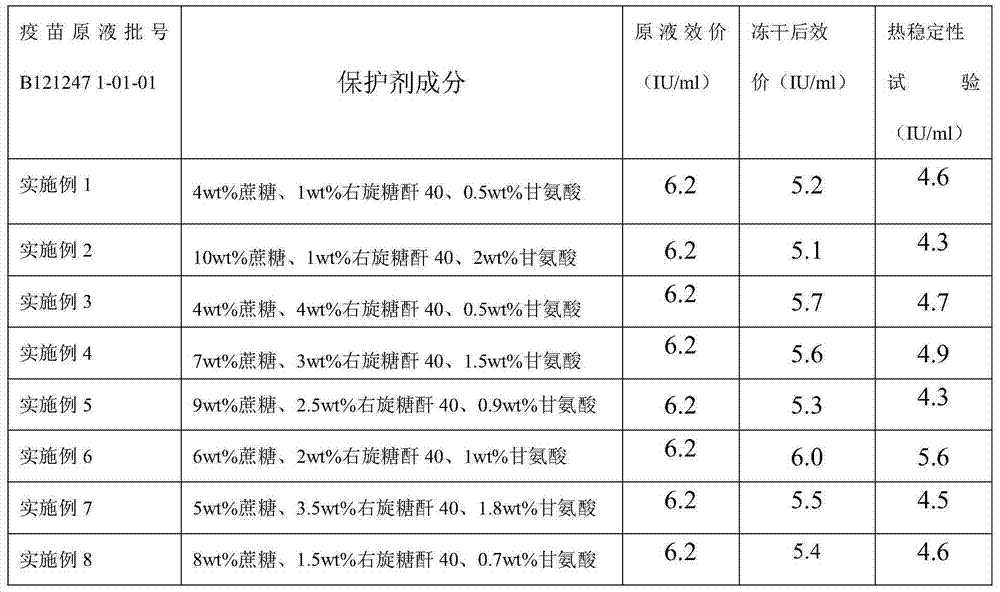
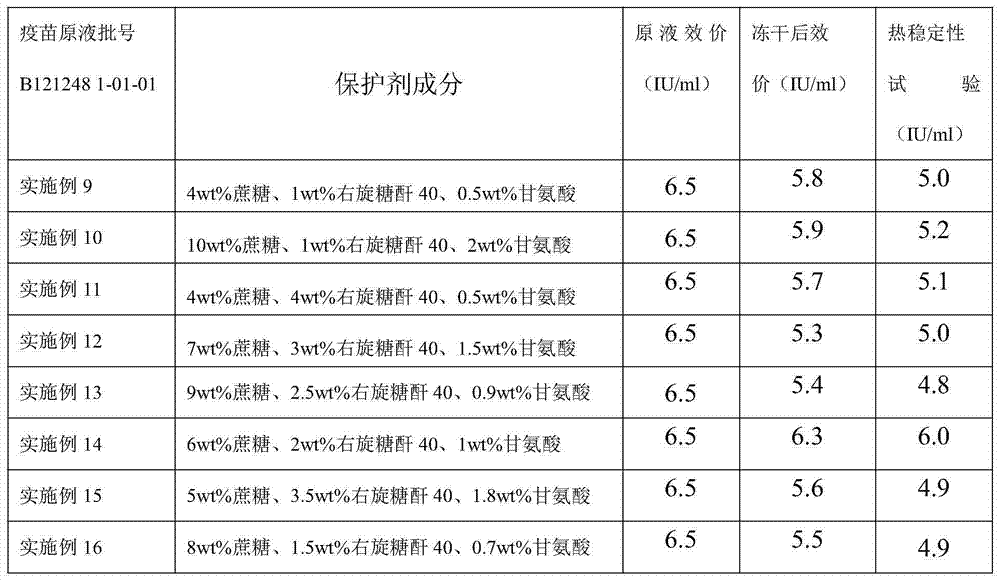
The invention discloses a freeze-dried rabies vaccine for human use and a preparation method thereof. The preparation method comprises the following steps: adding cane sugar and dextran 40 into water for injection respectively, stirring to a fully-dissolved state, and performing steam sterilization at the temperature of 115 DEG C under the pressure of 0.09-0.10 MPa for 45 minutes; adding glycine into water for injection of which the temperature is 15-30 DEG C, stirring to a fully-dissolved state, and degerming and filtering with a microfiltration membrane of 0.22 mu m; preparing a dose of which the total protein content does not surpass 80 mu g according to the measured protein content or antigen content of an early vaccine stock solution, and adding cane sugar of which the final concentration is 4-10 percent by weight, dextran 40 of which the final concentration is 1-4 percent by weight and glycine of which the final concentration is 0.5-2 percent by weight to obtain a semi-finished product; performing split charging on the semi-finished product, semi-plugging, putting into a freezer drier box, setting a freeze drying parameter, and performing freeze drying; performing pre-freezing, vacuum pumping, sublimation, secondary drying and vacuum plugging, and ending freeze drying to obtain the freeze-dried rabies vaccine for human use. Active ingredients in the vaccine disclosed by the invention can be well protected, the vaccine is high in thermal stability, and the period of validity can be at least up to 24 months.
Owner:DALIAN HISSEN BIO-PHARM CO LTD
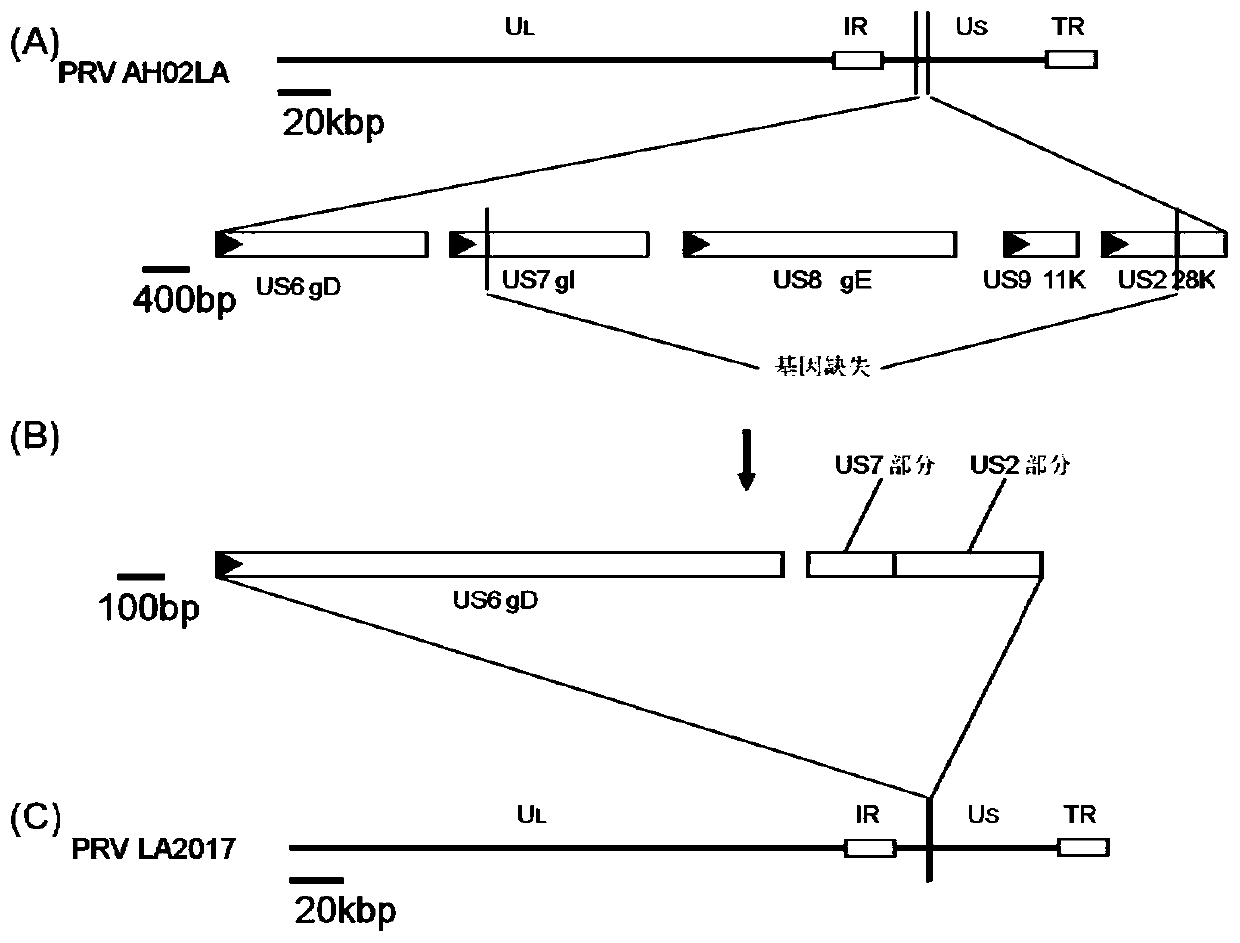
Pseudorabies virus passage attenuated strain and application thereof
ActiveCN110387354ALow toxicityImprove securityViral antigen ingredientsInactivation/attenuationAttenuated strainToxin
The invention provides a pseudorabies virus passage attenuated strain and an application thereof, and belongs to the field of vaccines of animal medicine. The pseudorabies virus passage attenuated strain is a pseudorabies virus LA2017 strain, and the preservation number is CGMCC No.18170. The invention also provides an application of the pseudorabies virus passage attenuated strain to preparationof pseudorabies vaccines, and a vaccine using the pseudorabies virus passage attenuated strain as an active component. The LA2017 strain is a natural deletion attenuated strain, the virulence is notably reduced, and when new-born piggies are inoculated with the LA2017 strain, no adverse reactions are caused. The strain is obtained by a passage attenuated method, so that the biological security risk is low. After weaned highly-susceptible piggies are subjected to primary inoculation of the vaccines prepared from the LA2017 strain for 7 days, 100% of protective effects can be achieved, and the immunization persistent period can achieve 5 months. The vaccines not only can prevent pathogenesis but also can prevent toxin expelling, and purification of pseudorabies virus variation strains can beextremely facilitated.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Rabies vaccine
ActiveUS10682426B2Easy to identifyFaster and strong attackSsRNA viruses negative-senseVirus peptidesAntigenPharmaceutical drug
Owner:CUREVAC AG
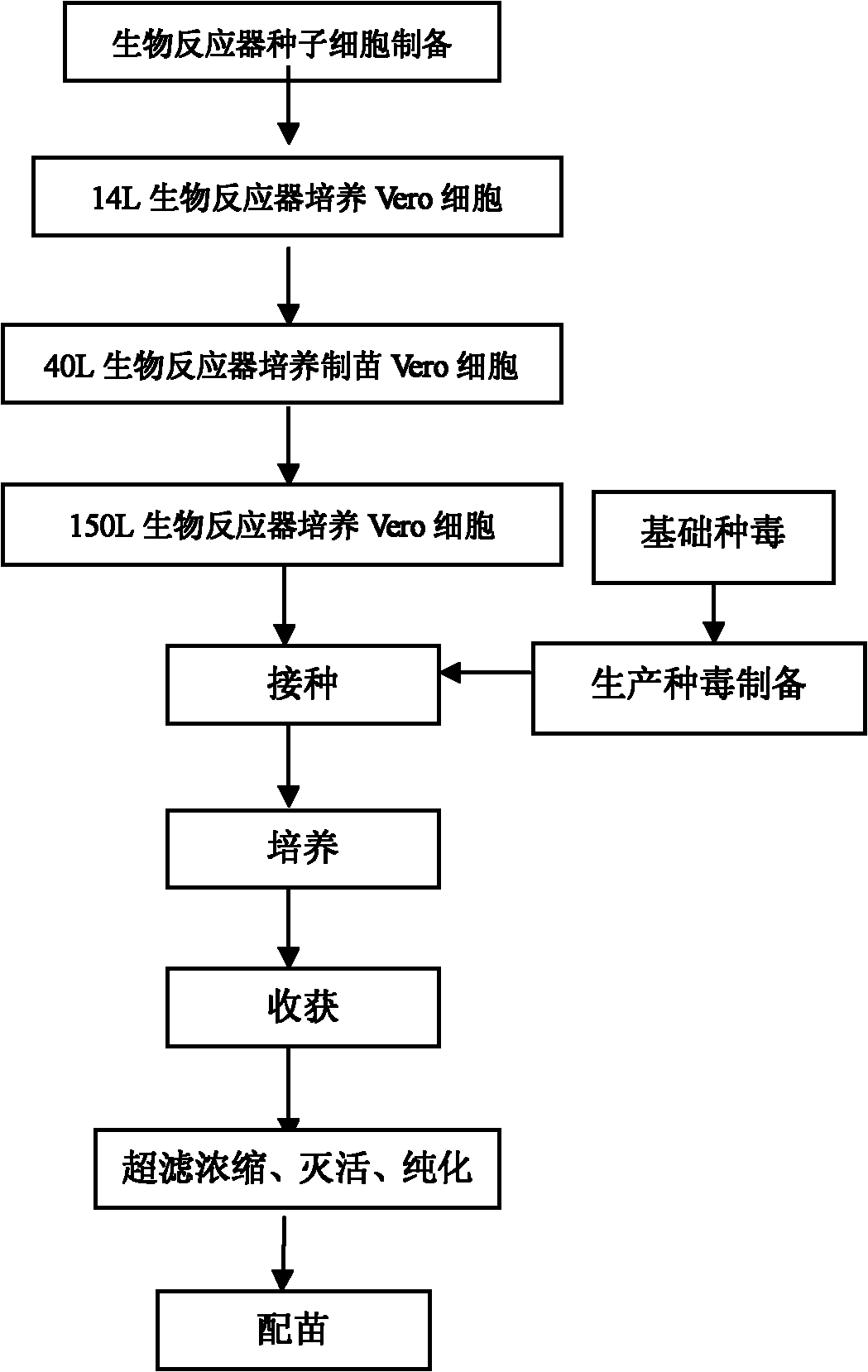
Method for producing rabies vaccine by applying bioreactor and sheet carrier
InactiveCN102240400AHigh densityHigh viral titeMicroorganism based processesAntiviralsFixed bedVolumetric Mass Density
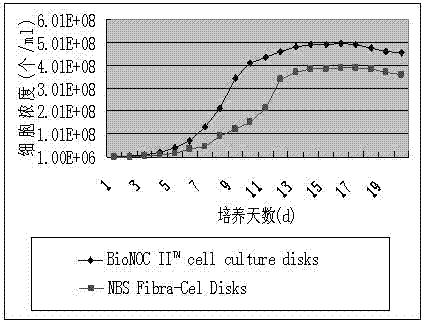
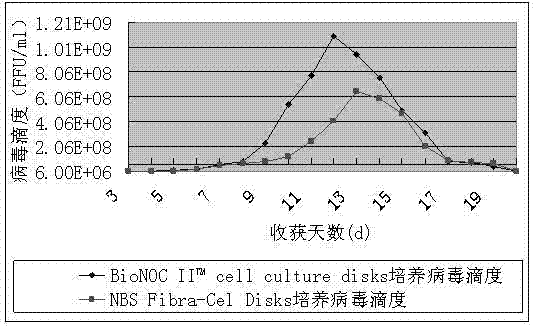
The invention relates to a method for producing rabies vaccines by applying a bioreactor and a sheet carrier, belonging to the technical field of biological products. The method comprises the following steps of: culturing cells by taking BioNOC IITM cell culture disks of the Taiwan SaiYu cell technology Co., Ltd as carriers in a Celligen 310 type 14 L bioreactor with a fixed bed basket-type stirring system; preparing rabies viruses with high titer through specific jar cell density, added viruses and the set parameters of the Celligen 310 type 14 L bioreactor; and producing the rabies vaccines. The method disclosed by the invention has the advantages of high density of cultured cells, high titer of the rabies viruses, high yield and low production cost; the Celligen 310 type 14 L bioreactor is enlarged in surface area used for cell adherence growth by adding the BioNOC IITM cell culture disks and then achieves the cell culture density at 5*108 / ml by creating good culture conditions; according to the method disclosed by the invention, the titer of the rabies viruses is correspondingly, greatly logarithmically enhanced and can maximally reach 1*109 FFU / ml, and therefore production cost is greatly reduced; in addition, the method disclosed by the invention is suitable for the large-scale production of rabies vaccines.
Owner:长春生物制品研究所有限责任公司
Antirabies virus IgG (Immunoglobulin G) antibody ELISA (Enzyme Linked Immunosorbent Assay) detection kit for dogs
ActiveCN101936998AMake up for the shortcomings of low sensitivityHigh sensitivityMaterial analysisPositive controlSerum protein albumin
The invention relates to an antirabies virus IgG (Immunoglobulin G) antibody kit for dogs. In the kit, an enzyme label plate is coated with anti-rabies virus monoclonal antibodies in advance, wherein a coating buffer solution is 0.05M of carbonate buffer solution with pH of 9.6, and the coating amount is 0.1-1ug per hole; 1-10% of BSA (Bull Serum Albumin) or skimmed milk in percentage by mass concentration is used as a blocking buffer; rabies virus purified antigens are coated after blocking, wherein the coating amount is 0.1-1ug per hole; 0.01mol / L PBS (Phosphate-Buffered Saline) with pH 7.2-7.4 containing 0.1-10% of BSA and 0.01-0.05% of NaN3 in percentage by mass concentration is used as a sample diluent; a horse radish peroxidase-rabbit anti-rabies IgG enzyme conjugate is used as an enzyme conjugate; 0.01mol / L PBS with pH 7.2-7.4 containing tween-20 with volume concentration of 0.05% is used as a washing concentrate; an enzyme substrate A solution is a 3,3'-5,5'-tetramethyl benzidine solution, and an enzyme substrate B solution is a hydrogen peroxide solution; a 1 mol / L H2SO4 solution is adopted as a stop solution; and a positive control and a negative control are arranged in the kit. With 100% of specificity and 1:640 of sensitivity, the kit is used for the immune effect evaluation of experimental dogs, pet dogs and common dogs which are inoculated with rabies vaccines.
Owner:WUHAN CHOPPER BIOLOGY
Method for removing residual DNA in hydrophobia vaccine product by utilizing ultrasound combined with EDTA solution
ActiveCN101780276AEffective removal of contentBreak through the quality bottleneckAntiviralsAntibody medical ingredientsAntigenHybrid protein
The invention provides a method for removing residual DNA in a hydrophobia vaccine product by utilizing ultrasound combined with EDTA solution. The method solves the problems that a method for extracting hydrophobia vaccine through concentration, purification and the like can only remove dissociative DNA in a certain percentage and cannot remove host DNA combined with antigen protein and clinical adverse reactions commonly occur. The method has main points of: adding the EDTA solution into hydrophobia vaccine concentrate; performing ultrasonic treatment on the hydrophobia vaccine by using ultrasonic wave to make the host DNA broken more easily under the action of the ultrasound; and removing the host DNA through chromatography and purification. The method has the advantages that: on the premise of ensuring the valence of the vaccine, the quality of a vaccine product is improved, mass hybrid protein and the host DNA are removed so that the residual DNA content of the vaccine product is less than 100 pg / dose, and the quality problem in the hydrophobia vaccine industry at present is solved.
Owner:LIAONING YISHENG BIOLOGY PHARMACY
Method for industrially producing animal rabies vaccine by utilizing bioreactor
InactiveCN102038947AHigh titerHigh degree of automation controlMicroorganism based processesAntiviralsUltrafiltrationBottle
The invention provides a method for industrially producing an animal rabies vaccine by utilizing a bioreactor, comprising the following steps of: (1) sterilizing a micro-carrier and the bioreactor, adding a cell growth solution, inoculating Vero cells for culturing, inoculating rabies viruses after the cell on the micro-carrier forms a compact single layer, and continuously culturing to propagate the viruses; (2) inoculating for 18h and then continuously harvesting a virus solution; (3) carrying out ultrafiltration concentration and virus inactivation on the harvested virus solution; and (4) purifying and inactivating the viruses through a column chromatography method to prepare the vaccine. In the invention, the high density culturing of the cells is carried out by utilizing a bioreactor micro-carrier culturing technology to produce the animal rabies vaccine. Compared with the traditional rotating bottle production method, the automation control degree is high, the production can be monitored in real time, the labor power and the cost are reduced, the production land is less, the scale is easy to expand, the produced virus titer is high, the difference among batches is less, the product quality is stable, and the side reaction is less.
Owner:WUHAN CHOPPER BIOLOGY
Rabies vaccine
InactiveUS20060275776A1Sufficient neutralizing antibody titerReduced rabies virus vaccine antigen concentrationSsRNA viruses negative-senseViral antigen ingredientsRabiesReduced dose
The invention provides for an immunogenic rabies vaccine comprising a reduced vaccine dose and methods of pre- and post-exposure immunization with a reduced dose. The concentration of rabies vaccine antigen per dose is preferably less than 2.5 IU / mL.
Owner:CHIRON BEHRING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com