Human diploid cell rabies vaccine virus seed and preparation method thereof
A technology for human diploid cells and rabies vaccine, applied in the biological field, can solve the problems of high vaccine cost, low virus titer, high price, etc., and achieve improved safety, major social and economic benefits, and good immunogenicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
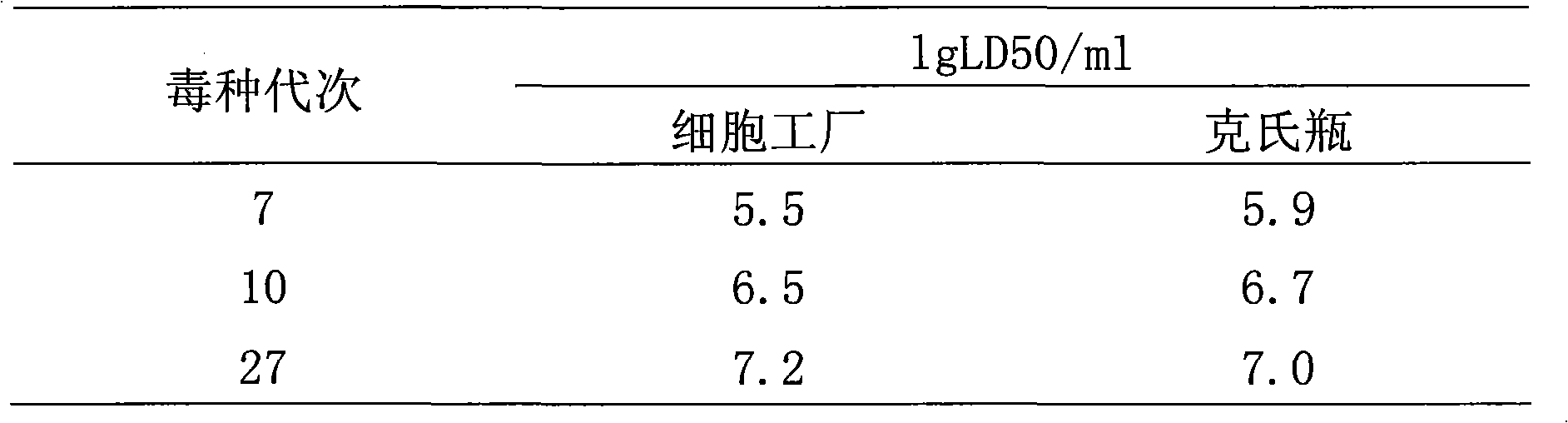
[0020] Human diploid cells (KMB17) were cultured and expanded at 35°C to 37°C with a cell nutrient solution consisting of Eagle's solution, milk protein and 2%-15% newborn bovine serum. When the cells grew into a dense monolayer, they were digested with trypsin-EDTA, the cells were collected, and CTN-1V 5 The strain was inoculated into human diploid cells (KMB17) by the suspension cell inoculation method at a dose of 0.005 to 1.0 MOI, cultured at 32 ° C to 37 ° C, pH 7.2 to 8.0, and the virus was harvested after culturing for 2 to 20 days. CTN-DK1, and continued passage in the same way. when passaged
[0021] At the time of CTN-DK20, the virus with higher titer was screened by the terminal dilution method and continued to be passaged for 9 generations, named CTN-DK29, and then screened by the terminal dilution method with CTN-DK29 and passed down for 4 generations, named CTN-DK33 , and then screened by terminal dilution method with CTN-DK33 and passed to 47 generations. CTN...
Embodiment 2
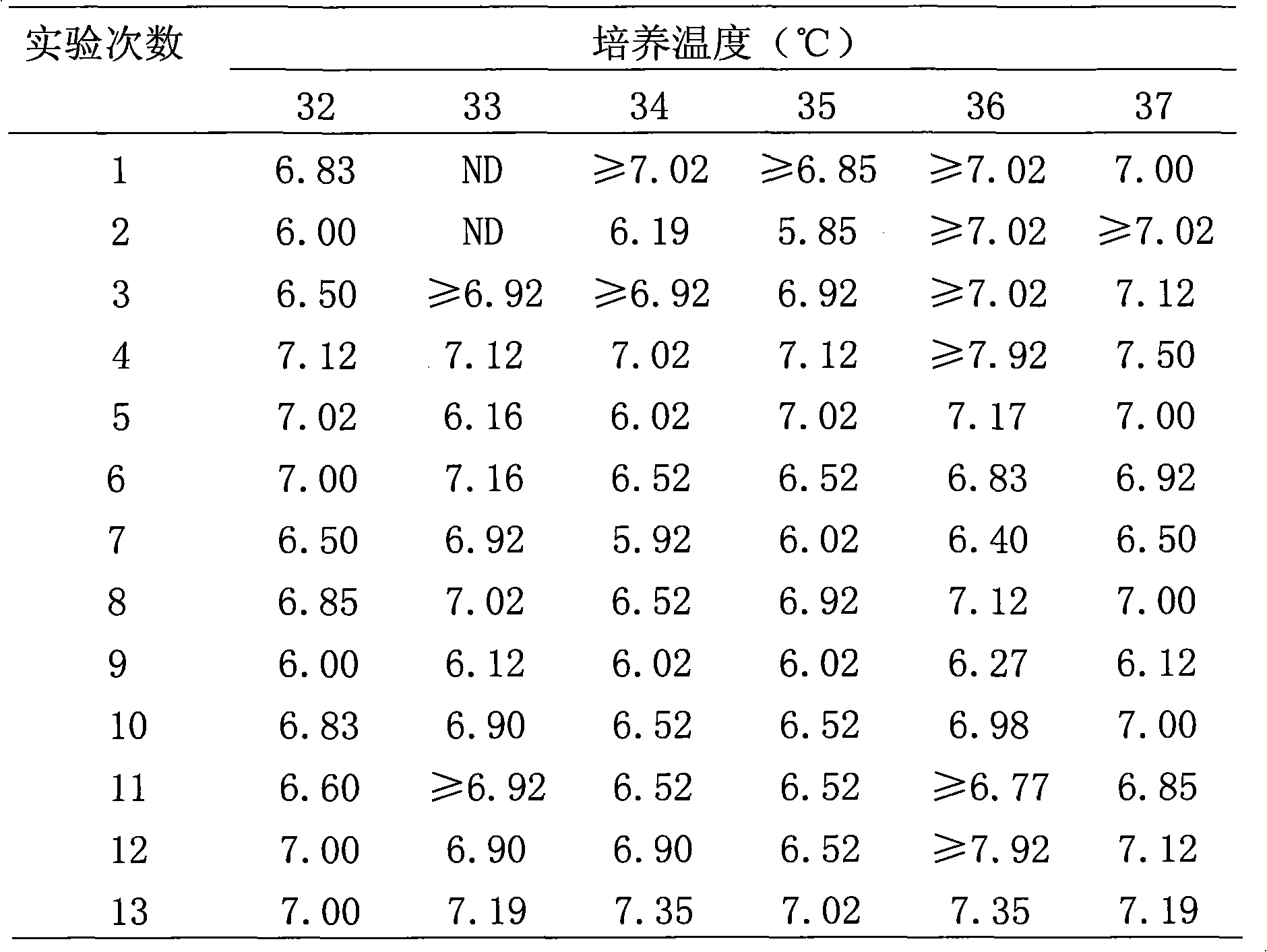
[0023] Using the suspension cell inoculation method, take the cells that have grown into a monolayer, routinely digest them with trypsin, collect the cells, and then suspend them in a growth medium (Eagle lactalbumin culture medium containing 10% to 15% newborn calf serum) in an appropriate proportion cell. The virus was inoculated into the suspension cells, cultured at 37°C until the cells grew into a monolayer, the original culture medium was removed, and the maintenance medium (Eagle lactalbumin medium containing 2% to 5% newborn calf serum) was replaced. Incubate at 33°C, 34°C, 35°C, 36°C, and 37°C, and harvest the virus solution in 5 to 7 days. The harvested virus liquid was detected by rabies virus titer titration test (LD50). The results are shown in Table 1.
[0024] Table 1 Virus titers (lgLD50 / ml) of virus species at different culture temperatures in human diploid cells (KMB17)
[0025]
[0026] The above data were tested by T test, 0.2
Embodiment 3
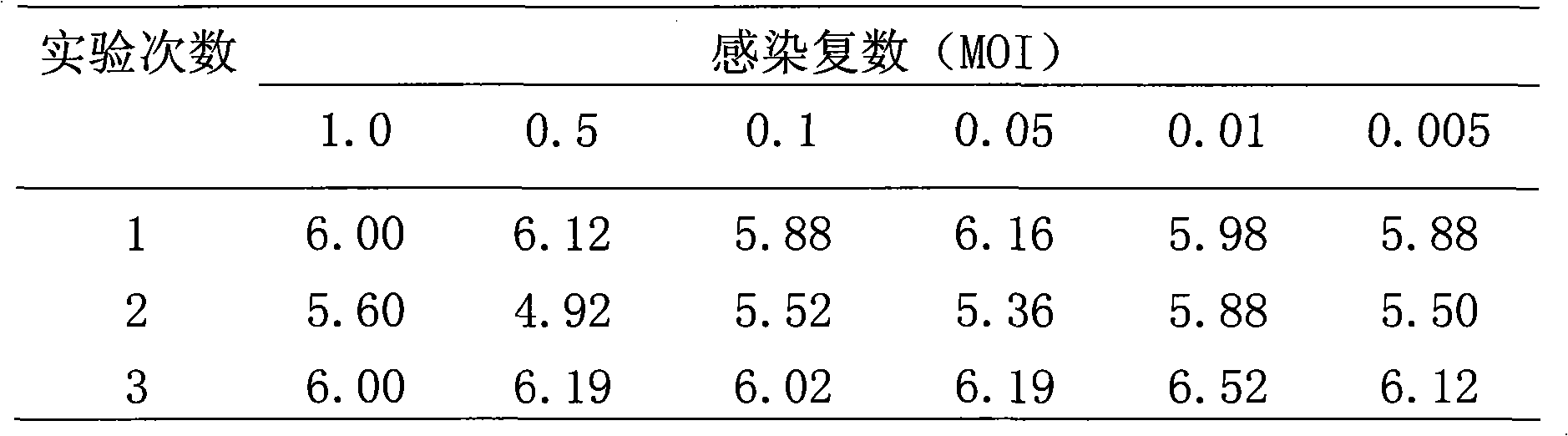
[0028] In order to determine the optimal inoculation amount of the virus species, experiments were carried out to infect human diploid cells (KMB17) with different multiplicity of infection (MOI) of the immobilized rabies virus CTN-DK strain. The method is to make a cell suspension of KMB-17 cells grown in log phase and count them. The titrated virus was inoculated into suspension cells at MOI of 1.0, 0.5, 0.1, 0.05, 0.01, 0.005, cultured at a suitable temperature, and the virus liquid was harvested after culturing for a certain period of time. The harvested virus liquid was detected by rabies virus titer titration test (LD50). The results are shown in Table 2.
[0029]Table 2 Virus titers (lgLD50 / ml) of human diploid cells (KMB17) infected with different MOIs of virus species
[0030]
[0031] The results showed that there was no significant difference in the yield of cells infected with different virus numbers. Therefore, it is more appropriate to use 0.005-1.0 MOI to i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com