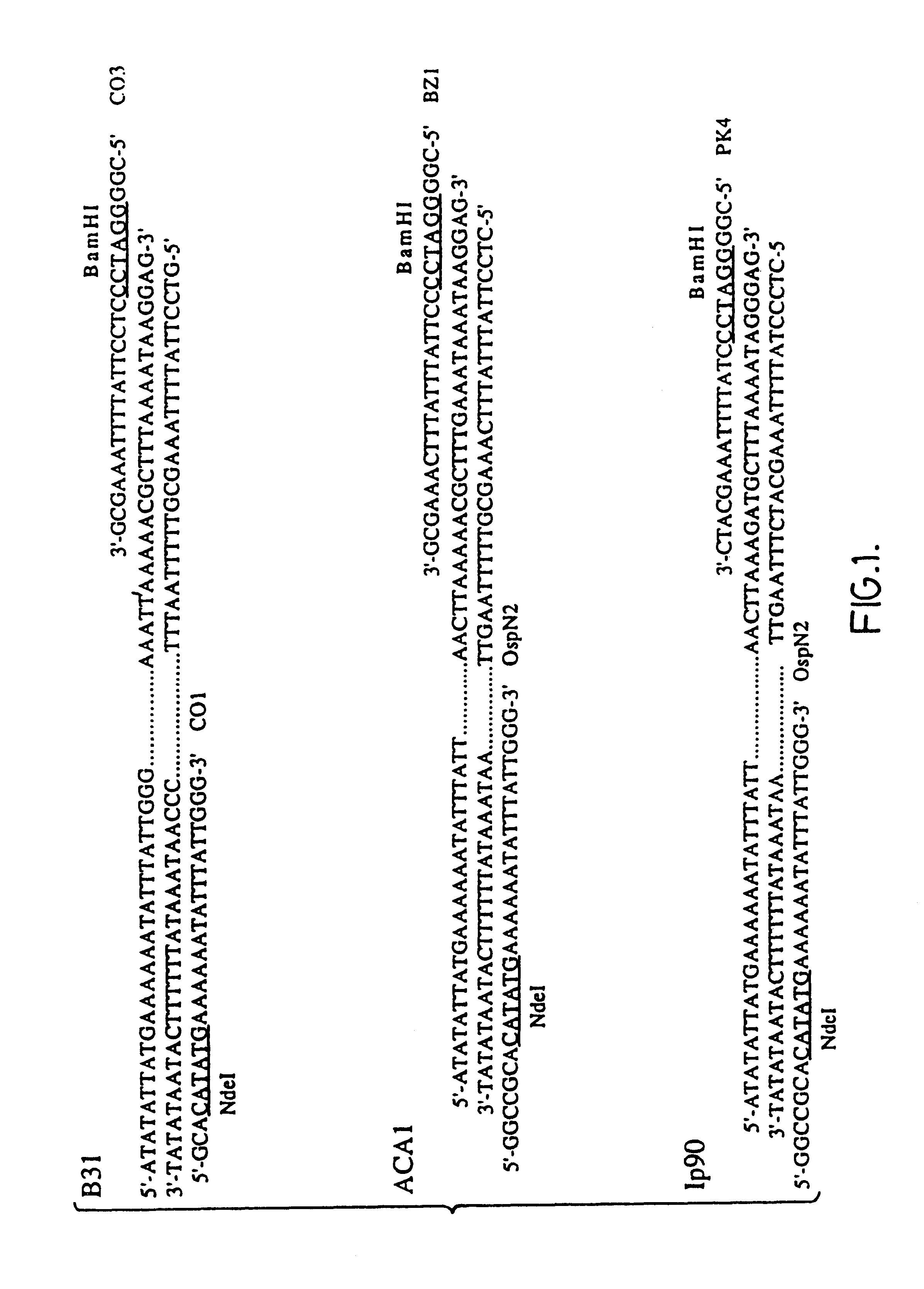
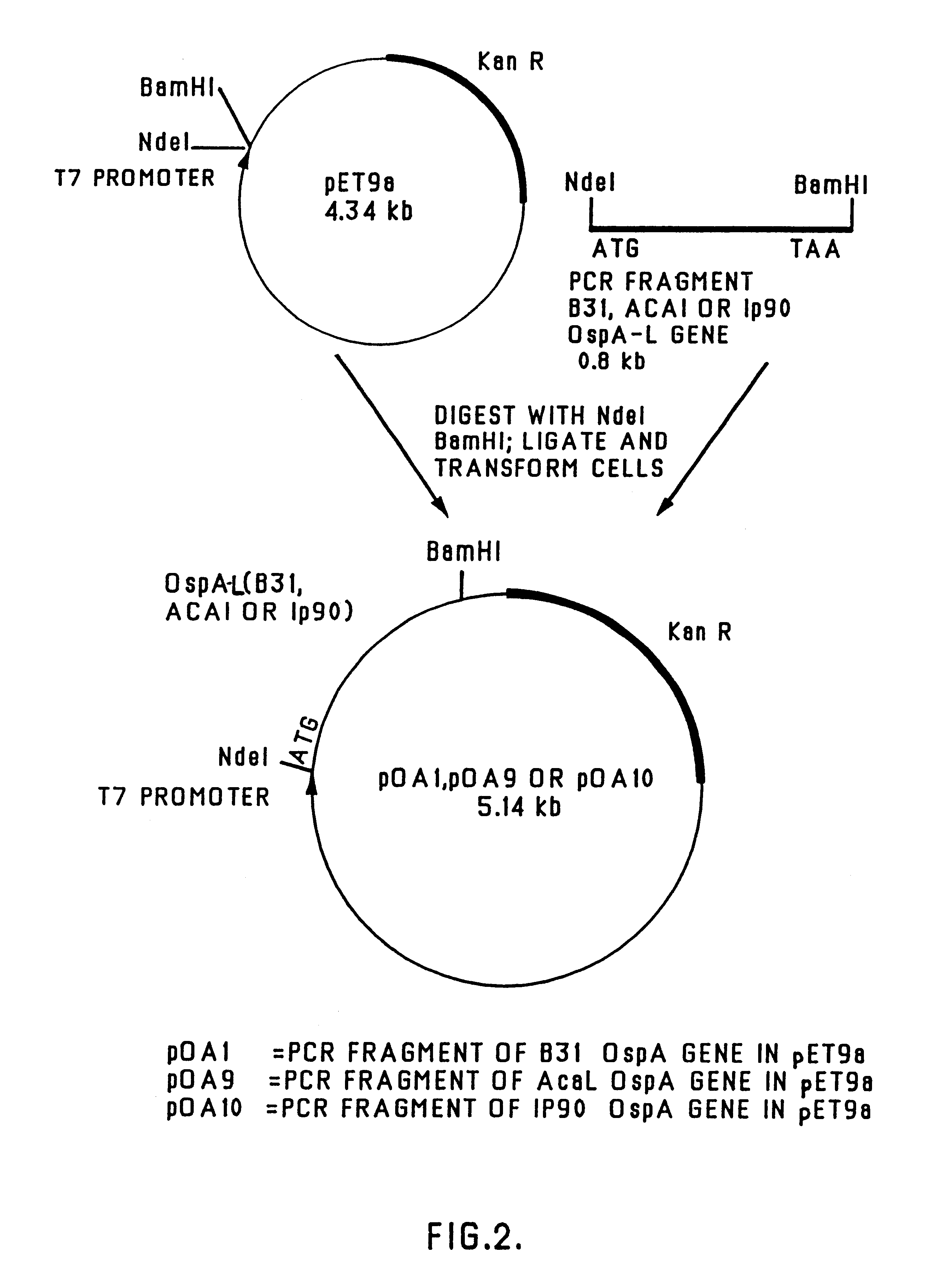
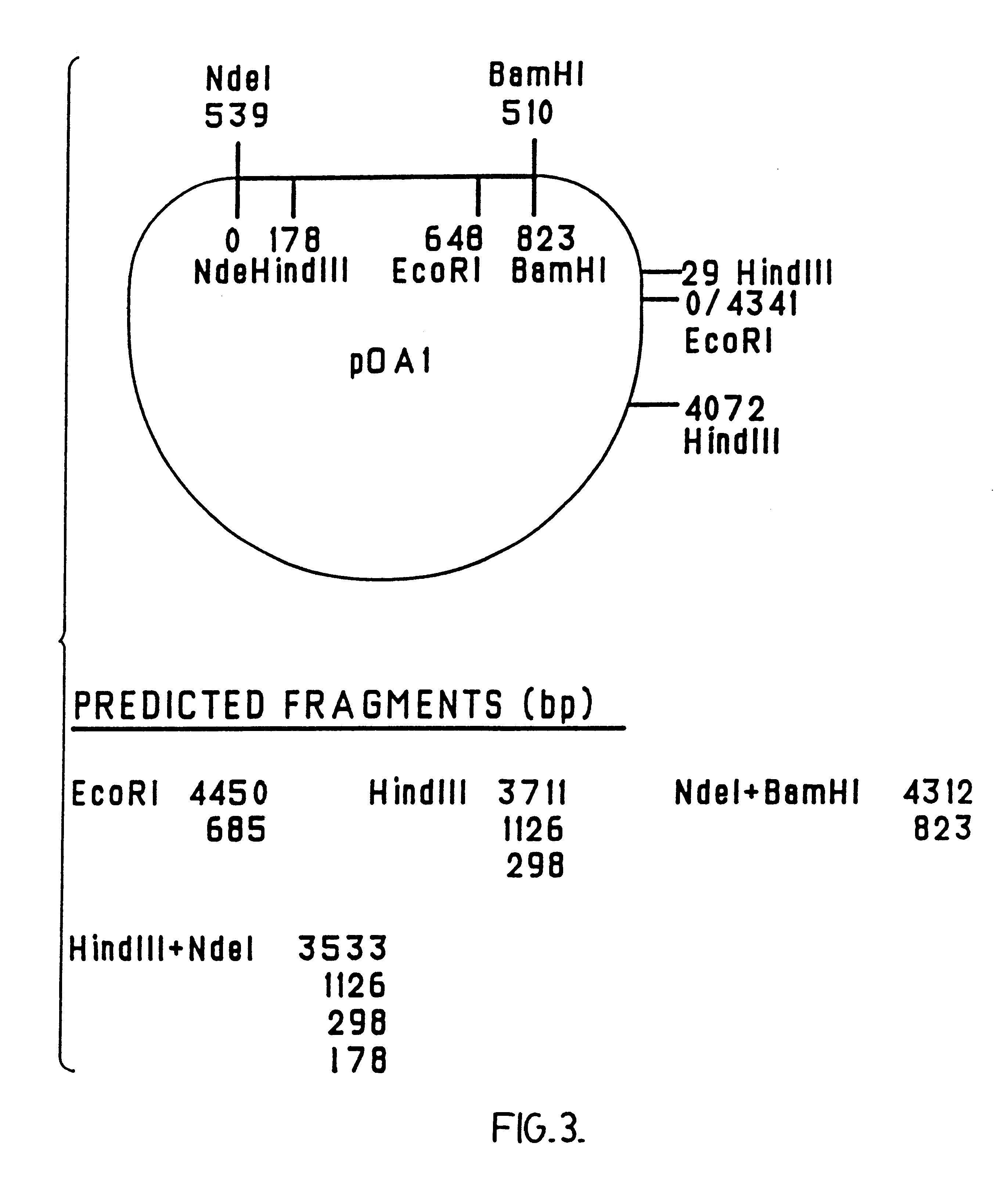
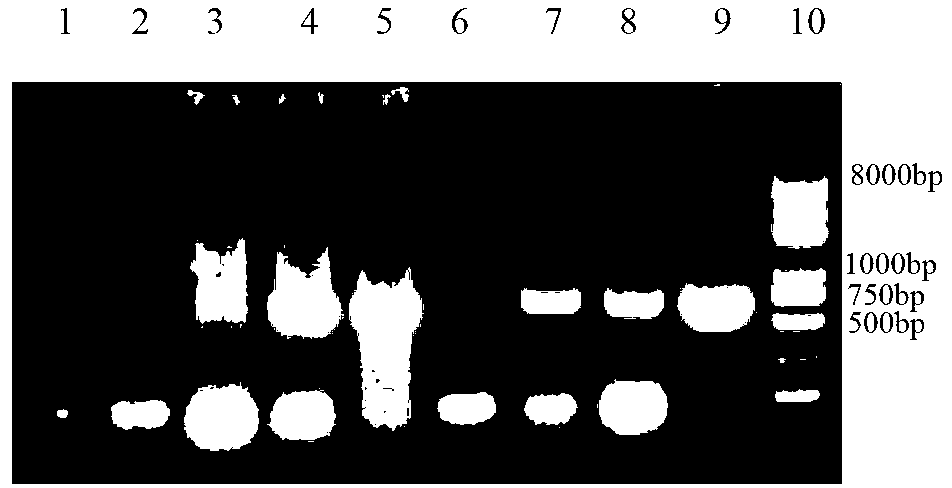
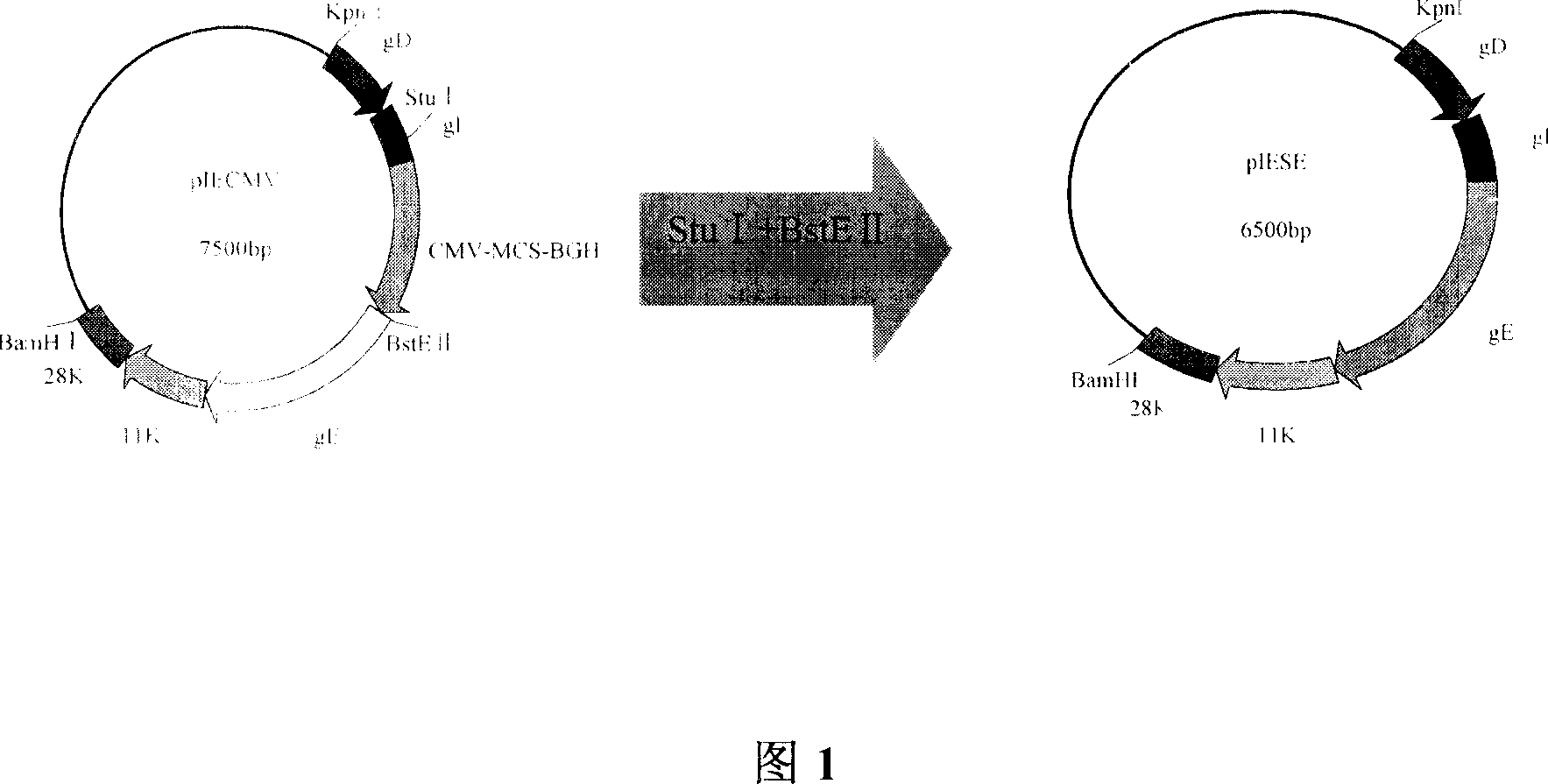
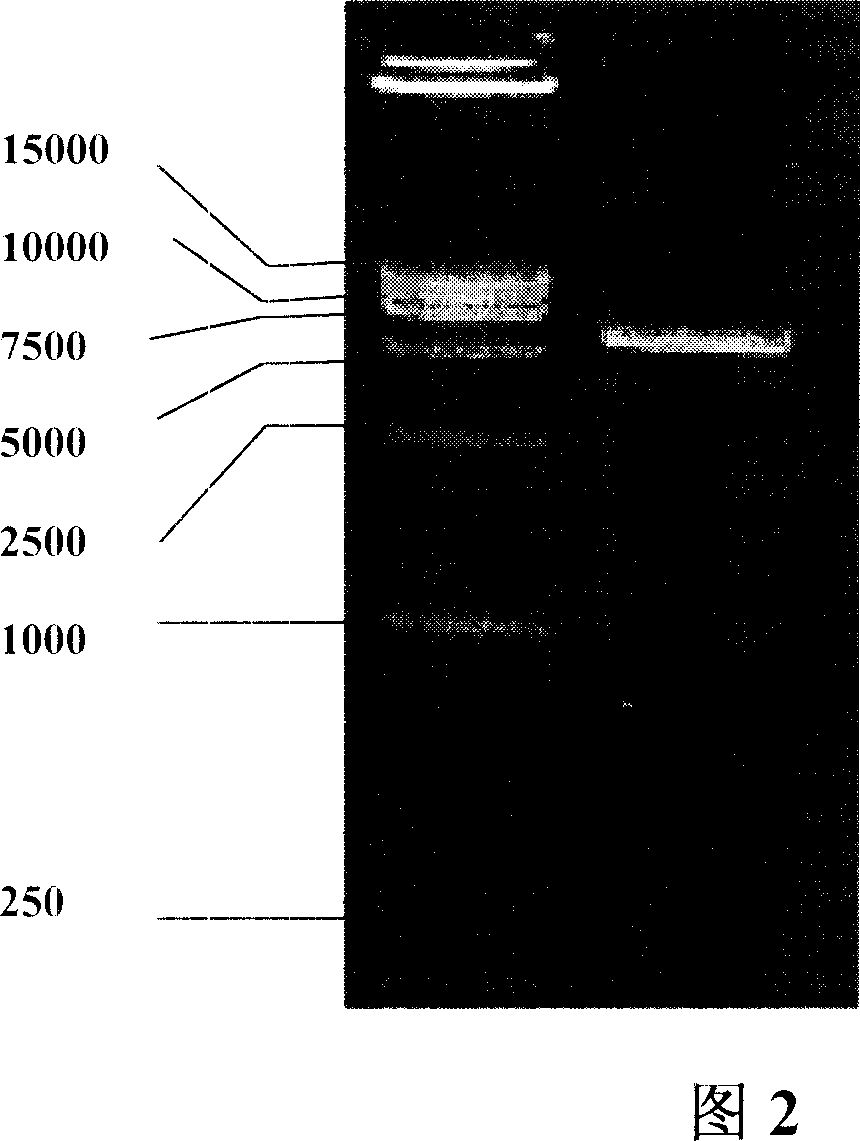
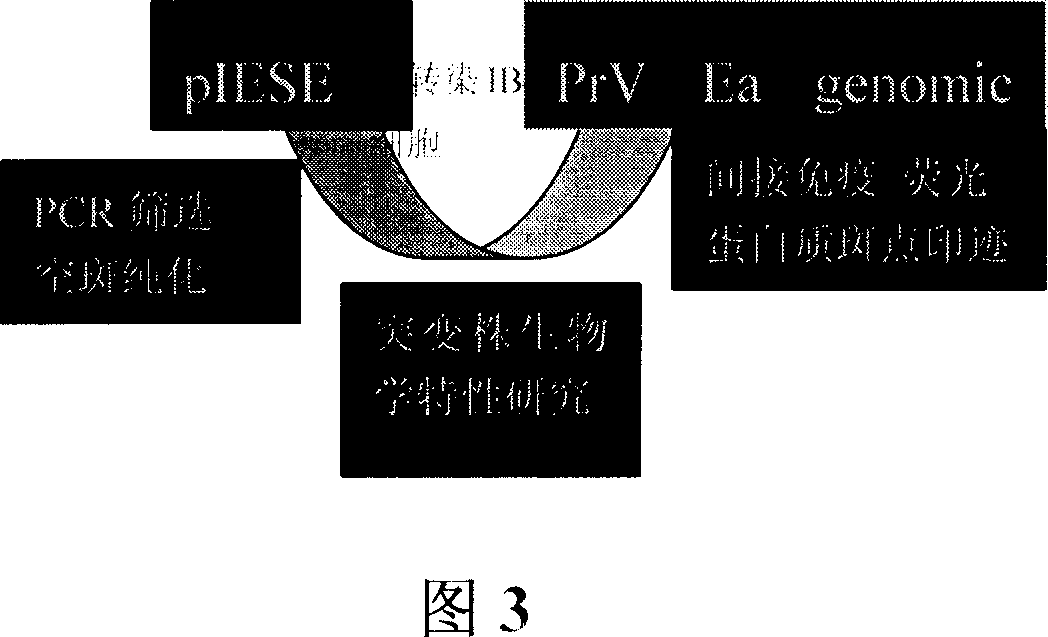
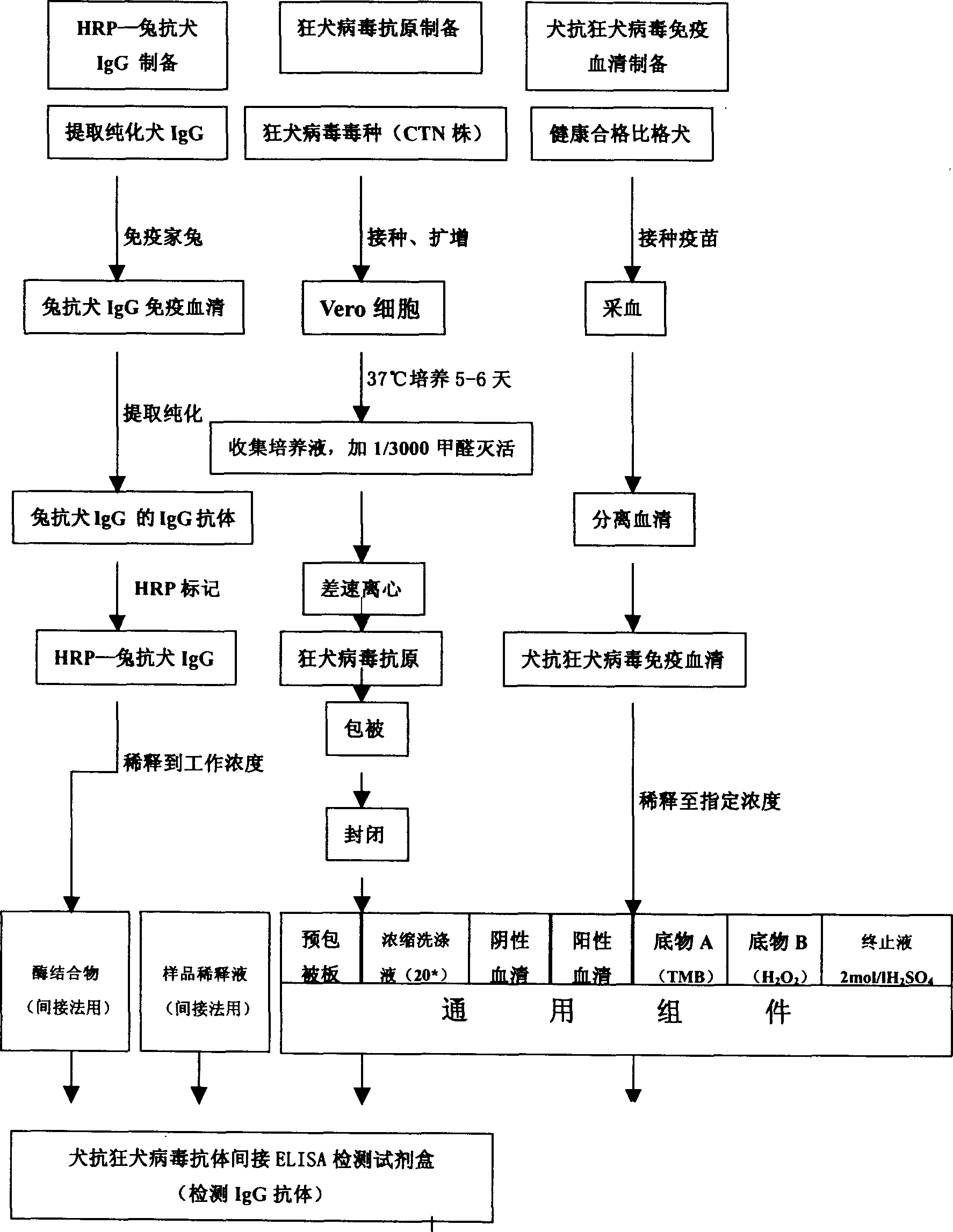
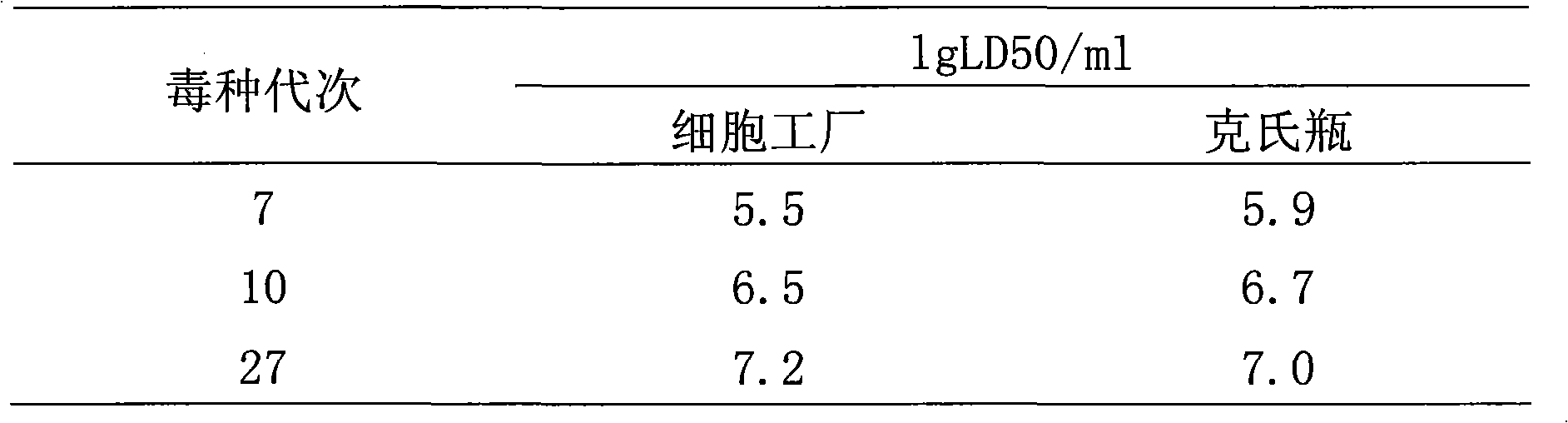
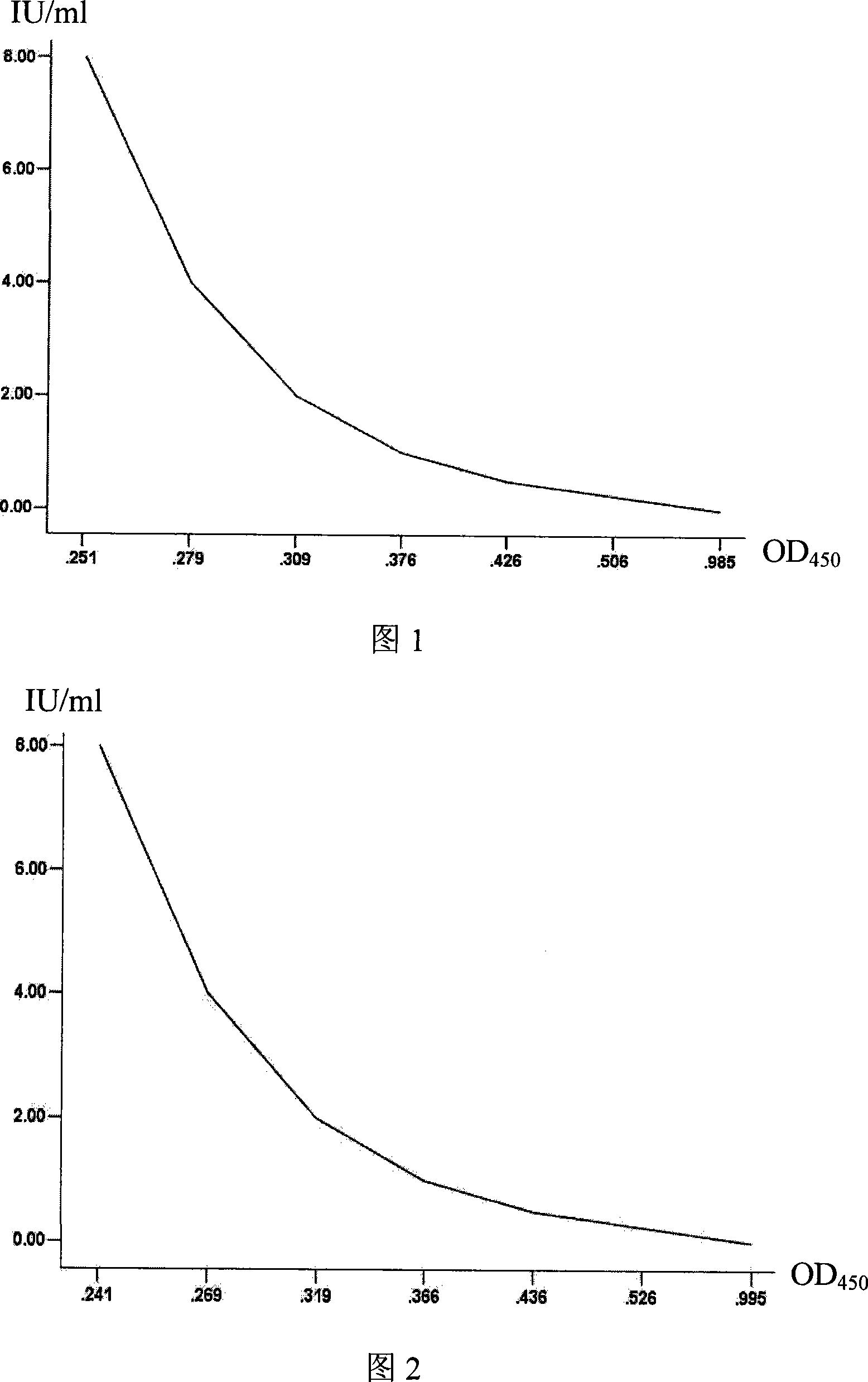
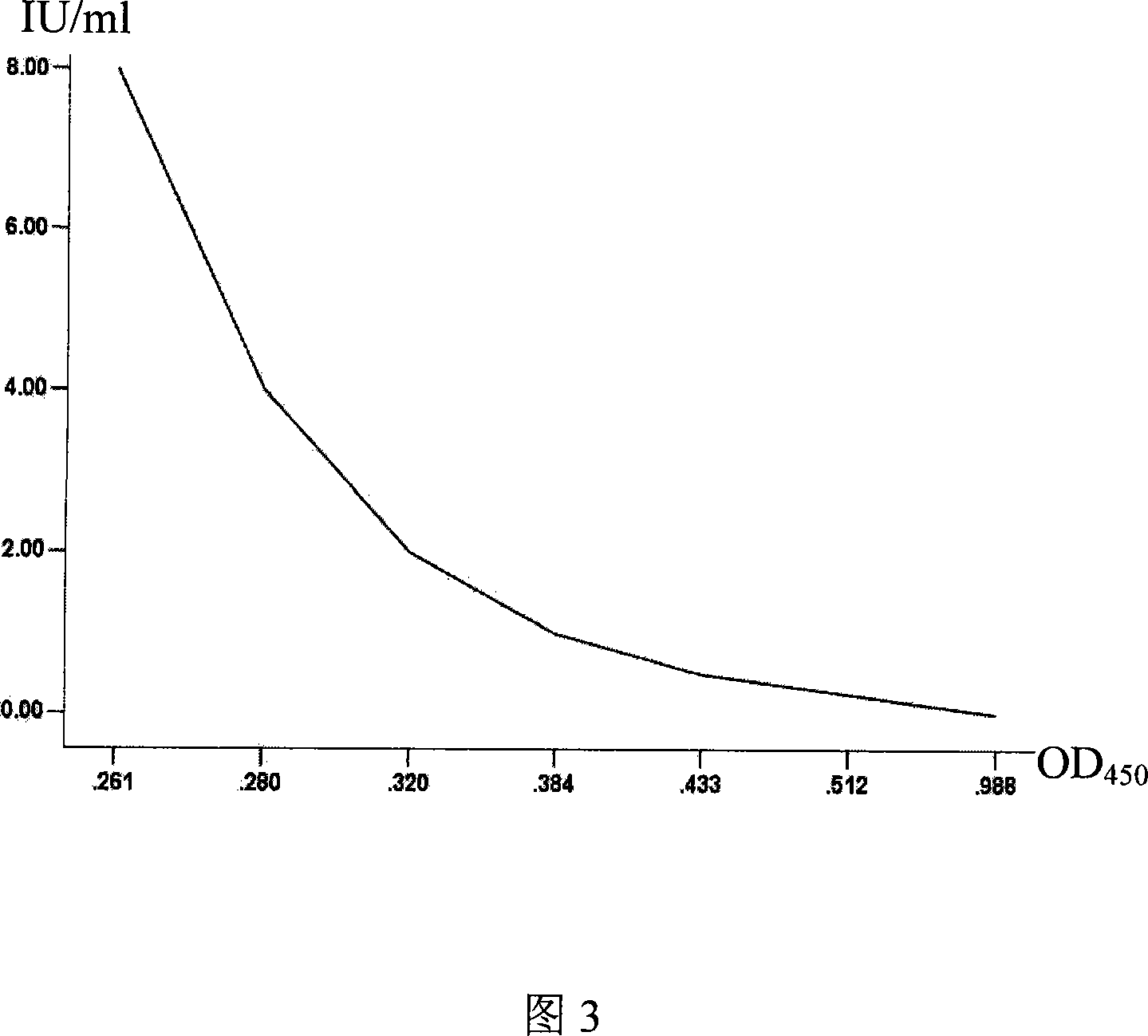
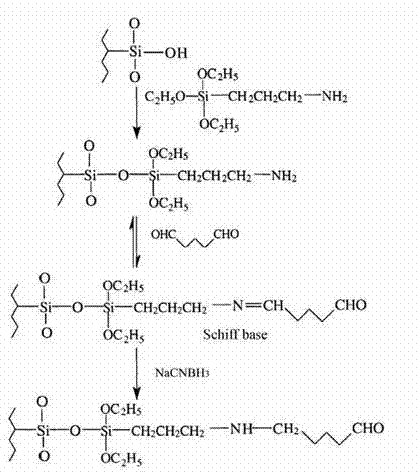
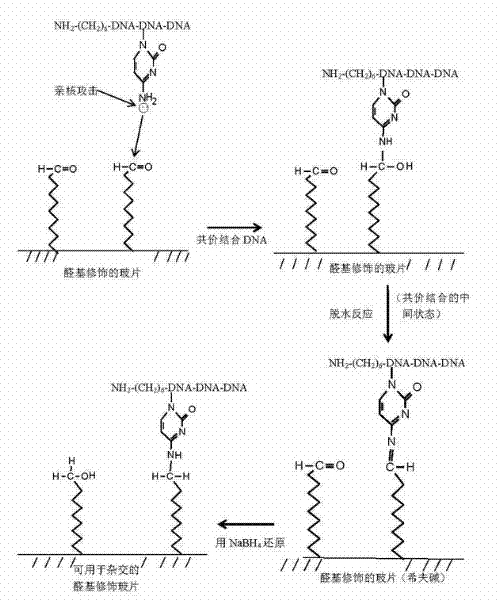
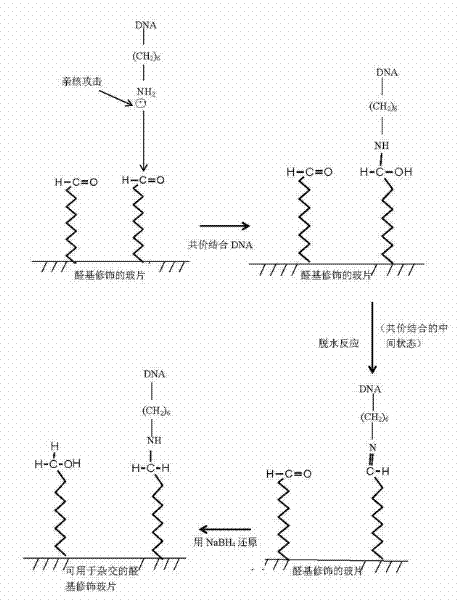
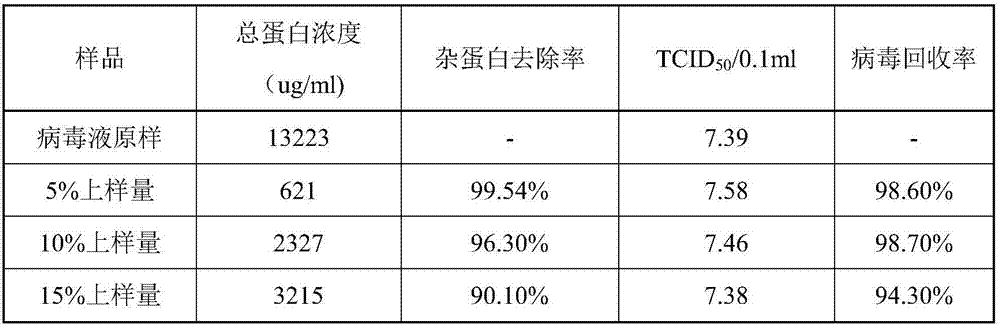
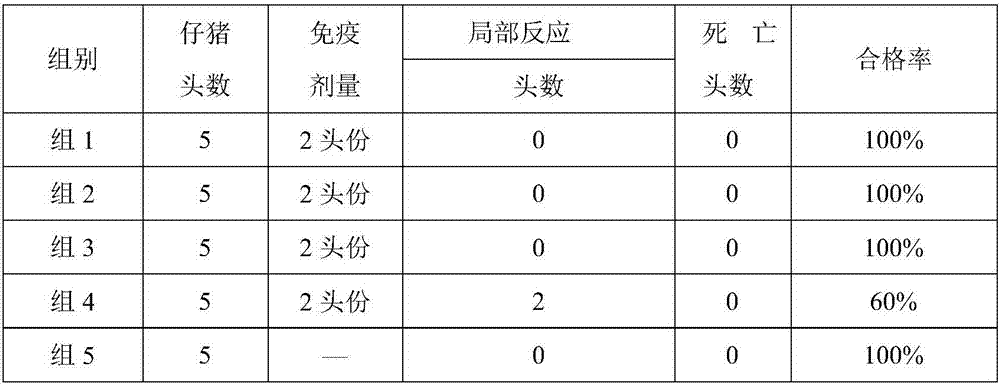
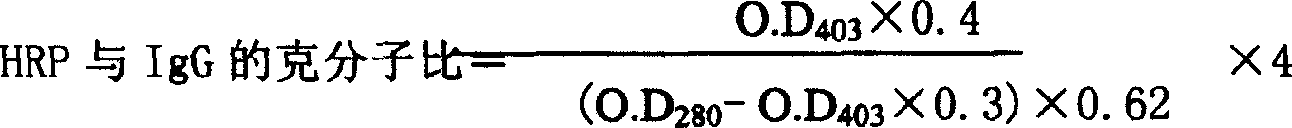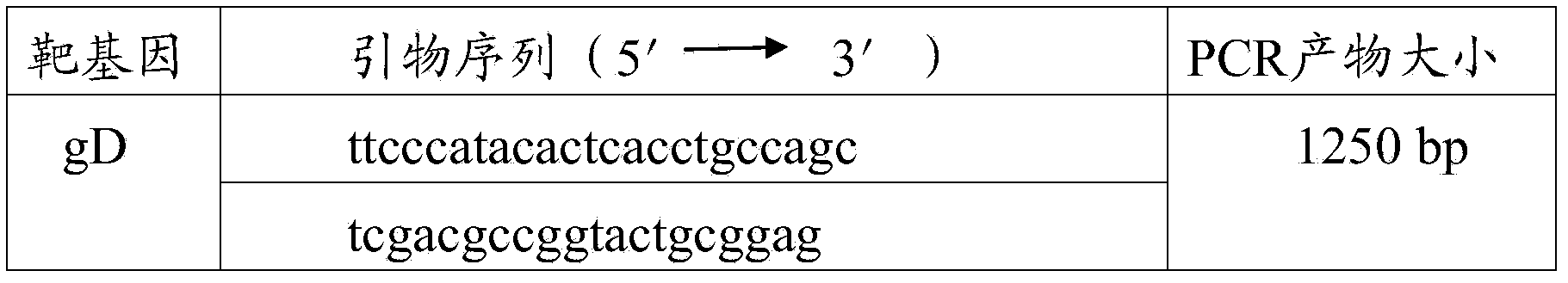Patents
Literature
358 results about "Rabies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A viral disease which spreads by the saliva of infected animals and leads to brain inflammation.
Lyme combination compositions and uses
InactiveUS6368603B1Safe and efficacious in dogNo exacerbation of diseaseAntibacterial agentsNanotechAntigenRabies
Disclosed and claimed are compositions containing a Borrelia burgdorferi antigen, and methods for making and using them. The antigen can be OspA. The compositions can contain at least one additional antigen from a pathogen other than Borrelia burgdorferi. The compositions are useful for eliciting an immunological response in a host mammal susceptible to Lyme Disease and to the mammalian pathogen other than Borrelia burgdorferi. Suitable host mammals include dogs, pups, horses, and, the additional antigen can be of a canine, equine or feline pathogen, such as rabies, canine distemper, adenovirus, coronavirus, parainfluenza and parvovirus. No significant efficacy interference is observed.
Owner:MERIAL LTD
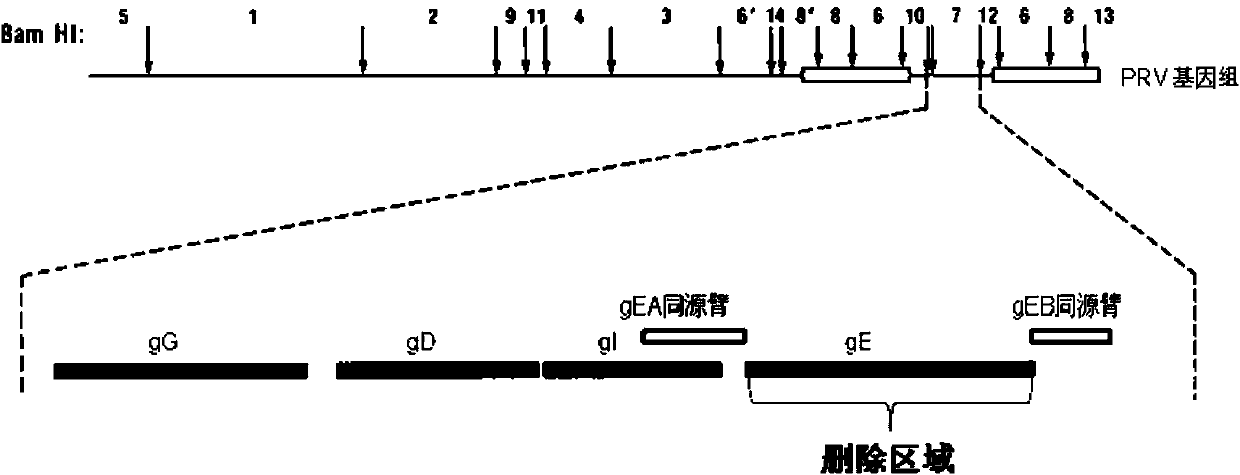
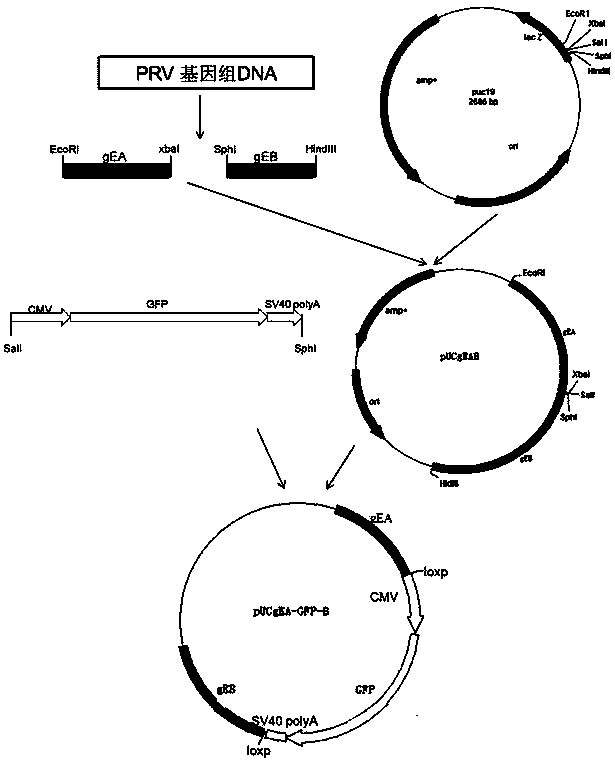
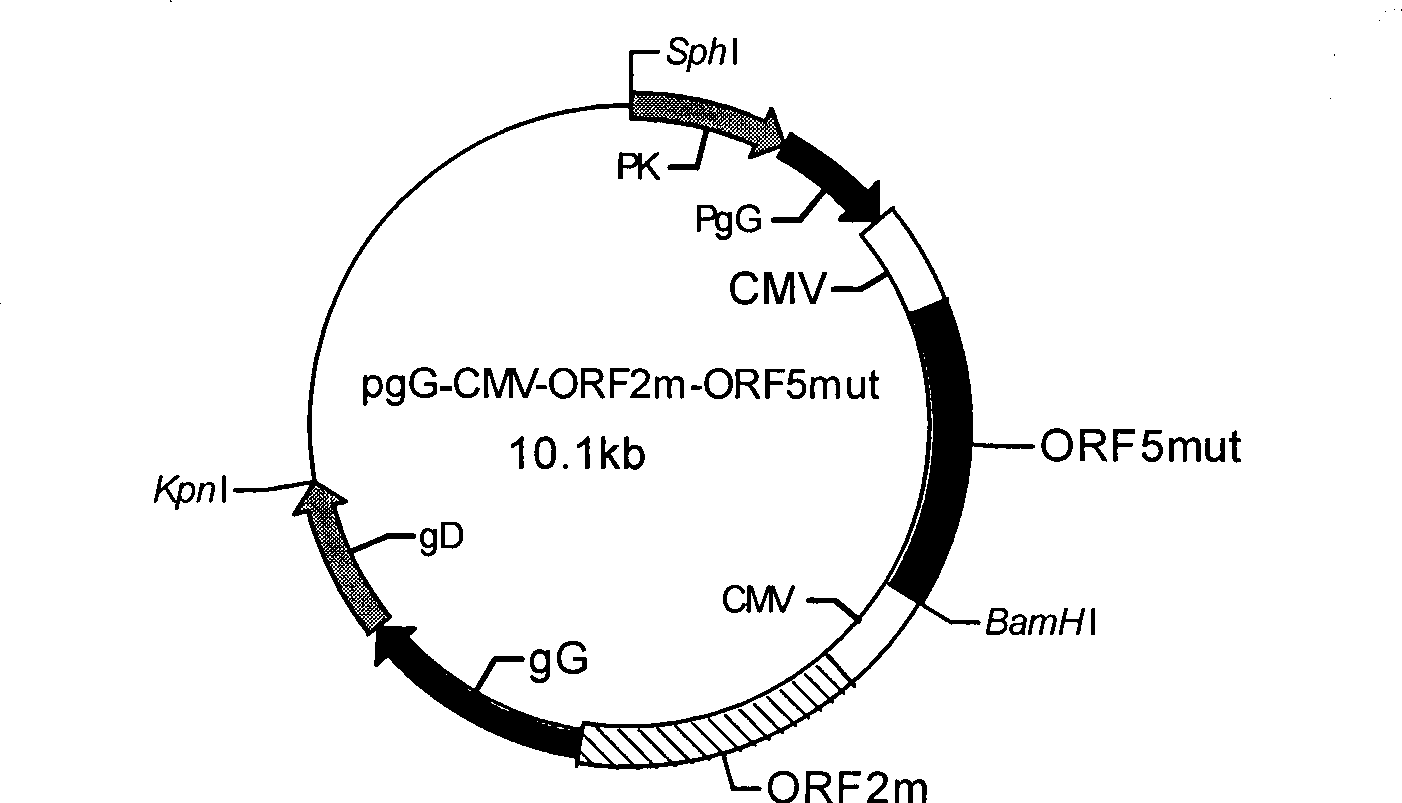
Porcine pseudorabies virus virulent strain, and gene deletion vaccine strain thereof and applications thereof
ActiveCN102994458AEffective preventionEffective therapeuticMicroorganism based processesAntiviralsRabiesMicrobacterium
The invention discloses a porcine pseudorabies virus virulent strain, and a gene deletion vaccine strain thereof and applications thereof. The porcine pseudorabies virus virulent strain is named as HeN1, the microbial preservation number of the porcine pseudorabies virus virulent strain is CGMCC NO.6656, the deleted gE gene obtaines the gene deletion vaccine strain rPRV-gE-EGFP+ on the basis of the virulent strain HeN1, and the microbial preservation number is CGMCC NO.6657. The virulent strain can be prepared into inactivated vaccine (single vaccine or combined vaccine), the gene deletion vaccine strain rPRV-gE-EGFP+ can be prepared into activated vaccine or inactivated vaccine (single vaccine or combined vaccine) and the like, so that porcine pseudorabies can be effectively prevented or cured, or the gene deletion vaccine strain rPRV-gE-EGFP+ can be prepared into a diagnosis reagent for diagnosing the porcine pseudorabies. The gene deletion vaccine strain rPRV-gE-EGFP+ has the advantages of being good in safety, high in protection efficiency, beneficial to differential diagnosis.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Porcine pseudorabies virus (PRV) variant PRV-ZJ01 and application thereof
ActiveCN103627678AImprove securityImproving immunogenicityMicroorganism based processesAntiviralsRabiesEngineering
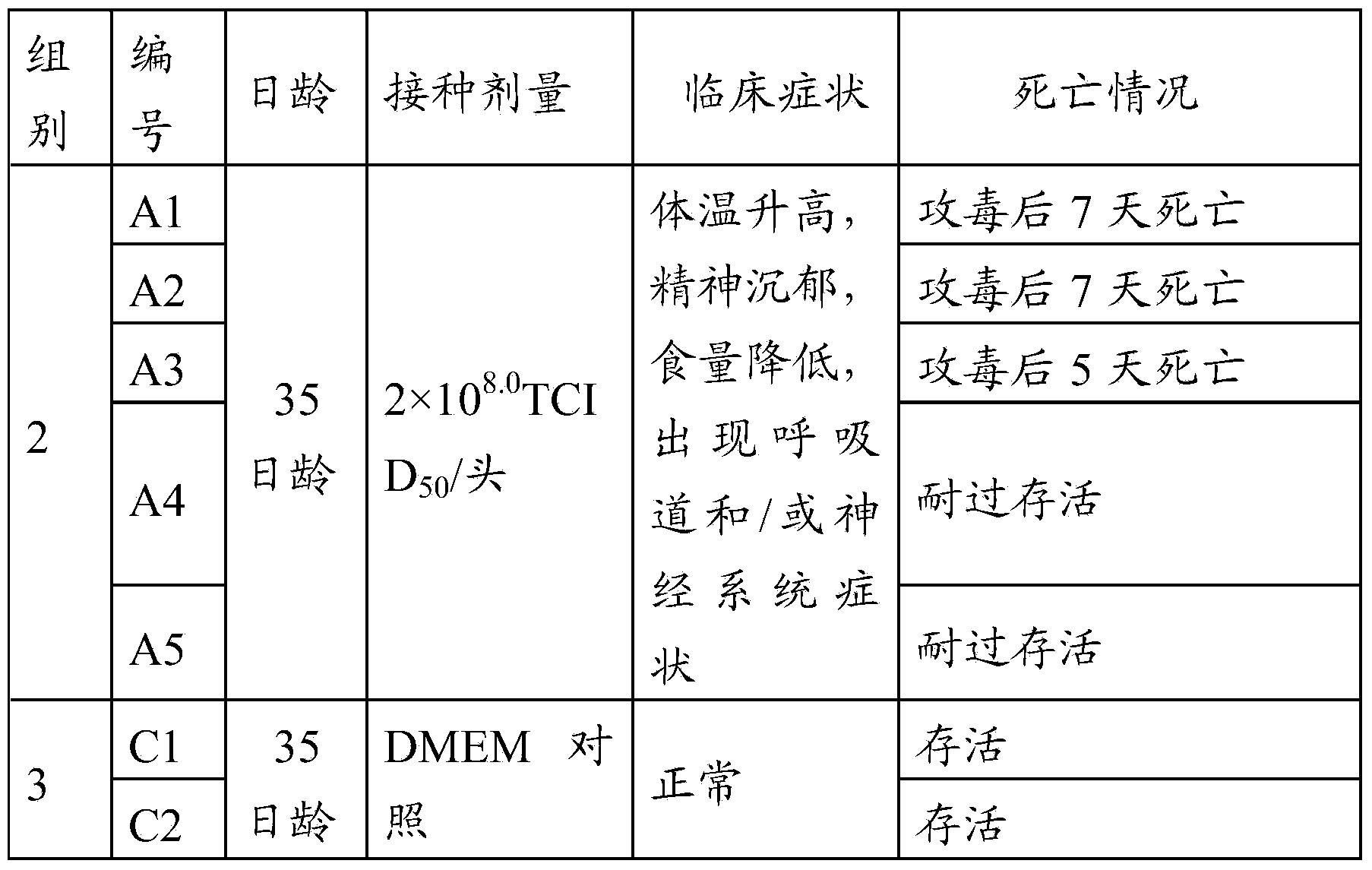
The invention relates to the technical field of porcine pseudorabies viruses (PRVs) and in particular relates to a porcine PRV variant PRV-ZJ01 with collection number of CGMCCNo.8170 and an application of the porcine PRV variant PRV-ZJ01 in preparation of vaccines. The porcine PRV variant PRV-ZJ01 has the beneficial effects that a water-soluble inactivated vaccine is prepared by adopting a PRV-ZJ01 variant virus solution and is subjected to a swine immune protection test with live vaccines of Bartha-K61, Bucharest and HB-98 strains and the results show that the inactivated vaccine of the ZJ01 strain has relatively high safety and has the immune protection efficiency obviously higher than that of immunity groups of the live vaccines of the Bartha-K61, Bucharest and HB-98 strains, and the live vaccines of the Bartha-K61, Bucharest and HB-98 strains can not provide full protection for the ZJ01 very virulent strain; the inactivated vaccine of ZJ01 has relatively good immune protection effects on the PRV variant and the traditional strains; infected with 10<6.0>TCID50 (Tissue culture infectious dose 50) / ml nasal drops of the PRV-ZJ01 variant, all the 85-day-old non-immune swine can become ill and die; results prove that the virulence of the virus strain is obviously enhanced, the antigenicity is varied and the virus strain has relatively good immunogenicity after being inactivated and can be used for research and development of the vaccine of the virus strain and the diagnostic methods.
Owner:NANJING AGRICULTURAL UNIVERSITY
Porcine pseudorabies virus gene deletion strain, vaccine composition, and preparation method and application of vaccine composition
ActiveCN103923884ASymptoms relieved or improvedMicroorganism based processesAntiviralsVirus antigenTGE VACCINE
The invention provides a porcine pseudorabies virus gene deletion strain, a vaccine composition, and a preparation method and an application of the vaccine composition. The vaccine composition comprises an immunizing dose of an attenuated livetotivirus antigen and an inactivated totivirus antigen of the porcine pseudorabies virus gene deletion strain or its culture. The vaccine composition can effectively induce the antibody production, can effectively protect pigs, and can be used as a marking vaccine to effectively differentiate wild strains and vaccine strains.
Owner:PU LIKE BIO ENG
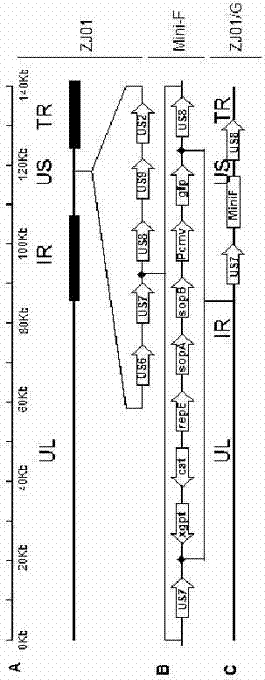
gE- and gI-deleted porcine pseudorabies virus variant strain and use thereof
The invention relates to the technical field of porcine pseudorabies viruses and especially relates to a gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G and a use thereof. The gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G has the accession number of CGMCC No.7957. The invention discloses the use of the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G in vaccine preparation. After the New Zealand big white rabbit is inoculated with the 106.0TCID50 recombinant viruses, clinical symptoms such as pruritus are not caused. An oil-in-water inactivated vaccine prepared from the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G is injected into a piglet and after four weeks, the BELISA antibody is produced but the gE antibody does not exist, and the immunization protection efficiency is 100%. After immunization on sows, the piglets produced by the sows get immunization protection and the efficiency of PRV variant virus and traditional virus immunization protection is 100%. It is proved that the ZJ011G recombinant virus has good immunogenicity and can be used for vaccine preparation.
Owner:JIANGSU NANNONG HI TECH
Recombinant porcine pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus genetic engineering strain and application
InactiveCN101457215AAvoid infectionPrevention of swine pseudorabiesViral antigen ingredientsMicroorganism based processesAnimal virusAntigen
The invention belongs to the animal virus genetic engineering technique field, especially to a recombinant porcine pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus gene engineering strain construction, a vaccine preparation and applications. The recombinant porcine pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus gene engineering strain E001 lacks the pseudorabies virus main virulence gene TK, glycoprotein genes gG, gE and gI, does not express the functional glycoprotein gG and gE / gI of the pseudorabies virus; and simultaneously expresses a GP5m / M protein antigen of the porcine propagate and breath complex virus classical strain and the porcine propagate and breath complex virus internal variant GP5 protein and porcine circovirus ORF2. The strain can stimulate the porcine to produce protective immunity reaction for resisting pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus, effectively prevent the infection of the pseudorabies virus, the porcine propagate and breath complex virus and porcine circovirus. The invention also discloses a preparation and application of a tervalence genetic engineering vaccine.
Owner:HUAZHONG AGRI UNIV
Rabies cure
InactiveUS20110020279A1Inhibit rabies virus multiplicationAvoid spreadingSsRNA viruses negative-senseOrganic active ingredientsSubarachnoid spacePresent method
This invention is for a method of treatment of rabies once the patient develops signs and symptoms of rabies with the intent to save the patients from death and disability using insulin combined with various anti rabies viral therapeutic, pharmaceutical, biochemical, and biological agents or compounds with added supportive therapies administered through OM, SAS, IVB, IV, and IA routes. An embodiment provides devices for intranasal delivery of therapeutic agents to olfactory mucosal area. Another embodiment uses the technology to deliver the therapeutic, pharmaceutical, biochemical, and biological agents or compounds to the subarachnoid space and ventricular system by using continuous catheters and Ommaya reservoir at the same time. The present method incorporates breaking the blood brain barrier to allow the entry of the anti rabies therapeutic agents into the neuropile. Additionally, an embodiment incorporates cooling of the brain and inducing hibernation to preserve the brain from damage due to rabies.
Owner:SHANTHA TOTADA R
Pseudo-rabies gE/gI-gene loss poison strain, killed vaccine containing it and use
ActiveCN1940063AStrong targetingFacilitate chemical processingViral antigen ingredientsViruses/bacteriophagesRabiesPseudorabies Virus PRV
A recombinant Pseudorabies virus PrV gene engineering strain WKQ-001, inactivated vaccine containing the poisonous strain and its use are disclosed. It can be used to discriminate and diagnose artificial immunity pig or natural infectious pig. It's safe and doesn't contain exogenous gene.
Owner:HUAZHONG AGRI UNIV
IgG kit for detecting streetvirus of dogs using indirect enzyme immunosorbent assay and preparation method thereof
The invention refers to a kind of detecting reagent box and the manufacturing method, concretely refers to the reagent which is indirect enzyme immune sorption experiment for detecting rabies virus IgG and the manufacturing method. The reagent box compositions are: beforehand enclosed rabies virus antigen enzyme label board, sample diluting solution, HRP-rabies resisting IgG enzyme compound, condensed washer solvent, substrate and stopping liquid. The specificity of the reagent can reach 100%; the sensitivity is 1:640; the accuracy (the variation coefficient) is 6.98%. The reagent uses indirect ELISA to detect the rabies virus IgG antibody.
Owner:湖北省预防医学科学院
Rabies antibody gold immunochromatography assay testing indicator paper and preparation technique
This invention provides one dog rabies virus antigen glue gold immune chromatography test paper and its process, which comprises the following steps: according to antigen antibody immune combination basic principle to label the sheep IgG by glue gold label covering on the glass fiber film as combination pad; covering the purification rabies virus antigen and gene engineer expressed antigen and rabit IgG covering on the NC film as test line and quality control line; when testing the antibody, forming antibody combination to expose red band.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Porcine pseudorabies virus strain as well as inactivated vaccine and applications thereof
ActiveCN103305474APromote rapid proliferationHigh titerMicroorganism based processesAntiviralsLaboratory cultureVirus strain
The invention discloses a porcine pseudorabies virus strain as well as an inactivated vaccine and applications thereof, belonging to the field of separation and application of the porcine pseudorabies virus strain. The invention firstly provides a porcine pseudorabies virus BJ strain separated from diseased pig tissues, and the microbial preservation number of the porcine pseudorabies virus BJ strain is CGMCC (China General Microbiological Culture Collection Center) No.7351. The invention discloses a method for preparing the inactivated vaccine by applying the porcine pseudorabies virus BJ strain. The method comprises the steps of culturing a virus strain to obtain a virus solution; adding an inactivator, and inactivating and concentrating the virus solution; and evenly mixing an adjuvant and the virus solution, and emulsifying to obtain the inactivated vaccine. The technological parameters of the inactivated vaccine preparation method are further optimized, and the immune protection efficacy and safety of the inactivated vaccine can be improved. Shown by the immune protection efficacy and safety tests, the porcine pseudorabies inactivated vaccine prepared has good immune protection efficacy and safety, and can be clinically used for preventing or treating porcine pseudorabies.
Owner:泰州博莱得利生物科技有限公司 +2
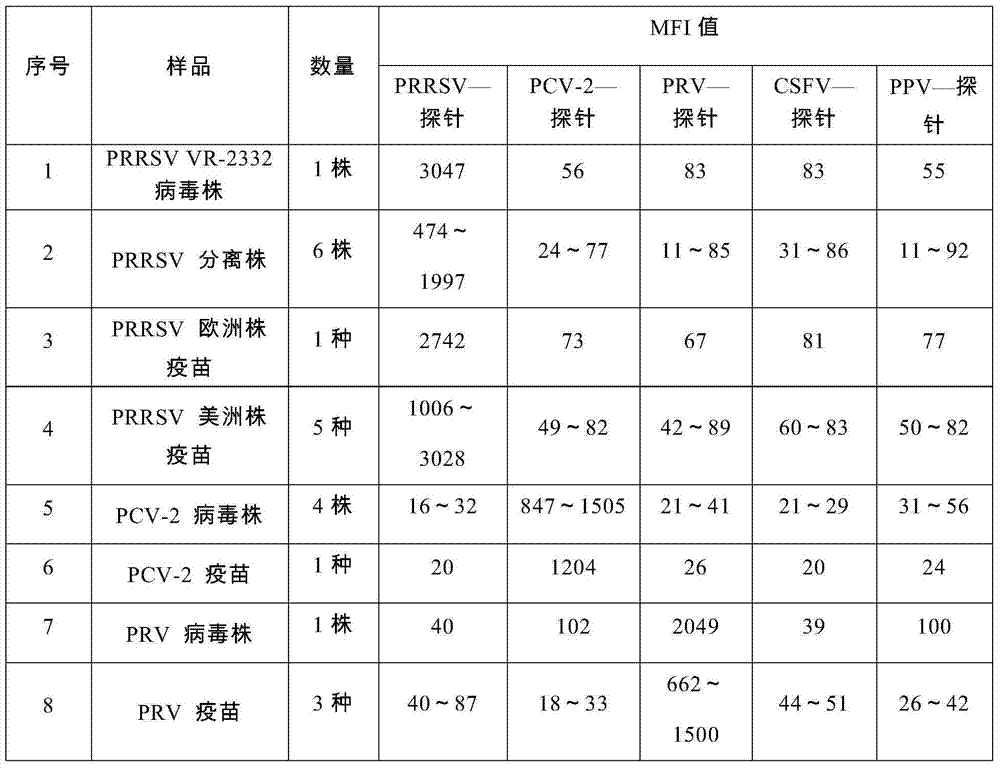
Nucleic acids of liquid-phase gene chip for synchronously detecting five porcine viruses and detection method thereof
ActiveCN104328218AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVMultiplex
The invention provides a set of nucleic acids of a liquid-phase gene chip for synchronously detecting five porcine viruses, which comprise forward and reverse primers and hybrid probes for porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus type 2 (PCV2), porcine pseudorabies virus (PRV), classical swine fever virus (CSFV) and porcine parvovirus (PPV). The invention also provides a multiplex liquid-phase chip high-flux molecular biology detection method of the five porcine viruses. According to the method, porcine virus nucleic acids in the sample to be detected are extracted to perform multiplex unsymmetric nucleic acid amplification / multiplex liquid-phase gene chip (suspension chip) combined detection, thereby synchronously and accurately detecting and identifying the five porcine viruses in the sample to be detected. The method has the advantages of high specificity, high sensitivity, high stability, high flux and high detection speed, and is simple to operate.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Preparation method of recombinant pseudorabies virus for expressing reverse neural circuit tracing of green fluorescin with high sensitivity, and application
ActiveCN106995824AImprove efficiencyRealize visualizationMicrobiological testing/measurementPreparing sample for investigationGreen fluorescent proteinViral Vaccine
The invention discloses a preparation method of a recombinant pseudorabies virus for expressing reverse neural circuit tracing of green fluorescin with high sensitivity, and application, including (1) the preparation of the recombinant pseudorabies virus for expressing green fluorescin with high sensitivity; (2) the application to marking of a neural circuit. The recombinant pseudorabies virus for expressing the green fluorescin with high sensitivity is successfully prepared by using a platform. The recombinant pseudorabies virus for expressing reverse neural circuit tracing of green fluorescin with high sensitivity is successfully obtained. Wide application value is realized in aspects of neural circuit marking, medicine screening platform building, medicine inhibition virus action mechanism, viral vaccine and diagnostic reagent invention and development, animal model building, virus replication, pathogenic mechanism analysis and the like.
Owner:衠奥生物技术(武汉)有限公司
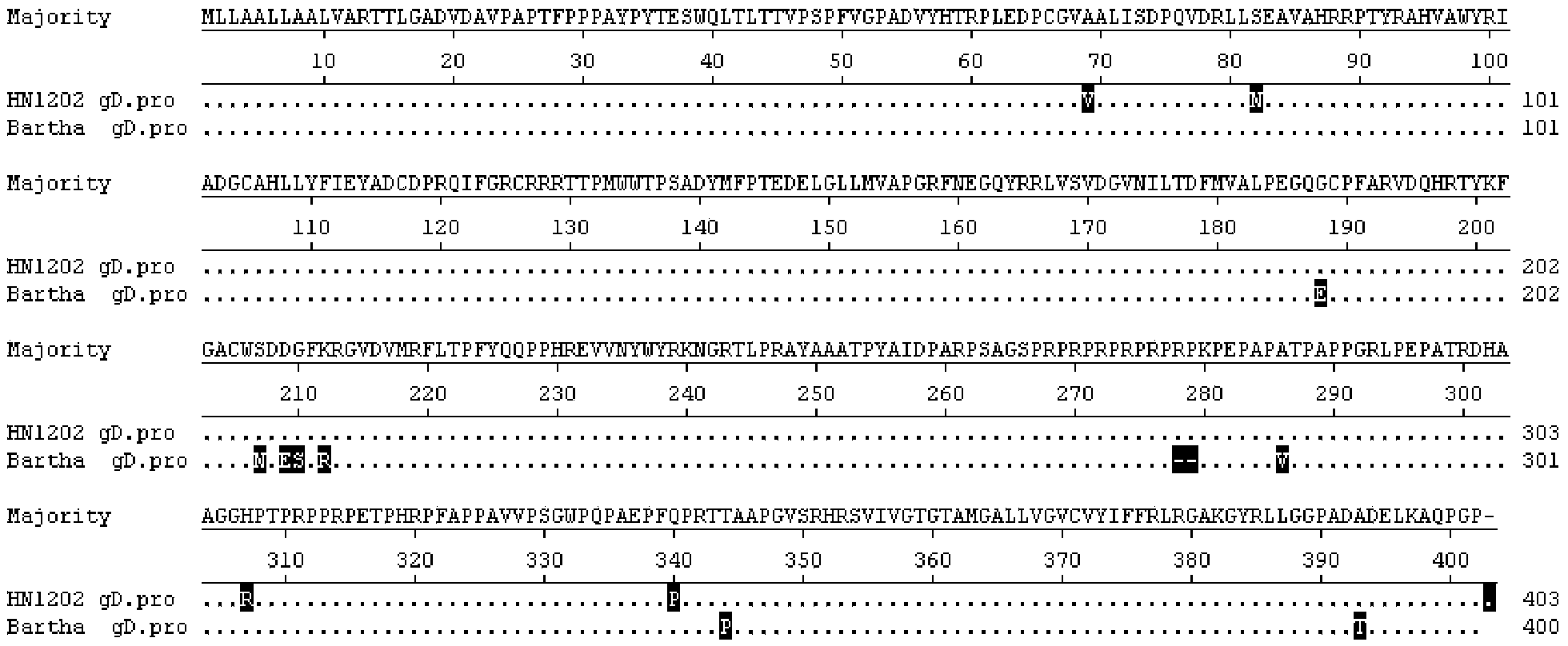
Detection reagent and method for identifying porcine pseudorabies virus vaccine strain and wild strain
InactiveCN104561374AHigh detection sensitivityGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseVirology
The invention provides a detection reagent and a method for identifying a porcine pseudorabies virus vaccine strains and a wild strain, and belongs to the field of animal pathogen detection. The detection reagent comprises two primer pairs as shown in SEQ ID NO.1-4 and probes as shown in SEQ ID NO.5-6. The invention further provides a method for identifying a porcine pseudorabies virus vaccine strain gD gene and a wild strain gE gene by using the detection reagent. The porcine pseudorabies virus vaccine strains and the wild strain can be simultaneously identified through dual fluorogenic quantitative PCR, high-flux detection on large-scale samples can be achieved, the detection reagent has the characteristics of rapidness, specificity, sensitivity, accuracy, simplicity and convenience, provides an effective tool for surveying porcine pseudorabies infection sources and tracing infection environments and disease sources, and has relatively good application value in prevention and control on porcine pseudorabies.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Porcine pseudorabies virus strain and porcine pseudorabies inactivated vaccine prepared by using same
ActiveCN102344912APromote rapid proliferationHigh titerMicroorganism based processesAntiviralsPig farmsTonsil
The invention discloses a porcine pseudorabies virus strain and a porcine pseudorabies inactivated vaccine prepared by using the same. The porcine pseudorabies virus strain is separated from the brain, tonsil and other tissues of a still birth of a sow on a pig farm by virtue of subculture adaptation and has a microbial preservation number of GCMCC (China General Microbiological Culture Collection Center) No.5013. The virus strain separated in the invention has quick proliferation and high titer on ST (scheduled tribe) cells, can induce an animal to generate a high-titer neutralizing antibody, and has excellent immunogenicity. The invention also discloses a preparation method of the porcine pseudorabies inactivated vaccine, and the method comprises the following steps of: culturing the porcine pseudorabies virus strain to obtain a virus solution; adding an inactivator to inactivate the virus solution; and preparing an oil phase and a water phase, and emulsifying, thus obtaining the porcine pseudorabies inactivated vaccine. In the invention, each process parameter of the inactivated vaccine is optimized. Immune protective efficacy and safety tests prove that the inactivated vaccine has excellent immunogenicity and safety.
Owner:哈药集团生物疫苗有限公司
Vector system
InactiveUS20040071675A1Eliminate the effects ofInhibition of activationBiocideNervous disorderVector systemRabies
There is provided the use of a vector system comprising at least part of a rabies g protein, to transduce a TH positive neuron. There is also provided the use of a rabies G vector system to transduce a target site, in which the vector system travels to the target site by retrograde transport, which may comprise the step of administration of the vector system to an administration site which is distant from the target site.
Owner:OXFORD BIOMEDICA (UK) LTD
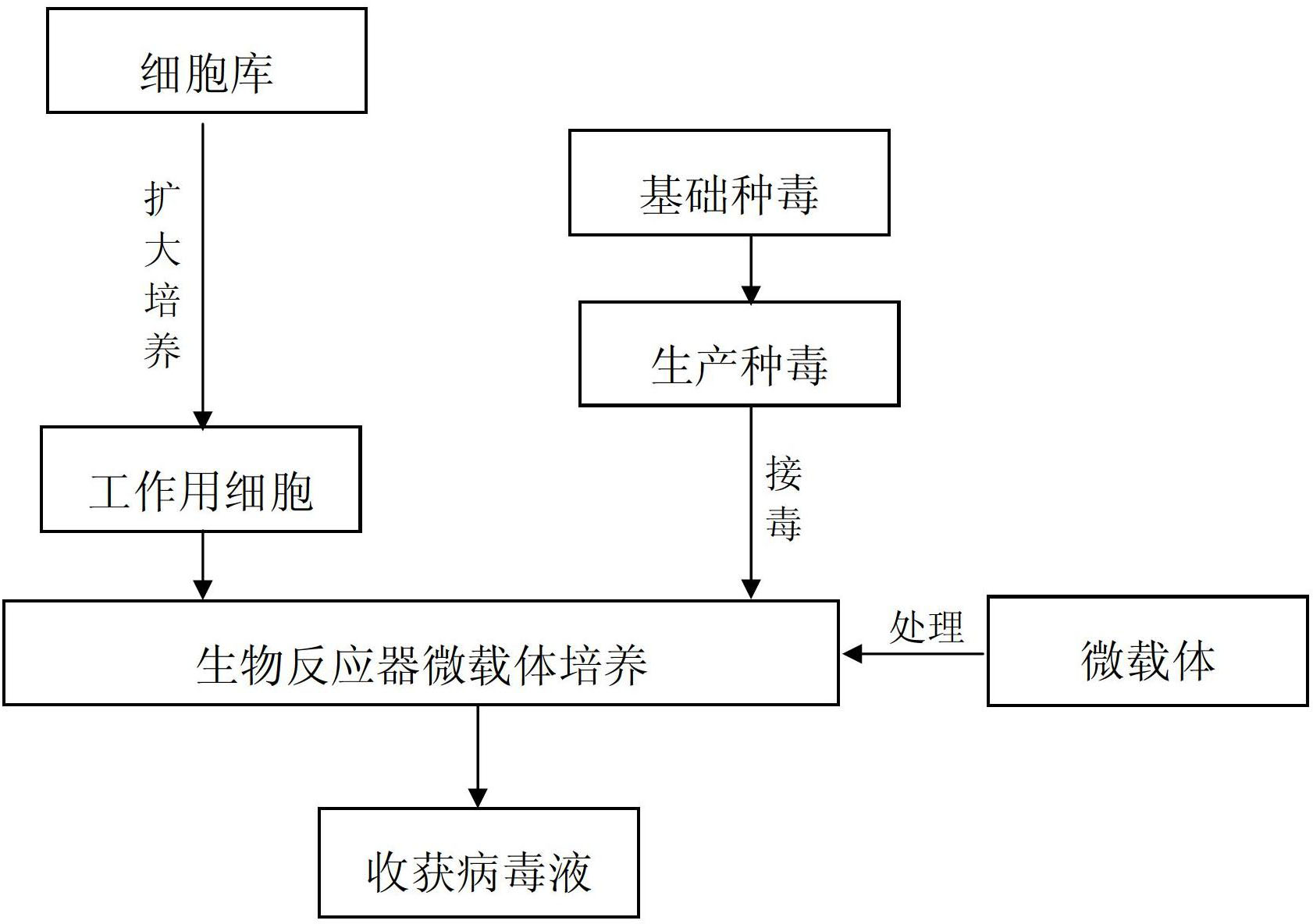
Method for generating porcine pseudorabies virus by culturing ST cell in microcarrier of bioreactor
InactiveCN102690791AEasy to harvestReduce pollutionMicroorganism based processesAntiviralsTISSUE CULTURE INFECTIOUS DOSE 50%Volumetric Mass Density
The invention discloses a method for generating a porcine pseudorabies virus by culturing an ST cell in a microcarrier of a bioreactor. The method comprises the following steps: (1) selecting the ST cell as a cell line for making vaccine; (2), transferring and culturing the ST cell; (3) reproducing a porcine pseudorabies virus cell virus seed; (4) culturing the ST cell in the microcarrier of the bioreactor in a suspension way; (5) reproducing porcine pseudorabies virus liquid; and (6) treating the obtained virus liquid. The method has the advantages that the cell vitality is more than and equal to 90%, the living cell density is more than and equal to 1*10<7> cells / mL, and the virus liquid tissue culture infectious dose 50 (TCID50) is more than and equal to 10<8.0>. The production process is intensive and smooth, the dimension is easy to amplify, the production period is short, a small space is occupied, little pollution is caused to the environment and is easy to treat, the virus liquid is convenient to obtain, the quality is easy to balance and stabilize, the production cost can be reduced obviously, and the yield and the quality of the vaccines are improved.
Owner:哈药集团生物疫苗有限公司
Swine pseudorabies virus strain, vaccine composition, preparation method and application thereof
ActiveCN104328090ASymptoms relieved or improvedMicroorganism based processesAntiviralsAntigenAdjuvant
The invention provides a vaccine composition. The vaccine composition comprises an immune amount of a swine pseudorabies virus antigen and a veterinary medicine-acceptable adjuvant. The vaccine composition can induce effective immune reactions in the swine and dog. A piglet immunized by the vaccine composition has short body temperature-raising time and substantially normal appetite, without clinical symptoms. The vaccine composition presents good immune protection.
Owner:PU LIKE BIO ENG
Human diploid cell rabies vaccine virus seed and preparation method thereof
ActiveCN102093983ASafeEffectiveMicroorganism based processesViruses/bacteriophagesBiotechnologyRabies
The invention relates to the field of biotechnology, in particular to a virus seed for producing vaccines for preventing human rabies by utilizing human diploid cells (KMB17) and a preparation method thereof. In the invention, a rabies fixed virus CTN-1V5 strain is continuously subcultured in the human diploid cells (KMB17), and a terminal dilution method is used for screening viruses with highertiter, thereby obtaining a rabies virus strain which is suitable for the human diploid cells (KMB17) and has good immunogenicity and heredity stability, and culturing a rabies vaccine virus seed (CTN-DK strain) capable of efficiently reproducing in the human diploid cells (KMB17). By using the virus seed for producing a human diploid cell (KMB17) rabies vaccine, the risk caused by residual heterogonous DNA (deoxyribonucleic acid) in the vaccine which is currently used at home can be effectively avoided, and the safety and practicability of the rabies vaccine in China are further improved, thus the invention has great social and economic benefits.
Owner:ZHEJIANG PUKANG BIOTECH
Diploid cell rabies vaccine and method for preparing purified rabies vaccine
InactiveCN101352570AIncreased antigenic activityHigh purityAntiviralsTissue cultureSerum free mediaSerum ige
The invention relates to a preparation method for a diploid cell rabies vaccine and a purified rabies vaccine, and the method uses a serum-free medium to culture human diploid cell rabies purified vaccine, thus greatly reducing anaphylactic reaction and being beneficial to purification.
Owner:崔栋
Preparation method of swine pseudorabies vaccine
The invention relates to a preparation method of a swine pseudorabies vaccine, which comprises the following steps of: culturing a virus by using a swine testicle cell, and when one layer of cells grows, inoculating a swine pseudorabies virus; then adding into a cell maintenance medium, statically or rotatably culturing, when the cell suffers from more than 80 percent of pathological changes, harvesting a cell culture, repeatedly freeze-thawing to obtain a cell venom containing supernate, and mixing the cell venom qualified in toxic valence detection with formaldehyde for inactivating; and mixing with an emulsifying agent for emulsifying to obtain the swine pseudorabies vaccine. Compared with the prior art, a strain used for preparing the vaccine has the advantages of stronger toxicity and high virus valence; the swine pseudorabies vaccine has good immunogenicity and long immunization period; and the preparation method has the advantages of reasonable process and lower cost, thereby greatly lowering the cost load of the fish breeding and poultry raising industry.
Owner:SHANGHAI ELITE AGRI SCI TECH GROUP +1
Kit for testing neutralizing antibody racing ELISA in human and animal rabies
InactiveCN101251537AAccurate quantitative determinationEasy to operateBiological testingAntigenRabies
The invention discloses a reagent box for detecting hydrophobia neutralizing antibody competition ELISA of human beings and animals, wherein the reagent box can easily, quickly, accurately and quantitatively detect the hydrophobia neutralizing antibody in blood serums of human beings and animals by marking the hydrophobia neutralizing antibody, the standard serum and the envelope antigen. By using hydrophobia virosome or virus glycoprotein to coat enzyme synapticulae, the enzyme labeling hydrophobia neutralizing antibody is mixed with the blood serum to be tested and the standard serum respectively according to a certain ratio and reacts with the hydrophobia virus glycoprotein antigen coated on the enzyme synapticulae, a standard curve is drawn according to the OD value of the standard blood serum reaction and the known neutralizing titer after the color development, and the titer of the corresponding neutralizing antibody is obtained from the standard curve according to the OD value of the reaction of the blood serum to be tested. The reagent box has the advantages of accurately and quantitatively detecting the neutralizing antibody of the hydrophobia virus, along with simple operation and short time; moreover, the test result of the invention keeps a good consistence with test results of neutralizing test methods recommended by WHO and OIE.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Pig virus gene chip and detection method thereof
ActiveCN102605099ARich varietyAvoid false positivesNucleotide librariesMicrobiological testing/measurementClassical swine fever virus CSFVMultiplex
The invention provides a pig virus gene chip and a detection method thereof. The gene chip comprises a probe fixedly arranged on a substrate carrier, wherein the probe is selected from the characteristic segments of the following viruses: a classical swine fever virus (CSFV), a porcine reproductive and respiratory comprehensive virus (PRRSV), a pseudorabies virus (PRV), a porcine circovirus (PCV) and a porcine parvovirus (PPV). The types of the viruses detected by the method are various and the viruses cover common porcine viruses substantially. Random primer polymerase chain reaction (PCR) and multiplex-PCR are adopted to mark, so that false positive which is easy to cause when a PCR result is detected by gel electrophoresis is avoided, and high-flux accurate detection with short time is realized. The substrate carrier is a glass sheet which is subjected to aldehyde treatment, so that the combination between the probe and a target is facilitated, and higher noise is not brought to detection.
Owner:BEIJING UNIV OF AGRI
Method for large-scale production of porcine pseudorabies inactivated vaccine
ActiveCN107267466AHigh purityLess side effectsViral antigen ingredientsInactivation/attenuationMolecular sieveAdjuvant
The invention belongs to the technical field of vaccines and relates to a method for large-scale production of a porcine pseudorabies inactivated vaccine. The method comprises preparing a virus solution of porcine pseudorabies (XF-1 strain), carrying out continuous flow centrifugation, hollow fiber column clarification filtration, hollow fiber column ultrafiltration concentration and Sepharose 4FF molecular sieve gel chromatography purification treatment to obtain purified porcine pseudorabies viruses, adding a formaldehyde solution having a final concentration of 0.4% (v / v) into the purified porcine pseudorabies viruses, carrying out inactivation at 37 DEG C for 48h, and carrying out emulsification with a 201 adjuvant to obtain the porcine pseudorabies inactivated vaccine. The porcine pseudorabies inactivated vaccine can well prevent highly pathogenic mutant pseudorabies prevailing in the market.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Porcine pseudorabies virus vaccine
ActiveCN104826103AEffective immune protectionMeet the standard of non-virulence reversionAntiviralsAntibody medical ingredientsAntigenVirulent characteristics
The invention aims at providing a porcine pseudorabies virus vaccine. The porcine pseudorabies virus vaccine comprises an antigen and a protective agent, wherein the antigen contains an attenuated virus strain which is prepared after deleting virulence genes by a porcine pseudorabies virus strain with the collection number of CGMCC No. 10266. The prepared vaccine can effectively prevent porcine pseudorabies; furthermore, because the porcine pseudorabies virus as the antigen is a gene-deleted strain, by continuous passage of horizontally transmitted infections in mouse bodies, no virulence reversion occurs, and the genetic stability is realized, thereby being in line with the standard of having no virulence reversion in the porcine pseudorabies virus deleted vaccine strain; and the prepared vaccine can provide effective immune protection, and has great commercialization development prospects.
Owner:SHANDONG SINDER TECH
Human diploid cell rabies vaccine and preparation method thereof
ActiveCN102671192AContains less impuritiesPerfect purification processMicroorganism based processesAntiviralsRabiesDiploid cells
The invention discloses a human diploid cell rabies vaccine and a preparation method thereof, and belongs to the field of vaccines. A CDKHBP-1 strain is inoculated into a human diploid cell WI-38, and the rabies vaccine is obtained through separation and purification. The human diploid cell does not contain any contaminants or oncogenicity, and is obvious superior to an animal cell serving as a medium for producing the vaccine; and the method is suitable for large-scale industrial production, a purification process is perfect, and the vaccine contains a few impurities and has high purity and immunogenicity.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Preparation method of rabies vaccine for human and its use
InactiveCN1824302AReduction in inflammatory nodulesLittle side effectsAntiviralsAntibody medical ingredientsRabiesAdjuvant
A human rabies vaccine is prepared from the existing rabies vaccine without adjuvant through adsorbing it by the aluminum hydroxide nanoparticles. Its adsorbed capacity is increased by 10-20 time. It can be used for emergency inoculation for preventing rabies as it has the fast release effect.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Subunit vaccine of porcine pseudorabies virus and preparation method
ActiveUS20180256705A1Improve protectionImprove expression levelViral antigen ingredientsAntibody mimetics/scaffoldsImmune effectsDisease
The present disclosure provides a PRV gB protein fragment, or a conservative variant or active fragment thereof, the gB protein fragment has a high level of expression, the subunit vaccine antigen prepared from the gB protein fragment has a better immune effect than a subunit vaccine antigen prepared from gB protein. The invention also provides a preparation method of a subunit vaccine by using the gB protein fragment alone, or the gB protein fragment together with gD protein. This vaccine has a simple preparation method and provides excellent protection against disease caused by the porcine pseudorabies virus.
Owner:PU LIKE BIO ENG
Human vaccine for preventing hydrophobia and tetanus
ActiveCN102671194AIncreased potencyImprove immunityAntibacterial agentsBacterial antigen ingredientsRabiesTetanus toxoids
The invention discloses a human vaccine for preventing hydrophobia and tetanus, which belongs to the field of vaccines. The vaccine consists of a hydrophobia vaccine and a tetanus toxin, wherein the hydrophobia vaccine is obtained by inoculating a CDKHBP-1 strain onto a human diploid cell WI-38 and purifying. By using the hydrophobia vaccine and the tetanus toxin prepared with the preparation method disclosed by the invention together, immunity to hydrophobia and tetanus can be realized; and moreover, the tetanus toxin can be used for remarkably enhancing valence effect of the hydrophobia vaccine, plays a role in enhancing immunity, and is convenient for administration.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Porcine pseudorabies virus, and vaccine composition and applications thereof
ActiveCN102952785ARaise antibody levelsLong durationMicroorganism based processesAntiviralsPig farmsAdjuvant
The invention provides a p porcine pseudorabies virus and vaccine composition and applications thereof, belonging to the field of biotechnology. The microbial preservation number of the porcine pseudorabies virus PRV-JS strain is CGMCCNO.6604. The invention further provides a vaccine composition which comprises an inactivated porcine pseudorabies virus PRV-JS strain and adjuvant acceptable on veteriary pharmacy. The vaccine composition further comprises a carrier acceptable on the veterinary pharmacy. The porcine pseudorabies virus PRV-JS strain is screened from porcine pseudorabies prevalent strains separated from all pig farms and has good immunogenicity, and can be used as inactivated vaccine production virus seeds or virus seeds for testing. After being immunized by the vaccine composition, pigs have higher produced antibody level and the lasting period is long. The vaccine composition prepared by adopting the porcine pseudorabies virus PRV-JS strain can be used for preventing the sow abortionbreeding difficulty and mortality syndromeboar infertility caused by the porcine pseudorabies virus, boar infertility and pseudorabies of other pigs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com