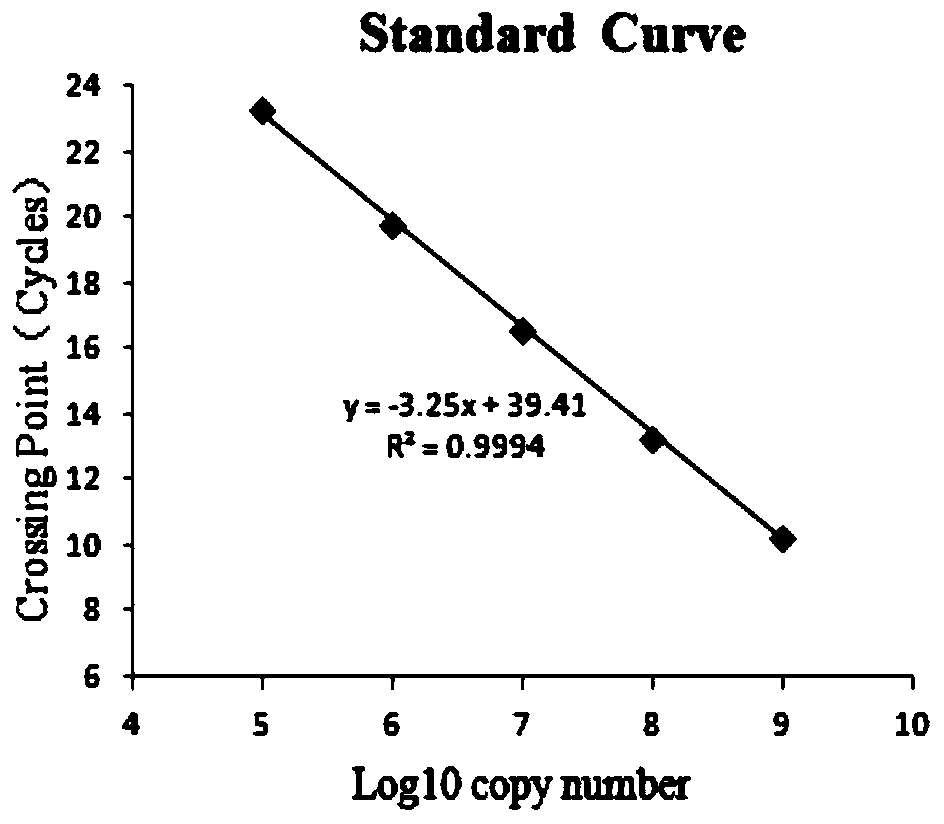
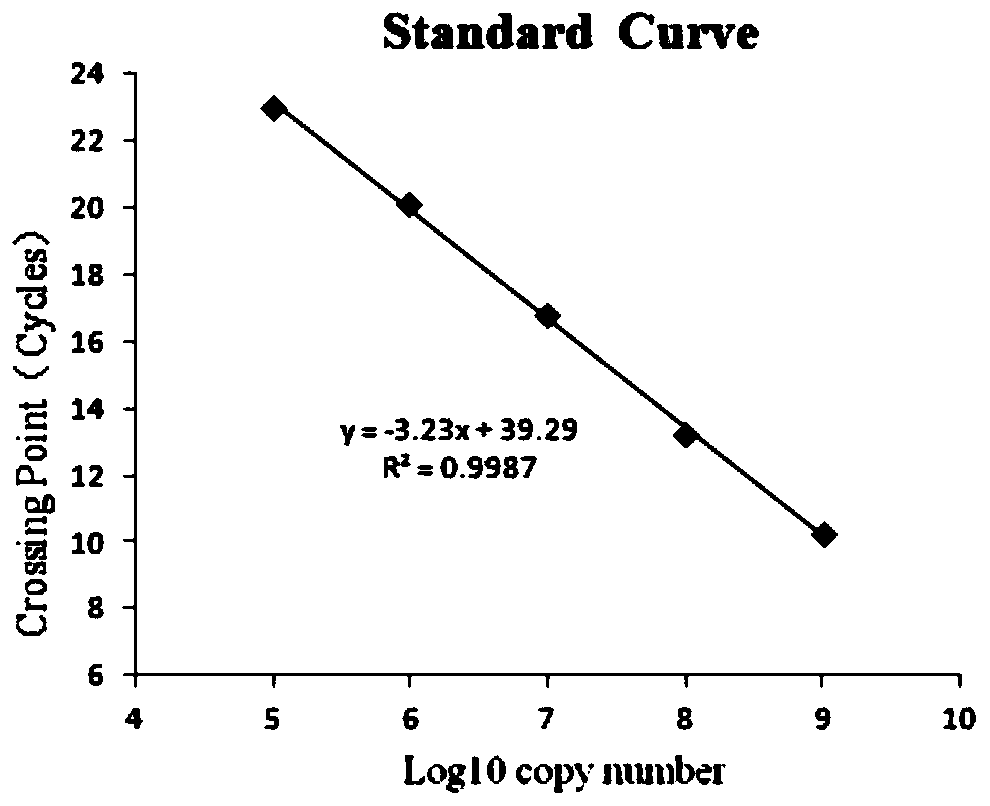
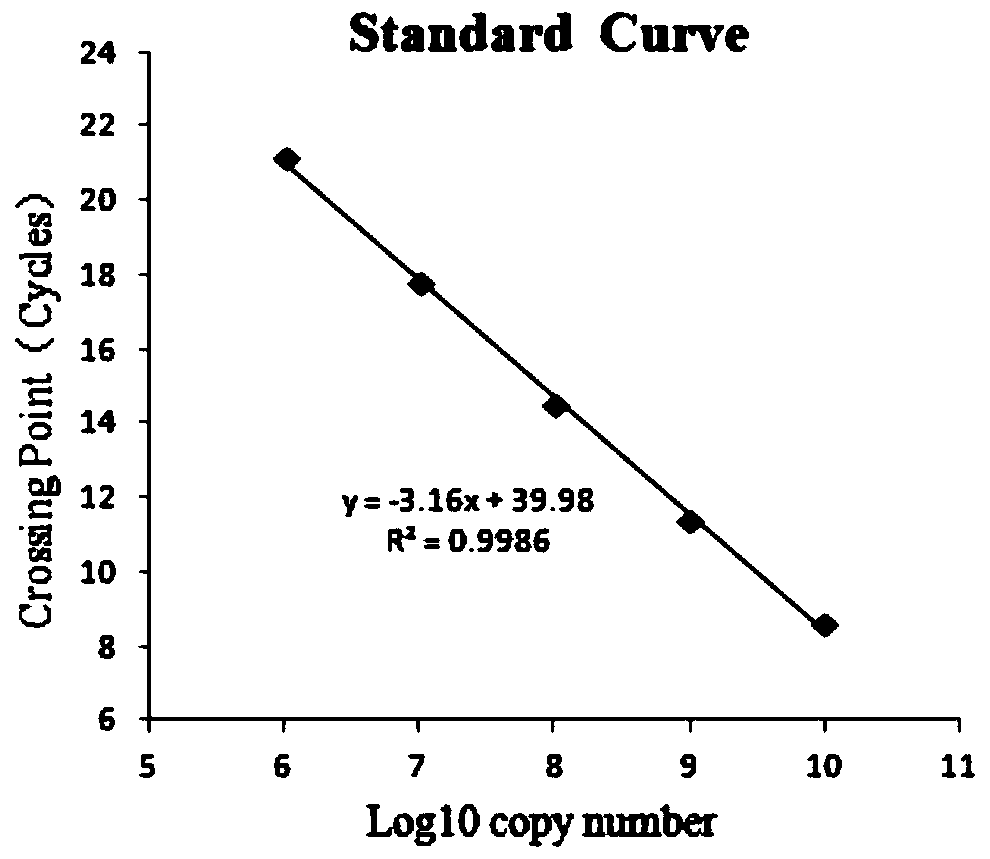
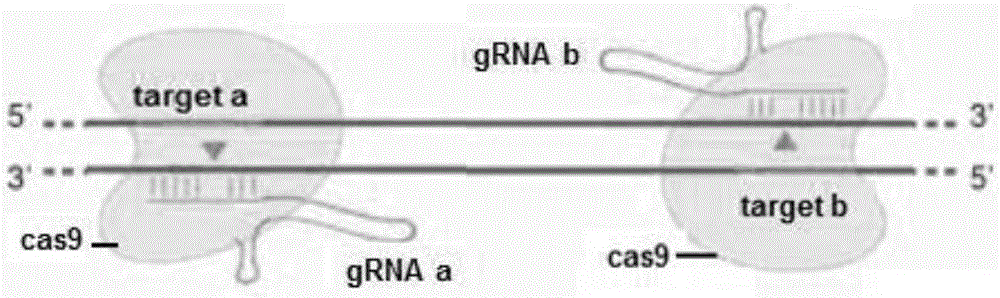
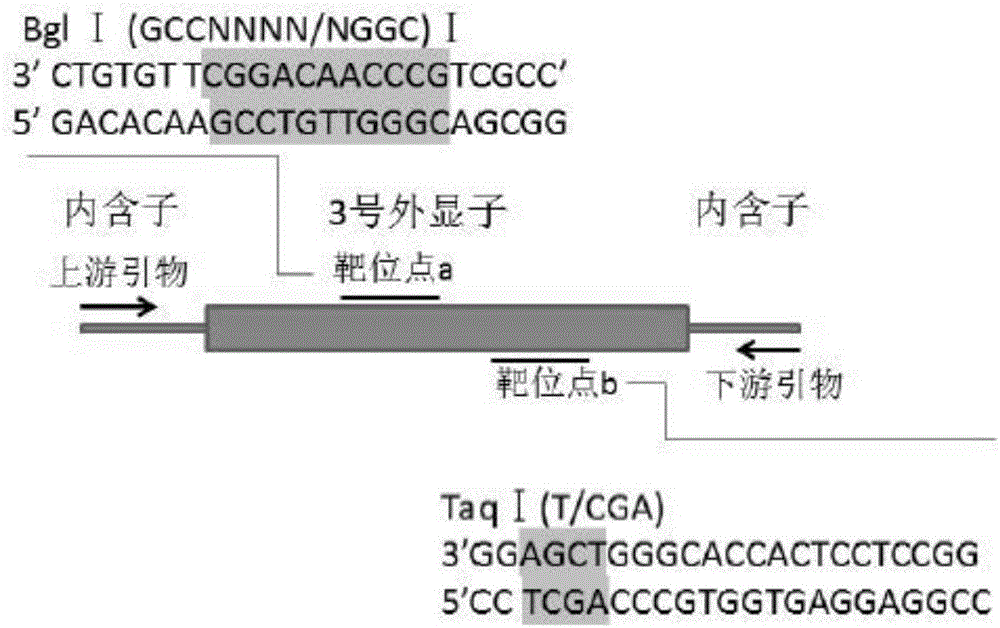
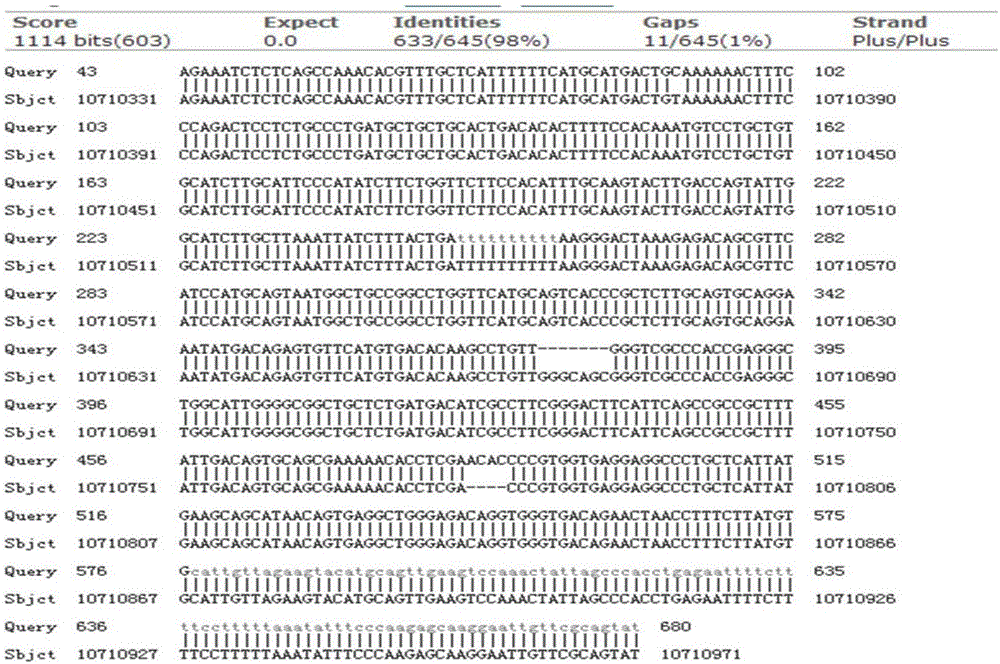
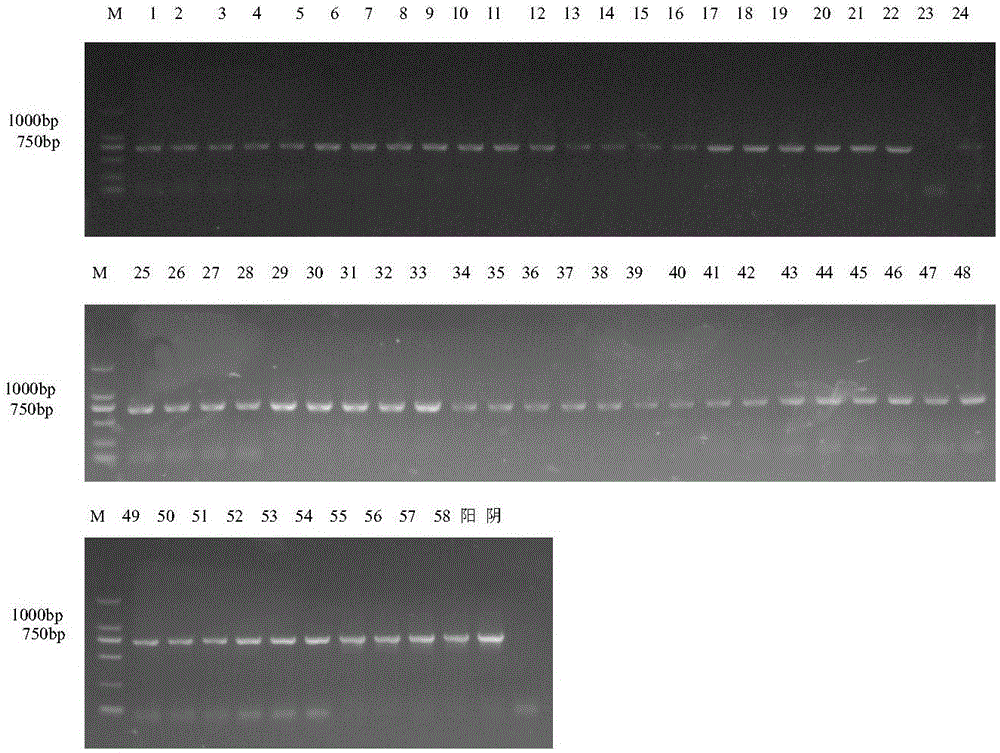
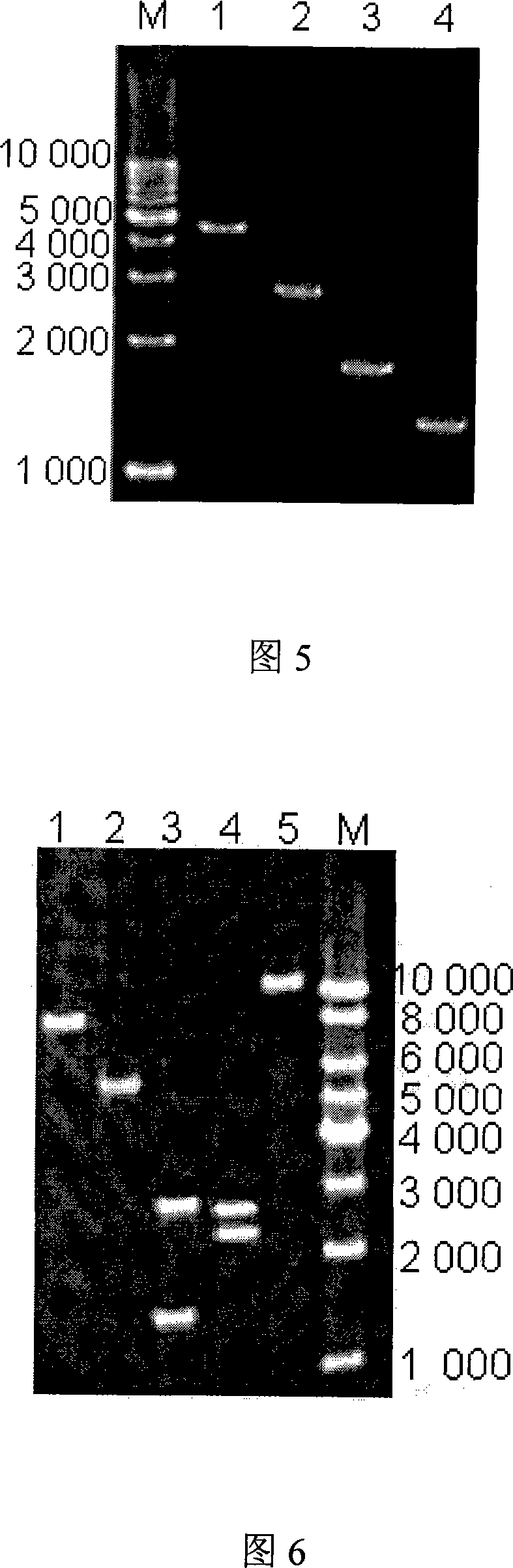
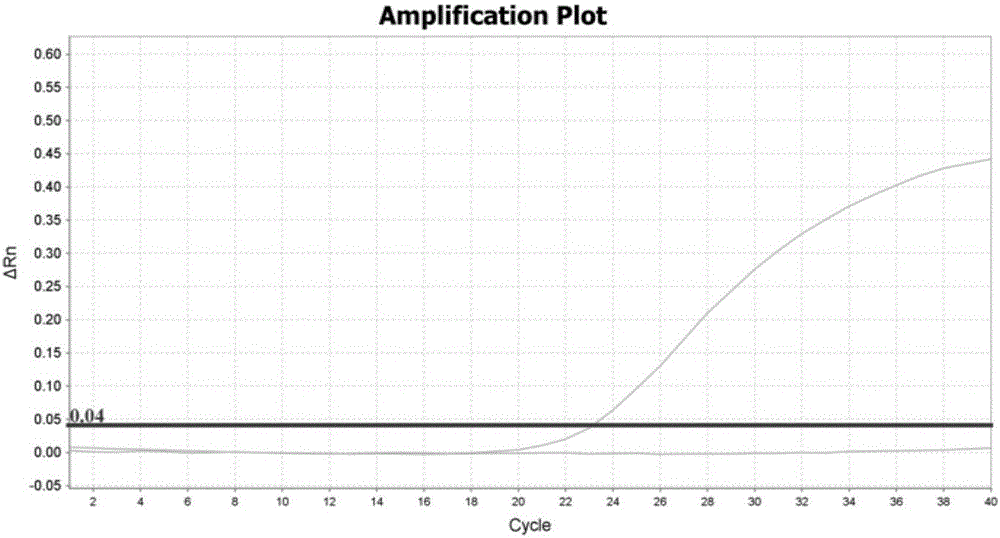
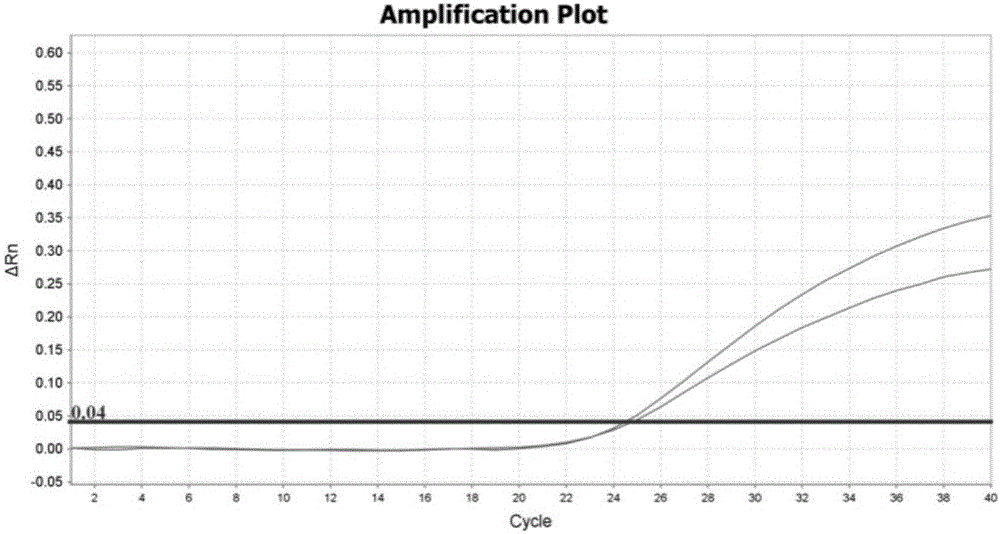
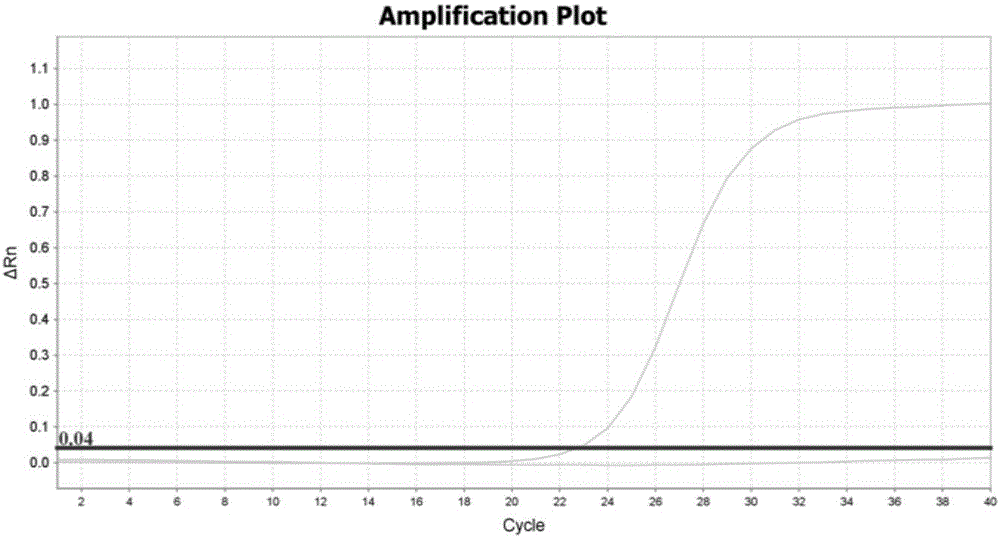
Patents
Literature
200 results about "Gene deletion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In genetics, a deletion (also called gene deletion, deficiency, or deletion mutation) (sign: Δ) is a mutation (a genetic aberration) in which a part of a chromosome or a sequence of DNA is lost during DNA replication. Any number of nucleotides can be deleted, from a single base to an entire piece of chromosome.
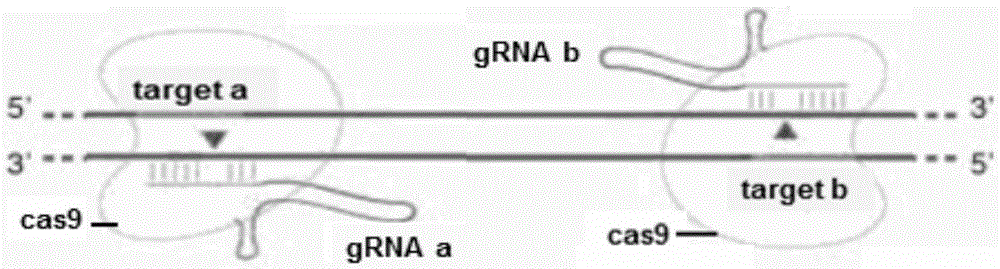
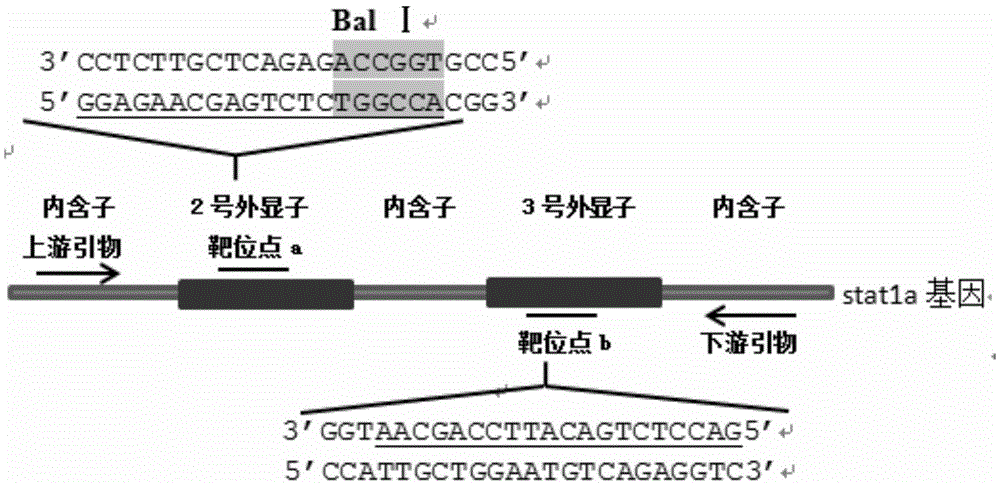
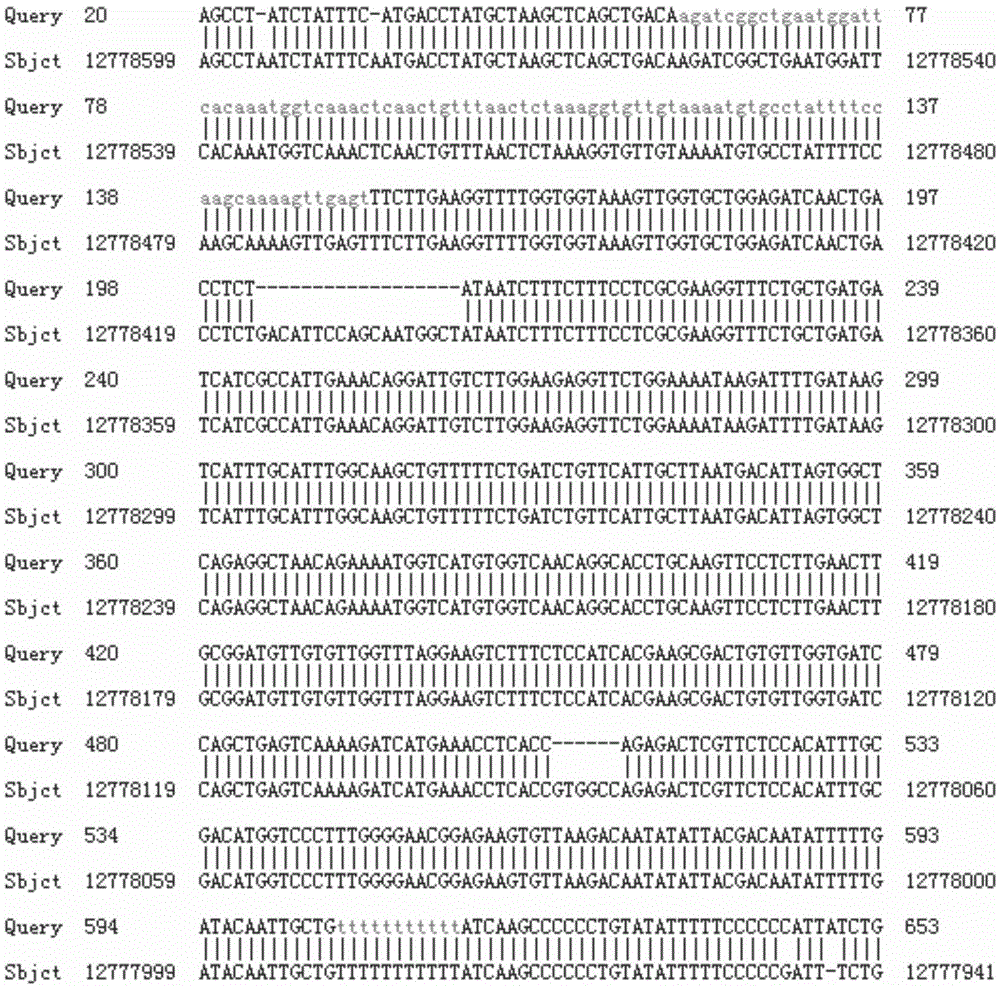
Method for breeding stat1a (signal transducer and activator of transcription 1) gene-deleted zebra fish through gene knockout
InactiveCN105647969AInefficient shooting techniqueLow costMicrobiological testing/measurementPeptidesFish embryoEmbryo
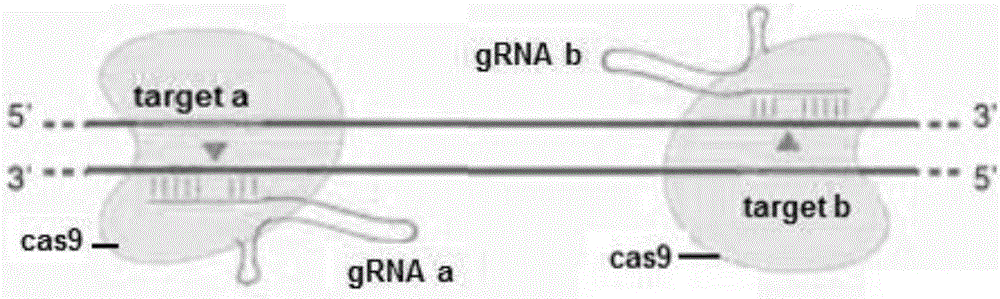
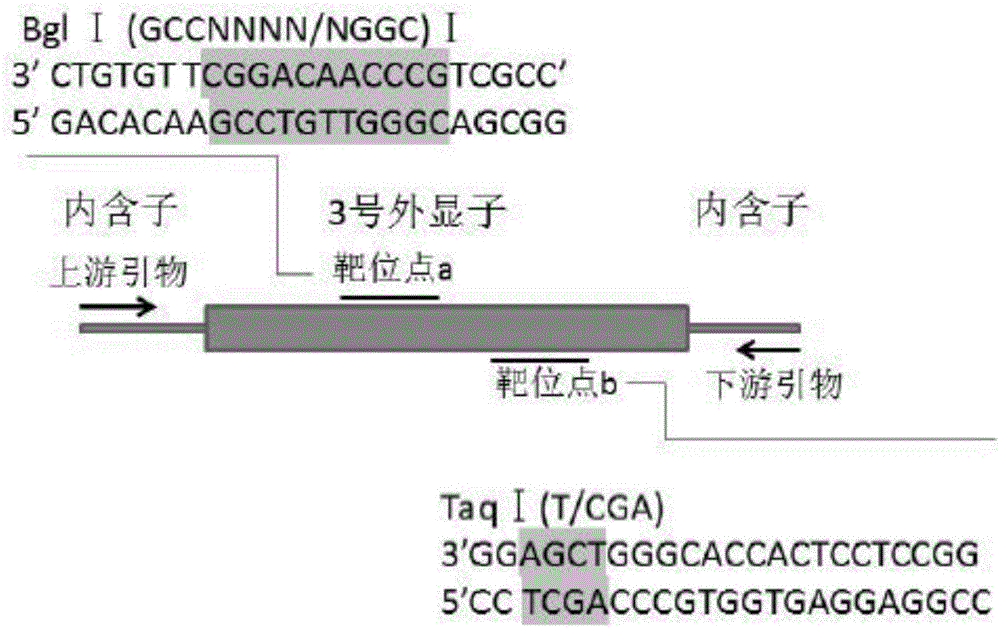
A method for breeding stat1a (signal transducer and activator of transcription 1) gene-deleted zebra fish through gene knockout comprises steps as follows: design of a CRISPR / Cas9 gene knockout target site: a gRNA expression carrier is established and gRNA is synthesized in vitro; micro-injection of a zebra fish embryo; detection of effectiveness of the target site with a T7E1 method through Sanger sequencing; tail cutting identification according to the identification steps after two months of injection; TA cloning of a target sequence; Sanger sequencing of plasmids; obtaining of heritable F1 generation of a zebra fish mutant; obtaining of F2 generation homozygote of the zebra fish mutant, F3 generation pure line inheritance of the gene-deleted zebra fish with the above method, and obtaining of a new zebra fish strain.
Owner:HUNAN NORMAL UNIVERSITY
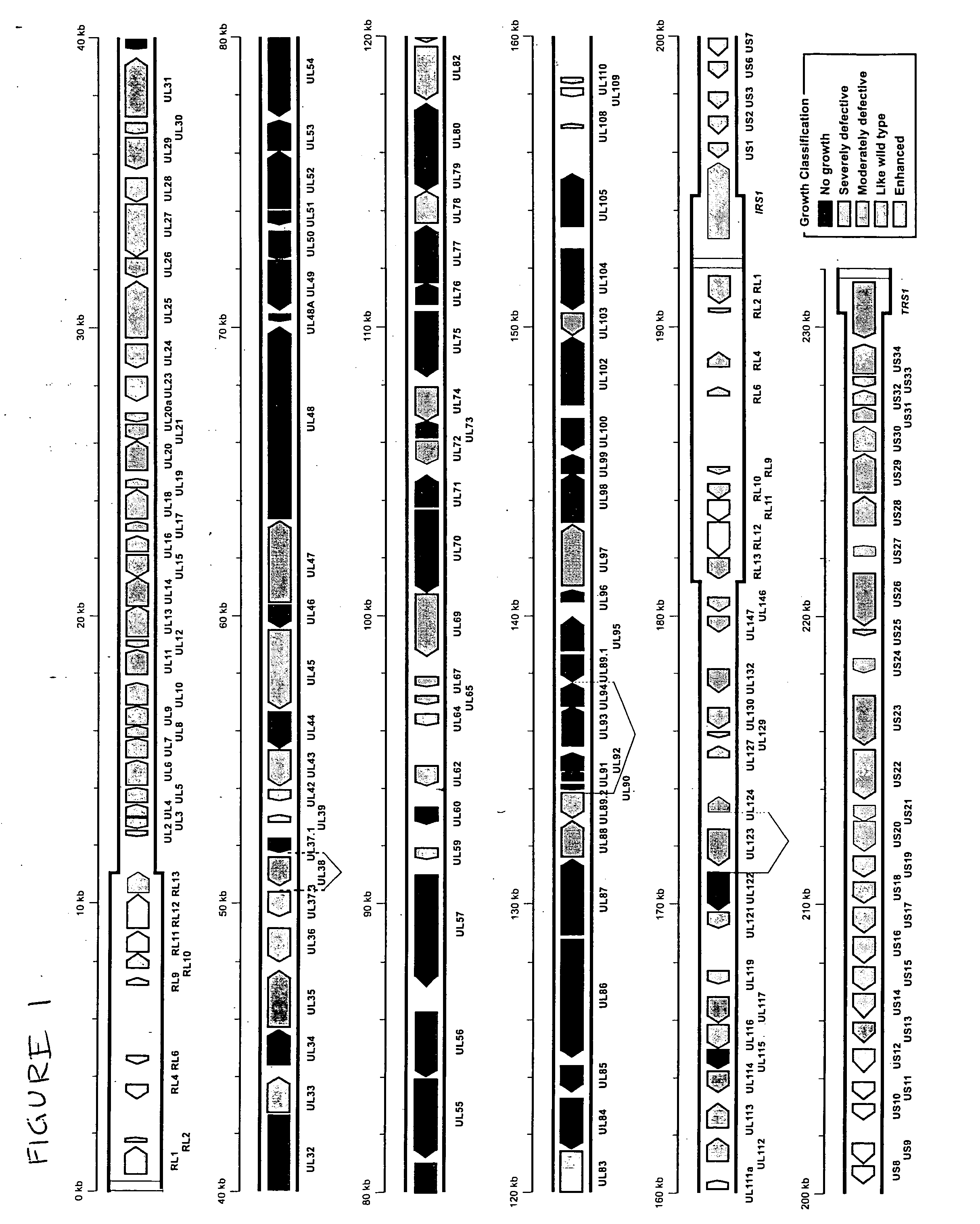
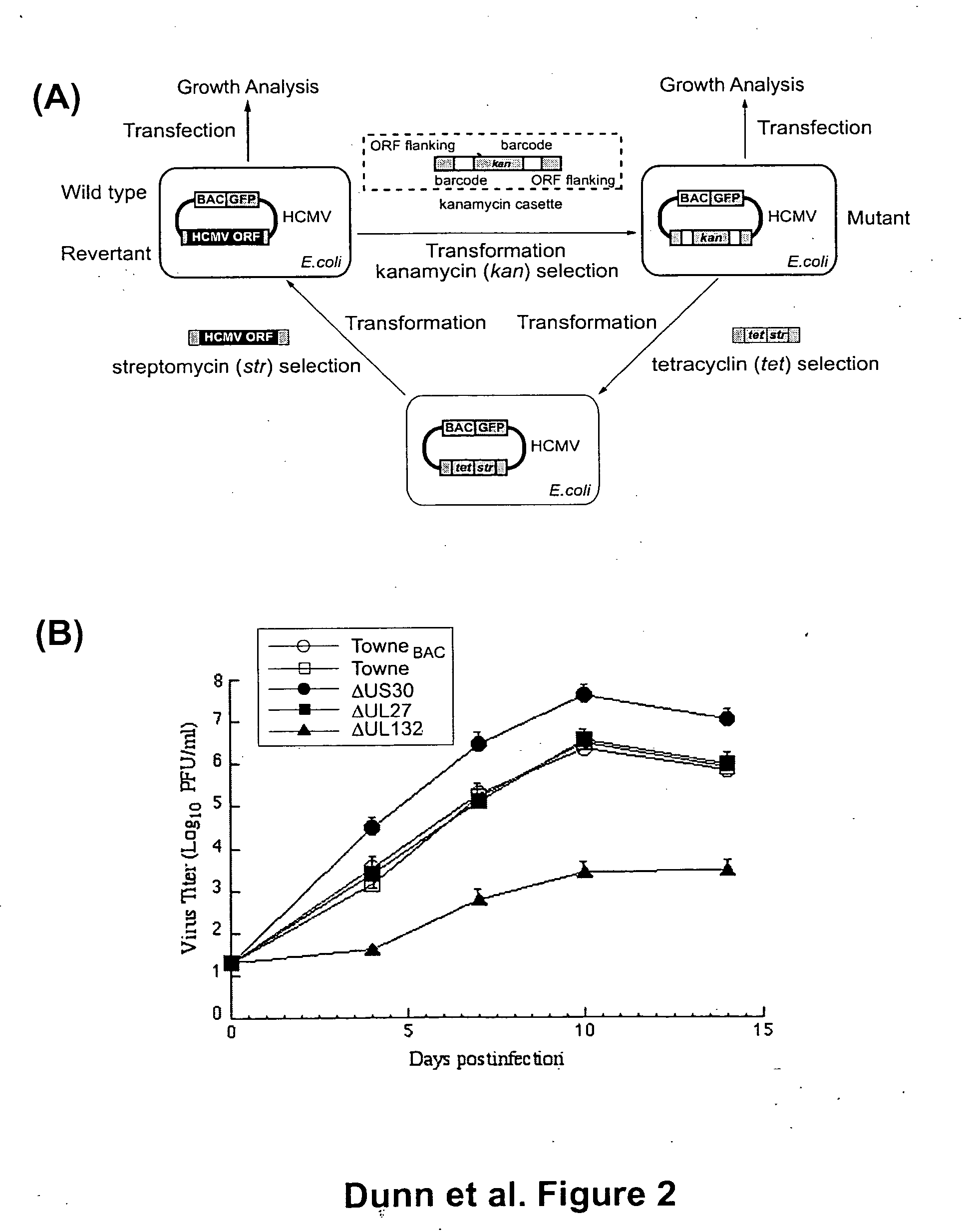
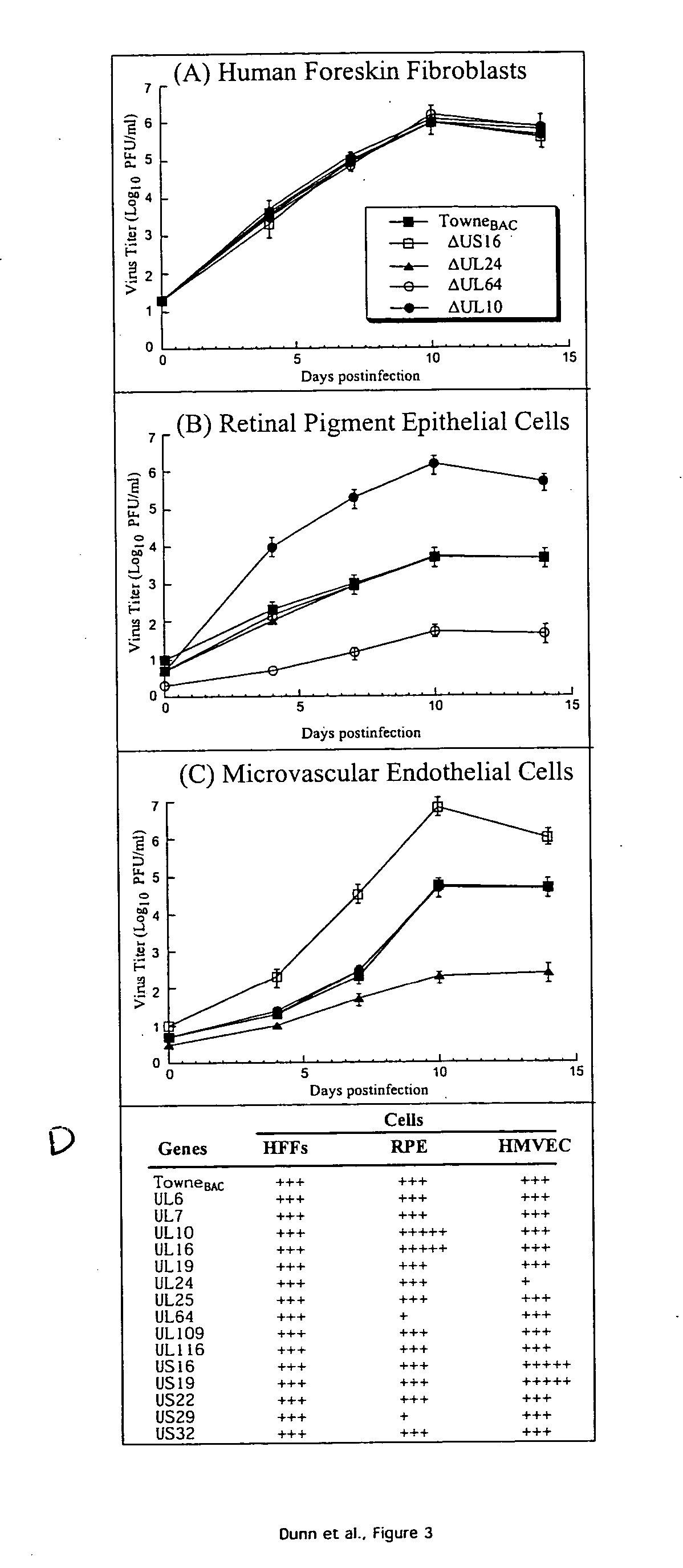
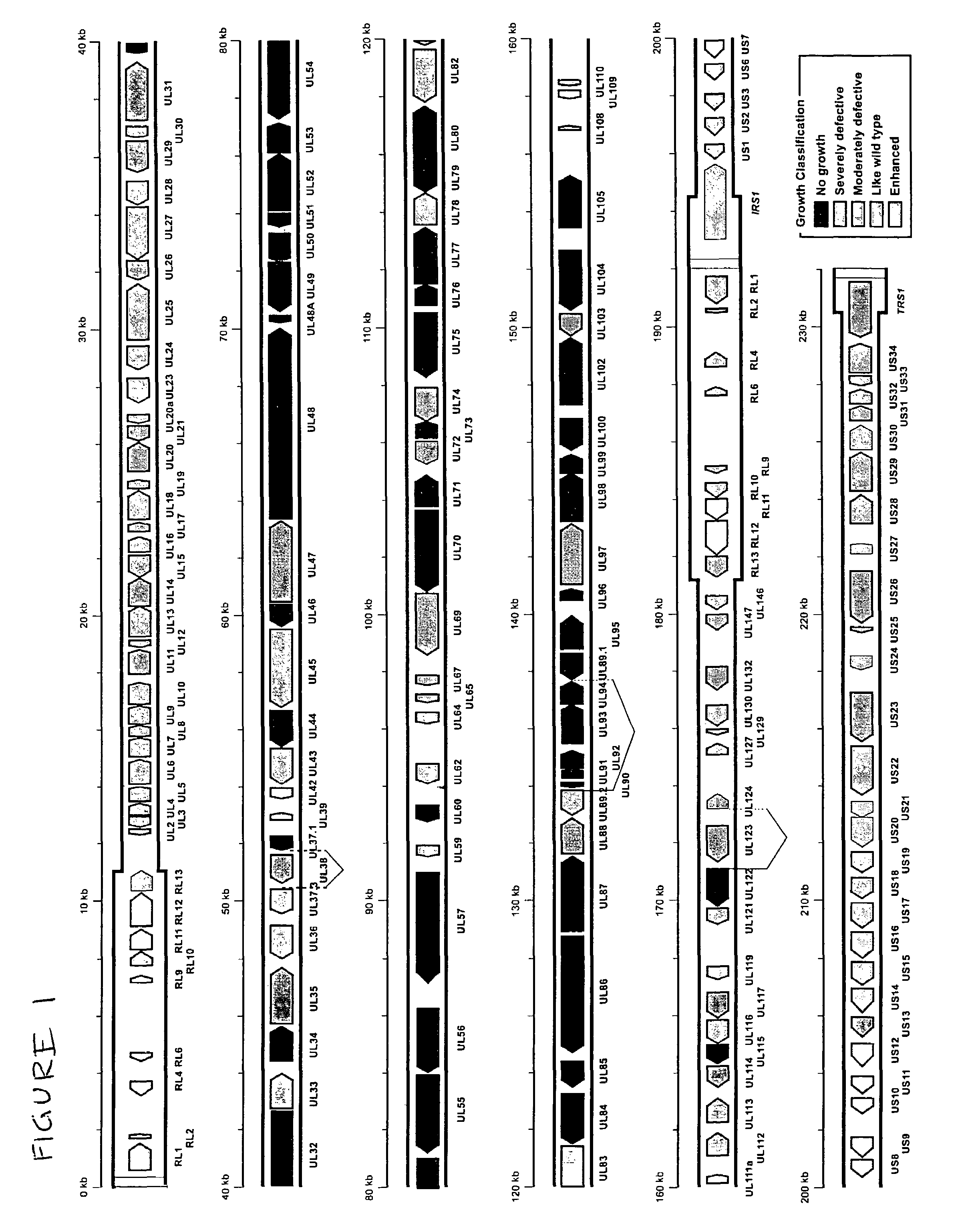
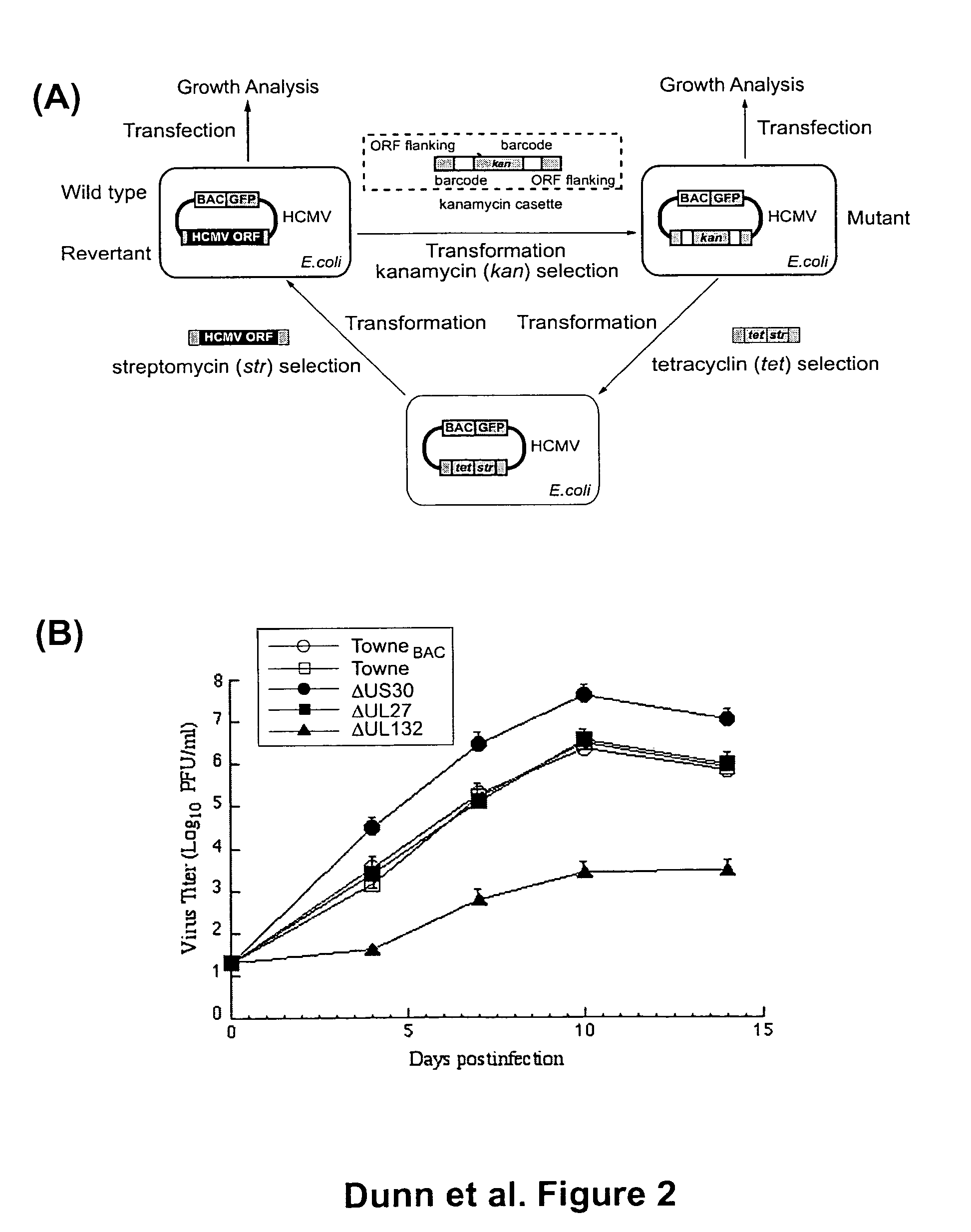
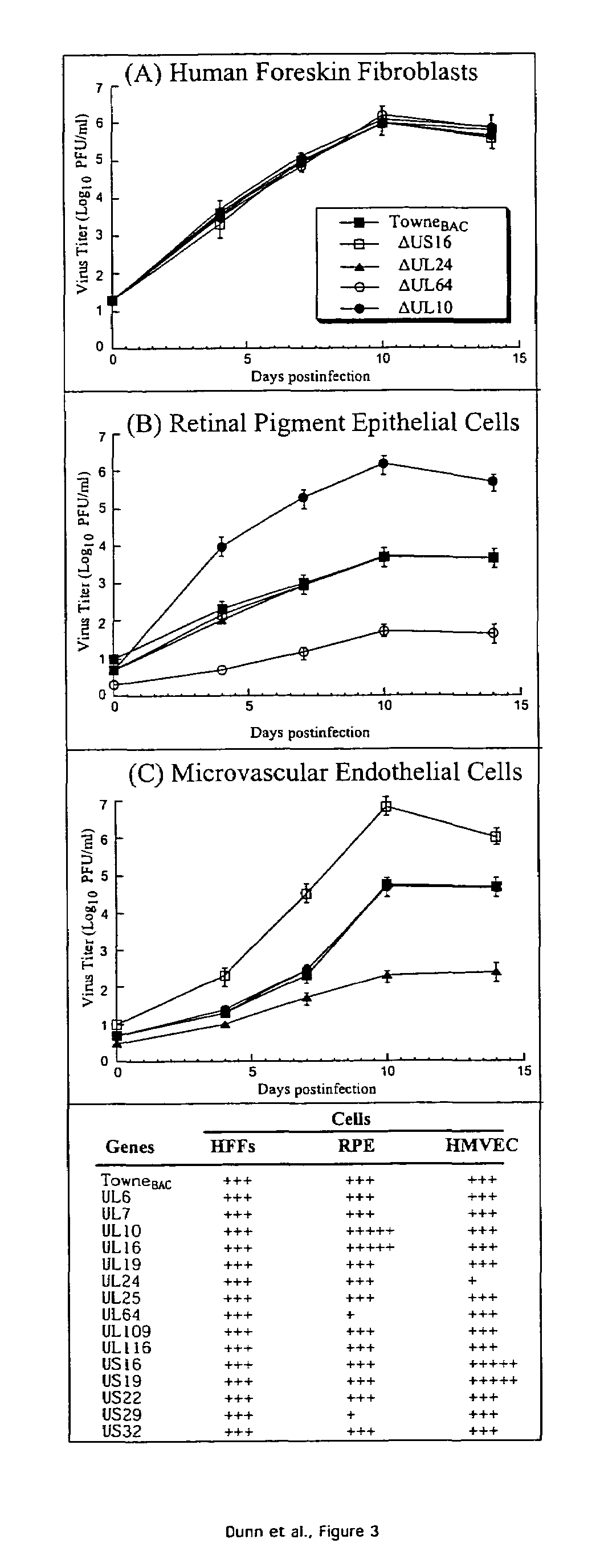
Cytomegalovirus gene function and methods for developing antivirals, anti-CMV vaccines, and CMV-based vectors
ActiveUS20050064394A1Enhance their long-term survivabilitySugar derivativesMicrobiological testing/measurementSurvivabilityORFS
A global functional analysis of HCMV genes is performed by constructing virus gene-deletion mutants and examining their growth phenotypes in different natural HCMV host cells. This systematic analysis of the HCMV genome identified 45 viral ORFs essential for viral replication and characterizes of 115 growth-dispensable viral genes. Of particular interest is the finding that HCMV encodes genes (temperance factors) that repress its own replication on a cell type-specific basis. In addition to HCMV, pathogen temperance may be a strategy employed by other infectious agents to enhance their long-term survivability within their respective host population.
Owner:RGT UNIV OF CALIFORNIA
Method for breeding tcf25 gene deletion type zebra fish through gene knockout
InactiveCN107988268AEfficient and More Precise SilenceEasy to makeHydrolasesMicroinjection basedDiseaseGenotype Analysis
The invention relates to the technical field of gene knockout, and particularly discloses a method for breeding a tcf25 gene deletion type zebra fish through gene knockout. The method comprises the steps of through a CRISPR / Cas9 gene editing technology, designing an appropriate targeting gene locus on a tcf25 gene of the zebra fish, synthesizing in vitro to obtain specific sgRNA and Cas9-mRNA, microinjecting into a cell of the zebra fish, and after culturing an embryo for 60h, selecting the embryo for carrying out genotyping, so that the effectiveness of the selected locus is verified. The method provided by the invention is lower in off-target rate, removes the tcf25 gene through interference, is beneficial to further revealing the whole process of cardiac morphogenesis and a molecular mechanism regulating the process through researching functions through a genetics means, and is of great importance on understanding a cardiac disease pathology and researching and developing a new therapeutic schedule medically.
Owner:HUNAN NORMAL UNIVERSITY
Recombinant porcine pseudorabies virus TK/gE double-gene deletion strain
The invention separates a porcine pseudorabies virus GDXX strain, and a separating strain is served as a female parent for deleting a thymidylate kinase (TK) gene and a gE gene through a molecular biology means in sequence, thus obtaining recombinant virus PRV / TK- / gE-. The recombinant virus provided by the invention is used as a vaccine strain for preparing safe and efficient porcine pseudorabies live vaccine.
Owner:GUANGDONG WENS DAHUANONG BIOTECH +1
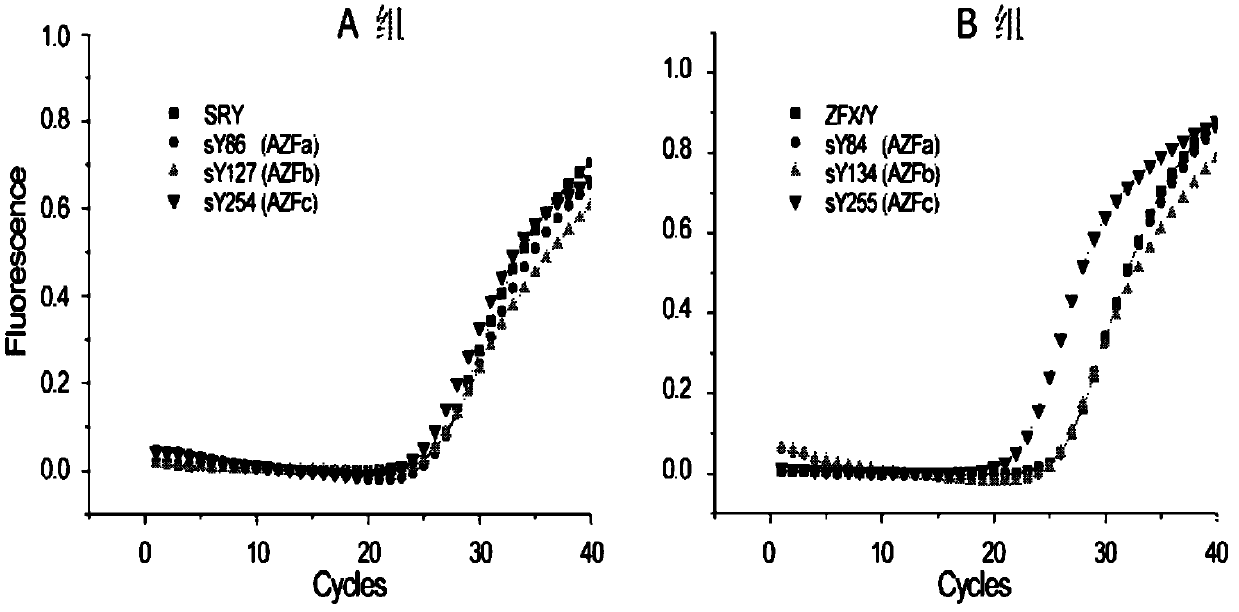
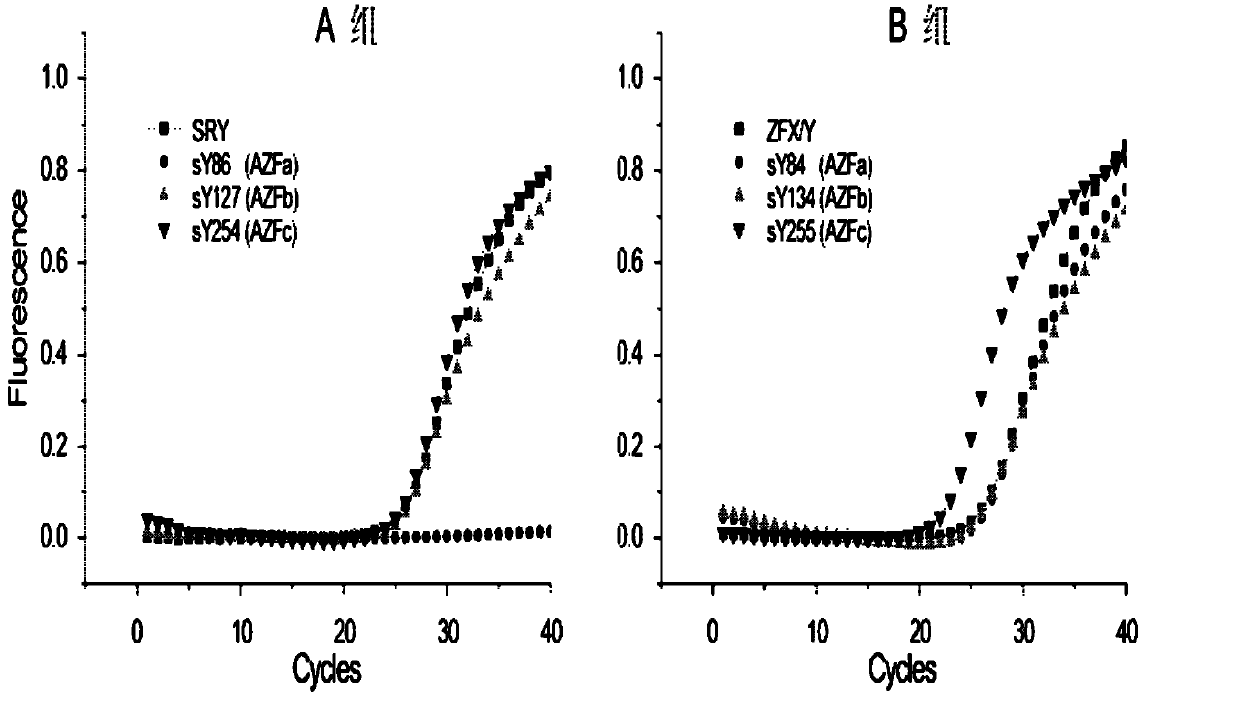
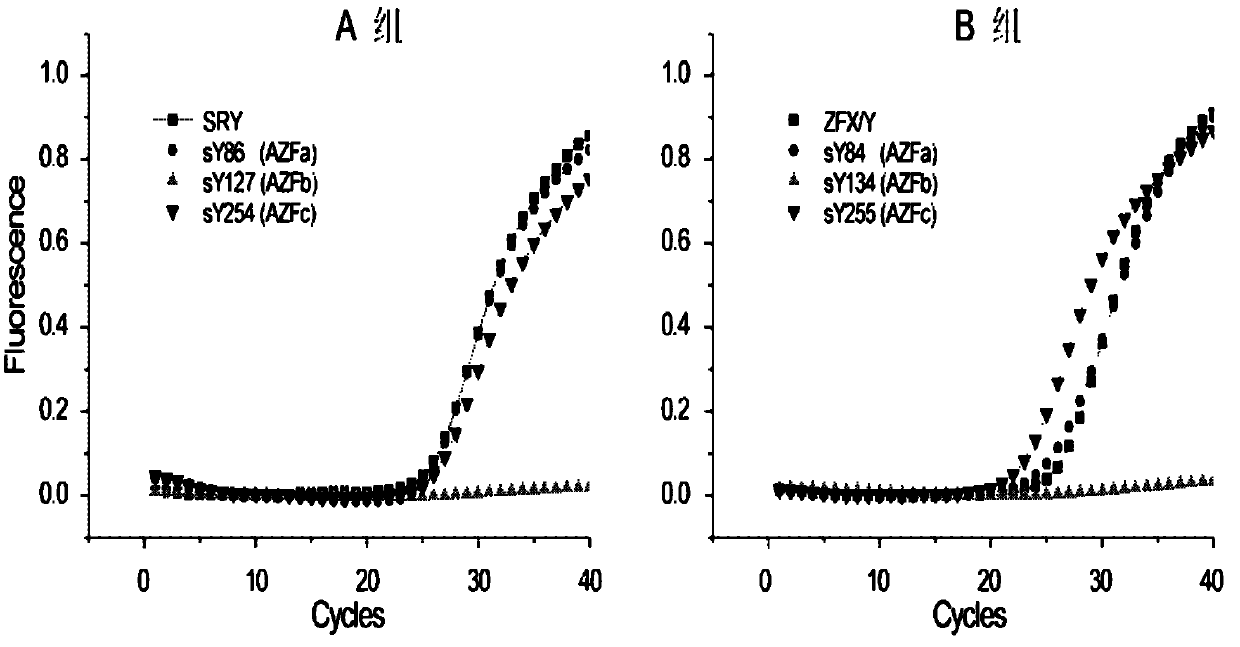
Multiple real-time fluorescent PCR detection kit for Y chromosome microdeletion
ActiveCN102703578ANo pollution concernsReal-time observation of experimental resultsMicrobiological testing/measurementY chromosome microdeletionFluorescence
The invention relates to the chromosome deletion detection field, and especially relates to a multiple real-time fluorescent PCR detection kit for Y chromosome microdeletion. The kit detects gene deletion at sY84 and sY86 loci of a Y chromosome AZFa subregion, sY127 and sY134 loci of a Y chromosome AZFb subregion, and sY254 and sY255 loci of a Y chromosome AZFc subregion by real-time fluorescent PCR. The kit realizes a stable and high-efficient multiple PCR reaction system by using homologous tailed primers and universal primers, adopts nucleic acid probes labeled by different fluorescent genes to indicate the presence of target fragments, and thus realizes the fastest simultaneous detection of sequences on 6 AZF deletion hotspots and 2 internally controlled genes by two tubes. The kit of the invention can be used for detection of male Y chromosome microdeletion genes, and has the advantages of high speed, accuracy, high throughput, low cost, no pollution, and the like.
Owner:郭奇伟
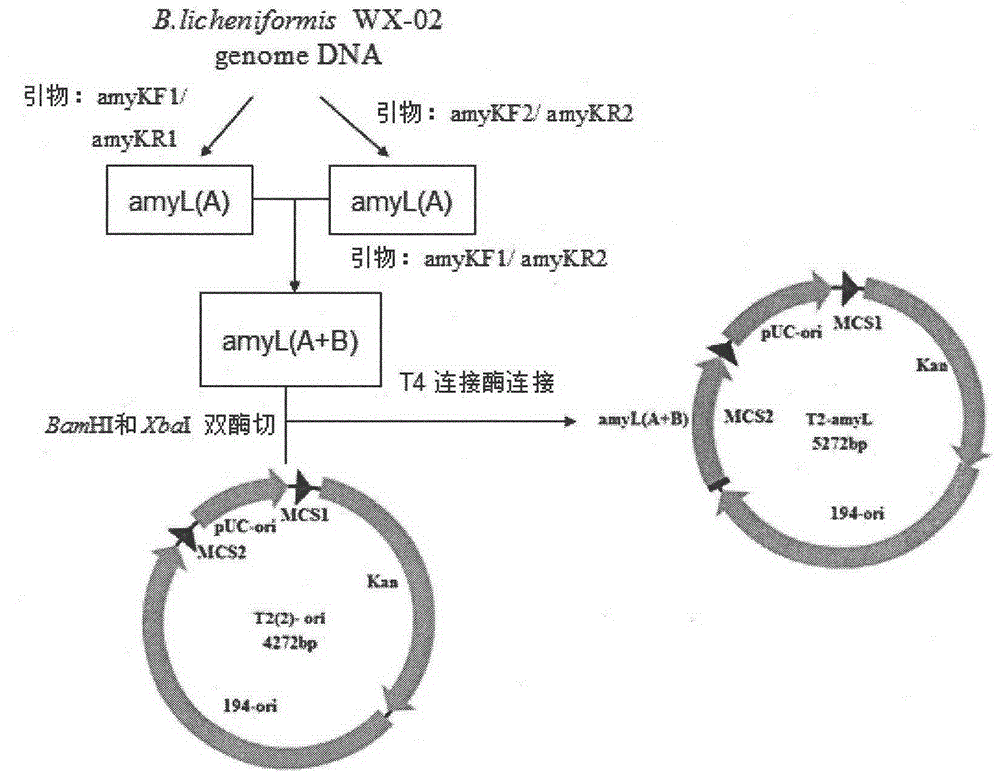
Bacillus licheniformis expression host
ActiveCN104630123AReduced activityIncrease enzyme activityBacteriaHydrolasesBacillus licheniformisProtein target
The invention discloses a bacillus licheniformis host bacterium BL10 with multiple gene deletion, the accession number of which is CCTCCNO:M2013400. The host bacterium derives from a bacillus licheniformis WX-02 which partially or completely has deletion of ten genes. The ten genes comprises eight protease genes (mpr encoding a metalloprotease; vpr encoding a serine protease; aprX encoding an intracellular serine protease; epr encoding a minimal extracellular protease; bpr encoding a bacillus peptidase F; wprA encoding a protease combined with cell walls; aprE encoding an extracellular alkaline serine protease; and bprA encoding a bacillus peptidase F) and two extracellular secretory protein genes (hag encoding flagellin; and amyL encoding alpha-amylase). The BL10 has completely no extracellular protease activity and can reduce the degradation effect of a protease on a target protein. When the target protein is expressed by the expression host, the expression level is higher, and the host bacterium benefits protein expression enhancement.
Owner:HUAZHONG AGRI UNIV
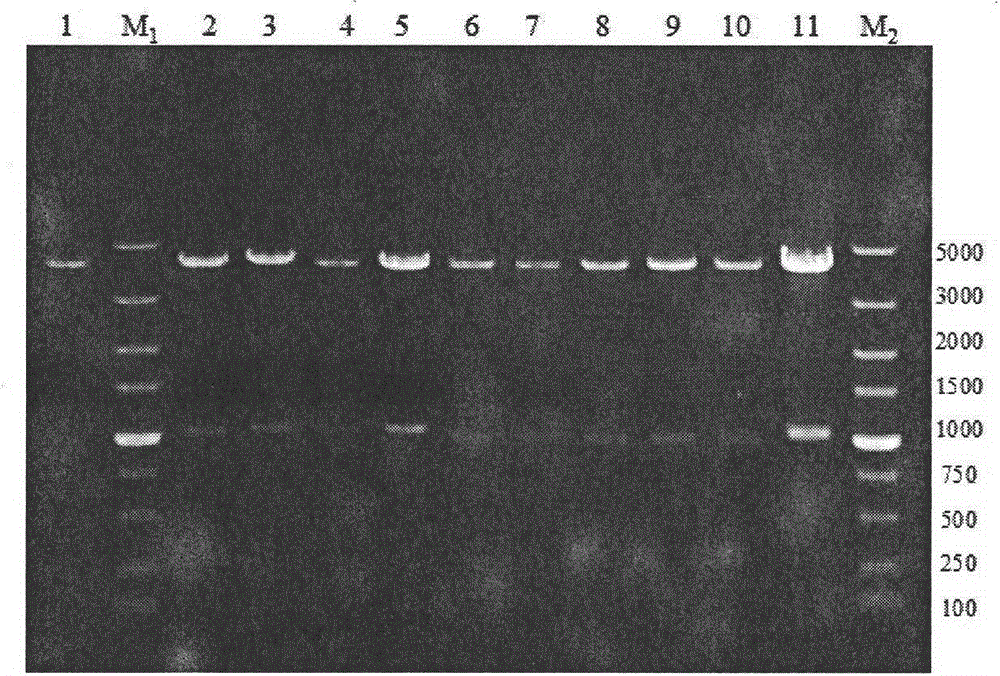
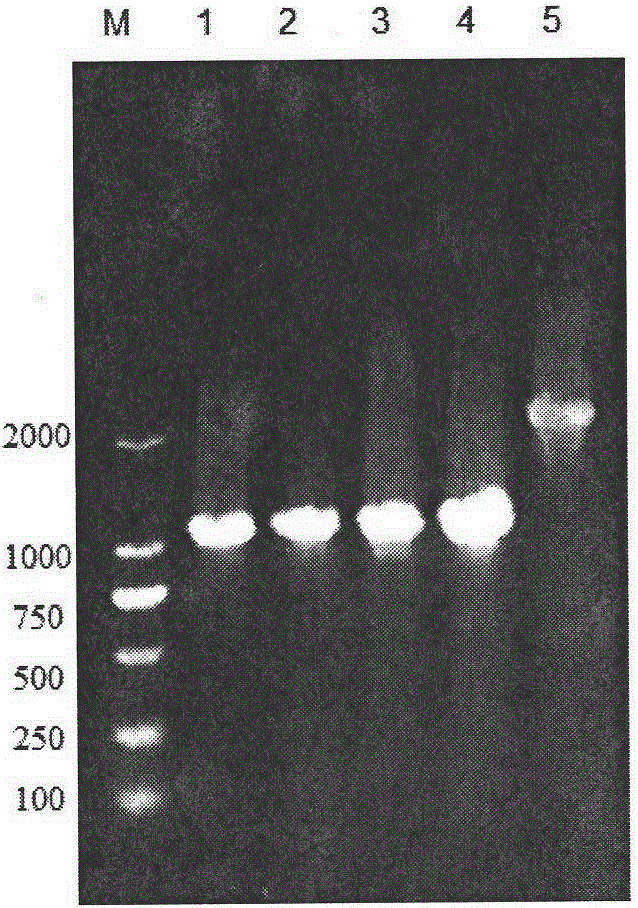
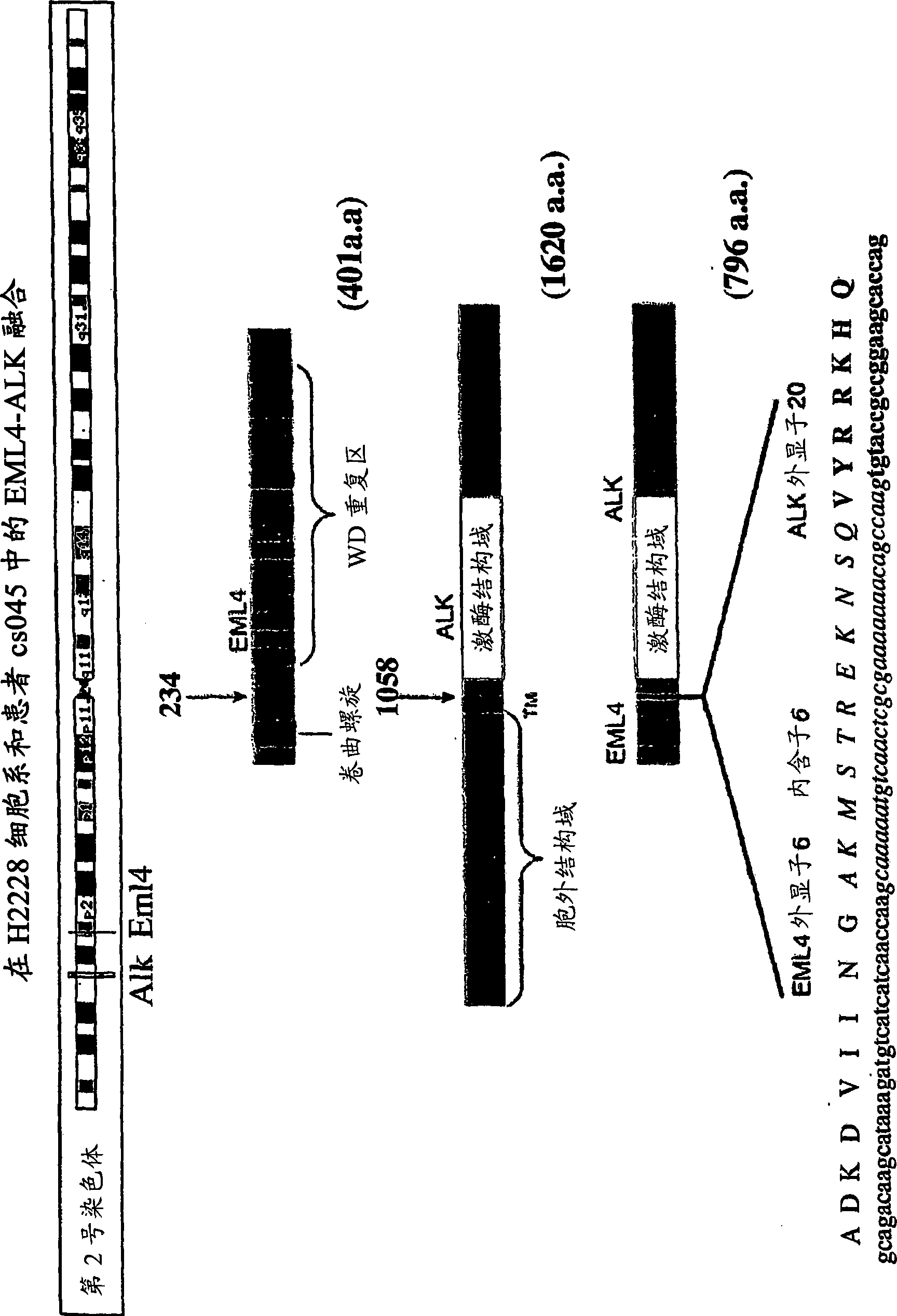
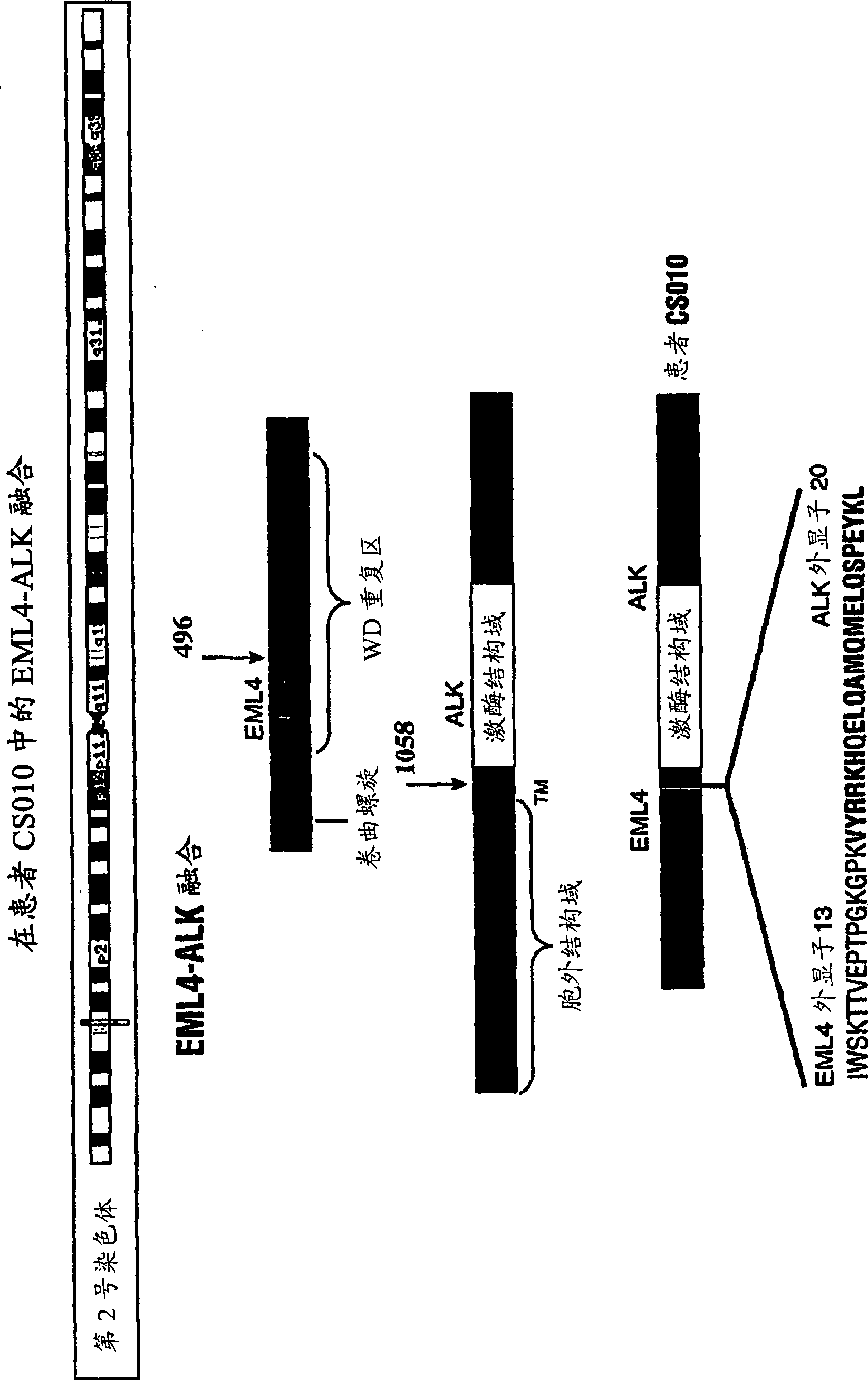
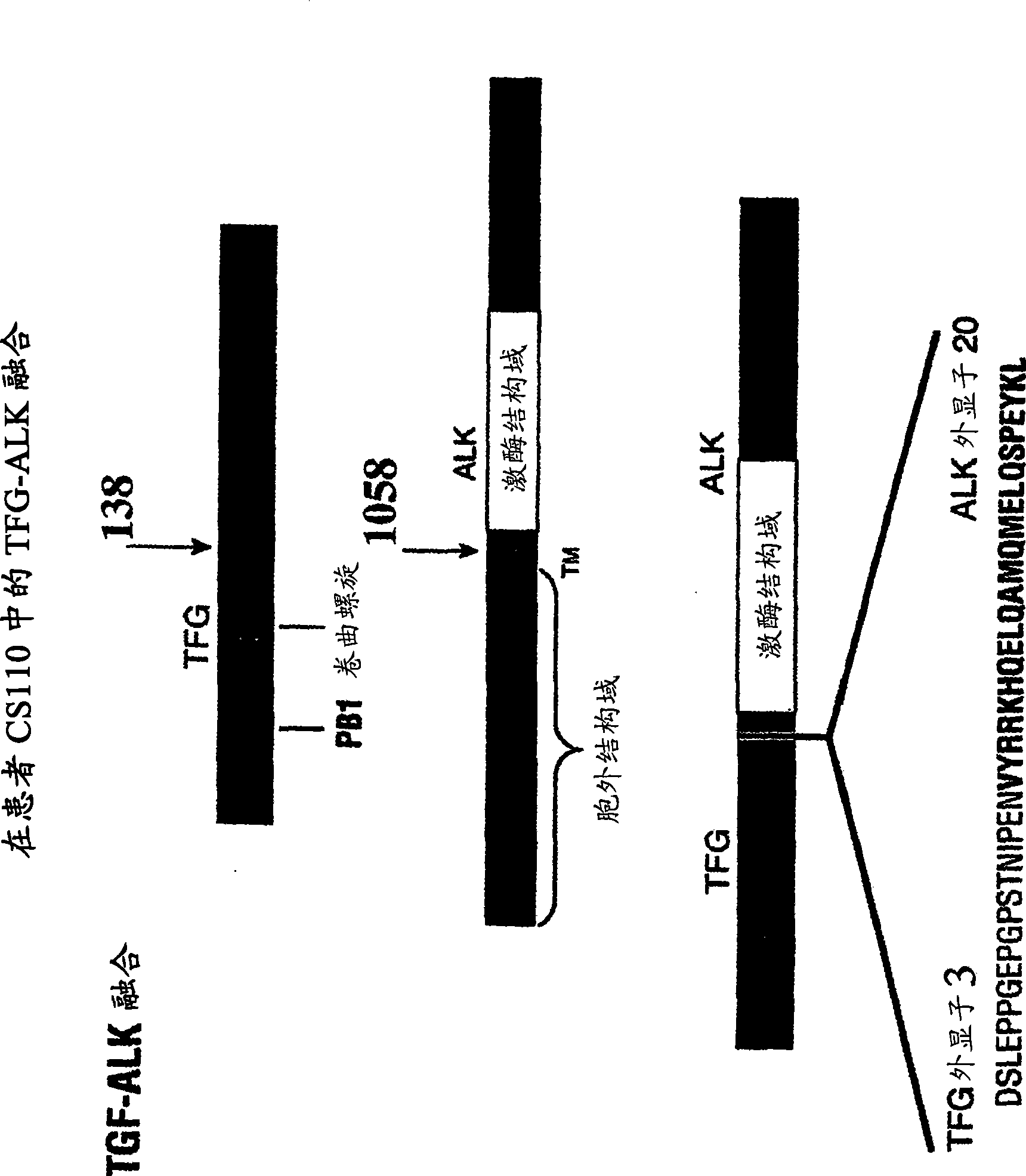
Gene defect and mutant ALK kinase in human entity tumour
ActiveCN101466721APeptide/protein ingredientsAntibody mimetics/scaffoldsADAMTS ProteinsProtein translocation
In accordance with the invention, novel gene deletions and translocations involving chromosome 2 resulting in fusion proteins combining part of Anaplastic Lymphoma Kinase (ALK) kinase with part of a secondary protein have now been identified in human solid tumors, e.g. non-small cell lung carcinoma (NSCLC). Secondary proteins include Echinoderm Microtubule- Associated Protein-Like 4 (EML-4) and TRK- Fusion Gene (TFG). The EML4-ALK fusion protein, which retains ALK tyrosine kinase activity, was confirmed to drive the proliferation and survival of NSCLC characterized by this mutation. The invention therefore provides, in part, isolated polynucleotides and vectors encoding the disclosed mutant ALK kinase polypeptides, probes for detecting it, isolated mutant polypeptides, recombinant polypeptides, and reagents for detecting the fusion and truncated polypeptides. The disclosed identification of this new fusion protein enables new methods for determining the presence of these mutant ALK kinase polypeptides in a biological sample, methods for screening for compounds that inhibit the proteins, and methods for inhibiting the progression of a cancer characterized by the mutant polynucleotides or polypeptides, which are also provided by the invention.
Owner:CELL SIGNALING TECHNOLOGY
Wnt16 gene deletion type zebra fish
InactiveCN106191110AShorten the growth cycleGood medical researchNucleic acid vectorVector-based foreign material introductionLiving bodyGenome
The invention discloses a wnt16 gene deletion type zebra fish. Compared with the prior art, specific genes in the genome of a living body can be muted more efficiently and precisely; moreover, the manufacturing is simple, the cost is lower, multiple sites on target genes can be sheared at the same time, and any number of single gene can be muted. The wnt16 gene deletion type zebra fish can be applied to research related with gene and skeleton growth and other research for finding whether deletion of wnt16 gene is related with growth of other organs such as heart or not, and thus the wnt16 gene deletion type zebra fish has a good medical research value. At the same time, the growth period of zebra fishes without wnt16 gene is obviously shortened, and the wnt16 gene deletion type zebra fish also has a good commercial value therefore.
Owner:HUNAN NORMAL UNIVERSITY
Gene deletion system for security control of transgene monocotyledon
InactiveCN101624594AImprove deletion efficiencyVector-based foreign material introductionAngiosperms/flowering plantsMultiple cloning siteTissue specific
The invention relates to a gene deletion system for the security control of transgene monocotyledon, comprising two homodromous loxP and FRT fused specificity recognition sites loxP-FRT, wherein a section of multiple clone site sequence for inserting a target gene to be led in plant is contained between the two specificity recognition sites. The gene deletion system can completely delete foreign genes in a specific plant organ by a plant expression vector which is formed by leading a monocotyledon tissue-specific promoter sequence, and can achieve the deletion efficiency of 99.8 percent, thereby being used for preparing the safe transgene monocotyledon.
Owner:SOUTHWEST UNIVERSITY
Kit for testing tiny gene loss of male Y chromosome, and testing method
InactiveCN101092648ALow costThe result is stableMicrobiological testing/measurementDNA/RNA fragmentationY chromosome microdeletionBiology
This invention relates to test kit and method for detecting male Y chromosome microdeletion genes. The test kit contains 30 primers for amplifying AZF of Y chromosome, and 2 primers for amplifying male-specific Sry gene. The test kit can be used for detecting male Y chromosome microdeletion genes, and has such advantages as high efficiency, low cost and stable result.
Owner:亚能生物技术(深圳)有限公司
Triple PCR detection primer and kit for rapidly distinguishing African swine fever virus wild strains from gene deletion strains
ActiveCN110551853AReduce testing costsShorten detection timeMicrobiological testing/measurementMicroorganism based processesAgricultural scienceAfrican swine fever
The invention discloses a triple PCR detection primer set for rapidly distinguishing African swine fever virus wild strains from CD2V and / or 360-505R gene deletion strains. Nucleotide sequences of three pairs of detection primers are as shown in SEQ ID NO: 1-6. Three pairs of primers are utilized to amplify three genes of African swine fever viruses CD2V, P72 and 360-505R at a time, so that the detection cost for identifying different genes is reduced, and the detection time for identifying different genes is shortened; and moreover, three genes with different lengths can be amplified only byone-time PCR reaction, and whether gene deletion exists in the strains or not can be distinguished. The three genes can be identified by one-time PCR amplification only, the cost is reduced by about 2 / 3 for a traditional method for respectively amplifying and detecting the samples of the three genes, and the triple PCR detection primer set for rapidly distinguishing the African swine fever virus wild strains from the CD2V and / or 360-505R gene deletion strains has wide market prospect.
Owner:SOUTH CHINA AGRI UNIV +1
Protein expression strains
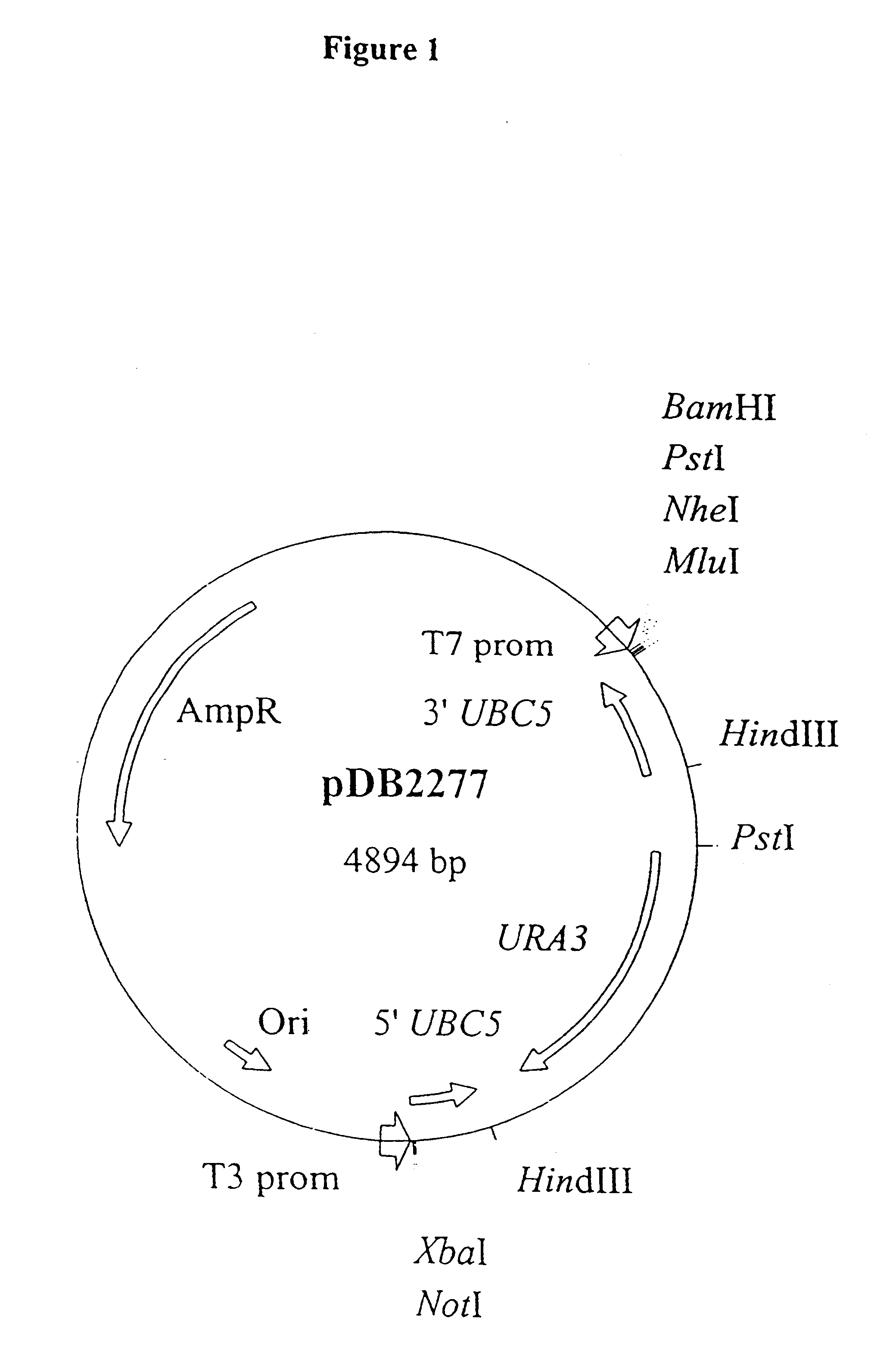
InactiveUS6379924B1Reduced activityReduction in translatable UBC mRNAFungiHaemoglobins/myoglobinsActive proteinPlasmid
The use of a means to vary Ubc4p or Ubc5p activity in a fungal cell to control the copy number of a plasmid in the cell. The level of Ubc4p or Ubc5p activity may be reduced / abolished (for example by gene deletion, mutagenesis to provide a less active protein, production of antisense mRNA or production of competitive peptides) to raise the copy number and increase yield of a protein encoded by the plasmid.
Owner:ALBUMEDIX AS
Triple fluorescent quantitative PCR detection material and kit for distinguishing African swine fever virus wild strains from CD2V and/or 360-505R gene deleted strains
ActiveCN111074000AHigh SensitivityThe detection method is accurateMicrobiological testing/measurementMicroorganism based processesNucleotideNucleotide sequencing
The invention discloses a triple fluorescent quantitative PCR detection material and kit for distinguishing African swine fever virus wild strains from CD2V and / or 360-505R gene deleted strains. The detection material comprises primers and probes, and the nucleotide sequences are shown as SEQ ID NO: 1-9. According to the invention, triple fluorescent quantitative PCR amplification is carried out on African swine fever virus P72, CD2V and 360-505R genes by utilizing three groups of primer probes, so that the detection cost and the detection time for identifying different genes are reduced; whether gene deletion exists in the strain or not can be distinguished; the detection material and kit have high specificity and sensitivity; and for traditional qPCR enabling only one gene to be amplified each time, the provided mode has the following advantages: three genes are amplified at the same time in each PCR reaction, and the cost is reduced by about 2 / 3.
Owner:SOUTH CHINA AGRI UNIV +1
Method for breeding zebra fish with wnt16 gene deletion through gene knockout
InactiveCN106191112AShorten the growth cycleGood medical researchPeptidesNucleic acid vectorState of artLiving body
The invention discloses a method for breeding zebra fish with wnt16 gene deletion through gene knockout. Compared with the prior art, the method has the advantages that specific genes can be more efficiently and more accurately silenced in a genome of a living body, in addition, the preparation is simple, the cost is low, moreover, a plurality of sites on a target gene can be simultaneously shorn, and single genes of any number can be silenced. The method is used for carrying out the relevant researches on gene and skeletal development, also can be used for carrying out the exploration research of other aspects, is used for detecting that whether the deletion of the wnt16 gene has relevance with the development of other organs, such as the heart, or not, and has a very good medical research value, meanwhile, the zebra fish with the wnt16 gene knocked out has the obviously shortened growth cycle, and thus the commercial value is good.
Owner:HUNAN NORMAL UNIVERSITY
Porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of cell and attenuation via drug screening and application thereof
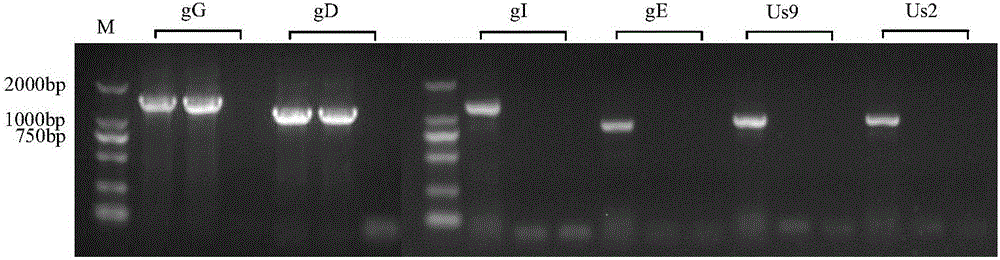
ActiveCN106282128AImprove securityImprove protection efficiencyViral antigen ingredientsMicroorganism based processesLarge fragmentVariant strain
The invention discloses a porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of a cell and attenuation via drug screening and an application thereof. The attenuated vaccine strain is prepared through the following steps: based on a porcine pseudorabies virus variant strain (named as strain HeN1, of which the microbial preservation serial No. is CGMCC No. 6656), firstly, carrying out low-temperature passage and screening on a Vero cell to obtain large fragments of deleted viruses including gI, gE, Us9, Us2 and part of inverted repeated sequence which exist in zone US through, and then making the TK gene thereof partially deleted through drug screening. The gene-deleted attenuated vaccine strain is named as strain PRV TP, of which the microbial preservation serial No. is CGMCC No. 12300. A live vaccine or an inactivated vaccine (a single vaccine or combined vaccine) can be prepared from the attenuated vaccine strain disclosed by the invention, and can prevent porcine pseudorabies effectively, and a reagent for diagnosing or treating porcine pseudorabies can be prepared from the attenuated vaccine strain too. According to the porcine pseudorabies virus gene deletion attenuated vaccine strain for passage via low temperature of a cell and attenuation via drug screening and the application thereof, the porcine pseudorabies attenuated vaccine strain PRV TP has the advantages of good safety, efficient protection, convenient differential diagnosis and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Chicken Marek's disease Meq gene deleted vaccine strain, construction method thereof, and application thereof
ActiveCN102363769ANon-pathogenicGenetic stabilityMicrobiological testing/measurementMicroorganism based processesOncogeneWild strain
The invention discloses a chicken Marek's disease (MD) Meq gene deleted vaccine strain, a construction method thereof, and an application thereof. The chicken Marek's disease rMS delta Meq gene deleted vaccine strain has a microbe reservation number of CGMCC No. 4612. According to the invention, on a basis of a parent strain which is MDV MS strain, a 468bp base sequence on a front part of a main oncogene is deleted thorough two times of homologous recombination, such that the chicken Marek's disease Meq gene deleted vaccine strain is obtained. The invention also relates to the application of the gene deleted vaccine strain in preparation of medicine used for controlling chicken Marek's disease, and in detection and disgnosis methods used for distinguishing a vaccine strain and a chicken Marek's disease wild strain, wherein the methods are designed aiming at the deleted gene sequence of the gene deleted strain. The gene deleted vaccine strain provided by the invention has good safety and good immuno-protection effect upon chicken Marek's diseases. The vaccine strain can be used for preparing monovalent vaccines or combined vaccines used for controlling chicken Marek's disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Food grade lactic acid bacteria active carrier Group A rotavirus vaccine and preparation method thereof
InactiveCN103656633AWill not spreadNo horizontal transferViral antigen ingredientsDigestive systemEscherichia coliSerotype
The invention discloses food grade lactic acid bacteria active carrier Group A rotavirus vaccine and a preparation method thereof. The food grade lactic acid bacteria active carrier Group A rotavirus vaccine is characterized in that VP6 antigen protein from Group A virus, common serotype VP7 antigen protein (P serotype) and VP4 antigen protein (G serotype-expressed separately in the form of VP5* and VP8* protein subunit), vaccine adjuvant escherichia coli thermal unstable toxin B (LTB) and cholera toxin subunit B (CTB) are expressed and secreted by thyA gene deletion lactic acid bacteria cell or shown by the cell wall. Expressions of antigen protein and vaccine adjuvant protein are controlled by inducible or constitutive promoter, protein expression cassette is integrated onto the chromosome of the expression host lactic acid bacteria strain, and external antibiotics resistance gene introduced in gene manipulation is removed. The lactic acid bacteria active carrier rotavirus vaccine has the advantages of having wide serotype covering range, being easy to produce in large scale, and being safe and convenient to use without a refrigerator and a needle tubing.
Owner:刘占良 +2
Recombinant oncolytic vaccinia virus and preparation method and application thereof
InactiveCN108165536AInhibitory activityIncrease lethalityUnknown materialsImmunoglobulinsWilms' tumorTumor cells
The invention discloses a recombinant oncolytic vaccinia virus and a preparation method and application thereof. The thymidine kinase (TK) region of the virus comprises a coding sequence of a PD1 full-length antibody shown in the formula of SEQ ID NO.1. Through effective combination of the anti-tumor effect of gene therapy and the oncolytic effect of virus treatment, the oncolytic vaccinia virus for efficiently expressing the PD1 full-length antibody gene is prepared. When the oncolytic vaccinia virus produces the oncolytic effect of splitting tumor cells, the PD1 full-length antibody is efficiently expressed, the activity of PD1 on the surfaces of T cells is inhibited, the T cell immune response is activated and the dual antitumor effects are produced. Compared with the single gene therapy or viral therapy, the method using the recombinant oncolytic vaccinia virus improves the effects of killing malignant B cell lymphoma. Through virus replication-related gene deletion, the TK regionof the vaccinia virus genome is deleted so that specific replication of the viruses in the abnormally proliferating tumor cells is ensured and the viruses can not replicate in normal cells, so that the safety of the oncolytic vaccinia virus vector is greatly improved.
Owner:ZHEJIANG UNIV
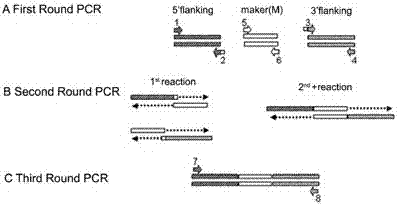
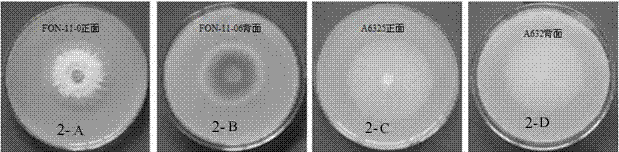
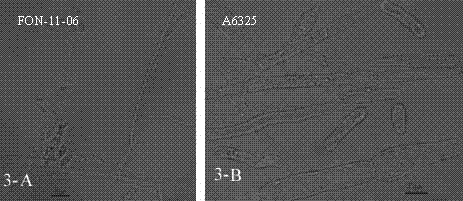
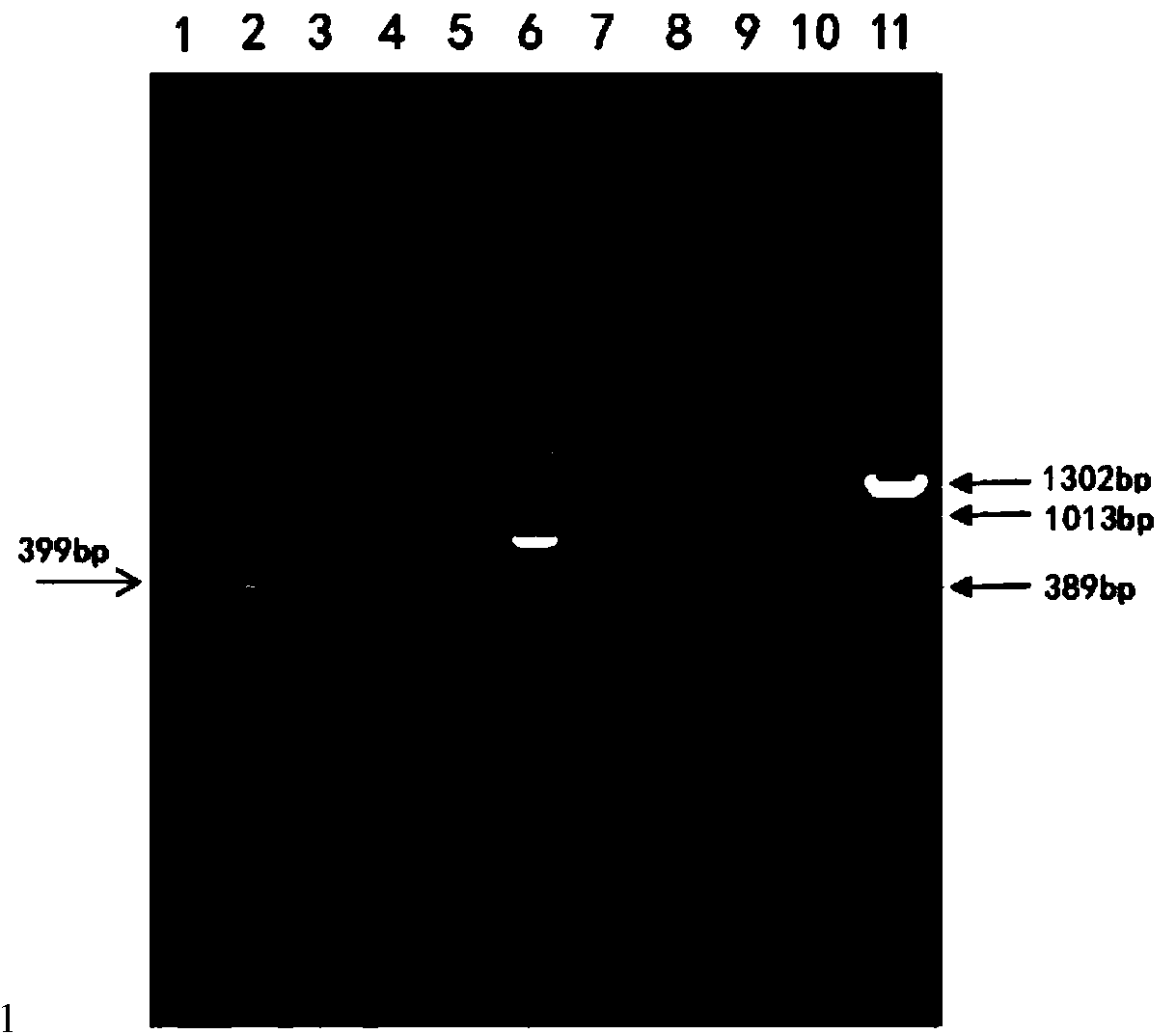
Watermelon oxysporum pathogenic FonAGL3 gene as well as deleted DNA fragment, deletion mutant and application thereof
InactiveCN107299105AClear control functionEffectiveness of Good Fusarium Wilt ControlBiocideFungiHygromycin BTreatment effect
The invention discloses a watermelon oxysporum pathogenic FonAGL3 gene as well as a deleted DNA fragment, a deletion mutant and application thereof, and aims to solve the technical problem of biological prevention and treatment on watermelon oxysporum. A pathogenic gene FonAGL3 derived from watermelon fusarium oxysporum is analyzed and screened by inserting watermelon oxysporum T-DNA into a mutant library, by utilizing the homologous gene replacement principle, DNA fragments of the target gene FonAGL3 are replaced by gene DNA fragments of a resistance gene hygromycin B (HPH), a FonAGL3 gene deletion mutant is obtained by establishing a gene deletion carrier and implementing genetic transformation of a wild strain FON-11-06, and the FonAGL3 mutant bacterium has a good wilt prevention and treatment effect and is environmental-friendly and low in prevention and treatment cost.
Owner:河南省农业科学院园艺研究所 +1
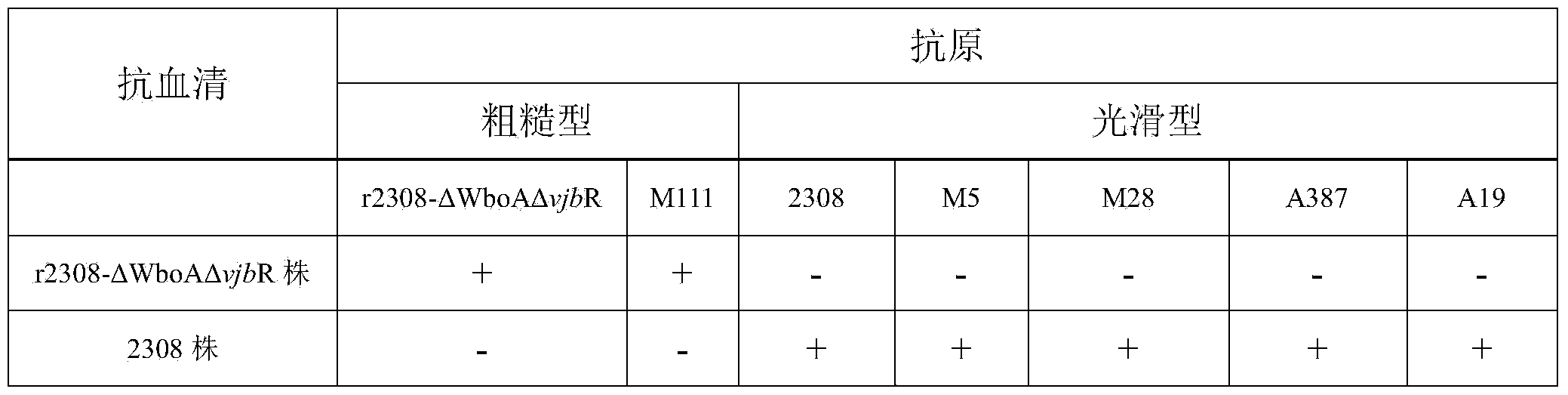
Dual-gene deletion rough type bovine brucellosis and production method for vaccine thereof
InactiveCN104031874AAntibacterial agentsBacterial antigen ingredientsResistant genesBrucella Vaccine
The invention relates to dual-gene deletion rough type bovine brucellosis and a production method for a vaccine thereof. A recombinant bacterial strain deletes a WboA gene and a vjbR gene of a bovine brucellosis 2308 strain by virtue of a non-resistance gene screening technology. Due to deletion of two genes, on the one hand, a condition (cased by WboA gene deletion) for forming an O chain in a smooth type bovine brucellosis cell wall LPS (lipopolysaccharide) structure is lost, and colonial morphology is changed into a rough type from a smooth type; and on the other hand, due to deletion of vjbR gene, toxicity of recombinant bacteria and viability in a cell are descended, so that infection ability of bovine brucellosis on a target animal is lowered, and therefore, safety of the vaccine is further improved. By using the bacterial strain to produce the vaccine, a current situation that a bovine brucellosis vaccine immune animal and a wild strain-infected animal are difficult to distinguish is changed, and safety of the existing vaccine is effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
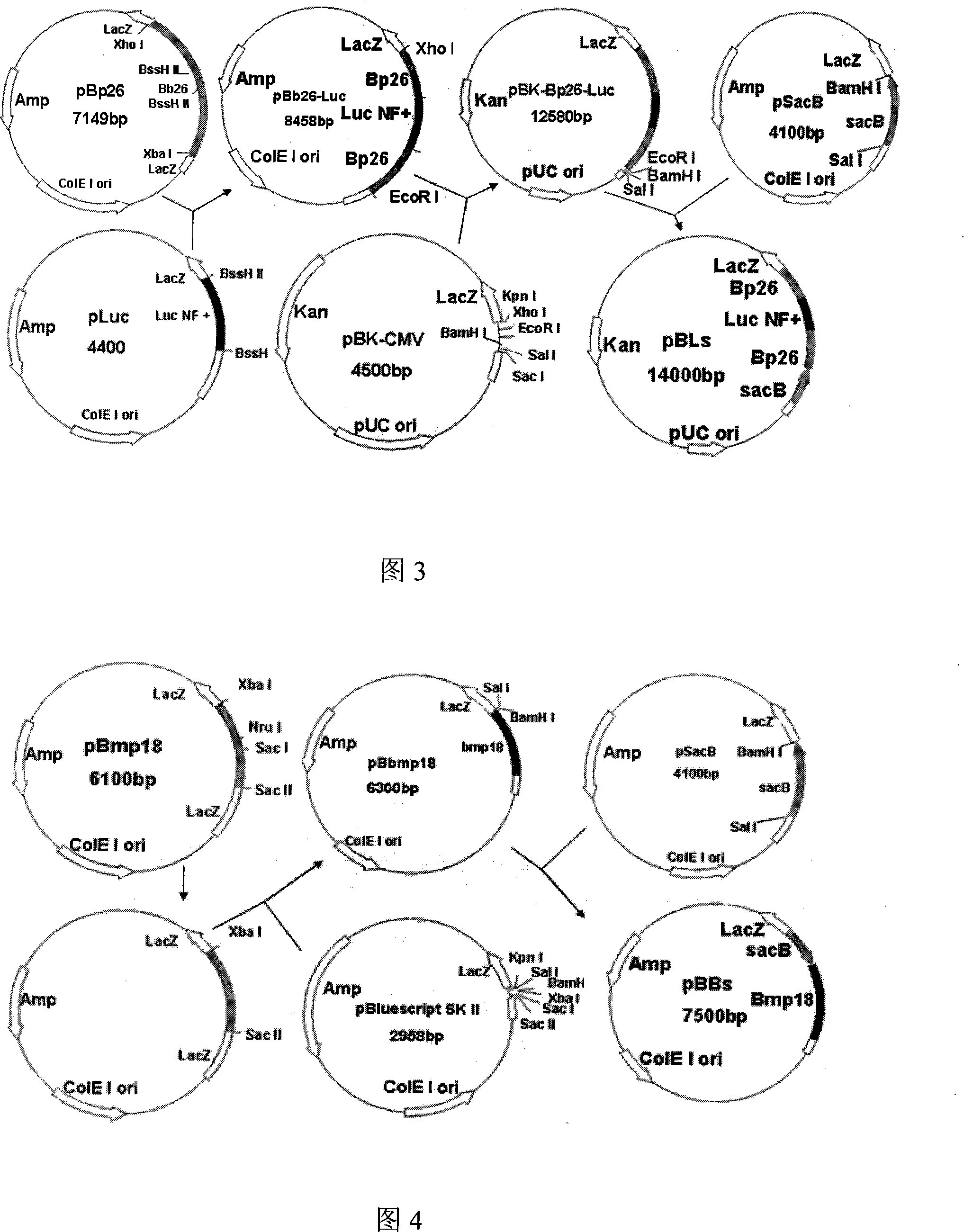
Brucella molecule marking and virulence deletion attenuated vaccine and preparation method
InactiveCN101185756AWide application of practical valueAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsVaccine Immunogenicity
The invention relates to a Brucella vaccine, in particular to the molecular marker and virulence gene deletion of Brucella vaccine strain. The study uses luciferase modified gene (Luc NF plus) to replace the partial fragment of Bp26 gene of Brucella attenuated vaccine S19 strain by constructing suicide plasmid and adopting the method of targeted homologous recombination (gene targeting), so as to damage the expression of the immunogenicity protein BP26 and construct the gene deletion mutant strain Delta S19-1 of the Brucella Bp26. The BMP18 protein is one of the main virulence factors of Brucella. The invention adopts the same method to exclude the Bmp 18 gene of Delta S19-1, so as to lead the Delta S19-1 not to express the Bmp 18 protein and the Brucella virulence gene deletion mutant strain Delta S19-2 is constructed. The invention solves the problems that the conventional Brucella vaccine can not distinguish between the artificial immunization and the wild bacteria infection of people and animals, the virus is strong and the vaccine is easy to cause the illness of inoculated people and animals. The invention has important significance and practical application value of the monitoring, diagnosis, purification and all the controls of Brucella.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Kit for simultaneously detecting multisite mutation of genes CYP2C19 and CYP2D6
InactiveCN105861703AHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDeletion mutationCoincidence
The invention discloses a kit for simultaneously detecting multisite mutation of genes CYP2C19 and CYP2D6. A group of Taqman allele resolution analysis method-based specific primers and probes is obtained by meticulous designing, multiple verification, screening and optimization, and 8 functional variations of 4 types, i.e. single base displacement, deletion mutation, gene deletion and duplication mutation, of genes CYP2C19 and CYP2D6 can be detected; from DNA extraction to fluorescent PCR and then to result acquisition, 4 hours is required only, and the manual operation time is shorter than 2 hours. The kit containing the primers has the advantages of time saving, convenience, high sensitivity, capability of ensuring that the positive coincidence rate and negative coincidence rate of a sample are both over 99 percent, and the like.
Owner:钟诗龙
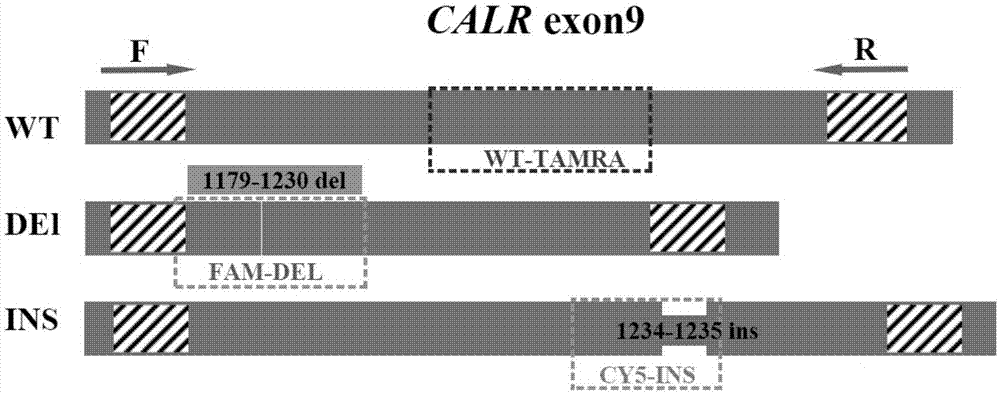
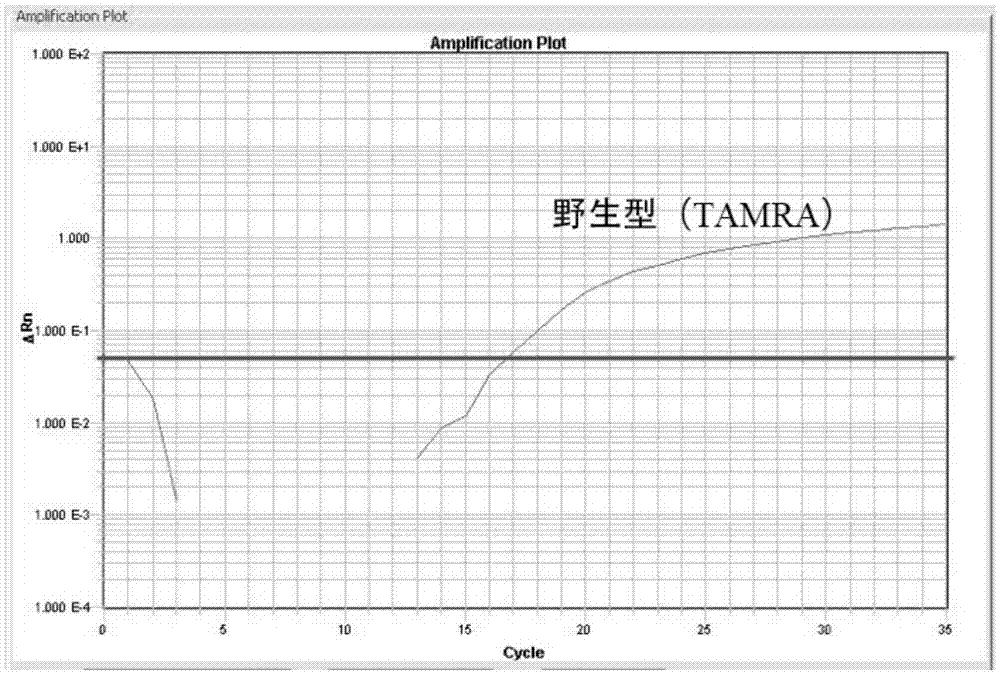
Detection method for CALR (Calreticulin) gene deletion and insertion mutation and kit
InactiveCN105441562AAccurate detectionImmediate observation of test resultsMicrobiological testing/measurementDNA/RNA fragmentationCalr geneSpecific detection
The invention provides a detection method for deletion and insertion mutation of L367fs*46 (c.1179-1230del) and K385fs*4(c.1234-1235insTTGTC) of the ninth exon of a CALR (Calreticulin) gene. Universal amplification primers are designed according to deletion and insertion mutation areas of the ninth exon of the CALR gene, specific detection fluorescence probes are designed according to amplified fragments, real-time fluorescence quantification PCR (polymerase chain reaction) is performed on a to-be-detected sample, and deletion and insertion mutation of the CALR gene are identified through multiple single-tube reactions. The invention further provides applications of the universal amplification primers and the specific detection fluorescence probes in preparation of myeloproliferative neoplasm detection reagents as well as a kit for detecting myeloproliferative neoplasms.
Owner:DIASYS DIAGNOSTIC SYST SHANGHAI
Cytomegalovirus gene function and methods for developing antivirals, anti-CMV vaccines, and CMV-based vectors
ActiveUS7407744B2Enhance their long-term survivabilitySugar derivativesMicrobiological testing/measurementSurvivabilityORFS
A global functional analysis of HCMV genes is performed by constructing virus gene-deletion mutants and examining their growth phenotypes in different natural HCMV host cells. This systematic analysis of the HCMV genome identified 45 viral ORFs essential for viral replication and characterizes of 115 growth-dispensable viral genes. Of particular interest is the finding that HCMV encodes genes (temperance factors) that repress its own replication on a cell type-specific basis. In addition to HCMV, pathogen temperance may be a strategy employed by other infectious agents to enhance their long-term survivability within their respective host population.
Owner:RGT UNIV OF CALIFORNIA
LAMP kit for detecting PRV and preparation method thereof
ActiveCN101775443AEasy to detectImprove the level of immune defenseMicrobiological testing/measurementFood safetyQuarantine
The invention relates to a LAMP kit for detecting PRV, an establishment method and an application thereof. The kit comprises a detection system consisting of LAMP reaction solution of four primers. Detection has verified that the kit can quickly and conveniently detect the PRV with high efficiency, high specificity and high sensitivity under 60 to 65DEG C isothermal condition, does not need any complicated instrument, can well meet the requirement on the field detection to the PRV, can distinguish PRV gE gene deletion vaccine and field strains, provides a novel technical platform for the safety detection of food, can well meet the urgent need on the field detection of the PRV, is used for the field detection of import and export quarantine, food health departments, livestock farms and the like, and is easy to be popularized and applied in a large scope.
Owner:天津市农业科学院
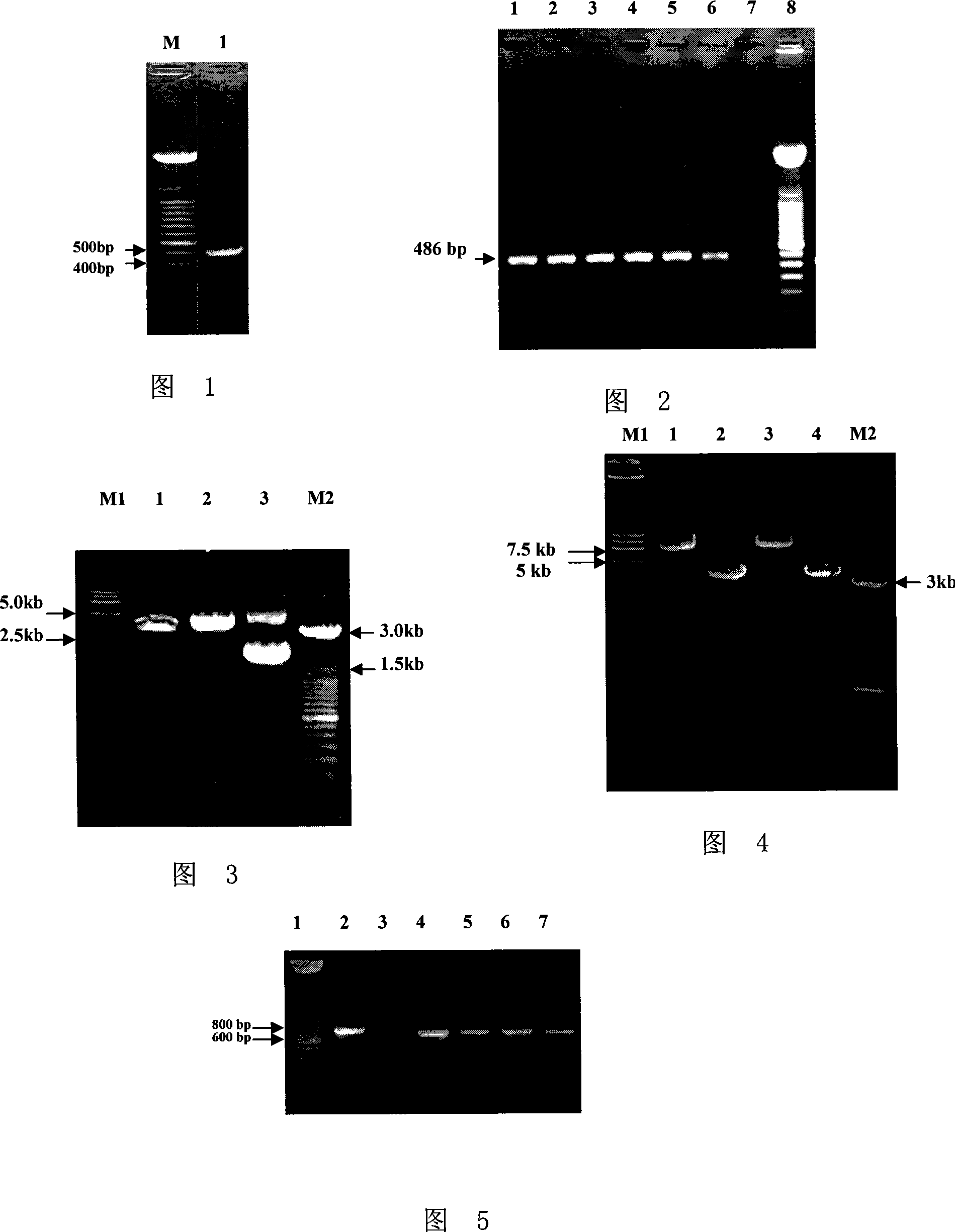
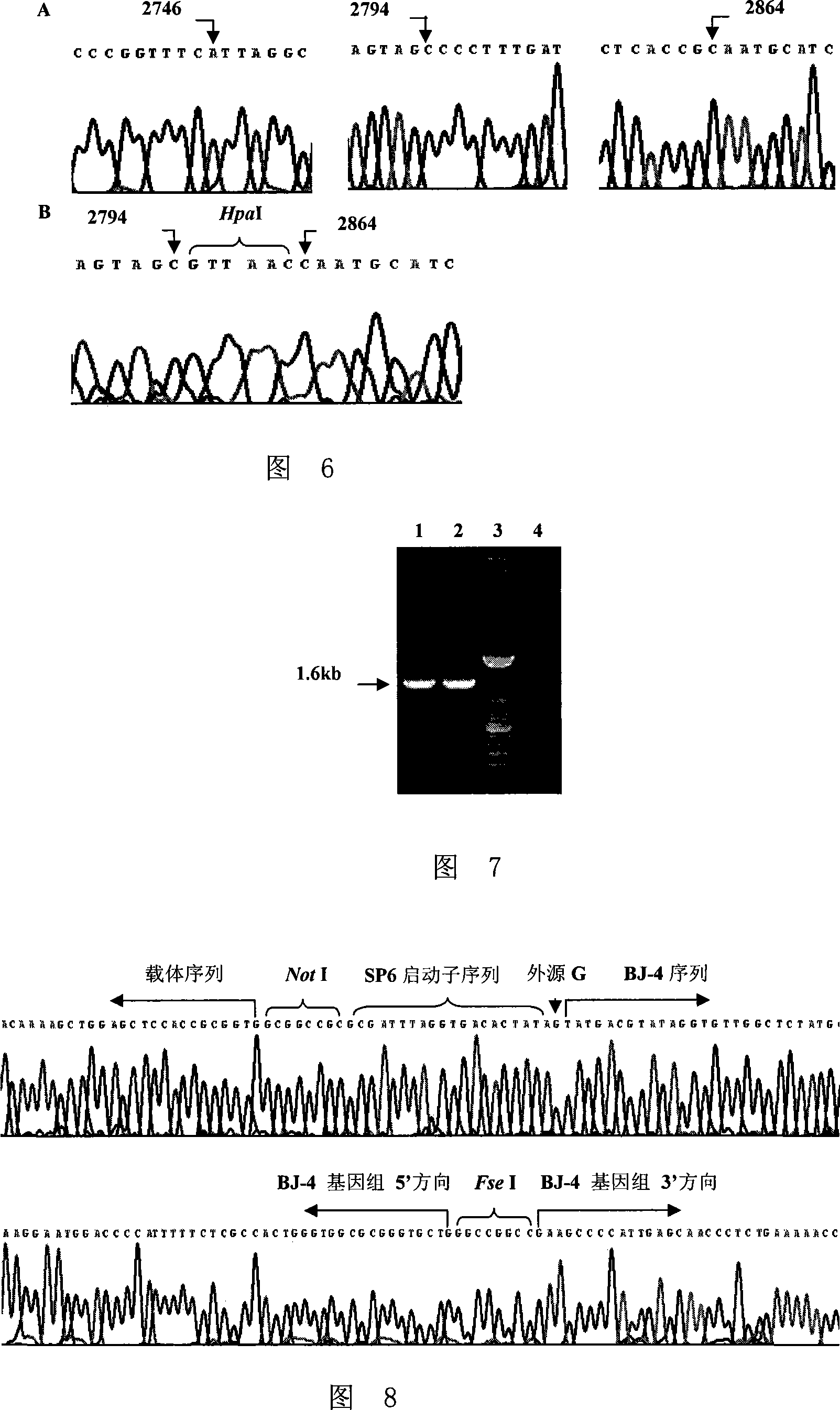
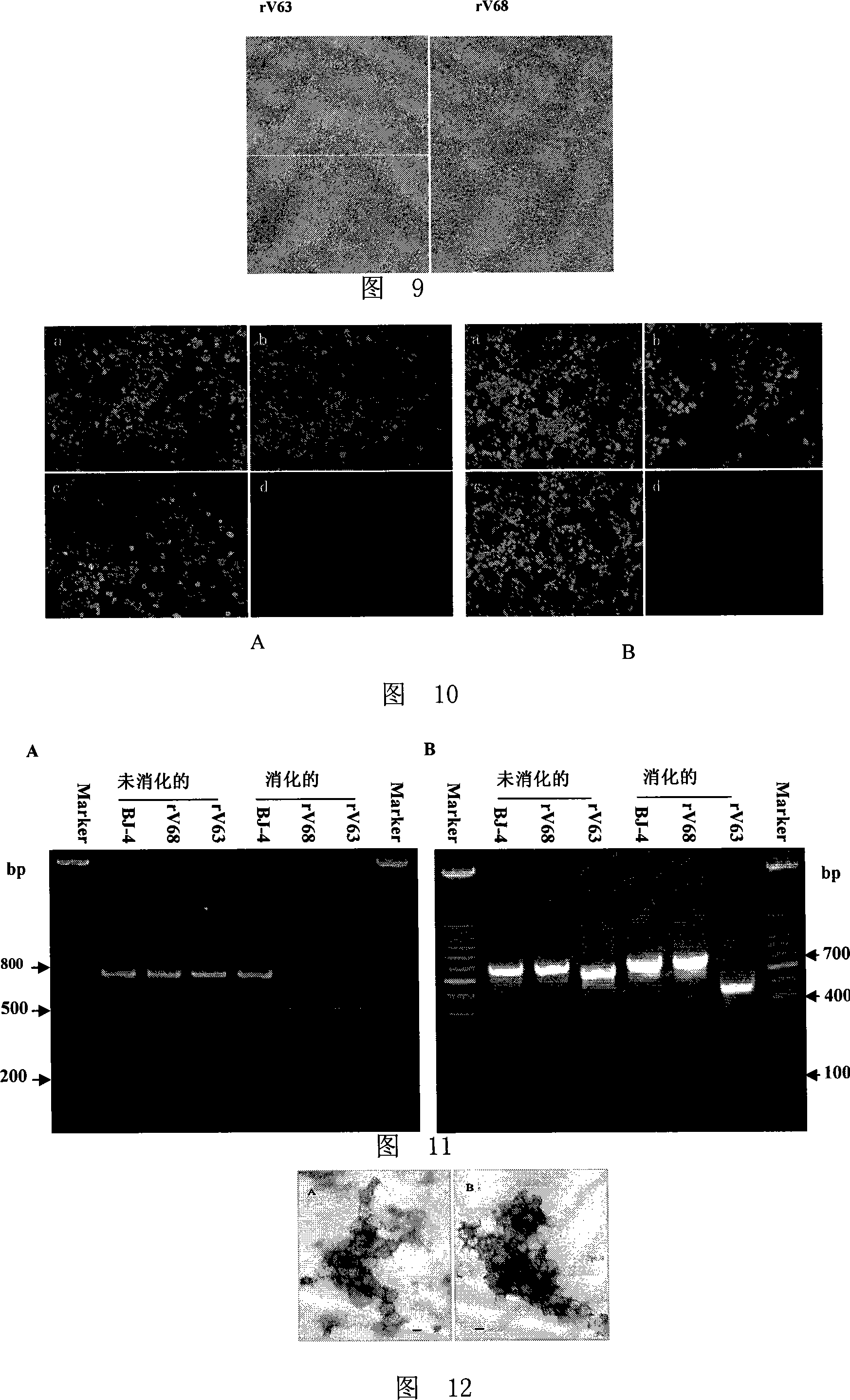
Method for constructing PRRSV gene deletion vaccine toxin strain by using Nsp2 gene deletion and uses thereof
InactiveCN101220351AIncreased neutralizing antibody levelsOvercome inhibitory factorsGenetic material ingredientsInactivation/attenuationNucleotideVaccine virus
The invention discloses a method for utilizing Nsp2 gene deletion for constructing a PRRSV gene deletion vaccine virus strain and the application thereof. The method for lowering the toxicity of the PRRSV BJ-4 strain of the invention is that a nucleotide segment with the length of 69nt in a Nsp2 coding region of the PRRSV BJ-4 strain genome is continuously deleted to obtain the virus strain with reduced toxicity; the nucleotide sequence of the 69nt nucleotide segment is shown as the 2795 to 2863 sites from the 5' tail end of GenBank Accession Number AF331831. The Nsp2 of the virus rV63 which is prepared by the method for lowering the toxicity of the PRRSV BJ-4 strain deletes 21 amino acids, which has the potential to develop a marker vaccine and can identify the diagnosis method of the deleted polypeptide. When in the development process of vaccine, the invention can utilizes a reverse genetic technical platform which is already established for carrying out the transformation of GP5 glycosylation sites, thus overcoming the suppression factors which affect the neutralizing epitope and further improving the level of the vaccine-induced neutralizing antibody.
Owner:CHINA AGRI UNIV
Methods and systems to detect large rearrangements in brca1/2
PendingUS20180340234A1High sensitivityMicrobiological testing/measurementBiostatisticsExonGene deletion
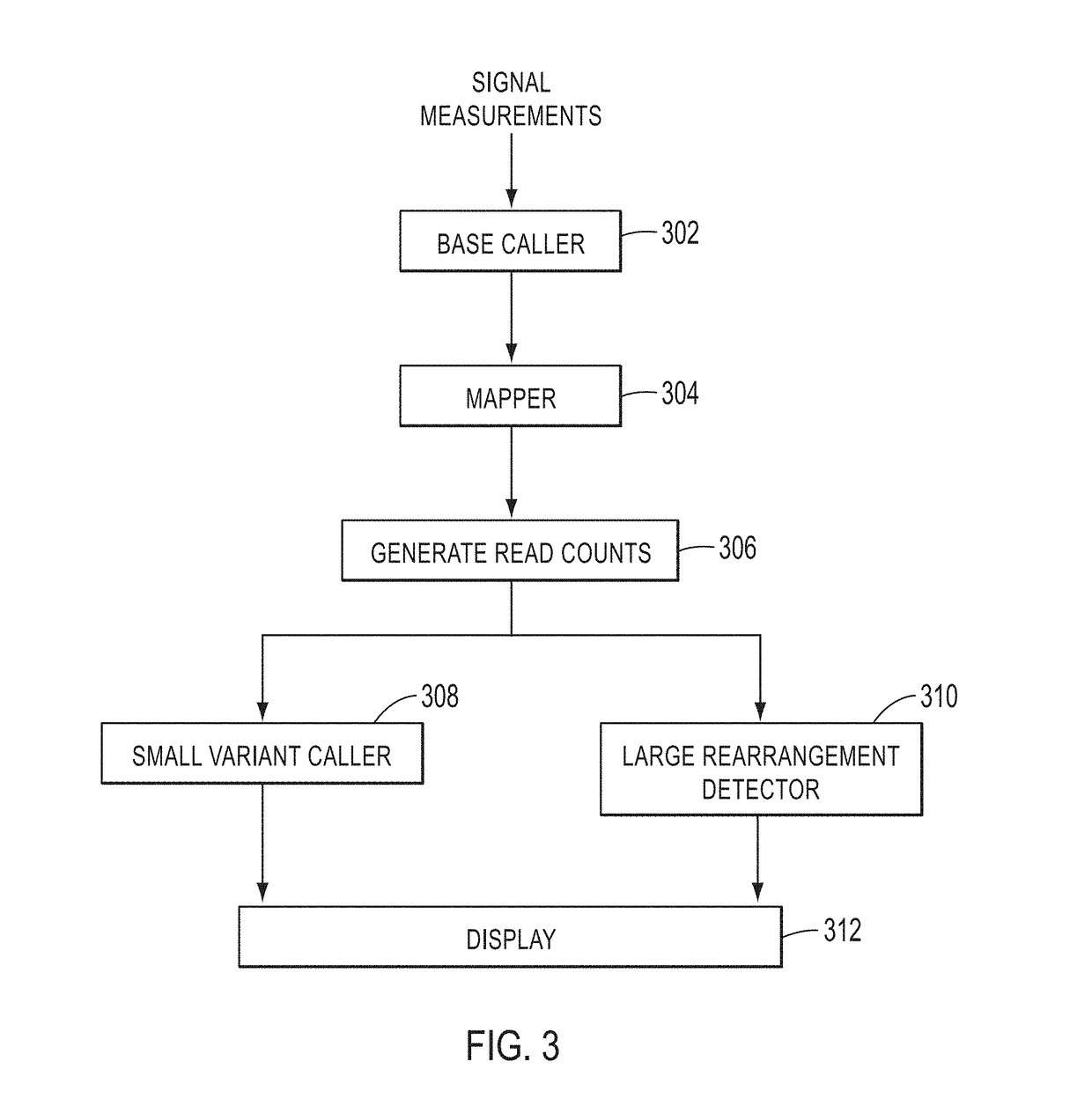
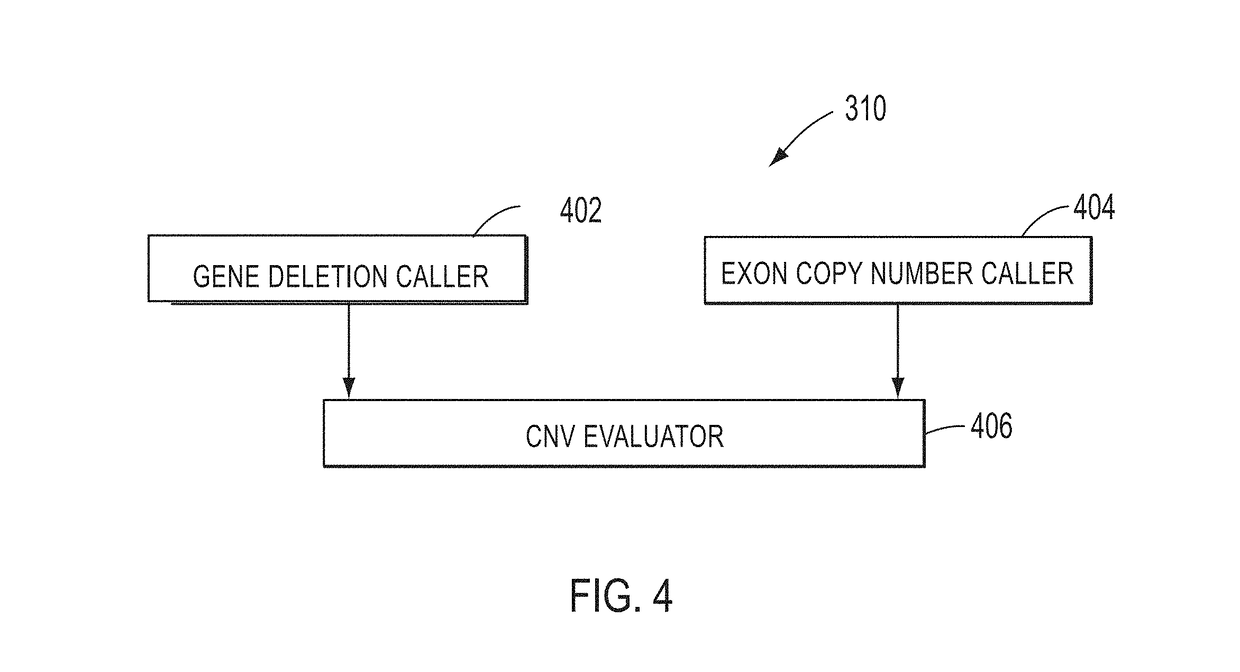
A method for detecting large rearrangements in BRCA1 and BRCA2 genes includes amplifying a nucleic acid sample in the presence of a primer pool to produce amplicons, where the primer pool includes target specific primers targeting regions of exons of the BRCA1 and BRCA2 genes. The method further includes sequencing the amplicons to generate a plurality of reads, mapping the reads to a reference sequence, determining a number of reads per amplicon for the amplicons associated with the exons of the BRCA and the BRCA2 genes, determining exon copy numbers for the exons of the BRCA1 and BRCA2 genes based on the number of reads per amplicon, detecting an exon deletion or duplication based on the exon copy numbers, and detecting a whole gene deletion of the BRCA1 or BRCA2 gene based on the number of reads per amplicon associated with the exons of the BRCA1 and BRCA2 genes.
Owner:LIFE TECH CORP
Triple fluorescent quantitative PCR detection primers and kit for identifying African swine fever wild strain and gene deletion strain
ActiveCN112646934ARapid identificationRapid epidemic monitoringMicrobiological testing/measurementDNA/RNA fragmentationClassical swine fever virus CSFVAfrican swine fever
The invention discloses triple fluorescent quantitative PCR (Polymerase Chain Reaction) detection primers and a kit for identifying an African swine fever virus wild strain and a gene deletion strain. The invention provides a group of specific primers and probes for detecting the African swine fever virus wild strain and the gene deletion strain, and the specific primers and probes are used for simultaneously detecting the B646L gene of the African swine fever virus wild strain and the EGFP and mCherry genes of the deleted strain through an RT-qPCR method, so that whether a sample is the African swine fever virus wild strain or the gene deletion strain can be identified. The detection method is high in sensitivity and strong in specificity, and the lowest detection concentrations of B646L, EGFP and mCherry gene positive standard plasmids are 5.94*10 <-9> ng / mu L, 9.67*10 <-9> ng / mu L and 5.85*10 <-9> ng / mu L respectively. The method is wide in detection range, can be used for detecting various African swine fever virus gene deletion strains, is rapid and efficient in detection process, is suitable for large-batch detection, provides a new means for identifying African swine fever virus wild strains and gene deletion strains, and can be used for rapidly identifying the African swine fever virus wild strains and gene deletion strains in production practice.
Owner:SOUTH CHINA AGRI UNIV
Infectious bovine rhinotracheitis virus recombinant strain, construction method and use thereof
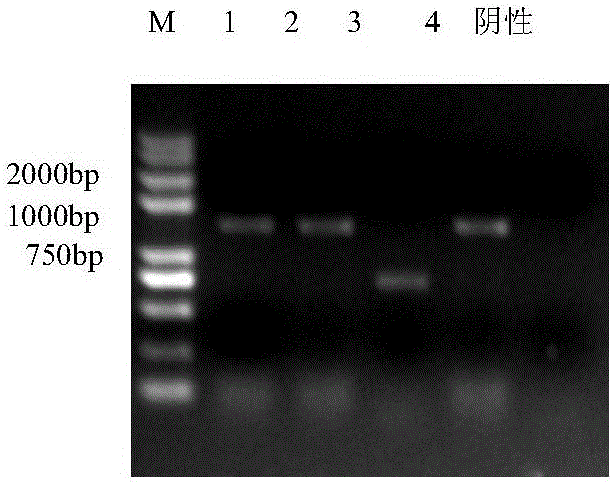
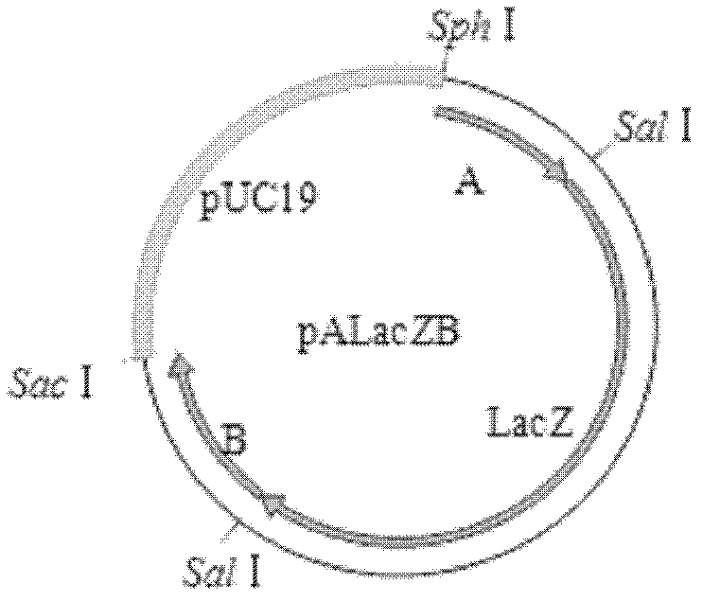
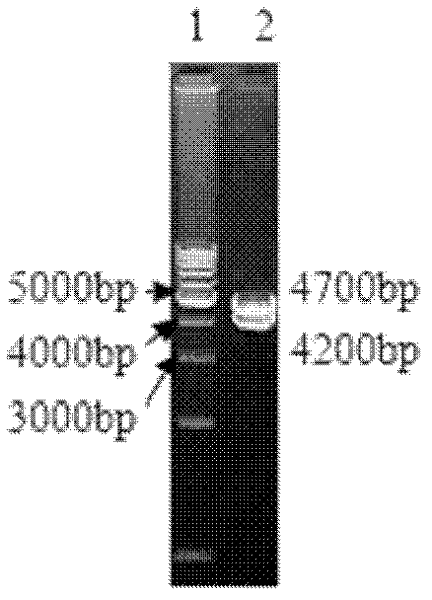
ActiveCN101353670AViruses/bacteriophagesAntibody medical ingredientsInfectious bovine rhinotracheitis virusTransfer vector
The invention discloses an IBRV recombinant transfer vector and a constructing method thereof, and also discloses a recombinant strain IBRV46 obtained by the recombinant transfer vector and an IBRV genome after being co-transfect. A gE gene in the recombinant transfer vector, including the 1134bp comprising an initiation codon ATG is deleted, the position where the gE gene is deleted is inserted with a LacZ expression cassette. The recombinant strain of the invention can be used for preparing a vaccine for preventing and curing IBRV and can also be used for preparing diagnostic reagent. According to the conventional molecular biological method or serological method, the strain-deleted infection of the gE gene is distinguished from the wild strain infection. Additionally, the strain can be further remodeled or the nonessential genes of the strain are deleted, therefore, a mutated strain is obtained, and the vaccine, etc., can be further prepared.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
A markerless gene deletion attenuated mutant strain of Vibrio alginolyticus wild strain, related preparations and applications
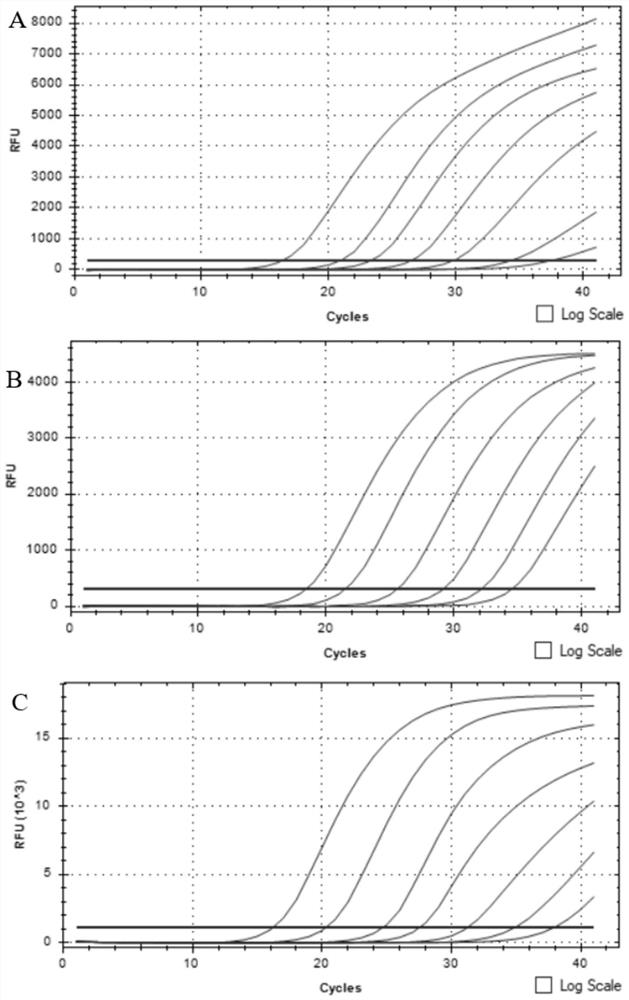
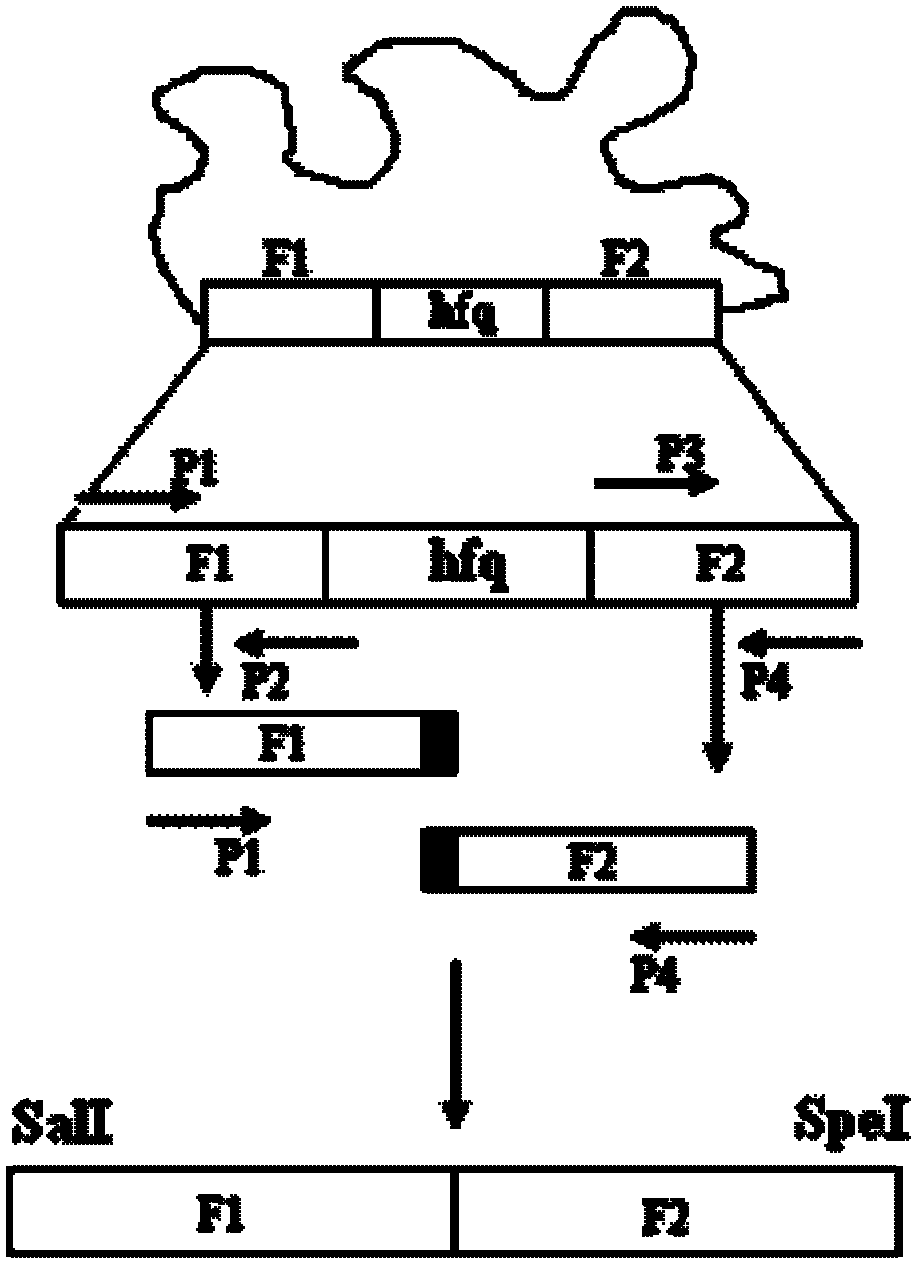
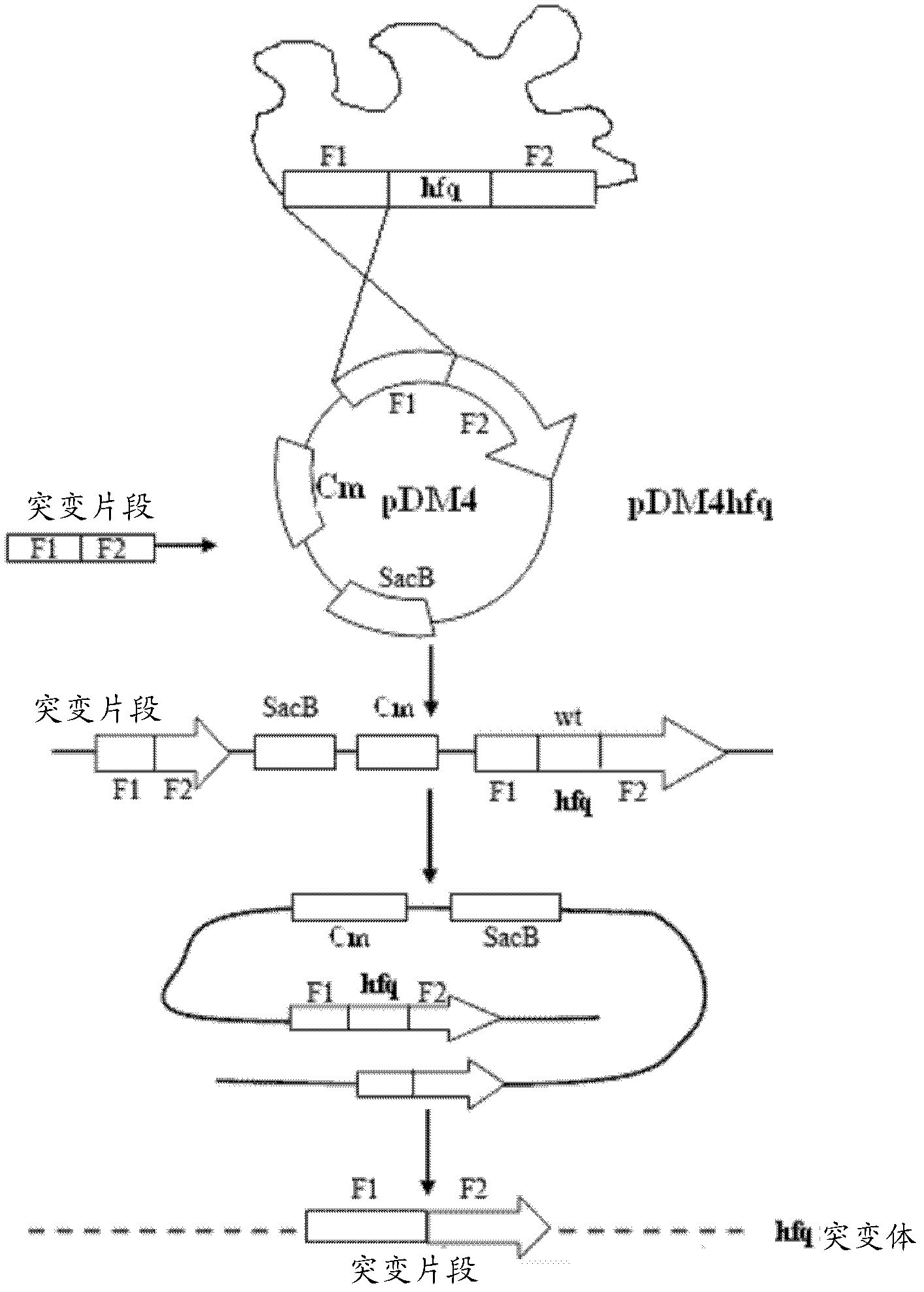
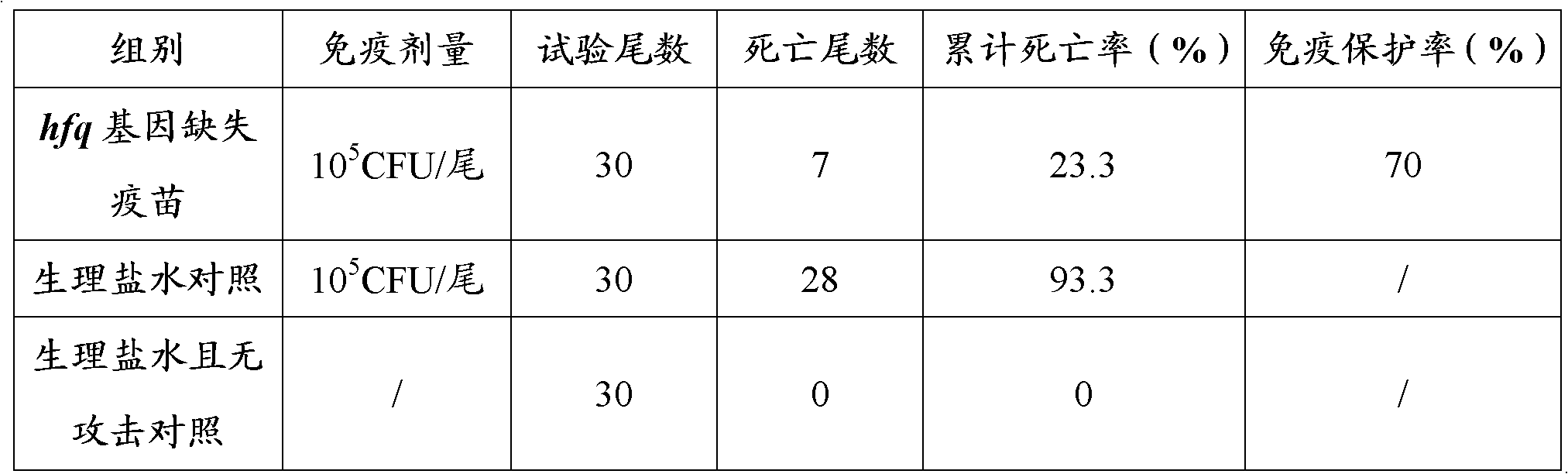
The invention relates to an unmarked gene-deleted and attenuated mutant strain of a vibrio alginolyticus wild strain, which is the attenuated active vaccine of the vibrio alginolyticus wild strain and in which the sRNA molecular chaperone gene hfq of the vibrio alginolyticus wild strain is deleted. The vibrio alginolyticus wild strain is EPGS020401, and the collection number of the vibrio alginolyticus wild strain is CCTCC NO.AB 209306. The invention also provides the preparation of the unmarked gene-deleted and attenuated mutant strain of the vibrio alginolyticus wild strain and the use thereof in prevention and control of vibrio alginolyticus disease in cultured fish. The unmarked gene-deleted and attenuated mutant strain of the vibrio alginolyticus wild strain or the preparation thereof, which are disclosed by the invention, eliminates universal potential environment and product safety risks of the conventional attenuated vaccine and is a safe, effective and economic vaccine for preventing and controlling vibrio alginolyticus diseases in cultured fish.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com