Gene defect and mutant ALK kinase in human entity tumour
A residue and amino acid technology, applied in the field of diagnosis and treatment, cancer detection, can solve problems such as side effects and troubles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0294] Identification of ALK kinase activity in solid tumors by global phosphopeptide profile
[0295] a. Distribution of human NSCLC cell lines
[0296] Kinase activation in 22 human NSCLC cell lines, including H2228, was examined using a recently described powerful technique for the isolation and mass spectrometric characterization of modified peptides from complex mixtures (the "IAP" technique, see Rush et al., supra). global phosphorylation profile. Using phosphotyrosine-specific antibodies ( Beverly, MA, 2003 / 04 Cat. #9411 ) implemented the IAP technique to isolate and subsequently characterize phosphotyrosine-containing peptides from extracts of NSCLC cell lines.
[0297] Specifically, the IAP approach was used to facilitate the identification of tyrosine kinases responsible for protein phosphorylation in each NSCLC cell line. In particular, atypical or abnormal kinase activity is considered.
[0298] cell culture
[0299] All cell culture reagents were purchas...
Embodiment 2
[0322] Isolation and sequencing of three ALK fusion genes
[0323] a. Sequencing in Human NSCLC Cell Lines
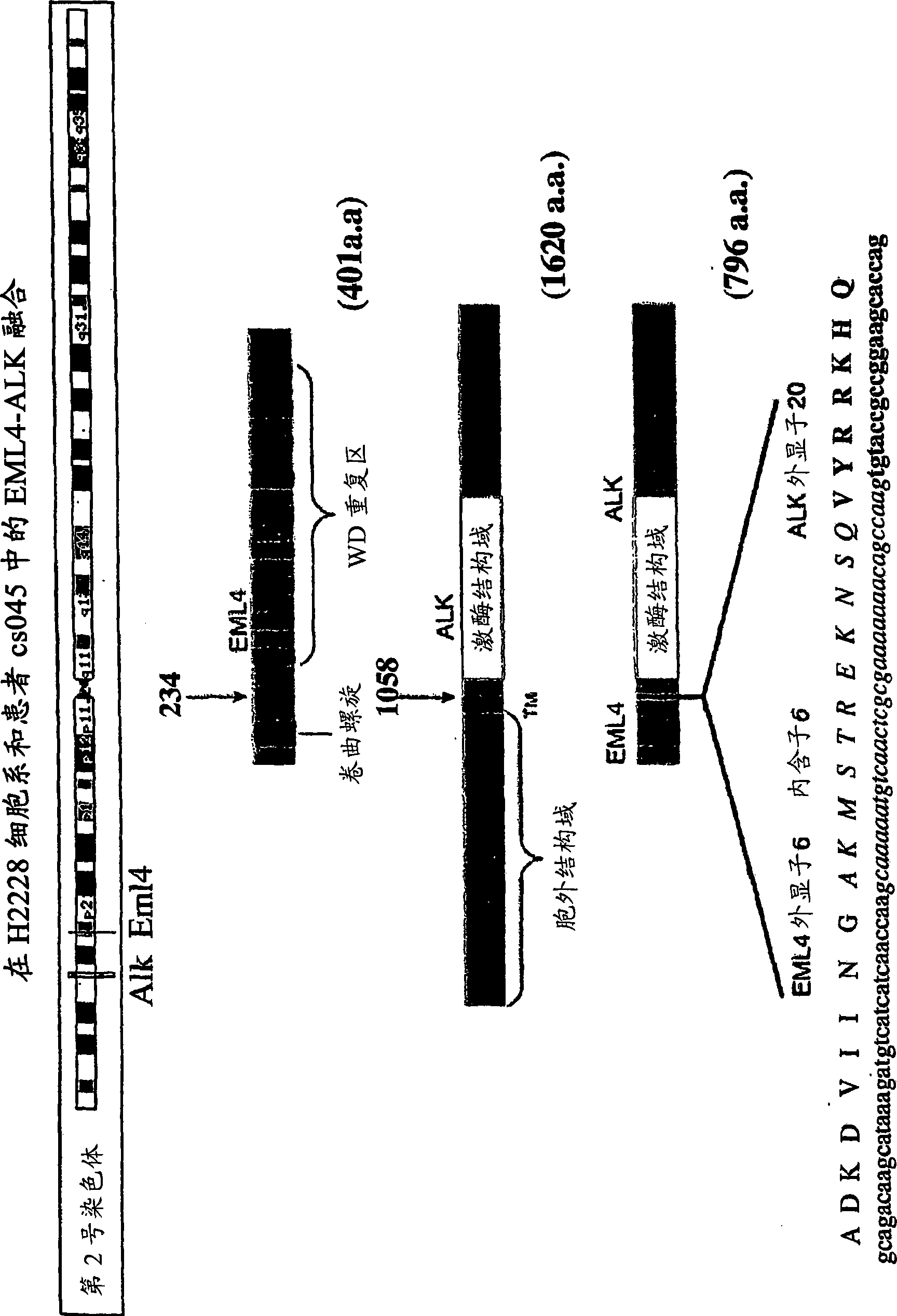
[0324] Given the high phosphorylation levels of ALK kinase detected in the NSCLC cell line H2228, rapid amplification of the 5' end of the cDNA at the sequence encoding the kinase domain of ALK was performed in order to determine the presence of chimeric ALK transcripts.
[0325] Rapid amplification of complementary DNA ends
[0326] RNeasy Mini Kit (Qiagen) was used to extract RNA from H2228 cell line. DNA was extracted by using DNeasy Tissue Kit (Qiagen). The cDNA ends were rapidly amplified with the aid of the 5' RACE system (Invitrogen), with primers ALK-GSP1 for cDNA synthesis and ALK-GSP2 and ALK-GSP3 for nested PCR reactions.
[0327] 5'RACE
[0328] Figure 5 (Panel A) shows the detection of the EML4-ALK fusion gene (short variant) by 5' RACE and the detection of the PCR amplification product after 2 rounds. PCR products were purified with a PCR purifi...
Embodiment 3
[0352] Inhibition of Growth of ALK Fusion-Expressing Mammalian Solid Tumors Using siRNA
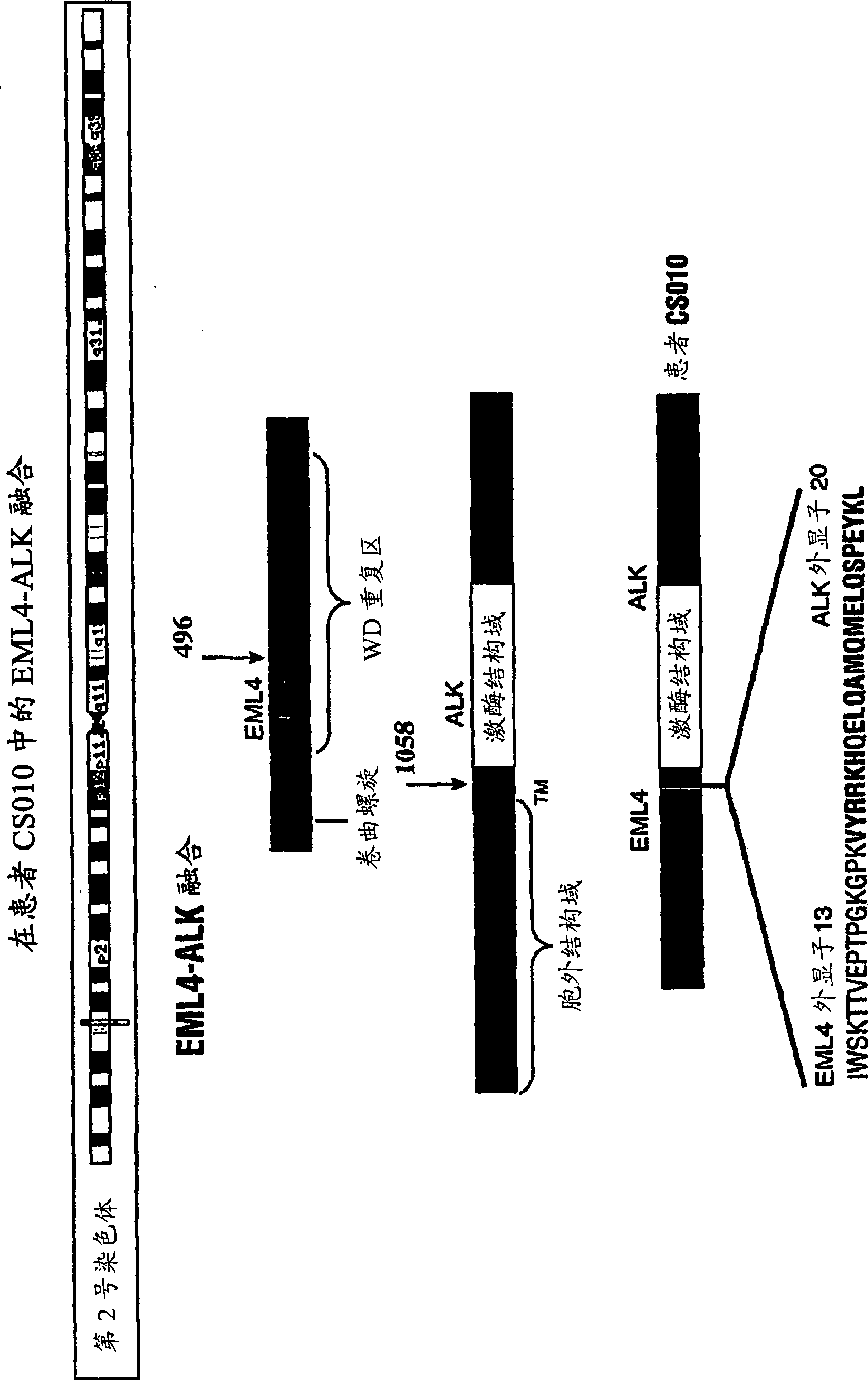
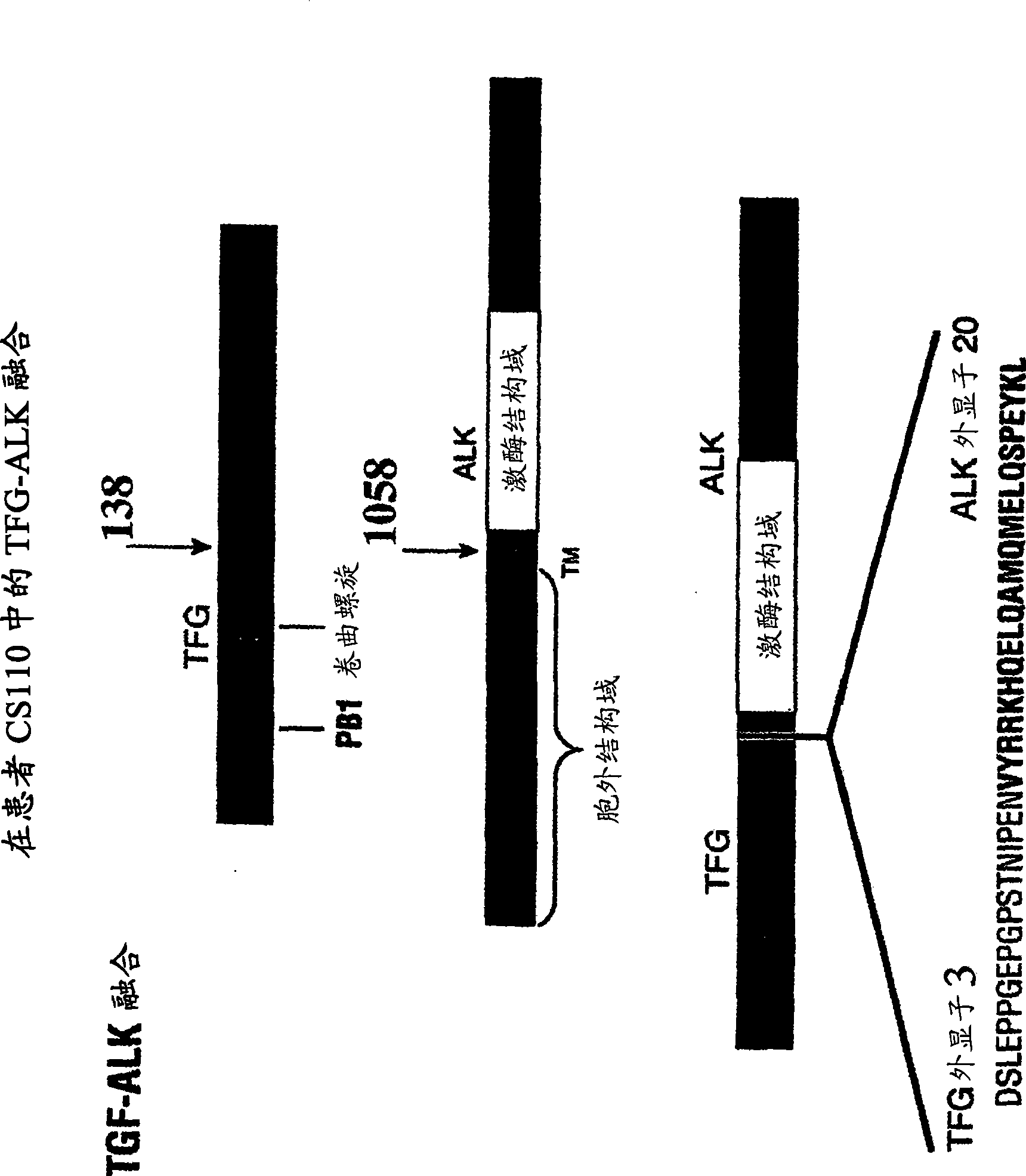
[0353] To confirm that truncated / fusion forms of ALK are driving cell growth and survival in the NSCLC cell line H2228 as well as in NSCLC tumor samples from patients CS010 / 11, CS045, and CS110, siRNA (relative to ALK) inhibition of these cells can be examined and tumor growth capacity.
[0354] ALK SMARTpool siRNA duplexes (proprietary target sequence - data not shown) can be purchased from, eg, Dharmacon Research, Inc. (Lafayette, CO). Non-specific SMARTpool siRNA was used as a control. Cells were transfected with siRNA by means of electroporation. Briefly, 2 × 10 7 Each cell (H2228) was pulsed once (20ms; 275V, K562 20ms; 285V), incubated at room temperature for 30 minutes, and then transferred to a T150 flask containing 30ml RPMI-1640 / 10% FBS.
[0355] With CellTiter 96 AQ ueous One Solution Cell Proliferation Assay (Promega) to determine the number of viable cells. IC was calc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com