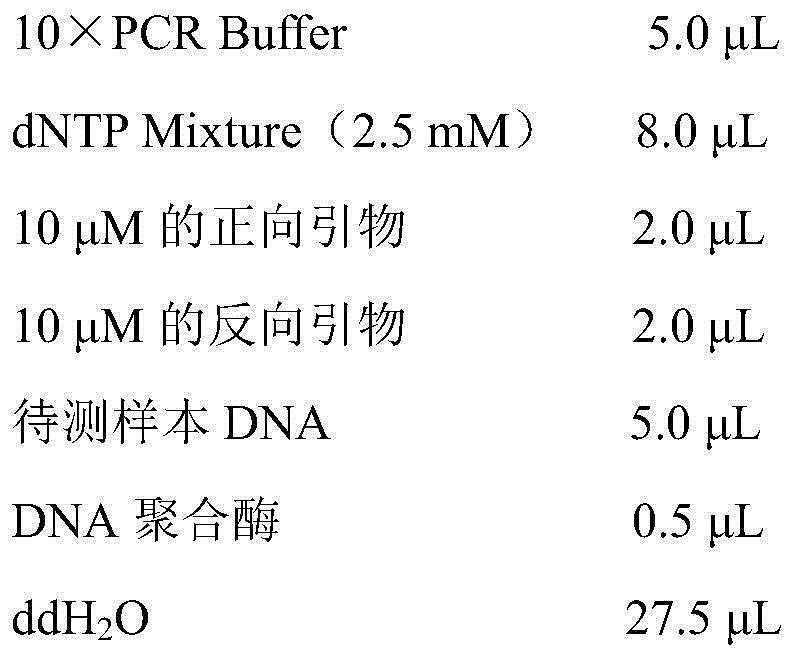
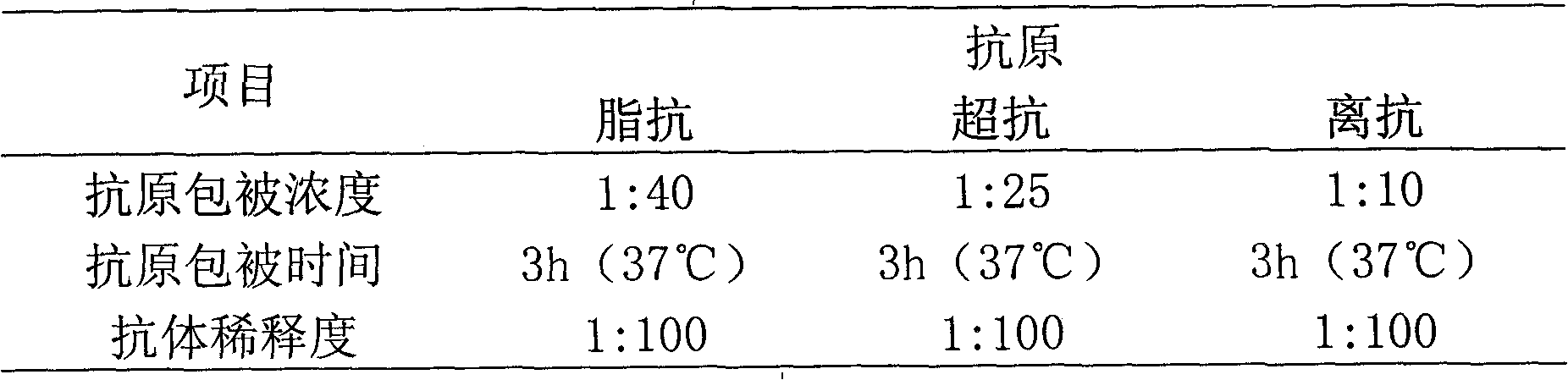
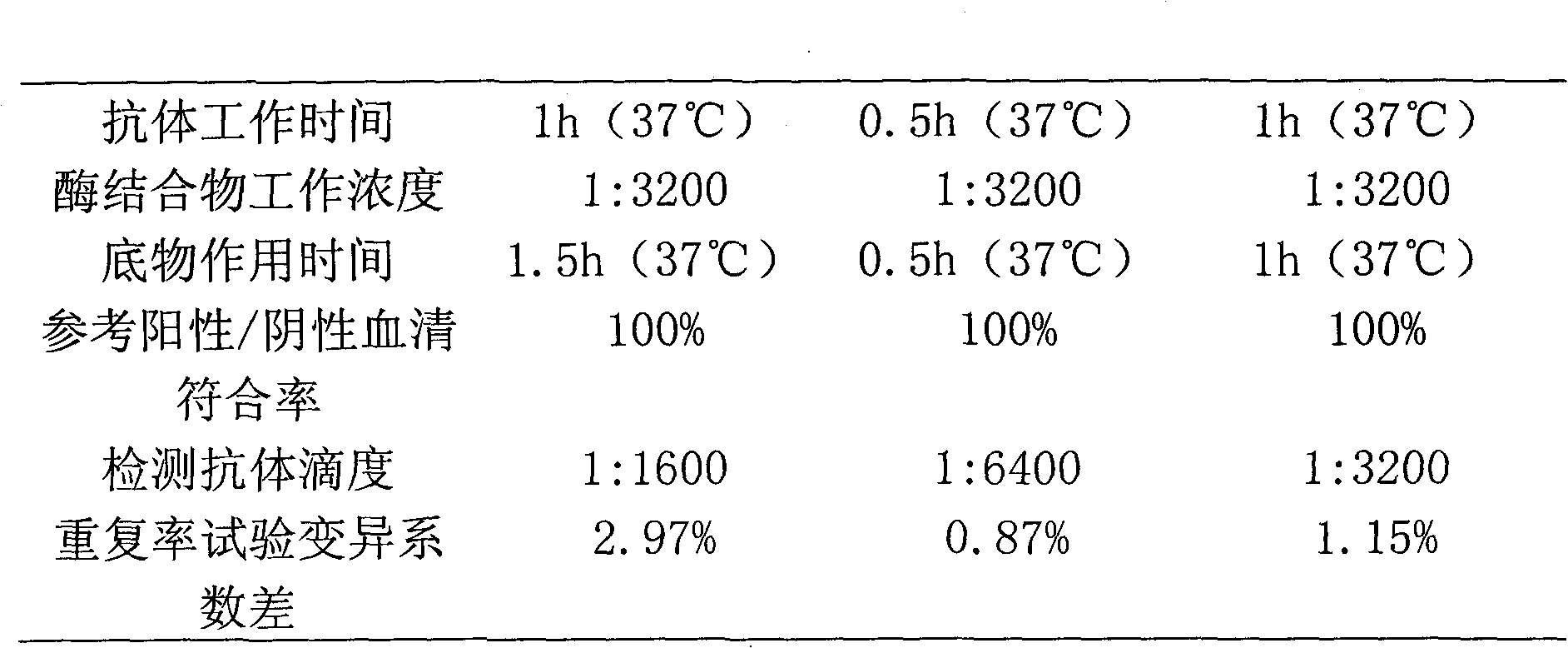
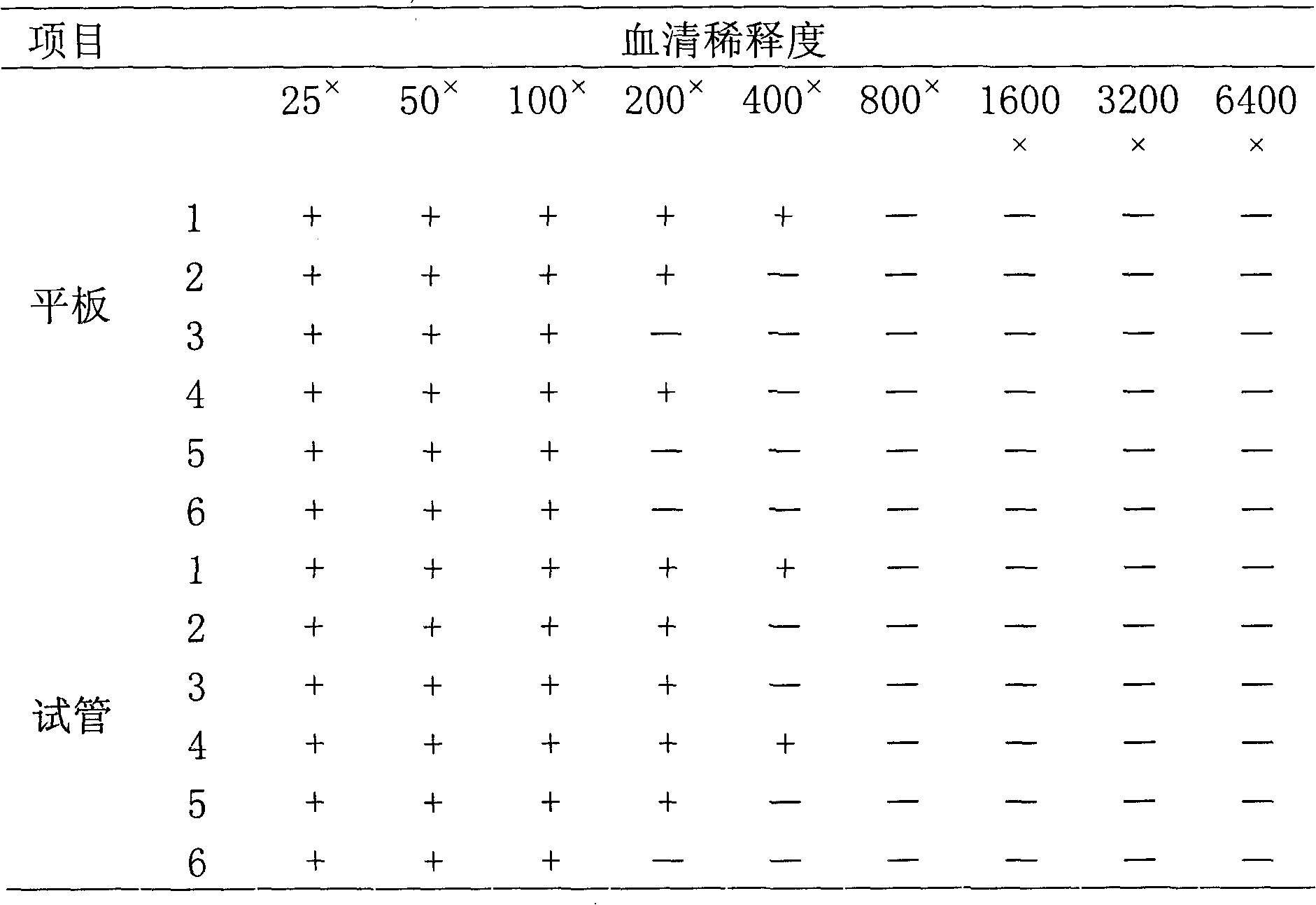
Patents
Literature
76 results about "Brucella Vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A bacterial vaccine for the prevention of brucellosis in man and animal. Brucella abortus vaccine is used for the immunization of cattle, sheep, and goats.
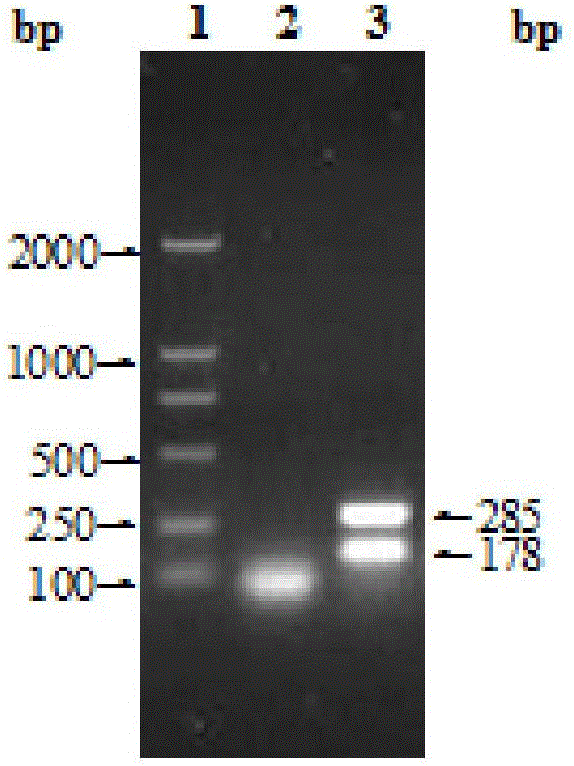
Kit for recognizing Brucella wild strain and vaccine strains A19 and S2
ActiveCN105018489AEasy to distinguishStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBacteroidesBrucella Vaccine
The invention relates to a kit for recognizing a Brucella wild strain and vaccine strains A19 and S2. The kit comprises a special primer pair SEQ ID No.1 and SEQ ID No.2 for recognizing the Brucella vaccine strain A19 and a special primer pair SEQ ID No.3 and SEQ ID No.4 for recognizing the Brucella vaccine strain S2. The kit is used for conducting further sequencing analysis on a PCR amplification product after the Brucella and other conventional bacterial strains are distributed on a PCR amplification-electrophoresis detection area, and the Brucella A19 and S2 vaccine strains in a clinic sample can be rapidly and effectively recognized and diagnosed according to the sequencing result.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Low virulent strain of Brucella and vaccine thereof
ActiveCN103981139AImprove immunityImprove securityAntibacterial agentsBacterial antigen ingredientsSerum igeImmune effects
The invention relates to a low virulent strain of Brucella and a vaccine thereof. According to a vaccine strain, a coarse low virulent strain RA343 of Brucella abortus is screened through a domestication and selection technology combining antibiotics and A-factor serum. The coarse low virulent strain is remarkably improved in safety and still remains a good immune effect on the Brucella is still maintained. A Brucella vaccine prepared by using the low virulent strain is to change the current situation that Brucella vaccine-immunized animals and wild strain-infected animals are hard to differentiate, and the safety of existing vaccine is effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Kit for identifying Brucella S2 vaccine strain and wild strain
InactiveCN105002173AEasy to distinguishStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBacteroidesSequence analysis
The invention relates to a kit for identifying a Brucella S2 vaccine strain and a wild strain. The kit comprises a specific primer pair SEQ ID No.1 and SEQ ID No.2 for identifying the Brucella vaccine strain S2. Sequencing analysis is further performed on a PCR amplification product after the kit is used for distributing Brucella and other conventional bacterial strains through a PCR amplification-electrophoresis detection area, and the Brucella S2 vaccine strain in a clinic sample can be rapidly and effectively identified and diagnosed according to the sequencing result.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Indirect enzyme linked immunosorbent assay (ELISA) of deer's brucellosis
InactiveCN102621304AStrong specificityIncreased sensitivityMaterial analysisSerum igeBrucella Vaccine
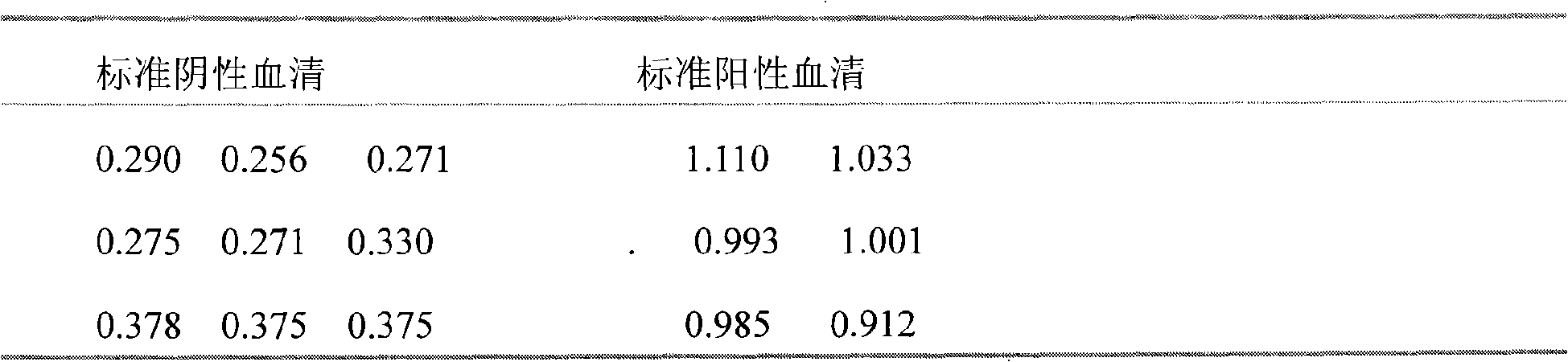
Indirect ELISA of deer's brucellosis belongs to the veterinary field and is used for diagnosing deer's brucellosis to improve the diagnosis level of the prior art. Biomaterials used in the assay of the invention comprise pig brucella vaccine 2 (S2) as an antigen, an enzyme labeled antibody rabbit anti-deer IgG-HRP, a standard positive serum, a standard negative serum and a serum to be assayed; and the assay comprises the following steps: coating the antigen, washing, adding the serum, adding the rabbit anti-deer IgG-HRP, adding a substrate liquid, terminating a reaction, testing by an enzyme-linked detector, adjusting to zero at 490nm, testing the OD value of each hole, and determining the result is positive when the OD value is equal to or greater than 0.32. According to the invention, the S2 is used as the antigen, the rabbit anti-deer IgG-HRP is used as the enzyme labeled antibody, and the indirect ELISA is used to diagnose the deer's brucellosis, so compared with previous diagnosis methods, the indirect ELISA of the invention has the advantages of good specificity, sensitivity and repeatability, and accurate diagnosis.
Owner:JILIN AGRICULTURAL UNIV
Dual-gene deletion rough type bovine brucellosis and production method for vaccine thereof
InactiveCN104031874AAntibacterial agentsBacterial antigen ingredientsResistant genesBrucella Vaccine
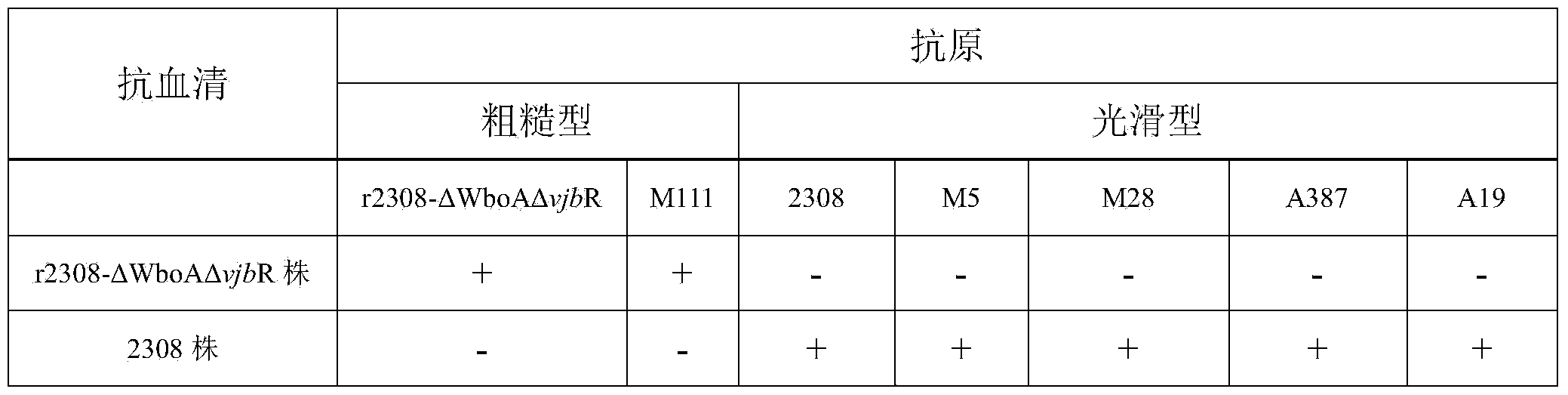
The invention relates to dual-gene deletion rough type bovine brucellosis and a production method for a vaccine thereof. A recombinant bacterial strain deletes a WboA gene and a vjbR gene of a bovine brucellosis 2308 strain by virtue of a non-resistance gene screening technology. Due to deletion of two genes, on the one hand, a condition (cased by WboA gene deletion) for forming an O chain in a smooth type bovine brucellosis cell wall LPS (lipopolysaccharide) structure is lost, and colonial morphology is changed into a rough type from a smooth type; and on the other hand, due to deletion of vjbR gene, toxicity of recombinant bacteria and viability in a cell are descended, so that infection ability of bovine brucellosis on a target animal is lowered, and therefore, safety of the vaccine is further improved. By using the bacterial strain to produce the vaccine, a current situation that a bovine brucellosis vaccine immune animal and a wild strain-infected animal are difficult to distinguish is changed, and safety of the existing vaccine is effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Brucella molecule marking and virulence deletion attenuated vaccine and preparation method
InactiveCN101185756AWide application of practical valueAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsVaccine Immunogenicity
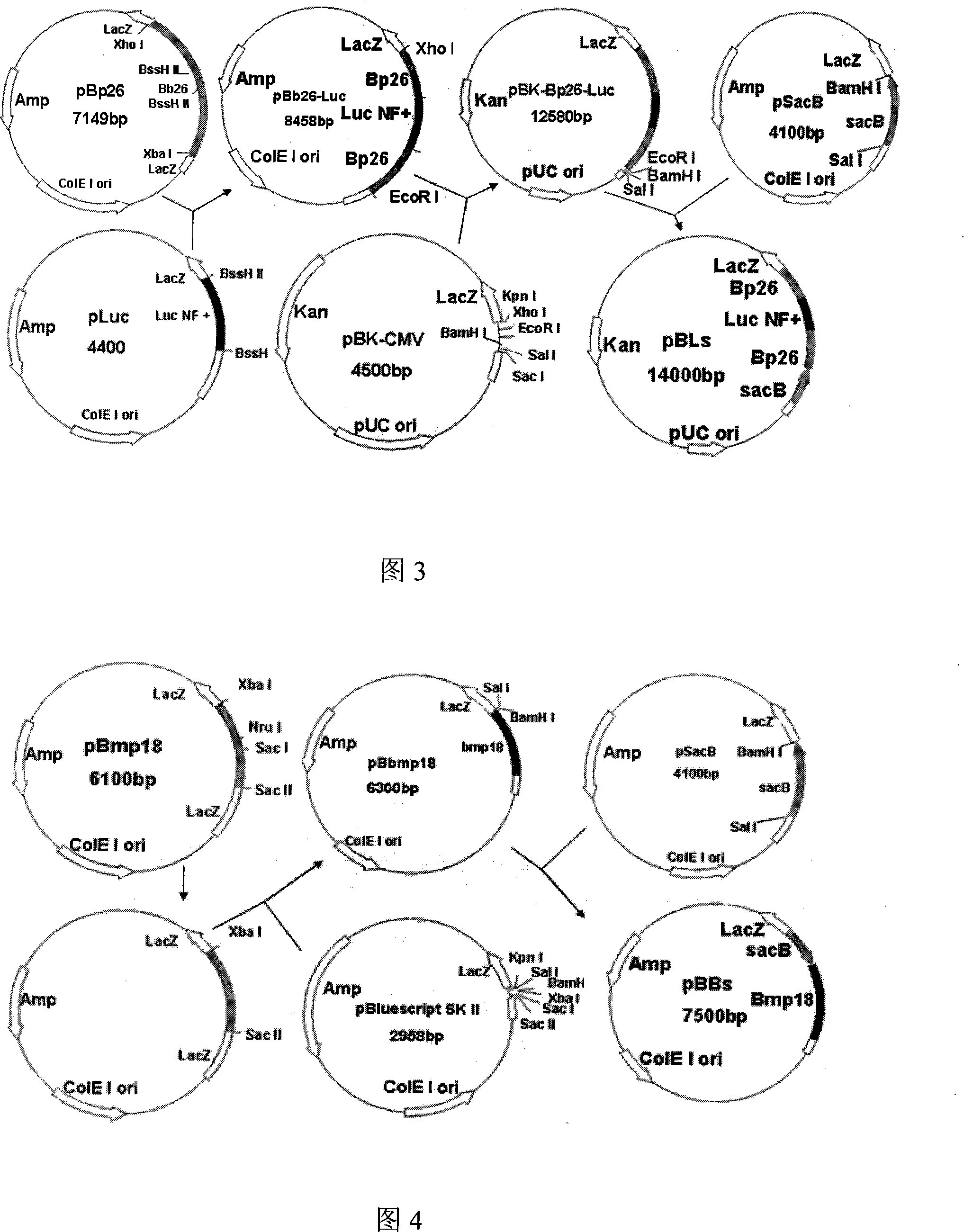
The invention relates to a Brucella vaccine, in particular to the molecular marker and virulence gene deletion of Brucella vaccine strain. The study uses luciferase modified gene (Luc NF plus) to replace the partial fragment of Bp26 gene of Brucella attenuated vaccine S19 strain by constructing suicide plasmid and adopting the method of targeted homologous recombination (gene targeting), so as to damage the expression of the immunogenicity protein BP26 and construct the gene deletion mutant strain Delta S19-1 of the Brucella Bp26. The BMP18 protein is one of the main virulence factors of Brucella. The invention adopts the same method to exclude the Bmp 18 gene of Delta S19-1, so as to lead the Delta S19-1 not to express the Bmp 18 protein and the Brucella virulence gene deletion mutant strain Delta S19-2 is constructed. The invention solves the problems that the conventional Brucella vaccine can not distinguish between the artificial immunization and the wild bacteria infection of people and animals, the virus is strong and the vaccine is easy to cause the illness of inoculated people and animals. The invention has important significance and practical application value of the monitoring, diagnosis, purification and all the controls of Brucella.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Recombinant brucella expressing VP1 gene of O-type foot-and-mouth disease virus and method for producing vaccines thereof
ActiveCN101979502AImprove securityStable genetic traitsAntibacterial agentsBacterial antigen ingredientsImmune effectsBrucella Vaccine
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Recombinant brucella for expressing Asia type-I foot and mouth disease virus VP1 genes and method for producing vaccines thereof
The invention relates to recombinant brucella for expressing Asia type-I foot and mouth disease virus VP1 genes and a method for producing vaccines thereof. Asia type-I foot and mouth disease virus (FMDV) Jiangsu strain VP1 genes are integrated into a brucella S2 strain genome, and the forming condition of an O chain in a smooth brucella cell wall LPS structure is simultaneously damaged so that the recombinant strain is changed into a rough type from a smooth type, the safety of the strain is further improved, but good immune effect on the brucella is still kept; and the recombinant strain is named as a recombinant brucella rS2-JS strain. The strain can express the Asia I FMDV VP1 protein, induces the generation of corresponding antibodies and has good basic immune effect on the Asia I FMD. The strain for producing the vaccines can change the current situation that brucella vaccine immunized animals and wild strain infected animals are difficult to distinguish, realizes cellular immunity of FMD vaccines at the same time, and provides a good vaccine for prevention and control of brucella diseases and FMD.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Rough type Brucella melitensis attenuated strain and vaccine thereof
ActiveCN104845916AImprove securityImprove immunityAntibacterial agentsBacterial antigen ingredientsImmune effectsBrucella Vaccine
The invention relates to a rough type Brucella melitensis attenuated strain and a vaccine thereof. The rough type Brucella melitensis attenuated strain RM57 is screened by using an antibiotics and M factor serum combined domesticating and screening technology. The safety of the rough type attenuated strain is remarkably improved, but the favorable immune effect for Brucella melitensis is stilled retained. If the attenuated strain is prepared into a Brucella melitensis vaccine, the situation that a Brucella melitensis vaccine immunized animal and a wild strain infected animal are difficult to distinguish is changed, and the safety of the existing vaccine is effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Brucella live vaccine and production method thereof
The invention relates to brucella and a production method of a brucella vaccine. In the vaccine, a recombinant brucella rS2-deltaWboA strain is taken as a production strain. A smooth recombinant strain is transformed into a rough recombinant strain by deleting base sequences among WboA genes 1-897bp of a brucella S2 strain and eliminating the forming condition of an O chain in a smooth brucella cell wall LPS (Lipopolysaccharide) structure with a non-resistant gene screening technology. The safety of the rough recombinant strain is improved remarkably, and a good immune effect on brucelliasis is still kept. Due to the adoption of the strain to the production of the vaccine, the current situation that a brucella vaccine immune animal cannot be distinguished from a wild strain infected animal easily is changed, and the safety of the conventional vaccine is effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Method of producing brucella vaccine antigen protein
InactiveCN101016541AAvoid poisonAvoid security issuesAntibacterial agentsBacterial antigen ingredientsEscherichia coliAntigen
The invention discloses a method to prepare antigen protein of abortion brucellosis subunit vaccine and usage, which comprises the following steps: abstracting bacteria total DNA from abortion brucellosis; using the total DNA as mold; adopting property primer; proceeding polyose chain reaction; cloning; getting OMP25 protein gene; transferring into pronucleus expressing carrier; getting restructuring express carrier; leading-in bacillus coli; getting reconstructing large intestine Escherichia; proceeding inducing expression; culturing reconstructing large intestine Escherichia; preparing antigen protein. This protein can be used to prepare abortion brucellosis subunit vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
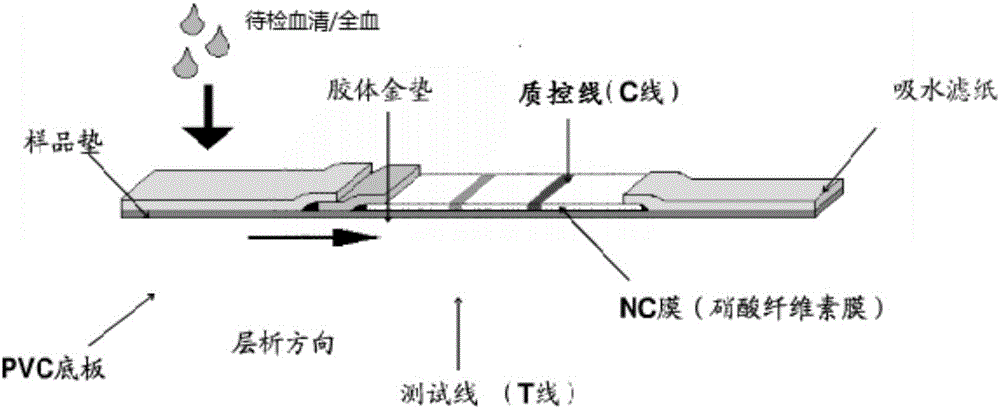
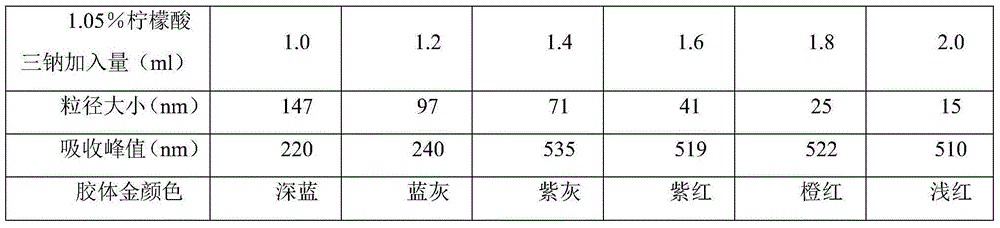
Colloidal gold test paper bar for antibody detection of sheep Brucella
The invention relates to a colloidal gold test paper bar for antibody detection of sheep Brucella. In the invention, lipopolysaccharide (LPS) extracted from a Brucella vaccine strain (S2) is taken as an envelope antigen of the colloidal gold test paper bar and improves the sensitivity of detection. Monoclonal-antibody-labeled colloidal gold of a mouse anti-sheep IgGFc terminal is prepared into a gold colloid pad of the colloidal gold test paper bar for antibody detection, and nonspecific adsorption of antibody protein by SPA in the tradition is changed. The monoclonal-antibody-labeled colloidal gold is taken as a colloidal gold labeled protein, so that specificity of detection is further improved. A set of serum for controlling quality of the test paper bar is prepared, and specificity and sensitivity of the test paper bar is ensured. The test paper bar can be used for on-site sampling, is convenient and rapid to use, and is quite suitable for application to a basic farm or a basic veterinarian.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Gene associated with toxicity of Brucella and application of gene in evaluation of toxicity of Brucella and preparation of low-toxicity Brucella
ActiveCN109652429ALow toxicityGrowth impactBacteriaMicrobiological testing/measurementKanamycinVirulent characteristics
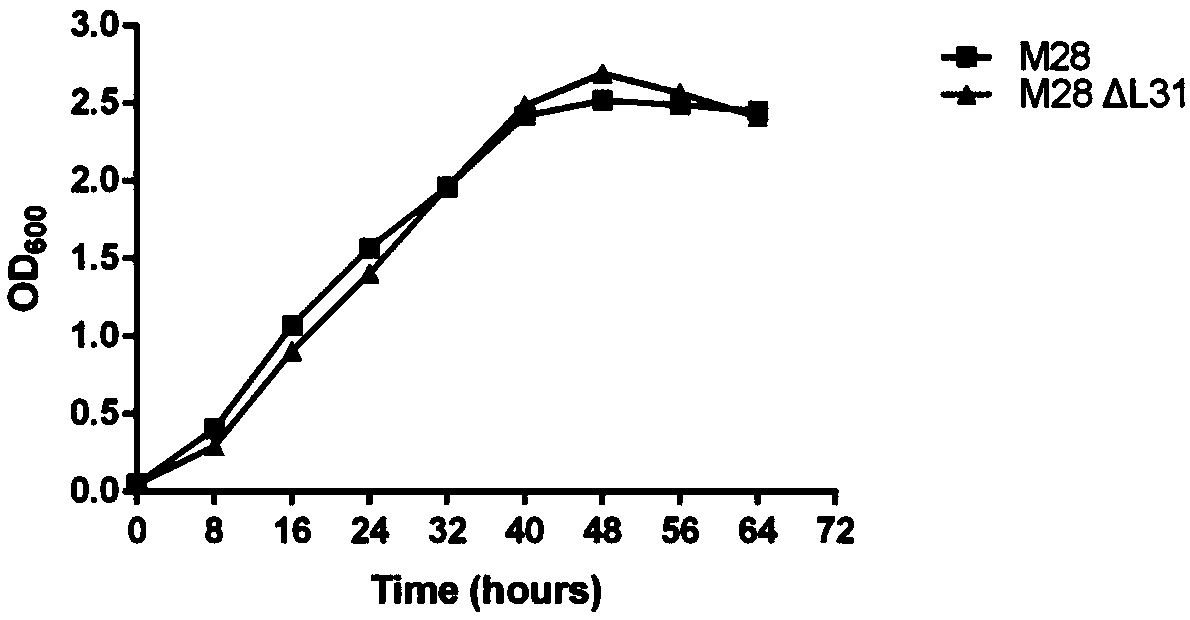
The invention discloses a gene associated with the toxicity of Brucella and application of the gene in evaluation of the toxicity of the Brucella and preparation of low-toxicity Brucella. By using a homologous recombination method, a ribosome gene L31 of the Brucella M28 is replaced by a kanamycin gene, and a Brucella L31 gene deletion strain M28deltaL31 is obtained. By using macrophages RAW264.7and a Babl / c mouse model, the influence of the Brucella L31 gene on the toxicity of the Brucella M28 is evaluated. Through results, it is shown that after the L31 gene is deleted, the growth of the M28 cannot be affected, but the duplication survival capacity of the high-toxicity strain M28 in the mouse macrophage system RAW264.7 and the mouse spleen can be obviously reduced, after the L31 gene issupplemented in the M28deltaL31, the toxicity can recover to the level of a wild strain, and it is proved that the gene is associated with the toxicity of the Brucella M28. The gene for predicting the toxicity can be used for research and development of new brucella vaccines, and the gene has a potential application value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Nucleic acid detection test strip for distinguishing Brucella melitensis vaccine strain S19 from natural infection strain
PendingCN110734993AInfection Effectively DistinguishesImprove featuresMicrobiological testing/measurementMicroorganism based processesForward primerNucleic acid detection
The invention relates to a nucleic acid detection test strip for distinguishing a Brucella melitensis vaccine strain S19 from a natural infection strain, relates to the field of Brucellosis detection,and solves the problems that a conventional pathogeny detection method cannot detect the Brucella melitensis vaccine strain S19 and the natural infection strain at the same time. The detection test strip comprises a Brucella melitensis forward primer of which the sequence is shown as SEQID NO:1, a Brucella melitensis reverse primer of which the sequence is shown as SEQID NO:2, and a Brucella melitensis probe of which the sequence is shown as SEQID NO:3. The sequence of the forward primer of the Brucella melitensis vaccine strain S19 is shown as SEQID NO:4, the sequence of the reverse primer of the Brucella melitensis vaccine strain S19 is shown as SEQID NO:5, and the sequence of the probe of the Brucella melitensis vaccine strain is shown as SEQID NO:6. The test strip can specially detectthe disferences between Brucella melitensis natural infection and artificial immunity, and has high specificity and sensibility.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Monogene deletion rough-type Brucella and production method of Brucella vaccine
ActiveCN104004697AStable genetic traitsNot low securityAntibacterial agentsBacterial antigen ingredientsImmune effectsBrucella Vaccine
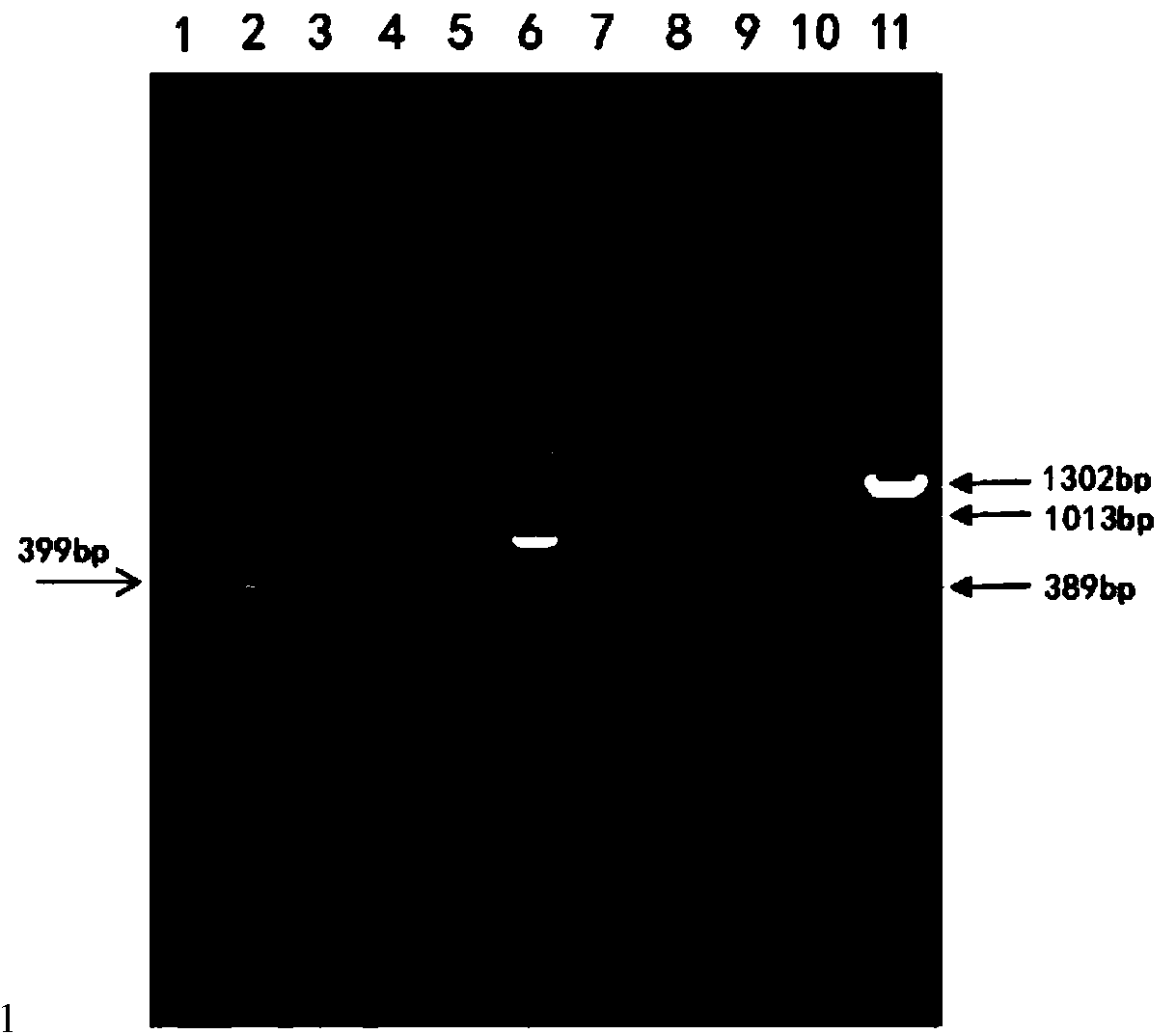
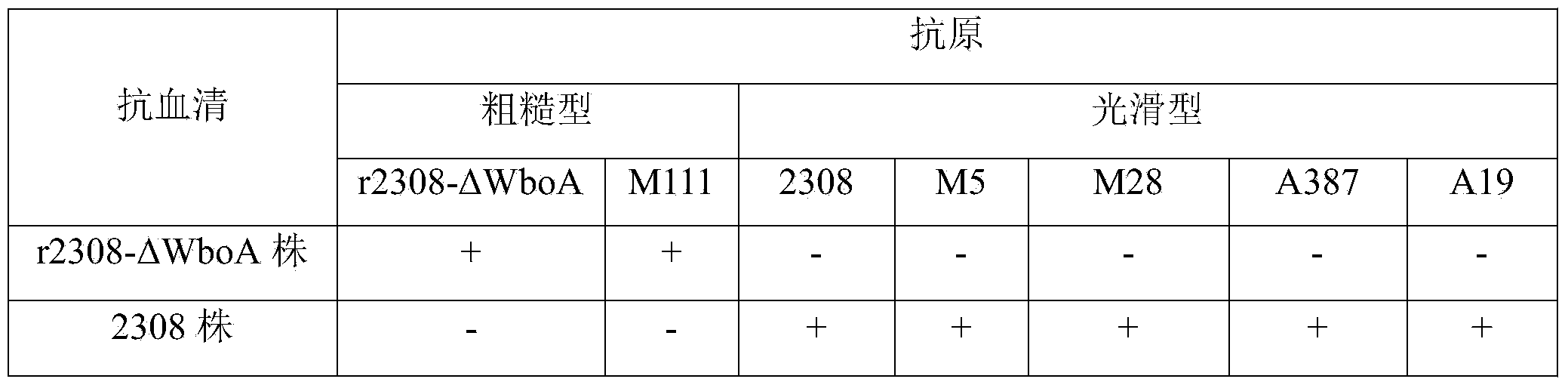
The invention relates to a monogene deletion rough-type Brucella and a production method of a Brucella vaccine. According to the recombinant strain, base sequences between 1-897bp of the WboA gene of a Brucella 2308 strain are deleted by virtue of a non-resistance gene screening technology so as to lose the conditions for the formation of O-chain in a smooth-type Brucella cell wall LPS (Lipopolysaccharide) structure, and thus the smooth-type Brucella is transformed into a rough -type Brucella. The safety of the rough-type recombinant strain is significantly improved, but a good immune effect on brucellosis is still maintained, the recombinant bovine Brucella is named Brucella r2308-delta WboA strain (CGMCC No.8885). The current situation that Brucella vaccine immune animals are hard to distinguish from wild-strain-infected animals is changed by a vaccine produced by the strain, and the safeties of existing vaccines are effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Brucella vaccine and method for preparing antigen protein used for same
InactiveCN101607081AWith large-scale industrial productionAvoid poisonAntibacterial agentsBacterial antigen ingredientsAntigenBacteroides
The invention discloses a brucella vaccine applied to livestock and a method for preparing antigen protein used for same. The antigen protein used for the brucella vaccine is brucella bcsap31 protein, and in particular brucella bcsp31 restructured protein. The method for preparing the restructured protein has steps of: taking bacterium total DNA extracted from the brucella as a template, designing a pair of primers according to ribotide complete sequence of brucella bcsp31 gene to carry out polymerase chain reaction, obtaining bcsp31 protein gene by clone and then transferring the bcsp31 protein gene to a yeast expression vector to obtain a restructured expression vector, importing the obtained restructured expression vector to yeast expression bacterium to obtain restructured pichia pastoris expression bacterium, and carrying out proliferation and induced expression of the restructured pichia pastoris expression bacterium so as to obtain the required antigen protein by separation and purification.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Abortus Brucella vaccine recombinant strain and application thereof in preparing vaccine
InactiveCN101575587APromote eradicationImprove purification effectAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsBiology
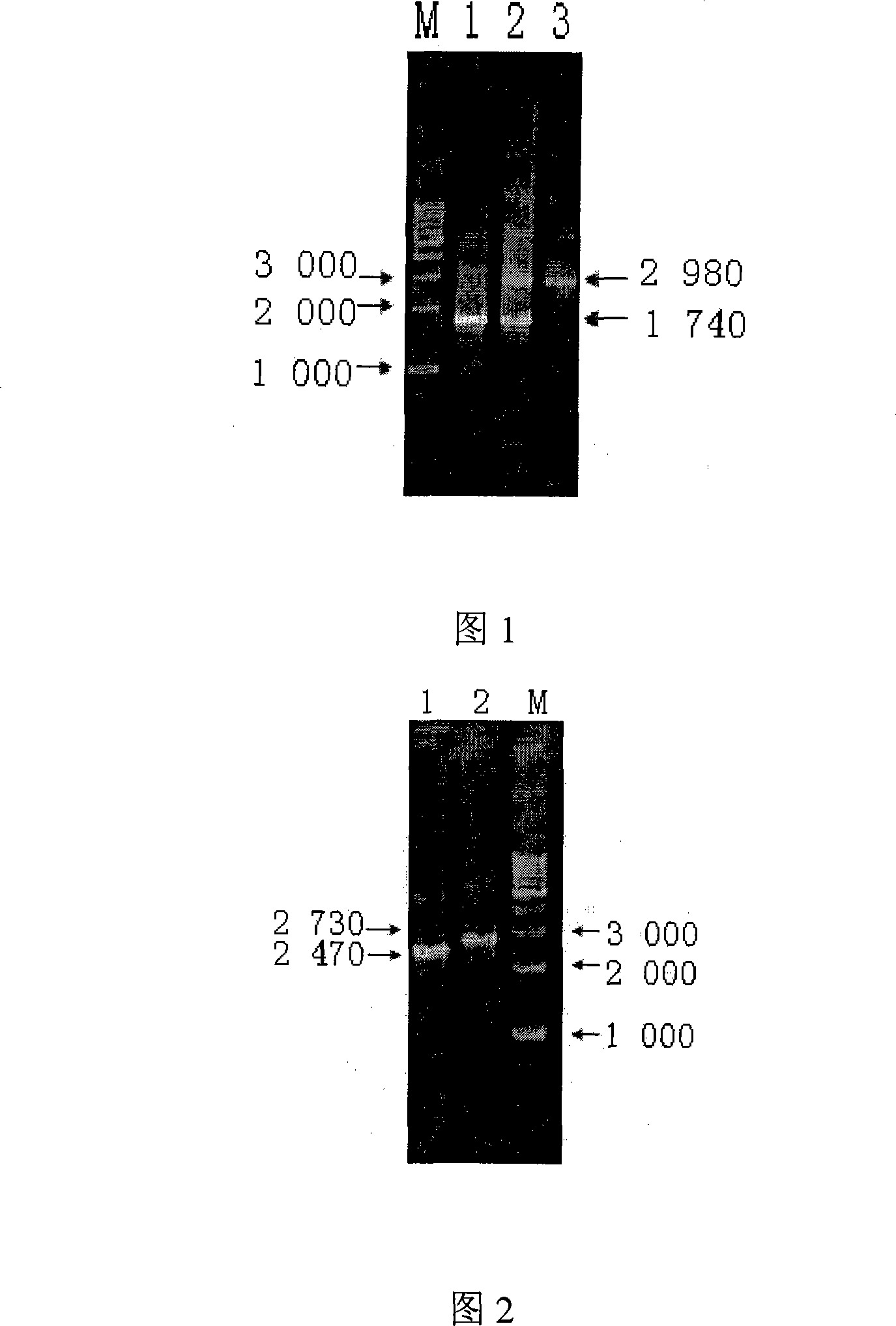
The invention discloses an abortus Brucella vaccine recombinant strain and an application thereof in preparing vaccine. The recombinant strain is a strain obtained by deactivating capsular polysaccharide synthesis protein gene and carboxyl uridine phosphate decarboxylase gene in the abortus Brucella strain S19. The obtained recombinant strain has small virulence and can be used as vaccines to immunize animals without needs of deactivation. The inoculated animal generates no lipopolysaccharide antibody and the virulent virus strain-infected animal can be distinguished from novel vaccine strain immunized animal by serological reaction. The recombinant strain is beneficial to improving the Brucella vaccine, researching diagnosis and identification reagents and developing the Brucella vaccine and matched diagnosis and identification reagents, and has extremely important significance in deracinating and purifying the global animal Brucella.
Owner:CHINA AGRI UNIV
Method for identifying brucella S2 vaccine strain by dual quantitative real-time PCR (polymerase chain reaction) and complete set of reagents used for method
ActiveCN111876502APrevent large outbreaksGuarantee normal developmentMicrobiological testing/measurementMicroorganism based processesNucleotideBrucellosis
The invention discloses a method for identifying a brucella S2 vaccine strain by dual quantitative real-time PCR (Polymerase Chain Reaction) and a complete set of reagents used for the method. The complete set of the reagents consist of a primer Bru-F1, a primer Bru-R1, a probe Bru-Probe1, a primer S2-F2, a primer S2-R2 and a probe S2-Probe2, and the nucleotide sequences of the primer Bru-F1, theprimer Bru-R1, the probe Bru-Probe1, the primer S2-F2, the primer S2-R2 and the probe S2-Probe2 are sequentially shown as SEQ ID NO: 1-SEQ ID NO: 6. Compared with other brucellosis nucleic acid detection methods, the method established by the invention directly performs qualitative and quantitative analysis and identification on the brucellosis S2 vaccine strain through fluorescence information, has the advantages of convenience, accuracy, sensitivity, specificity and the like, greatly shortens the detection time, has a great application value for prevention and control of brucellosis, and hasa wide application prospect. Epidemic situations can be controlled from the source, large-scale outbreak of the brucellosis epidemic situations is effectively prevented, and development of animal husbandry and public health safety are guaranteed.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
EryA gene deleted strain of brucella Rev.1, construction method and application thereof
InactiveCN112094792AAvoid interferenceEfficient purificationAntibacterial agentsBacterial antigen ingredientsBrucella VaccineBrucellosis
The invention discloses an eryA gene deleted strain of brucella Rev.1, a construction method and an application thereof, and belongs to the technical field of gene engineering. Compared with a wild brucella strain, the eryA gene deleted strain provided by the invention has the advantage that all eryA genes are deleted. When the eryA gene deleted strain is used as a brucella vaccine strain, the eryA gene can be detected to distinguish the wild brucella strain from the eryA gene deleted strain, so that natural infection with brucella can be distinguished from artificial immunization, thereby effectively purifying brucellosis. According to the construction method of the eryA gene deleted strain, PCR identification and single colony sequencing verification are adopted to obtain the pure eryA gene deleted strain of brucella Rev.1 with deletion of the eryA genes in high reliability.
Owner:JINYUBAOLING BIO PHARMA CO LTD +1
Method for detecting live attenuated Brucella vaccine strain and wild strain and application of method
PendingCN110564880AEasy to distinguishAccurate distinctionMicrobiological testing/measurementMicroorganism based processesBrucella VaccineMicrobiology
The invention provides a method for detecting a live attenuated Brucella vaccine strain and a wild strain and an application of the method, and belongs to the field of biotechnologies. The method fordetecting the live attenuated Brucella vaccine strain and the wild strain provided by the invention designs a primer according to a missing gene of the live attenuated Brucella vaccine strain, then adopts the primer to carry out PCR amplification on DNA of a sample to be detected, and judges the sample based on detection results. By the method, the primer is designed according to the missing geneof the live attenuated Brucella vaccine strain, namely the designed primer for amplification is a part or all of the missing gene, so that the primer effectively amplifies a gene of the wild strain without amplifying a gene of the live attenuated vaccine strain, thereby accurately and effectively distinguishing the live attenuated Brucella vaccine strain from the wild strain. The method has broaduniversality, high specificity and high accuracy.
Owner:天康生物制药有限公司
Application of protective antigen Omp25c to preparation of brucellosis vaccine
InactiveCN102038941AGood immune protectionBacterial antigen ingredientsAntinoxious agentsProtective antigenVirulent characteristics
The invention discloses protective antigen Omp25c protein of brucellosis and application thereof to preparation of a brucellosis vaccine. The test result shows that compared with a wild type vaccine strain, an omp25c mutant strain has reduced toxicity, so an omp25c gene plays a role in the toxicity of the vaccine strain. The immune level and the immune protection effect of body fluid and cells induced by an omp25c deletion strain are reduced, so the omp25c promotes the immune response and the immune protection effect of the vaccine strain. Accordingly, the omp25c protein is an important protective antigen of the brucellosis and can enhance the vaccine strain-induced immune response and the immune protection effect. The brucellosis vaccine is prepared from the omp25c protein serving as an active ingredient, so the immune protection effect of the vaccine can be improved.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Serum-based rapid brucella vaccine strain infection detection method
ActiveCN111307926AImprove throughputMaterial analysis by electric/magnetic meansBiostatisticsBrucella VaccineBrucellosis
The invention provides a serum-based rapid brucella vaccine strain infection detection method, and relates to the technical field of protein fingerprint spectrum detection. The invention provides characteristic protein combination for identifying serum infection of a brucella vaccine strain. The characteristic protein combination is used for constructing a standard detection model of vaccine strain infected brucellosis serum through a GA model algorithm of ClinProTools software, the recognition capability of the constructed model is 100%, and the cross validation value is 99.56%. MALDI-TOF MSmass spectrum data of to-be-detected serum are classified and analyzed by utilizing the standard detection model, and whether the to-be-detected serum is infected by a brucella vaccine strain or not can be accurately judged. The method is suitable for serum detection of high-risk groups infected by Brucella vaccine strains, is high in accuracy, good in repeatability and high in flux, detection of96 samples can be completed within 4 hours, the detection cost is low and the result is reliable, and the method has the broad application prospect.
Owner:ICDC CHINA CDC
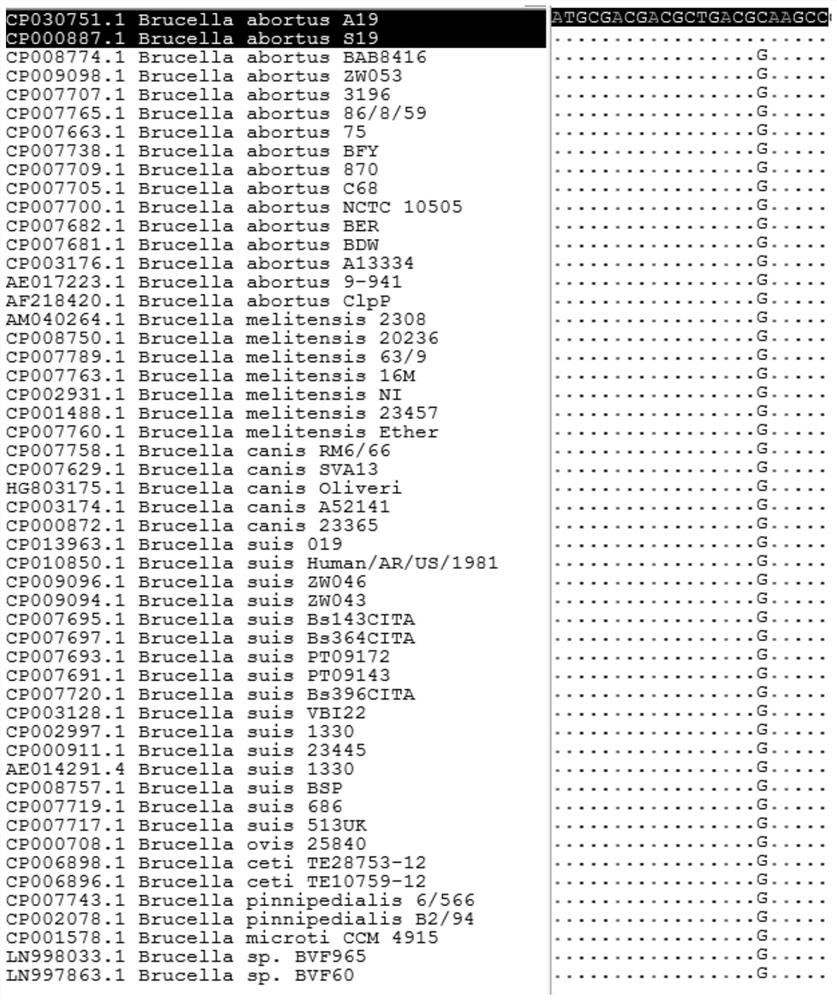
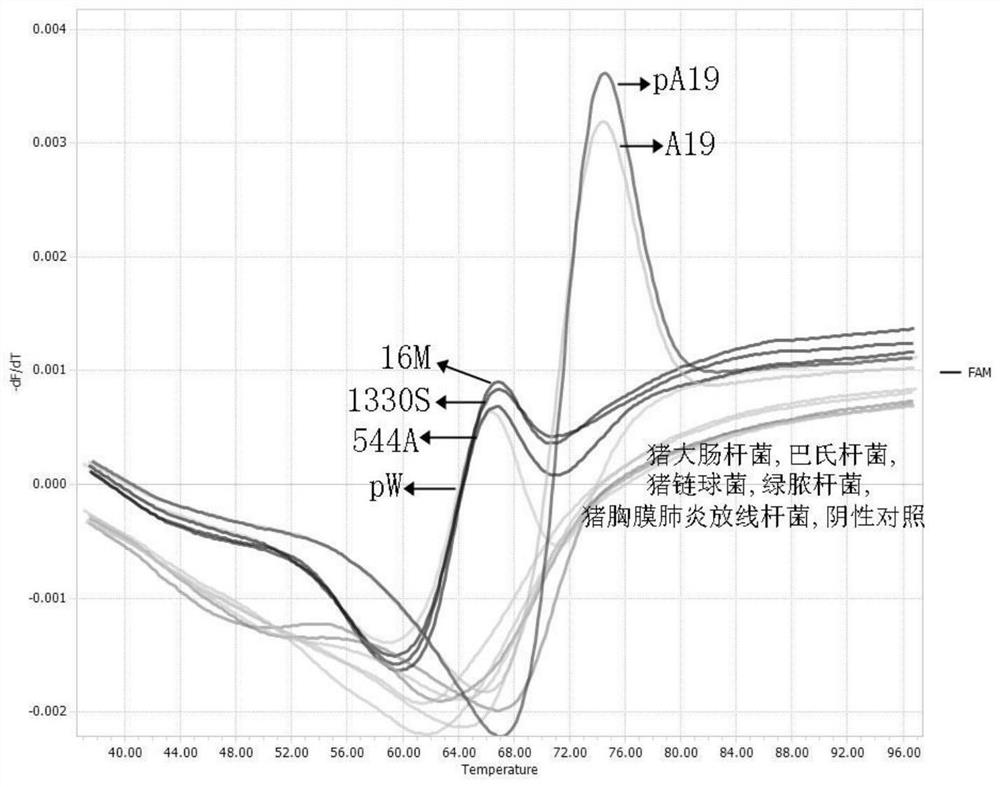
Primer and probe for identifying brucella vaccine strain A19 and wild strain, and application thereof
InactiveCN113025734AThe detection process is fastEasy to operateMicrobiological testing/measurementMicroorganism based processesBrucella VaccineNucleotide
The invention discloses a primer and a probe for identifying a brucella vaccine strain A19 and a wild strain, and application thereof, and belongs to the field of gene detection. The primer comprises an upstream primer as shown in SEQ ID NO: 1 and a downstream primer as shown in SEQ ID NO: 2. The nucleotide sequence of the probe is as shown in SEQ ID NO: 3. The primer and the probe are used for fluorescence detection, and rapid, high-throughput, high-sensitivity and specific region distribution of a vaccine strain and a wild strain of the Brucella A19 (S19) can be realized.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI +1
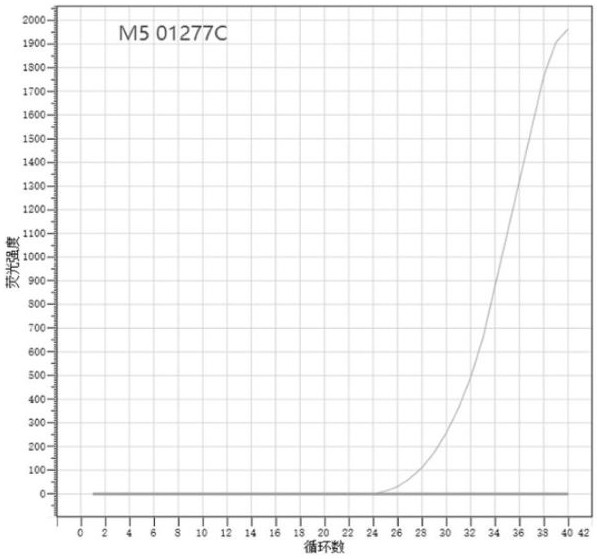
SNP molecular marker used for identifying Brucella vaccine strain M5, and application of SNP molecular marker
ActiveCN113481311AImprove featuresImprove accuracyMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention relates to the technical field of molecular markers, in particular to an SNP molecular marker used for identifying a Brucella vaccine strain M5, and application of the SNP molecular marker. The SNP molecular marker provided by the invention comprises a nucleotide sequence of which the polymorphism is A / C at the 37th site of a sequence as shown in SEQ ID NO.1, the polymorphism site of the SNP molecular marker is A and corresponds to the Brucella vaccine strain M5, and the polymorphism site of the SNP molecular marker is C and corresponds to a wild strain or other Brucella vaccine strains. The SNP molecular marker and a detection primer and a probe thereof can realize simple, convenient and efficient identification of the Brucella vaccine strain M5, the wild strain and other Brucella vaccine strains, are relatively high in accuracy, can be applied to Brucella disease pathogen monitoring and epidemiological analysis, and provide accurate technical support for Brucella disease prevention and control.
Owner:ICDC CHINA CDC
B.melitensis Delta UGPase and construction method thereof
InactiveCN106190897AImprove securityImproving immunogenicityBacteriaTransferasesMutation CarrierUridine diphosphate glucose pyrophosphorylase
The invention provides a B.melitensis Delta UGPase and a construction method thereof and belongs to the field of novel Brucella molecular marker vaccine research. The preservation number of the B.melitensis Delta UGPase is CGMCC No.11908, and a Brucella virulent strain M16 and UDP-glucose pyrophosphorylase (UGPase) gene related to virulence are lost. Upstream and downstream homologous arm sequences of the UGPase gene are amplified by PCR to obtain two gene segments, the gene segments and suicide carrier vector pBK-CMV-SacB are connected to pBK-CMV-SacB UGPase gene-NC losing mutation carriers after fusion and double enzyme digestion, the mixture is electro-transformed to Brucella for screening culture, and the B.melitensis Delta UGPase is finally obtained. The vaccine strain can be taken as a novel brucellosis vaccine candidate bacterial strain for controlling brucellosis spreading, and a basis is laid for novel Brucella vaccine development.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Serum-based brucella vaccine strain infection and wild strain infection rapid identification method
ActiveCN111007140ARapid infection screeningFacilitate infection screeningMaterial analysis by electric/magnetic meansSystems biologyBrucella VaccineRapid identification
Owner:ICDC CHINA CDC
Multivalent brucella vaccine for protection against mycobacterial infections and methods of using the same
ActiveUS20170246282A1Increase transcriptional activityHigh expressionAntibacterial agentsBacterial antigen ingredientsHeterologousBrucella Vaccine
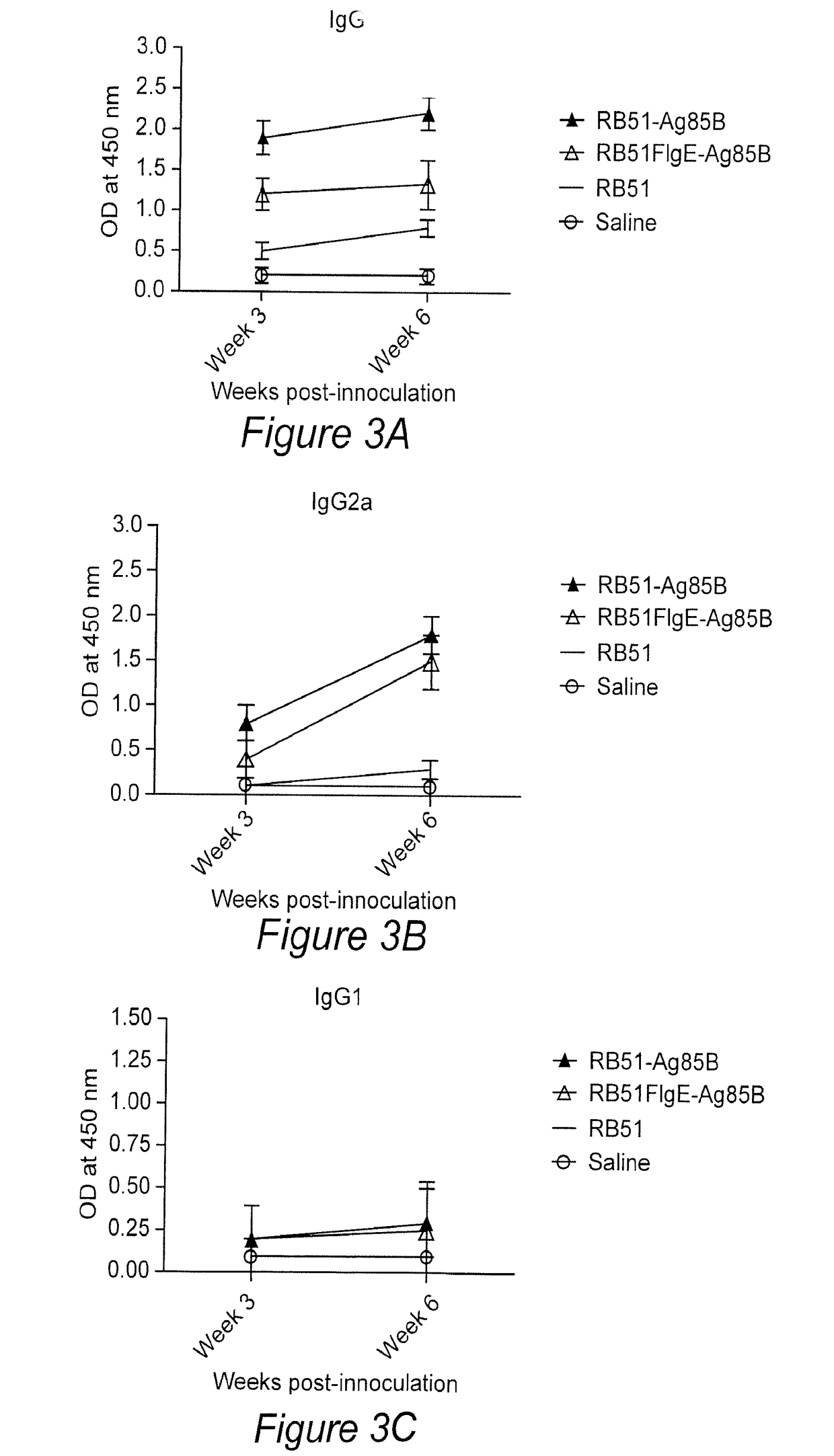
Provided herein is a multivalent Brucella vaccine expressing at least one heterologous M. tuberculosis antigen. The vaccines described herein serve as an environmentally safe bivalent vaccine for protection against Brucella and Mycobacterium infections simultaneously. In particular, a multivalent vaccine comprising a Brucella strain transformed with a vector that expresses at least one M. tuberculosis antigen, where the M. tuberculosis antigen(s) is codon optimized for the Brucella strain is provided. In some aspects, the Brucella strain is B. abortus strain RB51 leuB and the M. tuberculosis antigen is one or more of Ag85B, Rv2660c, and ESAT6.
Owner:VIRGINIA TECH INTPROP INC
Application of lipopolysaccharide extracted from brucella ovis vaccine strain M5 in preparation of product for diagnosing human brucellosis
ActiveCN111518225AEliminate infectivityNo infection hazardBiological testingImmunoassaysInfection diagnosisBrucella Vaccine
The invention discloses an application of lipopolysaccharide extracted from a brucella ovis vaccine strain M5 as a human brucellosis diagnosis antigen. According to the invention, the reactivity of anLPS antigen extracted from the brucella ovis vaccine strain M5 as a diagnosis antigen is obviously superior to that of an LPS antigen extracted from a brucella cattle strain S19 and a brucella pig strain S2 commonly used in a brucella antibody rapid detection kit on the market at present, therefore, the brucella ovis vaccine strain M5 can be used as a preferred source strain of a new brucella gold-labeled diagnosis LPS antigen, the extracted LPS has a good diagnosis value on human brucellosis, and a new way is provided for selection of a human brucellosis infection diagnosis antigen. The invention also finds that the antigenicity of the LPS antigen extracted from the brucella ovis vaccine strain M5 subjected to innocent treatment is not influenced.
Owner:新疆禹孚生物技术股份有限公司
Primer pair for detecting Brucella S2 vaccine strain, application thereof, and reagent kit
InactiveCN111778343ARealize detectionQuick checkMicrobiological testing/measurementMicroorganism based processesForward primerBrucella
The invention provides a primer pair for detecting a Brucella S2 vaccine strain, an application thereof, and a reagent kit, and belongs to the technical fields of bioengineering and molecular detection. The primer pair consists of a forward primer S2-up and a backward primer S2-down. The primer pair and the reagent kit provided by the invention are used for detection, the detection method is simple and quick, the repeatability is good, the susceptibility is high, the specificity is high, and detection of high-flux samples can be realized, so that quick, high-sensitive real-time fluorescent quantitation PCR detecetion on the Brucella S2 vaccine strain can be realized. A minor quantity of nucleic acid DNA of the Brucella S2 vaccine strain in samples to be detected such as blood can be detected, the detection limit is low, the specificity is high, other bacterial strains rather than the Brucella S2 vaccine strain can be distinguished, and therefore, the primer pair and the reagent kit have favorable practical application value.
Owner:青岛中创汇科生物科技有限公司
Abortus Brucella vaccine strain S19 marked recombinant strain and application thereof
InactiveCN101575589AReduced toxicityPromote eradicationAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsBrucella Vaccine
The invention discloses an abortus Brucella vaccine strain S19 marked recombinant strain and an application thereof. The abortus Brucella recombinant strain is a strain obtained by deactivating the glucosyltransferase coding gene. The obtained recombinant strain has small virulence and can be used as vaccines to immunize animals without needs of deactivation. When the recombinant strain is used for immunizing rats, the inoculated animal generates no lipopolysaccharide antibody and the virulent virus strain-infected animal can be distinguished from novel vaccine strain immunized animal by serological reaction. The recombinant strain is beneficial to improving the Brucella vaccine, researching diagnosis and identification reagents and developing the Brucella vaccine and matched diagnosis and identification reagents, and has extremely important significance in deracinating and purifying the global animal Brucella.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com