Patents
Literature
36 results about "C protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
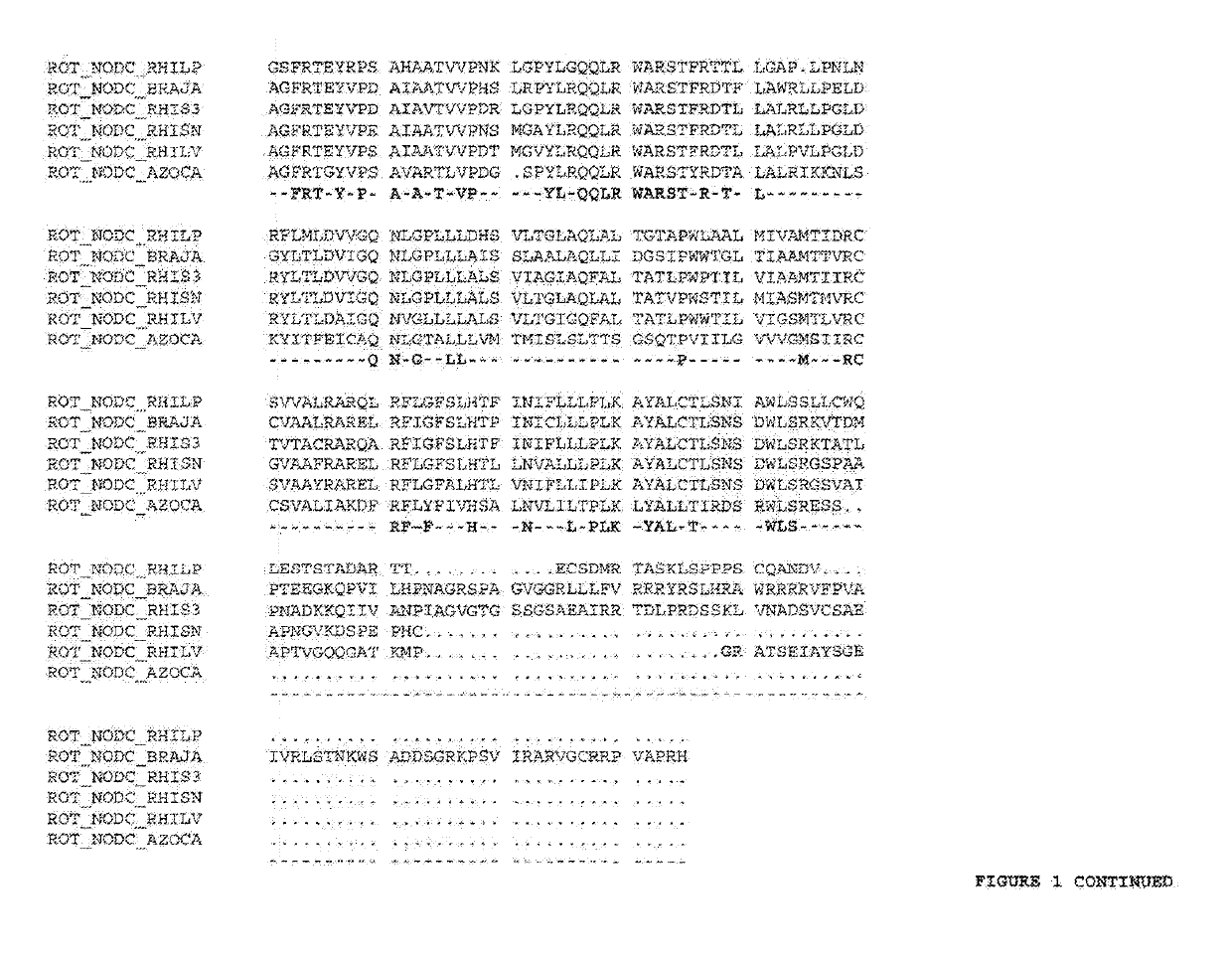
Protein C. Protein C (autoprothrombin IIA, blood coagulation factor XIV) is a zymogen, the activated form of which plays an important role in regulating anticoagulation, inflammation, cell death, and maintaining the permeability of blood vessel walls in humans and other animals. Activated protein C...
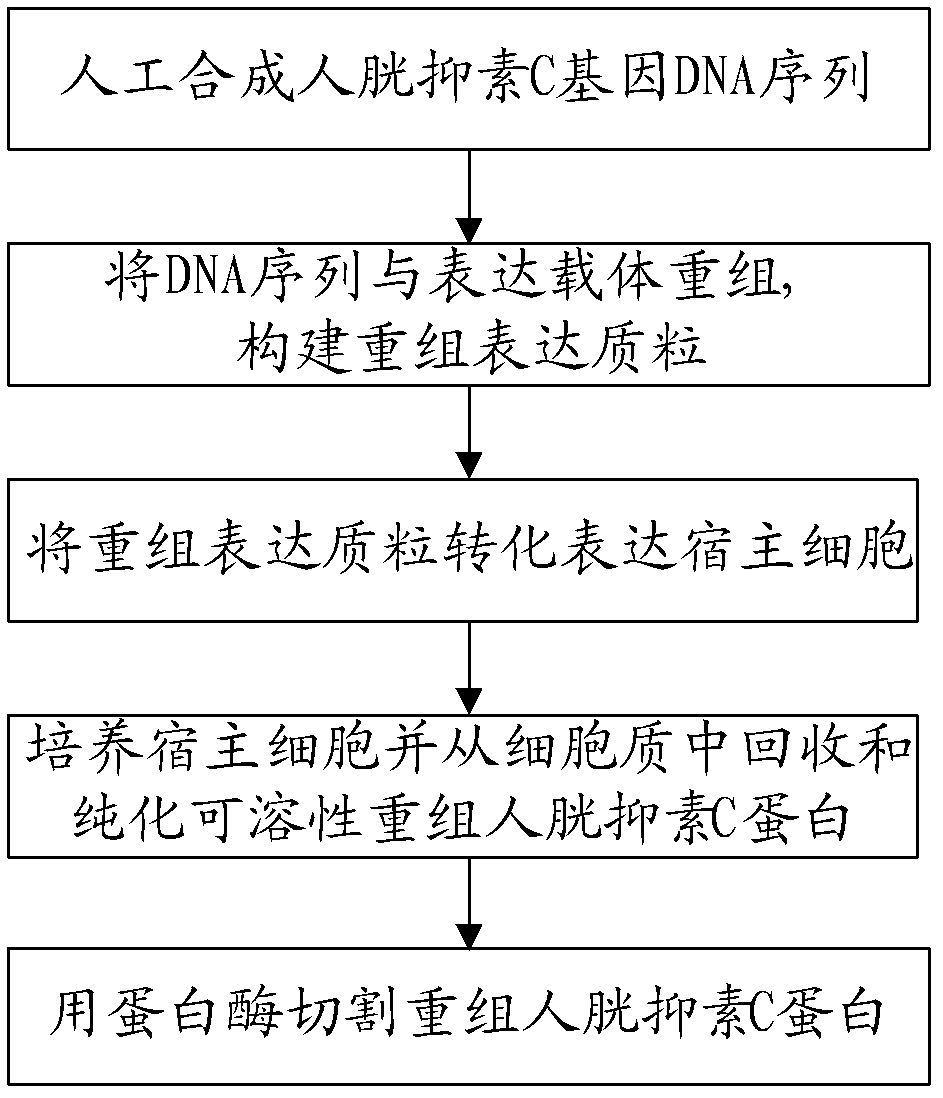
Recombined human cystatin-C protein with natural activity and preparation method thereof
ActiveCN103014047AImprove solubilitySolve the key problems of independent research and developmentMicroorganism based processesProtease inhibitorsBiotechnologyProteinase activity
The invention discloses a recombined human cystatin-C protein with natural activity and a preparation method thereof. According to the preparation method, lots of stable recombined human cystatin-C proteins are expressed and purified by employing a gene engineering technique, and the recombined human cystatin-C proteins are cut by utilizing the protease, so that the recombined human cystatin-C protein has high natural activity. The recombined human cystatin-C protein can be used for immune preparation of monoclonal antibodies and multi-antibody blood serum, and excellent raw materials are provided for preparing stable cystatin-C detection agents and quality control products thereof.
Owner:深圳前海菲鹏基因科技有限公司 +1
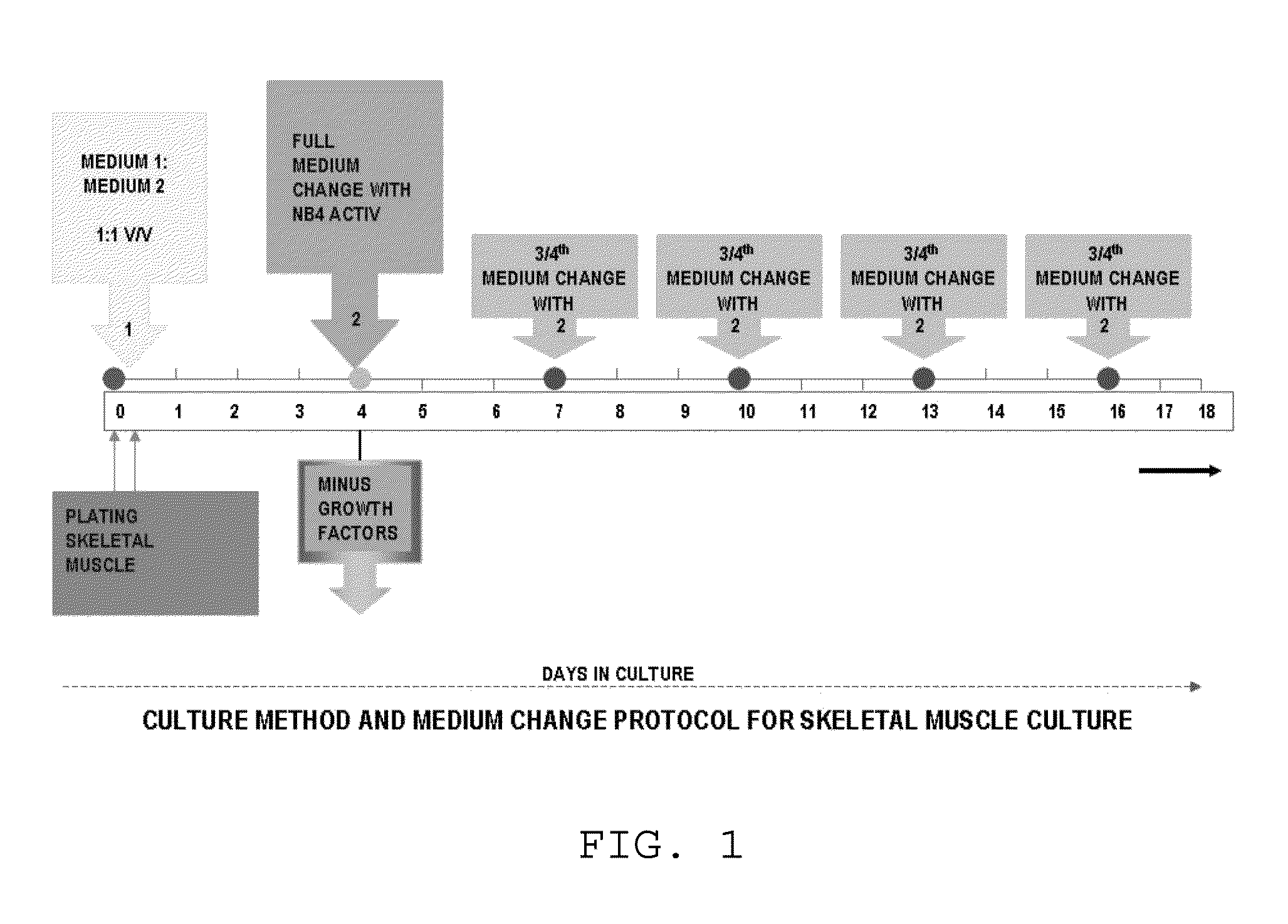
Method for culturing skeletal muscle for tissue engineering
ActiveUS9163216B1Small sizeReduce complexityCulture processNervous system cellsInsulin-like growth factorSerum free media
The invention provides a nutrient medium composition and associated methods for lengthening the useful life of a culture of muscle cells. Disclosed is a method of culturing mammalian muscle cells, including preparing one or more carriers coated with a covalently bonded monolayer of trimethoxy-silylpropyl-diethylenetriamine (DETA); verifying DETA monolayer formation by one or more associated optical parameters; suspending isolated fetal rat skeletal muscle cells in serum-free medium according to medium composition 1; plating the suspended cells onto the prepared carriers at a predetermined density; leaving the carriers undisturbed for cells to adhere to the DETA monolayer; covering the carriers with a mixture of medium 1 and medium 2; and incubating. A cell nutrient medium composition includes Neurobasal, an antibiotic-antimycotic composition, cholesterol, human TNF-alpha, PDGF BB, vasoactive intestinal peptides, insulin-like growth factor 1, NAP, r-Apolipoprotein E2, purified mouse Laminin, beta amyloid, human tenascin-C protein, rr-Sonic hedgehog Shh N-terminal, and rr-Agrin C terminal.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting haemophilus parasuis antibody
ActiveCN103941020AHigh homologyImproved conservatismBiological material analysisBiological testingToxin proteinCytolethal distending toxin
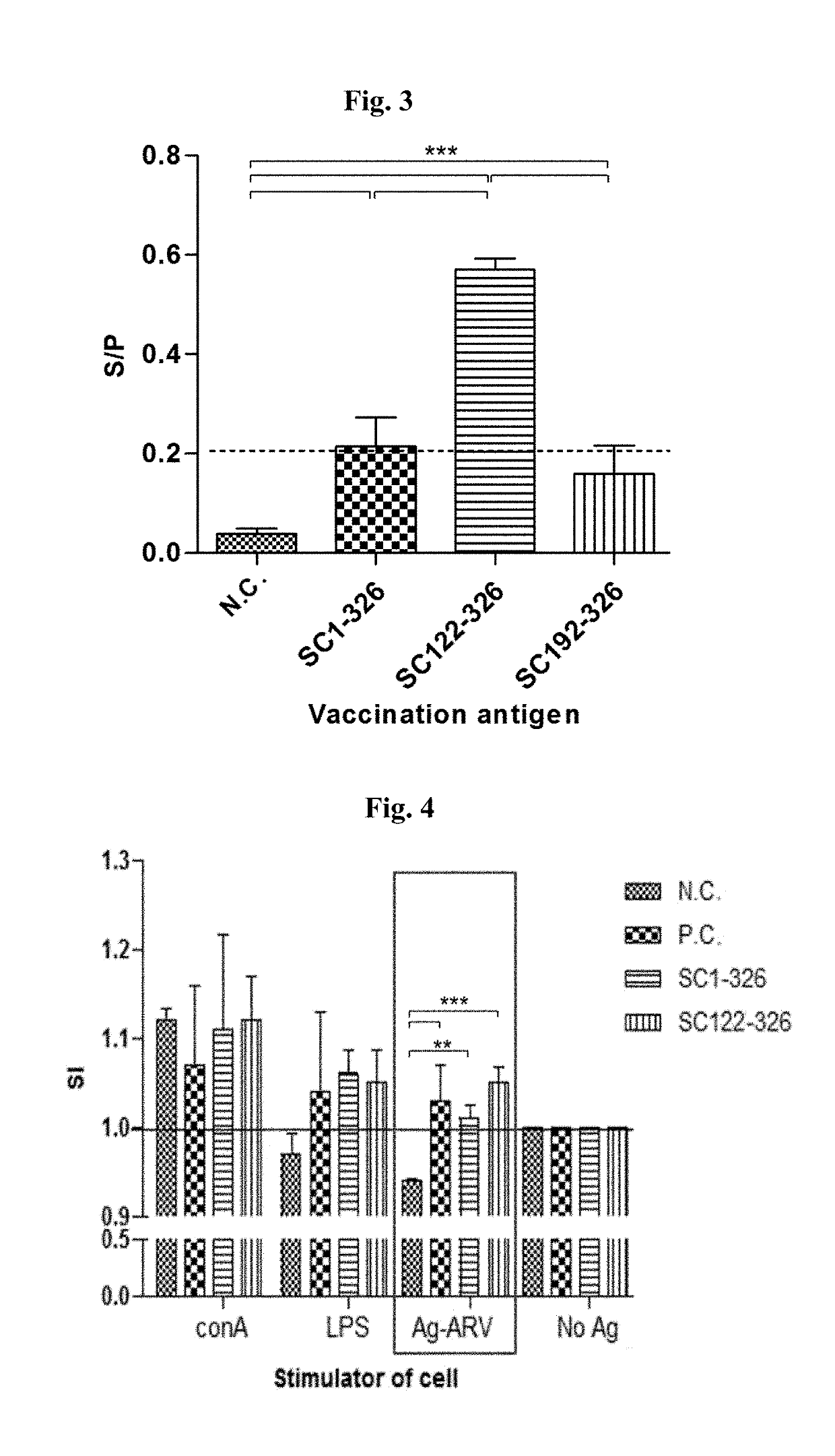
The invention discloses an indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting a haemophilus parasuis antibody. The kit consists of an ELISA coating plate with haemophilus parasuis cytolethal distending toxin)-C protein serving as a coating antigen, a to-be-detected sample dilution plate, a positive contrast serum, a negative contrast serum, 20-time concentrated washing liquid, a serum sample dilution solution, an enzyme-labeled antibody working solution, a developing solution and a terminating solution. A judgment standard is that if an S / P value is less than 0.200, a sample to be detected is negative; if the S / P value is greater than or equal to 0.200, the to-be-detected sample is positive; the S / P value is obtained according to a formula: S / P value=(the mean value of the to-be-detected sample OD450nm-the mean value of a negative contrast OD450nm) / (the mean value of a positive sample OD450nm-the mean value of the negative contrast OD450nm). Due to the specificity test, the sensitivity test, the repetitiveness test, the coincidence rate test, the test for comparing the kit disclosed by the invention with a kit on sale, the clinical application test and the like, the kit disclosed by the invention has the characteristics of high specificity, high sensitivity, high repetitiveness and the like and is high in coincidence rate to the same type of products on sale home and abroad; the indirect ELISA kit can be used for clinical large-scale detection and epidemiological investigation for the haemophilus parasuis antibodies.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
ELISA kit for detecting porcine toxigenic pasteurella multocida toxin antibody and application
InactiveCN101012446AHigh biosecurityNo biohazardBacteriaMicroorganism based processesPasteurella multocida toxinAntigen
The invention discloses an ELISA agent box and usage in the progressive atrophic rhinitis (PAR) as well as rPMT-C recombinant coliform BL21 / pET28b-C2115 (CCTCC NO: M207012) on the C' end of toxigenic pasteurellotic toxin toxA gene and expressing and purifying method of rPMT-C protein, wherein the agent box comprises the following parts: rPMT-C protein on the C' end of toxin toxA gene as enzyme target board, negative, positive standard serum, rabbit anti-swine lgG enzyme target diprevent, sample dilute, substrate display A liquid, substrate display B liquid, stopping liquid and washing liquid.
Owner:武汉科前动物生物制品有限贵任公司
Tumoricidal, bactericidal, or viricidal macrophage activation
Owner:KNEZEVICH CHARLES +1
Synthetic lipid mixtures for the preparation of a reconstituted surfactant
InactiveUS20080242589A1Peptide/protein ingredientsAlveolar/pulmonary surfactant peptidesDiseaseLipid formation
The invention relates to reconstituted surfactants consisting of artificial phospholipids and peptides able to lower the air-liquid surface tension, more particularly to reconstituted surfactants comprising special phospholipid mixtures and artificial peptides which are analogues of the natural surfactant SP-C protein for the treatment of respiratory distress syndrome (RDS) and other diseases relating to pulmonary surfactant dysfunctions.
Owner:CHIESI FARM SPA
Expression cassettes encoding modified human immunodeficiency virus type 1 subtype C envelope glycoproteins
Owner:STELLENBOSCH UNIVERSITY +1
Novel duck reovirus compound vaccine and preparation method of egg yolk antibody
ActiveCN111440815AGood clinical protective effectReduce manufacturing costEgg immunoglobulinsViral antigen ingredientsBiotechnologyAdjuvant
The invention aims to provide a novel duck reovirus compound vaccine and a preparation method of an egg yolk antibody. The preparation method comprises the following steps: mixing recombinant PVAX1-sigma B plasmid containing a novel duck reovirus sigma B protein gene and a novel duck reovirus sigma C protein according to a certain ratio, and performing emulsifying with a white oil adjuvant to prepare the compound vaccine. The average antibody titer of eggs collected 7-150 days after the three times of immunization reaches 1:1024 or above, and the highest antibody titer can reach 1:4096. The egg yolk antibody product prepared by extracting and purifying hyper-immune eggs can provide complete protection for ducks infected with the novel duck reovirus. The novel duck reovirus egg yolk antibody prepared by the method disclosed by the invention is definite in effect and low in cost, and has remarkable economic and social benefits.
Owner:WEIFANG HUAYING BIOTECH CO LTD
Polynucleotides encoding antigenic HIV type c polypeptides, polypeptides and uses thereof
InactiveUS20100316698A1Sufficient amountImprove efficiencyPowder deliveryFungiNucleotideDna immunization
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Optimized polypeptide for a subunit vaccine against avian reovirus
An isolated polypeptide comprising an amino acid sequence corresponding to the amino acid residues forming a full or partial alpha-helical domain, the hinge domain, the beta-triple spiral domain and afull or partial globular head domain of an avian reovirus sigma C protein, and lacking the amino acid sequence that is N-terminal to said alpha-helical domain is provided. Furthermore, a vaccine comprising, or a viral vector expressing, at least one of the isolated polypeptides of the present invention is provided.
Owner:GAVISH GALILEE BIO APPL
Resistance marker-free porcine actinobacillus pleuropneumoniae double-gene defective strain, construction method and application thereof
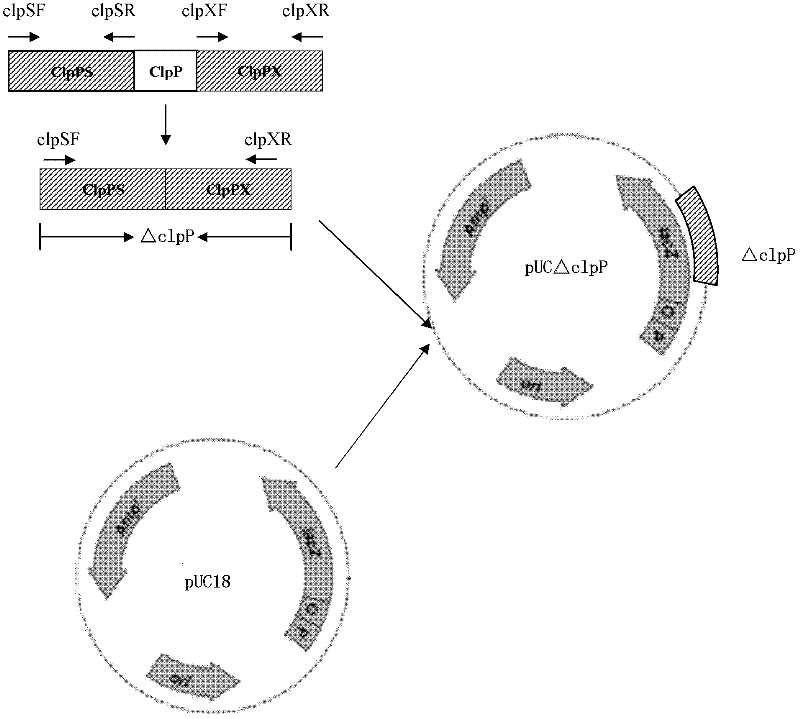
InactiveCN102517232AStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsBiotechnologyDifferential diagnosis
The invention discloses a resistance marker-free porcine actinobacillus pleuropneumoniae (APP) serum type 7 double-gene defective strain, and belongs to the technical field of bacterial gene engineering. The recombinant strain APPdeltaclpPdeltaapx II C of APP is obtained by inactivating ClpP protease in the APP and a coded gene of a hemolysin activated factor Apx II C by adopting a directional homologous recombination technology, and expression of the ClpP protease and the Apx II C protein is destroyed. The obtained double-gene defective strain has lower toxicity compared with a parent strain, is safe to animals, provides an important basis for transformation of porcine contagious pleuropneumonia (PCP) vaccines and research of matched identification and diagnosis reagents, and has great significance for promoting elimination and purification of the global PCP.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
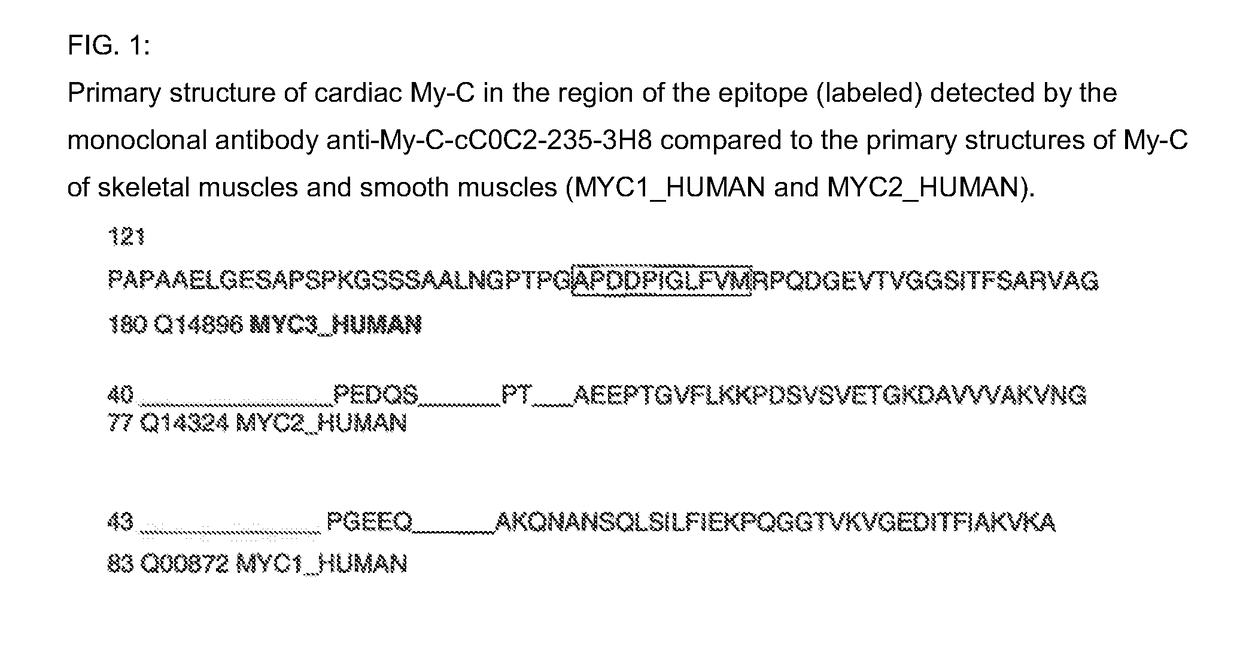
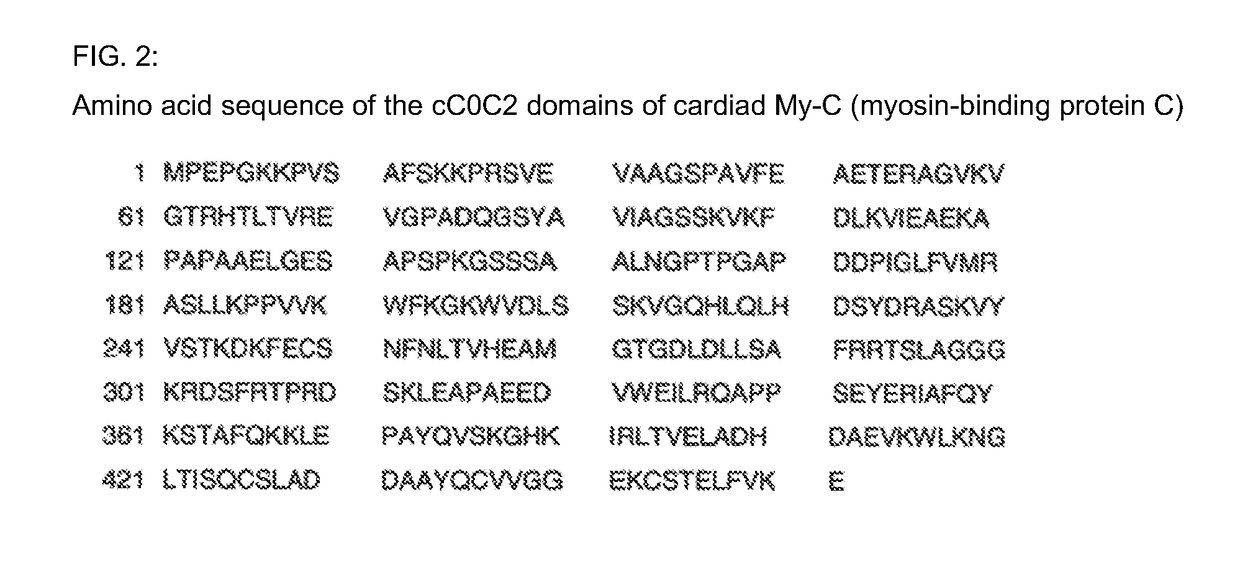
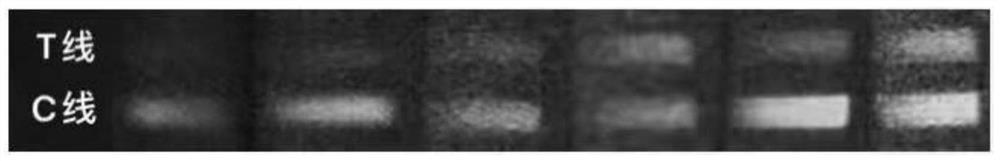
Hybridoma cell lines (my-c-cc0c2-235-3h8) and use thereof for producing a monoclonal antibody against the human cardiac myosin binding protein c (c-protein, mybpc3, cmybp-c or my-c)
ActiveUS20170088629A1Early diagnosis of cardiac infarctionsImmunoglobulins against animals/humansDisease diagnosisCardiac myosinBlood plasma
Monoclonal antibodies, which can be produced in vitro, against cardiac epitopes of the human My-C are produced by generating myeloma cell clones that produce such specific antibodies having epitope specificity. These monoclonal antibodies allow, among other things, the creation of an enzyme-linked immunosorbent assay (ELISA) for the specific, cross-reactivity-free quantitative determination of My-C in serum, plasma, whole blood or other body fluid. Specifically, a hybridoma cell clone producing a monoclonal antibody that detects and binds a cardiac epitope in the My-C is produced, which has no cross-reactivity with respect to the myosin-binding proteins of the skeletal muscles. The hybridoma cell line can be obtained by fusing myeloma cells with spleen cells of a test animal, in particular a mouse, immunized against recombinant My-C. The invention furthermore relates to epitope-specific antibodies produced by the hybridoma cell line, and to the use thereof.
Owner:KINGS COLLEGE LONDON
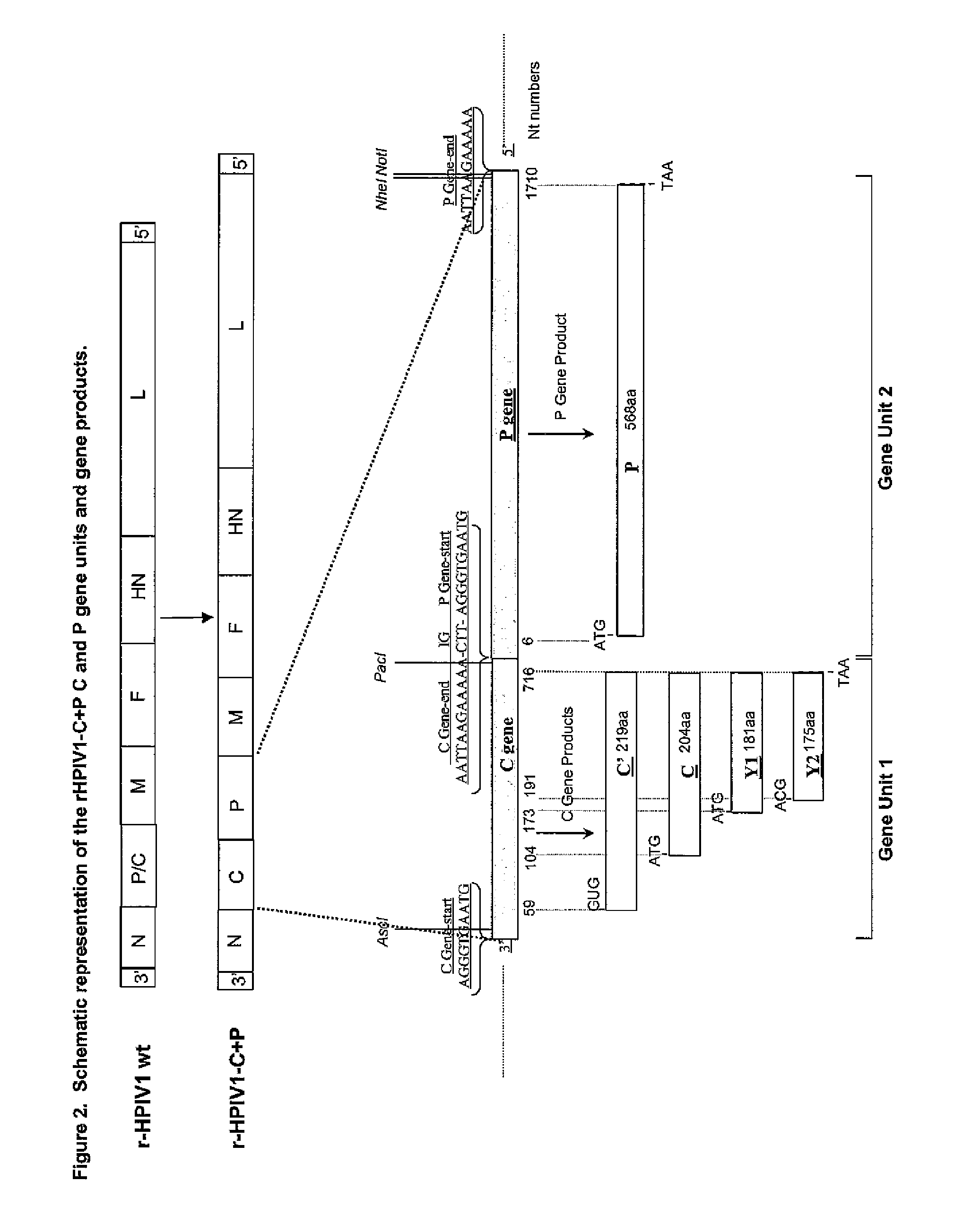
Human parainfluenza viruses having separated p and c genes
InactiveUS20100330120A1SsRNA viruses negative-senseSugar derivativesHuman Parainfluenza VirusPolynucleotide
The invention provides self replicating infectious recombinant paramyxoviruses where the P and C genes are separated rated. The recombinant paramyxoviruses preferably have one or more attenuating mutations and / or at least one temperature sensitive mutation and one non-temperature sensitive mutation. In some embodiments, the recombinant paramyxovirus has a separate variant polynucleotide encoding a C protein and a separate monocistronic polynucleotide encoding a P protein. Also provided are compositions and methods for using the recombinant paramyxoviruses as described herein.
Owner:UNITED STATES OF AMERICA
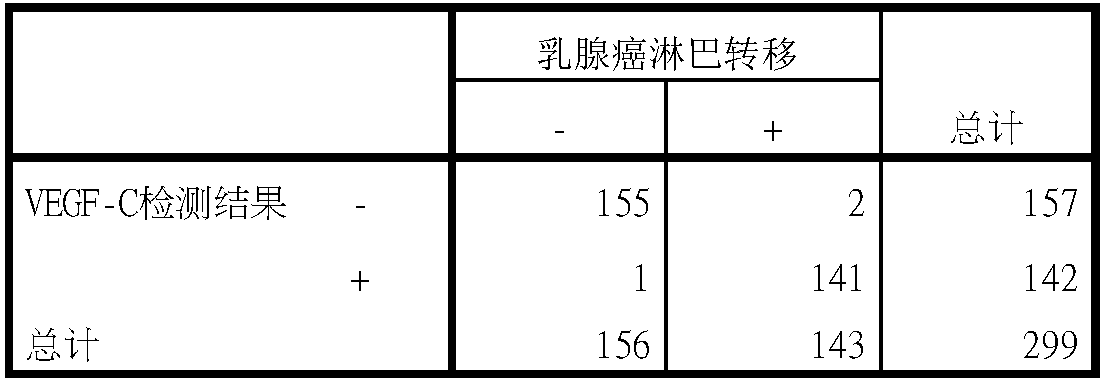
VEGF (vascular endothelial growth factor)-C monoclonal antibody and kit
InactiveCN108640994AHigh affinityStrong specificityBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainVascular endothelial growth factor
Owner:浙江众意生物科技有限公司
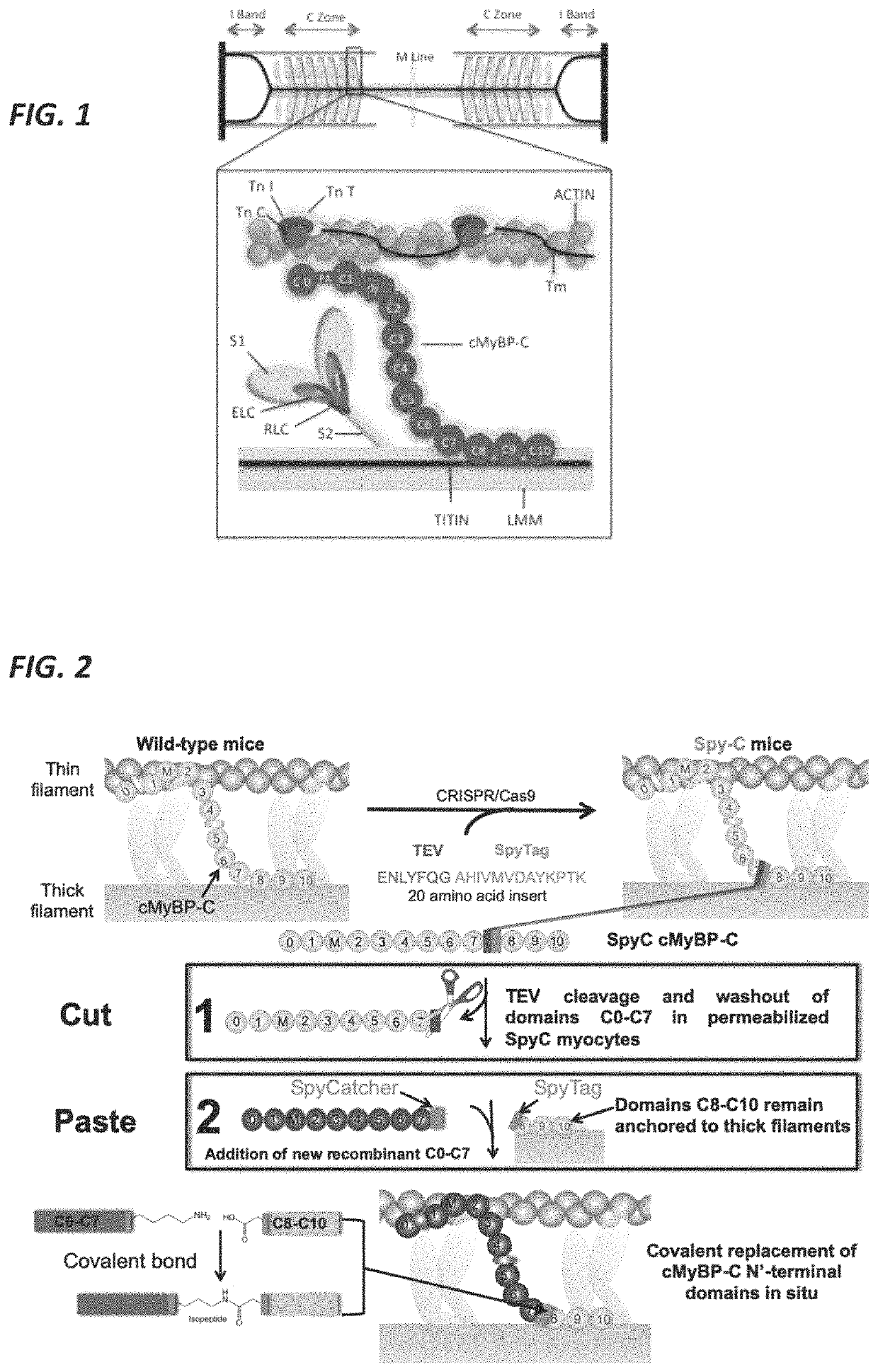
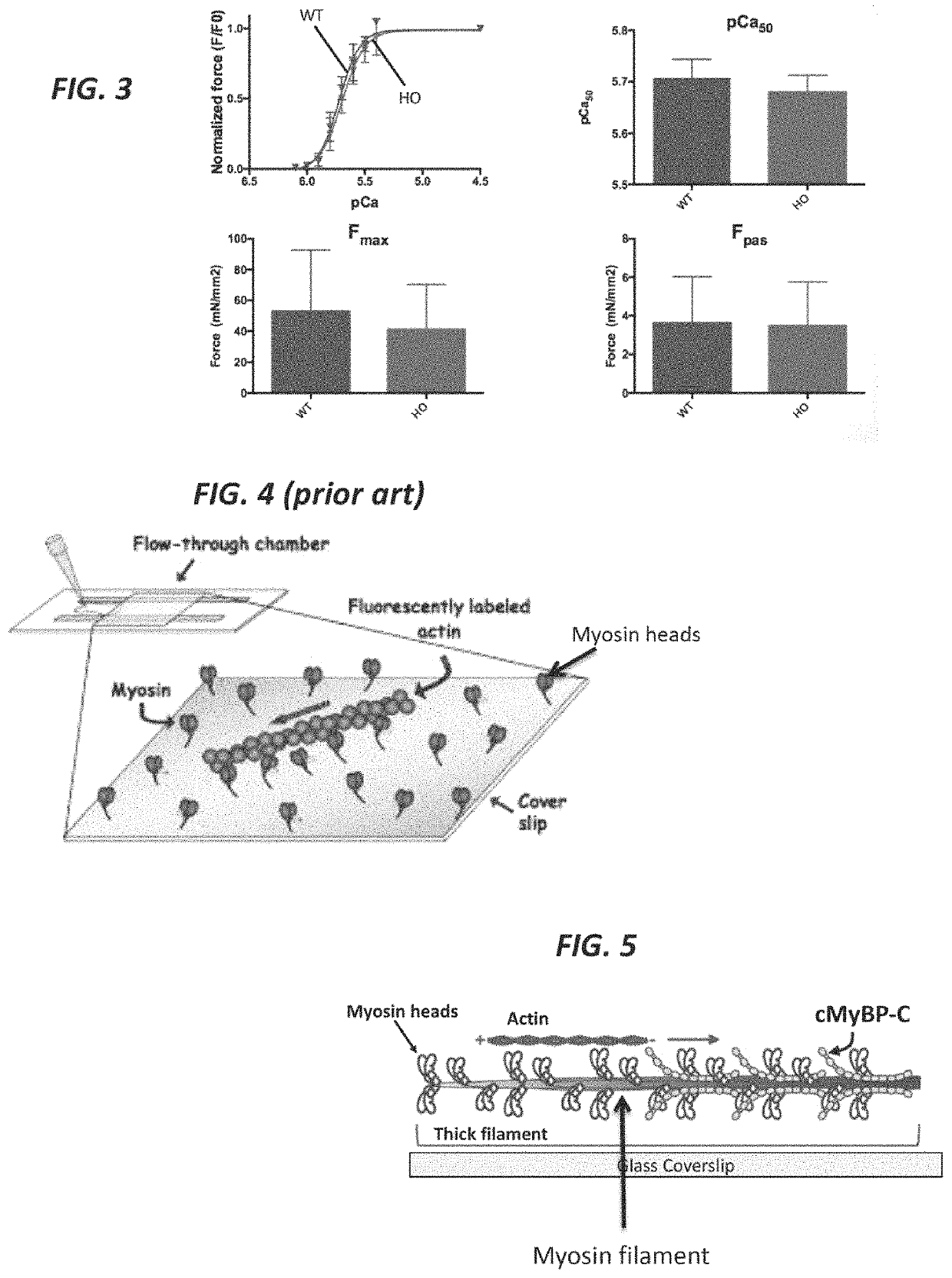
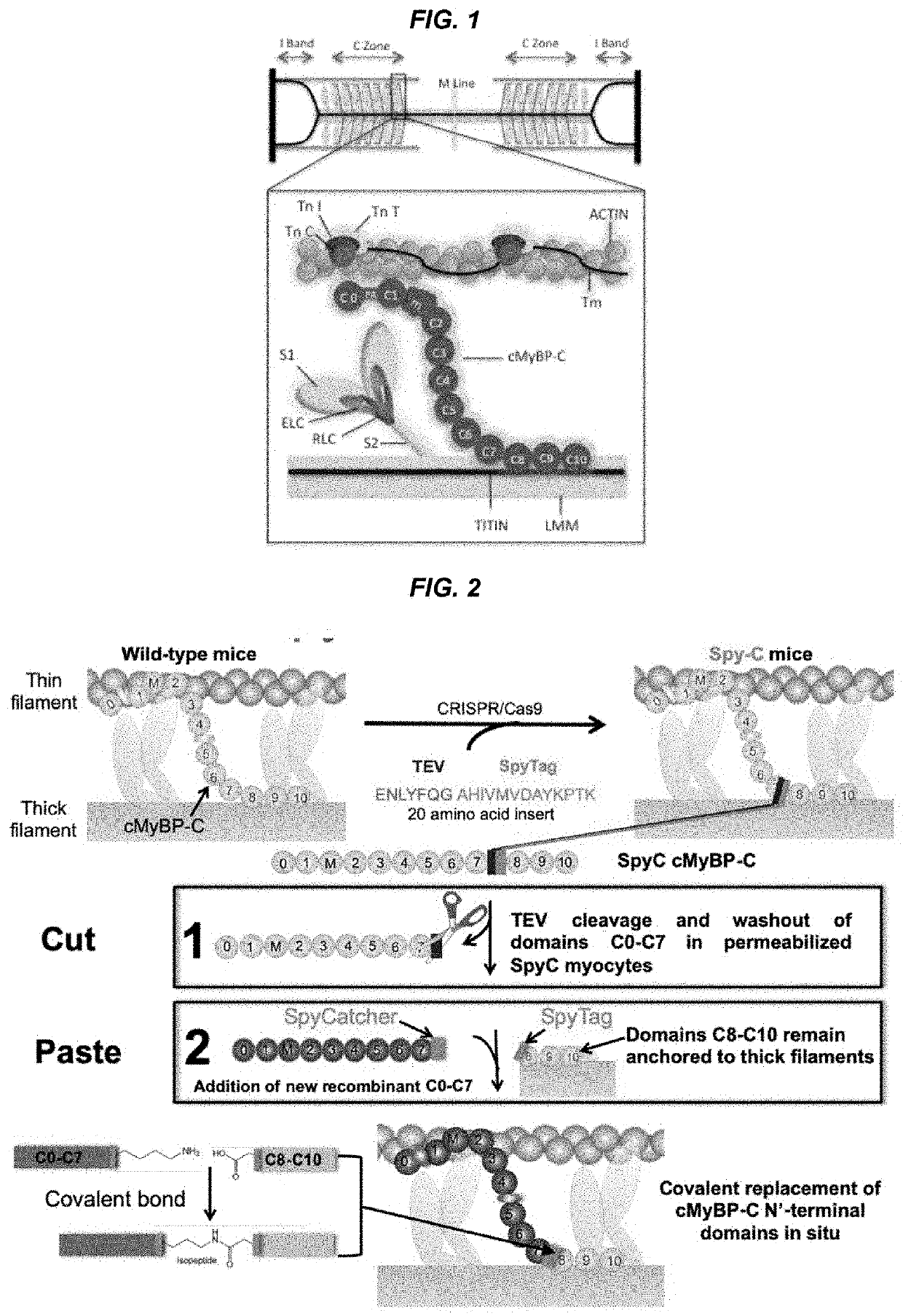
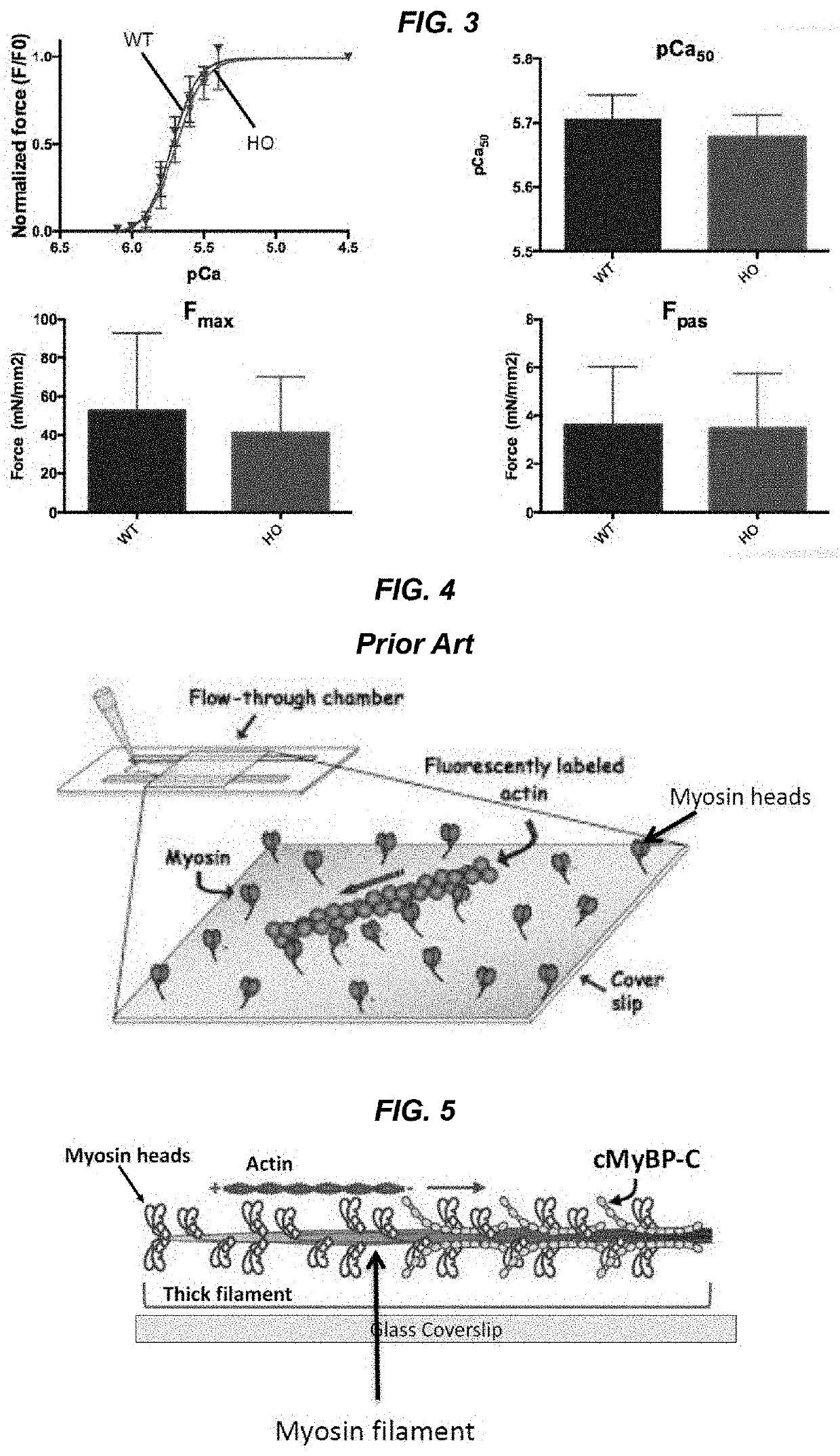
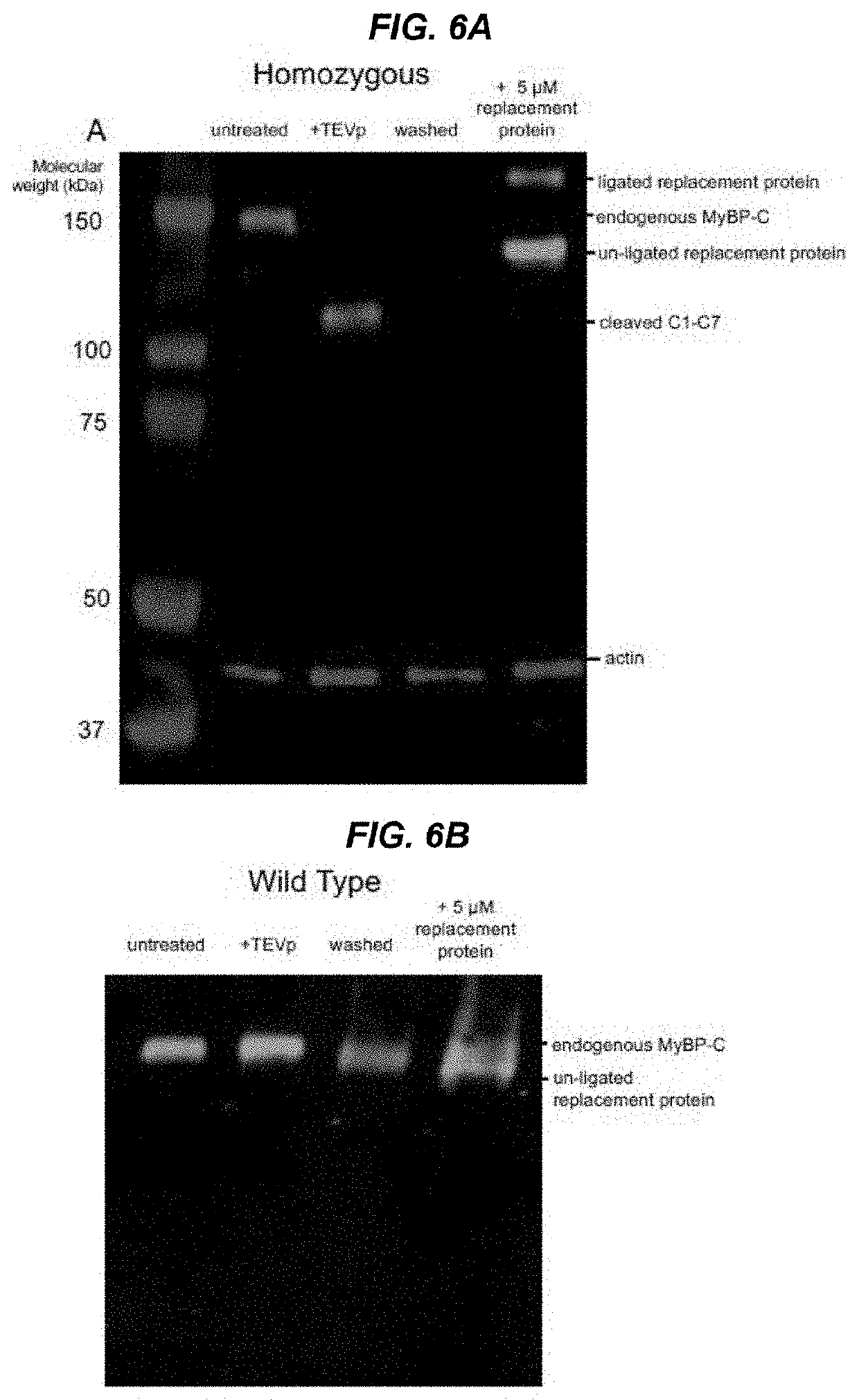
Methods and compositions for rapidly replacing cardiac myosin binding protein-C in sarcomeres
ActiveUS11242368B2Quick changeQuick exchangeStable introduction of DNAAnimals/human peptidesCardiac myosinCardiac muscle
Methods and compositions for rapidly replacing cMyBP-C in sarcomeres featuring the creation of Spy-C mice, which are mice genetically engineered to express cMyBP-C with a protease recognition site and SpyTag peptide introduced into the cMyBP-C gene. In permeabilized myocytes from the Spy-C mice, the cMyBP-C protein can be cleaved at the protease recognition site, and the N-Terminus of cMyBP-C can be removed while the C-terminus remains anchored to the thick filament. A new peptide featuring the SpyCatcher sequence can be covalently bonded to the remaining portion of cMyBP-C, thereby creating a modified cMyBP-C protein. The methods and compositions of the present invention allow for the reconstitution of full-length cMyBP-C at the precise position of native cMyBP-C in the sarcomere and allow for a variety of modifications to be introduced to cMyBP-C in situ.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Recombinant TsPKA-C protein and application thereof
The invention provides a recombinant TsPKA-C protein and application thereof and particularly relates to application of the recombinant TsPKA-C protein as taenia solium / cysticercerci detection antigen and application of the recombinant TsPKA-C protein to preparation of a porcine cysticercosis detection kit. The recombinant TsPKA-C protein can specifically detect taenia solium / cysticercerci and has no cross reaction with porcine thin-neck cysticercerci, trichinella sui and the like.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
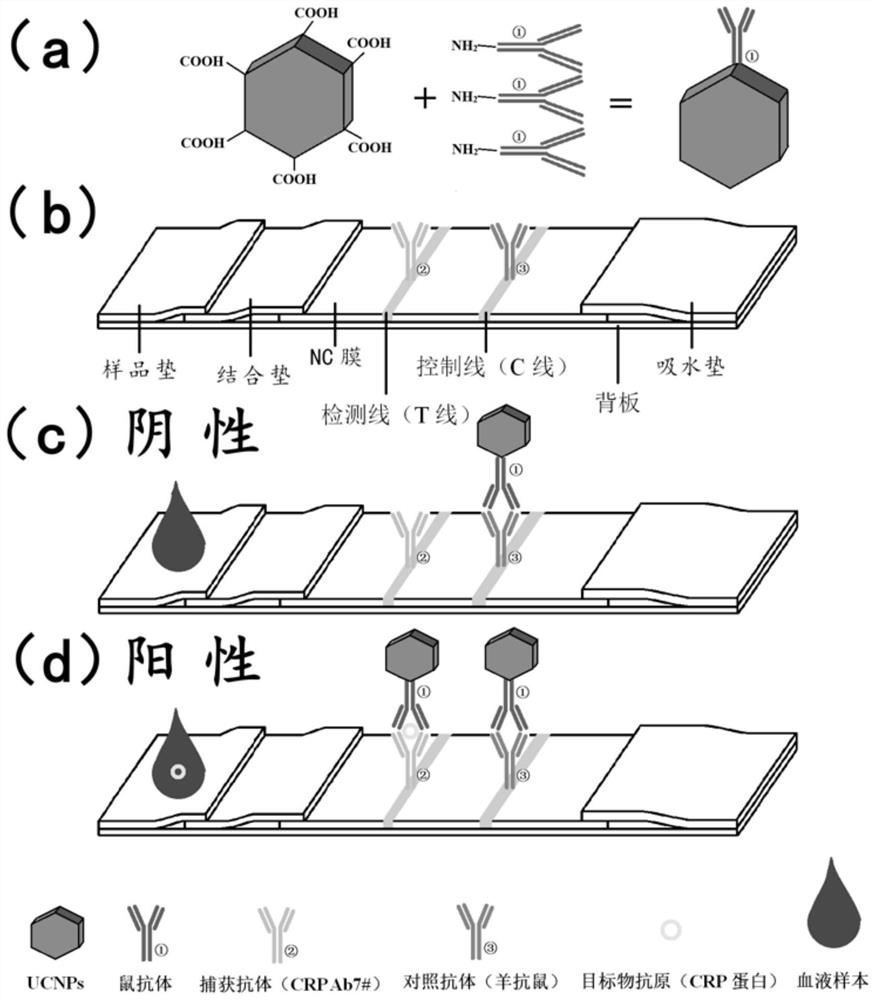
Preparation method of C protein detection test paper and handheld reading device
PendingCN113933518AGuaranteed accuracyAvoid detection performance impactBiological material analysisBiological testingWhole blood unitsBiology
The invention discloses a preparation method of C protein detection test paper, namely a handheld reading device. The preparation method comprises the following steps: pasting an NC membrane on a substrate of lateral flow test paper; fixing a prepared detection solution in a detection area on the NC membrane, and fixing the prepared contrast solution in a contrast area on the NC membrane; attaching a combination pad and a water absorption pad to the positions, located on the two sides of the NC membrane, of the substrate respectively, wherein the combination pad and the water absorption pad are both connected with the NC membrane, and a probe of a UCNPs-CRP antibody is arranged on the combination pad; and attaching a sample pad to the position, located on the other side of the combination pad, of the substrate, wherein the sample pad is connected with the combination pad. According to the invention, the UCNPs-CRP antibody probe is arranged on the combination pad, so that the influence of background fluorescence generated by a whole blood sample on the detection performance can be avoided; and the detection area and the control area are arranged on the NC membrane, so that the accuracy of a detection result can be ensured.
Owner:XIAN TECHNOLOGICAL UNIV
Hybridoma cell lines (my-c-cc0c2-259-1 a4) and use thereof for producing a monoclonal antibody against human cardiac myosin binding protein c (c-protein, mybpc3, cmybp-c or my-c)
ActiveCN106459927AImmunoglobulins against animals/humansBiological material analysisCardiac myosinMyosin-binding protein C
The aim of the invention is to produce, in vitro, monoclonal antibodies against cardiac epitopes of the human My-C by generating myeloma cell clones, which produce said specific antibodies with epitope specificity. Said monoclonal antibodies should, amongst other things, enable an ELISA (Enzyme-Linked Immuno Sorbent Assay) for the specific, cross-reactivity free quantitative determination of My-C in serum, plasma or full blood, to be formed. Said aim is achieved by generating a hybridome cell clone which produces a monoclonal antibody which recognizes and binds with a cardiac epitope in the My-C and which does not have the cross-reactivity with respect to the myosin binding proteins of the skeletal muscle. Said hybridome cell line can be obtained by fusing myelome cells with spleen cells of a test animal, in particular a mouse, immunized against recombined My-C. The invention also relates to epitope specific antibodies produced by the hybridome cell lines and to the use thereof.
Owner:KINGS COLLEGE LONDON
Methods for altering the reactivity of plant cell walls
InactiveUS20190153458A1Increase the amount addedImprove responsePolypeptide with localisation/targeting motifDyeing processPlant cellNodulations
Owner:BASF SE
Optimized polypeptide for a subunit vaccine against avian reovirus
An isolated polypeptide comprising an amino acid sequence corresponding to the amino acid residues forming a full or partial α-helical domain, the hinge domain, the β-triple spiral domain and a full or partial globular head domain of an avian reovirus sigma C protein, and lacking the amino acid sequence that is N-terminal to said α-helical domain is provided. Furthermore, a vaccine comprising, or a viral vector expressing, at least one of the isolated polypeptides of the present invention is provided.
Owner:GAVISH GALILEE BIO APPL
Methods and compositions for rapidly replacing cardiac myosin binding protein-c in sarcomeres
PendingUS20220119469A1Quick exchangeQuick changeAnimals/human peptidesMuscle proteinsCardiac myosinCardiac muscle
Methods and compositions for rapidly replacing cMyBP-C in sarcomeres featuring the creation of Spy-C mice, which are mice genetically engineered to express cMyBP-C with a protease recognition site and SpyTag peptide introduced into the cMyBP-C gene. In permeabilized myocytes from the Spy-C mice, the cMyBP-C protein can be cleaved at the protease recognition site, and the N-Terminus of cMyBP-C can be removed while the C-terminus remains anchored to the thick filament. A new peptide featuring the SpyCatcher sequence can be covalently bonded to the remaining portion of cMyBP-C, thereby creating a modified cMyBP-C protein. The methods and compositions of the present invention allow for the reconstitution of full-length cMyBP-C at the precise position of native cMyBP-C in the sarcomere and allow for a variety of modifications to be introduced to cMyBP-C in situ.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Method for preparing recombinant cystatin C
PendingCN114409800AImprove solubilityPromotes soluble expression efficiencyBacteriaMicroorganism based processesEscherichia coliEnterobacteriales
The invention discloses a method for preparing recombinant cystatin C. Specifically, gene engineering escherichia coli is adopted for recombinant fusion expression of Cys C protein, a specific tag sequence is fused at the N terminal of the expressed Cys C recombinant protein, so that soluble high-efficiency expression in an escherichia coli system is realized, and the protein is high in stability and has excellent immunological activity.
Owner:DAAN GENE CO LTD
Construction method of human embryonic stem cells
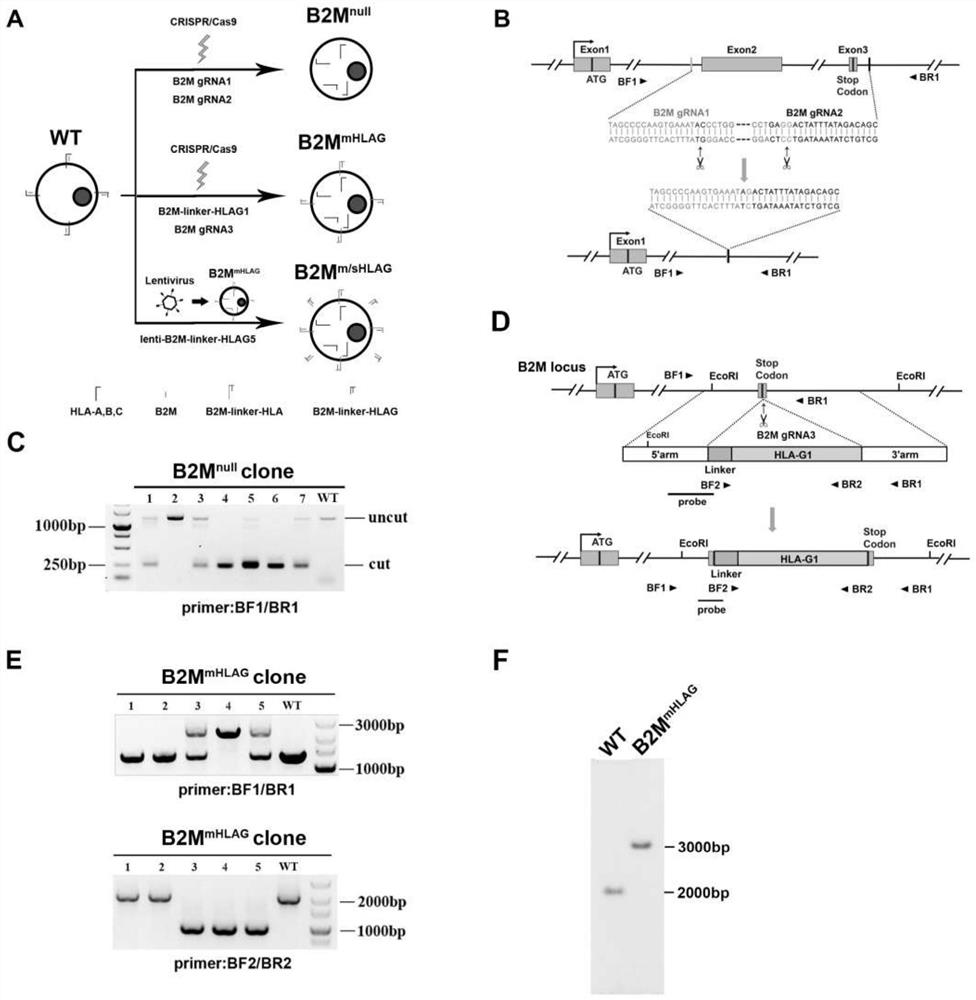
The invention relates to the technical field of biology, in particular to a construction method of human embryonic stem cells. The invention provides the construction method of the human embryonic stem cells, and the construction method comprises the following steps: exogenous polynucleotide for coding B2M-HLA-G1 fusion protein is integrated in a genome of the human embryonic stem cells, the B2M-HLA-G1 fusion protein comprises a first B2M fragment and an HLA-G1 fragment, and the human embryonic stem cells obtained by integration do not express free B2M protein. The endogenous HLA-A, B and C proteins of the human embryonic stem cell line constructed by the construction method of the human embryonic stem cells cannot reach the cell membrane surface, so that the cell line cannot be recognized by CD8 + T cells, the immunological rejection of allogeneic cells can be effectively prevented, and the pluripotency and proliferation ability of the embryonic stem cells are not influenced, and the cell can still be used as a target cell source for future cell transplantation.
Owner:TONGJI UNIV
ELISA kit for detecting porcine toxigenic pasteurella multocida toxin antibody and application
InactiveCN100523173CHigh biosecurityNo biohazardBacteriaMicroorganism based processesAntigenPasteurella multocida toxin
The invention belongs to the technical field of animal bacteriology and zoonotic disease detection, and in particular relates to an ELISA kit for detecting antibodies to toxin-producing Pasteurella multocida in pigs and its use in preventing and treating progressive atrophic rhinitis (progressive atrophic rhinitis, PAR). The kit includes a box body, a microtiter plate coated with the purified rPMT-C protein at the C' end of the toxA gene expressed as an antigen, negative and positive standard serum, rabbit anti-pig IgG enzyme-labeled secondary antibody, sample dilution Solution, substrate chromogenic solution A, substrate chromogenic solution B, stop solution and washing solution. Also disclosed is the preparation of a recombinant Escherichia coli BL21 / pET28b-C2115 (preservation number: CCTCC NO: M207012) expressing the rPMT-C protein at the C' end of the toxA gene of Pasteurella multocida toxA and the preparation of the rPMT-C protein expression and purification methods. The kit of the invention has the advantages of simple operation, fast and accurate diagnosis, low cost, etc., is suitable for large-scale clinical serological investigation of T+Pm, and can provide technical support for the purification of PAR in pigs.
Owner:武汉科前动物生物制品有限贵任公司
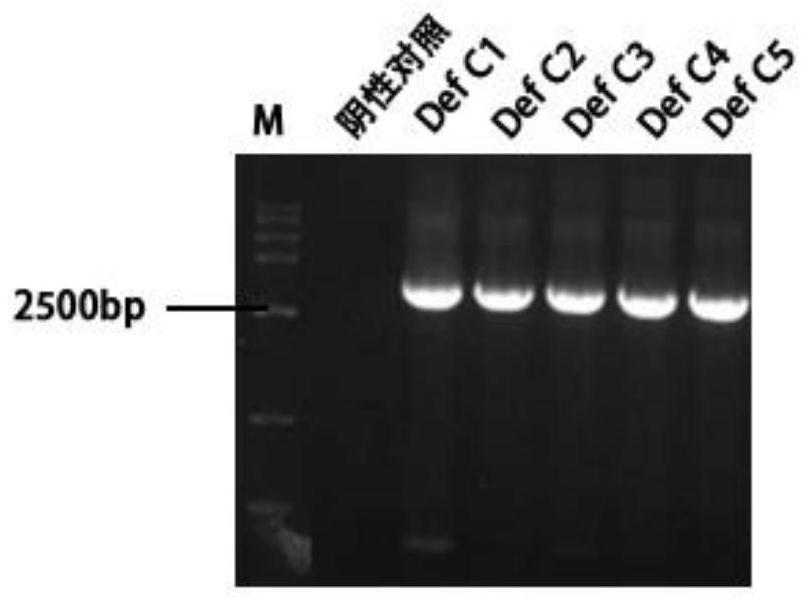
Application of Def C protein in resisting dengue virus and expression method of Def C protein
The invention provides an application of Def C protein in resisting dengue virus and an expression method of the Def C protein, and particularly provides an application of recombinant Def C protein of aedes albopictus or polypeptide thereof in preparation of drugs for resisting dengue virus. Further, the nucleotide sequence of the recombinant Def C protein is SEQ ID NO: 3, or obtained by substituting, deleting and / or adding one or more nucleotides to the nucleotide sequence as shown in SEQ ID NO: 3 for expressing protein with the same function. The amino acid sequence of the polypeptide is SEQ ID NO: 4. According to the invention, the new application of the Def C protein or the polypeptide thereof in preparation of drugs for resisting dengue virus is disclosed for the first time, thereby providing breakthrough for development of the anti-dengue-virus drug and vaccine preparation field, and having important application value and guiding significance for clinical treatment and related theoretical researches.
Owner:WENZHOU MEDICAL UNIV
A kind of recombinant human cystatin c protein with natural activity and its preparation method
ActiveCN103014047BImprove solubilitySolve the key problems of independent research and developmentMicroorganism based processesProtease inhibitorsAntiendomysial antibodiesGenetic engineering
The invention discloses a recombined human cystatin-C protein with natural activity and a preparation method thereof. According to the preparation method, lots of stable recombined human cystatin-C proteins are expressed and purified by employing a gene engineering technique, and the recombined human cystatin-C proteins are cut by utilizing the protease, so that the recombined human cystatin-C protein has high natural activity. The recombined human cystatin-C protein can be used for immune preparation of monoclonal antibodies and multi-antibody blood serum, and excellent raw materials are provided for preparing stable cystatin-C detection agents and quality control products thereof.
Owner:深圳前海菲鹏基因科技有限公司 +1
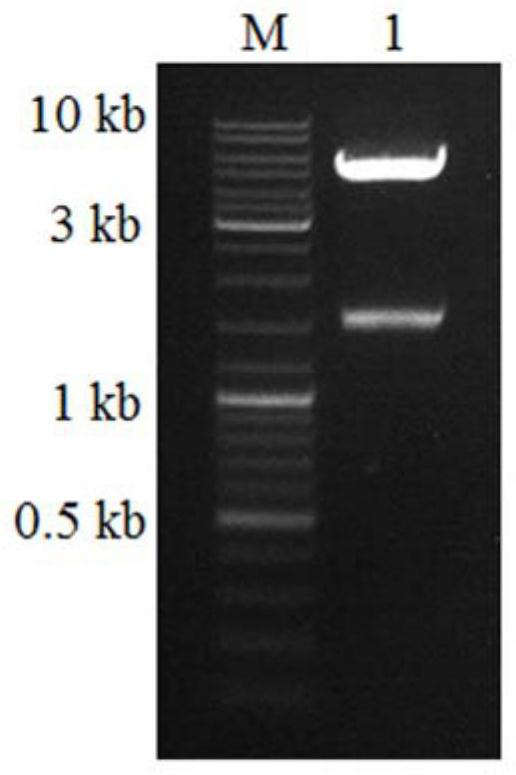
A kind of immunogenic cry1c recombinant protein, isolated nucleic acid molecule and application thereof
ActiveCN108314711BEfficient preparationEfficiently obtainedSerum immunoglobulinsImmunoglobulins against bacteriaImmunogenicityGenetic engineering
The invention provides an immunogenic cry1C recombinant protein, which relates to the technical field of genetic engineering, and its amino acid sequence is shown in SEQ ID No.1. The amino acid sequence encoding the cry1C recombinant protein is shorter than the amino acid sequence of the full-length cry1C protein, and the cry1C recombinant protein can immunoreact with the cry1C antibody, and can be used to rapidly and efficiently prepare the cry1C antibody. The present invention also provides a nucleic acid encoding the above immunogenic cry1C recombinant protein, a pair of primers capable of amplifying or detecting the nucleic acid molecule, a vector and a host cell including the nucleic acid molecule, and in addition, the present invention also provides a cry1C Antibody and preparation method thereof, the preparation method can obtain cry1C antibody rapidly and efficiently.
Owner:JILIN ACAD OF AGRI SCI
Method for recombinant production of horseshoe crab factor c protein in protozoa
The present invention provides a novel method for the recombinant production of Factor C protein from horseshoe crab using a parasitic protozoan expressing the Factor C protein. In particular, the present invention provides a parasitic protozoan host cell harbouring a polynucleotide encoding horseshoe crab Factor C protein, and a method for producing Factor C protein comprising culturing said parasitic protozoan host cell under conditions such that the cells express the horseshoe crab Factor C protein. Furthermore, the present invention provides recombinant Factor C protein produced by the novel method and its use in the detection and / or removal of endotoxin.
Owner:BIOMERIEUX DEUT GMBH
The beauveria bassiana analogue is applied as micromolecular agonist of APC/C
ActiveCN112569341AWide variety of sourcesEasy to expand and cultureCyclic peptide ingredientsAntineoplastic agentsDiseaseAgonist
The invention discloses an application of a beauveria bassiana analogue as a small molecular agonist of APC / C. The invention discloses the function of activating APC / C of the beauveria bassiana analogue with the natural source as shown in the formula I for the first time, and the beauveria bassiana analogue is a powerful agonist of a late-stage promoter compound (APC / C-Cdh1) or a late-stage promoter compound (APC / C-Cdc20). As a small molecular agonist of APC / C, the compound has important application value in the research fields of cell physiology change caused by APC / C protein activity disorder, targeted activation of APC / C to control cell proliferation disorder diseases and the like.
Owner:FUZHOU UNIV
Colorectal cancer diagnostic markers and colorectal cancer detection products and their applications
ActiveCN109557311BHigh sensitivityStrong specificityDisease diagnosisBiological testingProtein markersAids diagnostics
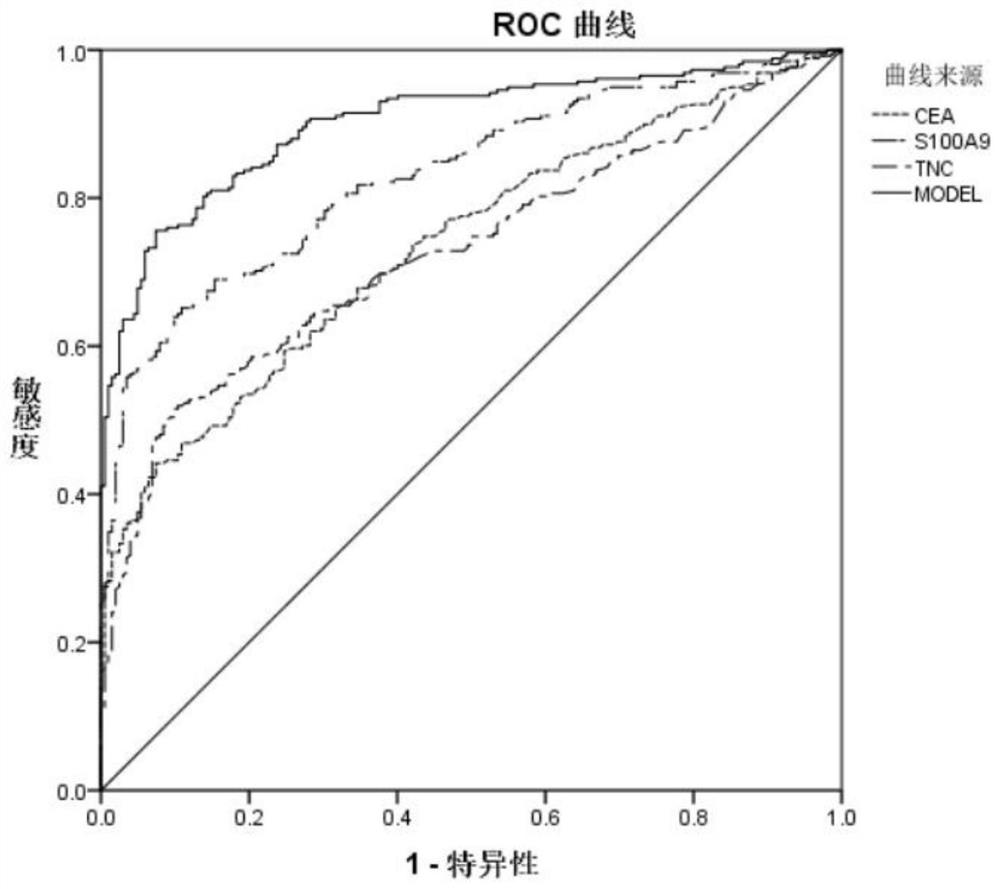
The invention relates to a colorectal cancer diagnostic marker, a colorectal cancer detection product and an application thereof. The colorectal cancer diagnostic marker includes multiple secreted proteins, and the multiple secreted proteins include one or two of S100A9 protein and tenascin-c protein, and CEA protein. With the characteristics of high sensitivity and strong specificity, the area under the ROC curve of the diagnostic model is 0.902, and the reliability of the diagnostic result is much higher than that of a single protein marker, which is of great significance for the diagnosis and treatment of colorectal cancer. The colorectal cancer detection product includes the above-mentioned colorectal diagnostic markers. The present invention also proposes the application of colorectal diagnostic markers in the preparation of products for auxiliary diagnosis of colorectal cancer.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com