Patents
Literature
57 results about "Dna immunization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Expression of HIV polypeptides and production of virus-like particles
InactiveUS6602705B1Sufficient amountImprove efficiencyOrganic active ingredientsSsRNA viruses positive-senseProteinDna immunization
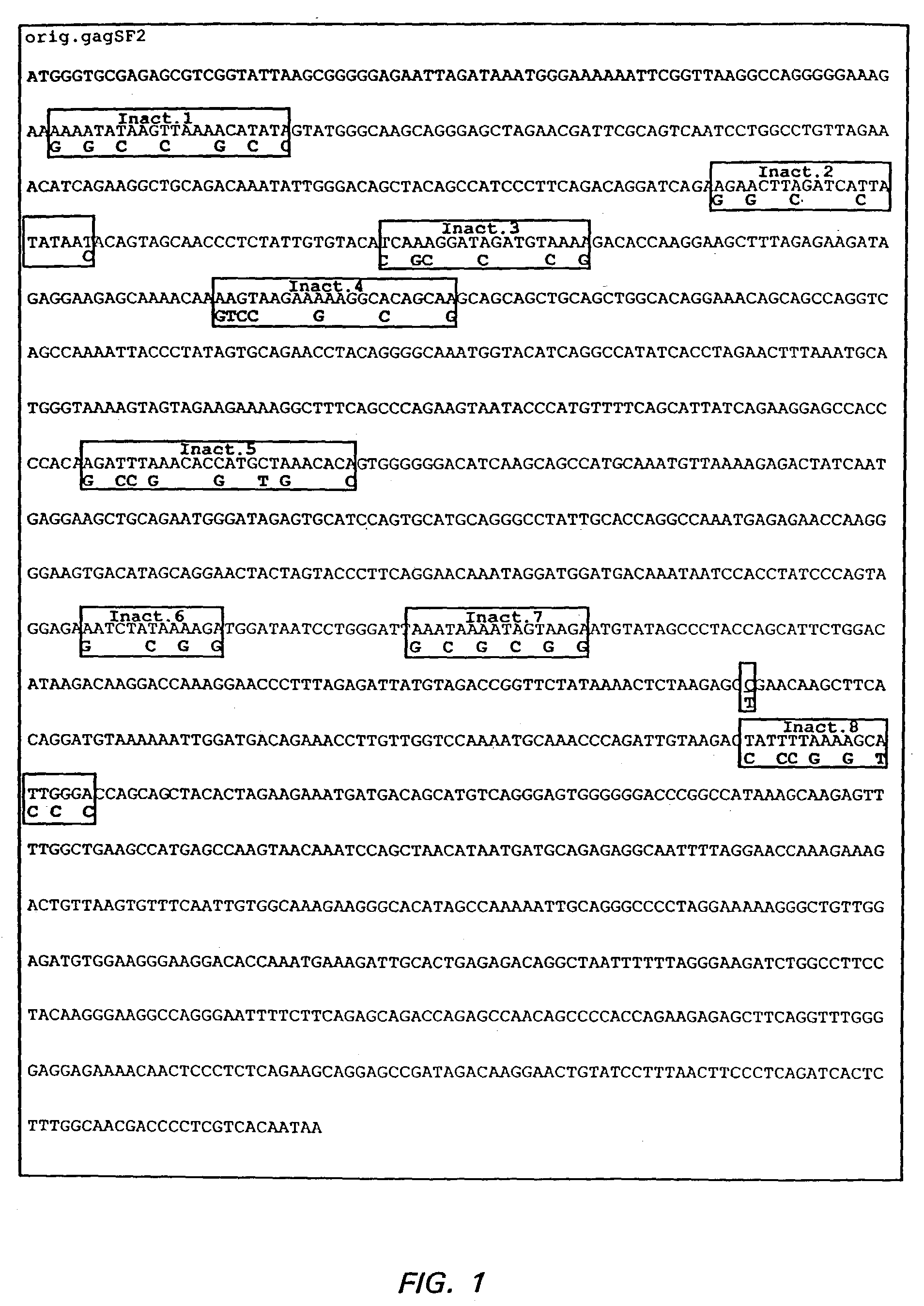
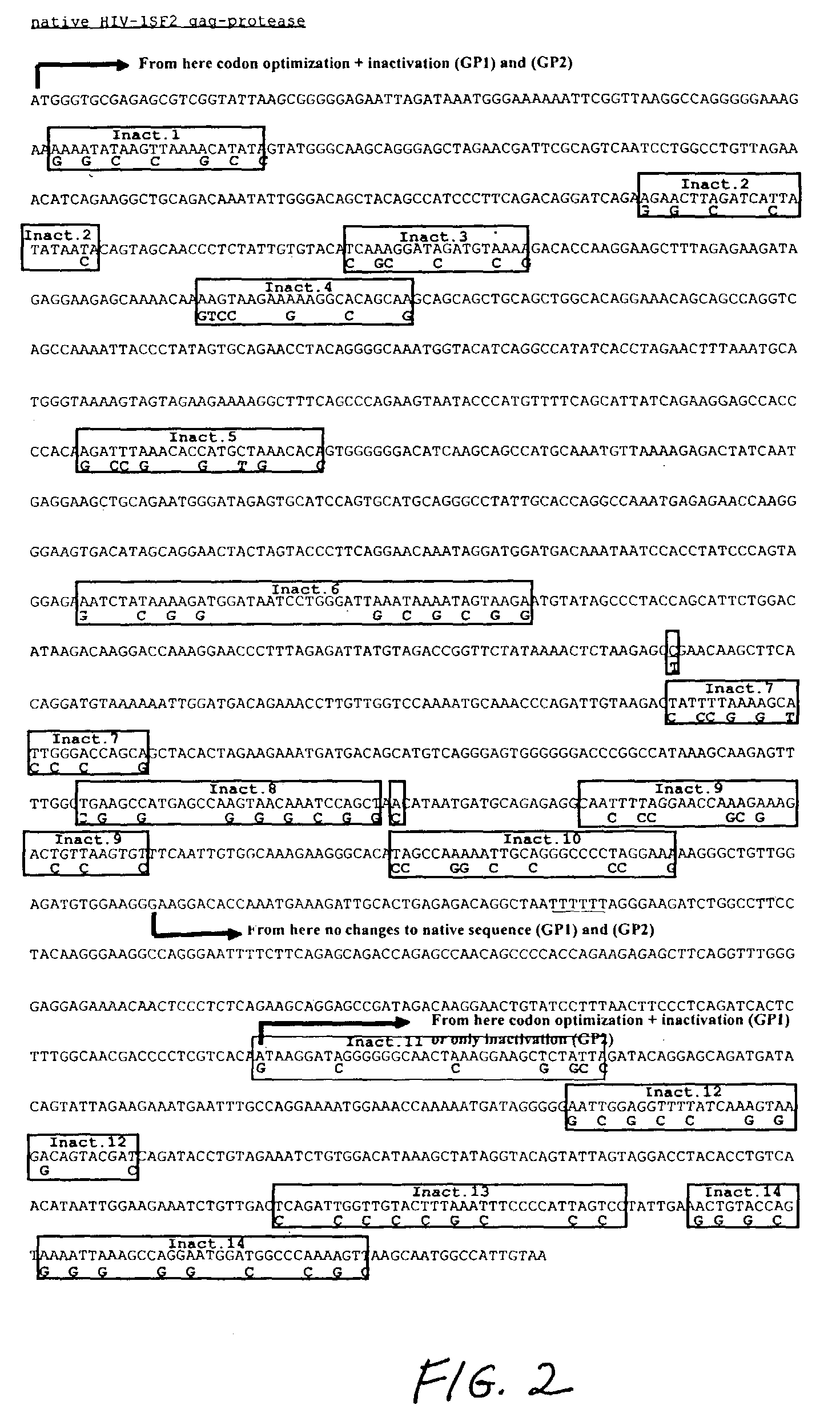
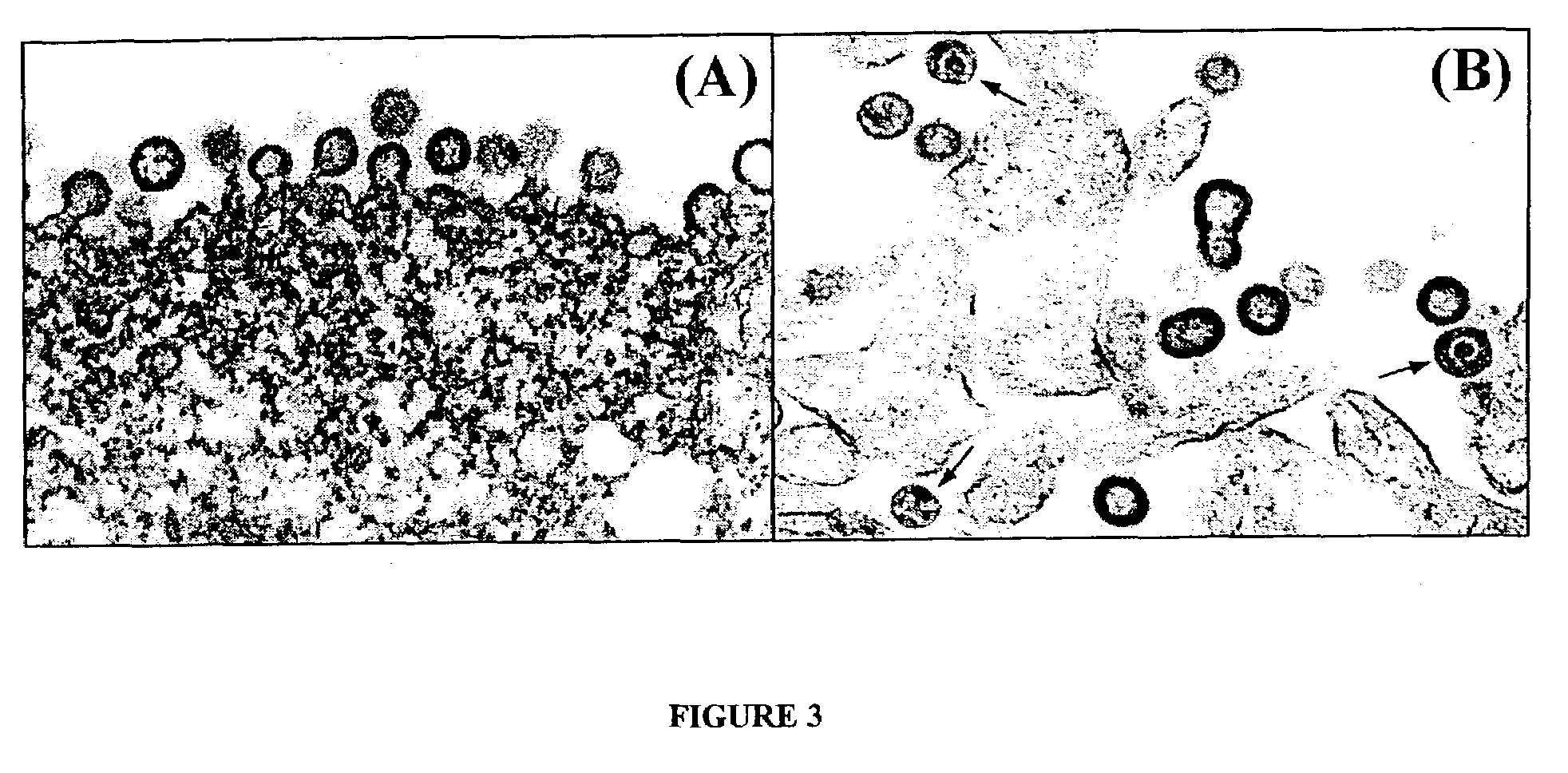
The present invention relates to the efficient expression of HIV polypeptides in a variety of cell types, including, but not limited to, mammalian, insect, and plant cells. Synthetic expression cassettes encoding the HIV Gag-containing polypeptides are described, as are uses of the expression cassettes in applications including DNA immunization, generation of packaging cell lines, and production of Env-, tat- or Gag-containing proteins. The invention provides methods of producing Virus-Like Particles (VLPs), as well as, uses of the VLPs including, but not limited to, vehicles for the presentation of antigens and stimulation of immune response in subjects to whom the VLPs are administered.
Owner:CHIRON CORP
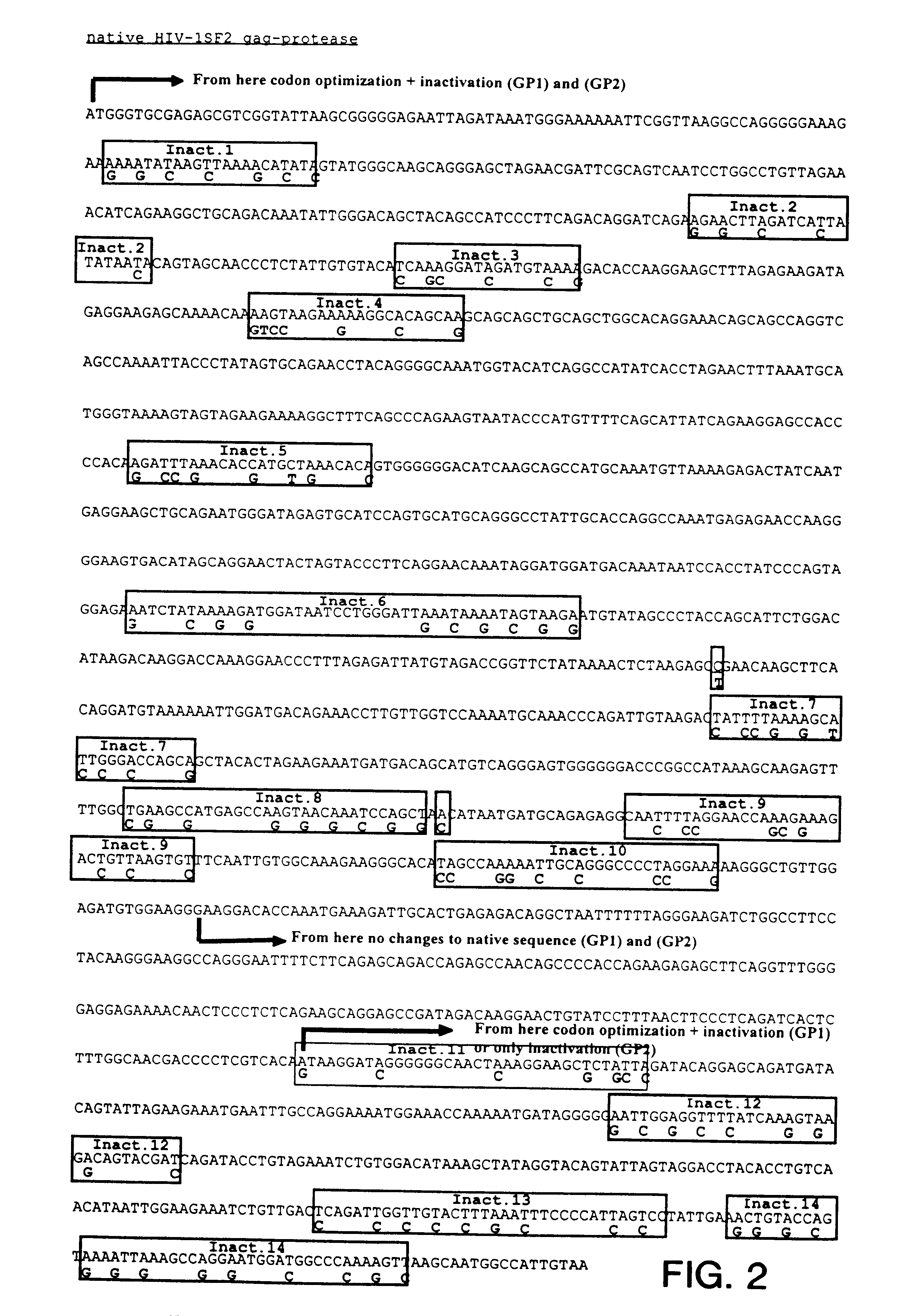
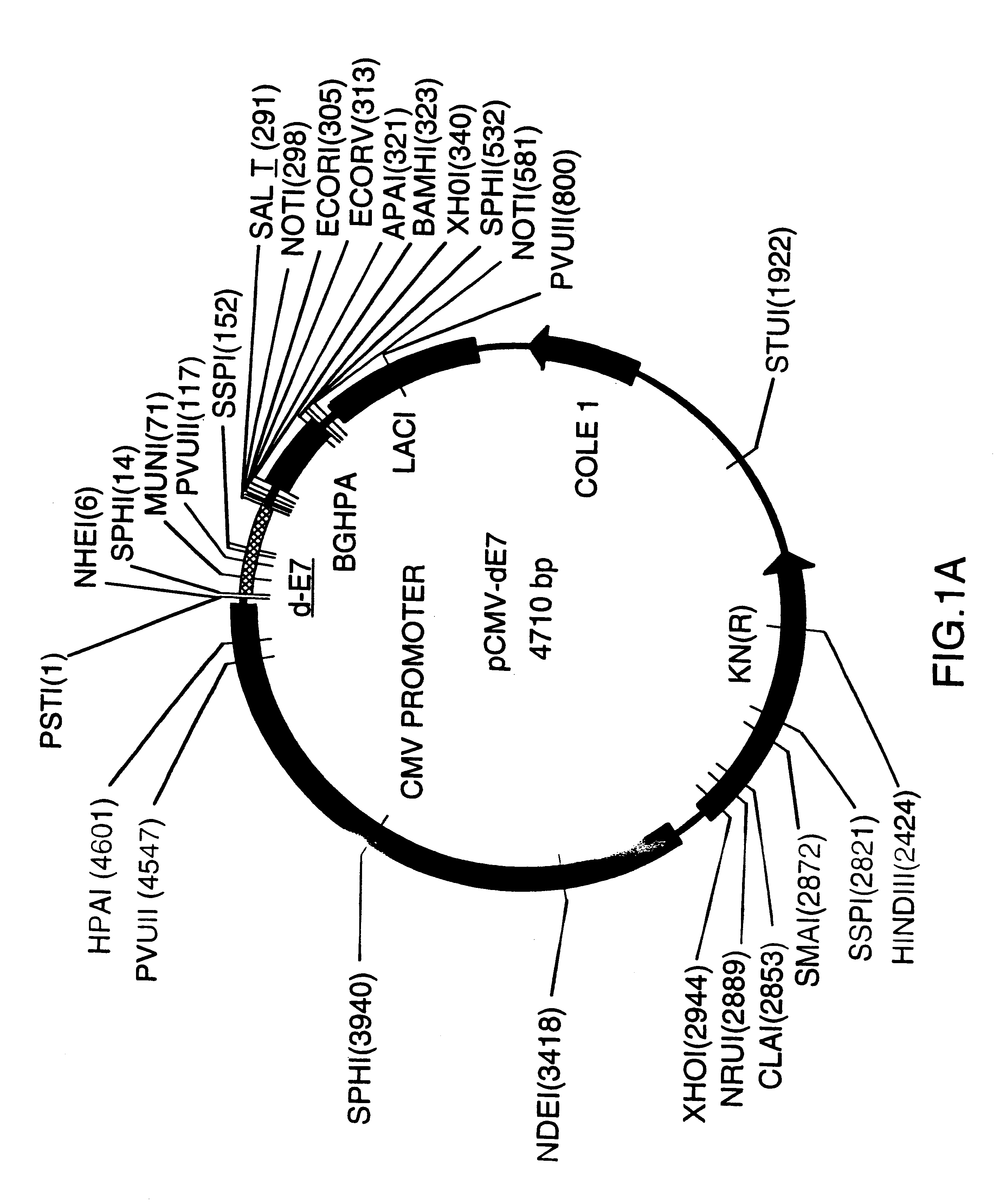
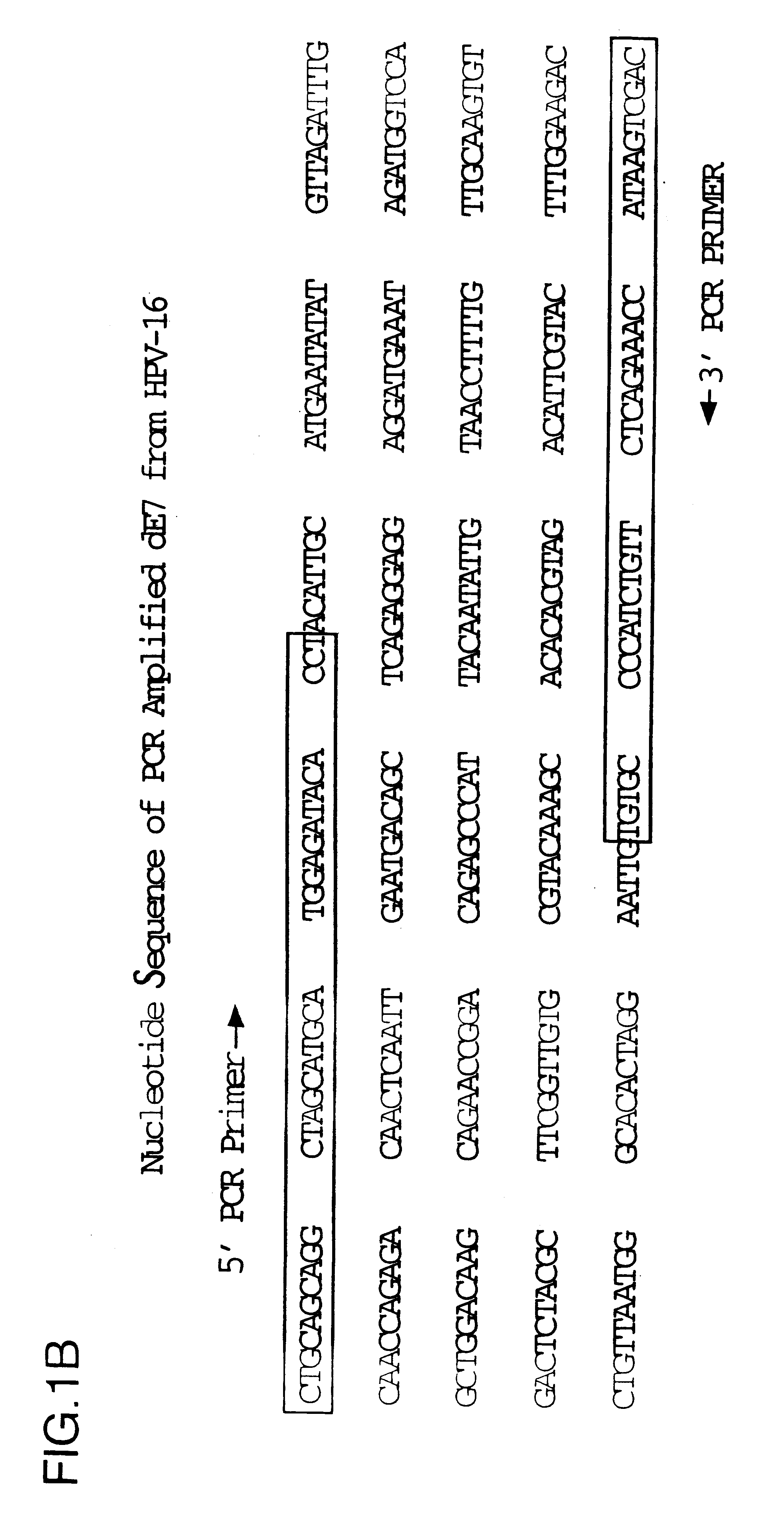
Vectors for DNA immunization against cervical cancer
InactiveUS6235523B1Improving immunogenicitySlow and sustained releaseOrganic active ingredientsVirusesAntigenDna immunization
Vectors for DNA immunization against cervical cancer comprise a nucleic acid molecule encoding at least one non-toxic T-cell epitope of the E6 and / or E7 antigens of a strain of human papilloma virus (HPV) associated with cervical cancer, such as HPV-16, and a promoter operatively coupled to the nucleic acid molecule for expression of the nucleic acid molecule in a host to which the vector is administered.
Owner:CONNAUGHT LAB
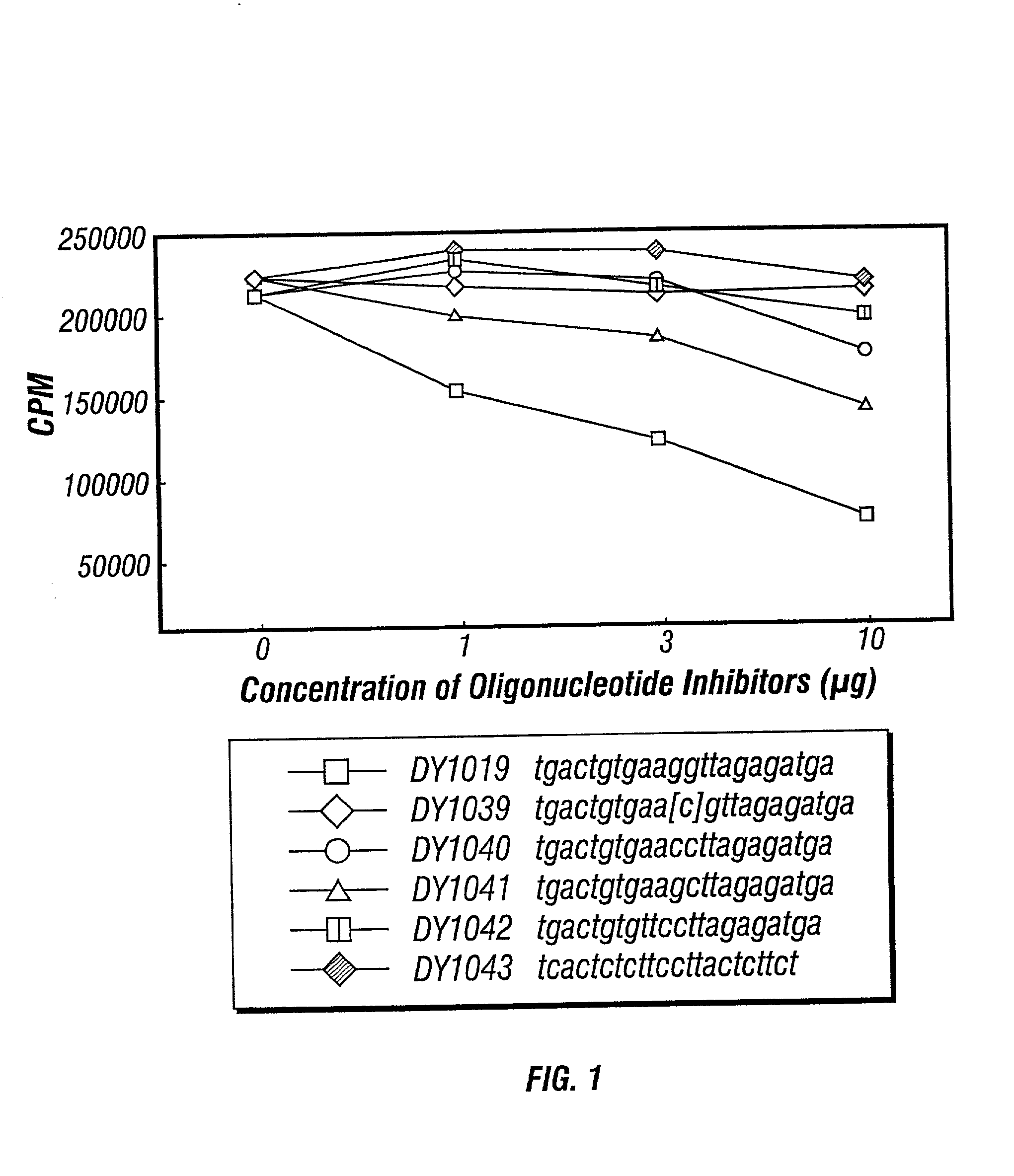
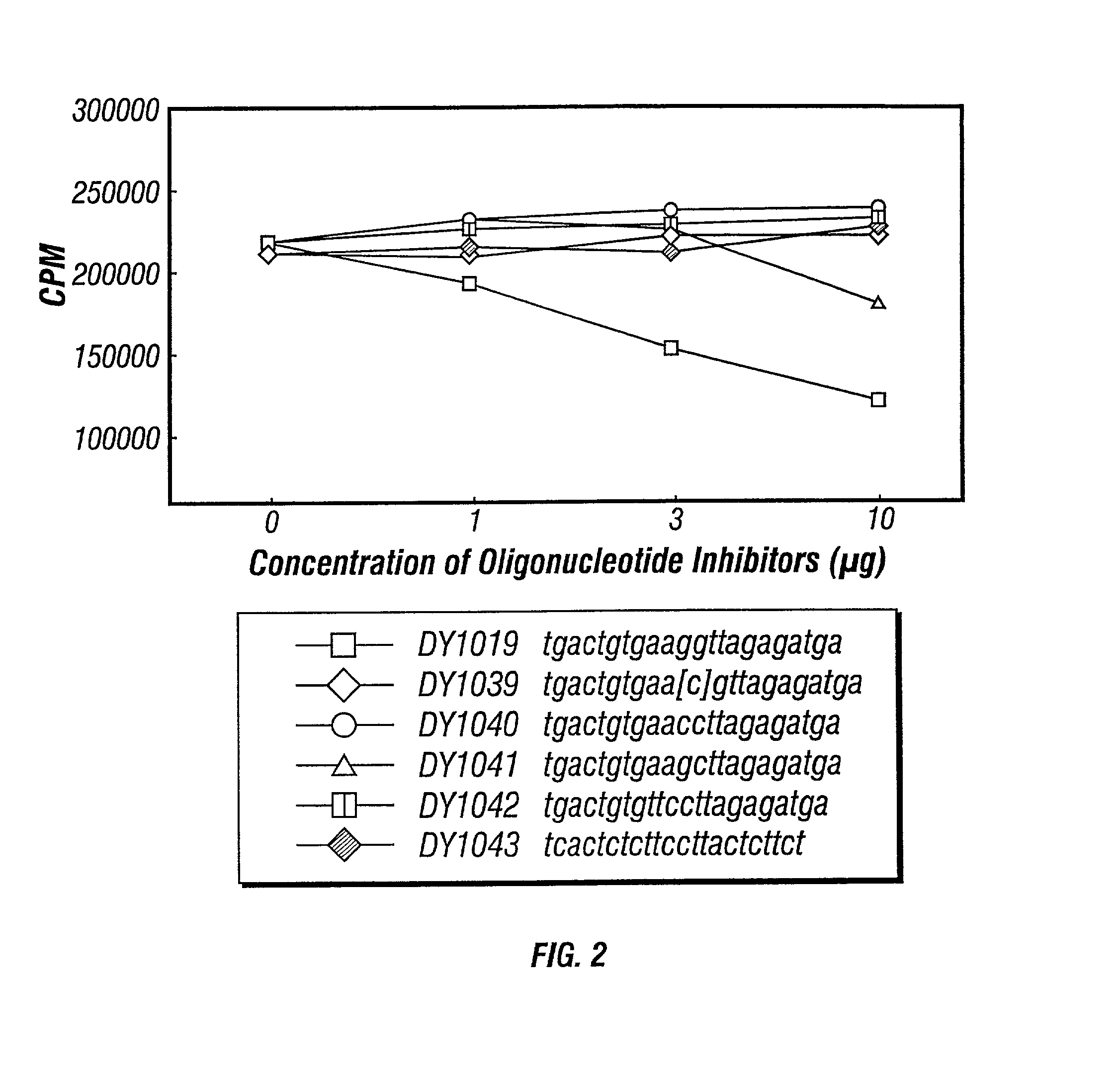
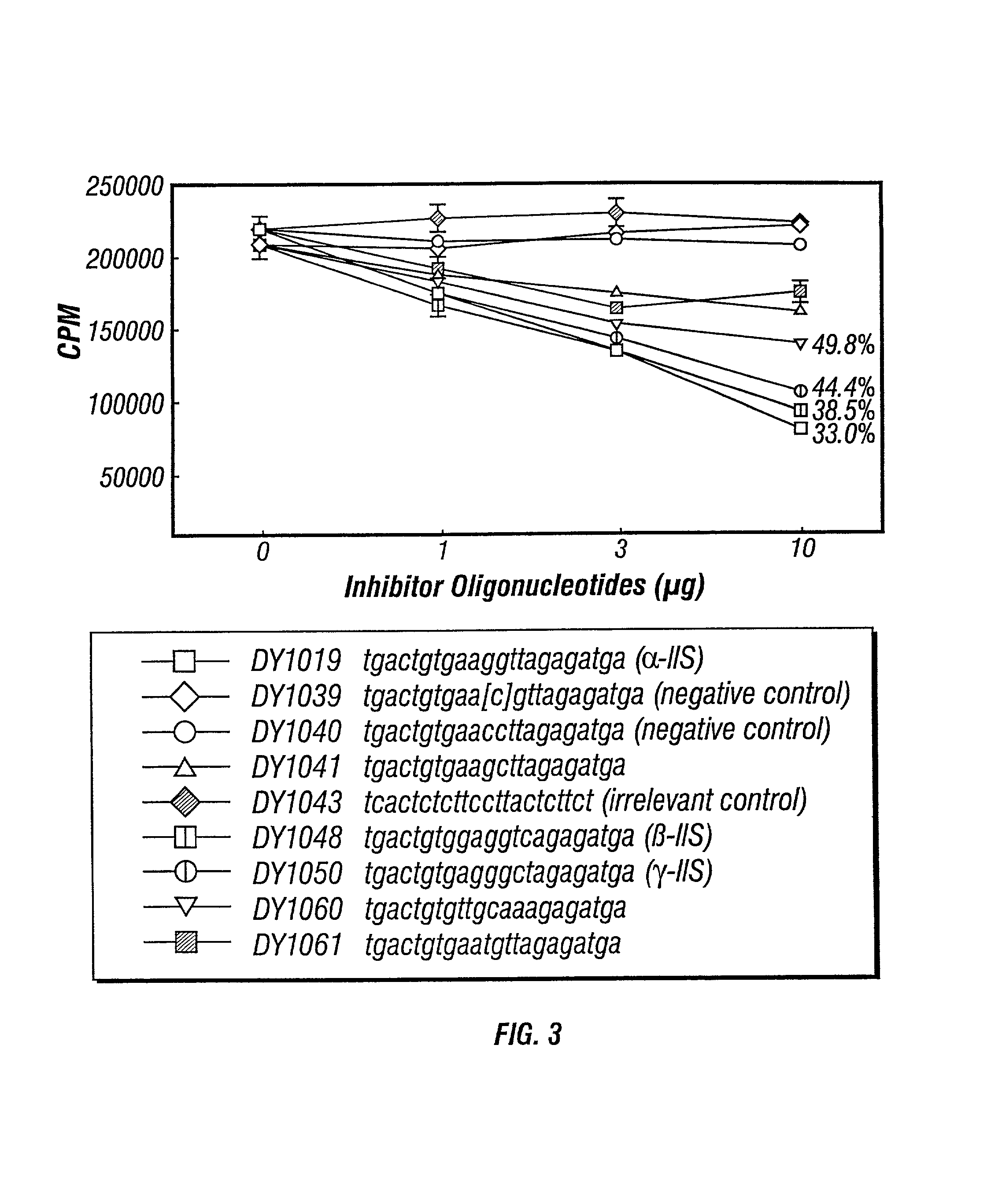
Inhibitors of DNA immunostimulatory sequence activity
InactiveUS20020086839A1Modulating immunostimulatory activityImprove responseOrganic active ingredientsPeptide/protein ingredientsAntigenDisease
The invention consists of oligonucleotides which inhibit the immunostimulatory activity of ISS-ODN (immunostimulatory sequence oligodeoxynucleotides) as well as methods for their identification and use. The oligonucleotides of the invention are useful in controlling therapeutically intended ISS-ODN adjuvant activity as well as undesired ISS-ODN activity exerted by recombinant expression vectors, such as those used for gene therapy and gene immunization. The oligonucleotides of the invention also have anti-inflammatory activity useful in reducing inflammation in response to infection of a host with ISS-ODN containing microbes, in controlling autoimmune disease and in boosting host Th2 type immune responses to an antigen. The invention also encompasses pharmaecutically useful conjugates of the oligonucleotides of the invention (including conjugate partners such as antigens and antibodies).
Owner:DYNAVAX TECH CORP +1
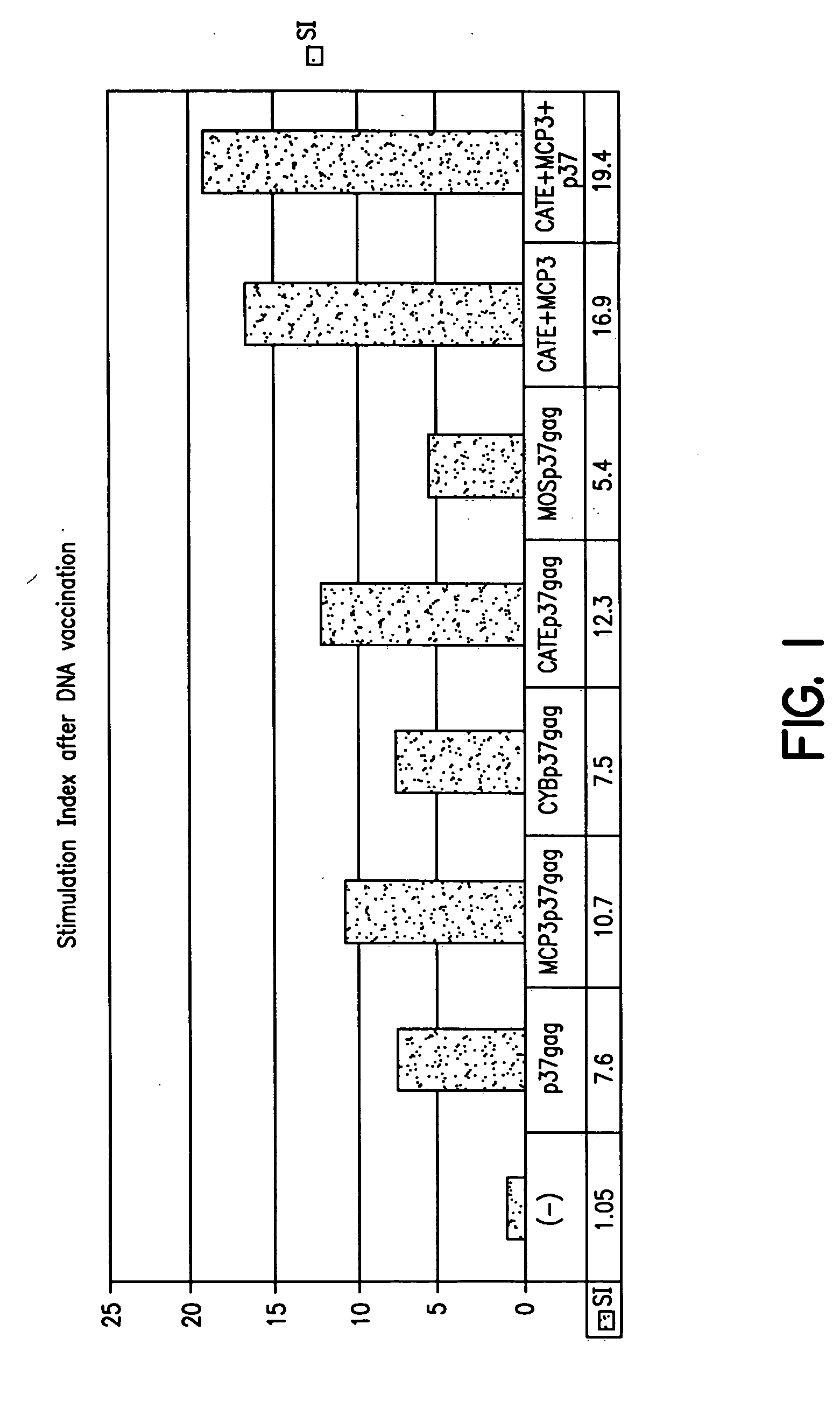
Expression vectors able to elicit improved immune response and methods of using same
The invention relates to nucleic acids (such as DNA immunization plasmids), encoding fusion proteins containing a destabilizing amino acid sequence attached to an amino acid sequence of interest, in which the immunogenicity of the amino acid sequence of interest is increased by the presence of the destabilizing amino acid sequence. The invention also relates to nucleic acids encoding secreted fusion proteins, such as those containing chemokines or cytokines, and an attached amino acid sequence of interest, in which the immunogenicity of the amino acid sequence of interest is increased as a result of being attached to the secretory sequence. The invention also relates methods of increasing the immunogenicity of the encoded proteins for use as vaccines or in gene therapy.
Owner:UNITED STATES OF AMERICA
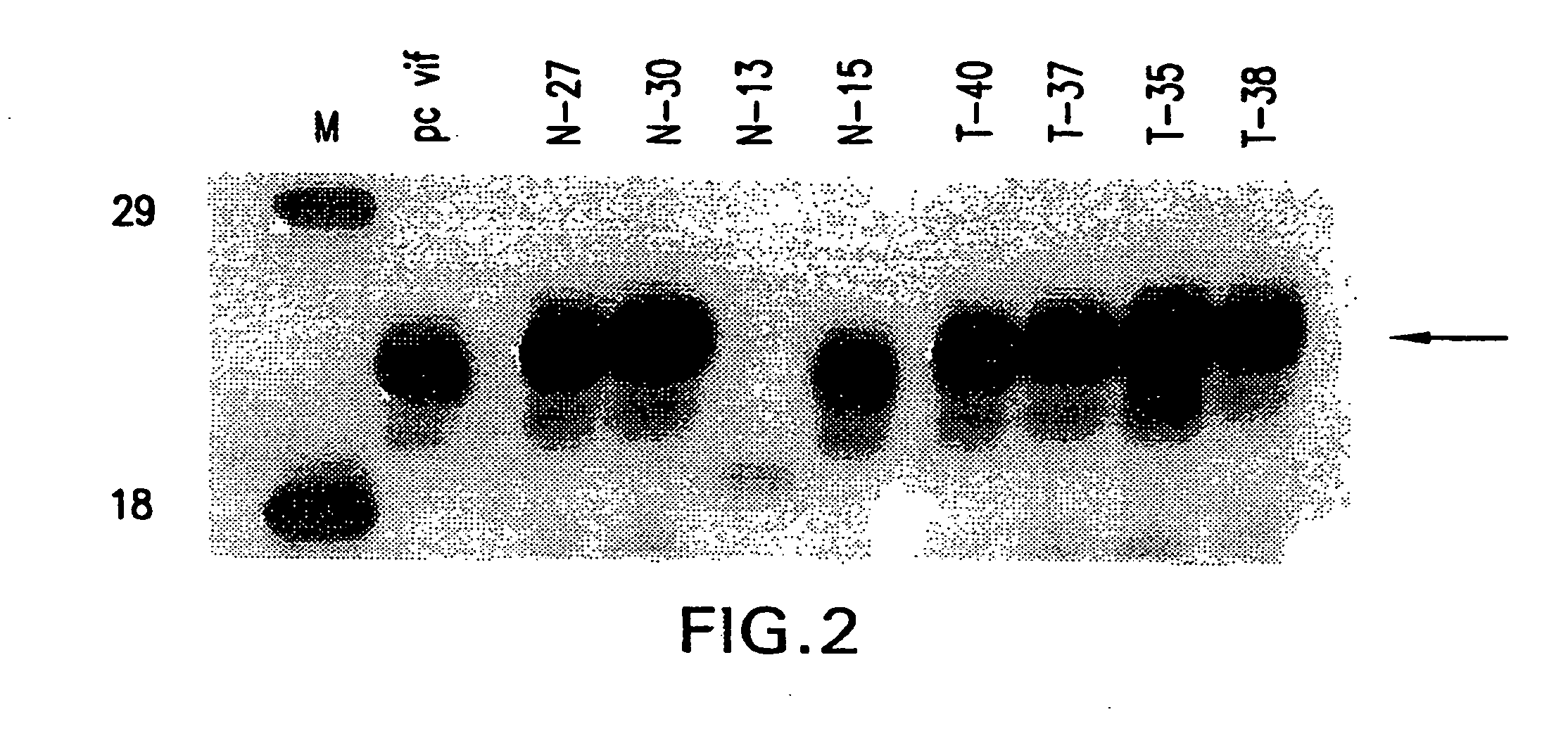
Attenuated vif DNA immunization cassettes for genetic vaccines
The present invention is directed to nucleic acid molecules encoding attenuated, non-functional virion infectivity factor (vif) proteins. The nucleic acid molecules of the invention are inserted into recombinant expression vectors and administered to mammals in order to induce a cellular and humoral immune response to the encoded protein product.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
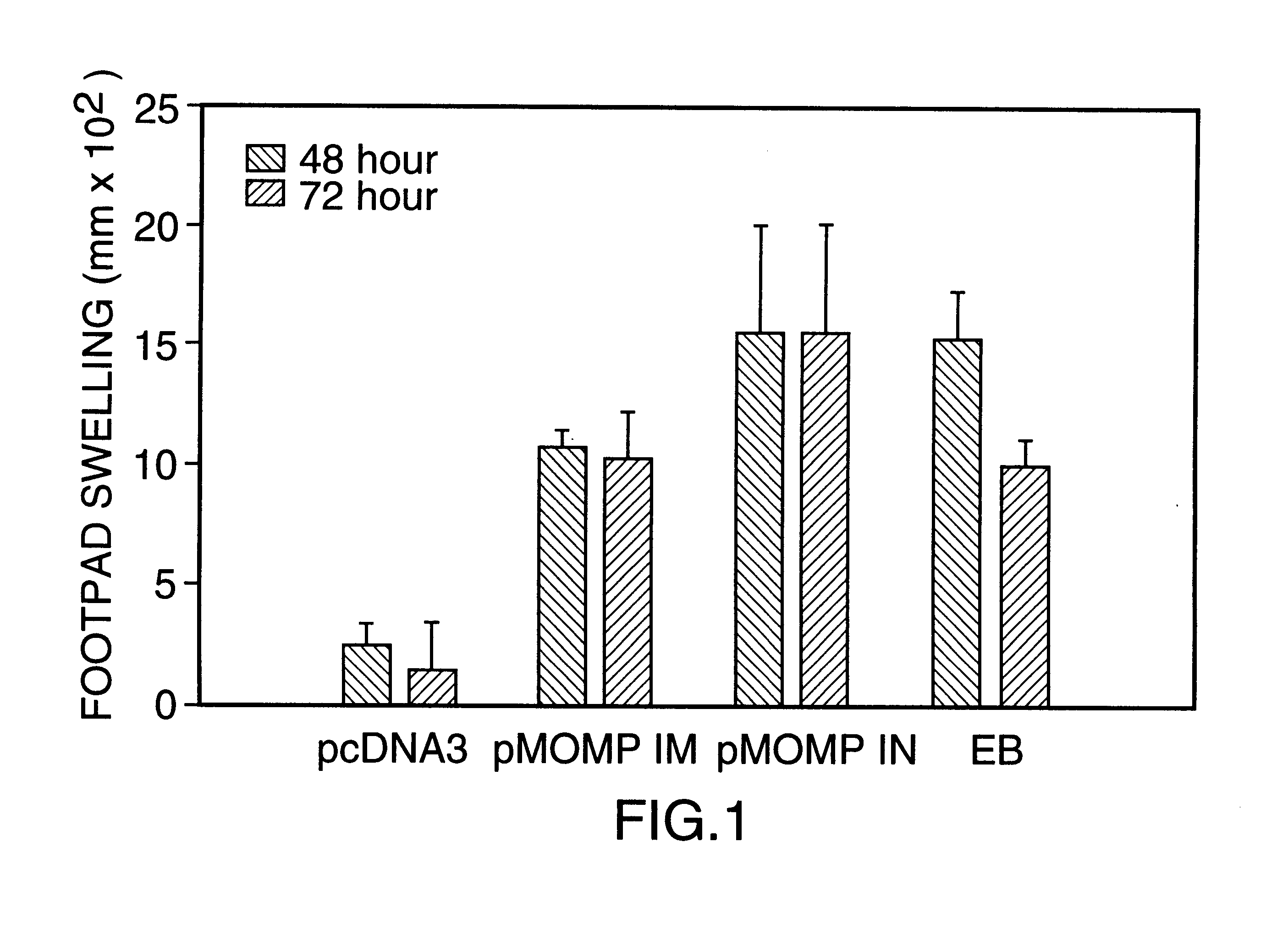
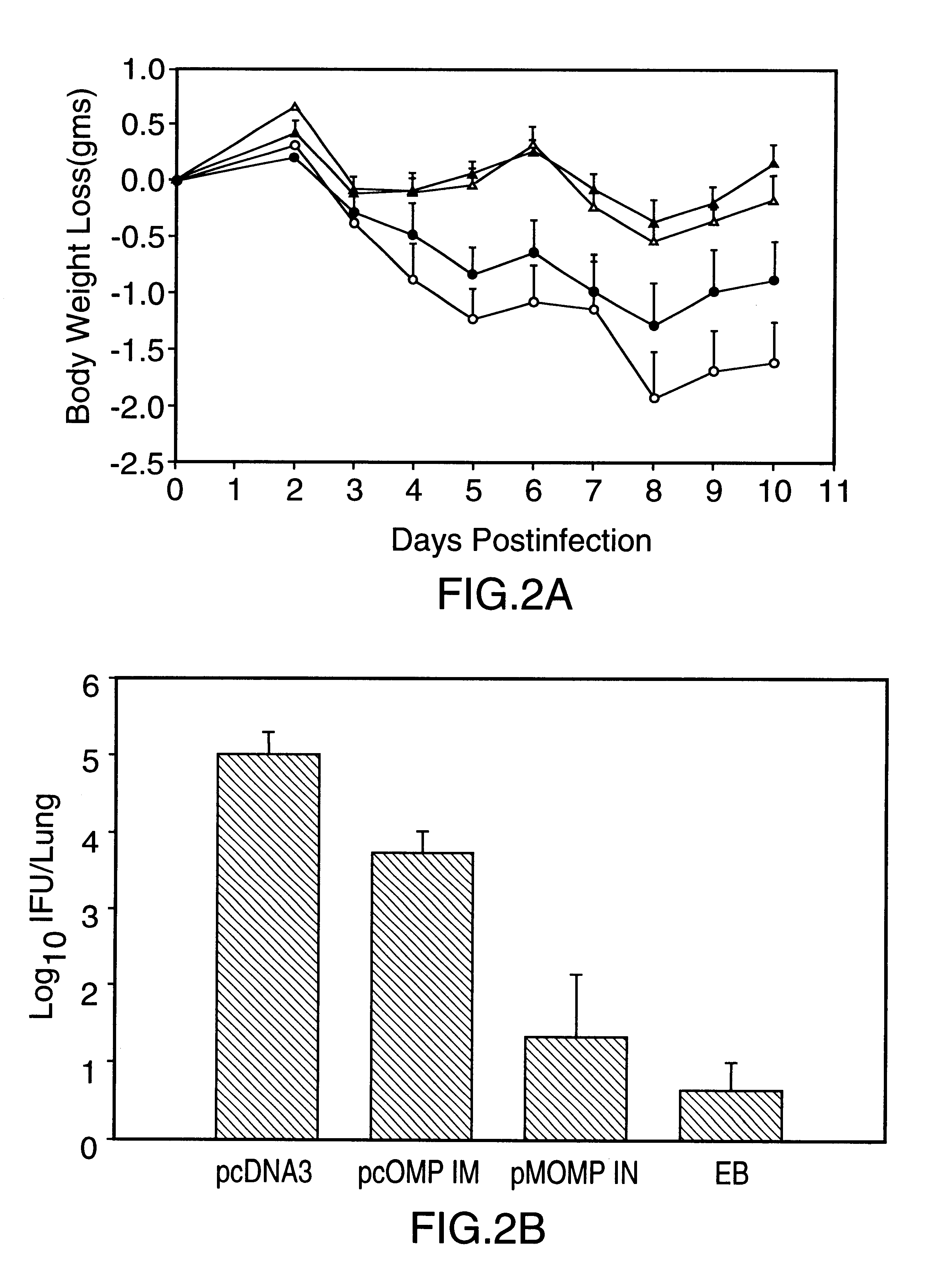
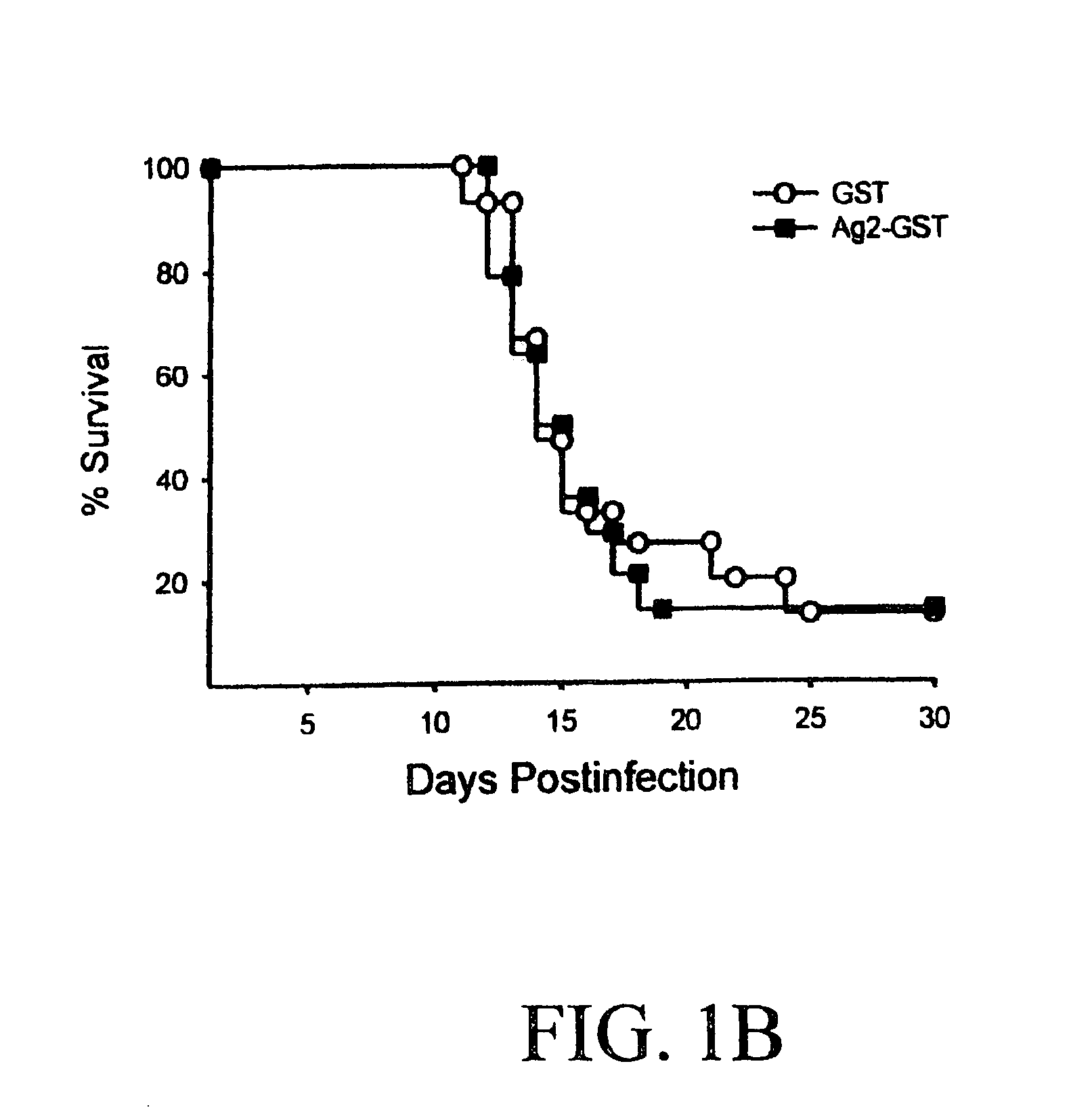
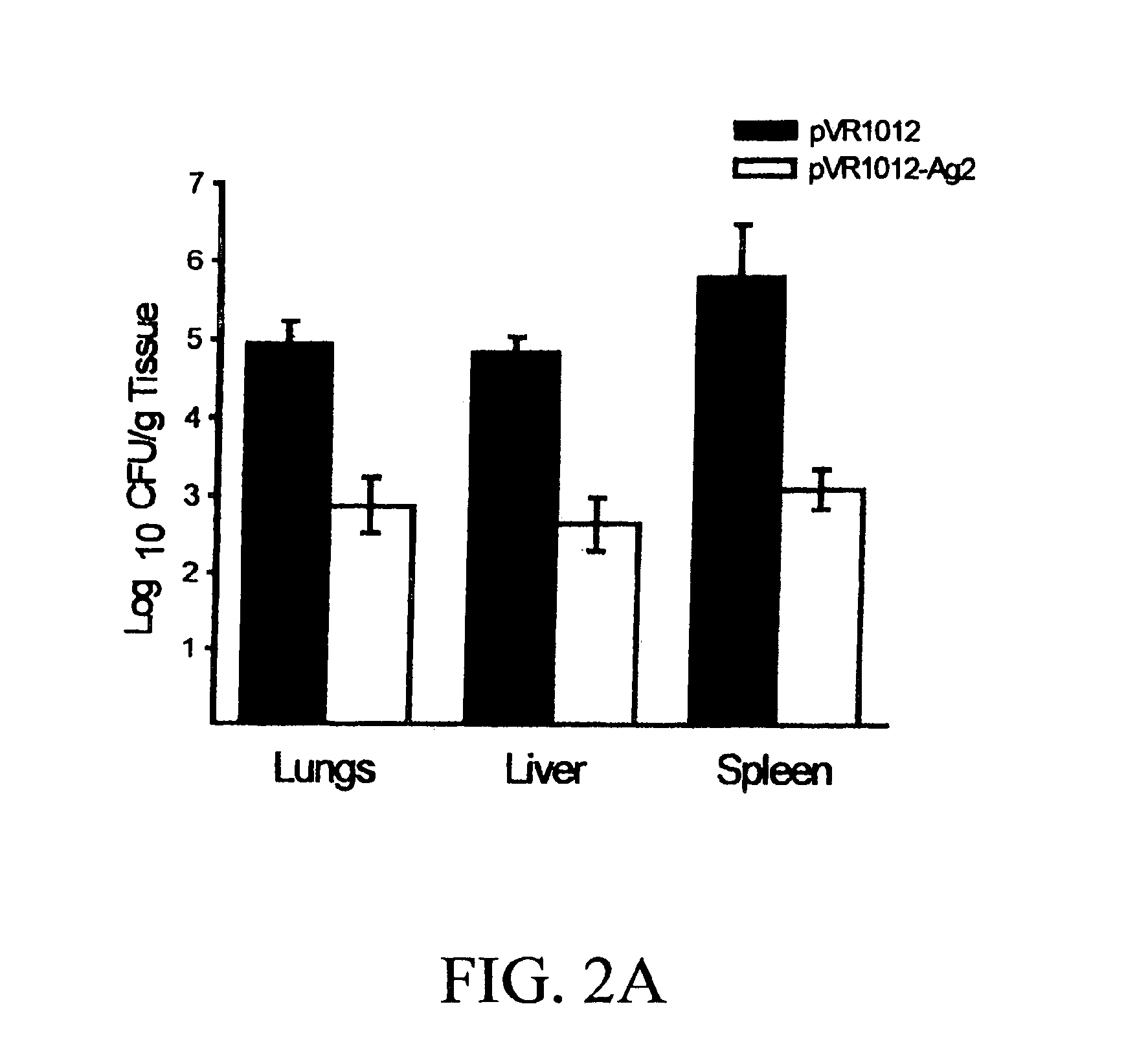
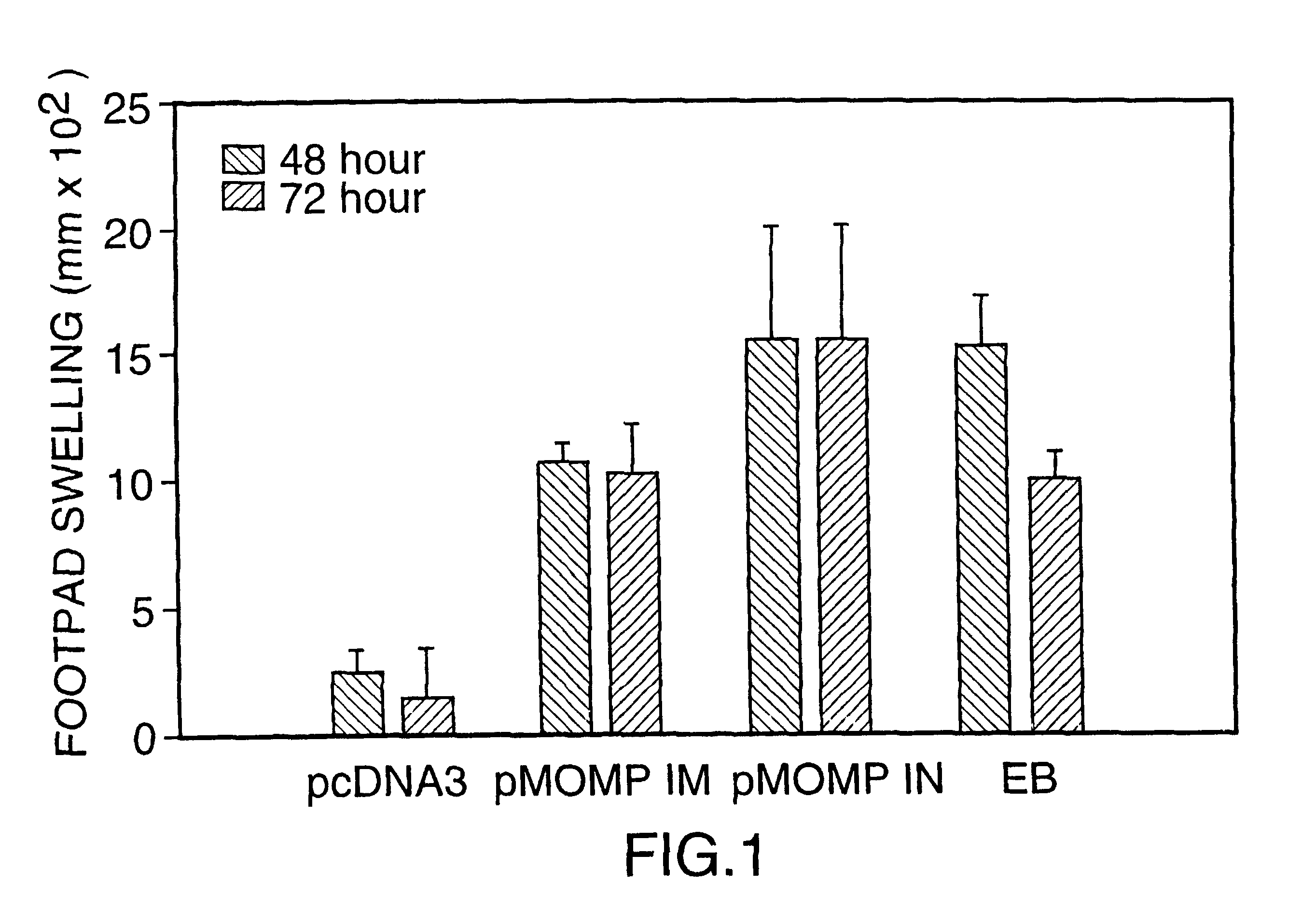
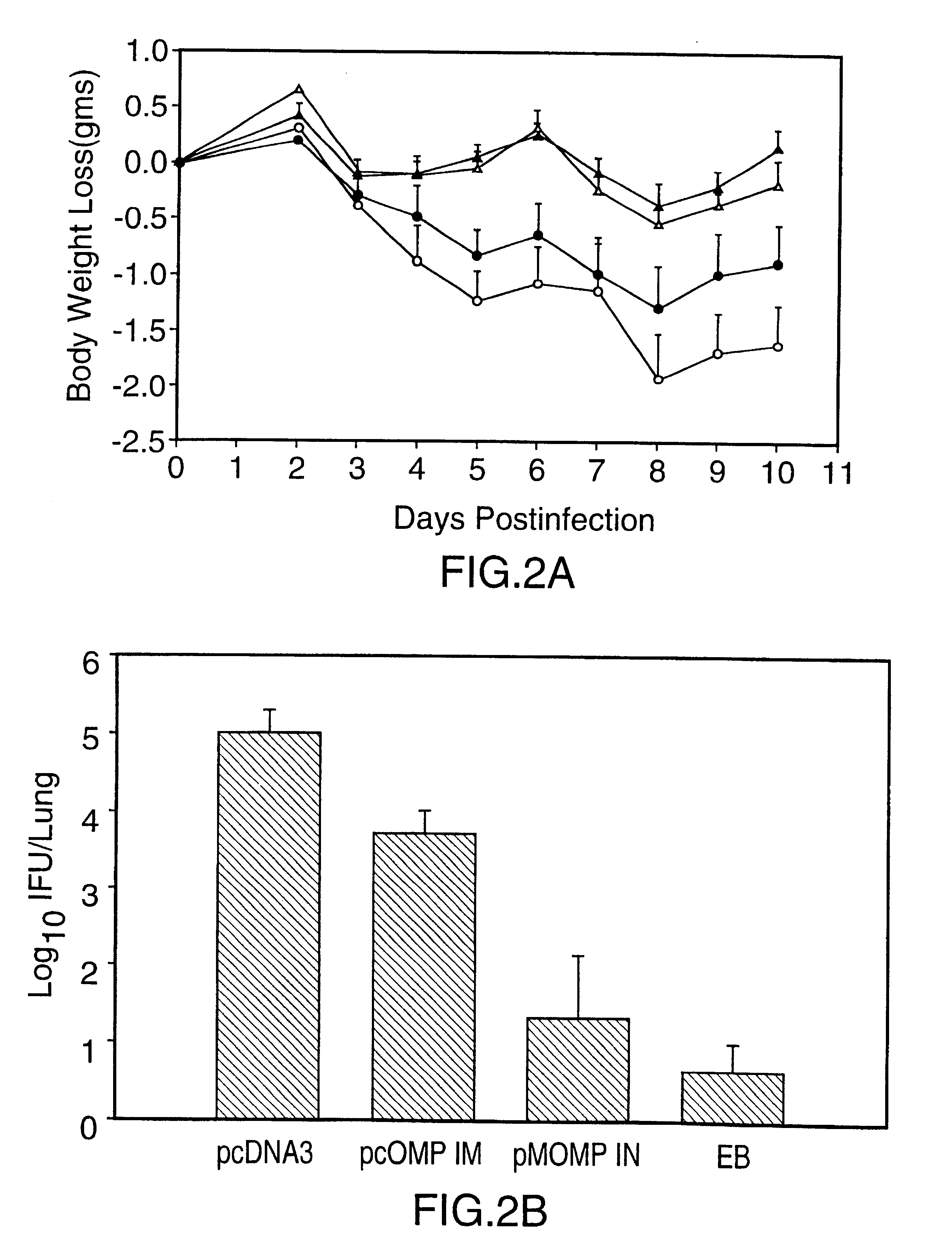
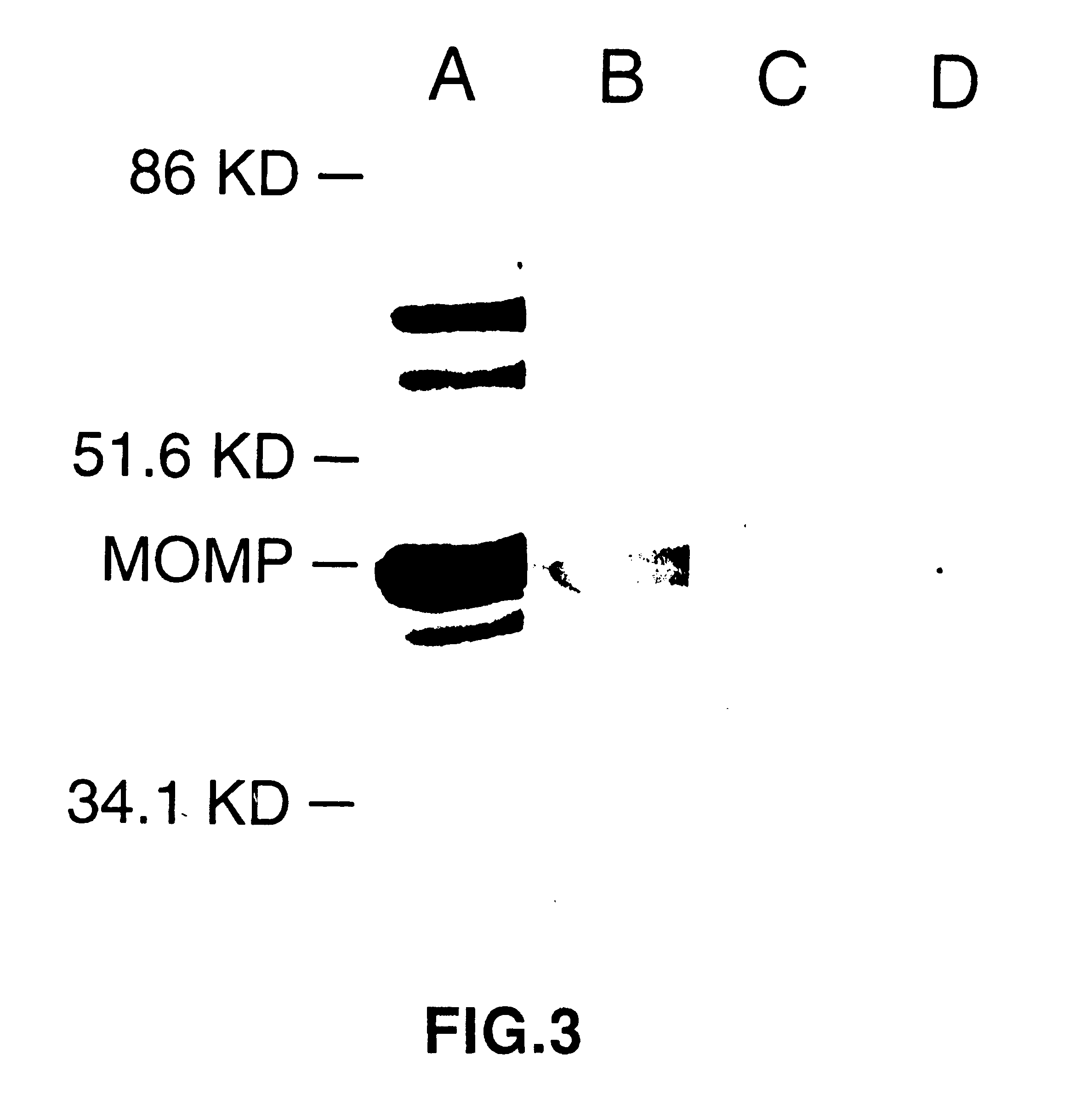
DNA immunization against chlaymdia infection
InactiveUS6235290B1Effectively induces protective immunityGood curative effectAntibacterial agentsVirusesDna immunizationIn vivo
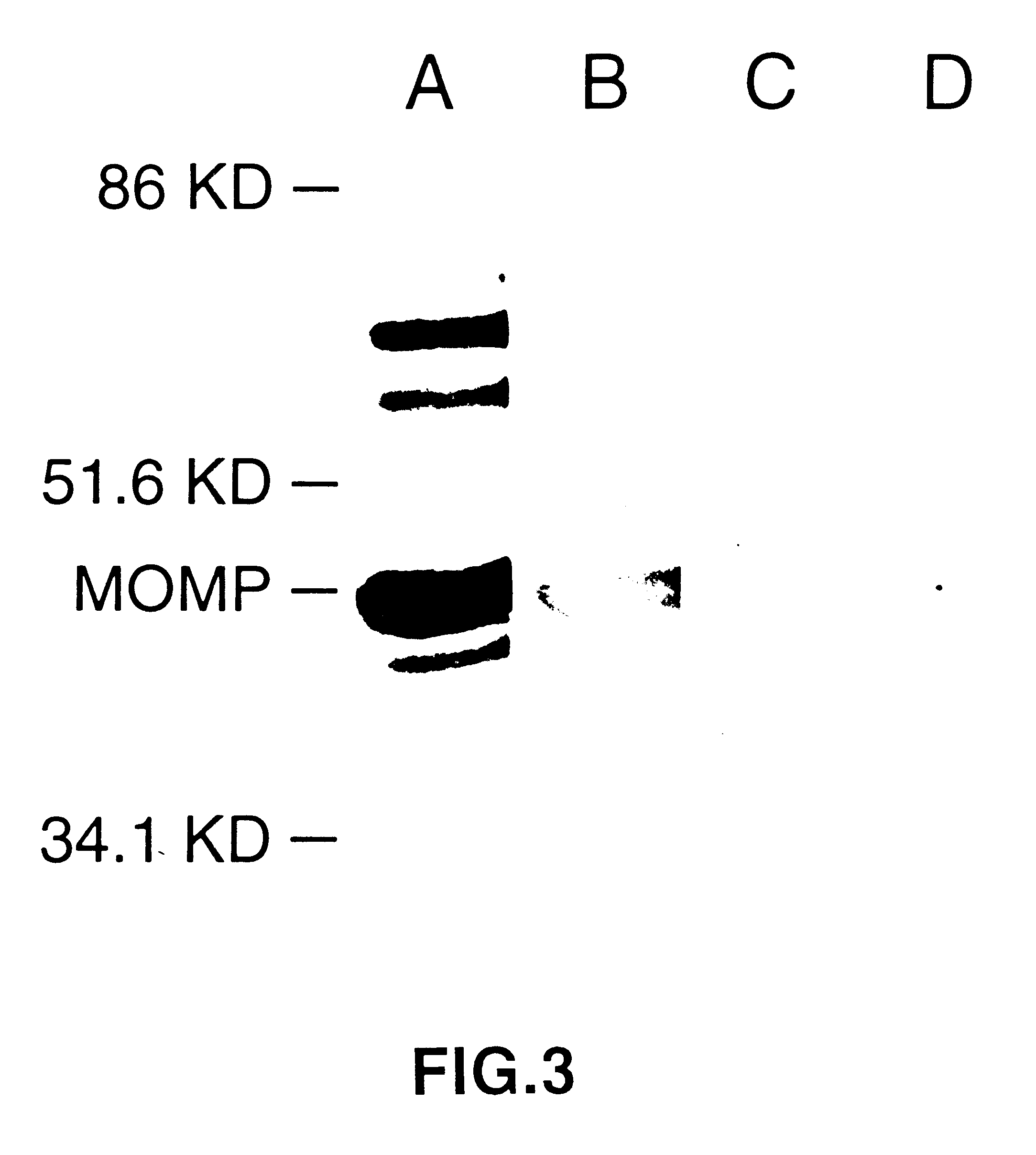
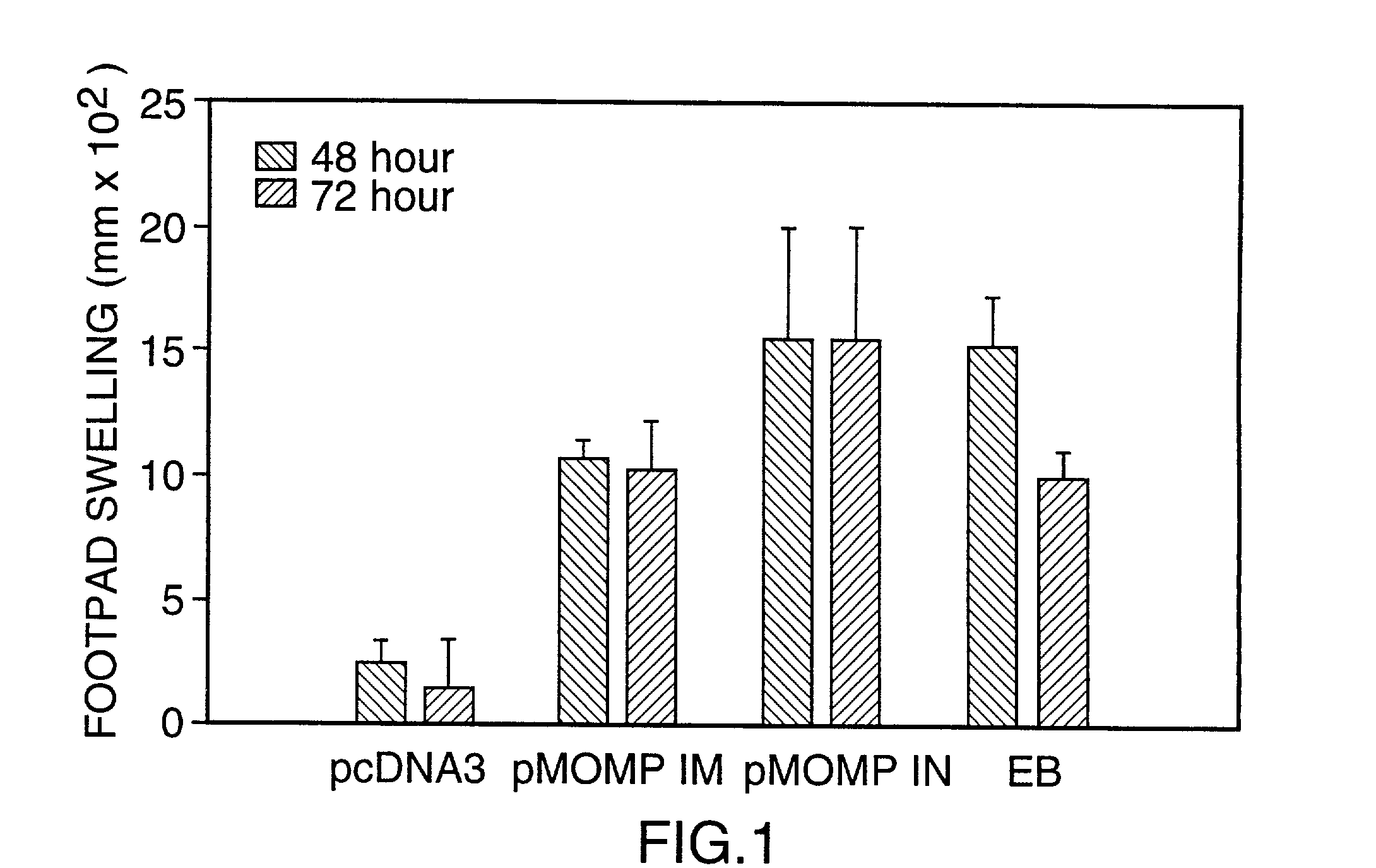
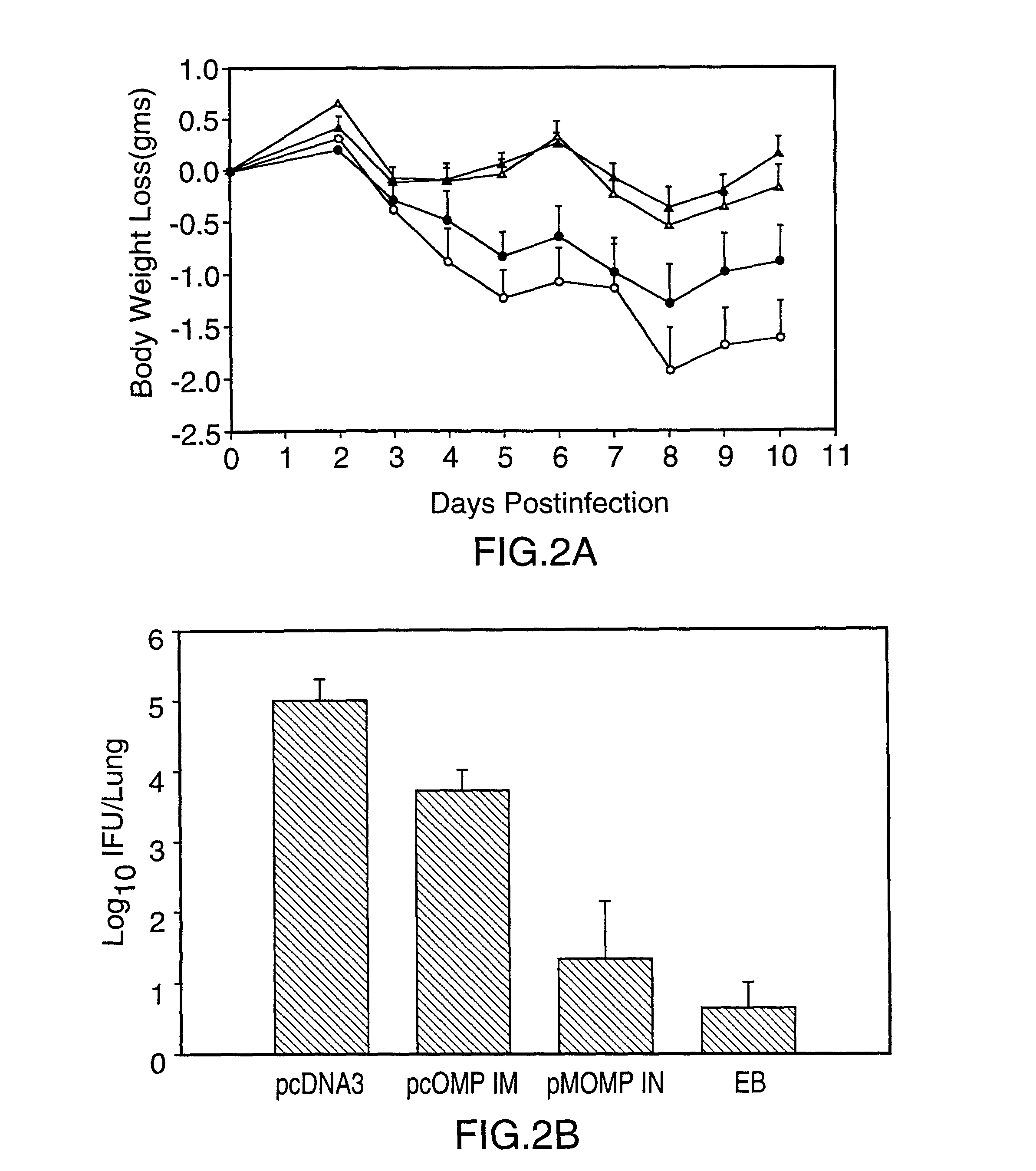
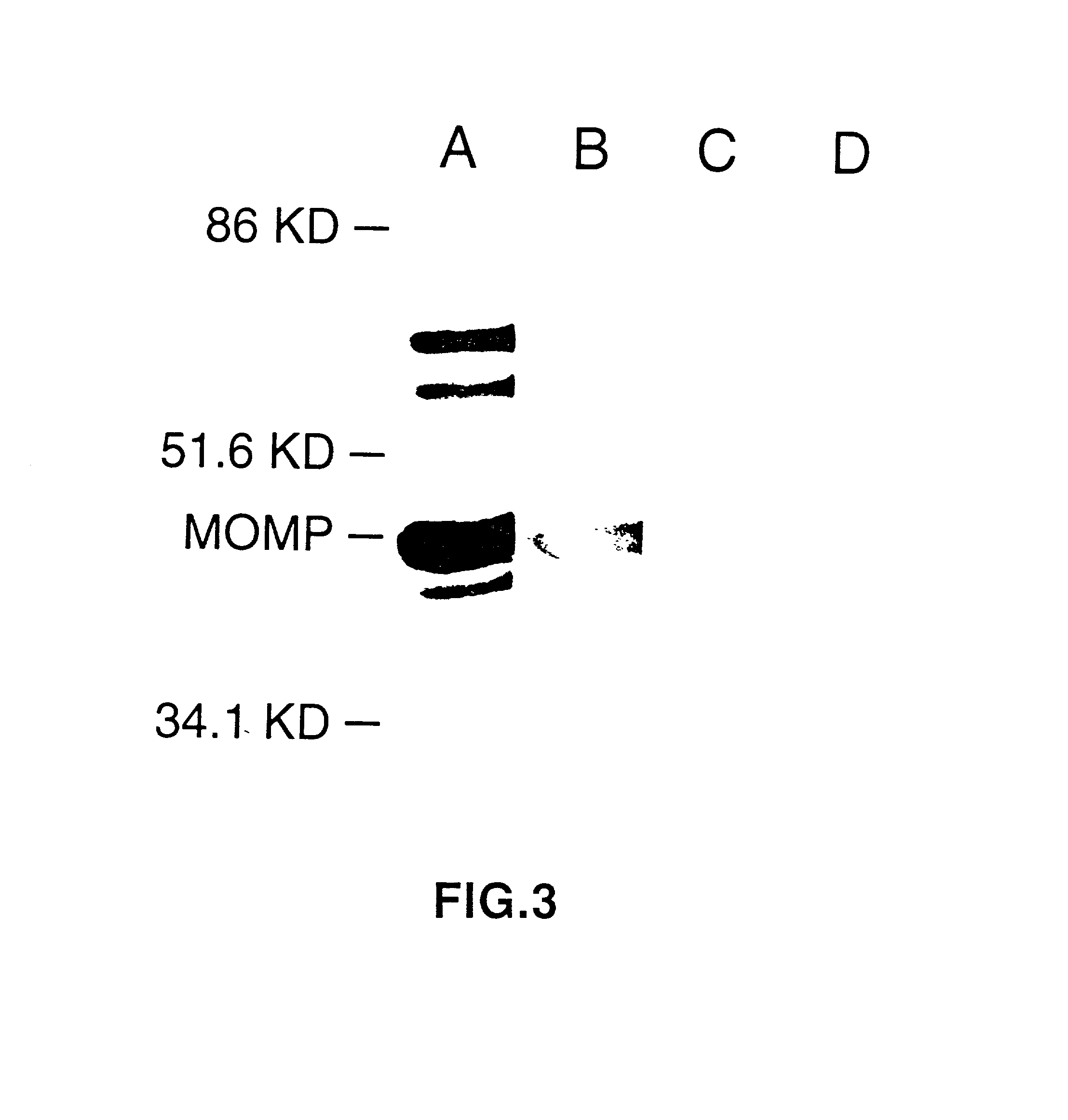
Nucleic acid, including DNA, immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The non-replicating vector may be formulated with a pharmaceutically-acceptable carrier for in vivo administration to the host.
Owner:MANITOBA UNIV OF THE
Peptide and DNA immunization against Coccidioides immitis infections
InactiveUS6923973B1Provide protectionCost-effectiveSugar derivativesMicrobiological testing/measurementCoccidioidomycosis infectionDNA construct
Disclosed are peptide and DNA compositions surprisingly found to be effective in generating immune responses against the pathogenic fungi Coccidioides spp., the causative agents of coccidioidomycosis and Valley Fever. The invention thus provides peptides and DNA constructs, combinations and related biological compositions, and prophylactic and therapeutic methods of using such components to generate effective immune responses against Coccidioides spp., including C. immitis.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
DNA immunization against chlamydia infection
InactiveUS6696421B2Significantly more efficient in inducing protective immunityRapid recruitment of dendritic cellsBiocideGenetic material ingredientsNucleotidePhylum Chlamydiae
Nucleic acid, including DNA, immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The non-replicating vector may be formulated with a pharmaceutically acceptable carrier for in vivo administration to the host.
Owner:UNIVERSITY OF MANITOBA
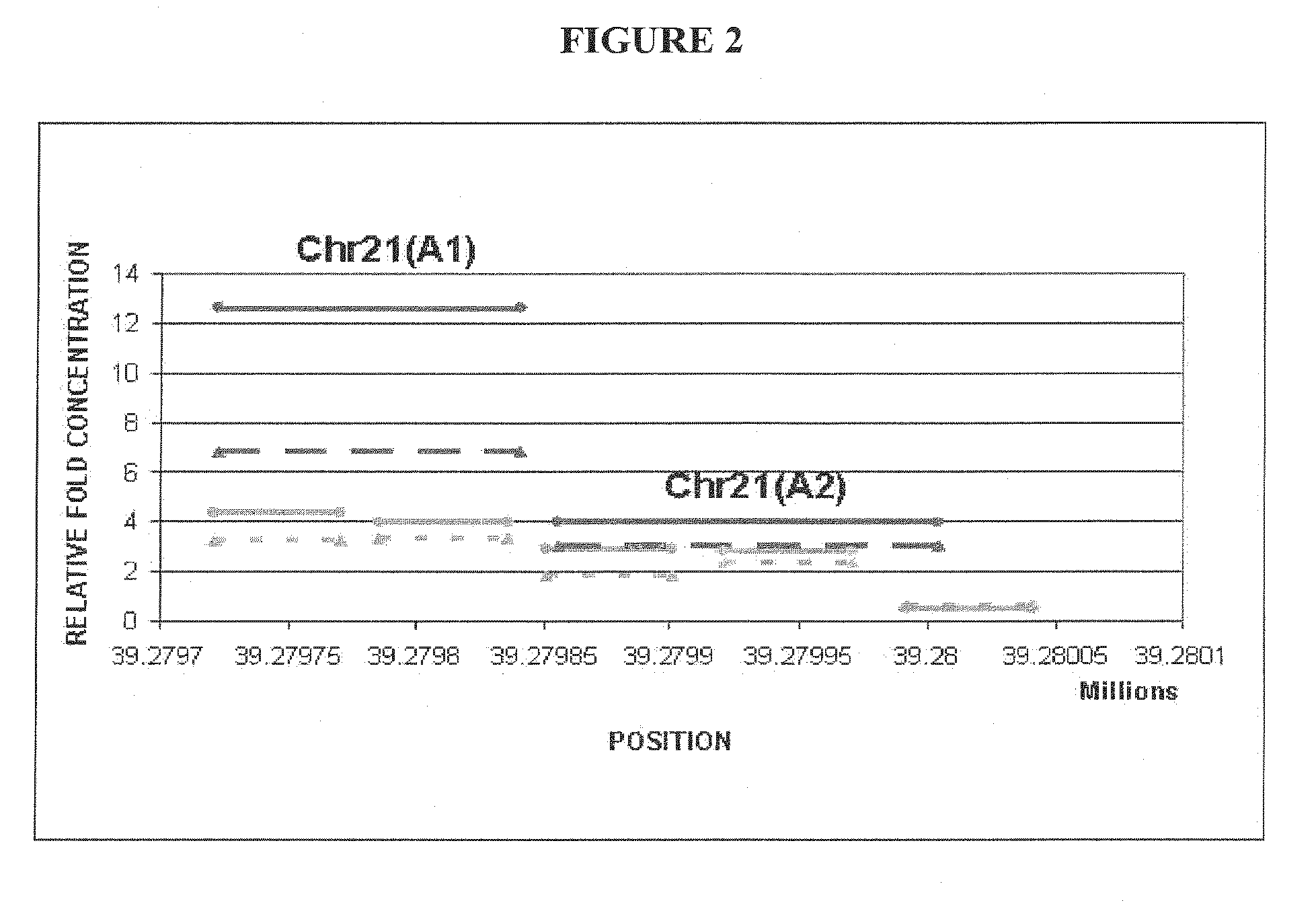
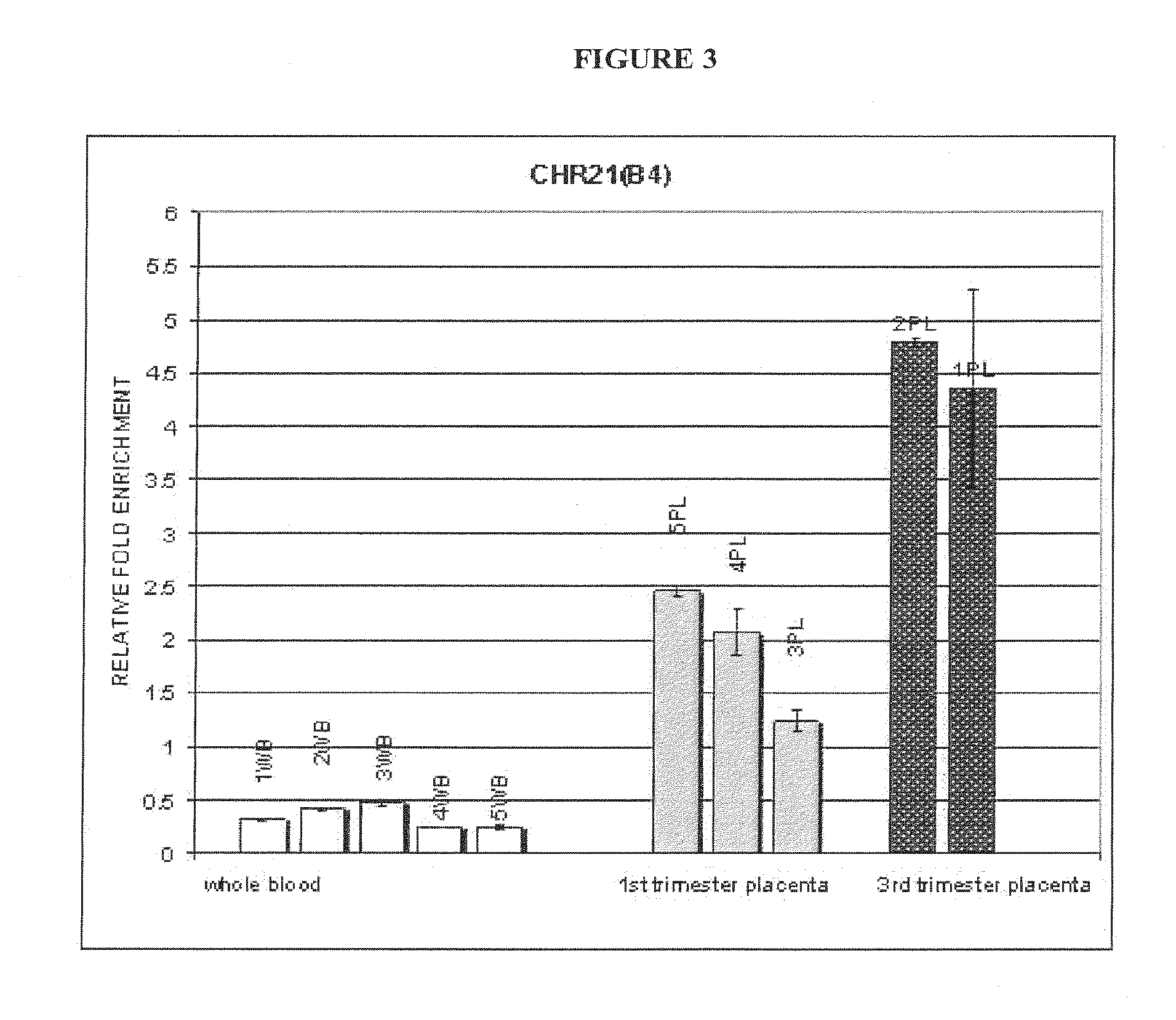
Methods and compositions for noninvasive prenatal diagnosis of fetal aneuploidies
ActiveUS20120282613A1Accurate predictionDiagnosing fetal aneuploidiesSugar derivativesMicrobiological testing/measurementMethylated DNA immunoprecipitationAdult female
The invention provides methods and compositions for noninvasive prenatal diagnosis of fetal aneuploidies. A large panel of differentially methylated regions (DMRs) have been identified. Certain of these DMRs are hypomethylated in adult female blood DNA and hypermethylated in fetal DNA, whereas others are hypermethylated in adult female blood DNA and hypomethylated in fetal DNA. Moreover, DMRs that are hypomethylated in adult female blood DNA and hypermethylated in fetal DNA have been shown to accurately predict a fetal aneuploidy in fetal DNA present in a maternal blood sample during pregnancy. In the methods of the invention, hypermethylated DNA is physically separated from hypomethylated DNA, preferably by methylated DNA immunoprecipitation.
Owner:NIPD GENETICS PUBLIC CO LTD
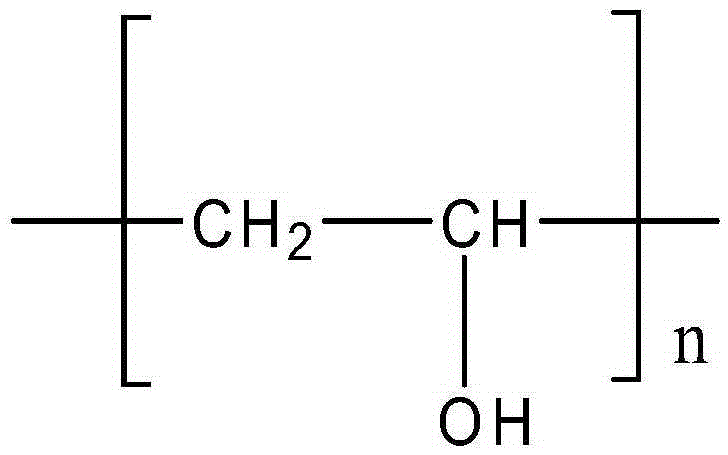
DNA immunity adsorbent and preparation method thereof
InactiveCN105289538ASimple manufacturing methodStable in natureOther blood circulation devicesOther chemical processesCross-linkPolyvinyl alcohol
The invention relates to DNA immunity adsorbent and a preparation method thereof. The DNA immunity adsorbent comprises an adsorbent carrier loaded with DNA. The DNA immunity adsorbent further comprises a cross-linking polyvinyl alcohol film mixed with DNA to achieve a film wrapping effect for the adsorbent. The preparation method includes the following step of sequentially conducting cross-linking and film wrapping or sequentially conducting film wrapping and cross-linking. The DNA immunity adsorbent has adsorption performance for anti-double stranded DNA antibodies in SLE patient plasma, and is superior to DNA immunity adsorbent prepared from a collodion wrapping film in general.
Owner:JAFRON BIOMEDICAL
Antibodies to complex targets
InactiveUS20160207996A1Improve production yieldLow production costImmunoglobulins against cell receptors/antigens/surface-determinantsWhole-cell/virus/DNA/RNA ingredientsEscherichia coliCamelid
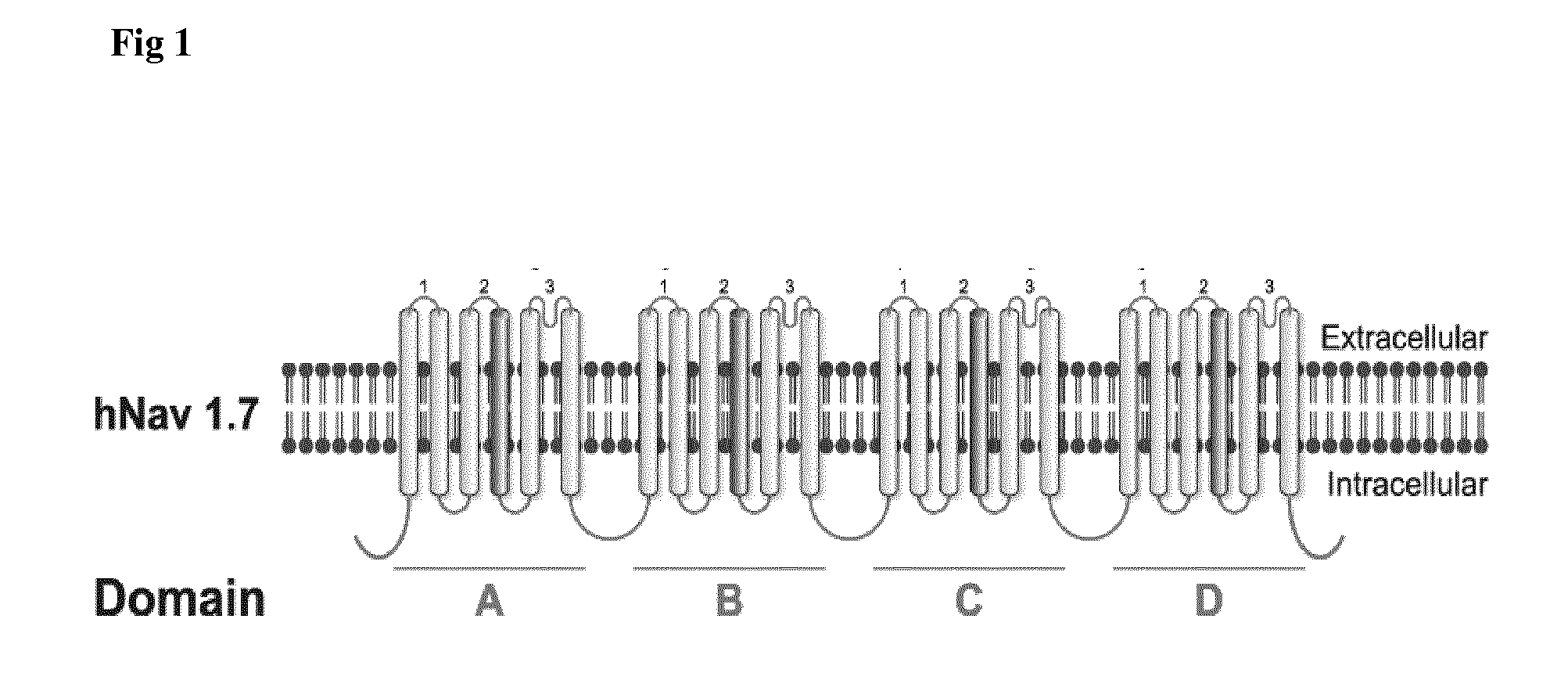
The present invention relates to antibodies and antigen binding fragments thereof derived from antibodies raised by DNA immunization of host animals, particularly camelids (e.g. llama). The antibodies and antigen binding fragments thereof bind to proteins which may be particularly large in size (at least 1115 amino acids in length), or have at least 8 transmembrane domains, or are naturally encoded by nucleotide sequences which are difficult to replicate in standard or common E. coli strains. The present invention also relates to antibodies and antigen binding fragments thereof which bind to ion channels, in particular the voltage-gated sodium channel Nav1.7. Methods of raising antibodies against particular protein targets by a process of DNA immunization are also provided.
Owner:ARGENX BVBA
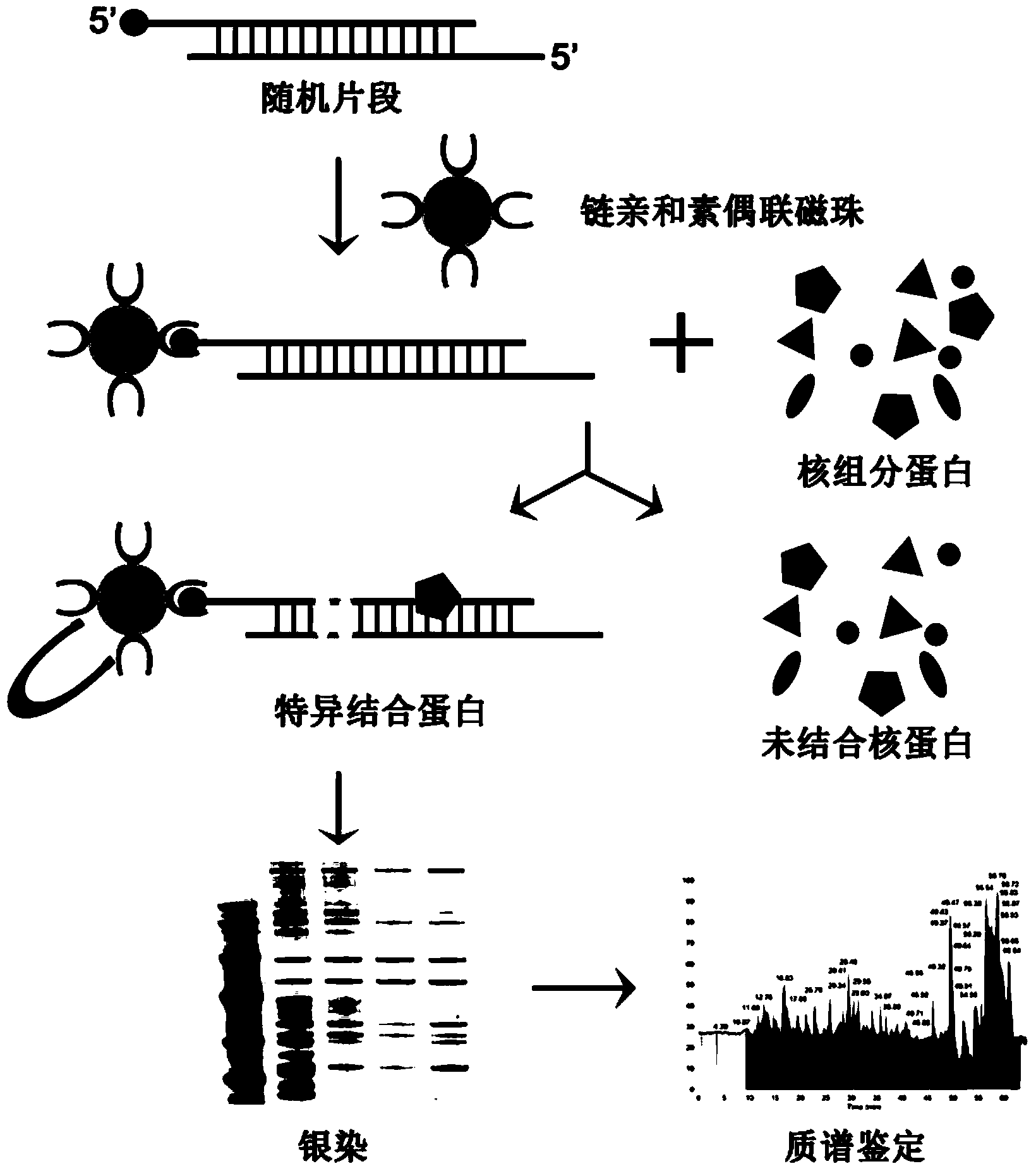
Methods for separating and identifying DNA (deoxyribonucleic acid) binding protein through DNA co-immunoprecipitation
ActiveCN103739664AImprove experimental efficiencyImprove stabilityMaterial analysis by electric/magnetic meansPeptide preparation methodsMolecular oncologyAdditive ingredient
The invention relates to methods for separating and identifying DNA (deoxyribonucleic acid) binding protein, in particular to methods for separating and identifying DNA binding protein through a DNA co-immunoprecipitation technology. The separating method comprises the steps of extracting nucleoprotein of a cell, mixing a target DNA molecule and nucleoprotein, adding a molecular entity capable of being bound with the target DNA molecule, separating to obtain a nucleoprotein ingredient bound with the target DNA molecule, further separating the obtained nucleoprotein ingredient, and obtaining the DNA binding protein. The identifying method further comprises the step of identifying the DNA binding protein obtained by separating. The methods have the characteristics of good stability and repeatability, and significantly improve the experiment efficiency of identifying the unknown DNA binding protein. The methods provide beneficial help for subjects such as molecular behavioristics, molecular oncology, cytobiology and biochemistry deeply researching into cytogene transcriptional control and genetic transcription.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
Bacterial delivery system
We describe a bacterial delivery system for the delivery of DNA and antigens into cells. We constructed an attenuated bacterial vector which enters mammalian cells and ruptures delivering functional plasmid DNA and antigens into the cell cytoplasm. This Shigella vector was designed to deliver DNA to colonic surfaces, thus opening the possibility of oral and other mucosal DNA immunization and gene therapy strategies. The attenuated Shigella is also useful as a vaccine for reducing disease symptoms caused by Shigella.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Expression vectors able to elicit improved immune response and methods of using same
The invention relates to nucleic acids (such as DNA immunization plasmids), encoding fusion proteins containing a destabilizing amino acid sequence attached to an amino acid sequence of interest, in which the immunogenicity of the amino acid sequence of interest is increased by the presence of the destabilizing amino acid sequence. The invention also relates to nucleic acids encoding secreted fusion proteins, such as those containing chemokines or cytokines, and an attached amino acid sequence of interest, in which the immunogenicity of the amino acid sequence of interest is increased as a result of being attached to the secretory sequence. The invention also relates methods of increasing the immunogenicity of the encoded proteins for use as vaccines or in gene therapy.
Owner:THE GOV OF THE USA REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES N I H
Monoclonal antibodies of avian influenza H5HA antigens
InactiveCN101892248AHigh expressionEasy to transcribeImmunoglobulins against virusesMicroorganism based processesAntigenAvian influenza virus
The invention discloses monoclonal antibodies of avian influenza H5HA antigens, belonging to the field of biotechnology. The invention provides H5-VN HA genes optimized by the codon of which the sequence is SEQ ID NO.1, and the sequence is used for preparing a monoclonal antibody 3B2IIG8F6 and a monoclonal antibody 4B10IIH4G12 of avian influenza H5-VN HA antigens through DNA immunization and a hybridoma cell CGMCC No.3648 and a hybridoma cell CGMCC No.3649 for secreting the two monoclonal antibodies. The H5-VN HA genes optimized by the codon, which is provided by the invention, can improve the expression of HA genes. In addition, the sequence optimization also enables mRNA to be more stable, and genes can be transcribed and translated more easily. The monoclonal antibodies of H5HA antigens, which are prepared by the invention, provide beneficial tools for research and clinical application of avian influenza viruses.
Owner:王世霞 +4
Personalized delivery vector-based immunotherapy and uses thereof
The invention provides a system of providing and creating personalized immunotherapeutic compositions for a subject having a disease or condition, including therapeutic immunotherapy delivery vectorsand methods of making the same comprising gene expression constructs expressing peptides associated with one or more neo-epitopes or peptides containing mutations that are specific to a subject's cancer or unhealthy tissue. A delivery vector of the invention includes bacterial vectors including Listeria bacterial vectors; or viral vectors, peptide immunotherapy vectors; or DNA immunotherapy vectors, comprising one or more fusion proteins comprising one or more peptides comprising one or more neo-epitopes present in disease-bearing biological samples obtained from the subject. This invention also provides methods of using the delivery vector for inducing an immune response against a disease or condition, including a tumor or cancer, or an infection, or an autoimmune disease or an organ transplant rejection in the subject.
Owner:ADVAXIS
DNA immunization against chlaymdia infection
InactiveUS6344202B1Effectively induces protective immunityGood curative effectOrganic active ingredientsVirusesNucleotideDna immunization
Nucleic acid, including DNA, for immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The non-replicating vector may be formulated with a pharmaceutically-acceptable carrier for in vivo administration to the host.
Owner:UNIVERSITY OF MANITOBA
Method for preparing a carbonized resin DNA immunoadsorbent
InactiveUS6262172B1Quality improvementAvoid damageOrganic active ingredientsGenetic material ingredientsBenzoyl peroxideSorbent
A method for preparing a carbonized resin DNA immunoadsorbent, which uses styrene, acrylonitrile, divinylbenzene, toluene, liquid paraffin, benzoyl peroxide and water solution of polyvinyl alcohol as raw materials, produces a DNA immunoadsorbent with 0.3-0.5 mg DNA per milliliter of resin using two-staged procedures for preparation of first carbonized resin, and secondly carbonized resin DNA immunoadsorbent. The resultant immunoadsorbent is high in adsorbent capacity, low in production cost, and can be synthesized without pyrogens. It therefore satisfies the demands of medical application for the treatment of systemic lupus erythematosus by immunoadsorption.
Owner:JAFRON BIOMEDICAL
DNA immunization against Chlaymdia infection
InactiveUS20020142001A1Significantly more efficient in inducing protective immunityRapid recruitment of dendritic cellsOrganic active ingredientsVirusesNucleotideDna immunization
Nucleic acid, including DNA, for immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The non-replicating vector may be formulated with a pharmaceutically-acceptable carrier for in vivo administration to the host.
Owner:UNIVERSITY OF MANITOBA
Self-replicating vector for DNA immunication against HIV
A nucleotide sequence encoding the HIV regulatory protein NEF, REV or TAT or an immunologically active fragment thereof is inserted into a vector comprising papilloma virus nucleotide sequences necessary and sufficient for long-term persistence. The resulting vectors are self-replicating and have a high copy number. They express the HIV genes in high amounts for a long period of time. The vectors elicit both a humoral and cell-mediated immune response and are therefore potential DNA immunization vaccines against HIV. The invention is directed to said vectors and vaccines and to a method for preparing the vectors. The invention is further directed to a host cell comprising the vector, to the use of the vector in the manufacture of a vaccine and to a method of preventing or treating HIV.
Owner:OY FINNISH IMMUNOTECH
High-throughput method of DNA immunogen preparation and immunization
InactiveUS20050277127A1Rapid and cost-effective preparationStrong immune responseMicrobiological testing/measurementCancer antigen ingredientsAdjuvantDna immunization
A high-throughput process of generating expression-competent, antigen-encoding immunogen DNA through amplification methodology including ligation-assisted PCR is described, as well as the use of the DNA for methods of DNA immunization. Also described is an adjuvant plasmid to enhance antibody production.
Owner:EPIOMICS
Expression cassettes encoding modified human immunodeficiency virus type 1 subtype C envelope glycoproteins
Owner:STELLENBOSCH UNIVERSITY +1
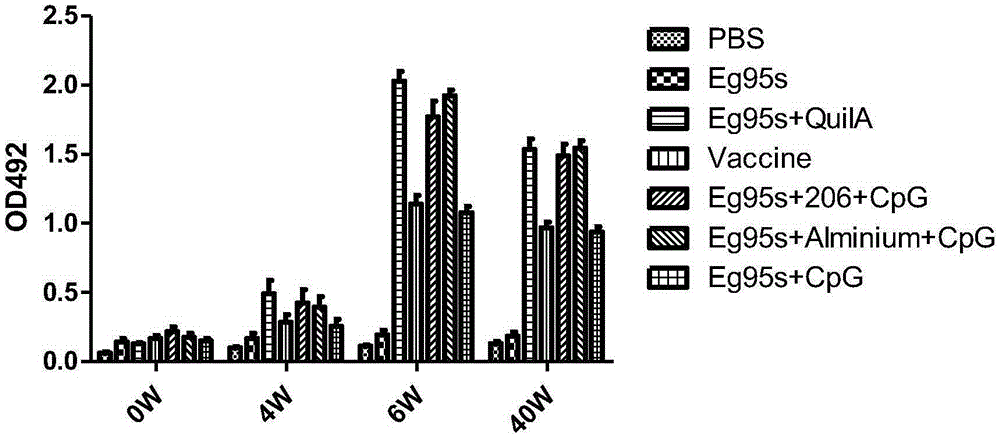
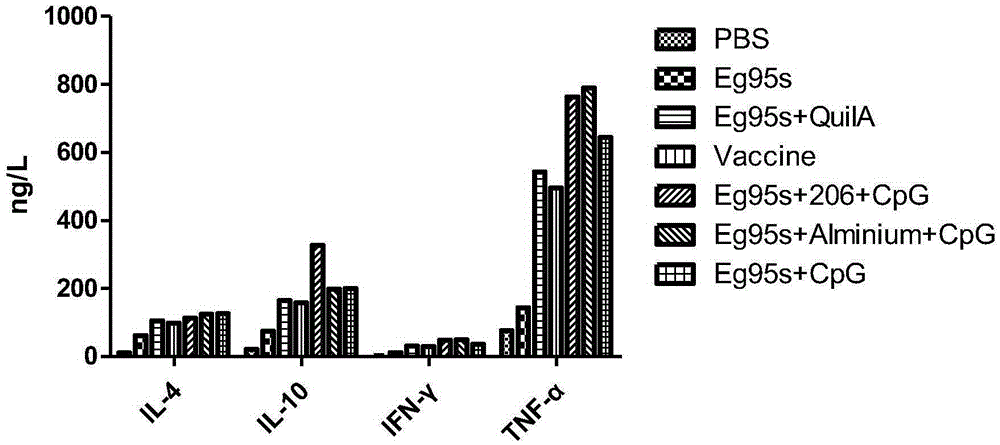
Novel echinococosis granulosis vaccine with CPG DNA (deoxyribonucleic acid) immune adjuvants
InactiveCN105267988AImprove immunityGood humoral immunityGenetic material ingredientsAntiparasitic agentsDna immunizationIntermediate host
The invention belongs to the technical field of vaccine development, and provides novel echinococosis granulosis vaccine for preventing intermediate hosts of echinococosis for human, livestock and the like. The novel echinococosis granulosis vaccine comprises soluble expression mixtures of Eg95 recombinant proteins, aluminum hydroxide and CpG immune adjuvants. The novel echinococosis granulosis vaccine has the advantage that excellent cellular immunity and humoral immunity of bodies can be effectively stimulated by the novel echinococosis granulosis vaccine.
Owner:BEIJING ZHONGNONG BIOLOGICAL ENG CO LTD
Improved DNA immunization with recombinase/transposase
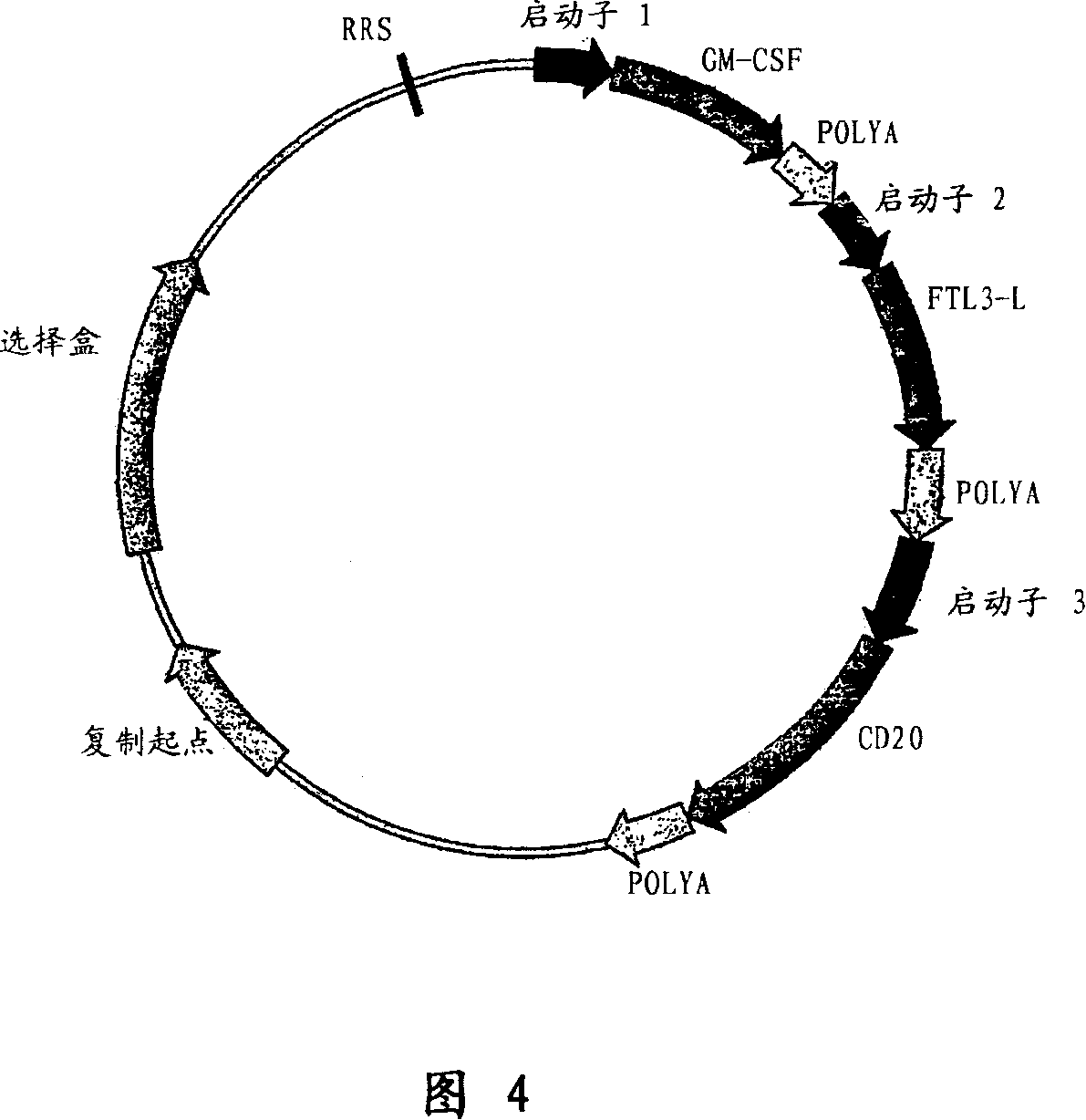
The present invention relates to improved methods to immunize / vaccinate or stimulate the immune system of animals, including humans, using vectors containing expression cassettes that encode for the DNA of one or more protein / peptide antigens and / or adjuvants, in particular, cytokines like GMCSF, Flt3L, interleukins, and the like, which can be encoded by DNA as well, also recombinase mediated integration. Adjuvants known to increase immune responses following DNA vaccination. In addition, the vectors contain one or more sites recognized by a recombinase / transposase, which catalyzes the insertion of the vector into the genome of transfected cells. Stable integration of the plasmid vector into the genome of transfected cells results in higher and longer-lasting expression of the encoded protein(s), and increases the immune response in the vaccinated animal. The present invention also relates to adjuvant compositions comprising the novel polypeptide, rabbit GMCSF, for boosting antibody production in rabbits.
Owner:人类多细胞治疗股份有限公司
Expression of HIV polypeptides and production of virus-like particles
InactiveUS7348177B2Sufficient amountImprove efficiencyOrganic active ingredientsBiocideVirus-like particlePlant cell
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
DNA immunoadsorbent, and preparation method thereof
ActiveCN108246264AHigh strengthReduce sheddingOther blood circulation devicesOther chemical processesImmunosorbentsPolystyrene
The invention discloses a DNA immunoadsorbent and a preparation method thereof. The preparation method comprises the following steps: 1) preparation of polystyrene-based macroporous adsorption resin containing aldehyde groups; and 2) grafting and immobilization of DNA ligand. The DNA immunosorbent prepared by using the method directly adopts the macroporous adsorption resin as a carrier and replaces activated carbon or carbonized resin with the macroporous adsorption resin; and the toughness of a resin skeleton is retained and the risk of falling of particles is greatly reduced due to the omitting of carbonization and activation procedures during production. In addition, a chemical grafting method is employed to directly immobilize the DNA ligand to the macroporous adsorption resin, and the DNA ligand linked by a chemical bond is firmly immobilized and hardly falls off; moreover, without embedding and covering of coating materials like collodion, DNAs immobilized on the surface of theresin and inside the pores of the resin can exert maximum adsorption effect.
Owner:JAFRON BIOMEDICAL
Method for preparing carbonized resin DNA immunoadsorbent
The invention provides a method for preparing carbonized resin DNA immunoadsorbent, in which the resin DNA immunoadsorbent having DNA of 0.3-0.5 mg / ml can be produced, taking styrene, acrylonitrile, divinylbenzene, toluene, white oil, benzoyl peroxide, and polyvinylalcohol auqeous solution as raw materials, by both phases of preparing carbonized resin and preparing carbonized resin DNA immunoadsorbent. The immunoadsorbent is better suitable to the clinical requirement on clinic, having high adsorbent ability and no heat source matter, as well as low manufacturing cost. The immunoadsorbent is popularized to treat systemic lupus erythematosus.
Owner:JAFRON BIOMEDICAL
Process for preparing hybridoma cell for secreting anti-human asparagine hydroxylase monoclonal antibodies
ActiveCN101307302AHigh titerGood immune effectTissue cultureMaterial analysisImmune cyclePlasmid dna
The invention relates to a preparation process for a hybridoma secreting a monoclonal antibody repelling human asparaginate hydroxylase. The method comprises the following steps: taking the recombinant enkaryon expression plasmid DNA of a human asparaginate hydroxylase HAAH coded gene, injecting the DNA into the skeletal muscle of a white rat for DNA immunity, carrying out human asparaginate hydroxylase HAAH recombinant protein immunity after 1 to 4 weeks, taking the splenic cell and myeloma cell of the immune white rat for cell fusion after 2 to 5 days, carrying out antibody detection, and cloning the positive cells in the antibody detection till obtaining the hybridoma secreting the monoclonal antibody repelling human asparaginate hydroxylase. The invention solves the technical problems of bad specificity, long immune cycle and low efficiency in the prior art. The hybridoma has clear immunogenicity, high purity, few non-specific reactions, and low use level of antigen with a total immune cycle of only 6 to 8 weeks.
Owner:SHANXI LIFEGEN
Immune plasmid, monoclonal antibody for detecting antimullerian hormone, hybridoma and preparation method and application thereof
ActiveCN107523586ASimple and fast operationAccurate detectionHormone peptidesTissue cultureAdditive ingredientActive agent
The invention discloses an immune plasmid, a monoclonal antibody for detecting antimullerian hormone, a hybridoma and a preparation method and application thereof. A toleragen which consists of proAMH and Mature AMH is creatively used for immunizing rats to prepare immune tolerance rats, high-immune-response rats are obtained through suitable immunogen and by a DNA immunizing mode, finally, the hybridoma capable of secreting a monoclonal antibody for detecting antimullerian hormone can be obtained by screening, the monoclonal antibody secreted by the hybridoma or the monoclonal antibody obtained by the immunogen by the other mode can be directly used for detecting a connection part of proAMH and Mature AMH in original AMH, and the circumstance that ingredients such as a surfactant are added to carry out pretreatment such as dissociation on samples is avoided. Operation is simple and convenient, the monoclonal antibody can adapt to different immunological detection platforms, and can rapidly and accurately detect original AMH protein in samples.
Owner:GUANGZHOU WONDFO BIOTECH
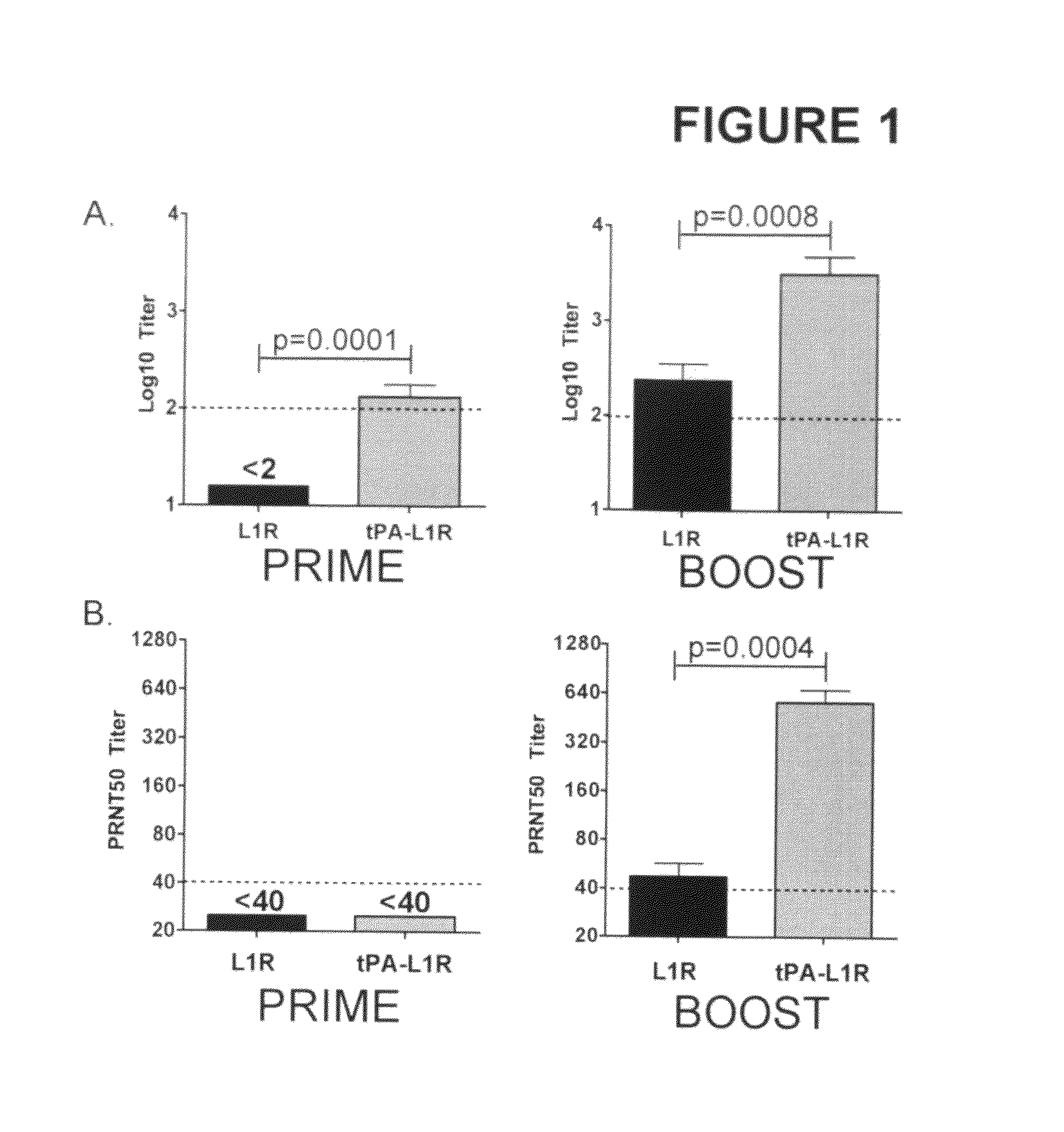
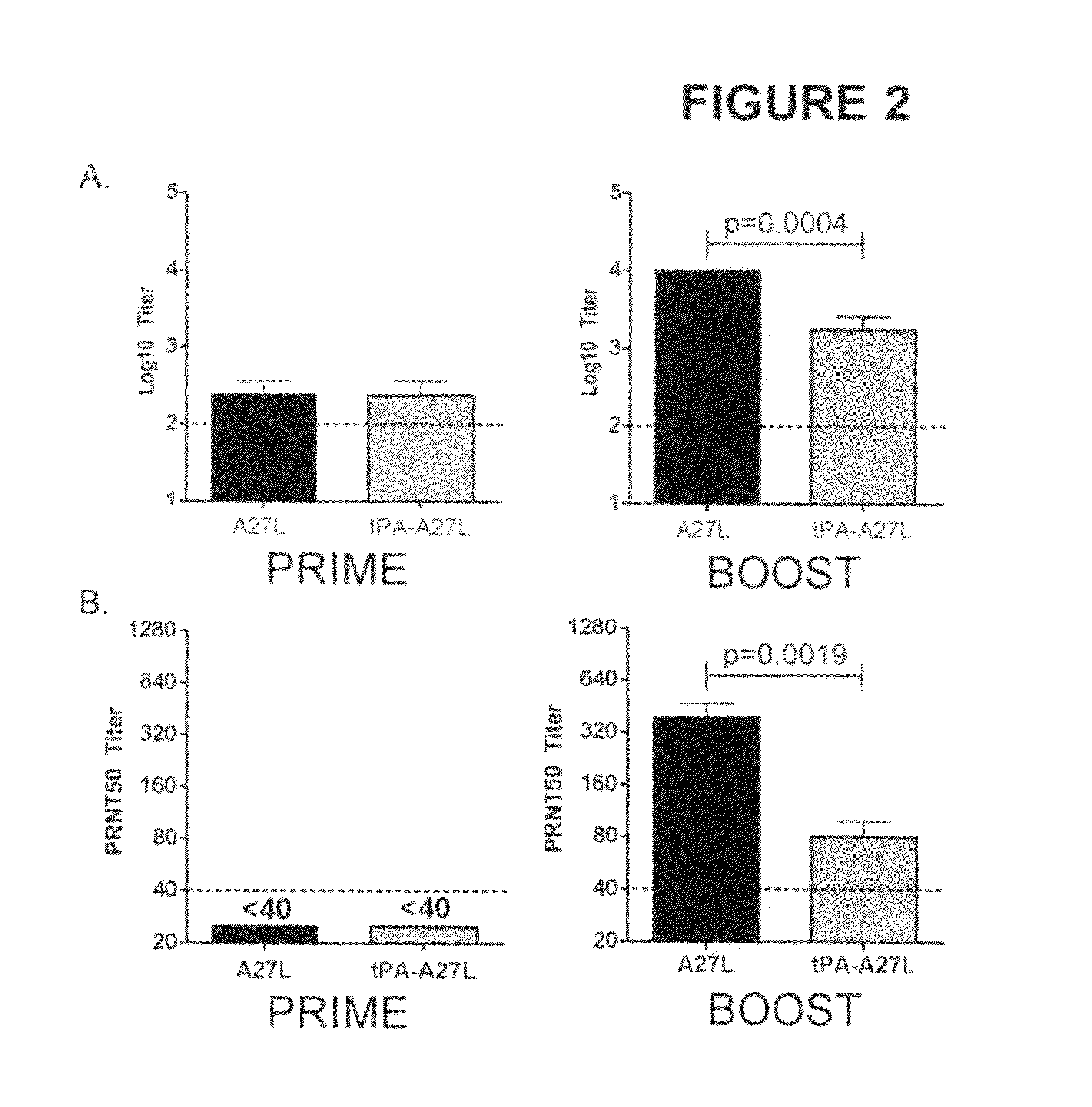
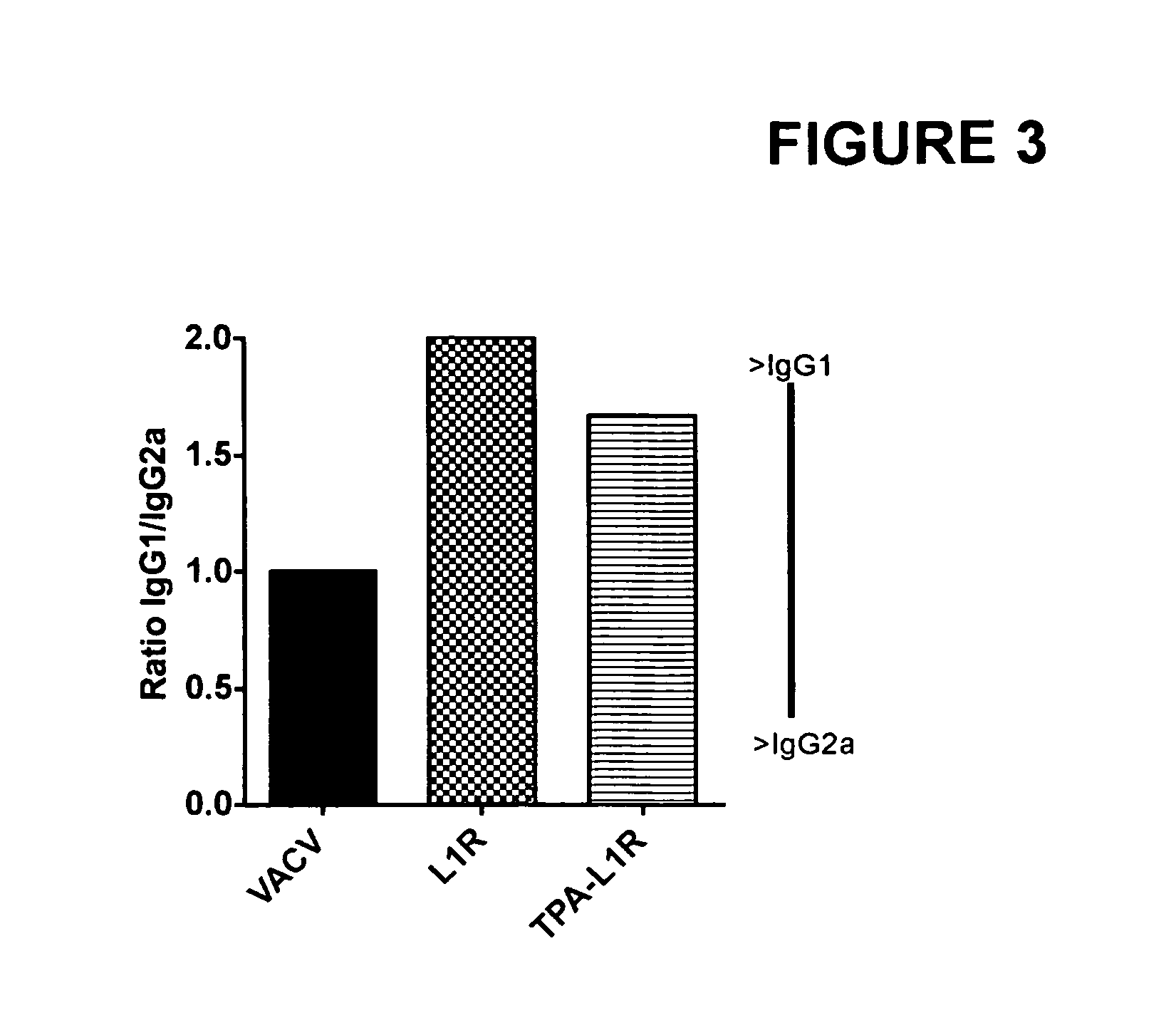
DNA immunogenic composition comprising a full-length modified poxvirus L1R gene fused to a tPA leader sequence
ActiveUS8513005B2Robust neutralizing antibody responseReduce the amount of solutionVirus peptidesAntiviralsReticulum cellDna immunization
The invention described here encompasses DNA and protein vaccines against poxviruses, and relevant immunogenic compositions, comprising at a minimum a nucleic acid encoding a modified full-length poxvirus L1R gene or its ortholog. The L1R gene is modified so that an endoplasmic reticulum-targeting sequence is operably linked on the 5′ end. Preferably the nucleic acid sequences for other poxviruses antigens are also included, such as A33R, B5R and / or A27L. These vaccines and compositions provide improved neutralizing antibody response elicited by molecular poxvirus vaccines, over known vaccines using unmodified L1R.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com