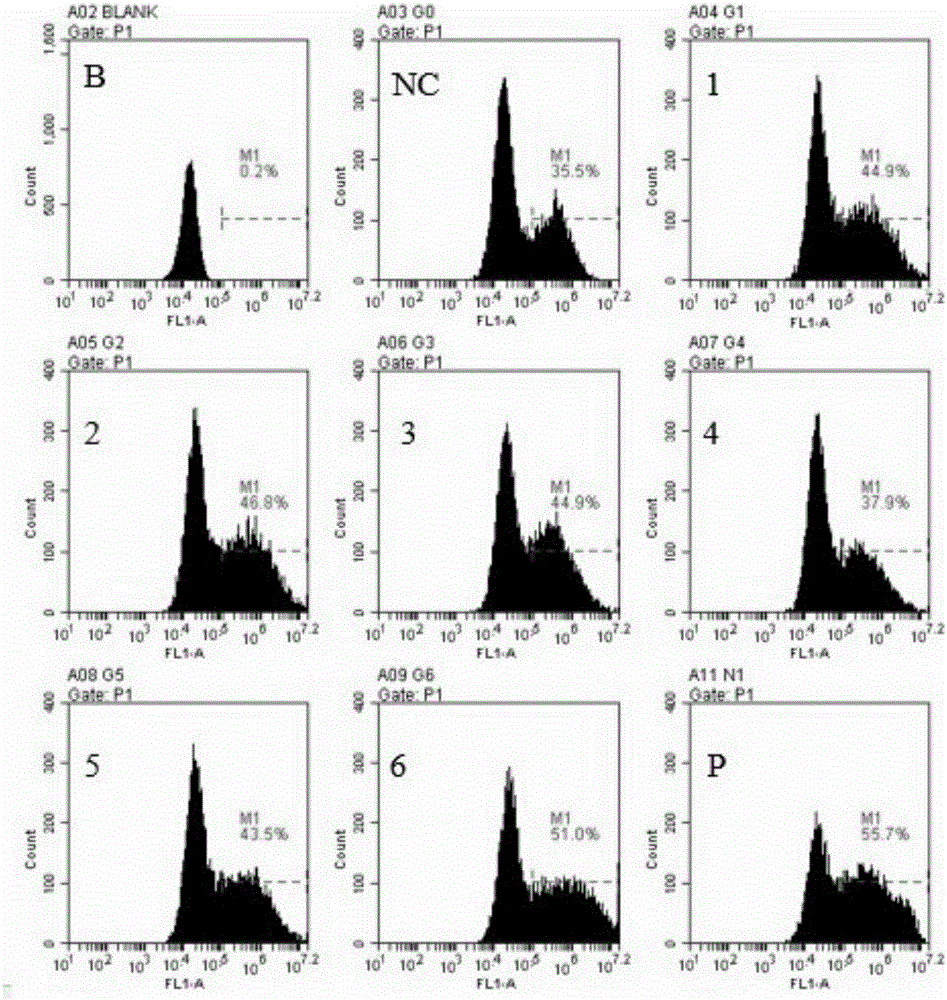
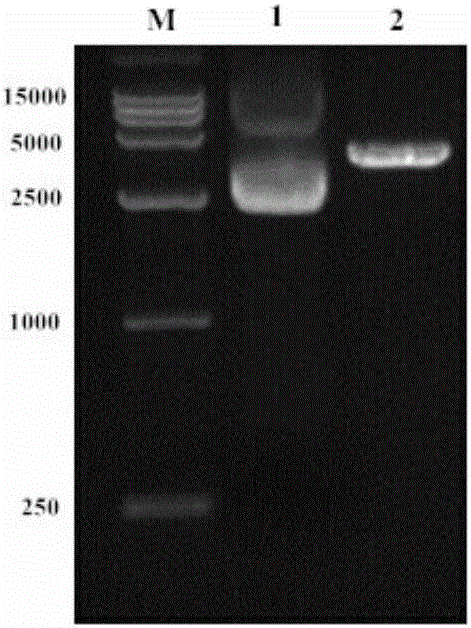
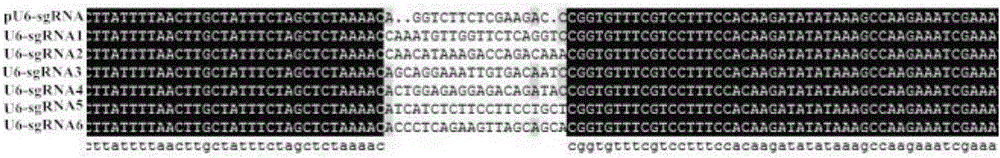
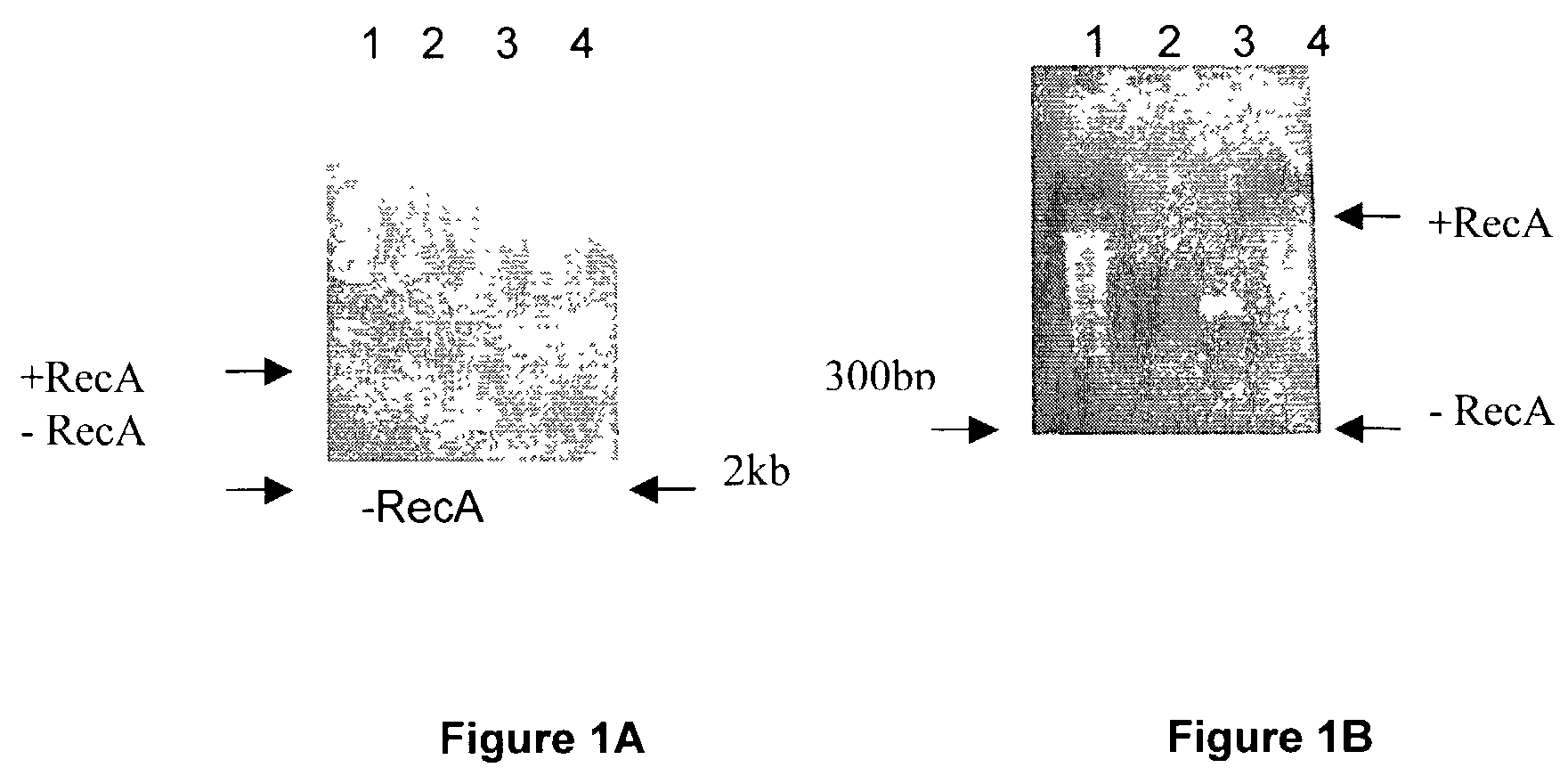
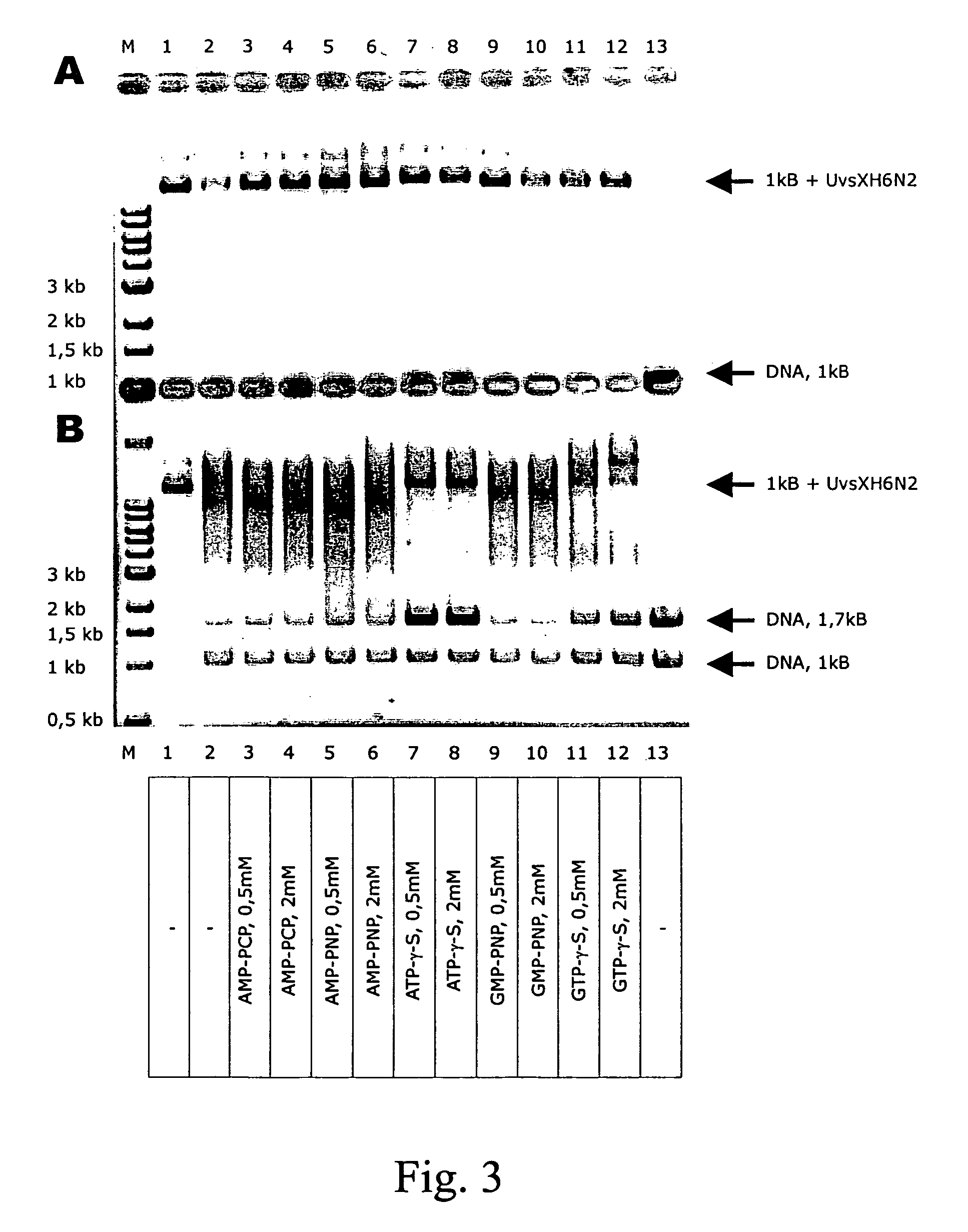
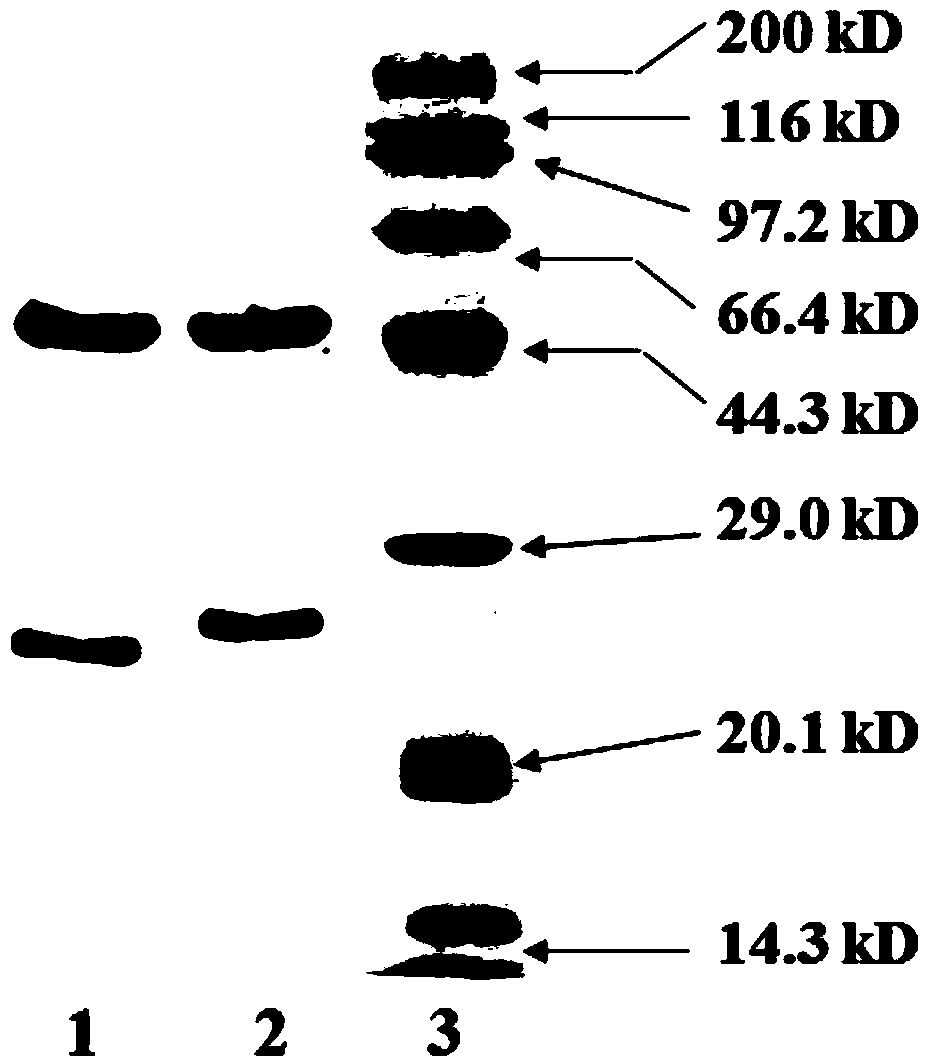
Patents
Literature
322 results about "Nucleoprotein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nucleoproteins are any proteins that are structurally associated with nucleic acids, either DNA or RNA. Typical nucleoproteins include ribosomes, nucleosomes, and viral nucleocapsid proteins.
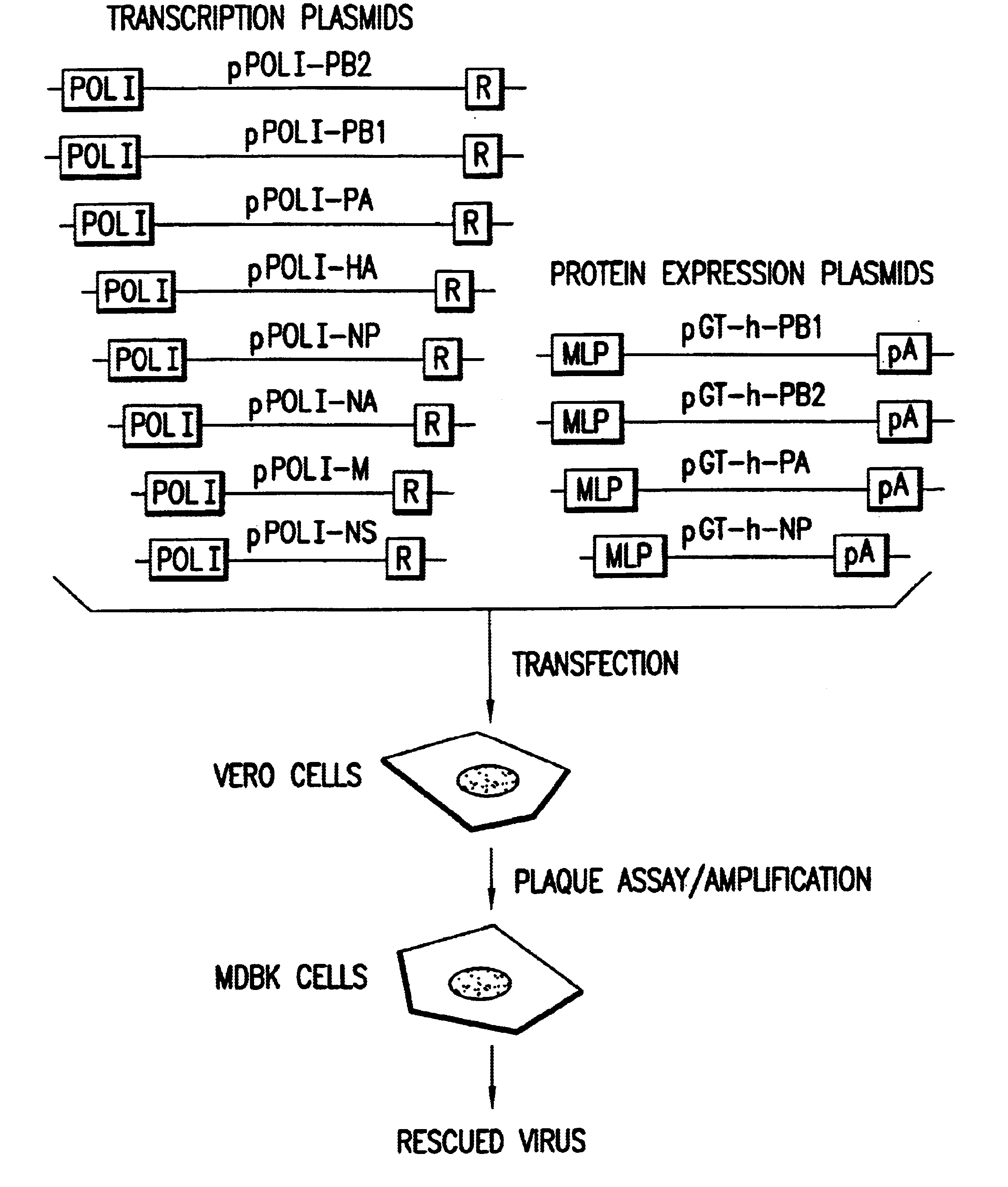
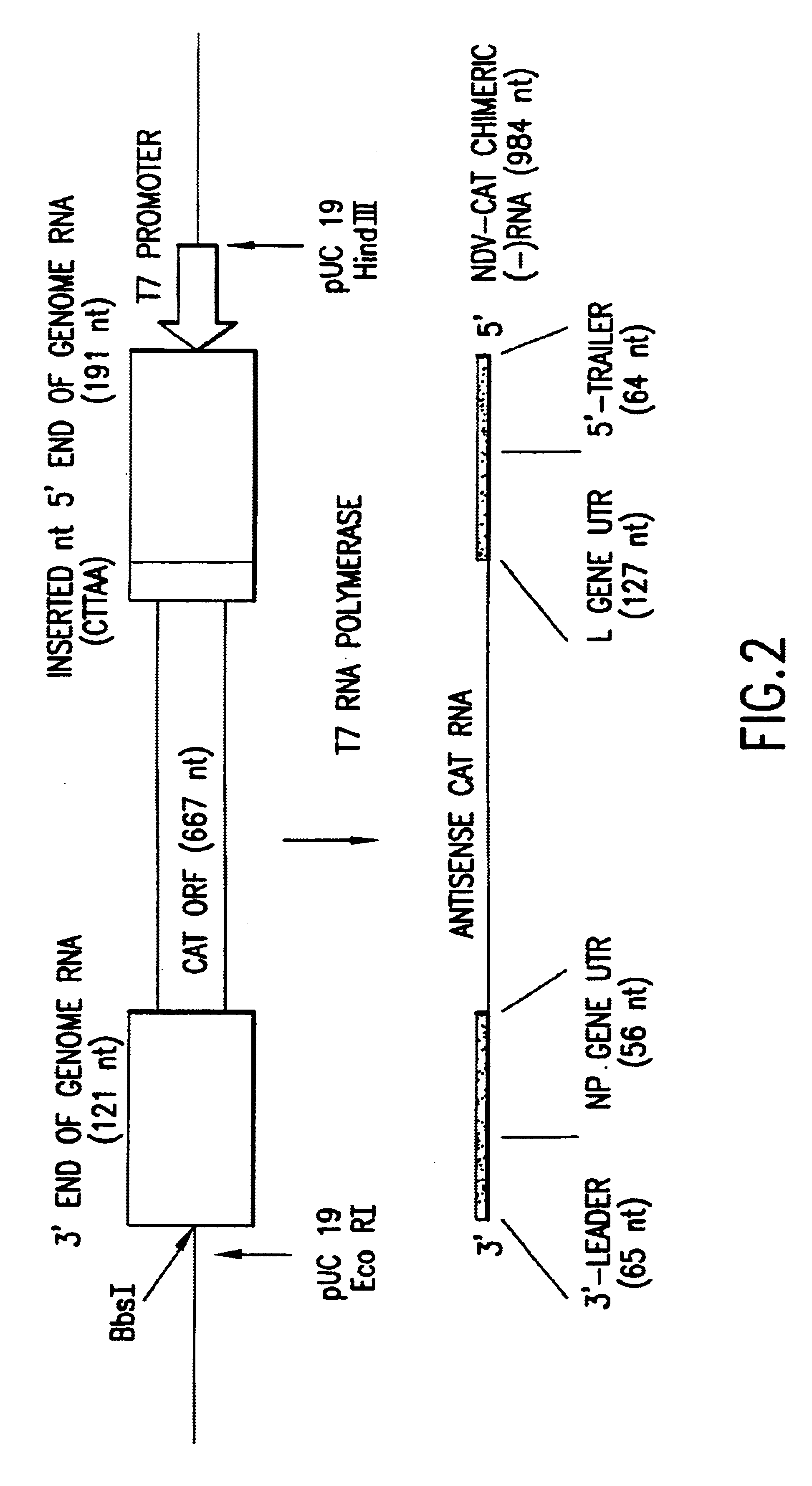
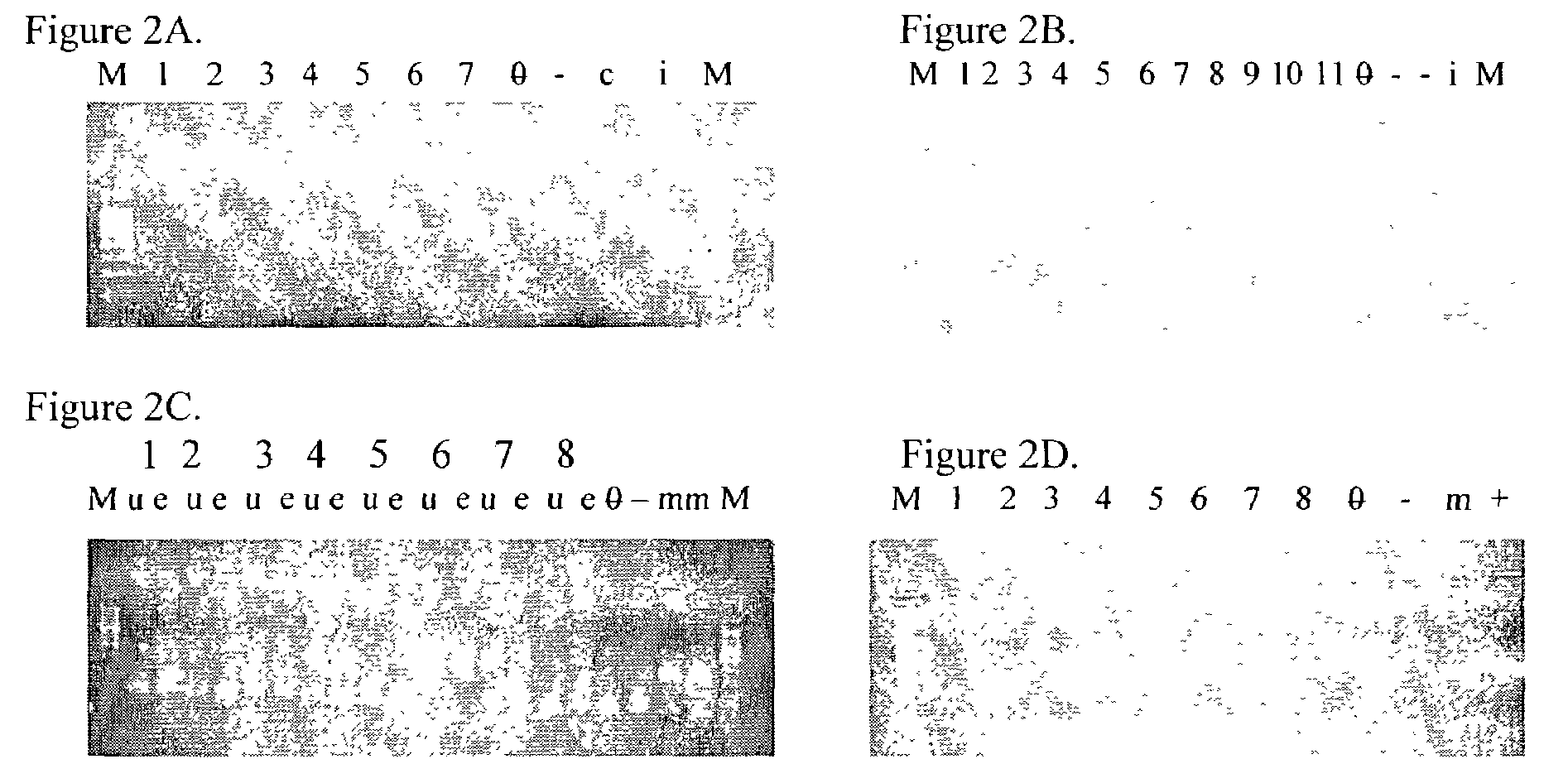
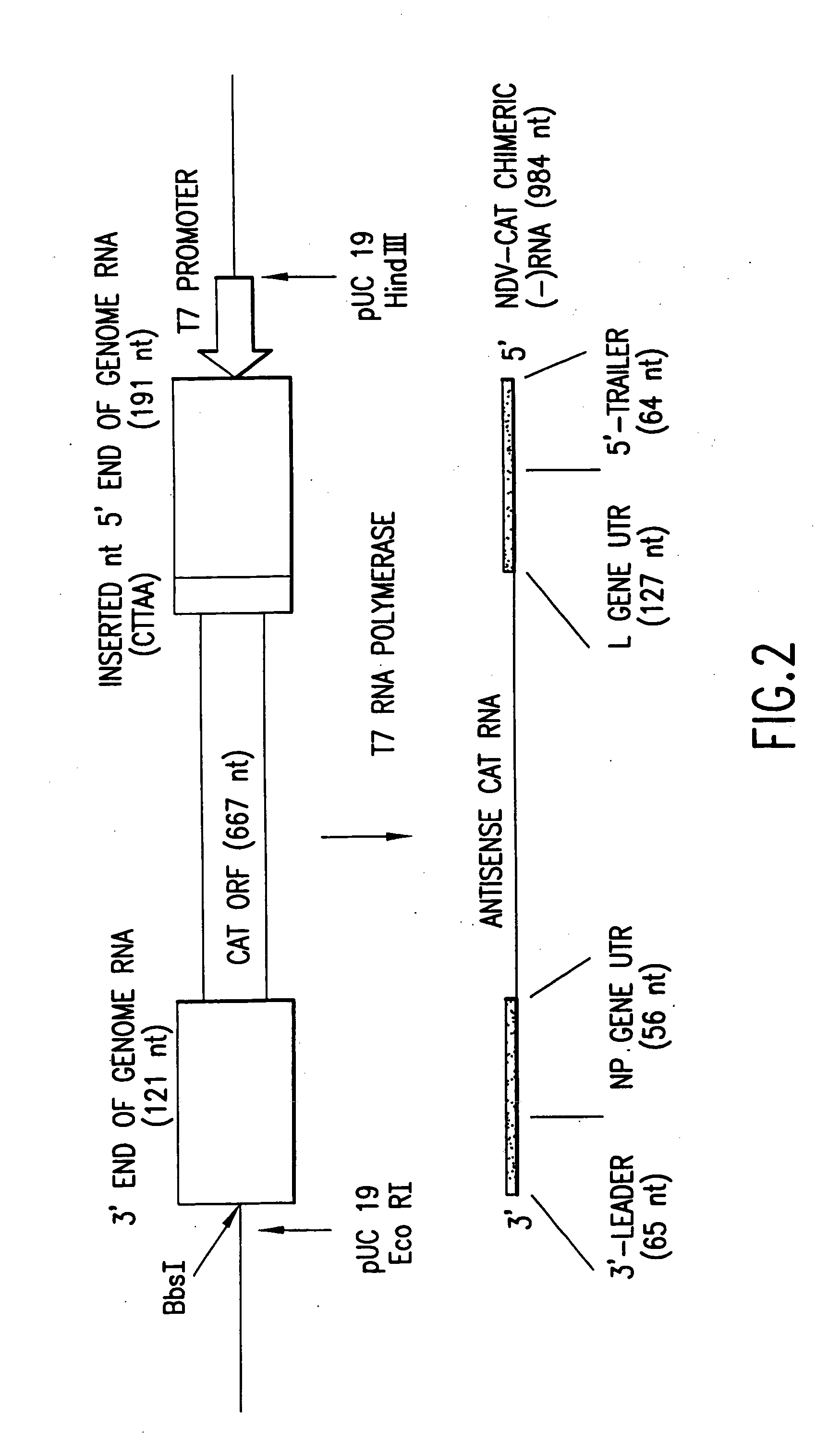
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Telomerase
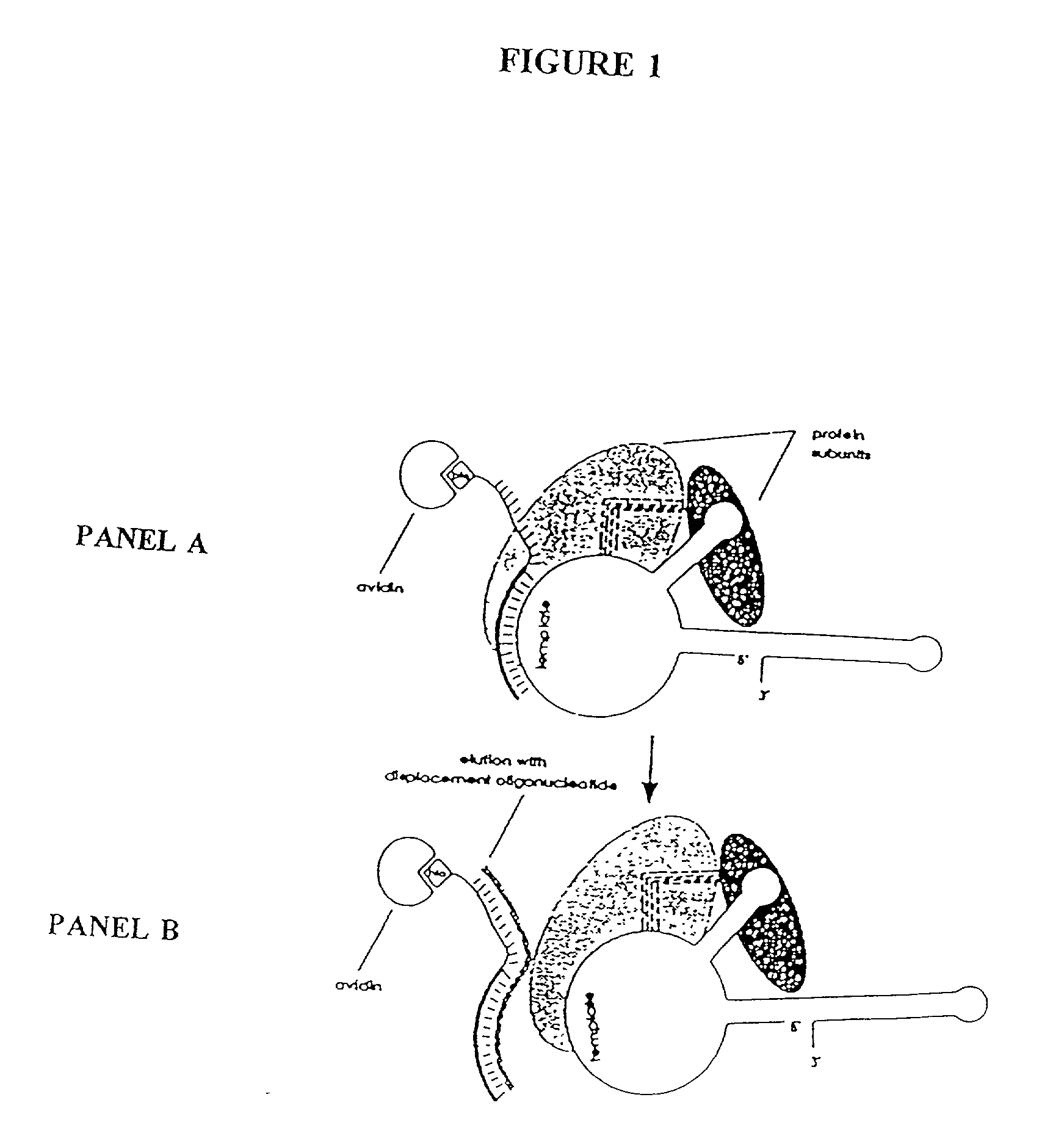
InactiveUS6261836B1Improve purification effectAvoid the needPeptide/protein ingredientsAntibody mimetics/scaffoldsTelomeraseRibonucleoprotein complex
The present invention is directed to telomerase nucleic acids and amino acids. In particular, the present invention is directed to nucleic acid and amino acid sequences encoding various telomerase protein subunits and motifs, including the 123 kDa and 43 kDa telomerase protein subunits of Euplotes aediculatus, and related sequences from Schizosaccharomyces, Saccharomyces sequences, and human telomerase. The present invention is also directed to polypeptides comprising these telomerase protein subunits, as well as functional polypeptides and ribonucleoproteins that contain these subunits.
Owner:GERON CORPORATION +1
Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer
InactiveUS20020106684A1Low tumor burdenImmunologic function is relatively intactSugar derivativesMicrobiological testing/measurementA lipoproteinNeoplasm
This invention relates to the use of tumor-derived or associated extracellular ribonucleic acid (RNA) found circulating in the plasma or serum fraction of blood for the detection, monitoring, or evaluation of cancer or premalignant conditions. Extracellular RNA may circulate as non-bound RNA, protein-bound RNA, lipid-RNA complexes, lipoprotein (proteolipid)-RNA complexes, protein-RNA complexes including within or in association with ribonucleoprotein complexes, nucleosomes, or within apoptotic bodies. Any intracellular RNA found in plasma or serum can additionally be detected by this invention. Specifically, this invention enables the extraction of circulating RNA from plasma or serum and utilizes nucleic acid amplification assays for the identification, detection, inference, monitoring, or evaluation of any neoplasm, benign, premalignant, or malignant, in humans or other animals, which might be associated with that RNA. Further, this invention allows the qualitative or quantitative detection of tumor-derived or associated extracellular RNA circulating in the plasma or serum of humans or animals with or without any prior knowledge of the presence of cancer or premalignant tissue.
Owner:ONCOMEDX
Peptide-based vaccine for influenza
A human synthetic peptide-based influenza vaccine for intranasal administration comprises a mixture of flagella containing at least four epitopes of influenza virus reactive with human cells, each expressed individually in Salmonella flagellin, said influenza virus epitopes being selected from the group consisting of: (i) one B-cell hemagglutinin (HA) epitope; (ii) one T-helper hemagglutinin (HA) or nucleo-protein (NP) epitope that can bind to many HLA molecules; and (iii) at least two cytotoxic lymphocyte (CTL) nucleoprotein (NP) or matrix protein (M) epitopes that are restricted to the most prevalent HLA molecules in different human populations.
Owner:YEDA RES & DEV CO LTD
Influenza vaccines
InactiveUS20080299151A1Less protectionBroad and efficient protective immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininMammal
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
Production of attenuated parainfluenza virus vaccines from cloned nucleotide sequences
InactiveUS7208161B1SsRNA viruses negative-senseSugar derivativesPolymerase LParainfluenza virus vaccine
Isolated polynucleotide molecules provide recombinant PIV genomes and antigenomes for production of recombinant PIV vaccines. The recombinant genome or antigenome can be expressed with a nucleoprotein (N), phosphoprotein (P), and a large (L) polymerase protein to produce isolated infectious PIV particles. The recombinant PIV genome and antigenome can be modified to produce desired changes, for example to incorporate attenuating mutations from biologically derived PIV mutants or to create chimeric PIV clones, to generate attenuated, immunogenic viruses for vaccine use.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA THE AS REPRESENTED BY THE DEPT OF
Animal sex control method based on Rbmy gene editing and application
ActiveCN105861554AIncrease productivityNucleic acid vectorFermentationControl animalPlant Germ Cells
The invention discloses an animal sex control method based on Rbmy gene editing and application. The method controls animal sex by using CRISPR / Cas9 system to specifically cut Rbmy gene on Y chromosome. Rbmy gene encodes a germ cell specific nucleoprotein, this protein is an important factor related to spermatogenesis, and by cutting Rbmy gene, it is possible to deactivate Rbmy gene, thus Y sperm or fertilized ovum with Y sperm is deactivated and cannot develop into a normal embryo, birth ratio of female animals is finally increased and sex control is achieved. Therefore, sex ratio of animal offspring can be controlled through the method so that the ratio of female animals with better economic value is increased and production efficiency and economic effect are greatly improved.
Owner:SOUTH CHINA AGRI UNIV
Method of generating a transgenic livestock animal
InactiveUS7199281B2Other foreign material introduction processesFermentationBiotechnologyHuman animal
The present invention provides methods of producing transgenic livestock animals. The methods generally involve first introducing a nucleoprotein made up of nucleic acid and a recombinase into a totipotent or pluripotent cell to produce a recombinant totipotent or pluripotent cell and then growing the recombinant totipotent or pluripotent cell to produce the transgenic livestock animal. The invention further provides kits for use in generating transgenic non-human animals of the invention.
Owner:RGT UNIV OF CALIFORNIA
Reverse genetic operation system of New castle disease LaSota vaccine strain and its applciation
The present invention is reverse genetic operation system of Newcastle disease Lasota low virulent vaccine strain and its application. The system includes one transcription plasmid including the genome cDNA sequence of the low virulent vaccine strain; one or several transcription aiding plasmids including the cDNA sequence coding the nucleoprotein of the low virulent vaccine strain, the cDNA sequence coding the phosphoprotein of the low virulent vaccine strain and the cDNA sequence coding the large polymerase protein of the low virulent vaccine strain; and the host cell of the Newcastle disease Lasota low virulent vaccine strain. Wild viral strain is obtained by means of the reverse genetic operation system. The present invention lays firm foundation for further development of Newcastle disease virus live carrier vaccine and Newcastle disease virus related research.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Replication-defective arenavirus vectors
ActiveUS20100297172A1Enhance protein expressionHigh expressionAntibacterial agentsSsRNA viruses negative-senseAntigenDisease
The invention relates to an infectious arenavirus particle that is engineered to contain a genome with the ability to amplify and express its genetic information in infected cells but unable to produce further infectious progeny particles in normal, not genetically engineered cells. One or more of the four arenavirus open reading frames glycoprotein (GP), nucleoprotein (NP), matrix protein Z and RNA-dependent RNA polymerase L are removed or mutated to prevent replication in normal cells but still allowing gene expression in arenavirus vector-infected cells, and foreign genes coding for an antigen or other protein of interest or nucleic acids modulating host gene expression are expressed under control of the arenavirus promoters, internal ribosome entry sites or under control of regulatory elements that can be read by the viral RNA-dependent RNA polymerase, cellular RNA polymerase I, RNA polymerase II or RNA polymerase III. The modified arenaviruses are useful as vaccines and therapeutic agents for a variety of diseases.
Owner:UNIV ZURICH
Entire gene sequence of severe fever with thrombocytopenia syndrome virus (SFTSV) and application
ActiveCN102070704AStrong specificityPeptide/protein ingredientsGenetic material ingredientsPolymerase LStructural protein
The invention relates to a severe fever with thrombocytopenia syndrome virus (SFTSV), an entire gene sequence represented by Hubei isolate HB29, amino acid sequences of coding proteins and application. The entire gene sequence of the virus is subjected to homology analysis. The virus belongs to bunyaviridae and comprises three gene segments, namely, L, M and S which represent polymerase and glycoprotein (Gn and Gc), nucleoprotein (NP) and non-structural proteins (NSs) of the virus respectively, and the three segments are all positioned on the branch of phlebovirus but farther from other viruses of phlebovirus. The entire gene sequence and the coding proteins of the virus can be used for developing drugs, vaccines or diagnostic reagents for preventing and treating the epidemic diseases caused by the SFTSV.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Method Enabling the Use of Extracellular Ribonucleic Acid (RNA) Extracted from Plasma or Serum to Detect, Monitor or Evaluate Cancer or Premalignant Conditions
InactiveUS20080261292A1Assess prognosisPredict prognosisSugar derivativesMicrobiological testing/measurementNeoplasmCirculating RNA
This invention relates to the use of tumor-derived or associated extracellular ribonucleic acid (RNA) found circulating in the plasma or serum fraction of blood for the detection, monitoring, or evaluation of cancer or premalignant conditions. Extracellular RNA may circulate as non-bound RNA, protein-bound RNA, lipid-RNA complexes, lipoprotein (proteolipid)-RNA complexes, protein-RNA complexes including within or in association with ribonucleoprotein complexes, nucleosomes, or within apoptotic bodies. Any intracellular RNA found in plasma or serum can additionally be detected by this invention. Specifically, this invention enables the extraction of circulating RNA from plasma or serum and utilizes nucleic acid amplification assays for the identification, detection, inference, monitoring, or evaluation of any neoplasm, benign, premalignant, or malignant, in humans or other animals, which might be associated with that RNA. Further, this invention allows the qualitative or quantitative detection of tumor-derived or associated extracellular RNA circulating in the plasma or serum of humans or animals with or without any prior knowledge of the presence of cancer or premalignant tissue.
Owner:ONCOMEDX
Compositions and Methods for Modifying a Predetermined Target Nucleic Acid Sequence
ActiveUS20150211023A1Cost-effective and reliableLess genotoxicOrganic active ingredientsHydrolasesNucleic acid sequencingIn vivo
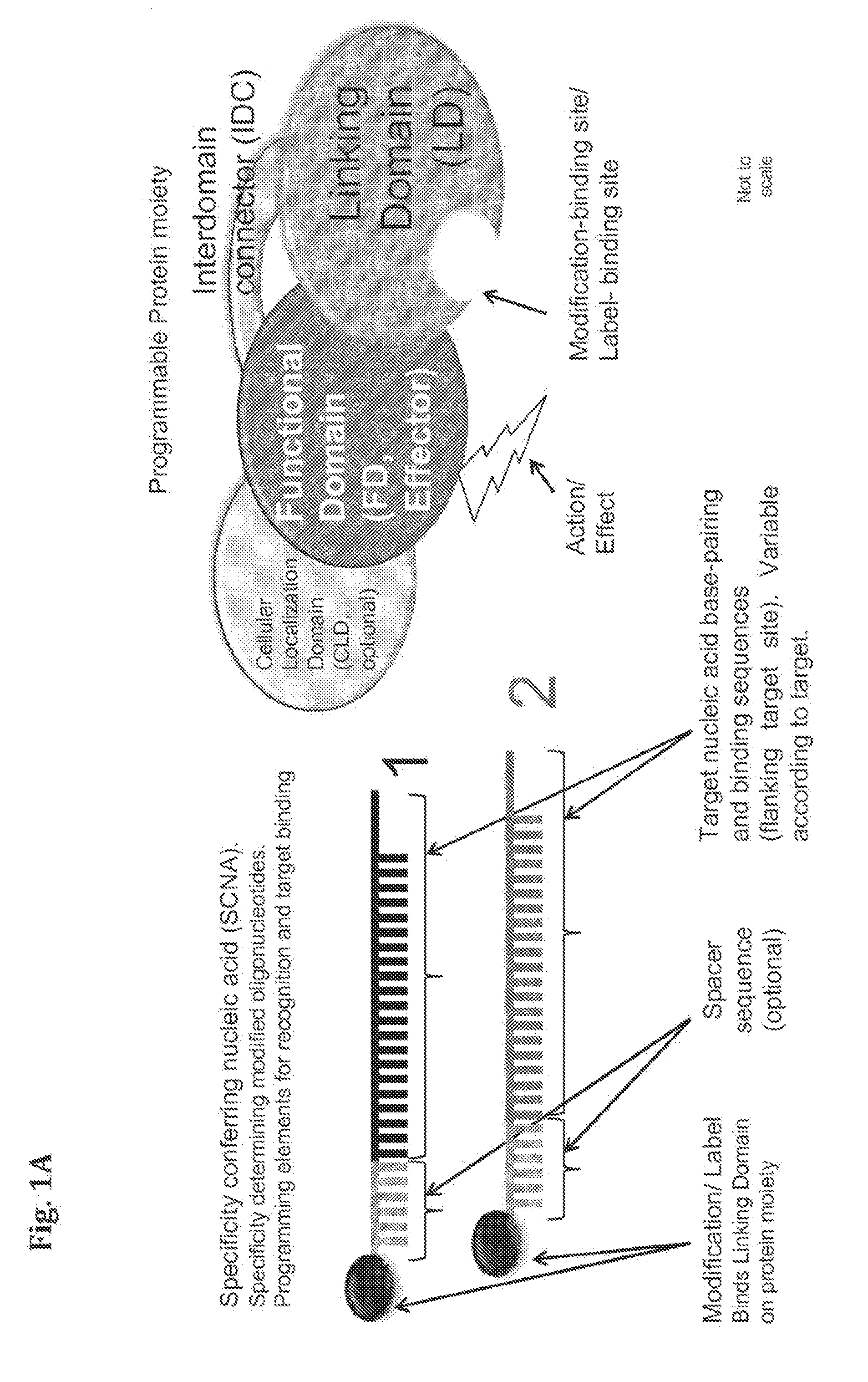
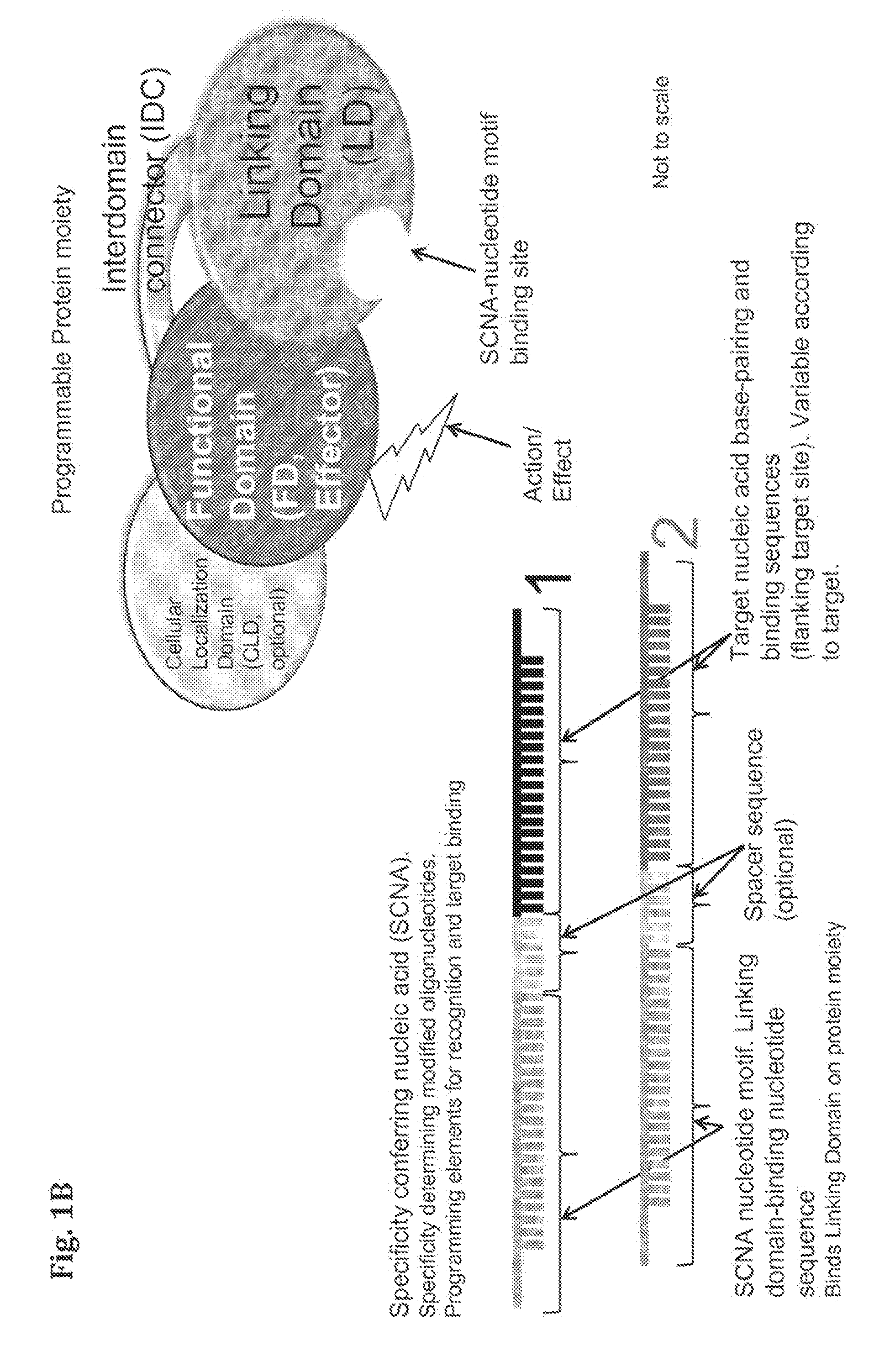
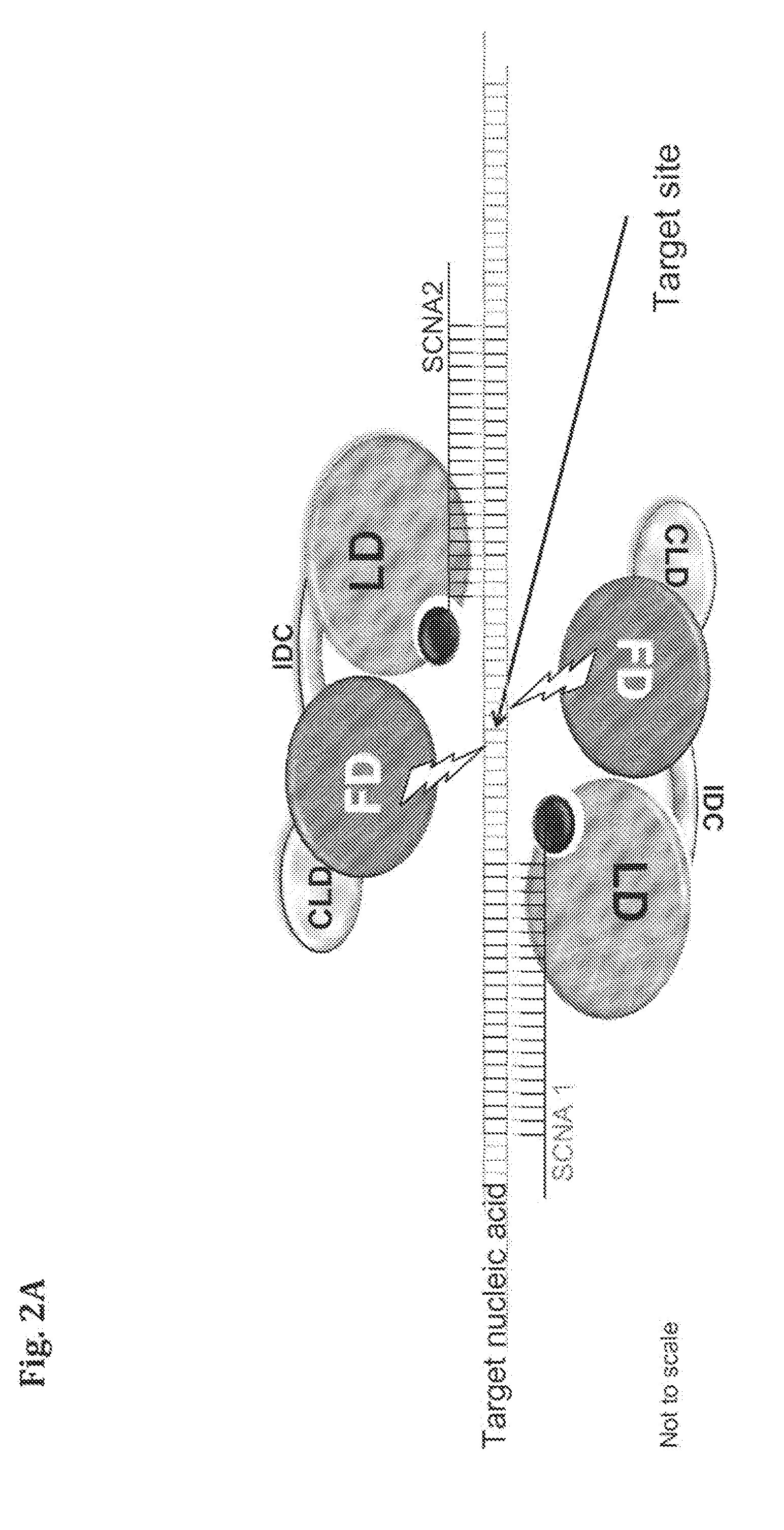
Provided herein are compositions and methods for modifying a predetermined nucleic acid sequence. A programmable nucleoprotein molecular complex containing a polypeptide moiety and a specificity conferring nucleic acid (SCNA) which assembles in-vivo, in a target cell, and is capable of interacting with the predetermined target nucleic acid sequence is provided. The programmable nucleoprotein molecular complex is capable of specifically modifying and / or editing a target site within the target nucleic acid sequence and / or modifying the function of the target nucleic acid sequence.
Owner:TARGETGENE BIOTECH
Compounds and methods for the treatment of viral infections
High throughput and virtual screening methods are disclosed that can identify potential anti-viral agents. The virtual screening methods identify agents that interact with a viral nucleoprotein binding site. The high throughput methods identify compounds that inhibit viral infection by binding to viral nucleoprotein. Also disclosed are pharmaceutical formulations useful for treating or preventing viral infections, especially influenza A.
Owner:VERSITECH LTD
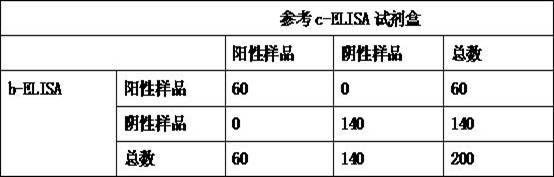
Kit for detecting antibody against Peste des petits ruminants virus b-ELISA and preparation method thereof
InactiveCN102419369AReduce economic costsLow costMaterial analysisViral antibodyEpidemiologic survey
The invention relates to the technical field of biology, particularly the field of viral antibody detection. A kit for detecting the antibody against Peste des petits ruminants virus b-ELISA comprises the following ingredients which are arranged respectively: Peste des petits ruminants nucleoprotein antigen, Peste des petits ruminants monoclonal antibody, diluent, strong positive serum, weak positive serum, negative serum, HRP sheep anti-mouse secondary antibody, 20 times the concentration of washing liquid, substrate liquid, stopping solution and enzyme-linked immunosorbent plate. The optimum proportion of each ingredient in the kit is determined by experiments. The kit can be used for rapid diagnosis and detection of animal Peste des petits ruminants virus antibody, especially for the antibody detection of a lot of samples in the epidemiological survey of Peste des petits ruminants. The detection method of Peste des petits ruminants virus b-ELISA has different detection principle and experiment operating procedures and the like from those of a c-ELISA detection method in a BIRAD laboratory. The Peste des petits ruminants nucleoprotein antigen and Peste des petits ruminants monoclonal antibody in the kit are self-developed. The detection sensitivity, singularity and other indexes of the kit are the same with those of the c-ELISA detection method in the internationally recognized BIRAD laboratory.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR +1
Composition and methods of making and using influenza proteins
InactiveUS20090196915A1Many symptomReduce the likelihood of infectionSsRNA viruses negative-sensePowder deliveryImmuno modulationBody fluid
The invention provides compositions of influenza proteins, such as matrix and nucleoprotein, that are presented to an individual's immune system as multimeric displays to induce an immune response. The compositions are optionally associated with any type of immunomodulatory compound (IMC) comprising an immunostimulatory sequences (ISS). The invention further provides compositions of influenza matrix and nucleoproteins that can induce cellular and / or humoral immune response. The invention also provides methods of making and using these compositions, e.g., as a vaccine, for ameliorating symptoms associated with infection with influenza virus or for reducing the risk of infection with influenza virus.
Owner:DYNAVAX TECH CORP
Novel vaccines against multiple subtypes of influenza virus
ActiveUS20090169505A1Elicit immune responseSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininMammal
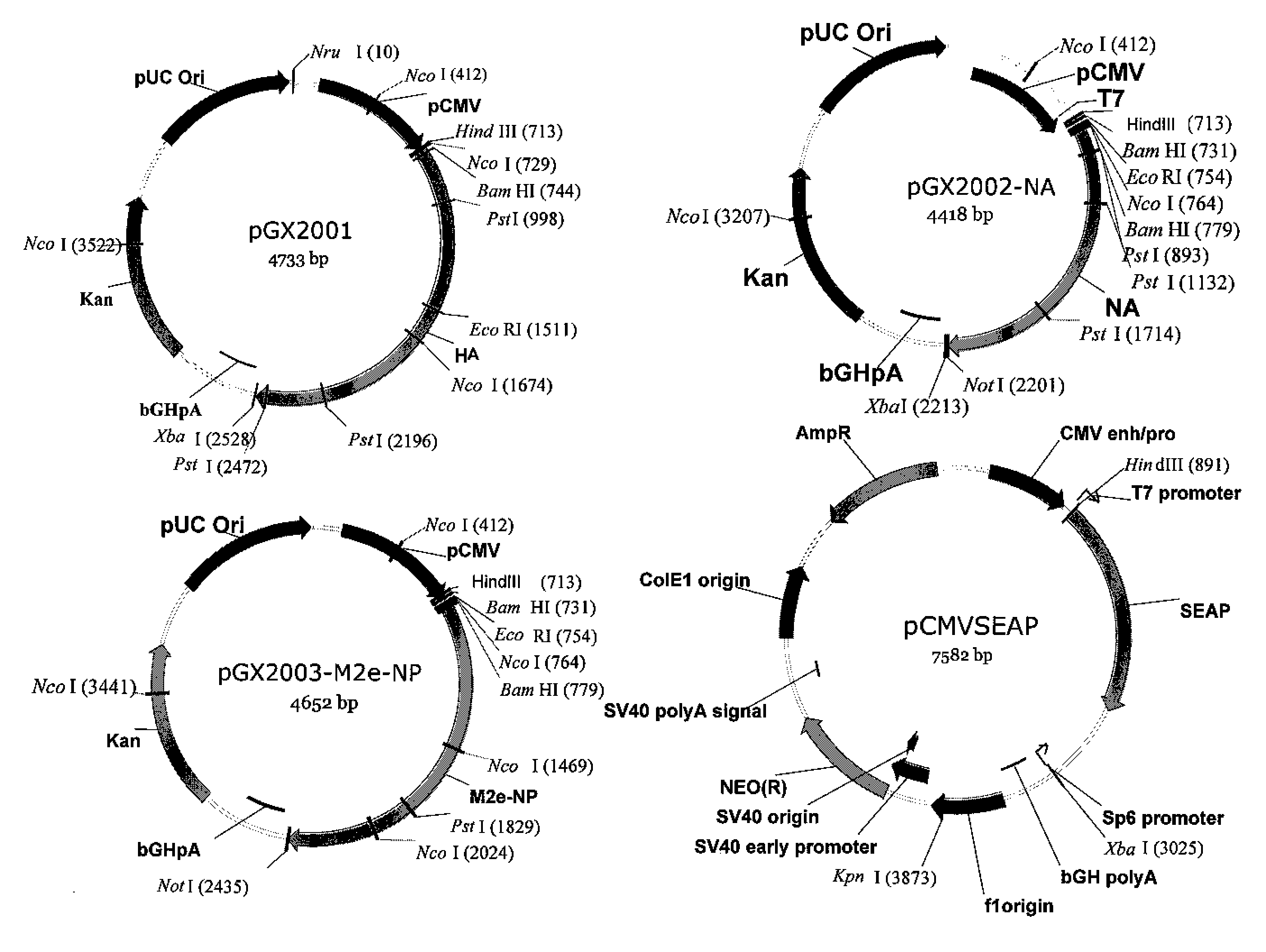
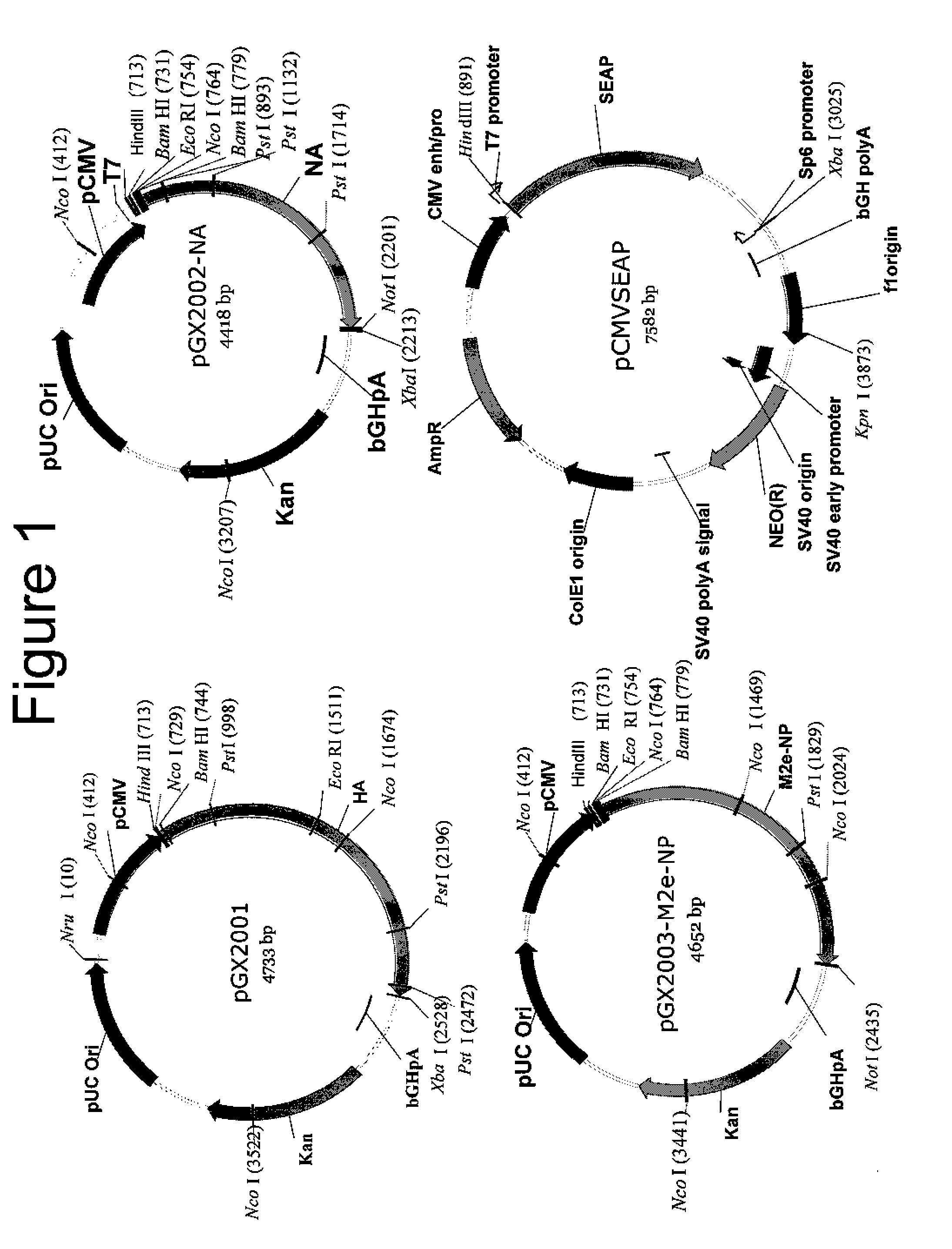
An aspect of the present invention is directed towards DNA plasmid vaccines capable of generating in a mammal an immune response against a plurality of influenza virus subtypes, comprising a DNA plasmid and a pharmaceutically acceptable excipient. The DNA plasmid is capable of expressing a consensus influenza antigen in a cell of the mammal in a quantity effective to elicit an immune response in the mammal, wherein the consensus influenza antigen comprises consensus hemagglutinin (HA), neuraminidase (NA), matrix protein, nucleoprotein, M2 ectodomain-nucleo-protein (M2e-NP), or a combination thereof. Preferably the consensus influenza antigen comprises HA, NA, M2e-NP, or a combination thereof. The DNA plasmid comprises a promoter operably linked to a coding sequence that encodes the consensus influenza antigen. Additionally, an aspect of the present invention includes methods of eliciting an immune response against a plurality of influenza virus subtypes in a mammal using the DNA plasmid vaccines provided.
Owner:VGX PHARMA +1
Novel telomerase
InactiveUS20030009019A1SpecificallyEfficiently catalyze endonucleolytic cleavageBacteriaPeptide/protein ingredientsTelomeraseRibonucleoprotein complex
Owner:UNIV OF COLORADO THE REGENTS OF
Antiviral agents and vaccines against influenza
InactiveUS20090208531A1SsRNA viruses negative-senseOrganic active ingredientsHemagglutininPrime boost
These vaccines target H5N1, H1, H3 and other subtypes of influenza and are designed to elicit neutralizing antibodies, as well as cellular immunity. The DNA vaccines express hemagglutinin (HA) or nucleoprotein (NP) proteins from influenza which are codon optimized and / or contain modifications to protease cleavage sites of HA which affect the normal function of the protein. Adenoviral constructs expressing the same inserts have been engineered for prime boost strategies. Protein-based vaccines based on protein production from insect or mammalian cells using foldon trimerization stabilization domains with or without cleavage sites to assist in purification of such proteins have been developed. Another embodiment of this invention is the work with HA pseudotyped lentiviral vectors which would be used to screen for neutralizing antibodies in patients and to screen for diagnostic and therapeutic antivirals such as monoclonal antibodies.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
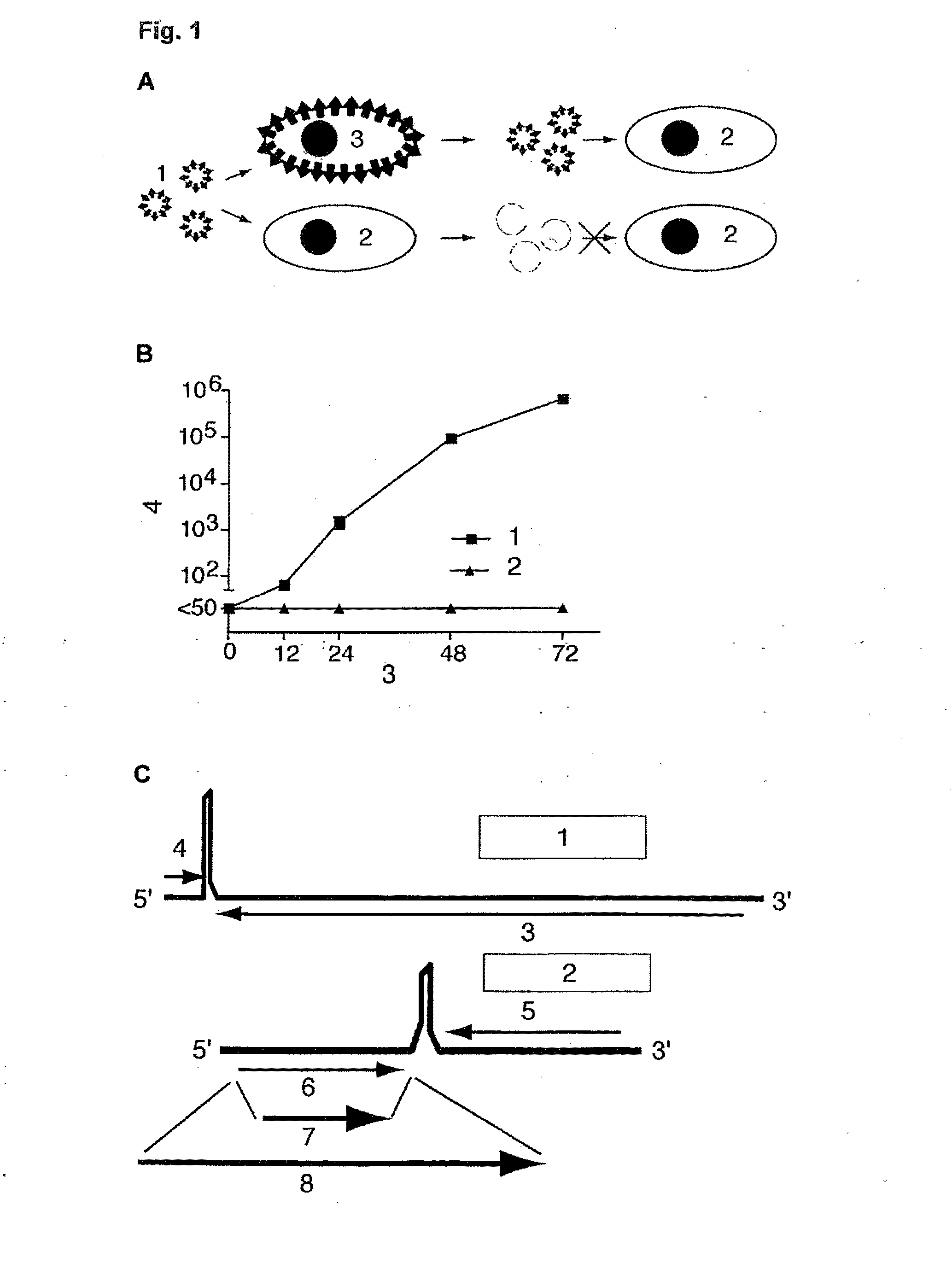
Helper-free rescue of recombinant negative strand RNA virus
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Modular transfection systems
InactiveUS7320859B2Suitable for transportationReduced diameter requirementsAntibody mimetics/scaffoldsGenetic material ingredientsModularityTherapeutic treatment
The present invention relates to a method for transfection of cells using at least one protein capable of forming nucleoprotein filaments, wherein the protein is initially modified with at least one functional component which influences one or more steps of the transfection, the nucleic acid to be transfected is then loaded with the modified protein, whereby the nucleic acid and the protein form a filament-like complex, and this complex is finally added to the cells to be transfected. The invention further relates to a transfection agent consisting of nucleoprotein filaments (NPF), with at least one nucleoprotein filament-forming protein being modified with at least one functional component for the transfection. Furthermore, the present invention relates to the use of the transfection agent according to the invention for producing a drug for gene therapeutic treatment of humans and animals. The present inventions also includes corresponding pharmaceutical preparations, especially for use in gene therapy as well as the use of such transfection agents as component in kits.
Owner:LONZA COLOGNE
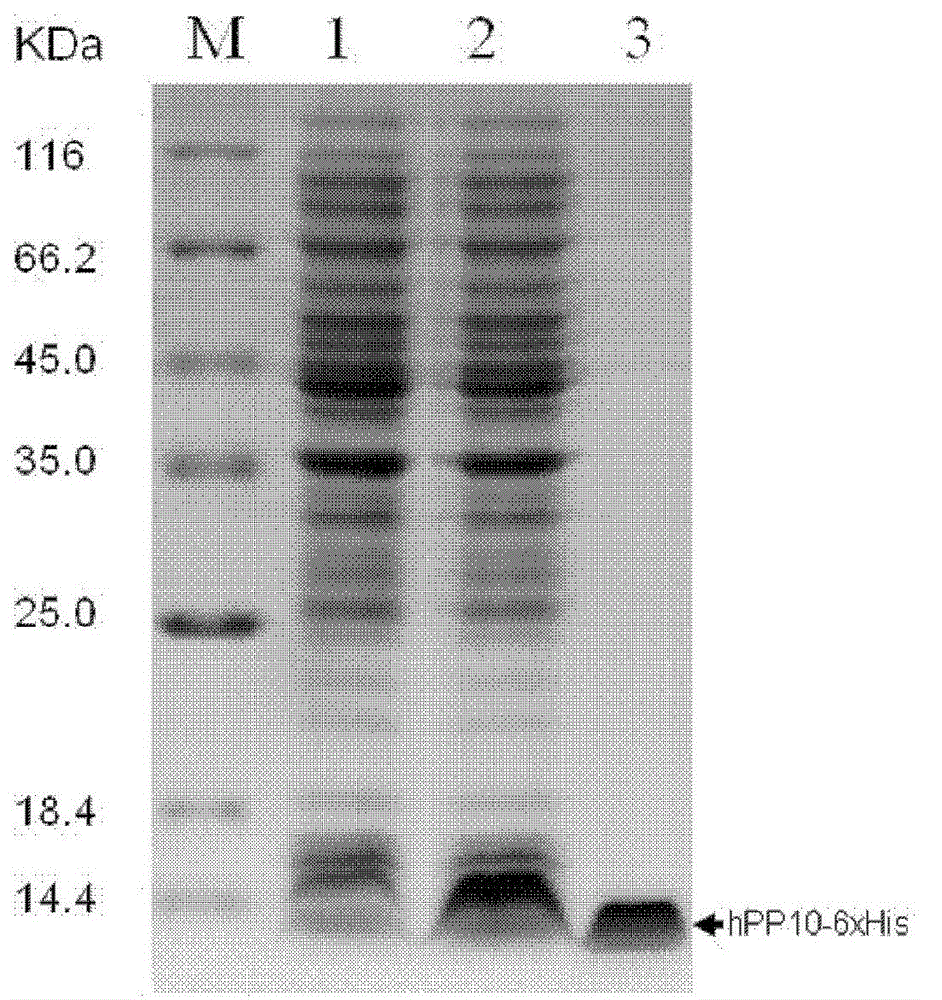
Production of cell-penetrating peptide hPP10 (human Pancreatic Polypeptide) and transfection method for mediated plasmid DNA (Deoxyribose Nucleic Acid) of hPP10
ActiveCN102863516ALess likely to have an immune responseReduce insecurityPeptide preparation methodsFermentationCell membranePlasmid dna
The invention relates to the field of biomedicine, in particular to production of a novel human cell-penetrating peptide hPP10 (human Pancreatic Polypeptide) and a transfection method for a mediated plasmid DNA (Deoxyribose Nucleic Acid) of the hPP10. The hPP10 is derived from human cell nucleoproteins; and one segment of polypeptide sequence of a lysine specific demethylated enzyme 4A has a cell penetrating function, can be used for carrying proteins, allows nucleic acids (DNA plasmids) to enter a plurality of types of cells in a transmembrane manner, and is a transmembrane transport carrier with an extremely good development prospect for bioactive molecules such as proteins, nucleic acids and the like.
Owner:深圳真实生物医药科技有限公司
Kit box used for detecting influenza virus in sample and detection method and application thereof
ActiveCN104062430ARealize the immune cascade effectSimple resultBiological material analysisImmunoglobulinsAvian influenza virusMonoclonal antibody
The invention provides a kit box used for detecting an influenza virus in a sample. The kit box comprises a buffer solution supply unit and a detection test strip. The detection test strip sequentially comprises a substrate supply area, a sample supply area and a detection area in the longitudinal direction. The substrate supply area comprises a substrate pad (3), a dry enzyme substrate is adsorbed to the substrate pad, and the substrate pad (3) makes contact with a nitrocellulose membrane (1). The sample supply area comprises an enzyme mark pad (2), a rat resistance avian influenza virus nucleoprotein antibody marking monoclonal antibody 1 is adsorbed to the enzyme mark pad (2), and the enzyme substrate can product a chromogenic reaction with enzymes marked on the marking monoclonal antibody 1. The detection area is provided with a rat resistance avian influenza virus nucleoprotein antibody fixed monoclonal antibody 2 in an immobilization mode. The invention further provides a method for detecting the influenza virus in the sample through the kit box. The kit box and the detection method of the kit box have the advantages of being rapid and accurate in detection, high in specificity, sensitive and good in stability.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Construction of nucleoprotein based assemblies comprising addressable components for nanoscale assembly and nanoprocessors
A nucleoprotein based nanoprocessor is described. The nanoprocessor includes one or more chimelic fusion proteins linked to a DNA scaffold. Both components of the fusion protein are enzymes.
Owner:CITY OF HOPE
Replication-defective arenavirus vectors
Owner:UNIV ZURICH
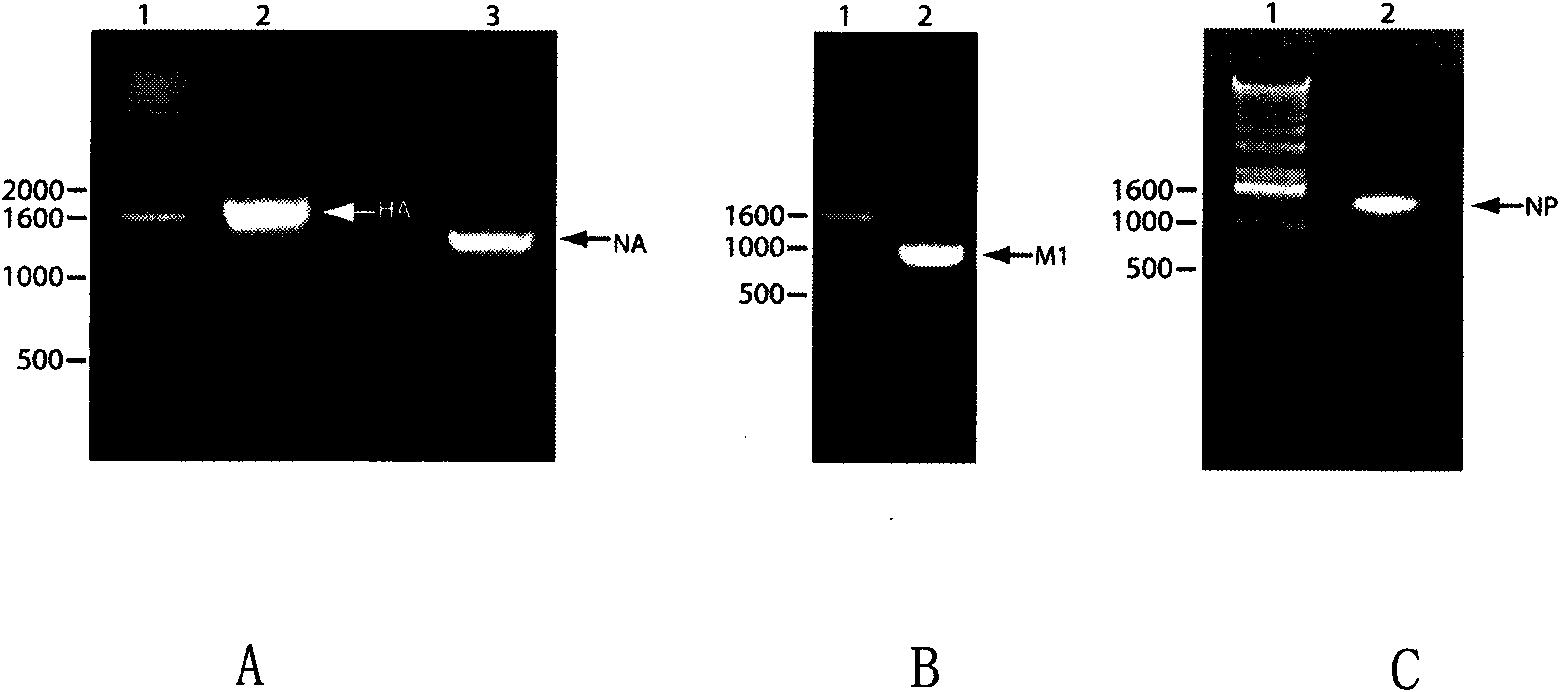
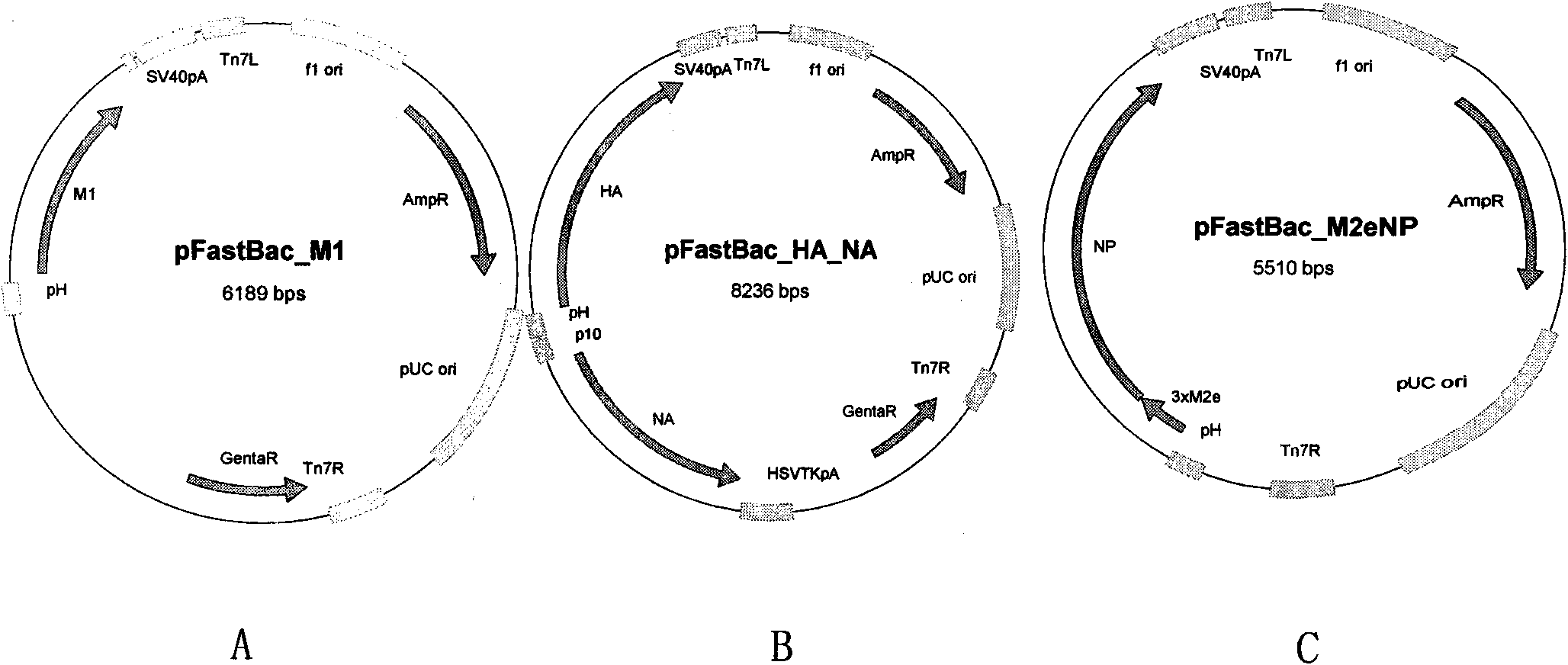
Broad-spectrum safe anti influenza A virus vaccine for animals
ActiveCN101643721AImprove immunityAnti-leakageMicroorganism based processesAntiviralsCell membraneMutant
Recombinant virus-like particle contains influenza A virus matrix protein M1, surface film proteins HA and NA and M2eNP fusion protein or protein obtained by modification of mutant of at least one protein and the rest proteins, and the recombinant virus-like particle is non-replicative; wherein the M2eNP fusion protein is polymer formed by one M2e polypeptide or a plurality of M2e polypeptides atthe external end of cell membrane of matrix protein M2 or is formed by fusion of nucleoprotein NP and polymer formed by one M2e polypeptide through artificial modification or a plurality of M2e polypeptides after modification; and the nucleoprotein NP is coupled with recombinant matrix protein M1 after recombination expression and embedded in the recombinant virus-like particle. The vaccine produced by the recombinant virus-like particle can be directly applied to various animals for prevention of infection and spread of influenza A virus. The vaccine is safe in use and obvious in immune effect. Production period is short, technical operation is simple, and no purification is required, thus the vaccine is low in cost.
Owner:许雁
Newcastle disease virus heat resistant live vaccine vector system and application thereof
ActiveCN104059942AHeat resistantHigh heat resistanceSsRNA viruses negative-senseMicroorganism based processesDiseasePBR322
The invention belongs to the field of virus genetic operation, and in particular to a Newcastle disease virus (NDV) heat resistant live vaccine vector system and application thereof. The Newcastle disease virus (NDV) heat resistant live vaccine vector system comprises a) a transcription plasmid, b) three auxiliary plasmids and c) host cells. The transcription plasmid is obtained by cloning genomic full-length cDNA of a NDV heat resistant vaccine strain to pBR322 vector; and the three auxiliary plasmids are obtained by cloning nucleoprotein, phosphoprotein and large polymerase protein gene of the NDV heat resistant vaccine strain to pcDNA3.1 vector. The artificial recombinant Newcastle disease virus has the characteristic of heat resistance, and the Newcastle disease virus (NDV) heat resistant live vaccine vector system is established for the first time. The artificial recombinant Newcastle disease virus has great application prospect in the aspects of research and development of multiple (multivalent) heat resistant genetic engineering live vaccines of the NDV, avian influenza and other major diseases of poultry, research on virus heat-resistant mechanism, and the like.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
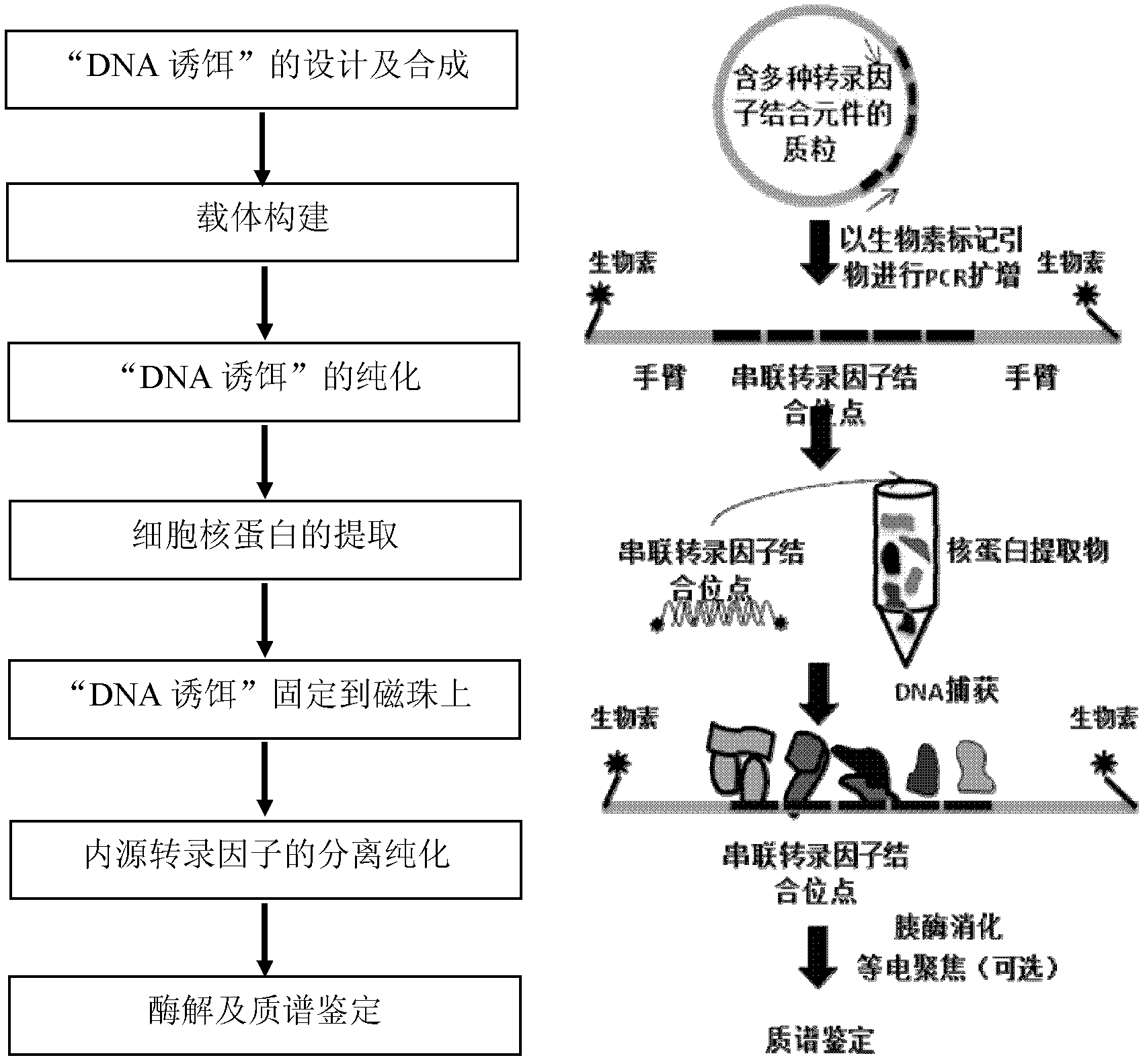
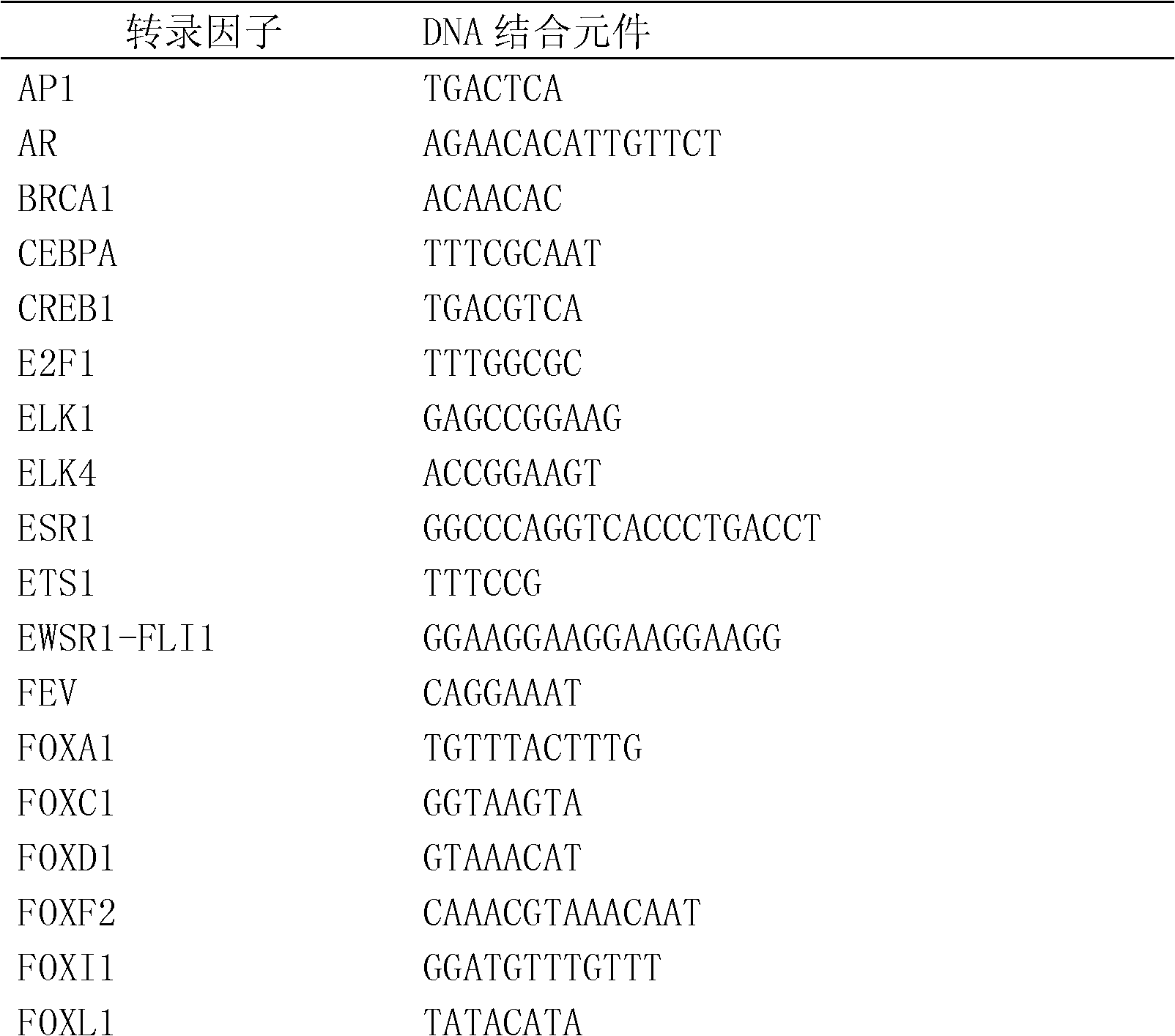
Method for enriching and separating endogenous transcription factors and compounds thereof and concatenated transcription factor response elements special for method
ActiveCN102517282AMicrobiological testing/measurementPeptide preparation methodsBiotin-streptavidin complexMagnetic bead
The invention discloses a method for enriching and separating endogenous transcription factors and compounds thereof and concatenated transcription factor response elements special for the method. The method includes of designing multi-copy double-stranded DNA (deoxyribonucleic acid) binding elements according to the characteristic that transcription factors are bound with sequence-specific DNA elements, adding double-stranded DNA arms labeled with biotin to two ends of each DNA binding element by the aid of molecular cloning technology to form 'DNA baits', coupling the biotinylated DNA baits which are purified onto magnetic beads enveloped by streptavidin when in separation and purification of the endogenous transcription factors and compounds thereof, incubating the magnetic beads together with the prepared nucleoprotein, and finally separating and purifying the endogenous transcription factors and the compounds thereof from the nucleoprotein. Further, after the endogenous transcription factors and the compounds thereof are separated and purified, protein identification and function analysis of the transcription factors can be performed subsequently.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA +1
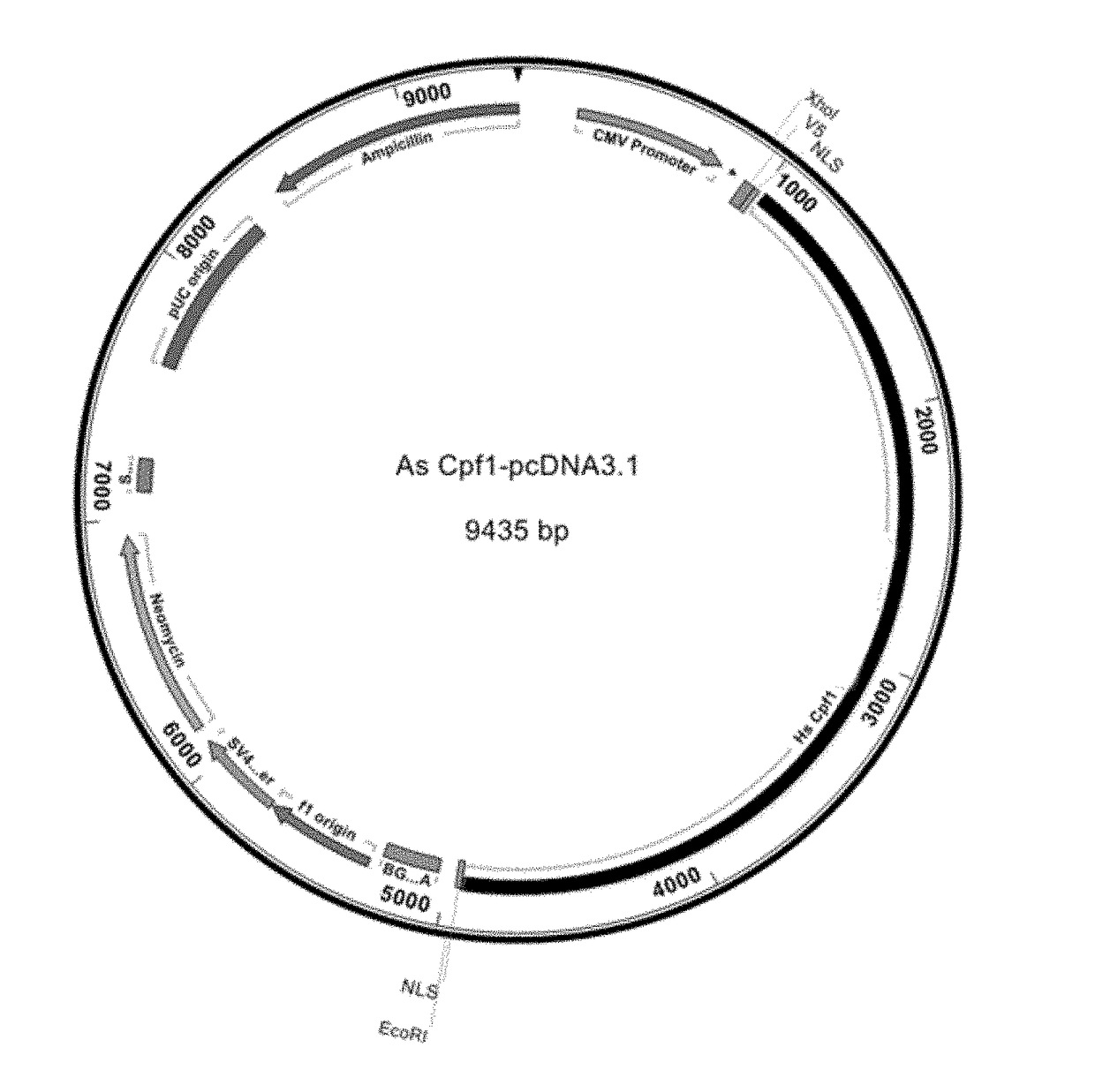
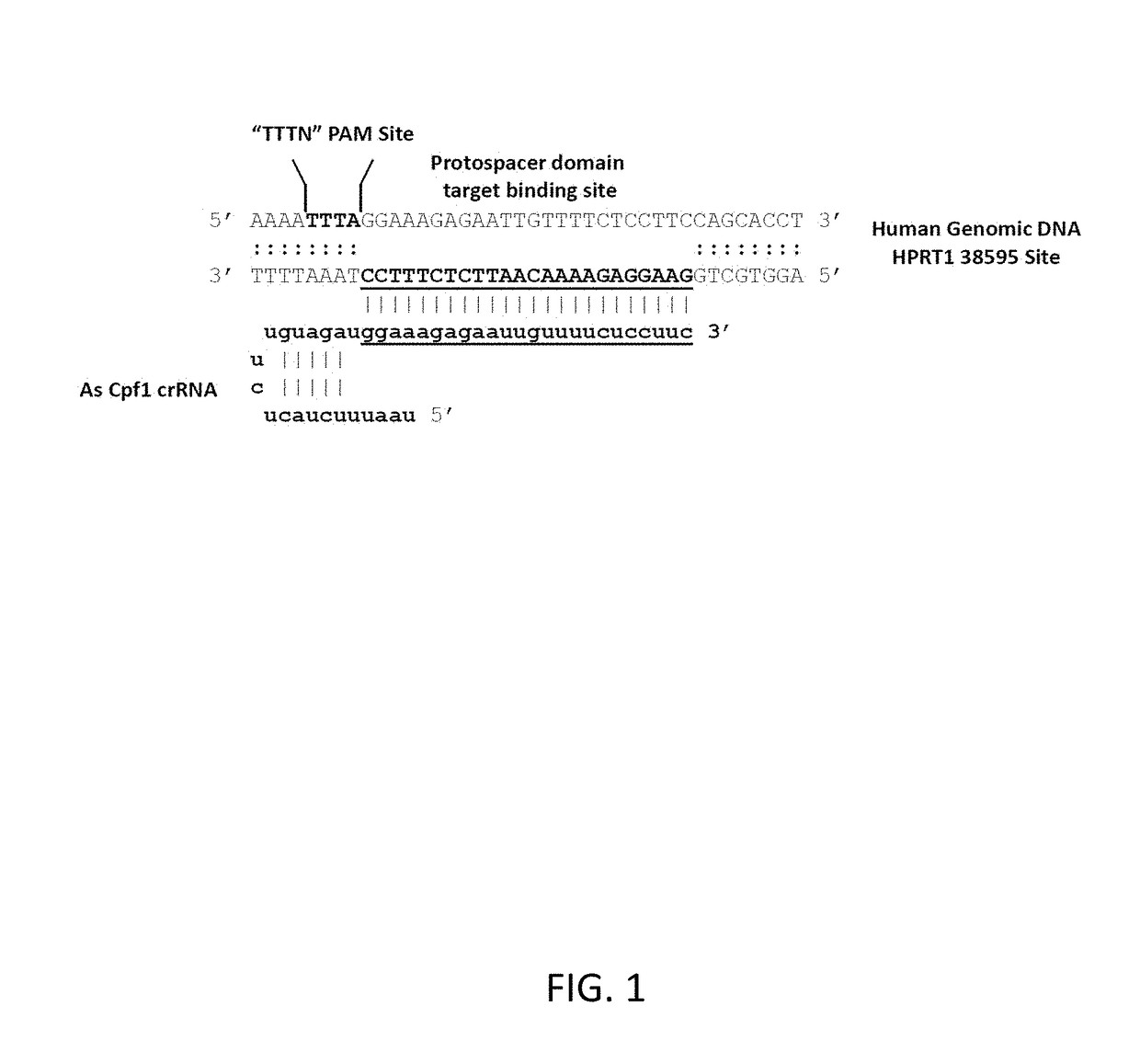
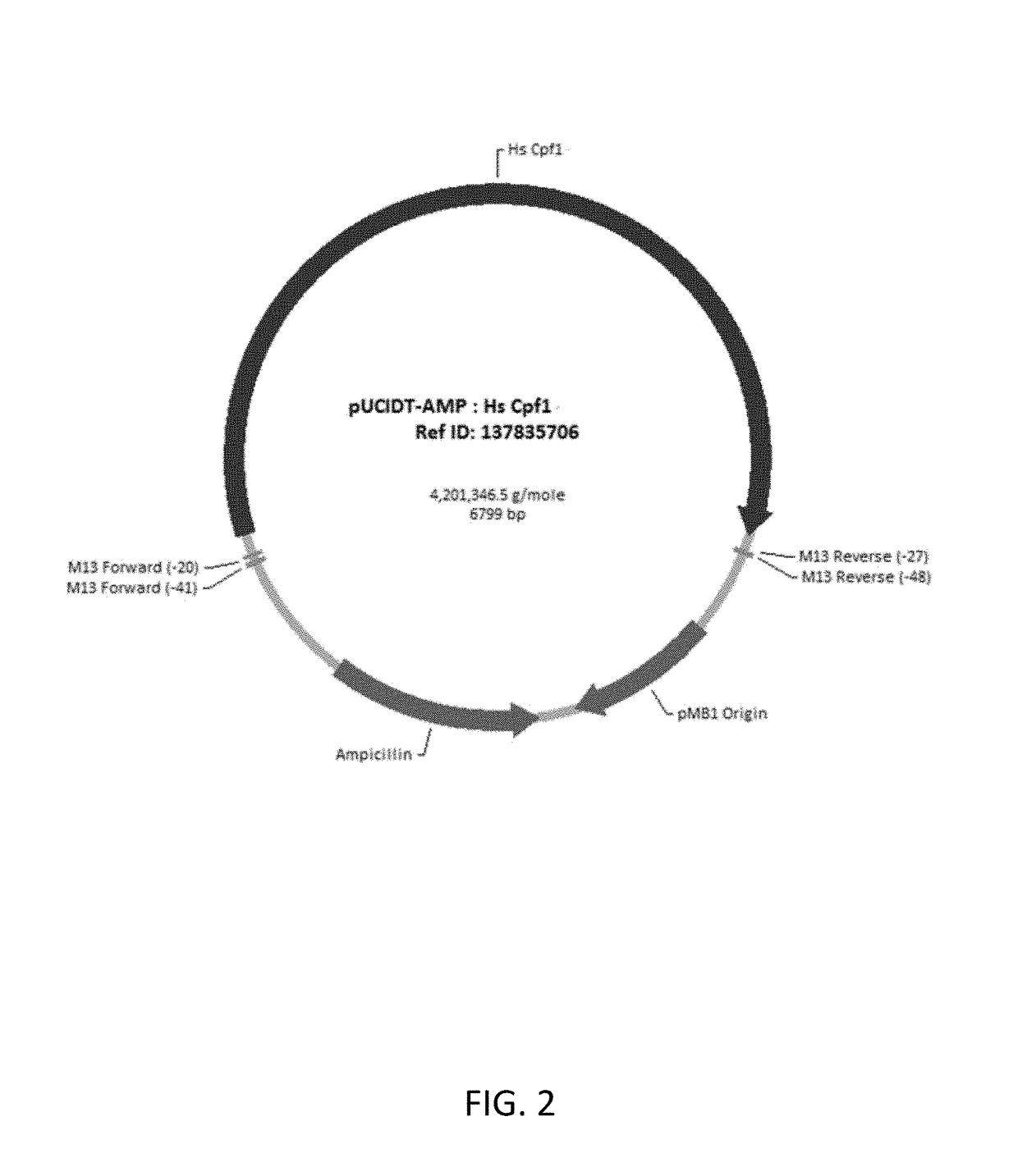
Crispr/cpf1 systems and methods
ActiveUS20180187176A1Improve purification effectHydrolasesStable introduction of DNARibonucleoprotein complexCRISPR/Cpf1
This invention pertains to recombinant AsCpf1 and LbCpf1 nucleic acids and polypeptides for use in CRISPR / Cpf1 endonuclease systems and mammalian cell lines encoding recombinant AsCpf1 or LbCpf1 polypeptides. The invention includes recombinant ribonucleoprotein complexes and CRSPR / Cpf1 endonuclease systems having a suitable AsCpf1 crRNA is selected from a length-truncated AsCpf1 crRNA, a chemically-modified AsCpf1 crRNA, or an AsCpf1 crRNA comprising both length truncations and chemical modifications. Methods of performing gene editing using these systems and reagents are also provided.
Owner:INTEGRATED DNA TECHNOLOGIES
Peste des petits ruminants virus (PPRV) reverse genetic operating system and application thereof
ActiveCN102071218ALow costReduce labor costsInactivation/attenuationVector-based foreign material introductionComplementary deoxyribonucleic acidFull length cdna
The invention relates to a peste des petits ruminants virus (PPRV) reverse genetic operating system and an application thereof. The PPRV reverse genetic operating system comprises a transcription plasmid and one or more helper plasmids, wherein the transcription plasmid can express the genome full-length cDNA (complementary deoxyribonucleic acid) sequence of the PPRV; and the helper plasmid(s) can express nucleoprotein (N), phosphoprotein (P) and polymerase large protein (L) of the PPRV, and virus replication-permitting host cells of the PPRV. By using the PPRV reverse genetic operating system, the recombined PPRV is successfully saved. The establishment of the PPRV reverse genetic operating technical platform provides an excellent technical platform for the development of PPRV live vector vaccines and the fundamental research related to PPRV.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com