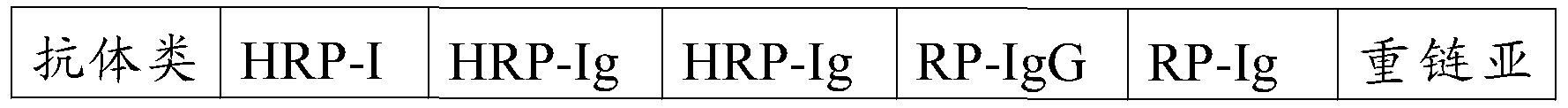Kit box used for detecting influenza virus in sample and detection method and application thereof
A technology for detecting influenza virus and samples, applied in chemical instruments and methods, measuring devices, instruments, etc., can solve the problems of low sensitivity and achieve the effects of high detection efficiency, convenient use, and simple results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
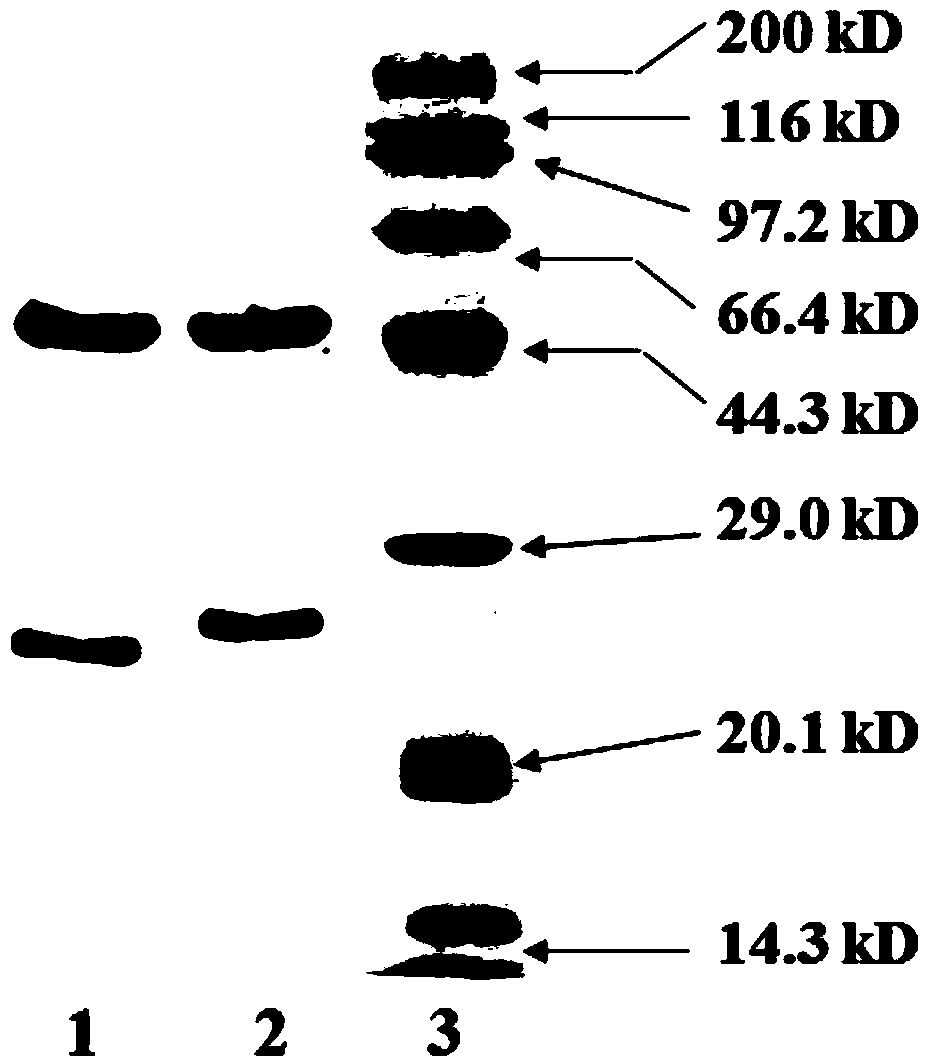
[0050] Example 1 Preparation, Purification, Identification and Testing of Anti-Influenza Virus Nucleoprotein Monoclonal Antibody
[0051] 1.1 Preparation and purification of anti-influenza virus nucleoprotein monoclonal antibody
[0052] The preparation and purification of anti-influenza virus nucleoprotein monoclonal antibody comprises the following steps:
[0053] 1.1.1 Animal immunization: Add 1 mL of inactivated virus of type A influenza virus A / California / 04 / 2009 (H1N1) strain and add the same amount of complete Freund's adjuvant and fully emulsify it, and immunize with the homologous myeloma cells used 6-8 weeks old BALB / c healthy mice, each multi-point injected with 500 μL of emulsified influenza A virus A / California / 04 / 2009 (H1N1) strain, boosted once every 2 weeks; its antiserum was measured by indirect ELISA :
[0054] After the completion of the process, the antiserum is measured by indirect ELISA, which consists of the following steps:
[0055] a. Coating:
[0...
Embodiment 2
[0100] Example 2 Pairing of Monoclonal Antibodies
[0101] 2.1 Selection of monoclonal antibody pairing mode
[0102] When screening monoclonal antibody pairing combinations, the following factors are mainly considered: first, the activity of the monoclonal antibody; second, whether there is a non-specific reaction between the monoclonal antibody and non-type A influenza virus; third, the color background.
[0103] 2.2 Detection of Monoclonal Antibody Activity
[0104] Select type A avian influenza virus strain A / Ck / HK / Yu22 / 02, dilute to 0.01HA, and test different matching modes. The results are shown in Table 2:
[0105] Table 2 Monoclonal antibody matching and activity detection
[0106]
[0107] Note: + means positive, - means negative.
[0108] The results showed that except for the Yu22 virus dilution of 0.01HA of monoclonal antibody 1 immobilized and monoclonal antibody 2 enzyme-labeled detection was negative, the other combinations were all positive results, but m...
Embodiment 3
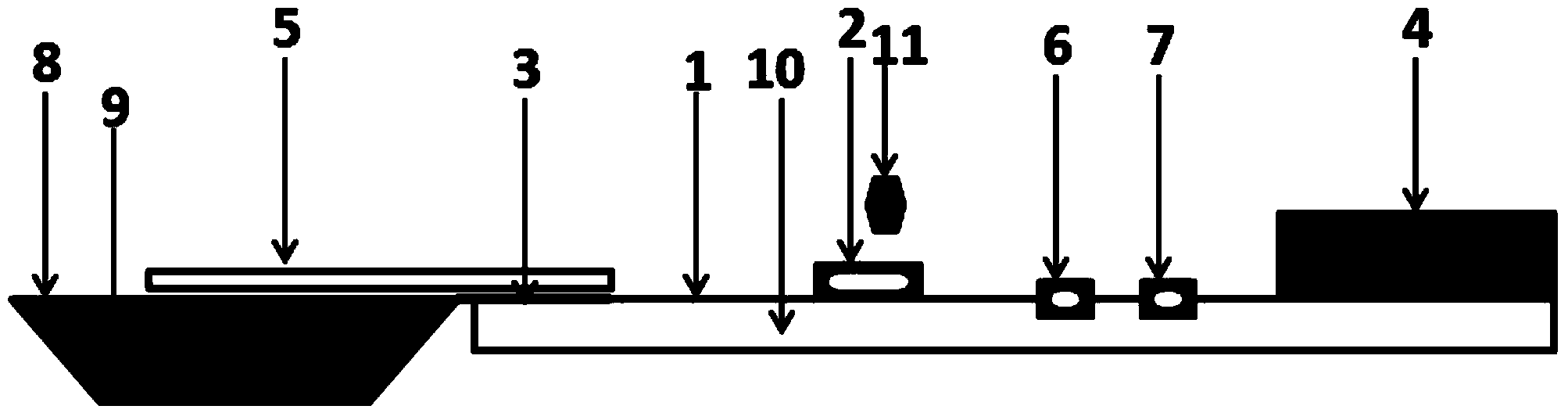
[0117] The structure and use of embodiment 3 type A influenza virus enzyme chromatography detection kit
[0118] 3.1 Immobilization of monoclonal antibodies
[0119] The monoclonal antibody 2 prepared in Example 1 was immobilized on the nitrocellulose membrane with a BioDot XYZ3050 three-dimensional spraying platform.
[0120] 3.2 Horseradish peroxidase (HRP) labeling of monoclonal antibodies
[0121] According to the monoclonal antibody 1 prepared in Example 1, according to Tijssen P et al (Tijssen P, Kurstak E. Highly efficient and simple methods for the preparation of peroxidase and active peroxidase antibody conjugates for enzymeimmunoassays [J]. -457) literature of anti-influenza A virus monoclonal antibody was labeled with horseradish peroxidase (HRP).
[0122] 3.3 Composition of the detection kit
[0123] The detection kit includes an enzyme chromatography detection test strip, a sample processing solution, a sample processing tube, and a sample preservation solution. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com