Patents
Literature
98 results about "Contagious disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A disease that can spread from one person to another through direct or indirect contact.
Self-contained device integrating nucleic acid extraction, amplification and detection
InactiveUS6153425AImprove accuracyHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsNucleic acid sequencingNucleic acid sequence
A self-contained device is described that integrates nucleic acid extraction, specific target amplification and detection into a single device. This integration permits rapid and accurate nucleic acid sequence detection. The invention may be used, for example, in the screening for nucleic acid sequences which may be indicative of genetic defects or contagious diseases, as well as for monitoring efficacy in the treatment of contagious diseases.
Owner:APPL BIOSYSTEMS INC
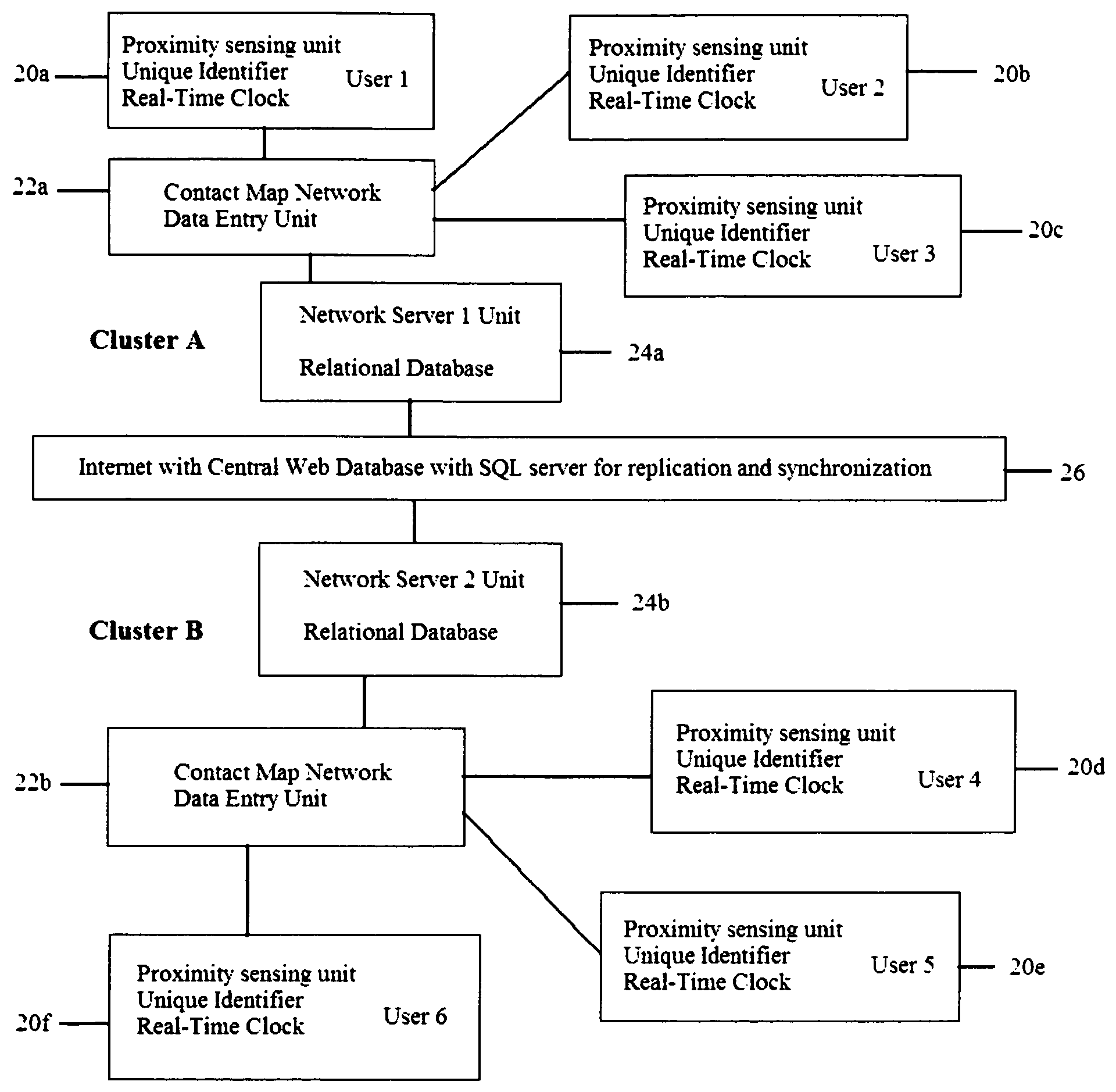
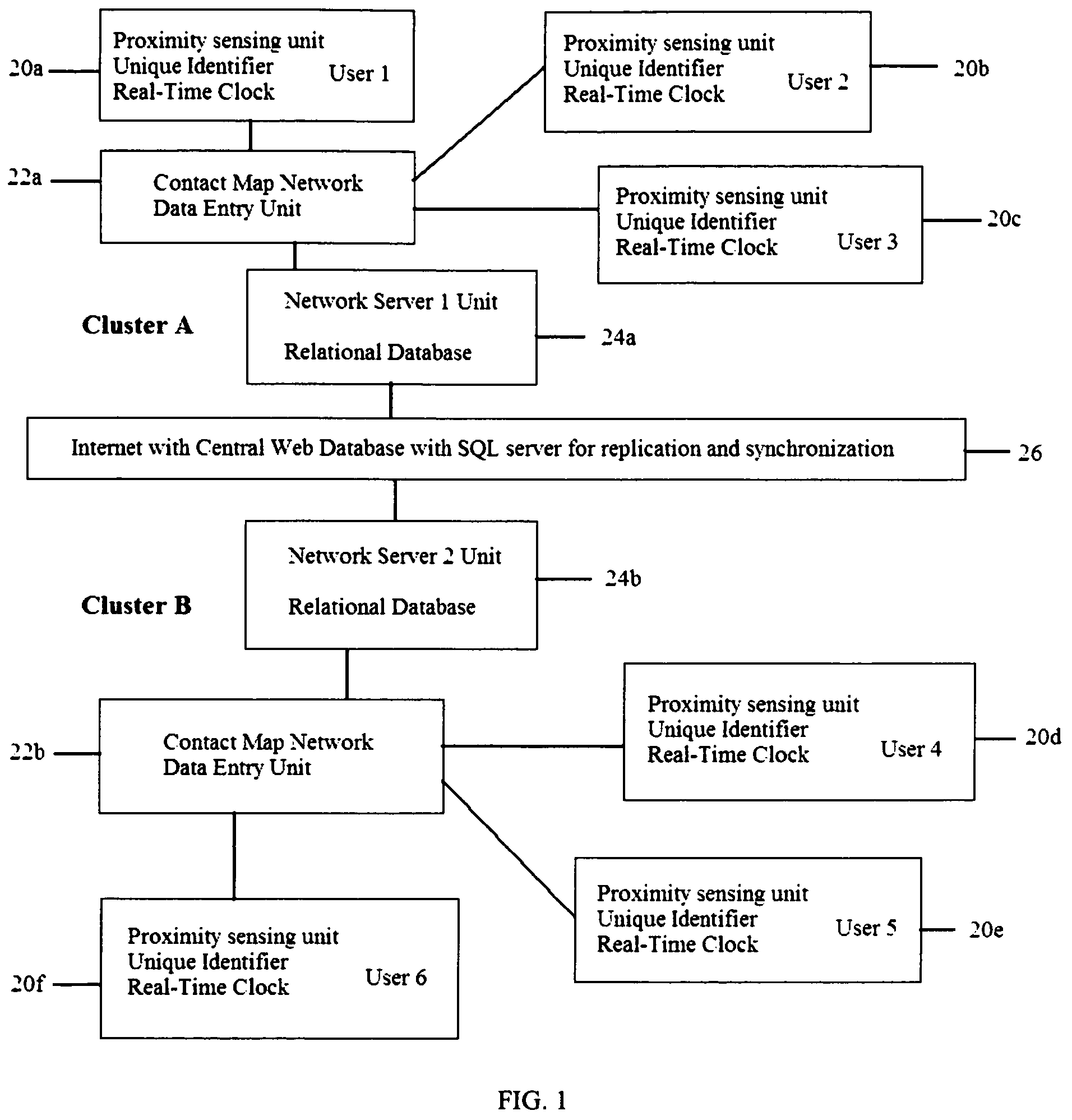
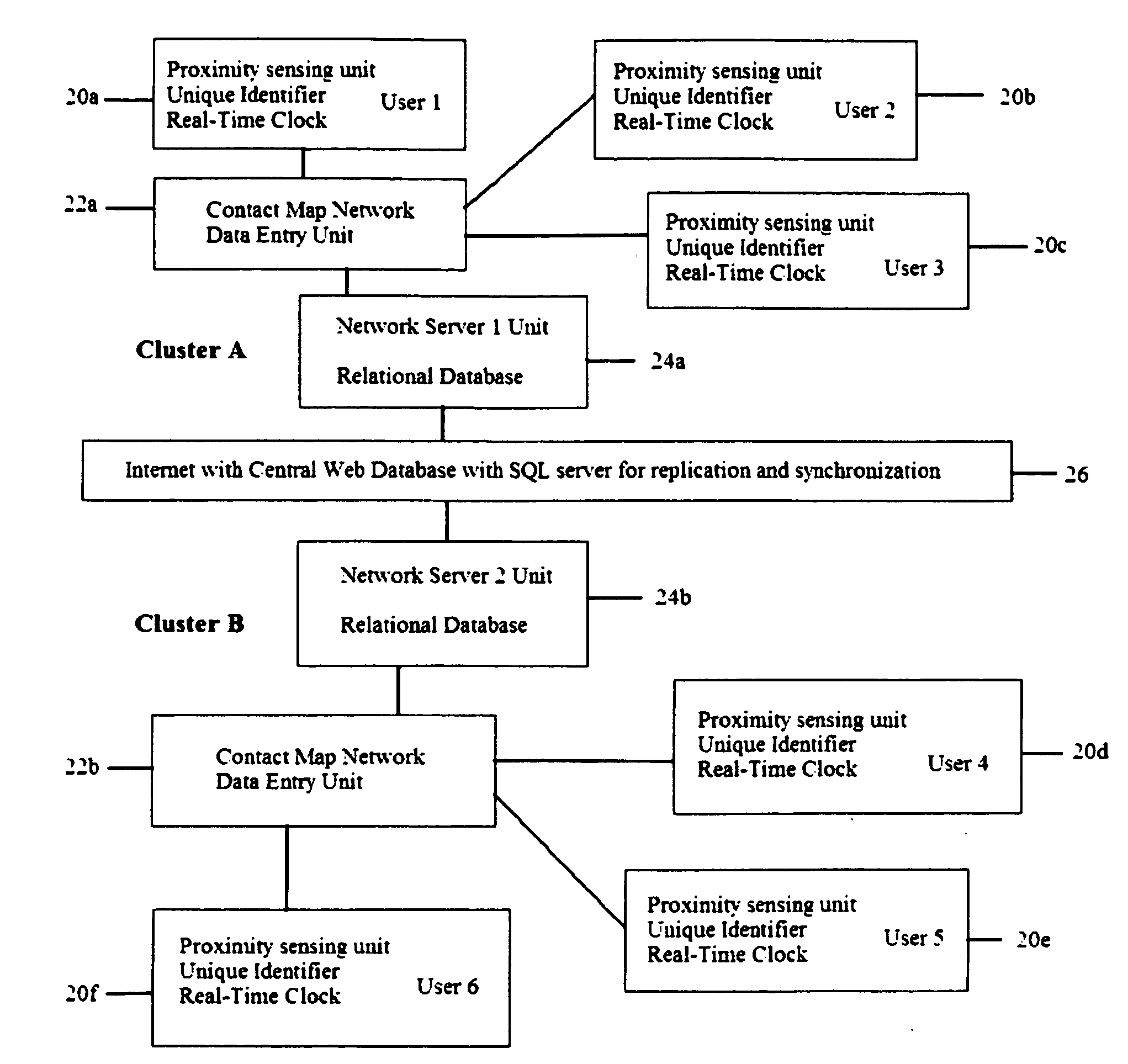
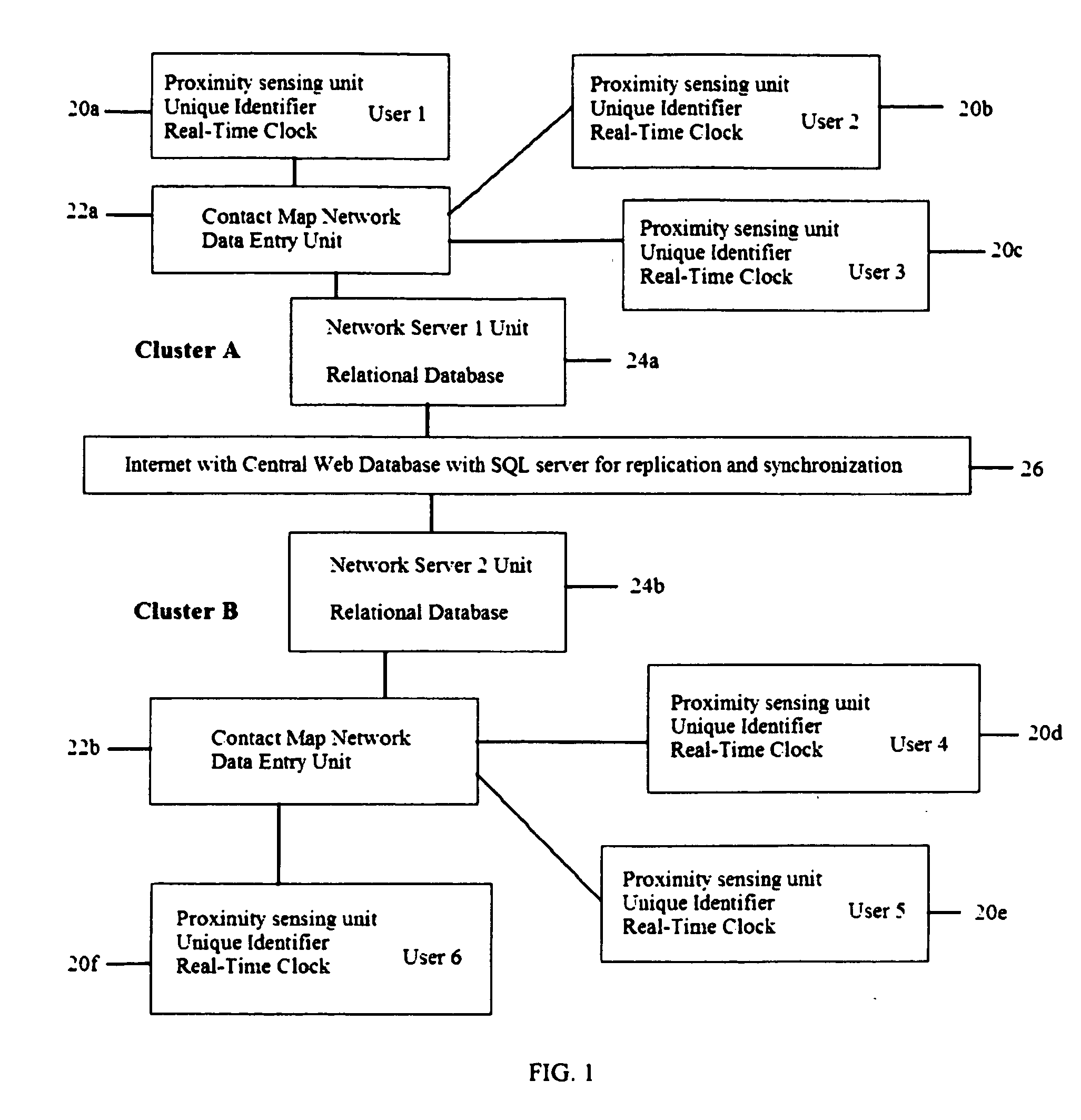
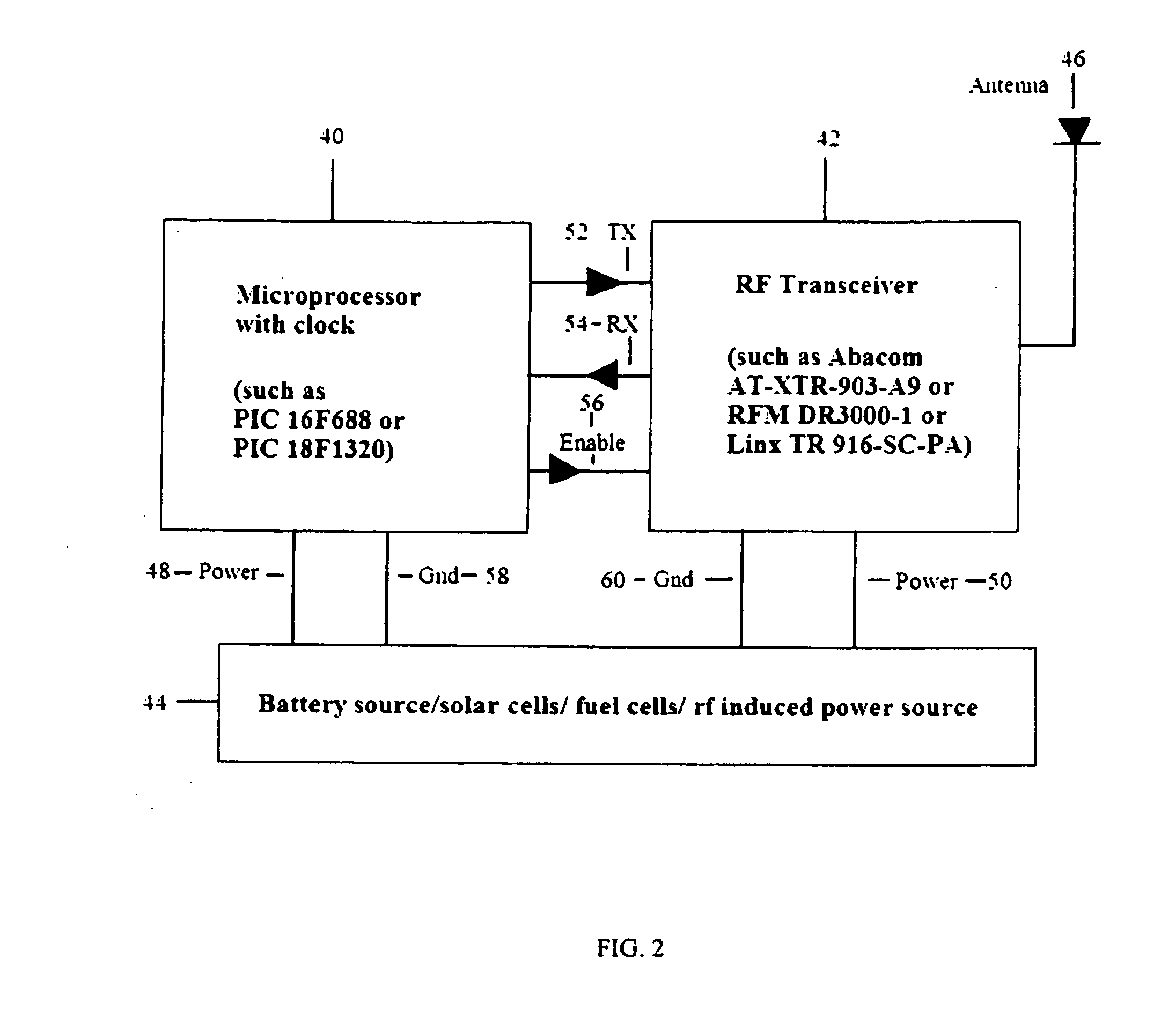
System and method for creating a proximity map of plurality of living beings and objects
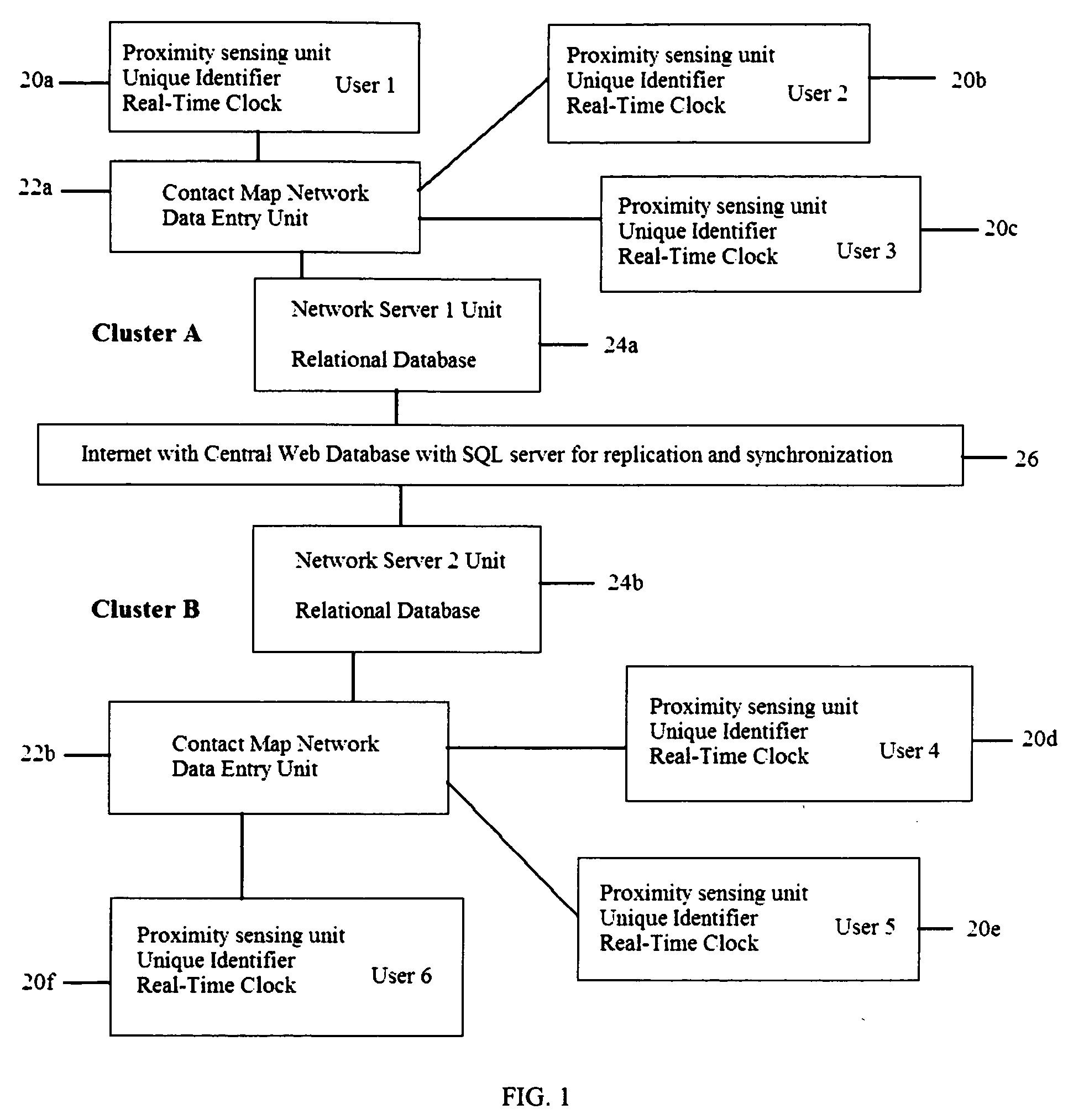
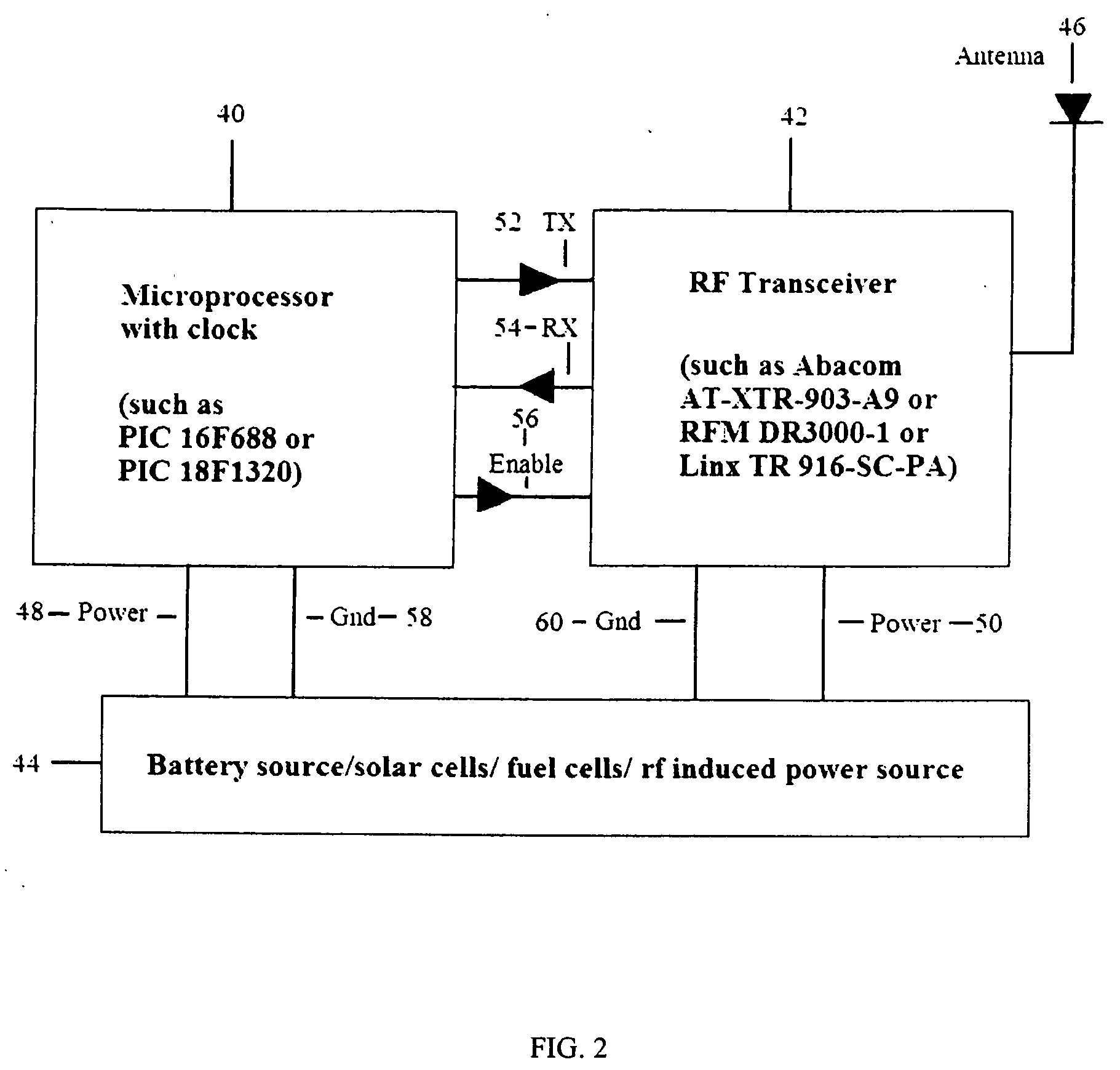
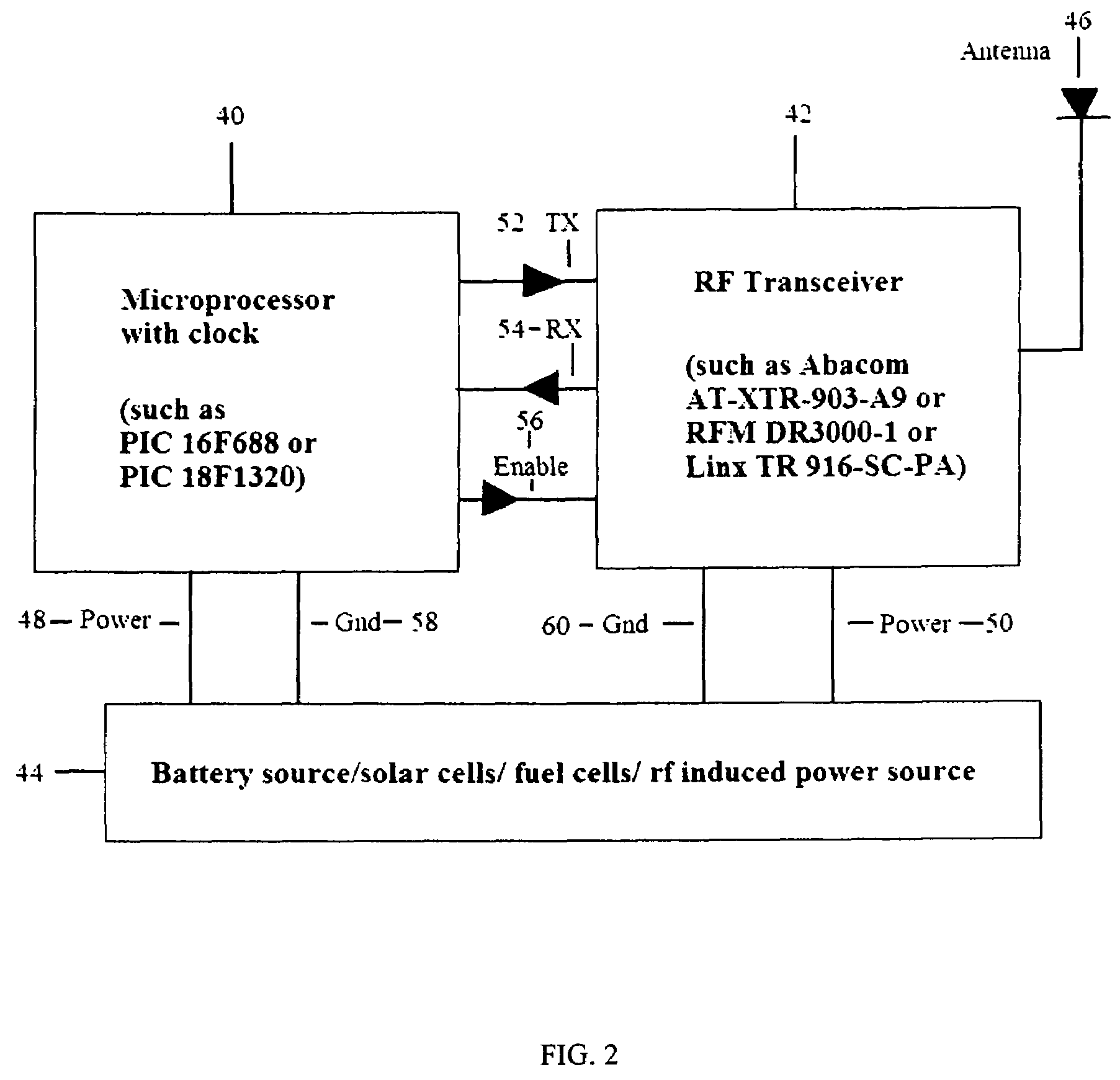
A proximity network map defines who and what objects have come in contact of each other including location and time. This map selects the list people who have come in contact with known infected people based on contagious disease epidemiology criteria to control its spread, or to prevent radiation poisoning, limit bio-chemicals exposure, etc. These people then undergo testing and quarantine procedures. It monitors hygiene practices and reduces nosocomial infections in hospitals and mitigates the pandemic flu threat by controlling contamination. It controls people interaction, information flow in a high security environment, control crime or gang activities. Each person or object carrying a proximity-sensing unit with unique ID records all units it encountered over a period of time. This information is stored with time stamp in a relational database and transferred to network servers. The databases are then replication throughout via a central web database server for retrieval and analysis.
Owner:WONG CHON MENG
Novel synthetic C-glycolipids, their synthesis and use to treat infections, cancer and autoimmune diseases
InactiveUS20050222048A1Esterified saccharide compoundsAntibacterial agentsAutoimmune diseaseGlycolipid
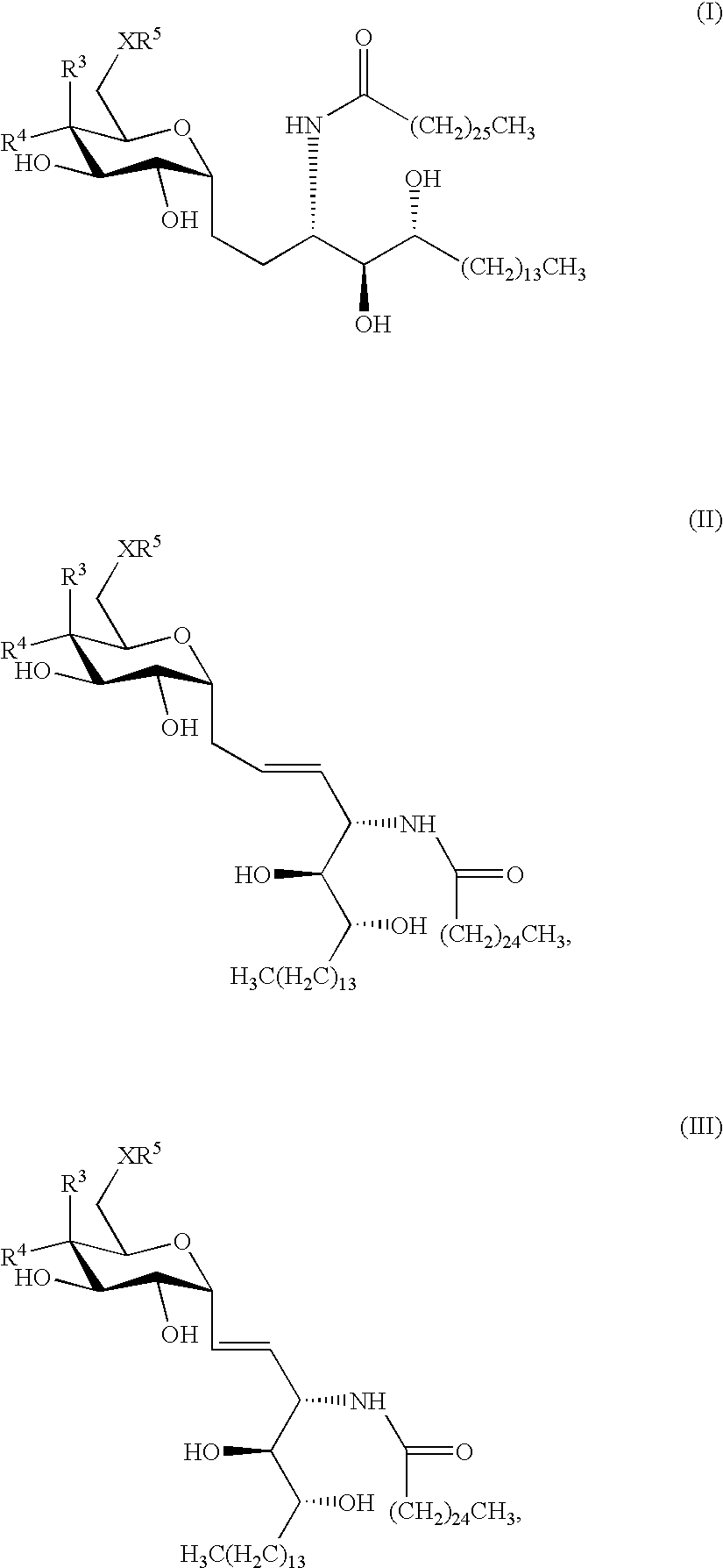
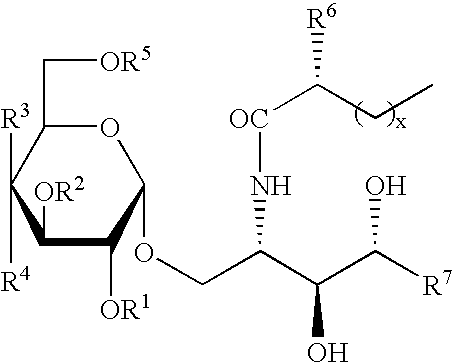
The invention is directed to novel compounds of formulae (I), (II) and (III): wherein X is O or NH; R3 is OH or a monosaccharide and R4 is hydrogen, or R3 is hydrogen and R4 is OH or a monosaccharide; R5 is hydrogen or a monosaccharide; and pharmaceutically acceptable salts or esters thereof. The invention is also directed to the use of the compounds both directly and as immune adjuvants for treating cancer, infectious diseases and autoimmune diseases. The invention is also directed to syntheses of the intermediates which can be used to make these novel compounds.
Owner:RES FOUND THE CITY UNIV OF NEW YORK +1
Method of and device for implementing contagious illness analysis and tracking
ActiveUS20200279339A1Efficient implementationQuality improvementImage enhancementImage analysisEmergency medicineBody fluid
A device is able to be used to detect an illness and / or symptoms of the illness in a user by utilizing a body fluid detector and / or other devices. The device is also able to determine when additional devices of users come within a specified distance of the device. An alert regarding a diagnosis and / or analysis of the symptoms of the illness is able to sent to a central server, a cloud device or another device to share the diagnosis and / or the analysis of the symptoms of the illness with the additional devices of users.
Owner:AKUTAGAWA CHRISTINE E +1
System and method for creating a proximity map of plurality of living beings and objects
A proximity network map defines who and what objects have come in contact of each other including location and time. This map selects the list people who have come in contact with known infected people based on contagious disease epidemiology criteria to control its spread, or to prevent radiation poisoning, limit bio-chemicals exposure, etc. These people then undergo testing and quarantine procedures. It monitors hygiene practices and reduces nosocomial infections in hospitals and mitigates the pandemic flu threat by controlling contamination. It controls people interaction, information flow in a high security environment, control crime or gang activities. Each person or object carrying a proximity-sensing unit with unique ID records all units it encountered over a period of time. This information is stored with time stamp in a relational database and transferred to network servers. The databases are then replication throughout via a central web database server for retrieval and analysis.
Owner:WONG CHON MENG
System and method for creating a proximity map of living beings and objects
A contact or proximity network map defines who and what objects have come in contact of each other including location and time. This map selects the list people who have come in contact with known infected people based on contagious disease epidemiology criteria for the purpose of control its spread, or to prevent radiation poisoning, limit bio-chemicals exposure, etc. This selected list of people then undergoes testing and quarantine procedures. It can monitor hygiene practices and reduce nosocomial infections in hospitals and mitigate the pandemic flu threat by controlling spread and control contamination. It controls people interaction, information flow in a high security environment, control crime or gang activities. Each person or object has a proximity-sensing device with unique ID, which is recorded by the encountered units during contact. This information is stored with time stamp in a relational database. It generates contact maps with real time replication of its database via a central web database for retrieval and analysis at any site.
Owner:WONG CHON MENG
Safety indwelled needle with wings for venous transfusion
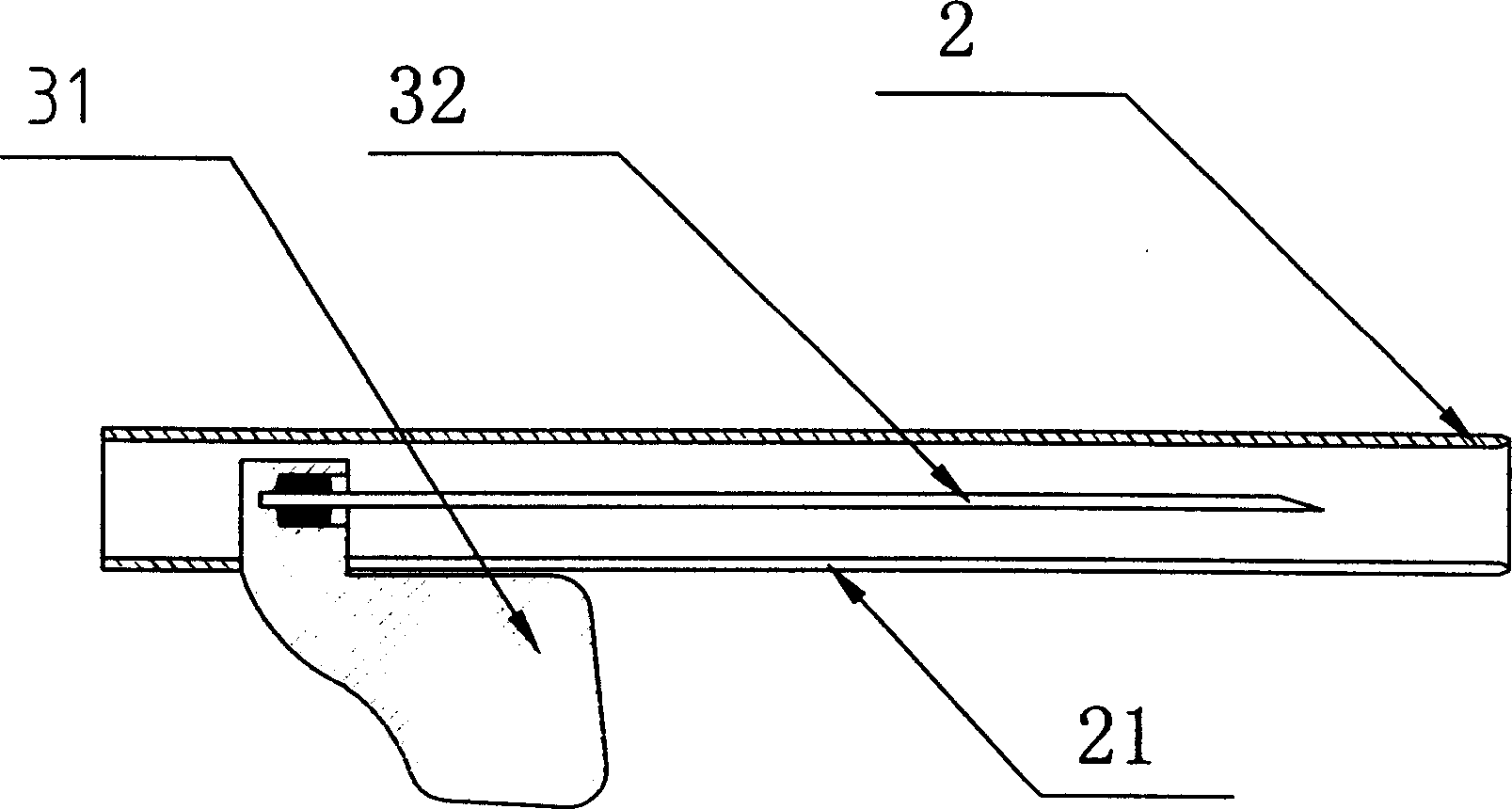
A safety type intravenous transfusion retention needle with wings is provided, which comprises puncture assembly, retention cannla assembly, a needle protection cap and a safety casing. The puncture assembly consists of puncture needle wings and puncture needle tubing. The retention assembly comprises a three-way rest, a soft tube, a joint, a sealing member and a retention cannula. The protection casing is a tubular casing, with a lock groove for positioning the root of the puncture wings is provided on the rear end of the casing, in addition, a guiding groove is cut along with the said safety casing length direction from the front end of the locking groove. The front end of the safety casing is connected with the first interface of said three-way rest and the root of the puncture needle wings is set inside of said guiding groove of the protection casing. The present invention can heal the needle in the safety protection casing in the needle tubling pulling out process, which enables the medical care personnel away from the infection of contagious disease due to the puncture accident. The invention has simple and compact structure and low cost.
Owner:ZHEJIANG KINDLY MEDICAL DEVICES
System and Method for Monitoring Outbreak of Contagious Diseases
InactiveUS20130132572A1Emergency connection handlingEpidemiological alert systemsOutbreakAd hoc communication
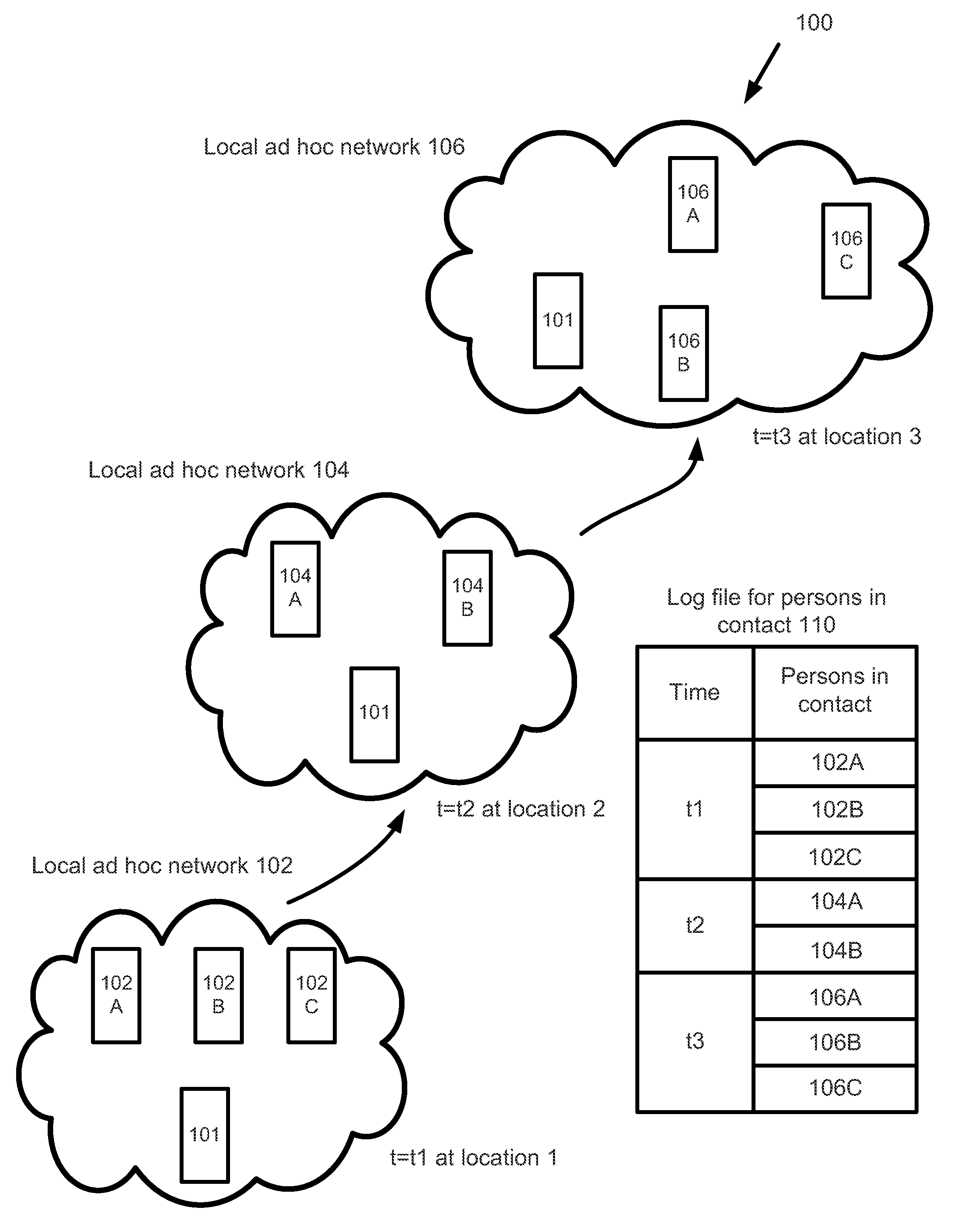
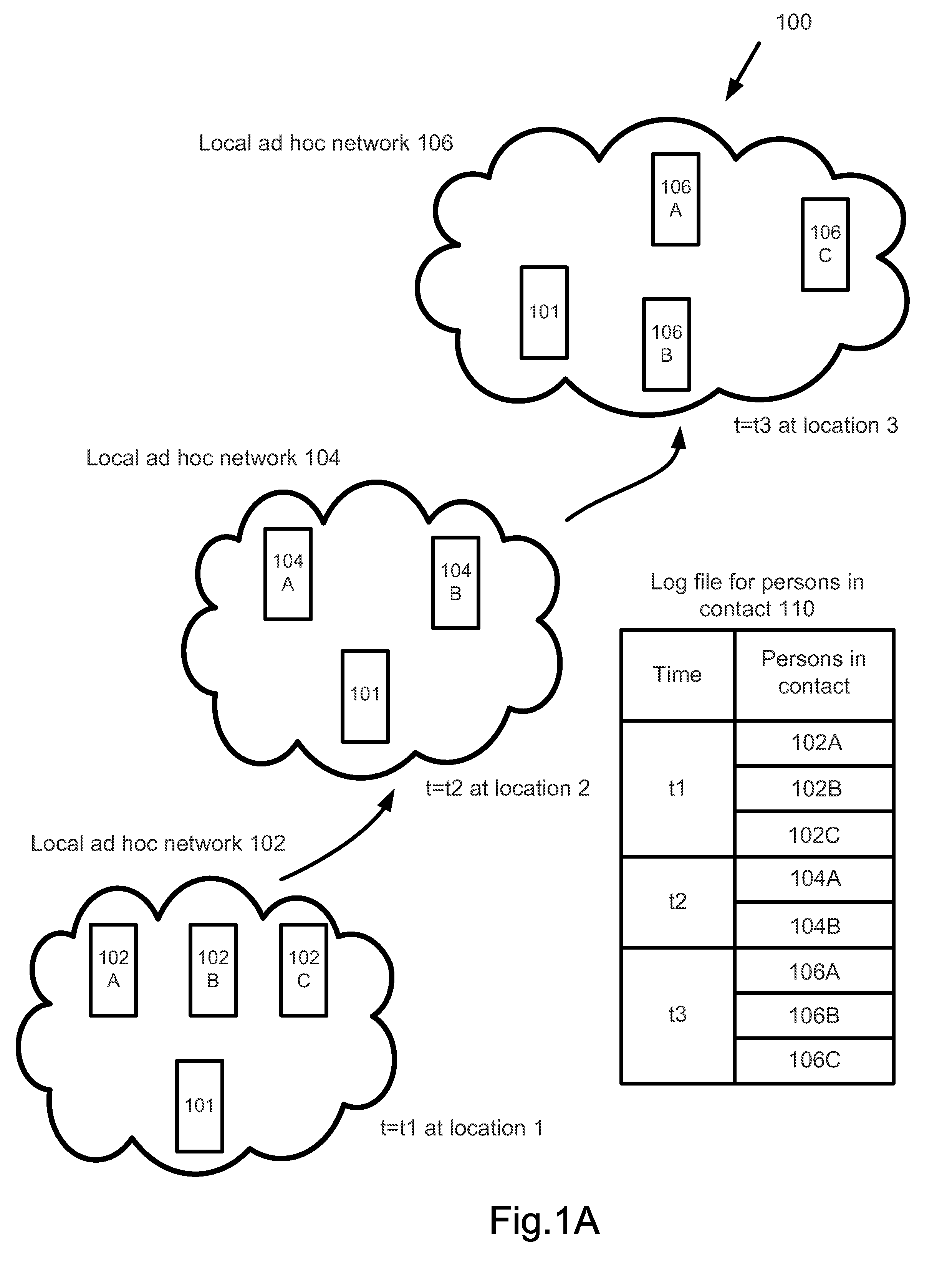
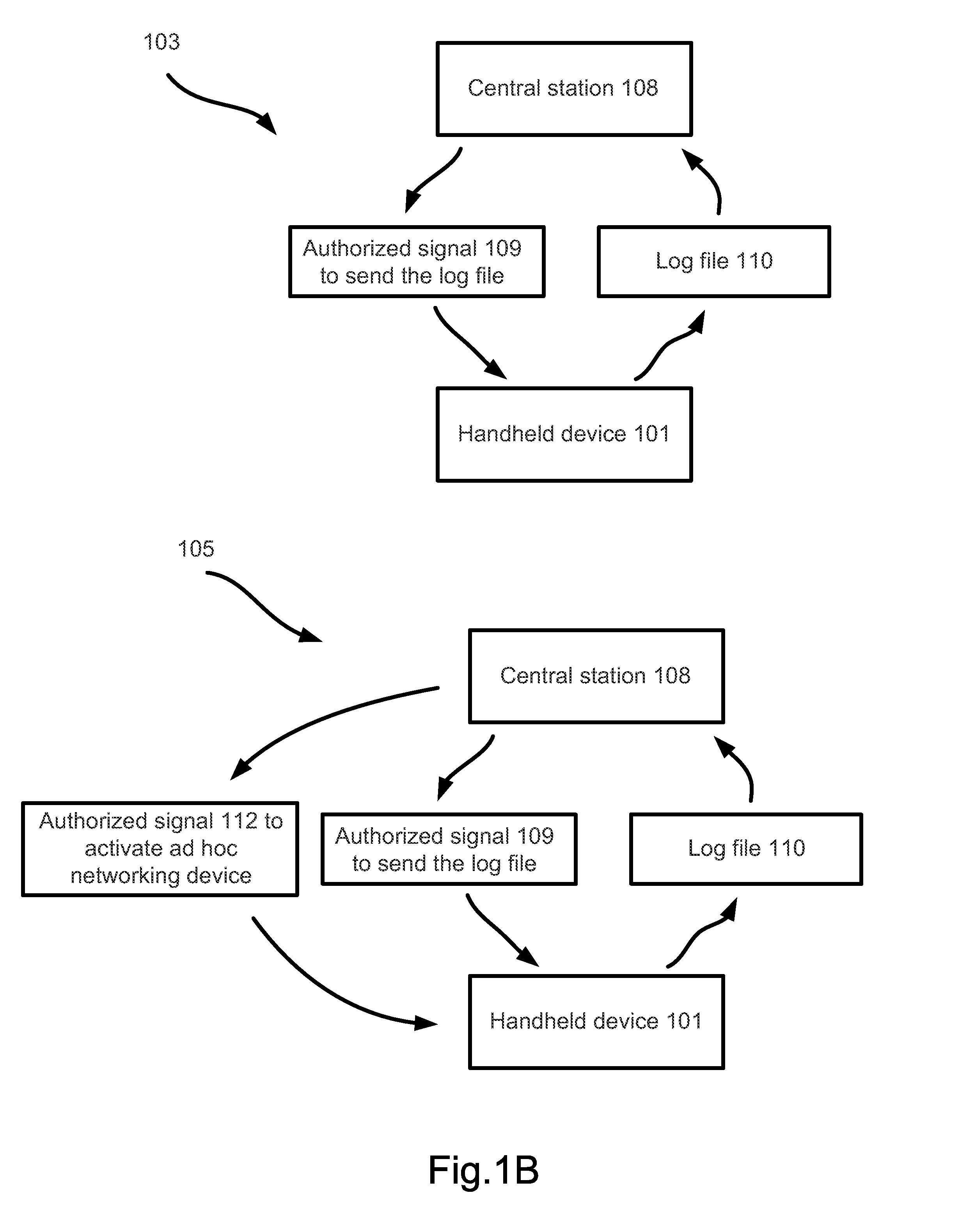
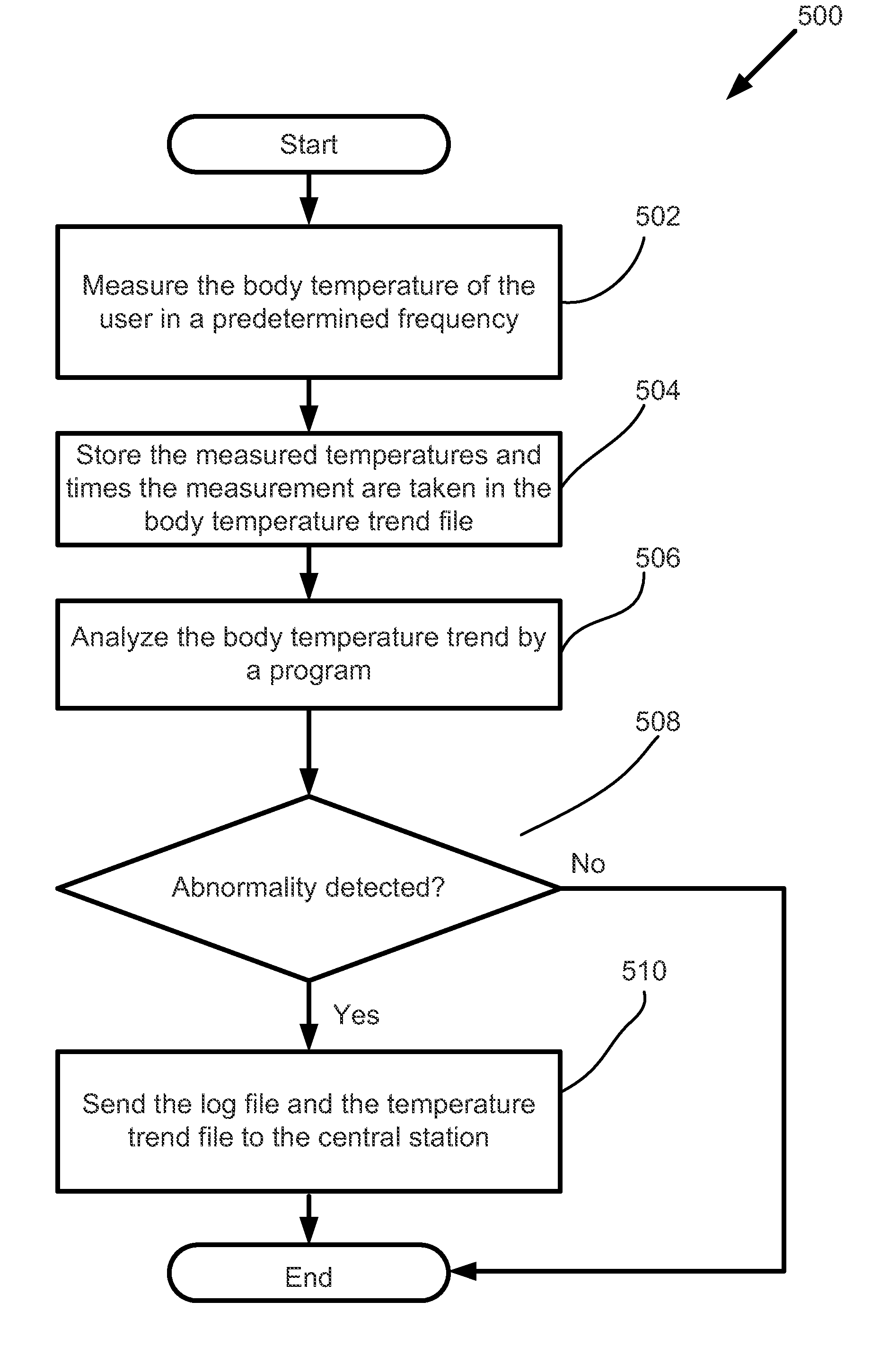
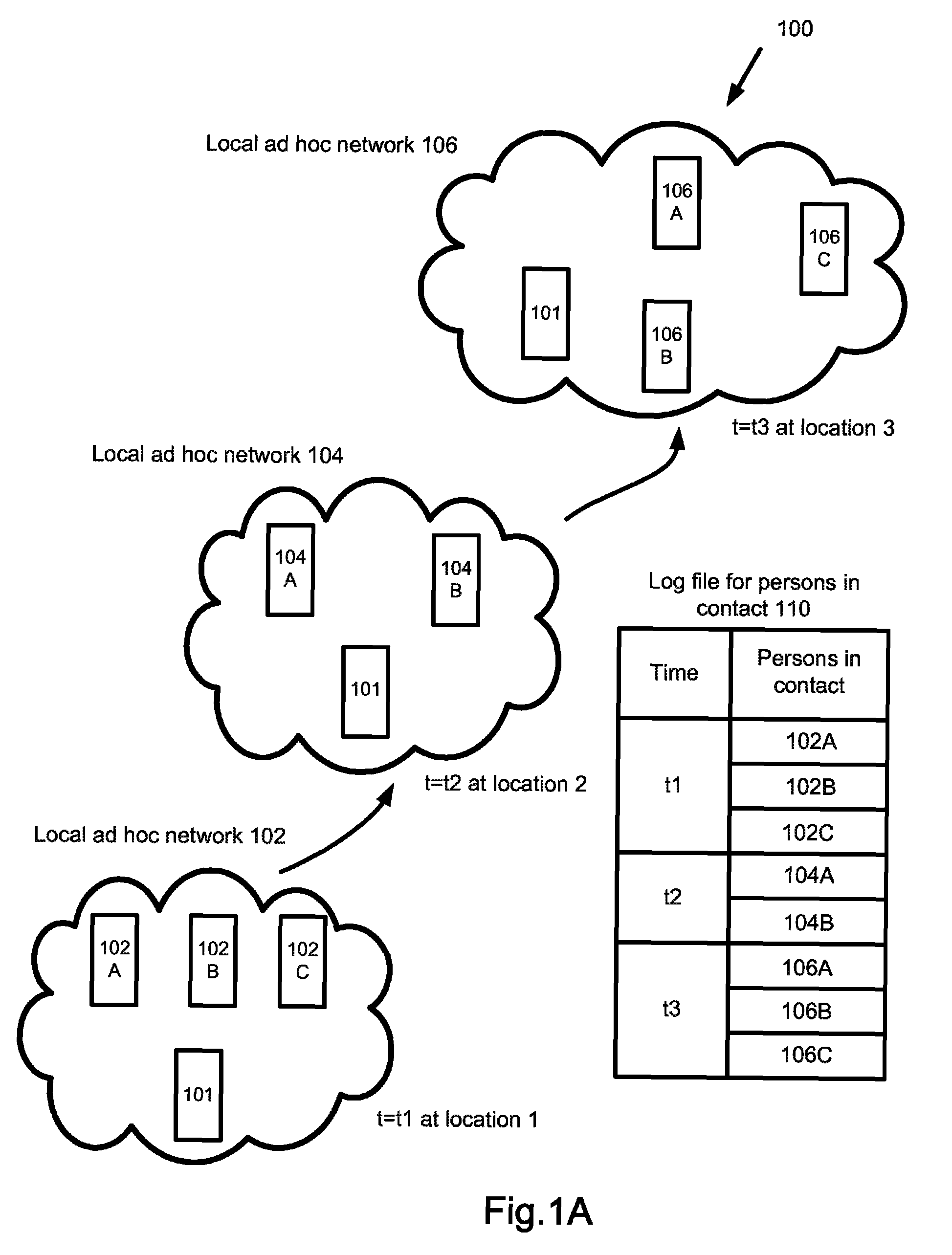
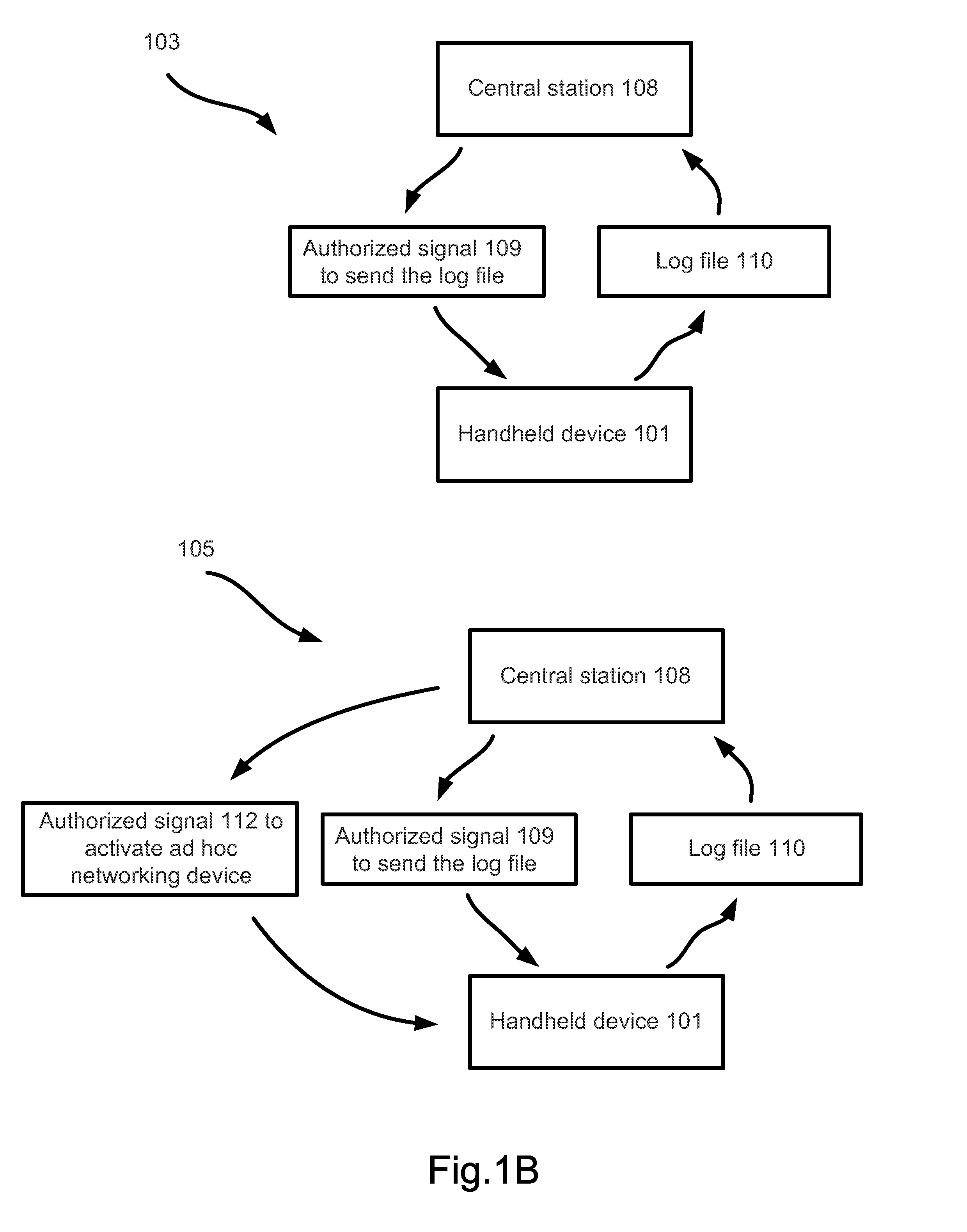
A surveillance system for monitoring outbreak of a contagious disease is disclosed. The system comprises a handheld computing and communication device with a short range ad hoc networking device. Handheld devices carried by persons in contacting with the device carried by a user form an ad hoc communication network at a location. Identities of all devices in the ad hoc network are broadcasted through the network. The user's device receives the identities and stores the received data in a log file. The log file may be sent to a central station after the device receives an authorized signal during an outbreak event of the contagious disease. The device may further include a body temperature automatic measuring system. The user's body temperature trend file may be sent together with the log file.
Owner:PAN YANG
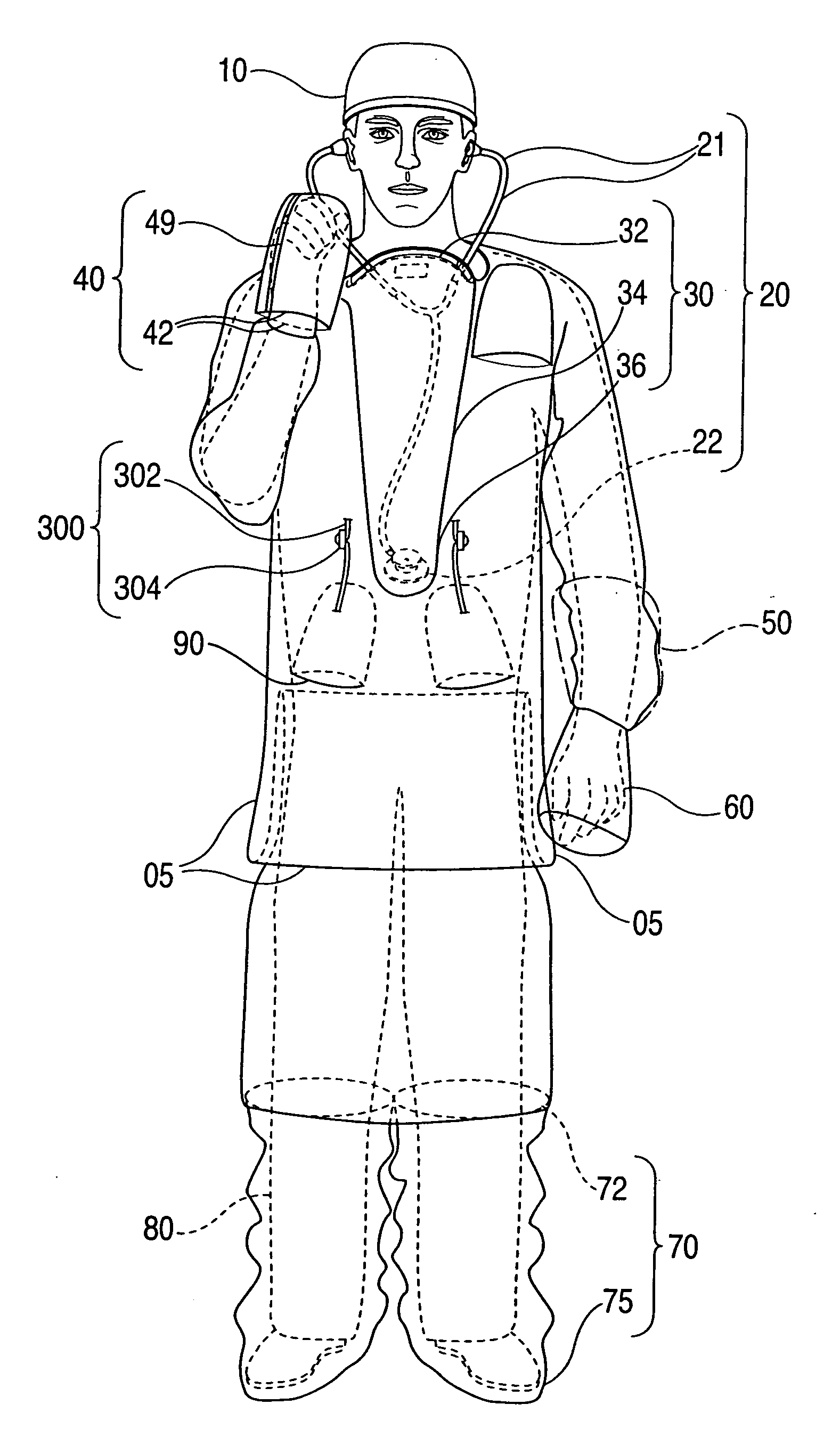
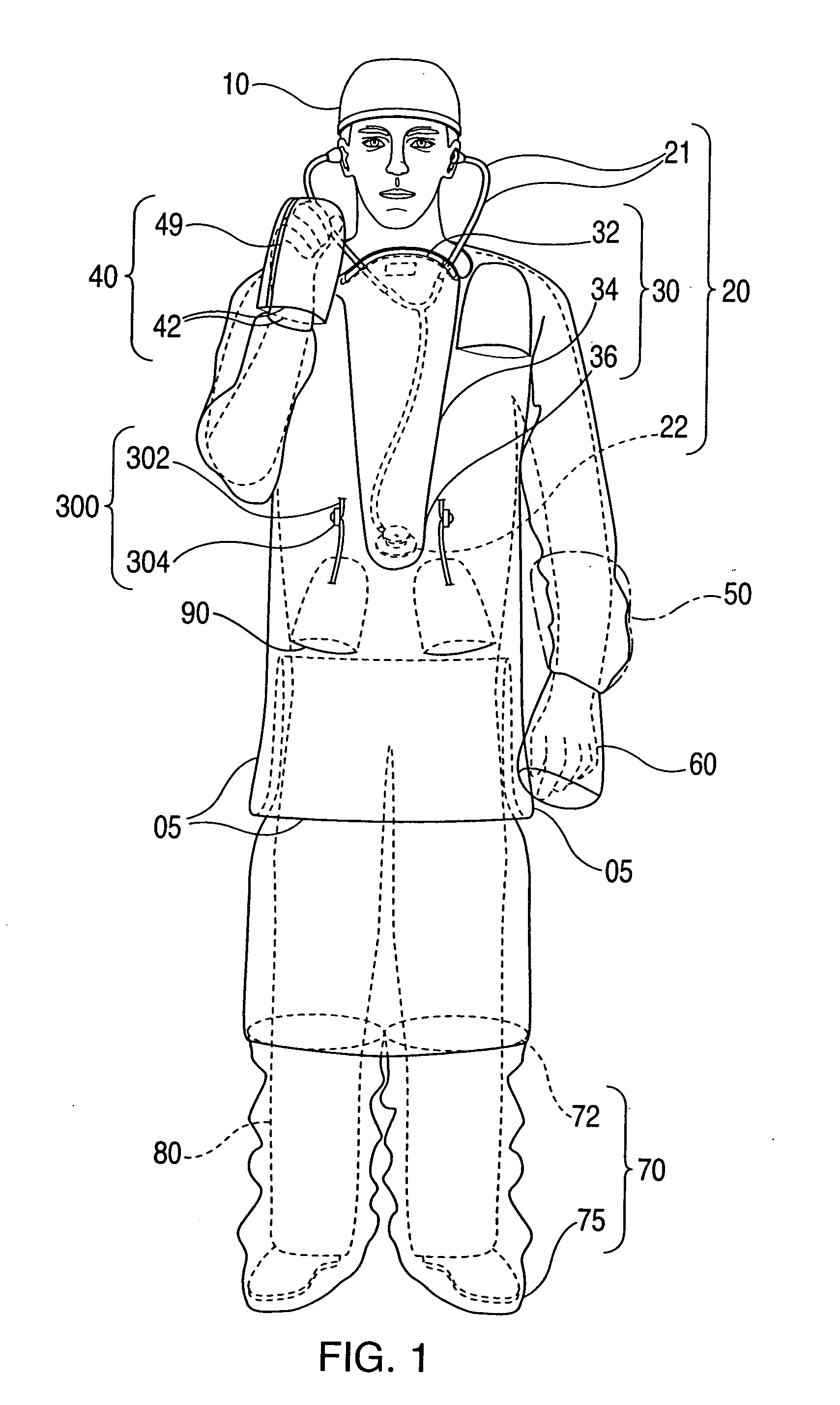
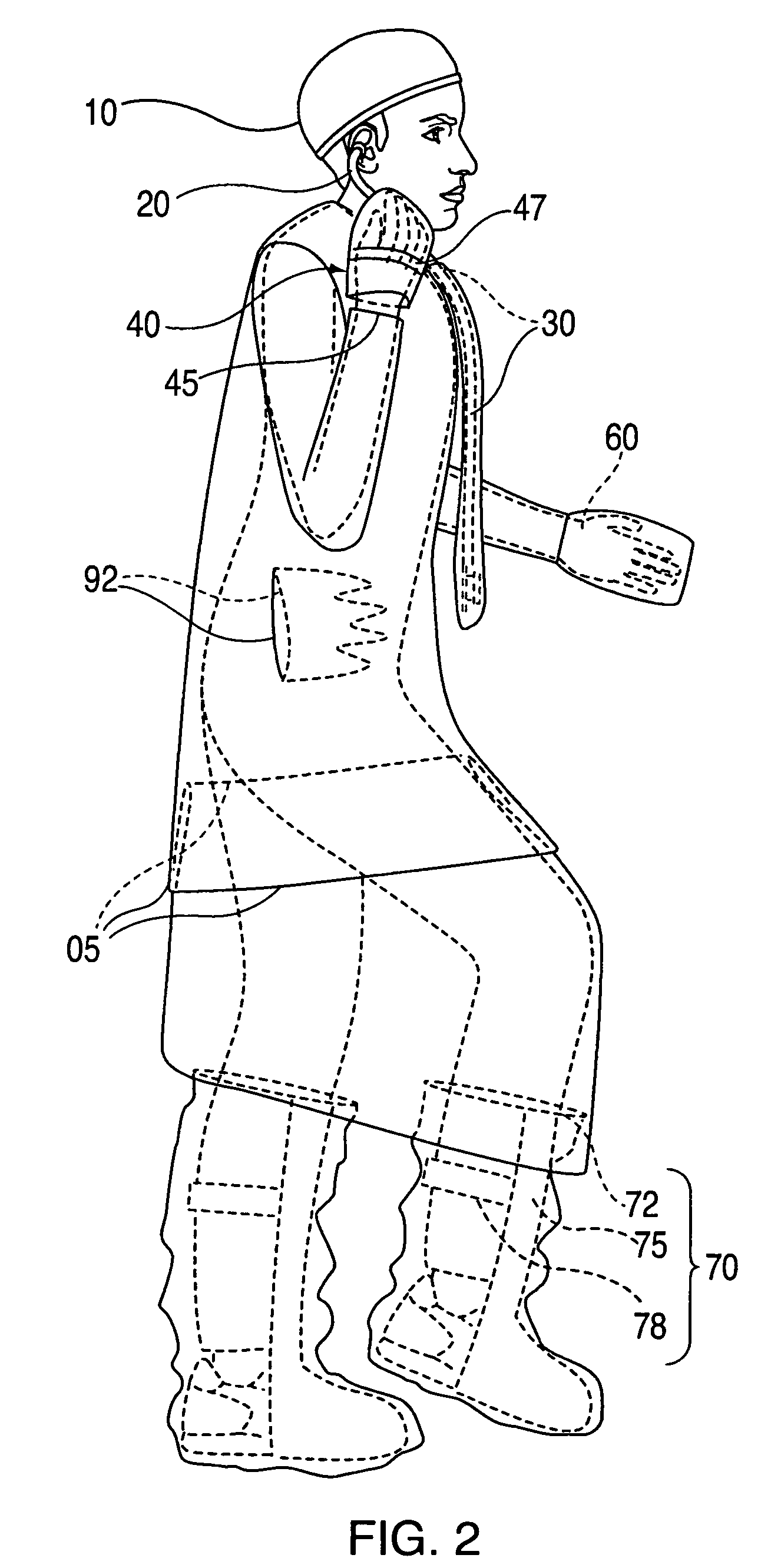
Advanced isolation gown
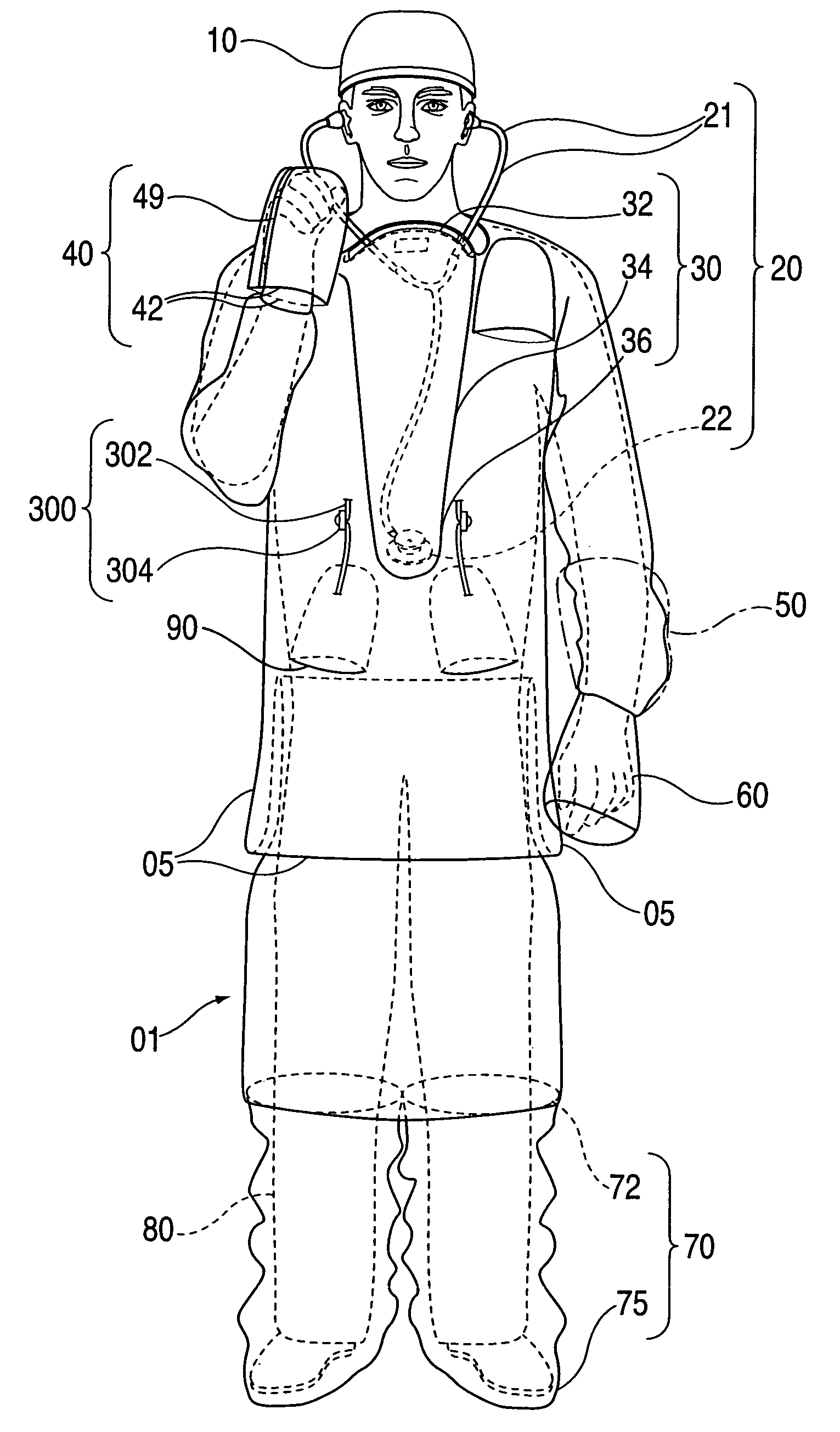
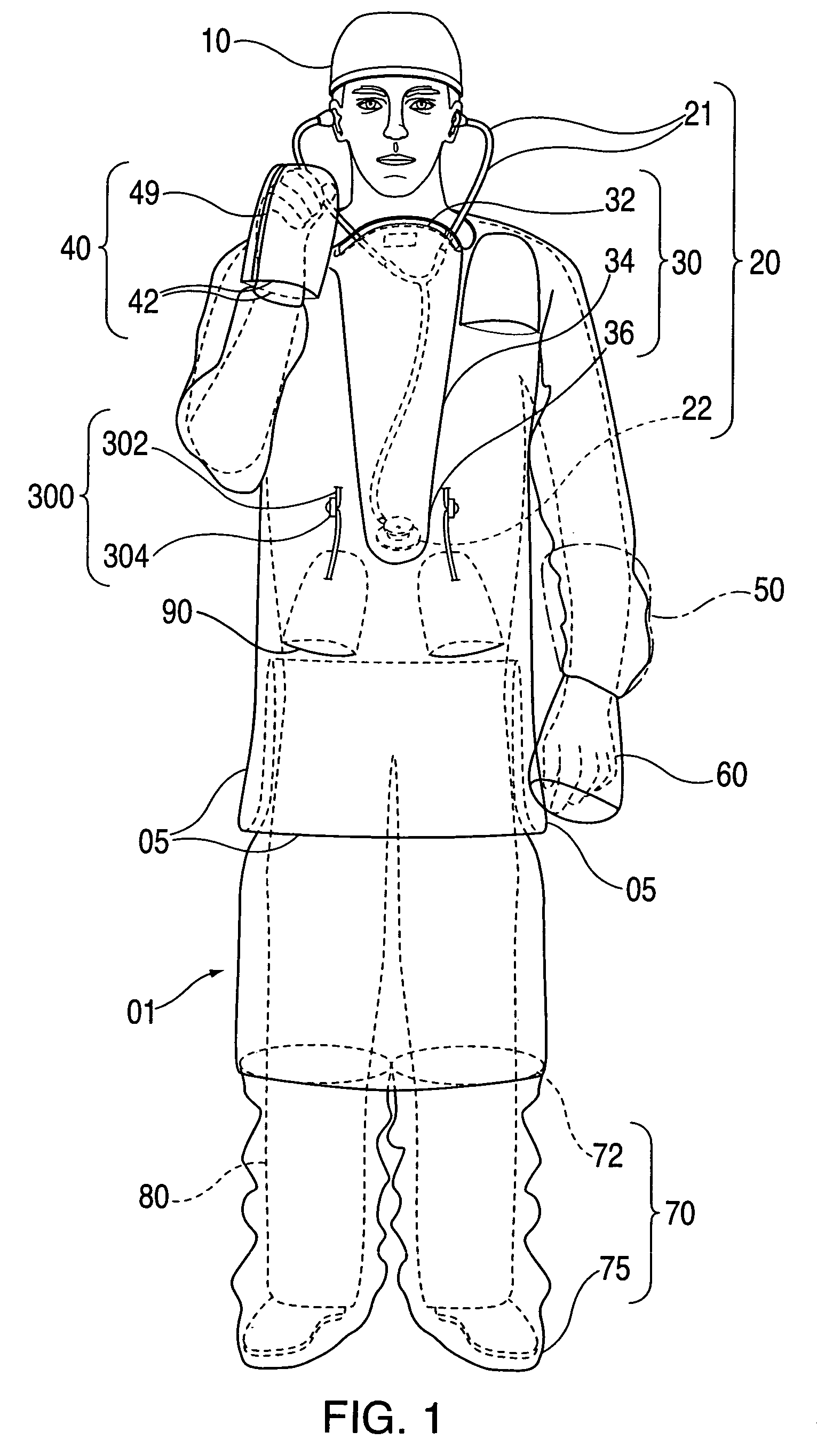
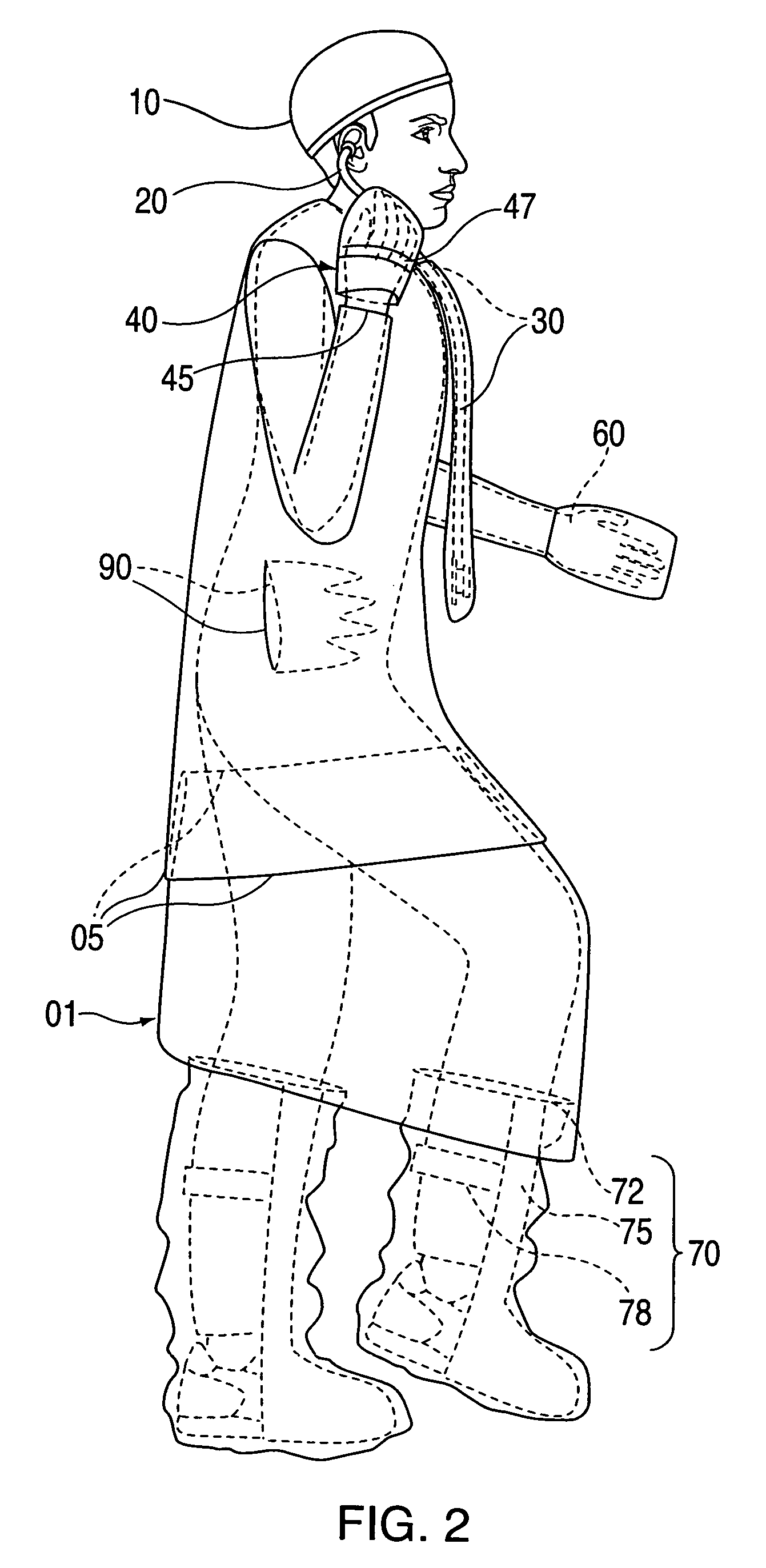
InactiveUS20090031474A1Low extremityAvoid pollutionBaby linensPyjamasIntensive care medicineContagious disease
A medical isolation gown is disclosed. The gown is to be used by medical personnel treating isolated patients with contagious diseases. The gown comprises slots and pouches that can be used to isolate the worker and his or her instruments from contamination when examining or attending the patient.
Owner:MEDICAL ISOLATION TECH
Method of Monitoring a Microorganism That Causes Infectious Disease of a Laboratory Animal
InactiveUS20070287147A1Improve throughputConducted easily and rapidlyMicrobiological testing/measurementBiological testingMicroorganismMicrobiology
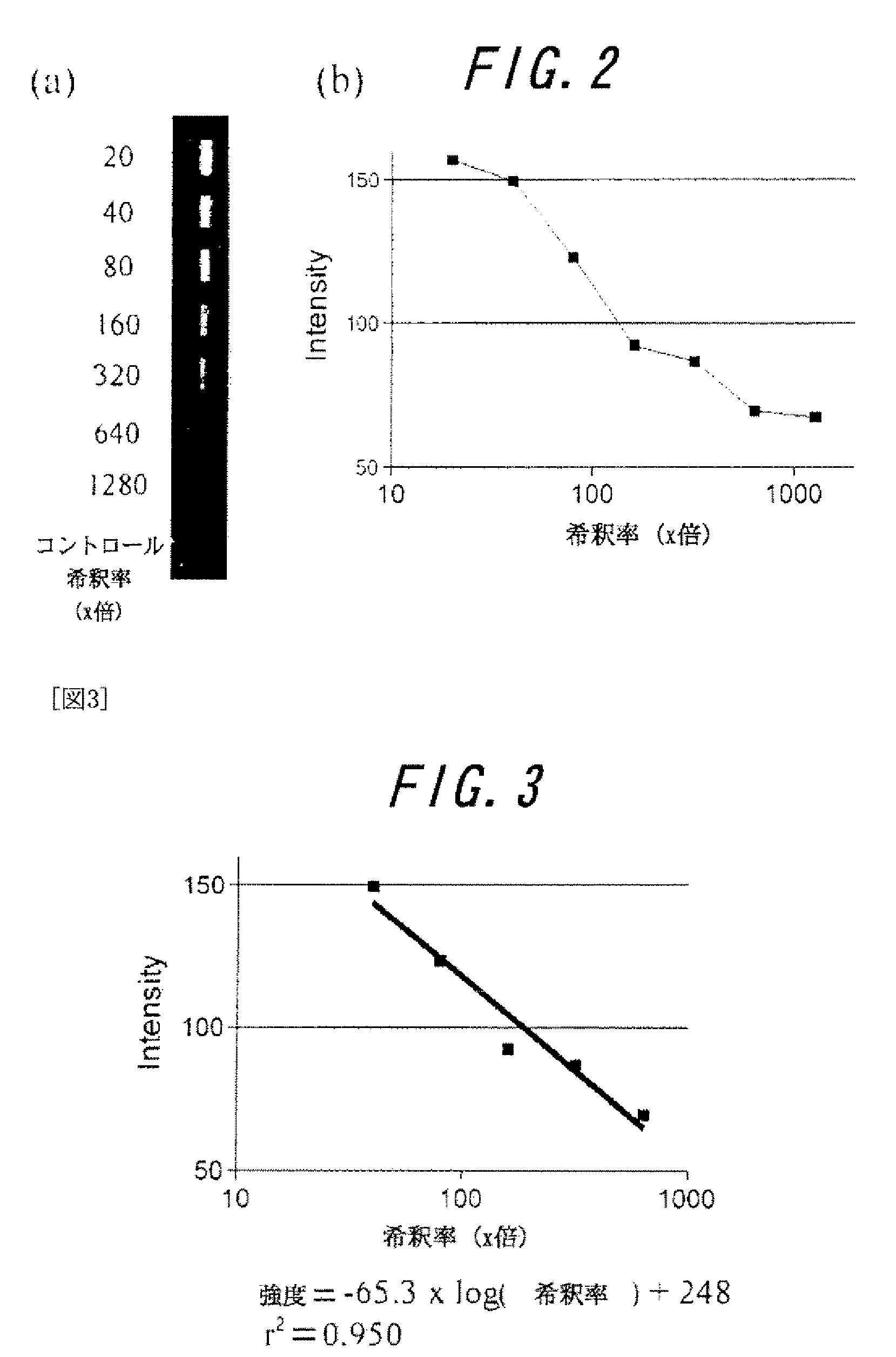
This invention provides a method to monitor a microorganism that causes infectious disease of a laboratory animal by using a micro flow channel chip immobilized with a molecular to be tested such as an antigen or an antibody of the microorganism that causes infectious disease, the method comprises flowing serum or body fluid taken from the laboratory animal through the minute flow channel of the micro flow channel chip and detecting the antigen antibody reaction on the chip. The method of this invention enabled medical inspection of an infectious disease of a laboratory animal and microorganism monitoring of a laboratory animal, by using minute amount of animal serum or body fluid in a closed system rapidly and sensitively.
Owner:NAGAMUNE TERUYUKI +1
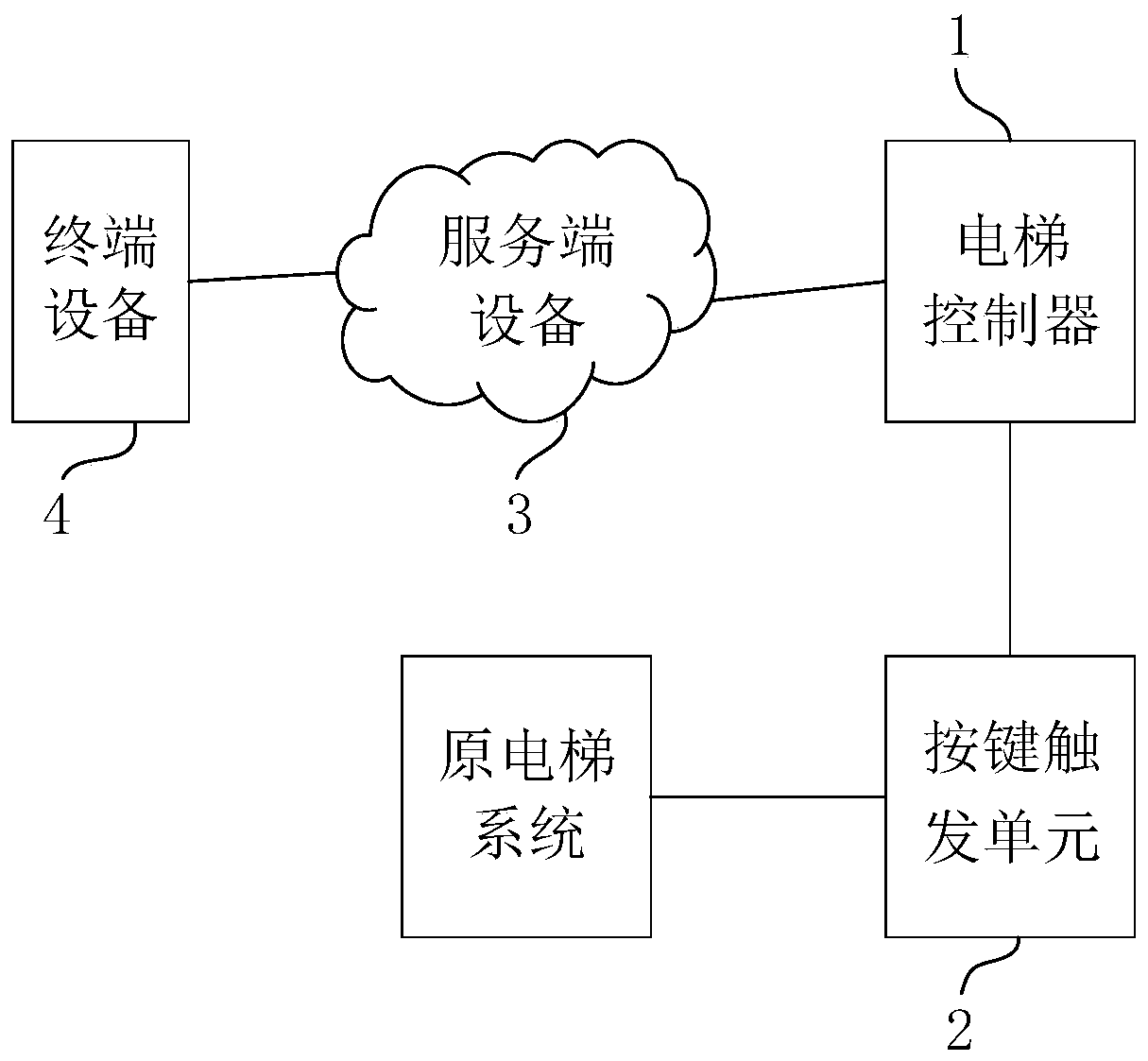
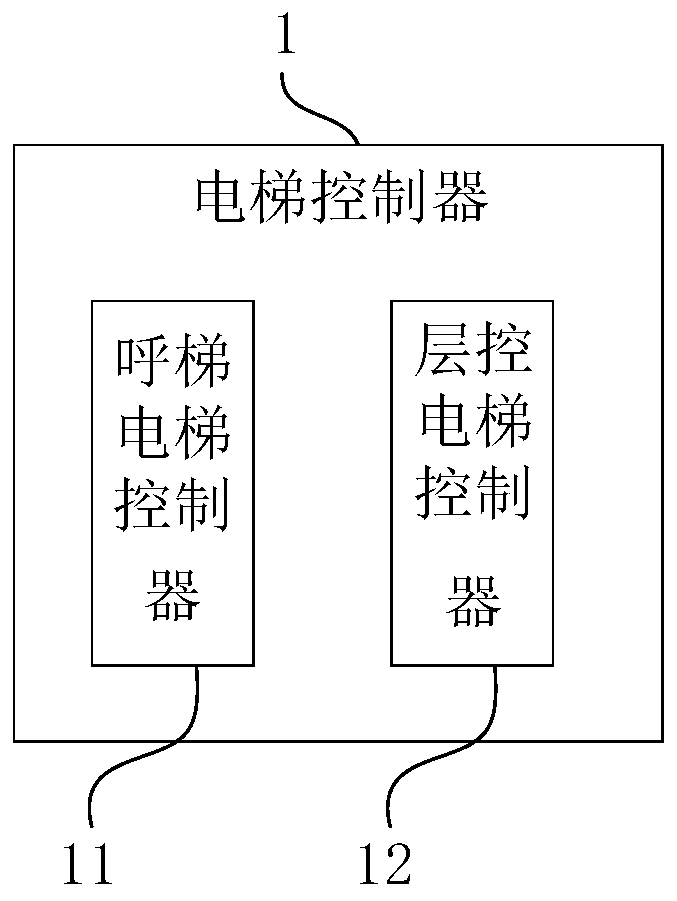
Method and system for taking public elevator without contact, control device and storage medium
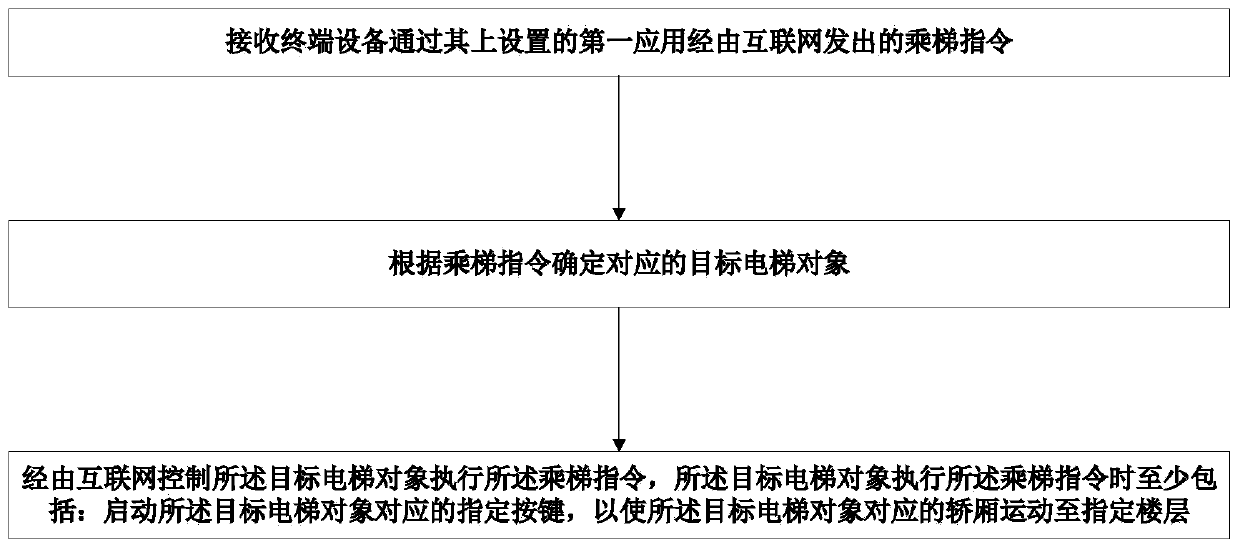
The invention provides a method and system for taking a public elevator without contact, a control device and a storage medium. The method applied to a cloud comprises the following steps of receivingan elevator taking instruction sent by terminal equipment and through the Internet by a first application set thereon; determining a corresponding target elevator object according to the elevator taking instruction; and controlling the target elevator object through the Internet to execute the elevator taking instruction, wherein the execution of the elevator taking instruction by the target elevator object comprises at least starting up a specified key corresponding to the target elevator object to make the car corresponding to the target elevator object move to a specified floor. The invention is conducive to the safe and healthy elevator use of elevator users, so as to solve the problem that contagious viruses spread by pressing the elevator buttons.
Owner:SHENZHEN WONGLONG INTELLIGENT TECH
Live attenuated antigenically marked classical swine fever virus
ActiveUS20080292653A1Effective protectionSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeStructural glycoprotein
Classical swine fever virus is a world-wide distributed highly-contagious disease affecting swine. The two main strategies for diseases control are prophylactic vaccination and non-vaccination stamping out policies. Marker vaccines are a promising strategy. Here we report the rational development of a doubly antigenic marker CSFV experimental live attenuated candidate strain vaccine (Flag / T4 virus). Flag / T virus (Flag / T4v) is based in the combination of two Brescia derived recombinant attenuated viruses: RB-C22 and T4. RB-C22v contains a 19mer insertion in the structural glycoprotein E1, while T4v posses mutated CSFV amino acid residues 830 to 834 in the structural glycoprotein E2, deleting the highly conserved epitope recognized by monoclonal antibody (mAb) WH303. Flag / T4 virus contains a positive foreign antigenic marker, due to the insertion of the highly antigenic epitope Flag in the 19mer insertion of E1, as well as a negative antigenic marker, the lack of reactivity with mAb WH303. Immunized with Flag / T4v induced a complete protection against the challenge with virulent strain Brescia both at 3 and 28 days post infection when nasally administered and since the second day post infection when intramuscularly administered. These results constitute an example of rational design of a CSFV antigenically marked LAV.
Owner:AGRI UNITED STATES OF AMERICA AS RESPRESENTED BY THE SEC OF THE +1
Organic composite Chinese medicine immunopotentiator/adjuvant and its prepn process
InactiveCN101019940AReduce severityImprove survival rateAnthropod material medical ingredientsAntiviralsAdjuvantPropolis
The present invention proposes one kind of organic composite Chinese medicine immunopotentiator / adjuvant for preventing and treating animal contagious diseases and its preparation process. The Chinese medicine immunopotentiator / adjuvant is prepared with propolis and eight kinds of Chinese medicinal materials, including astragalus root, fortune euonymus stem or leaf, Dangshen, glossy ganoderma, ginseng, etc. and through compounding the extracted liquid of the Chinese medicinal materials and the alcohol leaching liquid of propolis. It can enhance the immunity of available vaccine and strengthen animal's disease resistance.
Owner:广东省家禽科学研究所
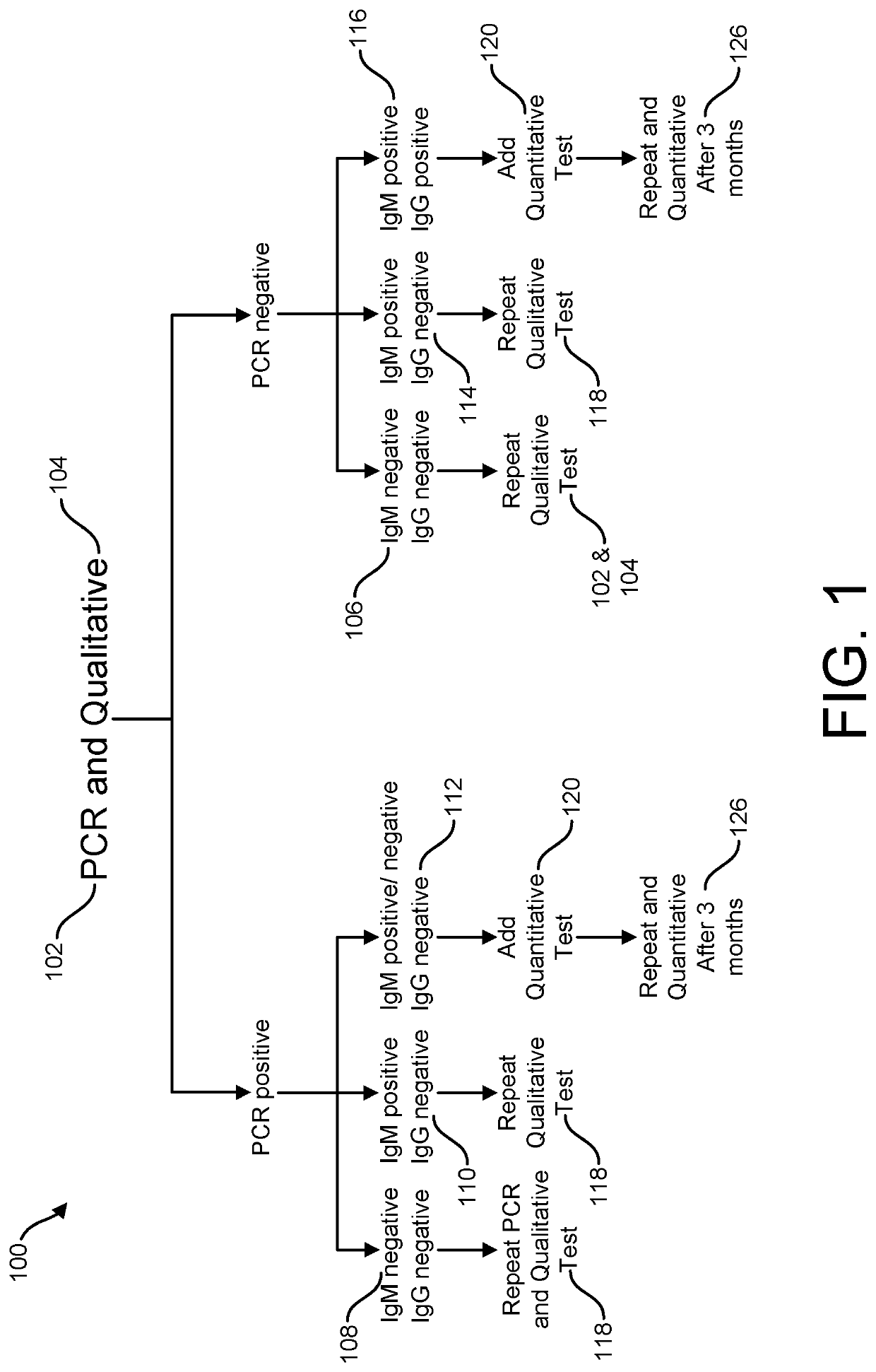
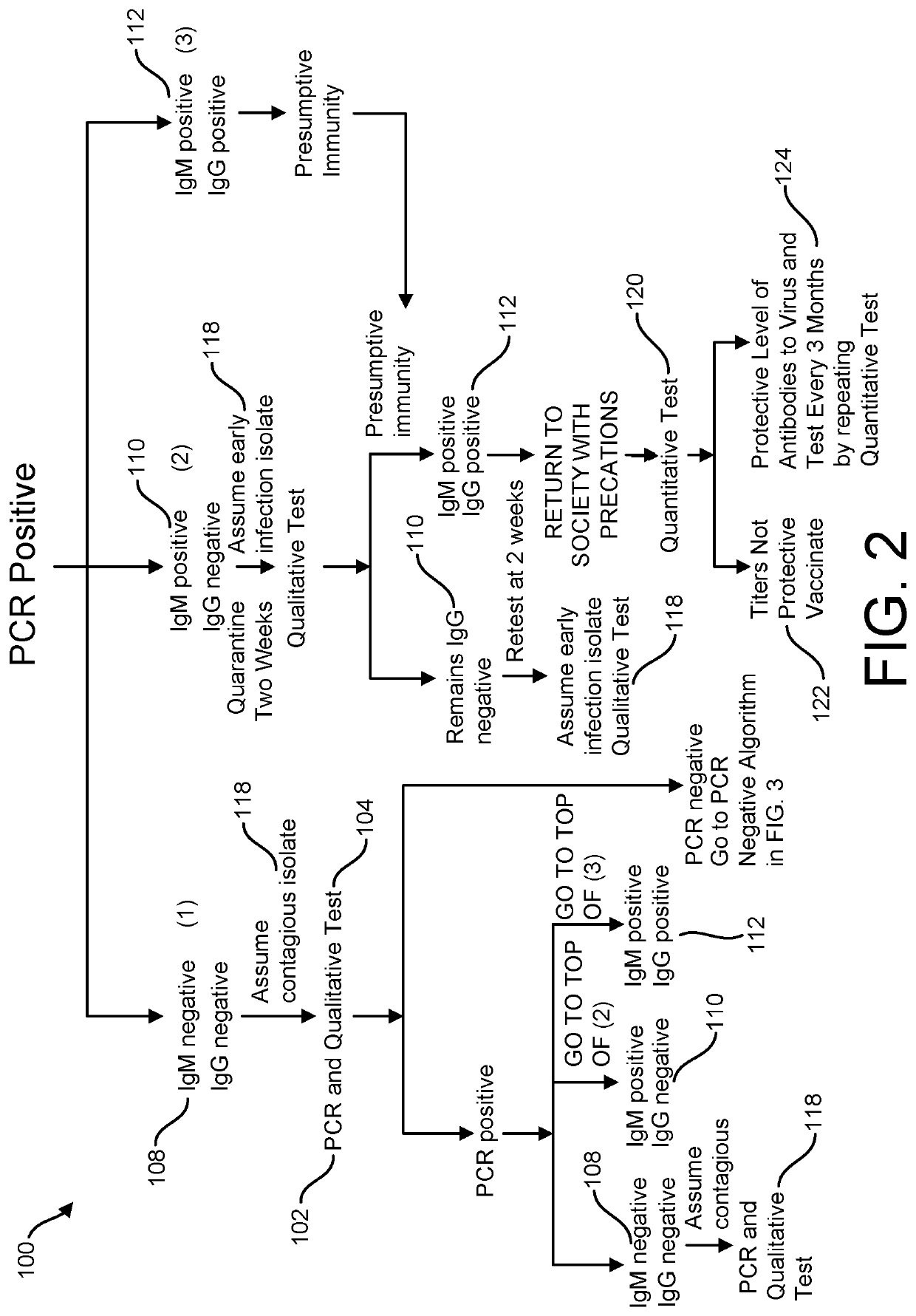
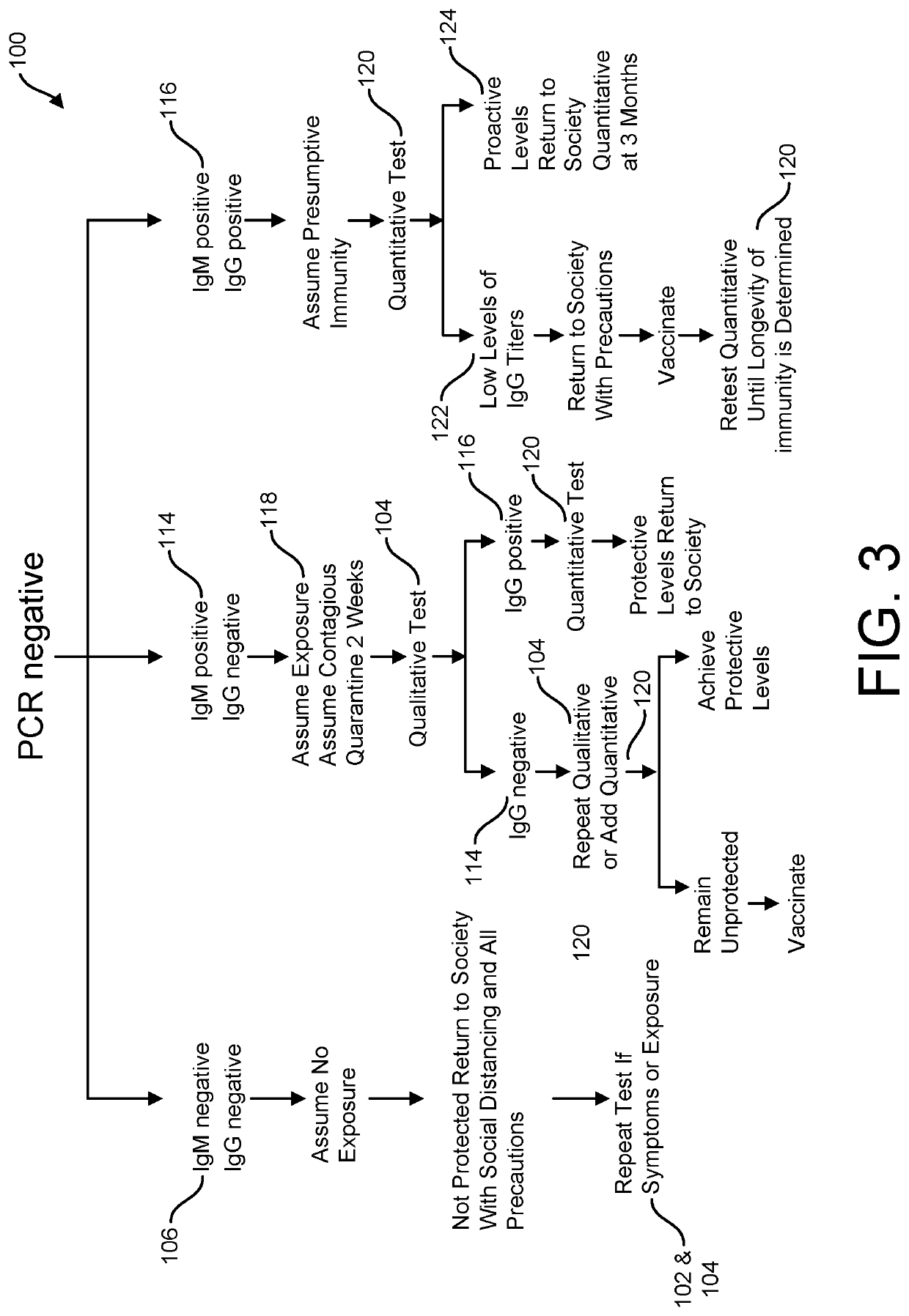
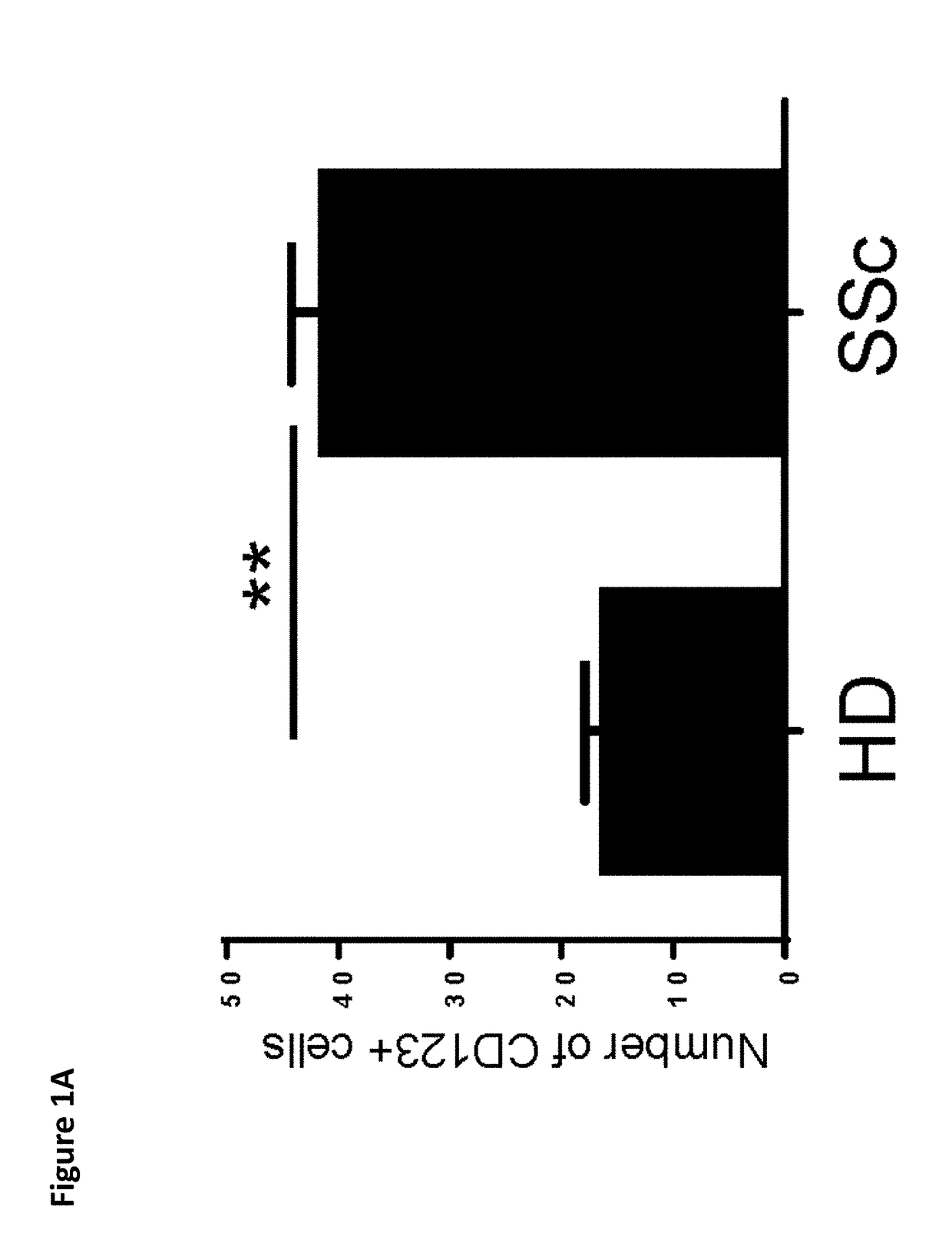
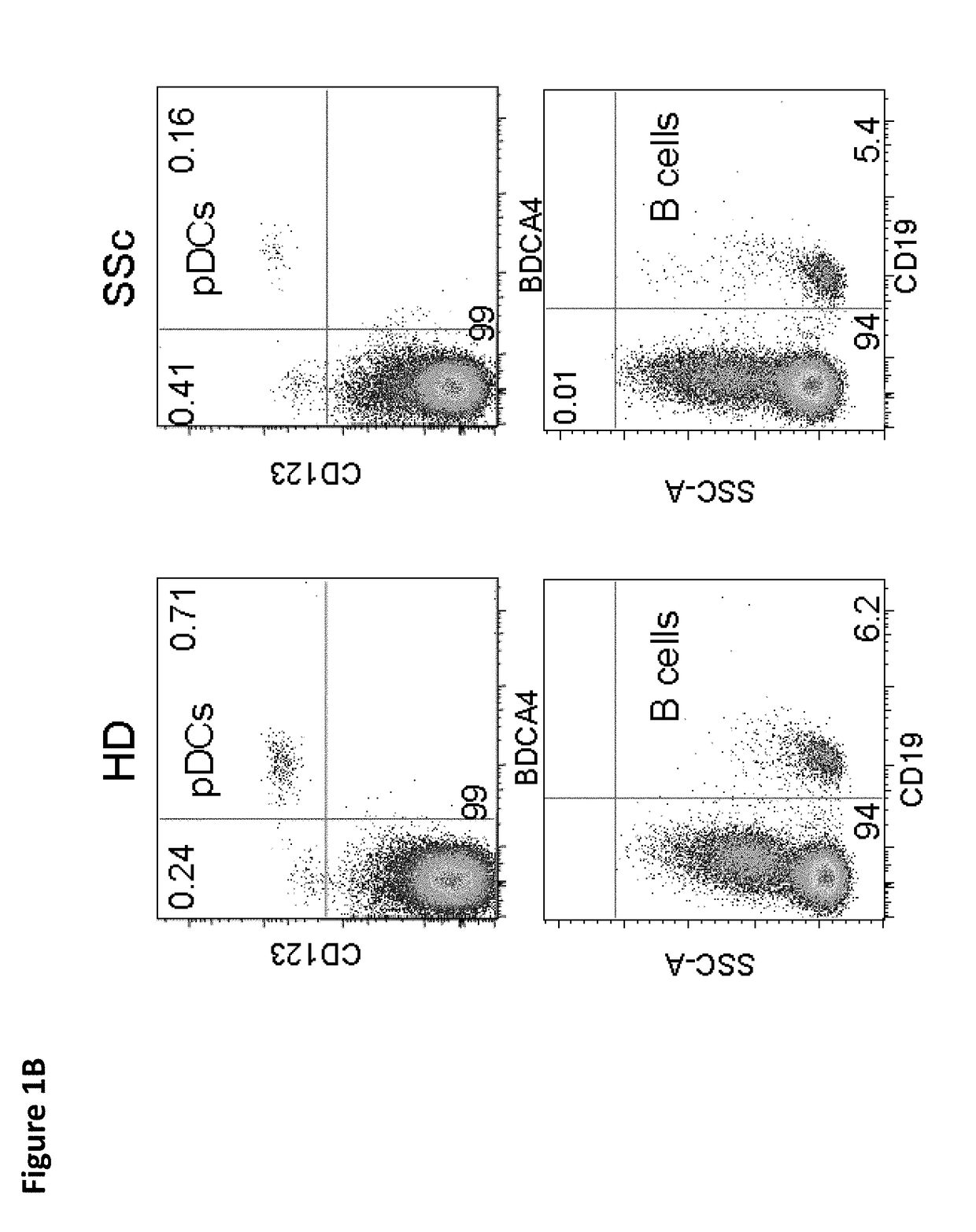
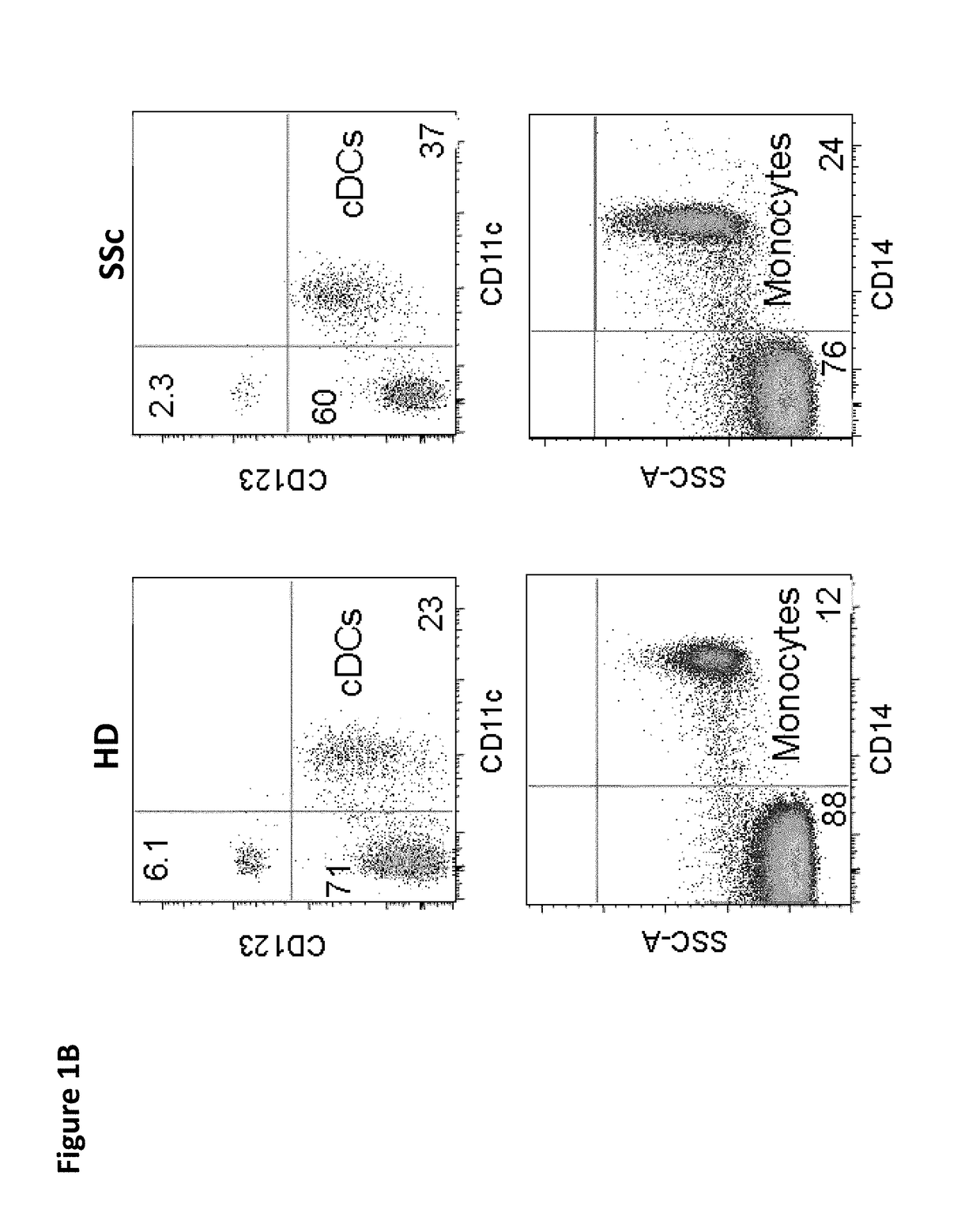
Risk Stratification for Contagious Disease
InactiveUS20200291490A1Intuitive imageHealth-index calculationMicrobiological testing/measurementReverse transcriptaseIntravenous gammaglobulin
A method of risk stratification for contagious disease is provided. The method performs a reverse transcriptase polymerase chain reaction (RT-PCR) test on a patient to determine the presence of a viral infection. The method next performs tests for the presence of antibody Immunoglobulin M (IgM) and Immunoglobulin G (IgG) assays. The method assigns the patient a level of readiness to return to society corresponding to the combination of the results of the qualitative RT-PCR, IgM, and IgG tests and quantitative IgG+ antibody testing. The levels of readiness to return to society may be verified by a QR code on a mobile device.
Owner:SENSIVA HEALTH LLC
Medication dispenser
This invention relates to a tamper resistant medication dispenser. The medication dispenser is programmed to dispense a specified dose of medication at a specified interval. The medication dispenser prevents direct access to the medication by the patient. The medication is dispensed to the patient dissolved in a large volume of liquid to prevent intravenous and intranasal abuse. The medication dispenser can be equipped with a self destruct mechanism when tampered with and have the ability to record video for subsequent monitoring, the actual medication ingestion of the medication infused liquid by the patient. It is especially useful for controlled substances which have high potential for abuse such as opioids, benzodiazepines and barbiturates and anesthetic agents and when patient compliance with medications is necessary such as medications for the treatment of contagious diseases.
Owner:SANDHU AMANPREET
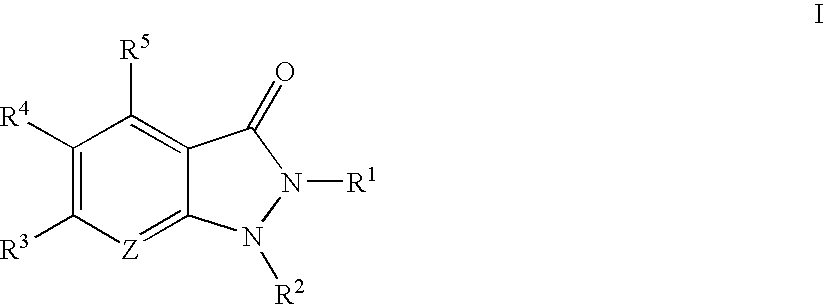
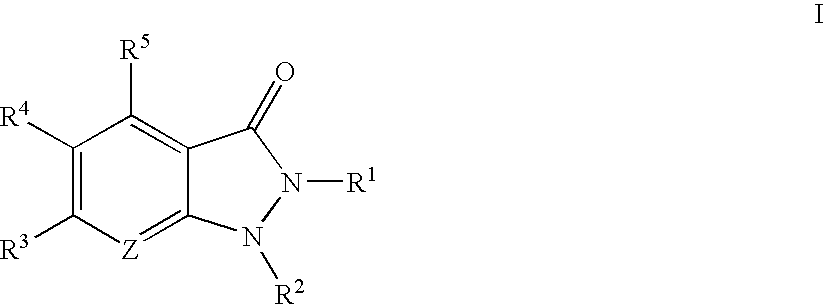
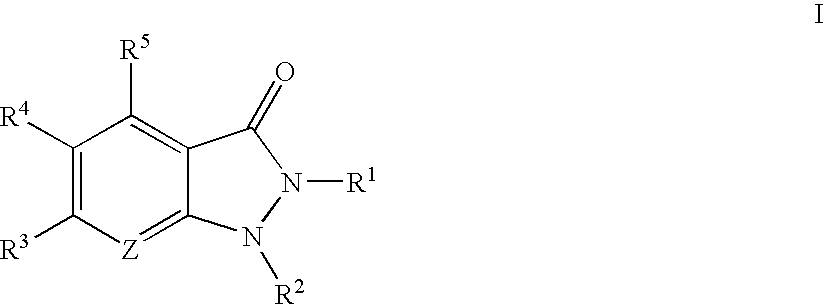
Indazolinone compositions useful as kinase inhibitors
The present invention provides compounds of formula I:These compounds, and pharmaceutically acceptable compositions thereof, are useful generally as kinase inhibitors, particularly as inhibitors of PRAK, GSK3, ERK2, CDK2, MK2, SRC, SYK, and Aurora-2. Accordingly, compounds and compositions of the invention are useful for treating or lessening the severity of a variety of disorders, including, but not limited to, heart disease, diabetes, Alzheimer's disease, immunodeficiency disorders, inflammatory diseases, allergic diseases, autoimmune diseases, destructive bone disorders such as osteoporosis, proliferative disorders, infectious diseases and viral diseases.
Owner:VERTEX PHARMA INC
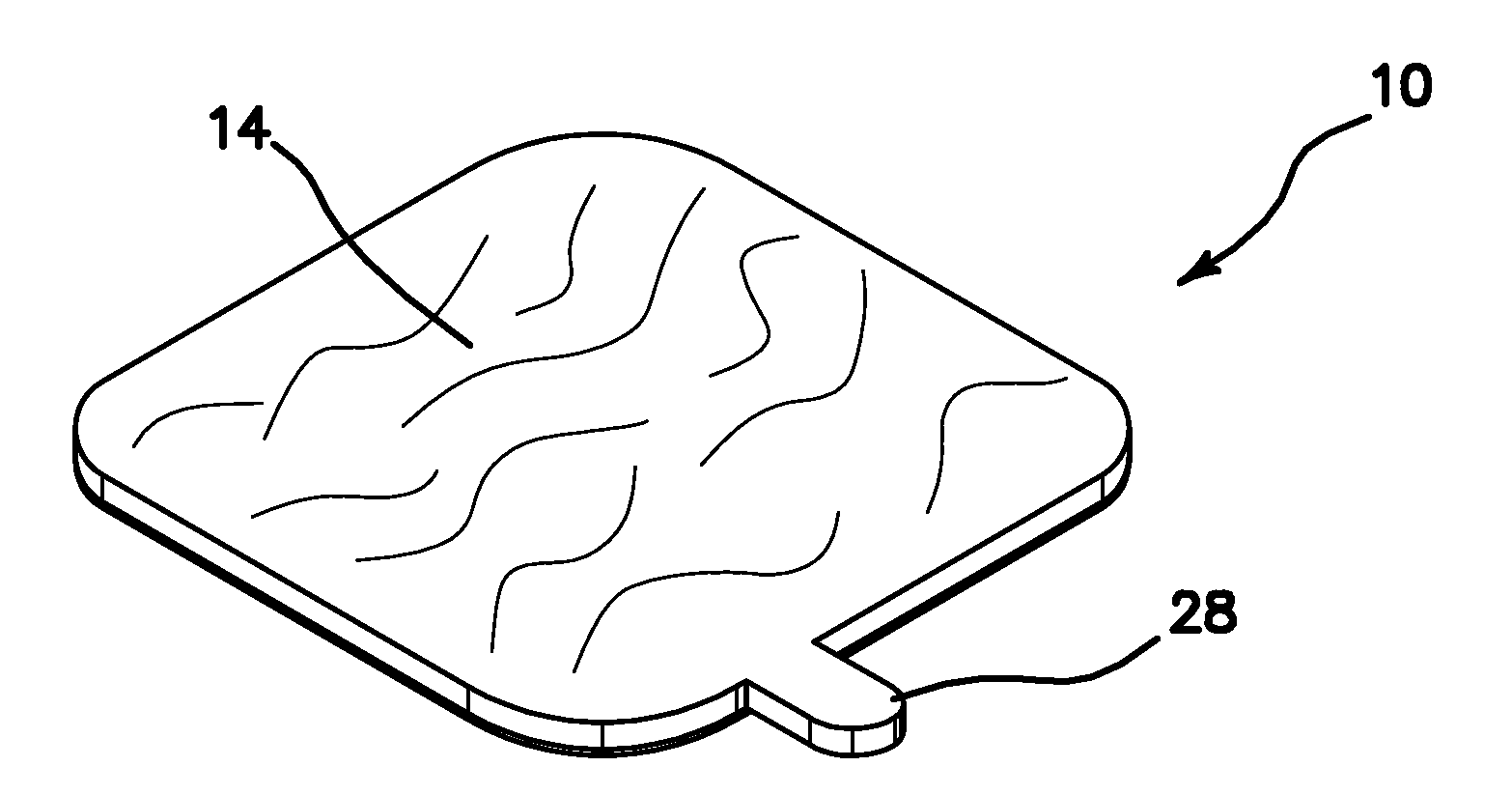
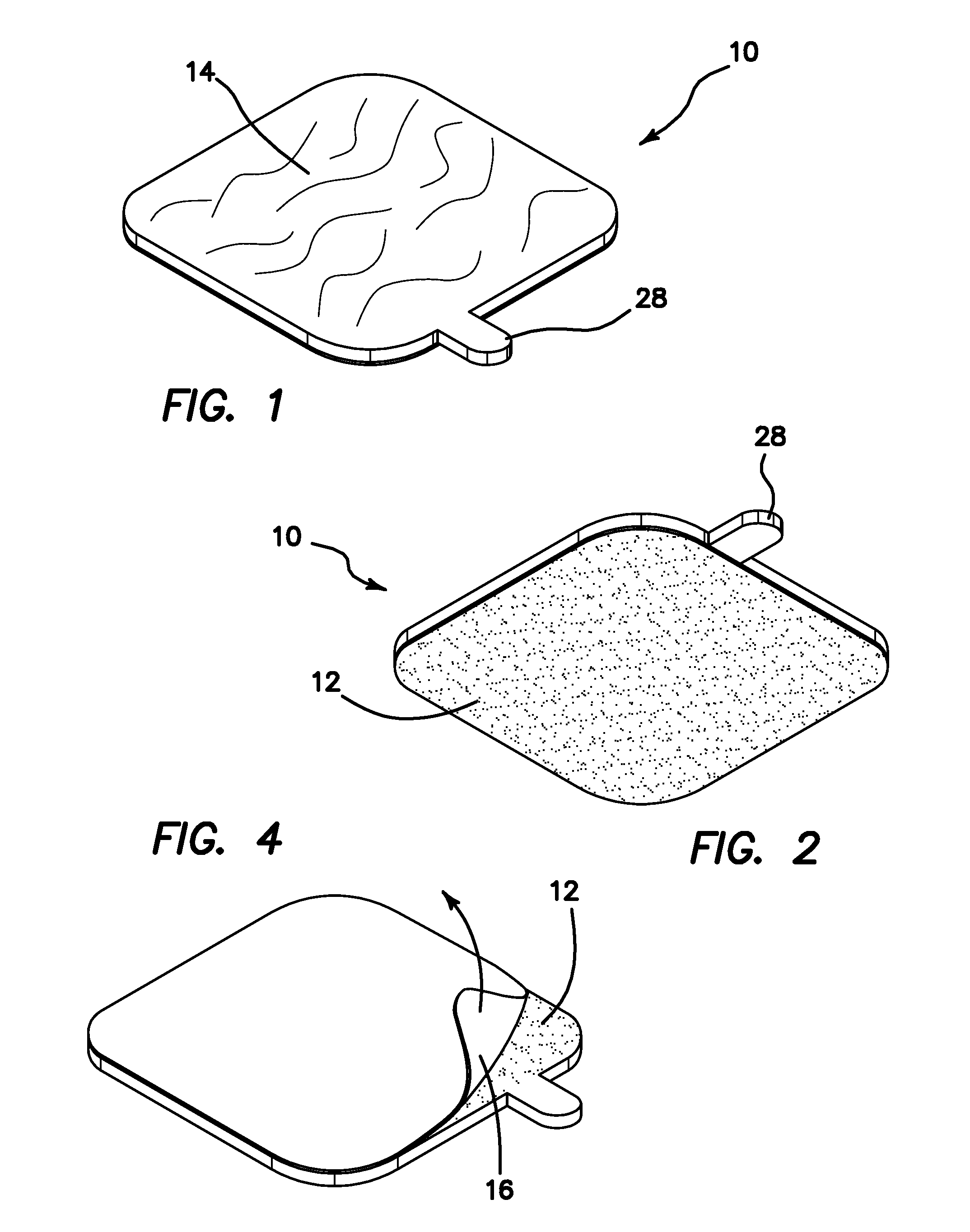
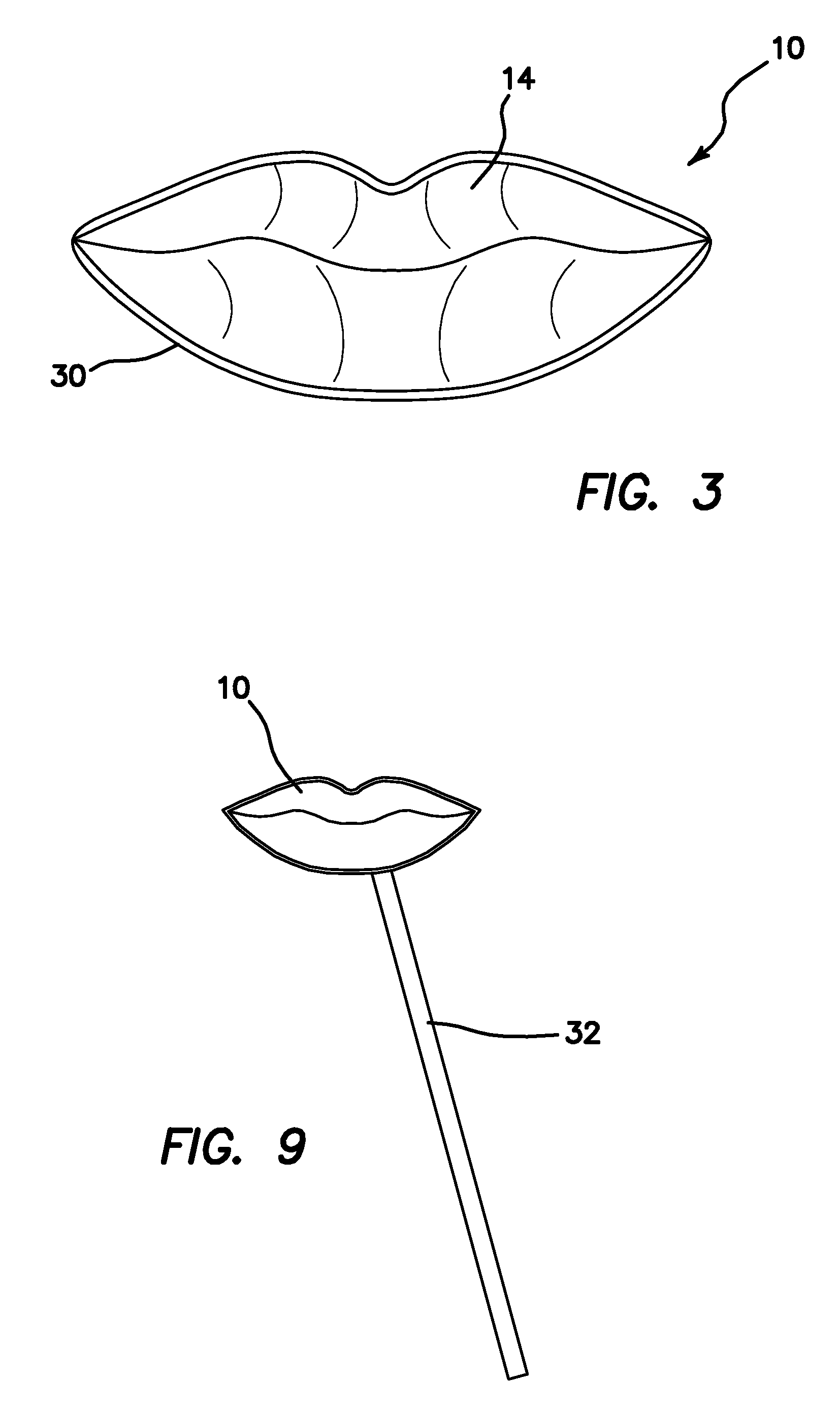
Sanitary Apparatus and Method for Sampling Cosmetics
InactiveUS20110067718A1Avoid spreadingMaintenance costCurling devicesOther accessoriesBiomedical engineeringContagious disease
A cosmetic patch for testing and evaluating a cosmetic product. The patch comprises a top surface colored and textured to match that of a user's features, preferably that of their lips. The reverse side of the patch comprises a weak adhesive surface to temporarily bond to the user's hand, forearm, or other object. When the user deposits a sample of the cosmetic product onto the textured surface of the patch, the user may then compare and evaluate the look of the cosmetic product on their particular features, for example their lips, without having to actually place the cosmetic product on their features and thus prevent the spread of contagious diseases and maintain costs at a minimum for the cosmetics vendor.
Owner:LEE BONNIE
Advanced isolation gown
InactiveUS7647648B2Low extremityAvoid pollutionBaby linensPyjamasIntensive care medicineContagious disease
Owner:MEDICAL ISOLATION TECH
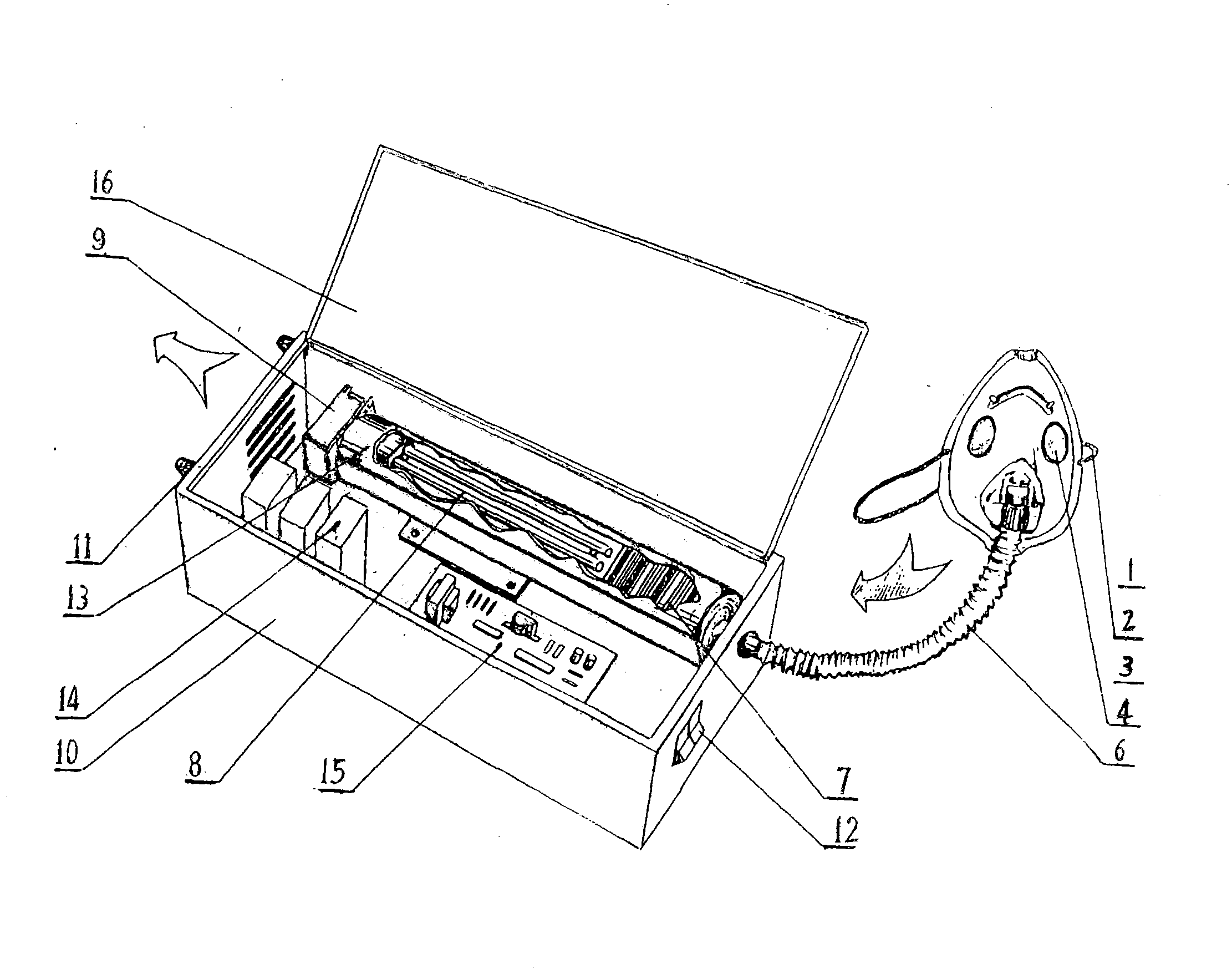
Respiration disinfecting machine
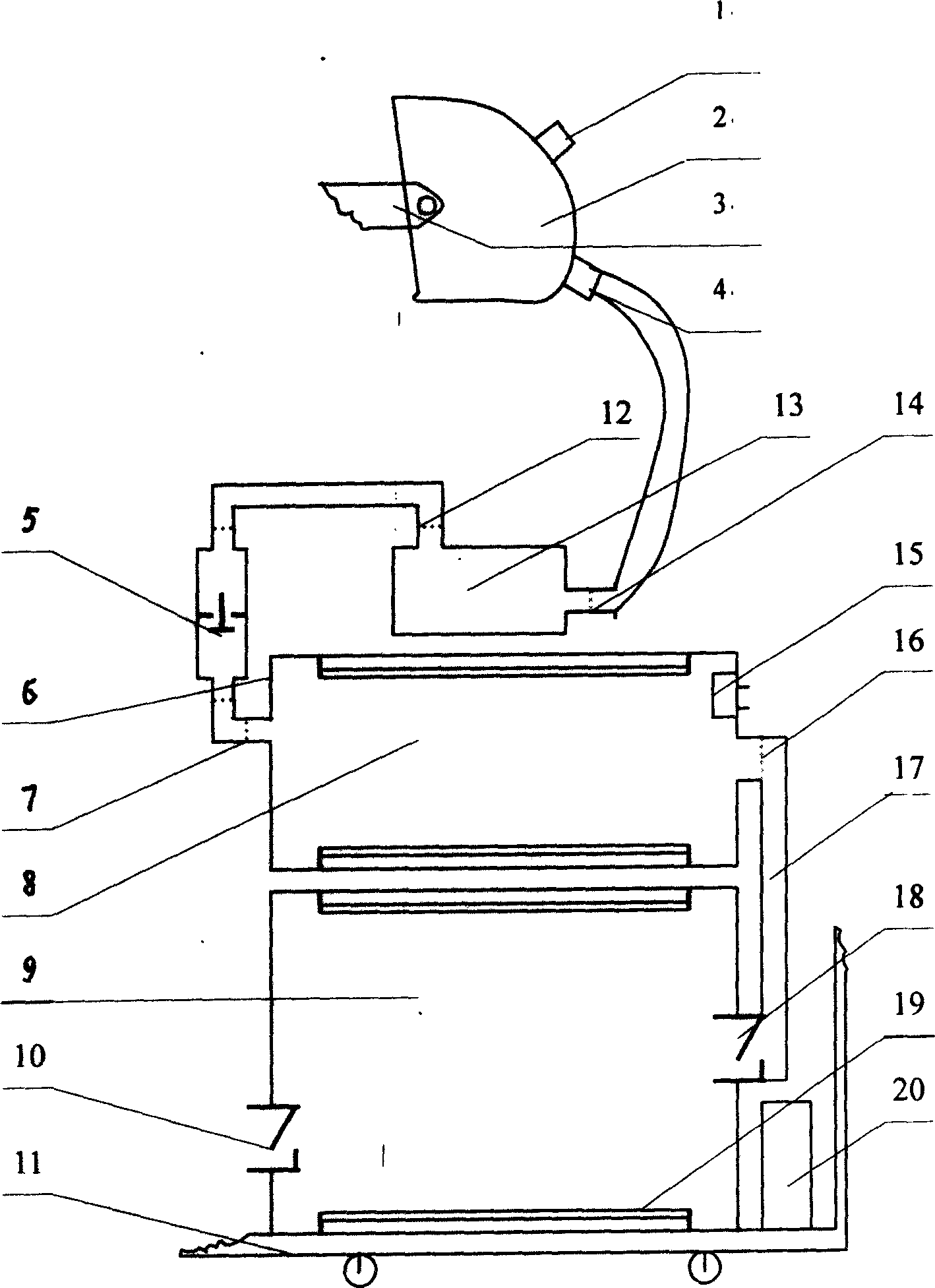
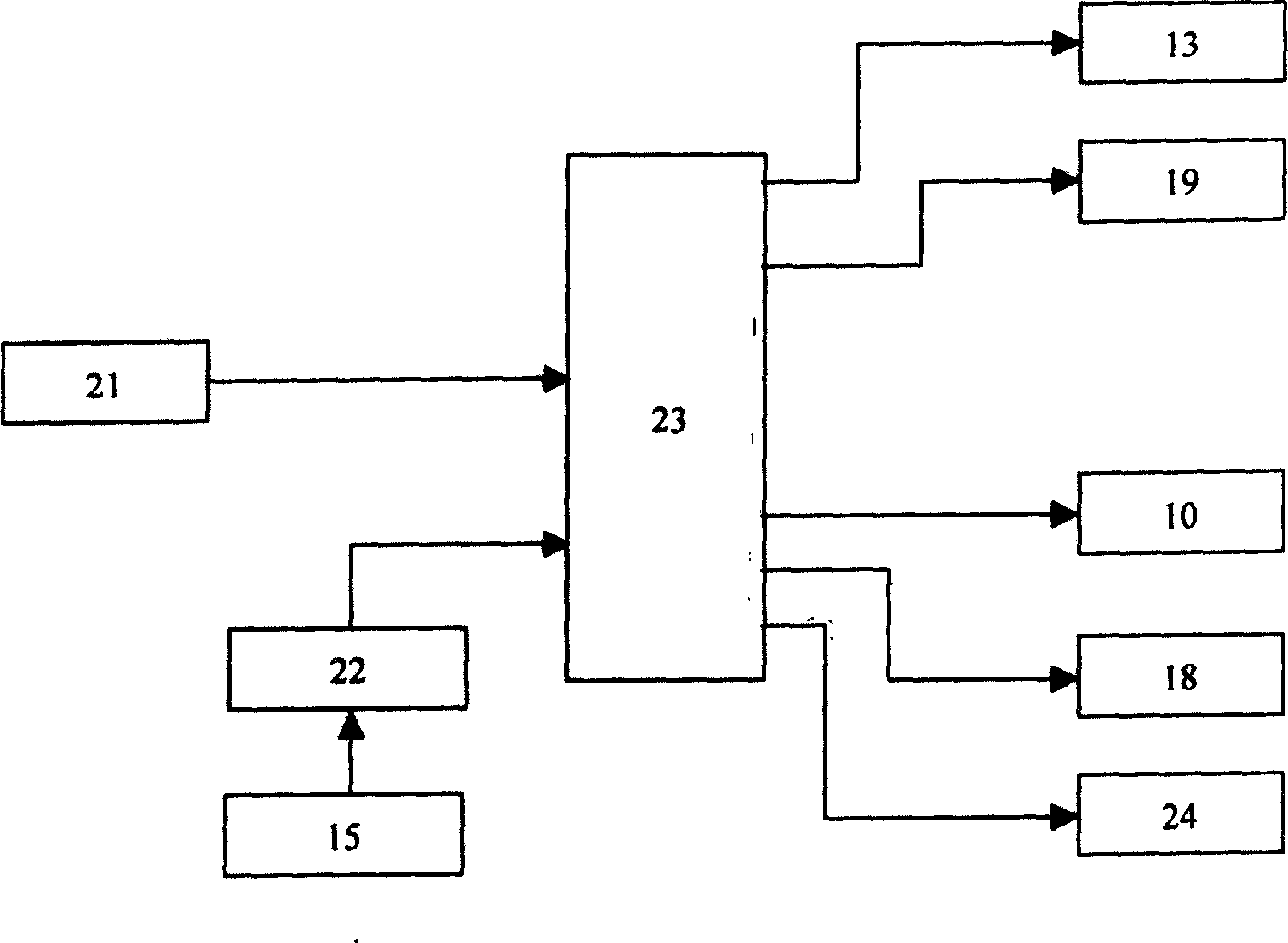
InactiveCN1569282AContagious noDisinfection SynchronizationFire rescueRespiratory apparatusEngineeringContagious disease
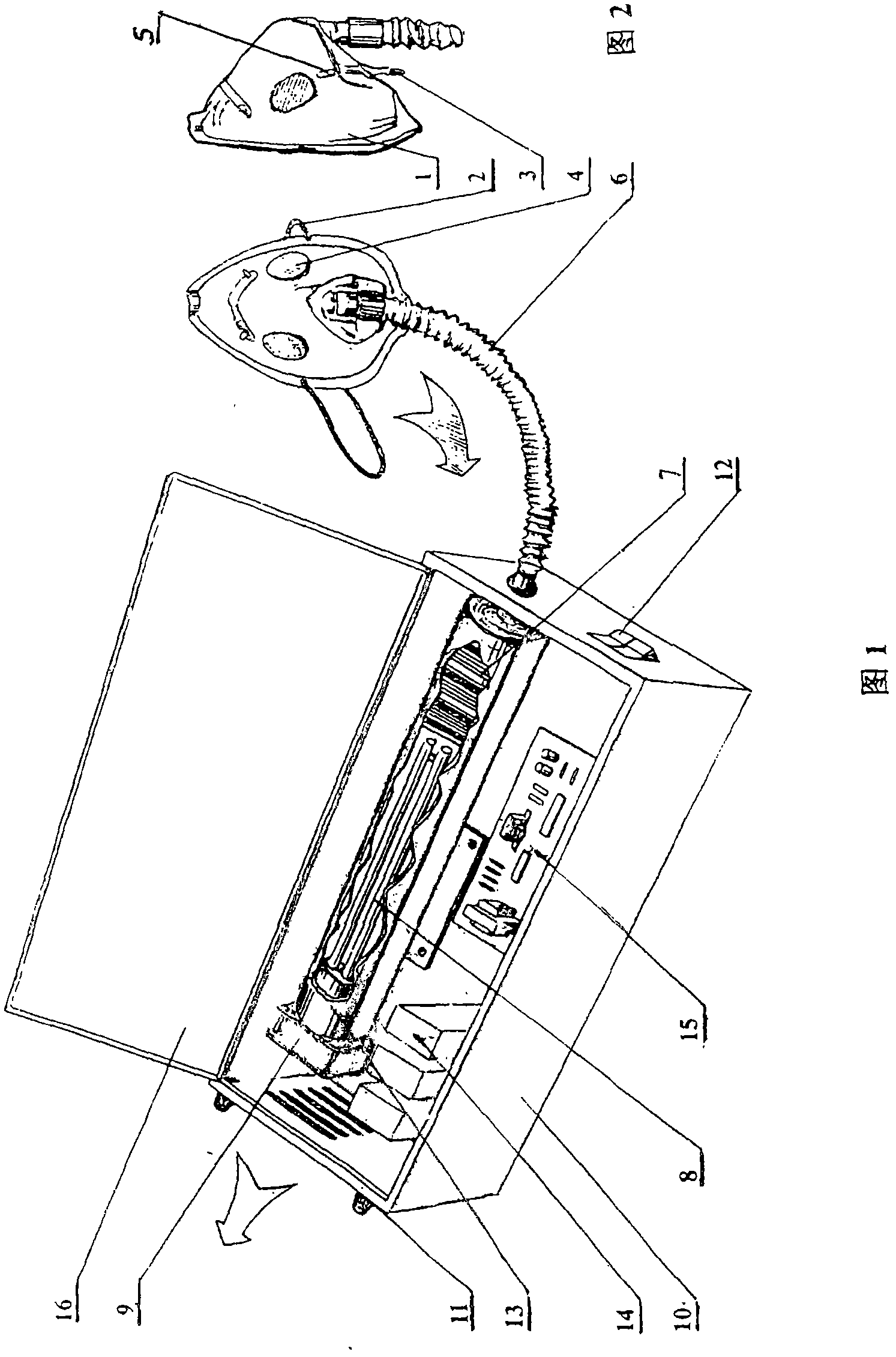
A respiration disinfecting machine is disclosed. It comprises a respiration mask 2, a disinfecting chamber, a control module 20 and engine casing 11. The disinfecting chamber is of multistage disinfecting chamber. A gas compression arrangement is set between the respiration mask 2 and the disinfecting chamber. The invention can prolong disinfecting time by making use of the gas compression arrangement and the multistage disinfecting chamber. It has advantages of simultaneous, dynamic, quick acting, and complete disinfecting to the air expired by the patients with respiratory tract contagious diseases.
Owner:刘爱彬
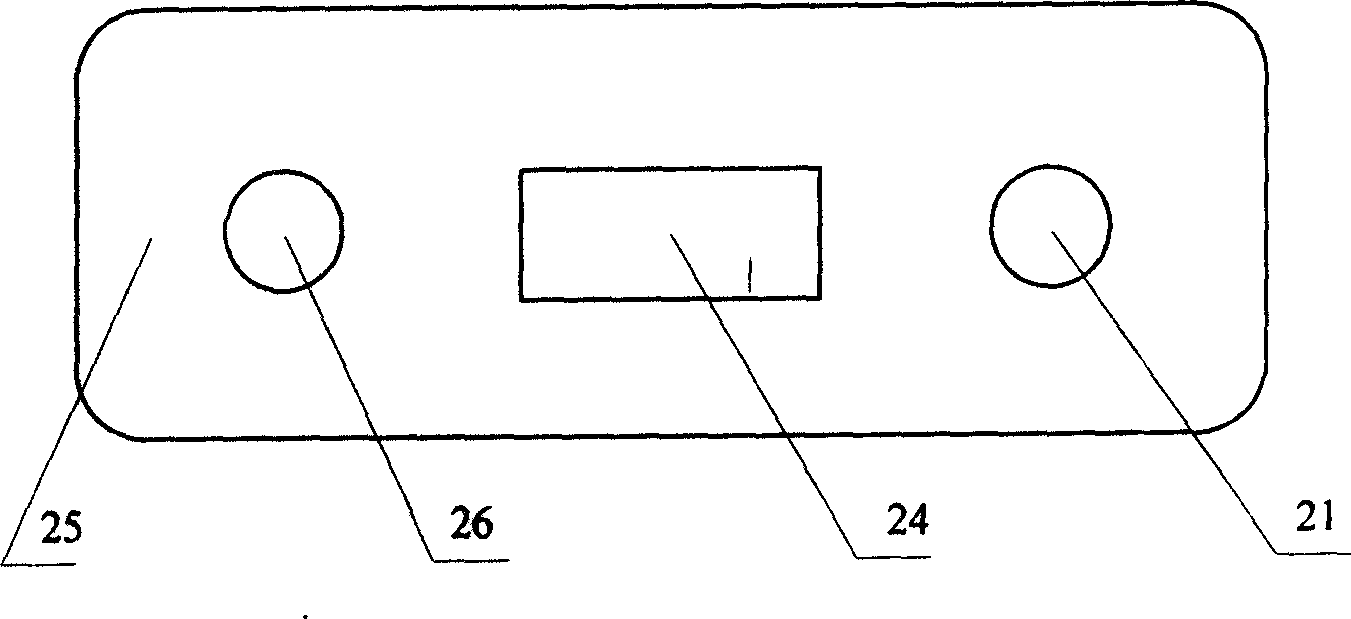
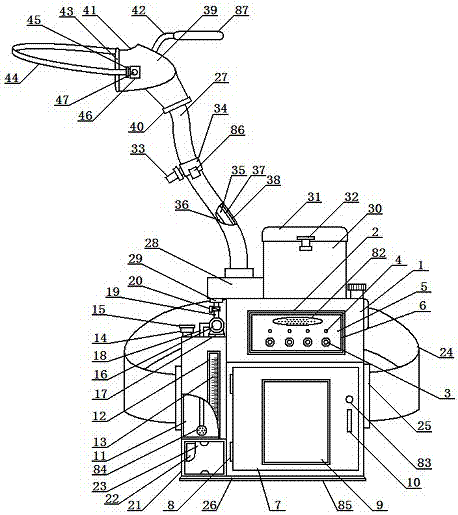
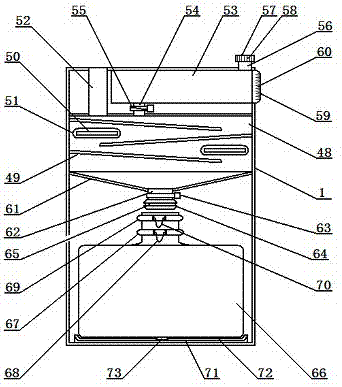
Portable specific vomit device for infectious disease
InactiveCN107875026AEasy to carryAvoid distributingMedical waste disposalSpittle receiving devicesRespiratorEngineering
The invention relates to a portable specific vomit device for an infectious disease and belongs to the technical field of medical utensils. The portable specific vomit device for the infectious disease comprises a box body. An operation panel is arranged on the front side of the box body; a flushing water box is arranged on the left side of the box body; the lower side of the flush water box is provided with a power supply box; an auxiliary back belt is mounted on the rear side of the box body; a foldable combination hose is mounted on the upper side of the box body; a moth mask accommodatingbox is formed in the right side of the foldable combination hose; a hose fixing clip is cooperated to the upper surface of the foldable combination hose; a sealing mouth mask is mounted on the upper side of the foldable combination hose; a disinfection and sterilizing box is disposed in the box body; a flushing protection plate is placed in the sealing mouth mask; a diversion flushing groove is formed in the right side of the flushing protection plate; and a flushing nozzle is formed in the upper side of the diversion flushing groove. The portable specific vomit device for the infectious disease is simply structured and convenient to use; an infectious disease patient can carry the device, so vomit can be flushed and sealed, and odor of the vomit and saliva splash can be prevented; and patient vomit can be sterilized and disinfected, so bacteria can be fully killed and cross infection can be avoided.
Owner:孙燕妮
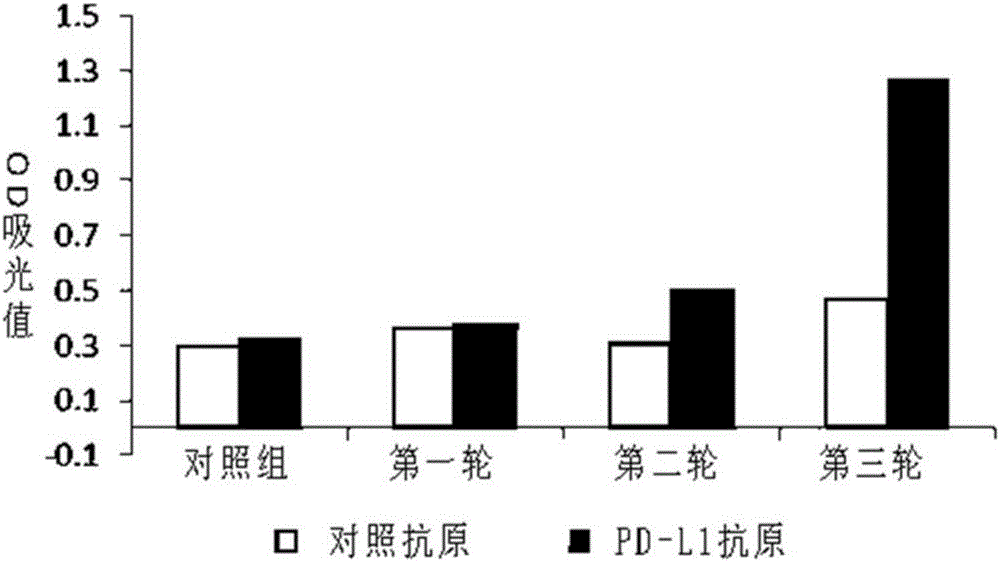
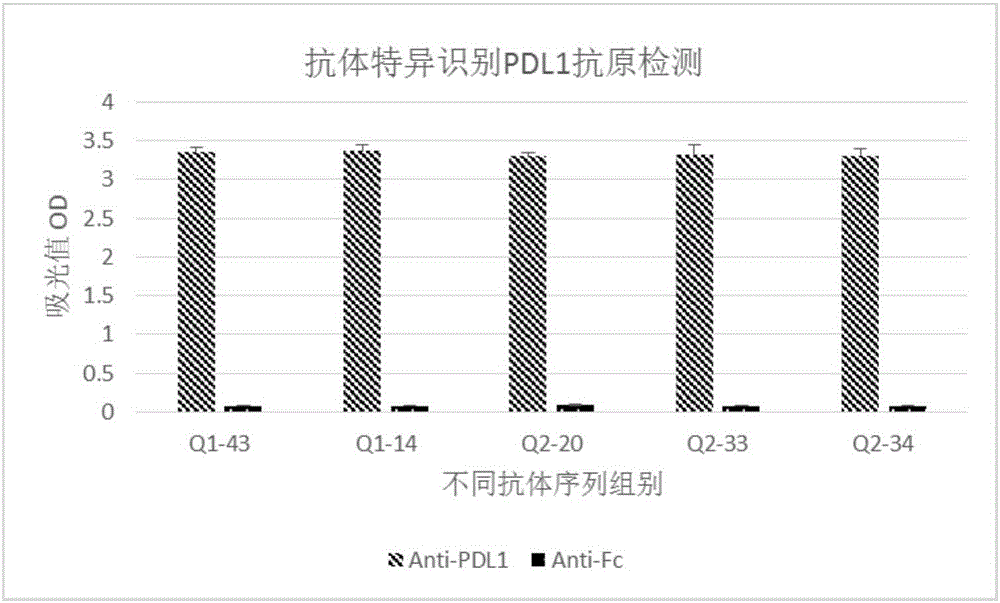
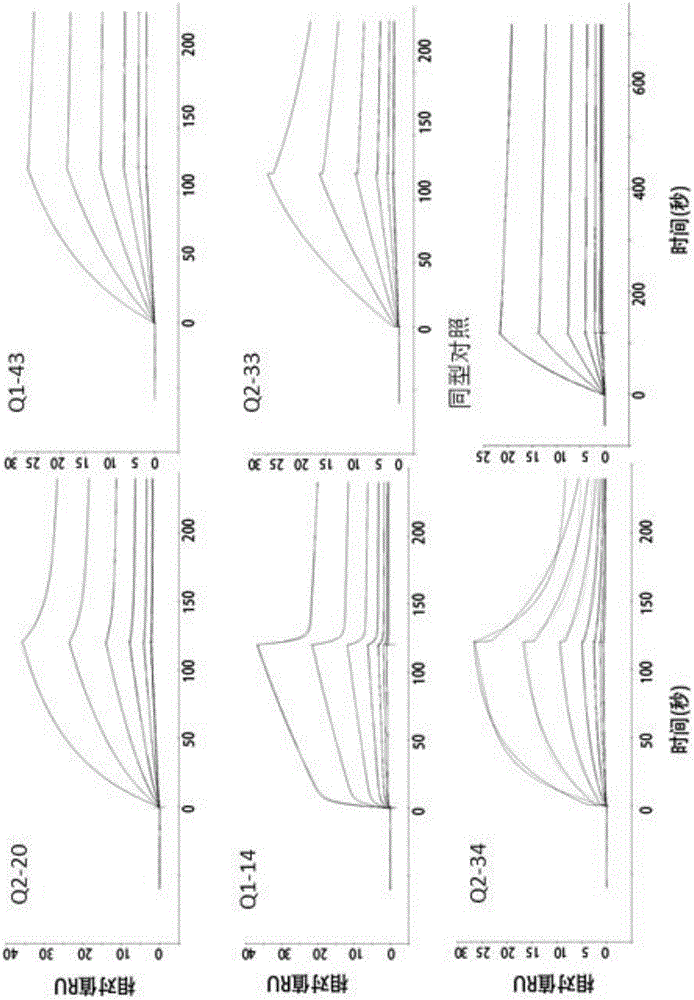
Humanized antibody or antibody fragment for PD-L1 extracellular fragments and application thereof, nucleotide sequence and carrier
ActiveCN106496327AStrong specificityImprove secretion capacityAntipyreticAnalgesicsComplementarity determining regionAntibody fragments
The invention discloses a humanized antibody or antibody fragment for PD-L1 extracellular fragments and application thereof, a nucleotide sequence and a carrier, belongs to the technical field of antibody humanization. The antibody or the antibody fragment comprises a heavy chain and a light chain. Each of the heavy chain and the light chain comprises a variable region. Each variable region comprises complementary determining regions. The complementary determining regions CDR1, CDR2 and CDR3 of the heavy chain are represented by HCDR1, HCDR2 and HCDR3 respectively. The complementary determining regions CDR1, CDR2 and CDR3 of the light chain are represented by LCDR1, LCDR2 and LCDR3 respectively. The antibody or the antibody fragment can improve immunity systems and promote lymphocyte to secrete interleukin-2, and is applied to preparation of diagnostic reagents and anti-tumor, anti-inflammation and anti-contagion drugs.
Owner:武汉科源安博生物技术有限公司
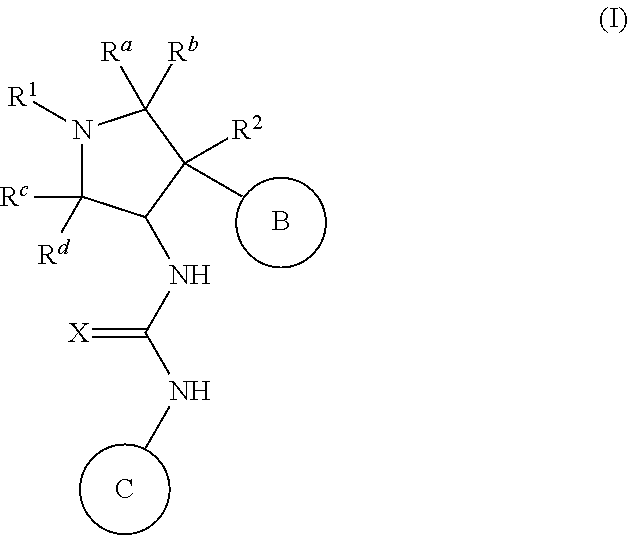
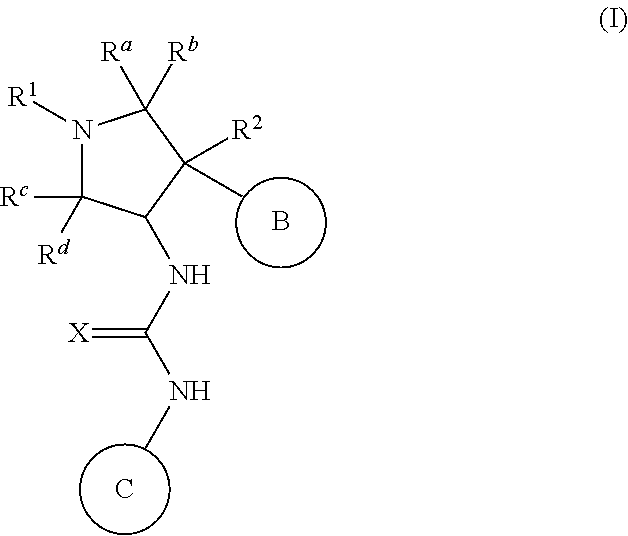
Pyrrolidinyl urea, thiourea, guanidine and cyanoguanidine compounds as trka kinase inhibitors
Compounds of Formula (I): or stereoisomers, tautomers, or pharmaceutically acceptable salts, or solvates or prodrugs thereof, where R1, R2, Ra, Rb, Rc, Rd, X, Ring B, and Ring C are as defined herein, and wherein Ring B moiety and the NH—C(═X)—NH moiety are in the trans configuration, are inhibitors of TrkA kinase and are useful in the treatment of diseases which can be treated with a TrkA kinase inhibitor such as pain, cancer, inflammation / inflammatory diseases, neurodegenerative diseases, certain infectious diseases, Sjogren's syndrome, endometriosis, diabetic peripheral neuropathy, prostatitis and pelvic pain syndrome.
Owner:ARRAY BIOPHARMA
Chinese medicinal composition for improving immunity of organisms, tonifying middle-jiao and qi and treating canine distemper
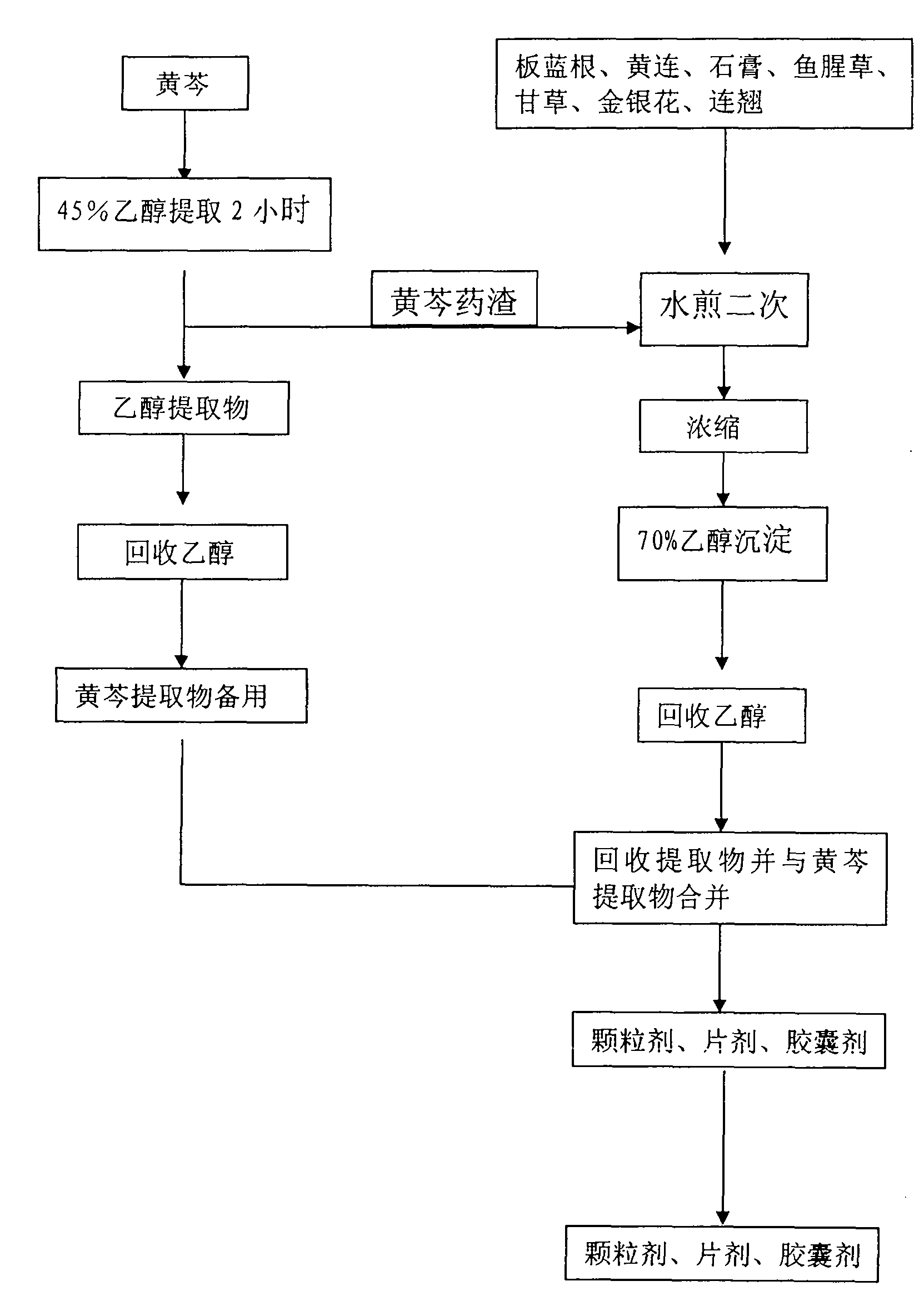
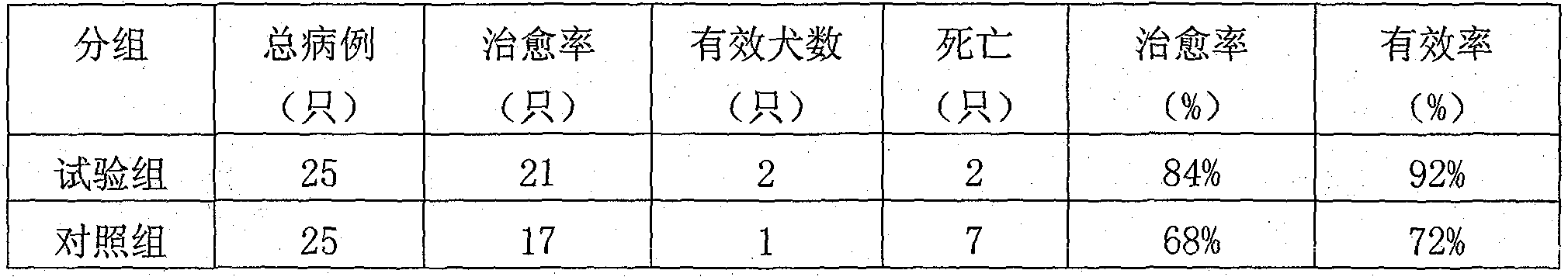
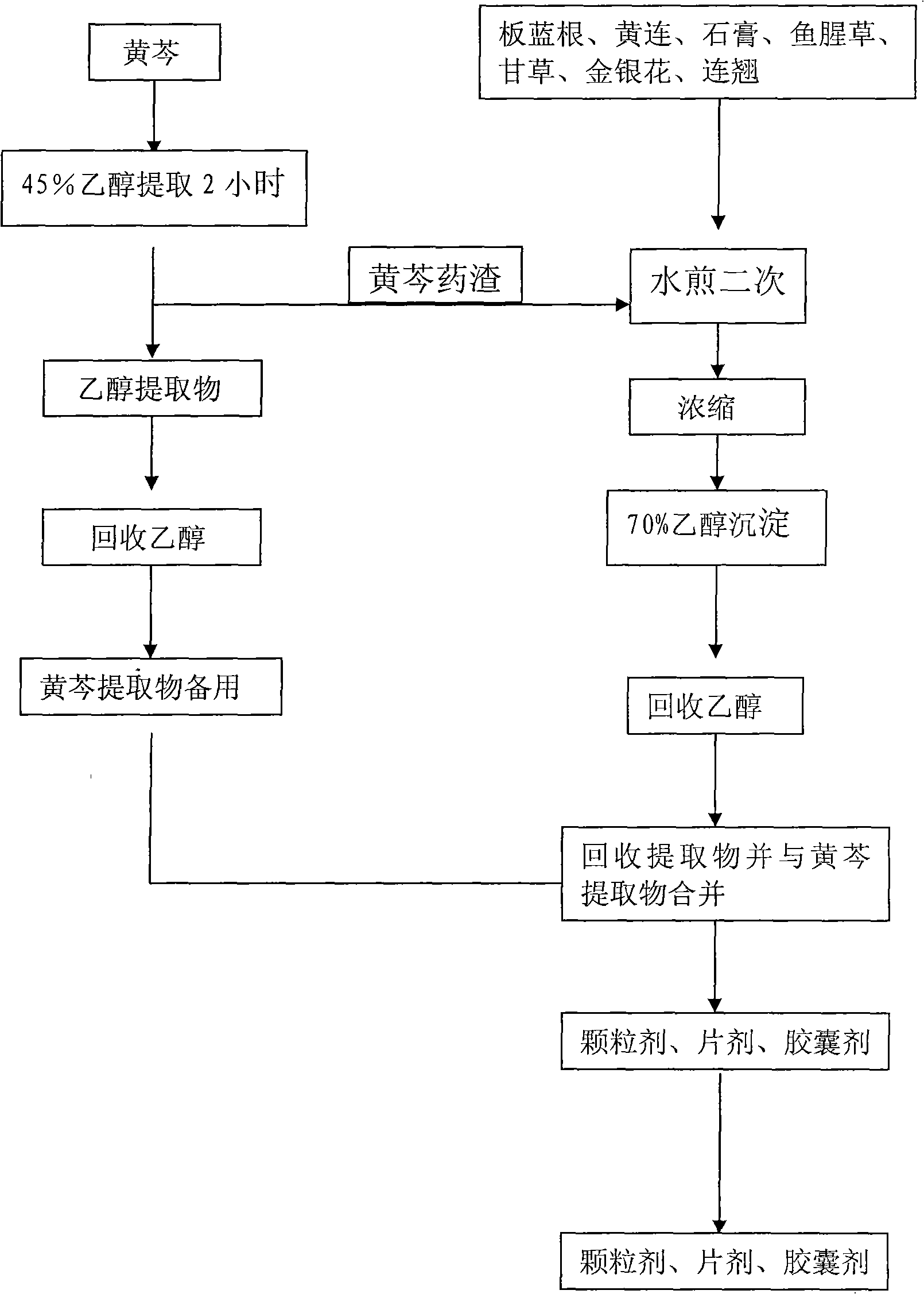
InactiveCN101904935AHigh cure rateDefinite curative effectAntiviralsAluminium/calcium/magnesium active ingredientsHouttuyniaBaical Skullcap Root
The invention relates to a Chinese medicinal composition for treating canine highly contagious disease canine distemper and a preparation method thereof. The Chinese medicinal composition mainly comprises indigowoad root, baical skullcap root, golden thread, gypsum, heartleaf houttuynia herb, liquoric root, honeysuckle flower, weeping forsythia and the like, has the effects of improving the immunity of organisms, tonifying middle-jiao and qi and treating the canine distemper, and is mainly used for dogs. The composition keeps the combined effect of the formula of a traditional Chinese medicine for treatment based on syndrome differentiation by combining modern Chinese medicine development technology according to the theory of traditional Chinese medicine. The composition has the effects of resisting viruses, tonifying middle-jiao and qi, and enhancing the immunity of the organisms, treats both symptoms and root causes, is prepared into tablets, has the advantages of convenient administration, high palatability, and definite treatment effect through clinical verification, and is a pollution-free Chinese medicinal composition for treating the canine distemper.
Owner:TIANJIN RINGPU BIO TECH
Traditional Chinese medicine for preventing contagious diseases of swine
InactiveCN102755552AThe effect of preventing swine fever is remarkableThe plague effect is significantAntiviralsPlant ingredientsBiotechnologySide effect
The invention relates to the technical field of veterinary traditional Chinese medicine, in particular to a traditional Chinese medicine for preventing contagious diseases of swine. The traditional Chinese medicine comprises the following raw material medicines in part by weight: 5 parts of reed rhizome, 8 parts of baical skullcap root, 3 parts of atractylis ovata, 15 parts of mung beans, 30 parts of radix isatidis, 10 parts of Chinese yam, 2 parts of peach kernels, 6 parts of rhubarb, 8 parts of evodia rutaecarpa, 12 parts of flower bud of lily magnolia, 15 parts of burdock, and 8 parts of notopterygium root. The traditional Chinese medicine provided by the invention has a remarkable effect for preventing contagious diseases of swine, has no any toxic or side effects, has no obvious side effects on food intake of pigs, has a positive effect on food conversion ratio, is low in dose and cost, and facilitates the increase of income of pig breeders.
Owner:张伟 +2
Traditional Chinese medical composition for treatment of sheep pox
InactiveCN102895418AEffective treatmentHeat-clearing and detoxifyingAntiviralsDermatological disorderMortality rateSemiaquilegia
The invention provides a traditional Chinese medical composition for treatment of sheep pox. The traditional Chinese medical composition consists of the following components by weight: 1-120 parts of honeysuckle, 1-120 parts of wild chrysanthemum, 1-120 parts of dandelion, 1-120 parts of herba violae, 1-115 parts of radix scutellariae, 1-115 parts of radix isatidis, 1-110 parts of cimicifugae foetidae, 1-115 parts of lithospermum, 1-110 parts of semiaquilegia root and 1-110 parts of liquorice. According to a method of Chinese veterinary dialectical treatment, the honeysuckle, the wild chrysanthemum, the dandelion, the herba violae, the radix scutellariae, the radix isatidis, the cimicifugae foetidae, the lithospermum, the semiaquilegia root and the liquorice are used together, a contagious disease caused by a sheep pox virus, namely sheep pox, can be treated effectively, typical smallpox on the body skin and sometimes the mucosa can be alleviated effectively, and the high mortality caused by fever of ill sheep can be reduced significantly.
Owner:高影
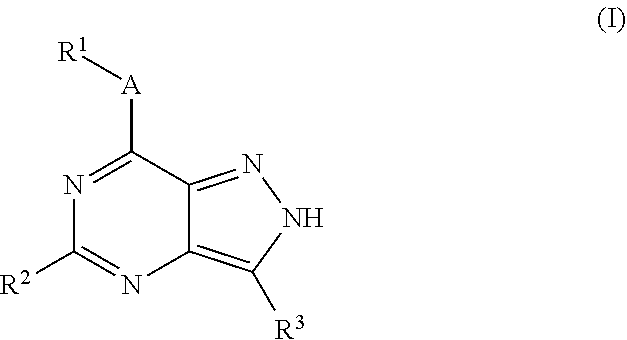
Substituted pyrazolo{4,3-D}pyrimidines as kinase inhibitors
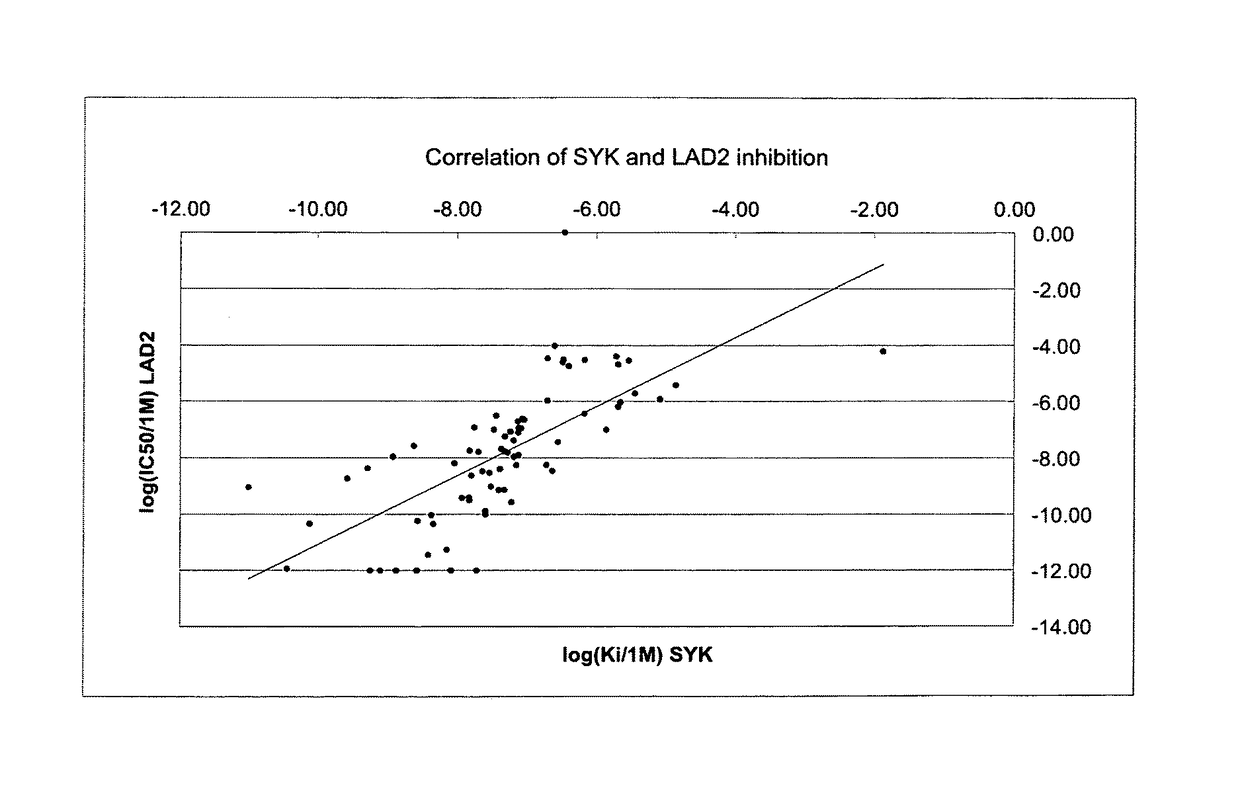
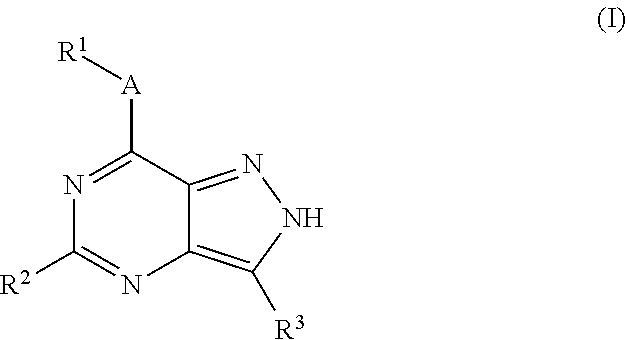
ActiveUS9802937B2Improve propertiesReduce formationBiocideSenses disorderAutoimmune conditionAutoimmune disease
The present invention relates to novel compounds of formula (I)that are capable of inhibiting one or more kinases, especially SYK (Spleen Tyrosine Kinase), LRRK2 (Leucine-rich repeat kinase 2) and / or MYLK (Myosin light chain kinase) or mutants thereof. The compounds find applications in the treatment of a variety of diseases. These diseases include autoimmune diseases, inflammatory diseases, bone diseases, metabolic diseases, neurological and neurodegenerative diseases, cancer, cardiovascular diseases, allergies, asthma, alzheimer's disease, parkinson's disease, skin disorders, eye diseases, infectious diseases and hormone-related diseases.
Owner:ORIGENIS
Methods of treating cancer, infectious disease, and autoimmune disease using cxc chemokines
ActiveUS20180193382A1Increase productionIncreasing chemokineOrganic active ingredientsPeptide/protein ingredientsAutoimmune conditionCXCL10
The current invention is related to the prevention and treatment of diseases including cancer, autoimmune disease, and infectious disease using CXC chemokines and the receptors to which they agonize. It has been found that certain chemokines, including CXCL4, CXCL9, CXCL10, and CXCL12 have various effects on toll-like receptors in various cell types and these can be utilized for disease treatment and prevention.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
System and method for monitoring outbreak of contagious diseases
InactiveUS8645538B2Emergency connection handlingEpidemiological alert systemsOutbreakAd hoc communication
A surveillance system for monitoring outbreak of a contagious disease is disclosed. The system comprises a handheld computing and communication device with a short range ad hoc networking device. Handheld devices carried by persons in contacting with the device carried by a user form an ad hoc communication network at a location. Identities of all devices in the ad hoc network are broadcasted through the network. The user's device receives the identities and stores the received data in a log file. The log file may be sent to a central station after the device receives an authorized signal during an outbreak event of the contagious disease. The device may further include a body temperature automatic measuring system. The user's body temperature trend file may be sent together with the log file.
Owner:PAN YANG
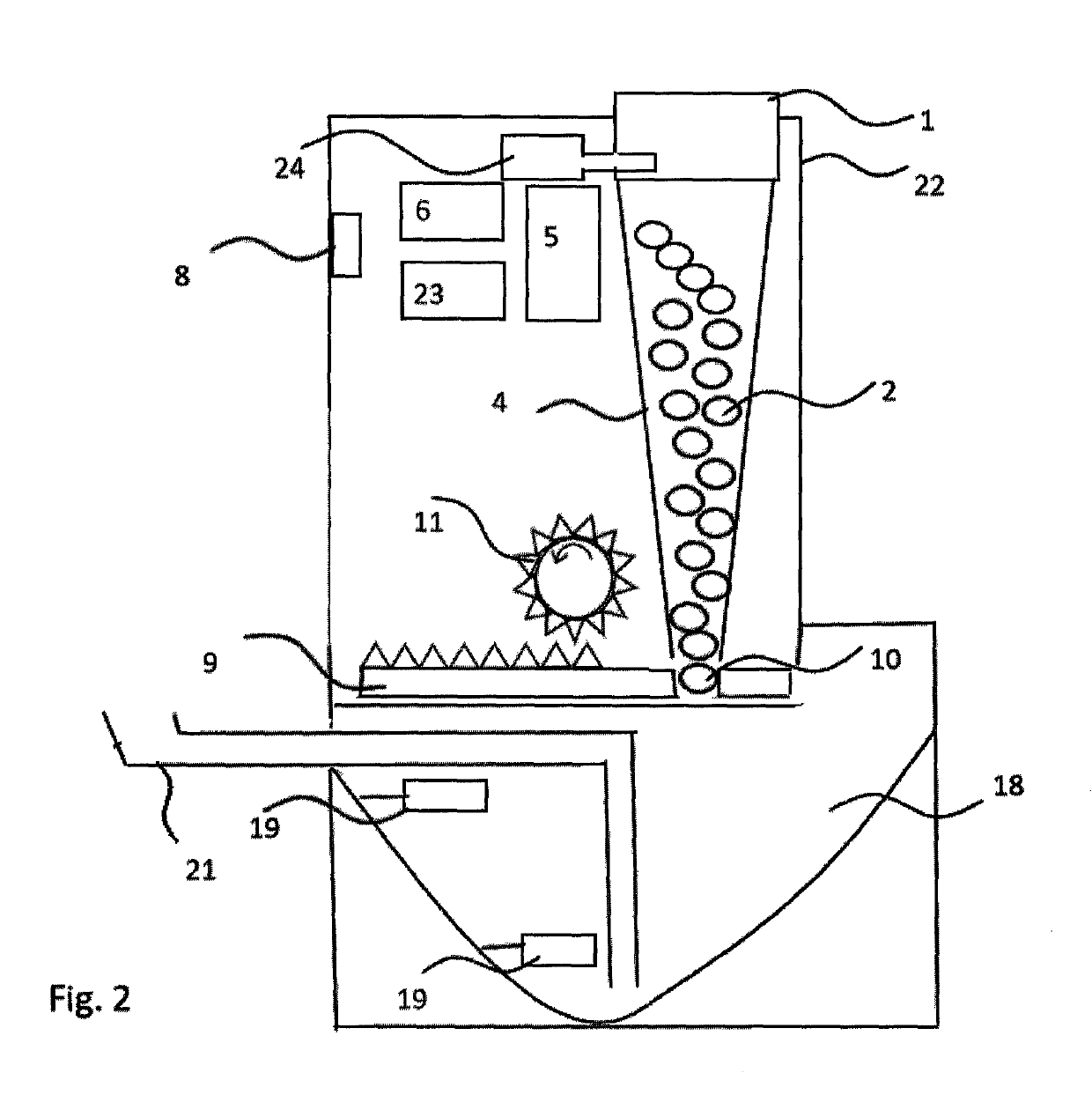
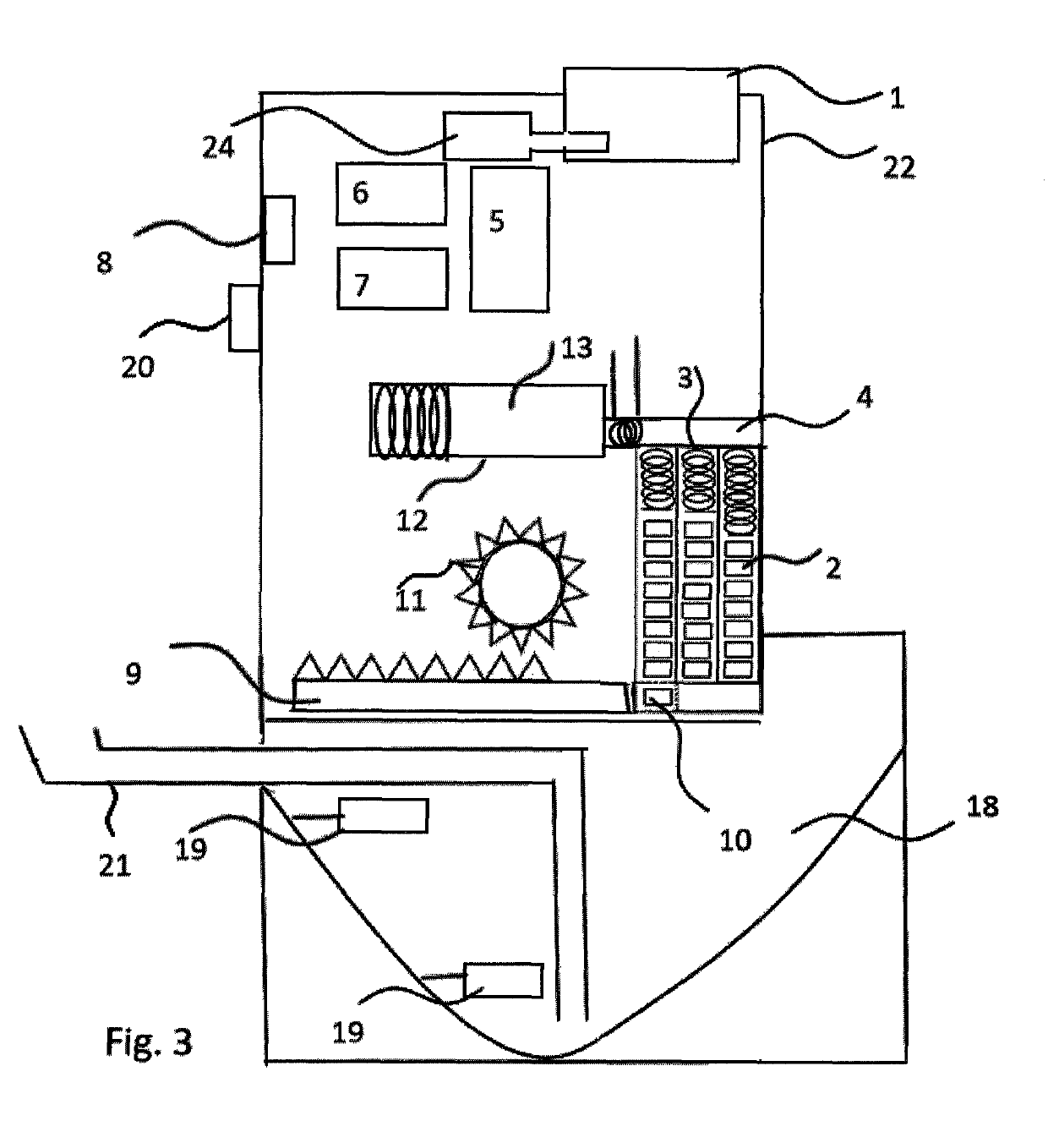
Infectious disease isolating and sterilizing equipment
InactiveCN102512740AGuarantee the safety of lifeImprove isolationRespiratory masksMedical waste disposalConstant powerUltraviolet
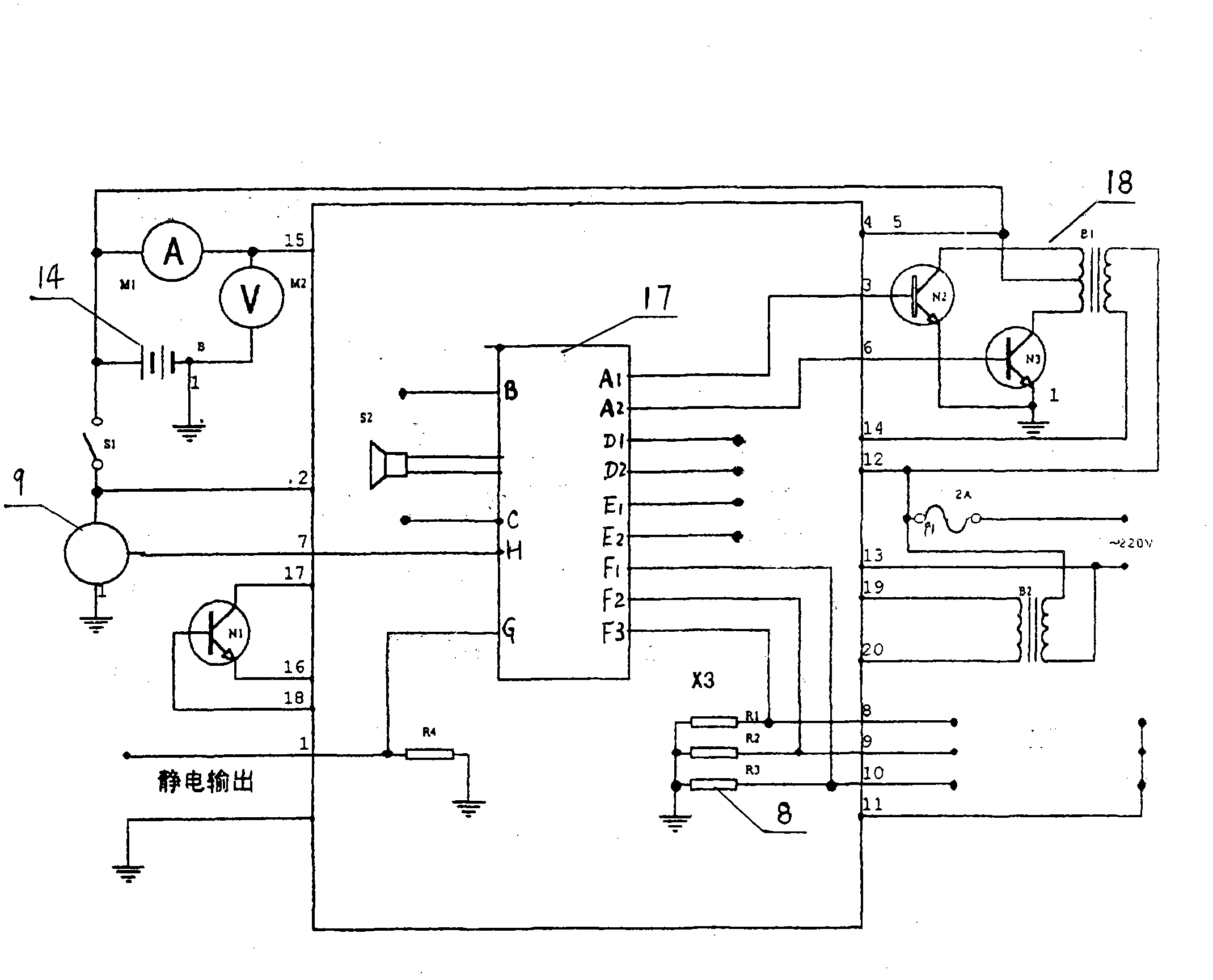
The invention relates to medical-care equipment for isolating and sterilizing a patient and protecting other people from being infected. The equipment consists of a mask, a sterilizing device, an electronic control device and constant power supply facilities, wherein the sterilizing device, the electronic control device and the constant power supply facilities are arranged in a sterilizing tank; the sterilizing device consists of a high-voltage electrostatic field, an ultraviolet sterilizing lamp and an electric fan; and expired gas goes first through absorption by the electrostatic field in the sterilizing device, then through radiation by an ultraviolet sterilizing lamp for sterilization, disinfection and purification and finally through the electrical fan before being discharged into the atmosphere. The results of clinic nursing trials in hospitals indicate that the isolating and sterilizing equipment is capable of disinfecting and sterilizing and has good isolating effect and high working efficiency. The equipment is applicable to treatment in hospital and nursing at home, particularly emergent rescue, patient transfer and rescue of patients in danger.
Owner:赵洪涛
Live attenuated antigenically marked classical swine fever virus
ActiveUS8133495B2SsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeStructural glycoprotein
Owner:AGRI UNITED STATES OF AMERICA AS RESPRESENTED BY THE SEC OF THE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com