Patents
Literature
11930results about "Infusion needles" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
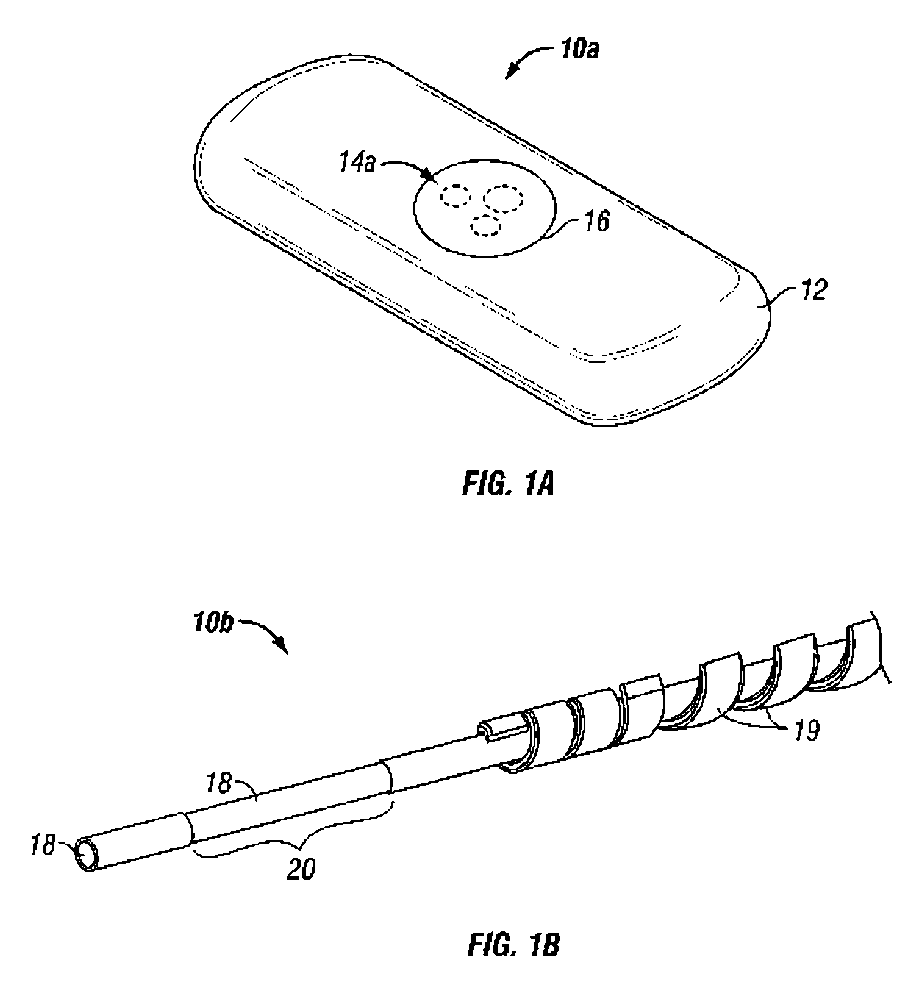
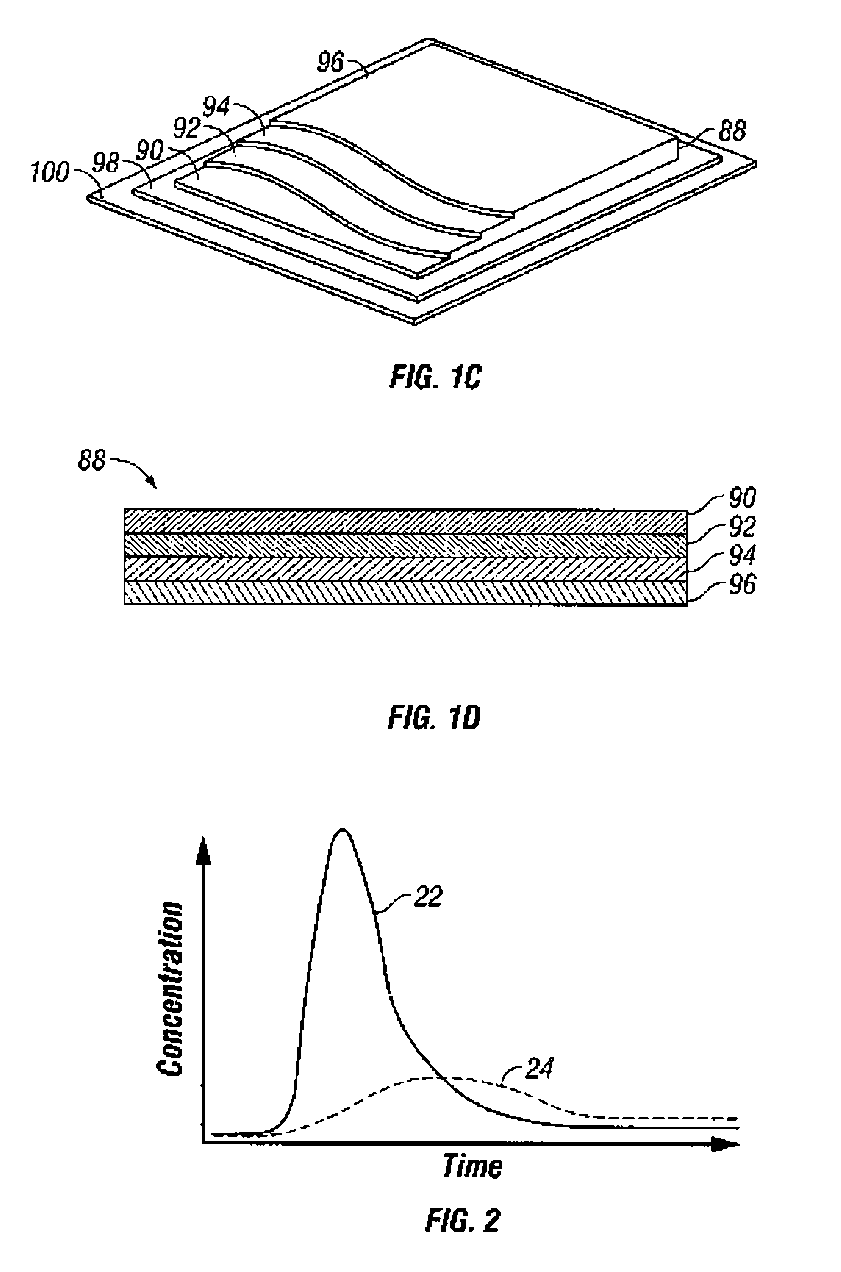
Methods and systems for inserting a transcutaneous analyte sensor
The present invention relates generally to systems and methods for measuring an analyte in a host. More particularly, the present invention relates to systems and methods for transcutaneous measurement of glucose in a host.
Owner:DEXCOM INC
Intubation device for enteral feeding
An intubation device is provided for use with a guide apparatus having a track that is adapted to be associated with an endoscope such that bending of the track is substantially decoupled from bending of the endoscope. The intubation device includes an elongated, flexible tube and a mating member attached to the tube and adapted to slidingly engage the track external of the endoscope. The intubation device further includes a tissue bolster disposed on the proximal portion of the tube and changeable between a collapsed and an expanded configuration. The tube is positionable inside the upper gastrointestinal tract of a patient such that the proximal end of the tube is externalized through the gastric and abdominal walls of the patient, and wherein the tissue bolster is securable against the inner gastric wall when the tissue bolster is in the expanded configuration.
Owner:ETHICON ENDO SURGERY INC
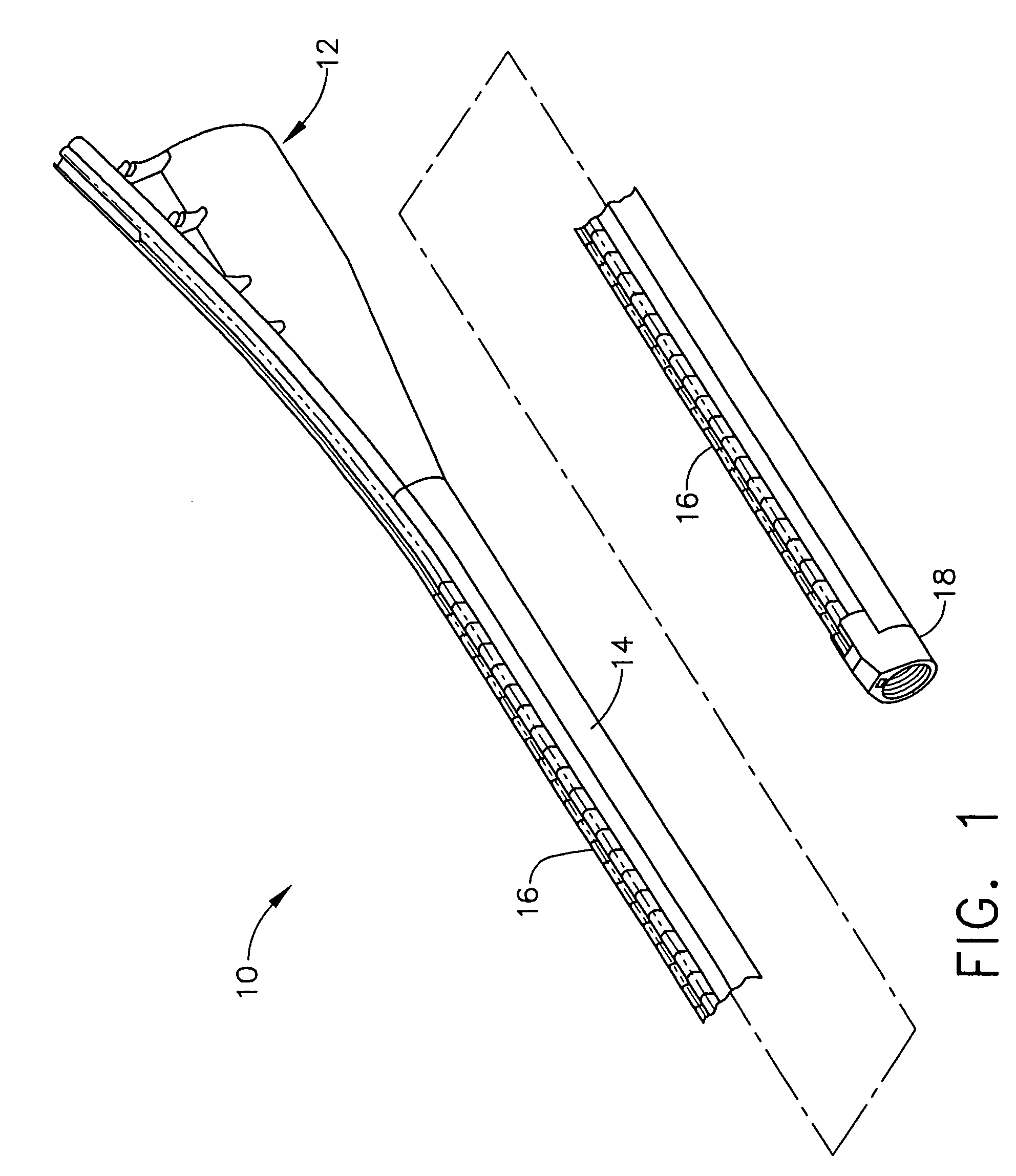
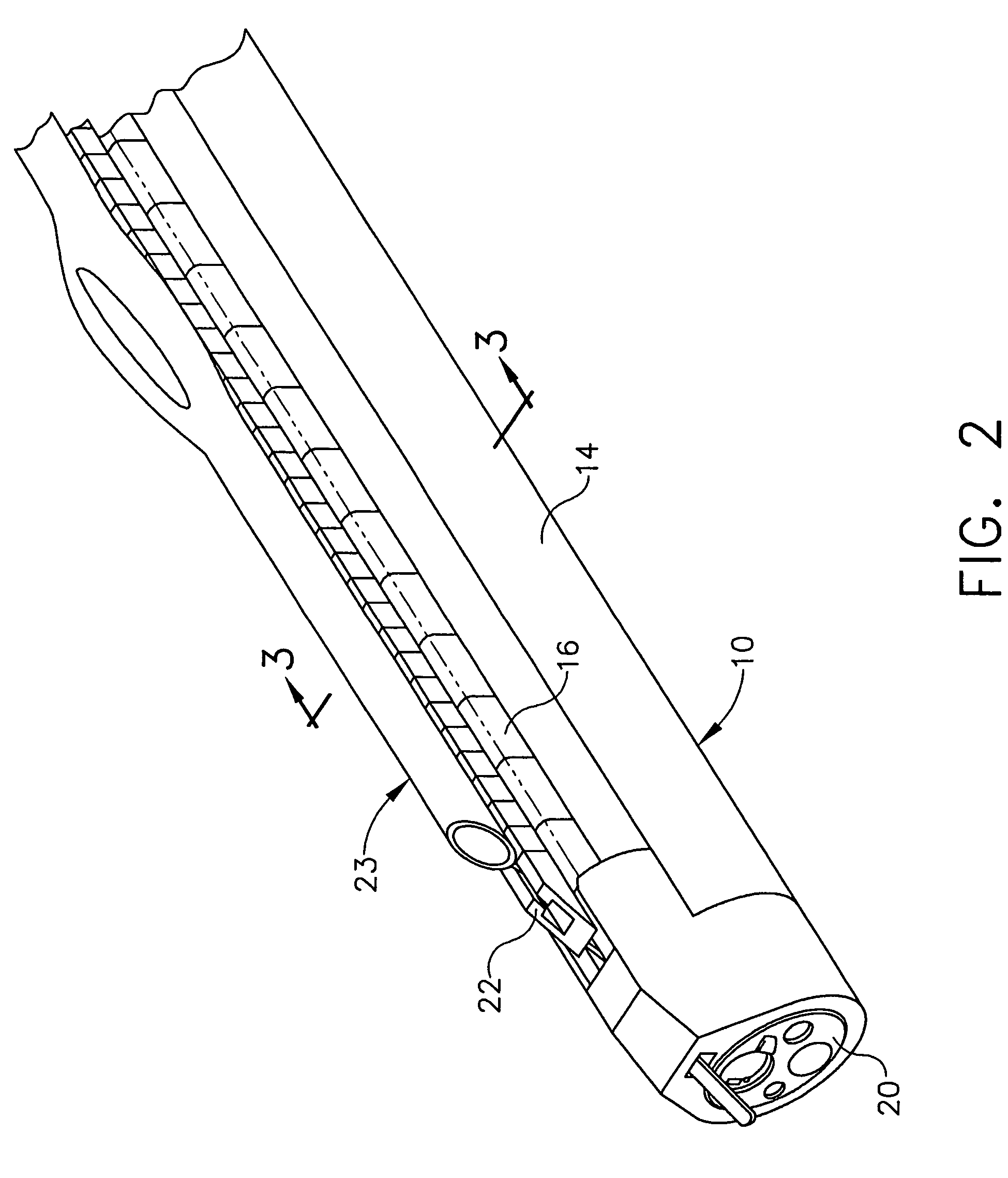
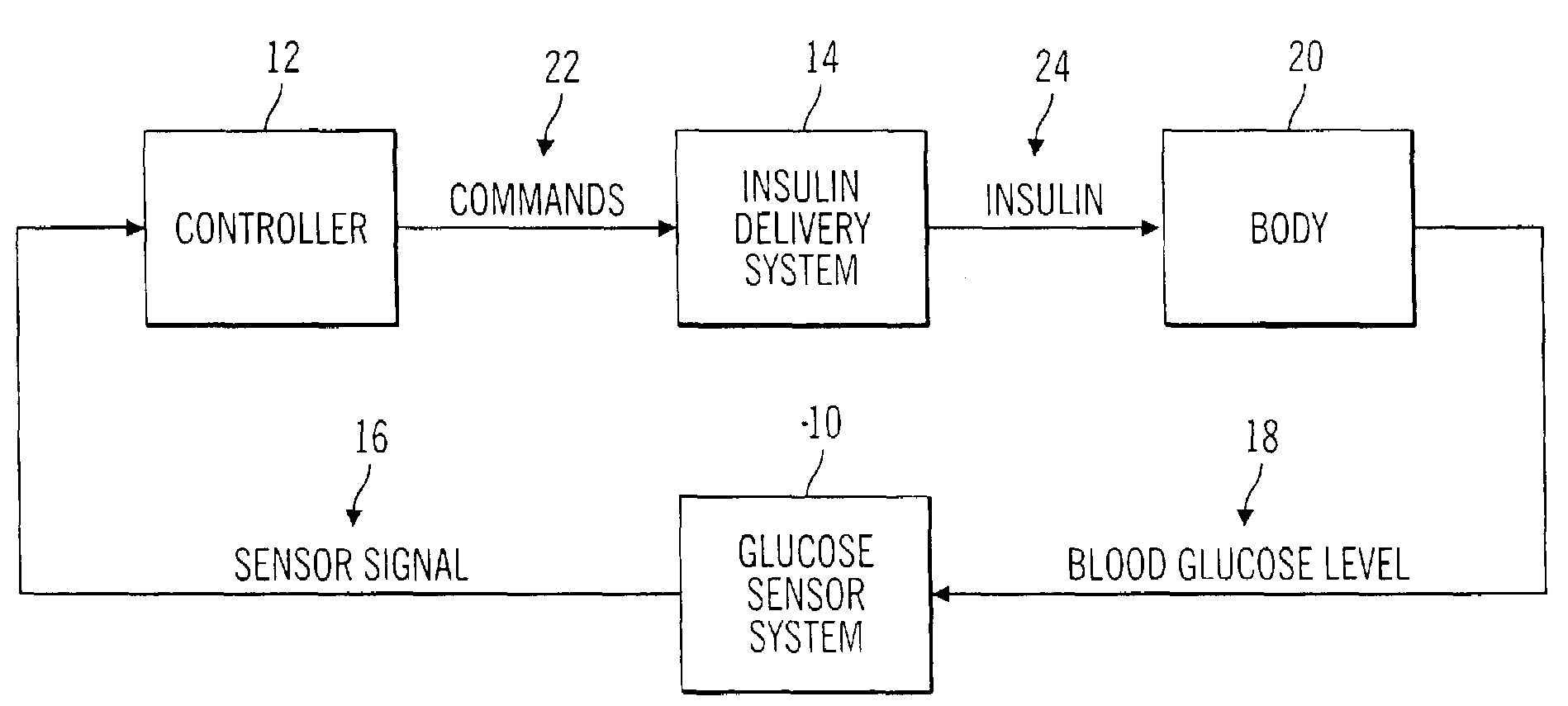
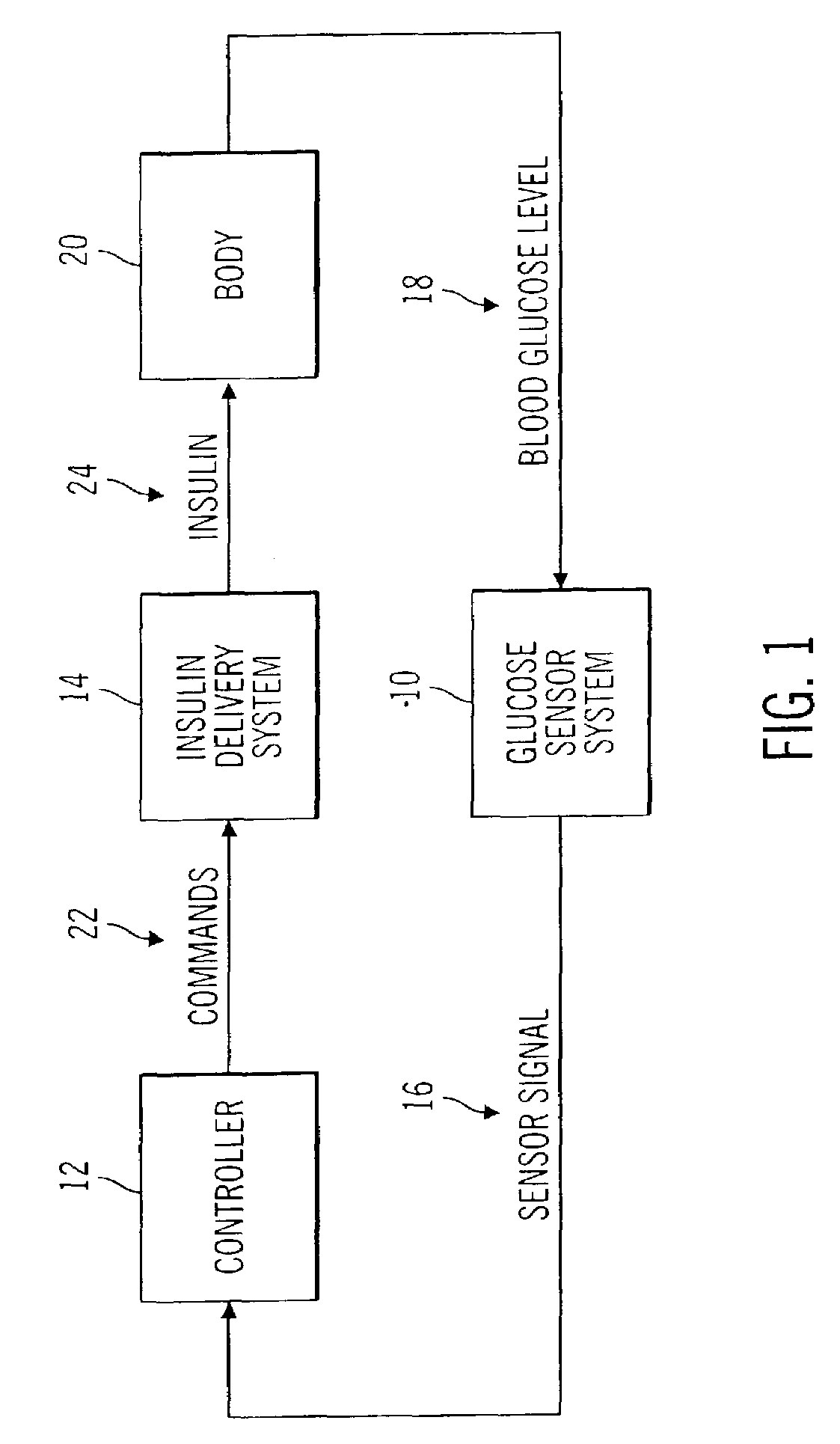
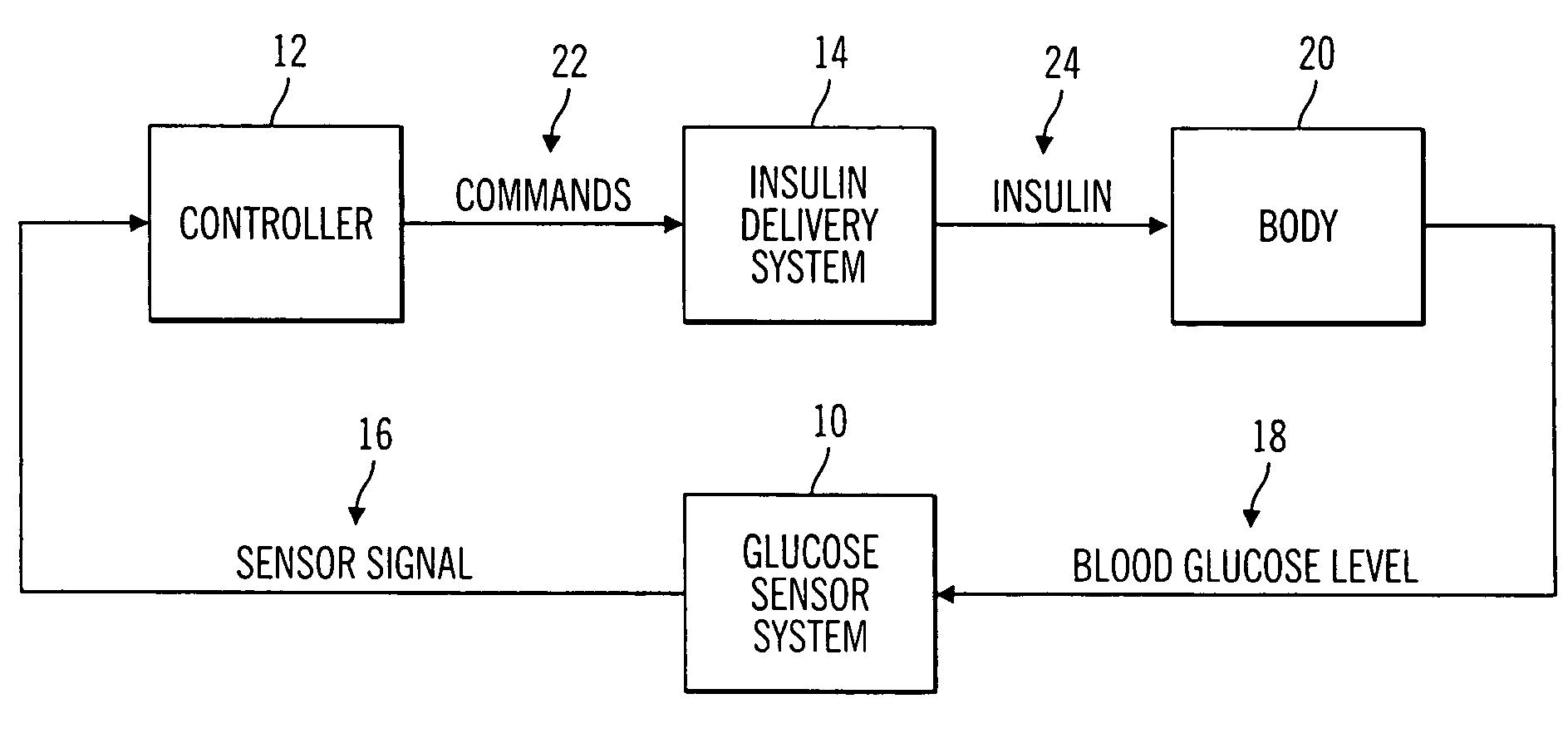
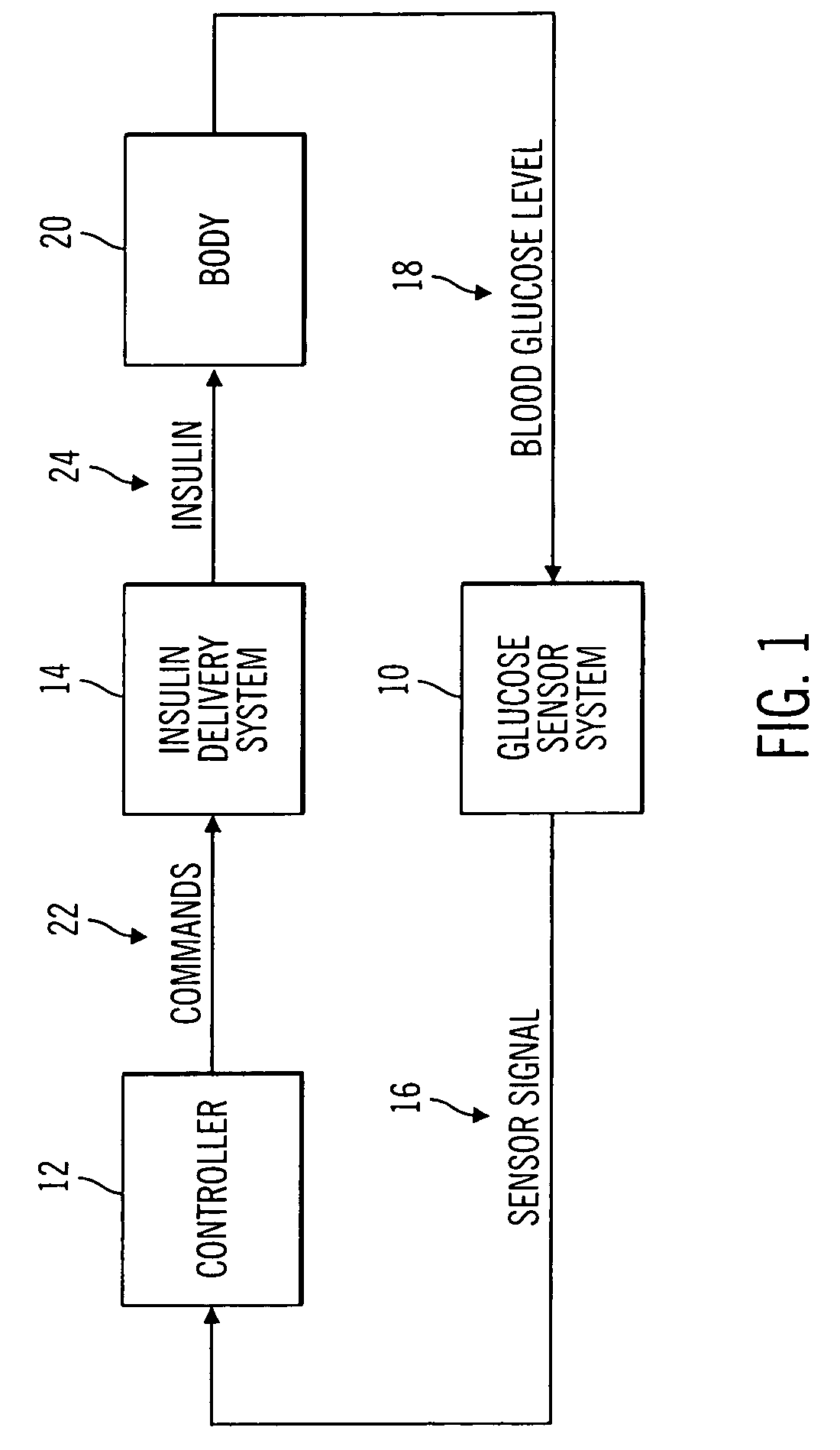
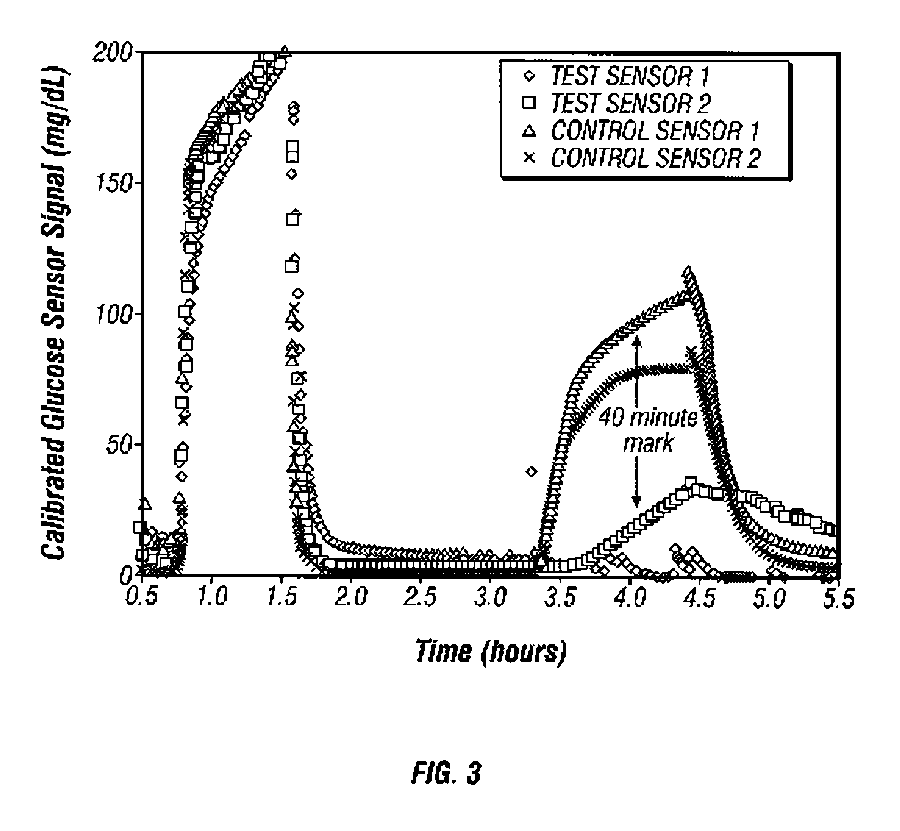
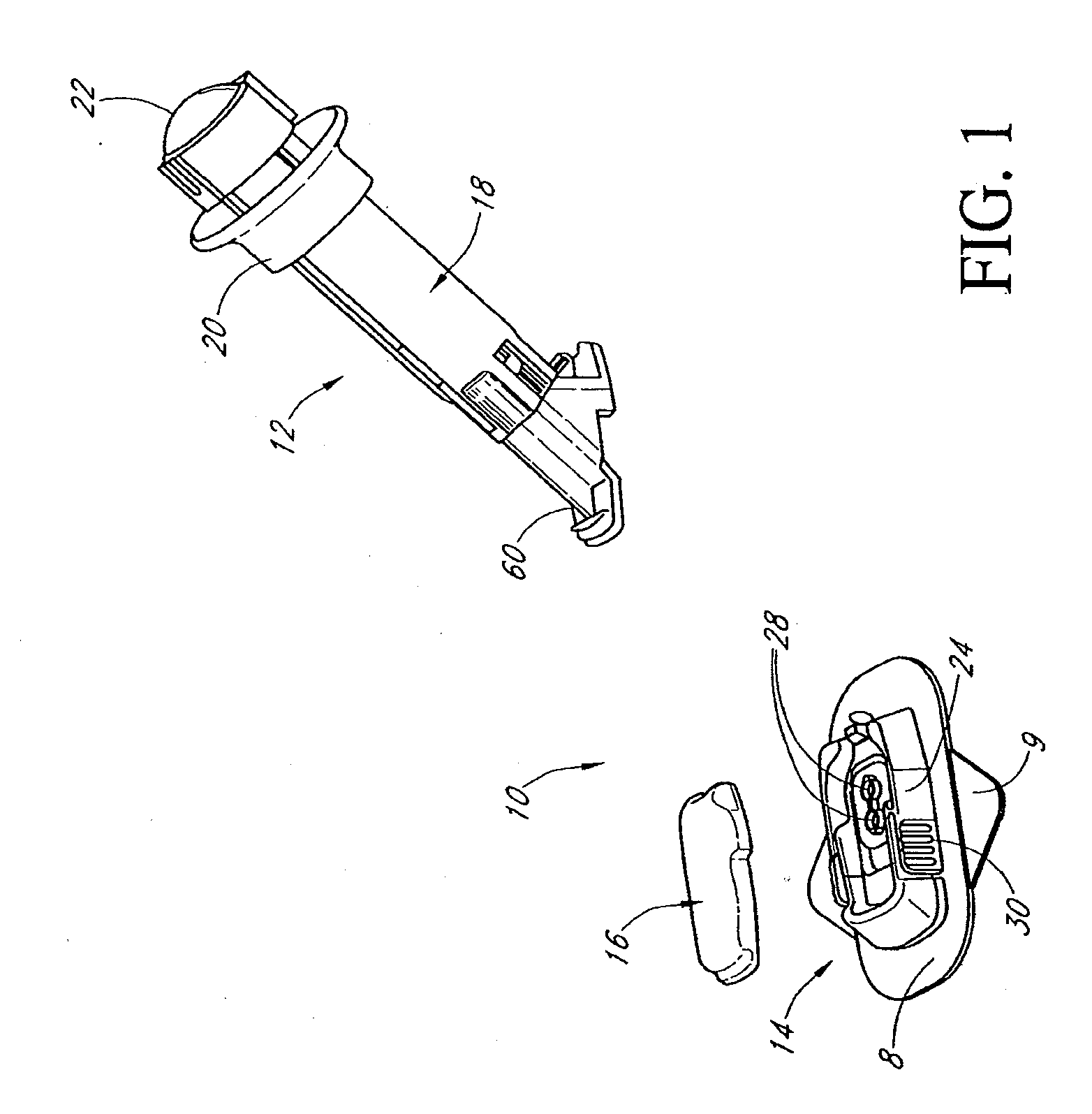
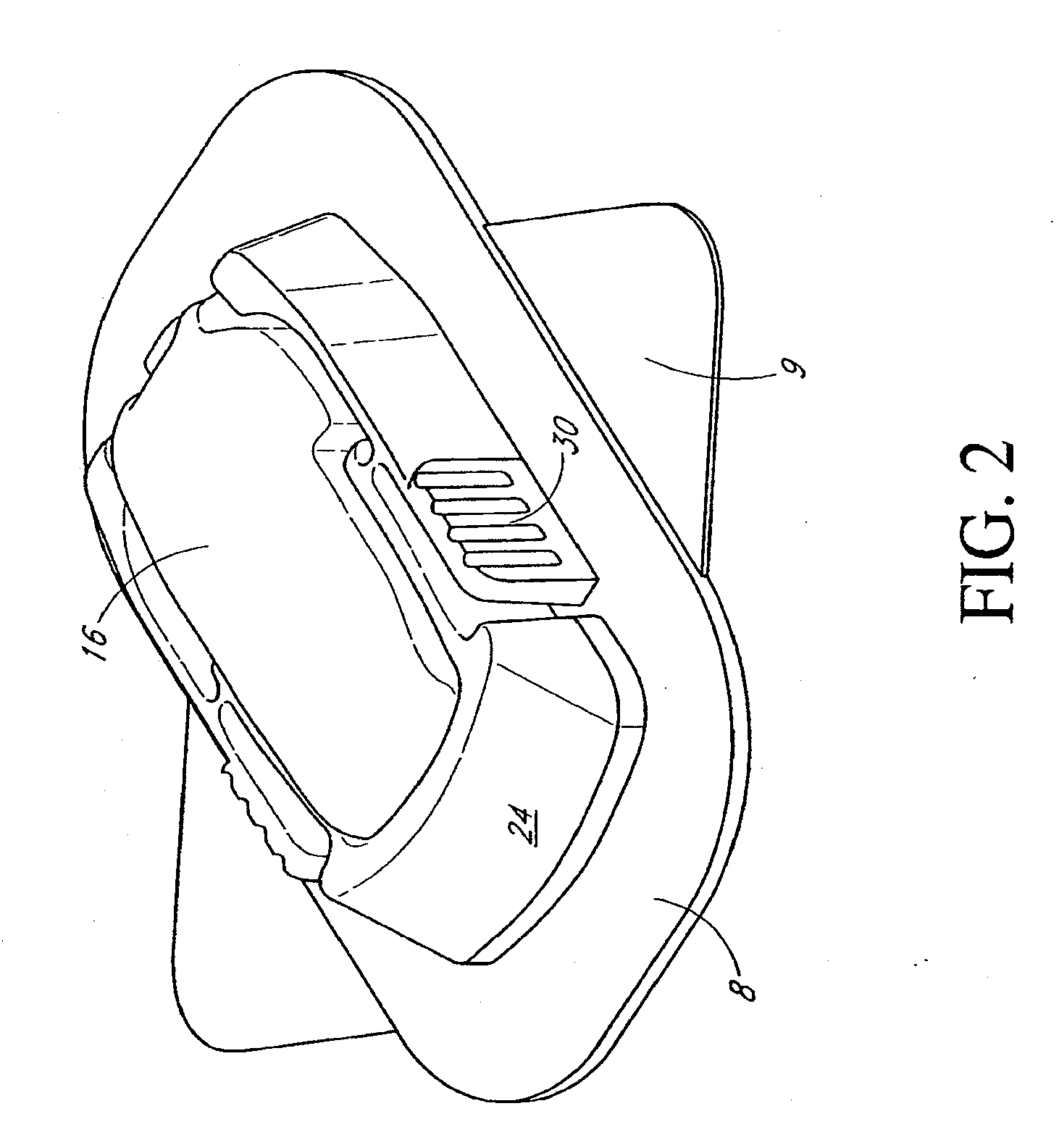
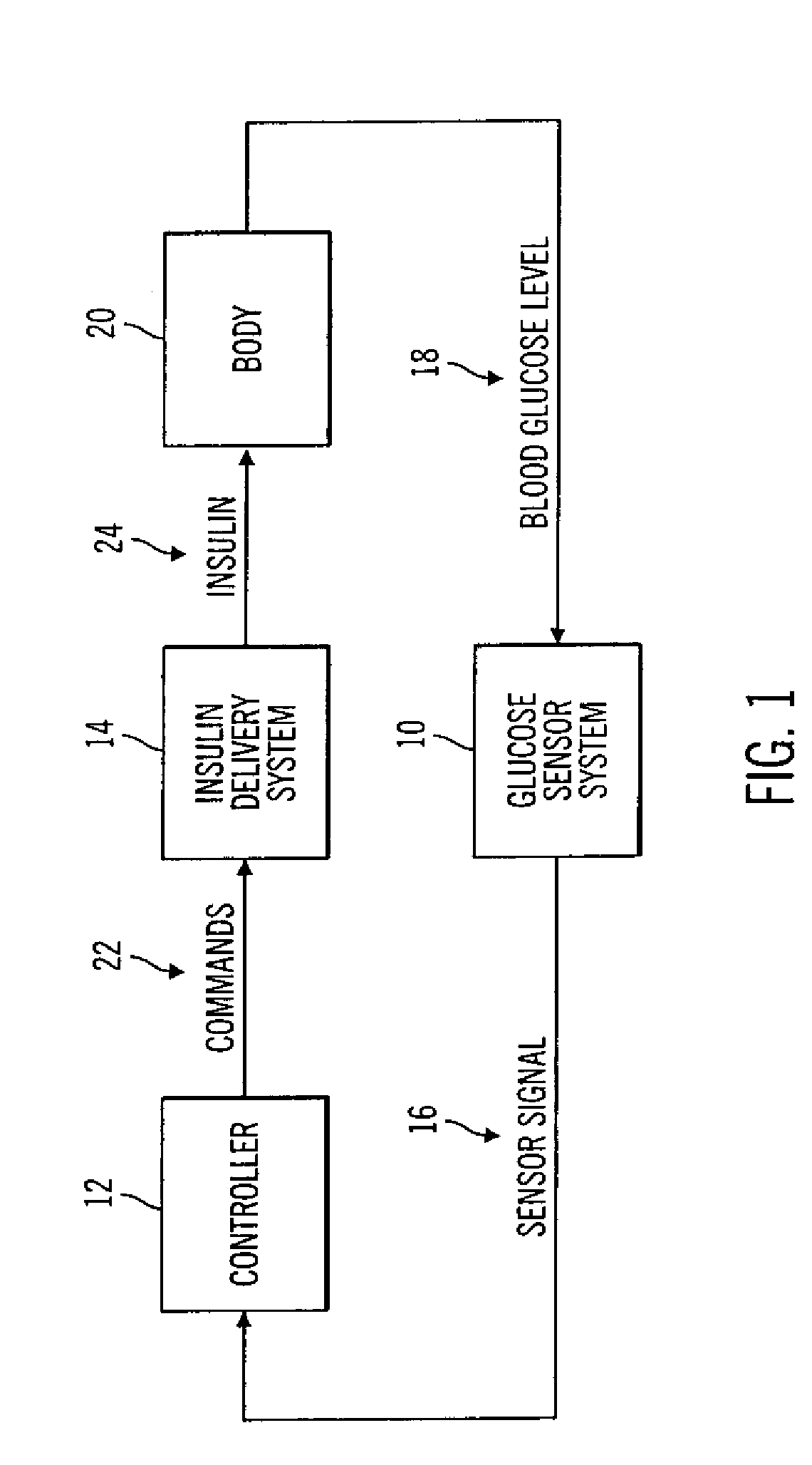
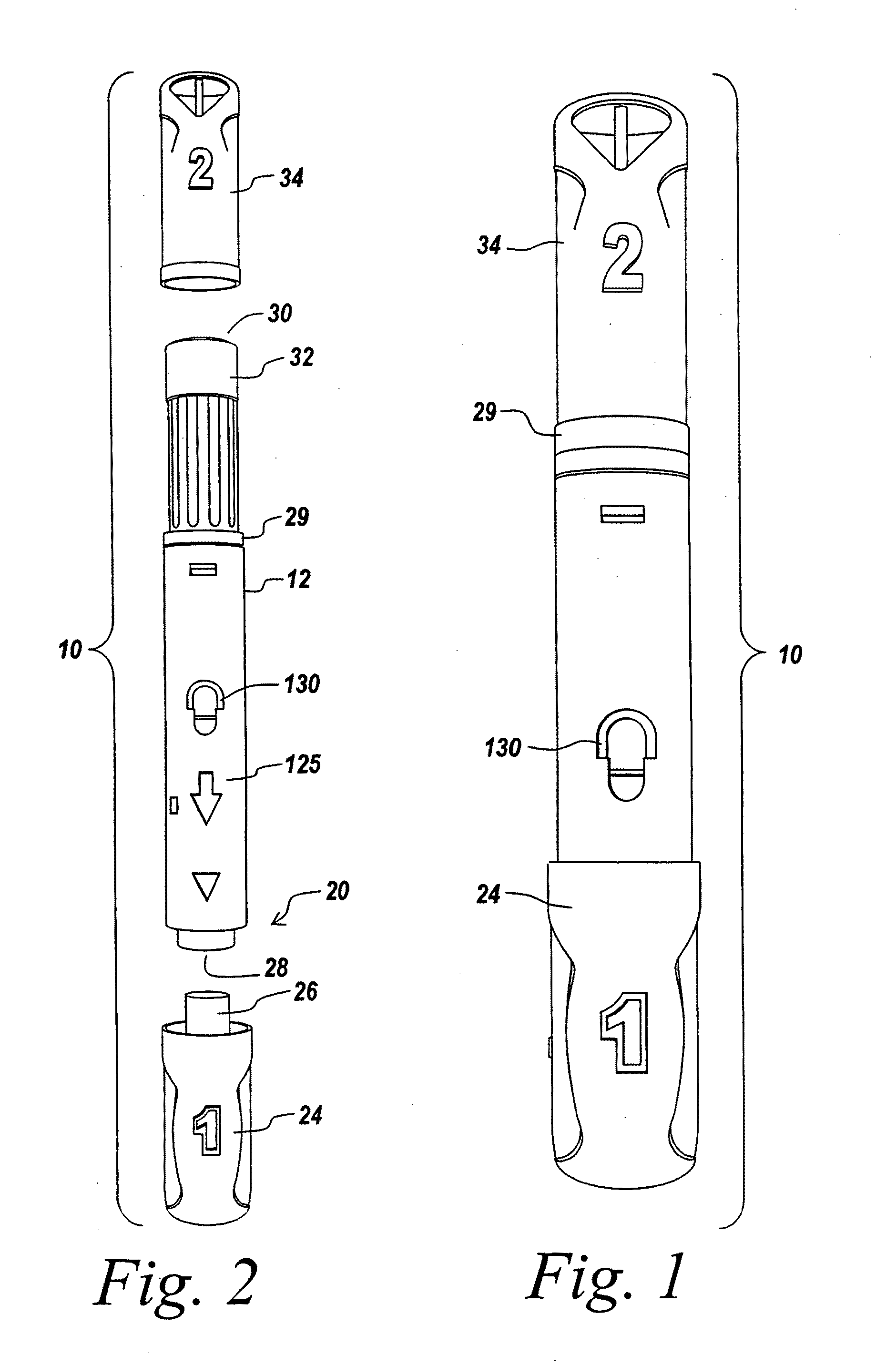
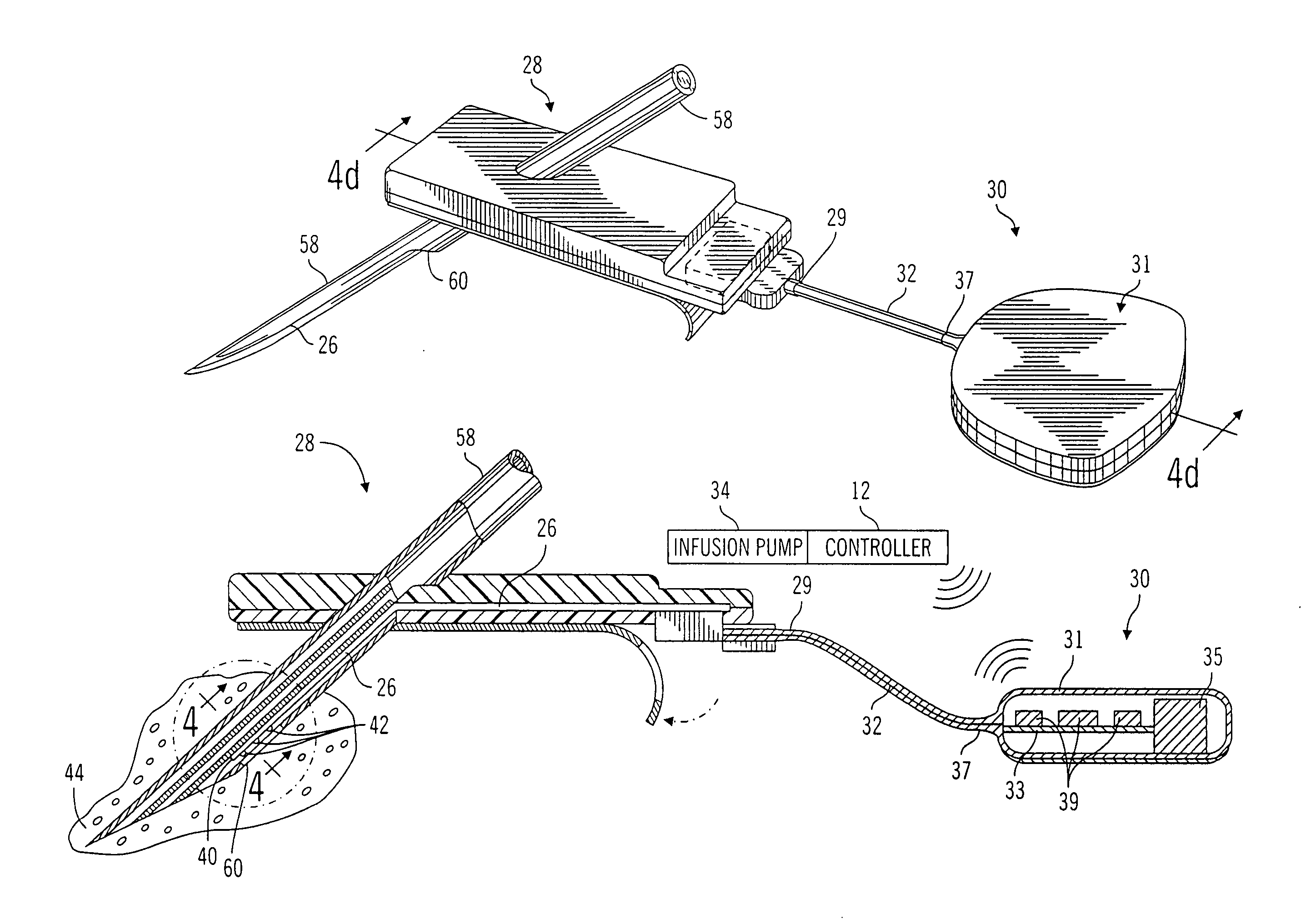
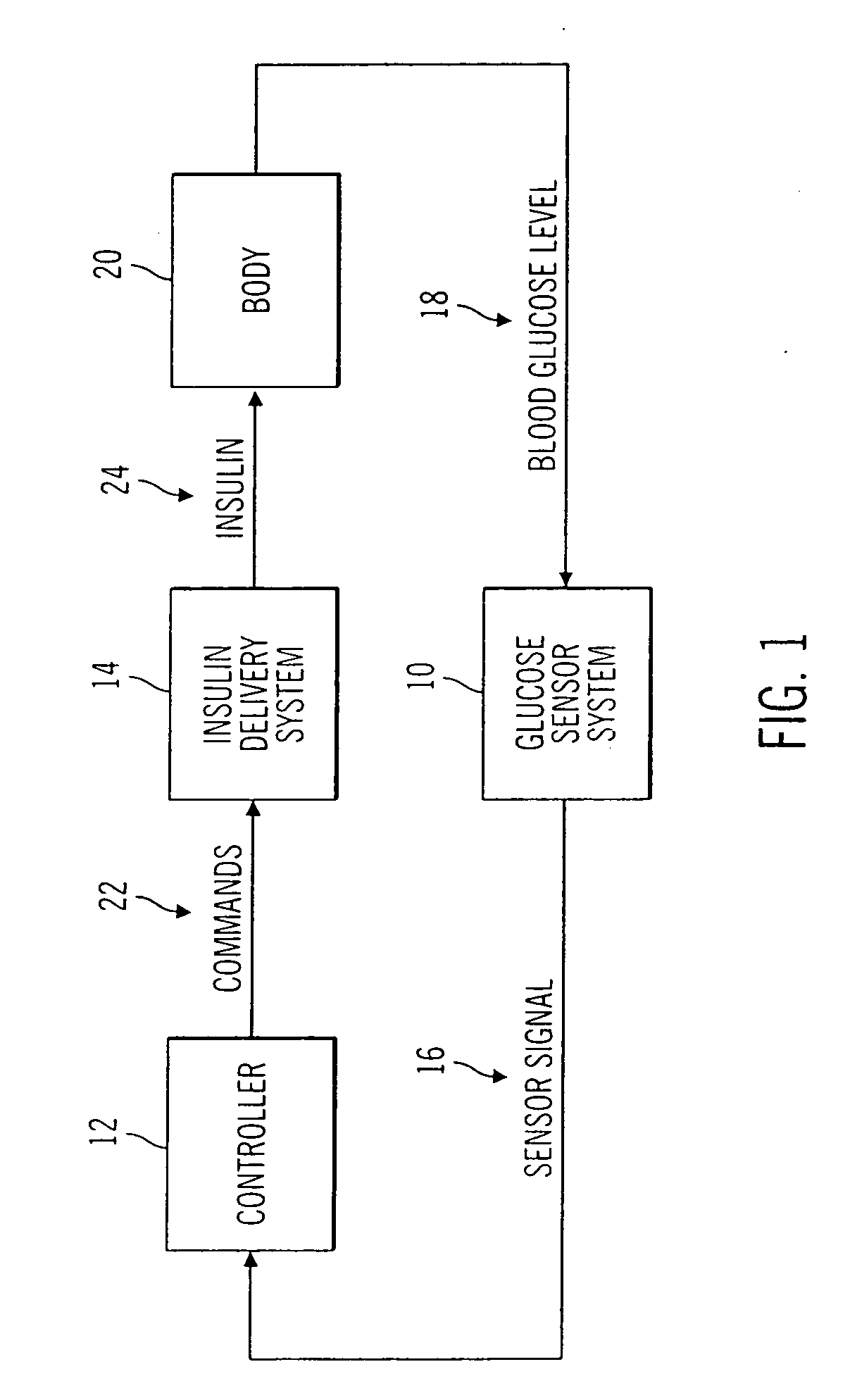
Closed loop system for controlling insulin infusion
InactiveUS7267665B2Other blood circulation devicesMedical devicesInsulin infusionConcentrations glucose
A closed loop infusion system controls the rate that fluid is infused into the body of a user. The closed loop infusion system includes a sensor system, a controller, and a delivery system. The sensor system includes a sensor for monitoring a condition of the user. The sensor produces a sensor signal, which is representative of the condition of the user. The sensor signal is used to generate a controller input. The controller uses the controller input to generate commands to operate the delivery system. The delivery system infuses a liquid into the user at a rate dictated by the commands from the controller. Preferably, the sensor system monitors the glucose concentration in the body of the user, and the liquid infused by the delivery system into the body of the user includes insulin.
Owner:MEDTRONIC MIMIMED INC
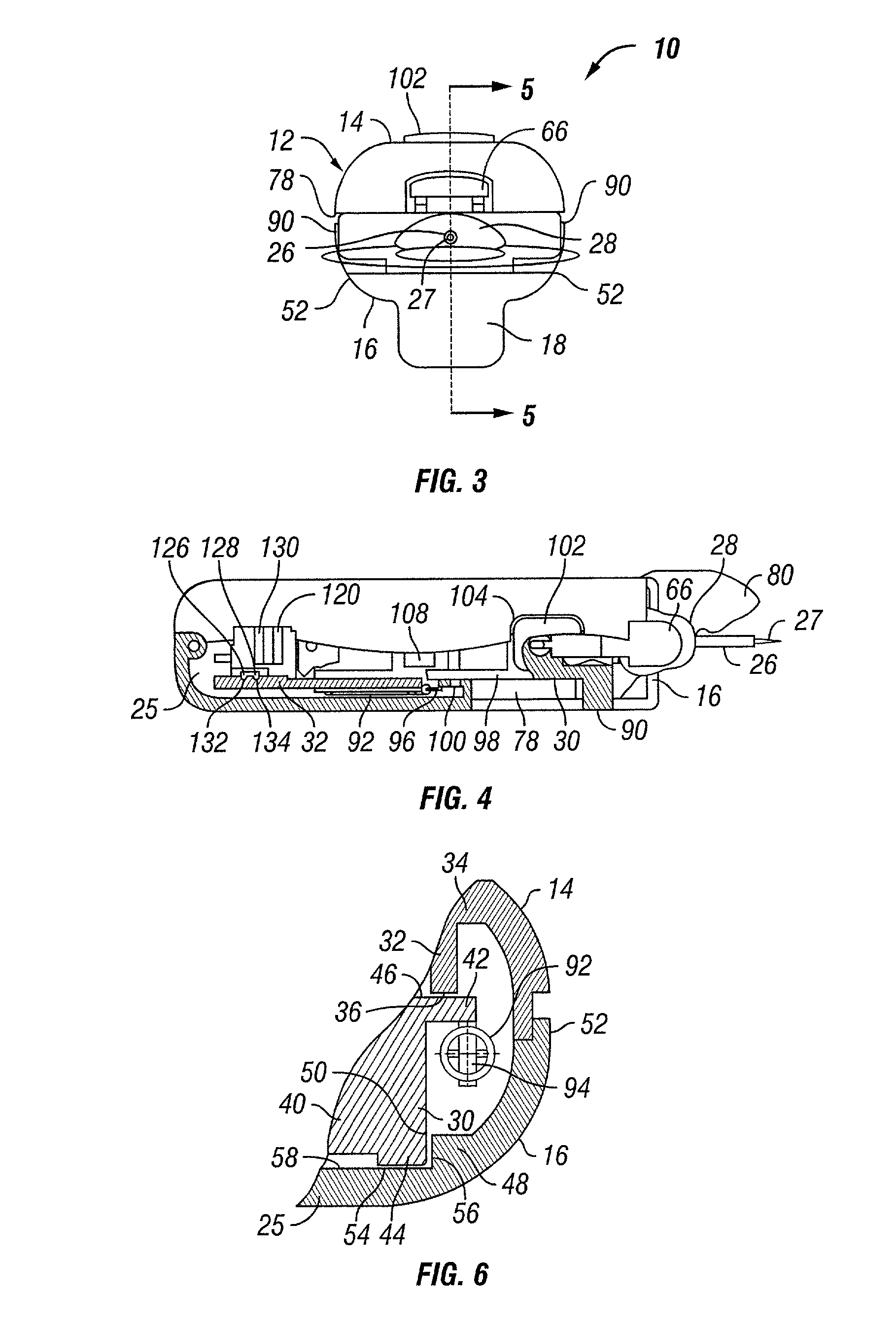
Analyte sensor method of making the same
A sensor and method of making the same for implantation in a body that includes a substrate with notches cut in the substrate to form a necked down region in the substrate; and at least one sensor electrode formed from one or more conductive layers. Preferably, the thickness of the substrate ranges from approximately 25μ to 350μ, but the thickness of the substrate can range from 5μ to 750μ. The sensor may be incorporated in to a sensor assembly includes a slotted needle having a slot. The notches creating the necked down region allow the substrate to slide into the slotted needle, which that has the slot narrow enough to permit passage of the necked down region. However, a non-necked down region of the substrate is prevented from pulling out of the slotted needle through the slot. The slot of the slotted needle may also permit the necked down region of the substrate to slide down the slot.
Owner:MEDTRONIC MIMIMED INC
Closed-loop method for controlling insulin infusion
A closed loop infusion system controls the rate that fluid is infused into the body of a user. The closed loop infusion system includes a sensor system, a controller, and a delivery system. The sensor system includes a sensor for monitoring a condition of the user. The sensor produces a sensor signal, which is representative of the condition of the user. The sensor signal is used to generate a controller input. The controller uses the controller input to generate commands to operate the delivery system. The delivery system infuses a liquid into the user at a rate dictated by the commands from the controller. Preferably, the sensor system monitors the glucose concentration in the body of the user, and the liquid infused by the delivery system into the body of the user includes insulin.
Owner:MEDTRONIC MIMIMED INC
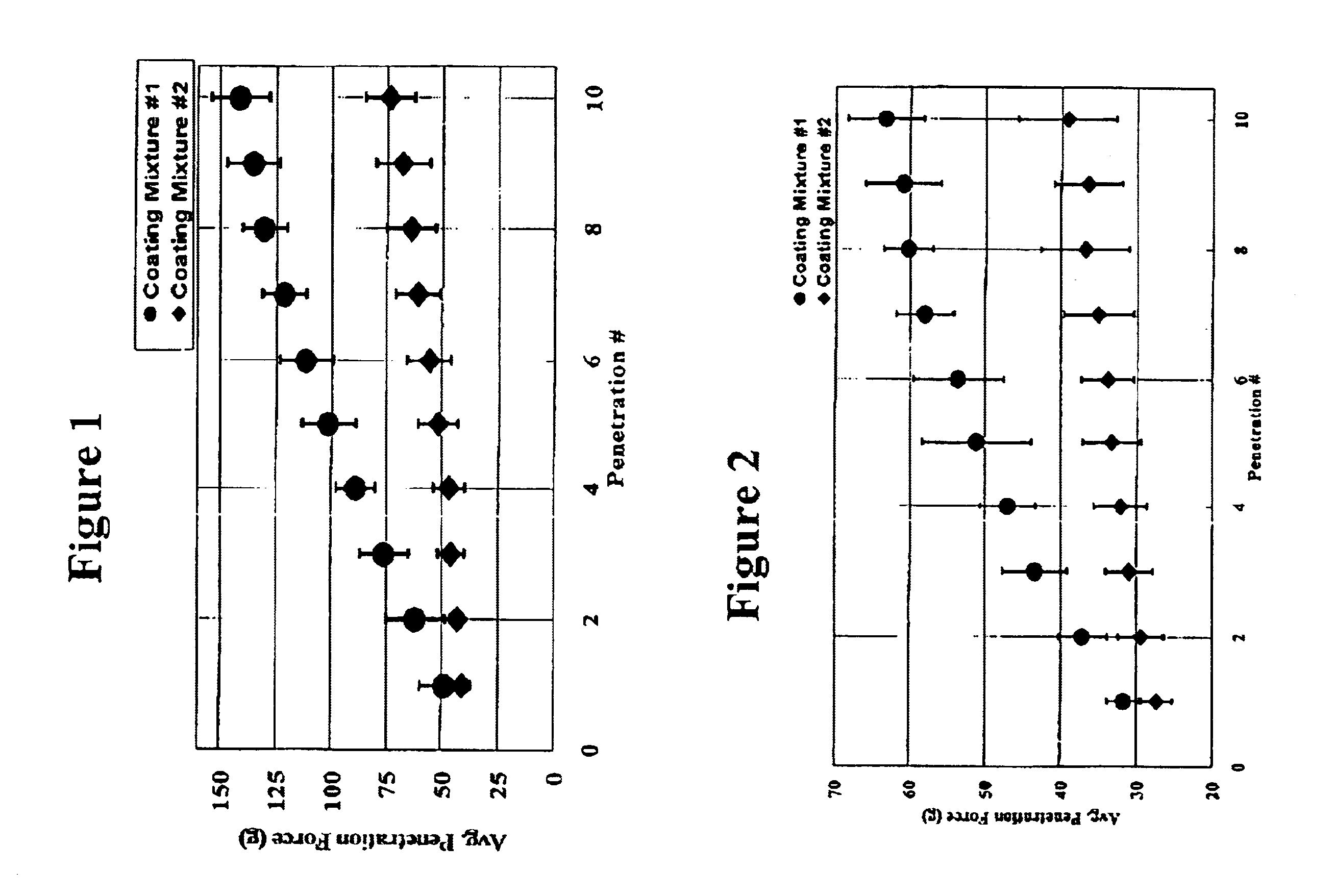
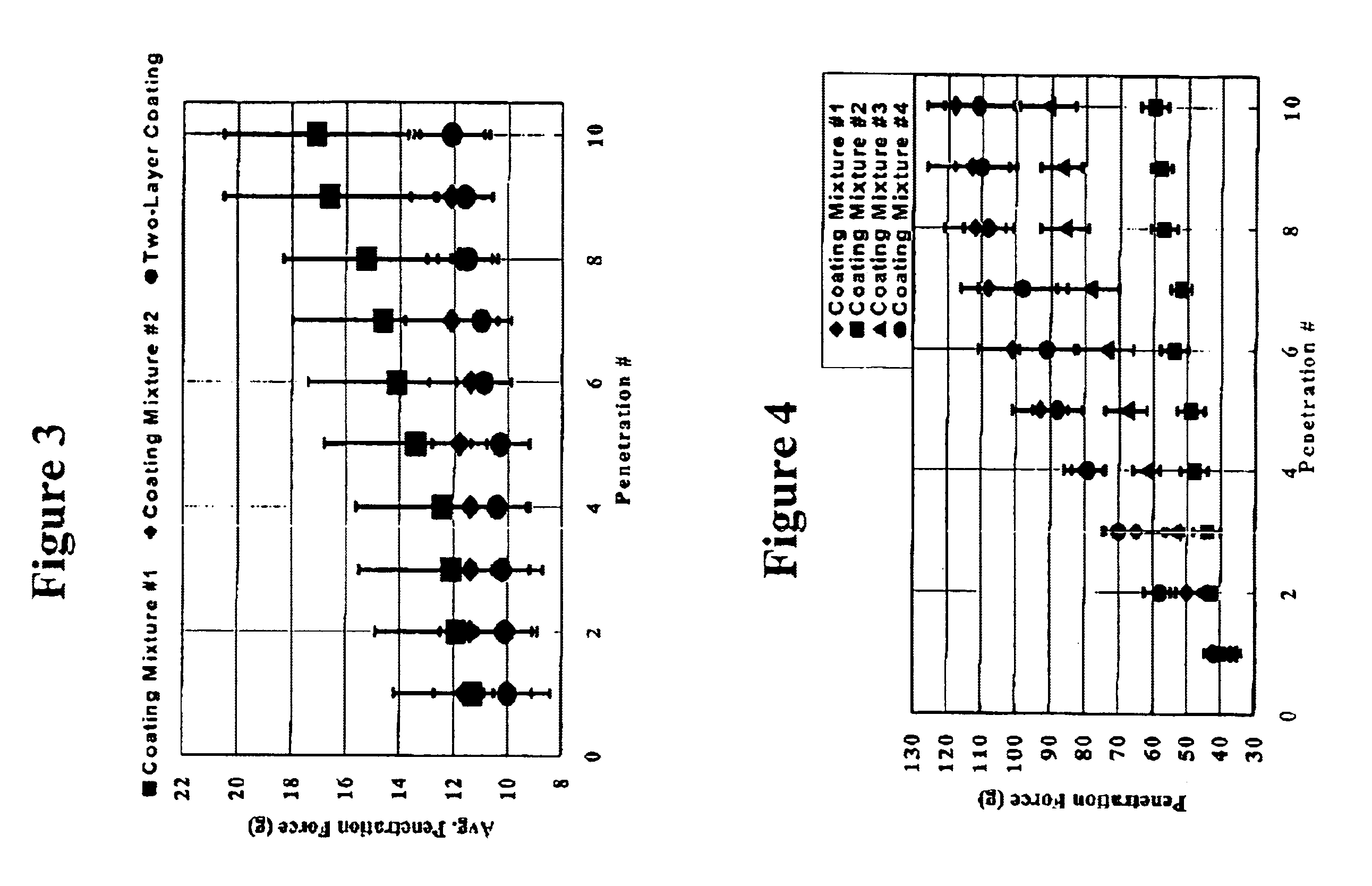
Medical devices having durable and lubricious polymeric coating
A medical device having a contact surface exposed repeatedly to bodily tissue is disclosed. The contact surface is coated with a silicone polymer and one or more non-silicone hydrophobic polymers. The preferred medical device is a surgical needle, and the preferred coating is a polydimethylsiloxane and polypropylene wax hydrocarbon mixture. The incorporation of the non-silicone hydrophobic polymer increases the durability of the coating on the device without sacrificing lubricity.
Owner:ETHICON INC
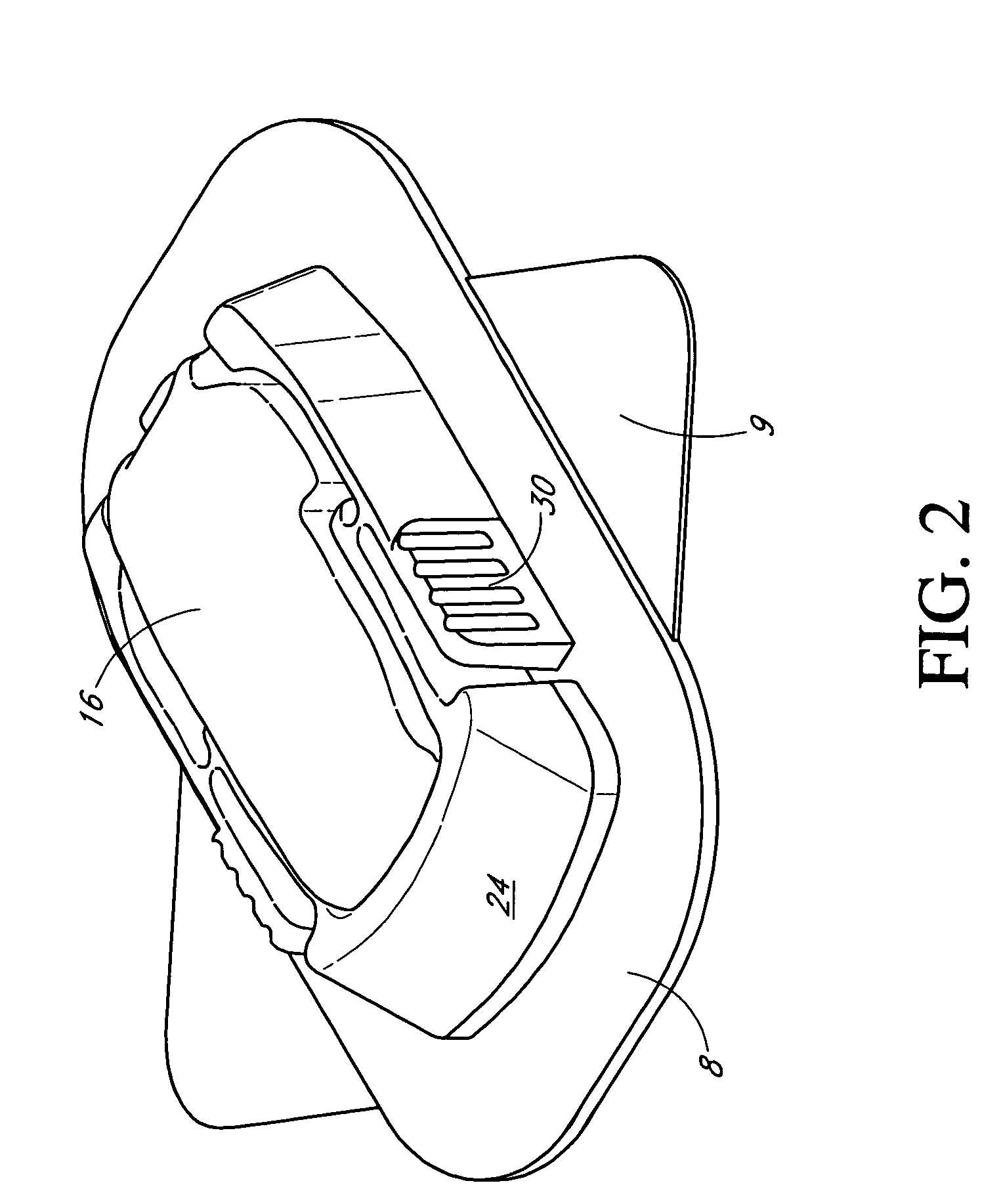
Subcutaneous self attaching injection port with integral moveable retention members
InactiveUS7862546B2Improve overall utilizationMedical devicesNon-surgical orthopedic devicesInjection portEngineering
A self attaching injection port has integral moveable fasteners which are moveable from a undeployed state to a deployed state engaging tissue. The fasteners may be disposed radially or tangentially, and rotated to pierce the fascia. The fasteners may be rigid or elastically deformable.
Owner:ETHICON ENDO SURGERY INC
Afinity domain for analyte sensor
InactiveUS20050176136A1Reduce impactBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteSorbent
Abstract of the DisclosureThe preferred embodiments provide a membrane system, particularly for use on an electrochemical sensor, wherein the membrane system includes an affinity domain that dampens the effects of target interferant(s) on the sensor. The affinity domain can be layer, surface, region, and / or portion of the membrane system formed using sorbents that have an affinity for the target interferant. The sorbents can be adapted to adsorb the interferants, for example using adsorbents such as chromatography packing materials. The sorbents can also be adapted to absorb the interferants by imprinting a molecular structure on the material that forms the affinity domain such that target interferants bind to the imprinted surfaces at the molecular level.
Owner:DEXCOM
Method and device for tissue removal and for delivery of a therapeutic agent or bulking agent
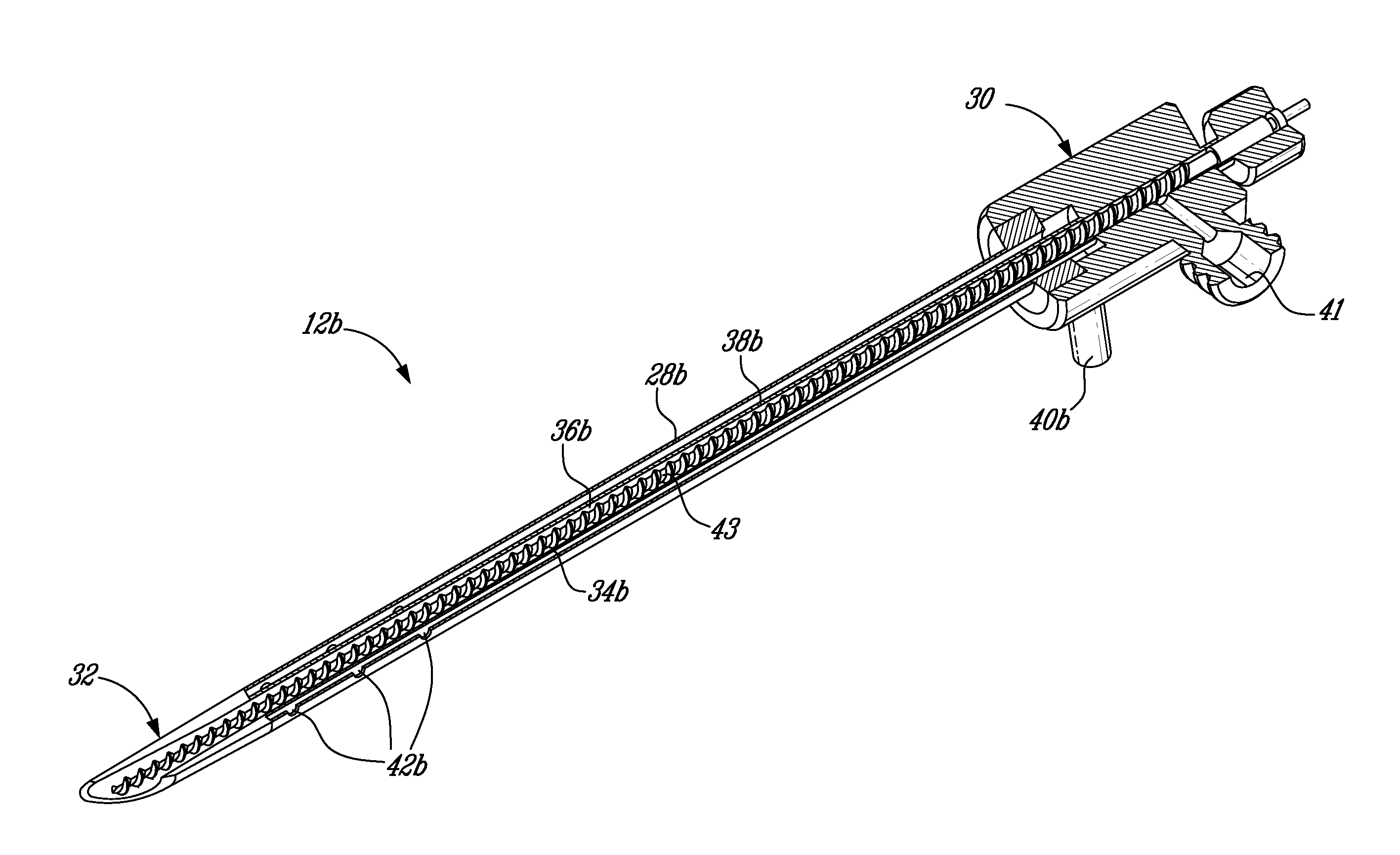
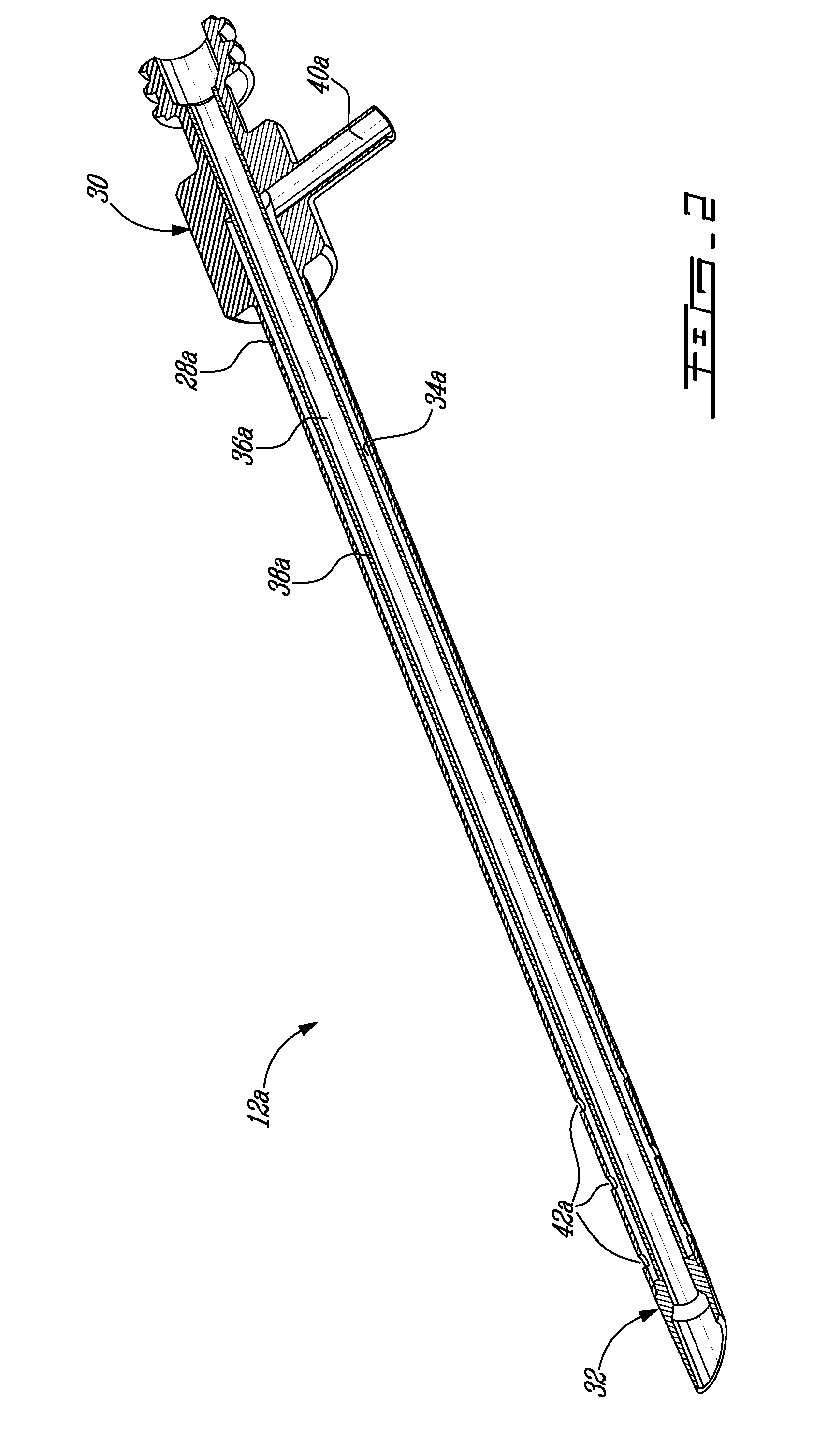
InactiveUS7806871B2Efficient removalMinimize damageInfusion syringesMedical devicesSurgeryMedical device
According to an aspect of the present invention, a medical device is provided, which comprises the following: (a) a hollow elongate body (e.g., a elongate cylinder, such as a needle) having distal and proximal ends; and (b) a rotatable member comprising a tissue morselizer and an elongate shaft (e.g., an auger-like tissue-drilling bit). The device (i) advances material (e.g., morselated tissue) toward the proximal end of the hollow elongate body when the shaft is rotated in a first direction, and (ii) advances material (e.g., a therapeutic agent and / or a bulking agent) toward the distal end of the hollow elongate body when the shaft is rotated in a second direction that is opposite the first direction. The invention also provides a method of treatment for morselizing and removing tissue from within the patient and creating a void within the patient and introducing a therapeutic agent and / or a bulking agent into the void.
Owner:BOSTON SCI SCIMED INC
Coupling for injection devices
InactiveUS8574199B2Reduce the slack occurringAmpoule syringesMedical devicesCouplingInjection device
Owner:NOVO NORDISK AS
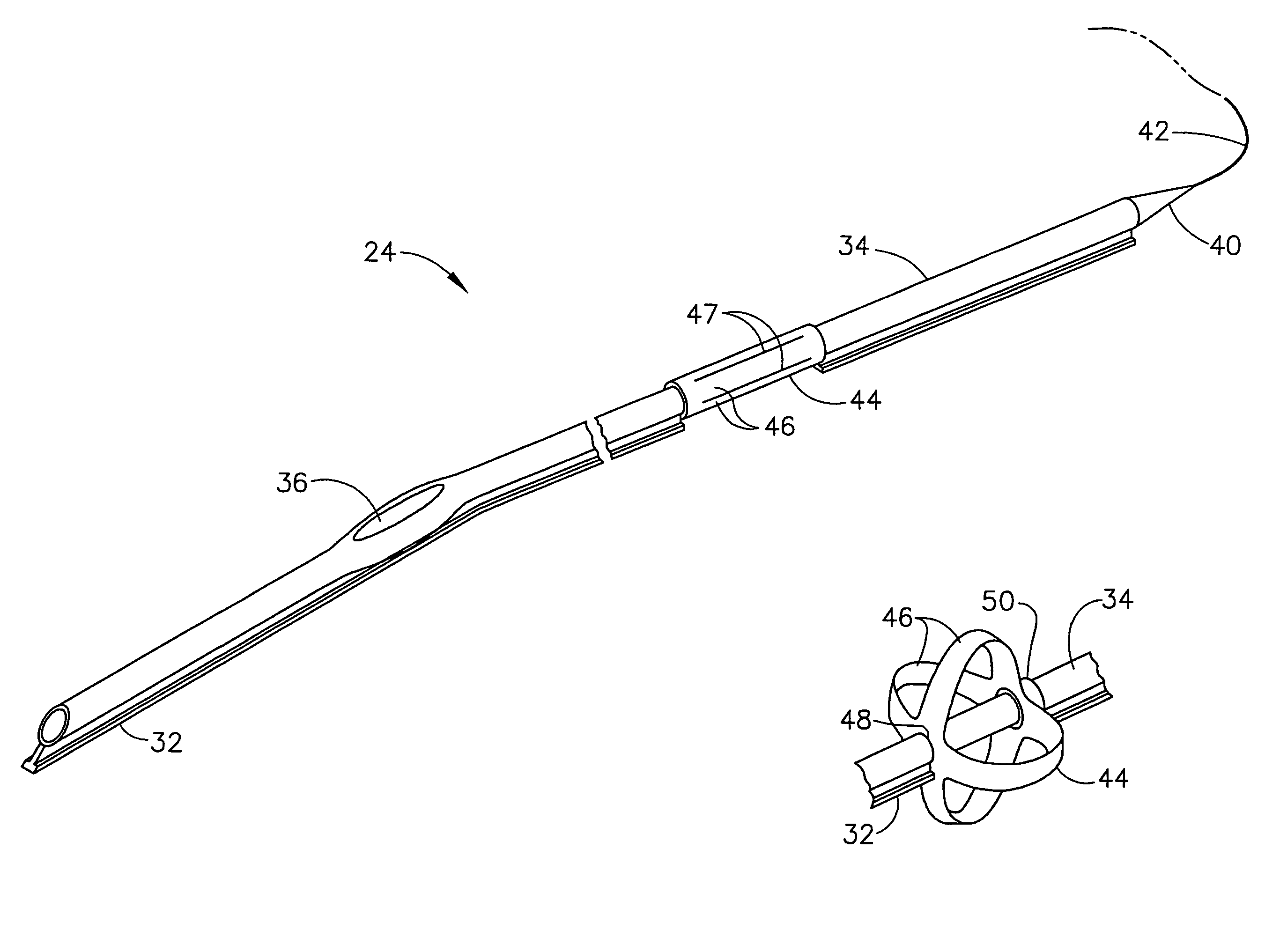
Laparoscopic port assembly
Various embodiments of a laparoscopic trocar assembly are disclosed. The port assemblies include inserted parts that protect the patient's tissues at the point of deployment. The port assemblies include seals for maintaining pneumoperitoneum both when instrument are being used and when instruments are not inserted.
Owner:COVIDIEN LP
Medical devices with enhanced ultrasonic visibilty
InactiveUS20070197954A1Ultrasonic/sonic/infrasonic diagnosticsElectrotherapyTip positionSolid tissue
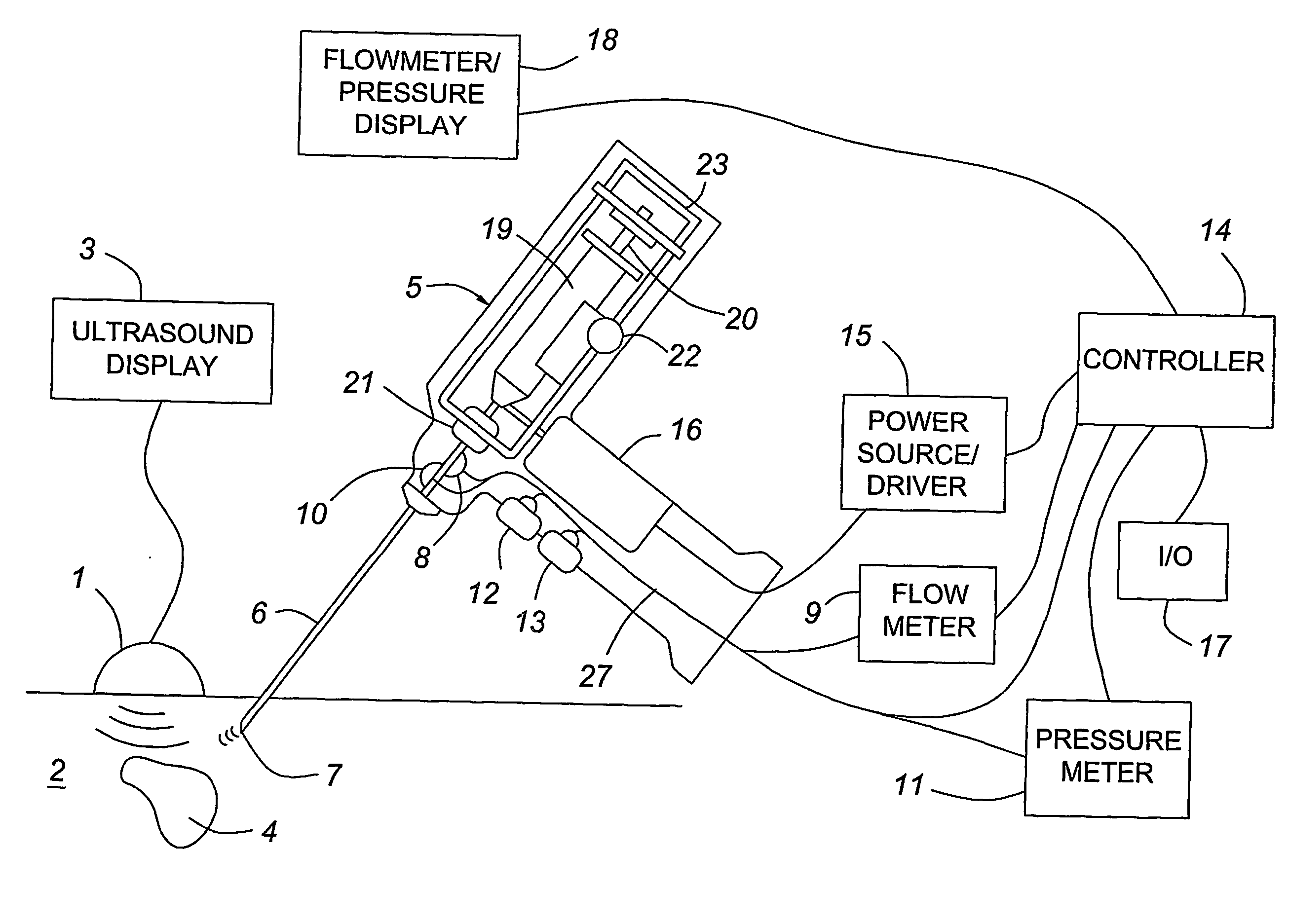
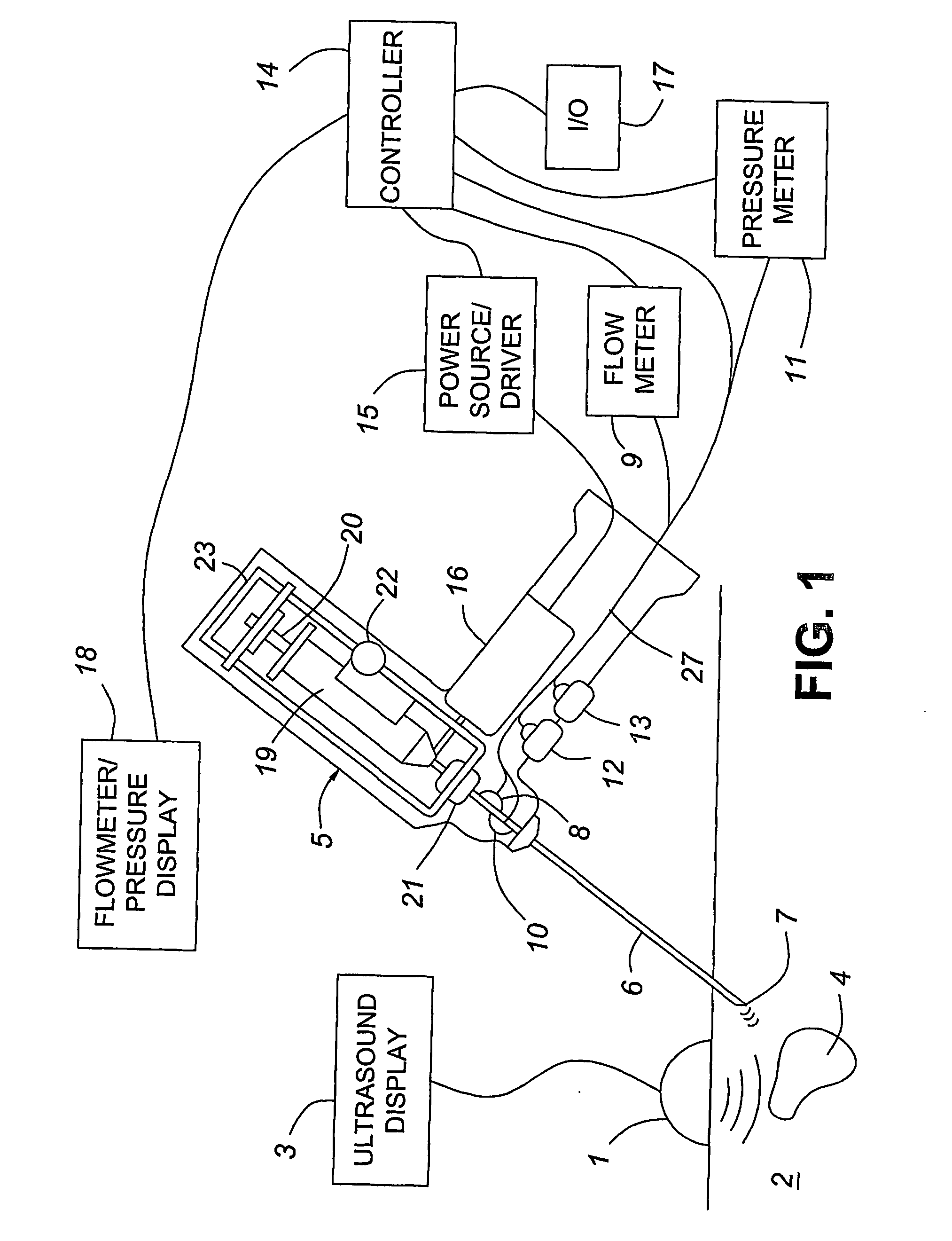
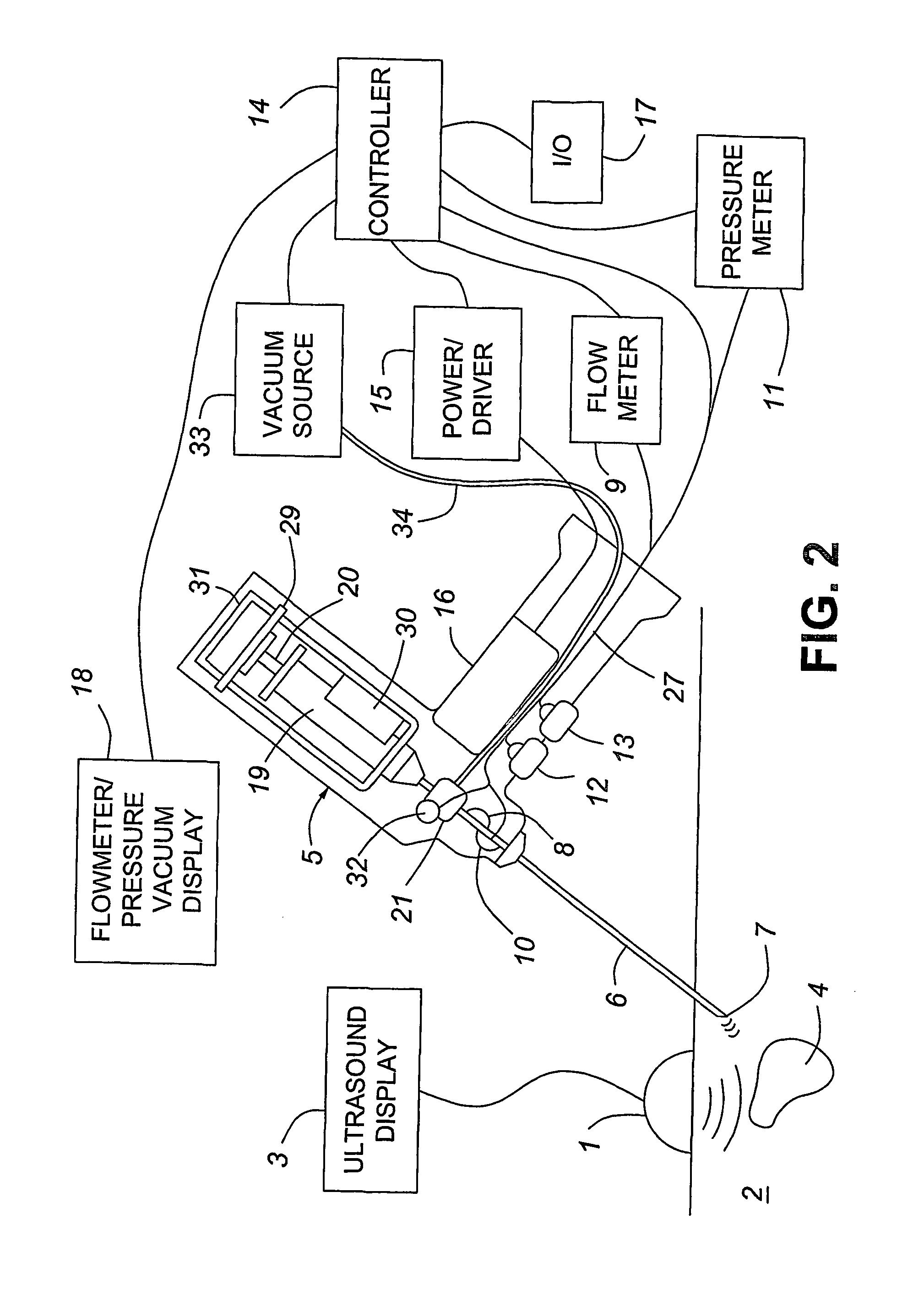
A medical device having enhanced ultrasonic visibility is provided. The device permits localized drug delivery, probe positioning, fluid drainage, biopsy, or ultrasound pulse delivery, through the real-time ultrasound monitoring of the needle tip position within a patient. The device permits controlled dispersion of a drug into solid tissue, the lodging of particles into solid tissue, and drug delivery into specific blood vessels. As a needle is inserted, a fluid that contrasts echogenically with the organ environment is injected into the patient. The fluid travels a brief distance before being slowed and stopped by the patient's tissue and this fluid flow will be detectable by ultrasound. The needle position during insertion will be monitored using ultrasound until it is at the desired point of action. A therapeutic drug is then delivered or a probe inserted
Owner:ARTENGA
Fabrication of biocompatible polymeric composites
InactiveUS6147135ASure easyImprove performanceSuture equipmentsCosmetic preparationsFiberPolymer science
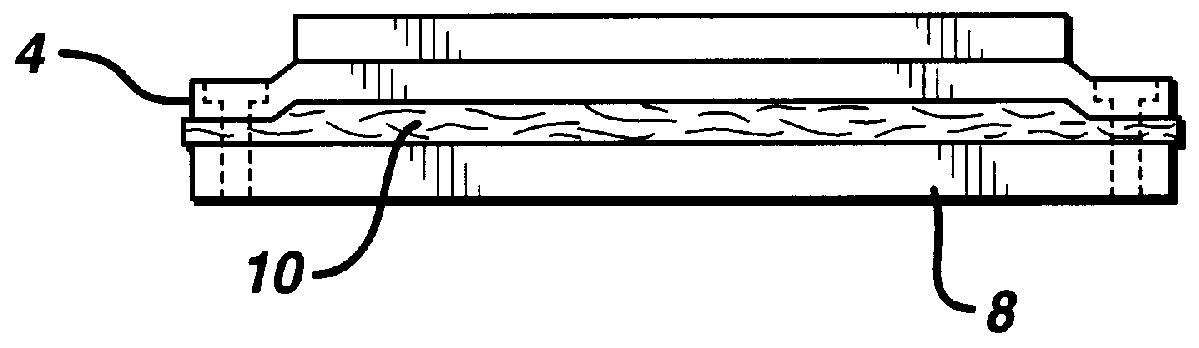
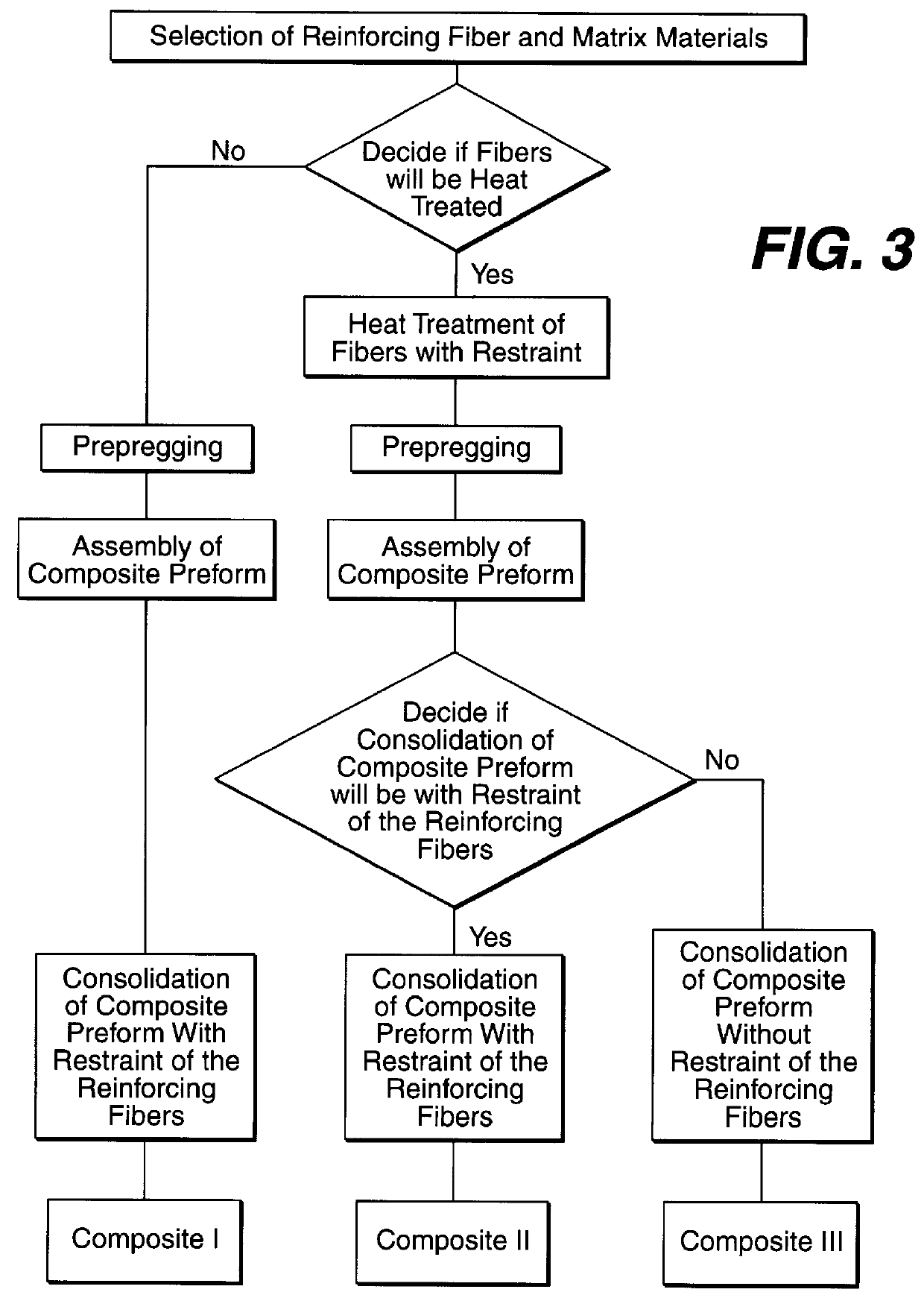
Composite materials formed from biocompatible polymer fibers and biodegradable polymers are disclosed. The heat treatment conditions for the reinforcing fibers are described so that the mechanical properties of the fibers can be retained during composite consolidation process. The processing conditions and set-ups to consolidations are constrained to the temperatures lower than fiber heat treatment temperatures. The reinforcing fibers are restrained under tension so that the minimum relaxation occurs during consolidation process.
Owner:ETHICON INC
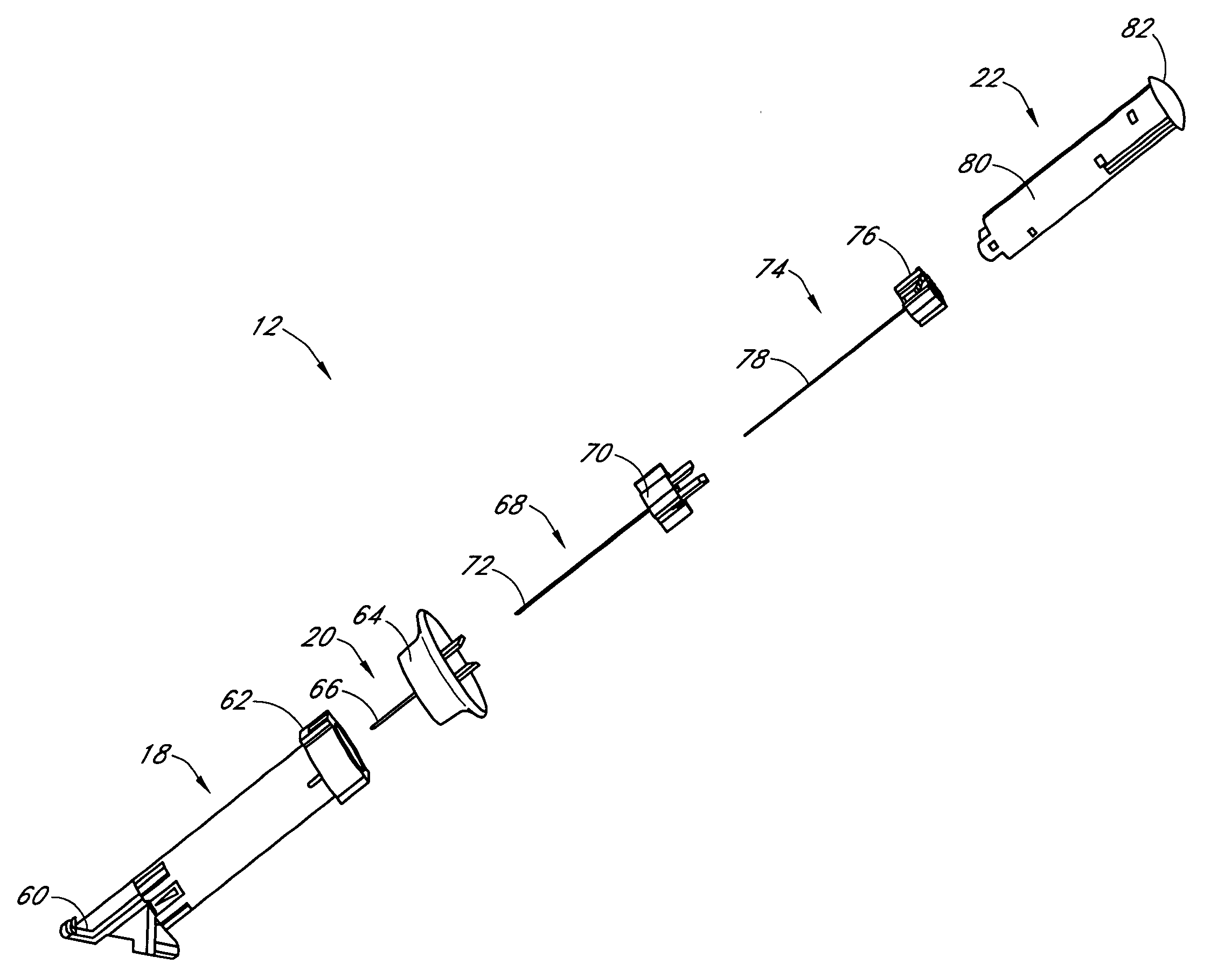
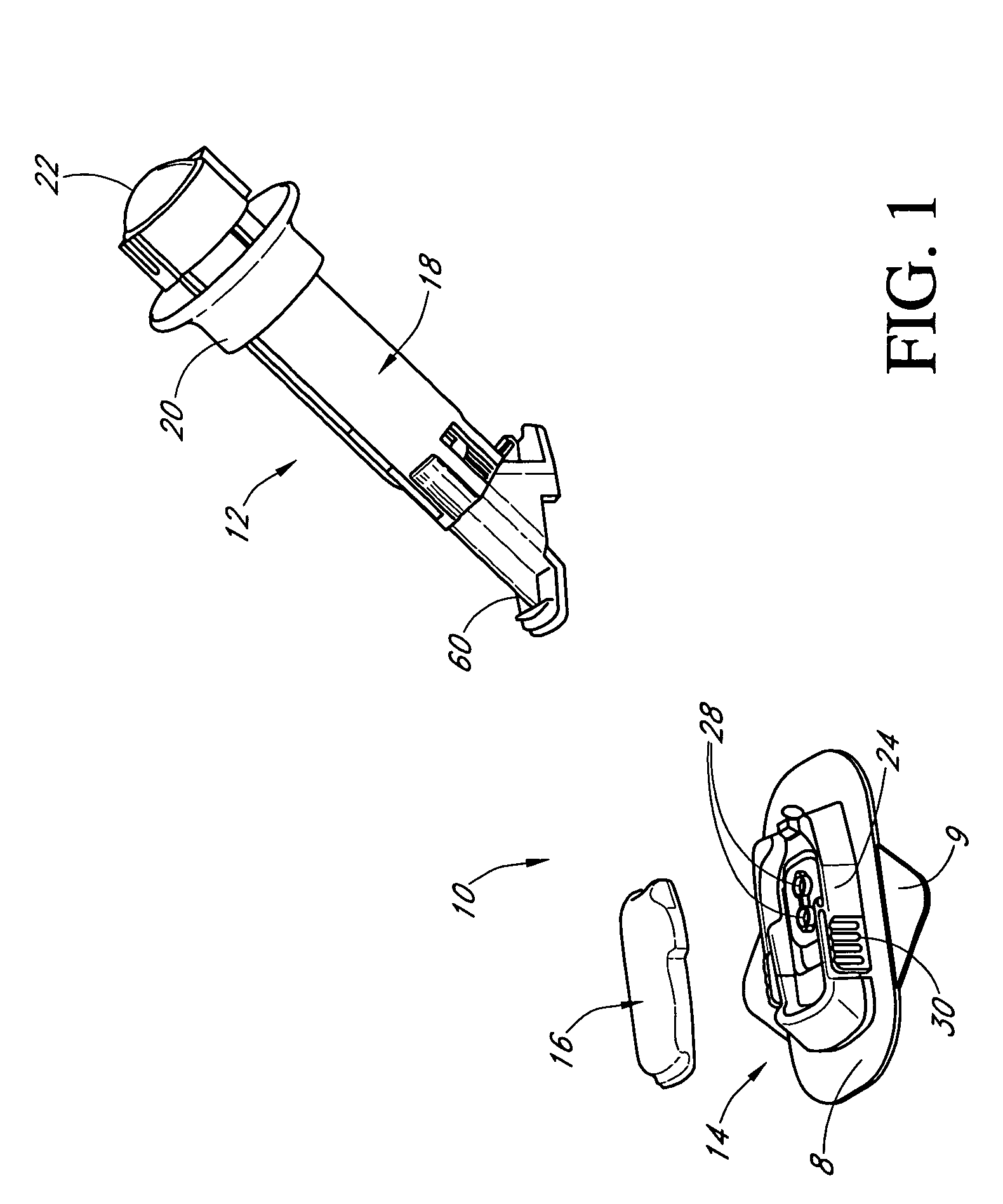
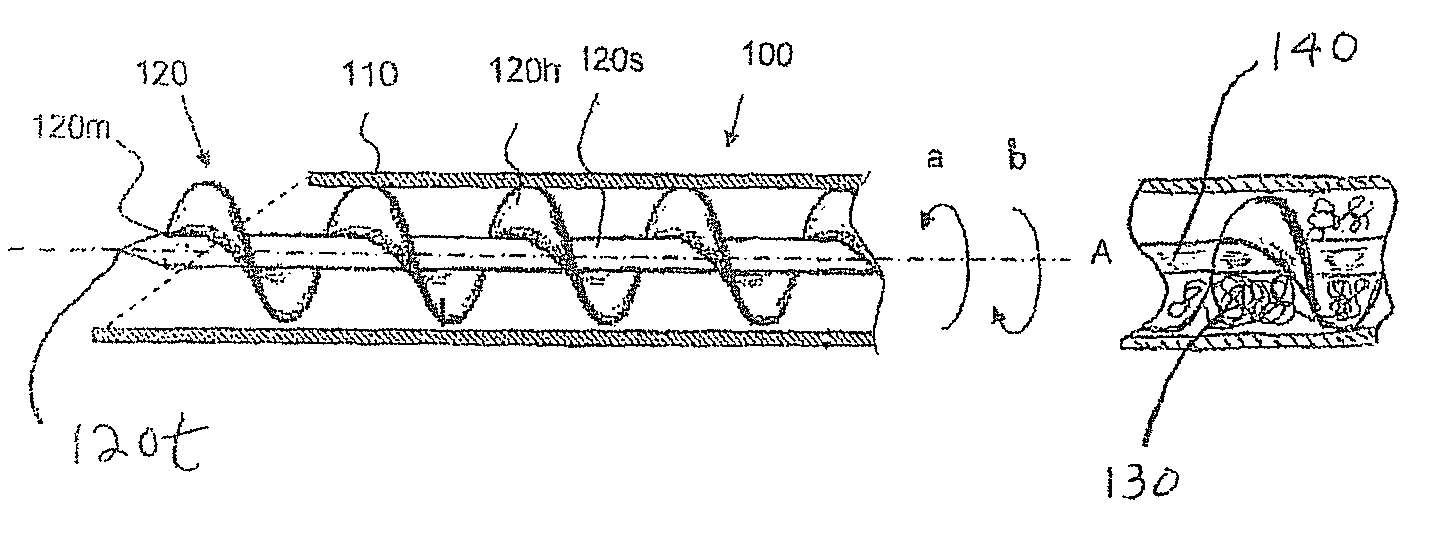
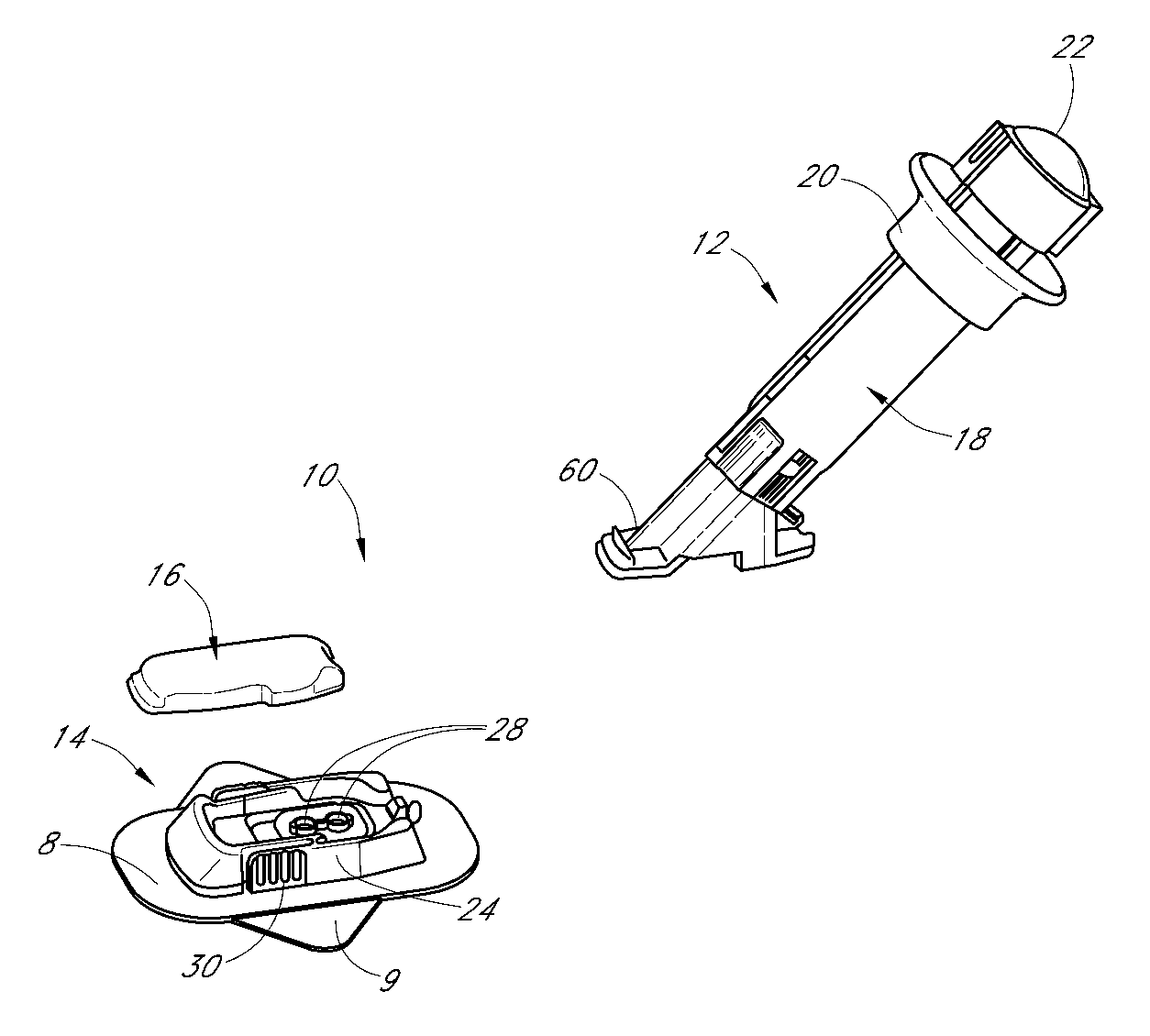
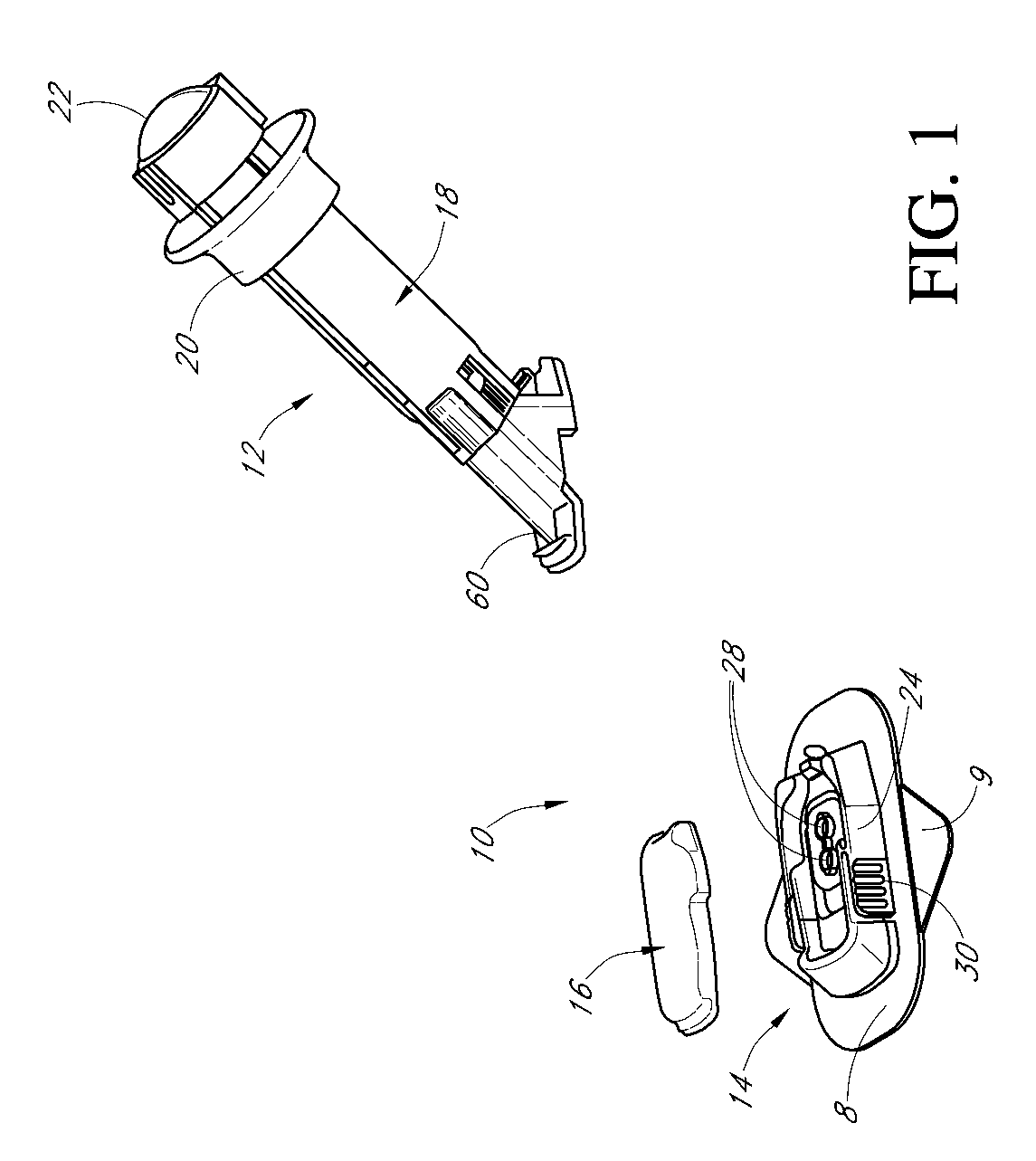
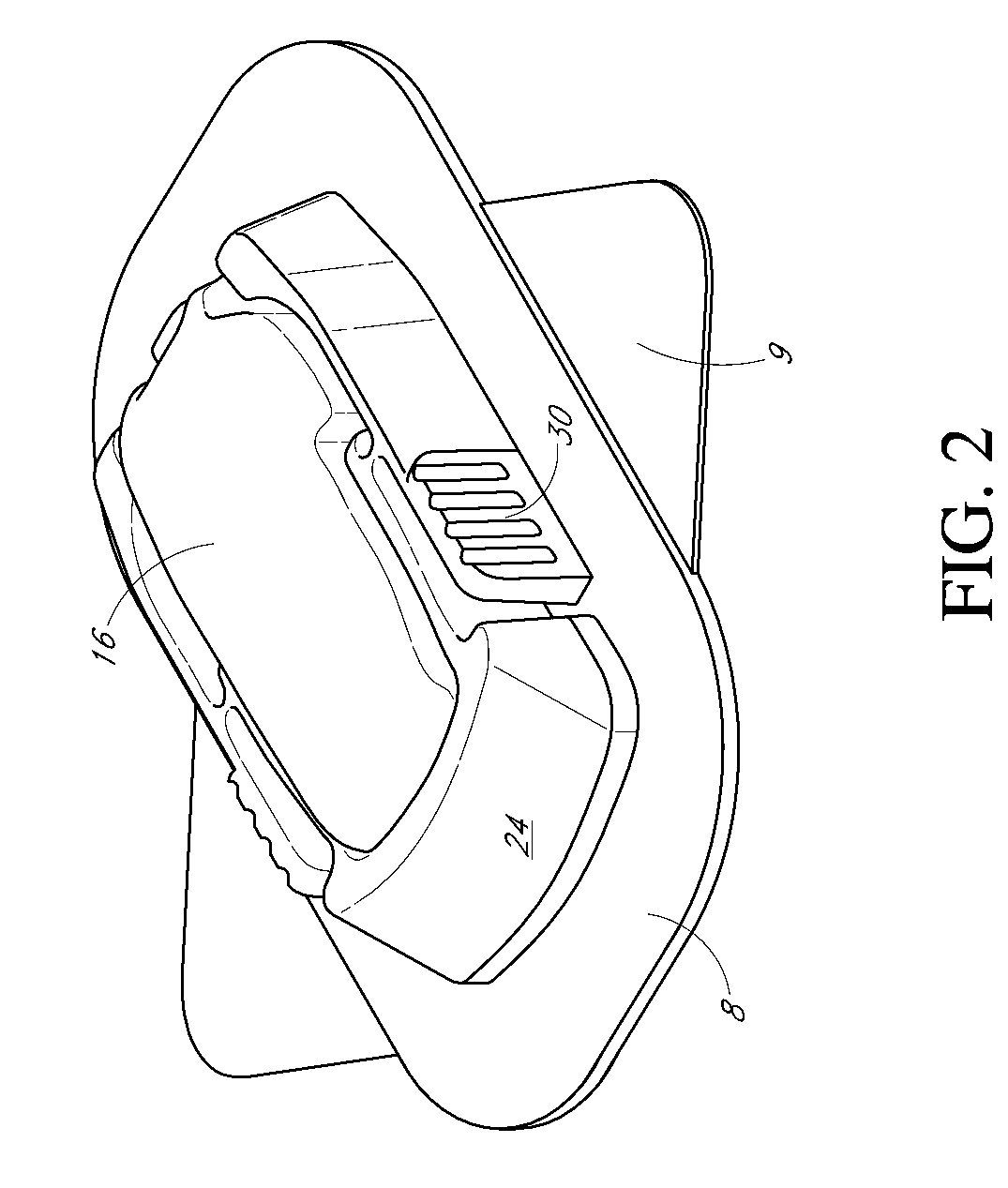
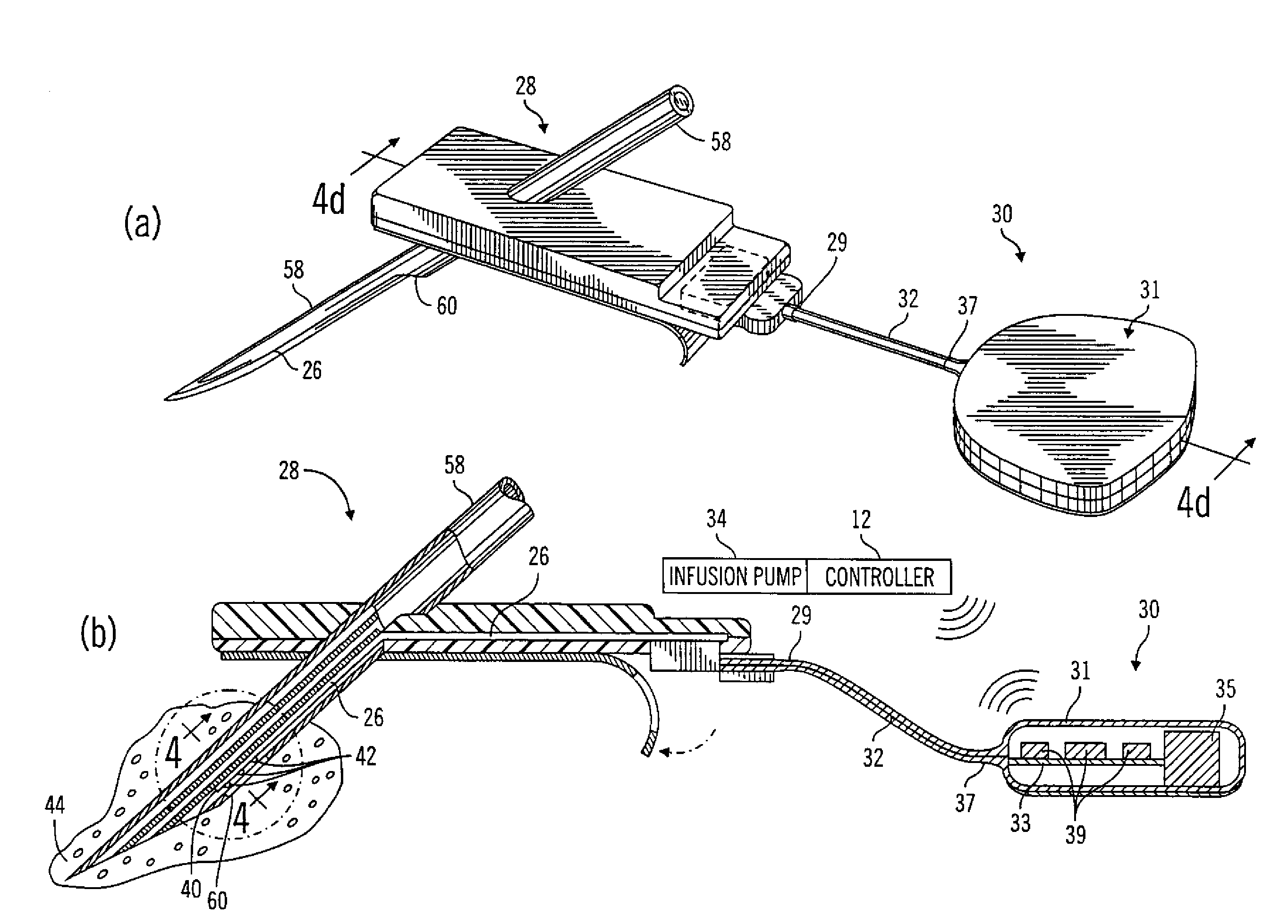
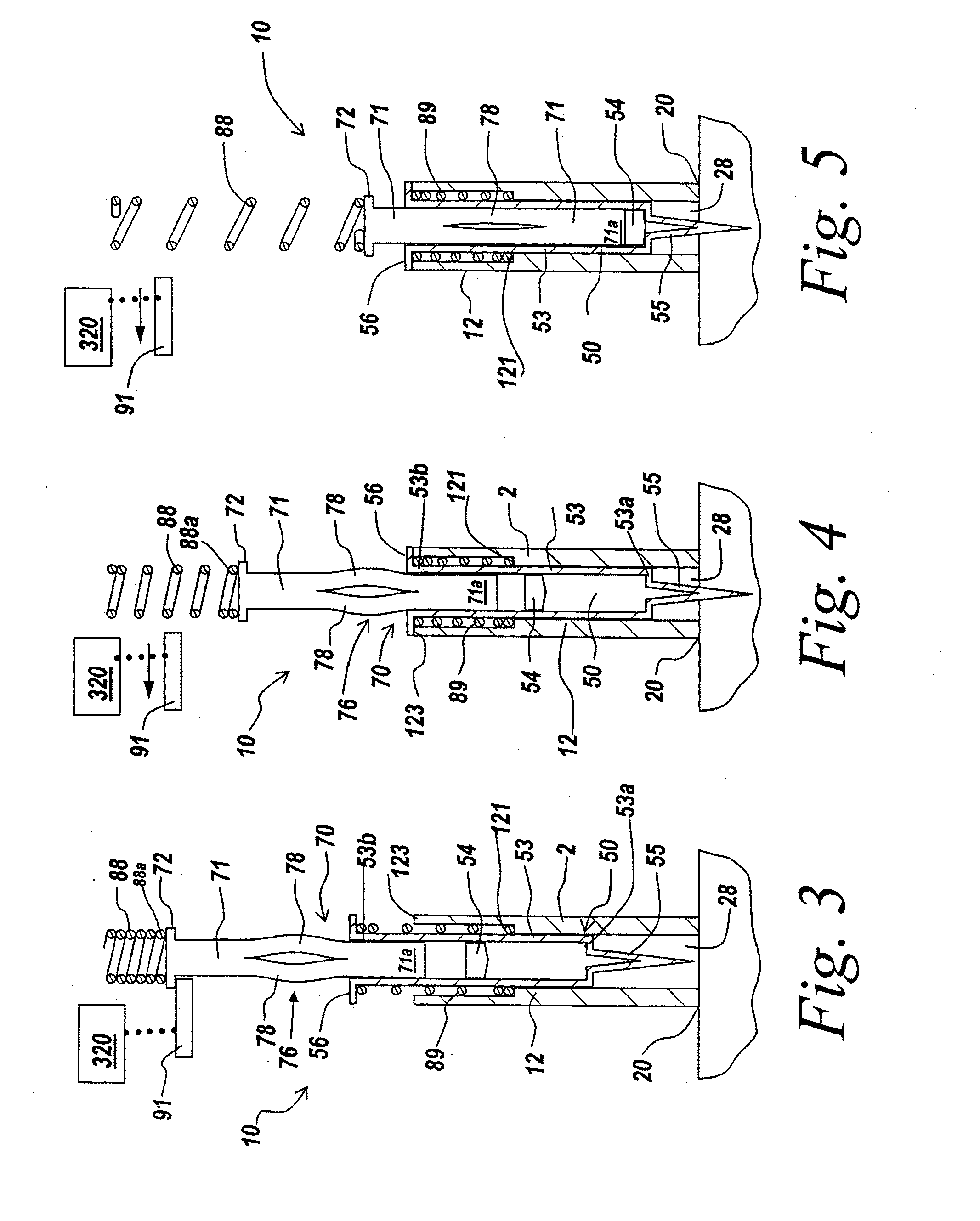
Insertion device for an insertion set and method of using the same
InactiveUS6997907B2Minimal patient discomfortAccurate placementAutomatic syringesCatheterBiomedical engineeringInsertion device
An insertion set for insertion through the skin of a patient includes at least one piercing member adapted to be secured to an insertion device and a set housing to be removable from the insertion device while maintaining installation of the insertion set. At least a portion of the at least one piercing member is insertable through the skin. The set housing is coupled to the at least one piercing member and is adapted so that it is shaped to fit an insertion device to orient the at least one piercing member for placement through the skin of at least a portion of the at least one piercing member at a predetermined angle relative to the skin to install the insertion set. The at least one piercing member is adapted to be retainable by the insertion device during removal from the insertion set.
Owner:MEDTRONIC MIMIMED INC
Drug solution filling plastic ampoule and process for producing the same
A drug solution filling plastic ampoule having gas, steam and light ray barrier properties, a drug permeation preventing capability and an absorption / adsorption preventing capability, and a production method for the plastic ampoule. The drug solution filling plastic ampoule includes a container body, a fusion-bonded portion which seals a mouth of the container body, and a wrench-off holder tab connected to the fusion-bonded portion. The ampoule is formed from a parison including two or more layers, at least one of which is a functional layer having at least one characteristic property selected from the group consisting of a gas permeation preventing capability, a steam permeation preventing capability, a light ray permeation preventing capability, a drug permeation preventing capability and a drug absorption / adsorption preventing capability.
Owner:OTSUKA PHARM FAB INC
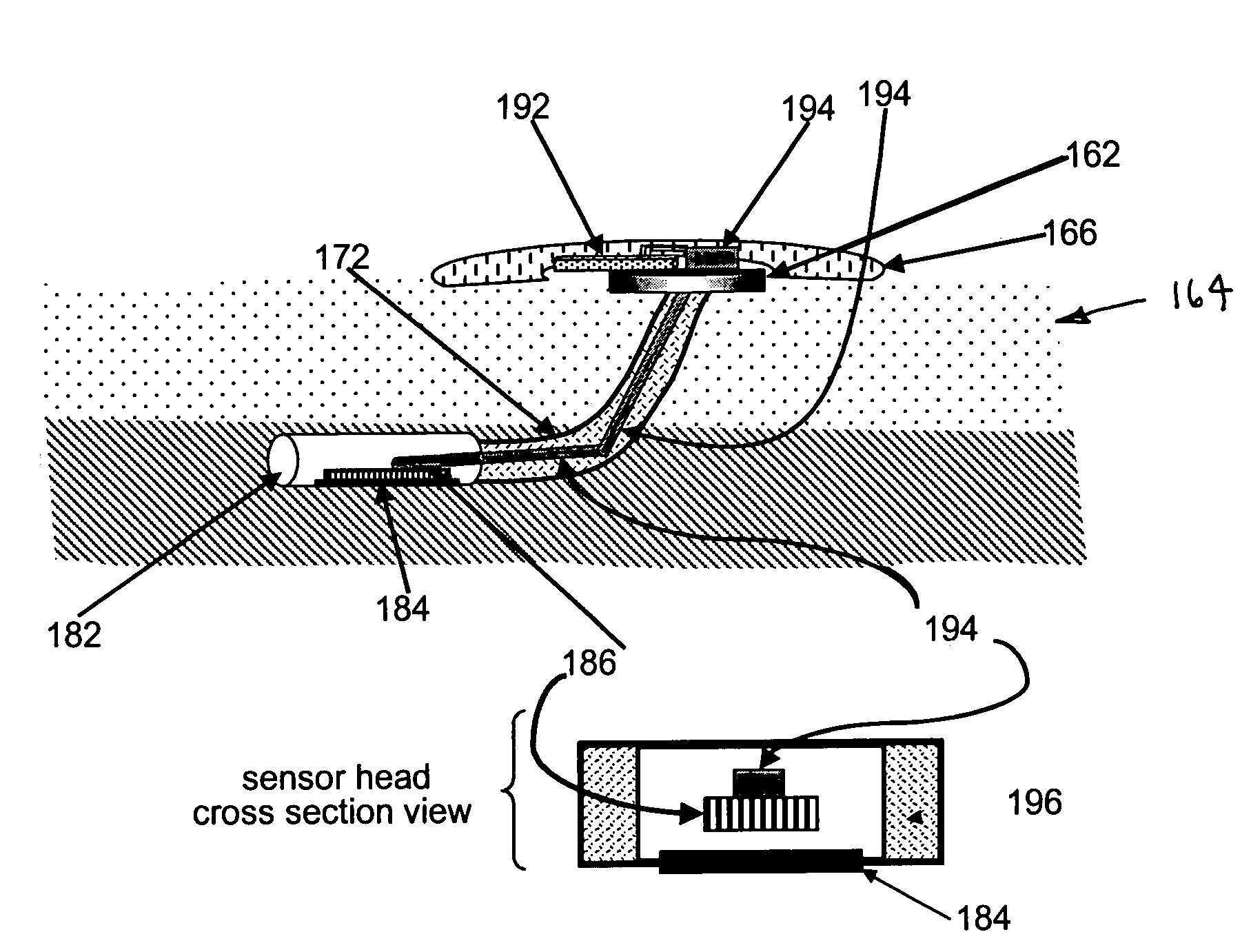
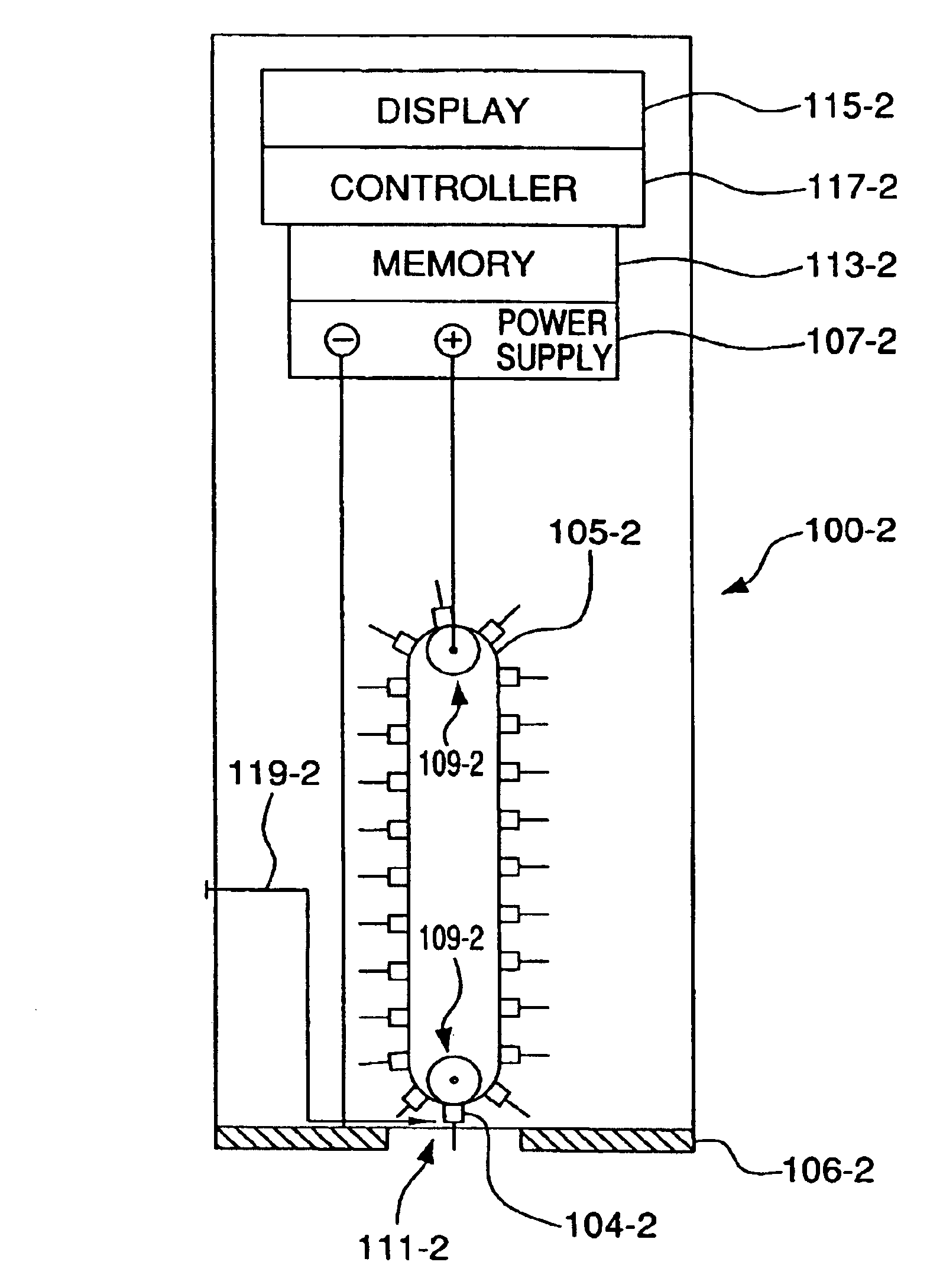
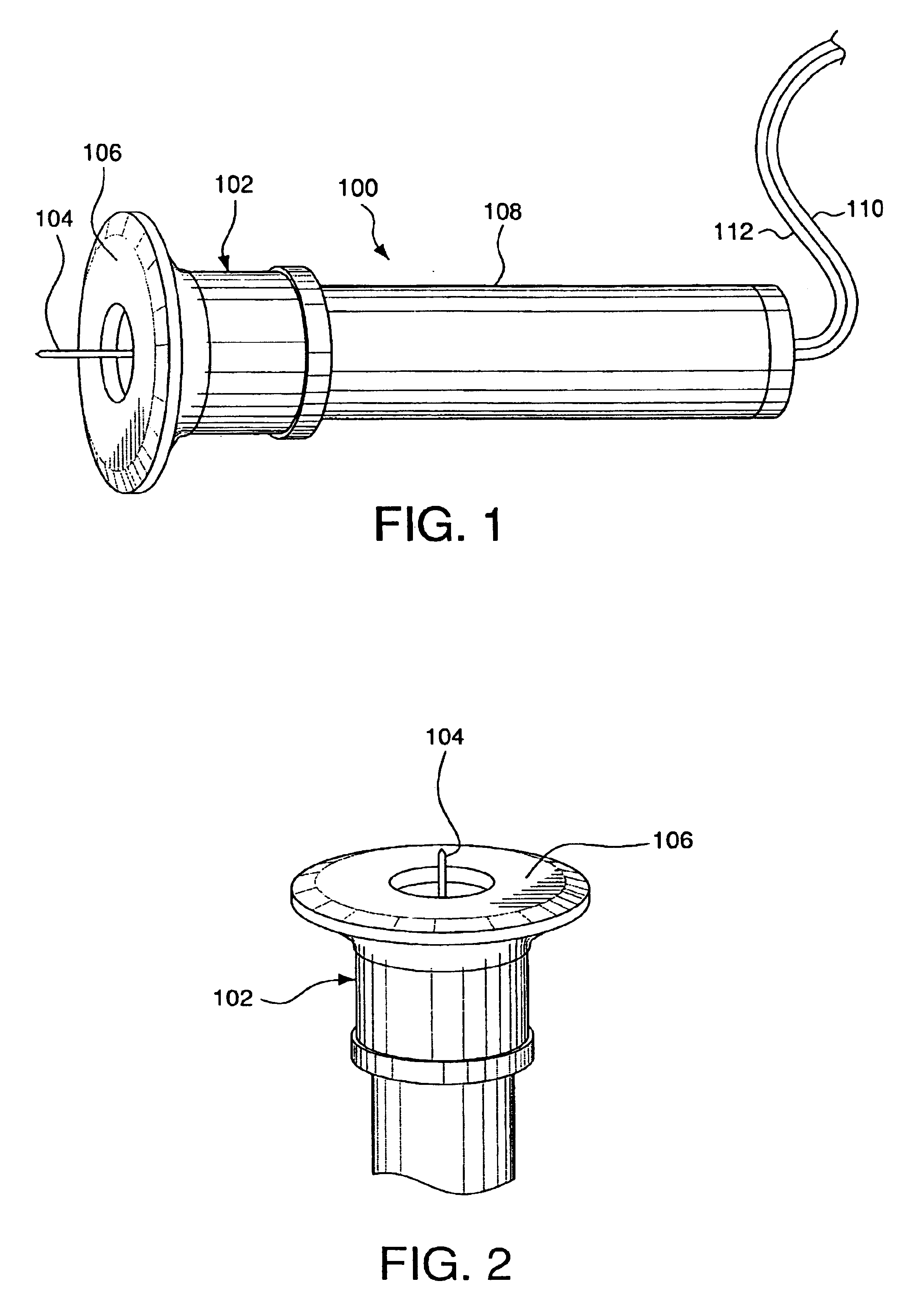
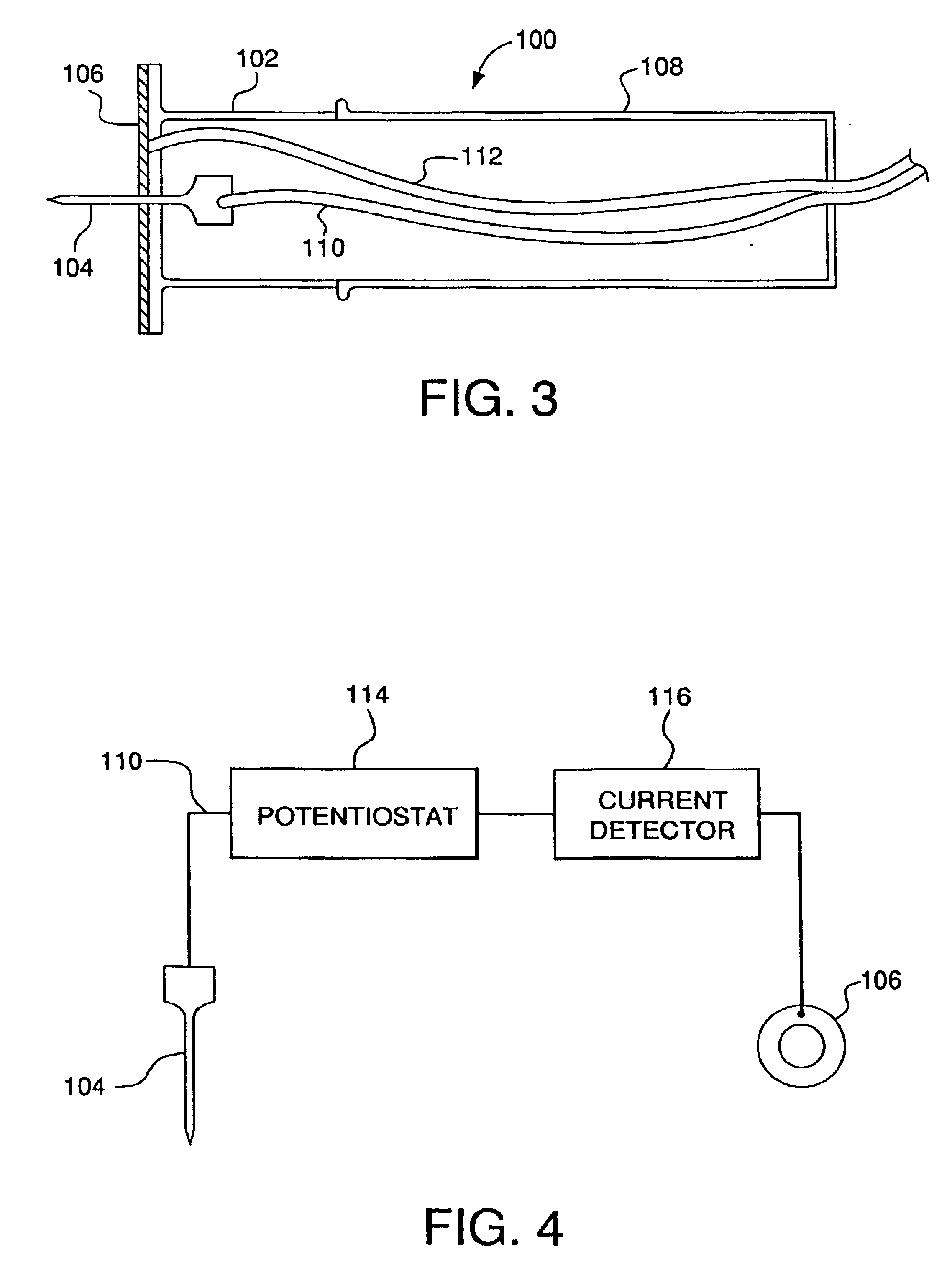
Transcutaneous analyte sensor
The present invention relates generally to systems and methods for measuring an analyte in a host. More particularly, the present invention relates to systems and methods for transcutaneous measurement of glucose in a host.
Owner:DEXCOM
Flexible instrument
A method of performing a medical procedure on a patient comprises conveying control signals from a remote controller to a drive unit, intravenously introducing the catheter into a heart of the patient (e.g., via the vena cava into the right atrium), and creating a puncture within a wall between two chambers (e.g., the left and right atria) of the heart. The method further comprises creating a puncture within a wall between two chambers of the heart, and operating the drive unit in accordance with the control signals to advance a working catheter within the guide catheter through the puncture.
Owner:HANSEN MEDICAL INC
Transcutaneous inserter for low-profile infusion sets
A low-profile inserter for an angled infusion set comprises an inserter housing having a bottom wall, a retainer slidably connected to the inserter housing for movement between retracted and extended positions in a direction substantially parallel with the bottom wall, and a base member connected to the inserter housing. The retainer is adapted to releasably receive a cannula assembly, including a cannula connected to a cannula housing. The base member has a lower surface that is adapted to contact a skin outer surface. The lower surface and bottom wall together form an acute angle. With this arrangement, the cannula can be inserted subcutaneously at the acute angle with respect to the skin outer surface.
Owner:LIFESCAN IP HLDG LLC
Gateway platform for biological monitoring and delivery of therapeutic compounds
InactiveUS7044911B2Improve data qualityReduce variationBioelectric signal measurementDrug and medicationsEngineeringBiomonitoring
The invention relates to methods and devices for remote or distributed continuous monitoring of physiologically relevant states. The invention provides for methods to automatically detect deviations or other states in physiological parameters and automatically alert a measured subject, user or other authorized party. The device provides for a universal platform for sensors, and further provides for the automatic compensation or distribution of devices or bioactive agents at appropriate levels and / or intervals in response to deviations or other states sensed in various physiological parameters.
Owner:PHILOMETRON
Minimally-invasive system and method for monitoring analyte levels
InactiveUS6952604B2Improve responseImprove signal and performanceCatheterSensorsAnalyteStratum corneum
A minimally-invasive analyte detecting device and method for using the same. The system and method employ a device having an active electrode optionally coated with a substance, and a counter-electrode that is configured at least partially surround the active electrode. The configuration of the auxiliary electrode and active electrode improves the current flow through the device and increases the sensitivity of the device. When the device is placed against the patient's skin, the active electrode is adapted to enter through the stratum corneum of a patient to a depth less than a depth in the dermis at which nerve endings reside. An electric potential is applied to the active electrode and the analyte level is determined based on the amount of current or charge flowing through the device.
Owner:BECTON DICKINSON & CO
Transcutaneous analyte sensor
The present invention relates generally to systems and methods for measuring an analyte in a host. More particularly, the present invention relates to systems and methods for transcutaneous measurement of glucose in a host.
Owner:DEXCOM INC
Closed-Loop Method for Controlling Insulin Infusion
InactiveUS20080188796A1Other blood circulation devicesDrug and medicationsInsulin infusionConcentrations glucose
A closed loop infusion system controls the rate that fluid is infused into the body of a user. The closed loop infusion system includes a sensor system, a controller, and a delivery system. The sensor system includes a sensor for monitoring a condition of the user. The sensor produces a sensor signal, which is representative of the condition of the user. The sensor signal is used to generate a controller input. The controller uses the controller input to generate commands to operate the delivery system. The delivery system infuses a liquid into the user at a rate dictated by the commands from the controller. Preferably, the sensor system monitors the glucose concentration in the body of the user, and the liquid infused by the delivery system into the body of the user includes insulin.
Owner:MEDTRONIC MIMIMED INC
Adhesive Patch Systems and Methods
ActiveUS20080269687A1High bonding strengthReduced adhesion strengthInfusion syringesFiltering accessoriesAdhesion strengthUltimate tensile strength
Various embodiments of the present invention are directed to patches for medical devices. In various embodiments, an adhesive patch of a medical device may have selective areas with adhesive material of varying adhesion strengths. In other embodiments, an adhesive patch of a medical device may include adhesive material that may be activated by a catalyst to increase or decrease the adhesion strength of the adhesive material. In further embodiments, a medical device may include a pierceable membrane containing an agent, the pierceable membrane positioned to be pierced by a needle and to cause some of the agent to be carried to the user-patient.
Owner:MEDTRONIC MIMIMED INC
Infusion set
ActiveUS7879010B2Minimise and rule out dangerReduce stepsGuide needlesInfusion syringesMedicineInfusion set
A device for inserting a cannula into tissue, including a cannula, a protective element which can accommodate said cannula, an operating element for moving the cannula out of the protective element, and a holder fixedly connected to the cannula. The invention encompasses a system for connecting a liquid supply to the cannula.
Owner:ROCHE DIABETES CARE INC
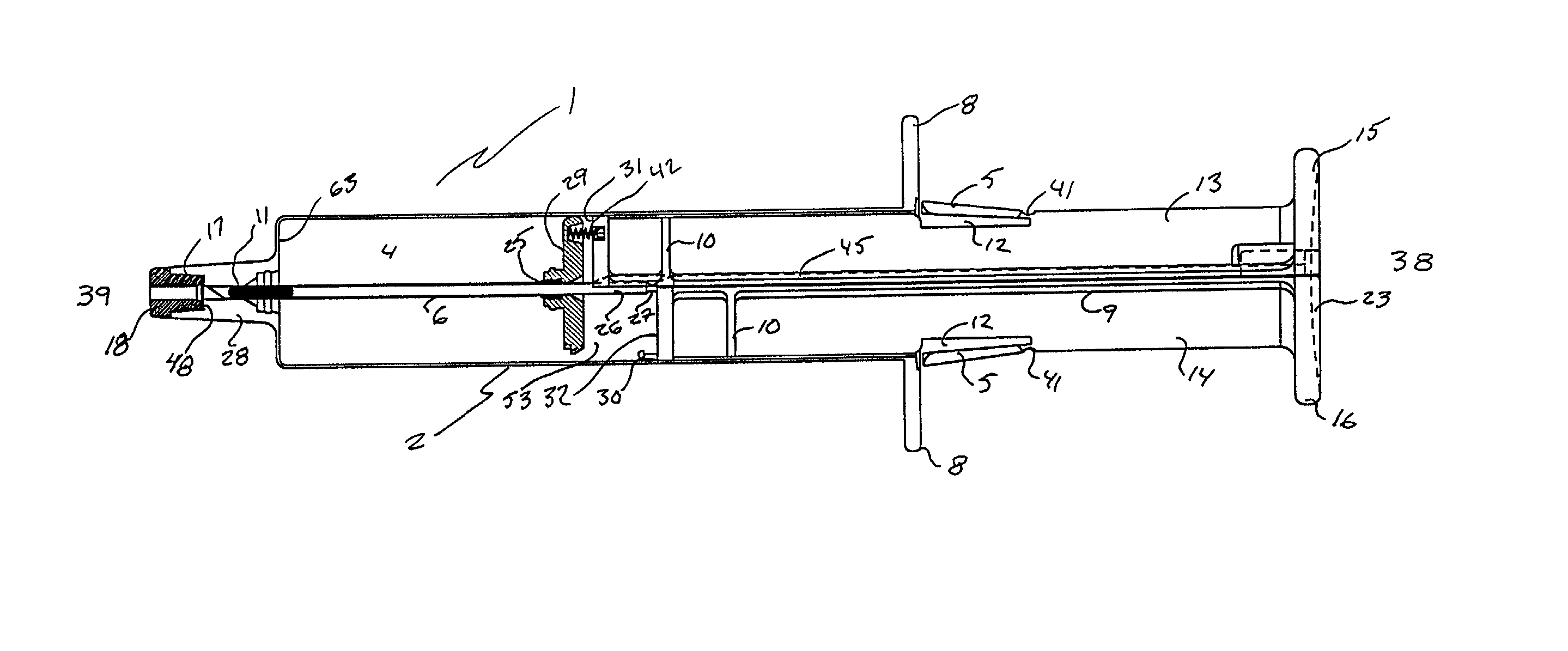
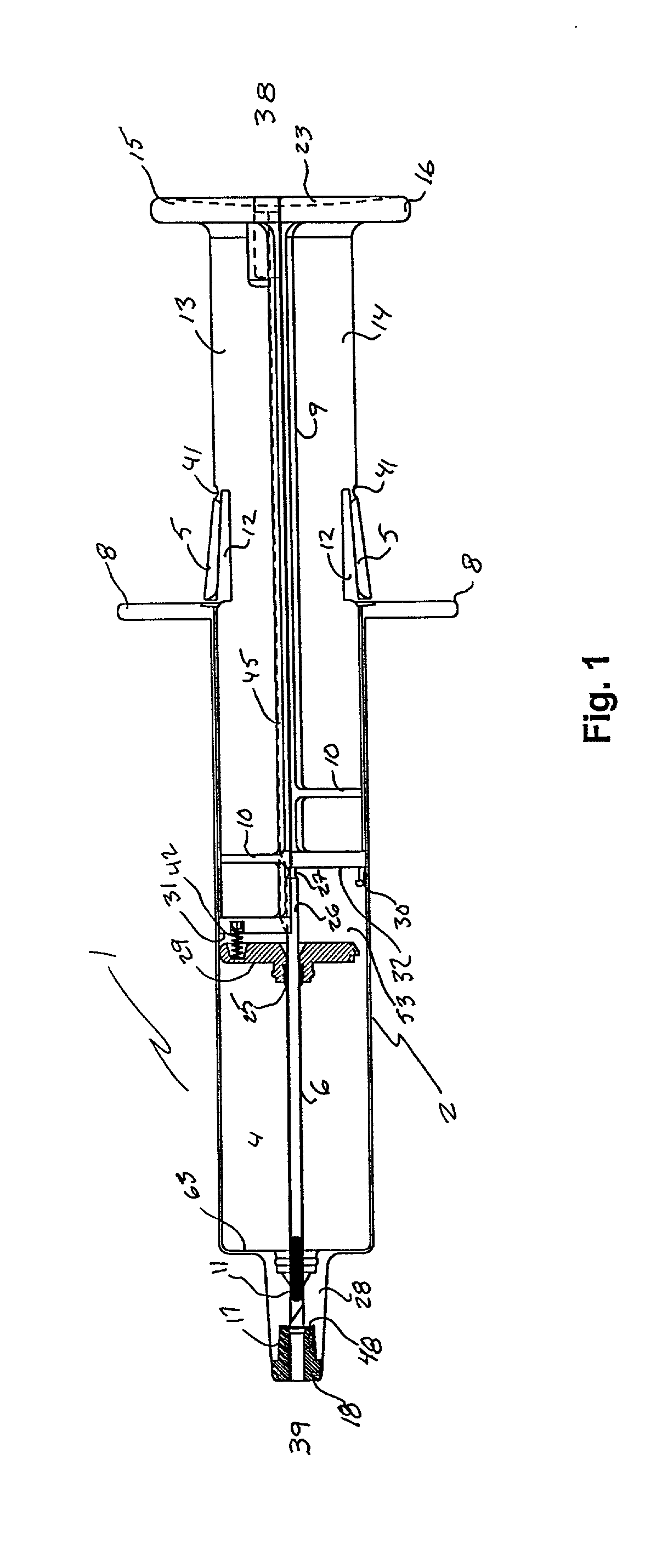
Automatic injection device
ActiveUS20100160894A1Easy to useReduce anxietyPeptide/protein ingredientsAntipyreticHypodermoclysisSubcutaneous injection
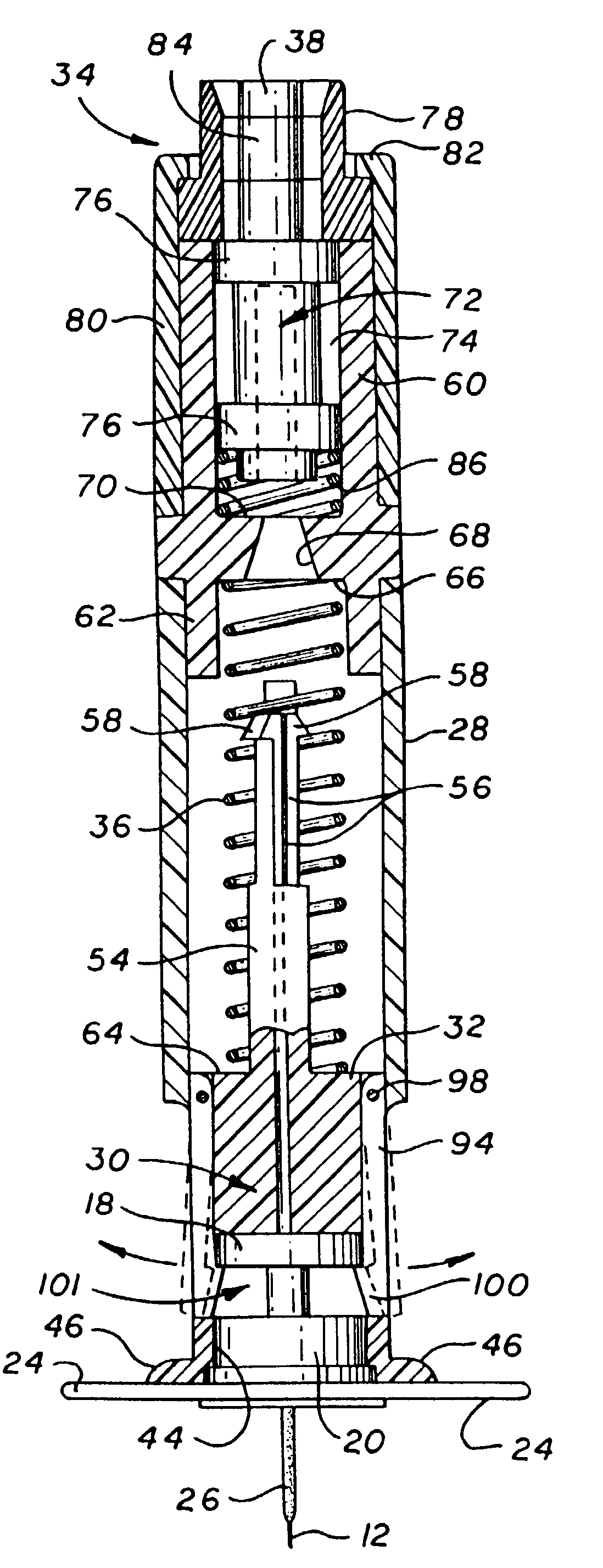
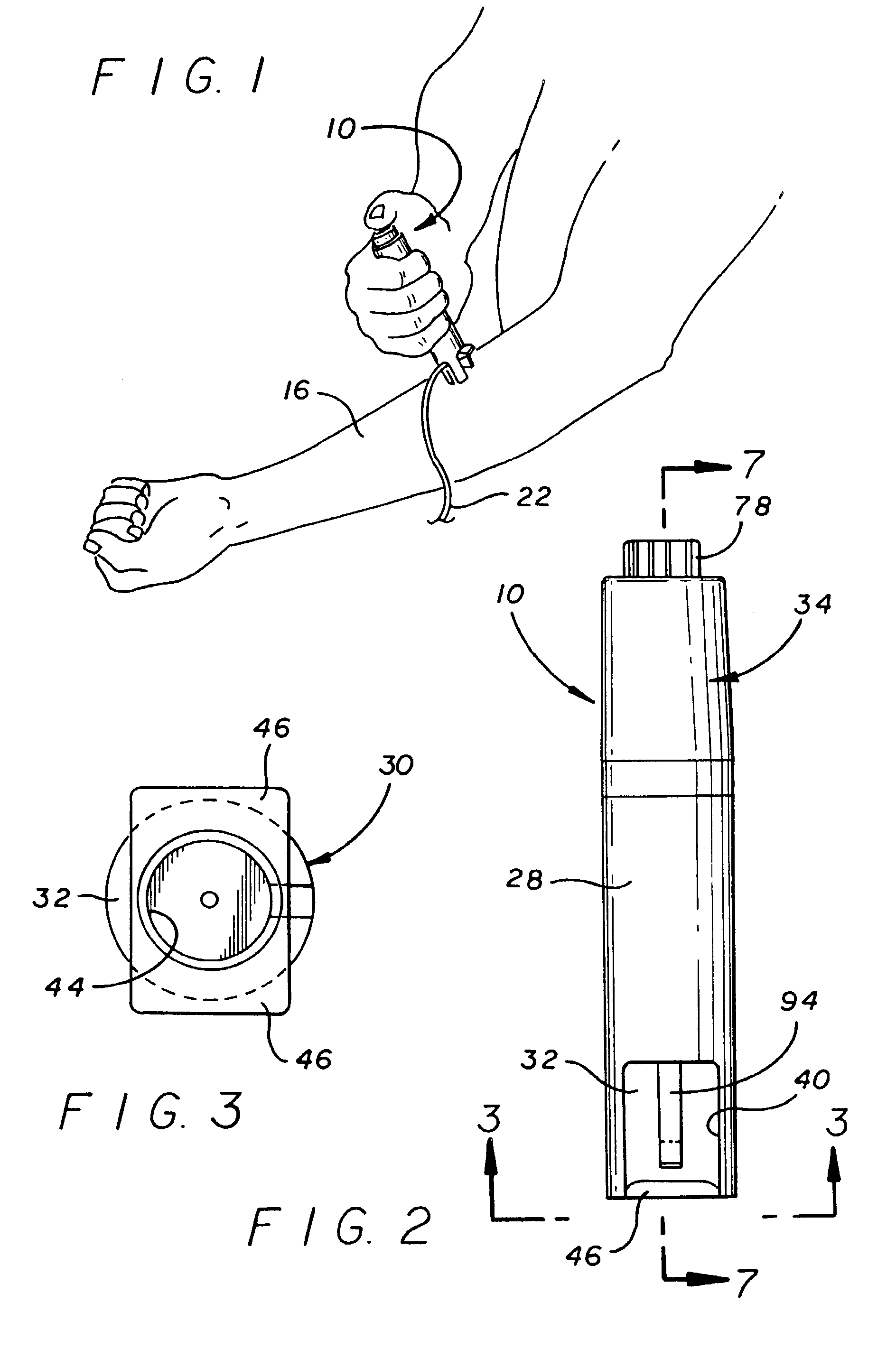
The invention provides an automatic injection device for providing a subcutaneous injection of a substance into a user, comprising: a housing having an open first end and a second end; a syringe movably disposed in the housing, the syringe including a barrel portion for holding the substance, a hollow needle in fluid communication with the barrel portion for ejecting the substance from the syringe, and a bung for sealing the barrel portion and selectively applying pressure to the substance to force the substance through the hollow needle; a plunger for first moving the syringe towards the first end such that the needle projects from the first end and subsequently applying pressure to the bung, the plunger including a rod connected at a first end to the bung, a compressible expanded central portion and a flange between a second end of the rod and the compressible expanded central portion; and a biasing mechanism for biasing the plunger towards the first open end of the housing, the biasing mechanism disposed about the second end of the rod between the flange and the second end of the housing. The present invention also provides methods and kits for using an automatic injection device, and methods and kits for promoting an automatic injection device comprising a medication based on advantageous properties of the device as compared to a pre-filled syringe. The invention also provides methods and kits for training a recipient on use of the automatic injection device.
Owner:ABBVIE BIOTECHNOLOGY LTD
Closed loop system for controlling insulin infusion
InactiveUS20060224109A1Limit accuracy of signalOther blood circulation devicesMedical devicesInsulin infusionConcentrations glucose
A closed loop infusion system controls the rate that fluid is infused into the body of a user. The closed loop infusion system includes a sensor system, a controller, and a delivery system. The sensor system includes a sensor for monitoring a condition of the user. The sensor produces a sensor signal, which is representative of the condition of the user. The sensor signal is used to generate a controller input. The controller uses the controller input to generate commands to operate the delivery system. The delivery system infuses a liquid into the user at a rate dictated by the commands from the controller. Preferably, the sensor system monitors the glucose concentration in the body of the user, and the liquid infused by the delivery system into the body of the user includes insulin.
Owner:MEDTRONIC MIMIMED INC
Fluid delivery systems and methods
ActiveUS20070228071A1Great degreePrevent backflowFlexible member pumpsMedical devicesDelivery systemBiomedical engineering
A method of dispensing fluid includes three processes. A first one of these processes includes pumping fluid into a resilient variable-volume dispensing chamber. The dispensing chamber is in series with a normally present finite fluid impedance and an output. The impedance is sufficient so as to cause expansion of the dispensing chamber as it receives pumped fluid even while some fluid flows through the output. Another one of these processes includes repeatedly measuring a parameter related to volume of the dispensing chamber over time. A third one of these processes includes controlling the pumping of fluid based on repeated measurements of the parameter to produce a desired fluid flow through the output. A corresponding system for dispensing fluid implements these processes.
Owner:DEKA PROD LLP
Integrated cement delivery system for bone augmentation procedures and methods
A cement delivery system for vertebroplasty including a rigid cannula having a tubular inner wall defining a central conduit to deliver bone cement and a tubular outer wall extending around the inner wall and spaced apart therefrom to define a peripheral conduit for aspirating bone fluids. Aspirating means communicate with a proximal outlet port of the peripheral conduit to create a pressure gradient between the central conduit and the peripheral conduit to provide a hydraulic force guiding the displacement of bone fluid and the flow of cement. Also, a bone cement delivery system including a rigid cannula having a tubular inner wall defining a central conduit, a tubular outer wall extending around the inner wall and spaced apart therefrom to define a peripheral conduit, and a tubular middle wall extending between the inner and peripheral walls and spaced apart therefrom to define a middle conduit around the central conduit.
Owner:BAROUD GAMAL DR
Endovascular thin film devices and methods for treating and preventing stroke
InactiveUS6605111B2Treating and preventing ischemic and hemorrhagic strokeInhibit migrationStentsCatheterIn situ polymerizationProsthesis
Owner:NEW YORK UNIV
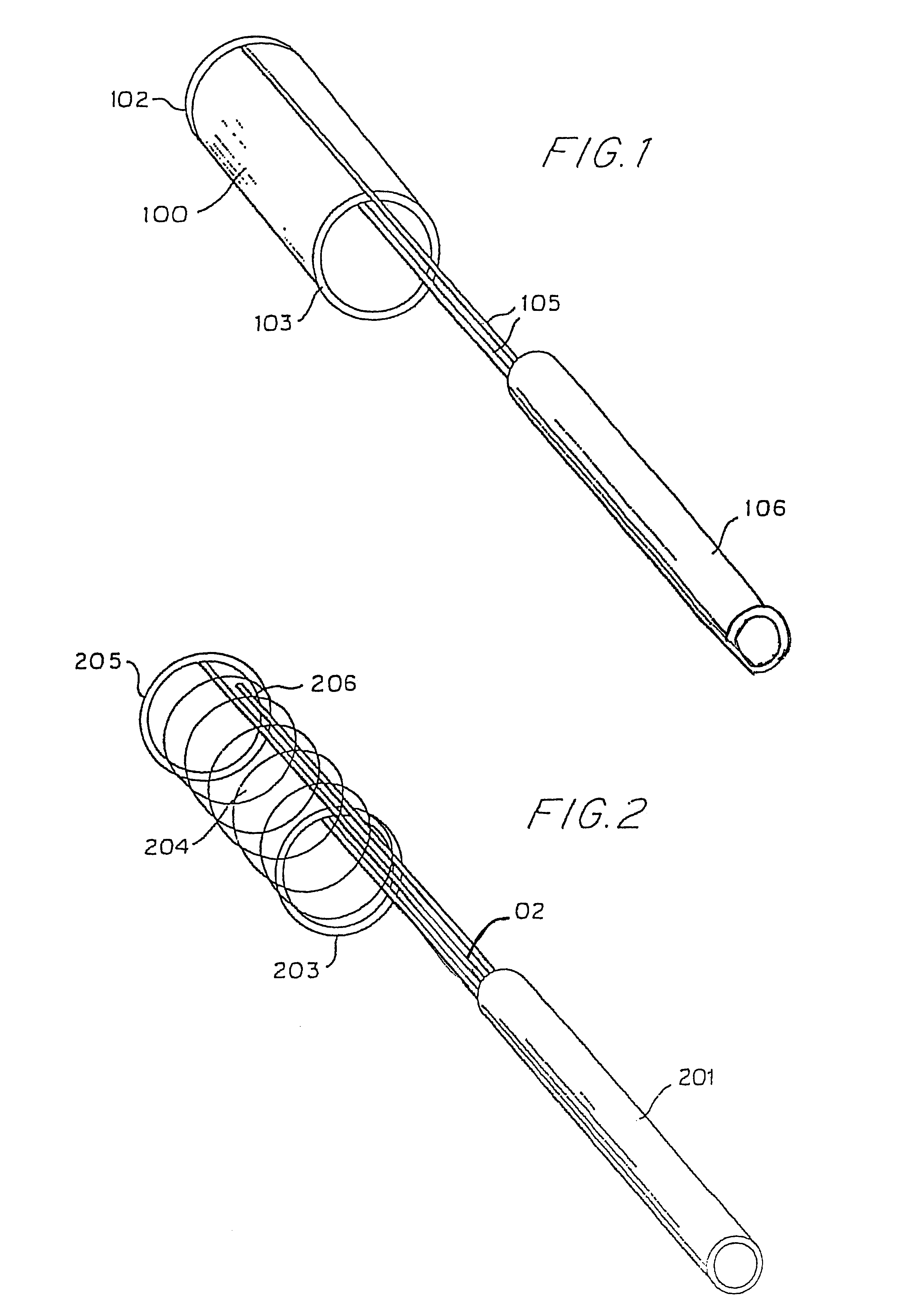
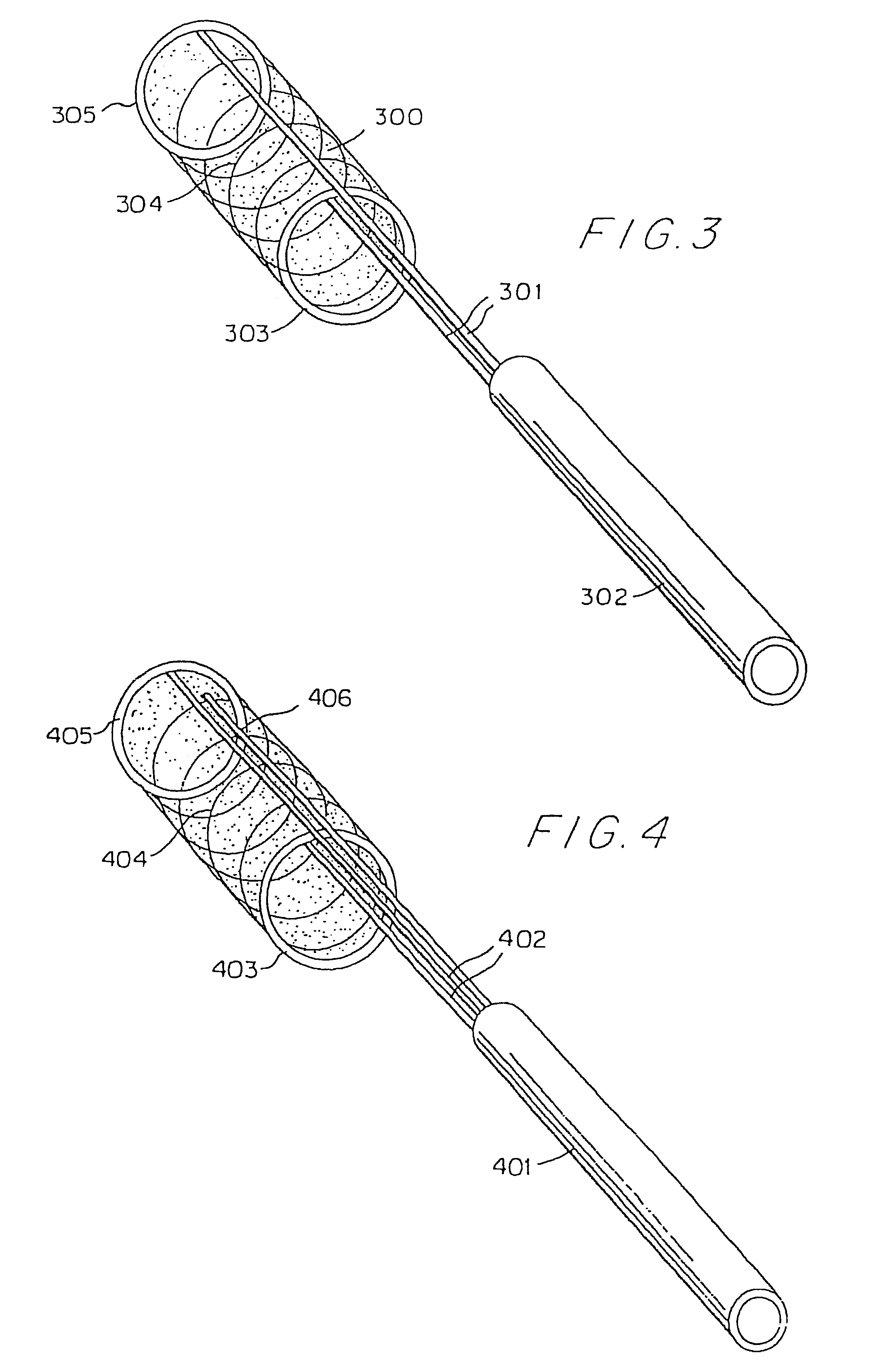
Hypodermic implant device
InactiveUS20030004457A1Avoid spreadingLess-costly disposalMedical devicesInfusion needlesSubcutaneous implantationImplanted device
A hypodermic implant device, comprising a barrel; an upper piston segment engaged with an inner wall of the barrel; an axially aligned needle extendably distally out of the barrel for insertion into an object when the upper piston segment is moved distally within the body; a lower piston segment push rod in alignment with the needle, so that upon distal movement of the lower piston segment, the push rod moves distally through the needle expelling a releasable implant housed in the needle; and a means for retraction of the needle into a needle receptacle in the barrel.
Owner:GRAY PLANT MOOTY MOOTY & BENNETT PA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com