Patents
Literature
1955 results about "Bung" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
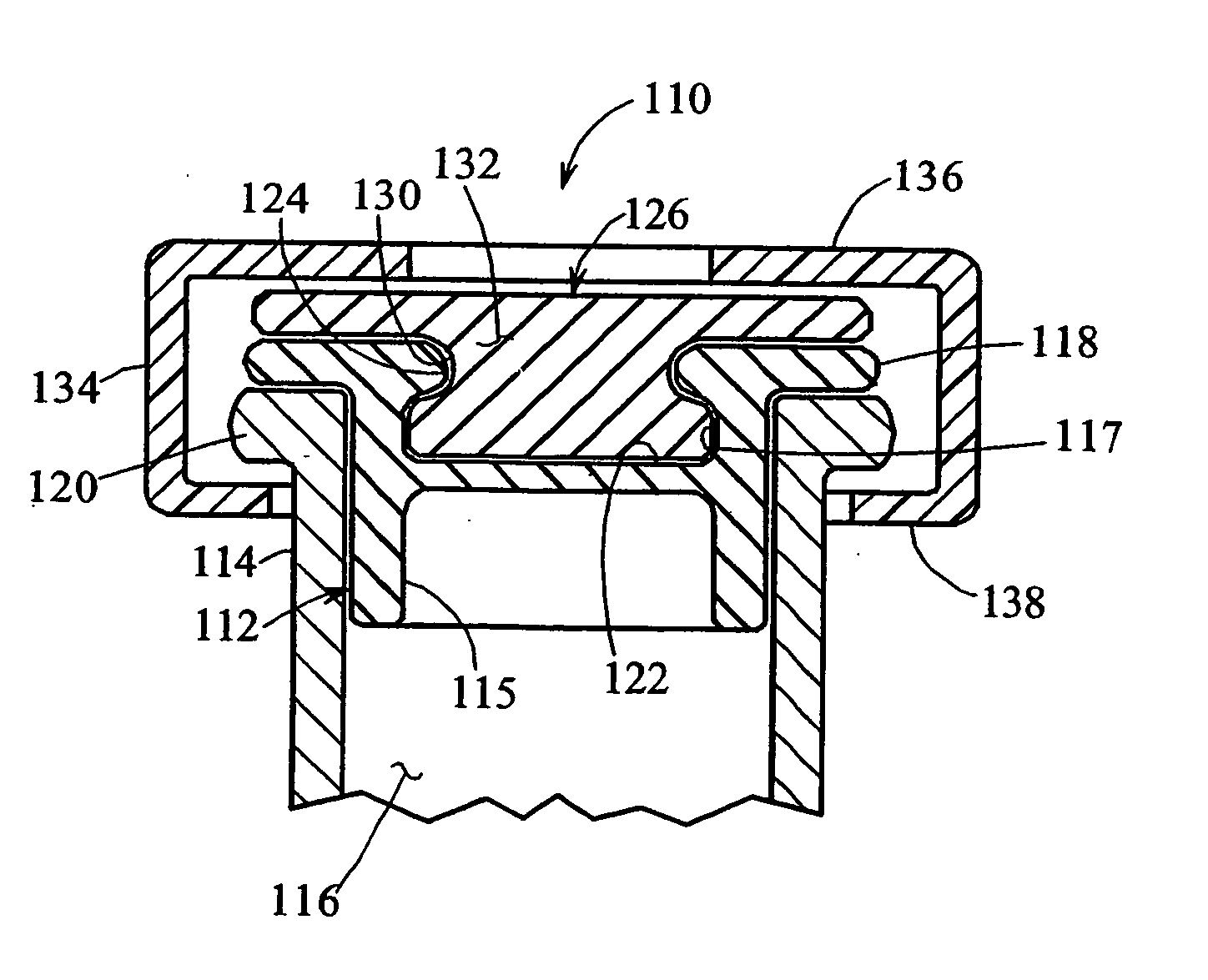
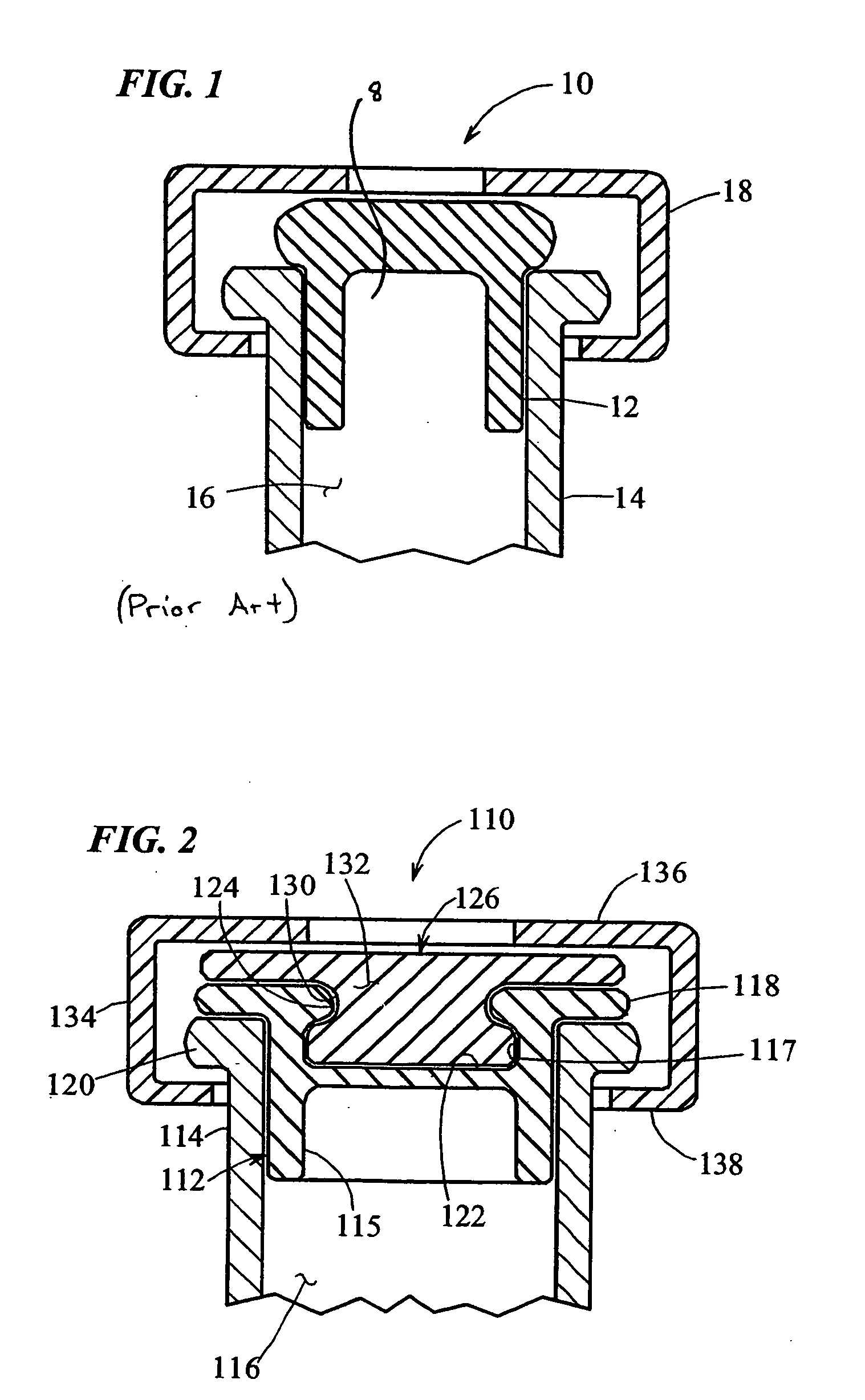
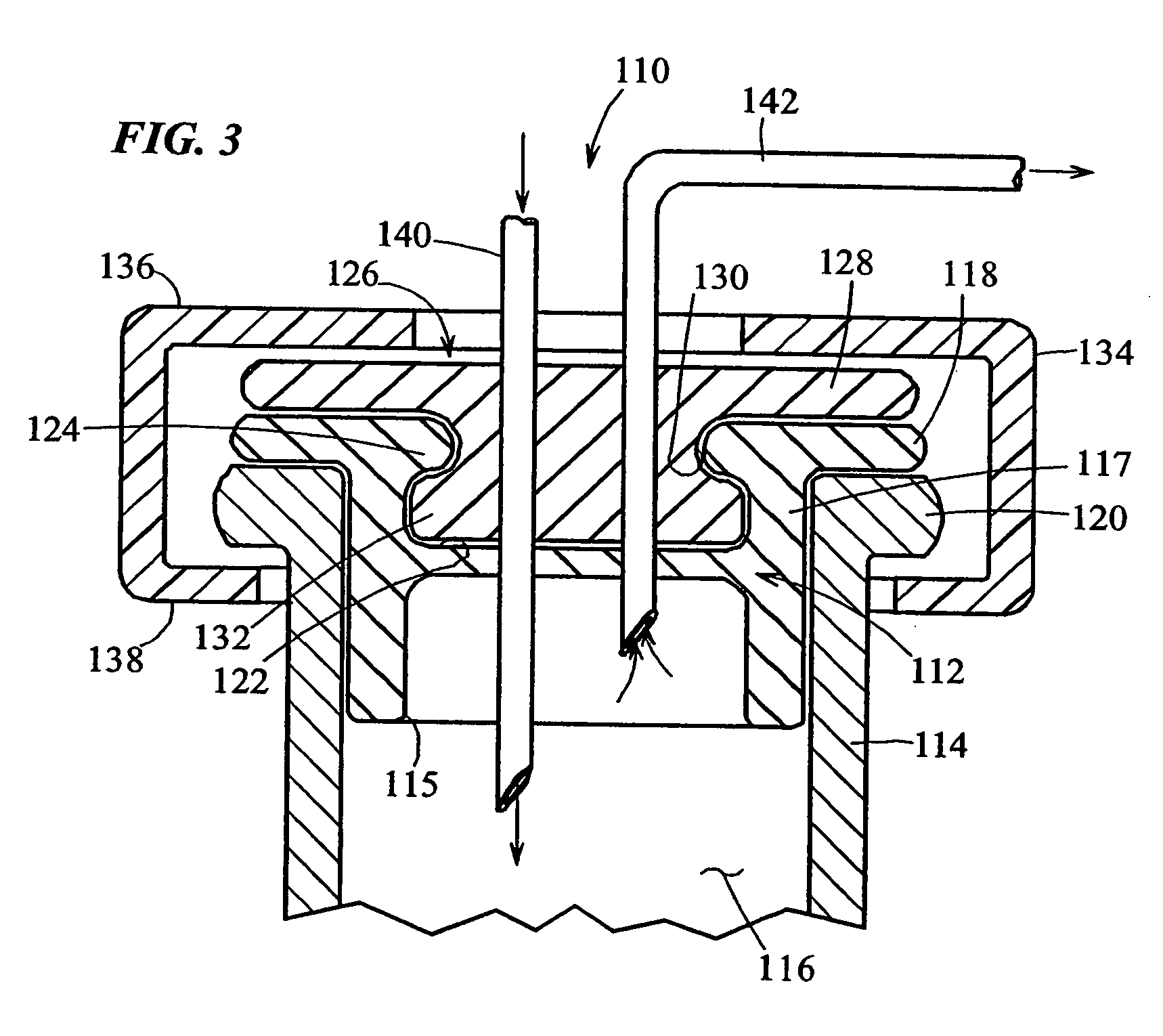
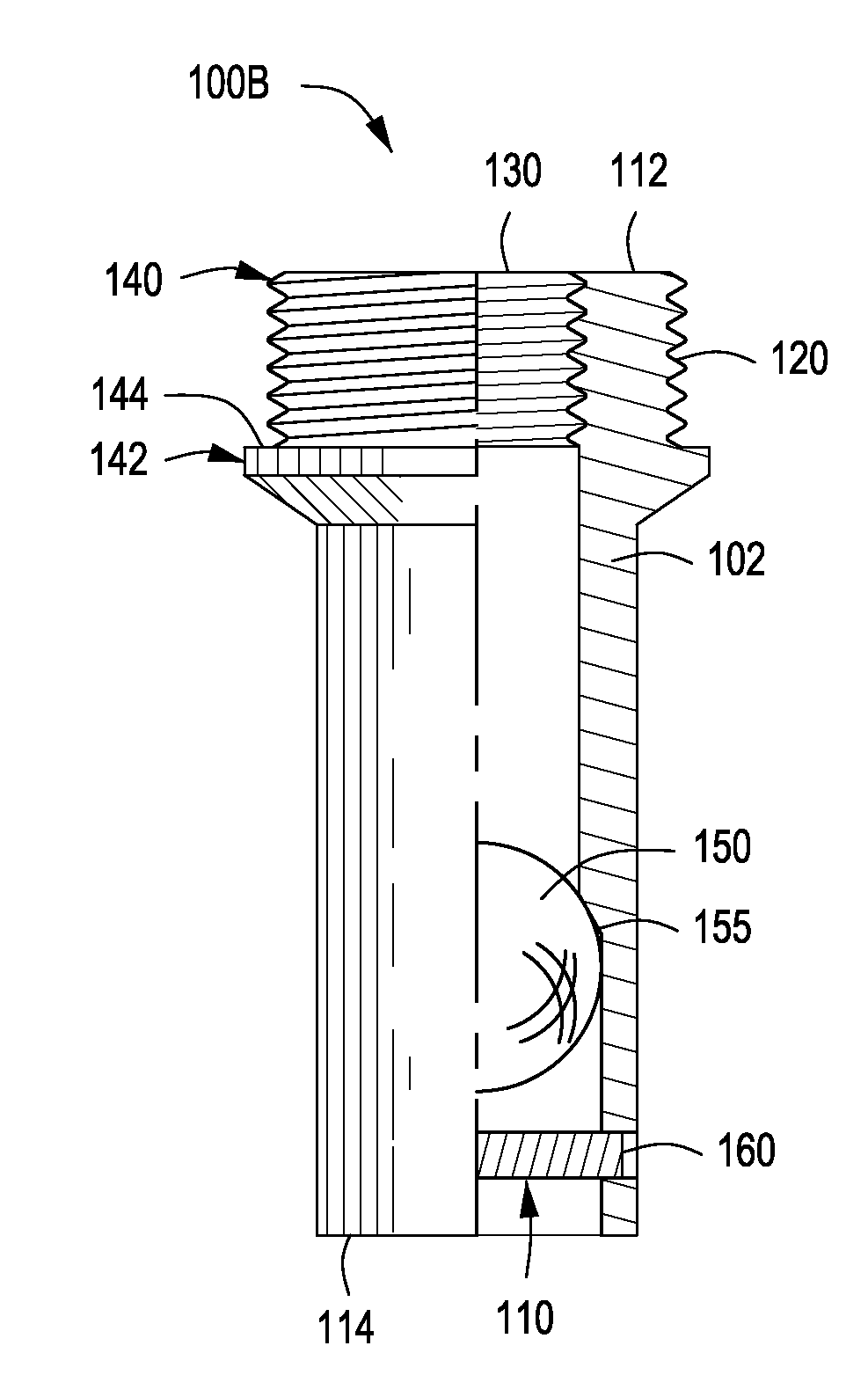
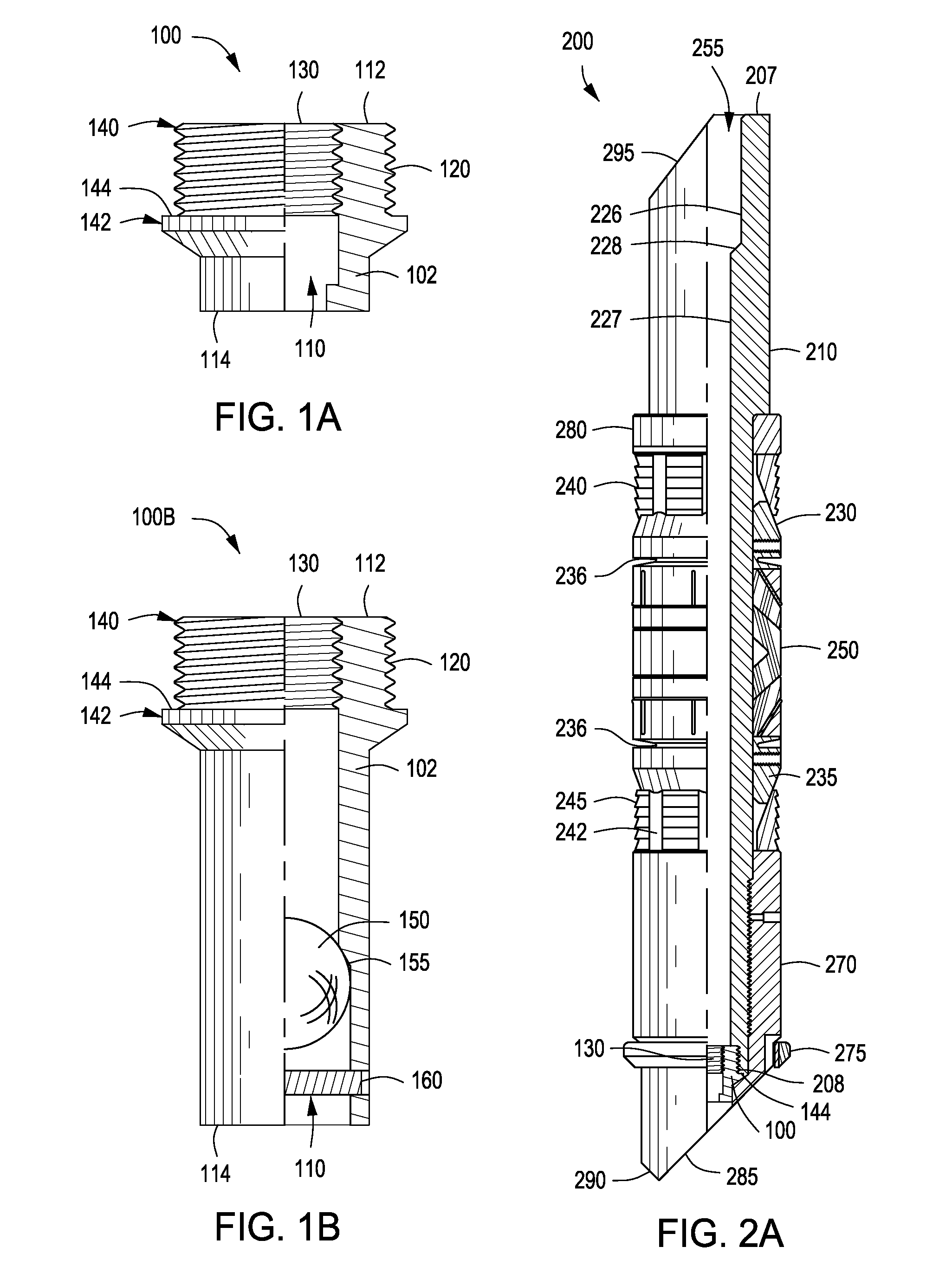
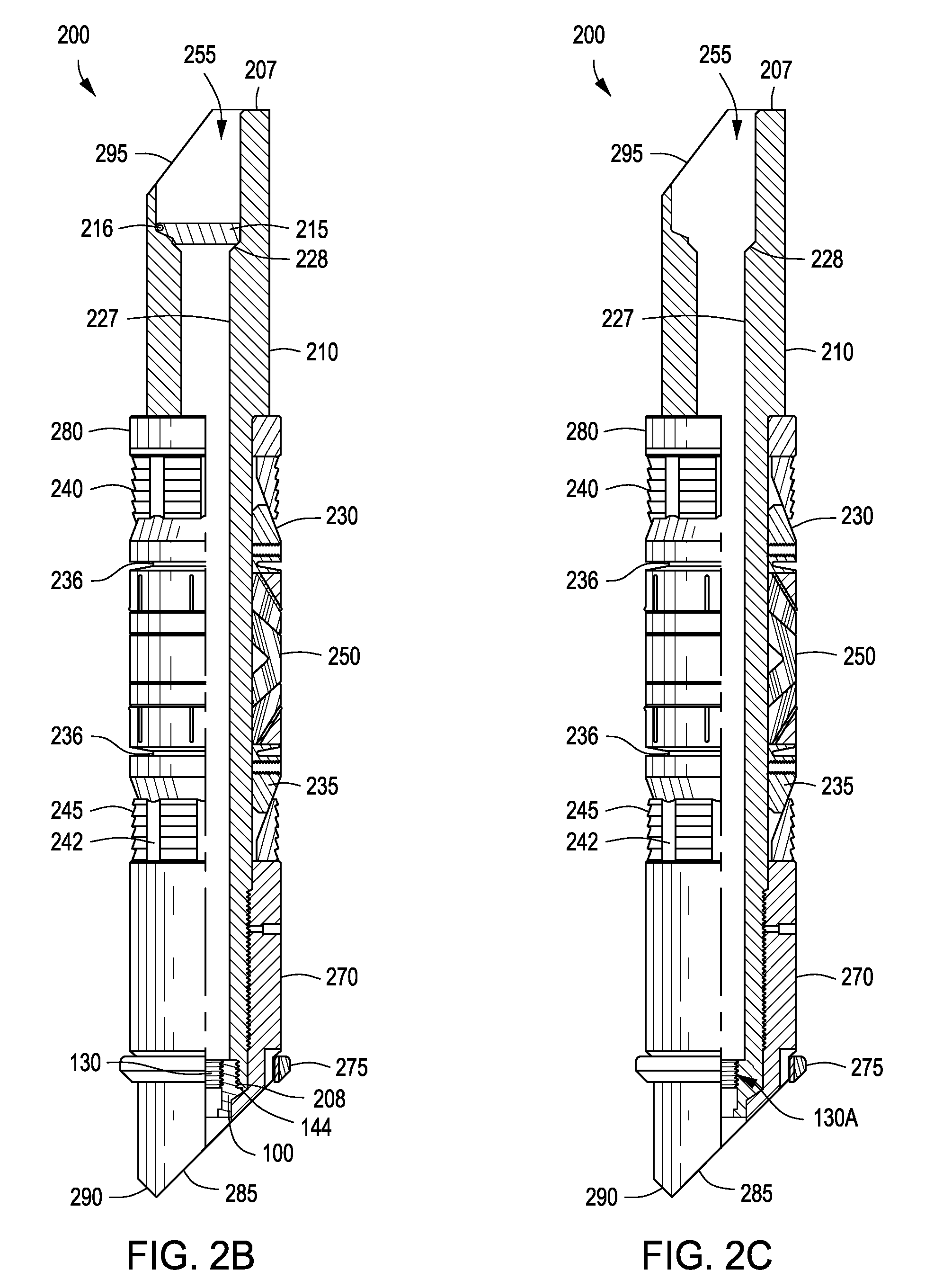
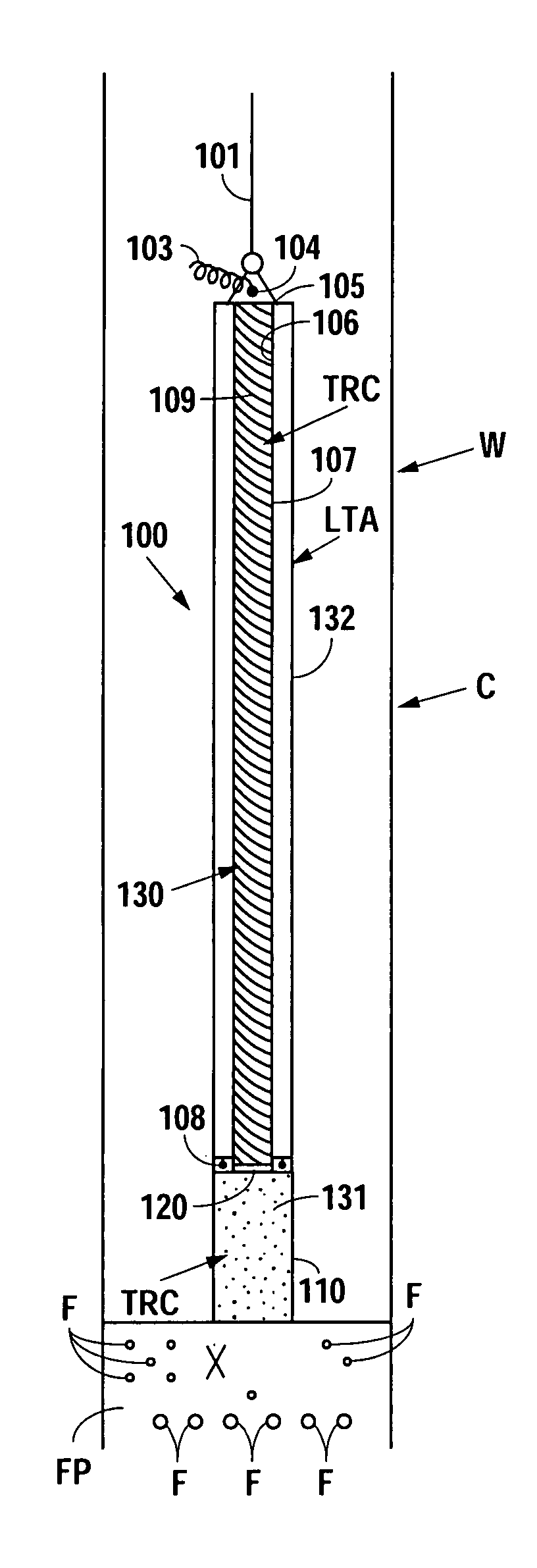
A bung, stopper or cork is a truncated cylindrical or conical closure to seal a container, such as a bottle, tube or barrel. Unlike a lid, which encloses a container from the outside without displacing the inner volume, a bung is partially inserted inside the container to act as a seal.
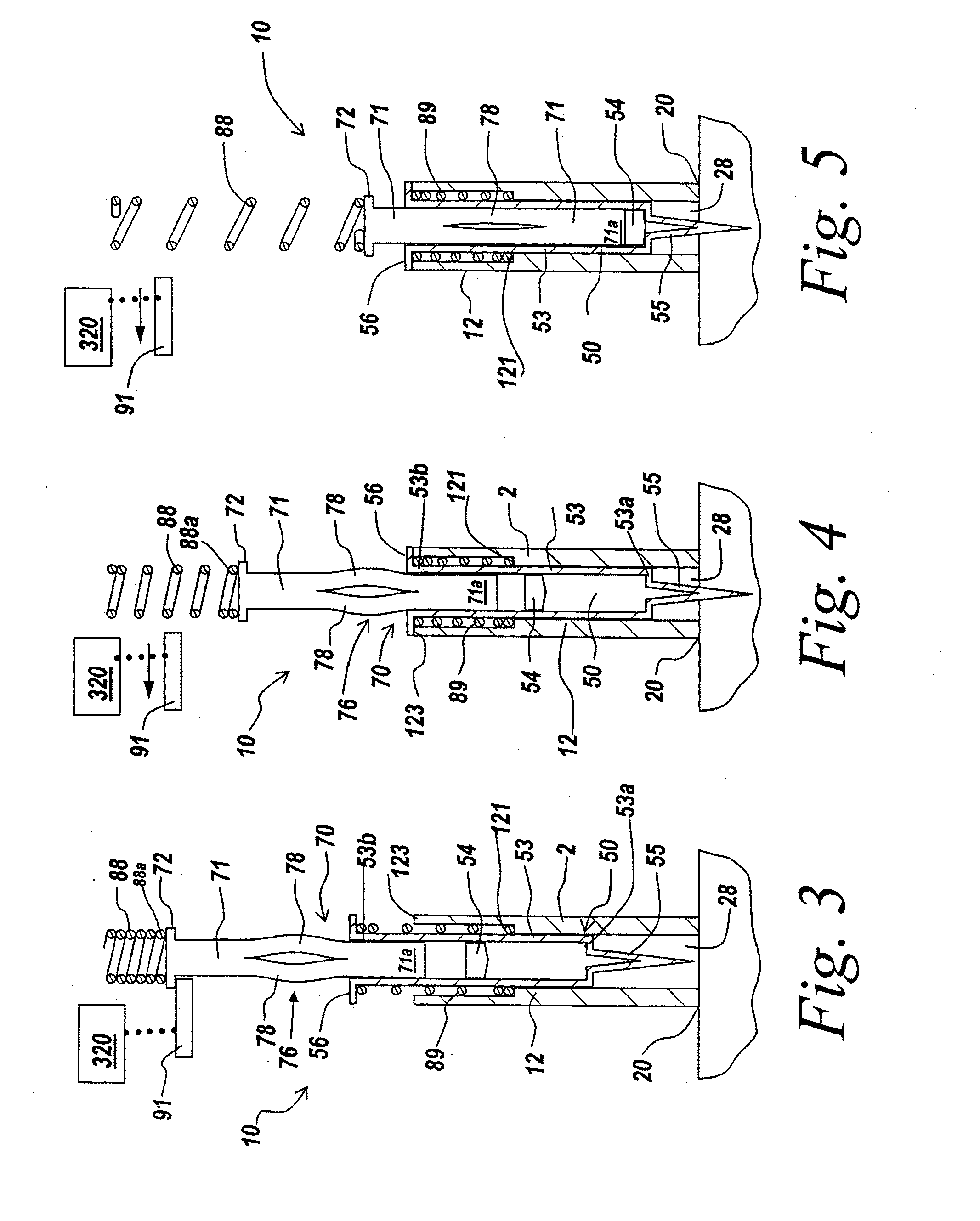
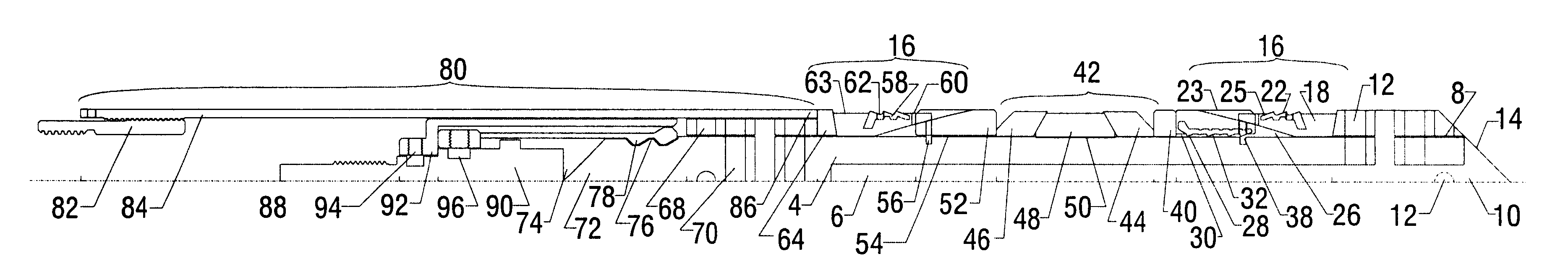
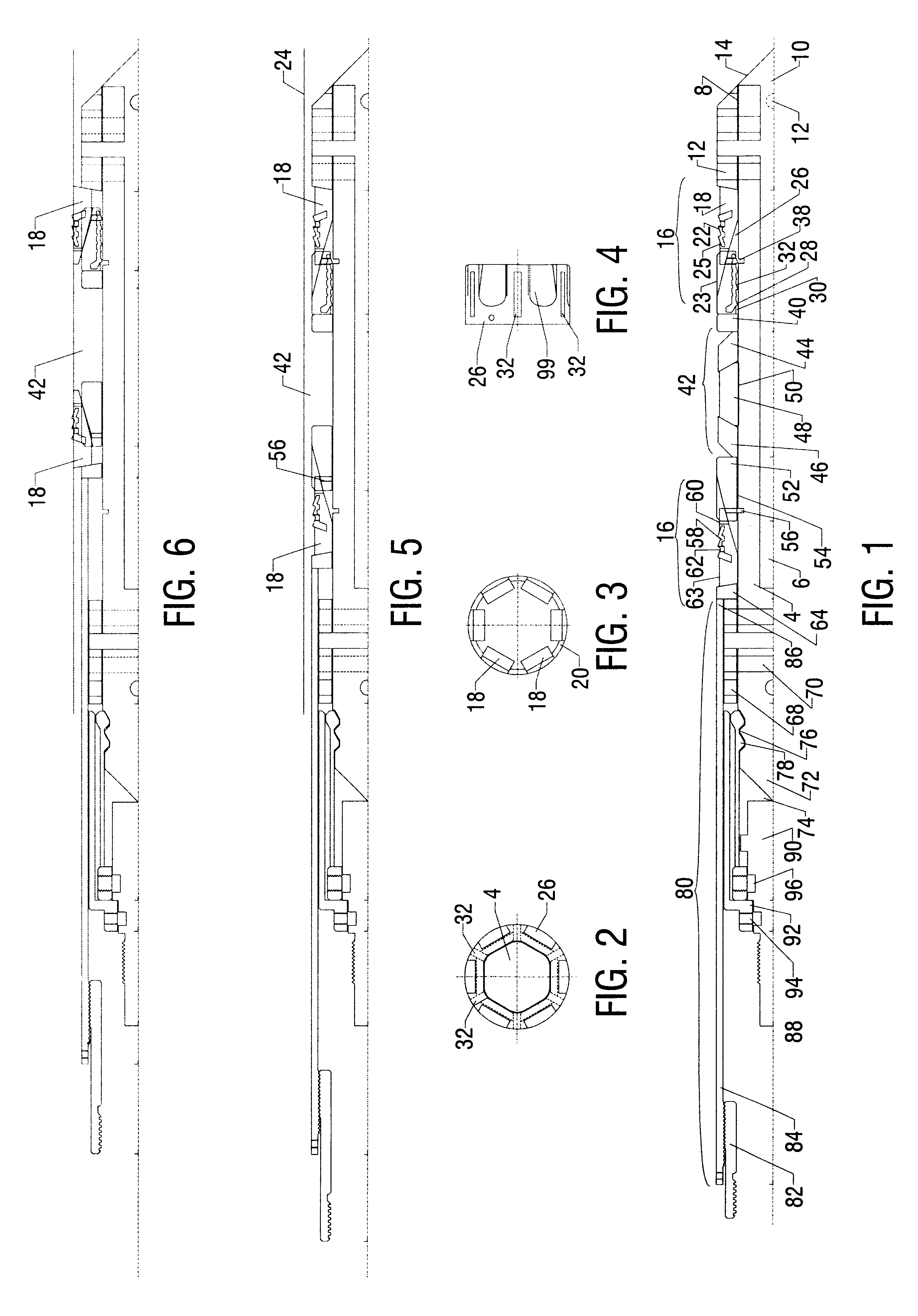
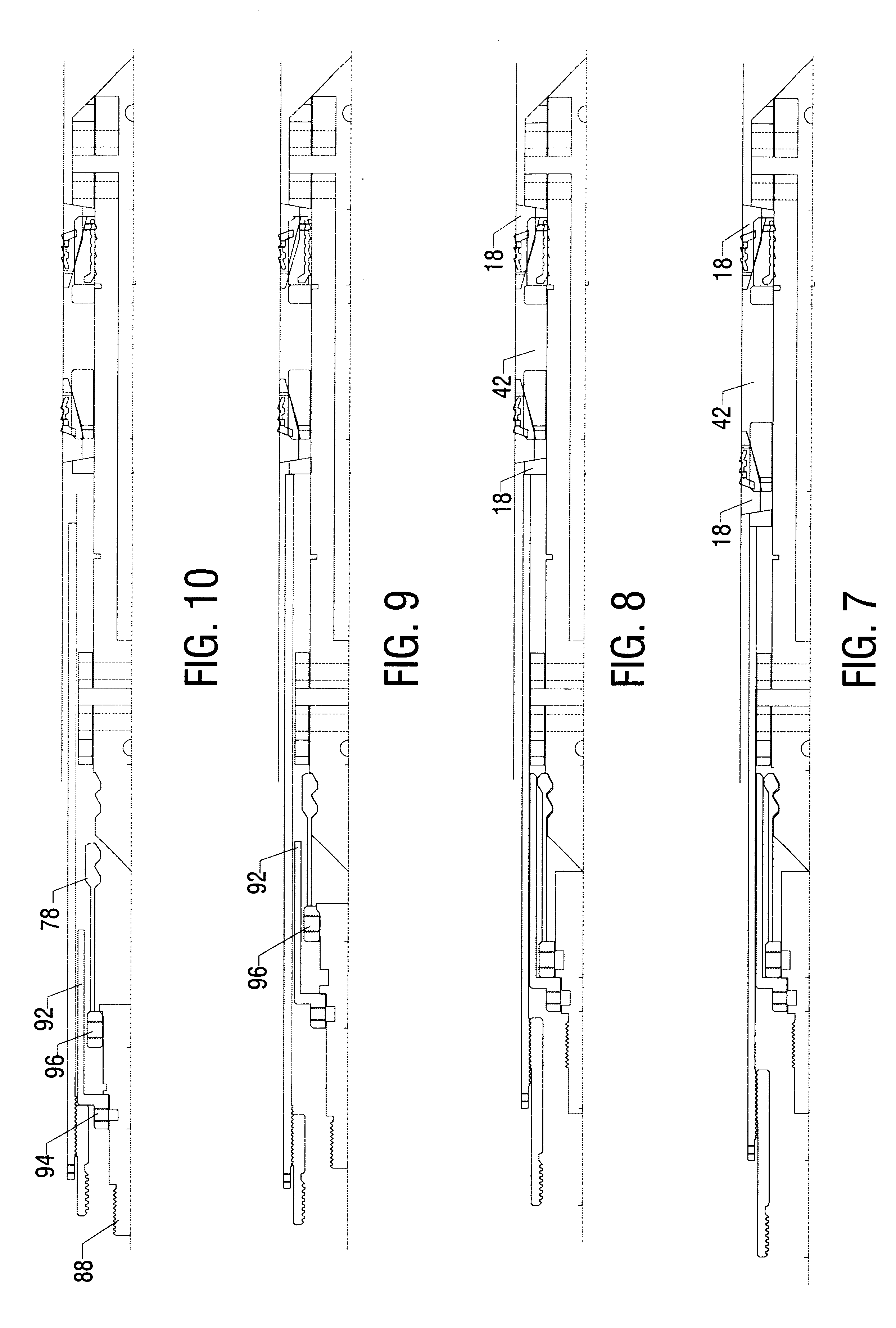
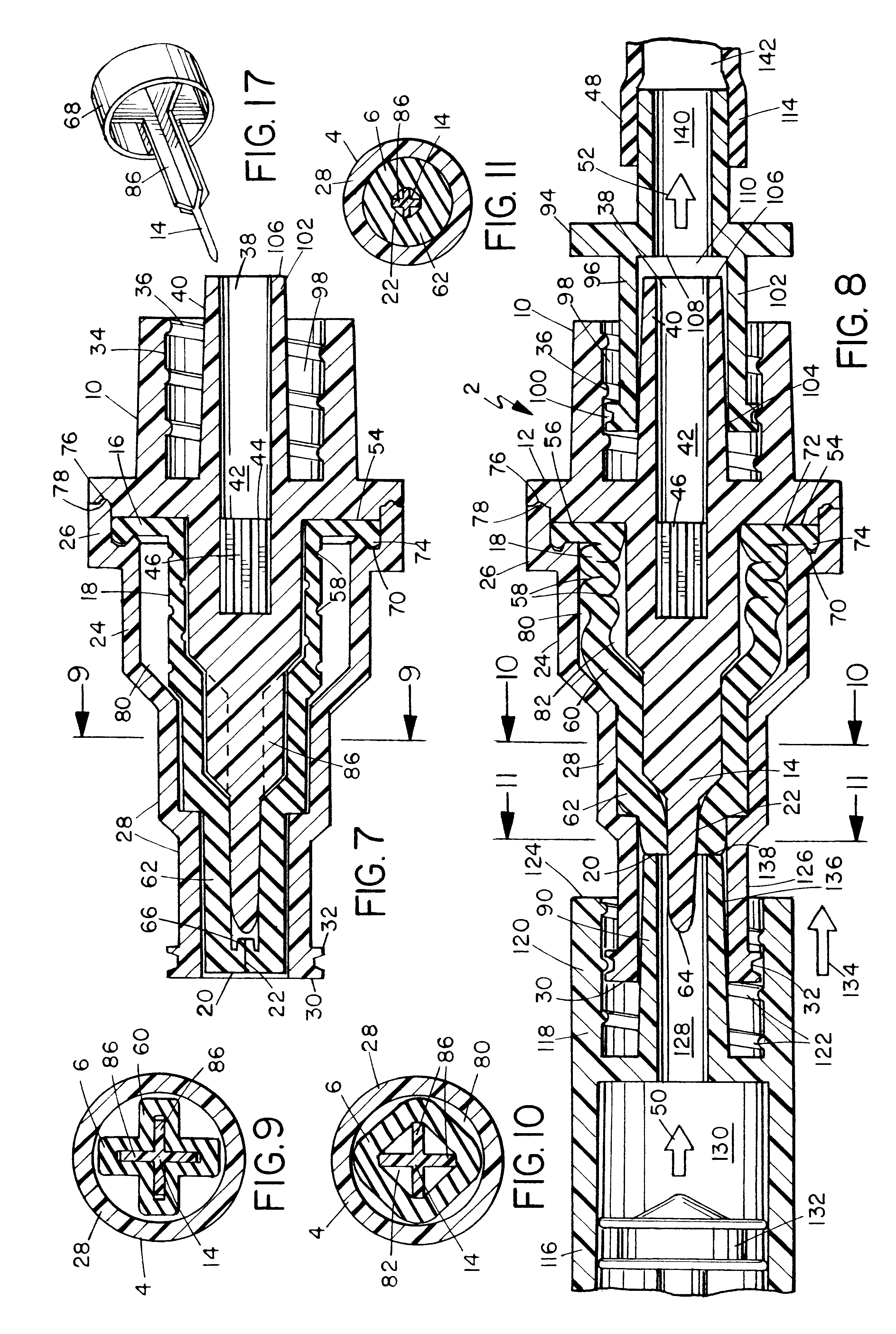
Electrosurgical system and method
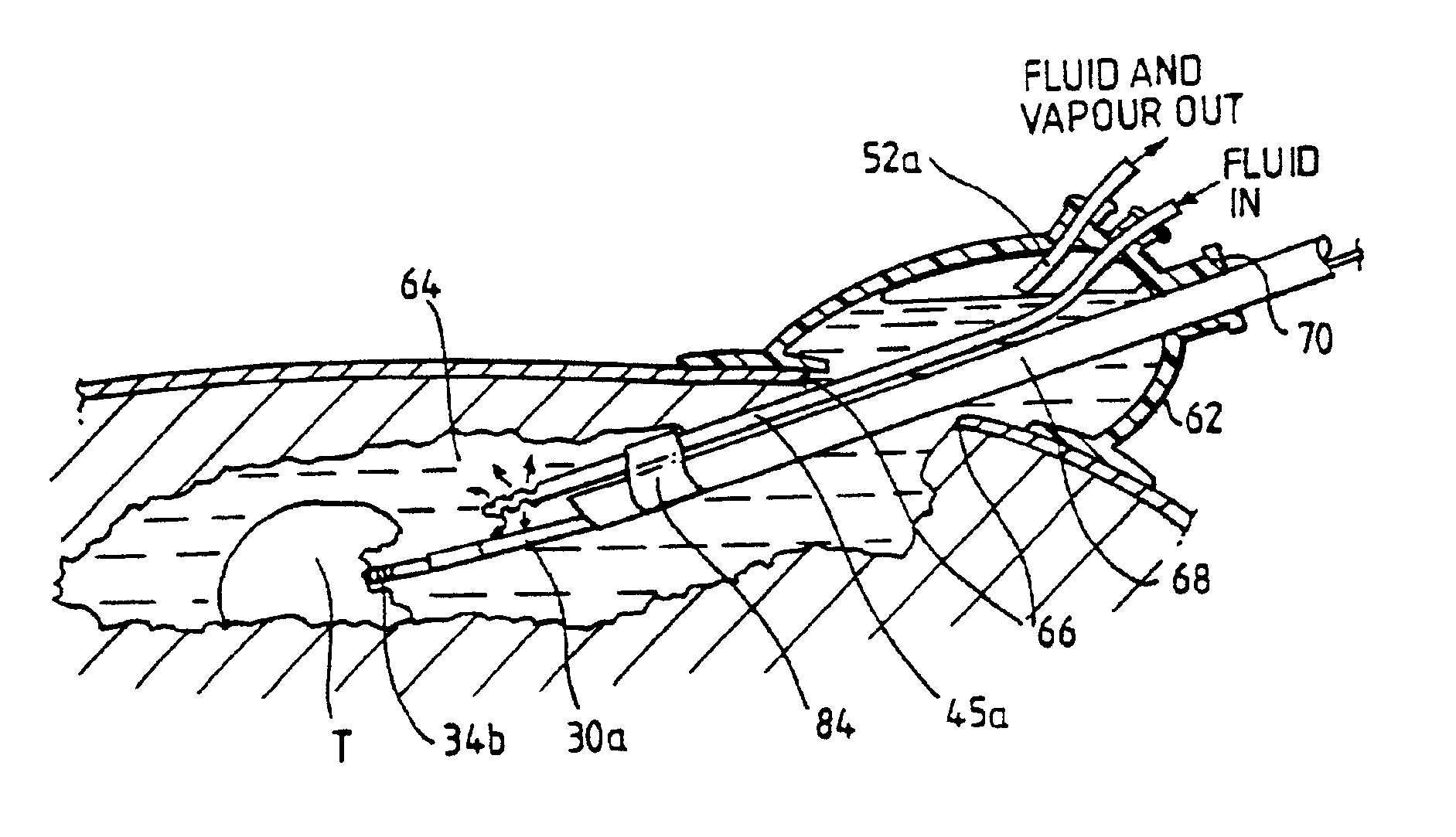
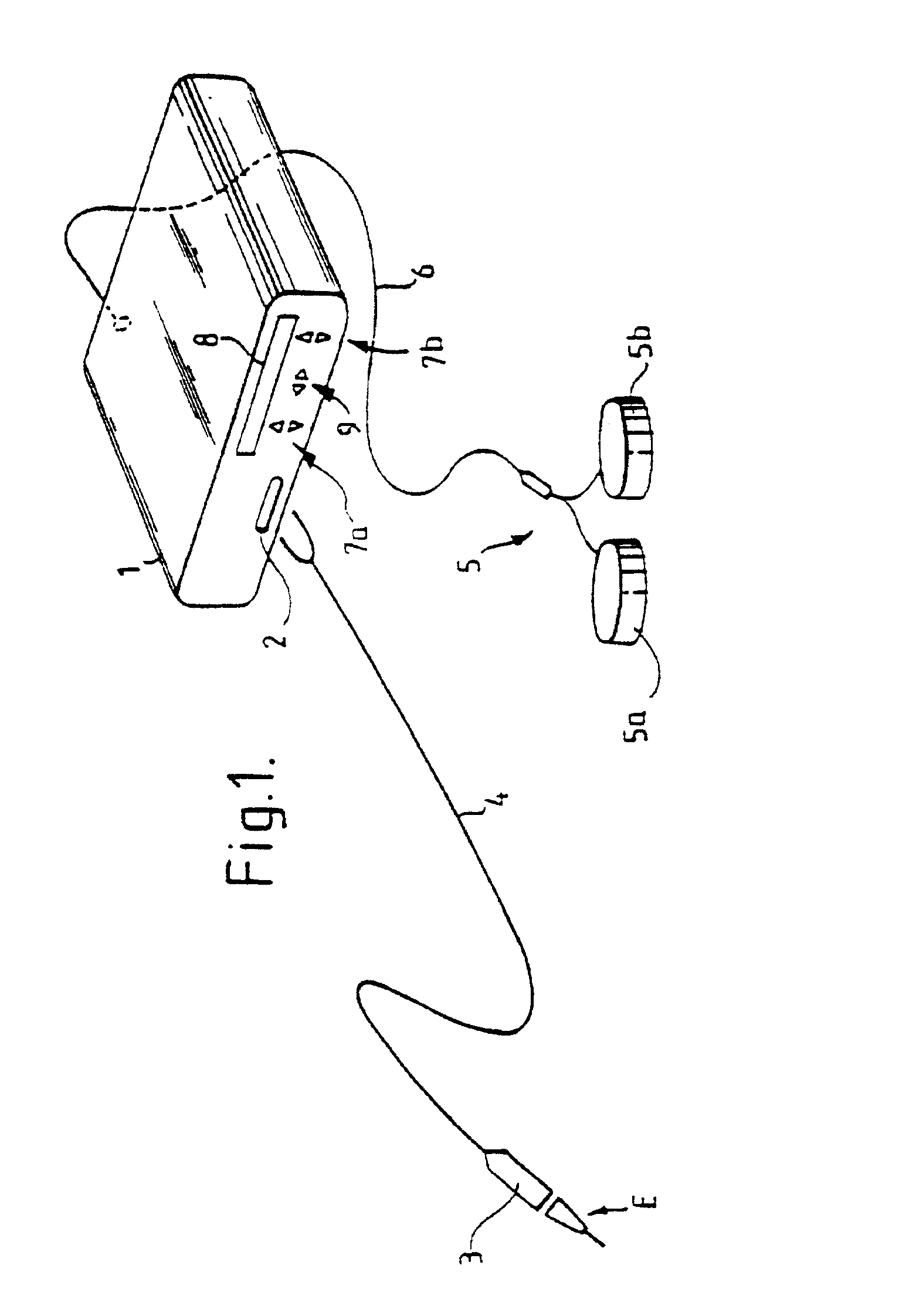
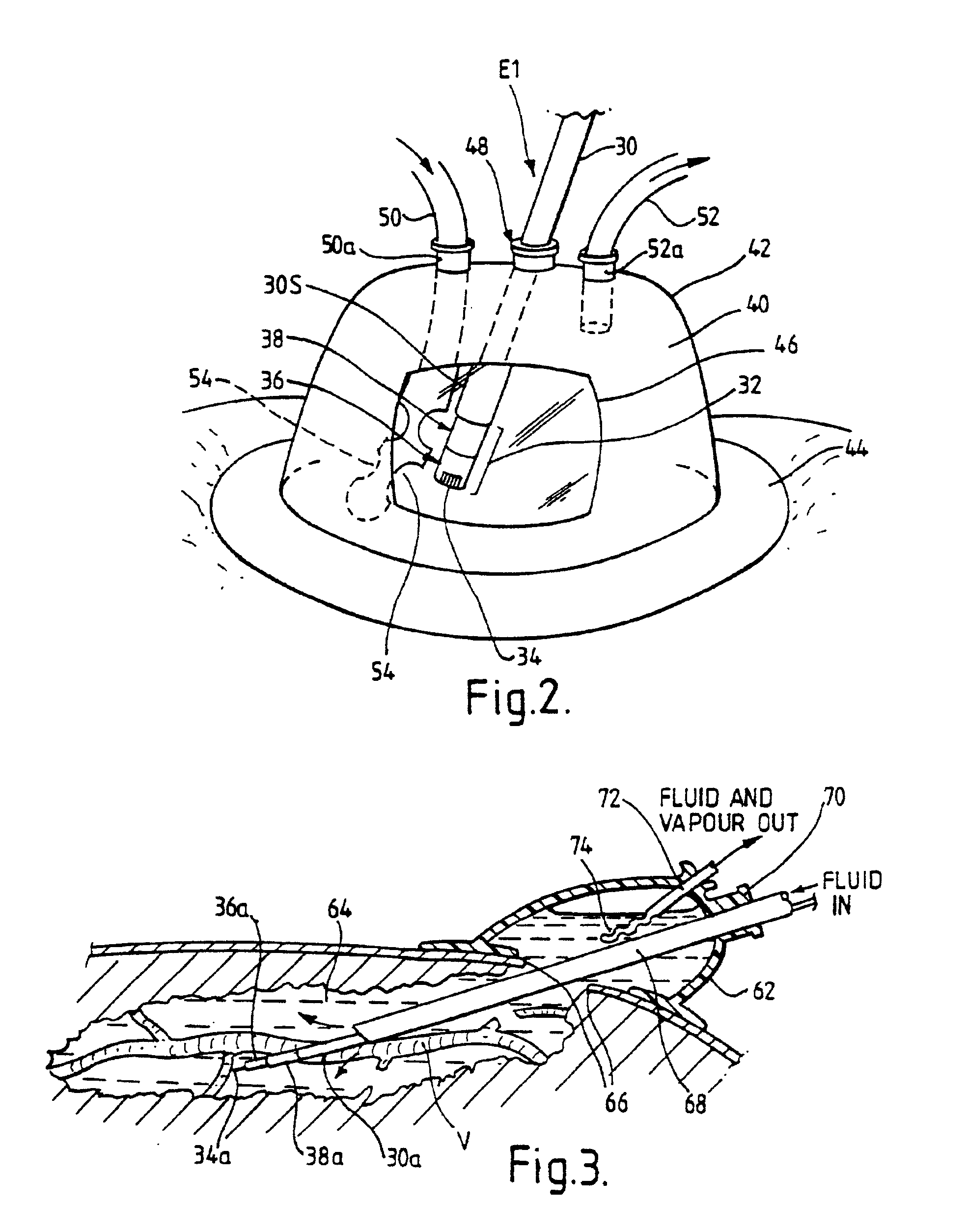
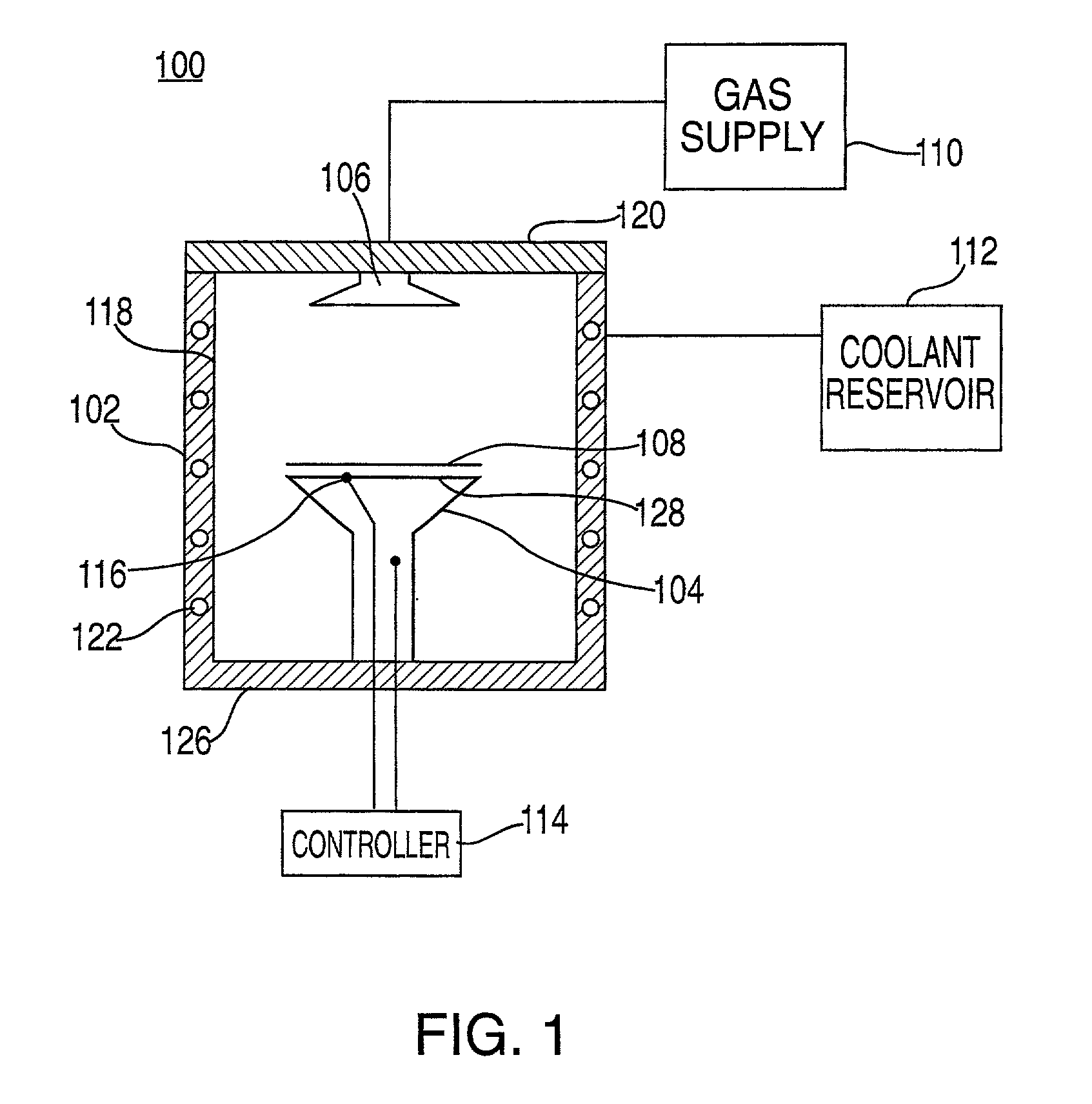
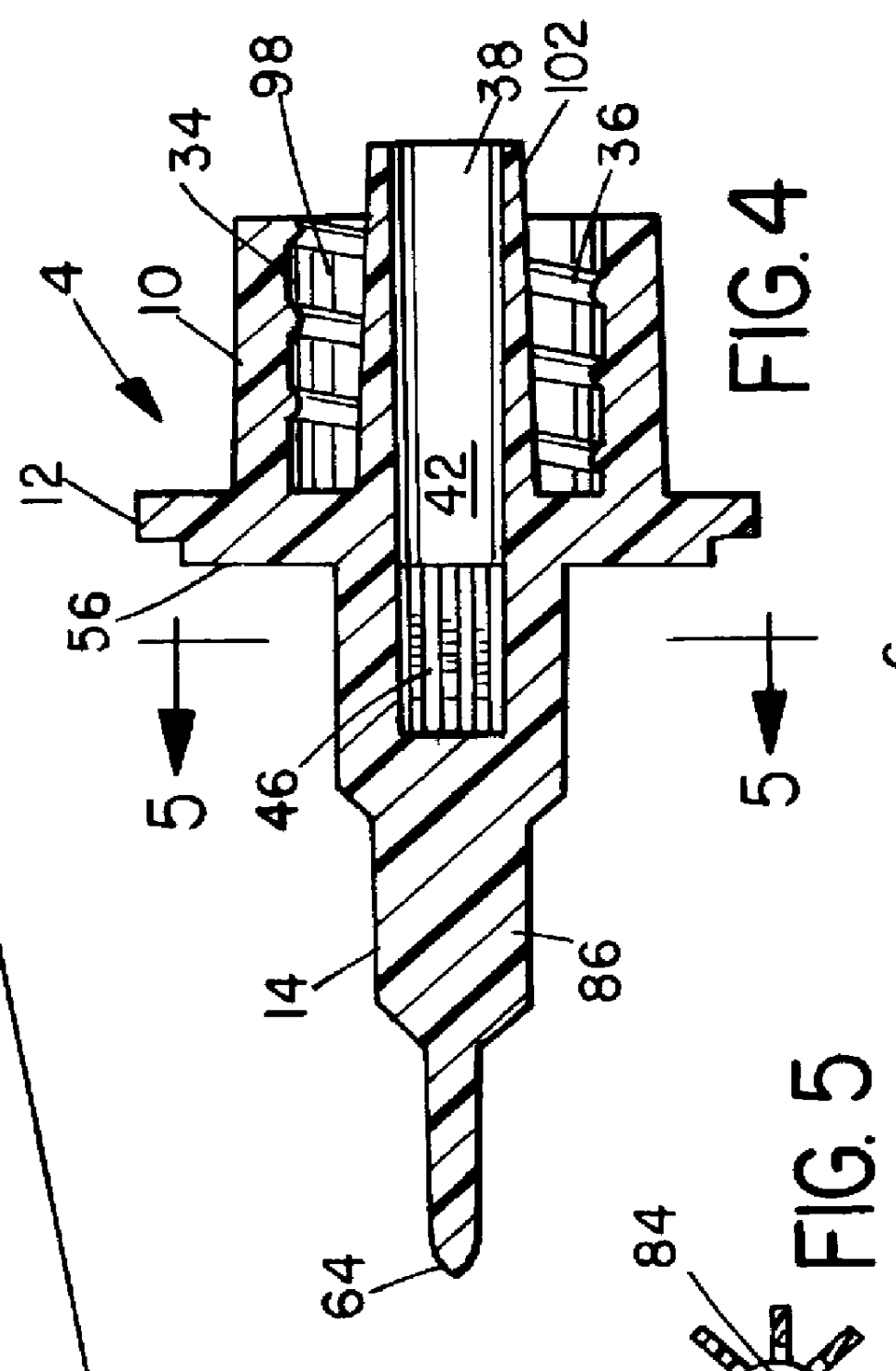
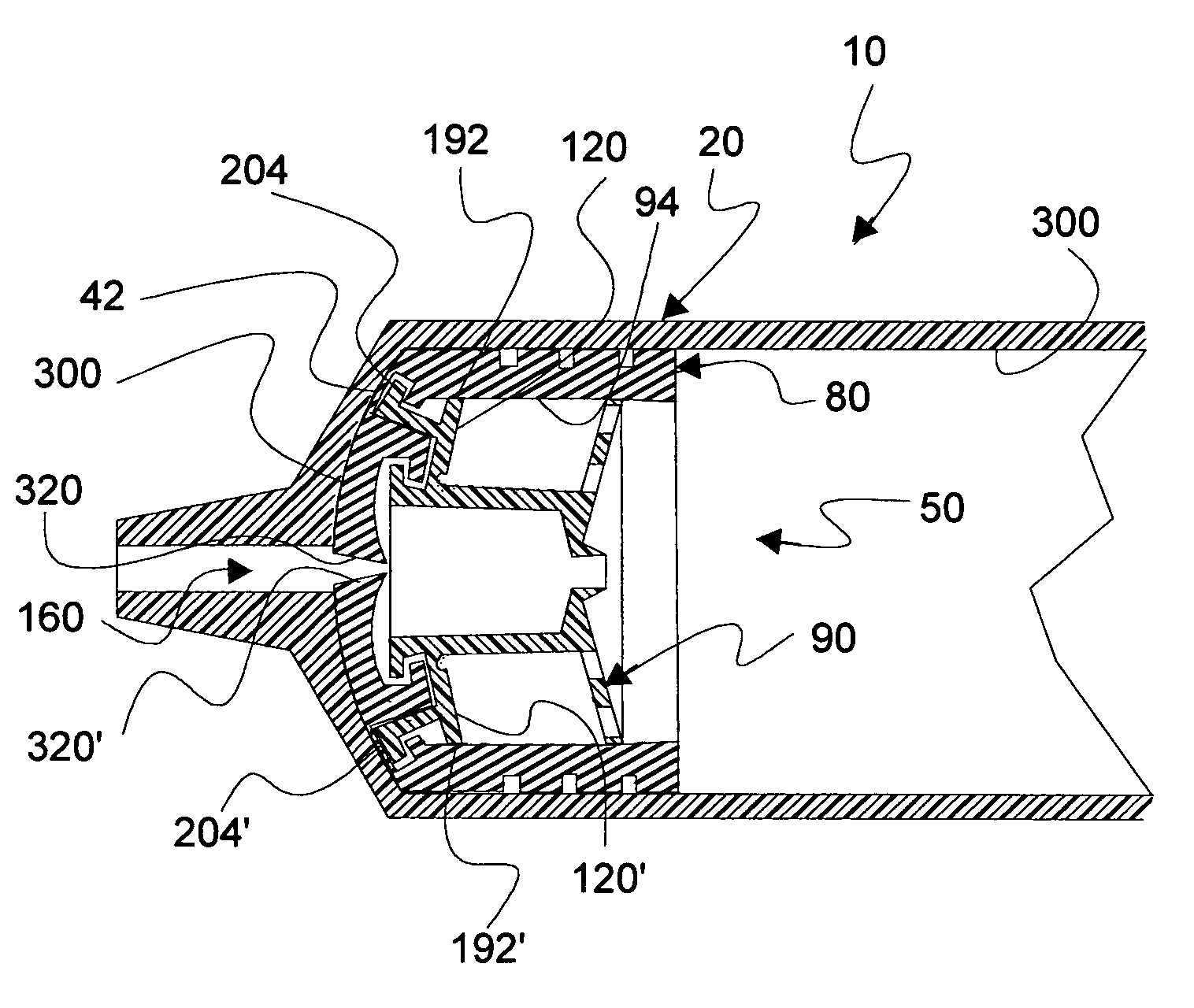
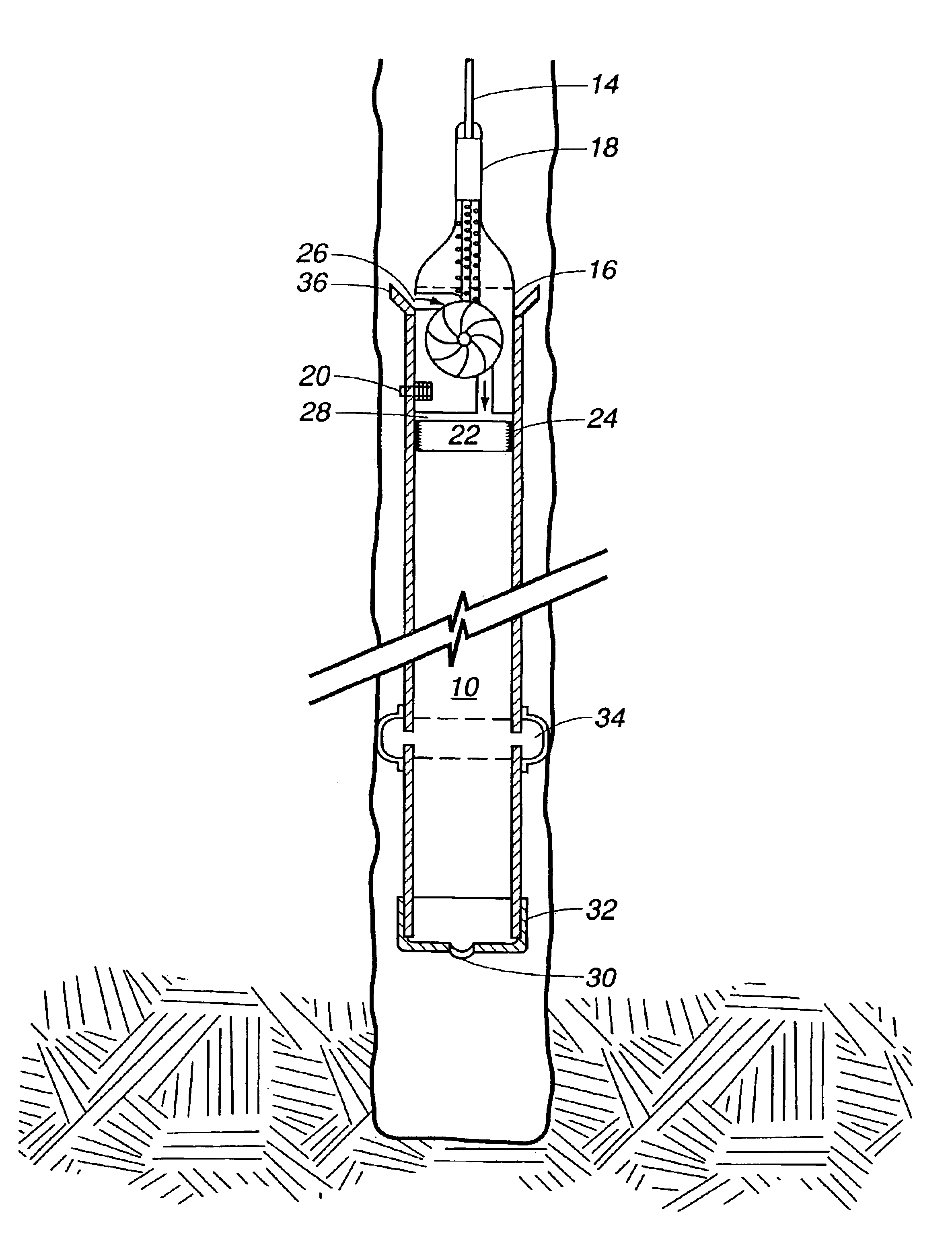
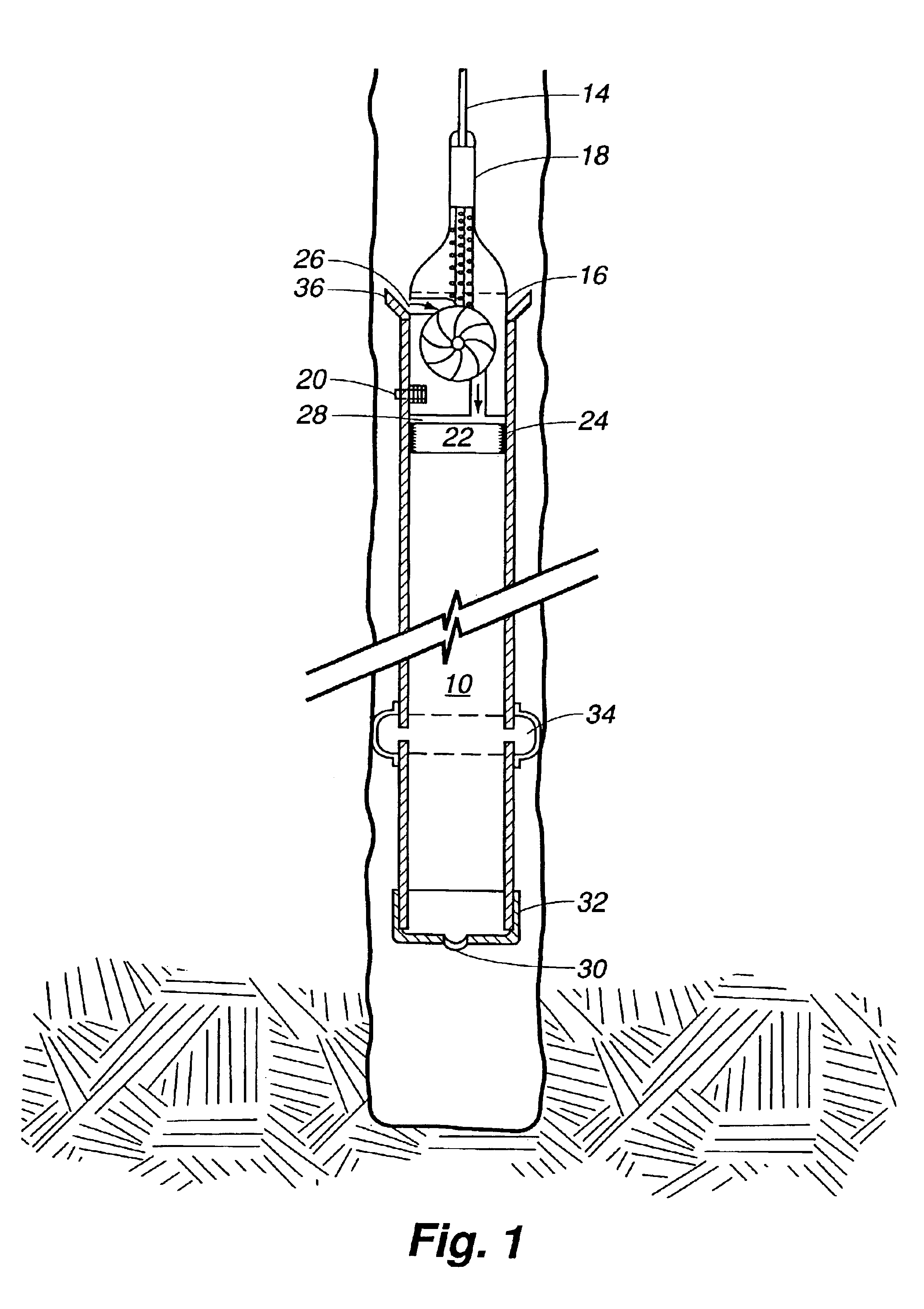
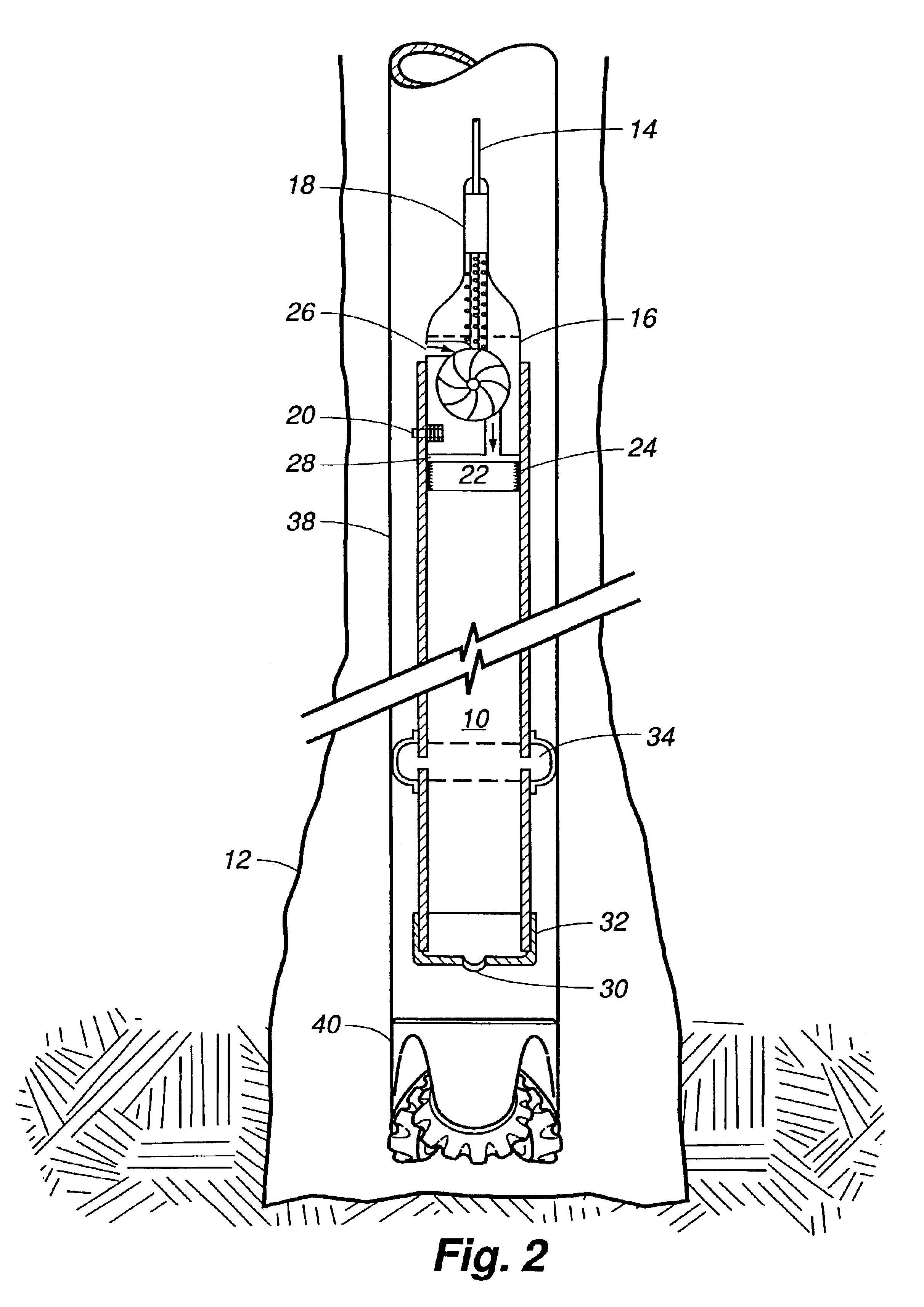
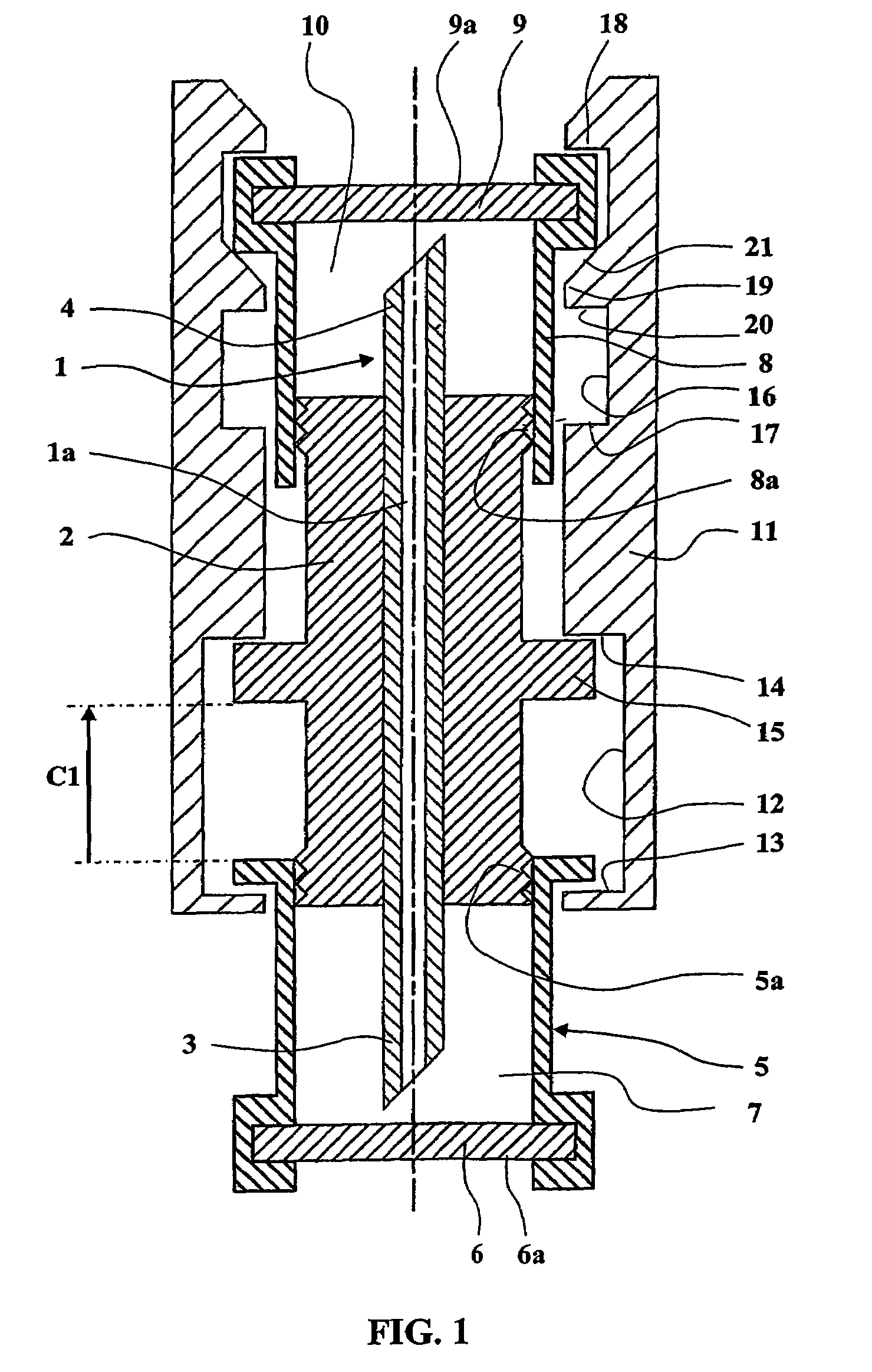
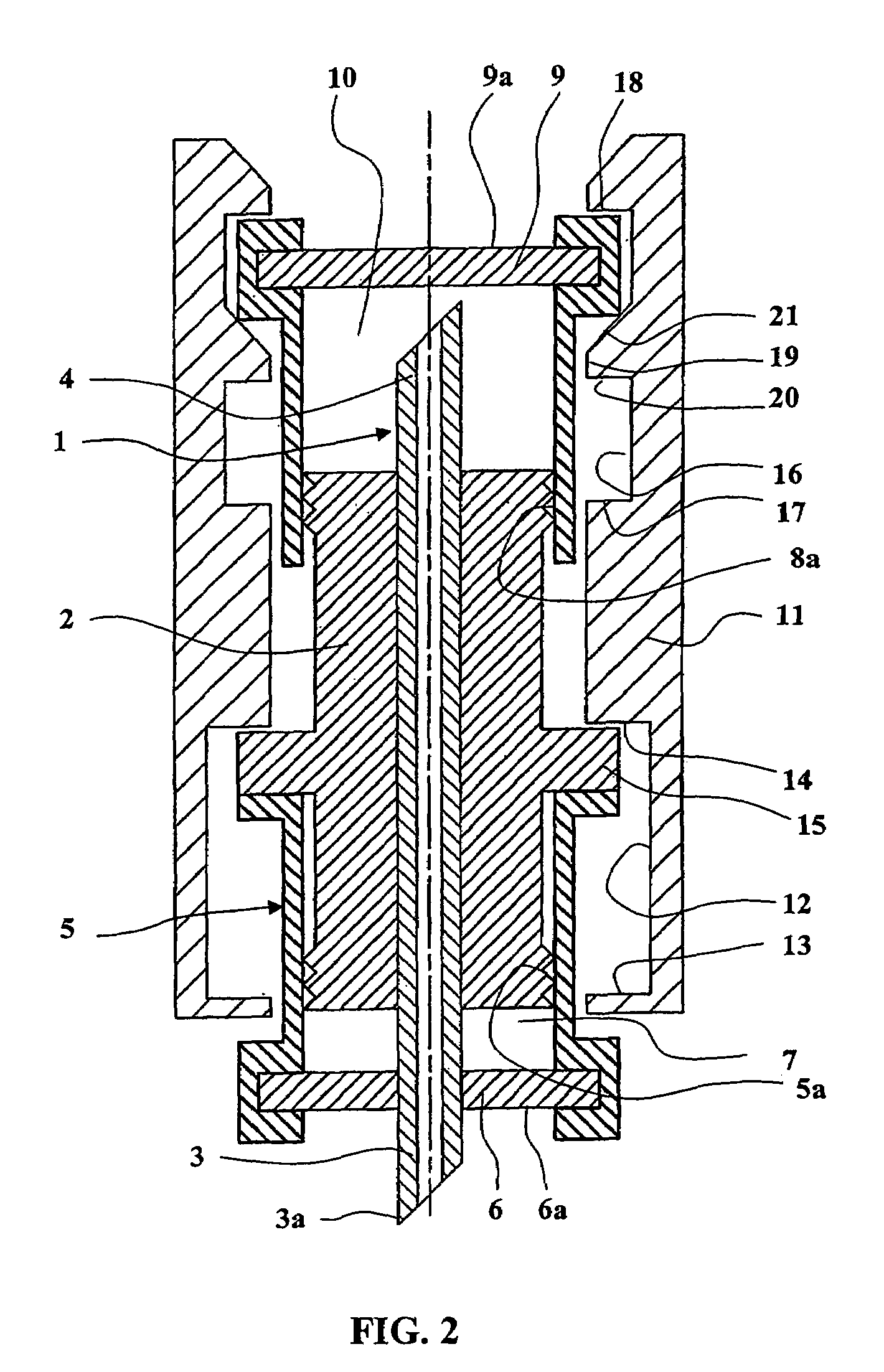
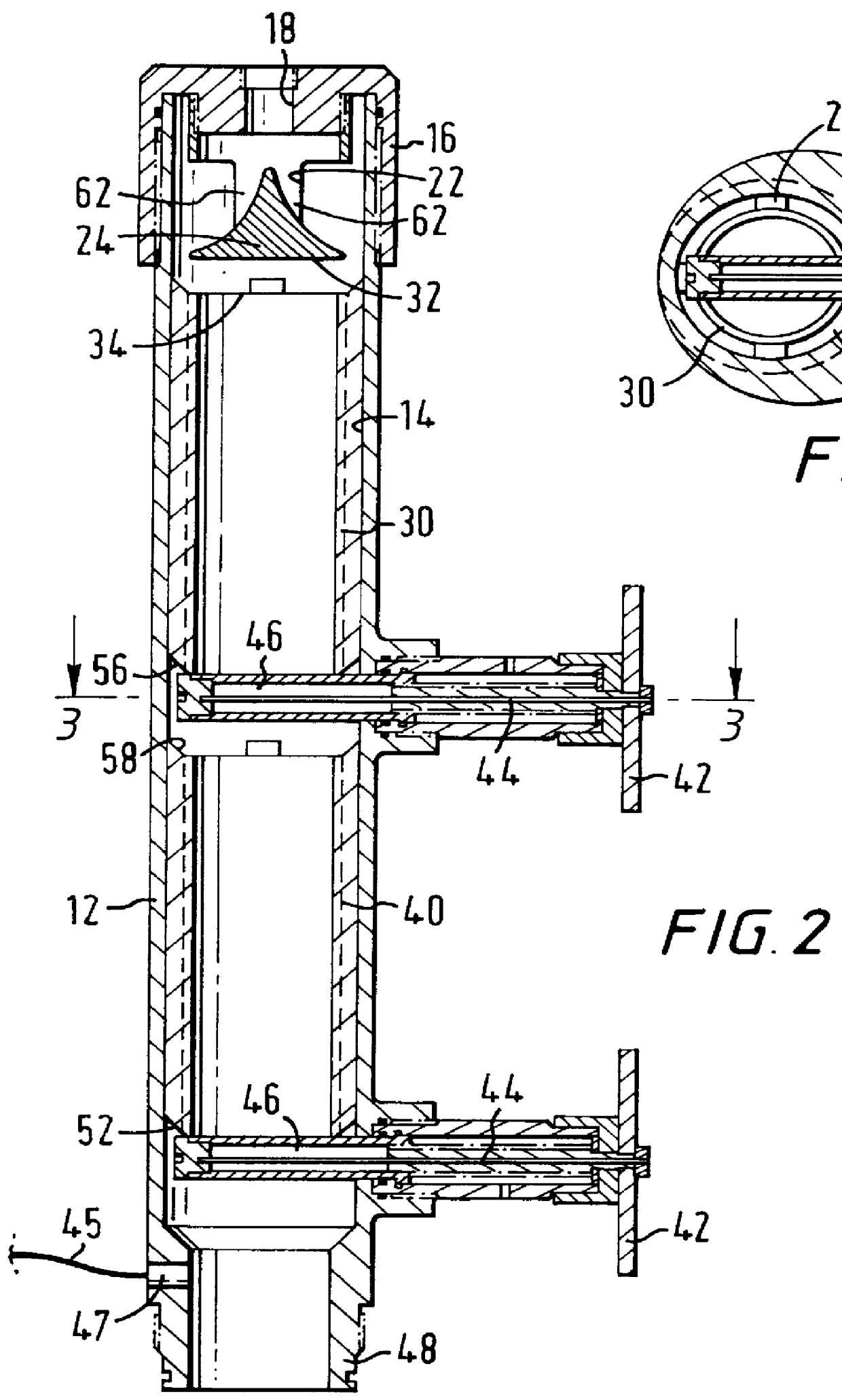
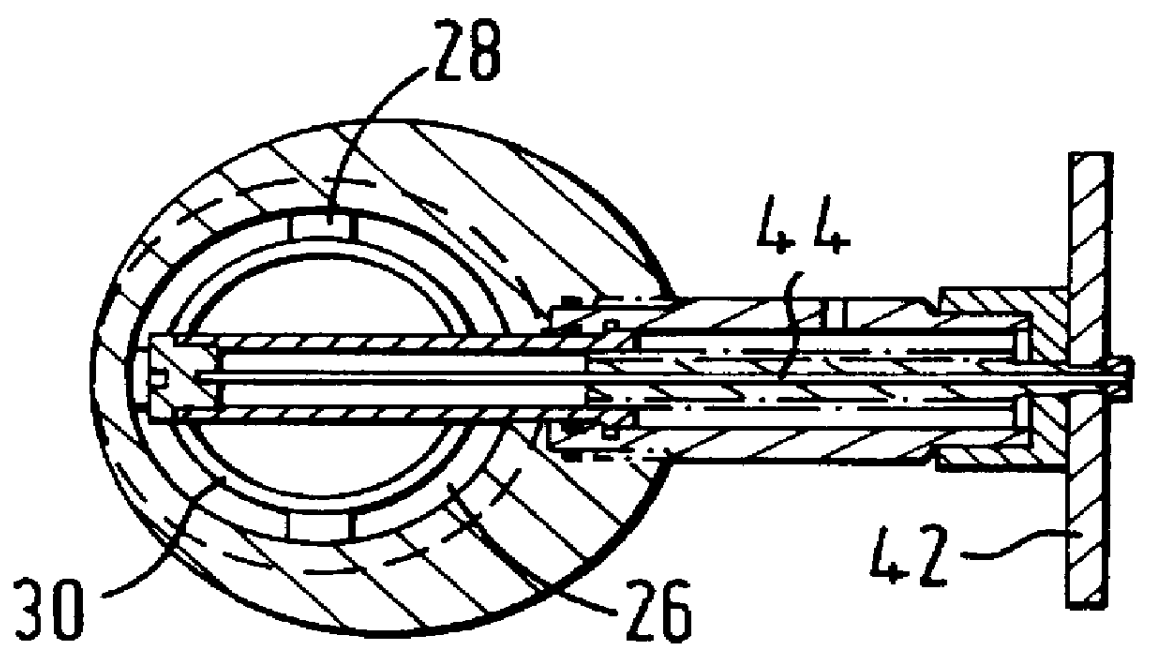
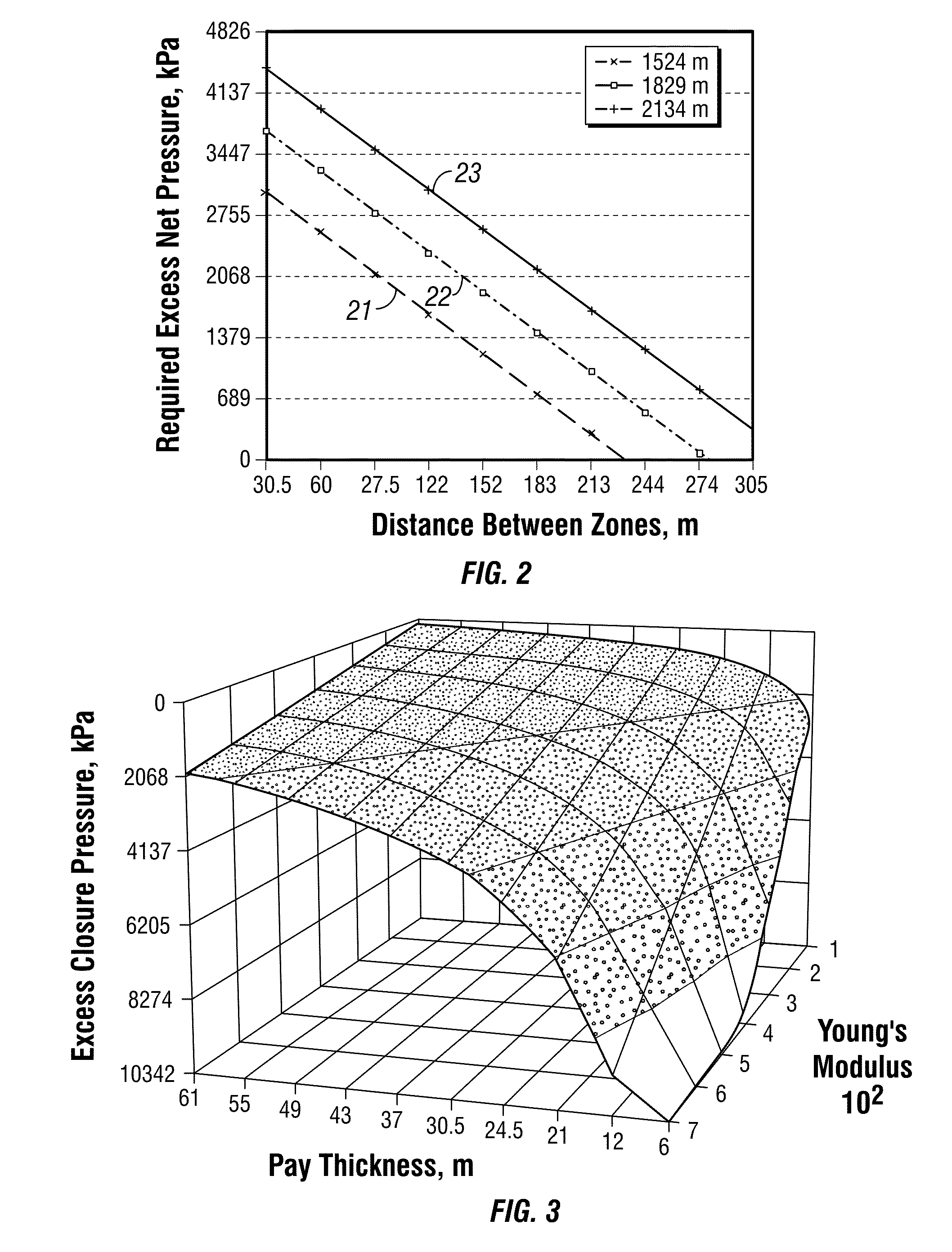
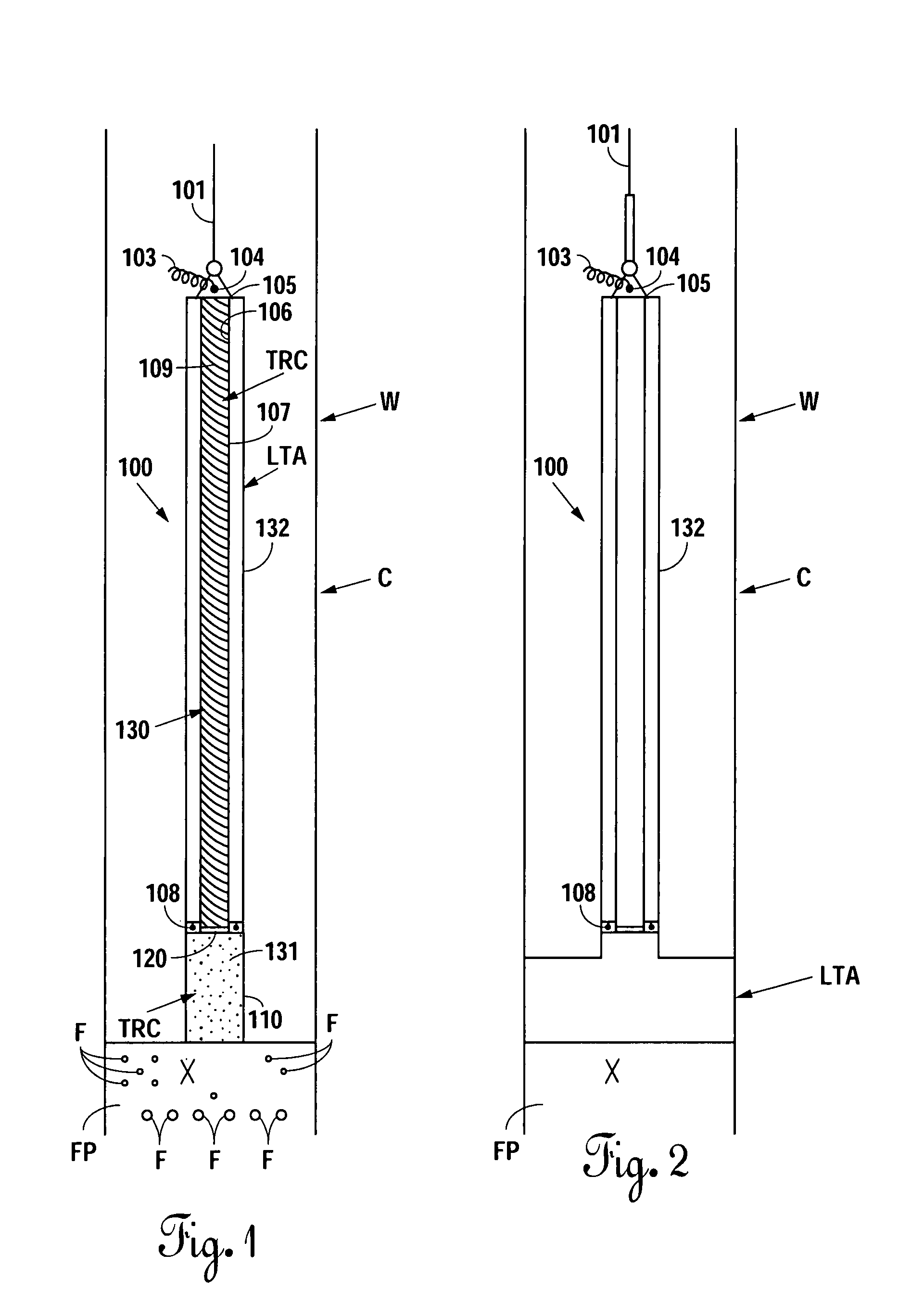
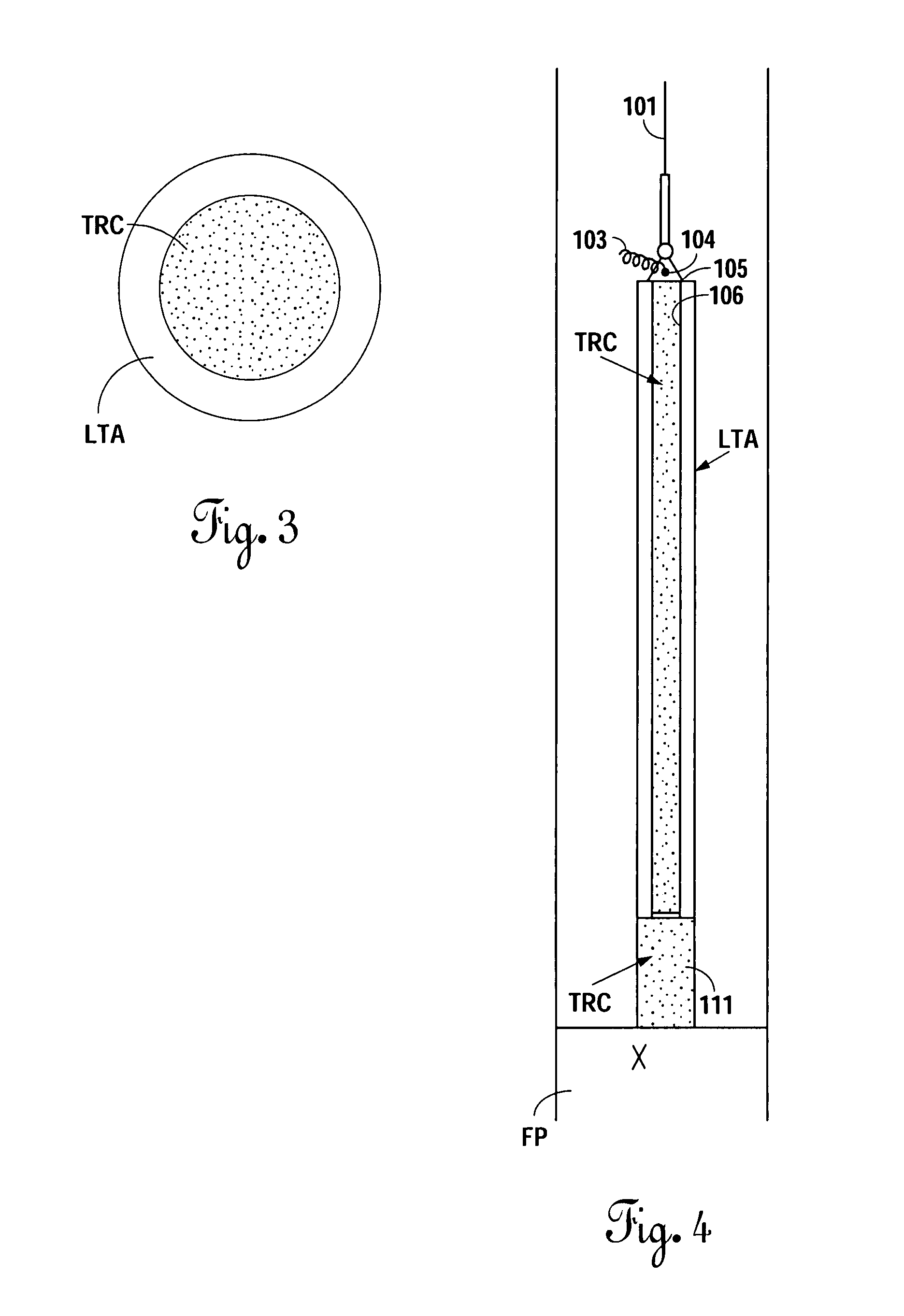
An electrosurgical system comprises a radio frequency generator (1), an electrosurgical instrument (E1), and a fluid enclosure (42). The generator (1) has a radio frequency output for delivery of power to the electrosurgical instrument (E1) when immersed in an electrically-conductive fluid. The electrosurgical instrument (E1) has an electrode assembly (32) at the distal end thereof, the electrode assembly comprising a tissue treatment electrode (34), and a return electrode (38) axially spaced therefrom in such a manner as to define, in use, a conductive fluid path that completes an electrical circuit between the tissue treatment electrode and the return electrode. The fluid enclosure (42) is adapted to surround an operation site on the skin of a patient or an incision leading to a cavity surgically created within the patient's body. The fluid enclosure (42) includes sealing means (44) for sealing against the patient's tissue, and the fluid enclosure includes at least one port (50a, 52a) through which the electrosurgical (E1) is insertable, and through which the electrically-conductive fluid can enter and / or leave the enclosure. The fluid enclosure device of the present invention can also be used to treat tumours within the colon. The enclosure, which includes a proximal and a distal bung, is inserted into the colon in a deflated condition and then inflated with a conductive fluid or gas. The colon can be supported against the pressure of the fluid or gas with a pressure sleeve that has been inserted to surround the region of the colon being treated. An electrosurgical instrument is then inserted into the colon and manipulated to vaporize the tumor.
Owner:GYRUS MEDICAL LTD
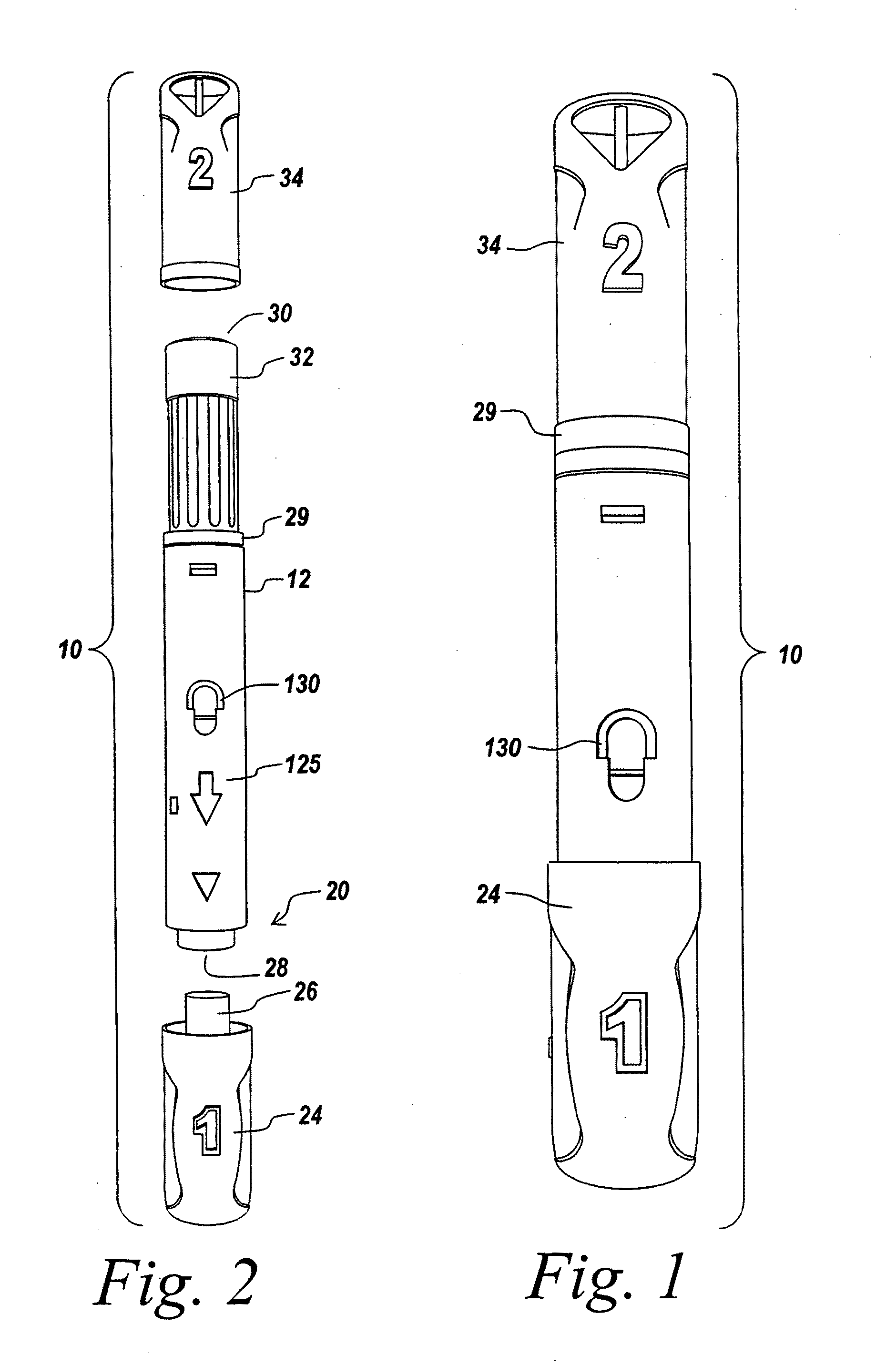
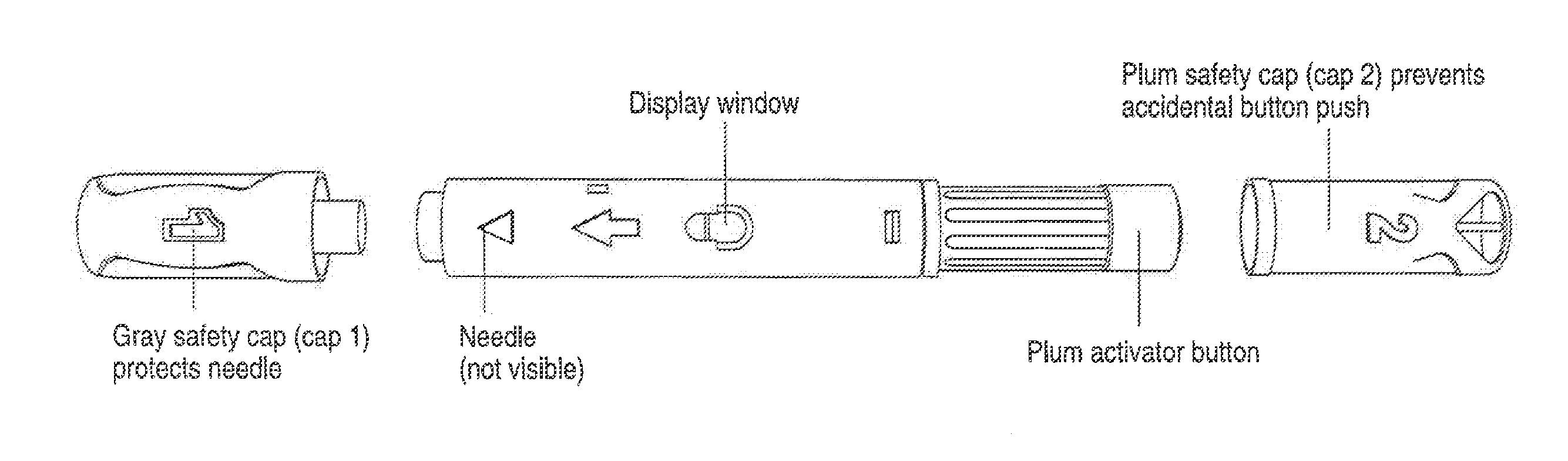
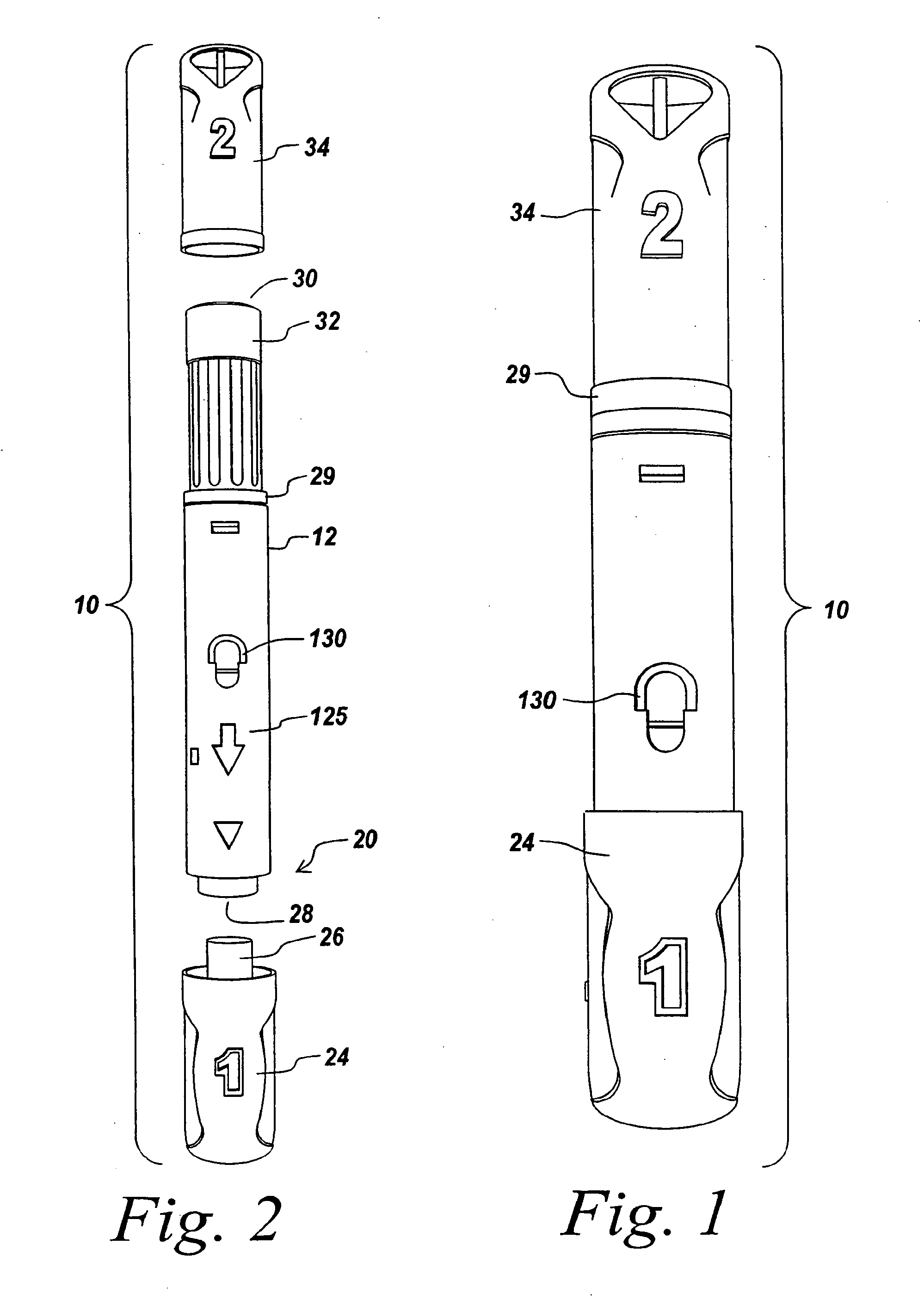
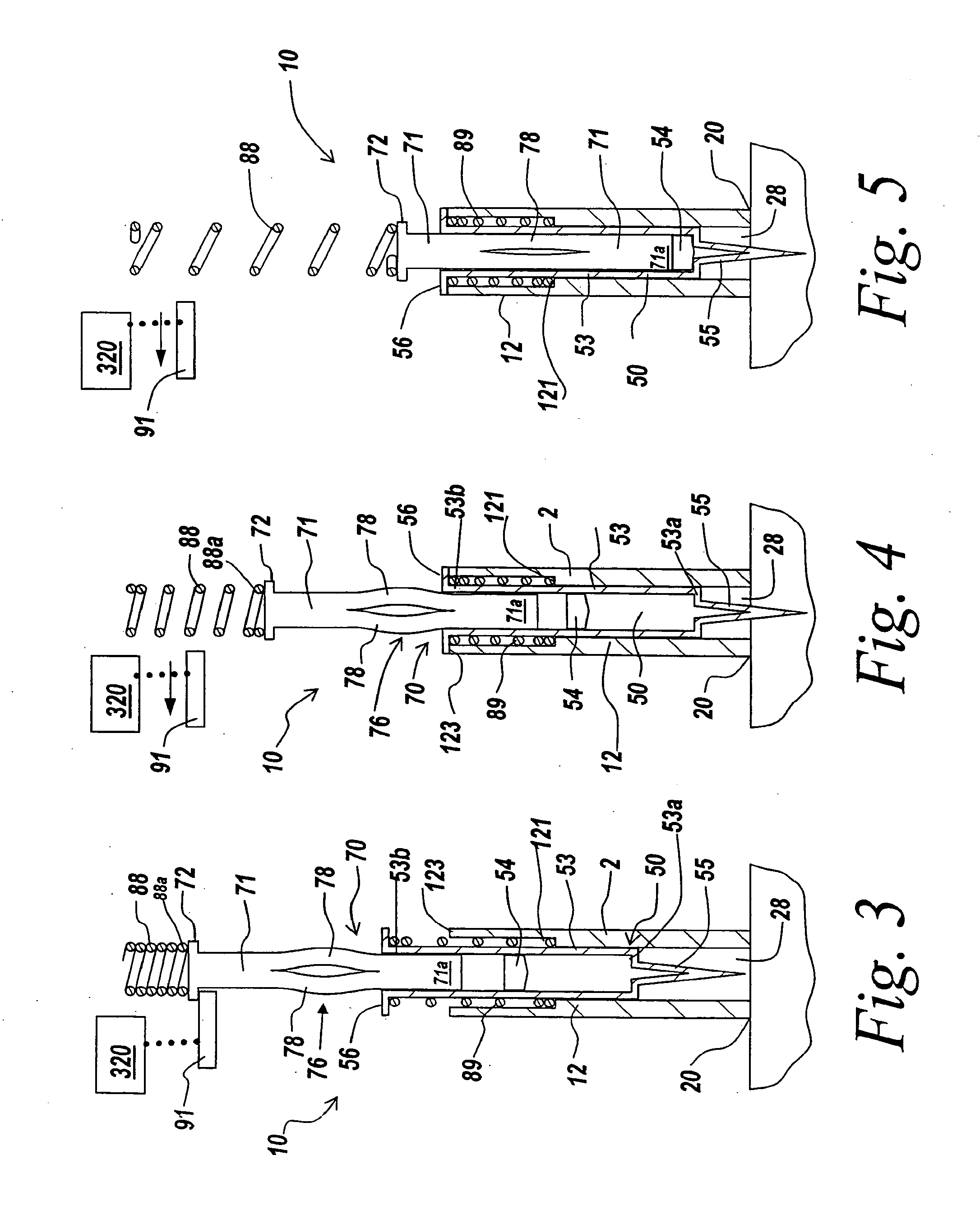
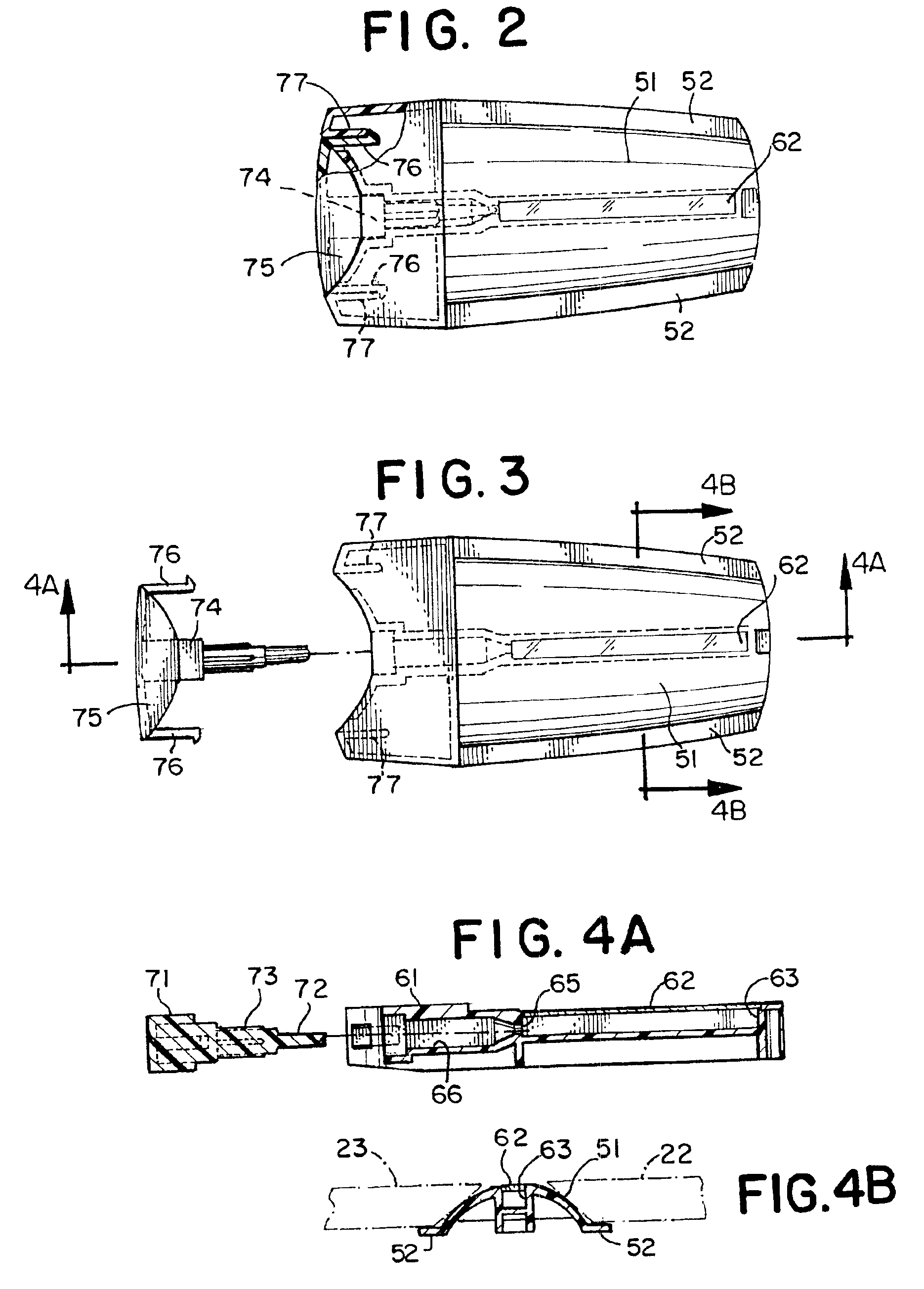
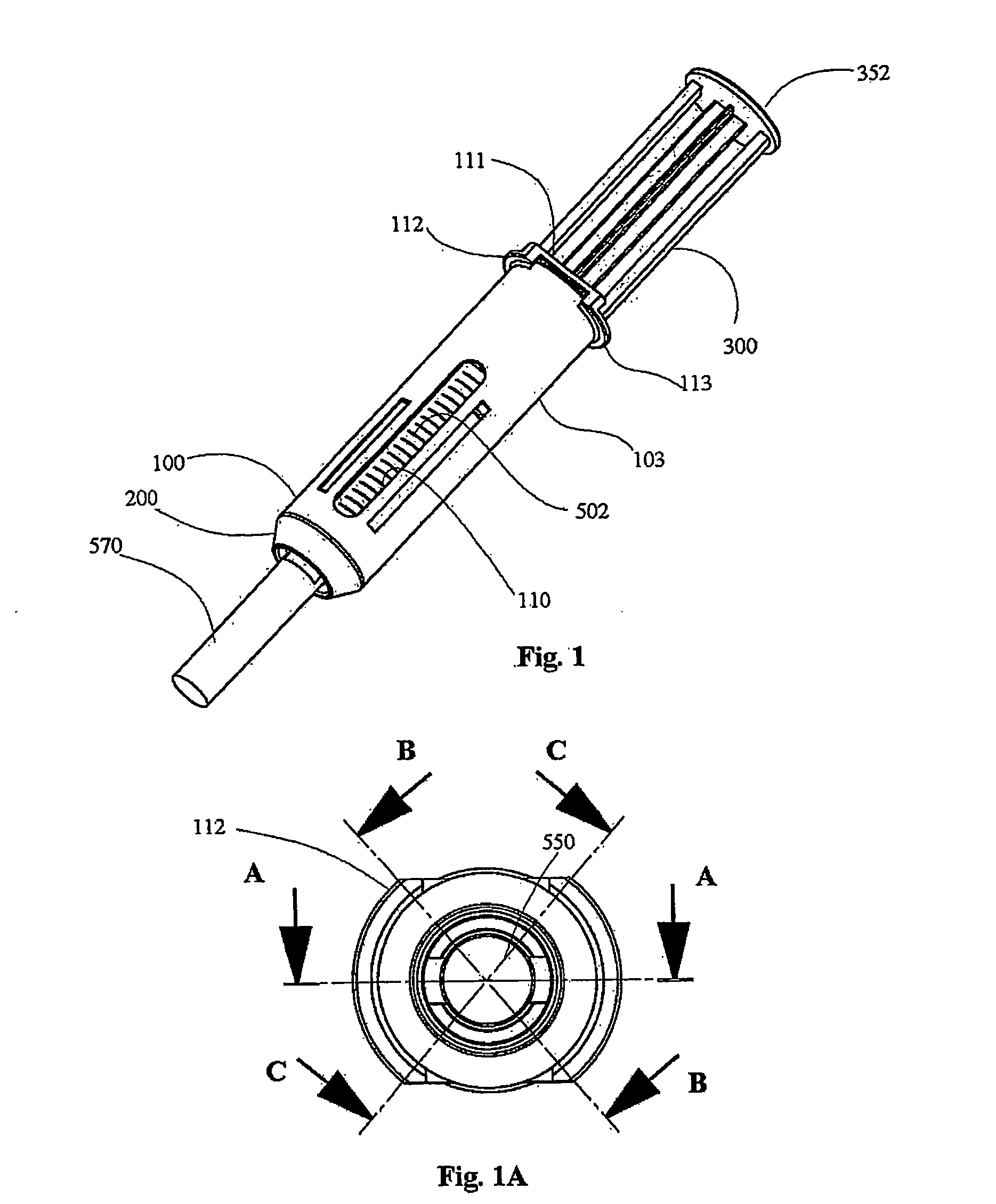
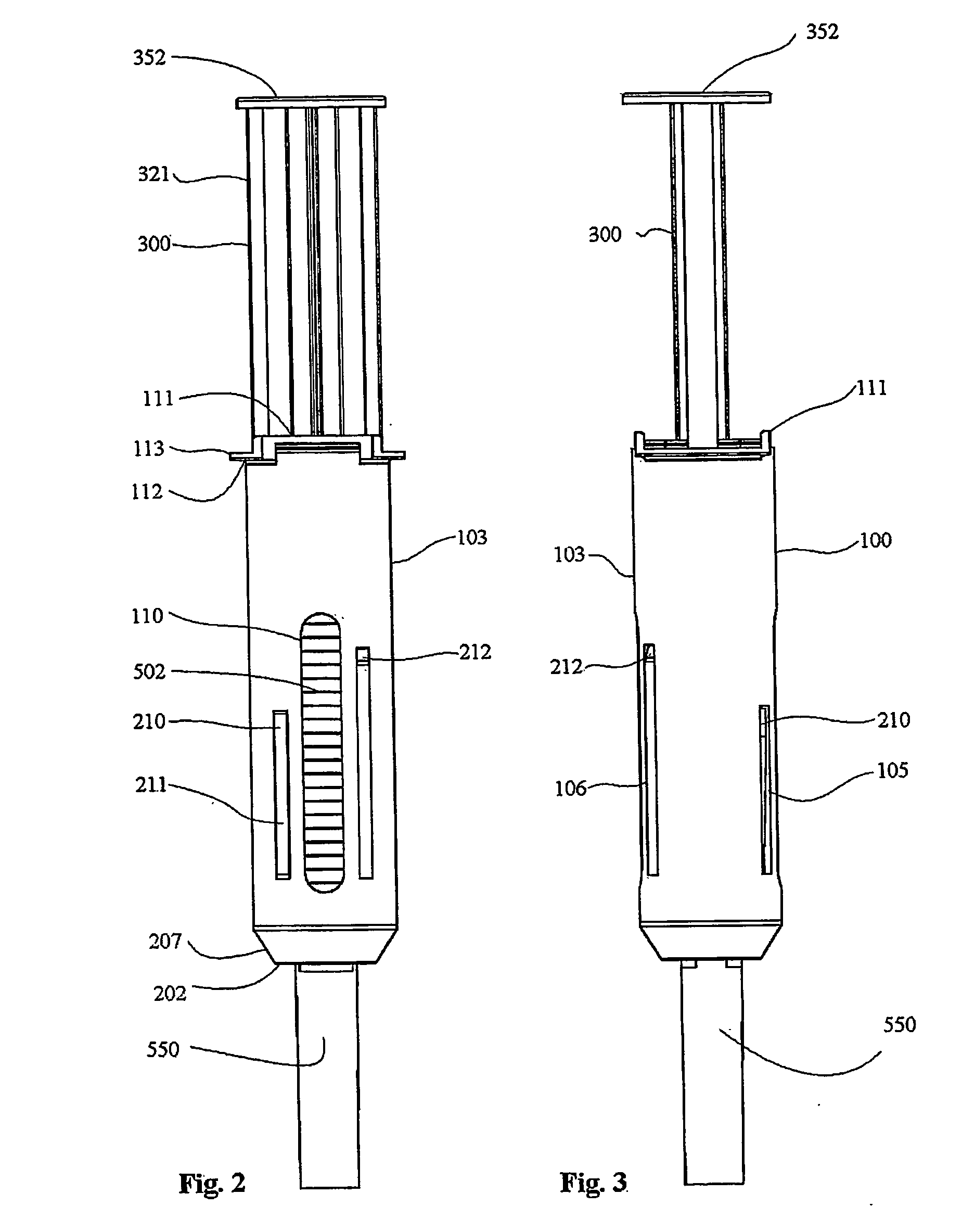
Automatic injection device
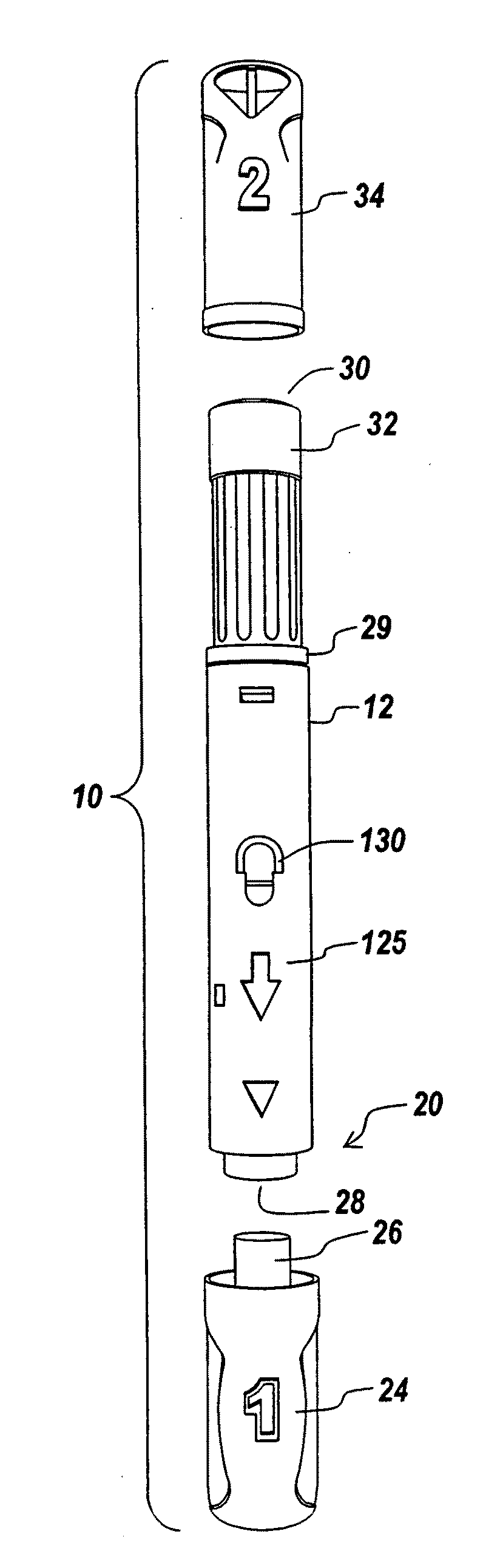
ActiveUS20100160894A1Easy to useReduce anxietyPeptide/protein ingredientsAntipyreticHypodermoclysisSubcutaneous injection
The invention provides an automatic injection device for providing a subcutaneous injection of a substance into a user, comprising: a housing having an open first end and a second end; a syringe movably disposed in the housing, the syringe including a barrel portion for holding the substance, a hollow needle in fluid communication with the barrel portion for ejecting the substance from the syringe, and a bung for sealing the barrel portion and selectively applying pressure to the substance to force the substance through the hollow needle; a plunger for first moving the syringe towards the first end such that the needle projects from the first end and subsequently applying pressure to the bung, the plunger including a rod connected at a first end to the bung, a compressible expanded central portion and a flange between a second end of the rod and the compressible expanded central portion; and a biasing mechanism for biasing the plunger towards the first open end of the housing, the biasing mechanism disposed about the second end of the rod between the flange and the second end of the housing. The present invention also provides methods and kits for using an automatic injection device, and methods and kits for promoting an automatic injection device comprising a medication based on advantageous properties of the device as compared to a pre-filled syringe. The invention also provides methods and kits for training a recipient on use of the automatic injection device.
Owner:ABBVIE BIOTECHNOLOGY LTD
Apparatus and methods for sealing a vascular puncture
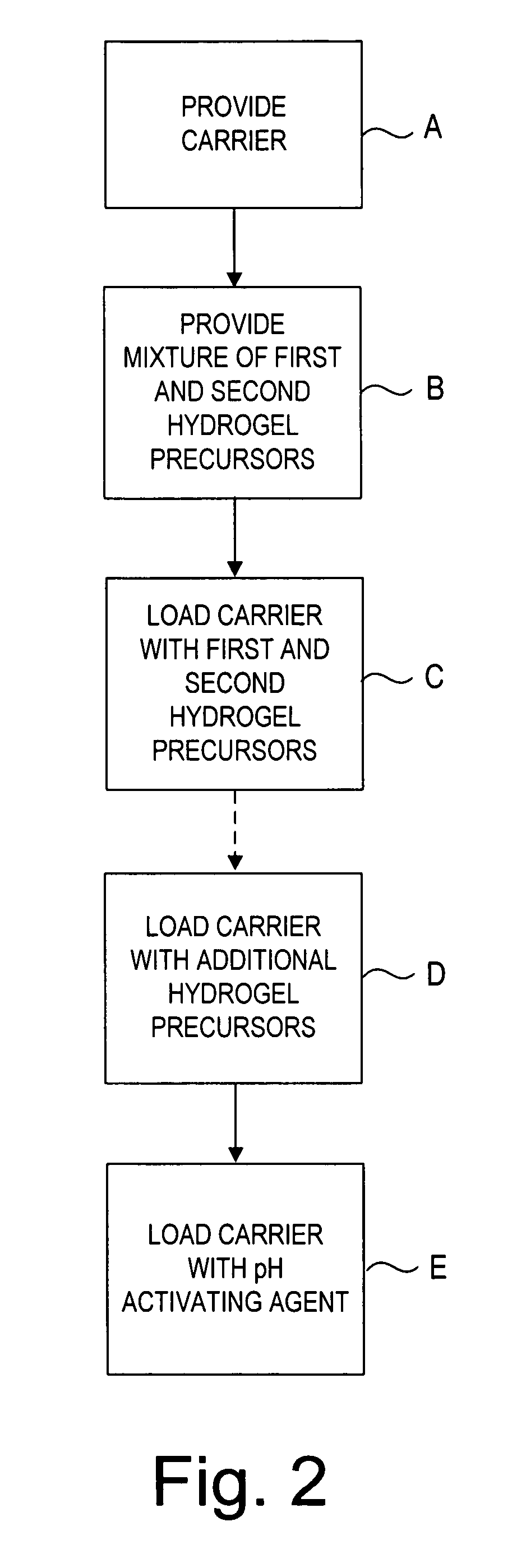
Apparatus for sealing a puncture communicating with a blood vessel includes a porous carrier formed from lyophilized hydrogel or other material. The plug may include at least first and second hydrogel precursors and a pH adjusting agent carried by the porous carrier in an unreactive state prior to exposure to an aqueous physiological environment. Once exposed to bodily fluids, the carrier expands as the lyophilized material hydrates to enhance and facilitate rapid hemostasis of the puncture. When the plug is placed into the puncture, the natural wetting of the plug by bodily fluids (e.g., blood) causes the first and second precursors to react and cross-link into an adhesive or “sticky” hydrogel that aids in retaining the plug in place within the puncture.
Owner:INCEPT LLC
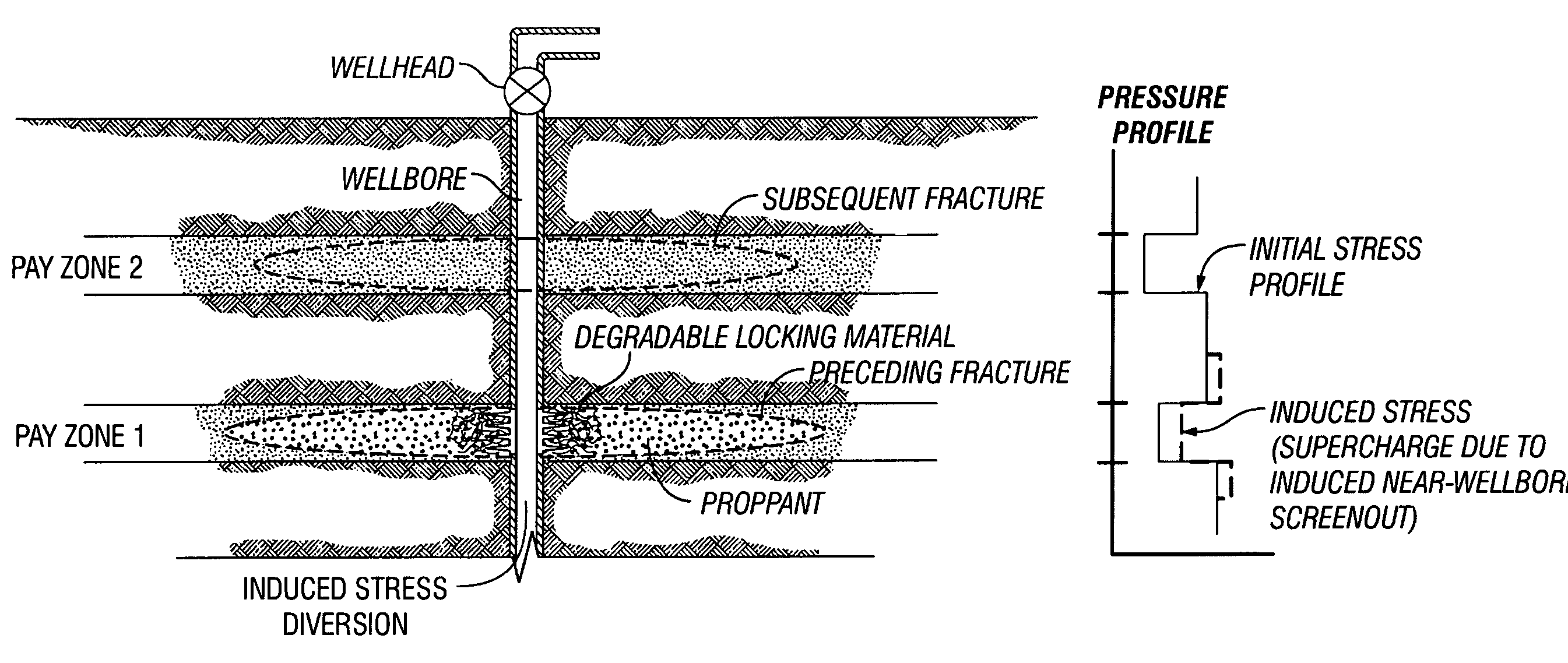
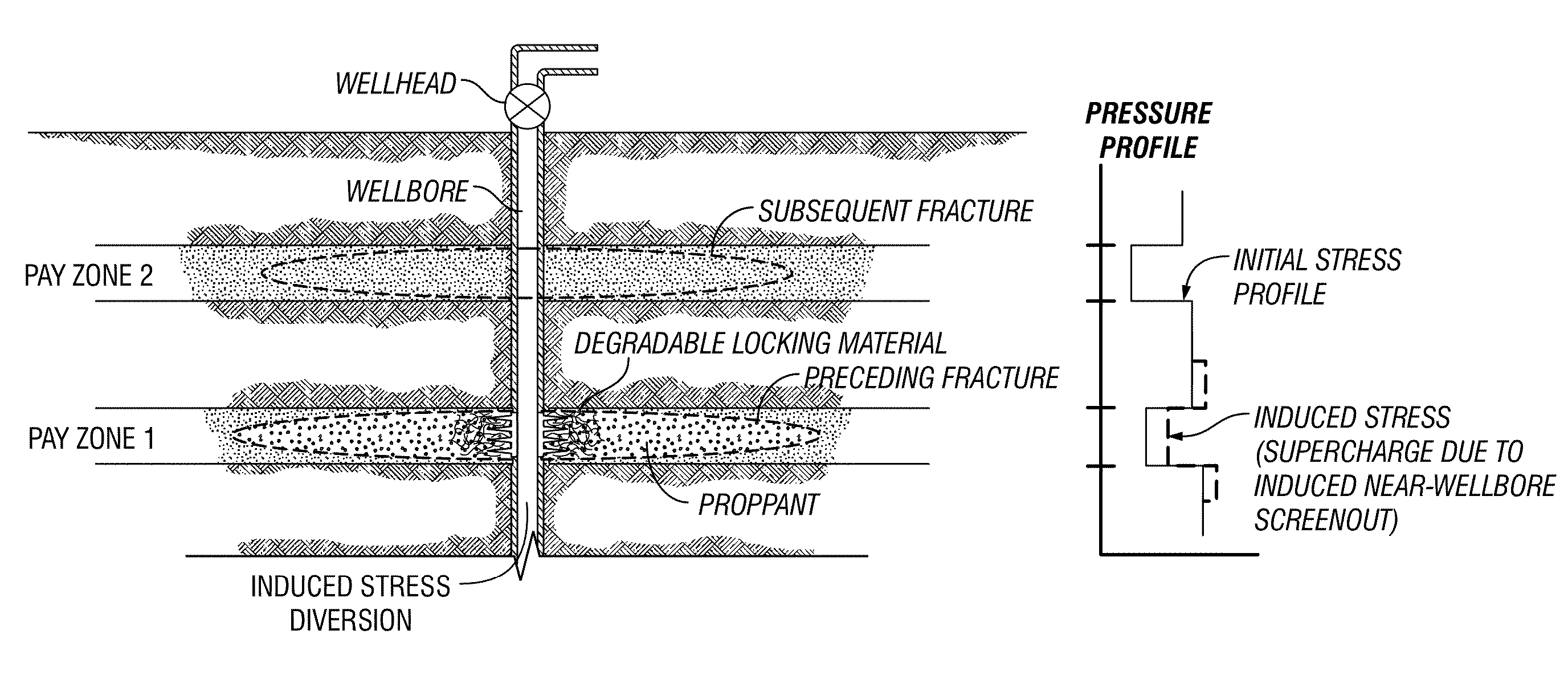
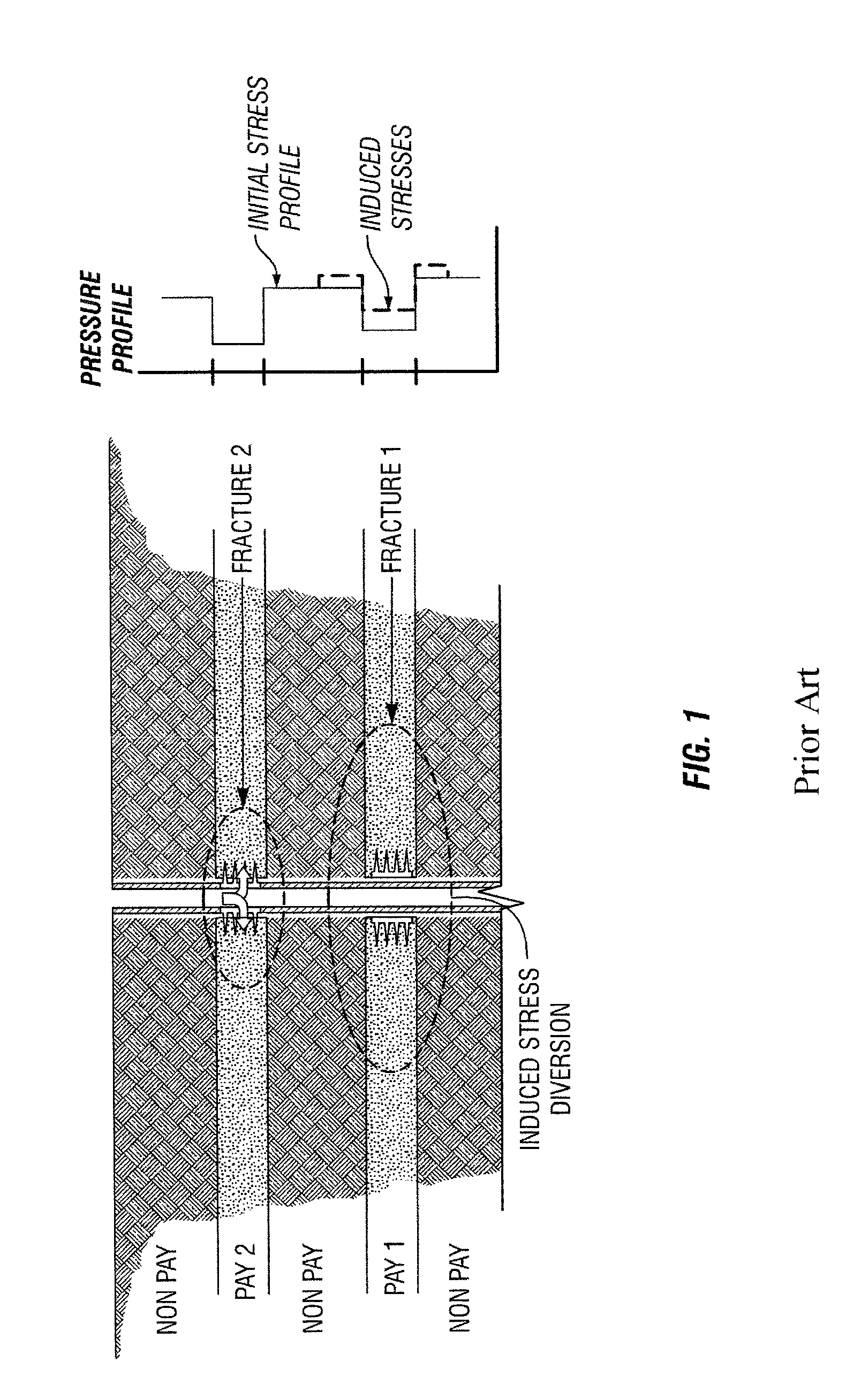
Degradable Material Assisted Diversion
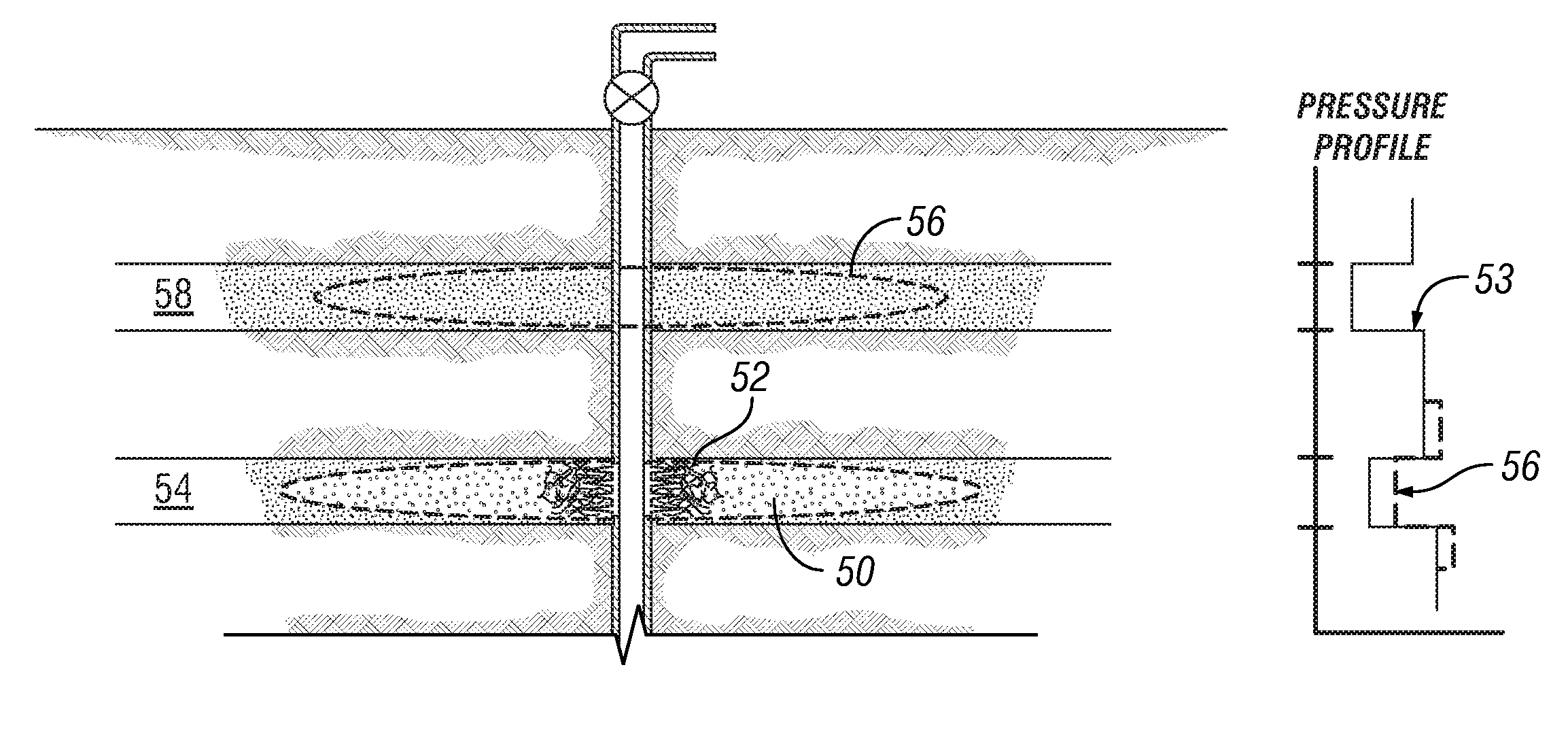
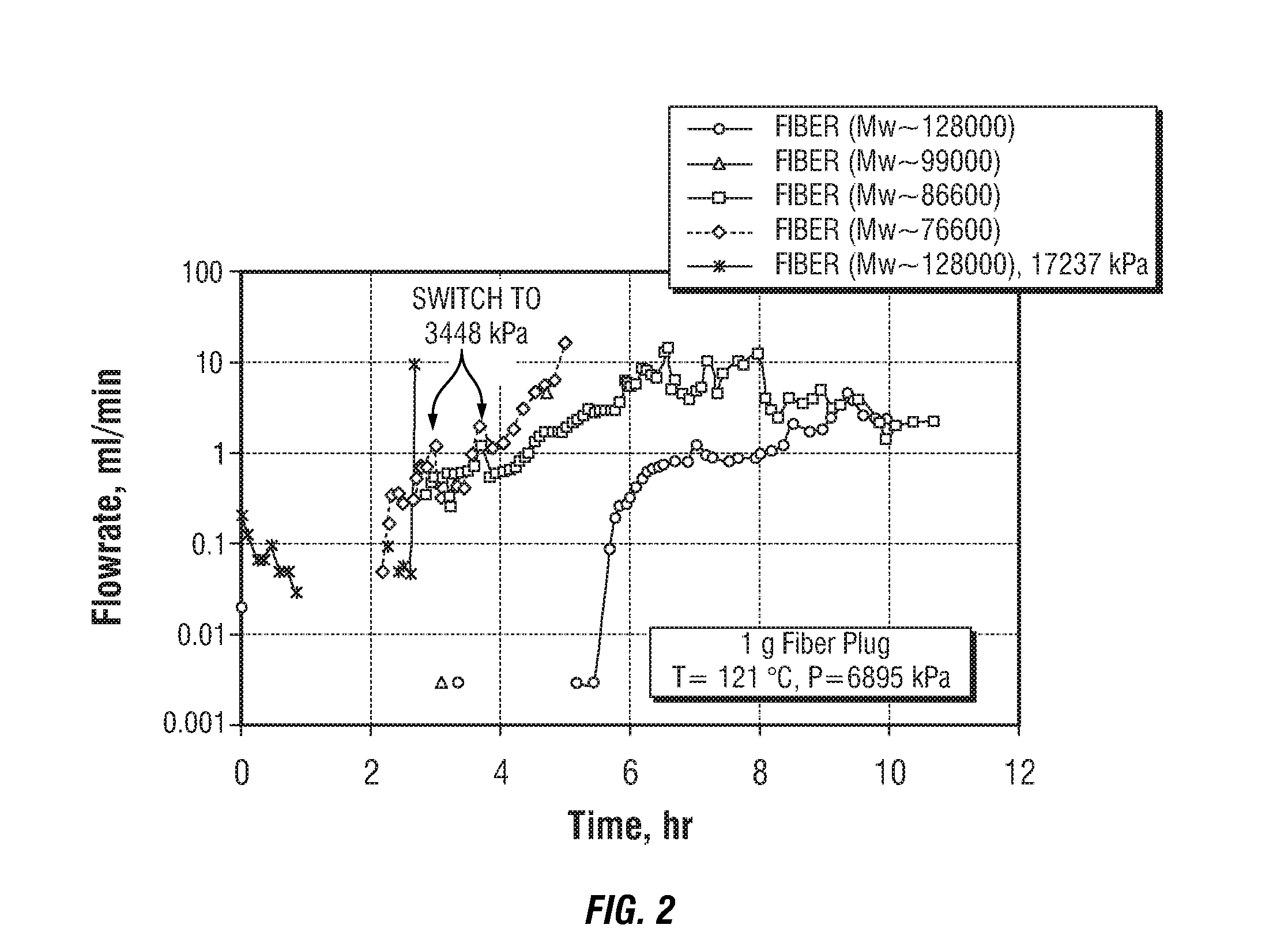
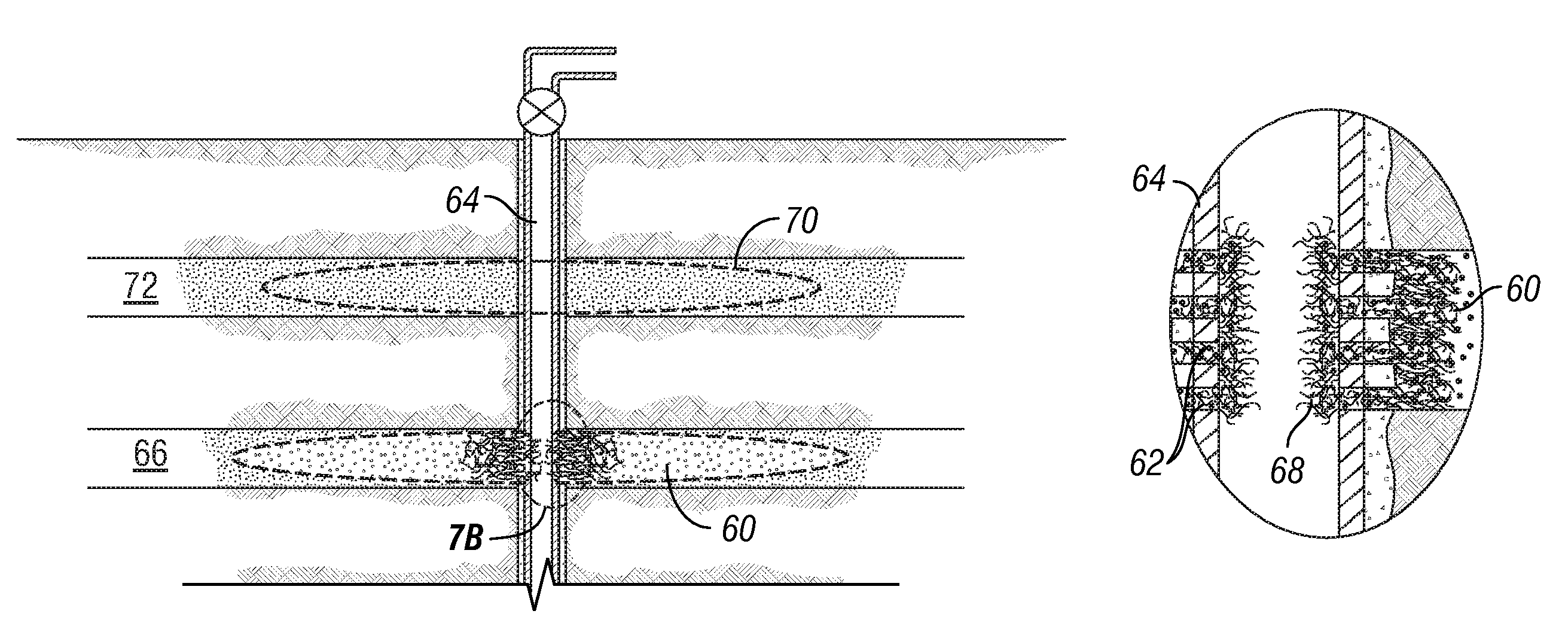
Degradable material assisted diversion (DMAD) methods for well treatment, DMAD treatment fluids, and removable plugs for DMAD in downhole operations. A slurry of solid degradable material is injected into the well, a plug of the degradable material is formed, a downhole operation is performed around the plug diverter, and the plug is then degraded for removal. Degradation triggers can be temperature or chemical reactants, with optional accelerators or retarders to provide the desired timing for plug removal. In multilayer formation DMAD fracturing, the plug isolates a completed fracture while additional layers are sequentially fractured and plugged, and then the plugs are removed for flowback from the fractured layers. In DMAD fluids, an aqueous slurry can have a solids phase including a degradable material and a fluid phase including a viscoelastic surfactant. The solids phase can be a mixture of fibers and a particulate material.
Owner:SCHLUMBERGER TECH CORP
Pedestal with a thermally controlled platen
InactiveUS20010004880A1Liquid surface applicatorsSemiconductor/solid-state device manufacturingEngineeringThermal transmittance
A workpiece support having dichotomy of thermal paths therethrough is provided for controlling the temperature of a workpiece support thereon. In one embodiment, a workpiece support includes a platen body having a plug centrally disposed in a workpiece support surface of the platen body. A lower surface of the plug defines a void between the plug and a bottom of the bore. The void creates a dichotomy of thermal paths through the platen body thus controlling the temperature of a wafer support surface. Alternatively, the plug and platen body may be fabricated from materials having different rates of thermal conductivity to created the dichotomy of thermal paths in addition to or in absence of the void.
Owner:APPLIED MATERIALS INC
Needleless valve
A needless valve is described which avoids the suctioning problems of the prior needleless devices upon deactivation and which preferably provides a positive self-purging effect. The valve is self-purging at the end of an administration cycle, avoiding clogging of attached catheters or other devices, and ensures that substantially all of liquid received into the valve is delivered to the receiver. The valve is also extremely simple in design and easy to construct and assemble, since it consists of only three pieces. The valve has a base with a connector for fluid communication attachment to tubing or other device, a solid elongated fluid channeling rod, and an internal fluid flow conduit; a flexible hollow expandable and contractible plug fitting over and moveable along the rod; and a tubular housing fitting over the plug and attached to the base. When the valve is activated by insertion of a nozzle of a fluid source, the rod and plug wall cooperate so that as the plug retracts along the rod, it is stretched and its interior expanded. Upon deactivation, the plug contracts, the interior volume decreases, and the resiling plug wall forces residual fliud within the valve to be expelled through the outlet, purging the residual fluid from the valve. No negative pressure is formed, so no suctioning of blood or other fluid from a patient or receiver occurs.
Owner:CAREFUSION 303 INC
Degradable material assisted diversion or isolation
A method for well treatment by forming a temporary plug in a fracture, a perforation, a wellbore, or more than one of these locations, in a well penetrating a subterranean formation is provided, in which the method of well treatment includes: injecting a slurry comprising a degradable material, allowing the degradable material to form a plug in a perforation, a fracture, or a wellbore in a well penetrating a formation; performing a downhole operation; and allowing the degradable material to degrade after a selected time such that the plug disappears.
Owner:SCHLUMBERGER TECH CORP
Automatic injection device
ActiveUS20120107783A1Easy to useReduce anxietyAntipyreticAutomatic syringesSubcutaneous injectionInjection device
Owner:ABBVIE BIOTECHNOLOGY LTD
Drillable bridge plug
A method and apparatus for use in a subterranean well. The apparatus typically includes a subterranean plug including a mandrel having an outer surface and a non-circular cross-section and a packing element arranged about the mandrel, the packing element having a non-cylindrical inner surface matching the mandrel outer surface such that concentric rotation between the mandrel and the packing element is precluded. The apparatus is substantially non-metallic to facilitate quick drill-out of the plug. The apparatus is alternatively adaptable as a cement retainer.
Owner:BJ SERICES
Honeycomb filter for clarifying exhaust gases
InactiveUS20050153099A1Increased durabilityPhysical/chemical process catalystsInternal combustion piston enginesParticulatesHoneycomb
An object of the present invention is to provide a honeycomb filter for purifying exhaust gases that is free from a gap formed between a plug and a partition wall and cracks generated in the plug and a portion of the partition wall contacting the plug, and is superior in durability. The present invention provides a honeycomb filter for purifying exhaust gases which has a structure in which: a columnar body made of porous ceramic comprises a number of through holes, said through holes being placed in parallel with one another in the length direction with a wall portion interposed therebetween; predetermined through holes of said through holes are filled with plugs at one end of said columnar body, while the through holes that have not been filled with said plugs at said one end are filled with plugs at the other end of said columnar body; and a part or all of said wall portion functions as a filter for collecting particulates, wherein the porosity of the columnar body is in a range from 20 to 80%, and the porosity of the plug is 90% or less and is also set to 0.15 to 4.0 times as much as the porosity of the columnar body.
Owner:IBIDEN CO LTD
Needleless valve
A needless valve is described which avoids the suctioning problems of the prior needleless devices upon deactivation and which preferably provides a positive self-purging effect. The valve is self-purging at the end of an administration cycle, avoiding clogging of attached catheters or other devices, and ensures that substantially all of liquid received into the valve is delivered to the receiver. The valve is also extremely simple in design and easy to construct and assemble, since it consists of only three pieces. The valve has a base with a connector for fluid communication attachment to tubing or other device, a solid elongated fluid channeling rod, and an internal fluid flow conduit; a flexible hollow expandable and contractible plug fitting over and moveable along the rod; and a tubular housing fitting over the plug and attached to the base. When the valve is activated by insertion of a nozzle of a fluid source, the rod and plug wall cooperate so that as the plug retracts along the rod, it is stretched and its interior expanded. Upon deactivation, the plug contracts, the interior volume decreases, and the resiling plug wall forces residual fluid within the valve to be expelled through the outlet, purging the residual fluid from the valve. No negative pressure is formed, so no suctioning of blood or other fluid from a patient or receiver occurs.
Owner:CAREFUSION 303 INC
Closure device with rapidly dissolving anchor
Closure devices with a rapidly dissolving anchor, systems delivering closure devices, and methods for making and using the same. An example closure device for closing an opening in a body lumen may include a plug, a rapidly dissolving anchor, and a suture coupling the plug to the anchor. The rapidly dissolving anchor may be configured to dissolve within the body lumen within about 30 days or less. At least a portion of the plug may be disposed adjacent an exterior surface of the body lumen. At least a portion of the rapidly dissolving anchor may be disposed within the body lumen.
Owner:BOSTON SCI SCIMED INC
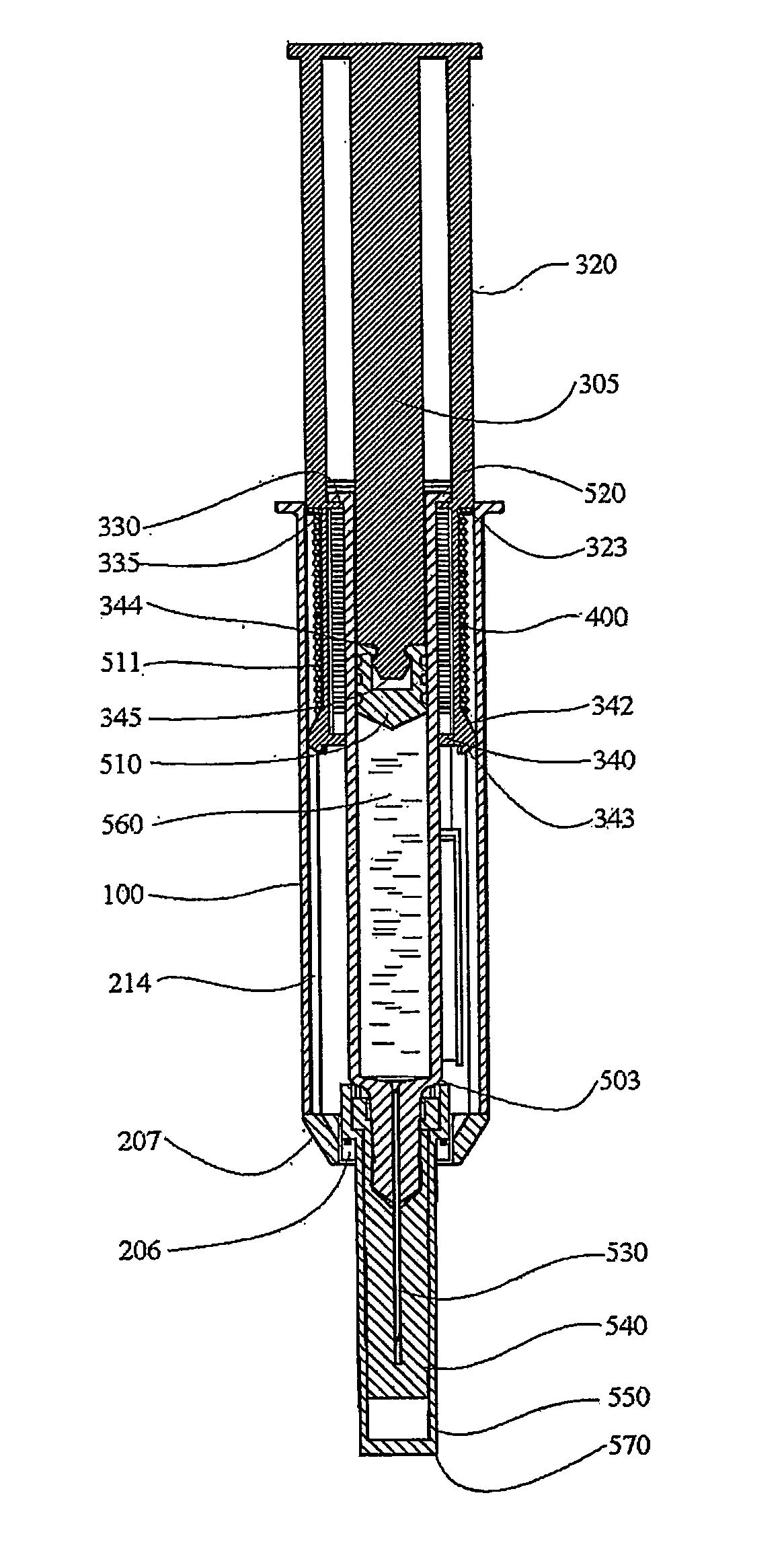
Multi-chamber, sequential dose dispensing syringe
A valve assembly is disclosed which effectively partitions a syringe into proximal and distal chambers to provide a multi-chamber, sequentially dispensing syringe apparatus. The valve assembly may be effectively used with a variety of standard, currently available commercial syringes and pre-filled syringes. Incorporated in the valve assembly is a valved stopper having a valve (which may be a slit valve), an impact sensor which opens the valve upon impact between the valve assembly and internal distal end of the syringe and a gas separator which separates liquid from gas disposed in the proximal chamber to assure gas is not delivered therefrom. The valve assembly is displaced as a plunger of the syringe is displaced via communication through fluid in the proximal chamber of the syringe. The actuator has a latching feature which latches the valve to an open state after being opened by the impact sensor. The gas separator has a proximally disposed orifice which facilitates priming. The valve assembly may be made from two parts. One part, a valved stopper, may be molded from the same rubber based material used in syringe plunger stoppers. The second part, the valve actuator, may be injection molded as a single part. A synthetic resinous material which is compatible with manufacture of living hinges my be used for the second part. Multiple valve assemblies may be used in a single syringe barrel.
Owner:INFUSIVE TECH LLC
Degradable material assisted diversion
Degradable material assisted diversion (DMAD) methods for well treatment, DMAD treatment fluids, and removable plugs for DMAD in downhole operations. A slurry of solid degradable material is injected into the well, a plug of the degradable material is formed, a downhole operation is performed around the plug diverter, and the plug is then degraded for removal. Degradation triggers can be temperature or chemical reactants, with optional accelerators or retarders to provide the desired timing for plug removal. In multilayer formation DMAD fracturing, the plug isolates a completed fracture while additional layers are sequentially fractured and plugged, and then the plugs are removed for flowback from the fractured layers. In DMAD fluids, an aqueous slurry can have a solids phase including a degradable material and a fluid phase including a viscoelastic surfactant. The solids phase can be a mixture of fibers and a particulate material.
Owner:SCHLUMBERGER TECH CORP
Tamper-proof enclosure for a circuit card
InactiveUS6970360B2Improve reliabilityImprove securitySemiconductor/solid-state device detailsSolid-state devicesInternal pressureTamper resistance
A tamper-proof enclosure for an electrical card, such as a high speed communications card, includes an enclosure in which the card is mounted. The enclosure has a wall with an opening, and a cup member is attached to the wall at the opening. A bus that is connected to the card extends through a passage in the cup member and through the opening in the wall. A security mesh is wrapped around the enclosure. The cup member is filled with liquid resin, which is also coated onto the security mesh. After the resin is cured, the resin in the cup member forms a plug that seals the security mesh from inner pressure when the enclosure is heated to an elevated temperature. The resin is preferably polyamide.
Owner:INT BUSINESS MASCH CORP
Degradable material assisted diversion or isolation
A method for well treatment by forming a temporary plug in a fracture, a perforation, a wellbore, or more than one of these locations, in a well penetrating a subterranean formation is provided, in which the method of well treatment includes: injecting a slurry comprising a degradable material, allowing the degradable material to form a plug in a perforation, a fracture, or a wellbore in a well penetrating a formation; performing a downhole operation; and allowing the degradable material to degrade after a selected time such that the plug disappears.
Owner:SCHLUMBERGER TECH CORP
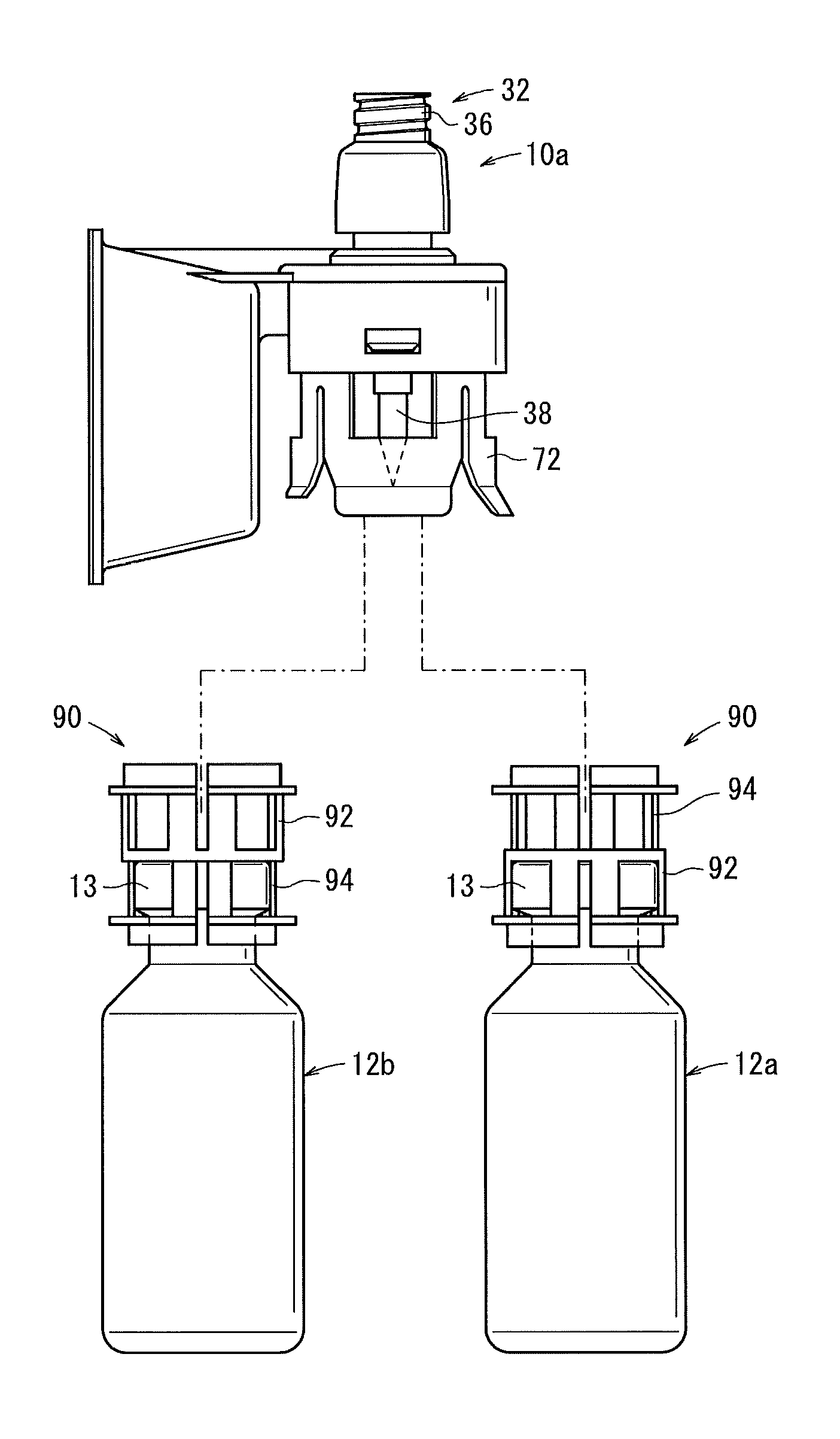
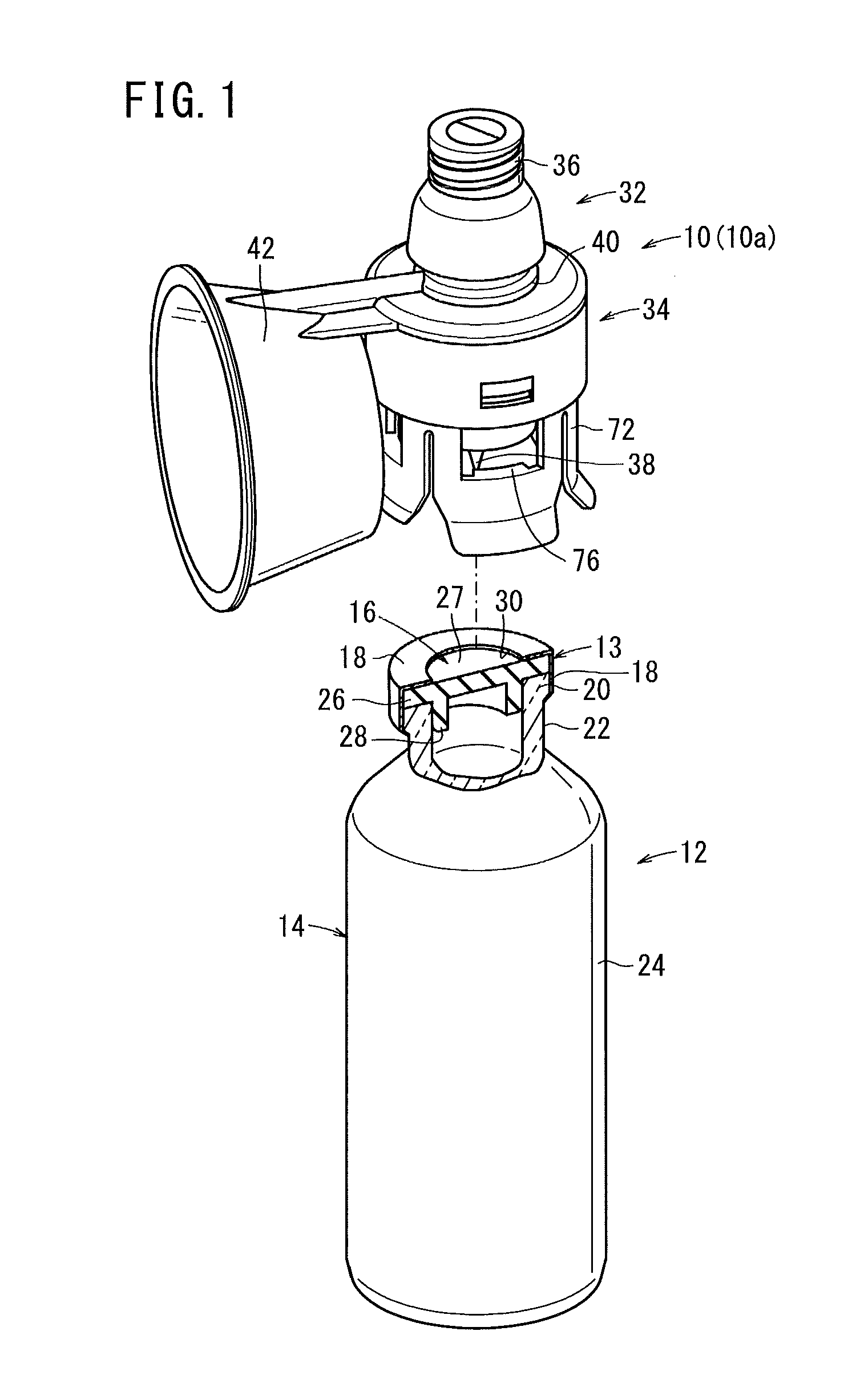
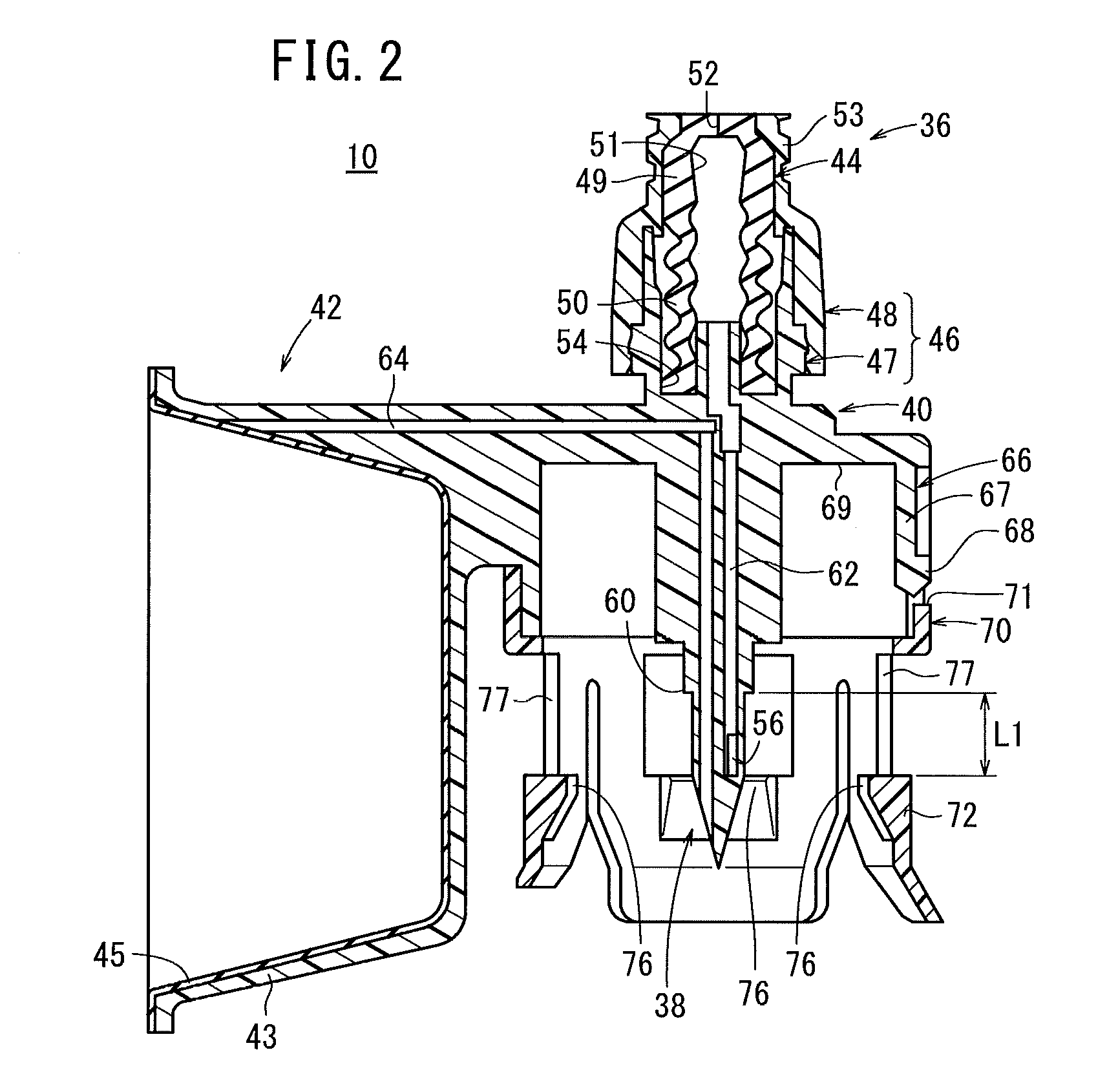
Sealed containers and methods of making and filling same
Disclosed is a uniquely configured medicament vial assembly which includes a storage vial, a stopper member and a securing ring. The vial assembly is configured to improve healthcare worker safety by providing a shielded gripping location to aid in the reduction of accidental needle sticks. The storage vial has a body portion which defines an interior chamber for storing a predetermined medicament and a neck portion through which medicament is received into and withdrawn from the interior chamber. The stopper member is inserted into the mouth of the vial and establishes a first seal. The securing ring is engaged with the mouth of the vial and adapted and configured for retaining the stopper member within the vial mouth and effectuating a second seal. The securing ring is formed from a thermoplastic and / or elastic material. Preferably, the securing ring is formed by molding the thermoplastic and / or elastic material over a portion of the storage vial and stopper member when engaged within the vial mouth.
Owner:MEDINSTILL DEV
Canister, sealing method and composition for sealing a borehole
Owner:TRIAD NAT SECURITY LLC
Fluid transfer devices with sealing arrangement
The present invention is directed toward fluid transfer devices including a vial adapter having a top wall and a cannula with a cannula tip, and an elastic O-ring like sealing element sealingly encircling the cannula and initially disposed towards the cannula tip and spaced apart from the top wall, the sealing element being brought into initial contact with the vial stopper subsequent to the cannula tip contacting the vial stopper at a puncture site and thereafter being slidingly urged towards the top wall and continuously sealing the puncture site during snap fit mounting the vial adapter on the vial.
Owner:WEST PHARM SERVICES IL LTD
Container providing a controlled hydrated environment
InactiveUS7036667B2Prevent leakageOther accessoriesContainer/bottle contructionPre-conditionAdhesive
A container is provided for shipping and storing a pre-wetted and pre-conditioned microfluidic “sipper” chip. The container contains both dry compartments and wet compartments. A base contains a fluid-filled reservoir configured to house the capillaries. The opening of the reservoir is sealed with an O-ring. The plastic mount of the chip rests on the base in a dry compartment. The upper surface of the chip contains several wells containing fluid. A gasket is provided with plugs configured to be disposed within and seal the wells. Alternatively, the wells are first sealed with a foil film adhered to the well openings with an adhesive and a gasket is disposed between the foil and a cover, which is removably attached to the base. When the cover is closed, the gasket and O-ring seal the wet compartments to prevent leakage and to slow evaporation.
Owner:CAPLIPER LIFE SCI INC
Vial adapter
ActiveUS8702675B2Bubbling can be restrained from occurringEasy to distinguishDiagnosticsSurgeryVIAL ADAPTERBiomedical engineering
A vial adapter is provided with a fitting portion which can be fitted into the head portion of a vial, and a hollow needle with a side hole. The base end portion of the needle is provided with a stopper contact portion which contacts the top surface of a stopper when the fitting portion is fitted in the head portion of the vial. When the fitting portion is fitted in the head portion of the vial, the stopper contact portion contacts the top surface of the stopper, and the fitting portion is fixed to the head portion of the vial by a claw engaging with the head portion of the vial, and further, the region of the side hole nearest the base end portion of the needle is positioned either roughly at the bottom surface of the stopper or inside of the stopper.
Owner:TERUMO KK
Perforating connector with sterile connection
A connector according to the invention comprises a hollow needle fastened to a body. A first tubular receptacle provided with a first foil surrounds a first end section of the needle. A second tubular receptacle provided with a second foil surrounds a second end section of the needle. The tubular receptacles are able to slide on the body. The holding sleeve moves the body toward the first foil over a first piercing stroke to pierce it, and then moves the second foil toward the body over a second piercing stroke to pierce it. In this way there may be obtained simultaneous piercing of the foils and foils or stoppers of containers such as flasks or sachets pressed against the foils, to provide a connection under optimum conditions of sterility.
Owner:P2A MEDICAL
Method of using a cartridge for containing a specimen sample for optical analysis
InactiveUS6861259B2Without riskMaterial analysis by optical meansLaboratory glasswaresTest chamberEngineering
A cartridge for holding a test specimen with an extremely small volume. The cartridge has a test chamber and a vestibule through which the test fluids are inserted into the test chamber. The cartridge has a stopper having a pair of seals, the first of which seals the test chamber inlet between the vestibule and the test chamber, and the second of which seals the mouth of the vestibule so that when the stopper is in place, the test chamber is closed to the admission of air or other contaminants and the vestibule is similarly closed against escape of the overflow from the test chamber.
Owner:MENARINI SILICON BIOSYSTEMS SPA
Syringe with automatically triggered safety sleeve
A medical injection device is provided which includes a shield system and a syringe. The shield system includes a housing, a shield telescopically received in the housing slidably coupled to the housing and a driver that pushes the syringe stopper to cause drug injection. The driver is equipped with sensing elements that automatically detect empty syringe. A spring resiliently urges the shield from a retracted position to an extended position shielding the needle. The syringe is coupled to the housing. The shield is positioned externally to the syringe and the driver. The shield includes flexible latches to keep its position relative to the housing during storage and use. The axial movement of the driver in respect to the syringe causes an automatic release of the spring by sensors when the syringe is empty, allowing the spring to move and lock the shield in the extended position after use.
Owner:WEST PHARMA SERVICES OF DELAWARE
Bottom set downhole plug
A plug for isolating a wellbore. The plug can include a body having a first end and a second end, wherein the body is formed from one or more composite materials and adapted to receive a setting tool through the first end thereof, at least one malleable element disposed about the body, at least one slip disposed about the body, at least one conical member disposed about the body, and one or more shearable threads disposed on an inner surface of the body, adjacent the second end thereof, wherein the one or more shearable threads are adapted to receive at least a portion of a setting tool that enters the body through the first end thereof, and wherein the one or more shearable threads are adapted to engage the setting tool when disposed through the body and adapted to release the setting tool when exposed to a predetermined axial force.
Owner:NINE DOWNHOLE TECHNOLOLGIES LLC
Cementing systems for wellbores
InactiveUS6056053AReduced inner body thicknessEasy to bendCleaning apparatusFluid removalWell cementingMetallic materials
A new wellbore cementing system has been developed which includes, in certain embodiments, a plug container with a flow diverter for diverting a portion of flowing fluid away from plugs in the plug container; a plug set system with internal sleeves or dart receivers with shearable parts for shearing to selectively release plugs-all in certain embodiments made of non-metal material and / or plastic; a swivel equalizer with internal valving to isolate a plug set (or any other item) from torque and to relieve pressure below the swivel equalizer; and unique burst tube systems for selectively controlling fluid flow and plug system operation. In one aspect a plug nose is tapered to correspond to a taper of a landing ring so that wedge-locking of the nose and ring effects desired non-rotation of the plug during drilling. In one aspect plug fin bending is facilitated by reducing plug body thickness so that an alternate fluid flow path is provided for cementing. In one aspect a new float valve system is provided with a top baffle that prevents debris etc. from shutting off fluid flow to a float valve.
Owner:WEATHERFORD TECH HLDG LLC
Degradable material assisted diversion or isolation
A method for well treatment by forming a temporary plug in a fracture, a perforation, a wellbore, or more than one of these locations, in a well penetrating a subterranean formation is provided, in which the method of well treatment includes: injecting a slurry comprising a degradable material, allowing the degradable material to form a plug in a perforation, a fracture, or a wellbore in a well penetrating a formation; performing a downhole operation; and allowing the degradable material to degrade after a selected time such that the plug disappears.
Owner:SCHLUMBERGER TECH CORP
Drainage apparatus and method
InactiveUS20070078444A1The process is simple and convenientGreat suctionInfusion syringesSurgeryThoracic cavityPressure difference
An apparatus for removing body fluids from a body cavity by suction, such as a thorax, gastric or any other human body cavity or a wound, comprises means (9) to increase the pressure difference between a pressure in a drainage lumen (3) and a pressure in the atmosphere when an auxiliary lumen (5) is open. The apparatus enables removal of clots or other plugs of the catheter and drainage tube in an efficient and not patient disturbing way.
Owner:MEDELA HLDG AG
Bottom set downhole plug
A plug for isolating a wellbore. The plug can include a body having a first end and a second end, wherein the body is formed from one or more composite materials and adapted to receive a setting tool through the first end thereof, at least one malleable element disposed about the body, at least one slip disposed about the body, at least one conical member disposed about the body, and one or more shearable threads disposed on an inner surface of the body, adjacent the second end thereof, wherein the one or more shearable threads are adapted to receive at least a portion of a setting tool that enters the body through the first end thereof, and wherein the one or more shearable threads are adapted to engage the setting tool when disposed through the body and adapted to release the setting tool when exposed to a predetermined axial force.
Owner:NINE DOWNHOLE TECHNOLOLGIES LLC
Subterranean well secondary plugging tool for repair of a first plug
A secondary plugging tool is disclosed for use in a subterranean plug, such as in a plugged and / or abandoned well. The repaired plug may be of a cementicious material, or a mechanically, hydraulically or electrically set plug or packer. The plugging tool includes an outer housing containing an eutectic metal alloy. A thermitic reaction charge is contained within chambers within an inner tubular member and a lower housing. The lower end of the outer housing being ported circumferentially there around, the thermitic reaction charge activates the eutectic metal charge such that the eutectic charge melts and pours out of the outer housing and across and upon the initial plug to repair any failure areas therein.
Owner:CINARUCO INT CALLE AGUILINO DE LA GUARDIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com