Patents
Literature
2065results about How to "Dissolve fast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
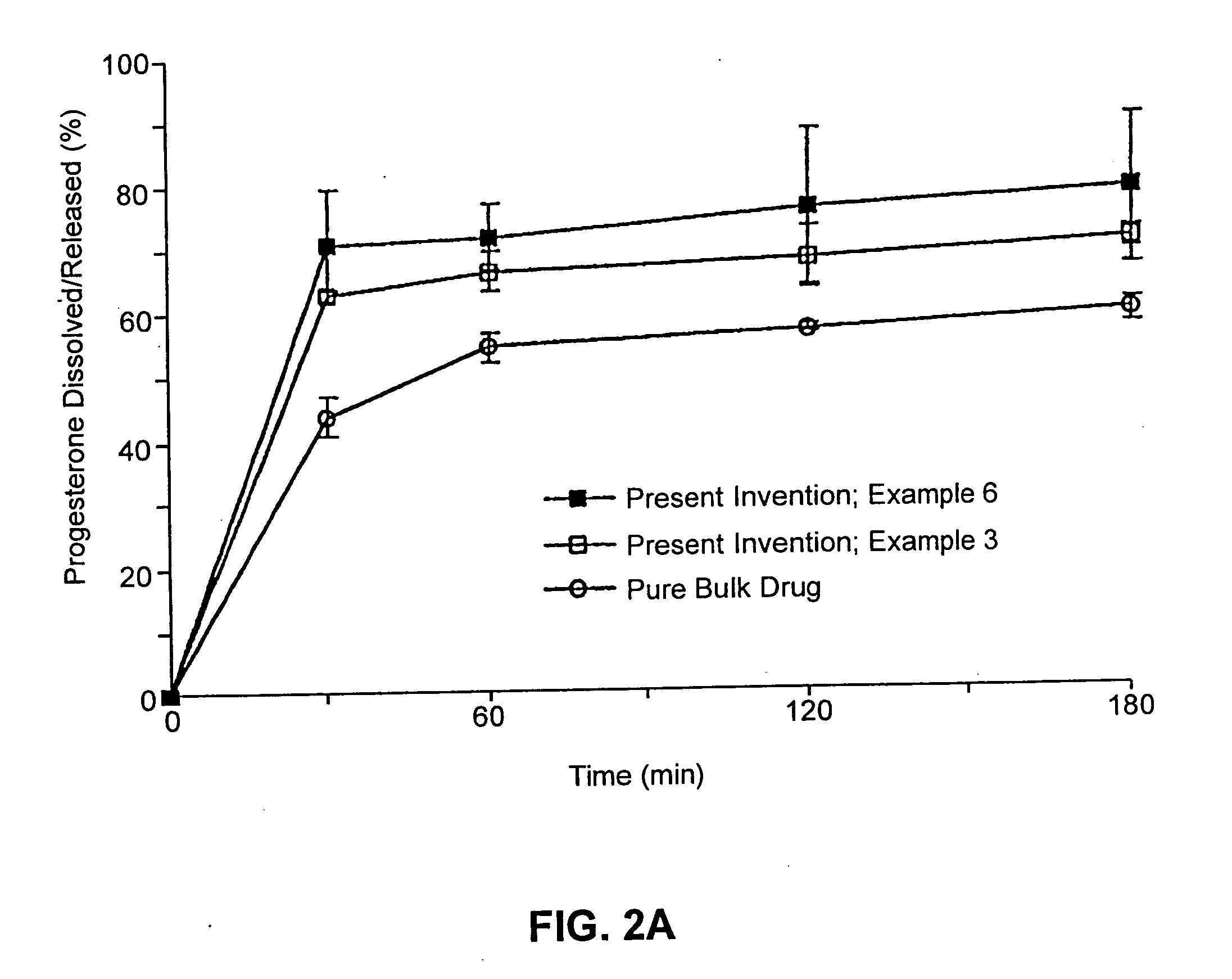
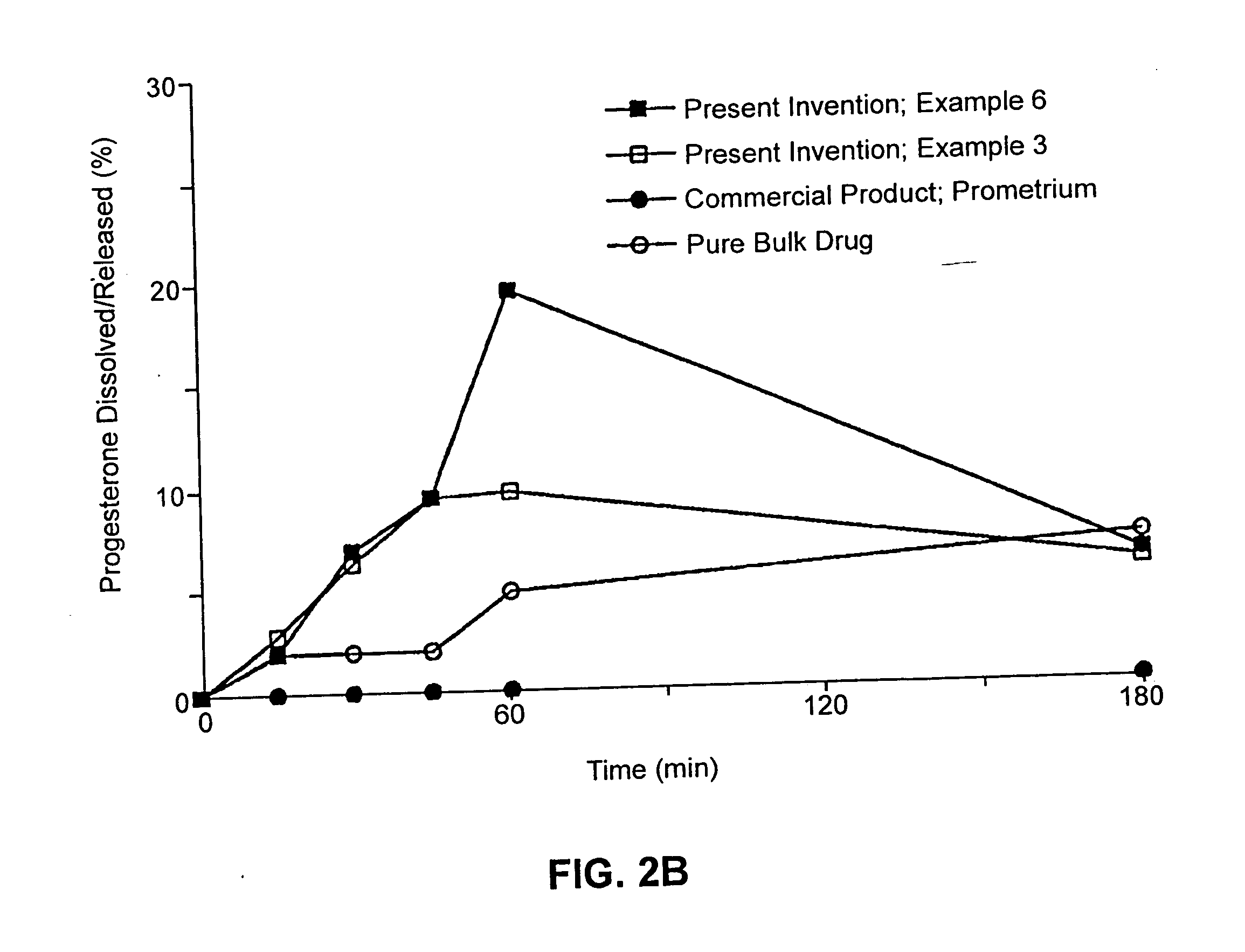
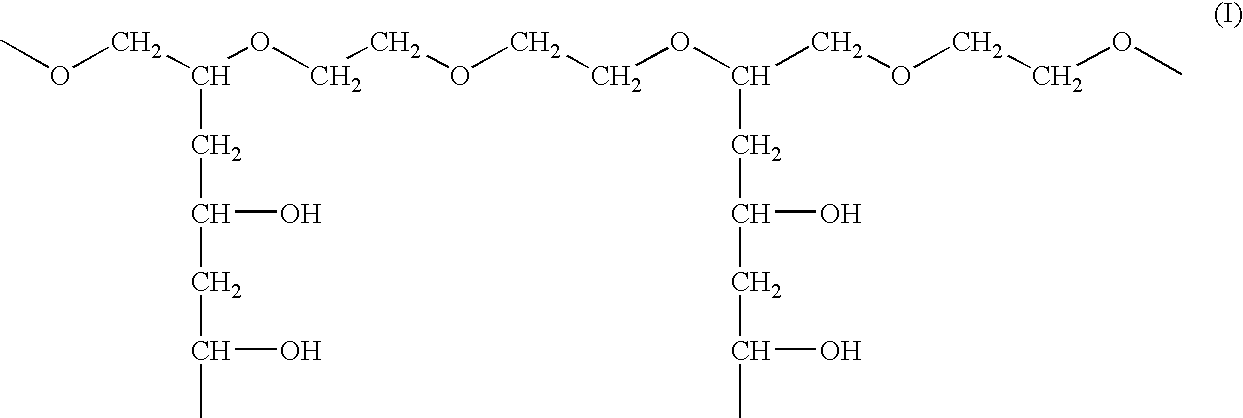
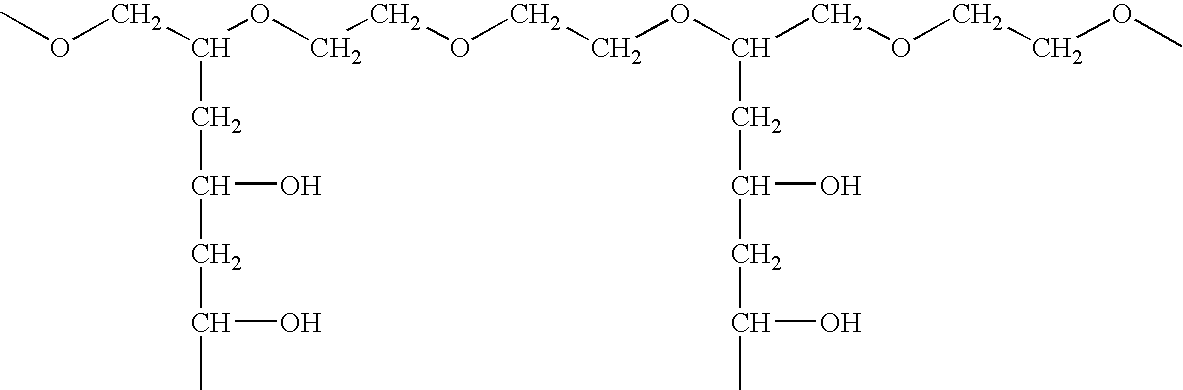
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20060034937A1Good chemical stabilityPromote absorptionGranular deliveryMicrocapsulesDiagnostic agentMedicine
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Solid solution perforator for drug delivery and other applications
InactiveUS6945952B2Fast biodegradationBarrier property can be diminished and controlledSurgeryMicroneedlesDrug reservoirDrugs solution
A solid drug solution perforator (SSP) system and an associated drug reservoir are provided for delivering therapeutic, prophylactic and / or cosmetic compounds, for nutrient delivery and for drug targeting. For drug delivery, the SSP system includes an active drug ingredient and a matrix of perforator material that biodegrades or dissolves quickly upon contact with a patient's body. The SSP system provides a skin barrier perforator and a controller for prompt initiation and cut-off drug delivery. In a preferred method of transdermal drug delivery, an SSP system containing a selected drug penetrates into an epidermis or dermis, and the drug is promptly released from the (dissolving) SSP system perforator. An additional drug is optionally delivered from a patch reservoir through skin pores created by insertion of the perforator. Formulation and fabrication procedures for the SSP and associated reservoir are also provided. An SSP system can be fabricated with variety of shapes and dimensions.
Owner:THERAJECT INC.
Orally dissolving films
ActiveUS20060198873A1Improve complianceRapid and sustained and combination nicotine craving reliefBiocideNervous disorderActive agentPharmacology
Rapidly dissolving, oral film preparations for rapid release of an active agent in the oral cavity, in particular, rapidly dissolving oral films comprising a nicotine active which achieve good transbuccal absorption and provide nicotine craving relief to an individual are disclosed herein.
Owner:GLAXO SMITHKLINE LLC
Fast dissolving orally consumable films
InactiveUS6923981B2Dissolve fastGood curative effectAntibacterial agentsCosmetic preparationsPolymer sciencePullulan
Physiologically acceptable films, including edible films, are disclosed. The films include a water soluble film-forming polymer such as pullulan. Edible films are disclosed that include pullulan and antimicrobially effective amounts of the essential oils thymol, methyl salicylate, eucalyptol and menthol. The edible films are effective at killing the plaque-producing germs that cause dental plaque, gingivitis and bad breath. The film can also contain pharmaceutically active agents. Methods for producing the films are also disclosed.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Drospirenone for hormone replacement therapy
InactiveUS20020132801A1Dissolve fastImprove bioavailabilityOrganic active ingredientsBiocideDrospirenoneBULK ACTIVE INGREDIENT
Drospirenone for Hormone Replacement Therapy A pharmaceutical composition comprising as a first active ingredient an estrogen, such as estradiol or estradiol valerate, in sufficient amounts to treat disorders and symptoms associated with deficient endogenous levels of estrogen in women, and as a second active ingredient 6beta,7beta; 15beta; 16beta-dimethylene-3-oxo-17alpha-preg-4-ene-21,17-carbolactone (drospirenone, DRSP) in sufficient amounts to protect the endometrium from the adverse effects of estrogen is useful for, amongst others, treating peri-menopausal, menopausal and post-menopausal women. This composition may be used for hormone replacement therapy and may be administered as a multi-phased pharmaceutical preparation. This combination therapy may comprise continuous, sequential or interrupted administration, or combinations thereof, of DRSP and estrogen, each optionally in micronized form.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
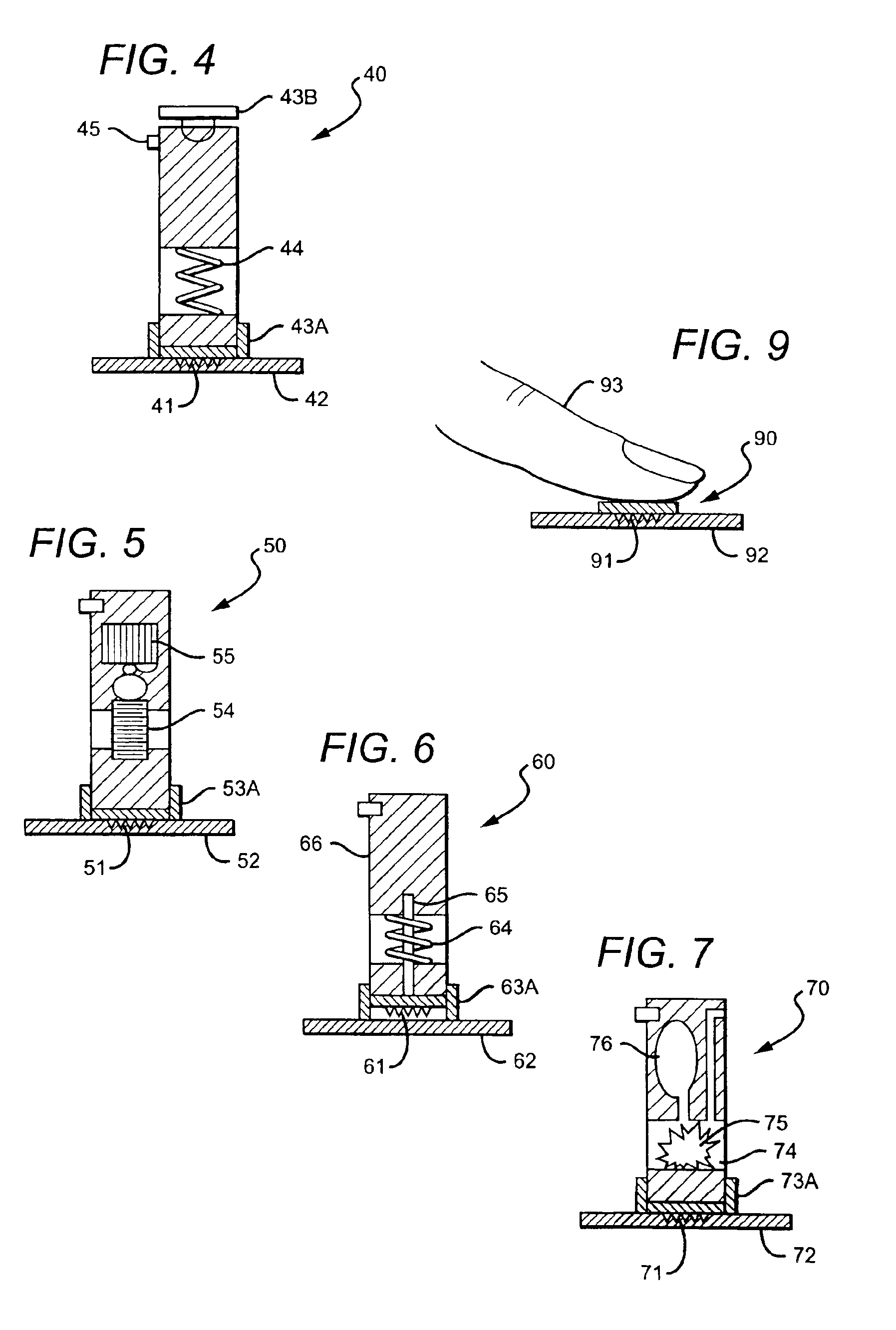
Closure device with rapidly dissolving anchor
Closure devices with a rapidly dissolving anchor, systems delivering closure devices, and methods for making and using the same. An example closure device for closing an opening in a body lumen may include a plug, a rapidly dissolving anchor, and a suture coupling the plug to the anchor. The rapidly dissolving anchor may be configured to dissolve within the body lumen within about 30 days or less. At least a portion of the plug may be disposed adjacent an exterior surface of the body lumen. At least a portion of the rapidly dissolving anchor may be disposed within the body lumen.
Owner:BOSTON SCI SCIMED INC
Venous valvuloplasty device and method
InactiveUS6932838B2ResilienceImprove anchoring abilityAnnuloplasty ringsStaplesBiomedical engineeringVALVE PORT
A device 10 and method for replacing or restoring competence to incompetent valves. The device 10 is inserted percutaneously or surgically and is preferably constructed of a material capable of promoting cellular ingrowth such that, eventually, native biologic tissue completely covers the device 10 insulating the blood flow therefrom. The material is preferably bioabsorbable over time, allowing the device to harbor the regeneration of a valve structure and to later become absorbed by the body. The device is sized and arranged to mimic the valve it is replacing or repairing.
Owner:TRICARDIA
Solubilization of algae and algal materials
InactiveUS20100068772A1Speed up the processIncrease ratingsMicroorganism lysisWaste based fuelLipid formationAlcohol
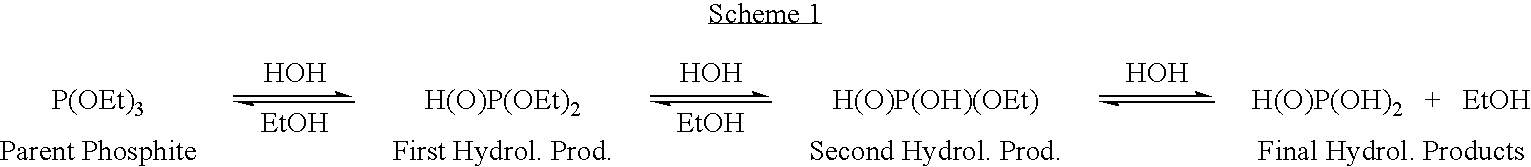
Methods for solubilizing algae or algal material are provided to facilitate the recovery of oil or lipids, as well as hydrocarbons and carbohydrates, from algae or algal material. The methods involve contacting algae or algal material with an oxoacid ester or thioacid ester of phosphorus or a mixture of an oxoacid of phosphorus and / or an alcohol to form a mixture thereof under conditions effective to solubilize the algae or algal material. These methods optionally further comprise bioconversion of the solubilized algae or algal material to form a composition suitable for recovery of oils and non-oil chemicals.
Owner:CIRIS ENERGY INC
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20090074859A1Dissolve fastReduce deliveryAntibacterial agentsPowder deliveryDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Edible film
InactiveUS20050089548A1Not easily friableEasy to handleMixingPharmaceutical non-active ingredientsMedicineActive agent
An edible film that rapidly disintegrates when placed in the mouth to release an active agent, the film consisting of a hydrocolloid film-forming material and microparticles containing active agent
Owner:GIVAUDAN SA
Nanoparticulate tacrolimus formulations
InactiveUS20060159766A1Dissolve fastImprove complianceMaterial nanotechnologyBiocideMedicineNanoparticle
The present invention is directed to nanoparticulate tacrolimus compositions. The composition comprising tacrolimus particles having an effective average particle size of less than about 2000 nm and at least one surface stabilizer.
Owner:ELAN PHRMA INT LTD
Supporting plate, apparatus, and method for stripping supporting plate
InactiveUS20070062644A1Fast supplyShort timeSemiconductor/solid-state device manufacturingDomestic articlesAdhesiveMechanical engineering
A supporting plate which makes it possible to easily strip the supporting plate from a substrate in a short period of time after thinning the substrate. In the supporting plate to which a circuit-formed surface of a substrate is bonded with an adhesive, a first penetrating hole is formed in a substantially central portion of the supporting plate in the thickness direction, grooves connecting with the first penetrating hole are formed on a surface of the supporting plate to be contacted with an adhesive, and a second penetrating hole connecting with the grooves is formed in a peripheral portion of the supporting plate in the thickness direction.
Owner:TOKYO OHKA KOGYO CO LTD
Oral pharmaceutical compositions in timed-release particle form and fast-disintegrating tablets containing this composition
InactiveUS20050287211A1Reduce solubilityProne to feverAntibacterial agentsOrganic active ingredientsWater solubleLag time
The present invention relates to an oral pharmaceutical composition in particle form, which comprises particles that contain a drug at the core of the pharmaceutical composition in particle form; a middle layer that contains two types of water-soluble components, an insolubilizer and an insolubilizing substance; and an outer layer for controlling water penetration that contains a water-insoluble substance. The present invention makes it possible to provide a pharmaceutical composition in particle form for oral use with which initial drug release is suppressed, the drug is quickly released thereafter, and lag time can be controlled as needed, and fast-disintegrating tablets containing this composition.
Owner:ASTELLAS PHARMA INC
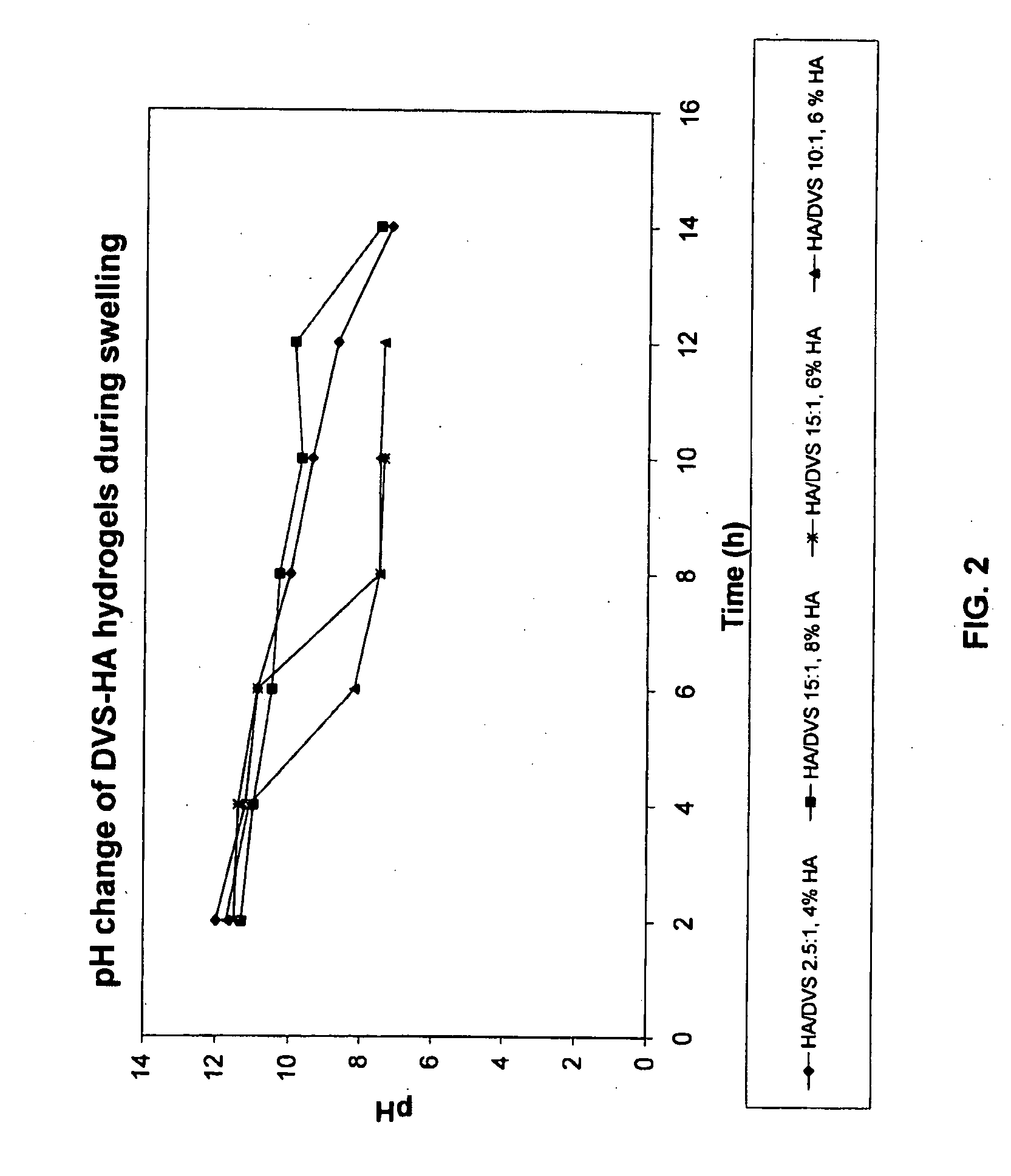
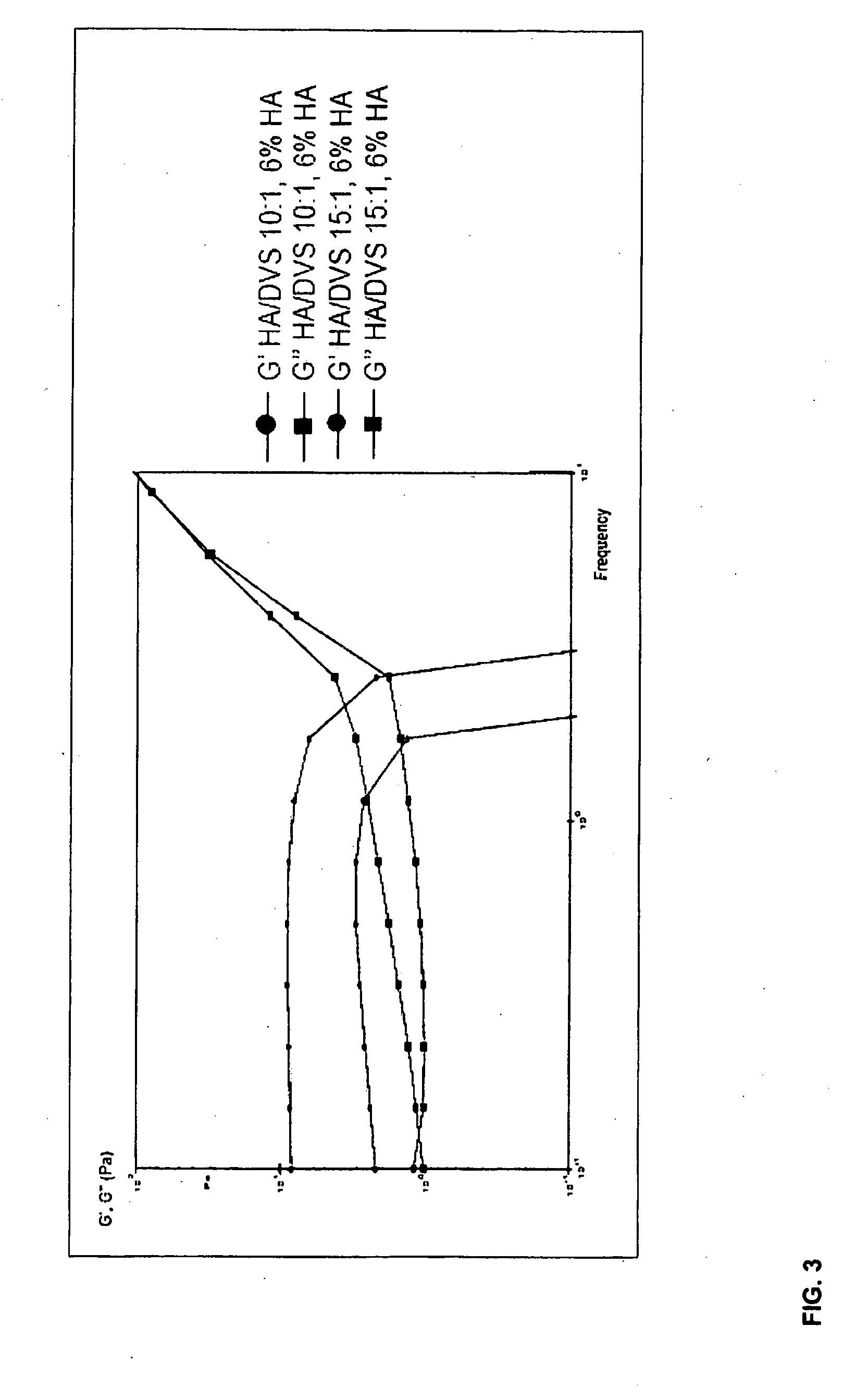
Method of cross-linking hyaluronic acid with divinulsulfone
ActiveUS20090155362A1Improve homogeneityGood flexibilityBiocideOrganic active ingredientsCross-linkHyaluronic acid
The present invention relates to methods of producing a homogenous hydrogel comprising hyaluronic acid, or salt thereof, crosslinked with divinylsulfone (DVS), said method comprising the steps of (a) providing an alkaline solution of hyaluronic acid, or salt thereof; (b) adding DVS to the solution of step (a), whereby the hyaluronic acid, or salt thereof, is crosslinked with the DVS to form a gel; (c) treating the gel of step (b) with a buffer, wherein the gel swells and forms a hydrogel comprising hyaluronic acid, or salt thereof, crosslinked with DVS.
Owner:HYAMEDIX IVS
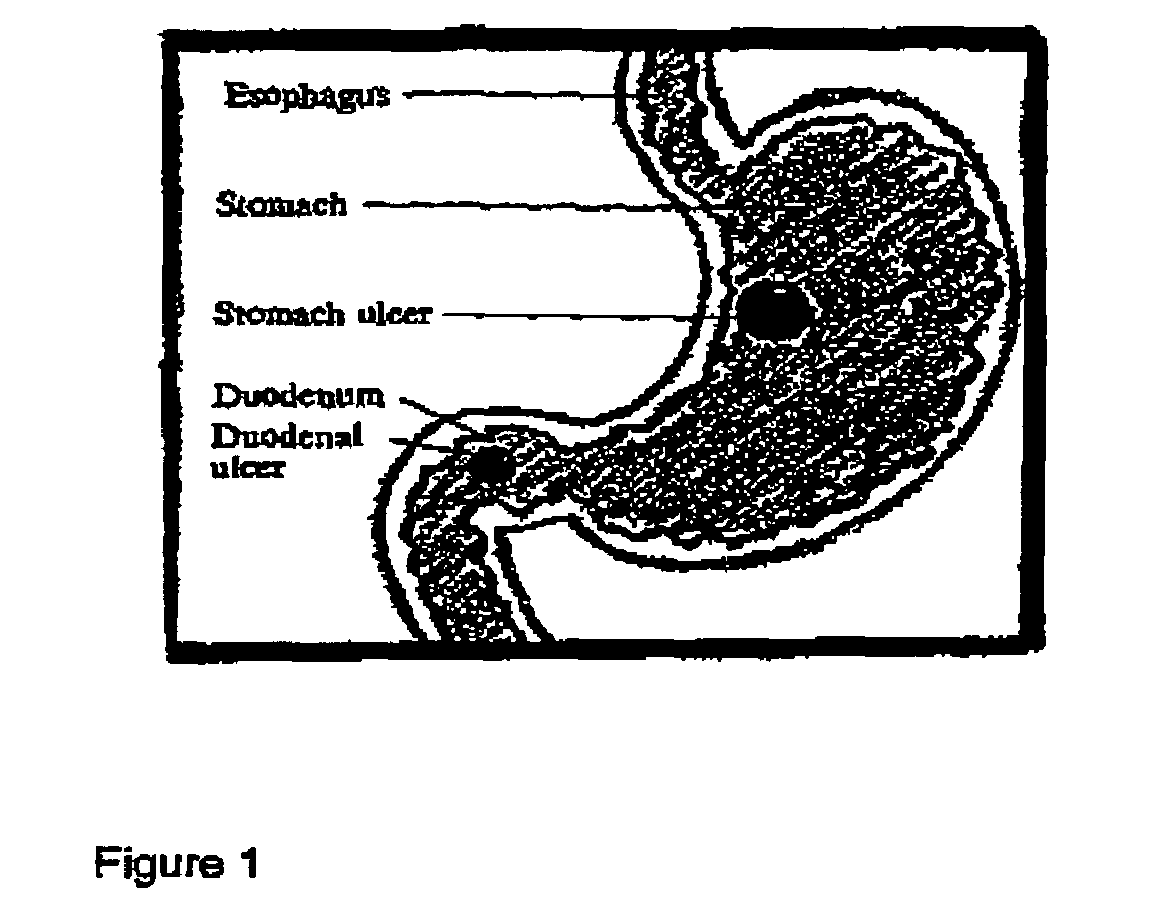
Methods for treating ulcers and gastroesophageal reflux disease
InactiveUS7238357B2Easy to changeChange effectBacterial antigen ingredientsPeptide/protein ingredientsRefluxOral medication
Methods for treating peptic ulcers and methods for treating gastroesophageal reflux disease by oral administration of a botulinum toxin.
Owner:ALLERGAN INC
Lyophilized solid taxane composition, a process for preparing said solid composition, a pharmaceutical formulation and a kit for said formulation
InactiveUS20090215882A1High level of chemical degradationImprove solid solubilityOrganic active ingredientsBiocideDocetaxel-PNPDocetaxel
A lyophilized solid composition of taxane (preferably docetaxel and paclitaxel), is suitable to prepare a pharmaceutical formulation to be administered to mammals, particularly humans, comprising a taxane, a tensoactive, a lyophilizing excipient, and acid; also essentially free from organic solvents. The solid composition is free from polysorbate 80 and polyoxyethylated castor oil; it is sterile; it is soluble in aqueous solutions in the absence of organic solvent and it has an apparent density from 0.05 g / ml to 0.45 g / ml. A procedure of double lyophilization obtains a solid composition of taxane. A pharmaceutical formulation of a taxane comprises a solid composition of lyophilized taxane and a solubilizing composition. A kit comprises the compositions and a syringe.
Owner:ERIOCHEM SA
Fast dissolving orally consumable films containing a sweetener
InactiveUS20030211136A1Fast deliverySufficient toleranceBiocideCosmetic preparationsActive agentSweetness
A consumable film adapted to adhere to and dissolve in the oral cavity of a warm-blooded animal including humans, comprising at least one water soluble polymer, a taste masking effective amount of a sweetener, and a pharmaceutically active agent having a sufficiently unpleasant taste that it is desirably masked by the sweetener.
Owner:MCNEIL PPC INC
Oral fast dissolving films for erectile dysfunction bioactive agents
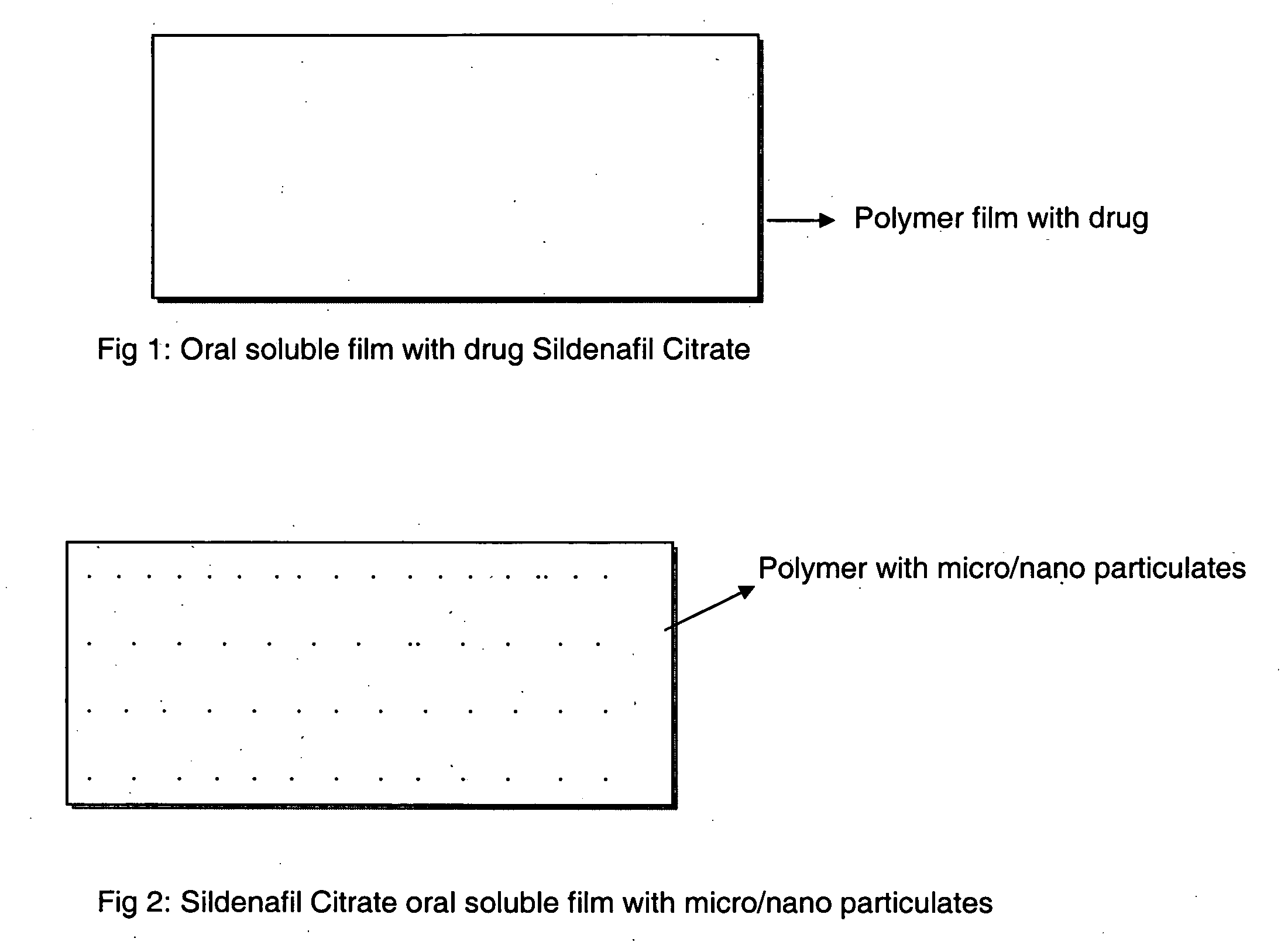
InactiveUS20090047330A1Improved ease of handlingIncrease usageBiocideAnimal repellantsVardenafilActive agent
A novel edible polymer based film dosage form manufactured using natural, synthetic, semisynthetic, pharmaceutically acceptable polymers addressing the issues of swallowing difficulties (Dysphagia and Dynaphagia), of tablet or capsule dosage forms and handling and storage difficulties associated with liquid dosage forms, that also includes materials such as emulsifying agents, suspending agents, buffering agents, effervescence agents, colorants, flavorants, sweeteners and specified amounts of bioactive agents, for erectile dysfunction. A flexible film dosage form containing sildenafil citrate, tadalafil or Vardenafil is presented. The film system is enabled to be used in various applications such as oral, mucosal and external environments.
Owner:BANGALORE RAMESH
Adhesive bioerodible transmucosal drug delivery system
InactiveUS20070207192A1Suitable bioadhesive capabilityRapid onsetAntibacterial agentsBiocideWhole bodyIrritation
The present invention is directed to a mucoadhesive delivery system for the local or systemic administration of a pharmaceutical agent. The delivery system of the invention effectively and facilely enables transport of the pharmaceutical agent through mucosal membranes and into the vasculattire of the mucosa. The delivery system includes an at least partially water soluble bioadhesive layer and an at least partially water soluble backing layer. Incorporated within either or both of these layers are the pharmaceutical agent and a mucosal penetration enhancing agent. The mucosal penetration enhancing agent displays localized tissue irritation properties. The mucoadhesive delivery system may be in the form of a gel, film, disc or patch. It may be applied to any mucosal membrane of a patient including but not limited to those of the buccal and nasal cavities, throat, eye, vagina, alimentary tract and peritoneum.
Owner:ARIUS TWO
Drospirenone for hormone replacement therapy
InactiveUS20030144258A1Dissolve fastImprove bioavailabilityBiocideOrganic active ingredientsDrospirenoneBULK ACTIVE INGREDIENT
A pharmaceutical composition comprising as a first active ingredient an estrogen, such as estradiol or estradiol valerate, in sufficient amounts to treat disorders and symptoms associated with deficient endogenous levels of estrogen in women, and as a second active ingredient 6beta,7beta; 15beta; 16beta-dimethylene-3-oxo-17alpha-preg-4-ene-21,17-carbolactone (drospirenone, DRSP) in sufficient amounts to protect the endometrium from the adverse effects of estrogen is useful for, amongst others, treating peri-menopausal, menopausal and post-menopausal women. This composition may be used for hormone replacement therapy and may be administered as a multi-phased pharmaceutical preparation. This combination therapy may comprise continuous, sequential or interrupted administration, or combinations thereof, of DRSP and estrogen, each optionally in micronized form.
Owner:SCHERING AG
Immediate release formulations and dosage forms of gamma-hydroxybutyrate
ActiveUS20110111027A1Reduce brittlenessDissolve fastBiocideNervous disorderOral medicationHigh weight
The present invention provides a solid immediate release dosage form adapted for oral administration of GHB. The solid immediate release dosage form includes an immediate release formulation comprising a relatively high weight-percentage of GHB with a bioavailability similar to that of a liquid GHB dosage form.
Owner:JAZZ PHARMA
Water soluble package and liquid contents thereof
InactiveUS6451750B2AvoidanceDissolve fastInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsHydrogen ion bindingHydrogen
A water soluble package formed from a copolymeric polyvinyl alcohol film, wherein the comonomer comprises a carboxylate function, the package containing a substantially non-aqueous liquid composition which comprises: at least one ionic ingredient with an exchangeable hydrogen ion; and a molar excess (with respect to the amount of exchangeable hydrogen ions in the at least one ionic ingredient) of a stabilizing compound effective for combining with the exchangeable hydrogen ions to hinder the formation of lactones within the film, but can be as low as 95 mole % if the stabilizing compound comprises an inorganic base and / or ammonium hydroxide.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Water soluble humic acid multi-element solid fertilizer and production method thereof
InactiveCN101570456ADissolve fastNot easy to agglomerateAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersSoil scienceWater soluble
The invention discloses a water soluble fertilizer and a production method thereof, in particular to a water soluble humic acid multi-element solid fertilizer and a production method thereof. The water soluble humic acid multi-element solid fertilizer is made by mixing water soluble humic acid, water soluble nitrogenous fertilizer, water soluble phosphorous fertilizer, water soluble potassic fertilizer and water soluble complex trace elements. Compared with the prior art, the water soluble humic acid multi-element solid fertilizer can reduce the transporting and packing cost, can be rapidly dissolved without agglomeration when in use, is applied along with water, and has the advantages of strong points, high nutrient content, complete nutrition, good water-solubility, combination of organic and inorganic matters as well as high effect of fertilizer and utilization rate. The utilization rate of product nitrogen reaches 60%, the utilization rate of phosphorus is 30-35%, and the utilization rate of potassium is 60-70%, so that the fertilizer application utilization rate is increased by more than 10% compared with the conventional fertilizer.
Owner:新疆三赢农业科技发展有限公司
Compressed composition comprising magnesium salt
InactiveUS20050220865A1Stable dissolution profileDisintegrates quicklyBiocidePill deliveryInorganic saltsCellulose
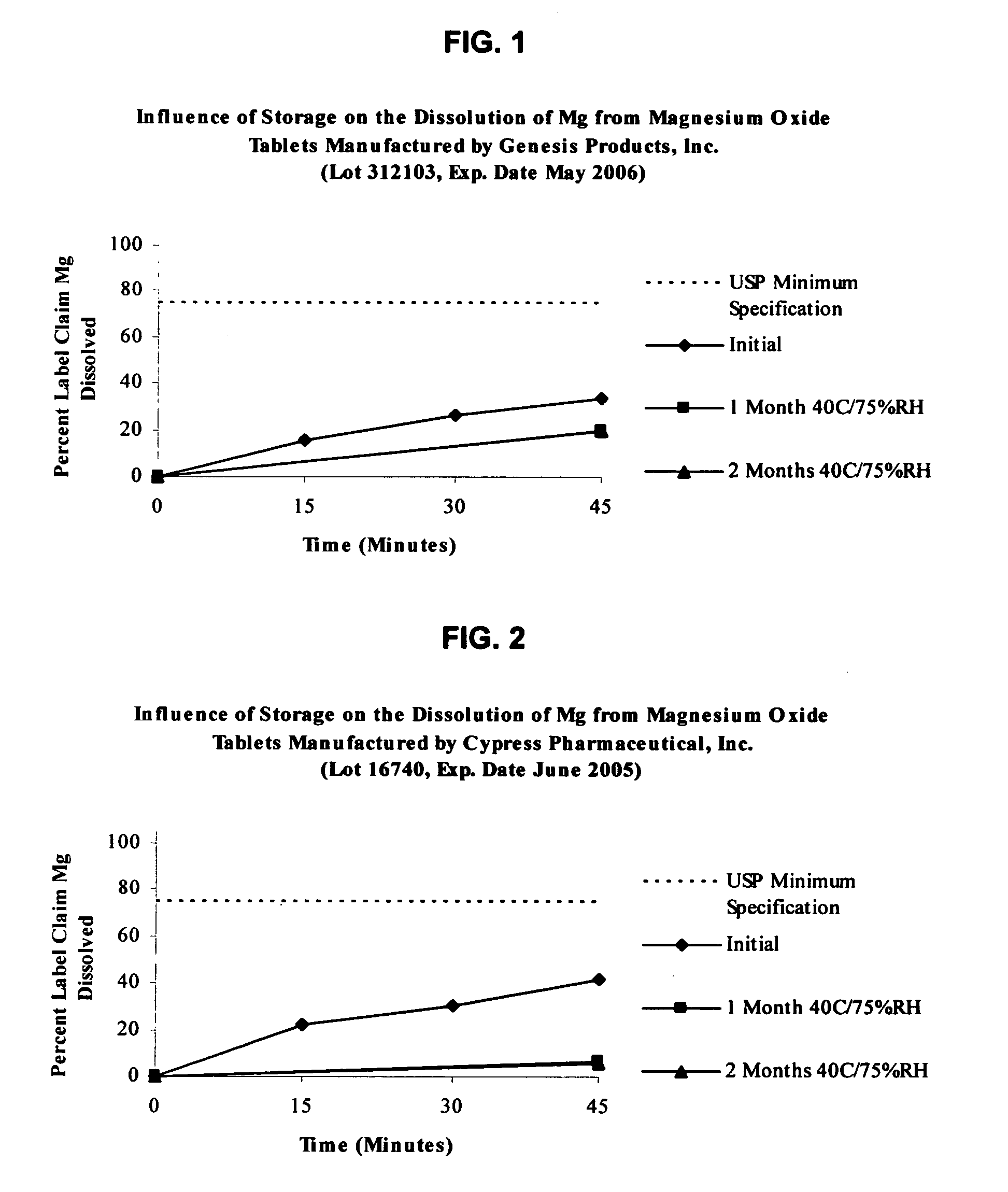
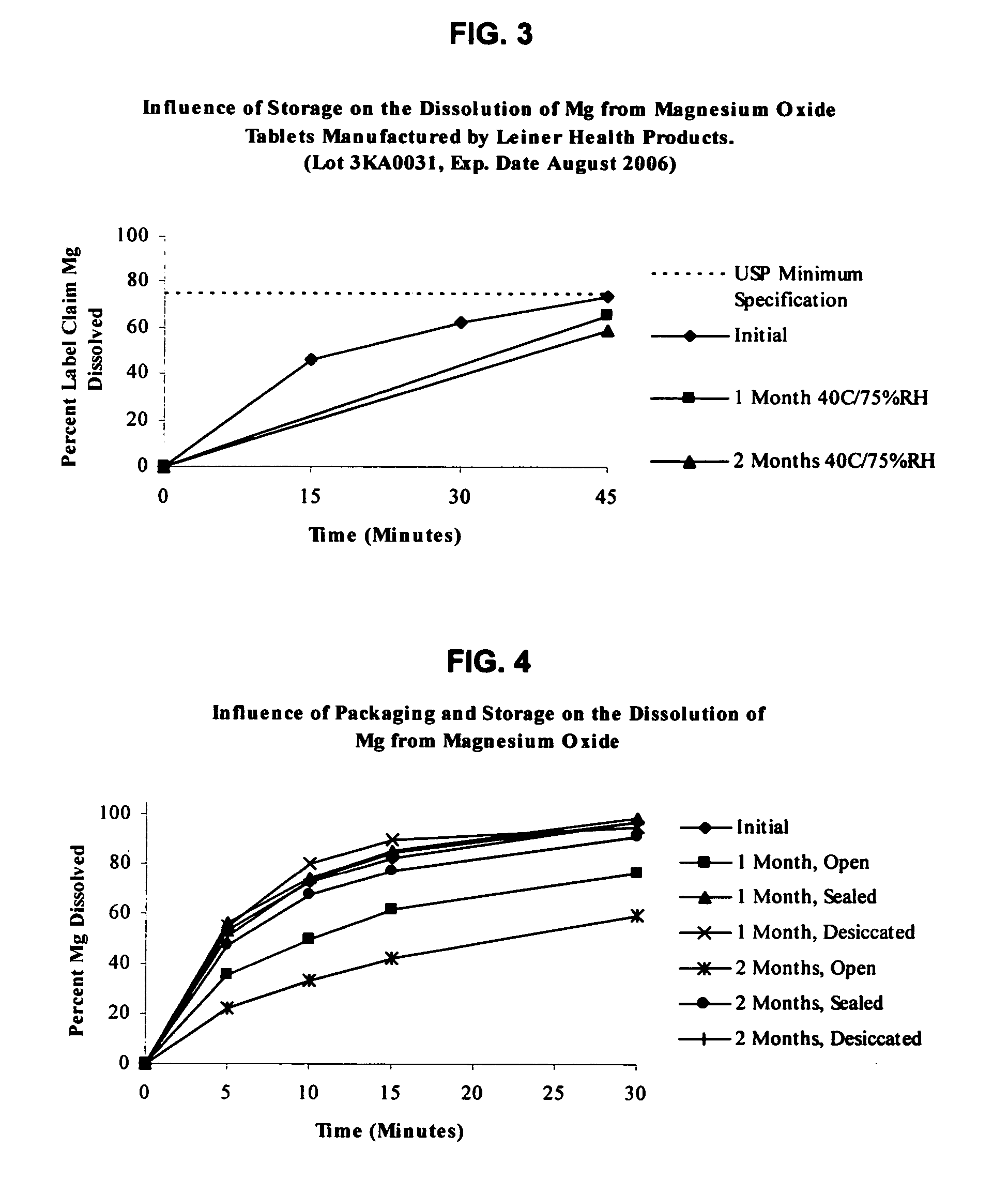
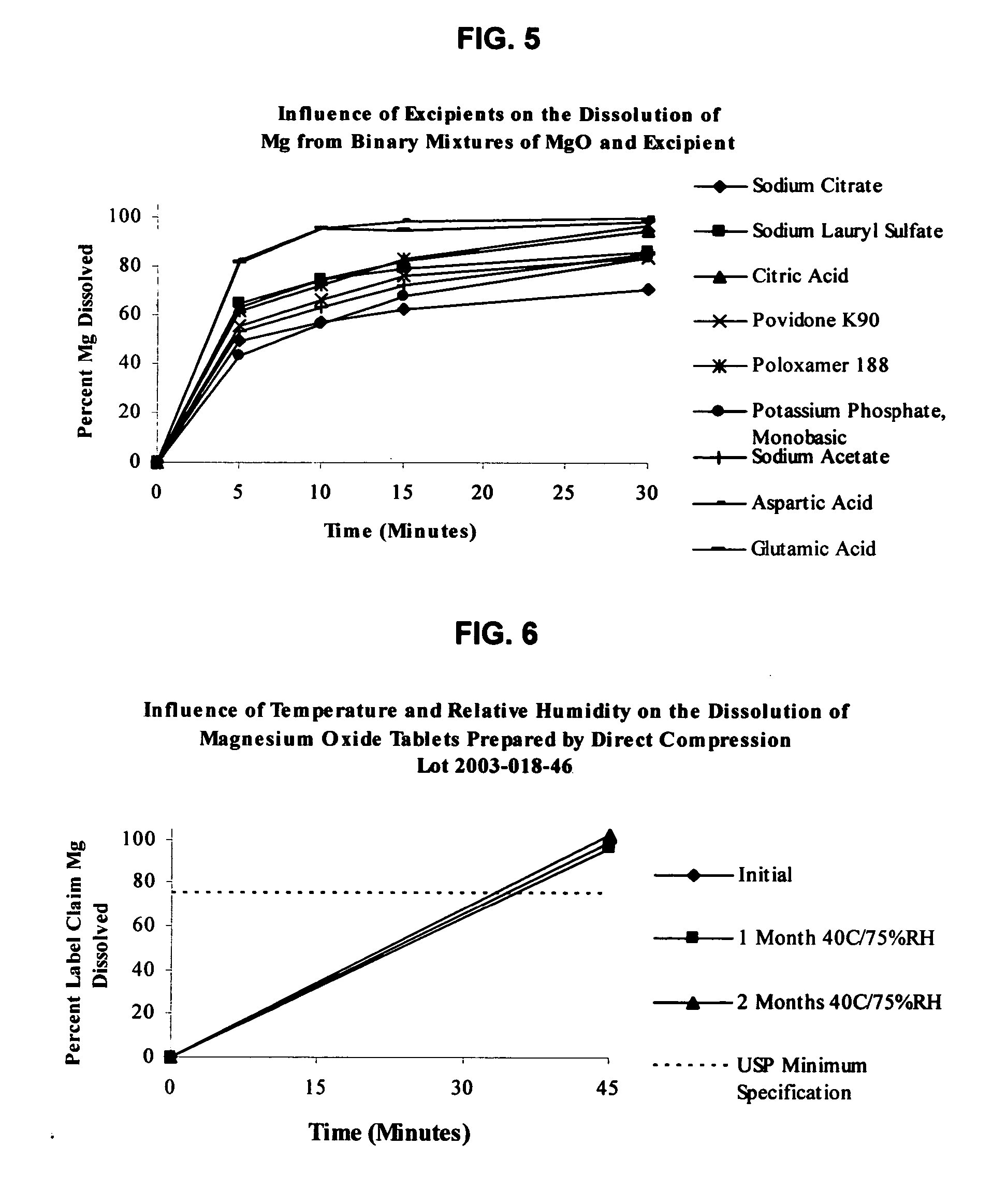
An oral solid compress composition comprising a magnesium salt is provided. The composition provides a rapid dissolution of the magnesium salt, wherein not less than 75% of the magnesium salt dissolves within 45 minutes after placement in hydrochloric acid (0.1 N, 900 mL) as per USP Method <711>. In a particular embodiment, the magnesium salt is an inorganic salt such as MgO, Mg(OH)2, MgCl2, and others. The composition can be prepared by dry granulation, direct compression or another suitable process. The composition provides a substantially stable dissolution profile for the magnesium salt so that the dissolution profile changes only minimally even after an extended period of storage under pharmaceutically acceptable conditions when packaged in a sealed container-enclosure system. The solid composition may also exclude a cellulose-based composition. The compressed composition can be prepared and stored under anhydrous conditions.
Owner:BLAINE PHARMA
In vitro methods for evaluating the in vivo effectiveness of dosage forms of microparticulate of nanoparticulate active agent compositions
Owner:ALKERMES PHARMA IRELAND LTD
Water soluble package and liquid contents therof
InactiveUS20020013243A1AvoidanceDissolve fastNon-ionic surface-active compoundsOrganic detergent compounding agentsHydrogen ion bindingHydrogen
A water soluble package formed from a copolymeric polyvinyl alcohol film, wherein the comonomer comprises a carboxylate function, the package containing a substantially non-aqueous liquid composition which comprises: at least one ionic ingredient with an exchangeable hydrogen ion; and a molar excess (with respect to the amount of exchangeable hydrogen ions in the at least one ionic ingredient) of a stabilizing compound effective for combining with the exchangeable hydrogen ions to hinder the formation of lactones within the film, but can be as low as 95 mole % if the stabilizing compound comprises an inorganic base and / or ammonium hydroxide.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Porous materials embedded with nanoparticles, methods of fabrication and uses thereof
InactiveUS20100231433A1Easy to implementReduce labor intensityShielding materialsCeramicwareNanometreNanoparticle
The present invention relates to porous structures embedded with nanoparticles, methods of forming the structures, and methods of using the structures. In most general form, the invention relates to porous materials embedded with nanoparticles having characteristics, such as magnetic, enabling to align or arrange the nanoparticles in the material by exposure, e.g. to a magnetic field. Therefore, a method according to the invention provides manufacturing materials having variable magnetic and electromagnetic properties which can be adapted during manufacture for various applications, such as electromagnetic wave absorbers, lens, concentrators, etc.
Owner:TISHIN ALEKSANDR METTALINOVICH +1
Lyophilization process and products obtained thereby
A lyophilization process which comprises dissolving a material in one or more solvents for said material to form a solution; forcing said material at least partially out of solution by combining the solution and a non-solvent for the material, which non-solvent is miscible with the solvent or solvents used and wherein said non-solvent is volatilizable under freeze-drying conditions. In addition, for hydrophobic and / or lipophilic materials, the anti-solvent can be omitted, and the solution of the material in the solvent can be subjected directly to freeze drying. The lyophilizates can then be reconstituted with typical aqueous diluent in the case of hydrophilic materials. Hydrophobic and or lipophilic materials can be initially reconstituted with propylene glycol and / or polyethyleneglycol to form a high concentration solution therein and this is further diluted for use with a diluent of Intralipid, plasma, serum, or even whole blood.
Owner:SCIDOSE PHARMA +1
Method for cleaning glass substrate
InactiveUS6568995B1High smoothnessIncrease degreeInorganic/elemental detergent compounding agentsPigmenting treatmentChemistryAqueous solution
After a polishing process of polishing a glass substrate with an abrasive containing lanthanoid oxides, the glass substrate is subjected to the first and second washing processes. In the first washing process, the polished substrate is washed with a washing solution containing acid and a reducing agent, wherein the acid includes at least nitric acid. In the second washing process, the washed substrate is treated with an aqueous solution of an alkaline detergent. The substrate is suitable for a recording medium.
Owner:HOYA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com