Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
a technology of active ingredients and solid carriers, applied in the direction of powder delivery, granular delivery, inorganic non-active ingredients, etc., can solve the problems of inability to dissolve, and inability to fully dissolv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Coated Beads
[0258]Compositions according to the present invention were prepared as follows. The specific components used are detailed in Examples 2-5.
[0259]A spraying solution of the coating materials was prepared by dissolving the desired amount of the active ingredient and mixing with the hydrophilic and / or lipophilic surfactants in an organic solvent or a mixture of organic solvents. The organic solvent used for the coating solution was a mixture of methylene chloride and isopropyl alcohol in a 3:1 to 1:1 weight ratio.
[0260]Commercially available sugar beads (30 / 35 mesh size) were coated in a conventional coating pan having a spray gun (Campbell Hausfield, DH 7500) with a nozzle diameter of 1.2 mm and an air pressure of 25 psi. The bed temperature was maintained at approximately 32° C. during the spraying process. Appropriate amounts of talc were sprinkled on the beads during the spraying process to reduce the agglomeration of coated beads. When the spraying proces...
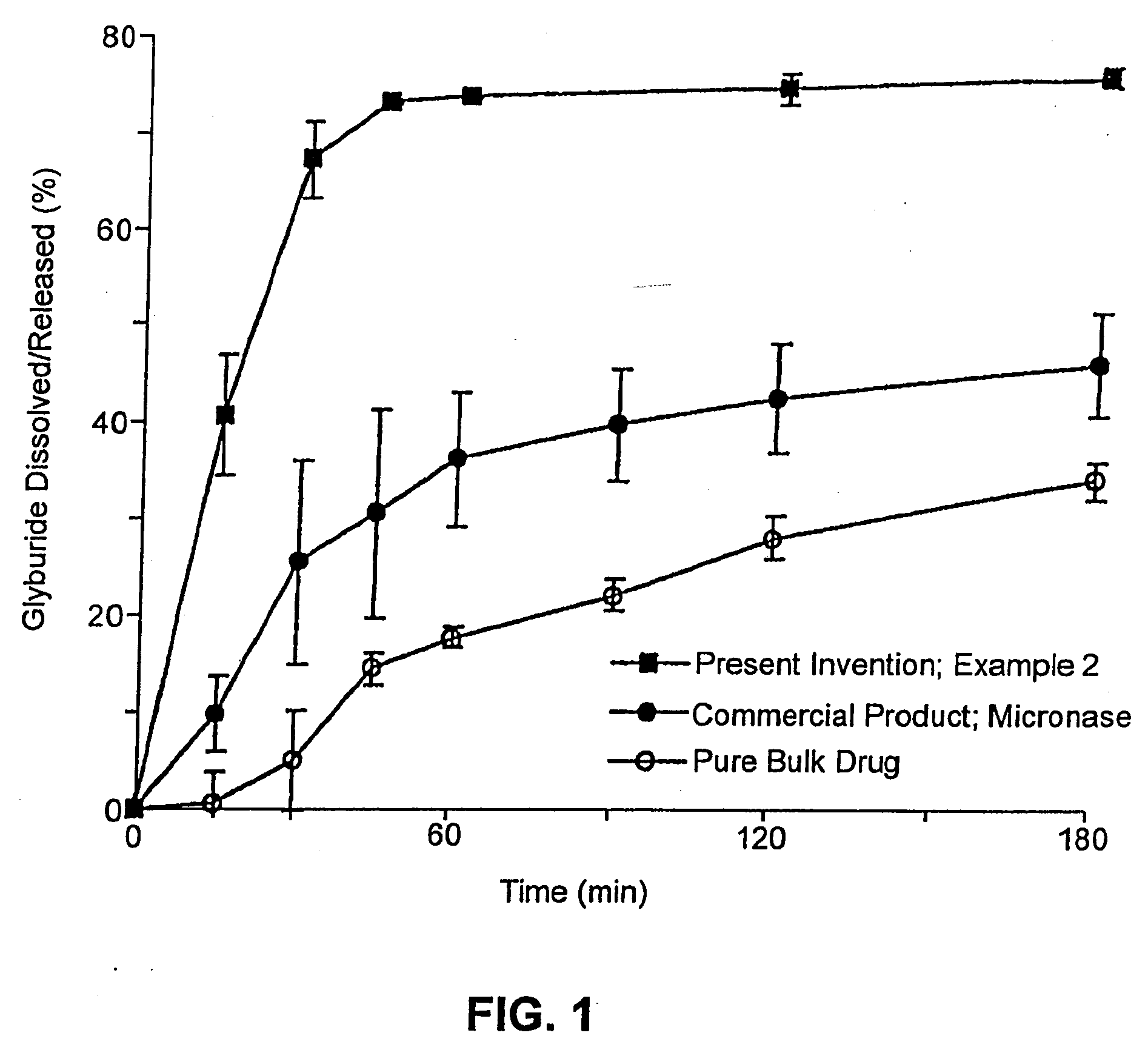
example 2
Composition I
[0261]A pharmaceutical composition was prepared according to the method of Example 1, having a substrate particle, an active ingredient (glyburide), and a mixture of a hydrophilic surfactant (PEG-40 stearate) and a lipophilic surfactant (glycerol monolaurate). The components and their amounts were as follows:
ComponentWeight (g)% (w / w)Glyburide10.8PEG-40 stearate3325.2Glycerol monolaurate1713.0Nonpareil seed (30 / 35 mesh)8061.1
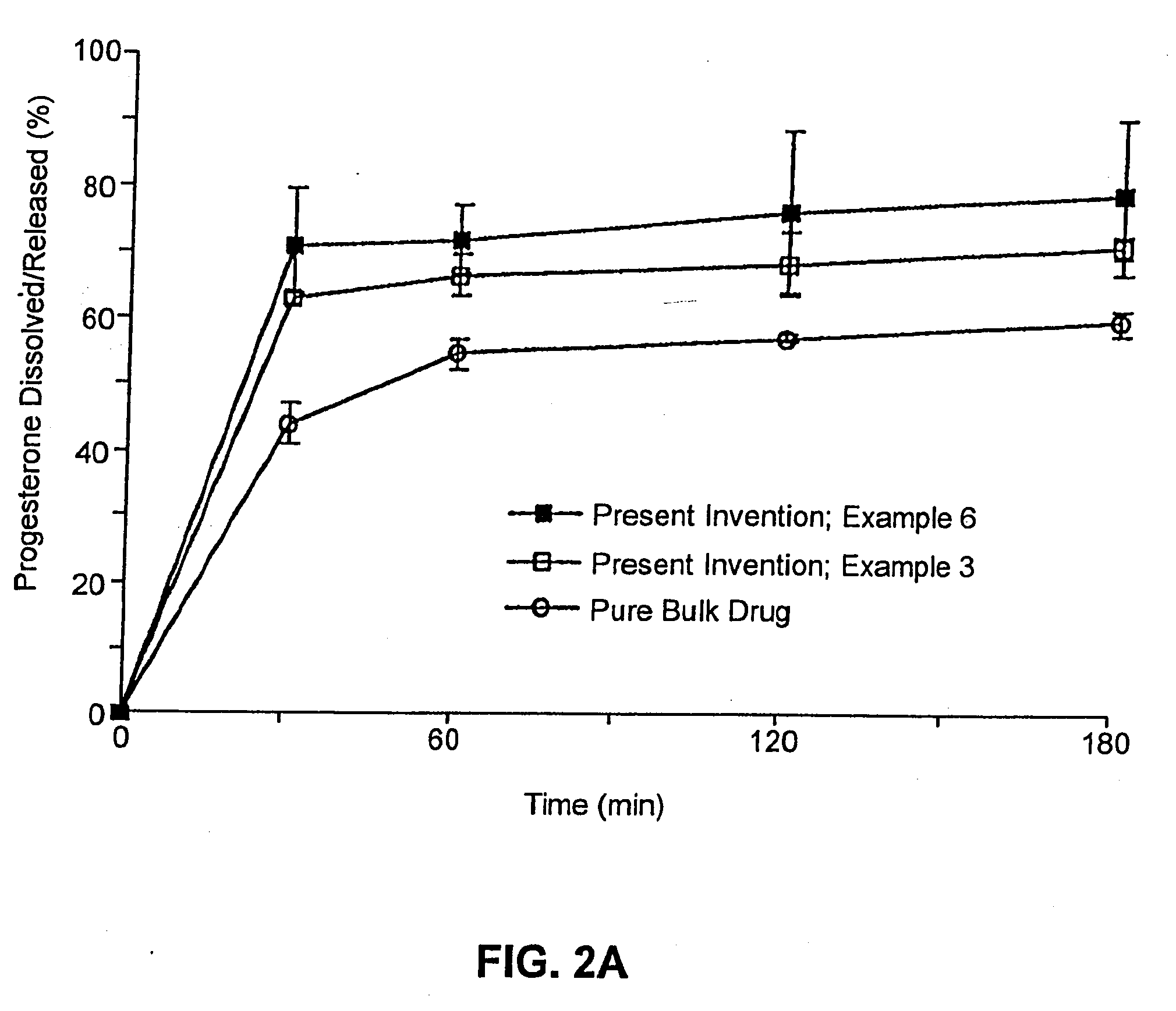
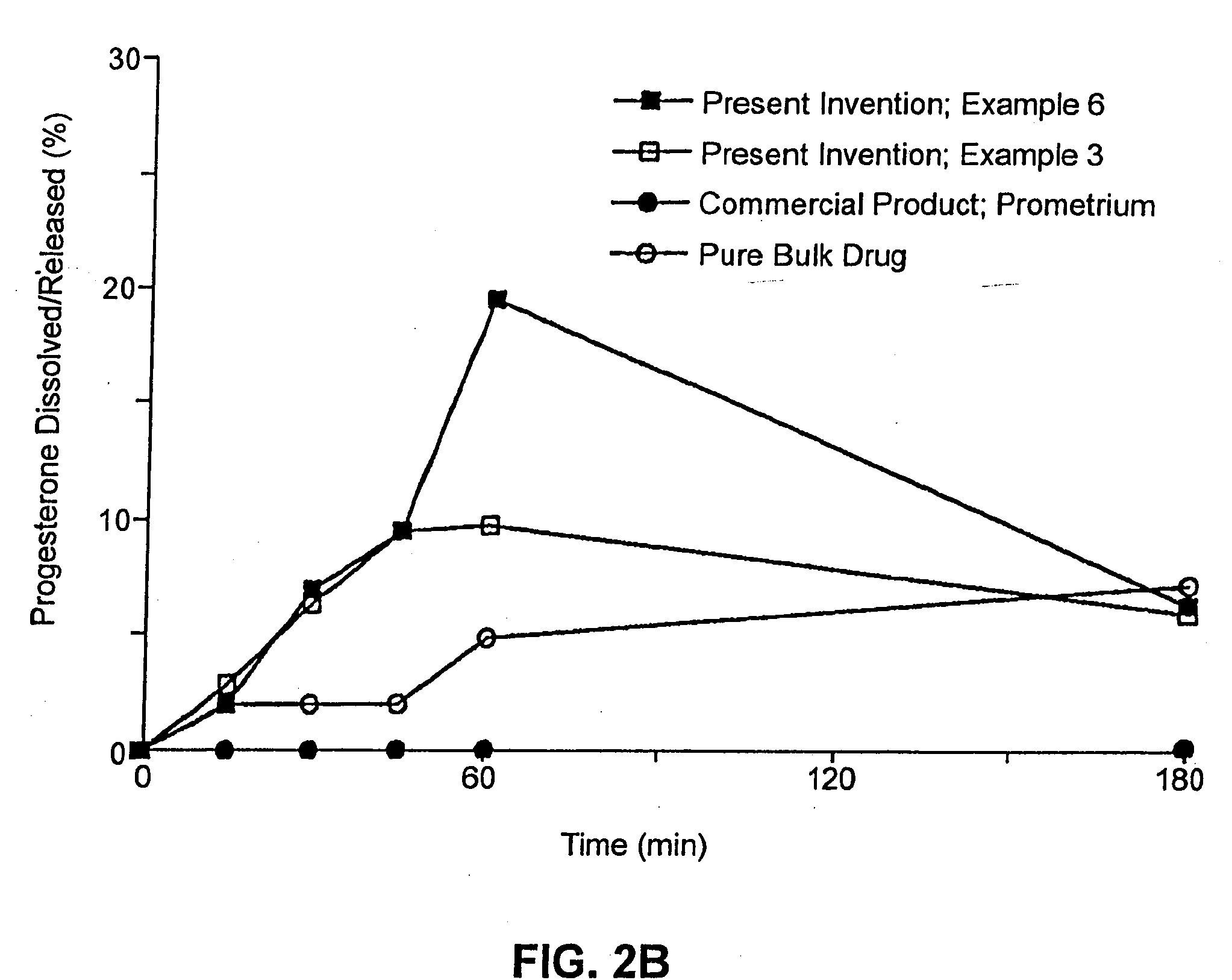
example 3
Composition II
[0262]A pharmaceutical composition was prepared according to the method of Example 1, having a substrate particle, an active ingredient (progesterone), a mixture of a hydrophilic surfactant (Solulan C-24) and two lipophilic components (deoxycholic acid and distilled monoglycerides). The components and their amounts were as follows:
ComponentWeight (g)% (w / w)Progesterone128.6Solulan C-24 (Amerchol)*3222.9Distilled monoglycerides85.7Deoxycholic acid85.7Nonpareil seed (30 / 35 mesh)8057.1*PEG-24 cholesterol ether
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| hydrophilic | aaaaa | aaaaa |
| lipophilic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com