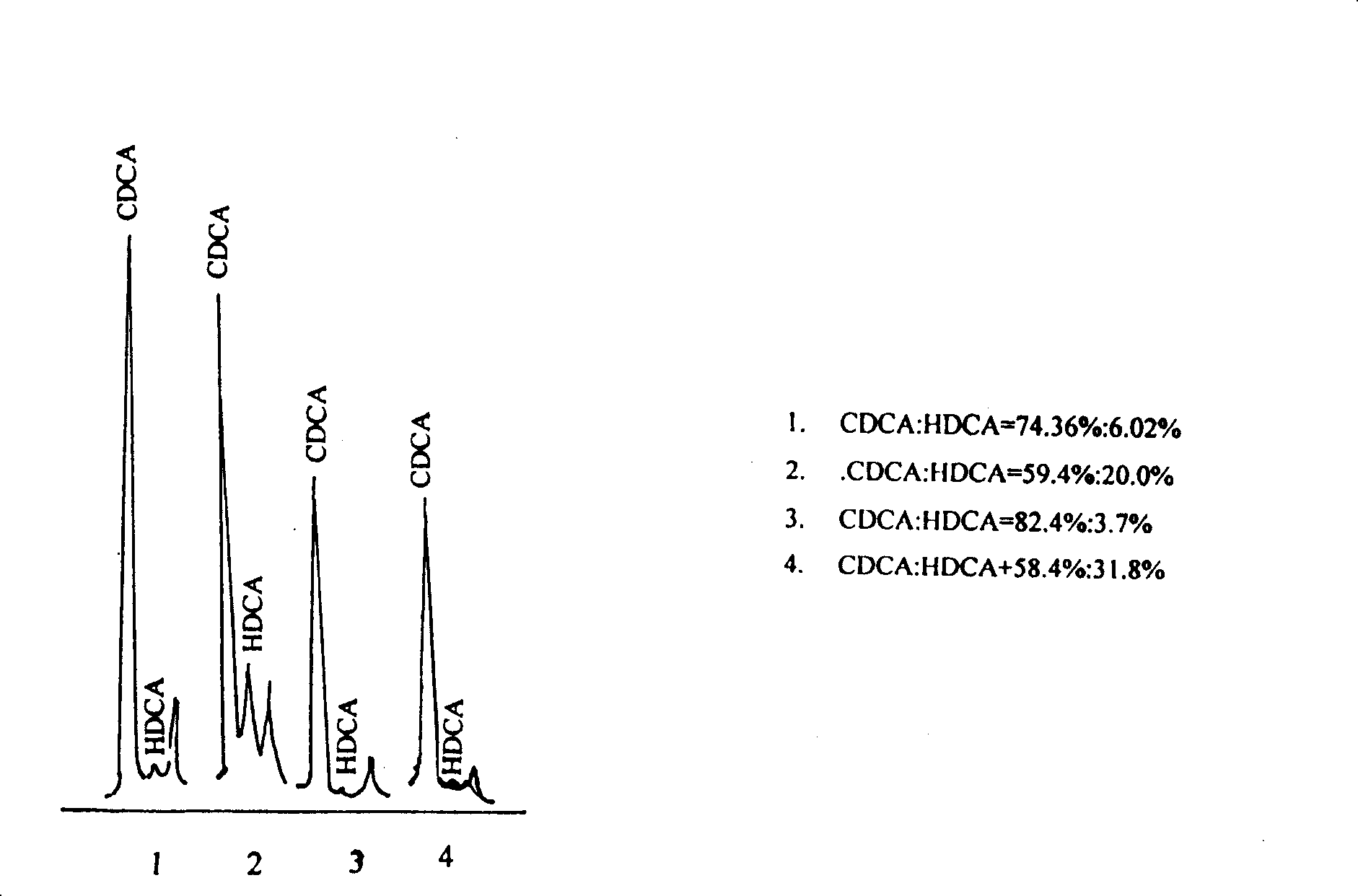
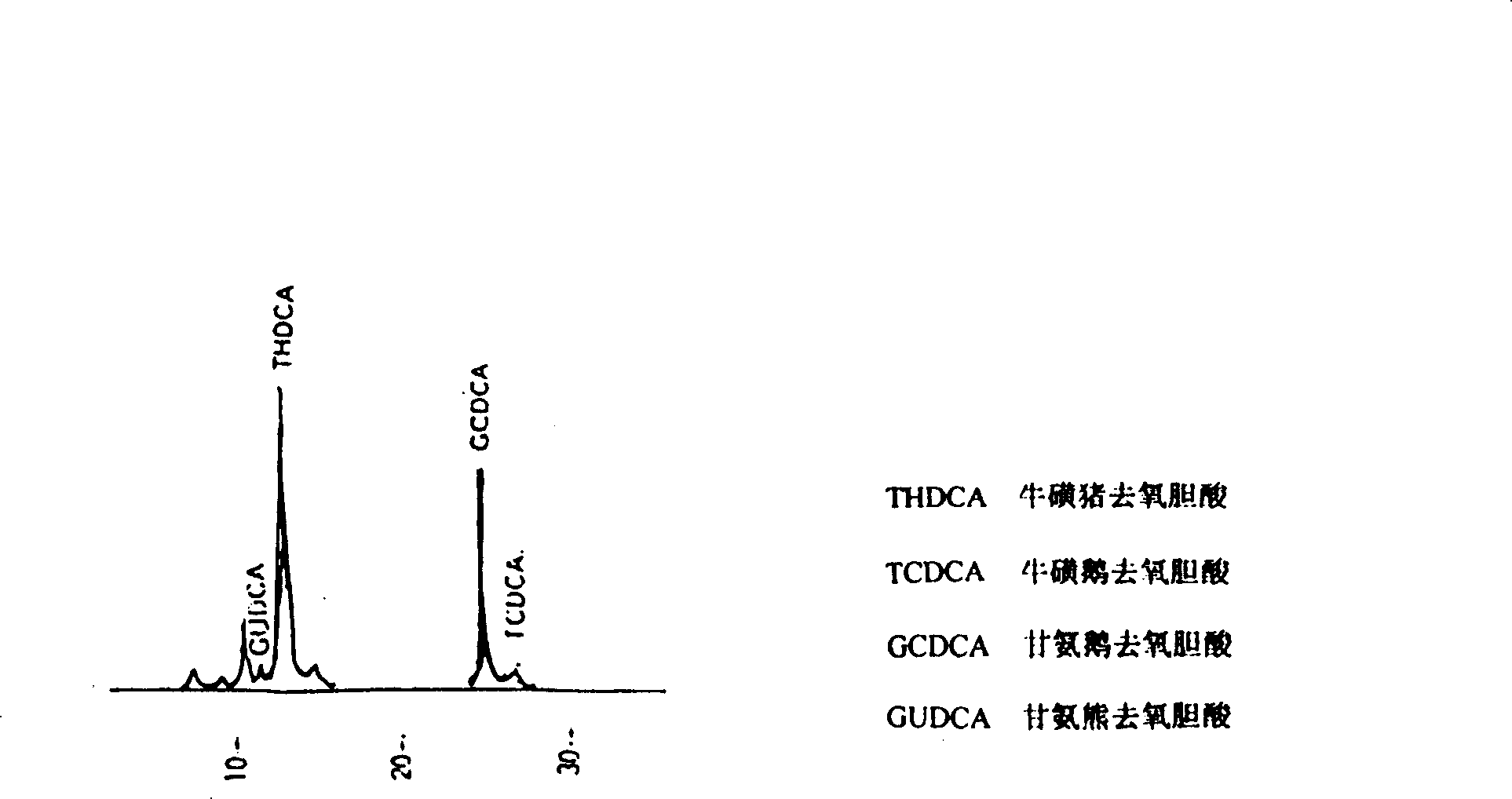
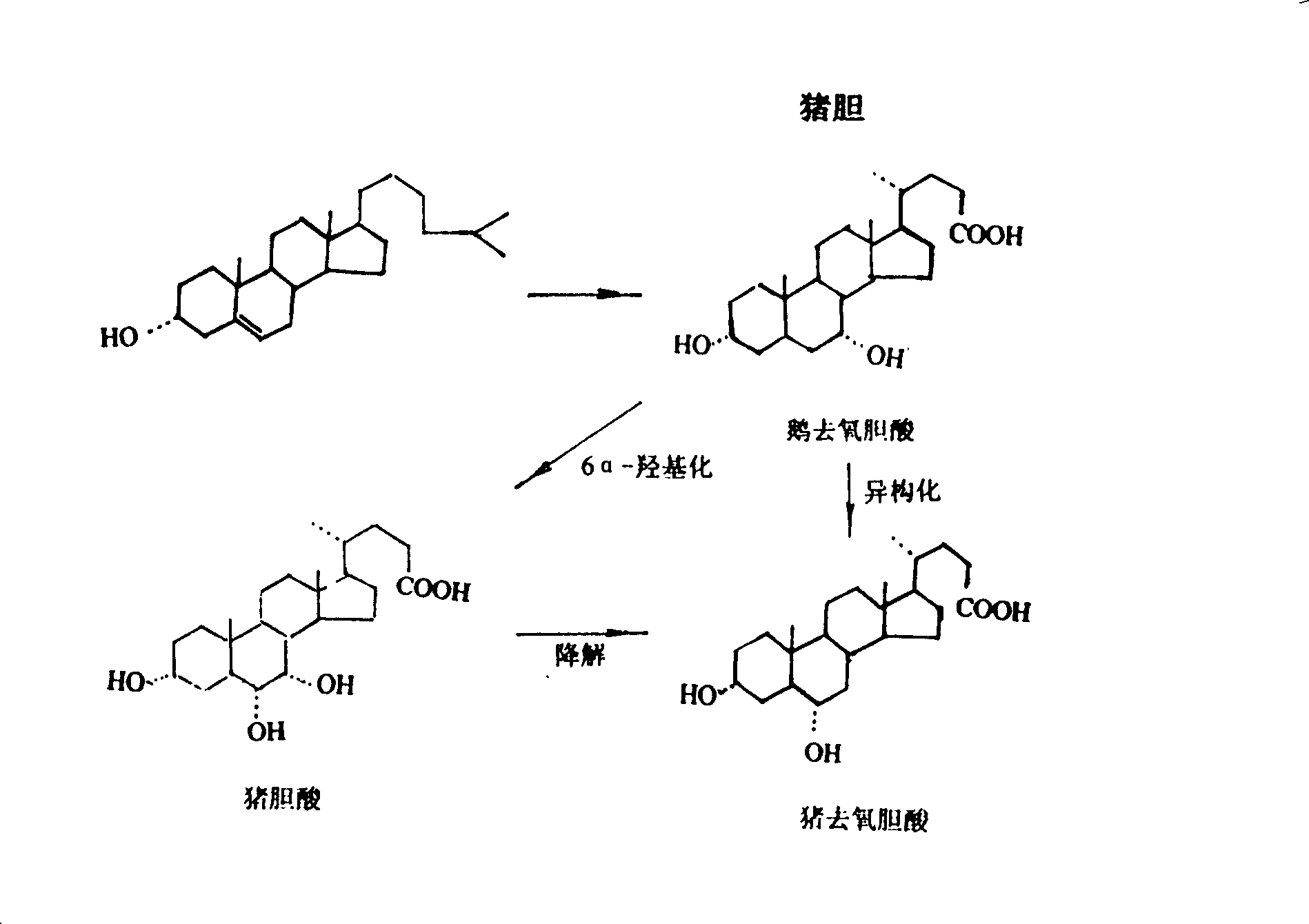
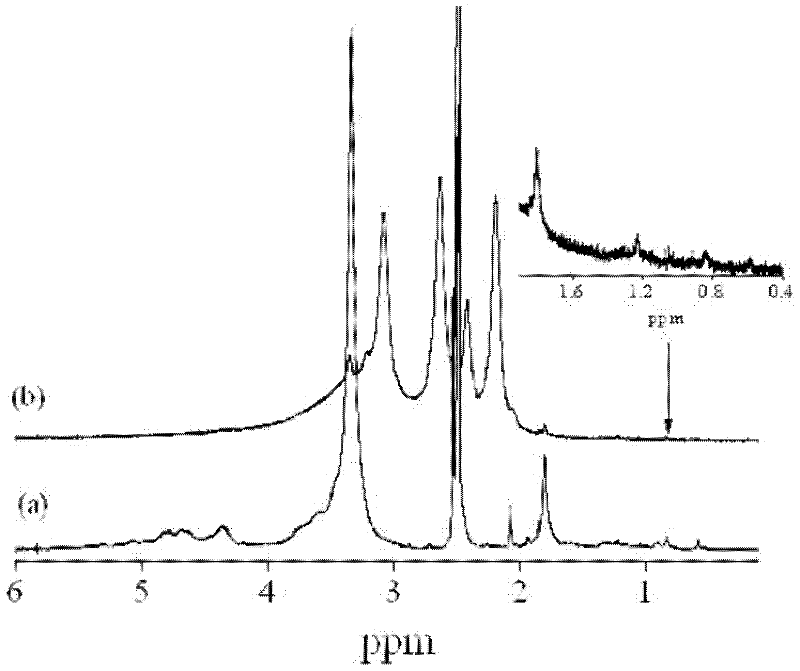
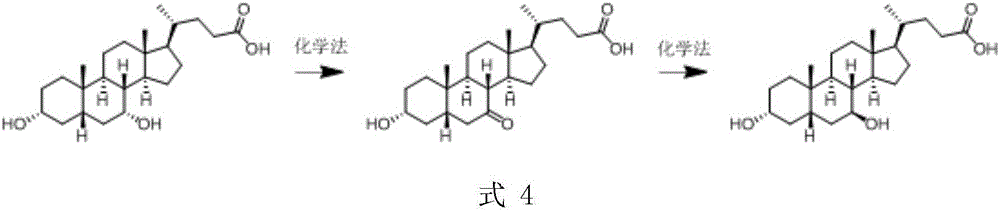
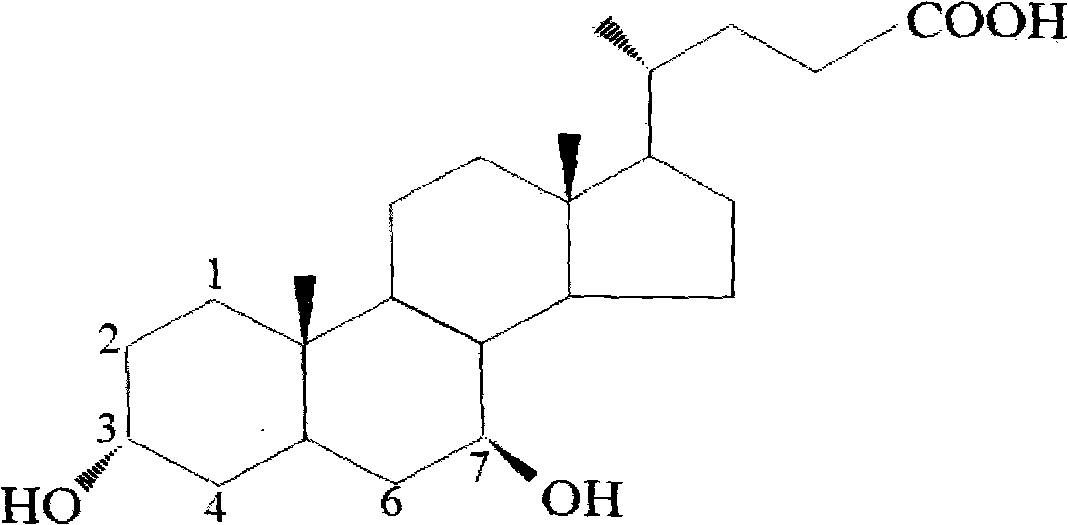
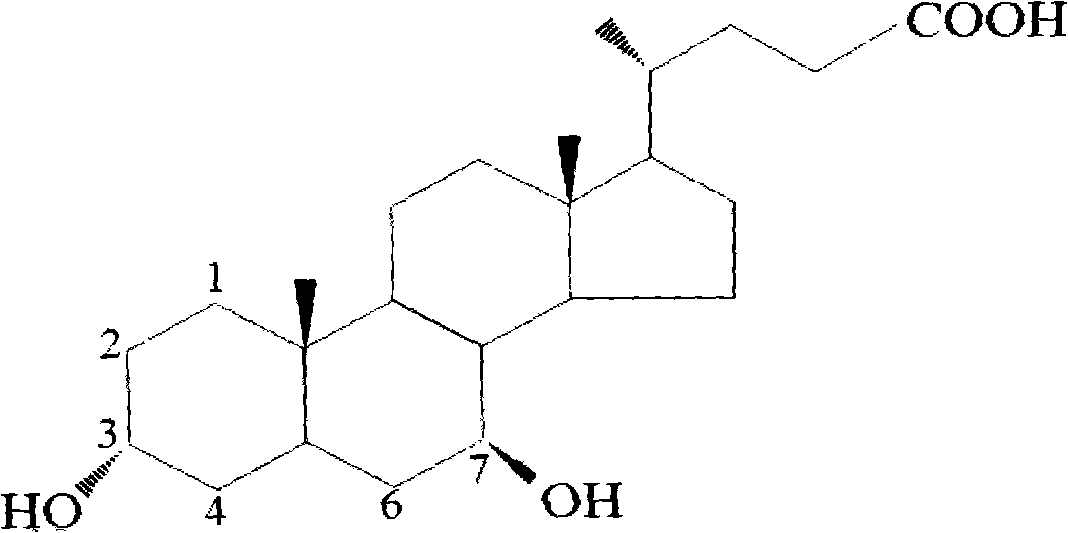
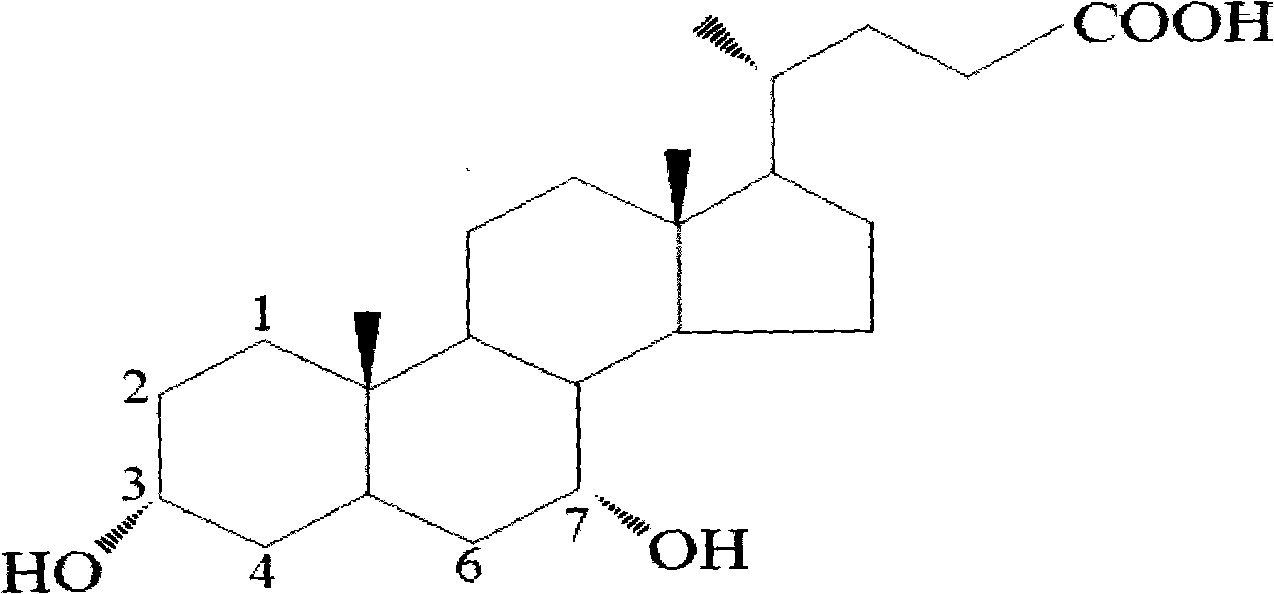
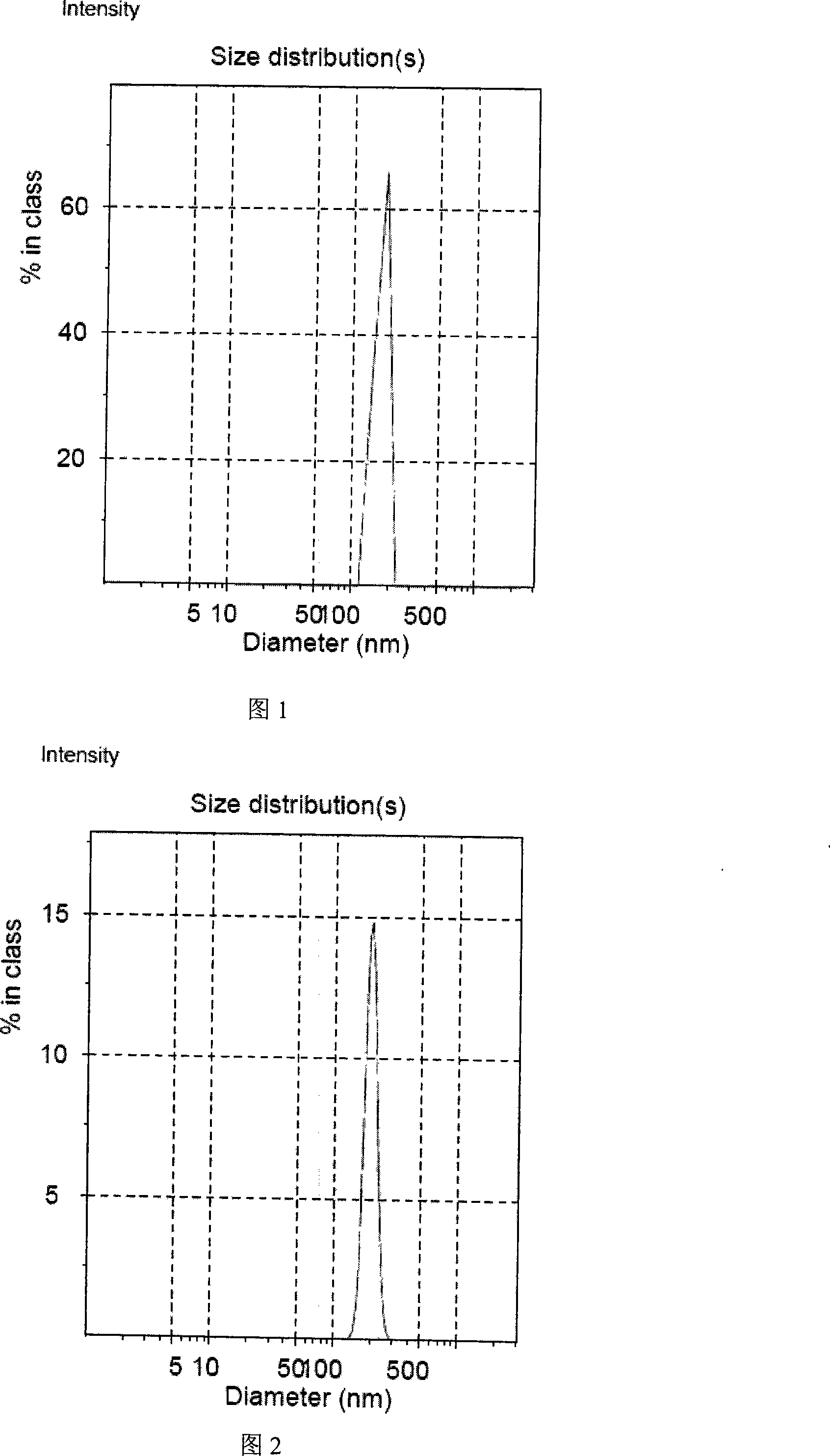
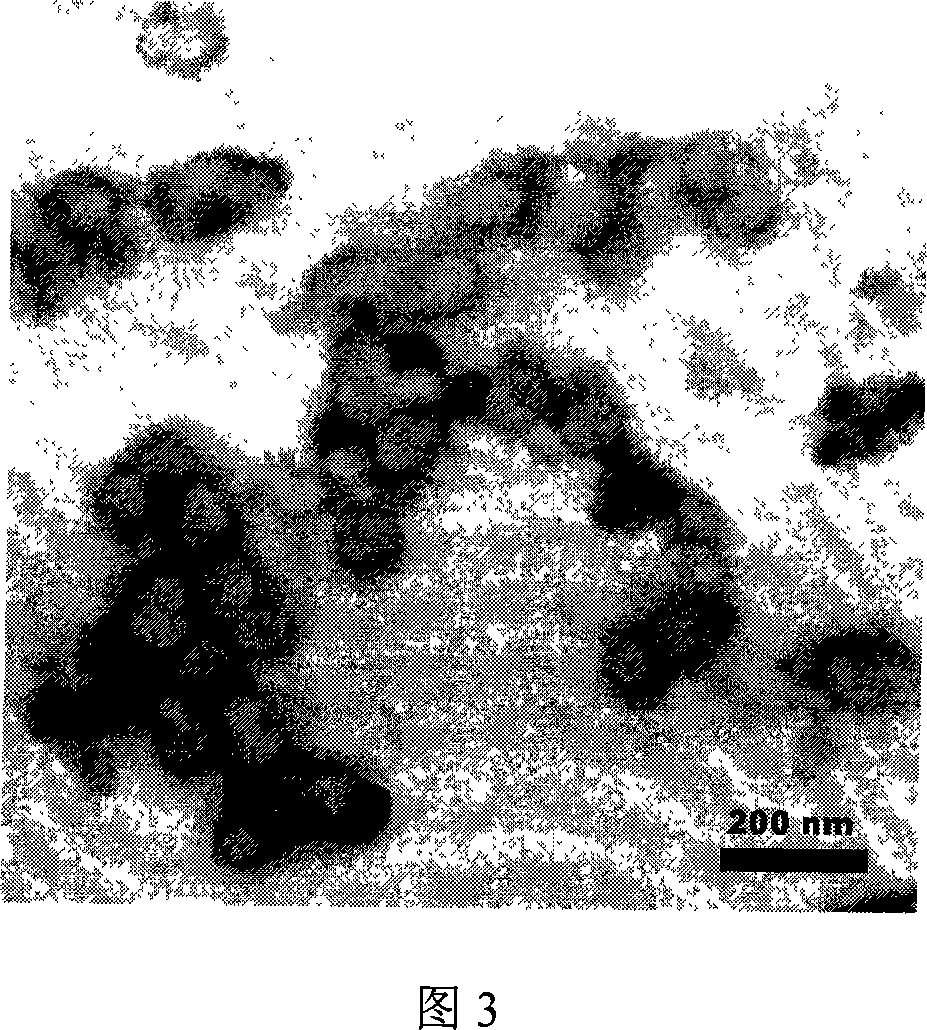
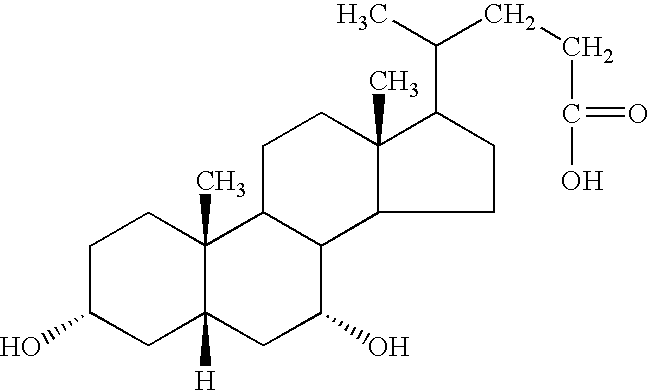
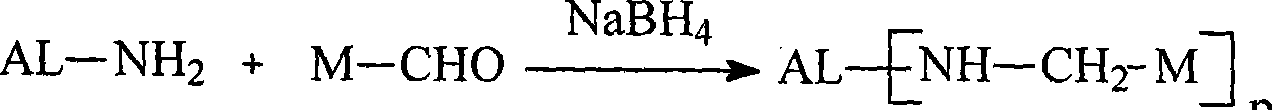Patents
Literature
485 results about "Deoxycholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
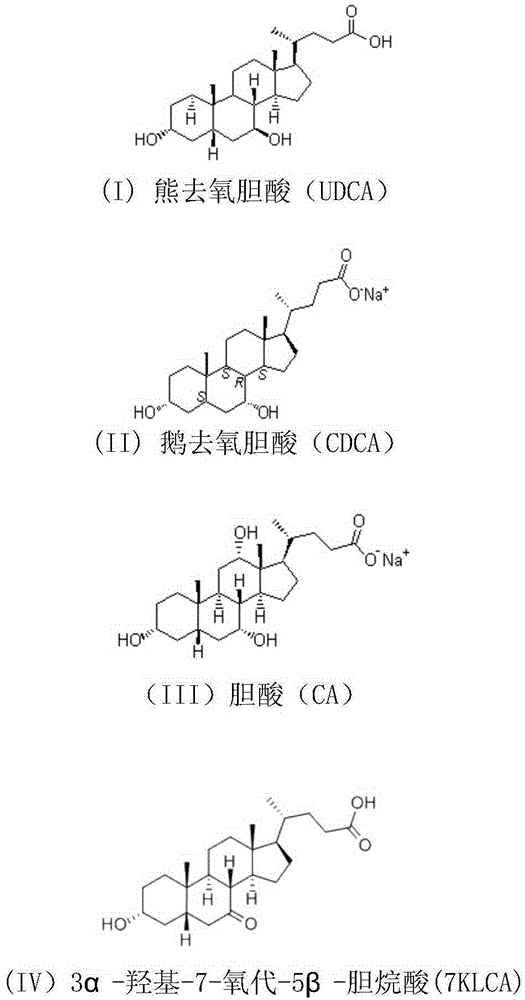
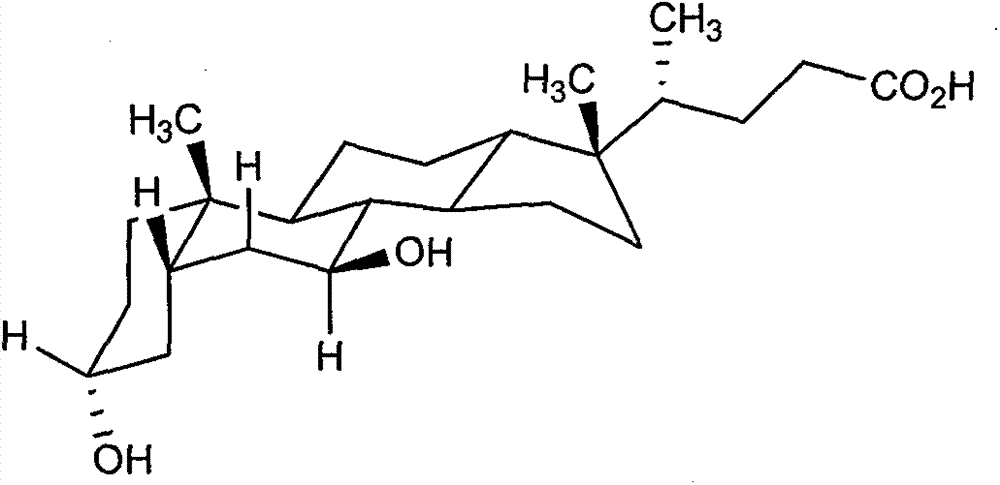
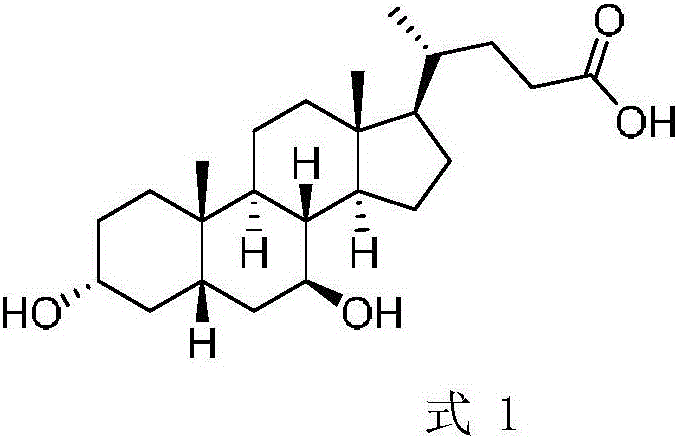
Deoxycholic acid (conjugate base deoxycholate), also known as cholanoic acid, Kybella, Celluform Plus, Belkyra, and 3α,12α-dihydroxy-5β-cholan-24-oic acid, is a bile acid. Deoxycholic acid is one of the secondary bile acids, which are metabolic byproducts of intestinal bacteria. The two primary bile acids secreted by the liver are cholic acid and chenodeoxycholic acid. Bacteria metabolize chenodeoxycholic acid into the secondary bile acid lithocholic acid, and they metabolize cholic acid into deoxycholic acid. There are additional secondary bile acids, such as ursodeoxycholic acid. Deoxycholic acid is soluble in alcohol and acetic acid. When pure, it comes in a white to off-white crystalline powder form.
Deoxycholic acid liposome-based dermatological topical preparation
InactiveUS20060222695A1Simple and rapidly transdermally deployableEffective treatmentLiposomal deliveryEmulsionTopical preparation
A dermatological topical preparation such a cream, a lotion, an emulsion, a paste, an ointment and the likes including liposomes carrying lipo-dissolving substances encapsulated by the liposomes wall or incorporated with the liposomes wall components. The lipo-dissolving substance is released by the liposomes into the target adipose tissue or its proximity after penetration of the superficial skin layers by the liposomes carrying the lipo-dissolving substance.
Owner:Z & Z MEDICAL HLDG
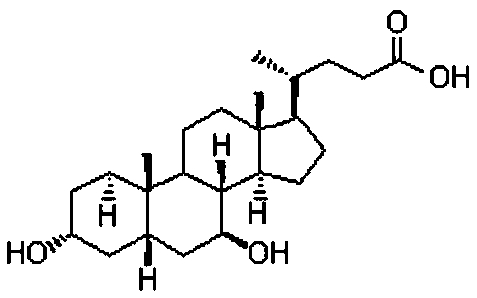
Method for preparing binding-form ursodesoxycholic acid by two-step enzymatic method
ActiveCN102994604ASimple preparation processImprove conversion rateFermentationChenodeoxycholic acidSolution state
The invention relates to a method for preparing binding-form ursodesoxycholic acid by a two-step enzymatic method, belonging to the field of biotechnology. Under the water solution state, a substrate, namely binding-form chenodeoxycholic acid, is converted into the binding-form ursodesoxycholic acid in the presence of 7alpha-HSDH and 7beta-HSDH, the separation is not needed in the intermediate step, thus the method disclosed by the invention is very simple, and furthermore, the conversion efficiency is very high. Especially chicken bile, duck bile or goose bile in a mixture form can be subjected to reaction without separating and purifying the binding-form chenodeoxycholic acid. The preparation process is simple and easy to implement, and the new, simple and convenient preparation method is provided for the binding-form ursodesoxycholic acid.
Owner:SHANGHAI KAIBAO PHARMA
Method for catalyzing chenodeoxycholic acids to compound ursodesoxycholic acids through efficient whole-cells
ActiveCN105368828AFermentation methods are cheap and readily availableSuitable for industrial productionBacteriaMicroorganism based processesChemical synthesisLactate dehydrogenase
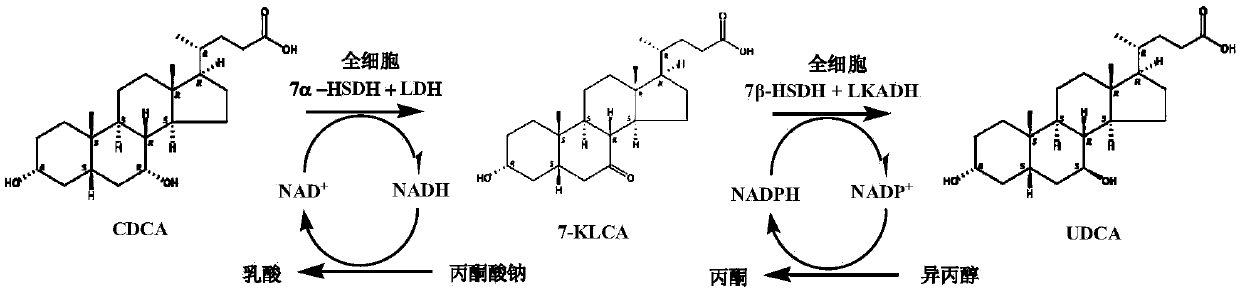
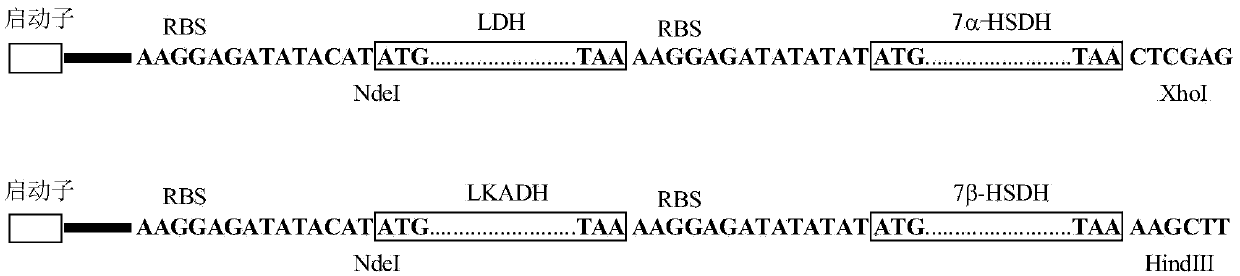
The invention provides a method for catalyzing chenodeoxycholic acids to compound ursodesoxycholic acids through efficient whole-cells. A 7a-hydroxysteroid dehydrogenase (7a-HSDH) and a lactic dehydrogenase (LDH) for regeneration of coenzyme nicotinamide adenine dinucleotide (NAD) are efficiently co-expressed in escherichia coli, escherichia coli whole cells are used to catalyze chenodeoxycholic acids (CDCA) to generate 3 alpha (Alpha)-hydroxyl-7-oxo-5bata (Beta)- cholanic acids (7-KLCA), and a reaction liquid which is obtained by catalyzing the chenodeoxycholic acids through whole cells is adjusted to be 7-KLCA crude products. Reconstitution cells can be easily obtained in low cost through a fermentation process, are better than a chemical synthesis method in production cost and product quality, and are suitable for commercial process.
Owner:苏州天绿生物制药有限公司
Compositions comprising deoxycholic acid and salts thereof suitable for use in treating fat deposits
The present application is directed to an aqueous pharmaceutical composition comprising from about 0.4% w / v to less than about 2% w / v of a salt of deoxycholic acid, wherein the composition is maintained at a pH from about 8.1 to about 8.5 such that the composition is stabilized against precipitation. Also disclosed herein, are methods for stabilizing an aqueous pharmaceutical composition comprising from about 0.4% w / v to less than about 2% w / v of a salt of deoxycholic acid against precipitation, said method comprising maintaining pH of the solution from about 8.1 to about 8.5.
Owner:ALLERGAN SALES LLC
Berberine electrostatic composite and preparation method of berberine electrostatic composite
ActiveCN102702190AIncrease biofilm permeabilityImprove oral bioavailabilitySteroidsCarboxylic acid salt preparationChemical synthesisFatty acid
The invention discloses a berberine electrostatic composite and a preparation method of the berberine electrostatic composite, which are applied to medical and health industries. The berberine electrostatic composite is prepared from anionic surfactant and berberine or berberine salt by compositing; the molar ratio of the anionic surfactant to the berberine or berberine salt is 1 to 0.5-10; the molar ratio of anion to the berberine salt is 1 to 1; the anionic surfactant is one or more of alkyl sulfate or alkyl sulfonate or fatty acid salt; the alkyl sulfate is one or more of sodium cholesteryl sulfate, lauryl sodium sulfate and deoxysodium cholate; the alkyl sulfonate is one or more of sodium heptanesulfonate and sodium dodecyl sulfate; the fatty acid salt is one or more of sodium caprate and sodium enanthate, and the berberine and berberine salt are obtained by chemical synthesis or by extracting traditional Chinese medicine. The berberine is prepared into the berberine electrostatic composite to increase the biological membrane penetrating ability of the berberine and improve the oral bioavailability of the berberine, and the berberine electrostatic composite plays a significant treatment role on important chronic metabolic disorder diseases such as diabetes mellitus, complication of the diabetes mellitus, hyperlipidemia and the like.
Owner:NORTHEAST PHARMA GRP
Method for producing ursodeoxycholic acid by using swine bladder as raw material
The invention provides a process of separating anthrodsoxycholic acid by using fresh swine bile as raw material, the invention further provides a process for preparing ursodeoxycholic acid by using fresh swine bile as raw material, which comprises extracting conjugated bile acid from swine bile after acidification, removing foreign materials such as aliphatic acid and protein and the like, preparing purified pig bile acid through hydrolysis (saponification) and hydroperoxide decolourization, extracting anthrodsoxycholic acid through solution immersion cleaning, preparing ursodeoxycholic acid through oxidization and reduction of anthrodsoxycholic acid which is obtained through separating. The process of the invention improves the preparation technology of ursodeoxycholic acid, and achieves positive effects on the aspects of technology simplification and output increase.
Owner:苏州天绿生物制药有限公司
Amphipathic chitosan derivative and preparation method and application thereof
InactiveCN102241790ARich sourcesPreparation reaction conditions are mildOrganic active ingredientsGenetic material ingredientsChemical reactionClick chemistry
The invention discloses an amphipathic chitosan derivative PAMAM-Cs-DCA (Poly(amido amine)-chitosan-deoxycholic acid). The PAMAM-Cs-DCA is prepared by sequentially grafting a PAMAM unit and a deoxycholic acid unit on a main chain of chitosan by click chemical reaction and amidation reaction. The preparation method has mild reaction conditions, high efficiency and selectivity. The invention also discloses an application of the amphipathic chitosan derivative in preparing an anticancer drug carrier: the amphipathic chitosan derivative forms nanomicelle which takes the PAMAM unit and chitosan asa hydrophilic shell and takes the DCA unit as a hydrophobic core by self assembly in a water solution, wherein hydrophobic anticancer drugs can be coated in the core, and the shell can be compounded with pDNA (plasmid deoxyribonucleic acid) to realize co-transmission of the drugs and genes. Due to the unique molecular structure, the amphipathic chitosan derivative has potential application valuesin the fields of gene therapy, controlled release of drugs, tissue engineering and the like.
Owner:SUN YAT SEN UNIV
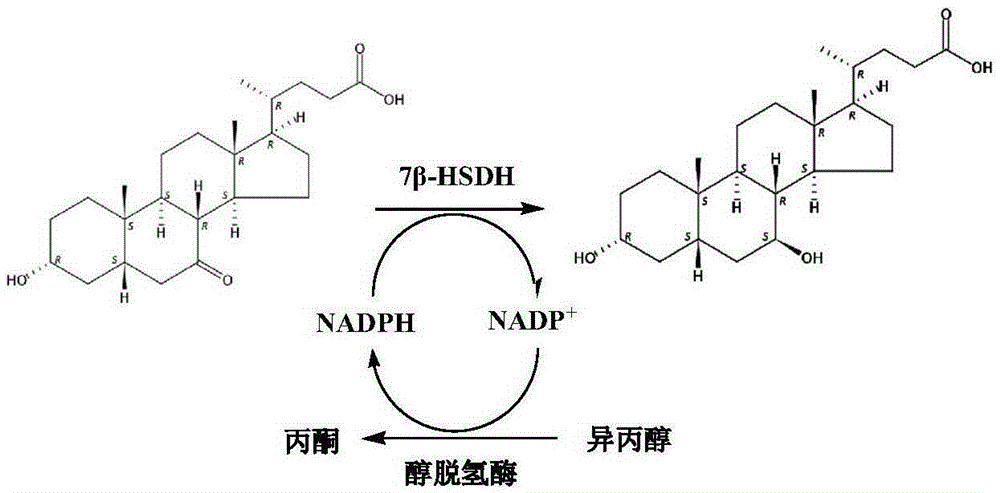
Mutant of 7 beta-hydroxyl steroid dehydrogenase, application of mutant and synthesis method
ActiveCN105274070ASuitable for industrial productionEasy to controlOxidoreductasesFermentationChemical synthesisCholic acid
The invention provides a mutant of 7 beta-hydroxyl steroid dehydrogenase, application of the mutant and a synthesis method. The mutant of the 7 beta-hydroxyl steroid dehydrogenase is characterized in that amino acid sequences of the mutant are Seq ID NO:4, and coded nucleotide sequences are Seq ID NO:3; or amino acid sequences of the mutant are Seq ID NO:6, and coded nucleotide sequences are Seq ID NO:5. The mutant, the application and the synthesis method have the advantages that cholic acid compounds, particularly ursodeoxycholic acid, can be catalytically synthesized by the efficient 7 beta-hydroxyl steroid dehydrogenase, mutant enzymes of the 7 beta-hydroxyl steroid dehydrogenase and coenzyme regeneration systems, accordingly, the substrate concentration can reach 100 g / L, the conversion rate is 99.2-99.5%, and the weight yield can reach 94-96%; and the enzymes can be inexpensively and easily obtained by the aid of a fermentation process, accordingly, the production cost and the product quality are superior to the production cost and the product quality of chemical synthesis methods, and the mutant and the synthesis method are applicable to industrial production.
Owner:苏州天绿生物制药有限公司
Preparation method of ursodeoxycholic acid
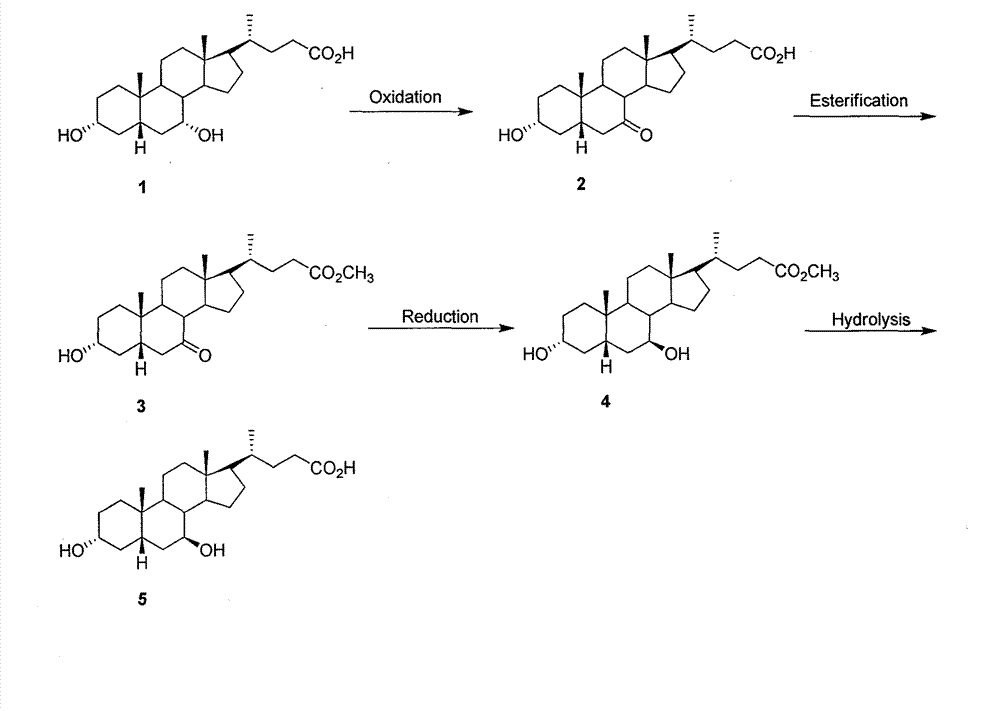
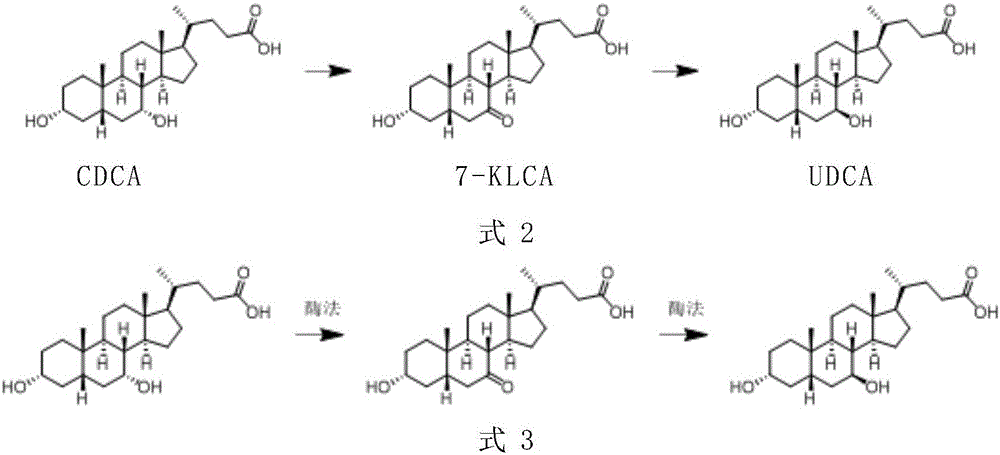
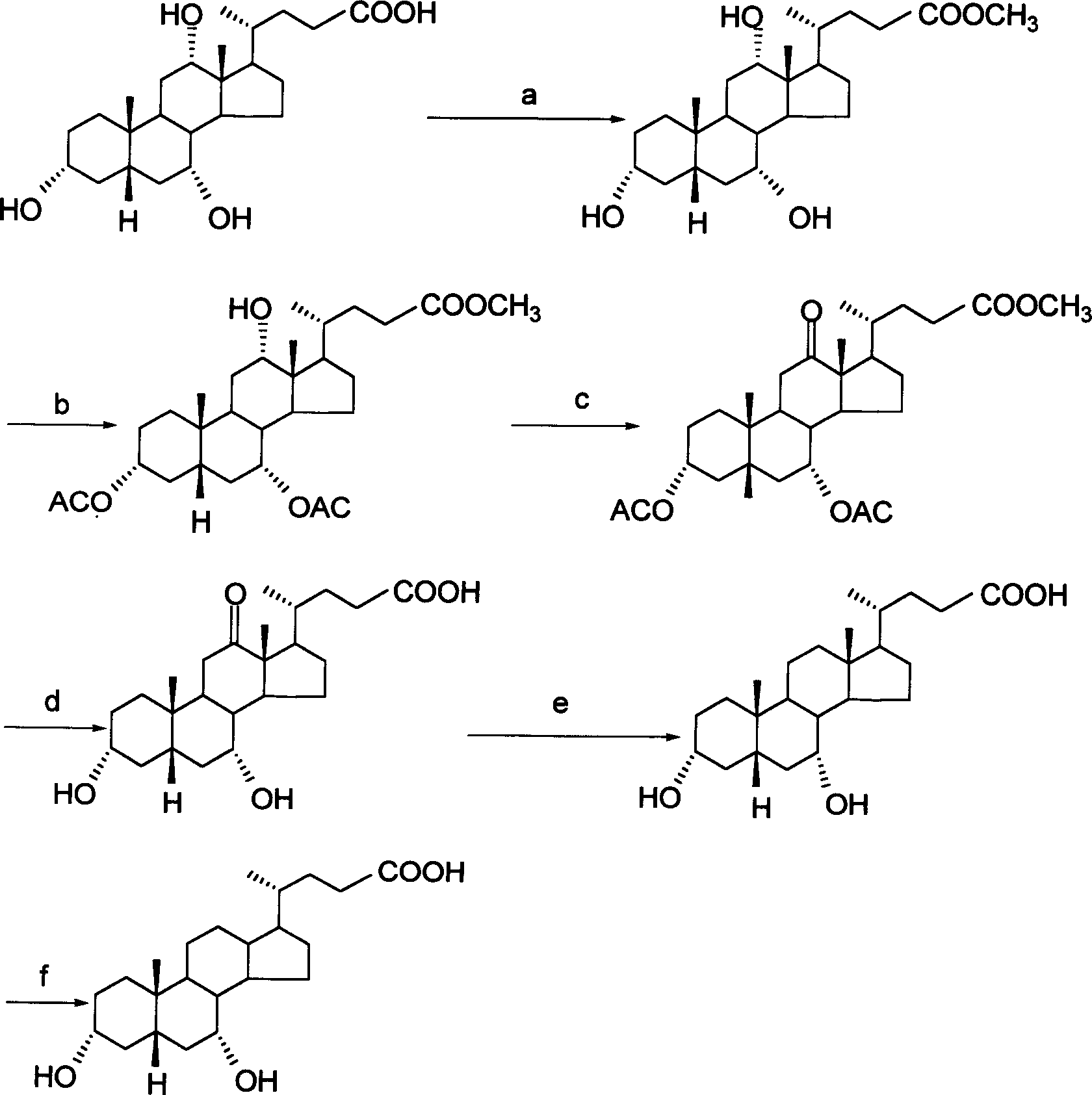
The invention provides a preparation method of ursodeoxycholic acid. Commercial available chenodeoxycholic acid is taken as raw materials, the ursodeoxycholic acid is obtained by four steps including selective oxidation, esterification, deoxidation and hydrolyzation, and the total yield is 85.7%. In a mixture of acetone and water, NBS is used for selective oxidation of hydroxy at C- 7 bit of the chenodeoxycholic acid, and the selective oxidation possesses excellent selectivity and high yield. NaBH14 / CeC13 may be used to deoxidize carbonyl at C-7 bit into hydroxy, and the ratio of alpha / beta is as high as 5 / 95.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing cheodexycholic acid
The invention relates to an industrialized preparing method of extracting and purifying chenodeoxycholic acid from fowl and animal biles. Its process includes the steps of saponifying bile by lye, preparing calcium salt deposit of total bile acid, decolorizing by oxydol and active carbon, converting calcium salt deposit of chenodeoxycholic acid by sodium carbonate and HCl, purifying by the marcoporous absorbing resin, and so on. It has advantages of friend environment, safety and innocuity, low cost and be easy for large-scale operation.
Owner:EAST CHINA UNIV OF SCI & TECH
A chemical-enzyme method of preparing ursodeoxycholic acid
ActiveCN106086149AHigh ee valueEliminate enzyme inactivationSteroidsFermentationChenodeoxycholic acidOxidizing agent
A chemical-enzyme method of preparing ursodeoxycholic acid is disclosed. The method adopts chenodeoxycholic acid as an initial substrate, and prepares the ursodeoxycholic acid through a chemical process and a bio-enzyme process in order, wherein 7-KLCA reductase is adopted as a biological catalyst. A situation that an oxidant residual in a process of preparing 7-ketolithocholic acid through a chemical manner causes later enzyme inactivation in the prior art does not occur. A product prepared by the method is high in ee value and low in comprehensive cost.
Owner:ENZYMEWORKS
Preparation method of ursodesoxycholic acid
ActiveCN101987860AAvoid disadvantagesHigh puritySteroidsBulk chemical productionCholic acidChenodeoxycholic acid
The invention discloses a preparation method of ursodesoxycholic acid, which comprises the flowing steps of: a. carrying out 3-bit esterification on chenodeoxycholic acid to obtain 3-esterification protected chenodeoxycholic acid; b. carrying out 7-bit oxidation reaction on 3-bit ester to obtain 3-estergroup-7-oxocompounds; c. carrying out hydrolysis reaction or reduction reaction on the 3-estergroup-7-oxocompounds; d, carrying out 7-bit reduction reaction on 3a-hydroxy-7-oxo-5 beta-cholic acid or hydrolysis reaction on 3a-estergroup-7 beta- hydroxyl-5 beta-cholic acid to obtain crude products of the ursodesoxycholic acid; e. forming salt through the crude products of the ursodesoxycholic acid and organic base; and f. carrying out steps such as water adding dissolution, acid adding crystallization and the like on ursodesoxycholate to obtain pure ursodesoxycholic acid products with the purity higher than 99.0 percent. The invention aims at overcoming the defects in the prior art to provide a novel method for preparing the ursodesoxycholic acid, and the ursodesoxycholic acid prepared by the method has high yield and high purity.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
High efficiency technique for extracting bilirubin and bile acid by using animal bile as raw material
The present invention relates to process of extracting cholic acid, deoxycholic acid and bilirubin from bile of pig, ox and sheep. Bile of pig, ox and sheep consists of water in about 97 %, bile acid in about 2.5 % and bilirubin in about 0.4 %; and contains also phospholipid, cholesterol, Na, K, Ca, phosphate, carbonate, small amount of protein, and other components. Fresh bile is treated through cooling, filtering to defat, basic hydrolysis, acidification and organic solvent extracting to obtain bilirubin; the rest solution is further treated through deep saponification, acidification and organic solvent precipitation to obtain the mixture of cholic acid and deoxycholic acid; and the mixture is re-crystallization separated to obtain high purity cholic acid and deoxycholic acid.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Production method for extracting chenodeoxycholic acid using chicken gall
InactiveCN1850846AEasy to separateSimple processUnknown materialsSteroidsCholic acidChenodeoxycholic acid
The feature of the invention includes: adopting frozen chicken gallbladder slicing, heating to 70 degree centigrade, adding 10% weight of the bile boiling for 24 hours, reflux cooling, adjusting the pH value by hydrochloric acid to gain cream, washing to neutrality; adding 95% alcohol and 10% diatomite, reflux cooling and adding active carbon, reflux, cooling and filtering, adding petrol to the filtrate to take degreasing, reflux, standing, separating alcohol liquid and washing to neutrality in reaction kettle, adding 95% alcohol, after resolving, adding barium chloride and active carbon, reflux, hot filtering, concentrating white crystal from the filtrate, washing, and adding water and sodium carbonate water solution, heating and reflux, filtering, adjusting the pH value, drying. The invention has simple technology, low cost, and could drastically separate cholesterol, lithocholic acid, and cholic acid from gall, and improves the purity.
Owner:辽宁百隆生物工程有限公司
Method for preparing high-purity hyodeoxycholic acid by pig bile
InactiveCN101037463AReduce the difficulty of purificationLow impurity contentDigestive systemUnknown materialsBenzeneHyodeoxycholic acid
The invention relates to a method for extracting the hyodeoxycholic acid from the leftovers of pig bile or extracted bilirubin, including saponify the leftovers with alkali, adjusting pH value, acetic ester extraction, decoloring with active carbon; concentrating the mother liquid to a proper volume, precipitating deposit, separating the deposit and drying to get coarse hyodeoxycholic acid; esterifying the coarse product, then doing an addition reaction with benzene, separating the methylhyodeoxycholanate-benzene addition compound; decomposing the addition compound with alkali, adjust pH value, getting the purified deoxycholic acid. The obtained mother liquid can be further extracted to get precious chenodeoxycholic acid. The craft has a lot of material, a low pollution, safety and no poison, a low cost, a high product purity and is suitable for industry production in a large scale.
Owner:JIANGSU UNIV
Folic acid-modified O-carboxymethyl chitosan-deoxycholic acid complex and preparation method and application thereof
InactiveCN102319436AGood biocompatibilityPromote degradationPharmaceutical delivery mechanismPharmaceutical non-active ingredientsO carboxymethyl chitosanSide effect
The invention discloses a folic acid-modified O-carboxymethyl chitosan-deoxycholic acid complex which is an active-targeting nano-micelle carrier material prepared by using folic acid, O-carboxymethyl chitosan and deoxycholic acid as raw materials; O-carboxymethyl chitosan is used as a carrier material; deoxycholic acid is used for hydrophobicity reconstruction; folic acid is used for surface modification; and folic acid-mediated tumor tissue-targeting polymer micelle is prepared. Drug-loaded nano-micelle is prepared by a self-assembly method with paclitaxel as a model drug; experiment demonstrates that the drug-loaded nano-micelle has high drug load and encapsulation efficiency, has sustained release effect, is intaken into tumor cells through a folic acid acceptor approach, can increase the distribution of the drug in tumor tissue, thus improves curative effect, reduces toxic and side effect, and reaches the purpose of targeting treatment. The invention provides an ideal and novel drug carrier and preparation form for hard-soluble antitumor drugs.
Owner:SHANDONG UNIV
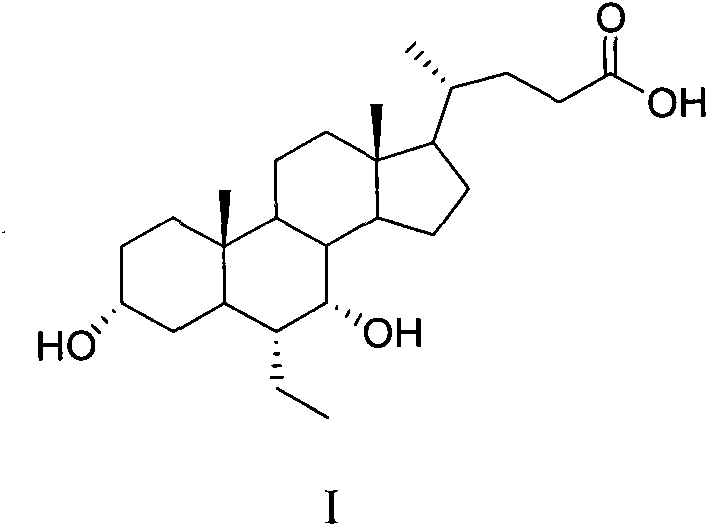
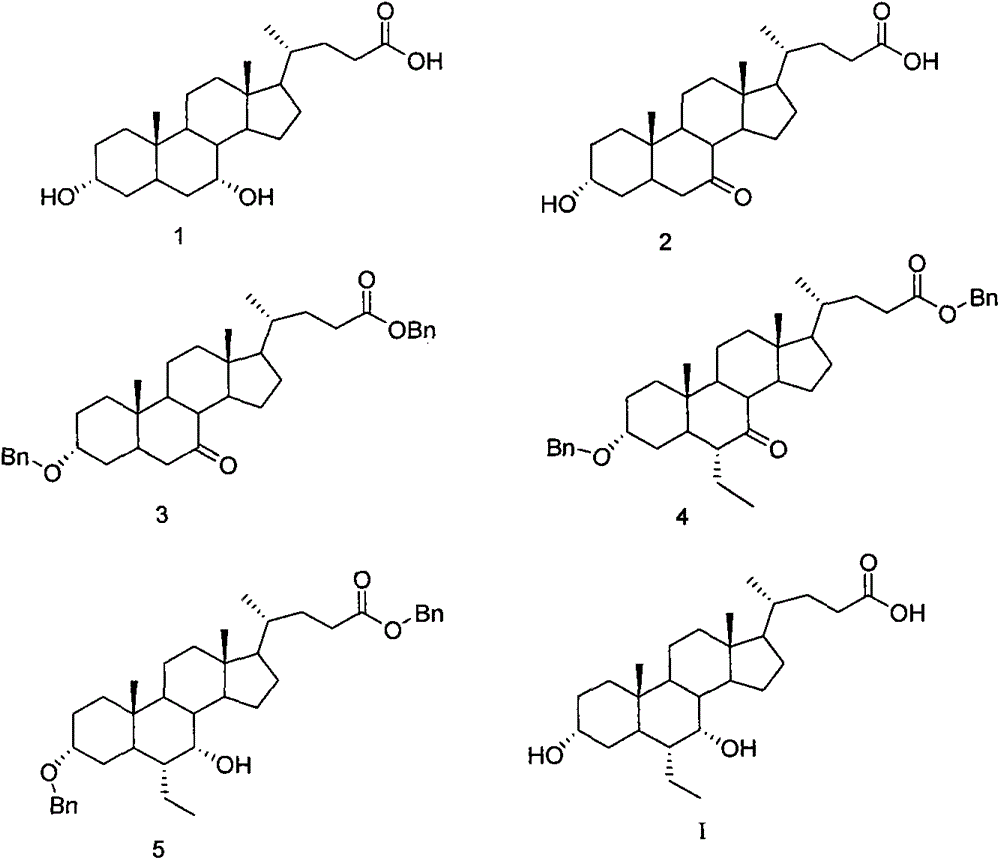
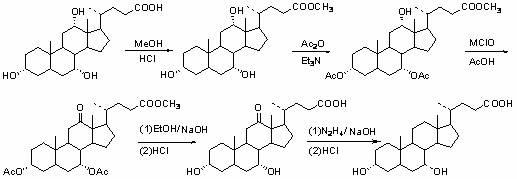
A preparing method of a chenodeoxycholic acid derivative
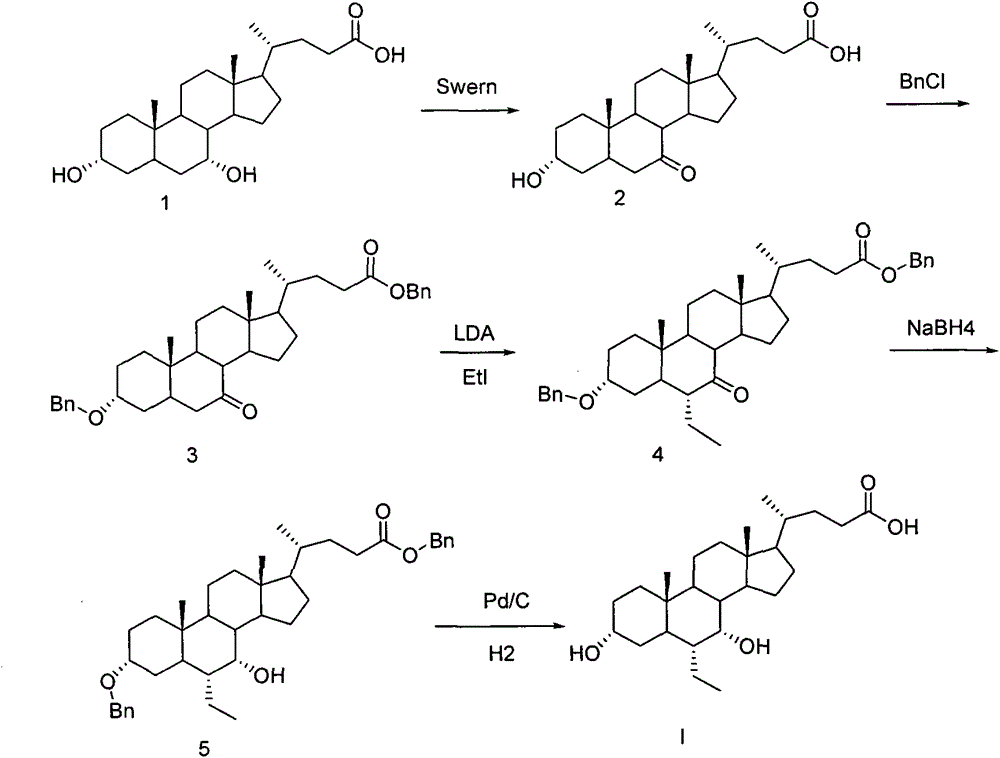
The invention relates to a preparing method of a chenodeoxycholic acid derivative, and particularly relates to a preparing method of a compound shown as a formula I. The method includes (1) subjecting a compound shown as a formula 1 to Swern oxidation to obtain a compound shown as a formula 2; (2) subjecting the compound shown as the formula 2 to hydroxy protection to obtain a compound shown as a formula 3; (3) bringing the compound shown as the formula 3 into contact with iodoethane to obtain a compound shown as a formula 4; (4) subjecting the compound shown as the formula 4 to a reduction reaction to obtain a compound shown as a formula 5; and (5) bringing the compound shown as the formula 5 into contact with hydrogen under the existence of palladium / carbon that is a catalyst to obtain the compound shown as the formula I. The method is short in steps, simple in operation, and suitable for industrial production, and raw materials are easily available.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
Nanoformulation of vitamin d derivatives and/or vitamin d metabolites
A nanoformulation that includes loaded nanoparticles. Each nanoparticle includes a modified chitosan polymer encapsulating at least one vitamin D derivative, at least one vitamin D metabolite, or combinations thereof. The modified chitosan polymer includes chitosan covalently linked to at least one entity selected from the group consisting of fatty acids (omega-3-fattay acids), amino acids, deoxycholic acid, alginate, arginine-alginate, hyaluronic acid, collagen, collagen-hydroxyapatite, poly(lactic-co-glycolic acid) (PLGA), and combinations thereof. A structure includes a medium and the nanoformulation, wherein the nanoparticles are dispersed in the medium. A method of using the nanoformulation to treat a disorder and improve efficacy of current therapies where resistance develop in a patient includes administering to the patient a therapeutically effective amount of the nanoformulation for treating the disorder. A nano-cosmetic formulation, comprising a cosmetic includes the nanoformulation, wherein the modified chitosan polymer encapsulates the at least one vitamin D derivative, and wherein the at least one vitamin D derivative encompasses 0.1 to 20.0 wt % of the nano-cosmetic formulation's total weight.
Owner:MOUSA SHAKER A
Method for producing high-purity chenodeoxy cholic acid from poultry and livestock bile
InactiveCN1775798ASimple extraction processSimple production processAntiinfectivesSteroids preparationCholic acidBile Juice
The invention discloses a method to manufacturing high purity chemocholic acid from fowl bile that belongs to refinement chemical and medicine field. It includes the following steps: a. saponification reaction: mixing the fowl bile and sodium hydroxide and reacting for 20-30 hours under the certain temperature; b. neutral reaction: adding dilute hydrochloric acid after saponification reaction and gaining raw cholic acid after filtering; c. column chromatography: adding dilute sodium hydroxide into raw cholic acid and injecting into chromatography column, uniting the eluent; super filtering: taking super filtering by super filtering film; e. gaining chemocholic acid that the purity is over 96% after adding dilute acid, filtering and drying. The invention has simple technology, high purity, low cost and environment protection.
Owner:SHANDONG BOERDE BIOLOGICAL SCI & TECH
Biological degradable albumin derivant, pharmacy composition, preparation and application of the same
InactiveCN101220093AFlat surfaceImprove uniformitySerum albuminPharmaceutical delivery mechanismWater insolubleWater insoluble drug
The invention relates to a bio-degradable alb derivative and the related pharmaceutical combinations; wherein, the derivative introduces alkyl, or fatty acyl or deoxycholic acid in the alb skeleton to enable the amphiphilic property and form a nano-micelle by self-organization in water, and can enwrap the drugs through the double effects of the hydrophobic group, the alb molecular chain and the drugs, thereby substantially improving the drug enwrapping ability of the alb and prolonging the stability time. The excipient can be used as the carrier for organic drugs, water-insoluble drugs or dugs with poor water solubility and the carrier of amphipathic drugs, and for the administration inside the vein or muscle injection and oral administration. The preparation method of the bio-degradable alb derivative and the related pharmaceutical combinations is simple and of mature techniques, which is suitable for large scale continuous production.
Owner:CHINA PHARM UNIV
Use of chenodeoxycholic acid for reducing adipose tissue
Use of chenodeoxycholic acid is disclosed to reduce adipose tissue and thereby reduce weight in mammals. The chenodeoxycholic acid can be administered orally through the use of a tablet, pill, capsule or liquid suspension.
Owner:DOX
Separation purification preparation method of chenodeoxycholic acid in pig's bile
InactiveCN1869044ASimple and fast operationLow costUnknown materialsSteroidsCholic acidChenodeoxycholic acid
A process for separating the chenodeoxycholic acid from pig's gall and purifying it includes such steps as preparing general cholic acid from the mother liquid generated by extracting the cholerythrin from pig's gall, saponifying, regulating pH value to obtain crude chenodeoxycholic acid, decoloring, defatting, preparing the deposit of barium chenodeoxycholate, reacting on potassium carbonate to remove Ba, regulating pH value, and purifying by silicon gel column.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for preparing chenodeoxycholic acid in pig bile by esterification method
InactiveCN102372757AEasy to prepareHigh extraction ratioSteroidsAcetic anhydrideChenodeoxycholic acid
The invention discloses a method for preparing chenodeoxycholic acid in pig bile by an esterification method, which comprises the following steps that: methanol, concentrated sulfuric acid and sodium hydroxide are used for esterification; acetic anhydride is used for acidylation; sherwood oil is used for extraction; the primary crystallization and the secondary crystallization are realized through ethanol and the sherwood oil; the sodium hydroxide is used for hydrolytic acidification; ethyl acetate and active carbon are used for crystallization; and the chenodeoxycholic acid is obtained through drying. The chenodeoxycholic acid is prepared by the method disclosed by the invention, the preparation method is simple, safety and environment protection are realized, the method can be used for large-scale industrial production, the content of the extracted chenodeoxycholic acid is higher or equal to 98 percent, the extraction rate is high, and the purity requirement on products in markets can be met.
Owner:ANHUI KEBAO BIOLOGICAL ENG CO LTD
Method for separating and purifying chenodeoxycholic acid from duck gall
The invention provides a method for separating and purifying chenodeoxycholic acid from duck gall, and concretely comprises to the following steps: dissolving and extracting a duck gall paste crude product, extracting ethyl acetate, and ultrafiltering and removing impurities. The method has the following advantages that 1) the raw material source of chenodeoxycholic acid can be enlarged, the method for separating and purifying chenodeoxycholic acid from the duck gall is provided; and 2) the content of chenodeoxycholic acid extracted from the duck gall by a traditional method is low with about 60%. The HPLC content of chenodeoxycholic acid obtained by using the method of the invention can reach more than 96%, and the extraction efficiency of chenodeoxycholic acid is enhanced.
Owner:苏州天绿生物制药有限公司 +1
Chenodeoxycholic acid synthesis method
The invention relates to a chenodeoxycholic acid (3 alpha, 7 alpha-dihydroxyl-5 belta-cholestane-24-acid) chemical synthesis method, belonging to the field of organic chemical synthesis. Chenodeoxycholic acid is prepared by the following steps of: (1) preparing cholate; (2) preparing 3 alpha, 7 alpha-acetyl-12 alpha-hydroxy cholate; (3) preparing 3 alpha, 7 alpha-acetyl-12-oxo-chenodeoxycholic acid ester; (4) preparing 12-oxo-chenodeoxycholic acid; and (5) preparing chenodeoxycholic acid. The application of the method for synthesizing chenodeoxycholic acid has the advantages of high yield rate, low cost and no pollution and is particularly convenient for industrial production.
Owner:ZHENGZHOU UNIV
Method for extracting chenodeoxycholic acid from bile of fowl
InactiveCN101948496AQuality is not affectedImprove versatilitySteroidsCompound aChenodeoxycholic acid
A method for extracting chenodeoxycholic acid from bile of fowl comprises the following steps of: acquiring rough bile acid or rough bile acid calcium salt from fresh or frozen bile of fowl through saponification reaction, heating the bile acid resin solution together with the aqueous solution of organic nitrogenous compound A, removing the majority of hydrophilic impurities from the rough bile acid by using the organic nitrogenous compound A, reacting the acquired bile acid resin solution with organic nitrogenous compound B, dissolving the sediment, filtering, decolorizing and refining, so as to acquire chenodeoxycholic acid with a purity about 95%. The method of the invention has simple operation, the generality of the production equipment is high, and the chemic matter is harmless, therefore the invention is fit for industrialization of chenodeoxycholic acid.
Owner:HUNAN CREDIT CHEM
Method for preparing chenodeoxycholic acid
The invention relates to a method of distilling the chenodeoxychoilc acid from the offcuts which be gained by distilling the bilirubin from the bile of the pig. With the offcuts of the alkali saponification, the deposit is treated with the oxidation treatment by means of the hydrogen peroxide, the filtrate is treated by the dilute sulfuric acid and decolored by means of the active carbon and solved, crystaled using the ethyl acetate again and again; the coordinate production of the hyodeoxycholic acid is purified, the mother liquor is concentrated to the cream and melted using the alkali and have the salifying treatment with the chloride of barium, so the cholate is formed, the cholate is treated to take off barium when the cholate suspending the aqueous solution containing the sodium carbonate, then it is acidificated with the dilute sulfuric acid and is separated ,deposited and dried to gain the crude chenodeoxycholic acid which is decolored using the active carbon and is melted, rimed and dried using the ethyl acetate. Then the finished production of the chenodeoxycholic acid form.The whole technics has some merits of the abundant material, the amity of the environment, the safety and innocuity, producing many outputs from the single stuff and easy to the mass production. The invention is used for distilling the chenodeoxycholic acid.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
Synthesis method of chenodeoxycholic acid
InactiveCN1869043AHigh puritySimple preparation processSteroidsChenodeoxycholic acidSynthesis methods
A process for synthesizing chenodeoxycholic acid includes such steps as preparing the ester used for cholic acid, preparing diacetyl-12a-hydroxy-methyl cholate, preparing 3a, 7a-diacetyloxy-12-oxy-methyl cholanic acid, preparing 12-oxy- chenodeoxycholic acid, preparing chenodeoxycholic acid, and purifying.
Owner:SHENYANG SUNSHINE PHARMA
Freeze dried polyene lecithin powder for injection and its prepn
ActiveCN1771914AImprove solubilityImprove drug activityPowder deliveryOrganic active ingredientsPolyene phosphatidylcholineSodium dehydrocholate
The present invention discloses one kind of freeze dried polyene phosphatidylcholine powder for injection and its preparation process. It features that the preparation contains polyene phosphatidylcholine and solubilizer sodium deoxycholate, sodium dehydrocholate or sodium cholate in the weight ratio of 0.5-1.5.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
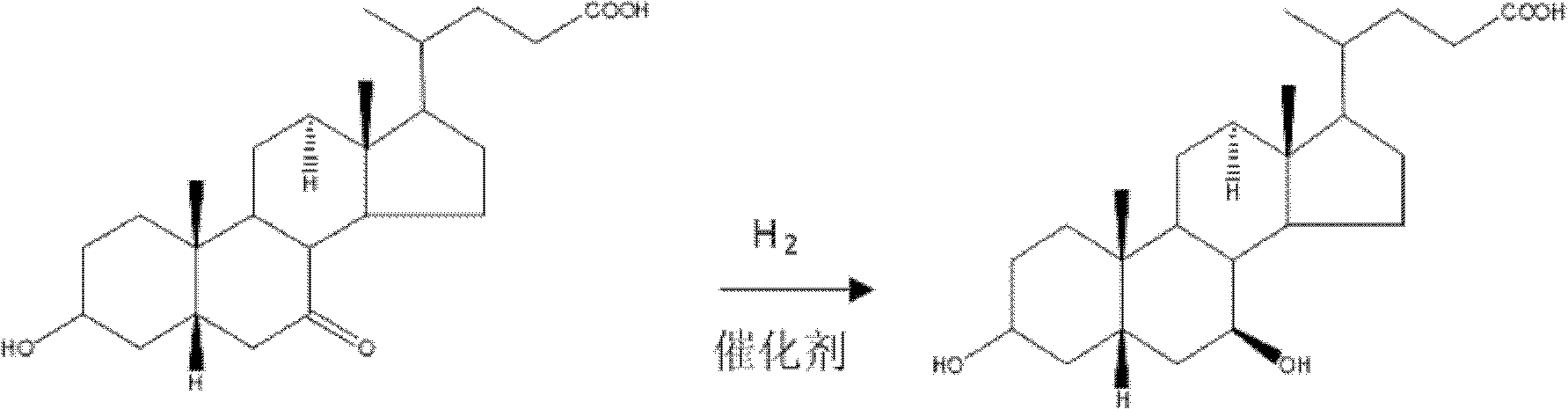
Method for preparing ursodesoxycholic acid by chiral catalytic hydrogenation of 7-ketodesoxycholic acid
InactiveCN102070693AReduce generationReduced purification stepsSteroidsChenodeoxycholic acidDistillation
The invention discloses a method for preparing an ursodesoxycholic acid by the chiral catalytic hydrogenation of a 7-ketodesoxycholic acid, which is characterized by comprising the following steps of: performing oxidation to prepare the 7-ketodesoxycholic acid from a chenodeoxycholic acid serving as an initiative raw material by using a common method; dissolving the 7-ketodesoxycholic acid into a solvent, adding a chiral catalyst, maintaining the pressure of 0 to 20 MPa under alkali condition, introducing nitrogen to perform hydrogenation reduction reaction at 10 to 80 DEG C, and performing distillation after the reaction is finished to remove the solvent; adding purified water in a volume which is 10 to 100 times that of a hydrogenation reduction reaction product, and adding acid liquor to crystallize the hydrogenation reduction reaction product; and separating solids from liquid, and performing washing and drying to obtain solid powder which is the ursodesoxycholic acid. The method for preparing the ursodesoxycholic acid by the chiral catalytic hydrogenation of the 7-ketodesoxycholic acid aims to overcome the shortcomings of the prior art, and ensures a short production flow, high yield and high quality.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com