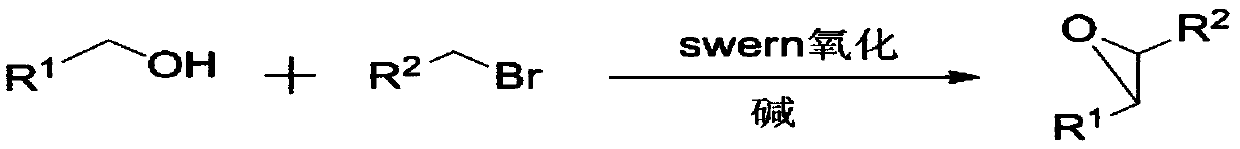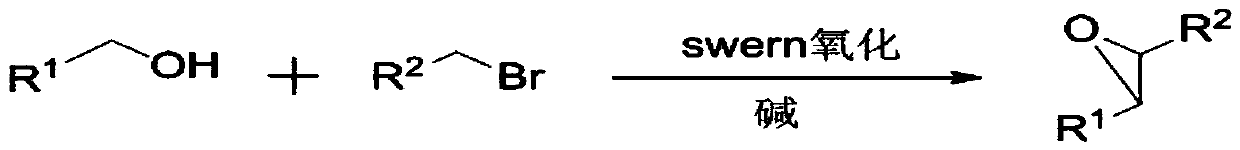Patents
Literature
45 results about "Swern oxidation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. The reaction is known for its mild character and wide tolerance of functional groups.
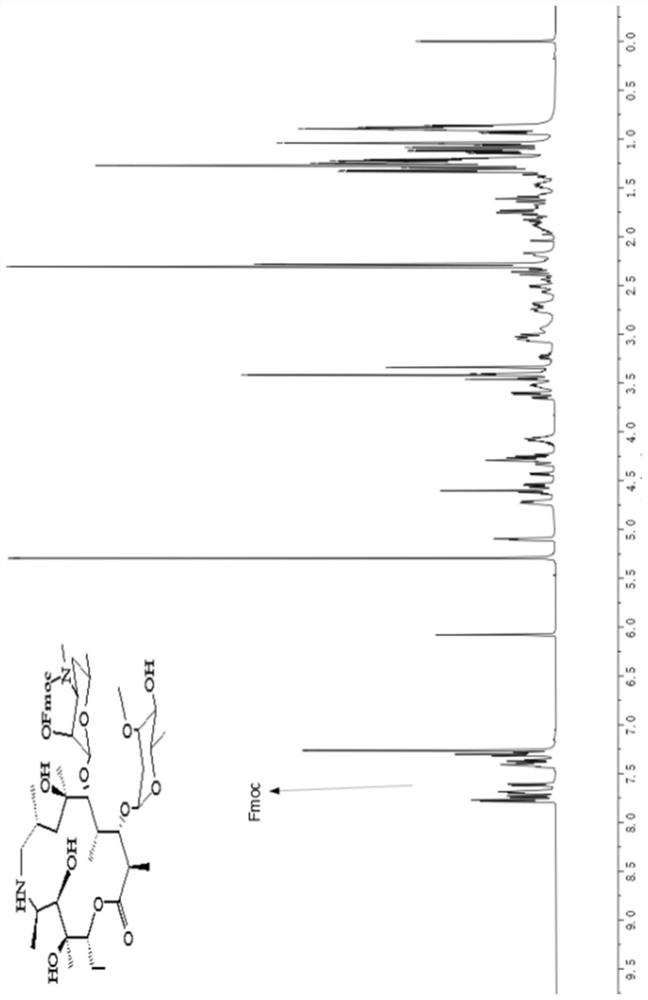
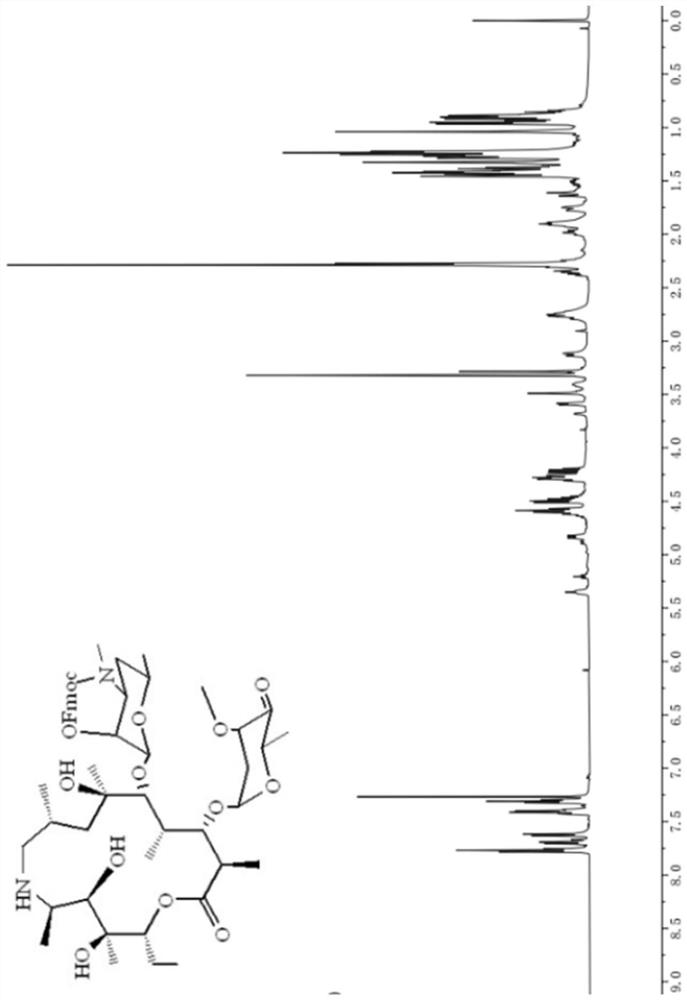
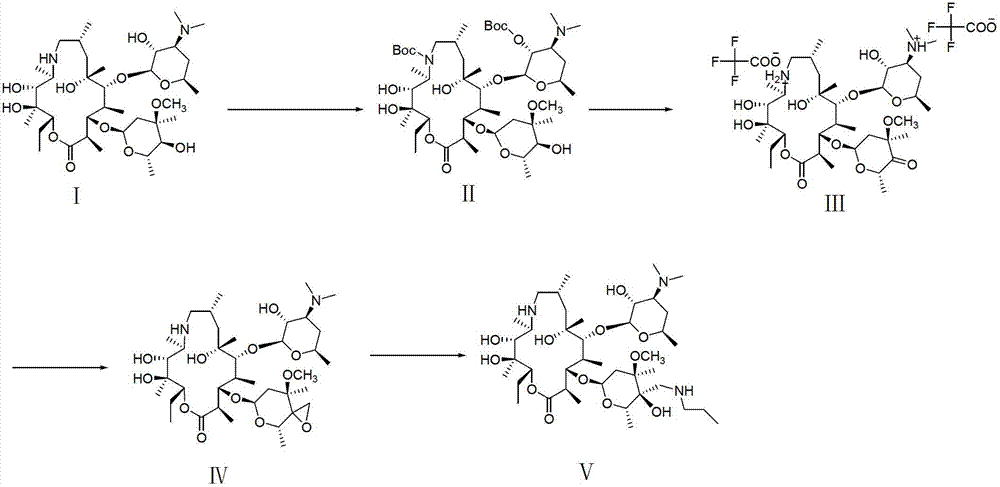
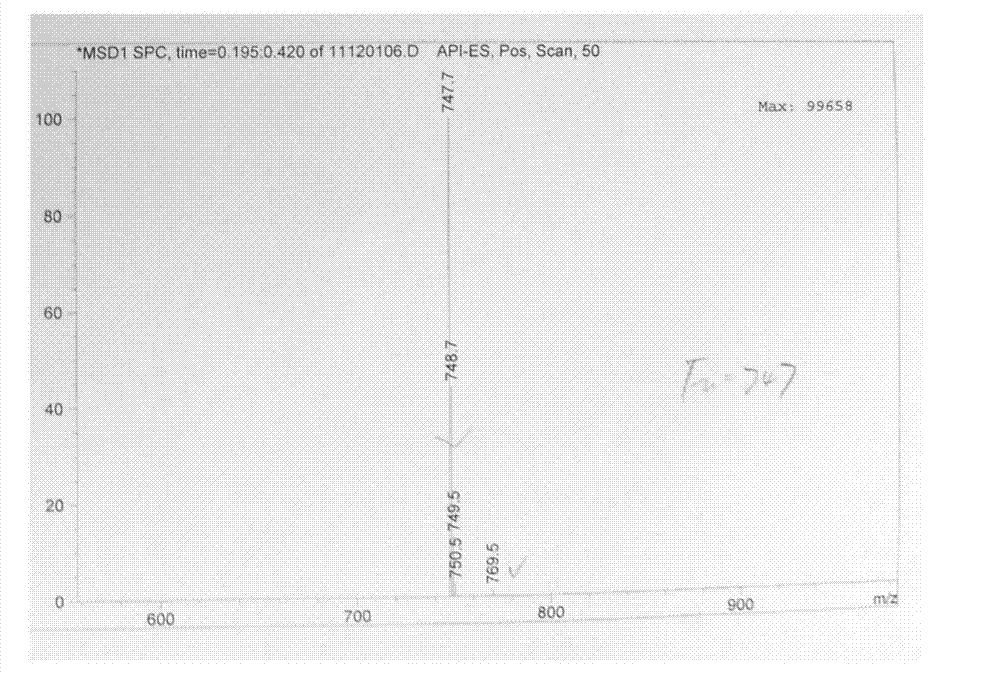
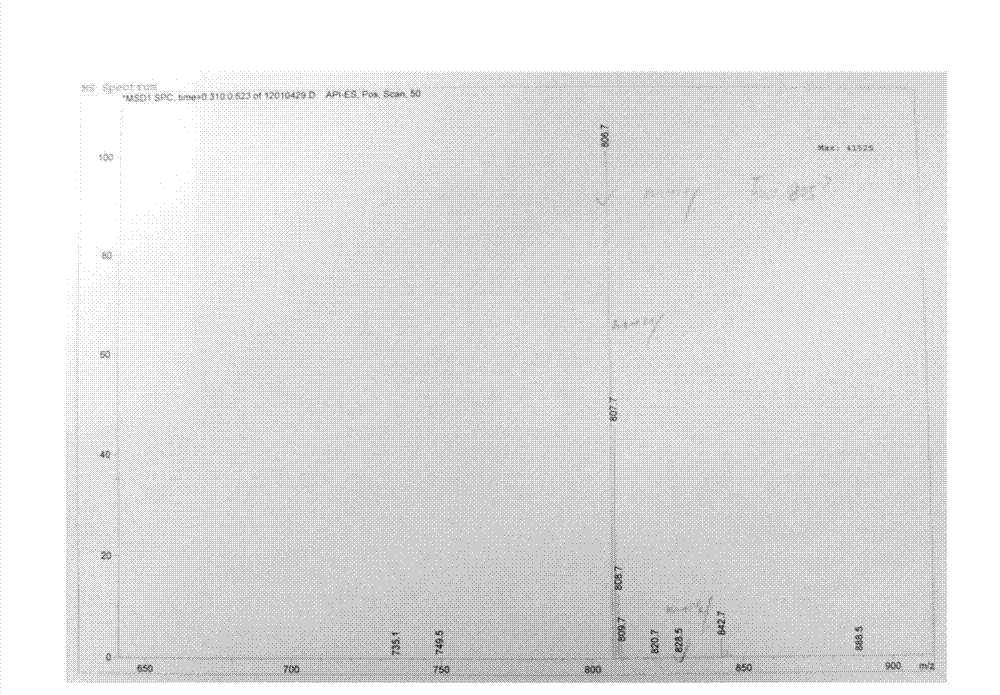
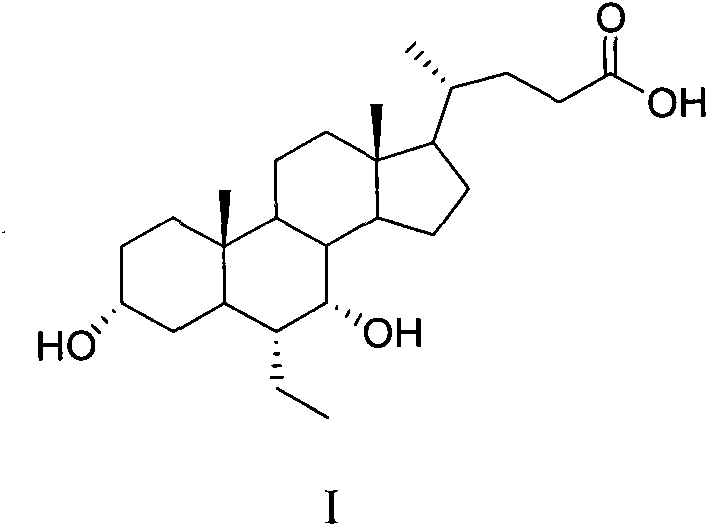
Tulathromycin intermediate and preparation method thereof, as well as preparation method of tulathromycin
ActiveCN102786569AReduce manufacturing costMild conditionsSugar derivativesSugar derivatives preparationEpoxyTert-Butyloxycarbonyl protecting group
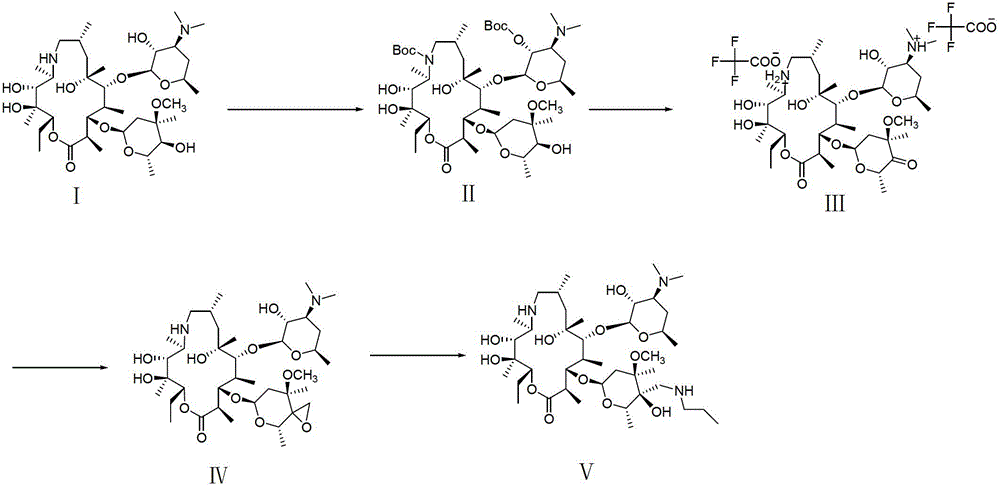
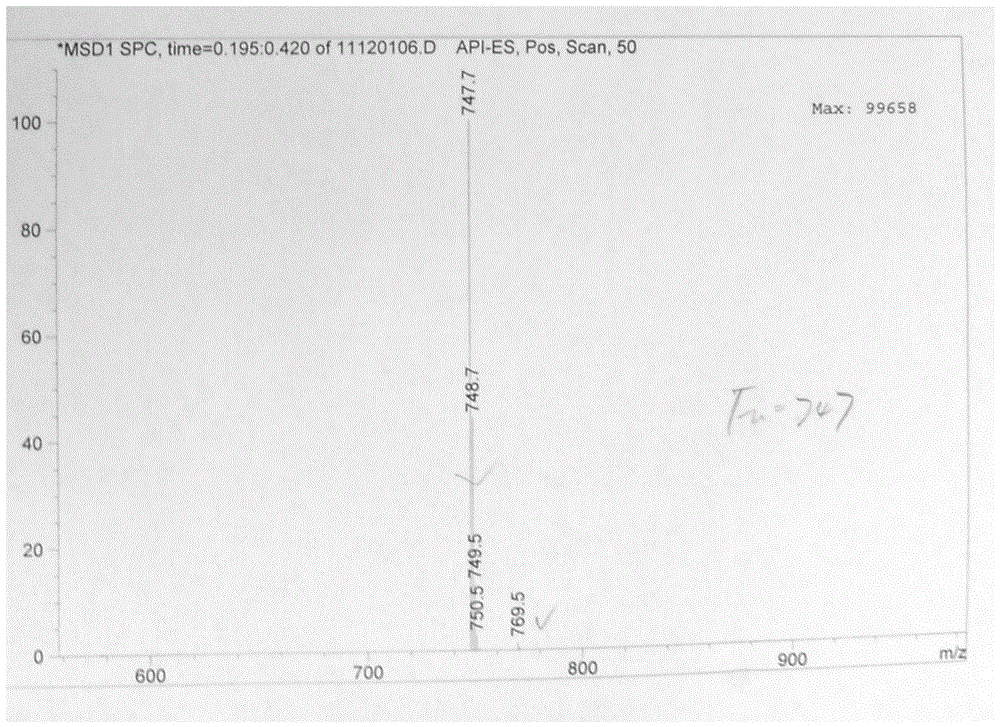
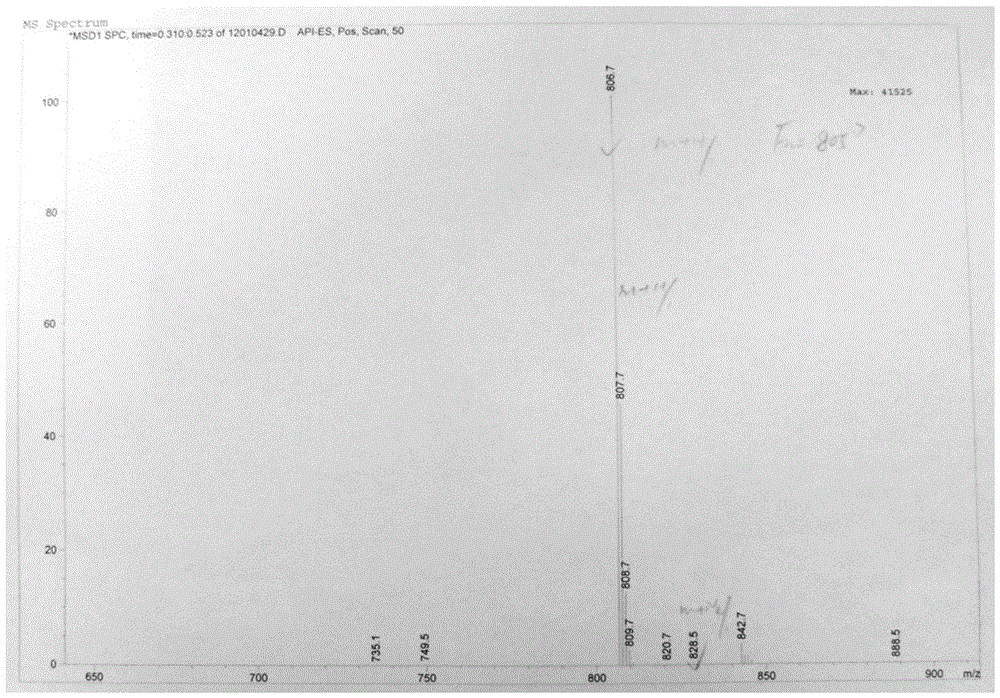
The invention provides a tulathromycin intermediate, a preparation method of the tulathromycin intermediate, and a preparation method of the tulathromycin. The preparation method of the tulathromycin has the advantages of mild condition, convenience for operation, and low cost. The preparation method of the tulathromycin comprises the following steps of: using azithromycin A as a raw material; protecting 2'-hydroxy and 6'-amino in the azithromycin A through di-tert-butyl dicarbonate so as to obtain double-protective azithromycin A; carrying out Swern oxidation to 4''-hydroxy to the double-protective azithromycin A; salifying along with trifluoroacetic acid; and synchronously removing boc t-butyloxycarbonyl to obtain the azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl; and then reacting with trimethylsulfonium bromide to obtain 4''-epoxy compound; and finally carrying out nucleophilic addition on the 4''-epoxy compound by n-propylamine so as to obtain the phosphate of tulathromycin; and further neutralizing via alkaline to obtain the target compound tulathromycin; and synchronously obtaining the tulathromycin intermediate of azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
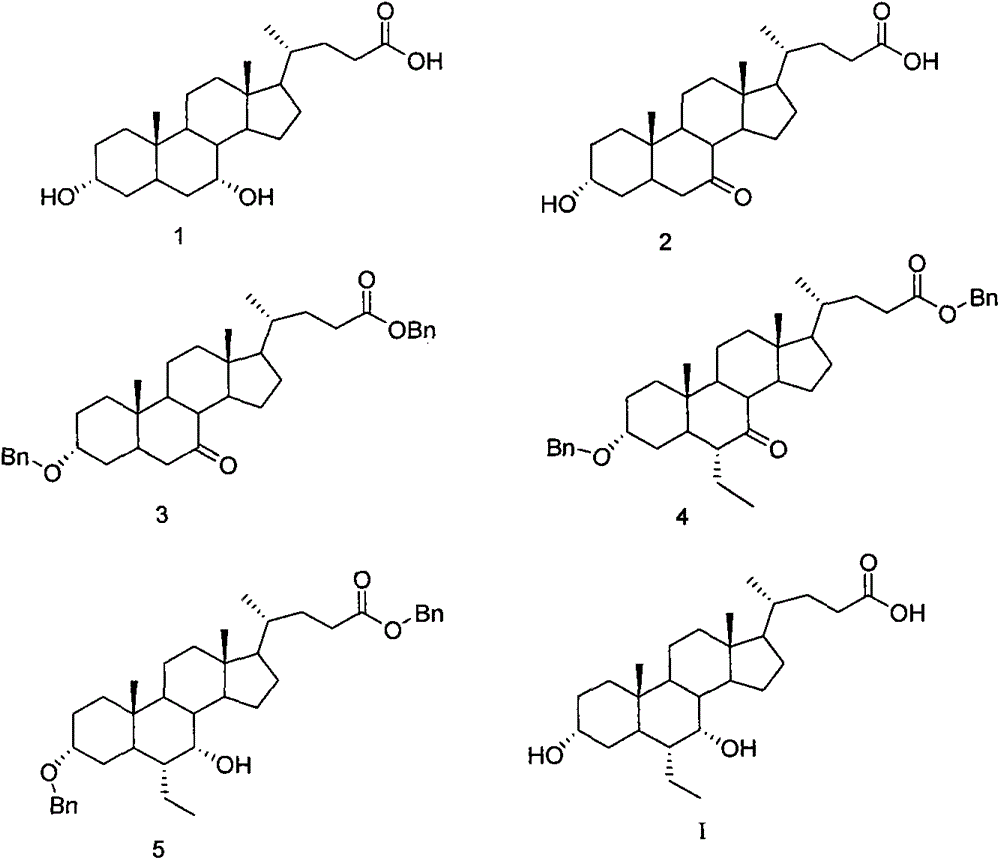
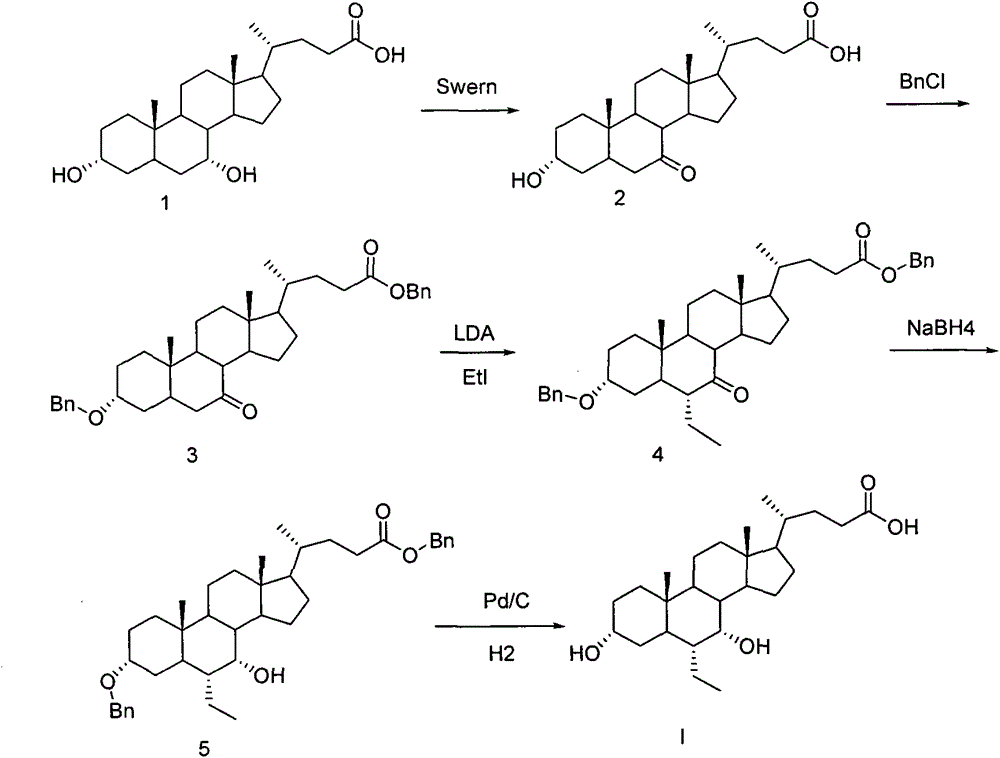
A preparing method of a chenodeoxycholic acid derivative
The invention relates to a preparing method of a chenodeoxycholic acid derivative, and particularly relates to a preparing method of a compound shown as a formula I. The method includes (1) subjecting a compound shown as a formula 1 to Swern oxidation to obtain a compound shown as a formula 2; (2) subjecting the compound shown as the formula 2 to hydroxy protection to obtain a compound shown as a formula 3; (3) bringing the compound shown as the formula 3 into contact with iodoethane to obtain a compound shown as a formula 4; (4) subjecting the compound shown as the formula 4 to a reduction reaction to obtain a compound shown as a formula 5; and (5) bringing the compound shown as the formula 5 into contact with hydrogen under the existence of palladium / carbon that is a catalyst to obtain the compound shown as the formula I. The method is short in steps, simple in operation, and suitable for industrial production, and raw materials are easily available.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
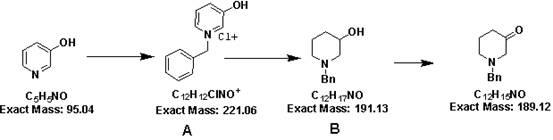
Synthesizing method of 1-benzyl-piperidone hydrochloride
The invention discloses a synthesizing method of 1-benzyl-piperidone hydrochloride. The method comprises steps that: (1) 3-hydroxypyridine is added to an organic solvent, and is refluxed under a temperature of 100 to 110 DEG C; benzyl halide is dropped into the solution, and the refluxing reaction is continued for 2 to 4 hours, such that a product A is obtained; (2) the product A is added to an alcohol organic solvent; sodium borohydride is added to the solution under an ice bath; the temperature of the solution is recovered to room temperature; the solution is stirred for 10 to 15 hours; the reaction liquid is quenched by using water; the alcohol organic solvent is removed; the pH value of the solution is regulated to 1-2 by using strong acid; the solution is extracted by using an extractant, and a water phase is preserved; the pH value of the solution is regulated to 13-14 by using an alkali solution, such that a product B is obtained; (3) through a Swerns oxidation reaction, hydroxyl groups in the product B are oxidized into ketone groups; the product is washed; an organic phase is dried, filtered, and condensed; an ethyl acetate hydrochloride solution is added to the product until the pH value is 1-2; the product is stirred and cooled; when solid is completely precipitated, the product is filtered and dried by baking, such that 1-benzyl-piperidone hydrochloride is obtained. The invention is advantaged in low cost and safe operation.
Owner:兰州博实生化科技有限责任公司
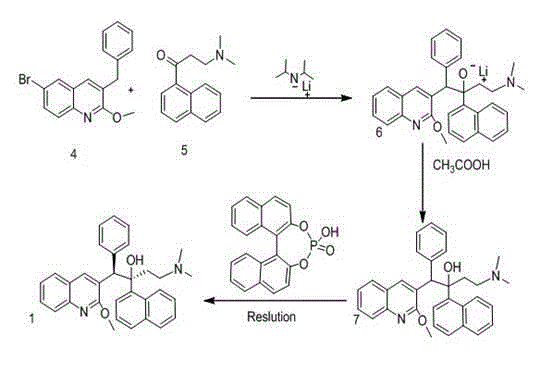
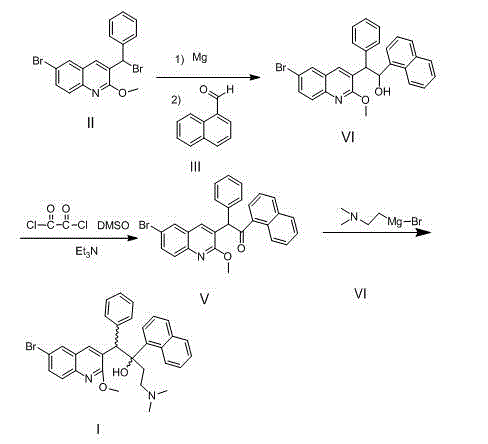
New synthesis route and method of bedaquiline racemate
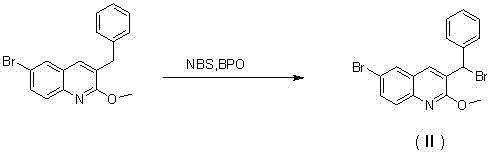
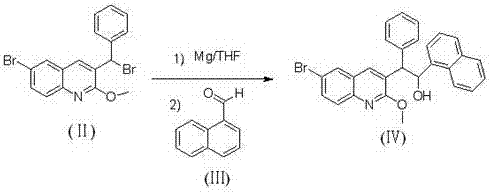
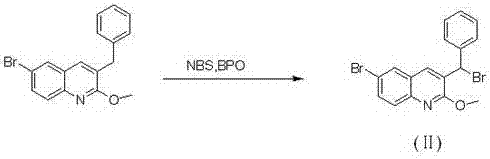
The invention relates to a new synthesis route and method of a bedaquiline racemate, and belongs to the medical technical field. The method comprises the steps: (1) carrying out a reaction of a starting material 3-bromobenzyl-6-bromo-2-methoxyquinoline (II) with metal magnesium in THF to prepare a Grignard reagent, then adding 1-naphthaldehyde (III) in the prepared Grignard reagent, carrying out a reflux reaction to obtain a compound (IV) 2-(6-bromo-2-methoxyquinoline-3-yl)-1-(naphthalen-1-yl)-2-phenylethanol; (2) undergoing swern oxidation of the compound (IV) to obtain a carbonyl compound 2-(6-bromo-2-methoxyquinoline-3-yl)-1-(naphthalen-1-yl)-2-phenyl ethyl ketone (a compound V); and (3) carrying out a Grignard reaction of the compound (V) with the Grignard reagent (VI) to obtain the bedaquiline racemate (I). The method is simple in route, is mild in reaction conditions, low in cost, high in yield, and suitable for industrialized production application.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
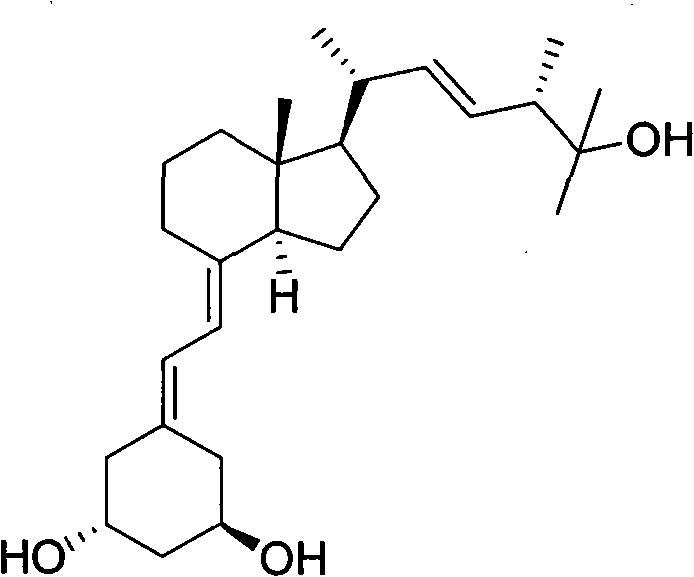
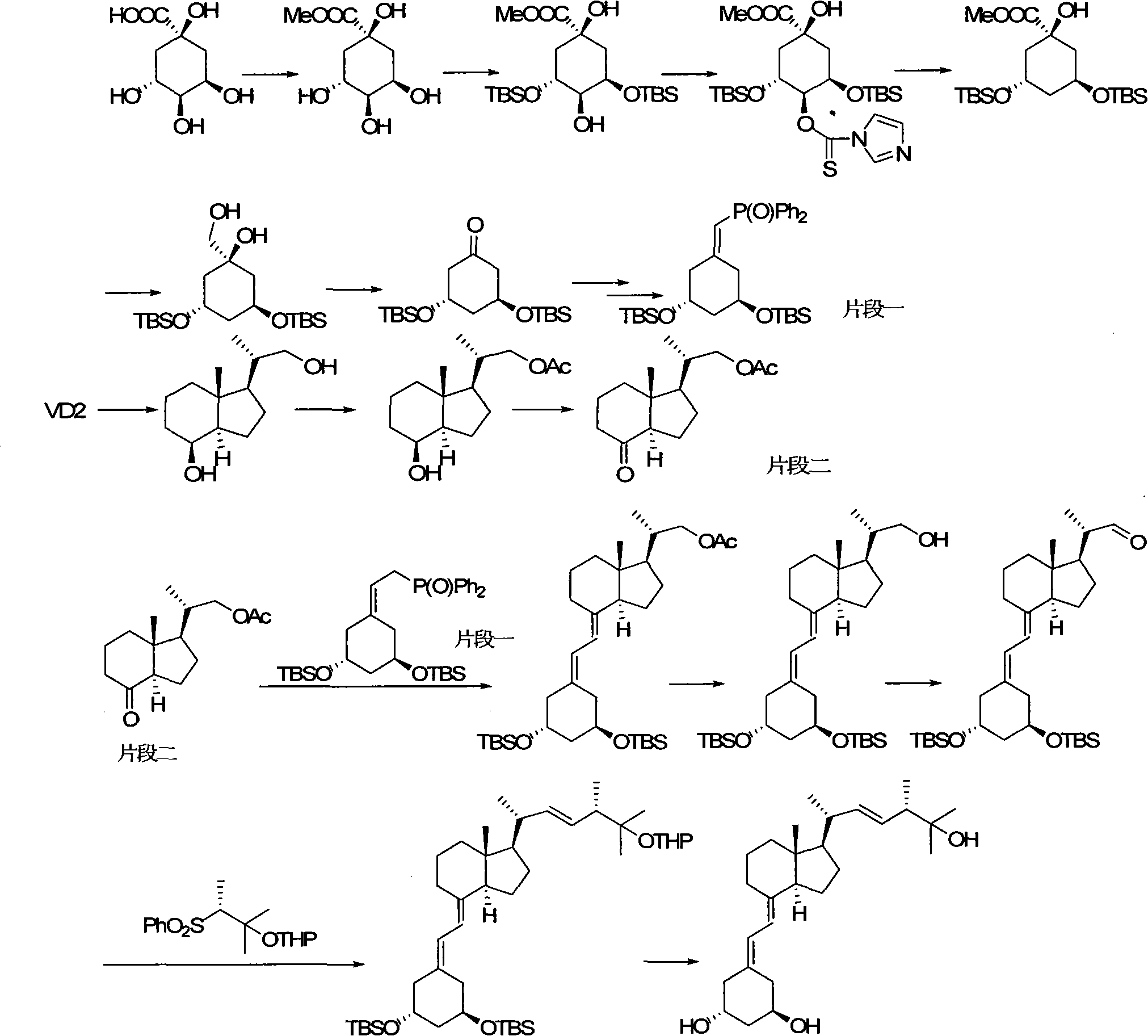
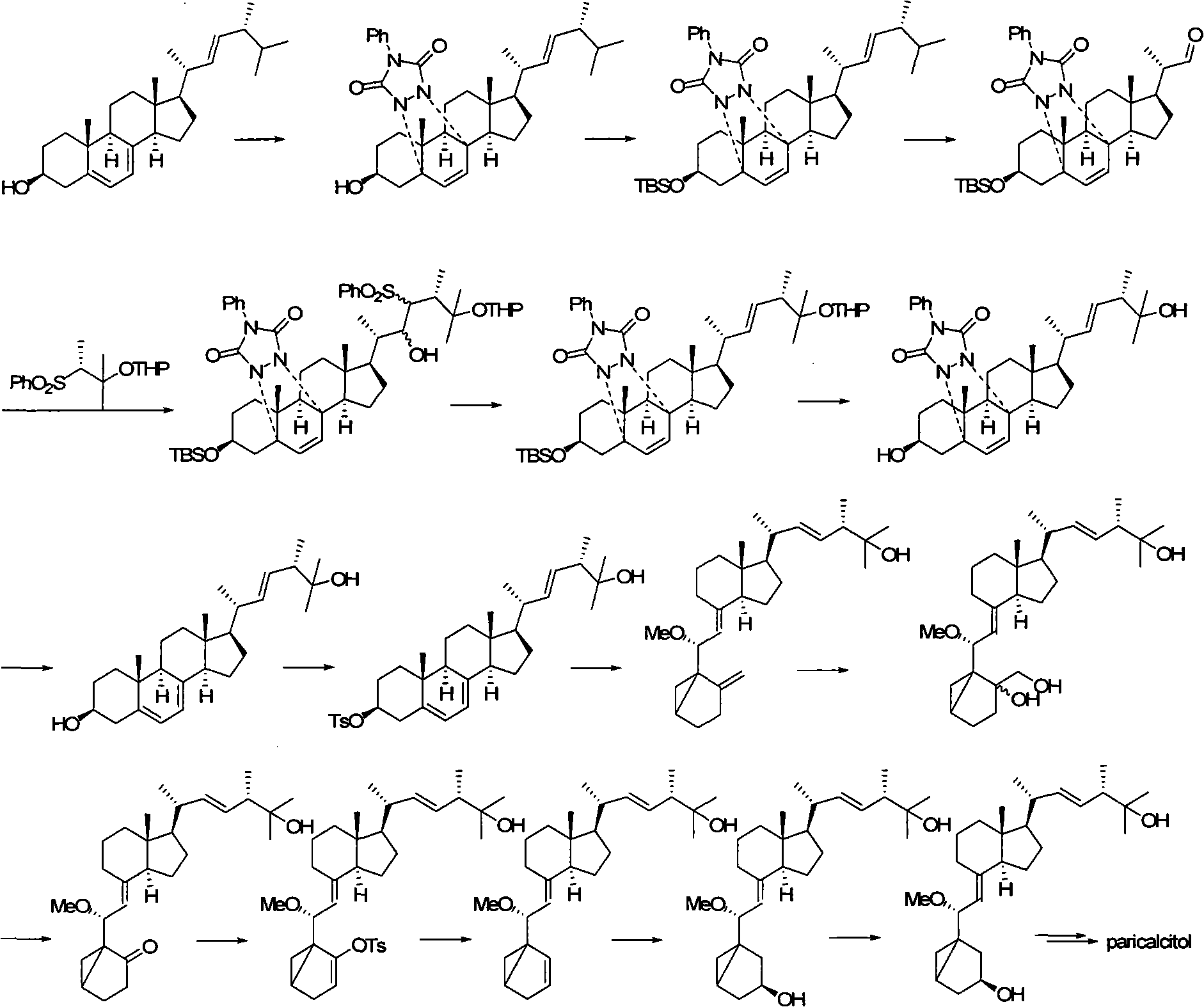
Preparation method of paricalcitol
InactiveCN101880253AEasy to operateLess regional selectivityOrganic chemistryBulk chemical productionSide chainDouble bond
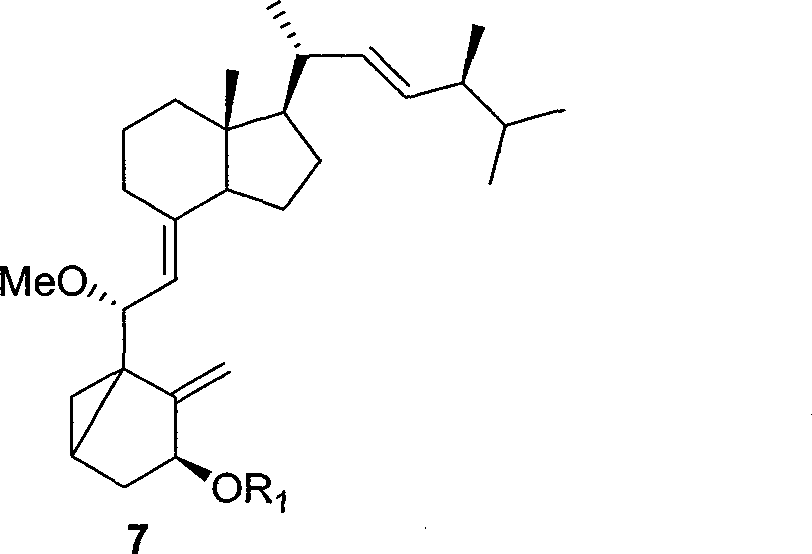
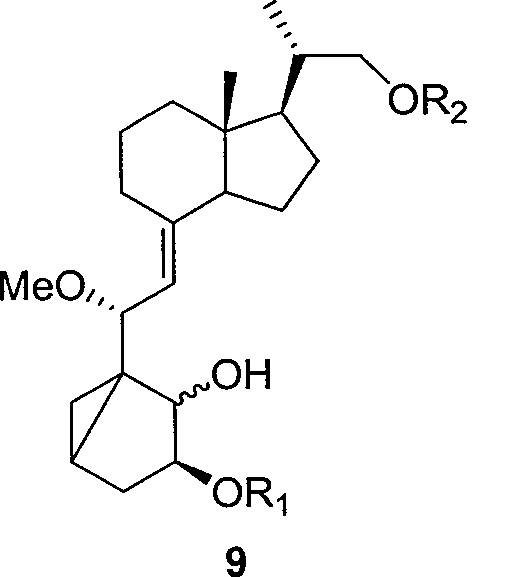
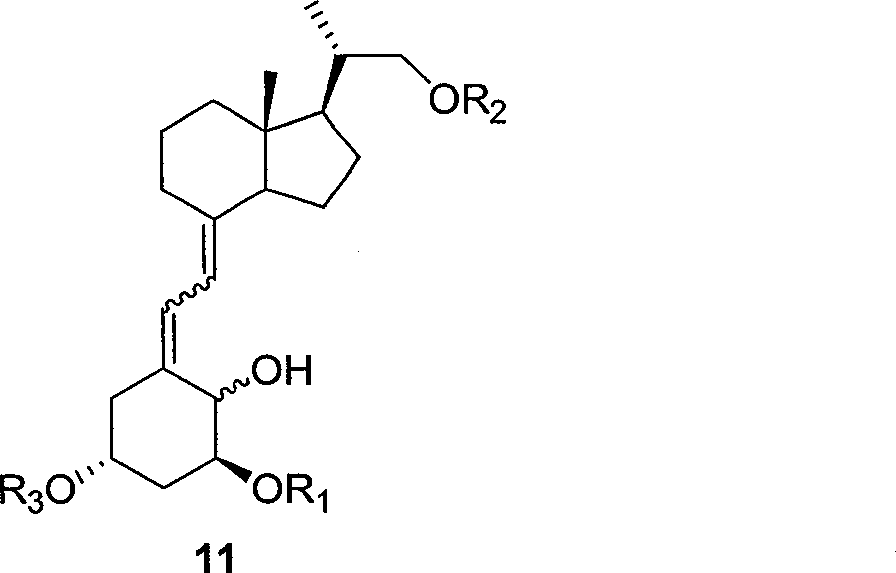
The invention relates to a preparation method of paricalcitol, which is characterized in that after hydroxyl in the vitamin D2 is protected by p-toluenesulfonates, in the presence of alkali, intramolecular cyclization reaction happens in methanol to generate a compound 5; the compound 5 undergoes allylic oxidation and hydroxyl is protected to obtain a key intermediate 7; in the presence of ozone,the side chains and exocyclic terminal double bonds of the key intermediate 7 are cut off to obtain a compound 8; the primary hydroxyl in the compound 8 is selectively protected, a three-membered ring is opened in the presence of acid and then hydroxyl is protected to obtain a key intermediate 11; after the secondary hydroxyl in the key intermediate 11 is protected by sulphonate, a compound 12 isobtained through reduction by LiAlH4; a compound 13 is obtained after the compound 12 is subjected to Swern oxidation and carries out Wittig reaction with the compound 12 to obtain a compound 14; andthe target compound can be obtained by removing the protective group in the compound 14. The reagents used in the method are simple and are convenient to operate, the reactions concerning regioselectivity and stereoselectivity are few, the route is shorter and 12 steps of reactions are carried out.
Owner:CHONGQING TAIHAO PHARM CO LTD
Preparation and application of novel Swern reagent
ActiveCN105585540AInhibition releaseRaise the reaction temperatureOrganic compound preparationCarbonyl group formation/introductionMorpholineReaction temperature
The invention discloses 4-(2-(2-methyl sulfoxide)ethyl)-4-nitrobenzene)morpholine shown in the formula (I) and preparation and application thereof. A preparation method includes the steps that 2-(2-chlorine-5 nitro)phenethyl alcohol shown in the formula (II) and morpholine are mixed to prepare 2-(2-morpholine-5-nitrobenzene)ethanol shown in the formula (III); bis(trichloromethyl)carbonate ester, a sodium methyl mercaptide aqueous solution and an aqueous hydrogen peroxide solution are sequentially added dropwise to 2-(2-morpholine-5-nitrobenzene)ethanol shown in the formula (III), and finally 4-(2-methyl sulfoxide)ethyl)-4-nitrobenzene)morpholine is prepared. According to the application of 4-(2-methyl sulfoxide)ethyl)-4-nitrobenzene)morpholine, the obtained Swern reagent reacts with an alcohol compound shown in the formula (IV), and aldehyde or ketone is prepared after after-treatment. The defects of an existing Swern oxidation method are overcome, generation of a stink byproduct dimethyl sulfide and toxic carbon monoxide is avoided from the source, the reaction temperature is increased to be -30 DEG C to 0 DEG C, and an odorless byproduct novel sulfur ether can be recycled and reused. The formulas are shown in the description.
Owner:ZHEJIANG UNIV OF TECH
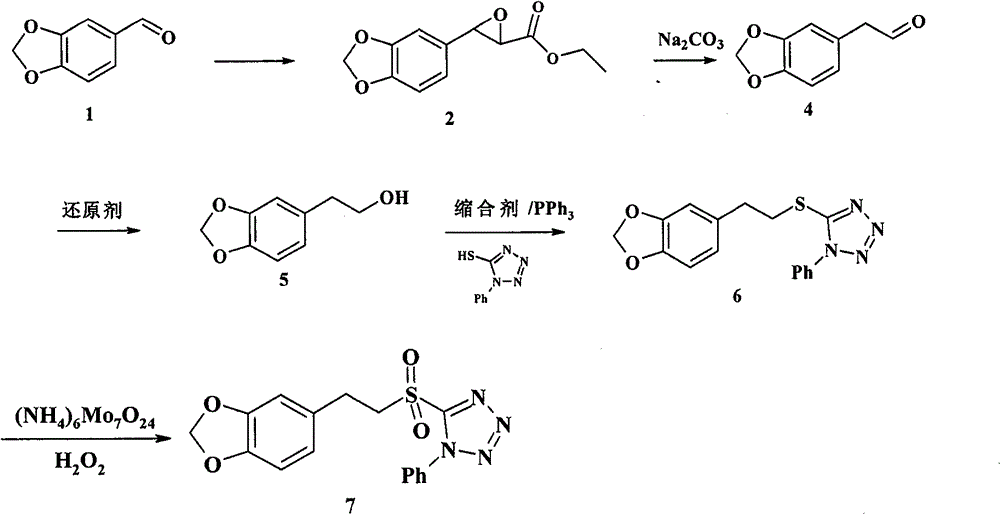
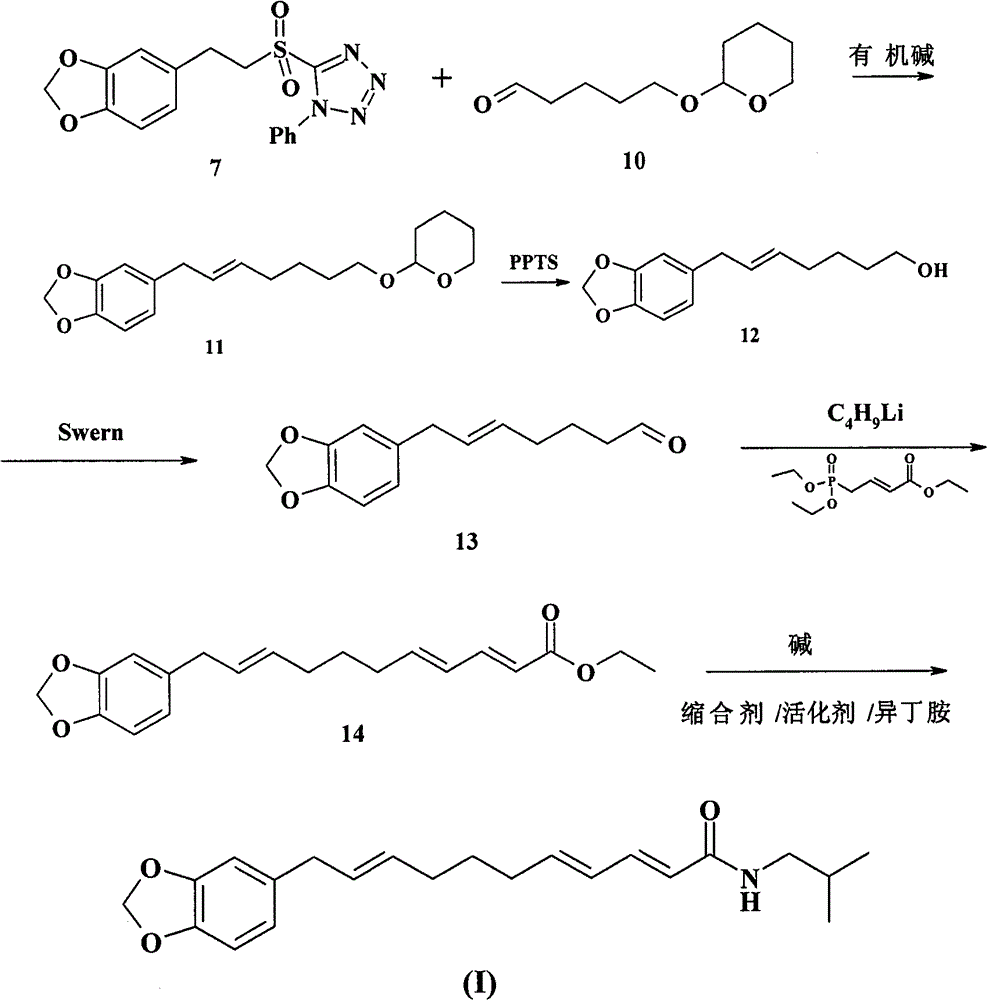
Chemical synthesis method of laetispicine
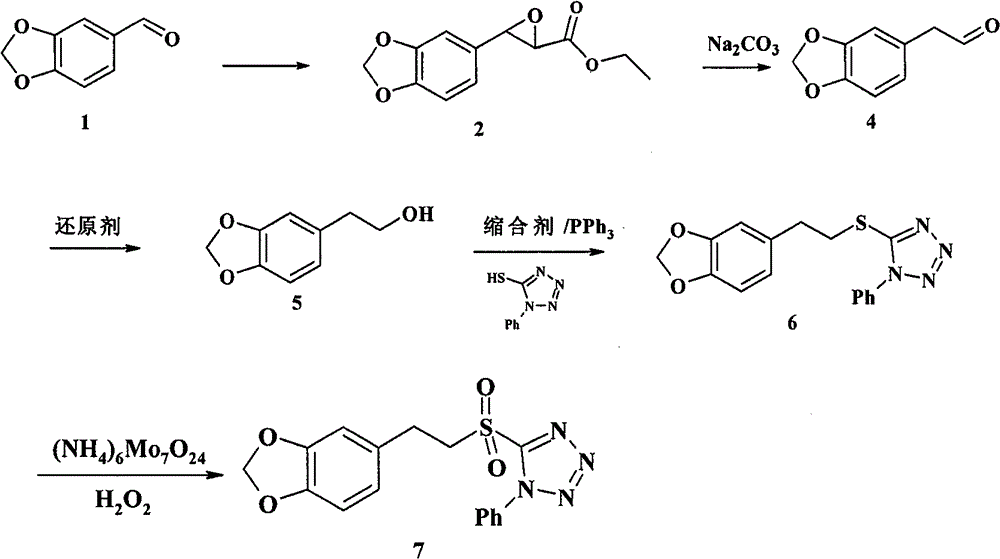
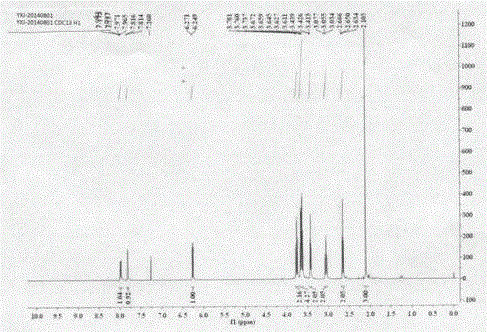
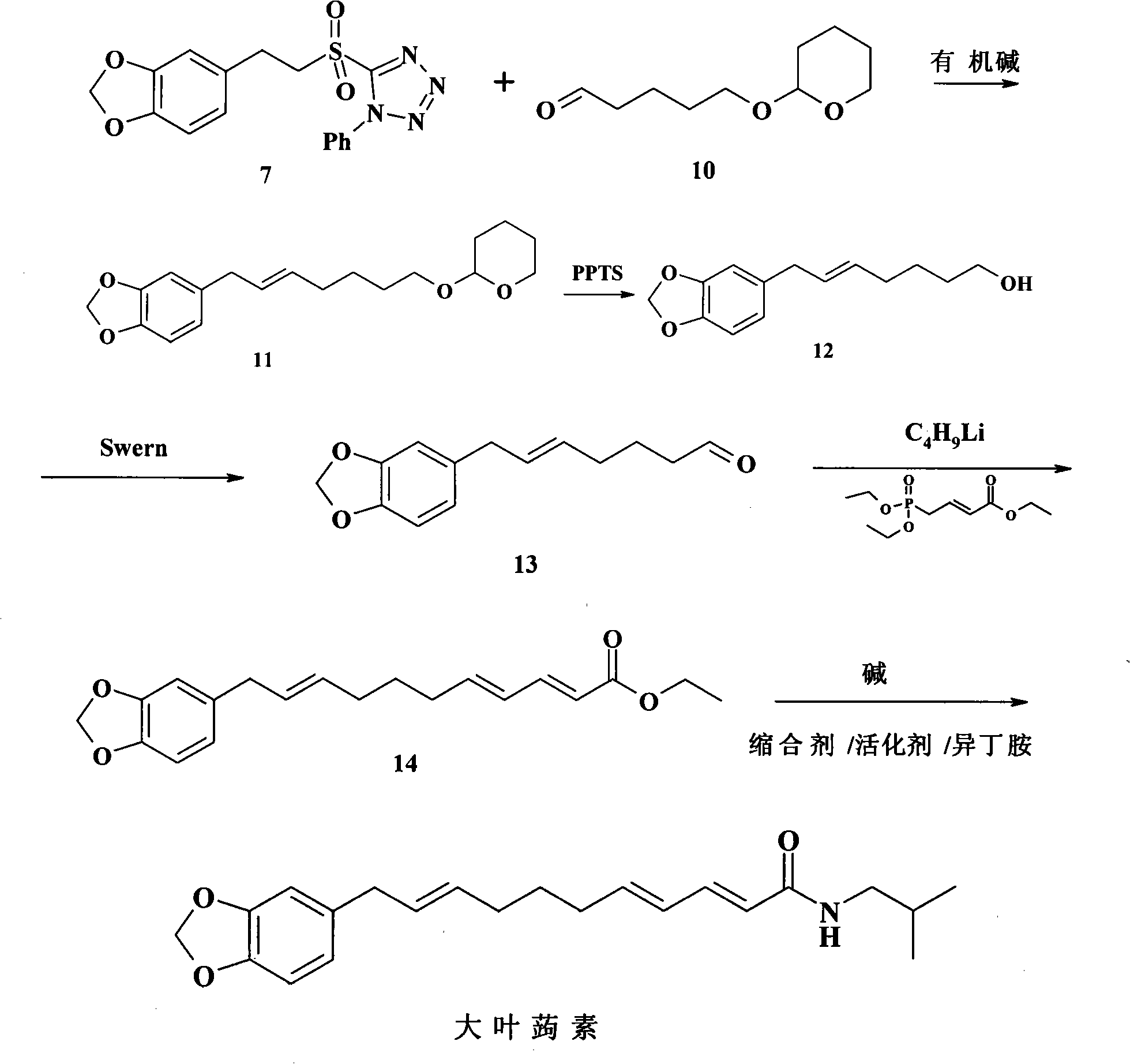
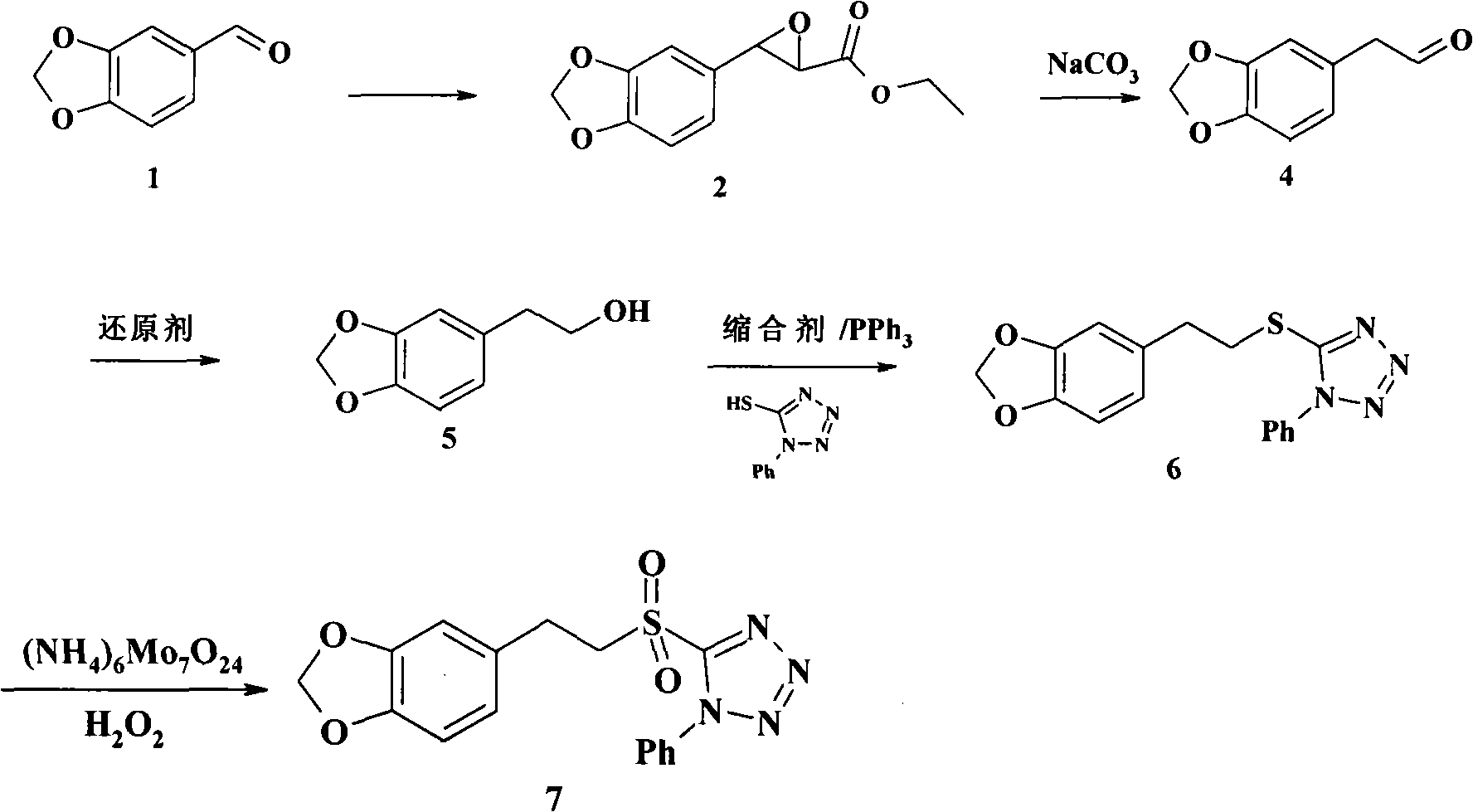
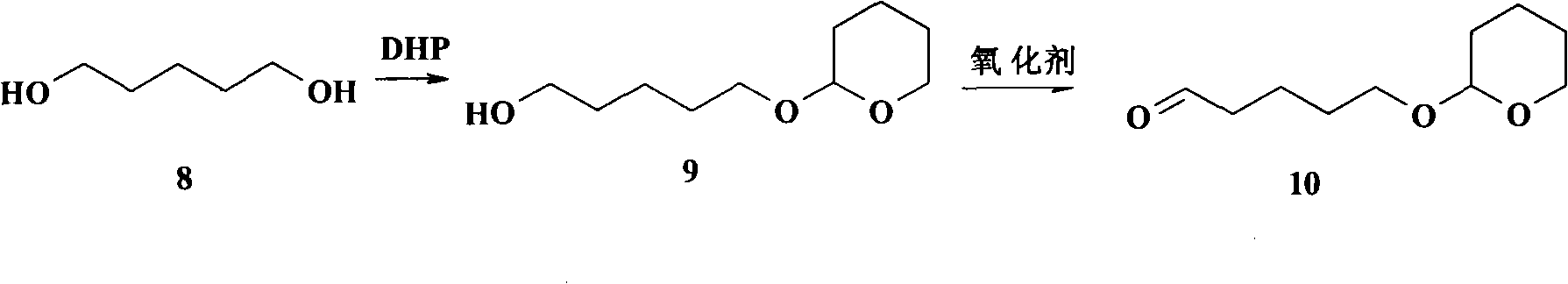
The invention relates to a chemical synthesis method of natural product laetispicine. The method comprises the following steps of: obtaining a key intermediate parent nucleus part 1-phenyl-5-(3,4-methylene dioxybenzene ethyl) sulfonyl tetrazole by using piperonal as a raw material through the steps of Darzens condensation, hydrolysis, decarboxylation, reduction, condensation and oxidation; obtaining a key intermediate part 5-(tetrahydropyrane-2-ol) valeraldehyde by using pentanediol as a raw material through the steps of hydroxyl protection and oxidation; and then condensing the parent nucleus part and a side chain part and obtaining the target product laetispicine through deprotection, Swern oxidation, a Wittig-Horner reaction, hydrolysis and amidation. It is known from a hydrogen spectrum, a carbon spectrum and an infrared spectrum, the laetispicine synthesized by the invention is the same as natural laetispicine.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Method for synthesizing natural product Tarchonanthuslactone isomer
InactiveCN104402852ANovel and reasonable designRaw materials are cheap and easy to getOrganic chemistrySwern oxidationNatural product
The invention provides a method for synthesizing a natural product Tarchonanthuslactone isomer. According to the method, methyl-3-hydroxybutyrate is used as a raw material, and PMB ester protection, LiALH4 reduction, Swern oxidation, Mukaiyama Aldol reaction, ester hydrolysis, Yanaguchi cyclization, de-protection and condensation are performed, so that the natural product Tarchonanthuslactone isomer is obtained. The synthetic route is novel and reasonable in design, the raw materials are cheap and easy to obtain, the operation process is simple and convenient, the reaction condition is mild, the rate is high, side reaction is less, the operation is simple and convenient, and the synthesizing cost is greatly reduced.
Owner:JIANGXI SCI & TECH NORMAL UNIV
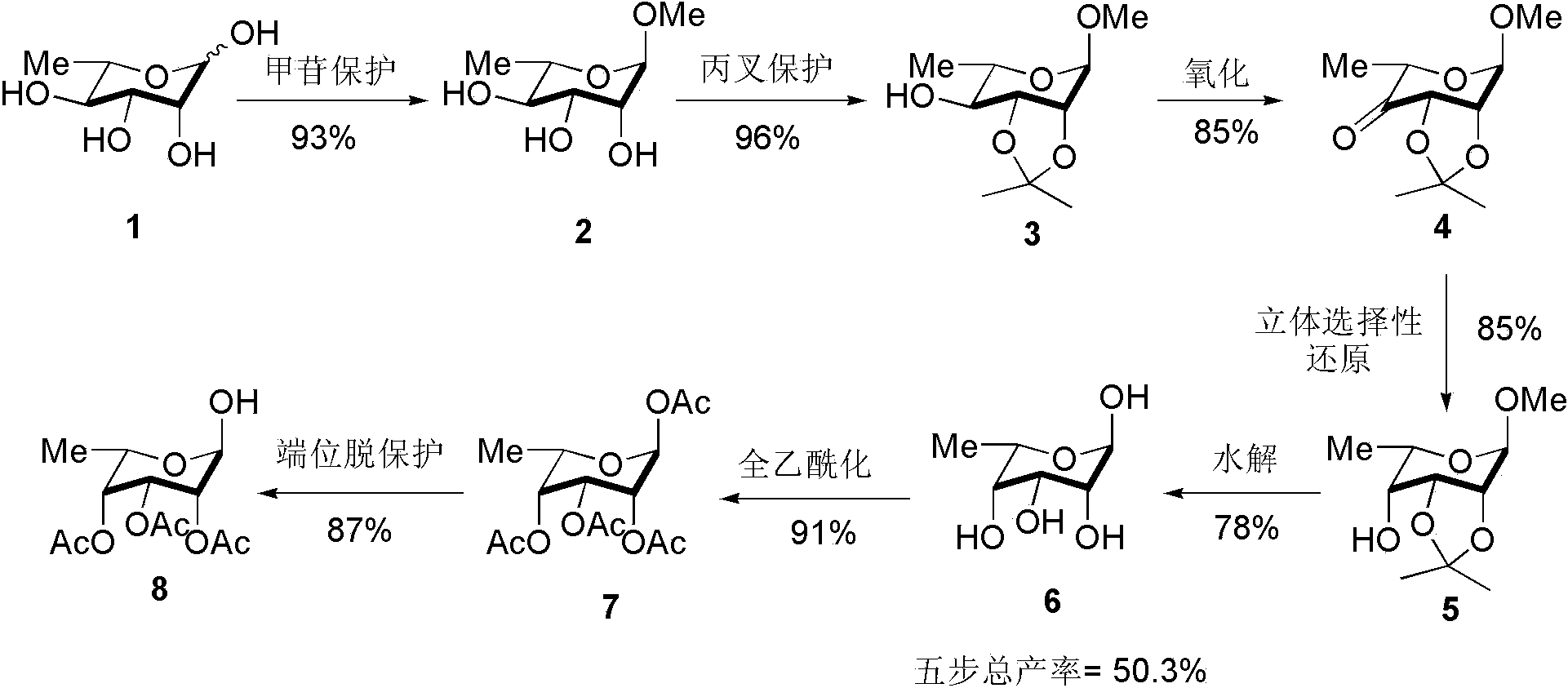
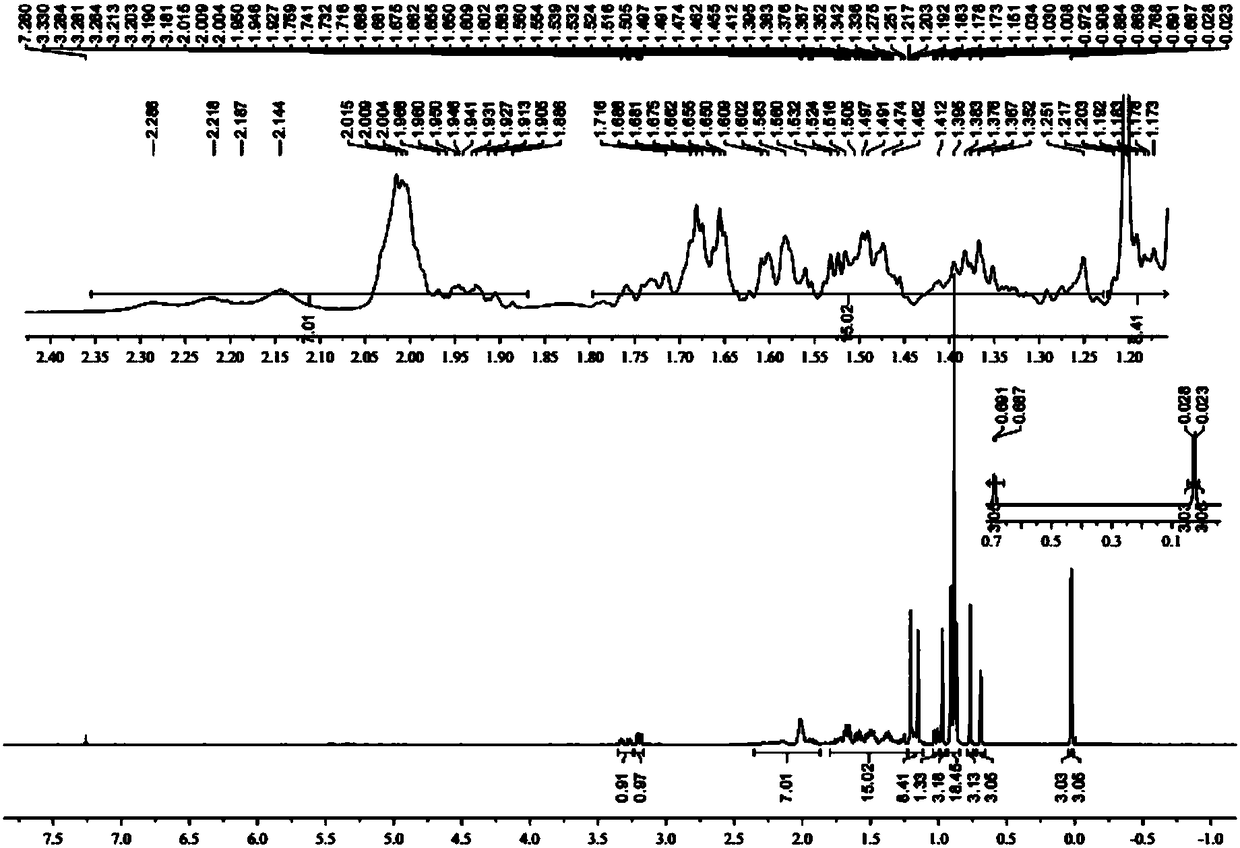
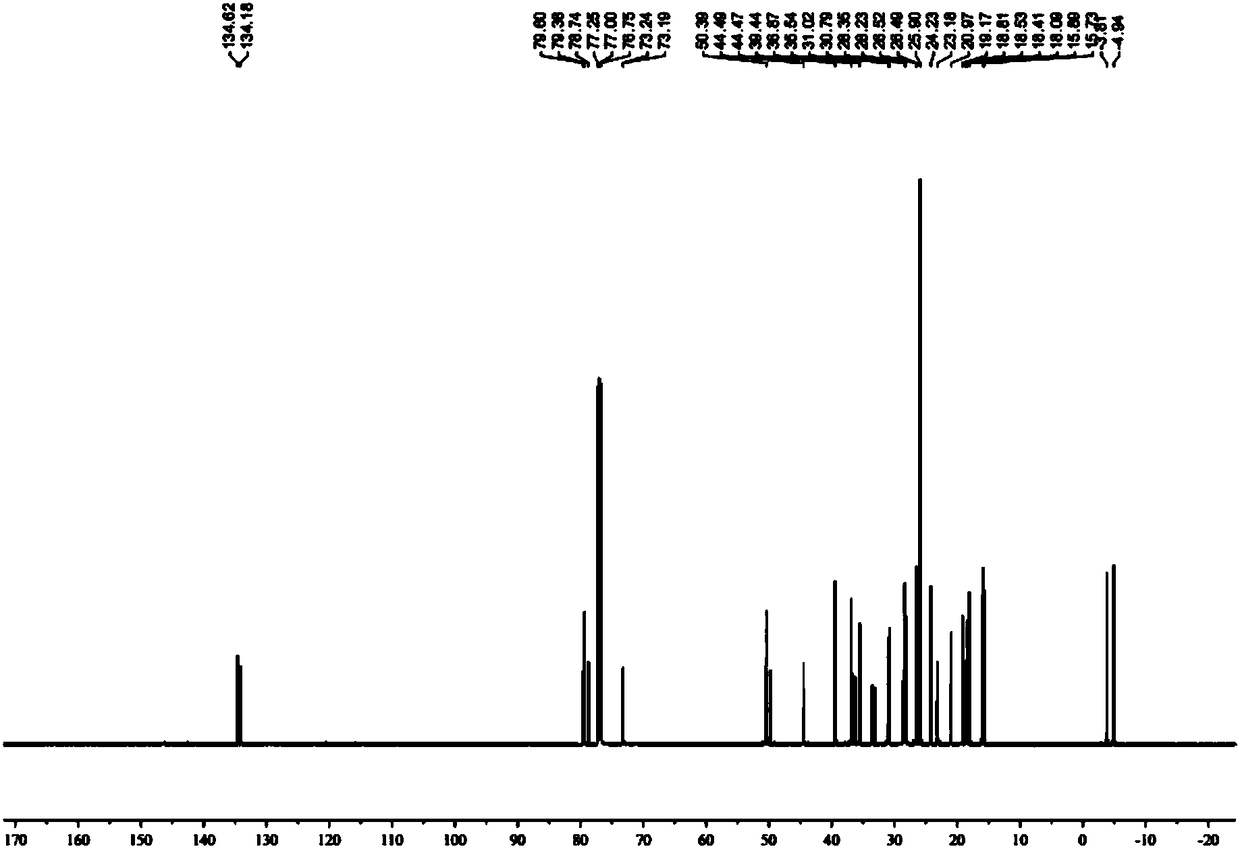
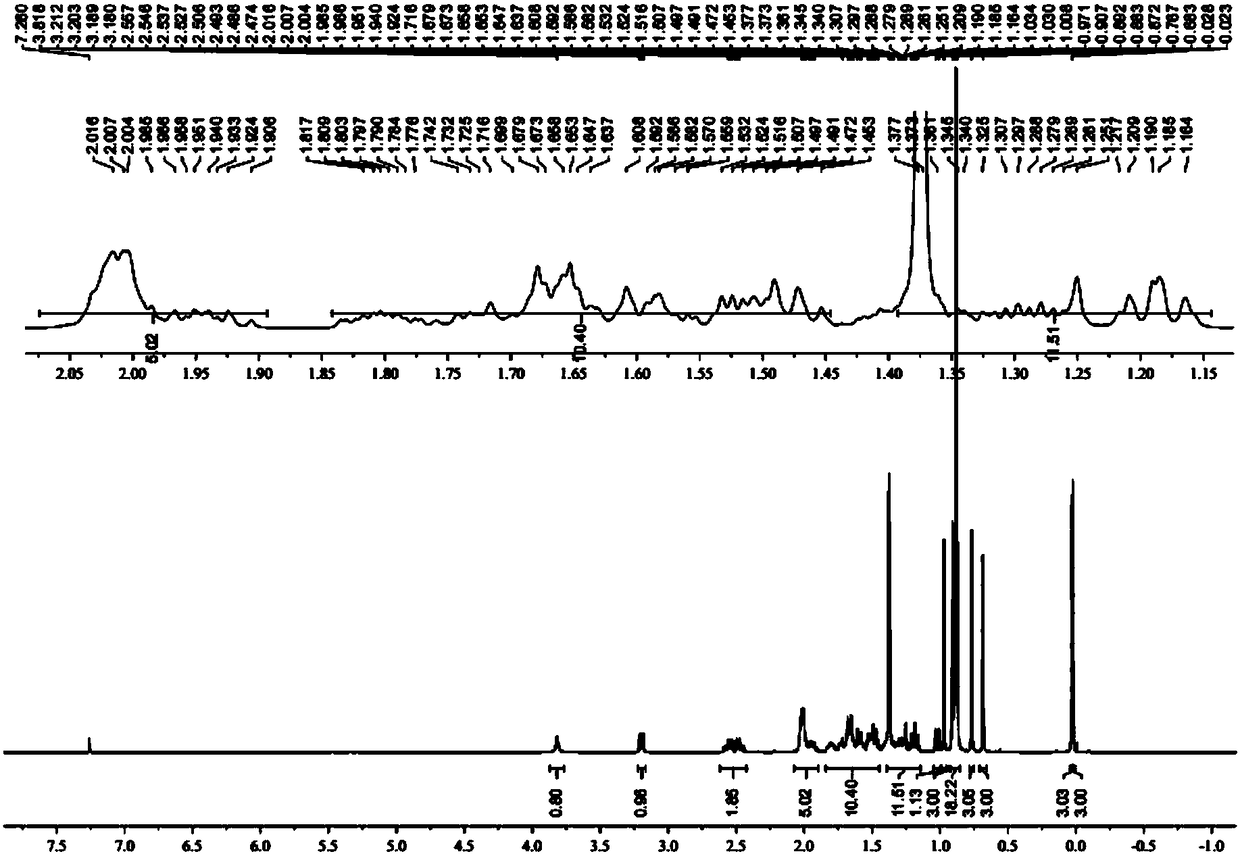
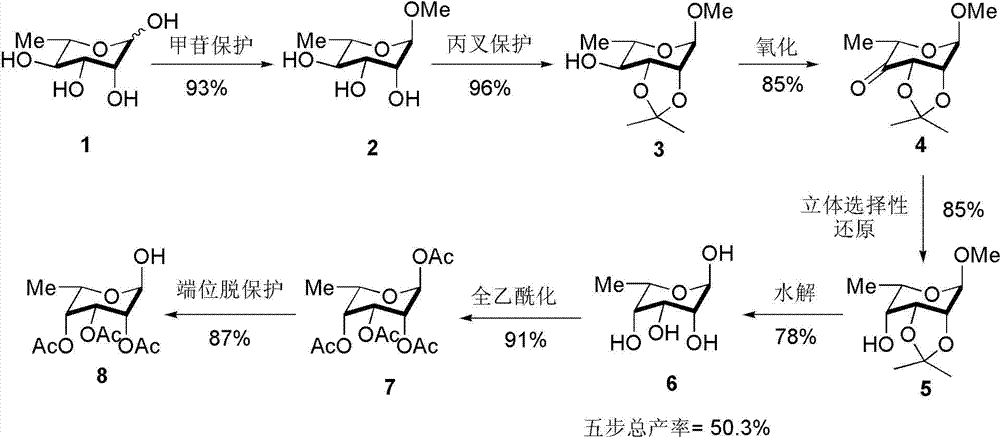
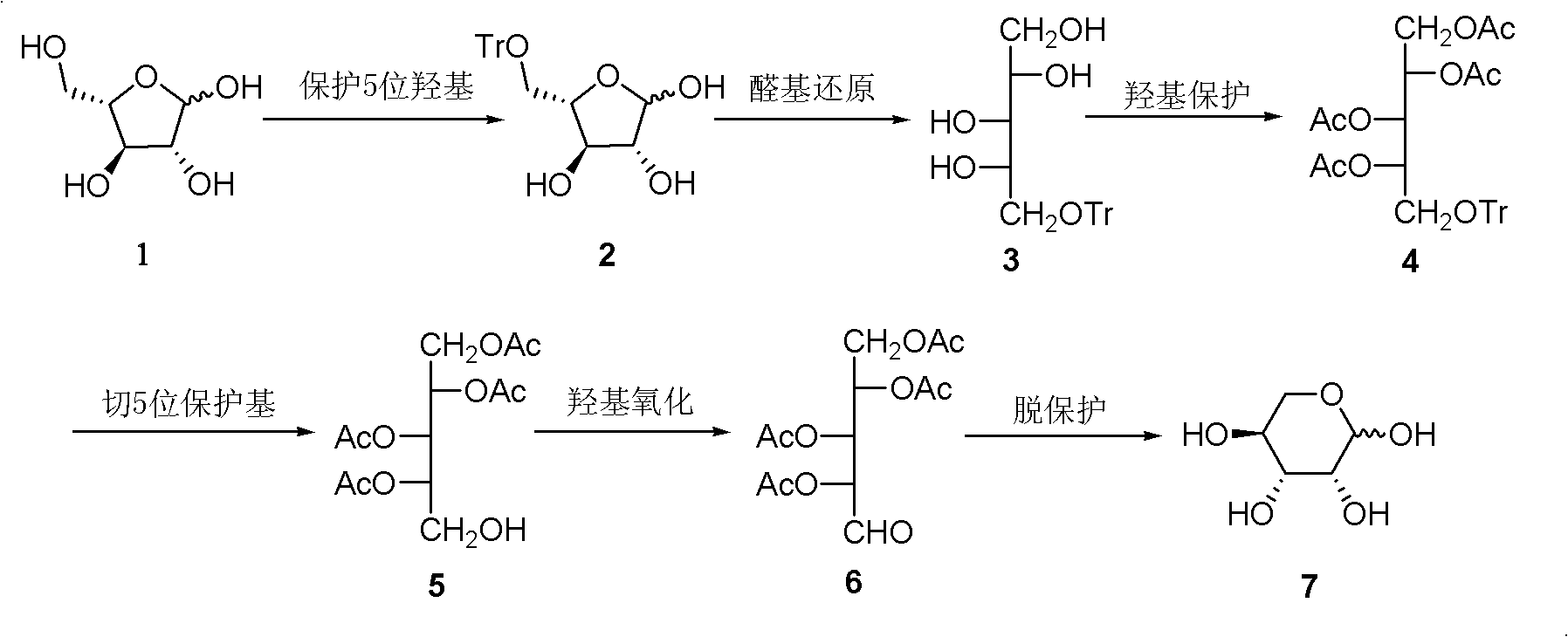
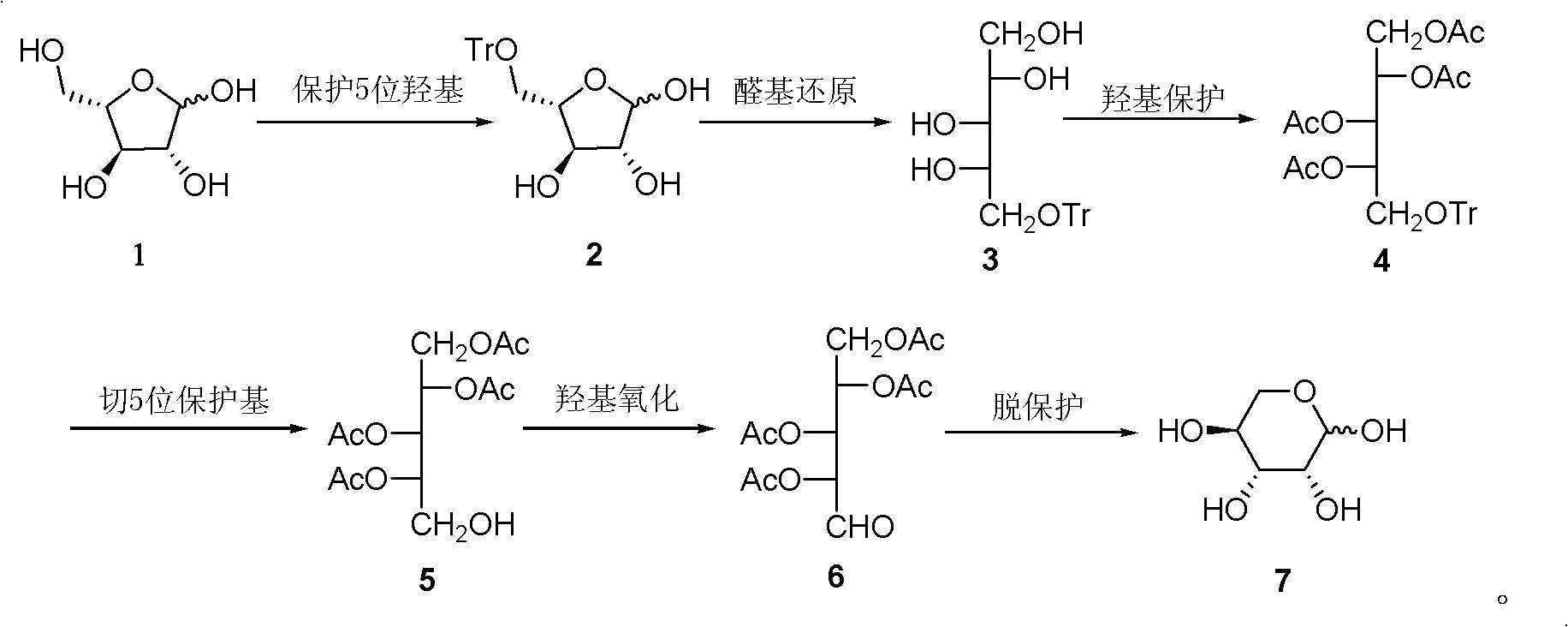
A kind of preparation method of L-lyxose
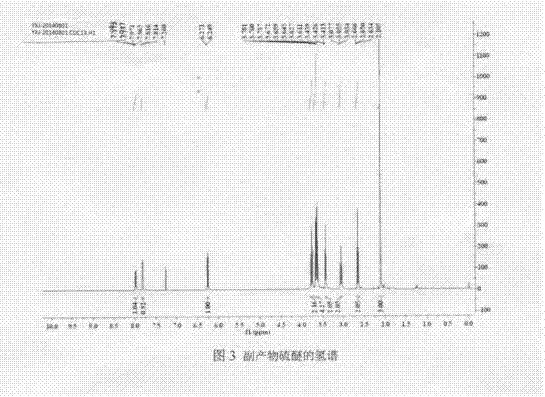
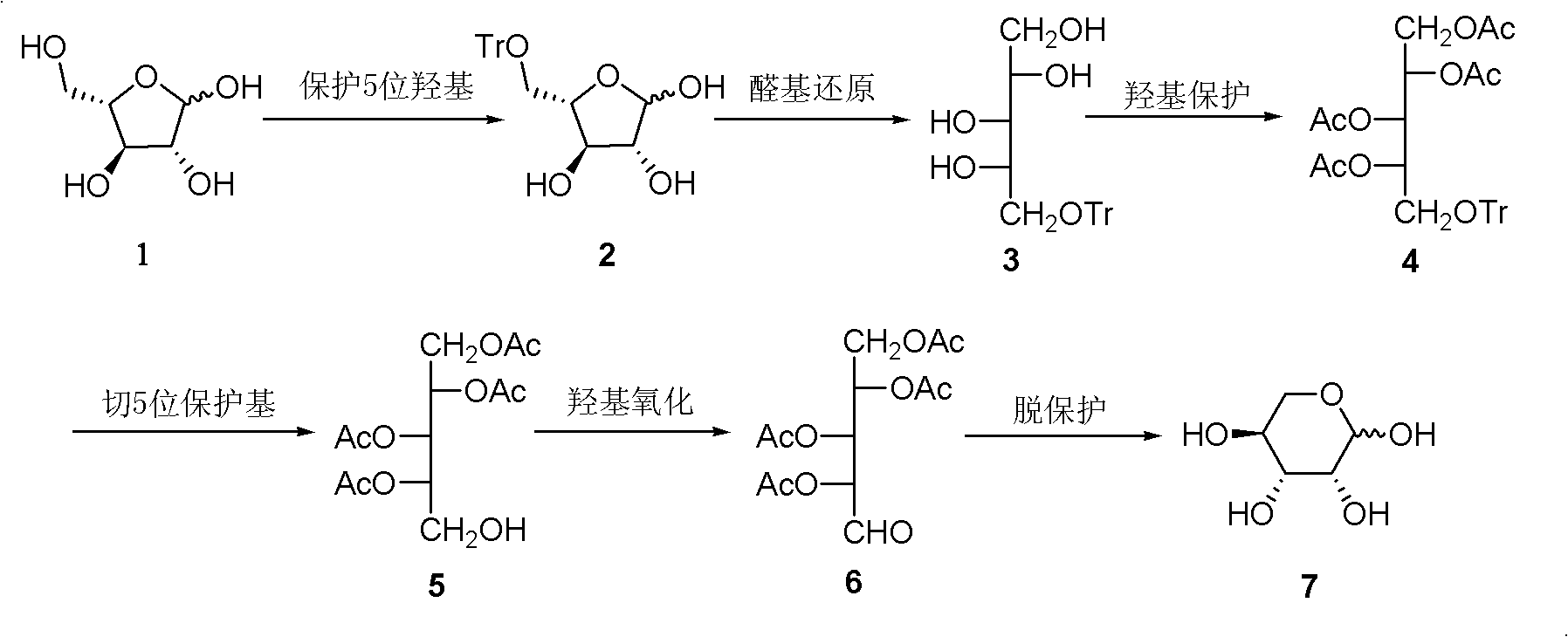
InactiveCN102286030AHigh purityRaw materials are cheap and easy to getSugar derivativesSugar derivatives preparationAcetic acidSwern oxidation
The invention relates to a preparation method of L-lyxose and relates to a five-carbon sugar. The invention provides a preparation method of L-lyxose and the method has cheap and easily available raw materials, fewer reagents and simple process. The technical scheme of the preparation method uses L-arabinose as a raw material; L-arabinose is subjected to hydroxyl protection and is reduced into a sugar alcohol, then the molecular configuration is changed under Swern oxidation condition, and finally the product L-lyxose with mirror symmetry conversion is obtained. The method comprises the following steps: synthesizing 5-O-triphenylmethyl-L-arabinose (2); synthesizing 5-O-triphenylmethyl-L-arabitol (3); synthesizing 5-O-triphenylmethyl-1,2,3,4-tetraacetylarabitol (4); synthesizing L-arabitol-1,2,3,4-tetraacetate (5); synthesizing L-lyxose-2,3,4,5-tetraacetate (6); and synthesizing L-lyxose (7).
Owner:XIAMEN UNIV
Preparation method of paricalcitol
InactiveCN101880253BEasy to operateShort routeOrganic chemistryBulk chemical productionSide chainDouble bond
The invention relates to a preparation method of paricalcitol, which is characterized in that after hydroxyl in the vitamin D2 is protected by p-toluenesulfonates, in the presence of alkali, intramolecular cyclization reaction happens in methanol to generate a compound 5; the compound 5 undergoes allylic oxidation and hydroxyl is protected to obtain a key intermediate 7; in the presence of ozone,the side chains and exocyclic terminal double bonds of the key intermediate 7 are cut off to obtain a compound 8; the primary hydroxyl in the compound 8 is selectively protected, a three-membered ring is opened in the presence of acid and then hydroxyl is protected to obtain a key intermediate 11; after the secondary hydroxyl in the key intermediate 11 is protected by sulphonate, a compound 12 isobtained through reduction by LiAlH4; a compound 13 is obtained after the compound 12 is subjected to Swern oxidation and carries out Wittig reaction with the compound 12 to obtain a compound 14; andthe target compound can be obtained by removing the protective group in the compound 14. The reagents used in the method are simple and are convenient to operate, the reactions concerning regioselectivity and stereoselectivity are few, the route is shorter and 12 steps of reactions are carried out.
Owner:CHONGQING TAIHAO PHARM CO LTD
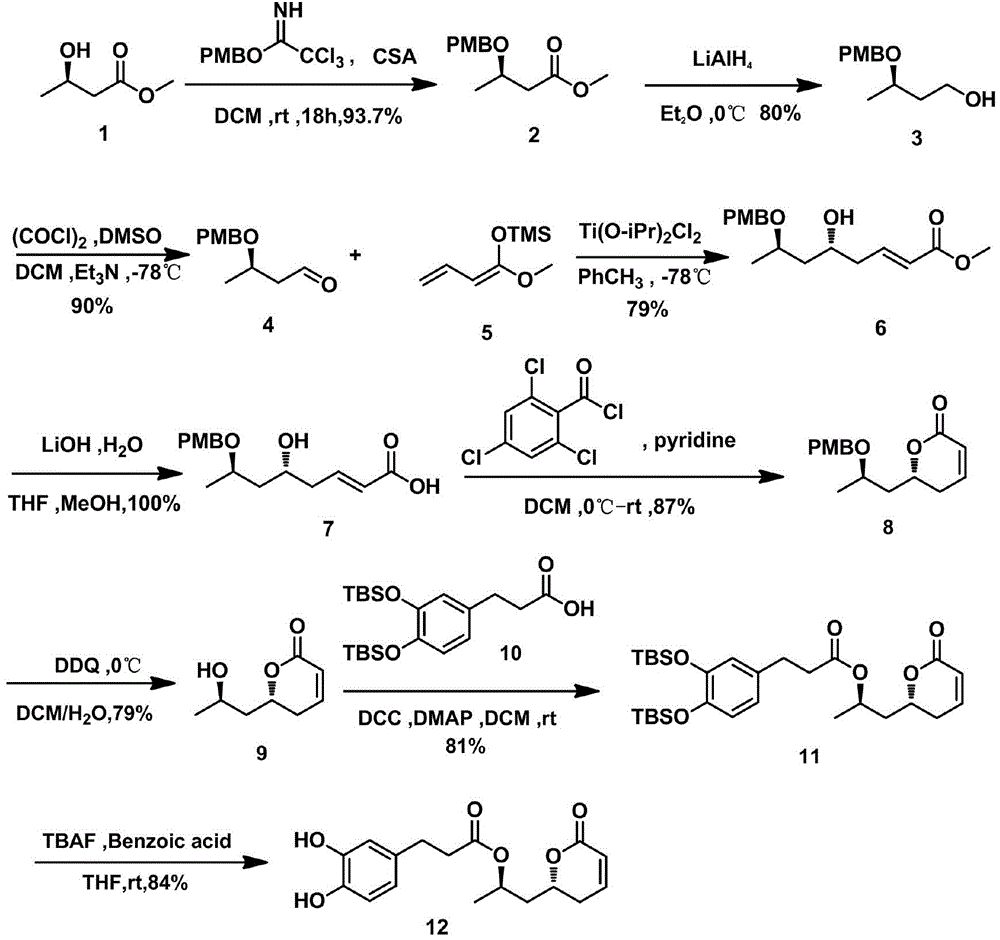
Preparation method of tert-butyl-5-(hydroxymethyl)-7-oxa-2-azaspiro[3.5]nonane-2-formate
InactiveCN110551133AShort synthetic routeEasy to operateOrganic chemistryBulk chemical productionLithiumSwern oxidation
The invention relates to a preparation method of tert-butyl-5-(hydroxymethyl)-7-oxa-2-azaspiro[3.5]nonane-2-formate, and mainly solves the technical problem that no proper industrial synthesis methodexists at present. The product is synthesized by six steps, and the preparation method comprises the steps: a first step, a compound 1 is subjected to Swern oxidation reaction to generate a compound 2; a second step, a compound 3 is obtained through a Horner-Wadsworth-Emmons reaction; a third step, a compound 4 is obtained through Michael addition; a fourth step, a compound 5 is obtained through reduction of the compound 4 with lithium tetrahydroaluminum; a fifth step, the compound 5 is dehydrated and retained with a ring to obtain a compound 6 under the action of sodium hydrogen; and a sixthstep, hydrogenation is preformed to remove a protective group, and then a protective group is added to obtain the target compound 7. The obtained compound is a useful intermediate or product for synthesis of many drugs.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
Method of preparing 6-deoxy-L-talose
InactiveCN103833796AHigh yieldAvoid pollutionEsterified saccharide compoundsSugar derivativesChemical synthesisKetone
The invention belongs to the field of chemical synthesis. The invention relates to a method of synthesis of 6-deoxy-L-talose,including: 1) performing glucoside protection of 1-positon hydroxyl of L-rhamnose in methanol solution by utilizing cation exchange resin (732H+type) to obtain a compound; 2) performing isopropylidene protection of hydroxyls at 2 and 3 positions of the compound to obtain a compound; 3) oxidizing 4-position hydroxyl of the compound to a ketone carbonyl group by utilizing a Swern oxidation system, so as to obtain a compound; 4) performing stereoselective reduction of 4-position carbonyl of the compound by utilizing sodium borohydride or sodium cyanoborohydride, so as to obtain a precursor compound with an opposite configuration to the raw materials, of 6-deoxy-L-talose; 5) refluxing the compound in sulfuric acid aqueous solution to hydrolyze to obtain 6-deoxy-L-talose. The preparing method using easily-available L-rhamnose having low price as the initial raw material, is a simple method, and is utility and efficient.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Preparation method of anticancer drug lanosterol derivative
InactiveCN108929363AHigh purityGood industrial use valueSilicon compound active ingredientsSteroids preparationLanosterolAcetic anhydride
The invention belongs to the technical field of drug synthesis, and relates to a preparation method of a novel anticancer drug lanosterol derivative. According to the method, an intermediate LD-a is generated by taking lanosterol as a starting raw material under the protection of tert-butyl dimethyl silicon (TBS); a Sharpless asymmetric dihydroxylation reaction is carried out on the intermediate LD-a under the action of osmium tetroxide (OsO4), and a vicinal diol compound LD-b is generated; Swern oxidation is carried out on the LD-b compound under the a triethylamine alkaline condition, and aalpha-hydroxy-ketone compound LD-c is generated; an acylation reaction is carried out on the LD-c with acetic anhydride, and an intermediate LD-d is obtained; the intermediate LD-d is subjected to TBSremoval protection, and an intermediate LD-e is obtained; and finally, the target lanosterol derivative LD is obtained through epoxidation. According to the invention, the reaction conditions of allthe steps are relatively mild, the synthetic steps are short, the use of highly toxic or expensive reagents is avoided, the prepared product is high in purity, and the preparation method can be applied to industrial production.
Owner:INST OF DONGGUAN SUN YAT SEN UNIV +1
Preparation method for trace tritiated deoxynivalenol
InactiveCN103421019AReduce Radiation DamageSynthetic conditions are suitableOrganic chemistrySolventHydroxy compound
The invention relates to a preparation method for trace tritiated biotoxin deoxynivalenol. The method comprises the following steps: using the deoxynivalenol as a raw materials, and phenylboronic acid to protect 7 alpha-position and 15-position hydroxies, and oxidizing 3-position hydroxyl through Swern Oxidation; using isopropyl alcohol as a resolvent, and adopting tritiated sodium boro-hydride to selectively reduce the 3-position carboxide, so as to obtain tritiated deoxynivalenol. The method has the advantages that the raw materials are easy to obtain, the reaction condition is mild, the operation is easy, and the obtained tritiated compound is clear in labeling position and stable; the chemical purity and the radiochemical purity of the product are not less than 98 percent, and the specific activity is 2.52 Ci / mmol. The method provides an important material foundation for carrying out poison mechanism and food safety evaluation of the deoxynivalenol in a deep-going way.
Owner:HUAZHONG AGRI UNIV
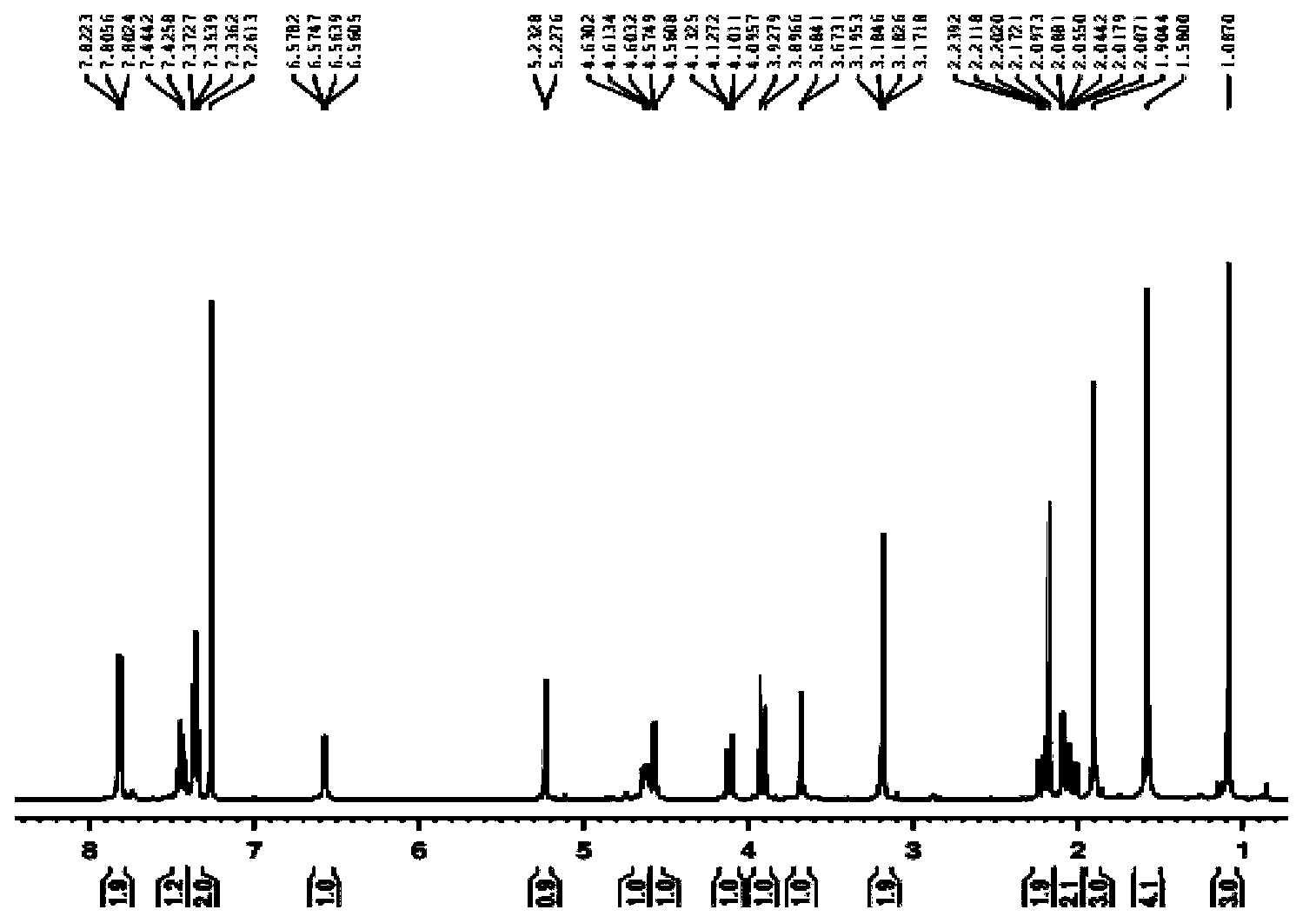
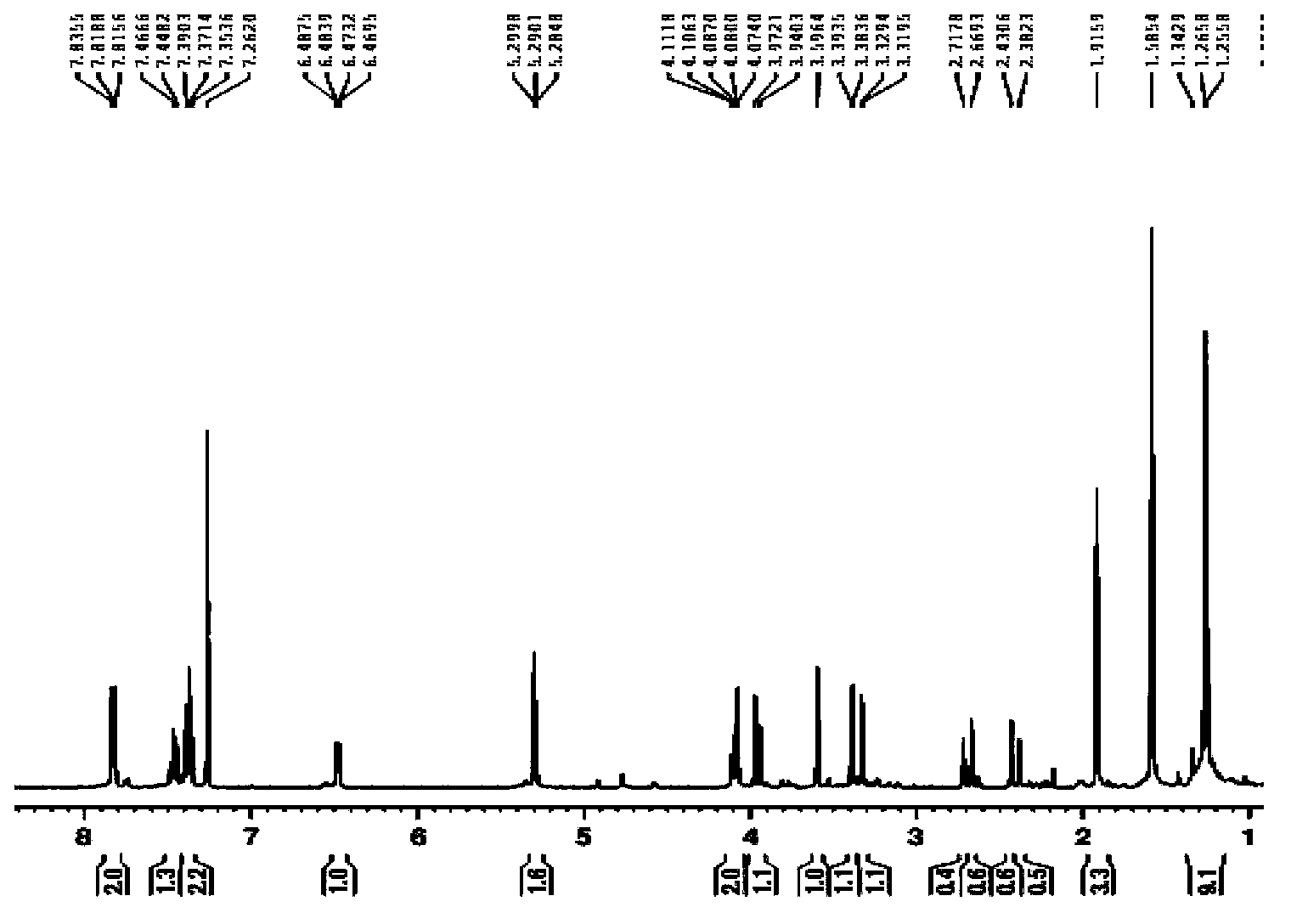
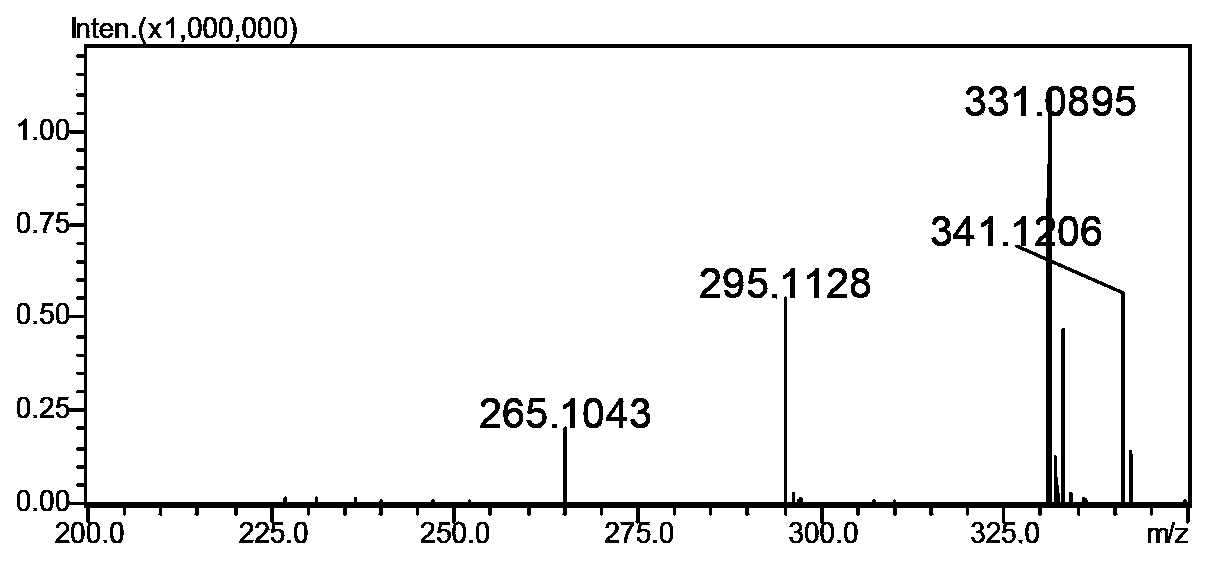
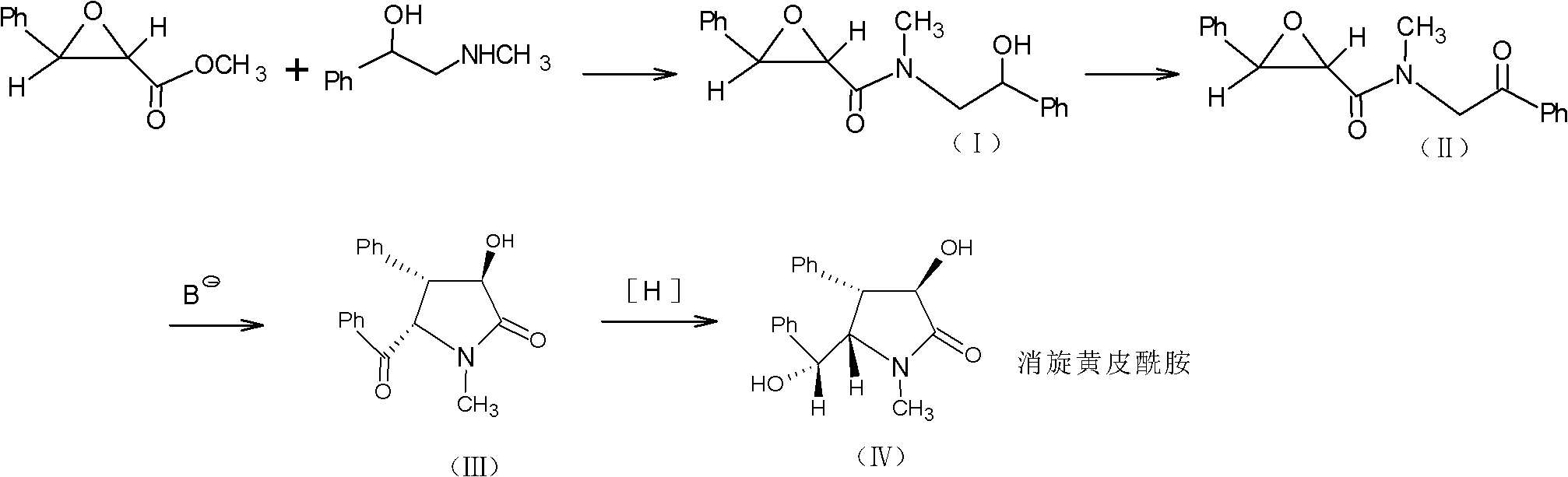
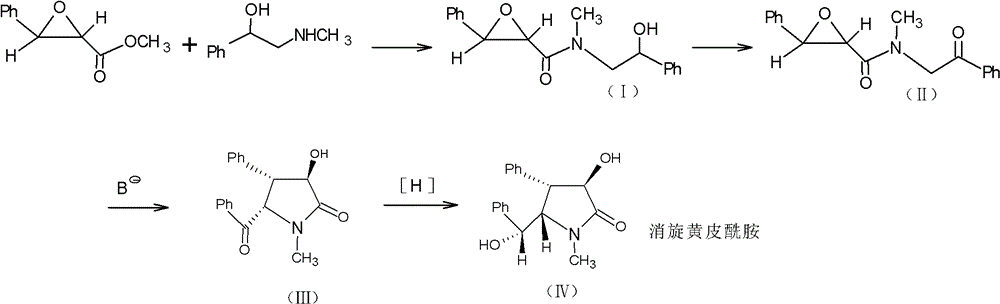
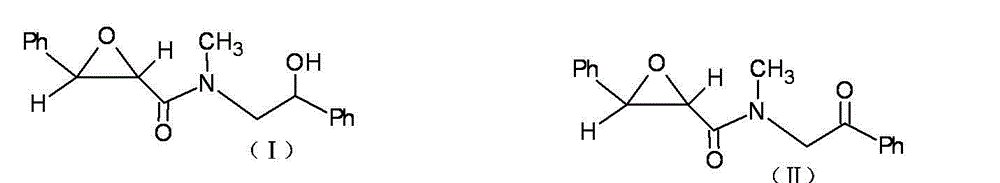
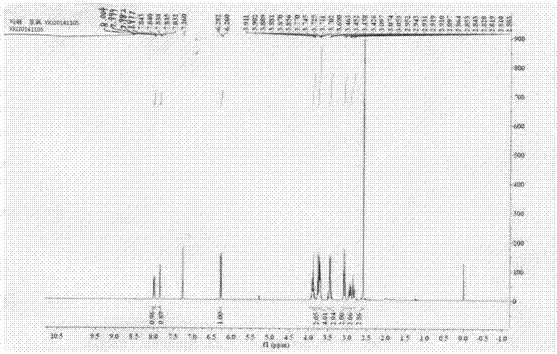
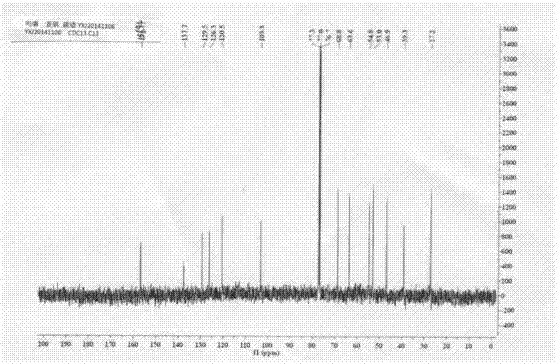
Method for preparing clausenamide intermediate by Swern oxidation process
The invention relates to a method for preparing a clausenamide intermediate by a Swern oxidation process and belongs to the field of pharmaceutical chemicals. The method comprises a step of oxidizing N-methyl-N-(2-hydroxy-2-phenyl)ethyl-3-phenylglycidylamide (I) compound under an alkaline condition in a solvent to obtain a N-methyl-N-benzoylmethyl-3-phenylglycidylamide(II) compound.
Owner:广州诺浩医药科技有限公司
Tyramycin intermediate and preparation method thereof and preparation method of telamycin
ActiveCN102786569BReduce manufacturing costMild conditionsSugar derivativesSugar derivatives preparationEpoxyPhosphate
The invention provides a tulathromycin intermediate, a preparation method of the tulathromycin intermediate, and a preparation method of the tulathromycin. The preparation method of the tulathromycin has the advantages of mild condition, convenience for operation, and low cost. The preparation method of the tulathromycin comprises the following steps of: using azithromycin A as a raw material; protecting 2'-hydroxy and 6'-amino in the azithromycin A through di-tert-butyl dicarbonate so as to obtain double-protective azithromycin A; carrying out Swern oxidation to 4''-hydroxy to the double-protective azithromycin A; salifying along with trifluoroacetic acid; and synchronously removing boc t-butyloxycarbonyl to obtain the azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl; and then reacting with trimethylsulfonium bromide to obtain 4''-epoxy compound; and finally carrying out nucleophilic addition on the 4''-epoxy compound by n-propylamine so as to obtain the phosphate of tulathromycin; and further neutralizing via alkaline to obtain the target compound tulathromycin; and synchronously obtaining the tulathromycin intermediate of azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
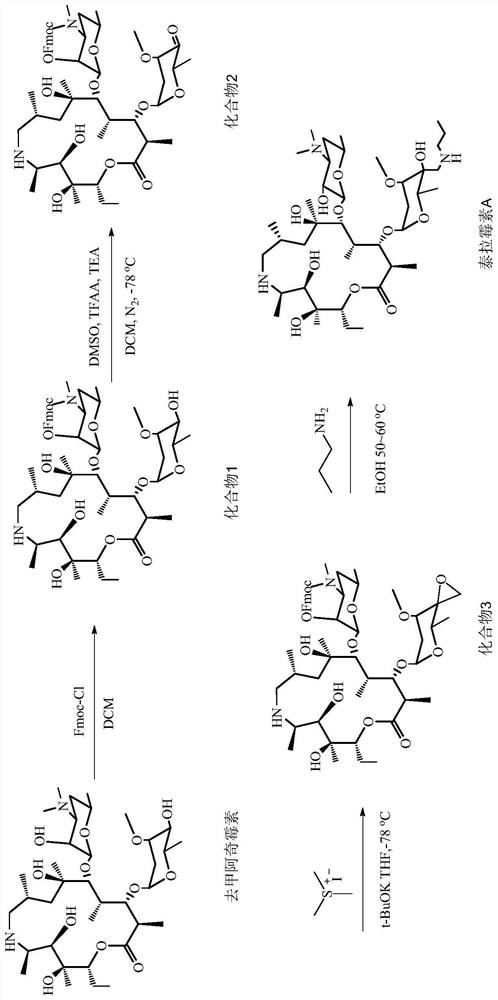
Tulathromycin synthesis method
PendingCN113493483AHigh selectivityHigh yieldSugar derivativesSugar derivatives preparationIodidePropylamine
Owner:SHANGHAI INST OF TECH
Chemical synthesis method of laetispicine
A sulphonyl tetrazole compound 7 with the following structure, its preparation method and its use for preparing laetispicine. The said sulphonyl tetrazole compound 7, (1-phenyl-5-(3,4-methylenedioxyphenyl ethyl) sulphonyl tetrazole), is obtained from piperonal as raw material and by steps of Darzens condensation, hydrolysis, decarboxylation, reduction, condensation and oxidation. Laetispicine is prepared from the said compound as raw material, by condensation with 9-carbonyl-2E-4E-nonadienoic acid isobutyramide (compound 14), or by reaction with 5-(tetrahydropyran-2-oxo) n-pentanal (compound 10), deprotection, Swern oxidation, Wittig-Horner reaction, hydrolysis and amidation. As shown in H NMR, C NMR, MS and IR, the laetispicine prepared from the above-said compound is consistent with the laetispicine extracted from natural products.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
A synthetic route and method of a bentelinoline anti -rotor body
The invention relates to a new synthesis route and method of a bedaquiline racemate, belonging to the technical field of medicine. Its steps include: (1) starting material 3-bromobenzyl-6-bromo-2-methoxyquinoline (II) reacts with metal magnesium in THF to prepare Grignard reagent, and then 1-naphthaldehyde (III ) is added in the prepared Grignard reagent, and the reflux reaction obtains the compound (Ⅳ) "2-(6-bromo-2-methoxyquinoline-3-yl)-1-(naphthalene-1-yl)-2- Phenyl alcohol "; (2) make compound (IV) undergo swern oxidation to obtain carbonyl compound 2-(6-bromo-2-methoxyquinoline-3-yl)-1-(naphthalene-1-yl)-2 ‑Phenyl ethyl ketone (compound V); (3) Compound (V) reacts with Grignard reagent (VI) to obtain bedaquiline racemate (I). The method of the invention has simple and convenient route, mild reaction conditions, low cost and high yield, and is suitable for industrial production and application.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Method for preparing clausenamide intermediate by Swern oxidation process
The invention relates to a method for preparing a clausenamide intermediate by a Swern oxidation process and belongs to the field of pharmaceutical chemicals. The method comprises a step of oxidizing N-methyl-N-(2-hydroxy-2-phenyl)ethyl-3-phenylglycidylamide (I) compound under an alkaline condition in a solvent to obtain a N-methyl-N-benzoylmethyl-3-phenylglycidylamide(II) compound.
Owner:广州诺浩医药科技有限公司
Preparation and application of a swern reagent
ActiveCN105585540BRaise the reaction temperatureInhibition releaseOrganic compound preparationCarbonyl group formation/introductionMorpholineReaction temperature
The invention discloses a 4-(2-(2-(methylsulfoxide)ethyl)-4-nitrophenyl)morpholine represented by formula (I), and discloses its preparation and application. Its preparation method is as follows: 2-(2-chloro-5 nitro)phenethyl alcohol and morpholine as shown in formula (II) are mixed to obtain 2-(2-morpholine-5 as shown in formula (III) -Nitrophenyl) ethanol; Then in (III), add dropwise two (trichloromethyl) carbonates successively, sodium methyl mercaptide aqueous solution, hydrogen peroxide aqueous solution, finally make 4-(2-(2-(form (sulfoxide)ethyl)-4-nitrophenyl)morpholine. Its application is to react the obtained swern reagent with the alcohol compound shown in formula (IV), and prepare the aldehyde or ketone shown in formula (V) through post-treatment. The present invention overcomes the shortcomings of the existing Swern oxidation method, avoids the generation of foul-smelling by-products dimethyl sulfide and toxic carbon monoxide from the source, increases the reaction temperature to -30°C to 0°C, and has no odor The by-product new sulfide can be recovered and reused.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of L-lyxose
InactiveCN102286030BHigh purityRaw materials are cheap and easy to getSugar derivativesSugar derivatives preparationAcetic acidSwern oxidation
The invention relates to a preparation method of L-lyxose and relates to a five-carbon sugar. The invention provides a preparation method of L-lyxose and the method has cheap and easily available raw materials, fewer reagents and simple process. The technical scheme of the preparation method uses L-arabinose as a raw material; L-arabinose is subjected to hydroxyl protection and is reduced into a sugar alcohol, then the molecular configuration is changed under Swern oxidation condition, and finally the product L-lyxose with mirror symmetry conversion is obtained. The method comprises the following steps: synthesizing 5-O-triphenylmethyl-L-arabinose (2); synthesizing 5-O-triphenylmethyl-L-arabitol (3); synthesizing 5-O-triphenylmethyl-1,2,3,4-tetraacetylarabitol (4); synthesizing L-arabitol-1,2,3,4-tetraacetate (5); synthesizing L-lyxose-2,3,4,5-tetraacetate (6); and synthesizing L-lyxose (7).
Owner:XIAMEN UNIV
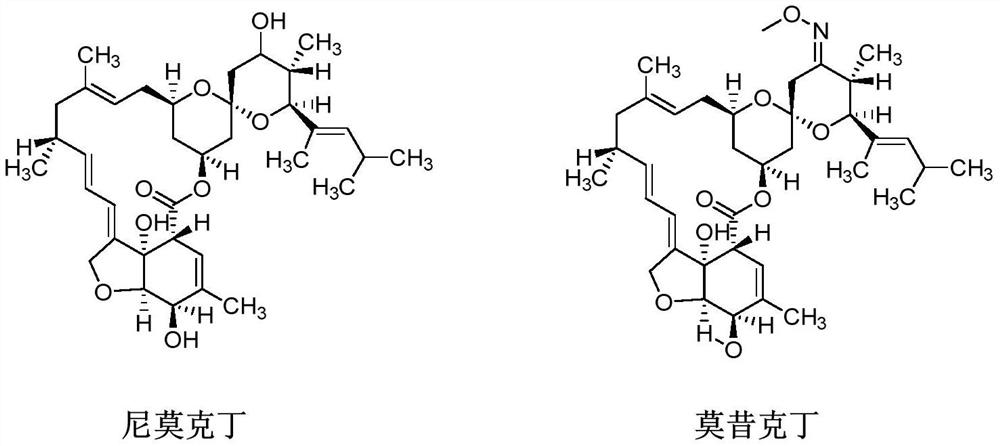
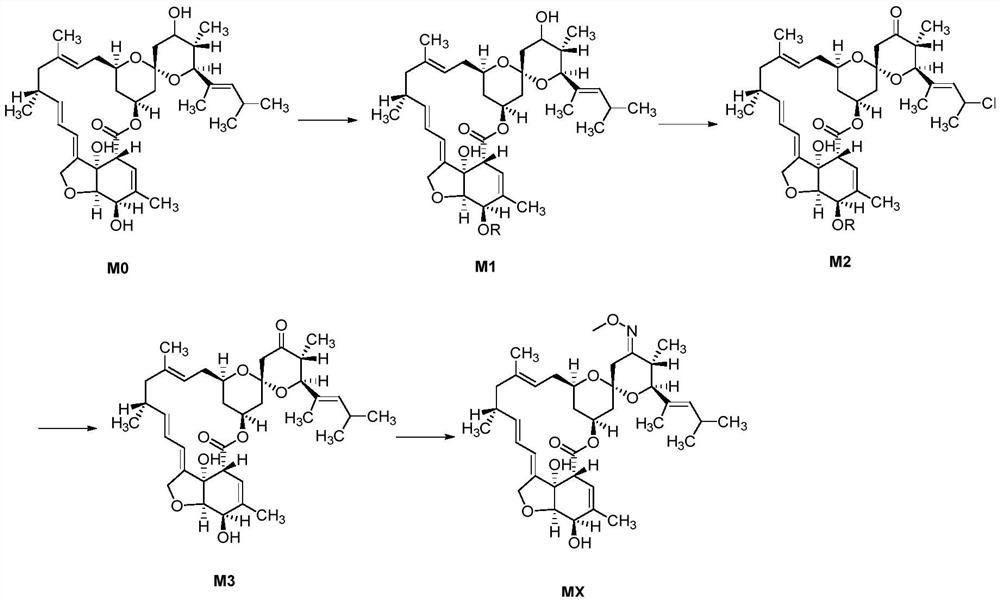
A kind of method of synthesizing moxidectin
ActiveCN111825693BGood choiceMild oxidation conditionsOrganic chemistrySwern oxidationChemical reaction
The invention provides a method for synthesizing moxidectin, comprising steps: 1) adding a protective substance to nimoctin M0 for reaction, and protecting the 3' hydroxyl of nimoctin M0 to obtain an upper-protected intermediate M1; 2) Using Dess Martin oxidant to oxidize the protected intermediate M1 to obtain the oxidized intermediate M2; 3) deprotect the oxidized intermediate M2 to obtain the deprotected intermediate M3; 4) subject the deprotected intermediate M3 to an oxime chemical reaction to obtain moxidectin MX. The present invention uses Dess Martin oxidant to replace the Swern oxidation system in the prior art and PCC / PDC oxidation to oxidize the protected intermediate M1 to obtain the key intermediate M2 of moxidectin. The oxidation condition is mild and can be carried out at room temperature. , no need to use low temperature; and the oxidation reaction has good selectivity and high yield, and the obtained oxidation intermediate M2 has high purity.
Owner:LIVZON NEW NORTH RIVER PHARMA
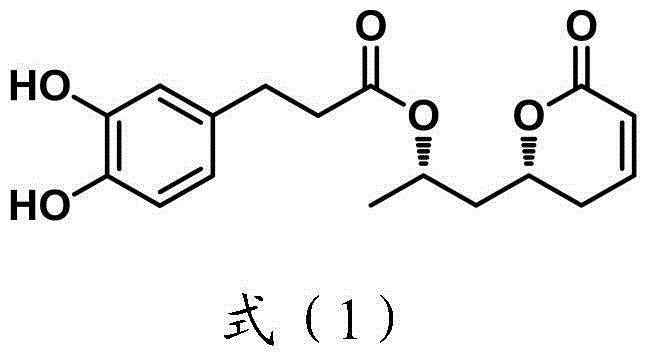
Process for making 3-aryloxy, 4-arylfuran-2-ones useful as inhibitors of cox-2
Described is a process of preparing 3-aryl, 4-aryloxy furan-5- ones which are useful as inhibitors of cyclooxygenase-2(COX-2). Such compounds are useful as anti-inflammatory agents. The process is directed to an asymmetric synthesis which involves: a trisubstituted styrene derivative preparation via Horner-Wadsworth-Emmons reaction and subsequent one pot trifluoromethylation of the allylic alcohol; preparation of the alpha -hydroxyl ketone using Sharpless assymmetric dihydroxylation and Swern oxidation; the esterification of the alpha -hydroxyl ketone with the phenoxy acetic acid; and the Dieckman condensation of the resulting ester.
Owner:MERCK & CO INC
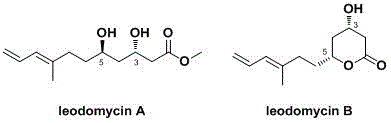
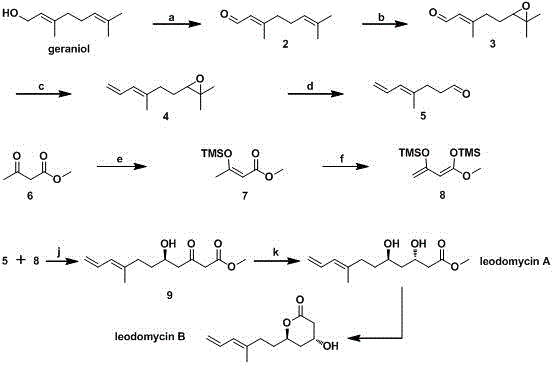
Stereoselective synthesis of ieodomycina and b
InactiveCN103755568BInnovative design ideasAsymmetric Total SynthesisOrganic compound preparationCarboxylic acid esters preparationTitanium isopropoxideMeta-Chloroperoxybenzoic acid
Owner:JIANGXI SCI & TECH NORMAL UNIV
Process for preparing 6-deoxy-l-talose
InactiveCN103833796BHigh yieldAvoid pollutionEsterified saccharide compoundsSugar derivativesChemical synthesisKetone
The invention belongs to the field of chemical synthesis. The invention relates to a method of synthesis of 6-deoxy-L-talose,including: 1) performing glucoside protection of 1-positon hydroxyl of L-rhamnose in methanol solution by utilizing cation exchange resin (732H+type) to obtain a compound; 2) performing isopropylidene protection of hydroxyls at 2 and 3 positions of the compound to obtain a compound; 3) oxidizing 4-position hydroxyl of the compound to a ketone carbonyl group by utilizing a Swern oxidation system, so as to obtain a compound; 4) performing stereoselective reduction of 4-position carbonyl of the compound by utilizing sodium borohydride or sodium cyanoborohydride, so as to obtain a precursor compound with an opposite configuration to the raw materials, of 6-deoxy-L-talose; 5) refluxing the compound in sulfuric acid aqueous solution to hydrolyze to obtain 6-deoxy-L-talose. The preparing method using easily-available L-rhamnose having low price as the initial raw material, is a simple method, and is utility and efficient.
Owner:JIANGXI SCI & TECH NORMAL UNIV
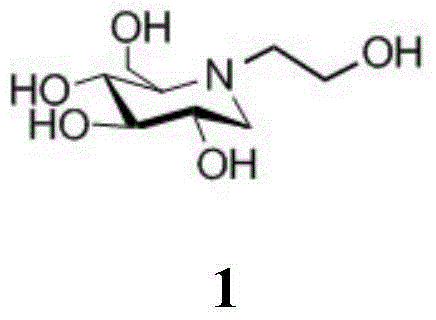
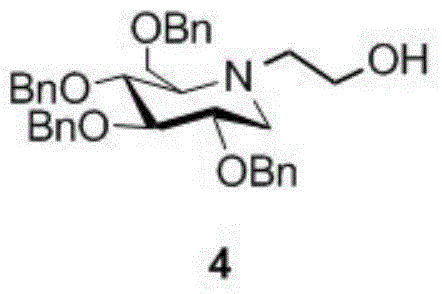
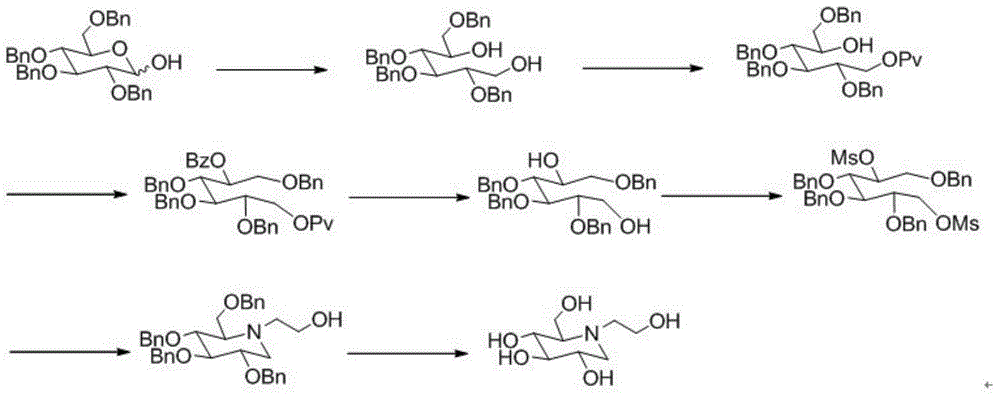
A preparing method of a miglitol intermediate
InactiveCN104693109AReduce separation and purification stepsReduce lossesOrganic chemistryMiglitolEnantiomer
The invention belongs to the field of medicine synthesis, and relates to a preparing method of a miglitol intermediate. The method includes performing Swern oxidation by adopting tetrabenzyl glucose diol as a raw material to obtain a tetrabenzyl glucose dicarbonyl derivative, and directly subjecting the oxidation product without separation to a double reductive amination reaction with ethanolamine and sodium cyanoborohydride under the existence of a dehydrant to prepare tetrabenzyl miglitol that is an important intermediate for preparation of miglitol. The method is mainly advantageous in that: the tetrabenzyl glucose dicarbonyl derivative which is instable is directly used for the reaction in the next step without separation; proper reaction conditions are selected in the double reductive amination reaction step to directly obtain an optically pure target product 2-position of which is in an R configuration; steps of separation and purification for an enantiomer mixture are omitted, and loss caused by the steps is omitted; and the method is short in reaction time and high in yield.
Owner:SHAANXI NORMAL UNIV +1
A novel compound 6,6-dimethyltetrahydropyran-2-methanol and its preparation method
InactiveCN107746396BEasy to handle and purifyMild responseOrganic chemistryTrimethylsilyl chlorideSwern oxidation
The invention discloses a novel compound that is 6,6-dimethyl tetrahydropyran-2-methanol and a preparing method thereof. The method includes preparing benzyloxy ethanol by utilizing a sodium alkoxideprocess; then oxidizing the benzyloxy ethanol into benzyloxy acetaldehyde by utilizing a swern oxidation process; reacting the benzyloxy acetaldehyde and allyltributyltin prepared by utilizing a Grignard reaction to obtain 1-(benzyloxy)-4-penten-2-ol; subjecting the 1-(benzyloxy)-4-penten-2-ol and acetone to cyclization under catalysis of trimethylchlorosilane and potassium iodide to obtain 4-iodo-6,6-dimethyl tetrahydropyran-2-methanol; and subjecting the 4-iodo-6,6-dimethyl tetrahydropyran-2-methanol to hydrogenation to remove iodine to obtain the target product that is the 6,6-dimethyl tetrahydropyran-2-methanol. According to the method, reactions are relatively mild, products can be easily treated and purified, and the method is suitable for batch preparation, and therefore the methodhas important application value.
Owner:HENAN VOCATIONAL & TECHN COLLEGE OF COMM
Method used for direct synthesis of epoxy compounds from alcohol
ActiveCN110283146AAchieve direct epoxidationThe synthetic route is simpleOrganic chemistryBulk chemical productionEpoxySwern oxidation
The invention discloses a method used for direct synthesis of an epoxy compounds from an alcohol. According to the method, an alcohol is taken as a raw material, Swern oxidation is adopted to synthesize an aldehyde, a bromo-hydrocarbon and an alkali are added into the aldehyde directly to construct epoxy functional groups, and generate the epoxy compound. According to the method, one-pot method is adopted to realize direct epoxidation of the alcohol, the synthesis route is simple, the preparation process is easy to control, no catalyst is needed in the process, substrate suitable range is wide, reagents are cheap and easily available, preparation conditions are mild, reaction yield is high, and the method is suitable for synthesis of epoxy compounds.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
A kind of preparation method of anticancer drug lanosterol derivative
InactiveCN108929363BHas anticancer effectReduce usageSilicon compound active ingredientsSteroids preparationLanosterolAcetic anhydride
Owner:INST OF DONGGUAN SUN YAT SEN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Preparation method of tert-butyl-5-(hydroxymethyl)-7-oxa-2-azaspiro[3.5]nonane-2-formate Preparation method of tert-butyl-5-(hydroxymethyl)-7-oxa-2-azaspiro[3.5]nonane-2-formate](https://images-eureka.patsnap.com/patent_img/51b27d37-112c-4035-b5cd-9f3e6db271b5/DEST_PATH_S.png)
![Preparation method of tert-butyl-5-(hydroxymethyl)-7-oxa-2-azaspiro[3.5]nonane-2-formate Preparation method of tert-butyl-5-(hydroxymethyl)-7-oxa-2-azaspiro[3.5]nonane-2-formate](https://images-eureka.patsnap.com/patent_img/51b27d37-112c-4035-b5cd-9f3e6db271b5/938717DEST_PATH_S.png)