Preparation method of paricalcitol
A technology for paricalcitol and compounds, which is applied in the field of preparation of paricalcitol, can solve the problems of unenvironmental protection, low yield of Julia coupling olefination, poor selectivity, etc., and achieves convenient operation and reagents. Simple, short route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] For further elaborating the technical means and effects that the present invention takes to reach the intended invention purpose, below in conjunction with accompanying drawing and preferred embodiment, to its specific implementation, method, Steps, features and effects thereof are described in detail below.
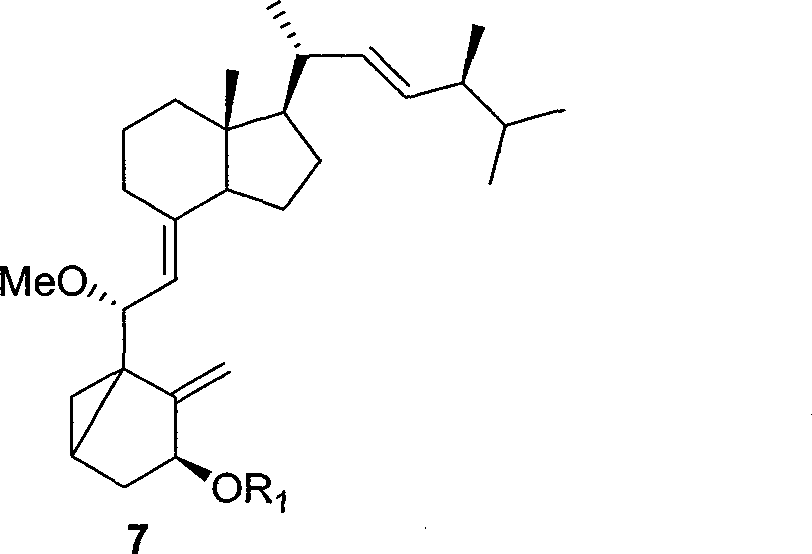
[0037] The method of the present invention uses vitamin D2 as a starting material, after protecting the three-membered ring, protecting the hydroxyl group to obtain compound 7,
[0038]
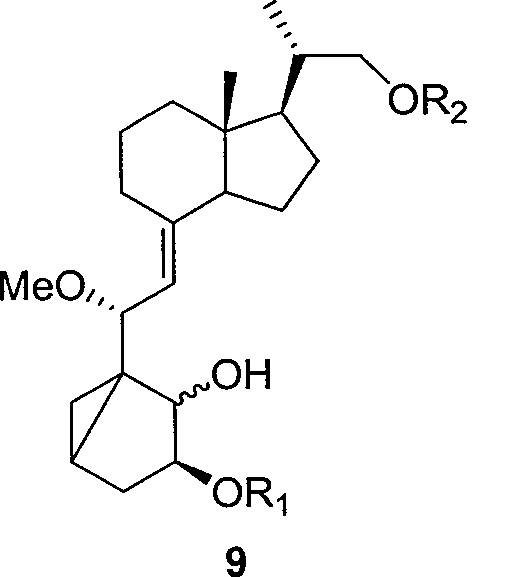
[0039] R 1 Can be acetyl, benzoyl, t-butyldimethylsilyl, triethylsilyl, methoxymethyl. Compound 7 was cut off the double bonds on the outer ring and the side chain by one ozonation reaction, and compound 8 was obtained by reduction, and compound 9 was obtained by selective esterification.
[0040]
[0041] R 2 Can be acetyl, benzoyl.
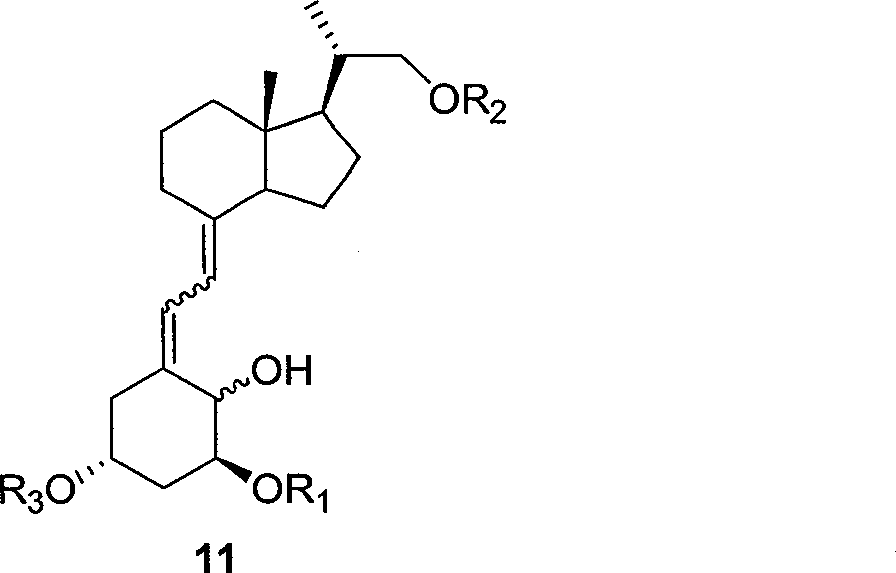
[0042] Compound 9 was ring-opened to obtain compound 10, and the hydroxyl group was selectively protected to obtain compound 11.
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com