Patents
Literature
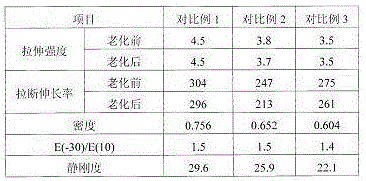
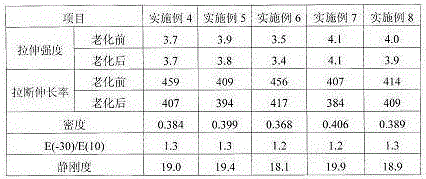
867 results about "Benzenesulfinic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Scale inhibitor for an aqueous system
InactiveUS20030091467A1Less-expensive to useInhibition formationOther chemical processesScale removal and water softeningSulfonateTricarboxylic acid
This invention relates to a composition and a method for inhibiting the formation of scale, particularly complex scale, in an aqueous system where more than one type of scale is present. The scale typically present in the aqueous system includes silica, silicates, and carbonates. The scale inhibiting composition comprises (1) 2-phosphonobutane-1,2,4-tricarboxylic acid and salts thereof, and (2) a copolymer of (1) one or more allyloxybenzenesulfonate monomers, and (2) one or more water-soluble acrylic monomers, substituted acrylic monomers, or mixtures thereof.
Owner:SOLENIS TECH CAYMAN
Quick-setting cationic aqueous emulsions using pre-treated rubber modified asphalt cement
Cationic aqueous emulsions of rubber modified asphalt cement (RMAC) useable for paving, seal coat, slurry seal, roofing, coating and other applications. First, a RMAC is prepared by combining 45 to 90% by weight asphalt with about 5% to about 55% by weight solid recycled rubber (e.g., crumb rubber from used vehicle tires) under conditions that cause at least a substantial portion of the solid rubber to become liquified or otherwise incorporated into the asphalt. The RMAC may be treated with dodecyl benzenesulfonic acid (DDBSA) which causes reaction(s) to occur and results in a lowering of the viscosity of the RMAC. The RMAC (or DDBSA-treated RMAC) may then be emulsified in an aqueous medium to provide an aqueous emulsion.
Owner:SABUR DIANA +1
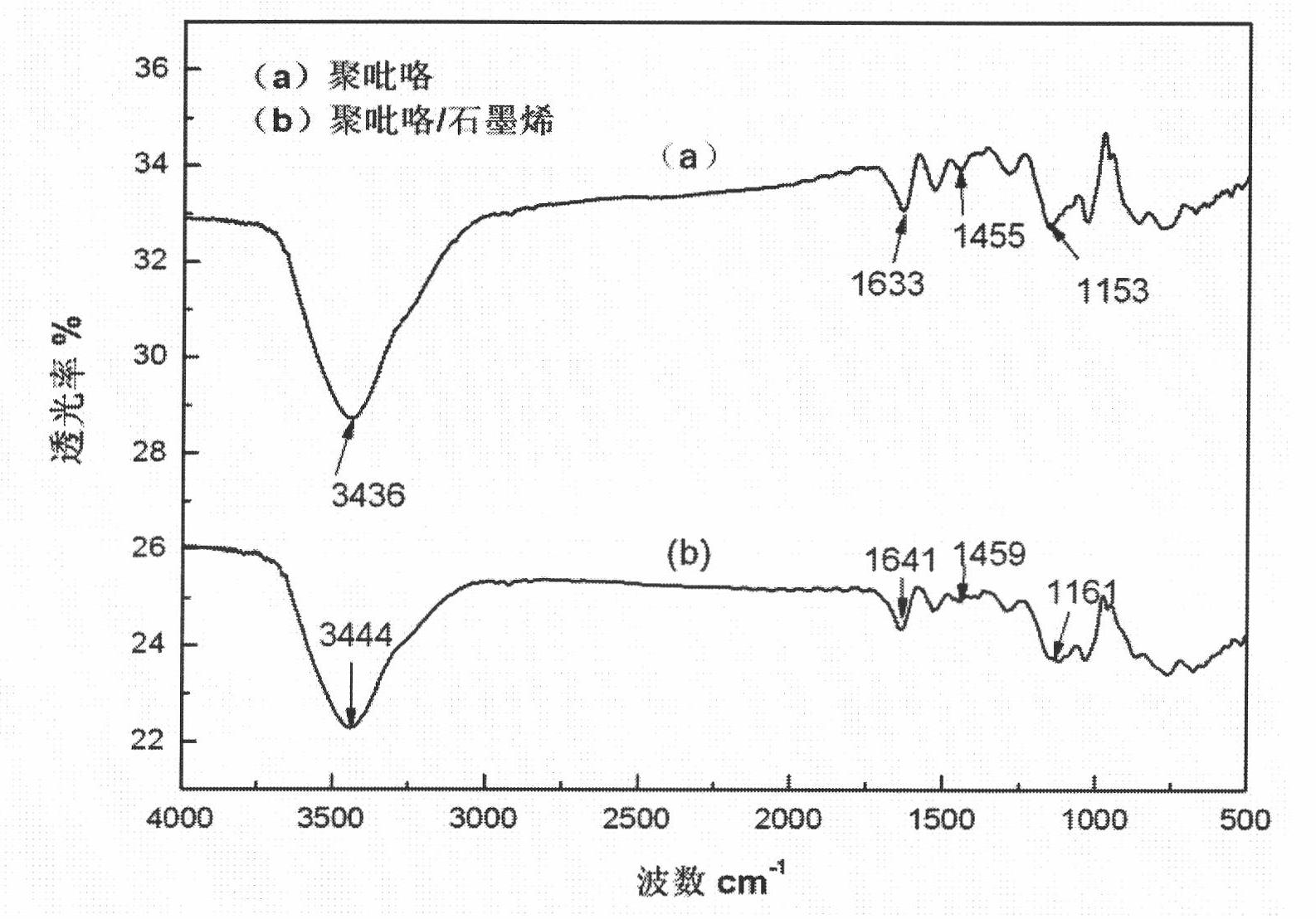
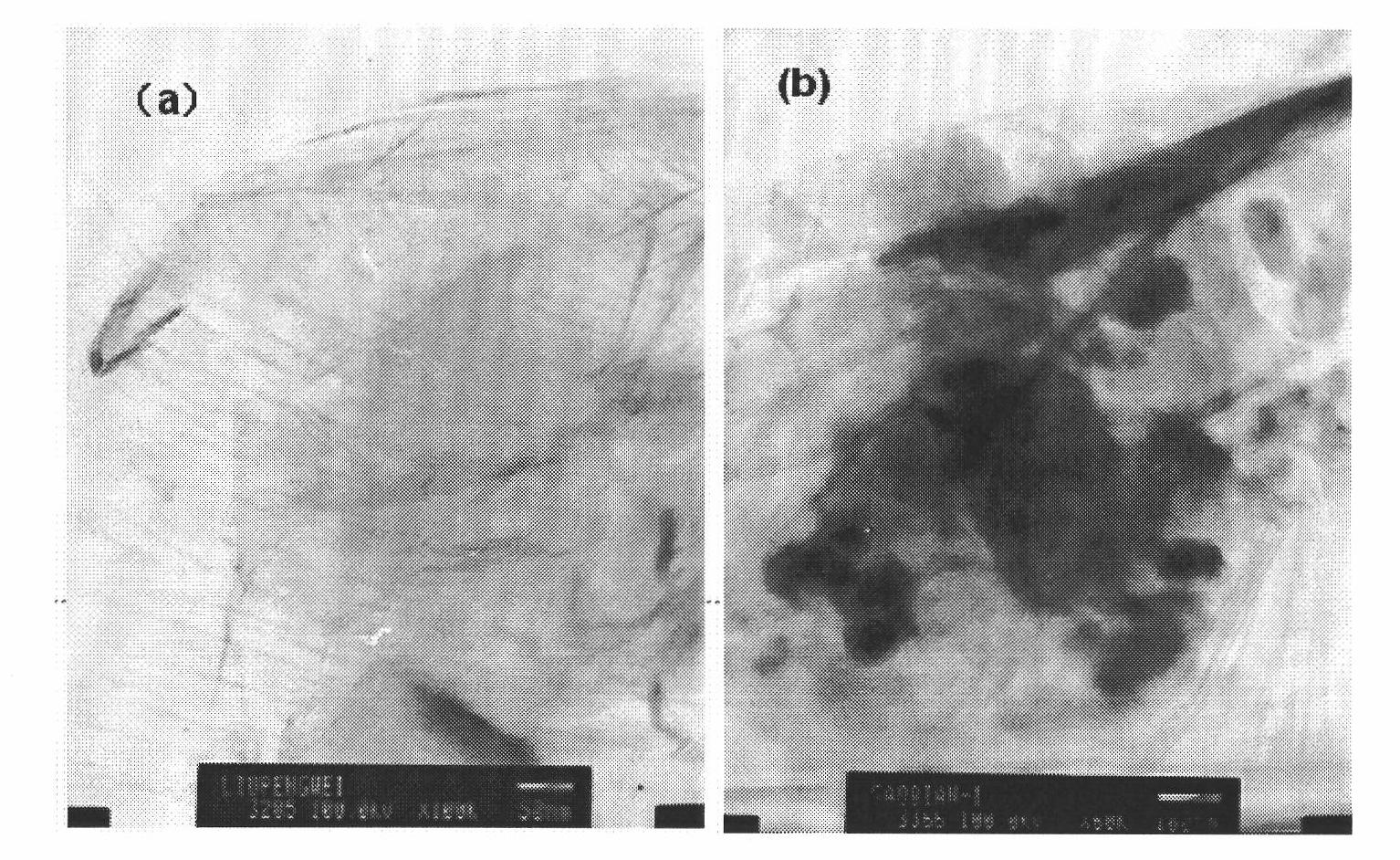
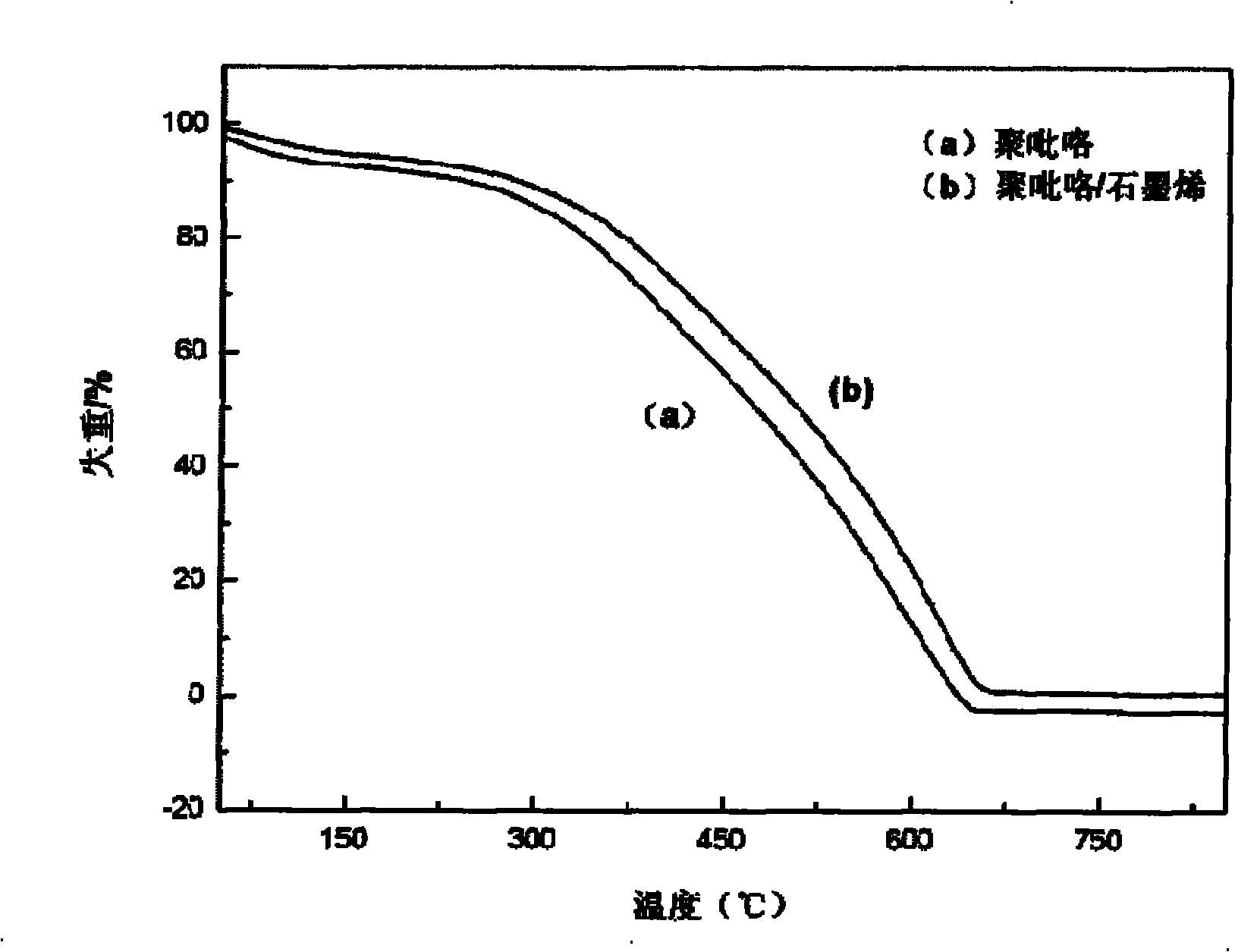
Polypyrrole/graphene nano composite and preparation method thereof
The invention discloses a polypyrrole / graphene nano composite which belongs to the technical field of composites. In the invention, ethanol is taken as a medium, para-toluenesulfonic acid is taken as a surface active agent, polyethylene glycol-400 is taken as phase transfer catalyst, FeCl3*6H2O is taken as an evocating agent, and under ultrasound conditions, pyrrole monomers are polymerized in situ on graphene, so as to obtain the polypyrrole / graphene nano composite. In the composite of the invention, through analysis by electron microscopy, the pyrrole monomers are polymerized in situ and evenly cover the grapheme, and the graphene and the polypyrrole are tightly combined in a nano level; and thermogravimetric analysis and electric conductivity study show that the composite has better heat stability, electric conductivity and processability and can be used in the fields of sensors, electron devices and biomedicine.
Owner:NORTHWEST NORMAL UNIVERSITY
Conductive graphene printing ink and preparation method thereof
The invention provides a conductive graphene printing ink. The conductive graphene printing ink is composed of 0.001-80wt% of graphene, 1-60wt% of a linking material, 0.1-30wt% of an assistant, and the balance solvent. The invention also provides a preparation method of the conductive graphene printing ink. The conductive graphene printing ink has the advantages of good toughness, good die molding performance, good adhesion and good impact resistance; chemically doped graphene and chemically modified graphene in the graphene have good conductive, mechanical and thermal performances. The molecules of the chemically doped graphene comprise one or more of polyaniline, polyacetylene, polythiophene, polyparaphenylene and polypyrrole, and the functional groups of the chemically modified graphene comprise one or more of an anilino group, a pyrryl group, an imidazolyl group, a benzenesulfonic acid group, a thienyl group, a furyl group, a phenyl group, a hydroxy group, an ester group and derivative groups thereof, so the conductive performance, the mechanical performances and the dispersion stability of graphene in the printing ink are improved.
Owner:ZHUHAI LETONG NEW MATERIAL TECH CO LTD
Separators for electrochemical cells
InactiveUS20130171500A1Improve securityHybrid capacitor separatorsElectrolytic capacitorsElectrical batteryPyrrolidinones
Provided are separators for use in batteries and capacitors comprising (a) at least 50% by weight of an aluminum oxide and (b) an organic polymer, wherein the aluminum oxide is surface modified by treatment with an organic acid to form a modified aluminum oxide, and wherein the treatment provides dispersibility of the aluminum oxide in aprotic solvents such as N-methyl pyrrolidone. Preferably, the organic acid is a sulfonic acid, such as p-toluenesulfonic acid. Also preferably, the organic polymer is a fluorinated polymer, such as polyvinylidene fluoride. Also provided are electrochemical cells and capacitors comprising such separators.
Owner:OPTODOT CORP
Water-based environment-friendly finishing paint and preparation method thereof
InactiveCN101712833AGood anti-corrosion decoration effectHigh hardnessEpoxy resin coatingsWater basedEpoxy
The invention discloses a water-based environment-friendly finishing paint, which is prepared from the following compositions in part by weight: 20 to 70 parts of water borne acrylic resin solution of which the solid content is 30 to 80 percent and / or 20 to 70 parts of water borne epoxy resin solution of which the solid content is 30 to 80 percent, 4 to 10 parts of water borne amino resin solution of which the solid content is 60 to 98 percent; 1 to 5 parts of organic amine, 0.2 to 2 parts of wetting and leveling agent, 0.2 to 2 parts of thickener, 0.1 to 0.8 part of defoaming agent, 1 to 5 parts of adhesion promoter, 0.2 to 5 parts of catalyst, 10 to 30 parts of alcohol diluting solvent and / or 1 to 5 parts of nontoxic ether diluting solvent, and 20 to 50 parts of deionized water, wherein the catalyst is one or more of ammonias, alcohols and benzenesulfonic acids. The water-based environment-friendly finishing paint has the advantages of wide applicable range, high hardness, good flexibility, strong adhesion, good scratchproof performance, and environmental protection. Simultaneously, the invention also discloses a method for preparing the water-based environment-friendly finishing paint.
Owner:重庆亢石新材料科技有限公司
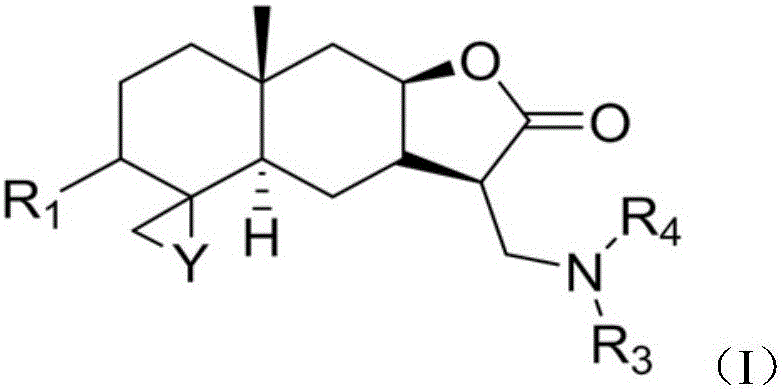
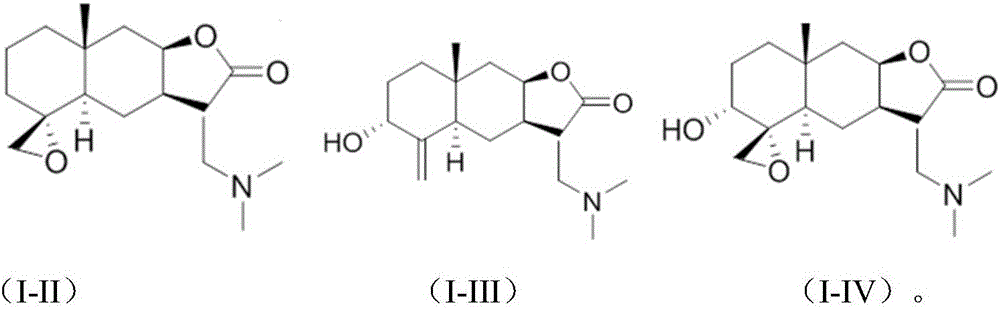
SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)
ActiveUS20170183346A1Safe and efficient and cost-effective and readily scalableSafe and efficient and and readily methodOrganic chemistryPyridine3-Methylbutanoic acid
Provided herein are processes for the preparation of (S)-(2R,3R,11bR-3-isobuty-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl 2-amino-3-methylbutanoate di(4-methylbenzenesulfonate), or a solvate, hydrate, or polymorph thereof.
Owner:NEUROCRINE BIOSCI INC
Agomelatine benzenesulfonic acid compound and preparation method thereof
The invention relates to agomelatine benzenesulfonic acid compound of a formula I and a preparation method thereof; the agomelatine benzenesulfonic acid compound obtained by the invention is good in product stability, high in purity and is suitable for application demand when finished drug is prepared; preparation technology is also very simple; and a high-purity product can be obtained without special operation, wherein in the formula (I), R=CH3, H.
Owner:SHANGHAI RIGHTHAND PHARMTECH
High performance water reducing agent in new type comb shaped molecular structure
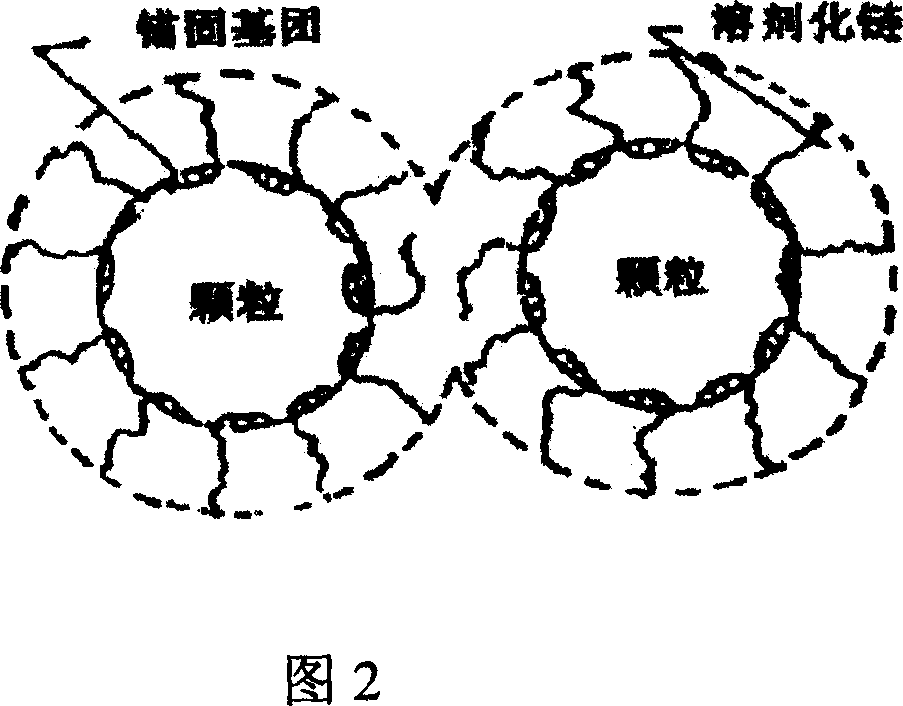
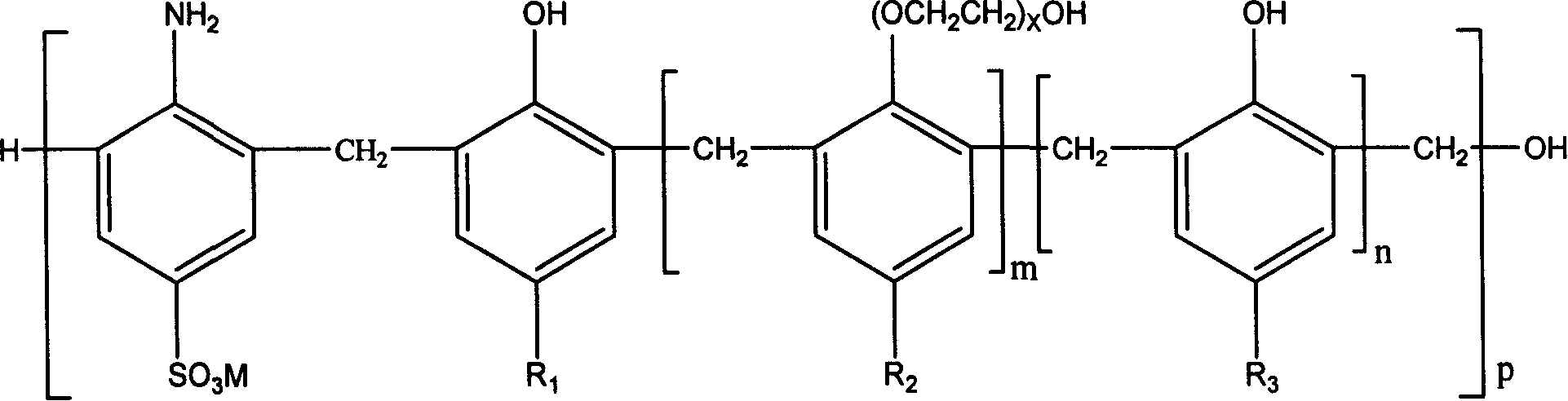
This invention relates to a method for preparing high-performance aminosulfonic acid-series water reducer with polyether-grafted comb-like molecular structure for cement. The high-performance aminosulfonic acid-series water reducer is prepared from: p-aminobenzenesulfonic acid 100 parts, NaOH 115-140 parts, phenol 10-100 parts, modified polyether 0-90 parts, formaldehyde 300-600 parts, and water 3000-10000 parts. The method comprises: dissolving p-aminobenzenesulfonic acid, NaOH, phenol and modified polyether in water at 5-50 deg.C, heating to 50-120 deg.C, dropping formaldehyde, and keeping the temperature for 1-9 h. The high-performance aminosulfonic acid-series water reducer can effectively inhibit the collapse loss of concrete. The high-performance aminosulfonic acid-series water reducer has high workability, and water cannot infiltrate into concrete containing it.
Owner:CCCC WUHAN HARBOR ENG DESIGN & RES
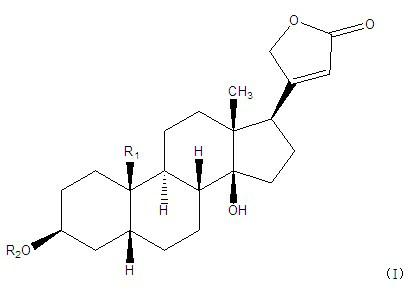
Cardiac glycoside compounds and antitumor application thereof
InactiveCN102219821AStrongly inhibits proliferative activityOrganic active ingredientsGlycoside steroidsBenzoic acidO-Phosphoric Acid
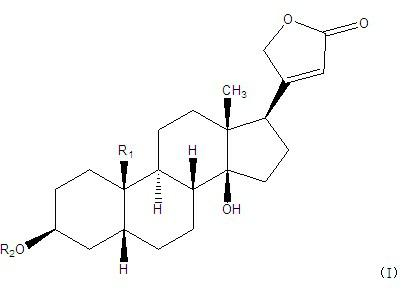
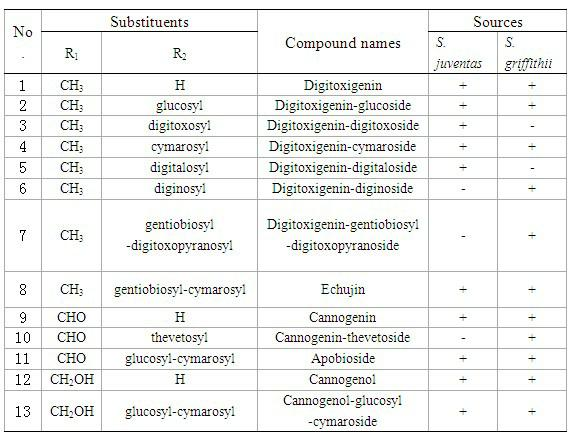
The invention belongs to the technical field of medicaments and in particular relates to cardiac glycoside compounds with a general formula (I), as well as derivatives, stereoisomers, a racemic mixture or a non-racemic mixture of the stereoisomers and pharmaceutically acceptable acid addition salts or solvates, wherein R1 is CH3, CHO or CH2OH; R2 is H or a linear saccharide chain or a branched saccharide chain, the linear saccharide chain or the branched saccharide chain is formed by saccharides; and these acids comprise inorganic acids like hydrochloric acid, sulfuric acid, hydrobromic acid, phosphoric acid, nitric acid, carbonic acid and the like as well as organic acids like methanoic acid, acetic acid, succinic acid, citric acid, lactic acid, fumaric acid, tartaric acid, benzoic acid, p-methyl-benzenesulfonic acid, methylsulphonic acid, naphthalenesulfonic acid, gluconic acid and the like. The cardiac glycoside compounds can be obtained by separation from plants, in particular Streptocaulon plant juventas or Streptocaulon griffithii, by using multiple conventional separating means or obtained through synthesis, semisynthesis or bioconversion means. These compounds have excellent inhibiting and treatment effects on multiple tumor cells.
Owner:SHENYANG PHARMA UNIVERSITY
Developing roller, electrophotographic process cartridge, and electrophotographic image forming apparatus
ActiveUS8913930B2Improve charging effectHigh quality imagingSynthetic resin layered productsElectrographic process apparatusHydrogen atomEngineering
Provided is the following developer carrying member. The member has high charge-providing performance even under a high-temperature, high-humidity environment, and its surface layer hardly peels off its elastic layer even after long-term standing under the high-temperature, high-humidity environment. The developer carrying member comprises a mandrel, an elastic layer including a silicone rubber, and a surface layer covering a surface of the elastic layer, and the surface layer comprises a binder resin, and a copolymer having structural units of formula (1) and formula (2). R1 represents an alkyl group having 10-18 carbon atoms, R2 represents a methyl group or a hydrogen atom, R3 represents an alkylene group having 1-4 carbon atoms, X− represents a chloride ion, a bromide ion, or a p-toluenesulfonic acid ion, R4 represents a methyl group or a hydrogen atom, and R5 represents an alkylene group having 1-4 carbon atoms.
Owner:CANON KK
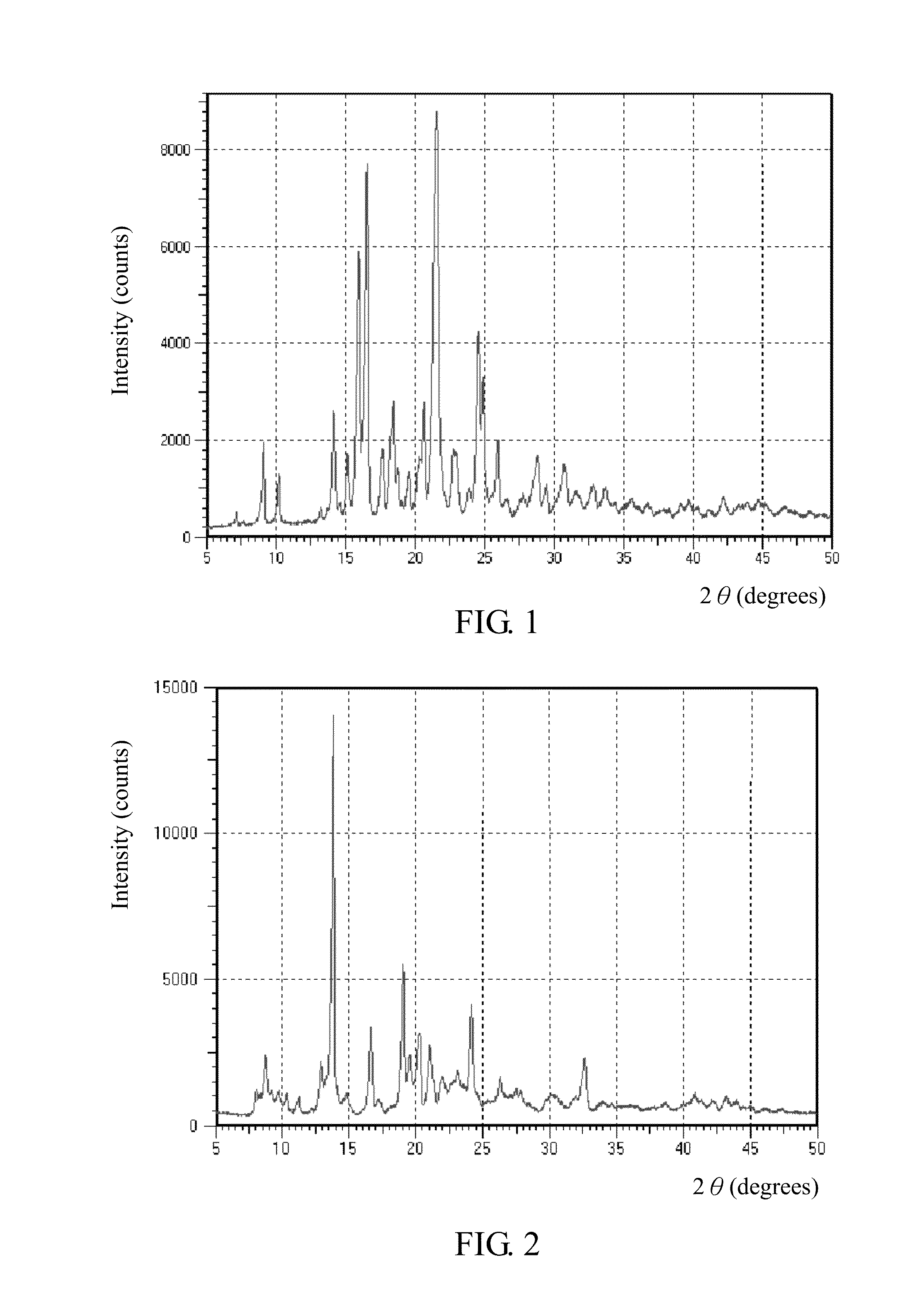
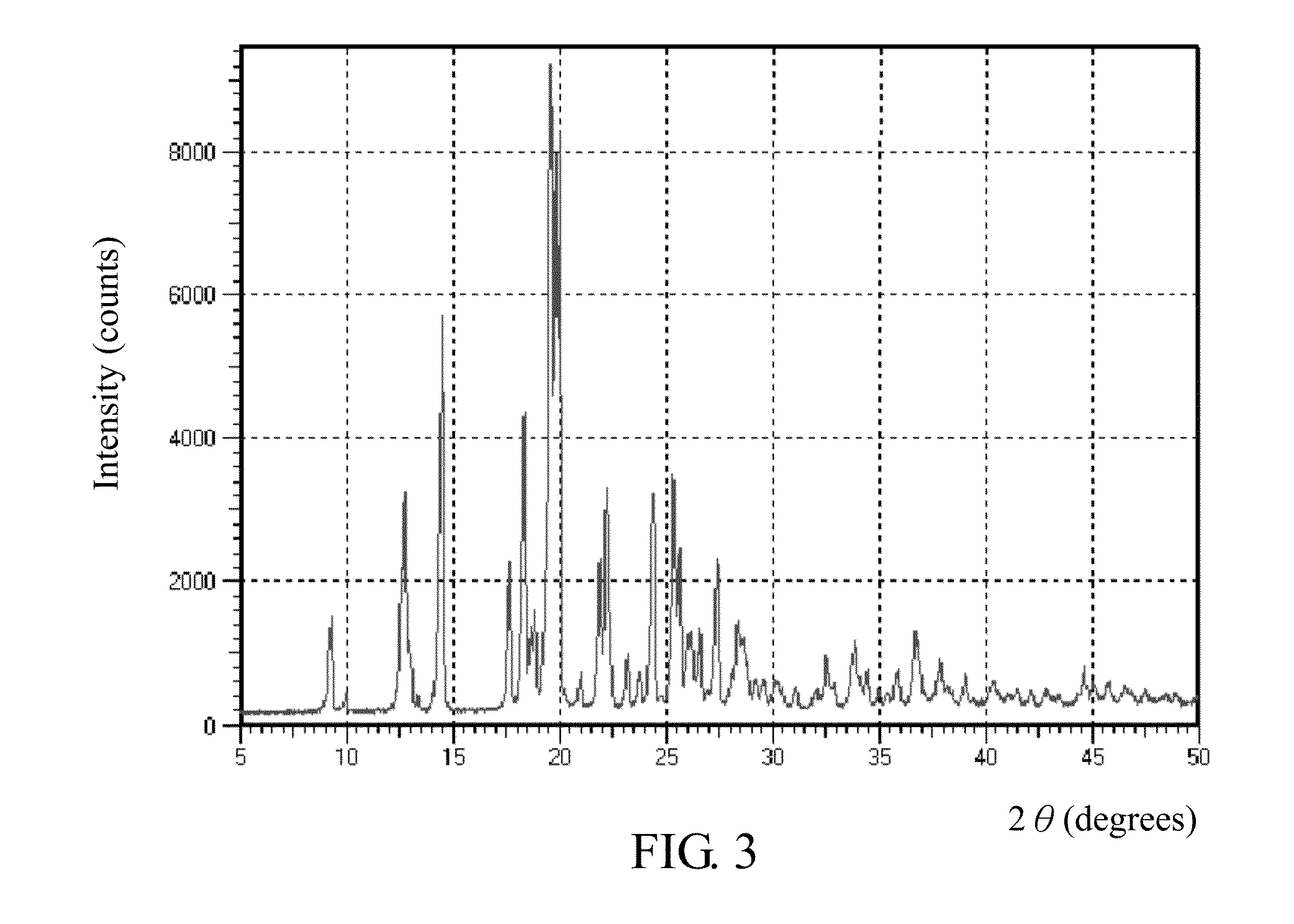
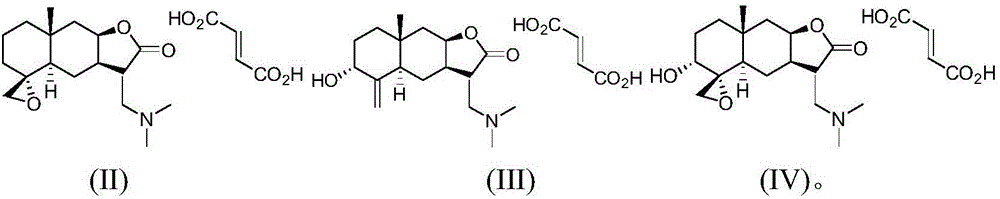
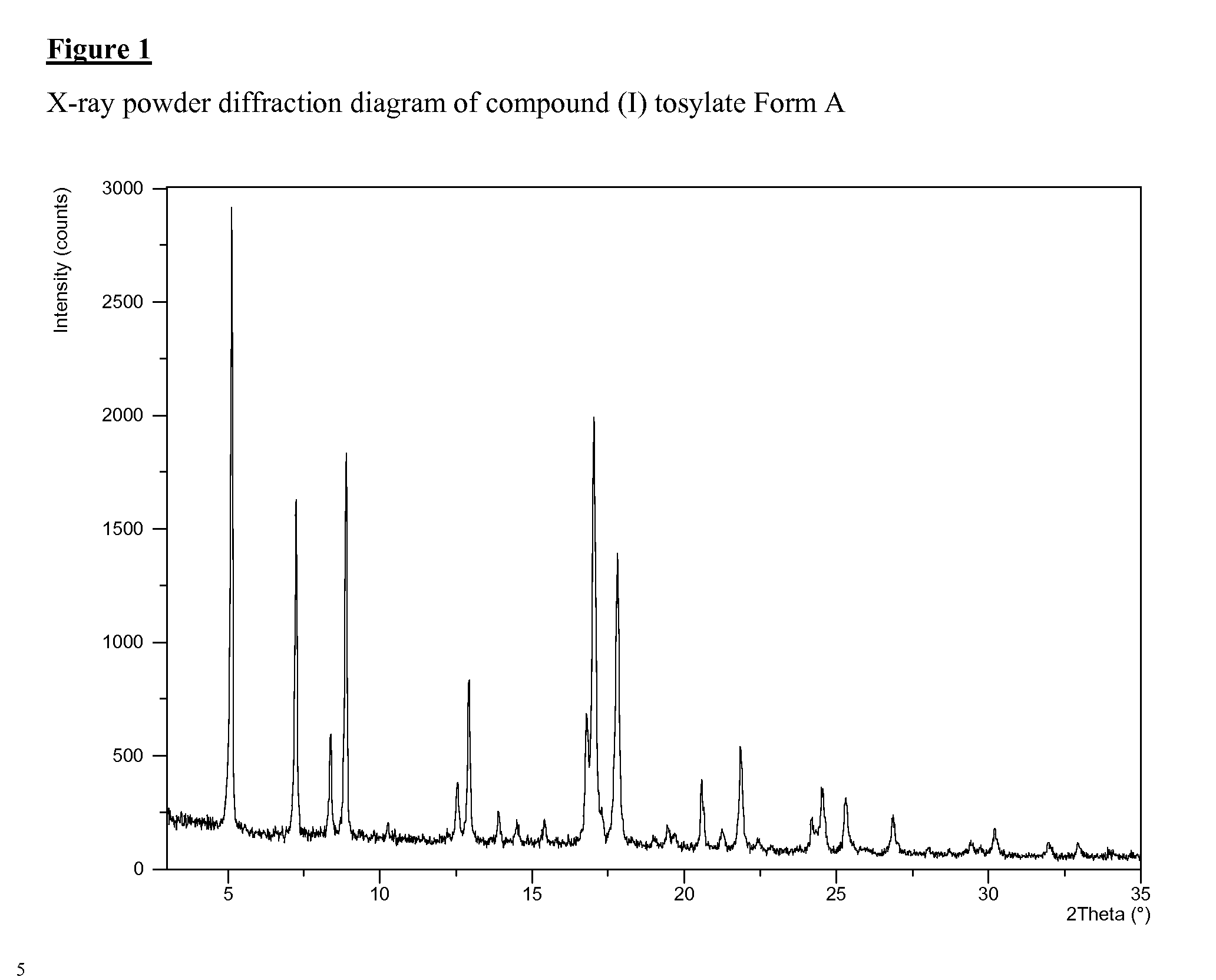
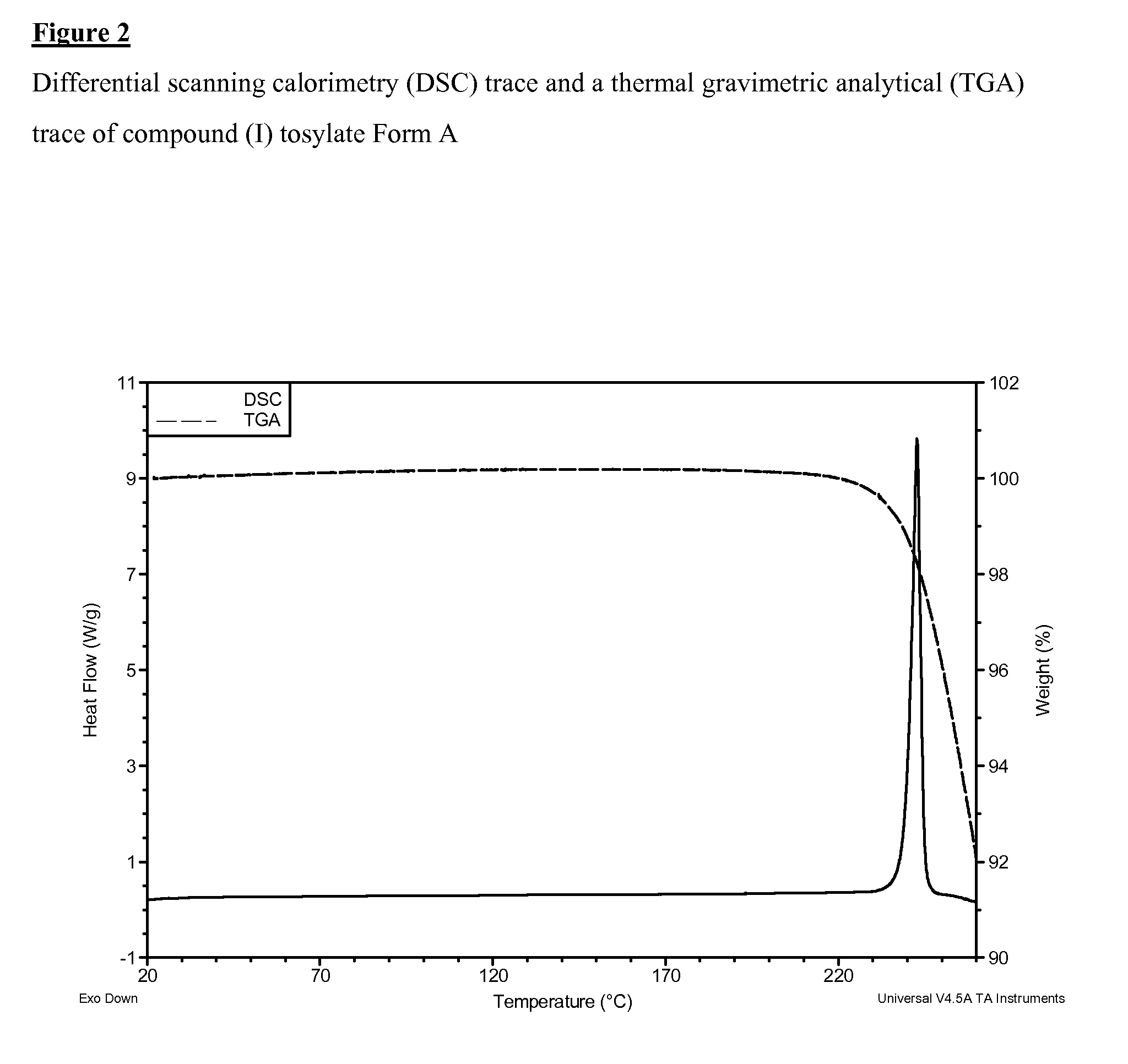
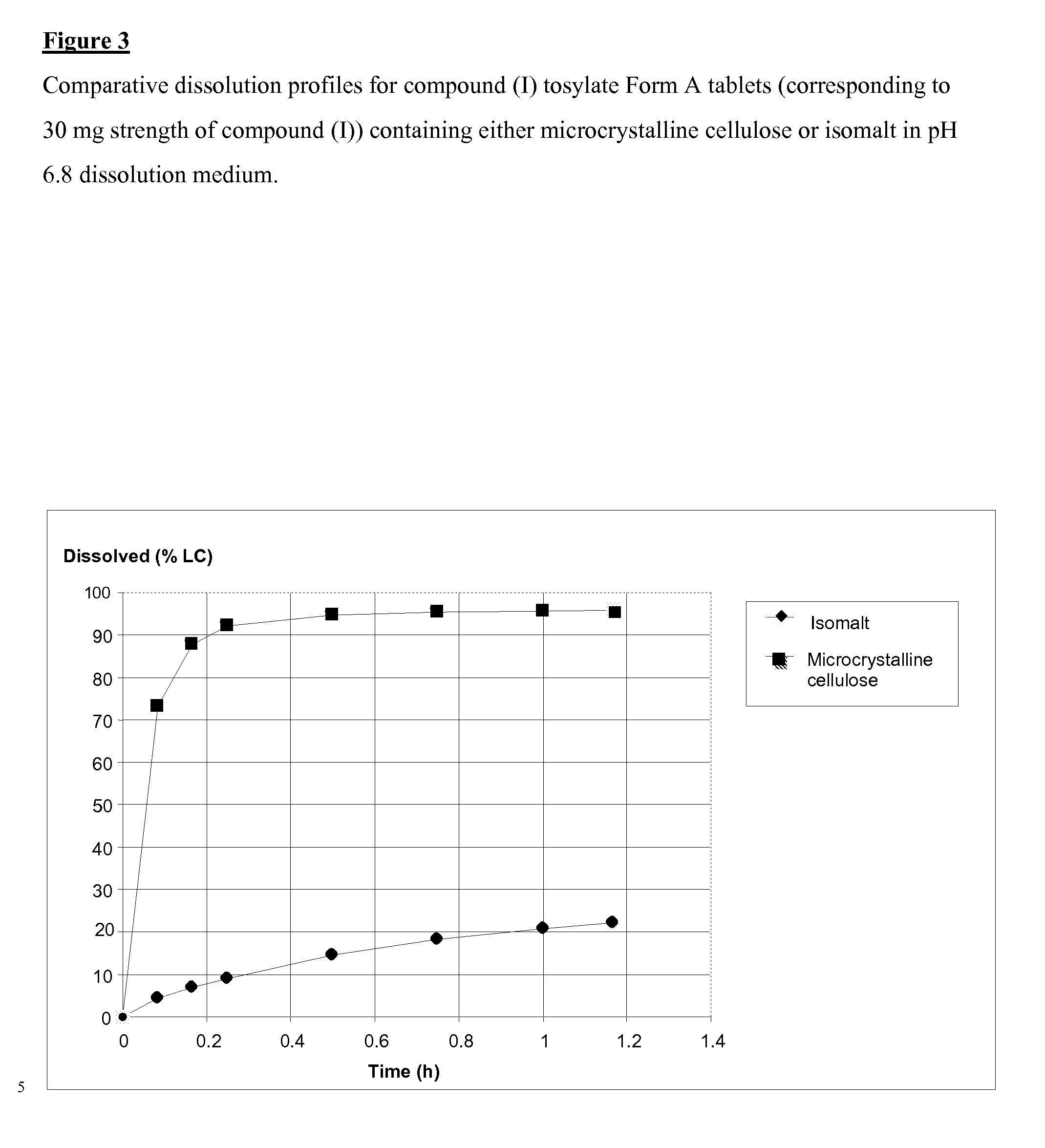
SALTS OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YL-METHYL)-IMIDAZO[1,2-b][1,2,4]TRIAZIN-2-YL]BENZAMIDE AND PROCESSES RELATED TO PREPARING THE SAME
ActiveUS20090291956A1Inhibit proliferative activityInhibit growthBiocideOrganic chemistryKetoneC-Met
The present invention is directed to dihydrochloric acid and dibenzenesulfonic acid salts of the c-Met kinase inhibitor 2-fluoro-N-methyl-4-[7-(quinolin-6-ylmethyl)-imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide, and pharmaceutical compositions thereof, useful in the treatment of cancer and other diseases related to the dysregulation of kinase pathways. The present invention further relates to processes and intermediates for preparing 2-fluoro-N-methyl-4-[7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide, and salts thereof.
Owner:INCYTE
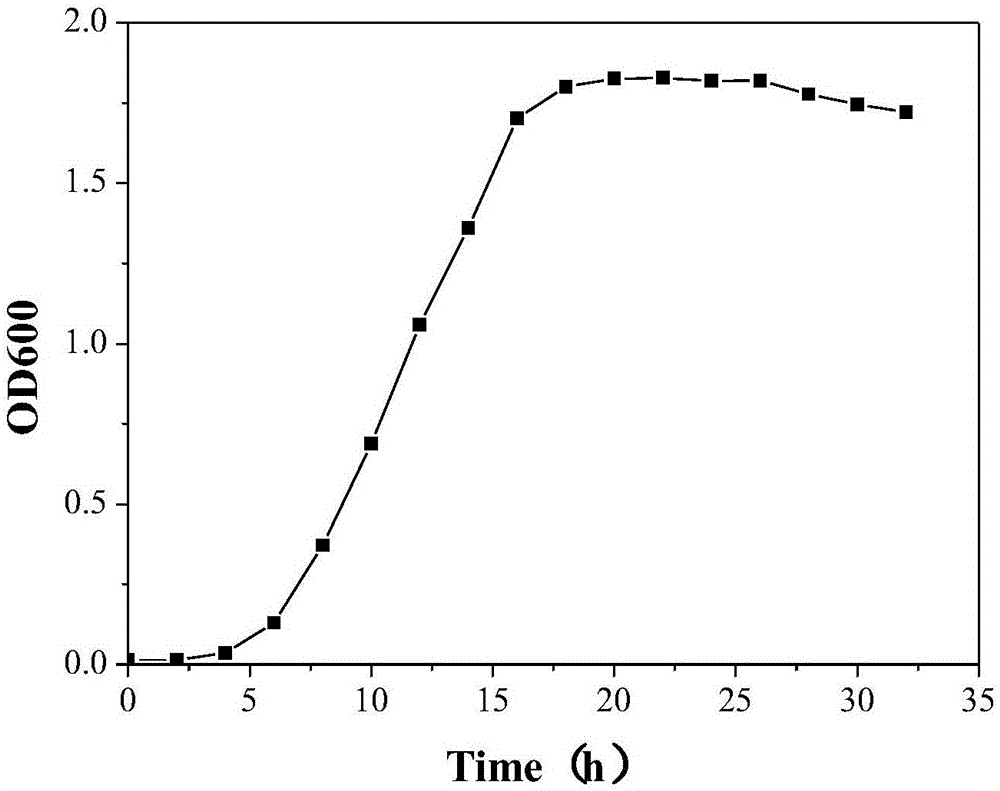
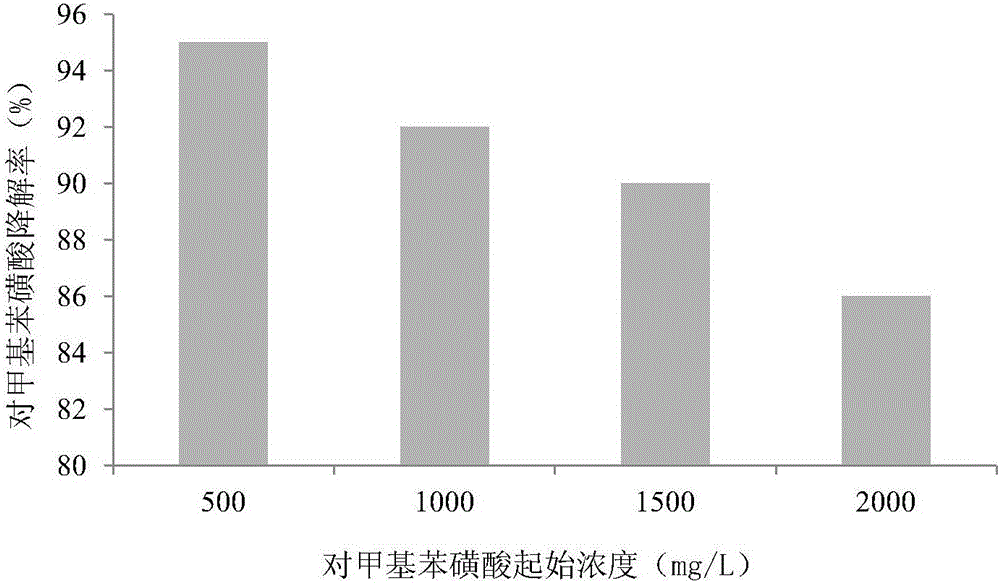
Rhodococcus ruber PTA-2, immobilization and applications thereof
ActiveCN106591172AReduce manufacturing costEasy to useBacteriaWater contaminantsMicroorganismWastewater
The invention discloses Rhodococcus ruber PTA-2, which is classified and named Rhodococcus ruber PTA-2, is preserved in the China General Microbiological Culture Collection Center on February 9, 2015, and has the preservation number of CGMCC NO.10538. According to the present invention, the Rhodococcus ruber PTA-2 can grow by adopting p-toluenesulfonic acid as the sole carbon source, wherein the degradation rate on the 500 mg / L p-toluenesulfonic acid is 96%; the immobilized pellets prepared from the Rhodococcus ruber PTA-2 provides the good p-toluenesulfonic acid degradation effect, wherein the degradation rate on the 500 mg / L p-toluenesulfonic acid is more than 90%; and the production cost is low, the use is convenient, and the Rhodococcus ruber PTA-2 is suitable for promotion and application in the p-toluenesulfonic acid-containing wastewater treatment.
Owner:JIANGSU NANZI ENVIRONMENTAL PROTECTION SCI & TECH
Arctigenin pro-drug, preparation method and use thereof
InactiveCN101284823AImprove oral bioavailabilityGood water solubilityOrganic active ingredientsOrganic chemistryGlutaric acidPhosphate
The invention relates to the following general formula compound (I) and use of the general formula compound in preparing anti-inflammatory and anti-endotoxin medicines. In formula I, R is an acid solubilizing side chain of hydroxide radical in an arctigenin molecule, and can be an acid group or side chain imported through alkylation reaction or acylating reaction, such as sulphonic acid ester, sulfuric acid ester, organic phosphate, succinate, propionic ether, butyric ester, semi-succinate, semi-glutaric acid ester, semi-tartrate, semi-phthalic acid ester, m-benzene sulfonic acid ester and so on.
Owner:YANTAI TARGET DRUG RES
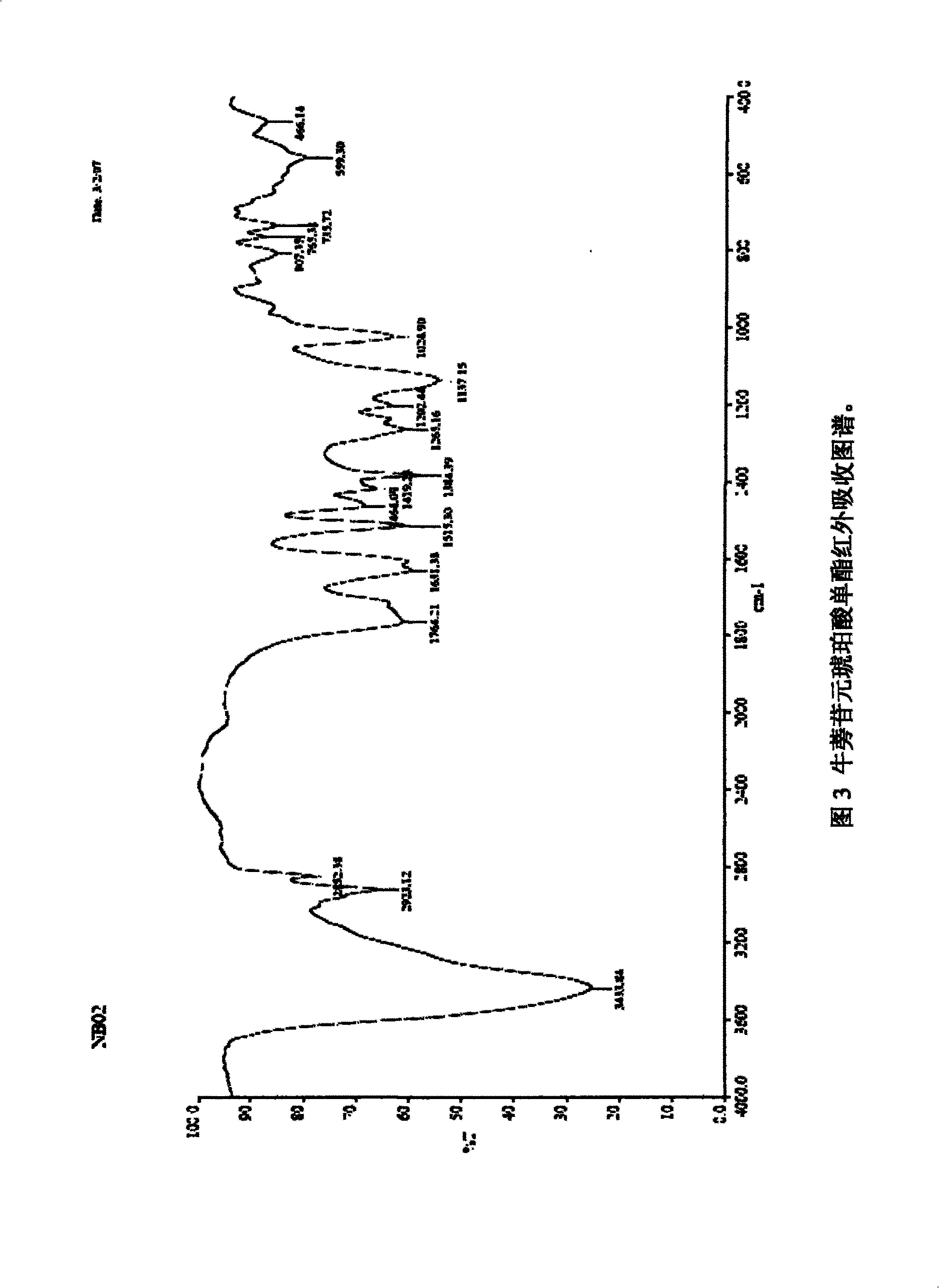
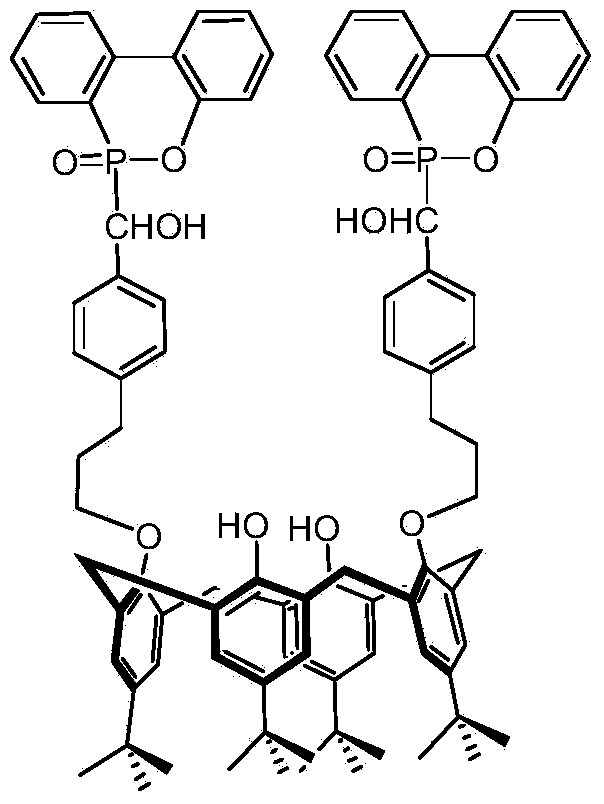
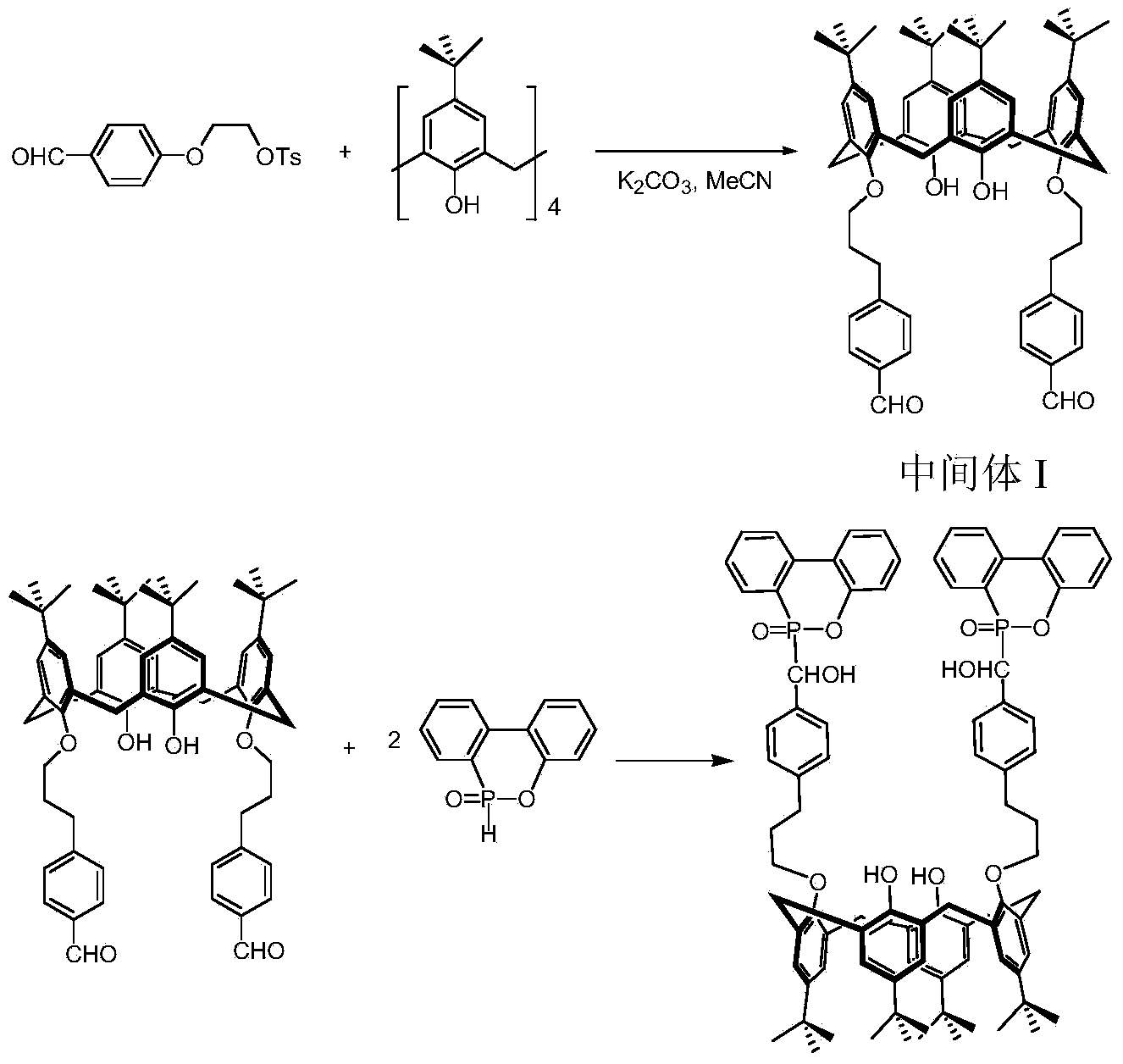
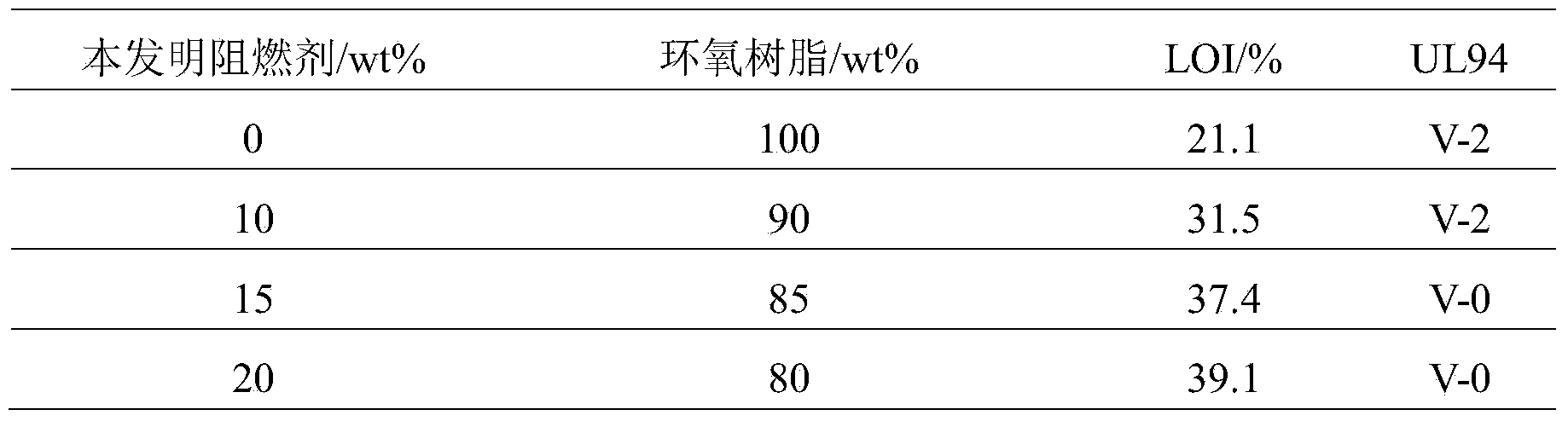
Oxa-phosphaphenanthrene fire retardant as well as preparation method and application of oxa-phosphaphenanthrene fire retardant
ActiveCN104231309AEasy to operateAdvanced technologyGroup 5/15 element organic compoundsEpoxyPolymer science
The invention discloses an oxa-phosphaphenanthrene fire retardant as well as a preparation method and application of the oxa-phosphaphenanthrene fire retardant. The fire retardant has a calyx[4]arene structure; p-tert-butyl calyx[4]arene and p-tosylate ethoxy p-benzaldehyde are prepared into an intermediate I in the presence of K2CO3 and acetonitrile, and the intermediate I reacts with DOPO to obtain a target compound; the compound is white in appearance, has the melting point of 198-200 DEG C and the purity of 98.5% and is good in thermal stability and high in flame retarding rate; the used raw materials are easily available, and a process is advanced and easy for industrialized production. The flame retardant not only can serve as a reactive flame retardant to be used in thermosetting resin such as epoxy resin and polyurethane but also can serve as an additive flame retardant to be used for engineering plastics with relatively high requirement on the heat resistance of the flame retardant.
Owner:湖北同广和新材料有限公司
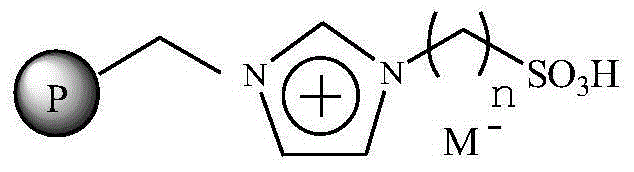
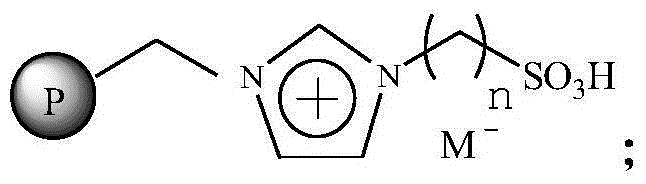
Immobilized ionic liquid catalyst and application thereof
InactiveCN106391112AHigh catalytic activityNot easy to inactivateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateTriflic acid
The invention relates to an immobilized ionic liquid catalyst and application thereof. The immobilized ionic liquid catalyst has a general structural formula as defined in the specification. In the general structural formula, P is a nanogel resin matrix; n is an integer in a range of 2 to 12; and M<-> is a negative ion selected from a group consisting of a trifluoromethanesulfonate group, a p-toluenesulfonate group, a benzenesulfonate group, a methanesulfonate group, a tetrafluoroborate group and a hexafluorophosphate group. The immobilized ionic liquid catalyst can be applied to industrial olefine acid addition for preparation of corresponding esters.
Owner:CHINA PETROLEUM & CHEM CORP +1
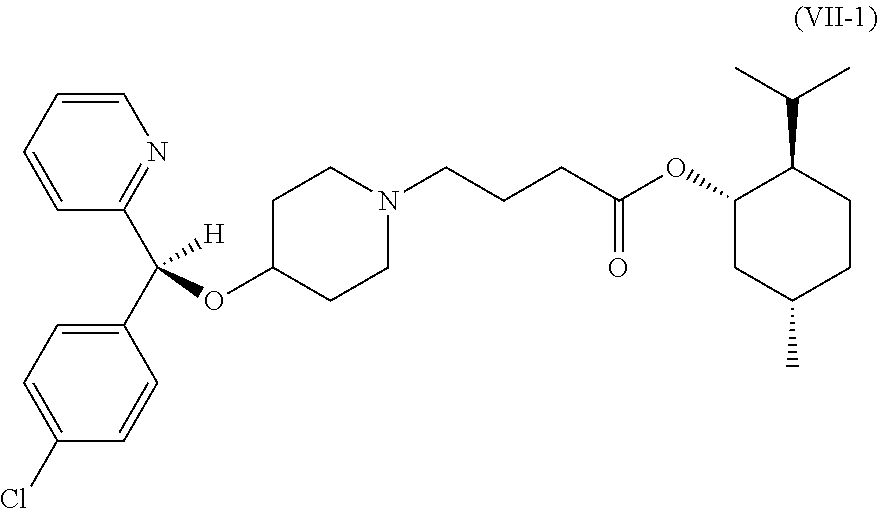
Method of synthesizing bepotastine or benzenesulfonic acid salt thereof and intermediates used therein
InactiveUS20140046068A1High optical puritySafe and effective and low-costOrganic chemistryAlpha hydroxy acidBenzenesulfonic acid
The present invention relates to a novel method of synthesizing bepotastine or its benzenesulfonic acid salt and novel intermediates used therein. The present invention uses L-α-hydroxy acid for chiral resolution to form an L-α-hydroxy acid salt of a compound represented by the following formula (VII-1), so as to synthesize bepotastine or its benzenesulfonic acid salt in high optical purity.
Owner:EVERLIGHT USA INC
Emulsifying agent having stabilizing effect in clethodim emulsifiable concentrates and preparation method thereof
InactiveCN102318602ASolve the problem of high decomposition rate after storageDilution stability qualifiedBiocideAnimal repellantsEpoxyPhenyl Ethers
The invention discloses an emulsifying agent having a stabilizing effect, which is one or a mixture of several of polyoxyethylene lauryl ether, polyoxyethylene nonyl phenyl ether, alkylaryl polyethenoxy ether, polyoxyethylated fatty acid, epoxy chloropropane, PO-EO block polyether, alkylphenol polyoxyethylene formaldehyde condensation product, calcium dodecyl benzene sulfonate, dodecyl benzenesulfonic acid, 2,6-di-tert-butyl-4-methyl phenol and tween-80. By adopting a method of combining a particular stabilizer with a reasonable formula, the problem of high decomposition rate after the clethodim is stored can be solved; and the characteristics are stable, the clethodim is free from having the layering and precipitation problems during the storage process, and the dilution stabilization of the emulsifible concentrates is qualified. The emulsifying agent is particularly suitable for preparing clethodim emulsifiable concentrates. By utilizing the clethodim emulsifiable concentrates which is prepared by the emulsifying agent having the stabilizing effect, the resource can be saved, so the clethodim can be maximally used; and the clethodim emulsifiable concentrates which is prepared by the emulsifying agent having the stabilizing effect have an excellent effect on preventing the gramineous weed, have no residual influence on the aftercrop crops and have good safety.
Owner:南京扬子鸿利源化学品有限责任公司
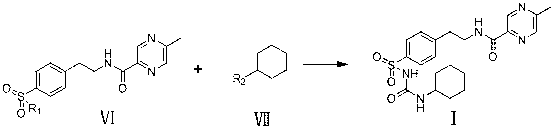
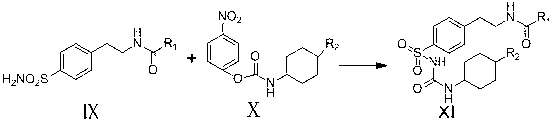
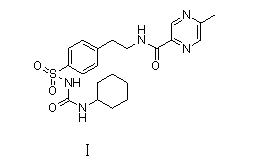
Novel synthesis route of glipizide
The invention relates to a novel synthesis route of glipizide, which is characterized by comprising the following steps of protecting 4-(2-amino ethyl)benzenesulfonic acid ammonia (II) by Boc anhydride to obtain a compound (III); reacting the compound (III) with cyclohexyl isocyanate to obtain a compound (IV); carrying out deprotection on the compound (IV) to obtain a compound (V); and reacting the compound (V) with 2-methyl-5-pyrazine carboxylic acid to obtain the glipizide (I) with the single impurity which is less than or equal to 0.5% and the high purity which is more than or equal to 99%. The process is simple, the yield is high, the purity is high and the single impurity is low; and the process is environment-friendly and industrial production is easy to realize.
Owner:WUHAN WUYAO PHARMA
Isoalantolactone derivative and salt thereof
InactiveCN106478569AImprove tumor inhibition rateProlong lifeOrganic active ingredientsAntipyreticBenzoic acidPhosphomolybdic acid
The invention relates to isoalantolactone derivatives and salts thereof and provides an isoalantolactone derivative as shown in a formula (I). Salification acids are selected from inorganic acids or organic acids; the inorganic acids are selected from hydrofluoric acid, hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, nitric acid, phosphoric acid, carbonic acid, boric acid, seleninic acid, phosphomolybdic acid, phosphorous acid and sulphurous acid; the organic acids are selected from citric acid, maleic acid, D-malic acid, L-malic acid, DL-malic acid, L-lactic acid, D-lactic acid, DL-acid, oxalic acid, methylsulfonic acid, pentanoic acid, oleic acid, lauric acid, p-toluene sulfonic acid, 1-naphthalenesulfonic acid, 2-naphthalenesulfonic acid, phthalic acid, tartaric acid, malonic acid, succinic acid, fumaric acid, glycolic acid, mercaptan acid, glycine, sarcosine, sulfonic acid, nicotinic acid, methylpyridine acid, isonicotinic acid, benzoic acid or substituted benzoic acid.
Owner:NANKAI UNIV
Scale inhibitor for an aqueous system
InactiveUS6846452B2Less-expensive to useInhibition formationOther chemical processesScale removal and water softeningSulfonateTricarboxylic acid
Owner:SOLENIS TECH CAYMAN
Microporous rubber base plate and preparation process thereof
The invention belongs to the technical field of microporous rubber and discloses a microporous rubber base plate and a preparation process thereof. The microporous rubber base plate has the major technical characteristic that the microporous rubber base plate body is prepared from the following components in parts by weight: 100 parts of ethylene-propylene-diene rubber, 6-18 parts of polyethylene glycol mixture, 60-100 parts of a filling material, 30-60 parts of alkane oil, 6-12 parts of a foaming agent, 2.5-5.5 parts of a vulcanizing agent and 2-5.5 parts of an accelerant. According to the microporous rubber base plate, an evenly diffused multi-phase structure is formed by mechanically blending low-Mooney EPDM and high-Mooney EPDM, and in combination with benzenesulfonic acid formed in the reaction process of neutralizing the foaming agent 4,4-oxodibenzenesulfonyl hydrazide by use of the polyethylene glycol mixture, the foaming ratio can be increased. In addition, accessories carbon black excellent in reinforcement property and porous diatomite filler excellent in aging property and light in weight are adopted, and consequently, the microporous rubber base plate has such properties as high strength, low rigidity and low temperature dependency.
Owner:HENGSHUI ZHONGTIEJIAN ENG RUBBER
Novel salt 628
ActiveUS20100216843A1Improved physical propertyHigh solubilityBiocideOrganic chemistryPhenyl groupTrifluoromethyl
6-Methyl-5-(1-methyl-1H-pyrazol-5-yl)-N-{[5-(methylsulfonyl)pyridin-2-yl]methyl}-2-oxo-1-[3-(trifluoromethyl)phenyl]-1,2-dihydropyridine-3-carboxamide 4-methylbenzenesulfonate and a novel crystalline form thereof are disclosed together with processes for preparing such salt and form, pharmaceutical compositions comprising such a salt and form, and the methods of treatment using such a salt and form.
Owner:ASTRAZENECA AB
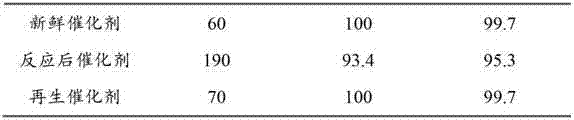
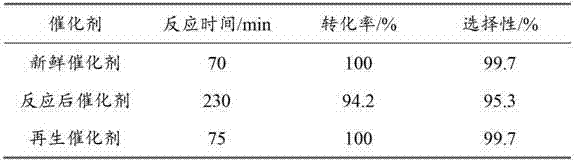
Regeneration method of Pd/C catalyst and application of regenerated Pd/C catalyst
InactiveCN103191759ARestore activitySelectivity unchangedChemical recyclingSulfonic acid preparationAir atmosphereOrganic chemistry
The invention discloses a regeneration method of a Pd / C catalyst and an application of the regenerated Pd / C catalyst. The Pd / C catalyst is inactivated due to poisoning by sulfur-containing substances during the process of catalyzing the hydrogenation synthesis of m-aminobenzenesulfonic acid from m-nitrobenzenesulfonic acid; and the regeneration method comprises the steps of drying and oxidizing the inactivated Pd / C catalyst in an air atmosphere at the temperature of 50-140 DEG C to obtain the regenerated Pd / C catalyst. The Pd / C catalyst obtained by regeneration according to the regeneration method disclosed by the invention can be applied to the reaction of catalyzing the hydrogenation synthesis of the m-aminobenzenesulfonic acid from the m-nitrobenzenesulfonic acid. The method disclosed by the invention is environment-friendly and pollution-free, can effectively restore the activity of the Pd / C catalyst which is inactivated due to the positioning of the sulfur-containing substances during the process of catalyzing the hydrogenation synthesis of the m-aminobenzenesulfonic acid from the m-nitrobenzenesulfonic acid, can ensure that the catalyst can be mechanically used for more than 80 times in a circulating manner, and can keep the activity of the catalyst and the selectivity of the target product unchanged.
Owner:ZHEJIANG UNIV OF TECH +1
Method for preparing 9-oxo-10(9H)-acridineacetic acid
ActiveCN103396362ASmooth responseResponse is smooth and easy to controlOrganic chemistryBenzoic acidPtru catalyst
The invention discloses a method for preparing 9-oxo-10(9H)-acridineacetic acid, and belongs to the field of organic synthesis. The method comprises the following steps: (1) under the effects of a metal carbonate, a catalyst and a solvent, performing a condensation reaction on o-chlorobenzoic acid (a) and aniline (b) to prepare an intermediate c; (2) under the effects of toluene and p-toluenesulfonic acid, performing a cyclization reaction on the intermediate c by refluxing and dehydrating to prepare an intermediate d; (3) dissolving the intermediate d in dimethyl formamide , successively adding 60% NaH, KI and ethyl chloroacetate, reacting at room temperature to obtain a wet intermediate e; and (4) hydrolyzing the wet intermediate d with NaOH to obtain a crude product f, and refining the crude product f to obtain the 9-oxo-10(9H)-acridineacetic acid product. The method has the advantages of low cost consumption, small pollution during reaction, smooth and easy-controlling reaction and high product yield, and has better industrial application value.
Owner:ZHENGZHOU SIGMA CHEM
Synthetic method for 2-methyl-3-trifluoromethyl phenylamine
ActiveCN102491906AWide variety of sourcesReduce manufacturing costOrganic compound preparationAmino compound preparationMethylanilinePtru catalyst
The invention discloses a synthetic method for 2-methyl-3-trifluoromethyl phenylamine. The method comprises the following steps: 1) preparing 3-nitro-4-methyl benzenesulfonic acid; 2) preparing 3-nitro-4-methyl-5-bromobenzenesulfonic acid; 3) preparing 2-nitro-6-bromotoluene; 4) preparing 2-nitro-6-trichloromethyl toluene; 5) preparing 2-nitro-6-trifluoromethyl toluene; 6) preparing 2-methyl-3-trifluoromethyl phenylamine, that is, adding 5% palladium-charcoal and methanol in 2-nitro-6-trifluoromethyl toluene obtained in step 5), carrying out hydrogenation reduction at a temperature of 35 DEG C and removing a catalyst so as to obtain 2-methyl-3-trifluoromethyl phenylamine. According to the invention, p-toluenesulfonic acid is used as a starting material, and therefore, disadvantages of theprior art are overcome and yield of a target product is improved.
Owner:QILU ANIMAL HEALTH PROD +1
Low temperature quick-drying amino paint for automobile chassis and production method thereof
ActiveCN101434809AImprove efficiencyImprove coating efficiencyPolyester coatingsBenzoic acidPropanoic acid
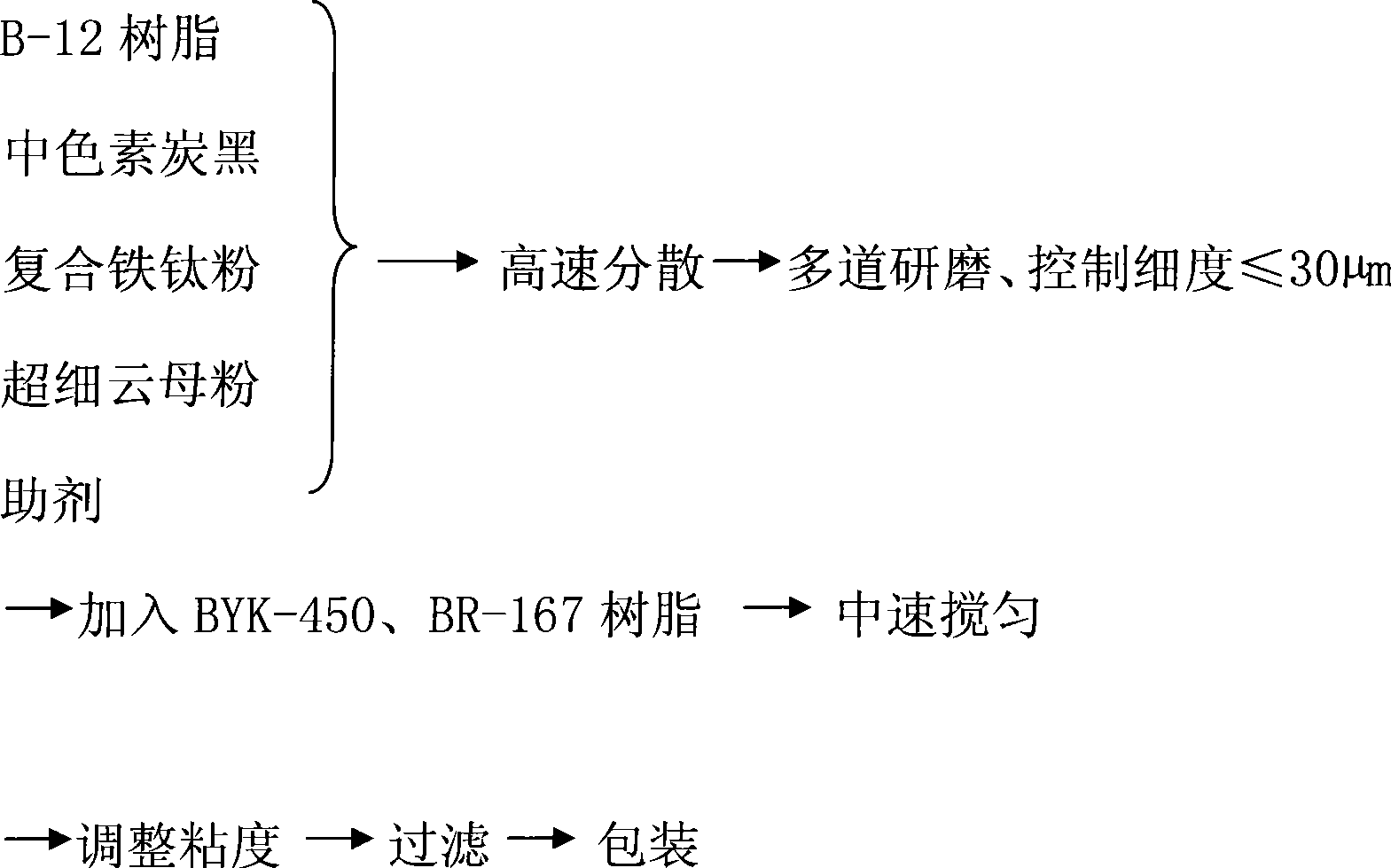
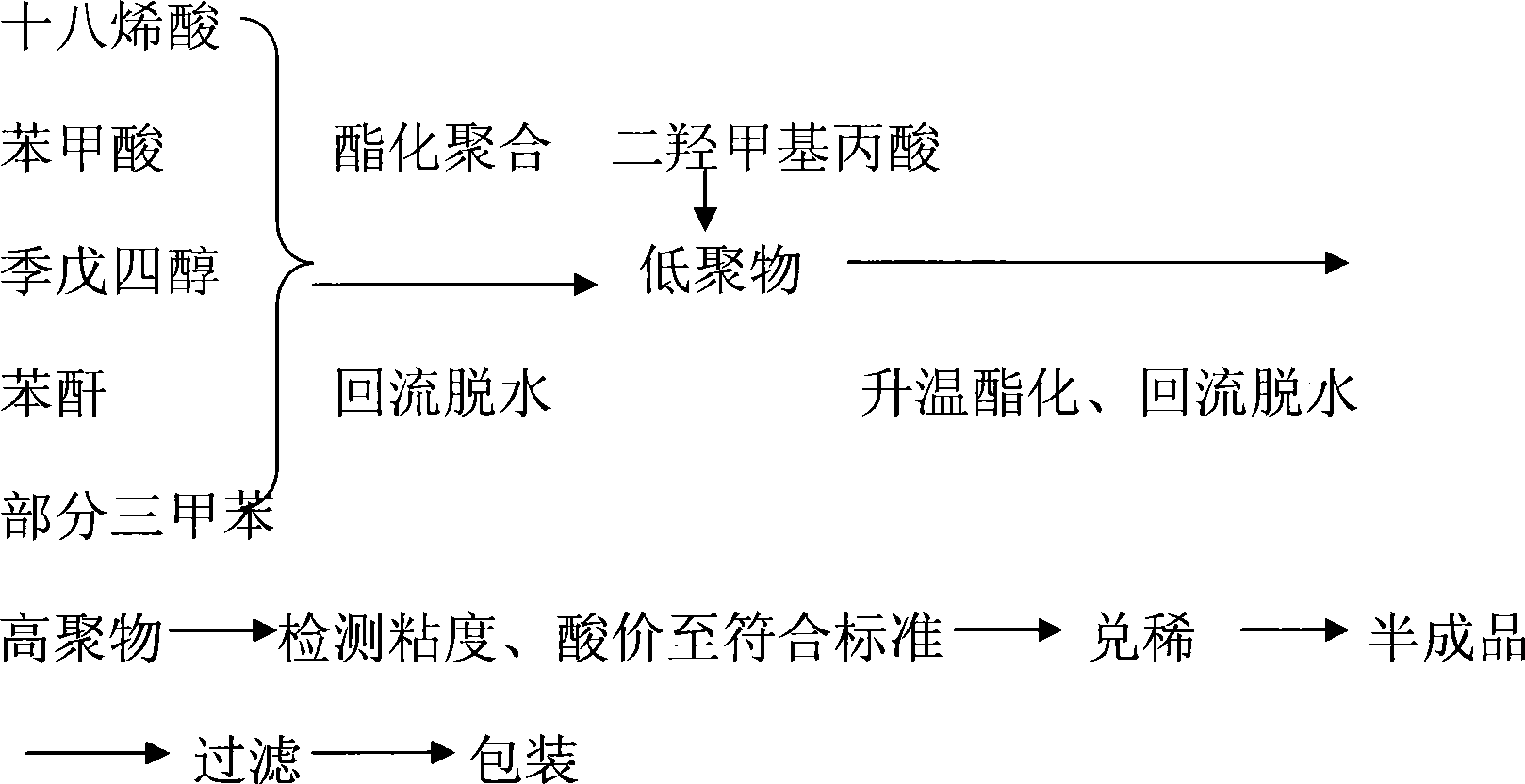
The invention provides a low-temperature fast-dry amino automobile chassis paint and a production method thereof, wherein, the paint consists of B-12 resin, medium pigment carbon black, compound ferrotitanium powder, ultra-fine mica powder, an auxiliary agent, sealed paratoluenesulfonic acid BYK-450 and BR-167 amino resin; B-12 resin is prepared by octadecenoic acid, benzoic acid, pentaerythritol, phthalic anhydride, dimethylol propionic acid, and trimethyl benzene. The automobile chassis paint adopts special B-12 resin synthetic route to lead the polymer chain to have more active functional groups simultaneously to be matched with the blocked paratoluenesulfonic acid, reduces the baking temperature of the existing amino paint from 130 DEG C to 90 DEG C and reduces the curing film-forming time from 1 hour to half an hour. As the paint film has high crosslinking density, compound ferrotitanium powder and squamous anti-rust pigment are matched for use, and the polyaniline is used for improving the anticorrosive potentials, thus leading the salt spray resistance of the paint to achieve 150 hours and improving the protection performance and the coating efficiency.
Owner:ANQING LINGHU PAINT
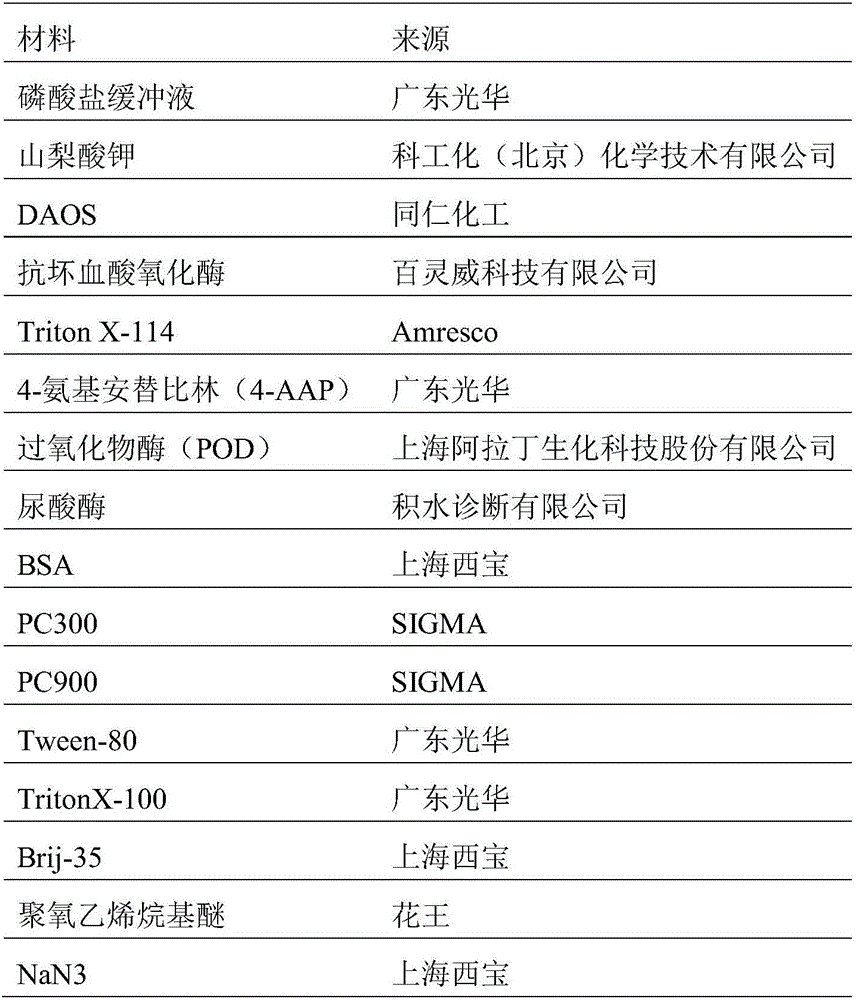
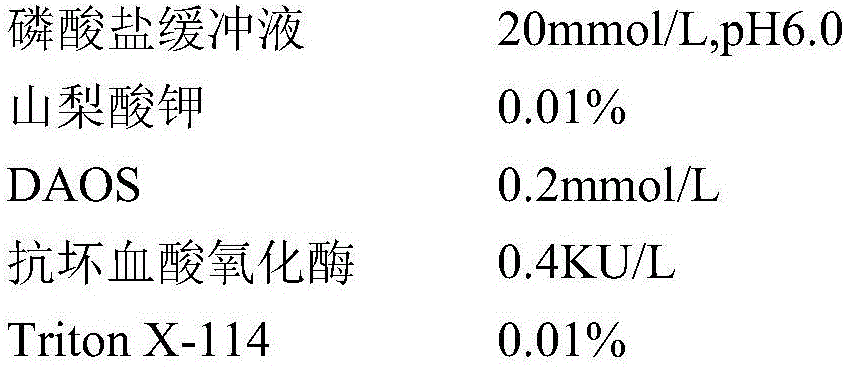
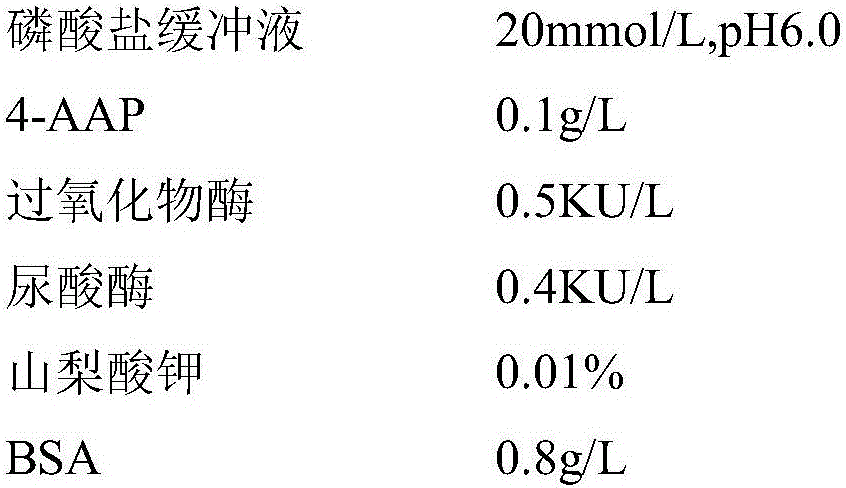
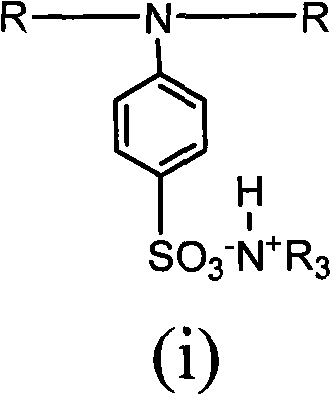
Kit and method for determining uric acid
ActiveCN106367472AMicrobiological testing/measurementBiological material analysisEnzymePhenolsulfonate
The invention relates to a kit and method for determining uric acid. The kit comprises a reagent set 1 and a reagent set 2, wherein the reagent set 1 comprises a chromogen; the reagent set 2 comprises 4-aminoantipyrine, peroxidase and uricase; and the chromogen is or comprises N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline. The kit and method can lower the interference caused by calcium phenolsulfonate and / or etamsylate in the sample in the uric acid detection process.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
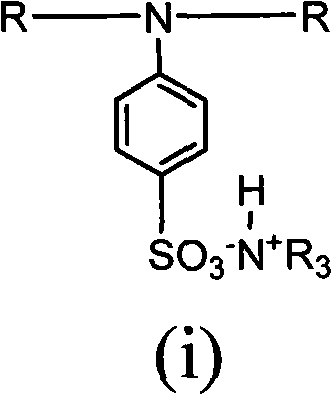
Aqueous macromolecular photoinitiator and preparation method thereof
The invention relates to an aqueous macromolecular photoinitiator and a preparation method thereof. The general formula of the aqueous macromolecular photoinitiator is (i). The aqueous macromolecular photoinitiator prepared by the method has the photoinitiating efficiency equivalent to a corresponding micro-molecular photoinitiator and has better compatibility with an aqueous light-cured resin; carboxylate ions and sulfosalt ions exist in a molecular chain simultaneously, so the resin has better water solubility than an aqueous macromolecular photoinitiator only containing carboxylate ions; and in addition, the price of an introduced chain extender (4-aminobenzenesulfonic acid) used for sulfonic acid group is lower than that of a conventional aqueous functional monomer (dihydromethylpropionic acid), so that the cost of products is reduced and the industrial production is facilitated. At the same time, in the method, the chain is extended by using the functional monomer with photoinitiating function, so the content of photoinitiating radicals in the resin per gram is improved and the aqueous macromolecular photoinitiator has better photoinitiating efficiency than the aqueous macromolecular photoinitiator taking end radicals as the photoinitiating radicals.
Owner:SHANGHAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)
![SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE) SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)](https://images-eureka.patsnap.com/patent_img/af5a93d0-aa6a-4674-8cd4-51408de0087f/US20170183346A1-20170629-C00001.png)
![SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE) SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)](https://images-eureka.patsnap.com/patent_img/af5a93d0-aa6a-4674-8cd4-51408de0087f/US20170183346A1-20170629-C00002.png)
![SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE) SYNTHETIC METHODS FOR PREPARATION OF (S)-(2R,3R,11bR)-3-ISOBUTYL-9,10-DIMETHOXY-2,3,4,6,7,11b-HEXAHYDRO-1H-PYRIDO[2,1-a]ISOQUINOLIN-2-YL 2-AMINO-3-METHYLBUTANOATE DI(4-METHYLBENZENESULFONATE)](https://images-eureka.patsnap.com/patent_img/af5a93d0-aa6a-4674-8cd4-51408de0087f/US20170183346A1-20170629-C00003.png)
![SALTS OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YL-METHYL)-IMIDAZO[1,2-b][1,2,4]TRIAZIN-2-YL]BENZAMIDE AND PROCESSES RELATED TO PREPARING THE SAME SALTS OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YL-METHYL)-IMIDAZO[1,2-b][1,2,4]TRIAZIN-2-YL]BENZAMIDE AND PROCESSES RELATED TO PREPARING THE SAME](https://images-eureka.patsnap.com/patent_img/f86edc4f-d7a1-4901-998d-640bf82cd6b1/US20090291956A1-20091126-D00000.png)
![SALTS OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YL-METHYL)-IMIDAZO[1,2-b][1,2,4]TRIAZIN-2-YL]BENZAMIDE AND PROCESSES RELATED TO PREPARING THE SAME SALTS OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YL-METHYL)-IMIDAZO[1,2-b][1,2,4]TRIAZIN-2-YL]BENZAMIDE AND PROCESSES RELATED TO PREPARING THE SAME](https://images-eureka.patsnap.com/patent_img/f86edc4f-d7a1-4901-998d-640bf82cd6b1/US20090291956A1-20091126-D00001.png)
![SALTS OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YL-METHYL)-IMIDAZO[1,2-b][1,2,4]TRIAZIN-2-YL]BENZAMIDE AND PROCESSES RELATED TO PREPARING THE SAME SALTS OF 2-FLUORO-N-METHYL-4-[7-(QUINOLIN-6-YL-METHYL)-IMIDAZO[1,2-b][1,2,4]TRIAZIN-2-YL]BENZAMIDE AND PROCESSES RELATED TO PREPARING THE SAME](https://images-eureka.patsnap.com/patent_img/f86edc4f-d7a1-4901-998d-640bf82cd6b1/US20090291956A1-20091126-D00002.png)