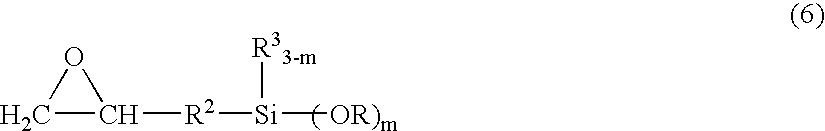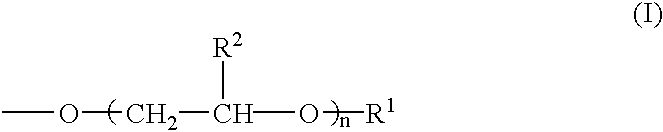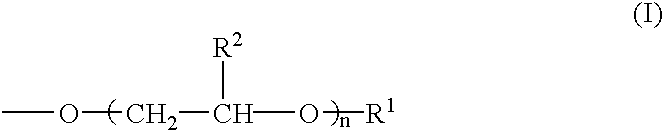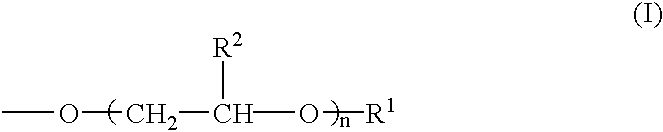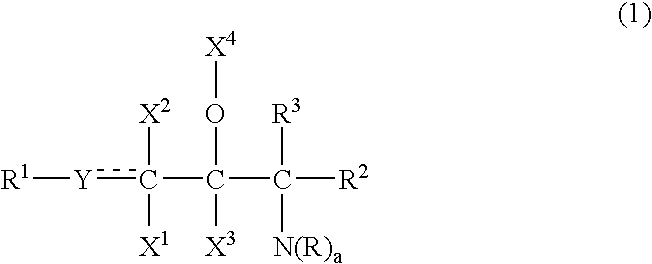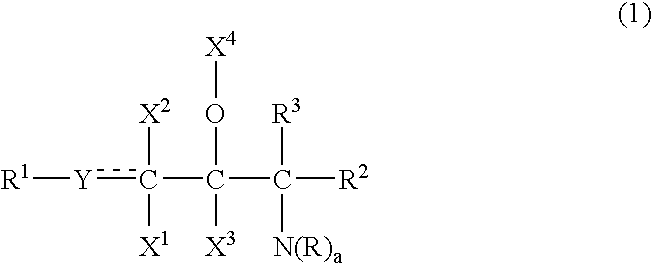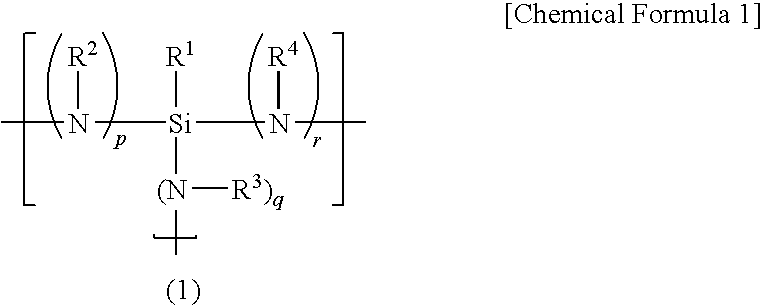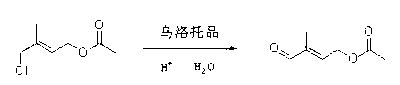Patents
Literature
573 results about "Acetoxy group" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
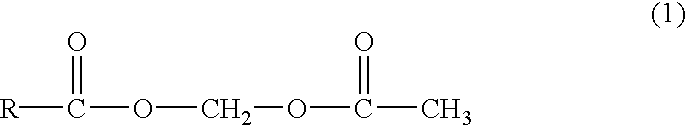
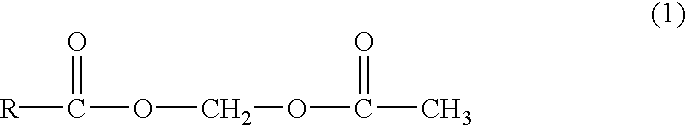
Acetoxy group, abbreviated AcO or OAc, is a chemical functional group of the structure CH₃-C(=O)-O-. It differs from the acetyl group CH₃-C(=O)- by the presence of one additional oxygen atom. The name acetoxy is the short form of acetyl-oxy.
Precursors and flowable CVD methods for making low-K films to fill surface features
A method for depositing a silicon-containing film, the method comprising: placing a substrate comprising at least one surface feature into a flowable CVD reactor which is at a temperature of from about −20° C. to about 400° C.; introducing into the reactor at least one silicon-containing compound having at least one acetoxy group to at least partially react the at least one silicon-containing compound to form a flowable liquid oligomer wherein the flowable liquid oligomer forms a silicon oxide coating on the substrate and at least partially fills at least a portion of the at least one surface feature. Once cured, the silicon oxide coating has a low k and excellent mechanical properties.
Owner:VERSUM MATERIALS US LLC
Structural modification of 19-norprogesterone I: 17-α-substituted-11-β-substituted-4-aryl and 21-substituted 19-norpregnadienedione as new antiprogestational agents
The present invention relates, inter alia, to compounds having the general formula: in which: R1 is a member selected from the group consisting of —OCH3, —SCH3, —N(CH3)2, —NHCH3, —NC4H8, —NC5H10, —NC4H8O, —CHO, —CH(OH)CH3, —C(O)CH3, —O(CH2)2N(CH3)2, and —O(CH2)2NC5H10; R2 is a member selected from the group consisting of hydrogen, halogen, alkyl, acyl, hydroxy, alkoxy (e.g., methoxy, ethoxy, vinyloxy, ethynyloxy, cyclopropyloxy, etc.), acyloxy (e.g., acetoxy, glycinate, etc.), alkylcarbonate, cypionyloxy, S-alkyl, —SCN, S-acyl and —OC(O)R6, wherein R6 is a functional group including, but not limited to, alkyl (e.g., methyl, ethyl, etc.), alkoxy ester (e.g., —CH2OCH3) and alkoxy (—OCH3); R3 is a member selected from the group consisting of alkyl, hydroxy, alkoxy and acyloxy; R4 is a member selected from the group consisting of hydrogen and alkyl; and X is a member selected from the group consisting of ═O and ═N—OR5, wherein R5 is a member selected from the group consisting of hydrogen and alkyl.In addition to providing the compounds of Formula I, the present invention provides methods wherein the compounds of Formula I are advantageously used, inter alia, to antagonize endogenous progesterone; to induce menses; to treat endometriosis; to treat dysmenorrhea; to treat endocrine hormone-dependent tumors; to treat meningiomas; to treat uterine leiomyomas; to treat uterine fibroids; to inhibit uterine endometrial proliferation; to induce cervical ripening; to induce labor; and for contraception.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF
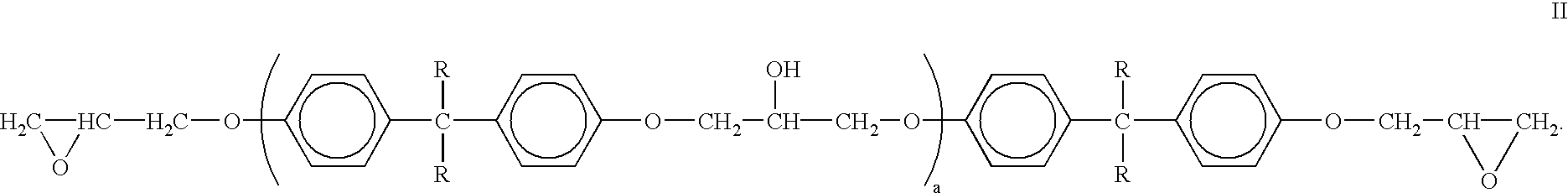
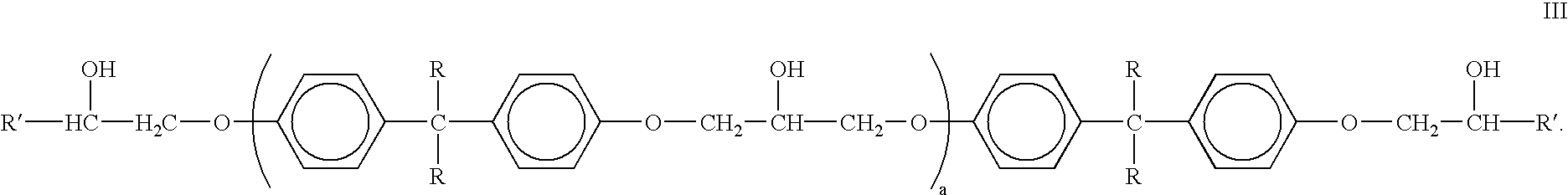
Absorbent article comprising a synthetic polymer derived from a renewable resource and methods of producing said article
An element of an absorbent article is provided. The element has a bio-based content of at least about 50% based on the total weight of the element, and comprises a synthetic polymer derived from a renewable resource via a first intermediate compound selected from the group consisting of crotonic acid, propiolactone, ethylene oxide, i-propanol, butanol, butyric acid, propionic acid, 2-acetoxypropanoic acid, methyl 2-acetoxypropanoate, methyl lactate, ethyl lactate, polyhydroxybutyrate, and a polyhydroxyalkanoate comprising 3-hydroxypropionate monomers. An absorbent article comprising the element and a method of making an element for an absorbent article also are provided.
Owner:THE PROCTER & GAMBLE COMPANY
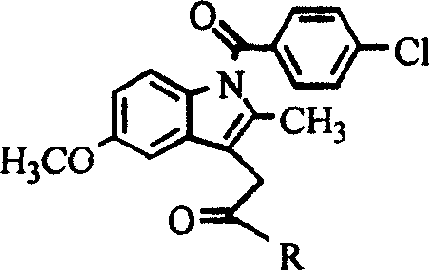
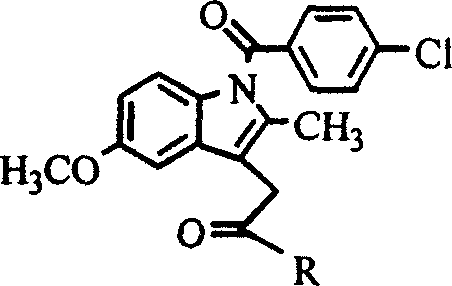
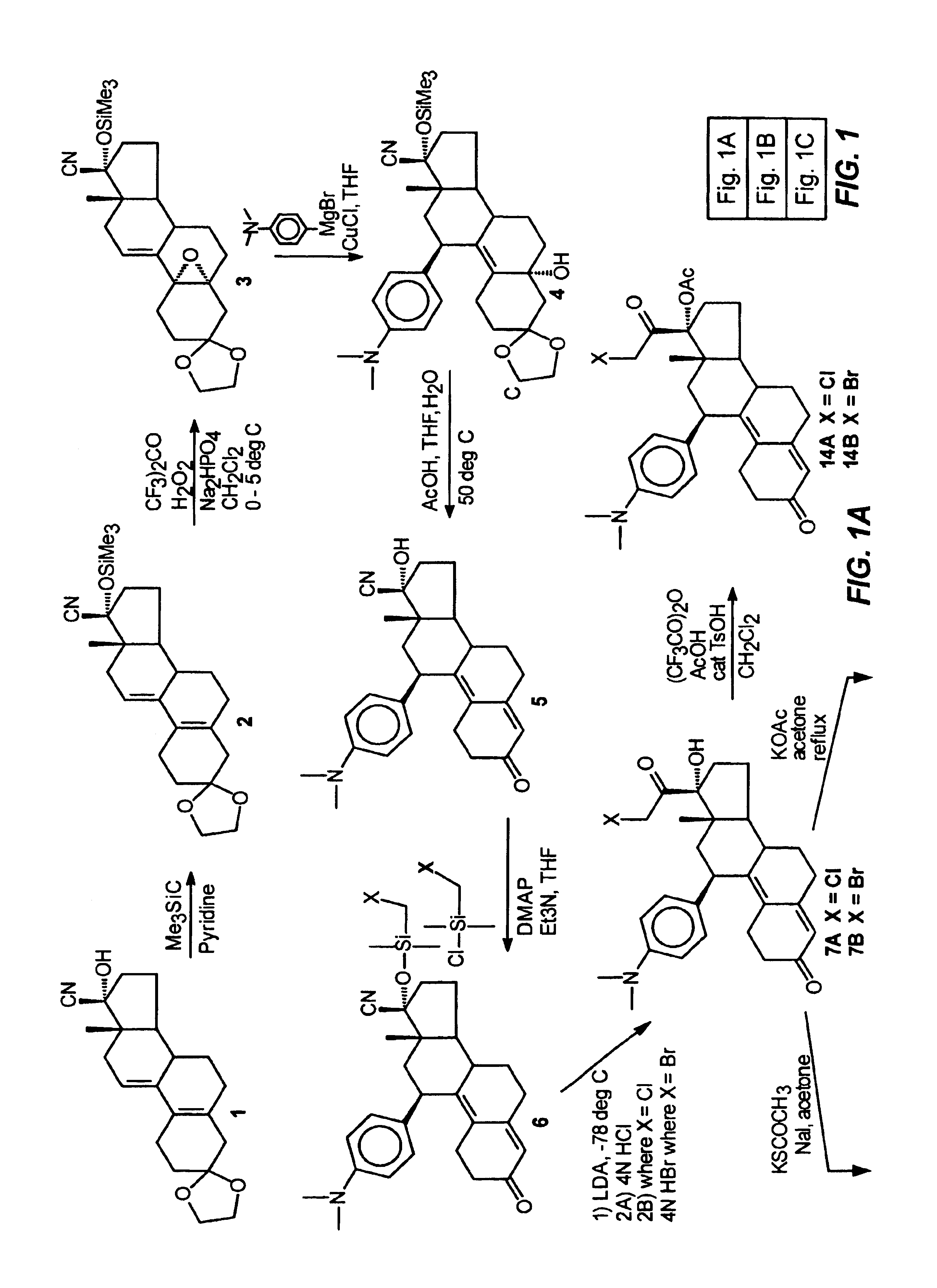
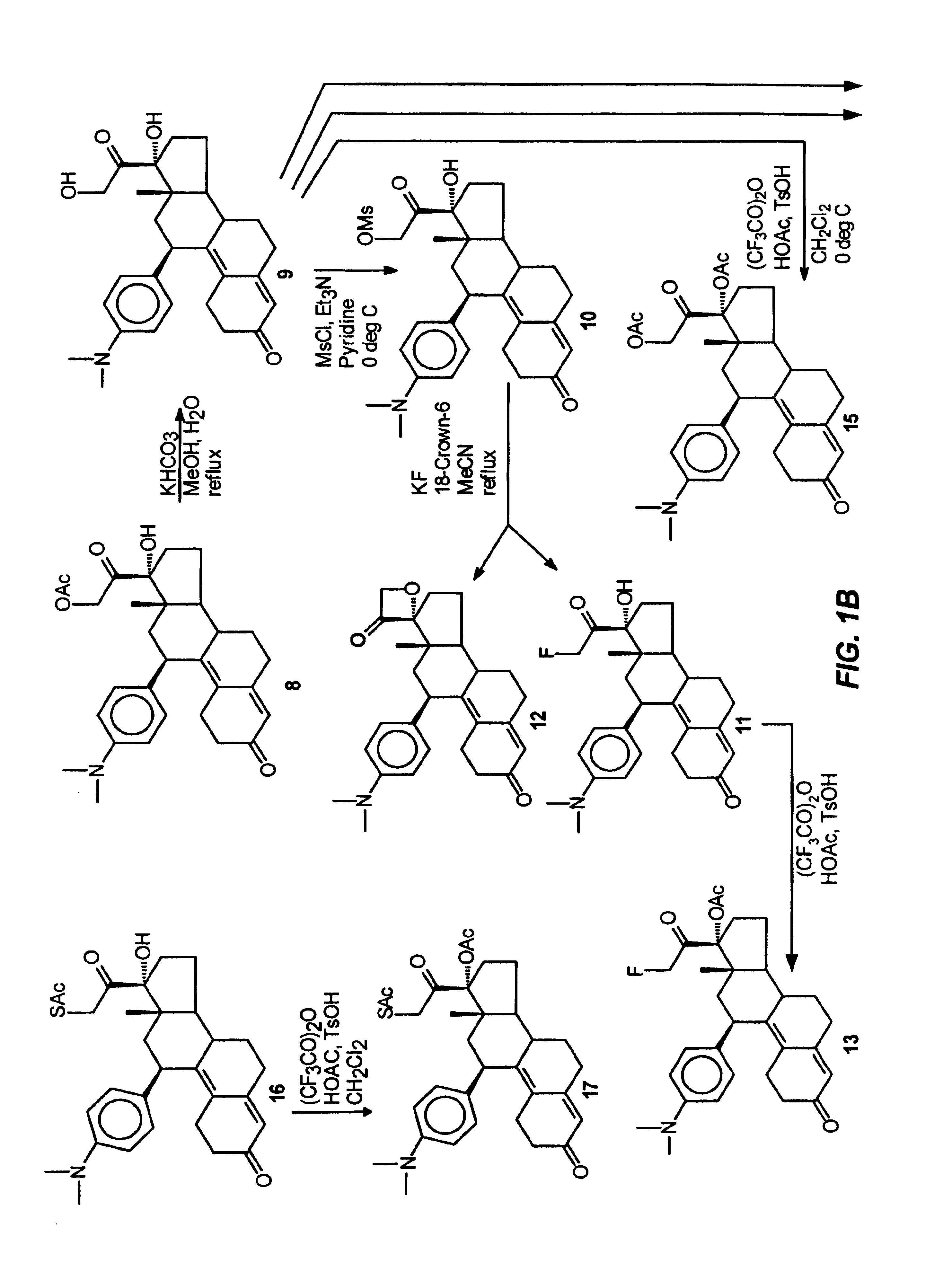
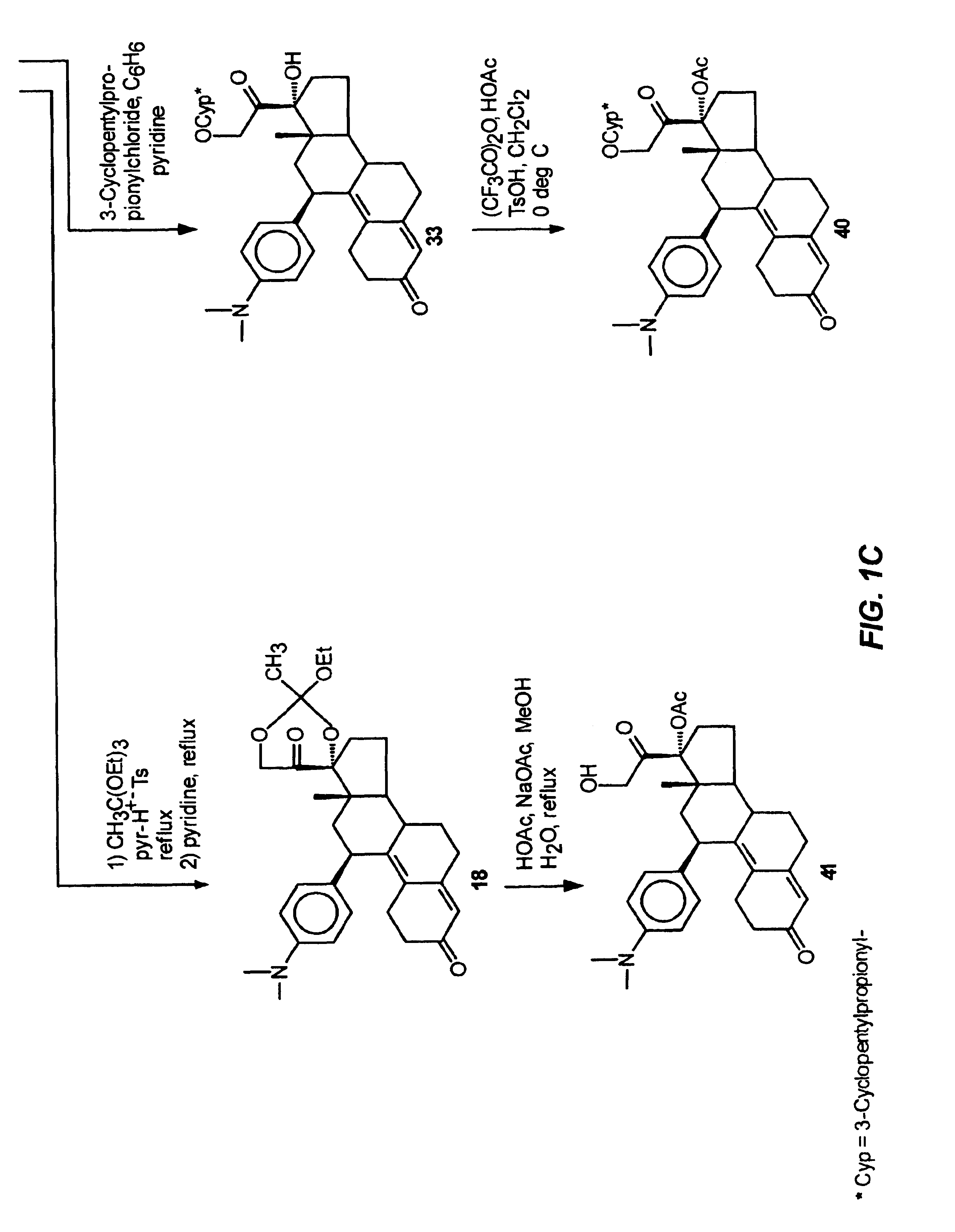
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Surface-modifying agents for wettability modification
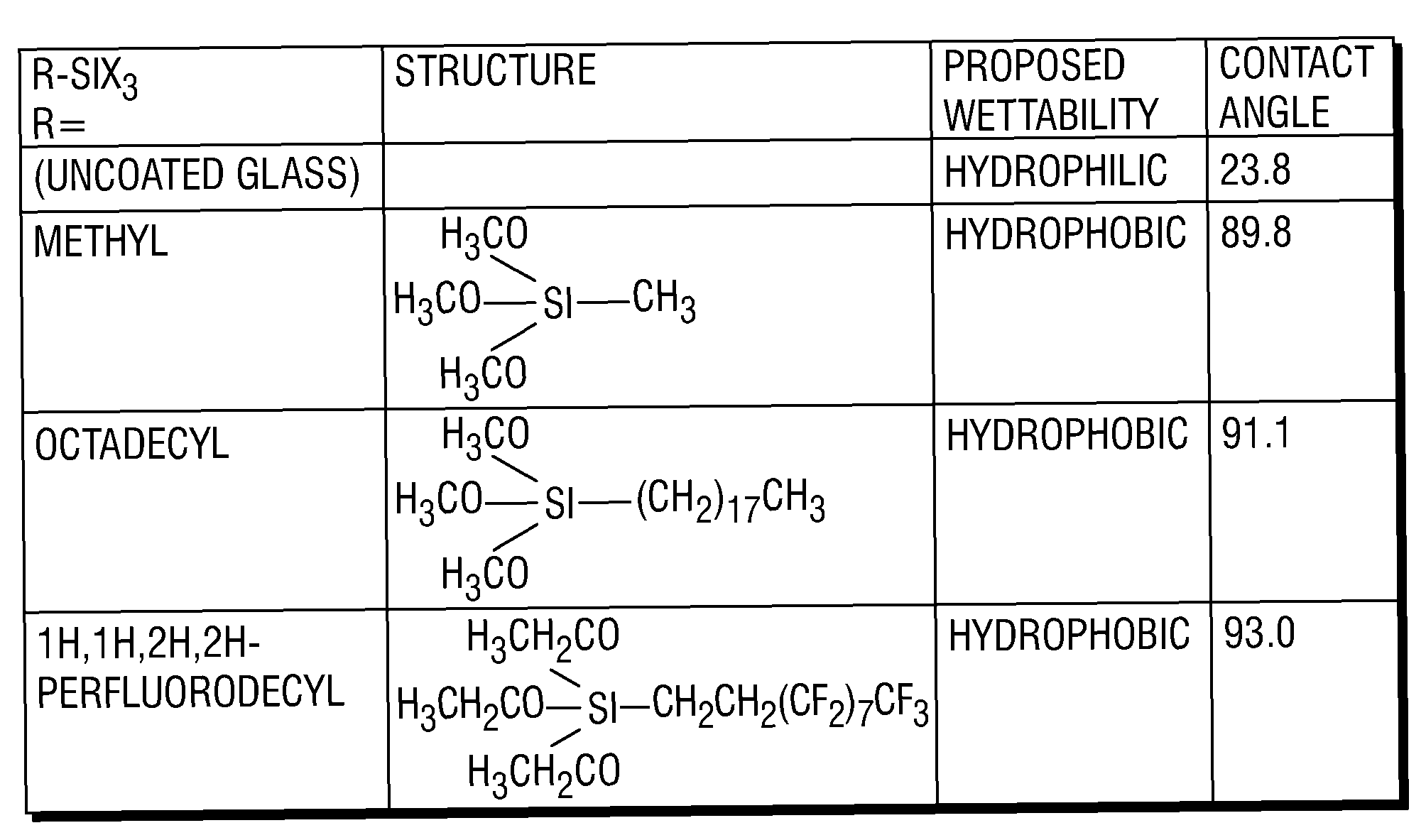
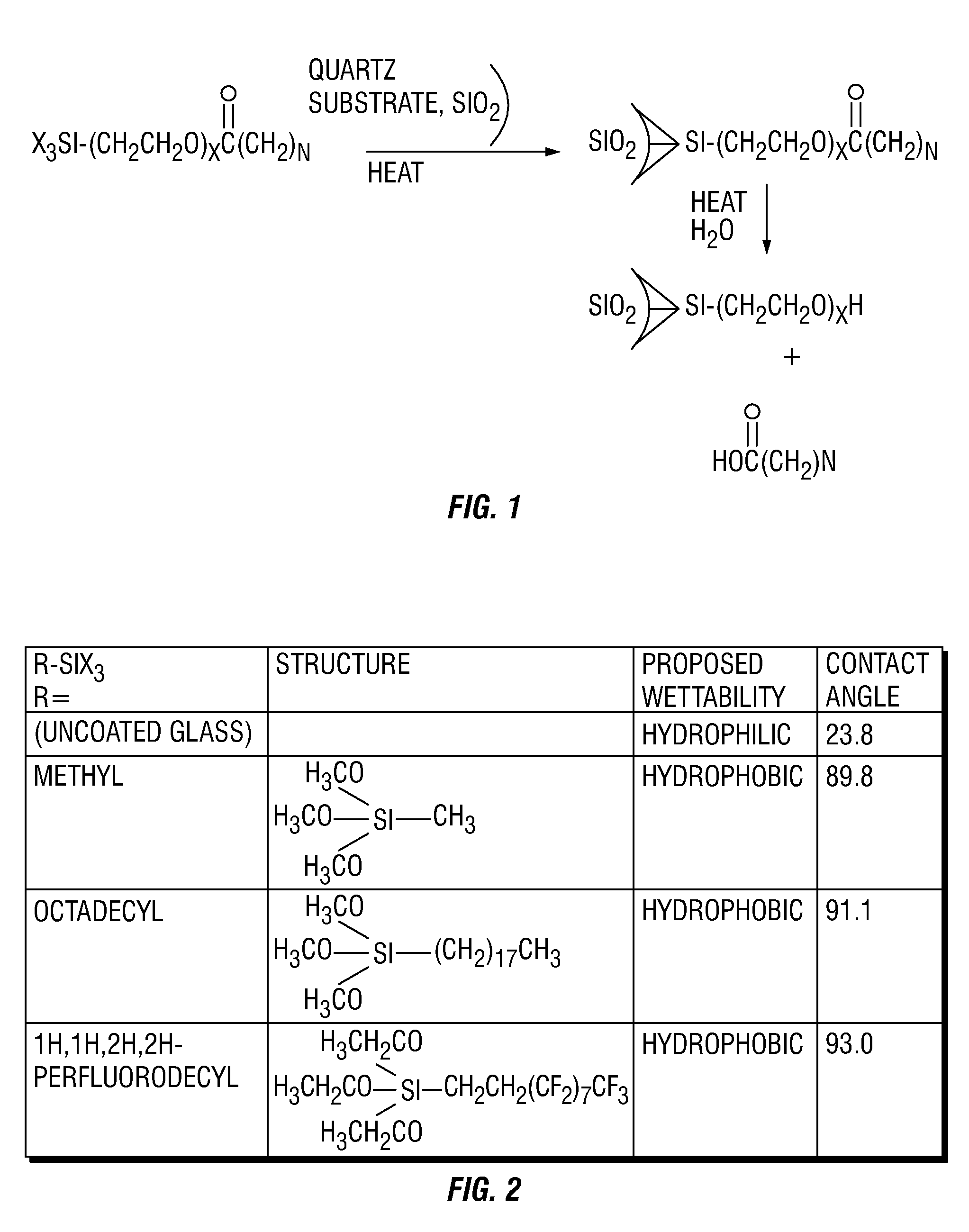
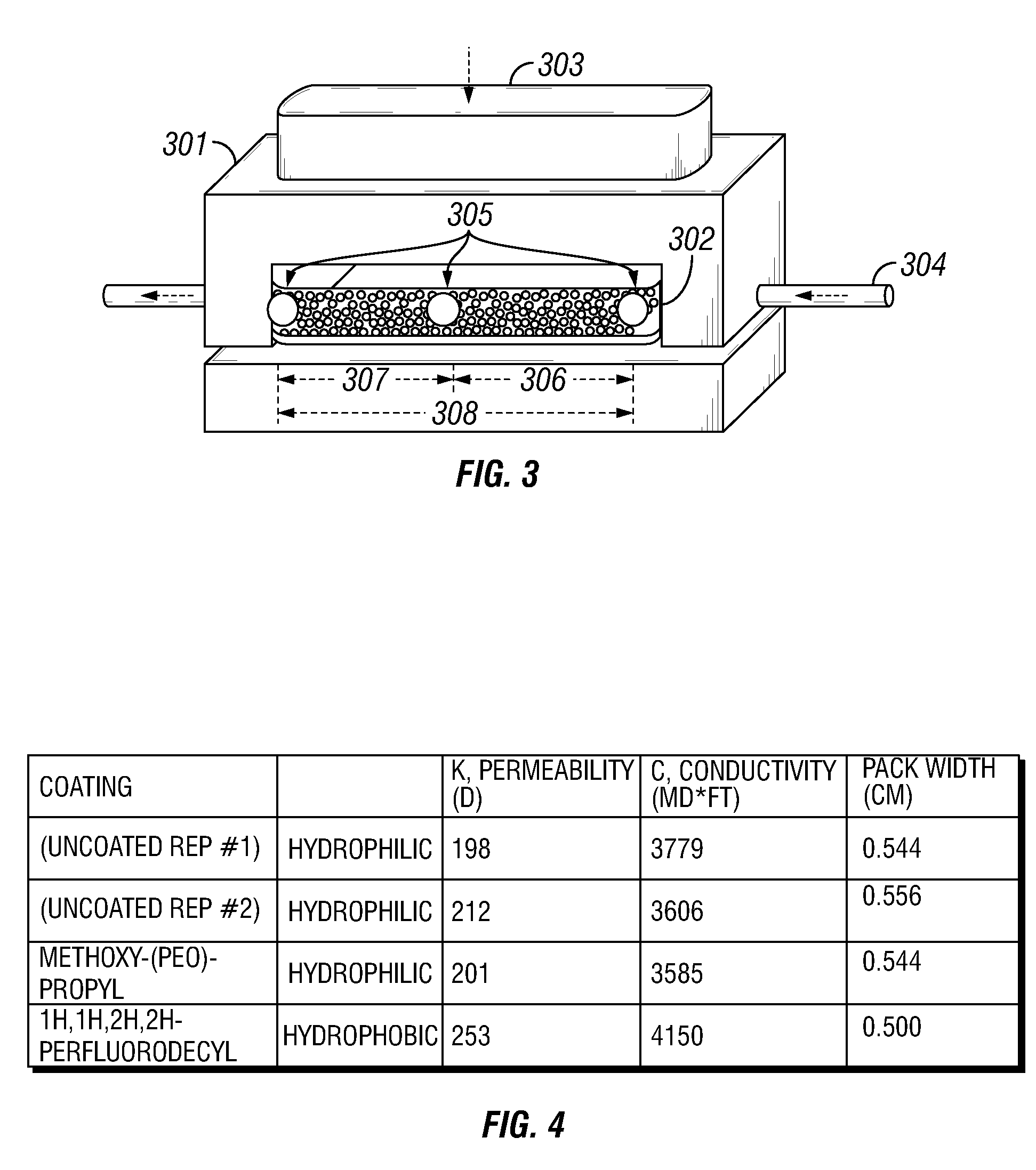
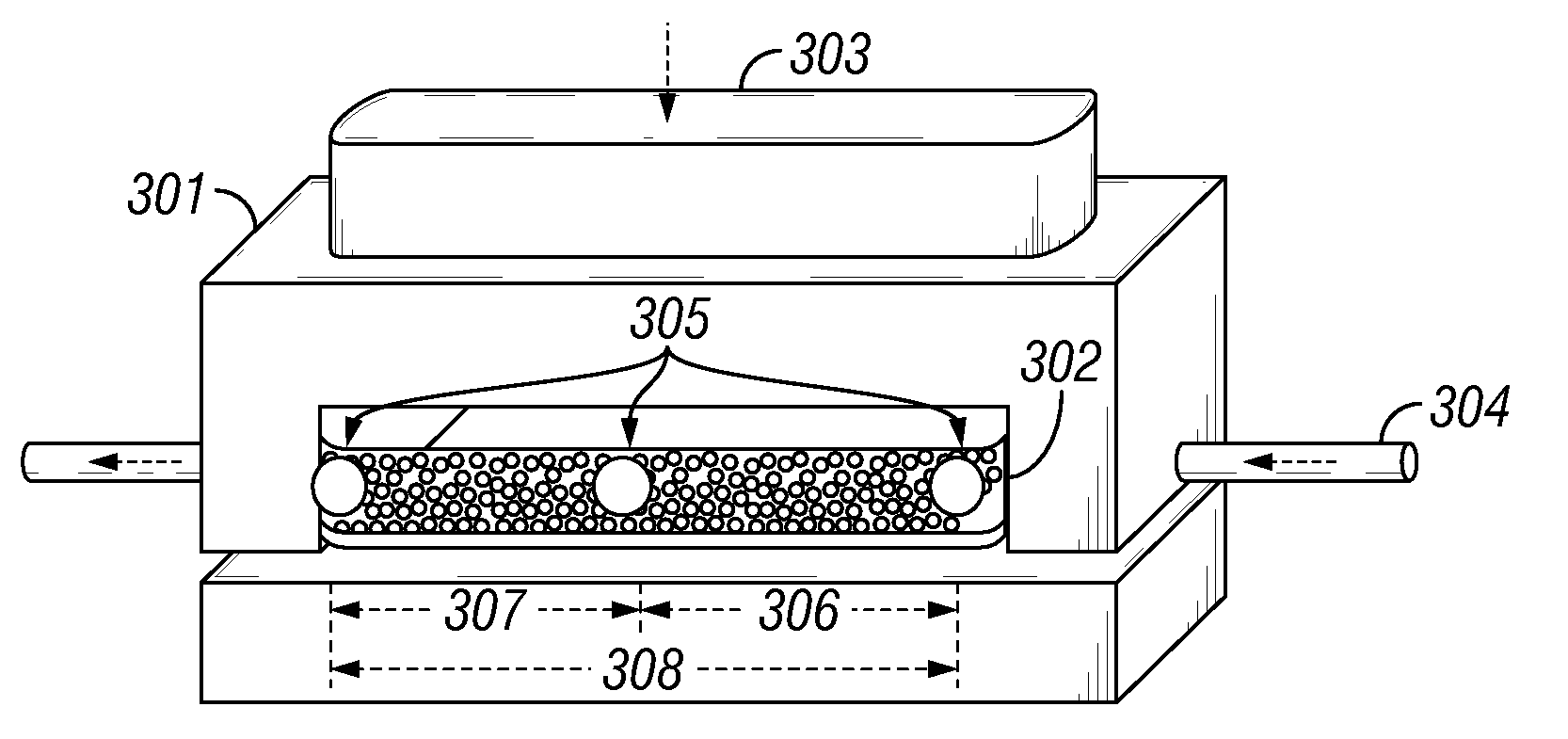
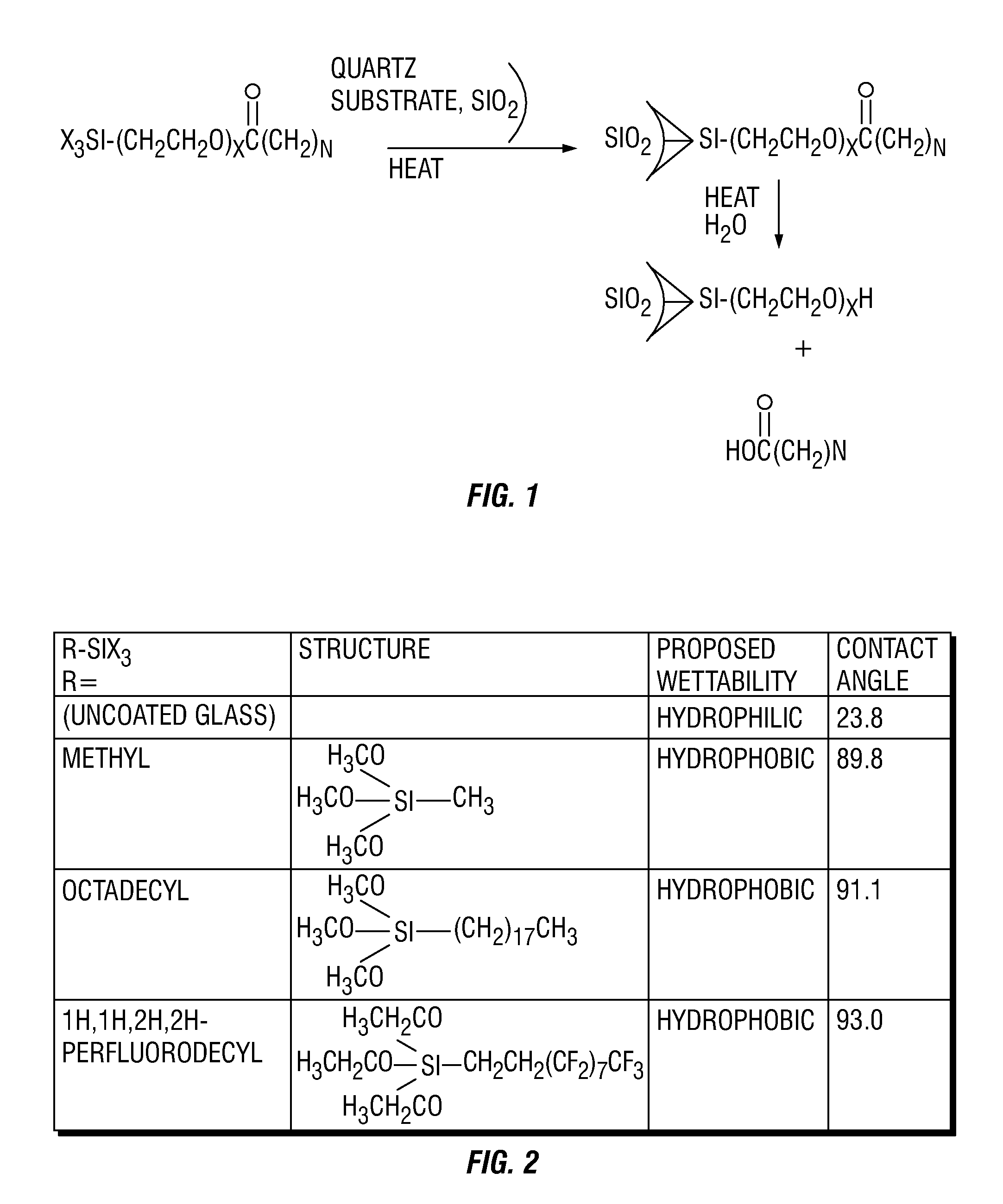
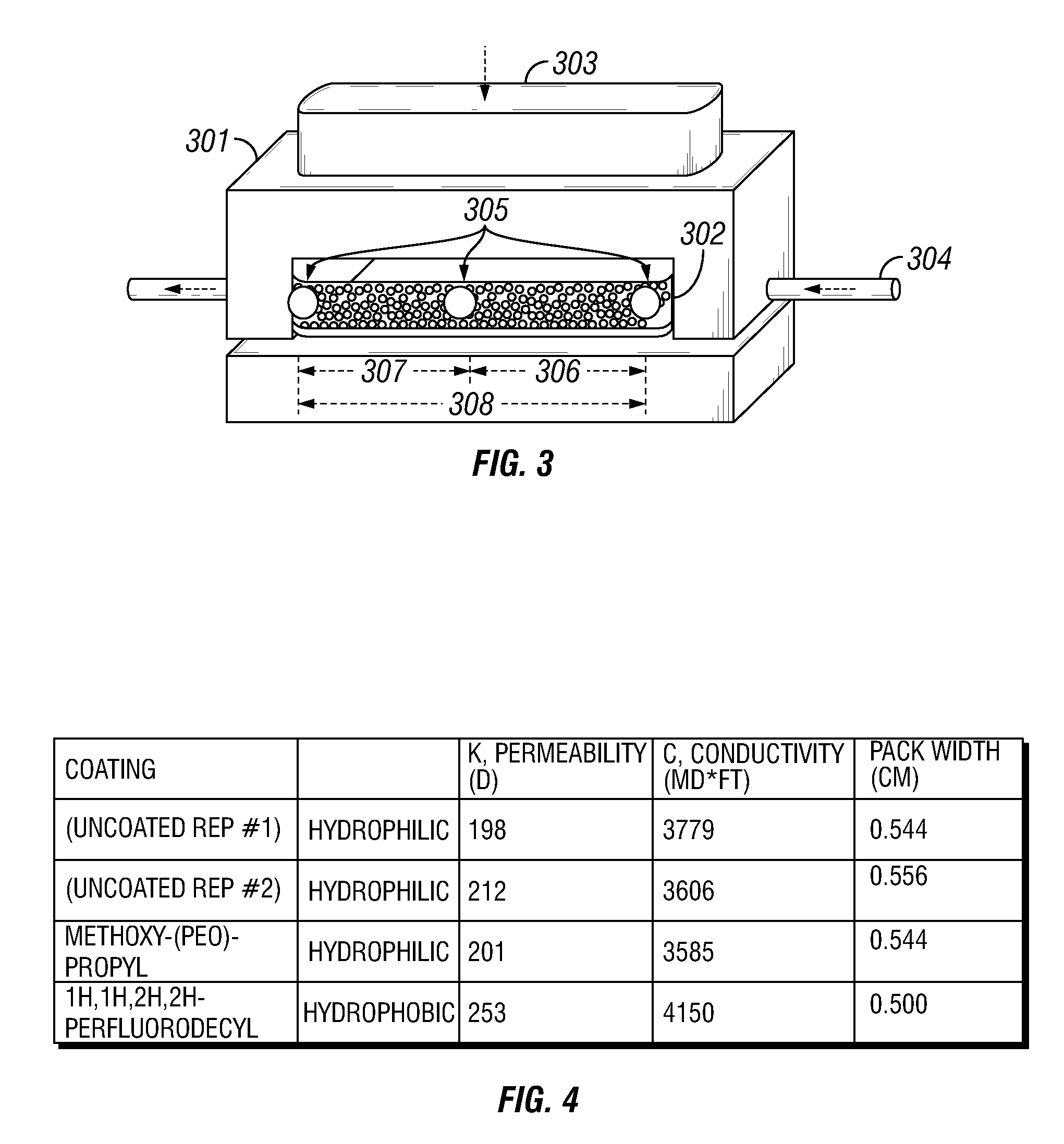
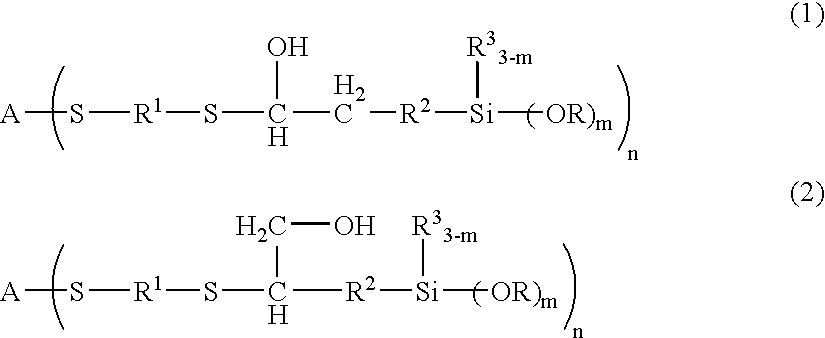
A method and composition for treating a subterranean formation with a fluid, including forming a fluid including a particulate and an organosilane with the chemical formula RnSiX4-n, wherein n is equal to 1, 2, or 3, R is an organic functional group, and X is a halogen, alkoxy, or acetoxy group, introducing the fluid into a subterranean formation with exposed surfaces, and modifying the wettability of a surface of the particulate or subterranean formation or both. A method and composition for treating a subterranean formation with a fluid including forming a fluid comprising a particulate and an organosilane, introducing the fluid into a subterranean formation with exposed surfaces, and modifying the wettability of the proppant or surfaces or both, wherein the wettability modification degrades.
Owner:SCHLUMBERGER TECH CORP
Surface-Modifying Agents for Wettability Modification
A method and composition for treating a subterranean formation with a fluid, including forming a fluid including a particulate and an organosilane with the chemical formula RnSiX4-n, wherein n is equal to 1, 2, or 3, R is an organic functional group, and X is a halogen, alkoxy, or acetoxy group, introducing the fluid into a subterranean formation with exposed surfaces, and modifying the wettability of a surface of the particulate or subterranean formation or both. A method and composition for treating a subterranean formation with a fluid including forming a fluid comprising a particulate and an organosilane, introducing the fluid into a subterranean formation with exposed surfaces, and modifying the wettability of the proppant or surfaces or both, wherein the wettability modification degrades. A method and composition for producing hydrocarbon from a subterranean formation, including providing a wellbore in a subterranean formation, forming a fluid including a particulate and an organosilane with the chemical formula RnSiX4-x, wherein n is equal to 1, 2, or 3, R is an organic functional group, and X is a halogen, alkoxy, or acetoxy group, introducing the fluid into the subterranean formation with exposed surfaces, modifying the wettability of a surface of the particulate or the subterranean formation or both, and producing hydrocarbon from the wellbore in the subterranean formation.
Owner:SCHLUMBERGER TECH CORP
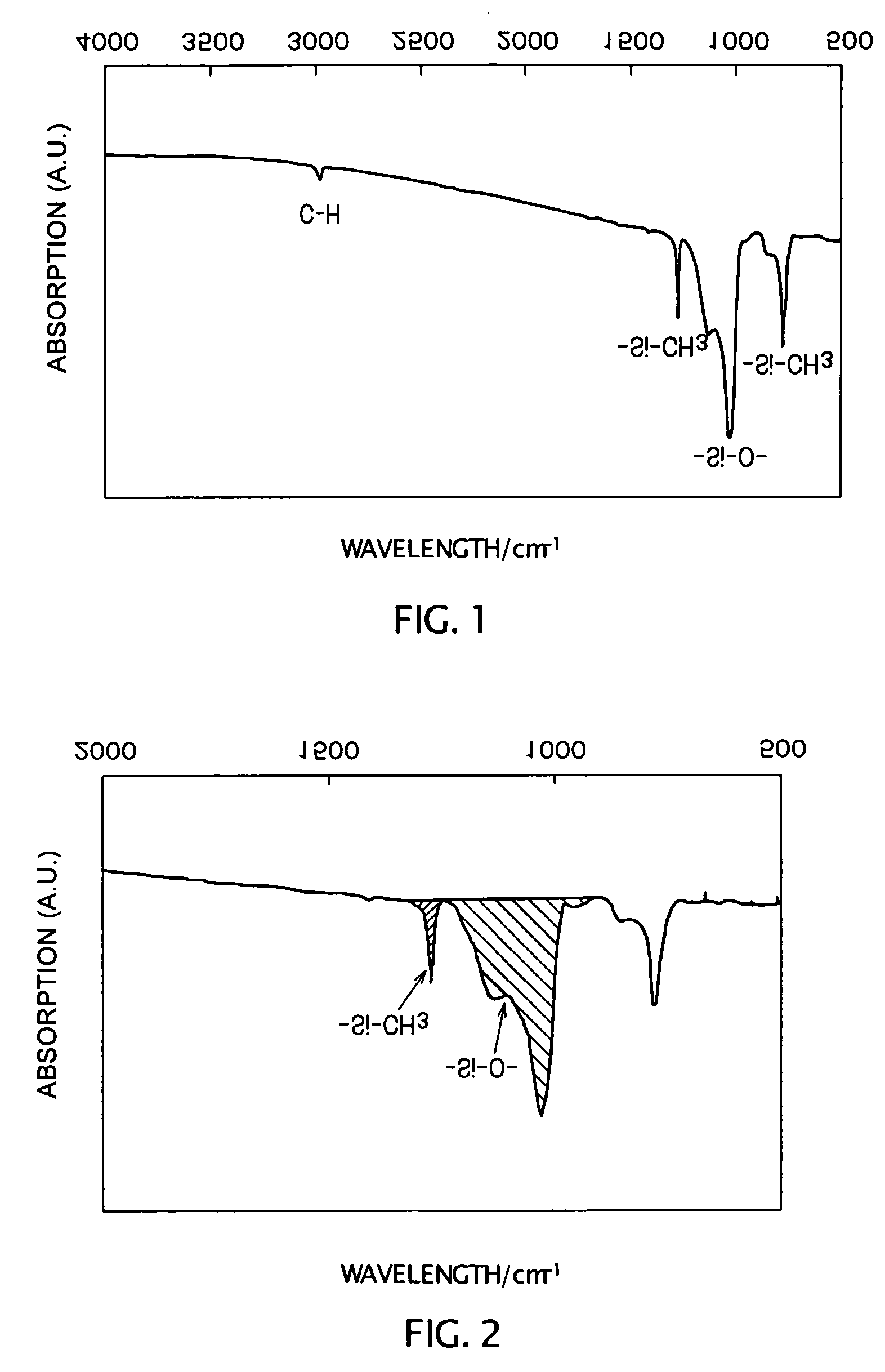
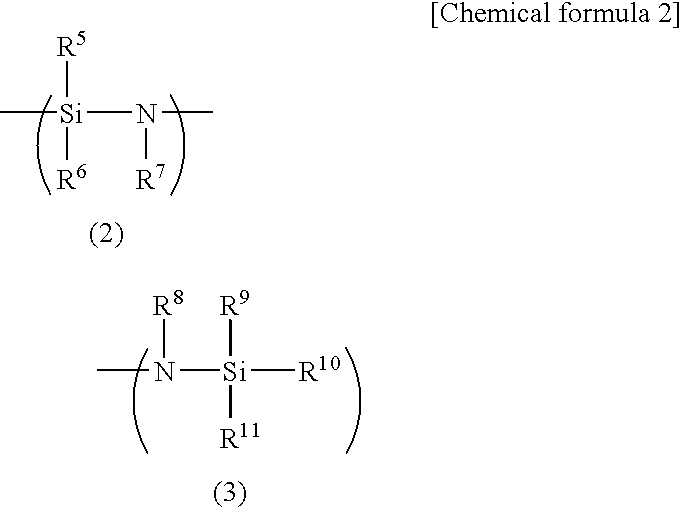
Curing resin, method for producing same and curing resin composition
InactiveUS20060079605A1Increasing advantageous effectHigh viscosityOrganic non-macromolecular adhesiveCellulose adhesivesMethacrylateSilylene
An object of the present invention is to provide a curable resin having a silyl group at the terminal that is also moisture-hardening resin which has lower in viscosity when it is used and provides superior adhesiveness when it is utilized for adhesive, sealant, and paint. The object of the invention could be achieved by a curable resin that has silicon atom-containing groups having a silicon atom bound to one or more hydrolysable groups such as alkoxy groups, acetoxy groups and oxim groups at the ends of the molecule, thioether and hydroxyl groups in the molecule, and a polymer having a polyoxyalkylene, (meth)acrylic ester, or hydrocarbon polymer as the main chain skeleton.
Owner:KONISHI CO
Novel acetyloxymethyl esters and methods for using the same
Novel acetyloxymethyl esters are disclosed. Methods of treating an illness, including cancer, hemological disorders and inherited metabolic disorders, and treating or ameliorating other conditions using these compounds are also disclosed. The compounds are effective in the inhibition of histone deacetylase.
Owner:ERRANT GENE THERAPEUTICS
Coated optical fibers using adhesion promoters, and methods for making and using same
InactiveUS20030129397A1Improve adhesionNot affecting cure speedLiquid surface applicatorsOptical fibre with multilayer core/claddingPolymer scienceSilylene
The present invention provides a radiation curable coating composition for forming a polymeric coating on a glass optical fiber, the composition comprising a mixture of: a base radiation curable liquid composition capable of forming a polymeric coating; at least one adhesion promoter selected from the group consisting of bis-silyl amines, diacrylated silane tertiary amine, acetoxy functional silanes, trifunctional isocyanurates and mixtures thereof, and 0 to about 10 percent by weight of one or more photoinitiators. The present invention also includes a coated optical fiber, a method for making an optical fiber and compositions containing adhesion promoters that do not undergo free radical reaction with base radiation curable pre-polymer of the composition.
Owner:BOODEN LTDZA
Methods and compositions for treating cancer
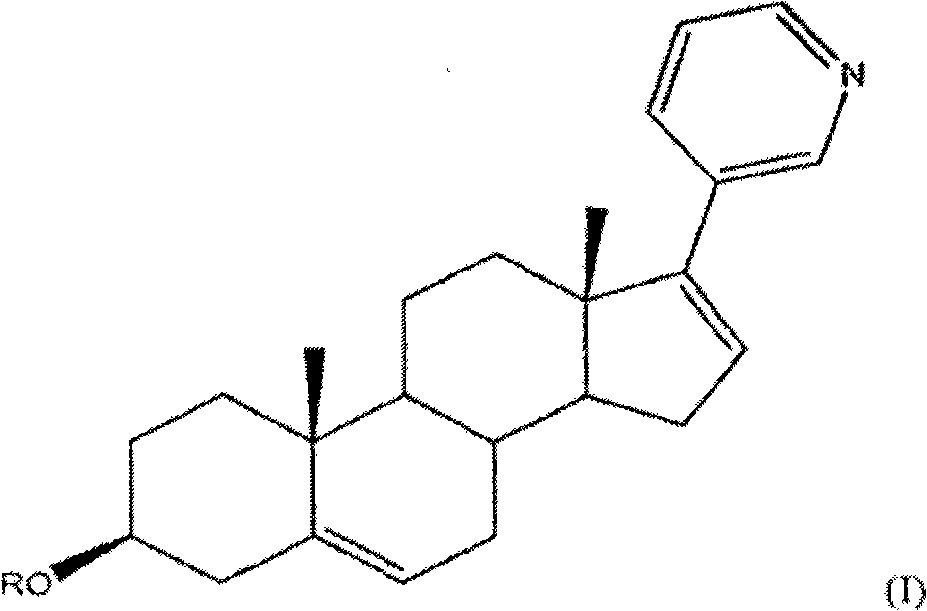
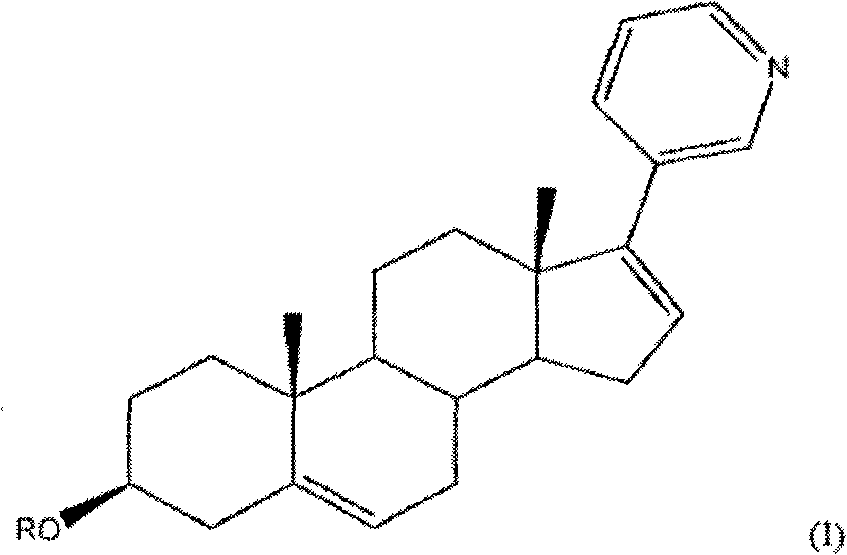
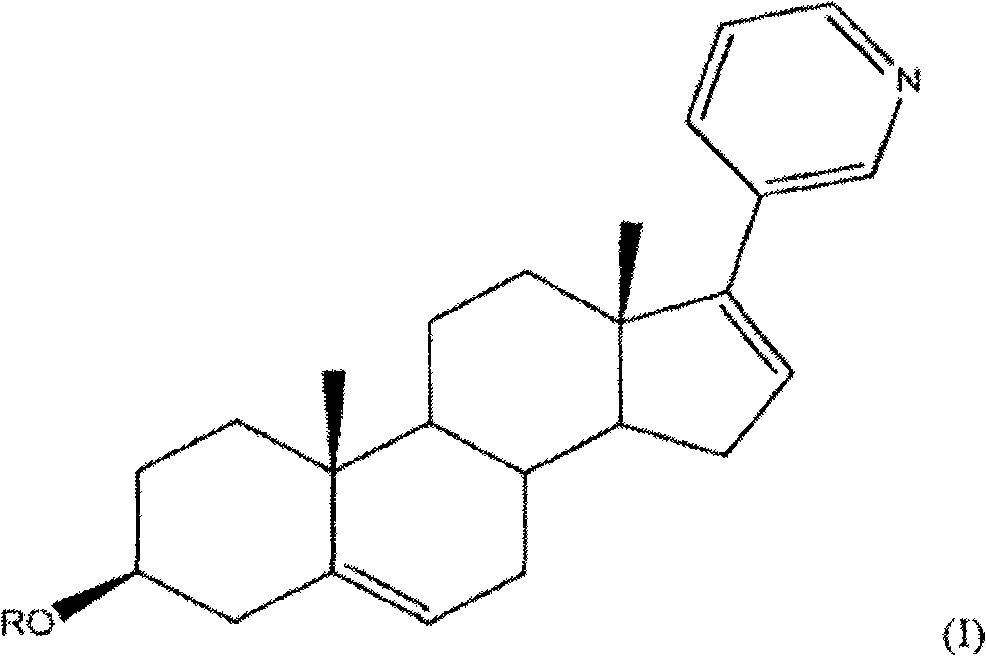
Methods and compositions for treating cancer are described herein. More particularly, the methods for treating cancer comprise administering a 17a-hydroxylase / C17, 20-lyase inhibitor, such as abiraterone acetate (i.e., 3beta-acetoxy-17-(3-pyridyl) androsta-5, 16-diene), in combination with at least one additional therapeutic agent such as an anti-cancer agent or a steroid. Furthermore, disclosed are compositions comprising a 17a- hydroxylase / C17, 20-lyase inhibitor, and at least one additional therapeutic agent, such as an anti-cancer agent or a steroid.
Owner:库伽尔生物科技公司
Terminal modified organic silicone coating flatting agent and preparation method thereof
The invention relates to a coating flatting agent, in particular to a preparation of polyether terminal modified polydimethylsiloxane and an application of the polyether terminal modified polydimethylsiloxane serving as the flatting agent in coatings. The structural formula of a terminal modified organic silicone coating flatting agent is shown as follows, wherein m is equal to 0-100; and R is equal to - (CH2) 3-O-(C2H4O) x (C3H6O) y-R', wherein the x is equal to 0-100, the y is equal to 0-100, and R' is hydrogen (H) or alkyl, acetoxy group and epoxy group with one to ten carbon atoms. The flatting agent has the advantages that the surface tension of the coatings is reduced, the wettability of substrates is improved, the flowing and flatting of the coatings are increased, the surface defects are eliminated, the smoothness is improved, the surface friction coefficient of the coatings is lowered, and the scrath resistance and anti-blocking are improved.
Owner:RUNHE ORGANICSILICONE NEW MATERIAL
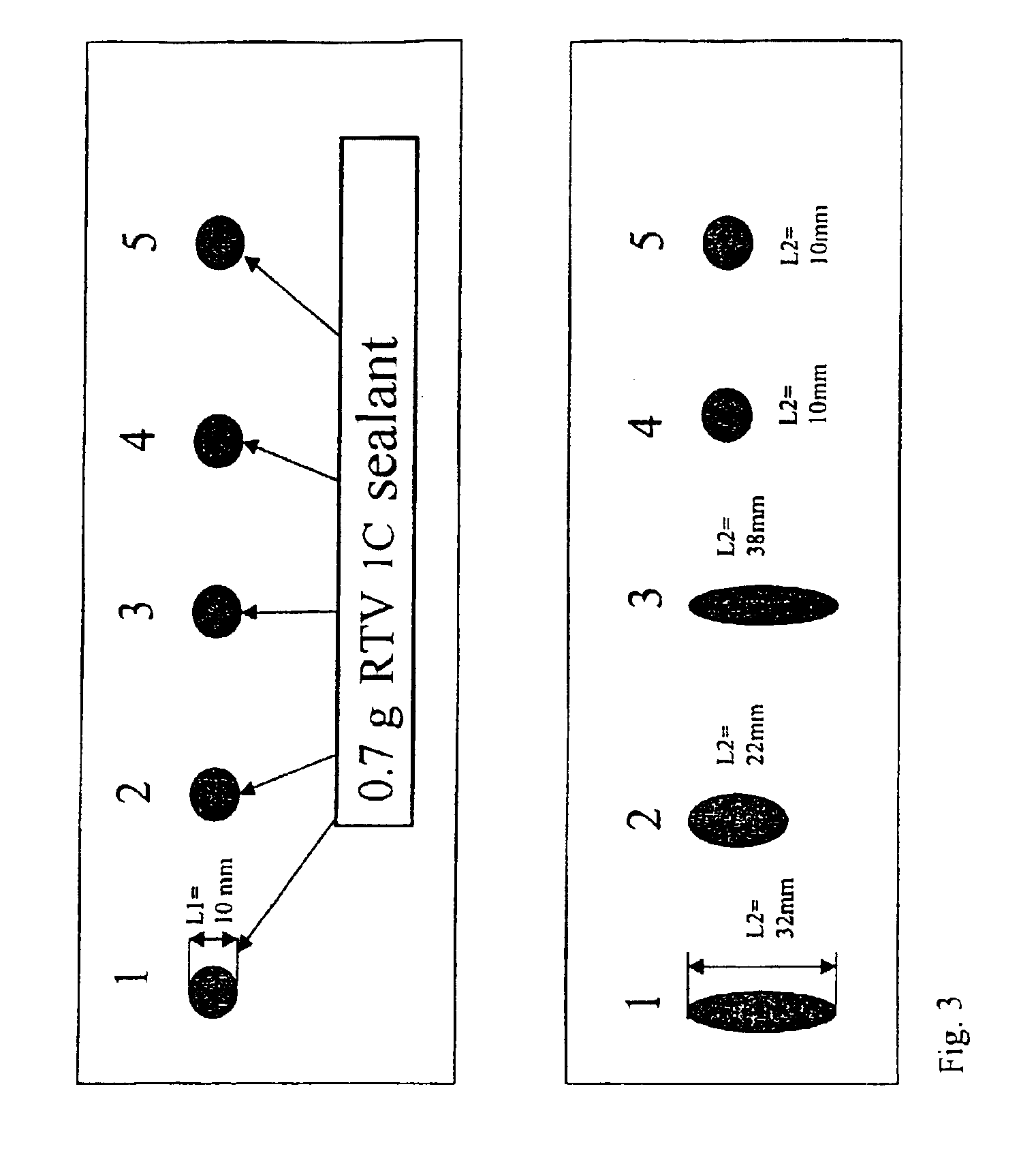
Room temperature crosslinking, one component silicone rubber formulation with hydrophobic silica
InactiveUS6956080B2Improve the level ofHigh whitenessPigmenting treatmentOther chemical processesPolystyreneHydrophobic silica
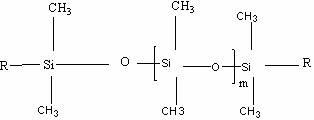
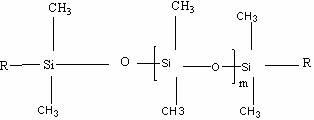
A room temperature crosslinking, one component (RTV 1C) silicone rubber formulation with hydrophobic, silica featuring extremely low water absorption, a high level of whiteness, and fine-tunable, storage-stable rheological properties with a high reinforcing action in the silicone rubber vulcanizate contains A) from 0.5 to 60% by weight of a hydrophobic silica having the following properties: a carbon content of >3.1%; a methanol wettability of >60%; a reflectance of >94%; a BET / CTAB ratio of from >1 to <3; a DBP absorption of <230 g / 100 g; a BET surface area of from 50 to 110 m2 / g; a CTAB surface area of >30 m2 / g; a water vapor absorption at 30° C. at an ambient humidity of 30 of <1.3; a water vapor absorption at 30° C. at an ambient humidity of 70 of <1.7; and B) 40-99.5% by weight of an organopolysiloxane of the formulaZnSiR3-n—O—[SiR2O]x—SiR3-n—Z′nwherein each R represents independently alkyl, acetoxy, oxime, alkoxy, amido, aryl or alkenyl radicals, each having from 1 to 50 carbon atoms, each unsubstituted or substituted identically or differently by O, S, F, Cl, Br or I; or R represents independently polystyrene, polyvinyl acetate, polyacrylate, polymethacrylate, or polyacrylonitrile radicals, each having 50-10 000 repeating units; wherein each Z represents independently OH, Cl, Br, acetoxy, amino, amido, amineoxy, oxime, alkoxy, alkenyloxy, acyloxy or phosphate radicals, wherein said acetoxy, amino, amido, amineoxy, oxime, alkoxy, alkenyloxy and acyloxy radicals each have up to 20 carbon atoms; wherein each Z′ represents independently oxime, alkoxy, amido or acetoxy radicals; wherein n is 1-3; and wherein x is 100-15 000.
Owner:EVONIK DEGUSSA GMBH
Silane coupling agent treated-silica and rubber composition containing the same
InactiveUS20070078202A1Dispersibility wetWet performanceSilicaSynthetic resin layered productsArylPolymer science
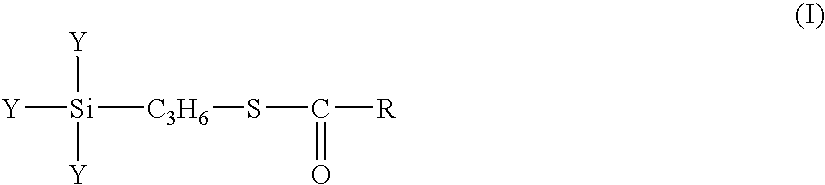
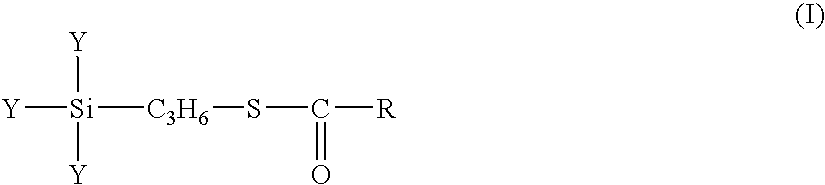
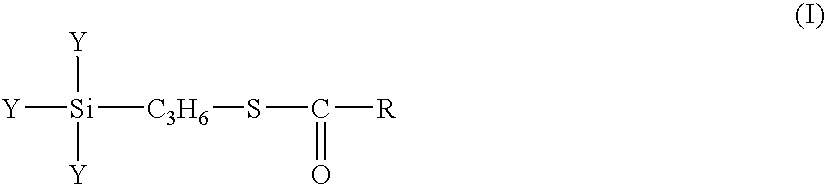
A rubber component containing at least one a diene-based rubber in an amount of 100 parts by weight and a surface-treated silica treated, on the surface thereof, with at least one silane coupling agent X represented by formula (I): wherein Y independently indicate a methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy or acetoxy group, R indicates a C1 to C18 hydrocarbon group selected from a linear, cyclic or branched alkyl group, alkenyl group, aryl group, and aralkyl group and a rubber composition containing the same.
Owner:THE YOKOHAMA RUBBER CO LTD +1
Hydrophilic modification method and heat exchanger treated thereby
InactiveUS20030037914A1Improve the level ofWeak affinityPretreated surfacesCorrosion preventionHydrogen atomPlate heat exchanger
A hydrophilic modification method comprising a step of forming a coat having a solid coat amount of 0.02 to 3 g / m2 on a heat exchanger with a modifier for hydrophilicity after treatment for rust prevention thereof wherein said modifier for hydrophilicity comprises a modified polyvinyl alcohol (A) having, on a side chain thereof, a group represented by the formula (I): in the formula, n represents an integer of 1 to 500, R1 represents a hydrogen atom or an alkyl group containing 1 to 4 carbon atoms, and R2 represents a hydrogen atom or a methyl group, and at least one member (B) selected from the group consisting of phosphorus compound salts and boron compound salts of Ca, Al, Mg, Fe and Zn, said group represented by the formula (I) accounting for 0.01 to 20 mole percent relative to hydroxyl and acetoxy groups contained in said modified polyvinyl alcohol.
Owner:NIPPON PAINT SURF CHEM +1
Ceramide emulsions
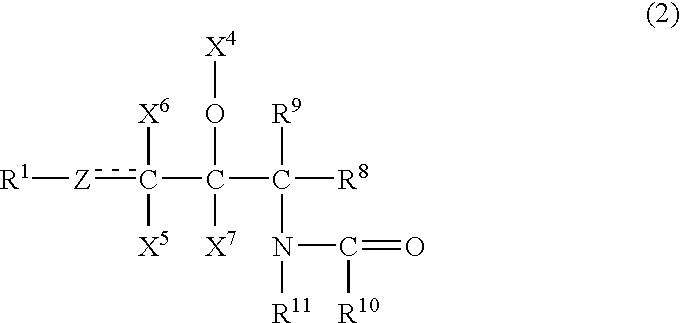
Provided is a ceramide emulsion containing the following components (A), (B) and (C) and not containing an acrylic acid polymer or a phospholipid: (A) sphingosines represented by Formula (1): (wherein R1 represents a hydrocarbon group having 4 to 30 carbon atoms which is optionally substituted with a hydroxyl group and the like; Y represents CH2, CH or O; X1, X2 and X3 represent H, a hydroxyl group or an acetoxy group; X4 represents H, an acetyl group or a glyceryl group or, together with an adjacent O atom, forms an oxo group; R2 and R3 represent H, a hydroxyl group, a hydroxymethyl group or an acetoxymethyl group; groups “R”s are H or an amidino group or represent a hydrocarbon group having 1 to 8 carbon atoms which may have a substituent such as a hydroxyl group and the like; and “a” is 2 or 3), (B) an acidic compound selected from inorganic acids and organic acids having a molecular weight of 200 or less, and (C) ceramides represented by Formula (2): (wherein R7 represents a hydrocarbon group having 4 to 30 carbon atoms which is optionally substituted with a hydroxyl group and the like; Z represents CH2, CH or O; X5, X6 and X7 represent H, a hydroxyl group or an acetoxy group, X4 represents H, an acetyl group or a glyceryl group or, together with an adjacent O atom, forms an oxo group; R8 and R9 represent H, a hydroxyl group, a hydroxymethyl group or an acetoxymethyl group; R10 represents a hydrocarbon group having 5 to 60 carbon atoms which is optionally substituted with a hydroxyl group and the like; and R11 represents H or a hydrocarbon group having 1 to 8 carbon atoms which optionally have a substituent such as a hydroxyl group and the like).
Owner:KAO CORP
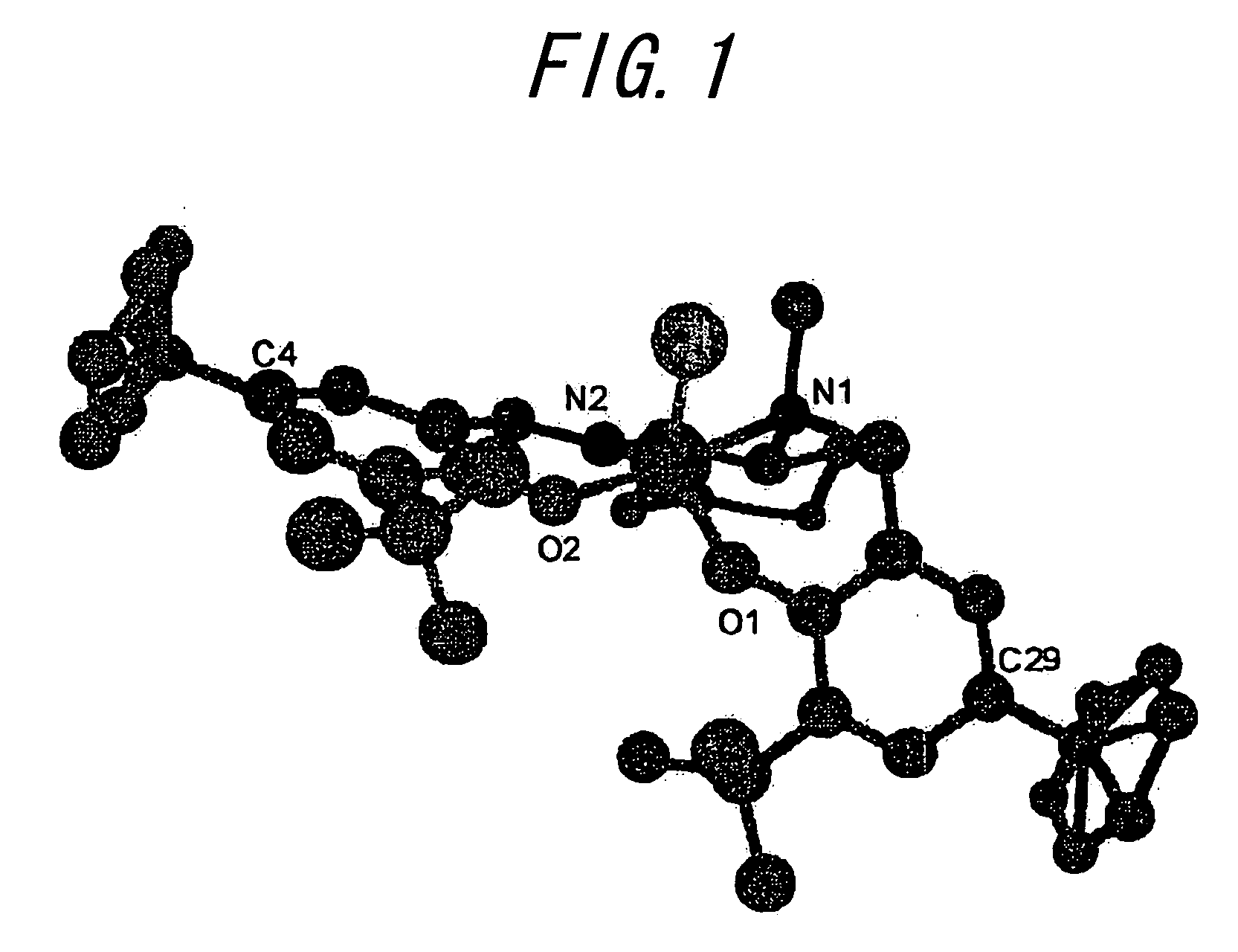
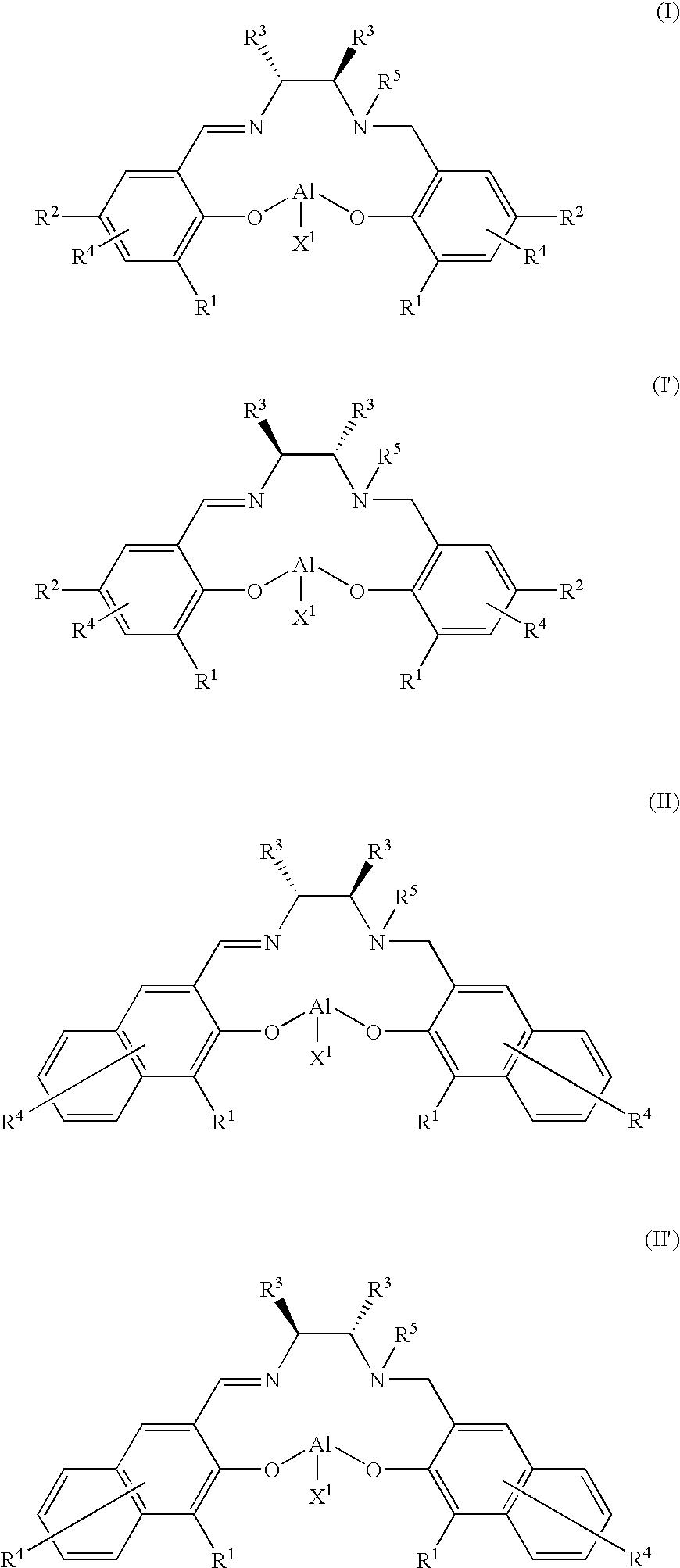
Optically Active Alpha-Hydroxyphosphonic Acid, Its Derivatives and Production Method thereof, Optically Active Aluminum (Salalen) Complex and Production Method Thereof, and Production Method of Salalen Ligand
InactiveUS20090099381A1High enantioselectivityEfficient CatalysisIsocyanic acid derivatives preparationOrganic compound preparationTosylhydrazoneEnantio selectivity
The present invention relates to a production method capable of producing an optically active α-hydroxyphosphonic acid and its derivatives with sufficiently high enantioselectivity not only for aromatic aldehydes but also for aliphatic aldehydes, and more specifically to a method of producing an optically active α-hydroxyphosphonic acid and its derivatives, characterized in that an optically active aluminum(salalen) complex represented by any one of the following formulae (I), (I′), (II) and (II′):[wherein R1s are each alkyl group or aryl group independently; R2s are each alkyl group or aryl group independently; R3s are each alkyl group or aryl group independently, and two R3s may bond with each other to form a ring; R4s are each hydrogen atom, halogen atom, alkyl group, alkoxy group, nitro group, or cyano group independently; R5 is alkyl group; and X1 is halogen atom, alkyl group, alkoxy group, acetoxy group or toluenesulfonyloxy group] is used as a catalyst to asymmetrically hydrophosphonylate an aldehyde with phosphonic acid or its derivatives.
Owner:JAPAN SCI & TECH CORP
Room temperature crosslinkage and curable styrene-acrylate emulsion, and its preparation method
ActiveCN103130948AImprove corrosion resistanceImprove mechanical propertiesAnti-corrosive paintsEpoxy resin coatingsSolventChemistry
The invention discloses a room temperature crosslinkable and curable styrene-acrylate emulsion, and its preparation method. The styrene-acrylate emulsion adopts a non-APEO emulsifier to emulsify, proper amounts of phosphate groups, hydroxy groups, siloxane groups, epoxide groups and acetylacetoxy groups are introduced to a system to make the adhesion of a film of a styrene-acrylate emulsion anti-corrosion coating reach first order, and the coating cures at 25DEG C for 6 days to form the film, so the salt mist resistance is not less than 240h, the impact resistance is 50Kg / cm, and excellent anti-corrosion performance and mechanical performances are possessed; and the substitution of traditional organic solvents by a water solvent reduces the VOC discharge, accords with a 8E' principle, and reaches environmental protection and energy saving purposes.
Owner:广东优贝精细化工有限公司
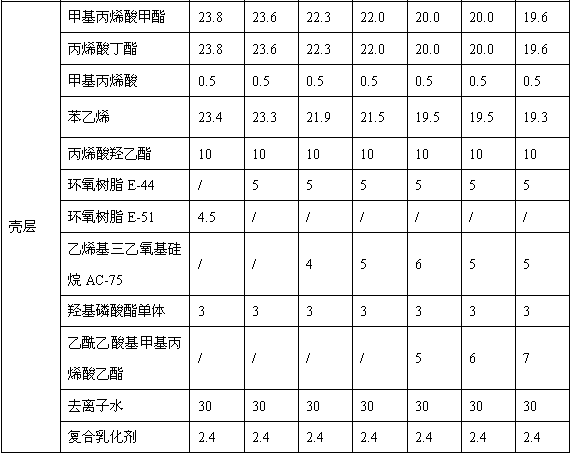
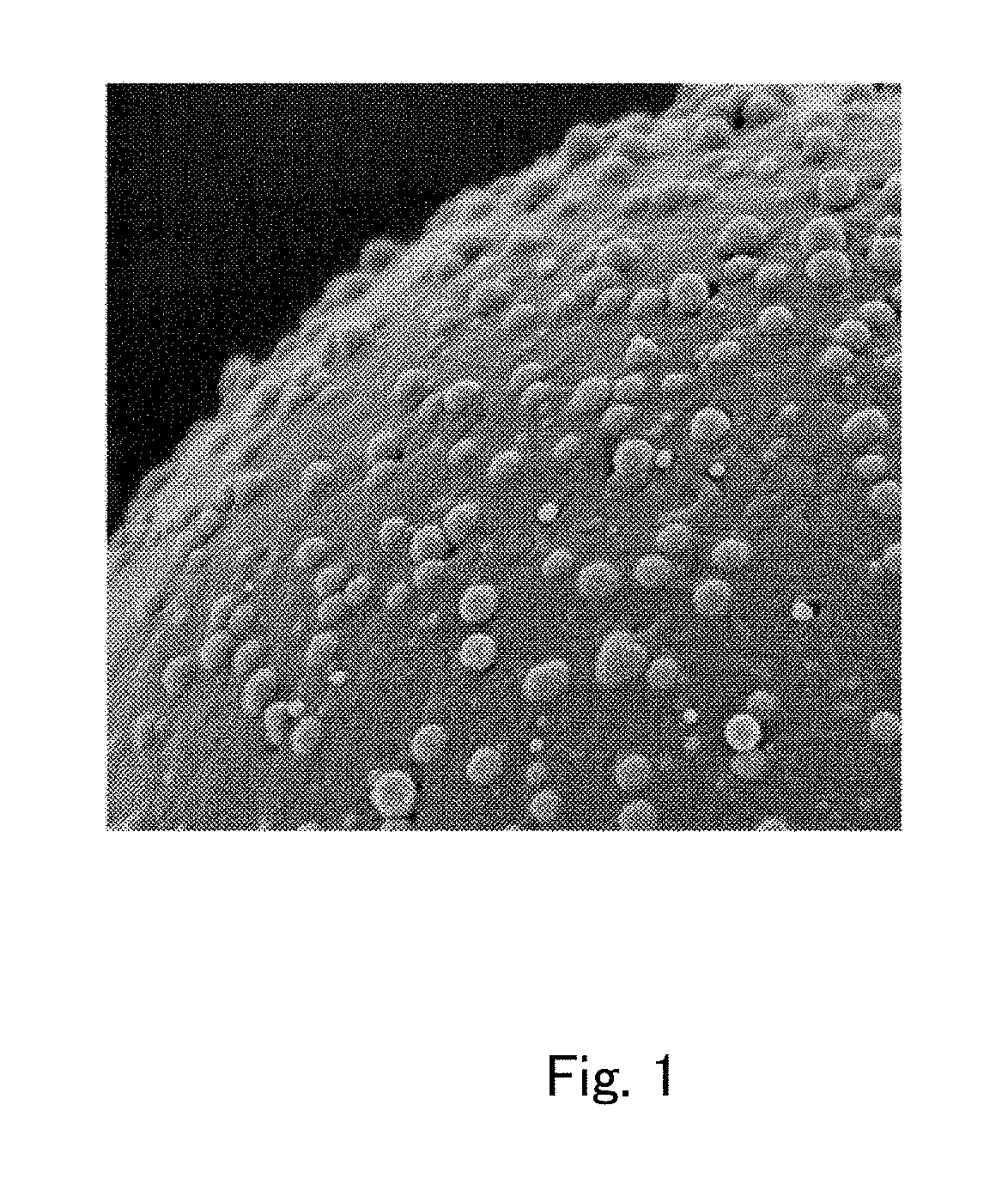
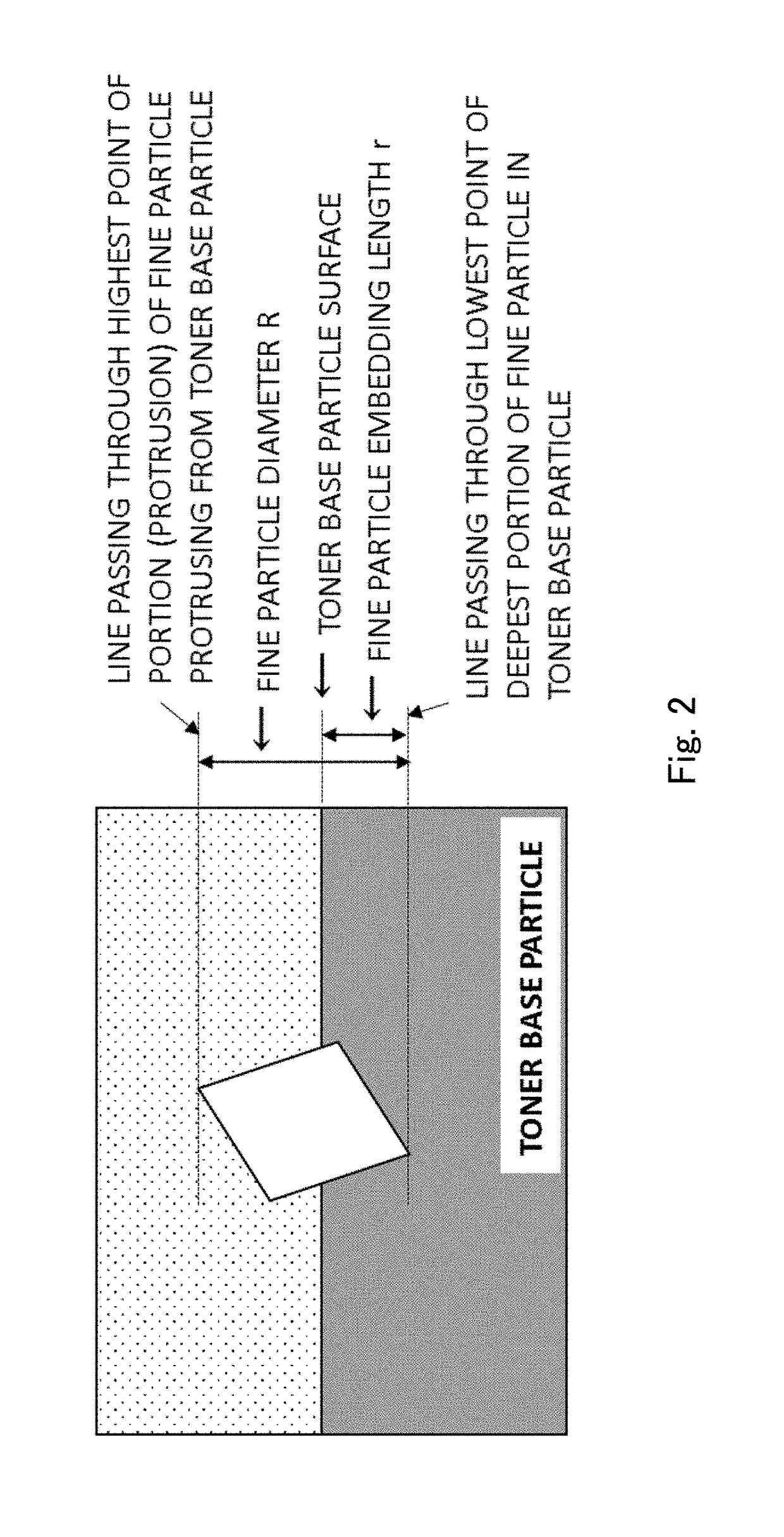
Toner
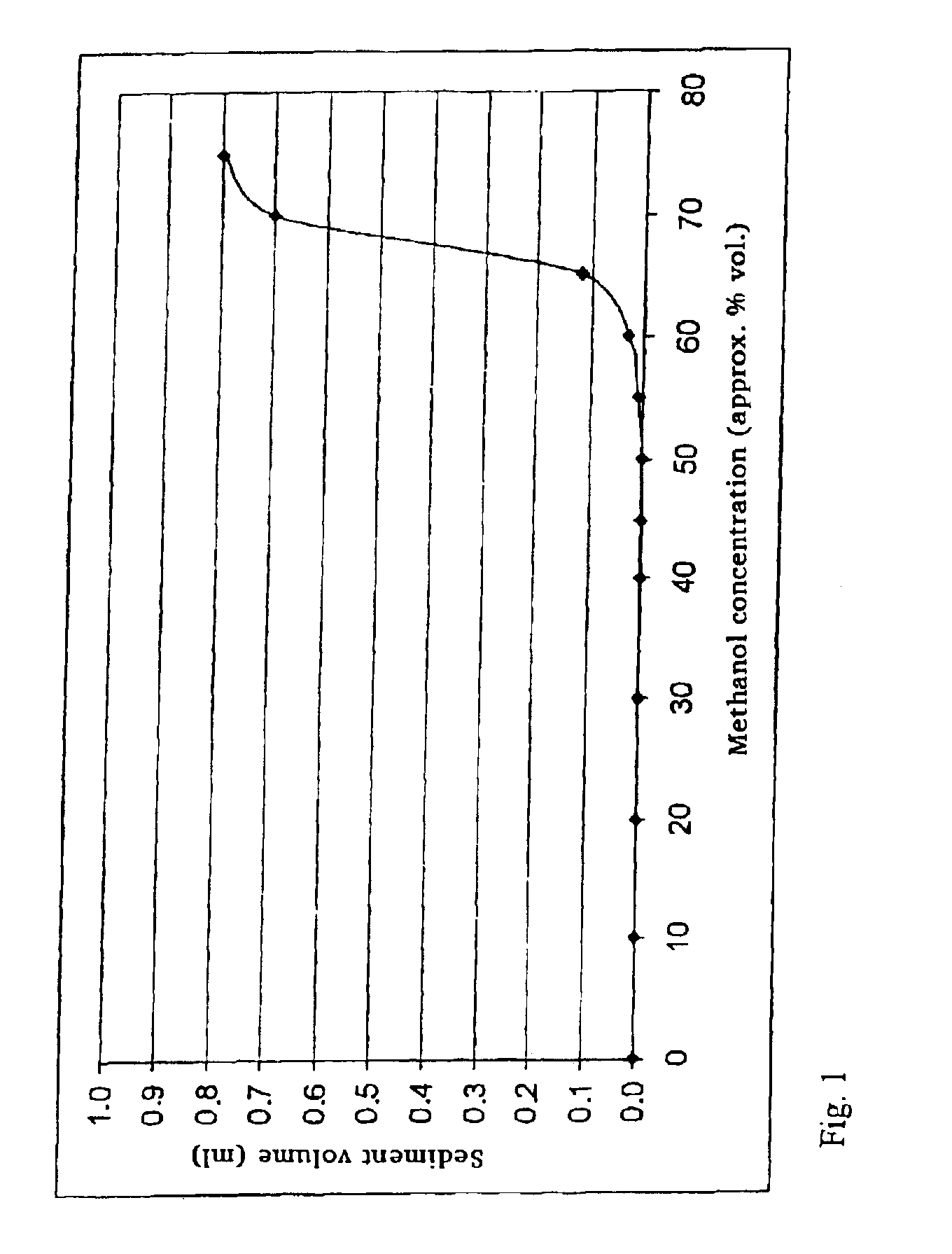
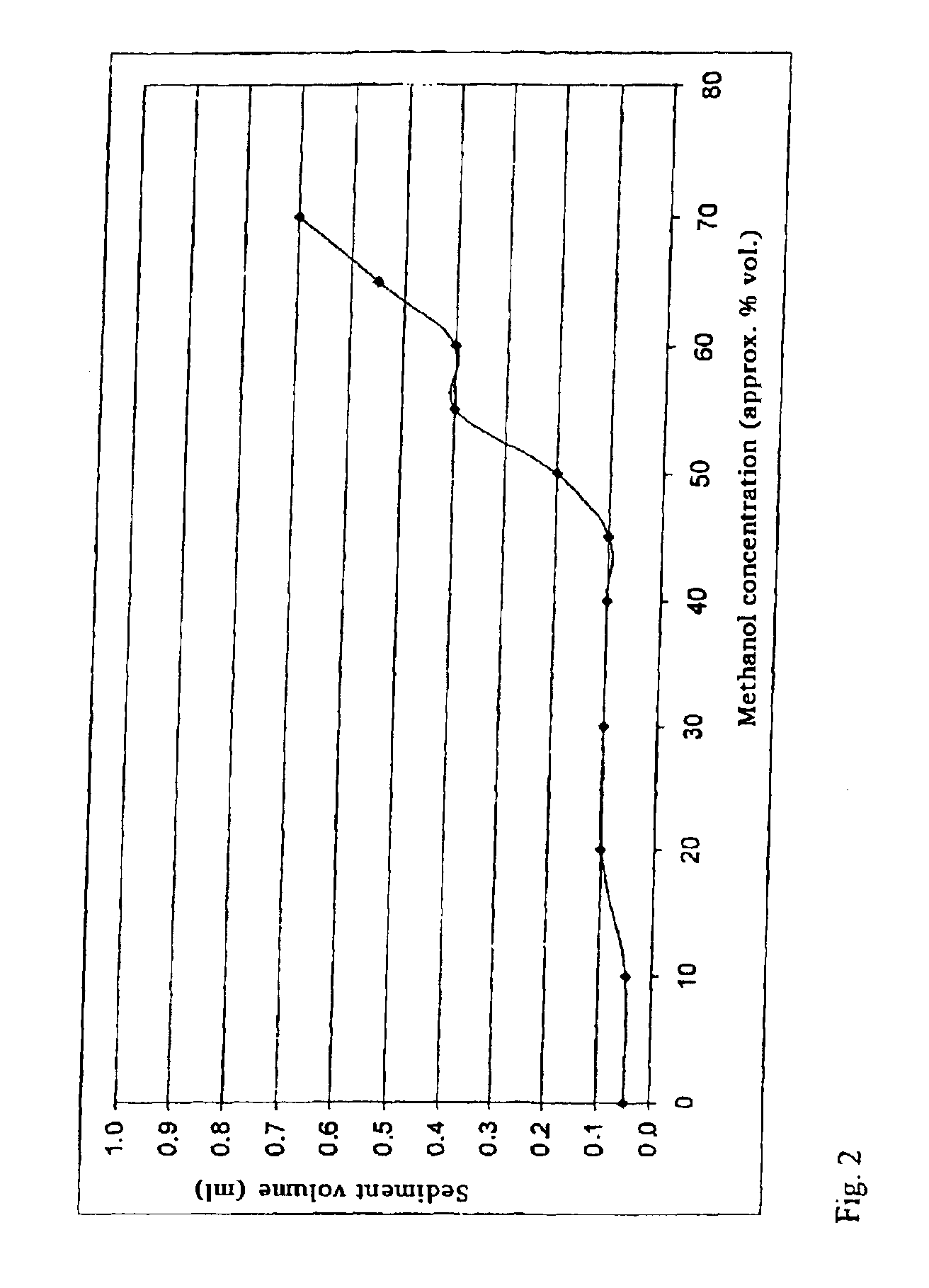
Provided is a toner comprising a toner particle including a toner base particle and fine particles present on a surface of the toner base particle, wherein each of the fine particles includes a core fine particle and a condensate of at least one organosilicon compound selected from the group consisting of organosilicon compounds represented by specific structural formulas, the condensate coating the surface of the core fine particle, and in a wettability test of the toner with respect to a methanol / water mixed solvent, a methanol concentration, when a transmittance of light having a wavelength of 780 nm is 50%, is 5.0 to 20.0% by volume:in above Formulas, each of Ra, Rb and Rc independently represents an alkyl group, an alkenyl group, an acetoxy group, an acyl group, an aryl group, or a methacryloxyalkyl group, R1 to R5 each independently represents a halogen atom or an alkoxy group.
Owner:CANON KK
Preparation method for imipenem medicine intermediate 4AA
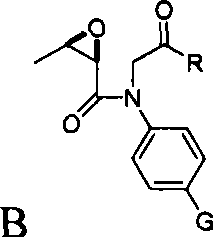
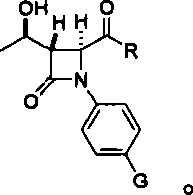
InactiveCN102936262ANo pollution problemRaw materials are cheap and easy to getGroup 4/14 element organic compoundsEpoxyAniline
The invention discloses a preparation method for imipenem medicine intermediate 4AA. The preparation method comprises making 4-substituted aniline into an intermediate A, perform epoxidation on L-threonine to produce (2R, 3R)-epoxy butyric acid; enabling (2R, 3R)-epoxy butyric acid and the intermediate A to undergo a coupling reaction, obtaining an intermediate B, enabling the intermediate B to undergo a cyclization reaction, obtaining an intermediate C, enabling the intermediate C to undergo a hydroxyl protection reaction, obtaining an intermediate D, enabling the intermediate D to be oxidized to form an acetoxy group, and enabling an oxidized product to undergo an ozonation reaction, wherein G is H, F, Cl, Br, a methoxy group, oxethyl or an amino group; and R is H, straight chain alkyl of C1-C6, cyclopropyl, isopropyl, tert-butyl, a phenyl group, p-chlorophenyl, o-chlorophenyl, p-bromophenyl, o-bromophenyl, p-methoxyphenyl, o-methoxyphenyl or m-methoxyphenyl. According to the preparation method, raw materials are cheap and easy to obtain, reaction conditions are mild, the conversion rate and the yield rate are high, and the preparation method is suitable for industrial production.
Owner:ASYMCHEM LAB TIANJIN +4
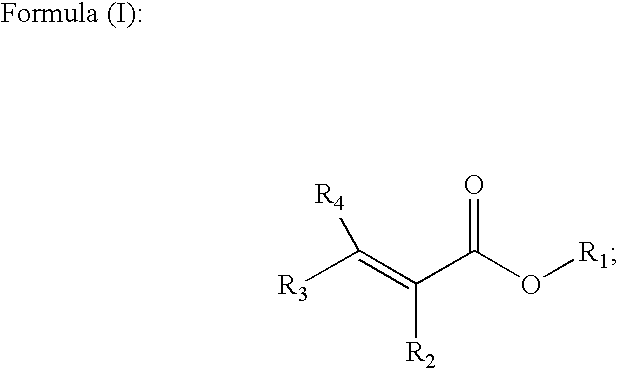
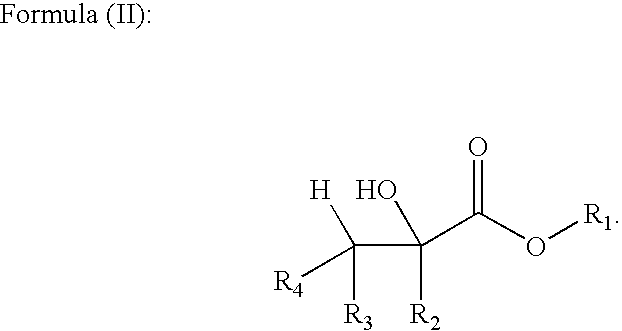
Methods of forming alpha, beta-unsaturated acids and esters
ActiveUS6992209B2Fatty acid chemical modificationFatty acids production/refiningAlpha hydroxy acidAcrylic acid
The invention includes a method of forming an alpha, beta-unsaturated compound. A carboxylic acid is mixed with an alpha-hydroxy acid or an alpha-hydroxy ester and is esterified to form an alpha-acyloxy derivative. The alpha-acyloxy derivative is transformed into an alpha, beta-unsaturated derivative. The invention additionally includes a process of forming an acrylate. Lactic acid or a lactic acid ester is reacted with a first portion of acetic acid in the presence of a first catalyst to produce the corresponding 2-acetoxy propionic acid or ester. A non-reacted portion of the acetic acid is recycled. The 2-acetoxy propionic acid or ester is transferred to a second vessel containing a second catalyst, and acetic acid is liberated from the 2-acetoxy propionic acid or ester to produce a corresponding acrylic acid or acrylate ester. The acid or ester is subsequently esterified by reaction with an alcohol to form a desired acrylate ester.
Owner:BATTELLE MEMORIAL INST
Coating composition and low dielectric siliceous material produced by using same
InactiveUS7754003B2Lower levelHigh mechanical strengthFibre treatmentPretreated surfacesOrganic solventChemical resistance
Owner:MERCK PATENT GMBH
Method for obtaining high-purity 17α-acetoxy-11β-(4-n,n-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione
The invention discloses a method of acquiring high-purity 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione, comprising the following steps: a) putting the acquired crude 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione in a proper solvent system to generate a pure 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione solid; b) separating the 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione solid; and c) carrying out recrystallization on the acquired solid. The compound used as a new oral emergency contraception can be taken in 120 h after unprotected sexual intercourse of women without a reduction of emergency contraception effect with the delay of the time of using drugs, and has good safety and survivability simultaneously.
Owner:SICHUAN UNIV
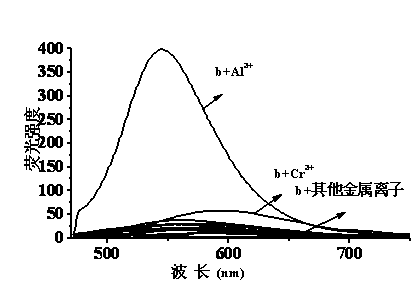
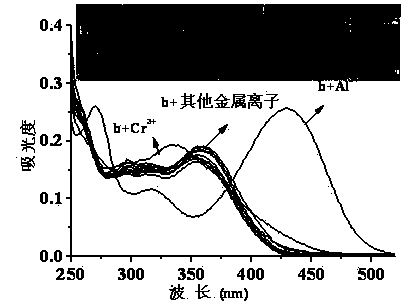
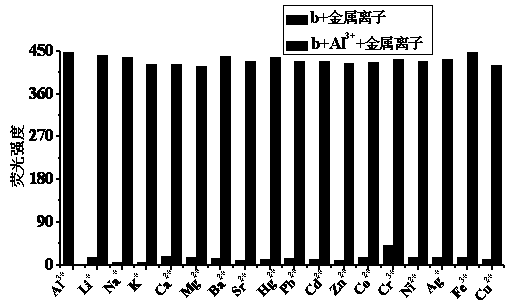
Quinaldine derivative b fluorescent and colorimetric reagent as well as preparation method and application thereof
InactiveCN103641779AOrganic chemistryColor/spectral properties measurementsOrganic synthesisBenzaldehyde
The invention discloses a quinaldine derivative b fluorescent and colorimetric reagent as well as a preparation method and an application thereof and belongs to the field of organic synthesis and analytical chemistry. 8-hydroxyquinaldine serving as a raw material has a Knoevenagel condensation reaction with 2,4-dihydroxy benzaldehyde to obtain an intermediate a, wherein the chemical name of the intermediate a is (E)-2-(2,4-didiacetoxylphenyl)ethenyl-8-acetoxyquinoline; the intermediate a is hydrolyzed in a mixed medium of pyridine and water to obtain a derivative b, wherein the chemical name of the derivative b is (E)-2-(2,4-dihydroxylphenyl)ethenyl-8-hydroxyquinoline. The reaction materials are easily available; the synthesis method is simple; a target product can be obtained by two-step reaction. In a special solvent medium, the derivative b can act as fluorescent and colorimetric reagent for detecting Al<3+> and F-. The structural formula of the derivative is shown in the specification.
Owner:GUIZHOU UNIV
Emulsified composition
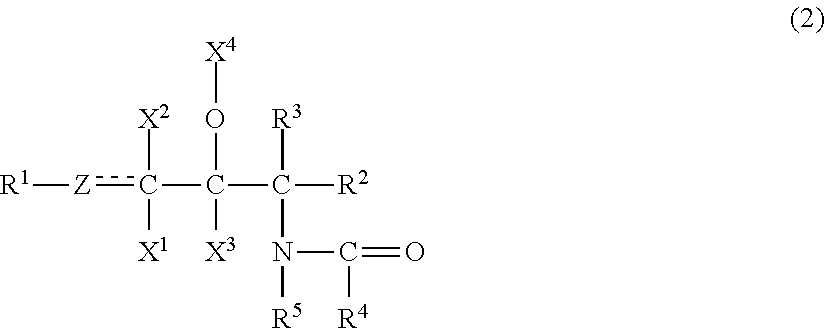
ActiveUS20120108661A1High steric regularityImprove water retentionCosmetic preparationsBiocideAlcohol sugarsMethyl group
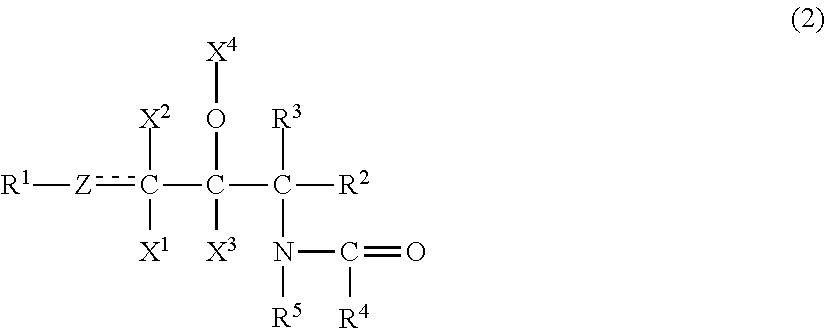
The emulsion composition of the present invention contains(A) 0.001 to 10 wt. % of an organic compound having two or more hydroxyl groups, an inorganic value of 220 to 450, and an organic value of 300 to 1,000;(B) 0.001 to 10 wt. % of an organic compound having one hydroxyl group, an inorganic value of 100 to 200, and an organic value of 280 to 700;(C) 0.001 to 10 wt. % of a compound represented by formula (2):wherein R1 is a C4 to C30 hydrocarbon group; Z is a methylene group, a methine group, or an oxygen atom; X1, X2, X3 is a hydrogen atom, a hydroxyl group, or an acetoxy group; X4 is a hydrogen atom, an acetyl group, or a glyceryl group; each of R2 and R3 a hydrogen atom, a hydroxyl group, a hydroxymethyl group, or an acetoxymethyl group; R4 is a C5 to C60 hydrocarbon group; and R5 is a hydrogen atom or a hydrocarbon group containing 1 to 30 carbon atoms in total;(D) 0.00012 to 10 wt. % of at least one compound selected from the group consisting of a nonionic surfactant having a polyoxyethylene group and an HLB of 10 or higher, an ionic surfactant, and a sphingosine salt;(E) 0.003 to 15 wt. % of at least one compound selected from the group consisting of a sugar alcohol selected from the group consisting of erythritol, threitol, xylitol, and mannitol, a disaccharide, and a trisaccharide; and(F) water.
Owner:KAO CORP
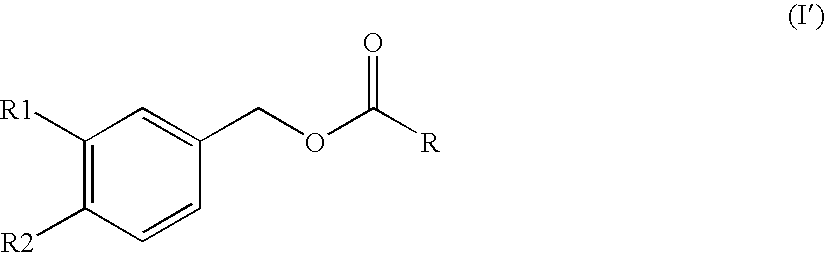
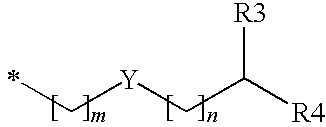
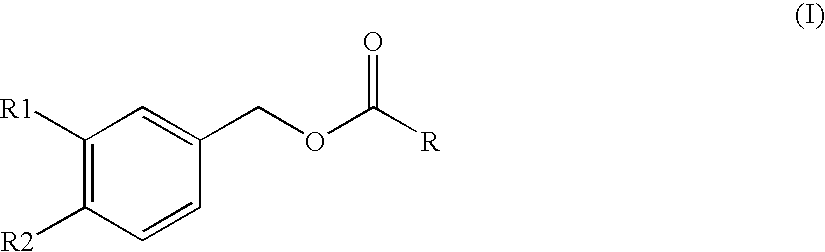
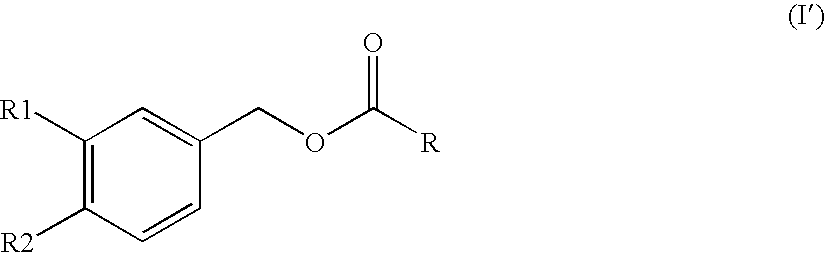
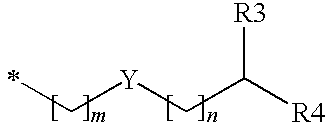
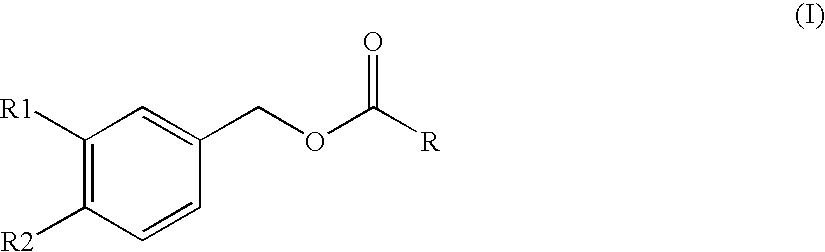
Substituted benzyl ester derivative and use thereof
The present invention relates to a pharmaceutical composition, a food composition or a cosmetic composition, containing one or more kinds of a compound represented by the following formula (I′)wherein R1 is a hydrogen atom, a hydroxyl group, a methoxy group or an ethoxy group, R2 is a hydroxyl group, a methoxy group or an acetoxy group, or R1 and R2 in combination optionally form a methylenedioxy group,R is represented by the following formulawherein Y is an ethylene group or a vinylene group, m and n are each an integer of 0 to 7, which satisfy m+n=2 to 8, and R3 and R4 are each independently a hydrogen atom, a methyl group or an ethyl group,provided that,(1) when R1 is a methoxy group, then R2 is not a hydroxyl group; and(2) when R1 is a hydroxyl group, then R2 is not a hydroxyl group and an acetoxy group.According to the present invention, a stable capsinoid derivative is provided, and a pharmaceutical composition, a food composition, a cosmetic composition and the like containing the derivative as an active ingredient can be provided.
Owner:AJINOMOTO CO INC
Method for hydroformylation of vinyl acetate
ActiveCN106565476AHigh selectivityImprove conversion ratePreparation by carbon monoxide or formate reactionFormylation reactionReaction temperature
The invention relates to a method for hydroformylation of vinyl acetate. The method comprises the step of enabling the vinyl acetate to be in contact with carbon monoxide and hydrogen gas in the presence of a solvent and a catalyst system, wherein the catalyst system contains a rhodium complex and a phosphine ligand; the rhodium complex is one or more selected from rhodium(triphenylphosphine)carbonyl acetylacetonate, rhodium acetylacetone and chlorocarbonylbis(triphenylphosphine)rhodium; and the phosphine ligand is one or more selected from phosphine-containing organic matters represented by the following formulae shown in the description, wherein Ar in the formulae (1, 3 and 4) is substituted or unsubstituted aryl, M in the formula (2) is hydrogen, halogen element or substituted or unsubstituted alkyl, and t in the formula (3) is an integer from 2 to 10. According to the method, the selectivity to 3-acetoxypropanal in a hydroformylation reaction process of the vinyl acetate can be improved while a relatively high conversion ratio is guaranteed under the conditions of relatively low reaction temperature and reaction pressure.
Owner:CHINA PETROLEUM & CHEM CORP +1
Novel method for preparing rocuronium bromide
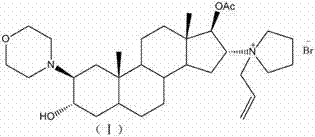
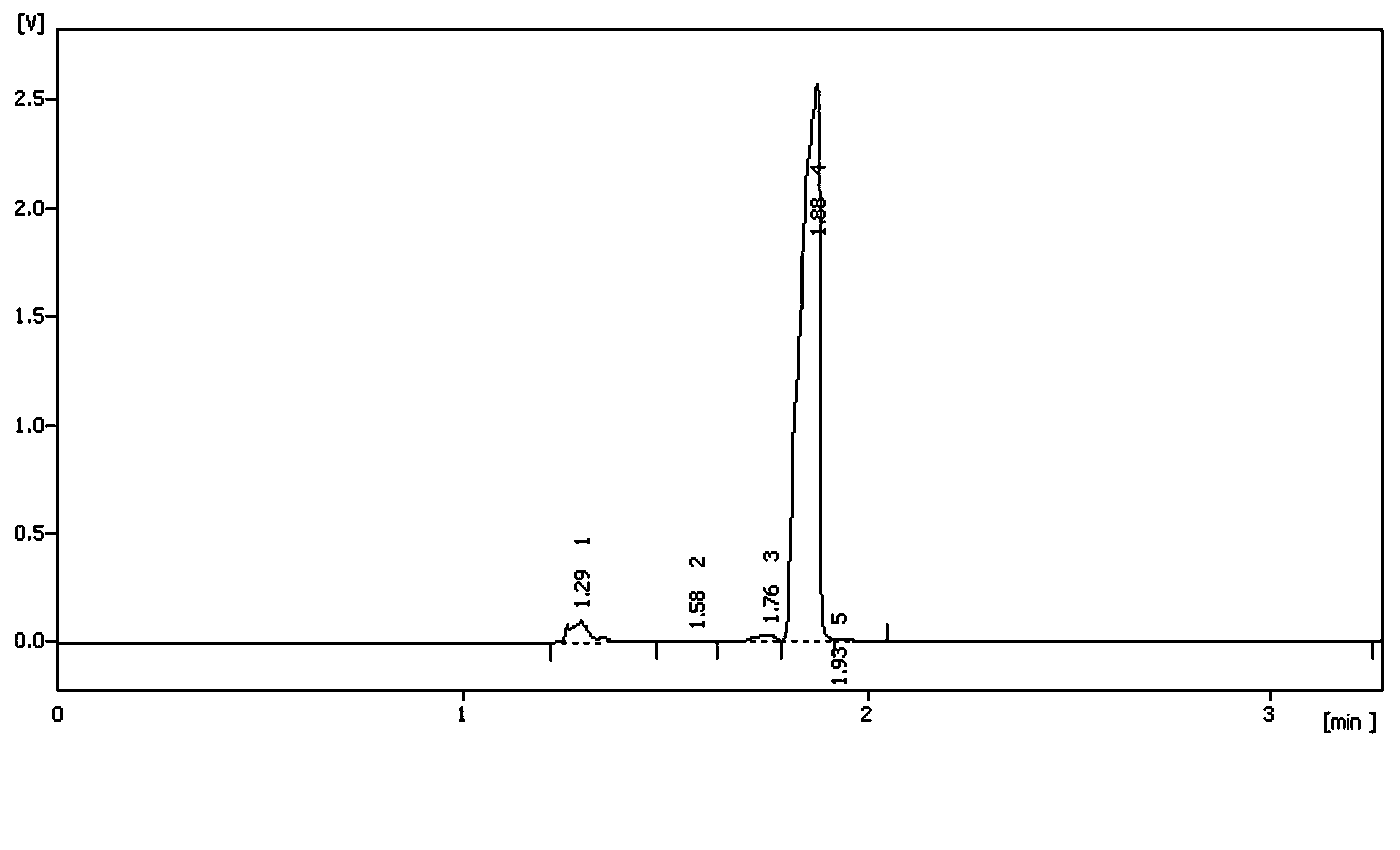
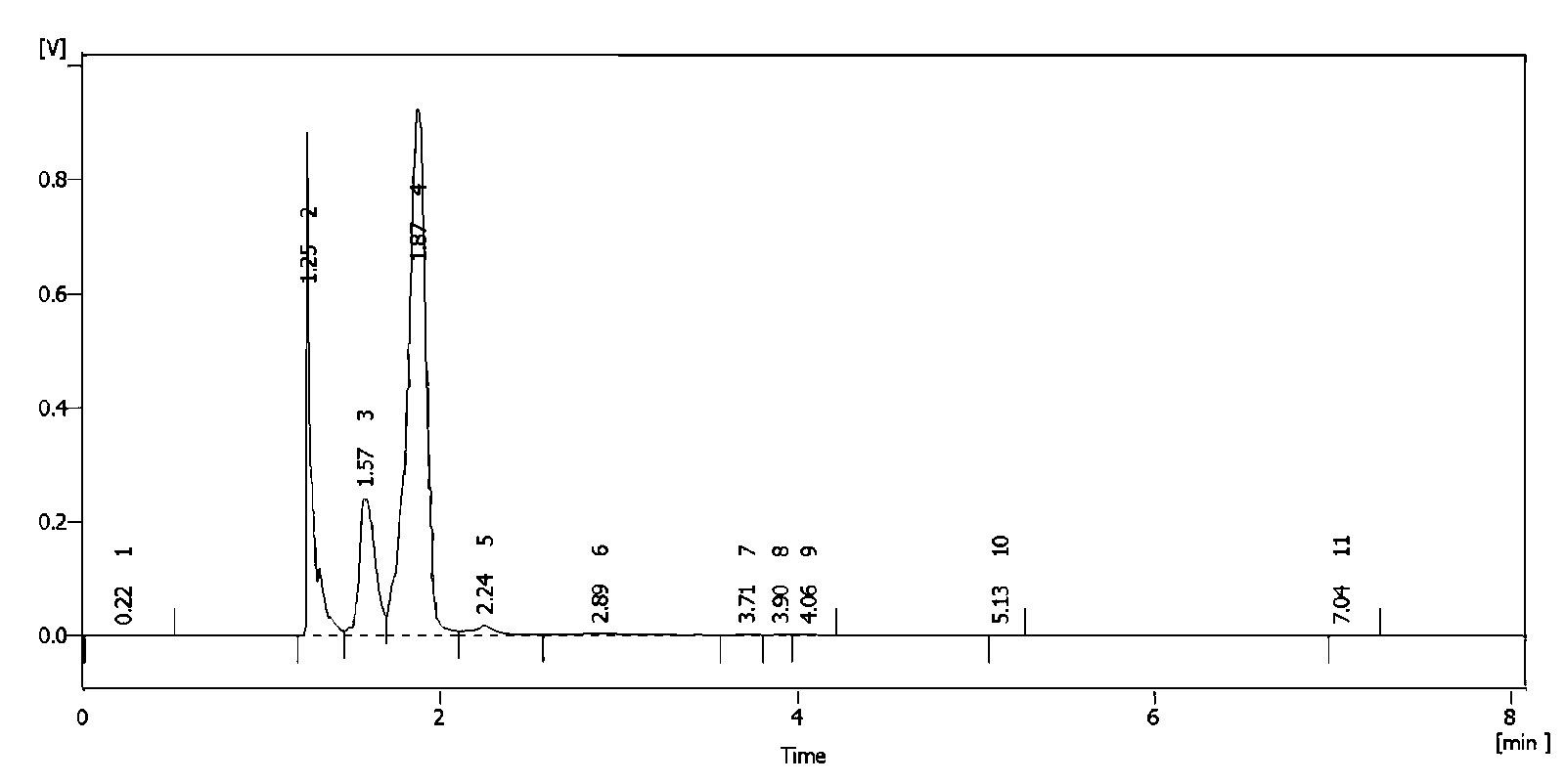
The invention relates to a novel method for preparing rocuronium bromide 1-[17beta-acetoxyl-3alpha-hydroxyl-2beta-(4-morpholinyl)-androstane-16beta-yl]-1-(2-propenyl) pyrrole bromide, the problem of chemoselectivity of pyrrolidine open epoxy in an original line is solved, generation of byproducts is avoided, reaction yield is greatly improved, the production cost is reduced, column chromatography separation is avoided, and aftertreatment purification is implemented easily.
Owner:JIANGSU QINGJIANG PHARMA
Method for preparing 4-acetoxy-2-methyl-2-butenal
ActiveCN103012131ALow costEmission reductionOrganic compound preparationCarboxylic acid esters preparationTrans esterificationButene
The invention discloses a method for preparing 4-acetoxy-2-methyl-2-butenal. The method comprises the following steps of: carrying out a methyl esterification reaction, an ester exchange reaction and an oxidizing reaction on 1-acetoxy-4-chloro-3-methyl-2-butene to obtain a product of 4-acetoxy-2-methyl-2-butene-1-ol. According to the method, the problems of raw material cost and environment friendliness are comprehensively considered, the 4-acetoxy-2-methyl-2-butenal is synthesized through chloride ester through a three-step reaction which is mild in conditions and high in yield, an easily recovered organic solvent is adopted in the reaction of each step, the pollutant discharge is greatly reduced, the pollution problem is effectively solved due to the improvement of a synthetic route, the yield is improved, the raw material cost is reduced, the effective resource treatment is realized, and the method is suitable for industrial production.
Owner:GUANGZHOU LEADER BIO TECH
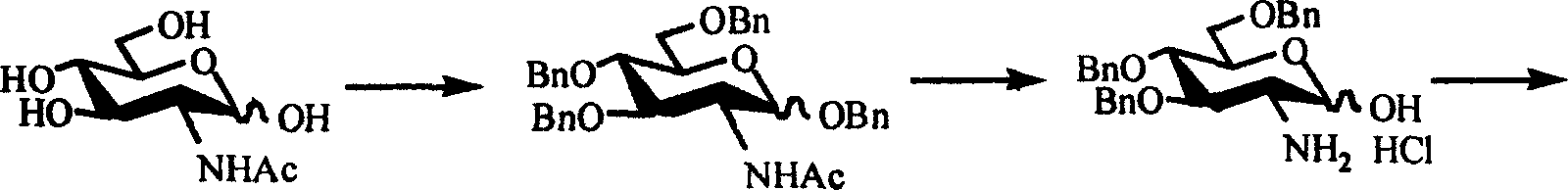
Acetamido glucose devicative of indomethacin and its synthetic method and use
InactiveCN1616474AAvoid disadvantagesOrganic active ingredientsSugar derivativesGlucose polymersD-Glucose
The present invention relates to acetamido glucose derivatives of indomethacin and their synthesis process and use. The kinds of acetamido glucose derivatives of indomethacin prepared with indomethacin and acetamido glucose are 1-O-[1-(4-chloro-benzoyl)-5 -methoxy-2-methyl-3-indolyl-acetoxy]-2-acetylamino-2-deoxy-alpha-D-glucose and 6-O-[1-(4-chloro-benzoyl)-5-methoxy-2-methyl-3-indolyl-acetoxy]-2-acetylamino-2-deoxy-alpha-D-glucose. The derivatives of the present invention has the activity of inhibiting mouse ear dropsy caused by croton oil and may be used in preparing antiphlogistic medicine.
Owner:OCEAN UNIV OF CHINA
Substituted benzyl ester derivative and use thereof
The present invention relates to a pharmaceutical composition, a food composition or a cosmetic composition, containing one or more kinds of a compound represented by the following formula (I′)wherein R1 is a hydrogen atom, a hydroxyl group, a methoxy group or an ethoxy group, R2 is a hydroxyl group, a methoxy group or an acetoxy group, or R1 and R2 in combination optionally form a methylenedioxy group,R is represented by the following formulawherein Y is an ethylene group or a vinylene group, m and n are each an integer of 0 to 7, which satisfy m+n=2 to 8, and R3 and R4 are each independently a hydrogen atom, a methyl group or an ethyl group,provided that,(1) when R1 is a methoxy group, then R2 is not a hydroxyl group; and(2) when R1 is a hydroxyl group, then R2 is not a hydroxyl group and an acetoxy group.According to the present invention, a stable capsinoid derivative is provided, and a pharmaceutical composition, a food composition, a cosmetic composition and the like containing the derivative as an active ingredient can be provided.
Owner:AJINOMOTO CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)