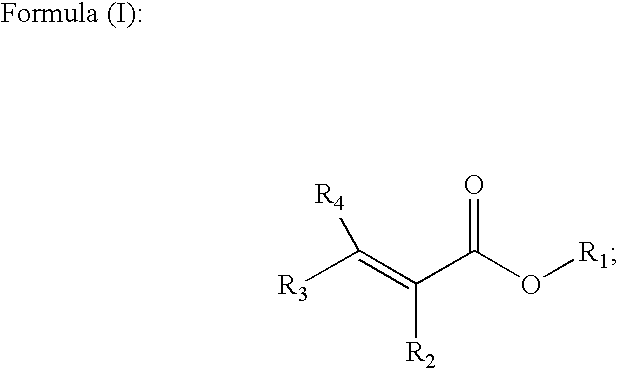
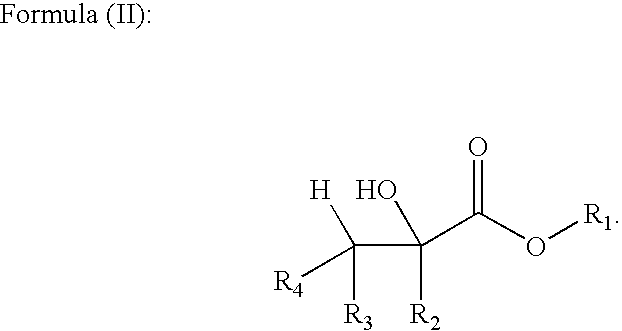
Methods of forming alpha, beta-unsaturated acids and esters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Batch Production of Alpha-Acyloxy Products
[0041]Anhydrous methyl lactate (1 ml) was combined with glacial acetic acid (15 ml) in a reaction vessel to form a solution. Concentrated sulfuric acid (0.1 ml) was added and the resulting solution was heated to a reflux temperature of about 73° C. at a pressure of 150 mm Hg. The resulting condensate was dripped into a soxlet containing SiO2 (disposed between the reaction vessel and a reflux condenser) to remove water from the vapor and recycle dried acetic acid back into the reaction vessel. After one hour, the reaction was terminated by cooling the solution to room temperature and venting the vessel to atmospheric pressure. Nuclear magnetic resonance (NMR) analysis of the solution indicated a product ratio of 2-acetoxy propionic acid to its methyl ester (methyl 2-acetoxy propionate) of about 1:1. Methyl acetate was also formed as a byproduct. The combined acid and methyl ester product yield was approximately 95% of theoretical (estimated b...
example 2
Production of Alpha-Acyloxy Products Utilizing Continuous Flow Simulation
[0044]In a distillation reactor at atmospheric pressure, an acid solution formed by combining 30 ml of glacial acetic acid with 0.5 ml concentrated sulfuric acid was maintained at approximately 114° C. A reactant solution was prepared by combining 5.0 ml of an 85% lactic acid solution, by weight (15% water) with 20 ml of glacial acetic acid, and was added dropwise to the distillation reactor. The resulting distillate was collected and analyzed by NMR. After 1 hour, the 2-acetoxy propionic acid product yield was greater than about 90%. A small amount of dimer or polymer was also detected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
| Elevation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com