Patents
Literature
769 results about "Hemagglutinin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
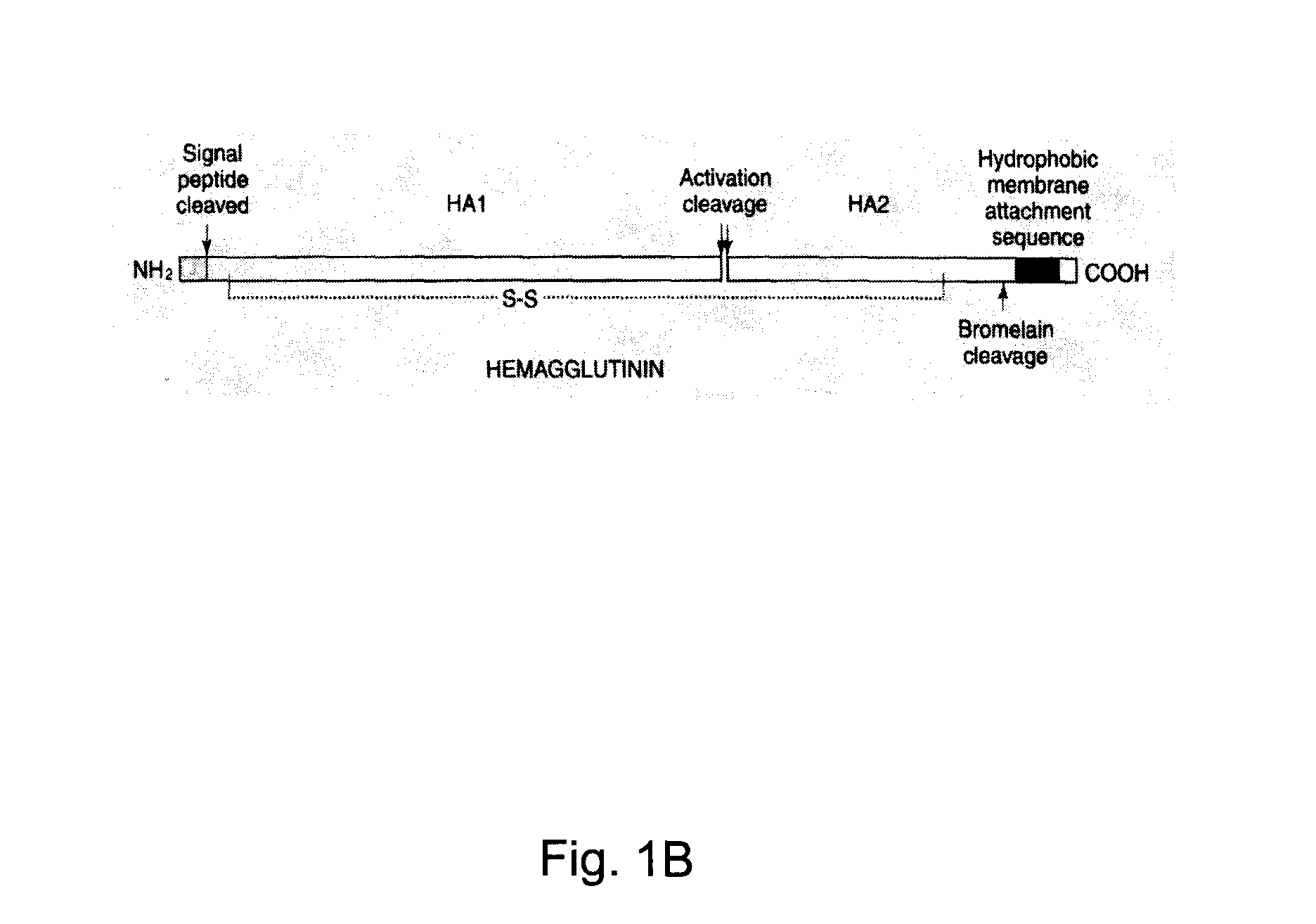
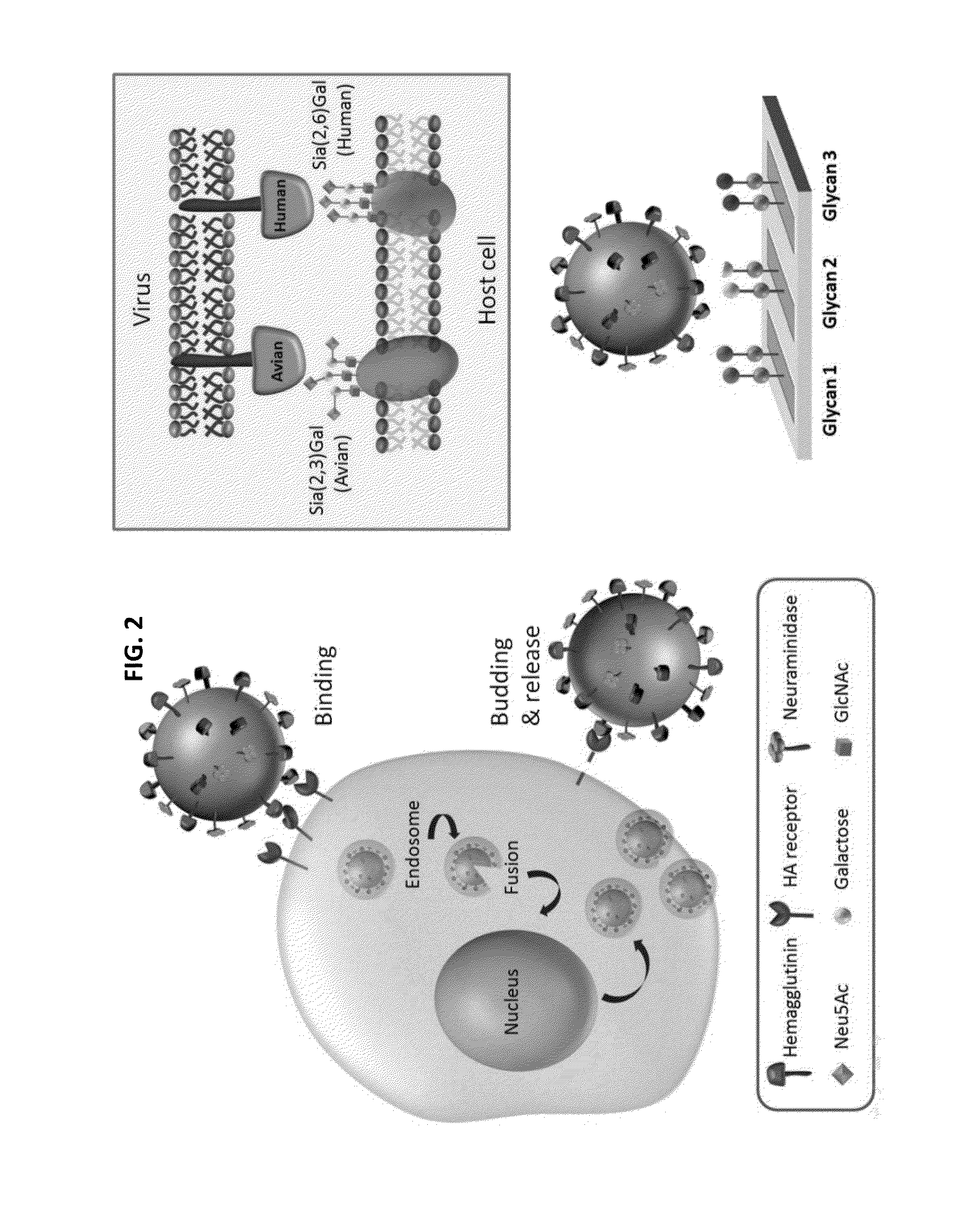
Hemagglutinin or haemagglutinin (British English; both /ˌhɛməˈɡluːtɪn/) refers to glycoproteins which cause red blood cells (RBCs) to agglutinate. This process is called hemagglutination or haemagglutination.
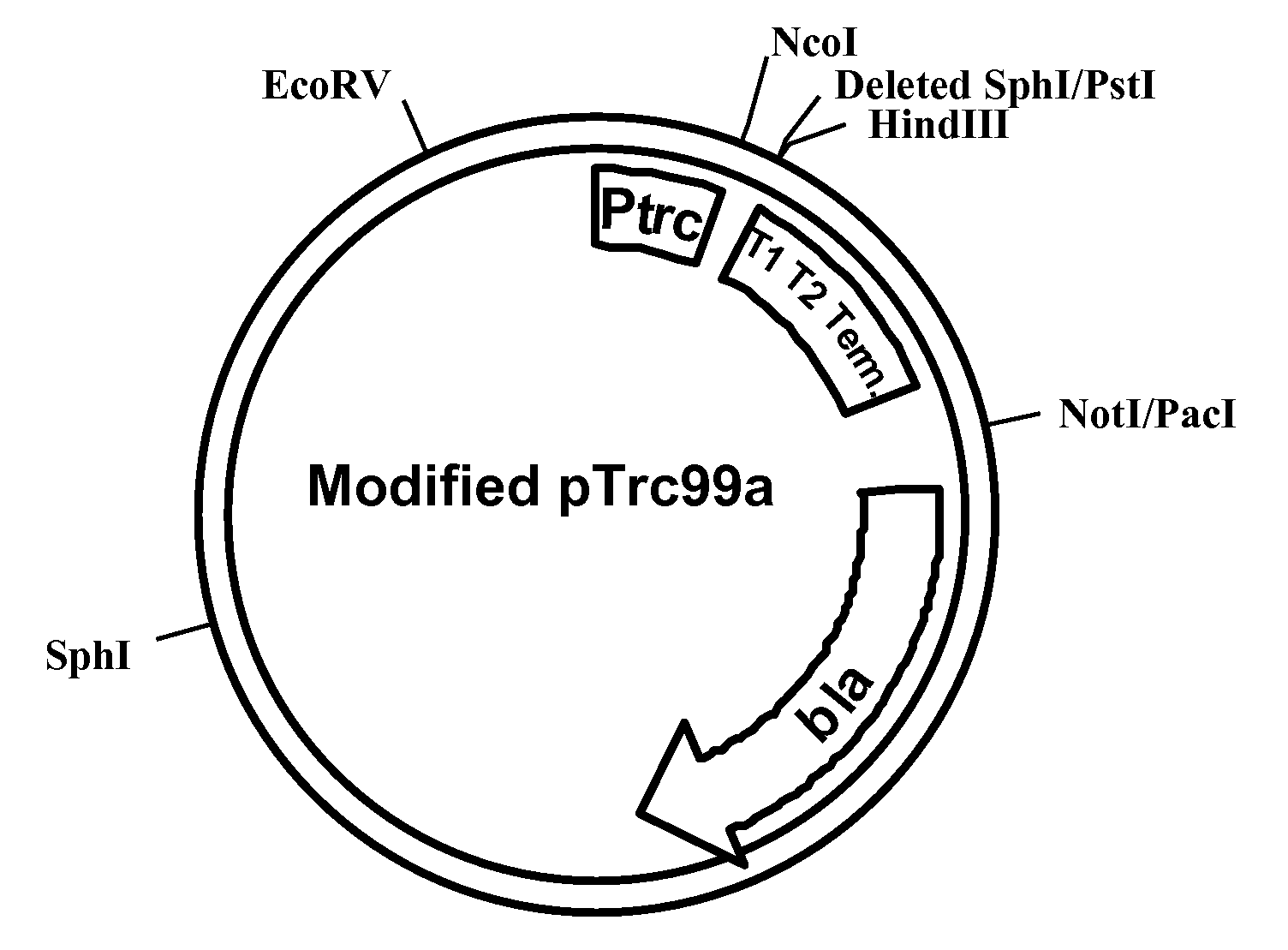
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
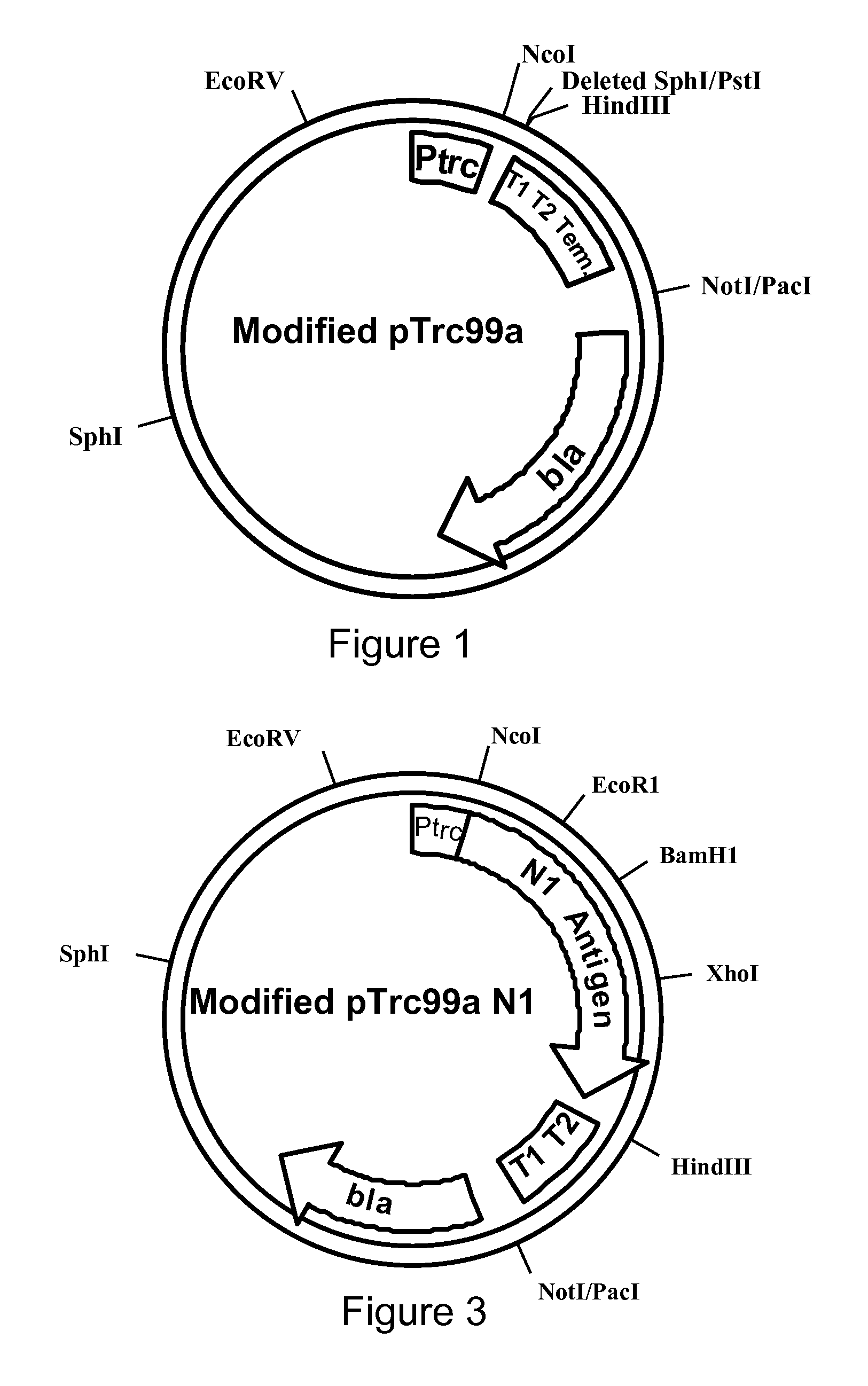
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050042229A1Efficient productionSsRNA viruses negative-senseHydrolasesHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
DNA transfection system for the generation of infectious influenza virus
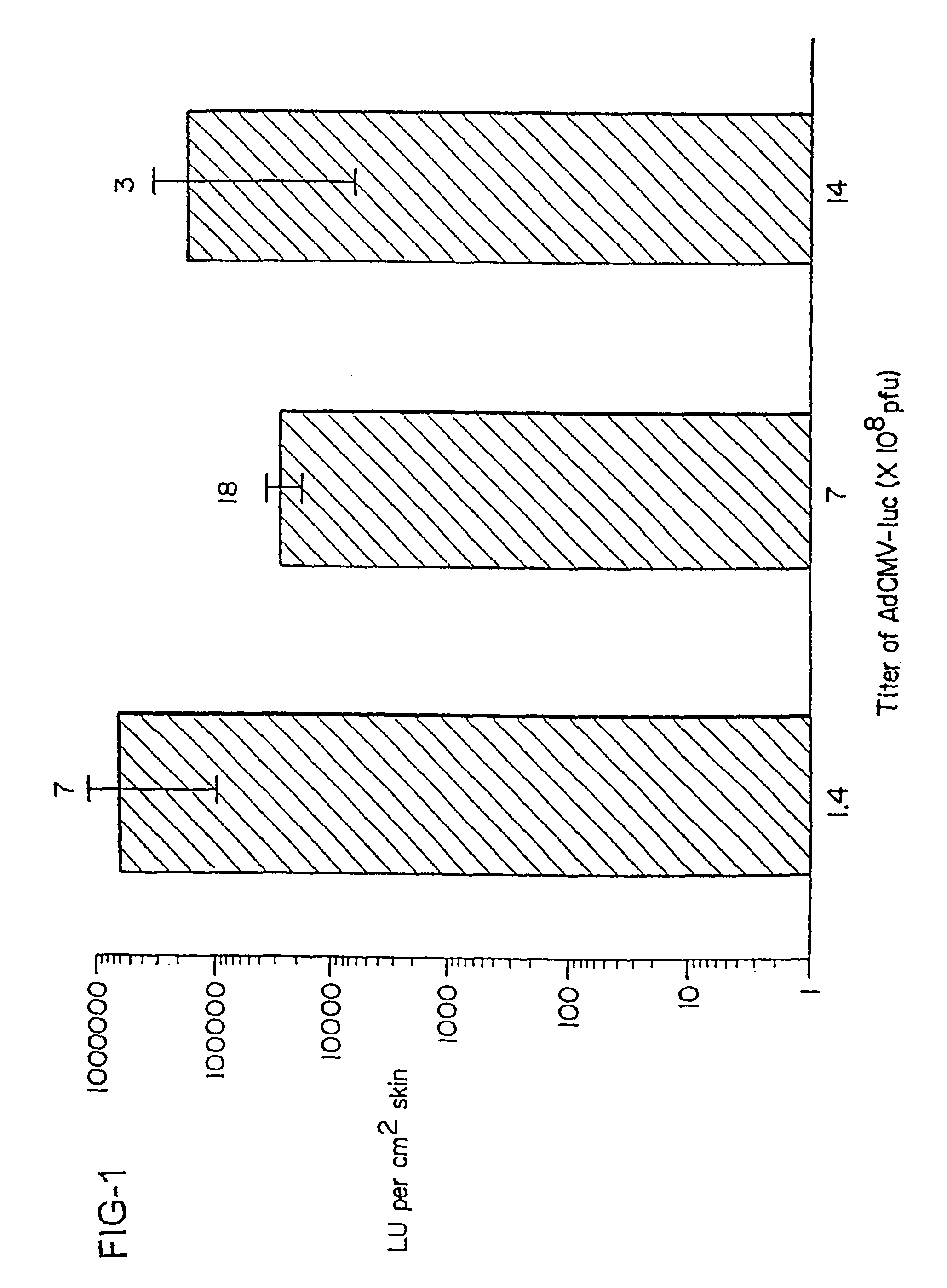
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuramimidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Poxvirus-canine distemper virus (CDV) or measles virus recombinants and compositions and methods employing the recombinants
InactiveUS6309647B1Improve securityImprove security levelSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininCanine distemper virus Antigen
Attenuated recombinant viruses containing DNA coding for a canine distemper virus antigen or measles M or N antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: canine distemper virus fusion protein, canine distemper virus hemagglutinin glycoprotein, canine distemper nucleocaspid protein, canine distemper matrix protein, measles virus nucleocaspid protein, and measles virus matrix protein. The recombinant viruses and gene products therefrom are useful for eliciting protection against canine distemper virus and / or measles virus, and, the gene products and antibodies elicited thereby are useful in assays. Additionally, DNA from the recombinants is used for probes or for generating PCR primers.
Owner:AVENTIS PASTEUR LTD
Combination vaccine
ActiveUS20160166678A1SsRNA viruses negative-senseSugar derivativesHemagglutininFamily Orthomyxoviridae
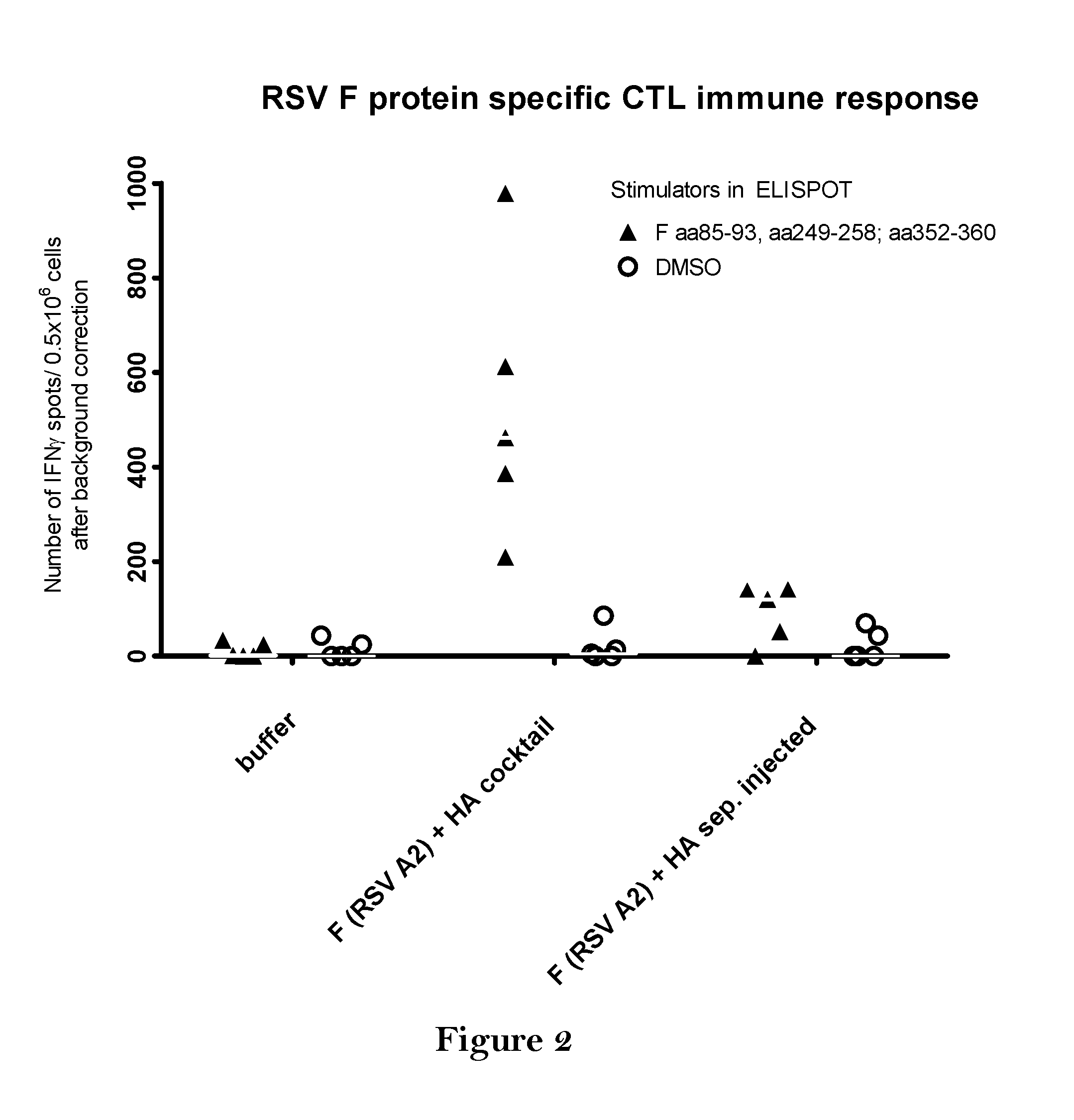
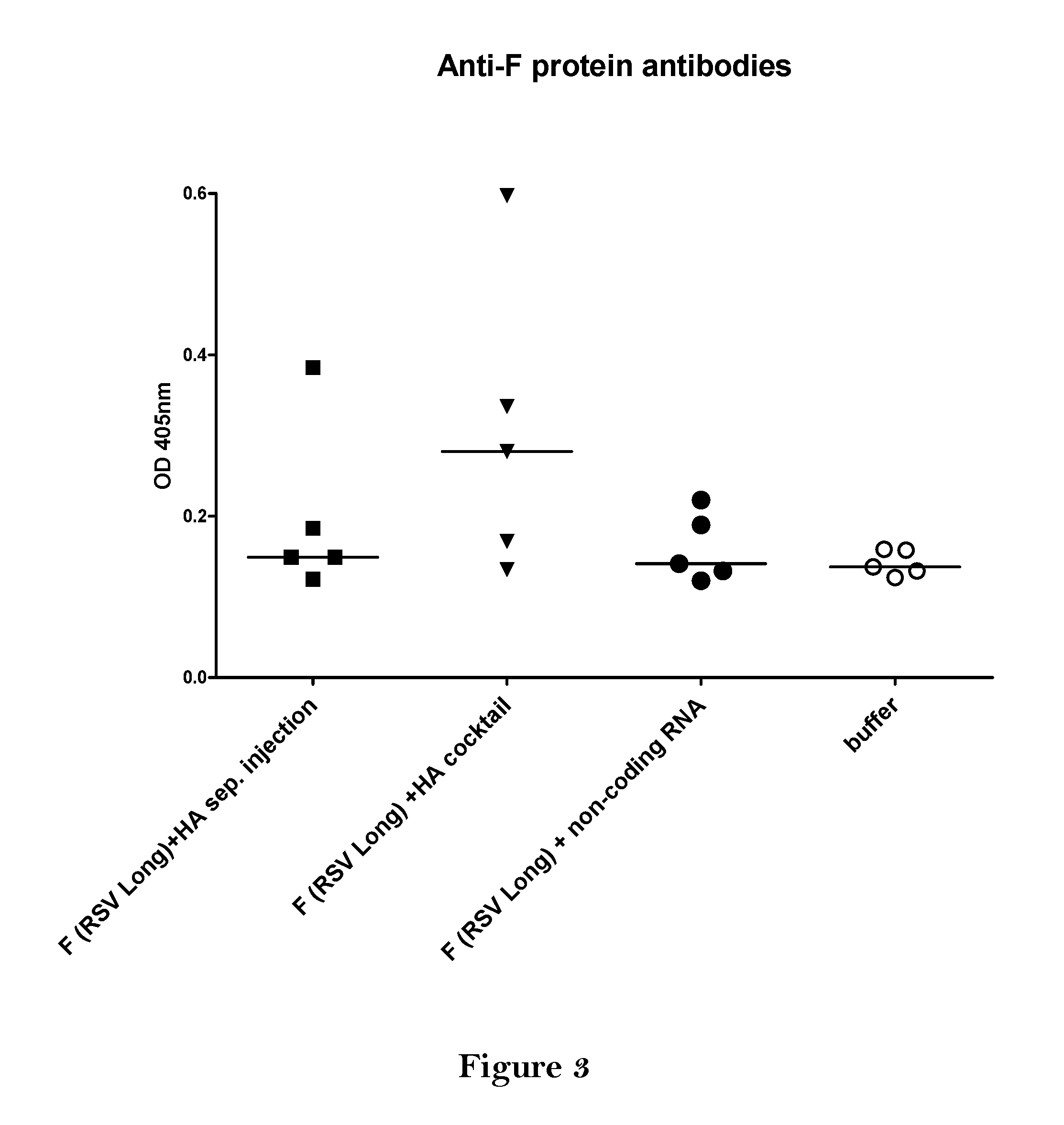
The present invention relates to a vaccine, especially a combination vaccine providing at least a first and a second antigenic function, the combination vaccine comprising at least one RNA encoding at least one or more proteins or fragments, variants or derivatives of proteins awarding antigenic function, wherein the first antigenic function being a Fusion (F) protein or a fragment, variant or derivative of a Fusion (F) protein derived from the virus family Paramyxoviridae and the second antigenic function being an Hemagglutinin (HA) protein or a fragment, variant or derivative of an Hemagglutinin (HA) protein derived from the virus family Orthomyxoviridae. Furthermore, the present invention is directed to a kit or kit of parts comprising the components of said combination vaccine and to said combination vaccine for use in a method of prophylactic or therapeutic treatment of diseases, particularly in the prevention or treatment of infectious diseases like RSV and influenza.
Owner:CUREVAC SE
Influenza virus vaccines and uses thereof
ActiveUS20100297174A1Reduce severityImprove survivalSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Owner:MT SINAI SCHOOL OF MEDICINE
Synthetic nanocarrier vaccines comprising peptides obtained or derived from human influenza a virus hemagglutinin
InactiveUS20120064110A1SsRNA viruses negative-senseVirus peptidesHemagglutininHuman Influenza A Virus
This invention relates to compositions and methods that can be used immunize a subject against influenza. Generally, the compositions and methods include peptides obtained or derived from human influenza A virus hemagglutinin.
Owner:SELECTA BIOSCI
Influenza Hemagglutinin And Neuraminidase Variants
InactiveUS20080069821A1Extended half-lifeReducing/increasing polypeptide antigenicityFungiVirusesHemagglutininNeuraminidase
Owner:MEDIMMUNE LLC +1
DNA transfection system for the generation of infectious influenza virus
InactiveUS20050186563A1Improve effectivenessElicit protective immunitySsRNA viruses negative-senseFungiDual promoterSingle-Stranded RNA
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuraminidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Compositions that include hemagglutinin, methods of making and methods of use thereof
InactiveUS20070224205A1Avoid serious illnessAvoid deathSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininArginine
Compositions comprise a flagellin component or a Toll-like Receptor agonist component that is at least a portion of a flagellin or a Toll-like Receptor agonist, wherein the flagellin component or Toll-like Receptor agonist component includes at least one cysteine residue and whereby the flagellin component or Toll-like Receptor agonist component activates a Toll-like Receptor 5 or Toll-like Receptor. Compositions comprise a flagellin component that is at least a portion of a flagellin, wherein at least one lysine of the flagellin component has been substituted with at least one arginine, serine and histidine, whereby the flagellin component activates Toll-like Receptor 5. Compositions can further include an antigen, such as an influenza antigen, a flavivirus antigen, a pathogen-related antigen, a bacterial capsular antigen and a carrier protein. The compositions are used to stimulate an immune response and a protective immune response in a subject.
Owner:VAXINNATE
Influenza hemagglutinin and neuraminidase variants
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Peptide-based vaccine for influenza
A human synthetic peptide-based influenza vaccine for intranasal administration comprises a mixture of flagella containing at least four epitopes of influenza virus reactive with human cells, each expressed individually in Salmonella flagellin, said influenza virus epitopes being selected from the group consisting of: (i) one B-cell hemagglutinin (HA) epitope; (ii) one T-helper hemagglutinin (HA) or nucleo-protein (NP) epitope that can bind to many HLA molecules; and (iii) at least two cytotoxic lymphocyte (CTL) nucleoprotein (NP) or matrix protein (M) epitopes that are restricted to the most prevalent HLA molecules in different human populations.
Owner:YEDA RES & DEV CO LTD
Influenza virus-like particles (VLPS) comprising hemagglutinin produced within a plant
InactiveUS20100239610A1Enhance immune responseEasy to captureSsRNA viruses negative-senseVirus peptidesHemagglutininLipid formation
A method for synthesizing influenza virus-like particles (VLPs) within a plant or a portion of a plant is provided. The method involves expression of influenza HA in plants and the purification by size exclusion chromatography. The invention is also directed towards a VLP comprising influenza HA protein and plants lipids. The invention is also directed to a nucleic acid encoding influenza HA as well as vectors. The VLPs may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
Neutralizing Anti-influenza a virus antibodies and uses thereof
ActiveUS20100080813A1Neutralization is narrowLimited successBiocidePeptide/protein ingredientsHemagglutininAntigen Binding Fragment
The invention relates to antibodies, and antigen binding fragments thereof, that bind to hemagglutinin and neutralize a group 1 subtype and a group 2 subtype of influenza A virus. The invention also relates to nucleic acids that encode, immortalized B cells and cultured single plasma cells that produce, and to epitopes that bind to such antibodies and antibody fragments. In addition, the invention relates to the use of the antibodies, antibody fragments, and epitopes in screening methods as well as in the diagnosis, treatment and prevention of influenza A virus infection.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE
Compositions that include hemagglutinin, methods of making and methods of use thereof
InactiveUS20070253982A1Stimulate immune responseCost effectiveSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininToll-like receptor
Owner:VAXINNATE
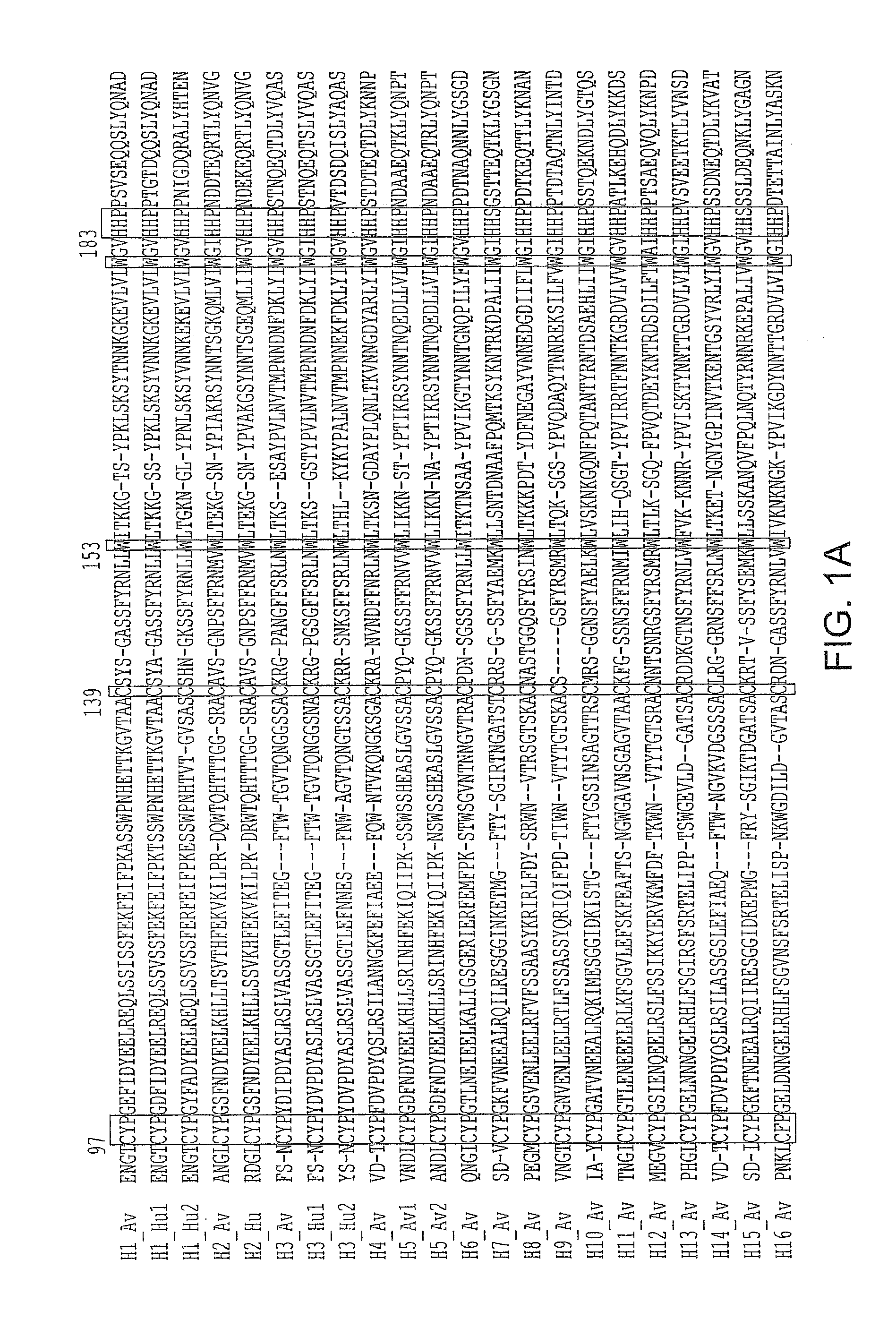
Hemagglutinin polypeptides, and reagents and methods relating thereto
InactiveUS20080241918A1Immunoglobulins against blood coagulation factorsPeptide/protein ingredientsHemagglutininGlycan
The present invention provides a system for analyzing interactions between glycans and interaction partners that bind to them. The present invention also provides HA polypeptides that bind to umbrella-topology glycans, and reagents and methods relating thereto.
Owner:MASSACHUSETTS INST OF TECH
Influenza hemagglutinin and neuraminidase variants
InactiveUS20060008473A1SsRNA viruses negative-senseViral antigen ingredientsHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising (avian pandemic) influenza hemagglutinin and neuraminidase variants are provided.
Owner:GOVERNMENT OF THE US SEC THE DEPT OF HEALTH SERVICES NAT INSTITTUTE OF HEALTH
Influenza vaccines
InactiveUS20080299151A1Less protectionBroad and efficient protective immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininMammal
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
Live bacterial vaccines for viral infection prophylaxis or treatment
InactiveUS20080124355A1Enhance immune responseSsRNA viruses negative-senseBacterial antigen ingredientsHemagglutininBacteroides
The present invention provides a vaccine, method of use, and kit employing genetically isolated and stabilized, live attenuated bacterial strains including Salmonella that express one or more avian influenza antigens for use in live vaccine compositions that can be orally administered to an individual to protect against avian influenza. Genetic stabilization may be achieved through deletion of IS200 elements and bacteria phage and prophage elements. The bacterial strains may be genetically isolated from external phage infection by constitutive expression of a P22 phage repressor. Nucleic acid sequences encoding antigenic hemagglutinin and neuraminidase avian influenza proteins, having at least one modified codon for optimum expression when transferred into a prokaryotic microorganism for improved immunogenicity
Owner:AVIEX TECH
Vaccines Including Antigen From Four Strains of Influenza Virus
InactiveUS20140178429A1Reduce in quantityFacilitate matterSsRNA viruses negative-senseViral antigen ingredientsHemagglutininAdjuvant
Owner:SEQIRUS UK LTD
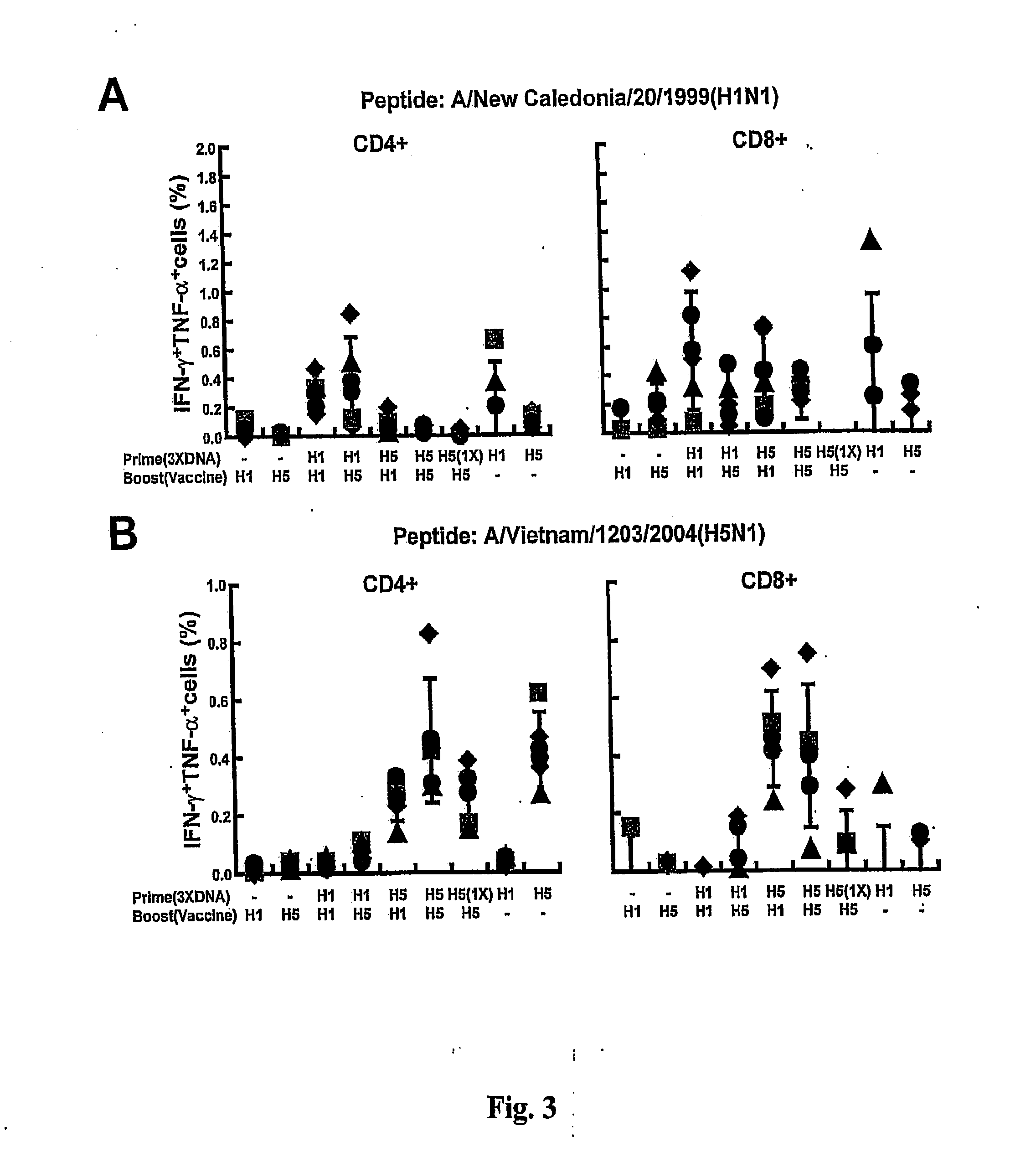
DNA prime/activated vaccine boost immunization to influenza virus
InactiveUS20110177122A1Enhance immune responseStimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsHemagglutininEpitope
The present invention relates to a combination of a priming composition and a boosting composition to prime and boost an immune response in a subject whereby the immune response resulting from administration of the priming composition to the subject is capable of being boosted. The priming composition comprises a DNA plasmid that comprises a nucleic acid molecule encoding an influenza virus hemagglutinin (HA) or an epitope-bearing domain thereof. The boosting composition comprises an influenza vaccine. The present invention also relates to a method to use such a combination to vaccinate a subject and to enhance an immune response to an influenza vaccine administered alone. Such a combination can elicit an immune response not only against at least one influenza virus strain from which the priming composition or boosting composition is derived but also to at least one heterologous influenza virus strain.
Owner:UNITED STATES OF AMERICA
Prophylactic and therapeutic influenza vaccines, antigens, compositions and methods
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by influenza virus. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and subunit vaccine compositions. In some embodiments, influenza antigens include hemagglutinin polypeptides neuraminidase polypeptides, and / or combinations thereof.
Owner:FRAUNHOFER USA
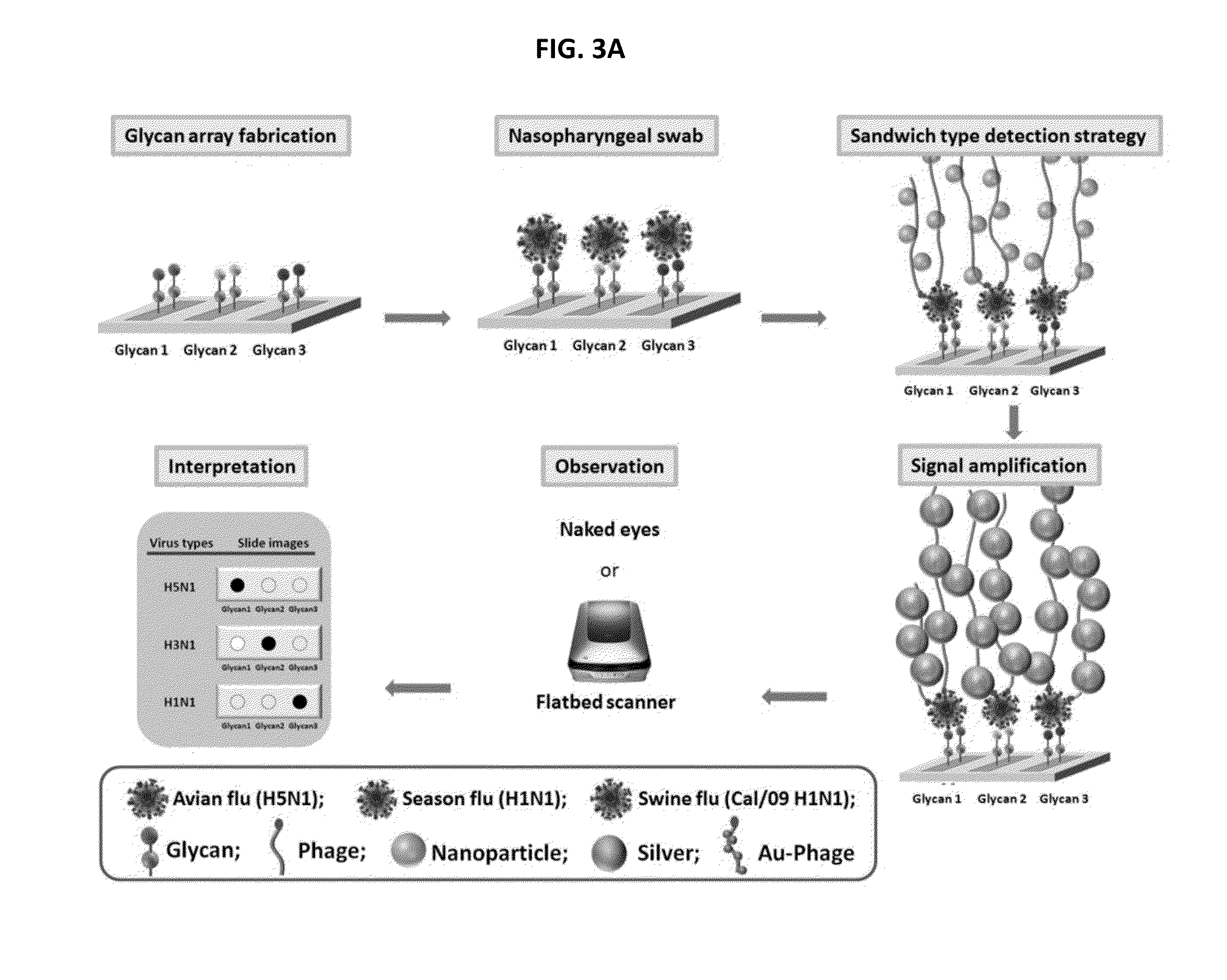
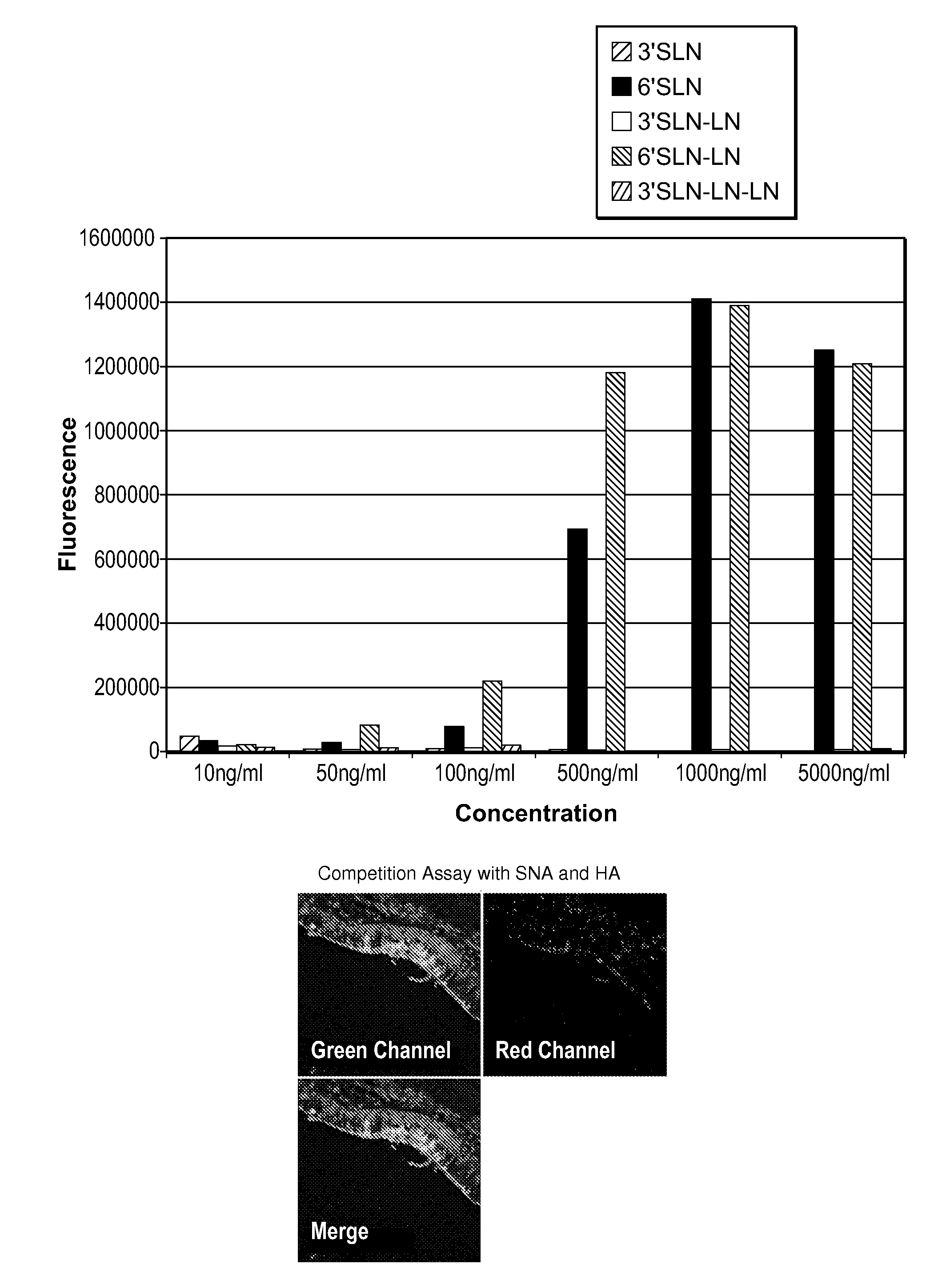
Glycan arrays for high throughput screening of viruses
ActiveUS20150160217A1Improve signal-to-noise ratioThe process is simple and fastSugar derivativesMicrobiological testing/measurementHemagglutininHigh-Throughput Screening Methods
Glycan arrays that can detect and distinguish between various sub-types and strains of influenza virus are provided. Methods for using the glycan arrays with assays using nanoparticle amplification technique are disclosed. Sandwich assays using gold nanoparticles conjugated to phage particles comprising influenza virus-specific antibodies for detecting multiple serotypes using a single reaction are provided. Plurality of glycans directed to specific target HA of influenza virus comprises the array. Detector molecules comprising noble metals conjugated to (a) phage display particles expressing antibodies against hemagglutinin and (b) neuraminidase binding agents are disclosed.
Owner:ACAD SINIC
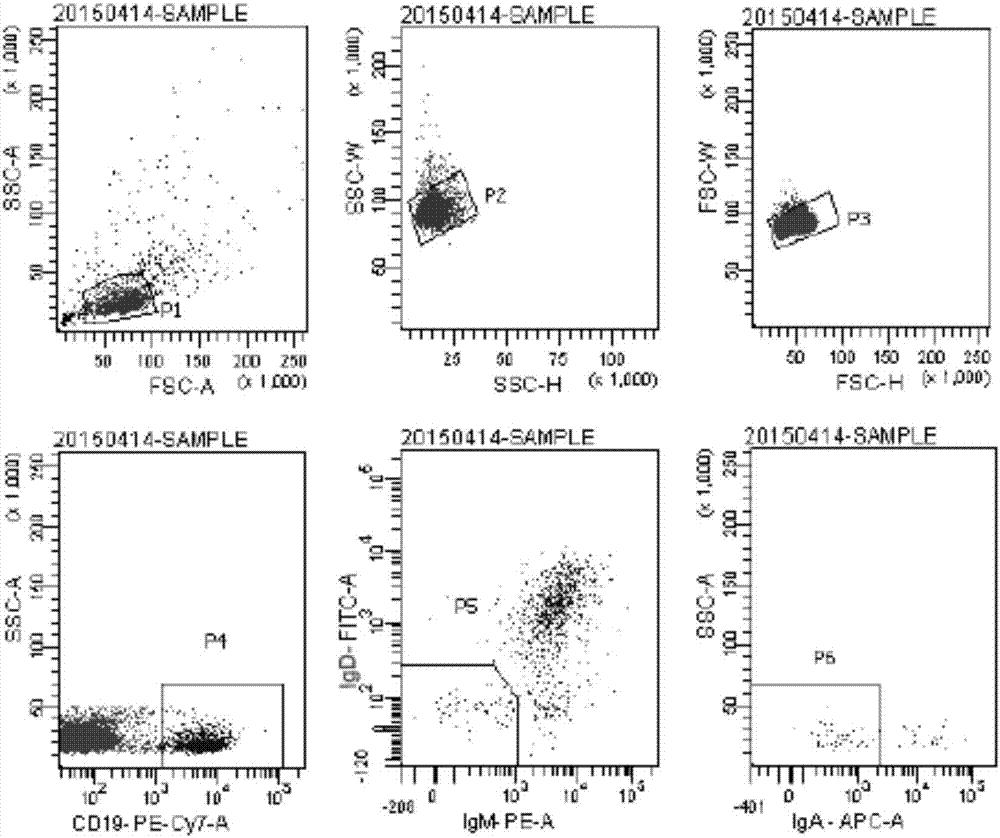
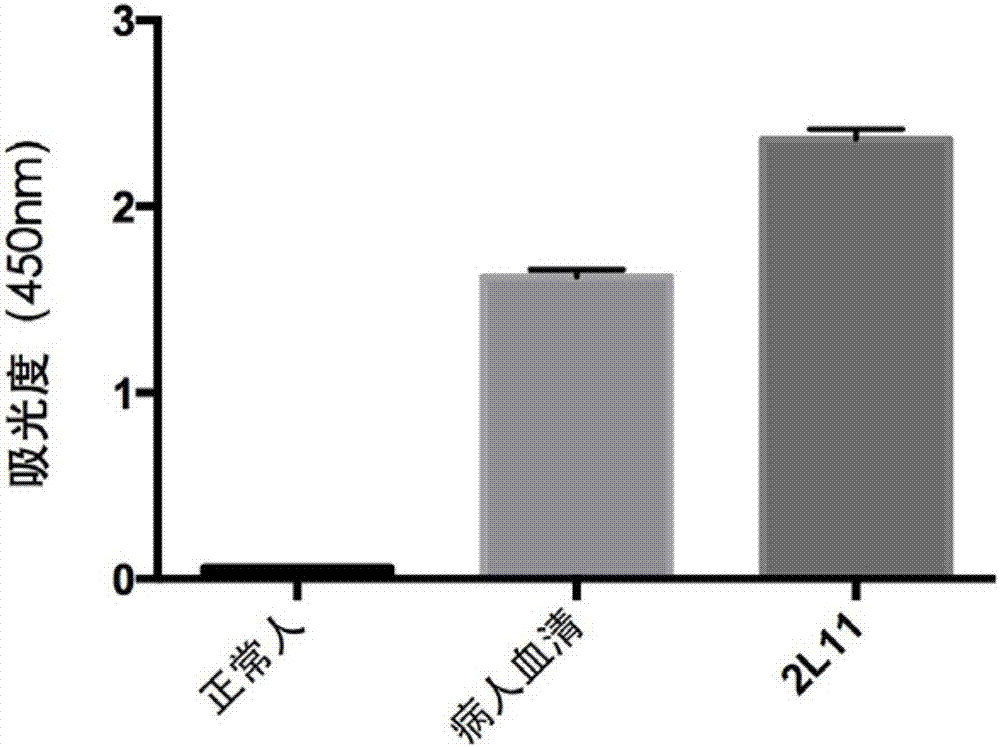
An anti-H7N9 completely humanized monoclonal antibody 2L11, and a preparing method and applications thereof
ActiveCN107353340AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininHeavy chain
The invention relates to an anti-H7N9 completely humanized monoclonal antibody 2L11, and a preparing method and applications thereof. The amino acid sequence of the heavy chain variable region of the antibody is shown as SEQ ID NO:2, or an amino acid sequence having similar functions and obtained by subjecting the sequence shown as the SEQ ID NO:2 to replacement, deletion or addition of one or more amino acids; and / or the amino acid sequence of the light chain variable region of the antibody is shown as SEQ ID NO:4, or an amino acid sequence having similar functions and obtained by subjecting the sequence shown as the SEQ ID NO:4 to replacement, deletion or addition of one or more amino acids. The antibody 2L11 can be bonded in a targeted manner with hemagglutinin HA of H7N9 viruses. Compared with mouse-sourced antibodies, genes of the completely humanized antibody are completely derived from human genes and do not comprise components of other species, and therefore no side or toxic effect such as anti-mouse antibodies is generated in a human body, and the antibody 2L11 has better biocompatibility, and is more suitable and has higher potential as a macro-molecule medicine treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH
Influenza nucleic acid molecules and vaccines made therefrom
ActiveUS20110182938A1SsRNA viruses negative-senseOrganic active ingredientsHemagglutininInfluenza A antigen
Provided herein are nucleic acid sequences that encode novel consensus amino acid sequences of HA hemagglutinin, as well as genetic constructs / vectors and vaccines expressing the sequences. Also provided herein are methods for generating an immune response against one or more Influenza A serotypes using the vaccines that are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
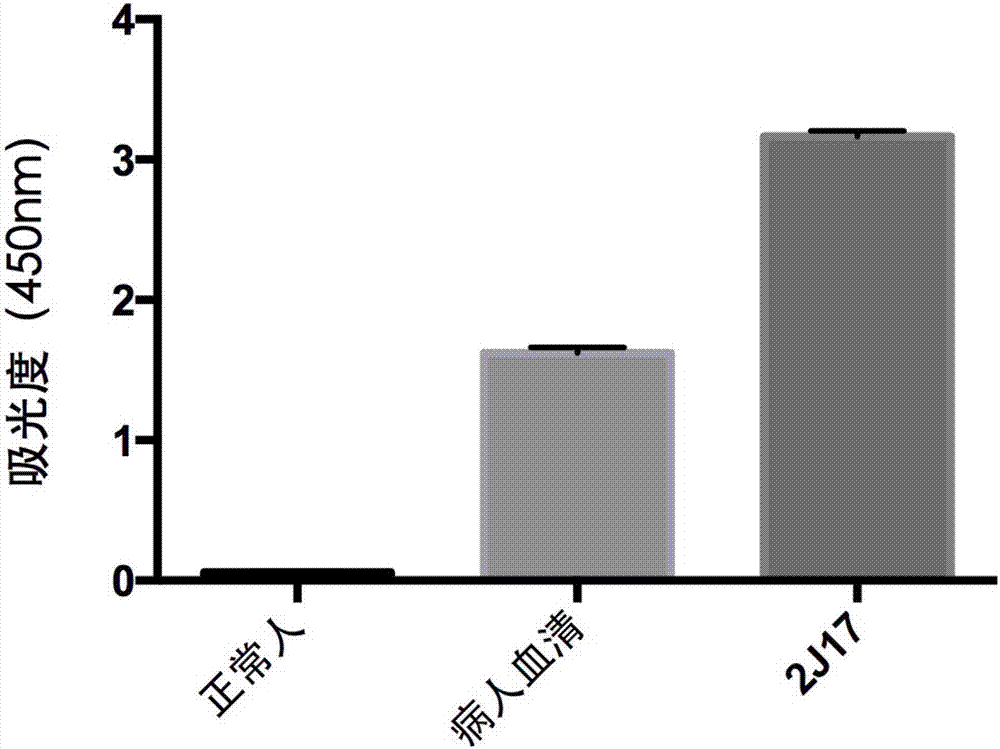
Anti-H7N9 all-human-derived monoclonal antibody 2J17, and preparation method and application thereof
ActiveCN107337732AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininSide effect
The invention relates to an anti-H7N9 all-human-derived monoclonal antibody 2J17, and a preparation method and application thereof. The heavy chain variable region amino acid sequence of the antibody is disclosed as SEQ ID NO:2, or an amino acid sequence with the same function, which is formed by performing substitution, deletion or addition of one or more amino acids on the sequence; and / or the light chain variable region amino acid sequence of the antibody is disclosed as SEQ ID NO:4, or an amino acid sequence with the same function, which is formed by performing substitution, deletion or addition of one or more amino acids on the sequence. The antibody 2J17 can be bound with hemagglutinin HA of the H7N9 virus in a targeted way. Compared with the rat-derived antibody, genes of the all-human-derived antibody are completely derived from human genes without components of other species, so that the all-human-derived antibody does not have rat antiantibody resistance and other toxic or side effects in the human body, has better biocompatibility, and has higher adaptability and potential to become the macromolecular drug for treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI +1
Monoclonal antibodies binding to avian influenza virus subtype h5 haemagglutinin and use thereof
InactiveUS20090068637A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHemagglutininAvian influenza virus
The present application provides monoclonal antibodies that specifically bind to the hemagglutinin of avian influenza virus subtype H5, as well as monoclonal antibodies capable of blocking at least 50% of the hemagglutinin binding activity of these monoclonal antibodies. Such antibodies are useful, for example, in the detection, diagnosis, prevention, and treatment of avian influenza virus. Also provided herein are hybridoma cell lines, isolated nucleic acid molecules, and short peptides related to the monoclonal antibodies provided herein, and pharmaceutical compositions and kits containing the monoclonal antibodies provided herein.
Owner:HX DIAGNOSTICS INC
Ii-key enhanced vaccine potency
InactiveUS20080095798A1Improve effectivenessEasy to manageAntibacterial agentsSsRNA viruses negative-senseHemagglutininVaccine Potency
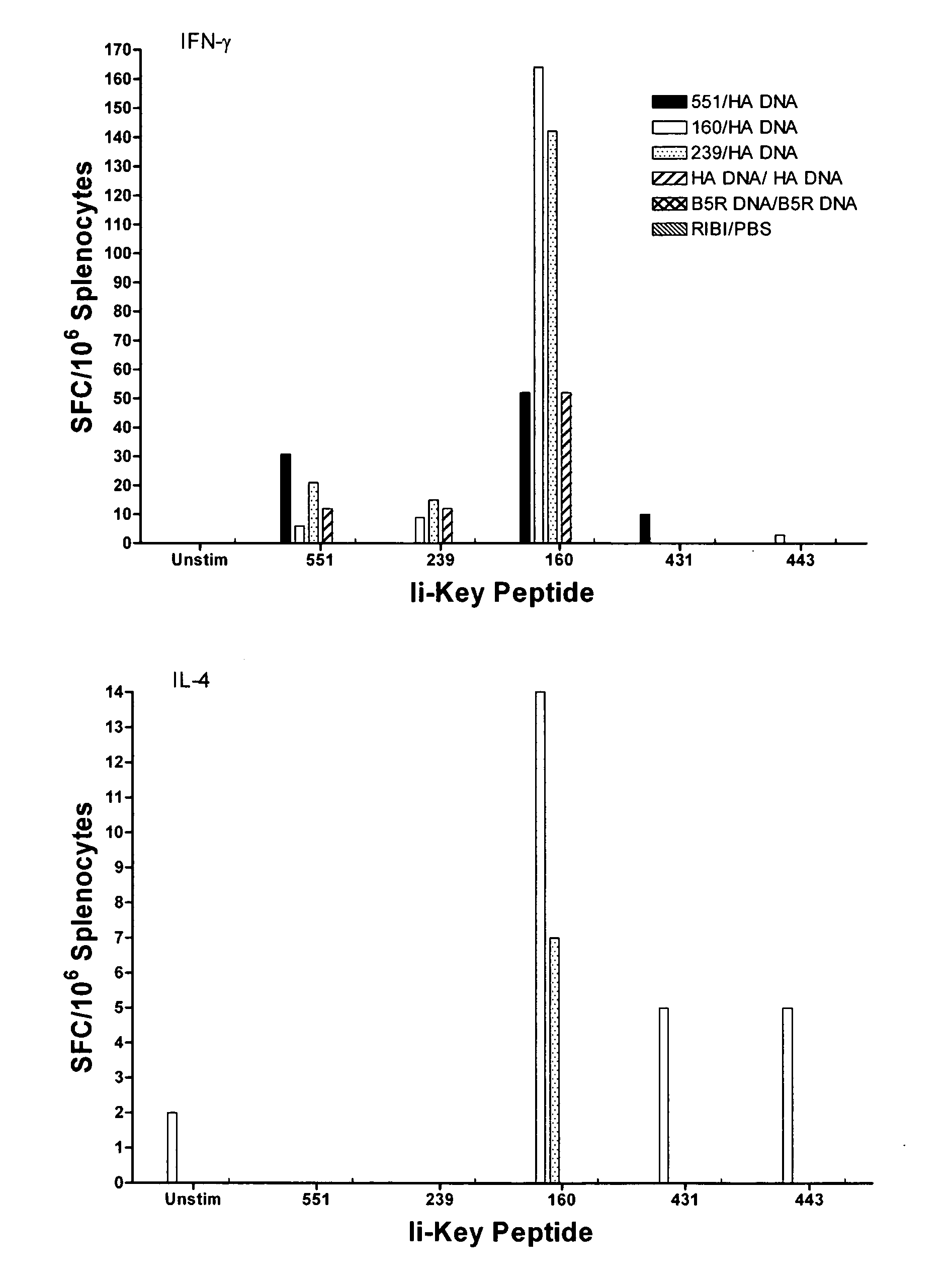
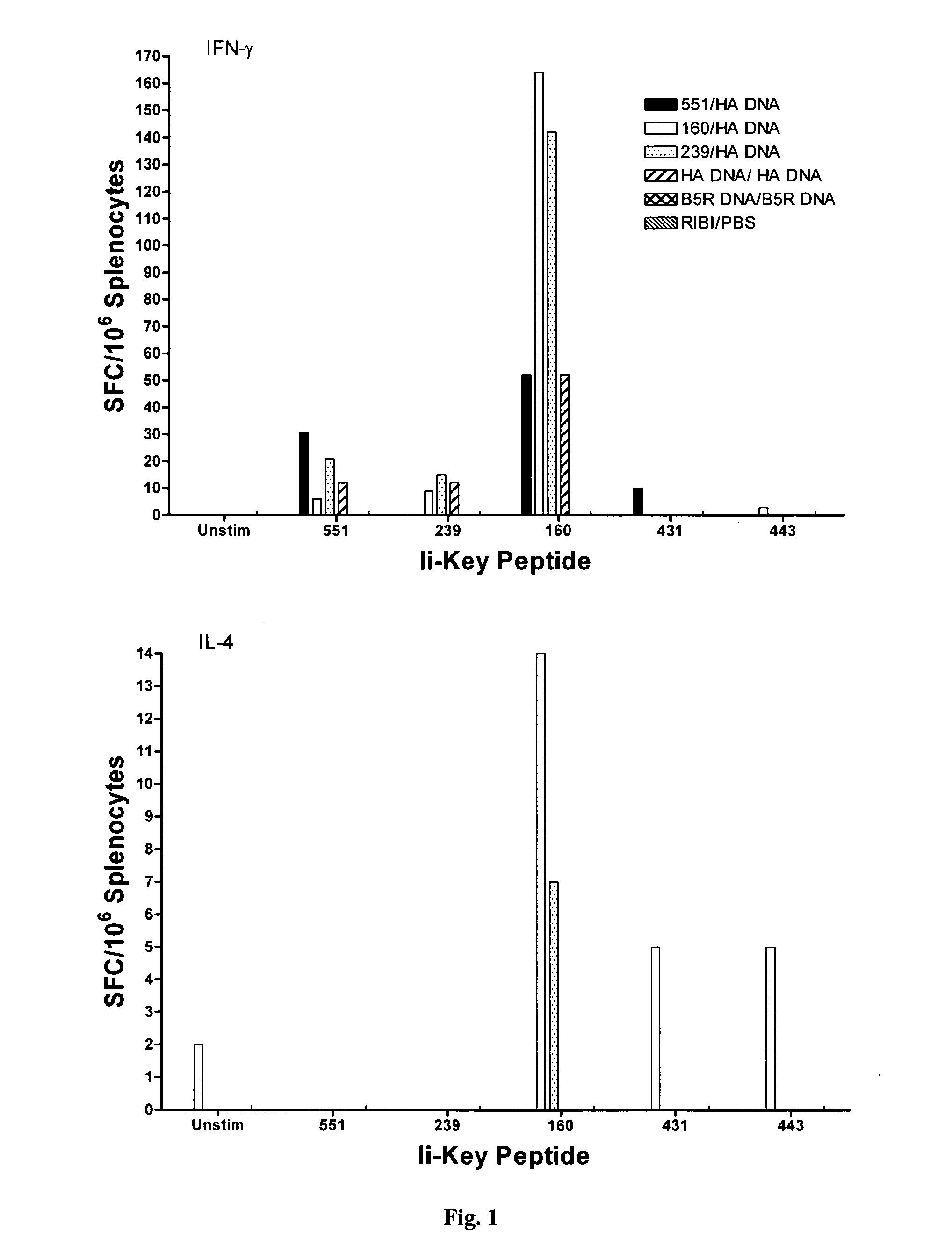
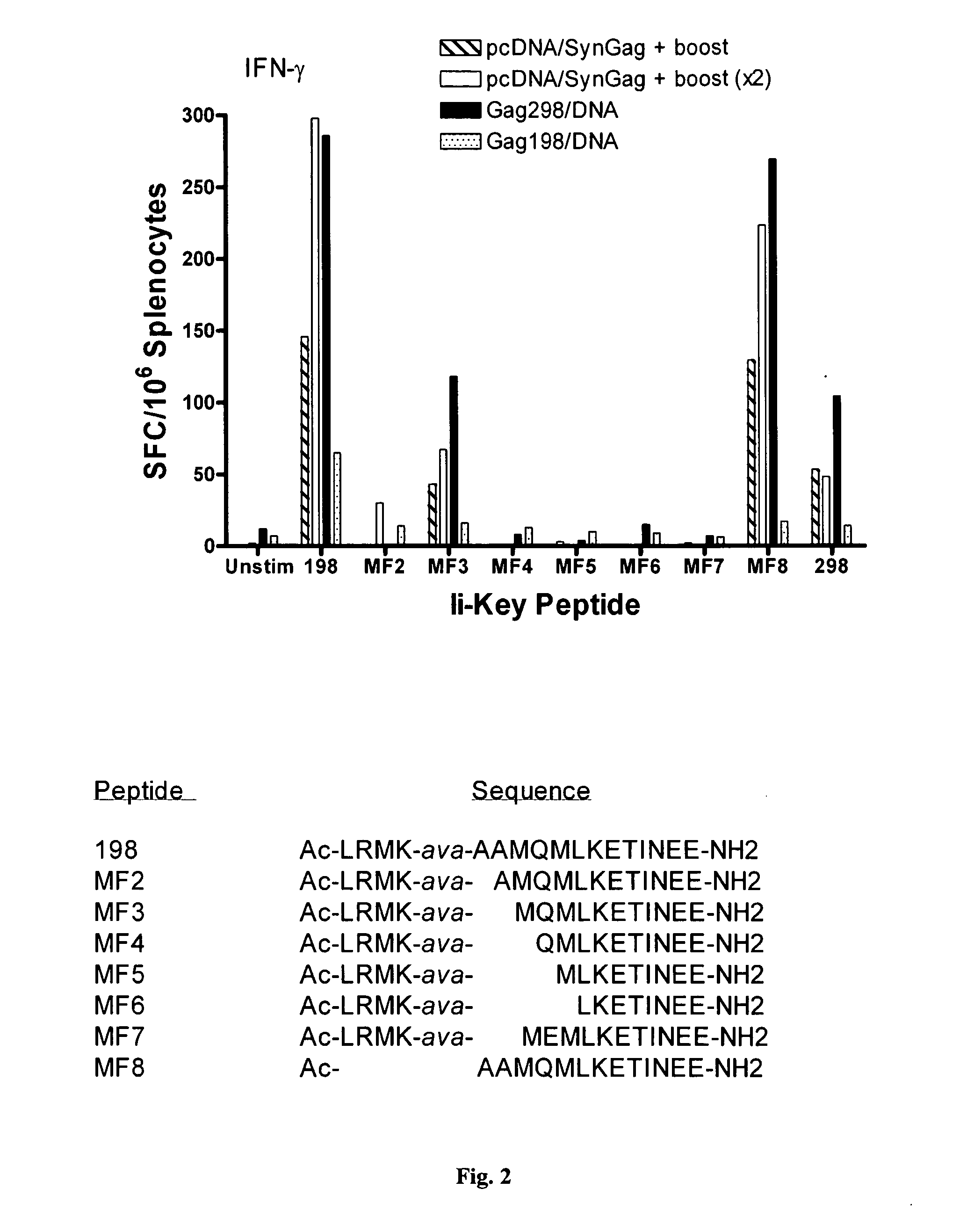
Disclosed is a method for increasing vaccine potency whereby a subject's immune system is first primed with an Ii-Key hybrid peptide construct before the subject subsequently receives a vaccine for a pathogen of interest. The vaccine may be comprised of a protein or portion thereof that is encoded by the genome of the pathogen. The vaccine may also be a DNA vaccine comprised of DNA encoding a protein of the pathogen. The Ii-Key hybrid peptide construct includes the LRMK residues of Ii-Key protein and an MHC Class II epitope of the protein or portion thereof which is used in the vaccine. The Ii-Key construct may be administered in the form of a nucleic acid construct encoding the Ii-Key hybrid peptide. Priming with Ii-Key peptides enhances the immunogenicity of rHA protein and HA and HIV DNA vaccines. Methods are described relating to the use of Ii-Key hybrid constructs in vaccine protocols wherein the pathogen is HIV or Influenza A, including H5N1. Methods and compositions are described wherein the MHC Class II epitope of the Ii-Key hybrid is hemagglutinin encoded by Influenza A or the Gag protein encoded by HIV.
Owner:ANTIGEN EXPRESS
Hemagglutinin Polypeptides, and Reagents and Methods Relating Thereto
InactiveUS20090269342A1High affinityStrong specificityVirusesPeptide/protein ingredientsHemagglutininGlycan
The present invention provides a system for analyzing interactions between glycans and interaction partners that bind to them. The present invention also provides HA polypeptides that bind to umbrella-topology glycans, and reagents and methods relating thereto.
Owner:MASSACHUSETTS INST OF TECH
Hemagglutinin polypeptides, and reagents and methods relating thereto
InactiveUS20090081193A1High affinityStrong specificityOrganic active ingredientsBacteriaHemagglutininSyndecan binding
The present invention provides a system for analyzing interactions between glycans and interaction partners that bind to them. The present invention also provides HA polypeptides that bind to umbrella-topology glycans, and reagents and methods relating thereto.
Owner:MASSACHUSETTS INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com