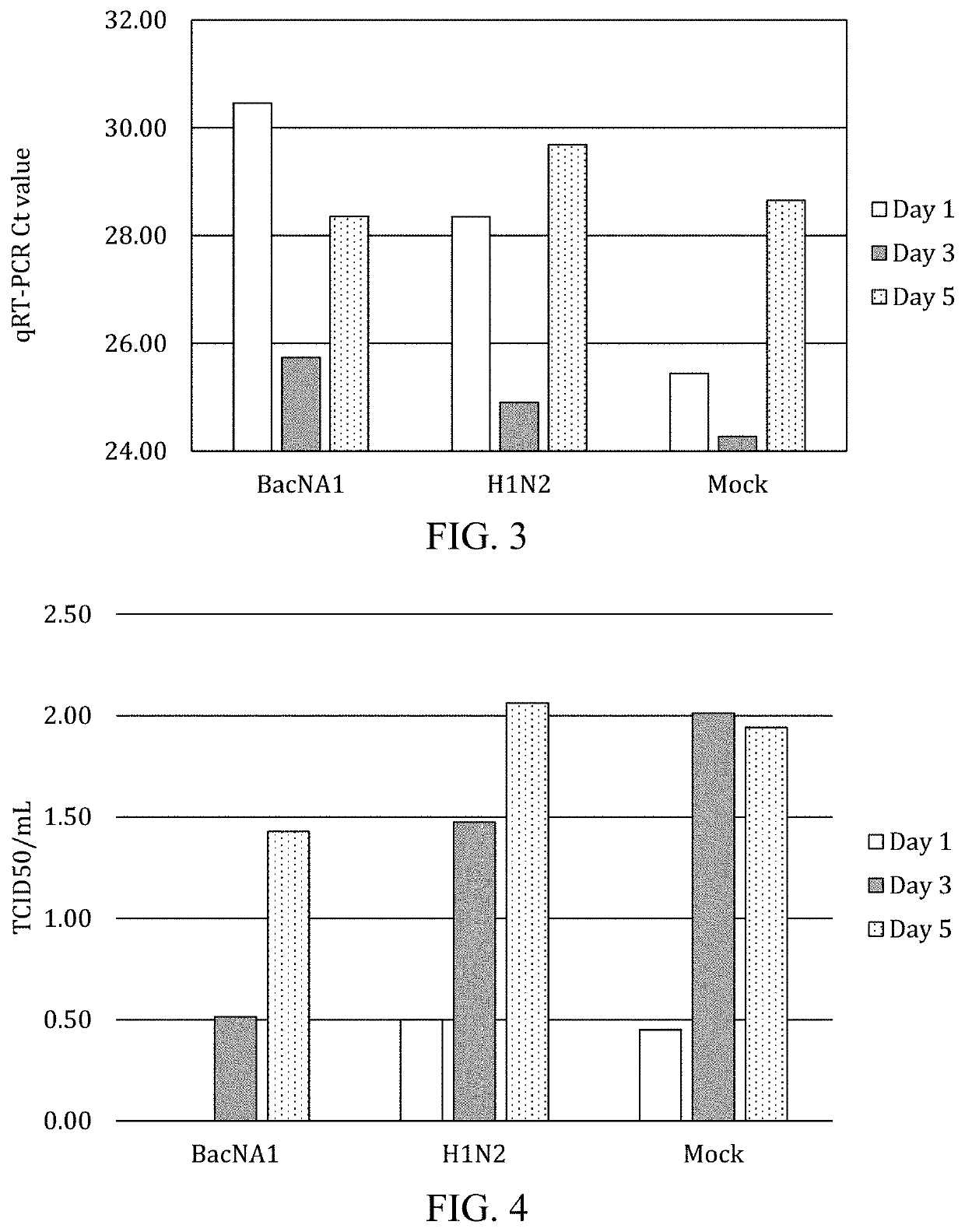
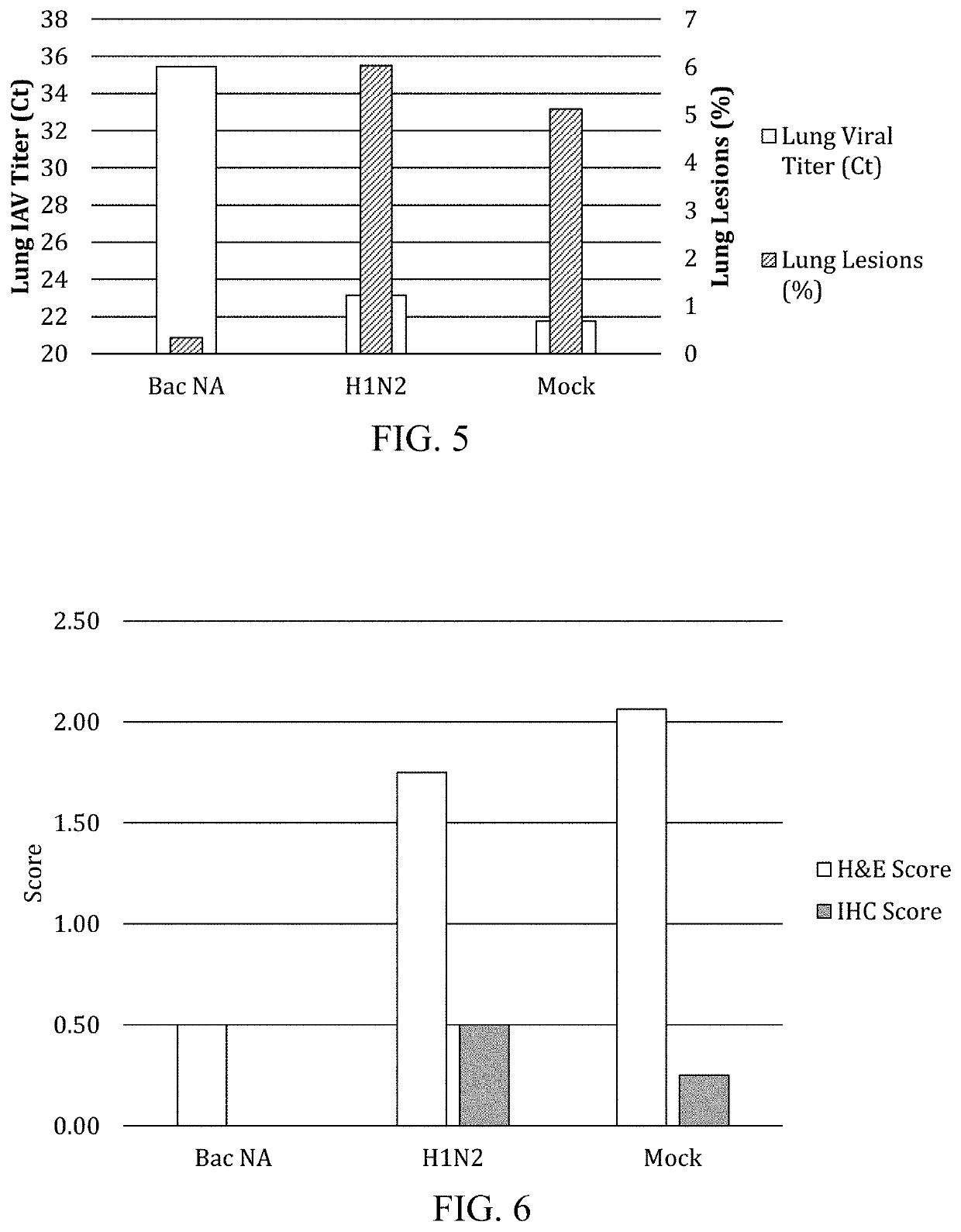
Patents
Literature
74 results about "Influenza A antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antiviral compounds and methods of making and using thereof
ActiveUS20110212975A1Organic active ingredientsOrganic chemistryPiperazinePharmaceutical preservatives
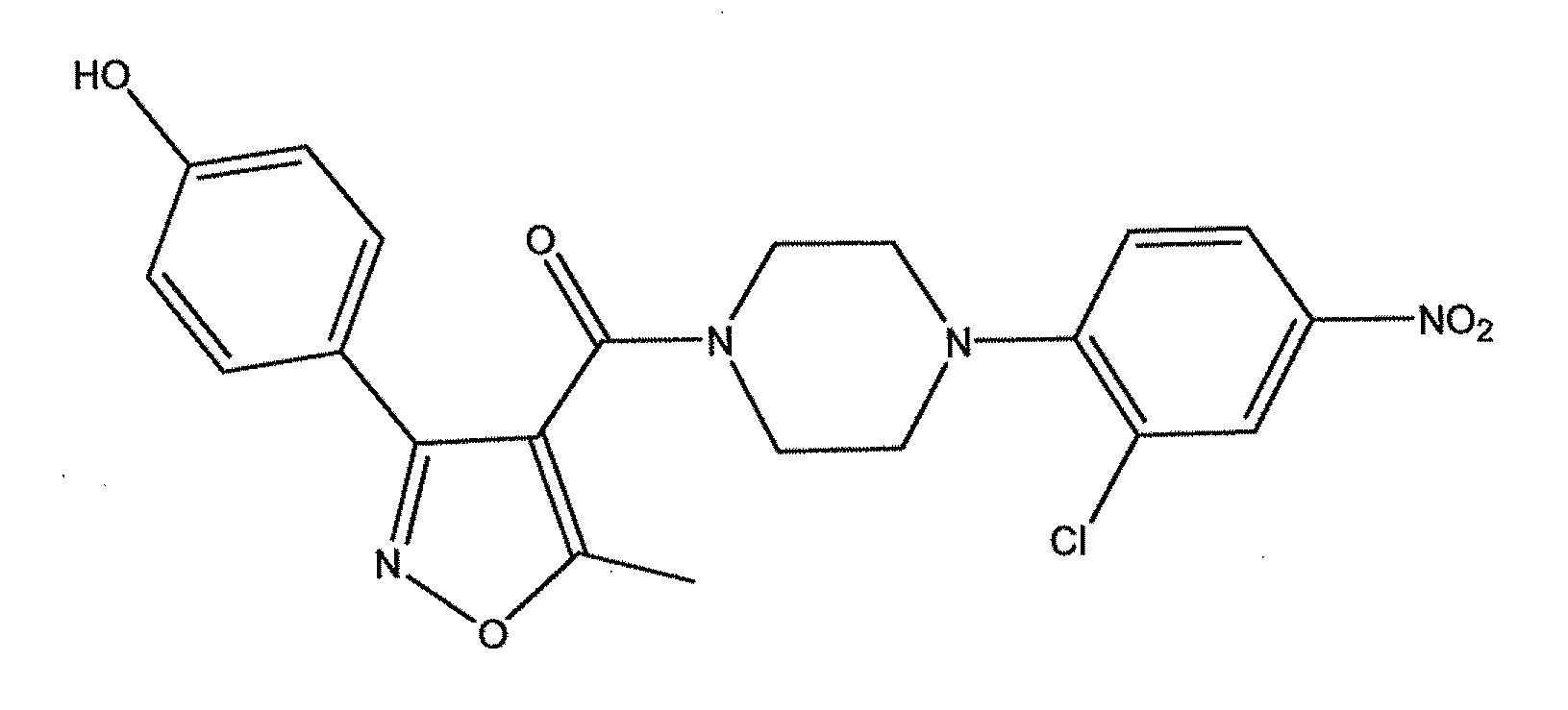
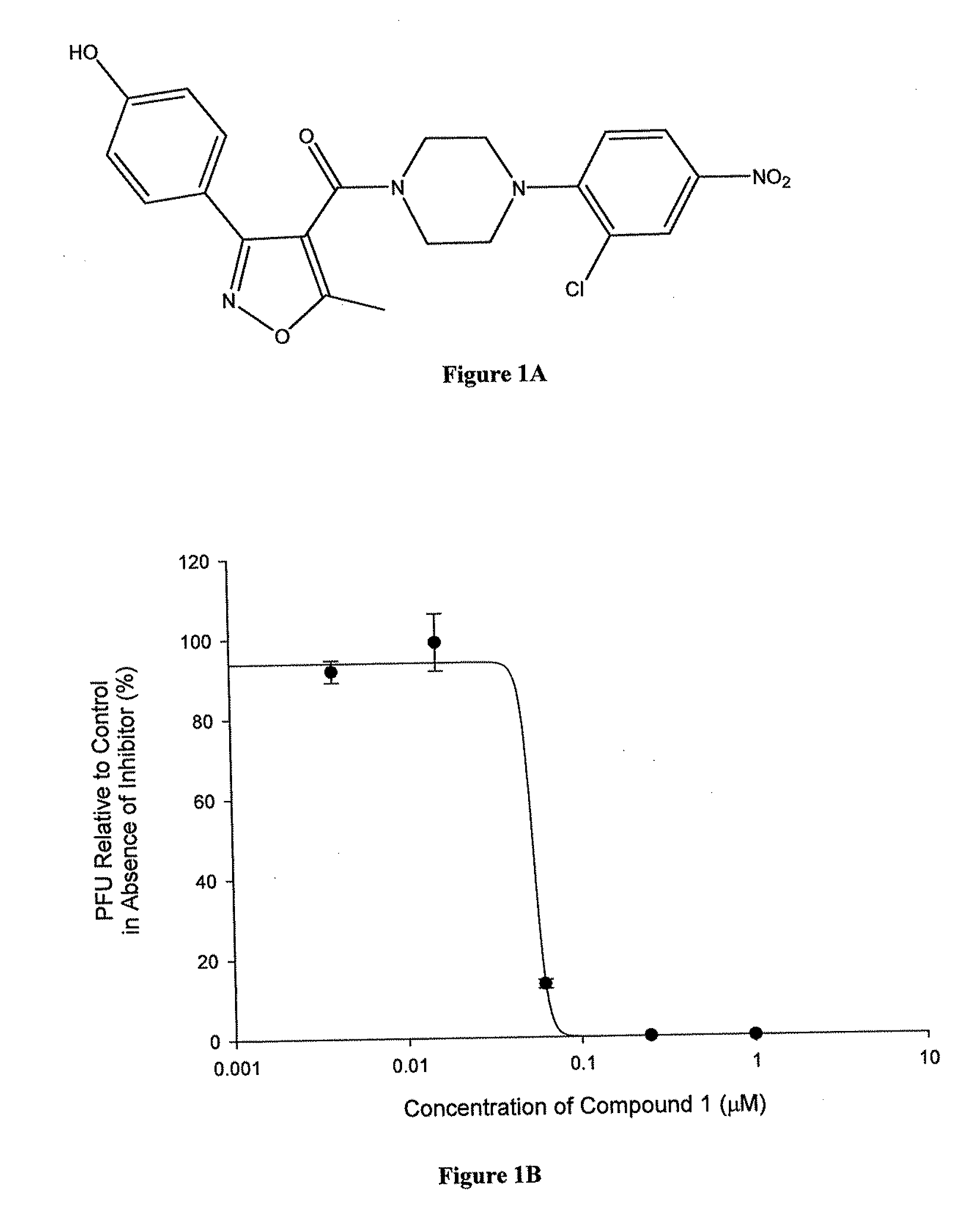
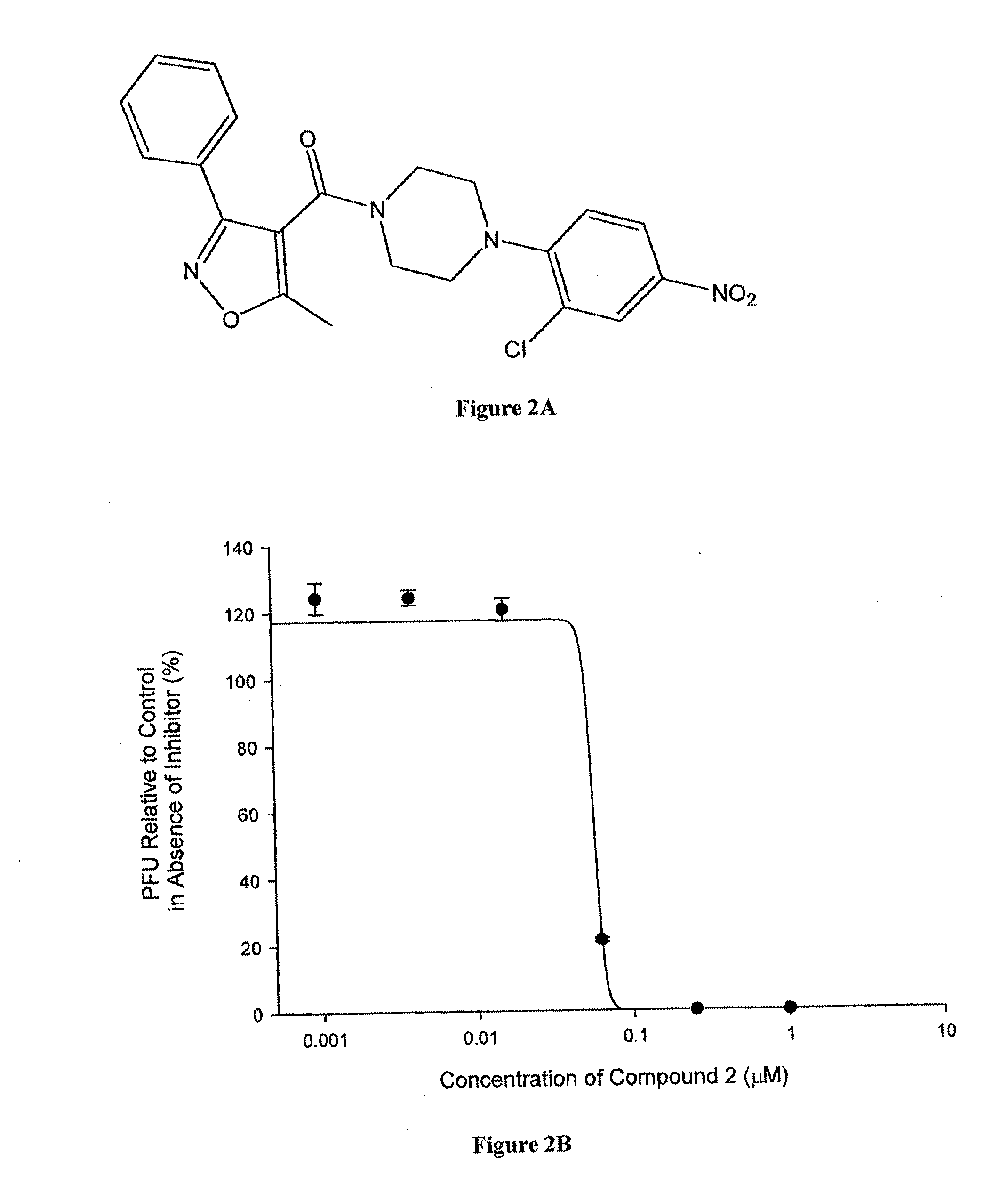
Compounds which exhibit antiviral activity, particularly against influenza virus, and methods of making and using thereof are described herein. In one embodiment, the compounds are heterocyclic amides containing piperazine and isozazole rings and optionally substituted with one or more substituents. The compounds can be formulated with one or more pharmaceutically acceptable excipients to form compositions suitable for enteral or parenteral administration. The compounds are preferably used to treat or prevent Influenza A infections, such as H1N1, H2N2, H3N2, H5N1, H7N7, H1N2, H9N2, H7N2, H7N3, and H10N7.
Owner:VERSITECH LTD
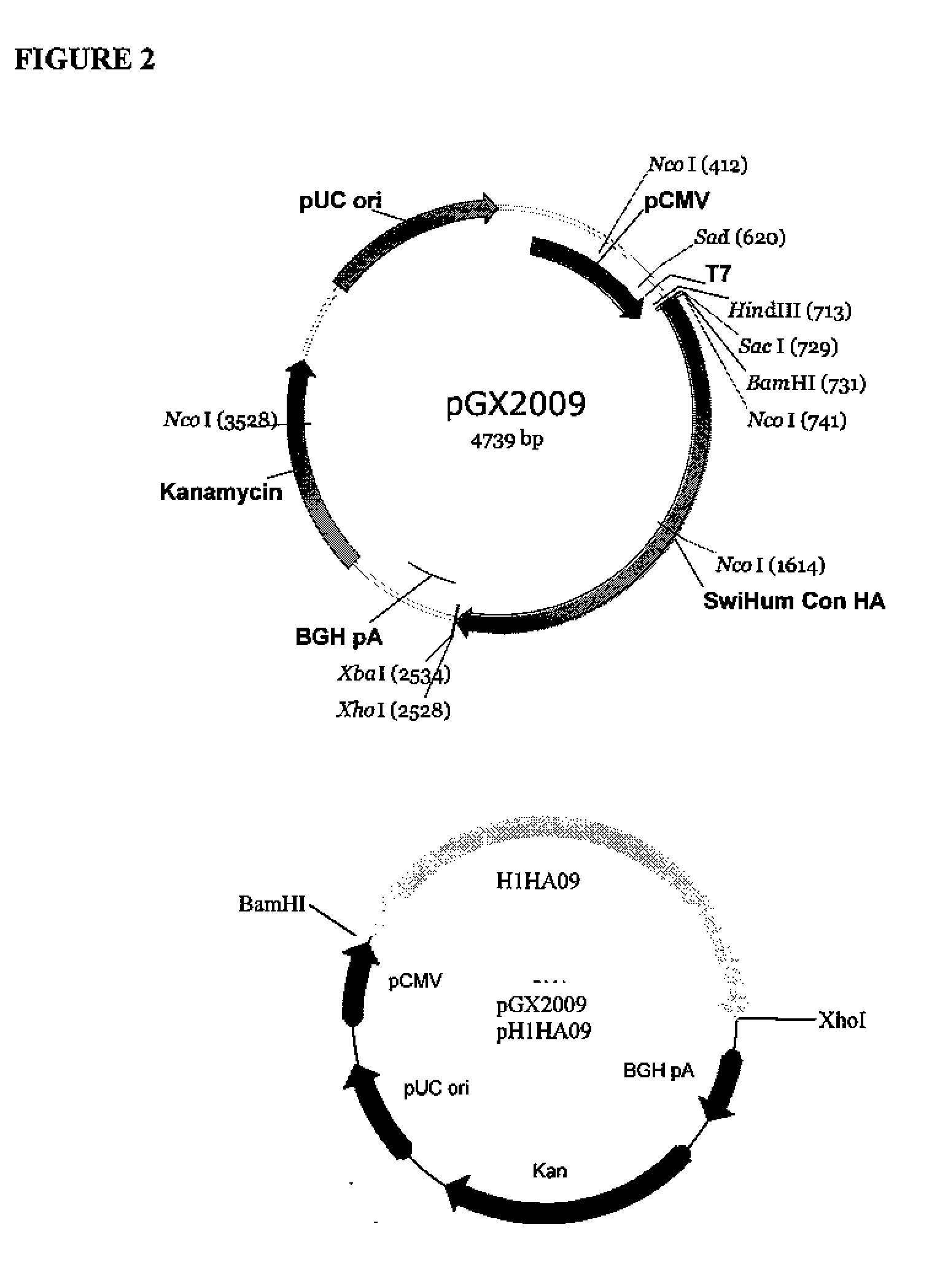
Influenza nucleic acid molecules and vaccines made therefrom
ActiveUS20110182938A1SsRNA viruses negative-senseOrganic active ingredientsHemagglutininInfluenza A antigen
Provided herein are nucleic acid sequences that encode novel consensus amino acid sequences of HA hemagglutinin, as well as genetic constructs / vectors and vaccines expressing the sequences. Also provided herein are methods for generating an immune response against one or more Influenza A serotypes using the vaccines that are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
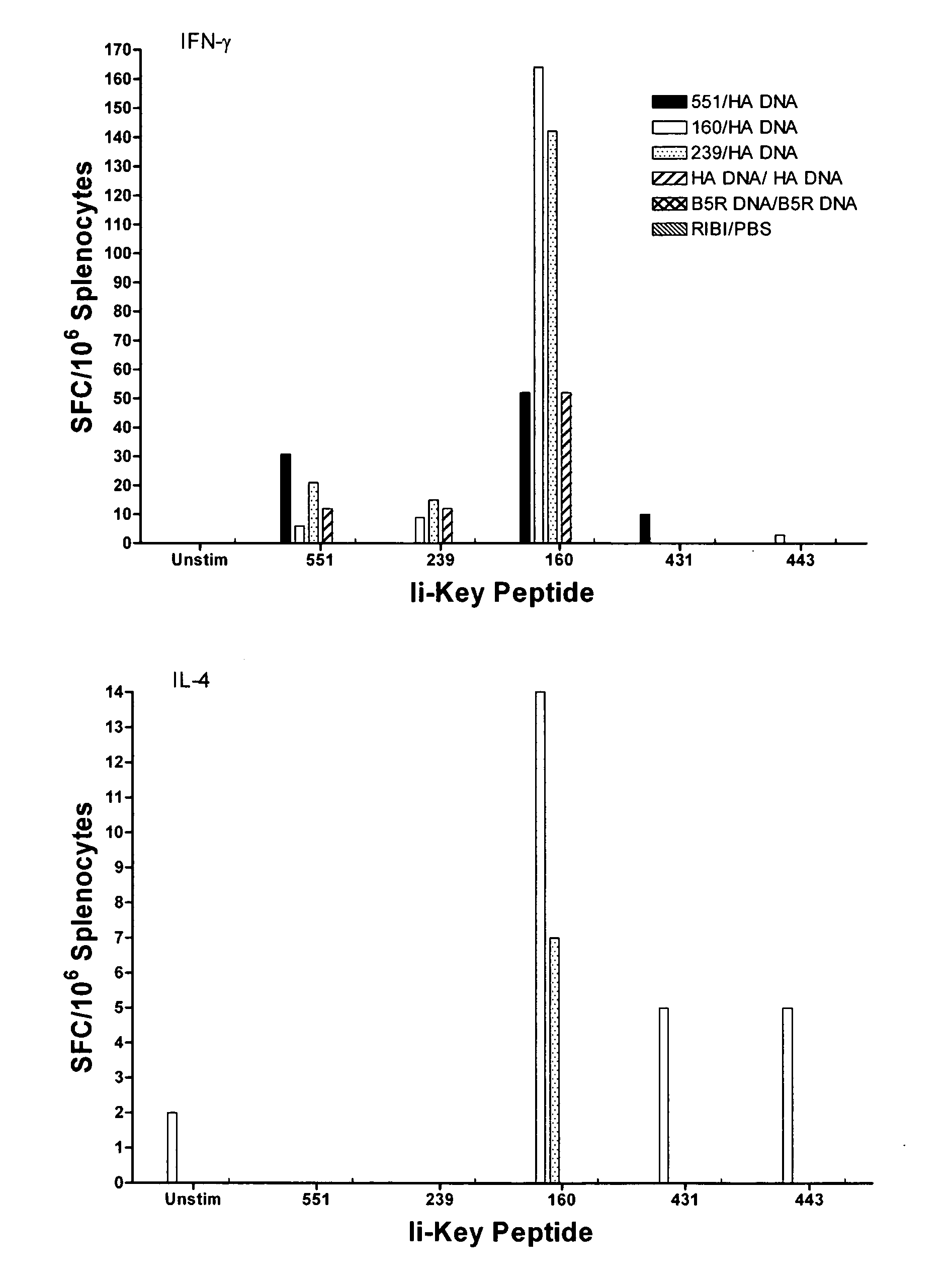
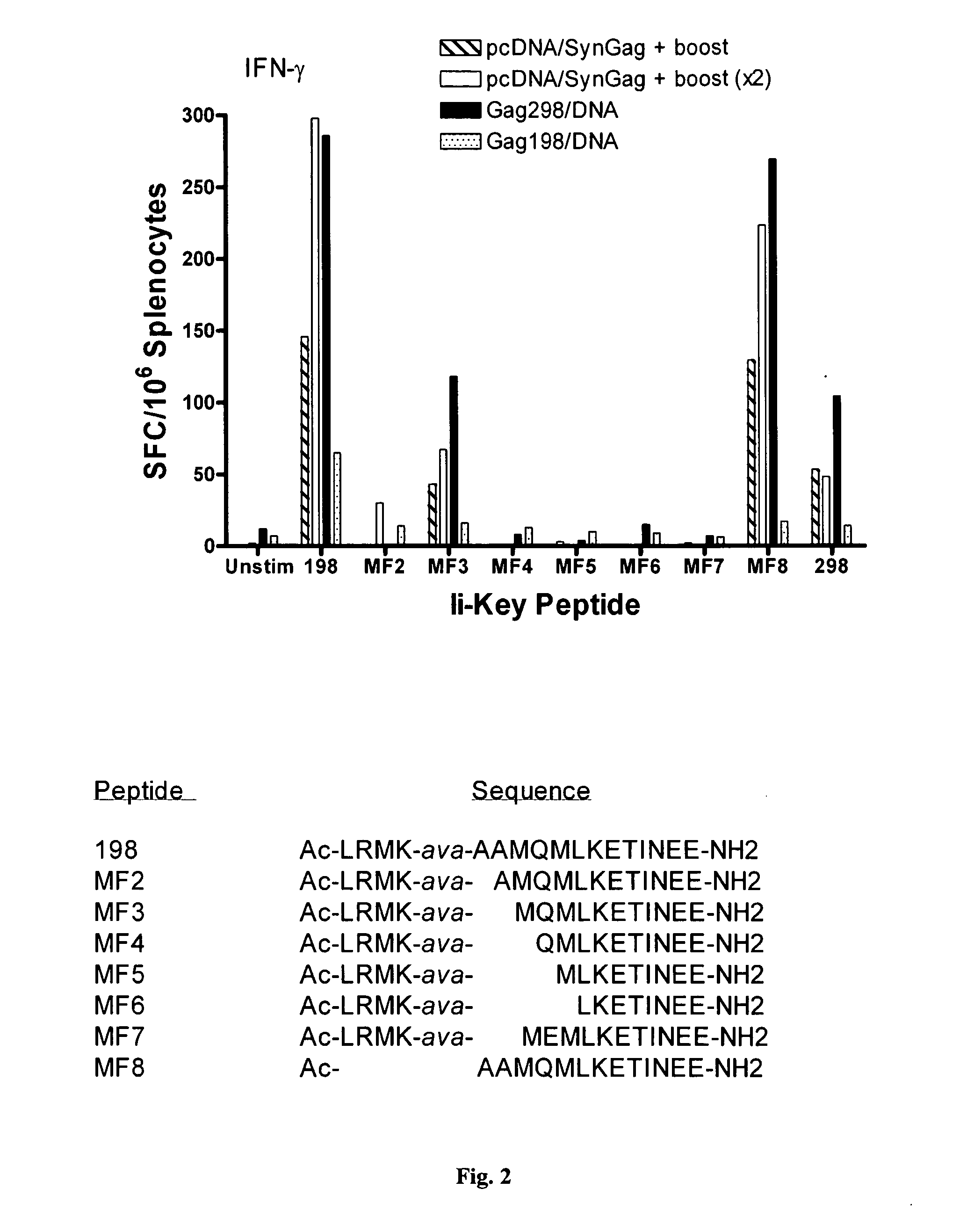
Ii-key enhanced vaccine potency
InactiveUS20080095798A1Improve effectivenessEasy to manageAntibacterial agentsSsRNA viruses negative-senseHemagglutininVaccine Potency
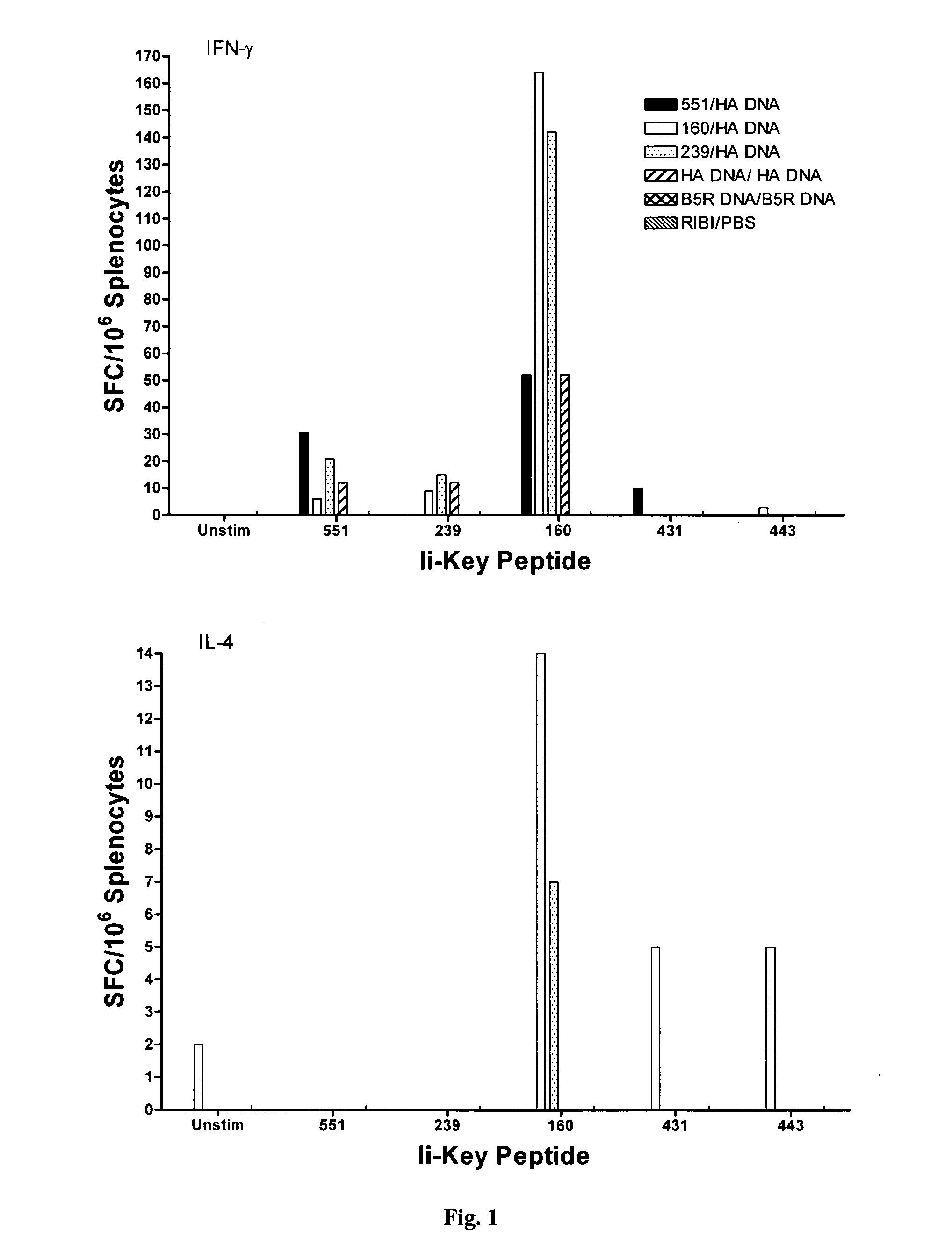
Disclosed is a method for increasing vaccine potency whereby a subject's immune system is first primed with an Ii-Key hybrid peptide construct before the subject subsequently receives a vaccine for a pathogen of interest. The vaccine may be comprised of a protein or portion thereof that is encoded by the genome of the pathogen. The vaccine may also be a DNA vaccine comprised of DNA encoding a protein of the pathogen. The Ii-Key hybrid peptide construct includes the LRMK residues of Ii-Key protein and an MHC Class II epitope of the protein or portion thereof which is used in the vaccine. The Ii-Key construct may be administered in the form of a nucleic acid construct encoding the Ii-Key hybrid peptide. Priming with Ii-Key peptides enhances the immunogenicity of rHA protein and HA and HIV DNA vaccines. Methods are described relating to the use of Ii-Key hybrid constructs in vaccine protocols wherein the pathogen is HIV or Influenza A, including H5N1. Methods and compositions are described wherein the MHC Class II epitope of the Ii-Key hybrid is hemagglutinin encoded by Influenza A or the Gag protein encoded by HIV.
Owner:ANTIGEN EXPRESS
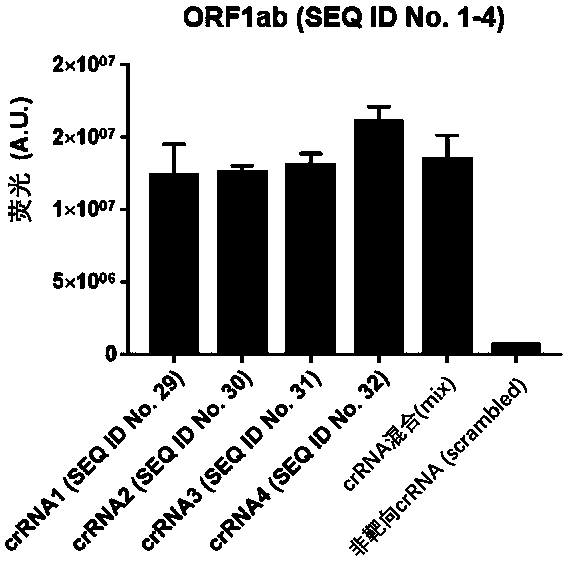
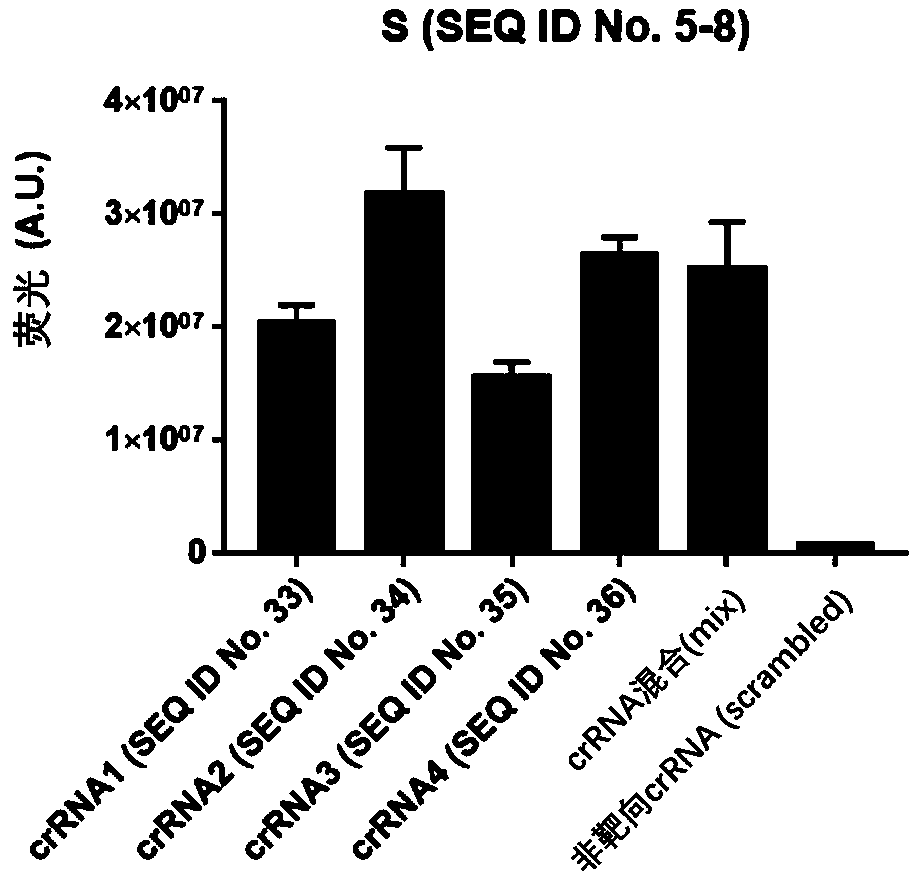
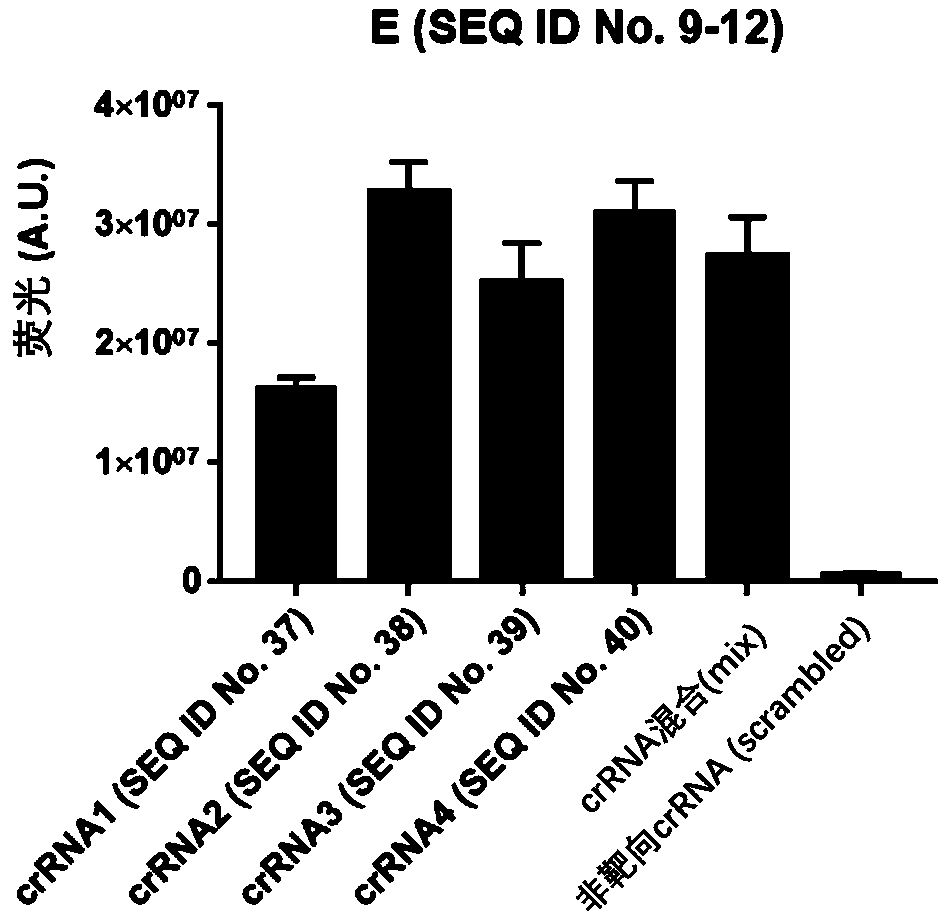
Test kit for detecting nucleic acid of respiratory tract pathogens, detection method and application
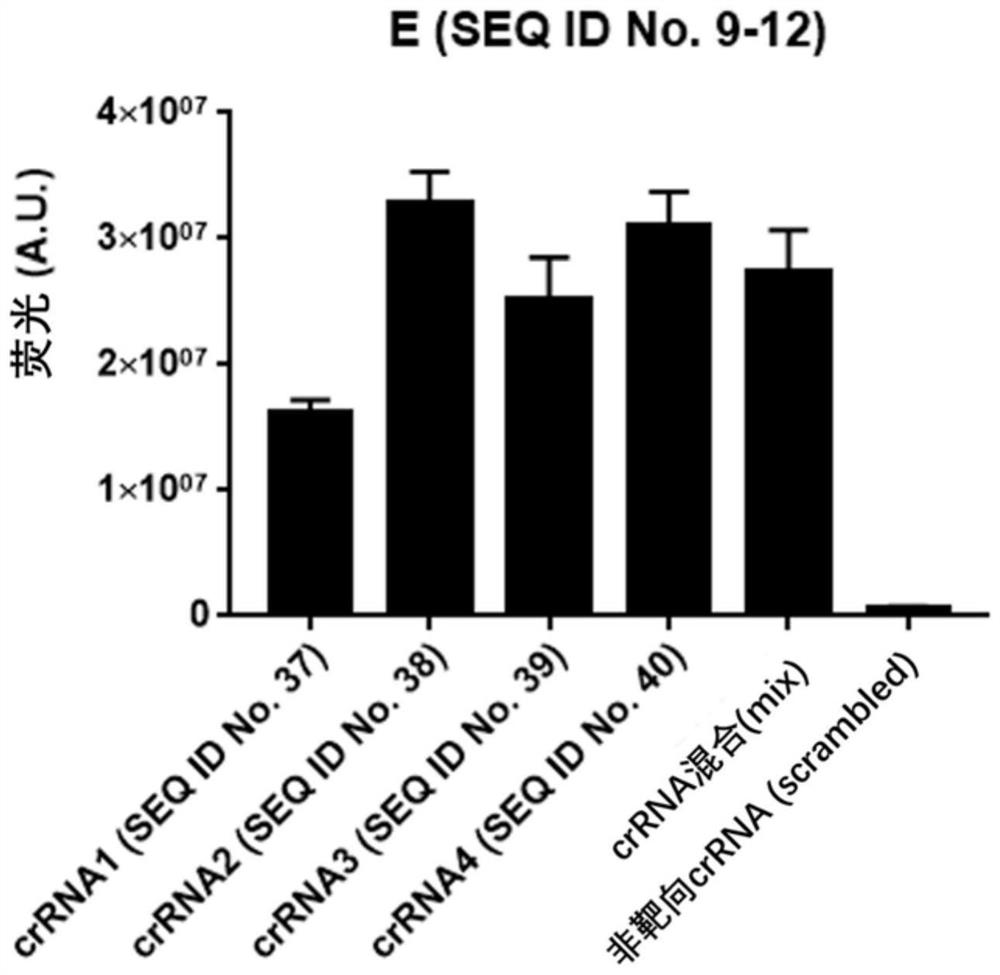
ActiveCN111534643AEfficient detection/diagnosisRapid Test/DiagnosisMicrobiological testing/measurementAgainst vector-borne diseasesInfluenza A antigenMedicine
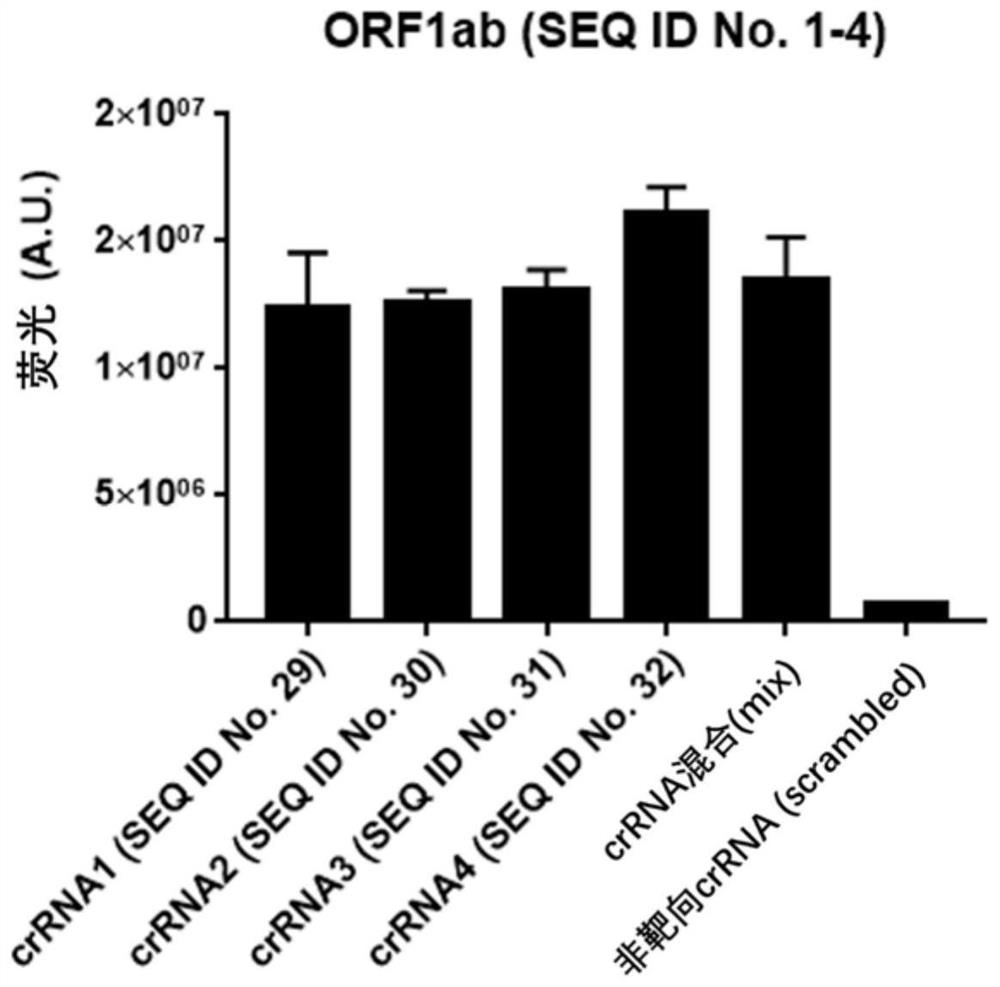
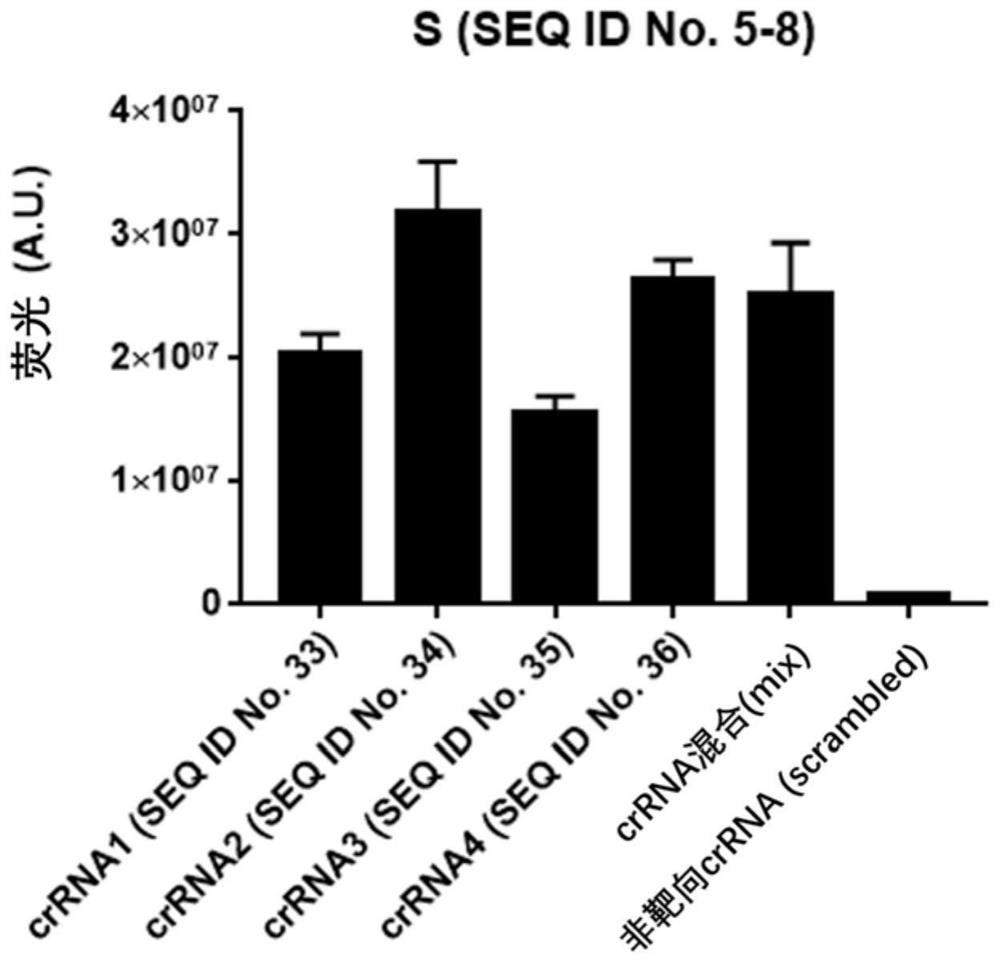
The invention provides a test kit for detecting nucleic acid of respiratory tract pathogens. The kit comprises a CRISPR-Cas detection system: crRNA, a primer pair, Cas protein and a nucleic acid probethat are described in the invention. The invention further provides a detection method of the nucleic acid of the respiratory tract pathogens, a combination of the crRNA and the primer pair and application of the combination. The test kit and the detection method disclosed by the invention can be used for directly observing results by naked eyes without depending on a large instrument, and detection can be realized under a mild condition, so that the detection is more convenient; the test kit and the detection method provided by the invention can be used for efficiently and rapidly detecting / diagnosing the respiratory tract pathogens (such as flu A, flu B and COVID-19), have relatively high specificity and sensitivity, and can be used for detecting and screening the respiratory tract pathogens, for example, rapidly distinguishing various respiratory tract pathogens including the flu A, the flu B and the COVID-19.
Owner:SHANGHAI TECH UNIV
Copper ion compositions and methods of treatment for conditions caused by coronavirus and influenza
Provided herein are formulations containing copper ions and methods of treating underlying infections and conditions caused by coronavirus, particularly COVID-19, and influenzas, particularly influenza A and / or influenza B using such formulations. Methods of treating the underlying viruses and their resultant conditions using topical copper ion treatments are provided. A topical treatment in its basic form comprises a biocompatible copper ion solution or suspension obtained by leaching of the copper ions from copper metal. The copper ion solution or suspension may be combined with various carriers to form the copper ion treatment including creams or solutions. Methods of making the copper ion solution or suspension from solid copper metal in a biocompatible solution are also provided.
Owner:CDA RES GROUP
Probe and primer composition for simultaneously detecting human novel coronavirus, influenza A virus and influenza B virus
PendingCN111411172ASimple and fast operationImprove adaptabilityMicrobiological testing/measurementMicroorganism based processesInfluenza A antigenInfluenza Viruses Type A
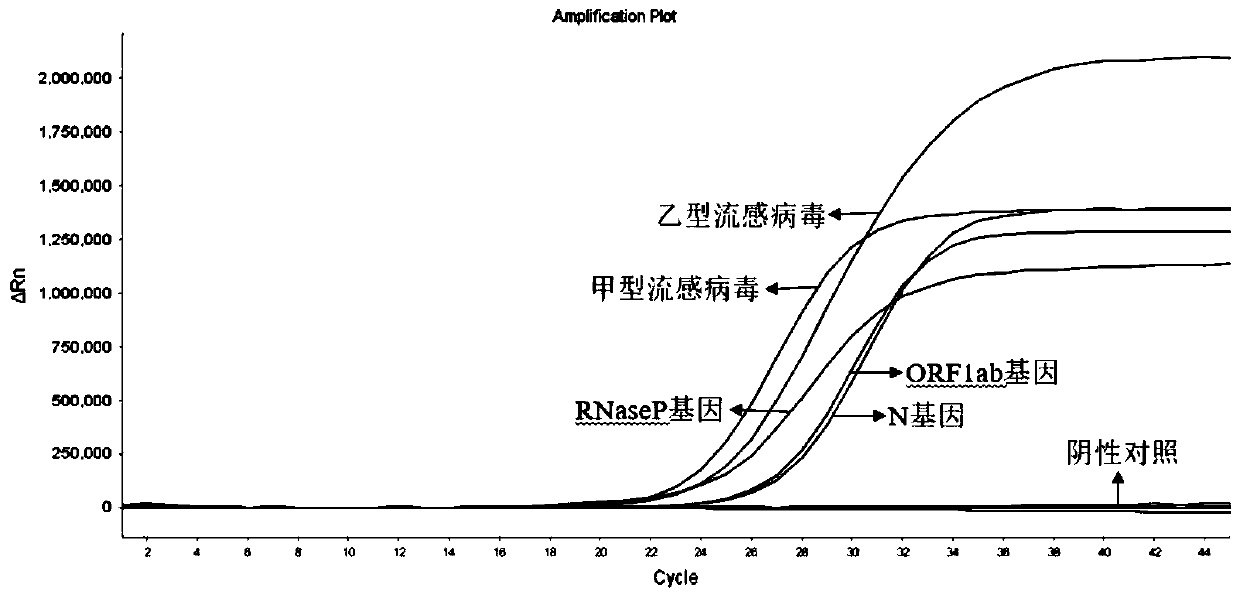
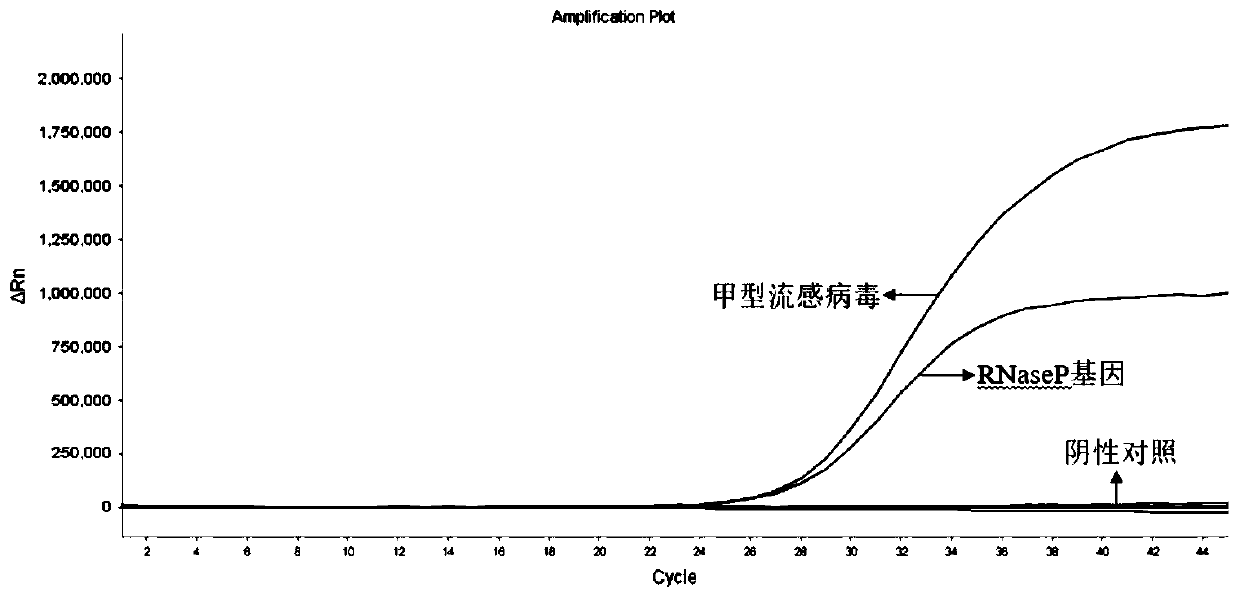
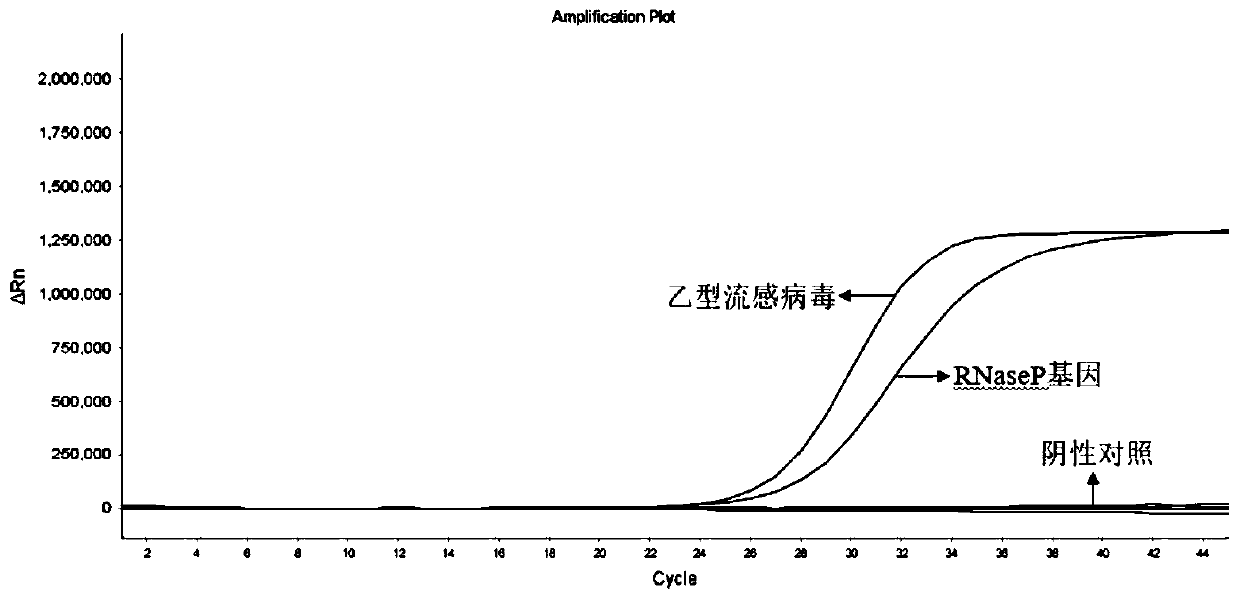
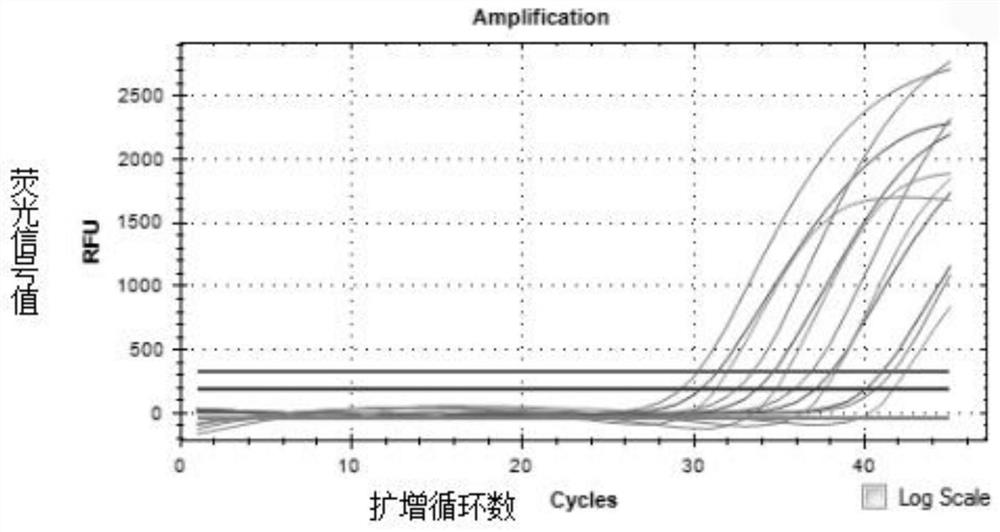
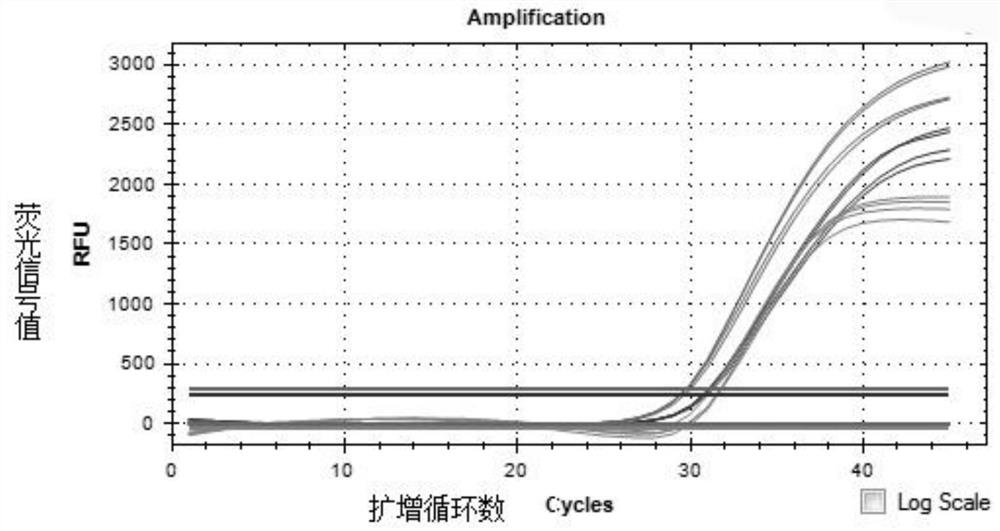
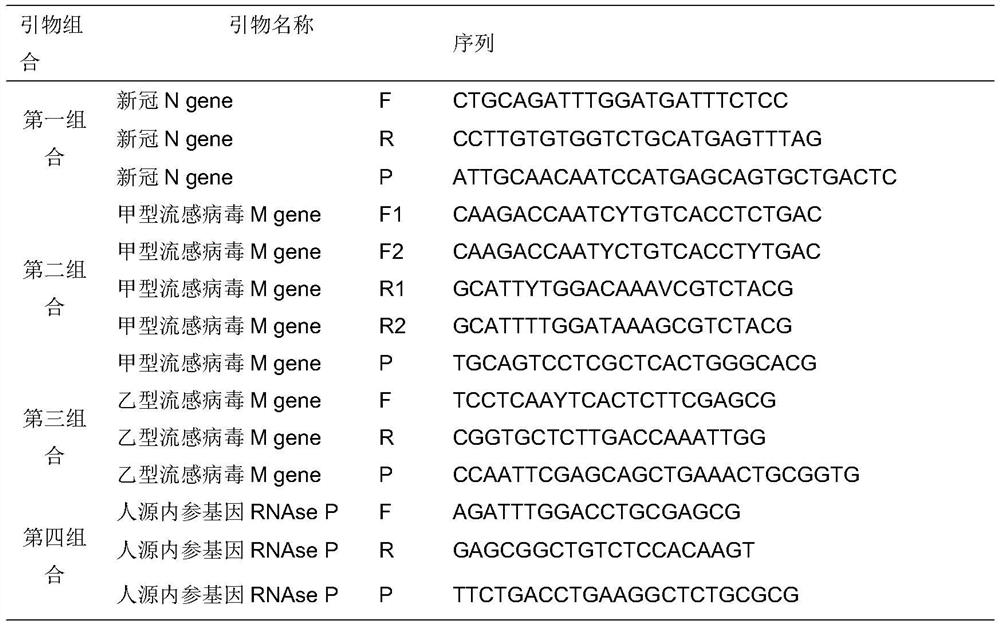
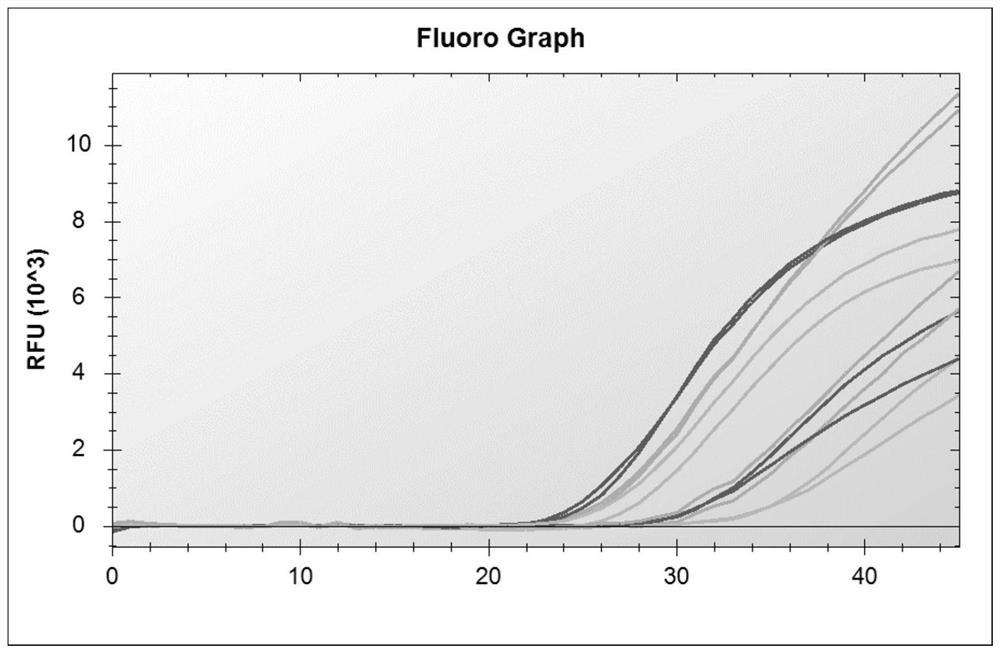
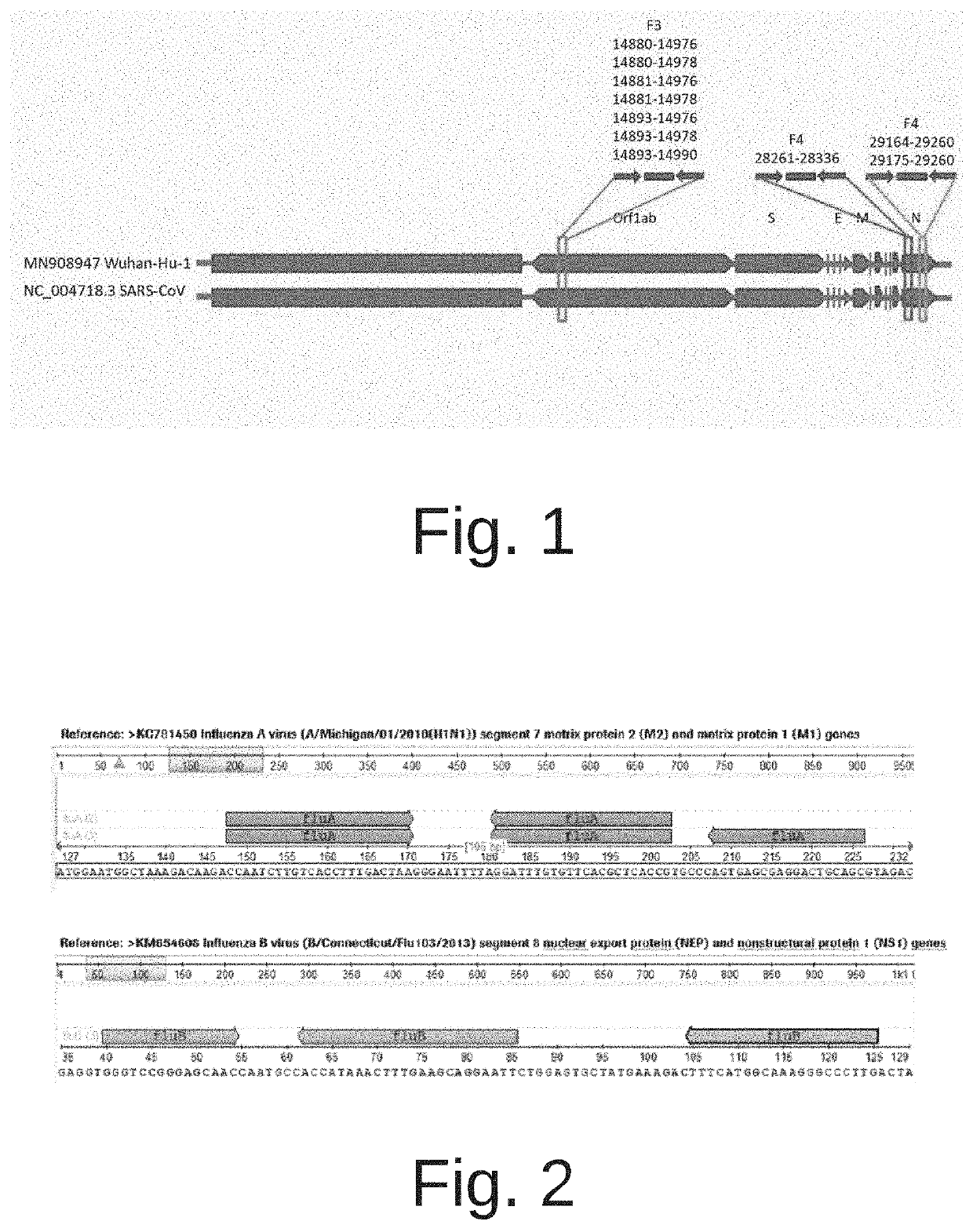
The invention discloses a probe and primer composition for simultaneously detecting human novel coronavirus, influenza A virus and influenza B virus. 10 specific primers and 5 probes are designed mainly according to genome specificity of M gene of influenza A virus, NS gene of influenza B virus, ORF1ab gene, N genes and internal standard gene RNaseP of novel coronavirus (2019-nCoV), and influenzaA virus, influenza B virus and novel coronavirus 2019-nCoV are distinguished according to amplification curves of different channels by utilizing a real-time fluorescence PCR amplification technology.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Corona virus disease 2019 screening method and system based on deep learning
PendingCN111653356AImprove accuracyFast diagnosisEpidemiological alert systemsCharacter and pattern recognitionDiseaseInfluenza A antigen
The invention discloses a corona virus disease 2019 screening method and system based on deep learning. The corona virus disease 2019 screening method comprises the steps: detecting a lung lesion areaof CT by using a deep learning detection model, and conveying the lung lesion area of CT into a three-classification network, wherein three classifications comprise COVID-19, influenza A and non-infection symptoms; and through calculation processing, outputting a CT diagnosis result and a disease probability. According to the method, based on deep learning, characteristics of CT images are automatically learned to distinguish COVIDI-19, influenza A and healthy people, so that the method is high in accuracy, the overall accuracy of current testing reaches 86.7%, the diagnosis speed is high, and only 30-60 S is needed for one set of CT according to different slice numbers; and a user can upload a CT image file and calculate and output a CT diagnosis result and the disease probability through the corona virus disease 2019 screening system, so that operation is convenient, the speed is high, and the detection rate of COVID-19 is greatly increased.
Owner:ZHEJIANG UNIV
Pharmaceutical Compositions Comprising A Pancreatic Enzyme Preparation With Viral Infectivity Reduced Below A Significant Level And Methods Of Preparing And Using The Same
InactiveUS20140017223A1Reduce alkylation activitySure easySsRNA viruses positive-sensePeptide/protein ingredientsAmylasePorcine circovirus
The present invention provides for pharmaceutical compositions comprising pancreatic enzyme preparations (PEPs) with viral infectivity reduced below significant levels and having high enzymatic activity. The PEPs can comprise lipases, proteases, amylases, non-enveloped viruses (e.g., porcine parvovirus (PPV), porcine circovirus type 2 (PCV-2), porcine encephalomyocarditis virus (EMCV)), and enveloped viruses (e.g., vesicular stomatitis virus (VSV), and influenza A (IFA)). The present invention also includes methods of treating pancreatic insufficiency by administering these pharmaceutical compositions and methods of making the same by treating the PEP with beta-propiolactone (BPL) to reduce viral infectivity.
Owner:APTALIS PHARMA CANADA
Detection of influenza virus
The present application describes methods for detecting influenza A and / or influenza B and / or distinguishing between pathogenic and seasonal influenza A subtypes. Many of these preferred formats employ pan-specific antibodies (i.e., that react with all or at least multiple strains within an influenza type) to detect presence of influenza A and / or influenza B and PDZ domains in combination with panspecific antibodies to influenza A to distinguish pathogenic and seasonal influenza A subtypes.
Owner:AVC ROYALTY FUND I
Target sequence for detecting RNA of influenza A H1N1 viruses and kit
InactiveCN102071260AMicrobiological testing/measurementMicroorganism based processesNegative controlReaction tube
The invention provides a target sequence for detecting the RNA of influenza A H1N1 viruses (2009) and a kit. The invention is characterized in that: specific primer probes designed on the basis of the two nucleotide target sequences of influenza A H1N1 viruses are adopted to detect the RNA of the influenza A H1N1 viruses (2009) by a one-step fluorescent reverse transcription-polymerase chain reaction (RT-PCR) in a single reaction tube, and detect the existence of the RNA of the influenza A H1N1 viruses in a sample by the fluorescent signal intensity and circulating threshold of an amplification template. The kit comprises the following components: PCR buffer solution, RT / Taq mixed enzymes, a negative reference and a positive reference. The kit is used by two steps, namely, a sample processing step and an amplification detection step. The operation of the kit is simple, convenient and quick, and the sensitivity of the kit is high. The kit can be widely used disease control and quick detection of influenza A H1N1 viruses (2009) in clinic.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Copper ion compositions and methods of treatment for conditions caused by coronavirus and influenza
Provided herein are formulations containing copper ions and methods of treating underlying infections and conditions caused by coronavirus, particularly COVID-19, and influenzas, particularly influenza A and / or influenza B using such formulations. Methods of treating the underlying viruses and their resultant conditions using topical copper ion treatments are provided. A topical treatment in its basic form comprises a biocompatible copper ion solution or suspension obtained by leaching of the copper ions from copper metal. The copper ion solution or suspension may be combined with various carriers to form the copper ion treatment including creams or solutions. Methods of making the copper ion solution or suspension from solid copper metal in a biocompatible solution are also provided.
Owner:CDA RES GROUP
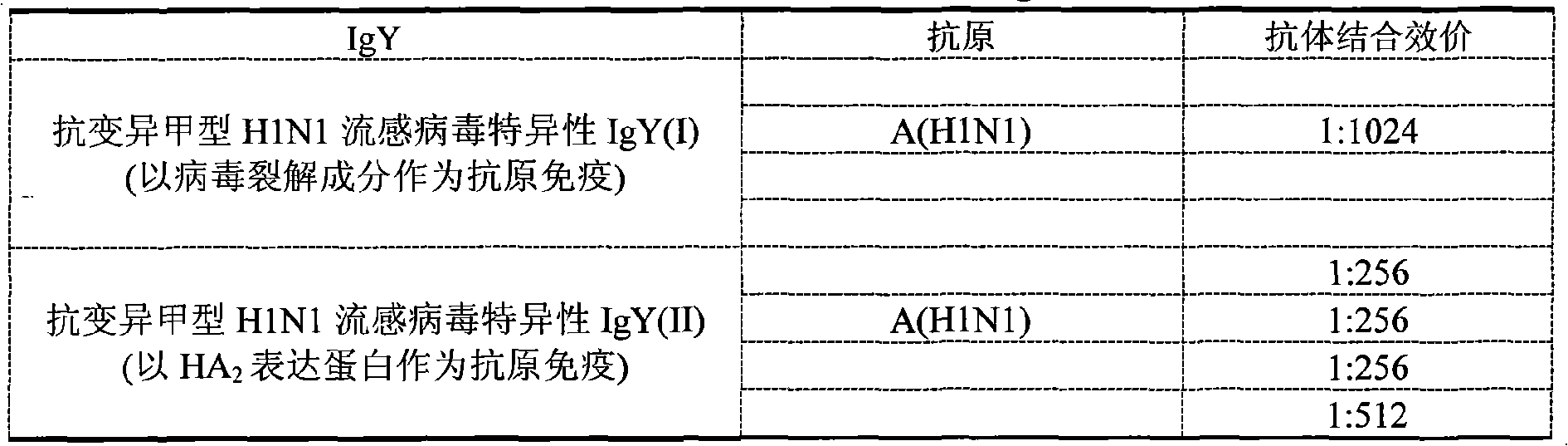
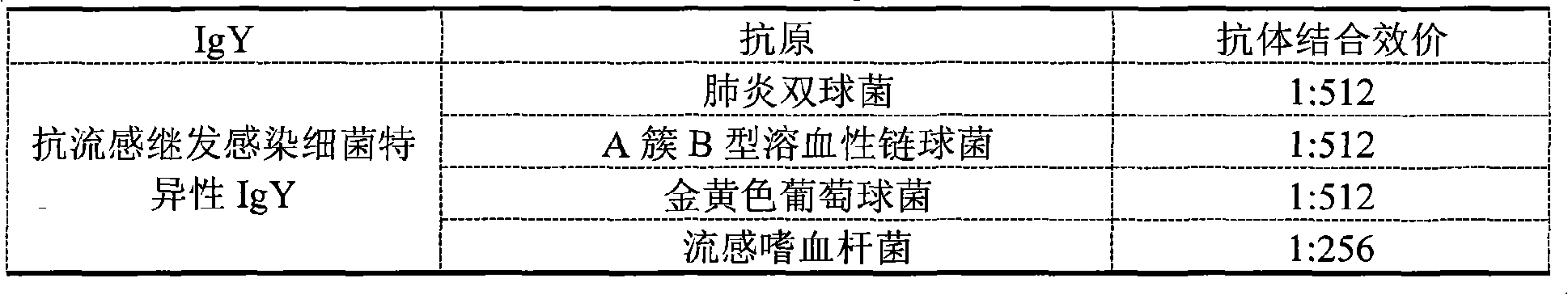
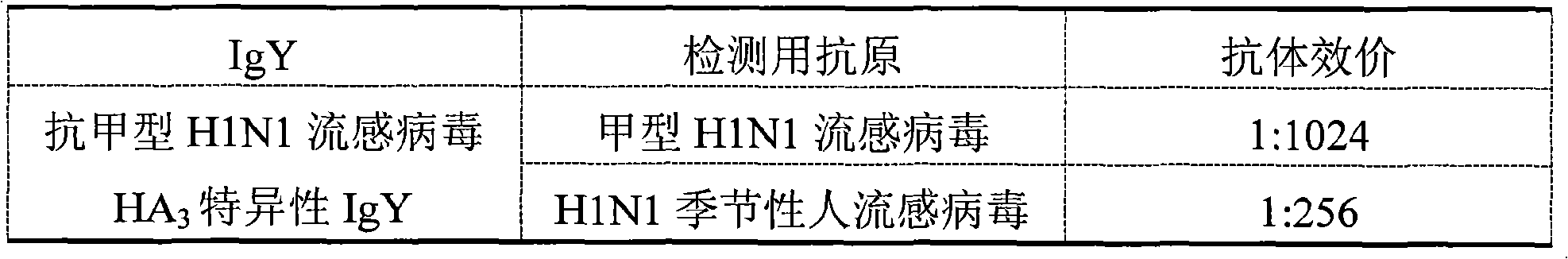
Preparation method of anti-influenza A H1N1 virus specific IgY and related formulation thereof
InactiveCN101555281AGood for immunomodulation and suppressionEliminate viral infectionsEgg immunoglobulinsImmunoglobulins against bacteriaAntigenBacilli
The invention discloses a preparation method of an anti-influenza A H1N1 virus specific IgY and related formulations thereof. The method comprises the steps of preparing a plurality of types of complex antigens aiming at influenza A H1N1, using the complex antigens for immunizing laying hen, utilizing the immune egg yolk for preparing specific IgY crude extract dry powder, conducting purification, removing all types of bacterial virus by filtration, and then preparing the anti-variant influenza A H1N1 virus specific IgY, anti-influenza secondary infection bacterial specific IgY, or anti-variant influenza A H1N1 virus specific complex IgY. In addition, the invention makes the anti-variant influenza A H1N1 virus specific IgY into nanoliposomes, and the obtained nanoliposome anti-variant influenza A H1N1 virus specific IgY enhances healing efficacy. The anti-influenza A H1N1 virus specific IgY can be utilized for preparing various formulations which then can prevent and teat the influenza A H1N1 of human and animals fundamentally; and the invention is more convenient, more economic, safer and more effective than the control method used at the present time.
Owner:深圳雅臣生物科技有限公司
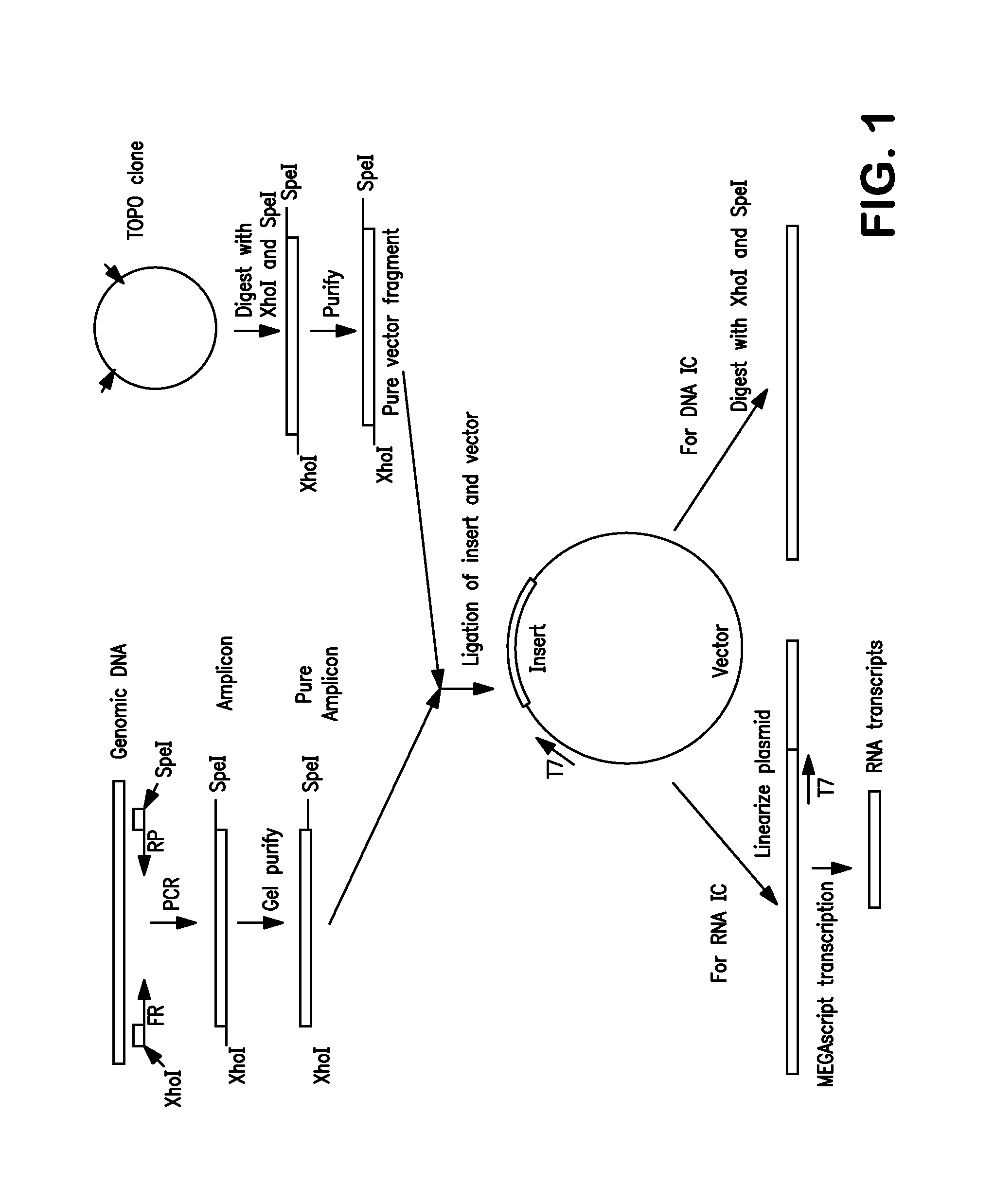
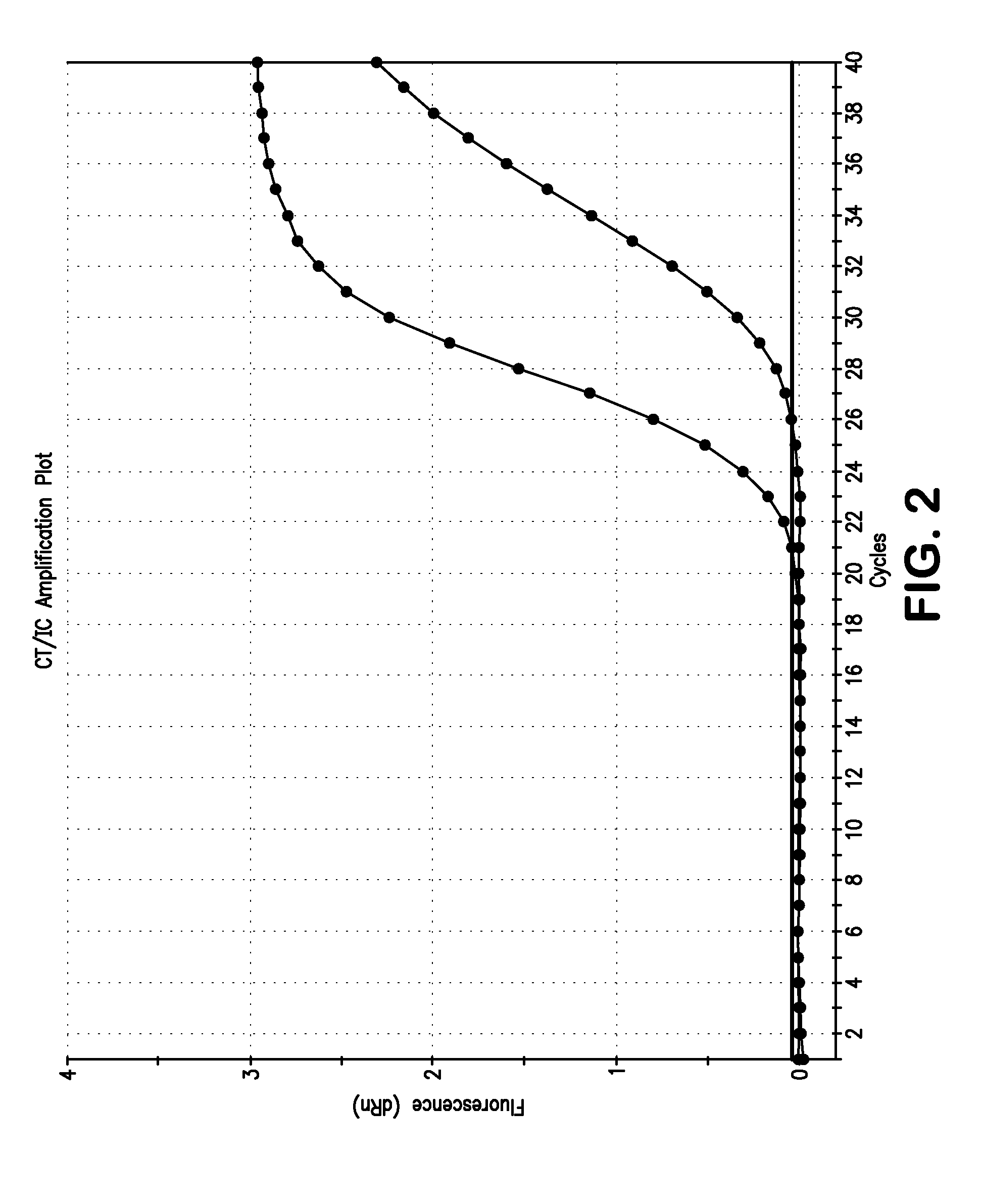
Non-Competitive Internal Controls for Use in Nucleic Acid Tests
InactiveUS20110003309A1Sugar derivativesMicrobiological testing/measurementNucleic acid testHuman immunodeficiency
Provided are non-competitive internal controls for use in nucleic acid tests (NATs), which are obtained from the organisms Methanobacterium thermoautrophicum (MET) and Zea mays (Corn). The non-competitive internal controls have utility in DNA and RNA NATs selected from Influenza A, Influenza B, parainfluenza viruses 1 to 4 (PIV-1 to PIV-4), respiratory syncytial virus type A (RSV A), RSV B, human metapneumovirus (hMPV), Chlamydia trachomatis (CT), and Neisseria gonorrhea (GC), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human Immunodeficiency Virus I (HIV-1), and Severe Acute Respiratory Syndrome (SARS).
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
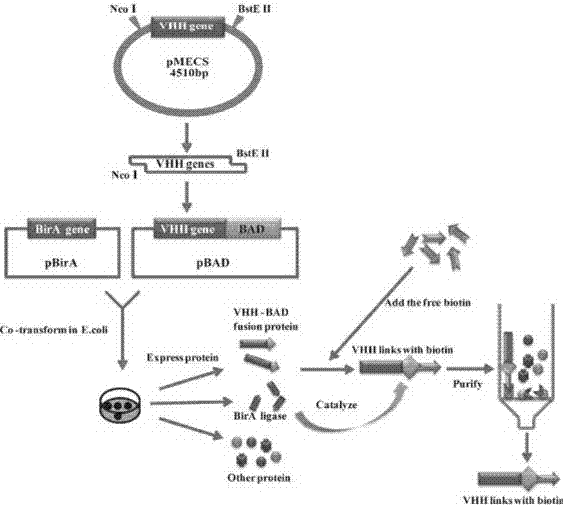
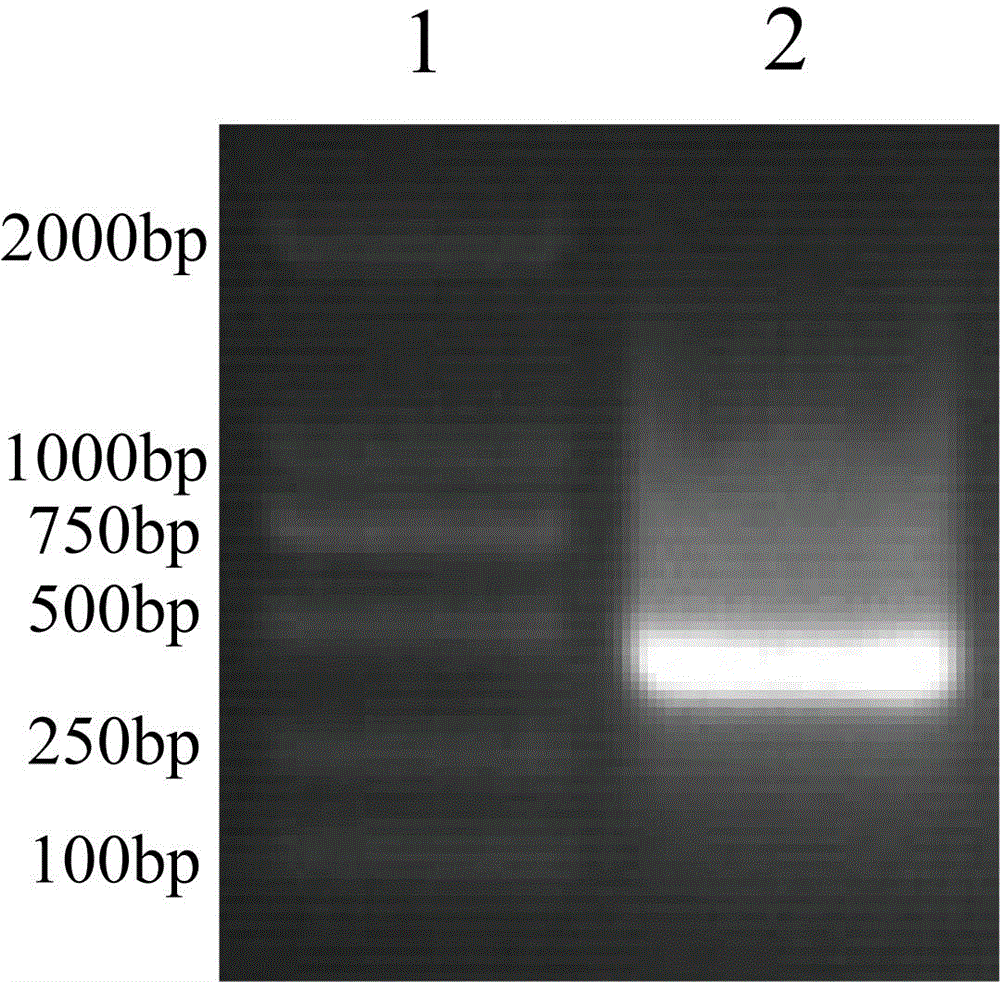
Nanometer antibody for specifically aiming at H3N2 influenza A virus and application thereof in diagnosis
ActiveCN103936852AQuality improvementStrong specificityBacteriaImmunoglobulins against virusesEscherichia coliAntigen
The invention discloses a nanometer antibody for specifically aiming at an H3N2 influenza A virus, a gene sequence for encoding the nanometer antibody and an application of the nanometer antibody in diagnosis, solving the problem that the production and detection of a high-purity high-affinity high-sensitivity antibody can not be obtained in the research process of the traditional H3N2 influenza A diagnostic reagent. The nanometer antibody for specifically aiming at the H3N2 influenza A virus, which is disclosed by the invention, can be used for fast detecting the antigen by being efficiently expressed in escherichia coli and being improved and is applied to the research of an H3N2 influenza A fast diagnosis kit.
Owner:北京科卫临床诊断试剂有限公司
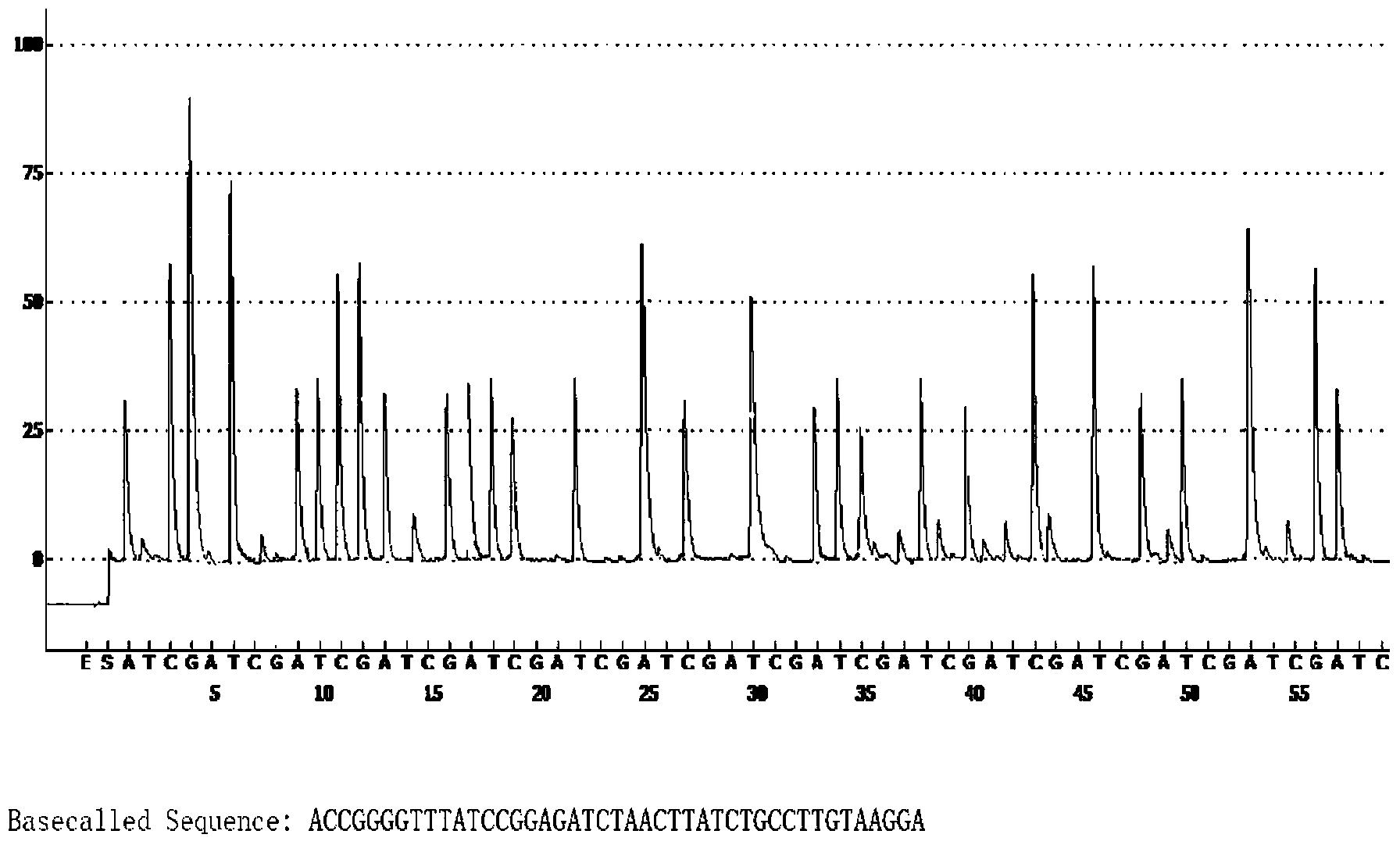
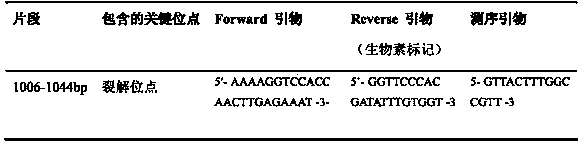
Method for detecting pathogenicity of influenza A (H1N1) virus based on pyrosequencing
The invention relates to a method for detecting the pathogenicity of an influenza A (H1N1) virus based on pyrosequencing. The method comprises the steps of carrying out RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification on a hemagglutinin (HA) gene of an H1N1 virus; carrying out pyrosequencing on a PCR amplification product to judge whether the cleavage site of the HA gene of the influenza A (H1N1) virus has a mutation, wherein the pyrosequencing is carried out in an SQA (Sequence Analysis) mode, and a nucleotide sampling sequence is shown as AGCT. The mutation of the cleavage site of the HA gene of the influenza A (H1N1) virus can be detected at rather high accuracy through comparing a detected result with the sequence of the cleavage site of the HA gene of a standard strain of the influenza A / H1N1 virus. The invention provides a simple and rapid experimental scheme for determining the virulence, pathogenicity and host range of the virus, the complex experiment steps related to complete genome sequencing are omitted, the variation direction of the virus can also be accurately mastered, the virus is conveniently monitored, an infection source is isolated, a transmission route is cut off, and the further development of an epidemic situation is stopped.
Owner:山东国际旅行卫生保健中心
Nanometer antibody for avian influenza virus H7N2, and application of nanometer antibody
ActiveCN106188283AGood linear relationshipBacteriaMicroorganism based processesEscherichia coliEpitope
The invention discloses a nanometer antibody in accordance with epitope of avian influenza virus H7N2, and a gene sequence encoding the nanometer antibody, and besides, further discloses a host cell capable of expressing the nanometer antibody for the influenza virus H7N2. The nanometer antibody for the avian influenza virus H7N2 and the encoding sequence of the nanometer antibody are obtained for the first time, the nanometer antibody can be efficiently expressed in escherichia coli, can specifically recognize the avian influenza virus H7N2, is high in detection sensitivity, presents favorable linear relationship when being used for detecting the avian influenza virus H7N2, and has the application value of diagnosing the avian influenza virus H7N2.
Owner:北京科卫临床诊断试剂有限公司
Kit for detecting nucleic acid of respiratory tract pathogen, detection method and application
ActiveCN111748651AEfficient detection/diagnosisRapid Test/DiagnosisMicrobiological testing/measurementAgainst vector-borne diseasesInfluenza A antigenNucleic Acid Probes
The invention provides a kit for detecting nucleic acid of respiratory tract pathogens. The kit comprises a CRISPR-Cas detection system: crRNA, Cas protein and a nucleic acid probe as shown in the invention. The invention also provides a detection method of the nucleic acid of the respiratory tract pathogen, a separated nucleic acid, crRNA, a primer pair and application thereof. The kit and the detection method disclosed by the invention do not depend on large instruments, enable the results to be directly observed by naked eyes, can realize detection under mild conditions, and are more convenient in detection; the kit and the detection method provided by the invention can be used for efficiently and quickly detecting / diagnosing respiratory pathogens (such as influenza A, influenza B and novel coronavirus); the kit is high in specificity and sensitivity, and can be used for detecting and screening respiratory pathogens, such as rapidly distinguishing various respiratory pathogens including influenza A, influenza B and novel coronavirus.
Owner:SHANGHAI TECH UNIV
AAV mediated influenza vaccines
A non-replicating recombinant adeno-associated associated virus (rAAV) having an AAV capsid having packaged therein a vector genome which comprises AAV inverted terminal repeat sequences and at least one nucleic acid sequence encoding four different immunoglobulin regions (a), (b), (c) and (d) is provided. The rAAV-expressed immunoglobulins are useful for providing passive immunization against influenza A and influenza B. Also described herein are compositions containing the rAAV. Methods of vaccinating patients against influenza are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Fully-premixed freeze-drying multi-fluorescent PCR detection kit for novel coronavirus, influenza A virus and influenza B virus and detection method thereof
PendingCN112760415ALow level of operation requiredQuick filterMicrobiological testing/measurementAgainst vector-borne diseasesFreeze-dryingRespiratory pathogen
The invention discloses a multi-fluorescent PCR rapid detection kit for novel coronavirus, influenza A virus and influenza B virus. The kit comprises freeze-dried solid RT-PCR Mix, liquid redissolution Buffer, freeze-dried solid positive control and freeze-dried solid negative control, wherein the freeze-dried solid RT-PCR Mix contains a primer group corresponding to primer sequences of a SARS-CoV-2 specific gene ORF1ab, an influenza A M gene, an influenza B M gene and a human reference gene RNAse P. The kit combines a multi-fluorescent quantitative PCR technology and a freeze-drying process, utilizes three pairs of special primers and human reference genes to amplify specific sequences of three pathogens in vitro, and performs real-time detection in combination with a fluorescent probe. The detection method is simple and convenient to operate, has low requirements for the operation level of detection personnel, and can detect three common respiratory pathogens at a time, the detection time and the detection cost are greatly saved, rapid screening of large-batch samples is realized, the whole detection process only takes 40 minutes to 1 hours, and results are accurate and reliable.
Owner:青岛巴特菲科技发展有限公司
Capsule for treating H1N1 influenza A and preparation method of capsule
InactiveCN104667242AEasy to acceptStrong targetingAntiviralsCapsule deliveryGelsemium elegansTreatment effect
The invention discloses a capsule for treating H1N1 influenza A and a preparation method of the capsule. The capsule comprises the following active ingredients: gelsemium elegans, ginger, pteris multifda poir, salomonia cantoniensis lour, hispid arthraxon, white strawberry, Chinese angelica, small-leaved premna roots, herba schizonepetae, cowry shells, litsea cubeba, dandelion, notopterygium roots, Indian lettuce roots, potentilla chinensis and myrobalan. A preparation process of the capsule comprises the steps of grinding, mixing to form gelatin liquid, preparing a soft film, pressing into a finished product and the like. Compared with the prior art, the capsule has effects of strengthening body resistance, eliminating pathogenic factors, relieving an exterior syndrome, eliminating dampness, clearing a throat, clearing away heat, diffusing a lung, clearing away toxic materials and strengthening a spleen, and has better pertinency, strong practicability and an ideal treatment effect.
Owner:JINAN HONGFEI BIOTECH
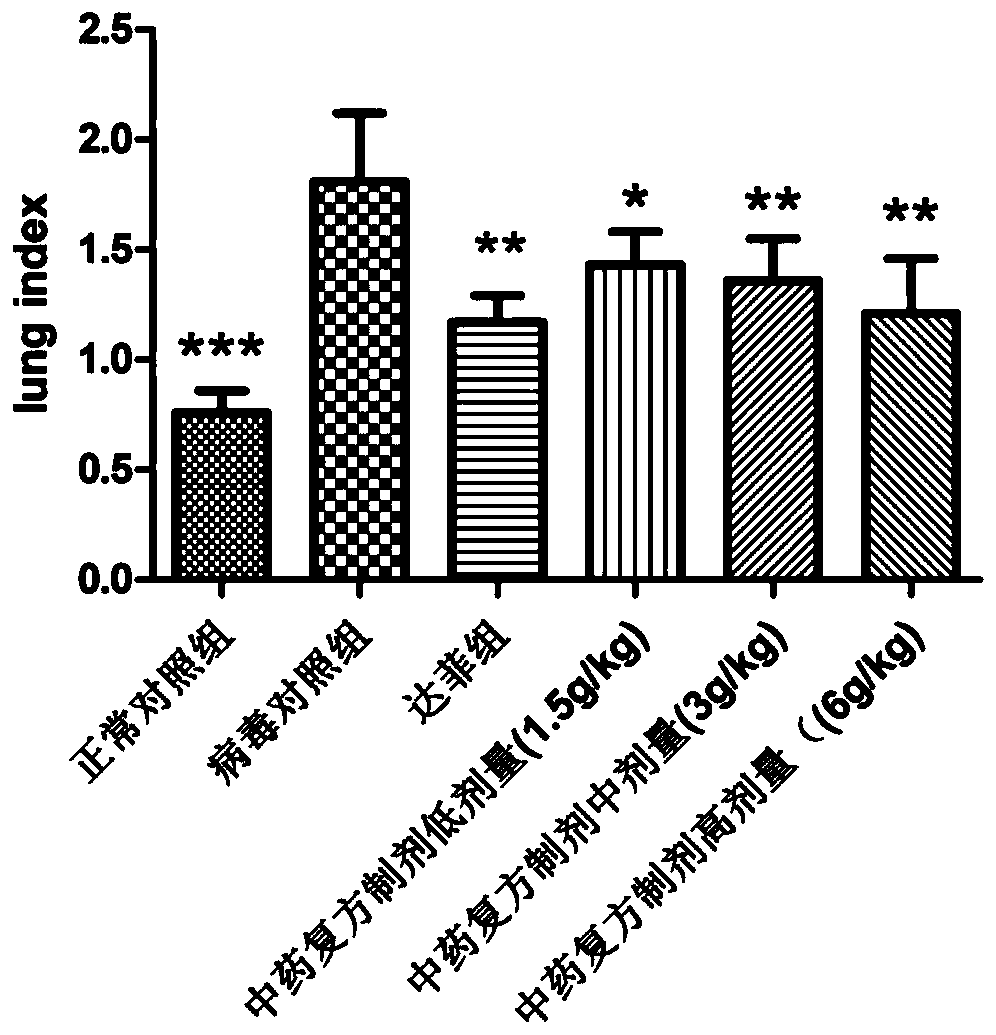
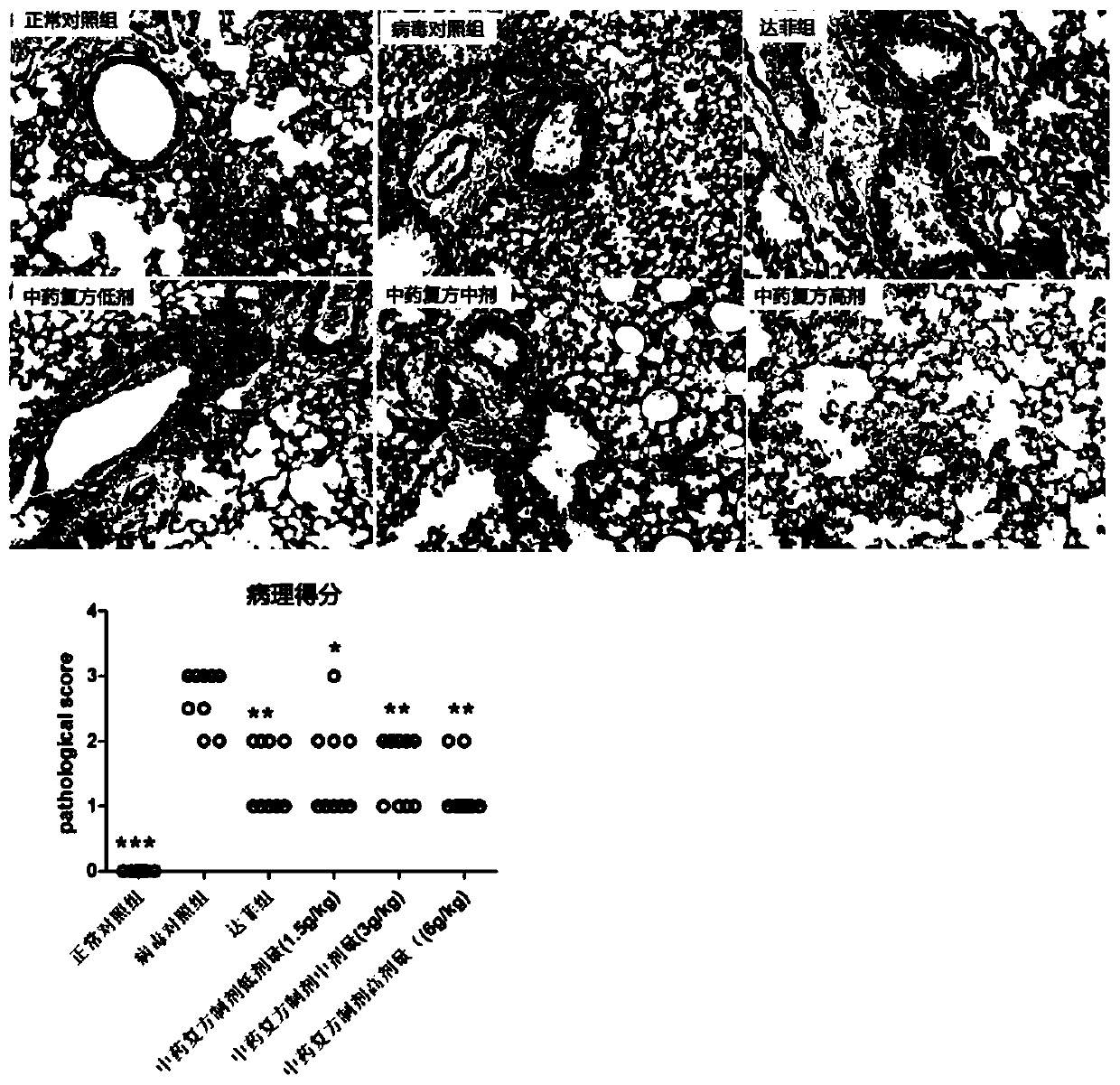
Traditional Chinese medicine composite preparation and preparation method and application thereof
ActiveCN110801486AImprove survival rateReduce inflammatory damageAntiviralsRespiratory disorderLicorice rootsWolfiporia extensa
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Polypeptides for treating and/or limiting influenza infection
The present invention provides polypeptides according to the general formulas disclosed herein, which recognize and are strong binders to Influenza A hemagglutinin and can be used, for example, to treat and / or limit development of an influenza infection. The present invention further provides isolated nucleic acids encoding the polypeptides of the invention, recombinant expression vectors comprising the nucleic acids encoding the polypeptides of the invention operatively linked to a suitable control sequence, and recombinant host cells comprising the recombinant expression vectors of the invention. The present invention also provides antibodies that selectively bind to the polypeptides of the invention, and pharmaceutical compositions comprising one or more polypeptides according to the invention and a pharmaceutically acceptable carrier. Additionally, the present invention provides methods for treating and / or limiting an influenza infection, methods for diagnosing an influenza infection, or monitoring progression of an influenza infection, methods for identifying candidate influenza vaccines, and methods for identifying candidate compounds for treating, limiting, and / or diagnosing influenza infection.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION
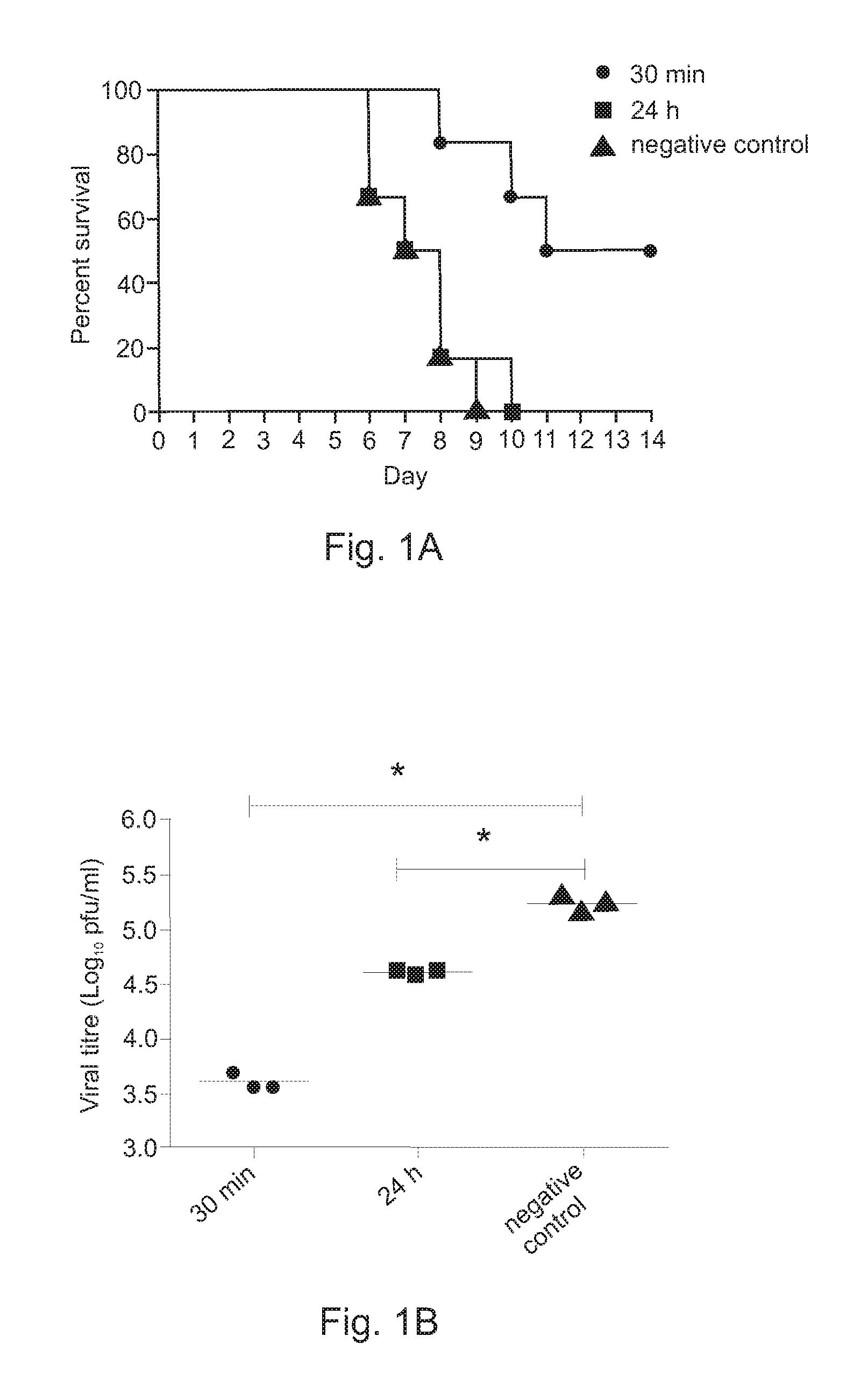
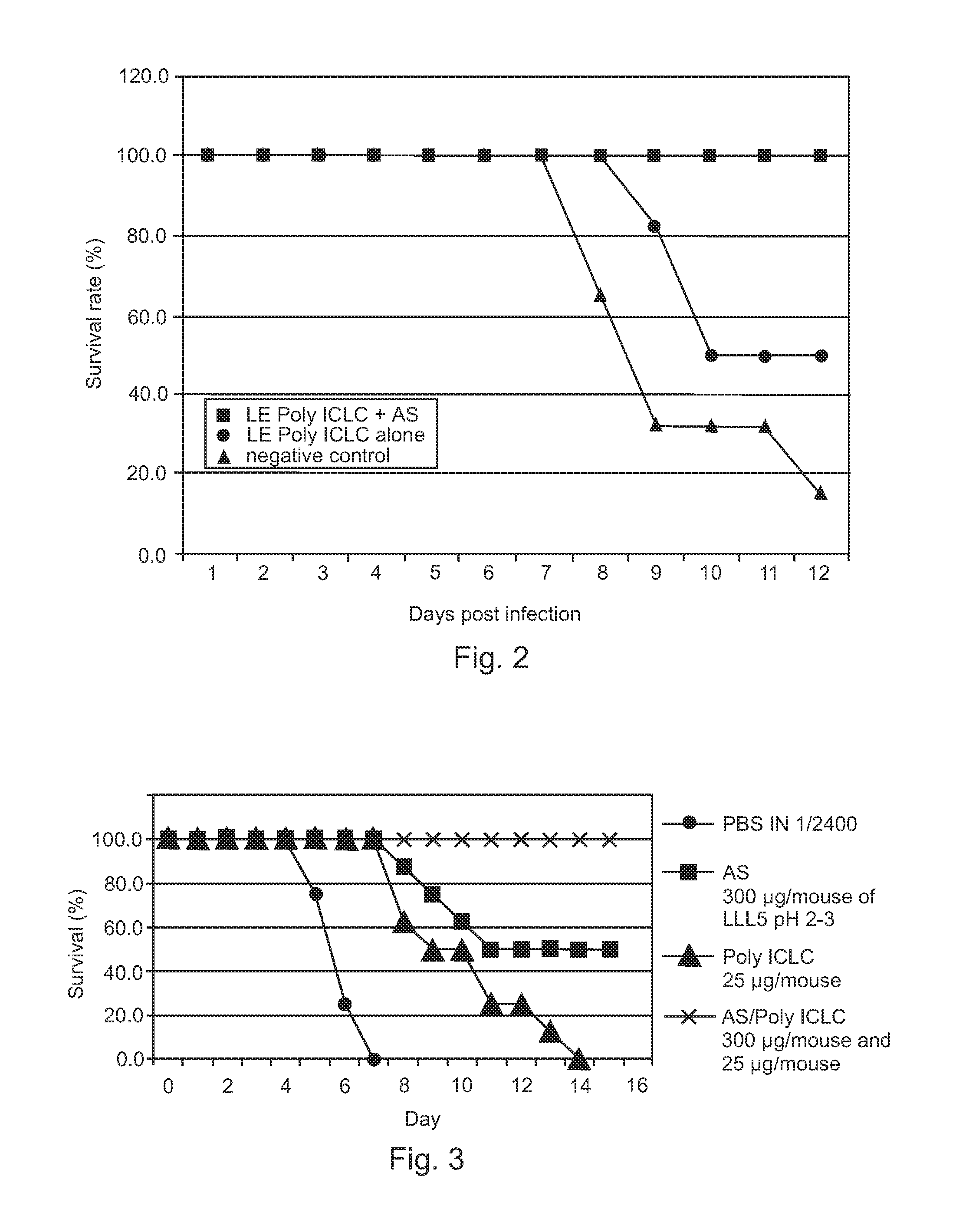
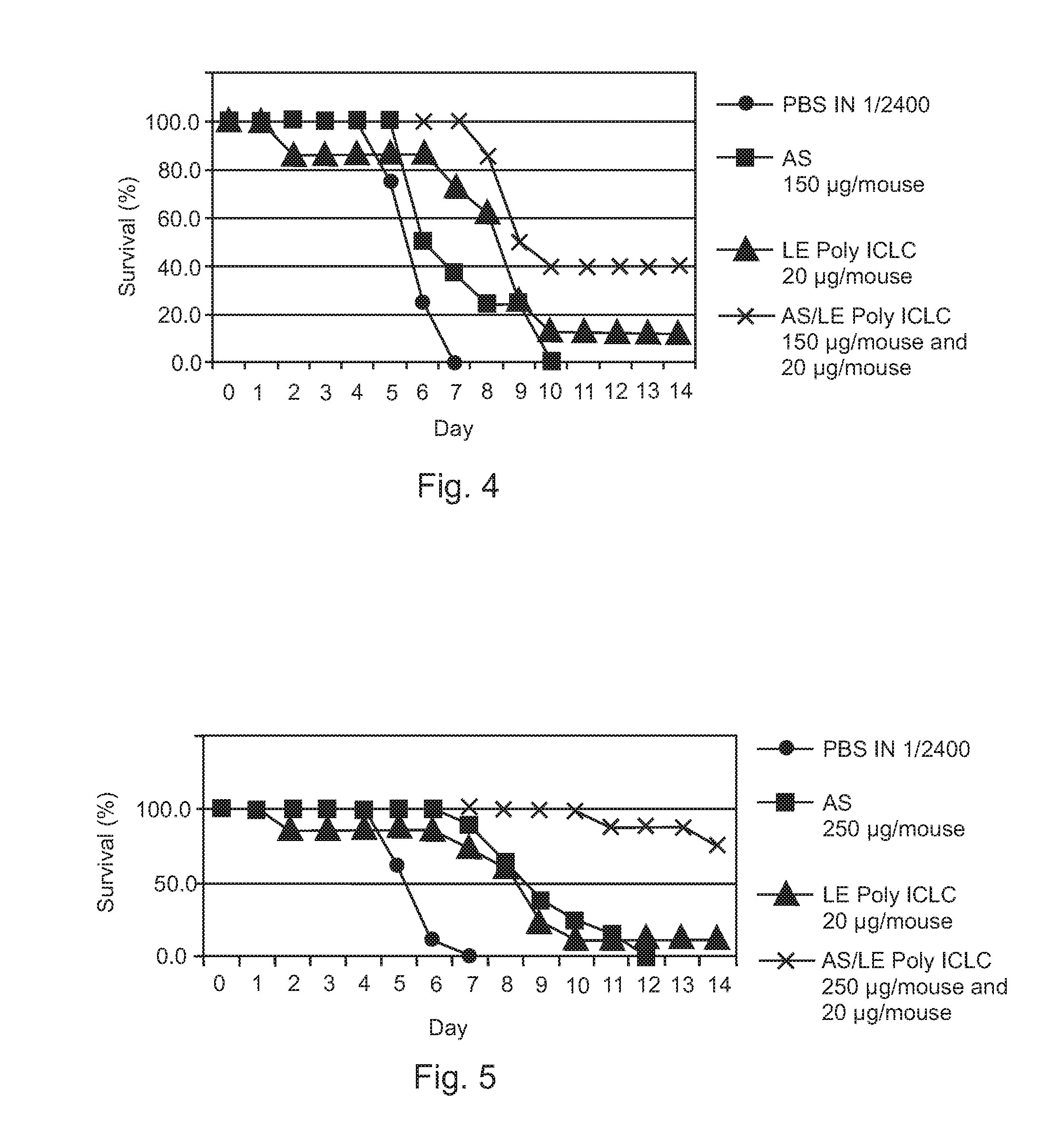
Post-exposure therapy of influenza a infections
InactiveUS20120195960A1Excellent survival prospectGood initiativeSsRNA viruses negative-senseOrganic active ingredientsH5N1 virusLiposome
Poly ICLC or liposome-encapsulated Poly ICLC (LE Poly ICLC) in combination with antisense oligonucleotides (AS) act synergistically in post-exposure prophylaxis or therapy of influenza infections, especially H5N1 virus infections.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
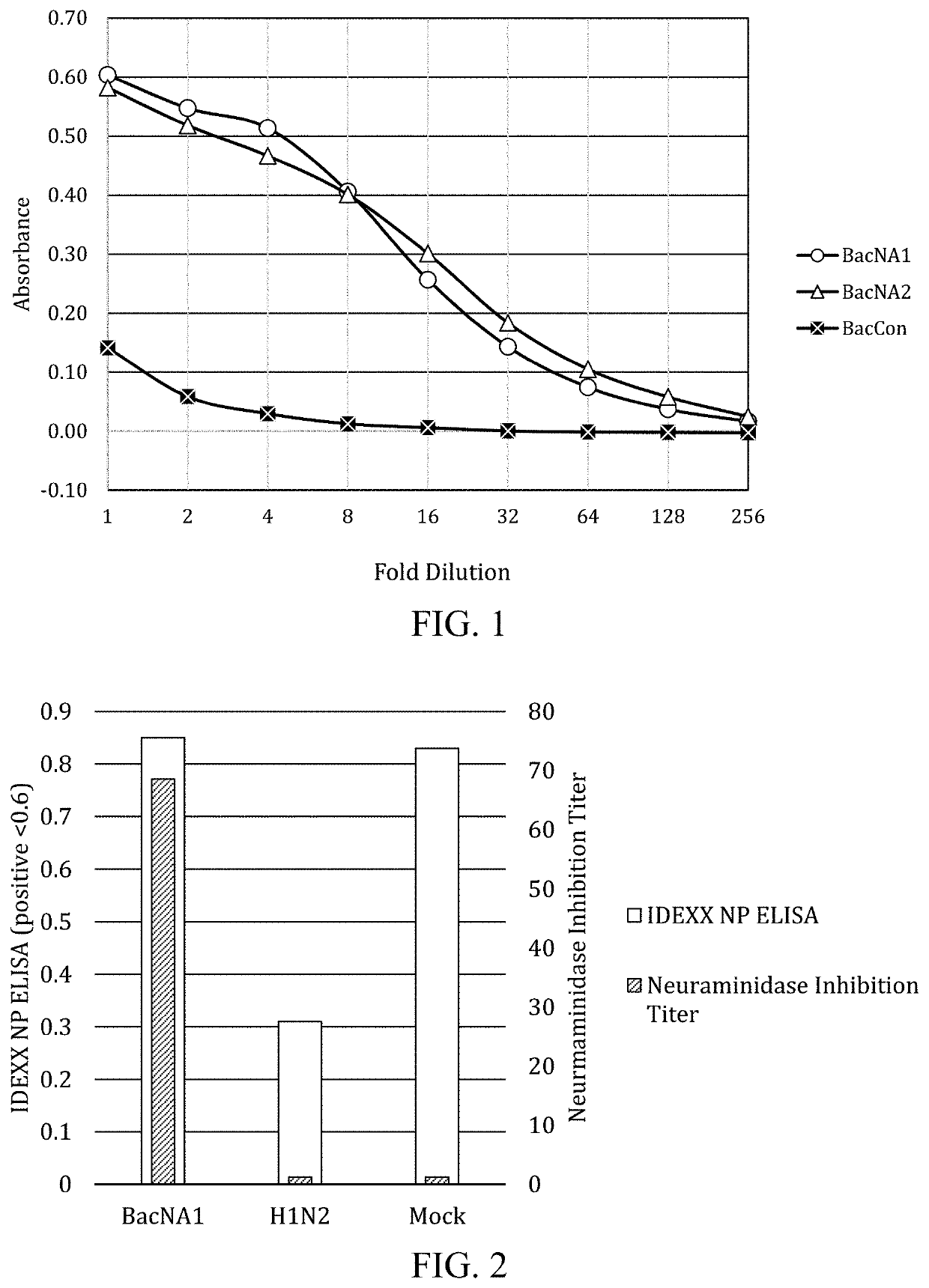
Universal influenza vaccine
ActiveUS11351241B2SsRNA viruses negative-senseViral antigen ingredientsAdjuvantAntiendomysial antibodies
Immunogenic compositions for inducing a universal immune response to influenza, and particularly influenza A, by eliciting anti-neuraminidase antibodies which provide protection against heterologous influenza infection. Compositions comprising recombinant baculovirus expression vectors expressing neuraminidase in cultured insect cells dispersed in a pharmaceutically-acceptable carrier comprising insect cell culture media, and optional adjuvant. Methods of inducing immune responses against influenza, and particularly influenza A, by eliciting anti-neuraminidase antibodies in a host animal susceptible to infection.
Owner:CAMBRIDGE TECH LLC
Multi-primer and kit for rapidly detecting influenza A, influenza B and novel coronavirus
ActiveCN112359145AStrong specificityNo cross reactionMicrobiological testing/measurementAgainst vector-borne diseasesInfluenza A antigenPcr method
The invention provides multiple primers and a kit for rapidly detecting influenza A, influenza B and novel coronavirus and relates to the field of molecular biological detection technology and molecular diagnosis. According to the real-time fluorescence PCR method based on reverse transcription heat convection, a specific multiple rapid diagnosis system is developed for respective conserved regions of influenza A virus, influenza B virus and novel coronavirus, and meanwhile, human endogenous genes are detected for sample quality control. The multiple primers and the kit are good in specificityand high in sensitivity, have the characteristics of simplicity, convenience, quickness and practicability, and meet detection requirements of influenza A viruses, influenza B viruses and novel coronaviruses in entry and exit and on-site environments.
Owner:广州领上源生物科技有限公司
COMPOSITIONS AND METHODS FOR THE SIMULTANEOUS DETECTION OF INFLUENZA A, INFLUENZA B, AND SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-CoV-2)
PendingUS20220042117A1Enlarge regionMinimizing the potential for cross-contaminationMicrobiological testing/measurementInfluenza A antigenCare setting
Methods for the rapid detection of the presence or absence of SARS-CoV-2 in biological or non-biological samples are described. These methods are adapted to be performed rapidly in a point-of-care setting. The methods can include performing an amplifying step, a hybridizing step, and a detecting step. Specifically, primers and probes targeting SARS-CoV-2 are provided that are designed for the detection of this target. Additionally, kits and reaction vessels containing primers and probes targeting SARS-CoV-2 are provided. Additionally, methods, kits and reaction vessels for the simultaneous rapid detection of the presence or absence of SARS-CoV-2, influenza A, and influenza B in biological or non-biological samples are described.
Owner:ROCHE MOLECULAR SYST INC
Cells for detection of influenza and parainfluenza viruses
InactiveUS6991899B2Improve productivityHigh sensitivityHydrolasesMicrobiological testing/measurementAntigenAntiviral drug
The invention provides cell lines which are useful for the rapid detection and production of influenza and parainfluenza viruses. In particular, the invention relates to transgenic mink lung cells which show increased sensitivity to infection by influenza A, influenza B, or parainfluenza 3 viruses, or which are capable of enhanced productivity of infectious virions. The invention is suitable for use in culturing clinical influenza and parainfluenza virus isolates and for the production of influenza and parainfluenza virus for vaccine formulations, as antigen preparations for diagnostic applications, and for screening antiviral drugs.
Owner:UNIVERSITY HOSPITALS OF CLEVELAND CLEVELAND
Antibody pairs for use in a rapid influenza a diagnostic test
Owner:GLAXOSMITHKLINE CONSUMER HEALTHCARE HLDG US
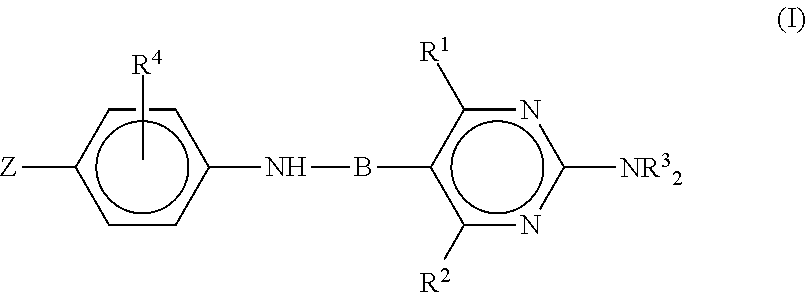
Anti-influenza compounds
The present invention provides pyrimidinyl compounds of formula (I) and pharmaceutically acceptable salts thereof. These compounds may be used for the inhibition of influenza. In particular, the compounds of the invention may be used for the treatment or prophylaxis of influenza A, most particularly H1N1 or H5N1 influenza. The compounds of the invention can also be used for the treatment or prophylaxis of a disease caused by Vibrio cholerae, Clostridium perfringens, Streptococcus pneumoniae, Arthrobacter sialophilus, an orthomyxovirus, a paramyxovirus, a parainfluenza virus, mumps virus, Newcastle disease virus, fowl plague virus or Sendai virus.
Owner:VERSITECH LTD +1
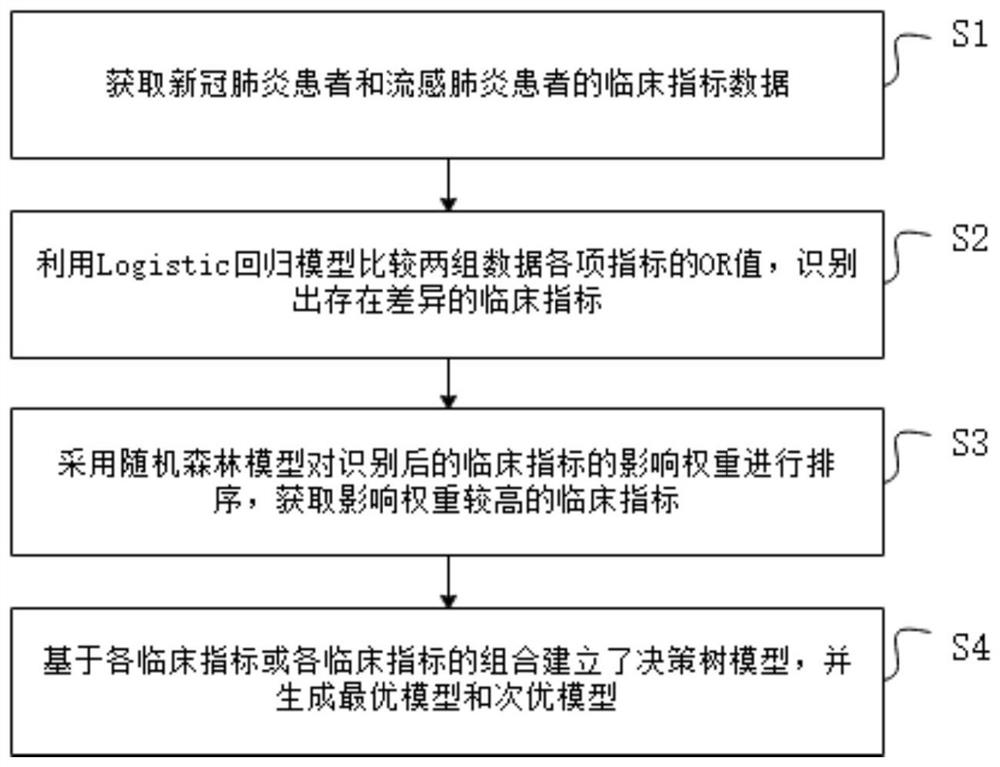
COVID-19 and influenza pneumonia preliminary screening method and system and equipment
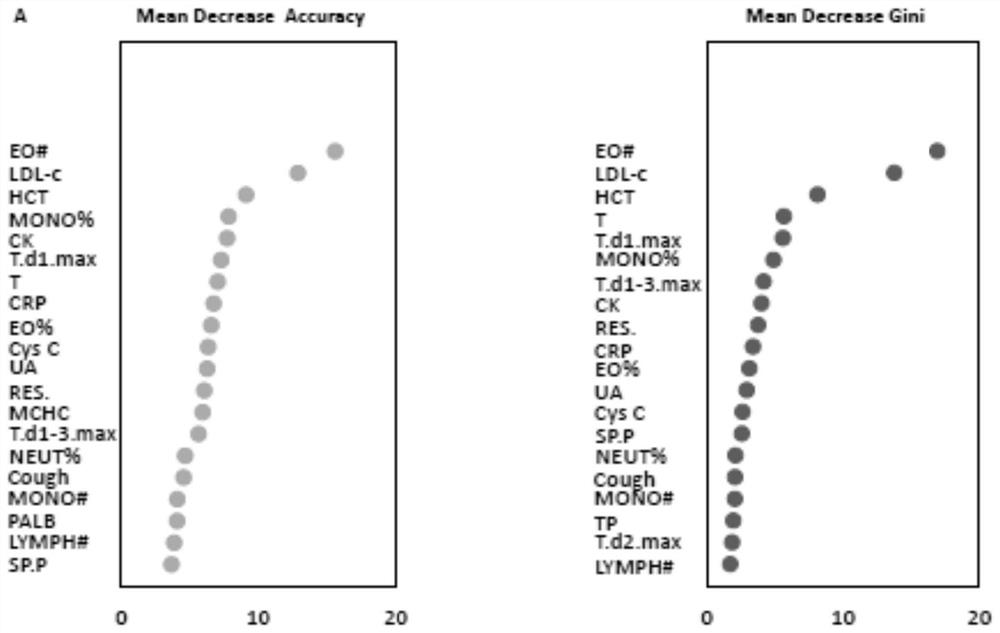
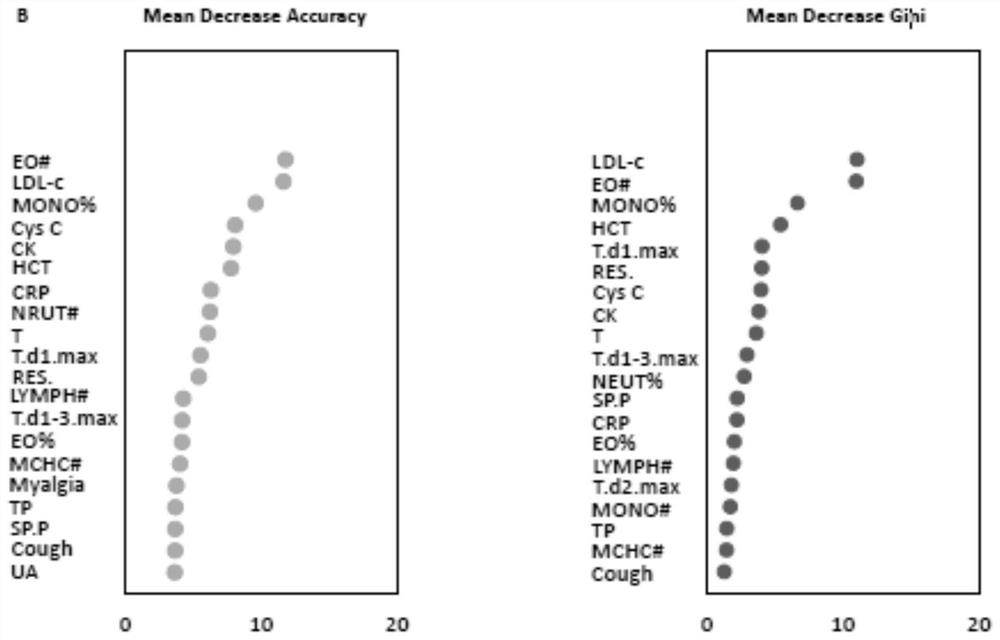
The invention provides a COVID-19 and influenza pneumonia preliminary screening method and system and equipment. Based on clinical symptom signs, blood routine examination indexes and blood biochemical examination indexes of a patient, a tool capable of rapidly identifying and distinguishing the COVID-19 and the influenza A pneumonia is constructed; and finally, an optimal model and a suboptimal model are constructed according to the diagnosis capability. The optimal model has a rapid and accurate preliminary screening capability; the suboptimal model provides a preliminary screening identification tool constructed based on personal symptom signs and blood routine examination when underdeveloped area medical resources cannot undertake blood biochemical detection, so that assistance is provided for effective investment of medical and public health prevention and control resources.
Owner:李智敏 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com